User login
Rates, predictors, and variability of interhospital transfers
Clinical question: What is the national frequency of interhospital transfers, and are there any patient or hospital factors that predict these transfers?
Background: Interhospital patient transfers may be due to the need for a specialized service, but the factors and patterns have not been well studied.
Setting: All acute care hospitals in the United States.
Synopsis: Using data from the 2013 Centers for Medicare & Medicaid Services and the 2013 American Hospital Association, this study showed that 1.5% of the 6.6 million eligible beneficiaries underwent interhospital transfer (IHT). Patient and hospital characteristics that increased the odds of IHT included age 74-85 years, nonblack race, higher comorbidity, lower diagnosis-related group weight, fewer recent hospitalizations, and hospitalization in the Northeast region of the United States. Lower case mix index was associated with increased odds of IHT. Rates of IHT remain variable, after adjusting for patient and hospital characteristics. This study was restricted to the Medicare population so did not represent all populations. IHT from the emergency room was not assessed, and those who were transferred more than once (to another hospital and back) were not included.
Bottom line: A large number of Medicare patients undergo IHT nationally, and the rate varies widely based on patient factors, geography, and other factors unrelated to patient or hospital characteristics.
Citation: Mueller SK, Jie Zheng, Orav EJ, Schnipper JL. Rates, predictors, and variability of interhospital transfers: A national evaluation. J Hosp Med. 2017;6:435-42.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Clinical question: What is the national frequency of interhospital transfers, and are there any patient or hospital factors that predict these transfers?
Background: Interhospital patient transfers may be due to the need for a specialized service, but the factors and patterns have not been well studied.
Setting: All acute care hospitals in the United States.
Synopsis: Using data from the 2013 Centers for Medicare & Medicaid Services and the 2013 American Hospital Association, this study showed that 1.5% of the 6.6 million eligible beneficiaries underwent interhospital transfer (IHT). Patient and hospital characteristics that increased the odds of IHT included age 74-85 years, nonblack race, higher comorbidity, lower diagnosis-related group weight, fewer recent hospitalizations, and hospitalization in the Northeast region of the United States. Lower case mix index was associated with increased odds of IHT. Rates of IHT remain variable, after adjusting for patient and hospital characteristics. This study was restricted to the Medicare population so did not represent all populations. IHT from the emergency room was not assessed, and those who were transferred more than once (to another hospital and back) were not included.
Bottom line: A large number of Medicare patients undergo IHT nationally, and the rate varies widely based on patient factors, geography, and other factors unrelated to patient or hospital characteristics.
Citation: Mueller SK, Jie Zheng, Orav EJ, Schnipper JL. Rates, predictors, and variability of interhospital transfers: A national evaluation. J Hosp Med. 2017;6:435-42.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Clinical question: What is the national frequency of interhospital transfers, and are there any patient or hospital factors that predict these transfers?
Background: Interhospital patient transfers may be due to the need for a specialized service, but the factors and patterns have not been well studied.
Setting: All acute care hospitals in the United States.
Synopsis: Using data from the 2013 Centers for Medicare & Medicaid Services and the 2013 American Hospital Association, this study showed that 1.5% of the 6.6 million eligible beneficiaries underwent interhospital transfer (IHT). Patient and hospital characteristics that increased the odds of IHT included age 74-85 years, nonblack race, higher comorbidity, lower diagnosis-related group weight, fewer recent hospitalizations, and hospitalization in the Northeast region of the United States. Lower case mix index was associated with increased odds of IHT. Rates of IHT remain variable, after adjusting for patient and hospital characteristics. This study was restricted to the Medicare population so did not represent all populations. IHT from the emergency room was not assessed, and those who were transferred more than once (to another hospital and back) were not included.
Bottom line: A large number of Medicare patients undergo IHT nationally, and the rate varies widely based on patient factors, geography, and other factors unrelated to patient or hospital characteristics.
Citation: Mueller SK, Jie Zheng, Orav EJ, Schnipper JL. Rates, predictors, and variability of interhospital transfers: A national evaluation. J Hosp Med. 2017;6:435-42.
Dr. Xu is assistant professor and hospitalist, Icahn School of Medicine of the Mount Sinai Health System, New York.
Insulin Pump Therapy: Who, Why, and How
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
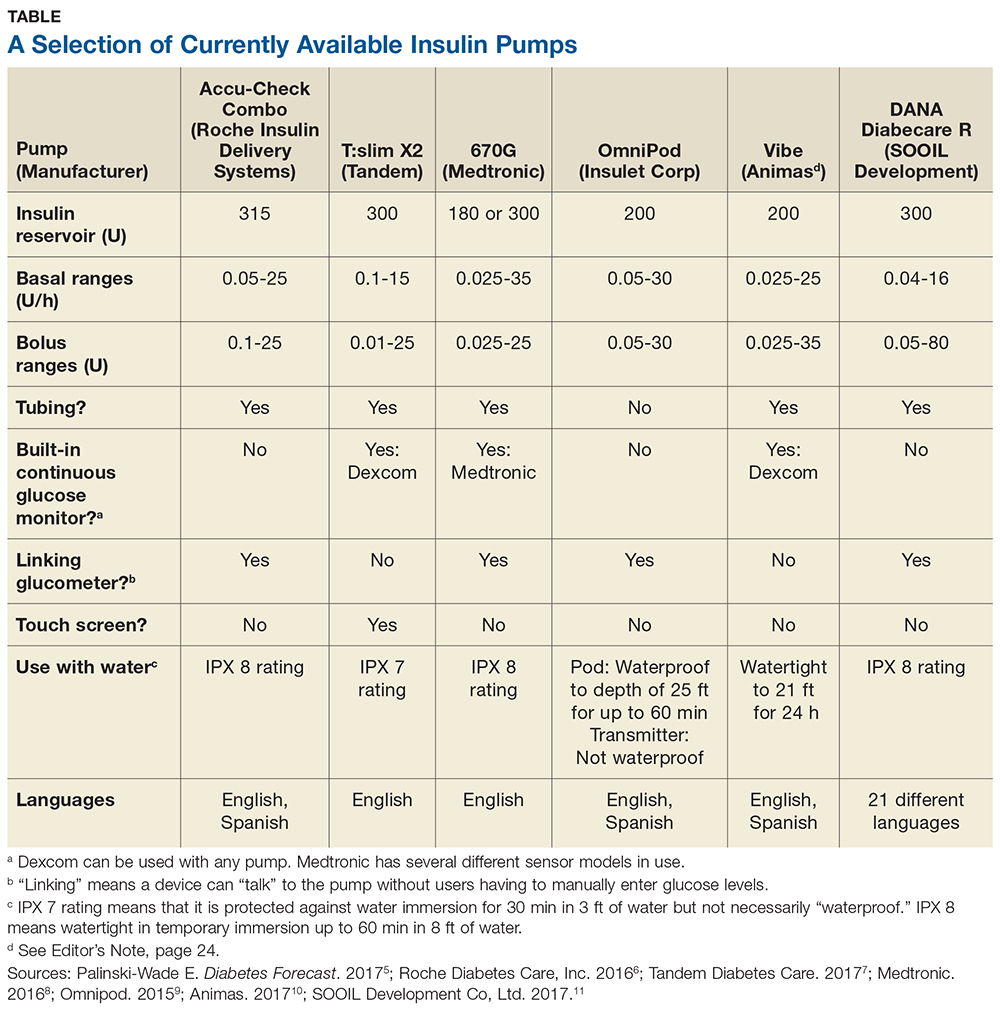
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
With new technology available to aid patients, diabetes management in the 21st century is moving beyond metformin. Among these advances are insulin pumps, which are not just for the young and tech-savvy. In fact, in 2016, the American Diabetes Association (ADA) revised their Standards in Medical Care to recommend patients 65 and older continue to use their insulin pumps and continuous glucose monitoring devices (CGMs), rather than forego technology for more traditional treatment options.1
Insulin pumps enhance or mimic the role of the pancreas by providing a background, or basal, rate of insulin, as well as boluses for food or glucose corrections. A small catheter is inserted under the skin—in the same areas used for injections (eg, arm, thigh, abdomen)—to release insulin.2
While the benefits of technology cannot always be quantified, there are data to suggest insulin pumps can reduce A1C by 1.1% in patients with type 2 diabetes. In tandem with CGMs, insulin pumps have been shown to be cost effective in those with a history of severe hypoglycemia.3,4
Q When should patients consider using an insulin pump?
Patients with type 1 or type 2 diabetes can benefit from an insulin pump. In particular, they can be useful for patients who
Are tired of multiple daily injections. Insulin is still bolused at mealtime—just electronically.
Require a tailored approach. Multiple basal settings can be programmed to reflect activity and work level; some patients need more insulin on active days and less on sedentary days.
Have an on-the-go lifestyle. Insulin pumps replace multiple daily injections, as noted, which helps when patients miss manual injections due to accessibility issues.
Value discretion. Those who wear restrictive uniforms for work or simply desire privacy may benefit from an insulin pump model that can bolus via remote control, without physical access to the pump.
Have found other treatments suboptimal. Some insurers allow patients to try a pump before a decision is made.
Experience hypoglycemia unawareness. Some pumps work with CGMs to suspend insulin delivery with a low glucose level; proper use of a pump can help to restore patient awareness of their condition.
Are sensitive to insulin. Select pumps can deliver insulin at a rate of one-hundredth of a unit at a time.
Experience the dawn phenomenon or Somogyi effect. Patients with high early-morning glucose levels can adjust their rates to combat hyperglycemia, and those with overnight lows and rebounding hyperglycemia can adjust their basal rates or nighttime snacking settings to prevent this occurrence.
Q Who would be an ideal candidate?
Motivated patients who want to attain glycemic control and adhere to the recommendations of their care team are ideally suited to insulin pump use. Insurance companies want to ensure patient safety, so before approving coverage for an insulin pump, they may require patients to demonstrate their willingness to adjust their lifestyle, work with their diabetes educator and/or provider team, and test routinely in the weeks or months leading up to the final decision—all expected behavior while using pump therapy.
Q How do you initiate insulin pump therapy?
With any new treatment, clear communication is key. Patients should schedule specific appointments with their provider and diabetes team to know what is expected from both parties during this process.
Pump selection should be individualized choice, depending on the patient’s goals, lifestyle, and a thorough review of the pros and cons of each pump. When a selection has been made, patients can begin testing—at least four times daily, before meals and at bedtime, as required by most insurers. Representatives from the pump’s manufacturer can be a helpful resource for questions about the particular pump, as well as a liaison to the insurance company if clarifications are needed.
Each practice is different, but once insurance coverage is determined, the patient may be asked to review his/her food log with the team. Those who count carbohydrates may be assessed for their ability to accurately measure and record this information, since it improves the accuracy of insulin boluses and effectiveness of treatment. Patients who do not count carbs should be advised of alternative options, such as capping meals at a certain carbohydrate amount (eg, 60 to 75 g, based on labels) or carbohydrate exchanges (eg, if a typical serving size is 15 g, patients may have 3 servings per meal).
The comfort level of the practice and the care team, as well as the patient, may influence how pump therapy is initiated. Some care teams may decide to do a trial run with saline for safety, to ensure the patient is using the pump properly before advancing to insulin.
Q What are some features to consider when selecting a pump?
The practical reality is that individual practices and providers are unlikely to offer every possible insulin pump; a practice may not have the software needed to download data from every type of pump. Patients must be comfortable with their choice of pump—but so must providers. A clinician may be more familiar and/or comfortable with a particular pump (or pumps), based in part on his/her relationship with the manufacturer. If the provider feels sufficiently educated, he or she is better equipped to advise the patient on usage.
Some of the insulin pumps available in the United States are described in the table.5-11 Note that there are many common features, such as 24-hour toll-free assistance hotlines; child button lockouts; full training; temporary basal rate options; programmable reminders; downloadable glucose data; low insulin warnings; low battery warnings; and user-set active insulin times. Other features vary and may influence a patient’s choice of pump. These include color vs black-and-white screen (which can impact patients with impaired vision); tubeless versus insulin tubes; insulin cartridge size; compatibility and integration with CGMs; various degrees of water resistance; and hypoglycemia suspension.12
Q Does insurance cover insulin pumps?
Insurance coverage varies and may be offered on a case-by-case basis. Also, some insurers have preferred insulin pumps just as they have a preferred formulary.
Some insurance companies may require patients to use multiple daily injections for at least six months prior to pump approval. Prior authorization for a pump trial (of a specific duration) may be required; after trial completion, another prior authorization may be needed before approval is granted. During the trial, the patient will need to demonstrate competency in self-management with the pump, motivation to continue use, and commitment to making dietary and lifestyle changes. Some insurers may want to see A1C lowered to less than 7%, although this stipulation may be assessed by case, particularly in patients with risk factors for poor glycemic control (eg, recurrent hypoglycemia, severe hypoglycemic episode, dawn phenomenon, large glycemic excursions, or pregnancy). Others will require patients to complete a comprehensive diabetes education program within two years prior to pump initiation.13,14
For Medicare-qualified patients, pump supplies may not be covered; they may have to rely on supplemental insurance or switch therapies if insulin pump usage is not financially feasible. Under “original Medicare,” patients pay 20% of the Medicare-approved amount after the part B deductible for pump equipment (which is categorized as durable medical equipment). Medicare does pay for 80% of the insulin cost, but coverage can differ by case.15
Q What are CGMs, and are they required?
CGMs are small, external devices that sample glucose from the interstitial fluid using an electrode under the skin. The electrode transmits information to a display device, which can alert patients of patient-specific glucose values: if a high or low glucose value is reached; if the glucose value is predicted to change; or the rate of glucose value change.
CGMs do not completely replace testing glucose levels, as they typically need to be calibrated two or more times throughout the day (though upcoming technology may reduce that to one or no calibrations). During calibration, patients manually check their glucose levels and enter readings into the CGM to ensure accuracy.
CGMs are not required, but they can enhance a patient’s insulin pump experience. Some CGMs “talk” directly with insulin pumps, so users do not have to manually input glucose levels. However, while there is even a CGM on the market that allows insulin dosing without a finger-prick, most sensors encourage patients to dose insulin based on manual glucose readings rather than CGM readings. A notable exception is a CGM “talking” to an insulin pump with a safety feature that can temporarily shut off the pump if low glucose levels are detected or predicted.16-18
Q What are the latest advances in insulin pumps?
In September 2016, the FDA approved Medtronic’s hybrid closed-loop system for use by patients (ages 14 and older) with type 1 diabetes who use 8 U or more of insulin daily. This new insulin pump system, which uses a CGM, is sometimes referred to as an “artificial pancreas.” It tracks glucose levels every five minutes, increasing or decreasing basal insulin rates according to a glucose-based algorithm (though users still bolus at mealtimes by entering carbohydrates and calibrating their sensor).19,20 Approval was based on results from a study of patients ages 14 to 75 with type 1 diabetes, which showed a reduction in A1C from 7.4% to 6.9%, without severe hypoglycemia or diabetic ketoacidosis. The percentage of patients in target range (defined as 70-180 mg/dL) increased from 66.7% at baseline to 72.2% at the end of the study.21 Medtronic officially launched the device in June 2017.
Other manufacturers are not far behind. Tandem is currently participating in an NIH-funded International Diabetes Closed Loop (IDCL) Trial of combined technology from Tandem Diabetes Care, Dexcom, and TypeZero (a software company). The company projects a launch date of late 2018 for their product.22
Editor’s Note: At press time, Animas had announced that it will be discontinuing its insulin pumps in the United States. More information, including a transition plan for patients, is available at www.animaspatientsupport.com.
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
1. American Diabetes Association. Standards of Medical Care in Diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S112.
2. American Diabetes Association. Insulin pumps. www.diabetes.org/living-with-diabetes/treatment-and-care/medication/insulin/insulin-pumps.html. Accessed October 2, 2017.
3. Aronson R, Cohen O, Conget I, et al; OpT2mis Study Group. OpT2mise: a randomized controlled trial to compare insulin pump therapy with multiple daily injections in the treatment of type 2 diabetes—research design and methods. Diabetes Technol Ther. 2014;16:414-420.
4. Ly TT, Brnabic AJ, Eggleston A, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561-569.
5. Palinski-Wade E. Everything you need to know about insulin pumps. Diabetes Forecast. March/April 2017: Consumer Guide. www.diabetesforecast.org/2017/mar-apr/insulin-pumps-101.html. Accessed October 2, 2017.
6. Roche Diabetes Care, Inc. ACCU-CHECK Spirit Combo User’s Manual (2016). www.accu-chek.com/download/file/fid/17481. Accessed October 2, 2017.
7. Tandem Diabetes Care. t:slim Insulin Pump User Guide (2017). https://www.tandemdiabetes.com/docs/default-source/product-documents/tslim-insulin-pump/updated-t-slim-user-guide.pdf. Accessed October 2, 2017.
8. Medtronic. Using the Minimed 630G Insulin Pump (2016). www.medtronicdiabetes.com/sites/default/files/library/download-library/workbooks/950M15270-011.pdf. Accessed October 2, 2017.
9. Omnipod. UST400 user guide: insulin management system (2015). https://www.myomnipod.com/sites/default/files/pdf/ust400_user_guide_EN.pdf. Accessed October 2, 2017.
10. Animas. Diabetes training resources (2017). www.animas.com/diabetes-education-and-training/diabetes-resources. Accessed October 2, 2017.
11. SOOIL Development Co, Ltd. DANA Diabecare R (2017). http://sooil.com/eng/product. Accessed October 2, 2017.
12. Integrated Diabetes Services. Top insulin pump comparisons (2016). http://integrateddiabetes.com/insulin-pump-comparisons. Accessed October 2, 2017.
13. MassHealth. Guidelines for medical necessity determination for ambulatory infusion pumps (insulin pumps) (2011). www.mass.gov/eohhs/docs/masshealth/guidelines/insulin-pump-guideline.pdf. Accessed October 2, 2017.
14. Anthem. Clinical UM Guideline (2017). www.anthem.com/medicalpolicies/guidelines/gl_pw_a053532.htm. Accessed October 2, 2017.
15. Centers for Medicare & Medicaid Services. Your Medicare coverage: insulin pumps and supplies. www.medicare.gov/coverage/infusion-pumps.html. Accessed October 2, 2017.
16. Dexcom. What is continuous glucose monitoring? (2017). www.dexcom.com/continuous-glucose-monitoring. Accessed October 2, 2017.
17. Medtronic. Continuous glucose monitoring (2017). www.medtronicdiabetes.com/treatments/continuous-glucose-monitoring. Accessed October 2, 2017.
18. Medtronic. SmartGuard low management suspend quick reference (2017). www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/smartguard-quick-reference. Accessed October 2, 2017.
19. FDA. FDA approves first automated insulin delivery device for type 1 diabetes [press release]. September 28, 2016. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm. Accessed October 2, 2017.
20. FDA. Medical devices: the 670G System - P160017 (2016). www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm522764.htm. Accessed October 2, 2017.
21. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. .
Moderate psoriasis: the new frontier for systemic therapies
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
GENEVA – Apremilast showed substantial efficacy for patients with truly moderate psoriasis as defined by an involved body surface area of 5%-10% in the first-of-its-kind UNVEIL trial, Bruce Strober, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Patients with moderate psoriasis constitute a very large and underserved population, said Dr. Strober, professor and chair of the department of dermatology at the University of Connecticut, Farmington. “I would say the moderate psoriasis group defined by 5%-10% psoriasis-involved body surface area represents a gray area in our treatment. Often, people with this degree of psoriasis receive no treatment or are relegated to topical monotherapy, yet unfortunately do not respond to those treatments. Nevertheless, clinical trials for systemic therapies, including biologics, exclude this population solely because they’re under 10% involved body surface area,” he noted.
“A large percentage of my patients who are on biologic therapy have less than 10% involved body surface area and would have a PASI [Psoriasis Area Severity Index] score, if I were to measure it, of under 12. Therefore they couldn’t get into a typical registration study for moderate to severe psoriasis,” he continued.
The UNVEIL trial included a 16-week, double-blind, placebo-controlled phase in which 221 systemic therapy–naive patients with plaque psoriasis on 5%-10% of their body surface area (BSA) were randomized 2:1 to apremilast at 30 mg twice a day or placebo. Thereafter, the placebo group was switched over to apremilast and the trial continued in open-label fashion out to 52 weeks. Apremilast (Otezla) is approved only for use in moderate to severe psoriasis.
At baseline, participants had a mean 15-year duration of psoriasis, an involved BSA of 7.1%, a PASI score of 8.1, a static Physician’s Global Assessment (PGA) score of 3 on a 0-5 scale, and a Dermatology Life Quality Index (DLQI) score of 11.
UNVEIL not only targeted a new population for a modern systemic therapy, it also debuted as its primary endpoint a novel metric for disease severity. Because PASI score is a relatively crude measure of change in a population with moderate psoriasis, Dr. Strober and his coinvestigators developed and employed as the primary outcome measure PGA+BSA, which can range from 15 to 30.
The mean baseline PGA+BSA was 21.8. At week 16, the placebo group averaged a 10% decrease in this metric, while the apremilast group showed a 48% reduction. At week 52, the group switched from placebo to apremilast at 16 weeks had a 42% improvement in PGA+BSA and patients on apremilast for the full study had a 49% improvement.
“So you can assume about half of patients with moderate psoriasis will experience a 50% reduction in the product of PGA+BSA on apremilast,” Dr. Strober observed.
At week 52, a PGA score of 0 or 1, meaning clear or almost clear, was present in 36% of the switchover group and 29% of patients on apremilast for 52 weeks.
A 75% improvement in PGA+BSA, or a PGA+BSA–75 response, occurred at week 52 in 45% of the switchover group and 37% of those on apremilast for the entire study.
The mean improvement in the DLQI at week 16 was 2.4 points in the placebo arm and twice that amount with apremilast. At 52 weeks, the switchover group averaged a 5.1-point reduction in DLQI from baseline and the full-time apremilast group had a 4.3-point reduction.
The incidence of apremilast-related adverse events didn’t increase over time. The main issues were diarrhea, nausea, and headache, as is the case when the oral drug is prescribed for its approved indication in patients with moderate to severe psoriasis.
In an interview, Dr. Strober said that despite the encouraging results of UNVEIL, the study is not by itself sufficient evidence to win an expanded indication for apremilast from regulatory agencies and the drug’s manufacturer, Celgene, is not interested in pursuing that course.
UNVEIL, a phase 4 study, was funded by Celgene. Dr. Strober reported serving as a consultant to and/or receiving research funding from that company and more than a dozen others.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Roughly half of apremilast-treated patients with moderate psoriasis as defined by an involved body surface area of 5%-10% experienced a 50% reduction in a novel outcome metric: the product of the Physician’s Global Assessment plus the involved body surface area.
Data source: This 52-week study of 221 patients with truly moderate psoriasis featured a 16-week, double-blind, placebo-controlled phase, after which everyone continued on open-label apremilast out to 51 weeks.
Disclosures: The UNVEIL study was sponsored by Celgene. The presenter reported receiving research funding from and serving as a consultant to that company and numerous others.
Red Scaly Rash Following Tattoo Application
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
The Diagnosis: Isomorphic Psoriasis
Tattooing has become an increasingly popular trend among young people. Currently, there are no guidelines in the United States regulating the production of tattoo ink and pigments.1 Henna tattooing, a form of temporary skin painting, also has risks of allergic contact dermatitis from paraphenylenediamine dye.2 Complications following tattoo application include an allergic contact dermatitis to tattoo pigments, infection, granulomatous and lichenoid reactions, and skin disease localized to the tattooed area.3
Localized dermatosis arising in a traumatized area, or the Koebner phenomenon, was first described by Heinrich Koebner in 1877.4 He described the formation of psoriasiform lesions at the site of cutaneous trauma.5 These isomorphic lesions can occur in 25% of patients with psoriasis after trauma to the skin such as tattooing.6 Other dermatologic diseases that can present as an isomorphic response to tattooing include lichen planus, Darier disease, vitiligo, and autoimmune bullous disease.3,5,6
Various causes of trauma such as burns, insect bites, physical trauma, and needle trauma have been shown to produce new psoriatic lesions.6 The time period from trauma to formation of psoriasiform lesions usually ranges from 10 to 20 days; however, an initial reaction can occur as early as 3 days or as long as 2 years after trauma.4 Although the pathophysiology of the isomorphic response is not well known, it has been shown that nerve growth factor has a role. Raychaudhuri et al7 demonstrated the upregulation of nerve growth factor in the development of a psoriatic lesion, influencing keratinocyte proliferation, angiogenesis, and T-cell activation.
Physical trauma such as tattooing has been shown to cause an isomorphic response in psoriasis. We describe a case of isomorphic psoriasis in a patient after tattoo application. Our patient had a several-month history of well-controlled psoriasis prior to obtaining the new tattoo. Several days after receiving the tattoo, the patient reported an increase in psoriatic lesions, including at the site of the tattoo. The trauma causing the isomorphic response could have been either a response to the tattoo pigment or needle injury to the skin.6
Psoriasis and isomorphic lesions can be treated with topical corticosteroids as well as systemic and biologic agents. Our patient was treated with triamcinolone cream with good response.8
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.
- Haugh IM, Laumann SL, Laumann AE. Regulation of tattoo ink production and the tattoo business in the US. Curr Probl Dermatol. 2015;48:248-252.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Bassi A, Campolmi P, Cannarozzo G, et al. Tattoo-associated skin reaction: the importance of an early diagnosis and proper treatment [published online July 23, 2014]. Biomed Res Int. 2014;2014:354608.
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16:241-248.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Orzan OA, Popa LG, Vexler ES, et al. Tattoo-induced psoriasis. J Med Life. 2014;7:65-68.
- Raychaudhuri SP, Jiang WY, Raychaudhuri SK. Revisiting the Koebner phenomenon: role of NGF and its receptor system in the pathogenesis of psoriasis. Am J Pathol. 2008;172:961-971.
- Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(1 suppl 1):S3-S16.

A 26-year-old man presented with a mildly pruritic red scaly rash on the right arm of 3 weeks' duration. He reported having a tattoo placed on previously normal skin on the right lateral arm prior to the development of the rash. Two weeks after receiving the tattoo, he developed scaling and redness of the skin involved in the tattoo. He also had similar papules and plaques over the rest of his body. Physical examination showed well-demarcated, erythematous, scaly papules and plaques following the design of a black-pigmented tattoo on the lateral aspect of the right arm. There also were similar erythematous scaly plaques scattered over both arms and the trunk. He denied any pain or blister formation of the involved areas.
Pediatric thyroid nodules: Experienced radiologists best ultrasound risk stratification
VICTORIA, B.C. – Ultrasound risk criteria for adults are no match for the training, skill, and gut instinct of an experienced radiologist when evaluating pediatric thyroid nodules, results of a retrospective cohort study reported at the annual meeting of the American Thyroid Association suggest.
“In 2015, the ATA commissioned a pediatric task force that developed valuable guidelines specific to our pediatric patients. These guidelines recommend performing an FNA [fine-needle aspiration biopsy] in any nodule with a concerning clinical history or a concerning ultrasound feature,” commented first author Ana L. Creo, MD, a pediatric endocrinology fellow at the Mayo Clinic in Rochester, Minn.
She and her colleagues analyzed findings from diagnostic ultrasound in 112 patients aged under 21 years who had 145 thyroid nodules that were ultimately assessed histologically or cytologically.
Results showed that the radiologists’ overall impression and the ATA risk-stratification system for adults had the same high sensitivity, picking up 9 out of 10 malignant cases, she reported. But the radiologists’ overall impression had much higher specificity, correctly classifying 8 out of 10 benign cases, versus about 5 out of 10 for the risk stratification.
“These findings may have implications in trying to avoid unnecessary FNAs, particularly in our population,” Dr. Creo summarized. “Our million dollar question is trying to get in the heads of the radiologists to figure out what really goes into that overall impression. And if we can apply a specific score to that, I think that would be most clinically useful.”
“Based upon these results, further work is needed to determine the usefulness of the adult ATA ultrasound risk stratification in children, moving towards an ultrasound-based stratification system specific to our pediatric patients,” she concluded.
One session cochair, Catherine A. Dinauer, MD, a pediatric endocrinologist and clinician at the Yale Pediatric Thyroid Center, New Haven, Conn., commented, “It seems as though we’re pretty good at picking up which nodules are malignant, but I still feel like so many of the nodules that are benign are suspicious by ultrasound. Trying to tease out what is it about those benign ones may allow us to figure out in which ones we could avoid biopsy. That’s where we see we are not that good at it.”
Nodule attributes that might help in this regard include subtypes of microcalcifications, irregular margins, and position of the nodule in the gland relative to the skin, she proposed.
The other session cochair, Yaron Tomer, MD, chair of the department of medicine and the Anita and Jack Saltz Chair in Diabetes Research at the Montefiore Medical Center, New York, stressed knowing one’s radiologist and questioned the generalizability of the findings.
“You have to know your own radiologist well and how well you can trust them. Probably, the investigators chose some of the top radiologists in their institution and maybe even in the nation, so we have to be careful as to whether this applies to places that don’t have access to such great radiologists,” he commented. “But I think even if the guidelines are not perfect, they are the best we have right now.”
Study details
The investigators studied pediatric patients (mean age, 15.5 years) with thyroid nodules who underwent initial ultrasound at the Mayo Clinic during 1996-2015, had at least a year of follow-up, and for whom histology or cytology results were available. Those with a known genetic tumor syndrome or a history of radiation exposure were excluded.
Two blinded radiologists assessed nodule ultrasound features using the Thyroid Imaging and Reporting Data System (TIRADS) (J Am Coll Radiol. 2015;12[12 Pt A]:1272-9) and then rendered their overall impression: malignant, indeterminate, or benign.
Next, an independent reviewer assigned each nodule an ATA adult risk category (Thyroid. 2016;26:1-133): high, intermediate, low, or very low suspicion.
Finally, both measures were compared against the reference standard of the nodule’s histology or cytology results.
Ultimately, 34% of the patients had malignant nodules, Dr. Creo reported. “This is likely quite a bit elevated from the true prevalence due to our intentional study design requiring follow-up, likely excluding some patients with benign nodules,” she commented.
Patients with benign and malignant nodules did not differ significantly on any of a variety of sociodemographic and clinical factors, such as family history and mode of detection.
For comparison of sensitivity, the investigators combined the ATA risk categories of high and intermediate suspicion and combined the overall radiologists’ impression of malignant and indeterminate. “We felt this best answered the practical clinical question of how many malignant nodules would be missed if FNA was not performed, assuming FNA would typically be performed if the ATA risk stratification was high or intermediate or if the radiologist’s overall impression was malignant or indeterminate,” Dr. Creo explained.
Results here showed that the ATA risk stratification and the radiologists’ overall impression had the same high sensitivity of 90%.
For comparison of specificity, the investigators compared the ATA risk category of high suspicion with the radiologists’ overall impression of malignant.
Results showed that the overall impression had specificity of 80%, whereas the risk category had a specificity of only about 52%. Findings were similar when analyses instead used ATA high suspicion and intermediate suspicion combined.
“The key ultrasound characteristics that drove the diagnosis included having a solid component, calcifications, irregular margins, and hypoechogenicity – all similar to those seen in pediatric studies and similar to those in adult studies as well,” Dr. Creo noted.
Compared with benign nodules, malignant nodules significantly more often had a greater than 75% solid component (84% vs. 64%; P = .01), contained calcifications (60% vs. 18%; P less than .0001), had irregular margins (70% vs. 46%; P = .0073), and were hypoechogenic (74% vs. 51%; P = .0073). Notably, size and presence of halo did not differ significantly.
“Our study adds to previous work in that it had a relatively large pediatric sample size, used strict inclusion criteria with at least a year of follow-up to increase the validity of the diagnosis, and had precise definitions of the ultrasound features,” concluded Dr. Creo, who disclosed that she had no relevant conflicts of interest.
At the same time, the study had limitations, such as its use of a referral population, likely loss to follow-up of some patients with benign nodules, and possible clustering effect. “Lastly, we had the benefit of extremely experienced pediatric radiologists, and their overall diagnostic accuracy may not universally apply across all radiologists,” she said.
VICTORIA, B.C. – Ultrasound risk criteria for adults are no match for the training, skill, and gut instinct of an experienced radiologist when evaluating pediatric thyroid nodules, results of a retrospective cohort study reported at the annual meeting of the American Thyroid Association suggest.
“In 2015, the ATA commissioned a pediatric task force that developed valuable guidelines specific to our pediatric patients. These guidelines recommend performing an FNA [fine-needle aspiration biopsy] in any nodule with a concerning clinical history or a concerning ultrasound feature,” commented first author Ana L. Creo, MD, a pediatric endocrinology fellow at the Mayo Clinic in Rochester, Minn.
She and her colleagues analyzed findings from diagnostic ultrasound in 112 patients aged under 21 years who had 145 thyroid nodules that were ultimately assessed histologically or cytologically.
Results showed that the radiologists’ overall impression and the ATA risk-stratification system for adults had the same high sensitivity, picking up 9 out of 10 malignant cases, she reported. But the radiologists’ overall impression had much higher specificity, correctly classifying 8 out of 10 benign cases, versus about 5 out of 10 for the risk stratification.
“These findings may have implications in trying to avoid unnecessary FNAs, particularly in our population,” Dr. Creo summarized. “Our million dollar question is trying to get in the heads of the radiologists to figure out what really goes into that overall impression. And if we can apply a specific score to that, I think that would be most clinically useful.”
“Based upon these results, further work is needed to determine the usefulness of the adult ATA ultrasound risk stratification in children, moving towards an ultrasound-based stratification system specific to our pediatric patients,” she concluded.
One session cochair, Catherine A. Dinauer, MD, a pediatric endocrinologist and clinician at the Yale Pediatric Thyroid Center, New Haven, Conn., commented, “It seems as though we’re pretty good at picking up which nodules are malignant, but I still feel like so many of the nodules that are benign are suspicious by ultrasound. Trying to tease out what is it about those benign ones may allow us to figure out in which ones we could avoid biopsy. That’s where we see we are not that good at it.”
Nodule attributes that might help in this regard include subtypes of microcalcifications, irregular margins, and position of the nodule in the gland relative to the skin, she proposed.
The other session cochair, Yaron Tomer, MD, chair of the department of medicine and the Anita and Jack Saltz Chair in Diabetes Research at the Montefiore Medical Center, New York, stressed knowing one’s radiologist and questioned the generalizability of the findings.
“You have to know your own radiologist well and how well you can trust them. Probably, the investigators chose some of the top radiologists in their institution and maybe even in the nation, so we have to be careful as to whether this applies to places that don’t have access to such great radiologists,” he commented. “But I think even if the guidelines are not perfect, they are the best we have right now.”
Study details
The investigators studied pediatric patients (mean age, 15.5 years) with thyroid nodules who underwent initial ultrasound at the Mayo Clinic during 1996-2015, had at least a year of follow-up, and for whom histology or cytology results were available. Those with a known genetic tumor syndrome or a history of radiation exposure were excluded.
Two blinded radiologists assessed nodule ultrasound features using the Thyroid Imaging and Reporting Data System (TIRADS) (J Am Coll Radiol. 2015;12[12 Pt A]:1272-9) and then rendered their overall impression: malignant, indeterminate, or benign.
Next, an independent reviewer assigned each nodule an ATA adult risk category (Thyroid. 2016;26:1-133): high, intermediate, low, or very low suspicion.
Finally, both measures were compared against the reference standard of the nodule’s histology or cytology results.
Ultimately, 34% of the patients had malignant nodules, Dr. Creo reported. “This is likely quite a bit elevated from the true prevalence due to our intentional study design requiring follow-up, likely excluding some patients with benign nodules,” she commented.
Patients with benign and malignant nodules did not differ significantly on any of a variety of sociodemographic and clinical factors, such as family history and mode of detection.
For comparison of sensitivity, the investigators combined the ATA risk categories of high and intermediate suspicion and combined the overall radiologists’ impression of malignant and indeterminate. “We felt this best answered the practical clinical question of how many malignant nodules would be missed if FNA was not performed, assuming FNA would typically be performed if the ATA risk stratification was high or intermediate or if the radiologist’s overall impression was malignant or indeterminate,” Dr. Creo explained.
Results here showed that the ATA risk stratification and the radiologists’ overall impression had the same high sensitivity of 90%.
For comparison of specificity, the investigators compared the ATA risk category of high suspicion with the radiologists’ overall impression of malignant.
Results showed that the overall impression had specificity of 80%, whereas the risk category had a specificity of only about 52%. Findings were similar when analyses instead used ATA high suspicion and intermediate suspicion combined.
“The key ultrasound characteristics that drove the diagnosis included having a solid component, calcifications, irregular margins, and hypoechogenicity – all similar to those seen in pediatric studies and similar to those in adult studies as well,” Dr. Creo noted.
Compared with benign nodules, malignant nodules significantly more often had a greater than 75% solid component (84% vs. 64%; P = .01), contained calcifications (60% vs. 18%; P less than .0001), had irregular margins (70% vs. 46%; P = .0073), and were hypoechogenic (74% vs. 51%; P = .0073). Notably, size and presence of halo did not differ significantly.
“Our study adds to previous work in that it had a relatively large pediatric sample size, used strict inclusion criteria with at least a year of follow-up to increase the validity of the diagnosis, and had precise definitions of the ultrasound features,” concluded Dr. Creo, who disclosed that she had no relevant conflicts of interest.
At the same time, the study had limitations, such as its use of a referral population, likely loss to follow-up of some patients with benign nodules, and possible clustering effect. “Lastly, we had the benefit of extremely experienced pediatric radiologists, and their overall diagnostic accuracy may not universally apply across all radiologists,” she said.
VICTORIA, B.C. – Ultrasound risk criteria for adults are no match for the training, skill, and gut instinct of an experienced radiologist when evaluating pediatric thyroid nodules, results of a retrospective cohort study reported at the annual meeting of the American Thyroid Association suggest.
“In 2015, the ATA commissioned a pediatric task force that developed valuable guidelines specific to our pediatric patients. These guidelines recommend performing an FNA [fine-needle aspiration biopsy] in any nodule with a concerning clinical history or a concerning ultrasound feature,” commented first author Ana L. Creo, MD, a pediatric endocrinology fellow at the Mayo Clinic in Rochester, Minn.
She and her colleagues analyzed findings from diagnostic ultrasound in 112 patients aged under 21 years who had 145 thyroid nodules that were ultimately assessed histologically or cytologically.
Results showed that the radiologists’ overall impression and the ATA risk-stratification system for adults had the same high sensitivity, picking up 9 out of 10 malignant cases, she reported. But the radiologists’ overall impression had much higher specificity, correctly classifying 8 out of 10 benign cases, versus about 5 out of 10 for the risk stratification.
“These findings may have implications in trying to avoid unnecessary FNAs, particularly in our population,” Dr. Creo summarized. “Our million dollar question is trying to get in the heads of the radiologists to figure out what really goes into that overall impression. And if we can apply a specific score to that, I think that would be most clinically useful.”
“Based upon these results, further work is needed to determine the usefulness of the adult ATA ultrasound risk stratification in children, moving towards an ultrasound-based stratification system specific to our pediatric patients,” she concluded.
One session cochair, Catherine A. Dinauer, MD, a pediatric endocrinologist and clinician at the Yale Pediatric Thyroid Center, New Haven, Conn., commented, “It seems as though we’re pretty good at picking up which nodules are malignant, but I still feel like so many of the nodules that are benign are suspicious by ultrasound. Trying to tease out what is it about those benign ones may allow us to figure out in which ones we could avoid biopsy. That’s where we see we are not that good at it.”
Nodule attributes that might help in this regard include subtypes of microcalcifications, irregular margins, and position of the nodule in the gland relative to the skin, she proposed.
The other session cochair, Yaron Tomer, MD, chair of the department of medicine and the Anita and Jack Saltz Chair in Diabetes Research at the Montefiore Medical Center, New York, stressed knowing one’s radiologist and questioned the generalizability of the findings.
“You have to know your own radiologist well and how well you can trust them. Probably, the investigators chose some of the top radiologists in their institution and maybe even in the nation, so we have to be careful as to whether this applies to places that don’t have access to such great radiologists,” he commented. “But I think even if the guidelines are not perfect, they are the best we have right now.”
Study details
The investigators studied pediatric patients (mean age, 15.5 years) with thyroid nodules who underwent initial ultrasound at the Mayo Clinic during 1996-2015, had at least a year of follow-up, and for whom histology or cytology results were available. Those with a known genetic tumor syndrome or a history of radiation exposure were excluded.
Two blinded radiologists assessed nodule ultrasound features using the Thyroid Imaging and Reporting Data System (TIRADS) (J Am Coll Radiol. 2015;12[12 Pt A]:1272-9) and then rendered their overall impression: malignant, indeterminate, or benign.
Next, an independent reviewer assigned each nodule an ATA adult risk category (Thyroid. 2016;26:1-133): high, intermediate, low, or very low suspicion.
Finally, both measures were compared against the reference standard of the nodule’s histology or cytology results.
Ultimately, 34% of the patients had malignant nodules, Dr. Creo reported. “This is likely quite a bit elevated from the true prevalence due to our intentional study design requiring follow-up, likely excluding some patients with benign nodules,” she commented.
Patients with benign and malignant nodules did not differ significantly on any of a variety of sociodemographic and clinical factors, such as family history and mode of detection.
For comparison of sensitivity, the investigators combined the ATA risk categories of high and intermediate suspicion and combined the overall radiologists’ impression of malignant and indeterminate. “We felt this best answered the practical clinical question of how many malignant nodules would be missed if FNA was not performed, assuming FNA would typically be performed if the ATA risk stratification was high or intermediate or if the radiologist’s overall impression was malignant or indeterminate,” Dr. Creo explained.
Results here showed that the ATA risk stratification and the radiologists’ overall impression had the same high sensitivity of 90%.
For comparison of specificity, the investigators compared the ATA risk category of high suspicion with the radiologists’ overall impression of malignant.
Results showed that the overall impression had specificity of 80%, whereas the risk category had a specificity of only about 52%. Findings were similar when analyses instead used ATA high suspicion and intermediate suspicion combined.
“The key ultrasound characteristics that drove the diagnosis included having a solid component, calcifications, irregular margins, and hypoechogenicity – all similar to those seen in pediatric studies and similar to those in adult studies as well,” Dr. Creo noted.
Compared with benign nodules, malignant nodules significantly more often had a greater than 75% solid component (84% vs. 64%; P = .01), contained calcifications (60% vs. 18%; P less than .0001), had irregular margins (70% vs. 46%; P = .0073), and were hypoechogenic (74% vs. 51%; P = .0073). Notably, size and presence of halo did not differ significantly.
“Our study adds to previous work in that it had a relatively large pediatric sample size, used strict inclusion criteria with at least a year of follow-up to increase the validity of the diagnosis, and had precise definitions of the ultrasound features,” concluded Dr. Creo, who disclosed that she had no relevant conflicts of interest.
At the same time, the study had limitations, such as its use of a referral population, likely loss to follow-up of some patients with benign nodules, and possible clustering effect. “Lastly, we had the benefit of extremely experienced pediatric radiologists, and their overall diagnostic accuracy may not universally apply across all radiologists,” she said.
AT ATA 2017
Key clinical point:
Major finding: Radiologists’ overall impression and ATA risk stratification had the same good sensitivity (90% for each), but the former had higher specificity (80% vs. 52%).
Data source: A retrospective cohort study of 112 patients younger than 21 who had 145 thyroid nodules.
Disclosures: Dr. Creo disclosed that she had no relevant conflicts of interest.
Low vitamin D levels linked to increased ESRD risk in SLE patients
SAN DIEGO – , results from a single-center cohort study showed.
“We had previously proved that vitamin D supplementation helped lupus activity,” lead study author Michelle Petri, MD, MPH, said in an interview in advance of the annual meeting of the American College of Rheumatology. “Now, we prove that it specifically helps renal activity as measured by the urine protein. By helping to reduce urine protein, it helps to prevent permanent renal damage and end-stage renal disease.”
The first measure of vitamin D typically occurred in late 2009 or 2010 for existing patients and at the first visit of new patients after that. The researchers categorized patients based on their first measure of vitamin D as less than 20 ng/mL or 20 ng/mL or higher. At the first visit when vitamin D was measured, 27.3% had levels of 25-hydroxyvitamin D less than 20 ng/mL. The mean age of patients was 47.3 years, 92% were female, 50% were white, and 41% were African American.
In the study, Dr. Petri and her associates used the Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index to calculate the risk of lifetime organ damage. After adjusting for age, gender, and ethnicity, low levels of vitamin D were significantly associated with increased risk of renal damage (RR, 1.66; P = .0206) and total organ damage (RR, 1.17; P = .0245), they found.
Skin damage was another concern, with an adjusted relative risk of 1.22, though it was not statistically significant (P = .3561). The investigators observed no association between low vitamin D and musculoskeletal damage, including osteoporotic fractures.
“There is a lot of interest in lupus right now, due to [singer Selena] Gomez’s kidney transplant for lupus nephritis,” said Dr. Petri, who also directs the Johns Hopkins Lupus Center. “So, I think there is interest in how to prevent the need for kidney transplant. Vitamin D helps kidney lupus – and we only need to achieve a level of 40 ng/mL, [which is] safe and easy to do.” She acknowledged the study’s single-center design as a limitation but underscored its large sample size as a strength.
The Hopkins Lupus Cohort is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Petri disclosed having received research support from Anthera, GlaxoSmithKline, EMD Serono, Eli Lilly, Bristol-Myers Squibb, Amgen, United Rheumatology, Global Academy, and Exagen.
SAN DIEGO – , results from a single-center cohort study showed.
“We had previously proved that vitamin D supplementation helped lupus activity,” lead study author Michelle Petri, MD, MPH, said in an interview in advance of the annual meeting of the American College of Rheumatology. “Now, we prove that it specifically helps renal activity as measured by the urine protein. By helping to reduce urine protein, it helps to prevent permanent renal damage and end-stage renal disease.”
The first measure of vitamin D typically occurred in late 2009 or 2010 for existing patients and at the first visit of new patients after that. The researchers categorized patients based on their first measure of vitamin D as less than 20 ng/mL or 20 ng/mL or higher. At the first visit when vitamin D was measured, 27.3% had levels of 25-hydroxyvitamin D less than 20 ng/mL. The mean age of patients was 47.3 years, 92% were female, 50% were white, and 41% were African American.
In the study, Dr. Petri and her associates used the Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index to calculate the risk of lifetime organ damage. After adjusting for age, gender, and ethnicity, low levels of vitamin D were significantly associated with increased risk of renal damage (RR, 1.66; P = .0206) and total organ damage (RR, 1.17; P = .0245), they found.
Skin damage was another concern, with an adjusted relative risk of 1.22, though it was not statistically significant (P = .3561). The investigators observed no association between low vitamin D and musculoskeletal damage, including osteoporotic fractures.
“There is a lot of interest in lupus right now, due to [singer Selena] Gomez’s kidney transplant for lupus nephritis,” said Dr. Petri, who also directs the Johns Hopkins Lupus Center. “So, I think there is interest in how to prevent the need for kidney transplant. Vitamin D helps kidney lupus – and we only need to achieve a level of 40 ng/mL, [which is] safe and easy to do.” She acknowledged the study’s single-center design as a limitation but underscored its large sample size as a strength.
The Hopkins Lupus Cohort is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Petri disclosed having received research support from Anthera, GlaxoSmithKline, EMD Serono, Eli Lilly, Bristol-Myers Squibb, Amgen, United Rheumatology, Global Academy, and Exagen.
SAN DIEGO – , results from a single-center cohort study showed.
“We had previously proved that vitamin D supplementation helped lupus activity,” lead study author Michelle Petri, MD, MPH, said in an interview in advance of the annual meeting of the American College of Rheumatology. “Now, we prove that it specifically helps renal activity as measured by the urine protein. By helping to reduce urine protein, it helps to prevent permanent renal damage and end-stage renal disease.”
The first measure of vitamin D typically occurred in late 2009 or 2010 for existing patients and at the first visit of new patients after that. The researchers categorized patients based on their first measure of vitamin D as less than 20 ng/mL or 20 ng/mL or higher. At the first visit when vitamin D was measured, 27.3% had levels of 25-hydroxyvitamin D less than 20 ng/mL. The mean age of patients was 47.3 years, 92% were female, 50% were white, and 41% were African American.
In the study, Dr. Petri and her associates used the Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index to calculate the risk of lifetime organ damage. After adjusting for age, gender, and ethnicity, low levels of vitamin D were significantly associated with increased risk of renal damage (RR, 1.66; P = .0206) and total organ damage (RR, 1.17; P = .0245), they found.
Skin damage was another concern, with an adjusted relative risk of 1.22, though it was not statistically significant (P = .3561). The investigators observed no association between low vitamin D and musculoskeletal damage, including osteoporotic fractures.
“There is a lot of interest in lupus right now, due to [singer Selena] Gomez’s kidney transplant for lupus nephritis,” said Dr. Petri, who also directs the Johns Hopkins Lupus Center. “So, I think there is interest in how to prevent the need for kidney transplant. Vitamin D helps kidney lupus – and we only need to achieve a level of 40 ng/mL, [which is] safe and easy to do.” She acknowledged the study’s single-center design as a limitation but underscored its large sample size as a strength.
The Hopkins Lupus Cohort is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Petri disclosed having received research support from Anthera, GlaxoSmithKline, EMD Serono, Eli Lilly, Bristol-Myers Squibb, Amgen, United Rheumatology, Global Academy, and Exagen.
AT ACR 2017
Key clinical point: Supplemental vitamin D should be part of the treatment plan for patients with systemic lupus erythematosus (SLE).
Major finding: SLE patients with low vitamin D levels face a significantly increased risk of renal damage (relatve risk, 1.66; P = .0206) and total organ damage (RR, 1.17; P = .0245).
Study details: A single-center cohort study of 1,392 patients with SLE.
Disclosures: The Hopkins Lupus Cohort is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Petri disclosed having received research support from Anthera, GlaxoSmithKline, EMD Serono, Eli Lilly, Bristol-Myers Squibb, Amgen, United Rheumatology, Global Academy, and Exagen.
FDA approves first Erdheim-Chester disease treatment
The kinase inhibitor – marketed as Zelboraf – was approved on Nov. 6. It is the first approved treatment for ECD and is already on the market as a treatment for patients with unresectable or metastatic melanoma with BRAF V600E mutation.
The FDA expedited approval of the drug under the Priority Review and Breakthrough Therapy programs. The drug also received an orphan status designation, which makes the sponsor eligible for incentives such as tax credits for clinical testing.
The agency based its approval on results from 22 patients with BRAF-V600-mutation positive ECD. Half of the patients (11) experienced a partial reduction in tumor size and 1 patient experienced a complete response, according to the FDA. Initial results from the phase 2, open-label VE-BASKET study were published in 2015 (N Engl J Med. 2015 Aug 20;373[8]:726-36).
Common side effects of vemurafenib include arthralgias, maculopapular rash, alopecia, fatigue, prolonged QT interval, and papilloma. Severe side effects include development of new cancers, growth of tumors in patients with BRAF wild-type melanoma, anaphylaxis and DRESS syndrome, severe skin reactions, heart abnormalities, hepatotoxicity, photosensitivity, uveitis, radiation sensitization and radiation recall, and Dupuytren’s contracture and plantar fascial fibromatosis. The drug is also considered teratogenic and women should be advised to use contraception while taking it, according to the FDA.
The full prescribing information is available at zelboraf.com.
[email protected]
On Twitter @maryellenny
The kinase inhibitor – marketed as Zelboraf – was approved on Nov. 6. It is the first approved treatment for ECD and is already on the market as a treatment for patients with unresectable or metastatic melanoma with BRAF V600E mutation.
The FDA expedited approval of the drug under the Priority Review and Breakthrough Therapy programs. The drug also received an orphan status designation, which makes the sponsor eligible for incentives such as tax credits for clinical testing.
The agency based its approval on results from 22 patients with BRAF-V600-mutation positive ECD. Half of the patients (11) experienced a partial reduction in tumor size and 1 patient experienced a complete response, according to the FDA. Initial results from the phase 2, open-label VE-BASKET study were published in 2015 (N Engl J Med. 2015 Aug 20;373[8]:726-36).
Common side effects of vemurafenib include arthralgias, maculopapular rash, alopecia, fatigue, prolonged QT interval, and papilloma. Severe side effects include development of new cancers, growth of tumors in patients with BRAF wild-type melanoma, anaphylaxis and DRESS syndrome, severe skin reactions, heart abnormalities, hepatotoxicity, photosensitivity, uveitis, radiation sensitization and radiation recall, and Dupuytren’s contracture and plantar fascial fibromatosis. The drug is also considered teratogenic and women should be advised to use contraception while taking it, according to the FDA.
The full prescribing information is available at zelboraf.com.
[email protected]
On Twitter @maryellenny
The kinase inhibitor – marketed as Zelboraf – was approved on Nov. 6. It is the first approved treatment for ECD and is already on the market as a treatment for patients with unresectable or metastatic melanoma with BRAF V600E mutation.
The FDA expedited approval of the drug under the Priority Review and Breakthrough Therapy programs. The drug also received an orphan status designation, which makes the sponsor eligible for incentives such as tax credits for clinical testing.
The agency based its approval on results from 22 patients with BRAF-V600-mutation positive ECD. Half of the patients (11) experienced a partial reduction in tumor size and 1 patient experienced a complete response, according to the FDA. Initial results from the phase 2, open-label VE-BASKET study were published in 2015 (N Engl J Med. 2015 Aug 20;373[8]:726-36).
Common side effects of vemurafenib include arthralgias, maculopapular rash, alopecia, fatigue, prolonged QT interval, and papilloma. Severe side effects include development of new cancers, growth of tumors in patients with BRAF wild-type melanoma, anaphylaxis and DRESS syndrome, severe skin reactions, heart abnormalities, hepatotoxicity, photosensitivity, uveitis, radiation sensitization and radiation recall, and Dupuytren’s contracture and plantar fascial fibromatosis. The drug is also considered teratogenic and women should be advised to use contraception while taking it, according to the FDA.
The full prescribing information is available at zelboraf.com.
[email protected]
On Twitter @maryellenny
Teach your adolescent patients about normal menses, so they know when it’s abnormal
CHICAGO – , according to S. Paige Hertweck, MD, chief of gynecology at Norton Children’s Hospital in Louisville, Ky.
“Remember to use the menstrual cycle as a vital sign,” Dr. Hertweck told attendees at the American Academy of Pediatrics annual meeting. “Even within the first year of menarche, most girls have a period at least every 90 days, so work up those who don’t.”
The median age of menarche is 12.4 years, typically beginning within 2-3 years of breast budding at Tanner Stage 4 breast development, she said. By 15 years of age, 98% of girls have begun menstruation.
Girls’ cycles typically last 21-45 days, an average of 32.2 days during their first year of menstruation, with flow for 7 days or less, requiring an average of 3-6 pads and/or tampons per day. Dr. Hertweck recommends you write down these features of normal menstruation so that your patients can tell you when their cycle is abnormal or menses doesn’t return.
“Cycle length is more variable for teens versus women 20-40 years old,” she said. However, “it’s not true that ‘anything goes’ for cycle length” in teens, she added. “Cycles that are consistently outside the range of 21-45 days are statistically uncommon.” Hence the need to evaluate causes of amenorrhea in girls whose cycles exceed 90 days.
Possible causes of amenorrhea include pregnancy, polycystic ovary syndrome, thyroid abnormalities, hyperprolactinemia, primary ovarian insufficiency, or hypogonadal amenorrhea, typically stimulated by the first instance of anorexia, Crohn’s disease, celiac disease, or a gluten intolerance.
Primary amenorrhea
Dr. Hertweck listed five benchmarks that indicate primary amenorrhea requiring evaluation. Those indicators include girls who have no menarche by age 15 years or within 3 years of breast budding, no breast development by age 13 years, or no menses by age 14 years with hirsutism or with a history of excessive exercise or of an eating disorder.
You can start by examining what normal menstruation relies on: an intact central nervous system with a functioning pituitary, an ovarian response, and a normal uterus, cervix, and vagina. You should check the patient’s follicle-stimulating hormone, thyroid-stimulating hormone, and prolactin levels to assess CNS functioning, and estradiol levels to assess ovarian response. A genital exam with a pelvic ultrasound can reveal any possible defects in the uterus, cervix, or vagina.
The presence of breasts without a uterus indicates normal estrogen production, so the missing uterus could be a congenital defect or result from androgen insensitivity, Dr. Hertweck explained. In those without breasts, gonadal dysgenesis or gonadal enzymatic deficiency may explain no estrogen production. If the patient has both breasts and a uterus, you should rule out pregnancy first and then track CNS changes via FSH, TSH, and prolactin levels.
Premature ovarian insufficiency
Approximately 1% of females experience premature ovarian insufficiency, which can be diagnosed as early as age 14 years and should be suspected in a patient with a uterus but without breasts who has low estradiol levels, CNS failure identified by a high FSH level, and gonadal failure.
Formal diagnosis requires two separate instances of FSH elevation, and chromosomal testing should be done to rule out gonadal dysgenesis. You also should test the serum anti-Müllerian hormone biomarker (readings above 8 are concerning) and look for two possible causes. The FMR1 (Fragile X) premutation carrier status could be a cause, or presence of 21-hydroxylase and/or adrenal antibodies indicate autoimmune polyglandular syndrome.
Catching premature ovarian insufficiency early enough may allow patients to preserve some fertility if they still have oocytes present. Aside from this, girls will need hormone replacement therapy to fulfill developmental emotional and physical needs, such as bone growth and overall health. Despite a history of treating teens with premature ovarian insufficiency like adults, you should follow the practice guidelines specific to adolescents by the American College of Obstetricians and Gynecologists committee opinion statement (Obstet Gynecol. 2014;123:193-7).
Menorrhagia: heavy menstrual bleeding
Even though average blood loss is estimated at 30 mL per period, that number means little in clinical practice because patients cannot measure the actual amount of menses. Better indicators of abnormally greater flow include flow lasting longer than 7 days, finding clots larger than a quarter, changing menstrual products every 1-2 hours, leaking onto clothing such that patients need to take extra clothes to school, and any heavy periods that occur with easy bruising or with a family history of bleeding disorders.
First-line treatment for heavy menstrual bleeding in teens is hormonal contraception, either combination oral contraceptive pills, the transdermal patch, or the intravaginal ring, which can be combined with other therapies.
An alternative for those under age 18 (per Food and Drug Administration labeling) is oral tranexamic acid, found in a crossover trial with an oral contraceptive pill to be just as effective at reducing average blood loss and improving quality of life, but with fewer side effects and better compliance. Before prescribing anything for heavy menstrual bleeding, however, you must consider possible causes and rule some out that require different management.
Aside from pregnancy, one potential cause of menorrhagia is infection such as chlamydia or gonorrhea, which should be considered even in those with a negative sexual history, Dr. Hertweck said. Other possible causes include an immature hypothalamic-pituitary-ovarian axis, polycystic ovary syndrome (even with low hemoglobin), malignancy with a hormone-producing tumor, hypothalamic dysfunction (often stimulated by eating disorders, obesity, rapid weight loss, or gluten intolerance), or coagulopathy.
“Teens with menorrhagia may need to be screened for a bleeding disorder,” Dr. Hertweck said. At a minimum, she recommends checking complete blood count, ferritin, and TSH. “The most common bleeding disorders associated with heavy menstrual bleeding include platelet function disorders and von Willebrand.”
Up to half of teen girls with menorrhagia who visit a hematologist or multidisciplinary clinic receive a diagnosis of a bleeding disorder, Dr. Hertweck said. And up to half of those with menorrhagia at menarche may have von Willebrand, as do one in six adolescents who go to the emergency department because of heavy menstrual bleeding.
Von Willebrand syndrome
Von Willebrand syndrome is a deficiency or dysfunction of von Willebrand factor (vWF), a protein with binding sites for platelets, collagen, and factor VIII that “serves as a bridge between platelets and injury sites in vessel walls” and “protects factor VIII from rapid proteolytic degradation,” Dr. Hertweck said. Von Willebrand syndrome is the most common inherited congenital bleeding disorder. Although acquired von Willebrand syndrome is rare, it has grown in incidence among those with complex cardiovascular, hematologic, or immunologic disorders.
“Correct diagnosis is complex and not always straightforward,” Dr. Hertweck said, but “a positive response to questions in four categories is highly sensitive.” They are as follows:
• Menses lasting at least 7 days and interfering with a person’s daily activities.
• “History of treatment for anemia.
• Family history of a diagnosed bleeding disorder.
• History of excessive bleeding after tooth extraction, delivery, miscarriage, or surgery.
Diagnostic assays include platelet concentration of vWF antigen, an activity test of vWF-platelet binding, and factor VIII activity. However, you often need to repeat diagnostic testing because vWF antigens vary according to race, blood type, age, acute phase response, and menstrual cycle timing, Dr. Hertweck said.
“Remember to draw von Willebrand testing only during the first 3 days of the menstrual cycle when estrogen levels are at the nadir,” she said.
Because estrogen increases vWF, treatment for von Willebrand syndrome should be progestin only, either oral pills, medroxyprogesterone acetate (MPA, or Depo-Provera injections), or an etonogestrel implant.
Dr. Hertweck presented several cases of abnormal menstruation and extreme conditions such as severe menorrhagia. Outside of von Willebrand in such patients, possible platelet disorders could include Glanzmann thrombasthenia (a platelet function disorder that is caused by an abnormality in the genes for glycoproteins IIb/IIIa) and platelet storage pool disorder, both of which should be diagnosed by a hematologist.
Dr. Hertweck reported having a research grant from Merck related to contraceptive implants in adolescents.
CHICAGO – , according to S. Paige Hertweck, MD, chief of gynecology at Norton Children’s Hospital in Louisville, Ky.
“Remember to use the menstrual cycle as a vital sign,” Dr. Hertweck told attendees at the American Academy of Pediatrics annual meeting. “Even within the first year of menarche, most girls have a period at least every 90 days, so work up those who don’t.”
The median age of menarche is 12.4 years, typically beginning within 2-3 years of breast budding at Tanner Stage 4 breast development, she said. By 15 years of age, 98% of girls have begun menstruation.
Girls’ cycles typically last 21-45 days, an average of 32.2 days during their first year of menstruation, with flow for 7 days or less, requiring an average of 3-6 pads and/or tampons per day. Dr. Hertweck recommends you write down these features of normal menstruation so that your patients can tell you when their cycle is abnormal or menses doesn’t return.
“Cycle length is more variable for teens versus women 20-40 years old,” she said. However, “it’s not true that ‘anything goes’ for cycle length” in teens, she added. “Cycles that are consistently outside the range of 21-45 days are statistically uncommon.” Hence the need to evaluate causes of amenorrhea in girls whose cycles exceed 90 days.
Possible causes of amenorrhea include pregnancy, polycystic ovary syndrome, thyroid abnormalities, hyperprolactinemia, primary ovarian insufficiency, or hypogonadal amenorrhea, typically stimulated by the first instance of anorexia, Crohn’s disease, celiac disease, or a gluten intolerance.
Primary amenorrhea
Dr. Hertweck listed five benchmarks that indicate primary amenorrhea requiring evaluation. Those indicators include girls who have no menarche by age 15 years or within 3 years of breast budding, no breast development by age 13 years, or no menses by age 14 years with hirsutism or with a history of excessive exercise or of an eating disorder.
You can start by examining what normal menstruation relies on: an intact central nervous system with a functioning pituitary, an ovarian response, and a normal uterus, cervix, and vagina. You should check the patient’s follicle-stimulating hormone, thyroid-stimulating hormone, and prolactin levels to assess CNS functioning, and estradiol levels to assess ovarian response. A genital exam with a pelvic ultrasound can reveal any possible defects in the uterus, cervix, or vagina.
The presence of breasts without a uterus indicates normal estrogen production, so the missing uterus could be a congenital defect or result from androgen insensitivity, Dr. Hertweck explained. In those without breasts, gonadal dysgenesis or gonadal enzymatic deficiency may explain no estrogen production. If the patient has both breasts and a uterus, you should rule out pregnancy first and then track CNS changes via FSH, TSH, and prolactin levels.
Premature ovarian insufficiency
Approximately 1% of females experience premature ovarian insufficiency, which can be diagnosed as early as age 14 years and should be suspected in a patient with a uterus but without breasts who has low estradiol levels, CNS failure identified by a high FSH level, and gonadal failure.
Formal diagnosis requires two separate instances of FSH elevation, and chromosomal testing should be done to rule out gonadal dysgenesis. You also should test the serum anti-Müllerian hormone biomarker (readings above 8 are concerning) and look for two possible causes. The FMR1 (Fragile X) premutation carrier status could be a cause, or presence of 21-hydroxylase and/or adrenal antibodies indicate autoimmune polyglandular syndrome.
Catching premature ovarian insufficiency early enough may allow patients to preserve some fertility if they still have oocytes present. Aside from this, girls will need hormone replacement therapy to fulfill developmental emotional and physical needs, such as bone growth and overall health. Despite a history of treating teens with premature ovarian insufficiency like adults, you should follow the practice guidelines specific to adolescents by the American College of Obstetricians and Gynecologists committee opinion statement (Obstet Gynecol. 2014;123:193-7).
Menorrhagia: heavy menstrual bleeding
Even though average blood loss is estimated at 30 mL per period, that number means little in clinical practice because patients cannot measure the actual amount of menses. Better indicators of abnormally greater flow include flow lasting longer than 7 days, finding clots larger than a quarter, changing menstrual products every 1-2 hours, leaking onto clothing such that patients need to take extra clothes to school, and any heavy periods that occur with easy bruising or with a family history of bleeding disorders.
First-line treatment for heavy menstrual bleeding in teens is hormonal contraception, either combination oral contraceptive pills, the transdermal patch, or the intravaginal ring, which can be combined with other therapies.
An alternative for those under age 18 (per Food and Drug Administration labeling) is oral tranexamic acid, found in a crossover trial with an oral contraceptive pill to be just as effective at reducing average blood loss and improving quality of life, but with fewer side effects and better compliance. Before prescribing anything for heavy menstrual bleeding, however, you must consider possible causes and rule some out that require different management.
Aside from pregnancy, one potential cause of menorrhagia is infection such as chlamydia or gonorrhea, which should be considered even in those with a negative sexual history, Dr. Hertweck said. Other possible causes include an immature hypothalamic-pituitary-ovarian axis, polycystic ovary syndrome (even with low hemoglobin), malignancy with a hormone-producing tumor, hypothalamic dysfunction (often stimulated by eating disorders, obesity, rapid weight loss, or gluten intolerance), or coagulopathy.
“Teens with menorrhagia may need to be screened for a bleeding disorder,” Dr. Hertweck said. At a minimum, she recommends checking complete blood count, ferritin, and TSH. “The most common bleeding disorders associated with heavy menstrual bleeding include platelet function disorders and von Willebrand.”
Up to half of teen girls with menorrhagia who visit a hematologist or multidisciplinary clinic receive a diagnosis of a bleeding disorder, Dr. Hertweck said. And up to half of those with menorrhagia at menarche may have von Willebrand, as do one in six adolescents who go to the emergency department because of heavy menstrual bleeding.
Von Willebrand syndrome
Von Willebrand syndrome is a deficiency or dysfunction of von Willebrand factor (vWF), a protein with binding sites for platelets, collagen, and factor VIII that “serves as a bridge between platelets and injury sites in vessel walls” and “protects factor VIII from rapid proteolytic degradation,” Dr. Hertweck said. Von Willebrand syndrome is the most common inherited congenital bleeding disorder. Although acquired von Willebrand syndrome is rare, it has grown in incidence among those with complex cardiovascular, hematologic, or immunologic disorders.
“Correct diagnosis is complex and not always straightforward,” Dr. Hertweck said, but “a positive response to questions in four categories is highly sensitive.” They are as follows:
• Menses lasting at least 7 days and interfering with a person’s daily activities.
• “History of treatment for anemia.
• Family history of a diagnosed bleeding disorder.
• History of excessive bleeding after tooth extraction, delivery, miscarriage, or surgery.
Diagnostic assays include platelet concentration of vWF antigen, an activity test of vWF-platelet binding, and factor VIII activity. However, you often need to repeat diagnostic testing because vWF antigens vary according to race, blood type, age, acute phase response, and menstrual cycle timing, Dr. Hertweck said.
“Remember to draw von Willebrand testing only during the first 3 days of the menstrual cycle when estrogen levels are at the nadir,” she said.
Because estrogen increases vWF, treatment for von Willebrand syndrome should be progestin only, either oral pills, medroxyprogesterone acetate (MPA, or Depo-Provera injections), or an etonogestrel implant.
Dr. Hertweck presented several cases of abnormal menstruation and extreme conditions such as severe menorrhagia. Outside of von Willebrand in such patients, possible platelet disorders could include Glanzmann thrombasthenia (a platelet function disorder that is caused by an abnormality in the genes for glycoproteins IIb/IIIa) and platelet storage pool disorder, both of which should be diagnosed by a hematologist.
Dr. Hertweck reported having a research grant from Merck related to contraceptive implants in adolescents.
CHICAGO – , according to S. Paige Hertweck, MD, chief of gynecology at Norton Children’s Hospital in Louisville, Ky.
“Remember to use the menstrual cycle as a vital sign,” Dr. Hertweck told attendees at the American Academy of Pediatrics annual meeting. “Even within the first year of menarche, most girls have a period at least every 90 days, so work up those who don’t.”
The median age of menarche is 12.4 years, typically beginning within 2-3 years of breast budding at Tanner Stage 4 breast development, she said. By 15 years of age, 98% of girls have begun menstruation.
Girls’ cycles typically last 21-45 days, an average of 32.2 days during their first year of menstruation, with flow for 7 days or less, requiring an average of 3-6 pads and/or tampons per day. Dr. Hertweck recommends you write down these features of normal menstruation so that your patients can tell you when their cycle is abnormal or menses doesn’t return.
“Cycle length is more variable for teens versus women 20-40 years old,” she said. However, “it’s not true that ‘anything goes’ for cycle length” in teens, she added. “Cycles that are consistently outside the range of 21-45 days are statistically uncommon.” Hence the need to evaluate causes of amenorrhea in girls whose cycles exceed 90 days.
Possible causes of amenorrhea include pregnancy, polycystic ovary syndrome, thyroid abnormalities, hyperprolactinemia, primary ovarian insufficiency, or hypogonadal amenorrhea, typically stimulated by the first instance of anorexia, Crohn’s disease, celiac disease, or a gluten intolerance.
Primary amenorrhea
Dr. Hertweck listed five benchmarks that indicate primary amenorrhea requiring evaluation. Those indicators include girls who have no menarche by age 15 years or within 3 years of breast budding, no breast development by age 13 years, or no menses by age 14 years with hirsutism or with a history of excessive exercise or of an eating disorder.
You can start by examining what normal menstruation relies on: an intact central nervous system with a functioning pituitary, an ovarian response, and a normal uterus, cervix, and vagina. You should check the patient’s follicle-stimulating hormone, thyroid-stimulating hormone, and prolactin levels to assess CNS functioning, and estradiol levels to assess ovarian response. A genital exam with a pelvic ultrasound can reveal any possible defects in the uterus, cervix, or vagina.
The presence of breasts without a uterus indicates normal estrogen production, so the missing uterus could be a congenital defect or result from androgen insensitivity, Dr. Hertweck explained. In those without breasts, gonadal dysgenesis or gonadal enzymatic deficiency may explain no estrogen production. If the patient has both breasts and a uterus, you should rule out pregnancy first and then track CNS changes via FSH, TSH, and prolactin levels.
Premature ovarian insufficiency
Approximately 1% of females experience premature ovarian insufficiency, which can be diagnosed as early as age 14 years and should be suspected in a patient with a uterus but without breasts who has low estradiol levels, CNS failure identified by a high FSH level, and gonadal failure.
Formal diagnosis requires two separate instances of FSH elevation, and chromosomal testing should be done to rule out gonadal dysgenesis. You also should test the serum anti-Müllerian hormone biomarker (readings above 8 are concerning) and look for two possible causes. The FMR1 (Fragile X) premutation carrier status could be a cause, or presence of 21-hydroxylase and/or adrenal antibodies indicate autoimmune polyglandular syndrome.
Catching premature ovarian insufficiency early enough may allow patients to preserve some fertility if they still have oocytes present. Aside from this, girls will need hormone replacement therapy to fulfill developmental emotional and physical needs, such as bone growth and overall health. Despite a history of treating teens with premature ovarian insufficiency like adults, you should follow the practice guidelines specific to adolescents by the American College of Obstetricians and Gynecologists committee opinion statement (Obstet Gynecol. 2014;123:193-7).
Menorrhagia: heavy menstrual bleeding
Even though average blood loss is estimated at 30 mL per period, that number means little in clinical practice because patients cannot measure the actual amount of menses. Better indicators of abnormally greater flow include flow lasting longer than 7 days, finding clots larger than a quarter, changing menstrual products every 1-2 hours, leaking onto clothing such that patients need to take extra clothes to school, and any heavy periods that occur with easy bruising or with a family history of bleeding disorders.
First-line treatment for heavy menstrual bleeding in teens is hormonal contraception, either combination oral contraceptive pills, the transdermal patch, or the intravaginal ring, which can be combined with other therapies.
An alternative for those under age 18 (per Food and Drug Administration labeling) is oral tranexamic acid, found in a crossover trial with an oral contraceptive pill to be just as effective at reducing average blood loss and improving quality of life, but with fewer side effects and better compliance. Before prescribing anything for heavy menstrual bleeding, however, you must consider possible causes and rule some out that require different management.
Aside from pregnancy, one potential cause of menorrhagia is infection such as chlamydia or gonorrhea, which should be considered even in those with a negative sexual history, Dr. Hertweck said. Other possible causes include an immature hypothalamic-pituitary-ovarian axis, polycystic ovary syndrome (even with low hemoglobin), malignancy with a hormone-producing tumor, hypothalamic dysfunction (often stimulated by eating disorders, obesity, rapid weight loss, or gluten intolerance), or coagulopathy.
“Teens with menorrhagia may need to be screened for a bleeding disorder,” Dr. Hertweck said. At a minimum, she recommends checking complete blood count, ferritin, and TSH. “The most common bleeding disorders associated with heavy menstrual bleeding include platelet function disorders and von Willebrand.”
Up to half of teen girls with menorrhagia who visit a hematologist or multidisciplinary clinic receive a diagnosis of a bleeding disorder, Dr. Hertweck said. And up to half of those with menorrhagia at menarche may have von Willebrand, as do one in six adolescents who go to the emergency department because of heavy menstrual bleeding.
Von Willebrand syndrome
Von Willebrand syndrome is a deficiency or dysfunction of von Willebrand factor (vWF), a protein with binding sites for platelets, collagen, and factor VIII that “serves as a bridge between platelets and injury sites in vessel walls” and “protects factor VIII from rapid proteolytic degradation,” Dr. Hertweck said. Von Willebrand syndrome is the most common inherited congenital bleeding disorder. Although acquired von Willebrand syndrome is rare, it has grown in incidence among those with complex cardiovascular, hematologic, or immunologic disorders.
“Correct diagnosis is complex and not always straightforward,” Dr. Hertweck said, but “a positive response to questions in four categories is highly sensitive.” They are as follows:
• Menses lasting at least 7 days and interfering with a person’s daily activities.
• “History of treatment for anemia.
• Family history of a diagnosed bleeding disorder.
• History of excessive bleeding after tooth extraction, delivery, miscarriage, or surgery.
Diagnostic assays include platelet concentration of vWF antigen, an activity test of vWF-platelet binding, and factor VIII activity. However, you often need to repeat diagnostic testing because vWF antigens vary according to race, blood type, age, acute phase response, and menstrual cycle timing, Dr. Hertweck said.
“Remember to draw von Willebrand testing only during the first 3 days of the menstrual cycle when estrogen levels are at the nadir,” she said.
Because estrogen increases vWF, treatment for von Willebrand syndrome should be progestin only, either oral pills, medroxyprogesterone acetate (MPA, or Depo-Provera injections), or an etonogestrel implant.
Dr. Hertweck presented several cases of abnormal menstruation and extreme conditions such as severe menorrhagia. Outside of von Willebrand in such patients, possible platelet disorders could include Glanzmann thrombasthenia (a platelet function disorder that is caused by an abnormality in the genes for glycoproteins IIb/IIIa) and platelet storage pool disorder, both of which should be diagnosed by a hematologist.
Dr. Hertweck reported having a research grant from Merck related to contraceptive implants in adolescents.
EXPERT ANALYSIS FROM AAP 2017
Return to Activities After Patellofemoral Arthroplasty
Take-Home Points
- PFA improved knee function and pain scores in patients with isolated patellofemoral arthritis.
- The majority (84.2%) of patients undergoing PFA were female.
- Regardless of age or gender, 72.2% of patients returned to their desired preoperative activity after PFA, and 52.8% returned at the same or higher level.
- The rate of conversion from PFA to TKA was 6.3%.
- PFA is an alternative to TKA in active patients with isolated patellofemoral arthritis.
Compared with total knee arthroplasty (TKA), single-compartment knee arthroplasty may provide better physiologic function, faster recovery, and higher rates of return to activities in patients with unicompartmental knee disease.1-3 In 1955, McKeever4 introduced patellar arthroplasty for surgical management of isolated patellofemoral arthritis. In 1979, Lubinus5 improved on the technique and design by adding a femoral component. Since then, implants and techniques have been developed to effect better clinical outcomes. Patellofemoral arthroplasty (PFA) has many advantages over TKA in the treatment of patellofemoral arthritis. PFA is less invasive, requires shorter tourniquet times, has faster recovery, and spares the tibiofemoral compartment, leaving more native bone for potential conversion to TKA. Regarding activity and function, the resurfacing arthroplasty (vs TKA) allows maintenance of nearly normal knee kinematics.
Despite these advantages, the broader orthopedic surgery community has only cautiously accepted PFA. The procedure has high complication rates. Persistent instability, malalignment, wear, impingement, and tibiofemoral arthritis progression can occur after PFA.6 Although first-generation PFA prostheses often failed because of mechanical problems, loosening, maltracking, or instability,7 the most common indication for PFA revision has been, according to a recent large retrospective study,8 unexplained pain. More than 10 to 15 years after PFA, tibiofemoral arthritis may be the primary mechanism of failure.9 Nevertheless, compared with standard TKA for isolated patellofemoral arthritis, modern PFA does not have significantly different clinical outcomes, including complication and revision rates.6Numerous patient factors influence functional prognosis before and after knee arthroplasty, regardless of surgical technique and implant used. Age, comorbidities, athletic status, mental health, pain, functional limitations, excessive caution, “artificial joint”–related worries, and rehabilitation protocol all influence function.10 Return to activity and other quality-of-life indices are important aspects of postoperative patient satisfaction.
Methods
We conducted a retrospective cohort study to describe functional status after PFA for patellofemoral arthritis. We identified 48 consecutive PFAs (39 patients) performed by a team of 2 orthopedic surgeons (specialists in treating patellofemoral pathology) between 2009 and 2014.
Three validated patient-reported outcome measures (PROMs) were used to determine preoperative (baseline) and postoperative functional status: Kujala score, Lysholm score, and International Knee Documentation Committee (IKDC) score. The Kujala score is a measure of knee function specific to the patellofemoral joint; the Lysholm score focuses on activities related to the knee; and the IKDC score is a general measure of knee function. Charts were reviewed to extract patients’ clinical data, including preoperative outcome scores, medical history, physical examination data, intraoperative characteristics, and postoperative course. By telephone, patients answered questions about their postoperative clinical course and completed final follow-up questionnaires. They were also asked which sporting or fitness activity they had preferred before surgery and whether they were able to return to that activity after surgery.
Statistical analysis included the study population’s descriptive statistics. Means and SDs were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Paired t tests were used to analyze changes in PROM scores. For comparison of differences between characteristics of patients who did and did not return to their previous activity level, independent-samples t tests were used for continuous variables. Chi-square tests or Fisher exact tests were used to compare discrete variables. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 22.0 (IBM).
Results
Postoperative knee-specific PROM scores and general pain score (reported by the patient on a scale of 0-10) were statistically significantly improved (P < .001 for all measures) over preoperative scores (Table 4).
After surgery, 1 patient (2.6%) developed a pulmonary embolus, which was successfully identified and treated without incident. Five patients (10.4%) had another surgery on the same knee. Three patients (6.3%) underwent conversion to TKA: 1 for continued symptoms in the setting of newly diagnosed inflammatory arthritis, 1 for arthritic pain, and 1 for patellofemoral instability. Two patients (4.2%) underwent irrigation and débridement: 1 for hematoma and 1 for suspected (culture-negative) infection.
Discussion
Historically, the literature evaluating knee arthroplasty outcomes has focused on implant survivorship, pain relief, and patient satisfaction. Since the advent of partial knee arthroplasty options, more attention has been given to functional outcomes and return to activities after single-compartment knee resurfacing. TKA remains the gold standard by which newer, less invasive surgical options are measured. In a large prospective study, 97% of patients (age, >55 years) who had TKA for patellofemoral arthritis reported good or excellent clinical results, the majority being excellent.11 Post-TKA functional status and activity levels may not be rated as highly. After TKA, many patients switch to lower impact sports or reduce or stop their participation in sports.12 A small study of competitive adult tennis players found high levels of post-TKA satisfaction, ability to resume playing tennis, pain relief, and increased or continued enjoyment in playing.13 In a study of 355 patients (417 knees) who had underwent TKA, improvement in Knee Society function score showed a moderate correlation to an increase in weighted activity score (R = 0.362).14
Unicondylar knee arthroplasty (UKA) is becoming a popular treatment option for single-compartment tibiofemoral arthritis. A systematic review of 18 original studies of patients with knee osteoarthritis found that overall return to sports varied from 36% to 89% after TKA and from 75% to 100% after UKA.15 In another study, return-to-sports rates were similar for UKA (87%) and TKA (83%); the only significant difference was UKA patients returned quicker.16 The authors of a large meta-analysis conceded that significant heterogeneity of data prevented them from drawing definitive conclusions, but UKA patients seemed to return to low- and high-impact sports 2 weeks faster than their TKA counterparts.10 Overall, UKA and TKA patients (age, 51-71 years) had comparable return-to-sports rates at an average of 4 years after surgery.10 A smaller study corroborated faster return to sports for UKA over TKA patients and also found that, compared with TKA patients, UKA patients participated in sports more regularly and over a longer period.17 On the other hand, Walton and colleagues18 found similar return-to-sports rates but higher frequency of and satisfaction with sports participation in UKA over TKA patients.
A large retrospective study found no differences in rates of return to sports after TKA, UKA, patellar resurfacing, hip resurfacing, and total hip arthroplasty.19 Pain was the most common barrier to return. UKA patients who returned to sports tended to be younger than those who did not.20 Naal and colleagues3 found that 95% of UKA patients returned to their activities—hiking, walking, cycling, and swimming being most common. Although 90.3% of patients said surgery maintained or improved their ability to participate in sports, participation in high-impact sports (eg, running) decreased after surgery.
Outcomes of PFA vary because of evolving patient selection, implant design, surgical technique, and return-to-activity expectations.21,22 Most PFA outcome studies focus on implant survivorship, complication rates, and postoperative knee scores.23-28 PFA studies focused on return to activities are limited. Kooijman and colleagues7 and Mertl and colleagues29 reported good or excellent clinical results of PFA in 86% and 82% of patients, respectively. Neither study included a comprehensive analysis of postoperative functional status. Similarly, De Cloedt and colleagues30 reported good PFA outcomes in 43% of patients with degenerative joint disease and in 83% of patients with instability. Specific activity status was not described. Dahm and colleagues31 and Farr and colleagues32 suggested postoperative pain resolution motivates some PFA patients not only to resume preoperative activities but to start participating in new, higher level activities after pain has subsided. However, the studies did not examine the characteristics of patients who returned to baseline activities and did not examine return-to-sports rates.
Study Strengths and Limitations
Our study focused on the PFA patient population of a surgical team of 2 fellowship-trained orthopedic surgeons (specialists in treating patellofemoral pathology). Although generalization of our findings to other surgeons and different implants may be limited, the study design standardized treatment in a way that makes these findings more reliable. The 100% follow-up strengthens these findings as well. Last, though the patient population was relatively small, it was consistent with or larger than the PFA patient groups studied previously.
Conclusion
In this study, PROM and pain scores were significantly improved after PFA. That almost 75% of patients returned to their preferred activities and >50% of patients returned at the same or a higher activity level provides useful information for preoperative discussions with patients who want to remain active after PFA. Prospective studies are needed to evaluate the longevity and durability of PFA, particularly in active patients.
1. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
2. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
3. Naal FD, Fischer M, Preuss A, et al. Return to sports and recreational activity after unicompartmental knee arthroplasty. Am J Sports Med. 2007;35(10):1688-1695.
4. McKeever DC. Patellar prosthesis. J Bone Joint Surg Am. 1955;37(5):1074-1084.
5. Lubinus HH. Patella glide bearing total replacement. Orthopedics. 1979;2(2):119-127.
6. Dy CJ, Franco N, Ma Y, Mazumdar M, McCarthy MM, Gonzalez Della Valle A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2174-2190.
7. Kooijman HJ, Driessen AP, van Horn JR. Long-term results of patellofemoral arthroplasty. A report of 56 arthroplasties with 17 years of follow-up. J Bone Joint Surg Br. 2003;85(6):836-840.
8. Baker PN, Refaie R, Gregg P, Deehan D. Revision following patello-femoral arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2047-2053.
9. Lonner JH, Bloomfield MR. The clinical outcome of patellofemoral arthroplasty. Orthop Clin North Am. 2013;44(3):271-280.
10. Papalia R, Del Buono A, Zampogna B, Maffulli N, Denaro V. Sport activity following joint arthroplasty: a systematic review. Br Med Bull. 2012;101:81-103.
11. Mont MA, Haas S, Mullick T, Hungerford DS. Total knee arthroplasty for patellofemoral arthritis. J Bone Joint Surg Am. 2002;84(11):1977-1981.
12. Chatterji U, Ashworth MJ, Lewis PL, Dobson PJ. Effect of total knee arthroplasty on recreational and sporting activity. ANZ J Surg. 2005;75(6):405-408.
13. Mont MA, Rajadhyaksha AD, Marxen JL, Silberstein CE, Hungerford DS. Tennis after total knee arthroplasty. Am J Sports Med. 2002;30(2):163-166.
14. Marker DR, Mont MA, Seyler TM, McGrath MS, Kolisek FR, Bonutti PM. Does functional improvement following TKA correlate to increased sports activity? Iowa Orthop J. 2009;29:11-16.
15. Witjes S, Gouttebarge V, Kuijer PP, van Geenen RC, Poolman RW, Kerkhoffs GM. Return to sports and physical activity after total and unicondylar knee arthroplasty: a systematic review and meta-analysis. Sports Med. 2016;46(2):269-292.
16. Ho JC, Stitzlein RN, Green CJ, Stoner T, Froimson MI. Return to sports activity following UKA and TKA. J Knee Surg. 2016;29(3):254-259.
17. Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979.
18. Walton NP, Jahromi I, Lewis PL, Dobson PJ, Angel KR, Campbell DG. Patient-perceived outcomes and return to sport and work: TKA versus mini-incision unicompartmental knee arthroplasty. J Knee Surg. 2006;19(2):112-116.
19. Wylde V, Blom A, Dieppe P, Hewlett S, Learmonth I. Return to sport after joint replacement. J Bone Joint Surg Br. 2008;90(7):920-923.
20. Pietschmann MF, Wohlleb L, Weber P, et al. Sports activities after medial unicompartmental knee arthroplasty Oxford III—what can we expect? Int Orthop. 2013;37(1):31-37.
21. Lonner JH. Patellofemoral arthroplasty. Orthopedics. 2010;33(9):653.
22. Lustig S. Patellofemoral arthroplasty. Orthop Traumatol Surg Res. 2014;100(1 suppl):S35-S43.
23. Krajca-Radcliffe JB, Coker TP. Patellofemoral arthroplasty. A 2- to 18-year followup study. Clin Orthop Relat Res. 1996;(330):143-151.
24. Mihalko WM, Boachie-Adjei Y, Spang JT, Fulkerson JP, Arendt EA, Saleh KJ. Controversies and techniques in the surgical management of patellofemoral arthritis. Instr Course Lect. 2008;57:365-380.
25. Lonner JH. Patellofemoral arthroplasty: pros, cons, and design considerations. Clin Orthop Relat Res. 2004;(428):158-165.
26. Lonner JH. Patellofemoral arthroplasty: the impact of design on outcomes. Orthop Clin North Am. 2008;39(3):347-354.
27. Farr J 2nd, Barrett D. Optimizing patellofemoral arthroplasty. Knee. 2008;15(5):339-347.
28. Leadbetter WB, Seyler TM, Ragland PS, Mont MA. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88(suppl 4):122-137.
29. Mertl P, Van FT, Bonhomme P, Vives P. Femoropatellar osteoarthritis treated by prosthesis. Retrospective study of 50 implants [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83(8):712-718.
30. De Cloedt P, Legaye J, Lokietek W. Femoro-patellar prosthesis. A retrospective study of 45 consecutive cases with a follow-up of 3-12 years [in French]. Acta Orthop Belg. 1999;65(2):170-175.
31. Dahm DL, Al-Rayashi W, Dajani K, Shah JP, Levy BA, Stuart MJ. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop. 2010;39(10):487-491.
32. Farr J, Arendt E, Dahm D, Daynes J. Patellofemoral arthroplasty in the athlete. Clin Sports Med. 2014;33(3):547-552.
Take-Home Points
- PFA improved knee function and pain scores in patients with isolated patellofemoral arthritis.
- The majority (84.2%) of patients undergoing PFA were female.
- Regardless of age or gender, 72.2% of patients returned to their desired preoperative activity after PFA, and 52.8% returned at the same or higher level.
- The rate of conversion from PFA to TKA was 6.3%.
- PFA is an alternative to TKA in active patients with isolated patellofemoral arthritis.
Compared with total knee arthroplasty (TKA), single-compartment knee arthroplasty may provide better physiologic function, faster recovery, and higher rates of return to activities in patients with unicompartmental knee disease.1-3 In 1955, McKeever4 introduced patellar arthroplasty for surgical management of isolated patellofemoral arthritis. In 1979, Lubinus5 improved on the technique and design by adding a femoral component. Since then, implants and techniques have been developed to effect better clinical outcomes. Patellofemoral arthroplasty (PFA) has many advantages over TKA in the treatment of patellofemoral arthritis. PFA is less invasive, requires shorter tourniquet times, has faster recovery, and spares the tibiofemoral compartment, leaving more native bone for potential conversion to TKA. Regarding activity and function, the resurfacing arthroplasty (vs TKA) allows maintenance of nearly normal knee kinematics.
Despite these advantages, the broader orthopedic surgery community has only cautiously accepted PFA. The procedure has high complication rates. Persistent instability, malalignment, wear, impingement, and tibiofemoral arthritis progression can occur after PFA.6 Although first-generation PFA prostheses often failed because of mechanical problems, loosening, maltracking, or instability,7 the most common indication for PFA revision has been, according to a recent large retrospective study,8 unexplained pain. More than 10 to 15 years after PFA, tibiofemoral arthritis may be the primary mechanism of failure.9 Nevertheless, compared with standard TKA for isolated patellofemoral arthritis, modern PFA does not have significantly different clinical outcomes, including complication and revision rates.6Numerous patient factors influence functional prognosis before and after knee arthroplasty, regardless of surgical technique and implant used. Age, comorbidities, athletic status, mental health, pain, functional limitations, excessive caution, “artificial joint”–related worries, and rehabilitation protocol all influence function.10 Return to activity and other quality-of-life indices are important aspects of postoperative patient satisfaction.
Methods
We conducted a retrospective cohort study to describe functional status after PFA for patellofemoral arthritis. We identified 48 consecutive PFAs (39 patients) performed by a team of 2 orthopedic surgeons (specialists in treating patellofemoral pathology) between 2009 and 2014.
Three validated patient-reported outcome measures (PROMs) were used to determine preoperative (baseline) and postoperative functional status: Kujala score, Lysholm score, and International Knee Documentation Committee (IKDC) score. The Kujala score is a measure of knee function specific to the patellofemoral joint; the Lysholm score focuses on activities related to the knee; and the IKDC score is a general measure of knee function. Charts were reviewed to extract patients’ clinical data, including preoperative outcome scores, medical history, physical examination data, intraoperative characteristics, and postoperative course. By telephone, patients answered questions about their postoperative clinical course and completed final follow-up questionnaires. They were also asked which sporting or fitness activity they had preferred before surgery and whether they were able to return to that activity after surgery.
Statistical analysis included the study population’s descriptive statistics. Means and SDs were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Paired t tests were used to analyze changes in PROM scores. For comparison of differences between characteristics of patients who did and did not return to their previous activity level, independent-samples t tests were used for continuous variables. Chi-square tests or Fisher exact tests were used to compare discrete variables. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 22.0 (IBM).
Results
Postoperative knee-specific PROM scores and general pain score (reported by the patient on a scale of 0-10) were statistically significantly improved (P < .001 for all measures) over preoperative scores (Table 4).
After surgery, 1 patient (2.6%) developed a pulmonary embolus, which was successfully identified and treated without incident. Five patients (10.4%) had another surgery on the same knee. Three patients (6.3%) underwent conversion to TKA: 1 for continued symptoms in the setting of newly diagnosed inflammatory arthritis, 1 for arthritic pain, and 1 for patellofemoral instability. Two patients (4.2%) underwent irrigation and débridement: 1 for hematoma and 1 for suspected (culture-negative) infection.
Discussion
Historically, the literature evaluating knee arthroplasty outcomes has focused on implant survivorship, pain relief, and patient satisfaction. Since the advent of partial knee arthroplasty options, more attention has been given to functional outcomes and return to activities after single-compartment knee resurfacing. TKA remains the gold standard by which newer, less invasive surgical options are measured. In a large prospective study, 97% of patients (age, >55 years) who had TKA for patellofemoral arthritis reported good or excellent clinical results, the majority being excellent.11 Post-TKA functional status and activity levels may not be rated as highly. After TKA, many patients switch to lower impact sports or reduce or stop their participation in sports.12 A small study of competitive adult tennis players found high levels of post-TKA satisfaction, ability to resume playing tennis, pain relief, and increased or continued enjoyment in playing.13 In a study of 355 patients (417 knees) who had underwent TKA, improvement in Knee Society function score showed a moderate correlation to an increase in weighted activity score (R = 0.362).14
Unicondylar knee arthroplasty (UKA) is becoming a popular treatment option for single-compartment tibiofemoral arthritis. A systematic review of 18 original studies of patients with knee osteoarthritis found that overall return to sports varied from 36% to 89% after TKA and from 75% to 100% after UKA.15 In another study, return-to-sports rates were similar for UKA (87%) and TKA (83%); the only significant difference was UKA patients returned quicker.16 The authors of a large meta-analysis conceded that significant heterogeneity of data prevented them from drawing definitive conclusions, but UKA patients seemed to return to low- and high-impact sports 2 weeks faster than their TKA counterparts.10 Overall, UKA and TKA patients (age, 51-71 years) had comparable return-to-sports rates at an average of 4 years after surgery.10 A smaller study corroborated faster return to sports for UKA over TKA patients and also found that, compared with TKA patients, UKA patients participated in sports more regularly and over a longer period.17 On the other hand, Walton and colleagues18 found similar return-to-sports rates but higher frequency of and satisfaction with sports participation in UKA over TKA patients.
A large retrospective study found no differences in rates of return to sports after TKA, UKA, patellar resurfacing, hip resurfacing, and total hip arthroplasty.19 Pain was the most common barrier to return. UKA patients who returned to sports tended to be younger than those who did not.20 Naal and colleagues3 found that 95% of UKA patients returned to their activities—hiking, walking, cycling, and swimming being most common. Although 90.3% of patients said surgery maintained or improved their ability to participate in sports, participation in high-impact sports (eg, running) decreased after surgery.
Outcomes of PFA vary because of evolving patient selection, implant design, surgical technique, and return-to-activity expectations.21,22 Most PFA outcome studies focus on implant survivorship, complication rates, and postoperative knee scores.23-28 PFA studies focused on return to activities are limited. Kooijman and colleagues7 and Mertl and colleagues29 reported good or excellent clinical results of PFA in 86% and 82% of patients, respectively. Neither study included a comprehensive analysis of postoperative functional status. Similarly, De Cloedt and colleagues30 reported good PFA outcomes in 43% of patients with degenerative joint disease and in 83% of patients with instability. Specific activity status was not described. Dahm and colleagues31 and Farr and colleagues32 suggested postoperative pain resolution motivates some PFA patients not only to resume preoperative activities but to start participating in new, higher level activities after pain has subsided. However, the studies did not examine the characteristics of patients who returned to baseline activities and did not examine return-to-sports rates.
Study Strengths and Limitations
Our study focused on the PFA patient population of a surgical team of 2 fellowship-trained orthopedic surgeons (specialists in treating patellofemoral pathology). Although generalization of our findings to other surgeons and different implants may be limited, the study design standardized treatment in a way that makes these findings more reliable. The 100% follow-up strengthens these findings as well. Last, though the patient population was relatively small, it was consistent with or larger than the PFA patient groups studied previously.
Conclusion
In this study, PROM and pain scores were significantly improved after PFA. That almost 75% of patients returned to their preferred activities and >50% of patients returned at the same or a higher activity level provides useful information for preoperative discussions with patients who want to remain active after PFA. Prospective studies are needed to evaluate the longevity and durability of PFA, particularly in active patients.
Take-Home Points
- PFA improved knee function and pain scores in patients with isolated patellofemoral arthritis.
- The majority (84.2%) of patients undergoing PFA were female.
- Regardless of age or gender, 72.2% of patients returned to their desired preoperative activity after PFA, and 52.8% returned at the same or higher level.
- The rate of conversion from PFA to TKA was 6.3%.
- PFA is an alternative to TKA in active patients with isolated patellofemoral arthritis.
Compared with total knee arthroplasty (TKA), single-compartment knee arthroplasty may provide better physiologic function, faster recovery, and higher rates of return to activities in patients with unicompartmental knee disease.1-3 In 1955, McKeever4 introduced patellar arthroplasty for surgical management of isolated patellofemoral arthritis. In 1979, Lubinus5 improved on the technique and design by adding a femoral component. Since then, implants and techniques have been developed to effect better clinical outcomes. Patellofemoral arthroplasty (PFA) has many advantages over TKA in the treatment of patellofemoral arthritis. PFA is less invasive, requires shorter tourniquet times, has faster recovery, and spares the tibiofemoral compartment, leaving more native bone for potential conversion to TKA. Regarding activity and function, the resurfacing arthroplasty (vs TKA) allows maintenance of nearly normal knee kinematics.
Despite these advantages, the broader orthopedic surgery community has only cautiously accepted PFA. The procedure has high complication rates. Persistent instability, malalignment, wear, impingement, and tibiofemoral arthritis progression can occur after PFA.6 Although first-generation PFA prostheses often failed because of mechanical problems, loosening, maltracking, or instability,7 the most common indication for PFA revision has been, according to a recent large retrospective study,8 unexplained pain. More than 10 to 15 years after PFA, tibiofemoral arthritis may be the primary mechanism of failure.9 Nevertheless, compared with standard TKA for isolated patellofemoral arthritis, modern PFA does not have significantly different clinical outcomes, including complication and revision rates.6Numerous patient factors influence functional prognosis before and after knee arthroplasty, regardless of surgical technique and implant used. Age, comorbidities, athletic status, mental health, pain, functional limitations, excessive caution, “artificial joint”–related worries, and rehabilitation protocol all influence function.10 Return to activity and other quality-of-life indices are important aspects of postoperative patient satisfaction.
Methods
We conducted a retrospective cohort study to describe functional status after PFA for patellofemoral arthritis. We identified 48 consecutive PFAs (39 patients) performed by a team of 2 orthopedic surgeons (specialists in treating patellofemoral pathology) between 2009 and 2014.
Three validated patient-reported outcome measures (PROMs) were used to determine preoperative (baseline) and postoperative functional status: Kujala score, Lysholm score, and International Knee Documentation Committee (IKDC) score. The Kujala score is a measure of knee function specific to the patellofemoral joint; the Lysholm score focuses on activities related to the knee; and the IKDC score is a general measure of knee function. Charts were reviewed to extract patients’ clinical data, including preoperative outcome scores, medical history, physical examination data, intraoperative characteristics, and postoperative course. By telephone, patients answered questions about their postoperative clinical course and completed final follow-up questionnaires. They were also asked which sporting or fitness activity they had preferred before surgery and whether they were able to return to that activity after surgery.
Statistical analysis included the study population’s descriptive statistics. Means and SDs were reported for continuous variables, and frequencies and percentages were reported for categorical variables. Paired t tests were used to analyze changes in PROM scores. For comparison of differences between characteristics of patients who did and did not return to their previous activity level, independent-samples t tests were used for continuous variables. Chi-square tests or Fisher exact tests were used to compare discrete variables. Statistical significance was set at P ≤ .05. All analyses were performed with SPSS Version 22.0 (IBM).
Results
Postoperative knee-specific PROM scores and general pain score (reported by the patient on a scale of 0-10) were statistically significantly improved (P < .001 for all measures) over preoperative scores (Table 4).
After surgery, 1 patient (2.6%) developed a pulmonary embolus, which was successfully identified and treated without incident. Five patients (10.4%) had another surgery on the same knee. Three patients (6.3%) underwent conversion to TKA: 1 for continued symptoms in the setting of newly diagnosed inflammatory arthritis, 1 for arthritic pain, and 1 for patellofemoral instability. Two patients (4.2%) underwent irrigation and débridement: 1 for hematoma and 1 for suspected (culture-negative) infection.
Discussion
Historically, the literature evaluating knee arthroplasty outcomes has focused on implant survivorship, pain relief, and patient satisfaction. Since the advent of partial knee arthroplasty options, more attention has been given to functional outcomes and return to activities after single-compartment knee resurfacing. TKA remains the gold standard by which newer, less invasive surgical options are measured. In a large prospective study, 97% of patients (age, >55 years) who had TKA for patellofemoral arthritis reported good or excellent clinical results, the majority being excellent.11 Post-TKA functional status and activity levels may not be rated as highly. After TKA, many patients switch to lower impact sports or reduce or stop their participation in sports.12 A small study of competitive adult tennis players found high levels of post-TKA satisfaction, ability to resume playing tennis, pain relief, and increased or continued enjoyment in playing.13 In a study of 355 patients (417 knees) who had underwent TKA, improvement in Knee Society function score showed a moderate correlation to an increase in weighted activity score (R = 0.362).14
Unicondylar knee arthroplasty (UKA) is becoming a popular treatment option for single-compartment tibiofemoral arthritis. A systematic review of 18 original studies of patients with knee osteoarthritis found that overall return to sports varied from 36% to 89% after TKA and from 75% to 100% after UKA.15 In another study, return-to-sports rates were similar for UKA (87%) and TKA (83%); the only significant difference was UKA patients returned quicker.16 The authors of a large meta-analysis conceded that significant heterogeneity of data prevented them from drawing definitive conclusions, but UKA patients seemed to return to low- and high-impact sports 2 weeks faster than their TKA counterparts.10 Overall, UKA and TKA patients (age, 51-71 years) had comparable return-to-sports rates at an average of 4 years after surgery.10 A smaller study corroborated faster return to sports for UKA over TKA patients and also found that, compared with TKA patients, UKA patients participated in sports more regularly and over a longer period.17 On the other hand, Walton and colleagues18 found similar return-to-sports rates but higher frequency of and satisfaction with sports participation in UKA over TKA patients.
A large retrospective study found no differences in rates of return to sports after TKA, UKA, patellar resurfacing, hip resurfacing, and total hip arthroplasty.19 Pain was the most common barrier to return. UKA patients who returned to sports tended to be younger than those who did not.20 Naal and colleagues3 found that 95% of UKA patients returned to their activities—hiking, walking, cycling, and swimming being most common. Although 90.3% of patients said surgery maintained or improved their ability to participate in sports, participation in high-impact sports (eg, running) decreased after surgery.
Outcomes of PFA vary because of evolving patient selection, implant design, surgical technique, and return-to-activity expectations.21,22 Most PFA outcome studies focus on implant survivorship, complication rates, and postoperative knee scores.23-28 PFA studies focused on return to activities are limited. Kooijman and colleagues7 and Mertl and colleagues29 reported good or excellent clinical results of PFA in 86% and 82% of patients, respectively. Neither study included a comprehensive analysis of postoperative functional status. Similarly, De Cloedt and colleagues30 reported good PFA outcomes in 43% of patients with degenerative joint disease and in 83% of patients with instability. Specific activity status was not described. Dahm and colleagues31 and Farr and colleagues32 suggested postoperative pain resolution motivates some PFA patients not only to resume preoperative activities but to start participating in new, higher level activities after pain has subsided. However, the studies did not examine the characteristics of patients who returned to baseline activities and did not examine return-to-sports rates.
Study Strengths and Limitations
Our study focused on the PFA patient population of a surgical team of 2 fellowship-trained orthopedic surgeons (specialists in treating patellofemoral pathology). Although generalization of our findings to other surgeons and different implants may be limited, the study design standardized treatment in a way that makes these findings more reliable. The 100% follow-up strengthens these findings as well. Last, though the patient population was relatively small, it was consistent with or larger than the PFA patient groups studied previously.
Conclusion
In this study, PROM and pain scores were significantly improved after PFA. That almost 75% of patients returned to their preferred activities and >50% of patients returned at the same or a higher activity level provides useful information for preoperative discussions with patients who want to remain active after PFA. Prospective studies are needed to evaluate the longevity and durability of PFA, particularly in active patients.
1. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
2. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
3. Naal FD, Fischer M, Preuss A, et al. Return to sports and recreational activity after unicompartmental knee arthroplasty. Am J Sports Med. 2007;35(10):1688-1695.
4. McKeever DC. Patellar prosthesis. J Bone Joint Surg Am. 1955;37(5):1074-1084.
5. Lubinus HH. Patella glide bearing total replacement. Orthopedics. 1979;2(2):119-127.
6. Dy CJ, Franco N, Ma Y, Mazumdar M, McCarthy MM, Gonzalez Della Valle A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2174-2190.
7. Kooijman HJ, Driessen AP, van Horn JR. Long-term results of patellofemoral arthroplasty. A report of 56 arthroplasties with 17 years of follow-up. J Bone Joint Surg Br. 2003;85(6):836-840.
8. Baker PN, Refaie R, Gregg P, Deehan D. Revision following patello-femoral arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2047-2053.
9. Lonner JH, Bloomfield MR. The clinical outcome of patellofemoral arthroplasty. Orthop Clin North Am. 2013;44(3):271-280.
10. Papalia R, Del Buono A, Zampogna B, Maffulli N, Denaro V. Sport activity following joint arthroplasty: a systematic review. Br Med Bull. 2012;101:81-103.
11. Mont MA, Haas S, Mullick T, Hungerford DS. Total knee arthroplasty for patellofemoral arthritis. J Bone Joint Surg Am. 2002;84(11):1977-1981.
12. Chatterji U, Ashworth MJ, Lewis PL, Dobson PJ. Effect of total knee arthroplasty on recreational and sporting activity. ANZ J Surg. 2005;75(6):405-408.
13. Mont MA, Rajadhyaksha AD, Marxen JL, Silberstein CE, Hungerford DS. Tennis after total knee arthroplasty. Am J Sports Med. 2002;30(2):163-166.
14. Marker DR, Mont MA, Seyler TM, McGrath MS, Kolisek FR, Bonutti PM. Does functional improvement following TKA correlate to increased sports activity? Iowa Orthop J. 2009;29:11-16.
15. Witjes S, Gouttebarge V, Kuijer PP, van Geenen RC, Poolman RW, Kerkhoffs GM. Return to sports and physical activity after total and unicondylar knee arthroplasty: a systematic review and meta-analysis. Sports Med. 2016;46(2):269-292.
16. Ho JC, Stitzlein RN, Green CJ, Stoner T, Froimson MI. Return to sports activity following UKA and TKA. J Knee Surg. 2016;29(3):254-259.
17. Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979.
18. Walton NP, Jahromi I, Lewis PL, Dobson PJ, Angel KR, Campbell DG. Patient-perceived outcomes and return to sport and work: TKA versus mini-incision unicompartmental knee arthroplasty. J Knee Surg. 2006;19(2):112-116.
19. Wylde V, Blom A, Dieppe P, Hewlett S, Learmonth I. Return to sport after joint replacement. J Bone Joint Surg Br. 2008;90(7):920-923.
20. Pietschmann MF, Wohlleb L, Weber P, et al. Sports activities after medial unicompartmental knee arthroplasty Oxford III—what can we expect? Int Orthop. 2013;37(1):31-37.
21. Lonner JH. Patellofemoral arthroplasty. Orthopedics. 2010;33(9):653.
22. Lustig S. Patellofemoral arthroplasty. Orthop Traumatol Surg Res. 2014;100(1 suppl):S35-S43.
23. Krajca-Radcliffe JB, Coker TP. Patellofemoral arthroplasty. A 2- to 18-year followup study. Clin Orthop Relat Res. 1996;(330):143-151.
24. Mihalko WM, Boachie-Adjei Y, Spang JT, Fulkerson JP, Arendt EA, Saleh KJ. Controversies and techniques in the surgical management of patellofemoral arthritis. Instr Course Lect. 2008;57:365-380.
25. Lonner JH. Patellofemoral arthroplasty: pros, cons, and design considerations. Clin Orthop Relat Res. 2004;(428):158-165.
26. Lonner JH. Patellofemoral arthroplasty: the impact of design on outcomes. Orthop Clin North Am. 2008;39(3):347-354.
27. Farr J 2nd, Barrett D. Optimizing patellofemoral arthroplasty. Knee. 2008;15(5):339-347.
28. Leadbetter WB, Seyler TM, Ragland PS, Mont MA. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88(suppl 4):122-137.
29. Mertl P, Van FT, Bonhomme P, Vives P. Femoropatellar osteoarthritis treated by prosthesis. Retrospective study of 50 implants [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83(8):712-718.
30. De Cloedt P, Legaye J, Lokietek W. Femoro-patellar prosthesis. A retrospective study of 45 consecutive cases with a follow-up of 3-12 years [in French]. Acta Orthop Belg. 1999;65(2):170-175.
31. Dahm DL, Al-Rayashi W, Dajani K, Shah JP, Levy BA, Stuart MJ. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop. 2010;39(10):487-491.
32. Farr J, Arendt E, Dahm D, Daynes J. Patellofemoral arthroplasty in the athlete. Clin Sports Med. 2014;33(3):547-552.
1. Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res. 1991;(273):151-156.
2. Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145-150.
3. Naal FD, Fischer M, Preuss A, et al. Return to sports and recreational activity after unicompartmental knee arthroplasty. Am J Sports Med. 2007;35(10):1688-1695.
4. McKeever DC. Patellar prosthesis. J Bone Joint Surg Am. 1955;37(5):1074-1084.
5. Lubinus HH. Patella glide bearing total replacement. Orthopedics. 1979;2(2):119-127.
6. Dy CJ, Franco N, Ma Y, Mazumdar M, McCarthy MM, Gonzalez Della Valle A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2174-2190.
7. Kooijman HJ, Driessen AP, van Horn JR. Long-term results of patellofemoral arthroplasty. A report of 56 arthroplasties with 17 years of follow-up. J Bone Joint Surg Br. 2003;85(6):836-840.
8. Baker PN, Refaie R, Gregg P, Deehan D. Revision following patello-femoral arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2047-2053.
9. Lonner JH, Bloomfield MR. The clinical outcome of patellofemoral arthroplasty. Orthop Clin North Am. 2013;44(3):271-280.
10. Papalia R, Del Buono A, Zampogna B, Maffulli N, Denaro V. Sport activity following joint arthroplasty: a systematic review. Br Med Bull. 2012;101:81-103.
11. Mont MA, Haas S, Mullick T, Hungerford DS. Total knee arthroplasty for patellofemoral arthritis. J Bone Joint Surg Am. 2002;84(11):1977-1981.
12. Chatterji U, Ashworth MJ, Lewis PL, Dobson PJ. Effect of total knee arthroplasty on recreational and sporting activity. ANZ J Surg. 2005;75(6):405-408.
13. Mont MA, Rajadhyaksha AD, Marxen JL, Silberstein CE, Hungerford DS. Tennis after total knee arthroplasty. Am J Sports Med. 2002;30(2):163-166.
14. Marker DR, Mont MA, Seyler TM, McGrath MS, Kolisek FR, Bonutti PM. Does functional improvement following TKA correlate to increased sports activity? Iowa Orthop J. 2009;29:11-16.
15. Witjes S, Gouttebarge V, Kuijer PP, van Geenen RC, Poolman RW, Kerkhoffs GM. Return to sports and physical activity after total and unicondylar knee arthroplasty: a systematic review and meta-analysis. Sports Med. 2016;46(2):269-292.
16. Ho JC, Stitzlein RN, Green CJ, Stoner T, Froimson MI. Return to sports activity following UKA and TKA. J Knee Surg. 2016;29(3):254-259.
17. Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):973-979.
18. Walton NP, Jahromi I, Lewis PL, Dobson PJ, Angel KR, Campbell DG. Patient-perceived outcomes and return to sport and work: TKA versus mini-incision unicompartmental knee arthroplasty. J Knee Surg. 2006;19(2):112-116.
19. Wylde V, Blom A, Dieppe P, Hewlett S, Learmonth I. Return to sport after joint replacement. J Bone Joint Surg Br. 2008;90(7):920-923.
20. Pietschmann MF, Wohlleb L, Weber P, et al. Sports activities after medial unicompartmental knee arthroplasty Oxford III—what can we expect? Int Orthop. 2013;37(1):31-37.
21. Lonner JH. Patellofemoral arthroplasty. Orthopedics. 2010;33(9):653.
22. Lustig S. Patellofemoral arthroplasty. Orthop Traumatol Surg Res. 2014;100(1 suppl):S35-S43.
23. Krajca-Radcliffe JB, Coker TP. Patellofemoral arthroplasty. A 2- to 18-year followup study. Clin Orthop Relat Res. 1996;(330):143-151.
24. Mihalko WM, Boachie-Adjei Y, Spang JT, Fulkerson JP, Arendt EA, Saleh KJ. Controversies and techniques in the surgical management of patellofemoral arthritis. Instr Course Lect. 2008;57:365-380.
25. Lonner JH. Patellofemoral arthroplasty: pros, cons, and design considerations. Clin Orthop Relat Res. 2004;(428):158-165.
26. Lonner JH. Patellofemoral arthroplasty: the impact of design on outcomes. Orthop Clin North Am. 2008;39(3):347-354.
27. Farr J 2nd, Barrett D. Optimizing patellofemoral arthroplasty. Knee. 2008;15(5):339-347.
28. Leadbetter WB, Seyler TM, Ragland PS, Mont MA. Indications, contraindications, and pitfalls of patellofemoral arthroplasty. J Bone Joint Surg Am. 2006;88(suppl 4):122-137.
29. Mertl P, Van FT, Bonhomme P, Vives P. Femoropatellar osteoarthritis treated by prosthesis. Retrospective study of 50 implants [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1997;83(8):712-718.
30. De Cloedt P, Legaye J, Lokietek W. Femoro-patellar prosthesis. A retrospective study of 45 consecutive cases with a follow-up of 3-12 years [in French]. Acta Orthop Belg. 1999;65(2):170-175.
31. Dahm DL, Al-Rayashi W, Dajani K, Shah JP, Levy BA, Stuart MJ. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop. 2010;39(10):487-491.
32. Farr J, Arendt E, Dahm D, Daynes J. Patellofemoral arthroplasty in the athlete. Clin Sports Med. 2014;33(3):547-552.
Genotype-guided warfarin dosing reduced adverse events in arthroplasty patients
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
Warfarin is the most commonly used anticoagulant in the world, and a significant cause of emergency department visits and hospitalizations, especially among older patients. Walking the fine line between dosing too little and too much is not an easy task – especially since warfarin response is influenced by diet, comorbidities, interactions with other medications and – as studies over the last 20 years have confirmed – many genetic variants.
Also, the practicality of genotyping every patient who needs anticoagulation therapy must be questioned. Based on the results of GIFT, 26 patients would need to be genotyped to prevent one event, typically an INR of 4 or greater. Although the cost of genotyping continues to decline, health insurers and publicly funded health systems have not yet been convinced that genotype-guided warfarin prescribing is a cost-effective strategy.
The benefits of genotyping would likely be less in patients with atrial fibrillation, for example, as they run a lower risk of VTE than do arthroplasty patients. The GIFT surgeries were all elective, so there was plenty of time to get back genotyping results before starting warfarin. That is a luxury not afforded to many patients in need of anticoagulation.
It’s possible, however, that the benefits of genotyping might be larger in the real world. GIFT was conducted at academic medical centers and used a clinical dosing algorithm as comparator. As a result, adverse event rates were likely lower in the comparison group than would be expected in other clinical settings with less-intense INR monitoring or empirically based initiation regimens.
Still, GIFT’s results are gaining global attention. Based on prepublication results of the GIFT trial, the Clinical Pharmacogenetics Implementation Consortium (CPIC), an international research network that develops consensus recommendations about the use of pharmacogenomic test results, recently published guidelines about genotype-guided dosing for warfarin. The group now recommends using genotype-guided warfarin dosing based on CYP2C9*2, CYP2C9*3, and VKORC1 variants for adult patients of non-African ancestry. It also recommends that patients with combinations of high-risk variants would benefit from an alternative anticoagulant strategy, because of likely greater risks of poor INR control and bleeding.
A single pharmacogenomic test covering many common variants relevant to multiple prescribing decisions over time is far more likely to be a cost-effective approach; however, there is no evidence for this proposition. Until then, it might be simpler and less expensive to use clinical dosing algorithms to reduce the risks of anticoagulation.
Jon D. Emery, PhD, is the Herman Professor of Primary Care Cancer Research at the University of Melbourne and Western Health, Melbourne. He made these remarks in an accompanying editorial (JAMA 2017;318;110-2 doi: 10.1001/jama.2017.11465 ).
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
The difference in the composite endpoint (major bleeding within 30 days, international normalized ratio [INR] of 4 or greater within 30 days, venous thromboembolism within 60 days, or death within 30 days) in the Genetic Informatics Trial of Warfarin to Prevent Deep Vein Thrombosis (GIFT) trial was mainly driven by a significant difference in episodes of elevated INR, reported Brian F. Gage, MD, and his colleagues (JAMA 2017;318[12]:1115-1124. doi: 10.1001/jama.2017.11469).
A total of 1,597 patients completed the trial. Of 808 patients in the genotype-guided group, 10.8% met one of the endpoints. Of 789 in the clinically guided warfarin dosing group, 14.7% met at least 1 of the endpoints. There were no deaths in the study.
“Widespread use of genotype-guided dosing will depend on reimbursement, regulations, and logistics. Although several commercial platforms for warfarin-related genes have been approved by the Food and Drug Administration and the European Medicines Agency, routine genotyping is not yet recommended,” wrote Dr. Gage of Washington University, St. Louis, and his coauthors.
The Centers for Medicare and Medicaid Services used its Coverage with Evidence Development program to fund genotyping in this trial and will review the results to determine future coverage, the researchers added.
In GIFT, patients were randomized to an 11-day regimen of warfarin guided either by a clinical algorithm or by their individual genotype. The team tested for four polymorphisms known to affect warfarin metabolism: VKORC1-1639G>A, CYP2C9*2, CYP2C9*3, and CYP4F2 V433M. The treatment goal was an INR of 1.8-2. After 11 days, physicians could administer warfarin according to their own judgment.
The absolute difference of 3.9% in the composite endpoint was largely driven by a 2.8% absolute difference in the rate of an INR of 4 or greater. The rate difference between the two groups was 0.8% for major bleeding, and 0.7% for VTE.
About 41% of the cohort was considered to be at high risk of bleeding complications, and this group accrued the highest benefit from genotype-based dosing. Among them, the composite endpoint was 11.5% compared with 15.2% in the clinical algorithm group – an absolute difference of 3.76%.
The benefit was consistent among black patients, and those with CYP2C9.
By day 90, one VTE had occurred in each group. An intracranial hemorrhage occurred in one patient in the clinically guided group, 2 months after stopping warfarin.
The clinical benefit of genotype-based dosing influenced 90-day outcomes as well, with the composite endpoint occurring in 11% of the genotype group and 15% of the clinically guided group (absolute difference 3.9%).
Among the 1,588 patients who had their percentage of time in the therapeutic range (PTTR) calculated, genotyping improved PTTR time by 3.4% overall. The effect was especially strong from days 4 to 14, when it improved PTTR by 5.7% relative to clinical guidance.
Three other studies have examined the effect of a genotype-based warfarin dosing regimen, Dr. Gage and his coauthors noted: Two found no benefit, and a third found that such guidance improved INR control. GIFT has several advantages over those trials, which the authors said lend credence to its results.
“Compared with previous studies, this trial was larger, used genotype-guided dosing for a longer duration, and incorporated more genes into the dosing algorithm …The longer period of genotype-guided dosing likely prevented cases of supratherapeutic INR that were common in these trials,” they wrote.
Dr. Gage reported no financial disclosures, but several coauthors reported ties with pharmaceutical and imaging companies.
FROM JAMA
Key clinical point:
Major finding: Genotype-guided dosing reduced adverse events – primarily elevated INRs – by almost 4% compared to clinically based warfarin dosing.
Data source: A randomized trial comprising 1,650 elderly patients undergoing elective knee or hip arthroplasty.
Disclosures: Dr. Gage had no financial disclosures, but several of his coauthors noted relationships with pharmaceutical and imaging companies.