User login
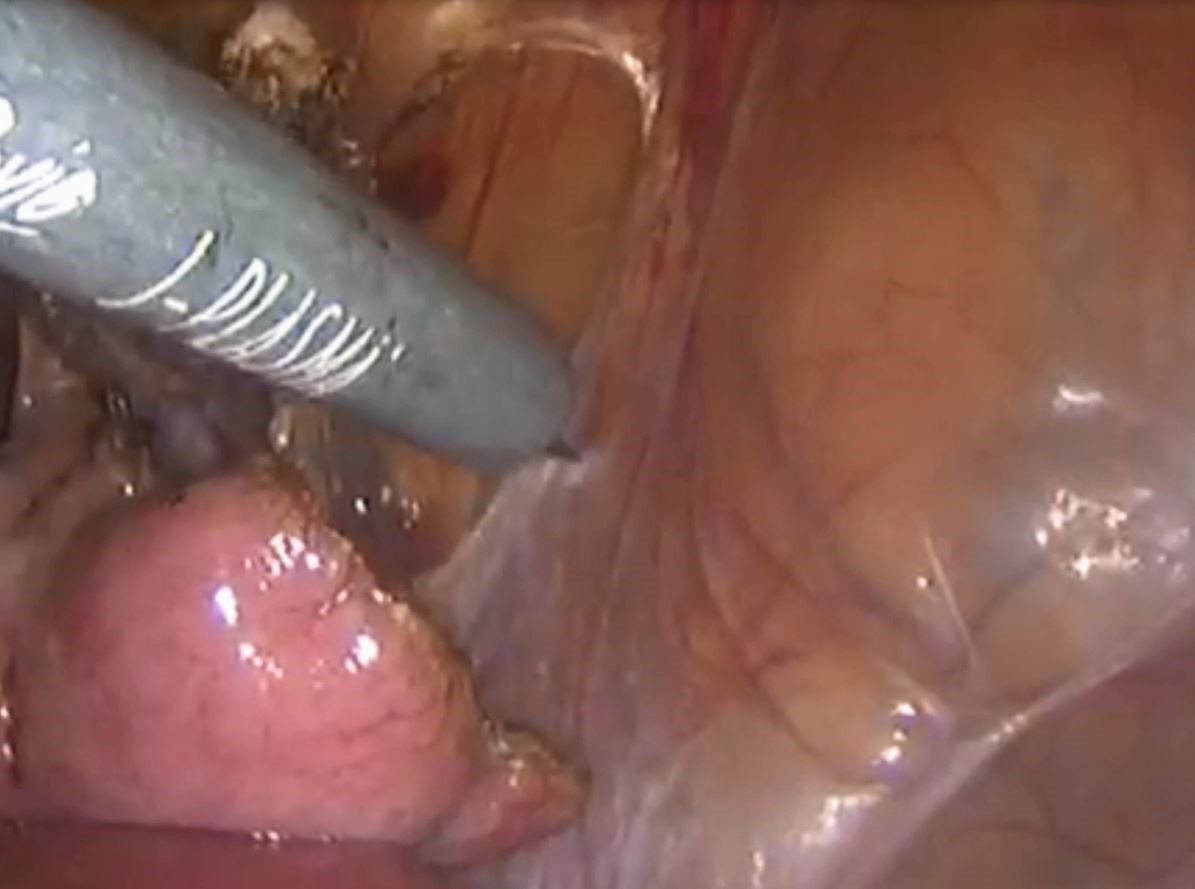
Laparoscopic management of chronic pelvic pain utilizing J-Plasma



VIDEO: MBSAQIP data looks at sleeve gastrectomy outcomes
Surgeon Matthew A. Hutter, MD, FACS, discusses the first report from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) focused on laparoscopic sleeve gastrectomy. The study (Ann Surg. 2016;264[3]:464-73) looked at outcomes, methods, and complications of this procedure based on a database of nearly 190,000 patients, more than 1,600 surgeons, and 720 centers. Dr. Hutter said that is high-quality data that offers surgeons good information on the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Surgeon Matthew A. Hutter, MD, FACS, discusses the first report from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) focused on laparoscopic sleeve gastrectomy. The study (Ann Surg. 2016;264[3]:464-73) looked at outcomes, methods, and complications of this procedure based on a database of nearly 190,000 patients, more than 1,600 surgeons, and 720 centers. Dr. Hutter said that is high-quality data that offers surgeons good information on the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Surgeon Matthew A. Hutter, MD, FACS, discusses the first report from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) focused on laparoscopic sleeve gastrectomy. The study (Ann Surg. 2016;264[3]:464-73) looked at outcomes, methods, and complications of this procedure based on a database of nearly 190,000 patients, more than 1,600 surgeons, and 720 centers. Dr. Hutter said that is high-quality data that offers surgeons good information on the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FDA approves Cinvanti for chemo-induced nausea and vomiting
The Food and Drug Administration has approved aprepitant injectable emulsion for the prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV), Heron Therapeutics announced Nov. 9.
“CINV remains a high unmet medical need in the oncology community, and five full days of CINV coverage continues to be our goal,” Heron CEO Jeffrey Patton, MD, said in a statement. “NK1 receptor antagonists are recommended for routine use with [highly emetogenic chemotherapy] and are a recommended option with [moderately emetogenic chemotherapy]. Despite this, NK1 receptor antagonists are underutilized in CINV.”
Aprepitant injectable emulsion is indicated specifically for acute and delayed nausea and vomiting associated with initial and repeated courses of highly emetogenic chemotherapy, including high-dose cisplatin. Treatment is a single dose of 130 mg via intravenous infusion on day 1, approximately 30 minutes before chemotherapy is initiated. It is also indicated for use in moderately emetogenic chemotherapy; treatment of these patients is 100 mg on day 1, followed by oral aprepitant on days 2 and 3.
The most common adverse reactions with single-dose aprepitant injectable emulsion were headache and fatigue.
Aprepitant injectable emulsion will be marketed as Cinvanti and is expected to be available in January 2018, according to the company.
The Food and Drug Administration has approved aprepitant injectable emulsion for the prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV), Heron Therapeutics announced Nov. 9.
“CINV remains a high unmet medical need in the oncology community, and five full days of CINV coverage continues to be our goal,” Heron CEO Jeffrey Patton, MD, said in a statement. “NK1 receptor antagonists are recommended for routine use with [highly emetogenic chemotherapy] and are a recommended option with [moderately emetogenic chemotherapy]. Despite this, NK1 receptor antagonists are underutilized in CINV.”
Aprepitant injectable emulsion is indicated specifically for acute and delayed nausea and vomiting associated with initial and repeated courses of highly emetogenic chemotherapy, including high-dose cisplatin. Treatment is a single dose of 130 mg via intravenous infusion on day 1, approximately 30 minutes before chemotherapy is initiated. It is also indicated for use in moderately emetogenic chemotherapy; treatment of these patients is 100 mg on day 1, followed by oral aprepitant on days 2 and 3.
The most common adverse reactions with single-dose aprepitant injectable emulsion were headache and fatigue.
Aprepitant injectable emulsion will be marketed as Cinvanti and is expected to be available in January 2018, according to the company.
The Food and Drug Administration has approved aprepitant injectable emulsion for the prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV), Heron Therapeutics announced Nov. 9.
“CINV remains a high unmet medical need in the oncology community, and five full days of CINV coverage continues to be our goal,” Heron CEO Jeffrey Patton, MD, said in a statement. “NK1 receptor antagonists are recommended for routine use with [highly emetogenic chemotherapy] and are a recommended option with [moderately emetogenic chemotherapy]. Despite this, NK1 receptor antagonists are underutilized in CINV.”
Aprepitant injectable emulsion is indicated specifically for acute and delayed nausea and vomiting associated with initial and repeated courses of highly emetogenic chemotherapy, including high-dose cisplatin. Treatment is a single dose of 130 mg via intravenous infusion on day 1, approximately 30 minutes before chemotherapy is initiated. It is also indicated for use in moderately emetogenic chemotherapy; treatment of these patients is 100 mg on day 1, followed by oral aprepitant on days 2 and 3.
The most common adverse reactions with single-dose aprepitant injectable emulsion were headache and fatigue.
Aprepitant injectable emulsion will be marketed as Cinvanti and is expected to be available in January 2018, according to the company.
Some children with HIV may not get enough medical care
, according to researchers at the Centers for Disease Control and Prevention
The investigators examined claims cohorts of children aged 13 years and younger who had been diagnosed with HIV to track their retention of medical care over a 36-month period, starting in 2010. They found that rates of retention in care – defined as at least one visit in every 6-month period – were lower than expected.
However, because rates of AIDS diagnoses and deaths among children are low nationally – an estimated 2,477 children younger than 13 years had HIV in 2014 – it may be that “failure to meet the retention in care definition ... does not necessarily mean loss to follow-up,” cautioned Mary R. Tanner, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, and her coinvestigators (MMWR. 2017 Oct 6:66[39];1033-8).
In addition, in the Medicaid claims cohort, 59% of the children who were not retained in care during the first 24 months (but who remained in the study) were in care during months 25-36.
The researchers used the 2010-2014 MarketScan Multi-State Medicaid and MarketScan Commercial Claims and Encounters databases to make cohorts of Medicaid and commercial claims of 163 and 129 children, respectively. They tracked retention of care for these children starting from the first reported ICD-9-CM code for HIV or AIDS. One reason for the current study is that the National HIV Surveillance System, which has a goal of increasing retention in care, does not track children with HIV infection in its progress indicators.
In the first 24 months, 60% of the Medicaid cohort and 69% of the commercial claims cohort were retained in care. For children who remained in the study after month 24, the investigators further divided the cohorts into subgroups of those who remained in care thus far and those who did not. A total of 93% of those in the Medicaid cohort who remained in care during the first 24 months stayed in care in months 25-36, while 59% of those who didn’t remain in care in the first 24 months did remain in care during months 25-36.
For the subgroups in the commercial claims cohort, the same numbers were 85% and 32%.
Noting many possible limitations to the current study, the investigators nevertheless remarked that “overall, the fact that greater than 25% of children with diagnosed HIV infection did not meet the retention in care definition suggests that portions of this medically vulnerable population are not receiving the recommended frequency of medical care.”
, according to researchers at the Centers for Disease Control and Prevention
The investigators examined claims cohorts of children aged 13 years and younger who had been diagnosed with HIV to track their retention of medical care over a 36-month period, starting in 2010. They found that rates of retention in care – defined as at least one visit in every 6-month period – were lower than expected.
However, because rates of AIDS diagnoses and deaths among children are low nationally – an estimated 2,477 children younger than 13 years had HIV in 2014 – it may be that “failure to meet the retention in care definition ... does not necessarily mean loss to follow-up,” cautioned Mary R. Tanner, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, and her coinvestigators (MMWR. 2017 Oct 6:66[39];1033-8).
In addition, in the Medicaid claims cohort, 59% of the children who were not retained in care during the first 24 months (but who remained in the study) were in care during months 25-36.
The researchers used the 2010-2014 MarketScan Multi-State Medicaid and MarketScan Commercial Claims and Encounters databases to make cohorts of Medicaid and commercial claims of 163 and 129 children, respectively. They tracked retention of care for these children starting from the first reported ICD-9-CM code for HIV or AIDS. One reason for the current study is that the National HIV Surveillance System, which has a goal of increasing retention in care, does not track children with HIV infection in its progress indicators.
In the first 24 months, 60% of the Medicaid cohort and 69% of the commercial claims cohort were retained in care. For children who remained in the study after month 24, the investigators further divided the cohorts into subgroups of those who remained in care thus far and those who did not. A total of 93% of those in the Medicaid cohort who remained in care during the first 24 months stayed in care in months 25-36, while 59% of those who didn’t remain in care in the first 24 months did remain in care during months 25-36.
For the subgroups in the commercial claims cohort, the same numbers were 85% and 32%.
Noting many possible limitations to the current study, the investigators nevertheless remarked that “overall, the fact that greater than 25% of children with diagnosed HIV infection did not meet the retention in care definition suggests that portions of this medically vulnerable population are not receiving the recommended frequency of medical care.”
, according to researchers at the Centers for Disease Control and Prevention
The investigators examined claims cohorts of children aged 13 years and younger who had been diagnosed with HIV to track their retention of medical care over a 36-month period, starting in 2010. They found that rates of retention in care – defined as at least one visit in every 6-month period – were lower than expected.
However, because rates of AIDS diagnoses and deaths among children are low nationally – an estimated 2,477 children younger than 13 years had HIV in 2014 – it may be that “failure to meet the retention in care definition ... does not necessarily mean loss to follow-up,” cautioned Mary R. Tanner, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, and her coinvestigators (MMWR. 2017 Oct 6:66[39];1033-8).
In addition, in the Medicaid claims cohort, 59% of the children who were not retained in care during the first 24 months (but who remained in the study) were in care during months 25-36.
The researchers used the 2010-2014 MarketScan Multi-State Medicaid and MarketScan Commercial Claims and Encounters databases to make cohorts of Medicaid and commercial claims of 163 and 129 children, respectively. They tracked retention of care for these children starting from the first reported ICD-9-CM code for HIV or AIDS. One reason for the current study is that the National HIV Surveillance System, which has a goal of increasing retention in care, does not track children with HIV infection in its progress indicators.
In the first 24 months, 60% of the Medicaid cohort and 69% of the commercial claims cohort were retained in care. For children who remained in the study after month 24, the investigators further divided the cohorts into subgroups of those who remained in care thus far and those who did not. A total of 93% of those in the Medicaid cohort who remained in care during the first 24 months stayed in care in months 25-36, while 59% of those who didn’t remain in care in the first 24 months did remain in care during months 25-36.
For the subgroups in the commercial claims cohort, the same numbers were 85% and 32%.
Noting many possible limitations to the current study, the investigators nevertheless remarked that “overall, the fact that greater than 25% of children with diagnosed HIV infection did not meet the retention in care definition suggests that portions of this medically vulnerable population are not receiving the recommended frequency of medical care.”
FROM MMWR
Generic clofarabine injection hits the market
The generic clofarabine injection for treatment of children with relapsed or refractory acute lymphoblastic leukemia (ALL) is now available on the U.S. market.
The generic version of the drug was approved by the Food and Drug Administration in May 2017 for children up to age 21 years with relapsed or refractory ALL after at least two prior regimens.
The injection, marketed by Mylan N.V., is available in 20 mg/20 mL (1 mg/mL) single-dose vials. It is a generic version of Genzyme’s Clolar.
[email protected]
On Twitter @maryellenny
The generic clofarabine injection for treatment of children with relapsed or refractory acute lymphoblastic leukemia (ALL) is now available on the U.S. market.
The generic version of the drug was approved by the Food and Drug Administration in May 2017 for children up to age 21 years with relapsed or refractory ALL after at least two prior regimens.
The injection, marketed by Mylan N.V., is available in 20 mg/20 mL (1 mg/mL) single-dose vials. It is a generic version of Genzyme’s Clolar.
[email protected]
On Twitter @maryellenny
The generic clofarabine injection for treatment of children with relapsed or refractory acute lymphoblastic leukemia (ALL) is now available on the U.S. market.
The generic version of the drug was approved by the Food and Drug Administration in May 2017 for children up to age 21 years with relapsed or refractory ALL after at least two prior regimens.
The injection, marketed by Mylan N.V., is available in 20 mg/20 mL (1 mg/mL) single-dose vials. It is a generic version of Genzyme’s Clolar.
[email protected]
On Twitter @maryellenny
FDA approves first two-dose HBV vaccine
When the Food and Drug Administration approved Heplisav-B Nov. 9, it marked the first new vaccine for hepatitis B virus (HBV) to be sanctioned in over 25 years.
Heplisav-B is the only two-dose regimen that protects against all known subtypes of HBV in adults 18 years and older, according to a statement released by Dynavax Technologies, the creator of the drug.
“Heplisav-B is the first FDA-approved product for Dynavax and demonstrates our ability to develop innovative products and progress them from discovery to commercialization,” according to Eddie Gray, chief executive officer of Dynavax. “We expect that it will become an essential tool in the public health community’s fight to prevent hepatitis B [infection], and we look forward to making Heplisav-B available to clinicians and their adult patients.”
Incidence of HBV has increased sharply from 2012 to 2015 in the United States, with reported cases rising from 2,895 to 3,370, according to the Centers for Disease Control and Prevention.
From 2014 to 2015, acute HBV infection increased 20.7%, according to the CDC report.
The new vaccine’s approval came after review of safety and efficacy data from three phase 3 trials comparing Heplisav-B with Engerix-B, another HBV vaccine currently available, that is given in a three-dose regimen.
In one study of 2,032 patients between the ages of 18 and 55 years, seroprotection rate in the Heplisav-B group (1,511) was 95%, compared with 81.5% in the Engerix-B group (521).
Heplisav-B patients were given a two-dose regimen of the drug at 0 and 1 months, followed by a placebo at 6 months, while investigators administered Engerix-B at all three intervals in the comparator subjects.
The FDA’s decision to green-light the new vaccine follows a recommendation for approval from the FDA’s Vaccines and Related Biological Products Advisory Committee, held at the end of July this year.
During the advisory committee meeting, members were concerned about an increased relative risk for acute myocardial infarction of 6.97 in Heplisav-B patients (14), compared with Engerix-B patients (1).
The recommendation for approval came with the caveat of conducting postmarketing analysis for the risk of AMI in Heplisav-B patients, which Dynavax is conducting through the Kaiser Permanente system in California.
“To evaluate the risk of AMIs, the study will enroll 25,000 Heplisav-B patients and 25,000 Engerix-B patients over approximately 10 months and follow them for 1 year after vaccination,” according to a statement from Dynavax. “In addition we will evaluate the rate of immune-mediated diseases in these patients in an additional 5,000 Heplisav-B recipients and 5,000 Engerix-B recipients.”
Dynavax is currently set to introduce Heplisav-B commercially in the United States in 2018, with the cost of the drug set to be released soon.
“Dynavax is in the process of finalizing the price of a two-dose series of Heplisav-B, and they plan to disclose it shortly after approval,” according to the company. “Their pricing and access strategy will be aimed at ensuring that populations at risk of infection are able to access this new vaccine, while recognizing the value it brings to the health care system with a two-dose regimen and higher rates of protection compared to Engerix-B.”
[email protected]
On Twitter @eaztweets
When the Food and Drug Administration approved Heplisav-B Nov. 9, it marked the first new vaccine for hepatitis B virus (HBV) to be sanctioned in over 25 years.
Heplisav-B is the only two-dose regimen that protects against all known subtypes of HBV in adults 18 years and older, according to a statement released by Dynavax Technologies, the creator of the drug.
“Heplisav-B is the first FDA-approved product for Dynavax and demonstrates our ability to develop innovative products and progress them from discovery to commercialization,” according to Eddie Gray, chief executive officer of Dynavax. “We expect that it will become an essential tool in the public health community’s fight to prevent hepatitis B [infection], and we look forward to making Heplisav-B available to clinicians and their adult patients.”
Incidence of HBV has increased sharply from 2012 to 2015 in the United States, with reported cases rising from 2,895 to 3,370, according to the Centers for Disease Control and Prevention.
From 2014 to 2015, acute HBV infection increased 20.7%, according to the CDC report.
The new vaccine’s approval came after review of safety and efficacy data from three phase 3 trials comparing Heplisav-B with Engerix-B, another HBV vaccine currently available, that is given in a three-dose regimen.
In one study of 2,032 patients between the ages of 18 and 55 years, seroprotection rate in the Heplisav-B group (1,511) was 95%, compared with 81.5% in the Engerix-B group (521).
Heplisav-B patients were given a two-dose regimen of the drug at 0 and 1 months, followed by a placebo at 6 months, while investigators administered Engerix-B at all three intervals in the comparator subjects.
The FDA’s decision to green-light the new vaccine follows a recommendation for approval from the FDA’s Vaccines and Related Biological Products Advisory Committee, held at the end of July this year.
During the advisory committee meeting, members were concerned about an increased relative risk for acute myocardial infarction of 6.97 in Heplisav-B patients (14), compared with Engerix-B patients (1).
The recommendation for approval came with the caveat of conducting postmarketing analysis for the risk of AMI in Heplisav-B patients, which Dynavax is conducting through the Kaiser Permanente system in California.
“To evaluate the risk of AMIs, the study will enroll 25,000 Heplisav-B patients and 25,000 Engerix-B patients over approximately 10 months and follow them for 1 year after vaccination,” according to a statement from Dynavax. “In addition we will evaluate the rate of immune-mediated diseases in these patients in an additional 5,000 Heplisav-B recipients and 5,000 Engerix-B recipients.”
Dynavax is currently set to introduce Heplisav-B commercially in the United States in 2018, with the cost of the drug set to be released soon.
“Dynavax is in the process of finalizing the price of a two-dose series of Heplisav-B, and they plan to disclose it shortly after approval,” according to the company. “Their pricing and access strategy will be aimed at ensuring that populations at risk of infection are able to access this new vaccine, while recognizing the value it brings to the health care system with a two-dose regimen and higher rates of protection compared to Engerix-B.”
[email protected]
On Twitter @eaztweets
When the Food and Drug Administration approved Heplisav-B Nov. 9, it marked the first new vaccine for hepatitis B virus (HBV) to be sanctioned in over 25 years.
Heplisav-B is the only two-dose regimen that protects against all known subtypes of HBV in adults 18 years and older, according to a statement released by Dynavax Technologies, the creator of the drug.
“Heplisav-B is the first FDA-approved product for Dynavax and demonstrates our ability to develop innovative products and progress them from discovery to commercialization,” according to Eddie Gray, chief executive officer of Dynavax. “We expect that it will become an essential tool in the public health community’s fight to prevent hepatitis B [infection], and we look forward to making Heplisav-B available to clinicians and their adult patients.”
Incidence of HBV has increased sharply from 2012 to 2015 in the United States, with reported cases rising from 2,895 to 3,370, according to the Centers for Disease Control and Prevention.
From 2014 to 2015, acute HBV infection increased 20.7%, according to the CDC report.
The new vaccine’s approval came after review of safety and efficacy data from three phase 3 trials comparing Heplisav-B with Engerix-B, another HBV vaccine currently available, that is given in a three-dose regimen.
In one study of 2,032 patients between the ages of 18 and 55 years, seroprotection rate in the Heplisav-B group (1,511) was 95%, compared with 81.5% in the Engerix-B group (521).
Heplisav-B patients were given a two-dose regimen of the drug at 0 and 1 months, followed by a placebo at 6 months, while investigators administered Engerix-B at all three intervals in the comparator subjects.
The FDA’s decision to green-light the new vaccine follows a recommendation for approval from the FDA’s Vaccines and Related Biological Products Advisory Committee, held at the end of July this year.
During the advisory committee meeting, members were concerned about an increased relative risk for acute myocardial infarction of 6.97 in Heplisav-B patients (14), compared with Engerix-B patients (1).
The recommendation for approval came with the caveat of conducting postmarketing analysis for the risk of AMI in Heplisav-B patients, which Dynavax is conducting through the Kaiser Permanente system in California.
“To evaluate the risk of AMIs, the study will enroll 25,000 Heplisav-B patients and 25,000 Engerix-B patients over approximately 10 months and follow them for 1 year after vaccination,” according to a statement from Dynavax. “In addition we will evaluate the rate of immune-mediated diseases in these patients in an additional 5,000 Heplisav-B recipients and 5,000 Engerix-B recipients.”
Dynavax is currently set to introduce Heplisav-B commercially in the United States in 2018, with the cost of the drug set to be released soon.
“Dynavax is in the process of finalizing the price of a two-dose series of Heplisav-B, and they plan to disclose it shortly after approval,” according to the company. “Their pricing and access strategy will be aimed at ensuring that populations at risk of infection are able to access this new vaccine, while recognizing the value it brings to the health care system with a two-dose regimen and higher rates of protection compared to Engerix-B.”
[email protected]
On Twitter @eaztweets
Lung injury risk higher with apheresis blood products
SAN DIEGO – , according to research presented at the annual meeting of the American Association of Blood Banks.
Compared with other RBC products, those derived from apheresis significantly increased pulmonary cell interleukin (IL)–6 and IL-8 production, and this was further exacerbated by cell stretching. Conversely, red cell–filtered products appeared to be the least likely to cause cell injury.
“Several studies have shown that red blood cell transfusion is associated with acute lung injury, and transfusion induces leakage in ICU patients,” said lead study author Mathijs Wirtz, MD, of the Academic Medical Center, Amsterdam.
ICU patients who did not receive any transfusions had significantly lower leakage than those who were transfused. “There also seems to be a synergy between transfusion and mechanical ventilation,” Dr. Wirtz said.
Studies have also shown that there are differences in the prevalence of transfusion-related acute lung injury, when comparing Europe to the United States. Storage and manufacturing methods do differ between Europe and the United States, Dr. Wirtz noted. “This led to our hypothesis that lung injury inflicted by red blood cell transfusion is influenced by manufacturing methods.”
In this study, Dr. Wirtz and his colleagues investigated the response of pulmonary cells to the different methods of manufacturing RBC products. Using type A or B blood obtained from eight donors, a variety of RBC products were manufactured for the study, including whole-blood filtered, red-cell filtered, apheresis derived, and whole-blood derived.
For measuring thrombin generation and analyzing extracellular vesicles (EV), supernatants were prepared after 4-5 days of storage for fresh and 41-42 days for stored. The researchers selected A549 type II alveolar cells to seed onto flexible membranes, which were then incubated with RBC supernatant also stretched 25% using a cell stretcher.
After 24 hours, the production of IL-8 and IL-6 was measured.
Both fresh and stored supernatants that were derived from apheresis significantly increased the production of IL-6 and IL-8 in pulmonary cells, compared with nonincubated controls and most of the other RBC products. The production of IL-6 and IL-8 was exacerbated by cell stretching.
Average IL-6 production in nonstretched cells was 91 pg/mL for fresh and 87 pg/mL for expired (P less than .05 vs. control and other RBC products). For stretched cells, it was 130 pg/mL and 150 pg/mL (P less than .05 vs. control). For controls, mean nonstretched and stretched production was 21 pg/mL and 85 pg/mL.
Mean IL-8 production in nonstretched cells was 2,100 pg/mL for fresh and 1,900 pg/mL for stored (P less than .05 vs. control and other RBC products). For stretched cells, the means were 4,100 pg/mL for fresh and 5,200 pg/mL for stored (P less than .05 vs. control).
The average nonstretched and stretched control IL-8 production was 1,200 pg/mL for fresh and 4,300 pg/mL for stored.
Products derived from apheresis also demonstrated a significantly higher ability to generate thrombin, compared with other RBC products, and a significantly increased number of RBC-derived EVs, compared with filtered red cell and whole blood–derived products (P less than .05).
However, incubated stretched cells from stored whole blood–filtered products had higher IL-8 production (16,000 pg/mL), compared with other products and stretched controls. The lowest mean levels of IL-6 were observed in supernatants derived from red cell–filtered products (nonstretched fresh and expired, 12 pg/mL and 8 pg/mL; stretched, 40 pg/mL and 36 pg/mL) and they did not appear to activate pulmonary cells. Levels of EVs were also low, compared with other blood products.
“We can conclude that manufacturing methods contribute to the differences in inducing lung injury, and especially the apheresis-derived products, which induced the most consistent injury in our model,” Dr. Wirtz said. “The red cell–filtered products appeared to be the safest.”
Dr. Wirtz had no disclosures.
SAN DIEGO – , according to research presented at the annual meeting of the American Association of Blood Banks.
Compared with other RBC products, those derived from apheresis significantly increased pulmonary cell interleukin (IL)–6 and IL-8 production, and this was further exacerbated by cell stretching. Conversely, red cell–filtered products appeared to be the least likely to cause cell injury.
“Several studies have shown that red blood cell transfusion is associated with acute lung injury, and transfusion induces leakage in ICU patients,” said lead study author Mathijs Wirtz, MD, of the Academic Medical Center, Amsterdam.
ICU patients who did not receive any transfusions had significantly lower leakage than those who were transfused. “There also seems to be a synergy between transfusion and mechanical ventilation,” Dr. Wirtz said.
Studies have also shown that there are differences in the prevalence of transfusion-related acute lung injury, when comparing Europe to the United States. Storage and manufacturing methods do differ between Europe and the United States, Dr. Wirtz noted. “This led to our hypothesis that lung injury inflicted by red blood cell transfusion is influenced by manufacturing methods.”
In this study, Dr. Wirtz and his colleagues investigated the response of pulmonary cells to the different methods of manufacturing RBC products. Using type A or B blood obtained from eight donors, a variety of RBC products were manufactured for the study, including whole-blood filtered, red-cell filtered, apheresis derived, and whole-blood derived.
For measuring thrombin generation and analyzing extracellular vesicles (EV), supernatants were prepared after 4-5 days of storage for fresh and 41-42 days for stored. The researchers selected A549 type II alveolar cells to seed onto flexible membranes, which were then incubated with RBC supernatant also stretched 25% using a cell stretcher.
After 24 hours, the production of IL-8 and IL-6 was measured.
Both fresh and stored supernatants that were derived from apheresis significantly increased the production of IL-6 and IL-8 in pulmonary cells, compared with nonincubated controls and most of the other RBC products. The production of IL-6 and IL-8 was exacerbated by cell stretching.
Average IL-6 production in nonstretched cells was 91 pg/mL for fresh and 87 pg/mL for expired (P less than .05 vs. control and other RBC products). For stretched cells, it was 130 pg/mL and 150 pg/mL (P less than .05 vs. control). For controls, mean nonstretched and stretched production was 21 pg/mL and 85 pg/mL.
Mean IL-8 production in nonstretched cells was 2,100 pg/mL for fresh and 1,900 pg/mL for stored (P less than .05 vs. control and other RBC products). For stretched cells, the means were 4,100 pg/mL for fresh and 5,200 pg/mL for stored (P less than .05 vs. control).
The average nonstretched and stretched control IL-8 production was 1,200 pg/mL for fresh and 4,300 pg/mL for stored.
Products derived from apheresis also demonstrated a significantly higher ability to generate thrombin, compared with other RBC products, and a significantly increased number of RBC-derived EVs, compared with filtered red cell and whole blood–derived products (P less than .05).
However, incubated stretched cells from stored whole blood–filtered products had higher IL-8 production (16,000 pg/mL), compared with other products and stretched controls. The lowest mean levels of IL-6 were observed in supernatants derived from red cell–filtered products (nonstretched fresh and expired, 12 pg/mL and 8 pg/mL; stretched, 40 pg/mL and 36 pg/mL) and they did not appear to activate pulmonary cells. Levels of EVs were also low, compared with other blood products.
“We can conclude that manufacturing methods contribute to the differences in inducing lung injury, and especially the apheresis-derived products, which induced the most consistent injury in our model,” Dr. Wirtz said. “The red cell–filtered products appeared to be the safest.”
Dr. Wirtz had no disclosures.
SAN DIEGO – , according to research presented at the annual meeting of the American Association of Blood Banks.
Compared with other RBC products, those derived from apheresis significantly increased pulmonary cell interleukin (IL)–6 and IL-8 production, and this was further exacerbated by cell stretching. Conversely, red cell–filtered products appeared to be the least likely to cause cell injury.
“Several studies have shown that red blood cell transfusion is associated with acute lung injury, and transfusion induces leakage in ICU patients,” said lead study author Mathijs Wirtz, MD, of the Academic Medical Center, Amsterdam.
ICU patients who did not receive any transfusions had significantly lower leakage than those who were transfused. “There also seems to be a synergy between transfusion and mechanical ventilation,” Dr. Wirtz said.
Studies have also shown that there are differences in the prevalence of transfusion-related acute lung injury, when comparing Europe to the United States. Storage and manufacturing methods do differ between Europe and the United States, Dr. Wirtz noted. “This led to our hypothesis that lung injury inflicted by red blood cell transfusion is influenced by manufacturing methods.”
In this study, Dr. Wirtz and his colleagues investigated the response of pulmonary cells to the different methods of manufacturing RBC products. Using type A or B blood obtained from eight donors, a variety of RBC products were manufactured for the study, including whole-blood filtered, red-cell filtered, apheresis derived, and whole-blood derived.
For measuring thrombin generation and analyzing extracellular vesicles (EV), supernatants were prepared after 4-5 days of storage for fresh and 41-42 days for stored. The researchers selected A549 type II alveolar cells to seed onto flexible membranes, which were then incubated with RBC supernatant also stretched 25% using a cell stretcher.
After 24 hours, the production of IL-8 and IL-6 was measured.
Both fresh and stored supernatants that were derived from apheresis significantly increased the production of IL-6 and IL-8 in pulmonary cells, compared with nonincubated controls and most of the other RBC products. The production of IL-6 and IL-8 was exacerbated by cell stretching.
Average IL-6 production in nonstretched cells was 91 pg/mL for fresh and 87 pg/mL for expired (P less than .05 vs. control and other RBC products). For stretched cells, it was 130 pg/mL and 150 pg/mL (P less than .05 vs. control). For controls, mean nonstretched and stretched production was 21 pg/mL and 85 pg/mL.
Mean IL-8 production in nonstretched cells was 2,100 pg/mL for fresh and 1,900 pg/mL for stored (P less than .05 vs. control and other RBC products). For stretched cells, the means were 4,100 pg/mL for fresh and 5,200 pg/mL for stored (P less than .05 vs. control).
The average nonstretched and stretched control IL-8 production was 1,200 pg/mL for fresh and 4,300 pg/mL for stored.
Products derived from apheresis also demonstrated a significantly higher ability to generate thrombin, compared with other RBC products, and a significantly increased number of RBC-derived EVs, compared with filtered red cell and whole blood–derived products (P less than .05).
However, incubated stretched cells from stored whole blood–filtered products had higher IL-8 production (16,000 pg/mL), compared with other products and stretched controls. The lowest mean levels of IL-6 were observed in supernatants derived from red cell–filtered products (nonstretched fresh and expired, 12 pg/mL and 8 pg/mL; stretched, 40 pg/mL and 36 pg/mL) and they did not appear to activate pulmonary cells. Levels of EVs were also low, compared with other blood products.
“We can conclude that manufacturing methods contribute to the differences in inducing lung injury, and especially the apheresis-derived products, which induced the most consistent injury in our model,” Dr. Wirtz said. “The red cell–filtered products appeared to be the safest.”
Dr. Wirtz had no disclosures.
From AABB17
Key clinical point: The method of manufacturing blood products can markedly influence the interaction of RBC products with lung cells, especially in patients on mechanical ventilation.
Major finding: Apheresis-derived products are the most consistent in causing injuries, while red cell–filtered products appear to be the safest in avoiding lung injury.
Data source: An experimental study that investigated different manufacturing methods of RBC products and the response of pulmonary cells in an in vitro model of mechanical ventilation.
Disclosures: Dr. Wirtz had no disclosures.
MMR deficiency testing remains low in colorectal cancer patients
Overall utilization of mismatch repair (MMR) deficiency testing is poor among patients with colorectal cancer, and utilization also remains low among young adults despite national guidelines calling for universal testing, according to an analysis of cases from the National Cancer Database.
The findings suggest that interventions that target groups at risk for nonadherence to guidelines may be warranted, wrote Talha Shaikh, MD, and colleagues at Fox Chase Cancer Center, Philadelphia. The report was published in JAMA Oncology (2017 Nov 9. doi: 10.1001/jamaoncol.2017.3580).
Of 152,993 adults with colorectal cancer (CRC) who were included in the study, only 28% underwent MMR deficiency testing, and of 17,218 aged 30-49 years, only 43% were tested. The proportion of patients tested in both groups increased between 2010 and 2012 (from about 22% to 33%, and from about 36% to 48%, respectively).
After the researchers controlled for all other covariates, factors significantly associated with being tested were higher educational level (odds ratio, 1.38), later diagnosis year (OR, 1.81), early-stage disease (OR, 1.24), and number of regional lymph nodes examined (OR, 1.44 for 12 or more lymph nodes). Factors associated with underuse of testing were older age (OR, 0.31), insurance status (Medicare, Medicaid, uninsured; ORs, 0.89, 0.83, and 0.78, respectively), research facility type (nonacademic vs. academic; OR, 0.44), rectosigmoid or rectal tumor location (OR, 0.76), unknown grade (OR, 0.61), and nonreceipt of definitive surgery (OR, 0.33).
MMR deficiency occurs in up to 15% of sporadic CRC and is a feature of Lynch syndrome, which occurs most often in patients under age 50 years. National guidelines have long recommended routine MMR deficiency testing for CRC patients in that age group, and universal testing has been recommended since 2014.
“Although the proportions of patients tested increased during the study period, our results suggest that underutilization of MMR deficiency testing was significant and pervasive, even among young patients with CRC with a well-established risk of Lynch syndrome. Our study ... identifies significant groups at risk for potential nonadherence to newly implemented universal testing guidelines moving forward,” the investigators said, noting that the associations between type and utilization of patient testing and socioeconomic status, insurance status, and cancer program location are of particular concern.
Ongoing analyses to track progress toward “closing this important clinical service gap,” will be needed, they concluded.
This study was funded by a grant from the National Institutes of Health, National Cancer Institute. The authors reported having no conflicts of interest.
The findings by Shaikh et al. are sobering, given the overwhelming published evidence regarding the importance of MMR deficiency testing, and they underscore a need to determine the causes of the low testing rates, according to Stanley R. Hamilton, MD.
Importantly, they also highlight areas that are “potentially actionable.” For example, the higher frequency of testing among those with higher educational levels, and underuse of testing in older patients and those from nonacademic facilities suggest that better education of physicians and patients about the value of testing could improve adherence to guidelines, Dr. Hamilton wrote in an editorial (JAMA Oncol. 2017 Nov 9. doi: 10.1001/jamaoncol.2017.3574).
Further, the lower frequency of testing among certain demographic groups suggests a need to address underserved and underresourced patient populations, he said, concluding that efforts must continue to meet the goal of universal testing and that those efforts must be accompanied by studies to evaluate the clinical utility of testing in reducing CRC mortality.
Dr. Hamilton is with the University of Texas MD Anderson Cancer Center, Houston. He is a member of the Fred Hutchinson Cancer Research Scientific Advisory Committee, a consultant for LOXO Oncology, and a member of the HalioDx Scientific Advisory Committee. He has a financial relationship with The Johns Hopkins University School of Medicine and with Merck.
The findings by Shaikh et al. are sobering, given the overwhelming published evidence regarding the importance of MMR deficiency testing, and they underscore a need to determine the causes of the low testing rates, according to Stanley R. Hamilton, MD.
Importantly, they also highlight areas that are “potentially actionable.” For example, the higher frequency of testing among those with higher educational levels, and underuse of testing in older patients and those from nonacademic facilities suggest that better education of physicians and patients about the value of testing could improve adherence to guidelines, Dr. Hamilton wrote in an editorial (JAMA Oncol. 2017 Nov 9. doi: 10.1001/jamaoncol.2017.3574).
Further, the lower frequency of testing among certain demographic groups suggests a need to address underserved and underresourced patient populations, he said, concluding that efforts must continue to meet the goal of universal testing and that those efforts must be accompanied by studies to evaluate the clinical utility of testing in reducing CRC mortality.
Dr. Hamilton is with the University of Texas MD Anderson Cancer Center, Houston. He is a member of the Fred Hutchinson Cancer Research Scientific Advisory Committee, a consultant for LOXO Oncology, and a member of the HalioDx Scientific Advisory Committee. He has a financial relationship with The Johns Hopkins University School of Medicine and with Merck.
The findings by Shaikh et al. are sobering, given the overwhelming published evidence regarding the importance of MMR deficiency testing, and they underscore a need to determine the causes of the low testing rates, according to Stanley R. Hamilton, MD.
Importantly, they also highlight areas that are “potentially actionable.” For example, the higher frequency of testing among those with higher educational levels, and underuse of testing in older patients and those from nonacademic facilities suggest that better education of physicians and patients about the value of testing could improve adherence to guidelines, Dr. Hamilton wrote in an editorial (JAMA Oncol. 2017 Nov 9. doi: 10.1001/jamaoncol.2017.3574).
Further, the lower frequency of testing among certain demographic groups suggests a need to address underserved and underresourced patient populations, he said, concluding that efforts must continue to meet the goal of universal testing and that those efforts must be accompanied by studies to evaluate the clinical utility of testing in reducing CRC mortality.
Dr. Hamilton is with the University of Texas MD Anderson Cancer Center, Houston. He is a member of the Fred Hutchinson Cancer Research Scientific Advisory Committee, a consultant for LOXO Oncology, and a member of the HalioDx Scientific Advisory Committee. He has a financial relationship with The Johns Hopkins University School of Medicine and with Merck.
Overall utilization of mismatch repair (MMR) deficiency testing is poor among patients with colorectal cancer, and utilization also remains low among young adults despite national guidelines calling for universal testing, according to an analysis of cases from the National Cancer Database.
The findings suggest that interventions that target groups at risk for nonadherence to guidelines may be warranted, wrote Talha Shaikh, MD, and colleagues at Fox Chase Cancer Center, Philadelphia. The report was published in JAMA Oncology (2017 Nov 9. doi: 10.1001/jamaoncol.2017.3580).
Of 152,993 adults with colorectal cancer (CRC) who were included in the study, only 28% underwent MMR deficiency testing, and of 17,218 aged 30-49 years, only 43% were tested. The proportion of patients tested in both groups increased between 2010 and 2012 (from about 22% to 33%, and from about 36% to 48%, respectively).
After the researchers controlled for all other covariates, factors significantly associated with being tested were higher educational level (odds ratio, 1.38), later diagnosis year (OR, 1.81), early-stage disease (OR, 1.24), and number of regional lymph nodes examined (OR, 1.44 for 12 or more lymph nodes). Factors associated with underuse of testing were older age (OR, 0.31), insurance status (Medicare, Medicaid, uninsured; ORs, 0.89, 0.83, and 0.78, respectively), research facility type (nonacademic vs. academic; OR, 0.44), rectosigmoid or rectal tumor location (OR, 0.76), unknown grade (OR, 0.61), and nonreceipt of definitive surgery (OR, 0.33).
MMR deficiency occurs in up to 15% of sporadic CRC and is a feature of Lynch syndrome, which occurs most often in patients under age 50 years. National guidelines have long recommended routine MMR deficiency testing for CRC patients in that age group, and universal testing has been recommended since 2014.
“Although the proportions of patients tested increased during the study period, our results suggest that underutilization of MMR deficiency testing was significant and pervasive, even among young patients with CRC with a well-established risk of Lynch syndrome. Our study ... identifies significant groups at risk for potential nonadherence to newly implemented universal testing guidelines moving forward,” the investigators said, noting that the associations between type and utilization of patient testing and socioeconomic status, insurance status, and cancer program location are of particular concern.
Ongoing analyses to track progress toward “closing this important clinical service gap,” will be needed, they concluded.
This study was funded by a grant from the National Institutes of Health, National Cancer Institute. The authors reported having no conflicts of interest.
Overall utilization of mismatch repair (MMR) deficiency testing is poor among patients with colorectal cancer, and utilization also remains low among young adults despite national guidelines calling for universal testing, according to an analysis of cases from the National Cancer Database.
The findings suggest that interventions that target groups at risk for nonadherence to guidelines may be warranted, wrote Talha Shaikh, MD, and colleagues at Fox Chase Cancer Center, Philadelphia. The report was published in JAMA Oncology (2017 Nov 9. doi: 10.1001/jamaoncol.2017.3580).
Of 152,993 adults with colorectal cancer (CRC) who were included in the study, only 28% underwent MMR deficiency testing, and of 17,218 aged 30-49 years, only 43% were tested. The proportion of patients tested in both groups increased between 2010 and 2012 (from about 22% to 33%, and from about 36% to 48%, respectively).
After the researchers controlled for all other covariates, factors significantly associated with being tested were higher educational level (odds ratio, 1.38), later diagnosis year (OR, 1.81), early-stage disease (OR, 1.24), and number of regional lymph nodes examined (OR, 1.44 for 12 or more lymph nodes). Factors associated with underuse of testing were older age (OR, 0.31), insurance status (Medicare, Medicaid, uninsured; ORs, 0.89, 0.83, and 0.78, respectively), research facility type (nonacademic vs. academic; OR, 0.44), rectosigmoid or rectal tumor location (OR, 0.76), unknown grade (OR, 0.61), and nonreceipt of definitive surgery (OR, 0.33).
MMR deficiency occurs in up to 15% of sporadic CRC and is a feature of Lynch syndrome, which occurs most often in patients under age 50 years. National guidelines have long recommended routine MMR deficiency testing for CRC patients in that age group, and universal testing has been recommended since 2014.
“Although the proportions of patients tested increased during the study period, our results suggest that underutilization of MMR deficiency testing was significant and pervasive, even among young patients with CRC with a well-established risk of Lynch syndrome. Our study ... identifies significant groups at risk for potential nonadherence to newly implemented universal testing guidelines moving forward,” the investigators said, noting that the associations between type and utilization of patient testing and socioeconomic status, insurance status, and cancer program location are of particular concern.
Ongoing analyses to track progress toward “closing this important clinical service gap,” will be needed, they concluded.
This study was funded by a grant from the National Institutes of Health, National Cancer Institute. The authors reported having no conflicts of interest.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Only 28% of patients overall, and only 43% of younger patients, underwent MMR deficiency testing.
Data source: 152,993 cases from the National Cancer Database.
Disclosures: This study was funded by a grant from the National Institutes of Health, National Cancer Institute. The authors reported having no conflicts of interest.
TP53 mutations could help stratify MCL patients
TP53 mutations identified a phenotypically distinct and aggressive form of mantle cell lymphoma (MCL) that did not respond to standard-of-care treatments, according to results from 183 patients younger than 66 years from the Nordic MCL2 and MCL3 trials.
, and that patients with the mutations should be considered for experimental trials of novel agents, wrote Christian W. Eskelund of the department of hematology at Rigshospitalet, Copenhagen, and colleagues.
The researchers collected DNA from the Nordic MCL2 and MCL3 trials and 183 samples were of sufficient quality for genetic analyses. They examined the prognostic value of eight recurrently mutated and two recurrently deleted genes. Only TP53 mutations showed an independent prognostic effect for overall survival (hazard ratio 6.2; P less than .0001) in multivariate Cox regression analyses.
“Our data show that TP53 mutations identify a unique MCL subtype associated with high-risk baseline characteristics, dismal response to standard treatment, and poor clinical outcome,” the researchers wrote.
Read the full study in Blood (2017 Oct 26;130[17]:1903-10).
[email protected]
On Twitter @maryellenny
TP53 mutations identified a phenotypically distinct and aggressive form of mantle cell lymphoma (MCL) that did not respond to standard-of-care treatments, according to results from 183 patients younger than 66 years from the Nordic MCL2 and MCL3 trials.
, and that patients with the mutations should be considered for experimental trials of novel agents, wrote Christian W. Eskelund of the department of hematology at Rigshospitalet, Copenhagen, and colleagues.
The researchers collected DNA from the Nordic MCL2 and MCL3 trials and 183 samples were of sufficient quality for genetic analyses. They examined the prognostic value of eight recurrently mutated and two recurrently deleted genes. Only TP53 mutations showed an independent prognostic effect for overall survival (hazard ratio 6.2; P less than .0001) in multivariate Cox regression analyses.
“Our data show that TP53 mutations identify a unique MCL subtype associated with high-risk baseline characteristics, dismal response to standard treatment, and poor clinical outcome,” the researchers wrote.
Read the full study in Blood (2017 Oct 26;130[17]:1903-10).
[email protected]
On Twitter @maryellenny
TP53 mutations identified a phenotypically distinct and aggressive form of mantle cell lymphoma (MCL) that did not respond to standard-of-care treatments, according to results from 183 patients younger than 66 years from the Nordic MCL2 and MCL3 trials.
, and that patients with the mutations should be considered for experimental trials of novel agents, wrote Christian W. Eskelund of the department of hematology at Rigshospitalet, Copenhagen, and colleagues.
The researchers collected DNA from the Nordic MCL2 and MCL3 trials and 183 samples were of sufficient quality for genetic analyses. They examined the prognostic value of eight recurrently mutated and two recurrently deleted genes. Only TP53 mutations showed an independent prognostic effect for overall survival (hazard ratio 6.2; P less than .0001) in multivariate Cox regression analyses.
“Our data show that TP53 mutations identify a unique MCL subtype associated with high-risk baseline characteristics, dismal response to standard treatment, and poor clinical outcome,” the researchers wrote.
Read the full study in Blood (2017 Oct 26;130[17]:1903-10).
[email protected]
On Twitter @maryellenny
FROM BLOOD
Concerning rise of staphylococcal scalded skin syndrome has U.S. doctors on alert
The rate of staphylococcal scalded skin syndrome (SSSS) appears to be on the rise among children in the United States, according to analysis of the Nationwide Inpatient Sample.
Alanna Staiman of Northwestern University, Chicago, and her associates evaluated 6,149,864 pediatric admissions from between 2008 and 2012 included in the Nationwide Inpatient Sample, and they identified 589 hospitalizations with a diagnosis of SSSS. They found that the SSSS annual incidence rate among U.S. children was 7.67 cases per million (Br J Dermatol. 2017 Oct 27. doi: 10.1111/bjd.16097). The estimated annual incidence rate was higher among children younger than 2 years of age at 45.1 cases per million, with a rate of 20.9 cases per million in children aged 1 year.
There were several factors associated with SSSS, including the state of residence, time of year, and sex of the patient. In particular, patients in Midwestern and Southern states experienced the highest rates of SSSS. The times of year associated with the highest rates of SSSS were summer and autumn. Female children also experienced higher rates of SSSS than their male counterparts. Conversely, those who were of certain racial backgrounds and had a certain socioeconomic status had lower rates of SSSS, including patients who were black, whose families were in the second quartile of household incomes, whose families had public insurance, and those who had more chronic conditions.
The cost and length of stay (LOS) for SSSS was not insignificant; the investigators noted that these were more pronounced among black patients possibly because darker skin pigments might mask erythema and therefore delay diagnosis. Patients with SSSS can expect to have greater LOS than those without (3.2 vs. 2.4 days, respectively) and incur higher hospital costs ($4,624 vs. $1,872).
“The adjusted in-hospital mortality of SSSS was low (0.33%)” in this study, said Ms. Staiman and her associates. They added that this was consistent with findings in other studies.
There were several comorbidities frequently associated with SSSS. These included skin infections, cellulitis, pharyngitis, upper respiratory tract infection, and other respiratory infections. Patients diagnosed with SSSS also were likely to have a fungal or viral infection.
“SSSS poses a significant health care burden, with increased LOS and costs of care per hospitalization and increasing prevalence over the 2008-2012 study period” the investigators wrote. Future work must be done to further understand how to reduce SSSS, they added.
The researchers had no conflicts of interest. Funding for the study was provided by a grant from the Agency for Healthcare Research and Quality and from the Dermatology Foundation.
The rate of staphylococcal scalded skin syndrome (SSSS) appears to be on the rise among children in the United States, according to analysis of the Nationwide Inpatient Sample.
Alanna Staiman of Northwestern University, Chicago, and her associates evaluated 6,149,864 pediatric admissions from between 2008 and 2012 included in the Nationwide Inpatient Sample, and they identified 589 hospitalizations with a diagnosis of SSSS. They found that the SSSS annual incidence rate among U.S. children was 7.67 cases per million (Br J Dermatol. 2017 Oct 27. doi: 10.1111/bjd.16097). The estimated annual incidence rate was higher among children younger than 2 years of age at 45.1 cases per million, with a rate of 20.9 cases per million in children aged 1 year.
There were several factors associated with SSSS, including the state of residence, time of year, and sex of the patient. In particular, patients in Midwestern and Southern states experienced the highest rates of SSSS. The times of year associated with the highest rates of SSSS were summer and autumn. Female children also experienced higher rates of SSSS than their male counterparts. Conversely, those who were of certain racial backgrounds and had a certain socioeconomic status had lower rates of SSSS, including patients who were black, whose families were in the second quartile of household incomes, whose families had public insurance, and those who had more chronic conditions.
The cost and length of stay (LOS) for SSSS was not insignificant; the investigators noted that these were more pronounced among black patients possibly because darker skin pigments might mask erythema and therefore delay diagnosis. Patients with SSSS can expect to have greater LOS than those without (3.2 vs. 2.4 days, respectively) and incur higher hospital costs ($4,624 vs. $1,872).
“The adjusted in-hospital mortality of SSSS was low (0.33%)” in this study, said Ms. Staiman and her associates. They added that this was consistent with findings in other studies.
There were several comorbidities frequently associated with SSSS. These included skin infections, cellulitis, pharyngitis, upper respiratory tract infection, and other respiratory infections. Patients diagnosed with SSSS also were likely to have a fungal or viral infection.
“SSSS poses a significant health care burden, with increased LOS and costs of care per hospitalization and increasing prevalence over the 2008-2012 study period” the investigators wrote. Future work must be done to further understand how to reduce SSSS, they added.
The researchers had no conflicts of interest. Funding for the study was provided by a grant from the Agency for Healthcare Research and Quality and from the Dermatology Foundation.
The rate of staphylococcal scalded skin syndrome (SSSS) appears to be on the rise among children in the United States, according to analysis of the Nationwide Inpatient Sample.
Alanna Staiman of Northwestern University, Chicago, and her associates evaluated 6,149,864 pediatric admissions from between 2008 and 2012 included in the Nationwide Inpatient Sample, and they identified 589 hospitalizations with a diagnosis of SSSS. They found that the SSSS annual incidence rate among U.S. children was 7.67 cases per million (Br J Dermatol. 2017 Oct 27. doi: 10.1111/bjd.16097). The estimated annual incidence rate was higher among children younger than 2 years of age at 45.1 cases per million, with a rate of 20.9 cases per million in children aged 1 year.
There were several factors associated with SSSS, including the state of residence, time of year, and sex of the patient. In particular, patients in Midwestern and Southern states experienced the highest rates of SSSS. The times of year associated with the highest rates of SSSS were summer and autumn. Female children also experienced higher rates of SSSS than their male counterparts. Conversely, those who were of certain racial backgrounds and had a certain socioeconomic status had lower rates of SSSS, including patients who were black, whose families were in the second quartile of household incomes, whose families had public insurance, and those who had more chronic conditions.
The cost and length of stay (LOS) for SSSS was not insignificant; the investigators noted that these were more pronounced among black patients possibly because darker skin pigments might mask erythema and therefore delay diagnosis. Patients with SSSS can expect to have greater LOS than those without (3.2 vs. 2.4 days, respectively) and incur higher hospital costs ($4,624 vs. $1,872).
“The adjusted in-hospital mortality of SSSS was low (0.33%)” in this study, said Ms. Staiman and her associates. They added that this was consistent with findings in other studies.
There were several comorbidities frequently associated with SSSS. These included skin infections, cellulitis, pharyngitis, upper respiratory tract infection, and other respiratory infections. Patients diagnosed with SSSS also were likely to have a fungal or viral infection.
“SSSS poses a significant health care burden, with increased LOS and costs of care per hospitalization and increasing prevalence over the 2008-2012 study period” the investigators wrote. Future work must be done to further understand how to reduce SSSS, they added.
The researchers had no conflicts of interest. Funding for the study was provided by a grant from the Agency for Healthcare Research and Quality and from the Dermatology Foundation.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point:
Major finding: The annual incidence rate of SSSS was 7.67 per million U.S. children.
Data source: Analysis of the U.S. Nationwide Inpatient Sample of 6,149,864 pediatric admissions from 2008 to 2012, including 589 cases of SSSS.
Disclosures: The researchers had no conflicts of interest. Funding for the study was provided by a grant from the Agency for Healthcare Research and Quality and from the Dermatology Foundation.