User login
First-episode psychosis is a ‘brain attack,’ and LAIs can prevent recurrence, expert says
washington – As data mount confirming the neurodegenerative effects of psychotic episodes in schizophrenia, one expert urges psychiatrists to think of psychosis as a “brain attack” which, like heart attacks, must be prevented from recurring.
“Schizophrenia doesn’t have to be progressive neurodegenerative unless patients relapse again and again, but that happens all the time because we give our patients pills they don’t take as prescribed. There are many reasons for poor adherence,” Henry A. Nasrallah, MD, said at the meeting held by Global Academy for Medical Education.
In a presentation dedicated to the emerging science reshaping views on how schizophrenia occurs, how it can be prevented, and why it is a syndrome with genetic etiologies, Dr. Nasrallah emphasized that there now exist enough data to show that timely intervention with LAIs reliably prevent relapse in most patients, thereby averting progressive neurodegeneration and subsequent disability in people who develop schizophrenia.
“Researchers now stage schizophrenia. Just like cancer, the more advanced the stage, the worse the outcome,” said Dr. Nasrallah, the Sydney W. Souers Endowed Chair and professor of psychiatry and behavioral neuroscience at Saint Louis University, told his audience. “The additional damaging effects of the second episode is what leads to clinical deterioration and can start the process of treatment resistance. But if no psychotic episodes are allowed to recur after the first episode, many patients can return to their baseline functioning, such as school or work.”
The field still is clarifying the neurodevelopmental aspects of schizophrenia, including genetic and in utero adverse events that disrupt brain development, as well as the appropriate types and timing of intervention in the prodromal phase. However, Dr. Nasrallah explained, science already has demonstrated how the neurotoxic effects of psychosis in the brain of a person with schizophrenia lead to brain tissue degradation with every psychotic episode. The result is a progressive decline in social and vocational functioning.
Psychosis is associated with activation of microglia, which are monocytic cells that cross the blood-brain barrier during fetal life, settling in the brain and ultimately comprising 10%-15% of all brain cells. Once activated, they trigger an immune response, leading to neuroinflammation and oxidative stress (free radicals). However, Dr. Nasrallah said, rather than protect the brain, these processes destroy gray and white matter – particularly in the cortical region – degrading the brain and leaving it more compromised, especially if another episode of psychosis occurs.
Another factor in the vulnerability of the brain in schizophrenia is mitochondrial dysfunction. As mitochondria are the primary source of antioxidants – such as glutathione – the deficit in antioxidants increases oxidative stress, furthering the brain’s vulnerability to tissue loss.
Among other biological processes thought to be implicated in neurodegeneration with schizophrenia, Dr. Nasrallah said, are impairment of antiapoptotic signaling, glutamate excitotoxicity, hypercortisolemia, and gamma-aminobutyric acid hypofunction.
The overall effect of these neurotoxic blows is a brain that experiences white and gray matter pathologies, leading to impaired neuroplasticity with increasing levels of white matter disconnectivity, Dr. Nasrallah said.
He pointed out that studies have shown a loss of 1% of total brain volume and 3% of gray matter volume with the first psychotic episode. Cerebral ventricles expand by about 7%. “The different parts of the brain no longer communicate properly with each other across myelinated fibers, which is postulated as an explanation for cognitive impairment, thought disorder, negative symptoms, and lack of insight – all of which can contribute to nonadherence to treatment.”
Prompt treatment of the first episode of psychosis and starting the patient on an LAI can protect the brain from another destructive round of neuroinflammation and oxidative stress. He recommends that his patients take omega-3 fatty acids to expedite the anti-inflammatory response of the antipsychotic drug. First-generation antipsychotics are not neuroprotective, according to Dr. Nasrallah, whose own research shows that haloperidol is itself neurotoxic and kills neurons – although it is an efficacious antipsychotic.
Updated American Psychiatric Association practice guidelines for treating first-episode psychosis, published in 2010, do not recommend LAIs as a first-line treatment. Neither do the National Institute of Mental Health’s Schizophrenia Patient Outcomes Research Team treatment recommendations and summary statements, published in 2009. Similarly, neither document stresses atypicals over first-generation antipsychotics.
However, Dr. Nasrallah cited a randomized, controlled study from the University of California, Los Angeles, showing that in 86 patients with first-episode psychosis who were given the same antipsychotic in either oral or LAI form, at the end of 1-year follow-up, the researchers reported a 650% higher relapse rate in the oral group (33%), compared with the LAI group (5%) (JAMA Psychiatry. 2015 Aug;72[8]:822-9).
“I tell my residents to behave like cardiologists,” Dr. Nasrallah said. “When cardiologists have a patient who experienced the first heart attack, they make it an absolute goal never to let the patient have another myocardial infarction because the first one permanently killed part of the myocardium, and a second heart attack will either kill the person or make him need a heart transplant. They implement a multifaceted intervention to achieve that goal. We psychiatrists should regard [the first episode of psychosis] as a ‘brain attack,’ and we should never let patients have another one again. The best intervention we have for this is starting an atypical LAI in first-episode patients.”
Global Academy and this news organization are owned by the same company. Dr. Nasrallah has conducted Food and Drug Administration clinical trials with LAIs and has several industry ties, including with Alkermes, Janssen, and Otsuka.
[email protected]
On Twitter @whitneymcknight
washington – As data mount confirming the neurodegenerative effects of psychotic episodes in schizophrenia, one expert urges psychiatrists to think of psychosis as a “brain attack” which, like heart attacks, must be prevented from recurring.
“Schizophrenia doesn’t have to be progressive neurodegenerative unless patients relapse again and again, but that happens all the time because we give our patients pills they don’t take as prescribed. There are many reasons for poor adherence,” Henry A. Nasrallah, MD, said at the meeting held by Global Academy for Medical Education.
In a presentation dedicated to the emerging science reshaping views on how schizophrenia occurs, how it can be prevented, and why it is a syndrome with genetic etiologies, Dr. Nasrallah emphasized that there now exist enough data to show that timely intervention with LAIs reliably prevent relapse in most patients, thereby averting progressive neurodegeneration and subsequent disability in people who develop schizophrenia.
“Researchers now stage schizophrenia. Just like cancer, the more advanced the stage, the worse the outcome,” said Dr. Nasrallah, the Sydney W. Souers Endowed Chair and professor of psychiatry and behavioral neuroscience at Saint Louis University, told his audience. “The additional damaging effects of the second episode is what leads to clinical deterioration and can start the process of treatment resistance. But if no psychotic episodes are allowed to recur after the first episode, many patients can return to their baseline functioning, such as school or work.”
The field still is clarifying the neurodevelopmental aspects of schizophrenia, including genetic and in utero adverse events that disrupt brain development, as well as the appropriate types and timing of intervention in the prodromal phase. However, Dr. Nasrallah explained, science already has demonstrated how the neurotoxic effects of psychosis in the brain of a person with schizophrenia lead to brain tissue degradation with every psychotic episode. The result is a progressive decline in social and vocational functioning.
Psychosis is associated with activation of microglia, which are monocytic cells that cross the blood-brain barrier during fetal life, settling in the brain and ultimately comprising 10%-15% of all brain cells. Once activated, they trigger an immune response, leading to neuroinflammation and oxidative stress (free radicals). However, Dr. Nasrallah said, rather than protect the brain, these processes destroy gray and white matter – particularly in the cortical region – degrading the brain and leaving it more compromised, especially if another episode of psychosis occurs.
Another factor in the vulnerability of the brain in schizophrenia is mitochondrial dysfunction. As mitochondria are the primary source of antioxidants – such as glutathione – the deficit in antioxidants increases oxidative stress, furthering the brain’s vulnerability to tissue loss.
Among other biological processes thought to be implicated in neurodegeneration with schizophrenia, Dr. Nasrallah said, are impairment of antiapoptotic signaling, glutamate excitotoxicity, hypercortisolemia, and gamma-aminobutyric acid hypofunction.
The overall effect of these neurotoxic blows is a brain that experiences white and gray matter pathologies, leading to impaired neuroplasticity with increasing levels of white matter disconnectivity, Dr. Nasrallah said.
He pointed out that studies have shown a loss of 1% of total brain volume and 3% of gray matter volume with the first psychotic episode. Cerebral ventricles expand by about 7%. “The different parts of the brain no longer communicate properly with each other across myelinated fibers, which is postulated as an explanation for cognitive impairment, thought disorder, negative symptoms, and lack of insight – all of which can contribute to nonadherence to treatment.”
Prompt treatment of the first episode of psychosis and starting the patient on an LAI can protect the brain from another destructive round of neuroinflammation and oxidative stress. He recommends that his patients take omega-3 fatty acids to expedite the anti-inflammatory response of the antipsychotic drug. First-generation antipsychotics are not neuroprotective, according to Dr. Nasrallah, whose own research shows that haloperidol is itself neurotoxic and kills neurons – although it is an efficacious antipsychotic.
Updated American Psychiatric Association practice guidelines for treating first-episode psychosis, published in 2010, do not recommend LAIs as a first-line treatment. Neither do the National Institute of Mental Health’s Schizophrenia Patient Outcomes Research Team treatment recommendations and summary statements, published in 2009. Similarly, neither document stresses atypicals over first-generation antipsychotics.
However, Dr. Nasrallah cited a randomized, controlled study from the University of California, Los Angeles, showing that in 86 patients with first-episode psychosis who were given the same antipsychotic in either oral or LAI form, at the end of 1-year follow-up, the researchers reported a 650% higher relapse rate in the oral group (33%), compared with the LAI group (5%) (JAMA Psychiatry. 2015 Aug;72[8]:822-9).
“I tell my residents to behave like cardiologists,” Dr. Nasrallah said. “When cardiologists have a patient who experienced the first heart attack, they make it an absolute goal never to let the patient have another myocardial infarction because the first one permanently killed part of the myocardium, and a second heart attack will either kill the person or make him need a heart transplant. They implement a multifaceted intervention to achieve that goal. We psychiatrists should regard [the first episode of psychosis] as a ‘brain attack,’ and we should never let patients have another one again. The best intervention we have for this is starting an atypical LAI in first-episode patients.”
Global Academy and this news organization are owned by the same company. Dr. Nasrallah has conducted Food and Drug Administration clinical trials with LAIs and has several industry ties, including with Alkermes, Janssen, and Otsuka.
[email protected]
On Twitter @whitneymcknight
washington – As data mount confirming the neurodegenerative effects of psychotic episodes in schizophrenia, one expert urges psychiatrists to think of psychosis as a “brain attack” which, like heart attacks, must be prevented from recurring.
“Schizophrenia doesn’t have to be progressive neurodegenerative unless patients relapse again and again, but that happens all the time because we give our patients pills they don’t take as prescribed. There are many reasons for poor adherence,” Henry A. Nasrallah, MD, said at the meeting held by Global Academy for Medical Education.
In a presentation dedicated to the emerging science reshaping views on how schizophrenia occurs, how it can be prevented, and why it is a syndrome with genetic etiologies, Dr. Nasrallah emphasized that there now exist enough data to show that timely intervention with LAIs reliably prevent relapse in most patients, thereby averting progressive neurodegeneration and subsequent disability in people who develop schizophrenia.
“Researchers now stage schizophrenia. Just like cancer, the more advanced the stage, the worse the outcome,” said Dr. Nasrallah, the Sydney W. Souers Endowed Chair and professor of psychiatry and behavioral neuroscience at Saint Louis University, told his audience. “The additional damaging effects of the second episode is what leads to clinical deterioration and can start the process of treatment resistance. But if no psychotic episodes are allowed to recur after the first episode, many patients can return to their baseline functioning, such as school or work.”
The field still is clarifying the neurodevelopmental aspects of schizophrenia, including genetic and in utero adverse events that disrupt brain development, as well as the appropriate types and timing of intervention in the prodromal phase. However, Dr. Nasrallah explained, science already has demonstrated how the neurotoxic effects of psychosis in the brain of a person with schizophrenia lead to brain tissue degradation with every psychotic episode. The result is a progressive decline in social and vocational functioning.
Psychosis is associated with activation of microglia, which are monocytic cells that cross the blood-brain barrier during fetal life, settling in the brain and ultimately comprising 10%-15% of all brain cells. Once activated, they trigger an immune response, leading to neuroinflammation and oxidative stress (free radicals). However, Dr. Nasrallah said, rather than protect the brain, these processes destroy gray and white matter – particularly in the cortical region – degrading the brain and leaving it more compromised, especially if another episode of psychosis occurs.
Another factor in the vulnerability of the brain in schizophrenia is mitochondrial dysfunction. As mitochondria are the primary source of antioxidants – such as glutathione – the deficit in antioxidants increases oxidative stress, furthering the brain’s vulnerability to tissue loss.
Among other biological processes thought to be implicated in neurodegeneration with schizophrenia, Dr. Nasrallah said, are impairment of antiapoptotic signaling, glutamate excitotoxicity, hypercortisolemia, and gamma-aminobutyric acid hypofunction.
The overall effect of these neurotoxic blows is a brain that experiences white and gray matter pathologies, leading to impaired neuroplasticity with increasing levels of white matter disconnectivity, Dr. Nasrallah said.
He pointed out that studies have shown a loss of 1% of total brain volume and 3% of gray matter volume with the first psychotic episode. Cerebral ventricles expand by about 7%. “The different parts of the brain no longer communicate properly with each other across myelinated fibers, which is postulated as an explanation for cognitive impairment, thought disorder, negative symptoms, and lack of insight – all of which can contribute to nonadherence to treatment.”
Prompt treatment of the first episode of psychosis and starting the patient on an LAI can protect the brain from another destructive round of neuroinflammation and oxidative stress. He recommends that his patients take omega-3 fatty acids to expedite the anti-inflammatory response of the antipsychotic drug. First-generation antipsychotics are not neuroprotective, according to Dr. Nasrallah, whose own research shows that haloperidol is itself neurotoxic and kills neurons – although it is an efficacious antipsychotic.
Updated American Psychiatric Association practice guidelines for treating first-episode psychosis, published in 2010, do not recommend LAIs as a first-line treatment. Neither do the National Institute of Mental Health’s Schizophrenia Patient Outcomes Research Team treatment recommendations and summary statements, published in 2009. Similarly, neither document stresses atypicals over first-generation antipsychotics.
However, Dr. Nasrallah cited a randomized, controlled study from the University of California, Los Angeles, showing that in 86 patients with first-episode psychosis who were given the same antipsychotic in either oral or LAI form, at the end of 1-year follow-up, the researchers reported a 650% higher relapse rate in the oral group (33%), compared with the LAI group (5%) (JAMA Psychiatry. 2015 Aug;72[8]:822-9).
“I tell my residents to behave like cardiologists,” Dr. Nasrallah said. “When cardiologists have a patient who experienced the first heart attack, they make it an absolute goal never to let the patient have another myocardial infarction because the first one permanently killed part of the myocardium, and a second heart attack will either kill the person or make him need a heart transplant. They implement a multifaceted intervention to achieve that goal. We psychiatrists should regard [the first episode of psychosis] as a ‘brain attack,’ and we should never let patients have another one again. The best intervention we have for this is starting an atypical LAI in first-episode patients.”
Global Academy and this news organization are owned by the same company. Dr. Nasrallah has conducted Food and Drug Administration clinical trials with LAIs and has several industry ties, including with Alkermes, Janssen, and Otsuka.
[email protected]
On Twitter @whitneymcknight
AT SUMMIT IN NEUROLOGY & PSYCHIATRY
Savolitinib improves PFS in some with MET-driven papillary renal cell carcinoma
Savolitinib, a highly selective inhibitor of c-Met receptor tyrosine kinase, improved progression-free survival in a small portion of patients with MET-driven advanced renal papillary cell cancer.
The molecule, being developed by Hutchison China MediTech Limited and AstraZeneca, extended progression-free survival by about 5 months in these patients, compared with those whose tumors were not MET driven (6.2 vs. 1.4 months, respectively), reported Toni Choueiri, MD, and his colleagues (J Clin Oncol 2017. doi: 10.1200/JCO.2017.72.2967).
Of 109 patients in the phase II study, 44 (40%) had MET-driven disease. The cancer was MET independent in 46 (42%); MET status was unknown in the remainder.
Half of those with MET-driven tumors experienced stable disease, and 61% experienced tumor shrinkage ranging from 0.7% to 66%. Tumor shrinkage occurred in 20% of those with MET-independent tumors. These also responded less vigorously (shrinkage 0.5%-20%).
Of the eight patients exhibiting a partial response, six were still responding to treatment at the study’s end, with response ranging from 2 to 16 months. Two patients experienced progressive disease after 1.8 and 2.8 months. By the end of the study, disease progression had occurred in 75% of the MET-driven cases, 96% of the MET-independent cases, and 74% of cases whose MET status was unknown.
“These results confirm that savolitinib … holds promise as a personalized treatment for patients with metastatic MET-driven papillary renal cell carcinoma,” the investigators wrote. The data also support the June launch of the phase III SAVOIR trial, they noted.
SAVOIR will enroll about 180 patients with MET-driven renal papillary cancer confirmed by a next-generation molecular sequencing developed by the companies for savolitinib response. Patients will be randomized to continuous treatment with savolitinib 600 mg orally, once daily, or intermittent treatment with sunitinib 50 mg orally once daily (4 weeks on/2 weeks off), on a 6-week cycle.
Dr. Choueiri has been a consultant for and received research funding from numerous pharmaceutical companies, including AstraZeneca.
[email protected]
On Twitter @Alz_gal
Savolitinib, a highly selective inhibitor of c-Met receptor tyrosine kinase, improved progression-free survival in a small portion of patients with MET-driven advanced renal papillary cell cancer.
The molecule, being developed by Hutchison China MediTech Limited and AstraZeneca, extended progression-free survival by about 5 months in these patients, compared with those whose tumors were not MET driven (6.2 vs. 1.4 months, respectively), reported Toni Choueiri, MD, and his colleagues (J Clin Oncol 2017. doi: 10.1200/JCO.2017.72.2967).
Of 109 patients in the phase II study, 44 (40%) had MET-driven disease. The cancer was MET independent in 46 (42%); MET status was unknown in the remainder.
Half of those with MET-driven tumors experienced stable disease, and 61% experienced tumor shrinkage ranging from 0.7% to 66%. Tumor shrinkage occurred in 20% of those with MET-independent tumors. These also responded less vigorously (shrinkage 0.5%-20%).
Of the eight patients exhibiting a partial response, six were still responding to treatment at the study’s end, with response ranging from 2 to 16 months. Two patients experienced progressive disease after 1.8 and 2.8 months. By the end of the study, disease progression had occurred in 75% of the MET-driven cases, 96% of the MET-independent cases, and 74% of cases whose MET status was unknown.
“These results confirm that savolitinib … holds promise as a personalized treatment for patients with metastatic MET-driven papillary renal cell carcinoma,” the investigators wrote. The data also support the June launch of the phase III SAVOIR trial, they noted.
SAVOIR will enroll about 180 patients with MET-driven renal papillary cancer confirmed by a next-generation molecular sequencing developed by the companies for savolitinib response. Patients will be randomized to continuous treatment with savolitinib 600 mg orally, once daily, or intermittent treatment with sunitinib 50 mg orally once daily (4 weeks on/2 weeks off), on a 6-week cycle.
Dr. Choueiri has been a consultant for and received research funding from numerous pharmaceutical companies, including AstraZeneca.
[email protected]
On Twitter @Alz_gal
Savolitinib, a highly selective inhibitor of c-Met receptor tyrosine kinase, improved progression-free survival in a small portion of patients with MET-driven advanced renal papillary cell cancer.
The molecule, being developed by Hutchison China MediTech Limited and AstraZeneca, extended progression-free survival by about 5 months in these patients, compared with those whose tumors were not MET driven (6.2 vs. 1.4 months, respectively), reported Toni Choueiri, MD, and his colleagues (J Clin Oncol 2017. doi: 10.1200/JCO.2017.72.2967).
Of 109 patients in the phase II study, 44 (40%) had MET-driven disease. The cancer was MET independent in 46 (42%); MET status was unknown in the remainder.
Half of those with MET-driven tumors experienced stable disease, and 61% experienced tumor shrinkage ranging from 0.7% to 66%. Tumor shrinkage occurred in 20% of those with MET-independent tumors. These also responded less vigorously (shrinkage 0.5%-20%).
Of the eight patients exhibiting a partial response, six were still responding to treatment at the study’s end, with response ranging from 2 to 16 months. Two patients experienced progressive disease after 1.8 and 2.8 months. By the end of the study, disease progression had occurred in 75% of the MET-driven cases, 96% of the MET-independent cases, and 74% of cases whose MET status was unknown.
“These results confirm that savolitinib … holds promise as a personalized treatment for patients with metastatic MET-driven papillary renal cell carcinoma,” the investigators wrote. The data also support the June launch of the phase III SAVOIR trial, they noted.
SAVOIR will enroll about 180 patients with MET-driven renal papillary cancer confirmed by a next-generation molecular sequencing developed by the companies for savolitinib response. Patients will be randomized to continuous treatment with savolitinib 600 mg orally, once daily, or intermittent treatment with sunitinib 50 mg orally once daily (4 weeks on/2 weeks off), on a 6-week cycle.
Dr. Choueiri has been a consultant for and received research funding from numerous pharmaceutical companies, including AstraZeneca.
[email protected]
On Twitter @Alz_gal
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: A partial response occurred in 18% of these patients, who gained about 5 months of progression-free disease compared with those with MET-independent tumors.
Data source: A phase II trial comprising 109 patients.
Disclosures: Dr. Choueiri has been a consultant for and received research funds from AstraZeneca, which is developing the drug.
Multiply recurrent C. difficile infection is on the rise
A retrospective cohort study of Clostridium difficile infection (CDI), the most common health care–associated infection, found that multiply recurrent CDI (mrCDI) is increasing in incidence, disproportionately to the overall increase in CDI.
Researchers from the University of Pennsylvania, Philadelphia, worked with a database of more than 38 million individuals with private health insurance between January 2001 and December 2012.
Cases of CDI and mrCDI in the study population were determined through ICD-9 diagnosis codes, and prescriptions for treatment. To meet the definition of mrCDI, there had to be at least three courses of treatment lasting at least 14 days each.
In the study population, 45,341 persons developed CDI, of whom 1,669 had mrCDI. The median age was 46 years, and 58.9% were female. Between 2001 and 2012, CDI incidence increased by 42.7% (P = .004), while mrCDI incidence increased by 188.8% (P less than .001).
With increases in CDI and mrCDI incidence, and with the effectiveness of standard antibiotic treatment decreasing with each recurrence, “demand for new antimicrobial therapies and FMT [fecal microbiota transplantation] can be expected to increase considerably in the coming years,” wrote Gene K. Ma, MD, and his coauthors.
As for FMT, the researchers noted that its likely greater demand in the future (as suggested by their study results) highlights the importance of establishing the long-term safety of the procedure (Ann Intern Med. 2017 Jul. doi: 10.7326/M16-2733).
The retrospective cohort study was based on administrative data rather than laboratory data, Sameer D. Saini, MD, MS, and Akbar K. Waljee, MD, noted in an editorial accompanying the study. Further, with Medicare patients excluded from the study (because Medicare data were not available for the full time period studied for private insurance data), the data may not be of relevance to patients older than age 65 years.
But the general conclusion that both CDI and mrCDI are on the rise is a crucial matter. “We must first have a better understanding of mrCDI, its scope and epidemiology, and its associated risk factors. The study by Ma and colleagues begins this important work. A better understanding of the epidemiology of mrCDI is a critical first step toward developing a sound strategy to address this growing public health challenge.”
Dr. Saini and Dr. Waljee are with the VA Ann Arbor (Michigan) Center for Clinical Management. Their editorial accompanied the study in Annals of Internal Medicine (2017 Jul. doi: 10.7326/M17-1565).
The retrospective cohort study was based on administrative data rather than laboratory data, Sameer D. Saini, MD, MS, and Akbar K. Waljee, MD, noted in an editorial accompanying the study. Further, with Medicare patients excluded from the study (because Medicare data were not available for the full time period studied for private insurance data), the data may not be of relevance to patients older than age 65 years.
But the general conclusion that both CDI and mrCDI are on the rise is a crucial matter. “We must first have a better understanding of mrCDI, its scope and epidemiology, and its associated risk factors. The study by Ma and colleagues begins this important work. A better understanding of the epidemiology of mrCDI is a critical first step toward developing a sound strategy to address this growing public health challenge.”
Dr. Saini and Dr. Waljee are with the VA Ann Arbor (Michigan) Center for Clinical Management. Their editorial accompanied the study in Annals of Internal Medicine (2017 Jul. doi: 10.7326/M17-1565).
The retrospective cohort study was based on administrative data rather than laboratory data, Sameer D. Saini, MD, MS, and Akbar K. Waljee, MD, noted in an editorial accompanying the study. Further, with Medicare patients excluded from the study (because Medicare data were not available for the full time period studied for private insurance data), the data may not be of relevance to patients older than age 65 years.
But the general conclusion that both CDI and mrCDI are on the rise is a crucial matter. “We must first have a better understanding of mrCDI, its scope and epidemiology, and its associated risk factors. The study by Ma and colleagues begins this important work. A better understanding of the epidemiology of mrCDI is a critical first step toward developing a sound strategy to address this growing public health challenge.”
Dr. Saini and Dr. Waljee are with the VA Ann Arbor (Michigan) Center for Clinical Management. Their editorial accompanied the study in Annals of Internal Medicine (2017 Jul. doi: 10.7326/M17-1565).
A retrospective cohort study of Clostridium difficile infection (CDI), the most common health care–associated infection, found that multiply recurrent CDI (mrCDI) is increasing in incidence, disproportionately to the overall increase in CDI.
Researchers from the University of Pennsylvania, Philadelphia, worked with a database of more than 38 million individuals with private health insurance between January 2001 and December 2012.
Cases of CDI and mrCDI in the study population were determined through ICD-9 diagnosis codes, and prescriptions for treatment. To meet the definition of mrCDI, there had to be at least three courses of treatment lasting at least 14 days each.
In the study population, 45,341 persons developed CDI, of whom 1,669 had mrCDI. The median age was 46 years, and 58.9% were female. Between 2001 and 2012, CDI incidence increased by 42.7% (P = .004), while mrCDI incidence increased by 188.8% (P less than .001).
With increases in CDI and mrCDI incidence, and with the effectiveness of standard antibiotic treatment decreasing with each recurrence, “demand for new antimicrobial therapies and FMT [fecal microbiota transplantation] can be expected to increase considerably in the coming years,” wrote Gene K. Ma, MD, and his coauthors.
As for FMT, the researchers noted that its likely greater demand in the future (as suggested by their study results) highlights the importance of establishing the long-term safety of the procedure (Ann Intern Med. 2017 Jul. doi: 10.7326/M16-2733).
A retrospective cohort study of Clostridium difficile infection (CDI), the most common health care–associated infection, found that multiply recurrent CDI (mrCDI) is increasing in incidence, disproportionately to the overall increase in CDI.
Researchers from the University of Pennsylvania, Philadelphia, worked with a database of more than 38 million individuals with private health insurance between January 2001 and December 2012.
Cases of CDI and mrCDI in the study population were determined through ICD-9 diagnosis codes, and prescriptions for treatment. To meet the definition of mrCDI, there had to be at least three courses of treatment lasting at least 14 days each.
In the study population, 45,341 persons developed CDI, of whom 1,669 had mrCDI. The median age was 46 years, and 58.9% were female. Between 2001 and 2012, CDI incidence increased by 42.7% (P = .004), while mrCDI incidence increased by 188.8% (P less than .001).
With increases in CDI and mrCDI incidence, and with the effectiveness of standard antibiotic treatment decreasing with each recurrence, “demand for new antimicrobial therapies and FMT [fecal microbiota transplantation] can be expected to increase considerably in the coming years,” wrote Gene K. Ma, MD, and his coauthors.
As for FMT, the researchers noted that its likely greater demand in the future (as suggested by their study results) highlights the importance of establishing the long-term safety of the procedure (Ann Intern Med. 2017 Jul. doi: 10.7326/M16-2733).
FROM ANNALS OF INTERNAL MEDICINE
Hospitalist burnout
Some things I’ve been thinking about:
- Physician well-being, morale, and burnout seem to be getting more attention in both the medical and the lay press.
- Leaders from 10 prestigious health systems and the CEO of the American Medical Association wrote a March 2017 post in the Health Affairs Blog titled “Physician Burnout is a Public Health Crisis: A Message To Our Fellow Health Care CEOs.”
- I’m now regularly hearing and reading mention of the “Quadruple Aim.” The “Triple Aim,” first described in 2008, is the pursuit of excellence in 1) patient experience – both quality of care and patient satisfaction; 2) population health; and 3) cost reduction. The November/December 2014 Annals of Family Medicine included an article recommending “that the Triple Aim be expanded to a Quadruple Aim by adding the goal of improving the work life of health care providers, including clinicians and staff.”1
- The CEO at a community hospital near me chose to make addressing physician burnout one of his top priorities and tied success in the effort to his own compensation bonus.
- In the course of my consulting work with hospitalist groups across the country, I’ve noticed a meaningful increase in the number of our colleagues who seem deeply unhappy with their work and/or burned out. The “Hospitalist Morale Index” may be a worthwhile way for a group to conduct an assessment.2
- I’m concerned that many other hospital care givers, including RNs, social workers, and others, are experiencing levels of distress and/or burnout that might be similar to that of physicians. From where I sit, they seem to be getting less attention, and I can’t tell if that is just because I’m not as immersed in their world or if it reflects reality. It’s pretty disappointing if it’s the latter.
For the most part, I think the causes of hospitalist distress and burnout are very similar to that of doctors in other specialties, and interventions to address the problem can be similar across specialties. Yet, each specialty probably differs in ways that are important to keep in mind.
Hospitalists also bear a huge burden related to observation status. Doctors in most other specialties rarely face complex decisions regarding whether observation is the right choice and are not so often the target of patient/family frustration and anger related to it.
Those seeking to address hospitalist burnout and well-being specifically should keep in mind these uniquely hospitalist issues. I think of them as a chronic disease to manage and mitigate, since “curing” them (making them go away entirely) is probably impossible for the foreseeable future.
What to do?
An Internet search on physician burnout, or other terms related to well-being, will yield more articles with advice to address the problem than you’ll ever have time to read. Trying to read all of them would likely lead to burnout! I think interventions can be divided into two broad categories: organizational efforts and personal efforts.
Like the 10 CEOs mentioned above, health care leaders should acknowledge physician distress and burnout as a meaningful issue that can impede organizational performance and that investments to address it can have a meaningful return on investment. The Health Affairs Blog post listed 11 things the CEOs committed to doing. It’s a list anyone working on this issue should review.
Doctors at The Mayo Clinic have published a great deal of research on physician burnout. In the March 7, 2017, JAMA, (summarized in a YouTube video) they describe several worthwhile organizational changes, as well as some personal strategies.3 They wrote about their experiences with interventions such as a deliberate curriculum to train doctors in self-care (self-reflection, mindfulness, etc.) in a series of one-hour lectures over several months.4 In November 2016, they published a meta-analysis of interventions to address burnout.5
In total, all of the worthwhile recommendations to address burnout leave me feeling like they’re a lot of work, and any individual intervention may not be as helpful as hoped, so that the best way to approach this is with a collection of interventions. In many ways, it is similar to the problem of readmissions: There is a lot of research out there, it’s hard to prove that any single intervention really works, and success lies in implementing a broad set of interventions. And success doesn’t equate to eliminating readmissions, only reducing them.
Coda: Is a sabbatical uniquely valuable for hospitalists?
I think a sabbatical might be a good idea for hospitalists. It also seems practical for other doctors, such as radiologists, anesthesiologists, and ED doctors, who don’t have 1:1 continuity relationships with patients. However, it is problematic for primary care doctors and specialists who need to maintain continuity relationships with patients and referring doctors that could be disrupted by a lengthy absence.
I’m not sure a sabbatical would reduce burnout much on its own, but, if properly structured, it seems very likely to reduce staffing turnover, and the sabbatical could be spent in ways that help rejuvenate interest and satisfaction in our work rather than simply taking a long vacation to travel and play golf, etc. It should probably be at least 3 months and better if it lasts a year. A common arrangement is that a doctor becomes eligible for the sabbatical after 10 years and is paid half of her usual compensation while away. I’d like to see more hospitalist groups do this.
Dr. Nelson has had a career in clinical practice as a hospitalist starting in 1988. He is cofounder and past president of SHM and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses. Write to [email protected].
References
1. Bodenheimer, T and Sninsky, C. From Triple to Quadruple Aim: Care of the Patient Requires Care of the Provider. Ann Fam Med. Nov/Dec 2014.
2. Chandra, S et al. Introducing the Hospitalist Morale Index: A New Tool That May Be Relevant for Improving Provider Retention. JHM. June 2016.
3. Shanafelt, T, Dyrbye, L, West, C. Addressing Physician Burnout: The Way Forward. JAMA. March 7, 2017.
4. West, C et al. Intervention to Promote Physician Well-being, Job Satisfaction, and Professionalism: A Randomized Clinical Trial. JAMA Intern Med. 2014;174(4):527-33.
5. West, C et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. The Lancet. Nov 5, 2016.
Some things I’ve been thinking about:
- Physician well-being, morale, and burnout seem to be getting more attention in both the medical and the lay press.
- Leaders from 10 prestigious health systems and the CEO of the American Medical Association wrote a March 2017 post in the Health Affairs Blog titled “Physician Burnout is a Public Health Crisis: A Message To Our Fellow Health Care CEOs.”
- I’m now regularly hearing and reading mention of the “Quadruple Aim.” The “Triple Aim,” first described in 2008, is the pursuit of excellence in 1) patient experience – both quality of care and patient satisfaction; 2) population health; and 3) cost reduction. The November/December 2014 Annals of Family Medicine included an article recommending “that the Triple Aim be expanded to a Quadruple Aim by adding the goal of improving the work life of health care providers, including clinicians and staff.”1
- The CEO at a community hospital near me chose to make addressing physician burnout one of his top priorities and tied success in the effort to his own compensation bonus.
- In the course of my consulting work with hospitalist groups across the country, I’ve noticed a meaningful increase in the number of our colleagues who seem deeply unhappy with their work and/or burned out. The “Hospitalist Morale Index” may be a worthwhile way for a group to conduct an assessment.2
- I’m concerned that many other hospital care givers, including RNs, social workers, and others, are experiencing levels of distress and/or burnout that might be similar to that of physicians. From where I sit, they seem to be getting less attention, and I can’t tell if that is just because I’m not as immersed in their world or if it reflects reality. It’s pretty disappointing if it’s the latter.
For the most part, I think the causes of hospitalist distress and burnout are very similar to that of doctors in other specialties, and interventions to address the problem can be similar across specialties. Yet, each specialty probably differs in ways that are important to keep in mind.
Hospitalists also bear a huge burden related to observation status. Doctors in most other specialties rarely face complex decisions regarding whether observation is the right choice and are not so often the target of patient/family frustration and anger related to it.
Those seeking to address hospitalist burnout and well-being specifically should keep in mind these uniquely hospitalist issues. I think of them as a chronic disease to manage and mitigate, since “curing” them (making them go away entirely) is probably impossible for the foreseeable future.
What to do?
An Internet search on physician burnout, or other terms related to well-being, will yield more articles with advice to address the problem than you’ll ever have time to read. Trying to read all of them would likely lead to burnout! I think interventions can be divided into two broad categories: organizational efforts and personal efforts.
Like the 10 CEOs mentioned above, health care leaders should acknowledge physician distress and burnout as a meaningful issue that can impede organizational performance and that investments to address it can have a meaningful return on investment. The Health Affairs Blog post listed 11 things the CEOs committed to doing. It’s a list anyone working on this issue should review.
Doctors at The Mayo Clinic have published a great deal of research on physician burnout. In the March 7, 2017, JAMA, (summarized in a YouTube video) they describe several worthwhile organizational changes, as well as some personal strategies.3 They wrote about their experiences with interventions such as a deliberate curriculum to train doctors in self-care (self-reflection, mindfulness, etc.) in a series of one-hour lectures over several months.4 In November 2016, they published a meta-analysis of interventions to address burnout.5
In total, all of the worthwhile recommendations to address burnout leave me feeling like they’re a lot of work, and any individual intervention may not be as helpful as hoped, so that the best way to approach this is with a collection of interventions. In many ways, it is similar to the problem of readmissions: There is a lot of research out there, it’s hard to prove that any single intervention really works, and success lies in implementing a broad set of interventions. And success doesn’t equate to eliminating readmissions, only reducing them.
Coda: Is a sabbatical uniquely valuable for hospitalists?
I think a sabbatical might be a good idea for hospitalists. It also seems practical for other doctors, such as radiologists, anesthesiologists, and ED doctors, who don’t have 1:1 continuity relationships with patients. However, it is problematic for primary care doctors and specialists who need to maintain continuity relationships with patients and referring doctors that could be disrupted by a lengthy absence.
I’m not sure a sabbatical would reduce burnout much on its own, but, if properly structured, it seems very likely to reduce staffing turnover, and the sabbatical could be spent in ways that help rejuvenate interest and satisfaction in our work rather than simply taking a long vacation to travel and play golf, etc. It should probably be at least 3 months and better if it lasts a year. A common arrangement is that a doctor becomes eligible for the sabbatical after 10 years and is paid half of her usual compensation while away. I’d like to see more hospitalist groups do this.
Dr. Nelson has had a career in clinical practice as a hospitalist starting in 1988. He is cofounder and past president of SHM and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses. Write to [email protected].
References
1. Bodenheimer, T and Sninsky, C. From Triple to Quadruple Aim: Care of the Patient Requires Care of the Provider. Ann Fam Med. Nov/Dec 2014.
2. Chandra, S et al. Introducing the Hospitalist Morale Index: A New Tool That May Be Relevant for Improving Provider Retention. JHM. June 2016.
3. Shanafelt, T, Dyrbye, L, West, C. Addressing Physician Burnout: The Way Forward. JAMA. March 7, 2017.
4. West, C et al. Intervention to Promote Physician Well-being, Job Satisfaction, and Professionalism: A Randomized Clinical Trial. JAMA Intern Med. 2014;174(4):527-33.
5. West, C et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. The Lancet. Nov 5, 2016.
Some things I’ve been thinking about:
- Physician well-being, morale, and burnout seem to be getting more attention in both the medical and the lay press.
- Leaders from 10 prestigious health systems and the CEO of the American Medical Association wrote a March 2017 post in the Health Affairs Blog titled “Physician Burnout is a Public Health Crisis: A Message To Our Fellow Health Care CEOs.”
- I’m now regularly hearing and reading mention of the “Quadruple Aim.” The “Triple Aim,” first described in 2008, is the pursuit of excellence in 1) patient experience – both quality of care and patient satisfaction; 2) population health; and 3) cost reduction. The November/December 2014 Annals of Family Medicine included an article recommending “that the Triple Aim be expanded to a Quadruple Aim by adding the goal of improving the work life of health care providers, including clinicians and staff.”1
- The CEO at a community hospital near me chose to make addressing physician burnout one of his top priorities and tied success in the effort to his own compensation bonus.
- In the course of my consulting work with hospitalist groups across the country, I’ve noticed a meaningful increase in the number of our colleagues who seem deeply unhappy with their work and/or burned out. The “Hospitalist Morale Index” may be a worthwhile way for a group to conduct an assessment.2
- I’m concerned that many other hospital care givers, including RNs, social workers, and others, are experiencing levels of distress and/or burnout that might be similar to that of physicians. From where I sit, they seem to be getting less attention, and I can’t tell if that is just because I’m not as immersed in their world or if it reflects reality. It’s pretty disappointing if it’s the latter.
For the most part, I think the causes of hospitalist distress and burnout are very similar to that of doctors in other specialties, and interventions to address the problem can be similar across specialties. Yet, each specialty probably differs in ways that are important to keep in mind.
Hospitalists also bear a huge burden related to observation status. Doctors in most other specialties rarely face complex decisions regarding whether observation is the right choice and are not so often the target of patient/family frustration and anger related to it.
Those seeking to address hospitalist burnout and well-being specifically should keep in mind these uniquely hospitalist issues. I think of them as a chronic disease to manage and mitigate, since “curing” them (making them go away entirely) is probably impossible for the foreseeable future.
What to do?
An Internet search on physician burnout, or other terms related to well-being, will yield more articles with advice to address the problem than you’ll ever have time to read. Trying to read all of them would likely lead to burnout! I think interventions can be divided into two broad categories: organizational efforts and personal efforts.
Like the 10 CEOs mentioned above, health care leaders should acknowledge physician distress and burnout as a meaningful issue that can impede organizational performance and that investments to address it can have a meaningful return on investment. The Health Affairs Blog post listed 11 things the CEOs committed to doing. It’s a list anyone working on this issue should review.
Doctors at The Mayo Clinic have published a great deal of research on physician burnout. In the March 7, 2017, JAMA, (summarized in a YouTube video) they describe several worthwhile organizational changes, as well as some personal strategies.3 They wrote about their experiences with interventions such as a deliberate curriculum to train doctors in self-care (self-reflection, mindfulness, etc.) in a series of one-hour lectures over several months.4 In November 2016, they published a meta-analysis of interventions to address burnout.5
In total, all of the worthwhile recommendations to address burnout leave me feeling like they’re a lot of work, and any individual intervention may not be as helpful as hoped, so that the best way to approach this is with a collection of interventions. In many ways, it is similar to the problem of readmissions: There is a lot of research out there, it’s hard to prove that any single intervention really works, and success lies in implementing a broad set of interventions. And success doesn’t equate to eliminating readmissions, only reducing them.
Coda: Is a sabbatical uniquely valuable for hospitalists?
I think a sabbatical might be a good idea for hospitalists. It also seems practical for other doctors, such as radiologists, anesthesiologists, and ED doctors, who don’t have 1:1 continuity relationships with patients. However, it is problematic for primary care doctors and specialists who need to maintain continuity relationships with patients and referring doctors that could be disrupted by a lengthy absence.
I’m not sure a sabbatical would reduce burnout much on its own, but, if properly structured, it seems very likely to reduce staffing turnover, and the sabbatical could be spent in ways that help rejuvenate interest and satisfaction in our work rather than simply taking a long vacation to travel and play golf, etc. It should probably be at least 3 months and better if it lasts a year. A common arrangement is that a doctor becomes eligible for the sabbatical after 10 years and is paid half of her usual compensation while away. I’d like to see more hospitalist groups do this.
Dr. Nelson has had a career in clinical practice as a hospitalist starting in 1988. He is cofounder and past president of SHM and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses. Write to [email protected].
References
1. Bodenheimer, T and Sninsky, C. From Triple to Quadruple Aim: Care of the Patient Requires Care of the Provider. Ann Fam Med. Nov/Dec 2014.
2. Chandra, S et al. Introducing the Hospitalist Morale Index: A New Tool That May Be Relevant for Improving Provider Retention. JHM. June 2016.
3. Shanafelt, T, Dyrbye, L, West, C. Addressing Physician Burnout: The Way Forward. JAMA. March 7, 2017.
4. West, C et al. Intervention to Promote Physician Well-being, Job Satisfaction, and Professionalism: A Randomized Clinical Trial. JAMA Intern Med. 2014;174(4):527-33.
5. West, C et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. The Lancet. Nov 5, 2016.
Rash in HIV-positive man
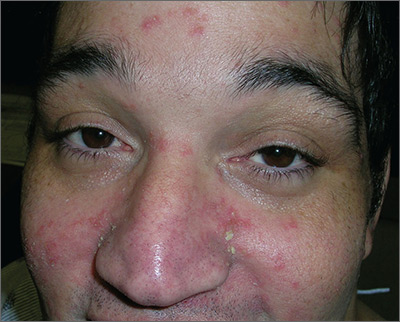
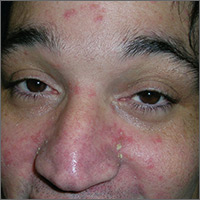
Taking into consideration the patient’s 2 predisposing risk factors (AIDS and dementia), the FP diagnosed seborrheic dermatitis. The FP also noted seborrheic dermatitis on the scalp. The scalp is frequently involved in cases of seborrheic dermatitis on the face.
Seborrheic dermatitis is a common, chronic, relapsing dermatitis affecting sebum-rich areas of the body. Its presentation may vary from mild erythema to greasy scale, and rarely, to erythroderma. Patients with seborrheic dermatitis may be colonized with certain species of lipophilic yeast of the genus Malassezia (also known as Pityrosporum). Malassezia, however, is considered normal skin flora because it is also found in unaffected people.
Some evidence suggests that an overgrowth of Malassezia may produce different irritants or metabolites on affected skin, leading to inflammation. It’s postulated that an overgrowth of Malassezia leads to the inflammatory dermatitis in seborrhea.
The goals of treatment are to diminish the fungal overgrowth and treat the inflammatory response. Thus, the mainstay of treatment is ongoing topical antifungals in shampoos and other preparations, along with a topical steroid to treat the inflammation.
The FP prescribed 2% ketoconazole shampoo to be used twice weekly and suggested that a selenium-containing shampoo be used on the other days of the week, as well as a 2.5% hydrocortisone cream to be applied twice daily to the face until the dermatitis resolved. The FP also prescribed a topical antifungal, cyclopirox 1% cream, to be applied twice daily on an ongoing basis to keep the Malassezia under control. At a one-month follow-up, the patient’s skin and scalp were cleared.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Hancock M, Bae Y, Usatine R. Seborrheic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 871-877.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Taking into consideration the patient’s 2 predisposing risk factors (AIDS and dementia), the FP diagnosed seborrheic dermatitis. The FP also noted seborrheic dermatitis on the scalp. The scalp is frequently involved in cases of seborrheic dermatitis on the face.
Seborrheic dermatitis is a common, chronic, relapsing dermatitis affecting sebum-rich areas of the body. Its presentation may vary from mild erythema to greasy scale, and rarely, to erythroderma. Patients with seborrheic dermatitis may be colonized with certain species of lipophilic yeast of the genus Malassezia (also known as Pityrosporum). Malassezia, however, is considered normal skin flora because it is also found in unaffected people.
Some evidence suggests that an overgrowth of Malassezia may produce different irritants or metabolites on affected skin, leading to inflammation. It’s postulated that an overgrowth of Malassezia leads to the inflammatory dermatitis in seborrhea.
The goals of treatment are to diminish the fungal overgrowth and treat the inflammatory response. Thus, the mainstay of treatment is ongoing topical antifungals in shampoos and other preparations, along with a topical steroid to treat the inflammation.
The FP prescribed 2% ketoconazole shampoo to be used twice weekly and suggested that a selenium-containing shampoo be used on the other days of the week, as well as a 2.5% hydrocortisone cream to be applied twice daily to the face until the dermatitis resolved. The FP also prescribed a topical antifungal, cyclopirox 1% cream, to be applied twice daily on an ongoing basis to keep the Malassezia under control. At a one-month follow-up, the patient’s skin and scalp were cleared.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Hancock M, Bae Y, Usatine R. Seborrheic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 871-877.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Taking into consideration the patient’s 2 predisposing risk factors (AIDS and dementia), the FP diagnosed seborrheic dermatitis. The FP also noted seborrheic dermatitis on the scalp. The scalp is frequently involved in cases of seborrheic dermatitis on the face.
Seborrheic dermatitis is a common, chronic, relapsing dermatitis affecting sebum-rich areas of the body. Its presentation may vary from mild erythema to greasy scale, and rarely, to erythroderma. Patients with seborrheic dermatitis may be colonized with certain species of lipophilic yeast of the genus Malassezia (also known as Pityrosporum). Malassezia, however, is considered normal skin flora because it is also found in unaffected people.
Some evidence suggests that an overgrowth of Malassezia may produce different irritants or metabolites on affected skin, leading to inflammation. It’s postulated that an overgrowth of Malassezia leads to the inflammatory dermatitis in seborrhea.
The goals of treatment are to diminish the fungal overgrowth and treat the inflammatory response. Thus, the mainstay of treatment is ongoing topical antifungals in shampoos and other preparations, along with a topical steroid to treat the inflammation.
The FP prescribed 2% ketoconazole shampoo to be used twice weekly and suggested that a selenium-containing shampoo be used on the other days of the week, as well as a 2.5% hydrocortisone cream to be applied twice daily to the face until the dermatitis resolved. The FP also prescribed a topical antifungal, cyclopirox 1% cream, to be applied twice daily on an ongoing basis to keep the Malassezia under control. At a one-month follow-up, the patient’s skin and scalp were cleared.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Hancock M, Bae Y, Usatine R. Seborrheic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 871-877.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Daily 150-mg aspirin dose slashes preterm preeclampsia risk
While low-dose aspirin has been shown for decades to decrease the risk of preterm preeclampsia, yet to be established are the optimal dose and time of day to administer aspirin; how preeclampsia risk is best assessed; and at what gestational week it is best to start aspirin.
New findings from a randomized, double-blind, placebo-controlled trial show that a daily 150-mg dose of aspirin, taken at night, significantly reduced incidence of preterm preeclampsia in women identified as being at high risk in the first trimester (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1704559).
After 156 women dropped out of the study or were lost to follow-up, investigators had results from 798 women in the aspirin group and 822 in the placebo group, with about 80% of subjects having taken aspirin or placebo as directed. The study’s primary outcome was delivery with preeclampsia before 37 weeks, seen in 13 women (1.6%) in the aspirin group, compared with 35 (4.3%) in the placebo group (odds ratio 0.38; 95% confidence interval, 0.20 to 0.74; P = .004).
Other decisions incorporated into the study design, including those about aspirin dosage (higher than the currently recommended 60-80 mg for this population), taking aspirin at night, and the gestational age at which to start aspirin, were based on results from prior trials, studies, and meta-analyses, the researchers said.
Screening at 11-13 weeks’ gestation identifies less than 40% of term preeclampsia, studies have shown, and aspirin did not reduce term preeclampsia in this study, the investigators said.
The European Union Seventh Framework Program and the Fetal Medicine Foundation (UK) sponsored the study. None of the investigators had any relevant financial disclosures.
While low-dose aspirin has been shown for decades to decrease the risk of preterm preeclampsia, yet to be established are the optimal dose and time of day to administer aspirin; how preeclampsia risk is best assessed; and at what gestational week it is best to start aspirin.
New findings from a randomized, double-blind, placebo-controlled trial show that a daily 150-mg dose of aspirin, taken at night, significantly reduced incidence of preterm preeclampsia in women identified as being at high risk in the first trimester (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1704559).
After 156 women dropped out of the study or were lost to follow-up, investigators had results from 798 women in the aspirin group and 822 in the placebo group, with about 80% of subjects having taken aspirin or placebo as directed. The study’s primary outcome was delivery with preeclampsia before 37 weeks, seen in 13 women (1.6%) in the aspirin group, compared with 35 (4.3%) in the placebo group (odds ratio 0.38; 95% confidence interval, 0.20 to 0.74; P = .004).
Other decisions incorporated into the study design, including those about aspirin dosage (higher than the currently recommended 60-80 mg for this population), taking aspirin at night, and the gestational age at which to start aspirin, were based on results from prior trials, studies, and meta-analyses, the researchers said.
Screening at 11-13 weeks’ gestation identifies less than 40% of term preeclampsia, studies have shown, and aspirin did not reduce term preeclampsia in this study, the investigators said.
The European Union Seventh Framework Program and the Fetal Medicine Foundation (UK) sponsored the study. None of the investigators had any relevant financial disclosures.
While low-dose aspirin has been shown for decades to decrease the risk of preterm preeclampsia, yet to be established are the optimal dose and time of day to administer aspirin; how preeclampsia risk is best assessed; and at what gestational week it is best to start aspirin.
New findings from a randomized, double-blind, placebo-controlled trial show that a daily 150-mg dose of aspirin, taken at night, significantly reduced incidence of preterm preeclampsia in women identified as being at high risk in the first trimester (N Engl J Med. 2017 June 28. doi: 10.1056/NEJMoa1704559).
After 156 women dropped out of the study or were lost to follow-up, investigators had results from 798 women in the aspirin group and 822 in the placebo group, with about 80% of subjects having taken aspirin or placebo as directed. The study’s primary outcome was delivery with preeclampsia before 37 weeks, seen in 13 women (1.6%) in the aspirin group, compared with 35 (4.3%) in the placebo group (odds ratio 0.38; 95% confidence interval, 0.20 to 0.74; P = .004).
Other decisions incorporated into the study design, including those about aspirin dosage (higher than the currently recommended 60-80 mg for this population), taking aspirin at night, and the gestational age at which to start aspirin, were based on results from prior trials, studies, and meta-analyses, the researchers said.
Screening at 11-13 weeks’ gestation identifies less than 40% of term preeclampsia, studies have shown, and aspirin did not reduce term preeclampsia in this study, the investigators said.
The European Union Seventh Framework Program and the Fetal Medicine Foundation (UK) sponsored the study. None of the investigators had any relevant financial disclosures.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Among patients taking aspirin, 1.6% developed preterm preeclampsia, compared with 4.3% of those taking placebo (odds ratio 0.38; 95% confidence interval, 0.20 to 0.74; P = .004).
Data source: A randomized, international, multicenter trial enrolling nearly 1,800 women identified through screening as being at high risk of preeclampsia.
Disclosures: The European Union Seventh Framework Program and the Fetal Medicine Foundation (UK) sponsored the study. None of the investigators had any relevant financial disclosures.
Atypical Femoral Fracture Due to Daily Ibandronate
As more people are surviving breast cancer, more will be given bisphosphonate (BP) for bone metastases—and may be at risk for treatment adverse effects, such as atypical femoral fracture (AFF). Clinicians from Royal Victoria Hospital, Belfast, United Kingdom, describe what they learned in the first reported case of AFF caused by daily ibandronate.
The patient, a 55-year-old woman, had been diagnosed and treated for breast cancer. Twelve years later, she began having hip and lower back pain and was diagnosed with bony metastatic spread. She was started on hormone-suppressing therapy as well as daily ibandronic acid. Four years later, she began having new lower limb and groin pain. Radiography, bone scans and other tests indicated further metastatic spread to her left femur.
While she was waiting for scheduled radiotherapy, she fell, fracturing her left femur. Radiographs revealed AFF, presumed secondary to her BP therapy rather than metastasis. She underwent intramedullary nail fixation.
Evidence suggests that AFFs are stress fractures, the clinicians say. Repetitive loading on bone can lead to micro cracks, which are even more vulnerable to stress when BPs suppress the normal bone repair process.
Atypical femoral fracture is rare. Most cases have been patients receiving intravenous BP; to the clinicians’ knowledge this is the first report of AFF with an oral BP prescribed for bone metastatic breast cancer. But incidence of AFF grows with prolonged treatment, and the risk is raised with oral dosing, which is much higher in cancer cases, the clinicians note, compared with osteoporosis.
Because patients often have prodromal pain before an overt break, the clinicians suggest asking all oncology patients on BP about pain in thigh, hip, or groin. Bone scans and magnetic resonance imaging may reveal an imminent fracture. The clinicians caution that AFFs can be bilateral, so the opposite side also should be imaged.
Source:
Espey R, Grimes S, Heyburn G, Kealey WD. BMJ Case Rep. 2017;2017. pii: bcr-2016-217489.
doi: 10.1136/bcr-2016-217489.
As more people are surviving breast cancer, more will be given bisphosphonate (BP) for bone metastases—and may be at risk for treatment adverse effects, such as atypical femoral fracture (AFF). Clinicians from Royal Victoria Hospital, Belfast, United Kingdom, describe what they learned in the first reported case of AFF caused by daily ibandronate.
The patient, a 55-year-old woman, had been diagnosed and treated for breast cancer. Twelve years later, she began having hip and lower back pain and was diagnosed with bony metastatic spread. She was started on hormone-suppressing therapy as well as daily ibandronic acid. Four years later, she began having new lower limb and groin pain. Radiography, bone scans and other tests indicated further metastatic spread to her left femur.
While she was waiting for scheduled radiotherapy, she fell, fracturing her left femur. Radiographs revealed AFF, presumed secondary to her BP therapy rather than metastasis. She underwent intramedullary nail fixation.
Evidence suggests that AFFs are stress fractures, the clinicians say. Repetitive loading on bone can lead to micro cracks, which are even more vulnerable to stress when BPs suppress the normal bone repair process.
Atypical femoral fracture is rare. Most cases have been patients receiving intravenous BP; to the clinicians’ knowledge this is the first report of AFF with an oral BP prescribed for bone metastatic breast cancer. But incidence of AFF grows with prolonged treatment, and the risk is raised with oral dosing, which is much higher in cancer cases, the clinicians note, compared with osteoporosis.
Because patients often have prodromal pain before an overt break, the clinicians suggest asking all oncology patients on BP about pain in thigh, hip, or groin. Bone scans and magnetic resonance imaging may reveal an imminent fracture. The clinicians caution that AFFs can be bilateral, so the opposite side also should be imaged.
Source:
Espey R, Grimes S, Heyburn G, Kealey WD. BMJ Case Rep. 2017;2017. pii: bcr-2016-217489.
doi: 10.1136/bcr-2016-217489.
As more people are surviving breast cancer, more will be given bisphosphonate (BP) for bone metastases—and may be at risk for treatment adverse effects, such as atypical femoral fracture (AFF). Clinicians from Royal Victoria Hospital, Belfast, United Kingdom, describe what they learned in the first reported case of AFF caused by daily ibandronate.
The patient, a 55-year-old woman, had been diagnosed and treated for breast cancer. Twelve years later, she began having hip and lower back pain and was diagnosed with bony metastatic spread. She was started on hormone-suppressing therapy as well as daily ibandronic acid. Four years later, she began having new lower limb and groin pain. Radiography, bone scans and other tests indicated further metastatic spread to her left femur.
While she was waiting for scheduled radiotherapy, she fell, fracturing her left femur. Radiographs revealed AFF, presumed secondary to her BP therapy rather than metastasis. She underwent intramedullary nail fixation.
Evidence suggests that AFFs are stress fractures, the clinicians say. Repetitive loading on bone can lead to micro cracks, which are even more vulnerable to stress when BPs suppress the normal bone repair process.
Atypical femoral fracture is rare. Most cases have been patients receiving intravenous BP; to the clinicians’ knowledge this is the first report of AFF with an oral BP prescribed for bone metastatic breast cancer. But incidence of AFF grows with prolonged treatment, and the risk is raised with oral dosing, which is much higher in cancer cases, the clinicians note, compared with osteoporosis.
Because patients often have prodromal pain before an overt break, the clinicians suggest asking all oncology patients on BP about pain in thigh, hip, or groin. Bone scans and magnetic resonance imaging may reveal an imminent fracture. The clinicians caution that AFFs can be bilateral, so the opposite side also should be imaged.
Source:
Espey R, Grimes S, Heyburn G, Kealey WD. BMJ Case Rep. 2017;2017. pii: bcr-2016-217489.
doi: 10.1136/bcr-2016-217489.
First Cancer Treatment Based on Biomarkers Is Approved
In an “important first,” the FDA has fast-tracked a cancer treatment based on a biomarker rather than on the location of the tumor.
Pembrolizumab (Keytruda) has already been approved for certain patients with metastatic melanoma, metastatic non-small cell lung cancer, and other cancers. With the new approval, pembrolizumab is now indicated for adult and pediatric patients with unresectable or metastatic solid tumors that have a biomarker known as microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR). Microsatellite instability-high and dMMR tumors have abnormalities that affect the proper repair of DNA inside the cell. Pembrolizumab blocks a cellular pathway (PD-1/PD-L1—proteins found in immune cells and some cancer cells), which may help the immune system fight the cancer cells.
Tumors with the biomarkers are most commonly found in colorectal, endometrial, and gastrointestinal cancers. About 5% of patients with metastatic colorectal cancer have MSI-H or dMMR tumors. Less commonly, they are found in cancers in the breast, prostate, bladder, thyroid gland, and other locations.
In 5 clinical trials, of 149 patients treated with pembrolizumab for 15 cancer types, 40% had a complete or partial response. For most of those patients, the response lasted ≥ 6 months.
In an “important first,” the FDA has fast-tracked a cancer treatment based on a biomarker rather than on the location of the tumor.
Pembrolizumab (Keytruda) has already been approved for certain patients with metastatic melanoma, metastatic non-small cell lung cancer, and other cancers. With the new approval, pembrolizumab is now indicated for adult and pediatric patients with unresectable or metastatic solid tumors that have a biomarker known as microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR). Microsatellite instability-high and dMMR tumors have abnormalities that affect the proper repair of DNA inside the cell. Pembrolizumab blocks a cellular pathway (PD-1/PD-L1—proteins found in immune cells and some cancer cells), which may help the immune system fight the cancer cells.
Tumors with the biomarkers are most commonly found in colorectal, endometrial, and gastrointestinal cancers. About 5% of patients with metastatic colorectal cancer have MSI-H or dMMR tumors. Less commonly, they are found in cancers in the breast, prostate, bladder, thyroid gland, and other locations.
In 5 clinical trials, of 149 patients treated with pembrolizumab for 15 cancer types, 40% had a complete or partial response. For most of those patients, the response lasted ≥ 6 months.
In an “important first,” the FDA has fast-tracked a cancer treatment based on a biomarker rather than on the location of the tumor.
Pembrolizumab (Keytruda) has already been approved for certain patients with metastatic melanoma, metastatic non-small cell lung cancer, and other cancers. With the new approval, pembrolizumab is now indicated for adult and pediatric patients with unresectable or metastatic solid tumors that have a biomarker known as microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR). Microsatellite instability-high and dMMR tumors have abnormalities that affect the proper repair of DNA inside the cell. Pembrolizumab blocks a cellular pathway (PD-1/PD-L1—proteins found in immune cells and some cancer cells), which may help the immune system fight the cancer cells.
Tumors with the biomarkers are most commonly found in colorectal, endometrial, and gastrointestinal cancers. About 5% of patients with metastatic colorectal cancer have MSI-H or dMMR tumors. Less commonly, they are found in cancers in the breast, prostate, bladder, thyroid gland, and other locations.
In 5 clinical trials, of 149 patients treated with pembrolizumab for 15 cancer types, 40% had a complete or partial response. For most of those patients, the response lasted ≥ 6 months.
Oral combo produces deep, durable responses in MM
An all-oral treatment regimen can produce deep and durable responses in patients with newly diagnosed multiple myeloma (MM), according to a speaker at the 22nd Congress of the European Hematology Association (EHA).
In a phase 1/2 study, patients with newly diagnosed MM received treatment with ixazomib, lenalidomide, and dexamethasone for up to 12 cycles, followed by maintenance with ixazomib alone.
The overall response rate was 88% for the entire cohort and 80% among patients who did not proceed to transplant.
The median progression-free survival was 35.4 months in the entire cohort and 29.4 months in patients who did not proceed to transplant.
Most patients did not complete all 12 cycles of induction, but 38% proceeded to maintenance, and 12% remain on study treatment at a median follow-up of 56 months.
Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, presented these results at the EHA Congress as abstract S408. The research was sponsored by Millennium Pharmaceuticals, Inc., a subsidiary of Takeda Pharmaceutical Company Limited.
Patients
The trial included 65 patients. Their median age was 66 (range, 34-86), and 55% were male. Eight percent of patients had high-risk cytogenetics.
ECOG performance status was 0 for 43% of patients, 1 for 52%, and 2 for 5%. ISS stage was 1 for 43% of patients, 2 for 43%, and 3 for 14%.
Treatment
Patients received ixazomib (1.68 - 3.95 mg/m2 in phase 1 and 4.0 mg in phase 2 on days 1, 8, and 15) plus lenalidomide (25 mg on days 1-21), and dexamethasone (40 mg on days 1, 8, 15, and 22) for up to twelve, 28-day cycles. Patients could then receive ixazomib maintenance until disease progression or unacceptable toxicity.
Twenty-three patients (35%) discontinued induction to undergo hematopoietic stem cell transplant (HSCT).
Thirty-seven patients discontinued study treatment for other reasons—17 of them during induction. Twenty-one patients discontinued due to progressive disease, 9 due to adverse events (AEs), 5 due to patient withdrawal, 1 due to unsatisfactory response, and 1 due to other reasons.
Twenty-five patients completed induction and went on to receive ixazomib maintenance. Five patients remain on study treatment.
Safety
Seventy-eight percent of patients had a grade 3 or higher AE, 46% had a serious AE, 57% had an AE leading to dose reduction, and 15% had an AE leading to discontinuation of any of the study drugs. There were 2 on-study deaths.
The most common grade 3 or higher AEs were neutropenia, thrombocytopenia, diarrhea, back pain, vomiting, nausea, peripheral neuropathy, and rashes/eruptions/exanthems.
Efficacy
The overall response rate was 88% for the entire cohort and 80% among patients who did not go on to receive HSCT.
The complete response (CR) rates were 23% and 32%, respectively. Thirty four percent and 32%, respectively, had a very good partial response (VGPR). And 30% and 17%, respectively, had a partial response (PR).
Thirty-two percent of patients who received maintenance (n=8) had an improvement in their response during the maintenance phase. Two patients went from a VGPR to a stringent CR, 3 went from a VGPR to a CR, 2 went from a VGPR to a near CR, and 1 went from a PR to a VGPR.
Sixteen patients were evaluated for minimal residual disease (MRD). Of the patients with a stringent CR/CR, 89% (8/9) were MRD-negative.
The median progression-free survival was 35.4 months in the entire cohort and 29.4 months in patients who did not proceed to HSCT. The 4-year overall survival rate was 84% and 82%, respectively.
“Long-term follow-up, a median follow-up of 56 months, with the combination of ixazomib, lenalidomide, and dexamethasone demonstrates that this is an efficacious regimen for newly diagnosed myeloma patients, with excellent tolerability, and something that can be maintained for a long period of time,” Dr Kumar said. “These results form the basis for an ongoing phase 3 program.” ![]()
An all-oral treatment regimen can produce deep and durable responses in patients with newly diagnosed multiple myeloma (MM), according to a speaker at the 22nd Congress of the European Hematology Association (EHA).
In a phase 1/2 study, patients with newly diagnosed MM received treatment with ixazomib, lenalidomide, and dexamethasone for up to 12 cycles, followed by maintenance with ixazomib alone.
The overall response rate was 88% for the entire cohort and 80% among patients who did not proceed to transplant.
The median progression-free survival was 35.4 months in the entire cohort and 29.4 months in patients who did not proceed to transplant.
Most patients did not complete all 12 cycles of induction, but 38% proceeded to maintenance, and 12% remain on study treatment at a median follow-up of 56 months.
Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, presented these results at the EHA Congress as abstract S408. The research was sponsored by Millennium Pharmaceuticals, Inc., a subsidiary of Takeda Pharmaceutical Company Limited.
Patients
The trial included 65 patients. Their median age was 66 (range, 34-86), and 55% were male. Eight percent of patients had high-risk cytogenetics.
ECOG performance status was 0 for 43% of patients, 1 for 52%, and 2 for 5%. ISS stage was 1 for 43% of patients, 2 for 43%, and 3 for 14%.
Treatment
Patients received ixazomib (1.68 - 3.95 mg/m2 in phase 1 and 4.0 mg in phase 2 on days 1, 8, and 15) plus lenalidomide (25 mg on days 1-21), and dexamethasone (40 mg on days 1, 8, 15, and 22) for up to twelve, 28-day cycles. Patients could then receive ixazomib maintenance until disease progression or unacceptable toxicity.
Twenty-three patients (35%) discontinued induction to undergo hematopoietic stem cell transplant (HSCT).
Thirty-seven patients discontinued study treatment for other reasons—17 of them during induction. Twenty-one patients discontinued due to progressive disease, 9 due to adverse events (AEs), 5 due to patient withdrawal, 1 due to unsatisfactory response, and 1 due to other reasons.
Twenty-five patients completed induction and went on to receive ixazomib maintenance. Five patients remain on study treatment.
Safety
Seventy-eight percent of patients had a grade 3 or higher AE, 46% had a serious AE, 57% had an AE leading to dose reduction, and 15% had an AE leading to discontinuation of any of the study drugs. There were 2 on-study deaths.
The most common grade 3 or higher AEs were neutropenia, thrombocytopenia, diarrhea, back pain, vomiting, nausea, peripheral neuropathy, and rashes/eruptions/exanthems.
Efficacy
The overall response rate was 88% for the entire cohort and 80% among patients who did not go on to receive HSCT.
The complete response (CR) rates were 23% and 32%, respectively. Thirty four percent and 32%, respectively, had a very good partial response (VGPR). And 30% and 17%, respectively, had a partial response (PR).
Thirty-two percent of patients who received maintenance (n=8) had an improvement in their response during the maintenance phase. Two patients went from a VGPR to a stringent CR, 3 went from a VGPR to a CR, 2 went from a VGPR to a near CR, and 1 went from a PR to a VGPR.
Sixteen patients were evaluated for minimal residual disease (MRD). Of the patients with a stringent CR/CR, 89% (8/9) were MRD-negative.
The median progression-free survival was 35.4 months in the entire cohort and 29.4 months in patients who did not proceed to HSCT. The 4-year overall survival rate was 84% and 82%, respectively.
“Long-term follow-up, a median follow-up of 56 months, with the combination of ixazomib, lenalidomide, and dexamethasone demonstrates that this is an efficacious regimen for newly diagnosed myeloma patients, with excellent tolerability, and something that can be maintained for a long period of time,” Dr Kumar said. “These results form the basis for an ongoing phase 3 program.” ![]()
An all-oral treatment regimen can produce deep and durable responses in patients with newly diagnosed multiple myeloma (MM), according to a speaker at the 22nd Congress of the European Hematology Association (EHA).
In a phase 1/2 study, patients with newly diagnosed MM received treatment with ixazomib, lenalidomide, and dexamethasone for up to 12 cycles, followed by maintenance with ixazomib alone.
The overall response rate was 88% for the entire cohort and 80% among patients who did not proceed to transplant.
The median progression-free survival was 35.4 months in the entire cohort and 29.4 months in patients who did not proceed to transplant.
Most patients did not complete all 12 cycles of induction, but 38% proceeded to maintenance, and 12% remain on study treatment at a median follow-up of 56 months.
Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, presented these results at the EHA Congress as abstract S408. The research was sponsored by Millennium Pharmaceuticals, Inc., a subsidiary of Takeda Pharmaceutical Company Limited.
Patients
The trial included 65 patients. Their median age was 66 (range, 34-86), and 55% were male. Eight percent of patients had high-risk cytogenetics.
ECOG performance status was 0 for 43% of patients, 1 for 52%, and 2 for 5%. ISS stage was 1 for 43% of patients, 2 for 43%, and 3 for 14%.
Treatment
Patients received ixazomib (1.68 - 3.95 mg/m2 in phase 1 and 4.0 mg in phase 2 on days 1, 8, and 15) plus lenalidomide (25 mg on days 1-21), and dexamethasone (40 mg on days 1, 8, 15, and 22) for up to twelve, 28-day cycles. Patients could then receive ixazomib maintenance until disease progression or unacceptable toxicity.
Twenty-three patients (35%) discontinued induction to undergo hematopoietic stem cell transplant (HSCT).
Thirty-seven patients discontinued study treatment for other reasons—17 of them during induction. Twenty-one patients discontinued due to progressive disease, 9 due to adverse events (AEs), 5 due to patient withdrawal, 1 due to unsatisfactory response, and 1 due to other reasons.
Twenty-five patients completed induction and went on to receive ixazomib maintenance. Five patients remain on study treatment.
Safety
Seventy-eight percent of patients had a grade 3 or higher AE, 46% had a serious AE, 57% had an AE leading to dose reduction, and 15% had an AE leading to discontinuation of any of the study drugs. There were 2 on-study deaths.
The most common grade 3 or higher AEs were neutropenia, thrombocytopenia, diarrhea, back pain, vomiting, nausea, peripheral neuropathy, and rashes/eruptions/exanthems.
Efficacy
The overall response rate was 88% for the entire cohort and 80% among patients who did not go on to receive HSCT.
The complete response (CR) rates were 23% and 32%, respectively. Thirty four percent and 32%, respectively, had a very good partial response (VGPR). And 30% and 17%, respectively, had a partial response (PR).
Thirty-two percent of patients who received maintenance (n=8) had an improvement in their response during the maintenance phase. Two patients went from a VGPR to a stringent CR, 3 went from a VGPR to a CR, 2 went from a VGPR to a near CR, and 1 went from a PR to a VGPR.
Sixteen patients were evaluated for minimal residual disease (MRD). Of the patients with a stringent CR/CR, 89% (8/9) were MRD-negative.
The median progression-free survival was 35.4 months in the entire cohort and 29.4 months in patients who did not proceed to HSCT. The 4-year overall survival rate was 84% and 82%, respectively.
“Long-term follow-up, a median follow-up of 56 months, with the combination of ixazomib, lenalidomide, and dexamethasone demonstrates that this is an efficacious regimen for newly diagnosed myeloma patients, with excellent tolerability, and something that can be maintained for a long period of time,” Dr Kumar said. “These results form the basis for an ongoing phase 3 program.” ![]()
Mapping the genomic landscape of T-ALL
Researchers say they have uncovered new details of the genomic landscape of T-lineage acute lymphoblastic leukemia (T-ALL).
The team believes their findings will aid the development of drugs to target newly discovered mutations and enable researchers to engineer better mouse models to probe the leukemia’s aberrant biological machinery.
Charles Mullighan, MD, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported their findings in Nature Genetics.
“This first comprehensive and systematic analysis in a large group of patients revealed many new mutations that are biologically significant as well as new drug targets that could be clinically important,” Dr Mullighan said.
“Leukemias typically arise from multiple genetic changes that work together. Most previous studies have not had the breadth of genomic data in enough patients to identify the constellations of mutations and recognize their associations.”
Dr Mullighan and his colleagues sequenced the genomes of 264 children and young adults with T-ALL. This revealed 106 driver genes, half of which had not been identified in childhood T-ALL.
“We went into this study knowing that we didn’t know the full genomic landscape of T-ALL,” said Stephen Hunger, MD, of the Children’s Hospital of Philadelphia in Pennsylvania.
“But we were surprised that over half of the new targets and mutations were previously unrecognized. It was particularly unexpected and very striking that some mutations were exclusively found in some subtypes of T-ALL but not others.”
The researchers’ analysis confirmed that T-ALL was driven by mutations in known signaling pathways, including JAK-STAT, Ras, and PTEN-PI3K. However, the study also revealed new mutations in those known pathways.
In addition, the researchers identified cases in which the same T-ALL subtype had mutations in different pathways triggered by the same founding mutation.
“We believe this finding suggests we can target such subtypes with an inhibitor drug for one of the pathways, and it’s likely to be effective,” Dr Mullighan said.
He and his colleagues also believe the mutations uncovered in this study will enable researchers to create mouse models that more accurately reflect human T-ALL.
“We now have a launching pad, if you will, to design mouse models that include multiple genetic mutations to more faithfully reflect the leukemias we see in humans,” Dr Mullighan said.
Data from this study are available to researchers through the St. Jude PeCan data portal and the TARGET Data Matrix. ![]()
Researchers say they have uncovered new details of the genomic landscape of T-lineage acute lymphoblastic leukemia (T-ALL).
The team believes their findings will aid the development of drugs to target newly discovered mutations and enable researchers to engineer better mouse models to probe the leukemia’s aberrant biological machinery.
Charles Mullighan, MD, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported their findings in Nature Genetics.
“This first comprehensive and systematic analysis in a large group of patients revealed many new mutations that are biologically significant as well as new drug targets that could be clinically important,” Dr Mullighan said.
“Leukemias typically arise from multiple genetic changes that work together. Most previous studies have not had the breadth of genomic data in enough patients to identify the constellations of mutations and recognize their associations.”
Dr Mullighan and his colleagues sequenced the genomes of 264 children and young adults with T-ALL. This revealed 106 driver genes, half of which had not been identified in childhood T-ALL.
“We went into this study knowing that we didn’t know the full genomic landscape of T-ALL,” said Stephen Hunger, MD, of the Children’s Hospital of Philadelphia in Pennsylvania.
“But we were surprised that over half of the new targets and mutations were previously unrecognized. It was particularly unexpected and very striking that some mutations were exclusively found in some subtypes of T-ALL but not others.”
The researchers’ analysis confirmed that T-ALL was driven by mutations in known signaling pathways, including JAK-STAT, Ras, and PTEN-PI3K. However, the study also revealed new mutations in those known pathways.
In addition, the researchers identified cases in which the same T-ALL subtype had mutations in different pathways triggered by the same founding mutation.
“We believe this finding suggests we can target such subtypes with an inhibitor drug for one of the pathways, and it’s likely to be effective,” Dr Mullighan said.
He and his colleagues also believe the mutations uncovered in this study will enable researchers to create mouse models that more accurately reflect human T-ALL.
“We now have a launching pad, if you will, to design mouse models that include multiple genetic mutations to more faithfully reflect the leukemias we see in humans,” Dr Mullighan said.
Data from this study are available to researchers through the St. Jude PeCan data portal and the TARGET Data Matrix. ![]()
Researchers say they have uncovered new details of the genomic landscape of T-lineage acute lymphoblastic leukemia (T-ALL).
The team believes their findings will aid the development of drugs to target newly discovered mutations and enable researchers to engineer better mouse models to probe the leukemia’s aberrant biological machinery.
Charles Mullighan, MD, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted this research and reported their findings in Nature Genetics.
“This first comprehensive and systematic analysis in a large group of patients revealed many new mutations that are biologically significant as well as new drug targets that could be clinically important,” Dr Mullighan said.
“Leukemias typically arise from multiple genetic changes that work together. Most previous studies have not had the breadth of genomic data in enough patients to identify the constellations of mutations and recognize their associations.”
Dr Mullighan and his colleagues sequenced the genomes of 264 children and young adults with T-ALL. This revealed 106 driver genes, half of which had not been identified in childhood T-ALL.
“We went into this study knowing that we didn’t know the full genomic landscape of T-ALL,” said Stephen Hunger, MD, of the Children’s Hospital of Philadelphia in Pennsylvania.
“But we were surprised that over half of the new targets and mutations were previously unrecognized. It was particularly unexpected and very striking that some mutations were exclusively found in some subtypes of T-ALL but not others.”
The researchers’ analysis confirmed that T-ALL was driven by mutations in known signaling pathways, including JAK-STAT, Ras, and PTEN-PI3K. However, the study also revealed new mutations in those known pathways.
In addition, the researchers identified cases in which the same T-ALL subtype had mutations in different pathways triggered by the same founding mutation.
“We believe this finding suggests we can target such subtypes with an inhibitor drug for one of the pathways, and it’s likely to be effective,” Dr Mullighan said.
He and his colleagues also believe the mutations uncovered in this study will enable researchers to create mouse models that more accurately reflect human T-ALL.
“We now have a launching pad, if you will, to design mouse models that include multiple genetic mutations to more faithfully reflect the leukemias we see in humans,” Dr Mullighan said.
Data from this study are available to researchers through the St. Jude PeCan data portal and the TARGET Data Matrix. ![]()