User login
Can medical scribes improve quality measure documentation?
ABSTRACT
Purpose To avoid disruption of administrative and clinical workflow in an increasingly complex system of health information technology, health care systems and providers have started using medical scribes. The purpose of this study was to investigate the impact of medical scribes on patient satisfaction, physician satisfaction, and quality measure documentation in a family medicine office.
Methods We reviewed 1000 electronic health records for documentation of specified quality measures in the family medicine setting, before and after the use of medical scribes. We surveyed 150 patients on attitude, comfort, and acceptance of medical scribes during their visit. Five physicians shared their perceptions related to productivity, efficiency, and overall job satisfaction on working with medical scribes.
Results Documentation of 4 quality measures improved with the use of scribes, demonstrating statistical significance: fall risk assessment (odds ratio [OR] = 5.5; P = .02), follow-up tobacco screen (OR = 6.4; P = .01), follow-up body mass index plan (OR = 6.2; P < .01), and follow-up blood pressure plan (OR = 39.6; P < .01). Patients reported comfort with scribes in the examination room (96%, n = 144), a more focused health care provider (76%, n = 113), increased efficiency (74%, n = 109), and a higher degree of satisfaction with the office visit (61%, n = 90). Physicians believed they were providing better care and developing better relationships with patients while spending less time documenting and experiencing less stress.
Conclusions Use of medical scribes in a primary care setting was associated with higher patient and physician satisfaction. Patients felt comfortable with a medical scribe in the room, attested to their professionalism, and understood their purpose during the visit. The use of medical scribes in this primary care setting improved documentation of 4 quality measures.
[polldaddy:10339849]
The widespread implementation and adoption of electronic health records (EHRs) continues to increase, primarily motivated by federal incentives through the Centers for Medicare and Medicaid Services to positively impact patient care. Physician use of the EHR in the exam room has the potential to affect the patient-physician relationship, patient satisfaction, physician satisfaction, physician productivity, and physician reimbursement. In the United States, the Health Information Technology for Economic and Clinical Health Act of 2009 established incentive programs to promote meaningful use of EHRs in primary care.1 Integrating EHRs into physician practice, adoption of meaningful use, and the increasing challenge of pay-for-performance quality measures have generated additional hours of administrative work for health care providers. These intrusions on routine clinical care, while hypothesized to improve care, have diminished physician satisfaction, increased stress, and contributed to physician burnout.2
The expanded role of clinicians incentivized to capture metrics for value-based care introduces an unprecedented level of multitasking required at the point of care. In a clinical setting, multitasking undermines the core clinical activities of observation, communication, problem solving, and, ultimately, the development of trusting relationships.3,4 EHR documentation creates a barrier to patient engagement and may contribute to patients feeling isolated when unable to view data being entered.5,6
Potential benefits of scribes. One means of increasing physician satisfaction and productivity may be the integration of medical scribes into health care systems. Medical scribes do not operate independently but are able to document activities or receive dictation critical for patient management—eg, recording patient histories, documenting physical examination findings and procedures, and following up on lab reports.7
Continue to: In a 2015 systematic review...
In a 2015 systematic review, Shultz and Holmstrom found that medical scribes in specialty settings may improve clinician satisfaction, productivity, time-related efficiency, revenue, and patient-clinician interactions.8 The use of scribes in one study increased the number of patients seen and time saved by emergency physicians, thereby increasing physician productivity.9 Studies have also shown that physicians were more satisfied during scribe engagement, related to increased time spent with patients, decreased work-related stress, and increased overall workplace satisfaction.10-12
Studies on the use of medical scribes have mainly focused on physician satisfaction and productivity; however, the data on patient satisfaction are limited. Data about the use of the medical scribe in the primary care setting are also limited. The aim of our research was threefold. We wanted to evaluate the effects of using a medical scribe on: (1) patient satisfaction, (2) documentation of primary care pay-for-performance quality measures, and (3) physicians’ perceptions of the use of scribes in the primary care setting.
METHODS
Data collection
This study was conducted at Family Practice Group in Arlington, Massachusetts, where 5 part-time physicians and 3 full-time physician assistants see approximately 400 patients each week. The representative patient population is approximately 80% privately insured, 10% Medicaid, and 10% Medicare. The EHR system is eClinicalWorks.
The scribes were undergraduate college students who were interested in careers as health care professionals. They had no scribe training or experience working in a medical office. These scribes underwent 4 hours of training in EHR functionality, pay-for-performance quality measures, and risk coding (using appropriate medical codes that capture the patient’s level of medical complexity). The Independent Physician Association affiliated with Family Practice Group provided this training at no cost to the practice. The 3 scribes worked full-time with the 5 part-time physicians in the study. Scribes were not required to have had a medical background prior to entering the program.
After the aforementioned training, scribes began working full-time with physicians during patient visits and continued learning on the job through feedback from supervising physicians. Scribes documented the patient encounters, recording medical and social histories and physical exam findings, and transcribing discussions of treatment plans and physicians’ instructions to patients.
Continue to: We reviewed patient EHRs...
We reviewed patient EHRs of 5 family physicians over 2 time periods: the 3 months prior to having a medical scribe and the 3 months after beginning to work with a medical scribe. Chart data extraction occurred from 4/11/13 to 8/28/14. We reviewed 1000 patient EHRs—100 EHRs each for the 5 participating physicians before and after scribe use. Selected EHRs ran chronologically from the start of each 3-month period. Reviewing EHRs at 3 months after the onset of the medical scribe program allowed time for the scribes to be fully integrated into the practice and confident in their job responsibilities. Chart review was performed by an office administrator who was blinded as to whether documentation had been done with or without a scribe present during the visit.
Eight quality measures were evaluated in chart review. These measures were drawn from the Healthcare Effectiveness Data and Information Set (HEDIS), a tool used to measure performance in medical care and service.
We surveyed 30 patients of each of the 5 providers, yielding a total of 150 survey responses. A medical assistant gave surveys to patients in the exam room following each office visit, to be completed anonymously and privately. Patients were told that surveys would take less than 2 minutes to complete. Office visits included episodic visits, physical exams, and chronic disease management.
After the trial period, we surveyed participating physicians regarding medical scribe assistance with documentation. We also asked the physicians 3 open-ended questions regarding their experiences with their medical scribe.
This study was reviewed and approved (IRB Approval #11424) by the Tufts Health Science Campus Institutional Review Board.
Continue to: Data analysis
Data analysis
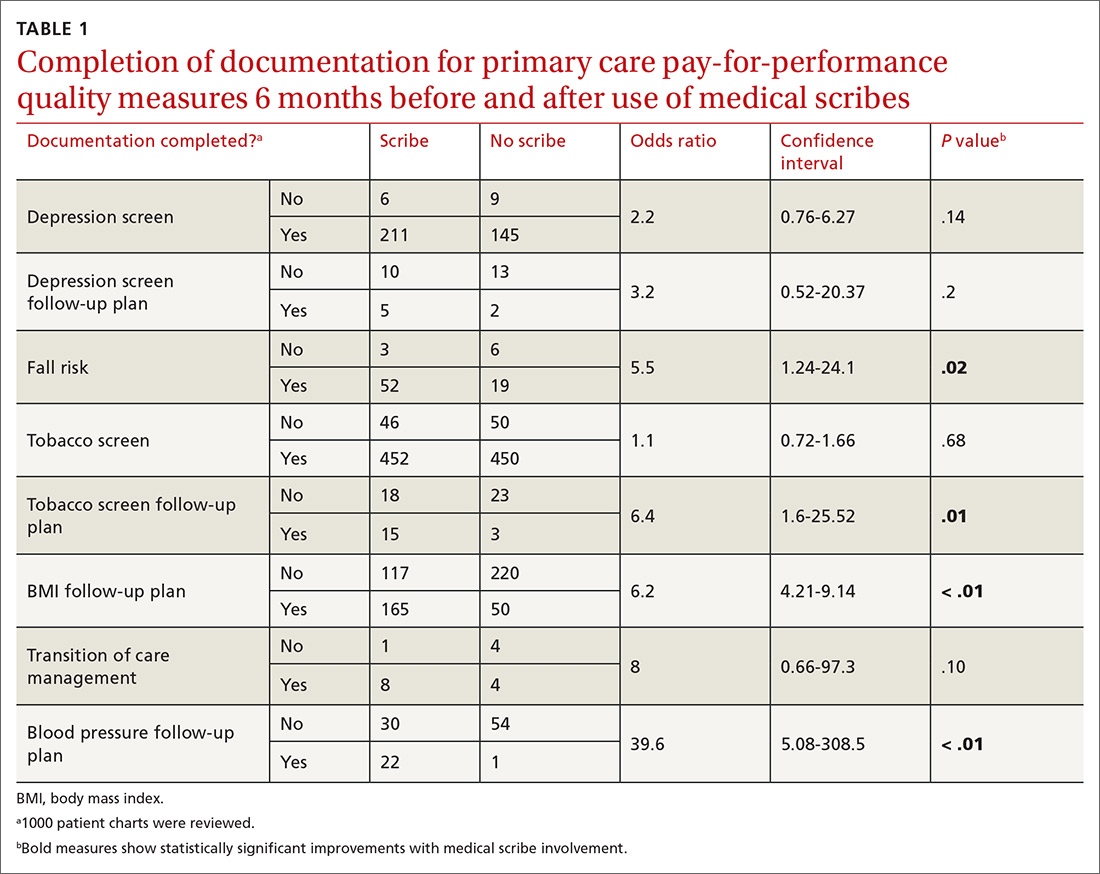
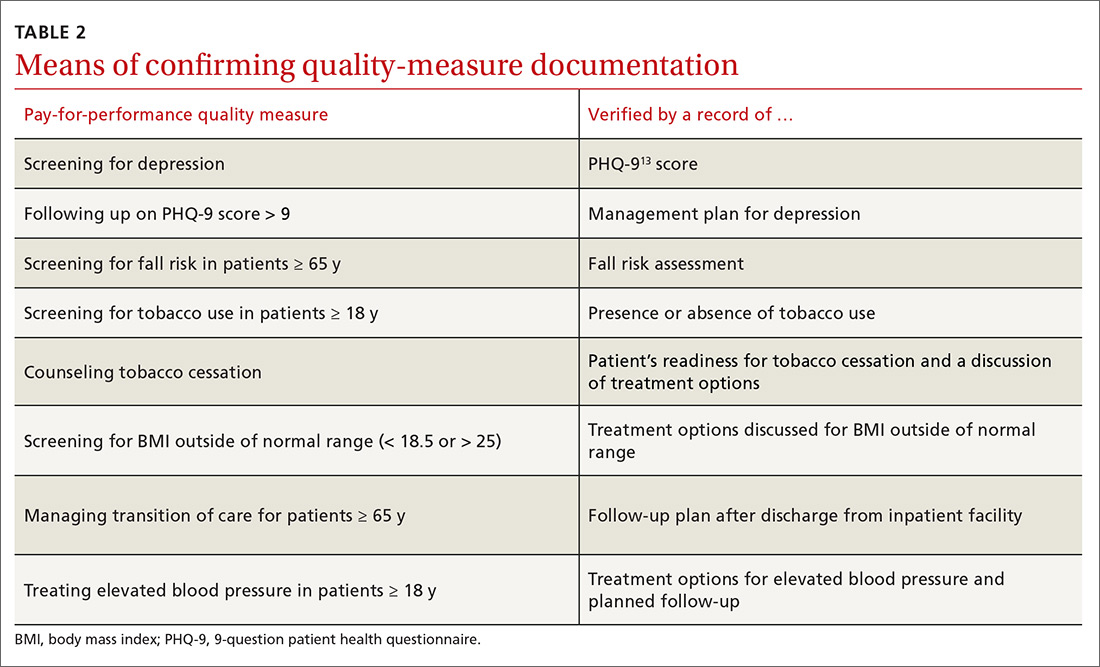
During chart review, we assessed the rate at which documentation was completed for 8 quality outcome measures commonly used in the primary care setting (TABLE 1), before and after the introduction of medical scribes. These quality measures and pertinent descriptors are listed in TABLE 2.13 Presence or absence of documentation on all quality measures was noted for all applicable patients.
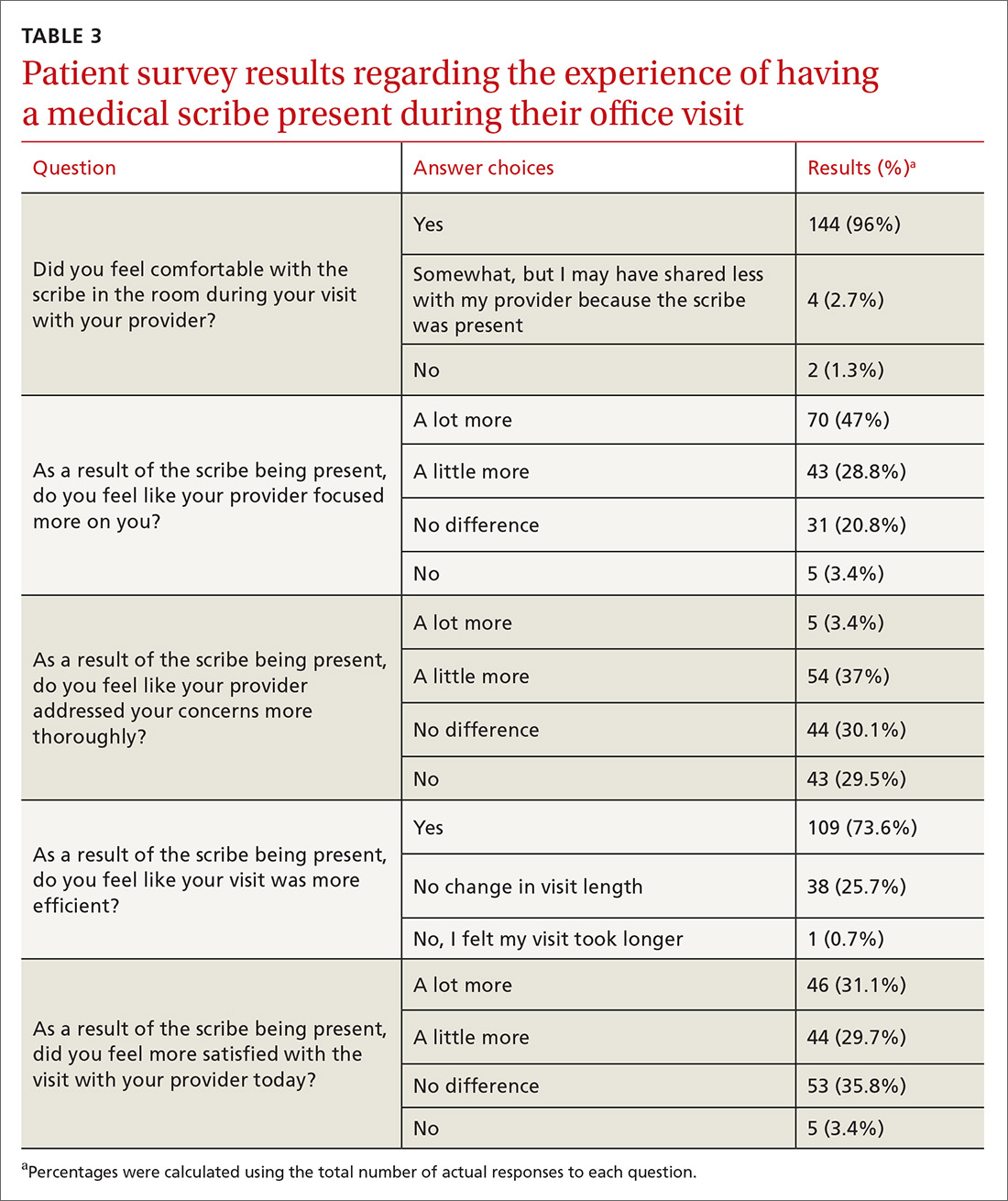
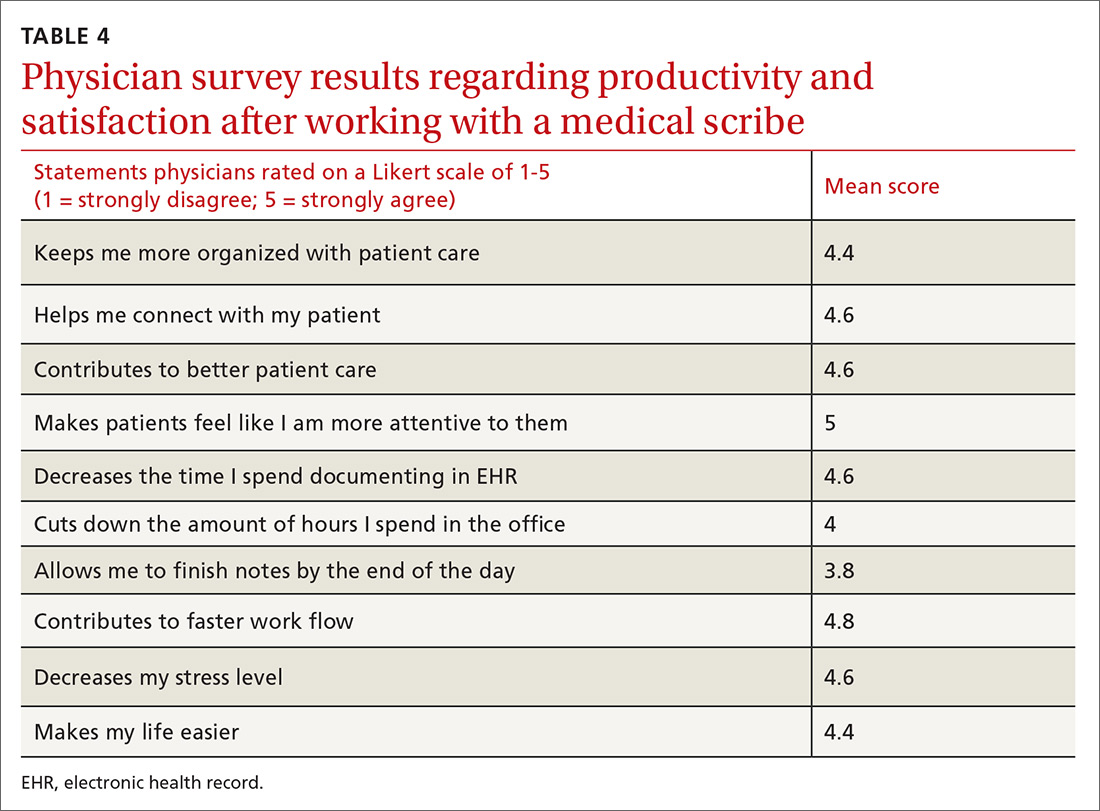
One hundred fifty patients were surveyed immediately after their office visit on their perceptions of medical scribes, including their attitude toward, comfort with, and acceptance of medical scribes (TABLE 3). Five participating physicians were surveyed to assess their perceptions related to productivity and job satisfaction with the use of medical scribes (TABLE 4), and regarding time saved and additional patients seen. Those who collected and analyzed the data from the surveys were blinded to patient and physician identifiers.
Statistical analysis
Using chi-squared tests, we compared the number of positive documentations for the 8 outcome measures before and after the use of medical scribes. Two-sided P values < .05 were considered statistically significant. All statistical analyses were performed with the use of STATA version 9 (StataCorp LP. College Station, Tex).
Physician survey data were calculated on a Likert scale, with a score of 1 corresponding to “strongly disagree,” 2 “disagree,” 3 “neither agree nor disagree,” 4 “agree,” and 5 “strongly agree.” Using the 5 answers generated from the 5 physicians, we calculated the mean for each question.
RESULTS
Continue to: We established at the beginning...
We established at the beginning of the study a target of obtaining surveys from 30 patients of each of the 5 physicians (total of 150). Response rates for surveys were 100% for both the 150 patients and the 5 physicians. No patients declined to complete the survey, although some did not answer every question.
Patients generally had positive experiences with medical scribes (TABLE 3). The majority of patients (96%, n = 144) felt comfortable with the scribe in the room during the visit with their provider. Patients felt that the provider focused on them “a little to a lot more” (75.8%, n = 113) and thought their visit was more efficient (73.6%, n = 109) as a result of the scribe being present vs not being present. Most patients were more satisfied with their office visit with the scribe being present (60.8%, n = 90).
Physicians felt that working with a medical scribe helped them connect with their patients, made patients feel that their physician was more attentive to them, contributed to better patient care, decreased the time they spent documenting in EHR, and contributed to faster work flow (TABLE 4). The physicians also believed they had saved a mean of 1.5 hours each day with the use of a medical scribe, and that they did not have to change their schedule in any way to accommodate additional patients as a result of having a scribe.
DISCUSSION
Documentation of fall risk assessment, follow-up tobacco screening, follow-up BMI plan, and follow-up blood pressure plan all demonstrated statistically significant increases with the use of medical scribes compared with practice before scribes. Follow-up depression screen and transition of care management had relatively high ORs (3.2 and 8, respectively), but did not yield statistically significant values, in part due to small sample sizes as the number of patients who were hospitalized and the number of patients who screened positive for depression were relatively small out of the total group of 1000 patients. The use of scribes had little effect on depression screen and tobacco screen. This is likely due to the fact that there were already effective office systems in place at the practice that alerted medical assistants to complete these screens for each appropriate patient.
We found that the use of medical scribes in a primary care setting was associated with both higher patient and physician satisfaction. Although the 5 physicians in this study chose not to see additional patients when using a medical scribe, they believed they were saving, on average, 1.5 hours of time each day with the use of a scribe. All 5 physicians reported that medical scribes enabled them to provide better patient care and to help patients feel as though they had more of the physician’s attention. Patient respondents attested to their provider focusing on them more during the visit. According to patient surveys, 40.4% of respondents felt that physicians addressed their concerns more thoroughly during the visit, while the remainder of patients did not.
Continue to: Some concerns...
Some concerns of introducing medical scribes into a health care system include possible patient discomfort with a third party being present during the visit and the cost of employing medical scribes. In this study, the vast majority of patients (96%) felt comfortable with a scribe in the room. Future research could compare patient discomfort due to the presence of a medical scribe with patient discomfort due to a physician using a computer during the visit.
Limitations of this study include the small sample size of both physicians and patients; a lack of validated measures for calculating productivity, time/efficiency, and overall satisfaction; and short time periods leading up to and following the introduction of medical scribes. In addition, EHRs of patients were chosen sequentially and not randomly, which could be a confounder. Participating physicians were aware of being studied; therefore, documentation could have been affected by the Hawthorne effect. The study also was limited to one family medicine site. Although improved documentation of primary care pay-for-performance quality measures was reported, wide confidence intervals and small patient numbers hindered generalizability of findings.
Additional studies are needed with a robust analytic plan sufficient to demonstrate baseline provider familiarity with EHRs, accuracy of medical scribe documentation, and improved documentation of pay-for-performance quality measures. Additional investigation regarding the variable competency of different medical scribes could be useful in measuring the effects of the scribe on a variety of outcomes related to both the physician and patient.
It is possible that the improved documentation yielded by the use of medical scribes could generate billing codes that reimburse physicians at a higher level (eg, a higher ratio of 99214 to 99213), leading to increased pay. Future research could aim to quantify this source of increased revenue. Furthermore, investigations could aim to quantify the revenue that medical scribes generate via improved quality measure pay-for-performance documentation.
CORRESPONDENCE
Jessica Platt, MD, 195 Canal Street, Malden, MA 02148; [email protected].
1. Blumenthal D. Wiring the health system—origins and provisions of a new federal program. N Engl J Med. 2011;365:2323-2329.
2. Welp A, Meier LL, Manser T. Emotional exhaustion and workload predict clinician-rated and objective patient safety. Front Psychol. 2015;5:1573.
3. Beasley JW, Wetterneck TB, Temte J, et al. Information chaos in primary care: implications for physician performance and patient safety. J Am Board Fam Med. 2011;24:745-751.
4. Sinsky CA, Beasley JW. Texting while doctoring: a patient safety hazard. Ann Intern Med. 2013;159:782-783.
5. Montague E, Asan O. Dynamic modeling of patient and physician eye gaze to understand the effects of electronic health records on doctor-patient communication and attention. Int J Med Inform. 2014;83:225-234.
6. Asan O, Montague E. Technology-mediated information sharing between patients and clinicians in primary care encounters. Behav Inf Technol. 2014;33:259-270.
7. The Joint Commission. Documentation assistance provided by scribes. https://www.jointcommission.org/standards_information/jcfaqdetails.aspx?StandardsFAQId=1908. Accessed June 4, 2019.
8. Shultz CG, Holmstrom HL. The use of medical scribes in health care settings: a systematic review and future directions. J Am Board Fam Med. 2015;28:371-381.
9. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
10. Conn J. Getting it in writing: Docs using scribes to ease the transition to EHRs. Mod Healthc. 2010;40:30,32.
11. Koshy S, Feustel PJ, Hong M, et al. Scribes in an ambulatory urology practice: patient and physician satisfaction. J Urol. 2010;184:258-262.
12. Allen B, Banapoor B, Weeks E, et al. An assessment of emergency department throughput and provider satisfaction after the implementation of a scribe program. Adv Emerg Med. 2014. https://www.hindawi.com/journals/aem/2014/517319/. Accessed June 4, 2019.
13. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report Version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737-1744.
ABSTRACT
Purpose To avoid disruption of administrative and clinical workflow in an increasingly complex system of health information technology, health care systems and providers have started using medical scribes. The purpose of this study was to investigate the impact of medical scribes on patient satisfaction, physician satisfaction, and quality measure documentation in a family medicine office.
Methods We reviewed 1000 electronic health records for documentation of specified quality measures in the family medicine setting, before and after the use of medical scribes. We surveyed 150 patients on attitude, comfort, and acceptance of medical scribes during their visit. Five physicians shared their perceptions related to productivity, efficiency, and overall job satisfaction on working with medical scribes.
Results Documentation of 4 quality measures improved with the use of scribes, demonstrating statistical significance: fall risk assessment (odds ratio [OR] = 5.5; P = .02), follow-up tobacco screen (OR = 6.4; P = .01), follow-up body mass index plan (OR = 6.2; P < .01), and follow-up blood pressure plan (OR = 39.6; P < .01). Patients reported comfort with scribes in the examination room (96%, n = 144), a more focused health care provider (76%, n = 113), increased efficiency (74%, n = 109), and a higher degree of satisfaction with the office visit (61%, n = 90). Physicians believed they were providing better care and developing better relationships with patients while spending less time documenting and experiencing less stress.
Conclusions Use of medical scribes in a primary care setting was associated with higher patient and physician satisfaction. Patients felt comfortable with a medical scribe in the room, attested to their professionalism, and understood their purpose during the visit. The use of medical scribes in this primary care setting improved documentation of 4 quality measures.
[polldaddy:10339849]
The widespread implementation and adoption of electronic health records (EHRs) continues to increase, primarily motivated by federal incentives through the Centers for Medicare and Medicaid Services to positively impact patient care. Physician use of the EHR in the exam room has the potential to affect the patient-physician relationship, patient satisfaction, physician satisfaction, physician productivity, and physician reimbursement. In the United States, the Health Information Technology for Economic and Clinical Health Act of 2009 established incentive programs to promote meaningful use of EHRs in primary care.1 Integrating EHRs into physician practice, adoption of meaningful use, and the increasing challenge of pay-for-performance quality measures have generated additional hours of administrative work for health care providers. These intrusions on routine clinical care, while hypothesized to improve care, have diminished physician satisfaction, increased stress, and contributed to physician burnout.2
The expanded role of clinicians incentivized to capture metrics for value-based care introduces an unprecedented level of multitasking required at the point of care. In a clinical setting, multitasking undermines the core clinical activities of observation, communication, problem solving, and, ultimately, the development of trusting relationships.3,4 EHR documentation creates a barrier to patient engagement and may contribute to patients feeling isolated when unable to view data being entered.5,6
Potential benefits of scribes. One means of increasing physician satisfaction and productivity may be the integration of medical scribes into health care systems. Medical scribes do not operate independently but are able to document activities or receive dictation critical for patient management—eg, recording patient histories, documenting physical examination findings and procedures, and following up on lab reports.7
Continue to: In a 2015 systematic review...
In a 2015 systematic review, Shultz and Holmstrom found that medical scribes in specialty settings may improve clinician satisfaction, productivity, time-related efficiency, revenue, and patient-clinician interactions.8 The use of scribes in one study increased the number of patients seen and time saved by emergency physicians, thereby increasing physician productivity.9 Studies have also shown that physicians were more satisfied during scribe engagement, related to increased time spent with patients, decreased work-related stress, and increased overall workplace satisfaction.10-12
Studies on the use of medical scribes have mainly focused on physician satisfaction and productivity; however, the data on patient satisfaction are limited. Data about the use of the medical scribe in the primary care setting are also limited. The aim of our research was threefold. We wanted to evaluate the effects of using a medical scribe on: (1) patient satisfaction, (2) documentation of primary care pay-for-performance quality measures, and (3) physicians’ perceptions of the use of scribes in the primary care setting.
METHODS
Data collection
This study was conducted at Family Practice Group in Arlington, Massachusetts, where 5 part-time physicians and 3 full-time physician assistants see approximately 400 patients each week. The representative patient population is approximately 80% privately insured, 10% Medicaid, and 10% Medicare. The EHR system is eClinicalWorks.
The scribes were undergraduate college students who were interested in careers as health care professionals. They had no scribe training or experience working in a medical office. These scribes underwent 4 hours of training in EHR functionality, pay-for-performance quality measures, and risk coding (using appropriate medical codes that capture the patient’s level of medical complexity). The Independent Physician Association affiliated with Family Practice Group provided this training at no cost to the practice. The 3 scribes worked full-time with the 5 part-time physicians in the study. Scribes were not required to have had a medical background prior to entering the program.
After the aforementioned training, scribes began working full-time with physicians during patient visits and continued learning on the job through feedback from supervising physicians. Scribes documented the patient encounters, recording medical and social histories and physical exam findings, and transcribing discussions of treatment plans and physicians’ instructions to patients.
Continue to: We reviewed patient EHRs...
We reviewed patient EHRs of 5 family physicians over 2 time periods: the 3 months prior to having a medical scribe and the 3 months after beginning to work with a medical scribe. Chart data extraction occurred from 4/11/13 to 8/28/14. We reviewed 1000 patient EHRs—100 EHRs each for the 5 participating physicians before and after scribe use. Selected EHRs ran chronologically from the start of each 3-month period. Reviewing EHRs at 3 months after the onset of the medical scribe program allowed time for the scribes to be fully integrated into the practice and confident in their job responsibilities. Chart review was performed by an office administrator who was blinded as to whether documentation had been done with or without a scribe present during the visit.
Eight quality measures were evaluated in chart review. These measures were drawn from the Healthcare Effectiveness Data and Information Set (HEDIS), a tool used to measure performance in medical care and service.
We surveyed 30 patients of each of the 5 providers, yielding a total of 150 survey responses. A medical assistant gave surveys to patients in the exam room following each office visit, to be completed anonymously and privately. Patients were told that surveys would take less than 2 minutes to complete. Office visits included episodic visits, physical exams, and chronic disease management.
After the trial period, we surveyed participating physicians regarding medical scribe assistance with documentation. We also asked the physicians 3 open-ended questions regarding their experiences with their medical scribe.
This study was reviewed and approved (IRB Approval #11424) by the Tufts Health Science Campus Institutional Review Board.
Continue to: Data analysis
Data analysis
During chart review, we assessed the rate at which documentation was completed for 8 quality outcome measures commonly used in the primary care setting (TABLE 1), before and after the introduction of medical scribes. These quality measures and pertinent descriptors are listed in TABLE 2.13 Presence or absence of documentation on all quality measures was noted for all applicable patients.

One hundred fifty patients were surveyed immediately after their office visit on their perceptions of medical scribes, including their attitude toward, comfort with, and acceptance of medical scribes (TABLE 3). Five participating physicians were surveyed to assess their perceptions related to productivity and job satisfaction with the use of medical scribes (TABLE 4), and regarding time saved and additional patients seen. Those who collected and analyzed the data from the surveys were blinded to patient and physician identifiers.

Statistical analysis
Using chi-squared tests, we compared the number of positive documentations for the 8 outcome measures before and after the use of medical scribes. Two-sided P values < .05 were considered statistically significant. All statistical analyses were performed with the use of STATA version 9 (StataCorp LP. College Station, Tex).

Physician survey data were calculated on a Likert scale, with a score of 1 corresponding to “strongly disagree,” 2 “disagree,” 3 “neither agree nor disagree,” 4 “agree,” and 5 “strongly agree.” Using the 5 answers generated from the 5 physicians, we calculated the mean for each question.

RESULTS
Continue to: We established at the beginning...
We established at the beginning of the study a target of obtaining surveys from 30 patients of each of the 5 physicians (total of 150). Response rates for surveys were 100% for both the 150 patients and the 5 physicians. No patients declined to complete the survey, although some did not answer every question.
Patients generally had positive experiences with medical scribes (TABLE 3). The majority of patients (96%, n = 144) felt comfortable with the scribe in the room during the visit with their provider. Patients felt that the provider focused on them “a little to a lot more” (75.8%, n = 113) and thought their visit was more efficient (73.6%, n = 109) as a result of the scribe being present vs not being present. Most patients were more satisfied with their office visit with the scribe being present (60.8%, n = 90).
Physicians felt that working with a medical scribe helped them connect with their patients, made patients feel that their physician was more attentive to them, contributed to better patient care, decreased the time they spent documenting in EHR, and contributed to faster work flow (TABLE 4). The physicians also believed they had saved a mean of 1.5 hours each day with the use of a medical scribe, and that they did not have to change their schedule in any way to accommodate additional patients as a result of having a scribe.
DISCUSSION
Documentation of fall risk assessment, follow-up tobacco screening, follow-up BMI plan, and follow-up blood pressure plan all demonstrated statistically significant increases with the use of medical scribes compared with practice before scribes. Follow-up depression screen and transition of care management had relatively high ORs (3.2 and 8, respectively), but did not yield statistically significant values, in part due to small sample sizes as the number of patients who were hospitalized and the number of patients who screened positive for depression were relatively small out of the total group of 1000 patients. The use of scribes had little effect on depression screen and tobacco screen. This is likely due to the fact that there were already effective office systems in place at the practice that alerted medical assistants to complete these screens for each appropriate patient.
We found that the use of medical scribes in a primary care setting was associated with both higher patient and physician satisfaction. Although the 5 physicians in this study chose not to see additional patients when using a medical scribe, they believed they were saving, on average, 1.5 hours of time each day with the use of a scribe. All 5 physicians reported that medical scribes enabled them to provide better patient care and to help patients feel as though they had more of the physician’s attention. Patient respondents attested to their provider focusing on them more during the visit. According to patient surveys, 40.4% of respondents felt that physicians addressed their concerns more thoroughly during the visit, while the remainder of patients did not.
Continue to: Some concerns...
Some concerns of introducing medical scribes into a health care system include possible patient discomfort with a third party being present during the visit and the cost of employing medical scribes. In this study, the vast majority of patients (96%) felt comfortable with a scribe in the room. Future research could compare patient discomfort due to the presence of a medical scribe with patient discomfort due to a physician using a computer during the visit.
Limitations of this study include the small sample size of both physicians and patients; a lack of validated measures for calculating productivity, time/efficiency, and overall satisfaction; and short time periods leading up to and following the introduction of medical scribes. In addition, EHRs of patients were chosen sequentially and not randomly, which could be a confounder. Participating physicians were aware of being studied; therefore, documentation could have been affected by the Hawthorne effect. The study also was limited to one family medicine site. Although improved documentation of primary care pay-for-performance quality measures was reported, wide confidence intervals and small patient numbers hindered generalizability of findings.
Additional studies are needed with a robust analytic plan sufficient to demonstrate baseline provider familiarity with EHRs, accuracy of medical scribe documentation, and improved documentation of pay-for-performance quality measures. Additional investigation regarding the variable competency of different medical scribes could be useful in measuring the effects of the scribe on a variety of outcomes related to both the physician and patient.
It is possible that the improved documentation yielded by the use of medical scribes could generate billing codes that reimburse physicians at a higher level (eg, a higher ratio of 99214 to 99213), leading to increased pay. Future research could aim to quantify this source of increased revenue. Furthermore, investigations could aim to quantify the revenue that medical scribes generate via improved quality measure pay-for-performance documentation.
CORRESPONDENCE
Jessica Platt, MD, 195 Canal Street, Malden, MA 02148; [email protected].
ABSTRACT
Purpose To avoid disruption of administrative and clinical workflow in an increasingly complex system of health information technology, health care systems and providers have started using medical scribes. The purpose of this study was to investigate the impact of medical scribes on patient satisfaction, physician satisfaction, and quality measure documentation in a family medicine office.
Methods We reviewed 1000 electronic health records for documentation of specified quality measures in the family medicine setting, before and after the use of medical scribes. We surveyed 150 patients on attitude, comfort, and acceptance of medical scribes during their visit. Five physicians shared their perceptions related to productivity, efficiency, and overall job satisfaction on working with medical scribes.
Results Documentation of 4 quality measures improved with the use of scribes, demonstrating statistical significance: fall risk assessment (odds ratio [OR] = 5.5; P = .02), follow-up tobacco screen (OR = 6.4; P = .01), follow-up body mass index plan (OR = 6.2; P < .01), and follow-up blood pressure plan (OR = 39.6; P < .01). Patients reported comfort with scribes in the examination room (96%, n = 144), a more focused health care provider (76%, n = 113), increased efficiency (74%, n = 109), and a higher degree of satisfaction with the office visit (61%, n = 90). Physicians believed they were providing better care and developing better relationships with patients while spending less time documenting and experiencing less stress.
Conclusions Use of medical scribes in a primary care setting was associated with higher patient and physician satisfaction. Patients felt comfortable with a medical scribe in the room, attested to their professionalism, and understood their purpose during the visit. The use of medical scribes in this primary care setting improved documentation of 4 quality measures.
[polldaddy:10339849]
The widespread implementation and adoption of electronic health records (EHRs) continues to increase, primarily motivated by federal incentives through the Centers for Medicare and Medicaid Services to positively impact patient care. Physician use of the EHR in the exam room has the potential to affect the patient-physician relationship, patient satisfaction, physician satisfaction, physician productivity, and physician reimbursement. In the United States, the Health Information Technology for Economic and Clinical Health Act of 2009 established incentive programs to promote meaningful use of EHRs in primary care.1 Integrating EHRs into physician practice, adoption of meaningful use, and the increasing challenge of pay-for-performance quality measures have generated additional hours of administrative work for health care providers. These intrusions on routine clinical care, while hypothesized to improve care, have diminished physician satisfaction, increased stress, and contributed to physician burnout.2
The expanded role of clinicians incentivized to capture metrics for value-based care introduces an unprecedented level of multitasking required at the point of care. In a clinical setting, multitasking undermines the core clinical activities of observation, communication, problem solving, and, ultimately, the development of trusting relationships.3,4 EHR documentation creates a barrier to patient engagement and may contribute to patients feeling isolated when unable to view data being entered.5,6
Potential benefits of scribes. One means of increasing physician satisfaction and productivity may be the integration of medical scribes into health care systems. Medical scribes do not operate independently but are able to document activities or receive dictation critical for patient management—eg, recording patient histories, documenting physical examination findings and procedures, and following up on lab reports.7
Continue to: In a 2015 systematic review...
In a 2015 systematic review, Shultz and Holmstrom found that medical scribes in specialty settings may improve clinician satisfaction, productivity, time-related efficiency, revenue, and patient-clinician interactions.8 The use of scribes in one study increased the number of patients seen and time saved by emergency physicians, thereby increasing physician productivity.9 Studies have also shown that physicians were more satisfied during scribe engagement, related to increased time spent with patients, decreased work-related stress, and increased overall workplace satisfaction.10-12
Studies on the use of medical scribes have mainly focused on physician satisfaction and productivity; however, the data on patient satisfaction are limited. Data about the use of the medical scribe in the primary care setting are also limited. The aim of our research was threefold. We wanted to evaluate the effects of using a medical scribe on: (1) patient satisfaction, (2) documentation of primary care pay-for-performance quality measures, and (3) physicians’ perceptions of the use of scribes in the primary care setting.
METHODS
Data collection
This study was conducted at Family Practice Group in Arlington, Massachusetts, where 5 part-time physicians and 3 full-time physician assistants see approximately 400 patients each week. The representative patient population is approximately 80% privately insured, 10% Medicaid, and 10% Medicare. The EHR system is eClinicalWorks.
The scribes were undergraduate college students who were interested in careers as health care professionals. They had no scribe training or experience working in a medical office. These scribes underwent 4 hours of training in EHR functionality, pay-for-performance quality measures, and risk coding (using appropriate medical codes that capture the patient’s level of medical complexity). The Independent Physician Association affiliated with Family Practice Group provided this training at no cost to the practice. The 3 scribes worked full-time with the 5 part-time physicians in the study. Scribes were not required to have had a medical background prior to entering the program.
After the aforementioned training, scribes began working full-time with physicians during patient visits and continued learning on the job through feedback from supervising physicians. Scribes documented the patient encounters, recording medical and social histories and physical exam findings, and transcribing discussions of treatment plans and physicians’ instructions to patients.
Continue to: We reviewed patient EHRs...
We reviewed patient EHRs of 5 family physicians over 2 time periods: the 3 months prior to having a medical scribe and the 3 months after beginning to work with a medical scribe. Chart data extraction occurred from 4/11/13 to 8/28/14. We reviewed 1000 patient EHRs—100 EHRs each for the 5 participating physicians before and after scribe use. Selected EHRs ran chronologically from the start of each 3-month period. Reviewing EHRs at 3 months after the onset of the medical scribe program allowed time for the scribes to be fully integrated into the practice and confident in their job responsibilities. Chart review was performed by an office administrator who was blinded as to whether documentation had been done with or without a scribe present during the visit.
Eight quality measures were evaluated in chart review. These measures were drawn from the Healthcare Effectiveness Data and Information Set (HEDIS), a tool used to measure performance in medical care and service.
We surveyed 30 patients of each of the 5 providers, yielding a total of 150 survey responses. A medical assistant gave surveys to patients in the exam room following each office visit, to be completed anonymously and privately. Patients were told that surveys would take less than 2 minutes to complete. Office visits included episodic visits, physical exams, and chronic disease management.
After the trial period, we surveyed participating physicians regarding medical scribe assistance with documentation. We also asked the physicians 3 open-ended questions regarding their experiences with their medical scribe.
This study was reviewed and approved (IRB Approval #11424) by the Tufts Health Science Campus Institutional Review Board.
Continue to: Data analysis
Data analysis
During chart review, we assessed the rate at which documentation was completed for 8 quality outcome measures commonly used in the primary care setting (TABLE 1), before and after the introduction of medical scribes. These quality measures and pertinent descriptors are listed in TABLE 2.13 Presence or absence of documentation on all quality measures was noted for all applicable patients.

One hundred fifty patients were surveyed immediately after their office visit on their perceptions of medical scribes, including their attitude toward, comfort with, and acceptance of medical scribes (TABLE 3). Five participating physicians were surveyed to assess their perceptions related to productivity and job satisfaction with the use of medical scribes (TABLE 4), and regarding time saved and additional patients seen. Those who collected and analyzed the data from the surveys were blinded to patient and physician identifiers.

Statistical analysis
Using chi-squared tests, we compared the number of positive documentations for the 8 outcome measures before and after the use of medical scribes. Two-sided P values < .05 were considered statistically significant. All statistical analyses were performed with the use of STATA version 9 (StataCorp LP. College Station, Tex).

Physician survey data were calculated on a Likert scale, with a score of 1 corresponding to “strongly disagree,” 2 “disagree,” 3 “neither agree nor disagree,” 4 “agree,” and 5 “strongly agree.” Using the 5 answers generated from the 5 physicians, we calculated the mean for each question.

RESULTS
Continue to: We established at the beginning...
We established at the beginning of the study a target of obtaining surveys from 30 patients of each of the 5 physicians (total of 150). Response rates for surveys were 100% for both the 150 patients and the 5 physicians. No patients declined to complete the survey, although some did not answer every question.
Patients generally had positive experiences with medical scribes (TABLE 3). The majority of patients (96%, n = 144) felt comfortable with the scribe in the room during the visit with their provider. Patients felt that the provider focused on them “a little to a lot more” (75.8%, n = 113) and thought their visit was more efficient (73.6%, n = 109) as a result of the scribe being present vs not being present. Most patients were more satisfied with their office visit with the scribe being present (60.8%, n = 90).
Physicians felt that working with a medical scribe helped them connect with their patients, made patients feel that their physician was more attentive to them, contributed to better patient care, decreased the time they spent documenting in EHR, and contributed to faster work flow (TABLE 4). The physicians also believed they had saved a mean of 1.5 hours each day with the use of a medical scribe, and that they did not have to change their schedule in any way to accommodate additional patients as a result of having a scribe.
DISCUSSION
Documentation of fall risk assessment, follow-up tobacco screening, follow-up BMI plan, and follow-up blood pressure plan all demonstrated statistically significant increases with the use of medical scribes compared with practice before scribes. Follow-up depression screen and transition of care management had relatively high ORs (3.2 and 8, respectively), but did not yield statistically significant values, in part due to small sample sizes as the number of patients who were hospitalized and the number of patients who screened positive for depression were relatively small out of the total group of 1000 patients. The use of scribes had little effect on depression screen and tobacco screen. This is likely due to the fact that there were already effective office systems in place at the practice that alerted medical assistants to complete these screens for each appropriate patient.
We found that the use of medical scribes in a primary care setting was associated with both higher patient and physician satisfaction. Although the 5 physicians in this study chose not to see additional patients when using a medical scribe, they believed they were saving, on average, 1.5 hours of time each day with the use of a scribe. All 5 physicians reported that medical scribes enabled them to provide better patient care and to help patients feel as though they had more of the physician’s attention. Patient respondents attested to their provider focusing on them more during the visit. According to patient surveys, 40.4% of respondents felt that physicians addressed their concerns more thoroughly during the visit, while the remainder of patients did not.
Continue to: Some concerns...
Some concerns of introducing medical scribes into a health care system include possible patient discomfort with a third party being present during the visit and the cost of employing medical scribes. In this study, the vast majority of patients (96%) felt comfortable with a scribe in the room. Future research could compare patient discomfort due to the presence of a medical scribe with patient discomfort due to a physician using a computer during the visit.
Limitations of this study include the small sample size of both physicians and patients; a lack of validated measures for calculating productivity, time/efficiency, and overall satisfaction; and short time periods leading up to and following the introduction of medical scribes. In addition, EHRs of patients were chosen sequentially and not randomly, which could be a confounder. Participating physicians were aware of being studied; therefore, documentation could have been affected by the Hawthorne effect. The study also was limited to one family medicine site. Although improved documentation of primary care pay-for-performance quality measures was reported, wide confidence intervals and small patient numbers hindered generalizability of findings.
Additional studies are needed with a robust analytic plan sufficient to demonstrate baseline provider familiarity with EHRs, accuracy of medical scribe documentation, and improved documentation of pay-for-performance quality measures. Additional investigation regarding the variable competency of different medical scribes could be useful in measuring the effects of the scribe on a variety of outcomes related to both the physician and patient.
It is possible that the improved documentation yielded by the use of medical scribes could generate billing codes that reimburse physicians at a higher level (eg, a higher ratio of 99214 to 99213), leading to increased pay. Future research could aim to quantify this source of increased revenue. Furthermore, investigations could aim to quantify the revenue that medical scribes generate via improved quality measure pay-for-performance documentation.
CORRESPONDENCE
Jessica Platt, MD, 195 Canal Street, Malden, MA 02148; [email protected].
1. Blumenthal D. Wiring the health system—origins and provisions of a new federal program. N Engl J Med. 2011;365:2323-2329.
2. Welp A, Meier LL, Manser T. Emotional exhaustion and workload predict clinician-rated and objective patient safety. Front Psychol. 2015;5:1573.
3. Beasley JW, Wetterneck TB, Temte J, et al. Information chaos in primary care: implications for physician performance and patient safety. J Am Board Fam Med. 2011;24:745-751.
4. Sinsky CA, Beasley JW. Texting while doctoring: a patient safety hazard. Ann Intern Med. 2013;159:782-783.
5. Montague E, Asan O. Dynamic modeling of patient and physician eye gaze to understand the effects of electronic health records on doctor-patient communication and attention. Int J Med Inform. 2014;83:225-234.
6. Asan O, Montague E. Technology-mediated information sharing between patients and clinicians in primary care encounters. Behav Inf Technol. 2014;33:259-270.
7. The Joint Commission. Documentation assistance provided by scribes. https://www.jointcommission.org/standards_information/jcfaqdetails.aspx?StandardsFAQId=1908. Accessed June 4, 2019.
8. Shultz CG, Holmstrom HL. The use of medical scribes in health care settings: a systematic review and future directions. J Am Board Fam Med. 2015;28:371-381.
9. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
10. Conn J. Getting it in writing: Docs using scribes to ease the transition to EHRs. Mod Healthc. 2010;40:30,32.
11. Koshy S, Feustel PJ, Hong M, et al. Scribes in an ambulatory urology practice: patient and physician satisfaction. J Urol. 2010;184:258-262.
12. Allen B, Banapoor B, Weeks E, et al. An assessment of emergency department throughput and provider satisfaction after the implementation of a scribe program. Adv Emerg Med. 2014. https://www.hindawi.com/journals/aem/2014/517319/. Accessed June 4, 2019.
13. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report Version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737-1744.
1. Blumenthal D. Wiring the health system—origins and provisions of a new federal program. N Engl J Med. 2011;365:2323-2329.
2. Welp A, Meier LL, Manser T. Emotional exhaustion and workload predict clinician-rated and objective patient safety. Front Psychol. 2015;5:1573.
3. Beasley JW, Wetterneck TB, Temte J, et al. Information chaos in primary care: implications for physician performance and patient safety. J Am Board Fam Med. 2011;24:745-751.
4. Sinsky CA, Beasley JW. Texting while doctoring: a patient safety hazard. Ann Intern Med. 2013;159:782-783.
5. Montague E, Asan O. Dynamic modeling of patient and physician eye gaze to understand the effects of electronic health records on doctor-patient communication and attention. Int J Med Inform. 2014;83:225-234.
6. Asan O, Montague E. Technology-mediated information sharing between patients and clinicians in primary care encounters. Behav Inf Technol. 2014;33:259-270.
7. The Joint Commission. Documentation assistance provided by scribes. https://www.jointcommission.org/standards_information/jcfaqdetails.aspx?StandardsFAQId=1908. Accessed June 4, 2019.
8. Shultz CG, Holmstrom HL. The use of medical scribes in health care settings: a systematic review and future directions. J Am Board Fam Med. 2015;28:371-381.
9. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
10. Conn J. Getting it in writing: Docs using scribes to ease the transition to EHRs. Mod Healthc. 2010;40:30,32.
11. Koshy S, Feustel PJ, Hong M, et al. Scribes in an ambulatory urology practice: patient and physician satisfaction. J Urol. 2010;184:258-262.
12. Allen B, Banapoor B, Weeks E, et al. An assessment of emergency department throughput and provider satisfaction after the implementation of a scribe program. Adv Emerg Med. 2014. https://www.hindawi.com/journals/aem/2014/517319/. Accessed June 4, 2019.
13. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report Version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737-1744.
How to incorporate HIV PrEP into your practice
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
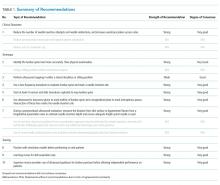
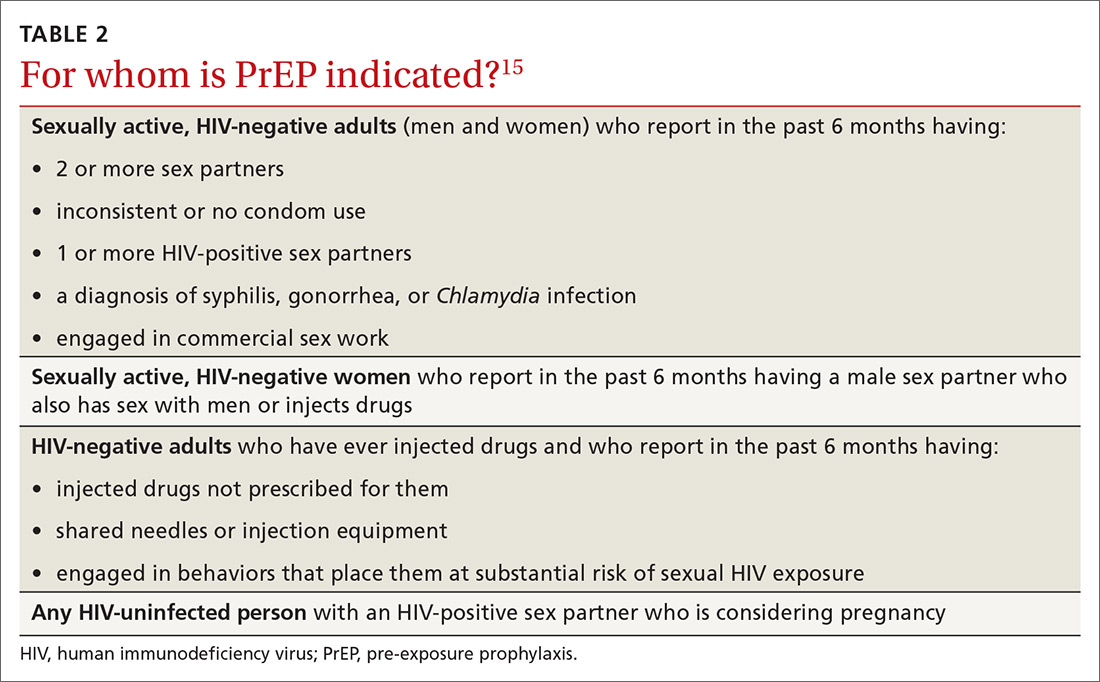
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).
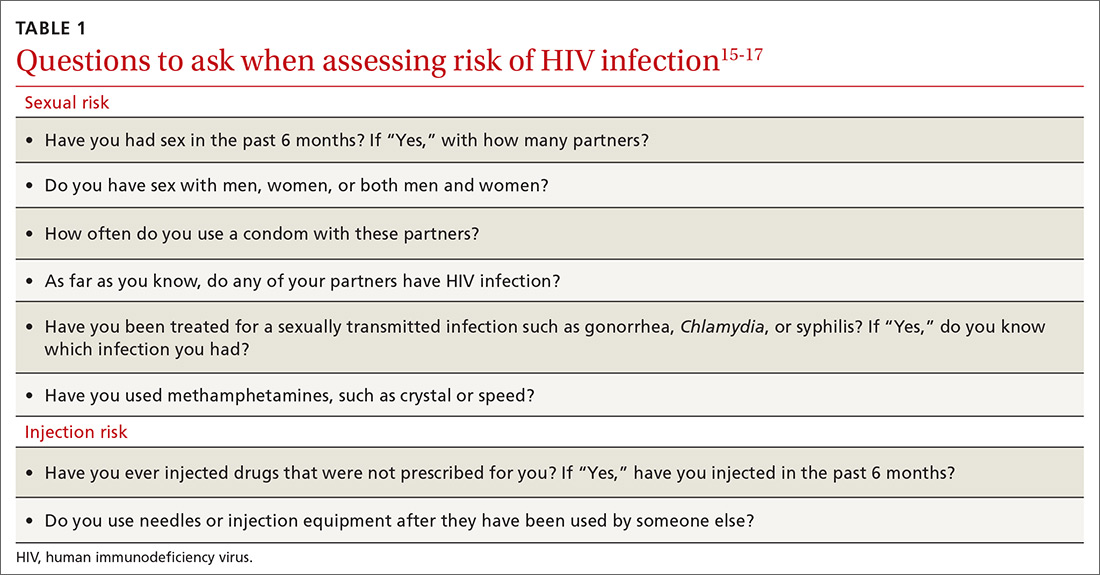
Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15
What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
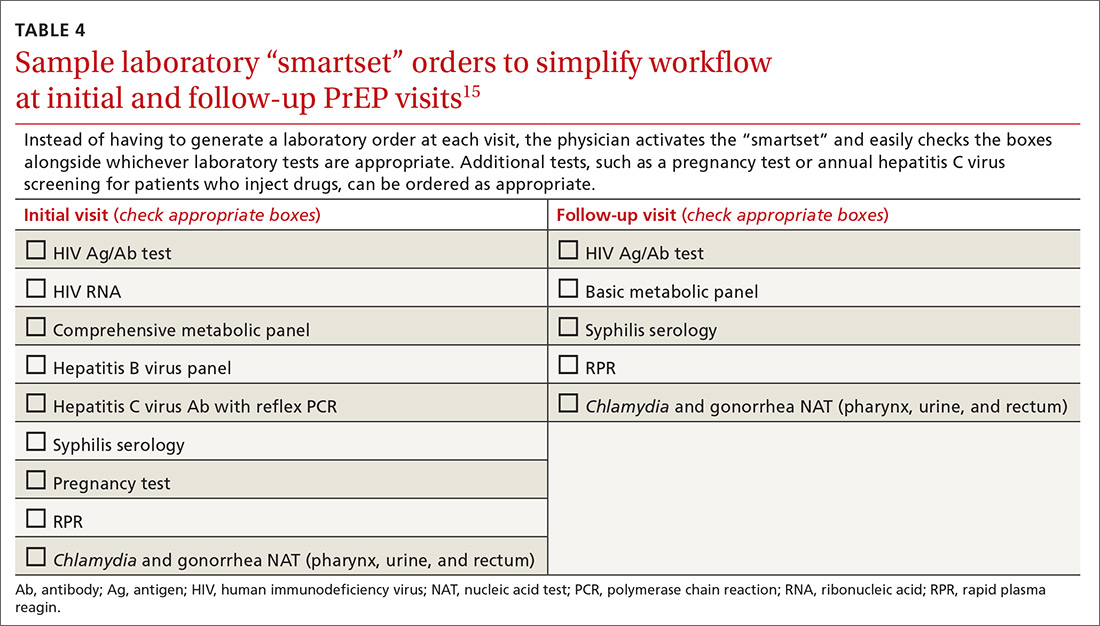
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.
Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
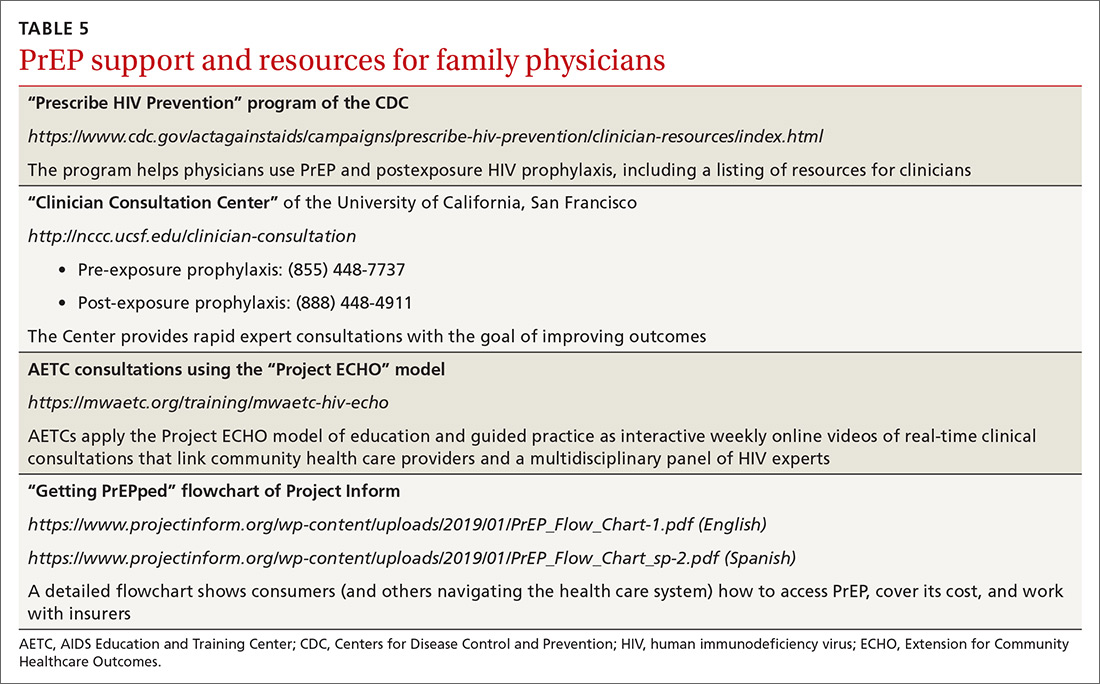
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.
Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).

Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15

What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.

Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.

Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).

Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15

What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.

Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.

Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
PRACTICE RECOMMENDATIONS
› Actively screen and identify HIV-negative patients who are a candidate for pre-exposure prophylaxis (PrEP); commit to talking to the most easily identifiable subsets of these patients, such as men who have sex with men and transgender patients. B
› Recognize that PrEP is indicated for patients who: are sexually active with inconsistent condom use and multiple recent sex partners; have recently been given a diagnosis of a sexually transmitted infection; or have a sexual or injection partner known to be HIV-infected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Allergy immunotherapy: Who, what, when … and how safe?
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).
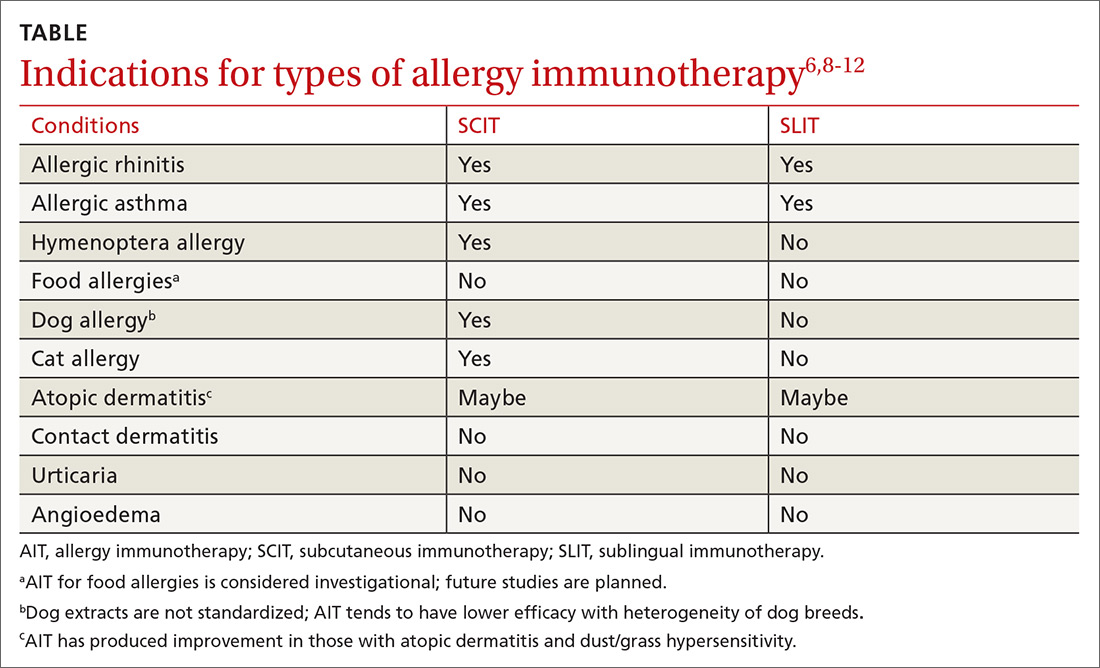
AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).

AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
The prevalence of allergic disease in the general population is quite high; 8.3% of adults and children have asthma and 11.4% of children have skin allergies.1 Food allergies are present in 8% of children and 5% of adults,2 and up to 10% of anaphylactic reactions in the United States are due to stinging insects.3
Moderate-to-severe food and environmental allergies can negatively affect multiple organ systems and significantly impact morbidity and mortality.4 Quality of life and the financial well-being of patients with allergic diseases, as well as that of their families, can also be significantly impacted by these conditions.4,5 High prevalence and burden of disease mandate that family physicians (FPs) stay up-to-date on the full array of treatment options for allergic diseases. What follows are 6 common questions about allergy immunotherapy (AIT) and the evidence-based answers that will help you to identify and treat appropriate candidates, as well as educate them along the way.

Who is a candidate for AIT?
Patients with moderate-to-severe immunoglobulin (Ig)E-mediated allergies whose symptoms are not adequately controlled by medications and allergen trigger avoidance are candidates for AIT.6-8 Skin prick/puncture testing provides the most reliable and cost-effective confirmation of allergies that are suspected, based on patient history and clinical assessment for allergic symptoms.9 Life-threatening reactions to skin prick/puncture testing are rare.9 While in vitro (laboratory) testing for IgE levels to specific antigens may be more convenient for patients and less invasive than skin prick/puncture testing, it is also less sensitive and less reliable at quantifying the severity of sensitization.9
What constitutes AIT?
AIT is a disease-modifying treatment that, along with allergen avoidance, can provide long-term remission of allergic disease in certain circumstances.6,7 Consistent gradual exposure to an allergen helps to dampen the inflammatory reaction driven by T cells and B cells, producing clinical tolerance or desensitization that persists after the discontinuation of AIT.8 While subcutaneous immunotherapy (SCIT) is the most widely known type of AIT (ie, allergy shots), there are additional ways that AIT can be administered. These include sublingual immunotherapy (SLIT), venom immunotherapy (VIT), and oral immunotherapy (OIT). The selection of the route of administration depends on the exact nature and symptoms of the allergic condition being treated (TABLE6,8-12).

AIT involves 2 phases
The first phase is the induction or buildup phase during which patients are given gradually increasing amounts of allergen to induce a protective immunologic response.6 After 8 to 28 weeks, the maintenance phase begins, during which continued, consistent allergen exposure is designed to prevent relapse of, and facilitate continued remission of, allergy symptoms.6 The maintenance phase of AIT can last 24 to 48 months.6,10 Certain patients may qualify for an expedited AIT regimen called cluster or rush immunotherapy.6
Conventional schedules for AIT involve increasing the dose of allergen given at each visit (1-3 doses/wk), whereas rush dosing involves multiple, increasing doses given in a single extended visit to reach therapeutic desensitization faster.6 AIT has been shown to produce a 2.7- to 13.7-fold overall improvement in hypersensitivity reactions.10
Length of therapy must be individualized
Experts recommend that the length of treatment with AIT be customized for each patient based on the severity of pretreatment allergy symptoms, the benefit experienced with AIT, the inconvenience of AIT to the patient, and the anticipated impact of symptom relapse.6,10 There are no physiologic symptoms or objective tests that predict which patients will remain in remission after discontinuing AIT; thus, a joint task force of allergy experts suggests that the decision to restart AIT in patients who have a relapse in allergic symptoms should be made based on the same factors used to determine the duration of the maintenance phase.6
Continue to: These allergans are appropriate for AIT
These allergens are appropriate for AIT
Allergens may be described in terms of mechanism and chronicity of exposure. While avoidance of offending allergens is recommended for those who are sensitized, avoidance is not always possible.6,7,9,13 AIT has been studied as a therapeutic modality to prevent exposure-related symptoms associated with each of the following types of allergens.6,7,9,11,14
Inhalant allergens circulate in disturbed and undisturbed air and may be seasonal (eg, pollen), perennial (eg, cat/dog allergens), and/or occupational.9 They can derive from the indoors (eg, cockroach, cat, dog, dust mite) or outdoors (eg, tree, grass, or weed pollen ),6,7,9,11 and serve as triggers for many allergic diseases such as allergic rhinitis (AR), allergic rhinoconjunctivitis, allergic dermatitis, and asthma.7,13
Food allergens. Sensitization to food allergens may produce a range of symptoms.6,7 One person may experience nothing more than tingling of the lips when eating a peach, while another may experience throat tightness and anaphylaxis due to the aroma of shellfish cooking.
Occupational allergens. Exposure to occupational allergens varies depending on the setting. Those who work in health care or with animals can be exposed to allergens (eg, latex and animal proteins, respectively) that can cause skin or respiratory hypersensitivity reactions. Occupational allergens can also include chemicals; workers in agriculture or housekeeping may be particularly at risk.
Insect allergens. Envenomation by stinging insects of the order Hymenoptera (bees, yellow jackets, hornets, wasps, fire ants) most commonly causes a pruritic, painful local reaction, but patients sensitized to Hymenoptera venom experience systemic allergic reactions that range from mild to life-threatening.3,6,7
Continue to: When should you use AIT?
When should you use AIT?
Allergic rhinitis (AR). AR can be triggered by exposure to indoor or outdoor inhalant allergens. Research has shown AIT to be an effective treatment for AR and the conjunctivitis caused by inhaled environmental allergens.15-17 AIT results in improved symptom control and decreased use of rescue medication (standardized mean difference [SMD] -0.32; 95% confidence interval [CI], -0.23 to -0.33, favoring AIT intervention) in patients with seasonal or perennial AR.15-17
SCIT effectiveness has been demonstrated in sensitized patients who have symptoms associated with pollen, animal allergens, dust mites, and mold/fungi,15,16 and SCIT may be effective for the treatment of symptoms associated with cockroach exposure.11 SLIT is approved by the US Food and Drug Administration (FDA) for the treatment of several pollen allergens with efficacy rates similar to those of SCIT and with no significant difference in adverse events (AEs).8,15,16 Direct comparison studies of SCIT and SLIT preparations for treating grass allergy, while of low quality, showed comparable reductions in allergic rhinoconjunctival symptoms.15
Asthma. AIT (SCIT and SLIT) has been shown to be effective and safe in patients with mild-to-moderate asthma associated with inhalant allergens. Asthma should be controlled prior to initiation of AIT.6,8,10 Well-known allergic triggers for asthma exacerbation include indoor inhaled allergens (eg, house dust mite, animal dander, cockroach), outdoor inhalant allergens (plants, pollen), and occupational inhaled allergens (silkworm, weevil).11,13
In one meta-analysis of 796 patients with asthma from 19 different randomized controlled trials, SCIT significantly decreased asthma-related symptom scores (SMD = -0.94; 95% CI, -1.58 to -0.29; P = .004), as well as asthma medication scores (SMD = -1.06; 95% CI, -1.70 to -0.42; P = .001).18 While AIT has not been shown to improve lung function, meta-analyses have shown that adults with asthma treated with AIT experience fewer/less severe exacerbations and use less rescue medication when compared with those taking placebo.19,20 Furthermore, studies have shown that SCIT and SLIT reduce asthma symptoms and asthma medication use compared with placebo or usual care in the pediatric population.20
As helpful as AIT can be for some patients with mild-to-moderate asthma, patients with severe asthma experience more severe adverse reactions with AIT.21 Therefore, most experts recommend against administering AIT to patients with severe asthma.6,8,21
Continue to: Stinging insects
Stinging insects. VIT is used for patients with hypersensitivity to the venom from insects of the order Hymenoptera (see previous list of insects).3,11,22 A meta-analysis concluded, based on limited evidence from low-quality studies, that VIT has the potential to substantially reduce the incidence of severe allergic reactions in patients with Hymenoptera sensitivity with 72% of patients benefitting from VIT (number needed to treat [NNT] = 1.4).22 VIT reduces the risk of a systemic reaction, as well as the size and duration of large local reactions (LLRs).6,22 Immunotherapy for stinging insects also has been shown to improve disease-specific quality of life (risk difference = 1.41 strongly favoring VIT).6,22
Insect allergens. Research has shown AIT to be an effective therapy for many allergens even though the potency and effectiveness for some allergens are not standardized or regulated.6,7,11,14 For example, AIT is available for some inhaled insect allergens; however, because the extracts are not standardized, AIT produces inconsistent outcomes.11,14 As another example, certain occupations lead to exposure to inhaled insect allergens such as silkworm and weevils. AIT is not indicated for either because available silkworm extracts are used only for allergy testing.11 There are no extracts to test for or treat weevil allergy.11
Food. IgE-mediated food allergy can result in oral allergy syndrome, angioedema, urticaria, and/or anaphylaxis.2,7,8 There is some evidence that AIT raises the threshold of reactivity in children with IgE-mediated food allergies.6,7,23-25 But the studies available for meta-analyses (some of which involved OIT) were deemed to be of low quality due to a high risk of bias and a small number of participants.24,25 AIT for food allergies is associated with a substantially increased incidence of moderate adverse reactions, including upper respiratory, gastrointestinal, and skin symptoms, with a probability of 46% during the buildup phase and a number needed to harm (NNH) of 2.1 (95% CI, 1.8-2.5; P < .0001).6,25 Therefore, experts consider AIT in any form for food hypersensitivity to be investigational.6,10
But preliminary data from a recent phase 3 trial of OIT for peanut allergy involving 499 children and teens are promising; 67.2% tolerated the food challenge of ≥ 600 mg of peanut protein at the completion of peanut OIT without dose-limiting symptoms (difference = 63.2 percentage points; 95% CI, 53-73.3; P < .001).26 More than twice as many participants in the placebo group vs the treatment group experienced AEs that were moderate (59% vs 25%, respectively) or severe (11% vs 5%, respectively).
There are ongoing trials of SCIT, SLIT, and OIT using modified food allergens to make participants less allergic while maintaining immunogenicity.2,27 Additional trials include adjunctive treatments like probiotics to create safer, more effective options for children with food allergies.2,27 Keep in mind that children with food allergies often have concomitant allergies (eg, inhalant allergies) that can benefit from AIT.
Continue to: Other clinical practice strategies include...
Other clinical practice strategies include the introduction of extensively heated (baked) milk and egg products, which benefit the majority of milk- and egg-allergic children.2,28 An American Academy of Allergy, Asthma and Immunology (AAAAI)-sponsored Task Force and the European Academy of Allergy and Clinical Immunology (EAACI) support exclusive breastfeeding for the first 4 to 6 months of life to decrease the risk of developing food allergies.6,7
Atopic dermatitis (AD). AD is an IgE-mediated skin disease that affects children and adults. AD is associated with asthma, AR, and food allergy.13 Early studies showed that AIT reduced topical corticosteroid use and improved the SCORAD (SCORing Atopic Dermatitis; see www.scorad.corti.li/) score.10 However, Cochrane reviews of studies involving children and adults with AD undergoing AIT via SCIT, SLIT, or OIT routes found that AIT was not effective in treating AD when accounting for the quality and heterogeneity of the studies.12,29 In addition, there were no significant differences in SCORAD scores.10,12
Contact allergens. Contact allergens, including plant resins (eg, poison ivy) and metals (eg, nickel) cause local dermatitis through a cell-mediated, delayed hypersensitivity response. AIT is not indicated for contact dermatitis.6,9
Why use AIT?
First, AIT has been shown to modify disease. Second, because of its disease-modifying properties, AIT may provide cost savings over standard drug treatment in patients with asthma and AR.17,20,30 In fact, individual studies have demonstrated ≥ 80% cost savings of AIT over standard drug regimens, although meta-analyses have been unable to demonstrate the same.30,31
In addition, initial studies suggested that AIT might help to prevent the development of new allergen sensitizations.32 One meta-analysis found that AIT decreased the short-term risk of developing asthma in children with AR; however, subsequent studies showed that AIT did not have efficacy in preventing new allergic disease.31,33
Continue to: How do you administer AIT?
How do you administer AIT?
FPs may be asked to administer AIT to their patients. Patients will typically have weekly office visits during the induction phase of AIT and should have appointments every 6 to 12 months during the maintenance phase.6,8
Collaboration with an allergy specialist is wise for dosing schedules and possibly for information regarding adverse reactions during administration. It is essential that AIT be administered by clinicians who are knowledgeable about the signs and symptoms of minor allergic reactions (eg, pruritus, mild erythema, and swelling at the administration site) and severe ones (eg, angioedema, shock, anaphylaxis), as well as who have immediate access to emergency medications and resuscitation, should it be needed.6-8,34
Most (86%) adverse reactions will occur within 30 minutes of administration of AIT; hence, the recommendation is to observe patients for 30 minutes following AIT administration.6,7,34 Continual training and “mock” severe reaction responses are beneficial for staff administering AIT to ensure appropriate equipment is available and that appropriate procedures are followed. Late-phase reactions can occur and usually present within 6 to 12 hours of administration; thus, it is essential for patients to be educated on the signs and symptoms of adverse reactions and on symptomatic and emergent treatment.9,34
Rush immunotherapy regimens for inhalant allergens are associated with increased AEs; therefore, pretreatment with antihistamines, leukotriene antagonists, the monoclonal antibody omalizumab, corticosteroids, or combinations of these agents is often used.6,34 In contrast to inhaled allergens, rush VIT has not been associated with an increased risk of adverse reactions in meta-analyses.6,22,34 Most experts recommend that AIT be discontinued if anaphylaxis occurs.8,34
Is AIT safe?
AIT is a proven safe and effective disease-modifying treatment option.6-8,31,35 Even when AIT is initiated within the season of increased allergen exposure, meta-analyses reveal no increase in adverse events in patients undergoing AIT.35 Given the lack of high-quality evidence confirming the safety of AIT in the following specific situations, both the AAAAI and EAACI have concluded that these conditions/situations are absolute contraindications for AIT due to the risk of severe reactions by activation of underlying disease8,21,36:
- severe asthma;
- acquired immune deficiency syndrome (AIDS); and
- initiation of AIT during pregnancy.
Continue to: Patients with a history of transplantation...
Patients with a history of transplantation, cancer in remission, human immunodeficiency virus (HIV) without AIDS, and cardiovascular disease have been safely treated with AIT with a < 1.5% incidence of serious adverse events.6,21,36 It is possible to give patients taking beta-blockers and/or angiotensin converting enzyme inhibitors (ACEIs) AIT with appropriate consideration. Both classes of drugs can interfere with emergency treatment, so one should consider substitution with an agent from another class if possible during AIT.6,8,20,34 Patients taking ACEIs receiving VIT had substantially increased adverse reactions compared with other forms of AIT; thus, individual risks and benefits must be weighed carefully before initiating VIT.6,34
Looking ahead
Studies evaluating the indications for AIT in oral allergy syndrome, food allergy, latex allergy, AD, and venom allergy are ongoing.2,7,10,26 Although the incidence of severe adverse allergy reactions during AIT is rare, there are investigations of using various immune-modifying agents to improve the safety and efficacy of AIT.37 Application of allergen preparation using skin patches, intralymphatic injections, and chemically modified allergens to make them less immunologically reactive are being researched to further improve safety profiles and make AIT less time consuming.38 In Europe and the United States, there is a call for more rigid studies using standardized SLIT preparations. This will allow for an increased number of AIT studies with decreased heterogeneity.
CORRESPONDENCE
Dellyse Bright, MD, Carolinas Medical Center Family Medicine Residency Program, Atrium Health, 2001 Vail Avenue, Suite 400B, Charlotte, NC 28207; [email protected].
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
1. US Department of Health and Human Services. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD. May 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf#035. Accessed May 1, 2019.
2. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.e1.
3. Tankersley MS, Ledford DK. Stinging insect allergy: state of the art 2015. J Allergy Clin Immunol Pract. 2015;3:315-322.
4. Gupta R, Holdford D, Bilaver L, et al. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026-1031.
5. Hamad A, Burks WA. Emerging approaches to food desensitization in children. Curr Allergy Asthma Rep. 2017;17:32.
6. Cox L, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-S55.
7. Agache I, Akdis CA, Chivato T, et al. European Academy of Allergy and Clinical Immunology (EAACI) White Paper on Research, Innovation, and Quality of Care. http://www.eaaci.org/documents/EAACI_White_Paper.pdf. Accessed May 1, 2019.
8. Greenhawt M, Oppenheimer J, Nelson M, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276-282.e2.
9. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(suppl 3):S1-S148.
10. Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288-1296.e3.
11. Khurana T, Bridgewater JL, Rabin RL. Allergenic extracts to diagnose and treat sensitivity to insect venoms and inhaled allergens. Ann Allergy Asthma Immunol. 2017;118:531-536.
12. Tam H, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev. 2016;2:CD008774.
13. National Heart, Lung, and Blood Institute. National asthma education and prevention program. Expert panel report 3: Guideline for the Diagnosis and Management of Asthma. August 28, 2007. https://www.nhlbi.nih.gov/sites/default/files/media/docs/asthgdln_1.pdf. Accessed May 2, 2019.
14. Ridolo E, Montagni M, Incorvala C, et al. Orphan immunotherapies for allergic diseases. Ann Allergy Asthma Immunol. 2016;116:194-198.
15. Nelson H, Cartier S, Allen-Ramey F, et al. Network meta-analysis shows commercialized subcutaneous and sublingual grass products have comparable efficacy. J Allergy Clin Immunol Pract. 2015;3:256-266.e3.
16. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339-349.e10.
17. Cox L. The role of allergen immunotherapy in the management of allergic rhinitis. Am J Rhinol Allergy. 2016;30:48-53.
18. Lu Y, Xu L, Xia M, et al. The efficacy and safety of subcutaneous immunotherapy in mite-sensitized subjects with asthma: a meta-analysis. Respir Care. 2015;60:269-278.
19. Mener DJ, Lin SY. The role of allergy immunotherapy in the treatment of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:215-220.
20. Dominguez-Ortega J, Delgado J, Blanco C, et al. Specific allergen immunotherapy for the treatment of allergic asthma: a review of current evidence. J Investig Allergol Clin Immunol. 2017;27(suppl 1):1-35.
21. Larenas-Linnemann DE, Hauswirth DW, Calabria CW, et al. American Academy of Allergy, Asthma & Immunology membership experience with allergen immunotherapy safety in patients with specific medical conditions. Allergy Asthma Proc. 2016;37:112-122.
22. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta-analysis. Allergy. 2017;72:342-365.
23. Pajno GB, Caminiti L, Chiera F, et al. Safety profile of oral immunotherapy with cow’s milk and hen egg: a 10-year experience in controlled trials. Allergy Asthma Proc. 2016;37:400-403.
24. Yepes-Nunez JJ, Zhang Y, Roque i Figuls M, et al. Immunotherapy (oral and sublingual) for food allergy to fruits. Cochrane Database Syst Rev. 2015;11:CD010522.
25. Nurmatov U, Dhami S, Arasi S, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133-1147.
26. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. 2018;379:1991-2001.
27. Lanser BJ, Wright BL, Orgel KA, et al. Current options for the treatment of food allergy. Pediatr Clin North Am. 2015;62:1531-1549.
28. Nowak-Wegrzyn A. Using food and nutrition strategies to induce tolerance in food- allergic children. Nestle Nutrition Institute Workshop Series. 2016;85:25-53.
29. Tam HH, Calderon MA, Manikam L, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a Cochrane systematic review. Allergy. 2016;71:1345-1356.
30. Cox L. Allergy immunotherapy in reducing healthcare cost. Curr Opin Otolaryngol Head Neck Surg. 2015;23:247-254.
31. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18-29.
32. Di Bona D, Plaia A, Leto-Barone MS, et al. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017;72:691-704.
33. Di Lorenzo G, Leto-Barone MS, La Piana S, et al. The effect of allergen immunotherapy in the onset of new sensitizations: a meta-analysis. Int Forum Allergy Rhinol. 2017;7:660-669.
34. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477-480.
35. Creticos PS, Bernstein DI, Casale TB, et al. Coseasonal initiation of allergen immunotherapy: a systematic review. J Allergy Clin Immunol Pract. 2016;4:1194-1204.e4.
36. Pitsios C, Demoly P, Bilo MB, et al. Clinical contraindications to allergen immunotherapy: an EAAACI position paper. Allergy. 2015;70:897-909.
37. Klimek L, Pfaar O, Bousquet J, et al. Allergen immunotherapy in allergic rhinitis: current use and future trends. Expert Rev Clin Immunol. 2017;13:897-906.
38. Nelson HS. Allergen immunotherapy now and in the future. Allergy Asthma Proc. 2016;37:268-272.
PRACTICE RECOMMENDATIONS
› Diagnose allergies that are amenable to allergy immunotherapy (AIT) using skin prick/puncture allergy testing in conjunction with clinical symptoms, triggers, and exposure. A
› Do not use AIT for urticaria, angioedema, drug hypersensitivity, or latex allergy. A
› Do not initiate AIT during pregnancy or in patients with acquired immune deficiency syndrome or severe asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Recommendations on the Use of Ultrasound Guidance for Adult Lumbar Puncture: A Position Statement of the Society of Hospital Medicine
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Approximately 400,000 lumbar punctures (LPs) are performed in the United States annually for either diagnostic workup or therapeutic relief.1 Lumbar punctures are increasingly being performed in the United States, with an estimated 97,000 LPs performed on Medicare fee-for-service beneficiaries in 2011 alone, which is an increase of approximately 4,000 LPs in the same population from 1991.2 Approximately 273,612 LPs were performed on hospitalized patients in the United States in 2010,1 and the inpatient hospital setting is the most common site for LPs.2,3
Many LPs are referred to radiologists who have access to imaging guidance to aid with needle insertion.2 However, referrals to radiology delay performance of LPs, and delayed diagnosis of acute bacterial meningitis, the most common yet serious condition for which LPs are performed, is associated with increased morbidity and mortality.4-8 Furthermore, although initiating empiric antibiotic treatment for suspected acute bacterial meningitis is recommended in some cases, doing so routinely can cause false-negative cerebrospinal fluid (CSF) culture results, complicating decisions about de-escalation and duration of antibiotics that could have been safely avoided by promptly performing an LP.9
Delaying the performance of LP has been associated with increased mortality.10 Demonstration of proficiency in performance of lumbar puncture is considered a core competency for hospitalists,11 and with the increasing availability of point-of-care ultrasound, hospitalists can use ultrasound to guide performance of LPs at the bedside.12 However, 30% of patients requiring LP in emergency departments have difficult-to-palpate lumbar spine landmarks,13 and lumbar puncture performed based on palpation of landmarks alone has been reported to fail or be traumatic in 28% of patients.14 Use of ultrasound guidance for lumbar puncture has been shown in randomized controlled trials to improve procedural success rates, while reducing the time to successful LP, needle passes, patient pain scores, and risk of a traumatic LP.15-17
The purpose of this position statement is to review the literature and present consensus-based recommendations on the performance of ultrasound-guided LP in adult patients. This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP. Similar to previously published Society of Hospital Medicine (SHM) position statements,12,18,19 this document presents recommendations with supporting evidence for the clinical outcomes, techniques, and training for using ultrasound guidance for LP. A manuscript describing the technique of ultrasound guidance for LPs has been previously published by some of the authors of this position statement.20
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the six working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. Updated searches were conducted in November 2016, January 2018, and October 2018. The search strings are included in Appendix 3. All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide LP were selected. In addition, the following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. Moreover, studies focusing on the use of ultrasound guidance for spinal nerve root injections, regional anesthesia, and assessment of lumbar spine anatomy alone were excluded. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided LP were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into the draft recommendations.
The Research and Development (RAND) Appropriateness Method that required panel judgment and consensus was used.21 The 27 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (REDCap™) in February 2018 and April 2018 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale. The three zones of the 9-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” A strong recommendation required 80% of the votes within one integer of the median, following the RAND rules. Disagreement was defined as >30% of panelists voting outside of the zone of the median.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording of each recommendation (Table 2). The revised consensus-based recommendations underwent internal and external reviews by POCUS experts from different subspecialties. The final review of this position statement was performed by members of the SHM POCUS Task Force, SHM Education Committee, and SHM Executive Committee. The SHM Executive Committee endorsed this position statement in June 2018 before submission to the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 4,389 references were pooled from four different sources: a search by a certified medical librarian in December 2015 (3,212 citations) that was updated in November 2016 (380 citations), January 2018 (282 citations), and October 2018 (274 citations); working group members’ personal bibliographies and searches (31 citations); and a search focusing on ultrasound-guided LP training (210 citations). A total of 232 full-text articles were reviewed, and the final selection included 77 articles that were abstracted into a data table and incorporated into the draft recommendations. Details of the literature search strategy are presented in Appendix 3.
RECOMMENDATIONS
Four domains (clinical outcomes, technique, training, and knowledge gaps) with 16 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation were drafted citing supporting evidence. After two rounds of panel voting, five recommendations did not achieve agreement based on the RAND rules, one recommendation was combined with another recommendation during peer review, and 10 statements received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Nine statements were approved as strong recommendations, and one was approved as a conditional recommendation. Therefore, the final recommendation count was 10. The strength of the recommendation and degree of consensus for each recommendation are summarized in Table 1.
Terminology
LP is a procedure in which a spinal needle is introduced into the subarachnoid space for the purpose of collecting CSF for diagnostic evaluation and/or therapeutic relief.
Throughout this document, the phrases “ultrasound-guided” and “ultrasound guidance” refer to the use of ultrasound to mark a needle insertion site immediately before performing the procedure. This is also known as static ultrasound guidance. Real-time or dynamic ultrasound guidance refers to direct visualization of the needle tip as it traverses through the skin and soft tissues to reach the ligamentum flavum. Any reference to real-time ultrasound guidance is explicitly stated.
Clinical outcomes
1) When ultrasound equipment is available, along with providers who are appropriately trained to use it, we recommend that ultrasound guidance should be used for site selection of LPs to reduce the number of needle insertion attempts and needle redirections and increase the overall procedure success rates, especially in patients who are obese or have difficult-to-palpate landmarks.
Rationale. LPs have historically been performed by selecting a needle insertion site based on palpation of anatomical landmarks. However, an estimated 30% of patients requiring LP in emergency departments have lumbar spine landmarks that are difficult to palpate, most commonly due to obesity.13 Furthermore, lumbar puncture performed based on palpation of landmarks alone has been reported to fail in 28% of patients.14
Ultrasound can be used at the bedside to elucidate the lumbar spine anatomy to guide performance of LP or epidural catheterization. Since the early 2000s, randomized studies comparing the use of ultrasound guidance (ultrasound-guided) versus anatomical landmarks (landmark-guided) to map the lumbar spine for epidural catheterization have emerged. It is important to recognize that the exact same ultrasound technique is used for site marking of LP, epidural catheterization, and spinal anesthesia—the key difference is how deep the needle tip is inserted. Therefore, data from these three ultrasound-guided procedures are often pooled. Currently, at least 33 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP, epidural catheterization, or spinal anesthesia have been published.22-49 We present three meta-analyses below that pooled data primarily from randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP or spinal anesthesia.
In 2013, Shaikh et al. published the first meta-analysis with 14 randomized controlled studies comparing ultrasound-guided vs landmark-guided site selection for LP (n = 5) or epidural catheterization (n = 9). The pooled data showed that use of ultrasound guidance decreased the proportion of failed procedures (risk ratio 0.21, 95% CI 0.10-0.43) with an absolute risk reduction of 6.3% (95% CI 4.1%-8.4%) and a number needed to treat of 16 (95% CI 12-25) to prevent one failed procedure. In addition, the use of ultrasound reduced the mean number of attempts by 0.44 (95% CI 0.24-0.64) and reduced the mean number of needle redirections by 1.00 (95% CI 0.75-1.24). The reduction in risk of a failed procedure was similar for LPs (risk ratio 0.19 [95% CI 0.07-0.56]) and epidural catheterizations (risk ratio 0.23 [95% CI 0.09-0.60]).16
A similar meta-analysis published by Perlas et al. in 2016 included a total of 31 studies, both randomized controlled and cohort studies, evaluating the use of ultrasound guidance for LP, spinal anesthesia, and epidural catheterization.50 The goal of this systematic review and meta-analysis was to establish clinical practice recommendations. The authors concluded (1) the data consistently suggest that ultrasound is more accurate than palpation for lumbar interspace identification, (2) ultrasound allows accurate measurement of the needle insertion depth to reach the epidural space with a mean difference of <3 mm compared with the actual needle insertion depth, and (3) ultrasound increases the efficacy of lumbar epidural or spinal anesthesia by decreasing the mean number of needle passes for success by 0.75 (95% CI 0.44-1.07) and reducing the risk of a failed procedure (risk ratio 0.51 [95% CI 0.32-0.80]), both in patients with normal surface anatomy and in those with technically difficult surface anatomy due to obesity, scoliosis, or previous spine surgery.
Compared to the two earlier meta-analyses that included studies of both LP and spinal anesthesia procedures, the meta-analysis conducted by Gottlieb et al. in 2018 pooled data from 12 randomized controlled studies of ultrasound guidance for LPs only. For the primary outcome, pooled data from both adult and pediatric studies demonstrated higher procedural success rates with ultrasound-guided vs landmark-guided LPs (90% vs 81%) with an odds ratio of 2.1 (95% CI 0.66-7.44) in favor of ultrasound; however, there were no statistically significant differences when the adult and pediatric subgroups were analyzed separately, probably due to underpowering. For the secondary outcomes, data from the adult subgroup showed that use of ultrasound guidance was associated with fewer traumatic LPs (OR 0.28, 95% CI 0.14-0.59), shorter time to procedural success (adjusted mean difference –3.03 minutes, 95% CI –3.54 to –2.52), fewer number of needle passes (adjusted mean difference –0.81 passes, 95% CI –1.57 to –0.05), and lower patient pain scores (adjusted mean difference –2.53, 95% CI –3.89 to –1.17).
At least 12 randomized controlled studies have been published comparing the use of ultrasound guidance vs landmarks for the performance of LP or spinal anesthesia in adult patients, which were not included in the abovementioned meta-analyses. These individual studies demonstrated similar benefits of using ultrasound guidance: reduced needle insertion attempts, reduced needle redirections, and increased overall procedural success rates.17,31,37,40,41,43-49
It is important to recognize that four randomized controlled studies did not demonstrate any benefits of ultrasound guidance on the number of attempts or procedural success rates,23,33,41,51 and three of these studies were included in the abovementioned meta-analyses.23,33,51 Limitations of these negative studies include potential selection bias, inadequate sample sizes, and varying levels of operator skills in procedures, ultrasound guidance, or both. One study included emergency medicine residents as operators with varying degrees of ultrasound skills, and more importantly, patient enrollment occurred by convenience sampling, which may have introduced selection bias. Furthermore, most of the patients were not obese (median BMI of 27 kg/m2), and it is unclear why 10 years lapsed from data collection until publication.33 Another study with three experienced anesthesiologists as operators performing spinal anesthesia enrolled only patients who were not obese (mean BMI of 29 kg/m2) and had easily palpable bony landmarks—two patient characteristics associated with the least benefit of using ultrasound guidance in other studies.23 Another negative study had one experienced anesthesiologist marking obstetric patients with ultrasound, but junior residents performing the actual procedure in the absence of the anesthesiologist who had marked the patient.41
In general, the greatest benefit of using ultrasound guidance for LP has been demonstrated in obese patients.24,32,34,35,52,53 Benefits have been shown in specific obese patient populations, including obstetric,31,54,55 orthopedic,24,56,57 and emergency department patients.30
By increasing the procedural success rates with the use of ultrasound at the bedside, fewer patients may be referred to interventional radiology for fluoroscopic-guided LP, decreasing the patient exposure to ionizing radiation. A randomized study (n = 112) that compared site marking with ultrasound guidance versus fluoroscopic guidance for epidural steroid injections found the two techniques to be equivalent with respect to mean procedure time, number of needle insertion attempts, or needle passes.58 Another randomized study found that the performance time of ultrasound guidance was two minutes shorter (P < .05) than fluoroscopic guidance.59
Techniques
2) We recommend that ultrasound should be used to more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients.
Rationale. Traditionally, an imaginary line connecting the iliac crests (intercristal line, Tuffier’s line, or Jacoby’s line) was considered to identify the L4 vertebra or the L4-L5 interspinous space in the midline; however, studies have revealed this traditional landmark to be much less accurate than previously thought. In general, palpating the iliac crests to mark the intercristal line identifies an interspinous space that is one space cephalad (ie, the L2-L3 interspinous space) but can range from L1-L2 to L4-L5.46,60-64 If an LP is inadvertently performed in the L1-L2 interspinous space, the risk of spinal cord injury is higher than that when performed in a more distal interspinous space.
A study by Margarido et al. with 45 patients with a mean BMI of 30 kg/m2 found that the intercristal line was located above the L4-L5 interspinous space in 100% of patients. More importantly, the intercristal line was above L2-L3 in 36% of patients and above L1-L2 in 4% of patients. It is important to note that patients with scoliosis or previous spine surgery were excluded from this study, and all examinations were performed by two experienced anesthesiologists with patients in a sitting position—all factors that would favor accurate palpation and marking of the iliac crests.60
In a study of nonobese patients (mean BMI 28 kg/m2) undergoing spinal anesthesia, Duniec et al. compared the lumbar level identified by palpation versus ultrasound and found discordance between the two techniques in 36% of patients; 18% were one space too cephalad, 16% were one space too caudal, and 2% were off by two interspinous spaces.61 Another study found discordance in 64% of patients (mean BMI 28 kg/m2) when comparing the interspinous level where spinal anesthesia had been performed by palpation versus a post-procedural ultrasound examination. This study revealed that the interspinous space was more cephalad in 50% of patients with 6% of punctures performed in the L1-L2 interspace.62 A similar study compared the accuracy of palpation vs ultrasound to identify the L3-L4 interspinous space in obese (mean BMI 34 kg/m2) versus nonobese (mean BMI 27 kg/m2) patients. This study found marking a space above L3-L4 in 51% of obese and 40% of nonobese patients and marking of the L1-L2 interspace in 7% of obese and 4% of nonobese patients.64
A study comparing palpation vs ultrasound found that 68% of obese patients with a BMI of >30 kg/m2 had difficult-to-palpate lumbar spine landmarks, but with the use of ultrasound, landmarks were identified in 76% of all patients, including obese and nonobese, with difficult-to-palpate landmarks.65
3) We suggest using ultrasound for selecting and marking a needle insertion site just before performing LPs in either a lateral decubitus or sitting position. The patient should remain in the same position after marking the needle insertion site.
Rationale. Ultrasound mapping of the lumbar spine can be performed in either a lateral decubitus or sitting position. Selecting and marking a needle insertion site should be performed at the bedside just before performing the procedure. The patient must remain in the same position in the interim between marking and inserting the needle, as a slight change in position can alter the needle trajectory, lowering the LP success rate. Although performing LPs in a lateral decubitus position has the advantage of accurately measuring the opening pressure, misalignment of the shoulder and pelvic girdles and bowing of the bed in a lateral decubitus position may lower LP success rates.
One randomized study comparing ultrasound-guided spinal anesthesia in a lateral decubitus versus sitting position found no difference in the number of needle insertion attempts or measurement of the skin-dura distance; however, the needle insertion depth was 0.73 cm greater in a lateral decubitus vs sitting position (P = .002).66 Procedural success rates of LP with ultrasound guidance have not been directly compared in a sitting versus lateral decubitus position, although the overall procedural success rates were higher in one study that allowed the operator to choose either sitting or lateral decubitus position when ultrasound was used.32
4) We recommend that a low-frequency transducer, preferably a curvilinear array transducer, should be used to evaluate the lumbar spine and mark a needle insertion site in most patients. A high-frequency linear array transducer may be used in nonobese patients.
Rationale. Low-frequency transducers emit sound waves that penetrate deep tissues, allowing visualization of bones and ligaments of the lumbar spine. A high-frequency linear transducer offers better resolution but shallower penetration to approximately 6-9 cm, limiting its use for site marking in overweight and obese patients. In obese patients, the ligamentum flavum is often deeper than 6 cm, which requires a low-frequency transducer to be visualized.
Most of the randomized controlled studies demonstrating benefits of using ultrasound guidance compared with landmark guidance for performance of LP, epidural anesthesia, or spinal anesthesia have used a low-frequency, curvilinear transducer.22,24,26-28,31,34-36,39,43-45,67 Two randomized controlled trials used a high-frequency linear transducer for site marking of lumbar procedures.30,32,37 Using a high-frequency linear transducer has been described in real-time, ultrasound-guided LPs, the advantage being better needle visualization with a linear transducer.29 Detection of blood vessels by color flow Doppler may be another advantage of using a high-frequency linear transducer, although a study by Grau et al. showed that use of color flow Doppler with a low-frequency curvilinear transducer permitted visualization of interspinous vessels as small as 0.5 mm in size.68
5) We recommend that ultrasound should be used to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad, sequentially identifying the lumbar spine interspaces.Rationale. Although no studies have directly compared different ultrasound scanning protocols to map the lumbar spine, starting at the level of the sacrum and sliding the transducer cephalad to sequentially identify the lumbar interspinous spaces is the most commonly described technique in studies demonstrating improved clinical outcomes with the use of ultrasound.24,31,34,37,39,40,45,56,57,67 Because the sacrum can be easily recognized, identifying it first is most beneficial in patients with few or no palpable landmarks.
All five lumbar spinous processes and interspinous spaces can be mapped from the sacrum using either a midline or a paramedian approach, and the widest interspinous space can be selected. In a midline approach, either a transverse or a longitudinal view is obtained. The transducer is centered on the sacrum and slid cephalad from L5 to L1 to identify each spinous process and interspinous space. In a paramedian approach, longitudinal paramedian views are obtained from the L5–sacrum interspace to the L1–L2 interspace, and each interspinous space is identified as the transducer is slid cephalad. Both these approaches are effective for mapping the lumbar spine. Whether the entire lumbar spine is mapped, and whether a midline or a paramedian approach is utilized, will depend on the operator’s preference.
6) We recommend that ultrasound should be used in a transverse plane to mark the midline of the lumbar spine and a longitudinal plane to mark the interspinous spaces. The intersection of these two lines marks the needle insertion site.
Rationale. The most common technique described in comparative studies of ultrasound vs landmarks includes visualization of the lumbar spine in two planes, a transverse plane to identify the midline and a longitudinal plane to identify the interspinous spaces. The majority of randomized controlled studies that demonstrated a reduction in the number of needle insertion attempts and an increase in the procedural success rates have used this technique (see Clinical Outcomes).22,24,28,32,35-37,43,44 Marking the midline and interspinous space(s) for LP may be performed in any order, starting with either the transverse or longitudinal plane first.
The midline of the spine is marked by placing the transducer in a transverse plane over the lumbar spine, centering over the spinous processes that have a distinct hyperechoic tip and a prominent acoustic shadow deep to the bone, and drawing a line perpendicular to the center of the transducer delineating the midline. The midline should be marked over a minimum of two or three spinous processes.
To identify the interspinous spaces, the transducer is aligned longitudinally over the midline. The transducer is slid along the midline to identify the widest interspinous space. Once the transducer is centered over the widest interspinous space, a line perpendicular to the center of the transducer is drawn to mark the interspinous space. The intersection of the lines marking the spinal midline and the selected interspinous space identifies the needle entry point.
To visualize the ligamentum flavum from a paramedian view, the transducer is oriented longitudinally over the midline, slid approximately 1 cm laterally, and tilted approximately 15 degrees aiming the ultrasound beam toward the midline. The skin–ligamentum flavum distance is most reliably measured from a paramedian view. Alternatively, in some patients, the ligamentum flavum may be visualized in the midline and the depth can be measured.
7) We recommend that ultrasound should be used during a preprocedural evaluation to measure the distance from the skin surface to the ligamentum flavum from a longitudinal paramedian view to estimate the needle insertion depth and ensure that a spinal needle of adequate length is used.
Rationale. The distance from the skin to the ligamentum flavum can be measured using ultrasound during preprocedural planning. Knowing the depth to the ligamentum flavum preprocedurally allows the operator to procure a spinal needle of adequate length, anticipate the insertion depth before CSF can be obtained, determine the depth to which a local anesthetic will need to be injected, and decide whether the anticipated difficulty of the procedure warrants referral to or consultation with another specialist.
The skin–ligamentum flavum distance can be measured from a transverse midline view or a longitudinal paramedian view. A longitudinal paramedian view provides an unobstructed view of the ligamentum flavum due to less shadowing from bony structures compared with a midline view. Several studies have demonstrated a strong correlation between the skin–ligamentum flavum distance measured by ultrasound and the actual needle insertion depth in both midline and paramedian views.28,34,36,53,54,57,69,70
A meta-analysis that included 13 comparative studies evaluating the correlation between ultrasound-measured depth and actual needle insertion depth to reach the epidural or intrathecal space consistently demonstrated a strong correlation between the measured and actual depth.50 A few studies have reported near-perfect Pearson correlation coefficients of 0.98.55,71,72 The pooled correlation was 0.91 (95% CI 0.87-0.94). All studies measured the depth from the skin to the ventral side of the ligamentum flavum or the intrathecal space from either a longitudinal paramedian view (n = 4) or a transverse midline view (n = 9). Eight of the more recent studies evaluated the accuracy of the ultrasound measurements and found the depth measurements by ultrasound to be accurate within 1-13 mm of the actual needle insertion depth, with seven of the eight studies reporting a mean difference of ≤3 mm.50
Measurement of the distance between the skin and the ligamentum flavum generally underestimates the needle insertion depth. One study reported that measurement of the skin–ligamentum flavum distance underestimates the needle insertion depth by 7.6 mm to obtain CSF, whereas measurement of the skin–posterior longitudinal ligament distance overestimates the needle insertion depth by 2.5 mm.57 A well-accepted contributor to underestimation of the depth measurements using ultrasound is compression of the skin and soft tissues by the transducer, and therefore, pressure on the skin must be released before freezing an image and measuring the depth to the subarachnoid space.
Training
8) We recommend that novices should undergo simulation-based training, where available, before attempting ultrasound-guided LPs on actual patients.
Rationale. Similar to training for other bedside procedures, dedicated training sessions, including didactics, supervised practice on patients, and simulation-based practice, should be considered when teaching novices to perform ultrasound-guided LP. Simulation-based training facilitates acquisition of knowledge and skills to perform invasive bedside procedures, including LP.73 Simulation-based training has been commonly incorporated into procedure training for trainees using an immersive experience, such as a “boot camp,”74-77 or a standardized curriculum,78,79 and has demonstrated improvements in post-course procedural knowledge, technical skills, and operator confidence. Two of these studies included training in the use of ultrasound guidance for LP. These studies showed that simulation-based practice improved skill acquisition and confidence.80,81 Simulation using novel computer software may improve skill acquisition in the use of ultrasound guidance for LP.82
9) We recommend that training in ultrasound-guided LPs should be adapted based on prior ultrasound experience, as learning curves will vary.Rationale. The learning curve to achieve competency in the use of ultrasound guidance for LP has not been well studied. The rate of attaining competency in identifying lumbar spine structures using ultrasound will vary by provider based on prior skills in ultrasound-guided procedures.83 Thus, providers with prior ultrasound experience may require less training than those without such experience to achieve competency. However, extensive experience in performing landmark-guided LPs does not necessarily translate into rapid acquisition of skills to perform the procedure with ultrasound guidance. A study of practicing anesthesiologists with no prior ultrasound experience demonstrated that 20 supervised trials of ultrasound-guided spinal anesthesia were insufficient to achieve competency.84 Although minimums may be a necessary step to gain competence, using them as a sole means to define competence does not account for variable learning curves.12 Based on a national survey of 21 hospitalist procedure experts, the mean current vs suggested minimums for initial and ongoing hospital privileging for LPs were 1.8 vs 6.9 and 2.2 vs 4.6 annually in one report.85
A fundamental question that needs to be answered is how to define competency in the use of ultrasound guidance for LP, including the specific skills and knowledge that must be mastered. At a minimum, providers must be able to identify lumbar spinous processes and distinguish them from the sacrum, identify the lumbar interspinous spaces and their corresponding levels, and estimate the depth from the skin to the ligamentum flavum from the midline and paramedian planes. Novice operators may benefit from practicing lumbar spine mapping of nonobese patients using a high-frequency linear transducer that generates high-resolution images and facilitates recognition of lumbar spine structures.
10) We recommend that novice providers should be supervised when performing ultrasound-guided LPs before performing the procedure independently on patients.
Rationale: Demonstration of competency in the use of ultrasound to identify lumbar spine anatomy should be achieved before routinely performing the procedure independently on patients.18 All providers will require a variable period of supervised practice to demonstrate the appropriate technique, followed by a period of unsupervised practice before competency is achieved. Supervised practice with guidance and feedback has been shown to significantly improve providers’ ability to delineate lumbar spine anatomy.86
KNOWLEDGE GAPS
The process of producing these guidelines revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for LP.
First, it is unclear whether the use of ultrasound guidance for LP reduces postprocedural back pain and whether it improves patient satisfaction. Several studies have evaluated postprocedural back pain28,30,32,33,52 and patient satisfaction28,29,33,51 with the use of ultrasound guidance, but these studies have found inconsistent results. Some of these results were probably due to insufficient statistical power or confounding variables. Furthermore, benefits have been demonstrated in certain subgroups, such as overweight patients or those with anatomical abnormalities, as was found in two studies.52,87 Use of ultrasound guidance for spinal anesthesia has been shown to reduce postprocedural headache28 and improve patient satisfaction51, although similar benefit has not been demonstrated in patients undergoing LP.
Second, the effect of using ultrasound guidance on the frequency of traumatic LPs is an area of uncertainty. A “traumatic tap” is defined as an inadvertent puncture of an epidural vein during passage of the spinal needle through the dura. It remains difficult to discern in these studies whether red blood cells detected in the CSF resulted from puncture of an epidural vein or from needle trauma of the skin and soft tissues. Despite this uncertainty, at least seven randomized controlled studies have assessed the effect of ultrasound guidance on traumatic LPs. The meta-analysis by Shaikh et al. included five randomized controlled studies that assessed the effect of ultrasound guidance on the reporting of traumatic taps. The study found a reduced risk of traumatic taps (risk ratio 0.27 [95% CI 0.11-0.67]), an absolute risk reduction of 5.9% (95% CI 2.3%-9.5%), and a number needed to treat of 17 (95% CI 11-44) to prevent one traumatic tap.16 Similarly, the meta-analysis by Gottlieb et al. showed a lower risk of traumatic taps among adults undergoing LP with ultrasound guidance in five randomized controlled studies with an odds ratio of 0.28 (95% CI 0.14-0.59). The meta-analysis by Gottlieb et al. included two adult studies that were not included by Shaikh et al.
Third, several important questions about the technique of ultrasound-guided LP remain unanswered. In addition to the static technique, a dynamic technique with real-time needle tracking has been described to perform ultrasound-guided LP, epidural catheterization, and spinal anesthesia. A pilot study by Grau et al. found that ultrasound used either statically or dynamically had fewer insertion attempts and needle redirections than use of landmarks alone.29 Three other pilot studies showed successful spinal anesthesia in almost all patients88-90 and one large study demonstrated successful spinal anesthesia with real-time ultrasound guidance in 97 of 100 patients with a median of three needle passes.91 Furthermore, a few industry-sponsored studies with small numbers of patients have described the use of novel needle tracking systems that facilitate needle visualization during real-time ultrasound-guided LP.92,93 However, to our knowledge, no comparative studies of static versus dynamic guidance using novel needle tracking systems in human subjects have been published, and any potential role for these novel needle tracking systems has not yet been defined.
Finally, the effects of using ultrasound guidance on clinical decision-making, timeliness, and cost-effectiveness of LP have not yet been explored but could have important clinical practice implications.
CONCLUSION
Randomized controlled trials have demonstrated that using ultrasound guidance for LPs can reduce the number of needle insertion attempts and needle redirections and increase the overall procedural success rates. Ultrasound can more accurately identify the lumbar spine level than physical examination in both obese and nonobese patients, although the greatest benefit of using ultrasound guidance for LPs has been shown in obese patients.
Ultrasound permits assessment of the interspinous space width and measurement of the ligamentum flavum depth to select an optimal needle insertion site and adequate length spinal needle. Although the use of real-time ultrasound guidance has been described, the use of static ultrasound guidance for LP site marking remains the standard technique.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators from Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Paul Mayo, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclosures
The authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1).
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
1. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023.
2. Kroll H, Duszak R, Jr, Nsiah E, et al. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. AJR Am J Roentgenol. 2015;204(1):15-19. https://doi.org/10.2214/AJR.14.12622.
3. Vickers A, Donnelly JP, Moore JX, Wang HE. 263EMF epidemiology of lumbar punctures in hospitalized patients in United States. Ann Emerg Med. 2017;70(4):S104. https://doi.org/10.1016/j.annemergmed.2017.07.241.
4. Køster-Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57(6):449-454. https://doi.org/10.1016/j.jinf.2008.09.033.
5. Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med. 1998;129(11):862-869. https://doi.org/10.7326/0003-4819-129-11_Part_1-199812010-00004.
6. Lepur D, Barsić B. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection. 2007;35(4):225-231. https://doi.org/10.1007/s15010-007-6202-0.
7. Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98(4):291-298. https://doi.org/10.1093/qjmed/hci047.
8. Auburtin M, Wolff M, Charpentier J, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34(11):2758-2765. https://doi.org/10.1097/01.CCM.0000239434.26669.65.
9. Michael B, Menezes BF, Cunniffe J, et al. Effect of delayed lumbar punctures on the diagnosis of acute bacterial meningitis in adults. Emerg Med J. 2010;27(6):433-438. https://doi.org/10.1136/emj.2009.075598.
10. Glimåker M, Johansson B, Grindborg Ö, et al. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis. 2015;60(8):1162-1169. https://doi.org/10.1093/cid/civ011.
11. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 Revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715.
12. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079.
13. Shah KH, McGillicuddy D, Spear J, Edlow JA. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25(6):608-611. https://doi.org/10.1016/j.ajem.2006.11.025.
14. Williams P, Tait G, Wijeratne T. Success rate of elective lumbar puncture at a major Melbourne neurology unit. Surg Neurol Int. 2018;9:12. https://doi.org/10.4103/sni.sni_426_17.
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2018;26(1). https://doi.org/10.1111/acem.13558.
16. Shaikh F, Brzezinski J, Alexander S, et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ. 2013;346:f1720. https://doi.org/10.1136/bmj.f1720.
17. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
18. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
19. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
20. Soni NJ, Franco-Sadud R, Schnobrich D, et al. Ultrasound guidance for lumbar puncture. Neurol Clin Pract. 2016;6(4):358-368. https://doi.org/10.1212/CPJ.0000000000000265.
21. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The Rand/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: Rand Corp; 2001.
22. Abdelhamid SA, Mansour MA. Ultrasound-guided intrathecal anesthesia: does scanning help? Egypt J Anaesth. 2013;29(4):389-394. https://doi.org/10.1016/j.egja.2013.06.003.
23. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided spinal anaesthesia in obstetrics: is there an advantage over the landmark technique in patients with easily palpable spines? Int J Obstet Anesth. 2014;23(3):213-216. https://doi.org/10.1016/j.ijoa.2014.03.001.
24. Chin KJ, Perlas A, Chan V, et al. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94-101. https://doi.org/10.1097/ALN.0b013e31821a8ad4.
25. Cho YC, Koo DH, Oh SK, et al. Comparison of ultrasound-assisted lumbar puncture with lumbar puncture using palpation of landmarks in aged patients in an emergency center. J Korean Soc Emerg Med. 2009;20(3):304.
26. Grau T, Leipold RW, Conradi R, Martin E. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45(6):766-771. https://doi.org/10.1034/j.1399-6576.2001.045006766.x.
27. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Ultrasound imaging facilitates localization of the epidural space during combined spinal and epidural anesthesia. Reg Anesth Pain Med. 2001;26(1):64-67. https://doi.org/10.1053/rapm.2001.19633.
28. Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14(3):169-175. https://doi.org/10.1016/S0952-8180(01)00378-6.
29. Grau T, Leipold RW, Fatehi S, Martin E, Motsch J. Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol. 2004;21(1):25-31. https://doi.org/10.1017/S026502150400105X.
30. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
31. Nassar M, Abdelazim IA. Pre-puncture ultrasound guided epidural insertion before vaginal delivery. J Clin Monit Comput. 2015;29(5):573-577. https://doi.org/10.1007/s10877-014-9634-y.
32. Nomura JT, Leech SJ, Shenbagamurthi S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26(10):1341-1348. https://doi.org/10.7863/jum.2007.26.10.1341.
33. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
34. Sahin T, Balaban O, Sahin L, Solak M, Toker K. A randomized controlled trial of preinsertion ultrasound guidance for spinal anaesthesia in pregnancy: outcomes among obese and lean parturients: ultrasound for spinal anesthesia in pregnancy. J Anesth. 2014;28(3):413-419. https://doi.org/10.1007/s00540-013-1726-1.
35. Wang Q, Yin C, Wang TL. Ultrasound facilitates identification of combined spinal-epidural puncture in obese parturients. Chin Med J (Engl). 2012;125(21):3840-3843. PubMed
36. Vallejo MC, Phelps AL, Singh S, Orebaugh SL, Sah N. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373-378. https://doi.org/10.1016/j.ijoa.2010.04.002.
37. Darrieutort-Laffite C, Bart G, Planche L, et al. Usefulness of a pre-procedure ultrasound scanning of the lumbar spine before epidural injection in patients with a presumed difficult puncture: a randomized controlled trial. Joint Bone Spine. 2015;82(5):356-361. https://doi.org/10.1016/j.jbspin.2015.02.001.
38. Vosko MR, Brunner C, Schreiber S. Lumbar puncture with ultrasound study (lupus study)-international prospective randomized multicentre trial. Int J Stroke. 2017;12(1):22. https://doi.org/10.1055/s-0037-1606991.
39. Urfalioğlu A, Bilal B, Öksüz G, et al. Comparison of the landmark and ultrasound methods in cesarean sections performed under spinal anesthesia on obese pregnants. J Matern Fetal Neonatal Med. 2017;30(9):1051-1056. https://doi.org/10.1080/14767058.2016.1199677.
40. Tawfik MM, Atallah MM, Elkharboutly WS, Allakkany NS, Abdelkhalek M. Does preprocedural ultrasound increase the first-pass success rate of epidural catheterization before cesarean delivery? A randomized controlled trial. Anesth Analg. 2017;124(3):851-856. https://doi.org/10.1213/ANE.0000000000001325.
41. Turkstra TP, Marmai KL, Armstrong KP, Kumar K, Singh SI. Preprocedural ultrasound assessment does not improve trainee performance of spinal anesthesia for obstetrical patients: a randomized controlled trial. J Clin Anesth. 2017;37:21-24. https://doi.org/10.1016/j.jclinane.2016.10.034.
42. Chong SE, Mohd Nikman A, Saedah A, et al. Real-time ultrasound-guided paramedian spinal anaesthesia: evaluation of the efficacy and the success rate of single needle pass. Br J Anaesth. 2017;118(5):799-801. https://doi.org/10.1093/bja/aex108.
43. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to identify the lumbar space in women with impalpable bony landmarks presenting for elective caesarean delivery under spinal anaesthesia: a randomised trial. Int J Obstet Anesth. 2016;28:12-16. https://doi.org/10.1016/j.ijoa.2016.07.007.
44. Ekinci M, Alici HA, Ahiskalioglu A, et al. The use of ultrasound in planned cesarean delivery under spinal anesthesia for patients having nonprominent anatomic landmarks. J Clin Anesth. 2017;37:82-85. https://doi.org/10.1016/j.jclinane.2016.10.014.
45. Perna P, Gioia A, Ragazzi R, Volta CA, Innamorato M. Can pre-procedure neuroaxial ultrasound improve the identification of the potential epidural space when compared with anatomical landmarks? A prospective randomized study. Minerva Anestesiol. 2017;83(1):41-49. https://doi.org/10.23736/S0375-9393.16.11399-9.
46. Chin A, Crooke B, Heywood L, et al. A randomised controlled trial comparing needle movements during combined spinal-epidural anaesthesia with and without ultrasound assistance. Anaesthesia. 2018;73(4):466-473. https://doi.org/10.1111/anae.14206.
47. Dhanger S, Vinayagam S, Vaidhyanathan B, Rajesh IJ, Tripathy DK. Comparison of landmark versus pre-procedural ultrasonography-assisted midline approach for identification of subarachnoid space in elective caesarean section: a randomised controlled trial. Indian J Anaesth. 2018;62(4):280-284. https://doi.org/10.4103/ija.IJA_488_17.
48. Evans DP, Tozer J, Joyce M, Vitto MJ. Comparison of ultrasound-guided and landmark-based lumbar punctures in inexperienced resident physicians. J Ultrasound Med. 2019;38(3):613-620. https://doi.org/10.1002/jum.14728.
49. Srinivasan KK, Leo AM, Iohom G, Loughnane F, Lee PJ. Pre-procedure ultrasound-guided paramedian spinal anaesthesia at L5-S1: is this better than landmark-guided midline approach? A randomised controlled trial. Indian J Anaesth. 2018;62(1):53-60. https://doi.org/10.4103/ija.IJA_448_17.
50. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med. 2016;41(2):251-260. https://doi.org/10.1097/AAP.0000000000000184.
51. Lim YC, Choo CY, Tan KT. A randomised controlled trial of ultrasound-assisted spinal anaesthesia. Anaesth Intensive Care. 2014;42(2):191-198. https://doi.org/10.1177/0310057X1404200205.
52. Honarbakhsh S, Osman C, Teo JTH, Gabriel C. Ultrasound-guided lumbar puncture as a diagnostic aid to reduce number of attempts and complication rates. Ultrasound. 2013;21(4):170-175. https://doi.org/10.1177/1742271X13504332.
53. Sahota JS, Carvalho JC, Balki M, Fanning N, Arzola C. Ultrasound estimates for midline epidural punctures in the obese parturient: paramedian sagittal oblique is comparable to transverse median plane. Anesth Analg. 2013;116(4):829-835. https://doi.org/10.1213/ANE.0b013e31827f55f0.
54. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108(6):1876-1881. https://doi.org/10.1213/ane.0b013e3181a323f6.
55. Wallace DH, Currie JM, Gilstrap LC, Santos R. Indirect sonographic guidance for epidural anesthesia in obese pregnant patients. Reg Anesth. 1992;17(4):233-236. PubMed
56. Srinivasan KK, Iohom G, Loughnane F, Lee PJ. Conventional landmark-guided midline versus preprocedure ultrasound-guided paramedian techniques in spinal anesthesia. Anesth Analg. 2015;21(4):1089-1096. https://doi.org/10.1213/ANE.0000000000000911.
57. Chin KJ, Perlas A, Singh M, et al. An ultrasound-assisted approach facilitates spinal anesthesia for total joint arthroplasty. Can J Anaesth. 2009;56(9):643-650. https://doi.org/10.1007/s12630-009-9132-8.
58. Evansa I, Logina I, Vanags I, Borgeat A. Ultrasound versus fluoroscopic-guided epidural steroid injections in patients with degenerative spinal diseases: a randomised study. Eur J Anaesthesiol. 2015;32(4):262-268. https://doi.org/10.1097/EJA.0000000000000103.
59. Park Y, Lee JH, Park KD, et al. Ultrasound-guided vs fluoroscopy-guided caudal epidural steroid injection for the treatment of unilateral lower lumbar radicular pain: a prospective, randomized, single-blind clinical study. Am J Phys Med Rehabil. 2013;92(7):575-586. https://doi.org/10.1097/PHM.0b013e318292356b.
60. Margarido CB, Mikhael R, Arzola C, Balki M, Carvalho JC. The intercristal line determined by palpation is not a reliable anatomical landmark for neuraxial anesthesia. Can J Anaesth. 2011;58(3):262-266. https://doi.org/10.1007/s12630-010-9432-z.
61. Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1-6. https://doi.org/10.5603/AIT.2013.0001.
62. Schlotterbeck H, Schaeffer R, Dow WA, et al. Ultrasonographic control of the puncture level for lumbar neuraxial block in obstetric anaesthesia. Br J Anaesth. 2008;100(2):230-234. https://doi.org/10.1093/bja/aem371.
63. Whitty R, Moore M, Macarthur A. Identification of the lumbar interspinous spaces: palpation versus ultrasound. Anesth Analg. 2008;106(2):538-540, table of contents. https://doi.org/10.1213/ane.0b013e31816069d9.
64. Locks Gde F, Almeida MC, Pereira AA. Use of the ultrasound to determine the level of lumbar puncture in pregnant women. Rev Bras Anestesiol. 2010;60(1):13-19. https://doi.org/10.1016/S0034-7094(10)70002-7.
65. Stiffler KA, Jwayyed S, Wilber ST, Robinson A. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25(3):331-334. https://doi.org/10.1016/j.ajem.2006.07.010.
66. Gulay U, Meltem T, Nadir SS, Aysin A. Ultrasound-guided evaluation of the lumbar subarachnoid space in lateral and sitting positions in pregnant patients to receive elective cesarean operation. Pak J Med Sci. 2015;31(1):76-81. https://doi.org/10.12669/pjms.311.5647.
67. Kawaguchi R, Yamauchi M, Sugino S, Yamakage M. Ultrasound-aided ipsilateral-dominant epidural block for total hip arthroplasty: a randomised controlled single-blind study. Eur J Anaesthesiol. 2011;28(2):137-140. https://doi.org/10.1097/EJA.0b013e3283423457.
68. Grau T, Leipold RW, Horter J, Martin E, Motsch J. Colour Doppler imaging of the interspinous and epidural space. Eur J Anaesthesiol. 2001;18(11):706-712. https://doi.org/10.1097/00003643-200111000-00002.
69. Arzola C, Davies S, Rofaeel A, Carvalho JC. Ultrasound using the transverse approach to the lumbar spine provides reliable landmarks for labor epidurals. Anesth Analg. 2007;104(5):1188-92, tables of contents. https://doi.org/10.1213/01.ane.0000250912.66057.41.
70. Chauhan AK, Bhatia R, Agrawal S. Lumbar epidural depth using transverse ultrasound scan and its correlation with loss of resistance technique: a prospective observational study in Indian population. Saudi J Anaesth. 2018;12(2):279-282. https://doi.org/10.4103/sja.SJA_679_17.
71. Gnaho A, Nguyen V, Villevielle T, et al. Assessing the depth of the subarachnoid space by ultrasound. Rev Bras Anestesiol. 2012;62(4):520-530. https://doi.org/10.1016/S0034-7094(12)70150-2.
72. Cork RC, Kryc JJ, Vaughan RW. Ultrasonic localization of the lumbar epidural space. Anesthesiology. 1980;52(6):513-516. https://doi.org/10.1097/00000542-198006000-00013.
73. Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. https://doi.org/10.1212/WNL.0b013e31825dd39d.
74. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. https://doi.org/10.3109/0142159X.2010.509412.
75. Wayne DB, Cohen ER, Singer BD, et al. Progress toward improving medical school graduates’ skills via a “boot camp” curriculum. Simul Healthc. 2014;9(1):33-39. https://doi.org/10.1097/SIH.0000000000000001.
76. Cohen ER, Barsuk JH, Moazed F, et al. Making July safer: simulation-based mastery learning during intern boot camp. Acad Med. 2013;88(2):233-239. https://doi.org/10.1097/ACM.0b013e31827bfc0a.
77. Martin R, Gannon D, Riggle J, et al. A comprehensive workshop using simulation to train internal medicine residents in bedside procedures performed by internists. Chest. 2012;142(4):545A. https://doi.org/10.1378/chest.1390093.
78. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
79. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1.
80. Restrepo CG, Baker MD, Pruitt CM, Gullett JP, Pigott DC. Ability of pediatric emergency medicine physicians to identify anatomic landmarks with the assistance of ultrasound prior to lumbar puncture in a simulated obese model. Pediatr Emerg Care. 2015;31(1):15-19. https://doi.org/10.1097/PEC.0000000000000330.
81. VanderWielen BA, Harris R, Galgon RE, VanderWielen LM, Schroeder KM. Teaching sonoanatomy to anesthesia faculty and residents: utility of hands-on gel phantom and instructional video training models. J Clin Anesth. 2015;27(3):188-194. https://doi.org/10.1016/j.jclinane.2014.07.007.
82. Keri Z, Sydor D, Ungi T, et al. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: a randomized controlled trial. Can J Anaesth. 2015;62(7):777-784. https://doi.org/10.1007/s12630-015-0367-2.
83. Deacon AJ, Melhuishi NS, Terblanche NC. CUSUM method for construction of trainee spinal ultrasound learning curves following standardised teaching. Anaesth Intensive Care. 2014;42(4):480-486. https://doi.org/10.1177/0310057X1404200409.
84. Margarido CB, Arzola C, Balki M, Carvalho JC. Anesthesiologists’ learning curves for ultrasound assessment of the lumbar spine. Can J Anaesth. 2010;57(2):120-126. https://doi.org/10.1007/s12630-009-9219-2.
85. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
86. Terblanche NC, Arzola C, Wills KE, et al. Standardised training program in spinal ultrasound for epidural insertion: protocol driven versus non-protocol driven teaching approach. Anaesth Intensive Care. 2014;42(4):460-466. https://doi.org/10.1177/0310057X1404200406.
87. Mofidi M, Mohammadi M, Saidi H, et al. Ultrasound guided lumbar puncture in emergency department: time saving and less complications. J Res Med Sci. 2013;18(4):303-307. PubMed
88. Karmakar MK, Li X, Ho AM, Kwok WH, Chui PT. Real-time ultrasound-guided paramedian epidural access: evaluation of a novel in-plane technique. Br J Anaesth. 2009;102(6):845-854. https://doi.org/10.1093/bja/aep079.
89. Tran D, Kamani AA, Al-Attas E, et al. Single-operator real-time ultrasound-guidance to aim and insert a lumbar epidural needle. Can J Anaesth. 2010;57(4):313-321. https://doi.org/10.1007/s12630-009-9252-1.
90. Liu Y, Qian W, Ke XJ, Mei W. Real-time ultrasound-guided spinal anesthesia using a new paramedian transverse approach. Curr Med Sci. 2018;38(5):910-913. https://doi.org/10.1007/s11596-018-1961-7.
91. Conroy PH, Luyet C, McCartney CJ, McHardy PG. Real-time ultrasound-guided spinal anaesthesia: a prospective observational study of a new approach. Anesthesiol Res Pract. 2013;2013:525818. https://doi.org/10.1155/2013/525818.
92. Brinkmann S, Tang R, Sawka A, Vaghadia H. Single-operator real-time ultrasound-guided spinal injection using SonixGPS™: a case series. Can J Anaesth. 2013;60(9):896-901. https://doi.org/10.1007/s12630-013-9984-9.
93. Niazi AU, Chin KJ, Jin R, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS ultrasound guidance system: a feasibility study. Acta Anaesthesiol Scand. 2014;58(7):875-881. https://doi.org/10.1111/aas.12353.
© 2019 Society of Hospital Medicine
Ultrasound Guidance for Lumbar Puncture: A Consideration, Not an Obligation
Recognizing the increasingly important role of point-of-care ultrasound (POCUS) in advancing clinical care, the Society of Hospital Medicine (SHM) has published a valuable series of position statements to guide hospitalists and administrators on the safe and effective use of POCUS.1 In this issue of the Journal of Hospital Medicine, Soni et al. present a series of consensus-based recommendations on ultrasound guidance for lumbar puncture (LP).2 Among these are the recommendations that ultrasound “should be used” to map the lumbar spine and to select an appropriate puncture site to reduce insertion attempts, reduce needle redirections, and increase overall procedural success.
At first glance, the recommendations appear definitive. However, not immediately obvious is the authors’ clarification that “This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP.” Even with the authors’ caveat, this nuance may not be readily apparent to the readers who review only the executive summary of the guidelines or who omit the context provided in the background of the position statement.
The directive language of this position statement may be a result of an unmerited amplification. The SHM POCUS Task Force employed the Research and Development Appropriateness Method to quantify the degree of consensus and the strength of the recommendation assigned,3 reaching “very good” consensus for each of the recommendations espoused in its position statement. Procedurally, this implies that ≥80% of the 27 voting members rated each published recommendation statement as “appropriate”. Using wording assigned a priori by the committee to each level of consensus, appropriateness became magnified to the declaration “should be used”. In this manner, the strength of the recommendations in this position statement is not necessarily based on the experts’ convictions related to ultrasound-guided LP, nor the strength of the supporting evidence.
In the case of ultrasound-guided LP, we might choose different descriptors than “appropriate” or “should be used”. The evidence base for ultrasound guidance for LP, though growing, may be insufficient as a foundation to a position statement and is certainly insufficient to create a new standard of care for hospitalists. Although the SHM POCUS Task Force completed a thoughtful literature review, no systematic approach (eg, GRADE methodology4) was used to rate the quality of evidence. Furthermore, the literature reviewed was drawn predominantly from anesthesia and emergency medicine sources—not readily generalizable to the hospitalist. Notably, these studies examined all neuraxial procedures (most commonly epidural and spinal anesthesia), which employ different techniques and tools than LP and are performed by clinicians with vastly different procedural training backgrounds than most hospitalists. Altogether, this creates the potential for a gap between true evidence quality and the strength of recommendation.
At a high level, although the technique for ultrasound mapping of the lumbar spine may be similar, the use of ultrasound has been less well studied specifically for LP. When considering LP alone, the available literature is inadequate to recommend uniform ultrasound guidance. A 2018 meta-analysis by Gottlieb et al. included 12 studies focusing only on LP, totaling N = 957 patients.5 This showed some favorability of ultrasound guidance, with a success rate of 90% using ultrasound, 81.4% with a landmark-based approach, and an odds ratio of 2.22 favoring ultrasound guidance (95% CI: 1.03-4.77). Unfortunately, when focusing only on adult patients, the advantage of POCUS diminished, with 91.4% success in the ultrasound group, 87.7% success in the landmark group, and a nonsignificant odds ratio of 2.10 (95% CI: 0.66-7.44).
Unequivocally, POCUS has established itself as a transformative technology for the guidance of invasive bedside procedures, bringing increased procedural success, improved safety, and decreased complication rates.6 For some procedures, particularly central venous catheterization, ultrasound guidance is a clear standard of care.7,8 For LP, the greatest benefit has been observed in patients with anticipated procedural challenges, most commonly obese patients in whom landmarks are not easily palpable.9 Moreover, the harms ultrasound seeks to prevent are substantially different. The primary risk of deferring ultrasound guidance for LP is most often a failed procedure, whereas for other common ultrasound-guided procedures, the harms may include significant vascular injury, pneumothorax, or bowel perforation. Differences in the relative harms make risk-benefit assessments harder to quantify and studies harder to carry out.
Sonographic guidance for LP has a role in clinical practice and should always be considered. However, at present, there exist no guidelines in any other specialty regarding the routine use of ultrasound-guided LP, including anesthesia, emergency medicine, neurology, or interventional radiology.10-15 As a result, a conservative interpretation of the POCUS Task Force’s findings would be to consider the use of ultrasound guidance for LP in patients where landmark identification is particularly challenging, but not to consider it a standard requirement for accreditation, training, or practice as of yet. Saying “more studies are required” can be a cop-out in some cases, but in this situation, the old adage does seem to apply.
We have great respect for the work of the SHM POCUS Task Force in advancing the use of POCUS in hospital medicine. Though ultrasound is not currently mandated as a care standard for the performance of LP, we all can agree that POCUS does confer advantages for this procedure, particularly in a well-selected patient population. To continue to provide care of the highest quality, hospitalists must be encouraged to elevate their practice with POCUS and be supported with the equipment, training, credentialing, and quality assurance structures necessary to integrate bedside ultrasound safely and effectively into their diagnostic and procedural practice.
Disclosures
No conflicts of interest to disclose.
Funding
None.
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3079.
2. Soni NJ, Franco-Sadud R, Dobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
3. Fitch, K, Bernstein SJ, Aguilar MD et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation, 2001.
4. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;334(7650):924-926. PubMed
5. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
6. Moore CL, Copel JA. Point of care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
7. Shojania K, Duncan B, McDonald K, Wachter RM. Making health care safer: a critical analysis of patient safety practices. Rockville, MD: Agency for Healthcare Research and Quality, 2001. Evidence Report/Technology Assessment No. 43; AHRQ publication 01-E058. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catherization. Cochrane Database Syst Rev. 2015;Art. No.: 1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
9. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
11. Neal JM, Brull R, Horn JL, et al. The Second American Society of Regional Anesthesia and Pain Medicine Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia: executive summary. Reg Anesth Pain Med. 2016;41(2):181-194. doi: 10.1097/AAP.0000000000000331.
12. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270-300. doi: 10.1097/ALN.0000000000000935.
13. Engelborghs S, Sebastiaan E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111-126. doi: 10.1016/j.dadm.2017.04.007.
14. American College of Radiology. ACR-SPR-SRU Practice Parameter for the Performing and Interpreting Diagnostic Ultrasound Examinations. 2017; Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-perf-interpret.pdf. Accessed April 15, 2019.
15. American College of Radiology. ACR-AIUM-SPR-SRU Practice Parameter for the Performance of an Ultrasound Examination of the Neonatal and Infant Spine. 2016/ Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-NeonatalSpine.pdf. Accessed April 15, 2019.
Recognizing the increasingly important role of point-of-care ultrasound (POCUS) in advancing clinical care, the Society of Hospital Medicine (SHM) has published a valuable series of position statements to guide hospitalists and administrators on the safe and effective use of POCUS.1 In this issue of the Journal of Hospital Medicine, Soni et al. present a series of consensus-based recommendations on ultrasound guidance for lumbar puncture (LP).2 Among these are the recommendations that ultrasound “should be used” to map the lumbar spine and to select an appropriate puncture site to reduce insertion attempts, reduce needle redirections, and increase overall procedural success.
At first glance, the recommendations appear definitive. However, not immediately obvious is the authors’ clarification that “This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP.” Even with the authors’ caveat, this nuance may not be readily apparent to the readers who review only the executive summary of the guidelines or who omit the context provided in the background of the position statement.
The directive language of this position statement may be a result of an unmerited amplification. The SHM POCUS Task Force employed the Research and Development Appropriateness Method to quantify the degree of consensus and the strength of the recommendation assigned,3 reaching “very good” consensus for each of the recommendations espoused in its position statement. Procedurally, this implies that ≥80% of the 27 voting members rated each published recommendation statement as “appropriate”. Using wording assigned a priori by the committee to each level of consensus, appropriateness became magnified to the declaration “should be used”. In this manner, the strength of the recommendations in this position statement is not necessarily based on the experts’ convictions related to ultrasound-guided LP, nor the strength of the supporting evidence.
In the case of ultrasound-guided LP, we might choose different descriptors than “appropriate” or “should be used”. The evidence base for ultrasound guidance for LP, though growing, may be insufficient as a foundation to a position statement and is certainly insufficient to create a new standard of care for hospitalists. Although the SHM POCUS Task Force completed a thoughtful literature review, no systematic approach (eg, GRADE methodology4) was used to rate the quality of evidence. Furthermore, the literature reviewed was drawn predominantly from anesthesia and emergency medicine sources—not readily generalizable to the hospitalist. Notably, these studies examined all neuraxial procedures (most commonly epidural and spinal anesthesia), which employ different techniques and tools than LP and are performed by clinicians with vastly different procedural training backgrounds than most hospitalists. Altogether, this creates the potential for a gap between true evidence quality and the strength of recommendation.
At a high level, although the technique for ultrasound mapping of the lumbar spine may be similar, the use of ultrasound has been less well studied specifically for LP. When considering LP alone, the available literature is inadequate to recommend uniform ultrasound guidance. A 2018 meta-analysis by Gottlieb et al. included 12 studies focusing only on LP, totaling N = 957 patients.5 This showed some favorability of ultrasound guidance, with a success rate of 90% using ultrasound, 81.4% with a landmark-based approach, and an odds ratio of 2.22 favoring ultrasound guidance (95% CI: 1.03-4.77). Unfortunately, when focusing only on adult patients, the advantage of POCUS diminished, with 91.4% success in the ultrasound group, 87.7% success in the landmark group, and a nonsignificant odds ratio of 2.10 (95% CI: 0.66-7.44).
Unequivocally, POCUS has established itself as a transformative technology for the guidance of invasive bedside procedures, bringing increased procedural success, improved safety, and decreased complication rates.6 For some procedures, particularly central venous catheterization, ultrasound guidance is a clear standard of care.7,8 For LP, the greatest benefit has been observed in patients with anticipated procedural challenges, most commonly obese patients in whom landmarks are not easily palpable.9 Moreover, the harms ultrasound seeks to prevent are substantially different. The primary risk of deferring ultrasound guidance for LP is most often a failed procedure, whereas for other common ultrasound-guided procedures, the harms may include significant vascular injury, pneumothorax, or bowel perforation. Differences in the relative harms make risk-benefit assessments harder to quantify and studies harder to carry out.
Sonographic guidance for LP has a role in clinical practice and should always be considered. However, at present, there exist no guidelines in any other specialty regarding the routine use of ultrasound-guided LP, including anesthesia, emergency medicine, neurology, or interventional radiology.10-15 As a result, a conservative interpretation of the POCUS Task Force’s findings would be to consider the use of ultrasound guidance for LP in patients where landmark identification is particularly challenging, but not to consider it a standard requirement for accreditation, training, or practice as of yet. Saying “more studies are required” can be a cop-out in some cases, but in this situation, the old adage does seem to apply.
We have great respect for the work of the SHM POCUS Task Force in advancing the use of POCUS in hospital medicine. Though ultrasound is not currently mandated as a care standard for the performance of LP, we all can agree that POCUS does confer advantages for this procedure, particularly in a well-selected patient population. To continue to provide care of the highest quality, hospitalists must be encouraged to elevate their practice with POCUS and be supported with the equipment, training, credentialing, and quality assurance structures necessary to integrate bedside ultrasound safely and effectively into their diagnostic and procedural practice.
Disclosures
No conflicts of interest to disclose.
Funding
None.
Recognizing the increasingly important role of point-of-care ultrasound (POCUS) in advancing clinical care, the Society of Hospital Medicine (SHM) has published a valuable series of position statements to guide hospitalists and administrators on the safe and effective use of POCUS.1 In this issue of the Journal of Hospital Medicine, Soni et al. present a series of consensus-based recommendations on ultrasound guidance for lumbar puncture (LP).2 Among these are the recommendations that ultrasound “should be used” to map the lumbar spine and to select an appropriate puncture site to reduce insertion attempts, reduce needle redirections, and increase overall procedural success.
At first glance, the recommendations appear definitive. However, not immediately obvious is the authors’ clarification that “This position statement does not mandate that hospitalists use ultrasound guidance for LP, nor does it establish ultrasound guidance as the standard of care for LP.” Even with the authors’ caveat, this nuance may not be readily apparent to the readers who review only the executive summary of the guidelines or who omit the context provided in the background of the position statement.
The directive language of this position statement may be a result of an unmerited amplification. The SHM POCUS Task Force employed the Research and Development Appropriateness Method to quantify the degree of consensus and the strength of the recommendation assigned,3 reaching “very good” consensus for each of the recommendations espoused in its position statement. Procedurally, this implies that ≥80% of the 27 voting members rated each published recommendation statement as “appropriate”. Using wording assigned a priori by the committee to each level of consensus, appropriateness became magnified to the declaration “should be used”. In this manner, the strength of the recommendations in this position statement is not necessarily based on the experts’ convictions related to ultrasound-guided LP, nor the strength of the supporting evidence.
In the case of ultrasound-guided LP, we might choose different descriptors than “appropriate” or “should be used”. The evidence base for ultrasound guidance for LP, though growing, may be insufficient as a foundation to a position statement and is certainly insufficient to create a new standard of care for hospitalists. Although the SHM POCUS Task Force completed a thoughtful literature review, no systematic approach (eg, GRADE methodology4) was used to rate the quality of evidence. Furthermore, the literature reviewed was drawn predominantly from anesthesia and emergency medicine sources—not readily generalizable to the hospitalist. Notably, these studies examined all neuraxial procedures (most commonly epidural and spinal anesthesia), which employ different techniques and tools than LP and are performed by clinicians with vastly different procedural training backgrounds than most hospitalists. Altogether, this creates the potential for a gap between true evidence quality and the strength of recommendation.
At a high level, although the technique for ultrasound mapping of the lumbar spine may be similar, the use of ultrasound has been less well studied specifically for LP. When considering LP alone, the available literature is inadequate to recommend uniform ultrasound guidance. A 2018 meta-analysis by Gottlieb et al. included 12 studies focusing only on LP, totaling N = 957 patients.5 This showed some favorability of ultrasound guidance, with a success rate of 90% using ultrasound, 81.4% with a landmark-based approach, and an odds ratio of 2.22 favoring ultrasound guidance (95% CI: 1.03-4.77). Unfortunately, when focusing only on adult patients, the advantage of POCUS diminished, with 91.4% success in the ultrasound group, 87.7% success in the landmark group, and a nonsignificant odds ratio of 2.10 (95% CI: 0.66-7.44).
Unequivocally, POCUS has established itself as a transformative technology for the guidance of invasive bedside procedures, bringing increased procedural success, improved safety, and decreased complication rates.6 For some procedures, particularly central venous catheterization, ultrasound guidance is a clear standard of care.7,8 For LP, the greatest benefit has been observed in patients with anticipated procedural challenges, most commonly obese patients in whom landmarks are not easily palpable.9 Moreover, the harms ultrasound seeks to prevent are substantially different. The primary risk of deferring ultrasound guidance for LP is most often a failed procedure, whereas for other common ultrasound-guided procedures, the harms may include significant vascular injury, pneumothorax, or bowel perforation. Differences in the relative harms make risk-benefit assessments harder to quantify and studies harder to carry out.
Sonographic guidance for LP has a role in clinical practice and should always be considered. However, at present, there exist no guidelines in any other specialty regarding the routine use of ultrasound-guided LP, including anesthesia, emergency medicine, neurology, or interventional radiology.10-15 As a result, a conservative interpretation of the POCUS Task Force’s findings would be to consider the use of ultrasound guidance for LP in patients where landmark identification is particularly challenging, but not to consider it a standard requirement for accreditation, training, or practice as of yet. Saying “more studies are required” can be a cop-out in some cases, but in this situation, the old adage does seem to apply.
We have great respect for the work of the SHM POCUS Task Force in advancing the use of POCUS in hospital medicine. Though ultrasound is not currently mandated as a care standard for the performance of LP, we all can agree that POCUS does confer advantages for this procedure, particularly in a well-selected patient population. To continue to provide care of the highest quality, hospitalists must be encouraged to elevate their practice with POCUS and be supported with the equipment, training, credentialing, and quality assurance structures necessary to integrate bedside ultrasound safely and effectively into their diagnostic and procedural practice.
Disclosures
No conflicts of interest to disclose.
Funding
None.
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3079.
2. Soni NJ, Franco-Sadud R, Dobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
3. Fitch, K, Bernstein SJ, Aguilar MD et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation, 2001.
4. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;334(7650):924-926. PubMed
5. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
6. Moore CL, Copel JA. Point of care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
7. Shojania K, Duncan B, McDonald K, Wachter RM. Making health care safer: a critical analysis of patient safety practices. Rockville, MD: Agency for Healthcare Research and Quality, 2001. Evidence Report/Technology Assessment No. 43; AHRQ publication 01-E058. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catherization. Cochrane Database Syst Rev. 2015;Art. No.: 1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
9. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
11. Neal JM, Brull R, Horn JL, et al. The Second American Society of Regional Anesthesia and Pain Medicine Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia: executive summary. Reg Anesth Pain Med. 2016;41(2):181-194. doi: 10.1097/AAP.0000000000000331.
12. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270-300. doi: 10.1097/ALN.0000000000000935.
13. Engelborghs S, Sebastiaan E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111-126. doi: 10.1016/j.dadm.2017.04.007.
14. American College of Radiology. ACR-SPR-SRU Practice Parameter for the Performing and Interpreting Diagnostic Ultrasound Examinations. 2017; Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-perf-interpret.pdf. Accessed April 15, 2019.
15. American College of Radiology. ACR-AIUM-SPR-SRU Practice Parameter for the Performance of an Ultrasound Examination of the Neonatal and Infant Spine. 2016/ Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-NeonatalSpine.pdf. Accessed April 15, 2019.
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3079.
2. Soni NJ, Franco-Sadud R, Dobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940.
3. Fitch, K, Bernstein SJ, Aguilar MD et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation, 2001.
4. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;334(7650):924-926. PubMed
5. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
6. Moore CL, Copel JA. Point of care ultrasonography. N Engl J Med. 2011;364(8):749-757. https://doi.org/10.1056/NEJMra0909487.
7. Shojania K, Duncan B, McDonald K, Wachter RM. Making health care safer: a critical analysis of patient safety practices. Rockville, MD: Agency for Healthcare Research and Quality, 2001. Evidence Report/Technology Assessment No. 43; AHRQ publication 01-E058. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catherization. Cochrane Database Syst Rev. 2015;Art. No.: 1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
9. Peterson MA, Pisupati D, Heyming TW, Abele JA, Lewis RJ. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21(2):130-136. https://doi.org/10.1111/acem.12305.
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. https://doi.org/10.1016/j.annemergmed.2016.08.457.
11. Neal JM, Brull R, Horn JL, et al. The Second American Society of Regional Anesthesia and Pain Medicine Evidence-Based Medicine Assessment of Ultrasound-Guided Regional Anesthesia: executive summary. Reg Anesth Pain Med. 2016;41(2):181-194. doi: 10.1097/AAP.0000000000000331.
12. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2016;124(2):270-300. doi: 10.1097/ALN.0000000000000935.
13. Engelborghs S, Sebastiaan E, Struyfs H, et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimers Dement. 2017;8:111-126. doi: 10.1016/j.dadm.2017.04.007.
14. American College of Radiology. ACR-SPR-SRU Practice Parameter for the Performing and Interpreting Diagnostic Ultrasound Examinations. 2017; Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-perf-interpret.pdf. Accessed April 15, 2019.
15. American College of Radiology. ACR-AIUM-SPR-SRU Practice Parameter for the Performance of an Ultrasound Examination of the Neonatal and Infant Spine. 2016/ Available at https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-NeonatalSpine.pdf. Accessed April 15, 2019.
© 2019 Society of Hospital Medicine
Improving Respiratory Rate Accuracy in the Hospital: A Quality Improvement Initiative
Respiratory rate (RR) is an essential vital sign that is routinely measured for hospitalized adults. It is a strong predictor of adverse events.1,2 Therefore, RR is a key component of several widely used risk prediction scores, including the systemic inflammatory response syndrome (SIRS).3
Despite its clinical utility, RR is inaccurately measured.4-7 One reason for the inaccurate measurement of RR is that RR measurement, in contrast to that of other vital signs, is not automated. The gold-standard technique for measuring RR is the visual assessment of a resting patient. Thus, RR measurement is perceived as time-consuming. Clinical staff instead frequently approximate RR through brief observation.8-11
Given its clinical importance and widespread inaccuracy, we conducted a quality improvement (QI) initiative to improve RR accuracy.
METHODS
Design and Setting
We conducted an interdisciplinary QI initiative by using the plan–do–study–act (PDSA) methodology from July 2017 to February 2018. The initiative was set in a single adult 28-bed medical inpatient unit of a large, urban, safety-net hospital consisting of general internal medicine and hematology/oncology patients. Routine vital sign measurements on this unit occur at four- or six-hour intervals per physician orders and are performed by patient-care assistants (PCAs) who are nonregistered nursing support staff. PCAs use a vital signs cart equipped with automated tools to measure vital signs except for RR, which is manually assessed. PCAs are trained on vital sign measurements during a two-day onboarding orientation and four to six weeks of on-the-job training by experienced PCAs. PCAs are directly supervised by nursing operations managers. Formal continuing education programs for PCAs or performance audits of their clinical duties did not exist prior to our QI initiative.
Intervention
Intervention development addressing several important barriers and workflow inefficiencies was based on the direct observation of PCA workflow and information gathering by engaging stakeholders, including PCAs, nursing operations management, nursing leadership, and hospital administration (PDSA cycles 1-7 in Table). Our modified PCA vital sign workflow incorporated RR measurement during the approximate 30 seconds needed to complete automated blood pressure measurement as previously described.12 Nursing administration purchased three stopwatches (each $5 US) to attach to vital signs carts. One investigator (NK) participated in two monthly one-hour meetings, and three investigators (NK, KB, and SD) participated in 19 daily 15-minute huddles to conduct stakeholder engagement and educate and retrain PCAs on proper technique (total of 6.75 hours).
Evaluation
The primary aim of this QI initiative was to improve RR accuracy, which was evaluated using two distinct but complementary analyses: the prospective comparison of PCA-recorded RRs with gold-standard recorded RRs and the retrospective comparison of RRs recorded in electronic health records (EHR) on the intervention unit versus two control units. The secondary aims were to examine time to complete vital sign measurement and to assess whether the intervention was associated with a reduction in the incidence of SIRS specifically due to tachypnea.
Respiratory Rate Accuracy
PCA-recorded RRs were considered accurate if the RR was within ±2 breaths of a gold-standard RR measurement performed by a trained study member (NK or KB). We conducted gold-standard RR measurements for 100 observations pre- and postintervention within 30 minutes of PCA measurement to avoid Hawthorne bias.
We assessed the variability of recorded RRs in the EHR for all patients in the intervention unit as a proxy for accuracy. We hypothesized on the basis of prior research that improving the accuracy of RR measurement would increase the variability and normality of distribution in RRs.13 This is an approach that we have employed previously.7 The EHR cohort included consecutive hospitalizations by patients who were admitted to either the intervention unit or to one of two nonintervention general medicine inpatient units that served as concurrent controls. We grouped hospitalizations into a preintervention phase from March 1, 2017-July 22, 2017, a planning phase from July 23, 2017-December 3, 2017, and a postintervention phase from December 21, 2017-February 28, 2018. Hospitalizations during the two-week teaching phase from December 3, 2017-December 21, 2017 were excluded. We excluded vital signs obtained in the emergency department or in a location different from the patient’s admission unit. We qualitatively assessed RR distribution using histograms as we have done previously.7
We examined the distributions of RRs recorded in the EHR before and after intervention by individual PCAs on the intervention floor to assess for fidelity and adherence in the PCA uptake of the intervention.
Time
We compared the time to complete vital sign measurement among convenience samples of 50 unique observations pre- and postintervention using the Wilcoxon rank sum test.
SIRS Incidence
Since we hypothesized that improved RR accuracy would reduce falsely elevated RRs but have no impact on the other three SIRS criteria, we assessed changes in tachypnea-specific SIRS incidence, which was defined a priori as the presence of exactly two concurrent SIRS criteria, one of which was an elevated RR.3 We examined changes using a difference-in-differences approach with three different units of analysis (per vital sign measurement, hospital-day, and hospitalization; see footnote for Appendix Table 1 for methodological details. All analyses were conducted using STATA 12.0 (StataCorp, College Station, Texas).
RESULTS
Respiratory Rate Accuracy
Prior to the intervention, the median PCA RR was 18 (IQR 18-20) versus 12 (IQR 12-18) for the gold-standard RR (Appendix Figure 1), with only 36% of PCA measurements considered accurate. After the intervention, the median PCA-recorded RR was 14 (IQR 15-20) versus 14 (IQR 14-20) for the gold-standard RR and a RR accuracy of 58% (P < .001).
For our analyses on RR distribution using EHR data, we included 143,447 unique RRs (Appendix Table 2). After the intervention, the normality of the distribution of RRs on the intervention unit had increased, whereas those of RRs on the control units remained qualitatively similar pre- and postintervention (Appendix Figure 2).
Notable differences existed among the 11 individual PCAs (Figure) despite observing increased variability in PCA-recorded RRs postintervention. Some PCAs (numbers 2, 7, and 10) shifted their narrow RR interquartile range lower by several breaths/minute, whereas most other PCAs had a reduced median RR and widened interquartile range.
Time
Before the intervention, the median time to complete vital sign measurements was 2:36 (IQR 2:04-3:20). After the intervention, the time to complete vital signs decreased to 1:55 (IQR, 1:40-2:22; P < .001), which was 41 less seconds on average per vital sign set.
SIRS Incidence
The intervention was associated with a 3.3% reduction (95% CI, –6.4% to –0.005%) in tachypnea-specific SIRS incidence per hospital-day and a 7.8% reduction (95% CI, –13.5% to –2.2%) per hospitalization (Appendix Table 1). We also observed a modest reduction in overall SIRS incidence after the intervention (2.9% less per vital sign check, 4.6% less per hospital-day, and 3.2% less per hospitalization), although these reductions were not statistically significant.
DISCUSSION
Our QI initiative improved the absolute RR accuracy by 22%, saved PCAs 41 seconds on average per vital sign measurement, and decreased the absolute proportion of hospitalizations with tachypnea-specific SIRS by 7.8%. Our intervention is a novel, interdisciplinary, low-cost, low-effort, low-tech approach that addressed known challenges to accurate RR measurement,8,9,11 as well as the key barriers identified in our initial PDSA cycles. Our approach includes adding a time-keeping device to vital sign carts and standardizing a PCA vital sign workflow with increased efficiency. Lastly, this intervention is potentially scalable because stakeholder engagement, education, and retraining of the entire PCA staff for the unit required only 6.75 hours.
While our primary goal was to improve RR accuracy, our QI initiative also improved vital sign efficiency. By extrapolating our findings to an eight-hour PCA shift caring for eight patients who require vital sign checks every four hours, we estimated that our intervention would save approximately 16:24 minutes per PCA shift. This newfound time could be repurposed for other patient-care tasks or could be spent ensuring the accuracy of other vital signs given that accurate monitoring may be neglected because of time constraints.11 Additionally, the improvement in RR accuracy reduced falsely elevated RRs and thus lowered SIRS incidence specifically due to tachypnea. Given that EHR-based sepsis alerts are often based on SIRS criteria, improved RR accuracy may also improve alarm fatigue by reducing the rate of false-positive alerts.14
This initiative is not without limitations. Generalizability to other hospitals and even other units within the same hospital is uncertain. However, because this initiative was conducted within a safety-net hospital, we anticipate at least similar, if not increased, success in better-resourced hospitals. Second, the long-term durability of our intervention is unclear, although EHR RR variability remained steady for two months after our intervention (data not shown).
To ensure long-term sustainability and further improve RR accuracy, future PDSA cycles could include electing a PCA “vital signs champion” to reiterate the importance of RRs in clinical decision-making and ensure adherence to the modified workflow. Nursing champions act as persuasive change agents that disseminate and implement healthcare change,15 which may also be true of PCA champions. Additionally, future PDSA cycles can obviate the need for labor-intensive manual audits by leveraging EHR-based auditing to target education and retraining interventions to PCAs with minimal RR variability to optimize workflow adherence.
In conclusion, through a multipronged QI initiative we improved RR accuracy, increased the efficiency of vital sign measurement, and decreased SIRS incidence specifically due to tachypnea by reducing the number of falsely elevated RRs. This novel, low-cost, low-effort, low-tech approach can readily be implemented and disseminated in hospital inpatient settings.
Acknowledgments
The authors would like to acknowledge the meaningful contributions of Mr. Sudarshaan Pathak, RN, Ms. Shirly Koduvathu, RN, and Ms. Judy Herrington MSN, RN in this multidisciplinary initiative. We thank Mr. Christopher McKintosh, RN for his support in data acquisition. Lastly, the authors would like to acknowledge all of the patient-care assistants involved in this QI initiative.
Disclosures
Dr. Makam reports grants from NIA/NIH, during the conduct of the study. All other authors have nothing to disclose.
Funding
This work is supported in part by the Agency for Healthcare Research and Quality-funded UT Southwestern Center for Patient-Centered Outcomes Research (R24HS022418). OKN is funded by the National Heart, Lung, and Blood Institute (K23HL133441), and ANM is funded by the National Institute on Aging (K23AG052603).
1. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. https://doi.org/10.1007/BF02600071.
2. Hodgetts TJ, Kenward G, Vlachonikolis IG, Payne S, Castle N. The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team. Resuscitation. 2002;54(2):125-131. https://doi.org/10.1016/S0300-9572(02)00100-4.
3. Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481-1483.
4. Lovett PB, Buchwald JM, Sturmann K, Bijur P. The vexatious vital: neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage. Ann Emerg Med. 2005;45(1):68-76. https://doi.org/10.1016/j.annemergmed.2004.06.016.
5. Chen J, Hillman K, Bellomo R, et al. The impact of introducing medical emergency team system on the documentations of vital signs. Resuscitation. 2009;80(1):35-43. https://doi.org/10.1016/j.resuscitation.2008.10.009.
6. Leuvan CH, Mitchell I. Missed opportunities? An observational study of vital sign measurements. Crit Care Resusc. 2008;10(2):111-115.
7. Badawy J, Nguyen OK, Clark C, Halm EA, Makam AN. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual Saf. 2017;26(10):832-836. https://doi.org/10.1136/bmjqs-2017-006671.
8. Chua WL, Mackey S, Ng EK, Liaw SY. Front line nurses’ experiences with deteriorating ward patients: a qualitative study. Int Nurs Rev. 2013;60(4):501-509. https://doi.org/10.1111/inr.12061.
9. De Meester K, Van Bogaert P, Clarke SP, Bossaert L. In-hospital mortality after serious adverse events on medical and surgical nursing units: a mixed methods study. J Clin Nurs. 2013;22(15-16):2308-2317. https://doi.org/10.1111/j.1365-2702.2012.04154.x.
10. Cheng AC, Black JF, Buising KL. Respiratory rate: the neglected vital sign. Med J Aust. 2008;189(9):531. https://doi.org/10.5694/j.1326-5377.2008.tb02163.x.
11. Mok W, Wang W, Cooper S, Ang EN, Liaw SY. Attitudes towards vital signs monitoring in the detection of clinical deterioration: scale development and survey of ward nurses. Int J Qual Health Care. 2015;27(3):207-213. https://doi.org/10.1093/intqhc/mzv019.
12. Keshvani N, Berger K, Nguyen OK, Makam AN. Roadmap for improving the accuracy of respiratory rate measurements. BMJ Qual Saf. 2018;27(8):e5. https://doi.org/10.1136/bmjqs-2017-007516.
13. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. https://doi.org/10.1378/chest.12-1837.
14. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: a systematic review. J Hosp Med. 2015;10(6):396-402. https://doi.org/10.1002/jhm.2347.
15. Ploeg J, Skelly J, Rowan M, et al. The role of nursing best practice champions in diffusing practice guidelines: a mixed methods study. Worldviews Evid Based Nurs. 2010;7(4):238-251. https://doi.org/10.1111/j.1741-6787.2010.00202.x.
Respiratory rate (RR) is an essential vital sign that is routinely measured for hospitalized adults. It is a strong predictor of adverse events.1,2 Therefore, RR is a key component of several widely used risk prediction scores, including the systemic inflammatory response syndrome (SIRS).3
Despite its clinical utility, RR is inaccurately measured.4-7 One reason for the inaccurate measurement of RR is that RR measurement, in contrast to that of other vital signs, is not automated. The gold-standard technique for measuring RR is the visual assessment of a resting patient. Thus, RR measurement is perceived as time-consuming. Clinical staff instead frequently approximate RR through brief observation.8-11
Given its clinical importance and widespread inaccuracy, we conducted a quality improvement (QI) initiative to improve RR accuracy.
METHODS
Design and Setting
We conducted an interdisciplinary QI initiative by using the plan–do–study–act (PDSA) methodology from July 2017 to February 2018. The initiative was set in a single adult 28-bed medical inpatient unit of a large, urban, safety-net hospital consisting of general internal medicine and hematology/oncology patients. Routine vital sign measurements on this unit occur at four- or six-hour intervals per physician orders and are performed by patient-care assistants (PCAs) who are nonregistered nursing support staff. PCAs use a vital signs cart equipped with automated tools to measure vital signs except for RR, which is manually assessed. PCAs are trained on vital sign measurements during a two-day onboarding orientation and four to six weeks of on-the-job training by experienced PCAs. PCAs are directly supervised by nursing operations managers. Formal continuing education programs for PCAs or performance audits of their clinical duties did not exist prior to our QI initiative.
Intervention
Intervention development addressing several important barriers and workflow inefficiencies was based on the direct observation of PCA workflow and information gathering by engaging stakeholders, including PCAs, nursing operations management, nursing leadership, and hospital administration (PDSA cycles 1-7 in Table). Our modified PCA vital sign workflow incorporated RR measurement during the approximate 30 seconds needed to complete automated blood pressure measurement as previously described.12 Nursing administration purchased three stopwatches (each $5 US) to attach to vital signs carts. One investigator (NK) participated in two monthly one-hour meetings, and three investigators (NK, KB, and SD) participated in 19 daily 15-minute huddles to conduct stakeholder engagement and educate and retrain PCAs on proper technique (total of 6.75 hours).
Evaluation
The primary aim of this QI initiative was to improve RR accuracy, which was evaluated using two distinct but complementary analyses: the prospective comparison of PCA-recorded RRs with gold-standard recorded RRs and the retrospective comparison of RRs recorded in electronic health records (EHR) on the intervention unit versus two control units. The secondary aims were to examine time to complete vital sign measurement and to assess whether the intervention was associated with a reduction in the incidence of SIRS specifically due to tachypnea.
Respiratory Rate Accuracy
PCA-recorded RRs were considered accurate if the RR was within ±2 breaths of a gold-standard RR measurement performed by a trained study member (NK or KB). We conducted gold-standard RR measurements for 100 observations pre- and postintervention within 30 minutes of PCA measurement to avoid Hawthorne bias.
We assessed the variability of recorded RRs in the EHR for all patients in the intervention unit as a proxy for accuracy. We hypothesized on the basis of prior research that improving the accuracy of RR measurement would increase the variability and normality of distribution in RRs.13 This is an approach that we have employed previously.7 The EHR cohort included consecutive hospitalizations by patients who were admitted to either the intervention unit or to one of two nonintervention general medicine inpatient units that served as concurrent controls. We grouped hospitalizations into a preintervention phase from March 1, 2017-July 22, 2017, a planning phase from July 23, 2017-December 3, 2017, and a postintervention phase from December 21, 2017-February 28, 2018. Hospitalizations during the two-week teaching phase from December 3, 2017-December 21, 2017 were excluded. We excluded vital signs obtained in the emergency department or in a location different from the patient’s admission unit. We qualitatively assessed RR distribution using histograms as we have done previously.7
We examined the distributions of RRs recorded in the EHR before and after intervention by individual PCAs on the intervention floor to assess for fidelity and adherence in the PCA uptake of the intervention.
Time
We compared the time to complete vital sign measurement among convenience samples of 50 unique observations pre- and postintervention using the Wilcoxon rank sum test.
SIRS Incidence
Since we hypothesized that improved RR accuracy would reduce falsely elevated RRs but have no impact on the other three SIRS criteria, we assessed changes in tachypnea-specific SIRS incidence, which was defined a priori as the presence of exactly two concurrent SIRS criteria, one of which was an elevated RR.3 We examined changes using a difference-in-differences approach with three different units of analysis (per vital sign measurement, hospital-day, and hospitalization; see footnote for Appendix Table 1 for methodological details. All analyses were conducted using STATA 12.0 (StataCorp, College Station, Texas).
RESULTS
Respiratory Rate Accuracy
Prior to the intervention, the median PCA RR was 18 (IQR 18-20) versus 12 (IQR 12-18) for the gold-standard RR (Appendix Figure 1), with only 36% of PCA measurements considered accurate. After the intervention, the median PCA-recorded RR was 14 (IQR 15-20) versus 14 (IQR 14-20) for the gold-standard RR and a RR accuracy of 58% (P < .001).
For our analyses on RR distribution using EHR data, we included 143,447 unique RRs (Appendix Table 2). After the intervention, the normality of the distribution of RRs on the intervention unit had increased, whereas those of RRs on the control units remained qualitatively similar pre- and postintervention (Appendix Figure 2).
Notable differences existed among the 11 individual PCAs (Figure) despite observing increased variability in PCA-recorded RRs postintervention. Some PCAs (numbers 2, 7, and 10) shifted their narrow RR interquartile range lower by several breaths/minute, whereas most other PCAs had a reduced median RR and widened interquartile range.
Time
Before the intervention, the median time to complete vital sign measurements was 2:36 (IQR 2:04-3:20). After the intervention, the time to complete vital signs decreased to 1:55 (IQR, 1:40-2:22; P < .001), which was 41 less seconds on average per vital sign set.
SIRS Incidence
The intervention was associated with a 3.3% reduction (95% CI, –6.4% to –0.005%) in tachypnea-specific SIRS incidence per hospital-day and a 7.8% reduction (95% CI, –13.5% to –2.2%) per hospitalization (Appendix Table 1). We also observed a modest reduction in overall SIRS incidence after the intervention (2.9% less per vital sign check, 4.6% less per hospital-day, and 3.2% less per hospitalization), although these reductions were not statistically significant.
DISCUSSION
Our QI initiative improved the absolute RR accuracy by 22%, saved PCAs 41 seconds on average per vital sign measurement, and decreased the absolute proportion of hospitalizations with tachypnea-specific SIRS by 7.8%. Our intervention is a novel, interdisciplinary, low-cost, low-effort, low-tech approach that addressed known challenges to accurate RR measurement,8,9,11 as well as the key barriers identified in our initial PDSA cycles. Our approach includes adding a time-keeping device to vital sign carts and standardizing a PCA vital sign workflow with increased efficiency. Lastly, this intervention is potentially scalable because stakeholder engagement, education, and retraining of the entire PCA staff for the unit required only 6.75 hours.
While our primary goal was to improve RR accuracy, our QI initiative also improved vital sign efficiency. By extrapolating our findings to an eight-hour PCA shift caring for eight patients who require vital sign checks every four hours, we estimated that our intervention would save approximately 16:24 minutes per PCA shift. This newfound time could be repurposed for other patient-care tasks or could be spent ensuring the accuracy of other vital signs given that accurate monitoring may be neglected because of time constraints.11 Additionally, the improvement in RR accuracy reduced falsely elevated RRs and thus lowered SIRS incidence specifically due to tachypnea. Given that EHR-based sepsis alerts are often based on SIRS criteria, improved RR accuracy may also improve alarm fatigue by reducing the rate of false-positive alerts.14
This initiative is not without limitations. Generalizability to other hospitals and even other units within the same hospital is uncertain. However, because this initiative was conducted within a safety-net hospital, we anticipate at least similar, if not increased, success in better-resourced hospitals. Second, the long-term durability of our intervention is unclear, although EHR RR variability remained steady for two months after our intervention (data not shown).
To ensure long-term sustainability and further improve RR accuracy, future PDSA cycles could include electing a PCA “vital signs champion” to reiterate the importance of RRs in clinical decision-making and ensure adherence to the modified workflow. Nursing champions act as persuasive change agents that disseminate and implement healthcare change,15 which may also be true of PCA champions. Additionally, future PDSA cycles can obviate the need for labor-intensive manual audits by leveraging EHR-based auditing to target education and retraining interventions to PCAs with minimal RR variability to optimize workflow adherence.
In conclusion, through a multipronged QI initiative we improved RR accuracy, increased the efficiency of vital sign measurement, and decreased SIRS incidence specifically due to tachypnea by reducing the number of falsely elevated RRs. This novel, low-cost, low-effort, low-tech approach can readily be implemented and disseminated in hospital inpatient settings.
Acknowledgments
The authors would like to acknowledge the meaningful contributions of Mr. Sudarshaan Pathak, RN, Ms. Shirly Koduvathu, RN, and Ms. Judy Herrington MSN, RN in this multidisciplinary initiative. We thank Mr. Christopher McKintosh, RN for his support in data acquisition. Lastly, the authors would like to acknowledge all of the patient-care assistants involved in this QI initiative.
Disclosures
Dr. Makam reports grants from NIA/NIH, during the conduct of the study. All other authors have nothing to disclose.
Funding
This work is supported in part by the Agency for Healthcare Research and Quality-funded UT Southwestern Center for Patient-Centered Outcomes Research (R24HS022418). OKN is funded by the National Heart, Lung, and Blood Institute (K23HL133441), and ANM is funded by the National Institute on Aging (K23AG052603).
Respiratory rate (RR) is an essential vital sign that is routinely measured for hospitalized adults. It is a strong predictor of adverse events.1,2 Therefore, RR is a key component of several widely used risk prediction scores, including the systemic inflammatory response syndrome (SIRS).3
Despite its clinical utility, RR is inaccurately measured.4-7 One reason for the inaccurate measurement of RR is that RR measurement, in contrast to that of other vital signs, is not automated. The gold-standard technique for measuring RR is the visual assessment of a resting patient. Thus, RR measurement is perceived as time-consuming. Clinical staff instead frequently approximate RR through brief observation.8-11
Given its clinical importance and widespread inaccuracy, we conducted a quality improvement (QI) initiative to improve RR accuracy.
METHODS
Design and Setting
We conducted an interdisciplinary QI initiative by using the plan–do–study–act (PDSA) methodology from July 2017 to February 2018. The initiative was set in a single adult 28-bed medical inpatient unit of a large, urban, safety-net hospital consisting of general internal medicine and hematology/oncology patients. Routine vital sign measurements on this unit occur at four- or six-hour intervals per physician orders and are performed by patient-care assistants (PCAs) who are nonregistered nursing support staff. PCAs use a vital signs cart equipped with automated tools to measure vital signs except for RR, which is manually assessed. PCAs are trained on vital sign measurements during a two-day onboarding orientation and four to six weeks of on-the-job training by experienced PCAs. PCAs are directly supervised by nursing operations managers. Formal continuing education programs for PCAs or performance audits of their clinical duties did not exist prior to our QI initiative.
Intervention
Intervention development addressing several important barriers and workflow inefficiencies was based on the direct observation of PCA workflow and information gathering by engaging stakeholders, including PCAs, nursing operations management, nursing leadership, and hospital administration (PDSA cycles 1-7 in Table). Our modified PCA vital sign workflow incorporated RR measurement during the approximate 30 seconds needed to complete automated blood pressure measurement as previously described.12 Nursing administration purchased three stopwatches (each $5 US) to attach to vital signs carts. One investigator (NK) participated in two monthly one-hour meetings, and three investigators (NK, KB, and SD) participated in 19 daily 15-minute huddles to conduct stakeholder engagement and educate and retrain PCAs on proper technique (total of 6.75 hours).
Evaluation
The primary aim of this QI initiative was to improve RR accuracy, which was evaluated using two distinct but complementary analyses: the prospective comparison of PCA-recorded RRs with gold-standard recorded RRs and the retrospective comparison of RRs recorded in electronic health records (EHR) on the intervention unit versus two control units. The secondary aims were to examine time to complete vital sign measurement and to assess whether the intervention was associated with a reduction in the incidence of SIRS specifically due to tachypnea.
Respiratory Rate Accuracy
PCA-recorded RRs were considered accurate if the RR was within ±2 breaths of a gold-standard RR measurement performed by a trained study member (NK or KB). We conducted gold-standard RR measurements for 100 observations pre- and postintervention within 30 minutes of PCA measurement to avoid Hawthorne bias.
We assessed the variability of recorded RRs in the EHR for all patients in the intervention unit as a proxy for accuracy. We hypothesized on the basis of prior research that improving the accuracy of RR measurement would increase the variability and normality of distribution in RRs.13 This is an approach that we have employed previously.7 The EHR cohort included consecutive hospitalizations by patients who were admitted to either the intervention unit or to one of two nonintervention general medicine inpatient units that served as concurrent controls. We grouped hospitalizations into a preintervention phase from March 1, 2017-July 22, 2017, a planning phase from July 23, 2017-December 3, 2017, and a postintervention phase from December 21, 2017-February 28, 2018. Hospitalizations during the two-week teaching phase from December 3, 2017-December 21, 2017 were excluded. We excluded vital signs obtained in the emergency department or in a location different from the patient’s admission unit. We qualitatively assessed RR distribution using histograms as we have done previously.7
We examined the distributions of RRs recorded in the EHR before and after intervention by individual PCAs on the intervention floor to assess for fidelity and adherence in the PCA uptake of the intervention.
Time
We compared the time to complete vital sign measurement among convenience samples of 50 unique observations pre- and postintervention using the Wilcoxon rank sum test.
SIRS Incidence
Since we hypothesized that improved RR accuracy would reduce falsely elevated RRs but have no impact on the other three SIRS criteria, we assessed changes in tachypnea-specific SIRS incidence, which was defined a priori as the presence of exactly two concurrent SIRS criteria, one of which was an elevated RR.3 We examined changes using a difference-in-differences approach with three different units of analysis (per vital sign measurement, hospital-day, and hospitalization; see footnote for Appendix Table 1 for methodological details. All analyses were conducted using STATA 12.0 (StataCorp, College Station, Texas).
RESULTS
Respiratory Rate Accuracy
Prior to the intervention, the median PCA RR was 18 (IQR 18-20) versus 12 (IQR 12-18) for the gold-standard RR (Appendix Figure 1), with only 36% of PCA measurements considered accurate. After the intervention, the median PCA-recorded RR was 14 (IQR 15-20) versus 14 (IQR 14-20) for the gold-standard RR and a RR accuracy of 58% (P < .001).
For our analyses on RR distribution using EHR data, we included 143,447 unique RRs (Appendix Table 2). After the intervention, the normality of the distribution of RRs on the intervention unit had increased, whereas those of RRs on the control units remained qualitatively similar pre- and postintervention (Appendix Figure 2).
Notable differences existed among the 11 individual PCAs (Figure) despite observing increased variability in PCA-recorded RRs postintervention. Some PCAs (numbers 2, 7, and 10) shifted their narrow RR interquartile range lower by several breaths/minute, whereas most other PCAs had a reduced median RR and widened interquartile range.
Time
Before the intervention, the median time to complete vital sign measurements was 2:36 (IQR 2:04-3:20). After the intervention, the time to complete vital signs decreased to 1:55 (IQR, 1:40-2:22; P < .001), which was 41 less seconds on average per vital sign set.
SIRS Incidence
The intervention was associated with a 3.3% reduction (95% CI, –6.4% to –0.005%) in tachypnea-specific SIRS incidence per hospital-day and a 7.8% reduction (95% CI, –13.5% to –2.2%) per hospitalization (Appendix Table 1). We also observed a modest reduction in overall SIRS incidence after the intervention (2.9% less per vital sign check, 4.6% less per hospital-day, and 3.2% less per hospitalization), although these reductions were not statistically significant.
DISCUSSION
Our QI initiative improved the absolute RR accuracy by 22%, saved PCAs 41 seconds on average per vital sign measurement, and decreased the absolute proportion of hospitalizations with tachypnea-specific SIRS by 7.8%. Our intervention is a novel, interdisciplinary, low-cost, low-effort, low-tech approach that addressed known challenges to accurate RR measurement,8,9,11 as well as the key barriers identified in our initial PDSA cycles. Our approach includes adding a time-keeping device to vital sign carts and standardizing a PCA vital sign workflow with increased efficiency. Lastly, this intervention is potentially scalable because stakeholder engagement, education, and retraining of the entire PCA staff for the unit required only 6.75 hours.
While our primary goal was to improve RR accuracy, our QI initiative also improved vital sign efficiency. By extrapolating our findings to an eight-hour PCA shift caring for eight patients who require vital sign checks every four hours, we estimated that our intervention would save approximately 16:24 minutes per PCA shift. This newfound time could be repurposed for other patient-care tasks or could be spent ensuring the accuracy of other vital signs given that accurate monitoring may be neglected because of time constraints.11 Additionally, the improvement in RR accuracy reduced falsely elevated RRs and thus lowered SIRS incidence specifically due to tachypnea. Given that EHR-based sepsis alerts are often based on SIRS criteria, improved RR accuracy may also improve alarm fatigue by reducing the rate of false-positive alerts.14
This initiative is not without limitations. Generalizability to other hospitals and even other units within the same hospital is uncertain. However, because this initiative was conducted within a safety-net hospital, we anticipate at least similar, if not increased, success in better-resourced hospitals. Second, the long-term durability of our intervention is unclear, although EHR RR variability remained steady for two months after our intervention (data not shown).
To ensure long-term sustainability and further improve RR accuracy, future PDSA cycles could include electing a PCA “vital signs champion” to reiterate the importance of RRs in clinical decision-making and ensure adherence to the modified workflow. Nursing champions act as persuasive change agents that disseminate and implement healthcare change,15 which may also be true of PCA champions. Additionally, future PDSA cycles can obviate the need for labor-intensive manual audits by leveraging EHR-based auditing to target education and retraining interventions to PCAs with minimal RR variability to optimize workflow adherence.
In conclusion, through a multipronged QI initiative we improved RR accuracy, increased the efficiency of vital sign measurement, and decreased SIRS incidence specifically due to tachypnea by reducing the number of falsely elevated RRs. This novel, low-cost, low-effort, low-tech approach can readily be implemented and disseminated in hospital inpatient settings.
Acknowledgments
The authors would like to acknowledge the meaningful contributions of Mr. Sudarshaan Pathak, RN, Ms. Shirly Koduvathu, RN, and Ms. Judy Herrington MSN, RN in this multidisciplinary initiative. We thank Mr. Christopher McKintosh, RN for his support in data acquisition. Lastly, the authors would like to acknowledge all of the patient-care assistants involved in this QI initiative.
Disclosures
Dr. Makam reports grants from NIA/NIH, during the conduct of the study. All other authors have nothing to disclose.
Funding
This work is supported in part by the Agency for Healthcare Research and Quality-funded UT Southwestern Center for Patient-Centered Outcomes Research (R24HS022418). OKN is funded by the National Heart, Lung, and Blood Institute (K23HL133441), and ANM is funded by the National Institute on Aging (K23AG052603).
1. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. https://doi.org/10.1007/BF02600071.
2. Hodgetts TJ, Kenward G, Vlachonikolis IG, Payne S, Castle N. The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team. Resuscitation. 2002;54(2):125-131. https://doi.org/10.1016/S0300-9572(02)00100-4.
3. Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481-1483.
4. Lovett PB, Buchwald JM, Sturmann K, Bijur P. The vexatious vital: neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage. Ann Emerg Med. 2005;45(1):68-76. https://doi.org/10.1016/j.annemergmed.2004.06.016.
5. Chen J, Hillman K, Bellomo R, et al. The impact of introducing medical emergency team system on the documentations of vital signs. Resuscitation. 2009;80(1):35-43. https://doi.org/10.1016/j.resuscitation.2008.10.009.
6. Leuvan CH, Mitchell I. Missed opportunities? An observational study of vital sign measurements. Crit Care Resusc. 2008;10(2):111-115.
7. Badawy J, Nguyen OK, Clark C, Halm EA, Makam AN. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual Saf. 2017;26(10):832-836. https://doi.org/10.1136/bmjqs-2017-006671.
8. Chua WL, Mackey S, Ng EK, Liaw SY. Front line nurses’ experiences with deteriorating ward patients: a qualitative study. Int Nurs Rev. 2013;60(4):501-509. https://doi.org/10.1111/inr.12061.
9. De Meester K, Van Bogaert P, Clarke SP, Bossaert L. In-hospital mortality after serious adverse events on medical and surgical nursing units: a mixed methods study. J Clin Nurs. 2013;22(15-16):2308-2317. https://doi.org/10.1111/j.1365-2702.2012.04154.x.
10. Cheng AC, Black JF, Buising KL. Respiratory rate: the neglected vital sign. Med J Aust. 2008;189(9):531. https://doi.org/10.5694/j.1326-5377.2008.tb02163.x.
11. Mok W, Wang W, Cooper S, Ang EN, Liaw SY. Attitudes towards vital signs monitoring in the detection of clinical deterioration: scale development and survey of ward nurses. Int J Qual Health Care. 2015;27(3):207-213. https://doi.org/10.1093/intqhc/mzv019.
12. Keshvani N, Berger K, Nguyen OK, Makam AN. Roadmap for improving the accuracy of respiratory rate measurements. BMJ Qual Saf. 2018;27(8):e5. https://doi.org/10.1136/bmjqs-2017-007516.
13. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. https://doi.org/10.1378/chest.12-1837.
14. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: a systematic review. J Hosp Med. 2015;10(6):396-402. https://doi.org/10.1002/jhm.2347.
15. Ploeg J, Skelly J, Rowan M, et al. The role of nursing best practice champions in diffusing practice guidelines: a mixed methods study. Worldviews Evid Based Nurs. 2010;7(4):238-251. https://doi.org/10.1111/j.1741-6787.2010.00202.x.
1. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. https://doi.org/10.1007/BF02600071.
2. Hodgetts TJ, Kenward G, Vlachonikolis IG, Payne S, Castle N. The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team. Resuscitation. 2002;54(2):125-131. https://doi.org/10.1016/S0300-9572(02)00100-4.
3. Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481-1483.
4. Lovett PB, Buchwald JM, Sturmann K, Bijur P. The vexatious vital: neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage. Ann Emerg Med. 2005;45(1):68-76. https://doi.org/10.1016/j.annemergmed.2004.06.016.
5. Chen J, Hillman K, Bellomo R, et al. The impact of introducing medical emergency team system on the documentations of vital signs. Resuscitation. 2009;80(1):35-43. https://doi.org/10.1016/j.resuscitation.2008.10.009.
6. Leuvan CH, Mitchell I. Missed opportunities? An observational study of vital sign measurements. Crit Care Resusc. 2008;10(2):111-115.
7. Badawy J, Nguyen OK, Clark C, Halm EA, Makam AN. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual Saf. 2017;26(10):832-836. https://doi.org/10.1136/bmjqs-2017-006671.
8. Chua WL, Mackey S, Ng EK, Liaw SY. Front line nurses’ experiences with deteriorating ward patients: a qualitative study. Int Nurs Rev. 2013;60(4):501-509. https://doi.org/10.1111/inr.12061.
9. De Meester K, Van Bogaert P, Clarke SP, Bossaert L. In-hospital mortality after serious adverse events on medical and surgical nursing units: a mixed methods study. J Clin Nurs. 2013;22(15-16):2308-2317. https://doi.org/10.1111/j.1365-2702.2012.04154.x.
10. Cheng AC, Black JF, Buising KL. Respiratory rate: the neglected vital sign. Med J Aust. 2008;189(9):531. https://doi.org/10.5694/j.1326-5377.2008.tb02163.x.
11. Mok W, Wang W, Cooper S, Ang EN, Liaw SY. Attitudes towards vital signs monitoring in the detection of clinical deterioration: scale development and survey of ward nurses. Int J Qual Health Care. 2015;27(3):207-213. https://doi.org/10.1093/intqhc/mzv019.
12. Keshvani N, Berger K, Nguyen OK, Makam AN. Roadmap for improving the accuracy of respiratory rate measurements. BMJ Qual Saf. 2018;27(8):e5. https://doi.org/10.1136/bmjqs-2017-007516.
13. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. https://doi.org/10.1378/chest.12-1837.
14. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: a systematic review. J Hosp Med. 2015;10(6):396-402. https://doi.org/10.1002/jhm.2347.
15. Ploeg J, Skelly J, Rowan M, et al. The role of nursing best practice champions in diffusing practice guidelines: a mixed methods study. Worldviews Evid Based Nurs. 2010;7(4):238-251. https://doi.org/10.1111/j.1741-6787.2010.00202.x.
© 2019 Society of Hospital Medicine
Adverse Events Experienced by Patients Hospitalized without Definite Medical Acuity: A Retrospective Cohort Study
Evidence exists that physicians consider what may be called “social” or “nonmedical” factors (lack of social support or barriers to access) in hospital admission decision-making and that patients are hospitalized even in the absence of a level of medical acuity warranting admission.1-3 Although hospitalization is associated with the risk of adverse events (AEs),4 whether this risk is related to the medical acuity of admission remains unclear. Our study sought to quantify the AEs experienced by patients hospitalized without definite medical acuity compared with those experienced by patients hospitalized with a definite medically appropriate indication for admission.
METHODS
Setting and Database Used for Analysis
This study was conducted at an urban, safety-net, public teaching hospital. At our site, calls for medical admissions are always answered by a hospital medicine attending physician (“triage physician”) who works collaboratively with the referring physician to facilitate appropriate disposition. Many of these discussions occur via telephone, but the triage physician may also assess the patient directly if needed. This study involved 24 triage physicians who directly assessed the patient in 65% of the cases.
At the time of each admission call, the triage physician logs the following information into a central triage database: date and time of call, patient location, reason for admission, assessment of appropriateness for medical floor, contributing factors to admission decision-making, and patient disposition.
Admission Appropriateness Group Designation
To be considered for inclusion in this study, calls must have originated from the emergency department and resulted in admission to the general medicine floor on either a resident teaching or hospitalist service from February 1, 2018 to June 1, 2018. This time frame was selected to avoid the start of a new academic cycle in late June that may confound AE rates.
The designation of appropriateness was determined by the triage physician’s logged response to triage database questions at the time of the admission call. Of the 748 admissions meeting inclusion criteria, 513 (68.6%) were considered definitely appropriate on the basis of the triage physician’s response to the question “Based ONLY on the medical reason for hospitalization, in your opinion, how appropriate is this admission to the medicine floor service?” Furthermore, 169 (22.6%) were considered without definite medical acuity on the basis of the triage physician’s indication that “severity of medical problems alone may not require inpatient hospitalization” (Appendix Figure 1).
Study Design
Following a retrospective cohort study design, we systematically sampled 150 admissions from those “admitted without definite medical acuity” to create the exposure group and 150 from the “definitely medically appropriate” admissions to create the nonexposure group. Our sampling method involved selecting every third record until reaching the target sample size. This method and group sizes were determined prior to beginning data collection. Given the expected incidence of 33% AEs in the unexposed group (consistent with previous reports of AEs using the trigger tool5), we anticipated that a total sample size of 300 would be appropriate to capture a relative risk of at least 1.5 with 80% power and 95% confidence level.
Chart review was performed to capture patient demographics, admission characteristics, and hospitalization outcomes. We captured emergency severity index (ESI)6, a validated, reliable triage assessment score assigned by our emergency department, as a measurement of acute illness and calculated the Charlson comorbidity index (CCI)7 as a measurement of chronic comorbidity.
Identification of Adverse Events
We measured AEs by using the Institute for Healthcare Improvement Global Trigger Tool,8,9 which is estimated to identify up to 10 times more AEs than other methods, such as voluntary reporting.5 This protocol includes 28 triggers in the Cares and Medication Modules that serve as indicators that an AE may have occurred. The presence of a trigger is not necessarily an AE but a clue for further analysis. Two investigators (AS and CS) independently systematically searched for the presence of triggers within each patient chart. Trigger identification prompted in-depth analysis to confirm the occurrence of an AE and to characterize its severity by using the National Coordinating Council for Medication Error Reporting and Prevention categorization.10 An AE was coded when independent reviewers identified evidence of a preventable or nonpreventable “noxious and unintended event occurring in association with medical care.”9 By definition, any AEs identified were patient harms. Findings were reviewed weekly to ensure agreement, and discrepancies were adjudicated by a third investigator (MB).
All study data were collected by using REDCap electronic data capture tools hosted at the University of Washington.11 The University of Washington Institutional Review Board granted approval for this study.
Study Outcome and Statistical Analysis
The primary outcome was AEs per group with results calculated in three ways: AEs per 1,000 patient-days, AEs per 100 admissions, and percent of admissions with an AE. The risk ratio (RR) for the percent of admissions with an AE and the incidence rate ratio (IRR) for AEs per 1,000 patient-days were calculated for the comparison of significance.
Other data were analyzed by using Pearson’s chi square for categorical data, Student t test for normally distributed quantitative data, and Wilcoxon rank-sum (Mann–Whitney) for the length of stay (due to skew). Analyses were conducted using STATA (version 15.1, College Station, TX).
This work follows standards for reporting observational students as outlined in the STROBE statement.12
RESULTS
Patient Demographics
Both groups were predominantly white/non-Hispanic, male, and English-speaking (Table 1). More patients without definite medical acuity were covered by public insurance (78.9% vs 69.8%, P = .010) and discharged to homelessness (34.8% vs 22.6%, P = .041).
Measures of Illness
Patients considered definitely medically appropriate had lower ESI scores, indicative of more acute presentation, than those without definite medical acuity (2.73 [95% CI 2.64-2.81] vs 2.87 [95% CI 2.78-2.95], P = .026). There was no difference in CCI scores (Table 1).
Reason for Admission and Outcomes
Admissions considered definitely medically appropriate more frequently had an identified diagnosis/syndrome (66% vs 53%) or objective measurement (8.7% vs 2.7%) listed as the reason for admission, whereas patients admitted without definite medical acuity more freuqently had undifferentiated symptoms (34.7% vs 24%) or other/disposition (6% vs 1.3%) listed. The most common factors that triage physicians cited as contributing to the decision to admit patients without definite medical acuity included homelessness (34%), lack of outpatient social support (32%), and substance use disorder (25%). More details are available in Appendix Tables 1 and 2.
Admissions without definite medical acuity were longer than those with definite medical acuity (6.6 vs 6.0 days, P = .038), but there was no difference in emergency department readmissions within 48 hours or hospital readmissions within 30 days (Table 1).
Adverse Events
We identified 76 AEs in 41 admissions without definite medical acuity (range 0-10 AEs per admission) and 63 AEs in 44 definitely medically appropriate admissions (range 0-4 AEs per admission). The percentage of admissions with AE (27.3% vs 29.3%; RR 0.93, 95% CI 0.65-1.34, P = .70) and the rate of AE/1,000 patient-days (76.8 vs 70.4; IRR 1.09, 95% CI 0.77-1.55, P = .61) did not show statistically significant differences. The distribution of AE severity was similar between the two groups (Table 2). Most identified AEs caused temporary harm to the patient and were rated at severity levels E or F. Severe AEs, including at least one level I (patient death), occurred in both groups. The complete listing of positive triggers leading to adverse event identification by group and severity is available in Appendix Table 3.
DISCUSSION
By using a robust, standardized method, we found that patients admitted without definite medical acuity experienced the same number of inpatient AEs as patients admitted for definitely medically appropriate reasons. While the groups were relatively similar overall in terms of demographics and chronic comorbidity, we found evidence of social vulnerability in the group admitted without definite medical acuity in the form of increased rates of homelessness, triage physician concern regarding the lack of outpatient social support, and disposition-related reasons for admission. That both groups suffered harm―including patient death―while admitted to the hospital is striking, in particular for those patients who were admitted because of the lack of suitable outpatient options.
The potential limitations to the generalizability of this work include the single-site, safety-net setting and the use of individual physician determination of admission appropriateness. The proportion of admissions without definite medical acuity reported here is similar to that reported by previously published admission decision-making studies,2,3 and the rate of AEs observed is similar to rates measured in other studies using the trigger tool methodology.5,13 These similarities suggest some commonality across settings. Our study treats triage physician assessment as the marker of difference in defining the two groups and is an inherently subjective assessment that is reflective of real-world, holistic decision-making. Notably, the triage physician assessment was corroborated by corresponding differences in the ESI score, an acute triage assessment completed by a clinician outside of our team.
This study adds foundational knowledge to the risk/benefit discussion surrounding the decision to admit. Physician admission decisions are likely influenced by concern for the safety of vulnerable patients. Our results suggest that considering the risk of hospitalization itself in this decision-making remains important.
1. Mushlin AI, Appel FA. Extramedical factors in the decision to hospitalize medical patients. Am J Public Health. 1976;66(2):170-172. https://doi.org/10.2105/AJPH.66.2.170.
2. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of noncritically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
3. Pope I, Burn H, Ismail SA, Harris T, McCoy D. A qualitative study exploring the factors influencing admission to hospital from the emergency department. BMJ Open. 2017;7(8):e011543. https://doi.org/10.1136/bmjopen-2016-011543.
4. Levinson DR. Adverse Events in Hospitals: National Incidence among Medicare Beneficiaries. 2010. https://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf. Accessed May 20, 2019.
5. Classen DC, Resar R, Griffin F, et al. ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
6. Wuerz RC, Milne LW, Eitel DR, Travers D, Gilboy N. Reliability and validity of a new five-level triage instrument. Acad Emerg Med. 2000;7(3):236-242.https://doi.org/10.1111/j.1553-2712.2000.tb01066.x.
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
8. Resar RK, Rozich JD, Classen D. Methodology and rationale for the measurement of harm with trigger tools. Qual Saf Health Care. 2003;12(2):ii39-ii45. https://doi.org/10.1136/qhc.12.suppl_2.ii39.
9. Griffen FA, Resar RK. IHI Global Trigger Tool for Measuring Adverse Events (Second Edition). Cambridge, Massachusetts: Institute for Healthcare Improvement; 2009.
10. National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) Index for Categorizing Errors. https://www.nccmerp.org/types-medication-errors Accessed May 20, 2019.
11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577.
13. Kennerly DA, Kudyakov R, da Graca B, et al. Characterization of adverse events detected in a large health care delivery system using an enhanced global trigger tool over a five-year interval. Health Serv Res. 2014;49(5):1407-1425. https://doi.org/10.1111/1475-6773.12163.
Evidence exists that physicians consider what may be called “social” or “nonmedical” factors (lack of social support or barriers to access) in hospital admission decision-making and that patients are hospitalized even in the absence of a level of medical acuity warranting admission.1-3 Although hospitalization is associated with the risk of adverse events (AEs),4 whether this risk is related to the medical acuity of admission remains unclear. Our study sought to quantify the AEs experienced by patients hospitalized without definite medical acuity compared with those experienced by patients hospitalized with a definite medically appropriate indication for admission.
METHODS
Setting and Database Used for Analysis
This study was conducted at an urban, safety-net, public teaching hospital. At our site, calls for medical admissions are always answered by a hospital medicine attending physician (“triage physician”) who works collaboratively with the referring physician to facilitate appropriate disposition. Many of these discussions occur via telephone, but the triage physician may also assess the patient directly if needed. This study involved 24 triage physicians who directly assessed the patient in 65% of the cases.
At the time of each admission call, the triage physician logs the following information into a central triage database: date and time of call, patient location, reason for admission, assessment of appropriateness for medical floor, contributing factors to admission decision-making, and patient disposition.
Admission Appropriateness Group Designation
To be considered for inclusion in this study, calls must have originated from the emergency department and resulted in admission to the general medicine floor on either a resident teaching or hospitalist service from February 1, 2018 to June 1, 2018. This time frame was selected to avoid the start of a new academic cycle in late June that may confound AE rates.
The designation of appropriateness was determined by the triage physician’s logged response to triage database questions at the time of the admission call. Of the 748 admissions meeting inclusion criteria, 513 (68.6%) were considered definitely appropriate on the basis of the triage physician’s response to the question “Based ONLY on the medical reason for hospitalization, in your opinion, how appropriate is this admission to the medicine floor service?” Furthermore, 169 (22.6%) were considered without definite medical acuity on the basis of the triage physician’s indication that “severity of medical problems alone may not require inpatient hospitalization” (Appendix Figure 1).
Study Design
Following a retrospective cohort study design, we systematically sampled 150 admissions from those “admitted without definite medical acuity” to create the exposure group and 150 from the “definitely medically appropriate” admissions to create the nonexposure group. Our sampling method involved selecting every third record until reaching the target sample size. This method and group sizes were determined prior to beginning data collection. Given the expected incidence of 33% AEs in the unexposed group (consistent with previous reports of AEs using the trigger tool5), we anticipated that a total sample size of 300 would be appropriate to capture a relative risk of at least 1.5 with 80% power and 95% confidence level.
Chart review was performed to capture patient demographics, admission characteristics, and hospitalization outcomes. We captured emergency severity index (ESI)6, a validated, reliable triage assessment score assigned by our emergency department, as a measurement of acute illness and calculated the Charlson comorbidity index (CCI)7 as a measurement of chronic comorbidity.
Identification of Adverse Events
We measured AEs by using the Institute for Healthcare Improvement Global Trigger Tool,8,9 which is estimated to identify up to 10 times more AEs than other methods, such as voluntary reporting.5 This protocol includes 28 triggers in the Cares and Medication Modules that serve as indicators that an AE may have occurred. The presence of a trigger is not necessarily an AE but a clue for further analysis. Two investigators (AS and CS) independently systematically searched for the presence of triggers within each patient chart. Trigger identification prompted in-depth analysis to confirm the occurrence of an AE and to characterize its severity by using the National Coordinating Council for Medication Error Reporting and Prevention categorization.10 An AE was coded when independent reviewers identified evidence of a preventable or nonpreventable “noxious and unintended event occurring in association with medical care.”9 By definition, any AEs identified were patient harms. Findings were reviewed weekly to ensure agreement, and discrepancies were adjudicated by a third investigator (MB).
All study data were collected by using REDCap electronic data capture tools hosted at the University of Washington.11 The University of Washington Institutional Review Board granted approval for this study.
Study Outcome and Statistical Analysis
The primary outcome was AEs per group with results calculated in three ways: AEs per 1,000 patient-days, AEs per 100 admissions, and percent of admissions with an AE. The risk ratio (RR) for the percent of admissions with an AE and the incidence rate ratio (IRR) for AEs per 1,000 patient-days were calculated for the comparison of significance.
Other data were analyzed by using Pearson’s chi square for categorical data, Student t test for normally distributed quantitative data, and Wilcoxon rank-sum (Mann–Whitney) for the length of stay (due to skew). Analyses were conducted using STATA (version 15.1, College Station, TX).
This work follows standards for reporting observational students as outlined in the STROBE statement.12
RESULTS
Patient Demographics
Both groups were predominantly white/non-Hispanic, male, and English-speaking (Table 1). More patients without definite medical acuity were covered by public insurance (78.9% vs 69.8%, P = .010) and discharged to homelessness (34.8% vs 22.6%, P = .041).
Measures of Illness
Patients considered definitely medically appropriate had lower ESI scores, indicative of more acute presentation, than those without definite medical acuity (2.73 [95% CI 2.64-2.81] vs 2.87 [95% CI 2.78-2.95], P = .026). There was no difference in CCI scores (Table 1).
Reason for Admission and Outcomes
Admissions considered definitely medically appropriate more frequently had an identified diagnosis/syndrome (66% vs 53%) or objective measurement (8.7% vs 2.7%) listed as the reason for admission, whereas patients admitted without definite medical acuity more freuqently had undifferentiated symptoms (34.7% vs 24%) or other/disposition (6% vs 1.3%) listed. The most common factors that triage physicians cited as contributing to the decision to admit patients without definite medical acuity included homelessness (34%), lack of outpatient social support (32%), and substance use disorder (25%). More details are available in Appendix Tables 1 and 2.
Admissions without definite medical acuity were longer than those with definite medical acuity (6.6 vs 6.0 days, P = .038), but there was no difference in emergency department readmissions within 48 hours or hospital readmissions within 30 days (Table 1).
Adverse Events
We identified 76 AEs in 41 admissions without definite medical acuity (range 0-10 AEs per admission) and 63 AEs in 44 definitely medically appropriate admissions (range 0-4 AEs per admission). The percentage of admissions with AE (27.3% vs 29.3%; RR 0.93, 95% CI 0.65-1.34, P = .70) and the rate of AE/1,000 patient-days (76.8 vs 70.4; IRR 1.09, 95% CI 0.77-1.55, P = .61) did not show statistically significant differences. The distribution of AE severity was similar between the two groups (Table 2). Most identified AEs caused temporary harm to the patient and were rated at severity levels E or F. Severe AEs, including at least one level I (patient death), occurred in both groups. The complete listing of positive triggers leading to adverse event identification by group and severity is available in Appendix Table 3.
DISCUSSION
By using a robust, standardized method, we found that patients admitted without definite medical acuity experienced the same number of inpatient AEs as patients admitted for definitely medically appropriate reasons. While the groups were relatively similar overall in terms of demographics and chronic comorbidity, we found evidence of social vulnerability in the group admitted without definite medical acuity in the form of increased rates of homelessness, triage physician concern regarding the lack of outpatient social support, and disposition-related reasons for admission. That both groups suffered harm―including patient death―while admitted to the hospital is striking, in particular for those patients who were admitted because of the lack of suitable outpatient options.
The potential limitations to the generalizability of this work include the single-site, safety-net setting and the use of individual physician determination of admission appropriateness. The proportion of admissions without definite medical acuity reported here is similar to that reported by previously published admission decision-making studies,2,3 and the rate of AEs observed is similar to rates measured in other studies using the trigger tool methodology.5,13 These similarities suggest some commonality across settings. Our study treats triage physician assessment as the marker of difference in defining the two groups and is an inherently subjective assessment that is reflective of real-world, holistic decision-making. Notably, the triage physician assessment was corroborated by corresponding differences in the ESI score, an acute triage assessment completed by a clinician outside of our team.
This study adds foundational knowledge to the risk/benefit discussion surrounding the decision to admit. Physician admission decisions are likely influenced by concern for the safety of vulnerable patients. Our results suggest that considering the risk of hospitalization itself in this decision-making remains important.
Evidence exists that physicians consider what may be called “social” or “nonmedical” factors (lack of social support or barriers to access) in hospital admission decision-making and that patients are hospitalized even in the absence of a level of medical acuity warranting admission.1-3 Although hospitalization is associated with the risk of adverse events (AEs),4 whether this risk is related to the medical acuity of admission remains unclear. Our study sought to quantify the AEs experienced by patients hospitalized without definite medical acuity compared with those experienced by patients hospitalized with a definite medically appropriate indication for admission.
METHODS
Setting and Database Used for Analysis
This study was conducted at an urban, safety-net, public teaching hospital. At our site, calls for medical admissions are always answered by a hospital medicine attending physician (“triage physician”) who works collaboratively with the referring physician to facilitate appropriate disposition. Many of these discussions occur via telephone, but the triage physician may also assess the patient directly if needed. This study involved 24 triage physicians who directly assessed the patient in 65% of the cases.
At the time of each admission call, the triage physician logs the following information into a central triage database: date and time of call, patient location, reason for admission, assessment of appropriateness for medical floor, contributing factors to admission decision-making, and patient disposition.
Admission Appropriateness Group Designation
To be considered for inclusion in this study, calls must have originated from the emergency department and resulted in admission to the general medicine floor on either a resident teaching or hospitalist service from February 1, 2018 to June 1, 2018. This time frame was selected to avoid the start of a new academic cycle in late June that may confound AE rates.
The designation of appropriateness was determined by the triage physician’s logged response to triage database questions at the time of the admission call. Of the 748 admissions meeting inclusion criteria, 513 (68.6%) were considered definitely appropriate on the basis of the triage physician’s response to the question “Based ONLY on the medical reason for hospitalization, in your opinion, how appropriate is this admission to the medicine floor service?” Furthermore, 169 (22.6%) were considered without definite medical acuity on the basis of the triage physician’s indication that “severity of medical problems alone may not require inpatient hospitalization” (Appendix Figure 1).
Study Design
Following a retrospective cohort study design, we systematically sampled 150 admissions from those “admitted without definite medical acuity” to create the exposure group and 150 from the “definitely medically appropriate” admissions to create the nonexposure group. Our sampling method involved selecting every third record until reaching the target sample size. This method and group sizes were determined prior to beginning data collection. Given the expected incidence of 33% AEs in the unexposed group (consistent with previous reports of AEs using the trigger tool5), we anticipated that a total sample size of 300 would be appropriate to capture a relative risk of at least 1.5 with 80% power and 95% confidence level.
Chart review was performed to capture patient demographics, admission characteristics, and hospitalization outcomes. We captured emergency severity index (ESI)6, a validated, reliable triage assessment score assigned by our emergency department, as a measurement of acute illness and calculated the Charlson comorbidity index (CCI)7 as a measurement of chronic comorbidity.
Identification of Adverse Events
We measured AEs by using the Institute for Healthcare Improvement Global Trigger Tool,8,9 which is estimated to identify up to 10 times more AEs than other methods, such as voluntary reporting.5 This protocol includes 28 triggers in the Cares and Medication Modules that serve as indicators that an AE may have occurred. The presence of a trigger is not necessarily an AE but a clue for further analysis. Two investigators (AS and CS) independently systematically searched for the presence of triggers within each patient chart. Trigger identification prompted in-depth analysis to confirm the occurrence of an AE and to characterize its severity by using the National Coordinating Council for Medication Error Reporting and Prevention categorization.10 An AE was coded when independent reviewers identified evidence of a preventable or nonpreventable “noxious and unintended event occurring in association with medical care.”9 By definition, any AEs identified were patient harms. Findings were reviewed weekly to ensure agreement, and discrepancies were adjudicated by a third investigator (MB).
All study data were collected by using REDCap electronic data capture tools hosted at the University of Washington.11 The University of Washington Institutional Review Board granted approval for this study.
Study Outcome and Statistical Analysis
The primary outcome was AEs per group with results calculated in three ways: AEs per 1,000 patient-days, AEs per 100 admissions, and percent of admissions with an AE. The risk ratio (RR) for the percent of admissions with an AE and the incidence rate ratio (IRR) for AEs per 1,000 patient-days were calculated for the comparison of significance.
Other data were analyzed by using Pearson’s chi square for categorical data, Student t test for normally distributed quantitative data, and Wilcoxon rank-sum (Mann–Whitney) for the length of stay (due to skew). Analyses were conducted using STATA (version 15.1, College Station, TX).
This work follows standards for reporting observational students as outlined in the STROBE statement.12
RESULTS
Patient Demographics
Both groups were predominantly white/non-Hispanic, male, and English-speaking (Table 1). More patients without definite medical acuity were covered by public insurance (78.9% vs 69.8%, P = .010) and discharged to homelessness (34.8% vs 22.6%, P = .041).
Measures of Illness
Patients considered definitely medically appropriate had lower ESI scores, indicative of more acute presentation, than those without definite medical acuity (2.73 [95% CI 2.64-2.81] vs 2.87 [95% CI 2.78-2.95], P = .026). There was no difference in CCI scores (Table 1).
Reason for Admission and Outcomes
Admissions considered definitely medically appropriate more frequently had an identified diagnosis/syndrome (66% vs 53%) or objective measurement (8.7% vs 2.7%) listed as the reason for admission, whereas patients admitted without definite medical acuity more freuqently had undifferentiated symptoms (34.7% vs 24%) or other/disposition (6% vs 1.3%) listed. The most common factors that triage physicians cited as contributing to the decision to admit patients without definite medical acuity included homelessness (34%), lack of outpatient social support (32%), and substance use disorder (25%). More details are available in Appendix Tables 1 and 2.
Admissions without definite medical acuity were longer than those with definite medical acuity (6.6 vs 6.0 days, P = .038), but there was no difference in emergency department readmissions within 48 hours or hospital readmissions within 30 days (Table 1).
Adverse Events
We identified 76 AEs in 41 admissions without definite medical acuity (range 0-10 AEs per admission) and 63 AEs in 44 definitely medically appropriate admissions (range 0-4 AEs per admission). The percentage of admissions with AE (27.3% vs 29.3%; RR 0.93, 95% CI 0.65-1.34, P = .70) and the rate of AE/1,000 patient-days (76.8 vs 70.4; IRR 1.09, 95% CI 0.77-1.55, P = .61) did not show statistically significant differences. The distribution of AE severity was similar between the two groups (Table 2). Most identified AEs caused temporary harm to the patient and were rated at severity levels E or F. Severe AEs, including at least one level I (patient death), occurred in both groups. The complete listing of positive triggers leading to adverse event identification by group and severity is available in Appendix Table 3.
DISCUSSION
By using a robust, standardized method, we found that patients admitted without definite medical acuity experienced the same number of inpatient AEs as patients admitted for definitely medically appropriate reasons. While the groups were relatively similar overall in terms of demographics and chronic comorbidity, we found evidence of social vulnerability in the group admitted without definite medical acuity in the form of increased rates of homelessness, triage physician concern regarding the lack of outpatient social support, and disposition-related reasons for admission. That both groups suffered harm―including patient death―while admitted to the hospital is striking, in particular for those patients who were admitted because of the lack of suitable outpatient options.
The potential limitations to the generalizability of this work include the single-site, safety-net setting and the use of individual physician determination of admission appropriateness. The proportion of admissions without definite medical acuity reported here is similar to that reported by previously published admission decision-making studies,2,3 and the rate of AEs observed is similar to rates measured in other studies using the trigger tool methodology.5,13 These similarities suggest some commonality across settings. Our study treats triage physician assessment as the marker of difference in defining the two groups and is an inherently subjective assessment that is reflective of real-world, holistic decision-making. Notably, the triage physician assessment was corroborated by corresponding differences in the ESI score, an acute triage assessment completed by a clinician outside of our team.
This study adds foundational knowledge to the risk/benefit discussion surrounding the decision to admit. Physician admission decisions are likely influenced by concern for the safety of vulnerable patients. Our results suggest that considering the risk of hospitalization itself in this decision-making remains important.
1. Mushlin AI, Appel FA. Extramedical factors in the decision to hospitalize medical patients. Am J Public Health. 1976;66(2):170-172. https://doi.org/10.2105/AJPH.66.2.170.
2. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of noncritically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
3. Pope I, Burn H, Ismail SA, Harris T, McCoy D. A qualitative study exploring the factors influencing admission to hospital from the emergency department. BMJ Open. 2017;7(8):e011543. https://doi.org/10.1136/bmjopen-2016-011543.
4. Levinson DR. Adverse Events in Hospitals: National Incidence among Medicare Beneficiaries. 2010. https://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf. Accessed May 20, 2019.
5. Classen DC, Resar R, Griffin F, et al. ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
6. Wuerz RC, Milne LW, Eitel DR, Travers D, Gilboy N. Reliability and validity of a new five-level triage instrument. Acad Emerg Med. 2000;7(3):236-242.https://doi.org/10.1111/j.1553-2712.2000.tb01066.x.
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
8. Resar RK, Rozich JD, Classen D. Methodology and rationale for the measurement of harm with trigger tools. Qual Saf Health Care. 2003;12(2):ii39-ii45. https://doi.org/10.1136/qhc.12.suppl_2.ii39.
9. Griffen FA, Resar RK. IHI Global Trigger Tool for Measuring Adverse Events (Second Edition). Cambridge, Massachusetts: Institute for Healthcare Improvement; 2009.
10. National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) Index for Categorizing Errors. https://www.nccmerp.org/types-medication-errors Accessed May 20, 2019.
11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577.
13. Kennerly DA, Kudyakov R, da Graca B, et al. Characterization of adverse events detected in a large health care delivery system using an enhanced global trigger tool over a five-year interval. Health Serv Res. 2014;49(5):1407-1425. https://doi.org/10.1111/1475-6773.12163.
1. Mushlin AI, Appel FA. Extramedical factors in the decision to hospitalize medical patients. Am J Public Health. 1976;66(2):170-172. https://doi.org/10.2105/AJPH.66.2.170.
2. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of noncritically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
3. Pope I, Burn H, Ismail SA, Harris T, McCoy D. A qualitative study exploring the factors influencing admission to hospital from the emergency department. BMJ Open. 2017;7(8):e011543. https://doi.org/10.1136/bmjopen-2016-011543.
4. Levinson DR. Adverse Events in Hospitals: National Incidence among Medicare Beneficiaries. 2010. https://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf. Accessed May 20, 2019.
5. Classen DC, Resar R, Griffin F, et al. ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood). 2011;30(4):581-589. https://doi.org/10.1377/hlthaff.2011.0190.
6. Wuerz RC, Milne LW, Eitel DR, Travers D, Gilboy N. Reliability and validity of a new five-level triage instrument. Acad Emerg Med. 2000;7(3):236-242.https://doi.org/10.1111/j.1553-2712.2000.tb01066.x.
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383. https://doi.org/10.1016/0021-9681(87)90171-8.
8. Resar RK, Rozich JD, Classen D. Methodology and rationale for the measurement of harm with trigger tools. Qual Saf Health Care. 2003;12(2):ii39-ii45. https://doi.org/10.1136/qhc.12.suppl_2.ii39.
9. Griffen FA, Resar RK. IHI Global Trigger Tool for Measuring Adverse Events (Second Edition). Cambridge, Massachusetts: Institute for Healthcare Improvement; 2009.
10. National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) Index for Categorizing Errors. https://www.nccmerp.org/types-medication-errors Accessed May 20, 2019.
11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. https://doi.org/10.1016/j.jbi.2008.08.010.
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577.
13. Kennerly DA, Kudyakov R, da Graca B, et al. Characterization of adverse events detected in a large health care delivery system using an enhanced global trigger tool over a five-year interval. Health Serv Res. 2014;49(5):1407-1425. https://doi.org/10.1111/1475-6773.12163.
© 2020 Society of Hospital Medicine
Breathing New Life into Vital Sign Measurement
As you review the electronic health record before rounds in the morning, you notice a red exclamation mark in the chart of a patient who was admitted two days ago for an acute chronic obstructive pulmonary disease (COPD) exacerbation. The patient’s respiratory rate (RR) this morning is recorded at 24 breaths per minute (bpm). His RR last evening was 16 bpm and he remains on two liters per minute of supplemental oxygen. No one has notified you that he is getting worse, but you stop by the room to confirm that he is clinically stable.
During rounds, the resident states “The respiratory rate is recorded as 24 bpm, which is high, but I never trust the respiratory rate.” You silently agree and confirm your mistrust of the recorded RR.
Elevated RR has been associated with numerous poor outcomes, including mortality after myocardial infarction1 and death and readmission after acute COPD exacerbation.2 Furthermore, RR is used in models to predict mortality and intensive care unit admission,3 as well as in models to identify and predict mortality from sepsis.4 Recorded RRs are frequency inaccurate,5 and medical staff lack confidence in recorded RR values.6 Based on this evidence, you feel justified in your mistrust of recorded RR values. You might even believe that until a high-tech RR monitoring system is invented and implemented at your hospital, human error will forever prevent you from knowing your patients’ true RRs.
However, there is hope. In this issue of the Journal of Hospital Medicine, Keshvani et al.7 describe a successful quality improvement project where they employed plan–do–study–act methodology in a single inpatient unit to improve the accuracy of recorded RR. Before their project, only 36% of RR measurements were accurate, and there was considerable heterogeneity in the RR measurement technique. To address this problem, an interdisciplinary team of patient care assistants (PCAs), nurses, physicians, and hospital administration developed a plan to identify barriers, improve workflow, and educate stakeholders in RR recording.
The authors created a low-cost, “low-tech” intervention that consisted of training and educating PCAs on the correct technique and the importance of RR measurement, modifying workflow to incorporate RR measurement into a 30-second period of automated blood pressure measurement, and adding stopwatches to the vital sign carts. The RR measurements obtained by PCAs were compared with the RR measurements obtained by trained team members to assess for accuracy. PCA-obtained RR measurements were also compared with two control units, both before and after the intervention. Secondary outcomes included time to complete vital sign measurements and the incidence of systemic inflammatory response syndrome (SIRS)
The intervention improved the accuracy of PCA-obtained RRs from 36% to 58% and decreased the median RR from 18 to 14 breaths per minute. The implementation also resulted in a more normal distribution of RR in the intervention unit compared with the control unit. Interestingly, this intervention did not increase the time spent in obtaining vital signs—in fact, the time to complete vital signs decreased from a median of 2:26 to 1:55 minutes. In addition, tachypnea-specific SIRS incidence was reduced by 7.8% per hospitalization. An important implication of this finding is that reducing the false-positive rate of SIRS could possibly decrease unnecessary testing, medical interventions, and alert fatigue.
This project shows that meaningful interventions need not be expensive or overly technologic to have very real clinical effects. It would be very easy for a system to advocate for funding to purchase advanced monitors that purport to remove human error from the situation rather than trying first to improve human performance. Certainly, there is a role for advanced technologies—but improvement need not wait for, or be completely predicated on, these new technologies. The first barrier often expressed when evaluating a potential improvement initiative is that “we don’t have time for that”. This project demonstrates that innovations to improve care can also benefit the care team and improve workflow. Certainly, this project is not definitive and should be replicated elsewhere, but it is an important first step.
In an era where technology is expanding rapidly and the pace of innovation is breathtaking, we have an obligation to ensure that we are getting the basics right. Further, we must not take core tasks—such as vital signs, physical examination, and medication reconciliation—for granted, nor should we accept that they are as they will be. We discuss and debate the merits of advanced imaging, artificial intelligence, and machine learning—which are certainly exciting advances—but we must occasionally pause, breathe, and examine our practice to make sure that we do not overlook things that are truly vital to our patients’ care.
Disclosures
The authors have nothing to disclose.
1. Barthel P, Wensel R, Bauer A, et al. Respiratory rate predicts outcome after acute myocardial infarction: a prospective cohort study. Eur Heart J. 2013;34(22):1644-1650. https://doi.org/10.1093/eurheartj/ehs420.
2. Flattet Y, Garin N, Serratrice J, Arnaud P, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467-475. https://doi.org/10.2147/COPD.S122382.
3. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified early warning score in medical admissions. QJM. 2001;94(10):521-526. https://doi.org/10.1093/qjmed/94.10.521.
4. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762-774. https://doi.org/10.1001/jama.2016.0288.
5. Badawy J, Nguyen OK, Clark C, Halm EA, Makam AN. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual Saf. 2017;26(10):832-836. https://doi.org/10.1136/bmjqs-2017-006671.
6. Philip K, Richardson R, Cohen M. Staff perceptions of respiratory rate measurement in a general hospital. Br J Nurs. 2013;22(10):570-574. https://doi.org/10.12968/bjon.2013.22.10.570.
7. Keshvani N, Berger K, Gupta A, DePaola S, Nguyen O, Makam A. Improving respiratory rate accuracy in the hospital: a quality improvement initiative [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(11):673-677. https://doi.org/10.12788/jhm.3232.
As you review the electronic health record before rounds in the morning, you notice a red exclamation mark in the chart of a patient who was admitted two days ago for an acute chronic obstructive pulmonary disease (COPD) exacerbation. The patient’s respiratory rate (RR) this morning is recorded at 24 breaths per minute (bpm). His RR last evening was 16 bpm and he remains on two liters per minute of supplemental oxygen. No one has notified you that he is getting worse, but you stop by the room to confirm that he is clinically stable.
During rounds, the resident states “The respiratory rate is recorded as 24 bpm, which is high, but I never trust the respiratory rate.” You silently agree and confirm your mistrust of the recorded RR.
Elevated RR has been associated with numerous poor outcomes, including mortality after myocardial infarction1 and death and readmission after acute COPD exacerbation.2 Furthermore, RR is used in models to predict mortality and intensive care unit admission,3 as well as in models to identify and predict mortality from sepsis.4 Recorded RRs are frequency inaccurate,5 and medical staff lack confidence in recorded RR values.6 Based on this evidence, you feel justified in your mistrust of recorded RR values. You might even believe that until a high-tech RR monitoring system is invented and implemented at your hospital, human error will forever prevent you from knowing your patients’ true RRs.
However, there is hope. In this issue of the Journal of Hospital Medicine, Keshvani et al.7 describe a successful quality improvement project where they employed plan–do–study–act methodology in a single inpatient unit to improve the accuracy of recorded RR. Before their project, only 36% of RR measurements were accurate, and there was considerable heterogeneity in the RR measurement technique. To address this problem, an interdisciplinary team of patient care assistants (PCAs), nurses, physicians, and hospital administration developed a plan to identify barriers, improve workflow, and educate stakeholders in RR recording.
The authors created a low-cost, “low-tech” intervention that consisted of training and educating PCAs on the correct technique and the importance of RR measurement, modifying workflow to incorporate RR measurement into a 30-second period of automated blood pressure measurement, and adding stopwatches to the vital sign carts. The RR measurements obtained by PCAs were compared with the RR measurements obtained by trained team members to assess for accuracy. PCA-obtained RR measurements were also compared with two control units, both before and after the intervention. Secondary outcomes included time to complete vital sign measurements and the incidence of systemic inflammatory response syndrome (SIRS)
The intervention improved the accuracy of PCA-obtained RRs from 36% to 58% and decreased the median RR from 18 to 14 breaths per minute. The implementation also resulted in a more normal distribution of RR in the intervention unit compared with the control unit. Interestingly, this intervention did not increase the time spent in obtaining vital signs—in fact, the time to complete vital signs decreased from a median of 2:26 to 1:55 minutes. In addition, tachypnea-specific SIRS incidence was reduced by 7.8% per hospitalization. An important implication of this finding is that reducing the false-positive rate of SIRS could possibly decrease unnecessary testing, medical interventions, and alert fatigue.
This project shows that meaningful interventions need not be expensive or overly technologic to have very real clinical effects. It would be very easy for a system to advocate for funding to purchase advanced monitors that purport to remove human error from the situation rather than trying first to improve human performance. Certainly, there is a role for advanced technologies—but improvement need not wait for, or be completely predicated on, these new technologies. The first barrier often expressed when evaluating a potential improvement initiative is that “we don’t have time for that”. This project demonstrates that innovations to improve care can also benefit the care team and improve workflow. Certainly, this project is not definitive and should be replicated elsewhere, but it is an important first step.
In an era where technology is expanding rapidly and the pace of innovation is breathtaking, we have an obligation to ensure that we are getting the basics right. Further, we must not take core tasks—such as vital signs, physical examination, and medication reconciliation—for granted, nor should we accept that they are as they will be. We discuss and debate the merits of advanced imaging, artificial intelligence, and machine learning—which are certainly exciting advances—but we must occasionally pause, breathe, and examine our practice to make sure that we do not overlook things that are truly vital to our patients’ care.
Disclosures
The authors have nothing to disclose.
As you review the electronic health record before rounds in the morning, you notice a red exclamation mark in the chart of a patient who was admitted two days ago for an acute chronic obstructive pulmonary disease (COPD) exacerbation. The patient’s respiratory rate (RR) this morning is recorded at 24 breaths per minute (bpm). His RR last evening was 16 bpm and he remains on two liters per minute of supplemental oxygen. No one has notified you that he is getting worse, but you stop by the room to confirm that he is clinically stable.
During rounds, the resident states “The respiratory rate is recorded as 24 bpm, which is high, but I never trust the respiratory rate.” You silently agree and confirm your mistrust of the recorded RR.
Elevated RR has been associated with numerous poor outcomes, including mortality after myocardial infarction1 and death and readmission after acute COPD exacerbation.2 Furthermore, RR is used in models to predict mortality and intensive care unit admission,3 as well as in models to identify and predict mortality from sepsis.4 Recorded RRs are frequency inaccurate,5 and medical staff lack confidence in recorded RR values.6 Based on this evidence, you feel justified in your mistrust of recorded RR values. You might even believe that until a high-tech RR monitoring system is invented and implemented at your hospital, human error will forever prevent you from knowing your patients’ true RRs.
However, there is hope. In this issue of the Journal of Hospital Medicine, Keshvani et al.7 describe a successful quality improvement project where they employed plan–do–study–act methodology in a single inpatient unit to improve the accuracy of recorded RR. Before their project, only 36% of RR measurements were accurate, and there was considerable heterogeneity in the RR measurement technique. To address this problem, an interdisciplinary team of patient care assistants (PCAs), nurses, physicians, and hospital administration developed a plan to identify barriers, improve workflow, and educate stakeholders in RR recording.
The authors created a low-cost, “low-tech” intervention that consisted of training and educating PCAs on the correct technique and the importance of RR measurement, modifying workflow to incorporate RR measurement into a 30-second period of automated blood pressure measurement, and adding stopwatches to the vital sign carts. The RR measurements obtained by PCAs were compared with the RR measurements obtained by trained team members to assess for accuracy. PCA-obtained RR measurements were also compared with two control units, both before and after the intervention. Secondary outcomes included time to complete vital sign measurements and the incidence of systemic inflammatory response syndrome (SIRS)
The intervention improved the accuracy of PCA-obtained RRs from 36% to 58% and decreased the median RR from 18 to 14 breaths per minute. The implementation also resulted in a more normal distribution of RR in the intervention unit compared with the control unit. Interestingly, this intervention did not increase the time spent in obtaining vital signs—in fact, the time to complete vital signs decreased from a median of 2:26 to 1:55 minutes. In addition, tachypnea-specific SIRS incidence was reduced by 7.8% per hospitalization. An important implication of this finding is that reducing the false-positive rate of SIRS could possibly decrease unnecessary testing, medical interventions, and alert fatigue.
This project shows that meaningful interventions need not be expensive or overly technologic to have very real clinical effects. It would be very easy for a system to advocate for funding to purchase advanced monitors that purport to remove human error from the situation rather than trying first to improve human performance. Certainly, there is a role for advanced technologies—but improvement need not wait for, or be completely predicated on, these new technologies. The first barrier often expressed when evaluating a potential improvement initiative is that “we don’t have time for that”. This project demonstrates that innovations to improve care can also benefit the care team and improve workflow. Certainly, this project is not definitive and should be replicated elsewhere, but it is an important first step.
In an era where technology is expanding rapidly and the pace of innovation is breathtaking, we have an obligation to ensure that we are getting the basics right. Further, we must not take core tasks—such as vital signs, physical examination, and medication reconciliation—for granted, nor should we accept that they are as they will be. We discuss and debate the merits of advanced imaging, artificial intelligence, and machine learning—which are certainly exciting advances—but we must occasionally pause, breathe, and examine our practice to make sure that we do not overlook things that are truly vital to our patients’ care.
Disclosures
The authors have nothing to disclose.
1. Barthel P, Wensel R, Bauer A, et al. Respiratory rate predicts outcome after acute myocardial infarction: a prospective cohort study. Eur Heart J. 2013;34(22):1644-1650. https://doi.org/10.1093/eurheartj/ehs420.
2. Flattet Y, Garin N, Serratrice J, Arnaud P, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467-475. https://doi.org/10.2147/COPD.S122382.
3. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified early warning score in medical admissions. QJM. 2001;94(10):521-526. https://doi.org/10.1093/qjmed/94.10.521.
4. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762-774. https://doi.org/10.1001/jama.2016.0288.
5. Badawy J, Nguyen OK, Clark C, Halm EA, Makam AN. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual Saf. 2017;26(10):832-836. https://doi.org/10.1136/bmjqs-2017-006671.
6. Philip K, Richardson R, Cohen M. Staff perceptions of respiratory rate measurement in a general hospital. Br J Nurs. 2013;22(10):570-574. https://doi.org/10.12968/bjon.2013.22.10.570.
7. Keshvani N, Berger K, Gupta A, DePaola S, Nguyen O, Makam A. Improving respiratory rate accuracy in the hospital: a quality improvement initiative [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(11):673-677. https://doi.org/10.12788/jhm.3232.
1. Barthel P, Wensel R, Bauer A, et al. Respiratory rate predicts outcome after acute myocardial infarction: a prospective cohort study. Eur Heart J. 2013;34(22):1644-1650. https://doi.org/10.1093/eurheartj/ehs420.
2. Flattet Y, Garin N, Serratrice J, Arnaud P, Stirnemann J, Carballo S. Determining prognosis in acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:467-475. https://doi.org/10.2147/COPD.S122382.
3. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified early warning score in medical admissions. QJM. 2001;94(10):521-526. https://doi.org/10.1093/qjmed/94.10.521.
4. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762-774. https://doi.org/10.1001/jama.2016.0288.
5. Badawy J, Nguyen OK, Clark C, Halm EA, Makam AN. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual Saf. 2017;26(10):832-836. https://doi.org/10.1136/bmjqs-2017-006671.
6. Philip K, Richardson R, Cohen M. Staff perceptions of respiratory rate measurement in a general hospital. Br J Nurs. 2013;22(10):570-574. https://doi.org/10.12968/bjon.2013.22.10.570.
7. Keshvani N, Berger K, Gupta A, DePaola S, Nguyen O, Makam A. Improving respiratory rate accuracy in the hospital: a quality improvement initiative [published online ahead of print June 10, 2019]. J Hosp Med. 2019;14(11):673-677. https://doi.org/10.12788/jhm.3232.
© 2019 Society of Hospital Medicine
Varicella vaccine delivers doubled benefit to children
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
FROM PEDIATRICS
Key clinical point: Varicella vaccine is preventing pediatric zoster among children aged 2-17 years.
Major finding: Varicella-vaccinated children have a 78% lower incidence of pediatric zoster than do unvaccinated children.
Study details: The population-based cohort study included more than 6.3 million children.
Disclosures: Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
Source: Weinmann S et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2917.
Less Is More When It Comes to Ketorolac for Pain

A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.

A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).

A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.