User login
A new paradigm for pain?
The care of people with pain has been wrought with ineffective and unnecessary treatment, including the misuse of opioids, largely because we do not have an accurate conceptualization of pain. The absence of animal and human models of central nervous system (CNS) pain processing ensures that our understanding of pain will remain incomplete for the foreseeable future, but enough evidence exists to help family physicians develop an understanding of pain that goes beyond what we learned in medical school and that can help us more effectively treat patients with pain.
In this review, we will briefly discuss the established concepts of nociceptive and neuropathic pain. And then, with those concepts in mind, we will explore a third type of pain that for lack of a better term, we will call “pain for psychological reasons.” We hypothesize that this pain may be the consequence of changes in nervous system function that arise from developmental trauma, other traumatic experiences in a patient’s life, or mental health disorders. It is this third type of pain that may offer us insights into conditions such as fibromyalgia.
While we do not yet have validated diagnostic criteria for this third type of pain, we believe that there is enough information to present initial criteria so that one may distinguish it from nociceptive and neuropathic pain.
Nociceptive and neuropathic pain: The current paradigm
Nociceptive pain. The sensory pain experience, or nociceptive pain, is produced by noxious stimuli that either damage, or are capable of damaging, tissues (eg, burns, cuts, fractures, inflammation, and increased pressure in a hollow viscus). Noxious stimuli are detected at the molecular level by specific pain sensory receptors embedded in our tissues called nociceptors.
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps—transduction, transmission, modulation, and perception—which are described in “From periphery to brain: The process of nociceptive pain.”1-4
Neuropathic pain. While nociceptive pain can be easily traced from a peripheral nociceptive fiber to the brain and typically resolves when the nociceptive stimulus stops, neuropathic pain (NPP) results from changes to the function of the nervous system and is typically caused by injury to the nerves. Such changes, referred to as neuronal sensitization, may not quickly resolve, as is the case with postherpetic neuralgia. In fact, the changes can become permanent. NPP fundamentally differs from nociceptive pain because it results from changes in the central processing of pain that can lead a person to perceive pain sensations even in the absence of tissue pathology.
Common causes of NPP that persists even after tissue damage has healed include trauma (eg, amputation of a limb), ischemia (eg, pressure palsy), disease (eg, the metabolic injury of diabetes or the injury caused by a shingles infection), and drug treatment (eg, chemotherapy). The underlying mechanisms of NPP and the neuronal plasticity (the ability of the nervous system to rewire itself) that initiate and then maintain NPP are important areas of active research that may eventually lead to the development of more effective treatments.
Timing is critical. Neuroplastic changes in the nervous system following nerve injury are time-dependent. Synaptic plasticity can occur within seconds to minutes, while cellular plasticity occurs within hours to days. Synaptic and cellular plasticity happen relatively fast and may be reversible.
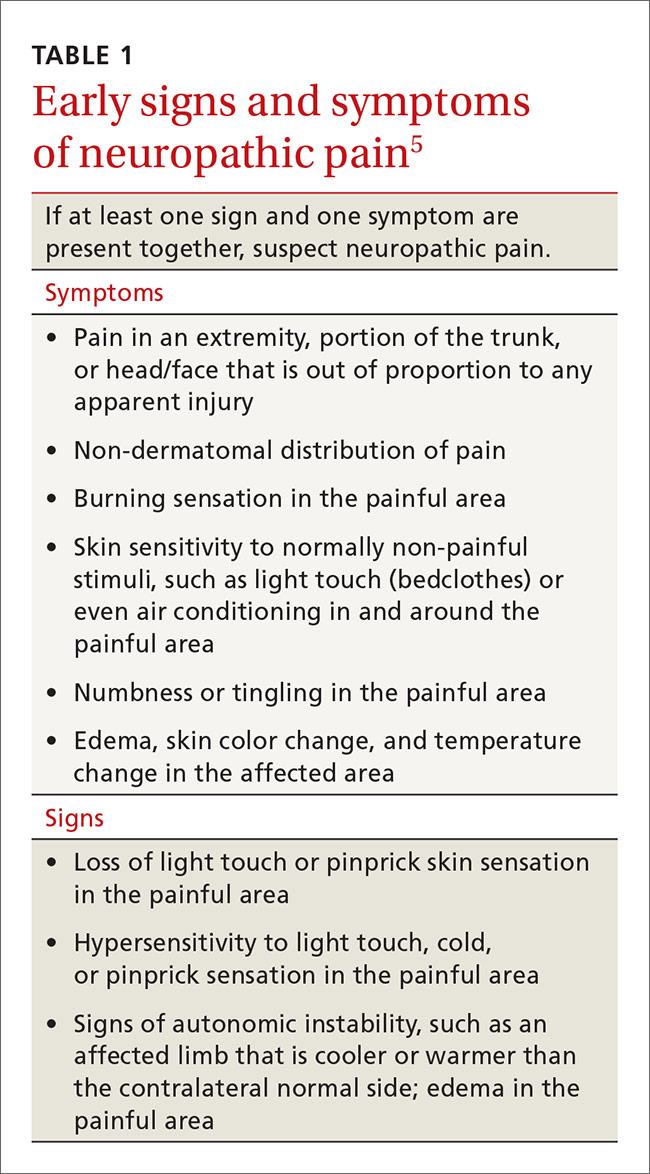
In contrast, systems plasticity (when new CNS neuronal connections are formed in response to nerve injury) takes place over the months and years following nerve injury and is often irreversible. When we recognize NPP and intervene before system neuroplastic changes occur, it may be possible to prevent pain from becoming chronic (TABLE 15). In cases of nerve injury, researchers have long suspected that early and aggressive pain treatment within the first few months that may include sympathetic and peripheral neural blockade reduces the likelihood that the patient will have chronic pain.6,7
It’s time to update our understanding of pain
The International Association for the Study of Pain (IASP)—a group of health care providers, scientists, and policymakers seeking to improve pain relief worldwide—notes in its definition of pain that the complaint, “I hurt” does not necessarily imply that there is a painful stimulus in the form of tissue injury.8 Yet most of us have been taught to think of pain solely as the result of tissue pathology, and we assume that emotional factors merely modify how the physical damage is perceived. This traditional concept of pain is incomplete. It leads clinicians to misdiagnose the cause of pain, initiate expensive and unnecessary treatment, engage in well-meaning but misguided prescribing behavior, and miss opportunities to help patients.
SIDEBAR
From periphery to brain: The process of nociceptive pain1-4
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps:
In transduction, nociceptors containing special molecular proteins respond to noxious modalities, such as thermal, mechanical, or chemical stimuli, and trigger nerve impulses in the nociceptive nerve fibers (nerves dedicated to pain sensation).
During transmission—the second stage of the process—information from the nociceptors in the periphery (skin, muscle, viscera) is relayed to the spinal cord mainly by 2 types of nociceptive neurons: C-fibers and A delta (Aδ) fibers. Both approach the spinal cord in a peripheral nerve and then enter the spinal cord in the dorsal root entry zone. Because Aδ fibers are thinly myelinated, they send impulses faster than unmyelinated C fibers. This is why when injury occurs, we first feel sharp, acute pain that then slowly diffuses into a duller ache.
Once the incoming signal is transmitted to the CNS at the spinal cord, primary afferent neurons synapse on second order neurons. From there, information travels on to the thalamus via multiple neurons that have the capacity to change their response patterns when activity of nociceptive fibers is sustained (as occurs in the setting of a tissue or nerve injury and perhaps in the setting of psychological trauma). This is known as modulation of the incoming nociceptive stimulus. During this step of the process, stimuli can be amplified, suppressed, or even transformed from one type to another (eg, a light touch can be modulated in such a way that it will be perceived as a burning sensation). Also, it is this step that is affected by many medications, by intrathecal drug infusions, and by spinal neurostimulators.
In perception, the thalamus then directs the pain sensation to multiple brain centers. At this step, the stimulus is finally consciously perceived as pain by the individual.
Cortical pain circuits can be activated without physical input (ie, no tissue damage, noxious stimuli, or nerve injury). This becomes important in understanding pain syndromes, such as fibromyalgia.
Pain in the absence of any pathophysiologic cause or injury
The clinician’s search for a pain diagnosis is typically predicated on the notion that there must be an underlying tissue injury of severity equal to the severity of the patient’s pain complaints. This approach to a pain evaluation rests on 2 assumptions that are not true for all patients:
- Pain is simply a sensory experience that is always caused by tissue damage of some type.
- The severity of the pain experienced by a patient should be tightly bound to the severity of the pain stimulus (ie, tissue damage).
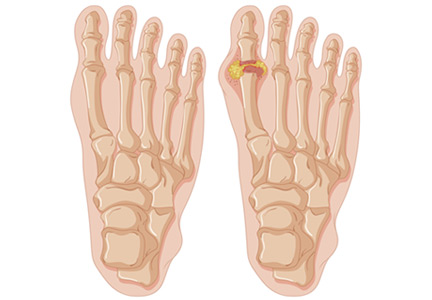
These assumptions are true of acute nociceptive pain, they may or may not be true for NPP, but they do not apply to the third type of pain—pain for psychological reasons. While tissue pathology in humans and animals with nociceptive pain is usually visible, measurable, and correlates with observed pain behaviors, the damage to nerve tissue and the ensuing changes in nervous system function with NPP are not always visible or able to be imaged. These changes produce pain that can appear more severe than expected based on a brief exam. Some of the time, however, characteristic symptoms and physical signs of NPP will be present, and perhaps electrodiagnostic or other tests will be abnormal, thus providing some objective sense of changes in nervous system function.
In contrast, pain behavior due to the third type of pain usually appears very much out of proportion, and unbound to, tissue pathology. Furthermore, the patient’s pain behaviors often reflect heightened emotional pain processing (TABLE 29). The resulting emotionally charged presentation can be alarming and suggestive of extreme tissue injury, but there may be absolutely no evidence of tissue injury or pathology.

Functional change in the CNS
There is evidence from experimental studies that psychologic factors change nervous system function. In one review, the authors concluded, “Pain…can vary widely between people and even within an individual depending on…the psychological state of the person.”10 In a second review, the authors concluded that our emotional state has an enormous influence on pain; a negative emotional state increases pain, whereas a positive state lowers pain.11
But can psychological factors induce long-term changes in nervous system function analogous to the systems neuroplasticity responsible for irreversible changes in NPP? And can psychologically induced changes in nervous system sensory processing lead to pain without any tissue or nerve damage?
We theorize that a functional change in the CNS can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents). (More on this in a bit.) When such changes occur, mildly painful stimuli are amplified and processed through overly sensitized, dysregulated, ramped-up emotional and somatosensory pain circuits in the brain. This is analogous to the functional changes in the nervous system that occur with NPP; however, when the nervous system changes are due to psychological factors, there may be no tissue or nerve injury.
Childhood trauma influences adult pain. One of the more compelling narratives emerging in health care has to do with the influence that childhood developmental trauma can have on health, including pain. In his chapter on the impact of early life trauma on health and disease, Lanius states:12
“Women were 50% more likely than men to have experienced 5 or more categories of adverse childhood experiences. We believe that here is a key to what in mainstream epidemiology appears as women’s natural proneness to ill-defined health problems like fibromyalgia, chronic fatigue syndrome, obesity, irritable bowel syndrome, and chronic non-malignant pain syndromes. In light of our findings, we now see these as medical constructs, artifacts resulting from medical blindness to social realities and ignorance of the impact of gender.”
Lanius12 suggests that adverse childhood experiences13 (trauma such as abuse and sexual assault) can lead to long-term changes within the nervous system, including areas of pain processing. My coauthor and I describe these changes here in terms of nervous system sensitization or dysregulation, and we believe that these changes lead to a bias toward hyperactivation of emotional pain circuits, which leads to the emotionally laden pain behaviors that often seem out of proportion to tissue pathology.
1. Dubner R, Gold M. The neurobiology of pain. Proc Natl Acad Sci U S A. 1999;96:7627-7630.
2. Markenson JA. Mechanisms of chronic pain. Am J Med. 1996;101:S6-S18.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968-971.
4. Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415-418.
5. Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332-338.
6. Fassoulaki A, Triga A, Melemeni A, et al. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005;101:1427-1432.
7. Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362-379.
8. International Association for the Study of Pain Web site. IASP Taxonomy. Available at: http://www.iasp-pain.org/Taxonomy. Accessed January 10, 2016.
9. Waddell G, McCulloch JA, Kummel E, et al. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
10. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502-511.
11. Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195-199.
12. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders, and sexual behavior: implications for healthcare. In: Lanius R, Vermetten E, eds. The Hidden Epidemic: The Impact of Early Life Trauma on Health and Disease. Cambridge University Press; 2010. Available at: http://www.unnaturalcauses.org/assets/uploads/file/ACE%20Study-Lanius.pdf. Accessed January 11, 2016.
13. Centers for Disease Control and Prevention. Injury prevention and control: Division of violence prevention. Adverse childhood experiences. Available at: http://www.cdc.gov/violenceprevention/acestudy/. Accessed January 11, 2016.
14. Kross E, Berman MG, Mischel W, et al. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci. 2011;108:6270-6275.
15. Geuze E, Westenberg HGM, Jochims A, et al. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76-85.
16. Mickleborough MJ, Daniels JK, Coupland NJ. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: an fMRI investigation. J Psychiatry Neurosci. 2011;36: 6-14.
17. Noll-Hussong M, Otti A, Laeer L, et al. Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychoso Res. 2010; 68:483-487.
The care of people with pain has been wrought with ineffective and unnecessary treatment, including the misuse of opioids, largely because we do not have an accurate conceptualization of pain. The absence of animal and human models of central nervous system (CNS) pain processing ensures that our understanding of pain will remain incomplete for the foreseeable future, but enough evidence exists to help family physicians develop an understanding of pain that goes beyond what we learned in medical school and that can help us more effectively treat patients with pain.
In this review, we will briefly discuss the established concepts of nociceptive and neuropathic pain. And then, with those concepts in mind, we will explore a third type of pain that for lack of a better term, we will call “pain for psychological reasons.” We hypothesize that this pain may be the consequence of changes in nervous system function that arise from developmental trauma, other traumatic experiences in a patient’s life, or mental health disorders. It is this third type of pain that may offer us insights into conditions such as fibromyalgia.
While we do not yet have validated diagnostic criteria for this third type of pain, we believe that there is enough information to present initial criteria so that one may distinguish it from nociceptive and neuropathic pain.
Nociceptive and neuropathic pain: The current paradigm
Nociceptive pain. The sensory pain experience, or nociceptive pain, is produced by noxious stimuli that either damage, or are capable of damaging, tissues (eg, burns, cuts, fractures, inflammation, and increased pressure in a hollow viscus). Noxious stimuli are detected at the molecular level by specific pain sensory receptors embedded in our tissues called nociceptors.
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps—transduction, transmission, modulation, and perception—which are described in “From periphery to brain: The process of nociceptive pain.”1-4
Neuropathic pain. While nociceptive pain can be easily traced from a peripheral nociceptive fiber to the brain and typically resolves when the nociceptive stimulus stops, neuropathic pain (NPP) results from changes to the function of the nervous system and is typically caused by injury to the nerves. Such changes, referred to as neuronal sensitization, may not quickly resolve, as is the case with postherpetic neuralgia. In fact, the changes can become permanent. NPP fundamentally differs from nociceptive pain because it results from changes in the central processing of pain that can lead a person to perceive pain sensations even in the absence of tissue pathology.
Common causes of NPP that persists even after tissue damage has healed include trauma (eg, amputation of a limb), ischemia (eg, pressure palsy), disease (eg, the metabolic injury of diabetes or the injury caused by a shingles infection), and drug treatment (eg, chemotherapy). The underlying mechanisms of NPP and the neuronal plasticity (the ability of the nervous system to rewire itself) that initiate and then maintain NPP are important areas of active research that may eventually lead to the development of more effective treatments.
Timing is critical. Neuroplastic changes in the nervous system following nerve injury are time-dependent. Synaptic plasticity can occur within seconds to minutes, while cellular plasticity occurs within hours to days. Synaptic and cellular plasticity happen relatively fast and may be reversible.
In contrast, systems plasticity (when new CNS neuronal connections are formed in response to nerve injury) takes place over the months and years following nerve injury and is often irreversible. When we recognize NPP and intervene before system neuroplastic changes occur, it may be possible to prevent pain from becoming chronic (TABLE 15). In cases of nerve injury, researchers have long suspected that early and aggressive pain treatment within the first few months that may include sympathetic and peripheral neural blockade reduces the likelihood that the patient will have chronic pain.6,7

It’s time to update our understanding of pain
The International Association for the Study of Pain (IASP)—a group of health care providers, scientists, and policymakers seeking to improve pain relief worldwide—notes in its definition of pain that the complaint, “I hurt” does not necessarily imply that there is a painful stimulus in the form of tissue injury.8 Yet most of us have been taught to think of pain solely as the result of tissue pathology, and we assume that emotional factors merely modify how the physical damage is perceived. This traditional concept of pain is incomplete. It leads clinicians to misdiagnose the cause of pain, initiate expensive and unnecessary treatment, engage in well-meaning but misguided prescribing behavior, and miss opportunities to help patients.
SIDEBAR
From periphery to brain: The process of nociceptive pain1-4
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps:
In transduction, nociceptors containing special molecular proteins respond to noxious modalities, such as thermal, mechanical, or chemical stimuli, and trigger nerve impulses in the nociceptive nerve fibers (nerves dedicated to pain sensation).
During transmission—the second stage of the process—information from the nociceptors in the periphery (skin, muscle, viscera) is relayed to the spinal cord mainly by 2 types of nociceptive neurons: C-fibers and A delta (Aδ) fibers. Both approach the spinal cord in a peripheral nerve and then enter the spinal cord in the dorsal root entry zone. Because Aδ fibers are thinly myelinated, they send impulses faster than unmyelinated C fibers. This is why when injury occurs, we first feel sharp, acute pain that then slowly diffuses into a duller ache.
Once the incoming signal is transmitted to the CNS at the spinal cord, primary afferent neurons synapse on second order neurons. From there, information travels on to the thalamus via multiple neurons that have the capacity to change their response patterns when activity of nociceptive fibers is sustained (as occurs in the setting of a tissue or nerve injury and perhaps in the setting of psychological trauma). This is known as modulation of the incoming nociceptive stimulus. During this step of the process, stimuli can be amplified, suppressed, or even transformed from one type to another (eg, a light touch can be modulated in such a way that it will be perceived as a burning sensation). Also, it is this step that is affected by many medications, by intrathecal drug infusions, and by spinal neurostimulators.
In perception, the thalamus then directs the pain sensation to multiple brain centers. At this step, the stimulus is finally consciously perceived as pain by the individual.
Cortical pain circuits can be activated without physical input (ie, no tissue damage, noxious stimuli, or nerve injury). This becomes important in understanding pain syndromes, such as fibromyalgia.
Pain in the absence of any pathophysiologic cause or injury
The clinician’s search for a pain diagnosis is typically predicated on the notion that there must be an underlying tissue injury of severity equal to the severity of the patient’s pain complaints. This approach to a pain evaluation rests on 2 assumptions that are not true for all patients:
- Pain is simply a sensory experience that is always caused by tissue damage of some type.
- The severity of the pain experienced by a patient should be tightly bound to the severity of the pain stimulus (ie, tissue damage).
These assumptions are true of acute nociceptive pain, they may or may not be true for NPP, but they do not apply to the third type of pain—pain for psychological reasons. While tissue pathology in humans and animals with nociceptive pain is usually visible, measurable, and correlates with observed pain behaviors, the damage to nerve tissue and the ensuing changes in nervous system function with NPP are not always visible or able to be imaged. These changes produce pain that can appear more severe than expected based on a brief exam. Some of the time, however, characteristic symptoms and physical signs of NPP will be present, and perhaps electrodiagnostic or other tests will be abnormal, thus providing some objective sense of changes in nervous system function.
In contrast, pain behavior due to the third type of pain usually appears very much out of proportion, and unbound to, tissue pathology. Furthermore, the patient’s pain behaviors often reflect heightened emotional pain processing (TABLE 29). The resulting emotionally charged presentation can be alarming and suggestive of extreme tissue injury, but there may be absolutely no evidence of tissue injury or pathology.

Functional change in the CNS
There is evidence from experimental studies that psychologic factors change nervous system function. In one review, the authors concluded, “Pain…can vary widely between people and even within an individual depending on…the psychological state of the person.”10 In a second review, the authors concluded that our emotional state has an enormous influence on pain; a negative emotional state increases pain, whereas a positive state lowers pain.11
But can psychological factors induce long-term changes in nervous system function analogous to the systems neuroplasticity responsible for irreversible changes in NPP? And can psychologically induced changes in nervous system sensory processing lead to pain without any tissue or nerve damage?
We theorize that a functional change in the CNS can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents). (More on this in a bit.) When such changes occur, mildly painful stimuli are amplified and processed through overly sensitized, dysregulated, ramped-up emotional and somatosensory pain circuits in the brain. This is analogous to the functional changes in the nervous system that occur with NPP; however, when the nervous system changes are due to psychological factors, there may be no tissue or nerve injury.
Childhood trauma influences adult pain. One of the more compelling narratives emerging in health care has to do with the influence that childhood developmental trauma can have on health, including pain. In his chapter on the impact of early life trauma on health and disease, Lanius states:12
“Women were 50% more likely than men to have experienced 5 or more categories of adverse childhood experiences. We believe that here is a key to what in mainstream epidemiology appears as women’s natural proneness to ill-defined health problems like fibromyalgia, chronic fatigue syndrome, obesity, irritable bowel syndrome, and chronic non-malignant pain syndromes. In light of our findings, we now see these as medical constructs, artifacts resulting from medical blindness to social realities and ignorance of the impact of gender.”
Lanius12 suggests that adverse childhood experiences13 (trauma such as abuse and sexual assault) can lead to long-term changes within the nervous system, including areas of pain processing. My coauthor and I describe these changes here in terms of nervous system sensitization or dysregulation, and we believe that these changes lead to a bias toward hyperactivation of emotional pain circuits, which leads to the emotionally laden pain behaviors that often seem out of proportion to tissue pathology.
The care of people with pain has been wrought with ineffective and unnecessary treatment, including the misuse of opioids, largely because we do not have an accurate conceptualization of pain. The absence of animal and human models of central nervous system (CNS) pain processing ensures that our understanding of pain will remain incomplete for the foreseeable future, but enough evidence exists to help family physicians develop an understanding of pain that goes beyond what we learned in medical school and that can help us more effectively treat patients with pain.
In this review, we will briefly discuss the established concepts of nociceptive and neuropathic pain. And then, with those concepts in mind, we will explore a third type of pain that for lack of a better term, we will call “pain for psychological reasons.” We hypothesize that this pain may be the consequence of changes in nervous system function that arise from developmental trauma, other traumatic experiences in a patient’s life, or mental health disorders. It is this third type of pain that may offer us insights into conditions such as fibromyalgia.
While we do not yet have validated diagnostic criteria for this third type of pain, we believe that there is enough information to present initial criteria so that one may distinguish it from nociceptive and neuropathic pain.
Nociceptive and neuropathic pain: The current paradigm
Nociceptive pain. The sensory pain experience, or nociceptive pain, is produced by noxious stimuli that either damage, or are capable of damaging, tissues (eg, burns, cuts, fractures, inflammation, and increased pressure in a hollow viscus). Noxious stimuli are detected at the molecular level by specific pain sensory receptors embedded in our tissues called nociceptors.
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps—transduction, transmission, modulation, and perception—which are described in “From periphery to brain: The process of nociceptive pain.”1-4
Neuropathic pain. While nociceptive pain can be easily traced from a peripheral nociceptive fiber to the brain and typically resolves when the nociceptive stimulus stops, neuropathic pain (NPP) results from changes to the function of the nervous system and is typically caused by injury to the nerves. Such changes, referred to as neuronal sensitization, may not quickly resolve, as is the case with postherpetic neuralgia. In fact, the changes can become permanent. NPP fundamentally differs from nociceptive pain because it results from changes in the central processing of pain that can lead a person to perceive pain sensations even in the absence of tissue pathology.
Common causes of NPP that persists even after tissue damage has healed include trauma (eg, amputation of a limb), ischemia (eg, pressure palsy), disease (eg, the metabolic injury of diabetes or the injury caused by a shingles infection), and drug treatment (eg, chemotherapy). The underlying mechanisms of NPP and the neuronal plasticity (the ability of the nervous system to rewire itself) that initiate and then maintain NPP are important areas of active research that may eventually lead to the development of more effective treatments.
Timing is critical. Neuroplastic changes in the nervous system following nerve injury are time-dependent. Synaptic plasticity can occur within seconds to minutes, while cellular plasticity occurs within hours to days. Synaptic and cellular plasticity happen relatively fast and may be reversible.
In contrast, systems plasticity (when new CNS neuronal connections are formed in response to nerve injury) takes place over the months and years following nerve injury and is often irreversible. When we recognize NPP and intervene before system neuroplastic changes occur, it may be possible to prevent pain from becoming chronic (TABLE 15). In cases of nerve injury, researchers have long suspected that early and aggressive pain treatment within the first few months that may include sympathetic and peripheral neural blockade reduces the likelihood that the patient will have chronic pain.6,7

It’s time to update our understanding of pain
The International Association for the Study of Pain (IASP)—a group of health care providers, scientists, and policymakers seeking to improve pain relief worldwide—notes in its definition of pain that the complaint, “I hurt” does not necessarily imply that there is a painful stimulus in the form of tissue injury.8 Yet most of us have been taught to think of pain solely as the result of tissue pathology, and we assume that emotional factors merely modify how the physical damage is perceived. This traditional concept of pain is incomplete. It leads clinicians to misdiagnose the cause of pain, initiate expensive and unnecessary treatment, engage in well-meaning but misguided prescribing behavior, and miss opportunities to help patients.
SIDEBAR
From periphery to brain: The process of nociceptive pain1-4
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps:
In transduction, nociceptors containing special molecular proteins respond to noxious modalities, such as thermal, mechanical, or chemical stimuli, and trigger nerve impulses in the nociceptive nerve fibers (nerves dedicated to pain sensation).
During transmission—the second stage of the process—information from the nociceptors in the periphery (skin, muscle, viscera) is relayed to the spinal cord mainly by 2 types of nociceptive neurons: C-fibers and A delta (Aδ) fibers. Both approach the spinal cord in a peripheral nerve and then enter the spinal cord in the dorsal root entry zone. Because Aδ fibers are thinly myelinated, they send impulses faster than unmyelinated C fibers. This is why when injury occurs, we first feel sharp, acute pain that then slowly diffuses into a duller ache.
Once the incoming signal is transmitted to the CNS at the spinal cord, primary afferent neurons synapse on second order neurons. From there, information travels on to the thalamus via multiple neurons that have the capacity to change their response patterns when activity of nociceptive fibers is sustained (as occurs in the setting of a tissue or nerve injury and perhaps in the setting of psychological trauma). This is known as modulation of the incoming nociceptive stimulus. During this step of the process, stimuli can be amplified, suppressed, or even transformed from one type to another (eg, a light touch can be modulated in such a way that it will be perceived as a burning sensation). Also, it is this step that is affected by many medications, by intrathecal drug infusions, and by spinal neurostimulators.
In perception, the thalamus then directs the pain sensation to multiple brain centers. At this step, the stimulus is finally consciously perceived as pain by the individual.
Cortical pain circuits can be activated without physical input (ie, no tissue damage, noxious stimuli, or nerve injury). This becomes important in understanding pain syndromes, such as fibromyalgia.
Pain in the absence of any pathophysiologic cause or injury
The clinician’s search for a pain diagnosis is typically predicated on the notion that there must be an underlying tissue injury of severity equal to the severity of the patient’s pain complaints. This approach to a pain evaluation rests on 2 assumptions that are not true for all patients:
- Pain is simply a sensory experience that is always caused by tissue damage of some type.
- The severity of the pain experienced by a patient should be tightly bound to the severity of the pain stimulus (ie, tissue damage).
These assumptions are true of acute nociceptive pain, they may or may not be true for NPP, but they do not apply to the third type of pain—pain for psychological reasons. While tissue pathology in humans and animals with nociceptive pain is usually visible, measurable, and correlates with observed pain behaviors, the damage to nerve tissue and the ensuing changes in nervous system function with NPP are not always visible or able to be imaged. These changes produce pain that can appear more severe than expected based on a brief exam. Some of the time, however, characteristic symptoms and physical signs of NPP will be present, and perhaps electrodiagnostic or other tests will be abnormal, thus providing some objective sense of changes in nervous system function.
In contrast, pain behavior due to the third type of pain usually appears very much out of proportion, and unbound to, tissue pathology. Furthermore, the patient’s pain behaviors often reflect heightened emotional pain processing (TABLE 29). The resulting emotionally charged presentation can be alarming and suggestive of extreme tissue injury, but there may be absolutely no evidence of tissue injury or pathology.

Functional change in the CNS
There is evidence from experimental studies that psychologic factors change nervous system function. In one review, the authors concluded, “Pain…can vary widely between people and even within an individual depending on…the psychological state of the person.”10 In a second review, the authors concluded that our emotional state has an enormous influence on pain; a negative emotional state increases pain, whereas a positive state lowers pain.11
But can psychological factors induce long-term changes in nervous system function analogous to the systems neuroplasticity responsible for irreversible changes in NPP? And can psychologically induced changes in nervous system sensory processing lead to pain without any tissue or nerve damage?
We theorize that a functional change in the CNS can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents). (More on this in a bit.) When such changes occur, mildly painful stimuli are amplified and processed through overly sensitized, dysregulated, ramped-up emotional and somatosensory pain circuits in the brain. This is analogous to the functional changes in the nervous system that occur with NPP; however, when the nervous system changes are due to psychological factors, there may be no tissue or nerve injury.
Childhood trauma influences adult pain. One of the more compelling narratives emerging in health care has to do with the influence that childhood developmental trauma can have on health, including pain. In his chapter on the impact of early life trauma on health and disease, Lanius states:12
“Women were 50% more likely than men to have experienced 5 or more categories of adverse childhood experiences. We believe that here is a key to what in mainstream epidemiology appears as women’s natural proneness to ill-defined health problems like fibromyalgia, chronic fatigue syndrome, obesity, irritable bowel syndrome, and chronic non-malignant pain syndromes. In light of our findings, we now see these as medical constructs, artifacts resulting from medical blindness to social realities and ignorance of the impact of gender.”
Lanius12 suggests that adverse childhood experiences13 (trauma such as abuse and sexual assault) can lead to long-term changes within the nervous system, including areas of pain processing. My coauthor and I describe these changes here in terms of nervous system sensitization or dysregulation, and we believe that these changes lead to a bias toward hyperactivation of emotional pain circuits, which leads to the emotionally laden pain behaviors that often seem out of proportion to tissue pathology.
1. Dubner R, Gold M. The neurobiology of pain. Proc Natl Acad Sci U S A. 1999;96:7627-7630.
2. Markenson JA. Mechanisms of chronic pain. Am J Med. 1996;101:S6-S18.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968-971.
4. Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415-418.
5. Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332-338.
6. Fassoulaki A, Triga A, Melemeni A, et al. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005;101:1427-1432.
7. Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362-379.
8. International Association for the Study of Pain Web site. IASP Taxonomy. Available at: http://www.iasp-pain.org/Taxonomy. Accessed January 10, 2016.
9. Waddell G, McCulloch JA, Kummel E, et al. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
10. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502-511.
11. Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195-199.
12. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders, and sexual behavior: implications for healthcare. In: Lanius R, Vermetten E, eds. The Hidden Epidemic: The Impact of Early Life Trauma on Health and Disease. Cambridge University Press; 2010. Available at: http://www.unnaturalcauses.org/assets/uploads/file/ACE%20Study-Lanius.pdf. Accessed January 11, 2016.
13. Centers for Disease Control and Prevention. Injury prevention and control: Division of violence prevention. Adverse childhood experiences. Available at: http://www.cdc.gov/violenceprevention/acestudy/. Accessed January 11, 2016.
14. Kross E, Berman MG, Mischel W, et al. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci. 2011;108:6270-6275.
15. Geuze E, Westenberg HGM, Jochims A, et al. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76-85.
16. Mickleborough MJ, Daniels JK, Coupland NJ. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: an fMRI investigation. J Psychiatry Neurosci. 2011;36: 6-14.
17. Noll-Hussong M, Otti A, Laeer L, et al. Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychoso Res. 2010; 68:483-487.
1. Dubner R, Gold M. The neurobiology of pain. Proc Natl Acad Sci U S A. 1999;96:7627-7630.
2. Markenson JA. Mechanisms of chronic pain. Am J Med. 1996;101:S6-S18.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968-971.
4. Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415-418.
5. Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332-338.
6. Fassoulaki A, Triga A, Melemeni A, et al. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005;101:1427-1432.
7. Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362-379.
8. International Association for the Study of Pain Web site. IASP Taxonomy. Available at: http://www.iasp-pain.org/Taxonomy. Accessed January 10, 2016.
9. Waddell G, McCulloch JA, Kummel E, et al. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
10. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502-511.
11. Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195-199.
12. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders, and sexual behavior: implications for healthcare. In: Lanius R, Vermetten E, eds. The Hidden Epidemic: The Impact of Early Life Trauma on Health and Disease. Cambridge University Press; 2010. Available at: http://www.unnaturalcauses.org/assets/uploads/file/ACE%20Study-Lanius.pdf. Accessed January 11, 2016.
13. Centers for Disease Control and Prevention. Injury prevention and control: Division of violence prevention. Adverse childhood experiences. Available at: http://www.cdc.gov/violenceprevention/acestudy/. Accessed January 11, 2016.
14. Kross E, Berman MG, Mischel W, et al. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci. 2011;108:6270-6275.
15. Geuze E, Westenberg HGM, Jochims A, et al. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76-85.
16. Mickleborough MJ, Daniels JK, Coupland NJ. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: an fMRI investigation. J Psychiatry Neurosci. 2011;36: 6-14.
17. Noll-Hussong M, Otti A, Laeer L, et al. Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychoso Res. 2010; 68:483-487.
From The Journal of Family Practice | 2016;65(9):598-600,602-605.
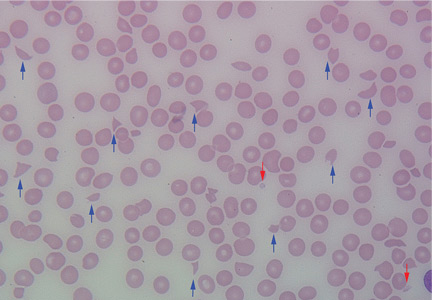
Erythrocytosis due to presumed polycythemia vera
A 40-year-old woman with hypertrophic obstructive cardiomyopathy presents to the hematology clinic for a second opinion regarding a history of headaches and fatigue for the past 10 years. She has been diagnosed with idiopathic erythrocytosis, presumed to be due to polycythemia vera. She periodically undergoes phlebotomy to keep her hematocrit below 41%, and this markedly improves her headaches. She denies shortness of breath, cough, fever, weight loss, joint pain, and visual or other neurologic symptoms. She has never reported pruritus related to bathing or exposure to water.
She does not smoke, drink alcohol, or use illicit drugs. She works as a pharmacy technician. She says her father died of cancer (no further details available) and describes a family history of gastrointestinal malignancy in her grandfather and paternal aunt. She takes aspirin, metoprolol, and spironolactone for her cardiomyopathy.
Physical examination reveals generalized plethora, more marked on her cheeks and face, and mild bilateral pitting pedal edema. No lymphadenopathy or hepatosplenomegaly can be palpated. Other systems, including the cardiac, respiratory, and nervous systems, are normal.
ERYTHROCYTOSIS AND POLYCYTHEMIA VERA
1. In patients with erythrocytosis, which of the following is not characteristic of polycythemia vera?
- Erythromelalgia and postbathing pruritus
- Splenomegaly
- History of thrombosis
- Gout
- Hematuria
Erythrocytosis—an abnormally high concentration of red blood cells in the peripheral blood—is a laboratory finding. It often reflects an increase in the total quantity or mass of red blood cells in the body (polycythemia) but can sometimes be due to decreased plasma volume (spurious polycythemia).1 Erythrocytosis can be caused by a number of diseases, hereditary and acquired, and can be classified as primary or secondary (Table 1).
Symptoms arise from an increase in the total blood volume and red blood cell mass, often leading to dilated capillaries and other blood vessels. Symptoms can occur regardless of the cause and classically include headache (often described as diffuse heaviness), dizziness, and a tendency for bleeding or thrombosis.2 Symptoms are relieved when the hematocrit is lowered.
Several features in the history and physical examination of a patient being evaluated for erythrocytosis can suggest an underlying cause. Smoking, chronic respiratory insufficiency, and congenital cyanotic heart disease point to secondary erythrocytosis and can usually be identified at the outset. A history of occupational exposure to carbon monoxide (such as engine exhaust) should be elicited carefully. A family history of erythrocytosis should raise suspicion of a heritable condition such as a hemoglobinopathy associated with increased oxygen affinity or rare forms of primary erythrocytosis associated with endogenous overproduction of erythropoietin or activating mutations of the erythropoietin receptor.3 Iatrogenic causes such as androgen supplementation, erythropoietin abuse, and postrenal-transplant erythrocytosis should also be considered.
Secretion of erythropoietin or erythropoietinlike proteins by a malignant neoplasm is a rare but important cause of erythrocytosis. For example, renal cell carcinoma may present with erythrocytosis secondary to excessive erythropoietin production, and hematuria can be an early symptom.
Polycythemia vera
Polycythemia vera, a myeloproliferative neoplasm, is characterized by increased red blood cell production independent of the mechanisms that normally regulate erythropoiesis. The bone marrow shows a panmyelosis that is often accompanied by leukocytosis or thrombocytosis, or both, in the peripheral blood.
Symptoms such as severe itching after exposure to hot water (aquagenic pruritus) and periodic attacks of redness, swelling, and pain in the hands or feet, or both (erythromelalgia), have been described in patients with polycythemia vera. Splenomegaly is relatively common, seen in approximately two-thirds of patients.4 Hyperuricemia (from increased cell turnover) and gout are also associated with polycythemia vera, as is a history of arterial and venous thrombosis.5
Hematuria is not commonly seen in polycythemia vera, although bleeding from the bladder, vagina, or uterus has been described.
CASE RESUMED: INITIAL LABORATORY TESTS
Results of our patient’s initial laboratory tests are:
- Hemoglobin 16.9 g/dL (reference range 11.5–15.5)
- Hematocrit 48.8% (36.0–46.0)
- Mean corpuscular volume 85.2 fL (80–100)
- Platelet count 328 × 109/L (150–400)
- White blood cell count 9.14 × 109/L (3.7–11.0)
- Absolute neutrophil count 5.95 × 109/L (1.45–7.5)
- Blood urea nitrogen 12 mg/dL (8–25)
- Creatinine 0.5 mg/dL (0.7–1.4)
- Lactate dehydrogenase 180 U/L (100–220)
- Uric acid 3.0 mg/dL (2.0–7.0)
- Thyroid-stimulating hormone 2.2 µU/mL (0.4–5.5).
The patient undergoes additional tests, including a serum erythropoietin level and hemoglobinopathy screen. Bone marrow aspiration and biopsy are performed, with cytogenetic analysis, chromosomal microarray analysis, and molecular testing for mutation of the Janus kinase 2 (JAK2) gene.
CONFIRMING SUSPECTED POLYCYTHEMIA VERA
2. In patients with suspected polycythemia vera, which of the following laboratory tests is most useful in making the diagnosis?
- Hemoglobin, hematocrit, and red blood cell mass
- Serum erythropoietin level
- Arterial blood gases with co-oximetry
- Testing for the JAK2 mutation
- Bone marrow aspiration and biopsy
The aim of the initial workup of erythrocytosis is to differentiate polycythemia vera from secondary causes of erythrocytosis.
Hemoglobin, hematocrit, red cell mass
Erythrocytosis is defined by an abnormal elevation in the hematocrit (> 48% in women or > 49% in men), hemoglobin concentration (> 16.0 g/dL in women or > 16.5 g/dL in men), or red blood cell mass. The red blood cell count should not be used as a surrogate for red blood cell mass, since some anemias (especially thalassemia minor) can be associated with an increase in the number of red blood cells but a low hemoglobin concentration.
Isotope dilution techniques to determine the red cell mass and plasma volume can differentiate true erythrocytosis from a spurious elevation due to a decrease in plasma volume.6,7 However, this is an expensive, time-consuming test that is not widely available and so is rarely performed.8
JAK2 mutation testing
The initial evaluation of a patient with erythrocytosis has changed significantly in the past 10 years with the discovery of the JAK2 gene and its role in the pathogenesis of polycythemia vera and other myeloproliferative neoplasms.
JAK2, located at 9p24, codes for a tyrosine kinase important for signal transduction in hematopoietic cells. Mutations in this gene have been shown to promote hypersensitivity to cytokines, including erythropoietin.9 The most common somatic mutation occurs within exon 14 at base pair 1849 and results in a phenylalanine-for-valine amino acid substitution in the JAK2 protein, designated V617F. Less commonly, mutations occur elsewhere in exons 12 to 15, with more than 50 different mutations described; nonpolymorphic mutations are assumed to have biologic effects similar to those of V617F.
Taken together, the JAK2 V617F and non-V617F mutations have a diagnostic sensitivity of 98% to 100% for polycythemia vera. For practical purposes, this means that the presence of a JAK2 mutation can be used as a clonal marker to distinguish polycythemia vera from reactive secondary causes of erythrocytosis. A JAK2 mutation is one of three major diagnostic criteria for polycythemia vera in the 2016 revision to the 2008 World Health Organization criteria (Table 2).10 Of note, this mutation is not specific for polycythemia vera and can also be found in other myeloproliferative neoplasms, including primary myelofibrosis and essential thrombocythemia.
Absence of a JAK2 mutation makes polycythemia vera unlikely, so this test is most useful in making the diagnosis.
Serum erythropoietin
Serum erythropoietin testing can be very useful to distinguish polycythemia vera from secondary erythrocytosis. Low levels suggest polycythemia vera, while high levels are seen in secondary processes.11
This test is best used along with JAK2 V617F mutation analysis as an initial step in evaluating patients with erythrocytosis. When JAK2 V617F mutation analysis is negative, a low serum erythropoietin level should prompt further testing for non-V617F JAK2 mutations, whereas a normal or elevated erythropoietin level should be evaluated further with tests to distinguish hereditary from acquired secondary causes of erythrocytosis.
Arterial blood gas analysis and co-oximetry
Arterial blood gas analysis can reveal hypoxemia, pointing to a cardiorespiratory process driving the erythrocytosis, whereas co-oximetry can be used to identify the presence and amount of carboxyhemoglobin in the blood.
Bone marrow biopsy
An increase in pleomorphic megakaryocytes in the bone marrow without stainable iron is often described as characteristic in polycythemia vera patients, but it is not diagnostic. Panmyelosis with increased cellularity is the norm but can be seen in other myeloproliferative neoplasms. The morphologic features of bone marrow are now included as one of the major diagnostic criteria for polycythemia vera (Table 2).
OUR PATIENT’S FURTHER WORKUP
Our patient’s erythropoietin level is 34.2 mIU/mL (reference range 4.7–28.6). Her oxygen saturation is 96%, and her carboxyhemoglobin level is 1.1% (0–5).
She undergoes bone marrow biopsy. Analysis finds that the marrow is normocellular (60%) with trilineage hematopoiesis and decreased stainable iron.
Cytogenetic analysis shows a 46,XX[20] karyotype. Chromosomal microarray analysis shows no pathogenic copy-number changes. There is no detectable JAK2 V617F or exon 12-to-15 mutation.
The patient’s erythrocytosis and abnormal hemoglobin electrophoresis study raise suspicion for a variant type of hemoglobin that has a higher affinity for oxygen than normal.
3. What is the next best step to evaluate this patient?
- Red-cell oxygen equilibrium curve to calculate the P50 (the partial pressure of oxygen that is required to saturate 50% of the hemoglobin.)
- High-performance liquid chromatography
- Globin gene DNA sequencing
- Testing 2,3-bisphosphoglycerate mutase (BPGM) activity
Nearly 200 mutational variants in alpha and beta globin chains that lead to an increased affinity of hemoglobin for oxygen have been reported.12 While not all mutations are clinically significant, increased oxygen affinity variants can lead to impaired oxygen delivery to tissues, especially the kidneys, resulting in a physiologic increase in erythropoietin and erythrocytosis.
In patients being evaluated for a high-oxygen-affinity hemoglobinopathy, a two-step approach has been outlined.13 The first involves measuring the oxygen-binding properties of a freshly collected sample of blood by directly measuring the oxygen saturation of the hemoglobin and pO2 using a co-oximeter. This information is used to create a red cell oxygen equilibrium curve and to calculate the P50. A low P50 correlates with an abnormally high affinity of hemoglobin for oxygen.
The second step is to identify the abnormal hemoglobin. High-performance liquid chromatography is now widely available as a screening test but does not detect all variants. For many years, sequencing of globin chain DNA has been a gold standard for identifying specific mutations. Subsequent to analyzing a catalog of known hemoglobin variants, mass spectrometry can serve as a screening and identification technique. Mass spectroscopy can also detect known rare variants with posttranslational modifications14 that are not recognized by DNA analysis. Mass spectroscopy and DNA sequencing are complementary techniques available only in specialized reference laboratories.
Erythrocytosis due to BPGM deficiency is very rare. Clinical and laboratory features mimic those of high-oxygen-affinity hemoglobin, but patients do not have a demonstrable mutation in alpha or beta globin genes. The level of BPGM is low, and the diagnosis is established by measuring BPGM levels and sequencing the BPGM gene.15
RESULTS OF THE ADDITIONAL WORKUP
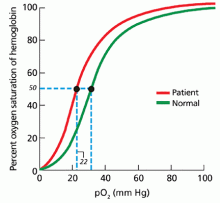
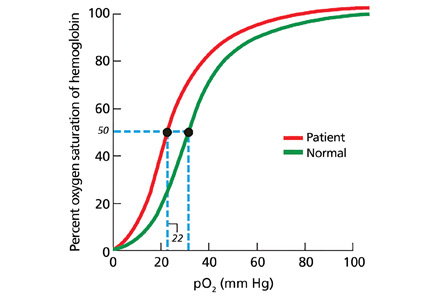
In our patient, hemoglobin electrophoresis reveals an abnormal hemoglobin variant. High-performance liquid chromatography reveals an abnormal peak that comprises approximately 23.7% of the total hemoglobin, consistent with an alpha globin variant. Further characterization (using a sample of venous blood) shows an oxygen dissociation P50 of 22 mm Hg (normal 24–30 mm Hg) (Figure 1).
Mass spectrometry identifies the variant as hemoglobin Tarrant. This variant is characterized by a substitution of asparagine for aspartic acid at position 126 of the alpha globin chain, a known site of contact between the alpha 1 and beta 1 chains.16 It has been seen in patients of Hispanic heritage and clinically correlates with mild erythrocytosis. Indeed, this woman’s mother was from Mexico.
EDUCATING PATIENTS
4. What should patients know about their high-oxygen-affinity hemoglobinopathy?
- High altitudes and air travel can be risky
- Pregnancy may have adverse outcomes
- Systemic anticoagulation may lower the risk of venous thromboembolism
- Periodic phlebotomy may help control symptoms
Most patients with high-oxygen-affinity hemoglobin do not require specific clinical management but only counseling and education about their condition. Establishing an accurate diagnosis is important in order to avoid further inappropriate, invasive, and expensive testing.
Although exposure to high altitudes may be associated with decreased ambient oxygen levels, hypoxia is usually not a problem because of hemoglobin’s high affinity for oxygen.
Impaired delivery of oxygen across the placenta may be anticipated in a mother with high-oxygen-affinity hemoglobin, but this has not been observed clinically.17
Compared with patients with polycythemia vera, patients with high-oxygen-affinity hemoglobin have fewer complications from hyperviscosity and thrombosis, even with comparable degrees of erythrocytosis.
Although patients usually do not require treatment, phlebotomy may be helpful for symptoms that can be attributed to the higher hemoglobin concentration.
Our patient continues to be seen in clinic for periodic blood counts and phlebotomy for her headaches, as required.
HEMOGLOBIN: RELAXED OR TENSE
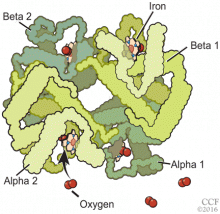
Normal adult hemoglobin is a tetramer composed of two pairs of globin polypeptide chains: alpha and beta (Figure 2). The intrinsic properties of the constituent globin chains and their allosteric conformation—as well as extrinsic factors including temperature, pH, and the binding of hydrogen ion and 2,3-BPG—play important roles in modifying the affinity of hemoglobin for oxygen. The major modulator of hemoglobin-oxygen affinity in human erythrocytes is 2,3-BPG.
The hemoglobin tetramer, consisting of two identical halves, alpha 1-beta 1 and alpha 2-beta 2, oscillates between two quaternary conformations, “relaxed” (fully oxygenated) and “tense” (fully deoxygenated).18 High-oxygen-affinity hemoglobins can result from factors that enhance the relaxed state, either by stabilizing the relaxed state or by destabilizing the tense state. Structural modifications in hemoglobin typically affect the main contacts involved in the transition from the deoxygenated to the oxygenated state, the 2,3-BPG binding sites, the heme pocket, or elongation of globin chains by various mutations. In hemoglobin Tarrant, the mutation prevents formation of noncovalent salt bridges in the alpha 1-beta 1 contact that normally stabilize the deoxygenated conformation of hemoglobin. As a result, the deoxygenated (tense) state is destabilized, shifting the allosteric equilibrium in favor of the oxygenated (relaxed) state with consequent high oxygen affinity.16
MORE ABOUT HIGH-OXYGEN-AFFINITY HEMOGLOBINS
The first case of erythrocytosis due to an abnormal hemoglobin was identified in 1966. This was an alpha chain variant with an arginine-to-leucine substitution at position 92, named hemoglobin Chesapeake.19
High-oxygen-affinity hemoglobin variants are usually transmitted as autosomal dominant traits. Patients are most often identified because of unexplained erythrocytosis detected on a routine blood cell count, as in our patient.
Not all high-oxygen-affinity hemoglobinopathies are associated with erythrocytosis. The degree of increased oxygen affinity may only be mild or the abnormal hemoglobin may be slightly unstable, thereby masking the usual clinical signs and symptoms.
Therapeutic phlebotomy should be used cautiously since it can decrease delivery of oxygen to tissues. A subset of patients whose symptoms are related to an elevated red cell mass may experience some relief, as did our patient.
- Kremyanskaya M, Mascarenhas J, Hoffman R. Why does my patient have erythrocytosis? Hematol Oncol Clin North Am 2012; 26:267–283.
- Keohane C, McMullin MF, Harrison C. The diagnosis and management of erythrocytosis. BMJ 2013; 347:f6667.
- Agarwal N, Gordeuk RV, Prchal JT. Genetic mechanisms underlying regulation of hemoglobin mass. Adv Exp Med Biol 2007; 618:195–210.
- Tefferi A. Polycythemia vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 2012; 87:285–293.
- Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia 2008; 22:2020–2028.
- Tefferi A, Spivak JL. Polycythemia vera: scientific advances and current practice. Semin Hematol 2005; 42:206–220.
- Ferrant A. What clinical and laboratory data are indicative of polycythemia and when are blood volume studies needed? Nouv Rev Fr Hematol 1994; 36:151–154.
- Fairbanks VF, Klee GG, Wiseman GA, et al. Measurement of blood volume and red cell mass: re-examination of 51Cr and 125I methods. Blood Cells Mol Dis 1996; 22:169–186; discussion 186a–186g.
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434:1144–1148.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127:2391–2405.
- Messinezy M, Westwood NB, El-Hemaidi I, Marsden JT, Sherwood RS, Pearson TC. Serum erythropoietin values in erythrocytosis and in primary thrombocythaemia. Br J Haematol 2002; 117:47–53.
- Hardison RC, Chui DHK, Giardine B, et al. HbVar: a relational database of human hemoglobin variants and thalassemia mutations at the globin gene server. Human Mutat 2002; 19:225–233.
- Percy MJ, Butt NN, Crotty GM, et al. Identification of high oxygen affinity hemoglobin variants in the investigation of patients with erythrocytosis. Haematologica 2009; 94:1321–1322.
- Kattamis AC, Kelly KM, Ohene-Frempong K, et al. Hb Osler [beta 145(HC2)Tyr-->Asp] results from posttranslational modification. Hemoglobin 1997; 21:109–120.
- Hoyer JD, Allen SL, Beutler E, Kubik K, West C, Fairbanks VF. Erythrocytosis due to bisphosphoglycerate mutase deficiency with concurrent glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. Am J Hematol 2004; 75:205–208.
- Moo-Penn WF, Jue DL, Johnson MH, Wilson SM, Therrell B Jr, Schmidt RM. Hemoglobin Tarrant: alpha126(H9) asp leads to asn. A new hemoglobin variant in the alpha1beta1 contact region showing high oxygen affinity and reduced cooperativity. Biochim Biophys Acta 1977; 490:443–451.
- Bard H, Peri KG, Gagnon C. The biologic implications of a rare hemoglobin mutant that decreases oxygen affinity. Pediatr Res 2001; 49:69–73.
- Wajcman H, Galacteros F. Hemoglobins with high oxygen affinity leading to erythrocytosis: new variants and concepts. Hemoglobin 2005; 29:91–106.
- Clegg JB, Naughton MA, Weatherall DJ. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol 1966; 19:91–108.
A 40-year-old woman with hypertrophic obstructive cardiomyopathy presents to the hematology clinic for a second opinion regarding a history of headaches and fatigue for the past 10 years. She has been diagnosed with idiopathic erythrocytosis, presumed to be due to polycythemia vera. She periodically undergoes phlebotomy to keep her hematocrit below 41%, and this markedly improves her headaches. She denies shortness of breath, cough, fever, weight loss, joint pain, and visual or other neurologic symptoms. She has never reported pruritus related to bathing or exposure to water.
She does not smoke, drink alcohol, or use illicit drugs. She works as a pharmacy technician. She says her father died of cancer (no further details available) and describes a family history of gastrointestinal malignancy in her grandfather and paternal aunt. She takes aspirin, metoprolol, and spironolactone for her cardiomyopathy.
Physical examination reveals generalized plethora, more marked on her cheeks and face, and mild bilateral pitting pedal edema. No lymphadenopathy or hepatosplenomegaly can be palpated. Other systems, including the cardiac, respiratory, and nervous systems, are normal.
ERYTHROCYTOSIS AND POLYCYTHEMIA VERA
1. In patients with erythrocytosis, which of the following is not characteristic of polycythemia vera?
- Erythromelalgia and postbathing pruritus
- Splenomegaly
- History of thrombosis
- Gout
- Hematuria
Erythrocytosis—an abnormally high concentration of red blood cells in the peripheral blood—is a laboratory finding. It often reflects an increase in the total quantity or mass of red blood cells in the body (polycythemia) but can sometimes be due to decreased plasma volume (spurious polycythemia).1 Erythrocytosis can be caused by a number of diseases, hereditary and acquired, and can be classified as primary or secondary (Table 1).
Symptoms arise from an increase in the total blood volume and red blood cell mass, often leading to dilated capillaries and other blood vessels. Symptoms can occur regardless of the cause and classically include headache (often described as diffuse heaviness), dizziness, and a tendency for bleeding or thrombosis.2 Symptoms are relieved when the hematocrit is lowered.
Several features in the history and physical examination of a patient being evaluated for erythrocytosis can suggest an underlying cause. Smoking, chronic respiratory insufficiency, and congenital cyanotic heart disease point to secondary erythrocytosis and can usually be identified at the outset. A history of occupational exposure to carbon monoxide (such as engine exhaust) should be elicited carefully. A family history of erythrocytosis should raise suspicion of a heritable condition such as a hemoglobinopathy associated with increased oxygen affinity or rare forms of primary erythrocytosis associated with endogenous overproduction of erythropoietin or activating mutations of the erythropoietin receptor.3 Iatrogenic causes such as androgen supplementation, erythropoietin abuse, and postrenal-transplant erythrocytosis should also be considered.
Secretion of erythropoietin or erythropoietinlike proteins by a malignant neoplasm is a rare but important cause of erythrocytosis. For example, renal cell carcinoma may present with erythrocytosis secondary to excessive erythropoietin production, and hematuria can be an early symptom.
Polycythemia vera
Polycythemia vera, a myeloproliferative neoplasm, is characterized by increased red blood cell production independent of the mechanisms that normally regulate erythropoiesis. The bone marrow shows a panmyelosis that is often accompanied by leukocytosis or thrombocytosis, or both, in the peripheral blood.
Symptoms such as severe itching after exposure to hot water (aquagenic pruritus) and periodic attacks of redness, swelling, and pain in the hands or feet, or both (erythromelalgia), have been described in patients with polycythemia vera. Splenomegaly is relatively common, seen in approximately two-thirds of patients.4 Hyperuricemia (from increased cell turnover) and gout are also associated with polycythemia vera, as is a history of arterial and venous thrombosis.5
Hematuria is not commonly seen in polycythemia vera, although bleeding from the bladder, vagina, or uterus has been described.
CASE RESUMED: INITIAL LABORATORY TESTS
Results of our patient’s initial laboratory tests are:
- Hemoglobin 16.9 g/dL (reference range 11.5–15.5)
- Hematocrit 48.8% (36.0–46.0)
- Mean corpuscular volume 85.2 fL (80–100)
- Platelet count 328 × 109/L (150–400)
- White blood cell count 9.14 × 109/L (3.7–11.0)
- Absolute neutrophil count 5.95 × 109/L (1.45–7.5)
- Blood urea nitrogen 12 mg/dL (8–25)
- Creatinine 0.5 mg/dL (0.7–1.4)
- Lactate dehydrogenase 180 U/L (100–220)
- Uric acid 3.0 mg/dL (2.0–7.0)
- Thyroid-stimulating hormone 2.2 µU/mL (0.4–5.5).
The patient undergoes additional tests, including a serum erythropoietin level and hemoglobinopathy screen. Bone marrow aspiration and biopsy are performed, with cytogenetic analysis, chromosomal microarray analysis, and molecular testing for mutation of the Janus kinase 2 (JAK2) gene.
CONFIRMING SUSPECTED POLYCYTHEMIA VERA
2. In patients with suspected polycythemia vera, which of the following laboratory tests is most useful in making the diagnosis?
- Hemoglobin, hematocrit, and red blood cell mass
- Serum erythropoietin level
- Arterial blood gases with co-oximetry
- Testing for the JAK2 mutation
- Bone marrow aspiration and biopsy
The aim of the initial workup of erythrocytosis is to differentiate polycythemia vera from secondary causes of erythrocytosis.
Hemoglobin, hematocrit, red cell mass
Erythrocytosis is defined by an abnormal elevation in the hematocrit (> 48% in women or > 49% in men), hemoglobin concentration (> 16.0 g/dL in women or > 16.5 g/dL in men), or red blood cell mass. The red blood cell count should not be used as a surrogate for red blood cell mass, since some anemias (especially thalassemia minor) can be associated with an increase in the number of red blood cells but a low hemoglobin concentration.
Isotope dilution techniques to determine the red cell mass and plasma volume can differentiate true erythrocytosis from a spurious elevation due to a decrease in plasma volume.6,7 However, this is an expensive, time-consuming test that is not widely available and so is rarely performed.8
JAK2 mutation testing
The initial evaluation of a patient with erythrocytosis has changed significantly in the past 10 years with the discovery of the JAK2 gene and its role in the pathogenesis of polycythemia vera and other myeloproliferative neoplasms.
JAK2, located at 9p24, codes for a tyrosine kinase important for signal transduction in hematopoietic cells. Mutations in this gene have been shown to promote hypersensitivity to cytokines, including erythropoietin.9 The most common somatic mutation occurs within exon 14 at base pair 1849 and results in a phenylalanine-for-valine amino acid substitution in the JAK2 protein, designated V617F. Less commonly, mutations occur elsewhere in exons 12 to 15, with more than 50 different mutations described; nonpolymorphic mutations are assumed to have biologic effects similar to those of V617F.
Taken together, the JAK2 V617F and non-V617F mutations have a diagnostic sensitivity of 98% to 100% for polycythemia vera. For practical purposes, this means that the presence of a JAK2 mutation can be used as a clonal marker to distinguish polycythemia vera from reactive secondary causes of erythrocytosis. A JAK2 mutation is one of three major diagnostic criteria for polycythemia vera in the 2016 revision to the 2008 World Health Organization criteria (Table 2).10 Of note, this mutation is not specific for polycythemia vera and can also be found in other myeloproliferative neoplasms, including primary myelofibrosis and essential thrombocythemia.
Absence of a JAK2 mutation makes polycythemia vera unlikely, so this test is most useful in making the diagnosis.
Serum erythropoietin
Serum erythropoietin testing can be very useful to distinguish polycythemia vera from secondary erythrocytosis. Low levels suggest polycythemia vera, while high levels are seen in secondary processes.11
This test is best used along with JAK2 V617F mutation analysis as an initial step in evaluating patients with erythrocytosis. When JAK2 V617F mutation analysis is negative, a low serum erythropoietin level should prompt further testing for non-V617F JAK2 mutations, whereas a normal or elevated erythropoietin level should be evaluated further with tests to distinguish hereditary from acquired secondary causes of erythrocytosis.
Arterial blood gas analysis and co-oximetry
Arterial blood gas analysis can reveal hypoxemia, pointing to a cardiorespiratory process driving the erythrocytosis, whereas co-oximetry can be used to identify the presence and amount of carboxyhemoglobin in the blood.
Bone marrow biopsy
An increase in pleomorphic megakaryocytes in the bone marrow without stainable iron is often described as characteristic in polycythemia vera patients, but it is not diagnostic. Panmyelosis with increased cellularity is the norm but can be seen in other myeloproliferative neoplasms. The morphologic features of bone marrow are now included as one of the major diagnostic criteria for polycythemia vera (Table 2).
OUR PATIENT’S FURTHER WORKUP
Our patient’s erythropoietin level is 34.2 mIU/mL (reference range 4.7–28.6). Her oxygen saturation is 96%, and her carboxyhemoglobin level is 1.1% (0–5).
She undergoes bone marrow biopsy. Analysis finds that the marrow is normocellular (60%) with trilineage hematopoiesis and decreased stainable iron.
Cytogenetic analysis shows a 46,XX[20] karyotype. Chromosomal microarray analysis shows no pathogenic copy-number changes. There is no detectable JAK2 V617F or exon 12-to-15 mutation.
The patient’s erythrocytosis and abnormal hemoglobin electrophoresis study raise suspicion for a variant type of hemoglobin that has a higher affinity for oxygen than normal.
3. What is the next best step to evaluate this patient?
- Red-cell oxygen equilibrium curve to calculate the P50 (the partial pressure of oxygen that is required to saturate 50% of the hemoglobin.)
- High-performance liquid chromatography
- Globin gene DNA sequencing
- Testing 2,3-bisphosphoglycerate mutase (BPGM) activity
Nearly 200 mutational variants in alpha and beta globin chains that lead to an increased affinity of hemoglobin for oxygen have been reported.12 While not all mutations are clinically significant, increased oxygen affinity variants can lead to impaired oxygen delivery to tissues, especially the kidneys, resulting in a physiologic increase in erythropoietin and erythrocytosis.
In patients being evaluated for a high-oxygen-affinity hemoglobinopathy, a two-step approach has been outlined.13 The first involves measuring the oxygen-binding properties of a freshly collected sample of blood by directly measuring the oxygen saturation of the hemoglobin and pO2 using a co-oximeter. This information is used to create a red cell oxygen equilibrium curve and to calculate the P50. A low P50 correlates with an abnormally high affinity of hemoglobin for oxygen.
The second step is to identify the abnormal hemoglobin. High-performance liquid chromatography is now widely available as a screening test but does not detect all variants. For many years, sequencing of globin chain DNA has been a gold standard for identifying specific mutations. Subsequent to analyzing a catalog of known hemoglobin variants, mass spectrometry can serve as a screening and identification technique. Mass spectroscopy can also detect known rare variants with posttranslational modifications14 that are not recognized by DNA analysis. Mass spectroscopy and DNA sequencing are complementary techniques available only in specialized reference laboratories.
Erythrocytosis due to BPGM deficiency is very rare. Clinical and laboratory features mimic those of high-oxygen-affinity hemoglobin, but patients do not have a demonstrable mutation in alpha or beta globin genes. The level of BPGM is low, and the diagnosis is established by measuring BPGM levels and sequencing the BPGM gene.15
RESULTS OF THE ADDITIONAL WORKUP
In our patient, hemoglobin electrophoresis reveals an abnormal hemoglobin variant. High-performance liquid chromatography reveals an abnormal peak that comprises approximately 23.7% of the total hemoglobin, consistent with an alpha globin variant. Further characterization (using a sample of venous blood) shows an oxygen dissociation P50 of 22 mm Hg (normal 24–30 mm Hg) (Figure 1).
Mass spectrometry identifies the variant as hemoglobin Tarrant. This variant is characterized by a substitution of asparagine for aspartic acid at position 126 of the alpha globin chain, a known site of contact between the alpha 1 and beta 1 chains.16 It has been seen in patients of Hispanic heritage and clinically correlates with mild erythrocytosis. Indeed, this woman’s mother was from Mexico.
EDUCATING PATIENTS
4. What should patients know about their high-oxygen-affinity hemoglobinopathy?
- High altitudes and air travel can be risky
- Pregnancy may have adverse outcomes
- Systemic anticoagulation may lower the risk of venous thromboembolism
- Periodic phlebotomy may help control symptoms
Most patients with high-oxygen-affinity hemoglobin do not require specific clinical management but only counseling and education about their condition. Establishing an accurate diagnosis is important in order to avoid further inappropriate, invasive, and expensive testing.
Although exposure to high altitudes may be associated with decreased ambient oxygen levels, hypoxia is usually not a problem because of hemoglobin’s high affinity for oxygen.
Impaired delivery of oxygen across the placenta may be anticipated in a mother with high-oxygen-affinity hemoglobin, but this has not been observed clinically.17
Compared with patients with polycythemia vera, patients with high-oxygen-affinity hemoglobin have fewer complications from hyperviscosity and thrombosis, even with comparable degrees of erythrocytosis.
Although patients usually do not require treatment, phlebotomy may be helpful for symptoms that can be attributed to the higher hemoglobin concentration.
Our patient continues to be seen in clinic for periodic blood counts and phlebotomy for her headaches, as required.
HEMOGLOBIN: RELAXED OR TENSE
Normal adult hemoglobin is a tetramer composed of two pairs of globin polypeptide chains: alpha and beta (Figure 2). The intrinsic properties of the constituent globin chains and their allosteric conformation—as well as extrinsic factors including temperature, pH, and the binding of hydrogen ion and 2,3-BPG—play important roles in modifying the affinity of hemoglobin for oxygen. The major modulator of hemoglobin-oxygen affinity in human erythrocytes is 2,3-BPG.
The hemoglobin tetramer, consisting of two identical halves, alpha 1-beta 1 and alpha 2-beta 2, oscillates between two quaternary conformations, “relaxed” (fully oxygenated) and “tense” (fully deoxygenated).18 High-oxygen-affinity hemoglobins can result from factors that enhance the relaxed state, either by stabilizing the relaxed state or by destabilizing the tense state. Structural modifications in hemoglobin typically affect the main contacts involved in the transition from the deoxygenated to the oxygenated state, the 2,3-BPG binding sites, the heme pocket, or elongation of globin chains by various mutations. In hemoglobin Tarrant, the mutation prevents formation of noncovalent salt bridges in the alpha 1-beta 1 contact that normally stabilize the deoxygenated conformation of hemoglobin. As a result, the deoxygenated (tense) state is destabilized, shifting the allosteric equilibrium in favor of the oxygenated (relaxed) state with consequent high oxygen affinity.16
MORE ABOUT HIGH-OXYGEN-AFFINITY HEMOGLOBINS
The first case of erythrocytosis due to an abnormal hemoglobin was identified in 1966. This was an alpha chain variant with an arginine-to-leucine substitution at position 92, named hemoglobin Chesapeake.19
High-oxygen-affinity hemoglobin variants are usually transmitted as autosomal dominant traits. Patients are most often identified because of unexplained erythrocytosis detected on a routine blood cell count, as in our patient.
Not all high-oxygen-affinity hemoglobinopathies are associated with erythrocytosis. The degree of increased oxygen affinity may only be mild or the abnormal hemoglobin may be slightly unstable, thereby masking the usual clinical signs and symptoms.
Therapeutic phlebotomy should be used cautiously since it can decrease delivery of oxygen to tissues. A subset of patients whose symptoms are related to an elevated red cell mass may experience some relief, as did our patient.
A 40-year-old woman with hypertrophic obstructive cardiomyopathy presents to the hematology clinic for a second opinion regarding a history of headaches and fatigue for the past 10 years. She has been diagnosed with idiopathic erythrocytosis, presumed to be due to polycythemia vera. She periodically undergoes phlebotomy to keep her hematocrit below 41%, and this markedly improves her headaches. She denies shortness of breath, cough, fever, weight loss, joint pain, and visual or other neurologic symptoms. She has never reported pruritus related to bathing or exposure to water.
She does not smoke, drink alcohol, or use illicit drugs. She works as a pharmacy technician. She says her father died of cancer (no further details available) and describes a family history of gastrointestinal malignancy in her grandfather and paternal aunt. She takes aspirin, metoprolol, and spironolactone for her cardiomyopathy.
Physical examination reveals generalized plethora, more marked on her cheeks and face, and mild bilateral pitting pedal edema. No lymphadenopathy or hepatosplenomegaly can be palpated. Other systems, including the cardiac, respiratory, and nervous systems, are normal.
ERYTHROCYTOSIS AND POLYCYTHEMIA VERA
1. In patients with erythrocytosis, which of the following is not characteristic of polycythemia vera?
- Erythromelalgia and postbathing pruritus
- Splenomegaly
- History of thrombosis
- Gout
- Hematuria
Erythrocytosis—an abnormally high concentration of red blood cells in the peripheral blood—is a laboratory finding. It often reflects an increase in the total quantity or mass of red blood cells in the body (polycythemia) but can sometimes be due to decreased plasma volume (spurious polycythemia).1 Erythrocytosis can be caused by a number of diseases, hereditary and acquired, and can be classified as primary or secondary (Table 1).
Symptoms arise from an increase in the total blood volume and red blood cell mass, often leading to dilated capillaries and other blood vessels. Symptoms can occur regardless of the cause and classically include headache (often described as diffuse heaviness), dizziness, and a tendency for bleeding or thrombosis.2 Symptoms are relieved when the hematocrit is lowered.
Several features in the history and physical examination of a patient being evaluated for erythrocytosis can suggest an underlying cause. Smoking, chronic respiratory insufficiency, and congenital cyanotic heart disease point to secondary erythrocytosis and can usually be identified at the outset. A history of occupational exposure to carbon monoxide (such as engine exhaust) should be elicited carefully. A family history of erythrocytosis should raise suspicion of a heritable condition such as a hemoglobinopathy associated with increased oxygen affinity or rare forms of primary erythrocytosis associated with endogenous overproduction of erythropoietin or activating mutations of the erythropoietin receptor.3 Iatrogenic causes such as androgen supplementation, erythropoietin abuse, and postrenal-transplant erythrocytosis should also be considered.
Secretion of erythropoietin or erythropoietinlike proteins by a malignant neoplasm is a rare but important cause of erythrocytosis. For example, renal cell carcinoma may present with erythrocytosis secondary to excessive erythropoietin production, and hematuria can be an early symptom.
Polycythemia vera
Polycythemia vera, a myeloproliferative neoplasm, is characterized by increased red blood cell production independent of the mechanisms that normally regulate erythropoiesis. The bone marrow shows a panmyelosis that is often accompanied by leukocytosis or thrombocytosis, or both, in the peripheral blood.
Symptoms such as severe itching after exposure to hot water (aquagenic pruritus) and periodic attacks of redness, swelling, and pain in the hands or feet, or both (erythromelalgia), have been described in patients with polycythemia vera. Splenomegaly is relatively common, seen in approximately two-thirds of patients.4 Hyperuricemia (from increased cell turnover) and gout are also associated with polycythemia vera, as is a history of arterial and venous thrombosis.5
Hematuria is not commonly seen in polycythemia vera, although bleeding from the bladder, vagina, or uterus has been described.
CASE RESUMED: INITIAL LABORATORY TESTS
Results of our patient’s initial laboratory tests are:
- Hemoglobin 16.9 g/dL (reference range 11.5–15.5)
- Hematocrit 48.8% (36.0–46.0)
- Mean corpuscular volume 85.2 fL (80–100)
- Platelet count 328 × 109/L (150–400)
- White blood cell count 9.14 × 109/L (3.7–11.0)
- Absolute neutrophil count 5.95 × 109/L (1.45–7.5)
- Blood urea nitrogen 12 mg/dL (8–25)
- Creatinine 0.5 mg/dL (0.7–1.4)
- Lactate dehydrogenase 180 U/L (100–220)
- Uric acid 3.0 mg/dL (2.0–7.0)
- Thyroid-stimulating hormone 2.2 µU/mL (0.4–5.5).
The patient undergoes additional tests, including a serum erythropoietin level and hemoglobinopathy screen. Bone marrow aspiration and biopsy are performed, with cytogenetic analysis, chromosomal microarray analysis, and molecular testing for mutation of the Janus kinase 2 (JAK2) gene.
CONFIRMING SUSPECTED POLYCYTHEMIA VERA
2. In patients with suspected polycythemia vera, which of the following laboratory tests is most useful in making the diagnosis?
- Hemoglobin, hematocrit, and red blood cell mass
- Serum erythropoietin level
- Arterial blood gases with co-oximetry
- Testing for the JAK2 mutation
- Bone marrow aspiration and biopsy
The aim of the initial workup of erythrocytosis is to differentiate polycythemia vera from secondary causes of erythrocytosis.
Hemoglobin, hematocrit, red cell mass
Erythrocytosis is defined by an abnormal elevation in the hematocrit (> 48% in women or > 49% in men), hemoglobin concentration (> 16.0 g/dL in women or > 16.5 g/dL in men), or red blood cell mass. The red blood cell count should not be used as a surrogate for red blood cell mass, since some anemias (especially thalassemia minor) can be associated with an increase in the number of red blood cells but a low hemoglobin concentration.
Isotope dilution techniques to determine the red cell mass and plasma volume can differentiate true erythrocytosis from a spurious elevation due to a decrease in plasma volume.6,7 However, this is an expensive, time-consuming test that is not widely available and so is rarely performed.8
JAK2 mutation testing
The initial evaluation of a patient with erythrocytosis has changed significantly in the past 10 years with the discovery of the JAK2 gene and its role in the pathogenesis of polycythemia vera and other myeloproliferative neoplasms.
JAK2, located at 9p24, codes for a tyrosine kinase important for signal transduction in hematopoietic cells. Mutations in this gene have been shown to promote hypersensitivity to cytokines, including erythropoietin.9 The most common somatic mutation occurs within exon 14 at base pair 1849 and results in a phenylalanine-for-valine amino acid substitution in the JAK2 protein, designated V617F. Less commonly, mutations occur elsewhere in exons 12 to 15, with more than 50 different mutations described; nonpolymorphic mutations are assumed to have biologic effects similar to those of V617F.
Taken together, the JAK2 V617F and non-V617F mutations have a diagnostic sensitivity of 98% to 100% for polycythemia vera. For practical purposes, this means that the presence of a JAK2 mutation can be used as a clonal marker to distinguish polycythemia vera from reactive secondary causes of erythrocytosis. A JAK2 mutation is one of three major diagnostic criteria for polycythemia vera in the 2016 revision to the 2008 World Health Organization criteria (Table 2).10 Of note, this mutation is not specific for polycythemia vera and can also be found in other myeloproliferative neoplasms, including primary myelofibrosis and essential thrombocythemia.
Absence of a JAK2 mutation makes polycythemia vera unlikely, so this test is most useful in making the diagnosis.
Serum erythropoietin
Serum erythropoietin testing can be very useful to distinguish polycythemia vera from secondary erythrocytosis. Low levels suggest polycythemia vera, while high levels are seen in secondary processes.11
This test is best used along with JAK2 V617F mutation analysis as an initial step in evaluating patients with erythrocytosis. When JAK2 V617F mutation analysis is negative, a low serum erythropoietin level should prompt further testing for non-V617F JAK2 mutations, whereas a normal or elevated erythropoietin level should be evaluated further with tests to distinguish hereditary from acquired secondary causes of erythrocytosis.
Arterial blood gas analysis and co-oximetry
Arterial blood gas analysis can reveal hypoxemia, pointing to a cardiorespiratory process driving the erythrocytosis, whereas co-oximetry can be used to identify the presence and amount of carboxyhemoglobin in the blood.
Bone marrow biopsy
An increase in pleomorphic megakaryocytes in the bone marrow without stainable iron is often described as characteristic in polycythemia vera patients, but it is not diagnostic. Panmyelosis with increased cellularity is the norm but can be seen in other myeloproliferative neoplasms. The morphologic features of bone marrow are now included as one of the major diagnostic criteria for polycythemia vera (Table 2).
OUR PATIENT’S FURTHER WORKUP
Our patient’s erythropoietin level is 34.2 mIU/mL (reference range 4.7–28.6). Her oxygen saturation is 96%, and her carboxyhemoglobin level is 1.1% (0–5).
She undergoes bone marrow biopsy. Analysis finds that the marrow is normocellular (60%) with trilineage hematopoiesis and decreased stainable iron.
Cytogenetic analysis shows a 46,XX[20] karyotype. Chromosomal microarray analysis shows no pathogenic copy-number changes. There is no detectable JAK2 V617F or exon 12-to-15 mutation.
The patient’s erythrocytosis and abnormal hemoglobin electrophoresis study raise suspicion for a variant type of hemoglobin that has a higher affinity for oxygen than normal.
3. What is the next best step to evaluate this patient?
- Red-cell oxygen equilibrium curve to calculate the P50 (the partial pressure of oxygen that is required to saturate 50% of the hemoglobin.)
- High-performance liquid chromatography
- Globin gene DNA sequencing
- Testing 2,3-bisphosphoglycerate mutase (BPGM) activity
Nearly 200 mutational variants in alpha and beta globin chains that lead to an increased affinity of hemoglobin for oxygen have been reported.12 While not all mutations are clinically significant, increased oxygen affinity variants can lead to impaired oxygen delivery to tissues, especially the kidneys, resulting in a physiologic increase in erythropoietin and erythrocytosis.
In patients being evaluated for a high-oxygen-affinity hemoglobinopathy, a two-step approach has been outlined.13 The first involves measuring the oxygen-binding properties of a freshly collected sample of blood by directly measuring the oxygen saturation of the hemoglobin and pO2 using a co-oximeter. This information is used to create a red cell oxygen equilibrium curve and to calculate the P50. A low P50 correlates with an abnormally high affinity of hemoglobin for oxygen.
The second step is to identify the abnormal hemoglobin. High-performance liquid chromatography is now widely available as a screening test but does not detect all variants. For many years, sequencing of globin chain DNA has been a gold standard for identifying specific mutations. Subsequent to analyzing a catalog of known hemoglobin variants, mass spectrometry can serve as a screening and identification technique. Mass spectroscopy can also detect known rare variants with posttranslational modifications14 that are not recognized by DNA analysis. Mass spectroscopy and DNA sequencing are complementary techniques available only in specialized reference laboratories.
Erythrocytosis due to BPGM deficiency is very rare. Clinical and laboratory features mimic those of high-oxygen-affinity hemoglobin, but patients do not have a demonstrable mutation in alpha or beta globin genes. The level of BPGM is low, and the diagnosis is established by measuring BPGM levels and sequencing the BPGM gene.15
RESULTS OF THE ADDITIONAL WORKUP
In our patient, hemoglobin electrophoresis reveals an abnormal hemoglobin variant. High-performance liquid chromatography reveals an abnormal peak that comprises approximately 23.7% of the total hemoglobin, consistent with an alpha globin variant. Further characterization (using a sample of venous blood) shows an oxygen dissociation P50 of 22 mm Hg (normal 24–30 mm Hg) (Figure 1).
Mass spectrometry identifies the variant as hemoglobin Tarrant. This variant is characterized by a substitution of asparagine for aspartic acid at position 126 of the alpha globin chain, a known site of contact between the alpha 1 and beta 1 chains.16 It has been seen in patients of Hispanic heritage and clinically correlates with mild erythrocytosis. Indeed, this woman’s mother was from Mexico.
EDUCATING PATIENTS
4. What should patients know about their high-oxygen-affinity hemoglobinopathy?
- High altitudes and air travel can be risky
- Pregnancy may have adverse outcomes
- Systemic anticoagulation may lower the risk of venous thromboembolism
- Periodic phlebotomy may help control symptoms
Most patients with high-oxygen-affinity hemoglobin do not require specific clinical management but only counseling and education about their condition. Establishing an accurate diagnosis is important in order to avoid further inappropriate, invasive, and expensive testing.
Although exposure to high altitudes may be associated with decreased ambient oxygen levels, hypoxia is usually not a problem because of hemoglobin’s high affinity for oxygen.
Impaired delivery of oxygen across the placenta may be anticipated in a mother with high-oxygen-affinity hemoglobin, but this has not been observed clinically.17
Compared with patients with polycythemia vera, patients with high-oxygen-affinity hemoglobin have fewer complications from hyperviscosity and thrombosis, even with comparable degrees of erythrocytosis.
Although patients usually do not require treatment, phlebotomy may be helpful for symptoms that can be attributed to the higher hemoglobin concentration.
Our patient continues to be seen in clinic for periodic blood counts and phlebotomy for her headaches, as required.
HEMOGLOBIN: RELAXED OR TENSE
Normal adult hemoglobin is a tetramer composed of two pairs of globin polypeptide chains: alpha and beta (Figure 2). The intrinsic properties of the constituent globin chains and their allosteric conformation—as well as extrinsic factors including temperature, pH, and the binding of hydrogen ion and 2,3-BPG—play important roles in modifying the affinity of hemoglobin for oxygen. The major modulator of hemoglobin-oxygen affinity in human erythrocytes is 2,3-BPG.
The hemoglobin tetramer, consisting of two identical halves, alpha 1-beta 1 and alpha 2-beta 2, oscillates between two quaternary conformations, “relaxed” (fully oxygenated) and “tense” (fully deoxygenated).18 High-oxygen-affinity hemoglobins can result from factors that enhance the relaxed state, either by stabilizing the relaxed state or by destabilizing the tense state. Structural modifications in hemoglobin typically affect the main contacts involved in the transition from the deoxygenated to the oxygenated state, the 2,3-BPG binding sites, the heme pocket, or elongation of globin chains by various mutations. In hemoglobin Tarrant, the mutation prevents formation of noncovalent salt bridges in the alpha 1-beta 1 contact that normally stabilize the deoxygenated conformation of hemoglobin. As a result, the deoxygenated (tense) state is destabilized, shifting the allosteric equilibrium in favor of the oxygenated (relaxed) state with consequent high oxygen affinity.16
MORE ABOUT HIGH-OXYGEN-AFFINITY HEMOGLOBINS
The first case of erythrocytosis due to an abnormal hemoglobin was identified in 1966. This was an alpha chain variant with an arginine-to-leucine substitution at position 92, named hemoglobin Chesapeake.19
High-oxygen-affinity hemoglobin variants are usually transmitted as autosomal dominant traits. Patients are most often identified because of unexplained erythrocytosis detected on a routine blood cell count, as in our patient.
Not all high-oxygen-affinity hemoglobinopathies are associated with erythrocytosis. The degree of increased oxygen affinity may only be mild or the abnormal hemoglobin may be slightly unstable, thereby masking the usual clinical signs and symptoms.
Therapeutic phlebotomy should be used cautiously since it can decrease delivery of oxygen to tissues. A subset of patients whose symptoms are related to an elevated red cell mass may experience some relief, as did our patient.
- Kremyanskaya M, Mascarenhas J, Hoffman R. Why does my patient have erythrocytosis? Hematol Oncol Clin North Am 2012; 26:267–283.
- Keohane C, McMullin MF, Harrison C. The diagnosis and management of erythrocytosis. BMJ 2013; 347:f6667.
- Agarwal N, Gordeuk RV, Prchal JT. Genetic mechanisms underlying regulation of hemoglobin mass. Adv Exp Med Biol 2007; 618:195–210.
- Tefferi A. Polycythemia vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 2012; 87:285–293.
- Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia 2008; 22:2020–2028.
- Tefferi A, Spivak JL. Polycythemia vera: scientific advances and current practice. Semin Hematol 2005; 42:206–220.
- Ferrant A. What clinical and laboratory data are indicative of polycythemia and when are blood volume studies needed? Nouv Rev Fr Hematol 1994; 36:151–154.
- Fairbanks VF, Klee GG, Wiseman GA, et al. Measurement of blood volume and red cell mass: re-examination of 51Cr and 125I methods. Blood Cells Mol Dis 1996; 22:169–186; discussion 186a–186g.
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434:1144–1148.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127:2391–2405.
- Messinezy M, Westwood NB, El-Hemaidi I, Marsden JT, Sherwood RS, Pearson TC. Serum erythropoietin values in erythrocytosis and in primary thrombocythaemia. Br J Haematol 2002; 117:47–53.
- Hardison RC, Chui DHK, Giardine B, et al. HbVar: a relational database of human hemoglobin variants and thalassemia mutations at the globin gene server. Human Mutat 2002; 19:225–233.
- Percy MJ, Butt NN, Crotty GM, et al. Identification of high oxygen affinity hemoglobin variants in the investigation of patients with erythrocytosis. Haematologica 2009; 94:1321–1322.
- Kattamis AC, Kelly KM, Ohene-Frempong K, et al. Hb Osler [beta 145(HC2)Tyr-->Asp] results from posttranslational modification. Hemoglobin 1997; 21:109–120.
- Hoyer JD, Allen SL, Beutler E, Kubik K, West C, Fairbanks VF. Erythrocytosis due to bisphosphoglycerate mutase deficiency with concurrent glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. Am J Hematol 2004; 75:205–208.
- Moo-Penn WF, Jue DL, Johnson MH, Wilson SM, Therrell B Jr, Schmidt RM. Hemoglobin Tarrant: alpha126(H9) asp leads to asn. A new hemoglobin variant in the alpha1beta1 contact region showing high oxygen affinity and reduced cooperativity. Biochim Biophys Acta 1977; 490:443–451.
- Bard H, Peri KG, Gagnon C. The biologic implications of a rare hemoglobin mutant that decreases oxygen affinity. Pediatr Res 2001; 49:69–73.
- Wajcman H, Galacteros F. Hemoglobins with high oxygen affinity leading to erythrocytosis: new variants and concepts. Hemoglobin 2005; 29:91–106.
- Clegg JB, Naughton MA, Weatherall DJ. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol 1966; 19:91–108.
- Kremyanskaya M, Mascarenhas J, Hoffman R. Why does my patient have erythrocytosis? Hematol Oncol Clin North Am 2012; 26:267–283.
- Keohane C, McMullin MF, Harrison C. The diagnosis and management of erythrocytosis. BMJ 2013; 347:f6667.
- Agarwal N, Gordeuk RV, Prchal JT. Genetic mechanisms underlying regulation of hemoglobin mass. Adv Exp Med Biol 2007; 618:195–210.
- Tefferi A. Polycythemia vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 2012; 87:285–293.
- Landolfi R, Di Gennaro L, Falanga A. Thrombosis in myeloproliferative disorders: pathogenetic facts and speculation. Leukemia 2008; 22:2020–2028.
- Tefferi A, Spivak JL. Polycythemia vera: scientific advances and current practice. Semin Hematol 2005; 42:206–220.
- Ferrant A. What clinical and laboratory data are indicative of polycythemia and when are blood volume studies needed? Nouv Rev Fr Hematol 1994; 36:151–154.
- Fairbanks VF, Klee GG, Wiseman GA, et al. Measurement of blood volume and red cell mass: re-examination of 51Cr and 125I methods. Blood Cells Mol Dis 1996; 22:169–186; discussion 186a–186g.
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434:1144–1148.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127:2391–2405.
- Messinezy M, Westwood NB, El-Hemaidi I, Marsden JT, Sherwood RS, Pearson TC. Serum erythropoietin values in erythrocytosis and in primary thrombocythaemia. Br J Haematol 2002; 117:47–53.
- Hardison RC, Chui DHK, Giardine B, et al. HbVar: a relational database of human hemoglobin variants and thalassemia mutations at the globin gene server. Human Mutat 2002; 19:225–233.
- Percy MJ, Butt NN, Crotty GM, et al. Identification of high oxygen affinity hemoglobin variants in the investigation of patients with erythrocytosis. Haematologica 2009; 94:1321–1322.
- Kattamis AC, Kelly KM, Ohene-Frempong K, et al. Hb Osler [beta 145(HC2)Tyr-->Asp] results from posttranslational modification. Hemoglobin 1997; 21:109–120.
- Hoyer JD, Allen SL, Beutler E, Kubik K, West C, Fairbanks VF. Erythrocytosis due to bisphosphoglycerate mutase deficiency with concurrent glucose-6-phosphate dehydrogenase (G-6-PD) deficiency. Am J Hematol 2004; 75:205–208.
- Moo-Penn WF, Jue DL, Johnson MH, Wilson SM, Therrell B Jr, Schmidt RM. Hemoglobin Tarrant: alpha126(H9) asp leads to asn. A new hemoglobin variant in the alpha1beta1 contact region showing high oxygen affinity and reduced cooperativity. Biochim Biophys Acta 1977; 490:443–451.
- Bard H, Peri KG, Gagnon C. The biologic implications of a rare hemoglobin mutant that decreases oxygen affinity. Pediatr Res 2001; 49:69–73.
- Wajcman H, Galacteros F. Hemoglobins with high oxygen affinity leading to erythrocytosis: new variants and concepts. Hemoglobin 2005; 29:91–106.
- Clegg JB, Naughton MA, Weatherall DJ. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol 1966; 19:91–108.
Are there alternatives to surgery for Zenker diverticulum?
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
ESOPHAGEAL POUCH IN SPACE BETWEEN MUSCLES
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
USUALLY PRESENTS WITH DYSPHAGIA
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
TREATMENT IS SURGERY OR ENDOSCOPY
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
Open surgery
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
- Diverticulectomy
- Diverticulopexy, in which the diverticulum is repositioned and sutured against the prevertebral fascia
- Diverticular inversion, in which the diverticulum is inverted into the esophageal lumen and then oversewn.4
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Endoscopic procedures
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Improved device on horizon
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
TAKE-HOME POINTS
- Zenker diverticulum is an outpouching of the esophageal mucosa. It is often asymptomatic. Patients with minimal symptoms and with no functional impairment can be followed conservatively.
- A head-to-head comparison of surgical and endoscopic therapy has not yet been done, and optimal patient selection criteria are lacking. Therefore, treatment recommendations are based primarily on expert opinion.
- We recommend flexible endoscopic therapy as initial treatment in patients with a small diverticulum, but this may be limited by the unavailability of a surgeon experienced in this technique.
- For a large diverticulum, we recommend an open cervical procedure involving excision of the diverticulum or a diverticulopexy for patients who are good surgical candidates.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.
- Ferreira LE, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus 2008; 21:1–8.
- Manno M, Manta R, Caruso A, et al. Alternative endoscopic treatment of Zenker’s diverticulum: a case series (with video). Gastrointest Endosc 2014; 79:168–170.
- Yuan Y, Zhao YF, Hu Y, Chen LQ. Surgical treatment of Zenker’s diverticulum. Dig Surg 2013; 30:207–218.
- Bizzotto A, Iacopini F, Landi R, Costamagna G. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngol Ital 2013; 33:219–229.
- Repici A, Pagano N, Fumagalli U, et. al. Transoral treatment of Zenker diverticulum: flexible endoscopy versus endoscopic stapling. A retrospective comparison of outcomes. Dis Esophagus 2011; 24:235–239.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol 2014; 12:1773–1782.
- Huberty V, El Bacha S, Blero D, Le Moine O, Hassid S, Devière J. Endoscopic treatment for Zenker’s diverticulum: long-term results (with video). Gastrointest Endosc 2013; 77:701–707.
- Case DJ, Baron TH. Flexible endoscopic management of Zenker diverticulum: the Mayo Clinic experience. Mayo Clin Proc 2010; 85:719–722.
- Al-Kadi AS, Maghrabi AA, Thomson D, Gillman LM, Dhalla S. Endoscopic treatment of Zenker diverticulum: results of a 7-year experience. J Am Coll Surg 2010; 211:239–243.
- Costamagna G, Iacopini F, Tringali A, et al. Flexible endoscopic Zenker’s diverticulotomy: cap-assisted technique vs diverticuloscope-assisted technique. Endoscopy 2007; 39:146–152.
Correction: Pancreatectomy and islet cell autotransplantation
The article “Total pancreatectomy and islet cell autotransplantation: Definitive treatment for chronic pancreatitis” (Arce KM, Lin YK, Stevens T, Walsh RM, Hatipoglu BA. Cleve Clin J Med 2016; 83:435–442) incorrectly stated that Paul Lacy and David Scharp performed research at the University of Washington at Seattle. They did their work at Washington University in St. Louis, Missouri.
The article “Total pancreatectomy and islet cell autotransplantation: Definitive treatment for chronic pancreatitis” (Arce KM, Lin YK, Stevens T, Walsh RM, Hatipoglu BA. Cleve Clin J Med 2016; 83:435–442) incorrectly stated that Paul Lacy and David Scharp performed research at the University of Washington at Seattle. They did their work at Washington University in St. Louis, Missouri.
The article “Total pancreatectomy and islet cell autotransplantation: Definitive treatment for chronic pancreatitis” (Arce KM, Lin YK, Stevens T, Walsh RM, Hatipoglu BA. Cleve Clin J Med 2016; 83:435–442) incorrectly stated that Paul Lacy and David Scharp performed research at the University of Washington at Seattle. They did their work at Washington University in St. Louis, Missouri.
An abnormal peripheral blood smear and altered mental status
A 72-year-old woman with type 2 diabetes mellitus, hypertension, and atrial fibrillation on anticoagulation was brought to the emergency department by her husband after 1 day of altered mental status with acute onset. Her husband reported that she had been minimally arousable, and the physical examination revealed that she was stuporous and withdrew extremities only from noxious stimuli.
Results of initial laboratory tests revealed a creatinine level of 2.4 mg/dL (reference range 0.7–1.4), hemoglobin 12.1 g/dL (12–16), platelet count 16 × 109/L (150–400), white blood cell count of 7.7 × 109/L (3.7–11), and international normalized ratio of 2.1. A peripheral blood smear is shown in Figure 1.
Computed tomography showed evidence of chronic small vascular ischemia. Magnetic resonance imaging of the brain showed numerous foci of restricted diffusion within the supratentorial and infratentorial areas, suggesting microembolic phenomena.
The peripheral blood smear was compatible with microangiopathic hemolytic anemia, which can occur in thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome, malignant hypertension, scleroderma, antiphospholipid antibody syndrome, systemic lupus erythematosus, eclampsia, renal allograft rejection, hematopoietic stem cell transplant, and severe sepsis.1,2
In addition to hemolytic anemia, the patient also had neurologic abnormalities, renal involvement, and thrombocytopenia. The hemolytic anemia and thrombocytopenia were sufficient to raise our suspicion of TTP and to consider initiation of plasma exchange. Only 5% of patients with TTP demonstrate the classic pentad of clinical features,1 ie, thrombocytopenia, microangiopathic hemolytic anemia, fluctuating neurologic signs, renal impairment, and fever.
In 1991, when plasma exchange was introduced for TTP, the survival rate of patients increased from 10% to 78%.1,3 Thus, the diagnosis of TTP is an urgent indication for plasma exchange. We normally do plasma exchange daily until the platelet levels improve.
Our patient received methylprednisone 125 mg intravenously every 12 hours and plasma exchange daily. After three cycles of plasma exchange, she regained normal consciousness, and her platelet count had increased to 20.5 × 109/L on the day of discharge from our hospital.
TTP is a life-threatening hematologic disorder. Evidence of microangiopathic hemolytic anemia on a peripheral blood smear is vital to the suspicion of TTP. The diagnosis should be confirmed by ADAMTS13 testing, which should show decreased activity (< 10%) or increased inhibition, or both. Rapid management with plasma exchange and steroids can lead to a satisfactory outcome.
Acknowledgment: We are particularly grateful to Dr. Vivian Arguello (Director of Flow Cytometry, Department of Pathology, Einstein Medical Center, Philadelphia) for her kind support with the blood smear image.
- George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood 2010; 116:4060–4069.
- Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 2008; 112:11–18.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med 1991; 325:393–397.
A 72-year-old woman with type 2 diabetes mellitus, hypertension, and atrial fibrillation on anticoagulation was brought to the emergency department by her husband after 1 day of altered mental status with acute onset. Her husband reported that she had been minimally arousable, and the physical examination revealed that she was stuporous and withdrew extremities only from noxious stimuli.
Results of initial laboratory tests revealed a creatinine level of 2.4 mg/dL (reference range 0.7–1.4), hemoglobin 12.1 g/dL (12–16), platelet count 16 × 109/L (150–400), white blood cell count of 7.7 × 109/L (3.7–11), and international normalized ratio of 2.1. A peripheral blood smear is shown in Figure 1.
Computed tomography showed evidence of chronic small vascular ischemia. Magnetic resonance imaging of the brain showed numerous foci of restricted diffusion within the supratentorial and infratentorial areas, suggesting microembolic phenomena.
The peripheral blood smear was compatible with microangiopathic hemolytic anemia, which can occur in thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome, malignant hypertension, scleroderma, antiphospholipid antibody syndrome, systemic lupus erythematosus, eclampsia, renal allograft rejection, hematopoietic stem cell transplant, and severe sepsis.1,2
In addition to hemolytic anemia, the patient also had neurologic abnormalities, renal involvement, and thrombocytopenia. The hemolytic anemia and thrombocytopenia were sufficient to raise our suspicion of TTP and to consider initiation of plasma exchange. Only 5% of patients with TTP demonstrate the classic pentad of clinical features,1 ie, thrombocytopenia, microangiopathic hemolytic anemia, fluctuating neurologic signs, renal impairment, and fever.
In 1991, when plasma exchange was introduced for TTP, the survival rate of patients increased from 10% to 78%.1,3 Thus, the diagnosis of TTP is an urgent indication for plasma exchange. We normally do plasma exchange daily until the platelet levels improve.
Our patient received methylprednisone 125 mg intravenously every 12 hours and plasma exchange daily. After three cycles of plasma exchange, she regained normal consciousness, and her platelet count had increased to 20.5 × 109/L on the day of discharge from our hospital.
TTP is a life-threatening hematologic disorder. Evidence of microangiopathic hemolytic anemia on a peripheral blood smear is vital to the suspicion of TTP. The diagnosis should be confirmed by ADAMTS13 testing, which should show decreased activity (< 10%) or increased inhibition, or both. Rapid management with plasma exchange and steroids can lead to a satisfactory outcome.
Acknowledgment: We are particularly grateful to Dr. Vivian Arguello (Director of Flow Cytometry, Department of Pathology, Einstein Medical Center, Philadelphia) for her kind support with the blood smear image.
A 72-year-old woman with type 2 diabetes mellitus, hypertension, and atrial fibrillation on anticoagulation was brought to the emergency department by her husband after 1 day of altered mental status with acute onset. Her husband reported that she had been minimally arousable, and the physical examination revealed that she was stuporous and withdrew extremities only from noxious stimuli.
Results of initial laboratory tests revealed a creatinine level of 2.4 mg/dL (reference range 0.7–1.4), hemoglobin 12.1 g/dL (12–16), platelet count 16 × 109/L (150–400), white blood cell count of 7.7 × 109/L (3.7–11), and international normalized ratio of 2.1. A peripheral blood smear is shown in Figure 1.
Computed tomography showed evidence of chronic small vascular ischemia. Magnetic resonance imaging of the brain showed numerous foci of restricted diffusion within the supratentorial and infratentorial areas, suggesting microembolic phenomena.
The peripheral blood smear was compatible with microangiopathic hemolytic anemia, which can occur in thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome, malignant hypertension, scleroderma, antiphospholipid antibody syndrome, systemic lupus erythematosus, eclampsia, renal allograft rejection, hematopoietic stem cell transplant, and severe sepsis.1,2
In addition to hemolytic anemia, the patient also had neurologic abnormalities, renal involvement, and thrombocytopenia. The hemolytic anemia and thrombocytopenia were sufficient to raise our suspicion of TTP and to consider initiation of plasma exchange. Only 5% of patients with TTP demonstrate the classic pentad of clinical features,1 ie, thrombocytopenia, microangiopathic hemolytic anemia, fluctuating neurologic signs, renal impairment, and fever.
In 1991, when plasma exchange was introduced for TTP, the survival rate of patients increased from 10% to 78%.1,3 Thus, the diagnosis of TTP is an urgent indication for plasma exchange. We normally do plasma exchange daily until the platelet levels improve.
Our patient received methylprednisone 125 mg intravenously every 12 hours and plasma exchange daily. After three cycles of plasma exchange, she regained normal consciousness, and her platelet count had increased to 20.5 × 109/L on the day of discharge from our hospital.
TTP is a life-threatening hematologic disorder. Evidence of microangiopathic hemolytic anemia on a peripheral blood smear is vital to the suspicion of TTP. The diagnosis should be confirmed by ADAMTS13 testing, which should show decreased activity (< 10%) or increased inhibition, or both. Rapid management with plasma exchange and steroids can lead to a satisfactory outcome.
Acknowledgment: We are particularly grateful to Dr. Vivian Arguello (Director of Flow Cytometry, Department of Pathology, Einstein Medical Center, Philadelphia) for her kind support with the blood smear image.
- George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood 2010; 116:4060–4069.
- Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 2008; 112:11–18.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med 1991; 325:393–397.
- George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood 2010; 116:4060–4069.
- Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 2008; 112:11–18.
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med 1991; 325:393–397.
A man with HIV and papules and nodules on the knees
A 39-year-old man with human immunodeficiency virus (HIV) infection and a CD4 cell count of 528 × 106/L without treatment was referred for evaluation of periarticular, indurated, erythematous papules and nodules on the knees and elbows and purpuric lesions on the ankles (Figure 1). He has had recurrent fever, arthralgia, and mild constitutional symptoms during the past month. He also reported a diagnosis of polyclonal immunoglobulin A gammopathy.
Punch biopsy of the purpuric lesions was performed, and histologic study showed leukocytoclastic vasculitis with eosinophilic necrosis of the epithelium.
Treatment with a systemic corticosteroid was started. The purpuric lesions disappeared after 3 weeks of therapy, but the nodules over the extensor surface of both knees showed no improvement (Figure 2). Subsequent biopsy of late-stage lesions (3 months after the start of therapy) demonstrated perivascular fibrosis with small, persistent foci of vasculitis, and confirmed the diagnosis of HIV-associated nodular erythema elevatum diutinum (EED). Antiretroviral therapy was started in addition to intralesional corticosteroids and topical dapsone 5% gel, with resolution of the lesions 1 month later.
ERYTHEMA ELEVATUM DIUTINUM
EED is an uncommon chronic dermatosis, classified as a fibrosing form of leukocytoclastic vasculitis and characterized clinically by violaceous papules, plaques, and nodules, usually distributed acrally and symmetrically over extensor surfaces. The histopathologic picture depends on the stage of the lesion. Features of leukocytoclastic vasculitis are found in early-stage lesions, while a fibrotic replacement of the dermis with small persistent foci of vasculitis is typical of late-stage lesions.
EED has clinical and histopathologic similarities to Sweet syndrome, but EED is distinguished from neutrophilic dermatosis by vasculitis.
In HIV-infected patients, it is important to include pruritic papular eruption in the differential diagnosis. It is characterized by chronic bilaterally symmetric pruritic papules on the trunk and extremities and is the most common cutaneous noninfectious manifestation of HIV.
The clinical presentation of EED may also be easily confused with Kaposi sarcoma and bacillary angiomatosis.2
AN EMERGING HIV-RELATED DERMATOSIS
EED is emerging as a specific HIV-associated dermatosis, with 20 cases reported in the medical literature as of this writing.3
The cause of EED is not known, but it is often associated with streptococcal infection, monoclonal IgA gammopathy, hematologic malignancy, cryoglobulinemia, rheumatoid arthritis, and autoimmune disease.3 The stimulus could be immune-complex deposition in blood vessels triggered by HIV infection, or by another infection acting as an antigenic stimulus.4 The nodular variant of EED is even rarer, but it evolves most often in HIV-positive individuals.5,6
Oral dapsone is the treatment of choice but is less effective in late-stage fibrotic lesions.7 Treatment courses tend to be long and recurrence is common.8 Intralesional, topical, and oral corticosteroids, topical dapsone 5% gel,9 tetracycline and nicotinamide, sulfonamides, colchicine, chloroquine, and surgical excision are other options.
Our patient’s presentation reminds us to consider EED in HIV-infected patients and illustrates the importance of histologic diagnosis to differentiate EED from assumed Kaposi sarcoma in patients with HIV. EED can also be the first clinical sign of HIV infection. It is important to rule out underlying disorders such as HIV infection, because directed therapy is often the best management.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol 1992; 26:38–44.
- Requena L, Sánchez Yus E, Martín L, Barat A, Arias D. Erythema elevatum diutinum in a patient with acquired immunodeficiency syndrome. Another clinical simulator of Kaposi’s sarcoma. Arch Dermatol 1991; 127:1819–1822.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol 2014; 28:1594–1602.
- Muratori S, Carrera C, Gorani A, Alessi E. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol 1999; 141:335–338.
- LeBoit PE, Cockerell CJ. Nodular lesions of erythema elevatum diutinum in patients infected with the human immunodeficiency virus. J Am Acad Dermatol 1993; 28:919–922.
- Rover PA, Bittencourt C, Discacciati MP, Zaniboni MC, Arruda LH, Cintra ML. Erythema elevatum diutinum as a first clinical manifestation for diagnosing HIV infection: case history. Sao Paulo Med J 2005; 123:201–203.
- Fakheri A, Gupta SM, White SM, Don PC, Weinberg JM. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis 2001; 68:41–42.
- Katz SI, Gallin JL, Hertz KC, Fauci AS, Lawley TJ. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore) 1977; 56:443–455.
- Frieling GW, Williams NL, Lim SJ, Rosenthal SI. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol 2013; 12:481–484.
A 39-year-old man with human immunodeficiency virus (HIV) infection and a CD4 cell count of 528 × 106/L without treatment was referred for evaluation of periarticular, indurated, erythematous papules and nodules on the knees and elbows and purpuric lesions on the ankles (Figure 1). He has had recurrent fever, arthralgia, and mild constitutional symptoms during the past month. He also reported a diagnosis of polyclonal immunoglobulin A gammopathy.
Punch biopsy of the purpuric lesions was performed, and histologic study showed leukocytoclastic vasculitis with eosinophilic necrosis of the epithelium.
Treatment with a systemic corticosteroid was started. The purpuric lesions disappeared after 3 weeks of therapy, but the nodules over the extensor surface of both knees showed no improvement (Figure 2). Subsequent biopsy of late-stage lesions (3 months after the start of therapy) demonstrated perivascular fibrosis with small, persistent foci of vasculitis, and confirmed the diagnosis of HIV-associated nodular erythema elevatum diutinum (EED). Antiretroviral therapy was started in addition to intralesional corticosteroids and topical dapsone 5% gel, with resolution of the lesions 1 month later.
ERYTHEMA ELEVATUM DIUTINUM
EED is an uncommon chronic dermatosis, classified as a fibrosing form of leukocytoclastic vasculitis and characterized clinically by violaceous papules, plaques, and nodules, usually distributed acrally and symmetrically over extensor surfaces. The histopathologic picture depends on the stage of the lesion. Features of leukocytoclastic vasculitis are found in early-stage lesions, while a fibrotic replacement of the dermis with small persistent foci of vasculitis is typical of late-stage lesions.
EED has clinical and histopathologic similarities to Sweet syndrome, but EED is distinguished from neutrophilic dermatosis by vasculitis.
In HIV-infected patients, it is important to include pruritic papular eruption in the differential diagnosis. It is characterized by chronic bilaterally symmetric pruritic papules on the trunk and extremities and is the most common cutaneous noninfectious manifestation of HIV.
The clinical presentation of EED may also be easily confused with Kaposi sarcoma and bacillary angiomatosis.2
AN EMERGING HIV-RELATED DERMATOSIS
EED is emerging as a specific HIV-associated dermatosis, with 20 cases reported in the medical literature as of this writing.3
The cause of EED is not known, but it is often associated with streptococcal infection, monoclonal IgA gammopathy, hematologic malignancy, cryoglobulinemia, rheumatoid arthritis, and autoimmune disease.3 The stimulus could be immune-complex deposition in blood vessels triggered by HIV infection, or by another infection acting as an antigenic stimulus.4 The nodular variant of EED is even rarer, but it evolves most often in HIV-positive individuals.5,6
Oral dapsone is the treatment of choice but is less effective in late-stage fibrotic lesions.7 Treatment courses tend to be long and recurrence is common.8 Intralesional, topical, and oral corticosteroids, topical dapsone 5% gel,9 tetracycline and nicotinamide, sulfonamides, colchicine, chloroquine, and surgical excision are other options.
Our patient’s presentation reminds us to consider EED in HIV-infected patients and illustrates the importance of histologic diagnosis to differentiate EED from assumed Kaposi sarcoma in patients with HIV. EED can also be the first clinical sign of HIV infection. It is important to rule out underlying disorders such as HIV infection, because directed therapy is often the best management.
A 39-year-old man with human immunodeficiency virus (HIV) infection and a CD4 cell count of 528 × 106/L without treatment was referred for evaluation of periarticular, indurated, erythematous papules and nodules on the knees and elbows and purpuric lesions on the ankles (Figure 1). He has had recurrent fever, arthralgia, and mild constitutional symptoms during the past month. He also reported a diagnosis of polyclonal immunoglobulin A gammopathy.
Punch biopsy of the purpuric lesions was performed, and histologic study showed leukocytoclastic vasculitis with eosinophilic necrosis of the epithelium.
Treatment with a systemic corticosteroid was started. The purpuric lesions disappeared after 3 weeks of therapy, but the nodules over the extensor surface of both knees showed no improvement (Figure 2). Subsequent biopsy of late-stage lesions (3 months after the start of therapy) demonstrated perivascular fibrosis with small, persistent foci of vasculitis, and confirmed the diagnosis of HIV-associated nodular erythema elevatum diutinum (EED). Antiretroviral therapy was started in addition to intralesional corticosteroids and topical dapsone 5% gel, with resolution of the lesions 1 month later.
ERYTHEMA ELEVATUM DIUTINUM
EED is an uncommon chronic dermatosis, classified as a fibrosing form of leukocytoclastic vasculitis and characterized clinically by violaceous papules, plaques, and nodules, usually distributed acrally and symmetrically over extensor surfaces. The histopathologic picture depends on the stage of the lesion. Features of leukocytoclastic vasculitis are found in early-stage lesions, while a fibrotic replacement of the dermis with small persistent foci of vasculitis is typical of late-stage lesions.
EED has clinical and histopathologic similarities to Sweet syndrome, but EED is distinguished from neutrophilic dermatosis by vasculitis.
In HIV-infected patients, it is important to include pruritic papular eruption in the differential diagnosis. It is characterized by chronic bilaterally symmetric pruritic papules on the trunk and extremities and is the most common cutaneous noninfectious manifestation of HIV.
The clinical presentation of EED may also be easily confused with Kaposi sarcoma and bacillary angiomatosis.2
AN EMERGING HIV-RELATED DERMATOSIS
EED is emerging as a specific HIV-associated dermatosis, with 20 cases reported in the medical literature as of this writing.3
The cause of EED is not known, but it is often associated with streptococcal infection, monoclonal IgA gammopathy, hematologic malignancy, cryoglobulinemia, rheumatoid arthritis, and autoimmune disease.3 The stimulus could be immune-complex deposition in blood vessels triggered by HIV infection, or by another infection acting as an antigenic stimulus.4 The nodular variant of EED is even rarer, but it evolves most often in HIV-positive individuals.5,6
Oral dapsone is the treatment of choice but is less effective in late-stage fibrotic lesions.7 Treatment courses tend to be long and recurrence is common.8 Intralesional, topical, and oral corticosteroids, topical dapsone 5% gel,9 tetracycline and nicotinamide, sulfonamides, colchicine, chloroquine, and surgical excision are other options.
Our patient’s presentation reminds us to consider EED in HIV-infected patients and illustrates the importance of histologic diagnosis to differentiate EED from assumed Kaposi sarcoma in patients with HIV. EED can also be the first clinical sign of HIV infection. It is important to rule out underlying disorders such as HIV infection, because directed therapy is often the best management.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol 1992; 26:38–44.
- Requena L, Sánchez Yus E, Martín L, Barat A, Arias D. Erythema elevatum diutinum in a patient with acquired immunodeficiency syndrome. Another clinical simulator of Kaposi’s sarcoma. Arch Dermatol 1991; 127:1819–1822.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol 2014; 28:1594–1602.
- Muratori S, Carrera C, Gorani A, Alessi E. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol 1999; 141:335–338.
- LeBoit PE, Cockerell CJ. Nodular lesions of erythema elevatum diutinum in patients infected with the human immunodeficiency virus. J Am Acad Dermatol 1993; 28:919–922.
- Rover PA, Bittencourt C, Discacciati MP, Zaniboni MC, Arruda LH, Cintra ML. Erythema elevatum diutinum as a first clinical manifestation for diagnosing HIV infection: case history. Sao Paulo Med J 2005; 123:201–203.
- Fakheri A, Gupta SM, White SM, Don PC, Weinberg JM. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis 2001; 68:41–42.
- Katz SI, Gallin JL, Hertz KC, Fauci AS, Lawley TJ. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore) 1977; 56:443–455.
- Frieling GW, Williams NL, Lim SJ, Rosenthal SI. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol 2013; 12:481–484.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol 1992; 26:38–44.
- Requena L, Sánchez Yus E, Martín L, Barat A, Arias D. Erythema elevatum diutinum in a patient with acquired immunodeficiency syndrome. Another clinical simulator of Kaposi’s sarcoma. Arch Dermatol 1991; 127:1819–1822.
- Momen SE, Jorizzo J, Al-Niaimi F. Erythema elevatum diutinum: a review of presentation and treatment. J Eur Acad Dermatol Venereol 2014; 28:1594–1602.
- Muratori S, Carrera C, Gorani A, Alessi E. Erythema elevatum diutinum and HIV infection: a report of five cases. Br J Dermatol 1999; 141:335–338.
- LeBoit PE, Cockerell CJ. Nodular lesions of erythema elevatum diutinum in patients infected with the human immunodeficiency virus. J Am Acad Dermatol 1993; 28:919–922.
- Rover PA, Bittencourt C, Discacciati MP, Zaniboni MC, Arruda LH, Cintra ML. Erythema elevatum diutinum as a first clinical manifestation for diagnosing HIV infection: case history. Sao Paulo Med J 2005; 123:201–203.
- Fakheri A, Gupta SM, White SM, Don PC, Weinberg JM. Erythema elevatum diutinum in a patient with human immunodeficiency virus. Cutis 2001; 68:41–42.
- Katz SI, Gallin JL, Hertz KC, Fauci AS, Lawley TJ. Erythema elevatum diutinum: skin and systemic manifestations, immunologic studies, and successful treatment with dapsone. Medicine (Baltimore) 1977; 56:443–455.
- Frieling GW, Williams NL, Lim SJ, Rosenthal SI. Novel use of topical dapsone 5% gel for erythema elevatum diutinum: safer and effective. J Drugs Dermatol 2013; 12:481–484.
‘Air-raising’: An air-fluid level in the right subphrenic region
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
A 39-year-old Filipino man presented with nausea, vomiting, and abdominal pain of 2 weeks’ duration. He did not report trauma, and he had no history of medical illness or surgery.
On arrival, his blood pressure was 123/83 mm Hg, pulse 122 beats per minute, respiratory rate 18 breaths per minute, and temperature 100.7°F (38.1°C). On physical examination, he exhibited marked tenderness of the right upper quadrant on palpation. The abdomen was otherwise soft with no guarding or rebound tenderness.
Results of initial laboratory testing were as follows:
- Leukocyte count 17.0 × 109/L (reference range 4.5–11.0)
- Serum glucose 558 mg/dL without ketoacidosis
- Aspartate aminotransferase 109 U/L (2–40)
- Alanine aminotranferase 28 U/L (2–50)
- Total serum bilirubin 4.0 mg/dL (0.0–1.5).
Plain chest radiography showed dramatic elevation of the right hemidiaphragm with a large subphrenic air-fluid level (Figure 1). Abdominal computed tomography (CT) demonstrated a multiloculated hepatic abscess 18 × 13.5 cm subjacent to the diaphragm (Figure 2). Cultures of blood and the abscess yielded Klebsiella pneumoniae. The patient recovered after percutaneous drainage and a course of ceftriaxone.
PRIMARY KLEBSIELLA LIVER ABSCESS
K pneumoniae, a gram-negative aerobic encapsulated bacillus of the normal human intestinal flora, is closely related to Escherichia coli, historically the most frequent bacterial cause of pyogenic liver abscess.1 Over the last 30 years, K pneumoniae has eclipsed E coli as the most common causative agent, with the epicenter of this trend being located in Taiwan and South Korea, perhaps because rates of fecal Klebsiella carriage in that region are particularly high.1,2
Concurrently, there has been increasing recognition—initially across Asia, but lately in Europe and the Western Hemisphere—of the so-called invasive Klebsiella liver abscess (KLA) syndrome, virtually unique to the hypervirulent K1 and K2 capsular serotypes of K pneumoniae prevalent in Asia.3–6 This community-acquired syndrome is characterized by hematogenous deposition of the organism at distant sites, such as the lung, soft tissues, central nervous system, and eyes. Impairment of phagocytic function, as occurs in diabetes mellitus, and the resistance to phagocytosis conferred by the K1 and K2 serotypes have been identified as predisposing factors for dissemination.7,8 The mucoid phenotype of K pneumoniae, very common in Asian isolates of the K1 and K2 serotypes, is also associated with hypervirulence and extrahepatic spread, presumably through evasion of phagocytosis and complement-mediated opsonization.2,9
Our patient’s risk factors for KLA were his Asian origin and uncontrolled diabetes. No evidence of remote infection was detected during his hospitalization.
HEMIDIAPHRAGM ELEVATION
Acquired hemidiaphragm elevation is most commonly unilateral and typically represents an incidental radiologic finding attributable to paralysis of the corresponding diaphragm after phrenic nerve injury caused by trauma, surgery, or infection. Unilateral diaphragmatic paralysis is classically confirmed by performing a fluoroscopic sniff test, which is positive if the affected hemidiaphragm is observed in real time to paradoxically move upward during forced inhalation.10 This condition is usually asymptomatic at rest but could cause exertional dyspnea and contribute to ventilatory failure when pulmonary disease coexists.11
Occasionally, as in our patient, hemidiaphragm elevation is part of the presentation of active abdominal pathology that displaces the corresponding hemidiaphragm cephalad by mass effect. Examples of such space-occupying abdominal lesions include infections, malignancy, hepatosplenomegaly, and pneumoperitoneum from a ruptured viscus. Pneumoperitoneum is suggested by the presence of an air crescent immediately subjacent to the affected hemidiaphragm on an upright radiograph accompanied by peritoneal signs.
Although there was subphrenic air on this patient’s initial chest radiograph, it was actually part of an air-fluid level without associated peritoneal signs. An air-fluid level is characterized by a sharp horizontal demarcation between the lighter gas component floating at the top and the heavier fluid component settling on the bottom (Figure 1). The subsequent CT excluded free intra-abdominal air while identifying a large hepatic abscess as the cause of hemidiaphragm elevation. In trauma victims, CT is also helpful in ruling out diaphragmatic rupture, which can have a similar radiographic appearance.12
Our patient’s presentation was a reminder that an elevated hemidiaphragm may reflect abdominal pathology and that subphrenic air in this context need not be either “free” or a surgical emergency. Drainage of the abscess restored the normal position of our patient’s right hemidiaphragm (Figure 3).
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
- Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess: changing trends over 42 years. Ann Surg 1996; 223:600–607.
- Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 2012; 12:13.
- Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 1998; 26:1434–1438.
- Pastagia M, Arumugam V. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 2008; 6:228–233.
- Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004; 39:1654–1659.
- Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013; 41:681–686.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLoS One 2011; 6:e23366.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012; 12:881–887.
- Gierada DS, Slone RM, Fleishman MJ. Imaging evaluation of the diaphragm. Chest Surg Clin North Am 1998; 8:237–280.
- Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med 2009; 30:315–320.
- Havens JM, Kelly E, Patel V. A 78-year-old man with an elevated hemidiaphragm following trauma. Chest 2008; 134:1336–1339.
The people behind the Journal really matter
Our goal at the Cleveland Clinic Journal of Medicine is to provide timely, readily digestible, and useful clinical information to our readers. To do so, we need authors who buy into our educational mission, but we also need conscientious peer reviewers and an editorial staff capable of turning “doctorese” into readily understood English.
Our physician deputy editors Pelin Batur and James Pile solicit articles, guide authors, draft and revise CME questions, and assist greatly in the peer review process. Our nonphysician editors edit manuscripts to achieve a consistent editorial style in all of our published papers, but they serve many other key functions. They manage the business of publishing a monthly journal at a time of drastically shrinking advertising revenue, they ensure that Journal content complies with rules for CME material published in print and online, and they keep up with continuous changes in online publishing. The Journal receives significant funding from Cleveland Clinic’s Education Institute, funding we need to pursue our role as an independent, peer-reviewed conveyer of clinical information.
I write this to emphasize that real people manage all of these tasks, and to gratefully acknowledge two people who are leaving the Journal: Mr. Joseph Dennehy and Dr. James Pile.
Long-time sales and marketing director Joe Dennehy played a key role in the Journal’s rise from relative obscurity about 20 years ago. This period was marked by many hospital-based journals closing up shop. Published since 1931, the Journal was relaunched in 1995 as a resource for postgraduate medical education with a national and international reach. Joe was brought in to guide and implement the marketing of the Journal as an independent, high-quality, clinical educational journal to increase its attractiveness as an advertising medium for the pharmaceutical industry so we could at least partly defray the significant expenses of publishing.
Joe is well known to medical publishers and media buyers, and over the past 20 years he became the face of the Journal in sales and marketing circles. In 2010, the Association of Medical Media recognized Joe’s achievements at its 18th Annual Nexus Representatives of the Year Awards, which acknowledge outstanding sales and marketing directors for their superior service, professionalism, and communication of ideas. Joe exemplifies these qualities and has been an inspiration to those of us who know and work with him. He has fully understood the sincerity of our mission and has never asked us to stray from it. Although Joe and his wife Holly live in New York, they are part of the Cleveland-based Journal family, and we will miss them. We wish them happiness and good health.
Dr. James Pile is an internal medicine hospitalist, an infectious disease specialist, and a superior medical educator. His work with us for the past several years has enhanced the Journal’s educational value for practicing hospitalists. His working familiarity with clinical leaders in the Society of Hospital Medicine has provided us with willing and skilled peer reviewers. Jim is now transitioning to a role as director of the internal medicine residency program at MetroHealth Medical Center, also here in Cleveland. He will continue to be a clinical resource for us as author and reviewer, but his ever-calm demeanor and clinical common sense will be hard to replace.
The Journal continues to evolve with the publishing times. We are assuming a greater presence in the digital world and adjusting to an environment of ever-diminishing advertising revenue. But with the work of our editorial team (I invite you to periodically glance at our masthead to note our team of writers, managers, and production staff), we intend to stay true to our educational mission. Our personal thanks to Joe and Jim for their contributions of the past, and to our current team in Scientific Publications for their ongoing and very personal commitment to providing the highest quality medical education that we can.
Our goal at the Cleveland Clinic Journal of Medicine is to provide timely, readily digestible, and useful clinical information to our readers. To do so, we need authors who buy into our educational mission, but we also need conscientious peer reviewers and an editorial staff capable of turning “doctorese” into readily understood English.
Our physician deputy editors Pelin Batur and James Pile solicit articles, guide authors, draft and revise CME questions, and assist greatly in the peer review process. Our nonphysician editors edit manuscripts to achieve a consistent editorial style in all of our published papers, but they serve many other key functions. They manage the business of publishing a monthly journal at a time of drastically shrinking advertising revenue, they ensure that Journal content complies with rules for CME material published in print and online, and they keep up with continuous changes in online publishing. The Journal receives significant funding from Cleveland Clinic’s Education Institute, funding we need to pursue our role as an independent, peer-reviewed conveyer of clinical information.
I write this to emphasize that real people manage all of these tasks, and to gratefully acknowledge two people who are leaving the Journal: Mr. Joseph Dennehy and Dr. James Pile.
Long-time sales and marketing director Joe Dennehy played a key role in the Journal’s rise from relative obscurity about 20 years ago. This period was marked by many hospital-based journals closing up shop. Published since 1931, the Journal was relaunched in 1995 as a resource for postgraduate medical education with a national and international reach. Joe was brought in to guide and implement the marketing of the Journal as an independent, high-quality, clinical educational journal to increase its attractiveness as an advertising medium for the pharmaceutical industry so we could at least partly defray the significant expenses of publishing.
Joe is well known to medical publishers and media buyers, and over the past 20 years he became the face of the Journal in sales and marketing circles. In 2010, the Association of Medical Media recognized Joe’s achievements at its 18th Annual Nexus Representatives of the Year Awards, which acknowledge outstanding sales and marketing directors for their superior service, professionalism, and communication of ideas. Joe exemplifies these qualities and has been an inspiration to those of us who know and work with him. He has fully understood the sincerity of our mission and has never asked us to stray from it. Although Joe and his wife Holly live in New York, they are part of the Cleveland-based Journal family, and we will miss them. We wish them happiness and good health.
Dr. James Pile is an internal medicine hospitalist, an infectious disease specialist, and a superior medical educator. His work with us for the past several years has enhanced the Journal’s educational value for practicing hospitalists. His working familiarity with clinical leaders in the Society of Hospital Medicine has provided us with willing and skilled peer reviewers. Jim is now transitioning to a role as director of the internal medicine residency program at MetroHealth Medical Center, also here in Cleveland. He will continue to be a clinical resource for us as author and reviewer, but his ever-calm demeanor and clinical common sense will be hard to replace.
The Journal continues to evolve with the publishing times. We are assuming a greater presence in the digital world and adjusting to an environment of ever-diminishing advertising revenue. But with the work of our editorial team (I invite you to periodically glance at our masthead to note our team of writers, managers, and production staff), we intend to stay true to our educational mission. Our personal thanks to Joe and Jim for their contributions of the past, and to our current team in Scientific Publications for their ongoing and very personal commitment to providing the highest quality medical education that we can.
Our goal at the Cleveland Clinic Journal of Medicine is to provide timely, readily digestible, and useful clinical information to our readers. To do so, we need authors who buy into our educational mission, but we also need conscientious peer reviewers and an editorial staff capable of turning “doctorese” into readily understood English.
Our physician deputy editors Pelin Batur and James Pile solicit articles, guide authors, draft and revise CME questions, and assist greatly in the peer review process. Our nonphysician editors edit manuscripts to achieve a consistent editorial style in all of our published papers, but they serve many other key functions. They manage the business of publishing a monthly journal at a time of drastically shrinking advertising revenue, they ensure that Journal content complies with rules for CME material published in print and online, and they keep up with continuous changes in online publishing. The Journal receives significant funding from Cleveland Clinic’s Education Institute, funding we need to pursue our role as an independent, peer-reviewed conveyer of clinical information.
I write this to emphasize that real people manage all of these tasks, and to gratefully acknowledge two people who are leaving the Journal: Mr. Joseph Dennehy and Dr. James Pile.
Long-time sales and marketing director Joe Dennehy played a key role in the Journal’s rise from relative obscurity about 20 years ago. This period was marked by many hospital-based journals closing up shop. Published since 1931, the Journal was relaunched in 1995 as a resource for postgraduate medical education with a national and international reach. Joe was brought in to guide and implement the marketing of the Journal as an independent, high-quality, clinical educational journal to increase its attractiveness as an advertising medium for the pharmaceutical industry so we could at least partly defray the significant expenses of publishing.
Joe is well known to medical publishers and media buyers, and over the past 20 years he became the face of the Journal in sales and marketing circles. In 2010, the Association of Medical Media recognized Joe’s achievements at its 18th Annual Nexus Representatives of the Year Awards, which acknowledge outstanding sales and marketing directors for their superior service, professionalism, and communication of ideas. Joe exemplifies these qualities and has been an inspiration to those of us who know and work with him. He has fully understood the sincerity of our mission and has never asked us to stray from it. Although Joe and his wife Holly live in New York, they are part of the Cleveland-based Journal family, and we will miss them. We wish them happiness and good health.
Dr. James Pile is an internal medicine hospitalist, an infectious disease specialist, and a superior medical educator. His work with us for the past several years has enhanced the Journal’s educational value for practicing hospitalists. His working familiarity with clinical leaders in the Society of Hospital Medicine has provided us with willing and skilled peer reviewers. Jim is now transitioning to a role as director of the internal medicine residency program at MetroHealth Medical Center, also here in Cleveland. He will continue to be a clinical resource for us as author and reviewer, but his ever-calm demeanor and clinical common sense will be hard to replace.
The Journal continues to evolve with the publishing times. We are assuming a greater presence in the digital world and adjusting to an environment of ever-diminishing advertising revenue. But with the work of our editorial team (I invite you to periodically glance at our masthead to note our team of writers, managers, and production staff), we intend to stay true to our educational mission. Our personal thanks to Joe and Jim for their contributions of the past, and to our current team in Scientific Publications for their ongoing and very personal commitment to providing the highest quality medical education that we can.
Dual antiplatelet therapy for acute coronary syndromes: How long to continue?
Percutaneous coronary intervention for acute coronary syndromes has evolved, and so, hand in hand, has antiplatelet therapy. With the advent of clopidogrel and newer agents, several studies demonstrated the benefits of dual antiplatelet therapy in preventing major vascular ischemic complications. The findings culminated in a guideline recommendation for at least 12 months of dual antiplatelet therapy after placement of a drug-eluting stent, when feasible—a class I recommendation (treatment should be given), level of evidence B (limited populations evaluated).1,2 But extending dual antiplatelet therapy beyond 12 months had no strong favorable evidence until the recent Dual Antiplatelet Therapy (DAPT) study3 shed light on this topic.
Here, we review the evidence thus far on the optimal duration of dual antiplatelet therapy in the secondary prevention of coronary artery disease.
PLATELETS IN ACUTE CORONARY SYNDROMES AND STENT THROMBOSIS
Acute coronary syndromes begin with fissuring or ulceration of a vulnerable atherosclerotic plaque, followed by thrombosis and occlusion, mediated by platelet adhesion, activation, and aggregation (Figure 1). Transient occlusion results in unstable angina or non-ST-elevation myocardial infarction, while total occlusion usually results in ST-elevation myocardial infarction.
Platelet aggregation is prominent among the mechanisms leading to stent thrombosis and vaso-occlusive ischemic complications after percutaneous coronary intervention. Thus, antiplatelet agents play a vital role in both primary and secondary prevention of cardiovascular events.4–6
Adhesion, activation, and aggregation
Adhesion. Disruption of the vascular endothelium as a result of vulnerable plaque fissuring or ulceration exposes subendothelial thrombogenic collagen and von Willebrand factor to blood. Collagen engages platelets through their glycoprotein (GP) Ia, IIa, and VI receptors, and von Willebrand factor binds platelets through the GP Ib-IX-V receptor.
Activation. Once platelets adhere to the subendothelium, they undergo a conformational change and become activated. Simultaneous release of various autocrine and paracrine mediators including adenosine diphosphate, serotonin, epinephrine, thromboxane, and various ligand-receptor interactions all contribute to the activation cascade. Adenosine diphosphate binds to the platelet receptor P2Y1, leading to an increase in intracellular calcium, and it binds to P2Y12, leading to a decrease in cyclic adenosine monophosphate, both of which cause GP IIb/IIIa receptor activation. Thromboxane A2 released by platelets by cyclo-oxygenase 1 binds to alpha or beta variant receptors and contributes to GP IIb/IIIa activation through elevation of intracellular calcium levels.
Aggregation and thrombosis. Exposure of tissue factor to plasma following plaque rupture activates the coagulation cascade via the extrinsic pathway, which generates thrombin, a powerful platelet activator that causes thrombus formation via fibrin. Thrombin binds to protease-activated receptors PAR-1 and PAR-4 on platelets, causing an increase in intracellular calcium and a decrease in cyclic adenosine monophosphate with subsequent GP IIb/IIIa activation. GP IIb/IIIa facilitates platelet aggregation by binding to fibrinogen and forming a stable platelet thrombus.
In the early stages of thrombus formation, platelets predominate (“white” thrombi); further organization with fibrin results in older “red” thrombi. The stages of thrombi vary in non-ST-elevation and ST-elevation myocardial infarction and are prognostic markers of death.4–8
PERCUTANEOUS INTERVENTION, RESTENOSIS, AND STENT THROMBOSIS
Percutaneous coronary intervention, the preferred means of revascularization for many patients, is performed emergently in patients with ST-elevation myocardial infarction, urgently in those with acute coronary syndromes without ST elevation, and electively in those with stable ischemic symptoms.
Percutaneous revascularization techniques have evolved from balloon angioplasty to bare-metal stents to drug-eluting stents, but each of these procedures has been associated with a periprocedural and postprocedural risk of thrombosis.
Balloon angioplasty was associated with vascular intimal injury, inciting elastic vascular recoil and smooth muscle cell proliferation leading to restenosis.
Bare-metal stents reduced the restenosis rate by eliminating vascular recoil, although restenosis still occurred within the stent because of neointimal proliferation of vascular smooth muscle cells. This was an important limitation, as both acute and subacute stent thrombosis were refractory to aggressive anticoagulation regimens that were associated with major bleeding complications and longer hospital length of stay. Stenting became mainstream practice only after the ISAR9 and STARS10 trials showed that dual antiplatelet therapy controlled stent thrombosis.
Drug-eluting stents coated with anti-proliferative and anti-inflammatory polymers markedly reduced in-stent restenosis rates by suppressing the initial vascular smooth-muscle proliferative response. However, they were still associated with late and very late stent thrombosis with incomplete endothelialization, even up to 40 months after implantation. Proposed mechanisms include incomplete stent apposition and inflammatory hypersensitivity reactions to the polymer coating. Incomplete stent apposition associated with low-velocity blood flow at the junction of the stent strut and vessel wall, together with delayed endothelialization, promotes platelet adhesion and aggregation, followed by thrombus formation.11
Second-generation drug-eluting stents have thinner struts and more biocompatible polymers and are thought to favor more complete re-endothelialization, reducing the rates of stent thrombosis.8,12,13
Predictors of early stent thrombosis
The Dutch Stent Thrombosis Registry and other studies looked at risk factors for stent thrombosis.14,15
Procedure-related factors included:
- Stent undersizing
- Residual uncovered dissections after angioplasty
- Longer stents
- Low flow after angioplasty (< 3 on the 0–3 Thrombolysis in Myocardial Infarction [TIMI] scale).
Lesion-related factors included:
- Intermediate coronary artery disease both proximal and distal to the culprit lesions
- Bifurcation lesions.
Patient-related factors included:
- Low left ventricular ejection fraction
- Diabetes mellitus
- Peripheral arterial disease Premature discontinuation of clopidogrel.
ANTIPLATELET AGENTS: MECHANISM OF ACTION
Various pathways play synergistic roles in platelet activation and aggregation and thrombus formation, and different antiplatelet agents inhibit these specific pathways, thus complementing each other and having additive effects (Figure 2, Table 1).5,16–21
Aspirin inhibits cyclo-oxygenase 1
Cyclo-oxygenase 1, found in platelets, endothelial cells, and other cells, catalyzes the conversion of arachidonic acid to thromboxane A2. Aspirin irreversibly inhibits cyclo-oxygenase 1 by acetylating its serine residue, preventing formation of thromboxane A2 and preventing platelet activation and aggregation.
P2Y12 ADP receptor antagonists
Clopidogrel and prasugrel are thienopyridine agents that irreversibly inhibit the P2Y12 receptor, thereby preventing binding of adenosine diphosphate and the subsequent platelet activation-aggregation cascade. They are both prodrugs and require conversion by cytochrome P450 enzymes to active metabolites. Prasugrel is 10 times more potent than clopidogrel due to more efficient formation of its active metabolite, and it achieves a comparable effect on platelet inhibition 30 minutes faster than the peak effect of clopidogrel at 6 hours. The overall peak inhibitory effect of prasugrel is twice that of clopidogrel.22
Ticagrelor, a cyclopentyl-triazolo-pyrimidine, directly and reversibly inhibits the P2Y12 ADP receptor. Unlike clopidogrel and prasugrel, it does not need to be converted to an active metabolite, and it noncompetitively inhibits P2Y12 at a site different from the adenosine diphosphate binding site.23 Like prasugrel, ticagrelor inhibits platelet function more rapidly and more completely than clopidogrel.
Cangrelor, an intravenously administered analogue of adenosine triphosphate, reversibly inhibits the P2Y12 receptor. It has undergone phase 3 trials but is not yet approved for clinical use.24
WHY DUAL ANTIPLATELET THERAPY?
Aspirin is good, clopidogrel is better
Aspirin has a well-validated role in both primary and secondary prevention of coronary and noncoronary atherosclerotic vascular disease.
The CAPRIE trial found clopidogrel monotherapy to be superior to aspirin monotherapy in patients with established atherosclerotic vascular disease.25
After stenting, short-term dual therapy is better than short-term warfarin
Thrombotic complications in the early postprocedural period were a major limitation of stenting, and existing anticoagulation regimens were ineffective in preventing them.26,27
The ISAR trial studied the benefit of combined antiplatelet vs anticoagulant therapy after stent placement. Patients randomized to receive combined aspirin plus ticlopidine (an early P2Y12 inhibitor) had significantly lower rates of primary cardiac, hemorrhagic, and vascular events at 30 days.9 Two other trials confirmed this finding.28,29
STARS10 also confirmed the benefit of aspirin and ticlopidine after stenting. Patients were randomly assigned to aspirin alone, aspirin plus warfarin, or aspirin plus ticlopidine after stent placement. The rate of stent thrombosis at 30 days was significantly lower in the dual antiplatelet group than in the other two groups. The dual antiplatelet group had a higher rate of bleeding than the aspirin-alone group, but the rate was similar to that of the aspirin-plus-warfarin group.
Long-term dual antiplatelet therapy is beneficial in several situations
ISAR and STARS were landmark trials that showed stent thrombosis could be reduced by dual antiplatelet therapy for a 30-day period. However, the long-term role of dual antiplatelet therapy was still unknown.
The CURE trial30–32 randomized patients presenting with acute coronary syndromes without ST elevation to receive clopidogrel plus aspirin or placebo plus aspirin for 3 to 12 months. The rate of the primary end point (cardiac death, nonfatal myocardial infarction, or stroke) was significantly lower in the clopidogrel-plus-aspirin group. A similar benefit of dual antiplatelet therapy was seen in the subgroup of patients who underwent percutaneous coronary intervention. Both pretreatment with clopidogrel plus aspirin for a median of 10 days prior to percutaneous intervention and continuing it for a mean of 9 months reduced major adverse cardiovascular events.
The CREDO trial20 found that the combination of clopidogrel and aspirin significantly reduced the incidence of death, myocardial infarction, or stroke at 1 year after percutaneous coronary intervention. A subgroup of patients in this trial who had a longer pretreatment interval with a loading clopidogrel dose showed a benefit at 28 days, which was not as evident with a shorter loading dose interval.
The CLARITY-TIMI 28 trial33,34 showed the advantage of adding clopidogrel to aspirin in patients receiving fibrinolytic therapy for ST-elevation myocardial infarction. Adding clopidogrel both improved the patency of the infarct-related artery and reduced ischemic complications. In patients who subsequently underwent percutaneous coronary intervention and stenting, clopidogrel pretreatment was associated with a significant decrease in ischemic complications before and after the procedure. There was no significant increase in bleeding complications in either group.
COMMIT/CCS 235 also showed the benefit of dual antiplatelet therapy in patients with ST-elevation myocardial infarction. Clopidogrel added to aspirin during the short-term in-hospital or postdischarge treatment period significantly reduced a composite end point of reinfarction, death, or stroke as well as death from any cause.
The CHARISMA trial36–38 aimed to determine if patients who were more stable (ie, no recent acute coronary syndrome event or percutaneous coronary intervention) would benefit. Overall, CHARISMA showed no benefit of adding clopidogrel to aspirin compared with aspirin alone in a broad population of patients with established vascular disease (secondary prevention) or risk factors for vascular disease (primary prevention).
But importantly, though no benefit was seen in the primary prevention group, the large subgroup of patients with established atherosclerotic vascular disease (12,153 of the 15,603 patients in the trial) did benefit from dual antiplatelet therapy.36,37 This subgroup showed an overall reduction in absolute risk of 1.5% (relative risk 0.88, P = .046) over a median follow-up of 27.6 months. This benefit was even more apparent in the 9,478 patients with prior myocardial infarction, stroke, or peripheral artery disease, for whom the relative risk reduction was 17.1% (P = .01) and the reduction in absolute risk 1.5%.38
These results are comparable to the 2% absolute risk reduction in the CURE trial for similar end points over 9 months. In both studies, there was no significant increase in the risk of major bleeding or intracranial bleeding in the clopidogrel-plus-aspirin groups, although minor bleeding was increased by dual antiplatelet therapy.
The rate of severe bleeding, which was the primary safety end point in CHARISMA, was not significantly different in the clopidogrel-plus-aspirin group compared with the placebo-plus-aspirin group (relative risk 1.25, 95% CI 0.97–1.61, P = .09).
Thus, although the CHARISMA findings were negative overall, the positive finding observed in the predominant subgroup of patients with established vascular disease can therefore be considered supportive of the results of the subsequent trials discussed below.
The PEGASUS-TIMI 54 trial39 studied the benefit of adding ticagrelor (60 or 90 mg) to low-dose aspirin in patients with stable coronary artery disease who had had a myocardial infarction 1 to 3 years earlier.
Confirming the results of the CHARISMA subgroup analysis, the incidence of the ischemic primary efficacy end point (a composite of cardiovascular death, myocardial infarction, and stroke) was significantly lower in both groups receiving ticagrelor plus aspirin compared with those receiving placebo plus aspirin. The Kaplan-Meier rate at 3 years for the ticagrelor 90 mg-plus-aspirin group was 7.85% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.85, 95% confidence interval [CI] 0.75–0.96, P = .008). The rate for the ticagrelor 60 mg-plus-aspirin group was 7.77% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.84, 95% CI 0.74–0.95, P = .004).
The rates of all TIMI major and minor bleeding, as well as bleeding requiring transfusion or discontinuation of the study drug, were significantly higher in both ticagrelor dosing groups than in the placebo group (P < .01 for both groups vs placebo). The rates of fatal bleeding and nonfatal intracranial hemorrhage were not significantly higher. Although there was an overall reduction in ischemic end points with the addition of ticagrelor, there was also a significantly higher incidence of bleeding in this group.
Comment. Thus, with or without percutaneous coronary intervention in acute coronary syndrome as well as in stable coronary artery disease, dual antiplatelet therapy was shown to improve outcomes and decrease ischemic complications compared with aspirin alone. It provided benefit in the setting of acute coronary syndrome (in the CURE trial) and percutaneous coronary intervention (in the CREDO trial) for up to 1 year.
Major questions remained to be addressed:
- Do the results of CREDO, which was performed before the current interventional era and the use of drug-eluting stents, reflect outcomes after current interventional practice?
- Could shorter periods of dual antiplatelet therapy be sufficient, especially with newer stents with less risk of late thrombosis?
- Does the benefit of dual antiplatelet therapy extend beyond the 1-year time period tested in those trials to date?
RECOMMENDATIONS FOR DOSING
The American College of Cardiology Foundation/American Heart Association guidelines for dosing of antiplatelet agents for non-ST-elevation myocardial infarction are summarized in Table 2, and those for ST-elevation myocardial infarction are summarized in Table 3.1,2
WOULD SHORTER THERAPY AFTER STENTING WORK AS WELL?
The American College of Cardiology Foundation/American Heart Association currently recommend dual antiplatelet therapy for at least 12 months after drug-eluting stent placement, with shorter courses appropriate for patients who develop excessive bleeding complications or who are at high risk of bleeding.
Four trials (Table 4) evaluated whether shorter durations of dual antiplatelet therapy would suffice: SECURITY,40 EXCELLENT,41 OPTIMIZE,42 and RESET.43 All of them showed that short-duration therapy was not inferior to standard-duration therapy.44 These studies were comparable in that:
- Patients were randomized at the time of percutaneous coronary intervention or within 24 hours of it.
- Most patients received a second-generation drug-eluting stent, with the following exceptions: in EXCELLENT,41 one-fourth of patients received a Cypher first-generation drug-eluting stent, and in RESET,43 approximately one-fourth of the patients received a sirolimus-eluting stent in the standard-duration group for short lesions. Those patients with longer lesions in the RESET standard-duration group received an everolimus drug-eluting stent.
- The second antiplatelet added to aspirin in all studies was clopidogrel, with the exception of the SECURITY trial, in which fewer than 2% of patients received ticagrelor or prasugrel.40
- All the trials except RESET excluded patients who had had a myocardial infarction within 72 hours, and thus most patients studied had a lower risk profile.
- All of the trials sought to study noninferiority of short- vs standard-duration dual antiplatelet therapy, defined as the occurrence of a primary end point at 1 year (a composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, target vessel failure or revascularization, or bleeding).
Their low-risk patient populations and infrequent end points rendered these studies underpowered to make definitive conclusions about the relative efficacy of 6-months vs 12-months of dual antiplatelet therapy.
WOULD LONGER THERAPY BE BETTER?
The PRODIGY trial45 assessed durations of dual antiplatelet therapy both shorter and longer than the conventional 1 year, randomizing patients undergoing placement of a bare-metal stent, first-generation drug-eluting stent, or second-generation drug-eluting stent to receive aspirin and clopidogrel for either 6 months or 24 months. The study showed no significant difference in primary outcomes in the short- or long-duration groups.
Other trials that compared the standard 12 months of dual antiplatelet therapy with extended duration beyond 12 months were DAPT,3 ARCTIC-Interruption,46 and DES-LATE.47 The trials were comparable in that:
- All patients were randomized after completing 12 months of dual antiplatelet therapy following drug-eluting stent placement.
- All patients who were included had been free of major cardiac ischemic events or bleeding during the 12 months following stent placement.
- The primary aim of all three studies was to compare primary end points in groups receiving aspirin alone vs extended dual antiplatelet therapy. The primary end point was a composite of death due to a cardiovascular cause, nonfatal myocardial infarction, stroke, or stent thrombosis.
- The principal safety end point was bleeding.
Although the two earlier studies (ARCTIC-Interruption and DES-LATE) did not show any benefit of extended dual antiplatelet therapy compared with the standard 12-month duration, the recent DAPT study did.
The DAPT study
The DAPT study3 was an international, multicenter, placebo-controlled, double-blind randomized trial designed to examine the benefit of dual antiplatelet therapy beyond 1 year in a patient population large enough to provide definitive assessment of benefit and risk.
A total of 9,961 patients who received drug-eluting stents were randomized after 12 months of dual antiplatelet therapy to receive either a thienopyridine (clopidogrel or prasugrel) plus aspirin or placebo plus aspirin. They were followed for an additional 18 months. The coprimary efficacy end points were stent thrombosis and a composite of death, myocardial infarction, or stroke, while the primary safety end point was moderate or severe bleeding. The patients were also observed from months 30 to 33 on aspirin alone after stopping the thienopyridine.
Results. Longer therapy substantially reduced the risks of stent thrombosis (hazard ratio [HR] 0.29, 95% confidence interval [CI] 0.17–0.48) and the composite ischemic end point (HR 0.71, 95% CI 0.59–0.85). Follow-up during the 3-month thienopyridine discontinuation phase starting at 30 months revealed convergence of the ischemic event-rate curves in the two groups, which suggested that continuing dual antiplatelet therapy beyond 30 months might have been beneficial. Myocardial infarction unrelated to stent thrombosis accounted for 55% of the treatment benefit of dual antiplatelet therapy.
The risk of bleeding was higher in the thienopyridine group during the treatment period (2.5% vs 1.6%, P = .001). There was also a higher rate of noncardiovascular mortality in the thienopyridine group, although this difference may have been due to chance.3,48
Why were the results different?
All three trials included first- and second-generation drug-eluting stents, with different proportions in different trials. In ARCTIC-Interruption,46 43% of the patients in the continuation group had a first-generation stent, as did 64% of the patients in the dual antiplatelet group of DES-LATE.47 In the DAPT trial,3 38% of the patients in the longer-duration arm had a first-generation stent, and in 26% of cases it was a paclitaxel-eluting stent.
Only clopidogrel was used as the second antiplatelet agent in DES-LATE, whereas prasugrel was used in 10% of patients in ARCTIC-Interruption and 35% in DAPT.
Yet none of these differences seem to explain the differences in outcome among the studies. ARCTIC-Interruption and DES-LATE did not show any benefit of continued dual antiplatelet therapy beyond 12 months. DAPT showed benefit of extended therapy with prasugrel or with clopidogrel, and with first-generation or second-generation drug-eluting stents. The most likely explanation for the different results was that DAPT was the only trial sufficiently powered to definitively assess the end points, including stent thrombosis.
A balance between ischemic efficacy and bleeding risk is the major consideration with any antithrombotic and antiplatelet therapy. In the three largest trials we discussed (the vascular disease subgroups of CHARISMA,38 PEGASUS,39 and DAPT3), comparison of the prespecified efficacy and safety end points of each trial suggests that dual antiplatelet therapy has a net benefit, particularly given the irreversible nature of ischemic end points.
In CHARISMA,38 60 cardiovascular deaths, myocardial infarctions, or strokes were prevented per year per 10,000 patients treated, at the cost of 28 excess moderate bleeding events.
In PEGASUS,39 42 cardiovascular deaths, myocardial infarctions, or strokes were prevented, at the cost of 79 excess bleeding events requiring transfusion.
In DAPT (a selected population who had tolerated dual antiplatelet therapy for 1 year), 106 deaths, myocardial infarctions, or stroke events were prevented, at the cost of 47 excess moderate bleeding events.3
Indirect comparisons between trials are problematic, given different end point definitions, populations, and background therapies. But their results suggest that less-intensive inhibition with clopidogrel as the second antiplatelet long-term (as in CHARISMA) may provide the best balance of benefit vs risk.
BALANCING RISK AND BENEFIT
The evidence is unequivocal that dual antiplatelet therapy suppresses coronary ischemic complications resulting from thrombosis at sites of spontaneous plaque rupture following acute coronary syndromes or mechanical plaque disruption and foreign body implantation associated with percutaneous coronary intervention.
Three large-scale trials (DAPT,3 PEGASUS,39 and the secondary prevention subgroup of CHARISMA38) showed that the protective effect of dual antiplatelet therapy continues with prolonged therapy in patients who have experienced an acute coronary syndrome event or have received a drug-eluting stent. That benefit seems to be due to the action of these therapies on the culprit vessel (the one that caused the acute coronary syndrome or the site of stenting), as well as nonculprit arteries, emphasizing that dual antiplatelet therapy protects against atherosclerosis progression and future plaque rupture events.
For the durations studied in the longest trials thus far, 30 months (DAPT3) and 36 months (PEGASUS39), event curves continue to diverge, indicating that the advantage of dual antiplatelet therapy may persist for an indefinite period of time. Thus, indefinite therapy with dual antiplatelet agents can be supported, particularly in patients with advanced coronary artery disease or those who have had multiple coronary events.
We believe that the balance of evidence suggests that smaller studies that failed to show a benefit of longer-term therapy were underpowered to do so.
The ischemic protection is associated with the adverse effect of increased bleeding risk. Unfortunately, there has been little success in guiding dual antiplatelet therapy based on ischemic vs bleeding risk, in part because the same factors that predict risk of ischemic complications seem to predict increased susceptibility to bleeding. Nevertheless, indirect comparisons between studies suggest that for longer-term therapy clopidogrel may be superior to ticagrelor or prasugrel: the absolute excess bleeding risk with dual antiplatelet therapy vs aspirin in the CHARISMA secondary prevention subgroup was less than that in PEGASUS, with similar absolute reductions in ischemic events. So while the TRITON-TIMI 3822 and PLATO23 trials support the superiority of prasugrel or ticagrelor over clopidogrel for the first year after acute coronary syndrome, subsequent years of therapy may best be provided with clopidogrel.
Some patients may have identifiable factors that place them at very high risk of bleeding—need for surgical procedures, need for anticoagulation, or occurrence of bleeding complications or excessive “nuisance bleeding.” In those patients, the data suggest that dual antiplatelet therapy could be discontinued after 6 months, or perhaps even 3 months in the highest bleeding risk circumstances after second-generation drug-eluting stent placement.
WOEST49 was an open-label randomized controlled trial that studied the safety of antiplatelet regimens in patients on anticoagulation requiring percutaneous coronary interventions. Patients were randomized to double therapy with anticoagulant and clopidogrel vs triple therapy with additional aspirin following percutaneous coronary intervention. The primary end point was bleeding events within 1 year. Clopidogrel without aspirin was associated with significantly fewer bleeding events compared with triple therapy, with no increase in adverse ischemic events. The strategy tested in the WOEST trial seems reasonable in the specific group of patients who require ongoing anticoagulant therapy after drug-eluting stent placement, recognizing that the trial was somewhat underpowered to make definitive conclusions, particularly in patients at high risk for stent thrombosis.
Based on the results of PEGASUS and the CHARISMA subgroup with established ischemic burden, in which dual antiplatelet therapy was started after an interruption following the index coronary event, it is also reasonable to restart long-term dual antiplatelet therapy in patients who require interruption for short-term indications such as a surgical procedure.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Amsterdam EA, Wenger NK, Brindis RG, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130:e344–e426.
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371:2155–2166.
- Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J 2010; 74:597–607.
- Papp J, Kenyeres P, Toth K. Clinical importance of antiplatelet drugs in cardiovascular diseases. Clin Hemorheol Microcirc 2013; 53:81–96.
- Showkathali R, Natarajan A. Antiplatelet and antithrombin strategies in acute coronary syndrome: state-of-the-art review. Curr Cardiol Rev 2012; 8:239–249.
- Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 2012; 72:2087–2116.
- Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv 2014; 7:1081–1092.
- Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006; 48:193–202.
- Nikam N, Steinberg TB, Steinberg DH. Advances in stent technologies and their effect on clinical efficacy and safety. Med Devices (Auckl) 2014; 7:165–178.
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O’Brien ER. The evolution of coronary stents: a brief review. Can J Cardiol 2014; 30:35–45.
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J 2015; 36:3320–3331.
- van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol 2009; 53:1399–1409.
- Berger JS. Aspirin, clopidogrel, and ticagrelor in acute coronary syndromes. Am J Cardiol 2013; 112:737–745.
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015; 12:30–47.
- Patrono C, Rocca B. The future of antiplatelet therapy in cardiovascular disease. Annu Rev Med 2010; 61:49–61.
- Park SJ, Kang SM, Park DW. Dual antiplatelet therapy after drug-eluting stents: defining the proper duration. Coron Artery Dis 2014; 25:83–89.
- Steinhubl SR, Berger PB, Mann JT 3rd, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Nusca A, Patti G. Platelet function and inhibition in ischemic heart disease. Curr Cardiol Rep 2012; 14:457–467.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Genereux P, Stone GW, Harrington RA, et al; CHAMPION PHOENIX Investigators. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: Insights from the CHAMPION PHOENIX Trial (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects Who Require Percutaneous Coronary Intervention). J Am Coll Cardiol 2014; 63:619–629.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Warren J, Baber U, Mehran R. Antiplatelet therapy after drug-eluting stent implantation. J Cardiol 2015; 65:98–104.
- Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the Multicenter Aspirin and Ticlopidine Trial After Intracoronary Stenting (MATTIS). Circulation 1998; 98:2126–2132.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001; 358:527–533.
- Morais J. Insights from CURE: using clopidogrel on top of standard therapy. Cerebrovasc Dis 2002; 13(suppl 1):17–21.
- Ferguson JJ. Clopidogrel plus aspirin in patients with acute myocardial infarction treated with fibrinolytic therapy—CLARITY-TIMI 28. Future Cardiol 2005; 1:605–610.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372:1791–1800.
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64:2086–2097.
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125:505–513.
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310:2510–2522.
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60:1340–1348.
- El-Hayek G, Messerli F, Bangalore S, et al. Meta-analysis of randomized clinical trials comparing short-term versus long-term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 2014; 114:236–242.
- Valgimigli M, Campo G, Monti M, et al; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012; 125:2015–2026.
- Collet JP, Silvain J, Barthelemy O, et al; ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014; 384:1577–1585.
- Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014; 129:304–312.
- Kwok CS, Bulluck H, Ryding AD, Loke YK. Benefits and harms of extending the duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stents: a meta-analysis. ScientificWorldJournal 2014; 2014:794078.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
Percutaneous coronary intervention for acute coronary syndromes has evolved, and so, hand in hand, has antiplatelet therapy. With the advent of clopidogrel and newer agents, several studies demonstrated the benefits of dual antiplatelet therapy in preventing major vascular ischemic complications. The findings culminated in a guideline recommendation for at least 12 months of dual antiplatelet therapy after placement of a drug-eluting stent, when feasible—a class I recommendation (treatment should be given), level of evidence B (limited populations evaluated).1,2 But extending dual antiplatelet therapy beyond 12 months had no strong favorable evidence until the recent Dual Antiplatelet Therapy (DAPT) study3 shed light on this topic.
Here, we review the evidence thus far on the optimal duration of dual antiplatelet therapy in the secondary prevention of coronary artery disease.
PLATELETS IN ACUTE CORONARY SYNDROMES AND STENT THROMBOSIS
Acute coronary syndromes begin with fissuring or ulceration of a vulnerable atherosclerotic plaque, followed by thrombosis and occlusion, mediated by platelet adhesion, activation, and aggregation (Figure 1). Transient occlusion results in unstable angina or non-ST-elevation myocardial infarction, while total occlusion usually results in ST-elevation myocardial infarction.
Platelet aggregation is prominent among the mechanisms leading to stent thrombosis and vaso-occlusive ischemic complications after percutaneous coronary intervention. Thus, antiplatelet agents play a vital role in both primary and secondary prevention of cardiovascular events.4–6
Adhesion, activation, and aggregation
Adhesion. Disruption of the vascular endothelium as a result of vulnerable plaque fissuring or ulceration exposes subendothelial thrombogenic collagen and von Willebrand factor to blood. Collagen engages platelets through their glycoprotein (GP) Ia, IIa, and VI receptors, and von Willebrand factor binds platelets through the GP Ib-IX-V receptor.
Activation. Once platelets adhere to the subendothelium, they undergo a conformational change and become activated. Simultaneous release of various autocrine and paracrine mediators including adenosine diphosphate, serotonin, epinephrine, thromboxane, and various ligand-receptor interactions all contribute to the activation cascade. Adenosine diphosphate binds to the platelet receptor P2Y1, leading to an increase in intracellular calcium, and it binds to P2Y12, leading to a decrease in cyclic adenosine monophosphate, both of which cause GP IIb/IIIa receptor activation. Thromboxane A2 released by platelets by cyclo-oxygenase 1 binds to alpha or beta variant receptors and contributes to GP IIb/IIIa activation through elevation of intracellular calcium levels.
Aggregation and thrombosis. Exposure of tissue factor to plasma following plaque rupture activates the coagulation cascade via the extrinsic pathway, which generates thrombin, a powerful platelet activator that causes thrombus formation via fibrin. Thrombin binds to protease-activated receptors PAR-1 and PAR-4 on platelets, causing an increase in intracellular calcium and a decrease in cyclic adenosine monophosphate with subsequent GP IIb/IIIa activation. GP IIb/IIIa facilitates platelet aggregation by binding to fibrinogen and forming a stable platelet thrombus.
In the early stages of thrombus formation, platelets predominate (“white” thrombi); further organization with fibrin results in older “red” thrombi. The stages of thrombi vary in non-ST-elevation and ST-elevation myocardial infarction and are prognostic markers of death.4–8
PERCUTANEOUS INTERVENTION, RESTENOSIS, AND STENT THROMBOSIS
Percutaneous coronary intervention, the preferred means of revascularization for many patients, is performed emergently in patients with ST-elevation myocardial infarction, urgently in those with acute coronary syndromes without ST elevation, and electively in those with stable ischemic symptoms.
Percutaneous revascularization techniques have evolved from balloon angioplasty to bare-metal stents to drug-eluting stents, but each of these procedures has been associated with a periprocedural and postprocedural risk of thrombosis.
Balloon angioplasty was associated with vascular intimal injury, inciting elastic vascular recoil and smooth muscle cell proliferation leading to restenosis.
Bare-metal stents reduced the restenosis rate by eliminating vascular recoil, although restenosis still occurred within the stent because of neointimal proliferation of vascular smooth muscle cells. This was an important limitation, as both acute and subacute stent thrombosis were refractory to aggressive anticoagulation regimens that were associated with major bleeding complications and longer hospital length of stay. Stenting became mainstream practice only after the ISAR9 and STARS10 trials showed that dual antiplatelet therapy controlled stent thrombosis.
Drug-eluting stents coated with anti-proliferative and anti-inflammatory polymers markedly reduced in-stent restenosis rates by suppressing the initial vascular smooth-muscle proliferative response. However, they were still associated with late and very late stent thrombosis with incomplete endothelialization, even up to 40 months after implantation. Proposed mechanisms include incomplete stent apposition and inflammatory hypersensitivity reactions to the polymer coating. Incomplete stent apposition associated with low-velocity blood flow at the junction of the stent strut and vessel wall, together with delayed endothelialization, promotes platelet adhesion and aggregation, followed by thrombus formation.11
Second-generation drug-eluting stents have thinner struts and more biocompatible polymers and are thought to favor more complete re-endothelialization, reducing the rates of stent thrombosis.8,12,13
Predictors of early stent thrombosis
The Dutch Stent Thrombosis Registry and other studies looked at risk factors for stent thrombosis.14,15
Procedure-related factors included:
- Stent undersizing
- Residual uncovered dissections after angioplasty
- Longer stents
- Low flow after angioplasty (< 3 on the 0–3 Thrombolysis in Myocardial Infarction [TIMI] scale).
Lesion-related factors included:
- Intermediate coronary artery disease both proximal and distal to the culprit lesions
- Bifurcation lesions.
Patient-related factors included:
- Low left ventricular ejection fraction
- Diabetes mellitus
- Peripheral arterial disease Premature discontinuation of clopidogrel.
ANTIPLATELET AGENTS: MECHANISM OF ACTION
Various pathways play synergistic roles in platelet activation and aggregation and thrombus formation, and different antiplatelet agents inhibit these specific pathways, thus complementing each other and having additive effects (Figure 2, Table 1).5,16–21
Aspirin inhibits cyclo-oxygenase 1
Cyclo-oxygenase 1, found in platelets, endothelial cells, and other cells, catalyzes the conversion of arachidonic acid to thromboxane A2. Aspirin irreversibly inhibits cyclo-oxygenase 1 by acetylating its serine residue, preventing formation of thromboxane A2 and preventing platelet activation and aggregation.
P2Y12 ADP receptor antagonists
Clopidogrel and prasugrel are thienopyridine agents that irreversibly inhibit the P2Y12 receptor, thereby preventing binding of adenosine diphosphate and the subsequent platelet activation-aggregation cascade. They are both prodrugs and require conversion by cytochrome P450 enzymes to active metabolites. Prasugrel is 10 times more potent than clopidogrel due to more efficient formation of its active metabolite, and it achieves a comparable effect on platelet inhibition 30 minutes faster than the peak effect of clopidogrel at 6 hours. The overall peak inhibitory effect of prasugrel is twice that of clopidogrel.22
Ticagrelor, a cyclopentyl-triazolo-pyrimidine, directly and reversibly inhibits the P2Y12 ADP receptor. Unlike clopidogrel and prasugrel, it does not need to be converted to an active metabolite, and it noncompetitively inhibits P2Y12 at a site different from the adenosine diphosphate binding site.23 Like prasugrel, ticagrelor inhibits platelet function more rapidly and more completely than clopidogrel.
Cangrelor, an intravenously administered analogue of adenosine triphosphate, reversibly inhibits the P2Y12 receptor. It has undergone phase 3 trials but is not yet approved for clinical use.24
WHY DUAL ANTIPLATELET THERAPY?
Aspirin is good, clopidogrel is better
Aspirin has a well-validated role in both primary and secondary prevention of coronary and noncoronary atherosclerotic vascular disease.
The CAPRIE trial found clopidogrel monotherapy to be superior to aspirin monotherapy in patients with established atherosclerotic vascular disease.25
After stenting, short-term dual therapy is better than short-term warfarin
Thrombotic complications in the early postprocedural period were a major limitation of stenting, and existing anticoagulation regimens were ineffective in preventing them.26,27
The ISAR trial studied the benefit of combined antiplatelet vs anticoagulant therapy after stent placement. Patients randomized to receive combined aspirin plus ticlopidine (an early P2Y12 inhibitor) had significantly lower rates of primary cardiac, hemorrhagic, and vascular events at 30 days.9 Two other trials confirmed this finding.28,29
STARS10 also confirmed the benefit of aspirin and ticlopidine after stenting. Patients were randomly assigned to aspirin alone, aspirin plus warfarin, or aspirin plus ticlopidine after stent placement. The rate of stent thrombosis at 30 days was significantly lower in the dual antiplatelet group than in the other two groups. The dual antiplatelet group had a higher rate of bleeding than the aspirin-alone group, but the rate was similar to that of the aspirin-plus-warfarin group.
Long-term dual antiplatelet therapy is beneficial in several situations
ISAR and STARS were landmark trials that showed stent thrombosis could be reduced by dual antiplatelet therapy for a 30-day period. However, the long-term role of dual antiplatelet therapy was still unknown.
The CURE trial30–32 randomized patients presenting with acute coronary syndromes without ST elevation to receive clopidogrel plus aspirin or placebo plus aspirin for 3 to 12 months. The rate of the primary end point (cardiac death, nonfatal myocardial infarction, or stroke) was significantly lower in the clopidogrel-plus-aspirin group. A similar benefit of dual antiplatelet therapy was seen in the subgroup of patients who underwent percutaneous coronary intervention. Both pretreatment with clopidogrel plus aspirin for a median of 10 days prior to percutaneous intervention and continuing it for a mean of 9 months reduced major adverse cardiovascular events.
The CREDO trial20 found that the combination of clopidogrel and aspirin significantly reduced the incidence of death, myocardial infarction, or stroke at 1 year after percutaneous coronary intervention. A subgroup of patients in this trial who had a longer pretreatment interval with a loading clopidogrel dose showed a benefit at 28 days, which was not as evident with a shorter loading dose interval.
The CLARITY-TIMI 28 trial33,34 showed the advantage of adding clopidogrel to aspirin in patients receiving fibrinolytic therapy for ST-elevation myocardial infarction. Adding clopidogrel both improved the patency of the infarct-related artery and reduced ischemic complications. In patients who subsequently underwent percutaneous coronary intervention and stenting, clopidogrel pretreatment was associated with a significant decrease in ischemic complications before and after the procedure. There was no significant increase in bleeding complications in either group.
COMMIT/CCS 235 also showed the benefit of dual antiplatelet therapy in patients with ST-elevation myocardial infarction. Clopidogrel added to aspirin during the short-term in-hospital or postdischarge treatment period significantly reduced a composite end point of reinfarction, death, or stroke as well as death from any cause.
The CHARISMA trial36–38 aimed to determine if patients who were more stable (ie, no recent acute coronary syndrome event or percutaneous coronary intervention) would benefit. Overall, CHARISMA showed no benefit of adding clopidogrel to aspirin compared with aspirin alone in a broad population of patients with established vascular disease (secondary prevention) or risk factors for vascular disease (primary prevention).
But importantly, though no benefit was seen in the primary prevention group, the large subgroup of patients with established atherosclerotic vascular disease (12,153 of the 15,603 patients in the trial) did benefit from dual antiplatelet therapy.36,37 This subgroup showed an overall reduction in absolute risk of 1.5% (relative risk 0.88, P = .046) over a median follow-up of 27.6 months. This benefit was even more apparent in the 9,478 patients with prior myocardial infarction, stroke, or peripheral artery disease, for whom the relative risk reduction was 17.1% (P = .01) and the reduction in absolute risk 1.5%.38
These results are comparable to the 2% absolute risk reduction in the CURE trial for similar end points over 9 months. In both studies, there was no significant increase in the risk of major bleeding or intracranial bleeding in the clopidogrel-plus-aspirin groups, although minor bleeding was increased by dual antiplatelet therapy.
The rate of severe bleeding, which was the primary safety end point in CHARISMA, was not significantly different in the clopidogrel-plus-aspirin group compared with the placebo-plus-aspirin group (relative risk 1.25, 95% CI 0.97–1.61, P = .09).
Thus, although the CHARISMA findings were negative overall, the positive finding observed in the predominant subgroup of patients with established vascular disease can therefore be considered supportive of the results of the subsequent trials discussed below.
The PEGASUS-TIMI 54 trial39 studied the benefit of adding ticagrelor (60 or 90 mg) to low-dose aspirin in patients with stable coronary artery disease who had had a myocardial infarction 1 to 3 years earlier.
Confirming the results of the CHARISMA subgroup analysis, the incidence of the ischemic primary efficacy end point (a composite of cardiovascular death, myocardial infarction, and stroke) was significantly lower in both groups receiving ticagrelor plus aspirin compared with those receiving placebo plus aspirin. The Kaplan-Meier rate at 3 years for the ticagrelor 90 mg-plus-aspirin group was 7.85% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.85, 95% confidence interval [CI] 0.75–0.96, P = .008). The rate for the ticagrelor 60 mg-plus-aspirin group was 7.77% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.84, 95% CI 0.74–0.95, P = .004).
The rates of all TIMI major and minor bleeding, as well as bleeding requiring transfusion or discontinuation of the study drug, were significantly higher in both ticagrelor dosing groups than in the placebo group (P < .01 for both groups vs placebo). The rates of fatal bleeding and nonfatal intracranial hemorrhage were not significantly higher. Although there was an overall reduction in ischemic end points with the addition of ticagrelor, there was also a significantly higher incidence of bleeding in this group.
Comment. Thus, with or without percutaneous coronary intervention in acute coronary syndrome as well as in stable coronary artery disease, dual antiplatelet therapy was shown to improve outcomes and decrease ischemic complications compared with aspirin alone. It provided benefit in the setting of acute coronary syndrome (in the CURE trial) and percutaneous coronary intervention (in the CREDO trial) for up to 1 year.
Major questions remained to be addressed:
- Do the results of CREDO, which was performed before the current interventional era and the use of drug-eluting stents, reflect outcomes after current interventional practice?
- Could shorter periods of dual antiplatelet therapy be sufficient, especially with newer stents with less risk of late thrombosis?
- Does the benefit of dual antiplatelet therapy extend beyond the 1-year time period tested in those trials to date?
RECOMMENDATIONS FOR DOSING
The American College of Cardiology Foundation/American Heart Association guidelines for dosing of antiplatelet agents for non-ST-elevation myocardial infarction are summarized in Table 2, and those for ST-elevation myocardial infarction are summarized in Table 3.1,2
WOULD SHORTER THERAPY AFTER STENTING WORK AS WELL?
The American College of Cardiology Foundation/American Heart Association currently recommend dual antiplatelet therapy for at least 12 months after drug-eluting stent placement, with shorter courses appropriate for patients who develop excessive bleeding complications or who are at high risk of bleeding.
Four trials (Table 4) evaluated whether shorter durations of dual antiplatelet therapy would suffice: SECURITY,40 EXCELLENT,41 OPTIMIZE,42 and RESET.43 All of them showed that short-duration therapy was not inferior to standard-duration therapy.44 These studies were comparable in that:
- Patients were randomized at the time of percutaneous coronary intervention or within 24 hours of it.
- Most patients received a second-generation drug-eluting stent, with the following exceptions: in EXCELLENT,41 one-fourth of patients received a Cypher first-generation drug-eluting stent, and in RESET,43 approximately one-fourth of the patients received a sirolimus-eluting stent in the standard-duration group for short lesions. Those patients with longer lesions in the RESET standard-duration group received an everolimus drug-eluting stent.
- The second antiplatelet added to aspirin in all studies was clopidogrel, with the exception of the SECURITY trial, in which fewer than 2% of patients received ticagrelor or prasugrel.40
- All the trials except RESET excluded patients who had had a myocardial infarction within 72 hours, and thus most patients studied had a lower risk profile.
- All of the trials sought to study noninferiority of short- vs standard-duration dual antiplatelet therapy, defined as the occurrence of a primary end point at 1 year (a composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, target vessel failure or revascularization, or bleeding).
Their low-risk patient populations and infrequent end points rendered these studies underpowered to make definitive conclusions about the relative efficacy of 6-months vs 12-months of dual antiplatelet therapy.
WOULD LONGER THERAPY BE BETTER?
The PRODIGY trial45 assessed durations of dual antiplatelet therapy both shorter and longer than the conventional 1 year, randomizing patients undergoing placement of a bare-metal stent, first-generation drug-eluting stent, or second-generation drug-eluting stent to receive aspirin and clopidogrel for either 6 months or 24 months. The study showed no significant difference in primary outcomes in the short- or long-duration groups.
Other trials that compared the standard 12 months of dual antiplatelet therapy with extended duration beyond 12 months were DAPT,3 ARCTIC-Interruption,46 and DES-LATE.47 The trials were comparable in that:
- All patients were randomized after completing 12 months of dual antiplatelet therapy following drug-eluting stent placement.
- All patients who were included had been free of major cardiac ischemic events or bleeding during the 12 months following stent placement.
- The primary aim of all three studies was to compare primary end points in groups receiving aspirin alone vs extended dual antiplatelet therapy. The primary end point was a composite of death due to a cardiovascular cause, nonfatal myocardial infarction, stroke, or stent thrombosis.
- The principal safety end point was bleeding.
Although the two earlier studies (ARCTIC-Interruption and DES-LATE) did not show any benefit of extended dual antiplatelet therapy compared with the standard 12-month duration, the recent DAPT study did.
The DAPT study
The DAPT study3 was an international, multicenter, placebo-controlled, double-blind randomized trial designed to examine the benefit of dual antiplatelet therapy beyond 1 year in a patient population large enough to provide definitive assessment of benefit and risk.
A total of 9,961 patients who received drug-eluting stents were randomized after 12 months of dual antiplatelet therapy to receive either a thienopyridine (clopidogrel or prasugrel) plus aspirin or placebo plus aspirin. They were followed for an additional 18 months. The coprimary efficacy end points were stent thrombosis and a composite of death, myocardial infarction, or stroke, while the primary safety end point was moderate or severe bleeding. The patients were also observed from months 30 to 33 on aspirin alone after stopping the thienopyridine.
Results. Longer therapy substantially reduced the risks of stent thrombosis (hazard ratio [HR] 0.29, 95% confidence interval [CI] 0.17–0.48) and the composite ischemic end point (HR 0.71, 95% CI 0.59–0.85). Follow-up during the 3-month thienopyridine discontinuation phase starting at 30 months revealed convergence of the ischemic event-rate curves in the two groups, which suggested that continuing dual antiplatelet therapy beyond 30 months might have been beneficial. Myocardial infarction unrelated to stent thrombosis accounted for 55% of the treatment benefit of dual antiplatelet therapy.
The risk of bleeding was higher in the thienopyridine group during the treatment period (2.5% vs 1.6%, P = .001). There was also a higher rate of noncardiovascular mortality in the thienopyridine group, although this difference may have been due to chance.3,48
Why were the results different?
All three trials included first- and second-generation drug-eluting stents, with different proportions in different trials. In ARCTIC-Interruption,46 43% of the patients in the continuation group had a first-generation stent, as did 64% of the patients in the dual antiplatelet group of DES-LATE.47 In the DAPT trial,3 38% of the patients in the longer-duration arm had a first-generation stent, and in 26% of cases it was a paclitaxel-eluting stent.
Only clopidogrel was used as the second antiplatelet agent in DES-LATE, whereas prasugrel was used in 10% of patients in ARCTIC-Interruption and 35% in DAPT.
Yet none of these differences seem to explain the differences in outcome among the studies. ARCTIC-Interruption and DES-LATE did not show any benefit of continued dual antiplatelet therapy beyond 12 months. DAPT showed benefit of extended therapy with prasugrel or with clopidogrel, and with first-generation or second-generation drug-eluting stents. The most likely explanation for the different results was that DAPT was the only trial sufficiently powered to definitively assess the end points, including stent thrombosis.
A balance between ischemic efficacy and bleeding risk is the major consideration with any antithrombotic and antiplatelet therapy. In the three largest trials we discussed (the vascular disease subgroups of CHARISMA,38 PEGASUS,39 and DAPT3), comparison of the prespecified efficacy and safety end points of each trial suggests that dual antiplatelet therapy has a net benefit, particularly given the irreversible nature of ischemic end points.
In CHARISMA,38 60 cardiovascular deaths, myocardial infarctions, or strokes were prevented per year per 10,000 patients treated, at the cost of 28 excess moderate bleeding events.
In PEGASUS,39 42 cardiovascular deaths, myocardial infarctions, or strokes were prevented, at the cost of 79 excess bleeding events requiring transfusion.
In DAPT (a selected population who had tolerated dual antiplatelet therapy for 1 year), 106 deaths, myocardial infarctions, or stroke events were prevented, at the cost of 47 excess moderate bleeding events.3
Indirect comparisons between trials are problematic, given different end point definitions, populations, and background therapies. But their results suggest that less-intensive inhibition with clopidogrel as the second antiplatelet long-term (as in CHARISMA) may provide the best balance of benefit vs risk.
BALANCING RISK AND BENEFIT
The evidence is unequivocal that dual antiplatelet therapy suppresses coronary ischemic complications resulting from thrombosis at sites of spontaneous plaque rupture following acute coronary syndromes or mechanical plaque disruption and foreign body implantation associated with percutaneous coronary intervention.
Three large-scale trials (DAPT,3 PEGASUS,39 and the secondary prevention subgroup of CHARISMA38) showed that the protective effect of dual antiplatelet therapy continues with prolonged therapy in patients who have experienced an acute coronary syndrome event or have received a drug-eluting stent. That benefit seems to be due to the action of these therapies on the culprit vessel (the one that caused the acute coronary syndrome or the site of stenting), as well as nonculprit arteries, emphasizing that dual antiplatelet therapy protects against atherosclerosis progression and future plaque rupture events.
For the durations studied in the longest trials thus far, 30 months (DAPT3) and 36 months (PEGASUS39), event curves continue to diverge, indicating that the advantage of dual antiplatelet therapy may persist for an indefinite period of time. Thus, indefinite therapy with dual antiplatelet agents can be supported, particularly in patients with advanced coronary artery disease or those who have had multiple coronary events.
We believe that the balance of evidence suggests that smaller studies that failed to show a benefit of longer-term therapy were underpowered to do so.
The ischemic protection is associated with the adverse effect of increased bleeding risk. Unfortunately, there has been little success in guiding dual antiplatelet therapy based on ischemic vs bleeding risk, in part because the same factors that predict risk of ischemic complications seem to predict increased susceptibility to bleeding. Nevertheless, indirect comparisons between studies suggest that for longer-term therapy clopidogrel may be superior to ticagrelor or prasugrel: the absolute excess bleeding risk with dual antiplatelet therapy vs aspirin in the CHARISMA secondary prevention subgroup was less than that in PEGASUS, with similar absolute reductions in ischemic events. So while the TRITON-TIMI 3822 and PLATO23 trials support the superiority of prasugrel or ticagrelor over clopidogrel for the first year after acute coronary syndrome, subsequent years of therapy may best be provided with clopidogrel.
Some patients may have identifiable factors that place them at very high risk of bleeding—need for surgical procedures, need for anticoagulation, or occurrence of bleeding complications or excessive “nuisance bleeding.” In those patients, the data suggest that dual antiplatelet therapy could be discontinued after 6 months, or perhaps even 3 months in the highest bleeding risk circumstances after second-generation drug-eluting stent placement.
WOEST49 was an open-label randomized controlled trial that studied the safety of antiplatelet regimens in patients on anticoagulation requiring percutaneous coronary interventions. Patients were randomized to double therapy with anticoagulant and clopidogrel vs triple therapy with additional aspirin following percutaneous coronary intervention. The primary end point was bleeding events within 1 year. Clopidogrel without aspirin was associated with significantly fewer bleeding events compared with triple therapy, with no increase in adverse ischemic events. The strategy tested in the WOEST trial seems reasonable in the specific group of patients who require ongoing anticoagulant therapy after drug-eluting stent placement, recognizing that the trial was somewhat underpowered to make definitive conclusions, particularly in patients at high risk for stent thrombosis.
Based on the results of PEGASUS and the CHARISMA subgroup with established ischemic burden, in which dual antiplatelet therapy was started after an interruption following the index coronary event, it is also reasonable to restart long-term dual antiplatelet therapy in patients who require interruption for short-term indications such as a surgical procedure.
Percutaneous coronary intervention for acute coronary syndromes has evolved, and so, hand in hand, has antiplatelet therapy. With the advent of clopidogrel and newer agents, several studies demonstrated the benefits of dual antiplatelet therapy in preventing major vascular ischemic complications. The findings culminated in a guideline recommendation for at least 12 months of dual antiplatelet therapy after placement of a drug-eluting stent, when feasible—a class I recommendation (treatment should be given), level of evidence B (limited populations evaluated).1,2 But extending dual antiplatelet therapy beyond 12 months had no strong favorable evidence until the recent Dual Antiplatelet Therapy (DAPT) study3 shed light on this topic.
Here, we review the evidence thus far on the optimal duration of dual antiplatelet therapy in the secondary prevention of coronary artery disease.
PLATELETS IN ACUTE CORONARY SYNDROMES AND STENT THROMBOSIS
Acute coronary syndromes begin with fissuring or ulceration of a vulnerable atherosclerotic plaque, followed by thrombosis and occlusion, mediated by platelet adhesion, activation, and aggregation (Figure 1). Transient occlusion results in unstable angina or non-ST-elevation myocardial infarction, while total occlusion usually results in ST-elevation myocardial infarction.
Platelet aggregation is prominent among the mechanisms leading to stent thrombosis and vaso-occlusive ischemic complications after percutaneous coronary intervention. Thus, antiplatelet agents play a vital role in both primary and secondary prevention of cardiovascular events.4–6
Adhesion, activation, and aggregation
Adhesion. Disruption of the vascular endothelium as a result of vulnerable plaque fissuring or ulceration exposes subendothelial thrombogenic collagen and von Willebrand factor to blood. Collagen engages platelets through their glycoprotein (GP) Ia, IIa, and VI receptors, and von Willebrand factor binds platelets through the GP Ib-IX-V receptor.
Activation. Once platelets adhere to the subendothelium, they undergo a conformational change and become activated. Simultaneous release of various autocrine and paracrine mediators including adenosine diphosphate, serotonin, epinephrine, thromboxane, and various ligand-receptor interactions all contribute to the activation cascade. Adenosine diphosphate binds to the platelet receptor P2Y1, leading to an increase in intracellular calcium, and it binds to P2Y12, leading to a decrease in cyclic adenosine monophosphate, both of which cause GP IIb/IIIa receptor activation. Thromboxane A2 released by platelets by cyclo-oxygenase 1 binds to alpha or beta variant receptors and contributes to GP IIb/IIIa activation through elevation of intracellular calcium levels.
Aggregation and thrombosis. Exposure of tissue factor to plasma following plaque rupture activates the coagulation cascade via the extrinsic pathway, which generates thrombin, a powerful platelet activator that causes thrombus formation via fibrin. Thrombin binds to protease-activated receptors PAR-1 and PAR-4 on platelets, causing an increase in intracellular calcium and a decrease in cyclic adenosine monophosphate with subsequent GP IIb/IIIa activation. GP IIb/IIIa facilitates platelet aggregation by binding to fibrinogen and forming a stable platelet thrombus.
In the early stages of thrombus formation, platelets predominate (“white” thrombi); further organization with fibrin results in older “red” thrombi. The stages of thrombi vary in non-ST-elevation and ST-elevation myocardial infarction and are prognostic markers of death.4–8
PERCUTANEOUS INTERVENTION, RESTENOSIS, AND STENT THROMBOSIS
Percutaneous coronary intervention, the preferred means of revascularization for many patients, is performed emergently in patients with ST-elevation myocardial infarction, urgently in those with acute coronary syndromes without ST elevation, and electively in those with stable ischemic symptoms.
Percutaneous revascularization techniques have evolved from balloon angioplasty to bare-metal stents to drug-eluting stents, but each of these procedures has been associated with a periprocedural and postprocedural risk of thrombosis.
Balloon angioplasty was associated with vascular intimal injury, inciting elastic vascular recoil and smooth muscle cell proliferation leading to restenosis.
Bare-metal stents reduced the restenosis rate by eliminating vascular recoil, although restenosis still occurred within the stent because of neointimal proliferation of vascular smooth muscle cells. This was an important limitation, as both acute and subacute stent thrombosis were refractory to aggressive anticoagulation regimens that were associated with major bleeding complications and longer hospital length of stay. Stenting became mainstream practice only after the ISAR9 and STARS10 trials showed that dual antiplatelet therapy controlled stent thrombosis.
Drug-eluting stents coated with anti-proliferative and anti-inflammatory polymers markedly reduced in-stent restenosis rates by suppressing the initial vascular smooth-muscle proliferative response. However, they were still associated with late and very late stent thrombosis with incomplete endothelialization, even up to 40 months after implantation. Proposed mechanisms include incomplete stent apposition and inflammatory hypersensitivity reactions to the polymer coating. Incomplete stent apposition associated with low-velocity blood flow at the junction of the stent strut and vessel wall, together with delayed endothelialization, promotes platelet adhesion and aggregation, followed by thrombus formation.11
Second-generation drug-eluting stents have thinner struts and more biocompatible polymers and are thought to favor more complete re-endothelialization, reducing the rates of stent thrombosis.8,12,13
Predictors of early stent thrombosis
The Dutch Stent Thrombosis Registry and other studies looked at risk factors for stent thrombosis.14,15
Procedure-related factors included:
- Stent undersizing
- Residual uncovered dissections after angioplasty
- Longer stents
- Low flow after angioplasty (< 3 on the 0–3 Thrombolysis in Myocardial Infarction [TIMI] scale).
Lesion-related factors included:
- Intermediate coronary artery disease both proximal and distal to the culprit lesions
- Bifurcation lesions.
Patient-related factors included:
- Low left ventricular ejection fraction
- Diabetes mellitus
- Peripheral arterial disease Premature discontinuation of clopidogrel.
ANTIPLATELET AGENTS: MECHANISM OF ACTION
Various pathways play synergistic roles in platelet activation and aggregation and thrombus formation, and different antiplatelet agents inhibit these specific pathways, thus complementing each other and having additive effects (Figure 2, Table 1).5,16–21
Aspirin inhibits cyclo-oxygenase 1
Cyclo-oxygenase 1, found in platelets, endothelial cells, and other cells, catalyzes the conversion of arachidonic acid to thromboxane A2. Aspirin irreversibly inhibits cyclo-oxygenase 1 by acetylating its serine residue, preventing formation of thromboxane A2 and preventing platelet activation and aggregation.
P2Y12 ADP receptor antagonists
Clopidogrel and prasugrel are thienopyridine agents that irreversibly inhibit the P2Y12 receptor, thereby preventing binding of adenosine diphosphate and the subsequent platelet activation-aggregation cascade. They are both prodrugs and require conversion by cytochrome P450 enzymes to active metabolites. Prasugrel is 10 times more potent than clopidogrel due to more efficient formation of its active metabolite, and it achieves a comparable effect on platelet inhibition 30 minutes faster than the peak effect of clopidogrel at 6 hours. The overall peak inhibitory effect of prasugrel is twice that of clopidogrel.22
Ticagrelor, a cyclopentyl-triazolo-pyrimidine, directly and reversibly inhibits the P2Y12 ADP receptor. Unlike clopidogrel and prasugrel, it does not need to be converted to an active metabolite, and it noncompetitively inhibits P2Y12 at a site different from the adenosine diphosphate binding site.23 Like prasugrel, ticagrelor inhibits platelet function more rapidly and more completely than clopidogrel.
Cangrelor, an intravenously administered analogue of adenosine triphosphate, reversibly inhibits the P2Y12 receptor. It has undergone phase 3 trials but is not yet approved for clinical use.24
WHY DUAL ANTIPLATELET THERAPY?
Aspirin is good, clopidogrel is better
Aspirin has a well-validated role in both primary and secondary prevention of coronary and noncoronary atherosclerotic vascular disease.
The CAPRIE trial found clopidogrel monotherapy to be superior to aspirin monotherapy in patients with established atherosclerotic vascular disease.25
After stenting, short-term dual therapy is better than short-term warfarin
Thrombotic complications in the early postprocedural period were a major limitation of stenting, and existing anticoagulation regimens were ineffective in preventing them.26,27
The ISAR trial studied the benefit of combined antiplatelet vs anticoagulant therapy after stent placement. Patients randomized to receive combined aspirin plus ticlopidine (an early P2Y12 inhibitor) had significantly lower rates of primary cardiac, hemorrhagic, and vascular events at 30 days.9 Two other trials confirmed this finding.28,29
STARS10 also confirmed the benefit of aspirin and ticlopidine after stenting. Patients were randomly assigned to aspirin alone, aspirin plus warfarin, or aspirin plus ticlopidine after stent placement. The rate of stent thrombosis at 30 days was significantly lower in the dual antiplatelet group than in the other two groups. The dual antiplatelet group had a higher rate of bleeding than the aspirin-alone group, but the rate was similar to that of the aspirin-plus-warfarin group.
Long-term dual antiplatelet therapy is beneficial in several situations
ISAR and STARS were landmark trials that showed stent thrombosis could be reduced by dual antiplatelet therapy for a 30-day period. However, the long-term role of dual antiplatelet therapy was still unknown.
The CURE trial30–32 randomized patients presenting with acute coronary syndromes without ST elevation to receive clopidogrel plus aspirin or placebo plus aspirin for 3 to 12 months. The rate of the primary end point (cardiac death, nonfatal myocardial infarction, or stroke) was significantly lower in the clopidogrel-plus-aspirin group. A similar benefit of dual antiplatelet therapy was seen in the subgroup of patients who underwent percutaneous coronary intervention. Both pretreatment with clopidogrel plus aspirin for a median of 10 days prior to percutaneous intervention and continuing it for a mean of 9 months reduced major adverse cardiovascular events.
The CREDO trial20 found that the combination of clopidogrel and aspirin significantly reduced the incidence of death, myocardial infarction, or stroke at 1 year after percutaneous coronary intervention. A subgroup of patients in this trial who had a longer pretreatment interval with a loading clopidogrel dose showed a benefit at 28 days, which was not as evident with a shorter loading dose interval.
The CLARITY-TIMI 28 trial33,34 showed the advantage of adding clopidogrel to aspirin in patients receiving fibrinolytic therapy for ST-elevation myocardial infarction. Adding clopidogrel both improved the patency of the infarct-related artery and reduced ischemic complications. In patients who subsequently underwent percutaneous coronary intervention and stenting, clopidogrel pretreatment was associated with a significant decrease in ischemic complications before and after the procedure. There was no significant increase in bleeding complications in either group.
COMMIT/CCS 235 also showed the benefit of dual antiplatelet therapy in patients with ST-elevation myocardial infarction. Clopidogrel added to aspirin during the short-term in-hospital or postdischarge treatment period significantly reduced a composite end point of reinfarction, death, or stroke as well as death from any cause.
The CHARISMA trial36–38 aimed to determine if patients who were more stable (ie, no recent acute coronary syndrome event or percutaneous coronary intervention) would benefit. Overall, CHARISMA showed no benefit of adding clopidogrel to aspirin compared with aspirin alone in a broad population of patients with established vascular disease (secondary prevention) or risk factors for vascular disease (primary prevention).
But importantly, though no benefit was seen in the primary prevention group, the large subgroup of patients with established atherosclerotic vascular disease (12,153 of the 15,603 patients in the trial) did benefit from dual antiplatelet therapy.36,37 This subgroup showed an overall reduction in absolute risk of 1.5% (relative risk 0.88, P = .046) over a median follow-up of 27.6 months. This benefit was even more apparent in the 9,478 patients with prior myocardial infarction, stroke, or peripheral artery disease, for whom the relative risk reduction was 17.1% (P = .01) and the reduction in absolute risk 1.5%.38
These results are comparable to the 2% absolute risk reduction in the CURE trial for similar end points over 9 months. In both studies, there was no significant increase in the risk of major bleeding or intracranial bleeding in the clopidogrel-plus-aspirin groups, although minor bleeding was increased by dual antiplatelet therapy.
The rate of severe bleeding, which was the primary safety end point in CHARISMA, was not significantly different in the clopidogrel-plus-aspirin group compared with the placebo-plus-aspirin group (relative risk 1.25, 95% CI 0.97–1.61, P = .09).
Thus, although the CHARISMA findings were negative overall, the positive finding observed in the predominant subgroup of patients with established vascular disease can therefore be considered supportive of the results of the subsequent trials discussed below.
The PEGASUS-TIMI 54 trial39 studied the benefit of adding ticagrelor (60 or 90 mg) to low-dose aspirin in patients with stable coronary artery disease who had had a myocardial infarction 1 to 3 years earlier.
Confirming the results of the CHARISMA subgroup analysis, the incidence of the ischemic primary efficacy end point (a composite of cardiovascular death, myocardial infarction, and stroke) was significantly lower in both groups receiving ticagrelor plus aspirin compared with those receiving placebo plus aspirin. The Kaplan-Meier rate at 3 years for the ticagrelor 90 mg-plus-aspirin group was 7.85% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.85, 95% confidence interval [CI] 0.75–0.96, P = .008). The rate for the ticagrelor 60 mg-plus-aspirin group was 7.77% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.84, 95% CI 0.74–0.95, P = .004).
The rates of all TIMI major and minor bleeding, as well as bleeding requiring transfusion or discontinuation of the study drug, were significantly higher in both ticagrelor dosing groups than in the placebo group (P < .01 for both groups vs placebo). The rates of fatal bleeding and nonfatal intracranial hemorrhage were not significantly higher. Although there was an overall reduction in ischemic end points with the addition of ticagrelor, there was also a significantly higher incidence of bleeding in this group.
Comment. Thus, with or without percutaneous coronary intervention in acute coronary syndrome as well as in stable coronary artery disease, dual antiplatelet therapy was shown to improve outcomes and decrease ischemic complications compared with aspirin alone. It provided benefit in the setting of acute coronary syndrome (in the CURE trial) and percutaneous coronary intervention (in the CREDO trial) for up to 1 year.
Major questions remained to be addressed:
- Do the results of CREDO, which was performed before the current interventional era and the use of drug-eluting stents, reflect outcomes after current interventional practice?
- Could shorter periods of dual antiplatelet therapy be sufficient, especially with newer stents with less risk of late thrombosis?
- Does the benefit of dual antiplatelet therapy extend beyond the 1-year time period tested in those trials to date?
RECOMMENDATIONS FOR DOSING
The American College of Cardiology Foundation/American Heart Association guidelines for dosing of antiplatelet agents for non-ST-elevation myocardial infarction are summarized in Table 2, and those for ST-elevation myocardial infarction are summarized in Table 3.1,2
WOULD SHORTER THERAPY AFTER STENTING WORK AS WELL?
The American College of Cardiology Foundation/American Heart Association currently recommend dual antiplatelet therapy for at least 12 months after drug-eluting stent placement, with shorter courses appropriate for patients who develop excessive bleeding complications or who are at high risk of bleeding.
Four trials (Table 4) evaluated whether shorter durations of dual antiplatelet therapy would suffice: SECURITY,40 EXCELLENT,41 OPTIMIZE,42 and RESET.43 All of them showed that short-duration therapy was not inferior to standard-duration therapy.44 These studies were comparable in that:
- Patients were randomized at the time of percutaneous coronary intervention or within 24 hours of it.
- Most patients received a second-generation drug-eluting stent, with the following exceptions: in EXCELLENT,41 one-fourth of patients received a Cypher first-generation drug-eluting stent, and in RESET,43 approximately one-fourth of the patients received a sirolimus-eluting stent in the standard-duration group for short lesions. Those patients with longer lesions in the RESET standard-duration group received an everolimus drug-eluting stent.
- The second antiplatelet added to aspirin in all studies was clopidogrel, with the exception of the SECURITY trial, in which fewer than 2% of patients received ticagrelor or prasugrel.40
- All the trials except RESET excluded patients who had had a myocardial infarction within 72 hours, and thus most patients studied had a lower risk profile.
- All of the trials sought to study noninferiority of short- vs standard-duration dual antiplatelet therapy, defined as the occurrence of a primary end point at 1 year (a composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, target vessel failure or revascularization, or bleeding).
Their low-risk patient populations and infrequent end points rendered these studies underpowered to make definitive conclusions about the relative efficacy of 6-months vs 12-months of dual antiplatelet therapy.
WOULD LONGER THERAPY BE BETTER?
The PRODIGY trial45 assessed durations of dual antiplatelet therapy both shorter and longer than the conventional 1 year, randomizing patients undergoing placement of a bare-metal stent, first-generation drug-eluting stent, or second-generation drug-eluting stent to receive aspirin and clopidogrel for either 6 months or 24 months. The study showed no significant difference in primary outcomes in the short- or long-duration groups.
Other trials that compared the standard 12 months of dual antiplatelet therapy with extended duration beyond 12 months were DAPT,3 ARCTIC-Interruption,46 and DES-LATE.47 The trials were comparable in that:
- All patients were randomized after completing 12 months of dual antiplatelet therapy following drug-eluting stent placement.
- All patients who were included had been free of major cardiac ischemic events or bleeding during the 12 months following stent placement.
- The primary aim of all three studies was to compare primary end points in groups receiving aspirin alone vs extended dual antiplatelet therapy. The primary end point was a composite of death due to a cardiovascular cause, nonfatal myocardial infarction, stroke, or stent thrombosis.
- The principal safety end point was bleeding.
Although the two earlier studies (ARCTIC-Interruption and DES-LATE) did not show any benefit of extended dual antiplatelet therapy compared with the standard 12-month duration, the recent DAPT study did.
The DAPT study
The DAPT study3 was an international, multicenter, placebo-controlled, double-blind randomized trial designed to examine the benefit of dual antiplatelet therapy beyond 1 year in a patient population large enough to provide definitive assessment of benefit and risk.
A total of 9,961 patients who received drug-eluting stents were randomized after 12 months of dual antiplatelet therapy to receive either a thienopyridine (clopidogrel or prasugrel) plus aspirin or placebo plus aspirin. They were followed for an additional 18 months. The coprimary efficacy end points were stent thrombosis and a composite of death, myocardial infarction, or stroke, while the primary safety end point was moderate or severe bleeding. The patients were also observed from months 30 to 33 on aspirin alone after stopping the thienopyridine.
Results. Longer therapy substantially reduced the risks of stent thrombosis (hazard ratio [HR] 0.29, 95% confidence interval [CI] 0.17–0.48) and the composite ischemic end point (HR 0.71, 95% CI 0.59–0.85). Follow-up during the 3-month thienopyridine discontinuation phase starting at 30 months revealed convergence of the ischemic event-rate curves in the two groups, which suggested that continuing dual antiplatelet therapy beyond 30 months might have been beneficial. Myocardial infarction unrelated to stent thrombosis accounted for 55% of the treatment benefit of dual antiplatelet therapy.
The risk of bleeding was higher in the thienopyridine group during the treatment period (2.5% vs 1.6%, P = .001). There was also a higher rate of noncardiovascular mortality in the thienopyridine group, although this difference may have been due to chance.3,48
Why were the results different?
All three trials included first- and second-generation drug-eluting stents, with different proportions in different trials. In ARCTIC-Interruption,46 43% of the patients in the continuation group had a first-generation stent, as did 64% of the patients in the dual antiplatelet group of DES-LATE.47 In the DAPT trial,3 38% of the patients in the longer-duration arm had a first-generation stent, and in 26% of cases it was a paclitaxel-eluting stent.
Only clopidogrel was used as the second antiplatelet agent in DES-LATE, whereas prasugrel was used in 10% of patients in ARCTIC-Interruption and 35% in DAPT.
Yet none of these differences seem to explain the differences in outcome among the studies. ARCTIC-Interruption and DES-LATE did not show any benefit of continued dual antiplatelet therapy beyond 12 months. DAPT showed benefit of extended therapy with prasugrel or with clopidogrel, and with first-generation or second-generation drug-eluting stents. The most likely explanation for the different results was that DAPT was the only trial sufficiently powered to definitively assess the end points, including stent thrombosis.
A balance between ischemic efficacy and bleeding risk is the major consideration with any antithrombotic and antiplatelet therapy. In the three largest trials we discussed (the vascular disease subgroups of CHARISMA,38 PEGASUS,39 and DAPT3), comparison of the prespecified efficacy and safety end points of each trial suggests that dual antiplatelet therapy has a net benefit, particularly given the irreversible nature of ischemic end points.
In CHARISMA,38 60 cardiovascular deaths, myocardial infarctions, or strokes were prevented per year per 10,000 patients treated, at the cost of 28 excess moderate bleeding events.
In PEGASUS,39 42 cardiovascular deaths, myocardial infarctions, or strokes were prevented, at the cost of 79 excess bleeding events requiring transfusion.
In DAPT (a selected population who had tolerated dual antiplatelet therapy for 1 year), 106 deaths, myocardial infarctions, or stroke events were prevented, at the cost of 47 excess moderate bleeding events.3
Indirect comparisons between trials are problematic, given different end point definitions, populations, and background therapies. But their results suggest that less-intensive inhibition with clopidogrel as the second antiplatelet long-term (as in CHARISMA) may provide the best balance of benefit vs risk.
BALANCING RISK AND BENEFIT
The evidence is unequivocal that dual antiplatelet therapy suppresses coronary ischemic complications resulting from thrombosis at sites of spontaneous plaque rupture following acute coronary syndromes or mechanical plaque disruption and foreign body implantation associated with percutaneous coronary intervention.
Three large-scale trials (DAPT,3 PEGASUS,39 and the secondary prevention subgroup of CHARISMA38) showed that the protective effect of dual antiplatelet therapy continues with prolonged therapy in patients who have experienced an acute coronary syndrome event or have received a drug-eluting stent. That benefit seems to be due to the action of these therapies on the culprit vessel (the one that caused the acute coronary syndrome or the site of stenting), as well as nonculprit arteries, emphasizing that dual antiplatelet therapy protects against atherosclerosis progression and future plaque rupture events.
For the durations studied in the longest trials thus far, 30 months (DAPT3) and 36 months (PEGASUS39), event curves continue to diverge, indicating that the advantage of dual antiplatelet therapy may persist for an indefinite period of time. Thus, indefinite therapy with dual antiplatelet agents can be supported, particularly in patients with advanced coronary artery disease or those who have had multiple coronary events.
We believe that the balance of evidence suggests that smaller studies that failed to show a benefit of longer-term therapy were underpowered to do so.
The ischemic protection is associated with the adverse effect of increased bleeding risk. Unfortunately, there has been little success in guiding dual antiplatelet therapy based on ischemic vs bleeding risk, in part because the same factors that predict risk of ischemic complications seem to predict increased susceptibility to bleeding. Nevertheless, indirect comparisons between studies suggest that for longer-term therapy clopidogrel may be superior to ticagrelor or prasugrel: the absolute excess bleeding risk with dual antiplatelet therapy vs aspirin in the CHARISMA secondary prevention subgroup was less than that in PEGASUS, with similar absolute reductions in ischemic events. So while the TRITON-TIMI 3822 and PLATO23 trials support the superiority of prasugrel or ticagrelor over clopidogrel for the first year after acute coronary syndrome, subsequent years of therapy may best be provided with clopidogrel.
Some patients may have identifiable factors that place them at very high risk of bleeding—need for surgical procedures, need for anticoagulation, or occurrence of bleeding complications or excessive “nuisance bleeding.” In those patients, the data suggest that dual antiplatelet therapy could be discontinued after 6 months, or perhaps even 3 months in the highest bleeding risk circumstances after second-generation drug-eluting stent placement.
WOEST49 was an open-label randomized controlled trial that studied the safety of antiplatelet regimens in patients on anticoagulation requiring percutaneous coronary interventions. Patients were randomized to double therapy with anticoagulant and clopidogrel vs triple therapy with additional aspirin following percutaneous coronary intervention. The primary end point was bleeding events within 1 year. Clopidogrel without aspirin was associated with significantly fewer bleeding events compared with triple therapy, with no increase in adverse ischemic events. The strategy tested in the WOEST trial seems reasonable in the specific group of patients who require ongoing anticoagulant therapy after drug-eluting stent placement, recognizing that the trial was somewhat underpowered to make definitive conclusions, particularly in patients at high risk for stent thrombosis.
Based on the results of PEGASUS and the CHARISMA subgroup with established ischemic burden, in which dual antiplatelet therapy was started after an interruption following the index coronary event, it is also reasonable to restart long-term dual antiplatelet therapy in patients who require interruption for short-term indications such as a surgical procedure.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Amsterdam EA, Wenger NK, Brindis RG, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130:e344–e426.
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371:2155–2166.
- Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J 2010; 74:597–607.
- Papp J, Kenyeres P, Toth K. Clinical importance of antiplatelet drugs in cardiovascular diseases. Clin Hemorheol Microcirc 2013; 53:81–96.
- Showkathali R, Natarajan A. Antiplatelet and antithrombin strategies in acute coronary syndrome: state-of-the-art review. Curr Cardiol Rev 2012; 8:239–249.
- Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 2012; 72:2087–2116.
- Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv 2014; 7:1081–1092.
- Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006; 48:193–202.
- Nikam N, Steinberg TB, Steinberg DH. Advances in stent technologies and their effect on clinical efficacy and safety. Med Devices (Auckl) 2014; 7:165–178.
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O’Brien ER. The evolution of coronary stents: a brief review. Can J Cardiol 2014; 30:35–45.
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J 2015; 36:3320–3331.
- van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol 2009; 53:1399–1409.
- Berger JS. Aspirin, clopidogrel, and ticagrelor in acute coronary syndromes. Am J Cardiol 2013; 112:737–745.
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015; 12:30–47.
- Patrono C, Rocca B. The future of antiplatelet therapy in cardiovascular disease. Annu Rev Med 2010; 61:49–61.
- Park SJ, Kang SM, Park DW. Dual antiplatelet therapy after drug-eluting stents: defining the proper duration. Coron Artery Dis 2014; 25:83–89.
- Steinhubl SR, Berger PB, Mann JT 3rd, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Nusca A, Patti G. Platelet function and inhibition in ischemic heart disease. Curr Cardiol Rep 2012; 14:457–467.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Genereux P, Stone GW, Harrington RA, et al; CHAMPION PHOENIX Investigators. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: Insights from the CHAMPION PHOENIX Trial (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects Who Require Percutaneous Coronary Intervention). J Am Coll Cardiol 2014; 63:619–629.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Warren J, Baber U, Mehran R. Antiplatelet therapy after drug-eluting stent implantation. J Cardiol 2015; 65:98–104.
- Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the Multicenter Aspirin and Ticlopidine Trial After Intracoronary Stenting (MATTIS). Circulation 1998; 98:2126–2132.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001; 358:527–533.
- Morais J. Insights from CURE: using clopidogrel on top of standard therapy. Cerebrovasc Dis 2002; 13(suppl 1):17–21.
- Ferguson JJ. Clopidogrel plus aspirin in patients with acute myocardial infarction treated with fibrinolytic therapy—CLARITY-TIMI 28. Future Cardiol 2005; 1:605–610.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372:1791–1800.
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64:2086–2097.
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125:505–513.
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310:2510–2522.
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60:1340–1348.
- El-Hayek G, Messerli F, Bangalore S, et al. Meta-analysis of randomized clinical trials comparing short-term versus long-term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 2014; 114:236–242.
- Valgimigli M, Campo G, Monti M, et al; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012; 125:2015–2026.
- Collet JP, Silvain J, Barthelemy O, et al; ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014; 384:1577–1585.
- Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014; 129:304–312.
- Kwok CS, Bulluck H, Ryding AD, Loke YK. Benefits and harms of extending the duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stents: a meta-analysis. ScientificWorldJournal 2014; 2014:794078.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Amsterdam EA, Wenger NK, Brindis RG, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130:e344–e426.
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371:2155–2166.
- Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J 2010; 74:597–607.
- Papp J, Kenyeres P, Toth K. Clinical importance of antiplatelet drugs in cardiovascular diseases. Clin Hemorheol Microcirc 2013; 53:81–96.
- Showkathali R, Natarajan A. Antiplatelet and antithrombin strategies in acute coronary syndrome: state-of-the-art review. Curr Cardiol Rev 2012; 8:239–249.
- Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 2012; 72:2087–2116.
- Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv 2014; 7:1081–1092.
- Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006; 48:193–202.
- Nikam N, Steinberg TB, Steinberg DH. Advances in stent technologies and their effect on clinical efficacy and safety. Med Devices (Auckl) 2014; 7:165–178.
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O’Brien ER. The evolution of coronary stents: a brief review. Can J Cardiol 2014; 30:35–45.
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J 2015; 36:3320–3331.
- van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol 2009; 53:1399–1409.
- Berger JS. Aspirin, clopidogrel, and ticagrelor in acute coronary syndromes. Am J Cardiol 2013; 112:737–745.
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015; 12:30–47.
- Patrono C, Rocca B. The future of antiplatelet therapy in cardiovascular disease. Annu Rev Med 2010; 61:49–61.
- Park SJ, Kang SM, Park DW. Dual antiplatelet therapy after drug-eluting stents: defining the proper duration. Coron Artery Dis 2014; 25:83–89.
- Steinhubl SR, Berger PB, Mann JT 3rd, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Nusca A, Patti G. Platelet function and inhibition in ischemic heart disease. Curr Cardiol Rep 2012; 14:457–467.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Genereux P, Stone GW, Harrington RA, et al; CHAMPION PHOENIX Investigators. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: Insights from the CHAMPION PHOENIX Trial (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects Who Require Percutaneous Coronary Intervention). J Am Coll Cardiol 2014; 63:619–629.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Warren J, Baber U, Mehran R. Antiplatelet therapy after drug-eluting stent implantation. J Cardiol 2015; 65:98–104.
- Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the Multicenter Aspirin and Ticlopidine Trial After Intracoronary Stenting (MATTIS). Circulation 1998; 98:2126–2132.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001; 358:527–533.
- Morais J. Insights from CURE: using clopidogrel on top of standard therapy. Cerebrovasc Dis 2002; 13(suppl 1):17–21.
- Ferguson JJ. Clopidogrel plus aspirin in patients with acute myocardial infarction treated with fibrinolytic therapy—CLARITY-TIMI 28. Future Cardiol 2005; 1:605–610.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372:1791–1800.
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64:2086–2097.
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125:505–513.
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310:2510–2522.
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60:1340–1348.
- El-Hayek G, Messerli F, Bangalore S, et al. Meta-analysis of randomized clinical trials comparing short-term versus long-term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 2014; 114:236–242.
- Valgimigli M, Campo G, Monti M, et al; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012; 125:2015–2026.
- Collet JP, Silvain J, Barthelemy O, et al; ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014; 384:1577–1585.
- Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014; 129:304–312.
- Kwok CS, Bulluck H, Ryding AD, Loke YK. Benefits and harms of extending the duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stents: a meta-analysis. ScientificWorldJournal 2014; 2014:794078.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
KEY POINTS
- The outcomes of patients with acute coronary syndrome events have been improving as percutaneous coronary intervention and its accompanying medical therapy have evolved.
- Newer, more potent antiplatelet agents are preferred over clopidogrel when possible.
- Two earlier studies showed no advantage of extended dual antiplatelet therapy over the standard 12-month duration, but the recent Dual Antiplatelet Therapy trial did.
- The protection against ischemia afforded by dual antiplatelet therapy comes at the price of increased risk of bleeding.
Gout and African Americans: Reducing disparities
Despite the historic association of gout with royalty and “rich eating,” gout disproportionately affects those of lower socioeconomic status.1 Risk factors for gout, including obesity, chronic kidney disease, diabetes, and hypertension, are more common in African Americans, resulting in a higher prevalence of the disease. In addition, African Americans are less likely to receive the aggressive treatment for gout advocated by professional societies, such as anti-inflammatory medications during flares and prophylactic urate-lowering therapy.
This article reviews the epidemiology of gout and its pathophysiology, risk factors, and optimal management, focusing on African Americans and strategies to reduce healthcare disparities in this patient population.
GOUT IS COMMON AND SERIOUS
Gout is the most common inflammatory arthritis in the United States today, affecting 4% of the adult US population.2 It is a chronic disease associated with high levels of uric acid, usually manifesting as intermittent attacks of painful monoarthritis, although multiple joints may be involved.
Despite a popular misconception that gout is merely an episodic nuisance, it is a serious disease that can significantly affect physical function and quality of life.3 A 2013 systematic review found that quality of life was significantly reduced in patients with gout, particularly those with polyarticular gout, tophi, comorbidities, and radiographic damage.4
Economic costs include decreased worker productivity and increased absences from work.5,6 From 2001 to 2005, an estimated 2 million visits were made to primary care providers due to gout.7 Because gout frequently coexists with diabetes, hypertension, coronary artery disease, and kidney disease, it is often overlooked during routine clinic visits.3
HIGH URIC ACID HAS MANY EFFECTS
Uric acid, a product of purine metabolism, is the key mediator of gouty arthritis. Accumulation of uric acid in joints and other tissues leads to an exuberant inflammatory response manifesting as a gouty attack. When uncontrolled, uric acid may crystallize in joints and other structures, leading to tophi formation, which can cause chronic deforming and erosive arthritis, known as chronic tophaceous gout.8
Although gout is considered to be a musculoskeletal disorder, recent evidence indicates that hyperuricemia plays an important role in the development of renal disease and contributes to cardiovascular morbidity and death.9 Several studies have found that reducing uric acid levels lowers cardiovascular mortality rates and retards the progression of chronic kidney disease.10
MEN AND CERTAIN RACIAL GROUPS MOST AFFECTED
Large-scale epidemiologic surveys have established that prevalence varies widely among population groups. Premenopausal women are less likely to be affected than men, presumably due to the effect of female hormones on renal tubular excretion of uric acid.11
Certain Asian populations (Filipinos, Taiwanese, Micronesians, the Maoris of New Zealand, Hmong Chinese immigrants in Minnesota) and African Americans have a high prevalence, while people from several sub-Saharan African countries have a very low prevalence. These differences are likely due to a variety of reasons, including genetic predisposition; diet (Table 1); risk factors such as obesity, diabetes, and hypertension; less access to healthcare resources; and inappropriate treatment.12,13
AFRICAN AMERICANS ARE A DIVERSE GROUP
“African Americans” are a highly heterogeneous group making up about 13% of the US population. Most scholars now consider racial identity largely a product of socioeconomic and political circumstances rather than a scientific concept.14 The US Census Bureau defines African Americans (or “blacks”) as those having origins in the black racial groups of Africa,15 but even this is problematic, since it combines people as diverse as descendants of 17th, 18th, and 19th century enslaved Africans with recent first-generation African immigrants, as well as with Caribbean- and Latino-Americans of African heritage.
In addition, many African Americans trace their ancestry to other racial and ethnic groups, especially European and Native American. A study of nine European DNA markers among 10 African American populations in the United States and one population of Jamaicans of African descent found a range of frequencies from 7% among Jamaicans to 23% among African Americans living in New Orleans.16
Although such diversity argues against generalizing healthcare needs for the entire African American community, some evidence indicates that genetic markers for gene-disease associations may be consistent across traditional racial boundaries.17
GENETIC FACTORS CANNOT EXPLAIN GOUT DISPARITIES
Several small studies have found evidence of genetic factors mediating the risk for hyperuricemia and gout. Twin studies indicate that hyperuricemia is highly heritable,18 although they do not show a concordance in the heritability of gout, suggesting that environmental factors also have an important role in developing clinical manifestations of the disease.12
Genome-wide association studies have identified 28 separate loci influencing uric acid levels, including genes for uric acid transporters (eg, SLC2A9 and ABCG2), and metabolic pathway regulators (eg, PDZK1, SCL22A12, and PRPSAP1), but the distribution among African Americans is largely unknown.19 One important exception is SLC2A9, a renal tubular transporter of uric acid: variants of SLC2A9 were found exclusively in African Americans, but the clinical significance of this association is unclear.20
Several epidemiologic studies have looked at gout in African Americans.
In the Coronary Artery Risk Development in Young Adults study,21 young African Americans had lower levels of uric acid than whites after adjusting for body mass index (BMI), glomerular filtration rate (GFR), diet, and medications. But after up to 20 years of follow-up, the risk of hyperuricemia was 2.3 times higher in African American women than in white women (95% confidence interval [CI] 1.34–3.99). Such differences were not found between African American and white men.
The National Health and Nutritional Examination Survey (NHANES) found that African American adolescents had lower uric acid levels than white adolescents after taking into account sugar intake, GFR, BMI, and onset of puberty.22
The Multiple Risk Factor Intervention Trial23 also found that among those with high cardiovascular risk, the incidence of hyperuricemia and gout was lower in African Americans than in whites.
COMORBID DISEASES MAY EXPLAIN DISPARITIES
Despite some evidence that African Americans have genetic factors that are protective against gout, they have a much greater frequency of acquired risk factors for gout, including obesity, physical inactivity, hypertension, diabetes mellitus, renal failure, high intake of seafood, elevated blood lead levels, and use of antihypertensive medications (Table 2).
Hochberg et al24 examined the incidence of gout in 352 African American and 571 white physicians and found higher rates in African Americans (9% vs 5%). The authors suggested different rates of hypertension as an explanation, although the use of antihypertensive medications such as diuretics that promote hyperuricemia confounds the strength of this conclusion.
AFRICAN AMERICANS NEED STANDARD TREATMENT . . .
Unlike for heart failure, in which subgroup analyses of large prospective studies have found different efficacies of medications in African Americans than in whites, no such data exist for gout. Although there is a higher risk of allopurinol hypersensitivity in ethnic groups that express the HLA-B*5801 polymorphism (eg, Han Chinese, Korean, Thai, Japanese, Portuguese), African Americans are not known to be at greater risk.25 There are also no special precautions for using febuxostat or probenecid in African Americans.
Absent any compelling reason, gout management should be the same regardless of ethnicity.26 Patients should be counseled on primary prevention measures such as dietary and behavioral modification and, if necessary, started on aggressive urate-lowering therapy.27
. . . BUT ARE LESS LIKELY TO HAVE GOUT APPROPRIATELY TREATED
African Americans with gout are less likely to receive urate-lowering therapy.28 According to the 2002 National Ambulatory Medical Care Survey in the United States, African Americans with gout are far less likely to receive allopurinol than whites (42% vs 80%; odds ratio [OR] 0.18; 95% CI 0.04–0.78).23 Even when therapy is prescribed, rates of nonadherence are greater in African Americans than in whites (OR 1.86, 95% CI 1.52–2.27), though the authors do not speculate why this is so.29 No studies have compared rates of prescribing febuxostat to African Americans vs whites.
African Americans are also less likely to receive ongoing routine care for their gout. A 2007 study30 of 663 veterans found that physicians were 1.41 times less likely to adhere to three selected quality indicators when dealing with nonwhite than white patients (95% CI 0.52–3.84). The three quality indicators studied were:
- Lowering of daily allopurinol dose to below 300 mg/day in the presence of renal insufficiency (no longer considered a quality measure)
- Monitoring of serum urate level at least once during the first 6 months of continued use of a xanthine oxidase inhibitor, such as allopurinol
- Monitoring of complete blood cell count and creatinine kinase at least every 6 months in patients with renal impairment receiving long-term prophylactic oral colchicine (> 0.5 mg/day for at least 6 months).
The finding was independent of age, comorbidity index, healthcare access, and utilization characteristics (eg, number of inpatient and outpatient visits, type of physician, most frequently seen physician).
LIFESTYLE MODIFICATION
Dietary modification is a useful initial step toward reducing uric acid levels (Table 1).27 The following measures are recommended:
Reduce alcohol intake. Alcoholic beverages, particularly beer, are strongly linked to hyperuricemia, according to a 2013 meta-analysis.31 Although alcohol consumption is lower in African Americans than in whites, mortality rates from cirrhosis and other alcohol-related diseases are 10% higher, suggesting metabolic differences that render African Americans more susceptible to the negative health effects of alcohol.32
Avoid sugary drinks. Sweetened beverages, especially those rich in fructose, are also implicated in hyperuricemia and gout. NHANES found an increase in serum uric acid of 0.33 mg/dL (95% CI, 0.11–0.73) in those consuming one to three sugar-sweetened drinks per day compared with nonconsumers, adjusting for total energy intake, age, sex, medications, hypertension, and GFR.33 A prospective study also found a relative risk of 1.85 for those who drink two or more sugar-sweetened beverages per day compared with those who drink fewer than one per month (95% CI 1.08–3.16).34
Unfortunately, African Americans consume a disproportionate amount of sugar from all sources: 17% of African Americans are considered heavy consumers vs 9% of whites.35
Limit foods rich in purines. Red meat, seafood, and some vegetables, including asparagus, spinach, peas, cauliflower, and mushrooms, are associated with increased serum uric acid levels. NHANES found that greater meat and seafood consumption was associated with increased uric acid levels. Choi et al36 found that the risk of gout increased by 21% with each additional daily serving of meat; the relative risk of developing gout was 1.41 (95% CI 1.07–1.86) in the fifth quintile of meat intake compared with the first quintile, and 1.51 (95% CI 1.17–1.95) in the fifth quintile of seafood consumption.
Despite these associations with high-purine food consumption and gout, many purine-rich foods may not contribute to hyperuricemia, and therefore a low-purine diet may not be protective. Interestingly, purine-rich vegetable protein intake is not associated with increased gout risk.37
Increase dairy consumption. Dairy in the diet is associated with a lower incidence of gout, with a decrease of 0.21 mg/dL (95% CI –0.37 to –0.04) in serum uric acid levels between the highest and lowest quintiles of dairy consumption.38 A randomized controlled trial found a 10% reduction in serum uric acid levels with milk consumption.39
Enjoy coffee. Coffee intake has been inversely correlated with gout. Daily intake of 4 to 5 cups of coffee is associated with a relative risk of 0.60 (95% CI 0.41–0.87) vs no coffee.40
Vitamin C and cherry juice41,42 have also been linked to lower gout risk, but the data are less robust.
Control weight. Primary care providers should advise patients to increase physical activity and maintain a healthy weight.
In a prospective study, Choi et al43 found that, in men, the risk of gout increased with the BMI. Compared with men with BMIs in the range of 21 to 22.9 kg/m2, the relative risks were:
- 1.95 (95% CI 1.44–2.65) at BMI 25 to 29.9 kg/m2
- 2.33 (95% CI 1.62–3.36) at BMI 30 to 34.9 kg/m2
- 2.97 (95% CI 1.73–5.10) at BMI > 35 kg/m2.
In addition, those who gained more than 13.6 kg since age 21 had a relative risk of 1.99 (95% CI 1.49–2.66) of developing gout compared with those whose weight remained within 1.8 kg.
For those who cannot achieve weight loss through conservative measures, bariatric surgery has shown promise. In a prospective study of 60 obese patients (BMI > 35 kg/m2) with gout and type 2 diabetes mellitus, uric acid steadily declined during the first year after bariatric surgery.44
TREATMENT OF ACUTE ATTACKS
Gout can be effectively managed in most patients. Behavioral and pharmacologic interventions are cheap and effective and have been shown to halt further damage to joints as well as retard the progression of renal disease and reduce cardiovascular morbidity.
During acute attacks, anti-inflammatory medications, principally glucocorticoids, nonsteroidal anti-inflammatory drugs, and colchicine, should be given promptly to reduce the intensity and duration of flares.
Although traditional teaching has been that urate-lowering therapy should not be initiated during an acute gout attack because it could prolong the duration of the attack, guidelines now permit it, based on studies showing that therapy does not prolong attacks.45,46
AGGRESSIVE URATE-LOWERING THERAPY FOR PROPHYLAXIS
Long-term treatment of gout is aimed at reducing uric acid levels by mitigating modifiable risk factors and through urate-lowering therapy.46 For many patients, conservative management with dietary and other behavioral changes is not sufficient to prevent further attacks of gout, necessitating urate-lowering therapy. Comorbid diseases such as obesity, hypertension, chronic kidney disease, and diabetes should also be addressed because they promote hyperuricemia and gouty attacks.47
A number of organizations have issued gout management guidelines over the past decade, including the American College of Rheumatology (ACR) in 2012, the European League Against Rheumatism (2006, updated in 2014), and the British Society of Rheumatology (2007). All recommend urate-lowering therapy to prevent gout flares.
The American and European guidelines recommend a target uric acid level below 6 mg/dL, and the British guidelines recommend a target below 5 mg/dL.48 For patients with palpable and visible tophi, the ACR guidelines state that lowering to below 5 mg/dL may be needed.46
First-line agents for urate-lowering therapy are xanthine oxidase inhibitors, which include allopurinol (costing $0.48 per generic 100-mg tablet or $0.92 per 300-mg tablet), and febuxostat ($5.38 per 40-mg or 80-mg tablet). For patients with contraindications or intolerances to allopurinol or febuxostat, probenecid ($1.15 per 500-mg tablet), which functions as a uricosuric agent (ie, increases urinary excretion of uric acid), may be used.
Losartan ($1.68 per 25-mg tablet) and fenofibrate ($1.91 per 48-mg tablet) are also often used to reduce uric acid levels, but they have only modest effects and are not approved for this indication in the United States.49
MANAGE CHRONIC CASES WITH CONTINUED THERAPY
ACR guidelines strongly emphasize continuing prophylaxis in case of ongoing gout activity (ie, detection of tophi on examination, recent gout attacks, or chronic gouty arthritis) (Table 3). The following durations have been proposed for prophylaxis:
- 6 months after an attack
- 6 months after achieving target uric acid level in patients with evidence of tophi
- 3 months after achieving target uric acid level in patients with resolved tophi.
Two randomized controlled trials support the use of the anti-inflammatory agent colchicine ($4.30 per tablet) for prophylaxis when initiating urate-lowering therapy.50,51
Monitor uric acid levels, renal function, adverse effects
The initial dosage of urate-lowering agents depends on the presence of kidney disease (Table 3).
Allopurinol is typically started at 50 mg to 100 mg oral daily, and titrated upward in increments of 100 mg depending on uric acid levels. According to the ACR guidelines, uric acid levels should be measured every 2 to 5 weeks.46 The Febuxostat vs Allopurinol Streamline Trial found that 97% of patients reached target uric acid levels within two titrations.52
Especially during the first months of therapy, physicians should be vigilant for adverse effects of allopurinol, including hypersensitivity reaction (rash, fever, Stevens-Johnson syndrome), hepatotoxicity, and myelotoxicity (bone marrow suppression), and for effects of febuxostat, such as rash, diarrhea, elevations in aminotransferase levels, and upper respiratory tract infections.53 Although the maximum acceptable dose of allopurinol is 800 mg even in chronic kidney disease, regularly monitoring for hypersensitivity reactions and other adverse effects is needed if doses are above 300 mg per day.46
Routine follow-up is essential
Adherence to therapy should be assessed at every visit. Patients should be counseled that gout is a chronic disease and that they should continue on urate-lowering therapy even if they are not having acute attacks. Adverse effects of medications should be monitored and addressed, although for allopurinol and febuxostat these are rare beyond the first few months of initiation and titration. If worsening of gout or uric acid levels occurs, therapy should be augmented and contributors to hyperuricemia reviewed. In refractory cases, rheumatology consultation may be needed; medications such as pegloticase ($16,800/mL) may be deemed necessary for severe tophaceous gout or for patients who need more rapid reduction of urates.49
STRATEGIES TO ADDRESS DISPARITIES
Creative approaches are needed to engage African American communities to reduce the burden of gout. No trials have been published evaluating methods for reducing health disparities with gout, but strategies for other chronic conditions may be applicable.
Incorporate guidelines better. Although setting and disseminating guidelines should ensure that care is standardized, studies have found that primary care physicians and rheumatologists frequently do not implement them.3,54 Reasons cited for poor adherence to gout guidelines include their relatively recent release, poor patient adherence, lack of measurement tools, and inadequate education of primary care physicians. Incorporating the guidelines as “best practice advisories” into electronic medical record systems has been proposed to improve their implementation.46
Use a team approach. Some quality improvement projects have used pharmacists and nurses to help implement gout guidelines. In two studies, empowering nurses and pharmacists to better educate patients and implement standardized protocols for titrating urate-lowering medications led to sustained improvements in maintaining serum uric acid levels less than 6 mg/dL.55–57
Multidisciplinary involvement by nutritionists, physicians, and community health workers have been found to help improve glycemic control in African Americans.58 Similar efforts can be undertaken to improve control of uric acid levels through dietary modification and improved compliance.
Address patient concerns. Substantial gaps exist in knowledge about gout between providers and the general population, although large studies specifically focusing on African Americans are lacking.59 Qualitative studies suggest that patient experience of gout may vary depending on race, with African Americans more likely than whites to rank the following concerns regarding gout as high: dietary restrictions, emotional burden, severe pain, the need for canes or crutches during flares, and gout bringing their day to a halt.60 Another study among African Americans with gout found concerns about the effectiveness of urate-lowering therapy, side effects of medications, polypharmacy, pill size, cost, refill issues, and forgetting to take medications regularly.61
- Hayward RA, Rathod T, Roddy E, Muller S, Hider SL, Mallen CD. The association of gout with socioeconomic status in primary care: a cross-sectional observational study. Rheumatology (Oxford) 2013; 52:2004–2008.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011; 63:3136–3141.
- Oderda GM, Shiozawa A, Walsh M, et al. Physician adherence to ACR gout treatment guidelines: perception versus practice. Postgrad Med 2014; 126:257–267.
- Chandratre P, Roddy E, Clarson L, Richardson J, Hider SL, Mallen CD. Health-related quality of life in gout: a systematic review. Rheumatology (Oxford) 2013; 52:2031–2040.
- Kleinman NL, Brook RA, Patel PA, et al. The impact of gout on work absence and productivity. Value Health 2007; 10:231–237.
- Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ 2011; 14:10–15.
- Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States. Arthritis Care Res (Hoboken) 2010; 62:460–464.
- Juraschek SP, Kovell LC, Miller ER 3rd, Gelber AC. Gout, urate-lowering therapy, and uric acid levels among adults in the United States. Arthritis Care Res (Hoboken) 2015; 67:588–592.
- Grassi D, Desideri G, Di Giacomantonio AV, Di Giosia P, Ferri C. Hyperuricemia and cardiovascular risk. High Blood Press Cardiovasc Prev 2014; 21:235–242.
- Karis E, Crittenden DB, Pillinger MH. Hyperuricemia, gout, and related comorbidities: cause and effect on a two-way street. South Med J 2014; 107:235–241.
- Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women—the Third National Health and Nutrition Examination Survey. Arthritis Res Ther 2008; 10:R116.
- MacFarlane LA, Kim SC. Gout: a review of nonmodifiable and modifiable risk factors. Rheum Dis Clin North Am 2014; 40:581–604.
- Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015 Nov; 11:649–662.
- Rivas-Drake D, Seaton EK, Markstrom C, et al; Ethnic and Racial Identity in the 21st Century Study Group. Ethnic and racial identity in adolescence: implications for psychosocial, academic, and health outcomes. Child Dev 2014; 85:40–57.
- US Commission on Civil Rights. Racial categorization in the 2010 census. www.usccr.gov/pubs/RC2010Web_Version.pdf. Accessed August 12, 2016.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Ioannidis JP, Ntzani EE, Trikalinos TA. ‘Racial’ differences in genetic effects for complex diseases. Nat Genet 2004; 36:1312–1218.
- Emmerson BT, Nagel SL, Duffy DL, Martin NG. Genetic control of the renal clearance of urate: a study of twins. Ann Rheum Dis 1992; 51:375–377.
- Merriman TR, Choi HK, Dalbeth N. The genetic basis of gout. Rheum Dis Clin North Am 2014; 40:279–290.
- Rule AD, de Andrade M, Matsumoto M, Mosley TH, Kardia S, Turner ST. Association between SLC2A9 transporter gene variants and uric acid phenotypes in African American and white families. Rheumatology (Oxford) 2011; 50: 871–878.
- Gaffo AL, Jacobs DR Jr, Lewis CE, Mikuls TR, Saag KG. Association between being African-American, serum urate levels and the risk of developing hyperuricemia: findings from the Coronary Artery Risk Development in Young Adults cohort. Arthritis Res Ther 2012; 14:R4.
- DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and Nutrition Survey 1999-2006. Metabolism 2012; 61: 554–561.
- Krishnan E. Gout in African Americans. Am J Med 2014; 127:858–864.
- Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum 1995; 38:628–632.
- Jarjour S, Barrette M, Normand V, Rouleau JL, Dubé MP, de Denus S. Genetic markers associated with cutaneous adverse drug reactions to allopurinol: a systematic review. Pharmacogenomics 2015; 16:755–767.
- Singh JA. Can racial disparities in optimal gout treatment be reduced? Evidence from a randomized trial. BMC Med 2012; 10:15.
- Nasser-Ghodsi N, Harrold LR. Overcoming adherence issues and other barriers to optimal care in gout. Curr Opin Rheumatol 2015; 27:134–138.
- Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol 2008; 35:498–501.
- Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis 2008; 67:609–613.
- Singh JA, Hodges JS, Toscano JP, Asch SM. Quality of care for gout in the US needs improvement. Arthritis Rheum 2007; 57:822–829.
- Wang M, Jiang X, Wu W, Zhang D. A meta-analysis of alcohol consumption and the risk of gout. Clin Rheumatol 2013; 32:1641–1648.
- Zapolski TC, Pedersen SL, McCarthy DM, Smith GT. Less drinking, yet more problems: understanding African American drinking and related problems. Psychol Bull 2014; 140:188–223.
- Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008; 59:109–116.
- Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 2008; 336:309–312.
- Schmidt LA. New unsweetened truths about sugar. JAMA Intern Med 2014; 174:525–526.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol 2010; 22:165–172.
- Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2005; 52:283–289.
- Dalbeth N, Wong S, Gamble GD, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis 2010; 69:1677–1682.
- Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: a prospective study. Arthritis Rheum 2007; 56:2049–2055.
- Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum 2012; 64:4004–4011.
- Schlesinger N, Schlesinger M. Previously reported prior studies of cherry juice concentrate for gout flare prophylaxis: comment on the article by Zhang et al. Arthritis Rheum 2013; 65:1135–1136.
- Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005; 165:742–748.
- Dalbeth N, Chen P, White M, et al. Impact of bariatric surgery on serum urate targets in people with morbid obesity and diabetes: a prospective longitudinal study. Ann Rheum Dis 2014; 73:797–802.
- Hill EM, Sky K, Sit M, Collamer A, Higgs J. Does starting allopurinol prolong acute treated gout? A randomized clinical trial. J Clin Rheumatol 2015; 21:120–125.
- Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012; 64:1431–1446.
- Puig JG, Martínez MA. Hyperuricemia, gout and the metabolic syndrome. Curr Opin Rheumatol 2008; 20:187–191.
- Wise E, Khanna PP. The impact of gout guidelines. Curr Opin Rheumatol 2015; 27:225–230.
- Becker MA. Urate-lowering medications. www.uptodate.com. Accessed August 15, 2016.
- Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004; 31:2429–2432.
- Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo-controlled study of probenecid-treated patients. Arthritis Rheum 1974; 17:609–614.
- Jennings CG, Mackenzie IS, Flynn R, et al; FAST study group. Up-titration of allopurinol in patients with gout. Semin Arthritis Rheum 2014; 44:25–30.
- Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010; 12: R63.
- Cottrell E, Crabtree V, Edwards JJ, Roddy E. Improvement in the management of gout is vital and overdue: an audit from a UK primary care medical practice. BMC Fam Pract 2013; 14:170.
- Goldfien RD, Ng MS, Yip G, et al. Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study. BMJ Open 2014; 4:e003627.
- Lim AY, Shen L, Tan CH, Lateef A, Lau TC, Teng GG. Achieving treat to target in gout: a clinical practice improvement project. Scand J Rheumatol 2012; 41:450–457.
- Rees F, Jenkins W, Doherty M. Patients with gout adhere to curative treatment if informed appropriately: proof-of-concept observational study. Ann Rheum Dis 2013; 72:826–830.
- Smalls BL, Walker RJ, Bonilha HS, Campbell JA, Egede LE. Community interventions to improve glycemic control in African Americans with type 2 diabetes: a systemic review. Glob J Health Sci 2015; 7:171–182.
- Harrold LR, Mazor KM, Peterson D, Naz N, Firneno C, Yood RA. Patients’ knowledge and beliefs concerning gout and its treatment: a population based study. BMC Musculoskelet Disord 2012; 13:180.
- Singh JA. The impact of gout on patient’s lives: a study of African American and Caucasian men and women with gout. Arthritis Res Ther 2014; 16:R132.
- Singh JA. Facilitators and barriers to adherence to urate-lowering therapy in African Americans with gout: a qualitative study. Arthritis Res Ther 2014; 16:R82.
Despite the historic association of gout with royalty and “rich eating,” gout disproportionately affects those of lower socioeconomic status.1 Risk factors for gout, including obesity, chronic kidney disease, diabetes, and hypertension, are more common in African Americans, resulting in a higher prevalence of the disease. In addition, African Americans are less likely to receive the aggressive treatment for gout advocated by professional societies, such as anti-inflammatory medications during flares and prophylactic urate-lowering therapy.
This article reviews the epidemiology of gout and its pathophysiology, risk factors, and optimal management, focusing on African Americans and strategies to reduce healthcare disparities in this patient population.
GOUT IS COMMON AND SERIOUS
Gout is the most common inflammatory arthritis in the United States today, affecting 4% of the adult US population.2 It is a chronic disease associated with high levels of uric acid, usually manifesting as intermittent attacks of painful monoarthritis, although multiple joints may be involved.
Despite a popular misconception that gout is merely an episodic nuisance, it is a serious disease that can significantly affect physical function and quality of life.3 A 2013 systematic review found that quality of life was significantly reduced in patients with gout, particularly those with polyarticular gout, tophi, comorbidities, and radiographic damage.4
Economic costs include decreased worker productivity and increased absences from work.5,6 From 2001 to 2005, an estimated 2 million visits were made to primary care providers due to gout.7 Because gout frequently coexists with diabetes, hypertension, coronary artery disease, and kidney disease, it is often overlooked during routine clinic visits.3
HIGH URIC ACID HAS MANY EFFECTS
Uric acid, a product of purine metabolism, is the key mediator of gouty arthritis. Accumulation of uric acid in joints and other tissues leads to an exuberant inflammatory response manifesting as a gouty attack. When uncontrolled, uric acid may crystallize in joints and other structures, leading to tophi formation, which can cause chronic deforming and erosive arthritis, known as chronic tophaceous gout.8
Although gout is considered to be a musculoskeletal disorder, recent evidence indicates that hyperuricemia plays an important role in the development of renal disease and contributes to cardiovascular morbidity and death.9 Several studies have found that reducing uric acid levels lowers cardiovascular mortality rates and retards the progression of chronic kidney disease.10
MEN AND CERTAIN RACIAL GROUPS MOST AFFECTED
Large-scale epidemiologic surveys have established that prevalence varies widely among population groups. Premenopausal women are less likely to be affected than men, presumably due to the effect of female hormones on renal tubular excretion of uric acid.11
Certain Asian populations (Filipinos, Taiwanese, Micronesians, the Maoris of New Zealand, Hmong Chinese immigrants in Minnesota) and African Americans have a high prevalence, while people from several sub-Saharan African countries have a very low prevalence. These differences are likely due to a variety of reasons, including genetic predisposition; diet (Table 1); risk factors such as obesity, diabetes, and hypertension; less access to healthcare resources; and inappropriate treatment.12,13
AFRICAN AMERICANS ARE A DIVERSE GROUP
“African Americans” are a highly heterogeneous group making up about 13% of the US population. Most scholars now consider racial identity largely a product of socioeconomic and political circumstances rather than a scientific concept.14 The US Census Bureau defines African Americans (or “blacks”) as those having origins in the black racial groups of Africa,15 but even this is problematic, since it combines people as diverse as descendants of 17th, 18th, and 19th century enslaved Africans with recent first-generation African immigrants, as well as with Caribbean- and Latino-Americans of African heritage.
In addition, many African Americans trace their ancestry to other racial and ethnic groups, especially European and Native American. A study of nine European DNA markers among 10 African American populations in the United States and one population of Jamaicans of African descent found a range of frequencies from 7% among Jamaicans to 23% among African Americans living in New Orleans.16
Although such diversity argues against generalizing healthcare needs for the entire African American community, some evidence indicates that genetic markers for gene-disease associations may be consistent across traditional racial boundaries.17
GENETIC FACTORS CANNOT EXPLAIN GOUT DISPARITIES
Several small studies have found evidence of genetic factors mediating the risk for hyperuricemia and gout. Twin studies indicate that hyperuricemia is highly heritable,18 although they do not show a concordance in the heritability of gout, suggesting that environmental factors also have an important role in developing clinical manifestations of the disease.12
Genome-wide association studies have identified 28 separate loci influencing uric acid levels, including genes for uric acid transporters (eg, SLC2A9 and ABCG2), and metabolic pathway regulators (eg, PDZK1, SCL22A12, and PRPSAP1), but the distribution among African Americans is largely unknown.19 One important exception is SLC2A9, a renal tubular transporter of uric acid: variants of SLC2A9 were found exclusively in African Americans, but the clinical significance of this association is unclear.20
Several epidemiologic studies have looked at gout in African Americans.
In the Coronary Artery Risk Development in Young Adults study,21 young African Americans had lower levels of uric acid than whites after adjusting for body mass index (BMI), glomerular filtration rate (GFR), diet, and medications. But after up to 20 years of follow-up, the risk of hyperuricemia was 2.3 times higher in African American women than in white women (95% confidence interval [CI] 1.34–3.99). Such differences were not found between African American and white men.
The National Health and Nutritional Examination Survey (NHANES) found that African American adolescents had lower uric acid levels than white adolescents after taking into account sugar intake, GFR, BMI, and onset of puberty.22
The Multiple Risk Factor Intervention Trial23 also found that among those with high cardiovascular risk, the incidence of hyperuricemia and gout was lower in African Americans than in whites.
COMORBID DISEASES MAY EXPLAIN DISPARITIES
Despite some evidence that African Americans have genetic factors that are protective against gout, they have a much greater frequency of acquired risk factors for gout, including obesity, physical inactivity, hypertension, diabetes mellitus, renal failure, high intake of seafood, elevated blood lead levels, and use of antihypertensive medications (Table 2).
Hochberg et al24 examined the incidence of gout in 352 African American and 571 white physicians and found higher rates in African Americans (9% vs 5%). The authors suggested different rates of hypertension as an explanation, although the use of antihypertensive medications such as diuretics that promote hyperuricemia confounds the strength of this conclusion.
AFRICAN AMERICANS NEED STANDARD TREATMENT . . .
Unlike for heart failure, in which subgroup analyses of large prospective studies have found different efficacies of medications in African Americans than in whites, no such data exist for gout. Although there is a higher risk of allopurinol hypersensitivity in ethnic groups that express the HLA-B*5801 polymorphism (eg, Han Chinese, Korean, Thai, Japanese, Portuguese), African Americans are not known to be at greater risk.25 There are also no special precautions for using febuxostat or probenecid in African Americans.
Absent any compelling reason, gout management should be the same regardless of ethnicity.26 Patients should be counseled on primary prevention measures such as dietary and behavioral modification and, if necessary, started on aggressive urate-lowering therapy.27
. . . BUT ARE LESS LIKELY TO HAVE GOUT APPROPRIATELY TREATED
African Americans with gout are less likely to receive urate-lowering therapy.28 According to the 2002 National Ambulatory Medical Care Survey in the United States, African Americans with gout are far less likely to receive allopurinol than whites (42% vs 80%; odds ratio [OR] 0.18; 95% CI 0.04–0.78).23 Even when therapy is prescribed, rates of nonadherence are greater in African Americans than in whites (OR 1.86, 95% CI 1.52–2.27), though the authors do not speculate why this is so.29 No studies have compared rates of prescribing febuxostat to African Americans vs whites.
African Americans are also less likely to receive ongoing routine care for their gout. A 2007 study30 of 663 veterans found that physicians were 1.41 times less likely to adhere to three selected quality indicators when dealing with nonwhite than white patients (95% CI 0.52–3.84). The three quality indicators studied were:
- Lowering of daily allopurinol dose to below 300 mg/day in the presence of renal insufficiency (no longer considered a quality measure)
- Monitoring of serum urate level at least once during the first 6 months of continued use of a xanthine oxidase inhibitor, such as allopurinol
- Monitoring of complete blood cell count and creatinine kinase at least every 6 months in patients with renal impairment receiving long-term prophylactic oral colchicine (> 0.5 mg/day for at least 6 months).
The finding was independent of age, comorbidity index, healthcare access, and utilization characteristics (eg, number of inpatient and outpatient visits, type of physician, most frequently seen physician).
LIFESTYLE MODIFICATION
Dietary modification is a useful initial step toward reducing uric acid levels (Table 1).27 The following measures are recommended:
Reduce alcohol intake. Alcoholic beverages, particularly beer, are strongly linked to hyperuricemia, according to a 2013 meta-analysis.31 Although alcohol consumption is lower in African Americans than in whites, mortality rates from cirrhosis and other alcohol-related diseases are 10% higher, suggesting metabolic differences that render African Americans more susceptible to the negative health effects of alcohol.32
Avoid sugary drinks. Sweetened beverages, especially those rich in fructose, are also implicated in hyperuricemia and gout. NHANES found an increase in serum uric acid of 0.33 mg/dL (95% CI, 0.11–0.73) in those consuming one to three sugar-sweetened drinks per day compared with nonconsumers, adjusting for total energy intake, age, sex, medications, hypertension, and GFR.33 A prospective study also found a relative risk of 1.85 for those who drink two or more sugar-sweetened beverages per day compared with those who drink fewer than one per month (95% CI 1.08–3.16).34
Unfortunately, African Americans consume a disproportionate amount of sugar from all sources: 17% of African Americans are considered heavy consumers vs 9% of whites.35
Limit foods rich in purines. Red meat, seafood, and some vegetables, including asparagus, spinach, peas, cauliflower, and mushrooms, are associated with increased serum uric acid levels. NHANES found that greater meat and seafood consumption was associated with increased uric acid levels. Choi et al36 found that the risk of gout increased by 21% with each additional daily serving of meat; the relative risk of developing gout was 1.41 (95% CI 1.07–1.86) in the fifth quintile of meat intake compared with the first quintile, and 1.51 (95% CI 1.17–1.95) in the fifth quintile of seafood consumption.
Despite these associations with high-purine food consumption and gout, many purine-rich foods may not contribute to hyperuricemia, and therefore a low-purine diet may not be protective. Interestingly, purine-rich vegetable protein intake is not associated with increased gout risk.37
Increase dairy consumption. Dairy in the diet is associated with a lower incidence of gout, with a decrease of 0.21 mg/dL (95% CI –0.37 to –0.04) in serum uric acid levels between the highest and lowest quintiles of dairy consumption.38 A randomized controlled trial found a 10% reduction in serum uric acid levels with milk consumption.39
Enjoy coffee. Coffee intake has been inversely correlated with gout. Daily intake of 4 to 5 cups of coffee is associated with a relative risk of 0.60 (95% CI 0.41–0.87) vs no coffee.40
Vitamin C and cherry juice41,42 have also been linked to lower gout risk, but the data are less robust.
Control weight. Primary care providers should advise patients to increase physical activity and maintain a healthy weight.
In a prospective study, Choi et al43 found that, in men, the risk of gout increased with the BMI. Compared with men with BMIs in the range of 21 to 22.9 kg/m2, the relative risks were:
- 1.95 (95% CI 1.44–2.65) at BMI 25 to 29.9 kg/m2
- 2.33 (95% CI 1.62–3.36) at BMI 30 to 34.9 kg/m2
- 2.97 (95% CI 1.73–5.10) at BMI > 35 kg/m2.
In addition, those who gained more than 13.6 kg since age 21 had a relative risk of 1.99 (95% CI 1.49–2.66) of developing gout compared with those whose weight remained within 1.8 kg.
For those who cannot achieve weight loss through conservative measures, bariatric surgery has shown promise. In a prospective study of 60 obese patients (BMI > 35 kg/m2) with gout and type 2 diabetes mellitus, uric acid steadily declined during the first year after bariatric surgery.44
TREATMENT OF ACUTE ATTACKS
Gout can be effectively managed in most patients. Behavioral and pharmacologic interventions are cheap and effective and have been shown to halt further damage to joints as well as retard the progression of renal disease and reduce cardiovascular morbidity.
During acute attacks, anti-inflammatory medications, principally glucocorticoids, nonsteroidal anti-inflammatory drugs, and colchicine, should be given promptly to reduce the intensity and duration of flares.
Although traditional teaching has been that urate-lowering therapy should not be initiated during an acute gout attack because it could prolong the duration of the attack, guidelines now permit it, based on studies showing that therapy does not prolong attacks.45,46
AGGRESSIVE URATE-LOWERING THERAPY FOR PROPHYLAXIS
Long-term treatment of gout is aimed at reducing uric acid levels by mitigating modifiable risk factors and through urate-lowering therapy.46 For many patients, conservative management with dietary and other behavioral changes is not sufficient to prevent further attacks of gout, necessitating urate-lowering therapy. Comorbid diseases such as obesity, hypertension, chronic kidney disease, and diabetes should also be addressed because they promote hyperuricemia and gouty attacks.47
A number of organizations have issued gout management guidelines over the past decade, including the American College of Rheumatology (ACR) in 2012, the European League Against Rheumatism (2006, updated in 2014), and the British Society of Rheumatology (2007). All recommend urate-lowering therapy to prevent gout flares.
The American and European guidelines recommend a target uric acid level below 6 mg/dL, and the British guidelines recommend a target below 5 mg/dL.48 For patients with palpable and visible tophi, the ACR guidelines state that lowering to below 5 mg/dL may be needed.46
First-line agents for urate-lowering therapy are xanthine oxidase inhibitors, which include allopurinol (costing $0.48 per generic 100-mg tablet or $0.92 per 300-mg tablet), and febuxostat ($5.38 per 40-mg or 80-mg tablet). For patients with contraindications or intolerances to allopurinol or febuxostat, probenecid ($1.15 per 500-mg tablet), which functions as a uricosuric agent (ie, increases urinary excretion of uric acid), may be used.
Losartan ($1.68 per 25-mg tablet) and fenofibrate ($1.91 per 48-mg tablet) are also often used to reduce uric acid levels, but they have only modest effects and are not approved for this indication in the United States.49
MANAGE CHRONIC CASES WITH CONTINUED THERAPY
ACR guidelines strongly emphasize continuing prophylaxis in case of ongoing gout activity (ie, detection of tophi on examination, recent gout attacks, or chronic gouty arthritis) (Table 3). The following durations have been proposed for prophylaxis:
- 6 months after an attack
- 6 months after achieving target uric acid level in patients with evidence of tophi
- 3 months after achieving target uric acid level in patients with resolved tophi.
Two randomized controlled trials support the use of the anti-inflammatory agent colchicine ($4.30 per tablet) for prophylaxis when initiating urate-lowering therapy.50,51
Monitor uric acid levels, renal function, adverse effects
The initial dosage of urate-lowering agents depends on the presence of kidney disease (Table 3).
Allopurinol is typically started at 50 mg to 100 mg oral daily, and titrated upward in increments of 100 mg depending on uric acid levels. According to the ACR guidelines, uric acid levels should be measured every 2 to 5 weeks.46 The Febuxostat vs Allopurinol Streamline Trial found that 97% of patients reached target uric acid levels within two titrations.52
Especially during the first months of therapy, physicians should be vigilant for adverse effects of allopurinol, including hypersensitivity reaction (rash, fever, Stevens-Johnson syndrome), hepatotoxicity, and myelotoxicity (bone marrow suppression), and for effects of febuxostat, such as rash, diarrhea, elevations in aminotransferase levels, and upper respiratory tract infections.53 Although the maximum acceptable dose of allopurinol is 800 mg even in chronic kidney disease, regularly monitoring for hypersensitivity reactions and other adverse effects is needed if doses are above 300 mg per day.46
Routine follow-up is essential
Adherence to therapy should be assessed at every visit. Patients should be counseled that gout is a chronic disease and that they should continue on urate-lowering therapy even if they are not having acute attacks. Adverse effects of medications should be monitored and addressed, although for allopurinol and febuxostat these are rare beyond the first few months of initiation and titration. If worsening of gout or uric acid levels occurs, therapy should be augmented and contributors to hyperuricemia reviewed. In refractory cases, rheumatology consultation may be needed; medications such as pegloticase ($16,800/mL) may be deemed necessary for severe tophaceous gout or for patients who need more rapid reduction of urates.49
STRATEGIES TO ADDRESS DISPARITIES
Creative approaches are needed to engage African American communities to reduce the burden of gout. No trials have been published evaluating methods for reducing health disparities with gout, but strategies for other chronic conditions may be applicable.
Incorporate guidelines better. Although setting and disseminating guidelines should ensure that care is standardized, studies have found that primary care physicians and rheumatologists frequently do not implement them.3,54 Reasons cited for poor adherence to gout guidelines include their relatively recent release, poor patient adherence, lack of measurement tools, and inadequate education of primary care physicians. Incorporating the guidelines as “best practice advisories” into electronic medical record systems has been proposed to improve their implementation.46
Use a team approach. Some quality improvement projects have used pharmacists and nurses to help implement gout guidelines. In two studies, empowering nurses and pharmacists to better educate patients and implement standardized protocols for titrating urate-lowering medications led to sustained improvements in maintaining serum uric acid levels less than 6 mg/dL.55–57
Multidisciplinary involvement by nutritionists, physicians, and community health workers have been found to help improve glycemic control in African Americans.58 Similar efforts can be undertaken to improve control of uric acid levels through dietary modification and improved compliance.
Address patient concerns. Substantial gaps exist in knowledge about gout between providers and the general population, although large studies specifically focusing on African Americans are lacking.59 Qualitative studies suggest that patient experience of gout may vary depending on race, with African Americans more likely than whites to rank the following concerns regarding gout as high: dietary restrictions, emotional burden, severe pain, the need for canes or crutches during flares, and gout bringing their day to a halt.60 Another study among African Americans with gout found concerns about the effectiveness of urate-lowering therapy, side effects of medications, polypharmacy, pill size, cost, refill issues, and forgetting to take medications regularly.61
Despite the historic association of gout with royalty and “rich eating,” gout disproportionately affects those of lower socioeconomic status.1 Risk factors for gout, including obesity, chronic kidney disease, diabetes, and hypertension, are more common in African Americans, resulting in a higher prevalence of the disease. In addition, African Americans are less likely to receive the aggressive treatment for gout advocated by professional societies, such as anti-inflammatory medications during flares and prophylactic urate-lowering therapy.
This article reviews the epidemiology of gout and its pathophysiology, risk factors, and optimal management, focusing on African Americans and strategies to reduce healthcare disparities in this patient population.
GOUT IS COMMON AND SERIOUS
Gout is the most common inflammatory arthritis in the United States today, affecting 4% of the adult US population.2 It is a chronic disease associated with high levels of uric acid, usually manifesting as intermittent attacks of painful monoarthritis, although multiple joints may be involved.
Despite a popular misconception that gout is merely an episodic nuisance, it is a serious disease that can significantly affect physical function and quality of life.3 A 2013 systematic review found that quality of life was significantly reduced in patients with gout, particularly those with polyarticular gout, tophi, comorbidities, and radiographic damage.4
Economic costs include decreased worker productivity and increased absences from work.5,6 From 2001 to 2005, an estimated 2 million visits were made to primary care providers due to gout.7 Because gout frequently coexists with diabetes, hypertension, coronary artery disease, and kidney disease, it is often overlooked during routine clinic visits.3
HIGH URIC ACID HAS MANY EFFECTS
Uric acid, a product of purine metabolism, is the key mediator of gouty arthritis. Accumulation of uric acid in joints and other tissues leads to an exuberant inflammatory response manifesting as a gouty attack. When uncontrolled, uric acid may crystallize in joints and other structures, leading to tophi formation, which can cause chronic deforming and erosive arthritis, known as chronic tophaceous gout.8
Although gout is considered to be a musculoskeletal disorder, recent evidence indicates that hyperuricemia plays an important role in the development of renal disease and contributes to cardiovascular morbidity and death.9 Several studies have found that reducing uric acid levels lowers cardiovascular mortality rates and retards the progression of chronic kidney disease.10
MEN AND CERTAIN RACIAL GROUPS MOST AFFECTED
Large-scale epidemiologic surveys have established that prevalence varies widely among population groups. Premenopausal women are less likely to be affected than men, presumably due to the effect of female hormones on renal tubular excretion of uric acid.11
Certain Asian populations (Filipinos, Taiwanese, Micronesians, the Maoris of New Zealand, Hmong Chinese immigrants in Minnesota) and African Americans have a high prevalence, while people from several sub-Saharan African countries have a very low prevalence. These differences are likely due to a variety of reasons, including genetic predisposition; diet (Table 1); risk factors such as obesity, diabetes, and hypertension; less access to healthcare resources; and inappropriate treatment.12,13
AFRICAN AMERICANS ARE A DIVERSE GROUP
“African Americans” are a highly heterogeneous group making up about 13% of the US population. Most scholars now consider racial identity largely a product of socioeconomic and political circumstances rather than a scientific concept.14 The US Census Bureau defines African Americans (or “blacks”) as those having origins in the black racial groups of Africa,15 but even this is problematic, since it combines people as diverse as descendants of 17th, 18th, and 19th century enslaved Africans with recent first-generation African immigrants, as well as with Caribbean- and Latino-Americans of African heritage.
In addition, many African Americans trace their ancestry to other racial and ethnic groups, especially European and Native American. A study of nine European DNA markers among 10 African American populations in the United States and one population of Jamaicans of African descent found a range of frequencies from 7% among Jamaicans to 23% among African Americans living in New Orleans.16
Although such diversity argues against generalizing healthcare needs for the entire African American community, some evidence indicates that genetic markers for gene-disease associations may be consistent across traditional racial boundaries.17
GENETIC FACTORS CANNOT EXPLAIN GOUT DISPARITIES
Several small studies have found evidence of genetic factors mediating the risk for hyperuricemia and gout. Twin studies indicate that hyperuricemia is highly heritable,18 although they do not show a concordance in the heritability of gout, suggesting that environmental factors also have an important role in developing clinical manifestations of the disease.12
Genome-wide association studies have identified 28 separate loci influencing uric acid levels, including genes for uric acid transporters (eg, SLC2A9 and ABCG2), and metabolic pathway regulators (eg, PDZK1, SCL22A12, and PRPSAP1), but the distribution among African Americans is largely unknown.19 One important exception is SLC2A9, a renal tubular transporter of uric acid: variants of SLC2A9 were found exclusively in African Americans, but the clinical significance of this association is unclear.20
Several epidemiologic studies have looked at gout in African Americans.
In the Coronary Artery Risk Development in Young Adults study,21 young African Americans had lower levels of uric acid than whites after adjusting for body mass index (BMI), glomerular filtration rate (GFR), diet, and medications. But after up to 20 years of follow-up, the risk of hyperuricemia was 2.3 times higher in African American women than in white women (95% confidence interval [CI] 1.34–3.99). Such differences were not found between African American and white men.
The National Health and Nutritional Examination Survey (NHANES) found that African American adolescents had lower uric acid levels than white adolescents after taking into account sugar intake, GFR, BMI, and onset of puberty.22
The Multiple Risk Factor Intervention Trial23 also found that among those with high cardiovascular risk, the incidence of hyperuricemia and gout was lower in African Americans than in whites.
COMORBID DISEASES MAY EXPLAIN DISPARITIES
Despite some evidence that African Americans have genetic factors that are protective against gout, they have a much greater frequency of acquired risk factors for gout, including obesity, physical inactivity, hypertension, diabetes mellitus, renal failure, high intake of seafood, elevated blood lead levels, and use of antihypertensive medications (Table 2).
Hochberg et al24 examined the incidence of gout in 352 African American and 571 white physicians and found higher rates in African Americans (9% vs 5%). The authors suggested different rates of hypertension as an explanation, although the use of antihypertensive medications such as diuretics that promote hyperuricemia confounds the strength of this conclusion.
AFRICAN AMERICANS NEED STANDARD TREATMENT . . .
Unlike for heart failure, in which subgroup analyses of large prospective studies have found different efficacies of medications in African Americans than in whites, no such data exist for gout. Although there is a higher risk of allopurinol hypersensitivity in ethnic groups that express the HLA-B*5801 polymorphism (eg, Han Chinese, Korean, Thai, Japanese, Portuguese), African Americans are not known to be at greater risk.25 There are also no special precautions for using febuxostat or probenecid in African Americans.
Absent any compelling reason, gout management should be the same regardless of ethnicity.26 Patients should be counseled on primary prevention measures such as dietary and behavioral modification and, if necessary, started on aggressive urate-lowering therapy.27
. . . BUT ARE LESS LIKELY TO HAVE GOUT APPROPRIATELY TREATED
African Americans with gout are less likely to receive urate-lowering therapy.28 According to the 2002 National Ambulatory Medical Care Survey in the United States, African Americans with gout are far less likely to receive allopurinol than whites (42% vs 80%; odds ratio [OR] 0.18; 95% CI 0.04–0.78).23 Even when therapy is prescribed, rates of nonadherence are greater in African Americans than in whites (OR 1.86, 95% CI 1.52–2.27), though the authors do not speculate why this is so.29 No studies have compared rates of prescribing febuxostat to African Americans vs whites.
African Americans are also less likely to receive ongoing routine care for their gout. A 2007 study30 of 663 veterans found that physicians were 1.41 times less likely to adhere to three selected quality indicators when dealing with nonwhite than white patients (95% CI 0.52–3.84). The three quality indicators studied were:
- Lowering of daily allopurinol dose to below 300 mg/day in the presence of renal insufficiency (no longer considered a quality measure)
- Monitoring of serum urate level at least once during the first 6 months of continued use of a xanthine oxidase inhibitor, such as allopurinol
- Monitoring of complete blood cell count and creatinine kinase at least every 6 months in patients with renal impairment receiving long-term prophylactic oral colchicine (> 0.5 mg/day for at least 6 months).
The finding was independent of age, comorbidity index, healthcare access, and utilization characteristics (eg, number of inpatient and outpatient visits, type of physician, most frequently seen physician).
LIFESTYLE MODIFICATION
Dietary modification is a useful initial step toward reducing uric acid levels (Table 1).27 The following measures are recommended:
Reduce alcohol intake. Alcoholic beverages, particularly beer, are strongly linked to hyperuricemia, according to a 2013 meta-analysis.31 Although alcohol consumption is lower in African Americans than in whites, mortality rates from cirrhosis and other alcohol-related diseases are 10% higher, suggesting metabolic differences that render African Americans more susceptible to the negative health effects of alcohol.32
Avoid sugary drinks. Sweetened beverages, especially those rich in fructose, are also implicated in hyperuricemia and gout. NHANES found an increase in serum uric acid of 0.33 mg/dL (95% CI, 0.11–0.73) in those consuming one to three sugar-sweetened drinks per day compared with nonconsumers, adjusting for total energy intake, age, sex, medications, hypertension, and GFR.33 A prospective study also found a relative risk of 1.85 for those who drink two or more sugar-sweetened beverages per day compared with those who drink fewer than one per month (95% CI 1.08–3.16).34
Unfortunately, African Americans consume a disproportionate amount of sugar from all sources: 17% of African Americans are considered heavy consumers vs 9% of whites.35
Limit foods rich in purines. Red meat, seafood, and some vegetables, including asparagus, spinach, peas, cauliflower, and mushrooms, are associated with increased serum uric acid levels. NHANES found that greater meat and seafood consumption was associated with increased uric acid levels. Choi et al36 found that the risk of gout increased by 21% with each additional daily serving of meat; the relative risk of developing gout was 1.41 (95% CI 1.07–1.86) in the fifth quintile of meat intake compared with the first quintile, and 1.51 (95% CI 1.17–1.95) in the fifth quintile of seafood consumption.
Despite these associations with high-purine food consumption and gout, many purine-rich foods may not contribute to hyperuricemia, and therefore a low-purine diet may not be protective. Interestingly, purine-rich vegetable protein intake is not associated with increased gout risk.37
Increase dairy consumption. Dairy in the diet is associated with a lower incidence of gout, with a decrease of 0.21 mg/dL (95% CI –0.37 to –0.04) in serum uric acid levels between the highest and lowest quintiles of dairy consumption.38 A randomized controlled trial found a 10% reduction in serum uric acid levels with milk consumption.39
Enjoy coffee. Coffee intake has been inversely correlated with gout. Daily intake of 4 to 5 cups of coffee is associated with a relative risk of 0.60 (95% CI 0.41–0.87) vs no coffee.40
Vitamin C and cherry juice41,42 have also been linked to lower gout risk, but the data are less robust.
Control weight. Primary care providers should advise patients to increase physical activity and maintain a healthy weight.
In a prospective study, Choi et al43 found that, in men, the risk of gout increased with the BMI. Compared with men with BMIs in the range of 21 to 22.9 kg/m2, the relative risks were:
- 1.95 (95% CI 1.44–2.65) at BMI 25 to 29.9 kg/m2
- 2.33 (95% CI 1.62–3.36) at BMI 30 to 34.9 kg/m2
- 2.97 (95% CI 1.73–5.10) at BMI > 35 kg/m2.
In addition, those who gained more than 13.6 kg since age 21 had a relative risk of 1.99 (95% CI 1.49–2.66) of developing gout compared with those whose weight remained within 1.8 kg.
For those who cannot achieve weight loss through conservative measures, bariatric surgery has shown promise. In a prospective study of 60 obese patients (BMI > 35 kg/m2) with gout and type 2 diabetes mellitus, uric acid steadily declined during the first year after bariatric surgery.44
TREATMENT OF ACUTE ATTACKS
Gout can be effectively managed in most patients. Behavioral and pharmacologic interventions are cheap and effective and have been shown to halt further damage to joints as well as retard the progression of renal disease and reduce cardiovascular morbidity.
During acute attacks, anti-inflammatory medications, principally glucocorticoids, nonsteroidal anti-inflammatory drugs, and colchicine, should be given promptly to reduce the intensity and duration of flares.
Although traditional teaching has been that urate-lowering therapy should not be initiated during an acute gout attack because it could prolong the duration of the attack, guidelines now permit it, based on studies showing that therapy does not prolong attacks.45,46
AGGRESSIVE URATE-LOWERING THERAPY FOR PROPHYLAXIS
Long-term treatment of gout is aimed at reducing uric acid levels by mitigating modifiable risk factors and through urate-lowering therapy.46 For many patients, conservative management with dietary and other behavioral changes is not sufficient to prevent further attacks of gout, necessitating urate-lowering therapy. Comorbid diseases such as obesity, hypertension, chronic kidney disease, and diabetes should also be addressed because they promote hyperuricemia and gouty attacks.47
A number of organizations have issued gout management guidelines over the past decade, including the American College of Rheumatology (ACR) in 2012, the European League Against Rheumatism (2006, updated in 2014), and the British Society of Rheumatology (2007). All recommend urate-lowering therapy to prevent gout flares.
The American and European guidelines recommend a target uric acid level below 6 mg/dL, and the British guidelines recommend a target below 5 mg/dL.48 For patients with palpable and visible tophi, the ACR guidelines state that lowering to below 5 mg/dL may be needed.46
First-line agents for urate-lowering therapy are xanthine oxidase inhibitors, which include allopurinol (costing $0.48 per generic 100-mg tablet or $0.92 per 300-mg tablet), and febuxostat ($5.38 per 40-mg or 80-mg tablet). For patients with contraindications or intolerances to allopurinol or febuxostat, probenecid ($1.15 per 500-mg tablet), which functions as a uricosuric agent (ie, increases urinary excretion of uric acid), may be used.
Losartan ($1.68 per 25-mg tablet) and fenofibrate ($1.91 per 48-mg tablet) are also often used to reduce uric acid levels, but they have only modest effects and are not approved for this indication in the United States.49
MANAGE CHRONIC CASES WITH CONTINUED THERAPY
ACR guidelines strongly emphasize continuing prophylaxis in case of ongoing gout activity (ie, detection of tophi on examination, recent gout attacks, or chronic gouty arthritis) (Table 3). The following durations have been proposed for prophylaxis:
- 6 months after an attack
- 6 months after achieving target uric acid level in patients with evidence of tophi
- 3 months after achieving target uric acid level in patients with resolved tophi.
Two randomized controlled trials support the use of the anti-inflammatory agent colchicine ($4.30 per tablet) for prophylaxis when initiating urate-lowering therapy.50,51
Monitor uric acid levels, renal function, adverse effects
The initial dosage of urate-lowering agents depends on the presence of kidney disease (Table 3).
Allopurinol is typically started at 50 mg to 100 mg oral daily, and titrated upward in increments of 100 mg depending on uric acid levels. According to the ACR guidelines, uric acid levels should be measured every 2 to 5 weeks.46 The Febuxostat vs Allopurinol Streamline Trial found that 97% of patients reached target uric acid levels within two titrations.52
Especially during the first months of therapy, physicians should be vigilant for adverse effects of allopurinol, including hypersensitivity reaction (rash, fever, Stevens-Johnson syndrome), hepatotoxicity, and myelotoxicity (bone marrow suppression), and for effects of febuxostat, such as rash, diarrhea, elevations in aminotransferase levels, and upper respiratory tract infections.53 Although the maximum acceptable dose of allopurinol is 800 mg even in chronic kidney disease, regularly monitoring for hypersensitivity reactions and other adverse effects is needed if doses are above 300 mg per day.46
Routine follow-up is essential
Adherence to therapy should be assessed at every visit. Patients should be counseled that gout is a chronic disease and that they should continue on urate-lowering therapy even if they are not having acute attacks. Adverse effects of medications should be monitored and addressed, although for allopurinol and febuxostat these are rare beyond the first few months of initiation and titration. If worsening of gout or uric acid levels occurs, therapy should be augmented and contributors to hyperuricemia reviewed. In refractory cases, rheumatology consultation may be needed; medications such as pegloticase ($16,800/mL) may be deemed necessary for severe tophaceous gout or for patients who need more rapid reduction of urates.49
STRATEGIES TO ADDRESS DISPARITIES
Creative approaches are needed to engage African American communities to reduce the burden of gout. No trials have been published evaluating methods for reducing health disparities with gout, but strategies for other chronic conditions may be applicable.
Incorporate guidelines better. Although setting and disseminating guidelines should ensure that care is standardized, studies have found that primary care physicians and rheumatologists frequently do not implement them.3,54 Reasons cited for poor adherence to gout guidelines include their relatively recent release, poor patient adherence, lack of measurement tools, and inadequate education of primary care physicians. Incorporating the guidelines as “best practice advisories” into electronic medical record systems has been proposed to improve their implementation.46
Use a team approach. Some quality improvement projects have used pharmacists and nurses to help implement gout guidelines. In two studies, empowering nurses and pharmacists to better educate patients and implement standardized protocols for titrating urate-lowering medications led to sustained improvements in maintaining serum uric acid levels less than 6 mg/dL.55–57
Multidisciplinary involvement by nutritionists, physicians, and community health workers have been found to help improve glycemic control in African Americans.58 Similar efforts can be undertaken to improve control of uric acid levels through dietary modification and improved compliance.
Address patient concerns. Substantial gaps exist in knowledge about gout between providers and the general population, although large studies specifically focusing on African Americans are lacking.59 Qualitative studies suggest that patient experience of gout may vary depending on race, with African Americans more likely than whites to rank the following concerns regarding gout as high: dietary restrictions, emotional burden, severe pain, the need for canes or crutches during flares, and gout bringing their day to a halt.60 Another study among African Americans with gout found concerns about the effectiveness of urate-lowering therapy, side effects of medications, polypharmacy, pill size, cost, refill issues, and forgetting to take medications regularly.61
- Hayward RA, Rathod T, Roddy E, Muller S, Hider SL, Mallen CD. The association of gout with socioeconomic status in primary care: a cross-sectional observational study. Rheumatology (Oxford) 2013; 52:2004–2008.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011; 63:3136–3141.
- Oderda GM, Shiozawa A, Walsh M, et al. Physician adherence to ACR gout treatment guidelines: perception versus practice. Postgrad Med 2014; 126:257–267.
- Chandratre P, Roddy E, Clarson L, Richardson J, Hider SL, Mallen CD. Health-related quality of life in gout: a systematic review. Rheumatology (Oxford) 2013; 52:2031–2040.
- Kleinman NL, Brook RA, Patel PA, et al. The impact of gout on work absence and productivity. Value Health 2007; 10:231–237.
- Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ 2011; 14:10–15.
- Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States. Arthritis Care Res (Hoboken) 2010; 62:460–464.
- Juraschek SP, Kovell LC, Miller ER 3rd, Gelber AC. Gout, urate-lowering therapy, and uric acid levels among adults in the United States. Arthritis Care Res (Hoboken) 2015; 67:588–592.
- Grassi D, Desideri G, Di Giacomantonio AV, Di Giosia P, Ferri C. Hyperuricemia and cardiovascular risk. High Blood Press Cardiovasc Prev 2014; 21:235–242.
- Karis E, Crittenden DB, Pillinger MH. Hyperuricemia, gout, and related comorbidities: cause and effect on a two-way street. South Med J 2014; 107:235–241.
- Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women—the Third National Health and Nutrition Examination Survey. Arthritis Res Ther 2008; 10:R116.
- MacFarlane LA, Kim SC. Gout: a review of nonmodifiable and modifiable risk factors. Rheum Dis Clin North Am 2014; 40:581–604.
- Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015 Nov; 11:649–662.
- Rivas-Drake D, Seaton EK, Markstrom C, et al; Ethnic and Racial Identity in the 21st Century Study Group. Ethnic and racial identity in adolescence: implications for psychosocial, academic, and health outcomes. Child Dev 2014; 85:40–57.
- US Commission on Civil Rights. Racial categorization in the 2010 census. www.usccr.gov/pubs/RC2010Web_Version.pdf. Accessed August 12, 2016.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Ioannidis JP, Ntzani EE, Trikalinos TA. ‘Racial’ differences in genetic effects for complex diseases. Nat Genet 2004; 36:1312–1218.
- Emmerson BT, Nagel SL, Duffy DL, Martin NG. Genetic control of the renal clearance of urate: a study of twins. Ann Rheum Dis 1992; 51:375–377.
- Merriman TR, Choi HK, Dalbeth N. The genetic basis of gout. Rheum Dis Clin North Am 2014; 40:279–290.
- Rule AD, de Andrade M, Matsumoto M, Mosley TH, Kardia S, Turner ST. Association between SLC2A9 transporter gene variants and uric acid phenotypes in African American and white families. Rheumatology (Oxford) 2011; 50: 871–878.
- Gaffo AL, Jacobs DR Jr, Lewis CE, Mikuls TR, Saag KG. Association between being African-American, serum urate levels and the risk of developing hyperuricemia: findings from the Coronary Artery Risk Development in Young Adults cohort. Arthritis Res Ther 2012; 14:R4.
- DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and Nutrition Survey 1999-2006. Metabolism 2012; 61: 554–561.
- Krishnan E. Gout in African Americans. Am J Med 2014; 127:858–864.
- Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum 1995; 38:628–632.
- Jarjour S, Barrette M, Normand V, Rouleau JL, Dubé MP, de Denus S. Genetic markers associated with cutaneous adverse drug reactions to allopurinol: a systematic review. Pharmacogenomics 2015; 16:755–767.
- Singh JA. Can racial disparities in optimal gout treatment be reduced? Evidence from a randomized trial. BMC Med 2012; 10:15.
- Nasser-Ghodsi N, Harrold LR. Overcoming adherence issues and other barriers to optimal care in gout. Curr Opin Rheumatol 2015; 27:134–138.
- Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol 2008; 35:498–501.
- Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis 2008; 67:609–613.
- Singh JA, Hodges JS, Toscano JP, Asch SM. Quality of care for gout in the US needs improvement. Arthritis Rheum 2007; 57:822–829.
- Wang M, Jiang X, Wu W, Zhang D. A meta-analysis of alcohol consumption and the risk of gout. Clin Rheumatol 2013; 32:1641–1648.
- Zapolski TC, Pedersen SL, McCarthy DM, Smith GT. Less drinking, yet more problems: understanding African American drinking and related problems. Psychol Bull 2014; 140:188–223.
- Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008; 59:109–116.
- Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 2008; 336:309–312.
- Schmidt LA. New unsweetened truths about sugar. JAMA Intern Med 2014; 174:525–526.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol 2010; 22:165–172.
- Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2005; 52:283–289.
- Dalbeth N, Wong S, Gamble GD, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis 2010; 69:1677–1682.
- Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: a prospective study. Arthritis Rheum 2007; 56:2049–2055.
- Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum 2012; 64:4004–4011.
- Schlesinger N, Schlesinger M. Previously reported prior studies of cherry juice concentrate for gout flare prophylaxis: comment on the article by Zhang et al. Arthritis Rheum 2013; 65:1135–1136.
- Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005; 165:742–748.
- Dalbeth N, Chen P, White M, et al. Impact of bariatric surgery on serum urate targets in people with morbid obesity and diabetes: a prospective longitudinal study. Ann Rheum Dis 2014; 73:797–802.
- Hill EM, Sky K, Sit M, Collamer A, Higgs J. Does starting allopurinol prolong acute treated gout? A randomized clinical trial. J Clin Rheumatol 2015; 21:120–125.
- Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012; 64:1431–1446.
- Puig JG, Martínez MA. Hyperuricemia, gout and the metabolic syndrome. Curr Opin Rheumatol 2008; 20:187–191.
- Wise E, Khanna PP. The impact of gout guidelines. Curr Opin Rheumatol 2015; 27:225–230.
- Becker MA. Urate-lowering medications. www.uptodate.com. Accessed August 15, 2016.
- Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004; 31:2429–2432.
- Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo-controlled study of probenecid-treated patients. Arthritis Rheum 1974; 17:609–614.
- Jennings CG, Mackenzie IS, Flynn R, et al; FAST study group. Up-titration of allopurinol in patients with gout. Semin Arthritis Rheum 2014; 44:25–30.
- Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010; 12: R63.
- Cottrell E, Crabtree V, Edwards JJ, Roddy E. Improvement in the management of gout is vital and overdue: an audit from a UK primary care medical practice. BMC Fam Pract 2013; 14:170.
- Goldfien RD, Ng MS, Yip G, et al. Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study. BMJ Open 2014; 4:e003627.
- Lim AY, Shen L, Tan CH, Lateef A, Lau TC, Teng GG. Achieving treat to target in gout: a clinical practice improvement project. Scand J Rheumatol 2012; 41:450–457.
- Rees F, Jenkins W, Doherty M. Patients with gout adhere to curative treatment if informed appropriately: proof-of-concept observational study. Ann Rheum Dis 2013; 72:826–830.
- Smalls BL, Walker RJ, Bonilha HS, Campbell JA, Egede LE. Community interventions to improve glycemic control in African Americans with type 2 diabetes: a systemic review. Glob J Health Sci 2015; 7:171–182.
- Harrold LR, Mazor KM, Peterson D, Naz N, Firneno C, Yood RA. Patients’ knowledge and beliefs concerning gout and its treatment: a population based study. BMC Musculoskelet Disord 2012; 13:180.
- Singh JA. The impact of gout on patient’s lives: a study of African American and Caucasian men and women with gout. Arthritis Res Ther 2014; 16:R132.
- Singh JA. Facilitators and barriers to adherence to urate-lowering therapy in African Americans with gout: a qualitative study. Arthritis Res Ther 2014; 16:R82.
- Hayward RA, Rathod T, Roddy E, Muller S, Hider SL, Mallen CD. The association of gout with socioeconomic status in primary care: a cross-sectional observational study. Rheumatology (Oxford) 2013; 52:2004–2008.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011; 63:3136–3141.
- Oderda GM, Shiozawa A, Walsh M, et al. Physician adherence to ACR gout treatment guidelines: perception versus practice. Postgrad Med 2014; 126:257–267.
- Chandratre P, Roddy E, Clarson L, Richardson J, Hider SL, Mallen CD. Health-related quality of life in gout: a systematic review. Rheumatology (Oxford) 2013; 52:2031–2040.
- Kleinman NL, Brook RA, Patel PA, et al. The impact of gout on work absence and productivity. Value Health 2007; 10:231–237.
- Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ 2011; 14:10–15.
- Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States. Arthritis Care Res (Hoboken) 2010; 62:460–464.
- Juraschek SP, Kovell LC, Miller ER 3rd, Gelber AC. Gout, urate-lowering therapy, and uric acid levels among adults in the United States. Arthritis Care Res (Hoboken) 2015; 67:588–592.
- Grassi D, Desideri G, Di Giacomantonio AV, Di Giosia P, Ferri C. Hyperuricemia and cardiovascular risk. High Blood Press Cardiovasc Prev 2014; 21:235–242.
- Karis E, Crittenden DB, Pillinger MH. Hyperuricemia, gout, and related comorbidities: cause and effect on a two-way street. South Med J 2014; 107:235–241.
- Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women—the Third National Health and Nutrition Examination Survey. Arthritis Res Ther 2008; 10:R116.
- MacFarlane LA, Kim SC. Gout: a review of nonmodifiable and modifiable risk factors. Rheum Dis Clin North Am 2014; 40:581–604.
- Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015 Nov; 11:649–662.
- Rivas-Drake D, Seaton EK, Markstrom C, et al; Ethnic and Racial Identity in the 21st Century Study Group. Ethnic and racial identity in adolescence: implications for psychosocial, academic, and health outcomes. Child Dev 2014; 85:40–57.
- US Commission on Civil Rights. Racial categorization in the 2010 census. www.usccr.gov/pubs/RC2010Web_Version.pdf. Accessed August 12, 2016.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Ioannidis JP, Ntzani EE, Trikalinos TA. ‘Racial’ differences in genetic effects for complex diseases. Nat Genet 2004; 36:1312–1218.
- Emmerson BT, Nagel SL, Duffy DL, Martin NG. Genetic control of the renal clearance of urate: a study of twins. Ann Rheum Dis 1992; 51:375–377.
- Merriman TR, Choi HK, Dalbeth N. The genetic basis of gout. Rheum Dis Clin North Am 2014; 40:279–290.
- Rule AD, de Andrade M, Matsumoto M, Mosley TH, Kardia S, Turner ST. Association between SLC2A9 transporter gene variants and uric acid phenotypes in African American and white families. Rheumatology (Oxford) 2011; 50: 871–878.
- Gaffo AL, Jacobs DR Jr, Lewis CE, Mikuls TR, Saag KG. Association between being African-American, serum urate levels and the risk of developing hyperuricemia: findings from the Coronary Artery Risk Development in Young Adults cohort. Arthritis Res Ther 2012; 14:R4.
- DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and Nutrition Survey 1999-2006. Metabolism 2012; 61: 554–561.
- Krishnan E. Gout in African Americans. Am J Med 2014; 127:858–864.
- Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum 1995; 38:628–632.
- Jarjour S, Barrette M, Normand V, Rouleau JL, Dubé MP, de Denus S. Genetic markers associated with cutaneous adverse drug reactions to allopurinol: a systematic review. Pharmacogenomics 2015; 16:755–767.
- Singh JA. Can racial disparities in optimal gout treatment be reduced? Evidence from a randomized trial. BMC Med 2012; 10:15.
- Nasser-Ghodsi N, Harrold LR. Overcoming adherence issues and other barriers to optimal care in gout. Curr Opin Rheumatol 2015; 27:134–138.
- Krishnan E, Lienesch D, Kwoh CK. Gout in ambulatory care settings in the United States. J Rheumatol 2008; 35:498–501.
- Solomon DH, Avorn J, Levin R, Brookhart MA. Uric acid lowering therapy: prescribing patterns in a large cohort of older adults. Ann Rheum Dis 2008; 67:609–613.
- Singh JA, Hodges JS, Toscano JP, Asch SM. Quality of care for gout in the US needs improvement. Arthritis Rheum 2007; 57:822–829.
- Wang M, Jiang X, Wu W, Zhang D. A meta-analysis of alcohol consumption and the risk of gout. Clin Rheumatol 2013; 32:1641–1648.
- Zapolski TC, Pedersen SL, McCarthy DM, Smith GT. Less drinking, yet more problems: understanding African American drinking and related problems. Psychol Bull 2014; 140:188–223.
- Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008; 59:109–116.
- Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 2008; 336:309–312.
- Schmidt LA. New unsweetened truths about sugar. JAMA Intern Med 2014; 174:525–526.
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 2004; 350:1093–1103.
- Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol 2010; 22:165–172.
- Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2005; 52:283–289.
- Dalbeth N, Wong S, Gamble GD, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis 2010; 69:1677–1682.
- Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: a prospective study. Arthritis Rheum 2007; 56:2049–2055.
- Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi HK. Cherry consumption and decreased risk of recurrent gout attacks. Arthritis Rheum 2012; 64:4004–4011.
- Schlesinger N, Schlesinger M. Previously reported prior studies of cherry juice concentrate for gout flare prophylaxis: comment on the article by Zhang et al. Arthritis Rheum 2013; 65:1135–1136.
- Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005; 165:742–748.
- Dalbeth N, Chen P, White M, et al. Impact of bariatric surgery on serum urate targets in people with morbid obesity and diabetes: a prospective longitudinal study. Ann Rheum Dis 2014; 73:797–802.
- Hill EM, Sky K, Sit M, Collamer A, Higgs J. Does starting allopurinol prolong acute treated gout? A randomized clinical trial. J Clin Rheumatol 2015; 21:120–125.
- Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012; 64:1431–1446.
- Puig JG, Martínez MA. Hyperuricemia, gout and the metabolic syndrome. Curr Opin Rheumatol 2008; 20:187–191.
- Wise E, Khanna PP. The impact of gout guidelines. Curr Opin Rheumatol 2015; 27:225–230.
- Becker MA. Urate-lowering medications. www.uptodate.com. Accessed August 15, 2016.
- Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol 2004; 31:2429–2432.
- Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo-controlled study of probenecid-treated patients. Arthritis Rheum 1974; 17:609–614.
- Jennings CG, Mackenzie IS, Flynn R, et al; FAST study group. Up-titration of allopurinol in patients with gout. Semin Arthritis Rheum 2014; 44:25–30.
- Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010; 12: R63.
- Cottrell E, Crabtree V, Edwards JJ, Roddy E. Improvement in the management of gout is vital and overdue: an audit from a UK primary care medical practice. BMC Fam Pract 2013; 14:170.
- Goldfien RD, Ng MS, Yip G, et al. Effectiveness of a pharmacist-based gout care management programme in a large integrated health plan: results from a pilot study. BMJ Open 2014; 4:e003627.
- Lim AY, Shen L, Tan CH, Lateef A, Lau TC, Teng GG. Achieving treat to target in gout: a clinical practice improvement project. Scand J Rheumatol 2012; 41:450–457.
- Rees F, Jenkins W, Doherty M. Patients with gout adhere to curative treatment if informed appropriately: proof-of-concept observational study. Ann Rheum Dis 2013; 72:826–830.
- Smalls BL, Walker RJ, Bonilha HS, Campbell JA, Egede LE. Community interventions to improve glycemic control in African Americans with type 2 diabetes: a systemic review. Glob J Health Sci 2015; 7:171–182.
- Harrold LR, Mazor KM, Peterson D, Naz N, Firneno C, Yood RA. Patients’ knowledge and beliefs concerning gout and its treatment: a population based study. BMC Musculoskelet Disord 2012; 13:180.
- Singh JA. The impact of gout on patient’s lives: a study of African American and Caucasian men and women with gout. Arthritis Res Ther 2014; 16:R132.
- Singh JA. Facilitators and barriers to adherence to urate-lowering therapy in African Americans with gout: a qualitative study. Arthritis Res Ther 2014; 16:R82.
KEY POINTS
- Gout is more common in African Americans mainly because of their higher prevalence of risk factors such as obesity, diabetes, chronic kidney disease, and hypertension.
- Gout significantly reduces quality of life, economic productivity, and physical function and increases the risks of cardiovascular and renal disease.
- Although professional guidelines and effective medications are widely available, studies have found low physician compliance with providing optimal gout treatment, especially for African American patients.
- Treatment for gout in African Americans is the same as for all patients. Acute attacks should be treated promptly with anti-inflammatory agents, and uric acid levels should be aggressively lowered with drug therapy and diet modification.