User login
Review clarifies depression, anxiety risk among hidradenitis suppurativa patients
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
and meta-analysis of more than 40,000 adults.
Previous studies of psychiatric comorbidities in HS patients have suggested an increased rate of depression, anxiety, and suicide risk, but have varied in methodology. “Therefore, the exact magnitude of the prevalence and odds of depression and anxiety in patients with HS is unclear,” wrote Myrela O. Machado, MD, of the division of dermatology, Women’s College Hospital, Toronto, and colleagues.
In a review of PubMed/MEDLINE, Embase, and PsycINFO electronic databases through July 2018, the researchers identified 10 studies comprising 40,307 adults with HS. The overall prevalence of depression was 16.9%, and in the studies that included a comparison group, the odds ratio for depression was 1.84 for HS patients, compared with controls who did not have HS. The overall prevalence of anxiety was 4.9%, but data were insufficient to determine an odds ratio for anxiety. The study was published in JAMA Dermatology.
All 10 studies assessed depression; 4 assessed anxiety. In subgroup analyses, the prevalence of depression was 11.9% in studies with a diagnosis based on clinical criteria and 26.8% in studies that used a screening instrument. The prevalence was 25.9% for outpatients with HS.
“Although our findings indicate that depression and anxiety may be common among people with HS, whether there is a causal relationship in those associations remains to be proved,” the researchers wrote.
However, they noted, “the consequences of depression and anxiety on HS-related outcomes have recently received more attention.”
The study findings were limited by several factors including the lack of data from structured diagnostic interviews, variation in methodological quality, and variation in comparison groups across studies, as well as a high level of heterogeneity across studies, the researchers noted. However, the results support the need to recognize and treat psychiatric conditions in HS patients, and to develop new management strategies, they said.
Dr. Machado had no financial conflicts to disclose. Several coauthors disclosed relationships with Galderma, LEO Pharma, Janssen, Novartis, AbbVie, Celgene, Naos, Lilly, Sanofi, Valeant, and La Roche-Posay.
SOURCE: Machado M et al. JAMA Dermatol. 2019 June 5. doi: 10.1001/jamadermatol.2019.0759.
FROM JAMA DERMATOLOGY
The benefits of first-trimester fetal heart evaluation
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.
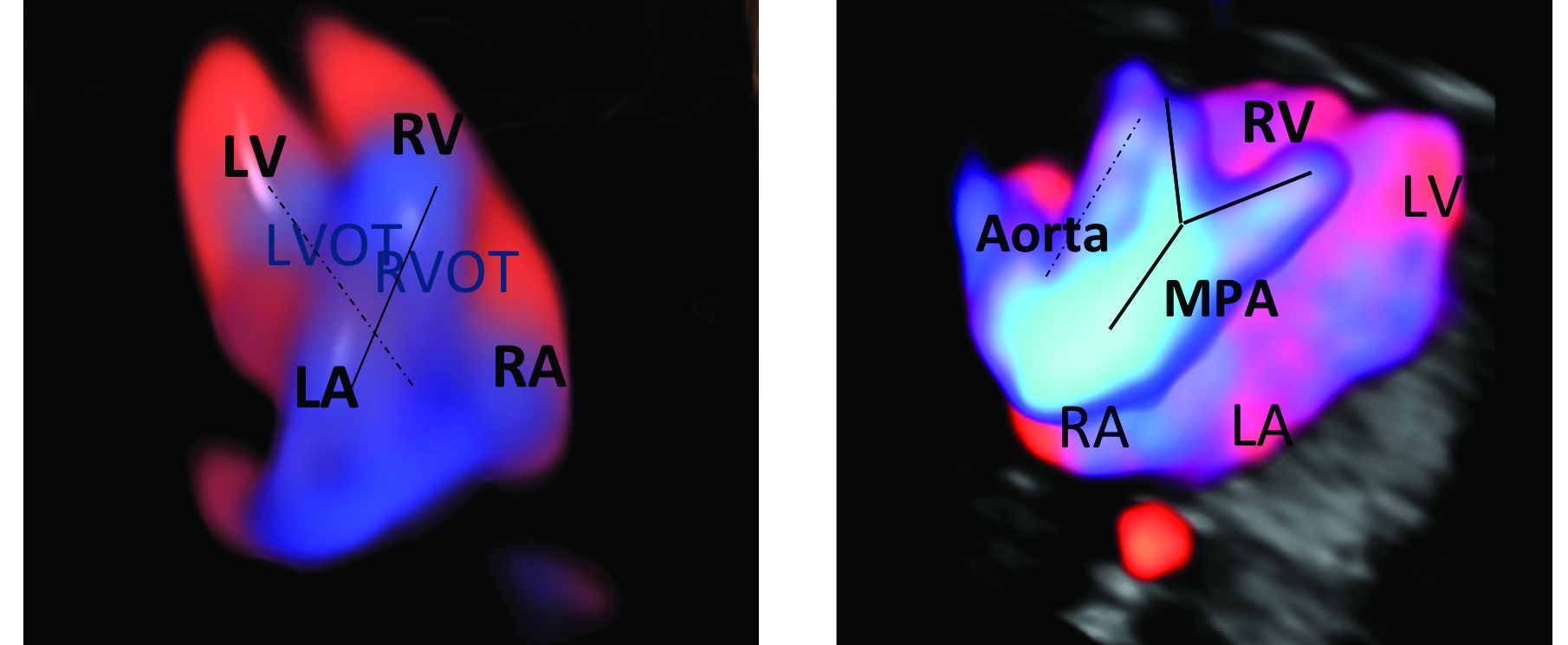
Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.
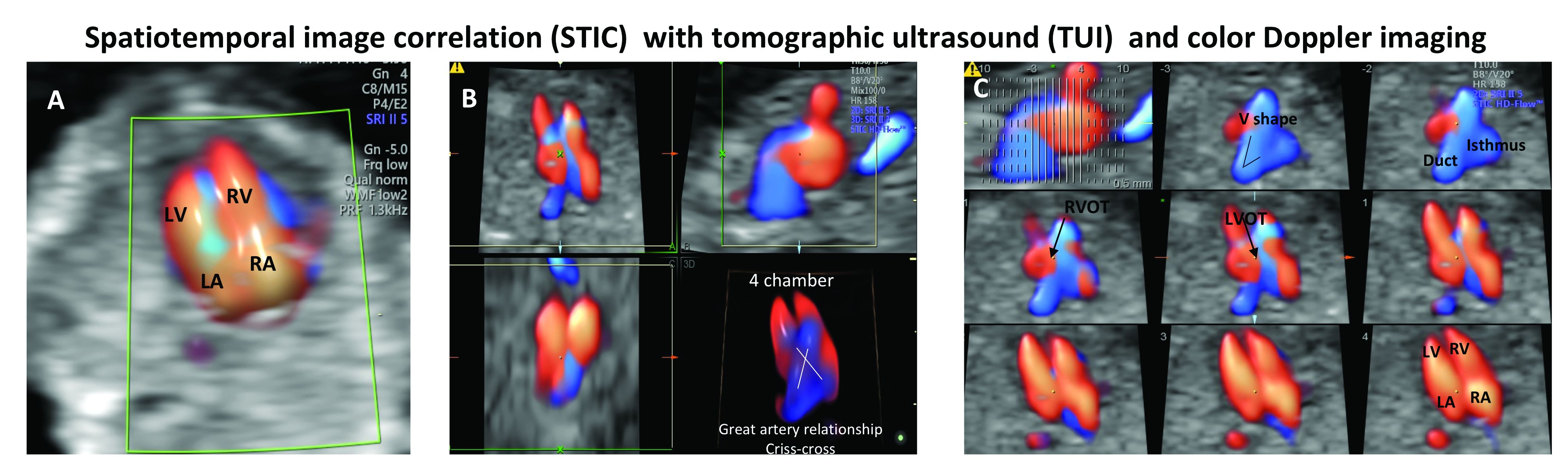
In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
Considering congenital heart defects early
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Low-dose CT lung cancer screening nets payoff in community setting too
Implementation of low-dose CT screening for lung cancer in community settings is successfully detecting the disease at an early, more treatable stage, suggests a survey of U.S. lung cancer screening centers.
Such screening led to a 20% reduction in lung cancer mortality in the National Lung Screening Trial (N Engl J Med. 2011;365:395-409), prompting the Centers for Medicare & Medicaid Services to start covering it in 2015. However, fewer than a third of trial practices were community based, and questions lingered regarding applicability of this screening in nonacademic settings.
Investigators working under senior author Jennifer C. King, PhD, senior director of science and research at the GO2 Foundation for Lung Cancer, Washington, administered a 21-question survey to 165 lung cancer screening centers designated as Screening Centers of Excellence asking about their 2016 program data and practices. Overall, 62% of the centers were community based, having no university or other academic affiliation.
Results reported in the Journal of Oncology Practice showed that more than half of 529 lung cancer diagnoses the centers made in 2016 were made at stage I or limited stage, with the same pattern seen for community and academic centers. Findings were similar when analyses instead considered Lung Imaging Reporting and Data System results for 40,000 low-dose CT scans performed that year.
Community and academic centers differed in how they addressed the CMS requirement for a prescreening shared decision making visit led by a health practitioner, with the former more commonly relying on only primary care providers (52% vs. 40%). The centers were similar in how they addressed the CMS requirement for smoking cessation services, with both using referral to a quitline, cessation counseling within the screening facility, and printed educational materials; however, academic centers more commonly followed up with current smokers (52% vs. 36%).
The main barriers to implementing screening were insurance and billing issues, lack of provider referral, lack of patient awareness, and internal work flow challenges, cited by more than half of centers overall. Community centers less often cited staffing and time limitations (35% vs. 53%) and insurance and billing issues (64% vs. 74%), but percentages were similar for other barriers.
“There has been concern about the ability of nonacademic centers to implement lung cancer screening as safely and effectively as academic medical centers,” Dr. King and coauthors wrote. “In this study, we not only demonstrate that lung cancer screening is happening in the community setting, but also that nonacademic screening programs are using similar protocols and are seeing similar findings as academic medical centers.
“These data indicate that responsible implementation is possible in the community and results in a meaningful stage shift for lung cancer diagnoses, and providers should support ongoing implementation of lung cancer screening efforts,” they concluded.
Dr. King reported that she receives honoraria from MedImmune, AstraZeneca, and Genentech; has a consulting or advisory role with GRAIL, Tesaro, AbbVie, and Foundation Medicine; and receives research funding from AstraZeneca. The study was supported by the GO2 Foundation for Lung Cancer.
SOURCE: King JC et al. J Oncol Pract. 2019 May 31. doi: 10.1200/JOP.18.00788.
Implementation of low-dose CT screening for lung cancer in community settings is successfully detecting the disease at an early, more treatable stage, suggests a survey of U.S. lung cancer screening centers.
Such screening led to a 20% reduction in lung cancer mortality in the National Lung Screening Trial (N Engl J Med. 2011;365:395-409), prompting the Centers for Medicare & Medicaid Services to start covering it in 2015. However, fewer than a third of trial practices were community based, and questions lingered regarding applicability of this screening in nonacademic settings.
Investigators working under senior author Jennifer C. King, PhD, senior director of science and research at the GO2 Foundation for Lung Cancer, Washington, administered a 21-question survey to 165 lung cancer screening centers designated as Screening Centers of Excellence asking about their 2016 program data and practices. Overall, 62% of the centers were community based, having no university or other academic affiliation.
Results reported in the Journal of Oncology Practice showed that more than half of 529 lung cancer diagnoses the centers made in 2016 were made at stage I or limited stage, with the same pattern seen for community and academic centers. Findings were similar when analyses instead considered Lung Imaging Reporting and Data System results for 40,000 low-dose CT scans performed that year.
Community and academic centers differed in how they addressed the CMS requirement for a prescreening shared decision making visit led by a health practitioner, with the former more commonly relying on only primary care providers (52% vs. 40%). The centers were similar in how they addressed the CMS requirement for smoking cessation services, with both using referral to a quitline, cessation counseling within the screening facility, and printed educational materials; however, academic centers more commonly followed up with current smokers (52% vs. 36%).
The main barriers to implementing screening were insurance and billing issues, lack of provider referral, lack of patient awareness, and internal work flow challenges, cited by more than half of centers overall. Community centers less often cited staffing and time limitations (35% vs. 53%) and insurance and billing issues (64% vs. 74%), but percentages were similar for other barriers.
“There has been concern about the ability of nonacademic centers to implement lung cancer screening as safely and effectively as academic medical centers,” Dr. King and coauthors wrote. “In this study, we not only demonstrate that lung cancer screening is happening in the community setting, but also that nonacademic screening programs are using similar protocols and are seeing similar findings as academic medical centers.
“These data indicate that responsible implementation is possible in the community and results in a meaningful stage shift for lung cancer diagnoses, and providers should support ongoing implementation of lung cancer screening efforts,” they concluded.
Dr. King reported that she receives honoraria from MedImmune, AstraZeneca, and Genentech; has a consulting or advisory role with GRAIL, Tesaro, AbbVie, and Foundation Medicine; and receives research funding from AstraZeneca. The study was supported by the GO2 Foundation for Lung Cancer.
SOURCE: King JC et al. J Oncol Pract. 2019 May 31. doi: 10.1200/JOP.18.00788.
Implementation of low-dose CT screening for lung cancer in community settings is successfully detecting the disease at an early, more treatable stage, suggests a survey of U.S. lung cancer screening centers.
Such screening led to a 20% reduction in lung cancer mortality in the National Lung Screening Trial (N Engl J Med. 2011;365:395-409), prompting the Centers for Medicare & Medicaid Services to start covering it in 2015. However, fewer than a third of trial practices were community based, and questions lingered regarding applicability of this screening in nonacademic settings.
Investigators working under senior author Jennifer C. King, PhD, senior director of science and research at the GO2 Foundation for Lung Cancer, Washington, administered a 21-question survey to 165 lung cancer screening centers designated as Screening Centers of Excellence asking about their 2016 program data and practices. Overall, 62% of the centers were community based, having no university or other academic affiliation.
Results reported in the Journal of Oncology Practice showed that more than half of 529 lung cancer diagnoses the centers made in 2016 were made at stage I or limited stage, with the same pattern seen for community and academic centers. Findings were similar when analyses instead considered Lung Imaging Reporting and Data System results for 40,000 low-dose CT scans performed that year.
Community and academic centers differed in how they addressed the CMS requirement for a prescreening shared decision making visit led by a health practitioner, with the former more commonly relying on only primary care providers (52% vs. 40%). The centers were similar in how they addressed the CMS requirement for smoking cessation services, with both using referral to a quitline, cessation counseling within the screening facility, and printed educational materials; however, academic centers more commonly followed up with current smokers (52% vs. 36%).
The main barriers to implementing screening were insurance and billing issues, lack of provider referral, lack of patient awareness, and internal work flow challenges, cited by more than half of centers overall. Community centers less often cited staffing and time limitations (35% vs. 53%) and insurance and billing issues (64% vs. 74%), but percentages were similar for other barriers.
“There has been concern about the ability of nonacademic centers to implement lung cancer screening as safely and effectively as academic medical centers,” Dr. King and coauthors wrote. “In this study, we not only demonstrate that lung cancer screening is happening in the community setting, but also that nonacademic screening programs are using similar protocols and are seeing similar findings as academic medical centers.
“These data indicate that responsible implementation is possible in the community and results in a meaningful stage shift for lung cancer diagnoses, and providers should support ongoing implementation of lung cancer screening efforts,” they concluded.
Dr. King reported that she receives honoraria from MedImmune, AstraZeneca, and Genentech; has a consulting or advisory role with GRAIL, Tesaro, AbbVie, and Foundation Medicine; and receives research funding from AstraZeneca. The study was supported by the GO2 Foundation for Lung Cancer.
SOURCE: King JC et al. J Oncol Pract. 2019 May 31. doi: 10.1200/JOP.18.00788.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
Could biosimilar switchbacks due to ‘inefficacy’ be subjective?
BIRMINGHAM, ENGLAND – Patients with rheumatoid arthritis (RA) who switch back to an original tumor necrosis factor inhibitor (TNFi) after using a biosimilar product often do so because of ineffectiveness, but this could be largely subjective, research suggests.
Data from the British Society for Rheumatology Biologics Register–Rheumatoid Arthritis (BSRBR-RA), presented at the annual conference of the British Society for Rheumatology, have shown that the majority (81%) of 760 patients who switched from an etanercept originator (ETN-O) product (Enbrel) to an etanercept biosimilar (ETN-B) product (Benepali or Erelzi) remained on the latter at an average of 2 years’ follow-up.
However, of those who switched back to an ETN-O (8%, n = 58), ineffectiveness was the primary reason for doing so in more than half of patients (53%). Of patients who stopped ETN treatment altogether after the biosimilar switch (11%, n = 84), 29% stopped because of inefficacy. Other important reasons for switching back or stopping treatment were adverse events in 28% and 54% of cases, respectively. The switch back to an ETN-O happened within a median time of 4 months (range, 2–6 months).
Patients switching back to an ETN-O tended to be slightly younger than all patients who had switched to an ETN-B (median age 60 vs. 64 years). They were more likely to be female (79% vs. 75%). Median disease activity score in 28 joints (DAS28) at baseline were highest in those who discontinued ETN (3.9 vs. 3.2 for those who switched back and 3.0 for all who had switched to a biosimilar).
It is not known what is driving the purported ineffectiveness, said research assistant Rebecca Davies of the Arthritis Research UK Epidemiology Unit at the University of Manchester (England).
“It could be due to patient factors and a nocebo effect,” she suggested. This is where “negative patient expectations cause treatment to have a more negative effect.” For example, she added, “if a patient is settled on the originator drug and had to mandatorily switch to a biosimilar, it might be that they are more heightened to symptoms.”
Another explanation could be that consultants may be “overcautious” to continuing the biosimilar in patients who had responded well to the originator product, she hypothesized.
“Our next steps are to look at disease activity between 4 and 9 months post switch to determine” the cause of the described ineffectiveness.
Educate patients to reduce switchbacks?
Meanwhile data presented by a team of researchers from Imperial College Healthcare NHS Trust in London, headed by Maresa Carulli, MD, suggested that patient education may be an important factor in tackling why patients want to switch back to biosimilar treatment.
Over a 20-month period among 202 patients who were switched to an ETN-B after they had been established on an ETN-O, 27 (13.4%) switched back.
“The majority of patients who switched back to Enbrel reported subjective worsening of disease symptoms with Benepali,” Dr. Carulli and coauthors said in their poster. Indeed, 78% of patients who switched back after an average of just under 6 months reported that their symptoms had become worse on the biosimilar product.
Analysis of the RA patients (n = 16) who switched back demonstrated that DAS28 scores had increased by more than 1.2 points during the period of the switch, but this was “mainly due to increases in visual analog scores [VAS],” they said.
The average change in DAS28 was an increase of 1.32 points during the switch period, the researchers noted. The average changes in DAS28 components were: +5 and +0.44 points for the tender and swollen joint counts, +9.89 points for the erythrocyte sedimentation rate, and +25.56 points for VAS.
Although further data are need, the results “may suggest that a subjective component contributes toward intolerance” of the biosimilar product, the researchers said.
“Consideration of patient education during initiation of biosimilar treatment could be a significant factor in improving compliance with it.”
The BSR receives restricted income from multiple U.K. pharmaceutical companies, which is used to fund the BSRBR-RA. The pharmaceutical company funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or any decision to submit manuscripts for publication.
Ms. Davies and Dr. Carulli and team declared having no competing interests.
SOURCES: Davies R et al. Rheumatology. 2019;58(suppl 3), Abstract 022; and Dahanayake C et al. Rheumatology. 2019;58(suppl 3), Abstract 102
BIRMINGHAM, ENGLAND – Patients with rheumatoid arthritis (RA) who switch back to an original tumor necrosis factor inhibitor (TNFi) after using a biosimilar product often do so because of ineffectiveness, but this could be largely subjective, research suggests.
Data from the British Society for Rheumatology Biologics Register–Rheumatoid Arthritis (BSRBR-RA), presented at the annual conference of the British Society for Rheumatology, have shown that the majority (81%) of 760 patients who switched from an etanercept originator (ETN-O) product (Enbrel) to an etanercept biosimilar (ETN-B) product (Benepali or Erelzi) remained on the latter at an average of 2 years’ follow-up.
However, of those who switched back to an ETN-O (8%, n = 58), ineffectiveness was the primary reason for doing so in more than half of patients (53%). Of patients who stopped ETN treatment altogether after the biosimilar switch (11%, n = 84), 29% stopped because of inefficacy. Other important reasons for switching back or stopping treatment were adverse events in 28% and 54% of cases, respectively. The switch back to an ETN-O happened within a median time of 4 months (range, 2–6 months).
Patients switching back to an ETN-O tended to be slightly younger than all patients who had switched to an ETN-B (median age 60 vs. 64 years). They were more likely to be female (79% vs. 75%). Median disease activity score in 28 joints (DAS28) at baseline were highest in those who discontinued ETN (3.9 vs. 3.2 for those who switched back and 3.0 for all who had switched to a biosimilar).
It is not known what is driving the purported ineffectiveness, said research assistant Rebecca Davies of the Arthritis Research UK Epidemiology Unit at the University of Manchester (England).
“It could be due to patient factors and a nocebo effect,” she suggested. This is where “negative patient expectations cause treatment to have a more negative effect.” For example, she added, “if a patient is settled on the originator drug and had to mandatorily switch to a biosimilar, it might be that they are more heightened to symptoms.”
Another explanation could be that consultants may be “overcautious” to continuing the biosimilar in patients who had responded well to the originator product, she hypothesized.
“Our next steps are to look at disease activity between 4 and 9 months post switch to determine” the cause of the described ineffectiveness.
Educate patients to reduce switchbacks?
Meanwhile data presented by a team of researchers from Imperial College Healthcare NHS Trust in London, headed by Maresa Carulli, MD, suggested that patient education may be an important factor in tackling why patients want to switch back to biosimilar treatment.
Over a 20-month period among 202 patients who were switched to an ETN-B after they had been established on an ETN-O, 27 (13.4%) switched back.
“The majority of patients who switched back to Enbrel reported subjective worsening of disease symptoms with Benepali,” Dr. Carulli and coauthors said in their poster. Indeed, 78% of patients who switched back after an average of just under 6 months reported that their symptoms had become worse on the biosimilar product.
Analysis of the RA patients (n = 16) who switched back demonstrated that DAS28 scores had increased by more than 1.2 points during the period of the switch, but this was “mainly due to increases in visual analog scores [VAS],” they said.
The average change in DAS28 was an increase of 1.32 points during the switch period, the researchers noted. The average changes in DAS28 components were: +5 and +0.44 points for the tender and swollen joint counts, +9.89 points for the erythrocyte sedimentation rate, and +25.56 points for VAS.
Although further data are need, the results “may suggest that a subjective component contributes toward intolerance” of the biosimilar product, the researchers said.
“Consideration of patient education during initiation of biosimilar treatment could be a significant factor in improving compliance with it.”
The BSR receives restricted income from multiple U.K. pharmaceutical companies, which is used to fund the BSRBR-RA. The pharmaceutical company funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or any decision to submit manuscripts for publication.
Ms. Davies and Dr. Carulli and team declared having no competing interests.
SOURCES: Davies R et al. Rheumatology. 2019;58(suppl 3), Abstract 022; and Dahanayake C et al. Rheumatology. 2019;58(suppl 3), Abstract 102
BIRMINGHAM, ENGLAND – Patients with rheumatoid arthritis (RA) who switch back to an original tumor necrosis factor inhibitor (TNFi) after using a biosimilar product often do so because of ineffectiveness, but this could be largely subjective, research suggests.
Data from the British Society for Rheumatology Biologics Register–Rheumatoid Arthritis (BSRBR-RA), presented at the annual conference of the British Society for Rheumatology, have shown that the majority (81%) of 760 patients who switched from an etanercept originator (ETN-O) product (Enbrel) to an etanercept biosimilar (ETN-B) product (Benepali or Erelzi) remained on the latter at an average of 2 years’ follow-up.
However, of those who switched back to an ETN-O (8%, n = 58), ineffectiveness was the primary reason for doing so in more than half of patients (53%). Of patients who stopped ETN treatment altogether after the biosimilar switch (11%, n = 84), 29% stopped because of inefficacy. Other important reasons for switching back or stopping treatment were adverse events in 28% and 54% of cases, respectively. The switch back to an ETN-O happened within a median time of 4 months (range, 2–6 months).
Patients switching back to an ETN-O tended to be slightly younger than all patients who had switched to an ETN-B (median age 60 vs. 64 years). They were more likely to be female (79% vs. 75%). Median disease activity score in 28 joints (DAS28) at baseline were highest in those who discontinued ETN (3.9 vs. 3.2 for those who switched back and 3.0 for all who had switched to a biosimilar).
It is not known what is driving the purported ineffectiveness, said research assistant Rebecca Davies of the Arthritis Research UK Epidemiology Unit at the University of Manchester (England).
“It could be due to patient factors and a nocebo effect,” she suggested. This is where “negative patient expectations cause treatment to have a more negative effect.” For example, she added, “if a patient is settled on the originator drug and had to mandatorily switch to a biosimilar, it might be that they are more heightened to symptoms.”
Another explanation could be that consultants may be “overcautious” to continuing the biosimilar in patients who had responded well to the originator product, she hypothesized.
“Our next steps are to look at disease activity between 4 and 9 months post switch to determine” the cause of the described ineffectiveness.
Educate patients to reduce switchbacks?
Meanwhile data presented by a team of researchers from Imperial College Healthcare NHS Trust in London, headed by Maresa Carulli, MD, suggested that patient education may be an important factor in tackling why patients want to switch back to biosimilar treatment.
Over a 20-month period among 202 patients who were switched to an ETN-B after they had been established on an ETN-O, 27 (13.4%) switched back.
“The majority of patients who switched back to Enbrel reported subjective worsening of disease symptoms with Benepali,” Dr. Carulli and coauthors said in their poster. Indeed, 78% of patients who switched back after an average of just under 6 months reported that their symptoms had become worse on the biosimilar product.
Analysis of the RA patients (n = 16) who switched back demonstrated that DAS28 scores had increased by more than 1.2 points during the period of the switch, but this was “mainly due to increases in visual analog scores [VAS],” they said.
The average change in DAS28 was an increase of 1.32 points during the switch period, the researchers noted. The average changes in DAS28 components were: +5 and +0.44 points for the tender and swollen joint counts, +9.89 points for the erythrocyte sedimentation rate, and +25.56 points for VAS.
Although further data are need, the results “may suggest that a subjective component contributes toward intolerance” of the biosimilar product, the researchers said.
“Consideration of patient education during initiation of biosimilar treatment could be a significant factor in improving compliance with it.”
The BSR receives restricted income from multiple U.K. pharmaceutical companies, which is used to fund the BSRBR-RA. The pharmaceutical company funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or any decision to submit manuscripts for publication.
Ms. Davies and Dr. Carulli and team declared having no competing interests.
SOURCES: Davies R et al. Rheumatology. 2019;58(suppl 3), Abstract 022; and Dahanayake C et al. Rheumatology. 2019;58(suppl 3), Abstract 102
REPORTING FROM BSR 2019
Carotid ultrasound may aid cardiovascular risk stratification of patients with psoriatic disease
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
according to findings from a retrospective study.
When added to the Framingham risk score, the measurement significantly improved its predictive ability, Curtis Sobchak, MD, and colleagues wrote in Arthritis & Rheumatology.
The findings indicate that carotid ultrasound could be a useful addition to cardiovascular risk stratification among these patients.
“Traditional algorithms do not consider other factors that may contribute to increased cardiovascular risk in rheumatic disease patients and tend to underestimate cardiovascular risk,” wrote Dr. Sobchak of the University of Toronto and coauthors.
“The advantage of ultrasound over other modalities for vascular imaging includes lack of radiation, low cost of the examination, and its widespread use in rheumatology for joint evaluation. Thus, this assessment could potentially be performed ‘at the bedside’ during consultation to provide immediate valuable information to complement clinical data from history, physical examination, and laboratory data,” they added.
The study retrospectively examined a prospective, observational cohort of 559 patients with psoriasis alone or psoriasis and psoriatic arthritis enrolled in the University of Toronto Psoriatic Disease Program. The investigators evaluated five ultrasound measures of atherosclerosis, including total plaque area (TPA), mean carotid intima-media thickness (cIMT), maximal cIMT, plaque category, and TPA category. Then they analyzed the risk relationship with major cardiovascular events (CVEs) classified as myocardial infarction, unstable angina, ischemic stroke, revascularization procedures, or cardiovascular-related death. Minor CVEs included stable angina, exacerbation of congestive heart failure, and transient ischemic attack over a mean follow-up close to 4 years.
The mean baseline TPA was 0.18 cm2 and mean cIMT was 639 mcm. Most patients had plaques, including 27.0% with unilateral and 31.5% with bilateral plaques.
The rate of a first CVE during the study period was 1.11 per 100 patient-years, and the rate of a first major CVE was 0.91 per 100 patient-years. The risk of each was significantly related to a higher baseline burden of atherosclerosis.
A multivariate analysis determined that increased TPA at baseline increased the risk of an event by nearly 200% (hazard ratio, 2.85). Mean cIMT was not an independent predictor in the final analysis, “suggesting that TPA is a stronger predictor for CVE than cIMT,” the authors wrote.
Finally, they examined the predictive value of atherosclerosis alone, as well as combined with the Framingham risk score. The 5-year model indicated that the bivariate model was slightly more accurate than the Framingham score alone (area under the curve, 0.84 vs. 0.81), although this was not a significant difference. The predictive value of the Framingham risk score plus maximal cIMT, mean cIMT, or TPA all significantly improved when they were calculated using only high-risk patients (those above the treatment threshold for dyslipidemia).
“To the best of our knowledge this is the first study to assess the utility of various measures of carotid atherosclerosis to predict CVE in patients with psoriasis and PsA [psoriatic arthritis]. ... Combining vascular imaging data with clinical and laboratory measures of traditional cardiovascular risk factors could improve accuracy of cardiovascular risk stratification in patients with psoriatic disease and facilitate earlier initiation of appropriate treatment to reduce CVE in this population,” the investigators wrote.
The study was supported in part by a Young Investigator Operating Grant from the Arthritis Society. Dr. Sobchak had no financial disclosures.
SOURCE: Sobchak C et al. Arthritis Rheumatol. 2019 Jun 5. doi: 10.1002/art.40925.
FROM ARTHRITIS & RHEUMATOLOGY
Jury still out on gemcitabine/nab-paclitaxel as treatment for pancreatic cancer
CHICAGO – While gemcitabine plus nab-paclitaxel did not extend independently assessed disease-free survival versus gemcitabine alone in a phase 3 pancreatic cancer study, overall survival data are “encouraging” thus far, and merit further follow-up, an investigator said at the annual meeting of the American Society of Clinical Oncology.
The APACT trial was the first adjuvant pancreatic cancer trial to use an endpoint of disease-free survival, independently assessed without knowledge of medical or clinical circumstances, said investigator Margaret A. Tempero, MD, director of the UCSF Pancreas Center, San Francisco.
With that specific method of assessment, median disease-free survival was 19.4 months for gemcitabine plus nab-paclitaxel versus 18.8 months for gemcitabine alone, a finding that had a “slight trend” in favor of the combination arm, Dr. Tempero said.
By contrast, a more standard investigator-assessed disease-free survival showed an improvement favoring gemcitabine plus nab-paclitaxel over gemcitabine alone, in line with preliminary overall survival data that also appear to show an advantage for the combination over gemcitabine, according to Dr. Tempero.
“We thought we were introducing more rigor into the trial, but I think we all recognize clinically that it is often difficult to differentiate or distinguish recurrence in the pancreatic bed from postsurgical changes,” she said in a discussion period, “but clearly, this is a lesson learned, and I think that would be helpful for the field going forward in avoiding that endpoint.”
Since the trial was launched, both modified FOLFIRINOX and gemcitabine plus capecitabine have become category 1 recommendations for adjuvant pancreatic cancer in clinical practice guidelines from the National Comprehensive Cancer Network.
The “jury is still out” on whether gemcitabine plus nab-paclitaxel will join that group, said Jiping Wang, MD, PhD, of Dana-Farber Cancer Institute, Boston, who discussed the results of the APACT trial in a podium presentation.
“We are still waiting for the final overall survival result of the APACT trial,” he said. “Hopefully, the nab-paclitaxel/gemcitabine combination provides an alternative treatment, especially for patients with R1 resection who cannot tolerate modified FOLFIRINOX.”
While gemcitabine plus capecitabine is a good choice for patients who aren’t able to tolerate modified FOLFIRINOX, available evidence suggests it may not benefit patients with R1 resection, according to Dr. Wang.
The phase 3 APACT trial included patients with surgically resected pancreatic cancer enrolled at 179 sites in 21 countries. A total of 866 patients were randomized 1:1 to receive gemcitabine plus nab-paclitaxel or gemcitabine alone for six cycles.
While median independently assessed disease-free survival was 19.4 months for gemcitabine/nab-paclitaxel and 18.8 months for gemcitabine (hazard ratio, 0.88; P = .1824), median investigator-assessed disease free survival was 16.6 months versus 13.7 months for those arms, respectively (HR, 0.82; P = .0168), according to reported results.
Median interim overall survival was 40.5 months for gemcitabine/nab-paclitaxel versus 36.2 months for gemcitabine (HR, 0.82; P = .045), though the presenter emphasized that longer follow-up is needed to clarify the role of this combination as adjuvant therapy for pancreatic adenocarcinoma.
“I don’t think it’s wrong in a patient who can’t tolerate modified FOLFIRINOX to use gemcitabine and nab-paclitaxel, but I’m not sure everybody would agree with that,” Dr. Tempero said after being asked in the study discussion period if she would advocate for use of this combination regimen in frail patients.
“I think when we get more mature overall survival data, it will be a lot easier to make those decisions,” she said.
Dr. Tempero reported disclosures related to Abbie, Advance Medical, Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, BioPharm Communications, Celgene, CPRIT, EcoR1 Capital, Eisai, FibroGen, Halozyme, Ignyta, Immunovia, Merck, Pharmacyclics, Pharmacyte Biotech, and Tocagen.
SOURCE: Tempero MA, et al. ASCO 2019. Abstract 4000.
CHICAGO – While gemcitabine plus nab-paclitaxel did not extend independently assessed disease-free survival versus gemcitabine alone in a phase 3 pancreatic cancer study, overall survival data are “encouraging” thus far, and merit further follow-up, an investigator said at the annual meeting of the American Society of Clinical Oncology.
The APACT trial was the first adjuvant pancreatic cancer trial to use an endpoint of disease-free survival, independently assessed without knowledge of medical or clinical circumstances, said investigator Margaret A. Tempero, MD, director of the UCSF Pancreas Center, San Francisco.
With that specific method of assessment, median disease-free survival was 19.4 months for gemcitabine plus nab-paclitaxel versus 18.8 months for gemcitabine alone, a finding that had a “slight trend” in favor of the combination arm, Dr. Tempero said.
By contrast, a more standard investigator-assessed disease-free survival showed an improvement favoring gemcitabine plus nab-paclitaxel over gemcitabine alone, in line with preliminary overall survival data that also appear to show an advantage for the combination over gemcitabine, according to Dr. Tempero.
“We thought we were introducing more rigor into the trial, but I think we all recognize clinically that it is often difficult to differentiate or distinguish recurrence in the pancreatic bed from postsurgical changes,” she said in a discussion period, “but clearly, this is a lesson learned, and I think that would be helpful for the field going forward in avoiding that endpoint.”
Since the trial was launched, both modified FOLFIRINOX and gemcitabine plus capecitabine have become category 1 recommendations for adjuvant pancreatic cancer in clinical practice guidelines from the National Comprehensive Cancer Network.
The “jury is still out” on whether gemcitabine plus nab-paclitaxel will join that group, said Jiping Wang, MD, PhD, of Dana-Farber Cancer Institute, Boston, who discussed the results of the APACT trial in a podium presentation.
“We are still waiting for the final overall survival result of the APACT trial,” he said. “Hopefully, the nab-paclitaxel/gemcitabine combination provides an alternative treatment, especially for patients with R1 resection who cannot tolerate modified FOLFIRINOX.”
While gemcitabine plus capecitabine is a good choice for patients who aren’t able to tolerate modified FOLFIRINOX, available evidence suggests it may not benefit patients with R1 resection, according to Dr. Wang.
The phase 3 APACT trial included patients with surgically resected pancreatic cancer enrolled at 179 sites in 21 countries. A total of 866 patients were randomized 1:1 to receive gemcitabine plus nab-paclitaxel or gemcitabine alone for six cycles.
While median independently assessed disease-free survival was 19.4 months for gemcitabine/nab-paclitaxel and 18.8 months for gemcitabine (hazard ratio, 0.88; P = .1824), median investigator-assessed disease free survival was 16.6 months versus 13.7 months for those arms, respectively (HR, 0.82; P = .0168), according to reported results.
Median interim overall survival was 40.5 months for gemcitabine/nab-paclitaxel versus 36.2 months for gemcitabine (HR, 0.82; P = .045), though the presenter emphasized that longer follow-up is needed to clarify the role of this combination as adjuvant therapy for pancreatic adenocarcinoma.
“I don’t think it’s wrong in a patient who can’t tolerate modified FOLFIRINOX to use gemcitabine and nab-paclitaxel, but I’m not sure everybody would agree with that,” Dr. Tempero said after being asked in the study discussion period if she would advocate for use of this combination regimen in frail patients.
“I think when we get more mature overall survival data, it will be a lot easier to make those decisions,” she said.
Dr. Tempero reported disclosures related to Abbie, Advance Medical, Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, BioPharm Communications, Celgene, CPRIT, EcoR1 Capital, Eisai, FibroGen, Halozyme, Ignyta, Immunovia, Merck, Pharmacyclics, Pharmacyte Biotech, and Tocagen.
SOURCE: Tempero MA, et al. ASCO 2019. Abstract 4000.
CHICAGO – While gemcitabine plus nab-paclitaxel did not extend independently assessed disease-free survival versus gemcitabine alone in a phase 3 pancreatic cancer study, overall survival data are “encouraging” thus far, and merit further follow-up, an investigator said at the annual meeting of the American Society of Clinical Oncology.
The APACT trial was the first adjuvant pancreatic cancer trial to use an endpoint of disease-free survival, independently assessed without knowledge of medical or clinical circumstances, said investigator Margaret A. Tempero, MD, director of the UCSF Pancreas Center, San Francisco.
With that specific method of assessment, median disease-free survival was 19.4 months for gemcitabine plus nab-paclitaxel versus 18.8 months for gemcitabine alone, a finding that had a “slight trend” in favor of the combination arm, Dr. Tempero said.
By contrast, a more standard investigator-assessed disease-free survival showed an improvement favoring gemcitabine plus nab-paclitaxel over gemcitabine alone, in line with preliminary overall survival data that also appear to show an advantage for the combination over gemcitabine, according to Dr. Tempero.
“We thought we were introducing more rigor into the trial, but I think we all recognize clinically that it is often difficult to differentiate or distinguish recurrence in the pancreatic bed from postsurgical changes,” she said in a discussion period, “but clearly, this is a lesson learned, and I think that would be helpful for the field going forward in avoiding that endpoint.”
Since the trial was launched, both modified FOLFIRINOX and gemcitabine plus capecitabine have become category 1 recommendations for adjuvant pancreatic cancer in clinical practice guidelines from the National Comprehensive Cancer Network.
The “jury is still out” on whether gemcitabine plus nab-paclitaxel will join that group, said Jiping Wang, MD, PhD, of Dana-Farber Cancer Institute, Boston, who discussed the results of the APACT trial in a podium presentation.
“We are still waiting for the final overall survival result of the APACT trial,” he said. “Hopefully, the nab-paclitaxel/gemcitabine combination provides an alternative treatment, especially for patients with R1 resection who cannot tolerate modified FOLFIRINOX.”
While gemcitabine plus capecitabine is a good choice for patients who aren’t able to tolerate modified FOLFIRINOX, available evidence suggests it may not benefit patients with R1 resection, according to Dr. Wang.
The phase 3 APACT trial included patients with surgically resected pancreatic cancer enrolled at 179 sites in 21 countries. A total of 866 patients were randomized 1:1 to receive gemcitabine plus nab-paclitaxel or gemcitabine alone for six cycles.
While median independently assessed disease-free survival was 19.4 months for gemcitabine/nab-paclitaxel and 18.8 months for gemcitabine (hazard ratio, 0.88; P = .1824), median investigator-assessed disease free survival was 16.6 months versus 13.7 months for those arms, respectively (HR, 0.82; P = .0168), according to reported results.
Median interim overall survival was 40.5 months for gemcitabine/nab-paclitaxel versus 36.2 months for gemcitabine (HR, 0.82; P = .045), though the presenter emphasized that longer follow-up is needed to clarify the role of this combination as adjuvant therapy for pancreatic adenocarcinoma.
“I don’t think it’s wrong in a patient who can’t tolerate modified FOLFIRINOX to use gemcitabine and nab-paclitaxel, but I’m not sure everybody would agree with that,” Dr. Tempero said after being asked in the study discussion period if she would advocate for use of this combination regimen in frail patients.
“I think when we get more mature overall survival data, it will be a lot easier to make those decisions,” she said.
Dr. Tempero reported disclosures related to Abbie, Advance Medical, Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, BioPharm Communications, Celgene, CPRIT, EcoR1 Capital, Eisai, FibroGen, Halozyme, Ignyta, Immunovia, Merck, Pharmacyclics, Pharmacyte Biotech, and Tocagen.
SOURCE: Tempero MA, et al. ASCO 2019. Abstract 4000.
REPORTING FROM ASCO 2019
Focus on science, not format
How JHM is improving the author experience
“No hassle” new manuscript submission process
Many authors have experienced the frustration of formatting a manuscript for submission to a medical journal. The process is time consuming and each journal has different requirements. This means that if you decide to submit your manuscript to one journal and later decide that another journal is a better fit, you may spend an hour (or several hours) reformatting to meet the new journal’s unique requirements.
To allow authors to spend more time on what matters to them, we’re pleased to introduce our “No Hassle” process for initial original research and brief report manuscript submissions to the Journal of Hospital Medicine. Our goal is to eliminate unnecessary and burdensome steps in the manuscript submission process. Thus, we have relaxed formatting requirements for initial manuscript submissions. Any conventional and readable manuscript format and reference style is acceptable.
Tables and figures can be embedded in the main document file or uploaded individually, depending on your preference. Funding and disclosures should be included on the title page but there is no need to submit completed disclosure or copyright forms unless we request a manuscript revision.
Timely decisions
We have all experienced the agony of waiting months on end for a journal to make a decision about our manuscript. The review process itself can take many months (or even longer). Furthermore, a manuscript may not be published for many more months (or even longer) following acceptance. At the Journal of Hospital Medicine, we commit to making timely decisions and publishing your accepted manuscript as fast as we can.
We currently reject approximately half of all original research and brief report manuscript submissions without formal peer review. We do this for two reasons. First, we want to ensure that we’re not overburdening our peer reviewers so we only ask them to review manuscripts that we are seriously considering for publication. Second, we want to ensure that we’re being respectful of our authors’ time. If we are unlikely to publish a manuscript based on lower priority scores assigned by me, as editor-in-chief, or other journal editors, we don’t want to subject your manuscript to a lengthy peer review, but would rather return the manuscript to you quickly for timely submission elsewhere.
Here are data that support our timely decision making:
- 1.3 days = our average time from manuscript submission to rejection without formal peer review (median, less than one day).
- 23 days = our average time from manuscript submission to first decision for manuscripts sent for peer review.
We also are working to improve our time to publication. Our goal is to publish accepted manuscripts within 120 days from initial submission to publication, and within 60 days from acceptance to publication.
Dissemination
Finally, little public knowledge is gleaned from medical research unless the study is published and widely read. The Journal of Hospital Medicine is at the leading edge of helping authors disseminate their work to a broader audience. Of course, we produce press releases and distribute those to many media outlets in partnership with the Society of Hospital Medicine. We also leverage social media to promote your article through tweets, visual abstracts, and, more recently, comics or graphic medicine abstracts. Some articles are even discussed on #JHMChat, our twitter-based journal club. This work is led by our exceptional Digital Media Editors, Dr. Vineet Arora (@FutureDocs), Dr. Charlie Wray (@WrayCharles), and Dr. Grace Farris (@gracefarris).
In summary, we are committed to making the Journal of Hospital Medicine even more author friendly. To that end, we’re making it easy for authors to submit their work, making timely disposition decisions, and facilitating dissemination of the work we publish.
Dr. Shah is chief metrics officer and director of the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. He is the current editor-in-chief of the Journal of Hospital Medicine.
How JHM is improving the author experience
How JHM is improving the author experience
“No hassle” new manuscript submission process
Many authors have experienced the frustration of formatting a manuscript for submission to a medical journal. The process is time consuming and each journal has different requirements. This means that if you decide to submit your manuscript to one journal and later decide that another journal is a better fit, you may spend an hour (or several hours) reformatting to meet the new journal’s unique requirements.
To allow authors to spend more time on what matters to them, we’re pleased to introduce our “No Hassle” process for initial original research and brief report manuscript submissions to the Journal of Hospital Medicine. Our goal is to eliminate unnecessary and burdensome steps in the manuscript submission process. Thus, we have relaxed formatting requirements for initial manuscript submissions. Any conventional and readable manuscript format and reference style is acceptable.
Tables and figures can be embedded in the main document file or uploaded individually, depending on your preference. Funding and disclosures should be included on the title page but there is no need to submit completed disclosure or copyright forms unless we request a manuscript revision.
Timely decisions
We have all experienced the agony of waiting months on end for a journal to make a decision about our manuscript. The review process itself can take many months (or even longer). Furthermore, a manuscript may not be published for many more months (or even longer) following acceptance. At the Journal of Hospital Medicine, we commit to making timely decisions and publishing your accepted manuscript as fast as we can.
We currently reject approximately half of all original research and brief report manuscript submissions without formal peer review. We do this for two reasons. First, we want to ensure that we’re not overburdening our peer reviewers so we only ask them to review manuscripts that we are seriously considering for publication. Second, we want to ensure that we’re being respectful of our authors’ time. If we are unlikely to publish a manuscript based on lower priority scores assigned by me, as editor-in-chief, or other journal editors, we don’t want to subject your manuscript to a lengthy peer review, but would rather return the manuscript to you quickly for timely submission elsewhere.
Here are data that support our timely decision making:
- 1.3 days = our average time from manuscript submission to rejection without formal peer review (median, less than one day).
- 23 days = our average time from manuscript submission to first decision for manuscripts sent for peer review.
We also are working to improve our time to publication. Our goal is to publish accepted manuscripts within 120 days from initial submission to publication, and within 60 days from acceptance to publication.
Dissemination
Finally, little public knowledge is gleaned from medical research unless the study is published and widely read. The Journal of Hospital Medicine is at the leading edge of helping authors disseminate their work to a broader audience. Of course, we produce press releases and distribute those to many media outlets in partnership with the Society of Hospital Medicine. We also leverage social media to promote your article through tweets, visual abstracts, and, more recently, comics or graphic medicine abstracts. Some articles are even discussed on #JHMChat, our twitter-based journal club. This work is led by our exceptional Digital Media Editors, Dr. Vineet Arora (@FutureDocs), Dr. Charlie Wray (@WrayCharles), and Dr. Grace Farris (@gracefarris).
In summary, we are committed to making the Journal of Hospital Medicine even more author friendly. To that end, we’re making it easy for authors to submit their work, making timely disposition decisions, and facilitating dissemination of the work we publish.
Dr. Shah is chief metrics officer and director of the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. He is the current editor-in-chief of the Journal of Hospital Medicine.
“No hassle” new manuscript submission process
Many authors have experienced the frustration of formatting a manuscript for submission to a medical journal. The process is time consuming and each journal has different requirements. This means that if you decide to submit your manuscript to one journal and later decide that another journal is a better fit, you may spend an hour (or several hours) reformatting to meet the new journal’s unique requirements.
To allow authors to spend more time on what matters to them, we’re pleased to introduce our “No Hassle” process for initial original research and brief report manuscript submissions to the Journal of Hospital Medicine. Our goal is to eliminate unnecessary and burdensome steps in the manuscript submission process. Thus, we have relaxed formatting requirements for initial manuscript submissions. Any conventional and readable manuscript format and reference style is acceptable.
Tables and figures can be embedded in the main document file or uploaded individually, depending on your preference. Funding and disclosures should be included on the title page but there is no need to submit completed disclosure or copyright forms unless we request a manuscript revision.
Timely decisions
We have all experienced the agony of waiting months on end for a journal to make a decision about our manuscript. The review process itself can take many months (or even longer). Furthermore, a manuscript may not be published for many more months (or even longer) following acceptance. At the Journal of Hospital Medicine, we commit to making timely decisions and publishing your accepted manuscript as fast as we can.
We currently reject approximately half of all original research and brief report manuscript submissions without formal peer review. We do this for two reasons. First, we want to ensure that we’re not overburdening our peer reviewers so we only ask them to review manuscripts that we are seriously considering for publication. Second, we want to ensure that we’re being respectful of our authors’ time. If we are unlikely to publish a manuscript based on lower priority scores assigned by me, as editor-in-chief, or other journal editors, we don’t want to subject your manuscript to a lengthy peer review, but would rather return the manuscript to you quickly for timely submission elsewhere.
Here are data that support our timely decision making:
- 1.3 days = our average time from manuscript submission to rejection without formal peer review (median, less than one day).
- 23 days = our average time from manuscript submission to first decision for manuscripts sent for peer review.
We also are working to improve our time to publication. Our goal is to publish accepted manuscripts within 120 days from initial submission to publication, and within 60 days from acceptance to publication.
Dissemination
Finally, little public knowledge is gleaned from medical research unless the study is published and widely read. The Journal of Hospital Medicine is at the leading edge of helping authors disseminate their work to a broader audience. Of course, we produce press releases and distribute those to many media outlets in partnership with the Society of Hospital Medicine. We also leverage social media to promote your article through tweets, visual abstracts, and, more recently, comics or graphic medicine abstracts. Some articles are even discussed on #JHMChat, our twitter-based journal club. This work is led by our exceptional Digital Media Editors, Dr. Vineet Arora (@FutureDocs), Dr. Charlie Wray (@WrayCharles), and Dr. Grace Farris (@gracefarris).
In summary, we are committed to making the Journal of Hospital Medicine even more author friendly. To that end, we’re making it easy for authors to submit their work, making timely disposition decisions, and facilitating dissemination of the work we publish.
Dr. Shah is chief metrics officer and director of the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. He is the current editor-in-chief of the Journal of Hospital Medicine.
Pregnancy mortality in the United States: Top causes of death and timing
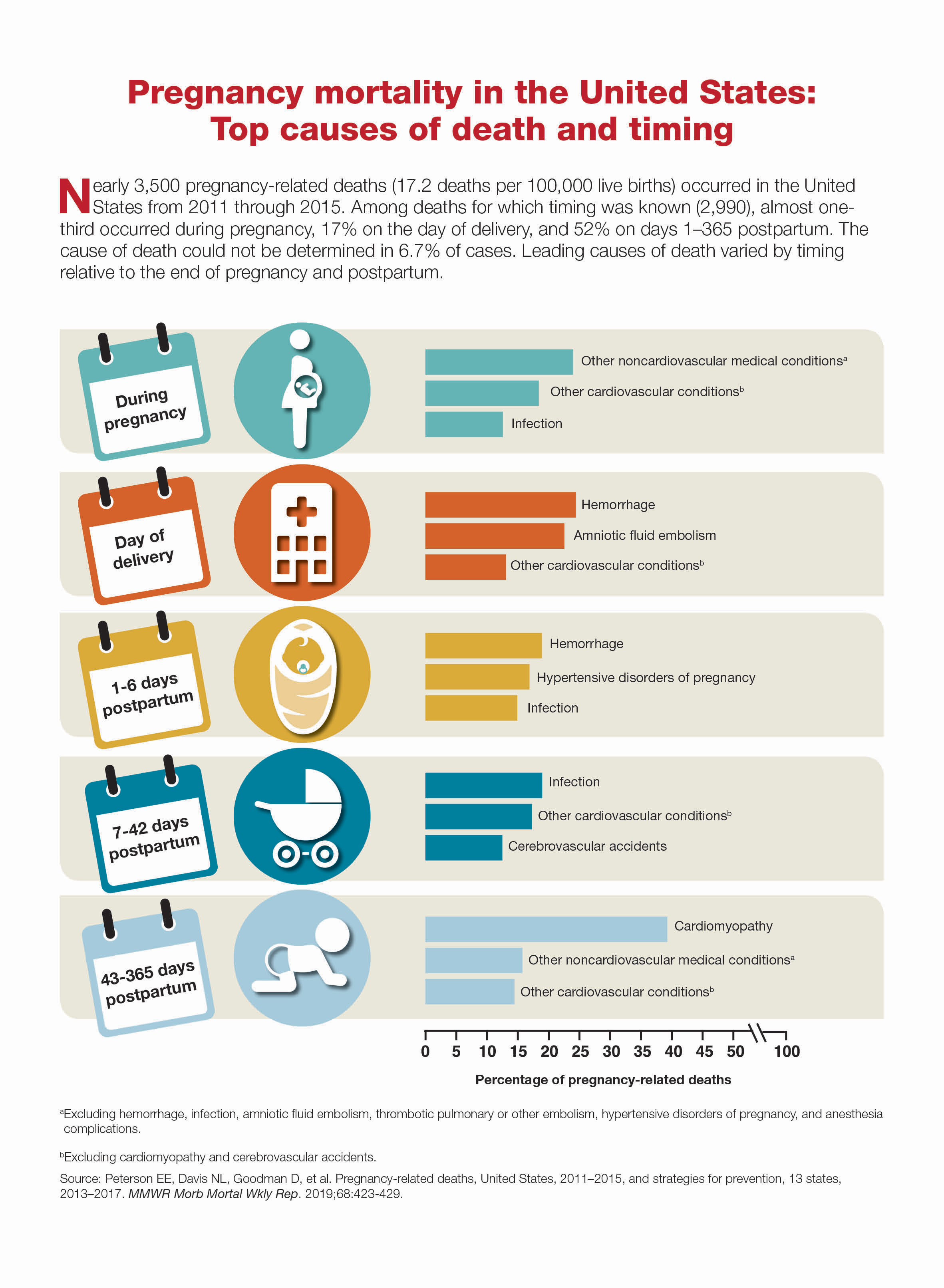


Novel molecular panel found to aid in eosinophilic gastritis diagnosis
SAN DIEGO – A new scoring system based on a set of 18 informative genes was sufficient to diagnose cases of eosinophilic gastritis, results from a molecular analysis showed.
The system, known as EG diagnostic panel 18 (EGDP18), “can provide clinicians with a better diagnostic classification of ambiguous cases of eosinophilic gastritis, indicating a strong correlation with disease severity,” lead study author Tetsuo Shoda, MD, PhD, said at the annual Digestive Disease Week®.“The EG molecular profile also strongly correlates with particular endoscopic and histological features, thus providing insight into pathogenesis for EG.”
In an effort to develop an EG diagnostic panel, to validate its utility for EG diagnosis and management, and to better understand disease pathogenesis, Dr. Shoda and his colleagues used RNA sequencing to generate genome-wide gene expression profiles from gastric biopsies. Next, they developed an EG diagnostic panel focusing on a set of 48 informative genes, and analyzed its performance in a discovery cohort (55 EG and 39 controls) and subsequently an independent validation cohort (67 EG and 27 controls). The EGDP score was calculated by summation of delta CT values of the most highly dysregulated 18 genes. For diagnosis, the researchers calculated the area under the receiver operating characteristic curve (AUC), and used Spearman correlation to analyze associations.
Dr. Shoda, a research fellow at Cincinnati Children’s Hospital Medical Center, reported that the EGDP18 score identified active EG patients in both cohorts (P less than .0001, AUC equal to or greater than 0.95). In the discovery cohort, a score of less than zero resulted in a sensitivity of 95.2%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 95.8%. In the validation cohort, a score of less than zero resulted in a sensitivity of 88.9%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 94.1%.
The researchers observed a significant inverse correlation between the EGDP18 score and gastric eosinophil counts cross-sectionally and longitudinally. The score also showed comparable levels and high correlation between the gastric antrum and body. In addition, when analyzed by EGDP18 score, 63% of ambiguous tissue eosinophils were found to be molecularly equivalent to active EG, “suggesting the capacity to offer an objective cutoff for EG diagnosis,” Dr. Shoda said.
The researchers reported having no financial disclosures.
SOURCE: Shoda T et al. DDW 2019, Abstract 165. doi: 10.1016/S0016-5085(19)36878-7.
SAN DIEGO – A new scoring system based on a set of 18 informative genes was sufficient to diagnose cases of eosinophilic gastritis, results from a molecular analysis showed.
The system, known as EG diagnostic panel 18 (EGDP18), “can provide clinicians with a better diagnostic classification of ambiguous cases of eosinophilic gastritis, indicating a strong correlation with disease severity,” lead study author Tetsuo Shoda, MD, PhD, said at the annual Digestive Disease Week®.“The EG molecular profile also strongly correlates with particular endoscopic and histological features, thus providing insight into pathogenesis for EG.”
In an effort to develop an EG diagnostic panel, to validate its utility for EG diagnosis and management, and to better understand disease pathogenesis, Dr. Shoda and his colleagues used RNA sequencing to generate genome-wide gene expression profiles from gastric biopsies. Next, they developed an EG diagnostic panel focusing on a set of 48 informative genes, and analyzed its performance in a discovery cohort (55 EG and 39 controls) and subsequently an independent validation cohort (67 EG and 27 controls). The EGDP score was calculated by summation of delta CT values of the most highly dysregulated 18 genes. For diagnosis, the researchers calculated the area under the receiver operating characteristic curve (AUC), and used Spearman correlation to analyze associations.
Dr. Shoda, a research fellow at Cincinnati Children’s Hospital Medical Center, reported that the EGDP18 score identified active EG patients in both cohorts (P less than .0001, AUC equal to or greater than 0.95). In the discovery cohort, a score of less than zero resulted in a sensitivity of 95.2%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 95.8%. In the validation cohort, a score of less than zero resulted in a sensitivity of 88.9%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 94.1%.
The researchers observed a significant inverse correlation between the EGDP18 score and gastric eosinophil counts cross-sectionally and longitudinally. The score also showed comparable levels and high correlation between the gastric antrum and body. In addition, when analyzed by EGDP18 score, 63% of ambiguous tissue eosinophils were found to be molecularly equivalent to active EG, “suggesting the capacity to offer an objective cutoff for EG diagnosis,” Dr. Shoda said.
The researchers reported having no financial disclosures.
SOURCE: Shoda T et al. DDW 2019, Abstract 165. doi: 10.1016/S0016-5085(19)36878-7.
SAN DIEGO – A new scoring system based on a set of 18 informative genes was sufficient to diagnose cases of eosinophilic gastritis, results from a molecular analysis showed.
The system, known as EG diagnostic panel 18 (EGDP18), “can provide clinicians with a better diagnostic classification of ambiguous cases of eosinophilic gastritis, indicating a strong correlation with disease severity,” lead study author Tetsuo Shoda, MD, PhD, said at the annual Digestive Disease Week®.“The EG molecular profile also strongly correlates with particular endoscopic and histological features, thus providing insight into pathogenesis for EG.”
In an effort to develop an EG diagnostic panel, to validate its utility for EG diagnosis and management, and to better understand disease pathogenesis, Dr. Shoda and his colleagues used RNA sequencing to generate genome-wide gene expression profiles from gastric biopsies. Next, they developed an EG diagnostic panel focusing on a set of 48 informative genes, and analyzed its performance in a discovery cohort (55 EG and 39 controls) and subsequently an independent validation cohort (67 EG and 27 controls). The EGDP score was calculated by summation of delta CT values of the most highly dysregulated 18 genes. For diagnosis, the researchers calculated the area under the receiver operating characteristic curve (AUC), and used Spearman correlation to analyze associations.
Dr. Shoda, a research fellow at Cincinnati Children’s Hospital Medical Center, reported that the EGDP18 score identified active EG patients in both cohorts (P less than .0001, AUC equal to or greater than 0.95). In the discovery cohort, a score of less than zero resulted in a sensitivity of 95.2%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 95.8%. In the validation cohort, a score of less than zero resulted in a sensitivity of 88.9%, a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 94.1%.
The researchers observed a significant inverse correlation between the EGDP18 score and gastric eosinophil counts cross-sectionally and longitudinally. The score also showed comparable levels and high correlation between the gastric antrum and body. In addition, when analyzed by EGDP18 score, 63% of ambiguous tissue eosinophils were found to be molecularly equivalent to active EG, “suggesting the capacity to offer an objective cutoff for EG diagnosis,” Dr. Shoda said.
The researchers reported having no financial disclosures.
SOURCE: Shoda T et al. DDW 2019, Abstract 165. doi: 10.1016/S0016-5085(19)36878-7.
REPORTING FROM DDW 2019