User login
Look for comorbidities associated with hidradenitis suppurativa
CHICAGO – Hidradenitis suppurativa in children is often associated with comorbidities, especially obesity and endocrine abnormalities, a retrospective review of cases showed.
“When treating hidradenitis suppurativa, it is imperative to not only treat the skin but also to look for associated comorbidities,” Maria del Carmen Liy-Wong, MD, said in an interview in advance of the World Congress for Pediatric Dermatology.
Of the 41 patients, 78% were girls; the mean age of onset was 11 years, and the mean age at diagnosis was 14 years. A positive family history was found in 24% of cases. The most common cutaneous lesions were papules and pustules (51%), followed by scars (39%), and 88% of patients reported associated tenderness and pain.
After using the Hurley severity grade to classify disease severity, the researchers found that 56% of cases were mild, 32% were moderate, and 12% were severe. Comorbidities were identified in 92% of the cases; the most common was obesity (73%), followed by endocrine abnormalities (29%) and menstrual irregularities (20%). The researchers also found that 70% of patients were treated with a combination of topical and systemic antibiotics, and that early onset of disease correlated with more severe disease (P = .03).
Dr. Liy-Wong acknowledged that the study’s retrospective design is a limitation of the analysis, but she said that a prospective evaluation in planned for the near future.
The study was supported in part by a grant from AbbVie. Dr. Liy-Wong reported having no relevant financial disclosures.
[email protected]
CHICAGO – Hidradenitis suppurativa in children is often associated with comorbidities, especially obesity and endocrine abnormalities, a retrospective review of cases showed.
“When treating hidradenitis suppurativa, it is imperative to not only treat the skin but also to look for associated comorbidities,” Maria del Carmen Liy-Wong, MD, said in an interview in advance of the World Congress for Pediatric Dermatology.
Of the 41 patients, 78% were girls; the mean age of onset was 11 years, and the mean age at diagnosis was 14 years. A positive family history was found in 24% of cases. The most common cutaneous lesions were papules and pustules (51%), followed by scars (39%), and 88% of patients reported associated tenderness and pain.
After using the Hurley severity grade to classify disease severity, the researchers found that 56% of cases were mild, 32% were moderate, and 12% were severe. Comorbidities were identified in 92% of the cases; the most common was obesity (73%), followed by endocrine abnormalities (29%) and menstrual irregularities (20%). The researchers also found that 70% of patients were treated with a combination of topical and systemic antibiotics, and that early onset of disease correlated with more severe disease (P = .03).
Dr. Liy-Wong acknowledged that the study’s retrospective design is a limitation of the analysis, but she said that a prospective evaluation in planned for the near future.
The study was supported in part by a grant from AbbVie. Dr. Liy-Wong reported having no relevant financial disclosures.
[email protected]
CHICAGO – Hidradenitis suppurativa in children is often associated with comorbidities, especially obesity and endocrine abnormalities, a retrospective review of cases showed.
“When treating hidradenitis suppurativa, it is imperative to not only treat the skin but also to look for associated comorbidities,” Maria del Carmen Liy-Wong, MD, said in an interview in advance of the World Congress for Pediatric Dermatology.
Of the 41 patients, 78% were girls; the mean age of onset was 11 years, and the mean age at diagnosis was 14 years. A positive family history was found in 24% of cases. The most common cutaneous lesions were papules and pustules (51%), followed by scars (39%), and 88% of patients reported associated tenderness and pain.
After using the Hurley severity grade to classify disease severity, the researchers found that 56% of cases were mild, 32% were moderate, and 12% were severe. Comorbidities were identified in 92% of the cases; the most common was obesity (73%), followed by endocrine abnormalities (29%) and menstrual irregularities (20%). The researchers also found that 70% of patients were treated with a combination of topical and systemic antibiotics, and that early onset of disease correlated with more severe disease (P = .03).
Dr. Liy-Wong acknowledged that the study’s retrospective design is a limitation of the analysis, but she said that a prospective evaluation in planned for the near future.
The study was supported in part by a grant from AbbVie. Dr. Liy-Wong reported having no relevant financial disclosures.
[email protected]
AT WCPD 2017
Key clinical point:
Major finding: Comorbidities were identified in 92% of the cases, with obesity (73%) the most common.
Data source: A retrospective review of clinical characteristics, degree of severity, comorbidities, and management of hidradenitis suppurativa in 41 patients followed between January 1995 and January 2015.
Disclosures: Dr. Liy-Wong reported having no relevant financial disclosures.
As designer drugs multiply, toxicologists spring into action
SAN DIEGO – Forensic toxicologist Donna Papsun spends her days with drugs, but she doesn’t see patients or try to make anyone better. Still, her work is crucial to every medical professional who needs to know which new illicit drugs their patients have been taking.
In Willow Grove, Pa., a suburb of Philadelphia, Ms. Papsun and her colleagues at NMS Labs develop screening tests for designer drugs that have just appeared on the black market or crossed the Drug Enforcement Administration’s (DEA) radar.
As Ms. Papsun told an audience at the annual meeting of the American Psychiatric Association, she faced a unique obstacle last summer, when an elephant tranquilizer called carfentanil, a derivative of fentanyl, began to make headlines. The obstacle? The U.S.-Canada border.
She wanted to develop a test for the opioid but couldn’t start until she got a reference sample of the controlled substance from carfentanil’s only manufacturer, a firm in Canada. It took months. “Crossing an international border caused all sorts of problems,” she said.
New designer drugs are constantly hitting the market. They’re often especially appealing – and especially risky – because routine drug tests can’t detect them, at least not yet.
In an interview, Ms. Papsun talked about the challenges of trying to keep up with the drug makers – and users.
“Designer drug testing developed back in 2008 will not catch anything that is seen in today’s designer drug market,” she said. “Designer drug testing requires constant attention, assessment, resources, and updating.”
Question: How long does it typically take to create a test for a new strain of illicit drug?
Answer: This can take anywhere from 3 to 9 months, and potentially longer, and it depends on many factors. Once a new drug has hit the market, we check to see if there is certified reference material available. If there is, then we can start to develop a test. Development includes identifying a successful chemical extraction technique – isolating the drug in question from biological matrix such as blood or urine – as well as a platform that reliably detects the drug without falsely reporting positives.
After development, the test has to go through a process called validation, which is a series of experiments to prove that the developed method works rigorously, day after day, and provides the same results. This is very important in forensic toxicology, because our results may be involved in criminal and civil litigation and must stand up to the rigors of court.
Q: What are some examples of the types of drugs that you’ve had to develop tests for?
A: Just in the past year, we have developed tests for new designer opioids (including carfentanil, furanylfentanyl, acrylfentanyl, and U-47700), designer benzodiazepines (including etizolam, diclazepam, flubromazolam, and flubromazepam) and new designer stimulants (including n-ethyl pentylone and dibutylone).
We have a synthetic cannabinoid test that was developed for the first time back in 2010. That test has been redeveloped several times since then, because we constantly have to update the test to keep up with the rapid changes in market availability of substances.
Q: What are some of the challenges that you face in terms of getting samples of human fluids that you can test for the drugs?
A: Most of the samples we see are from either death investigation cases or driving-under-the-influence cases. Samples from intoxications at hospitals are important, because those data help [us] understand the concentrations of drugs at which people can survive. But often, if the patients survive, their biological specimens are not forwarded for specialized toxicology testing. Most hospital systems do not have the analytical capabilities to detect designer drugs, and most lack the resources to seek out the causal agent for an intoxication or apparent overdose.
Q: At the APA meeting, you talked about the risk that you’ll hear about a strain from the DEA, develop a test and find out it’s obsolete because the drug isn’t used anymore. Does that happen very often?
A: Yes. The problem with designer drugs is that there are so many, so you can spend a lot of time, money, and other resources dedicated to developing and validating a test for a drug that may or may not even be popular.
As a business, you have to make decisions regarding prioritization: Do we build a test for a drug that has only been reported once, or do we focus our efforts on a substance that has been reported dozens of times?
We certainly have spent time and resources developing a test that became obsolete, or never reported a positive case. For example, we developed a test for desomorphine, and we have never chemically confirmed desomorphine in a biological specimen. It definitely is hit or miss, but we spend a lot of time and research a lot of different avenues to make educated decisions regarding the substances we develop tests for.
SAN DIEGO – Forensic toxicologist Donna Papsun spends her days with drugs, but she doesn’t see patients or try to make anyone better. Still, her work is crucial to every medical professional who needs to know which new illicit drugs their patients have been taking.
In Willow Grove, Pa., a suburb of Philadelphia, Ms. Papsun and her colleagues at NMS Labs develop screening tests for designer drugs that have just appeared on the black market or crossed the Drug Enforcement Administration’s (DEA) radar.
As Ms. Papsun told an audience at the annual meeting of the American Psychiatric Association, she faced a unique obstacle last summer, when an elephant tranquilizer called carfentanil, a derivative of fentanyl, began to make headlines. The obstacle? The U.S.-Canada border.
She wanted to develop a test for the opioid but couldn’t start until she got a reference sample of the controlled substance from carfentanil’s only manufacturer, a firm in Canada. It took months. “Crossing an international border caused all sorts of problems,” she said.
New designer drugs are constantly hitting the market. They’re often especially appealing – and especially risky – because routine drug tests can’t detect them, at least not yet.
In an interview, Ms. Papsun talked about the challenges of trying to keep up with the drug makers – and users.
“Designer drug testing developed back in 2008 will not catch anything that is seen in today’s designer drug market,” she said. “Designer drug testing requires constant attention, assessment, resources, and updating.”
Question: How long does it typically take to create a test for a new strain of illicit drug?
Answer: This can take anywhere from 3 to 9 months, and potentially longer, and it depends on many factors. Once a new drug has hit the market, we check to see if there is certified reference material available. If there is, then we can start to develop a test. Development includes identifying a successful chemical extraction technique – isolating the drug in question from biological matrix such as blood or urine – as well as a platform that reliably detects the drug without falsely reporting positives.
After development, the test has to go through a process called validation, which is a series of experiments to prove that the developed method works rigorously, day after day, and provides the same results. This is very important in forensic toxicology, because our results may be involved in criminal and civil litigation and must stand up to the rigors of court.
Q: What are some examples of the types of drugs that you’ve had to develop tests for?
A: Just in the past year, we have developed tests for new designer opioids (including carfentanil, furanylfentanyl, acrylfentanyl, and U-47700), designer benzodiazepines (including etizolam, diclazepam, flubromazolam, and flubromazepam) and new designer stimulants (including n-ethyl pentylone and dibutylone).
We have a synthetic cannabinoid test that was developed for the first time back in 2010. That test has been redeveloped several times since then, because we constantly have to update the test to keep up with the rapid changes in market availability of substances.
Q: What are some of the challenges that you face in terms of getting samples of human fluids that you can test for the drugs?
A: Most of the samples we see are from either death investigation cases or driving-under-the-influence cases. Samples from intoxications at hospitals are important, because those data help [us] understand the concentrations of drugs at which people can survive. But often, if the patients survive, their biological specimens are not forwarded for specialized toxicology testing. Most hospital systems do not have the analytical capabilities to detect designer drugs, and most lack the resources to seek out the causal agent for an intoxication or apparent overdose.
Q: At the APA meeting, you talked about the risk that you’ll hear about a strain from the DEA, develop a test and find out it’s obsolete because the drug isn’t used anymore. Does that happen very often?
A: Yes. The problem with designer drugs is that there are so many, so you can spend a lot of time, money, and other resources dedicated to developing and validating a test for a drug that may or may not even be popular.
As a business, you have to make decisions regarding prioritization: Do we build a test for a drug that has only been reported once, or do we focus our efforts on a substance that has been reported dozens of times?
We certainly have spent time and resources developing a test that became obsolete, or never reported a positive case. For example, we developed a test for desomorphine, and we have never chemically confirmed desomorphine in a biological specimen. It definitely is hit or miss, but we spend a lot of time and research a lot of different avenues to make educated decisions regarding the substances we develop tests for.
SAN DIEGO – Forensic toxicologist Donna Papsun spends her days with drugs, but she doesn’t see patients or try to make anyone better. Still, her work is crucial to every medical professional who needs to know which new illicit drugs their patients have been taking.
In Willow Grove, Pa., a suburb of Philadelphia, Ms. Papsun and her colleagues at NMS Labs develop screening tests for designer drugs that have just appeared on the black market or crossed the Drug Enforcement Administration’s (DEA) radar.
As Ms. Papsun told an audience at the annual meeting of the American Psychiatric Association, she faced a unique obstacle last summer, when an elephant tranquilizer called carfentanil, a derivative of fentanyl, began to make headlines. The obstacle? The U.S.-Canada border.
She wanted to develop a test for the opioid but couldn’t start until she got a reference sample of the controlled substance from carfentanil’s only manufacturer, a firm in Canada. It took months. “Crossing an international border caused all sorts of problems,” she said.
New designer drugs are constantly hitting the market. They’re often especially appealing – and especially risky – because routine drug tests can’t detect them, at least not yet.
In an interview, Ms. Papsun talked about the challenges of trying to keep up with the drug makers – and users.
“Designer drug testing developed back in 2008 will not catch anything that is seen in today’s designer drug market,” she said. “Designer drug testing requires constant attention, assessment, resources, and updating.”
Question: How long does it typically take to create a test for a new strain of illicit drug?
Answer: This can take anywhere from 3 to 9 months, and potentially longer, and it depends on many factors. Once a new drug has hit the market, we check to see if there is certified reference material available. If there is, then we can start to develop a test. Development includes identifying a successful chemical extraction technique – isolating the drug in question from biological matrix such as blood or urine – as well as a platform that reliably detects the drug without falsely reporting positives.
After development, the test has to go through a process called validation, which is a series of experiments to prove that the developed method works rigorously, day after day, and provides the same results. This is very important in forensic toxicology, because our results may be involved in criminal and civil litigation and must stand up to the rigors of court.
Q: What are some examples of the types of drugs that you’ve had to develop tests for?
A: Just in the past year, we have developed tests for new designer opioids (including carfentanil, furanylfentanyl, acrylfentanyl, and U-47700), designer benzodiazepines (including etizolam, diclazepam, flubromazolam, and flubromazepam) and new designer stimulants (including n-ethyl pentylone and dibutylone).
We have a synthetic cannabinoid test that was developed for the first time back in 2010. That test has been redeveloped several times since then, because we constantly have to update the test to keep up with the rapid changes in market availability of substances.
Q: What are some of the challenges that you face in terms of getting samples of human fluids that you can test for the drugs?
A: Most of the samples we see are from either death investigation cases or driving-under-the-influence cases. Samples from intoxications at hospitals are important, because those data help [us] understand the concentrations of drugs at which people can survive. But often, if the patients survive, their biological specimens are not forwarded for specialized toxicology testing. Most hospital systems do not have the analytical capabilities to detect designer drugs, and most lack the resources to seek out the causal agent for an intoxication or apparent overdose.
Q: At the APA meeting, you talked about the risk that you’ll hear about a strain from the DEA, develop a test and find out it’s obsolete because the drug isn’t used anymore. Does that happen very often?
A: Yes. The problem with designer drugs is that there are so many, so you can spend a lot of time, money, and other resources dedicated to developing and validating a test for a drug that may or may not even be popular.
As a business, you have to make decisions regarding prioritization: Do we build a test for a drug that has only been reported once, or do we focus our efforts on a substance that has been reported dozens of times?
We certainly have spent time and resources developing a test that became obsolete, or never reported a positive case. For example, we developed a test for desomorphine, and we have never chemically confirmed desomorphine in a biological specimen. It definitely is hit or miss, but we spend a lot of time and research a lot of different avenues to make educated decisions regarding the substances we develop tests for.
EXPERT ANALYSIS FROM APA
FDA approves new treatment for sickle cell disease
The US Food and Drug Administration (FDA) has granted approval for L-glutamine oral powder (Endari), the first treatment approved to treat sickle cell disease (SCD) in the US in nearly 20 years.
L-glutamine oral powder, a product developed by Emmaus Medical Inc., is intended to reduce severe complications of SCD in patients age 5 and older.
The FDA’s approval of L-glutamine was supported by efficacy data from a phase 3 trial.
The trial enrolled 230 adults and children with SCD, and they were randomized to receive L-glutamine or placebo.
Patients who received L-glutamine had fewer sickle cell crises, hospitalizations, cumulative hospital days, and cases of acute chest syndrome than patients who received placebo.
Results from this trial were presented at the 2014 ASH Annual Meeting.
The FDA approval of L-glutamine was also supported by safety data from 298 patients treated with L-glutamine and 111 patients treated with placebo in the phase 2 and phase 3 studies.
Based on these data, L-glutamine was considered well-tolerated in pediatric and adult patients. The most common adverse events (occurring in more than 10% of patients receiving L-glutamine) were constipation, nausea, headache, abdominal pain, cough, pain in extremity, back pain, and chest pain (non-cardiac). ![]()
The US Food and Drug Administration (FDA) has granted approval for L-glutamine oral powder (Endari), the first treatment approved to treat sickle cell disease (SCD) in the US in nearly 20 years.
L-glutamine oral powder, a product developed by Emmaus Medical Inc., is intended to reduce severe complications of SCD in patients age 5 and older.
The FDA’s approval of L-glutamine was supported by efficacy data from a phase 3 trial.
The trial enrolled 230 adults and children with SCD, and they were randomized to receive L-glutamine or placebo.
Patients who received L-glutamine had fewer sickle cell crises, hospitalizations, cumulative hospital days, and cases of acute chest syndrome than patients who received placebo.
Results from this trial were presented at the 2014 ASH Annual Meeting.
The FDA approval of L-glutamine was also supported by safety data from 298 patients treated with L-glutamine and 111 patients treated with placebo in the phase 2 and phase 3 studies.
Based on these data, L-glutamine was considered well-tolerated in pediatric and adult patients. The most common adverse events (occurring in more than 10% of patients receiving L-glutamine) were constipation, nausea, headache, abdominal pain, cough, pain in extremity, back pain, and chest pain (non-cardiac). ![]()
The US Food and Drug Administration (FDA) has granted approval for L-glutamine oral powder (Endari), the first treatment approved to treat sickle cell disease (SCD) in the US in nearly 20 years.
L-glutamine oral powder, a product developed by Emmaus Medical Inc., is intended to reduce severe complications of SCD in patients age 5 and older.
The FDA’s approval of L-glutamine was supported by efficacy data from a phase 3 trial.
The trial enrolled 230 adults and children with SCD, and they were randomized to receive L-glutamine or placebo.
Patients who received L-glutamine had fewer sickle cell crises, hospitalizations, cumulative hospital days, and cases of acute chest syndrome than patients who received placebo.
Results from this trial were presented at the 2014 ASH Annual Meeting.
The FDA approval of L-glutamine was also supported by safety data from 298 patients treated with L-glutamine and 111 patients treated with placebo in the phase 2 and phase 3 studies.
Based on these data, L-glutamine was considered well-tolerated in pediatric and adult patients. The most common adverse events (occurring in more than 10% of patients receiving L-glutamine) were constipation, nausea, headache, abdominal pain, cough, pain in extremity, back pain, and chest pain (non-cardiac). ![]()
Use of HbA1c in the Diagnosis of Diabetes in Adolescents
Study Overview
Objective. To examine the screening practices of family practitioners (FPs) and pediatricians for type 2 diabetes (T2D) in adolescents.
Design. Cross-sectional study.
Setting and participants. The researchers randomly sampled 700 pediatricians and 700 FPs who participated in direct patient care using the American Medical Association Physician Masterfile using a mail survey. Exclusion criteria included providers who were residents, hospital staff, retirees, or employed by federally owned medical facilities, certified with a subspecialty, or over age 70.
Main outcome measures. Providers were given a hypothetical case of an obese, female, teenaged patient with concurrent associated risk factors for T2D (family history of T2D, minority race, signs of insulin resistance) and asked what initial screening tests they would order. Respondents were then informed of the updated American Diabetes Association (ADA) guidelines that added hemoglobin A1c as a screening test to diagnose diabetes. The survey then asked if knowing this change in recommendation has changed or will change their screening practices in adolescents.
Main results. 1400 surveys were mailed. After 2 were excluded due to mailing issues, 52% of providers provided responses. Of these, 129 providers reported that they did not care for adolescents (age 10–17), resulting in 604 providers in the final sample, 398 pediatricians and 335 FPs.
The vast majority (92%) said they would screen the hypothetical case for diabetes, with most initially ordering a fasting test (fasting plasma glucose or 2-hour glucose tolerance test) (63%) or A1c test (58%). Of the 58% who planned to order HbA1c, only 35% ordered it in combination with a fasting test. HbA1c was significantly more likely to be ordered by pediatricians than by FPs (P = 0.001). After being presented with the new guidelines, 84% said then would now order HbA1c, a 27% increase.
Conclusion. In response to information about the new guidelines, providers were more likely to order A1c as part of initial testing. Due to the lower test performance in children and increased cost of the test, the use of HbA1c without fasting tests may result in missed diagnosis of T2D in adolescents as well as increased health care costs.
Commentary
Rates of childhood obesity continue to rise throughout the United States. Obese children are at risk for numerous comorbidities such as hypertension, hyperlipidemia, and T2D [1,2]. It is important for providers to use effective screening tools for risk assessment of prediabetes/T2DM in children.
The standard tests for diagnosing diabetes are the fasting plasma glucose test and the 2-hour plasma glucose test. While accurate, these tests are not convenient. In 2010, the ADA added an easier method of testing for T2D: an HbA1c, with results greater than or equal to 6.5% indicating diabetes [3]. However, this recommendation is controversial, given studies suggesting that HbA1c is not as reliable in children as it is in adults [4–6]. The ADA itself acknowledges that there are limited data in the pediatric population.
In this study, most providers were unaware of the 4-year-old revised guidelines offering the A1c option but are planning to apply the guidelines going forward. According to the study, this would result in a 27% increase in providers utilizing HbA1c.
Should increased uptake of A1c as an initial screening test be a concern? Using it in combination with other tests may be useful for assessing which adolescents will need further testing [3–6]. Additionally, by starting with a test that can be performed in the office with no regard to fasting time, it is possible that more cases of T2D will be found by primary care providers treating adolescents.
A weakness of the study is the potential for response bias related to mailed surveys. An additional weakness is that the researchers utilized only 1 hypothetical situation. Providing additional hypothetical situations may have allowed for further understanding of screening practices. The investigators also did not include nurse practitioners or physician assistants in their sample, a growing percentage of whom may care for adolescent populations at risk for T2D or be primary referral sources.
Applications for Clinical Practice
Providers can use HbA1c to screen for diabetes in nonfasting adolescents at risk for diabetes. While the test may not be as accurate in pediatric patients, utilizing HbA1C as directed by the ADA may aid in diagnosing patients that may otherwise miss follow-up appointments to complete a fasting test.
—Jennifer L. Nahum, MSN, CPNP-AC, PPCNP-BC, and Allison Squires, PhD, RN
1. Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103(6 Pt 1):1175–82.
2. Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 1996;128(5 Pt 1):608–15.
3. American Diabetes Association. Type 2 diabetes in children and adolescents. Pediatrics 2000 Mar;105(3 Pt 1):671–80.
4. Lee JM, Gebremariam A, Wu EL, et al. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care 2011;34:2597–602.
5. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
6. Lee JM, Wu EL, Tarini B, et al Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952.
Study Overview
Objective. To examine the screening practices of family practitioners (FPs) and pediatricians for type 2 diabetes (T2D) in adolescents.
Design. Cross-sectional study.
Setting and participants. The researchers randomly sampled 700 pediatricians and 700 FPs who participated in direct patient care using the American Medical Association Physician Masterfile using a mail survey. Exclusion criteria included providers who were residents, hospital staff, retirees, or employed by federally owned medical facilities, certified with a subspecialty, or over age 70.
Main outcome measures. Providers were given a hypothetical case of an obese, female, teenaged patient with concurrent associated risk factors for T2D (family history of T2D, minority race, signs of insulin resistance) and asked what initial screening tests they would order. Respondents were then informed of the updated American Diabetes Association (ADA) guidelines that added hemoglobin A1c as a screening test to diagnose diabetes. The survey then asked if knowing this change in recommendation has changed or will change their screening practices in adolescents.
Main results. 1400 surveys were mailed. After 2 were excluded due to mailing issues, 52% of providers provided responses. Of these, 129 providers reported that they did not care for adolescents (age 10–17), resulting in 604 providers in the final sample, 398 pediatricians and 335 FPs.
The vast majority (92%) said they would screen the hypothetical case for diabetes, with most initially ordering a fasting test (fasting plasma glucose or 2-hour glucose tolerance test) (63%) or A1c test (58%). Of the 58% who planned to order HbA1c, only 35% ordered it in combination with a fasting test. HbA1c was significantly more likely to be ordered by pediatricians than by FPs (P = 0.001). After being presented with the new guidelines, 84% said then would now order HbA1c, a 27% increase.
Conclusion. In response to information about the new guidelines, providers were more likely to order A1c as part of initial testing. Due to the lower test performance in children and increased cost of the test, the use of HbA1c without fasting tests may result in missed diagnosis of T2D in adolescents as well as increased health care costs.
Commentary
Rates of childhood obesity continue to rise throughout the United States. Obese children are at risk for numerous comorbidities such as hypertension, hyperlipidemia, and T2D [1,2]. It is important for providers to use effective screening tools for risk assessment of prediabetes/T2DM in children.
The standard tests for diagnosing diabetes are the fasting plasma glucose test and the 2-hour plasma glucose test. While accurate, these tests are not convenient. In 2010, the ADA added an easier method of testing for T2D: an HbA1c, with results greater than or equal to 6.5% indicating diabetes [3]. However, this recommendation is controversial, given studies suggesting that HbA1c is not as reliable in children as it is in adults [4–6]. The ADA itself acknowledges that there are limited data in the pediatric population.
In this study, most providers were unaware of the 4-year-old revised guidelines offering the A1c option but are planning to apply the guidelines going forward. According to the study, this would result in a 27% increase in providers utilizing HbA1c.
Should increased uptake of A1c as an initial screening test be a concern? Using it in combination with other tests may be useful for assessing which adolescents will need further testing [3–6]. Additionally, by starting with a test that can be performed in the office with no regard to fasting time, it is possible that more cases of T2D will be found by primary care providers treating adolescents.
A weakness of the study is the potential for response bias related to mailed surveys. An additional weakness is that the researchers utilized only 1 hypothetical situation. Providing additional hypothetical situations may have allowed for further understanding of screening practices. The investigators also did not include nurse practitioners or physician assistants in their sample, a growing percentage of whom may care for adolescent populations at risk for T2D or be primary referral sources.
Applications for Clinical Practice
Providers can use HbA1c to screen for diabetes in nonfasting adolescents at risk for diabetes. While the test may not be as accurate in pediatric patients, utilizing HbA1C as directed by the ADA may aid in diagnosing patients that may otherwise miss follow-up appointments to complete a fasting test.
—Jennifer L. Nahum, MSN, CPNP-AC, PPCNP-BC, and Allison Squires, PhD, RN
Study Overview
Objective. To examine the screening practices of family practitioners (FPs) and pediatricians for type 2 diabetes (T2D) in adolescents.
Design. Cross-sectional study.
Setting and participants. The researchers randomly sampled 700 pediatricians and 700 FPs who participated in direct patient care using the American Medical Association Physician Masterfile using a mail survey. Exclusion criteria included providers who were residents, hospital staff, retirees, or employed by federally owned medical facilities, certified with a subspecialty, or over age 70.
Main outcome measures. Providers were given a hypothetical case of an obese, female, teenaged patient with concurrent associated risk factors for T2D (family history of T2D, minority race, signs of insulin resistance) and asked what initial screening tests they would order. Respondents were then informed of the updated American Diabetes Association (ADA) guidelines that added hemoglobin A1c as a screening test to diagnose diabetes. The survey then asked if knowing this change in recommendation has changed or will change their screening practices in adolescents.
Main results. 1400 surveys were mailed. After 2 were excluded due to mailing issues, 52% of providers provided responses. Of these, 129 providers reported that they did not care for adolescents (age 10–17), resulting in 604 providers in the final sample, 398 pediatricians and 335 FPs.
The vast majority (92%) said they would screen the hypothetical case for diabetes, with most initially ordering a fasting test (fasting plasma glucose or 2-hour glucose tolerance test) (63%) or A1c test (58%). Of the 58% who planned to order HbA1c, only 35% ordered it in combination with a fasting test. HbA1c was significantly more likely to be ordered by pediatricians than by FPs (P = 0.001). After being presented with the new guidelines, 84% said then would now order HbA1c, a 27% increase.
Conclusion. In response to information about the new guidelines, providers were more likely to order A1c as part of initial testing. Due to the lower test performance in children and increased cost of the test, the use of HbA1c without fasting tests may result in missed diagnosis of T2D in adolescents as well as increased health care costs.
Commentary
Rates of childhood obesity continue to rise throughout the United States. Obese children are at risk for numerous comorbidities such as hypertension, hyperlipidemia, and T2D [1,2]. It is important for providers to use effective screening tools for risk assessment of prediabetes/T2DM in children.
The standard tests for diagnosing diabetes are the fasting plasma glucose test and the 2-hour plasma glucose test. While accurate, these tests are not convenient. In 2010, the ADA added an easier method of testing for T2D: an HbA1c, with results greater than or equal to 6.5% indicating diabetes [3]. However, this recommendation is controversial, given studies suggesting that HbA1c is not as reliable in children as it is in adults [4–6]. The ADA itself acknowledges that there are limited data in the pediatric population.
In this study, most providers were unaware of the 4-year-old revised guidelines offering the A1c option but are planning to apply the guidelines going forward. According to the study, this would result in a 27% increase in providers utilizing HbA1c.
Should increased uptake of A1c as an initial screening test be a concern? Using it in combination with other tests may be useful for assessing which adolescents will need further testing [3–6]. Additionally, by starting with a test that can be performed in the office with no regard to fasting time, it is possible that more cases of T2D will be found by primary care providers treating adolescents.
A weakness of the study is the potential for response bias related to mailed surveys. An additional weakness is that the researchers utilized only 1 hypothetical situation. Providing additional hypothetical situations may have allowed for further understanding of screening practices. The investigators also did not include nurse practitioners or physician assistants in their sample, a growing percentage of whom may care for adolescent populations at risk for T2D or be primary referral sources.
Applications for Clinical Practice
Providers can use HbA1c to screen for diabetes in nonfasting adolescents at risk for diabetes. While the test may not be as accurate in pediatric patients, utilizing HbA1C as directed by the ADA may aid in diagnosing patients that may otherwise miss follow-up appointments to complete a fasting test.
—Jennifer L. Nahum, MSN, CPNP-AC, PPCNP-BC, and Allison Squires, PhD, RN
1. Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103(6 Pt 1):1175–82.
2. Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 1996;128(5 Pt 1):608–15.
3. American Diabetes Association. Type 2 diabetes in children and adolescents. Pediatrics 2000 Mar;105(3 Pt 1):671–80.
4. Lee JM, Gebremariam A, Wu EL, et al. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care 2011;34:2597–602.
5. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
6. Lee JM, Wu EL, Tarini B, et al Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952.
1. Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103(6 Pt 1):1175–82.
2. Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 1996;128(5 Pt 1):608–15.
3. American Diabetes Association. Type 2 diabetes in children and adolescents. Pediatrics 2000 Mar;105(3 Pt 1):671–80.
4. Lee JM, Gebremariam A, Wu EL, et al. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care 2011;34:2597–602.
5. Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–11.
6. Lee JM, Wu EL, Tarini B, et al Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952.
Amplatzer devices outperform oral anticoagulation in atrial fib
PARIS – Percutaneous left atrial appendage closure with an Amplatzer device in patients with nonvalvular atrial fibrillation was associated with significantly lower rates of all-cause and cardiovascular mortality, compared with oral anticoagulation, in a large propensity score–matched observational registry study.
Left atrial appendage closure (LAAC) also bested oral anticoagulation (OAC) with warfarin or a novel oral anticoagulant (NOAC) in terms of net clinical benefit on the basis of the device therapy’s greater protection against stroke and systemic embolism coupled with a trend, albeit not statistically significant, for fewer bleeding events, Steffen Gloekler, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The Watchman LAAC device, commercially available both in Europe and the United States, has previously been shown to be superior to OAC in terms of efficacy and noninferior regarding safety. But there have been no randomized trials of an Amplatzer device versus OAC. This lack of data was the impetus for Dr. Gloekler and his coinvestigators to create a meticulously propensity-matched observational registry.
Five hundred consecutive patients with AF who received an Amplatzer Cardiac Plug or its second-generation version, the Amplatzer Amulet, during 2009-2014 were tightly matched to an equal number of AF patients on OAC based on age, sex, body mass index, left ventricular ejection fraction, renal function, coronary artery disease status, hemoglobin level, CHA2DS2-VASc score, and HAS-BLED score. During a mean 2.7 years, or 2,645 patient-years, of follow-up, the composite primary efficacy endpoint, composed of stroke, systemic embolism, and cardiovascular or unexplained death occurred in 5.6% of the LAAC group, compared with 7.8% of controls in the OAC arm, for a statistically significant 30% relative risk reduction. Disabling stroke occurred in 0.7% of Amplatzer patients versus 1.5% of controls. The ischemic stroke rate was 1.5% in the device therapy group and 2% in the OAC arm.
All-cause mortality occurred in 8.3% of Amplatzer patients and 11.6% of the OAC group, for a 28% relative risk reduction. The cardiovascular death rate was 4% in the Amplatzer group, compared with 6.5% of controls, for a 36% risk reduction.
The composite safety endpoint, comprising all major procedural adverse events and major or life-threatening bleeding during follow-up, occurred in 3.6% of the Amplatzer group and 4.6% of the OAC group, for a 20% relative risk reduction that is not significant at this point because of the low number of events. Major, life-threatening, or fatal bleeding occurred in 2% of Amplatzer recipients versus 5.5% of controls, added Dr. Gloekler of University Hospital in Bern, Switzerland.
The net clinical benefit, a composite of death, bleeding, or stroke, occurred in 8.1% of the Amplatzer group, compared with 10.9% of controls, for a significant 24% reduction in relative risk in favor of device therapy.
Of note, at 2.7 years of follow-up only 55% of the OAC group were still taking an anticoagulant: 38% of the original 500 patients were on warfarin, and 17% were taking a NOAC. At that point, 8% of the Amplatzer group were on any anticoagulation therapy.
Discussion of the study focused on that low rate of medication adherence in the OAC arm. Dr. Gloekler’s response was that, after looking at the literature, he was no longer surprised by the finding that only 55% of the control group were on OAC at follow-up.
“If you look in the literature, that’s exactly the real-world adherence for OACs. Even in all four certification trials for the NOACs, the rate of discontinuation was 30% after 2 years – and these were controlled studies. Ours was observational, and it depicts a good deal of the problem with any OAC in my eyes,” Dr. Gloekler said.
Patients on warfarin in the real-world Amplatzer registry study spent on average a mere 30% of time in the therapeutic international normalized ratio range of 2-3.
“That means 70% of the time patients are higher and have an increased bleeding risk or they are lower and don’t have adequate stroke protection,” he noted.
This prompted one observer to comment, “We either have to do a better job in our clinics with OAC or we have to occlude more appendages.”
A large pivotal U.S. trial aimed at winning FDA approval for the Amplatzer Amulet for LAAC is underway. Patients with AF are being randomized to the approved Watchman or investigational Amulet at roughly 100 U.S. and 50 foreign sites.
Dr. Gloekler reported receiving research funds for the registry from the Swiss Heart Foundation and Abbott.
PARIS – Percutaneous left atrial appendage closure with an Amplatzer device in patients with nonvalvular atrial fibrillation was associated with significantly lower rates of all-cause and cardiovascular mortality, compared with oral anticoagulation, in a large propensity score–matched observational registry study.
Left atrial appendage closure (LAAC) also bested oral anticoagulation (OAC) with warfarin or a novel oral anticoagulant (NOAC) in terms of net clinical benefit on the basis of the device therapy’s greater protection against stroke and systemic embolism coupled with a trend, albeit not statistically significant, for fewer bleeding events, Steffen Gloekler, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The Watchman LAAC device, commercially available both in Europe and the United States, has previously been shown to be superior to OAC in terms of efficacy and noninferior regarding safety. But there have been no randomized trials of an Amplatzer device versus OAC. This lack of data was the impetus for Dr. Gloekler and his coinvestigators to create a meticulously propensity-matched observational registry.
Five hundred consecutive patients with AF who received an Amplatzer Cardiac Plug or its second-generation version, the Amplatzer Amulet, during 2009-2014 were tightly matched to an equal number of AF patients on OAC based on age, sex, body mass index, left ventricular ejection fraction, renal function, coronary artery disease status, hemoglobin level, CHA2DS2-VASc score, and HAS-BLED score. During a mean 2.7 years, or 2,645 patient-years, of follow-up, the composite primary efficacy endpoint, composed of stroke, systemic embolism, and cardiovascular or unexplained death occurred in 5.6% of the LAAC group, compared with 7.8% of controls in the OAC arm, for a statistically significant 30% relative risk reduction. Disabling stroke occurred in 0.7% of Amplatzer patients versus 1.5% of controls. The ischemic stroke rate was 1.5% in the device therapy group and 2% in the OAC arm.
All-cause mortality occurred in 8.3% of Amplatzer patients and 11.6% of the OAC group, for a 28% relative risk reduction. The cardiovascular death rate was 4% in the Amplatzer group, compared with 6.5% of controls, for a 36% risk reduction.
The composite safety endpoint, comprising all major procedural adverse events and major or life-threatening bleeding during follow-up, occurred in 3.6% of the Amplatzer group and 4.6% of the OAC group, for a 20% relative risk reduction that is not significant at this point because of the low number of events. Major, life-threatening, or fatal bleeding occurred in 2% of Amplatzer recipients versus 5.5% of controls, added Dr. Gloekler of University Hospital in Bern, Switzerland.
The net clinical benefit, a composite of death, bleeding, or stroke, occurred in 8.1% of the Amplatzer group, compared with 10.9% of controls, for a significant 24% reduction in relative risk in favor of device therapy.
Of note, at 2.7 years of follow-up only 55% of the OAC group were still taking an anticoagulant: 38% of the original 500 patients were on warfarin, and 17% were taking a NOAC. At that point, 8% of the Amplatzer group were on any anticoagulation therapy.
Discussion of the study focused on that low rate of medication adherence in the OAC arm. Dr. Gloekler’s response was that, after looking at the literature, he was no longer surprised by the finding that only 55% of the control group were on OAC at follow-up.
“If you look in the literature, that’s exactly the real-world adherence for OACs. Even in all four certification trials for the NOACs, the rate of discontinuation was 30% after 2 years – and these were controlled studies. Ours was observational, and it depicts a good deal of the problem with any OAC in my eyes,” Dr. Gloekler said.
Patients on warfarin in the real-world Amplatzer registry study spent on average a mere 30% of time in the therapeutic international normalized ratio range of 2-3.
“That means 70% of the time patients are higher and have an increased bleeding risk or they are lower and don’t have adequate stroke protection,” he noted.
This prompted one observer to comment, “We either have to do a better job in our clinics with OAC or we have to occlude more appendages.”
A large pivotal U.S. trial aimed at winning FDA approval for the Amplatzer Amulet for LAAC is underway. Patients with AF are being randomized to the approved Watchman or investigational Amulet at roughly 100 U.S. and 50 foreign sites.
Dr. Gloekler reported receiving research funds for the registry from the Swiss Heart Foundation and Abbott.
PARIS – Percutaneous left atrial appendage closure with an Amplatzer device in patients with nonvalvular atrial fibrillation was associated with significantly lower rates of all-cause and cardiovascular mortality, compared with oral anticoagulation, in a large propensity score–matched observational registry study.
Left atrial appendage closure (LAAC) also bested oral anticoagulation (OAC) with warfarin or a novel oral anticoagulant (NOAC) in terms of net clinical benefit on the basis of the device therapy’s greater protection against stroke and systemic embolism coupled with a trend, albeit not statistically significant, for fewer bleeding events, Steffen Gloekler, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The Watchman LAAC device, commercially available both in Europe and the United States, has previously been shown to be superior to OAC in terms of efficacy and noninferior regarding safety. But there have been no randomized trials of an Amplatzer device versus OAC. This lack of data was the impetus for Dr. Gloekler and his coinvestigators to create a meticulously propensity-matched observational registry.
Five hundred consecutive patients with AF who received an Amplatzer Cardiac Plug or its second-generation version, the Amplatzer Amulet, during 2009-2014 were tightly matched to an equal number of AF patients on OAC based on age, sex, body mass index, left ventricular ejection fraction, renal function, coronary artery disease status, hemoglobin level, CHA2DS2-VASc score, and HAS-BLED score. During a mean 2.7 years, or 2,645 patient-years, of follow-up, the composite primary efficacy endpoint, composed of stroke, systemic embolism, and cardiovascular or unexplained death occurred in 5.6% of the LAAC group, compared with 7.8% of controls in the OAC arm, for a statistically significant 30% relative risk reduction. Disabling stroke occurred in 0.7% of Amplatzer patients versus 1.5% of controls. The ischemic stroke rate was 1.5% in the device therapy group and 2% in the OAC arm.
All-cause mortality occurred in 8.3% of Amplatzer patients and 11.6% of the OAC group, for a 28% relative risk reduction. The cardiovascular death rate was 4% in the Amplatzer group, compared with 6.5% of controls, for a 36% risk reduction.
The composite safety endpoint, comprising all major procedural adverse events and major or life-threatening bleeding during follow-up, occurred in 3.6% of the Amplatzer group and 4.6% of the OAC group, for a 20% relative risk reduction that is not significant at this point because of the low number of events. Major, life-threatening, or fatal bleeding occurred in 2% of Amplatzer recipients versus 5.5% of controls, added Dr. Gloekler of University Hospital in Bern, Switzerland.
The net clinical benefit, a composite of death, bleeding, or stroke, occurred in 8.1% of the Amplatzer group, compared with 10.9% of controls, for a significant 24% reduction in relative risk in favor of device therapy.
Of note, at 2.7 years of follow-up only 55% of the OAC group were still taking an anticoagulant: 38% of the original 500 patients were on warfarin, and 17% were taking a NOAC. At that point, 8% of the Amplatzer group were on any anticoagulation therapy.
Discussion of the study focused on that low rate of medication adherence in the OAC arm. Dr. Gloekler’s response was that, after looking at the literature, he was no longer surprised by the finding that only 55% of the control group were on OAC at follow-up.
“If you look in the literature, that’s exactly the real-world adherence for OACs. Even in all four certification trials for the NOACs, the rate of discontinuation was 30% after 2 years – and these were controlled studies. Ours was observational, and it depicts a good deal of the problem with any OAC in my eyes,” Dr. Gloekler said.
Patients on warfarin in the real-world Amplatzer registry study spent on average a mere 30% of time in the therapeutic international normalized ratio range of 2-3.
“That means 70% of the time patients are higher and have an increased bleeding risk or they are lower and don’t have adequate stroke protection,” he noted.
This prompted one observer to comment, “We either have to do a better job in our clinics with OAC or we have to occlude more appendages.”
A large pivotal U.S. trial aimed at winning FDA approval for the Amplatzer Amulet for LAAC is underway. Patients with AF are being randomized to the approved Watchman or investigational Amulet at roughly 100 U.S. and 50 foreign sites.
Dr. Gloekler reported receiving research funds for the registry from the Swiss Heart Foundation and Abbott.
AT EUROPCR
Key clinical point:
Major finding: The primary composite efficacy endpoint of stroke, systemic embolism, or cardiovascular or unexplained death during a mean 2.7 years of follow-up occurred in 5.6% of Amplatzer device recipients, a 30% reduction, compared with the 7.8% rate in the oral anticoagulation group.
Data source: This observational registry included 500 patients with atrial fibrillation who received an Amplatzer left atrial appendage closure device and an equal number of carefully matched AF patients on oral anticoagulation.
Disclosures: The study presenter reported receiving research funds for the registry from the Swiss Heart Foundation and Abbott.
Outpatient care 35% of mental health costs and growing
Outpatient care represents the largest share of mental health treatment expenditures, and it continues to get larger, while components such as retail drug prescriptions and inpatient care have declined, according to the National Center of Health Statistics.
In 2014, outpatient care took a $65.5-billion slice (about 35%) out of the $186-billion mental health care spending pie, compared with the $51.1 billion (27%) spent on retail drug prescriptions, which was the next-largest portion. Inpatient care was third with $30.3 billion in spending (16% of the total), followed by residential care at $23.2 billion (12%), and insurance administration at $15.9 billion (9%), the NCHS reported in “Health, United States, 2016.”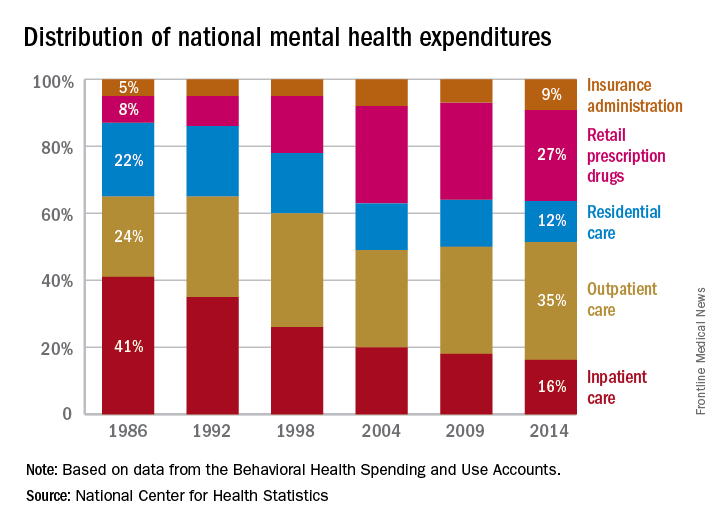
Outpatient care represents the largest share of mental health treatment expenditures, and it continues to get larger, while components such as retail drug prescriptions and inpatient care have declined, according to the National Center of Health Statistics.
In 2014, outpatient care took a $65.5-billion slice (about 35%) out of the $186-billion mental health care spending pie, compared with the $51.1 billion (27%) spent on retail drug prescriptions, which was the next-largest portion. Inpatient care was third with $30.3 billion in spending (16% of the total), followed by residential care at $23.2 billion (12%), and insurance administration at $15.9 billion (9%), the NCHS reported in “Health, United States, 2016.”
Outpatient care represents the largest share of mental health treatment expenditures, and it continues to get larger, while components such as retail drug prescriptions and inpatient care have declined, according to the National Center of Health Statistics.
In 2014, outpatient care took a $65.5-billion slice (about 35%) out of the $186-billion mental health care spending pie, compared with the $51.1 billion (27%) spent on retail drug prescriptions, which was the next-largest portion. Inpatient care was third with $30.3 billion in spending (16% of the total), followed by residential care at $23.2 billion (12%), and insurance administration at $15.9 billion (9%), the NCHS reported in “Health, United States, 2016.”
FDA approves first new drug for sickle cell in nearly 20 years
The approval was based on placebo-controlled phase II and phase III trials suggesting that L-glutamate offered moderate benefit to patients with this rare, serious, and potentially fatal blood disorder.
L-glutamine oral powder will be marketed under the brand name Endari by Emmaus Medical. The FDA granted the approval through its orphan drug pathway, which is reserved for treatments of rare diseases or conditions. The National Institutes of Health estimates that sickle cell disorder affects approximately 100,000 individuals in the United States. Previously, the only drug approved for treating sickle cell disorder was hydroxyurea, which the FDA green-lighted in 1998.
The randomized, placebo-controlled, phase III trial on which the approval of L-glutamine was based (GLUSCC09-01) comprised patients aged 5-58 years with sickle cell disease or beta-0 thalassemia who had at least two episodes of painful crises during the 12 months before screening. A total of 152 patients were randomly assigned to receive oral L-glutamine (0.3 mg/kg per day) for 48 weeks followed by a 3-week tapering period, while 78 patients received placebo. Patients who received L-glutamine averaged three hospital visits for painful crises for which they received parenteral narcotics or ketorolac, while the placebo group averaged four such hospital visits. Additionally, the time to second crisis was delayed by 79 days in the treatment group, compared with the placebo group (hazard ratio, 0.68).
L-glutamine also was associated with fewer hospital days (median 6.5 vs. 11 days) and fewer occurrences of potentially life-threatening acute chest syndrome (8.6% vs. 23.1%), investigators reported to the FDA’s Oncologic Drugs Advisory Committee during a meeting on May 24.
Safety studies of L-glutamine included phase II and phase III data from 187 patients who received L-glutamine and 111 patients who received placebo, the investigators reported. Based on these analyses, rates of sickle cell anemia with crisis were 66% in the treatment population and 72% in placebo recipients. Rates of acute chest syndrome were 7% and 19%, respectively. Treatment-emergent adverse events led patients to drop out of the studies in 2.7% and 0.9% of cases. The most common adverse events of L-glutamine therapy were constipation, nausea, headache, cough, pain in the extremities, back pain, chest pain, and abdominal pain.
The FDA advisory committee voted 10-3 in favor of approving L-glutamate after hearing from industry and FDA representatives, physicians who treat patients with sickle cell disorder, and patients and their family members at the May 24 meeting. “No” voters expressed concerns about differing drop-out rates between the study groups, but other committee members emphasized the severe impact of sickle cell disorder on quality of life and the crucial need for more treatments.
The FDA Orphan Products Grants Program provided some of the funding to develop the drug. The FDA committee members had no relevant conflicts of interests.
The approval was based on placebo-controlled phase II and phase III trials suggesting that L-glutamate offered moderate benefit to patients with this rare, serious, and potentially fatal blood disorder.
L-glutamine oral powder will be marketed under the brand name Endari by Emmaus Medical. The FDA granted the approval through its orphan drug pathway, which is reserved for treatments of rare diseases or conditions. The National Institutes of Health estimates that sickle cell disorder affects approximately 100,000 individuals in the United States. Previously, the only drug approved for treating sickle cell disorder was hydroxyurea, which the FDA green-lighted in 1998.
The randomized, placebo-controlled, phase III trial on which the approval of L-glutamine was based (GLUSCC09-01) comprised patients aged 5-58 years with sickle cell disease or beta-0 thalassemia who had at least two episodes of painful crises during the 12 months before screening. A total of 152 patients were randomly assigned to receive oral L-glutamine (0.3 mg/kg per day) for 48 weeks followed by a 3-week tapering period, while 78 patients received placebo. Patients who received L-glutamine averaged three hospital visits for painful crises for which they received parenteral narcotics or ketorolac, while the placebo group averaged four such hospital visits. Additionally, the time to second crisis was delayed by 79 days in the treatment group, compared with the placebo group (hazard ratio, 0.68).
L-glutamine also was associated with fewer hospital days (median 6.5 vs. 11 days) and fewer occurrences of potentially life-threatening acute chest syndrome (8.6% vs. 23.1%), investigators reported to the FDA’s Oncologic Drugs Advisory Committee during a meeting on May 24.
Safety studies of L-glutamine included phase II and phase III data from 187 patients who received L-glutamine and 111 patients who received placebo, the investigators reported. Based on these analyses, rates of sickle cell anemia with crisis were 66% in the treatment population and 72% in placebo recipients. Rates of acute chest syndrome were 7% and 19%, respectively. Treatment-emergent adverse events led patients to drop out of the studies in 2.7% and 0.9% of cases. The most common adverse events of L-glutamine therapy were constipation, nausea, headache, cough, pain in the extremities, back pain, chest pain, and abdominal pain.
The FDA advisory committee voted 10-3 in favor of approving L-glutamate after hearing from industry and FDA representatives, physicians who treat patients with sickle cell disorder, and patients and their family members at the May 24 meeting. “No” voters expressed concerns about differing drop-out rates between the study groups, but other committee members emphasized the severe impact of sickle cell disorder on quality of life and the crucial need for more treatments.
The FDA Orphan Products Grants Program provided some of the funding to develop the drug. The FDA committee members had no relevant conflicts of interests.
The approval was based on placebo-controlled phase II and phase III trials suggesting that L-glutamate offered moderate benefit to patients with this rare, serious, and potentially fatal blood disorder.
L-glutamine oral powder will be marketed under the brand name Endari by Emmaus Medical. The FDA granted the approval through its orphan drug pathway, which is reserved for treatments of rare diseases or conditions. The National Institutes of Health estimates that sickle cell disorder affects approximately 100,000 individuals in the United States. Previously, the only drug approved for treating sickle cell disorder was hydroxyurea, which the FDA green-lighted in 1998.
The randomized, placebo-controlled, phase III trial on which the approval of L-glutamine was based (GLUSCC09-01) comprised patients aged 5-58 years with sickle cell disease or beta-0 thalassemia who had at least two episodes of painful crises during the 12 months before screening. A total of 152 patients were randomly assigned to receive oral L-glutamine (0.3 mg/kg per day) for 48 weeks followed by a 3-week tapering period, while 78 patients received placebo. Patients who received L-glutamine averaged three hospital visits for painful crises for which they received parenteral narcotics or ketorolac, while the placebo group averaged four such hospital visits. Additionally, the time to second crisis was delayed by 79 days in the treatment group, compared with the placebo group (hazard ratio, 0.68).
L-glutamine also was associated with fewer hospital days (median 6.5 vs. 11 days) and fewer occurrences of potentially life-threatening acute chest syndrome (8.6% vs. 23.1%), investigators reported to the FDA’s Oncologic Drugs Advisory Committee during a meeting on May 24.
Safety studies of L-glutamine included phase II and phase III data from 187 patients who received L-glutamine and 111 patients who received placebo, the investigators reported. Based on these analyses, rates of sickle cell anemia with crisis were 66% in the treatment population and 72% in placebo recipients. Rates of acute chest syndrome were 7% and 19%, respectively. Treatment-emergent adverse events led patients to drop out of the studies in 2.7% and 0.9% of cases. The most common adverse events of L-glutamine therapy were constipation, nausea, headache, cough, pain in the extremities, back pain, chest pain, and abdominal pain.
The FDA advisory committee voted 10-3 in favor of approving L-glutamate after hearing from industry and FDA representatives, physicians who treat patients with sickle cell disorder, and patients and their family members at the May 24 meeting. “No” voters expressed concerns about differing drop-out rates between the study groups, but other committee members emphasized the severe impact of sickle cell disorder on quality of life and the crucial need for more treatments.
The FDA Orphan Products Grants Program provided some of the funding to develop the drug. The FDA committee members had no relevant conflicts of interests.
Tazemetostat active against follicular lymphoma with EZH2 mutation
LUGANO, SWITZERLAND – Tazemetostat, a first-in-class experimental agent that inhibits an oncogenic protein, shows efficacy in patients with heavily pretreated, relapsed/refractory follicular lymphoma (FL) and diffuse large B cell lymphoma (DLBCL), interim results from a phase II study suggest.
Among patients with relapsed/refractory FL who had mutations in EZH2 (enhancer of zeste homolog 2), a member of a family of proteins that are involved in epigenetic gene silencing, the overall response rate (ORR) was 92%, reported Franck Morschhauser, MD, PhD, of the University of Lille, France.
“What we observed is a four-fold increase in [ORR in] follicular lymphoma-mutated patients compared to wild-type patients, a two-fold increase in DLBCL patients mutated compared to wild-type patients,” he said.
“But if we had focused [only] on the actionable mutation, we would have missed those other complete responders in the wild-type setting,” he added.
EZH2, an epigenetic regulator of gene expression, had been shown in preclinical studies to play an important role in multiple forms of cancers, and activating mutations of EZH2 have been shown to be oncogenic drivers in approximately 20% of FL and germinal center B-cell–like DLBCL, Dr. Morschhauser explained.
EZH2 has also been shown to be over-expressed in leukemia-initiating cells in patients with chronic myeloid leukemia, and EZH2 inhibitors are being explored as a possible therapy for patients with chronic myeloid leukemia that has become resistant to tyrosine kinase inhibitors.
Large multicenter study
Dr. Morschhauser reported interim results from a global, multi-center open-label study of tazemetostat in six cohorts of patients with relapsed/refractory FL (two monotherapy cohorts of 45 patients each) or DLBCL (three monotherapy cohorts of 60 patients each). A sixth cohort consisting of 70 patients with DLBCL treated with tazemetostat and prednisolone was added in 2017.
In the ongoing trial, patients receive oral tazemetostat 800 mg twice daily until disease progression or withdrawal from study, and are being followed for ORR, progression-free survival, overall survival, duration-of response, safety, and pharmacokinetics.
The longest follow-up at the time of data cutoff was approximately 18 months. Among 13 evaluable patients with FL with EZH2 mutations, the ORR was 92%, including one complete response (CR) and 11 partial responses (PR). In contrast, the ORR for 54 patients with FL and wild-type EZH2 was 28%, consisting of three CRs and 11 PRs. One patient with mutated EZH2 and 23 with wild-type EZH2 had stable disease.
Among 17 patients with DLBCL and EZH2 mutations, the ORR was 29%, consisting of 5 PR. For 119 patients with wild-type EZH2, the ORR was 15%, consisting of 10 CR and 8 PR. Six patients with mutations and 22 with wild-type EZH2 had stable disease.
Among the patients with FL, 75% had significant reduction in tumor burden.
The time to response ranged from 2 months to 1 year, with a median of approximately 4 months.
The variability in time to response “makes it a little bit tricky to calculate response duration,” Dr. Morschhauser said.
The drug had a “favorable” safety profile, with treatment-related adverse events of grade 3 or greater in more than 5% of patients including thrombocytopenias in 6% of patients, anemias in 4%, and neutropenias in 6%. Treatment-emergent adverse events leading to dose reductions occurred in 4% of patients, and those leading to drug discontinuation or study withdrawal occurred in 12% of patients.
In a retrospective analysis, the investigators performed molecular profiling studies using next-generation sequencing to look for predictors of response to tazemetostat. They found that patients most likely to respond to tazemetostat were those with activating mutations in EZH2 and MYD88. In contrast, patients with mutations HIST1H1E or MYC were not likely to respond.
Thomas E. Witzig, MD, of the Mayo Clinic in Rochester, Minn., the invited discussant, said that the study is important because “it provides proof of principle that attacking the methylation issue, attacking one of these enzymes, is very important and can produce single-agent responses.
“It also demonstrates the value of mutation status, and this trial knowledge of that mutation status has actually changed the trial design, so that now they are only putting patients on with mutations,” he said.
The trial also raises the possibility of targeting other parts of the methylation pathway to treat cancer, he added.
The study was sponsored by Epizyme, the maker of tazemetostat. Dr. Morschhauser disclosed receiving honoraria from and serving on advisory boards for both companies. Dr. Witzig has disclosed grants for clinical trials from Novartis and Wyeth, and he has served on advisory boards for Cephalon, Novartis, and Wyeth.
LUGANO, SWITZERLAND – Tazemetostat, a first-in-class experimental agent that inhibits an oncogenic protein, shows efficacy in patients with heavily pretreated, relapsed/refractory follicular lymphoma (FL) and diffuse large B cell lymphoma (DLBCL), interim results from a phase II study suggest.
Among patients with relapsed/refractory FL who had mutations in EZH2 (enhancer of zeste homolog 2), a member of a family of proteins that are involved in epigenetic gene silencing, the overall response rate (ORR) was 92%, reported Franck Morschhauser, MD, PhD, of the University of Lille, France.
“What we observed is a four-fold increase in [ORR in] follicular lymphoma-mutated patients compared to wild-type patients, a two-fold increase in DLBCL patients mutated compared to wild-type patients,” he said.
“But if we had focused [only] on the actionable mutation, we would have missed those other complete responders in the wild-type setting,” he added.
EZH2, an epigenetic regulator of gene expression, had been shown in preclinical studies to play an important role in multiple forms of cancers, and activating mutations of EZH2 have been shown to be oncogenic drivers in approximately 20% of FL and germinal center B-cell–like DLBCL, Dr. Morschhauser explained.
EZH2 has also been shown to be over-expressed in leukemia-initiating cells in patients with chronic myeloid leukemia, and EZH2 inhibitors are being explored as a possible therapy for patients with chronic myeloid leukemia that has become resistant to tyrosine kinase inhibitors.
Large multicenter study
Dr. Morschhauser reported interim results from a global, multi-center open-label study of tazemetostat in six cohorts of patients with relapsed/refractory FL (two monotherapy cohorts of 45 patients each) or DLBCL (three monotherapy cohorts of 60 patients each). A sixth cohort consisting of 70 patients with DLBCL treated with tazemetostat and prednisolone was added in 2017.
In the ongoing trial, patients receive oral tazemetostat 800 mg twice daily until disease progression or withdrawal from study, and are being followed for ORR, progression-free survival, overall survival, duration-of response, safety, and pharmacokinetics.
The longest follow-up at the time of data cutoff was approximately 18 months. Among 13 evaluable patients with FL with EZH2 mutations, the ORR was 92%, including one complete response (CR) and 11 partial responses (PR). In contrast, the ORR for 54 patients with FL and wild-type EZH2 was 28%, consisting of three CRs and 11 PRs. One patient with mutated EZH2 and 23 with wild-type EZH2 had stable disease.
Among 17 patients with DLBCL and EZH2 mutations, the ORR was 29%, consisting of 5 PR. For 119 patients with wild-type EZH2, the ORR was 15%, consisting of 10 CR and 8 PR. Six patients with mutations and 22 with wild-type EZH2 had stable disease.
Among the patients with FL, 75% had significant reduction in tumor burden.
The time to response ranged from 2 months to 1 year, with a median of approximately 4 months.
The variability in time to response “makes it a little bit tricky to calculate response duration,” Dr. Morschhauser said.
The drug had a “favorable” safety profile, with treatment-related adverse events of grade 3 or greater in more than 5% of patients including thrombocytopenias in 6% of patients, anemias in 4%, and neutropenias in 6%. Treatment-emergent adverse events leading to dose reductions occurred in 4% of patients, and those leading to drug discontinuation or study withdrawal occurred in 12% of patients.
In a retrospective analysis, the investigators performed molecular profiling studies using next-generation sequencing to look for predictors of response to tazemetostat. They found that patients most likely to respond to tazemetostat were those with activating mutations in EZH2 and MYD88. In contrast, patients with mutations HIST1H1E or MYC were not likely to respond.
Thomas E. Witzig, MD, of the Mayo Clinic in Rochester, Minn., the invited discussant, said that the study is important because “it provides proof of principle that attacking the methylation issue, attacking one of these enzymes, is very important and can produce single-agent responses.
“It also demonstrates the value of mutation status, and this trial knowledge of that mutation status has actually changed the trial design, so that now they are only putting patients on with mutations,” he said.
The trial also raises the possibility of targeting other parts of the methylation pathway to treat cancer, he added.
The study was sponsored by Epizyme, the maker of tazemetostat. Dr. Morschhauser disclosed receiving honoraria from and serving on advisory boards for both companies. Dr. Witzig has disclosed grants for clinical trials from Novartis and Wyeth, and he has served on advisory boards for Cephalon, Novartis, and Wyeth.
LUGANO, SWITZERLAND – Tazemetostat, a first-in-class experimental agent that inhibits an oncogenic protein, shows efficacy in patients with heavily pretreated, relapsed/refractory follicular lymphoma (FL) and diffuse large B cell lymphoma (DLBCL), interim results from a phase II study suggest.
Among patients with relapsed/refractory FL who had mutations in EZH2 (enhancer of zeste homolog 2), a member of a family of proteins that are involved in epigenetic gene silencing, the overall response rate (ORR) was 92%, reported Franck Morschhauser, MD, PhD, of the University of Lille, France.
“What we observed is a four-fold increase in [ORR in] follicular lymphoma-mutated patients compared to wild-type patients, a two-fold increase in DLBCL patients mutated compared to wild-type patients,” he said.
“But if we had focused [only] on the actionable mutation, we would have missed those other complete responders in the wild-type setting,” he added.
EZH2, an epigenetic regulator of gene expression, had been shown in preclinical studies to play an important role in multiple forms of cancers, and activating mutations of EZH2 have been shown to be oncogenic drivers in approximately 20% of FL and germinal center B-cell–like DLBCL, Dr. Morschhauser explained.
EZH2 has also been shown to be over-expressed in leukemia-initiating cells in patients with chronic myeloid leukemia, and EZH2 inhibitors are being explored as a possible therapy for patients with chronic myeloid leukemia that has become resistant to tyrosine kinase inhibitors.
Large multicenter study
Dr. Morschhauser reported interim results from a global, multi-center open-label study of tazemetostat in six cohorts of patients with relapsed/refractory FL (two monotherapy cohorts of 45 patients each) or DLBCL (three monotherapy cohorts of 60 patients each). A sixth cohort consisting of 70 patients with DLBCL treated with tazemetostat and prednisolone was added in 2017.
In the ongoing trial, patients receive oral tazemetostat 800 mg twice daily until disease progression or withdrawal from study, and are being followed for ORR, progression-free survival, overall survival, duration-of response, safety, and pharmacokinetics.
The longest follow-up at the time of data cutoff was approximately 18 months. Among 13 evaluable patients with FL with EZH2 mutations, the ORR was 92%, including one complete response (CR) and 11 partial responses (PR). In contrast, the ORR for 54 patients with FL and wild-type EZH2 was 28%, consisting of three CRs and 11 PRs. One patient with mutated EZH2 and 23 with wild-type EZH2 had stable disease.
Among 17 patients with DLBCL and EZH2 mutations, the ORR was 29%, consisting of 5 PR. For 119 patients with wild-type EZH2, the ORR was 15%, consisting of 10 CR and 8 PR. Six patients with mutations and 22 with wild-type EZH2 had stable disease.
Among the patients with FL, 75% had significant reduction in tumor burden.
The time to response ranged from 2 months to 1 year, with a median of approximately 4 months.
The variability in time to response “makes it a little bit tricky to calculate response duration,” Dr. Morschhauser said.
The drug had a “favorable” safety profile, with treatment-related adverse events of grade 3 or greater in more than 5% of patients including thrombocytopenias in 6% of patients, anemias in 4%, and neutropenias in 6%. Treatment-emergent adverse events leading to dose reductions occurred in 4% of patients, and those leading to drug discontinuation or study withdrawal occurred in 12% of patients.
In a retrospective analysis, the investigators performed molecular profiling studies using next-generation sequencing to look for predictors of response to tazemetostat. They found that patients most likely to respond to tazemetostat were those with activating mutations in EZH2 and MYD88. In contrast, patients with mutations HIST1H1E or MYC were not likely to respond.
Thomas E. Witzig, MD, of the Mayo Clinic in Rochester, Minn., the invited discussant, said that the study is important because “it provides proof of principle that attacking the methylation issue, attacking one of these enzymes, is very important and can produce single-agent responses.
“It also demonstrates the value of mutation status, and this trial knowledge of that mutation status has actually changed the trial design, so that now they are only putting patients on with mutations,” he said.
The trial also raises the possibility of targeting other parts of the methylation pathway to treat cancer, he added.
The study was sponsored by Epizyme, the maker of tazemetostat. Dr. Morschhauser disclosed receiving honoraria from and serving on advisory boards for both companies. Dr. Witzig has disclosed grants for clinical trials from Novartis and Wyeth, and he has served on advisory boards for Cephalon, Novartis, and Wyeth.
AT 14-ICML
Key clinical point: The experimental drug tazemetostat induced responses in patients with heavily pretreated follicular lymphoma (FL) with mutations in EZH2.
Major finding: The overall response rate among patients with FL with mutated EZH2 was 92%.
Data source: Multicenter, open-label phase II study in patients with relapsed/refractory FL and diffuse large B cell lymphoma.
Disclosures: The study is sponsored by Epizyme. Dr. Morschhauser disclosed receiving honoraria from and serving on advisory boards for both companies. Dr. Witzig has disclosed grants for clinical trials from Novartis and Wyeth, and he has served on advisory boards for Cephalon, Novartis, and Wyeth.
Opioid prescribing drops nationally, remains high in some counties
Opioid prescribing in the United States declined overall between 2010 and 2015, but remained stable or increased in some counties, according to a report from the Centers for Disease Control and Prevention. The findings were published online in the CDC’s Morbidity and Mortality Weekly Report.
“The bottom line remains: We have too many people getting too many prescriptions at too high a dose,” Anne Schuchat, MD, acting director of the CDC, said in a July 6 teleconference.
CDC researchers calculated prescribing rates from 2006 to 2015 by dividing the number of opioid prescriptions by the population estimates from the U.S. census for each year and created quartiles using morphine milligram equivalent per capita to analyze opioid distribution. Annual opioid prescribing rates increased from 72 to 81 prescriptions per 100 persons from 2006 to 2010 and remained relatively constant from 2010 to 2012 before showing a 13% decrease to 71 prescriptions per 100 persons from 2012 to 2015 (MMWR. 2017 Jul 7;66[26]:697-704. doi: 10.15585/mmwr.mm6626a4).
But despite these overall declines, “We are now experiencing the highest overdose death rates ever recorded in the United States,” Dr. Schuchat said. Quartiles were created using MME per capita to characterize the distribution of opioids prescribed.
In the report, areas associated with higher opioid prescribing rates on a county level included small cities or towns, areas that had a higher proportion of white residents, areas with more doctors and dentists, and areas with more cases of arthritis, diabetes, or other disabilities, she said.
The findings suggest a need for more consistency among health care providers about prescription opioids, Dr. Schuchat said. “Clinical practice is all over the place, which is a sign that you need better standards; we hope the 2016 guidelines are a turning point for better prescribing,” she said.
The CDC’s guidelines on opioid prescribing were released in 2016. The guidelines recommend alternatives when possible. Clinicians should instead consider nonopioid therapy, other types of pain medication, and nondrug pain relief options, such as physical therapy and cognitive-behavioral therapy. Other concerns include the length and strength of opioid prescriptions. Even taking opioids for a few months increases the risk for addiction, Dr. Schuchat said.
“Physicians must continue to lead efforts to reverse the epidemic by using prescription drug–monitoring programs, eliminating stigma, prescribing the overdose reversal drug naloxone, and enhancing their education about safe opioid prescribing and effective pain management,” Patrice A. Harris, MD, chair of the American Medical Association Opioid Task Force, said in a statement in response to the report. “Our country must do more to provide evidence-based, comprehensive treatment for pain and for substance use disorders,” she said.
“We really encourage clinicians to look to the guidelines and the tools that are available,” Dr. Schuchat said. “We do know that internists and other primary care physicians prescribe most of the opioids, so it is important for them to be aware.” The CDC has developed a checklist and a mobile app that have been downloaded by thousands of clinicians so far, she noted. Changes in annual prescribing hold promise that practices can improve, she said.
The researchers reported no conflicts of interest.
Opioid prescribing in the United States declined overall between 2010 and 2015, but remained stable or increased in some counties, according to a report from the Centers for Disease Control and Prevention. The findings were published online in the CDC’s Morbidity and Mortality Weekly Report.
“The bottom line remains: We have too many people getting too many prescriptions at too high a dose,” Anne Schuchat, MD, acting director of the CDC, said in a July 6 teleconference.
CDC researchers calculated prescribing rates from 2006 to 2015 by dividing the number of opioid prescriptions by the population estimates from the U.S. census for each year and created quartiles using morphine milligram equivalent per capita to analyze opioid distribution. Annual opioid prescribing rates increased from 72 to 81 prescriptions per 100 persons from 2006 to 2010 and remained relatively constant from 2010 to 2012 before showing a 13% decrease to 71 prescriptions per 100 persons from 2012 to 2015 (MMWR. 2017 Jul 7;66[26]:697-704. doi: 10.15585/mmwr.mm6626a4).
But despite these overall declines, “We are now experiencing the highest overdose death rates ever recorded in the United States,” Dr. Schuchat said. Quartiles were created using MME per capita to characterize the distribution of opioids prescribed.
In the report, areas associated with higher opioid prescribing rates on a county level included small cities or towns, areas that had a higher proportion of white residents, areas with more doctors and dentists, and areas with more cases of arthritis, diabetes, or other disabilities, she said.
The findings suggest a need for more consistency among health care providers about prescription opioids, Dr. Schuchat said. “Clinical practice is all over the place, which is a sign that you need better standards; we hope the 2016 guidelines are a turning point for better prescribing,” she said.
The CDC’s guidelines on opioid prescribing were released in 2016. The guidelines recommend alternatives when possible. Clinicians should instead consider nonopioid therapy, other types of pain medication, and nondrug pain relief options, such as physical therapy and cognitive-behavioral therapy. Other concerns include the length and strength of opioid prescriptions. Even taking opioids for a few months increases the risk for addiction, Dr. Schuchat said.
“Physicians must continue to lead efforts to reverse the epidemic by using prescription drug–monitoring programs, eliminating stigma, prescribing the overdose reversal drug naloxone, and enhancing their education about safe opioid prescribing and effective pain management,” Patrice A. Harris, MD, chair of the American Medical Association Opioid Task Force, said in a statement in response to the report. “Our country must do more to provide evidence-based, comprehensive treatment for pain and for substance use disorders,” she said.
“We really encourage clinicians to look to the guidelines and the tools that are available,” Dr. Schuchat said. “We do know that internists and other primary care physicians prescribe most of the opioids, so it is important for them to be aware.” The CDC has developed a checklist and a mobile app that have been downloaded by thousands of clinicians so far, she noted. Changes in annual prescribing hold promise that practices can improve, she said.
The researchers reported no conflicts of interest.
Opioid prescribing in the United States declined overall between 2010 and 2015, but remained stable or increased in some counties, according to a report from the Centers for Disease Control and Prevention. The findings were published online in the CDC’s Morbidity and Mortality Weekly Report.
“The bottom line remains: We have too many people getting too many prescriptions at too high a dose,” Anne Schuchat, MD, acting director of the CDC, said in a July 6 teleconference.
CDC researchers calculated prescribing rates from 2006 to 2015 by dividing the number of opioid prescriptions by the population estimates from the U.S. census for each year and created quartiles using morphine milligram equivalent per capita to analyze opioid distribution. Annual opioid prescribing rates increased from 72 to 81 prescriptions per 100 persons from 2006 to 2010 and remained relatively constant from 2010 to 2012 before showing a 13% decrease to 71 prescriptions per 100 persons from 2012 to 2015 (MMWR. 2017 Jul 7;66[26]:697-704. doi: 10.15585/mmwr.mm6626a4).
But despite these overall declines, “We are now experiencing the highest overdose death rates ever recorded in the United States,” Dr. Schuchat said. Quartiles were created using MME per capita to characterize the distribution of opioids prescribed.
In the report, areas associated with higher opioid prescribing rates on a county level included small cities or towns, areas that had a higher proportion of white residents, areas with more doctors and dentists, and areas with more cases of arthritis, diabetes, or other disabilities, she said.
The findings suggest a need for more consistency among health care providers about prescription opioids, Dr. Schuchat said. “Clinical practice is all over the place, which is a sign that you need better standards; we hope the 2016 guidelines are a turning point for better prescribing,” she said.
The CDC’s guidelines on opioid prescribing were released in 2016. The guidelines recommend alternatives when possible. Clinicians should instead consider nonopioid therapy, other types of pain medication, and nondrug pain relief options, such as physical therapy and cognitive-behavioral therapy. Other concerns include the length and strength of opioid prescriptions. Even taking opioids for a few months increases the risk for addiction, Dr. Schuchat said.
“Physicians must continue to lead efforts to reverse the epidemic by using prescription drug–monitoring programs, eliminating stigma, prescribing the overdose reversal drug naloxone, and enhancing their education about safe opioid prescribing and effective pain management,” Patrice A. Harris, MD, chair of the American Medical Association Opioid Task Force, said in a statement in response to the report. “Our country must do more to provide evidence-based, comprehensive treatment for pain and for substance use disorders,” she said.
“We really encourage clinicians to look to the guidelines and the tools that are available,” Dr. Schuchat said. “We do know that internists and other primary care physicians prescribe most of the opioids, so it is important for them to be aware.” The CDC has developed a checklist and a mobile app that have been downloaded by thousands of clinicians so far, she noted. Changes in annual prescribing hold promise that practices can improve, she said.
The researchers reported no conflicts of interest.
FROM MMWR
FDA approves abatacept for adults with psoriatic arthritis
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.
The Food and Drug Administration has approved abatacept, a selective T-cell costimulation modulator, for treating adults with active psoriatic arthritis (PsA), the manufacturer, Bristol-Myers Squibb, has announced.
Approval of abatacept (Orencia) was based on two randomized, double-blind, placebo-controlled studies (PsA-I and PsA-II) in 594 adults with PsA for more than 7 years, according to the July 6 announcement. Patients had active PsA (at least three swollen joints and at least three tender joints), despite previous disease-modifying antirheumatic drug (DMARD) therapy and had one qualifying psoriatic skin lesion measuring at least 2 cm in diameter. The studies included patients treated with TNF inhibitors (TNFi) previously.
In the PsA-II trial, 424 patients received weekly doses of placebo or abatacept 25 mg administered subcutaneously (SC) without a loading dose for 24 weeks, followed by open-label abatacept at a dose of 125 mg SC weekly.
Compared with those on placebo, more patients treated with abatacept 10 mg/kg IV or 125 mg SC achieved an ACR 20 (American College of Rheumatology 20) response at 24 weeks: 47.5% vs. 19.0% and 39.4% vs. 22.3%, respectively (P less than .05).
Other results included a greater proportion of abatacept SC patients with at least a 0.35 decrease from baseline on the Health Assessment Questionnaire-Disability Index: 31% vs. 24% on placebo at 24 weeks. Responses were seen regardless of prior anti-TNFi treatment and regardless of concomitant non-biologic DMARD treatment. In addition, patients on abatacept IV and SC had improvements in enthesitis and dactylitis at 24 weeks.
The safety profile of abatacept in the two studies was “consistent with the safety profile” in rheumatoid arthritis, according to the company release.
Abatacept, initially approved in 2005, was previously approved for RA in adults and for juvenile idiopathic arthritis
Find the updated prescribing information for abatacept here.







