User login
Rotavirus vaccination herd effect benefits newborns and infants
Unvaccinated newborns and infants 42 days old or younger had significantly fewer rotavirus infections after the introduction of a universal mass vaccination (UMV) program, based on a decade of surveillance data from 11 pediatric care facilities in Austria.
“The present study aimed to investigate the long-term effect of UMV on rotavirus (RV)–associated hospitalizations, with particular focus on neonates and infants less than 6 weeks of age, comparing surveillance data between the prevaccination and postvaccination periods,” wrote Martina Prelog, MD, of University Hospital Wuerzburg (Germany), and her colleagues.
The data included 10,960 laboratory-confirmed cases of RV covering the periods before and after the initiation of the mass vaccination program.
Overall, hospitalizations for community-acquired RV infections dropped by almost 90% across all age groups. Among young infants, nosocomial RV infection rates were 28% prior to the vaccination program and 19% afterwards. However, overall nosocomial RV infection rates increased from 6% before the vaccination program to 13% after the program, and 6% of the cases were breakthrough infections, generally after incomplete RV vaccination.
“High numbers of documented cases and similar trends in all centers bolster the conclusion that UMV with RV vaccination may be associated with lower rates of RV hospitalization in unvaccinated neonates and young infants, supporting the beneficial role of UMV,” Dr. Prelog and her associates wrote.
Find the full study here in the Journal of Infectious Diseases (2016. doi: 10.1093/infdis/jiw186).
Unvaccinated newborns and infants 42 days old or younger had significantly fewer rotavirus infections after the introduction of a universal mass vaccination (UMV) program, based on a decade of surveillance data from 11 pediatric care facilities in Austria.
“The present study aimed to investigate the long-term effect of UMV on rotavirus (RV)–associated hospitalizations, with particular focus on neonates and infants less than 6 weeks of age, comparing surveillance data between the prevaccination and postvaccination periods,” wrote Martina Prelog, MD, of University Hospital Wuerzburg (Germany), and her colleagues.
The data included 10,960 laboratory-confirmed cases of RV covering the periods before and after the initiation of the mass vaccination program.
Overall, hospitalizations for community-acquired RV infections dropped by almost 90% across all age groups. Among young infants, nosocomial RV infection rates were 28% prior to the vaccination program and 19% afterwards. However, overall nosocomial RV infection rates increased from 6% before the vaccination program to 13% after the program, and 6% of the cases were breakthrough infections, generally after incomplete RV vaccination.
“High numbers of documented cases and similar trends in all centers bolster the conclusion that UMV with RV vaccination may be associated with lower rates of RV hospitalization in unvaccinated neonates and young infants, supporting the beneficial role of UMV,” Dr. Prelog and her associates wrote.
Find the full study here in the Journal of Infectious Diseases (2016. doi: 10.1093/infdis/jiw186).
Unvaccinated newborns and infants 42 days old or younger had significantly fewer rotavirus infections after the introduction of a universal mass vaccination (UMV) program, based on a decade of surveillance data from 11 pediatric care facilities in Austria.
“The present study aimed to investigate the long-term effect of UMV on rotavirus (RV)–associated hospitalizations, with particular focus on neonates and infants less than 6 weeks of age, comparing surveillance data between the prevaccination and postvaccination periods,” wrote Martina Prelog, MD, of University Hospital Wuerzburg (Germany), and her colleagues.
The data included 10,960 laboratory-confirmed cases of RV covering the periods before and after the initiation of the mass vaccination program.
Overall, hospitalizations for community-acquired RV infections dropped by almost 90% across all age groups. Among young infants, nosocomial RV infection rates were 28% prior to the vaccination program and 19% afterwards. However, overall nosocomial RV infection rates increased from 6% before the vaccination program to 13% after the program, and 6% of the cases were breakthrough infections, generally after incomplete RV vaccination.
“High numbers of documented cases and similar trends in all centers bolster the conclusion that UMV with RV vaccination may be associated with lower rates of RV hospitalization in unvaccinated neonates and young infants, supporting the beneficial role of UMV,” Dr. Prelog and her associates wrote.
Find the full study here in the Journal of Infectious Diseases (2016. doi: 10.1093/infdis/jiw186).
FROM THE JOURNAL OF INFECTIOUS DISEASES
Fatigue ... or chronic fatigue syndrome?
In an increasingly sleepless society inundated with caffeine drinks and social media, it is not surprising that 15%-30% of teens present with complaints of fatigue.1 As fatigue can be a symptom of many disorders, a careful work-up is important.
Fewer than 8 hours’ sleep, large intake of caffeine, and several hours on social media each day are linked with fatigue. Poor eating habits are more likely than not; nearly 21% of teens are overweight.2 Video games have also contributed to symptoms of fatigue by encouraging a sedentary life style.
The work-up for teens with fatigue requires a detailed history of present symptoms and a careful review of systems, which should specifically address weight gain or loss, menstrual change, palpitations, and respiratory changes. It is important to screen for signs of stress and depression.
The physical exam should identify systems that need further work-up, and a standard serum screen would include a complete blood cell count (CBC), complete metabolic panel (CMP), and a thyroid panel.
As many experienced physicians can attest, more often than not, this work-up results in normal findings. Suggesting that the basis for fatigue may be a psychiatric cause is usually not well received.
Another consideration is a diagnosis of chronic fatigue syndrome (CFS), a well-documented disorder with defined diagnostic criteria since 1994. Although CFS is more common in adults, research now reveals an annual incidence of 0.5% and a prevalence of 0.19%-1.29% in teens.3 The characteristics are severe and disabling new-onset fatigue lasting greater than 6 months and four of the following symptoms: impaired memory, sore throat, tender cervical and axillary lymph nodes, muscle pain, headaches, unrefreshing sleep, generalized malaise. Orthostatic intolerance has also been identified as a symptom.4
The cause of CFS is unknown, although its onset usually follows an illness.1 The diagnosis is challenging, as there is no definitive test. If the criteria are met, however, a diagnosis of CFS should be given. This allows the patient to feel validated and can foster a better physician-patient relationship.
Treatment for CFS is symptomatic. The first step is good “sleep hygiene.” Reducing or eliminating caffeine and promoting exercise and a healthy diet all contribute to better sleep. Also, the blue light emitted by electronic devices suppresses melatonin and makes it more difficult to fall asleep.5
Resuming normal activity and improving school attendance is the goal achieved through graded exercises, behavioral therapy, and management of pain with acetaminophen and NSAIDs. Tricyclic antidepressants have been studied in CFS but their effectiveness has not been proven.1 Significant improvements are seen in 50% of teens who are appropriately diagnosed and adhere to treatments.1
CFS is a debilitating disorder that can be frustrating to treat. Acknowledging CFS as a legitimate syndrome can aid in treatment by fostering a good physician-patient relationship.
References
1. Findlay, SM. “The Tired Teen: A Review of the Assessment and Management of the Adolescent with Sleepiness and Fatigue” Paediatr Child Health. 2008 Jan;13(1):37-42. Print.
2. Prevalence of Childhood Obesity in the United States, 2011-2012, CDC.
3. Nijhof SL, et al. “Adolescent Chronic Fatigue Syndrome: Prevalence, Incidence, and Morbidity” Pediatrics. 2011 May;127(5):e1169-e1175.
4. Orthostatic intolerance in adolescent chronic fatigue syndrome. Stewart JM, et al. Pediatrics. 1999 Jan;103(1):116-21.
5. “The impact of light from computer monitors on melatonin levels in college students” Figueiro MG, et al. Neuro Endocrinol Lett. 2011;32(2):158-163.
In an increasingly sleepless society inundated with caffeine drinks and social media, it is not surprising that 15%-30% of teens present with complaints of fatigue.1 As fatigue can be a symptom of many disorders, a careful work-up is important.
Fewer than 8 hours’ sleep, large intake of caffeine, and several hours on social media each day are linked with fatigue. Poor eating habits are more likely than not; nearly 21% of teens are overweight.2 Video games have also contributed to symptoms of fatigue by encouraging a sedentary life style.
The work-up for teens with fatigue requires a detailed history of present symptoms and a careful review of systems, which should specifically address weight gain or loss, menstrual change, palpitations, and respiratory changes. It is important to screen for signs of stress and depression.
The physical exam should identify systems that need further work-up, and a standard serum screen would include a complete blood cell count (CBC), complete metabolic panel (CMP), and a thyroid panel.
As many experienced physicians can attest, more often than not, this work-up results in normal findings. Suggesting that the basis for fatigue may be a psychiatric cause is usually not well received.
Another consideration is a diagnosis of chronic fatigue syndrome (CFS), a well-documented disorder with defined diagnostic criteria since 1994. Although CFS is more common in adults, research now reveals an annual incidence of 0.5% and a prevalence of 0.19%-1.29% in teens.3 The characteristics are severe and disabling new-onset fatigue lasting greater than 6 months and four of the following symptoms: impaired memory, sore throat, tender cervical and axillary lymph nodes, muscle pain, headaches, unrefreshing sleep, generalized malaise. Orthostatic intolerance has also been identified as a symptom.4
The cause of CFS is unknown, although its onset usually follows an illness.1 The diagnosis is challenging, as there is no definitive test. If the criteria are met, however, a diagnosis of CFS should be given. This allows the patient to feel validated and can foster a better physician-patient relationship.
Treatment for CFS is symptomatic. The first step is good “sleep hygiene.” Reducing or eliminating caffeine and promoting exercise and a healthy diet all contribute to better sleep. Also, the blue light emitted by electronic devices suppresses melatonin and makes it more difficult to fall asleep.5
Resuming normal activity and improving school attendance is the goal achieved through graded exercises, behavioral therapy, and management of pain with acetaminophen and NSAIDs. Tricyclic antidepressants have been studied in CFS but their effectiveness has not been proven.1 Significant improvements are seen in 50% of teens who are appropriately diagnosed and adhere to treatments.1
CFS is a debilitating disorder that can be frustrating to treat. Acknowledging CFS as a legitimate syndrome can aid in treatment by fostering a good physician-patient relationship.
References
1. Findlay, SM. “The Tired Teen: A Review of the Assessment and Management of the Adolescent with Sleepiness and Fatigue” Paediatr Child Health. 2008 Jan;13(1):37-42. Print.
2. Prevalence of Childhood Obesity in the United States, 2011-2012, CDC.
3. Nijhof SL, et al. “Adolescent Chronic Fatigue Syndrome: Prevalence, Incidence, and Morbidity” Pediatrics. 2011 May;127(5):e1169-e1175.
4. Orthostatic intolerance in adolescent chronic fatigue syndrome. Stewart JM, et al. Pediatrics. 1999 Jan;103(1):116-21.
5. “The impact of light from computer monitors on melatonin levels in college students” Figueiro MG, et al. Neuro Endocrinol Lett. 2011;32(2):158-163.
In an increasingly sleepless society inundated with caffeine drinks and social media, it is not surprising that 15%-30% of teens present with complaints of fatigue.1 As fatigue can be a symptom of many disorders, a careful work-up is important.
Fewer than 8 hours’ sleep, large intake of caffeine, and several hours on social media each day are linked with fatigue. Poor eating habits are more likely than not; nearly 21% of teens are overweight.2 Video games have also contributed to symptoms of fatigue by encouraging a sedentary life style.
The work-up for teens with fatigue requires a detailed history of present symptoms and a careful review of systems, which should specifically address weight gain or loss, menstrual change, palpitations, and respiratory changes. It is important to screen for signs of stress and depression.
The physical exam should identify systems that need further work-up, and a standard serum screen would include a complete blood cell count (CBC), complete metabolic panel (CMP), and a thyroid panel.
As many experienced physicians can attest, more often than not, this work-up results in normal findings. Suggesting that the basis for fatigue may be a psychiatric cause is usually not well received.
Another consideration is a diagnosis of chronic fatigue syndrome (CFS), a well-documented disorder with defined diagnostic criteria since 1994. Although CFS is more common in adults, research now reveals an annual incidence of 0.5% and a prevalence of 0.19%-1.29% in teens.3 The characteristics are severe and disabling new-onset fatigue lasting greater than 6 months and four of the following symptoms: impaired memory, sore throat, tender cervical and axillary lymph nodes, muscle pain, headaches, unrefreshing sleep, generalized malaise. Orthostatic intolerance has also been identified as a symptom.4
The cause of CFS is unknown, although its onset usually follows an illness.1 The diagnosis is challenging, as there is no definitive test. If the criteria are met, however, a diagnosis of CFS should be given. This allows the patient to feel validated and can foster a better physician-patient relationship.
Treatment for CFS is symptomatic. The first step is good “sleep hygiene.” Reducing or eliminating caffeine and promoting exercise and a healthy diet all contribute to better sleep. Also, the blue light emitted by electronic devices suppresses melatonin and makes it more difficult to fall asleep.5
Resuming normal activity and improving school attendance is the goal achieved through graded exercises, behavioral therapy, and management of pain with acetaminophen and NSAIDs. Tricyclic antidepressants have been studied in CFS but their effectiveness has not been proven.1 Significant improvements are seen in 50% of teens who are appropriately diagnosed and adhere to treatments.1
CFS is a debilitating disorder that can be frustrating to treat. Acknowledging CFS as a legitimate syndrome can aid in treatment by fostering a good physician-patient relationship.
References
1. Findlay, SM. “The Tired Teen: A Review of the Assessment and Management of the Adolescent with Sleepiness and Fatigue” Paediatr Child Health. 2008 Jan;13(1):37-42. Print.
2. Prevalence of Childhood Obesity in the United States, 2011-2012, CDC.
3. Nijhof SL, et al. “Adolescent Chronic Fatigue Syndrome: Prevalence, Incidence, and Morbidity” Pediatrics. 2011 May;127(5):e1169-e1175.
4. Orthostatic intolerance in adolescent chronic fatigue syndrome. Stewart JM, et al. Pediatrics. 1999 Jan;103(1):116-21.
5. “The impact of light from computer monitors on melatonin levels in college students” Figueiro MG, et al. Neuro Endocrinol Lett. 2011;32(2):158-163.
2016 Update on female sexual dysfunction
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.
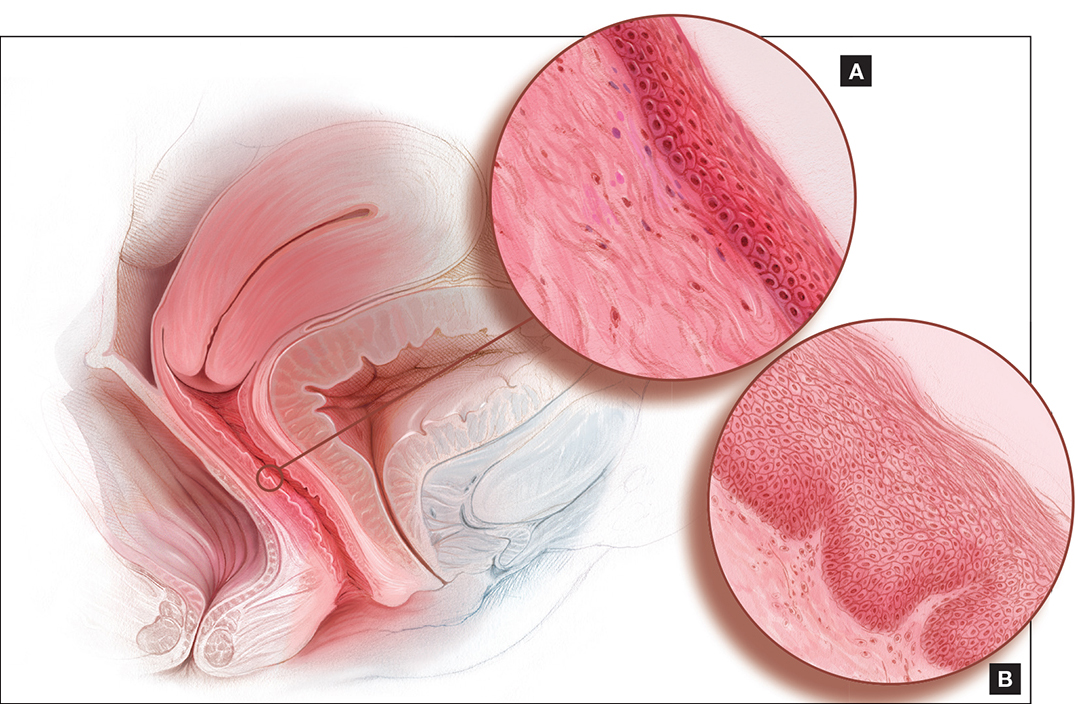
Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
In This Article
- This roundtable's expert panel
- Dyspareunia and low sexual desire in a breast cancer survivor
- Laser treatment and vaginal health
Peanut-allergic preschoolers benefit from oral immunotherapy
Early intervention oral immunotherapy (OIT) improved a majority of peanut-allergic preschoolers’ ability to eat peanut protein with no reaction, based on data from a randomized trial of 40 children aged 9-36 months.
“We postulated that targeting newly diagnosed young peanut-allergic children would provide the best opportunity to enhance the clinical effectiveness of OIT as an immunomodulatory and disease-modifying treatment by interrupting allergic priming before its full maturation,” wrote Brian P. Vickery, MD, of the University of North Carolina, Chapel Hill, and his colleagues.
The children received doses of either 300 mg/day or 3,000 mg/day of peanut protein for an average of 29 months. Overall, 78% of the 37 children in the intent-to-treat analysis met the primary endpoint of unresponsiveness to peanut protein 4 weeks after discontinuing oral immunotherapy (85% of the 300-mg group and 71% of the 3,000-mg group). Peanut-specific levels of IgE dropped significantly in the treatment group, and the treated children were 19 times more likely to eat 5 g of peanut protein without reaction than were 154 untreated matched controls.
Three children discontinued the study because of treatment-related adverse events, but no treatment-related severe adverse events, hospitalizations, or deaths were reported.
The findings suggest “that allergic responses may be more easily and durably corrected in young children, and that in this context, relatively low OIT doses are sufficiently potent in suppressing IgE responses and stimulating IgG4 production,” the researchers said.
Find the full study here in the Journal of Allergy and Clinical Immunology (2016 Aug. doi: 10.1016/j.jaci.2016.05.027).
Early intervention oral immunotherapy (OIT) improved a majority of peanut-allergic preschoolers’ ability to eat peanut protein with no reaction, based on data from a randomized trial of 40 children aged 9-36 months.
“We postulated that targeting newly diagnosed young peanut-allergic children would provide the best opportunity to enhance the clinical effectiveness of OIT as an immunomodulatory and disease-modifying treatment by interrupting allergic priming before its full maturation,” wrote Brian P. Vickery, MD, of the University of North Carolina, Chapel Hill, and his colleagues.
The children received doses of either 300 mg/day or 3,000 mg/day of peanut protein for an average of 29 months. Overall, 78% of the 37 children in the intent-to-treat analysis met the primary endpoint of unresponsiveness to peanut protein 4 weeks after discontinuing oral immunotherapy (85% of the 300-mg group and 71% of the 3,000-mg group). Peanut-specific levels of IgE dropped significantly in the treatment group, and the treated children were 19 times more likely to eat 5 g of peanut protein without reaction than were 154 untreated matched controls.
Three children discontinued the study because of treatment-related adverse events, but no treatment-related severe adverse events, hospitalizations, or deaths were reported.
The findings suggest “that allergic responses may be more easily and durably corrected in young children, and that in this context, relatively low OIT doses are sufficiently potent in suppressing IgE responses and stimulating IgG4 production,” the researchers said.
Find the full study here in the Journal of Allergy and Clinical Immunology (2016 Aug. doi: 10.1016/j.jaci.2016.05.027).
Early intervention oral immunotherapy (OIT) improved a majority of peanut-allergic preschoolers’ ability to eat peanut protein with no reaction, based on data from a randomized trial of 40 children aged 9-36 months.
“We postulated that targeting newly diagnosed young peanut-allergic children would provide the best opportunity to enhance the clinical effectiveness of OIT as an immunomodulatory and disease-modifying treatment by interrupting allergic priming before its full maturation,” wrote Brian P. Vickery, MD, of the University of North Carolina, Chapel Hill, and his colleagues.
The children received doses of either 300 mg/day or 3,000 mg/day of peanut protein for an average of 29 months. Overall, 78% of the 37 children in the intent-to-treat analysis met the primary endpoint of unresponsiveness to peanut protein 4 weeks after discontinuing oral immunotherapy (85% of the 300-mg group and 71% of the 3,000-mg group). Peanut-specific levels of IgE dropped significantly in the treatment group, and the treated children were 19 times more likely to eat 5 g of peanut protein without reaction than were 154 untreated matched controls.
Three children discontinued the study because of treatment-related adverse events, but no treatment-related severe adverse events, hospitalizations, or deaths were reported.
The findings suggest “that allergic responses may be more easily and durably corrected in young children, and that in this context, relatively low OIT doses are sufficiently potent in suppressing IgE responses and stimulating IgG4 production,” the researchers said.
Find the full study here in the Journal of Allergy and Clinical Immunology (2016 Aug. doi: 10.1016/j.jaci.2016.05.027).
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Congenital Zika virus associated with sensorineural hearing loss
Congenital Zika virus infection may be associated with sensorineural hearing loss, according to the latest Morbidity and Mortality Weekly Report published by the CDC.
“In the majority of cases of hearing loss associated with congenital viral infection, the damage to the auditory system is within the cochlea,” wrote the authors of the MMWR, led by Mariana C. Leal, PhD of the Hospital Agamenon Magalhães in Recife, Brazil. “It is likely that similar lesions account for the hearing deficit in children with congenital Zika virus infection” (MMWR. 2016 Aug 30.65:1-4)
Full auditory function evaluations were performed on 70 children born with microcephaly, all of whom had confirmed laboratory evidence of congenital Zika virus. One child with bilateral profound sensorineural hearing loss was excluded because the child had already received treatment with amikacin (a known ototoxic antibiotic) prior to evaluation for this study. All children were ages 0-10 months; investigators defined Zika-associated microcephaly as head circumference of 32 cm or lower at birth. Gestational ages at birth ranged from 37 weeks to 1 day shy of 42 weeks.
Of the 69 children included for analysis, four (5.8%) were found to have sensorineural hearing loss with no other potential cause, which the investigators noted is “within the range (6%-65%) reported for other congenital viral infections.” The investigators also stated that the auditory issues were mainly evident in children whose mothers experienced a rash illness during the first trimester of their pregnancy.
“Children with evidence of congenital Zika virus infection who have normal initial screening tests should receive regular follow-up, because onset of hearing loss associated with other congenital viral infections can be delayed and the loss can be progressive,” the authors noted.
No disclosures or funding sources were reported.
Congenital Zika virus infection may be associated with sensorineural hearing loss, according to the latest Morbidity and Mortality Weekly Report published by the CDC.
“In the majority of cases of hearing loss associated with congenital viral infection, the damage to the auditory system is within the cochlea,” wrote the authors of the MMWR, led by Mariana C. Leal, PhD of the Hospital Agamenon Magalhães in Recife, Brazil. “It is likely that similar lesions account for the hearing deficit in children with congenital Zika virus infection” (MMWR. 2016 Aug 30.65:1-4)
Full auditory function evaluations were performed on 70 children born with microcephaly, all of whom had confirmed laboratory evidence of congenital Zika virus. One child with bilateral profound sensorineural hearing loss was excluded because the child had already received treatment with amikacin (a known ototoxic antibiotic) prior to evaluation for this study. All children were ages 0-10 months; investigators defined Zika-associated microcephaly as head circumference of 32 cm or lower at birth. Gestational ages at birth ranged from 37 weeks to 1 day shy of 42 weeks.
Of the 69 children included for analysis, four (5.8%) were found to have sensorineural hearing loss with no other potential cause, which the investigators noted is “within the range (6%-65%) reported for other congenital viral infections.” The investigators also stated that the auditory issues were mainly evident in children whose mothers experienced a rash illness during the first trimester of their pregnancy.
“Children with evidence of congenital Zika virus infection who have normal initial screening tests should receive regular follow-up, because onset of hearing loss associated with other congenital viral infections can be delayed and the loss can be progressive,” the authors noted.
No disclosures or funding sources were reported.
Congenital Zika virus infection may be associated with sensorineural hearing loss, according to the latest Morbidity and Mortality Weekly Report published by the CDC.
“In the majority of cases of hearing loss associated with congenital viral infection, the damage to the auditory system is within the cochlea,” wrote the authors of the MMWR, led by Mariana C. Leal, PhD of the Hospital Agamenon Magalhães in Recife, Brazil. “It is likely that similar lesions account for the hearing deficit in children with congenital Zika virus infection” (MMWR. 2016 Aug 30.65:1-4)
Full auditory function evaluations were performed on 70 children born with microcephaly, all of whom had confirmed laboratory evidence of congenital Zika virus. One child with bilateral profound sensorineural hearing loss was excluded because the child had already received treatment with amikacin (a known ototoxic antibiotic) prior to evaluation for this study. All children were ages 0-10 months; investigators defined Zika-associated microcephaly as head circumference of 32 cm or lower at birth. Gestational ages at birth ranged from 37 weeks to 1 day shy of 42 weeks.
Of the 69 children included for analysis, four (5.8%) were found to have sensorineural hearing loss with no other potential cause, which the investigators noted is “within the range (6%-65%) reported for other congenital viral infections.” The investigators also stated that the auditory issues were mainly evident in children whose mothers experienced a rash illness during the first trimester of their pregnancy.
“Children with evidence of congenital Zika virus infection who have normal initial screening tests should receive regular follow-up, because onset of hearing loss associated with other congenital viral infections can be delayed and the loss can be progressive,” the authors noted.
No disclosures or funding sources were reported.
FROM THE CENTERS FOR DISEASE CONTROL AND PREVENTION
Key clinical point: Congenital Zika virus could be associated with sensorineural hearing loss in infants.
Major finding: 4 of 69 children (5.8%) with microcephaly and confirmed congenital Zika virus infection had sensorineural hearing loss without evidence of any other possible causes.
Data source: Retrospective analysis of 70 children born with microcephaly in Brazil from Nov. 2015 through May 2016.
Disclosures: No disclosures or funding source reported.
VIDEO: Functional noninvasive imaging cuts unnecessary angiography
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
The results from CE-MARC2 very nicely showed that imaging-guided angiography is as safe as compulsory angiography in the highest-risk subgroup of the enrolled patients, those with a pretest probability of 61%-90% for having coronary artery disease. Findings from the economic analysis of this study that remains pending will be crucial for eventually recommending one strategy over the other in this setting.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Udo Sechtem |
The 12-month rate of the hardest clinical endpoints measured in this study, cardiovascular deaths and MIs, was very low in this study: 1.3% in the patients managed with NICE guidance, 1% in those who first underwent cardiovascular MR, and 0.8% in the patients who first underwent myocardial perfusion scintigraphy. Despite this low risk, the patients in each of the three arms of the study underwent roughly 500 test procedures.
We should therefore consider a totally different approach. Instead of immediately performing a noninvasive test or the tests called for by the NICE guidelines, what about no testing at all. Instead, patients would first undergo optimal preventive and symptomatic medical treatments. If patients failed this strategy they then could be considered for revascularization. I propose a study that would compare imaging-guided conditional angiography, as tested in CE-MARC2, with symptom-guided conditional angiography. Functional, noninvasive testing for all needs to be compared against optimal management and symptom driven interventions.
Udo Sechtem, Dr Med, is head of cardiology at the Robert-Bosch-Hospital in Stuttgart, Germany. He made these comments as the designated discussant for the study. He had no disclosures.
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ROME – Functional, noninvasive cardiac imaging using cardiovascular MR or myocardial perfusion scintigraphy was significantly better than was a current and well regarded guideline-based approach to identifying patients with chest pain and suspected coronary artery disease who could safely avoid angiography, thereby cutting the rate of unnecessary angiography by about 75%.
Following the guideline formula adopted by the British National Institute for Health and Care Excellence (NICE) resulted in a 29% rate of unnecessary angiography compared with rates of 7.5% using cardiovascular MR (CMR) and 7.1% using myocardial perfusion scintigraphy (MPS) in a multicenter randomized trial with 1,202 patients, John P. Greenwood, MBChB, said at the annual congress of the European Society of Cardiology.
This universal use of a functional, noninvasive imaging strategy to guide angiography resulted in no significant penalty of missed coronary disease or subsequent coronary events. The rate of positive angiography findings was 12% among the 240 patients managed according to the NICE guidelines, 10% among 481 patients screened by CMR, and 9% among the 481 patients screened using MPS, reported Dr. Greenwood, professor of cardiology at the University of Leeds (England). The rate of major adverse coronary events after 12 months of follow-up were 3% following the NICE protocol and 4% when screening by CMR or with MPS.
Concurrently with Dr. Greenwood’s report, the findings from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease 2 (CE-MARC2) study appeared in an article online (JAMA. 2016 Aug 29. doi: 10.1001/jama.2016.12680).
“We showed that a functional test with CMR or MPS can reduce the rate of unnecessary coronary angiography. Cutting unnecessary angiography is really important to patients, and it may also cost effective,” he said, but cautioned that a formal cost analysis of the options tested in this study is still being run.
The NICE guidelines manage patients with chest pain that could be angina by their pretest probability of having coronary artery disease (CAD), and at the time the study was designed the NICE guidelines, issued in 2010, provided the most up-to-date expert guidance on how to triage these patients. The study enrolled patients with a pretest probability for CAD of 10%-90%; collectively their average probability was 50%. The patients participated in the study at one of six U.K. centers during November 2012 to March 2015. The average age was 56 years.
MPS is “probably the noninvasive imaging approach most commonly used worldwide to detect coronary ischemia,” Dr. Greenwood said. But he led an earlier study that showed that CMR, using a gadolinium-based tracing agent, works even better than MPS (in this study single photon emission CT) to predict a patient’s risk for major cardiac events. He said this superiority is probably because of the greater spatial resolution with CMR.
“The higher spatial resolution of CMR, about 5- to 10-fold greater that MPS, is less likely to produce false negative results,” he said in an interview. “We showed that CMR has higher diagnostic accuracy, is a better prognosticator, and is more cost effective” than MPS. Dr. Greenwood attributed the similar performance of CMR and MPS in CE-MARC2 to the study’s design, which led to fewer patients undergoing each of the two imaging methods and made CE-MARC2 underpowered to discern a difference in specificity. In his earlier study, which included 752 patients who underwent examination with both CMR and MPS, the negative predictive value of CMR was 91% compared with 79% with MPS.
CMR uses conventional MR machines, is now widely available, and is being widely used today as a first-line test in the United Kingdom and Europe, he added.
Dr. Greenwood believes that in his new study functional imaging outperformed the NICE guidelines because the pretest models used in the guidelines “tend to overestimate risk,” the factor that produces angiography overuse.
His report included two additional analyses that assessed the impact of CMR and MPS in the subgroup of patients with a high pretest probability for CAD, 61%-90%, and in the subgroup with a low pretest probability, 10%-29%. Among the patients with a high likelihood for CAD the two functional imaging methods cut the rate of unnecessary angiography by 95%, a statistically significant difference. Among those with a low likelihood functional imaging cut the rate 56%, a difference that did not reach statistical significance.
[email protected]
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2016
Key clinical point: Screening patients with suspected angina via cardiovascular MR or myocardial perfusion imaging substantially reduced the rate of unnecessary angiography compared with the screening algorithm currently endorsed by British national guidelines.
Major finding: The unnecessary angiography rate was 29% with the guideline algorithm, 7.5% with cardiovascular MR, and 7.1% with myocardial perfusion scintigraphy.
Data source: CE MARC2, a multicenter, randomized trial with 1,202 patients.
Disclosures: Dr. Greenwood had no disclosures.
Medical errors: Meeting ethical obligations and reducing liability with proper communication
In her position as Chief Medical Officer, Dr. Patrice Weiss leads efforts not only to assure clinical excellence from the more than 900 clinicians at the Carilion Clinic in Roanoke, Virginia, but also to improve patient experience. She lectures extensively on one of her passions in medicine: medical errors, and the concept of the second victim. OBG
OBG Management: What is the definition of a medical error?
Patrice M. Weiss, MD: Clinicians may be somewhat surprised to learn that there is no universal definition of a medical error that sets standardized nomenclature. The Institute of Medicine, in its landmark work To Err Is Human, adopted this definition: “failure of a planned action to be completed as intended, or the use of a wrong plan to achieve an aim.”1
In general terms, a medical error is an act of commission or omission, meaning that something was done or not done, that has negative consequences for the patient and is judged as wrong by our peers. An unanticipated outcome can be due to a medical error or can occur without a medical error. An unpredicted side effect, for instance—one that may have a low probability of drug–drug interaction or drug reaction occurrence—is an unexpected outcome. If the incidence of a drug reaction is 1 in 1,000 and your patient is that one, it does not necessarily mean that there was a medical error.
Often, if the outcome is unanticipated, patients and their families will assume, rightly or wrongly, that a medical error did occur.
OBG Management: Are physicians required to disclose medical errors?
Dr. Weiss: Yes. The Joint Commission’s standard principle states that the responsible licensed independent practitioner, or his or her designee, clearly explain the outcome of any treatment or procedure to the patient and, when appropriate, the patient’s family, whenever those outcomes differ significantly from the anticipated outcome.2
This can even include unanticipated outcomes that are not due to an error. Specifically speaking about medical errors, however, we do have the responsibility, both from this standard and from a professional and ethical standard to disclose what, why, and how the error occurred and what we are going to do to ensure it does not happen again.
OBG Management: How does a physician best communicate to a patient an unanticipated outcome that was not due to a medical error?
Dr. Weiss: Usually we as health care providers are more comfortable talking about unanticipated outcomes without medical errors. It is important to, when speaking with patients, be clear and concise, describing what you best know at the time, in language that patients can understand. I often jokingly say that, at a minimum, all of us in health care are bilingual: We speak our native language, and we speak “medicine.”
After describing unanticipated outcomes to patients and their families in terms they understand, affirm their understanding with a follow-up open-ended question. “Do you understand what I just said to you?” is ineffective. A better approach is saying, “Mrs. Jones, in your own words, will you describe back to me what your understanding is as to why this happened?” The answer received will allow you to know the patient’s level of understanding. It also will give you the opportunity to clear up points that are not clear or were misinterpreted. Do not leave patients feeling in a “lurch,” left to wonder or with a lack of understanding, or worse yet, with a sense that you are holding something back.
OBG Management: What is the best approach to disclosing an unanticipated outcome that was due to a medical error?
Dr. Weiss: First and foremost, you must be certain that a medical error did actually occur. There can be speculation at first, and that speculation should occur behind the scenes, with peer review or a root cause analysis on the event. Speculation should not enter into your conversation with the patient. Notional language can add to their anxiety, create mistrust on the patient’s part, and perhaps make a patient feel as if you are not giving the answers that he or she needs.
When it is believed that a medical error did occur, there are several things that need to be done:
- Gather as much information at the event as possible.
- Notify the hospital (ie, risk management or quality or patient safety). This is important because it is an organizational approach to medical errors when they do occur.
- Support the patient and the patient’s family through the entire process. Speaking to the patient and the family may be a part of ascertaining what happened and contributing factors. Clinicians have said to me, “Well, I can’t really go talk to the patient or the patient’s family right now because I don’t really know everything that happened.” Keep in mind, however, that the longer you wait to talk to the patient and family, the more time they have to speculate and to ask other people, perhaps those not involved and with no knowledge of, what happened.
OBG Management: What is the best timing and location for the disclosure conversation?
Dr. Weiss: The person who is responsible for the patient who was involved in the medical error needs to have the disclosure conversation. The conversation with the patient, and the family if the patient so desires, should occur as soon as possible. However, take into consideration the patient being awake, coherent, and not under the influence of medications. With those caveats, the best time to speak is when it is convenient for the patient. Do not plug this conversation into a 10-minute opening in your busy schedule. The conversation could take an hour, or it could take 15 minutes. It should not be conducted as a matter of convenience to the clinician.
In addition, often times the recovery room is not the best location—it is not private and confidential, and the patient is still groggy and will be unable to remember most of what is said or ask questions as needed.
OBG Management: You advocate a “TEAM” approach when speaking with the patient. What is TEAM?
Dr. Weiss: Disclosure conversations are not easy to have. The patient and the patient’s family are often upset. Medical errors challenge a physician’s humility and integrity, and they can lead to questioning of one’s own ability. I adopted the helpful pneumonic TEAM after first learning about it at what is now known as the Institute for Healthcare Communication. It refers to what parties need to be notified and who needs to be present with you when having a disclosure conversation. Of course, you want someone there who not only can serve as a witness but also can help facilitate the gathering of answers for questions that will be asked. The best person for this job could be the lead physician involved in the care of the patient, a hospital risk manager, a colleague, or the patient safety officer.
The “T” stands for truthful. When you begin the conversation, tell the patient at that point what you know to be true and what you know may have happened or definitely did not happen that contributed to the outcome. Again, do not speculate in answering the patient’s questions. A good approach is to say, “This is what I know happened. As of right now, this is what I know may have contributed or did contribute. We are going to be looking into this more thoroughly. As I learn more, you will be the first to know.”
These are not one-time conversations. As you do learn more, circle back and talk to the patient and family. This can be a dialogue that goes on for weeks or even months.
“E” equals empathy. Allow the patient and the patient’s family to ventilate. Try to understand what is it that they are most upset about, and try to soothe these upset feelings. For example, do not make the assumption that they are most upset about paying for a surgery in which there was a medical error. In fact, they really may be most upset about staying in the hospital 2 additional days, and they are going to now miss the visit of a relative, their child’s graduation, or something important to them.
Let patients talk. Do not interrupt them. Do not stand over them while they are in the bed; sit down at eye level with them. Talk in a voice and tone that the patient understands and try to soothe and empathetically relate to what is being said.
The “A” is important: Apology. There are 2 things that patients want when a medical error occurs: 1) to hear the clinician say, “I’m sorry”—and you should be sorry if a medical error occurred, and you should say that you are sorry this happened—and 2) what you or your organization is going to do so that this does not happen to the next person. Incorporate these 2 factors into the apology piece.
OBG Management: Can saying, “I’m sorry” expose a clinician unnecessarily to malpractice risk?
Dr. Weiss: Saying “I’m sorry,” of course, has come under a lot of scrutiny. There are various state laws, and you should be aware of your state’s apology laws. In many states an apology, with “I’m sorry,” cannot be used against a provider. However, there is not 100% absolution of the event if an apology occurs. In other words, “I’m sorry” cannot be held against you, but saying “I’m sorry” does not negate the error that occurred.
Even when practicing in a state in which there is not an apology law, however, and a clinician does apologize and that apology comes up in the legal setting of a true medical error, we would need to ask, is it really that bad that an apology was made on behalf of the medical error that was committed? Isn’t that compassion? Isn’t that empathy? Isn’t that showing that I as the physician care for the patient and the medical team cares for the patient?
Finally, abide by the disclosure policy and standards of your organization.
OBG Management: What does the “M” in TEAM stand for?
Dr. Weiss: Management. There may be times when a medical error occurs that the patient or the patient’s family are angry and upset to the point that they no longer want you to continue to care for them. Be empathetic and helpful by offering to assist them in finding someone else to continue to provide their care. Also let them know that you are more than happy to continue to care for them and assist them in their healing and restoration to health in any way that you can: “Of course the ongoing management of your care is your decision, and we will do whatever your wishes are.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed August 22, 2016.
- The Joint Commission on Health Care Accreditation, Patient Safety Standard RI.1.2.2.
In her position as Chief Medical Officer, Dr. Patrice Weiss leads efforts not only to assure clinical excellence from the more than 900 clinicians at the Carilion Clinic in Roanoke, Virginia, but also to improve patient experience. She lectures extensively on one of her passions in medicine: medical errors, and the concept of the second victim. OBG
OBG Management: What is the definition of a medical error?
Patrice M. Weiss, MD: Clinicians may be somewhat surprised to learn that there is no universal definition of a medical error that sets standardized nomenclature. The Institute of Medicine, in its landmark work To Err Is Human, adopted this definition: “failure of a planned action to be completed as intended, or the use of a wrong plan to achieve an aim.”1
In general terms, a medical error is an act of commission or omission, meaning that something was done or not done, that has negative consequences for the patient and is judged as wrong by our peers. An unanticipated outcome can be due to a medical error or can occur without a medical error. An unpredicted side effect, for instance—one that may have a low probability of drug–drug interaction or drug reaction occurrence—is an unexpected outcome. If the incidence of a drug reaction is 1 in 1,000 and your patient is that one, it does not necessarily mean that there was a medical error.
Often, if the outcome is unanticipated, patients and their families will assume, rightly or wrongly, that a medical error did occur.
OBG Management: Are physicians required to disclose medical errors?
Dr. Weiss: Yes. The Joint Commission’s standard principle states that the responsible licensed independent practitioner, or his or her designee, clearly explain the outcome of any treatment or procedure to the patient and, when appropriate, the patient’s family, whenever those outcomes differ significantly from the anticipated outcome.2
This can even include unanticipated outcomes that are not due to an error. Specifically speaking about medical errors, however, we do have the responsibility, both from this standard and from a professional and ethical standard to disclose what, why, and how the error occurred and what we are going to do to ensure it does not happen again.
OBG Management: How does a physician best communicate to a patient an unanticipated outcome that was not due to a medical error?
Dr. Weiss: Usually we as health care providers are more comfortable talking about unanticipated outcomes without medical errors. It is important to, when speaking with patients, be clear and concise, describing what you best know at the time, in language that patients can understand. I often jokingly say that, at a minimum, all of us in health care are bilingual: We speak our native language, and we speak “medicine.”
After describing unanticipated outcomes to patients and their families in terms they understand, affirm their understanding with a follow-up open-ended question. “Do you understand what I just said to you?” is ineffective. A better approach is saying, “Mrs. Jones, in your own words, will you describe back to me what your understanding is as to why this happened?” The answer received will allow you to know the patient’s level of understanding. It also will give you the opportunity to clear up points that are not clear or were misinterpreted. Do not leave patients feeling in a “lurch,” left to wonder or with a lack of understanding, or worse yet, with a sense that you are holding something back.
OBG Management: What is the best approach to disclosing an unanticipated outcome that was due to a medical error?
Dr. Weiss: First and foremost, you must be certain that a medical error did actually occur. There can be speculation at first, and that speculation should occur behind the scenes, with peer review or a root cause analysis on the event. Speculation should not enter into your conversation with the patient. Notional language can add to their anxiety, create mistrust on the patient’s part, and perhaps make a patient feel as if you are not giving the answers that he or she needs.
When it is believed that a medical error did occur, there are several things that need to be done:
- Gather as much information at the event as possible.
- Notify the hospital (ie, risk management or quality or patient safety). This is important because it is an organizational approach to medical errors when they do occur.
- Support the patient and the patient’s family through the entire process. Speaking to the patient and the family may be a part of ascertaining what happened and contributing factors. Clinicians have said to me, “Well, I can’t really go talk to the patient or the patient’s family right now because I don’t really know everything that happened.” Keep in mind, however, that the longer you wait to talk to the patient and family, the more time they have to speculate and to ask other people, perhaps those not involved and with no knowledge of, what happened.
OBG Management: What is the best timing and location for the disclosure conversation?
Dr. Weiss: The person who is responsible for the patient who was involved in the medical error needs to have the disclosure conversation. The conversation with the patient, and the family if the patient so desires, should occur as soon as possible. However, take into consideration the patient being awake, coherent, and not under the influence of medications. With those caveats, the best time to speak is when it is convenient for the patient. Do not plug this conversation into a 10-minute opening in your busy schedule. The conversation could take an hour, or it could take 15 minutes. It should not be conducted as a matter of convenience to the clinician.
In addition, often times the recovery room is not the best location—it is not private and confidential, and the patient is still groggy and will be unable to remember most of what is said or ask questions as needed.
OBG Management: You advocate a “TEAM” approach when speaking with the patient. What is TEAM?
Dr. Weiss: Disclosure conversations are not easy to have. The patient and the patient’s family are often upset. Medical errors challenge a physician’s humility and integrity, and they can lead to questioning of one’s own ability. I adopted the helpful pneumonic TEAM after first learning about it at what is now known as the Institute for Healthcare Communication. It refers to what parties need to be notified and who needs to be present with you when having a disclosure conversation. Of course, you want someone there who not only can serve as a witness but also can help facilitate the gathering of answers for questions that will be asked. The best person for this job could be the lead physician involved in the care of the patient, a hospital risk manager, a colleague, or the patient safety officer.
The “T” stands for truthful. When you begin the conversation, tell the patient at that point what you know to be true and what you know may have happened or definitely did not happen that contributed to the outcome. Again, do not speculate in answering the patient’s questions. A good approach is to say, “This is what I know happened. As of right now, this is what I know may have contributed or did contribute. We are going to be looking into this more thoroughly. As I learn more, you will be the first to know.”
These are not one-time conversations. As you do learn more, circle back and talk to the patient and family. This can be a dialogue that goes on for weeks or even months.
“E” equals empathy. Allow the patient and the patient’s family to ventilate. Try to understand what is it that they are most upset about, and try to soothe these upset feelings. For example, do not make the assumption that they are most upset about paying for a surgery in which there was a medical error. In fact, they really may be most upset about staying in the hospital 2 additional days, and they are going to now miss the visit of a relative, their child’s graduation, or something important to them.
Let patients talk. Do not interrupt them. Do not stand over them while they are in the bed; sit down at eye level with them. Talk in a voice and tone that the patient understands and try to soothe and empathetically relate to what is being said.
The “A” is important: Apology. There are 2 things that patients want when a medical error occurs: 1) to hear the clinician say, “I’m sorry”—and you should be sorry if a medical error occurred, and you should say that you are sorry this happened—and 2) what you or your organization is going to do so that this does not happen to the next person. Incorporate these 2 factors into the apology piece.
OBG Management: Can saying, “I’m sorry” expose a clinician unnecessarily to malpractice risk?
Dr. Weiss: Saying “I’m sorry,” of course, has come under a lot of scrutiny. There are various state laws, and you should be aware of your state’s apology laws. In many states an apology, with “I’m sorry,” cannot be used against a provider. However, there is not 100% absolution of the event if an apology occurs. In other words, “I’m sorry” cannot be held against you, but saying “I’m sorry” does not negate the error that occurred.
Even when practicing in a state in which there is not an apology law, however, and a clinician does apologize and that apology comes up in the legal setting of a true medical error, we would need to ask, is it really that bad that an apology was made on behalf of the medical error that was committed? Isn’t that compassion? Isn’t that empathy? Isn’t that showing that I as the physician care for the patient and the medical team cares for the patient?
Finally, abide by the disclosure policy and standards of your organization.
OBG Management: What does the “M” in TEAM stand for?
Dr. Weiss: Management. There may be times when a medical error occurs that the patient or the patient’s family are angry and upset to the point that they no longer want you to continue to care for them. Be empathetic and helpful by offering to assist them in finding someone else to continue to provide their care. Also let them know that you are more than happy to continue to care for them and assist them in their healing and restoration to health in any way that you can: “Of course the ongoing management of your care is your decision, and we will do whatever your wishes are.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In her position as Chief Medical Officer, Dr. Patrice Weiss leads efforts not only to assure clinical excellence from the more than 900 clinicians at the Carilion Clinic in Roanoke, Virginia, but also to improve patient experience. She lectures extensively on one of her passions in medicine: medical errors, and the concept of the second victim. OBG
OBG Management: What is the definition of a medical error?
Patrice M. Weiss, MD: Clinicians may be somewhat surprised to learn that there is no universal definition of a medical error that sets standardized nomenclature. The Institute of Medicine, in its landmark work To Err Is Human, adopted this definition: “failure of a planned action to be completed as intended, or the use of a wrong plan to achieve an aim.”1
In general terms, a medical error is an act of commission or omission, meaning that something was done or not done, that has negative consequences for the patient and is judged as wrong by our peers. An unanticipated outcome can be due to a medical error or can occur without a medical error. An unpredicted side effect, for instance—one that may have a low probability of drug–drug interaction or drug reaction occurrence—is an unexpected outcome. If the incidence of a drug reaction is 1 in 1,000 and your patient is that one, it does not necessarily mean that there was a medical error.
Often, if the outcome is unanticipated, patients and their families will assume, rightly or wrongly, that a medical error did occur.
OBG Management: Are physicians required to disclose medical errors?
Dr. Weiss: Yes. The Joint Commission’s standard principle states that the responsible licensed independent practitioner, or his or her designee, clearly explain the outcome of any treatment or procedure to the patient and, when appropriate, the patient’s family, whenever those outcomes differ significantly from the anticipated outcome.2
This can even include unanticipated outcomes that are not due to an error. Specifically speaking about medical errors, however, we do have the responsibility, both from this standard and from a professional and ethical standard to disclose what, why, and how the error occurred and what we are going to do to ensure it does not happen again.
OBG Management: How does a physician best communicate to a patient an unanticipated outcome that was not due to a medical error?
Dr. Weiss: Usually we as health care providers are more comfortable talking about unanticipated outcomes without medical errors. It is important to, when speaking with patients, be clear and concise, describing what you best know at the time, in language that patients can understand. I often jokingly say that, at a minimum, all of us in health care are bilingual: We speak our native language, and we speak “medicine.”
After describing unanticipated outcomes to patients and their families in terms they understand, affirm their understanding with a follow-up open-ended question. “Do you understand what I just said to you?” is ineffective. A better approach is saying, “Mrs. Jones, in your own words, will you describe back to me what your understanding is as to why this happened?” The answer received will allow you to know the patient’s level of understanding. It also will give you the opportunity to clear up points that are not clear or were misinterpreted. Do not leave patients feeling in a “lurch,” left to wonder or with a lack of understanding, or worse yet, with a sense that you are holding something back.
OBG Management: What is the best approach to disclosing an unanticipated outcome that was due to a medical error?
Dr. Weiss: First and foremost, you must be certain that a medical error did actually occur. There can be speculation at first, and that speculation should occur behind the scenes, with peer review or a root cause analysis on the event. Speculation should not enter into your conversation with the patient. Notional language can add to their anxiety, create mistrust on the patient’s part, and perhaps make a patient feel as if you are not giving the answers that he or she needs.
When it is believed that a medical error did occur, there are several things that need to be done:
- Gather as much information at the event as possible.
- Notify the hospital (ie, risk management or quality or patient safety). This is important because it is an organizational approach to medical errors when they do occur.
- Support the patient and the patient’s family through the entire process. Speaking to the patient and the family may be a part of ascertaining what happened and contributing factors. Clinicians have said to me, “Well, I can’t really go talk to the patient or the patient’s family right now because I don’t really know everything that happened.” Keep in mind, however, that the longer you wait to talk to the patient and family, the more time they have to speculate and to ask other people, perhaps those not involved and with no knowledge of, what happened.
OBG Management: What is the best timing and location for the disclosure conversation?
Dr. Weiss: The person who is responsible for the patient who was involved in the medical error needs to have the disclosure conversation. The conversation with the patient, and the family if the patient so desires, should occur as soon as possible. However, take into consideration the patient being awake, coherent, and not under the influence of medications. With those caveats, the best time to speak is when it is convenient for the patient. Do not plug this conversation into a 10-minute opening in your busy schedule. The conversation could take an hour, or it could take 15 minutes. It should not be conducted as a matter of convenience to the clinician.
In addition, often times the recovery room is not the best location—it is not private and confidential, and the patient is still groggy and will be unable to remember most of what is said or ask questions as needed.
OBG Management: You advocate a “TEAM” approach when speaking with the patient. What is TEAM?
Dr. Weiss: Disclosure conversations are not easy to have. The patient and the patient’s family are often upset. Medical errors challenge a physician’s humility and integrity, and they can lead to questioning of one’s own ability. I adopted the helpful pneumonic TEAM after first learning about it at what is now known as the Institute for Healthcare Communication. It refers to what parties need to be notified and who needs to be present with you when having a disclosure conversation. Of course, you want someone there who not only can serve as a witness but also can help facilitate the gathering of answers for questions that will be asked. The best person for this job could be the lead physician involved in the care of the patient, a hospital risk manager, a colleague, or the patient safety officer.
The “T” stands for truthful. When you begin the conversation, tell the patient at that point what you know to be true and what you know may have happened or definitely did not happen that contributed to the outcome. Again, do not speculate in answering the patient’s questions. A good approach is to say, “This is what I know happened. As of right now, this is what I know may have contributed or did contribute. We are going to be looking into this more thoroughly. As I learn more, you will be the first to know.”
These are not one-time conversations. As you do learn more, circle back and talk to the patient and family. This can be a dialogue that goes on for weeks or even months.
“E” equals empathy. Allow the patient and the patient’s family to ventilate. Try to understand what is it that they are most upset about, and try to soothe these upset feelings. For example, do not make the assumption that they are most upset about paying for a surgery in which there was a medical error. In fact, they really may be most upset about staying in the hospital 2 additional days, and they are going to now miss the visit of a relative, their child’s graduation, or something important to them.
Let patients talk. Do not interrupt them. Do not stand over them while they are in the bed; sit down at eye level with them. Talk in a voice and tone that the patient understands and try to soothe and empathetically relate to what is being said.
The “A” is important: Apology. There are 2 things that patients want when a medical error occurs: 1) to hear the clinician say, “I’m sorry”—and you should be sorry if a medical error occurred, and you should say that you are sorry this happened—and 2) what you or your organization is going to do so that this does not happen to the next person. Incorporate these 2 factors into the apology piece.
OBG Management: Can saying, “I’m sorry” expose a clinician unnecessarily to malpractice risk?
Dr. Weiss: Saying “I’m sorry,” of course, has come under a lot of scrutiny. There are various state laws, and you should be aware of your state’s apology laws. In many states an apology, with “I’m sorry,” cannot be used against a provider. However, there is not 100% absolution of the event if an apology occurs. In other words, “I’m sorry” cannot be held against you, but saying “I’m sorry” does not negate the error that occurred.
Even when practicing in a state in which there is not an apology law, however, and a clinician does apologize and that apology comes up in the legal setting of a true medical error, we would need to ask, is it really that bad that an apology was made on behalf of the medical error that was committed? Isn’t that compassion? Isn’t that empathy? Isn’t that showing that I as the physician care for the patient and the medical team cares for the patient?
Finally, abide by the disclosure policy and standards of your organization.
OBG Management: What does the “M” in TEAM stand for?
Dr. Weiss: Management. There may be times when a medical error occurs that the patient or the patient’s family are angry and upset to the point that they no longer want you to continue to care for them. Be empathetic and helpful by offering to assist them in finding someone else to continue to provide their care. Also let them know that you are more than happy to continue to care for them and assist them in their healing and restoration to health in any way that you can: “Of course the ongoing management of your care is your decision, and we will do whatever your wishes are.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed August 22, 2016.
- The Joint Commission on Health Care Accreditation, Patient Safety Standard RI.1.2.2.
- To err is human: Building a safer health system. Kohn LT, Corrigan JM, Donaldson MS, eds. Washington, DC: National Academy Press; 2000. http://www.nap.edu/books/0309068371/html. Accessed August 22, 2016.
- The Joint Commission on Health Care Accreditation, Patient Safety Standard RI.1.2.2.
In this Article
- Medical error disclosure requirements
- The TEAM approach to disclosure
- Saying “I’m sorry” and malpractice risk
ObGyn burnout: ACOG takes aim
Physician burnout is a serious issue, especially in the often high-pressure world of obstetrics and gynecology. In the July 2016 issue of OBG
Numerous studies show that burnout is associated with increased likelihood of physician suicide, decisions to leave the practice of medicine, and poorer health, including obesity. These statistics ring alarm bells that we cannot ignore, especially as they may signal real concern for patient care.
The top causes of physician burnout, according to a Medscape survey, are2:
- too many bureaucratic tasks
- spending too many hours at work
- increasing computerization of practice
- income not high enough.
Physician burnout is not a one-size-fits-all condition. Not everyone experiences it similarly. Likewise, there are many strategies you can use to prevent and overcome burnout.
Experts offer guidance on preventing burnout
The 3 ObGyn leaders profiled in this article offer insight, inspiration, and opportunities to help you regain the joy of practice. The American Medical Association (AMA) also offers an online practice-based initiative that includes strategies to help improve professional satisfaction.
Take burnout by the horns
As Assistant Dean for Graduate Medical Education and Professor of Clinical Biologic Sciences, Charles E. Schmidt College of Medicine, at Florida Atlantic University in Boca Raton, Florida, Roger P. Smith, MD, has developed an authoritative focus on the symptoms and effects of physician burnout. Recently, Dr. Smith taught a clinical seminar titled “Burnout: A constant threat” at the American College of Obstetricians and Gynecologists (ACOG) 2016 Annual Clinical and Scientific Meeting in Washington, DC, where he reviewed the causes and symptoms of burnout as well as strategies to minimize and avoid it. His work shows that burnout is pervasive and is becoming more so. Dr. Smith indicates that burnout is not any more common to one part of the country or one type of ObGyn care than any other, although it is becoming more commonly recognized among younger physicians.
As with other nonphysical ailments, symptoms of burnout may have affected ObGyns over many generations—symptoms that people did not discuss with their families or colleagues or that were easily dismissed.
Dr. Smith is opening the door for unreserved conversations about burnout in the physician community. He suggests ways for practices to focus on physician wellness and for individual ObGyns to recognize and take steps to overcome burnout signs. To hear Dr. Smith’s expert discussion on burnout, tune in to his audio interview, “Is burnout on the rise and what are the signs ObGyns should be on the lookout for?”
Develop a passion outside of medicine
Jeffrey M. Rothenberg, MD, MS, experienced serious burnout in 1997 after 3 infants in his high-risk obstetrical unit died in the same week. Like many ObGyns, he tries to not bring work-related issues home, but that week Dr. Rothenberg’s wife Joani, an accomplished art therapist, recognized her husband’s anguish. Soon, she enrolled him in a glass-blowing class to give him a needed break from the pressures of his practice. Dr. Rothenberg’s life was transformed. “Initially it was an escape,” he says about his venture into the art world. “Unlike the OR or in labor and delivery, no one could get hurt but me; it wasn’t life and death—it was something outside of what I do for a living and allowed me to disconnect from my chosen profession—if even for a little bit.”
Still a practicing ObGyn and currently the Designated Institutional Official at St. Vincent in Indianapolis, Dr. Rothenberg is now an accomplished glass artist with his work in public and private collections around the world. Because he feels so passionately that physicians should have a creative outlet, he helps medical students discover their own forms of self-expression through the Creative Arts Therapy Student Interest Group in the Office of Medical Student Service-Learning at Indiana University, where he serves as a mentor. There, he teaches medical students the importance of taking care of themselves as well as their patients. Dr. Rothenberg has made integrating the arts and humanities an integral part of the Indiana University School of Medicine curricula, and he helped establish the Visual Thinking Strategy program, which teaches nursing and medical students better communication skills using the power of observation gained through the arts. In honor of this work, Dr. Rothenberg received a 2015 Arnold P. Gold Foundation Humanism in Medicine Award.
For Dr. Rothenberg, glass has been the perfect medium. “It’s a very tactile art form, and I like doing things with my hands, as a surgeon—there’s a real affinity there—taking materials that are dangerous to touch and coaxing them into forms and shapes that are either artistic or functional is a challenge—but definitely stress reducing,” he says. He also found striking similarities between the operating room and the glass studio, which both involve teamwork, good communication skills, and repetition.
“I think that every healer needs an avocation in addition to his or her chosen vocation. Especially in medicine, both for patients and healers, we all deal with some amazingly complex and difficult situations—often at the intersection of life and death—that go beyond verbalizing and may have no right or wrong answer—just a bad and a worse choice. Given that backdrop, having another way to express yourself or an avenue for a kind of emotional catharsis... is very, very helpful. I think it’s important for our learners, in particular, to embrace the humanistic side of medicine. After all, medicine is so much more than just a science. It’s more of an art, and I think they can really bond with both patients and other providers alike if they have something else in common—something that they can talk about that is outside of medicine. We have very stressful jobs and we need to model to our trainees and to each other that it is important to take care of ourselves. It’s important for a healer to have a passion, a creative outlet,” he explains.
Be part of the solution
ACOG President Thomas Gellhaus, MD, has dedicated his life to advocating for his specialty and for patient care. His presidential initiatives hold great potential for preventing and overcoming burnout in ObGyns throughout the nation.
Join a mentoring program. Dr. Gellhaus encourages greater participation in mentoring—both as a mentor and a mentee—through an innovative mentor matching program created by Thomas Arnold, MD, and Tamara Helfer, MD, MBA, in ACOG District VI. This program allows physicians to share new ideas and aspirations, connect colleagues, and build lifelong bonds. Young ObGyns find an experienced and trusted advisor; more experienced ObGyns find satisfaction in helping others grow. Helping ObGyns avoid burnout is a key goal of this mentoring program, and Dr. Gellhaus is expanding the program to ACOG Fellows in all Districts.
The District VI mentoring program includes these goals:
- sharing skills, knowledge, and expertise
- demonstrating optimism and enthusiasm about the mentor-mentee relationship and the field of obstetrics and gynecology
- promoting ongoing learning and growth
- providing guidance and constructive feedback
- setting personal and professional goals
- celebrating accomplishments.
Dr. Arnold and Dr. Helfer point out that mentoring opportunities are also critical to help align practice with the future, especially working in collaborative groups, focusing on population health, and incorporating integrated learning. Learn more about bringing this mentorship program to your practice or ACOG section at http://www.acog.org/mentorshipprogram.
Consider going on a global health mission. Dr. Gellhaus and his family have participated in and led many medical missions over the years, braving oppressive heat and discomfort for the opportunity to bring health care to those who have none. He points out, “In areas where health care is out of reach, these missions help people return to economic productivity and retain their dignity.”
Under Dr. Gellhaus’ leadership, ACOG will help connect interested ObGyns with care needs across the globe through the organizations that need physician volunteers for short-term stays.
Already, ACOG is developing an ObGyn educational curriculum for Health Volunteers Overseas, a nonprofit organization that for 30 years has helped educate local health providers in villages in developing nations.
These short-term experiences not only can help physicians break out of their burnout ruts but also transform women’s lives and help build long-term relationships with physicians across the globe.
Become an advocate. Also under the leadership of Dr. Gellhaus, ACOG is investing in the imperative to help members thrive and lead health care into the next generation. Advocacy means getting politicians out of our exam rooms, reducing red tape, and improving payments and participation experiences for practicing ObGyns.
Dr. Gellhaus’ All-in for Advocacy program is designed to increase ACOG’s legislative power while expanding advocacy opportunities throughout the membership. Dr. Gellhaus said in his inaugural presidential address, “I’d like everyone to realize that caring for your patients does not end in the exam room or the surgical suite and everyone is affected by our legislative fights.”
All these initiatives offer alternatives to the pressure of day-to-day practice, and participation can bring back the joy of collegiality and making a difference.
AMA’s initiative to battle burnout
The AMA, too, has responded to physicians’ needs by developing its STEPS Forward interactive practice transformation series (www.stepsforward.org). A 2013 Rand study commissioned by the AMA found that “the satisfaction physicians derive from their work is eroding as they spend more time on grueling administrative rules, regulations and paperwork than caring for patients. The report noted that many physicians say that the bureaucratic obstacles to providing patients with high-quality care are major contributors to symptoms of burnout, including emotional fatigue, depersonalization, loss of enthusiasm and early retirement.”3,4
The STEPS Forward module Preventing Physician Burnout (www.stepsforward.org/modules/physician-burnout) can help identify if you are at risk for burnout and offers examples of how to implement changes that may restore your work satisfaction and work-life balance (TABLE).5 It details practice changes that can improve workflow and reduce administrative barriers, improving both patient and physician satisfaction.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600–1613.
- Peckham C. Medscape lifestyle report 2016: Bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=1. Published January 13, 2016. Accessed August 19, 2016.
- Friedberg MW, Chen PG, Van Busum KR, et al; RAND Corporation. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. 2013. http://www.rand.org/content/dam/rand/pubs/research_reports/RR400/RR439 /RAND_RR439.pdf. Accessed August 19, 2016.
- American Medical Association. AMA launches STEPS Forward to address physician burnout [news release]. http://www.ama-assn.org/ama/pub/news/news/2015/2015-06-08-ama-launches-steps-forward.page. Published June 8, 2015. Accessed August 19, 2016.
- Linzer M, Guzman-Corrales L, Poplau S; American Medical Association. STEPS Forward. Preventing physician burnout. https://www.stepsforward.org/modules/physician-burnout. Accessed August 19, 2016.
Physician burnout is a serious issue, especially in the often high-pressure world of obstetrics and gynecology. In the July 2016 issue of OBG
Numerous studies show that burnout is associated with increased likelihood of physician suicide, decisions to leave the practice of medicine, and poorer health, including obesity. These statistics ring alarm bells that we cannot ignore, especially as they may signal real concern for patient care.
The top causes of physician burnout, according to a Medscape survey, are2:
- too many bureaucratic tasks
- spending too many hours at work
- increasing computerization of practice
- income not high enough.
Physician burnout is not a one-size-fits-all condition. Not everyone experiences it similarly. Likewise, there are many strategies you can use to prevent and overcome burnout.
Experts offer guidance on preventing burnout
The 3 ObGyn leaders profiled in this article offer insight, inspiration, and opportunities to help you regain the joy of practice. The American Medical Association (AMA) also offers an online practice-based initiative that includes strategies to help improve professional satisfaction.
Take burnout by the horns
As Assistant Dean for Graduate Medical Education and Professor of Clinical Biologic Sciences, Charles E. Schmidt College of Medicine, at Florida Atlantic University in Boca Raton, Florida, Roger P. Smith, MD, has developed an authoritative focus on the symptoms and effects of physician burnout. Recently, Dr. Smith taught a clinical seminar titled “Burnout: A constant threat” at the American College of Obstetricians and Gynecologists (ACOG) 2016 Annual Clinical and Scientific Meeting in Washington, DC, where he reviewed the causes and symptoms of burnout as well as strategies to minimize and avoid it. His work shows that burnout is pervasive and is becoming more so. Dr. Smith indicates that burnout is not any more common to one part of the country or one type of ObGyn care than any other, although it is becoming more commonly recognized among younger physicians.
As with other nonphysical ailments, symptoms of burnout may have affected ObGyns over many generations—symptoms that people did not discuss with their families or colleagues or that were easily dismissed.
Dr. Smith is opening the door for unreserved conversations about burnout in the physician community. He suggests ways for practices to focus on physician wellness and for individual ObGyns to recognize and take steps to overcome burnout signs. To hear Dr. Smith’s expert discussion on burnout, tune in to his audio interview, “Is burnout on the rise and what are the signs ObGyns should be on the lookout for?”
Develop a passion outside of medicine
Jeffrey M. Rothenberg, MD, MS, experienced serious burnout in 1997 after 3 infants in his high-risk obstetrical unit died in the same week. Like many ObGyns, he tries to not bring work-related issues home, but that week Dr. Rothenberg’s wife Joani, an accomplished art therapist, recognized her husband’s anguish. Soon, she enrolled him in a glass-blowing class to give him a needed break from the pressures of his practice. Dr. Rothenberg’s life was transformed. “Initially it was an escape,” he says about his venture into the art world. “Unlike the OR or in labor and delivery, no one could get hurt but me; it wasn’t life and death—it was something outside of what I do for a living and allowed me to disconnect from my chosen profession—if even for a little bit.”
Still a practicing ObGyn and currently the Designated Institutional Official at St. Vincent in Indianapolis, Dr. Rothenberg is now an accomplished glass artist with his work in public and private collections around the world. Because he feels so passionately that physicians should have a creative outlet, he helps medical students discover their own forms of self-expression through the Creative Arts Therapy Student Interest Group in the Office of Medical Student Service-Learning at Indiana University, where he serves as a mentor. There, he teaches medical students the importance of taking care of themselves as well as their patients. Dr. Rothenberg has made integrating the arts and humanities an integral part of the Indiana University School of Medicine curricula, and he helped establish the Visual Thinking Strategy program, which teaches nursing and medical students better communication skills using the power of observation gained through the arts. In honor of this work, Dr. Rothenberg received a 2015 Arnold P. Gold Foundation Humanism in Medicine Award.
For Dr. Rothenberg, glass has been the perfect medium. “It’s a very tactile art form, and I like doing things with my hands, as a surgeon—there’s a real affinity there—taking materials that are dangerous to touch and coaxing them into forms and shapes that are either artistic or functional is a challenge—but definitely stress reducing,” he says. He also found striking similarities between the operating room and the glass studio, which both involve teamwork, good communication skills, and repetition.
“I think that every healer needs an avocation in addition to his or her chosen vocation. Especially in medicine, both for patients and healers, we all deal with some amazingly complex and difficult situations—often at the intersection of life and death—that go beyond verbalizing and may have no right or wrong answer—just a bad and a worse choice. Given that backdrop, having another way to express yourself or an avenue for a kind of emotional catharsis... is very, very helpful. I think it’s important for our learners, in particular, to embrace the humanistic side of medicine. After all, medicine is so much more than just a science. It’s more of an art, and I think they can really bond with both patients and other providers alike if they have something else in common—something that they can talk about that is outside of medicine. We have very stressful jobs and we need to model to our trainees and to each other that it is important to take care of ourselves. It’s important for a healer to have a passion, a creative outlet,” he explains.
Be part of the solution
ACOG President Thomas Gellhaus, MD, has dedicated his life to advocating for his specialty and for patient care. His presidential initiatives hold great potential for preventing and overcoming burnout in ObGyns throughout the nation.
Join a mentoring program. Dr. Gellhaus encourages greater participation in mentoring—both as a mentor and a mentee—through an innovative mentor matching program created by Thomas Arnold, MD, and Tamara Helfer, MD, MBA, in ACOG District VI. This program allows physicians to share new ideas and aspirations, connect colleagues, and build lifelong bonds. Young ObGyns find an experienced and trusted advisor; more experienced ObGyns find satisfaction in helping others grow. Helping ObGyns avoid burnout is a key goal of this mentoring program, and Dr. Gellhaus is expanding the program to ACOG Fellows in all Districts.
The District VI mentoring program includes these goals:
- sharing skills, knowledge, and expertise
- demonstrating optimism and enthusiasm about the mentor-mentee relationship and the field of obstetrics and gynecology
- promoting ongoing learning and growth
- providing guidance and constructive feedback
- setting personal and professional goals
- celebrating accomplishments.
Dr. Arnold and Dr. Helfer point out that mentoring opportunities are also critical to help align practice with the future, especially working in collaborative groups, focusing on population health, and incorporating integrated learning. Learn more about bringing this mentorship program to your practice or ACOG section at http://www.acog.org/mentorshipprogram.
Consider going on a global health mission. Dr. Gellhaus and his family have participated in and led many medical missions over the years, braving oppressive heat and discomfort for the opportunity to bring health care to those who have none. He points out, “In areas where health care is out of reach, these missions help people return to economic productivity and retain their dignity.”
Under Dr. Gellhaus’ leadership, ACOG will help connect interested ObGyns with care needs across the globe through the organizations that need physician volunteers for short-term stays.
Already, ACOG is developing an ObGyn educational curriculum for Health Volunteers Overseas, a nonprofit organization that for 30 years has helped educate local health providers in villages in developing nations.
These short-term experiences not only can help physicians break out of their burnout ruts but also transform women’s lives and help build long-term relationships with physicians across the globe.
Become an advocate. Also under the leadership of Dr. Gellhaus, ACOG is investing in the imperative to help members thrive and lead health care into the next generation. Advocacy means getting politicians out of our exam rooms, reducing red tape, and improving payments and participation experiences for practicing ObGyns.
Dr. Gellhaus’ All-in for Advocacy program is designed to increase ACOG’s legislative power while expanding advocacy opportunities throughout the membership. Dr. Gellhaus said in his inaugural presidential address, “I’d like everyone to realize that caring for your patients does not end in the exam room or the surgical suite and everyone is affected by our legislative fights.”
All these initiatives offer alternatives to the pressure of day-to-day practice, and participation can bring back the joy of collegiality and making a difference.
AMA’s initiative to battle burnout
The AMA, too, has responded to physicians’ needs by developing its STEPS Forward interactive practice transformation series (www.stepsforward.org). A 2013 Rand study commissioned by the AMA found that “the satisfaction physicians derive from their work is eroding as they spend more time on grueling administrative rules, regulations and paperwork than caring for patients. The report noted that many physicians say that the bureaucratic obstacles to providing patients with high-quality care are major contributors to symptoms of burnout, including emotional fatigue, depersonalization, loss of enthusiasm and early retirement.”3,4
The STEPS Forward module Preventing Physician Burnout (www.stepsforward.org/modules/physician-burnout) can help identify if you are at risk for burnout and offers examples of how to implement changes that may restore your work satisfaction and work-life balance (TABLE).5 It details practice changes that can improve workflow and reduce administrative barriers, improving both patient and physician satisfaction.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Physician burnout is a serious issue, especially in the often high-pressure world of obstetrics and gynecology. In the July 2016 issue of OBG
Numerous studies show that burnout is associated with increased likelihood of physician suicide, decisions to leave the practice of medicine, and poorer health, including obesity. These statistics ring alarm bells that we cannot ignore, especially as they may signal real concern for patient care.
The top causes of physician burnout, according to a Medscape survey, are2:
- too many bureaucratic tasks
- spending too many hours at work
- increasing computerization of practice
- income not high enough.
Physician burnout is not a one-size-fits-all condition. Not everyone experiences it similarly. Likewise, there are many strategies you can use to prevent and overcome burnout.
Experts offer guidance on preventing burnout
The 3 ObGyn leaders profiled in this article offer insight, inspiration, and opportunities to help you regain the joy of practice. The American Medical Association (AMA) also offers an online practice-based initiative that includes strategies to help improve professional satisfaction.
Take burnout by the horns
As Assistant Dean for Graduate Medical Education and Professor of Clinical Biologic Sciences, Charles E. Schmidt College of Medicine, at Florida Atlantic University in Boca Raton, Florida, Roger P. Smith, MD, has developed an authoritative focus on the symptoms and effects of physician burnout. Recently, Dr. Smith taught a clinical seminar titled “Burnout: A constant threat” at the American College of Obstetricians and Gynecologists (ACOG) 2016 Annual Clinical and Scientific Meeting in Washington, DC, where he reviewed the causes and symptoms of burnout as well as strategies to minimize and avoid it. His work shows that burnout is pervasive and is becoming more so. Dr. Smith indicates that burnout is not any more common to one part of the country or one type of ObGyn care than any other, although it is becoming more commonly recognized among younger physicians.
As with other nonphysical ailments, symptoms of burnout may have affected ObGyns over many generations—symptoms that people did not discuss with their families or colleagues or that were easily dismissed.
Dr. Smith is opening the door for unreserved conversations about burnout in the physician community. He suggests ways for practices to focus on physician wellness and for individual ObGyns to recognize and take steps to overcome burnout signs. To hear Dr. Smith’s expert discussion on burnout, tune in to his audio interview, “Is burnout on the rise and what are the signs ObGyns should be on the lookout for?”
Develop a passion outside of medicine
Jeffrey M. Rothenberg, MD, MS, experienced serious burnout in 1997 after 3 infants in his high-risk obstetrical unit died in the same week. Like many ObGyns, he tries to not bring work-related issues home, but that week Dr. Rothenberg’s wife Joani, an accomplished art therapist, recognized her husband’s anguish. Soon, she enrolled him in a glass-blowing class to give him a needed break from the pressures of his practice. Dr. Rothenberg’s life was transformed. “Initially it was an escape,” he says about his venture into the art world. “Unlike the OR or in labor and delivery, no one could get hurt but me; it wasn’t life and death—it was something outside of what I do for a living and allowed me to disconnect from my chosen profession—if even for a little bit.”
Still a practicing ObGyn and currently the Designated Institutional Official at St. Vincent in Indianapolis, Dr. Rothenberg is now an accomplished glass artist with his work in public and private collections around the world. Because he feels so passionately that physicians should have a creative outlet, he helps medical students discover their own forms of self-expression through the Creative Arts Therapy Student Interest Group in the Office of Medical Student Service-Learning at Indiana University, where he serves as a mentor. There, he teaches medical students the importance of taking care of themselves as well as their patients. Dr. Rothenberg has made integrating the arts and humanities an integral part of the Indiana University School of Medicine curricula, and he helped establish the Visual Thinking Strategy program, which teaches nursing and medical students better communication skills using the power of observation gained through the arts. In honor of this work, Dr. Rothenberg received a 2015 Arnold P. Gold Foundation Humanism in Medicine Award.
For Dr. Rothenberg, glass has been the perfect medium. “It’s a very tactile art form, and I like doing things with my hands, as a surgeon—there’s a real affinity there—taking materials that are dangerous to touch and coaxing them into forms and shapes that are either artistic or functional is a challenge—but definitely stress reducing,” he says. He also found striking similarities between the operating room and the glass studio, which both involve teamwork, good communication skills, and repetition.
“I think that every healer needs an avocation in addition to his or her chosen vocation. Especially in medicine, both for patients and healers, we all deal with some amazingly complex and difficult situations—often at the intersection of life and death—that go beyond verbalizing and may have no right or wrong answer—just a bad and a worse choice. Given that backdrop, having another way to express yourself or an avenue for a kind of emotional catharsis... is very, very helpful. I think it’s important for our learners, in particular, to embrace the humanistic side of medicine. After all, medicine is so much more than just a science. It’s more of an art, and I think they can really bond with both patients and other providers alike if they have something else in common—something that they can talk about that is outside of medicine. We have very stressful jobs and we need to model to our trainees and to each other that it is important to take care of ourselves. It’s important for a healer to have a passion, a creative outlet,” he explains.
Be part of the solution
ACOG President Thomas Gellhaus, MD, has dedicated his life to advocating for his specialty and for patient care. His presidential initiatives hold great potential for preventing and overcoming burnout in ObGyns throughout the nation.
Join a mentoring program. Dr. Gellhaus encourages greater participation in mentoring—both as a mentor and a mentee—through an innovative mentor matching program created by Thomas Arnold, MD, and Tamara Helfer, MD, MBA, in ACOG District VI. This program allows physicians to share new ideas and aspirations, connect colleagues, and build lifelong bonds. Young ObGyns find an experienced and trusted advisor; more experienced ObGyns find satisfaction in helping others grow. Helping ObGyns avoid burnout is a key goal of this mentoring program, and Dr. Gellhaus is expanding the program to ACOG Fellows in all Districts.
The District VI mentoring program includes these goals:
- sharing skills, knowledge, and expertise
- demonstrating optimism and enthusiasm about the mentor-mentee relationship and the field of obstetrics and gynecology
- promoting ongoing learning and growth
- providing guidance and constructive feedback
- setting personal and professional goals
- celebrating accomplishments.
Dr. Arnold and Dr. Helfer point out that mentoring opportunities are also critical to help align practice with the future, especially working in collaborative groups, focusing on population health, and incorporating integrated learning. Learn more about bringing this mentorship program to your practice or ACOG section at http://www.acog.org/mentorshipprogram.
Consider going on a global health mission. Dr. Gellhaus and his family have participated in and led many medical missions over the years, braving oppressive heat and discomfort for the opportunity to bring health care to those who have none. He points out, “In areas where health care is out of reach, these missions help people return to economic productivity and retain their dignity.”
Under Dr. Gellhaus’ leadership, ACOG will help connect interested ObGyns with care needs across the globe through the organizations that need physician volunteers for short-term stays.
Already, ACOG is developing an ObGyn educational curriculum for Health Volunteers Overseas, a nonprofit organization that for 30 years has helped educate local health providers in villages in developing nations.
These short-term experiences not only can help physicians break out of their burnout ruts but also transform women’s lives and help build long-term relationships with physicians across the globe.
Become an advocate. Also under the leadership of Dr. Gellhaus, ACOG is investing in the imperative to help members thrive and lead health care into the next generation. Advocacy means getting politicians out of our exam rooms, reducing red tape, and improving payments and participation experiences for practicing ObGyns.
Dr. Gellhaus’ All-in for Advocacy program is designed to increase ACOG’s legislative power while expanding advocacy opportunities throughout the membership. Dr. Gellhaus said in his inaugural presidential address, “I’d like everyone to realize that caring for your patients does not end in the exam room or the surgical suite and everyone is affected by our legislative fights.”
All these initiatives offer alternatives to the pressure of day-to-day practice, and participation can bring back the joy of collegiality and making a difference.
AMA’s initiative to battle burnout
The AMA, too, has responded to physicians’ needs by developing its STEPS Forward interactive practice transformation series (www.stepsforward.org). A 2013 Rand study commissioned by the AMA found that “the satisfaction physicians derive from their work is eroding as they spend more time on grueling administrative rules, regulations and paperwork than caring for patients. The report noted that many physicians say that the bureaucratic obstacles to providing patients with high-quality care are major contributors to symptoms of burnout, including emotional fatigue, depersonalization, loss of enthusiasm and early retirement.”3,4
The STEPS Forward module Preventing Physician Burnout (www.stepsforward.org/modules/physician-burnout) can help identify if you are at risk for burnout and offers examples of how to implement changes that may restore your work satisfaction and work-life balance (TABLE).5 It details practice changes that can improve workflow and reduce administrative barriers, improving both patient and physician satisfaction.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600–1613.
- Peckham C. Medscape lifestyle report 2016: Bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=1. Published January 13, 2016. Accessed August 19, 2016.
- Friedberg MW, Chen PG, Van Busum KR, et al; RAND Corporation. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. 2013. http://www.rand.org/content/dam/rand/pubs/research_reports/RR400/RR439 /RAND_RR439.pdf. Accessed August 19, 2016.
- American Medical Association. AMA launches STEPS Forward to address physician burnout [news release]. http://www.ama-assn.org/ama/pub/news/news/2015/2015-06-08-ama-launches-steps-forward.page. Published June 8, 2015. Accessed August 19, 2016.
- Linzer M, Guzman-Corrales L, Poplau S; American Medical Association. STEPS Forward. Preventing physician burnout. https://www.stepsforward.org/modules/physician-burnout. Accessed August 19, 2016.
- Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90(12):1600–1613.
- Peckham C. Medscape lifestyle report 2016: Bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=1. Published January 13, 2016. Accessed August 19, 2016.
- Friedberg MW, Chen PG, Van Busum KR, et al; RAND Corporation. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. 2013. http://www.rand.org/content/dam/rand/pubs/research_reports/RR400/RR439 /RAND_RR439.pdf. Accessed August 19, 2016.
- American Medical Association. AMA launches STEPS Forward to address physician burnout [news release]. http://www.ama-assn.org/ama/pub/news/news/2015/2015-06-08-ama-launches-steps-forward.page. Published June 8, 2015. Accessed August 19, 2016.
- Linzer M, Guzman-Corrales L, Poplau S; American Medical Association. STEPS Forward. Preventing physician burnout. https://www.stepsforward.org/modules/physician-burnout. Accessed August 19, 2016.
In this Article
- Developing outside passions
- Thomas Gellhaus, MD, encourages mentorship
- 7 steps to prevent burnout
When Should Concussed Students Return to Learn and Return to Play?
VANCOUVER—“Concussion is a public health epidemic,” said Amaal Starling, MD. “Neurologists are seeing more and more concussed patients every day.” At the 68th Annual Meeting of the American Academy of Neurology, Dr. Starling, who is an Assistant Professor of Neurology at the Mayo Clinic in Phoenix, provided a framework and a template for evaluating concussion in the outpatient setting.
“Prioritizing these patients into clinic is very important,” Dr. Starling said. She recommended expedited appointments for patients with a suspected concussion. “This will limit symptom exacerbation, provide an avenue for appropriate and quick symptomatic treatment, and prevent premature return to learn and return to play,” which may exacerbate symptoms and prolong recovery.
Outpatient Evaluation of Concussion
The patient history should always include the date of the injury and the injury description, which includes the mechanism of the injury, location of the impact, presence or absence of any whiplash injury, altered mental status or amnesia, as well as symptom progression. “How do the symptoms progress from the time of impact to the time the patient presents in the office?” Dr. Starling asked. “This will help you identify not only those immediate symptoms that occur, but also those delayed symptoms that can occur one to two days later. In addition, it will give you a time course of symptoms to determine if the patient has been worsening, improving, or has stayed about the same.”
Concussion has various symptoms that can be categorized in the following four domains: physical, cognitive, emotional, and sleep. The most frequently reported symptom is headache, followed by dizziness. To capture all of those symptom domains, Dr. Starling recommended using a postconcussion graded symptom checklist. “This can be effective at monitoring symptoms over time.”
It is also important to elicit risk factors for prolonged recovery. “If an individual has a personal history of migraine, they are at risk of having a prolonged recovery after the injury,” Dr. Starling said. “Even if they have no personal history of migraine, but if they have a family history of migraine, those individuals, per studies, have demonstrated a prolonged recovery after a concussion.” Other risk factors for a prolonged recovery include a history of learning disabilities, such as attention deficit disorder or dyslexia, and psychiatric disease, such as premorbid anxiety or depression.
A concussion history is also important because a prior concussion increases the risk of another concussion, as well as the risk of having a prolonged recovery. “Not only do you want to know how many concussions have occurred, but also the symptom duration and recovery course for those concussions.”
Since headache is the most common symptom after a concussion, it is important to evaluate headache when present. “In every headache history, it is important to look for red flags,” said Dr. Starling. She suggested using the mnemonic IFLOP to look for headache red flags in the setting of a concussion. IFLOP stands for Intractable vomiting, Focal neurologic symptoms and signs, changes in Level of awareness, Orthostatic headache, and Progressively worsening headache. When present, headache red flags should signal the need for neuroimaging. “For example, if someone is presenting with an orthostatic headache … I am concerned that they might have a CSF leak and I’ll want to get an MRI of the brain with and without contrast to look for diffuse pachymeningeal enhancement that we can see in that setting,” Dr. Starling said.
Management of a Concussed Patient
According to Dr. Starling, posttraumatic headaches should be treated according to their phenotypes. “If [the headache] has a migraine phenotype, treat it with migraine-specific medications. If it has a more cervicogenic phenotype, treat it that way.” The most common posttraumatic headache phenotype is migraine. That finding has been confirmed in the civilian as well as the military population. “But it is important to screen for other phenotypes that may also occur,” Dr. Starling advised.
Because patients with concussion seem to be at higher risk for medication overuse and medication overuse headache, a pre- and postinjury medication history is also important. “If they are using over-the-counter medications, you’ll want to know what they are using and how much.”
During the initial visit, it is also important to determine whether the patient has had any baseline testing. “If they had any computerized neurocognitive testing, obtain those results, Dr. Starling advised. “If they had a King-Devick test at baseline or pre season, obtain those results. If they have undergone gold-standard neuropsychometric testing or had a baseline neurologic examination or imaging, get those results so that you can compare postinjury [performance] to preinjury [performance].”
Regarding the physical examination in the outpatient setting, vitals are vital, Dr. Starling said. Many concussed athletes have autonomic dysfunction that looks like postural orthostatic tachycardia syndrome (POTS), although the prognosis is typically different. “When getting vitals, it is important to get orthostatic vitals—supine and then standing at one, five, and 10 minutes—to monitor for abnormal changes or an increase in the heart rate with standing.” The physical exam should also look for trigger points or any difficulties with range of motion of the neck. “These [findings] can give you avenues for therapeutic intervention,” Dr. Starling said. Additionally, the Dix–Hallpike maneuver can identify cases of benign paroxysmal positional vertigo, which can be treated with the Epley maneuver.
Mental status should be evaluated as part of a detailed neurologic examination. The Mini-Mental State Exam (MMSE), the Montreal Cognitive Assessment (MoCA), and the Kokmen are well-validated tools for the evaluation of mental status. The Standardized Assessment of Concussion (SAC) is another tool that was developed to assess mental status. The SAC was validated on the sideline and is used by a wide array of health care providers from athletic trainers to the team physicians.
During the cranial nerve examination, Dr. Starling tests for anosmia. “That is concerning for gross structural changes on the inferior surface of the frontal lobe where the olfactory nerve lies.” Abnormalities suggest a need for neuroimaging. A typical pupillary assessment with a swinging pen light test also is an essential part of the evaluation, but Dr. Starling commented that in her patients with mild traumatic brain injury or concussion, she has rarely found any clinically significant abnormalities with that test. “But that’s not true for the evaluation of extraocular movement,” she said. “I look for not only nystagmus, abnormalities of smooth pursuit, and horizontal and vertical saccades, but I also look for near point convergence. Near point convergence of greater than 6 cm is abnormal in the majority of individuals that we will evaluate for concussion.” When it is abnormal, it correlates with oculomotor abnormalities in function. So, these people have more difficulty with oculomotor function in day-to-day life—difficulties with reading and motion sensitivity.
The rest of the cranial nerve examination can also help identify subtle focal deficits. Upper motor neuron exam techniques also can detect subtle changes, and abnormalities can suggest a need for neuroimaging. Dr. Starling also recommended a good screening evaluation of balance, such as the timed tandem gait measure.
The Concussion Toolbox
No biomarkers or tests will yield a 100% accurate diagnosis of concussion. “However, studies have repeatedly demonstrated that if we use the tools that are available, and if we use a combination of them, we are nearing 100% sensitivity and specificity,” Dr. Starling said. In her return-to-play clinic, all patients undergo the King–Devick test, neuropsychologic testing, and objective vestibular testing. If patients report autonomic or orthostatic symptoms, they also undergo autonomic testing. “Unless I’m concerned about a skull fracture, I don’t get a CT scan of the head,” Dr. Starling said. “But we do obtain an MRI of the brain in individuals who have focal neurologic deficits, risk factors for prolonged recovery, or who have had prior concussions.” Dr. Starling recommended susceptibility-weighted imaging and diffusion tensor imaging.
Management priorities for patients with concussion include providing symptomatic treatment and preventing reinjury while the brain is healing. “Multidisciplinary symptoms require multidisciplinary treatment,” Dr. Starling said. “In my assessment, I’ll have a list of symptoms and a list of targeted approaches for each individual symptom.”
Posttraumatic Headache
“It is amazing how often I still see individuals two, four, eight weeks post injury who have never received a medication for posttraumatic headache because they’ve been told that the headache is a result of the concussion and as the concussion gets better, the headache will go away,” Dr. Starling said. Treating posttraumatic headache can relieve suffering and help the patient participate in active rehabilitation. Appropriate treatment can also prevent overuse of over-the-counter combination analgesics, which can complicate the problem. Experts in the headache community also suggest that there is a risk of chronification in untreated posttraumatic headache.
While there is a dearth of randomized, prospective, double-blind trials to guide the treatment of posttraumatic headache, “there still is an approach that you can use,” Dr. Starling said. Look for headache red flags first, then identify the phenotype and establish the headache history. If the patient had frequent migraines pre injury, it may be an indication for early initiation of a preventive medication. Initiating acute treatment early—within days—is also a priority, as is strictly monitoring for medication overuse. Also consider the comorbidities. “You don’t want to make comorbid symptoms worse,” Dr. Starling said. “For example, avoid topiramate in a patient who is having cognitive domain symptoms. Avoid sedating medications in someone who is having a lot of fatigue. Avoid steroids in a patient who is having a lot of emotional lability or difficulty with insomnia. Keep comorbid symptoms in mind when picking medications for posttraumatic headache.”
Return-to-Learn and Return-to-Play Decisions
Dr. Starling recommended symptom-limited cognitive and physical activity in the recovery phase, as opposed to total physical and cognitive rest. “There’s actually been a recent study that was done looking at strict rest,” she said. “The control group had one to two days of rest, followed by return to school and gradual return to activity. The intervention group had five days of strict rest.… The group with strict rest had higher symptom severity scores and had a longer symptom recovery. Exaggerated or extreme rest may not be the answer. Rather, we need to gradually reengage individuals back into life and give them a specific plan for graduated return to life, which includes both cognitive, as well as physical, activity.”
Return-to-learn protocols must be individualized, but there are some common goals. Dr. Starling recommended a short period of brain rest. “Not complete sensory deprivation, but rather symptom-limited brain rest. That should be followed by a brain warm-up phase where we initiate some time-limited and symptom-limited reading time—five to 10 minutes, as tolerated—and gradually increase that over time. After that, we reengage that individual back into school with extensive accommodations, which include the number of hours they are in school, as well as the curriculum, so a higher value on quality rather than quantity, and then a lot of environmental adjustments—perhaps a room that is quieter, a room where the lights are a little dimmer, they are allowed to wear a hat in class or sunglasses in class.” To avoid the sensory stimulation that characterizes school hallways between classes, which can make patients feel worse, Dr. Starling recommended that patients leave class five minutes early, spend the passing period in the nurse’s office, and then go to the next class five minutes late.
The next goal in recovery is full-day school with academic accommodations, and finally a return to learn without any accommodations. This requires an education specialist or a neuropsychologist who can get an individualized history from the patient as to what his or her day entails. A detailed recovery plan is then put into writing and provided to the patient and the school. The plan is then revised every one to two weeks as the patient recovers.
Dr. Starling suggested that physical activity could be initiated even when individuals are still having symptoms, but in a symptom-limited manner. “There have been studies looking at controlled exercise as a therapeutic approach for concussion,” she said. In an initial, nonrandomized pilot study, an exertion protocol seemed to improve symptoms, promote a faster rate of recovery, and normalize cerebral blood flow abnormalities during a cognitive task. “Although more rigorous studies are definitely needed, I think we are in the right paradigm,” Dr. Starling said. “After initial rest, but not complete sensory deprivation, active rehabilitation can be initiated, even in the presence of symptoms, as long as we have subthreshold activity.” This strategy, she said, is recommended to reduce symptom severity, speed recovery, and ensure full recovery.
“With active rehabilitation, we have to be prescriptive about what individual patients do. We want to make sure they are not exacerbating their symptoms.” At the Mayo Clinic, Dr. Starling and her team use written, as well as verbal, instructions. “We set in writing a goal heart rate that we want that individual patient to reach. In the clinical setting, we use a recumbent bike to determine a goal heart rate that is subthreshold to their symptoms. We then ask the patient to engage in activity up to that heart rate every day for the next couple of days. As they tolerate this, they can increase it [by] five to 10 beats per minute every three to seven days, and then we reevaluate this every one to two weeks to determine what the next step is.”
Once the exertion protocol is completed, a more sports-specific return-to-play protocol can be initiated. “During a concussion, the player can become deconditioned from their specific sport, so a sport-specific return to play protocol is important in that setting,” Dr. Starling said.
Recommending retirement from high-risk athletic activity is, of course, an individualized decision in which various components of the history come into play. According to Dr. Starling, the red flags for retirement include reduced threshold for concussion, neuroimaging abnormalities, persistent cognitive impairment, and debilitating refractory headaches.
VANCOUVER—“Concussion is a public health epidemic,” said Amaal Starling, MD. “Neurologists are seeing more and more concussed patients every day.” At the 68th Annual Meeting of the American Academy of Neurology, Dr. Starling, who is an Assistant Professor of Neurology at the Mayo Clinic in Phoenix, provided a framework and a template for evaluating concussion in the outpatient setting.
“Prioritizing these patients into clinic is very important,” Dr. Starling said. She recommended expedited appointments for patients with a suspected concussion. “This will limit symptom exacerbation, provide an avenue for appropriate and quick symptomatic treatment, and prevent premature return to learn and return to play,” which may exacerbate symptoms and prolong recovery.
Outpatient Evaluation of Concussion
The patient history should always include the date of the injury and the injury description, which includes the mechanism of the injury, location of the impact, presence or absence of any whiplash injury, altered mental status or amnesia, as well as symptom progression. “How do the symptoms progress from the time of impact to the time the patient presents in the office?” Dr. Starling asked. “This will help you identify not only those immediate symptoms that occur, but also those delayed symptoms that can occur one to two days later. In addition, it will give you a time course of symptoms to determine if the patient has been worsening, improving, or has stayed about the same.”
Concussion has various symptoms that can be categorized in the following four domains: physical, cognitive, emotional, and sleep. The most frequently reported symptom is headache, followed by dizziness. To capture all of those symptom domains, Dr. Starling recommended using a postconcussion graded symptom checklist. “This can be effective at monitoring symptoms over time.”
It is also important to elicit risk factors for prolonged recovery. “If an individual has a personal history of migraine, they are at risk of having a prolonged recovery after the injury,” Dr. Starling said. “Even if they have no personal history of migraine, but if they have a family history of migraine, those individuals, per studies, have demonstrated a prolonged recovery after a concussion.” Other risk factors for a prolonged recovery include a history of learning disabilities, such as attention deficit disorder or dyslexia, and psychiatric disease, such as premorbid anxiety or depression.
A concussion history is also important because a prior concussion increases the risk of another concussion, as well as the risk of having a prolonged recovery. “Not only do you want to know how many concussions have occurred, but also the symptom duration and recovery course for those concussions.”
Since headache is the most common symptom after a concussion, it is important to evaluate headache when present. “In every headache history, it is important to look for red flags,” said Dr. Starling. She suggested using the mnemonic IFLOP to look for headache red flags in the setting of a concussion. IFLOP stands for Intractable vomiting, Focal neurologic symptoms and signs, changes in Level of awareness, Orthostatic headache, and Progressively worsening headache. When present, headache red flags should signal the need for neuroimaging. “For example, if someone is presenting with an orthostatic headache … I am concerned that they might have a CSF leak and I’ll want to get an MRI of the brain with and without contrast to look for diffuse pachymeningeal enhancement that we can see in that setting,” Dr. Starling said.
Management of a Concussed Patient
According to Dr. Starling, posttraumatic headaches should be treated according to their phenotypes. “If [the headache] has a migraine phenotype, treat it with migraine-specific medications. If it has a more cervicogenic phenotype, treat it that way.” The most common posttraumatic headache phenotype is migraine. That finding has been confirmed in the civilian as well as the military population. “But it is important to screen for other phenotypes that may also occur,” Dr. Starling advised.
Because patients with concussion seem to be at higher risk for medication overuse and medication overuse headache, a pre- and postinjury medication history is also important. “If they are using over-the-counter medications, you’ll want to know what they are using and how much.”
During the initial visit, it is also important to determine whether the patient has had any baseline testing. “If they had any computerized neurocognitive testing, obtain those results, Dr. Starling advised. “If they had a King-Devick test at baseline or pre season, obtain those results. If they have undergone gold-standard neuropsychometric testing or had a baseline neurologic examination or imaging, get those results so that you can compare postinjury [performance] to preinjury [performance].”
Regarding the physical examination in the outpatient setting, vitals are vital, Dr. Starling said. Many concussed athletes have autonomic dysfunction that looks like postural orthostatic tachycardia syndrome (POTS), although the prognosis is typically different. “When getting vitals, it is important to get orthostatic vitals—supine and then standing at one, five, and 10 minutes—to monitor for abnormal changes or an increase in the heart rate with standing.” The physical exam should also look for trigger points or any difficulties with range of motion of the neck. “These [findings] can give you avenues for therapeutic intervention,” Dr. Starling said. Additionally, the Dix–Hallpike maneuver can identify cases of benign paroxysmal positional vertigo, which can be treated with the Epley maneuver.
Mental status should be evaluated as part of a detailed neurologic examination. The Mini-Mental State Exam (MMSE), the Montreal Cognitive Assessment (MoCA), and the Kokmen are well-validated tools for the evaluation of mental status. The Standardized Assessment of Concussion (SAC) is another tool that was developed to assess mental status. The SAC was validated on the sideline and is used by a wide array of health care providers from athletic trainers to the team physicians.
During the cranial nerve examination, Dr. Starling tests for anosmia. “That is concerning for gross structural changes on the inferior surface of the frontal lobe where the olfactory nerve lies.” Abnormalities suggest a need for neuroimaging. A typical pupillary assessment with a swinging pen light test also is an essential part of the evaluation, but Dr. Starling commented that in her patients with mild traumatic brain injury or concussion, she has rarely found any clinically significant abnormalities with that test. “But that’s not true for the evaluation of extraocular movement,” she said. “I look for not only nystagmus, abnormalities of smooth pursuit, and horizontal and vertical saccades, but I also look for near point convergence. Near point convergence of greater than 6 cm is abnormal in the majority of individuals that we will evaluate for concussion.” When it is abnormal, it correlates with oculomotor abnormalities in function. So, these people have more difficulty with oculomotor function in day-to-day life—difficulties with reading and motion sensitivity.
The rest of the cranial nerve examination can also help identify subtle focal deficits. Upper motor neuron exam techniques also can detect subtle changes, and abnormalities can suggest a need for neuroimaging. Dr. Starling also recommended a good screening evaluation of balance, such as the timed tandem gait measure.
The Concussion Toolbox
No biomarkers or tests will yield a 100% accurate diagnosis of concussion. “However, studies have repeatedly demonstrated that if we use the tools that are available, and if we use a combination of them, we are nearing 100% sensitivity and specificity,” Dr. Starling said. In her return-to-play clinic, all patients undergo the King–Devick test, neuropsychologic testing, and objective vestibular testing. If patients report autonomic or orthostatic symptoms, they also undergo autonomic testing. “Unless I’m concerned about a skull fracture, I don’t get a CT scan of the head,” Dr. Starling said. “But we do obtain an MRI of the brain in individuals who have focal neurologic deficits, risk factors for prolonged recovery, or who have had prior concussions.” Dr. Starling recommended susceptibility-weighted imaging and diffusion tensor imaging.
Management priorities for patients with concussion include providing symptomatic treatment and preventing reinjury while the brain is healing. “Multidisciplinary symptoms require multidisciplinary treatment,” Dr. Starling said. “In my assessment, I’ll have a list of symptoms and a list of targeted approaches for each individual symptom.”
Posttraumatic Headache
“It is amazing how often I still see individuals two, four, eight weeks post injury who have never received a medication for posttraumatic headache because they’ve been told that the headache is a result of the concussion and as the concussion gets better, the headache will go away,” Dr. Starling said. Treating posttraumatic headache can relieve suffering and help the patient participate in active rehabilitation. Appropriate treatment can also prevent overuse of over-the-counter combination analgesics, which can complicate the problem. Experts in the headache community also suggest that there is a risk of chronification in untreated posttraumatic headache.
While there is a dearth of randomized, prospective, double-blind trials to guide the treatment of posttraumatic headache, “there still is an approach that you can use,” Dr. Starling said. Look for headache red flags first, then identify the phenotype and establish the headache history. If the patient had frequent migraines pre injury, it may be an indication for early initiation of a preventive medication. Initiating acute treatment early—within days—is also a priority, as is strictly monitoring for medication overuse. Also consider the comorbidities. “You don’t want to make comorbid symptoms worse,” Dr. Starling said. “For example, avoid topiramate in a patient who is having cognitive domain symptoms. Avoid sedating medications in someone who is having a lot of fatigue. Avoid steroids in a patient who is having a lot of emotional lability or difficulty with insomnia. Keep comorbid symptoms in mind when picking medications for posttraumatic headache.”
Return-to-Learn and Return-to-Play Decisions
Dr. Starling recommended symptom-limited cognitive and physical activity in the recovery phase, as opposed to total physical and cognitive rest. “There’s actually been a recent study that was done looking at strict rest,” she said. “The control group had one to two days of rest, followed by return to school and gradual return to activity. The intervention group had five days of strict rest.… The group with strict rest had higher symptom severity scores and had a longer symptom recovery. Exaggerated or extreme rest may not be the answer. Rather, we need to gradually reengage individuals back into life and give them a specific plan for graduated return to life, which includes both cognitive, as well as physical, activity.”
Return-to-learn protocols must be individualized, but there are some common goals. Dr. Starling recommended a short period of brain rest. “Not complete sensory deprivation, but rather symptom-limited brain rest. That should be followed by a brain warm-up phase where we initiate some time-limited and symptom-limited reading time—five to 10 minutes, as tolerated—and gradually increase that over time. After that, we reengage that individual back into school with extensive accommodations, which include the number of hours they are in school, as well as the curriculum, so a higher value on quality rather than quantity, and then a lot of environmental adjustments—perhaps a room that is quieter, a room where the lights are a little dimmer, they are allowed to wear a hat in class or sunglasses in class.” To avoid the sensory stimulation that characterizes school hallways between classes, which can make patients feel worse, Dr. Starling recommended that patients leave class five minutes early, spend the passing period in the nurse’s office, and then go to the next class five minutes late.
The next goal in recovery is full-day school with academic accommodations, and finally a return to learn without any accommodations. This requires an education specialist or a neuropsychologist who can get an individualized history from the patient as to what his or her day entails. A detailed recovery plan is then put into writing and provided to the patient and the school. The plan is then revised every one to two weeks as the patient recovers.
Dr. Starling suggested that physical activity could be initiated even when individuals are still having symptoms, but in a symptom-limited manner. “There have been studies looking at controlled exercise as a therapeutic approach for concussion,” she said. In an initial, nonrandomized pilot study, an exertion protocol seemed to improve symptoms, promote a faster rate of recovery, and normalize cerebral blood flow abnormalities during a cognitive task. “Although more rigorous studies are definitely needed, I think we are in the right paradigm,” Dr. Starling said. “After initial rest, but not complete sensory deprivation, active rehabilitation can be initiated, even in the presence of symptoms, as long as we have subthreshold activity.” This strategy, she said, is recommended to reduce symptom severity, speed recovery, and ensure full recovery.
“With active rehabilitation, we have to be prescriptive about what individual patients do. We want to make sure they are not exacerbating their symptoms.” At the Mayo Clinic, Dr. Starling and her team use written, as well as verbal, instructions. “We set in writing a goal heart rate that we want that individual patient to reach. In the clinical setting, we use a recumbent bike to determine a goal heart rate that is subthreshold to their symptoms. We then ask the patient to engage in activity up to that heart rate every day for the next couple of days. As they tolerate this, they can increase it [by] five to 10 beats per minute every three to seven days, and then we reevaluate this every one to two weeks to determine what the next step is.”
Once the exertion protocol is completed, a more sports-specific return-to-play protocol can be initiated. “During a concussion, the player can become deconditioned from their specific sport, so a sport-specific return to play protocol is important in that setting,” Dr. Starling said.
Recommending retirement from high-risk athletic activity is, of course, an individualized decision in which various components of the history come into play. According to Dr. Starling, the red flags for retirement include reduced threshold for concussion, neuroimaging abnormalities, persistent cognitive impairment, and debilitating refractory headaches.
VANCOUVER—“Concussion is a public health epidemic,” said Amaal Starling, MD. “Neurologists are seeing more and more concussed patients every day.” At the 68th Annual Meeting of the American Academy of Neurology, Dr. Starling, who is an Assistant Professor of Neurology at the Mayo Clinic in Phoenix, provided a framework and a template for evaluating concussion in the outpatient setting.
“Prioritizing these patients into clinic is very important,” Dr. Starling said. She recommended expedited appointments for patients with a suspected concussion. “This will limit symptom exacerbation, provide an avenue for appropriate and quick symptomatic treatment, and prevent premature return to learn and return to play,” which may exacerbate symptoms and prolong recovery.
Outpatient Evaluation of Concussion
The patient history should always include the date of the injury and the injury description, which includes the mechanism of the injury, location of the impact, presence or absence of any whiplash injury, altered mental status or amnesia, as well as symptom progression. “How do the symptoms progress from the time of impact to the time the patient presents in the office?” Dr. Starling asked. “This will help you identify not only those immediate symptoms that occur, but also those delayed symptoms that can occur one to two days later. In addition, it will give you a time course of symptoms to determine if the patient has been worsening, improving, or has stayed about the same.”
Concussion has various symptoms that can be categorized in the following four domains: physical, cognitive, emotional, and sleep. The most frequently reported symptom is headache, followed by dizziness. To capture all of those symptom domains, Dr. Starling recommended using a postconcussion graded symptom checklist. “This can be effective at monitoring symptoms over time.”
It is also important to elicit risk factors for prolonged recovery. “If an individual has a personal history of migraine, they are at risk of having a prolonged recovery after the injury,” Dr. Starling said. “Even if they have no personal history of migraine, but if they have a family history of migraine, those individuals, per studies, have demonstrated a prolonged recovery after a concussion.” Other risk factors for a prolonged recovery include a history of learning disabilities, such as attention deficit disorder or dyslexia, and psychiatric disease, such as premorbid anxiety or depression.
A concussion history is also important because a prior concussion increases the risk of another concussion, as well as the risk of having a prolonged recovery. “Not only do you want to know how many concussions have occurred, but also the symptom duration and recovery course for those concussions.”
Since headache is the most common symptom after a concussion, it is important to evaluate headache when present. “In every headache history, it is important to look for red flags,” said Dr. Starling. She suggested using the mnemonic IFLOP to look for headache red flags in the setting of a concussion. IFLOP stands for Intractable vomiting, Focal neurologic symptoms and signs, changes in Level of awareness, Orthostatic headache, and Progressively worsening headache. When present, headache red flags should signal the need for neuroimaging. “For example, if someone is presenting with an orthostatic headache … I am concerned that they might have a CSF leak and I’ll want to get an MRI of the brain with and without contrast to look for diffuse pachymeningeal enhancement that we can see in that setting,” Dr. Starling said.
Management of a Concussed Patient
According to Dr. Starling, posttraumatic headaches should be treated according to their phenotypes. “If [the headache] has a migraine phenotype, treat it with migraine-specific medications. If it has a more cervicogenic phenotype, treat it that way.” The most common posttraumatic headache phenotype is migraine. That finding has been confirmed in the civilian as well as the military population. “But it is important to screen for other phenotypes that may also occur,” Dr. Starling advised.
Because patients with concussion seem to be at higher risk for medication overuse and medication overuse headache, a pre- and postinjury medication history is also important. “If they are using over-the-counter medications, you’ll want to know what they are using and how much.”
During the initial visit, it is also important to determine whether the patient has had any baseline testing. “If they had any computerized neurocognitive testing, obtain those results, Dr. Starling advised. “If they had a King-Devick test at baseline or pre season, obtain those results. If they have undergone gold-standard neuropsychometric testing or had a baseline neurologic examination or imaging, get those results so that you can compare postinjury [performance] to preinjury [performance].”
Regarding the physical examination in the outpatient setting, vitals are vital, Dr. Starling said. Many concussed athletes have autonomic dysfunction that looks like postural orthostatic tachycardia syndrome (POTS), although the prognosis is typically different. “When getting vitals, it is important to get orthostatic vitals—supine and then standing at one, five, and 10 minutes—to monitor for abnormal changes or an increase in the heart rate with standing.” The physical exam should also look for trigger points or any difficulties with range of motion of the neck. “These [findings] can give you avenues for therapeutic intervention,” Dr. Starling said. Additionally, the Dix–Hallpike maneuver can identify cases of benign paroxysmal positional vertigo, which can be treated with the Epley maneuver.
Mental status should be evaluated as part of a detailed neurologic examination. The Mini-Mental State Exam (MMSE), the Montreal Cognitive Assessment (MoCA), and the Kokmen are well-validated tools for the evaluation of mental status. The Standardized Assessment of Concussion (SAC) is another tool that was developed to assess mental status. The SAC was validated on the sideline and is used by a wide array of health care providers from athletic trainers to the team physicians.
During the cranial nerve examination, Dr. Starling tests for anosmia. “That is concerning for gross structural changes on the inferior surface of the frontal lobe where the olfactory nerve lies.” Abnormalities suggest a need for neuroimaging. A typical pupillary assessment with a swinging pen light test also is an essential part of the evaluation, but Dr. Starling commented that in her patients with mild traumatic brain injury or concussion, she has rarely found any clinically significant abnormalities with that test. “But that’s not true for the evaluation of extraocular movement,” she said. “I look for not only nystagmus, abnormalities of smooth pursuit, and horizontal and vertical saccades, but I also look for near point convergence. Near point convergence of greater than 6 cm is abnormal in the majority of individuals that we will evaluate for concussion.” When it is abnormal, it correlates with oculomotor abnormalities in function. So, these people have more difficulty with oculomotor function in day-to-day life—difficulties with reading and motion sensitivity.
The rest of the cranial nerve examination can also help identify subtle focal deficits. Upper motor neuron exam techniques also can detect subtle changes, and abnormalities can suggest a need for neuroimaging. Dr. Starling also recommended a good screening evaluation of balance, such as the timed tandem gait measure.
The Concussion Toolbox
No biomarkers or tests will yield a 100% accurate diagnosis of concussion. “However, studies have repeatedly demonstrated that if we use the tools that are available, and if we use a combination of them, we are nearing 100% sensitivity and specificity,” Dr. Starling said. In her return-to-play clinic, all patients undergo the King–Devick test, neuropsychologic testing, and objective vestibular testing. If patients report autonomic or orthostatic symptoms, they also undergo autonomic testing. “Unless I’m concerned about a skull fracture, I don’t get a CT scan of the head,” Dr. Starling said. “But we do obtain an MRI of the brain in individuals who have focal neurologic deficits, risk factors for prolonged recovery, or who have had prior concussions.” Dr. Starling recommended susceptibility-weighted imaging and diffusion tensor imaging.
Management priorities for patients with concussion include providing symptomatic treatment and preventing reinjury while the brain is healing. “Multidisciplinary symptoms require multidisciplinary treatment,” Dr. Starling said. “In my assessment, I’ll have a list of symptoms and a list of targeted approaches for each individual symptom.”
Posttraumatic Headache
“It is amazing how often I still see individuals two, four, eight weeks post injury who have never received a medication for posttraumatic headache because they’ve been told that the headache is a result of the concussion and as the concussion gets better, the headache will go away,” Dr. Starling said. Treating posttraumatic headache can relieve suffering and help the patient participate in active rehabilitation. Appropriate treatment can also prevent overuse of over-the-counter combination analgesics, which can complicate the problem. Experts in the headache community also suggest that there is a risk of chronification in untreated posttraumatic headache.
While there is a dearth of randomized, prospective, double-blind trials to guide the treatment of posttraumatic headache, “there still is an approach that you can use,” Dr. Starling said. Look for headache red flags first, then identify the phenotype and establish the headache history. If the patient had frequent migraines pre injury, it may be an indication for early initiation of a preventive medication. Initiating acute treatment early—within days—is also a priority, as is strictly monitoring for medication overuse. Also consider the comorbidities. “You don’t want to make comorbid symptoms worse,” Dr. Starling said. “For example, avoid topiramate in a patient who is having cognitive domain symptoms. Avoid sedating medications in someone who is having a lot of fatigue. Avoid steroids in a patient who is having a lot of emotional lability or difficulty with insomnia. Keep comorbid symptoms in mind when picking medications for posttraumatic headache.”
Return-to-Learn and Return-to-Play Decisions
Dr. Starling recommended symptom-limited cognitive and physical activity in the recovery phase, as opposed to total physical and cognitive rest. “There’s actually been a recent study that was done looking at strict rest,” she said. “The control group had one to two days of rest, followed by return to school and gradual return to activity. The intervention group had five days of strict rest.… The group with strict rest had higher symptom severity scores and had a longer symptom recovery. Exaggerated or extreme rest may not be the answer. Rather, we need to gradually reengage individuals back into life and give them a specific plan for graduated return to life, which includes both cognitive, as well as physical, activity.”
Return-to-learn protocols must be individualized, but there are some common goals. Dr. Starling recommended a short period of brain rest. “Not complete sensory deprivation, but rather symptom-limited brain rest. That should be followed by a brain warm-up phase where we initiate some time-limited and symptom-limited reading time—five to 10 minutes, as tolerated—and gradually increase that over time. After that, we reengage that individual back into school with extensive accommodations, which include the number of hours they are in school, as well as the curriculum, so a higher value on quality rather than quantity, and then a lot of environmental adjustments—perhaps a room that is quieter, a room where the lights are a little dimmer, they are allowed to wear a hat in class or sunglasses in class.” To avoid the sensory stimulation that characterizes school hallways between classes, which can make patients feel worse, Dr. Starling recommended that patients leave class five minutes early, spend the passing period in the nurse’s office, and then go to the next class five minutes late.
The next goal in recovery is full-day school with academic accommodations, and finally a return to learn without any accommodations. This requires an education specialist or a neuropsychologist who can get an individualized history from the patient as to what his or her day entails. A detailed recovery plan is then put into writing and provided to the patient and the school. The plan is then revised every one to two weeks as the patient recovers.
Dr. Starling suggested that physical activity could be initiated even when individuals are still having symptoms, but in a symptom-limited manner. “There have been studies looking at controlled exercise as a therapeutic approach for concussion,” she said. In an initial, nonrandomized pilot study, an exertion protocol seemed to improve symptoms, promote a faster rate of recovery, and normalize cerebral blood flow abnormalities during a cognitive task. “Although more rigorous studies are definitely needed, I think we are in the right paradigm,” Dr. Starling said. “After initial rest, but not complete sensory deprivation, active rehabilitation can be initiated, even in the presence of symptoms, as long as we have subthreshold activity.” This strategy, she said, is recommended to reduce symptom severity, speed recovery, and ensure full recovery.
“With active rehabilitation, we have to be prescriptive about what individual patients do. We want to make sure they are not exacerbating their symptoms.” At the Mayo Clinic, Dr. Starling and her team use written, as well as verbal, instructions. “We set in writing a goal heart rate that we want that individual patient to reach. In the clinical setting, we use a recumbent bike to determine a goal heart rate that is subthreshold to their symptoms. We then ask the patient to engage in activity up to that heart rate every day for the next couple of days. As they tolerate this, they can increase it [by] five to 10 beats per minute every three to seven days, and then we reevaluate this every one to two weeks to determine what the next step is.”
Once the exertion protocol is completed, a more sports-specific return-to-play protocol can be initiated. “During a concussion, the player can become deconditioned from their specific sport, so a sport-specific return to play protocol is important in that setting,” Dr. Starling said.
Recommending retirement from high-risk athletic activity is, of course, an individualized decision in which various components of the history come into play. According to Dr. Starling, the red flags for retirement include reduced threshold for concussion, neuroimaging abnormalities, persistent cognitive impairment, and debilitating refractory headaches.
Adult Photosensitivity Disorders
Review the PDF of the fact sheet on adult photosensitivity disorders with board-relevant, easy-to-review material. This month's fact sheet will review important disorders in the adult population where photosensitivity is a major feature.
Practice Questions
1. A 50-year-old woman with a history of alcoholism and new-onset diarrhea developed a painful, scaly, erythematous, and hyperpigmented eruption on the photoexposed areas on the chest and hands. A similar presentation can occur in patients on which medications?
a. azathioprine
b. fluorouracil
c. pyrazinamide
d. A and C only
e. all of the above
2. A college student presents with a streaky blistering rash on the arms and legs. He is on summer vacation and recently started a side job of mowing lawns. This phototoxic eruption requires which light spectrum?
a. 200–290 nm
b. 290–315 nm
c. 315–400 nm
d. 400–700 nm
e. none of the above
3. A middle-aged man with psoriasis complains of new onset of redness of the hands and face that occurs within hours of going outside. The patient may be taking which medications?
a. doxepin
b. NSAIDS
c. tar shampoo
d. terbinafine
e. A, B, and C
f. B, C, and D
4. A patient with metastatic melanoma was just started on vemurafenib. Which side effect is most likely to occur from this medication?
a. cough
b. myalgia
c. panniculitis
d. photosensitivity
e. squamous cell carcinoma
5. A 30-year-old black woman reports an itchy, flesh-colored, bumpy rash on the extensor forearms that appears 24 hours after sun exposure. There was no prior exposure to systemic or topical photoallergens. Which of the following is false regarding this condition?
a. classified as a type IV hypersensitivity reaction
b. condition improves with subsequent exposures (hardening)
c. histology is characterized by mucin deposition
d. rash is generally nonscarring
e. similar reaction localized to the helices may occur in adolescent boys
Answers to practice questions provided on next page
Practice Question Answers
1. A 50-year-old woman with a history of alcoholism and new-onset diarrhea developed a painful, scaly, erythematous, and hyperpigmented eruption on the photoexposed areas on the chest and hands. A similar presentation can occur in patients on which medications?
a. azathioprine
b. fluorouracil
c. pyrazinamide
d. A and C only
e. all of the above
2. A college student presents with a streaky blistering rash on the arms and legs. He is on summer vacation and recently started a side job of mowing lawns. This phototoxic eruption requires which light spectrum?
a. 200–290 nm
b. 290–315 nm
c. 315–400 nm
d. 400–700 nm
e. none of the above
3. A middle-aged man with psoriasis complains of new onset of redness of the hands and face that occurs within hours of going outside. The patient may be taking which medications?
a. doxepin
b. NSAIDS
c. tar shampoo
d. terbinafine
e. A, B, and C
f. B, C, and D
4. A patient with metastatic melanoma was just started on vemurafenib. Which side effect is most likely to occur from this medication?
a. cough
b. myalgia
c. panniculitis
d. photosensitivity
e. squamous cell carcinoma
5. A 30-year-old black woman reports an itchy, flesh-colored, bumpy rash on the extensor forearms that appears 24 hours after sun exposure. There was no prior exposure to systemic or topical photoallergens. Which of the following is false regarding this condition?
a. classified as a type IV hypersensitivity reaction
b. condition improves with subsequent exposures (hardening)
c. histology is characterized by mucin deposition
d. rash is generally nonscarring
e. similar reaction localized to the helices may occur in adolescent boys
Review the PDF of the fact sheet on adult photosensitivity disorders with board-relevant, easy-to-review material. This month's fact sheet will review important disorders in the adult population where photosensitivity is a major feature.
Practice Questions
1. A 50-year-old woman with a history of alcoholism and new-onset diarrhea developed a painful, scaly, erythematous, and hyperpigmented eruption on the photoexposed areas on the chest and hands. A similar presentation can occur in patients on which medications?
a. azathioprine
b. fluorouracil
c. pyrazinamide
d. A and C only
e. all of the above
2. A college student presents with a streaky blistering rash on the arms and legs. He is on summer vacation and recently started a side job of mowing lawns. This phototoxic eruption requires which light spectrum?
a. 200–290 nm
b. 290–315 nm
c. 315–400 nm
d. 400–700 nm
e. none of the above
3. A middle-aged man with psoriasis complains of new onset of redness of the hands and face that occurs within hours of going outside. The patient may be taking which medications?
a. doxepin
b. NSAIDS
c. tar shampoo
d. terbinafine
e. A, B, and C
f. B, C, and D
4. A patient with metastatic melanoma was just started on vemurafenib. Which side effect is most likely to occur from this medication?
a. cough
b. myalgia
c. panniculitis
d. photosensitivity
e. squamous cell carcinoma
5. A 30-year-old black woman reports an itchy, flesh-colored, bumpy rash on the extensor forearms that appears 24 hours after sun exposure. There was no prior exposure to systemic or topical photoallergens. Which of the following is false regarding this condition?
a. classified as a type IV hypersensitivity reaction
b. condition improves with subsequent exposures (hardening)
c. histology is characterized by mucin deposition
d. rash is generally nonscarring
e. similar reaction localized to the helices may occur in adolescent boys
Answers to practice questions provided on next page
Practice Question Answers
1. A 50-year-old woman with a history of alcoholism and new-onset diarrhea developed a painful, scaly, erythematous, and hyperpigmented eruption on the photoexposed areas on the chest and hands. A similar presentation can occur in patients on which medications?
a. azathioprine
b. fluorouracil
c. pyrazinamide
d. A and C only
e. all of the above
2. A college student presents with a streaky blistering rash on the arms and legs. He is on summer vacation and recently started a side job of mowing lawns. This phototoxic eruption requires which light spectrum?
a. 200–290 nm
b. 290–315 nm
c. 315–400 nm
d. 400–700 nm
e. none of the above
3. A middle-aged man with psoriasis complains of new onset of redness of the hands and face that occurs within hours of going outside. The patient may be taking which medications?
a. doxepin
b. NSAIDS
c. tar shampoo
d. terbinafine
e. A, B, and C
f. B, C, and D
4. A patient with metastatic melanoma was just started on vemurafenib. Which side effect is most likely to occur from this medication?
a. cough
b. myalgia
c. panniculitis
d. photosensitivity
e. squamous cell carcinoma
5. A 30-year-old black woman reports an itchy, flesh-colored, bumpy rash on the extensor forearms that appears 24 hours after sun exposure. There was no prior exposure to systemic or topical photoallergens. Which of the following is false regarding this condition?
a. classified as a type IV hypersensitivity reaction
b. condition improves with subsequent exposures (hardening)
c. histology is characterized by mucin deposition
d. rash is generally nonscarring
e. similar reaction localized to the helices may occur in adolescent boys
Review the PDF of the fact sheet on adult photosensitivity disorders with board-relevant, easy-to-review material. This month's fact sheet will review important disorders in the adult population where photosensitivity is a major feature.
Practice Questions
1. A 50-year-old woman with a history of alcoholism and new-onset diarrhea developed a painful, scaly, erythematous, and hyperpigmented eruption on the photoexposed areas on the chest and hands. A similar presentation can occur in patients on which medications?
a. azathioprine
b. fluorouracil
c. pyrazinamide
d. A and C only
e. all of the above
2. A college student presents with a streaky blistering rash on the arms and legs. He is on summer vacation and recently started a side job of mowing lawns. This phototoxic eruption requires which light spectrum?
a. 200–290 nm
b. 290–315 nm
c. 315–400 nm
d. 400–700 nm
e. none of the above
3. A middle-aged man with psoriasis complains of new onset of redness of the hands and face that occurs within hours of going outside. The patient may be taking which medications?
a. doxepin
b. NSAIDS
c. tar shampoo
d. terbinafine
e. A, B, and C
f. B, C, and D
4. A patient with metastatic melanoma was just started on vemurafenib. Which side effect is most likely to occur from this medication?
a. cough
b. myalgia
c. panniculitis
d. photosensitivity
e. squamous cell carcinoma
5. A 30-year-old black woman reports an itchy, flesh-colored, bumpy rash on the extensor forearms that appears 24 hours after sun exposure. There was no prior exposure to systemic or topical photoallergens. Which of the following is false regarding this condition?
a. classified as a type IV hypersensitivity reaction
b. condition improves with subsequent exposures (hardening)
c. histology is characterized by mucin deposition
d. rash is generally nonscarring
e. similar reaction localized to the helices may occur in adolescent boys
Answers to practice questions provided on next page
Practice Question Answers
1. A 50-year-old woman with a history of alcoholism and new-onset diarrhea developed a painful, scaly, erythematous, and hyperpigmented eruption on the photoexposed areas on the chest and hands. A similar presentation can occur in patients on which medications?
a. azathioprine
b. fluorouracil
c. pyrazinamide
d. A and C only
e. all of the above
2. A college student presents with a streaky blistering rash on the arms and legs. He is on summer vacation and recently started a side job of mowing lawns. This phototoxic eruption requires which light spectrum?
a. 200–290 nm
b. 290–315 nm
c. 315–400 nm
d. 400–700 nm
e. none of the above
3. A middle-aged man with psoriasis complains of new onset of redness of the hands and face that occurs within hours of going outside. The patient may be taking which medications?
a. doxepin
b. NSAIDS
c. tar shampoo
d. terbinafine
e. A, B, and C
f. B, C, and D
4. A patient with metastatic melanoma was just started on vemurafenib. Which side effect is most likely to occur from this medication?
a. cough
b. myalgia
c. panniculitis
d. photosensitivity
e. squamous cell carcinoma
5. A 30-year-old black woman reports an itchy, flesh-colored, bumpy rash on the extensor forearms that appears 24 hours after sun exposure. There was no prior exposure to systemic or topical photoallergens. Which of the following is false regarding this condition?
a. classified as a type IV hypersensitivity reaction
b. condition improves with subsequent exposures (hardening)
c. histology is characterized by mucin deposition
d. rash is generally nonscarring
e. similar reaction localized to the helices may occur in adolescent boys