User login
Countries with high malaria burden don’t receive research funding
A new study has revealed inequalities in malaria research funding in sub-Saharan Africa.
The study showed that some countries with a high malaria burden—such as Sierra Leone, Congo, Central African Republic, and Guinea—received little to no funding for malaria research in recent years.
However, other countries—such as Tanzania, Uganda, and Kenya—received close to $100 million in funding for malaria research.
Michael Head, PhD, of the University of Southampton in the UK, and his colleagues reported these findings in The Lancet Global Health.
“We have been able to provide a comprehensive overview of the landscape of funding for malaria in sub-Saharan Africa, a massive area where around 90% of worldwide malaria cases occur,” Dr Head said.
“We’ve shown that there are countries that are being neglected, and the global health community should reconsider strategies around resource allocation to reduce inequities and improve equality.”
For this study, Dr Head and his colleagues analyzed funding data spanning the period from 1997 to 2013. The data were sourced from 13 major public and philanthropic global health funders, as well as from funding databases.
The researchers ranked 45 countries according to the level of malaria research funding they received.
All of the countries studied received funding for malaria control, which includes investment for bed nets, public health schemes, and antimalarial drugs.
However, 8 of the 45 countries did not receive any funding related to malaria research. This included Central African Republic, Sierra Leone, and Congo—countries with a “reasonably high” malaria burden/mortality rate, according to Dr Head and his colleagues.
In all, there were 333 research awards, totaling $814.4 million. The countries that received the most research funding were Tanzania ($107.8 million), Uganda ($97.9 million), and Kenya ($92.9 million).
The 8 countries that received no research funding were Cape Verde, Botswana, Djibouti, Central African Republic, Mauritania, Congo, Chad, and Sierra Leone.
Dr Head and his colleagues suggested that the reason for the disparity in funding allocation could be, in part, due to the presence of established high-quality research infrastructure in countries such as Tanzania and Kenya, and political instability and poor healthcare infrastructures in lower-ranked nations such as Central African Republic or Sierra Leone.
“[N]ew investment in malaria research and development in these areas can encourage the development of improved health systems,” Dr Head said. “Many countries in sub-Saharan Africa simply do not have an established research infrastructure, and it is difficult for research funders to make investments in these settings.”
“Ultimately, however, there are neglected populations in these countries who suffer greatly from malaria and other diseases. Investments in health improve the wealth of a nation, and we need to be smarter with allocating limited resources to best help to reduce clear health inequalities.” ![]()
A new study has revealed inequalities in malaria research funding in sub-Saharan Africa.
The study showed that some countries with a high malaria burden—such as Sierra Leone, Congo, Central African Republic, and Guinea—received little to no funding for malaria research in recent years.
However, other countries—such as Tanzania, Uganda, and Kenya—received close to $100 million in funding for malaria research.
Michael Head, PhD, of the University of Southampton in the UK, and his colleagues reported these findings in The Lancet Global Health.
“We have been able to provide a comprehensive overview of the landscape of funding for malaria in sub-Saharan Africa, a massive area where around 90% of worldwide malaria cases occur,” Dr Head said.
“We’ve shown that there are countries that are being neglected, and the global health community should reconsider strategies around resource allocation to reduce inequities and improve equality.”
For this study, Dr Head and his colleagues analyzed funding data spanning the period from 1997 to 2013. The data were sourced from 13 major public and philanthropic global health funders, as well as from funding databases.
The researchers ranked 45 countries according to the level of malaria research funding they received.
All of the countries studied received funding for malaria control, which includes investment for bed nets, public health schemes, and antimalarial drugs.
However, 8 of the 45 countries did not receive any funding related to malaria research. This included Central African Republic, Sierra Leone, and Congo—countries with a “reasonably high” malaria burden/mortality rate, according to Dr Head and his colleagues.
In all, there were 333 research awards, totaling $814.4 million. The countries that received the most research funding were Tanzania ($107.8 million), Uganda ($97.9 million), and Kenya ($92.9 million).
The 8 countries that received no research funding were Cape Verde, Botswana, Djibouti, Central African Republic, Mauritania, Congo, Chad, and Sierra Leone.
Dr Head and his colleagues suggested that the reason for the disparity in funding allocation could be, in part, due to the presence of established high-quality research infrastructure in countries such as Tanzania and Kenya, and political instability and poor healthcare infrastructures in lower-ranked nations such as Central African Republic or Sierra Leone.
“[N]ew investment in malaria research and development in these areas can encourage the development of improved health systems,” Dr Head said. “Many countries in sub-Saharan Africa simply do not have an established research infrastructure, and it is difficult for research funders to make investments in these settings.”
“Ultimately, however, there are neglected populations in these countries who suffer greatly from malaria and other diseases. Investments in health improve the wealth of a nation, and we need to be smarter with allocating limited resources to best help to reduce clear health inequalities.” ![]()
A new study has revealed inequalities in malaria research funding in sub-Saharan Africa.
The study showed that some countries with a high malaria burden—such as Sierra Leone, Congo, Central African Republic, and Guinea—received little to no funding for malaria research in recent years.
However, other countries—such as Tanzania, Uganda, and Kenya—received close to $100 million in funding for malaria research.
Michael Head, PhD, of the University of Southampton in the UK, and his colleagues reported these findings in The Lancet Global Health.
“We have been able to provide a comprehensive overview of the landscape of funding for malaria in sub-Saharan Africa, a massive area where around 90% of worldwide malaria cases occur,” Dr Head said.
“We’ve shown that there are countries that are being neglected, and the global health community should reconsider strategies around resource allocation to reduce inequities and improve equality.”
For this study, Dr Head and his colleagues analyzed funding data spanning the period from 1997 to 2013. The data were sourced from 13 major public and philanthropic global health funders, as well as from funding databases.
The researchers ranked 45 countries according to the level of malaria research funding they received.
All of the countries studied received funding for malaria control, which includes investment for bed nets, public health schemes, and antimalarial drugs.
However, 8 of the 45 countries did not receive any funding related to malaria research. This included Central African Republic, Sierra Leone, and Congo—countries with a “reasonably high” malaria burden/mortality rate, according to Dr Head and his colleagues.
In all, there were 333 research awards, totaling $814.4 million. The countries that received the most research funding were Tanzania ($107.8 million), Uganda ($97.9 million), and Kenya ($92.9 million).
The 8 countries that received no research funding were Cape Verde, Botswana, Djibouti, Central African Republic, Mauritania, Congo, Chad, and Sierra Leone.
Dr Head and his colleagues suggested that the reason for the disparity in funding allocation could be, in part, due to the presence of established high-quality research infrastructure in countries such as Tanzania and Kenya, and political instability and poor healthcare infrastructures in lower-ranked nations such as Central African Republic or Sierra Leone.
“[N]ew investment in malaria research and development in these areas can encourage the development of improved health systems,” Dr Head said. “Many countries in sub-Saharan Africa simply do not have an established research infrastructure, and it is difficult for research funders to make investments in these settings.”
“Ultimately, however, there are neglected populations in these countries who suffer greatly from malaria and other diseases. Investments in health improve the wealth of a nation, and we need to be smarter with allocating limited resources to best help to reduce clear health inequalities.” ![]()
July 2017: Click for Credit
Here are 6 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. High-dose Oral Vitamin D3 Significantly Reduced Effects of Sunburn
To take the posttest, go to: http://bit.ly/2tmDiKc
Expires May 23, 2018
2. Women Less Likely to Be Diagnosed With Sleep Disorders
To take the posttest, go to: http://bit.ly/2rgLdne
Expires May 30, 2018
3. RA Treatment Delays Raise Risk for Long-term Disability
To take the posttest, go to: http://bit.ly/2tC0IGF
Expires May 30, 2018
4. Target Self-medication of Mood and Anxiety Symptoms
To take the posttest, go to: http://bit.ly/2vy5jel
Expires May 2, 2018
5. Two New Biomarkers for Breast Cancer Show Validity
To take the posttest, go to: http://bit.ly/2ve9H2L
Expires May 2, 2018
6. Time to Therapy for Gram-positive Bacteremia Reduced From 60 Hours to 4 Hours
To take the posttest, go to: http://bit.ly/2ssacIf
Expires May 25, 2018
Here are 6 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. High-dose Oral Vitamin D3 Significantly Reduced Effects of Sunburn
To take the posttest, go to: http://bit.ly/2tmDiKc
Expires May 23, 2018
2. Women Less Likely to Be Diagnosed With Sleep Disorders
To take the posttest, go to: http://bit.ly/2rgLdne
Expires May 30, 2018
3. RA Treatment Delays Raise Risk for Long-term Disability
To take the posttest, go to: http://bit.ly/2tC0IGF
Expires May 30, 2018
4. Target Self-medication of Mood and Anxiety Symptoms
To take the posttest, go to: http://bit.ly/2vy5jel
Expires May 2, 2018
5. Two New Biomarkers for Breast Cancer Show Validity
To take the posttest, go to: http://bit.ly/2ve9H2L
Expires May 2, 2018
6. Time to Therapy for Gram-positive Bacteremia Reduced From 60 Hours to 4 Hours
To take the posttest, go to: http://bit.ly/2ssacIf
Expires May 25, 2018
Here are 6 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. High-dose Oral Vitamin D3 Significantly Reduced Effects of Sunburn
To take the posttest, go to: http://bit.ly/2tmDiKc
Expires May 23, 2018
2. Women Less Likely to Be Diagnosed With Sleep Disorders
To take the posttest, go to: http://bit.ly/2rgLdne
Expires May 30, 2018
3. RA Treatment Delays Raise Risk for Long-term Disability
To take the posttest, go to: http://bit.ly/2tC0IGF
Expires May 30, 2018
4. Target Self-medication of Mood and Anxiety Symptoms
To take the posttest, go to: http://bit.ly/2vy5jel
Expires May 2, 2018
5. Two New Biomarkers for Breast Cancer Show Validity
To take the posttest, go to: http://bit.ly/2ve9H2L
Expires May 2, 2018
6. Time to Therapy for Gram-positive Bacteremia Reduced From 60 Hours to 4 Hours
To take the posttest, go to: http://bit.ly/2ssacIf
Expires May 25, 2018
Endo removes Opana ER from market
Even as it defended the product’s safety when appropriately used, Endo International withdrew from the market its long-acting opioid agonist Opana ER, in compliance with a June 8 Food and Drug Administration request. The company “continues to believe in the safety, efficacy, and favorable benefit-risk profile of Opana ER (oxymorphone hydrochloride extended release) when used as intended, and notes that the company has taken significant steps over the years to combat misuse and abuse,” according to a news release posted on Endo’s website. “Nevertheless, after careful consideration and consultation with the FDA following [its] June 2017 withdrawal request, the company has decided to voluntarily remove Opana ER from the market.”
In fact, the data showed a significant shift in the route of abuse of Opana ER from nasal to injection following the product’s reformulation. Injection abuse of reformulated Opana ER has been associated with a serious outbreak of HIV and hepatitis C, as well as cases of a thrombotic microangiopathy.
Endo said it will work with FDA to coordinate a smooth removal of the product, and insisted that the drug is safe and effective.
“Endo reiterates that neither the FDA’s withdrawal request nor Endo’s decision to voluntarily remove Opana ER from the market reflect a finding that the product is not safe or effective when taken as prescribed. To the contrary, Endo remains confident in the clinical research and other data demonstrating Opana ER’s safety and efficacy, as well as its favorable risk-benefit profile when used as intended in appropriate patients.”
Opana ER was first approved in 2006 for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. It was reformulated in 2012, with the intent of making it “resistant to physical and chemical manipulation for abuse by snorting or injecting,” according to the FDA release.
On Twitter @Alz_gal
Even as it defended the product’s safety when appropriately used, Endo International withdrew from the market its long-acting opioid agonist Opana ER, in compliance with a June 8 Food and Drug Administration request. The company “continues to believe in the safety, efficacy, and favorable benefit-risk profile of Opana ER (oxymorphone hydrochloride extended release) when used as intended, and notes that the company has taken significant steps over the years to combat misuse and abuse,” according to a news release posted on Endo’s website. “Nevertheless, after careful consideration and consultation with the FDA following [its] June 2017 withdrawal request, the company has decided to voluntarily remove Opana ER from the market.”
In fact, the data showed a significant shift in the route of abuse of Opana ER from nasal to injection following the product’s reformulation. Injection abuse of reformulated Opana ER has been associated with a serious outbreak of HIV and hepatitis C, as well as cases of a thrombotic microangiopathy.
Endo said it will work with FDA to coordinate a smooth removal of the product, and insisted that the drug is safe and effective.
“Endo reiterates that neither the FDA’s withdrawal request nor Endo’s decision to voluntarily remove Opana ER from the market reflect a finding that the product is not safe or effective when taken as prescribed. To the contrary, Endo remains confident in the clinical research and other data demonstrating Opana ER’s safety and efficacy, as well as its favorable risk-benefit profile when used as intended in appropriate patients.”
Opana ER was first approved in 2006 for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. It was reformulated in 2012, with the intent of making it “resistant to physical and chemical manipulation for abuse by snorting or injecting,” according to the FDA release.
On Twitter @Alz_gal
Even as it defended the product’s safety when appropriately used, Endo International withdrew from the market its long-acting opioid agonist Opana ER, in compliance with a June 8 Food and Drug Administration request. The company “continues to believe in the safety, efficacy, and favorable benefit-risk profile of Opana ER (oxymorphone hydrochloride extended release) when used as intended, and notes that the company has taken significant steps over the years to combat misuse and abuse,” according to a news release posted on Endo’s website. “Nevertheless, after careful consideration and consultation with the FDA following [its] June 2017 withdrawal request, the company has decided to voluntarily remove Opana ER from the market.”
In fact, the data showed a significant shift in the route of abuse of Opana ER from nasal to injection following the product’s reformulation. Injection abuse of reformulated Opana ER has been associated with a serious outbreak of HIV and hepatitis C, as well as cases of a thrombotic microangiopathy.
Endo said it will work with FDA to coordinate a smooth removal of the product, and insisted that the drug is safe and effective.
“Endo reiterates that neither the FDA’s withdrawal request nor Endo’s decision to voluntarily remove Opana ER from the market reflect a finding that the product is not safe or effective when taken as prescribed. To the contrary, Endo remains confident in the clinical research and other data demonstrating Opana ER’s safety and efficacy, as well as its favorable risk-benefit profile when used as intended in appropriate patients.”
Opana ER was first approved in 2006 for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time. It was reformulated in 2012, with the intent of making it “resistant to physical and chemical manipulation for abuse by snorting or injecting,” according to the FDA release.
On Twitter @Alz_gal
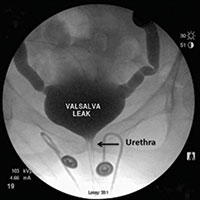
Video urodynamics for the evaluation of complex urinary symptoms
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
This video is brought to you by
FDA has the resources to answer questions about medicines and pregnancy
There are about 6 million pregnancies in the United States each year, and it’s estimated that 50% of pregnant women take at least one medicine. Physicians play an important role in helping their pregnant patients make informed health choices, especially when it comes to the safe use of medications.
- Emphasize that patients should always talk to their health care provider before taking any medicines, herbs, or vitamins. The website provides tips on how to start the conversation with patients about what medicines, herbs, or vitamins to avoid when pregnant.
- Encourage patients to check the drug label and other information that comes with their medicine to learn about possible risks for women who are pregnant or breastfeeding.
- Assist pregnant patients with changing medications as needed.
- Advise pregnant patients if they need to take more or less of their medicines.
- Advise patients about medicines that can and cannot be used when they start breastfeeding.
- Encourage patients to talk about any problems they have with their medicine.
- Report any serious problems your pregnant patients have had after taking a medicine to the FDA. It falls to physicians to report to the FDA any cases of serious side effects, problems with product quality, and product-use errors or with any of the following products: human drugs, medical devices, blood products and other biologics (except vaccines), and/or medical foods.
- Encourage patients to enroll in a Pregnancy Exposure Registry, if applicable at the FDA website, which collects information on pregnancy outcomes in women who already are taking medications. Observational studies of the patients that physicians help enroll in a pregnancy exposure registry can improve drug safety information for medicines used during pregnancy and can be used to update drug labeling. The observational studies included with the registries can also help physicians make medicine recommendations for use during pregnancy. The list includes contact information for each registry. Physicians can check online to see if there is a registry for their patients’ medicine.
The FDA website also provides information about the Pregnancy and Lactation Labeling Final Rule, which requires changes to the content and format for information presented in prescription drug labeling. The changes are implemented to help health care providers assess risk versus benefit and in subsequent counseling of pregnant women and nursing mothers.
The FDA offers free medicine safety and pregnancy resources for pregnant women, including downloadable infographics; a Medicines Record Keeper brochure in English, Spanish
Pregnancy is an exciting time for women, but they may have questions and concerns about how medicines they take will affect their babies. Our pregnancy website can help make a woman’s pregnancy happier and healthier.
Dr. Yao is the director of the division of pediatrics and maternal health, Office of Drug Evaluation IV, Center for Drug Evaluation and Research at the FDA, in Silver Spring, Md.
There are about 6 million pregnancies in the United States each year, and it’s estimated that 50% of pregnant women take at least one medicine. Physicians play an important role in helping their pregnant patients make informed health choices, especially when it comes to the safe use of medications.
- Emphasize that patients should always talk to their health care provider before taking any medicines, herbs, or vitamins. The website provides tips on how to start the conversation with patients about what medicines, herbs, or vitamins to avoid when pregnant.
- Encourage patients to check the drug label and other information that comes with their medicine to learn about possible risks for women who are pregnant or breastfeeding.
- Assist pregnant patients with changing medications as needed.
- Advise pregnant patients if they need to take more or less of their medicines.
- Advise patients about medicines that can and cannot be used when they start breastfeeding.
- Encourage patients to talk about any problems they have with their medicine.
- Report any serious problems your pregnant patients have had after taking a medicine to the FDA. It falls to physicians to report to the FDA any cases of serious side effects, problems with product quality, and product-use errors or with any of the following products: human drugs, medical devices, blood products and other biologics (except vaccines), and/or medical foods.
- Encourage patients to enroll in a Pregnancy Exposure Registry, if applicable at the FDA website, which collects information on pregnancy outcomes in women who already are taking medications. Observational studies of the patients that physicians help enroll in a pregnancy exposure registry can improve drug safety information for medicines used during pregnancy and can be used to update drug labeling. The observational studies included with the registries can also help physicians make medicine recommendations for use during pregnancy. The list includes contact information for each registry. Physicians can check online to see if there is a registry for their patients’ medicine.
The FDA website also provides information about the Pregnancy and Lactation Labeling Final Rule, which requires changes to the content and format for information presented in prescription drug labeling. The changes are implemented to help health care providers assess risk versus benefit and in subsequent counseling of pregnant women and nursing mothers.
The FDA offers free medicine safety and pregnancy resources for pregnant women, including downloadable infographics; a Medicines Record Keeper brochure in English, Spanish
Pregnancy is an exciting time for women, but they may have questions and concerns about how medicines they take will affect their babies. Our pregnancy website can help make a woman’s pregnancy happier and healthier.
Dr. Yao is the director of the division of pediatrics and maternal health, Office of Drug Evaluation IV, Center for Drug Evaluation and Research at the FDA, in Silver Spring, Md.
There are about 6 million pregnancies in the United States each year, and it’s estimated that 50% of pregnant women take at least one medicine. Physicians play an important role in helping their pregnant patients make informed health choices, especially when it comes to the safe use of medications.
- Emphasize that patients should always talk to their health care provider before taking any medicines, herbs, or vitamins. The website provides tips on how to start the conversation with patients about what medicines, herbs, or vitamins to avoid when pregnant.
- Encourage patients to check the drug label and other information that comes with their medicine to learn about possible risks for women who are pregnant or breastfeeding.
- Assist pregnant patients with changing medications as needed.
- Advise pregnant patients if they need to take more or less of their medicines.
- Advise patients about medicines that can and cannot be used when they start breastfeeding.
- Encourage patients to talk about any problems they have with their medicine.
- Report any serious problems your pregnant patients have had after taking a medicine to the FDA. It falls to physicians to report to the FDA any cases of serious side effects, problems with product quality, and product-use errors or with any of the following products: human drugs, medical devices, blood products and other biologics (except vaccines), and/or medical foods.
- Encourage patients to enroll in a Pregnancy Exposure Registry, if applicable at the FDA website, which collects information on pregnancy outcomes in women who already are taking medications. Observational studies of the patients that physicians help enroll in a pregnancy exposure registry can improve drug safety information for medicines used during pregnancy and can be used to update drug labeling. The observational studies included with the registries can also help physicians make medicine recommendations for use during pregnancy. The list includes contact information for each registry. Physicians can check online to see if there is a registry for their patients’ medicine.
The FDA website also provides information about the Pregnancy and Lactation Labeling Final Rule, which requires changes to the content and format for information presented in prescription drug labeling. The changes are implemented to help health care providers assess risk versus benefit and in subsequent counseling of pregnant women and nursing mothers.
The FDA offers free medicine safety and pregnancy resources for pregnant women, including downloadable infographics; a Medicines Record Keeper brochure in English, Spanish
Pregnancy is an exciting time for women, but they may have questions and concerns about how medicines they take will affect their babies. Our pregnancy website can help make a woman’s pregnancy happier and healthier.
Dr. Yao is the director of the division of pediatrics and maternal health, Office of Drug Evaluation IV, Center for Drug Evaluation and Research at the FDA, in Silver Spring, Md.
Septicemia admissions almost tripled from 2005 to 2014
Admissions for septicemia nearly tripled from 2005 to 2014, as it became the third most common diagnosis for hospital stays, according to the Agency for Healthcare Research and Quality.
There were over 1.5 million hospital stays with a principal diagnosis of septicemia in 2014, an increase of 192% over the 518,000 stays in 2005. The only diagnoses with more admissions in 2014 were pregnancy/childbirth with 4.1 million stays and newborns/neonates at almost 4 million, although both were down from 2005. That year, septicemia did not even rank among the top 10 diagnoses, the AHRQ reported.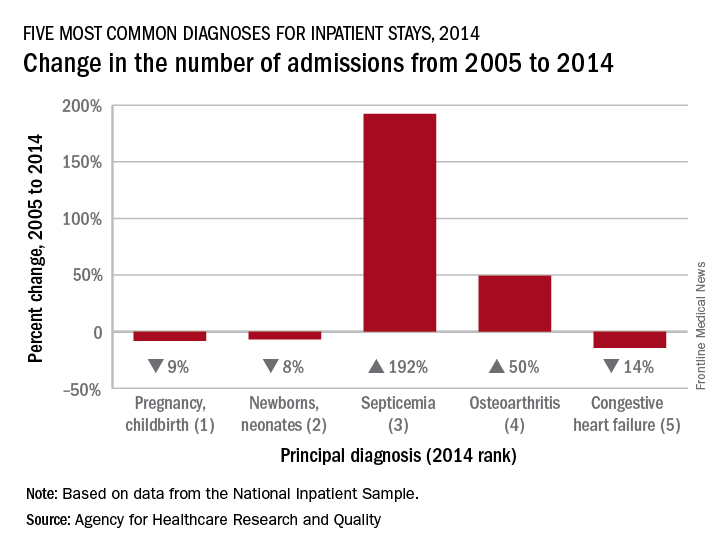
Pneumonia, which was the third most common diagnosis in 2005, dropped by 32% and ended up in sixth place in 2014, while admissions for coronary atherosclerosis, which was fourth in 2005, decreased by 63%, dropping out of the top 10, by 2014, the AHRQ said.
Septicemia was the most common diagnosis for inpatient stays among those aged 75 years and older and the second most common for those aged 65-74 and 45-64. The leading nonmaternal, nonneonatal diagnosis in the two youngest age groups, 0-17 and 18-44 years, was mood disorders, and the most common cause of admissions for those aged 45-64 and 65-74 was osteoarthritis, the AHRQ reported.
Admissions for septicemia nearly tripled from 2005 to 2014, as it became the third most common diagnosis for hospital stays, according to the Agency for Healthcare Research and Quality.
There were over 1.5 million hospital stays with a principal diagnosis of septicemia in 2014, an increase of 192% over the 518,000 stays in 2005. The only diagnoses with more admissions in 2014 were pregnancy/childbirth with 4.1 million stays and newborns/neonates at almost 4 million, although both were down from 2005. That year, septicemia did not even rank among the top 10 diagnoses, the AHRQ reported.
Pneumonia, which was the third most common diagnosis in 2005, dropped by 32% and ended up in sixth place in 2014, while admissions for coronary atherosclerosis, which was fourth in 2005, decreased by 63%, dropping out of the top 10, by 2014, the AHRQ said.
Septicemia was the most common diagnosis for inpatient stays among those aged 75 years and older and the second most common for those aged 65-74 and 45-64. The leading nonmaternal, nonneonatal diagnosis in the two youngest age groups, 0-17 and 18-44 years, was mood disorders, and the most common cause of admissions for those aged 45-64 and 65-74 was osteoarthritis, the AHRQ reported.
Admissions for septicemia nearly tripled from 2005 to 2014, as it became the third most common diagnosis for hospital stays, according to the Agency for Healthcare Research and Quality.
There were over 1.5 million hospital stays with a principal diagnosis of septicemia in 2014, an increase of 192% over the 518,000 stays in 2005. The only diagnoses with more admissions in 2014 were pregnancy/childbirth with 4.1 million stays and newborns/neonates at almost 4 million, although both were down from 2005. That year, septicemia did not even rank among the top 10 diagnoses, the AHRQ reported.
Pneumonia, which was the third most common diagnosis in 2005, dropped by 32% and ended up in sixth place in 2014, while admissions for coronary atherosclerosis, which was fourth in 2005, decreased by 63%, dropping out of the top 10, by 2014, the AHRQ said.
Septicemia was the most common diagnosis for inpatient stays among those aged 75 years and older and the second most common for those aged 65-74 and 45-64. The leading nonmaternal, nonneonatal diagnosis in the two youngest age groups, 0-17 and 18-44 years, was mood disorders, and the most common cause of admissions for those aged 45-64 and 65-74 was osteoarthritis, the AHRQ reported.
Bad news keeps piling up for Absorb coronary scaffold
PARIS – Device thrombosis occurred nearly four times more frequently in recipients of the Absorb everolimus-eluting bioresorbable vascular scaffold than with the Xience everolimus-eluting metallic stent during 2 years of prospective follow-up in the randomized AIDA trial.
AIDA (the Amsterdam Investigator-Initiated Absorb Strategy All-Comers Trial) was the first randomized trial designed to compare the Absorb scaffold to a drug-eluting metallic stent in a broad patient population reflecting routine real-world clinical practice. The disturbing AIDA finding follows upon earlier serious concerns raised regarding an increased risk of scaffold thrombosis – and the particularly worrisome complication of late thrombosis – in the ABSORB Japan and ABSORB II trials, Joanna J. Wykrzykowska, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The device was approved by the Food and Drug Administration in July 2016. In March 2017 the agency issued a safety alert regarding the Absorb scaffold after release of the 2-year data from the 2,008-patient ABSORB III trial showing a significantly higher rate of target-lesion failure than with the Xience stent. Both devices are marketed by Abbott Vascular.
AIDA was a single-blind multicenter Dutch trial that randomized 1,845 patients undergoing PCI, 55% of whom presented with acute coronary syndrome and 26% of whom had ST-elevation MI. The primary endpoint was target vessel failure, a composite of cardiac death, target vessel MI, or target vessel revascularization. The 2-year cumulative rate did not differ significantly between the two study arms: 11.7% in the scaffold group and 10.7% in the metallic stent recipients.
However, definite or probable device thrombosis occurred in 3.5% of the scaffold group compared with 0.9% of metallic stent recipients, for a highly significant 3.9-fold increased risk. This was associated with a significantly increased 2-year cumulative risk of MI: 5.5% versus 3.2%.
On the basis of this unsettling finding, coupled with the fact that ABSORB II investigators did not find any instance of very late scaffold thrombosis among 63 patients who remained on dual-antiplatelet therapy (DAPT) continuously for up to 3 years, Dr. Wykrzykowska and her coinvestigators have informed AIDA participants of their treatment assignment. They have also recommended that the Absorb recipients go on extended DAPT, even though there is no high-grade evidence as yet that this will prevent late scaffold thrombosis or that the drug-induced increased bleeding risk of prolonged DAPT might cancel or perhaps even outweigh the potential protection against device thrombosis.
On top of all this, implantation of the scaffold entails a longer procedure time and a greater volume of contrast material.
Discussant Mahmoud Hashemian, MD, observed that while bioresorbable vascular scaffolds are “physiologically ideal” because, unlike metallic stents, theoretically they leave no permanent implant to impede vasomotion and serve as a nidus for neoatherosclerosis, to date they have shown no real-world benefits over current-generation drug-eluting metallic stents, but only disadvantages.
“This doesn’t mean we have to feel hopeless. I’m not hopeless at all,” said Dr. Hashemian, an interventional cardiologist at Day General Hospital in Tehran. “I’m sure this [bioresorbable scaffolds] will be the future of our stents. But it needs more work. The company tells me they are going to launch a newer one, maybe next year, with thinner struts and more expandability.”
Asked about the likely mechanism of prolonged thrombosis risk with Absorb, Dr. Wykrzykowska was quick to say no one really knows at this point.
“Technique [predilation at a 1:1 balloon-to-artery ratio with an appropriately sized balloon] can obviously improve things in the short term for early events, but I don’t think we understand the biology of late events. We don’t understand the interaction between the device and the vessel. It’s extremely complex,” she said.
AIDA was funded by an unrestricted educational grant from Abbott Vascular. Dr. Wykrzykowska reported receiving consulting and lecture fees from the company.
PARIS – Device thrombosis occurred nearly four times more frequently in recipients of the Absorb everolimus-eluting bioresorbable vascular scaffold than with the Xience everolimus-eluting metallic stent during 2 years of prospective follow-up in the randomized AIDA trial.
AIDA (the Amsterdam Investigator-Initiated Absorb Strategy All-Comers Trial) was the first randomized trial designed to compare the Absorb scaffold to a drug-eluting metallic stent in a broad patient population reflecting routine real-world clinical practice. The disturbing AIDA finding follows upon earlier serious concerns raised regarding an increased risk of scaffold thrombosis – and the particularly worrisome complication of late thrombosis – in the ABSORB Japan and ABSORB II trials, Joanna J. Wykrzykowska, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The device was approved by the Food and Drug Administration in July 2016. In March 2017 the agency issued a safety alert regarding the Absorb scaffold after release of the 2-year data from the 2,008-patient ABSORB III trial showing a significantly higher rate of target-lesion failure than with the Xience stent. Both devices are marketed by Abbott Vascular.
AIDA was a single-blind multicenter Dutch trial that randomized 1,845 patients undergoing PCI, 55% of whom presented with acute coronary syndrome and 26% of whom had ST-elevation MI. The primary endpoint was target vessel failure, a composite of cardiac death, target vessel MI, or target vessel revascularization. The 2-year cumulative rate did not differ significantly between the two study arms: 11.7% in the scaffold group and 10.7% in the metallic stent recipients.
However, definite or probable device thrombosis occurred in 3.5% of the scaffold group compared with 0.9% of metallic stent recipients, for a highly significant 3.9-fold increased risk. This was associated with a significantly increased 2-year cumulative risk of MI: 5.5% versus 3.2%.
On the basis of this unsettling finding, coupled with the fact that ABSORB II investigators did not find any instance of very late scaffold thrombosis among 63 patients who remained on dual-antiplatelet therapy (DAPT) continuously for up to 3 years, Dr. Wykrzykowska and her coinvestigators have informed AIDA participants of their treatment assignment. They have also recommended that the Absorb recipients go on extended DAPT, even though there is no high-grade evidence as yet that this will prevent late scaffold thrombosis or that the drug-induced increased bleeding risk of prolonged DAPT might cancel or perhaps even outweigh the potential protection against device thrombosis.
On top of all this, implantation of the scaffold entails a longer procedure time and a greater volume of contrast material.
Discussant Mahmoud Hashemian, MD, observed that while bioresorbable vascular scaffolds are “physiologically ideal” because, unlike metallic stents, theoretically they leave no permanent implant to impede vasomotion and serve as a nidus for neoatherosclerosis, to date they have shown no real-world benefits over current-generation drug-eluting metallic stents, but only disadvantages.
“This doesn’t mean we have to feel hopeless. I’m not hopeless at all,” said Dr. Hashemian, an interventional cardiologist at Day General Hospital in Tehran. “I’m sure this [bioresorbable scaffolds] will be the future of our stents. But it needs more work. The company tells me they are going to launch a newer one, maybe next year, with thinner struts and more expandability.”
Asked about the likely mechanism of prolonged thrombosis risk with Absorb, Dr. Wykrzykowska was quick to say no one really knows at this point.
“Technique [predilation at a 1:1 balloon-to-artery ratio with an appropriately sized balloon] can obviously improve things in the short term for early events, but I don’t think we understand the biology of late events. We don’t understand the interaction between the device and the vessel. It’s extremely complex,” she said.
AIDA was funded by an unrestricted educational grant from Abbott Vascular. Dr. Wykrzykowska reported receiving consulting and lecture fees from the company.
PARIS – Device thrombosis occurred nearly four times more frequently in recipients of the Absorb everolimus-eluting bioresorbable vascular scaffold than with the Xience everolimus-eluting metallic stent during 2 years of prospective follow-up in the randomized AIDA trial.
AIDA (the Amsterdam Investigator-Initiated Absorb Strategy All-Comers Trial) was the first randomized trial designed to compare the Absorb scaffold to a drug-eluting metallic stent in a broad patient population reflecting routine real-world clinical practice. The disturbing AIDA finding follows upon earlier serious concerns raised regarding an increased risk of scaffold thrombosis – and the particularly worrisome complication of late thrombosis – in the ABSORB Japan and ABSORB II trials, Joanna J. Wykrzykowska, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The device was approved by the Food and Drug Administration in July 2016. In March 2017 the agency issued a safety alert regarding the Absorb scaffold after release of the 2-year data from the 2,008-patient ABSORB III trial showing a significantly higher rate of target-lesion failure than with the Xience stent. Both devices are marketed by Abbott Vascular.
AIDA was a single-blind multicenter Dutch trial that randomized 1,845 patients undergoing PCI, 55% of whom presented with acute coronary syndrome and 26% of whom had ST-elevation MI. The primary endpoint was target vessel failure, a composite of cardiac death, target vessel MI, or target vessel revascularization. The 2-year cumulative rate did not differ significantly between the two study arms: 11.7% in the scaffold group and 10.7% in the metallic stent recipients.
However, definite or probable device thrombosis occurred in 3.5% of the scaffold group compared with 0.9% of metallic stent recipients, for a highly significant 3.9-fold increased risk. This was associated with a significantly increased 2-year cumulative risk of MI: 5.5% versus 3.2%.
On the basis of this unsettling finding, coupled with the fact that ABSORB II investigators did not find any instance of very late scaffold thrombosis among 63 patients who remained on dual-antiplatelet therapy (DAPT) continuously for up to 3 years, Dr. Wykrzykowska and her coinvestigators have informed AIDA participants of their treatment assignment. They have also recommended that the Absorb recipients go on extended DAPT, even though there is no high-grade evidence as yet that this will prevent late scaffold thrombosis or that the drug-induced increased bleeding risk of prolonged DAPT might cancel or perhaps even outweigh the potential protection against device thrombosis.
On top of all this, implantation of the scaffold entails a longer procedure time and a greater volume of contrast material.
Discussant Mahmoud Hashemian, MD, observed that while bioresorbable vascular scaffolds are “physiologically ideal” because, unlike metallic stents, theoretically they leave no permanent implant to impede vasomotion and serve as a nidus for neoatherosclerosis, to date they have shown no real-world benefits over current-generation drug-eluting metallic stents, but only disadvantages.
“This doesn’t mean we have to feel hopeless. I’m not hopeless at all,” said Dr. Hashemian, an interventional cardiologist at Day General Hospital in Tehran. “I’m sure this [bioresorbable scaffolds] will be the future of our stents. But it needs more work. The company tells me they are going to launch a newer one, maybe next year, with thinner struts and more expandability.”
Asked about the likely mechanism of prolonged thrombosis risk with Absorb, Dr. Wykrzykowska was quick to say no one really knows at this point.
“Technique [predilation at a 1:1 balloon-to-artery ratio with an appropriately sized balloon] can obviously improve things in the short term for early events, but I don’t think we understand the biology of late events. We don’t understand the interaction between the device and the vessel. It’s extremely complex,” she said.
AIDA was funded by an unrestricted educational grant from Abbott Vascular. Dr. Wykrzykowska reported receiving consulting and lecture fees from the company.
AT EUROPCR
Key clinical point:
Major finding: During 2 years of prospective follow-up, definite or probable device thrombosis occurred in 3.5% of recipients of a bioresorbable vascular scaffold, compared with 0.9% of metallic stent recipients, for a highly significant 3.9-fold increased risk.
Data source: AIDA, a single-blind multicenter Dutch trial that randomized a broadly representative group of 1,845 patients undergoing PCI to the Absorb bioresorbable vascular scaffold or the Xience everolimus-eluting metallic stent.
Disclosures: The AIDA study was funded by an unrestricted educational grant from Abbott Vascular. The presenter reported receiving consulting and lecture fees from the company.
Reframe view of borderline personality disorder patients as survivors
WASHINGTON – A view of borderline personality disorder as neurobiological in nature can help clinicians extend their patience and empathy to these notoriously difficult-to-treat patients, according to an expert.
“Unfortunately, there are those who ... are just too frustrated by” [patients with borderline personality disorder], said Carmen V. Pinto, MD, an Elizabethtown, Ky.–based psychiatrist who specializes in treating patients with the disorder.
The diagnosis occurs in up to 2% of the population and is twice as common in women. About three-quarters of borderline personality disorder patients will attempt suicide at least once, and up to 1 in 10 complete suicide, said Dr. Pinto, assistant clinical professor of psychiatry at the University of Louisville (Ky.).
“There is an art to dealing with patients who are impulsive, and sometimes dangerous and scary,” Dr. Pinto said at Summit in Neurology & Psychiatry, held by Global Academy for Medical Education.
He emphasized the effect of unstable interpersonal relationships combined with frontolimbic brain abnormalities on the lives of patients with borderline personality disorder. In some cases, borderline personality disorder patients experienced traumatic events such as sexual abuse that was personal and ongoing – and occurred while the personality was being formed.
“These are normal people who have been exposed to abnormal stress,” Dr. Pinto said. “So you have to be careful, and see them not as victims but as survivors still suffering.”
Citing several lines of study into the neurobiochemical mechanisms of action in the disorder, Dr. Pinto discussed the role of disruption to the brain’s cortical-limbic circuit that serves as the brain’s basic stress response. In this population, an overactive amygdala and hypofunctioning frontal cortex continually signal threats even when none exist – exciting the sympathetic nervous system – as well as the endocrine and immune systems.
“The circuit gets overwhelmed, either because there really is too much to deal with, or there was poor functioning to start with, so the brain is going off like a pinball machine,” Dr. Pinto said. “If [the patients perceive] even the slightest offense, their limbic system lights up, and they have trouble turning it off, which is why it can be so difficult to deal with these folks in therapy.”
He explained the borderline personality patient’s typical negative valence as the result of this hyper-threat detection, often rooted in experiences in which people who professed to care for them acted contrary to their words, as in cases of incest. “They learn they can’t trust what is said, so they rely on nonverbal cues,” Dr. Pinto said. “They aren’t listening to what is said to them.”
Dr. Pinto cited a study of how this patient population had trouble distinguishing neutral faces from faces expressing anger or boredom, resulting in their believing that most people they encounter are unhappy with them in some way, causing them to retreat or have stressful relationships with others.
“Therapeutic neutrality is not the best way to approach this person, because they read it as ‘you are mad at me’ or ‘you’re bored with me’ or ‘you don’t like me,’ ” Dr. Pinto said, noting that he likes to reassure patients that they are doing the best they can with what they have. However, he warned against how often, in these patients’ quest to feel loved and accepted, they might ask for hugs or other contact. Rather than panic, use the opportunity to help the patient practice having healthy boundaries by explaining that not hugging them is designed to protect them, Dr. Pinto said.
Among the DSM-5 criteria for diagnosing this disorder is that the patient has an “enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture.” Because there are no imaging or laboratory tests to confirm a deviant inner experience, the clinician must turn to the patient for assessment while quelling the impulse to assign meanings to action and insight.
“You really have to be quiet and listen. They are the expert with their inner experience, and you have to let them tell you what that is,” he said, noting that the distortion of how they view themselves in relation to others is painful and pervasive, crossing all areas of their lives.
Because relationships are so difficult for these patients to develop, Dr. Pinto urged keeping in mind that it is possible they have no other therapeutic relationship in their lives. “That is a scary responsibility, to think they have no one else to talk to but you,” he said. This underscores the need for clinicians to protect themselves, too. “If I don’t feel safe, I can’t do a good job for them – and I tell them that,” Dr. Pinto said.
Global Academy and this news organization are owned by the same company. Dr. Pinto had no relevant disclosures, although he said he is a paid speaker for Otsuka, Lundbeck, Janssen, and other pharmaceutical companies.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – A view of borderline personality disorder as neurobiological in nature can help clinicians extend their patience and empathy to these notoriously difficult-to-treat patients, according to an expert.
“Unfortunately, there are those who ... are just too frustrated by” [patients with borderline personality disorder], said Carmen V. Pinto, MD, an Elizabethtown, Ky.–based psychiatrist who specializes in treating patients with the disorder.
The diagnosis occurs in up to 2% of the population and is twice as common in women. About three-quarters of borderline personality disorder patients will attempt suicide at least once, and up to 1 in 10 complete suicide, said Dr. Pinto, assistant clinical professor of psychiatry at the University of Louisville (Ky.).
“There is an art to dealing with patients who are impulsive, and sometimes dangerous and scary,” Dr. Pinto said at Summit in Neurology & Psychiatry, held by Global Academy for Medical Education.
He emphasized the effect of unstable interpersonal relationships combined with frontolimbic brain abnormalities on the lives of patients with borderline personality disorder. In some cases, borderline personality disorder patients experienced traumatic events such as sexual abuse that was personal and ongoing – and occurred while the personality was being formed.
“These are normal people who have been exposed to abnormal stress,” Dr. Pinto said. “So you have to be careful, and see them not as victims but as survivors still suffering.”
Citing several lines of study into the neurobiochemical mechanisms of action in the disorder, Dr. Pinto discussed the role of disruption to the brain’s cortical-limbic circuit that serves as the brain’s basic stress response. In this population, an overactive amygdala and hypofunctioning frontal cortex continually signal threats even when none exist – exciting the sympathetic nervous system – as well as the endocrine and immune systems.
“The circuit gets overwhelmed, either because there really is too much to deal with, or there was poor functioning to start with, so the brain is going off like a pinball machine,” Dr. Pinto said. “If [the patients perceive] even the slightest offense, their limbic system lights up, and they have trouble turning it off, which is why it can be so difficult to deal with these folks in therapy.”
He explained the borderline personality patient’s typical negative valence as the result of this hyper-threat detection, often rooted in experiences in which people who professed to care for them acted contrary to their words, as in cases of incest. “They learn they can’t trust what is said, so they rely on nonverbal cues,” Dr. Pinto said. “They aren’t listening to what is said to them.”
Dr. Pinto cited a study of how this patient population had trouble distinguishing neutral faces from faces expressing anger or boredom, resulting in their believing that most people they encounter are unhappy with them in some way, causing them to retreat or have stressful relationships with others.
“Therapeutic neutrality is not the best way to approach this person, because they read it as ‘you are mad at me’ or ‘you’re bored with me’ or ‘you don’t like me,’ ” Dr. Pinto said, noting that he likes to reassure patients that they are doing the best they can with what they have. However, he warned against how often, in these patients’ quest to feel loved and accepted, they might ask for hugs or other contact. Rather than panic, use the opportunity to help the patient practice having healthy boundaries by explaining that not hugging them is designed to protect them, Dr. Pinto said.
Among the DSM-5 criteria for diagnosing this disorder is that the patient has an “enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture.” Because there are no imaging or laboratory tests to confirm a deviant inner experience, the clinician must turn to the patient for assessment while quelling the impulse to assign meanings to action and insight.
“You really have to be quiet and listen. They are the expert with their inner experience, and you have to let them tell you what that is,” he said, noting that the distortion of how they view themselves in relation to others is painful and pervasive, crossing all areas of their lives.
Because relationships are so difficult for these patients to develop, Dr. Pinto urged keeping in mind that it is possible they have no other therapeutic relationship in their lives. “That is a scary responsibility, to think they have no one else to talk to but you,” he said. This underscores the need for clinicians to protect themselves, too. “If I don’t feel safe, I can’t do a good job for them – and I tell them that,” Dr. Pinto said.
Global Academy and this news organization are owned by the same company. Dr. Pinto had no relevant disclosures, although he said he is a paid speaker for Otsuka, Lundbeck, Janssen, and other pharmaceutical companies.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – A view of borderline personality disorder as neurobiological in nature can help clinicians extend their patience and empathy to these notoriously difficult-to-treat patients, according to an expert.
“Unfortunately, there are those who ... are just too frustrated by” [patients with borderline personality disorder], said Carmen V. Pinto, MD, an Elizabethtown, Ky.–based psychiatrist who specializes in treating patients with the disorder.
The diagnosis occurs in up to 2% of the population and is twice as common in women. About three-quarters of borderline personality disorder patients will attempt suicide at least once, and up to 1 in 10 complete suicide, said Dr. Pinto, assistant clinical professor of psychiatry at the University of Louisville (Ky.).
“There is an art to dealing with patients who are impulsive, and sometimes dangerous and scary,” Dr. Pinto said at Summit in Neurology & Psychiatry, held by Global Academy for Medical Education.
He emphasized the effect of unstable interpersonal relationships combined with frontolimbic brain abnormalities on the lives of patients with borderline personality disorder. In some cases, borderline personality disorder patients experienced traumatic events such as sexual abuse that was personal and ongoing – and occurred while the personality was being formed.
“These are normal people who have been exposed to abnormal stress,” Dr. Pinto said. “So you have to be careful, and see them not as victims but as survivors still suffering.”
Citing several lines of study into the neurobiochemical mechanisms of action in the disorder, Dr. Pinto discussed the role of disruption to the brain’s cortical-limbic circuit that serves as the brain’s basic stress response. In this population, an overactive amygdala and hypofunctioning frontal cortex continually signal threats even when none exist – exciting the sympathetic nervous system – as well as the endocrine and immune systems.
“The circuit gets overwhelmed, either because there really is too much to deal with, or there was poor functioning to start with, so the brain is going off like a pinball machine,” Dr. Pinto said. “If [the patients perceive] even the slightest offense, their limbic system lights up, and they have trouble turning it off, which is why it can be so difficult to deal with these folks in therapy.”
He explained the borderline personality patient’s typical negative valence as the result of this hyper-threat detection, often rooted in experiences in which people who professed to care for them acted contrary to their words, as in cases of incest. “They learn they can’t trust what is said, so they rely on nonverbal cues,” Dr. Pinto said. “They aren’t listening to what is said to them.”
Dr. Pinto cited a study of how this patient population had trouble distinguishing neutral faces from faces expressing anger or boredom, resulting in their believing that most people they encounter are unhappy with them in some way, causing them to retreat or have stressful relationships with others.
“Therapeutic neutrality is not the best way to approach this person, because they read it as ‘you are mad at me’ or ‘you’re bored with me’ or ‘you don’t like me,’ ” Dr. Pinto said, noting that he likes to reassure patients that they are doing the best they can with what they have. However, he warned against how often, in these patients’ quest to feel loved and accepted, they might ask for hugs or other contact. Rather than panic, use the opportunity to help the patient practice having healthy boundaries by explaining that not hugging them is designed to protect them, Dr. Pinto said.
Among the DSM-5 criteria for diagnosing this disorder is that the patient has an “enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual’s culture.” Because there are no imaging or laboratory tests to confirm a deviant inner experience, the clinician must turn to the patient for assessment while quelling the impulse to assign meanings to action and insight.
“You really have to be quiet and listen. They are the expert with their inner experience, and you have to let them tell you what that is,” he said, noting that the distortion of how they view themselves in relation to others is painful and pervasive, crossing all areas of their lives.
Because relationships are so difficult for these patients to develop, Dr. Pinto urged keeping in mind that it is possible they have no other therapeutic relationship in their lives. “That is a scary responsibility, to think they have no one else to talk to but you,” he said. This underscores the need for clinicians to protect themselves, too. “If I don’t feel safe, I can’t do a good job for them – and I tell them that,” Dr. Pinto said.
Global Academy and this news organization are owned by the same company. Dr. Pinto had no relevant disclosures, although he said he is a paid speaker for Otsuka, Lundbeck, Janssen, and other pharmaceutical companies.
[email protected]
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM SUMMIT IN NEUROLOGY & PSYCHIATRY
FDA approves new panel to identify mCRC patients for panitumumab treatment
The Food and Drug Administration has approved the Praxis Extended RAS Panel for the identification of metastatic colorectal cancer patients who can be treated with panitumumab.
The Praxis Extended RAS Panel is able to detect 56 specific mutations in the RAS genes of mCRC patients, and is the first next generation sequencing test approved by the FDA capable of testing more than one RAS gene mutation. If RAS mutations are not detected, then panitumumab is indicated, and if a mutation is detected, panitumumab is not indicated, according to the FDA statement.
“Panitumumab’s product labeling has been modified to align the indication for panitumumab and intended use for the Praxis Extended RAS Panel,” the FDA noted.
The Food and Drug Administration has approved the Praxis Extended RAS Panel for the identification of metastatic colorectal cancer patients who can be treated with panitumumab.
The Praxis Extended RAS Panel is able to detect 56 specific mutations in the RAS genes of mCRC patients, and is the first next generation sequencing test approved by the FDA capable of testing more than one RAS gene mutation. If RAS mutations are not detected, then panitumumab is indicated, and if a mutation is detected, panitumumab is not indicated, according to the FDA statement.
“Panitumumab’s product labeling has been modified to align the indication for panitumumab and intended use for the Praxis Extended RAS Panel,” the FDA noted.
The Food and Drug Administration has approved the Praxis Extended RAS Panel for the identification of metastatic colorectal cancer patients who can be treated with panitumumab.
The Praxis Extended RAS Panel is able to detect 56 specific mutations in the RAS genes of mCRC patients, and is the first next generation sequencing test approved by the FDA capable of testing more than one RAS gene mutation. If RAS mutations are not detected, then panitumumab is indicated, and if a mutation is detected, panitumumab is not indicated, according to the FDA statement.
“Panitumumab’s product labeling has been modified to align the indication for panitumumab and intended use for the Praxis Extended RAS Panel,” the FDA noted.
New on the streets: Drug for nerve pain boosts high for opioid abusers
ATHENS, OHIO – On April 5, Ciera Smith sat in a car parked on the gravel driveway of the Rural Women’s Recovery Program here with a choice to make: Go to jail or enter treatment for her addiction.
Ms. Smith, 22, started abusing drugs when she was 18 years old, enticed by the “good time” she and her friends found in smoking marijuana. She later turned to addictive painkillers, then antianxiety medications such as Xanax and eventually Suboxone, a narcotic often used to replace opioids when treating addiction.
Before stepping out of the car, she decided she needed one more high before treatment. She reached into her purse and then swallowed a handful of gabapentin pills.
Last December, Ohio’s Board of Pharmacy began reporting sales of gabapentin prescriptions in its regular monitoring of controlled substances. The drug, which is neither an opioid nor designated a controlled substance by federal authorities, is used to treat nerve pain. But the board found that it was the most prescribed medication on its list that month, surpassing oxycodone by more than 9 million doses. In February, the Ohio Substance Abuse Monitoring Network issued an alert regarding increasing misuse across the state.
And it’s not just in Ohio. Gabapentin’s ability to tackle multiple ailments has helped make it one of the most popular medications in the United States. In May, it was the fifth-most prescribed drug in the nation, according to GoodRx.
Gabapentin is approved by the Food and Drug Administration to treat epilepsy and pain related to nerve damage, called neuropathy. Also known by its brand name, Neurontin, the drug acts as a sedative. It is widely considered nonaddictive and touted by the federal Centers for Disease Control and Prevention as an alternative intervention to opiates for chronic pain. Generally, doctors prescribe no more than 1,800-2,400 mg of gabapentin per day, according to information on the Mayo Clinic’s website.
Gabapentin does not carry the same risk of lethal overdoses as opioids, but drug experts say the effects of using gabapentin for long periods of time or in very high quantities, particularly among such sensitive populations as pregnant women, are not well known.
As providers dole out the drug in mass quantities for conditions such as restless legs syndrome and alcoholism, it is being subverted to a drug of abuse. Gabapentin can enhance the euphoria caused by an opioid and stave off drug withdrawals. In addition, it can bypass the blocking effects of medications used for addiction treatment, enabling patients to get high while in recovery.
Athens, home to Ohio University, lies in the southeastern corner of the state, which has been ravaged by the opioid epidemic. Despite experience in combating illicit drug use, law enforcement officials and drug counselors say the addition of gabapentin adds a new obstacle.
“I don’t know if we have a clear picture of the risk,” said Joe Gay, executive director of Health Recovery Services, a network of substance abuse recovery centers headquartered in Athens.
‘Available to be abused’
A literature review published in 2016 in the journal Addiction found about a fifth of those who abuse opiates misuse gabapentin. A separate 2015 study of adults in Appalachian Kentucky who abused opiates found 15% of participants also misused gabapentin in the past 6 months “to get high.”
In the same year, the drug was involved in 109 overdose deaths in West Virginia, the Charleston Gazette-Mail reported.
Rachel Quivey, an Athens pharmacist, said she noticed signs of gabapentin misuse half a decade ago when patients began picking up the drug several days before their prescription ran out.
“Gabapentin is so readily available,” she said. “That, in my opinion, is where a lot of that danger is. It’s available to be abused.”
In May, Ms. Quivey’s pharmacy filled roughly 33 prescriptions of gabapentin per week, dispensing 90-120 pills for each client.
For customers who arrive with scripts demanding a high dosage of the drug, Ms. Quivey sometimes calls the doctor to discuss her concerns. But many of them aren’t aware of gabapentin misuse, she said.
Even as gabapentin gets restocked regularly on Ms. Quivey’s shelves, the drug’s presence is increasing on the streets of Athens. A 300-milligram pill sells for as little as 75 cents.
Yet, according to Chuck Haegele, field supervisor for the major crimes unit at the Athens City Police Department, law enforcement can do little to stop its spread. That’s because gabapentin is not categorized as a controlled substance. That designation places restrictions on who can possess and dispense the drug.
“There’s really not much we can do at this point,” he said. “If it’s not controlled … it’s not illegal for somebody that’s not prescribed it to possess it.”
Mr. Haegele said he heard about the drug less than 3 months ago when an officer accidentally received a text message from someone offering to sell it. The police force, he said, is still trying to assess the threat of gabapentin.
Little testing
Nearly anyone arrested and found to struggle with addiction in Athens is given the option to go through a drug-court program to get treatment. But officials said that some exploit the absence of routine exams for gabapentin to get high while testing clean.
Brice Johnson, a probation officer at Athens County Municipal Court, said participants in the municipal court’s substance abuse mentally ill program undergo gabapentin testing only when abuse is suspected. Screenings are not regularly done on every client because gabapentin abuse has not been a concern and the testing adds expense, he said.
The rehab program run through the county prosecutor’s office, called Fresh Start, does test for gabapentin. Its latest round of screenings detected the drug in 5 of its roughly 238 active participants, prosecutor Keller Blackburn said.
Linda Holley, a clinical supervisor at an Athens outpatient program run by the Health Recovery Services, said she suspects at least half of her clients on Suboxone treatment abuse gabapentin. But the center can’t afford to regularly test every participant.
Ms. Holley said she sees clients who are prescribed gabapentin but, because of health privacy laws, she can’t share their status as a person in recovery to an outside provider without written consent. The restrictions give clients in recovery an opportunity to get high using drugs they obtained legally and still pass a drug test.
“With the gabapentin, I wish there were more we could do, but our hands are tied,” she said. “We can’t do anything but educate the client and discourage” them from using such medications.
Ms. Smith visited two separate doctors to secure a prescription. As she rotated through drug court, Narcotics Anonymous meetings, jail for relapsing on cocaine, and house arrest enforced with an ankle bracelet, she said her gabapentin abuse wasn’t detected until she arrived at the residential recovery center.
Today, Ms. Smith sticks to the recovery process. Expecting a baby in early July, her successful completion of the program not only means sobriety but also allows her the opportunity to restore custody of her eldest daughter and raise her children.
She intends to relocate her family away from the friends and routines that helped lead her to addiction and said she will help guide her daughter away from making similar mistakes.
“All I can do is be there and give her the knowledge that I can about addiction,” Ms. Smith said, “and hope that she chooses to go on the right path.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
ATHENS, OHIO – On April 5, Ciera Smith sat in a car parked on the gravel driveway of the Rural Women’s Recovery Program here with a choice to make: Go to jail or enter treatment for her addiction.
Ms. Smith, 22, started abusing drugs when she was 18 years old, enticed by the “good time” she and her friends found in smoking marijuana. She later turned to addictive painkillers, then antianxiety medications such as Xanax and eventually Suboxone, a narcotic often used to replace opioids when treating addiction.
Before stepping out of the car, she decided she needed one more high before treatment. She reached into her purse and then swallowed a handful of gabapentin pills.
Last December, Ohio’s Board of Pharmacy began reporting sales of gabapentin prescriptions in its regular monitoring of controlled substances. The drug, which is neither an opioid nor designated a controlled substance by federal authorities, is used to treat nerve pain. But the board found that it was the most prescribed medication on its list that month, surpassing oxycodone by more than 9 million doses. In February, the Ohio Substance Abuse Monitoring Network issued an alert regarding increasing misuse across the state.
And it’s not just in Ohio. Gabapentin’s ability to tackle multiple ailments has helped make it one of the most popular medications in the United States. In May, it was the fifth-most prescribed drug in the nation, according to GoodRx.
Gabapentin is approved by the Food and Drug Administration to treat epilepsy and pain related to nerve damage, called neuropathy. Also known by its brand name, Neurontin, the drug acts as a sedative. It is widely considered nonaddictive and touted by the federal Centers for Disease Control and Prevention as an alternative intervention to opiates for chronic pain. Generally, doctors prescribe no more than 1,800-2,400 mg of gabapentin per day, according to information on the Mayo Clinic’s website.
Gabapentin does not carry the same risk of lethal overdoses as opioids, but drug experts say the effects of using gabapentin for long periods of time or in very high quantities, particularly among such sensitive populations as pregnant women, are not well known.
As providers dole out the drug in mass quantities for conditions such as restless legs syndrome and alcoholism, it is being subverted to a drug of abuse. Gabapentin can enhance the euphoria caused by an opioid and stave off drug withdrawals. In addition, it can bypass the blocking effects of medications used for addiction treatment, enabling patients to get high while in recovery.
Athens, home to Ohio University, lies in the southeastern corner of the state, which has been ravaged by the opioid epidemic. Despite experience in combating illicit drug use, law enforcement officials and drug counselors say the addition of gabapentin adds a new obstacle.
“I don’t know if we have a clear picture of the risk,” said Joe Gay, executive director of Health Recovery Services, a network of substance abuse recovery centers headquartered in Athens.
‘Available to be abused’
A literature review published in 2016 in the journal Addiction found about a fifth of those who abuse opiates misuse gabapentin. A separate 2015 study of adults in Appalachian Kentucky who abused opiates found 15% of participants also misused gabapentin in the past 6 months “to get high.”
In the same year, the drug was involved in 109 overdose deaths in West Virginia, the Charleston Gazette-Mail reported.
Rachel Quivey, an Athens pharmacist, said she noticed signs of gabapentin misuse half a decade ago when patients began picking up the drug several days before their prescription ran out.
“Gabapentin is so readily available,” she said. “That, in my opinion, is where a lot of that danger is. It’s available to be abused.”
In May, Ms. Quivey’s pharmacy filled roughly 33 prescriptions of gabapentin per week, dispensing 90-120 pills for each client.
For customers who arrive with scripts demanding a high dosage of the drug, Ms. Quivey sometimes calls the doctor to discuss her concerns. But many of them aren’t aware of gabapentin misuse, she said.
Even as gabapentin gets restocked regularly on Ms. Quivey’s shelves, the drug’s presence is increasing on the streets of Athens. A 300-milligram pill sells for as little as 75 cents.
Yet, according to Chuck Haegele, field supervisor for the major crimes unit at the Athens City Police Department, law enforcement can do little to stop its spread. That’s because gabapentin is not categorized as a controlled substance. That designation places restrictions on who can possess and dispense the drug.
“There’s really not much we can do at this point,” he said. “If it’s not controlled … it’s not illegal for somebody that’s not prescribed it to possess it.”
Mr. Haegele said he heard about the drug less than 3 months ago when an officer accidentally received a text message from someone offering to sell it. The police force, he said, is still trying to assess the threat of gabapentin.
Little testing
Nearly anyone arrested and found to struggle with addiction in Athens is given the option to go through a drug-court program to get treatment. But officials said that some exploit the absence of routine exams for gabapentin to get high while testing clean.
Brice Johnson, a probation officer at Athens County Municipal Court, said participants in the municipal court’s substance abuse mentally ill program undergo gabapentin testing only when abuse is suspected. Screenings are not regularly done on every client because gabapentin abuse has not been a concern and the testing adds expense, he said.
The rehab program run through the county prosecutor’s office, called Fresh Start, does test for gabapentin. Its latest round of screenings detected the drug in 5 of its roughly 238 active participants, prosecutor Keller Blackburn said.
Linda Holley, a clinical supervisor at an Athens outpatient program run by the Health Recovery Services, said she suspects at least half of her clients on Suboxone treatment abuse gabapentin. But the center can’t afford to regularly test every participant.
Ms. Holley said she sees clients who are prescribed gabapentin but, because of health privacy laws, she can’t share their status as a person in recovery to an outside provider without written consent. The restrictions give clients in recovery an opportunity to get high using drugs they obtained legally and still pass a drug test.
“With the gabapentin, I wish there were more we could do, but our hands are tied,” she said. “We can’t do anything but educate the client and discourage” them from using such medications.
Ms. Smith visited two separate doctors to secure a prescription. As she rotated through drug court, Narcotics Anonymous meetings, jail for relapsing on cocaine, and house arrest enforced with an ankle bracelet, she said her gabapentin abuse wasn’t detected until she arrived at the residential recovery center.
Today, Ms. Smith sticks to the recovery process. Expecting a baby in early July, her successful completion of the program not only means sobriety but also allows her the opportunity to restore custody of her eldest daughter and raise her children.
She intends to relocate her family away from the friends and routines that helped lead her to addiction and said she will help guide her daughter away from making similar mistakes.
“All I can do is be there and give her the knowledge that I can about addiction,” Ms. Smith said, “and hope that she chooses to go on the right path.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
ATHENS, OHIO – On April 5, Ciera Smith sat in a car parked on the gravel driveway of the Rural Women’s Recovery Program here with a choice to make: Go to jail or enter treatment for her addiction.
Ms. Smith, 22, started abusing drugs when she was 18 years old, enticed by the “good time” she and her friends found in smoking marijuana. She later turned to addictive painkillers, then antianxiety medications such as Xanax and eventually Suboxone, a narcotic often used to replace opioids when treating addiction.
Before stepping out of the car, she decided she needed one more high before treatment. She reached into her purse and then swallowed a handful of gabapentin pills.
Last December, Ohio’s Board of Pharmacy began reporting sales of gabapentin prescriptions in its regular monitoring of controlled substances. The drug, which is neither an opioid nor designated a controlled substance by federal authorities, is used to treat nerve pain. But the board found that it was the most prescribed medication on its list that month, surpassing oxycodone by more than 9 million doses. In February, the Ohio Substance Abuse Monitoring Network issued an alert regarding increasing misuse across the state.
And it’s not just in Ohio. Gabapentin’s ability to tackle multiple ailments has helped make it one of the most popular medications in the United States. In May, it was the fifth-most prescribed drug in the nation, according to GoodRx.
Gabapentin is approved by the Food and Drug Administration to treat epilepsy and pain related to nerve damage, called neuropathy. Also known by its brand name, Neurontin, the drug acts as a sedative. It is widely considered nonaddictive and touted by the federal Centers for Disease Control and Prevention as an alternative intervention to opiates for chronic pain. Generally, doctors prescribe no more than 1,800-2,400 mg of gabapentin per day, according to information on the Mayo Clinic’s website.
Gabapentin does not carry the same risk of lethal overdoses as opioids, but drug experts say the effects of using gabapentin for long periods of time or in very high quantities, particularly among such sensitive populations as pregnant women, are not well known.
As providers dole out the drug in mass quantities for conditions such as restless legs syndrome and alcoholism, it is being subverted to a drug of abuse. Gabapentin can enhance the euphoria caused by an opioid and stave off drug withdrawals. In addition, it can bypass the blocking effects of medications used for addiction treatment, enabling patients to get high while in recovery.
Athens, home to Ohio University, lies in the southeastern corner of the state, which has been ravaged by the opioid epidemic. Despite experience in combating illicit drug use, law enforcement officials and drug counselors say the addition of gabapentin adds a new obstacle.
“I don’t know if we have a clear picture of the risk,” said Joe Gay, executive director of Health Recovery Services, a network of substance abuse recovery centers headquartered in Athens.
‘Available to be abused’
A literature review published in 2016 in the journal Addiction found about a fifth of those who abuse opiates misuse gabapentin. A separate 2015 study of adults in Appalachian Kentucky who abused opiates found 15% of participants also misused gabapentin in the past 6 months “to get high.”
In the same year, the drug was involved in 109 overdose deaths in West Virginia, the Charleston Gazette-Mail reported.
Rachel Quivey, an Athens pharmacist, said she noticed signs of gabapentin misuse half a decade ago when patients began picking up the drug several days before their prescription ran out.
“Gabapentin is so readily available,” she said. “That, in my opinion, is where a lot of that danger is. It’s available to be abused.”
In May, Ms. Quivey’s pharmacy filled roughly 33 prescriptions of gabapentin per week, dispensing 90-120 pills for each client.
For customers who arrive with scripts demanding a high dosage of the drug, Ms. Quivey sometimes calls the doctor to discuss her concerns. But many of them aren’t aware of gabapentin misuse, she said.
Even as gabapentin gets restocked regularly on Ms. Quivey’s shelves, the drug’s presence is increasing on the streets of Athens. A 300-milligram pill sells for as little as 75 cents.
Yet, according to Chuck Haegele, field supervisor for the major crimes unit at the Athens City Police Department, law enforcement can do little to stop its spread. That’s because gabapentin is not categorized as a controlled substance. That designation places restrictions on who can possess and dispense the drug.
“There’s really not much we can do at this point,” he said. “If it’s not controlled … it’s not illegal for somebody that’s not prescribed it to possess it.”
Mr. Haegele said he heard about the drug less than 3 months ago when an officer accidentally received a text message from someone offering to sell it. The police force, he said, is still trying to assess the threat of gabapentin.
Little testing
Nearly anyone arrested and found to struggle with addiction in Athens is given the option to go through a drug-court program to get treatment. But officials said that some exploit the absence of routine exams for gabapentin to get high while testing clean.
Brice Johnson, a probation officer at Athens County Municipal Court, said participants in the municipal court’s substance abuse mentally ill program undergo gabapentin testing only when abuse is suspected. Screenings are not regularly done on every client because gabapentin abuse has not been a concern and the testing adds expense, he said.
The rehab program run through the county prosecutor’s office, called Fresh Start, does test for gabapentin. Its latest round of screenings detected the drug in 5 of its roughly 238 active participants, prosecutor Keller Blackburn said.
Linda Holley, a clinical supervisor at an Athens outpatient program run by the Health Recovery Services, said she suspects at least half of her clients on Suboxone treatment abuse gabapentin. But the center can’t afford to regularly test every participant.
Ms. Holley said she sees clients who are prescribed gabapentin but, because of health privacy laws, she can’t share their status as a person in recovery to an outside provider without written consent. The restrictions give clients in recovery an opportunity to get high using drugs they obtained legally and still pass a drug test.
“With the gabapentin, I wish there were more we could do, but our hands are tied,” she said. “We can’t do anything but educate the client and discourage” them from using such medications.
Ms. Smith visited two separate doctors to secure a prescription. As she rotated through drug court, Narcotics Anonymous meetings, jail for relapsing on cocaine, and house arrest enforced with an ankle bracelet, she said her gabapentin abuse wasn’t detected until she arrived at the residential recovery center.
Today, Ms. Smith sticks to the recovery process. Expecting a baby in early July, her successful completion of the program not only means sobriety but also allows her the opportunity to restore custody of her eldest daughter and raise her children.
She intends to relocate her family away from the friends and routines that helped lead her to addiction and said she will help guide her daughter away from making similar mistakes.
“All I can do is be there and give her the knowledge that I can about addiction,” Ms. Smith said, “and hope that she chooses to go on the right path.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.