User login
Inhibitor produces high response rate in relapsed/refractory FL
CHICAGO – The phosphoinositide 3-kinase–delta inhibitor ME-401, given with or without rituximab, produced an overall response rate of 80% in a phase 1b trial of patients with relapsed or refractory follicular lymphoma.
Response rates were similar between patients who received ME-401 alone and those who received it in combination with rituximab.
Response rates were also similar between patients on an intermittent dosing schedule and those on a continuous dosing schedule. However, intermittent dosing decreased the rate of delayed grade 3 adverse events (AEs).
“The idea that continuous inhibition of target is absolutely essential for activity of this class of drugs has not been proven,” said Andrew Zelenetz, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“The promising results of this somewhat novel intermittent schedule that we used with ME-401 suggests to me that we can maintain efficacy and reduce toxicity.”
Dr. Zelenetz and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Patients and dosing
Data were presented for 54 patients with relapsed/refractory follicular lymphoma enrolled on this study. The patients had a median age of 63.5 years, and 80% were male. They had received a median of 2 prior therapies (range, 1-10).
Initially, patients received ME-401 at 60 mg, 120 mg, or 180 mg once daily continuously on a 28-day cycle. However, the dose-escalation portion of the study was closed because response rates were comparable among the three doses, the safety profile was similar, and there were no dose-limiting toxicities.
Two additional groups of patients received ME-401 at 60 mg daily for two cycles, followed by an intermittent schedule (IS) of 60 mg on days 1-7, repeated every 28 days.
The researchers had observed delayed grade 3 AEs on the continuous schedule (CS), and they hypothesized that the IS might prevent these events. Patients could revert to the CS if they had stable disease or progressed on the IS.
“One of the advantages of this particular agent is the very long half-life,” Dr. Zelenetz said. “So, essentially, we have 2 weeks on drug and 2 weeks off [with the IS]. It takes about a week to clear the drug because it has about a 30-hour half-life.”
In all, 40 patients received ME-401 monotherapy, and 14 received ME-401 plus rituximab at 375 mg/m2 weekly for 4 weeks and then on day 1 of cycles 3-6. There were 31 patients who received ME-401 on the CS and 23 who received ME-401 on the IS.
Results
A total of 50 patients were evaluable for efficacy. The overall response rate in these patients was 80% (40/50), and 20% (10/50) achieved a complete response.
The overall response rate was 79% (30/38) in patients who received ME-401 alone, 83% (10/12) in those who received ME-401 plus rituximab, 83% (25/30) in patients on the CS, and 75% (15/20) in those on the IS.
The median duration of response and median progression-free survival have not been reached. The median follow-up for response duration is 8.8 months in the IS group and 8.3 months in the CS group. The median follow-up for progression-free survival is 5.5 months and 6.5 months, respectively.
A total of 18 patients on the IS (78%) were still on therapy at the data cutoff, as were 14 patients (45%) on the CS.
Seven patients (23%) on the CS and two patients (9%) on the IS discontinued treatment due to progression. Four patients in the IS group and two in the CS group who were switched to IS dosing reverted to CS dosing after experiencing progression.
Four CS patients (13%) discontinued treatment because of AEs, but none of the IS patients did.
AEs occurring in at least 15% of patients were diarrhea/colitis (40.7%), fatigue (35.2%), cough (33.3%), rash (24.1%), ALT increase (24.1%), nausea (24.1%), AST increase (22.2%), and decreased appetite (16.7%).
There were no grade 4-5 AEs. Grade 3 drug-related AEs of special interest (in the CS and IS groups, respectively) were diarrhea/colitis (16.1% and 8.7%), rash (12.9% and 0%), ALT increase (6.5% and 4.3%), AST increase (6.5% and 0%), pneumonia (6.5% and 0%), and mucositis (1.9% and 0%).
“[W]hile the grade 3 immune-related events seem to be very consistent in terms of class effects, they did seem to improve with transition to intermittent schedule,” said Carla Casulo, MD, of the University of Rochester (N.Y.), who reviewed this study in a poster discussion session.
“And I think that this novel design helps to create an opportunity to limit treatment and mitigate toxicity without necessarily compromising efficacy.”
The phase 1b trial is sponsored by MEI Pharma. Dr. Zelenetz reported relationships with MEI Pharma and several other companies. Dr. Casulo reported relationships with Gilead Sciences, Celgene, and Roche.
SOURCE: Zelenetz A et al. ASCO 2019, Abstract 7512.
CHICAGO – The phosphoinositide 3-kinase–delta inhibitor ME-401, given with or without rituximab, produced an overall response rate of 80% in a phase 1b trial of patients with relapsed or refractory follicular lymphoma.
Response rates were similar between patients who received ME-401 alone and those who received it in combination with rituximab.
Response rates were also similar between patients on an intermittent dosing schedule and those on a continuous dosing schedule. However, intermittent dosing decreased the rate of delayed grade 3 adverse events (AEs).
“The idea that continuous inhibition of target is absolutely essential for activity of this class of drugs has not been proven,” said Andrew Zelenetz, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“The promising results of this somewhat novel intermittent schedule that we used with ME-401 suggests to me that we can maintain efficacy and reduce toxicity.”
Dr. Zelenetz and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Patients and dosing
Data were presented for 54 patients with relapsed/refractory follicular lymphoma enrolled on this study. The patients had a median age of 63.5 years, and 80% were male. They had received a median of 2 prior therapies (range, 1-10).
Initially, patients received ME-401 at 60 mg, 120 mg, or 180 mg once daily continuously on a 28-day cycle. However, the dose-escalation portion of the study was closed because response rates were comparable among the three doses, the safety profile was similar, and there were no dose-limiting toxicities.
Two additional groups of patients received ME-401 at 60 mg daily for two cycles, followed by an intermittent schedule (IS) of 60 mg on days 1-7, repeated every 28 days.
The researchers had observed delayed grade 3 AEs on the continuous schedule (CS), and they hypothesized that the IS might prevent these events. Patients could revert to the CS if they had stable disease or progressed on the IS.
“One of the advantages of this particular agent is the very long half-life,” Dr. Zelenetz said. “So, essentially, we have 2 weeks on drug and 2 weeks off [with the IS]. It takes about a week to clear the drug because it has about a 30-hour half-life.”
In all, 40 patients received ME-401 monotherapy, and 14 received ME-401 plus rituximab at 375 mg/m2 weekly for 4 weeks and then on day 1 of cycles 3-6. There were 31 patients who received ME-401 on the CS and 23 who received ME-401 on the IS.
Results
A total of 50 patients were evaluable for efficacy. The overall response rate in these patients was 80% (40/50), and 20% (10/50) achieved a complete response.
The overall response rate was 79% (30/38) in patients who received ME-401 alone, 83% (10/12) in those who received ME-401 plus rituximab, 83% (25/30) in patients on the CS, and 75% (15/20) in those on the IS.
The median duration of response and median progression-free survival have not been reached. The median follow-up for response duration is 8.8 months in the IS group and 8.3 months in the CS group. The median follow-up for progression-free survival is 5.5 months and 6.5 months, respectively.
A total of 18 patients on the IS (78%) were still on therapy at the data cutoff, as were 14 patients (45%) on the CS.
Seven patients (23%) on the CS and two patients (9%) on the IS discontinued treatment due to progression. Four patients in the IS group and two in the CS group who were switched to IS dosing reverted to CS dosing after experiencing progression.
Four CS patients (13%) discontinued treatment because of AEs, but none of the IS patients did.
AEs occurring in at least 15% of patients were diarrhea/colitis (40.7%), fatigue (35.2%), cough (33.3%), rash (24.1%), ALT increase (24.1%), nausea (24.1%), AST increase (22.2%), and decreased appetite (16.7%).
There were no grade 4-5 AEs. Grade 3 drug-related AEs of special interest (in the CS and IS groups, respectively) were diarrhea/colitis (16.1% and 8.7%), rash (12.9% and 0%), ALT increase (6.5% and 4.3%), AST increase (6.5% and 0%), pneumonia (6.5% and 0%), and mucositis (1.9% and 0%).
“[W]hile the grade 3 immune-related events seem to be very consistent in terms of class effects, they did seem to improve with transition to intermittent schedule,” said Carla Casulo, MD, of the University of Rochester (N.Y.), who reviewed this study in a poster discussion session.
“And I think that this novel design helps to create an opportunity to limit treatment and mitigate toxicity without necessarily compromising efficacy.”
The phase 1b trial is sponsored by MEI Pharma. Dr. Zelenetz reported relationships with MEI Pharma and several other companies. Dr. Casulo reported relationships with Gilead Sciences, Celgene, and Roche.
SOURCE: Zelenetz A et al. ASCO 2019, Abstract 7512.
CHICAGO – The phosphoinositide 3-kinase–delta inhibitor ME-401, given with or without rituximab, produced an overall response rate of 80% in a phase 1b trial of patients with relapsed or refractory follicular lymphoma.
Response rates were similar between patients who received ME-401 alone and those who received it in combination with rituximab.
Response rates were also similar between patients on an intermittent dosing schedule and those on a continuous dosing schedule. However, intermittent dosing decreased the rate of delayed grade 3 adverse events (AEs).
“The idea that continuous inhibition of target is absolutely essential for activity of this class of drugs has not been proven,” said Andrew Zelenetz, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“The promising results of this somewhat novel intermittent schedule that we used with ME-401 suggests to me that we can maintain efficacy and reduce toxicity.”
Dr. Zelenetz and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Patients and dosing
Data were presented for 54 patients with relapsed/refractory follicular lymphoma enrolled on this study. The patients had a median age of 63.5 years, and 80% were male. They had received a median of 2 prior therapies (range, 1-10).
Initially, patients received ME-401 at 60 mg, 120 mg, or 180 mg once daily continuously on a 28-day cycle. However, the dose-escalation portion of the study was closed because response rates were comparable among the three doses, the safety profile was similar, and there were no dose-limiting toxicities.
Two additional groups of patients received ME-401 at 60 mg daily for two cycles, followed by an intermittent schedule (IS) of 60 mg on days 1-7, repeated every 28 days.
The researchers had observed delayed grade 3 AEs on the continuous schedule (CS), and they hypothesized that the IS might prevent these events. Patients could revert to the CS if they had stable disease or progressed on the IS.
“One of the advantages of this particular agent is the very long half-life,” Dr. Zelenetz said. “So, essentially, we have 2 weeks on drug and 2 weeks off [with the IS]. It takes about a week to clear the drug because it has about a 30-hour half-life.”
In all, 40 patients received ME-401 monotherapy, and 14 received ME-401 plus rituximab at 375 mg/m2 weekly for 4 weeks and then on day 1 of cycles 3-6. There were 31 patients who received ME-401 on the CS and 23 who received ME-401 on the IS.
Results
A total of 50 patients were evaluable for efficacy. The overall response rate in these patients was 80% (40/50), and 20% (10/50) achieved a complete response.
The overall response rate was 79% (30/38) in patients who received ME-401 alone, 83% (10/12) in those who received ME-401 plus rituximab, 83% (25/30) in patients on the CS, and 75% (15/20) in those on the IS.
The median duration of response and median progression-free survival have not been reached. The median follow-up for response duration is 8.8 months in the IS group and 8.3 months in the CS group. The median follow-up for progression-free survival is 5.5 months and 6.5 months, respectively.
A total of 18 patients on the IS (78%) were still on therapy at the data cutoff, as were 14 patients (45%) on the CS.
Seven patients (23%) on the CS and two patients (9%) on the IS discontinued treatment due to progression. Four patients in the IS group and two in the CS group who were switched to IS dosing reverted to CS dosing after experiencing progression.
Four CS patients (13%) discontinued treatment because of AEs, but none of the IS patients did.
AEs occurring in at least 15% of patients were diarrhea/colitis (40.7%), fatigue (35.2%), cough (33.3%), rash (24.1%), ALT increase (24.1%), nausea (24.1%), AST increase (22.2%), and decreased appetite (16.7%).
There were no grade 4-5 AEs. Grade 3 drug-related AEs of special interest (in the CS and IS groups, respectively) were diarrhea/colitis (16.1% and 8.7%), rash (12.9% and 0%), ALT increase (6.5% and 4.3%), AST increase (6.5% and 0%), pneumonia (6.5% and 0%), and mucositis (1.9% and 0%).
“[W]hile the grade 3 immune-related events seem to be very consistent in terms of class effects, they did seem to improve with transition to intermittent schedule,” said Carla Casulo, MD, of the University of Rochester (N.Y.), who reviewed this study in a poster discussion session.
“And I think that this novel design helps to create an opportunity to limit treatment and mitigate toxicity without necessarily compromising efficacy.”
The phase 1b trial is sponsored by MEI Pharma. Dr. Zelenetz reported relationships with MEI Pharma and several other companies. Dr. Casulo reported relationships with Gilead Sciences, Celgene, and Roche.
SOURCE: Zelenetz A et al. ASCO 2019, Abstract 7512.
REPORTING FROM ASCO 2019
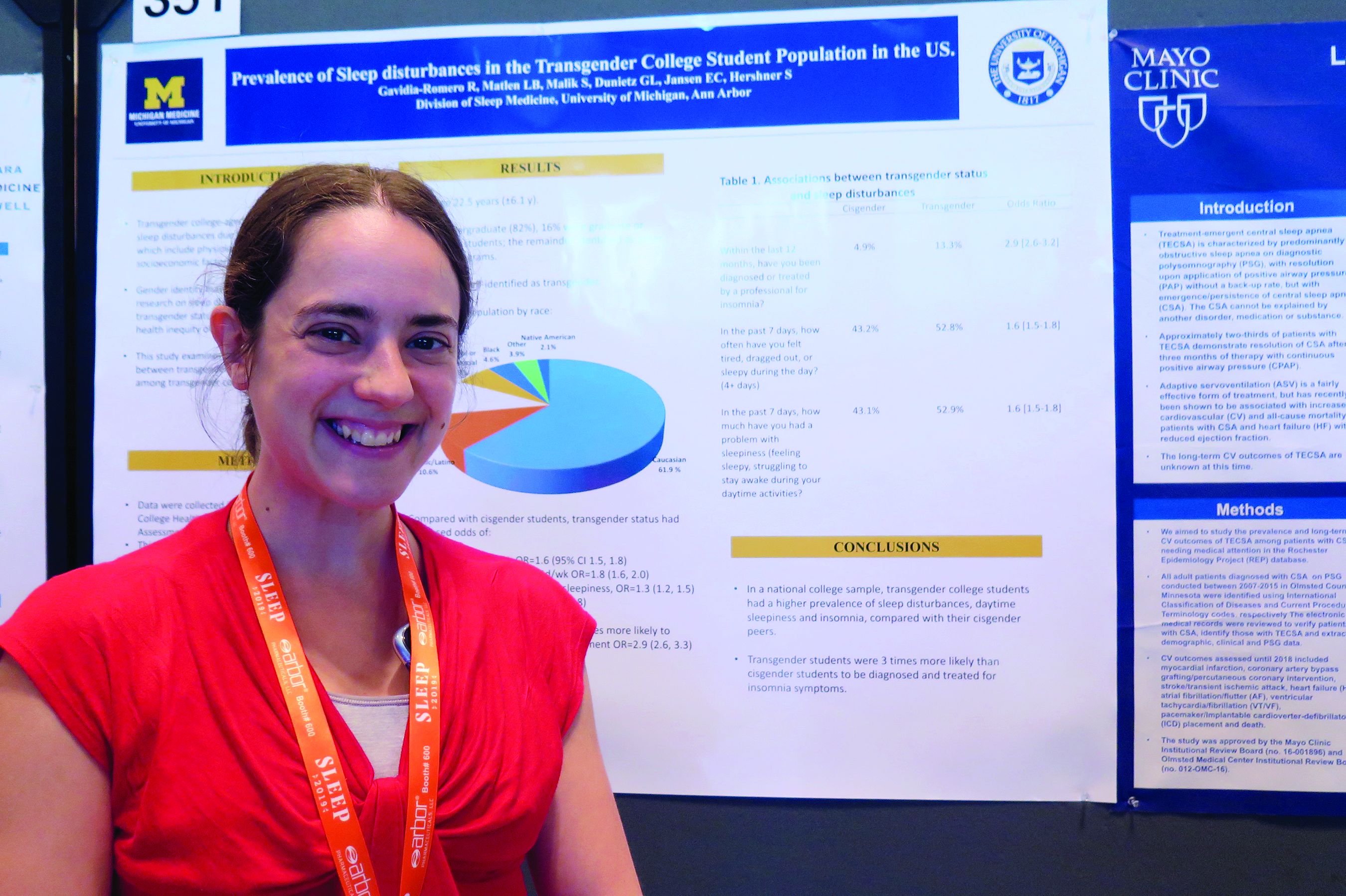
Insomnia common among transgender college students
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
REPORTING FROM SLEEP 2019
Waning pertussis immunity may be linked to acellular vaccine
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
FROM PEDIATRICS
Program meets social needs of 90% of patients
WASHINGTON – An innovative screening and referral model using electronic health records (EHRs) helped patients overcome social determinants of health (SDOH) by providing them with resources they had requested, according to a study.
“Our goal was to find whether this pilot program is feasible to systematically screen and refer patients for social needs in ambulatory care,” said Pablo Buitron de la Vega, MD, of Boston Medical Center (BMC) at the annual meeting of the Society of General Internal Medicine.
The program, called THRIVE, helps clinicians better understand patients’ social needs through a screening process to improve their health. THRIVE uses EHRs to document patient needs related to SDOH by using ICD-10 codes. This facilitates accurate data reporting and provides insight into social impacts on a patient. This component also matches SDOH needs to referral resources. In this pilot program, patients were screened during their primary care visits.
As of December 2018, THRIVE has demonstrated feasibility across all of its clinics. Eighty-two percent of patients were screened for social needs; 86% of patients received an ICD-10 code to document their social needs in their medical files for diagnosis and billing; and
The observational survey screened 50,532 unique patients seeking care at BMC between July 2017 and December 2018. In this population, 70% were underserved minorities (with 60% black patients and 10% Hispanic patients), 50% were below the federal poverty level, and 30% did not speak English as their primary language.
Of the screened population, 28% (13,975) of the patients identified having one or more social need. In addition, 19% (9,714) of patients requested help with one or more of their needs. The most prevalent needs were food insecurity, housing/shelter, and education, with 11% of patients listing each of these as a need.
The pilot program has been scaled up to include all patients presenting to 13 ambulatory clinics in family medicine, obstetrics and gynecology, infectious diseases, and pediatrics at Boston Medical Center.
WASHINGTON – An innovative screening and referral model using electronic health records (EHRs) helped patients overcome social determinants of health (SDOH) by providing them with resources they had requested, according to a study.
“Our goal was to find whether this pilot program is feasible to systematically screen and refer patients for social needs in ambulatory care,” said Pablo Buitron de la Vega, MD, of Boston Medical Center (BMC) at the annual meeting of the Society of General Internal Medicine.
The program, called THRIVE, helps clinicians better understand patients’ social needs through a screening process to improve their health. THRIVE uses EHRs to document patient needs related to SDOH by using ICD-10 codes. This facilitates accurate data reporting and provides insight into social impacts on a patient. This component also matches SDOH needs to referral resources. In this pilot program, patients were screened during their primary care visits.
As of December 2018, THRIVE has demonstrated feasibility across all of its clinics. Eighty-two percent of patients were screened for social needs; 86% of patients received an ICD-10 code to document their social needs in their medical files for diagnosis and billing; and
The observational survey screened 50,532 unique patients seeking care at BMC between July 2017 and December 2018. In this population, 70% were underserved minorities (with 60% black patients and 10% Hispanic patients), 50% were below the federal poverty level, and 30% did not speak English as their primary language.
Of the screened population, 28% (13,975) of the patients identified having one or more social need. In addition, 19% (9,714) of patients requested help with one or more of their needs. The most prevalent needs were food insecurity, housing/shelter, and education, with 11% of patients listing each of these as a need.
The pilot program has been scaled up to include all patients presenting to 13 ambulatory clinics in family medicine, obstetrics and gynecology, infectious diseases, and pediatrics at Boston Medical Center.
WASHINGTON – An innovative screening and referral model using electronic health records (EHRs) helped patients overcome social determinants of health (SDOH) by providing them with resources they had requested, according to a study.
“Our goal was to find whether this pilot program is feasible to systematically screen and refer patients for social needs in ambulatory care,” said Pablo Buitron de la Vega, MD, of Boston Medical Center (BMC) at the annual meeting of the Society of General Internal Medicine.
The program, called THRIVE, helps clinicians better understand patients’ social needs through a screening process to improve their health. THRIVE uses EHRs to document patient needs related to SDOH by using ICD-10 codes. This facilitates accurate data reporting and provides insight into social impacts on a patient. This component also matches SDOH needs to referral resources. In this pilot program, patients were screened during their primary care visits.
As of December 2018, THRIVE has demonstrated feasibility across all of its clinics. Eighty-two percent of patients were screened for social needs; 86% of patients received an ICD-10 code to document their social needs in their medical files for diagnosis and billing; and
The observational survey screened 50,532 unique patients seeking care at BMC between July 2017 and December 2018. In this population, 70% were underserved minorities (with 60% black patients and 10% Hispanic patients), 50% were below the federal poverty level, and 30% did not speak English as their primary language.
Of the screened population, 28% (13,975) of the patients identified having one or more social need. In addition, 19% (9,714) of patients requested help with one or more of their needs. The most prevalent needs were food insecurity, housing/shelter, and education, with 11% of patients listing each of these as a need.
The pilot program has been scaled up to include all patients presenting to 13 ambulatory clinics in family medicine, obstetrics and gynecology, infectious diseases, and pediatrics at Boston Medical Center.
REPORTING FROM SGIM 2019
Key clinical point: It is feasible to identify and help those affected by the social determinants of health within the primary care setting.
Major finding: 90% of the patients who reported having a social need received resources for all of their needs.
Study details: Observational survey. 13,975 identified one or more social needs, 9,714 patients requested help with one or more needs.
Disclosures: Study sponsored by Boston Medical Center.
Surgical Dermatoethics for the Trainee
It is an uncomfortable and unavoidable reality as physicians that for every procedure we learn, there must be a first time we perform it. As with any type of skill, it takes practice to become proficient. The unique challenge in medicine is that the practice involves performing procedures on real patients. We cannot avoid the hands-on nature of the training process; we can, however, approach its ethical challenges mindfully. Herein, I will discuss some of the ethical considerations in providing care as a trainee and identify potential barriers to best practices, particularly as they relate to procedural dermatology.
Tell Patients You Are in Training
In every patient encounter, we must introduce ourselves as a trainee. The principle of right to the truth dictates that we are transparent about our level of training and do not misrepresent ourselves to our patients. A statement released by the American Medical Association (AMA) Council on Ethical and Judicial Affairs asserts that “[p]atients should be informed of the identity and training status of individuals involved in their care.”1
Although straightforward in theory, this mandate is not always simple in practice. With patients unfamiliar with the health care system, it could be more onerous to clearly communicate training status than simply introducing oneself as a resident. A study conducted in the emergency department at Vanderbilt University Hospital (Nashville, Tennessee) found that many patients and their family members (N=430) did not understand the various roles and responsibilities of physicians in the teaching hospital setting. For example, 30% believed an attending physician requires supervision by a resident, and an additional 17% of those surveyed were not sure.2 The AMA requests we “refrain from using terms that may be confusing when describing the training status of the students,”1 which evidently is audience specific. Thus, as with any type of patient education, a thorough introduction may require assessment of understanding.
Disclosure of Experience Level With a Particular Procedure
There is a clear professional expectation that we disclose to patients that we are in training; however, a universal standard does not exist for disclosure of our exact level of experience in a particular procedure. Do we need to tell patients if it is our first time performing a given procedure? What if it is our tenth? Multiple studies have found that patients want specifics. In one study of bariatric surgery patients (N=108), 93% felt that they should always be informed if it was the first time a trainee was performing a particular procedure.3 A study conducted in the emergency department setting (N=202) also found that the majority of patients thought they should be informed if a resident was performing a procedure for the first time, but the distribution differed by procedure (66% for suturing vs 82% for lumbar puncture).4
Despite these findings, this degree of specificity is not always discussed with patients and perhaps does not need to be. LaRosa and Grant-Kels5 analyzed a hypothetical scenario in which a dermatology resident is to perform his first excision under attending supervision and concluded that broad disclosure of training status would suffice in the given scenario, as it would not be necessary to state that it was his first time performing an excision. It is unclear if the same conclusion could be drawn for all procedures and levels of experience. Outcome data would help inform the analysis, but the available data are from other specialties including general surgery, gynecology, and urology. Some studies demonstrate an increased risk of adverse outcomes with trainee involvement in procedures such as bariatric surgery and emergency general surgery, but the data are mixed and may not be generalizable to dermatologic procedures.6-8
The appropriate level of detail to disclose regarding a physician’s experience may need to be assessed on a case-by-case basis, and the principles of informed consent can help. Informed consent requires understanding of the diagnosis, the treatment options including nonintervention, and the risks and benefits of each alternative. In obtaining informed consent, we must disclose “any facts which are necessary to form the basis of an intelligent consent by the patient to the proposed treatment.”9 Providers must determine what aspects of a trainee’s experience level are relevant to the risk-benefit analysis in a given set of circumstances. Surely, there is a large degree of subjectivity in this determination as data are limited, but information deemed relevant must be shared. Information that is inconsequential, on the other hand, may be omitted. It could even be argued that more detailed information, especially if it may cause anxiety, would be detrimental to share. For example, we would not list the chemical name of every preservative in every vaccine we recommend for children if there is no evidence of inflicting harm. If the information has not been shown to have clinical impact or affect safety concerns, the anxiety may be undue.
Withholding Information Can Violate Ethical Principles
We must be careful not to withhold details of our experience level with a particular procedure for the wrong reasons. It would be wrong, for example, to withhold information simply to avoid causing anxiety, which could be seen as an invocation of therapeutic privilege, a controversial practice of withholding important information that poses a psychological threat to the patient. A classic example is the physician who defers disclosure of a terminal diagnosis to preserve hope. Although therapeutic privilege theoretically promotes the principle of beneficence, it violates the principles of autonomy and right to truth and therefore generally is regarded as unethically paternalistic in modern medical ethics.9
Patients Can Refuse Trainee Participation
It also is unethical to withhold information to obtain consent and avoid refusal of our care. Refusal of trainee participation is not uncommon. In the aforementioned study of bariatric surgery patients, 92.4% supported their procedure being performed at a teaching hospital, but only 56% would consent to a resident assisting staff during the procedure. A mere 33% of those patients would consent to a resident primarily performing with staff assisting.3 Although the proportion of patients who refuse certainly depends on the type of procedure among other factors, it is a reality in any teaching environment. The training paradigm in medicine depends on being able to practice procedures with supervision before we are independent providers. If patients refuse our care, our training suffers. However, the AMA maintains that “[p]atients are free to choose from whom they receive treatment,”1 and we must respect this aspect of patient autonomy.
Final Thoughts
When it comes to the performance of procedures, there are a few basic principles to keep in mind to provide ethical care to our patients while we are in training. Although we must accept that a crucial part of learning dermatologic procedures is hands on with real patients, we also need to come prepared having learned what we can through reading and practice with cadavers or skin substitutes. Procedures we execute as residents should be performed with adequate supervision, and as we progress through residency, we should be given increased autonomy and graded responsibility to prepare us for independent practice at graduation. Although it is the responsibility of the attending physician to provide appropriate oversight for the resident’s level of training, we should feel empowered to ask for help and have the humility to know when we need it.
- Medical student involvement in patient care: report of the council on ethical and judicial affairs. Virtual Mentor. 2001;3. doi:10.1001/virtualmentor.2001.3.3.code1-0103.
- Santen S, Hemphill RR, Prough E, et al. Do patients understand their physician’s level of training? a survey of emergency department patients. Acad Med. 2004;79:139-143.
- McClellan JM, Nelson D, Porta CR, et al. Bariatric surgery patient perceptions and willingness to consent to resident participation. Surg Obes Relat Dis. 2016;12:1065-1071.
- Santen SA, Hemphill RR, McDonald MF, et al. Patients’ willingness to allow residents to learn to practice medical procedures. Acad Med. 2004;79:144-147.
- LaRosa C, Grant-Kels JM. See one, do one, teach one: the ethical dilemma of residents performing their first procedure on patients. J Am Acad Dermatol. 2016;75:845-848.
- Can MF. The trainee effect on early postoperative surgical outcomes: reflects the effect of resident involvement or hospital capacity to overcome complications? J Invest Surg. 2017;31:67-68.
- Goldberg I, Yang J, Park J, et al. Surgical trainee impact on bariatric surgery safety [published online November 13, 2018]. Surg Endosc. doi:10.1007/s00464-018-6587-0.
- Kasotakis G, Lakha A, Sarkar B, et al. Trainee participation is associated with adverse outcomes in emergency general surgery: an analysis of the National Surgical Quality Improvement Program database. Ann Surg. 2014;3:483-490.
- Richard C, Lajeunesse Y, Lussier MT. Therapeutic privilege: between the ethics of lying and the practice of truth. J Med Ethics. 2010;36:353-357.
It is an uncomfortable and unavoidable reality as physicians that for every procedure we learn, there must be a first time we perform it. As with any type of skill, it takes practice to become proficient. The unique challenge in medicine is that the practice involves performing procedures on real patients. We cannot avoid the hands-on nature of the training process; we can, however, approach its ethical challenges mindfully. Herein, I will discuss some of the ethical considerations in providing care as a trainee and identify potential barriers to best practices, particularly as they relate to procedural dermatology.
Tell Patients You Are in Training
In every patient encounter, we must introduce ourselves as a trainee. The principle of right to the truth dictates that we are transparent about our level of training and do not misrepresent ourselves to our patients. A statement released by the American Medical Association (AMA) Council on Ethical and Judicial Affairs asserts that “[p]atients should be informed of the identity and training status of individuals involved in their care.”1
Although straightforward in theory, this mandate is not always simple in practice. With patients unfamiliar with the health care system, it could be more onerous to clearly communicate training status than simply introducing oneself as a resident. A study conducted in the emergency department at Vanderbilt University Hospital (Nashville, Tennessee) found that many patients and their family members (N=430) did not understand the various roles and responsibilities of physicians in the teaching hospital setting. For example, 30% believed an attending physician requires supervision by a resident, and an additional 17% of those surveyed were not sure.2 The AMA requests we “refrain from using terms that may be confusing when describing the training status of the students,”1 which evidently is audience specific. Thus, as with any type of patient education, a thorough introduction may require assessment of understanding.
Disclosure of Experience Level With a Particular Procedure
There is a clear professional expectation that we disclose to patients that we are in training; however, a universal standard does not exist for disclosure of our exact level of experience in a particular procedure. Do we need to tell patients if it is our first time performing a given procedure? What if it is our tenth? Multiple studies have found that patients want specifics. In one study of bariatric surgery patients (N=108), 93% felt that they should always be informed if it was the first time a trainee was performing a particular procedure.3 A study conducted in the emergency department setting (N=202) also found that the majority of patients thought they should be informed if a resident was performing a procedure for the first time, but the distribution differed by procedure (66% for suturing vs 82% for lumbar puncture).4
Despite these findings, this degree of specificity is not always discussed with patients and perhaps does not need to be. LaRosa and Grant-Kels5 analyzed a hypothetical scenario in which a dermatology resident is to perform his first excision under attending supervision and concluded that broad disclosure of training status would suffice in the given scenario, as it would not be necessary to state that it was his first time performing an excision. It is unclear if the same conclusion could be drawn for all procedures and levels of experience. Outcome data would help inform the analysis, but the available data are from other specialties including general surgery, gynecology, and urology. Some studies demonstrate an increased risk of adverse outcomes with trainee involvement in procedures such as bariatric surgery and emergency general surgery, but the data are mixed and may not be generalizable to dermatologic procedures.6-8
The appropriate level of detail to disclose regarding a physician’s experience may need to be assessed on a case-by-case basis, and the principles of informed consent can help. Informed consent requires understanding of the diagnosis, the treatment options including nonintervention, and the risks and benefits of each alternative. In obtaining informed consent, we must disclose “any facts which are necessary to form the basis of an intelligent consent by the patient to the proposed treatment.”9 Providers must determine what aspects of a trainee’s experience level are relevant to the risk-benefit analysis in a given set of circumstances. Surely, there is a large degree of subjectivity in this determination as data are limited, but information deemed relevant must be shared. Information that is inconsequential, on the other hand, may be omitted. It could even be argued that more detailed information, especially if it may cause anxiety, would be detrimental to share. For example, we would not list the chemical name of every preservative in every vaccine we recommend for children if there is no evidence of inflicting harm. If the information has not been shown to have clinical impact or affect safety concerns, the anxiety may be undue.
Withholding Information Can Violate Ethical Principles
We must be careful not to withhold details of our experience level with a particular procedure for the wrong reasons. It would be wrong, for example, to withhold information simply to avoid causing anxiety, which could be seen as an invocation of therapeutic privilege, a controversial practice of withholding important information that poses a psychological threat to the patient. A classic example is the physician who defers disclosure of a terminal diagnosis to preserve hope. Although therapeutic privilege theoretically promotes the principle of beneficence, it violates the principles of autonomy and right to truth and therefore generally is regarded as unethically paternalistic in modern medical ethics.9
Patients Can Refuse Trainee Participation
It also is unethical to withhold information to obtain consent and avoid refusal of our care. Refusal of trainee participation is not uncommon. In the aforementioned study of bariatric surgery patients, 92.4% supported their procedure being performed at a teaching hospital, but only 56% would consent to a resident assisting staff during the procedure. A mere 33% of those patients would consent to a resident primarily performing with staff assisting.3 Although the proportion of patients who refuse certainly depends on the type of procedure among other factors, it is a reality in any teaching environment. The training paradigm in medicine depends on being able to practice procedures with supervision before we are independent providers. If patients refuse our care, our training suffers. However, the AMA maintains that “[p]atients are free to choose from whom they receive treatment,”1 and we must respect this aspect of patient autonomy.
Final Thoughts
When it comes to the performance of procedures, there are a few basic principles to keep in mind to provide ethical care to our patients while we are in training. Although we must accept that a crucial part of learning dermatologic procedures is hands on with real patients, we also need to come prepared having learned what we can through reading and practice with cadavers or skin substitutes. Procedures we execute as residents should be performed with adequate supervision, and as we progress through residency, we should be given increased autonomy and graded responsibility to prepare us for independent practice at graduation. Although it is the responsibility of the attending physician to provide appropriate oversight for the resident’s level of training, we should feel empowered to ask for help and have the humility to know when we need it.
It is an uncomfortable and unavoidable reality as physicians that for every procedure we learn, there must be a first time we perform it. As with any type of skill, it takes practice to become proficient. The unique challenge in medicine is that the practice involves performing procedures on real patients. We cannot avoid the hands-on nature of the training process; we can, however, approach its ethical challenges mindfully. Herein, I will discuss some of the ethical considerations in providing care as a trainee and identify potential barriers to best practices, particularly as they relate to procedural dermatology.
Tell Patients You Are in Training
In every patient encounter, we must introduce ourselves as a trainee. The principle of right to the truth dictates that we are transparent about our level of training and do not misrepresent ourselves to our patients. A statement released by the American Medical Association (AMA) Council on Ethical and Judicial Affairs asserts that “[p]atients should be informed of the identity and training status of individuals involved in their care.”1
Although straightforward in theory, this mandate is not always simple in practice. With patients unfamiliar with the health care system, it could be more onerous to clearly communicate training status than simply introducing oneself as a resident. A study conducted in the emergency department at Vanderbilt University Hospital (Nashville, Tennessee) found that many patients and their family members (N=430) did not understand the various roles and responsibilities of physicians in the teaching hospital setting. For example, 30% believed an attending physician requires supervision by a resident, and an additional 17% of those surveyed were not sure.2 The AMA requests we “refrain from using terms that may be confusing when describing the training status of the students,”1 which evidently is audience specific. Thus, as with any type of patient education, a thorough introduction may require assessment of understanding.
Disclosure of Experience Level With a Particular Procedure
There is a clear professional expectation that we disclose to patients that we are in training; however, a universal standard does not exist for disclosure of our exact level of experience in a particular procedure. Do we need to tell patients if it is our first time performing a given procedure? What if it is our tenth? Multiple studies have found that patients want specifics. In one study of bariatric surgery patients (N=108), 93% felt that they should always be informed if it was the first time a trainee was performing a particular procedure.3 A study conducted in the emergency department setting (N=202) also found that the majority of patients thought they should be informed if a resident was performing a procedure for the first time, but the distribution differed by procedure (66% for suturing vs 82% for lumbar puncture).4
Despite these findings, this degree of specificity is not always discussed with patients and perhaps does not need to be. LaRosa and Grant-Kels5 analyzed a hypothetical scenario in which a dermatology resident is to perform his first excision under attending supervision and concluded that broad disclosure of training status would suffice in the given scenario, as it would not be necessary to state that it was his first time performing an excision. It is unclear if the same conclusion could be drawn for all procedures and levels of experience. Outcome data would help inform the analysis, but the available data are from other specialties including general surgery, gynecology, and urology. Some studies demonstrate an increased risk of adverse outcomes with trainee involvement in procedures such as bariatric surgery and emergency general surgery, but the data are mixed and may not be generalizable to dermatologic procedures.6-8
The appropriate level of detail to disclose regarding a physician’s experience may need to be assessed on a case-by-case basis, and the principles of informed consent can help. Informed consent requires understanding of the diagnosis, the treatment options including nonintervention, and the risks and benefits of each alternative. In obtaining informed consent, we must disclose “any facts which are necessary to form the basis of an intelligent consent by the patient to the proposed treatment.”9 Providers must determine what aspects of a trainee’s experience level are relevant to the risk-benefit analysis in a given set of circumstances. Surely, there is a large degree of subjectivity in this determination as data are limited, but information deemed relevant must be shared. Information that is inconsequential, on the other hand, may be omitted. It could even be argued that more detailed information, especially if it may cause anxiety, would be detrimental to share. For example, we would not list the chemical name of every preservative in every vaccine we recommend for children if there is no evidence of inflicting harm. If the information has not been shown to have clinical impact or affect safety concerns, the anxiety may be undue.
Withholding Information Can Violate Ethical Principles
We must be careful not to withhold details of our experience level with a particular procedure for the wrong reasons. It would be wrong, for example, to withhold information simply to avoid causing anxiety, which could be seen as an invocation of therapeutic privilege, a controversial practice of withholding important information that poses a psychological threat to the patient. A classic example is the physician who defers disclosure of a terminal diagnosis to preserve hope. Although therapeutic privilege theoretically promotes the principle of beneficence, it violates the principles of autonomy and right to truth and therefore generally is regarded as unethically paternalistic in modern medical ethics.9
Patients Can Refuse Trainee Participation
It also is unethical to withhold information to obtain consent and avoid refusal of our care. Refusal of trainee participation is not uncommon. In the aforementioned study of bariatric surgery patients, 92.4% supported their procedure being performed at a teaching hospital, but only 56% would consent to a resident assisting staff during the procedure. A mere 33% of those patients would consent to a resident primarily performing with staff assisting.3 Although the proportion of patients who refuse certainly depends on the type of procedure among other factors, it is a reality in any teaching environment. The training paradigm in medicine depends on being able to practice procedures with supervision before we are independent providers. If patients refuse our care, our training suffers. However, the AMA maintains that “[p]atients are free to choose from whom they receive treatment,”1 and we must respect this aspect of patient autonomy.
Final Thoughts
When it comes to the performance of procedures, there are a few basic principles to keep in mind to provide ethical care to our patients while we are in training. Although we must accept that a crucial part of learning dermatologic procedures is hands on with real patients, we also need to come prepared having learned what we can through reading and practice with cadavers or skin substitutes. Procedures we execute as residents should be performed with adequate supervision, and as we progress through residency, we should be given increased autonomy and graded responsibility to prepare us for independent practice at graduation. Although it is the responsibility of the attending physician to provide appropriate oversight for the resident’s level of training, we should feel empowered to ask for help and have the humility to know when we need it.
- Medical student involvement in patient care: report of the council on ethical and judicial affairs. Virtual Mentor. 2001;3. doi:10.1001/virtualmentor.2001.3.3.code1-0103.
- Santen S, Hemphill RR, Prough E, et al. Do patients understand their physician’s level of training? a survey of emergency department patients. Acad Med. 2004;79:139-143.
- McClellan JM, Nelson D, Porta CR, et al. Bariatric surgery patient perceptions and willingness to consent to resident participation. Surg Obes Relat Dis. 2016;12:1065-1071.
- Santen SA, Hemphill RR, McDonald MF, et al. Patients’ willingness to allow residents to learn to practice medical procedures. Acad Med. 2004;79:144-147.
- LaRosa C, Grant-Kels JM. See one, do one, teach one: the ethical dilemma of residents performing their first procedure on patients. J Am Acad Dermatol. 2016;75:845-848.
- Can MF. The trainee effect on early postoperative surgical outcomes: reflects the effect of resident involvement or hospital capacity to overcome complications? J Invest Surg. 2017;31:67-68.
- Goldberg I, Yang J, Park J, et al. Surgical trainee impact on bariatric surgery safety [published online November 13, 2018]. Surg Endosc. doi:10.1007/s00464-018-6587-0.
- Kasotakis G, Lakha A, Sarkar B, et al. Trainee participation is associated with adverse outcomes in emergency general surgery: an analysis of the National Surgical Quality Improvement Program database. Ann Surg. 2014;3:483-490.
- Richard C, Lajeunesse Y, Lussier MT. Therapeutic privilege: between the ethics of lying and the practice of truth. J Med Ethics. 2010;36:353-357.
- Medical student involvement in patient care: report of the council on ethical and judicial affairs. Virtual Mentor. 2001;3. doi:10.1001/virtualmentor.2001.3.3.code1-0103.
- Santen S, Hemphill RR, Prough E, et al. Do patients understand their physician’s level of training? a survey of emergency department patients. Acad Med. 2004;79:139-143.
- McClellan JM, Nelson D, Porta CR, et al. Bariatric surgery patient perceptions and willingness to consent to resident participation. Surg Obes Relat Dis. 2016;12:1065-1071.
- Santen SA, Hemphill RR, McDonald MF, et al. Patients’ willingness to allow residents to learn to practice medical procedures. Acad Med. 2004;79:144-147.
- LaRosa C, Grant-Kels JM. See one, do one, teach one: the ethical dilemma of residents performing their first procedure on patients. J Am Acad Dermatol. 2016;75:845-848.
- Can MF. The trainee effect on early postoperative surgical outcomes: reflects the effect of resident involvement or hospital capacity to overcome complications? J Invest Surg. 2017;31:67-68.
- Goldberg I, Yang J, Park J, et al. Surgical trainee impact on bariatric surgery safety [published online November 13, 2018]. Surg Endosc. doi:10.1007/s00464-018-6587-0.
- Kasotakis G, Lakha A, Sarkar B, et al. Trainee participation is associated with adverse outcomes in emergency general surgery: an analysis of the National Surgical Quality Improvement Program database. Ann Surg. 2014;3:483-490.
- Richard C, Lajeunesse Y, Lussier MT. Therapeutic privilege: between the ethics of lying and the practice of truth. J Med Ethics. 2010;36:353-357.
Resident Pearl
- As residents, we must gain experience performing procedures on real patients to enter independent practice as proficient dermatologists. It is important to be mindful of the ethical challenges inherent to the hands-on training process and to understand the ethical principles that guide best practices.
Fewer antibiotics prescribed with PCR than conventional stool testing
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
REPORTING FROM DDW 2019
USPSTF recommends PrEP combo for adults at high risk of HIV infection
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
FROM JAMA
Early TIPS shows superiority to standard care for advanced cirrhosis with acute variceal bleeding
Early TIPS “should therefore be preferred to the current standard of care,” reported lead author Yong Lv, MD, of the Fourth Military Medical University in Xi’an, China, and colleagues, referring to standard pharmaceutical and endoscopic therapy.
“[The current standard] approach has improved patient outcomes,” the investigators wrote in the Lancet Gastroenterology & Hepatology. “However, up to 10%-20% of patients still experience treatment failure, requiring further intensive management. In such patients, [TIPS] is successful in achieving hemostasis in 90%-100% of patients. However, 6-week mortality remains high [35%-55%]. This is probably because the severity of the underlying liver disease has worsened and additional organ dysfunction may have occurred after several failed endoscopic therapy attempts.”
Previous studies have explored earlier intervention with TIPS; however, according to the investigators, these trials were inconclusive for various reasons. For example, uncovered stents and an out-of-date control therapy were employed in a trial by Monescillo et al., while a study by Garcia-Pagan et al. lacked a primary survival endpoint and has been criticized for selection bias. “Thus, whether early TIPS confers a survival benefit in a broader population remains to be assessed,” the investigators wrote.
To this end, the investigators screened 373 patients with advanced cirrhosis (Child-Pugh class B or C) and acute variceal bleeding. Of these, 132 were eligible for inclusion based on age, disease severity, willingness to participate, comorbidities, and other factors. Patients were randomized 2:1 to receive either early TIPS or standard therapy. Within 12 hours of hospital admission for the initial bleeding episode, all patients received vasoactive drugs or endoscopic band ligation and prophylactic antibiotics. Control patients continued vasoactive drugs for up to 5 days, followed by propranolol, which was titrated to reduce resting heart rate by 25% but not less than 55 beats per minute. Elective endoscopic band ligation was performed within 1-2 weeks of initial endoscopic treatment, then approximately every 2 weeks until variceal eradication, and additionally if varices reappeared. TIPS was allowed as rescue therapy. In contrast, patients in the TIPS group underwent the procedure with conscious sedation and local anesthesia within 72 hours of diagnostic endoscopy, followed by approximately 1 week of antibiotics and vasoactive drugs. TIPS revision with angioplasty or another stent placement was performed in the event of shunt dysfunction or reemergence of portal hypertensive complications. The final dataset contained 127 patients, as 3 were excluded after randomization because of exclusionary diagnoses, 1 withdrew consent, and 1 died before TIPS placement.
The primary endpoint was transplantation-free survival. The secondary endpoints were new or worsening ascites based on ultrasound score or sustained ascites necessitating paracentesis, failure to control bleeding or rebleeding, overt hepatic encephalopathy, other complications of portal hypertension, and adverse events.
After a median follow-up of 24 months, data analysis showed a survival benefit associated with early TIPS based on multiple measures. Out of 84 patients in the TIPS group, 15 (18%) died during follow-up, compared with 15 (33%) in the control group. Actuarial transplantation-free survival was also better with TIPS instead of standard therapy at 6 weeks (99% vs. 84%), 1 year (86% vs. 73%), and 2 years (79% vs. 64%). The hazard ratio for transplantation-free survival was 0.50 in favor of TIPS (P = .04). These survival advantages were maintained regardless of hepatitis B virus status or Child-Pugh/Model for End-Stage Liver Disease score.
Similarly to transplantation-free survival, patients treated with TIPS were more likely to be free of uncontrolled bleeding or rebleeding at 1 year (89% vs. 66%) and 2 years (86% vs. 57%). The associated hazard ratio for this outcome favored early TIPS (HR, 0.26; P less than .0001), and univariate and multivariate analysis confirmed an independent protective role. In further support of superiority over standard therapy, patients treated with TIPS were more likely than those in the control group to be free of new or worsening ascites at 1 year (89% vs. 57%) and 2 years (81% vs. 54%).
No significant intergroup differences were found for rates of overt hepatic encephalopathy, hepatic hydrothorax, hepatorenal syndrome, spontaneous bacterial peritonitis, hepatocellular carcinoma, serious adverse events, or nonserious adverse events. At 1 and 3 months, patients in the TIPS group had a slight increase of median bilirubin concentrations and median international normalized ratio; however, these values normalized after 6 months. A similar temporal pattern was observed in early TIPS patients with regard to median Model for End-Stage Liver Disease score.
“[The transplantation-free survival benefit of early TIPS] was probably related to better control of factors contributing to death, such as failure to control bleeding or rebleeding or new or worsening ascites, without increasing the frequency and severity of overt hepatic encephalopathy and other adverse events,” the investigators concluded. “This study provides direct evidence and greater confidence in the recommendations of current guidelines that early TIPS should be performed in high-risk patients without contraindications.
“Future studies addressing whether early TIPS can be equally recommended in Child-Pugh B and C patients are warranted,” the investigators added.
The study was funded by the National Key Technology R&D Program, Boost Program of Xijing Hospital, Optimized Overall Project of Shaanxi Province, and National Natural Science Foundation of China. The investigators reported no conflicts of interest.
SOURCE: Lv Y et al. Lancet Gastroenterol Hepatol. 2019 May 29. doi: 10.1016/S2468-1253(19)30090-1.
Although the paper published by Lv et al. supports early transjugular intrahepatic portosystemic shunt (TIPS) for some patients with cirrhosis and variceal bleeding, Dominique Thabut, MD, and Marika Rudler, MD, pointed out that this conclusion cannot be applied to all patients.
“First ... the landscape of cirrhosis with acute variceal bleeding in China is different from that in Europe,” they wrote. “Second, the authors chose to include patients with Child-Pugh B disease without active bleeding at endoscopy [the largest group of patients in this trial]; such patients are not often seen in Europe. Last, a survival benefit was only observed when the Child-Pugh B and Child-Pugh C patients were combined, with and without active bleeding. Hence, this study does not permit conclusions to be made for patients with Child-Pugh B disease.”
“Overall, the authors should be congratulated for tackling the much debated issue of preemptive TIPS,” Dr. Thabut and Dr. Rudler wrote. “There is now no doubt about the benefit of preemptive TIPS in patients with Child-Pugh C disease. The beneficial effects of preemptive TIPS on ascites should push us to consider this approach in all patients, in the absence of contraindication.”
Dr. Tabut and Dr. Rudler, of the Institute of Cardiometabolism and Nutrition, Paris, made their remarks in an accompanying editorial (Lancet Gastroenterol Hepatol. 2019 May 29. doi: 10.1016/S2468-1253[19]30172-4). They reported no conflicts of interest.
Although the paper published by Lv et al. supports early transjugular intrahepatic portosystemic shunt (TIPS) for some patients with cirrhosis and variceal bleeding, Dominique Thabut, MD, and Marika Rudler, MD, pointed out that this conclusion cannot be applied to all patients.
“First ... the landscape of cirrhosis with acute variceal bleeding in China is different from that in Europe,” they wrote. “Second, the authors chose to include patients with Child-Pugh B disease without active bleeding at endoscopy [the largest group of patients in this trial]; such patients are not often seen in Europe. Last, a survival benefit was only observed when the Child-Pugh B and Child-Pugh C patients were combined, with and without active bleeding. Hence, this study does not permit conclusions to be made for patients with Child-Pugh B disease.”
“Overall, the authors should be congratulated for tackling the much debated issue of preemptive TIPS,” Dr. Thabut and Dr. Rudler wrote. “There is now no doubt about the benefit of preemptive TIPS in patients with Child-Pugh C disease. The beneficial effects of preemptive TIPS on ascites should push us to consider this approach in all patients, in the absence of contraindication.”
Dr. Tabut and Dr. Rudler, of the Institute of Cardiometabolism and Nutrition, Paris, made their remarks in an accompanying editorial (Lancet Gastroenterol Hepatol. 2019 May 29. doi: 10.1016/S2468-1253[19]30172-4). They reported no conflicts of interest.
Although the paper published by Lv et al. supports early transjugular intrahepatic portosystemic shunt (TIPS) for some patients with cirrhosis and variceal bleeding, Dominique Thabut, MD, and Marika Rudler, MD, pointed out that this conclusion cannot be applied to all patients.
“First ... the landscape of cirrhosis with acute variceal bleeding in China is different from that in Europe,” they wrote. “Second, the authors chose to include patients with Child-Pugh B disease without active bleeding at endoscopy [the largest group of patients in this trial]; such patients are not often seen in Europe. Last, a survival benefit was only observed when the Child-Pugh B and Child-Pugh C patients were combined, with and without active bleeding. Hence, this study does not permit conclusions to be made for patients with Child-Pugh B disease.”
“Overall, the authors should be congratulated for tackling the much debated issue of preemptive TIPS,” Dr. Thabut and Dr. Rudler wrote. “There is now no doubt about the benefit of preemptive TIPS in patients with Child-Pugh C disease. The beneficial effects of preemptive TIPS on ascites should push us to consider this approach in all patients, in the absence of contraindication.”
Dr. Tabut and Dr. Rudler, of the Institute of Cardiometabolism and Nutrition, Paris, made their remarks in an accompanying editorial (Lancet Gastroenterol Hepatol. 2019 May 29. doi: 10.1016/S2468-1253[19]30172-4). They reported no conflicts of interest.
Early TIPS “should therefore be preferred to the current standard of care,” reported lead author Yong Lv, MD, of the Fourth Military Medical University in Xi’an, China, and colleagues, referring to standard pharmaceutical and endoscopic therapy.
“[The current standard] approach has improved patient outcomes,” the investigators wrote in the Lancet Gastroenterology & Hepatology. “However, up to 10%-20% of patients still experience treatment failure, requiring further intensive management. In such patients, [TIPS] is successful in achieving hemostasis in 90%-100% of patients. However, 6-week mortality remains high [35%-55%]. This is probably because the severity of the underlying liver disease has worsened and additional organ dysfunction may have occurred after several failed endoscopic therapy attempts.”
Previous studies have explored earlier intervention with TIPS; however, according to the investigators, these trials were inconclusive for various reasons. For example, uncovered stents and an out-of-date control therapy were employed in a trial by Monescillo et al., while a study by Garcia-Pagan et al. lacked a primary survival endpoint and has been criticized for selection bias. “Thus, whether early TIPS confers a survival benefit in a broader population remains to be assessed,” the investigators wrote.
To this end, the investigators screened 373 patients with advanced cirrhosis (Child-Pugh class B or C) and acute variceal bleeding. Of these, 132 were eligible for inclusion based on age, disease severity, willingness to participate, comorbidities, and other factors. Patients were randomized 2:1 to receive either early TIPS or standard therapy. Within 12 hours of hospital admission for the initial bleeding episode, all patients received vasoactive drugs or endoscopic band ligation and prophylactic antibiotics. Control patients continued vasoactive drugs for up to 5 days, followed by propranolol, which was titrated to reduce resting heart rate by 25% but not less than 55 beats per minute. Elective endoscopic band ligation was performed within 1-2 weeks of initial endoscopic treatment, then approximately every 2 weeks until variceal eradication, and additionally if varices reappeared. TIPS was allowed as rescue therapy. In contrast, patients in the TIPS group underwent the procedure with conscious sedation and local anesthesia within 72 hours of diagnostic endoscopy, followed by approximately 1 week of antibiotics and vasoactive drugs. TIPS revision with angioplasty or another stent placement was performed in the event of shunt dysfunction or reemergence of portal hypertensive complications. The final dataset contained 127 patients, as 3 were excluded after randomization because of exclusionary diagnoses, 1 withdrew consent, and 1 died before TIPS placement.
The primary endpoint was transplantation-free survival. The secondary endpoints were new or worsening ascites based on ultrasound score or sustained ascites necessitating paracentesis, failure to control bleeding or rebleeding, overt hepatic encephalopathy, other complications of portal hypertension, and adverse events.
After a median follow-up of 24 months, data analysis showed a survival benefit associated with early TIPS based on multiple measures. Out of 84 patients in the TIPS group, 15 (18%) died during follow-up, compared with 15 (33%) in the control group. Actuarial transplantation-free survival was also better with TIPS instead of standard therapy at 6 weeks (99% vs. 84%), 1 year (86% vs. 73%), and 2 years (79% vs. 64%). The hazard ratio for transplantation-free survival was 0.50 in favor of TIPS (P = .04). These survival advantages were maintained regardless of hepatitis B virus status or Child-Pugh/Model for End-Stage Liver Disease score.
Similarly to transplantation-free survival, patients treated with TIPS were more likely to be free of uncontrolled bleeding or rebleeding at 1 year (89% vs. 66%) and 2 years (86% vs. 57%). The associated hazard ratio for this outcome favored early TIPS (HR, 0.26; P less than .0001), and univariate and multivariate analysis confirmed an independent protective role. In further support of superiority over standard therapy, patients treated with TIPS were more likely than those in the control group to be free of new or worsening ascites at 1 year (89% vs. 57%) and 2 years (81% vs. 54%).
No significant intergroup differences were found for rates of overt hepatic encephalopathy, hepatic hydrothorax, hepatorenal syndrome, spontaneous bacterial peritonitis, hepatocellular carcinoma, serious adverse events, or nonserious adverse events. At 1 and 3 months, patients in the TIPS group had a slight increase of median bilirubin concentrations and median international normalized ratio; however, these values normalized after 6 months. A similar temporal pattern was observed in early TIPS patients with regard to median Model for End-Stage Liver Disease score.
“[The transplantation-free survival benefit of early TIPS] was probably related to better control of factors contributing to death, such as failure to control bleeding or rebleeding or new or worsening ascites, without increasing the frequency and severity of overt hepatic encephalopathy and other adverse events,” the investigators concluded. “This study provides direct evidence and greater confidence in the recommendations of current guidelines that early TIPS should be performed in high-risk patients without contraindications.
“Future studies addressing whether early TIPS can be equally recommended in Child-Pugh B and C patients are warranted,” the investigators added.
The study was funded by the National Key Technology R&D Program, Boost Program of Xijing Hospital, Optimized Overall Project of Shaanxi Province, and National Natural Science Foundation of China. The investigators reported no conflicts of interest.
SOURCE: Lv Y et al. Lancet Gastroenterol Hepatol. 2019 May 29. doi: 10.1016/S2468-1253(19)30090-1.
Early TIPS “should therefore be preferred to the current standard of care,” reported lead author Yong Lv, MD, of the Fourth Military Medical University in Xi’an, China, and colleagues, referring to standard pharmaceutical and endoscopic therapy.
“[The current standard] approach has improved patient outcomes,” the investigators wrote in the Lancet Gastroenterology & Hepatology. “However, up to 10%-20% of patients still experience treatment failure, requiring further intensive management. In such patients, [TIPS] is successful in achieving hemostasis in 90%-100% of patients. However, 6-week mortality remains high [35%-55%]. This is probably because the severity of the underlying liver disease has worsened and additional organ dysfunction may have occurred after several failed endoscopic therapy attempts.”
Previous studies have explored earlier intervention with TIPS; however, according to the investigators, these trials were inconclusive for various reasons. For example, uncovered stents and an out-of-date control therapy were employed in a trial by Monescillo et al., while a study by Garcia-Pagan et al. lacked a primary survival endpoint and has been criticized for selection bias. “Thus, whether early TIPS confers a survival benefit in a broader population remains to be assessed,” the investigators wrote.
To this end, the investigators screened 373 patients with advanced cirrhosis (Child-Pugh class B or C) and acute variceal bleeding. Of these, 132 were eligible for inclusion based on age, disease severity, willingness to participate, comorbidities, and other factors. Patients were randomized 2:1 to receive either early TIPS or standard therapy. Within 12 hours of hospital admission for the initial bleeding episode, all patients received vasoactive drugs or endoscopic band ligation and prophylactic antibiotics. Control patients continued vasoactive drugs for up to 5 days, followed by propranolol, which was titrated to reduce resting heart rate by 25% but not less than 55 beats per minute. Elective endoscopic band ligation was performed within 1-2 weeks of initial endoscopic treatment, then approximately every 2 weeks until variceal eradication, and additionally if varices reappeared. TIPS was allowed as rescue therapy. In contrast, patients in the TIPS group underwent the procedure with conscious sedation and local anesthesia within 72 hours of diagnostic endoscopy, followed by approximately 1 week of antibiotics and vasoactive drugs. TIPS revision with angioplasty or another stent placement was performed in the event of shunt dysfunction or reemergence of portal hypertensive complications. The final dataset contained 127 patients, as 3 were excluded after randomization because of exclusionary diagnoses, 1 withdrew consent, and 1 died before TIPS placement.
The primary endpoint was transplantation-free survival. The secondary endpoints were new or worsening ascites based on ultrasound score or sustained ascites necessitating paracentesis, failure to control bleeding or rebleeding, overt hepatic encephalopathy, other complications of portal hypertension, and adverse events.
After a median follow-up of 24 months, data analysis showed a survival benefit associated with early TIPS based on multiple measures. Out of 84 patients in the TIPS group, 15 (18%) died during follow-up, compared with 15 (33%) in the control group. Actuarial transplantation-free survival was also better with TIPS instead of standard therapy at 6 weeks (99% vs. 84%), 1 year (86% vs. 73%), and 2 years (79% vs. 64%). The hazard ratio for transplantation-free survival was 0.50 in favor of TIPS (P = .04). These survival advantages were maintained regardless of hepatitis B virus status or Child-Pugh/Model for End-Stage Liver Disease score.
Similarly to transplantation-free survival, patients treated with TIPS were more likely to be free of uncontrolled bleeding or rebleeding at 1 year (89% vs. 66%) and 2 years (86% vs. 57%). The associated hazard ratio for this outcome favored early TIPS (HR, 0.26; P less than .0001), and univariate and multivariate analysis confirmed an independent protective role. In further support of superiority over standard therapy, patients treated with TIPS were more likely than those in the control group to be free of new or worsening ascites at 1 year (89% vs. 57%) and 2 years (81% vs. 54%).
No significant intergroup differences were found for rates of overt hepatic encephalopathy, hepatic hydrothorax, hepatorenal syndrome, spontaneous bacterial peritonitis, hepatocellular carcinoma, serious adverse events, or nonserious adverse events. At 1 and 3 months, patients in the TIPS group had a slight increase of median bilirubin concentrations and median international normalized ratio; however, these values normalized after 6 months. A similar temporal pattern was observed in early TIPS patients with regard to median Model for End-Stage Liver Disease score.
“[The transplantation-free survival benefit of early TIPS] was probably related to better control of factors contributing to death, such as failure to control bleeding or rebleeding or new or worsening ascites, without increasing the frequency and severity of overt hepatic encephalopathy and other adverse events,” the investigators concluded. “This study provides direct evidence and greater confidence in the recommendations of current guidelines that early TIPS should be performed in high-risk patients without contraindications.
“Future studies addressing whether early TIPS can be equally recommended in Child-Pugh B and C patients are warranted,” the investigators added.
The study was funded by the National Key Technology R&D Program, Boost Program of Xijing Hospital, Optimized Overall Project of Shaanxi Province, and National Natural Science Foundation of China. The investigators reported no conflicts of interest.
SOURCE: Lv Y et al. Lancet Gastroenterol Hepatol. 2019 May 29. doi: 10.1016/S2468-1253(19)30090-1.
FROM THE LANCET GASTROENTEROLOGY & HEPATOLOGY
Bringing QI training to an IM residency program
Consider a formal step-wise curriculum
For current and future hospitalists, there’s no doubt that knowledge of quality improvement (QI) fundamentals is an important component of a successful practice. One physician team set out to provide their trainees with that QI foundation and described the results.
“We believed that implementing a formal step-wise QI curriculum would not only meet the Accreditation Council of Graduate Medical Education (ACGME) requirements, but also increase residents’ knowledge of QI fundamentals and ultimately establish a culture of continuous improvement aiming to provide high-value care to our health care consumers,” said lead author J. Colt Cowdell, MD, MBA, of Mayo Clinic in Jacksonville, Fla.
Prior to any interventions, the team surveyed internal medicine residents regarding three unique patient scenarios and scored their answers. Residents were then assigned to one of five unique QI projects for the academic year in combination with a structured didactic QI curriculum.
After the structured progressive curriculum, in combination with team-based QI projects, residents were surveyed again. Results showed not only increased QI knowledge, but also improved patient safety and reduced waste.
“Keys to successful implementation included a thorough explanation of the need for this curriculum to the learners and ensuring that QI teams were multidisciplinary – residents, QI experts, nurses, techs, pharmacy, administrators, etc.,” said Dr. Cowdell.
For hospitalists in an academic setting, this work can provide a framework to incorporate QI into their residency programs. “I hope, if they have a passion for QI, they would seek out opportunities to mentor residents and help lead multidisciplinary team-based projects,” Dr. Cowdell said.
Reference
1. Cowdell, JC; Trautman, C; Lewis, M; Dawson, N. Integration of a Novel Quality Improvement Curriculum into an Internal Medicine Residency Program. Abstract published at Hospital Medicine 2018; April 8-11; Orlando, Fla. Abstract 54. https://www.shmabstracts.com/abstract/integration-of-a-novel-quality-improvement-curriculum-into-an-internal-medicine-residency-program/. Accessed Dec. 11, 2018.
Consider a formal step-wise curriculum
Consider a formal step-wise curriculum
For current and future hospitalists, there’s no doubt that knowledge of quality improvement (QI) fundamentals is an important component of a successful practice. One physician team set out to provide their trainees with that QI foundation and described the results.
“We believed that implementing a formal step-wise QI curriculum would not only meet the Accreditation Council of Graduate Medical Education (ACGME) requirements, but also increase residents’ knowledge of QI fundamentals and ultimately establish a culture of continuous improvement aiming to provide high-value care to our health care consumers,” said lead author J. Colt Cowdell, MD, MBA, of Mayo Clinic in Jacksonville, Fla.
Prior to any interventions, the team surveyed internal medicine residents regarding three unique patient scenarios and scored their answers. Residents were then assigned to one of five unique QI projects for the academic year in combination with a structured didactic QI curriculum.
After the structured progressive curriculum, in combination with team-based QI projects, residents were surveyed again. Results showed not only increased QI knowledge, but also improved patient safety and reduced waste.
“Keys to successful implementation included a thorough explanation of the need for this curriculum to the learners and ensuring that QI teams were multidisciplinary – residents, QI experts, nurses, techs, pharmacy, administrators, etc.,” said Dr. Cowdell.
For hospitalists in an academic setting, this work can provide a framework to incorporate QI into their residency programs. “I hope, if they have a passion for QI, they would seek out opportunities to mentor residents and help lead multidisciplinary team-based projects,” Dr. Cowdell said.
Reference
1. Cowdell, JC; Trautman, C; Lewis, M; Dawson, N. Integration of a Novel Quality Improvement Curriculum into an Internal Medicine Residency Program. Abstract published at Hospital Medicine 2018; April 8-11; Orlando, Fla. Abstract 54. https://www.shmabstracts.com/abstract/integration-of-a-novel-quality-improvement-curriculum-into-an-internal-medicine-residency-program/. Accessed Dec. 11, 2018.
For current and future hospitalists, there’s no doubt that knowledge of quality improvement (QI) fundamentals is an important component of a successful practice. One physician team set out to provide their trainees with that QI foundation and described the results.
“We believed that implementing a formal step-wise QI curriculum would not only meet the Accreditation Council of Graduate Medical Education (ACGME) requirements, but also increase residents’ knowledge of QI fundamentals and ultimately establish a culture of continuous improvement aiming to provide high-value care to our health care consumers,” said lead author J. Colt Cowdell, MD, MBA, of Mayo Clinic in Jacksonville, Fla.
Prior to any interventions, the team surveyed internal medicine residents regarding three unique patient scenarios and scored their answers. Residents were then assigned to one of five unique QI projects for the academic year in combination with a structured didactic QI curriculum.
After the structured progressive curriculum, in combination with team-based QI projects, residents were surveyed again. Results showed not only increased QI knowledge, but also improved patient safety and reduced waste.
“Keys to successful implementation included a thorough explanation of the need for this curriculum to the learners and ensuring that QI teams were multidisciplinary – residents, QI experts, nurses, techs, pharmacy, administrators, etc.,” said Dr. Cowdell.
For hospitalists in an academic setting, this work can provide a framework to incorporate QI into their residency programs. “I hope, if they have a passion for QI, they would seek out opportunities to mentor residents and help lead multidisciplinary team-based projects,” Dr. Cowdell said.
Reference
1. Cowdell, JC; Trautman, C; Lewis, M; Dawson, N. Integration of a Novel Quality Improvement Curriculum into an Internal Medicine Residency Program. Abstract published at Hospital Medicine 2018; April 8-11; Orlando, Fla. Abstract 54. https://www.shmabstracts.com/abstract/integration-of-a-novel-quality-improvement-curriculum-into-an-internal-medicine-residency-program/. Accessed Dec. 11, 2018.