User login
Seven days of opioids adequate for most hernia and other general surgery procedures
A 7-day limit on the initial opioid prescription may be sufficient for many common general surgery procedures, including hernia surgery and gynecologic procedures, findings of a large retrospective study suggest.
Rebecca E. Scully, MD, of the Center for Surgery and Public Health at Brigham and Women’s Hospital in Boston, and her associates examined opioid pain medication prescriptions and refills from records of the Military Health System Data Repository and the TRICARE insurance program of 215,140 opioid-naive patients. These patients were aged 18-64 years who underwent either cholecystectomy, appendectomy, inguinal hernia repair, anterior cruciate ligament reconstruction, rotator cuff tear repair, discectomy, mastectomy, or hysterectomy (JAMA Surg. 2017. doi: 10.1001/jamasurg.2017.3132). Only 20% of the covered individuals are active members of the U.S. military. The mean age was 40 years; 50% were male, and 60% were white.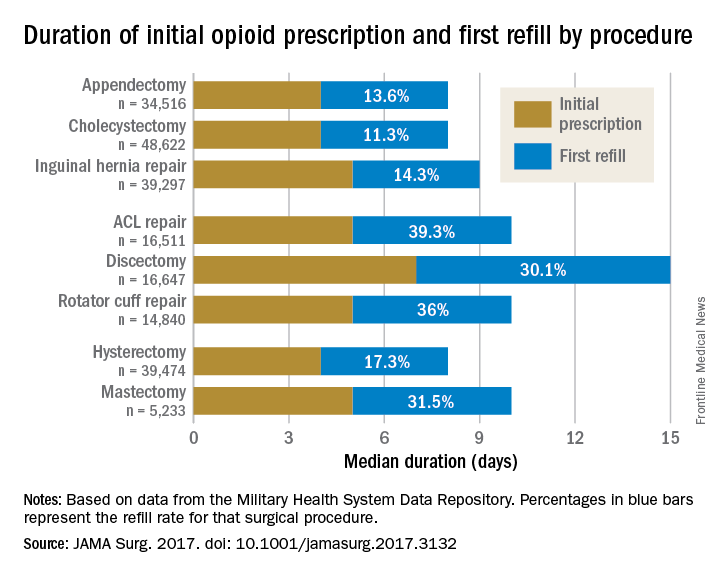
For appendectomy, cholecystectomy, and hysterectomy, the prescription was a median 4 days. For inguinal hernia repair, anterior cruciate ligament repair, rotator cuff repair, and mastectomy, the initial prescription was for 5 days. For discectomy, the median was 7 days.
Refill rates were the least at 11.3% for cholecystectomy and the most at 39.3% after anterior cruciate ligament repair. The time after the initial prescription until a refill was a median 6 days for appendectomy, cholecystectomy, and inguinal hernia repair, compared with a median 10 days for discectomy. The median duration of a refill prescription was 4 days for appendectomy, cholecystectomy, hernia repair, and hysterectomy versus 8 days for discectomy.
“Although 7 days appears to be more than adequate for many patients undergoing common general surgery and gynecologic procedures, prescription lengths likely should be extended to 10 days, particularly after common neurosurgical and musculoskeletal procedures, recognizing that as many as 40% of patients may still require one refill at a 7-day limit,” Dr. Scully and her associates said.
Although this study did not include rates of unused prescriptions or use of nonopioid pain relievers such as acetaminophen or NSAIDs, it did include a large population considered to be nationally representative “in many respects,” and it included a variety of procedures for which patients are commonly discharged to home, the researchers said.
The study was funded in part by the Department of Defense/Henry M. Jackson Foundation. The investigators had no conflict of interests. Adil H. Haider, MD, MPH, is deputy editor of JAMA Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
A 7-day limit on the initial opioid prescription may be sufficient for many common general surgery procedures, including hernia surgery and gynecologic procedures, findings of a large retrospective study suggest.
Rebecca E. Scully, MD, of the Center for Surgery and Public Health at Brigham and Women’s Hospital in Boston, and her associates examined opioid pain medication prescriptions and refills from records of the Military Health System Data Repository and the TRICARE insurance program of 215,140 opioid-naive patients. These patients were aged 18-64 years who underwent either cholecystectomy, appendectomy, inguinal hernia repair, anterior cruciate ligament reconstruction, rotator cuff tear repair, discectomy, mastectomy, or hysterectomy (JAMA Surg. 2017. doi: 10.1001/jamasurg.2017.3132). Only 20% of the covered individuals are active members of the U.S. military. The mean age was 40 years; 50% were male, and 60% were white.
For appendectomy, cholecystectomy, and hysterectomy, the prescription was a median 4 days. For inguinal hernia repair, anterior cruciate ligament repair, rotator cuff repair, and mastectomy, the initial prescription was for 5 days. For discectomy, the median was 7 days.
Refill rates were the least at 11.3% for cholecystectomy and the most at 39.3% after anterior cruciate ligament repair. The time after the initial prescription until a refill was a median 6 days for appendectomy, cholecystectomy, and inguinal hernia repair, compared with a median 10 days for discectomy. The median duration of a refill prescription was 4 days for appendectomy, cholecystectomy, hernia repair, and hysterectomy versus 8 days for discectomy.
“Although 7 days appears to be more than adequate for many patients undergoing common general surgery and gynecologic procedures, prescription lengths likely should be extended to 10 days, particularly after common neurosurgical and musculoskeletal procedures, recognizing that as many as 40% of patients may still require one refill at a 7-day limit,” Dr. Scully and her associates said.
Although this study did not include rates of unused prescriptions or use of nonopioid pain relievers such as acetaminophen or NSAIDs, it did include a large population considered to be nationally representative “in many respects,” and it included a variety of procedures for which patients are commonly discharged to home, the researchers said.
The study was funded in part by the Department of Defense/Henry M. Jackson Foundation. The investigators had no conflict of interests. Adil H. Haider, MD, MPH, is deputy editor of JAMA Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
A 7-day limit on the initial opioid prescription may be sufficient for many common general surgery procedures, including hernia surgery and gynecologic procedures, findings of a large retrospective study suggest.
Rebecca E. Scully, MD, of the Center for Surgery and Public Health at Brigham and Women’s Hospital in Boston, and her associates examined opioid pain medication prescriptions and refills from records of the Military Health System Data Repository and the TRICARE insurance program of 215,140 opioid-naive patients. These patients were aged 18-64 years who underwent either cholecystectomy, appendectomy, inguinal hernia repair, anterior cruciate ligament reconstruction, rotator cuff tear repair, discectomy, mastectomy, or hysterectomy (JAMA Surg. 2017. doi: 10.1001/jamasurg.2017.3132). Only 20% of the covered individuals are active members of the U.S. military. The mean age was 40 years; 50% were male, and 60% were white.
For appendectomy, cholecystectomy, and hysterectomy, the prescription was a median 4 days. For inguinal hernia repair, anterior cruciate ligament repair, rotator cuff repair, and mastectomy, the initial prescription was for 5 days. For discectomy, the median was 7 days.
Refill rates were the least at 11.3% for cholecystectomy and the most at 39.3% after anterior cruciate ligament repair. The time after the initial prescription until a refill was a median 6 days for appendectomy, cholecystectomy, and inguinal hernia repair, compared with a median 10 days for discectomy. The median duration of a refill prescription was 4 days for appendectomy, cholecystectomy, hernia repair, and hysterectomy versus 8 days for discectomy.
“Although 7 days appears to be more than adequate for many patients undergoing common general surgery and gynecologic procedures, prescription lengths likely should be extended to 10 days, particularly after common neurosurgical and musculoskeletal procedures, recognizing that as many as 40% of patients may still require one refill at a 7-day limit,” Dr. Scully and her associates said.
Although this study did not include rates of unused prescriptions or use of nonopioid pain relievers such as acetaminophen or NSAIDs, it did include a large population considered to be nationally representative “in many respects,” and it included a variety of procedures for which patients are commonly discharged to home, the researchers said.
The study was funded in part by the Department of Defense/Henry M. Jackson Foundation. The investigators had no conflict of interests. Adil H. Haider, MD, MPH, is deputy editor of JAMA Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
FROM JAMA SURGERY
Key clinical point:
Major finding: The initial opioid prescription was a median 4 days for appendectomy and cholecystectomy, a median 5 days for inguinal hernia repair and anterior cruciate ligament and rotator cuff repair, and a median 7 days for discectomy.
Data source: A study of opioid prescriptions in 215,140 surgery patients aged 18-64 years.
Disclosures: The study was funded in part by the Department of Defense/Henry M. Jackson Foundation. The investigators had no conflict of interests. Adil H. Haider, MD, MPH, is deputy editor of JAMA Surgery, but he was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Clinical hepatology debrief wraps up 2017 Liver Meeting
WASHINGTON – Research into alcoholic liver disease, drug-induced liver injury, the complications of chronic liver disease, and cholestatic liver diseases were among the clinical hepatology highlights presented at the annual meeting of the American Association for the Study of Liver Diseases.
This year’s debrief was given by Kris V. Kowdley, MD, of Swedish Medical Center in Seattle.
Over a median of 1.6 years of follow-up, 27% of patients resumed alcohol consumption post transplant with a median time to alcohol of 160 days, according to Brian Lee, MD, of the University of California, San Francisco. Younger age and lack of complete acceptance of their alcoholic hepatitis diagnosis were significant predictors of alcohol use post transplant while factors such as length of abstinence, race/ethnicity, insurance status, history of illicit drug use, and history of failed rehab attempts were not. Further, heavy drinking at presentation (more than 10 drinks per day), any alcohol use post transplant, and sustained alcohol use post transplant were significant predictors of posttransplant death.
Alcoholic hepatitis now “appears to be affecting more and more younger women, who present with a higher level of acuity,” Dr. Kowdley noted. He added that, while recent advances have decreased the absolute number of hepatitis C patients with decompensated liver disease who are listed for liver transplant, “the number is increasing rapidly in alcoholic liver disease, approaching the rate of patients being listed for hepatitis C.”
Unknown ingredients in herbal and dietary supplements continue to be of concern, Dr. Kowdley noted, as highlighted by Victor J. Navarro, MD, of Einstein Healthcare Network, Philadelphia, and his colleagues at the Drug-Induced Liver Injury Network (DILIN).
Investigators collected herbal and dietary supplements from patients enrolled in the DILIN prospective study and had chemical analysis performed by an outside laboratory. Labeled contents could not be verified in over half of the supplements collected and several unlabeled hepatotoxic ingredients were identified, Dr. Navarro and colleagues found (abstract 264).
“Even though we collect the supplements and review them with the patients, it’s not clear that we even know what it is that they are taking,” Dr. Kowdley commented.
Another DILIN study, this one presented by Jawad Ahmad, MD, of the Icahn School of Medicine at Mount Sinai, New York, “provided an opportunity for pause,” Dr. Kowdley said.
Dr. Ahmad and colleagues looked at hepatitis C virus (HCV) testing in DILIN patients and were able to correlate anti-HCV test results with HCV RNA tests results in more than 95% of 1,500 patients (abstract 16). About 7% of patients were HCV positive, and 23 cases of acute hepatitis were identified (16 with anti-HCV antibodies and HCV RNA, 7 with HCV RNA alone, and none with anti-HCV antibodies alone).
“So the take-home message here for me is, even if we think the patient has drug-induced liver injury, if they have not been tested for hepatitis C, especially if in the hospitalized setting … it is important to check not only the antibody test but also the RNA test,” Dr. Kowdley said.
Finally, in children, minocycline and valproate were the most commonly indicated agents in pediatric drug-induced liver injury, according to Frank DiPaola, MD, of the University of Michigan, and colleagues, on behalf of DILIN (abstract 13).
Dr. Kowdley also highlighted a couple of studies that addressed the complications of chronic liver disease.
The ADAPT-1 and ADAPT-2 trials (abstract 217) studied the use of avatrombopag, a thrombopoietin (TPO)–receptor agonist, to reduce severe thrombocytopenia in patients with chronic liver disease. Platelet transfusion is the current standard of care to reduce the risk of bleeding during invasive procedures in these patients; currently there are no drugs approved for this indication, Dr. Kowdley said.
Avatrombopag is an oral, small molecule TPO-receptor agonist, he said. “Because it binds to a different site on the TPO receptor than endogenous TPO, the effects are additive.”
In the phase 3 ADAPT-1 and ADAPT-2, the proportion of patients who did not require platelet transfusion or any rescue procedure for bleeding was significantly less in avatrombopag-treated patients than those receiving placebo. The effect was the same for patients with a low baseline platelet count (less than 40,000 platelets per mcL) as well as those with a high baseline platelet count (between 40,000/mcL and 50,000/mcL). Further, the proportion of patients who by procedure day achieved platelet count of at least 50,000/mcL was significantly higher in patients on the study drug.
Data on lusutrombopag, another TPO-receptor agonist, was presented as a late-breaker at the meeting, with very similar results in avoiding platelet transfusion, Dr. Kowdley noted.Two abstracts (502 and 219) focused on reducing ammonia levels in hospitalized cirrhosis patients with hepatic encephalitis.
Patients in the STOP-HE trial were randomized to either physician’s choice for standard of care or standard of care plus continuous infusion of ornithine phenylacetate for up to 5 days. Patients were assigned to one of three dosing groups (20 g, 15 g, or 10 g), based on severity of underlying liver disease; those with the most severe disease received the lowest dose.
Reduction in plasma ammonia levels correlated significantly with clinical improvement. At 48 hours, meaningful clinical improvement occurred in 84% of patients on ornithine phenylacetate, compared with 58% of placebo patients, according to Robert S. Rahimi, MD, of Baylor University, Dallas, and his colleagues.
“So, this may be an option for our hepatic encephalopathy patients who are admitted to the hospital and need acute treatment,” Dr. Kowdley said.
Dr. Kowdley finished up with two studies on primary biliary cholangitis (PBC).
Carla Murillo Perez, MD, of Toronto General Hospital and her colleagues in the Global PBC Study Group investigated the role of serum bilirubin in predicting transplant-free survival in patients with PBC (abstract 70).
When serum bilirubin levels from a previous study were input into a Cox regression analysis as a cubic spline function, then adjusted for factors such as age, sex, treatment with ursodeoxycholic acid, and year of diagnosis, the investigators found that patients with serum bilirubin levels of 0.7 times the upper limit of normal had a significantly increased risk of liver transplantation or death.
“We may want to be more sensitive in looking at bilirubin levels,” Dr. Kowdley said.
Another small but notable study presented by Gideon M. Hirschfield, MD, of the University of Birmingham (England), looked into whether a lower dose of seladelpar would safely and effectively lower alkaline phosphatase (AP) levels in PBC patients. A previous study of seladelpar at 50 mg and 200 mg doses indicated the drug’s effectiveness; however, the study was stopped because of the development of grade 3 alanine aminotransferase increases in a number of patients (Lancet Gastroenterol Hepatol. 2017;2;716-26).
Dr. Hirschfield and colleagues enrolled 24 patients and randomized 12 to seladelpar 5 mg and another 12 to 10 mg. The study cohort was mostly female, with an average age of 58 years. Most were either intolerant of or inadequately treated by ursodeoxycholic acid. AP levels were reduced significantly over time in both groups; however, differences between the groups were not significant, the investigators noted.
The Liver Meeting will be held in San Francisco in 2018, taking place Nov. 9-13. Many investigators in these trials reported relevant conflicts of interest; information is available (open access) in a supplement to Hepatology.
[email protected]
On Twitter @denisefulton
WASHINGTON – Research into alcoholic liver disease, drug-induced liver injury, the complications of chronic liver disease, and cholestatic liver diseases were among the clinical hepatology highlights presented at the annual meeting of the American Association for the Study of Liver Diseases.
This year’s debrief was given by Kris V. Kowdley, MD, of Swedish Medical Center in Seattle.
Over a median of 1.6 years of follow-up, 27% of patients resumed alcohol consumption post transplant with a median time to alcohol of 160 days, according to Brian Lee, MD, of the University of California, San Francisco. Younger age and lack of complete acceptance of their alcoholic hepatitis diagnosis were significant predictors of alcohol use post transplant while factors such as length of abstinence, race/ethnicity, insurance status, history of illicit drug use, and history of failed rehab attempts were not. Further, heavy drinking at presentation (more than 10 drinks per day), any alcohol use post transplant, and sustained alcohol use post transplant were significant predictors of posttransplant death.
Alcoholic hepatitis now “appears to be affecting more and more younger women, who present with a higher level of acuity,” Dr. Kowdley noted. He added that, while recent advances have decreased the absolute number of hepatitis C patients with decompensated liver disease who are listed for liver transplant, “the number is increasing rapidly in alcoholic liver disease, approaching the rate of patients being listed for hepatitis C.”
Unknown ingredients in herbal and dietary supplements continue to be of concern, Dr. Kowdley noted, as highlighted by Victor J. Navarro, MD, of Einstein Healthcare Network, Philadelphia, and his colleagues at the Drug-Induced Liver Injury Network (DILIN).
Investigators collected herbal and dietary supplements from patients enrolled in the DILIN prospective study and had chemical analysis performed by an outside laboratory. Labeled contents could not be verified in over half of the supplements collected and several unlabeled hepatotoxic ingredients were identified, Dr. Navarro and colleagues found (abstract 264).
“Even though we collect the supplements and review them with the patients, it’s not clear that we even know what it is that they are taking,” Dr. Kowdley commented.
Another DILIN study, this one presented by Jawad Ahmad, MD, of the Icahn School of Medicine at Mount Sinai, New York, “provided an opportunity for pause,” Dr. Kowdley said.
Dr. Ahmad and colleagues looked at hepatitis C virus (HCV) testing in DILIN patients and were able to correlate anti-HCV test results with HCV RNA tests results in more than 95% of 1,500 patients (abstract 16). About 7% of patients were HCV positive, and 23 cases of acute hepatitis were identified (16 with anti-HCV antibodies and HCV RNA, 7 with HCV RNA alone, and none with anti-HCV antibodies alone).
“So the take-home message here for me is, even if we think the patient has drug-induced liver injury, if they have not been tested for hepatitis C, especially if in the hospitalized setting … it is important to check not only the antibody test but also the RNA test,” Dr. Kowdley said.
Finally, in children, minocycline and valproate were the most commonly indicated agents in pediatric drug-induced liver injury, according to Frank DiPaola, MD, of the University of Michigan, and colleagues, on behalf of DILIN (abstract 13).
Dr. Kowdley also highlighted a couple of studies that addressed the complications of chronic liver disease.
The ADAPT-1 and ADAPT-2 trials (abstract 217) studied the use of avatrombopag, a thrombopoietin (TPO)–receptor agonist, to reduce severe thrombocytopenia in patients with chronic liver disease. Platelet transfusion is the current standard of care to reduce the risk of bleeding during invasive procedures in these patients; currently there are no drugs approved for this indication, Dr. Kowdley said.
Avatrombopag is an oral, small molecule TPO-receptor agonist, he said. “Because it binds to a different site on the TPO receptor than endogenous TPO, the effects are additive.”
In the phase 3 ADAPT-1 and ADAPT-2, the proportion of patients who did not require platelet transfusion or any rescue procedure for bleeding was significantly less in avatrombopag-treated patients than those receiving placebo. The effect was the same for patients with a low baseline platelet count (less than 40,000 platelets per mcL) as well as those with a high baseline platelet count (between 40,000/mcL and 50,000/mcL). Further, the proportion of patients who by procedure day achieved platelet count of at least 50,000/mcL was significantly higher in patients on the study drug.
Data on lusutrombopag, another TPO-receptor agonist, was presented as a late-breaker at the meeting, with very similar results in avoiding platelet transfusion, Dr. Kowdley noted.Two abstracts (502 and 219) focused on reducing ammonia levels in hospitalized cirrhosis patients with hepatic encephalitis.
Patients in the STOP-HE trial were randomized to either physician’s choice for standard of care or standard of care plus continuous infusion of ornithine phenylacetate for up to 5 days. Patients were assigned to one of three dosing groups (20 g, 15 g, or 10 g), based on severity of underlying liver disease; those with the most severe disease received the lowest dose.
Reduction in plasma ammonia levels correlated significantly with clinical improvement. At 48 hours, meaningful clinical improvement occurred in 84% of patients on ornithine phenylacetate, compared with 58% of placebo patients, according to Robert S. Rahimi, MD, of Baylor University, Dallas, and his colleagues.
“So, this may be an option for our hepatic encephalopathy patients who are admitted to the hospital and need acute treatment,” Dr. Kowdley said.
Dr. Kowdley finished up with two studies on primary biliary cholangitis (PBC).
Carla Murillo Perez, MD, of Toronto General Hospital and her colleagues in the Global PBC Study Group investigated the role of serum bilirubin in predicting transplant-free survival in patients with PBC (abstract 70).
When serum bilirubin levels from a previous study were input into a Cox regression analysis as a cubic spline function, then adjusted for factors such as age, sex, treatment with ursodeoxycholic acid, and year of diagnosis, the investigators found that patients with serum bilirubin levels of 0.7 times the upper limit of normal had a significantly increased risk of liver transplantation or death.
“We may want to be more sensitive in looking at bilirubin levels,” Dr. Kowdley said.
Another small but notable study presented by Gideon M. Hirschfield, MD, of the University of Birmingham (England), looked into whether a lower dose of seladelpar would safely and effectively lower alkaline phosphatase (AP) levels in PBC patients. A previous study of seladelpar at 50 mg and 200 mg doses indicated the drug’s effectiveness; however, the study was stopped because of the development of grade 3 alanine aminotransferase increases in a number of patients (Lancet Gastroenterol Hepatol. 2017;2;716-26).
Dr. Hirschfield and colleagues enrolled 24 patients and randomized 12 to seladelpar 5 mg and another 12 to 10 mg. The study cohort was mostly female, with an average age of 58 years. Most were either intolerant of or inadequately treated by ursodeoxycholic acid. AP levels were reduced significantly over time in both groups; however, differences between the groups were not significant, the investigators noted.
The Liver Meeting will be held in San Francisco in 2018, taking place Nov. 9-13. Many investigators in these trials reported relevant conflicts of interest; information is available (open access) in a supplement to Hepatology.
[email protected]
On Twitter @denisefulton
WASHINGTON – Research into alcoholic liver disease, drug-induced liver injury, the complications of chronic liver disease, and cholestatic liver diseases were among the clinical hepatology highlights presented at the annual meeting of the American Association for the Study of Liver Diseases.
This year’s debrief was given by Kris V. Kowdley, MD, of Swedish Medical Center in Seattle.
Over a median of 1.6 years of follow-up, 27% of patients resumed alcohol consumption post transplant with a median time to alcohol of 160 days, according to Brian Lee, MD, of the University of California, San Francisco. Younger age and lack of complete acceptance of their alcoholic hepatitis diagnosis were significant predictors of alcohol use post transplant while factors such as length of abstinence, race/ethnicity, insurance status, history of illicit drug use, and history of failed rehab attempts were not. Further, heavy drinking at presentation (more than 10 drinks per day), any alcohol use post transplant, and sustained alcohol use post transplant were significant predictors of posttransplant death.
Alcoholic hepatitis now “appears to be affecting more and more younger women, who present with a higher level of acuity,” Dr. Kowdley noted. He added that, while recent advances have decreased the absolute number of hepatitis C patients with decompensated liver disease who are listed for liver transplant, “the number is increasing rapidly in alcoholic liver disease, approaching the rate of patients being listed for hepatitis C.”
Unknown ingredients in herbal and dietary supplements continue to be of concern, Dr. Kowdley noted, as highlighted by Victor J. Navarro, MD, of Einstein Healthcare Network, Philadelphia, and his colleagues at the Drug-Induced Liver Injury Network (DILIN).
Investigators collected herbal and dietary supplements from patients enrolled in the DILIN prospective study and had chemical analysis performed by an outside laboratory. Labeled contents could not be verified in over half of the supplements collected and several unlabeled hepatotoxic ingredients were identified, Dr. Navarro and colleagues found (abstract 264).
“Even though we collect the supplements and review them with the patients, it’s not clear that we even know what it is that they are taking,” Dr. Kowdley commented.
Another DILIN study, this one presented by Jawad Ahmad, MD, of the Icahn School of Medicine at Mount Sinai, New York, “provided an opportunity for pause,” Dr. Kowdley said.
Dr. Ahmad and colleagues looked at hepatitis C virus (HCV) testing in DILIN patients and were able to correlate anti-HCV test results with HCV RNA tests results in more than 95% of 1,500 patients (abstract 16). About 7% of patients were HCV positive, and 23 cases of acute hepatitis were identified (16 with anti-HCV antibodies and HCV RNA, 7 with HCV RNA alone, and none with anti-HCV antibodies alone).
“So the take-home message here for me is, even if we think the patient has drug-induced liver injury, if they have not been tested for hepatitis C, especially if in the hospitalized setting … it is important to check not only the antibody test but also the RNA test,” Dr. Kowdley said.
Finally, in children, minocycline and valproate were the most commonly indicated agents in pediatric drug-induced liver injury, according to Frank DiPaola, MD, of the University of Michigan, and colleagues, on behalf of DILIN (abstract 13).
Dr. Kowdley also highlighted a couple of studies that addressed the complications of chronic liver disease.
The ADAPT-1 and ADAPT-2 trials (abstract 217) studied the use of avatrombopag, a thrombopoietin (TPO)–receptor agonist, to reduce severe thrombocytopenia in patients with chronic liver disease. Platelet transfusion is the current standard of care to reduce the risk of bleeding during invasive procedures in these patients; currently there are no drugs approved for this indication, Dr. Kowdley said.
Avatrombopag is an oral, small molecule TPO-receptor agonist, he said. “Because it binds to a different site on the TPO receptor than endogenous TPO, the effects are additive.”
In the phase 3 ADAPT-1 and ADAPT-2, the proportion of patients who did not require platelet transfusion or any rescue procedure for bleeding was significantly less in avatrombopag-treated patients than those receiving placebo. The effect was the same for patients with a low baseline platelet count (less than 40,000 platelets per mcL) as well as those with a high baseline platelet count (between 40,000/mcL and 50,000/mcL). Further, the proportion of patients who by procedure day achieved platelet count of at least 50,000/mcL was significantly higher in patients on the study drug.
Data on lusutrombopag, another TPO-receptor agonist, was presented as a late-breaker at the meeting, with very similar results in avoiding platelet transfusion, Dr. Kowdley noted.Two abstracts (502 and 219) focused on reducing ammonia levels in hospitalized cirrhosis patients with hepatic encephalitis.
Patients in the STOP-HE trial were randomized to either physician’s choice for standard of care or standard of care plus continuous infusion of ornithine phenylacetate for up to 5 days. Patients were assigned to one of three dosing groups (20 g, 15 g, or 10 g), based on severity of underlying liver disease; those with the most severe disease received the lowest dose.
Reduction in plasma ammonia levels correlated significantly with clinical improvement. At 48 hours, meaningful clinical improvement occurred in 84% of patients on ornithine phenylacetate, compared with 58% of placebo patients, according to Robert S. Rahimi, MD, of Baylor University, Dallas, and his colleagues.
“So, this may be an option for our hepatic encephalopathy patients who are admitted to the hospital and need acute treatment,” Dr. Kowdley said.
Dr. Kowdley finished up with two studies on primary biliary cholangitis (PBC).
Carla Murillo Perez, MD, of Toronto General Hospital and her colleagues in the Global PBC Study Group investigated the role of serum bilirubin in predicting transplant-free survival in patients with PBC (abstract 70).
When serum bilirubin levels from a previous study were input into a Cox regression analysis as a cubic spline function, then adjusted for factors such as age, sex, treatment with ursodeoxycholic acid, and year of diagnosis, the investigators found that patients with serum bilirubin levels of 0.7 times the upper limit of normal had a significantly increased risk of liver transplantation or death.
“We may want to be more sensitive in looking at bilirubin levels,” Dr. Kowdley said.
Another small but notable study presented by Gideon M. Hirschfield, MD, of the University of Birmingham (England), looked into whether a lower dose of seladelpar would safely and effectively lower alkaline phosphatase (AP) levels in PBC patients. A previous study of seladelpar at 50 mg and 200 mg doses indicated the drug’s effectiveness; however, the study was stopped because of the development of grade 3 alanine aminotransferase increases in a number of patients (Lancet Gastroenterol Hepatol. 2017;2;716-26).
Dr. Hirschfield and colleagues enrolled 24 patients and randomized 12 to seladelpar 5 mg and another 12 to 10 mg. The study cohort was mostly female, with an average age of 58 years. Most were either intolerant of or inadequately treated by ursodeoxycholic acid. AP levels were reduced significantly over time in both groups; however, differences between the groups were not significant, the investigators noted.
The Liver Meeting will be held in San Francisco in 2018, taking place Nov. 9-13. Many investigators in these trials reported relevant conflicts of interest; information is available (open access) in a supplement to Hepatology.
[email protected]
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Legislative landscape affecting rheumatology has potential wins but many challenges
SAN DIEGO – , but ongoing efforts to advocate for the specialty and patients are showing signs of paying off in some areas, Angus Worthing, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Worthing, who is chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area, encouraged rheumatologists to become involved in advocacy efforts and asked members of the audience at the meeting to visit the ACR’s advocacy website to learn how to help.
The ACR supports a group of bills that have been introduced in either the House or Senate that should have an effect on alleviating the projected shortage of rheumatologists across the United States through 2030. These bills will help, although much of the effort to address the shortage and maldistribution of rheumatologists across the United States will “probably be solved at the local level. It’s not going to be a federal solution. It will be relationships and treatment programs between primary care and rheumatology care that are very local,” Dr. Worthing said.
The Conrad State 30 and Physician Access Reauthorization Act (H.R. 2141, S. 898) aims to streamline visas for foreign physicians to practice in underserved areas.
The Resident Physician Shortage Reduction Act of 2017 (H.R. 2267) would increase for the first time since 1997 the number of graduate medical education residency slots in the United States.
The Ensuring Children’s Access to Specialty Care Act of 2017 (S. 989) allows pediatric subspecialists, including pediatric rheumatologists, to get access to the National Health Service Corps loan repayment program when they work in underserved areas.
More recently, in spring 2017 the American Medical Association played a big role in getting the Trump administration to reverse its stance on not allowing premium processing of H1-B visas for professionals such as physicians. If this had gone into effect, all the rheumatology fellows in training who were going to be practicing – some in underserved areas – might have been forced to return to their home country because of a lack of time to get their H1-B visa processed before finishing their fellowship, Dr. Worthing said.
Affordable Care Act (ACA)
- Provide sufficient, affordable, continuous coverage that encourages access to high-quality care for all.
- Prohibit exclusions based on preexisting conditions.
- Allow children to remain on parent’s insurance until age 26 years.
- Remove excessive administrative burdens that take focus away from patient care.
- Cap annual out-of-pocket costs and ban lifetime limits.
- Have affordable premiums, deductibles, and cost sharing.
- Continue the 10 essential health benefits that are required for ACA marketplace plans.
Alliance for Transparent & Affordable Prescriptions (ATAP)
The ACR convened this alliance along with the Coalition of State Rheumatology Organizations, the Global Healthy Living Foundation, the Association of Women in Rheumatology, the Rheumatology Nurses Society, and others to try to bring transparency to how pharmacy benefit managers (PBMs) operate in getting certain drugs on the formularies of payers. The ATAP recently had some success in making lawmakers aware of the PBM’s role in influencing drug prices via rebates to drug manufacturers. At a Congressional hearing in Oct. 2017, after many visits from rheumatologists and members of ATAP, the members of the Senate Committee on Health, Education, Labor, and Pensions “held the feet of these PBMs to the fire a little bit asking them about these rebates,” Dr. Worthing said, where at one point committee chair Sen. Lamar Alexander (R-Tenn.) asked, “ ‘Do we really need these rebates?’ ”
National Institutes of Health budget
After the National Institutes of Health received a $2 billion increase in funding for fiscal year 2017, the Trump administration proposed last summer to cut the NIH budget by 22%. Since then, however, bills to increase the NIH budget by $1.1 billion from the House and by $2 billion from the Senate have made their way through committees. But a budget must be passed by Congress and then signed by the president to make a potential budget increase a reality. Otherwise, a continuing resolution would leave the current level of funding in place through fiscal year 2018, Dr. Worthing noted.
Patients’ Access to Treatments Act of 2017 (H.R. 2999)
This bill has been raised for a fourth time after not making it past committees in previous Congresses, but the prospects for it passing appear somewhat better this time around, Dr. Worthing said. It would prevent insurance companies from putting drugs in specialty tiers that require patients to pay increasingly higher rates of coinsurance for the drugs on different tiers.
“It has been gathering momentum. We hope to get it across the finish line. And if we don’t get this across, then we’ll join with the coalition that rheumatology has formed around this issue of access to specialty treatments some other way, because this is a burning issue for us and our patients,” he said.
Medicare Access to Rehabilitation Services Act of 2017 (H.R. 807 and S. 253)
This bill would repeal the annual cap that was placed on rehabilitation services for patients covered by Medicare in 1997. The bill has bipartisan, majority support and has been gaining momentum for the past 4 years, Dr. Worthing said. It was advanced from both Senate and House committees in Oct. 2017.
SAN DIEGO – , but ongoing efforts to advocate for the specialty and patients are showing signs of paying off in some areas, Angus Worthing, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Worthing, who is chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area, encouraged rheumatologists to become involved in advocacy efforts and asked members of the audience at the meeting to visit the ACR’s advocacy website to learn how to help.
The ACR supports a group of bills that have been introduced in either the House or Senate that should have an effect on alleviating the projected shortage of rheumatologists across the United States through 2030. These bills will help, although much of the effort to address the shortage and maldistribution of rheumatologists across the United States will “probably be solved at the local level. It’s not going to be a federal solution. It will be relationships and treatment programs between primary care and rheumatology care that are very local,” Dr. Worthing said.
The Conrad State 30 and Physician Access Reauthorization Act (H.R. 2141, S. 898) aims to streamline visas for foreign physicians to practice in underserved areas.
The Resident Physician Shortage Reduction Act of 2017 (H.R. 2267) would increase for the first time since 1997 the number of graduate medical education residency slots in the United States.
The Ensuring Children’s Access to Specialty Care Act of 2017 (S. 989) allows pediatric subspecialists, including pediatric rheumatologists, to get access to the National Health Service Corps loan repayment program when they work in underserved areas.
More recently, in spring 2017 the American Medical Association played a big role in getting the Trump administration to reverse its stance on not allowing premium processing of H1-B visas for professionals such as physicians. If this had gone into effect, all the rheumatology fellows in training who were going to be practicing – some in underserved areas – might have been forced to return to their home country because of a lack of time to get their H1-B visa processed before finishing their fellowship, Dr. Worthing said.
Affordable Care Act (ACA)
- Provide sufficient, affordable, continuous coverage that encourages access to high-quality care for all.
- Prohibit exclusions based on preexisting conditions.
- Allow children to remain on parent’s insurance until age 26 years.
- Remove excessive administrative burdens that take focus away from patient care.
- Cap annual out-of-pocket costs and ban lifetime limits.
- Have affordable premiums, deductibles, and cost sharing.
- Continue the 10 essential health benefits that are required for ACA marketplace plans.
Alliance for Transparent & Affordable Prescriptions (ATAP)
The ACR convened this alliance along with the Coalition of State Rheumatology Organizations, the Global Healthy Living Foundation, the Association of Women in Rheumatology, the Rheumatology Nurses Society, and others to try to bring transparency to how pharmacy benefit managers (PBMs) operate in getting certain drugs on the formularies of payers. The ATAP recently had some success in making lawmakers aware of the PBM’s role in influencing drug prices via rebates to drug manufacturers. At a Congressional hearing in Oct. 2017, after many visits from rheumatologists and members of ATAP, the members of the Senate Committee on Health, Education, Labor, and Pensions “held the feet of these PBMs to the fire a little bit asking them about these rebates,” Dr. Worthing said, where at one point committee chair Sen. Lamar Alexander (R-Tenn.) asked, “ ‘Do we really need these rebates?’ ”
National Institutes of Health budget
After the National Institutes of Health received a $2 billion increase in funding for fiscal year 2017, the Trump administration proposed last summer to cut the NIH budget by 22%. Since then, however, bills to increase the NIH budget by $1.1 billion from the House and by $2 billion from the Senate have made their way through committees. But a budget must be passed by Congress and then signed by the president to make a potential budget increase a reality. Otherwise, a continuing resolution would leave the current level of funding in place through fiscal year 2018, Dr. Worthing noted.
Patients’ Access to Treatments Act of 2017 (H.R. 2999)
This bill has been raised for a fourth time after not making it past committees in previous Congresses, but the prospects for it passing appear somewhat better this time around, Dr. Worthing said. It would prevent insurance companies from putting drugs in specialty tiers that require patients to pay increasingly higher rates of coinsurance for the drugs on different tiers.
“It has been gathering momentum. We hope to get it across the finish line. And if we don’t get this across, then we’ll join with the coalition that rheumatology has formed around this issue of access to specialty treatments some other way, because this is a burning issue for us and our patients,” he said.
Medicare Access to Rehabilitation Services Act of 2017 (H.R. 807 and S. 253)
This bill would repeal the annual cap that was placed on rehabilitation services for patients covered by Medicare in 1997. The bill has bipartisan, majority support and has been gaining momentum for the past 4 years, Dr. Worthing said. It was advanced from both Senate and House committees in Oct. 2017.
SAN DIEGO – , but ongoing efforts to advocate for the specialty and patients are showing signs of paying off in some areas, Angus Worthing, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Worthing, who is chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area, encouraged rheumatologists to become involved in advocacy efforts and asked members of the audience at the meeting to visit the ACR’s advocacy website to learn how to help.
The ACR supports a group of bills that have been introduced in either the House or Senate that should have an effect on alleviating the projected shortage of rheumatologists across the United States through 2030. These bills will help, although much of the effort to address the shortage and maldistribution of rheumatologists across the United States will “probably be solved at the local level. It’s not going to be a federal solution. It will be relationships and treatment programs between primary care and rheumatology care that are very local,” Dr. Worthing said.
The Conrad State 30 and Physician Access Reauthorization Act (H.R. 2141, S. 898) aims to streamline visas for foreign physicians to practice in underserved areas.
The Resident Physician Shortage Reduction Act of 2017 (H.R. 2267) would increase for the first time since 1997 the number of graduate medical education residency slots in the United States.
The Ensuring Children’s Access to Specialty Care Act of 2017 (S. 989) allows pediatric subspecialists, including pediatric rheumatologists, to get access to the National Health Service Corps loan repayment program when they work in underserved areas.
More recently, in spring 2017 the American Medical Association played a big role in getting the Trump administration to reverse its stance on not allowing premium processing of H1-B visas for professionals such as physicians. If this had gone into effect, all the rheumatology fellows in training who were going to be practicing – some in underserved areas – might have been forced to return to their home country because of a lack of time to get their H1-B visa processed before finishing their fellowship, Dr. Worthing said.
Affordable Care Act (ACA)
- Provide sufficient, affordable, continuous coverage that encourages access to high-quality care for all.
- Prohibit exclusions based on preexisting conditions.
- Allow children to remain on parent’s insurance until age 26 years.
- Remove excessive administrative burdens that take focus away from patient care.
- Cap annual out-of-pocket costs and ban lifetime limits.
- Have affordable premiums, deductibles, and cost sharing.
- Continue the 10 essential health benefits that are required for ACA marketplace plans.
Alliance for Transparent & Affordable Prescriptions (ATAP)
The ACR convened this alliance along with the Coalition of State Rheumatology Organizations, the Global Healthy Living Foundation, the Association of Women in Rheumatology, the Rheumatology Nurses Society, and others to try to bring transparency to how pharmacy benefit managers (PBMs) operate in getting certain drugs on the formularies of payers. The ATAP recently had some success in making lawmakers aware of the PBM’s role in influencing drug prices via rebates to drug manufacturers. At a Congressional hearing in Oct. 2017, after many visits from rheumatologists and members of ATAP, the members of the Senate Committee on Health, Education, Labor, and Pensions “held the feet of these PBMs to the fire a little bit asking them about these rebates,” Dr. Worthing said, where at one point committee chair Sen. Lamar Alexander (R-Tenn.) asked, “ ‘Do we really need these rebates?’ ”
National Institutes of Health budget
After the National Institutes of Health received a $2 billion increase in funding for fiscal year 2017, the Trump administration proposed last summer to cut the NIH budget by 22%. Since then, however, bills to increase the NIH budget by $1.1 billion from the House and by $2 billion from the Senate have made their way through committees. But a budget must be passed by Congress and then signed by the president to make a potential budget increase a reality. Otherwise, a continuing resolution would leave the current level of funding in place through fiscal year 2018, Dr. Worthing noted.
Patients’ Access to Treatments Act of 2017 (H.R. 2999)
This bill has been raised for a fourth time after not making it past committees in previous Congresses, but the prospects for it passing appear somewhat better this time around, Dr. Worthing said. It would prevent insurance companies from putting drugs in specialty tiers that require patients to pay increasingly higher rates of coinsurance for the drugs on different tiers.
“It has been gathering momentum. We hope to get it across the finish line. And if we don’t get this across, then we’ll join with the coalition that rheumatology has formed around this issue of access to specialty treatments some other way, because this is a burning issue for us and our patients,” he said.
Medicare Access to Rehabilitation Services Act of 2017 (H.R. 807 and S. 253)
This bill would repeal the annual cap that was placed on rehabilitation services for patients covered by Medicare in 1997. The bill has bipartisan, majority support and has been gaining momentum for the past 4 years, Dr. Worthing said. It was advanced from both Senate and House committees in Oct. 2017.
AT ACR 2017
Obesity linked to RA disease activity, disability
SAN DIEGO – In what may be the largest study of its kind, British researchers have linked obesity to significantly higher odds of rheumatoid arthritis disease activity and disability.
“This study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis,” says Elena Nikiphorou, MD, of King’s College London, who presented the study findings at the annual meeting of the American College of Rheumatology. “Obesity reduced the odds of achieving remission or low disease activity by around 30%. And the odds of having disability were increased by 63%. This confirms what’s been shown in other, smaller studies.”
Despite obesity having been tied to decreased joint damage in established RA, Eric L. Matteson, MD, noted in an interview, that“the biomechanical effect of [being] overweight, especially on the weight-bearing joints” is one of the two “especially important” mechanisms explaining the link between RA and obesity. “The other is that fat cells produce inflammatory proteins, which contribute to the disease process and make it more difficult to treat,” said Dr. Matteson, a rheumatologist at the Mayo Clinic, Rochester, Minn.
“In my view the mechanical risk to the joint outweighs any possible ‘protective’ effect of RA,” Dr. Matteson added in an interview.
For the new study, Dr. Nikiphorou and colleagues compiled statistics from two consecutive United Kingdom RA inception cohorts. One tracked 1,465 patients for up to 25 years (median follow-up, 10 years), and the other tracked 1,236 patients for as many as 10 years (median follow-up, 6 years).
At baseline, 37.2% of 2,420 patients (90% of total) were overweight, and 21.3% were obese. Average body mass index (BMI) rose between the two consecutive studies from 25.5 to 27.6.
The researchers found that obesity was linked to lower likelihoods of remission and low disease activity status (odds ratio, 0.71; 95% confidence interval, 0.55-0.93 and OR, 0.69; 95% CI, 0.55-0.87, respectively.) After controlling for factors such as age and gender, they also saw slightly lower odds of remission in those with higher BMI (OR, 0.97; 95% CI, 0.95-0.99). But there was no statistically significant link between higher BMI and low disease activity status.
The study also connected obesity to higher odds of disability (OR, 1.63; 95% CI, 1.20-2.23). Furthermore, higher BMI was linked to higher odds of disability (OR, 1.04; 95% CI, 1.01-1.06).
Study lead author Dr. Nikiphorou, who spoke in an interview and in comments at an ACR press conference, said “this study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis. It creates a strong case for addressing BMI and addressing obesity, flagging it up to primary care.”
She added that rheumatologists too often focus on only rheumatoid conditions. “We place so much evidence on disease activity scores,” she said. “How often do we really address the patient in terms of other things going on, including obesity? What we can do is include discussion of BMI, exercise, nutrition.”
Dr. Nikiphorou and Dr. Matteson report no relevant disclosures. No specific study funding is reported.
This article was updated 11/10/17.
SAN DIEGO – In what may be the largest study of its kind, British researchers have linked obesity to significantly higher odds of rheumatoid arthritis disease activity and disability.
“This study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis,” says Elena Nikiphorou, MD, of King’s College London, who presented the study findings at the annual meeting of the American College of Rheumatology. “Obesity reduced the odds of achieving remission or low disease activity by around 30%. And the odds of having disability were increased by 63%. This confirms what’s been shown in other, smaller studies.”
Despite obesity having been tied to decreased joint damage in established RA, Eric L. Matteson, MD, noted in an interview, that“the biomechanical effect of [being] overweight, especially on the weight-bearing joints” is one of the two “especially important” mechanisms explaining the link between RA and obesity. “The other is that fat cells produce inflammatory proteins, which contribute to the disease process and make it more difficult to treat,” said Dr. Matteson, a rheumatologist at the Mayo Clinic, Rochester, Minn.
“In my view the mechanical risk to the joint outweighs any possible ‘protective’ effect of RA,” Dr. Matteson added in an interview.
For the new study, Dr. Nikiphorou and colleagues compiled statistics from two consecutive United Kingdom RA inception cohorts. One tracked 1,465 patients for up to 25 years (median follow-up, 10 years), and the other tracked 1,236 patients for as many as 10 years (median follow-up, 6 years).
At baseline, 37.2% of 2,420 patients (90% of total) were overweight, and 21.3% were obese. Average body mass index (BMI) rose between the two consecutive studies from 25.5 to 27.6.
The researchers found that obesity was linked to lower likelihoods of remission and low disease activity status (odds ratio, 0.71; 95% confidence interval, 0.55-0.93 and OR, 0.69; 95% CI, 0.55-0.87, respectively.) After controlling for factors such as age and gender, they also saw slightly lower odds of remission in those with higher BMI (OR, 0.97; 95% CI, 0.95-0.99). But there was no statistically significant link between higher BMI and low disease activity status.
The study also connected obesity to higher odds of disability (OR, 1.63; 95% CI, 1.20-2.23). Furthermore, higher BMI was linked to higher odds of disability (OR, 1.04; 95% CI, 1.01-1.06).
Study lead author Dr. Nikiphorou, who spoke in an interview and in comments at an ACR press conference, said “this study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis. It creates a strong case for addressing BMI and addressing obesity, flagging it up to primary care.”
She added that rheumatologists too often focus on only rheumatoid conditions. “We place so much evidence on disease activity scores,” she said. “How often do we really address the patient in terms of other things going on, including obesity? What we can do is include discussion of BMI, exercise, nutrition.”
Dr. Nikiphorou and Dr. Matteson report no relevant disclosures. No specific study funding is reported.
This article was updated 11/10/17.
SAN DIEGO – In what may be the largest study of its kind, British researchers have linked obesity to significantly higher odds of rheumatoid arthritis disease activity and disability.
“This study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis,” says Elena Nikiphorou, MD, of King’s College London, who presented the study findings at the annual meeting of the American College of Rheumatology. “Obesity reduced the odds of achieving remission or low disease activity by around 30%. And the odds of having disability were increased by 63%. This confirms what’s been shown in other, smaller studies.”
Despite obesity having been tied to decreased joint damage in established RA, Eric L. Matteson, MD, noted in an interview, that“the biomechanical effect of [being] overweight, especially on the weight-bearing joints” is one of the two “especially important” mechanisms explaining the link between RA and obesity. “The other is that fat cells produce inflammatory proteins, which contribute to the disease process and make it more difficult to treat,” said Dr. Matteson, a rheumatologist at the Mayo Clinic, Rochester, Minn.
“In my view the mechanical risk to the joint outweighs any possible ‘protective’ effect of RA,” Dr. Matteson added in an interview.
For the new study, Dr. Nikiphorou and colleagues compiled statistics from two consecutive United Kingdom RA inception cohorts. One tracked 1,465 patients for up to 25 years (median follow-up, 10 years), and the other tracked 1,236 patients for as many as 10 years (median follow-up, 6 years).
At baseline, 37.2% of 2,420 patients (90% of total) were overweight, and 21.3% were obese. Average body mass index (BMI) rose between the two consecutive studies from 25.5 to 27.6.
The researchers found that obesity was linked to lower likelihoods of remission and low disease activity status (odds ratio, 0.71; 95% confidence interval, 0.55-0.93 and OR, 0.69; 95% CI, 0.55-0.87, respectively.) After controlling for factors such as age and gender, they also saw slightly lower odds of remission in those with higher BMI (OR, 0.97; 95% CI, 0.95-0.99). But there was no statistically significant link between higher BMI and low disease activity status.
The study also connected obesity to higher odds of disability (OR, 1.63; 95% CI, 1.20-2.23). Furthermore, higher BMI was linked to higher odds of disability (OR, 1.04; 95% CI, 1.01-1.06).
Study lead author Dr. Nikiphorou, who spoke in an interview and in comments at an ACR press conference, said “this study emphasizes that obesity can have a profound effect on treatment goals in rheumatoid arthritis. It creates a strong case for addressing BMI and addressing obesity, flagging it up to primary care.”
She added that rheumatologists too often focus on only rheumatoid conditions. “We place so much evidence on disease activity scores,” she said. “How often do we really address the patient in terms of other things going on, including obesity? What we can do is include discussion of BMI, exercise, nutrition.”
Dr. Nikiphorou and Dr. Matteson report no relevant disclosures. No specific study funding is reported.
This article was updated 11/10/17.
AT ACR 2017
Key clinical point: Obesity may worsen the risk of disease activity and disability in rheumatoid arthritis.
Major finding: In an adjusted analysis, obese patients with RA were less likely to reach remission and low disease activity status (OR, 0.71; 95% CI, 0.55-0.93 and OR, 0.69; 95% CI, 0.55-0.87, respectively).
Data source: Two consecutive inception cohorts with a total of 1,236 RA patients followed for up to 25 years.
Disclosures: The lead study author reports no disclosures, and no other disclosures are reported. No specific study funding is reported.
Study examines intestinal microbiota role post liver transplant
WASHINGTON – During and after liver transplant, the reaction of the intestinal microbiota may be a critical determinant of outcomes; preliminary data from a cohort study may provide some clarification of what modulates gut microbiota post transplantation and shed light on predictive factors.
Anna-Catrin Uhlemann, MD, PhD, of Columbia University Medical Center, New York, noted that several studies in recent years sought to clarify influences on gut microbiota in people receiving liver transplants, but “there are still a number of important gaps in knowledge, including what exactly is the longitudinal evolution of the host transplant microbiome and what is the predictive value of pre- and early posttransplant dysbiosis on outcomes and complications.”
The researchers collected more than 1,000 samples to screen for colonization by the following MDR organisms: carbapenem-resistant Enterobacteriaceae (CRE), Enterobacteriaceae resistant to third-generation cephalosporins (ESBL), and vancomycin-resistant enterococci (VRE). Over the 1-year follow-up period, 19% (P =.031) of patients had CRE colonization associated with subsequent infection, 41% (P = .003) had ESBL colonization, and 46% (P = .021) had VRE colonization, Dr. Uhlemann said at the annual meeting of the American Association for the Study of Liver Diseases. The researchers then selected 484 samples for sequencing of the 16S ribosomal RNA gene to determine the composition of gut microbiota.
The study used two indexes to determine the alpha diversity of microbiota: the Chao index to estimate richness and the Shannon diversity index to determine the abundance of species in different settings. “We observed dynamic temporal evolution of alpha diversity and taxa abundance over the 1-year follow-up period,” Dr. Uhlemann said. “The diagnosis, the Child-Pugh class, and changes in perioperative antibiotics were important predictors of posttransplant alpha diversity.”
The study also found that Enterobacteriaceae and enterococci increased post transplant in general and as MDR organisms, and that a patient’s MDR status was an important modulator of the posttransplant microbiome, as was the lack of protective operational taxonomic units (OTUs).
The researchers evaluated the relative abundance of taxa and beta diversity. For example, pretransplant patients with a Model for End-stage Liver Disease (MELD) score greater than 25 showed enrichment of Enterobacteriaceae as well as different taxa of the Bacteroidiaceae, while those with MELD scores below 25 showed enrichment of Veillonellaceae. “The significance of this is not clear yet,” Dr. Uhlemann said.
Liver disease severity can also influence gut microbes. Those with Child-Pugh class C disease have the highest numbers in terms of richness and lowest in terms of diversity, Dr. Uhlemann said. “However, at the moment when we are looking at the differential abundance of the taxa, we don’t see quite as clear a pattern, although we noticed in the high group a higher abundance of Bacteroidiaceae,” she said.
Hepatitis B and C patients also presented divergent microbiota profiles. Hepatitis B virus patients “in general are always relatively healthy, and we actually see that these indices are relatively preserved,” Dr. Uhlemann said. “When we look at hepatitis C, however, we see that these patients are starting off quite low and then have an increase in alpha-diversity measures at around month 6.” A subset of patients with alcoholic liver disease also didn’t reach higher Chao and Shannon levels until 6 months after transplant.
“We also find that adjustment of periodic antibiotics for allergy or history of prior infection is significantly associated with a decrease in alpha diversity several months into the posttransplant course,” said Dr. Uhlemann. This is driven by an increase in the abundance of Enterococcaceae and Enterobacteriaceae. “And when we look at MDR colonization as a predictor of alpha diversity, we see that those who have MDR colonization, irrespective of the species, also have the lower alpha diversity.”
The researchers also started to look at pretransplant alpha diversity as a predictor of transplant outcomes, and while the analysis is still in progress, the Shannon indices were significantly different between patients who died and those who survived a year. “There was a trend for significant differences for posttransplant infection and the length of the hospital stay,” Dr. Uhlemann said. “However, we did not see any association with posttransplant ICU readmission, rejection, or VRE complications.”
She added that future analyses are needed to further evaluate the interaction between the clinical comorbidities in the microbiome and vice versa.
Dr. Uhlemann disclosed links to Merck.
WASHINGTON – During and after liver transplant, the reaction of the intestinal microbiota may be a critical determinant of outcomes; preliminary data from a cohort study may provide some clarification of what modulates gut microbiota post transplantation and shed light on predictive factors.
Anna-Catrin Uhlemann, MD, PhD, of Columbia University Medical Center, New York, noted that several studies in recent years sought to clarify influences on gut microbiota in people receiving liver transplants, but “there are still a number of important gaps in knowledge, including what exactly is the longitudinal evolution of the host transplant microbiome and what is the predictive value of pre- and early posttransplant dysbiosis on outcomes and complications.”
The researchers collected more than 1,000 samples to screen for colonization by the following MDR organisms: carbapenem-resistant Enterobacteriaceae (CRE), Enterobacteriaceae resistant to third-generation cephalosporins (ESBL), and vancomycin-resistant enterococci (VRE). Over the 1-year follow-up period, 19% (P =.031) of patients had CRE colonization associated with subsequent infection, 41% (P = .003) had ESBL colonization, and 46% (P = .021) had VRE colonization, Dr. Uhlemann said at the annual meeting of the American Association for the Study of Liver Diseases. The researchers then selected 484 samples for sequencing of the 16S ribosomal RNA gene to determine the composition of gut microbiota.
The study used two indexes to determine the alpha diversity of microbiota: the Chao index to estimate richness and the Shannon diversity index to determine the abundance of species in different settings. “We observed dynamic temporal evolution of alpha diversity and taxa abundance over the 1-year follow-up period,” Dr. Uhlemann said. “The diagnosis, the Child-Pugh class, and changes in perioperative antibiotics were important predictors of posttransplant alpha diversity.”
The study also found that Enterobacteriaceae and enterococci increased post transplant in general and as MDR organisms, and that a patient’s MDR status was an important modulator of the posttransplant microbiome, as was the lack of protective operational taxonomic units (OTUs).
The researchers evaluated the relative abundance of taxa and beta diversity. For example, pretransplant patients with a Model for End-stage Liver Disease (MELD) score greater than 25 showed enrichment of Enterobacteriaceae as well as different taxa of the Bacteroidiaceae, while those with MELD scores below 25 showed enrichment of Veillonellaceae. “The significance of this is not clear yet,” Dr. Uhlemann said.
Liver disease severity can also influence gut microbes. Those with Child-Pugh class C disease have the highest numbers in terms of richness and lowest in terms of diversity, Dr. Uhlemann said. “However, at the moment when we are looking at the differential abundance of the taxa, we don’t see quite as clear a pattern, although we noticed in the high group a higher abundance of Bacteroidiaceae,” she said.
Hepatitis B and C patients also presented divergent microbiota profiles. Hepatitis B virus patients “in general are always relatively healthy, and we actually see that these indices are relatively preserved,” Dr. Uhlemann said. “When we look at hepatitis C, however, we see that these patients are starting off quite low and then have an increase in alpha-diversity measures at around month 6.” A subset of patients with alcoholic liver disease also didn’t reach higher Chao and Shannon levels until 6 months after transplant.
“We also find that adjustment of periodic antibiotics for allergy or history of prior infection is significantly associated with a decrease in alpha diversity several months into the posttransplant course,” said Dr. Uhlemann. This is driven by an increase in the abundance of Enterococcaceae and Enterobacteriaceae. “And when we look at MDR colonization as a predictor of alpha diversity, we see that those who have MDR colonization, irrespective of the species, also have the lower alpha diversity.”
The researchers also started to look at pretransplant alpha diversity as a predictor of transplant outcomes, and while the analysis is still in progress, the Shannon indices were significantly different between patients who died and those who survived a year. “There was a trend for significant differences for posttransplant infection and the length of the hospital stay,” Dr. Uhlemann said. “However, we did not see any association with posttransplant ICU readmission, rejection, or VRE complications.”
She added that future analyses are needed to further evaluate the interaction between the clinical comorbidities in the microbiome and vice versa.
Dr. Uhlemann disclosed links to Merck.
WASHINGTON – During and after liver transplant, the reaction of the intestinal microbiota may be a critical determinant of outcomes; preliminary data from a cohort study may provide some clarification of what modulates gut microbiota post transplantation and shed light on predictive factors.
Anna-Catrin Uhlemann, MD, PhD, of Columbia University Medical Center, New York, noted that several studies in recent years sought to clarify influences on gut microbiota in people receiving liver transplants, but “there are still a number of important gaps in knowledge, including what exactly is the longitudinal evolution of the host transplant microbiome and what is the predictive value of pre- and early posttransplant dysbiosis on outcomes and complications.”
The researchers collected more than 1,000 samples to screen for colonization by the following MDR organisms: carbapenem-resistant Enterobacteriaceae (CRE), Enterobacteriaceae resistant to third-generation cephalosporins (ESBL), and vancomycin-resistant enterococci (VRE). Over the 1-year follow-up period, 19% (P =.031) of patients had CRE colonization associated with subsequent infection, 41% (P = .003) had ESBL colonization, and 46% (P = .021) had VRE colonization, Dr. Uhlemann said at the annual meeting of the American Association for the Study of Liver Diseases. The researchers then selected 484 samples for sequencing of the 16S ribosomal RNA gene to determine the composition of gut microbiota.
The study used two indexes to determine the alpha diversity of microbiota: the Chao index to estimate richness and the Shannon diversity index to determine the abundance of species in different settings. “We observed dynamic temporal evolution of alpha diversity and taxa abundance over the 1-year follow-up period,” Dr. Uhlemann said. “The diagnosis, the Child-Pugh class, and changes in perioperative antibiotics were important predictors of posttransplant alpha diversity.”
The study also found that Enterobacteriaceae and enterococci increased post transplant in general and as MDR organisms, and that a patient’s MDR status was an important modulator of the posttransplant microbiome, as was the lack of protective operational taxonomic units (OTUs).
The researchers evaluated the relative abundance of taxa and beta diversity. For example, pretransplant patients with a Model for End-stage Liver Disease (MELD) score greater than 25 showed enrichment of Enterobacteriaceae as well as different taxa of the Bacteroidiaceae, while those with MELD scores below 25 showed enrichment of Veillonellaceae. “The significance of this is not clear yet,” Dr. Uhlemann said.
Liver disease severity can also influence gut microbes. Those with Child-Pugh class C disease have the highest numbers in terms of richness and lowest in terms of diversity, Dr. Uhlemann said. “However, at the moment when we are looking at the differential abundance of the taxa, we don’t see quite as clear a pattern, although we noticed in the high group a higher abundance of Bacteroidiaceae,” she said.
Hepatitis B and C patients also presented divergent microbiota profiles. Hepatitis B virus patients “in general are always relatively healthy, and we actually see that these indices are relatively preserved,” Dr. Uhlemann said. “When we look at hepatitis C, however, we see that these patients are starting off quite low and then have an increase in alpha-diversity measures at around month 6.” A subset of patients with alcoholic liver disease also didn’t reach higher Chao and Shannon levels until 6 months after transplant.
“We also find that adjustment of periodic antibiotics for allergy or history of prior infection is significantly associated with a decrease in alpha diversity several months into the posttransplant course,” said Dr. Uhlemann. This is driven by an increase in the abundance of Enterococcaceae and Enterobacteriaceae. “And when we look at MDR colonization as a predictor of alpha diversity, we see that those who have MDR colonization, irrespective of the species, also have the lower alpha diversity.”
The researchers also started to look at pretransplant alpha diversity as a predictor of transplant outcomes, and while the analysis is still in progress, the Shannon indices were significantly different between patients who died and those who survived a year. “There was a trend for significant differences for posttransplant infection and the length of the hospital stay,” Dr. Uhlemann said. “However, we did not see any association with posttransplant ICU readmission, rejection, or VRE complications.”
She added that future analyses are needed to further evaluate the interaction between the clinical comorbidities in the microbiome and vice versa.
Dr. Uhlemann disclosed links to Merck.
AT THE LIVER MEETING 2017
Key clinical point: The presence or lack of specific modulators of gut microbiota may influence outcomes of liver transplantation.
Major finding: Over a 1-year follow-up period, 19% of patients had colonization with carbapenem-resistant Enterobacteriaceae, 41% had Enterobacteriaceae resistant to third-generation cephalosporins, and 46% had vancomycin-resistant enterococci associated with subsequent infections.
Data source: A prospective longitudinal cohort study of 323 patients, 125 of whom completed 1 year of follow-up.
Disclosures: Dr. Uhlemann disclosed receiving research funding from Merck.
Inside the Las Vegas crisis: Surgeons answered the call
SAN DIEGO– Long before the horrific night of Oct. 1, the three trauma centers in the Las Vegas region were ready for a mass casualty event. It was understood among hospital leaders that the city could be the scene of a disaster that would demand a coordinated response from the city’s health care centers.
Then came the deadliest mass shooting in modern American history, and the extensive preparation turned out to have been well worth the time and effort, according to four trauma surgeons who spoke about the medical response to the massacre during a session at the annual clinical congress of the American College of Surgeons.
The killing spree was unusual in a variety of ways, including the fact that it occurred at a site “that’s almost strategically surrounded by trauma centers,” Dr. Fildes said.
UMC is Nevada’s only level I trauma center, while Sunrise is a level II. St. Rose Dominican, in the neighboring city of Henderson is a level III. Only one other Nevada hospital, in Reno, is a verified trauma center.
While the trauma centers received hundreds of patients, “every hospital in the valley saw patients from this event,” Dr. Fildes said. “There were 22,000 people on scene, and when the shooting started, they extricated themselves and went to safety by one means or another. Some drove home to their neighborhood and sought care there. Some drove until they found an acute care facility, whether it was a trauma center or not. Others were transported by Uber or taxi. The drivers knew where the trauma centers were, and decided where to go based on how the patients looked.”
According to Dr. Fildes, Las Vegas–area hospitals kept in touch with each other by phone, and UMC accepted some transfers from other hospitals. “We were ready for transfers,” he said, “and we expected more than we got.”
The trauma centers faced a variety of challenges from confusion and false reports to overcrowding and a media onslaught.
“We knew there was a strong possibility this would happen where we live, so we practiced this,” said Sean Dort, MD, medical director of the hospital’s trauma center. “We have talked and walked through it.”
Indeed, all hospitals in the Las Vegas area take part in regional disaster drills twice a year, and UMC runs other drills during the year such as an active shooter drill, Dr. Fildes said in an interview.
Together, the three hospitals treated hundreds of patients. Three weeks later, a handful were still inpatients.
In the aftermath, Las Vegas trauma surgeons are focusing on missed opportunities and lessons learned.
Dr. Fildes said more attention needs to be paid to how to handle situations when tides of patients bring themselves to the emergency department. “The issue of self-delivery has to be reconsidered, restudied,” he said, and he suggested that it may be a good idea to equip taxis with bleeding control kits.
He said his hospital heard from a doctor who’d treated patients during the Pulse nightclub massacre in Orlando last year. “One of their lessons learned was to position all gurneys and wheelchairs near the intake triage area,” he said. “We did that, and it improved the movement of patients to areas of the hospital that were matched to the intensity of care that they required.”
At Sunrise, the flood of unidentified patients overwhelmed the hospital’s trauma patient alias system, and some names were repeated. “In the future, I think a better naming system should be employed,” said trauma surgeon Matthew S. Johnson, MD.
To that end, he said, the hospital has begun examining how hurricanes are named.
And when it comes to planning, he said, there’s no room for excuses or resistance. “Everyone knew their role,” he said. “You can’t start figuring this out when it happens. You have to push people through it when they don’t want to do it, and they’re busy.”
Dr. Fildes said that the UMC staff were physically and emotionally exhausted by the ordeal, but proud of what they were able to do for these patients, and that pride carried them through the experience. “We had support from all over the country; people sent banners with hundreds of signatures. Something like 1,100 pizzas were sent to the UMC staff, and dozens and dozens of surgeons from all over the country offered to come help us.”
Dr. Fildes noted that he is not easily surprised given his daily work, but he was impressed by the generosity and courage of the patients in this crisis situation.
He concluded that, “This was all made possible because of planning, training, commitment by staff and ultimately, the bravery of the patients.”
Dr. Dort, Dr. Fildes, Dr. Kuhls, and Dr. Johnson had no relevant financial disclosures.
I was at home and in bed with a book when my phone went off at 10:22 p.m. on that Sunday. It was a text message from one of my fellow residents who was on call at Sunrise: She wrote: “Mass casualty incident. Shooting on the Strip. You have to come now.”
There were multiple blood trails tracking from various parts of the ambulance bay into the ED. Medics were walking from bedside to bedside putting in lines. Two anesthesia attendings were frantically intubating patients. Two nurses were performing chest compressions.
I picked the nearest bed and started assessing patients. I placed 2 endotracheal tubes and black tagged 4 more patients within minutes of my arrival.
In the initial moments in the ER and in the OR, I focused on caring for the patient and blocked out any other thoughts or emotions. There was no time and no room for my horror or my tears.
As I went bedside to bedside in the ER, I was practically chanting in my head “airway, breathing, circulation, vital signs, other injuries.”
In the OR, I was working on controlling intra-abdominal bleeding from multiple sources, and again, my training became something of a mantra in my head. “Pack, control bleeding, assess injuries, repair.”
We saw well over 200 patients from the Route 91 shooting and operated on 95 of them within the first 24 hours.
Dylan Davey, MD, PhD, General Surgery Resident, PGY-4, Sunrise Hospital & Medical Center.
I was at home and in bed with a book when my phone went off at 10:22 p.m. on that Sunday. It was a text message from one of my fellow residents who was on call at Sunrise: She wrote: “Mass casualty incident. Shooting on the Strip. You have to come now.”
There were multiple blood trails tracking from various parts of the ambulance bay into the ED. Medics were walking from bedside to bedside putting in lines. Two anesthesia attendings were frantically intubating patients. Two nurses were performing chest compressions.
I picked the nearest bed and started assessing patients. I placed 2 endotracheal tubes and black tagged 4 more patients within minutes of my arrival.
In the initial moments in the ER and in the OR, I focused on caring for the patient and blocked out any other thoughts or emotions. There was no time and no room for my horror or my tears.
As I went bedside to bedside in the ER, I was practically chanting in my head “airway, breathing, circulation, vital signs, other injuries.”
In the OR, I was working on controlling intra-abdominal bleeding from multiple sources, and again, my training became something of a mantra in my head. “Pack, control bleeding, assess injuries, repair.”
We saw well over 200 patients from the Route 91 shooting and operated on 95 of them within the first 24 hours.
Dylan Davey, MD, PhD, General Surgery Resident, PGY-4, Sunrise Hospital & Medical Center.
I was at home and in bed with a book when my phone went off at 10:22 p.m. on that Sunday. It was a text message from one of my fellow residents who was on call at Sunrise: She wrote: “Mass casualty incident. Shooting on the Strip. You have to come now.”
There were multiple blood trails tracking from various parts of the ambulance bay into the ED. Medics were walking from bedside to bedside putting in lines. Two anesthesia attendings were frantically intubating patients. Two nurses were performing chest compressions.
I picked the nearest bed and started assessing patients. I placed 2 endotracheal tubes and black tagged 4 more patients within minutes of my arrival.
In the initial moments in the ER and in the OR, I focused on caring for the patient and blocked out any other thoughts or emotions. There was no time and no room for my horror or my tears.
As I went bedside to bedside in the ER, I was practically chanting in my head “airway, breathing, circulation, vital signs, other injuries.”
In the OR, I was working on controlling intra-abdominal bleeding from multiple sources, and again, my training became something of a mantra in my head. “Pack, control bleeding, assess injuries, repair.”
We saw well over 200 patients from the Route 91 shooting and operated on 95 of them within the first 24 hours.
Dylan Davey, MD, PhD, General Surgery Resident, PGY-4, Sunrise Hospital & Medical Center.
SAN DIEGO– Long before the horrific night of Oct. 1, the three trauma centers in the Las Vegas region were ready for a mass casualty event. It was understood among hospital leaders that the city could be the scene of a disaster that would demand a coordinated response from the city’s health care centers.
Then came the deadliest mass shooting in modern American history, and the extensive preparation turned out to have been well worth the time and effort, according to four trauma surgeons who spoke about the medical response to the massacre during a session at the annual clinical congress of the American College of Surgeons.
The killing spree was unusual in a variety of ways, including the fact that it occurred at a site “that’s almost strategically surrounded by trauma centers,” Dr. Fildes said.
UMC is Nevada’s only level I trauma center, while Sunrise is a level II. St. Rose Dominican, in the neighboring city of Henderson is a level III. Only one other Nevada hospital, in Reno, is a verified trauma center.
While the trauma centers received hundreds of patients, “every hospital in the valley saw patients from this event,” Dr. Fildes said. “There were 22,000 people on scene, and when the shooting started, they extricated themselves and went to safety by one means or another. Some drove home to their neighborhood and sought care there. Some drove until they found an acute care facility, whether it was a trauma center or not. Others were transported by Uber or taxi. The drivers knew where the trauma centers were, and decided where to go based on how the patients looked.”
According to Dr. Fildes, Las Vegas–area hospitals kept in touch with each other by phone, and UMC accepted some transfers from other hospitals. “We were ready for transfers,” he said, “and we expected more than we got.”
The trauma centers faced a variety of challenges from confusion and false reports to overcrowding and a media onslaught.
“We knew there was a strong possibility this would happen where we live, so we practiced this,” said Sean Dort, MD, medical director of the hospital’s trauma center. “We have talked and walked through it.”
Indeed, all hospitals in the Las Vegas area take part in regional disaster drills twice a year, and UMC runs other drills during the year such as an active shooter drill, Dr. Fildes said in an interview.
Together, the three hospitals treated hundreds of patients. Three weeks later, a handful were still inpatients.
In the aftermath, Las Vegas trauma surgeons are focusing on missed opportunities and lessons learned.
Dr. Fildes said more attention needs to be paid to how to handle situations when tides of patients bring themselves to the emergency department. “The issue of self-delivery has to be reconsidered, restudied,” he said, and he suggested that it may be a good idea to equip taxis with bleeding control kits.
He said his hospital heard from a doctor who’d treated patients during the Pulse nightclub massacre in Orlando last year. “One of their lessons learned was to position all gurneys and wheelchairs near the intake triage area,” he said. “We did that, and it improved the movement of patients to areas of the hospital that were matched to the intensity of care that they required.”
At Sunrise, the flood of unidentified patients overwhelmed the hospital’s trauma patient alias system, and some names were repeated. “In the future, I think a better naming system should be employed,” said trauma surgeon Matthew S. Johnson, MD.
To that end, he said, the hospital has begun examining how hurricanes are named.
And when it comes to planning, he said, there’s no room for excuses or resistance. “Everyone knew their role,” he said. “You can’t start figuring this out when it happens. You have to push people through it when they don’t want to do it, and they’re busy.”
Dr. Fildes said that the UMC staff were physically and emotionally exhausted by the ordeal, but proud of what they were able to do for these patients, and that pride carried them through the experience. “We had support from all over the country; people sent banners with hundreds of signatures. Something like 1,100 pizzas were sent to the UMC staff, and dozens and dozens of surgeons from all over the country offered to come help us.”
Dr. Fildes noted that he is not easily surprised given his daily work, but he was impressed by the generosity and courage of the patients in this crisis situation.
He concluded that, “This was all made possible because of planning, training, commitment by staff and ultimately, the bravery of the patients.”
Dr. Dort, Dr. Fildes, Dr. Kuhls, and Dr. Johnson had no relevant financial disclosures.
SAN DIEGO– Long before the horrific night of Oct. 1, the three trauma centers in the Las Vegas region were ready for a mass casualty event. It was understood among hospital leaders that the city could be the scene of a disaster that would demand a coordinated response from the city’s health care centers.
Then came the deadliest mass shooting in modern American history, and the extensive preparation turned out to have been well worth the time and effort, according to four trauma surgeons who spoke about the medical response to the massacre during a session at the annual clinical congress of the American College of Surgeons.
The killing spree was unusual in a variety of ways, including the fact that it occurred at a site “that’s almost strategically surrounded by trauma centers,” Dr. Fildes said.
UMC is Nevada’s only level I trauma center, while Sunrise is a level II. St. Rose Dominican, in the neighboring city of Henderson is a level III. Only one other Nevada hospital, in Reno, is a verified trauma center.
While the trauma centers received hundreds of patients, “every hospital in the valley saw patients from this event,” Dr. Fildes said. “There were 22,000 people on scene, and when the shooting started, they extricated themselves and went to safety by one means or another. Some drove home to their neighborhood and sought care there. Some drove until they found an acute care facility, whether it was a trauma center or not. Others were transported by Uber or taxi. The drivers knew where the trauma centers were, and decided where to go based on how the patients looked.”
According to Dr. Fildes, Las Vegas–area hospitals kept in touch with each other by phone, and UMC accepted some transfers from other hospitals. “We were ready for transfers,” he said, “and we expected more than we got.”
The trauma centers faced a variety of challenges from confusion and false reports to overcrowding and a media onslaught.
“We knew there was a strong possibility this would happen where we live, so we practiced this,” said Sean Dort, MD, medical director of the hospital’s trauma center. “We have talked and walked through it.”
Indeed, all hospitals in the Las Vegas area take part in regional disaster drills twice a year, and UMC runs other drills during the year such as an active shooter drill, Dr. Fildes said in an interview.
Together, the three hospitals treated hundreds of patients. Three weeks later, a handful were still inpatients.
In the aftermath, Las Vegas trauma surgeons are focusing on missed opportunities and lessons learned.
Dr. Fildes said more attention needs to be paid to how to handle situations when tides of patients bring themselves to the emergency department. “The issue of self-delivery has to be reconsidered, restudied,” he said, and he suggested that it may be a good idea to equip taxis with bleeding control kits.
He said his hospital heard from a doctor who’d treated patients during the Pulse nightclub massacre in Orlando last year. “One of their lessons learned was to position all gurneys and wheelchairs near the intake triage area,” he said. “We did that, and it improved the movement of patients to areas of the hospital that were matched to the intensity of care that they required.”
At Sunrise, the flood of unidentified patients overwhelmed the hospital’s trauma patient alias system, and some names were repeated. “In the future, I think a better naming system should be employed,” said trauma surgeon Matthew S. Johnson, MD.
To that end, he said, the hospital has begun examining how hurricanes are named.
And when it comes to planning, he said, there’s no room for excuses or resistance. “Everyone knew their role,” he said. “You can’t start figuring this out when it happens. You have to push people through it when they don’t want to do it, and they’re busy.”
Dr. Fildes said that the UMC staff were physically and emotionally exhausted by the ordeal, but proud of what they were able to do for these patients, and that pride carried them through the experience. “We had support from all over the country; people sent banners with hundreds of signatures. Something like 1,100 pizzas were sent to the UMC staff, and dozens and dozens of surgeons from all over the country offered to come help us.”
Dr. Fildes noted that he is not easily surprised given his daily work, but he was impressed by the generosity and courage of the patients in this crisis situation.
He concluded that, “This was all made possible because of planning, training, commitment by staff and ultimately, the bravery of the patients.”
Dr. Dort, Dr. Fildes, Dr. Kuhls, and Dr. Johnson had no relevant financial disclosures.
AT THE ACS CLINICAL CONGRESS
Review of Strategies to Reduce Central Line-Associated Bloodstream Infection (CLABSI) and Catheter-Associated Urinary Tract Infection (CAUTI) in Adult ICUs
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
© 2018 Society of Hospital Medicine
Fuller Road, Ann Arbor, MI 48105; Telephone: 734-845-3460; Fax: 734-845-3290, E-mail: [email protected]
Developing machines that detect disease
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Ustekinumab may reduce risk of nonmelanoma skin cancer
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Psoriasis patients on ustekinumab had an adjusted 65% reduction in the risk of developing nonmelanoma skin cancer compared with patients on methotrexate.
Data source: An analysis of 6,782 psoriasis patients participating in an international prospective observational registry evaluating the long-term safety and clinical outcomes of systemic therapies.
Disclosures: The PSOLAR registry is funded by ustekinumab manufacturer Janssen; the study presenter is a company employee.
Understanding the Causes of Venous Pressure
Developing an effective treatment plan for patients with venous disease hinges on an thorough clinical evaluation and a keen understanding of venous anatomy and physiology, both of which will be the focus of the Thursday morning session, “Venous Clinical Examination and Hemodynamics.”
“Less invasive treatments are now available because of the advent of new technologies,” said co-moderator Dr. Jose I. Almeida, director of the Miami Vein Center and voluntary professor of surgery at the University of Miami School of Medicine. “However, the proper treatment of patients with advanced venous disease has little to do with the new toys available on the shelf. Rather, it depends on a proper understanding of the fundamental problem: What causes increased venous pressure?”
Presentations will focus on answering that question. “Without an accurate physician assessment, one cannot apply an effective treatment plan for a patient with venous disease,” said Dr. Almeida. Session co-moderators include Dr. Lowell S. Kabnick, director of the New York University Vein Center, and Dr. Thomas W. Wakefield, vascular surgery section head at the University of Michigan Cardiovascular Center.
“The session begins with identifying symptoms and signs of venous disease, scoring the disease severity with a validated tool, and classifying the severity on a scale of one to six,” said Dr. Almeida, who starts off the session with his own review of the CEAP classification for venous disease – Clinical, Etiologic, Anatomic and Pathophysiologic – and the Venous Clinical Severity Score system. “Patient reported outcomes are now increasingly important to all stakeholders in venous disease space, and these principles will be summarized,” he said.
The session then moves into the anatomy and physiology of venous disorders. “Proper treatment hinges on the understanding of superficial reflux pathways and how to identify them with the ultrasound imaging,” said Dr. Almeida “Venous hypertension can also be caused by obstruction. The hemodynamics from the perspective of our European colleagues will be presented – and the controversial treatments used based on these hemodynamic principles will be reviewed.”
Dr. Bo G. Eklof of the University of Lund will provide the European perspective with an update on the Venous Symposium Consensus (SYMVein), a collaboration of the European Venous Forum and International Working Group to develop a consensus on venous symptoms.
Dr. Kabnick will then explore outcome assessment of central venous disease, and Dr. Wakefield will review the evidence on the pathophysiology of varicose veins. Dr. Seshadri Raju, vascular surgeon at St. Dominic Hospital will explore emerging concepts in venous flow and pressure.
“This will be the talk that ties in the concepts of flow and pressure and how each contributes to the final common pathway of venous disease—namely, venous hypertension,” Dr. Almeida said of Dr. Raju’s presentation.
In keeping with a deeper dive into venous anatomy, Dr. Neil M. Khilnani, associate professor of radiology at Weill Medical College of Cornell University, will discuss the role of duplex ultrasound in identifying reflux pathways, and Dr. Brajesh K. Lal, professor at University of Maryland School of Medicine, will present the latest evidence on venous return pathology. Then Dr. Byung-Boong Lee, professor of surgery at George Washington School of Medicine, will provide an update on the International Union of Phlebology consensus on chronic venous disease
Two presenters from the Riviera Vein Institute in the Principality of Monaco will close out the session. Research chair Dr. Sylvain Chastanet will present 10-year results of the ASVAL – the French acronym for selective ablation of varicose veins under local anesthesia – method for treating varicose veins. And Dr. Paul Pittaluga will wrap things up by analyzing the role of the saphenofemoral junction in planning treatment for varicose veins.
Developing an effective treatment plan for patients with venous disease hinges on an thorough clinical evaluation and a keen understanding of venous anatomy and physiology, both of which will be the focus of the Thursday morning session, “Venous Clinical Examination and Hemodynamics.”
“Less invasive treatments are now available because of the advent of new technologies,” said co-moderator Dr. Jose I. Almeida, director of the Miami Vein Center and voluntary professor of surgery at the University of Miami School of Medicine. “However, the proper treatment of patients with advanced venous disease has little to do with the new toys available on the shelf. Rather, it depends on a proper understanding of the fundamental problem: What causes increased venous pressure?”
Presentations will focus on answering that question. “Without an accurate physician assessment, one cannot apply an effective treatment plan for a patient with venous disease,” said Dr. Almeida. Session co-moderators include Dr. Lowell S. Kabnick, director of the New York University Vein Center, and Dr. Thomas W. Wakefield, vascular surgery section head at the University of Michigan Cardiovascular Center.
“The session begins with identifying symptoms and signs of venous disease, scoring the disease severity with a validated tool, and classifying the severity on a scale of one to six,” said Dr. Almeida, who starts off the session with his own review of the CEAP classification for venous disease – Clinical, Etiologic, Anatomic and Pathophysiologic – and the Venous Clinical Severity Score system. “Patient reported outcomes are now increasingly important to all stakeholders in venous disease space, and these principles will be summarized,” he said.
The session then moves into the anatomy and physiology of venous disorders. “Proper treatment hinges on the understanding of superficial reflux pathways and how to identify them with the ultrasound imaging,” said Dr. Almeida “Venous hypertension can also be caused by obstruction. The hemodynamics from the perspective of our European colleagues will be presented – and the controversial treatments used based on these hemodynamic principles will be reviewed.”
Dr. Bo G. Eklof of the University of Lund will provide the European perspective with an update on the Venous Symposium Consensus (SYMVein), a collaboration of the European Venous Forum and International Working Group to develop a consensus on venous symptoms.
Dr. Kabnick will then explore outcome assessment of central venous disease, and Dr. Wakefield will review the evidence on the pathophysiology of varicose veins. Dr. Seshadri Raju, vascular surgeon at St. Dominic Hospital will explore emerging concepts in venous flow and pressure.
“This will be the talk that ties in the concepts of flow and pressure and how each contributes to the final common pathway of venous disease—namely, venous hypertension,” Dr. Almeida said of Dr. Raju’s presentation.
In keeping with a deeper dive into venous anatomy, Dr. Neil M. Khilnani, associate professor of radiology at Weill Medical College of Cornell University, will discuss the role of duplex ultrasound in identifying reflux pathways, and Dr. Brajesh K. Lal, professor at University of Maryland School of Medicine, will present the latest evidence on venous return pathology. Then Dr. Byung-Boong Lee, professor of surgery at George Washington School of Medicine, will provide an update on the International Union of Phlebology consensus on chronic venous disease
Two presenters from the Riviera Vein Institute in the Principality of Monaco will close out the session. Research chair Dr. Sylvain Chastanet will present 10-year results of the ASVAL – the French acronym for selective ablation of varicose veins under local anesthesia – method for treating varicose veins. And Dr. Paul Pittaluga will wrap things up by analyzing the role of the saphenofemoral junction in planning treatment for varicose veins.
Developing an effective treatment plan for patients with venous disease hinges on an thorough clinical evaluation and a keen understanding of venous anatomy and physiology, both of which will be the focus of the Thursday morning session, “Venous Clinical Examination and Hemodynamics.”
“Less invasive treatments are now available because of the advent of new technologies,” said co-moderator Dr. Jose I. Almeida, director of the Miami Vein Center and voluntary professor of surgery at the University of Miami School of Medicine. “However, the proper treatment of patients with advanced venous disease has little to do with the new toys available on the shelf. Rather, it depends on a proper understanding of the fundamental problem: What causes increased venous pressure?”
Presentations will focus on answering that question. “Without an accurate physician assessment, one cannot apply an effective treatment plan for a patient with venous disease,” said Dr. Almeida. Session co-moderators include Dr. Lowell S. Kabnick, director of the New York University Vein Center, and Dr. Thomas W. Wakefield, vascular surgery section head at the University of Michigan Cardiovascular Center.
“The session begins with identifying symptoms and signs of venous disease, scoring the disease severity with a validated tool, and classifying the severity on a scale of one to six,” said Dr. Almeida, who starts off the session with his own review of the CEAP classification for venous disease – Clinical, Etiologic, Anatomic and Pathophysiologic – and the Venous Clinical Severity Score system. “Patient reported outcomes are now increasingly important to all stakeholders in venous disease space, and these principles will be summarized,” he said.
The session then moves into the anatomy and physiology of venous disorders. “Proper treatment hinges on the understanding of superficial reflux pathways and how to identify them with the ultrasound imaging,” said Dr. Almeida “Venous hypertension can also be caused by obstruction. The hemodynamics from the perspective of our European colleagues will be presented – and the controversial treatments used based on these hemodynamic principles will be reviewed.”
Dr. Bo G. Eklof of the University of Lund will provide the European perspective with an update on the Venous Symposium Consensus (SYMVein), a collaboration of the European Venous Forum and International Working Group to develop a consensus on venous symptoms.
Dr. Kabnick will then explore outcome assessment of central venous disease, and Dr. Wakefield will review the evidence on the pathophysiology of varicose veins. Dr. Seshadri Raju, vascular surgeon at St. Dominic Hospital will explore emerging concepts in venous flow and pressure.
“This will be the talk that ties in the concepts of flow and pressure and how each contributes to the final common pathway of venous disease—namely, venous hypertension,” Dr. Almeida said of Dr. Raju’s presentation.
In keeping with a deeper dive into venous anatomy, Dr. Neil M. Khilnani, associate professor of radiology at Weill Medical College of Cornell University, will discuss the role of duplex ultrasound in identifying reflux pathways, and Dr. Brajesh K. Lal, professor at University of Maryland School of Medicine, will present the latest evidence on venous return pathology. Then Dr. Byung-Boong Lee, professor of surgery at George Washington School of Medicine, will provide an update on the International Union of Phlebology consensus on chronic venous disease
Two presenters from the Riviera Vein Institute in the Principality of Monaco will close out the session. Research chair Dr. Sylvain Chastanet will present 10-year results of the ASVAL – the French acronym for selective ablation of varicose veins under local anesthesia – method for treating varicose veins. And Dr. Paul Pittaluga will wrap things up by analyzing the role of the saphenofemoral junction in planning treatment for varicose veins.