User login
An Unusual Case of Folliculitis Spinulosa Decalvans
Case Report
A 24-year-old man was referred to the dermatology department for evaluation of pustules, atrophic scars, and alopecia on the scalp of 6 years’ duration. Six years prior, erythema, scaling, and follicular keratotic papules had appeared on the superciliary arches, and he started to lose hair from the eyebrows. Three months later, he developed mildly pruritic and painful scaling and pustules on the scalp. These lesions resolved with atrophic scarring accompanied by alopecia. One year later, follicular keratotic papules developed on the cheeks, chest, abdomen, back, lateral upper arms, thighs, and axillae. Two years later, direct microscopy of the lesions on the scalp and fungal culture were negative. After 2 weeks of treatment with roxithromycin (0.15 g twice daily), the scalp pustules dried out and resolved; however, they recurred when the patient stopped taking the medication. Six months later, he was started on isotretinoin treatment (10 mg once daily) for half a year, but no improvement was seen. His parents were nonconsanguineous, and no other family members were affected.
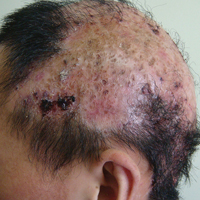
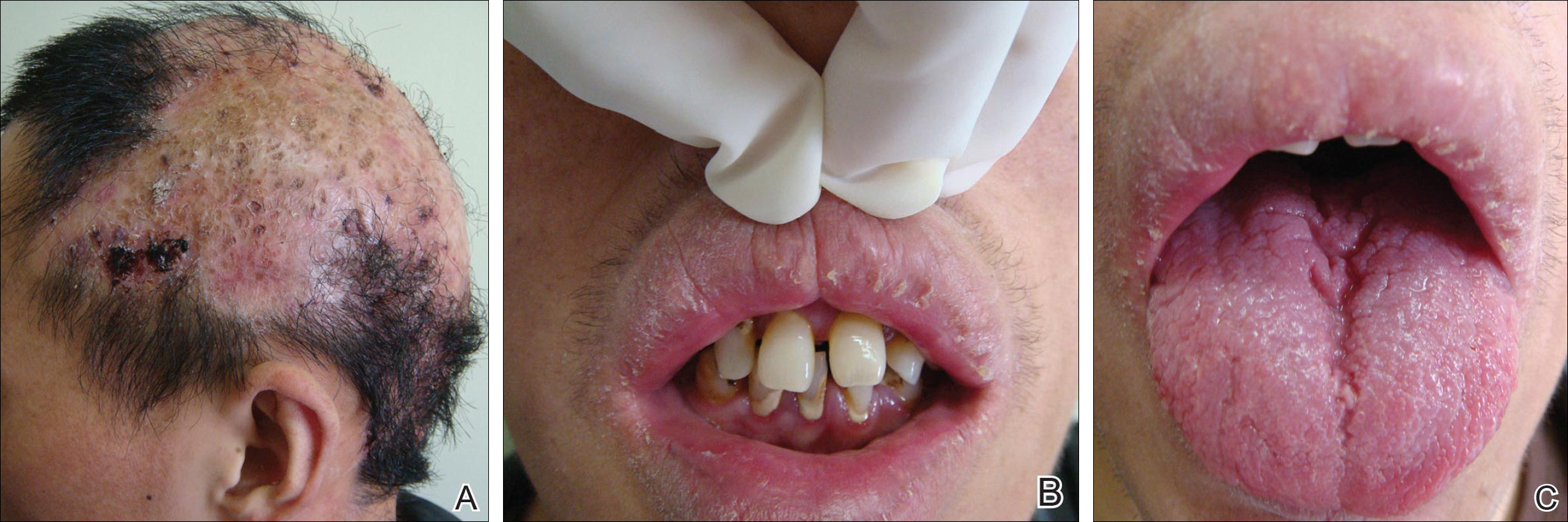
Dermatologic examination revealed large areas of atrophic scarring and alopecia on the scalp. Only a few solitary hairs remained on the top of the head, with the follicles surrounded by keratotic papules, pustules, and black scabs. There was sparse hair on the forehead and temples and scattered hair clusters in the occipital region near the hairline. These follicles also were associated with keratotic papules (Figure 1A). Erythema, scales, and follicular keratotic papules of the superciliary arches with sparse eyebrows and axillary hairs were noted. Follicular keratotic papules also were observed on the cheeks, axillae, chest, abdomen, back, lateral upper arms, and thighs. Dental examination revealed a large space between the upper anterior teeth and the lower anterior teeth. The upper anterior teeth were anteverted, there was congenital absence of right lower central incisors, and the anterior teeth were in deep overbite and overjet (Figure 1B). There was gingival atrophy and calculus dentalis in the upper and lower teeth. He had a fissured tongue with atrophic filiform papillae (Figure 1C).
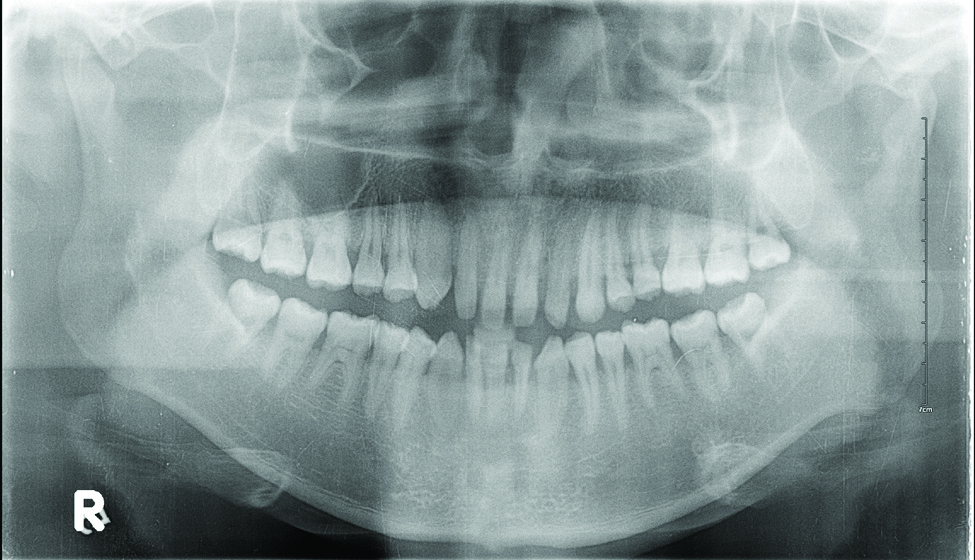
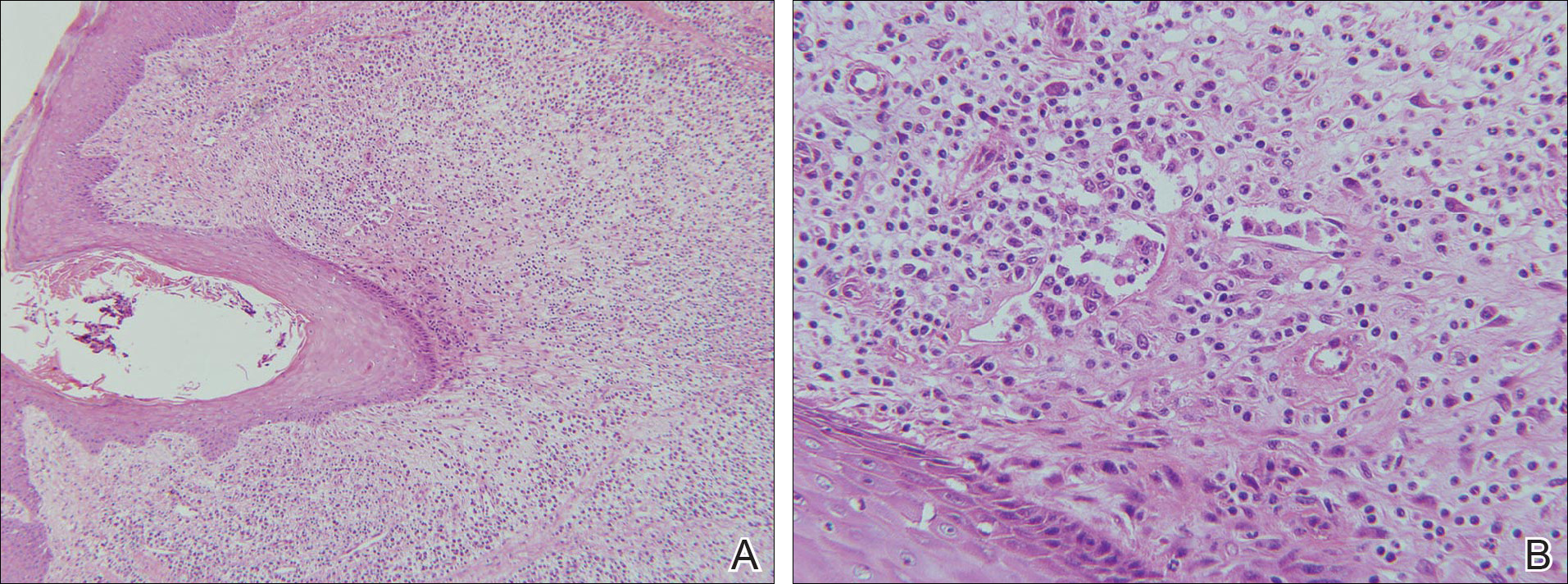
Laboratory testing of the blood, urine, stool, hepatic and renal function, and serum vitamin B2 and B12 levelswere all within reference range. A panoramic radiograph of the occlusal surface showed congenital absence of right lower central incisors (Figure 2), and a lateral projection of a cranial radiograph confirmed that the anterior teeth were in deep overbite and overjet. Direct microscopy and fungal culture of material collected from the dorsal tongue were negative. Direct microscopy and fungal culture of diseased hairs also were negative. A rapid plasma reagin test, Treponema pallidum hemagglutination assay, and human immunodeficiency virus test were negative. Staphylococcus aureus was isolated from the scalp pustules, and in vitro drug susceptibility testing showed that it was sensitive to clarithromycin and moxifloxacin. Pathological examination of a biopsy of the occipital skin lesions showed a thickened epidermal spinous layer and massive infiltration of plasma cells, neutrophils, and multinucleated giant cells around the hair follicles (Figure 3). Pathological examination of the skin lesions on the superciliary arch also showed infiltration of inflammatory cells in the dermis around the hair follicles.
Based on these findings, a diagnosis of folliculitis spinulosa decalvans (FSD) was made and the patient was started on clarithromycin (0.25 g twice daily), metronidazole (0.2 g 3 times daily), viaminate (50 mg 3 times daily), and fusidic acid cream (coating the affected area twice daily). When he returned for follow-up 1 month later, the pustules had disappeared and the black scabs had fallen off, leaving atrophic scars. The long-term efficacy of this regimen is still under observation.
Comment
Folliculitis spinulosa decalvans, along with keratosis follicularis spinulosa decalvans (KFSD), keratosis pilaris atrophicans faciei, and atrophoderma vermiculatum, belongs to a group of diseases that includes keratosis pilaris atrophicans. In 1994, Oranje et al1 suggested the term folliculitis spinulosa decalvans, with signs including persistent pustules, characteristic keratotic papules, and scarring alopecia of the scalp, which may be exacerbated at puberty. Staphylococcus aureus was isolated from the pustules in one study2; however, in another study, repeated cultures were negative.3 Although the main inheritance pattern of KFSD is X-linked, autosomal-dominant inheritance is more common in FSD. Furthermore, there are certain differences in the clinical manifestations of these 2 conditions. Therefore, it remains controversial if FSD is an independent disease or merely a subtype of KFSD.
Our patient’s symptoms manifested after puberty, primarily pustules as well as atrophic and scarring alopecia of the scalp and follicular keratotic papules on the head, face, trunk, lateral upper arms, and thighs. Pathologic examination showed massive infiltration of plasma cells, neutrophils, and multinucleated giant cells around the hair follicles. The clinical and histopathologic findings met the diagnostic criteria for FSD.
Folliculitis spinulosa decalvans is a rare clinical condition with few cases reported.3-5 In addition to the aforementioned characteristic clinical manifestations, our patient also had dental anomalies, a fissured tongue, and atrophy of the tongue papillae, which are not known to be associated with FSD. Dental anomalies are characteristic of patients with Down syndrome, ectodermal dysplasia, Papillon-Lefèvre syndrome, and other conditions.6 Fissured tongue is a normal variant that occurs in 5% to 11% of individuals. It also is a classic but nonspecific feature of Melkersson-Rosenthal syndrome and may occur in psoriasis, Down syndrome, acromegaly, and Sjögren syndrome.7 Atrophy of the tongue papillae is associated with anemia, pellagra, Sjögren syndrome, candidiasis, and other conditions.8 Because there are no known reports of associations between FSD and any of these oral manifestations, it is possible that they were unrelated to FSD in our patient.
Folliculitis spinulosa decalvans usually is recurrent and there is no consistently effective treatment for it. Kunte et al4 reported that dapsone (100 mg/d) led to resolution of scalp inflammation and pustules within 1 month. Romine et al2 reported that a 3-week course of dichloroxacillin (250 mg 4 times daily) induced disappearance of pustules around the hair follicles. However, Hallai et al5 reported a patient who was resistant to isotretinoin treatment. In our case, after 1 month of treatment with clarithromycin, metronidazole, viaminate, and fusidic acid cream, the pustules had resolved and the black scabs had fallen off, leaving atrophic scars. The long-term efficacy of this regimen is still under observation.
Conclusion
We report a case of FSD with dental anomalies, a fissured tongue, and atrophy of tongue papillae, none of which have previously been reported in association with FSD. We, therefore, believe that our patient’s oral manifestations are unrelated to FSD.
- Oranje AP, van Osch LD, Oosterwijk JC. Keratosis pilaris atrophicans. one heterogeneous disease or a symptom in different clinical entities? Arch Dermatol. 1994;13:500-502.
- Romine KA, Rothschild JG, Hansen RC. Cicatricial alopecia and keratosis pilaris. keratosis follicularis spinulosa decalvans. Arch Dermatol. 1997;13:381-384.
- Di Lernia V, Ricci C. Folliculitis spinulosa decalvans: an uncommon entity within the keratosis pilaris atrophicans spectrum. Pediatr Dermatol. 2006;23:255-258.
- Kunte C, Loeser C, Wolff H. Folliculitis spinulosa decalvans: successful therapy with dapsone. J Am Acad Dermatol. 1998;39(5, pt 2):891-892.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Scully C, Hegarty A. The oral cavity and lips. In: Burns T, Breathnach S, Cox N, et al. Rook’s Textbook of Dermatology. 8th ed. Oxford, England: Wiley-Blackwell; 2010:69.7-69.10.
- Wolff K, Goldsmith LA, Katz SI, et al. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies; 2007:643.
- Mulliken RA, Casner MJ. Oral manifestations of systemic disease. Emerg Med Clin North Am. 2000;18:565-575.
Case Report
A 24-year-old man was referred to the dermatology department for evaluation of pustules, atrophic scars, and alopecia on the scalp of 6 years’ duration. Six years prior, erythema, scaling, and follicular keratotic papules had appeared on the superciliary arches, and he started to lose hair from the eyebrows. Three months later, he developed mildly pruritic and painful scaling and pustules on the scalp. These lesions resolved with atrophic scarring accompanied by alopecia. One year later, follicular keratotic papules developed on the cheeks, chest, abdomen, back, lateral upper arms, thighs, and axillae. Two years later, direct microscopy of the lesions on the scalp and fungal culture were negative. After 2 weeks of treatment with roxithromycin (0.15 g twice daily), the scalp pustules dried out and resolved; however, they recurred when the patient stopped taking the medication. Six months later, he was started on isotretinoin treatment (10 mg once daily) for half a year, but no improvement was seen. His parents were nonconsanguineous, and no other family members were affected.
Dermatologic examination revealed large areas of atrophic scarring and alopecia on the scalp. Only a few solitary hairs remained on the top of the head, with the follicles surrounded by keratotic papules, pustules, and black scabs. There was sparse hair on the forehead and temples and scattered hair clusters in the occipital region near the hairline. These follicles also were associated with keratotic papules (Figure 1A). Erythema, scales, and follicular keratotic papules of the superciliary arches with sparse eyebrows and axillary hairs were noted. Follicular keratotic papules also were observed on the cheeks, axillae, chest, abdomen, back, lateral upper arms, and thighs. Dental examination revealed a large space between the upper anterior teeth and the lower anterior teeth. The upper anterior teeth were anteverted, there was congenital absence of right lower central incisors, and the anterior teeth were in deep overbite and overjet (Figure 1B). There was gingival atrophy and calculus dentalis in the upper and lower teeth. He had a fissured tongue with atrophic filiform papillae (Figure 1C).


Laboratory testing of the blood, urine, stool, hepatic and renal function, and serum vitamin B2 and B12 levelswere all within reference range. A panoramic radiograph of the occlusal surface showed congenital absence of right lower central incisors (Figure 2), and a lateral projection of a cranial radiograph confirmed that the anterior teeth were in deep overbite and overjet. Direct microscopy and fungal culture of material collected from the dorsal tongue were negative. Direct microscopy and fungal culture of diseased hairs also were negative. A rapid plasma reagin test, Treponema pallidum hemagglutination assay, and human immunodeficiency virus test were negative. Staphylococcus aureus was isolated from the scalp pustules, and in vitro drug susceptibility testing showed that it was sensitive to clarithromycin and moxifloxacin. Pathological examination of a biopsy of the occipital skin lesions showed a thickened epidermal spinous layer and massive infiltration of plasma cells, neutrophils, and multinucleated giant cells around the hair follicles (Figure 3). Pathological examination of the skin lesions on the superciliary arch also showed infiltration of inflammatory cells in the dermis around the hair follicles.

Based on these findings, a diagnosis of folliculitis spinulosa decalvans (FSD) was made and the patient was started on clarithromycin (0.25 g twice daily), metronidazole (0.2 g 3 times daily), viaminate (50 mg 3 times daily), and fusidic acid cream (coating the affected area twice daily). When he returned for follow-up 1 month later, the pustules had disappeared and the black scabs had fallen off, leaving atrophic scars. The long-term efficacy of this regimen is still under observation.
Comment
Folliculitis spinulosa decalvans, along with keratosis follicularis spinulosa decalvans (KFSD), keratosis pilaris atrophicans faciei, and atrophoderma vermiculatum, belongs to a group of diseases that includes keratosis pilaris atrophicans. In 1994, Oranje et al1 suggested the term folliculitis spinulosa decalvans, with signs including persistent pustules, characteristic keratotic papules, and scarring alopecia of the scalp, which may be exacerbated at puberty. Staphylococcus aureus was isolated from the pustules in one study2; however, in another study, repeated cultures were negative.3 Although the main inheritance pattern of KFSD is X-linked, autosomal-dominant inheritance is more common in FSD. Furthermore, there are certain differences in the clinical manifestations of these 2 conditions. Therefore, it remains controversial if FSD is an independent disease or merely a subtype of KFSD.
Our patient’s symptoms manifested after puberty, primarily pustules as well as atrophic and scarring alopecia of the scalp and follicular keratotic papules on the head, face, trunk, lateral upper arms, and thighs. Pathologic examination showed massive infiltration of plasma cells, neutrophils, and multinucleated giant cells around the hair follicles. The clinical and histopathologic findings met the diagnostic criteria for FSD.
Folliculitis spinulosa decalvans is a rare clinical condition with few cases reported.3-5 In addition to the aforementioned characteristic clinical manifestations, our patient also had dental anomalies, a fissured tongue, and atrophy of the tongue papillae, which are not known to be associated with FSD. Dental anomalies are characteristic of patients with Down syndrome, ectodermal dysplasia, Papillon-Lefèvre syndrome, and other conditions.6 Fissured tongue is a normal variant that occurs in 5% to 11% of individuals. It also is a classic but nonspecific feature of Melkersson-Rosenthal syndrome and may occur in psoriasis, Down syndrome, acromegaly, and Sjögren syndrome.7 Atrophy of the tongue papillae is associated with anemia, pellagra, Sjögren syndrome, candidiasis, and other conditions.8 Because there are no known reports of associations between FSD and any of these oral manifestations, it is possible that they were unrelated to FSD in our patient.
Folliculitis spinulosa decalvans usually is recurrent and there is no consistently effective treatment for it. Kunte et al4 reported that dapsone (100 mg/d) led to resolution of scalp inflammation and pustules within 1 month. Romine et al2 reported that a 3-week course of dichloroxacillin (250 mg 4 times daily) induced disappearance of pustules around the hair follicles. However, Hallai et al5 reported a patient who was resistant to isotretinoin treatment. In our case, after 1 month of treatment with clarithromycin, metronidazole, viaminate, and fusidic acid cream, the pustules had resolved and the black scabs had fallen off, leaving atrophic scars. The long-term efficacy of this regimen is still under observation.
Conclusion
We report a case of FSD with dental anomalies, a fissured tongue, and atrophy of tongue papillae, none of which have previously been reported in association with FSD. We, therefore, believe that our patient’s oral manifestations are unrelated to FSD.
Case Report
A 24-year-old man was referred to the dermatology department for evaluation of pustules, atrophic scars, and alopecia on the scalp of 6 years’ duration. Six years prior, erythema, scaling, and follicular keratotic papules had appeared on the superciliary arches, and he started to lose hair from the eyebrows. Three months later, he developed mildly pruritic and painful scaling and pustules on the scalp. These lesions resolved with atrophic scarring accompanied by alopecia. One year later, follicular keratotic papules developed on the cheeks, chest, abdomen, back, lateral upper arms, thighs, and axillae. Two years later, direct microscopy of the lesions on the scalp and fungal culture were negative. After 2 weeks of treatment with roxithromycin (0.15 g twice daily), the scalp pustules dried out and resolved; however, they recurred when the patient stopped taking the medication. Six months later, he was started on isotretinoin treatment (10 mg once daily) for half a year, but no improvement was seen. His parents were nonconsanguineous, and no other family members were affected.
Dermatologic examination revealed large areas of atrophic scarring and alopecia on the scalp. Only a few solitary hairs remained on the top of the head, with the follicles surrounded by keratotic papules, pustules, and black scabs. There was sparse hair on the forehead and temples and scattered hair clusters in the occipital region near the hairline. These follicles also were associated with keratotic papules (Figure 1A). Erythema, scales, and follicular keratotic papules of the superciliary arches with sparse eyebrows and axillary hairs were noted. Follicular keratotic papules also were observed on the cheeks, axillae, chest, abdomen, back, lateral upper arms, and thighs. Dental examination revealed a large space between the upper anterior teeth and the lower anterior teeth. The upper anterior teeth were anteverted, there was congenital absence of right lower central incisors, and the anterior teeth were in deep overbite and overjet (Figure 1B). There was gingival atrophy and calculus dentalis in the upper and lower teeth. He had a fissured tongue with atrophic filiform papillae (Figure 1C).


Laboratory testing of the blood, urine, stool, hepatic and renal function, and serum vitamin B2 and B12 levelswere all within reference range. A panoramic radiograph of the occlusal surface showed congenital absence of right lower central incisors (Figure 2), and a lateral projection of a cranial radiograph confirmed that the anterior teeth were in deep overbite and overjet. Direct microscopy and fungal culture of material collected from the dorsal tongue were negative. Direct microscopy and fungal culture of diseased hairs also were negative. A rapid plasma reagin test, Treponema pallidum hemagglutination assay, and human immunodeficiency virus test were negative. Staphylococcus aureus was isolated from the scalp pustules, and in vitro drug susceptibility testing showed that it was sensitive to clarithromycin and moxifloxacin. Pathological examination of a biopsy of the occipital skin lesions showed a thickened epidermal spinous layer and massive infiltration of plasma cells, neutrophils, and multinucleated giant cells around the hair follicles (Figure 3). Pathological examination of the skin lesions on the superciliary arch also showed infiltration of inflammatory cells in the dermis around the hair follicles.

Based on these findings, a diagnosis of folliculitis spinulosa decalvans (FSD) was made and the patient was started on clarithromycin (0.25 g twice daily), metronidazole (0.2 g 3 times daily), viaminate (50 mg 3 times daily), and fusidic acid cream (coating the affected area twice daily). When he returned for follow-up 1 month later, the pustules had disappeared and the black scabs had fallen off, leaving atrophic scars. The long-term efficacy of this regimen is still under observation.
Comment
Folliculitis spinulosa decalvans, along with keratosis follicularis spinulosa decalvans (KFSD), keratosis pilaris atrophicans faciei, and atrophoderma vermiculatum, belongs to a group of diseases that includes keratosis pilaris atrophicans. In 1994, Oranje et al1 suggested the term folliculitis spinulosa decalvans, with signs including persistent pustules, characteristic keratotic papules, and scarring alopecia of the scalp, which may be exacerbated at puberty. Staphylococcus aureus was isolated from the pustules in one study2; however, in another study, repeated cultures were negative.3 Although the main inheritance pattern of KFSD is X-linked, autosomal-dominant inheritance is more common in FSD. Furthermore, there are certain differences in the clinical manifestations of these 2 conditions. Therefore, it remains controversial if FSD is an independent disease or merely a subtype of KFSD.
Our patient’s symptoms manifested after puberty, primarily pustules as well as atrophic and scarring alopecia of the scalp and follicular keratotic papules on the head, face, trunk, lateral upper arms, and thighs. Pathologic examination showed massive infiltration of plasma cells, neutrophils, and multinucleated giant cells around the hair follicles. The clinical and histopathologic findings met the diagnostic criteria for FSD.
Folliculitis spinulosa decalvans is a rare clinical condition with few cases reported.3-5 In addition to the aforementioned characteristic clinical manifestations, our patient also had dental anomalies, a fissured tongue, and atrophy of the tongue papillae, which are not known to be associated with FSD. Dental anomalies are characteristic of patients with Down syndrome, ectodermal dysplasia, Papillon-Lefèvre syndrome, and other conditions.6 Fissured tongue is a normal variant that occurs in 5% to 11% of individuals. It also is a classic but nonspecific feature of Melkersson-Rosenthal syndrome and may occur in psoriasis, Down syndrome, acromegaly, and Sjögren syndrome.7 Atrophy of the tongue papillae is associated with anemia, pellagra, Sjögren syndrome, candidiasis, and other conditions.8 Because there are no known reports of associations between FSD and any of these oral manifestations, it is possible that they were unrelated to FSD in our patient.
Folliculitis spinulosa decalvans usually is recurrent and there is no consistently effective treatment for it. Kunte et al4 reported that dapsone (100 mg/d) led to resolution of scalp inflammation and pustules within 1 month. Romine et al2 reported that a 3-week course of dichloroxacillin (250 mg 4 times daily) induced disappearance of pustules around the hair follicles. However, Hallai et al5 reported a patient who was resistant to isotretinoin treatment. In our case, after 1 month of treatment with clarithromycin, metronidazole, viaminate, and fusidic acid cream, the pustules had resolved and the black scabs had fallen off, leaving atrophic scars. The long-term efficacy of this regimen is still under observation.
Conclusion
We report a case of FSD with dental anomalies, a fissured tongue, and atrophy of tongue papillae, none of which have previously been reported in association with FSD. We, therefore, believe that our patient’s oral manifestations are unrelated to FSD.
- Oranje AP, van Osch LD, Oosterwijk JC. Keratosis pilaris atrophicans. one heterogeneous disease or a symptom in different clinical entities? Arch Dermatol. 1994;13:500-502.
- Romine KA, Rothschild JG, Hansen RC. Cicatricial alopecia and keratosis pilaris. keratosis follicularis spinulosa decalvans. Arch Dermatol. 1997;13:381-384.
- Di Lernia V, Ricci C. Folliculitis spinulosa decalvans: an uncommon entity within the keratosis pilaris atrophicans spectrum. Pediatr Dermatol. 2006;23:255-258.
- Kunte C, Loeser C, Wolff H. Folliculitis spinulosa decalvans: successful therapy with dapsone. J Am Acad Dermatol. 1998;39(5, pt 2):891-892.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Scully C, Hegarty A. The oral cavity and lips. In: Burns T, Breathnach S, Cox N, et al. Rook’s Textbook of Dermatology. 8th ed. Oxford, England: Wiley-Blackwell; 2010:69.7-69.10.
- Wolff K, Goldsmith LA, Katz SI, et al. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies; 2007:643.
- Mulliken RA, Casner MJ. Oral manifestations of systemic disease. Emerg Med Clin North Am. 2000;18:565-575.
- Oranje AP, van Osch LD, Oosterwijk JC. Keratosis pilaris atrophicans. one heterogeneous disease or a symptom in different clinical entities? Arch Dermatol. 1994;13:500-502.
- Romine KA, Rothschild JG, Hansen RC. Cicatricial alopecia and keratosis pilaris. keratosis follicularis spinulosa decalvans. Arch Dermatol. 1997;13:381-384.
- Di Lernia V, Ricci C. Folliculitis spinulosa decalvans: an uncommon entity within the keratosis pilaris atrophicans spectrum. Pediatr Dermatol. 2006;23:255-258.
- Kunte C, Loeser C, Wolff H. Folliculitis spinulosa decalvans: successful therapy with dapsone. J Am Acad Dermatol. 1998;39(5, pt 2):891-892.
- Hallai N, Thompson I, Williams P, et al. Folliculitis spinulosa decalvans: failure to respond to oral isotretinoin. J Eur Acad Dermatol Venereol. 2006;20:223-224.
- Scully C, Hegarty A. The oral cavity and lips. In: Burns T, Breathnach S, Cox N, et al. Rook’s Textbook of Dermatology. 8th ed. Oxford, England: Wiley-Blackwell; 2010:69.7-69.10.
- Wolff K, Goldsmith LA, Katz SI, et al. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Companies; 2007:643.
- Mulliken RA, Casner MJ. Oral manifestations of systemic disease. Emerg Med Clin North Am. 2000;18:565-575.
Practice Points
- Folliculitis spinulosa decalvans (FSD) presents with persistent pustules, characteristic keratotic papules, and scarring alopecia of the scalp.
- In the case described here, oral manifestations also were present but are not characteristic of FSD.
Nevus Spilus: Is the Presence of Hair Associated With an Increased Risk for Melanoma?
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
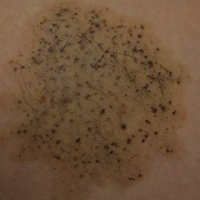
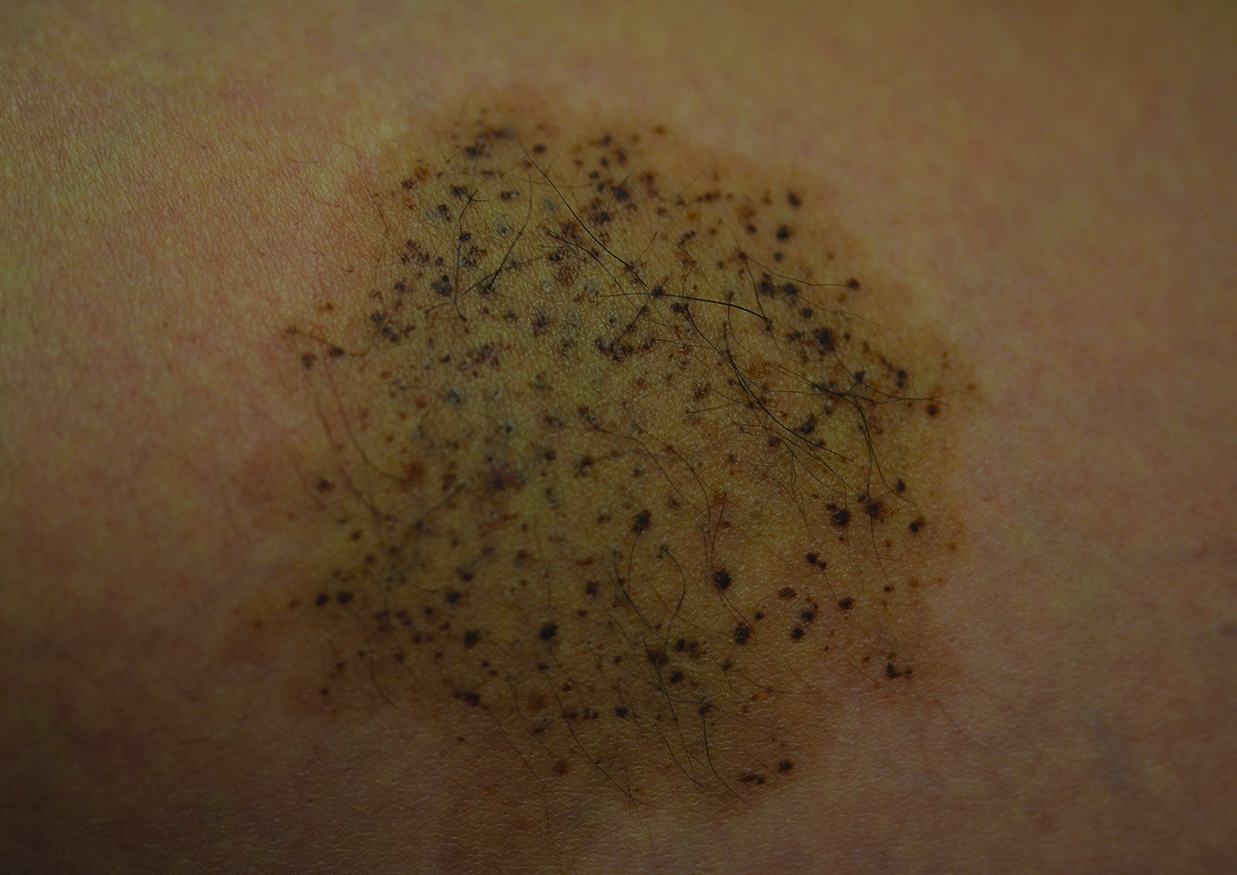
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.
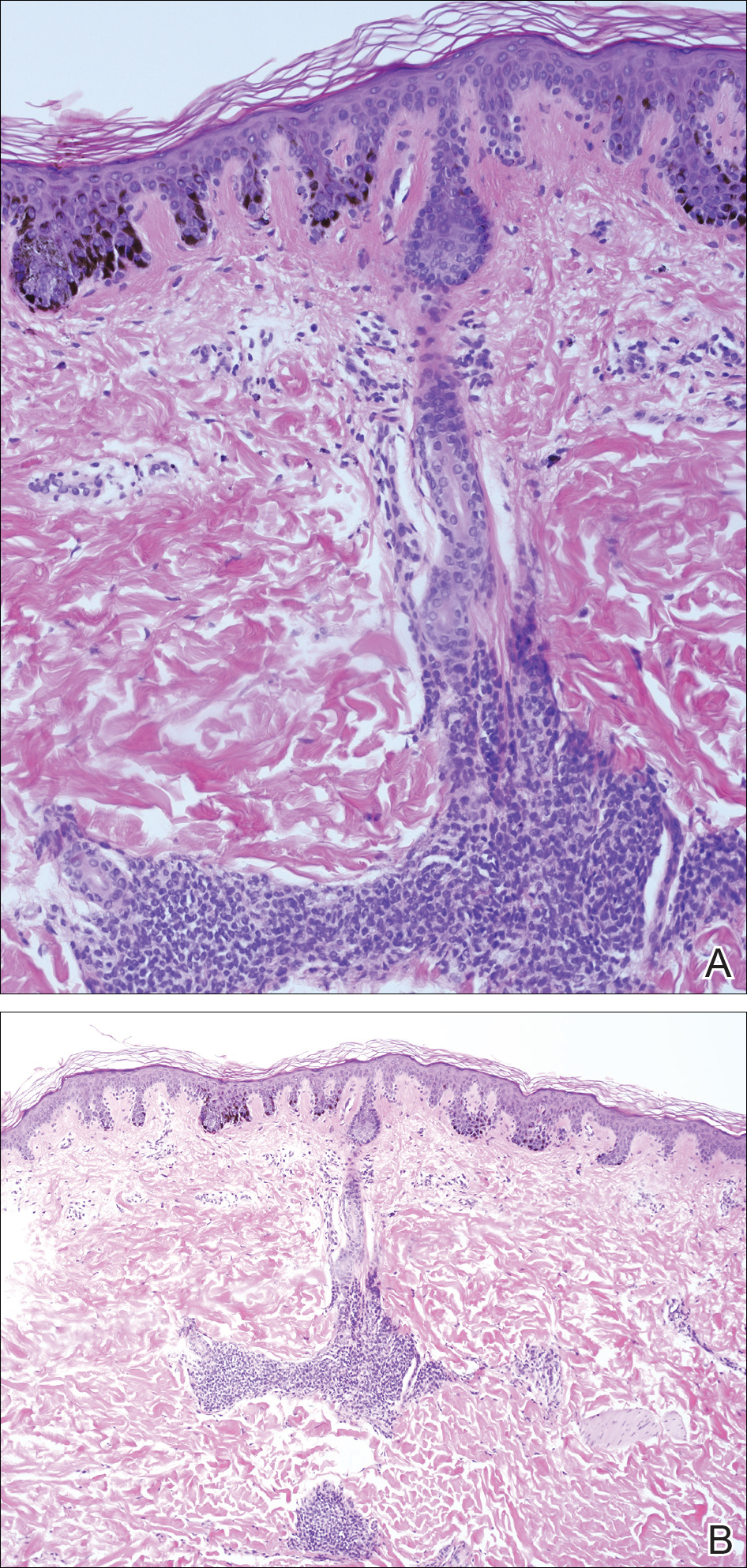
A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.
Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.

A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.

Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.

A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.

Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
Practice Points
- Nevus spilus (NS) appears as a café au lait macule studded with darker brown “moles.”
- Although melanoma has been described in NS, it is rare.
- There is no evidence that hairy NS are predisposed to melanoma.
The Translational Revolution in Atopic Dermatitis, and How It Also Translates to Other Inflammatory Skin Diseases
Atopic dermatitis (AD) is the most common inflammatory skin disease in both adults and children.1 Unfortunately, the current treatment armamentarium is largely confined to topical calcineurin inhibitors, topical and systemic steroids, phototherapy, cyclosporine (not approved by the US Food and Drug Administration for AD), and other oral immunosuppressants.2 The availability of partially helpful and highly toxic treatments creates a huge unmet need for more effective and safer treatments, particularly for patients with moderate to severe AD who often require systemic approaches.
Recent extensive translational (bench top to bedside and back) investigations in skin of AD patients has shown that skin phenotype is characterized by increased T-cell infiltration and related inflammatory cytokines as well as epidermal abnormalities (eg, hyperplasia, aberrant differentiation).3 Clinical improvement of AD has been demonstrated with broad T-cell targeted therapeutics, such as cyclosporine and narrowband UVB, coupled with decreases of T-cell infiltrates and inflammatory gene products as well as improvement of the pathologic epidermal phenotype.4,5
In the past, AD was conceptualized as a T helper cell TH2 (acute disease)/TH1 (chronic disease) bipolar cytokine disorder.6 Acute lesions are characterized by high TH2, TH22, and some TH17 signals, with intensification of these axes and TH1 augmentation orchestrating the chronic phenotype.7 The identification of the inflammatory pathways underlying AD has led to the development and testing of more than 10 broad or targeted therapeutics (Table).8 Phase 1 and phase 2 studies of dupilumab (targeting IL-4Rα) have shown not only tremendous AD improvement (~70%) but also tissue reversal of the immune and barrier abnormalities, including inflammatory cytokines and epidermal hyperplasia.9-11 As a result, other TH2 axis inhibitors (anti–IL-13/tralokinumab, anti–IL-31RA/CIM 331) are now in clinical trials. The identification of IL-22 in AD lesions has prompted trials with an anti–IL-22 (ILV 094) and an IL-12/IL-23p40 (ustekinumab) inhibitor.12 For psoriasis, ustekinumab showed 75% improvement in approximately 70% of patients,13 but for AD, despite clear clinical and molecular effects, differences compared to placebo were not statistically significant,12 probably due to underdosing of the drug in an excessively immune-activated disease14 as well as allowing topical steroids in patients, which may minimize the differences in treatment effect between drug and placebo.
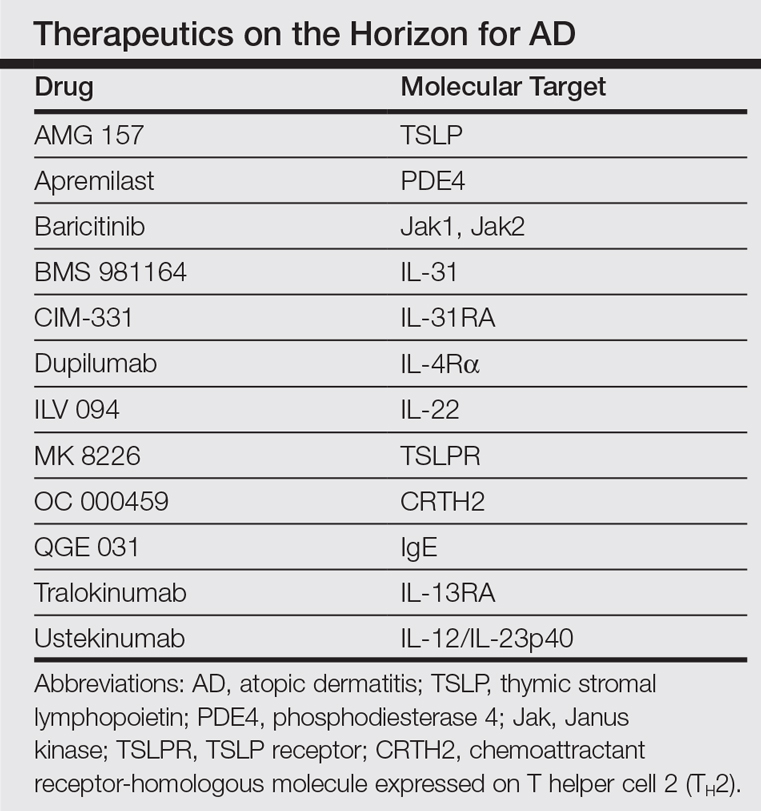
The developments seen in AD are now moving into other inflammatory skin diseases, particularly alopecia areata (AA), a T-cell–mediated disease that shares phenotypic similarities with AD and often is associated with it.15 There is a paucity of effective, remission-sustaining treatments of AA, particularly for patients with severe disease who rarely experience spontaneous hair regrowth and who have a limited response to topical interventions.16,17 Our clinical experience showed that successfully treating patients with concurrent AD and AA has led to hair regrowth. Inspired by these clinical observations and by results obtained in AD,9-12 studying AA skin showed an upregulation of not only the traditionally suspected culprit TH1 but also TH2 and TH9 axes, IL-23 cytokines, and phosphodiesterase 4.18 Subsequently, a pilot study of 3 patients with extensive AA treated with ustekinumab showed that all 3 patients not only experienced hair regrowth but also had a reduction in inflammatory markers in scalp lesions.19 Although these results are promising, AA is an immunologically complex disease and it is yet to be determined if therapeutically targeting 1 (eg, IL-4) rather than a wide array of cytokines can reverse disease phenotype. There are ongoing clinical trials directed at different pathogenic targets (eg, Jak inhibitors, IL-13 antagonist, IL-17 antagonist, phosphodiesterase 4 antagonist); some showed some efficacy in small studies.20,21
The finding of a commonly upregulated TH2 pathway in both AD and AA will pave the way for studies with TH2 antagonists in AA patients. Future targeted therapeutic studies will shed light on the pathogenic pathways of this devastating skin disease and answer the extensive unmet therapeutic need it presents.
- Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371-379; quiz 380-381.
- Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part
I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110-1118. - Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626-1634.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response [published online July 16, 2011]. J Allergy Clin Immunol. 2011;128:583-593.
- Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974-982.
- Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis [published online August 27, 2012]. J Allergy Clin Immunol. 2012;130:1344-1354.
- Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293-1300.
- Hamilton J, Ren H, Weinstein SP, et al. Dupilumab improved all domains of Eczema Area and Severity Index (EASI) and 5-D pruritus scale in adults with atopic dermatitis in a phase 2 study. J Invest Dermatol. 2014;134:S104.
- Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis [published online June 15, 2016]. Exp Dermatol. doi:10.1111/exd.13112.
- Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
- Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136:208-211.
- Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- Price VH, Hordinsky MK, Olsen EA, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395-402.
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update part II. treatment. J Am Acad Dermatol. 2010;62:191-202.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301-304.
- Xing LZ, Dai ZP, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748-751.
Atopic dermatitis (AD) is the most common inflammatory skin disease in both adults and children.1 Unfortunately, the current treatment armamentarium is largely confined to topical calcineurin inhibitors, topical and systemic steroids, phototherapy, cyclosporine (not approved by the US Food and Drug Administration for AD), and other oral immunosuppressants.2 The availability of partially helpful and highly toxic treatments creates a huge unmet need for more effective and safer treatments, particularly for patients with moderate to severe AD who often require systemic approaches.
Recent extensive translational (bench top to bedside and back) investigations in skin of AD patients has shown that skin phenotype is characterized by increased T-cell infiltration and related inflammatory cytokines as well as epidermal abnormalities (eg, hyperplasia, aberrant differentiation).3 Clinical improvement of AD has been demonstrated with broad T-cell targeted therapeutics, such as cyclosporine and narrowband UVB, coupled with decreases of T-cell infiltrates and inflammatory gene products as well as improvement of the pathologic epidermal phenotype.4,5
In the past, AD was conceptualized as a T helper cell TH2 (acute disease)/TH1 (chronic disease) bipolar cytokine disorder.6 Acute lesions are characterized by high TH2, TH22, and some TH17 signals, with intensification of these axes and TH1 augmentation orchestrating the chronic phenotype.7 The identification of the inflammatory pathways underlying AD has led to the development and testing of more than 10 broad or targeted therapeutics (Table).8 Phase 1 and phase 2 studies of dupilumab (targeting IL-4Rα) have shown not only tremendous AD improvement (~70%) but also tissue reversal of the immune and barrier abnormalities, including inflammatory cytokines and epidermal hyperplasia.9-11 As a result, other TH2 axis inhibitors (anti–IL-13/tralokinumab, anti–IL-31RA/CIM 331) are now in clinical trials. The identification of IL-22 in AD lesions has prompted trials with an anti–IL-22 (ILV 094) and an IL-12/IL-23p40 (ustekinumab) inhibitor.12 For psoriasis, ustekinumab showed 75% improvement in approximately 70% of patients,13 but for AD, despite clear clinical and molecular effects, differences compared to placebo were not statistically significant,12 probably due to underdosing of the drug in an excessively immune-activated disease14 as well as allowing topical steroids in patients, which may minimize the differences in treatment effect between drug and placebo.

The developments seen in AD are now moving into other inflammatory skin diseases, particularly alopecia areata (AA), a T-cell–mediated disease that shares phenotypic similarities with AD and often is associated with it.15 There is a paucity of effective, remission-sustaining treatments of AA, particularly for patients with severe disease who rarely experience spontaneous hair regrowth and who have a limited response to topical interventions.16,17 Our clinical experience showed that successfully treating patients with concurrent AD and AA has led to hair regrowth. Inspired by these clinical observations and by results obtained in AD,9-12 studying AA skin showed an upregulation of not only the traditionally suspected culprit TH1 but also TH2 and TH9 axes, IL-23 cytokines, and phosphodiesterase 4.18 Subsequently, a pilot study of 3 patients with extensive AA treated with ustekinumab showed that all 3 patients not only experienced hair regrowth but also had a reduction in inflammatory markers in scalp lesions.19 Although these results are promising, AA is an immunologically complex disease and it is yet to be determined if therapeutically targeting 1 (eg, IL-4) rather than a wide array of cytokines can reverse disease phenotype. There are ongoing clinical trials directed at different pathogenic targets (eg, Jak inhibitors, IL-13 antagonist, IL-17 antagonist, phosphodiesterase 4 antagonist); some showed some efficacy in small studies.20,21
The finding of a commonly upregulated TH2 pathway in both AD and AA will pave the way for studies with TH2 antagonists in AA patients. Future targeted therapeutic studies will shed light on the pathogenic pathways of this devastating skin disease and answer the extensive unmet therapeutic need it presents.
Atopic dermatitis (AD) is the most common inflammatory skin disease in both adults and children.1 Unfortunately, the current treatment armamentarium is largely confined to topical calcineurin inhibitors, topical and systemic steroids, phototherapy, cyclosporine (not approved by the US Food and Drug Administration for AD), and other oral immunosuppressants.2 The availability of partially helpful and highly toxic treatments creates a huge unmet need for more effective and safer treatments, particularly for patients with moderate to severe AD who often require systemic approaches.
Recent extensive translational (bench top to bedside and back) investigations in skin of AD patients has shown that skin phenotype is characterized by increased T-cell infiltration and related inflammatory cytokines as well as epidermal abnormalities (eg, hyperplasia, aberrant differentiation).3 Clinical improvement of AD has been demonstrated with broad T-cell targeted therapeutics, such as cyclosporine and narrowband UVB, coupled with decreases of T-cell infiltrates and inflammatory gene products as well as improvement of the pathologic epidermal phenotype.4,5
In the past, AD was conceptualized as a T helper cell TH2 (acute disease)/TH1 (chronic disease) bipolar cytokine disorder.6 Acute lesions are characterized by high TH2, TH22, and some TH17 signals, with intensification of these axes and TH1 augmentation orchestrating the chronic phenotype.7 The identification of the inflammatory pathways underlying AD has led to the development and testing of more than 10 broad or targeted therapeutics (Table).8 Phase 1 and phase 2 studies of dupilumab (targeting IL-4Rα) have shown not only tremendous AD improvement (~70%) but also tissue reversal of the immune and barrier abnormalities, including inflammatory cytokines and epidermal hyperplasia.9-11 As a result, other TH2 axis inhibitors (anti–IL-13/tralokinumab, anti–IL-31RA/CIM 331) are now in clinical trials. The identification of IL-22 in AD lesions has prompted trials with an anti–IL-22 (ILV 094) and an IL-12/IL-23p40 (ustekinumab) inhibitor.12 For psoriasis, ustekinumab showed 75% improvement in approximately 70% of patients,13 but for AD, despite clear clinical and molecular effects, differences compared to placebo were not statistically significant,12 probably due to underdosing of the drug in an excessively immune-activated disease14 as well as allowing topical steroids in patients, which may minimize the differences in treatment effect between drug and placebo.

The developments seen in AD are now moving into other inflammatory skin diseases, particularly alopecia areata (AA), a T-cell–mediated disease that shares phenotypic similarities with AD and often is associated with it.15 There is a paucity of effective, remission-sustaining treatments of AA, particularly for patients with severe disease who rarely experience spontaneous hair regrowth and who have a limited response to topical interventions.16,17 Our clinical experience showed that successfully treating patients with concurrent AD and AA has led to hair regrowth. Inspired by these clinical observations and by results obtained in AD,9-12 studying AA skin showed an upregulation of not only the traditionally suspected culprit TH1 but also TH2 and TH9 axes, IL-23 cytokines, and phosphodiesterase 4.18 Subsequently, a pilot study of 3 patients with extensive AA treated with ustekinumab showed that all 3 patients not only experienced hair regrowth but also had a reduction in inflammatory markers in scalp lesions.19 Although these results are promising, AA is an immunologically complex disease and it is yet to be determined if therapeutically targeting 1 (eg, IL-4) rather than a wide array of cytokines can reverse disease phenotype. There are ongoing clinical trials directed at different pathogenic targets (eg, Jak inhibitors, IL-13 antagonist, IL-17 antagonist, phosphodiesterase 4 antagonist); some showed some efficacy in small studies.20,21
The finding of a commonly upregulated TH2 pathway in both AD and AA will pave the way for studies with TH2 antagonists in AA patients. Future targeted therapeutic studies will shed light on the pathogenic pathways of this devastating skin disease and answer the extensive unmet therapeutic need it presents.
- Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371-379; quiz 380-381.
- Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part
I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110-1118. - Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626-1634.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response [published online July 16, 2011]. J Allergy Clin Immunol. 2011;128:583-593.
- Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974-982.
- Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis [published online August 27, 2012]. J Allergy Clin Immunol. 2012;130:1344-1354.
- Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293-1300.
- Hamilton J, Ren H, Weinstein SP, et al. Dupilumab improved all domains of Eczema Area and Severity Index (EASI) and 5-D pruritus scale in adults with atopic dermatitis in a phase 2 study. J Invest Dermatol. 2014;134:S104.
- Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis [published online June 15, 2016]. Exp Dermatol. doi:10.1111/exd.13112.
- Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
- Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136:208-211.
- Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- Price VH, Hordinsky MK, Olsen EA, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395-402.
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update part II. treatment. J Am Acad Dermatol. 2010;62:191-202.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301-304.
- Xing LZ, Dai ZP, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748-751.
- Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract. 2014;2:371-379; quiz 380-381.
- Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part
I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110-1118. - Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol. 2014;133:1626-1634.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response [published online July 16, 2011]. J Allergy Clin Immunol. 2011;128:583-593.
- Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974-982.
- Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis [published online August 27, 2012]. J Allergy Clin Immunol. 2012;130:1344-1354.
- Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293-1300.
- Hamilton J, Ren H, Weinstein SP, et al. Dupilumab improved all domains of Eczema Area and Severity Index (EASI) and 5-D pruritus scale in adults with atopic dermatitis in a phase 2 study. J Invest Dermatol. 2014;134:S104.
- Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis [published online June 15, 2016]. Exp Dermatol. doi:10.1111/exd.13112.
- Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
- Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol. 2015;136:208-211.
- Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581-591.
- Price VH, Hordinsky MK, Olsen EA, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395-402.
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update part II. treatment. J Am Acad Dermatol. 2010;62:191-202.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Guttman-Yassky E, Ungar B, Noda S, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. 2016;137:301-304.
- Xing LZ, Dai ZP, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Castela E, Le Duff F, Butori C, et al. Effects of low-dose recombinant interleukin 2 to promote T-regulatory cells in alopecia areata. JAMA Dermatol. 2014;150:748-751.
On-demand Pill Protocol Protects Against HIV

In most high-income countries, including the US, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 In the absence of a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the CDC began recommending daily use of tenofovir, disoproxil, fumarate, and emtricitabine (TDF-FTC) in high-risk individuals as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT], 46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably due to the difficulty in getting patients to take the medication daily.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 This is because, until now, no studies had demonstrated the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays study—a double-blind, multicenter study conducted in France and Canada—assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis proposed that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk for HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection—defined as having a history of unprotected anal sex with at least two partners in the past six months. Other inclusion criteria included an age of at least 18 and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance < 60 mL/min, alanine aminotransferase level more than 2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. Those who withdrew consent, were lost to follow-up, or acquired HIV-1 infection were excluded, and the remaining study participants were randomized to take TDF-FTC (n = 199) or placebo (n = 201) before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. Participants were instructed to take a loading dose of two pills of TDF-FTC or placebo with food two to 24 hours prior to intercourse, a third pill 24 hours later, and a fourth pill 24 hours after the third.
If there were multiple consecutive days of sexual intercourse, participants were to take one pill on each day of intercourse, followed by the two postexposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the PrEP; otherwise, they were to begin again with two pills two to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants four and eight weeks after enrollment, then every eight weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count, drug levels in plasma, and with an at-home, computer-assisted interview completed by participants prior to each visit.
Participants received counseling from a peer community member and were offered preventive services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took their assigned pills correctly. Participants were followed for a median of 9.3 months. Overall, 72% of participants took the study drugs (TDF-FTC or placebo), but 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. After 20 months, the study was unblinded and is now continuing as an open-label study because of the discontinuation of another PrEP study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk for HIV without PrEP. The open-label part of the study (iPrex-OLE) completed enrollment and data gathering in November 2013. The data analysis and results are pending.9
Eighty-six percent experienced relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with three of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group and two of the new cases were in the TDF-FTC group. This translated to an 86% relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group (NNT, 17 over 9.3 months).
The two cases in the TDF-FTC group occurred in participants found to be nonadherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain, which were seen more commonly in the treatment group than in the placebo group (14% vs 5%; number needed to harm, 11). There were also mild increases in serum creatinine level, but only two participants had a transient decrease in creatinine clearance to < 60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction nearly doubles
This is the first study to look at on-demand PrEP with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the increased benefit of on-demand PrEP is likely due to improved compliance with medication use.
CAVEATS
Can adherence be maintained?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we need to wait for the results of the open-label study to fully assess the risk for adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of the medication; the retail price of TDF-FTC is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(8):556-558.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/prepguide lines2014.pdf. Accessed August 9, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. iPrEx open-label extension. www.iprexnews.com. Accessed August 9, 2016.
10. GoodRx. Truvada. www.goodrx.com/truvada. Accessed August 9, 2016.

In most high-income countries, including the US, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 In the absence of a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the CDC began recommending daily use of tenofovir, disoproxil, fumarate, and emtricitabine (TDF-FTC) in high-risk individuals as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT], 46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably due to the difficulty in getting patients to take the medication daily.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 This is because, until now, no studies had demonstrated the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays study—a double-blind, multicenter study conducted in France and Canada—assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis proposed that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk for HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection—defined as having a history of unprotected anal sex with at least two partners in the past six months. Other inclusion criteria included an age of at least 18 and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance < 60 mL/min, alanine aminotransferase level more than 2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. Those who withdrew consent, were lost to follow-up, or acquired HIV-1 infection were excluded, and the remaining study participants were randomized to take TDF-FTC (n = 199) or placebo (n = 201) before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. Participants were instructed to take a loading dose of two pills of TDF-FTC or placebo with food two to 24 hours prior to intercourse, a third pill 24 hours later, and a fourth pill 24 hours after the third.
If there were multiple consecutive days of sexual intercourse, participants were to take one pill on each day of intercourse, followed by the two postexposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the PrEP; otherwise, they were to begin again with two pills two to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants four and eight weeks after enrollment, then every eight weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count, drug levels in plasma, and with an at-home, computer-assisted interview completed by participants prior to each visit.
Participants received counseling from a peer community member and were offered preventive services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took their assigned pills correctly. Participants were followed for a median of 9.3 months. Overall, 72% of participants took the study drugs (TDF-FTC or placebo), but 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. After 20 months, the study was unblinded and is now continuing as an open-label study because of the discontinuation of another PrEP study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk for HIV without PrEP. The open-label part of the study (iPrex-OLE) completed enrollment and data gathering in November 2013. The data analysis and results are pending.9
Eighty-six percent experienced relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with three of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group and two of the new cases were in the TDF-FTC group. This translated to an 86% relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group (NNT, 17 over 9.3 months).
The two cases in the TDF-FTC group occurred in participants found to be nonadherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain, which were seen more commonly in the treatment group than in the placebo group (14% vs 5%; number needed to harm, 11). There were also mild increases in serum creatinine level, but only two participants had a transient decrease in creatinine clearance to < 60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction nearly doubles
This is the first study to look at on-demand PrEP with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the increased benefit of on-demand PrEP is likely due to improved compliance with medication use.
CAVEATS
Can adherence be maintained?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we need to wait for the results of the open-label study to fully assess the risk for adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of the medication; the retail price of TDF-FTC is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(8):556-558.

In most high-income countries, including the US, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 In the absence of a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the CDC began recommending daily use of tenofovir, disoproxil, fumarate, and emtricitabine (TDF-FTC) in high-risk individuals as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT], 46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably due to the difficulty in getting patients to take the medication daily.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 This is because, until now, no studies had demonstrated the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays study—a double-blind, multicenter study conducted in France and Canada—assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis proposed that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk for HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection—defined as having a history of unprotected anal sex with at least two partners in the past six months. Other inclusion criteria included an age of at least 18 and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance < 60 mL/min, alanine aminotransferase level more than 2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. Those who withdrew consent, were lost to follow-up, or acquired HIV-1 infection were excluded, and the remaining study participants were randomized to take TDF-FTC (n = 199) or placebo (n = 201) before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. Participants were instructed to take a loading dose of two pills of TDF-FTC or placebo with food two to 24 hours prior to intercourse, a third pill 24 hours later, and a fourth pill 24 hours after the third.
If there were multiple consecutive days of sexual intercourse, participants were to take one pill on each day of intercourse, followed by the two postexposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the PrEP; otherwise, they were to begin again with two pills two to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants four and eight weeks after enrollment, then every eight weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count, drug levels in plasma, and with an at-home, computer-assisted interview completed by participants prior to each visit.
Participants received counseling from a peer community member and were offered preventive services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took their assigned pills correctly. Participants were followed for a median of 9.3 months. Overall, 72% of participants took the study drugs (TDF-FTC or placebo), but 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. After 20 months, the study was unblinded and is now continuing as an open-label study because of the discontinuation of another PrEP study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk for HIV without PrEP. The open-label part of the study (iPrex-OLE) completed enrollment and data gathering in November 2013. The data analysis and results are pending.9
Eighty-six percent experienced relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with three of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group and two of the new cases were in the TDF-FTC group. This translated to an 86% relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group (NNT, 17 over 9.3 months).
The two cases in the TDF-FTC group occurred in participants found to be nonadherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain, which were seen more commonly in the treatment group than in the placebo group (14% vs 5%; number needed to harm, 11). There were also mild increases in serum creatinine level, but only two participants had a transient decrease in creatinine clearance to < 60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction nearly doubles
This is the first study to look at on-demand PrEP with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the increased benefit of on-demand PrEP is likely due to improved compliance with medication use.
CAVEATS
Can adherence be maintained?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we need to wait for the results of the open-label study to fully assess the risk for adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of the medication; the retail price of TDF-FTC is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(8):556-558.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/prepguide lines2014.pdf. Accessed August 9, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. iPrEx open-label extension. www.iprexnews.com. Accessed August 9, 2016.
10. GoodRx. Truvada. www.goodrx.com/truvada. Accessed August 9, 2016.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/prepguide lines2014.pdf. Accessed August 9, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. iPrEx open-label extension. www.iprexnews.com. Accessed August 9, 2016.
10. GoodRx. Truvada. www.goodrx.com/truvada. Accessed August 9, 2016.
Roux-en-Y gastric bypass associated with long-term weight loss
Obese patients who underwent Roux-en-Y gastric bypass had higher percentages of weight loss at long-term follow-up, compared with obese patients who underwent other surgical procedures or who did not undergo surgery, according to a large, cohort study published in JAMA Surgery.
While prior research has clearly demonstrated that bariatric surgery is the most effective intervention for inducing weight loss among obese patients, the majority of those studies were short term; therefore, there is little known about the durability of weight loss following bariatric surgery, wrote Matthew Maciejewski, PhD, of Duke University, Durham, N.C., and Durham Veterans Affairs Medical Center and his associates.
This study compared the 10-year weight change in patients who underwent Roux-en-Y gastric bypass to patients who did not receive bariatric surgical intervention of any kind. A total of 1,787 patients who had undergone Roux-en-Y gastric bypass surgery were identified and matched by investigators to one or more patients with similar demographic characteristics (age, sex, race, body mass index, diabetes diagnosis). A total of 5,305 nonsurgical matches were selected for analysis. For the Roux-en-Y gastric bypass group, mean age was 52.1 years, and for the nonsurgical matches mean age was 52.2 years. Both groups were predominantly male (73.1% and 73.7%, respectively) and had high 10-year follow-up rates of 81.9% for surgical patients and 67.4% for nonsurgical matches (JAMA Surgery. 2016. doi: 10.1001/jamasurg.2016.2317).
The study’s primary outcome of percentage change in weight at 10-year follow-up, compared with baseline strongly favored Roux-en-Y gastric bypass over no surgical intervention. At the 10-year time point, patients who underwent Roux-en-Y gastric bypass had lost 21.3% more of their baseline weight than nonsurgical matches.
Remarkably, only 3.4% of patients who underwent Roux-en-Y gastric bypass were within 5% of their original baseline weight at 10 years while 55.5% of those who did not receive surgical intervention had regained most of their weight.
Additionally, investigators compared percentage change in weight at 4-year follow-up for obese patients who underwent either Roux-en-Y gastric bypass (n = 1,785), sleeve gastrectomy (n = 379), or adjustable gastric banding (n = 246). At this time point, patients who underwent Roux-en-Y gastric bypass had lost an average of 28% of their baseline weight while patients who underwent sleeve gastrectomy or adjustable gastric banding only lost 18% and 11% of their baseline weights, respectively.
“These results provide further evidence for the beneficial association between surgery and long-term weight loss that has been demonstrated in shorter-term studies of younger, predominantly female populations,” the investigators concluded.
This study was funded by the Department of Veterans Affairs. Dr. Maciejewski and four of his associates reported receiving financial compensation from or holding stock in various companies and institutions including the Department of Veterans Affairs.
On Twitter @jessnicolecraig
One of the most prevalent perceptions held by many is that most bariatric surgery patients will eventually gain all of their weight back. As illustrated in the article by Maciejewski et al., studies that follow up with a large cohort of bariatric surgery patients for more than a few years are limited and hampered by low rates of long-term follow-up. While the results of these studies generally imply that long-term weight loss is possible in most patients, poor follow-up leaves one to wonder whether this is a generalizable conclusion.
In the article by Maciejewski et al., it is remarkable that such a low number of gastric bypass patients (3%) regained weight back to within 5% of their baseline weight by 10 years. With the publication of the article by Maciejewski et al., the perception that all metabolic and bariatric surgery patients gain their weight back has become less valid.
Jon C. Gould, MD, is the chief of the division of general surgery at the Medical College of Wisconsin in Milwaukee. His comments were taken from his editorial accompanying the report by Dr. Maciejewski and his colleagues (JAMA Surgery. 2016. doi: 10.1001/jamasurg.2016.2301).
One of the most prevalent perceptions held by many is that most bariatric surgery patients will eventually gain all of their weight back. As illustrated in the article by Maciejewski et al., studies that follow up with a large cohort of bariatric surgery patients for more than a few years are limited and hampered by low rates of long-term follow-up. While the results of these studies generally imply that long-term weight loss is possible in most patients, poor follow-up leaves one to wonder whether this is a generalizable conclusion.
In the article by Maciejewski et al., it is remarkable that such a low number of gastric bypass patients (3%) regained weight back to within 5% of their baseline weight by 10 years. With the publication of the article by Maciejewski et al., the perception that all metabolic and bariatric surgery patients gain their weight back has become less valid.
Jon C. Gould, MD, is the chief of the division of general surgery at the Medical College of Wisconsin in Milwaukee. His comments were taken from his editorial accompanying the report by Dr. Maciejewski and his colleagues (JAMA Surgery. 2016. doi: 10.1001/jamasurg.2016.2301).
One of the most prevalent perceptions held by many is that most bariatric surgery patients will eventually gain all of their weight back. As illustrated in the article by Maciejewski et al., studies that follow up with a large cohort of bariatric surgery patients for more than a few years are limited and hampered by low rates of long-term follow-up. While the results of these studies generally imply that long-term weight loss is possible in most patients, poor follow-up leaves one to wonder whether this is a generalizable conclusion.
In the article by Maciejewski et al., it is remarkable that such a low number of gastric bypass patients (3%) regained weight back to within 5% of their baseline weight by 10 years. With the publication of the article by Maciejewski et al., the perception that all metabolic and bariatric surgery patients gain their weight back has become less valid.
Jon C. Gould, MD, is the chief of the division of general surgery at the Medical College of Wisconsin in Milwaukee. His comments were taken from his editorial accompanying the report by Dr. Maciejewski and his colleagues (JAMA Surgery. 2016. doi: 10.1001/jamasurg.2016.2301).
Obese patients who underwent Roux-en-Y gastric bypass had higher percentages of weight loss at long-term follow-up, compared with obese patients who underwent other surgical procedures or who did not undergo surgery, according to a large, cohort study published in JAMA Surgery.
While prior research has clearly demonstrated that bariatric surgery is the most effective intervention for inducing weight loss among obese patients, the majority of those studies were short term; therefore, there is little known about the durability of weight loss following bariatric surgery, wrote Matthew Maciejewski, PhD, of Duke University, Durham, N.C., and Durham Veterans Affairs Medical Center and his associates.
This study compared the 10-year weight change in patients who underwent Roux-en-Y gastric bypass to patients who did not receive bariatric surgical intervention of any kind. A total of 1,787 patients who had undergone Roux-en-Y gastric bypass surgery were identified and matched by investigators to one or more patients with similar demographic characteristics (age, sex, race, body mass index, diabetes diagnosis). A total of 5,305 nonsurgical matches were selected for analysis. For the Roux-en-Y gastric bypass group, mean age was 52.1 years, and for the nonsurgical matches mean age was 52.2 years. Both groups were predominantly male (73.1% and 73.7%, respectively) and had high 10-year follow-up rates of 81.9% for surgical patients and 67.4% for nonsurgical matches (JAMA Surgery. 2016. doi: 10.1001/jamasurg.2016.2317).
The study’s primary outcome of percentage change in weight at 10-year follow-up, compared with baseline strongly favored Roux-en-Y gastric bypass over no surgical intervention. At the 10-year time point, patients who underwent Roux-en-Y gastric bypass had lost 21.3% more of their baseline weight than nonsurgical matches.
Remarkably, only 3.4% of patients who underwent Roux-en-Y gastric bypass were within 5% of their original baseline weight at 10 years while 55.5% of those who did not receive surgical intervention had regained most of their weight.
Additionally, investigators compared percentage change in weight at 4-year follow-up for obese patients who underwent either Roux-en-Y gastric bypass (n = 1,785), sleeve gastrectomy (n = 379), or adjustable gastric banding (n = 246). At this time point, patients who underwent Roux-en-Y gastric bypass had lost an average of 28% of their baseline weight while patients who underwent sleeve gastrectomy or adjustable gastric banding only lost 18% and 11% of their baseline weights, respectively.
“These results provide further evidence for the beneficial association between surgery and long-term weight loss that has been demonstrated in shorter-term studies of younger, predominantly female populations,” the investigators concluded.
This study was funded by the Department of Veterans Affairs. Dr. Maciejewski and four of his associates reported receiving financial compensation from or holding stock in various companies and institutions including the Department of Veterans Affairs.
On Twitter @jessnicolecraig
Obese patients who underwent Roux-en-Y gastric bypass had higher percentages of weight loss at long-term follow-up, compared with obese patients who underwent other surgical procedures or who did not undergo surgery, according to a large, cohort study published in JAMA Surgery.
While prior research has clearly demonstrated that bariatric surgery is the most effective intervention for inducing weight loss among obese patients, the majority of those studies were short term; therefore, there is little known about the durability of weight loss following bariatric surgery, wrote Matthew Maciejewski, PhD, of Duke University, Durham, N.C., and Durham Veterans Affairs Medical Center and his associates.
This study compared the 10-year weight change in patients who underwent Roux-en-Y gastric bypass to patients who did not receive bariatric surgical intervention of any kind. A total of 1,787 patients who had undergone Roux-en-Y gastric bypass surgery were identified and matched by investigators to one or more patients with similar demographic characteristics (age, sex, race, body mass index, diabetes diagnosis). A total of 5,305 nonsurgical matches were selected for analysis. For the Roux-en-Y gastric bypass group, mean age was 52.1 years, and for the nonsurgical matches mean age was 52.2 years. Both groups were predominantly male (73.1% and 73.7%, respectively) and had high 10-year follow-up rates of 81.9% for surgical patients and 67.4% for nonsurgical matches (JAMA Surgery. 2016. doi: 10.1001/jamasurg.2016.2317).
The study’s primary outcome of percentage change in weight at 10-year follow-up, compared with baseline strongly favored Roux-en-Y gastric bypass over no surgical intervention. At the 10-year time point, patients who underwent Roux-en-Y gastric bypass had lost 21.3% more of their baseline weight than nonsurgical matches.
Remarkably, only 3.4% of patients who underwent Roux-en-Y gastric bypass were within 5% of their original baseline weight at 10 years while 55.5% of those who did not receive surgical intervention had regained most of their weight.
Additionally, investigators compared percentage change in weight at 4-year follow-up for obese patients who underwent either Roux-en-Y gastric bypass (n = 1,785), sleeve gastrectomy (n = 379), or adjustable gastric banding (n = 246). At this time point, patients who underwent Roux-en-Y gastric bypass had lost an average of 28% of their baseline weight while patients who underwent sleeve gastrectomy or adjustable gastric banding only lost 18% and 11% of their baseline weights, respectively.
“These results provide further evidence for the beneficial association between surgery and long-term weight loss that has been demonstrated in shorter-term studies of younger, predominantly female populations,” the investigators concluded.
This study was funded by the Department of Veterans Affairs. Dr. Maciejewski and four of his associates reported receiving financial compensation from or holding stock in various companies and institutions including the Department of Veterans Affairs.
On Twitter @jessnicolecraig
FROM JAMA SURGERY
Key clinical point: Roux-en-Y gastric bypass led to higher percentages of weight loss at long-term follow-up.
Major finding: Four years post treatment, patients who underwent Roux-en-Y gastric bypass lost an average of 28% of their baseline weight while patients who underwent sleeve gastrectomy or adjustable gastric banding lost only 18% and 11% of their baseline weights, respectively. At 10 years, patients who underwent Roux-en-Y had lost 21% more of their baseline weight than those who did not receive surgery.
Data source: Retrospective cohort study of 1,787 patients who had undergone Roux-en-Y gastric bypass surgery.
Disclosures: This study was funded by the Department of Veterans Affairs. Dr. Maciejewski and four of his associates reported receiving financial compensation from or holding stock in various companies and institutions including the Department of Veterans Affairs.
Dr. Benjamin Frizner Brings Post-Acute-Care Expertise to TH’s Editorial Board
Going to medical school at Universidad Autónoma de Guadalajara in Guadalajara, Mexico, could have been too much for Benjamin Frizner, MD, FHM.
Medicine is its own new language, as any first-year can tell you. Throw in learning Spanish? And a new culture? One could be forgiven for not excelling.
Dr. Frizner isn’t one of those people.
“The experience changed my life,” he says. “After I survived the first year, I knew I loved medicine.”
After medical school, Dr. Frizner had to complete a Fifth Pathway program, which formerly allowed students who completed four years at a foreign medical school to finish supervised clinical work at a U.S. medical school and become eligible as a U.S. resident.
He learned of hospital medicine during his residency at York Hospital in York, Pa., and, despite others suggesting hospital medicine was “something to do before you really figure out your career,” he enjoyed both working within the hospital walls and having a schedule that allowed 15 shifts a month and commensurate time off.
But as with his shift from undergraduate school in suburban Maryland to medical school in Mexico, Dr. Frizner likes a new challenge. So after a four-year stint as director of the hospitalist program at Saint Agnes Hospital in Baltimore, he took a job in August 2015 as director of the Ventilator Unit at FutureCare Irvington, a post-acute-care center in Baltimore staffed by CEP America.
“Post-acute care has become a new passion and chapter in my career,” he says, adding, “Skilled nursing facilities are extensions of the acute-care hospital and are just as challenging and fulfilling as hospitalist work.”
It’s a perspective Dr. Frizner will bring as one of eight new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: Why did you choose a career in medicine?
Answer: I enjoyed math and biology in college. I started out thinking I would be an engineer but fell in love with anatomy. I like solving problems and working with people. Internal medicine/hospital medicine is a perfect match, working to solve a patient’s diagnosis and helping families make difficult decisions about placement and palliative care.
Q: What do you like most about working as a hospitalist?
A: Interacting with all the different specialties, social work, case management, residents, ED docs. I really enjoy the camaraderie.
Q: What do you dislike most?
A: Hospital groups contribute immensely to patient flow, care, quality, process improvement, throughput, but hospitals always advertise the new specialist and never the excellent hospitalist group.
Q: What’s the best advice you ever received?
A: No matter what, do what is best for the patient. Everything else will take care of itself.
Q: What’s the worst advice you ever received?
A: Don’t worry about the contract; you don’t need to really look it over.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The pace of medicine continues to speed up. Residents have to hit the ground running with baseline case-management knowledge.
Q: What’s the biggest change you would like to see in HM?
A: I would like to see more hospitalists ascend into senior leadership in hospitals and healthcare systems.
Q: Why should group leaders continue to see patients?
A: It is important to maintain trust and respect with docs you are leading and managing. When I was a hospitalist director, I made sure I worked nights and weekends so I could understand the workload during those shifts and my team felt I was not just dumping on them.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care is most challenging?
A: Establishing trust with the patient and their family. But it has become second nature to me at this point. The secret is to introduce yourself, tell the patient and family you will take care of them in the hospital, communicate with their outpatient physician and that you are part of a 24-7 team of docs there to take care of the patient.
Q: What aspect of patient care is most rewarding?
A: Helping families navigate end-of-life decisions. It is the most stressful time in a family’s life, and I think it is the most rewarding and honorable part of practicing medicine.
Q: Are you on teaching service? If so, what aspect of teaching in the 21st century is most difficult? And what is most enjoyable?
A: I lead teaching rounds a few months a year when I was a hospitalist director, and I think the most difficult part is getting the residents to understand the workload will be a lot tougher when they get out into the real world. During their third year, residents need to practice efficiency and gauge their work ethic—not the kind of work ethic needed to pass the boards but the kind needed to stay in the ED and help your teammate out until the admissions are caught up or round on a few extra patients when there is a surge in the census.
Q: What is your biggest professional challenge?
A: [Getting others to] stop underestimating my skills and experience as a hospitalist and physician leader. I will complete an MBA through ACPE UMass this December. Learning basic accounting, business law, and finance has helped round out blind spots and build my confidence.
Q: What is your biggest professional reward?
A: Completing quality improvement projects such as increasing DVT prophylaxis, reducing CAUTI, and decreasing throughput times, which all help make the hospital course safer and efficient for the patient.
Q: What SHM event made the most lasting impression on you?
A: Seven years ago, I attended the Level I leadership academy at the Aria hotel in Las Vegas. The meeting opened my eyes to the world of leadership, management, and healthcare economics, which sparked my drive to eventually become a hospitalist director.
Q: What’s the best book you’ve read recently? Why?
A: David and Goliath by Malcolm Gladwell. As a foreign medical graduate, I was told there would be limits to what I could achieve in my career. Mr. Gladwell’s book is filled with stories of people who overcame difficult situations and went on to rise to the top of their fields.
Richard Quinn is a freelance writer in New Jersey.
Going to medical school at Universidad Autónoma de Guadalajara in Guadalajara, Mexico, could have been too much for Benjamin Frizner, MD, FHM.
Medicine is its own new language, as any first-year can tell you. Throw in learning Spanish? And a new culture? One could be forgiven for not excelling.
Dr. Frizner isn’t one of those people.
“The experience changed my life,” he says. “After I survived the first year, I knew I loved medicine.”
After medical school, Dr. Frizner had to complete a Fifth Pathway program, which formerly allowed students who completed four years at a foreign medical school to finish supervised clinical work at a U.S. medical school and become eligible as a U.S. resident.
He learned of hospital medicine during his residency at York Hospital in York, Pa., and, despite others suggesting hospital medicine was “something to do before you really figure out your career,” he enjoyed both working within the hospital walls and having a schedule that allowed 15 shifts a month and commensurate time off.
But as with his shift from undergraduate school in suburban Maryland to medical school in Mexico, Dr. Frizner likes a new challenge. So after a four-year stint as director of the hospitalist program at Saint Agnes Hospital in Baltimore, he took a job in August 2015 as director of the Ventilator Unit at FutureCare Irvington, a post-acute-care center in Baltimore staffed by CEP America.
“Post-acute care has become a new passion and chapter in my career,” he says, adding, “Skilled nursing facilities are extensions of the acute-care hospital and are just as challenging and fulfilling as hospitalist work.”
It’s a perspective Dr. Frizner will bring as one of eight new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: Why did you choose a career in medicine?
Answer: I enjoyed math and biology in college. I started out thinking I would be an engineer but fell in love with anatomy. I like solving problems and working with people. Internal medicine/hospital medicine is a perfect match, working to solve a patient’s diagnosis and helping families make difficult decisions about placement and palliative care.
Q: What do you like most about working as a hospitalist?
A: Interacting with all the different specialties, social work, case management, residents, ED docs. I really enjoy the camaraderie.
Q: What do you dislike most?
A: Hospital groups contribute immensely to patient flow, care, quality, process improvement, throughput, but hospitals always advertise the new specialist and never the excellent hospitalist group.
Q: What’s the best advice you ever received?
A: No matter what, do what is best for the patient. Everything else will take care of itself.
Q: What’s the worst advice you ever received?
A: Don’t worry about the contract; you don’t need to really look it over.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The pace of medicine continues to speed up. Residents have to hit the ground running with baseline case-management knowledge.
Q: What’s the biggest change you would like to see in HM?
A: I would like to see more hospitalists ascend into senior leadership in hospitals and healthcare systems.
Q: Why should group leaders continue to see patients?
A: It is important to maintain trust and respect with docs you are leading and managing. When I was a hospitalist director, I made sure I worked nights and weekends so I could understand the workload during those shifts and my team felt I was not just dumping on them.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care is most challenging?
A: Establishing trust with the patient and their family. But it has become second nature to me at this point. The secret is to introduce yourself, tell the patient and family you will take care of them in the hospital, communicate with their outpatient physician and that you are part of a 24-7 team of docs there to take care of the patient.
Q: What aspect of patient care is most rewarding?
A: Helping families navigate end-of-life decisions. It is the most stressful time in a family’s life, and I think it is the most rewarding and honorable part of practicing medicine.
Q: Are you on teaching service? If so, what aspect of teaching in the 21st century is most difficult? And what is most enjoyable?
A: I lead teaching rounds a few months a year when I was a hospitalist director, and I think the most difficult part is getting the residents to understand the workload will be a lot tougher when they get out into the real world. During their third year, residents need to practice efficiency and gauge their work ethic—not the kind of work ethic needed to pass the boards but the kind needed to stay in the ED and help your teammate out until the admissions are caught up or round on a few extra patients when there is a surge in the census.
Q: What is your biggest professional challenge?
A: [Getting others to] stop underestimating my skills and experience as a hospitalist and physician leader. I will complete an MBA through ACPE UMass this December. Learning basic accounting, business law, and finance has helped round out blind spots and build my confidence.
Q: What is your biggest professional reward?
A: Completing quality improvement projects such as increasing DVT prophylaxis, reducing CAUTI, and decreasing throughput times, which all help make the hospital course safer and efficient for the patient.
Q: What SHM event made the most lasting impression on you?
A: Seven years ago, I attended the Level I leadership academy at the Aria hotel in Las Vegas. The meeting opened my eyes to the world of leadership, management, and healthcare economics, which sparked my drive to eventually become a hospitalist director.
Q: What’s the best book you’ve read recently? Why?
A: David and Goliath by Malcolm Gladwell. As a foreign medical graduate, I was told there would be limits to what I could achieve in my career. Mr. Gladwell’s book is filled with stories of people who overcame difficult situations and went on to rise to the top of their fields.
Richard Quinn is a freelance writer in New Jersey.
Going to medical school at Universidad Autónoma de Guadalajara in Guadalajara, Mexico, could have been too much for Benjamin Frizner, MD, FHM.
Medicine is its own new language, as any first-year can tell you. Throw in learning Spanish? And a new culture? One could be forgiven for not excelling.
Dr. Frizner isn’t one of those people.
“The experience changed my life,” he says. “After I survived the first year, I knew I loved medicine.”
After medical school, Dr. Frizner had to complete a Fifth Pathway program, which formerly allowed students who completed four years at a foreign medical school to finish supervised clinical work at a U.S. medical school and become eligible as a U.S. resident.
He learned of hospital medicine during his residency at York Hospital in York, Pa., and, despite others suggesting hospital medicine was “something to do before you really figure out your career,” he enjoyed both working within the hospital walls and having a schedule that allowed 15 shifts a month and commensurate time off.
But as with his shift from undergraduate school in suburban Maryland to medical school in Mexico, Dr. Frizner likes a new challenge. So after a four-year stint as director of the hospitalist program at Saint Agnes Hospital in Baltimore, he took a job in August 2015 as director of the Ventilator Unit at FutureCare Irvington, a post-acute-care center in Baltimore staffed by CEP America.
“Post-acute care has become a new passion and chapter in my career,” he says, adding, “Skilled nursing facilities are extensions of the acute-care hospital and are just as challenging and fulfilling as hospitalist work.”
It’s a perspective Dr. Frizner will bring as one of eight new members of Team Hospitalist, The Hospitalist’s volunteer editorial advisory board.
Question: Why did you choose a career in medicine?
Answer: I enjoyed math and biology in college. I started out thinking I would be an engineer but fell in love with anatomy. I like solving problems and working with people. Internal medicine/hospital medicine is a perfect match, working to solve a patient’s diagnosis and helping families make difficult decisions about placement and palliative care.
Q: What do you like most about working as a hospitalist?
A: Interacting with all the different specialties, social work, case management, residents, ED docs. I really enjoy the camaraderie.
Q: What do you dislike most?
A: Hospital groups contribute immensely to patient flow, care, quality, process improvement, throughput, but hospitals always advertise the new specialist and never the excellent hospitalist group.
Q: What’s the best advice you ever received?
A: No matter what, do what is best for the patient. Everything else will take care of itself.
Q: What’s the worst advice you ever received?
A: Don’t worry about the contract; you don’t need to really look it over.
Q: What’s the biggest change you’ve seen in HM in your career?
A: The pace of medicine continues to speed up. Residents have to hit the ground running with baseline case-management knowledge.
Q: What’s the biggest change you would like to see in HM?
A: I would like to see more hospitalists ascend into senior leadership in hospitals and healthcare systems.
Q: Why should group leaders continue to see patients?
A: It is important to maintain trust and respect with docs you are leading and managing. When I was a hospitalist director, I made sure I worked nights and weekends so I could understand the workload during those shifts and my team felt I was not just dumping on them.
Q: As a hospitalist, seeing most of your patients for the very first time, what aspect of patient care is most challenging?
A: Establishing trust with the patient and their family. But it has become second nature to me at this point. The secret is to introduce yourself, tell the patient and family you will take care of them in the hospital, communicate with their outpatient physician and that you are part of a 24-7 team of docs there to take care of the patient.
Q: What aspect of patient care is most rewarding?
A: Helping families navigate end-of-life decisions. It is the most stressful time in a family’s life, and I think it is the most rewarding and honorable part of practicing medicine.
Q: Are you on teaching service? If so, what aspect of teaching in the 21st century is most difficult? And what is most enjoyable?
A: I lead teaching rounds a few months a year when I was a hospitalist director, and I think the most difficult part is getting the residents to understand the workload will be a lot tougher when they get out into the real world. During their third year, residents need to practice efficiency and gauge their work ethic—not the kind of work ethic needed to pass the boards but the kind needed to stay in the ED and help your teammate out until the admissions are caught up or round on a few extra patients when there is a surge in the census.
Q: What is your biggest professional challenge?
A: [Getting others to] stop underestimating my skills and experience as a hospitalist and physician leader. I will complete an MBA through ACPE UMass this December. Learning basic accounting, business law, and finance has helped round out blind spots and build my confidence.
Q: What is your biggest professional reward?
A: Completing quality improvement projects such as increasing DVT prophylaxis, reducing CAUTI, and decreasing throughput times, which all help make the hospital course safer and efficient for the patient.
Q: What SHM event made the most lasting impression on you?
A: Seven years ago, I attended the Level I leadership academy at the Aria hotel in Las Vegas. The meeting opened my eyes to the world of leadership, management, and healthcare economics, which sparked my drive to eventually become a hospitalist director.
Q: What’s the best book you’ve read recently? Why?
A: David and Goliath by Malcolm Gladwell. As a foreign medical graduate, I was told there would be limits to what I could achieve in my career. Mr. Gladwell’s book is filled with stories of people who overcame difficult situations and went on to rise to the top of their fields.
Richard Quinn is a freelance writer in New Jersey.
Rule identifies women at low risk of VTE recurrence

of women in a family
ROME—According to researchers, a clinical decision rule can identify women who, after their first unprovoked venous thromboembolism (VTE), have a low risk of VTE recurrence and might safely discontinue anticoagulant therapy.
The researchers evaluated the HERDOO2 rule, which is named after the risk factors the rule employs to determine the likelihood of VTE recurrence, in the REVERSE II trial.
Results from the trial were presented at ESC Congress 2016 (abstract 5721).
According to the HERDOO2 rule, the following risk factors must be considered to determine a patient’s risk of VTE recurrence:
- Hyperpigmentation, Edema, or Redness in either leg
- D-dimer >250 μg/mL on anticoagulants
- Obesity with body mass index >30 kg/m2
- Older than age 65.
Women (but not men) are considered at low risk of VTE recurrence if they have 0 to 1 of these risk factors.
In the REVERSE II trial, researchers tested the HERDOO2 rule in 2779 male and female patients with a first unprovoked VTE who had completed 5 to 12 months of anticoagulant therapy.
After drop-outs and exclusions, 622 women were considered low-risk, based on HERDOO2 criteria, and the majority of these women (n=591) discontinued anticoagulant therapy.
Thirty-one low-risk women continued anticoagulant therapy, as did 1802 men and high-risk women (with 2 or more HERDOO2 criteria). Three hundred and twenty-three men and high-risk women discontinued anticoagulant therapy.
After a year of follow-up, low-risk women who had discontinued anticoagulants had a 3% rate of recurrent VTE per patient year, and low-risk women who continued anticoagulant therapy had no VTEs.
Among the men and high-risk women, the rate of recurrent VTE per patient year was 8.1% in patients who discontinued therapy and 1.6% in patients who continued to receive anticoagulant therapy.
“This is an important finding as, using our rule, over half of women with unprovoked VTE can safely discontinue anticoagulants and be spared the burdens, costs, and risks of life-long anticoagulation,” said study investigator Marc Rodger, MD, of Ottawa Hospital and University of Ottawa in Ontario, Canada.
“Since current consensus guidelines suggest anticoagulants should be continued indefinitely in all patients with unprovoked VTE and non-high bleeding risk, our results are potentially practice-changing.”
Dr Rodger noted, however, that questions remain regarding anticoagulation duration after a first unprovoked VTE.
“One is whether indefinite anticoagulation is required for men and high-risk women, [which] was not the primary focus of our study,” he said. “The second is in the subgroup of post-menopausal women aged 50 and above.”
“In this group, even those who were considered low-risk according to the HERDOO2 rule had a higher than expected rate of recurrent VTE (5.7%) when they discontinued anticoagulants. As such, further validation of HERDOO2 is required in this subset of post-menopausal women.” ![]()

of women in a family
ROME—According to researchers, a clinical decision rule can identify women who, after their first unprovoked venous thromboembolism (VTE), have a low risk of VTE recurrence and might safely discontinue anticoagulant therapy.
The researchers evaluated the HERDOO2 rule, which is named after the risk factors the rule employs to determine the likelihood of VTE recurrence, in the REVERSE II trial.
Results from the trial were presented at ESC Congress 2016 (abstract 5721).
According to the HERDOO2 rule, the following risk factors must be considered to determine a patient’s risk of VTE recurrence:
- Hyperpigmentation, Edema, or Redness in either leg
- D-dimer >250 μg/mL on anticoagulants
- Obesity with body mass index >30 kg/m2
- Older than age 65.
Women (but not men) are considered at low risk of VTE recurrence if they have 0 to 1 of these risk factors.
In the REVERSE II trial, researchers tested the HERDOO2 rule in 2779 male and female patients with a first unprovoked VTE who had completed 5 to 12 months of anticoagulant therapy.
After drop-outs and exclusions, 622 women were considered low-risk, based on HERDOO2 criteria, and the majority of these women (n=591) discontinued anticoagulant therapy.
Thirty-one low-risk women continued anticoagulant therapy, as did 1802 men and high-risk women (with 2 or more HERDOO2 criteria). Three hundred and twenty-three men and high-risk women discontinued anticoagulant therapy.
After a year of follow-up, low-risk women who had discontinued anticoagulants had a 3% rate of recurrent VTE per patient year, and low-risk women who continued anticoagulant therapy had no VTEs.
Among the men and high-risk women, the rate of recurrent VTE per patient year was 8.1% in patients who discontinued therapy and 1.6% in patients who continued to receive anticoagulant therapy.
“This is an important finding as, using our rule, over half of women with unprovoked VTE can safely discontinue anticoagulants and be spared the burdens, costs, and risks of life-long anticoagulation,” said study investigator Marc Rodger, MD, of Ottawa Hospital and University of Ottawa in Ontario, Canada.
“Since current consensus guidelines suggest anticoagulants should be continued indefinitely in all patients with unprovoked VTE and non-high bleeding risk, our results are potentially practice-changing.”
Dr Rodger noted, however, that questions remain regarding anticoagulation duration after a first unprovoked VTE.
“One is whether indefinite anticoagulation is required for men and high-risk women, [which] was not the primary focus of our study,” he said. “The second is in the subgroup of post-menopausal women aged 50 and above.”
“In this group, even those who were considered low-risk according to the HERDOO2 rule had a higher than expected rate of recurrent VTE (5.7%) when they discontinued anticoagulants. As such, further validation of HERDOO2 is required in this subset of post-menopausal women.” ![]()

of women in a family
ROME—According to researchers, a clinical decision rule can identify women who, after their first unprovoked venous thromboembolism (VTE), have a low risk of VTE recurrence and might safely discontinue anticoagulant therapy.
The researchers evaluated the HERDOO2 rule, which is named after the risk factors the rule employs to determine the likelihood of VTE recurrence, in the REVERSE II trial.
Results from the trial were presented at ESC Congress 2016 (abstract 5721).
According to the HERDOO2 rule, the following risk factors must be considered to determine a patient’s risk of VTE recurrence:
- Hyperpigmentation, Edema, or Redness in either leg
- D-dimer >250 μg/mL on anticoagulants
- Obesity with body mass index >30 kg/m2
- Older than age 65.
Women (but not men) are considered at low risk of VTE recurrence if they have 0 to 1 of these risk factors.
In the REVERSE II trial, researchers tested the HERDOO2 rule in 2779 male and female patients with a first unprovoked VTE who had completed 5 to 12 months of anticoagulant therapy.
After drop-outs and exclusions, 622 women were considered low-risk, based on HERDOO2 criteria, and the majority of these women (n=591) discontinued anticoagulant therapy.
Thirty-one low-risk women continued anticoagulant therapy, as did 1802 men and high-risk women (with 2 or more HERDOO2 criteria). Three hundred and twenty-three men and high-risk women discontinued anticoagulant therapy.
After a year of follow-up, low-risk women who had discontinued anticoagulants had a 3% rate of recurrent VTE per patient year, and low-risk women who continued anticoagulant therapy had no VTEs.
Among the men and high-risk women, the rate of recurrent VTE per patient year was 8.1% in patients who discontinued therapy and 1.6% in patients who continued to receive anticoagulant therapy.
“This is an important finding as, using our rule, over half of women with unprovoked VTE can safely discontinue anticoagulants and be spared the burdens, costs, and risks of life-long anticoagulation,” said study investigator Marc Rodger, MD, of Ottawa Hospital and University of Ottawa in Ontario, Canada.
“Since current consensus guidelines suggest anticoagulants should be continued indefinitely in all patients with unprovoked VTE and non-high bleeding risk, our results are potentially practice-changing.”
Dr Rodger noted, however, that questions remain regarding anticoagulation duration after a first unprovoked VTE.
“One is whether indefinite anticoagulation is required for men and high-risk women, [which] was not the primary focus of our study,” he said. “The second is in the subgroup of post-menopausal women aged 50 and above.”
“In this group, even those who were considered low-risk according to the HERDOO2 rule had a higher than expected rate of recurrent VTE (5.7%) when they discontinued anticoagulants. As such, further validation of HERDOO2 is required in this subset of post-menopausal women.” ![]()
Antiplatelet monitoring doesn’t benefit high-risk patient group

Photo courtesy of NIH
ROME—Results of the ANTARCTIC trial suggest that monitoring platelet function to individualize antiplatelet therapy does not improve outcomes for elderly patients stented for an acute coronary syndrome.
These patients had a high risk of ischemic and bleeding complications, but the study showed no significant difference in the incidence of such complications between patients who were monitored and those who were not.
The findings challenge current international guidelines, which recommend platelet function testing in high-risk patients.
“Platelet function testing is still being used in many centers to measure the effect of antiplatelet drugs and adjust the choice of these drugs and their doses,” said study investigator Gilles Montalescot, MD, PhD, of Hôpital Pitié-Salpêtrière in Paris, France.
“Our study does not support this practice and these recommendations. Although measuring the effect of antiplatelet agents makes sense in order to choose the best
drugs or doses, this costly and more complex strategy does not appear to benefit patients, even when they present with extremely high risk of ischemic and bleeding events like those enrolled in ANTARCTIC.”
Results of the ANTARCTIC trial were presented at ESC Congress 2016 (abstract 2221) and published in The Lancet.
The study was funded by Eli Lilly and Company, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and Fondation Coeur et Recherche.
ANTARCTIC enrolled 877 patients, ages 75 and older, who presented with an acute coronary syndrome and underwent coronary stenting.
All patients were started on the antiplatelet agent prasugrel (5 mg), with 442 randomized to the conventional therapy (no adjustment) and 435 randomized to monitoring and treatment adjustment if needed.
Patients in the monitoring arm received 14 days of the daily 5 mg prasugrel dose, then underwent a platelet function test at day 14, followed by medication adjustment if the test showed high or low platelet reactivity. Additional monitoring was performed at day 28 in patients who needed treatment adjustment.
The primary endpoint of the trial was the composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, urgent revascularization, and bleeding complications at 1 year.
This endpoint occurred at a similar rate in both arms of the study—27.6% in the monitoring arm and 27.8% in the conventional therapy arm (hazard ratio=1.003; P=0.98).
Similarly, there was no significant difference between the arms with regard to the main secondary endpoint—a composite of cardiovascular death, myocardial infarction, stent thrombosis, and urgent revascularization.
This endpoint occurred in 9.9% of patients in the monitoring arm and 9.3% of patients in the conventional arm (hazard ratio=1.06; P=0.80).
“Platelet function monitoring led to a change of treatment in 44.8% of patients who were identified as being over- or under-treated, yet this strategy did not improve ischemic or safety outcomes,” Dr Montalescot noted.
“ANTARCTIC confirms the ARCTIC study in a different population with a different drug and has addressed the potential limitations of the ARCTIC study but finally reached the same conclusion. I expect there will be adjustments of guidelines and practice in light of this.” ![]()

Photo courtesy of NIH
ROME—Results of the ANTARCTIC trial suggest that monitoring platelet function to individualize antiplatelet therapy does not improve outcomes for elderly patients stented for an acute coronary syndrome.
These patients had a high risk of ischemic and bleeding complications, but the study showed no significant difference in the incidence of such complications between patients who were monitored and those who were not.
The findings challenge current international guidelines, which recommend platelet function testing in high-risk patients.
“Platelet function testing is still being used in many centers to measure the effect of antiplatelet drugs and adjust the choice of these drugs and their doses,” said study investigator Gilles Montalescot, MD, PhD, of Hôpital Pitié-Salpêtrière in Paris, France.
“Our study does not support this practice and these recommendations. Although measuring the effect of antiplatelet agents makes sense in order to choose the best
drugs or doses, this costly and more complex strategy does not appear to benefit patients, even when they present with extremely high risk of ischemic and bleeding events like those enrolled in ANTARCTIC.”
Results of the ANTARCTIC trial were presented at ESC Congress 2016 (abstract 2221) and published in The Lancet.
The study was funded by Eli Lilly and Company, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and Fondation Coeur et Recherche.
ANTARCTIC enrolled 877 patients, ages 75 and older, who presented with an acute coronary syndrome and underwent coronary stenting.
All patients were started on the antiplatelet agent prasugrel (5 mg), with 442 randomized to the conventional therapy (no adjustment) and 435 randomized to monitoring and treatment adjustment if needed.
Patients in the monitoring arm received 14 days of the daily 5 mg prasugrel dose, then underwent a platelet function test at day 14, followed by medication adjustment if the test showed high or low platelet reactivity. Additional monitoring was performed at day 28 in patients who needed treatment adjustment.
The primary endpoint of the trial was the composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, urgent revascularization, and bleeding complications at 1 year.
This endpoint occurred at a similar rate in both arms of the study—27.6% in the monitoring arm and 27.8% in the conventional therapy arm (hazard ratio=1.003; P=0.98).
Similarly, there was no significant difference between the arms with regard to the main secondary endpoint—a composite of cardiovascular death, myocardial infarction, stent thrombosis, and urgent revascularization.
This endpoint occurred in 9.9% of patients in the monitoring arm and 9.3% of patients in the conventional arm (hazard ratio=1.06; P=0.80).
“Platelet function monitoring led to a change of treatment in 44.8% of patients who were identified as being over- or under-treated, yet this strategy did not improve ischemic or safety outcomes,” Dr Montalescot noted.
“ANTARCTIC confirms the ARCTIC study in a different population with a different drug and has addressed the potential limitations of the ARCTIC study but finally reached the same conclusion. I expect there will be adjustments of guidelines and practice in light of this.” ![]()

Photo courtesy of NIH
ROME—Results of the ANTARCTIC trial suggest that monitoring platelet function to individualize antiplatelet therapy does not improve outcomes for elderly patients stented for an acute coronary syndrome.
These patients had a high risk of ischemic and bleeding complications, but the study showed no significant difference in the incidence of such complications between patients who were monitored and those who were not.
The findings challenge current international guidelines, which recommend platelet function testing in high-risk patients.
“Platelet function testing is still being used in many centers to measure the effect of antiplatelet drugs and adjust the choice of these drugs and their doses,” said study investigator Gilles Montalescot, MD, PhD, of Hôpital Pitié-Salpêtrière in Paris, France.
“Our study does not support this practice and these recommendations. Although measuring the effect of antiplatelet agents makes sense in order to choose the best
drugs or doses, this costly and more complex strategy does not appear to benefit patients, even when they present with extremely high risk of ischemic and bleeding events like those enrolled in ANTARCTIC.”
Results of the ANTARCTIC trial were presented at ESC Congress 2016 (abstract 2221) and published in The Lancet.
The study was funded by Eli Lilly and Company, Daiichi Sankyo, Stentys, Accriva Diagnostics, Medtronic, and Fondation Coeur et Recherche.
ANTARCTIC enrolled 877 patients, ages 75 and older, who presented with an acute coronary syndrome and underwent coronary stenting.
All patients were started on the antiplatelet agent prasugrel (5 mg), with 442 randomized to the conventional therapy (no adjustment) and 435 randomized to monitoring and treatment adjustment if needed.
Patients in the monitoring arm received 14 days of the daily 5 mg prasugrel dose, then underwent a platelet function test at day 14, followed by medication adjustment if the test showed high or low platelet reactivity. Additional monitoring was performed at day 28 in patients who needed treatment adjustment.
The primary endpoint of the trial was the composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, urgent revascularization, and bleeding complications at 1 year.
This endpoint occurred at a similar rate in both arms of the study—27.6% in the monitoring arm and 27.8% in the conventional therapy arm (hazard ratio=1.003; P=0.98).
Similarly, there was no significant difference between the arms with regard to the main secondary endpoint—a composite of cardiovascular death, myocardial infarction, stent thrombosis, and urgent revascularization.
This endpoint occurred in 9.9% of patients in the monitoring arm and 9.3% of patients in the conventional arm (hazard ratio=1.06; P=0.80).
“Platelet function monitoring led to a change of treatment in 44.8% of patients who were identified as being over- or under-treated, yet this strategy did not improve ischemic or safety outcomes,” Dr Montalescot noted.
“ANTARCTIC confirms the ARCTIC study in a different population with a different drug and has addressed the potential limitations of the ARCTIC study but finally reached the same conclusion. I expect there will be adjustments of guidelines and practice in light of this.” ![]()
BSIs costly for pediatric transplant, cancer patients

Staphylococcus infection
Photo by Bill Branson
Ambulatory bloodstream infections (BSIs) can be costly in young cancer patients and recipients of hematopoietic stem cell transplants, according to research published in Pediatric Blood & Cancer.
Among the 61 patients studied, the median cost for an ambulatory BSI was $40,852, and the median length of hospital stay was 7 days.
For patients who were hospitalized for BSI and other medical issues, the cost and length of stay were much higher.
“This issue has resonance beyond the pediatric stem cell transplant and oncology patient population,” said study author Amy Billett, MD, of the Dana–Farber Cancer Institute and Boston Children’s Hospital in Massachusetts.
“At a time when many aspects of care are being shifted to the home and of heightened attention to safety and cost, this is the new frontier. What we learn about preventing outpatient bloodstream infections in these patients could have broad relevance.”
To determine the economic and hospitalization impact of ambulatory BSIs, Dr Billet and her colleagues retrospectively analyzed data on outpatient BSIs at Dana-Farber/Boston Children’s that occurred between January 1, 2012, and December 31, 2013, and resulted in hospitalization.
The team identified 74 BSIs in 61 patients. Sixty-nine percent of these infections were classified as central-line-associated bloodstream infections.
In 43% of BSIs, the patient’s central line had to be surgically removed. In 15% of cases, the child was transferred to the intensive care unit. Four patients died during hospitalization, and 3 of these deaths were associated with the infections.
Most of the hospitalizations analyzed—62—were due solely to BSIs. The remainder involved at least 1 other medical issue.
The median total cost of BSIs was $40,852, and the median length of hospital stay was 7 days.
The median cost was $36,611 among patients who were hospitalized for BSIs alone (n=62) and $89,935 for patients who were hospitalized for other medical issues as well. The median lengths of hospital stay were 6 days and 15 days, respectively.
The top 3 drivers of cost for all BSIs were room and board (43%), non-chemotherapy medications (22%), and procedures (11%).
Room and board accounted for 42% of charges among patients who were hospitalized for BSIs alone and 44% among the other patients. Non-chemotherapy medications accounted for 20% and 25%, respectively. And procedures accounted for 11% and 10%, respectively.
“Behind these metrics are real and serious risks to patients’ health,” said study author Chris Wong, MD, of Dana-Farber/Boston Children’s.
“The bottom line is that the dollar cost and lengthy hospital stays signal complications that could become life-threatening or delay treatment of the children’s cancer. Reducing these infections is important both for cost containment and quality of care.” ![]()

Staphylococcus infection
Photo by Bill Branson
Ambulatory bloodstream infections (BSIs) can be costly in young cancer patients and recipients of hematopoietic stem cell transplants, according to research published in Pediatric Blood & Cancer.
Among the 61 patients studied, the median cost for an ambulatory BSI was $40,852, and the median length of hospital stay was 7 days.
For patients who were hospitalized for BSI and other medical issues, the cost and length of stay were much higher.
“This issue has resonance beyond the pediatric stem cell transplant and oncology patient population,” said study author Amy Billett, MD, of the Dana–Farber Cancer Institute and Boston Children’s Hospital in Massachusetts.
“At a time when many aspects of care are being shifted to the home and of heightened attention to safety and cost, this is the new frontier. What we learn about preventing outpatient bloodstream infections in these patients could have broad relevance.”
To determine the economic and hospitalization impact of ambulatory BSIs, Dr Billet and her colleagues retrospectively analyzed data on outpatient BSIs at Dana-Farber/Boston Children’s that occurred between January 1, 2012, and December 31, 2013, and resulted in hospitalization.
The team identified 74 BSIs in 61 patients. Sixty-nine percent of these infections were classified as central-line-associated bloodstream infections.
In 43% of BSIs, the patient’s central line had to be surgically removed. In 15% of cases, the child was transferred to the intensive care unit. Four patients died during hospitalization, and 3 of these deaths were associated with the infections.
Most of the hospitalizations analyzed—62—were due solely to BSIs. The remainder involved at least 1 other medical issue.
The median total cost of BSIs was $40,852, and the median length of hospital stay was 7 days.
The median cost was $36,611 among patients who were hospitalized for BSIs alone (n=62) and $89,935 for patients who were hospitalized for other medical issues as well. The median lengths of hospital stay were 6 days and 15 days, respectively.
The top 3 drivers of cost for all BSIs were room and board (43%), non-chemotherapy medications (22%), and procedures (11%).
Room and board accounted for 42% of charges among patients who were hospitalized for BSIs alone and 44% among the other patients. Non-chemotherapy medications accounted for 20% and 25%, respectively. And procedures accounted for 11% and 10%, respectively.
“Behind these metrics are real and serious risks to patients’ health,” said study author Chris Wong, MD, of Dana-Farber/Boston Children’s.
“The bottom line is that the dollar cost and lengthy hospital stays signal complications that could become life-threatening or delay treatment of the children’s cancer. Reducing these infections is important both for cost containment and quality of care.” ![]()

Staphylococcus infection
Photo by Bill Branson
Ambulatory bloodstream infections (BSIs) can be costly in young cancer patients and recipients of hematopoietic stem cell transplants, according to research published in Pediatric Blood & Cancer.
Among the 61 patients studied, the median cost for an ambulatory BSI was $40,852, and the median length of hospital stay was 7 days.
For patients who were hospitalized for BSI and other medical issues, the cost and length of stay were much higher.
“This issue has resonance beyond the pediatric stem cell transplant and oncology patient population,” said study author Amy Billett, MD, of the Dana–Farber Cancer Institute and Boston Children’s Hospital in Massachusetts.
“At a time when many aspects of care are being shifted to the home and of heightened attention to safety and cost, this is the new frontier. What we learn about preventing outpatient bloodstream infections in these patients could have broad relevance.”
To determine the economic and hospitalization impact of ambulatory BSIs, Dr Billet and her colleagues retrospectively analyzed data on outpatient BSIs at Dana-Farber/Boston Children’s that occurred between January 1, 2012, and December 31, 2013, and resulted in hospitalization.
The team identified 74 BSIs in 61 patients. Sixty-nine percent of these infections were classified as central-line-associated bloodstream infections.
In 43% of BSIs, the patient’s central line had to be surgically removed. In 15% of cases, the child was transferred to the intensive care unit. Four patients died during hospitalization, and 3 of these deaths were associated with the infections.
Most of the hospitalizations analyzed—62—were due solely to BSIs. The remainder involved at least 1 other medical issue.
The median total cost of BSIs was $40,852, and the median length of hospital stay was 7 days.
The median cost was $36,611 among patients who were hospitalized for BSIs alone (n=62) and $89,935 for patients who were hospitalized for other medical issues as well. The median lengths of hospital stay were 6 days and 15 days, respectively.
The top 3 drivers of cost for all BSIs were room and board (43%), non-chemotherapy medications (22%), and procedures (11%).
Room and board accounted for 42% of charges among patients who were hospitalized for BSIs alone and 44% among the other patients. Non-chemotherapy medications accounted for 20% and 25%, respectively. And procedures accounted for 11% and 10%, respectively.
“Behind these metrics are real and serious risks to patients’ health,” said study author Chris Wong, MD, of Dana-Farber/Boston Children’s.
“The bottom line is that the dollar cost and lengthy hospital stays signal complications that could become life-threatening or delay treatment of the children’s cancer. Reducing these infections is important both for cost containment and quality of care.” ![]()
Team uncovers potential treatments for Zika virus

Photo courtesy of
Muhammad Mahdi Karim
Researchers say they have identified compounds that might be used to inhibit Zika virus replication and reduce the ability of the virus to kill brain cells.
The compounds include emricasan (a drug being investigated as a treatment to reduce liver damage from hepatitis C virus), niclosamide (a drug approved in the US to combat parasitic infections), and an investigational cyclin-dependent kinase inhibitor known as PHA-690509.
The researchers described the anti-Zika activity of these compounds in Nature Medicine.
About the virus
The Zika virus has been reported in 60 countries and territories worldwide. Currently, there are no vaccines or effective treatments for the virus.
Research and anecdotal evidence have suggested infection with the Zika virus is related to fetal microcephaly, an abnormally small head resulting from an underdeveloped and/or damaged brain. The virus has also been linked with neurological diseases such as Guillain-Barré syndrome in infected adults.
The Zika virus is spread primarily through bites from infected Aedes aegypti mosquitoes, but it can also be transmitted from mother to child, through sexual contact, via blood transfusion, and possibly through other methods.
“The Zika virus poses a global health threat,” said study author Anton Simeonov, PhD, of the National Center for Advancing Translational Sciences in Bethesda, Maryland.
“While we await the development of effective vaccines, which can take a significant amount of time, our identification of repurposed small-molecule compounds may accelerate the translational process of finding a potential therapy.”
“It takes years, if not decades, to develop a new drug,” noted study author Hongjun Song, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland. “In this sort of global health emergency, we don’t have that kind of time.”
“So instead of using new drugs, we chose to screen existing drugs,” added Guo-li Ming, MD, PhD, also of Johns Hopkins. “In this way, we hope to create a therapy much more quickly.”
Identifying potential treatments
The researchers screened 6000 compounds, both investigational and approved (in the US), looking for drugs that might be effective against the Zika virus.
The team first exposed cell cultures to 3 strains of the virus—Ugandan, Cambodian, and Puerto Rican. Then, they introduced the various compounds and looked for indicators of cell death.
The researchers identified more than 100 promising compounds. The 3 lead compounds were emricasan, niclosamide, and PHA-690509.
These compounds were effective either in inhibiting the replication of Zika or in preventing the virus from killing brain cells. Emricasan prevents cell death, while niclosamide and PHA-690509 stop virus replication.
The researchers found that combining emricasan and PHA-690509 prevented both cell death and virus replication.
Dr Song cautioned that the 3 drugs “are very effective against Zika in the dish, but we don’t know if they can work in humans in the same way.”
For example, he noted that, although niclosamide can safely treat parasites in the human gastrointestinal tract, researchers have not yet determined if the drug can penetrate the central nervous system of adults or a fetus inside a carrier’s womb to treat the brain cells targeted by Zika.
Furthermore, it’s not clear if the drugs would address the wide range of effects of Zika infection.
“To address these questions, additional studies need to be done in animal models as well as humans to demonstrate their ability to treat Zika infection,” Dr Ming said. “So we could still be years away from finding a treatment that works.” ![]()

Photo courtesy of
Muhammad Mahdi Karim
Researchers say they have identified compounds that might be used to inhibit Zika virus replication and reduce the ability of the virus to kill brain cells.
The compounds include emricasan (a drug being investigated as a treatment to reduce liver damage from hepatitis C virus), niclosamide (a drug approved in the US to combat parasitic infections), and an investigational cyclin-dependent kinase inhibitor known as PHA-690509.
The researchers described the anti-Zika activity of these compounds in Nature Medicine.
About the virus
The Zika virus has been reported in 60 countries and territories worldwide. Currently, there are no vaccines or effective treatments for the virus.
Research and anecdotal evidence have suggested infection with the Zika virus is related to fetal microcephaly, an abnormally small head resulting from an underdeveloped and/or damaged brain. The virus has also been linked with neurological diseases such as Guillain-Barré syndrome in infected adults.
The Zika virus is spread primarily through bites from infected Aedes aegypti mosquitoes, but it can also be transmitted from mother to child, through sexual contact, via blood transfusion, and possibly through other methods.
“The Zika virus poses a global health threat,” said study author Anton Simeonov, PhD, of the National Center for Advancing Translational Sciences in Bethesda, Maryland.
“While we await the development of effective vaccines, which can take a significant amount of time, our identification of repurposed small-molecule compounds may accelerate the translational process of finding a potential therapy.”
“It takes years, if not decades, to develop a new drug,” noted study author Hongjun Song, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland. “In this sort of global health emergency, we don’t have that kind of time.”
“So instead of using new drugs, we chose to screen existing drugs,” added Guo-li Ming, MD, PhD, also of Johns Hopkins. “In this way, we hope to create a therapy much more quickly.”
Identifying potential treatments
The researchers screened 6000 compounds, both investigational and approved (in the US), looking for drugs that might be effective against the Zika virus.
The team first exposed cell cultures to 3 strains of the virus—Ugandan, Cambodian, and Puerto Rican. Then, they introduced the various compounds and looked for indicators of cell death.
The researchers identified more than 100 promising compounds. The 3 lead compounds were emricasan, niclosamide, and PHA-690509.
These compounds were effective either in inhibiting the replication of Zika or in preventing the virus from killing brain cells. Emricasan prevents cell death, while niclosamide and PHA-690509 stop virus replication.
The researchers found that combining emricasan and PHA-690509 prevented both cell death and virus replication.
Dr Song cautioned that the 3 drugs “are very effective against Zika in the dish, but we don’t know if they can work in humans in the same way.”
For example, he noted that, although niclosamide can safely treat parasites in the human gastrointestinal tract, researchers have not yet determined if the drug can penetrate the central nervous system of adults or a fetus inside a carrier’s womb to treat the brain cells targeted by Zika.
Furthermore, it’s not clear if the drugs would address the wide range of effects of Zika infection.
“To address these questions, additional studies need to be done in animal models as well as humans to demonstrate their ability to treat Zika infection,” Dr Ming said. “So we could still be years away from finding a treatment that works.” ![]()

Photo courtesy of
Muhammad Mahdi Karim
Researchers say they have identified compounds that might be used to inhibit Zika virus replication and reduce the ability of the virus to kill brain cells.
The compounds include emricasan (a drug being investigated as a treatment to reduce liver damage from hepatitis C virus), niclosamide (a drug approved in the US to combat parasitic infections), and an investigational cyclin-dependent kinase inhibitor known as PHA-690509.
The researchers described the anti-Zika activity of these compounds in Nature Medicine.
About the virus
The Zika virus has been reported in 60 countries and territories worldwide. Currently, there are no vaccines or effective treatments for the virus.
Research and anecdotal evidence have suggested infection with the Zika virus is related to fetal microcephaly, an abnormally small head resulting from an underdeveloped and/or damaged brain. The virus has also been linked with neurological diseases such as Guillain-Barré syndrome in infected adults.
The Zika virus is spread primarily through bites from infected Aedes aegypti mosquitoes, but it can also be transmitted from mother to child, through sexual contact, via blood transfusion, and possibly through other methods.
“The Zika virus poses a global health threat,” said study author Anton Simeonov, PhD, of the National Center for Advancing Translational Sciences in Bethesda, Maryland.
“While we await the development of effective vaccines, which can take a significant amount of time, our identification of repurposed small-molecule compounds may accelerate the translational process of finding a potential therapy.”
“It takes years, if not decades, to develop a new drug,” noted study author Hongjun Song, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland. “In this sort of global health emergency, we don’t have that kind of time.”
“So instead of using new drugs, we chose to screen existing drugs,” added Guo-li Ming, MD, PhD, also of Johns Hopkins. “In this way, we hope to create a therapy much more quickly.”
Identifying potential treatments
The researchers screened 6000 compounds, both investigational and approved (in the US), looking for drugs that might be effective against the Zika virus.
The team first exposed cell cultures to 3 strains of the virus—Ugandan, Cambodian, and Puerto Rican. Then, they introduced the various compounds and looked for indicators of cell death.
The researchers identified more than 100 promising compounds. The 3 lead compounds were emricasan, niclosamide, and PHA-690509.
These compounds were effective either in inhibiting the replication of Zika or in preventing the virus from killing brain cells. Emricasan prevents cell death, while niclosamide and PHA-690509 stop virus replication.
The researchers found that combining emricasan and PHA-690509 prevented both cell death and virus replication.
Dr Song cautioned that the 3 drugs “are very effective against Zika in the dish, but we don’t know if they can work in humans in the same way.”
For example, he noted that, although niclosamide can safely treat parasites in the human gastrointestinal tract, researchers have not yet determined if the drug can penetrate the central nervous system of adults or a fetus inside a carrier’s womb to treat the brain cells targeted by Zika.
Furthermore, it’s not clear if the drugs would address the wide range of effects of Zika infection.
“To address these questions, additional studies need to be done in animal models as well as humans to demonstrate their ability to treat Zika infection,” Dr Ming said. “So we could still be years away from finding a treatment that works.” ![]()