User login
Emergency Transfers: An Important Predictor of Adverse Outcomes in Hospitalized Children
Unrecognized in-hospital deterioration can result in tragic consequences for pediatric patients. The majority of deterioration events have antecedents such as increasingly abnormal vital signs and new concerns from nurses.1 Recent meta-analyses have shown that rapid response systems (RRSs), which include trigger mechanisms such as a pediatric early warning score (PEWS), are associated with a reduced rate of arrests and in-hospital mortality.2,3 Cardiopulmonary arrest rates are useful metrics to judge the effectiveness of the system to identify and respond to deteriorating adult patients; however, there are important challenges to their use as an outcome measure in pediatrics. Arrests, which have been relatively uncommon in pediatric patients, are now even less frequent since the adoption of a RRS in the majority of children’s hospitals.4,5 Several innovations in these systems will be context-dependent and hence best first evaluated in a single center, where arrests outside of the intensive care unit (ICU) may occur rarely. Identification of valid, more frequent proximal measures to arrests may better identify the risk factors for deterioration. This could potentially inform quality improvement efforts to mitigate clinical deterioration.
Bonafide et al. at the Children’s Hospital of Philadelphia developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had a >13-fold increased risk of in-hospital mortality.6 At Cincinnati Children’s Hospital Medical Center, an additional proximal outcome measure was developed for unrecognized clinical deterioration, now termed emergency transfers (ETs).7-9 An ET is defined as any patient transferred to the ICU where the patient received intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer.9 Improvement science work that aimed at increasing clinician situation awareness was associated with a reduction in ETs,8 but the association of ETs with mortality or other healthcare utilization outcomes is unknown. The objective of this study was to determine the predictive validity of an ET on in-hospital mortality, ICU length of stay (LOS), and overall hospital LOS.
METHODS
We conducted a case–control study at Cincinnati Children’s Hospital, a free-standing tertiary care children’s hospital. Our center has had an ICU-based RRS in place since 2005. In 2009, we eliminated the ICU consult such that each floor-to-ICU transfer is evaluated by the RRS. Nurses calculate a Monaghan PEWS every four hours on the majority of nursing units.
Patients of all ages cared for outside of the ICU at any point in their hospitalization from January 1, 2013, to July 31, 2017, were eligible for inclusion. There were no other exclusion criteria. The ICU included both the pediatric ICU and the cardiac ICU.
Cases
We identified all ET cases from an existing situation awareness database in which each RRS call is entered by the hospital nursing supervisor, whose role includes responding to each RRS activation. If the patient transfer meets the ET criteria, the nurse indicates this in the database. Each ET entry is later confirmed for assurance purposes by the nurse leader of the RRS committee (RG). For the purposes of this study, all records were again reviewed and validated using manual chart review in the electronic health record (Epic Systems, Verona, Wisconsin).
Controls
We identified nonemergent ICU transfers to serve as controls and matched those to ET in cases to limit the impact of confounders that may increase the likelihood of both an ET and a negative outcome such as ICU mortality. We identified up to three controls for each case from our database and matched in terms of age group (within five years of age), hospital unit before transfer, and time of year (within three months of ET). These variables were chosen to adjust for the impact of age, diversity of disease (as hospital units are generally organized by organ system of illness), and seasonality on outcomes.
Outcome Measures
Posttransfer LOS in the ICU, posttransfer hospital LOS, and in-hospital mortality were the primary outcome measures. Patient demographics, specific diagnoses, and number of medical conditions were a priori defined as covariates of interest. Data for each case and control were entered into a secure, web-based Research Electronic Data Capture (REDCap) database.
Analysis
Descriptive data were summarized using counts and percentages for categorical variables and medians and ranges for continuous variables due to nonnormal distributions. Chi-square test was used to compare in-hospital mortality between the ETs and the controls. The Wilcoxon rank-sum test was used to compare LOS between ETs and controls. All data analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
A total of 45 ETs were identified, and 110 controls were matched. Patient demographics were similar among all cases and controls (P > .05). Patients with ETs had a median age of seven years (interquartile range: 3-18 years), and 51% of them were males. The majority of patients among our examined cases were white (68%) and non-Hispanic (93%). There was no statistical difference in insurance between the ETs and the controls. When evaluating the hospital unit before the transfer, ETs occurred most commonly in the Cardiology (22%), Hematology/Oncology (22%), and Neuroscience (16%) units.
ETs stayed longer in the ICU than non-ETs [median of 4.9 days vs 2.2 days, P = .001; Figure (A)]. Similarly, ET cases had a significantly longer posttransfer hospital LOS [median of 35 days vs 21 days, P = .001; Figure (B)]. ETs had a 22% in-hospital mortality rate, compared with 9% in-hospital mortality in the matched controls (P = .02; Table).
DISCUSSION
Children who experienced an ET had a significantly longer ICU LOS, a longer posttransfer LOS, and a higher in-hospital mortality than the matched controls who were also transferred to the ICU. Researchers and improvement science teams at multiple hospitals have demonstrated that interventions targeting improved situation awareness can reduce ETs; we have demonstrated that reducing ETs may reduce subsequent adverse outcomes.8,10
These findings provide additional support for the use of the ET metric in children’s hospitals as a proximal measure for significant clinical deterioration. We found mortality rates that were overall high for a children’s hospital (22% in ET cases and 9% among controls) compared with a national average mortality rate of 2.3% in pediatric ICUs.11 This is likely due to the study sample containing a significant proportion of children with medical complexity.
Aoki et al. recently demonstrated that ETs, compared with non-ETs, were associated with longer LOS and higher mortality in a bivariate analysis.12 In our study, we found similar results with the important addition that these findings were robust when ETs were compared with matched controls who were likely at a higher risk of poor outcomes than ICU transfers in general. In addition, we demonstrated that ETs were associated with adverse outcomes in a United States children’s hospital with a mature, long-standing RRS process. As ETs are considerably more common than cardiac and respiratory arrests, use of the ET metric in children’s hospitals may enable more rapid learning and systems improvement implementations. We also found that most of the children with ETs present from units that care for children with substantial medical complexity, including Cardiology, Hematology/Oncology, and Neurosciences. Future work should continue to examine the relationship between medical complexity and ET risk.
The ET metric is complementary to the CDE measure developed by Bonafide et al. Both metrics capture potential events of unrecognized clinical deterioration, and both offer researchers the opportunity to better understand and improve their RRSs. Both ETs and CDEs are more common than arrests, and CDEs are more common than ETs. ETs, which by definition occur in the first hour of ICU care, are likely a more specific measure of unrecognized clinical deterioration. CDEs will capture therapies that may have been started up to 12 hours after transfer and thus are possibly more sensitive to identify unrecognized clinical deterioration. However, CDEs also may encompass some patients who arrived at the ICU after prompt recognition and then had a subacute deterioration in the ICU.
The maturity of the RRS and the bandwidth of teams to collect data may inform which metric(s) are best for individual centers. As ETs are less common and likely more specific to unrecognized clinical deterioration, they might be the first tracked as a center improves its RRS through QI methods. Alternatively, CDEs may be a useful metric for centers where unrecognized clinical deterioration is less common or in research studies where this more common outcome would lead to more power to detect the effect of interventions to improve care.
Our study had several limitations. Data collection was confined to one tertiary care children’s hospital with a high burden of complex cardiac and oncology care. The results may not generalize well to children hospitalized in smaller or community hospitals or in hospitals without a mature RRS. There is also the possibility of misclassification of covariates and outcomes, but any misclassification would likely be nondifferential and bias toward the null. Matching was not possible based on exact diagnosis, and the unit is a good but imperfect proxy for diagnosis grouping. At our center, overflow of patients into the Cardiology and Hematology/Oncology units is uncommon, mitigating this partially, although residual confounding may remain. The finding that ETs are associated with adverse outcomes does not necessarily mean that these events were preventable; however, it is important and encouraging that the rate of ETs has been reduced at two centers using improvement science interventions.8,10
CONCLUSION
Patients who experienced an ET had a significantly higher likelihood of in-hospital mortality, spent more time in the ICU, and had a longer hospital LOS posttransfer than matched controls. The use of the ET metric in children’s hospitals would allow for further analysis of such patients in hopes of identifying clinical characteristics that serve as predictors of deterioration. This may facilitate better risk stratification in the clinical system as well as enable more rapid learning and systems improvements targeted toward preventing unrecognized clinical deterioration.
Disclosures
Dr. Hussain, Dr. Sosa, Dr. Ambroggio, and Mrs. Gallagher have nothing to disclose. Patrick Brady reports grants from the Agency for Healthcare Research and Quality, outside the submitted work. The authors certify that this submission is not under review by any other publication. The author team has no conflicts of interest to disclose.
Funding
Ms. Hussain was supported by the Society of Hospital Medicine’s Student Hospitalist Scholar Grant Program in 2017. Dr. Brady receives career development support from AHRQ K08-HS023827. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the SHM, AHRQ, or NIH.
1. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388.
2. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. https://doi.org/10.1186/s13054-015-0973-y.
3. Bonafide CP, Roland D, Brady PW. Rapid response systems 20 years later: new approaches, old challenges. JAMA Pediatrics. 2016;170(8):729-730. https://doi.org/10.1001/jamapediatrics.2016.0398.
4. Hayes LW, Dobyns EL, DiGiovine B, et al. A multicenter collaborative approach to reducing pediatric codes outside the ICU. Pediatrics. 2012;129(3):e785-e791. https://doi.org/10.1542/peds.2011-0227.
5. Raymond TT, Bonafide CP, Praestgaard A, et al. Pediatric medical emergency team events and outcomes: a report of 3647 events from the American Heart Association’s get with the guidelines-resuscitation registry. Hosp Pediatr. 2016;6(2):57-64. https://doi.org/10.1542/hpeds.2015-0132.
6. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784.
7. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747.
8. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
9. Brady PW, Wheeler DS, Muething SE, Kotagal UR. Situation awareness: a new model for predicting and preventing patient deterioration. Hosp Pediatr. 2014;4(3):143-146. https://doi.org/10.1542/hpeds.2013-0119.
10. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042.
11. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med. 2017;18(10):915-923. https://doi.org/10.1097/PCC.0000000000001268.
12. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2018;55(2):213-215. https://doi.org/10.1111/jpc.14185.
Unrecognized in-hospital deterioration can result in tragic consequences for pediatric patients. The majority of deterioration events have antecedents such as increasingly abnormal vital signs and new concerns from nurses.1 Recent meta-analyses have shown that rapid response systems (RRSs), which include trigger mechanisms such as a pediatric early warning score (PEWS), are associated with a reduced rate of arrests and in-hospital mortality.2,3 Cardiopulmonary arrest rates are useful metrics to judge the effectiveness of the system to identify and respond to deteriorating adult patients; however, there are important challenges to their use as an outcome measure in pediatrics. Arrests, which have been relatively uncommon in pediatric patients, are now even less frequent since the adoption of a RRS in the majority of children’s hospitals.4,5 Several innovations in these systems will be context-dependent and hence best first evaluated in a single center, where arrests outside of the intensive care unit (ICU) may occur rarely. Identification of valid, more frequent proximal measures to arrests may better identify the risk factors for deterioration. This could potentially inform quality improvement efforts to mitigate clinical deterioration.
Bonafide et al. at the Children’s Hospital of Philadelphia developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had a >13-fold increased risk of in-hospital mortality.6 At Cincinnati Children’s Hospital Medical Center, an additional proximal outcome measure was developed for unrecognized clinical deterioration, now termed emergency transfers (ETs).7-9 An ET is defined as any patient transferred to the ICU where the patient received intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer.9 Improvement science work that aimed at increasing clinician situation awareness was associated with a reduction in ETs,8 but the association of ETs with mortality or other healthcare utilization outcomes is unknown. The objective of this study was to determine the predictive validity of an ET on in-hospital mortality, ICU length of stay (LOS), and overall hospital LOS.
METHODS
We conducted a case–control study at Cincinnati Children’s Hospital, a free-standing tertiary care children’s hospital. Our center has had an ICU-based RRS in place since 2005. In 2009, we eliminated the ICU consult such that each floor-to-ICU transfer is evaluated by the RRS. Nurses calculate a Monaghan PEWS every four hours on the majority of nursing units.
Patients of all ages cared for outside of the ICU at any point in their hospitalization from January 1, 2013, to July 31, 2017, were eligible for inclusion. There were no other exclusion criteria. The ICU included both the pediatric ICU and the cardiac ICU.
Cases
We identified all ET cases from an existing situation awareness database in which each RRS call is entered by the hospital nursing supervisor, whose role includes responding to each RRS activation. If the patient transfer meets the ET criteria, the nurse indicates this in the database. Each ET entry is later confirmed for assurance purposes by the nurse leader of the RRS committee (RG). For the purposes of this study, all records were again reviewed and validated using manual chart review in the electronic health record (Epic Systems, Verona, Wisconsin).
Controls
We identified nonemergent ICU transfers to serve as controls and matched those to ET in cases to limit the impact of confounders that may increase the likelihood of both an ET and a negative outcome such as ICU mortality. We identified up to three controls for each case from our database and matched in terms of age group (within five years of age), hospital unit before transfer, and time of year (within three months of ET). These variables were chosen to adjust for the impact of age, diversity of disease (as hospital units are generally organized by organ system of illness), and seasonality on outcomes.
Outcome Measures
Posttransfer LOS in the ICU, posttransfer hospital LOS, and in-hospital mortality were the primary outcome measures. Patient demographics, specific diagnoses, and number of medical conditions were a priori defined as covariates of interest. Data for each case and control were entered into a secure, web-based Research Electronic Data Capture (REDCap) database.
Analysis
Descriptive data were summarized using counts and percentages for categorical variables and medians and ranges for continuous variables due to nonnormal distributions. Chi-square test was used to compare in-hospital mortality between the ETs and the controls. The Wilcoxon rank-sum test was used to compare LOS between ETs and controls. All data analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
A total of 45 ETs were identified, and 110 controls were matched. Patient demographics were similar among all cases and controls (P > .05). Patients with ETs had a median age of seven years (interquartile range: 3-18 years), and 51% of them were males. The majority of patients among our examined cases were white (68%) and non-Hispanic (93%). There was no statistical difference in insurance between the ETs and the controls. When evaluating the hospital unit before the transfer, ETs occurred most commonly in the Cardiology (22%), Hematology/Oncology (22%), and Neuroscience (16%) units.
ETs stayed longer in the ICU than non-ETs [median of 4.9 days vs 2.2 days, P = .001; Figure (A)]. Similarly, ET cases had a significantly longer posttransfer hospital LOS [median of 35 days vs 21 days, P = .001; Figure (B)]. ETs had a 22% in-hospital mortality rate, compared with 9% in-hospital mortality in the matched controls (P = .02; Table).
DISCUSSION
Children who experienced an ET had a significantly longer ICU LOS, a longer posttransfer LOS, and a higher in-hospital mortality than the matched controls who were also transferred to the ICU. Researchers and improvement science teams at multiple hospitals have demonstrated that interventions targeting improved situation awareness can reduce ETs; we have demonstrated that reducing ETs may reduce subsequent adverse outcomes.8,10
These findings provide additional support for the use of the ET metric in children’s hospitals as a proximal measure for significant clinical deterioration. We found mortality rates that were overall high for a children’s hospital (22% in ET cases and 9% among controls) compared with a national average mortality rate of 2.3% in pediatric ICUs.11 This is likely due to the study sample containing a significant proportion of children with medical complexity.
Aoki et al. recently demonstrated that ETs, compared with non-ETs, were associated with longer LOS and higher mortality in a bivariate analysis.12 In our study, we found similar results with the important addition that these findings were robust when ETs were compared with matched controls who were likely at a higher risk of poor outcomes than ICU transfers in general. In addition, we demonstrated that ETs were associated with adverse outcomes in a United States children’s hospital with a mature, long-standing RRS process. As ETs are considerably more common than cardiac and respiratory arrests, use of the ET metric in children’s hospitals may enable more rapid learning and systems improvement implementations. We also found that most of the children with ETs present from units that care for children with substantial medical complexity, including Cardiology, Hematology/Oncology, and Neurosciences. Future work should continue to examine the relationship between medical complexity and ET risk.
The ET metric is complementary to the CDE measure developed by Bonafide et al. Both metrics capture potential events of unrecognized clinical deterioration, and both offer researchers the opportunity to better understand and improve their RRSs. Both ETs and CDEs are more common than arrests, and CDEs are more common than ETs. ETs, which by definition occur in the first hour of ICU care, are likely a more specific measure of unrecognized clinical deterioration. CDEs will capture therapies that may have been started up to 12 hours after transfer and thus are possibly more sensitive to identify unrecognized clinical deterioration. However, CDEs also may encompass some patients who arrived at the ICU after prompt recognition and then had a subacute deterioration in the ICU.
The maturity of the RRS and the bandwidth of teams to collect data may inform which metric(s) are best for individual centers. As ETs are less common and likely more specific to unrecognized clinical deterioration, they might be the first tracked as a center improves its RRS through QI methods. Alternatively, CDEs may be a useful metric for centers where unrecognized clinical deterioration is less common or in research studies where this more common outcome would lead to more power to detect the effect of interventions to improve care.
Our study had several limitations. Data collection was confined to one tertiary care children’s hospital with a high burden of complex cardiac and oncology care. The results may not generalize well to children hospitalized in smaller or community hospitals or in hospitals without a mature RRS. There is also the possibility of misclassification of covariates and outcomes, but any misclassification would likely be nondifferential and bias toward the null. Matching was not possible based on exact diagnosis, and the unit is a good but imperfect proxy for diagnosis grouping. At our center, overflow of patients into the Cardiology and Hematology/Oncology units is uncommon, mitigating this partially, although residual confounding may remain. The finding that ETs are associated with adverse outcomes does not necessarily mean that these events were preventable; however, it is important and encouraging that the rate of ETs has been reduced at two centers using improvement science interventions.8,10
CONCLUSION
Patients who experienced an ET had a significantly higher likelihood of in-hospital mortality, spent more time in the ICU, and had a longer hospital LOS posttransfer than matched controls. The use of the ET metric in children’s hospitals would allow for further analysis of such patients in hopes of identifying clinical characteristics that serve as predictors of deterioration. This may facilitate better risk stratification in the clinical system as well as enable more rapid learning and systems improvements targeted toward preventing unrecognized clinical deterioration.
Disclosures
Dr. Hussain, Dr. Sosa, Dr. Ambroggio, and Mrs. Gallagher have nothing to disclose. Patrick Brady reports grants from the Agency for Healthcare Research and Quality, outside the submitted work. The authors certify that this submission is not under review by any other publication. The author team has no conflicts of interest to disclose.
Funding
Ms. Hussain was supported by the Society of Hospital Medicine’s Student Hospitalist Scholar Grant Program in 2017. Dr. Brady receives career development support from AHRQ K08-HS023827. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the SHM, AHRQ, or NIH.
Unrecognized in-hospital deterioration can result in tragic consequences for pediatric patients. The majority of deterioration events have antecedents such as increasingly abnormal vital signs and new concerns from nurses.1 Recent meta-analyses have shown that rapid response systems (RRSs), which include trigger mechanisms such as a pediatric early warning score (PEWS), are associated with a reduced rate of arrests and in-hospital mortality.2,3 Cardiopulmonary arrest rates are useful metrics to judge the effectiveness of the system to identify and respond to deteriorating adult patients; however, there are important challenges to their use as an outcome measure in pediatrics. Arrests, which have been relatively uncommon in pediatric patients, are now even less frequent since the adoption of a RRS in the majority of children’s hospitals.4,5 Several innovations in these systems will be context-dependent and hence best first evaluated in a single center, where arrests outside of the intensive care unit (ICU) may occur rarely. Identification of valid, more frequent proximal measures to arrests may better identify the risk factors for deterioration. This could potentially inform quality improvement efforts to mitigate clinical deterioration.
Bonafide et al. at the Children’s Hospital of Philadelphia developed and validated the critical deterioration event (CDE) metric, demonstrating that children who were transferred to the ICU and who received noninvasive ventilation, intubation, or vasopressor initiation within 12 hours of transfer had a >13-fold increased risk of in-hospital mortality.6 At Cincinnati Children’s Hospital Medical Center, an additional proximal outcome measure was developed for unrecognized clinical deterioration, now termed emergency transfers (ETs).7-9 An ET is defined as any patient transferred to the ICU where the patient received intubation, inotropes, or three or more fluid boluses in the first hour after arrival or before transfer.9 Improvement science work that aimed at increasing clinician situation awareness was associated with a reduction in ETs,8 but the association of ETs with mortality or other healthcare utilization outcomes is unknown. The objective of this study was to determine the predictive validity of an ET on in-hospital mortality, ICU length of stay (LOS), and overall hospital LOS.
METHODS
We conducted a case–control study at Cincinnati Children’s Hospital, a free-standing tertiary care children’s hospital. Our center has had an ICU-based RRS in place since 2005. In 2009, we eliminated the ICU consult such that each floor-to-ICU transfer is evaluated by the RRS. Nurses calculate a Monaghan PEWS every four hours on the majority of nursing units.
Patients of all ages cared for outside of the ICU at any point in their hospitalization from January 1, 2013, to July 31, 2017, were eligible for inclusion. There were no other exclusion criteria. The ICU included both the pediatric ICU and the cardiac ICU.
Cases
We identified all ET cases from an existing situation awareness database in which each RRS call is entered by the hospital nursing supervisor, whose role includes responding to each RRS activation. If the patient transfer meets the ET criteria, the nurse indicates this in the database. Each ET entry is later confirmed for assurance purposes by the nurse leader of the RRS committee (RG). For the purposes of this study, all records were again reviewed and validated using manual chart review in the electronic health record (Epic Systems, Verona, Wisconsin).
Controls
We identified nonemergent ICU transfers to serve as controls and matched those to ET in cases to limit the impact of confounders that may increase the likelihood of both an ET and a negative outcome such as ICU mortality. We identified up to three controls for each case from our database and matched in terms of age group (within five years of age), hospital unit before transfer, and time of year (within three months of ET). These variables were chosen to adjust for the impact of age, diversity of disease (as hospital units are generally organized by organ system of illness), and seasonality on outcomes.
Outcome Measures
Posttransfer LOS in the ICU, posttransfer hospital LOS, and in-hospital mortality were the primary outcome measures. Patient demographics, specific diagnoses, and number of medical conditions were a priori defined as covariates of interest. Data for each case and control were entered into a secure, web-based Research Electronic Data Capture (REDCap) database.
Analysis
Descriptive data were summarized using counts and percentages for categorical variables and medians and ranges for continuous variables due to nonnormal distributions. Chi-square test was used to compare in-hospital mortality between the ETs and the controls. The Wilcoxon rank-sum test was used to compare LOS between ETs and controls. All data analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
A total of 45 ETs were identified, and 110 controls were matched. Patient demographics were similar among all cases and controls (P > .05). Patients with ETs had a median age of seven years (interquartile range: 3-18 years), and 51% of them were males. The majority of patients among our examined cases were white (68%) and non-Hispanic (93%). There was no statistical difference in insurance between the ETs and the controls. When evaluating the hospital unit before the transfer, ETs occurred most commonly in the Cardiology (22%), Hematology/Oncology (22%), and Neuroscience (16%) units.
ETs stayed longer in the ICU than non-ETs [median of 4.9 days vs 2.2 days, P = .001; Figure (A)]. Similarly, ET cases had a significantly longer posttransfer hospital LOS [median of 35 days vs 21 days, P = .001; Figure (B)]. ETs had a 22% in-hospital mortality rate, compared with 9% in-hospital mortality in the matched controls (P = .02; Table).
DISCUSSION
Children who experienced an ET had a significantly longer ICU LOS, a longer posttransfer LOS, and a higher in-hospital mortality than the matched controls who were also transferred to the ICU. Researchers and improvement science teams at multiple hospitals have demonstrated that interventions targeting improved situation awareness can reduce ETs; we have demonstrated that reducing ETs may reduce subsequent adverse outcomes.8,10
These findings provide additional support for the use of the ET metric in children’s hospitals as a proximal measure for significant clinical deterioration. We found mortality rates that were overall high for a children’s hospital (22% in ET cases and 9% among controls) compared with a national average mortality rate of 2.3% in pediatric ICUs.11 This is likely due to the study sample containing a significant proportion of children with medical complexity.
Aoki et al. recently demonstrated that ETs, compared with non-ETs, were associated with longer LOS and higher mortality in a bivariate analysis.12 In our study, we found similar results with the important addition that these findings were robust when ETs were compared with matched controls who were likely at a higher risk of poor outcomes than ICU transfers in general. In addition, we demonstrated that ETs were associated with adverse outcomes in a United States children’s hospital with a mature, long-standing RRS process. As ETs are considerably more common than cardiac and respiratory arrests, use of the ET metric in children’s hospitals may enable more rapid learning and systems improvement implementations. We also found that most of the children with ETs present from units that care for children with substantial medical complexity, including Cardiology, Hematology/Oncology, and Neurosciences. Future work should continue to examine the relationship between medical complexity and ET risk.
The ET metric is complementary to the CDE measure developed by Bonafide et al. Both metrics capture potential events of unrecognized clinical deterioration, and both offer researchers the opportunity to better understand and improve their RRSs. Both ETs and CDEs are more common than arrests, and CDEs are more common than ETs. ETs, which by definition occur in the first hour of ICU care, are likely a more specific measure of unrecognized clinical deterioration. CDEs will capture therapies that may have been started up to 12 hours after transfer and thus are possibly more sensitive to identify unrecognized clinical deterioration. However, CDEs also may encompass some patients who arrived at the ICU after prompt recognition and then had a subacute deterioration in the ICU.
The maturity of the RRS and the bandwidth of teams to collect data may inform which metric(s) are best for individual centers. As ETs are less common and likely more specific to unrecognized clinical deterioration, they might be the first tracked as a center improves its RRS through QI methods. Alternatively, CDEs may be a useful metric for centers where unrecognized clinical deterioration is less common or in research studies where this more common outcome would lead to more power to detect the effect of interventions to improve care.
Our study had several limitations. Data collection was confined to one tertiary care children’s hospital with a high burden of complex cardiac and oncology care. The results may not generalize well to children hospitalized in smaller or community hospitals or in hospitals without a mature RRS. There is also the possibility of misclassification of covariates and outcomes, but any misclassification would likely be nondifferential and bias toward the null. Matching was not possible based on exact diagnosis, and the unit is a good but imperfect proxy for diagnosis grouping. At our center, overflow of patients into the Cardiology and Hematology/Oncology units is uncommon, mitigating this partially, although residual confounding may remain. The finding that ETs are associated with adverse outcomes does not necessarily mean that these events were preventable; however, it is important and encouraging that the rate of ETs has been reduced at two centers using improvement science interventions.8,10
CONCLUSION
Patients who experienced an ET had a significantly higher likelihood of in-hospital mortality, spent more time in the ICU, and had a longer hospital LOS posttransfer than matched controls. The use of the ET metric in children’s hospitals would allow for further analysis of such patients in hopes of identifying clinical characteristics that serve as predictors of deterioration. This may facilitate better risk stratification in the clinical system as well as enable more rapid learning and systems improvements targeted toward preventing unrecognized clinical deterioration.
Disclosures
Dr. Hussain, Dr. Sosa, Dr. Ambroggio, and Mrs. Gallagher have nothing to disclose. Patrick Brady reports grants from the Agency for Healthcare Research and Quality, outside the submitted work. The authors certify that this submission is not under review by any other publication. The author team has no conflicts of interest to disclose.
Funding
Ms. Hussain was supported by the Society of Hospital Medicine’s Student Hospitalist Scholar Grant Program in 2017. Dr. Brady receives career development support from AHRQ K08-HS023827. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the SHM, AHRQ, or NIH.
1. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388.
2. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. https://doi.org/10.1186/s13054-015-0973-y.
3. Bonafide CP, Roland D, Brady PW. Rapid response systems 20 years later: new approaches, old challenges. JAMA Pediatrics. 2016;170(8):729-730. https://doi.org/10.1001/jamapediatrics.2016.0398.
4. Hayes LW, Dobyns EL, DiGiovine B, et al. A multicenter collaborative approach to reducing pediatric codes outside the ICU. Pediatrics. 2012;129(3):e785-e791. https://doi.org/10.1542/peds.2011-0227.
5. Raymond TT, Bonafide CP, Praestgaard A, et al. Pediatric medical emergency team events and outcomes: a report of 3647 events from the American Heart Association’s get with the guidelines-resuscitation registry. Hosp Pediatr. 2016;6(2):57-64. https://doi.org/10.1542/hpeds.2015-0132.
6. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784.
7. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747.
8. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
9. Brady PW, Wheeler DS, Muething SE, Kotagal UR. Situation awareness: a new model for predicting and preventing patient deterioration. Hosp Pediatr. 2014;4(3):143-146. https://doi.org/10.1542/hpeds.2013-0119.
10. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042.
11. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med. 2017;18(10):915-923. https://doi.org/10.1097/PCC.0000000000001268.
12. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2018;55(2):213-215. https://doi.org/10.1111/jpc.14185.
1. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. https://doi.org/10.1378/chest.98.6.1388.
2. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015;19:254. https://doi.org/10.1186/s13054-015-0973-y.
3. Bonafide CP, Roland D, Brady PW. Rapid response systems 20 years later: new approaches, old challenges. JAMA Pediatrics. 2016;170(8):729-730. https://doi.org/10.1001/jamapediatrics.2016.0398.
4. Hayes LW, Dobyns EL, DiGiovine B, et al. A multicenter collaborative approach to reducing pediatric codes outside the ICU. Pediatrics. 2012;129(3):e785-e791. https://doi.org/10.1542/peds.2011-0227.
5. Raymond TT, Bonafide CP, Praestgaard A, et al. Pediatric medical emergency team events and outcomes: a report of 3647 events from the American Heart Association’s get with the guidelines-resuscitation registry. Hosp Pediatr. 2016;6(2):57-64. https://doi.org/10.1542/hpeds.2015-0132.
6. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. https://doi.org/10.1542/peds.2011-2784.
7. Brady PW, Goldenhar LM. A qualitative study examining the influences on situation awareness and the identification, mitigation and escalation of recognised patient risk. BMJ Qual Saf. 2014;23(2):153-161. https://doi.org/10.1136/bmjqs-2012-001747.
8. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
9. Brady PW, Wheeler DS, Muething SE, Kotagal UR. Situation awareness: a new model for predicting and preventing patient deterioration. Hosp Pediatr. 2014;4(3):143-146. https://doi.org/10.1542/hpeds.2013-0119.
10. McClain Smith M, Chumpia M, Wargo L, Nicol J, Bugnitz M. Watcher initiative associated with decrease in failure to rescue events in pediatric population. Hosp Pediatr. 2017;7(12):710-715. https://doi.org/10.1542/hpeds.2017-0042.
11. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med. 2017;18(10):915-923. https://doi.org/10.1097/PCC.0000000000001268.
12. Aoki Y, Inata Y, Hatachi T, Shimizu Y, Takeuchi M. Outcomes of ‘unrecognised situation awareness failures events’ in intensive care unit transfer of children in a Japanese children’s hospital. J Paediatr Child Health. 2018;55(2):213-215. https://doi.org/10.1111/jpc.14185.
© 2019 Society of Hospital Medicine
Potentially Inappropriate Use of Intravenous Opioids in Hospitalized Patients
Recently released guidelines on safe opioid prescribing draw attention to the fact that physicians have the ability to curb the opioid epidemic through better adherence to prescribing guidelines and limiting opioid use when not clinically indicated.1,2 A consensus statement from the Society of Hospital Medicine includes 16 recommendations for improving the safety of opioid use in hospitalized patients, one of which is to use the oral route of administration whenever possible, reserving intravenous (IV) administration for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal (GI) malabsorption, or when immediate pain control and/or rapid dose titration is necessary.2 This recommendation was based on an increased risk of side effects, adverse events, and medication errors with IV compared with oral formulations.3-5 Furthermore, the reinforcement from opioids is inversely related to the rate of onset of action, and therefore opioids administered by an IV route may be more likely to lead to addiction.6-8
Choosing oral over IV opioids has several additional advantages. The cost of the IV formulation is more than oral; at our institution, the cost of IV morphine is 2.5-4.6 times greater than oral. Additional costs associated with IV administration include nursing time and equipment. Overall, transitioning patients from IV to oral medications could considerably lower costs of care.9 Ongoing need for an IV line may also lead to avoidable complications, including patient discomfort, infection, and thrombophlebitis. In addition, the recent national shortage of IV opioids has necessitated better stewardship of IV opioids.
Despite this recommendation, our observations suggest that patients often continue receiving IV opioids longer than clinically indicated. The goal of this study was to identify the incidence of potentially inappropriate IV opioid use in hospitalized patients.
METHODS
The present study was an observational study seeking to quantify the burden of potentially inappropriate IV opioid use and characteristics predicting potentially inappropriate use in the inpatient setting at a large academic medical center in Boston, Massachusetts, using retrospective review of medical records.
Definition of Potentially Inappropriate Use and Study Sample
We identified all hospitalizations during the month of February 2017 with any order for IV opioids using pharmacy charge data and performed chart reviews in this sample until we reached our prespecified study sample of 200 hospitalizations meeting inclusion/exclusion criteria further defined below.
We defined potentially inappropriate use of IV opioids as use of IV opioids for greater than 24 hours in a patient who could receive oral medications (evidenced by receipt of other orally administered medications during the same 24-hour period) and was not mechanically ventilated. This definition is consistent with recommendations in the recently released consensus statement from the Society of Hospital Medicine.2 We selected a time frame of 24 hours because IV pain medications may be indicated for initial immediate pain control and rapid dose titration; however, 24 hours should be sufficient time to determine opioid needs and transition to an oral regimen in patients without contraindications. After an initial IV dose, additional IV doses within 24 hours were considered appropriate, whereas IV doses thereafter were considered potentially inappropriate unless the patient had nil per os status, including medications. All IV opioids administered within 24 hours of a surgery or procedure were considered appropriate. Because it may be appropriate to continue IV opioids beyond 24 hours in patients with an active cancer diagnosis, in patients who have chosen comfort measures only, or in patients with GI dysfunction (including conditions such as small bowel obstruction, colitis, pancreatitis), we excluded these populations from the study sample. Patients admitted to the hospital for less than 24 hours were also excluded from the study, because they would not be at risk for the outcome of potentially inappropriate use. Doses of IV opioids administered for respiratory distress were considered to be appropriate. Given difficulty in identifying the appropriate time to transition from patient-controlled analgesia (PCA) to IV or per os (PO) opioids, days spent receiving opioids by PCA or continuous IV drip were excluded from the analysis.
We used Fisher’s exact test or the Chi-square test (in the setting of a multicategory variable) to calculate bivariable P values. We used multivariable logistic regression to identify independent predictors of receipt of at least one dose of potentially inappropriate IV opioids, using the hospitalization as the unit of analysis.
RESULTS
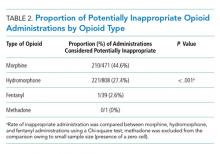
Of 630 hospitalizations with at least one order for IV opioids over a one-month period, we reviewed 502 charts, from which we excluded 76 hospitalizations with an active cancer diagnosis, 30 with comfort-focused care, 115 with GI dysfunction, and 108 with a hospitalization less than 24 hours in duration, resulting in 200 hospitalizations included in this analysis (some patients met multiple exclusion criteria). Table 1 outlines characteristics of the study population, stratified by appropriateness of IV opioid use. The study population was predominately white and had an average age of 56.3 years. The majority of patients were on a surgical service. Hydromorphone was the most commonly administered opioid. There were significant differences in the percentage of doses considered inappropriate between different types of opioids (P < .001), with morphine having the highest proportion of doses considered potentially inappropriate (Table 2).
Thirty-one percent of the cohort was administered at least one potentially inappropriate dose of IV opioids. A total of 432 of 1,319 (33%) IV doses were considered potentially inappropriate.
Predictors of Potentially Inappropriate Use
No significant associations were observed between potentially inappropriate IV opioid administration and age, sex, or admitting service (Table 1). Patients with an ethnicity described as other, unknown, or declined were less likely to have potentially inappropriate use.
DISCUSSION AND CONCLUSIONS
In this cohort of medical and surgical inpatients, we found that almost one-third received at least one potentially inappropriate IV opioid administration during their hospitalization, and one-third of all IV opioid administrations were potentially inappropriate based on current recommendations defining the appropriate use of IV versus oral opioids. Although this is a single-center analysis, to our knowledge, this is the first study to ascertain the rate of potentially inappropriate IV opioid administration in hospitalized patients. Our findings suggest that quality improvement initiatives are necessary to promote more guideline-concordant care in this realm.
Several factors may contribute to overuse. Requests from patients for immediate pain relief may at times drive prescription of the IV formulation. In addition, patients may expect the IV formulation because of precedents from prior interactions with the healthcare system. Both of these situations may be opportunities for patient education about the equivalent bioavailability of oral and IV formulations in patients with a functioning GI tract, as well as the relatively small difference in rate of onset between the two routes of administration (generally 15-20 minutes). When a patient’s pain is well controlled with IV medications, physicians may also fail to recognize the need to transition to PO medications, further prolonging unnecessary use. Finally, in patients with multiple, complex, or deteriorating medical conditions, transitioning to oral opioids may be deprioritized for the sake of addressing more urgent medical concerns.
This study highlights the potential for transitioning more patients to oral opioids, which should be feasible in the inpatient setting, where pain needs can often be anticipated in advance and oral medications can be administered earlier to overcome the short delay in the onset of action between the oral and IV routes. Oral medications also have the advantage of a longer duration of effect, which may provide overall improved pain control. At our institution, a recent shortage of IV opioids (which occurred after the data collection period for this study) and subsequent efforts to limit IV opioid use (via computerized prompts and active pharmacist consultation) resulted in an immediate 50% reduction in the daily number of IV opioid administrations, further supporting our conclusion that there is an opportunity to decrease inappropriate use of IV opioids.
There were no specific patient factors that contributed to potentially inappropriate use. Although the ethnicity category of other/unknown/declined was significantly less likely to receive opioids potentially inappropriately, given the heterogeneity of this group, it is difficult to draw conclusions on the clinical significance of this finding. Morphine was significantly more likely than other opioids to be administered inappropriately.
There are several limitations of this study. Because this was a retrospective review, our criteria for appropriate use may have resulted in some misclassification; as a result, we can comment only on potentially inappropriate use rather than on definitively inappropriate use. We attempted to use a conservative definition of appropriateness by automatically assuming all doses in the first 24 hours of administration to be appropriate, which could have resulted in underestimating potentially inappropriate use. Nonetheless, there may be instances in which a patient had suspected malabsorption that was not captured or a fluctuating ability to receive oral medications within a given 24-hour period (due to nausea, for example), resulting in outcome misclassification. In addition, we did not correlate findings with patient-reported pain scores. Because there is no clearly defined pain threshold at which IV opioids are indicated, we did not believe that would be useful in clarifying appropriate versus inappropriate use. That said, we believe that, most of the time, pain medications should be able to be titrated appropriately within 24 hours to avoid the need for immediate pain relief with IV opioids thereafter. Although there may be instances of patients who have breakthrough pain severe enough to require IV opioids despite adequate titration of oral medications, we believe this is likely to represent a small number of our population that received potentially inappropriate use. It is worth noting that even if we overestimated by 50%, such that the true rate of potentially inappropriate IV administrations is 15%, we believe this would still be a ripe target for quality improvement initiatives, given that tens of millions of hospitalized patients receive opioids each year in the United States.10 Finally, we were unable to quantify the number of providers involved in decision making for these patients, and the single-center nature and short time frame of the study limit generalizability; our analysis should be replicated at other hospitals.
In conclusion, in this sample of 200 medical and surgical hospitalizations receiving IV opioids at a large academic medical center, we identified potentially inappropriate IV administration in 31%, suggesting potential to improve value through improving prescribing practices.
Disclosures
None of the authors have conflicts to disclose.
Funding
Dr. Herzig is funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the views of the funding organizations.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315(15):1624-1645. https://doi.org/10.1001/jama.2016.1464.
2. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
3. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. https://doi.org/10.1155/2015/316275.
4. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. https://doi.org/10.2217/pmt.14.19.
5. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. https://doi.org/10.1097/01.JGP.0000229792.31009.da.
6. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. https://doi.org/10.1345/aph.1R521.
7. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. https://doi.org/10.1016/j.drugalcdep.2005.10.020.
8. O’Brien CP. Drug addiction and drug abuse. In: Hardman JG, ed. Goodman and Gilman’s Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001:621-642.
9. Lau BD, Pinto BL, Thiemann DR, Lehmann CU. Budget impact analysis of conversion from intravenous to oral medication when clinically eligible for oral intake. Clin Ther. 2011;33(11):1792-1796. https://doi.org/10.1016/j.clinthera.2011.09.030.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
Recently released guidelines on safe opioid prescribing draw attention to the fact that physicians have the ability to curb the opioid epidemic through better adherence to prescribing guidelines and limiting opioid use when not clinically indicated.1,2 A consensus statement from the Society of Hospital Medicine includes 16 recommendations for improving the safety of opioid use in hospitalized patients, one of which is to use the oral route of administration whenever possible, reserving intravenous (IV) administration for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal (GI) malabsorption, or when immediate pain control and/or rapid dose titration is necessary.2 This recommendation was based on an increased risk of side effects, adverse events, and medication errors with IV compared with oral formulations.3-5 Furthermore, the reinforcement from opioids is inversely related to the rate of onset of action, and therefore opioids administered by an IV route may be more likely to lead to addiction.6-8
Choosing oral over IV opioids has several additional advantages. The cost of the IV formulation is more than oral; at our institution, the cost of IV morphine is 2.5-4.6 times greater than oral. Additional costs associated with IV administration include nursing time and equipment. Overall, transitioning patients from IV to oral medications could considerably lower costs of care.9 Ongoing need for an IV line may also lead to avoidable complications, including patient discomfort, infection, and thrombophlebitis. In addition, the recent national shortage of IV opioids has necessitated better stewardship of IV opioids.
Despite this recommendation, our observations suggest that patients often continue receiving IV opioids longer than clinically indicated. The goal of this study was to identify the incidence of potentially inappropriate IV opioid use in hospitalized patients.
METHODS
The present study was an observational study seeking to quantify the burden of potentially inappropriate IV opioid use and characteristics predicting potentially inappropriate use in the inpatient setting at a large academic medical center in Boston, Massachusetts, using retrospective review of medical records.
Definition of Potentially Inappropriate Use and Study Sample
We identified all hospitalizations during the month of February 2017 with any order for IV opioids using pharmacy charge data and performed chart reviews in this sample until we reached our prespecified study sample of 200 hospitalizations meeting inclusion/exclusion criteria further defined below.
We defined potentially inappropriate use of IV opioids as use of IV opioids for greater than 24 hours in a patient who could receive oral medications (evidenced by receipt of other orally administered medications during the same 24-hour period) and was not mechanically ventilated. This definition is consistent with recommendations in the recently released consensus statement from the Society of Hospital Medicine.2 We selected a time frame of 24 hours because IV pain medications may be indicated for initial immediate pain control and rapid dose titration; however, 24 hours should be sufficient time to determine opioid needs and transition to an oral regimen in patients without contraindications. After an initial IV dose, additional IV doses within 24 hours were considered appropriate, whereas IV doses thereafter were considered potentially inappropriate unless the patient had nil per os status, including medications. All IV opioids administered within 24 hours of a surgery or procedure were considered appropriate. Because it may be appropriate to continue IV opioids beyond 24 hours in patients with an active cancer diagnosis, in patients who have chosen comfort measures only, or in patients with GI dysfunction (including conditions such as small bowel obstruction, colitis, pancreatitis), we excluded these populations from the study sample. Patients admitted to the hospital for less than 24 hours were also excluded from the study, because they would not be at risk for the outcome of potentially inappropriate use. Doses of IV opioids administered for respiratory distress were considered to be appropriate. Given difficulty in identifying the appropriate time to transition from patient-controlled analgesia (PCA) to IV or per os (PO) opioids, days spent receiving opioids by PCA or continuous IV drip were excluded from the analysis.
We used Fisher’s exact test or the Chi-square test (in the setting of a multicategory variable) to calculate bivariable P values. We used multivariable logistic regression to identify independent predictors of receipt of at least one dose of potentially inappropriate IV opioids, using the hospitalization as the unit of analysis.
RESULTS
Of 630 hospitalizations with at least one order for IV opioids over a one-month period, we reviewed 502 charts, from which we excluded 76 hospitalizations with an active cancer diagnosis, 30 with comfort-focused care, 115 with GI dysfunction, and 108 with a hospitalization less than 24 hours in duration, resulting in 200 hospitalizations included in this analysis (some patients met multiple exclusion criteria). Table 1 outlines characteristics of the study population, stratified by appropriateness of IV opioid use. The study population was predominately white and had an average age of 56.3 years. The majority of patients were on a surgical service. Hydromorphone was the most commonly administered opioid. There were significant differences in the percentage of doses considered inappropriate between different types of opioids (P < .001), with morphine having the highest proportion of doses considered potentially inappropriate (Table 2).
Thirty-one percent of the cohort was administered at least one potentially inappropriate dose of IV opioids. A total of 432 of 1,319 (33%) IV doses were considered potentially inappropriate.
Predictors of Potentially Inappropriate Use
No significant associations were observed between potentially inappropriate IV opioid administration and age, sex, or admitting service (Table 1). Patients with an ethnicity described as other, unknown, or declined were less likely to have potentially inappropriate use.
DISCUSSION AND CONCLUSIONS
In this cohort of medical and surgical inpatients, we found that almost one-third received at least one potentially inappropriate IV opioid administration during their hospitalization, and one-third of all IV opioid administrations were potentially inappropriate based on current recommendations defining the appropriate use of IV versus oral opioids. Although this is a single-center analysis, to our knowledge, this is the first study to ascertain the rate of potentially inappropriate IV opioid administration in hospitalized patients. Our findings suggest that quality improvement initiatives are necessary to promote more guideline-concordant care in this realm.
Several factors may contribute to overuse. Requests from patients for immediate pain relief may at times drive prescription of the IV formulation. In addition, patients may expect the IV formulation because of precedents from prior interactions with the healthcare system. Both of these situations may be opportunities for patient education about the equivalent bioavailability of oral and IV formulations in patients with a functioning GI tract, as well as the relatively small difference in rate of onset between the two routes of administration (generally 15-20 minutes). When a patient’s pain is well controlled with IV medications, physicians may also fail to recognize the need to transition to PO medications, further prolonging unnecessary use. Finally, in patients with multiple, complex, or deteriorating medical conditions, transitioning to oral opioids may be deprioritized for the sake of addressing more urgent medical concerns.
This study highlights the potential for transitioning more patients to oral opioids, which should be feasible in the inpatient setting, where pain needs can often be anticipated in advance and oral medications can be administered earlier to overcome the short delay in the onset of action between the oral and IV routes. Oral medications also have the advantage of a longer duration of effect, which may provide overall improved pain control. At our institution, a recent shortage of IV opioids (which occurred after the data collection period for this study) and subsequent efforts to limit IV opioid use (via computerized prompts and active pharmacist consultation) resulted in an immediate 50% reduction in the daily number of IV opioid administrations, further supporting our conclusion that there is an opportunity to decrease inappropriate use of IV opioids.
There were no specific patient factors that contributed to potentially inappropriate use. Although the ethnicity category of other/unknown/declined was significantly less likely to receive opioids potentially inappropriately, given the heterogeneity of this group, it is difficult to draw conclusions on the clinical significance of this finding. Morphine was significantly more likely than other opioids to be administered inappropriately.
There are several limitations of this study. Because this was a retrospective review, our criteria for appropriate use may have resulted in some misclassification; as a result, we can comment only on potentially inappropriate use rather than on definitively inappropriate use. We attempted to use a conservative definition of appropriateness by automatically assuming all doses in the first 24 hours of administration to be appropriate, which could have resulted in underestimating potentially inappropriate use. Nonetheless, there may be instances in which a patient had suspected malabsorption that was not captured or a fluctuating ability to receive oral medications within a given 24-hour period (due to nausea, for example), resulting in outcome misclassification. In addition, we did not correlate findings with patient-reported pain scores. Because there is no clearly defined pain threshold at which IV opioids are indicated, we did not believe that would be useful in clarifying appropriate versus inappropriate use. That said, we believe that, most of the time, pain medications should be able to be titrated appropriately within 24 hours to avoid the need for immediate pain relief with IV opioids thereafter. Although there may be instances of patients who have breakthrough pain severe enough to require IV opioids despite adequate titration of oral medications, we believe this is likely to represent a small number of our population that received potentially inappropriate use. It is worth noting that even if we overestimated by 50%, such that the true rate of potentially inappropriate IV administrations is 15%, we believe this would still be a ripe target for quality improvement initiatives, given that tens of millions of hospitalized patients receive opioids each year in the United States.10 Finally, we were unable to quantify the number of providers involved in decision making for these patients, and the single-center nature and short time frame of the study limit generalizability; our analysis should be replicated at other hospitals.
In conclusion, in this sample of 200 medical and surgical hospitalizations receiving IV opioids at a large academic medical center, we identified potentially inappropriate IV administration in 31%, suggesting potential to improve value through improving prescribing practices.
Disclosures
None of the authors have conflicts to disclose.
Funding
Dr. Herzig is funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the views of the funding organizations.
Recently released guidelines on safe opioid prescribing draw attention to the fact that physicians have the ability to curb the opioid epidemic through better adherence to prescribing guidelines and limiting opioid use when not clinically indicated.1,2 A consensus statement from the Society of Hospital Medicine includes 16 recommendations for improving the safety of opioid use in hospitalized patients, one of which is to use the oral route of administration whenever possible, reserving intravenous (IV) administration for patients who cannot take food or medications by mouth, patients suspected of gastrointestinal (GI) malabsorption, or when immediate pain control and/or rapid dose titration is necessary.2 This recommendation was based on an increased risk of side effects, adverse events, and medication errors with IV compared with oral formulations.3-5 Furthermore, the reinforcement from opioids is inversely related to the rate of onset of action, and therefore opioids administered by an IV route may be more likely to lead to addiction.6-8
Choosing oral over IV opioids has several additional advantages. The cost of the IV formulation is more than oral; at our institution, the cost of IV morphine is 2.5-4.6 times greater than oral. Additional costs associated with IV administration include nursing time and equipment. Overall, transitioning patients from IV to oral medications could considerably lower costs of care.9 Ongoing need for an IV line may also lead to avoidable complications, including patient discomfort, infection, and thrombophlebitis. In addition, the recent national shortage of IV opioids has necessitated better stewardship of IV opioids.
Despite this recommendation, our observations suggest that patients often continue receiving IV opioids longer than clinically indicated. The goal of this study was to identify the incidence of potentially inappropriate IV opioid use in hospitalized patients.
METHODS
The present study was an observational study seeking to quantify the burden of potentially inappropriate IV opioid use and characteristics predicting potentially inappropriate use in the inpatient setting at a large academic medical center in Boston, Massachusetts, using retrospective review of medical records.
Definition of Potentially Inappropriate Use and Study Sample
We identified all hospitalizations during the month of February 2017 with any order for IV opioids using pharmacy charge data and performed chart reviews in this sample until we reached our prespecified study sample of 200 hospitalizations meeting inclusion/exclusion criteria further defined below.
We defined potentially inappropriate use of IV opioids as use of IV opioids for greater than 24 hours in a patient who could receive oral medications (evidenced by receipt of other orally administered medications during the same 24-hour period) and was not mechanically ventilated. This definition is consistent with recommendations in the recently released consensus statement from the Society of Hospital Medicine.2 We selected a time frame of 24 hours because IV pain medications may be indicated for initial immediate pain control and rapid dose titration; however, 24 hours should be sufficient time to determine opioid needs and transition to an oral regimen in patients without contraindications. After an initial IV dose, additional IV doses within 24 hours were considered appropriate, whereas IV doses thereafter were considered potentially inappropriate unless the patient had nil per os status, including medications. All IV opioids administered within 24 hours of a surgery or procedure were considered appropriate. Because it may be appropriate to continue IV opioids beyond 24 hours in patients with an active cancer diagnosis, in patients who have chosen comfort measures only, or in patients with GI dysfunction (including conditions such as small bowel obstruction, colitis, pancreatitis), we excluded these populations from the study sample. Patients admitted to the hospital for less than 24 hours were also excluded from the study, because they would not be at risk for the outcome of potentially inappropriate use. Doses of IV opioids administered for respiratory distress were considered to be appropriate. Given difficulty in identifying the appropriate time to transition from patient-controlled analgesia (PCA) to IV or per os (PO) opioids, days spent receiving opioids by PCA or continuous IV drip were excluded from the analysis.
We used Fisher’s exact test or the Chi-square test (in the setting of a multicategory variable) to calculate bivariable P values. We used multivariable logistic regression to identify independent predictors of receipt of at least one dose of potentially inappropriate IV opioids, using the hospitalization as the unit of analysis.
RESULTS
Of 630 hospitalizations with at least one order for IV opioids over a one-month period, we reviewed 502 charts, from which we excluded 76 hospitalizations with an active cancer diagnosis, 30 with comfort-focused care, 115 with GI dysfunction, and 108 with a hospitalization less than 24 hours in duration, resulting in 200 hospitalizations included in this analysis (some patients met multiple exclusion criteria). Table 1 outlines characteristics of the study population, stratified by appropriateness of IV opioid use. The study population was predominately white and had an average age of 56.3 years. The majority of patients were on a surgical service. Hydromorphone was the most commonly administered opioid. There were significant differences in the percentage of doses considered inappropriate between different types of opioids (P < .001), with morphine having the highest proportion of doses considered potentially inappropriate (Table 2).
Thirty-one percent of the cohort was administered at least one potentially inappropriate dose of IV opioids. A total of 432 of 1,319 (33%) IV doses were considered potentially inappropriate.
Predictors of Potentially Inappropriate Use
No significant associations were observed between potentially inappropriate IV opioid administration and age, sex, or admitting service (Table 1). Patients with an ethnicity described as other, unknown, or declined were less likely to have potentially inappropriate use.
DISCUSSION AND CONCLUSIONS
In this cohort of medical and surgical inpatients, we found that almost one-third received at least one potentially inappropriate IV opioid administration during their hospitalization, and one-third of all IV opioid administrations were potentially inappropriate based on current recommendations defining the appropriate use of IV versus oral opioids. Although this is a single-center analysis, to our knowledge, this is the first study to ascertain the rate of potentially inappropriate IV opioid administration in hospitalized patients. Our findings suggest that quality improvement initiatives are necessary to promote more guideline-concordant care in this realm.
Several factors may contribute to overuse. Requests from patients for immediate pain relief may at times drive prescription of the IV formulation. In addition, patients may expect the IV formulation because of precedents from prior interactions with the healthcare system. Both of these situations may be opportunities for patient education about the equivalent bioavailability of oral and IV formulations in patients with a functioning GI tract, as well as the relatively small difference in rate of onset between the two routes of administration (generally 15-20 minutes). When a patient’s pain is well controlled with IV medications, physicians may also fail to recognize the need to transition to PO medications, further prolonging unnecessary use. Finally, in patients with multiple, complex, or deteriorating medical conditions, transitioning to oral opioids may be deprioritized for the sake of addressing more urgent medical concerns.
This study highlights the potential for transitioning more patients to oral opioids, which should be feasible in the inpatient setting, where pain needs can often be anticipated in advance and oral medications can be administered earlier to overcome the short delay in the onset of action between the oral and IV routes. Oral medications also have the advantage of a longer duration of effect, which may provide overall improved pain control. At our institution, a recent shortage of IV opioids (which occurred after the data collection period for this study) and subsequent efforts to limit IV opioid use (via computerized prompts and active pharmacist consultation) resulted in an immediate 50% reduction in the daily number of IV opioid administrations, further supporting our conclusion that there is an opportunity to decrease inappropriate use of IV opioids.
There were no specific patient factors that contributed to potentially inappropriate use. Although the ethnicity category of other/unknown/declined was significantly less likely to receive opioids potentially inappropriately, given the heterogeneity of this group, it is difficult to draw conclusions on the clinical significance of this finding. Morphine was significantly more likely than other opioids to be administered inappropriately.
There are several limitations of this study. Because this was a retrospective review, our criteria for appropriate use may have resulted in some misclassification; as a result, we can comment only on potentially inappropriate use rather than on definitively inappropriate use. We attempted to use a conservative definition of appropriateness by automatically assuming all doses in the first 24 hours of administration to be appropriate, which could have resulted in underestimating potentially inappropriate use. Nonetheless, there may be instances in which a patient had suspected malabsorption that was not captured or a fluctuating ability to receive oral medications within a given 24-hour period (due to nausea, for example), resulting in outcome misclassification. In addition, we did not correlate findings with patient-reported pain scores. Because there is no clearly defined pain threshold at which IV opioids are indicated, we did not believe that would be useful in clarifying appropriate versus inappropriate use. That said, we believe that, most of the time, pain medications should be able to be titrated appropriately within 24 hours to avoid the need for immediate pain relief with IV opioids thereafter. Although there may be instances of patients who have breakthrough pain severe enough to require IV opioids despite adequate titration of oral medications, we believe this is likely to represent a small number of our population that received potentially inappropriate use. It is worth noting that even if we overestimated by 50%, such that the true rate of potentially inappropriate IV administrations is 15%, we believe this would still be a ripe target for quality improvement initiatives, given that tens of millions of hospitalized patients receive opioids each year in the United States.10 Finally, we were unable to quantify the number of providers involved in decision making for these patients, and the single-center nature and short time frame of the study limit generalizability; our analysis should be replicated at other hospitals.
In conclusion, in this sample of 200 medical and surgical hospitalizations receiving IV opioids at a large academic medical center, we identified potentially inappropriate IV administration in 31%, suggesting potential to improve value through improving prescribing practices.
Disclosures
None of the authors have conflicts to disclose.
Funding
Dr. Herzig is funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the views of the funding organizations.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315(15):1624-1645. https://doi.org/10.1001/jama.2016.1464.
2. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
3. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. https://doi.org/10.1155/2015/316275.
4. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. https://doi.org/10.2217/pmt.14.19.
5. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. https://doi.org/10.1097/01.JGP.0000229792.31009.da.
6. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. https://doi.org/10.1345/aph.1R521.
7. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. https://doi.org/10.1016/j.drugalcdep.2005.10.020.
8. O’Brien CP. Drug addiction and drug abuse. In: Hardman JG, ed. Goodman and Gilman’s Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001:621-642.
9. Lau BD, Pinto BL, Thiemann DR, Lehmann CU. Budget impact analysis of conversion from intravenous to oral medication when clinically eligible for oral intake. Clin Ther. 2011;33(11):1792-1796. https://doi.org/10.1016/j.clinthera.2011.09.030.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315(15):1624-1645. https://doi.org/10.1001/jama.2016.1464.
2. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
3. Daoust R, Paquet J, Lavigne G, Piette E, Chauny JM. Impact of age, sex and route of administration on adverse events after opioid treatment in the emergency department: a retrospective study. Pain Res Manag. 2015;20(1):23-28. https://doi.org/10.1155/2015/316275.
4. Overdyk F, Dahan A, Roozekrans M, van der Schrier R, Aarts L, Niesters M. Opioid-induced respiratory depression in the acute care setting: a compendium of case reports. Pain Manag. 2014;4(4):317-325. https://doi.org/10.2217/pmt.14.19.
5. Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50-59. https://doi.org/10.1097/01.JGP.0000229792.31009.da.
6. Al-Qadheeb NS, O’Connor HH, White AC, et al. Antipsychotic prescribing patterns, and the factors and outcomes associated with their use, among patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting. Ann Pharmacother. 2013;47(2):181-188. https://doi.org/10.1345/aph.1R521.
7. Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(1):S4-S7. https://doi.org/10.1016/j.drugalcdep.2005.10.020.
8. O’Brien CP. Drug addiction and drug abuse. In: Hardman JG, ed. Goodman and Gilman’s Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001:621-642.
9. Lau BD, Pinto BL, Thiemann DR, Lehmann CU. Budget impact analysis of conversion from intravenous to oral medication when clinically eligible for oral intake. Clin Ther. 2011;33(11):1792-1796. https://doi.org/10.1016/j.clinthera.2011.09.030.
10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
© 2019 Society of Hospital Medicine
Repotrectinib highly active in ROS1-positive lung cancer
CHICAGO – The oral tyrosine kinase inhibitor (TKI) repotrectinib is safe and has demonstrated encouraging activity in patients with advanced ROS1 fusion-positive non-small cell lung cancer, early results of a phase 1/2 study show.
Objective response rates of 82% in 11 TKI-naive patients and 39% in 22 TKI-pretreated patients were seen after treatment with repotrectinib, a next-generation inhibitor of ROS1/TRK/ALK with a 90-fold greater potency for ROS1 versus crizotinib, according to an investigator in the study.
“The TRIDENT-1 study supports repotrectinib as a potential best-in-class ROS1 agent in advanced non–small cell lung cancer,” said investigator ByoungChul Cho, MD, PhD, of Yonsei Cancer Center in Seoul, South Korea, in a podium presentation at the annual meeting of the American Society of Clinical Oncology.
For the 11 TKI-naive patients, no median duration of response had yet been reached over a median follow-up duration of nearly 17 months, with individual response durations that ranged from 10.9 to 17.7 or more months in the 5 of 9 patients remaining in response, Dr. Cho reported.
“This is exciting, because this is the most promising data presented so far with ROS1 TKI in TKI-naive patient population,” Dr. Cho said.
Repotrectinib also showed a potential to overcome TKI resistance mutations, notably G2032R, which is the most common ROS1 resistance mutation after crizotinib treatment.
All five patients with ROS1 G2032R mutation experienced tumor regression, with a confirmed response rate of 40%, Dr. Cho said.
The TKI was relatively well tolerated with four dose-limiting toxicity events including grade 2-3 dizziness in three cases and grade 3 dyspnea and hypoxia in one case.
Of four grade 5 treatment-emergent adverse events, only one case was possibly related to the treatment, Dr. Cho said.
Based on this tolerability and preliminary activity, the pivotal phase 2 portion of TRIDENT-1 is set to begin in the second half of 2019.
Benjamin Besse, MD, PhD, of Paris-Sud University, Orsay, and Institut Gustave Roussy said these preliminary results were very encouraging.
“If we look at the global picture, repotrectinib is probably today the most potent TKI against ROS1,” Dr. Besse said in a podium discussion of the results. “We don’t know yet if this will translate in an improved progression-free survival.”
Close follow-up of adverse events are warranted in further investigations because of the potency of the drug, he added.
Turning Point Therapeutics sponsored the study. Dr. Cho reported disclosures related to TheraCanVac, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Champions Oncology, Dizal Pharma, Dong-A ST, Eli Lilly, Janssen, Mogam Institute, MSD, Novartis, Ono, Pfizer, Roche, Takeda, and Yuhan.
SOURCE: Cho BC et al. ASCO 2019, Abstract 9011.
CHICAGO – The oral tyrosine kinase inhibitor (TKI) repotrectinib is safe and has demonstrated encouraging activity in patients with advanced ROS1 fusion-positive non-small cell lung cancer, early results of a phase 1/2 study show.
Objective response rates of 82% in 11 TKI-naive patients and 39% in 22 TKI-pretreated patients were seen after treatment with repotrectinib, a next-generation inhibitor of ROS1/TRK/ALK with a 90-fold greater potency for ROS1 versus crizotinib, according to an investigator in the study.
“The TRIDENT-1 study supports repotrectinib as a potential best-in-class ROS1 agent in advanced non–small cell lung cancer,” said investigator ByoungChul Cho, MD, PhD, of Yonsei Cancer Center in Seoul, South Korea, in a podium presentation at the annual meeting of the American Society of Clinical Oncology.
For the 11 TKI-naive patients, no median duration of response had yet been reached over a median follow-up duration of nearly 17 months, with individual response durations that ranged from 10.9 to 17.7 or more months in the 5 of 9 patients remaining in response, Dr. Cho reported.
“This is exciting, because this is the most promising data presented so far with ROS1 TKI in TKI-naive patient population,” Dr. Cho said.
Repotrectinib also showed a potential to overcome TKI resistance mutations, notably G2032R, which is the most common ROS1 resistance mutation after crizotinib treatment.
All five patients with ROS1 G2032R mutation experienced tumor regression, with a confirmed response rate of 40%, Dr. Cho said.
The TKI was relatively well tolerated with four dose-limiting toxicity events including grade 2-3 dizziness in three cases and grade 3 dyspnea and hypoxia in one case.
Of four grade 5 treatment-emergent adverse events, only one case was possibly related to the treatment, Dr. Cho said.
Based on this tolerability and preliminary activity, the pivotal phase 2 portion of TRIDENT-1 is set to begin in the second half of 2019.
Benjamin Besse, MD, PhD, of Paris-Sud University, Orsay, and Institut Gustave Roussy said these preliminary results were very encouraging.
“If we look at the global picture, repotrectinib is probably today the most potent TKI against ROS1,” Dr. Besse said in a podium discussion of the results. “We don’t know yet if this will translate in an improved progression-free survival.”
Close follow-up of adverse events are warranted in further investigations because of the potency of the drug, he added.
Turning Point Therapeutics sponsored the study. Dr. Cho reported disclosures related to TheraCanVac, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Champions Oncology, Dizal Pharma, Dong-A ST, Eli Lilly, Janssen, Mogam Institute, MSD, Novartis, Ono, Pfizer, Roche, Takeda, and Yuhan.
SOURCE: Cho BC et al. ASCO 2019, Abstract 9011.
CHICAGO – The oral tyrosine kinase inhibitor (TKI) repotrectinib is safe and has demonstrated encouraging activity in patients with advanced ROS1 fusion-positive non-small cell lung cancer, early results of a phase 1/2 study show.
Objective response rates of 82% in 11 TKI-naive patients and 39% in 22 TKI-pretreated patients were seen after treatment with repotrectinib, a next-generation inhibitor of ROS1/TRK/ALK with a 90-fold greater potency for ROS1 versus crizotinib, according to an investigator in the study.
“The TRIDENT-1 study supports repotrectinib as a potential best-in-class ROS1 agent in advanced non–small cell lung cancer,” said investigator ByoungChul Cho, MD, PhD, of Yonsei Cancer Center in Seoul, South Korea, in a podium presentation at the annual meeting of the American Society of Clinical Oncology.
For the 11 TKI-naive patients, no median duration of response had yet been reached over a median follow-up duration of nearly 17 months, with individual response durations that ranged from 10.9 to 17.7 or more months in the 5 of 9 patients remaining in response, Dr. Cho reported.
“This is exciting, because this is the most promising data presented so far with ROS1 TKI in TKI-naive patient population,” Dr. Cho said.
Repotrectinib also showed a potential to overcome TKI resistance mutations, notably G2032R, which is the most common ROS1 resistance mutation after crizotinib treatment.
All five patients with ROS1 G2032R mutation experienced tumor regression, with a confirmed response rate of 40%, Dr. Cho said.
The TKI was relatively well tolerated with four dose-limiting toxicity events including grade 2-3 dizziness in three cases and grade 3 dyspnea and hypoxia in one case.
Of four grade 5 treatment-emergent adverse events, only one case was possibly related to the treatment, Dr. Cho said.
Based on this tolerability and preliminary activity, the pivotal phase 2 portion of TRIDENT-1 is set to begin in the second half of 2019.
Benjamin Besse, MD, PhD, of Paris-Sud University, Orsay, and Institut Gustave Roussy said these preliminary results were very encouraging.
“If we look at the global picture, repotrectinib is probably today the most potent TKI against ROS1,” Dr. Besse said in a podium discussion of the results. “We don’t know yet if this will translate in an improved progression-free survival.”
Close follow-up of adverse events are warranted in further investigations because of the potency of the drug, he added.
Turning Point Therapeutics sponsored the study. Dr. Cho reported disclosures related to TheraCanVac, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Champions Oncology, Dizal Pharma, Dong-A ST, Eli Lilly, Janssen, Mogam Institute, MSD, Novartis, Ono, Pfizer, Roche, Takeda, and Yuhan.
SOURCE: Cho BC et al. ASCO 2019, Abstract 9011.
REPORTING FROM ASCO 2019
AAD issues position statement addressing sexual, gender minority health
The individuals.
Some of the unique dermatologic issues faced by SGM individuals include a disproportionate risk for skin cancer in men who have sex with men; higher rates of sexually transmitted infections such as HIV, anogenital dysplasia, and anal cancer; and management of complications such as acne or scarring stemming from medical and/or surgical gender-affirming treatments for transgender individuals. In addition, racial and ethnic minority persons who identify as SGM or LGBTQ face additional stigma and health care disparities.
“While the precise role of dermatology remains controversial regarding anal cancer screening, treatment, and surveillance in these populations, comprehensive skin examinations as well as appropriate counseling and referrals may be linked to earlier detection and improved outcomes,” the statement notes.
The AAD has already taken some steps in advancing the care of SGM individuals, including dedicated educational sessions and workshops at AAD meetings, formation of the AAD LGBTQ/SGM Health Expert Resource Group, incorporation of LGBTQ/SGM content into online AAD basic dermatology curriculum modules, revision of the AAD position statement on isotretinoin, and forthcoming book chapters and CME articles for the Journal of the American Academy of Dermatology.
In order to further commit to the care of diverse populations, the AAD recognized a series of 11 positions in accordance with the association’s “core values of patient-first medicine and visionary leadership,” such as recognizing and affirming the identity and dignity of LGBTQ/SGM individuals, opposing all bias and discrimination, endorsing policies and initiatives that ensure nondiscrimination, and supporting training in cultural humility and structural competency.
“Adequate training of medical professionals regarding the unique health care needs of LGBTQ/SGM people and ongoing research into best care practices are necessary to provide care that facilitates trust and resilience while ensuring the ability of LGBTQ/SGM individuals to thrive,” the statement says.
Find the full position statement on the AAD website.
The individuals.
Some of the unique dermatologic issues faced by SGM individuals include a disproportionate risk for skin cancer in men who have sex with men; higher rates of sexually transmitted infections such as HIV, anogenital dysplasia, and anal cancer; and management of complications such as acne or scarring stemming from medical and/or surgical gender-affirming treatments for transgender individuals. In addition, racial and ethnic minority persons who identify as SGM or LGBTQ face additional stigma and health care disparities.
“While the precise role of dermatology remains controversial regarding anal cancer screening, treatment, and surveillance in these populations, comprehensive skin examinations as well as appropriate counseling and referrals may be linked to earlier detection and improved outcomes,” the statement notes.
The AAD has already taken some steps in advancing the care of SGM individuals, including dedicated educational sessions and workshops at AAD meetings, formation of the AAD LGBTQ/SGM Health Expert Resource Group, incorporation of LGBTQ/SGM content into online AAD basic dermatology curriculum modules, revision of the AAD position statement on isotretinoin, and forthcoming book chapters and CME articles for the Journal of the American Academy of Dermatology.
In order to further commit to the care of diverse populations, the AAD recognized a series of 11 positions in accordance with the association’s “core values of patient-first medicine and visionary leadership,” such as recognizing and affirming the identity and dignity of LGBTQ/SGM individuals, opposing all bias and discrimination, endorsing policies and initiatives that ensure nondiscrimination, and supporting training in cultural humility and structural competency.
“Adequate training of medical professionals regarding the unique health care needs of LGBTQ/SGM people and ongoing research into best care practices are necessary to provide care that facilitates trust and resilience while ensuring the ability of LGBTQ/SGM individuals to thrive,” the statement says.
Find the full position statement on the AAD website.
The individuals.
Some of the unique dermatologic issues faced by SGM individuals include a disproportionate risk for skin cancer in men who have sex with men; higher rates of sexually transmitted infections such as HIV, anogenital dysplasia, and anal cancer; and management of complications such as acne or scarring stemming from medical and/or surgical gender-affirming treatments for transgender individuals. In addition, racial and ethnic minority persons who identify as SGM or LGBTQ face additional stigma and health care disparities.
“While the precise role of dermatology remains controversial regarding anal cancer screening, treatment, and surveillance in these populations, comprehensive skin examinations as well as appropriate counseling and referrals may be linked to earlier detection and improved outcomes,” the statement notes.
The AAD has already taken some steps in advancing the care of SGM individuals, including dedicated educational sessions and workshops at AAD meetings, formation of the AAD LGBTQ/SGM Health Expert Resource Group, incorporation of LGBTQ/SGM content into online AAD basic dermatology curriculum modules, revision of the AAD position statement on isotretinoin, and forthcoming book chapters and CME articles for the Journal of the American Academy of Dermatology.
In order to further commit to the care of diverse populations, the AAD recognized a series of 11 positions in accordance with the association’s “core values of patient-first medicine and visionary leadership,” such as recognizing and affirming the identity and dignity of LGBTQ/SGM individuals, opposing all bias and discrimination, endorsing policies and initiatives that ensure nondiscrimination, and supporting training in cultural humility and structural competency.
“Adequate training of medical professionals regarding the unique health care needs of LGBTQ/SGM people and ongoing research into best care practices are necessary to provide care that facilitates trust and resilience while ensuring the ability of LGBTQ/SGM individuals to thrive,” the statement says.
Find the full position statement on the AAD website.
Checkpoint inhibitor rechallenge is possible for select patients
Rechallenge resulted in the recurrence of a grade 2 or higher immune-related adverse event (irAE) in 55% of rechallenged patients, but no deaths occurred, according to Audrey Simonaggio, MD, of the department of drug development at Gustave Roussy, Villejuif, France, and colleagues.
In those rechallenged patients who had a second irAE, the second event was not more severe than the first. “The rechallenge should first be assessed in a multidisciplinary team meeting with regard to each patient’s individual risk-reward ratio. ... We recommend close monitoring,” the researchers wrote in a study published in JAMA Oncology.
As there are no specific recommendations to guide the decision to rechallenge, the usefulness of the rechallenge was considered. The readministration could be delayed if the patient was in complete or excellent partial response. The existence of other therapeutic alternatives was also important as was the patient’s clinical state. Rechallenge was considered possible only after the grade of the initial irAE returned to 0 or 1.
“Because of life-threatening risk, we did not support rechallenge for cardiac (myocarditis) and neurologic irAEs [such] as Guillain-Barré syndrome, encephalitis, and severe myositis,” they said. CT scans were used to guide the decision to rechallenge in those with initial lung adverse events.
The cohort study included 93 consecutive adult patients who were referred over an 18-month period to the ImmunoTOX assessment board at the Gustave Roussy cancer center and followed for at least 1 year. The cohort was balanced for gender and ranged in age from 33 to 85 years, with a median age of 62.5 years. Melanoma was the predominant tumor (33%), followed by lung (16%), colorectal (9%), and lymphoma (9%).
The initial immune-related adverse event was a grade 2 event in 46% of patients, grade 3 in 39%, and grade 4 in 15%. Events included hepatitis (18%), skin toxicity (15%), pneumonitis (14%), colitis (12%), and arthralgia (7.5%). A rechallenge with the same anti–PD-1 or anti–PD-L1 was conducted in 43% of patients.
When compared with patients who were not rechallenged, there was no difference in median patient age, time to initial immune-related adverse event (five vs. three treatment cycles), event severity, or steroid use. With a median follow-up period of 14 months, the same or a different immune-related adverse event occurred in 22 patients (55%). A shorter time to the initial event was linked to the occurrence of a second event (9 vs. 15 weeks; P = .04).
“However, we did observe a trend toward a higher recurrence rate after a more severe initial irAE and a trend toward more frequent recurrence in patients treated with corticosteroids after the initial irAE,” the researchers wrote. “An anti–PD-1or anti–PD-L1 rechallenge after a grade 4 irAE should always be considered with caution.” Three of the five patients with these events were being treated for lymphoma, they said.
“As long as patients are closely monitored, anti–PD-1 or anti–PD-L1 rechallenge appears to have an acceptable toxic effect profile. Myocarditis and neurologic toxic effect should remain a contraindication. Rechallenge conditions require further investigation in a prospective clinical trial. ... Well-powered, prospective studies with a larger number of patients would be required to generate information on putative risk factors for the recurrence of irAEs. Our results highlighted the value of a review board, like ImmunoTOX, with intention to build a large irAE database and then establish evidence-based guidelines on the safety of a rechallenge,” the researchers concluded.
The study was supported by the Gustave Roussy cancer center and the Gustave Roussy immunotherapy program. Dr. Simonaggio had no relevant disclosures; several coauthors reported consultancy fees and research support from multiple drug companies.
SOURCE: Simonaggio A et al. JAMA Oncol. 2019 Jun 6. doi:10.1001/jamaoncol.2019.1022.
Rechallenge resulted in the recurrence of a grade 2 or higher immune-related adverse event (irAE) in 55% of rechallenged patients, but no deaths occurred, according to Audrey Simonaggio, MD, of the department of drug development at Gustave Roussy, Villejuif, France, and colleagues.
In those rechallenged patients who had a second irAE, the second event was not more severe than the first. “The rechallenge should first be assessed in a multidisciplinary team meeting with regard to each patient’s individual risk-reward ratio. ... We recommend close monitoring,” the researchers wrote in a study published in JAMA Oncology.
As there are no specific recommendations to guide the decision to rechallenge, the usefulness of the rechallenge was considered. The readministration could be delayed if the patient was in complete or excellent partial response. The existence of other therapeutic alternatives was also important as was the patient’s clinical state. Rechallenge was considered possible only after the grade of the initial irAE returned to 0 or 1.
“Because of life-threatening risk, we did not support rechallenge for cardiac (myocarditis) and neurologic irAEs [such] as Guillain-Barré syndrome, encephalitis, and severe myositis,” they said. CT scans were used to guide the decision to rechallenge in those with initial lung adverse events.
The cohort study included 93 consecutive adult patients who were referred over an 18-month period to the ImmunoTOX assessment board at the Gustave Roussy cancer center and followed for at least 1 year. The cohort was balanced for gender and ranged in age from 33 to 85 years, with a median age of 62.5 years. Melanoma was the predominant tumor (33%), followed by lung (16%), colorectal (9%), and lymphoma (9%).
The initial immune-related adverse event was a grade 2 event in 46% of patients, grade 3 in 39%, and grade 4 in 15%. Events included hepatitis (18%), skin toxicity (15%), pneumonitis (14%), colitis (12%), and arthralgia (7.5%). A rechallenge with the same anti–PD-1 or anti–PD-L1 was conducted in 43% of patients.
When compared with patients who were not rechallenged, there was no difference in median patient age, time to initial immune-related adverse event (five vs. three treatment cycles), event severity, or steroid use. With a median follow-up period of 14 months, the same or a different immune-related adverse event occurred in 22 patients (55%). A shorter time to the initial event was linked to the occurrence of a second event (9 vs. 15 weeks; P = .04).
“However, we did observe a trend toward a higher recurrence rate after a more severe initial irAE and a trend toward more frequent recurrence in patients treated with corticosteroids after the initial irAE,” the researchers wrote. “An anti–PD-1or anti–PD-L1 rechallenge after a grade 4 irAE should always be considered with caution.” Three of the five patients with these events were being treated for lymphoma, they said.
“As long as patients are closely monitored, anti–PD-1 or anti–PD-L1 rechallenge appears to have an acceptable toxic effect profile. Myocarditis and neurologic toxic effect should remain a contraindication. Rechallenge conditions require further investigation in a prospective clinical trial. ... Well-powered, prospective studies with a larger number of patients would be required to generate information on putative risk factors for the recurrence of irAEs. Our results highlighted the value of a review board, like ImmunoTOX, with intention to build a large irAE database and then establish evidence-based guidelines on the safety of a rechallenge,” the researchers concluded.
The study was supported by the Gustave Roussy cancer center and the Gustave Roussy immunotherapy program. Dr. Simonaggio had no relevant disclosures; several coauthors reported consultancy fees and research support from multiple drug companies.
SOURCE: Simonaggio A et al. JAMA Oncol. 2019 Jun 6. doi:10.1001/jamaoncol.2019.1022.
Rechallenge resulted in the recurrence of a grade 2 or higher immune-related adverse event (irAE) in 55% of rechallenged patients, but no deaths occurred, according to Audrey Simonaggio, MD, of the department of drug development at Gustave Roussy, Villejuif, France, and colleagues.
In those rechallenged patients who had a second irAE, the second event was not more severe than the first. “The rechallenge should first be assessed in a multidisciplinary team meeting with regard to each patient’s individual risk-reward ratio. ... We recommend close monitoring,” the researchers wrote in a study published in JAMA Oncology.
As there are no specific recommendations to guide the decision to rechallenge, the usefulness of the rechallenge was considered. The readministration could be delayed if the patient was in complete or excellent partial response. The existence of other therapeutic alternatives was also important as was the patient’s clinical state. Rechallenge was considered possible only after the grade of the initial irAE returned to 0 or 1.
“Because of life-threatening risk, we did not support rechallenge for cardiac (myocarditis) and neurologic irAEs [such] as Guillain-Barré syndrome, encephalitis, and severe myositis,” they said. CT scans were used to guide the decision to rechallenge in those with initial lung adverse events.
The cohort study included 93 consecutive adult patients who were referred over an 18-month period to the ImmunoTOX assessment board at the Gustave Roussy cancer center and followed for at least 1 year. The cohort was balanced for gender and ranged in age from 33 to 85 years, with a median age of 62.5 years. Melanoma was the predominant tumor (33%), followed by lung (16%), colorectal (9%), and lymphoma (9%).
The initial immune-related adverse event was a grade 2 event in 46% of patients, grade 3 in 39%, and grade 4 in 15%. Events included hepatitis (18%), skin toxicity (15%), pneumonitis (14%), colitis (12%), and arthralgia (7.5%). A rechallenge with the same anti–PD-1 or anti–PD-L1 was conducted in 43% of patients.
When compared with patients who were not rechallenged, there was no difference in median patient age, time to initial immune-related adverse event (five vs. three treatment cycles), event severity, or steroid use. With a median follow-up period of 14 months, the same or a different immune-related adverse event occurred in 22 patients (55%). A shorter time to the initial event was linked to the occurrence of a second event (9 vs. 15 weeks; P = .04).
“However, we did observe a trend toward a higher recurrence rate after a more severe initial irAE and a trend toward more frequent recurrence in patients treated with corticosteroids after the initial irAE,” the researchers wrote. “An anti–PD-1or anti–PD-L1 rechallenge after a grade 4 irAE should always be considered with caution.” Three of the five patients with these events were being treated for lymphoma, they said.
“As long as patients are closely monitored, anti–PD-1 or anti–PD-L1 rechallenge appears to have an acceptable toxic effect profile. Myocarditis and neurologic toxic effect should remain a contraindication. Rechallenge conditions require further investigation in a prospective clinical trial. ... Well-powered, prospective studies with a larger number of patients would be required to generate information on putative risk factors for the recurrence of irAEs. Our results highlighted the value of a review board, like ImmunoTOX, with intention to build a large irAE database and then establish evidence-based guidelines on the safety of a rechallenge,” the researchers concluded.
The study was supported by the Gustave Roussy cancer center and the Gustave Roussy immunotherapy program. Dr. Simonaggio had no relevant disclosures; several coauthors reported consultancy fees and research support from multiple drug companies.
SOURCE: Simonaggio A et al. JAMA Oncol. 2019 Jun 6. doi:10.1001/jamaoncol.2019.1022.
FROM JAMA ONCOLOGY
FDA approves Nucala’s new at-home formulations
, according to a press release from the drug’s developer. The biologic will now be available as an autoinjector and as a prefilled safety syringe.
The 100-mg subcutaneous mepolizumab injection is indicated as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and the three-dose 100-mg subcutaneous injections are indicated for the rare eosinophilic granulomatosis and polyangiitis, with the biologic administered every 4 weeks in either context. The release emphasizes that mepolizumab is not approved for acute bronchospasm or status asthmaticus. Health care professionals should first determine whether self-assisted administration or administration provided by a caregiver is appropriate, and then they should provide patients and/or caregivers with proper training in how to do so.
The approval is based on two open-label, single-arm, phase 3a studies that demonstrated successful administration was possible with these options among patients with severe eosinophilic asthma, at rates of 89%-95% in one study and 100% in the other. These results were followed by those of an open-label, parallel group, single-dose study that confirmed the pharmacokinetic and pharmacodynamic profiles of these new means of administration were comparable with those currently approved.
Mepolizumab is not indicated for those with a history of hypersensitivity to either mepolizumab or to the formulation’s excipients, such as anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, or rash. Any reductions of inhaled corticosteroids after initiation of mepolizumab should be gradual and under the supervision of a health care professional. Some infections by herpes zoster have been observed. The most common adverse reactions (occurring in 3% or more of patients and more often than with placebo) during the first 24 weeks of treatment were headache (19%), injection site reaction (8%), back pain (5%), fatigue (5%), influenza (3%), urinary tract infection (3%), abdominal pain upper (3%), pruritus (3%), eczema (3%), and muscle spasm (3%). Full prescribing information can be found on the FDA website.
, according to a press release from the drug’s developer. The biologic will now be available as an autoinjector and as a prefilled safety syringe.
The 100-mg subcutaneous mepolizumab injection is indicated as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and the three-dose 100-mg subcutaneous injections are indicated for the rare eosinophilic granulomatosis and polyangiitis, with the biologic administered every 4 weeks in either context. The release emphasizes that mepolizumab is not approved for acute bronchospasm or status asthmaticus. Health care professionals should first determine whether self-assisted administration or administration provided by a caregiver is appropriate, and then they should provide patients and/or caregivers with proper training in how to do so.
The approval is based on two open-label, single-arm, phase 3a studies that demonstrated successful administration was possible with these options among patients with severe eosinophilic asthma, at rates of 89%-95% in one study and 100% in the other. These results were followed by those of an open-label, parallel group, single-dose study that confirmed the pharmacokinetic and pharmacodynamic profiles of these new means of administration were comparable with those currently approved.
Mepolizumab is not indicated for those with a history of hypersensitivity to either mepolizumab or to the formulation’s excipients, such as anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, or rash. Any reductions of inhaled corticosteroids after initiation of mepolizumab should be gradual and under the supervision of a health care professional. Some infections by herpes zoster have been observed. The most common adverse reactions (occurring in 3% or more of patients and more often than with placebo) during the first 24 weeks of treatment were headache (19%), injection site reaction (8%), back pain (5%), fatigue (5%), influenza (3%), urinary tract infection (3%), abdominal pain upper (3%), pruritus (3%), eczema (3%), and muscle spasm (3%). Full prescribing information can be found on the FDA website.
, according to a press release from the drug’s developer. The biologic will now be available as an autoinjector and as a prefilled safety syringe.
The 100-mg subcutaneous mepolizumab injection is indicated as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and the three-dose 100-mg subcutaneous injections are indicated for the rare eosinophilic granulomatosis and polyangiitis, with the biologic administered every 4 weeks in either context. The release emphasizes that mepolizumab is not approved for acute bronchospasm or status asthmaticus. Health care professionals should first determine whether self-assisted administration or administration provided by a caregiver is appropriate, and then they should provide patients and/or caregivers with proper training in how to do so.
The approval is based on two open-label, single-arm, phase 3a studies that demonstrated successful administration was possible with these options among patients with severe eosinophilic asthma, at rates of 89%-95% in one study and 100% in the other. These results were followed by those of an open-label, parallel group, single-dose study that confirmed the pharmacokinetic and pharmacodynamic profiles of these new means of administration were comparable with those currently approved.
Mepolizumab is not indicated for those with a history of hypersensitivity to either mepolizumab or to the formulation’s excipients, such as anaphylaxis, angioedema, bronchospasm, hypotension, urticaria, or rash. Any reductions of inhaled corticosteroids after initiation of mepolizumab should be gradual and under the supervision of a health care professional. Some infections by herpes zoster have been observed. The most common adverse reactions (occurring in 3% or more of patients and more often than with placebo) during the first 24 weeks of treatment were headache (19%), injection site reaction (8%), back pain (5%), fatigue (5%), influenza (3%), urinary tract infection (3%), abdominal pain upper (3%), pruritus (3%), eczema (3%), and muscle spasm (3%). Full prescribing information can be found on the FDA website.
Back to the Future: Integrating Technology to Improve Patient-Provider Interactions
The advent of electronic medical records (EMRs) is arguably the most important technological revolution in modern medicine. The transition from paper documentation to EMRs has improved organization of medical records, consolidating all physician notes, orders, consultations, laboratory test results, and radiologic studies into a single accessible location.1 However, this revolution has led to mixed consequences for patients, especially in the outpatient setting. The use of EMRs can facilitate questions, clarification, and discussion between patients and health care providers, prompted by the sections of the EMR. Unfortunately, patients too often encounter pressed-for-time, documentation-focused providers who may not even look up from the computer. Provider behaviors such as making eye contact, stopping typing during discussion of sensitive topics, and allowing patients to view the computer screen and using it as an educational tool are important for patients to have a positive care experience.2 We envision further integration of current and future technology to overcome the challenges of outpatient care. We use a hypothetical patient encounter to illustrate what the future may hold.
Hypothetical Patient Encounter
An established patient, Ms. PS, comes to the dermatology clinic for a follow-up appointment and walks into an examination room (Figure). Prior to entering the room, the provider, Dr. FT, reviews Ms. PS’s history via a dermatology-specific EMR and reads that Ms. PS has a 1.5-year history of psoriasis and is considering other therapeutic options.
Upon entering the room, Dr. FT tells Ms. PS that the visit is being recorded and transcribed. A large interactive screen is a key component of the examination room. A remote medical assistant is virtually present via video to transcribe and document the patient-provider interaction. There is potential for artificial intelligence to replace the remote medical assistant in the future. Wearable technology, including a smartwatch and Bluetooth headphones, allow the provider to record audio of the visit as well as through microphones on the interactive screen.
As the interaction begins, Ms. PS reports that her psoriasis is poorly controlled with her current regimen of topical steroids. Dr. FT inquires about Ms. PS’s current symptoms and psychosocial well-being. Dr. FT then performs a skin examination and is easily able to evaluate her skin vs prior visits, as clinical images from prior visits are automatically displayed on the interactive screen. Dr. FT also closely examines Ms. PS’s nails and conducts a joint examination, reminded by a notification on his wearable technology. After capturing clinical images of Ms. PS’s skin and nails with a secure EMR-connected tablet, Dr. FT briefly steps out of the room to allow Ms. PS to get dressed and feel more comfortable in the discussion to follow.
Once he reenters the examination room, Dr. FT initiates a discussion on next steps. Ms. PS’s pathology report and clinical images are displayed on the interactive screen, along with her most recent laboratory test results, which were completed prior to the visit in anticipation of changing therapies. Dr. FT presents Ms. PS with several evidence-based therapeutic options for psoriasis, and she expresses interest in methotrexate. Following the discussion, the remote medical assistant displays information about methotrexate on the interactive screen, including evidence for treatment of psoriasis, contraindications, laboratory monitoring requirements, and possible adverse effects for both the patient and provider to review together. Dr. FT reviews the laboratory test results displayed on the screen, specifically her transaminase levels, and confirms that methotrexate is an appropriate therapeutic option. After a full discussion of risks and benefits, Ms. PS chooses to initiate methotrexate treatment. Reminded by a notification on his wearable technology, Dr. FT follows evidence-based dosing guidelines and sends the prescription electronically to Ms. PS’s pharmacy, which concludes Ms. PS’s visit.
Analysis of the Patient Encounter
In this interaction, Dr. FT was able to fully engage with the patient, unencumbered by the demands of documentation. There were only a few instances when the provider looked at or touched the interactive screen. Furthermore, joint decision-making was optimized by allowing both the patient and provider to review diagnostic test results and current evidence-based therapeutic guidelines together through the interactive screen. Ms. PS goes home feeling satisfied that she received her provider’s complete attention and that they selected a therapeutic option supported by evidence. After the visit, the remote medial assistant’s transcript populates a patient note template, which Dr. FT reviews and amends to create the final note. Reducing the time required to write patient notes increases the speed at which Dr. FT can complete patient encounters and may improve clinic flow and productivity. In addition, a patient summary is generated from Dr. FT’s final note, with an emphasis on patient instructions, and is sent to Ms. PS.
Final Thoughts
Our proposed integration of currently available and future technology can help minimize documentation burdens on providers and improve patient-provider communication in the age of the EMR, thus optimizing patient satisfaction and outcomes.
- Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. 2016;(suppl 1):S48-S61.
- Alkureishi MA, Lee WW, Lyons M, et al. Impact of electronic medical record use on the patient-doctor relationship and communication: a systematic review. J Gen Intern Med. 2016;31:548-560.
The advent of electronic medical records (EMRs) is arguably the most important technological revolution in modern medicine. The transition from paper documentation to EMRs has improved organization of medical records, consolidating all physician notes, orders, consultations, laboratory test results, and radiologic studies into a single accessible location.1 However, this revolution has led to mixed consequences for patients, especially in the outpatient setting. The use of EMRs can facilitate questions, clarification, and discussion between patients and health care providers, prompted by the sections of the EMR. Unfortunately, patients too often encounter pressed-for-time, documentation-focused providers who may not even look up from the computer. Provider behaviors such as making eye contact, stopping typing during discussion of sensitive topics, and allowing patients to view the computer screen and using it as an educational tool are important for patients to have a positive care experience.2 We envision further integration of current and future technology to overcome the challenges of outpatient care. We use a hypothetical patient encounter to illustrate what the future may hold.
Hypothetical Patient Encounter
An established patient, Ms. PS, comes to the dermatology clinic for a follow-up appointment and walks into an examination room (Figure). Prior to entering the room, the provider, Dr. FT, reviews Ms. PS’s history via a dermatology-specific EMR and reads that Ms. PS has a 1.5-year history of psoriasis and is considering other therapeutic options.

Upon entering the room, Dr. FT tells Ms. PS that the visit is being recorded and transcribed. A large interactive screen is a key component of the examination room. A remote medical assistant is virtually present via video to transcribe and document the patient-provider interaction. There is potential for artificial intelligence to replace the remote medical assistant in the future. Wearable technology, including a smartwatch and Bluetooth headphones, allow the provider to record audio of the visit as well as through microphones on the interactive screen.
As the interaction begins, Ms. PS reports that her psoriasis is poorly controlled with her current regimen of topical steroids. Dr. FT inquires about Ms. PS’s current symptoms and psychosocial well-being. Dr. FT then performs a skin examination and is easily able to evaluate her skin vs prior visits, as clinical images from prior visits are automatically displayed on the interactive screen. Dr. FT also closely examines Ms. PS’s nails and conducts a joint examination, reminded by a notification on his wearable technology. After capturing clinical images of Ms. PS’s skin and nails with a secure EMR-connected tablet, Dr. FT briefly steps out of the room to allow Ms. PS to get dressed and feel more comfortable in the discussion to follow.
Once he reenters the examination room, Dr. FT initiates a discussion on next steps. Ms. PS’s pathology report and clinical images are displayed on the interactive screen, along with her most recent laboratory test results, which were completed prior to the visit in anticipation of changing therapies. Dr. FT presents Ms. PS with several evidence-based therapeutic options for psoriasis, and she expresses interest in methotrexate. Following the discussion, the remote medical assistant displays information about methotrexate on the interactive screen, including evidence for treatment of psoriasis, contraindications, laboratory monitoring requirements, and possible adverse effects for both the patient and provider to review together. Dr. FT reviews the laboratory test results displayed on the screen, specifically her transaminase levels, and confirms that methotrexate is an appropriate therapeutic option. After a full discussion of risks and benefits, Ms. PS chooses to initiate methotrexate treatment. Reminded by a notification on his wearable technology, Dr. FT follows evidence-based dosing guidelines and sends the prescription electronically to Ms. PS’s pharmacy, which concludes Ms. PS’s visit.
Analysis of the Patient Encounter
In this interaction, Dr. FT was able to fully engage with the patient, unencumbered by the demands of documentation. There were only a few instances when the provider looked at or touched the interactive screen. Furthermore, joint decision-making was optimized by allowing both the patient and provider to review diagnostic test results and current evidence-based therapeutic guidelines together through the interactive screen. Ms. PS goes home feeling satisfied that she received her provider’s complete attention and that they selected a therapeutic option supported by evidence. After the visit, the remote medial assistant’s transcript populates a patient note template, which Dr. FT reviews and amends to create the final note. Reducing the time required to write patient notes increases the speed at which Dr. FT can complete patient encounters and may improve clinic flow and productivity. In addition, a patient summary is generated from Dr. FT’s final note, with an emphasis on patient instructions, and is sent to Ms. PS.
Final Thoughts
Our proposed integration of currently available and future technology can help minimize documentation burdens on providers and improve patient-provider communication in the age of the EMR, thus optimizing patient satisfaction and outcomes.
The advent of electronic medical records (EMRs) is arguably the most important technological revolution in modern medicine. The transition from paper documentation to EMRs has improved organization of medical records, consolidating all physician notes, orders, consultations, laboratory test results, and radiologic studies into a single accessible location.1 However, this revolution has led to mixed consequences for patients, especially in the outpatient setting. The use of EMRs can facilitate questions, clarification, and discussion between patients and health care providers, prompted by the sections of the EMR. Unfortunately, patients too often encounter pressed-for-time, documentation-focused providers who may not even look up from the computer. Provider behaviors such as making eye contact, stopping typing during discussion of sensitive topics, and allowing patients to view the computer screen and using it as an educational tool are important for patients to have a positive care experience.2 We envision further integration of current and future technology to overcome the challenges of outpatient care. We use a hypothetical patient encounter to illustrate what the future may hold.
Hypothetical Patient Encounter
An established patient, Ms. PS, comes to the dermatology clinic for a follow-up appointment and walks into an examination room (Figure). Prior to entering the room, the provider, Dr. FT, reviews Ms. PS’s history via a dermatology-specific EMR and reads that Ms. PS has a 1.5-year history of psoriasis and is considering other therapeutic options.

Upon entering the room, Dr. FT tells Ms. PS that the visit is being recorded and transcribed. A large interactive screen is a key component of the examination room. A remote medical assistant is virtually present via video to transcribe and document the patient-provider interaction. There is potential for artificial intelligence to replace the remote medical assistant in the future. Wearable technology, including a smartwatch and Bluetooth headphones, allow the provider to record audio of the visit as well as through microphones on the interactive screen.
As the interaction begins, Ms. PS reports that her psoriasis is poorly controlled with her current regimen of topical steroids. Dr. FT inquires about Ms. PS’s current symptoms and psychosocial well-being. Dr. FT then performs a skin examination and is easily able to evaluate her skin vs prior visits, as clinical images from prior visits are automatically displayed on the interactive screen. Dr. FT also closely examines Ms. PS’s nails and conducts a joint examination, reminded by a notification on his wearable technology. After capturing clinical images of Ms. PS’s skin and nails with a secure EMR-connected tablet, Dr. FT briefly steps out of the room to allow Ms. PS to get dressed and feel more comfortable in the discussion to follow.
Once he reenters the examination room, Dr. FT initiates a discussion on next steps. Ms. PS’s pathology report and clinical images are displayed on the interactive screen, along with her most recent laboratory test results, which were completed prior to the visit in anticipation of changing therapies. Dr. FT presents Ms. PS with several evidence-based therapeutic options for psoriasis, and she expresses interest in methotrexate. Following the discussion, the remote medical assistant displays information about methotrexate on the interactive screen, including evidence for treatment of psoriasis, contraindications, laboratory monitoring requirements, and possible adverse effects for both the patient and provider to review together. Dr. FT reviews the laboratory test results displayed on the screen, specifically her transaminase levels, and confirms that methotrexate is an appropriate therapeutic option. After a full discussion of risks and benefits, Ms. PS chooses to initiate methotrexate treatment. Reminded by a notification on his wearable technology, Dr. FT follows evidence-based dosing guidelines and sends the prescription electronically to Ms. PS’s pharmacy, which concludes Ms. PS’s visit.
Analysis of the Patient Encounter
In this interaction, Dr. FT was able to fully engage with the patient, unencumbered by the demands of documentation. There were only a few instances when the provider looked at or touched the interactive screen. Furthermore, joint decision-making was optimized by allowing both the patient and provider to review diagnostic test results and current evidence-based therapeutic guidelines together through the interactive screen. Ms. PS goes home feeling satisfied that she received her provider’s complete attention and that they selected a therapeutic option supported by evidence. After the visit, the remote medial assistant’s transcript populates a patient note template, which Dr. FT reviews and amends to create the final note. Reducing the time required to write patient notes increases the speed at which Dr. FT can complete patient encounters and may improve clinic flow and productivity. In addition, a patient summary is generated from Dr. FT’s final note, with an emphasis on patient instructions, and is sent to Ms. PS.
Final Thoughts
Our proposed integration of currently available and future technology can help minimize documentation burdens on providers and improve patient-provider communication in the age of the EMR, thus optimizing patient satisfaction and outcomes.
- Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. 2016;(suppl 1):S48-S61.
- Alkureishi MA, Lee WW, Lyons M, et al. Impact of electronic medical record use on the patient-doctor relationship and communication: a systematic review. J Gen Intern Med. 2016;31:548-560.
- Evans RS. Electronic health records: then, now, and in the future. Yearb Med Inform. 2016;(suppl 1):S48-S61.
- Alkureishi MA, Lee WW, Lyons M, et al. Impact of electronic medical record use on the patient-doctor relationship and communication: a systematic review. J Gen Intern Med. 2016;31:548-560.
Practice Points
- Electronic medical records afford many benefits, but documentation burdens on health care providers can impede positive patient-provider interactions.
- Integration of current and future technology can shift the focus back to the patient and facilitate shared decision-making.
The impact of HM19 on my practice
As an academic nurse practitioner hospitalist with faculty and leadership roles, I found that HM19 had many important and helpful topics that apply directly to my practice.
The “Onboarding Best Practices” session provided specific examples and tips for clinical ramp up, enculturation, and orienting staff to an academic career. As a result of this talk, I began the process of establishing a formal enculturation activity for new hires that includes a panel of senior advanced practice provider (APP) hospitalists to give career path advice.
The “Adaptive Leadership for Hospitalists” workshop provided the opportunity to practice emotional intelligence and effective communication in managing routine and difficult leadership interactions. The “Practice Models/Models of Care for Optimal Integration of NPs and PAs” presentation provided insight into variable team structures at other institutions that could be considered for improved efficiency in my group. The “Academic NP/PA” session provided ideas for how to apply for faculty positions in academic institutions. It also gave APPs who have faculty appointment specific illustrations of using current educational, quality improvement, and research projects to promote. I particularly found the “What Mentorship Has Meant to Me” talk significant. It gave practical essential advice on making sure there is chemistry and trust when seeking a mentor and staying engaged to be a successful mentee.
APPs, whether practicing in academic, private, or community settings, should attend the SHM Annual Conference. SHM is very inclusive and proud of APPs as colleagues and leaders. There are topics that directly apply to the needs of APP hospitalists – including career advancement – and that create excitement for APP practice in hospital medicine.
The Annual Conference also provides the very unique opportunity to meet and establish relationships with APP and physician colleagues and leaders nationwide. These relationships lend to career advancing opportunities for collaboration in clinical excellence, education, quality improvement, research, and leadership.
Dr. Apodaca is assistant professor and nurse practitioner hospitalist at the University of New Mexico. She is one of the first APPNP/PAs to receive faculty appointment at UNM. She serves as codirector of the UNM APP Hospital Medicine Fellowship and director of the APP Hospital Medicine Team. She is also the president of the New Mexico Chapter of SHM and is the first APP at her institution to achieve designation as a Fellow in Hospital Medicine.
As an academic nurse practitioner hospitalist with faculty and leadership roles, I found that HM19 had many important and helpful topics that apply directly to my practice.
The “Onboarding Best Practices” session provided specific examples and tips for clinical ramp up, enculturation, and orienting staff to an academic career. As a result of this talk, I began the process of establishing a formal enculturation activity for new hires that includes a panel of senior advanced practice provider (APP) hospitalists to give career path advice.
The “Adaptive Leadership for Hospitalists” workshop provided the opportunity to practice emotional intelligence and effective communication in managing routine and difficult leadership interactions. The “Practice Models/Models of Care for Optimal Integration of NPs and PAs” presentation provided insight into variable team structures at other institutions that could be considered for improved efficiency in my group. The “Academic NP/PA” session provided ideas for how to apply for faculty positions in academic institutions. It also gave APPs who have faculty appointment specific illustrations of using current educational, quality improvement, and research projects to promote. I particularly found the “What Mentorship Has Meant to Me” talk significant. It gave practical essential advice on making sure there is chemistry and trust when seeking a mentor and staying engaged to be a successful mentee.
APPs, whether practicing in academic, private, or community settings, should attend the SHM Annual Conference. SHM is very inclusive and proud of APPs as colleagues and leaders. There are topics that directly apply to the needs of APP hospitalists – including career advancement – and that create excitement for APP practice in hospital medicine.
The Annual Conference also provides the very unique opportunity to meet and establish relationships with APP and physician colleagues and leaders nationwide. These relationships lend to career advancing opportunities for collaboration in clinical excellence, education, quality improvement, research, and leadership.
Dr. Apodaca is assistant professor and nurse practitioner hospitalist at the University of New Mexico. She is one of the first APPNP/PAs to receive faculty appointment at UNM. She serves as codirector of the UNM APP Hospital Medicine Fellowship and director of the APP Hospital Medicine Team. She is also the president of the New Mexico Chapter of SHM and is the first APP at her institution to achieve designation as a Fellow in Hospital Medicine.
As an academic nurse practitioner hospitalist with faculty and leadership roles, I found that HM19 had many important and helpful topics that apply directly to my practice.
The “Onboarding Best Practices” session provided specific examples and tips for clinical ramp up, enculturation, and orienting staff to an academic career. As a result of this talk, I began the process of establishing a formal enculturation activity for new hires that includes a panel of senior advanced practice provider (APP) hospitalists to give career path advice.
The “Adaptive Leadership for Hospitalists” workshop provided the opportunity to practice emotional intelligence and effective communication in managing routine and difficult leadership interactions. The “Practice Models/Models of Care for Optimal Integration of NPs and PAs” presentation provided insight into variable team structures at other institutions that could be considered for improved efficiency in my group. The “Academic NP/PA” session provided ideas for how to apply for faculty positions in academic institutions. It also gave APPs who have faculty appointment specific illustrations of using current educational, quality improvement, and research projects to promote. I particularly found the “What Mentorship Has Meant to Me” talk significant. It gave practical essential advice on making sure there is chemistry and trust when seeking a mentor and staying engaged to be a successful mentee.
APPs, whether practicing in academic, private, or community settings, should attend the SHM Annual Conference. SHM is very inclusive and proud of APPs as colleagues and leaders. There are topics that directly apply to the needs of APP hospitalists – including career advancement – and that create excitement for APP practice in hospital medicine.
The Annual Conference also provides the very unique opportunity to meet and establish relationships with APP and physician colleagues and leaders nationwide. These relationships lend to career advancing opportunities for collaboration in clinical excellence, education, quality improvement, research, and leadership.
Dr. Apodaca is assistant professor and nurse practitioner hospitalist at the University of New Mexico. She is one of the first APPNP/PAs to receive faculty appointment at UNM. She serves as codirector of the UNM APP Hospital Medicine Fellowship and director of the APP Hospital Medicine Team. She is also the president of the New Mexico Chapter of SHM and is the first APP at her institution to achieve designation as a Fellow in Hospital Medicine.
Tofacitinib upped herpes zoster risk in ulcerative colitis
Among patients with moderate to severe ulcerative colitis, a median of 1.4 years and up to 4.4 years of tofacitinib therapy was safe apart from a dose-related increase in risk of herpes zoster infection, according to an integrated analysis of data from five clinical trials.
Compared with placebo, a 5-mg twice-daily maintenance dose of tofacitinib (Xeljanz) produced a 2.1-fold greater risk of herpes zoster infection (95% confidence interval, 0.4-6.0), while a 10-mg, twice-daily dose produced a statistically significant 6.6-fold increase in incidence (95% CI, 3.2-12.2).
With the exception of the higher incidence rate of herpes zoster, “in the overall cohort, the safety profile of tofacitinib was generally similar to that of tumor necrosis factor inhibitor therapies,” wrote William J. Sandborn, MD, director of the inflammatory bowel disease center and professor of medicine, at the University of California, San Diego, and associates. The findings were published in Clinical Gastroenterology and Hepatology.
Tofacitinib is an oral, small-molecular Janus kinase inhibitor approved in the United States for treating moderate to severe ulcerative colitis, as well as rheumatoid and psoriatic arthritis. The recommended ulcerative colitis dose is 10 mg twice daily for at least 8 weeks (induction therapy) followed by 5 or 10 mg twice daily (maintenance). The safety of tofacitinib has been studied in patients with rheumatoid arthritis through 9 years of treatment. To begin a similar undertaking in ulcerative colitis, Dr. Sandborn and associates pooled data from three 8-week, double-blind, placebo-controlled induction trials, as well as one 52-week, double-blind, placebo-controlled maintenance trial and one ongoing open-label trial. All patients received twice-daily tofacitinib (5 mg or 10 mg) or placebo.
Among 1,157 tofacitinib recipients in the pooled analysis, 84% received an average of 10 mg twice daily. For every 100 person-years of tofacitinib exposure, there were an estimated 2.0 serious infections, 1.3 opportunistic infections, 4.1 herpes zoster infections, 1.4 malignancies (including nonmelanoma skin cancer, which had an incidence of 0.7), 0.2 major adverse cardiovascular events, and 0.2 gastrointestinal perforations. The likelihood of these events did not increase with time on tofacitinib, the researchers said.
Worsening ulcerative colitis was the most common serious adverse event for patients who received both induction and maintenance therapy. For patients on maintenance therapy, only herpes zoster infection had a higher incidence than placebo, which reached statistical significance at the 10-mg dose. These safety findings resemble those in rheumatoid arthritis trials of tofacitinib, and apart from herpes zoster, they also resemble safety data for vedolizumab (an integrin receptor antagonist), and anti-tumor necrosis factor agents in ulcerative colitis, the researchers wrote.
There were four deaths during the entire tofacitinib ulcerative colitis program, for an incidence rate of 0.2 per 100 person-years of exposure. All occurred in patients receiving 10 mg twice daily. Causes of death were dissecting aortic aneurysm, hepatic angiosarcoma, acute myeloid leukemia, and pulmonary embolism in a patient with cholangiocarcinoma that had metastasized to the peritoneum. Recently, concerns about pulmonary embolism have led the European Medicines Agency (EMA) to recommend against the use of 10-mg twice daily tofacitinib dose in patients at increased risk for pulmonary embolism.
“Compared with prior experience with tofacitinib in rheumatoid arthritis, no new or unexpected safety signals were identified,” the researchers concluded. “These
Pfizer makes tofacitinib, funded the individual trials, and paid for medical writing. Dr. Sandborn disclosed grants, personal fees, and nonfinancial support from Pfizer and many other pharmaceutical companies.
SOURCE: Sandborn WJ et al. Clin Gastroenterol Hepatol. 2018 Nov 23. doi: 10.1016/j.cgh.2018.11.035.
As new mechanisms of action become available for ulcerative colitis (UC) drugs, clinicians must weigh the risks versus benefits (i.e., safety vs. efficacy). In this article, Sandborn and colleagues provide additional information on the safety profile of tofacitinib. They report an increased risk of herpes zoster that was dose dependent (sixfold increase on 10 mg twice daily). The overall safety profile was reassuring, is similar to the rheumatoid arthritis population treated with tofacitinib, and is in line with the safety profile of anti-TNF antibodies (excluding the increase risk of zoster). With a nonlive zoster vaccine now available, some have advocated vaccinating all patients being started on tofacitinib. However, there is a theoretical risk of disease exacerbation and ongoing studies that will hopefully answer this important question.
David A. Schwartz, MD, professor of medicine, division of gastroenterology, hepatology and nutrition, Inflammatory Bowel Disease Center, Vanderbilt University, Nashville.
As new mechanisms of action become available for ulcerative colitis (UC) drugs, clinicians must weigh the risks versus benefits (i.e., safety vs. efficacy). In this article, Sandborn and colleagues provide additional information on the safety profile of tofacitinib. They report an increased risk of herpes zoster that was dose dependent (sixfold increase on 10 mg twice daily). The overall safety profile was reassuring, is similar to the rheumatoid arthritis population treated with tofacitinib, and is in line with the safety profile of anti-TNF antibodies (excluding the increase risk of zoster). With a nonlive zoster vaccine now available, some have advocated vaccinating all patients being started on tofacitinib. However, there is a theoretical risk of disease exacerbation and ongoing studies that will hopefully answer this important question.
David A. Schwartz, MD, professor of medicine, division of gastroenterology, hepatology and nutrition, Inflammatory Bowel Disease Center, Vanderbilt University, Nashville.
As new mechanisms of action become available for ulcerative colitis (UC) drugs, clinicians must weigh the risks versus benefits (i.e., safety vs. efficacy). In this article, Sandborn and colleagues provide additional information on the safety profile of tofacitinib. They report an increased risk of herpes zoster that was dose dependent (sixfold increase on 10 mg twice daily). The overall safety profile was reassuring, is similar to the rheumatoid arthritis population treated with tofacitinib, and is in line with the safety profile of anti-TNF antibodies (excluding the increase risk of zoster). With a nonlive zoster vaccine now available, some have advocated vaccinating all patients being started on tofacitinib. However, there is a theoretical risk of disease exacerbation and ongoing studies that will hopefully answer this important question.
David A. Schwartz, MD, professor of medicine, division of gastroenterology, hepatology and nutrition, Inflammatory Bowel Disease Center, Vanderbilt University, Nashville.
Among patients with moderate to severe ulcerative colitis, a median of 1.4 years and up to 4.4 years of tofacitinib therapy was safe apart from a dose-related increase in risk of herpes zoster infection, according to an integrated analysis of data from five clinical trials.
Compared with placebo, a 5-mg twice-daily maintenance dose of tofacitinib (Xeljanz) produced a 2.1-fold greater risk of herpes zoster infection (95% confidence interval, 0.4-6.0), while a 10-mg, twice-daily dose produced a statistically significant 6.6-fold increase in incidence (95% CI, 3.2-12.2).
With the exception of the higher incidence rate of herpes zoster, “in the overall cohort, the safety profile of tofacitinib was generally similar to that of tumor necrosis factor inhibitor therapies,” wrote William J. Sandborn, MD, director of the inflammatory bowel disease center and professor of medicine, at the University of California, San Diego, and associates. The findings were published in Clinical Gastroenterology and Hepatology.
Tofacitinib is an oral, small-molecular Janus kinase inhibitor approved in the United States for treating moderate to severe ulcerative colitis, as well as rheumatoid and psoriatic arthritis. The recommended ulcerative colitis dose is 10 mg twice daily for at least 8 weeks (induction therapy) followed by 5 or 10 mg twice daily (maintenance). The safety of tofacitinib has been studied in patients with rheumatoid arthritis through 9 years of treatment. To begin a similar undertaking in ulcerative colitis, Dr. Sandborn and associates pooled data from three 8-week, double-blind, placebo-controlled induction trials, as well as one 52-week, double-blind, placebo-controlled maintenance trial and one ongoing open-label trial. All patients received twice-daily tofacitinib (5 mg or 10 mg) or placebo.
Among 1,157 tofacitinib recipients in the pooled analysis, 84% received an average of 10 mg twice daily. For every 100 person-years of tofacitinib exposure, there were an estimated 2.0 serious infections, 1.3 opportunistic infections, 4.1 herpes zoster infections, 1.4 malignancies (including nonmelanoma skin cancer, which had an incidence of 0.7), 0.2 major adverse cardiovascular events, and 0.2 gastrointestinal perforations. The likelihood of these events did not increase with time on tofacitinib, the researchers said.
Worsening ulcerative colitis was the most common serious adverse event for patients who received both induction and maintenance therapy. For patients on maintenance therapy, only herpes zoster infection had a higher incidence than placebo, which reached statistical significance at the 10-mg dose. These safety findings resemble those in rheumatoid arthritis trials of tofacitinib, and apart from herpes zoster, they also resemble safety data for vedolizumab (an integrin receptor antagonist), and anti-tumor necrosis factor agents in ulcerative colitis, the researchers wrote.
There were four deaths during the entire tofacitinib ulcerative colitis program, for an incidence rate of 0.2 per 100 person-years of exposure. All occurred in patients receiving 10 mg twice daily. Causes of death were dissecting aortic aneurysm, hepatic angiosarcoma, acute myeloid leukemia, and pulmonary embolism in a patient with cholangiocarcinoma that had metastasized to the peritoneum. Recently, concerns about pulmonary embolism have led the European Medicines Agency (EMA) to recommend against the use of 10-mg twice daily tofacitinib dose in patients at increased risk for pulmonary embolism.
“Compared with prior experience with tofacitinib in rheumatoid arthritis, no new or unexpected safety signals were identified,” the researchers concluded. “These
Pfizer makes tofacitinib, funded the individual trials, and paid for medical writing. Dr. Sandborn disclosed grants, personal fees, and nonfinancial support from Pfizer and many other pharmaceutical companies.
SOURCE: Sandborn WJ et al. Clin Gastroenterol Hepatol. 2018 Nov 23. doi: 10.1016/j.cgh.2018.11.035.
Among patients with moderate to severe ulcerative colitis, a median of 1.4 years and up to 4.4 years of tofacitinib therapy was safe apart from a dose-related increase in risk of herpes zoster infection, according to an integrated analysis of data from five clinical trials.
Compared with placebo, a 5-mg twice-daily maintenance dose of tofacitinib (Xeljanz) produced a 2.1-fold greater risk of herpes zoster infection (95% confidence interval, 0.4-6.0), while a 10-mg, twice-daily dose produced a statistically significant 6.6-fold increase in incidence (95% CI, 3.2-12.2).
With the exception of the higher incidence rate of herpes zoster, “in the overall cohort, the safety profile of tofacitinib was generally similar to that of tumor necrosis factor inhibitor therapies,” wrote William J. Sandborn, MD, director of the inflammatory bowel disease center and professor of medicine, at the University of California, San Diego, and associates. The findings were published in Clinical Gastroenterology and Hepatology.
Tofacitinib is an oral, small-molecular Janus kinase inhibitor approved in the United States for treating moderate to severe ulcerative colitis, as well as rheumatoid and psoriatic arthritis. The recommended ulcerative colitis dose is 10 mg twice daily for at least 8 weeks (induction therapy) followed by 5 or 10 mg twice daily (maintenance). The safety of tofacitinib has been studied in patients with rheumatoid arthritis through 9 years of treatment. To begin a similar undertaking in ulcerative colitis, Dr. Sandborn and associates pooled data from three 8-week, double-blind, placebo-controlled induction trials, as well as one 52-week, double-blind, placebo-controlled maintenance trial and one ongoing open-label trial. All patients received twice-daily tofacitinib (5 mg or 10 mg) or placebo.
Among 1,157 tofacitinib recipients in the pooled analysis, 84% received an average of 10 mg twice daily. For every 100 person-years of tofacitinib exposure, there were an estimated 2.0 serious infections, 1.3 opportunistic infections, 4.1 herpes zoster infections, 1.4 malignancies (including nonmelanoma skin cancer, which had an incidence of 0.7), 0.2 major adverse cardiovascular events, and 0.2 gastrointestinal perforations. The likelihood of these events did not increase with time on tofacitinib, the researchers said.
Worsening ulcerative colitis was the most common serious adverse event for patients who received both induction and maintenance therapy. For patients on maintenance therapy, only herpes zoster infection had a higher incidence than placebo, which reached statistical significance at the 10-mg dose. These safety findings resemble those in rheumatoid arthritis trials of tofacitinib, and apart from herpes zoster, they also resemble safety data for vedolizumab (an integrin receptor antagonist), and anti-tumor necrosis factor agents in ulcerative colitis, the researchers wrote.
There were four deaths during the entire tofacitinib ulcerative colitis program, for an incidence rate of 0.2 per 100 person-years of exposure. All occurred in patients receiving 10 mg twice daily. Causes of death were dissecting aortic aneurysm, hepatic angiosarcoma, acute myeloid leukemia, and pulmonary embolism in a patient with cholangiocarcinoma that had metastasized to the peritoneum. Recently, concerns about pulmonary embolism have led the European Medicines Agency (EMA) to recommend against the use of 10-mg twice daily tofacitinib dose in patients at increased risk for pulmonary embolism.
“Compared with prior experience with tofacitinib in rheumatoid arthritis, no new or unexpected safety signals were identified,” the researchers concluded. “These
Pfizer makes tofacitinib, funded the individual trials, and paid for medical writing. Dr. Sandborn disclosed grants, personal fees, and nonfinancial support from Pfizer and many other pharmaceutical companies.
SOURCE: Sandborn WJ et al. Clin Gastroenterol Hepatol. 2018 Nov 23. doi: 10.1016/j.cgh.2018.11.035.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Tofacitinib therapy shows a dose-related increase in risk of herpes zoster in patients with ulcerative colitis.
Major finding: Compared with placebo, a 5-mg twice-daily maintenance dose of tofacitinib produced a 2.1-fold greater risk of herpes zoster infection (95% CI, 0.4-6.0), while a 10-mg twice-daily dose produced a statistically significant 6.6-fold increase in incidence (95% CI, 3.2–12.2).
Study details: Integrated safety analysis of five clinical trials (four randomized, double-blinded, and placebo-controlled) with 1,612.8 total years of exposure (median treatment duration, 1.4 years).
Disclosures: Pfizer makes tofacitinib, funded the individual trials, and paid for medical writing. Dr. Sandborn disclosed grants, personal fees, and nonfinancial support from Pfizer and many other pharmaceutical companies.
Source: Sandborn WJ et al. Clin Gastroenterol Hepatol. 2018 Nov 23. doi: 10.1016/j.cgh.2018.11.035.
Inducible nitric oxide synthase promotes insulin resistance in obesity
Obesity promotes the localization of inducible nitric oxide synthase (iNOS) in hepatic lysosomes, leading to a cascade of downstream effects that include excess lysosomal nitric oxide production, reduced hepatic autophagy, and insulin resistance, investigators reported.
“It is well known that in the context of obesity, chronic inflammation and lysosome dysfunction coexist in the liver,” wrote Qingwen Qian, PhD, of the University of Iowa in Iowa City and associates in Cellular and Molecular Gastroenterology and Hepatology. “Our studies suggest that lysosomal iNOS-mediated nitric oxide signaling disrupts hepatic lysosomal function, contributing to obesity-associated defective hepatic autophagy and insulin resistance.” They noted that the findings could hasten the development of new treatments for metabolic diseases.
Lysosomes recycle autophagocytosed intracellular and extracellular material, which is crucial to maintain several types of homeostasis within the liver. Each hepatocyte has about 250 lysosomes, which help regulate nutrient sensing, glycogen metabolism, cholesterol trafficking, and viral defense.
Activation of iNOS is a hallmark of inflammation, and iNOS levels are known to be elevated in the livers of patients with hepatitis C, alcoholic cirrhosis, and alpha 1-anti-trypsin disorder, the researchers wrote. “At the cellular level, iNOS produces pathological nitric oxide [NO], which triggers downstream effects, such as aberrant S-nitrosylation. These downstream effects can disrupt the function of organelles such as the mitochondria and the endoplasmic reticulum.”
Studies indicate that pathologic NO impairs lysosomal function in neurodegenerative diseases, cardiovascular disease, nonalcoholic fatty liver disease, and kidney disease, Dr. Qian and associates noted. But it was unclear whether NO in hepatocytes was generated by local iNOS or localized to lysosomes.
The researchers therefore studied cell cultures of primary murine hepatocytes by measuring their lysosomal activity, autophagy levels, and NO levels. They also studied a murine model of diet-induced obesity in which 60% of calories were from fat. They performed glucose tolerance tests by means of intraperitoneal glucose injections and studied the effects of insulin infusion. Finally, they performed immunohistology, immunohistochemistry, electron microscopy, and measurements of nitrosylated proteins and lysosomal arginine in frozen liver sections from the mice. Lysosomal arginine is required to catalyze NO production in the setting of inflammation as observed in obesity. In fact, concomitant stimulation of lysosomal arginine transport and activation of mTOR (an enzyme which tightly regulates transcription factor EB) was sufficient to stimulate lysosomal NO production in hepatocytes even in the absence of an inflammatory stimulus; pointing to a central role for these processes.
The researchers found that a NO scavenger diminished lysosomal NO production, while overexpression of both mTOR and a lysomal arginine transporter upregulated lysosomal NO production and suppressed autophagy. In mice with diet-induced obesity, deleting iNOS also improved nitrosative stress in hepatic lysosomes, promoted lysosomal biogenesis by activating transcription factor EB, enhanced lysosomal function and autophagy, and improved hepatic insulin sensitivity. Improved insulin sensitivity diminished, however, when the researchers suppressed transcription factor EB or autophagy-related 7 (Atg7).
Usually, iNOS is primarily expressed in hepatic Kupffer cells, but obesity increases the expression of iNOS in hepatocytes, which promotes hepatic insulin resistance and inflammation, the researchers commented. Unpublished data indicate that deleting iNOS initially protects against obesity-linked fatty liver steatosis and insulin resistance, but that these benefits weaken over time. “Nevertheless, our data showed that liver-specific iNOS suppression has a protective role,” they wrote. “Specifically, we showed that iNOS inactivates transcription factor EB, and that suppression of transcription factor EB and Atg7 diminishes the improved hepatic insulin sensitivity by iNOS deletion.” Transcription factor EB both regulates autophagy and is a “key player in lipid metabolism,” they added. It remains unclear whether the metabolic effects of iNOS solely relate to autophagy, they noted.
Funders included the American Heart Association, American Diabetes Association, and National Institutes of Health. The researchers reported having no conflicts of interest.
SOURCE: Qingwen Qian, et al. Cell Molec Gastroenterol Hepatol. 2019;8(1):95-110.
Understanding the mechanisms for how obesity affects cellular pathways is critical for identifying therapeutic targets to prevent its adverse consequences. The current study by Qian et al. identifies acquired lysosome dysfunction as a core cellular event that predisposes to insulin resistance in obesity. Lysosomes are degradative organelles that orchestrate cellular metabolism to facilitate homeostasis and confer stress resistance. Through a well-designed series of experiments conducted in a mouse model of diet-induced obesity, the authors demonstrate localization of inducible nitric oxide synthase (iNOS) to lysosomes in the livers of obese animals. This triggers excess local nitric oxide (NO) generation which leads to excessive nitrosylation of lysosomal proteins. A direct consequence of the resultant lysosome dysfunction is impaired autophagy, which is a critical cellular pathway for clearing away damaged organelles and proteins and generating energy under nutrient stress. Their studies also implicate lysosomal NO generation in suppressing the activity of transcription factor EB (TFEB), a master regulator of autophagy and lysosome biogenesis. Remarkably, genetic ablation of iNOS prevents the lysosome dysfunction and autophagy impairment, to attenuate obesity-induced insulin resistance.
Future studies will be required to assess the mechanisms for iNOS localization to the lysosomes and its interplay with the mammalian target of rapamycin (mTOR) signaling pathway in the face of sustained nutrient excess.
Abhinav Diwan, MD, is an associate professor of medicine, cell biology, and physiology at Washington University and associate division chief of cardiology at the John Cochran VA Medical Center, both in St. Louis. He has no conflicts.
Understanding the mechanisms for how obesity affects cellular pathways is critical for identifying therapeutic targets to prevent its adverse consequences. The current study by Qian et al. identifies acquired lysosome dysfunction as a core cellular event that predisposes to insulin resistance in obesity. Lysosomes are degradative organelles that orchestrate cellular metabolism to facilitate homeostasis and confer stress resistance. Through a well-designed series of experiments conducted in a mouse model of diet-induced obesity, the authors demonstrate localization of inducible nitric oxide synthase (iNOS) to lysosomes in the livers of obese animals. This triggers excess local nitric oxide (NO) generation which leads to excessive nitrosylation of lysosomal proteins. A direct consequence of the resultant lysosome dysfunction is impaired autophagy, which is a critical cellular pathway for clearing away damaged organelles and proteins and generating energy under nutrient stress. Their studies also implicate lysosomal NO generation in suppressing the activity of transcription factor EB (TFEB), a master regulator of autophagy and lysosome biogenesis. Remarkably, genetic ablation of iNOS prevents the lysosome dysfunction and autophagy impairment, to attenuate obesity-induced insulin resistance.
Future studies will be required to assess the mechanisms for iNOS localization to the lysosomes and its interplay with the mammalian target of rapamycin (mTOR) signaling pathway in the face of sustained nutrient excess.
Abhinav Diwan, MD, is an associate professor of medicine, cell biology, and physiology at Washington University and associate division chief of cardiology at the John Cochran VA Medical Center, both in St. Louis. He has no conflicts.
Understanding the mechanisms for how obesity affects cellular pathways is critical for identifying therapeutic targets to prevent its adverse consequences. The current study by Qian et al. identifies acquired lysosome dysfunction as a core cellular event that predisposes to insulin resistance in obesity. Lysosomes are degradative organelles that orchestrate cellular metabolism to facilitate homeostasis and confer stress resistance. Through a well-designed series of experiments conducted in a mouse model of diet-induced obesity, the authors demonstrate localization of inducible nitric oxide synthase (iNOS) to lysosomes in the livers of obese animals. This triggers excess local nitric oxide (NO) generation which leads to excessive nitrosylation of lysosomal proteins. A direct consequence of the resultant lysosome dysfunction is impaired autophagy, which is a critical cellular pathway for clearing away damaged organelles and proteins and generating energy under nutrient stress. Their studies also implicate lysosomal NO generation in suppressing the activity of transcription factor EB (TFEB), a master regulator of autophagy and lysosome biogenesis. Remarkably, genetic ablation of iNOS prevents the lysosome dysfunction and autophagy impairment, to attenuate obesity-induced insulin resistance.
Future studies will be required to assess the mechanisms for iNOS localization to the lysosomes and its interplay with the mammalian target of rapamycin (mTOR) signaling pathway in the face of sustained nutrient excess.
Abhinav Diwan, MD, is an associate professor of medicine, cell biology, and physiology at Washington University and associate division chief of cardiology at the John Cochran VA Medical Center, both in St. Louis. He has no conflicts.
Obesity promotes the localization of inducible nitric oxide synthase (iNOS) in hepatic lysosomes, leading to a cascade of downstream effects that include excess lysosomal nitric oxide production, reduced hepatic autophagy, and insulin resistance, investigators reported.
“It is well known that in the context of obesity, chronic inflammation and lysosome dysfunction coexist in the liver,” wrote Qingwen Qian, PhD, of the University of Iowa in Iowa City and associates in Cellular and Molecular Gastroenterology and Hepatology. “Our studies suggest that lysosomal iNOS-mediated nitric oxide signaling disrupts hepatic lysosomal function, contributing to obesity-associated defective hepatic autophagy and insulin resistance.” They noted that the findings could hasten the development of new treatments for metabolic diseases.
Lysosomes recycle autophagocytosed intracellular and extracellular material, which is crucial to maintain several types of homeostasis within the liver. Each hepatocyte has about 250 lysosomes, which help regulate nutrient sensing, glycogen metabolism, cholesterol trafficking, and viral defense.
Activation of iNOS is a hallmark of inflammation, and iNOS levels are known to be elevated in the livers of patients with hepatitis C, alcoholic cirrhosis, and alpha 1-anti-trypsin disorder, the researchers wrote. “At the cellular level, iNOS produces pathological nitric oxide [NO], which triggers downstream effects, such as aberrant S-nitrosylation. These downstream effects can disrupt the function of organelles such as the mitochondria and the endoplasmic reticulum.”
Studies indicate that pathologic NO impairs lysosomal function in neurodegenerative diseases, cardiovascular disease, nonalcoholic fatty liver disease, and kidney disease, Dr. Qian and associates noted. But it was unclear whether NO in hepatocytes was generated by local iNOS or localized to lysosomes.
The researchers therefore studied cell cultures of primary murine hepatocytes by measuring their lysosomal activity, autophagy levels, and NO levels. They also studied a murine model of diet-induced obesity in which 60% of calories were from fat. They performed glucose tolerance tests by means of intraperitoneal glucose injections and studied the effects of insulin infusion. Finally, they performed immunohistology, immunohistochemistry, electron microscopy, and measurements of nitrosylated proteins and lysosomal arginine in frozen liver sections from the mice. Lysosomal arginine is required to catalyze NO production in the setting of inflammation as observed in obesity. In fact, concomitant stimulation of lysosomal arginine transport and activation of mTOR (an enzyme which tightly regulates transcription factor EB) was sufficient to stimulate lysosomal NO production in hepatocytes even in the absence of an inflammatory stimulus; pointing to a central role for these processes.
The researchers found that a NO scavenger diminished lysosomal NO production, while overexpression of both mTOR and a lysomal arginine transporter upregulated lysosomal NO production and suppressed autophagy. In mice with diet-induced obesity, deleting iNOS also improved nitrosative stress in hepatic lysosomes, promoted lysosomal biogenesis by activating transcription factor EB, enhanced lysosomal function and autophagy, and improved hepatic insulin sensitivity. Improved insulin sensitivity diminished, however, when the researchers suppressed transcription factor EB or autophagy-related 7 (Atg7).
Usually, iNOS is primarily expressed in hepatic Kupffer cells, but obesity increases the expression of iNOS in hepatocytes, which promotes hepatic insulin resistance and inflammation, the researchers commented. Unpublished data indicate that deleting iNOS initially protects against obesity-linked fatty liver steatosis and insulin resistance, but that these benefits weaken over time. “Nevertheless, our data showed that liver-specific iNOS suppression has a protective role,” they wrote. “Specifically, we showed that iNOS inactivates transcription factor EB, and that suppression of transcription factor EB and Atg7 diminishes the improved hepatic insulin sensitivity by iNOS deletion.” Transcription factor EB both regulates autophagy and is a “key player in lipid metabolism,” they added. It remains unclear whether the metabolic effects of iNOS solely relate to autophagy, they noted.
Funders included the American Heart Association, American Diabetes Association, and National Institutes of Health. The researchers reported having no conflicts of interest.
SOURCE: Qingwen Qian, et al. Cell Molec Gastroenterol Hepatol. 2019;8(1):95-110.
Obesity promotes the localization of inducible nitric oxide synthase (iNOS) in hepatic lysosomes, leading to a cascade of downstream effects that include excess lysosomal nitric oxide production, reduced hepatic autophagy, and insulin resistance, investigators reported.
“It is well known that in the context of obesity, chronic inflammation and lysosome dysfunction coexist in the liver,” wrote Qingwen Qian, PhD, of the University of Iowa in Iowa City and associates in Cellular and Molecular Gastroenterology and Hepatology. “Our studies suggest that lysosomal iNOS-mediated nitric oxide signaling disrupts hepatic lysosomal function, contributing to obesity-associated defective hepatic autophagy and insulin resistance.” They noted that the findings could hasten the development of new treatments for metabolic diseases.
Lysosomes recycle autophagocytosed intracellular and extracellular material, which is crucial to maintain several types of homeostasis within the liver. Each hepatocyte has about 250 lysosomes, which help regulate nutrient sensing, glycogen metabolism, cholesterol trafficking, and viral defense.
Activation of iNOS is a hallmark of inflammation, and iNOS levels are known to be elevated in the livers of patients with hepatitis C, alcoholic cirrhosis, and alpha 1-anti-trypsin disorder, the researchers wrote. “At the cellular level, iNOS produces pathological nitric oxide [NO], which triggers downstream effects, such as aberrant S-nitrosylation. These downstream effects can disrupt the function of organelles such as the mitochondria and the endoplasmic reticulum.”
Studies indicate that pathologic NO impairs lysosomal function in neurodegenerative diseases, cardiovascular disease, nonalcoholic fatty liver disease, and kidney disease, Dr. Qian and associates noted. But it was unclear whether NO in hepatocytes was generated by local iNOS or localized to lysosomes.
The researchers therefore studied cell cultures of primary murine hepatocytes by measuring their lysosomal activity, autophagy levels, and NO levels. They also studied a murine model of diet-induced obesity in which 60% of calories were from fat. They performed glucose tolerance tests by means of intraperitoneal glucose injections and studied the effects of insulin infusion. Finally, they performed immunohistology, immunohistochemistry, electron microscopy, and measurements of nitrosylated proteins and lysosomal arginine in frozen liver sections from the mice. Lysosomal arginine is required to catalyze NO production in the setting of inflammation as observed in obesity. In fact, concomitant stimulation of lysosomal arginine transport and activation of mTOR (an enzyme which tightly regulates transcription factor EB) was sufficient to stimulate lysosomal NO production in hepatocytes even in the absence of an inflammatory stimulus; pointing to a central role for these processes.
The researchers found that a NO scavenger diminished lysosomal NO production, while overexpression of both mTOR and a lysomal arginine transporter upregulated lysosomal NO production and suppressed autophagy. In mice with diet-induced obesity, deleting iNOS also improved nitrosative stress in hepatic lysosomes, promoted lysosomal biogenesis by activating transcription factor EB, enhanced lysosomal function and autophagy, and improved hepatic insulin sensitivity. Improved insulin sensitivity diminished, however, when the researchers suppressed transcription factor EB or autophagy-related 7 (Atg7).
Usually, iNOS is primarily expressed in hepatic Kupffer cells, but obesity increases the expression of iNOS in hepatocytes, which promotes hepatic insulin resistance and inflammation, the researchers commented. Unpublished data indicate that deleting iNOS initially protects against obesity-linked fatty liver steatosis and insulin resistance, but that these benefits weaken over time. “Nevertheless, our data showed that liver-specific iNOS suppression has a protective role,” they wrote. “Specifically, we showed that iNOS inactivates transcription factor EB, and that suppression of transcription factor EB and Atg7 diminishes the improved hepatic insulin sensitivity by iNOS deletion.” Transcription factor EB both regulates autophagy and is a “key player in lipid metabolism,” they added. It remains unclear whether the metabolic effects of iNOS solely relate to autophagy, they noted.
Funders included the American Heart Association, American Diabetes Association, and National Institutes of Health. The researchers reported having no conflicts of interest.
SOURCE: Qingwen Qian, et al. Cell Molec Gastroenterol Hepatol. 2019;8(1):95-110.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Obesity promotes the localization of inducible nitric oxide synthase (iNOS) in hepatic lysosomes, leading to excess lysosomal nitric oxide production, reduced hepatic autophagy, and insulin resistance.
Major finding: In mice with diet-induced obesity, deleting iNOS improved nitrosative stress in hepatic lysosomes, promoted lysosomal biogenesis by activating transcription factor EB, enhanced lysosomal function and autophagy, and improved hepatic insulin sensitivity.
Study details: Studies of live primary murine hepatocytes, mice with diet-induced obesity, and liver sections from the mice.
Disclosures: Funders included the American Heart Association, American Diabetes Association, and National Institutes of Health. The researchers reported having no conflicts of interest.
Source: Qingwen Qian et al. Cell Molec Gastroenterol Hepatol. 2019;8(1):95-110.