User login
Are you up for the challenge? Dr. Salim Surani is!
Recently, the CHEST Foundation had the pleasure of sitting down with Salim Surani, MD, FCCP to get his perspective on the NetWorks Challenge and its impact. Dr. Surani initially got involved with CHEST at the Board level and is now a leader within the Council of NetWorks. “My hope was that I could work within my NetWork to help them become more involved with CHEST and the CHEST Foundation. Through this involvement, I believe we can help shape changes in chest medicine practice dynamics. In the Practice Operations NetWork, we strive to educate physicians in practice to ensure they are up to date with government regulations and how to navigate changes in a positive way, ultimately with the goal of impacting our patients’ lives for the better.”
When asked about his involvement with CHEST and the Foundation, he said “It just makes sense to be involved in an institution that is passionate about taking care of patients and clinicians. The CHEST Foundation has given tens of millions of dollars in funding for grants to help shape the future of the education, the future of research, and the future of better patient care.”
Dr. Surani has always been a strong advocate for the NetWorks Challenge. “There is nothing that has been more satisfying in my life than the opportunity to give. I have always believed that the biggest winner is the person who gives a gift. When you give something to the right cause, what you get in return is a tremendous amount of satisfaction, and it is that satisfaction which drives you – which gives you a feeling of purpose. I want others to get involved and participate. If you feel passionate about something, put your money where your mouth is. This is why I will be matching any gift of $500 or greater by 10% made to any NetWork during the NetWorks Challenge. This is an opportunity to multiply your donation before it goes to the CHEST Foundation so that grants and other awards can be larger in the coming years. The NetWorks Challenge helps fund our Diversity Travel Grants Program and provides additional travel grants to each participating NetWork.” Last year, Dr. Surani gave an additional $2,365.17 through his challenge match. Are you up for the challenge this year?
Visit chestfoundation.org/donate today to help shape the future of our discipline!
Recently, the CHEST Foundation had the pleasure of sitting down with Salim Surani, MD, FCCP to get his perspective on the NetWorks Challenge and its impact. Dr. Surani initially got involved with CHEST at the Board level and is now a leader within the Council of NetWorks. “My hope was that I could work within my NetWork to help them become more involved with CHEST and the CHEST Foundation. Through this involvement, I believe we can help shape changes in chest medicine practice dynamics. In the Practice Operations NetWork, we strive to educate physicians in practice to ensure they are up to date with government regulations and how to navigate changes in a positive way, ultimately with the goal of impacting our patients’ lives for the better.”
When asked about his involvement with CHEST and the Foundation, he said “It just makes sense to be involved in an institution that is passionate about taking care of patients and clinicians. The CHEST Foundation has given tens of millions of dollars in funding for grants to help shape the future of the education, the future of research, and the future of better patient care.”
Dr. Surani has always been a strong advocate for the NetWorks Challenge. “There is nothing that has been more satisfying in my life than the opportunity to give. I have always believed that the biggest winner is the person who gives a gift. When you give something to the right cause, what you get in return is a tremendous amount of satisfaction, and it is that satisfaction which drives you – which gives you a feeling of purpose. I want others to get involved and participate. If you feel passionate about something, put your money where your mouth is. This is why I will be matching any gift of $500 or greater by 10% made to any NetWork during the NetWorks Challenge. This is an opportunity to multiply your donation before it goes to the CHEST Foundation so that grants and other awards can be larger in the coming years. The NetWorks Challenge helps fund our Diversity Travel Grants Program and provides additional travel grants to each participating NetWork.” Last year, Dr. Surani gave an additional $2,365.17 through his challenge match. Are you up for the challenge this year?
Visit chestfoundation.org/donate today to help shape the future of our discipline!
Recently, the CHEST Foundation had the pleasure of sitting down with Salim Surani, MD, FCCP to get his perspective on the NetWorks Challenge and its impact. Dr. Surani initially got involved with CHEST at the Board level and is now a leader within the Council of NetWorks. “My hope was that I could work within my NetWork to help them become more involved with CHEST and the CHEST Foundation. Through this involvement, I believe we can help shape changes in chest medicine practice dynamics. In the Practice Operations NetWork, we strive to educate physicians in practice to ensure they are up to date with government regulations and how to navigate changes in a positive way, ultimately with the goal of impacting our patients’ lives for the better.”
When asked about his involvement with CHEST and the Foundation, he said “It just makes sense to be involved in an institution that is passionate about taking care of patients and clinicians. The CHEST Foundation has given tens of millions of dollars in funding for grants to help shape the future of the education, the future of research, and the future of better patient care.”
Dr. Surani has always been a strong advocate for the NetWorks Challenge. “There is nothing that has been more satisfying in my life than the opportunity to give. I have always believed that the biggest winner is the person who gives a gift. When you give something to the right cause, what you get in return is a tremendous amount of satisfaction, and it is that satisfaction which drives you – which gives you a feeling of purpose. I want others to get involved and participate. If you feel passionate about something, put your money where your mouth is. This is why I will be matching any gift of $500 or greater by 10% made to any NetWork during the NetWorks Challenge. This is an opportunity to multiply your donation before it goes to the CHEST Foundation so that grants and other awards can be larger in the coming years. The NetWorks Challenge helps fund our Diversity Travel Grants Program and provides additional travel grants to each participating NetWork.” Last year, Dr. Surani gave an additional $2,365.17 through his challenge match. Are you up for the challenge this year?
Visit chestfoundation.org/donate today to help shape the future of our discipline!
Clinical pulmonary medicine. Cardiovascular medicine and surgery. Chest infections. Interprofessional team.
Clinical Pulmonary Medicine
Pulmonary embolism in pregnancy: A diagnostic conundrum
Pulmonary embolism (PE) is the 6th leading cause of maternal mortality in the United States. The clinical signs and symptoms of PE are usually nonspecific and often overlap with the normal physiologic changes of pregnancy. Due to low specificity and sensitivity of D-dimer test, pregnant patients with suspected PE often undergo CT pulmonary angiography (CTPA) and ventilation-perfusion scanning, both of which can cause radiation exposure to mother and fetus.
To answer whether pregnancy-adapted YEARS algorithm (Van der Hulle T et al. Lancet. 2017;390[10091]:289) can be safely used to avoid diagnostic imaging, Artemis Study Investigators prospectively studied three criteria from YEARS algorithm in combination with a D-dimer level (Van der Pol et al. N Engl J Med. 2019;380[12]:1139. The three criteria included clinical signs of deep-vein thrombosis (DVT), hemoptysis, and PE as the most likely diagnosis. PE was considered ruled out when none of the three criteria were present and D-dimer was less than 1000 ng/mL or if one or more of the criteria were met and D-dimer was less than 500 ng/mL. Patients in whom D-dimer was greater than 1000 ng/mL or in those with D-dimer greater than 500 ng/mL and had 1 or more of the YEARS algorithm criteria present, PE could not be ruled out and underwent CTPA. A modification of the criteria was done only for patients who had clinical signs of DVT at baseline. These patients underwent compression ultrasonography and if a clot was found, CTPA was not performed and patients were started on anticoagulation therapy. Those with negative DVT studies were subclassified based on D-dimer levels as the study population above. Patients in whom pulmonary embolism was not ruled out underwent CTPA. Of these 299 patients, 16 (5.4%) were confirmed to have PE at baseline.
In the remaining 195 patients in whom PE was ruled out on the basis of study protocol, a 3-month follow-up diagnosed one patient (0.51%) with VTE. Using pregnancy-adapted YEARS algorithm, CTPA was avoided in 39% of the patients of which 65% were in their first trimester when the radiation exposure can be most harmful to the fetus.
Muhammad Adrish, MD, FCCP
Steering Committee Member
Munish Luthra, MD, FCCP
Steering Committee Member
Cardiovascular Medicine and Surgery
Physical examination of low cardiac output in the ICU
Rapid evaluation of shock requires identifying signs of tissue hypoperfusion and differentiating between cardiogenic, obstructive, hypovolemic, and vasodilatory etiologies. Cardiac abnormalities may also contribute to mixed shock states in a broad array of critically ill patients. Left ventricular dysfunction in inpatients correlates with physical exam, with a 2.0 positive likelihood ratio and 0.41 negative likelihood ratio (Simel DL, Rennie D, eds. The Rational Clinical Examination: Evidence-Based Clinical Diagnosis. 2009). Accurate clinical assessment of cardiac output, however, is a fraught endeavor. In a recently published large series of patients with unplanned ICU admission, atrial fibrillation, systolic blood pressure (BP) < 90, altered consciousness, capillary refill time >4.5 seconds at the sternum, or skin mottling over the knee predicted low cardiac output with specificity >90%. Of 280 patients with a cardiac index of < 2.2 L/min/m2, less than half had any one of these findings (Hiemstra, et al. Intensive Care Med. 2019;45[2]:190).
Regarding determination of shock etiology, in a small series of patients with systolic blood pressure < 90 mm Hg, physical exam findings of relatively warm skin temperature and rapid capillary refill had 89% sensitivity for vasodilatory shock, and jugular venous pressure ≥8 had 82% sensitivity for cardiogenic etiologies (Vazquez, et al. J Hosp Med. 2010;5[8]:471). Thus, while physical exam findings may inform bedside shock assessment, their accuracy is limited. Critical care physicians should consider additional assessment techniques, such as echocardiography or invasive hemodynamic monitoring, if diagnostic uncertainty persists (Vincent, et al. N Engl J Med. 2013;369[18]:1726).
Benjamin Kenigsberg, MD
Steering Committee Member
Dr. David Bowton and Dr. Steven Hollenberg contributed to the article.
Chest Infections
Lung infections in the transplant recipients
The increase in lung transplantation over the years led to lung transplant recipients presenting to pulmonologists outside of specialized centers. One of the most common presentations is for infections. Infections account for more than 25% of all posttransplant deaths (Yusen, et al. J Heart Lung Transplant. 2014;33[10]:1009.
Multiple factors contribute to this increased infection risk, including donor lung colonization, disruption of local host defenses, constant contact with environmental pathogens, and heavy immunosuppression (Redmund KF, et al. Proc Am Thorac Soc. 2009;6[1]:94).
The onset of infectious manifestations, from the time of transplantation, is variable, depending on the organism. Based on the time of onset, infections can be categorized into within the first month posttransplant, 1 to 6 months, and beyond 6 months, posttransplant. During the first month, because of allograft colonization, preexisting infections in the recipient, and surgical- and hospital-acquired nosocomial infections are more common. The first 6 months are where the patients are at the highest risk for opportunistic infections. As the immunosuppression is lowered after 6 months, the causative organisms tend to be more common pathogens (Green M. Am J Transplant. 2013;13 [suppl 4]:3-8).
An early, aggressive, empiric antimicrobial therapy initiation and proactive, invasive diagnostic approach with needed testing to identify the potential pathogen, is imperative in these patients. Early bronchoscopy with bronchoalveolar lavage remains the most sensitive test to identify pathogens. Therapy can then be tailored toward the identified pathogen.
As part of the Chest Infections NetWork, we would like to raise awareness of lung infections in unique subgroups, such as lung transplant recipients. Treating infections in such patients requires a high index of suspicion in the setting of an atypical presentation.
Raed Alalawi, MD, FCCP
Steering Committee Member
Interprofessional Team
Extracorporeal Membrane Oxygenation (ECMO) in Near Fatal Asthma
Near fatal asthma (NFA) is defined as acute severe asthma characterized by acute respiratory failure with hypercapnia and/or respiratory acidosis requiring ventilator support. NFA refractory to conventional medical management and ventilator therapy can lead to fatal outcomes. Near fatal asthma also carries substantial mortality if invasive ventilation is needed (Marquette CH, et al. Am Rev Respir Dis. 1992;146[1]:76). Use of sedatives can exacerbate bronchospasm, and positive pressure ventilation can exacerbate dynamic hyperinflation, impairing hemodynamics, and gas exchange, and leading to barotrauma. This approach seems contrary to the goals of management. Outside of conventional therapies, such as IV steroids and inhaled beta-agonists, the data supporting other therapies such as IV beta-agonists, MgSO4, methylxanthines, mucolytics, heliox, and volatile anesthetics are scant. In contrast, venovenous ECMO can provide adequate gas exchange and prevent lung injury induced by mechanical ventilation and may be an effective bridging strategy to avoid aggressive ventilation in refractory NFA (Hye Ju Yeo, et al. Critical Care. 2017;21[1]:297).
Use of early ECMO to permit spontaneous breathing while the circuit accomplishes required ventilation and oxygenation seems more ideal. Avoidance of mechanical ventilation not only prevents complications like barotrauma but also may reduce delirium, malnutrition, and neuromuscular dysfunction. Performing “awake” ECMO has successfully been described for obstructive airway disease (Langer T, et al. Critical Care. 2016;20[1]:150). Factors limiting this approach are the invasive nature of ECMO and the inherent risks of large cannula dislodgement; however, the safety of this has been demonstrated with ambulation of ECMO patients to receive physical therapy (Abrams D, et al. Ann Cardiothorac Surg. 2019;8[1]:44). Alternatively, extracorporeal carbon dioxide removal (ECCO2R) systems utilize smaller catheters to satisfactorily remove CO2 while oxygen supplementation could be achieved via nasal cannula (Pisani L, et al. Respiratory Care. 2018;63[9]:1174). Incorporation of ECMO in select cases of NFA, especially ECCO2R, should be considered as an early rather than rescue therapy for acute severe asthma refractory to conventional medical therapy.
Robert Baeten, DMSc, PA-C, FCCP
Steering Committee Member
Munish Luthra MD, FCCP
Steering Committee Member
Clinical Pulmonary Medicine
Pulmonary embolism in pregnancy: A diagnostic conundrum
Pulmonary embolism (PE) is the 6th leading cause of maternal mortality in the United States. The clinical signs and symptoms of PE are usually nonspecific and often overlap with the normal physiologic changes of pregnancy. Due to low specificity and sensitivity of D-dimer test, pregnant patients with suspected PE often undergo CT pulmonary angiography (CTPA) and ventilation-perfusion scanning, both of which can cause radiation exposure to mother and fetus.
To answer whether pregnancy-adapted YEARS algorithm (Van der Hulle T et al. Lancet. 2017;390[10091]:289) can be safely used to avoid diagnostic imaging, Artemis Study Investigators prospectively studied three criteria from YEARS algorithm in combination with a D-dimer level (Van der Pol et al. N Engl J Med. 2019;380[12]:1139. The three criteria included clinical signs of deep-vein thrombosis (DVT), hemoptysis, and PE as the most likely diagnosis. PE was considered ruled out when none of the three criteria were present and D-dimer was less than 1000 ng/mL or if one or more of the criteria were met and D-dimer was less than 500 ng/mL. Patients in whom D-dimer was greater than 1000 ng/mL or in those with D-dimer greater than 500 ng/mL and had 1 or more of the YEARS algorithm criteria present, PE could not be ruled out and underwent CTPA. A modification of the criteria was done only for patients who had clinical signs of DVT at baseline. These patients underwent compression ultrasonography and if a clot was found, CTPA was not performed and patients were started on anticoagulation therapy. Those with negative DVT studies were subclassified based on D-dimer levels as the study population above. Patients in whom pulmonary embolism was not ruled out underwent CTPA. Of these 299 patients, 16 (5.4%) were confirmed to have PE at baseline.
In the remaining 195 patients in whom PE was ruled out on the basis of study protocol, a 3-month follow-up diagnosed one patient (0.51%) with VTE. Using pregnancy-adapted YEARS algorithm, CTPA was avoided in 39% of the patients of which 65% were in their first trimester when the radiation exposure can be most harmful to the fetus.
Muhammad Adrish, MD, FCCP
Steering Committee Member
Munish Luthra, MD, FCCP
Steering Committee Member
Cardiovascular Medicine and Surgery
Physical examination of low cardiac output in the ICU
Rapid evaluation of shock requires identifying signs of tissue hypoperfusion and differentiating between cardiogenic, obstructive, hypovolemic, and vasodilatory etiologies. Cardiac abnormalities may also contribute to mixed shock states in a broad array of critically ill patients. Left ventricular dysfunction in inpatients correlates with physical exam, with a 2.0 positive likelihood ratio and 0.41 negative likelihood ratio (Simel DL, Rennie D, eds. The Rational Clinical Examination: Evidence-Based Clinical Diagnosis. 2009). Accurate clinical assessment of cardiac output, however, is a fraught endeavor. In a recently published large series of patients with unplanned ICU admission, atrial fibrillation, systolic blood pressure (BP) < 90, altered consciousness, capillary refill time >4.5 seconds at the sternum, or skin mottling over the knee predicted low cardiac output with specificity >90%. Of 280 patients with a cardiac index of < 2.2 L/min/m2, less than half had any one of these findings (Hiemstra, et al. Intensive Care Med. 2019;45[2]:190).
Regarding determination of shock etiology, in a small series of patients with systolic blood pressure < 90 mm Hg, physical exam findings of relatively warm skin temperature and rapid capillary refill had 89% sensitivity for vasodilatory shock, and jugular venous pressure ≥8 had 82% sensitivity for cardiogenic etiologies (Vazquez, et al. J Hosp Med. 2010;5[8]:471). Thus, while physical exam findings may inform bedside shock assessment, their accuracy is limited. Critical care physicians should consider additional assessment techniques, such as echocardiography or invasive hemodynamic monitoring, if diagnostic uncertainty persists (Vincent, et al. N Engl J Med. 2013;369[18]:1726).
Benjamin Kenigsberg, MD
Steering Committee Member
Dr. David Bowton and Dr. Steven Hollenberg contributed to the article.
Chest Infections
Lung infections in the transplant recipients
The increase in lung transplantation over the years led to lung transplant recipients presenting to pulmonologists outside of specialized centers. One of the most common presentations is for infections. Infections account for more than 25% of all posttransplant deaths (Yusen, et al. J Heart Lung Transplant. 2014;33[10]:1009.
Multiple factors contribute to this increased infection risk, including donor lung colonization, disruption of local host defenses, constant contact with environmental pathogens, and heavy immunosuppression (Redmund KF, et al. Proc Am Thorac Soc. 2009;6[1]:94).
The onset of infectious manifestations, from the time of transplantation, is variable, depending on the organism. Based on the time of onset, infections can be categorized into within the first month posttransplant, 1 to 6 months, and beyond 6 months, posttransplant. During the first month, because of allograft colonization, preexisting infections in the recipient, and surgical- and hospital-acquired nosocomial infections are more common. The first 6 months are where the patients are at the highest risk for opportunistic infections. As the immunosuppression is lowered after 6 months, the causative organisms tend to be more common pathogens (Green M. Am J Transplant. 2013;13 [suppl 4]:3-8).
An early, aggressive, empiric antimicrobial therapy initiation and proactive, invasive diagnostic approach with needed testing to identify the potential pathogen, is imperative in these patients. Early bronchoscopy with bronchoalveolar lavage remains the most sensitive test to identify pathogens. Therapy can then be tailored toward the identified pathogen.
As part of the Chest Infections NetWork, we would like to raise awareness of lung infections in unique subgroups, such as lung transplant recipients. Treating infections in such patients requires a high index of suspicion in the setting of an atypical presentation.
Raed Alalawi, MD, FCCP
Steering Committee Member
Interprofessional Team
Extracorporeal Membrane Oxygenation (ECMO) in Near Fatal Asthma
Near fatal asthma (NFA) is defined as acute severe asthma characterized by acute respiratory failure with hypercapnia and/or respiratory acidosis requiring ventilator support. NFA refractory to conventional medical management and ventilator therapy can lead to fatal outcomes. Near fatal asthma also carries substantial mortality if invasive ventilation is needed (Marquette CH, et al. Am Rev Respir Dis. 1992;146[1]:76). Use of sedatives can exacerbate bronchospasm, and positive pressure ventilation can exacerbate dynamic hyperinflation, impairing hemodynamics, and gas exchange, and leading to barotrauma. This approach seems contrary to the goals of management. Outside of conventional therapies, such as IV steroids and inhaled beta-agonists, the data supporting other therapies such as IV beta-agonists, MgSO4, methylxanthines, mucolytics, heliox, and volatile anesthetics are scant. In contrast, venovenous ECMO can provide adequate gas exchange and prevent lung injury induced by mechanical ventilation and may be an effective bridging strategy to avoid aggressive ventilation in refractory NFA (Hye Ju Yeo, et al. Critical Care. 2017;21[1]:297).
Use of early ECMO to permit spontaneous breathing while the circuit accomplishes required ventilation and oxygenation seems more ideal. Avoidance of mechanical ventilation not only prevents complications like barotrauma but also may reduce delirium, malnutrition, and neuromuscular dysfunction. Performing “awake” ECMO has successfully been described for obstructive airway disease (Langer T, et al. Critical Care. 2016;20[1]:150). Factors limiting this approach are the invasive nature of ECMO and the inherent risks of large cannula dislodgement; however, the safety of this has been demonstrated with ambulation of ECMO patients to receive physical therapy (Abrams D, et al. Ann Cardiothorac Surg. 2019;8[1]:44). Alternatively, extracorporeal carbon dioxide removal (ECCO2R) systems utilize smaller catheters to satisfactorily remove CO2 while oxygen supplementation could be achieved via nasal cannula (Pisani L, et al. Respiratory Care. 2018;63[9]:1174). Incorporation of ECMO in select cases of NFA, especially ECCO2R, should be considered as an early rather than rescue therapy for acute severe asthma refractory to conventional medical therapy.
Robert Baeten, DMSc, PA-C, FCCP
Steering Committee Member
Munish Luthra MD, FCCP
Steering Committee Member
Clinical Pulmonary Medicine
Pulmonary embolism in pregnancy: A diagnostic conundrum
Pulmonary embolism (PE) is the 6th leading cause of maternal mortality in the United States. The clinical signs and symptoms of PE are usually nonspecific and often overlap with the normal physiologic changes of pregnancy. Due to low specificity and sensitivity of D-dimer test, pregnant patients with suspected PE often undergo CT pulmonary angiography (CTPA) and ventilation-perfusion scanning, both of which can cause radiation exposure to mother and fetus.
To answer whether pregnancy-adapted YEARS algorithm (Van der Hulle T et al. Lancet. 2017;390[10091]:289) can be safely used to avoid diagnostic imaging, Artemis Study Investigators prospectively studied three criteria from YEARS algorithm in combination with a D-dimer level (Van der Pol et al. N Engl J Med. 2019;380[12]:1139. The three criteria included clinical signs of deep-vein thrombosis (DVT), hemoptysis, and PE as the most likely diagnosis. PE was considered ruled out when none of the three criteria were present and D-dimer was less than 1000 ng/mL or if one or more of the criteria were met and D-dimer was less than 500 ng/mL. Patients in whom D-dimer was greater than 1000 ng/mL or in those with D-dimer greater than 500 ng/mL and had 1 or more of the YEARS algorithm criteria present, PE could not be ruled out and underwent CTPA. A modification of the criteria was done only for patients who had clinical signs of DVT at baseline. These patients underwent compression ultrasonography and if a clot was found, CTPA was not performed and patients were started on anticoagulation therapy. Those with negative DVT studies were subclassified based on D-dimer levels as the study population above. Patients in whom pulmonary embolism was not ruled out underwent CTPA. Of these 299 patients, 16 (5.4%) were confirmed to have PE at baseline.
In the remaining 195 patients in whom PE was ruled out on the basis of study protocol, a 3-month follow-up diagnosed one patient (0.51%) with VTE. Using pregnancy-adapted YEARS algorithm, CTPA was avoided in 39% of the patients of which 65% were in their first trimester when the radiation exposure can be most harmful to the fetus.
Muhammad Adrish, MD, FCCP
Steering Committee Member
Munish Luthra, MD, FCCP
Steering Committee Member
Cardiovascular Medicine and Surgery
Physical examination of low cardiac output in the ICU
Rapid evaluation of shock requires identifying signs of tissue hypoperfusion and differentiating between cardiogenic, obstructive, hypovolemic, and vasodilatory etiologies. Cardiac abnormalities may also contribute to mixed shock states in a broad array of critically ill patients. Left ventricular dysfunction in inpatients correlates with physical exam, with a 2.0 positive likelihood ratio and 0.41 negative likelihood ratio (Simel DL, Rennie D, eds. The Rational Clinical Examination: Evidence-Based Clinical Diagnosis. 2009). Accurate clinical assessment of cardiac output, however, is a fraught endeavor. In a recently published large series of patients with unplanned ICU admission, atrial fibrillation, systolic blood pressure (BP) < 90, altered consciousness, capillary refill time >4.5 seconds at the sternum, or skin mottling over the knee predicted low cardiac output with specificity >90%. Of 280 patients with a cardiac index of < 2.2 L/min/m2, less than half had any one of these findings (Hiemstra, et al. Intensive Care Med. 2019;45[2]:190).
Regarding determination of shock etiology, in a small series of patients with systolic blood pressure < 90 mm Hg, physical exam findings of relatively warm skin temperature and rapid capillary refill had 89% sensitivity for vasodilatory shock, and jugular venous pressure ≥8 had 82% sensitivity for cardiogenic etiologies (Vazquez, et al. J Hosp Med. 2010;5[8]:471). Thus, while physical exam findings may inform bedside shock assessment, their accuracy is limited. Critical care physicians should consider additional assessment techniques, such as echocardiography or invasive hemodynamic monitoring, if diagnostic uncertainty persists (Vincent, et al. N Engl J Med. 2013;369[18]:1726).
Benjamin Kenigsberg, MD
Steering Committee Member
Dr. David Bowton and Dr. Steven Hollenberg contributed to the article.
Chest Infections
Lung infections in the transplant recipients
The increase in lung transplantation over the years led to lung transplant recipients presenting to pulmonologists outside of specialized centers. One of the most common presentations is for infections. Infections account for more than 25% of all posttransplant deaths (Yusen, et al. J Heart Lung Transplant. 2014;33[10]:1009.
Multiple factors contribute to this increased infection risk, including donor lung colonization, disruption of local host defenses, constant contact with environmental pathogens, and heavy immunosuppression (Redmund KF, et al. Proc Am Thorac Soc. 2009;6[1]:94).
The onset of infectious manifestations, from the time of transplantation, is variable, depending on the organism. Based on the time of onset, infections can be categorized into within the first month posttransplant, 1 to 6 months, and beyond 6 months, posttransplant. During the first month, because of allograft colonization, preexisting infections in the recipient, and surgical- and hospital-acquired nosocomial infections are more common. The first 6 months are where the patients are at the highest risk for opportunistic infections. As the immunosuppression is lowered after 6 months, the causative organisms tend to be more common pathogens (Green M. Am J Transplant. 2013;13 [suppl 4]:3-8).
An early, aggressive, empiric antimicrobial therapy initiation and proactive, invasive diagnostic approach with needed testing to identify the potential pathogen, is imperative in these patients. Early bronchoscopy with bronchoalveolar lavage remains the most sensitive test to identify pathogens. Therapy can then be tailored toward the identified pathogen.
As part of the Chest Infections NetWork, we would like to raise awareness of lung infections in unique subgroups, such as lung transplant recipients. Treating infections in such patients requires a high index of suspicion in the setting of an atypical presentation.
Raed Alalawi, MD, FCCP
Steering Committee Member
Interprofessional Team
Extracorporeal Membrane Oxygenation (ECMO) in Near Fatal Asthma
Near fatal asthma (NFA) is defined as acute severe asthma characterized by acute respiratory failure with hypercapnia and/or respiratory acidosis requiring ventilator support. NFA refractory to conventional medical management and ventilator therapy can lead to fatal outcomes. Near fatal asthma also carries substantial mortality if invasive ventilation is needed (Marquette CH, et al. Am Rev Respir Dis. 1992;146[1]:76). Use of sedatives can exacerbate bronchospasm, and positive pressure ventilation can exacerbate dynamic hyperinflation, impairing hemodynamics, and gas exchange, and leading to barotrauma. This approach seems contrary to the goals of management. Outside of conventional therapies, such as IV steroids and inhaled beta-agonists, the data supporting other therapies such as IV beta-agonists, MgSO4, methylxanthines, mucolytics, heliox, and volatile anesthetics are scant. In contrast, venovenous ECMO can provide adequate gas exchange and prevent lung injury induced by mechanical ventilation and may be an effective bridging strategy to avoid aggressive ventilation in refractory NFA (Hye Ju Yeo, et al. Critical Care. 2017;21[1]:297).
Use of early ECMO to permit spontaneous breathing while the circuit accomplishes required ventilation and oxygenation seems more ideal. Avoidance of mechanical ventilation not only prevents complications like barotrauma but also may reduce delirium, malnutrition, and neuromuscular dysfunction. Performing “awake” ECMO has successfully been described for obstructive airway disease (Langer T, et al. Critical Care. 2016;20[1]:150). Factors limiting this approach are the invasive nature of ECMO and the inherent risks of large cannula dislodgement; however, the safety of this has been demonstrated with ambulation of ECMO patients to receive physical therapy (Abrams D, et al. Ann Cardiothorac Surg. 2019;8[1]:44). Alternatively, extracorporeal carbon dioxide removal (ECCO2R) systems utilize smaller catheters to satisfactorily remove CO2 while oxygen supplementation could be achieved via nasal cannula (Pisani L, et al. Respiratory Care. 2018;63[9]:1174). Incorporation of ECMO in select cases of NFA, especially ECCO2R, should be considered as an early rather than rescue therapy for acute severe asthma refractory to conventional medical therapy.
Robert Baeten, DMSc, PA-C, FCCP
Steering Committee Member
Munish Luthra MD, FCCP
Steering Committee Member
Five traditional New Orleans dishes to try
What makes the traditional New Orleans food so special? The flair and broad history for these dishes unite the city and the love for all things tasty with its seafood, Creole, Cajun, and many other types of food options. We’ve picked five famous New Orleans dishes that you should try while you attend CHEST 2019.
GUMBO
As one of Louisiana’s quintessential dishes, you can find gumbo in restaurants, at events, and homes all over the state. Claiming both French and West African roots, there’s no one way to make gumbo, but it is usually served over rice and with a wide variety of other ingredients. With so many different recipes that each family and cook has perfected to be the “best,” most cooks tend to guard their recipes closely.
CRAWFISH ETOUFFEE
The word étouffée (pronounced eh-too-fey) comes from the French word “to smother.” This dish is a very thick stew full of crawfish (or shrimp) served over rice. It is also similar in some way to gumbo – same types of Creole seasonings, served over rice, and made with a roux – but it is often made with a “blonde” roux, which is lighter in color and gives an almost sweet flavor. It’s a taste that’s worth trying and claimed you won’t forget.
JAMBALAYA
Another famous and traditional New Orleans dish is jambalaya. This is a rice dish that is a culinary staple of the city with a history from the time when colonial Spanish settlers tried reconstructing their native paella from locally sourced ingredients. It typically contains a mix of meat, vegetables, spices, and rice, combined in a variety of ways.
PO-BOYS
This classic French bread sandwich is stuffed and slathered with sauce. Filled with lettuce, tomato, and pickles, it’s usually whatever filled with whatever meat you choose – roast beef, fried shrimp, oysters. This allows for many types of po-boy sandwiches. You tend to see very creative po-boys at the Oak Street Po-Boy Festival each year.
BEIGNETS
These pastries are more than just a doughnut and are famous for being a doughnut without the hole. As the city’s most popular sweet treat and staple, locals and visitors can enjoy beignets all year long, available 24-hours a day in New Orleans at more than one coffee hotspot.
What makes the traditional New Orleans food so special? The flair and broad history for these dishes unite the city and the love for all things tasty with its seafood, Creole, Cajun, and many other types of food options. We’ve picked five famous New Orleans dishes that you should try while you attend CHEST 2019.
GUMBO
As one of Louisiana’s quintessential dishes, you can find gumbo in restaurants, at events, and homes all over the state. Claiming both French and West African roots, there’s no one way to make gumbo, but it is usually served over rice and with a wide variety of other ingredients. With so many different recipes that each family and cook has perfected to be the “best,” most cooks tend to guard their recipes closely.
CRAWFISH ETOUFFEE
The word étouffée (pronounced eh-too-fey) comes from the French word “to smother.” This dish is a very thick stew full of crawfish (or shrimp) served over rice. It is also similar in some way to gumbo – same types of Creole seasonings, served over rice, and made with a roux – but it is often made with a “blonde” roux, which is lighter in color and gives an almost sweet flavor. It’s a taste that’s worth trying and claimed you won’t forget.
JAMBALAYA
Another famous and traditional New Orleans dish is jambalaya. This is a rice dish that is a culinary staple of the city with a history from the time when colonial Spanish settlers tried reconstructing their native paella from locally sourced ingredients. It typically contains a mix of meat, vegetables, spices, and rice, combined in a variety of ways.
PO-BOYS
This classic French bread sandwich is stuffed and slathered with sauce. Filled with lettuce, tomato, and pickles, it’s usually whatever filled with whatever meat you choose – roast beef, fried shrimp, oysters. This allows for many types of po-boy sandwiches. You tend to see very creative po-boys at the Oak Street Po-Boy Festival each year.
BEIGNETS
These pastries are more than just a doughnut and are famous for being a doughnut without the hole. As the city’s most popular sweet treat and staple, locals and visitors can enjoy beignets all year long, available 24-hours a day in New Orleans at more than one coffee hotspot.
What makes the traditional New Orleans food so special? The flair and broad history for these dishes unite the city and the love for all things tasty with its seafood, Creole, Cajun, and many other types of food options. We’ve picked five famous New Orleans dishes that you should try while you attend CHEST 2019.
GUMBO
As one of Louisiana’s quintessential dishes, you can find gumbo in restaurants, at events, and homes all over the state. Claiming both French and West African roots, there’s no one way to make gumbo, but it is usually served over rice and with a wide variety of other ingredients. With so many different recipes that each family and cook has perfected to be the “best,” most cooks tend to guard their recipes closely.
CRAWFISH ETOUFFEE
The word étouffée (pronounced eh-too-fey) comes from the French word “to smother.” This dish is a very thick stew full of crawfish (or shrimp) served over rice. It is also similar in some way to gumbo – same types of Creole seasonings, served over rice, and made with a roux – but it is often made with a “blonde” roux, which is lighter in color and gives an almost sweet flavor. It’s a taste that’s worth trying and claimed you won’t forget.
JAMBALAYA
Another famous and traditional New Orleans dish is jambalaya. This is a rice dish that is a culinary staple of the city with a history from the time when colonial Spanish settlers tried reconstructing their native paella from locally sourced ingredients. It typically contains a mix of meat, vegetables, spices, and rice, combined in a variety of ways.
PO-BOYS
This classic French bread sandwich is stuffed and slathered with sauce. Filled with lettuce, tomato, and pickles, it’s usually whatever filled with whatever meat you choose – roast beef, fried shrimp, oysters. This allows for many types of po-boy sandwiches. You tend to see very creative po-boys at the Oak Street Po-Boy Festival each year.
BEIGNETS
These pastries are more than just a doughnut and are famous for being a doughnut without the hole. As the city’s most popular sweet treat and staple, locals and visitors can enjoy beignets all year long, available 24-hours a day in New Orleans at more than one coffee hotspot.
Not another burnout article
Does this sound like your day?
You show up to work after a terrible night’s sleep. Your back is tense, and you do some kind of walking/stretching combo as you walk through the doors. Your focus fades during the mind-numbing routine of the morning shift sign out. As the day moves forward, you begin to feel resentful as you sign orders, see patients, and address your ICU team needs. You know that’s not right, that it’s not in line with who you want to be, but the irritation doesn’t go away.
Your lunchtime is filled with computer screens, notes, billing, and more billing. The previous feelings of irritation begin to boil into anger because more of your day is filled with bureaucratic demands and insurance reports rather than actually helping people. This isn’t what you signed up for. Years and years of training so you could be a paper pusher? The thought leads to rage ... or sometimes apathy on days you give in to the inevitable.
You finish your shift with admissions, procedures, code blues, and an overwhelming and exhausting night shift sign out. You feel like a hamster in a wheel. You’re going nowhere. What’s the point of all of this? You find yourself questioning why you went into medicine anyways ... yeah, that’s burnout.
I know what you’re thinking. You keep hearing about this, and it’s important to recognize, but then you hear the same old solutions: be more positive, find balance, do some yoga, take this resilience module, be mindful (what on earth does this mean anyways?), get some more sleep. Basically, it’s our problem. It’s our burden. If all of these were easy to understand and implement, don’t you think doctors and health-care providers would have done it already? I think you and I are a lot alike. These were my exact feelings. But stick with me on this one. I have a solution for you, albeit a little different. I’ll show you a more “positive” spin on the DIY.
I burned out early. After fellowship, I didn’t want to be a doctor anymore. I desperately sought to alter my career somehow. I looked into website development, something I had been good at in high school. I took a few refresher classes on my days off and started coding my own sites, but I had bills to pay. Big bills. Student loan bills. Luckily, my first job out of fellowship accepted many of my schedule demands, such as day shifts only, and after about a year, I recovered and remembered why I had loved medicine to begin with.
What is burnout?
Mind-body-soul exhaustion caused by excessive stress. Stress and burnout are closely related, but they’re more like distant cousins. Stress can be (and is) a normal part of our jobs. I bet you think you’re stressed, when you’re probably burned out. Critical care doctors have the highest rate of burnout among all physician subspecialties at >55%, and it is even higher in pediatric critical care. (Sessler C. https://www.mdedge.com/chestphysician/article/160951/society-news/turning-heat-icu-burnout). The main difference between stress and burnout is hope. With stress, you still feel like things can get better and you can get it all under control. Burnout feels hopeless.
What are the three core symptoms of burnout?
• Irritability and impatience with patients (depersonalization)
• Cynicism and difficulty concentrating (emotional exhaustion)
• What’s the point of all of this? Nothing I do matters or is appreciated (decreased self-efficacy)
We can talk about the symptoms of burnout all day, but what does that really look like? It looks like the day we described at the beginning. You know, the day that resonated with you and caused you to keep reading.
Why should we all be discussing this important topic?
Being burned out not only affects us on a soul level (achingly described above), but, more importantly, this can trickle down to our personal lives, family relationships, and how we care for our patients, with some studies showing that it affects our performance and, gulp, patient outcomes. That’s scary (Moss M et al. Crit Care Med. 2016;44[7]:1414).
Causes of burnout
There are many causes of burnout, and several studies have identified risk factors. A lack of control, conflicts with colleagues and leadership, and performing menial tasks can add to the irritation of a workday. This doesn’t even include the nature of our actual job as critical care doctors. We care for the sickest and are frequently involved in end-of-life care. Over time, the stress morphs into burnout. Female gender is also an independent risk factor for doctors (Pastores SM, et al. Crit Care Med. 2019;47[4]:550).
We’ve identified it. We’ve quantified it. But we’re not fixing it. In fact, there are only a few studies that have incorporated a needs assessment of doctors, paired with appropriate environmental intervention. A study done with primary care doctors in New York City clinics found that surveying a doctor’s “wish list” of interventions can help identify gaps in workflow, such as pairing one medical assistant with each attending (Linzer M, et al. J Gen Intern Med. 2015;30[8]:1105).
Without more data like this, we’re hamsters in a wheel. Luckily, organizations like CHEST have joined together with others to create the Critical Care Societies Collaborative and have an annual summit to discuss research strategies.
Solutions
Even millennials are sick of the mindful “chore” list. Yoga pants, yoga mats, crystals, chakras, meditation, and the list goes on and on. What millennials want are work-life integrations that are easy; workspaces that invite mindful behavior and daily rituals that excite and relax them. Co-working spaces like WeWork have designated self-care spaces.
Self-care is now essential, not an indulgence. I wasn’t sure how to create this space in my ICU, so I started small, with things I could carry with myself. The key is to find small rituals with big meanings. What could this look like for you? I began doing breathwork. Frankly, the idea came to me from my Apple® watch. It just started giving me these reminders one day, and I decided to take it seriously. I found that my mind and muscles eased after only 1 minute of breathing in and out slowly. This elevated my mood and was the refresher I needed in the afternoons. My body ached less after procedures.
I also got a little woo-woo (stay with me now) and began carrying around crystal stones. You don’t have to carry around crystals. Prayer books, religious symbols, your child’s toy car, anything can work if it has meaning for you, so when you see it or touch it during your day, you remember your big why. Why you’re serving people. Why you’re a doctor. I prefer the crystals over jewelry because it’s something unusual that I don’t expect to be sitting in my pocket. It’s always a nice gentle reminder of the love I have for my patients, my job, and humanity. When I put my hands in my pocket as I’m talking to yet another frustrated family member, my responses are more patient and calmer, which leads to a more productive conversation.
Lastly, I started what I call a new Pavlov home routine. When I’m done with work, I light a candle and write out three things I’m grateful for. Retrain your brain. Retrain your triggers. What’s your Pavlov’s bell going to be? Many of us come home hungry and stressed. Food then becomes linked to stress. This is not good. Link it with something else. Light a candle, count to 3, then blow it out. Use your kids to incorporate something fun. Use a toy with “super powers” to “beam” the bad feelings away. Taking a few extra minutes to shift gears has created a much happier home for me.
There are things that we can’t control. That’s called circumstances. We can’t control other people; we can’t control the hospital system; we can’t control our past. But the rest of everything we can control: our thoughts, feelings, and daily self-care rituals.
It reminds me of something my dad always said when I was a little girl. When crossing the street, you always look twice, oftentimes three. Why be so careful? It’s the pedestrian’s right of way after all. “Well..” he replied, “If a car hits you, nothing much happens to them, but your entire life will be destroyed, forever.”
Stop walking into traffic thinking everything will be ok. Take control of what you can.
Look, I get it. As health-care providers, we are an independent group. But just because you can do it alone, doesn’t mean you have to.
Choose one thing. Whether it be something I mentioned or something that came to your mind as you read this. Then, drop me a line at my personal email [email protected]. I will send you a reply to let you know I hear you and I’m in your corner.
Burnout happens.
But, so does joy, job satisfaction, and balance. Those things just take more effort.
Dr. Khan is Assistant Editor, Web and Multimedia, CHEST® journal.
Does this sound like your day?
You show up to work after a terrible night’s sleep. Your back is tense, and you do some kind of walking/stretching combo as you walk through the doors. Your focus fades during the mind-numbing routine of the morning shift sign out. As the day moves forward, you begin to feel resentful as you sign orders, see patients, and address your ICU team needs. You know that’s not right, that it’s not in line with who you want to be, but the irritation doesn’t go away.
Your lunchtime is filled with computer screens, notes, billing, and more billing. The previous feelings of irritation begin to boil into anger because more of your day is filled with bureaucratic demands and insurance reports rather than actually helping people. This isn’t what you signed up for. Years and years of training so you could be a paper pusher? The thought leads to rage ... or sometimes apathy on days you give in to the inevitable.
You finish your shift with admissions, procedures, code blues, and an overwhelming and exhausting night shift sign out. You feel like a hamster in a wheel. You’re going nowhere. What’s the point of all of this? You find yourself questioning why you went into medicine anyways ... yeah, that’s burnout.
I know what you’re thinking. You keep hearing about this, and it’s important to recognize, but then you hear the same old solutions: be more positive, find balance, do some yoga, take this resilience module, be mindful (what on earth does this mean anyways?), get some more sleep. Basically, it’s our problem. It’s our burden. If all of these were easy to understand and implement, don’t you think doctors and health-care providers would have done it already? I think you and I are a lot alike. These were my exact feelings. But stick with me on this one. I have a solution for you, albeit a little different. I’ll show you a more “positive” spin on the DIY.
I burned out early. After fellowship, I didn’t want to be a doctor anymore. I desperately sought to alter my career somehow. I looked into website development, something I had been good at in high school. I took a few refresher classes on my days off and started coding my own sites, but I had bills to pay. Big bills. Student loan bills. Luckily, my first job out of fellowship accepted many of my schedule demands, such as day shifts only, and after about a year, I recovered and remembered why I had loved medicine to begin with.
What is burnout?
Mind-body-soul exhaustion caused by excessive stress. Stress and burnout are closely related, but they’re more like distant cousins. Stress can be (and is) a normal part of our jobs. I bet you think you’re stressed, when you’re probably burned out. Critical care doctors have the highest rate of burnout among all physician subspecialties at >55%, and it is even higher in pediatric critical care. (Sessler C. https://www.mdedge.com/chestphysician/article/160951/society-news/turning-heat-icu-burnout). The main difference between stress and burnout is hope. With stress, you still feel like things can get better and you can get it all under control. Burnout feels hopeless.
What are the three core symptoms of burnout?
• Irritability and impatience with patients (depersonalization)
• Cynicism and difficulty concentrating (emotional exhaustion)
• What’s the point of all of this? Nothing I do matters or is appreciated (decreased self-efficacy)
We can talk about the symptoms of burnout all day, but what does that really look like? It looks like the day we described at the beginning. You know, the day that resonated with you and caused you to keep reading.
Why should we all be discussing this important topic?
Being burned out not only affects us on a soul level (achingly described above), but, more importantly, this can trickle down to our personal lives, family relationships, and how we care for our patients, with some studies showing that it affects our performance and, gulp, patient outcomes. That’s scary (Moss M et al. Crit Care Med. 2016;44[7]:1414).
Causes of burnout
There are many causes of burnout, and several studies have identified risk factors. A lack of control, conflicts with colleagues and leadership, and performing menial tasks can add to the irritation of a workday. This doesn’t even include the nature of our actual job as critical care doctors. We care for the sickest and are frequently involved in end-of-life care. Over time, the stress morphs into burnout. Female gender is also an independent risk factor for doctors (Pastores SM, et al. Crit Care Med. 2019;47[4]:550).
We’ve identified it. We’ve quantified it. But we’re not fixing it. In fact, there are only a few studies that have incorporated a needs assessment of doctors, paired with appropriate environmental intervention. A study done with primary care doctors in New York City clinics found that surveying a doctor’s “wish list” of interventions can help identify gaps in workflow, such as pairing one medical assistant with each attending (Linzer M, et al. J Gen Intern Med. 2015;30[8]:1105).
Without more data like this, we’re hamsters in a wheel. Luckily, organizations like CHEST have joined together with others to create the Critical Care Societies Collaborative and have an annual summit to discuss research strategies.
Solutions
Even millennials are sick of the mindful “chore” list. Yoga pants, yoga mats, crystals, chakras, meditation, and the list goes on and on. What millennials want are work-life integrations that are easy; workspaces that invite mindful behavior and daily rituals that excite and relax them. Co-working spaces like WeWork have designated self-care spaces.
Self-care is now essential, not an indulgence. I wasn’t sure how to create this space in my ICU, so I started small, with things I could carry with myself. The key is to find small rituals with big meanings. What could this look like for you? I began doing breathwork. Frankly, the idea came to me from my Apple® watch. It just started giving me these reminders one day, and I decided to take it seriously. I found that my mind and muscles eased after only 1 minute of breathing in and out slowly. This elevated my mood and was the refresher I needed in the afternoons. My body ached less after procedures.
I also got a little woo-woo (stay with me now) and began carrying around crystal stones. You don’t have to carry around crystals. Prayer books, religious symbols, your child’s toy car, anything can work if it has meaning for you, so when you see it or touch it during your day, you remember your big why. Why you’re serving people. Why you’re a doctor. I prefer the crystals over jewelry because it’s something unusual that I don’t expect to be sitting in my pocket. It’s always a nice gentle reminder of the love I have for my patients, my job, and humanity. When I put my hands in my pocket as I’m talking to yet another frustrated family member, my responses are more patient and calmer, which leads to a more productive conversation.
Lastly, I started what I call a new Pavlov home routine. When I’m done with work, I light a candle and write out three things I’m grateful for. Retrain your brain. Retrain your triggers. What’s your Pavlov’s bell going to be? Many of us come home hungry and stressed. Food then becomes linked to stress. This is not good. Link it with something else. Light a candle, count to 3, then blow it out. Use your kids to incorporate something fun. Use a toy with “super powers” to “beam” the bad feelings away. Taking a few extra minutes to shift gears has created a much happier home for me.
There are things that we can’t control. That’s called circumstances. We can’t control other people; we can’t control the hospital system; we can’t control our past. But the rest of everything we can control: our thoughts, feelings, and daily self-care rituals.
It reminds me of something my dad always said when I was a little girl. When crossing the street, you always look twice, oftentimes three. Why be so careful? It’s the pedestrian’s right of way after all. “Well..” he replied, “If a car hits you, nothing much happens to them, but your entire life will be destroyed, forever.”
Stop walking into traffic thinking everything will be ok. Take control of what you can.
Look, I get it. As health-care providers, we are an independent group. But just because you can do it alone, doesn’t mean you have to.
Choose one thing. Whether it be something I mentioned or something that came to your mind as you read this. Then, drop me a line at my personal email [email protected]. I will send you a reply to let you know I hear you and I’m in your corner.
Burnout happens.
But, so does joy, job satisfaction, and balance. Those things just take more effort.
Dr. Khan is Assistant Editor, Web and Multimedia, CHEST® journal.
Does this sound like your day?
You show up to work after a terrible night’s sleep. Your back is tense, and you do some kind of walking/stretching combo as you walk through the doors. Your focus fades during the mind-numbing routine of the morning shift sign out. As the day moves forward, you begin to feel resentful as you sign orders, see patients, and address your ICU team needs. You know that’s not right, that it’s not in line with who you want to be, but the irritation doesn’t go away.
Your lunchtime is filled with computer screens, notes, billing, and more billing. The previous feelings of irritation begin to boil into anger because more of your day is filled with bureaucratic demands and insurance reports rather than actually helping people. This isn’t what you signed up for. Years and years of training so you could be a paper pusher? The thought leads to rage ... or sometimes apathy on days you give in to the inevitable.
You finish your shift with admissions, procedures, code blues, and an overwhelming and exhausting night shift sign out. You feel like a hamster in a wheel. You’re going nowhere. What’s the point of all of this? You find yourself questioning why you went into medicine anyways ... yeah, that’s burnout.
I know what you’re thinking. You keep hearing about this, and it’s important to recognize, but then you hear the same old solutions: be more positive, find balance, do some yoga, take this resilience module, be mindful (what on earth does this mean anyways?), get some more sleep. Basically, it’s our problem. It’s our burden. If all of these were easy to understand and implement, don’t you think doctors and health-care providers would have done it already? I think you and I are a lot alike. These were my exact feelings. But stick with me on this one. I have a solution for you, albeit a little different. I’ll show you a more “positive” spin on the DIY.
I burned out early. After fellowship, I didn’t want to be a doctor anymore. I desperately sought to alter my career somehow. I looked into website development, something I had been good at in high school. I took a few refresher classes on my days off and started coding my own sites, but I had bills to pay. Big bills. Student loan bills. Luckily, my first job out of fellowship accepted many of my schedule demands, such as day shifts only, and after about a year, I recovered and remembered why I had loved medicine to begin with.
What is burnout?
Mind-body-soul exhaustion caused by excessive stress. Stress and burnout are closely related, but they’re more like distant cousins. Stress can be (and is) a normal part of our jobs. I bet you think you’re stressed, when you’re probably burned out. Critical care doctors have the highest rate of burnout among all physician subspecialties at >55%, and it is even higher in pediatric critical care. (Sessler C. https://www.mdedge.com/chestphysician/article/160951/society-news/turning-heat-icu-burnout). The main difference between stress and burnout is hope. With stress, you still feel like things can get better and you can get it all under control. Burnout feels hopeless.
What are the three core symptoms of burnout?
• Irritability and impatience with patients (depersonalization)
• Cynicism and difficulty concentrating (emotional exhaustion)
• What’s the point of all of this? Nothing I do matters or is appreciated (decreased self-efficacy)
We can talk about the symptoms of burnout all day, but what does that really look like? It looks like the day we described at the beginning. You know, the day that resonated with you and caused you to keep reading.
Why should we all be discussing this important topic?
Being burned out not only affects us on a soul level (achingly described above), but, more importantly, this can trickle down to our personal lives, family relationships, and how we care for our patients, with some studies showing that it affects our performance and, gulp, patient outcomes. That’s scary (Moss M et al. Crit Care Med. 2016;44[7]:1414).
Causes of burnout
There are many causes of burnout, and several studies have identified risk factors. A lack of control, conflicts with colleagues and leadership, and performing menial tasks can add to the irritation of a workday. This doesn’t even include the nature of our actual job as critical care doctors. We care for the sickest and are frequently involved in end-of-life care. Over time, the stress morphs into burnout. Female gender is also an independent risk factor for doctors (Pastores SM, et al. Crit Care Med. 2019;47[4]:550).
We’ve identified it. We’ve quantified it. But we’re not fixing it. In fact, there are only a few studies that have incorporated a needs assessment of doctors, paired with appropriate environmental intervention. A study done with primary care doctors in New York City clinics found that surveying a doctor’s “wish list” of interventions can help identify gaps in workflow, such as pairing one medical assistant with each attending (Linzer M, et al. J Gen Intern Med. 2015;30[8]:1105).
Without more data like this, we’re hamsters in a wheel. Luckily, organizations like CHEST have joined together with others to create the Critical Care Societies Collaborative and have an annual summit to discuss research strategies.
Solutions
Even millennials are sick of the mindful “chore” list. Yoga pants, yoga mats, crystals, chakras, meditation, and the list goes on and on. What millennials want are work-life integrations that are easy; workspaces that invite mindful behavior and daily rituals that excite and relax them. Co-working spaces like WeWork have designated self-care spaces.
Self-care is now essential, not an indulgence. I wasn’t sure how to create this space in my ICU, so I started small, with things I could carry with myself. The key is to find small rituals with big meanings. What could this look like for you? I began doing breathwork. Frankly, the idea came to me from my Apple® watch. It just started giving me these reminders one day, and I decided to take it seriously. I found that my mind and muscles eased after only 1 minute of breathing in and out slowly. This elevated my mood and was the refresher I needed in the afternoons. My body ached less after procedures.
I also got a little woo-woo (stay with me now) and began carrying around crystal stones. You don’t have to carry around crystals. Prayer books, religious symbols, your child’s toy car, anything can work if it has meaning for you, so when you see it or touch it during your day, you remember your big why. Why you’re serving people. Why you’re a doctor. I prefer the crystals over jewelry because it’s something unusual that I don’t expect to be sitting in my pocket. It’s always a nice gentle reminder of the love I have for my patients, my job, and humanity. When I put my hands in my pocket as I’m talking to yet another frustrated family member, my responses are more patient and calmer, which leads to a more productive conversation.
Lastly, I started what I call a new Pavlov home routine. When I’m done with work, I light a candle and write out three things I’m grateful for. Retrain your brain. Retrain your triggers. What’s your Pavlov’s bell going to be? Many of us come home hungry and stressed. Food then becomes linked to stress. This is not good. Link it with something else. Light a candle, count to 3, then blow it out. Use your kids to incorporate something fun. Use a toy with “super powers” to “beam” the bad feelings away. Taking a few extra minutes to shift gears has created a much happier home for me.
There are things that we can’t control. That’s called circumstances. We can’t control other people; we can’t control the hospital system; we can’t control our past. But the rest of everything we can control: our thoughts, feelings, and daily self-care rituals.
It reminds me of something my dad always said when I was a little girl. When crossing the street, you always look twice, oftentimes three. Why be so careful? It’s the pedestrian’s right of way after all. “Well..” he replied, “If a car hits you, nothing much happens to them, but your entire life will be destroyed, forever.”
Stop walking into traffic thinking everything will be ok. Take control of what you can.
Look, I get it. As health-care providers, we are an independent group. But just because you can do it alone, doesn’t mean you have to.
Choose one thing. Whether it be something I mentioned or something that came to your mind as you read this. Then, drop me a line at my personal email [email protected]. I will send you a reply to let you know I hear you and I’m in your corner.
Burnout happens.
But, so does joy, job satisfaction, and balance. Those things just take more effort.
Dr. Khan is Assistant Editor, Web and Multimedia, CHEST® journal.
Envisioning the future: The CHEST Environmental Scan
As a leader in education for pulmonary, critical care, and sleep medicine, staying ahead of trends in its professional fields and across educational delivery, in general, is critical to remaining relevant and to best serve the membership. The leadership of the American College of Chest Physicians (CHEST) developed a multifaceted program this year entitled, “CHEST Inspiration,” a series of programmatic initiatives aimed at stimulating and encouraging innovation within the association and recognizing individuals with great ideas that streamline current processes or disrupt ways of traditional thinking about everyday problems.
The CHEST Board of Regents recently completed one of the first components of the CHEST Inspiration program – the 2019 CHEST Environmental Scan. This article describes the development of the 2019 CHEST Environmental Scan and its fit with the other components of CHEST Inspiration program.
Environmental scanning is a formal process for tracking trends and occurrences in an organization’s internal and external environment that bear on its success--currently and in the future. The environmental scanning process examines both quantitative and qualitative factors and identifies a set of key environmental indicators believed to have the most important impact on the organization’s work.
The 2019 CHEST Environmental Scan is a synthesis of work that took place in January 2019 at the CHEST Environmental Summit, a special joint session of the Board of Regents (BOR) and the CHEST Foundation Board of Trustees (BOT). In that session attendees attempted to free themselves from the usual concentrated focus on the College and Foundation missions, goals, and strategies, recognizing that a possible (even likely) unintended consequence of a narrow focus is losing sight of the outside world and the forces there that—like it or not—influence and could even disrupt the programs and strategies of CHEST and the CHEST Foundation.
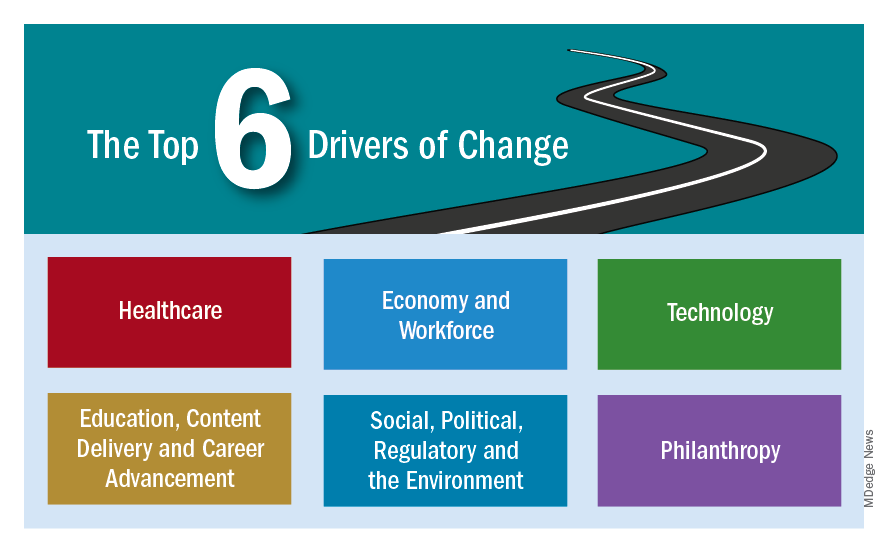
To facilitate the process, CHEST engaged a market research and consulting agency with expertise in environmental scans and a client base of nonprofit organizations and associations. The consultant conducted secondary research organized around six drivers of change selected by CHEST leadership:
• Health Care
• Economy and Workforce
• Technology
• Education, Content Delivery, and Career Advancement
• Social, Political, Regulatory, and the Environment
• Philanthropy
The leadership had the opportunity to review the consultant’s research findings prior to the Environmental Summit. Then, in the in-person BOT/BOR summit meeting, the consultant’s research findings were discussed and debated and were addressed with the following questions:
• How will this trend impact members? How will it change their work environment and what they need to know?
• How will this trend impact CHEST? What are the challenges and opportunities?
• What responses or actions should CHEST take?
• Does this insight require changes to our strategic plan?
The consultant synthesized the debates and discussions and prepared a draft document that shaped this year’s document.
The 2019 CHEST Environmental Scan, which will be undated periodically, will be used to:
• Inform members about external developments and put each in perspective
• Help leadership and staff determine future directions and program opportunities
• Keep the 5-year strategic plan fresh and relevant
The environmental scan will be published in six monthly installments in CHEST Physician, with each installment addressing one of the drivers of change. Most of the content is confirming rather than revolutionary in nature. Each installment will be accompanied comments from one of four leading physician experts who will put the content into perspective.
The two other components of the CHEST Inspiration program are to engage a group of experts from outside the field of medicine and health care who are innovative and successful in their own professions. This focus group of professionals from outside of our association will be held in conjunction with the June Board of Regents meeting. An additional component to stimulate innovative thinking and celebrate great ideas will be a new competitive event at the annual meeting. Dubbed “CHEST FISH Bowl (Furthering Innovation and Science for Health),” this event will launch this month, with contestants submitting video applications that feature their great idea, and winners in selected categories to be selected at CHEST 2019 in New Orleans. CHEST Physician will be your source for information about all the CHEST Inspiration programs through a new series of articles called “CHEST Inspiration: Pacing the Future.”
As a leader in education for pulmonary, critical care, and sleep medicine, staying ahead of trends in its professional fields and across educational delivery, in general, is critical to remaining relevant and to best serve the membership. The leadership of the American College of Chest Physicians (CHEST) developed a multifaceted program this year entitled, “CHEST Inspiration,” a series of programmatic initiatives aimed at stimulating and encouraging innovation within the association and recognizing individuals with great ideas that streamline current processes or disrupt ways of traditional thinking about everyday problems.

The CHEST Board of Regents recently completed one of the first components of the CHEST Inspiration program – the 2019 CHEST Environmental Scan. This article describes the development of the 2019 CHEST Environmental Scan and its fit with the other components of CHEST Inspiration program.
Environmental scanning is a formal process for tracking trends and occurrences in an organization’s internal and external environment that bear on its success--currently and in the future. The environmental scanning process examines both quantitative and qualitative factors and identifies a set of key environmental indicators believed to have the most important impact on the organization’s work.
The 2019 CHEST Environmental Scan is a synthesis of work that took place in January 2019 at the CHEST Environmental Summit, a special joint session of the Board of Regents (BOR) and the CHEST Foundation Board of Trustees (BOT). In that session attendees attempted to free themselves from the usual concentrated focus on the College and Foundation missions, goals, and strategies, recognizing that a possible (even likely) unintended consequence of a narrow focus is losing sight of the outside world and the forces there that—like it or not—influence and could even disrupt the programs and strategies of CHEST and the CHEST Foundation.
To facilitate the process, CHEST engaged a market research and consulting agency with expertise in environmental scans and a client base of nonprofit organizations and associations. The consultant conducted secondary research organized around six drivers of change selected by CHEST leadership:
• Health Care
• Economy and Workforce
• Technology
• Education, Content Delivery, and Career Advancement
• Social, Political, Regulatory, and the Environment
• Philanthropy
The leadership had the opportunity to review the consultant’s research findings prior to the Environmental Summit. Then, in the in-person BOT/BOR summit meeting, the consultant’s research findings were discussed and debated and were addressed with the following questions:
• How will this trend impact members? How will it change their work environment and what they need to know?
• How will this trend impact CHEST? What are the challenges and opportunities?
• What responses or actions should CHEST take?
• Does this insight require changes to our strategic plan?
The consultant synthesized the debates and discussions and prepared a draft document that shaped this year’s document.
The 2019 CHEST Environmental Scan, which will be undated periodically, will be used to:
• Inform members about external developments and put each in perspective
• Help leadership and staff determine future directions and program opportunities
• Keep the 5-year strategic plan fresh and relevant
The environmental scan will be published in six monthly installments in CHEST Physician, with each installment addressing one of the drivers of change. Most of the content is confirming rather than revolutionary in nature. Each installment will be accompanied comments from one of four leading physician experts who will put the content into perspective.
The two other components of the CHEST Inspiration program are to engage a group of experts from outside the field of medicine and health care who are innovative and successful in their own professions. This focus group of professionals from outside of our association will be held in conjunction with the June Board of Regents meeting. An additional component to stimulate innovative thinking and celebrate great ideas will be a new competitive event at the annual meeting. Dubbed “CHEST FISH Bowl (Furthering Innovation and Science for Health),” this event will launch this month, with contestants submitting video applications that feature their great idea, and winners in selected categories to be selected at CHEST 2019 in New Orleans. CHEST Physician will be your source for information about all the CHEST Inspiration programs through a new series of articles called “CHEST Inspiration: Pacing the Future.”
As a leader in education for pulmonary, critical care, and sleep medicine, staying ahead of trends in its professional fields and across educational delivery, in general, is critical to remaining relevant and to best serve the membership. The leadership of the American College of Chest Physicians (CHEST) developed a multifaceted program this year entitled, “CHEST Inspiration,” a series of programmatic initiatives aimed at stimulating and encouraging innovation within the association and recognizing individuals with great ideas that streamline current processes or disrupt ways of traditional thinking about everyday problems.

The CHEST Board of Regents recently completed one of the first components of the CHEST Inspiration program – the 2019 CHEST Environmental Scan. This article describes the development of the 2019 CHEST Environmental Scan and its fit with the other components of CHEST Inspiration program.
Environmental scanning is a formal process for tracking trends and occurrences in an organization’s internal and external environment that bear on its success--currently and in the future. The environmental scanning process examines both quantitative and qualitative factors and identifies a set of key environmental indicators believed to have the most important impact on the organization’s work.
The 2019 CHEST Environmental Scan is a synthesis of work that took place in January 2019 at the CHEST Environmental Summit, a special joint session of the Board of Regents (BOR) and the CHEST Foundation Board of Trustees (BOT). In that session attendees attempted to free themselves from the usual concentrated focus on the College and Foundation missions, goals, and strategies, recognizing that a possible (even likely) unintended consequence of a narrow focus is losing sight of the outside world and the forces there that—like it or not—influence and could even disrupt the programs and strategies of CHEST and the CHEST Foundation.
To facilitate the process, CHEST engaged a market research and consulting agency with expertise in environmental scans and a client base of nonprofit organizations and associations. The consultant conducted secondary research organized around six drivers of change selected by CHEST leadership:
• Health Care
• Economy and Workforce
• Technology
• Education, Content Delivery, and Career Advancement
• Social, Political, Regulatory, and the Environment
• Philanthropy
The leadership had the opportunity to review the consultant’s research findings prior to the Environmental Summit. Then, in the in-person BOT/BOR summit meeting, the consultant’s research findings were discussed and debated and were addressed with the following questions:
• How will this trend impact members? How will it change their work environment and what they need to know?
• How will this trend impact CHEST? What are the challenges and opportunities?
• What responses or actions should CHEST take?
• Does this insight require changes to our strategic plan?
The consultant synthesized the debates and discussions and prepared a draft document that shaped this year’s document.
The 2019 CHEST Environmental Scan, which will be undated periodically, will be used to:
• Inform members about external developments and put each in perspective
• Help leadership and staff determine future directions and program opportunities
• Keep the 5-year strategic plan fresh and relevant
The environmental scan will be published in six monthly installments in CHEST Physician, with each installment addressing one of the drivers of change. Most of the content is confirming rather than revolutionary in nature. Each installment will be accompanied comments from one of four leading physician experts who will put the content into perspective.
The two other components of the CHEST Inspiration program are to engage a group of experts from outside the field of medicine and health care who are innovative and successful in their own professions. This focus group of professionals from outside of our association will be held in conjunction with the June Board of Regents meeting. An additional component to stimulate innovative thinking and celebrate great ideas will be a new competitive event at the annual meeting. Dubbed “CHEST FISH Bowl (Furthering Innovation and Science for Health),” this event will launch this month, with contestants submitting video applications that feature their great idea, and winners in selected categories to be selected at CHEST 2019 in New Orleans. CHEST Physician will be your source for information about all the CHEST Inspiration programs through a new series of articles called “CHEST Inspiration: Pacing the Future.”
Antibody targeting ‘do not eat me’ signals is active in AML, MDS
CHICAGO – A novel antibody against CD47 – the “do not eat me” protein – is well tolerated and active in patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), according to initial results of a phase 1b study.
Combined with azacitidine, the antibody Hu5F9-G4 (5F9) produced an overall response rate of 64% in untreated AML (9 of 14 patients) and 91% in untreated MDS (10 of 11 patients), according to investigator David A. Sallman, MD, of Moffitt Cancer Center, Tampa, Fla.
With a median follow-up of 3.8 months, none of those patients had yet progressed on the 5F9/azacitidine combination, Dr. Sallman reported during a poster presentation at the annual meeting of the American Society of Clinical Oncology.
A maximum tolerated dose of 5F9 plus the hypomethylating agent was not reached in the study, according to the investigators.
“This was a well-tolerated and safe combination, with encouraging efficacy data in this small cohort that hasn’t been followed for too, too long,” Tara L. Lin, MD, of the University of Kansas Cancer Center, Kansas City, said during a poster discussion session.
“Most interesting is the fact that the combination seems to eliminate the leukemia stem cell population in those patients who respond,” she added.
The fact that 5F9 plus azacitidine eradicated leukemia stem cells in responding patients provides a mechanism for potential long-term durability of response, according to Dr. Sallman and his colleagues.
This first-in-class antibody targets CD47, a “do not eat me” macrophage checkpoint that is overexpressed on tumors, enabling immune invasion, they reported.
However, since CD47 is also expressed on older red blood cells, 5F9 is associated with transient anemia in the first cycle of treatment, Dr. Sallman told attendees at the poster discussion session.
“We do mitigate that with a priming dose of 5F9 that saturates these old red blood cells,” he said. “Over time, going along with the response, the patients have marked hemoglobin improvement, and we do not see worsening of other infection-related complications or cytopenias outside of anemia.”
Based on these results, expansion cohorts have been initiated in both AML and MDS, according to the investigators’ report.
When asked if 5F9 could be tolerable as part of more intensive regimens for fit patients, Dr. Sallman said there are a “whole host of combinations” that may possibly make sense.
“How chemotherapies and other novel agents impact these ‘eat me’ signals – I think some of that needs to be further investigated to come up with the most rational combination,” he said during a question and answer session.
Research funding for the study came from Forty Seven and the California Institute for Regenerative Medicine. Dr. Salman reported having no relationships to disclose. Study coauthors reported relationships with Abbvie, Agios, Celgene, Incyte, and Novartis, among other companies.
SOURCE: Sallman DA et al. ASCO 2019, Abstract 7009.
CHICAGO – A novel antibody against CD47 – the “do not eat me” protein – is well tolerated and active in patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), according to initial results of a phase 1b study.
Combined with azacitidine, the antibody Hu5F9-G4 (5F9) produced an overall response rate of 64% in untreated AML (9 of 14 patients) and 91% in untreated MDS (10 of 11 patients), according to investigator David A. Sallman, MD, of Moffitt Cancer Center, Tampa, Fla.
With a median follow-up of 3.8 months, none of those patients had yet progressed on the 5F9/azacitidine combination, Dr. Sallman reported during a poster presentation at the annual meeting of the American Society of Clinical Oncology.
A maximum tolerated dose of 5F9 plus the hypomethylating agent was not reached in the study, according to the investigators.
“This was a well-tolerated and safe combination, with encouraging efficacy data in this small cohort that hasn’t been followed for too, too long,” Tara L. Lin, MD, of the University of Kansas Cancer Center, Kansas City, said during a poster discussion session.
“Most interesting is the fact that the combination seems to eliminate the leukemia stem cell population in those patients who respond,” she added.
The fact that 5F9 plus azacitidine eradicated leukemia stem cells in responding patients provides a mechanism for potential long-term durability of response, according to Dr. Sallman and his colleagues.
This first-in-class antibody targets CD47, a “do not eat me” macrophage checkpoint that is overexpressed on tumors, enabling immune invasion, they reported.
However, since CD47 is also expressed on older red blood cells, 5F9 is associated with transient anemia in the first cycle of treatment, Dr. Sallman told attendees at the poster discussion session.
“We do mitigate that with a priming dose of 5F9 that saturates these old red blood cells,” he said. “Over time, going along with the response, the patients have marked hemoglobin improvement, and we do not see worsening of other infection-related complications or cytopenias outside of anemia.”
Based on these results, expansion cohorts have been initiated in both AML and MDS, according to the investigators’ report.
When asked if 5F9 could be tolerable as part of more intensive regimens for fit patients, Dr. Sallman said there are a “whole host of combinations” that may possibly make sense.
“How chemotherapies and other novel agents impact these ‘eat me’ signals – I think some of that needs to be further investigated to come up with the most rational combination,” he said during a question and answer session.
Research funding for the study came from Forty Seven and the California Institute for Regenerative Medicine. Dr. Salman reported having no relationships to disclose. Study coauthors reported relationships with Abbvie, Agios, Celgene, Incyte, and Novartis, among other companies.
SOURCE: Sallman DA et al. ASCO 2019, Abstract 7009.
CHICAGO – A novel antibody against CD47 – the “do not eat me” protein – is well tolerated and active in patients with acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), according to initial results of a phase 1b study.
Combined with azacitidine, the antibody Hu5F9-G4 (5F9) produced an overall response rate of 64% in untreated AML (9 of 14 patients) and 91% in untreated MDS (10 of 11 patients), according to investigator David A. Sallman, MD, of Moffitt Cancer Center, Tampa, Fla.
With a median follow-up of 3.8 months, none of those patients had yet progressed on the 5F9/azacitidine combination, Dr. Sallman reported during a poster presentation at the annual meeting of the American Society of Clinical Oncology.
A maximum tolerated dose of 5F9 plus the hypomethylating agent was not reached in the study, according to the investigators.
“This was a well-tolerated and safe combination, with encouraging efficacy data in this small cohort that hasn’t been followed for too, too long,” Tara L. Lin, MD, of the University of Kansas Cancer Center, Kansas City, said during a poster discussion session.
“Most interesting is the fact that the combination seems to eliminate the leukemia stem cell population in those patients who respond,” she added.
The fact that 5F9 plus azacitidine eradicated leukemia stem cells in responding patients provides a mechanism for potential long-term durability of response, according to Dr. Sallman and his colleagues.
This first-in-class antibody targets CD47, a “do not eat me” macrophage checkpoint that is overexpressed on tumors, enabling immune invasion, they reported.
However, since CD47 is also expressed on older red blood cells, 5F9 is associated with transient anemia in the first cycle of treatment, Dr. Sallman told attendees at the poster discussion session.
“We do mitigate that with a priming dose of 5F9 that saturates these old red blood cells,” he said. “Over time, going along with the response, the patients have marked hemoglobin improvement, and we do not see worsening of other infection-related complications or cytopenias outside of anemia.”
Based on these results, expansion cohorts have been initiated in both AML and MDS, according to the investigators’ report.
When asked if 5F9 could be tolerable as part of more intensive regimens for fit patients, Dr. Sallman said there are a “whole host of combinations” that may possibly make sense.
“How chemotherapies and other novel agents impact these ‘eat me’ signals – I think some of that needs to be further investigated to come up with the most rational combination,” he said during a question and answer session.
Research funding for the study came from Forty Seven and the California Institute for Regenerative Medicine. Dr. Salman reported having no relationships to disclose. Study coauthors reported relationships with Abbvie, Agios, Celgene, Incyte, and Novartis, among other companies.
SOURCE: Sallman DA et al. ASCO 2019, Abstract 7009.
REPORTING FROM ASCO 2019
Preprints come to medicine: medRxiv launches with safeguards
A new preprint server for the medical and health sciences – medRxiv – has launched, along with safeguards designed to mitigate the risk of non–peer-reviewed findings prematurely guiding clinical practice or misinforming the public.
The new repository of preprints is intended for researcher-to-researcher communication – and mainly to facilitate faster sharing of research findings before publication in peer-reviewed journals. Papers will not be scrutinized for study design or the strength of the science, but they will be screened by an external clinical scientist and – at least for now – by an editor funded by BMJ, the London-based publisher and one of the three cofounding organizations of medRxiv (pronounced “med archive”).
The server’s six-person leadership team – comprising leaders from BMJ and cofounders, Cold Spring Harbor Laboratory in New York, and Yale University in New Haven, Conn. – will make final decisions about whether to post papers that generate concerns.
“We’ve put in place more stringent screening procedures than existed for bioRxiv, [a biological preprint server launched in 2013 by Cold Spring Harbor Laboratory],”said Theodora Bloom, PhD, executive editor of BMJ. “We’ll specifically ask the question, is there a risk to public health or health-related behaviors if this preprint is posted and [turns out to be] wrong?”
Concerns that poor information will be disseminated to the public or that the public will misinterpret information published, were heard by the medRxiv founders as they “work-shopped the idea and talked with the community,” said Joseph Ross, MD, an associate professor of medicine and public health at Yale and codirector of the Yale Open Data Access (YODA) Project
“We’re taking a cautious approach, particularly in the early days as we learn from the process,” he said. “How a paper [could potentially influence clinical practice] will be a guiding question.”
The cofounders had several conversations, Dr. Ross said, with Howard Bauchner, MD, editor in chief of JAMA, who took a strong stance against preprints and shortcutting the peer review process in a 2017 editorial titled “The rush to publication: An editorial and scientific mistake.” (Dr. Ross is an associate editor at JAMA Internal Medicine. Dr. Bauchner was unavailable for comment on the safeguards built into medRvix.)
Aaron D. Viny, MD, a hematologist-oncologist at Memorial Sloan Kettering in New York, said he has mixed feelings about preprints and believes the stakes are higher with medRvix, given that it will house clinical content – including, he anticipates, single-institution, nonprospective outcome studies of off-label drug uses. “These aren’t bona fide clinical trials and may not have the best data,” he said.
Still, there are advantages for investigators – and for the progress of research – with earlier dissemination of findings, Dr. Viny said. He recently had a paper posted on bioRvix for the first time. The paper was undergoing revision for a peer-reviewed journal and was being presented at a national meeting at the time it was posted.
“We timed it [as such], so that not only were we presenting it at a national meeting, but it also got more Twitter buzz,” he said. “I thought it was a good body of work, and I was excited to discuss it online with the scientific community.”
Dr. Viny’s decision is common among preprint authors and reflects the values of the preprint server, Dr. Ross said. “When people are reading or hearing about [new findings] at a meeting, they can go to the papers to get more complete information.” And, he said, the investigators themselves can get more feedback than they otherwise would.
In addition to papers that are well on their way to publication in peer-reviewed journals, Dr. Ross anticipates that medRvix will house papers on qualitative studies and observational research that face more arduous publication paths. He said he expects to see research on medical education and hopes to see papers on “quality improvement work, which typically involve small interventions at a single institution, and have important insights but are hard to publish because of generalizability and controls.”
And while there has been a “positive shift” in the past 10 years in the publication of negative results in peer-reviewed literature, medRvix may well capture studies that have negative results “because they have challenges with recruitment or other [elements of study design],” Dr. Ross said. “There is still a lot that can be learned by the scientific community from these negative studies, but they’re very difficult to publish in a peer-reviewed journal.”
Road to preprints
BMJ has a history with preprints. The publisher established a preprint server for biomedical research in the late 1990s, but it never took off and was shut down in the early 2000s. “It just didn’t get the uptake,” said Dr. Bloom. “It’s hard to know exactly why.”
What is clear, she said, is what has changed in the past 20 years: Copious use of the Internet overall, a growing desire to stake out one’s research turf online, requests from funders to have preprints listed on grant applications, and disease outbreaks involving the Zika virus and Ebola that have highlighted the advantages of faster dissemination of research findings.
BMJ had begun discussions with John Inglis, PhD, of Cold Spring Harbor Laboratory about launching a preprint server for the medical sciences (building on the experience of bioRxiv) when they heard Harlan Krumholz, MD, professor of medicine at Yale and head of the YODA project, speak at the 2017 meeting of the International Congress on Peer Review & Scientific Publication. In his keynote address, Dr. Krumholz described Yale’s plans to launch a preprint server.
“We all felt it would be better working together than apart,” Dr. Bloom said.
Getting published
Each preprint on medRxiv will get a permanent DOI link and a disclaimer stating that preprints are not peer reviewed, should not be relied on to guide clinical practice, and should not be reported in the news media as established information.
Authors will be required to meet various standards and requirements common in the clinical and medical sciences, such as including details on ethics approvals, patient consent, funding sources and conflicts of interest, and trial registration numbers. They will have the option of adding a revision(s) of their preprint (each preprint will have a “history”), as well as the option of having their preprint marked as “withdrawn” if they can no longer stand by the findings or conclusions. Preprints will automatically be linked to final published papers.
Journals have wrestled with how to handle preprints. A look at several major peer-reviewed journals shows that they’ll consider articles that have appeared in early form as preprints (including the New England Journal of Medicine, according to media relations manager Jennifer Zeis), but there are caveats. JAMA, for instance, will look at whether submitted manuscripts add “meaningfully new” information above what the preprint disseminated.
Similarly, the American Society of Clinical Oncology (ASCO) will consider how preprints affect the “novelty” of the manuscript’s findings for its ASCO journal readers. Editors of the journal Blood will consider “public comments or coverage about [the] preprint” in its evaluation of the manuscript’s impact. Several of the major journals specify that preprints cannot be updated while manuscripts are under review.
A recent review of bioRxiv preprints shows that approximately two-thirds went on to peer-reviewed publication.
And according to the BMJ’s Dr. Bloom, “there is definitely evidence that preprints [overall] are getting cited [in the scientific literature] before peer-reviewed articles appear.”
The server medRvix began accepting manuscripts on June 6 and will go live on June 25. It will accept only research papers – not commentaries or case reports, Dr. Ross emphasized.
For now, Dr. Bloom said, the most immediate and “real question for us is, will clinical researchers embrace preprints? And if they do, can we continue to provide a light touch but rapid way to screen papers while ensuring the safety of what we’re posting?”
For his part, Dr. Viny is bracing for “public consumption” of medRxiv content, especially in the oncology community in which patients are often extraordinarily well educated about their disease and determined to learn about all possible treatment options. “My job as a clinician,” he said, “will be to contextualize the patient’s reference information.”
A new preprint server for the medical and health sciences – medRxiv – has launched, along with safeguards designed to mitigate the risk of non–peer-reviewed findings prematurely guiding clinical practice or misinforming the public.
The new repository of preprints is intended for researcher-to-researcher communication – and mainly to facilitate faster sharing of research findings before publication in peer-reviewed journals. Papers will not be scrutinized for study design or the strength of the science, but they will be screened by an external clinical scientist and – at least for now – by an editor funded by BMJ, the London-based publisher and one of the three cofounding organizations of medRxiv (pronounced “med archive”).
The server’s six-person leadership team – comprising leaders from BMJ and cofounders, Cold Spring Harbor Laboratory in New York, and Yale University in New Haven, Conn. – will make final decisions about whether to post papers that generate concerns.
“We’ve put in place more stringent screening procedures than existed for bioRxiv, [a biological preprint server launched in 2013 by Cold Spring Harbor Laboratory],”said Theodora Bloom, PhD, executive editor of BMJ. “We’ll specifically ask the question, is there a risk to public health or health-related behaviors if this preprint is posted and [turns out to be] wrong?”
Concerns that poor information will be disseminated to the public or that the public will misinterpret information published, were heard by the medRxiv founders as they “work-shopped the idea and talked with the community,” said Joseph Ross, MD, an associate professor of medicine and public health at Yale and codirector of the Yale Open Data Access (YODA) Project
“We’re taking a cautious approach, particularly in the early days as we learn from the process,” he said. “How a paper [could potentially influence clinical practice] will be a guiding question.”
The cofounders had several conversations, Dr. Ross said, with Howard Bauchner, MD, editor in chief of JAMA, who took a strong stance against preprints and shortcutting the peer review process in a 2017 editorial titled “The rush to publication: An editorial and scientific mistake.” (Dr. Ross is an associate editor at JAMA Internal Medicine. Dr. Bauchner was unavailable for comment on the safeguards built into medRvix.)
Aaron D. Viny, MD, a hematologist-oncologist at Memorial Sloan Kettering in New York, said he has mixed feelings about preprints and believes the stakes are higher with medRvix, given that it will house clinical content – including, he anticipates, single-institution, nonprospective outcome studies of off-label drug uses. “These aren’t bona fide clinical trials and may not have the best data,” he said.
Still, there are advantages for investigators – and for the progress of research – with earlier dissemination of findings, Dr. Viny said. He recently had a paper posted on bioRvix for the first time. The paper was undergoing revision for a peer-reviewed journal and was being presented at a national meeting at the time it was posted.
“We timed it [as such], so that not only were we presenting it at a national meeting, but it also got more Twitter buzz,” he said. “I thought it was a good body of work, and I was excited to discuss it online with the scientific community.”
Dr. Viny’s decision is common among preprint authors and reflects the values of the preprint server, Dr. Ross said. “When people are reading or hearing about [new findings] at a meeting, they can go to the papers to get more complete information.” And, he said, the investigators themselves can get more feedback than they otherwise would.
In addition to papers that are well on their way to publication in peer-reviewed journals, Dr. Ross anticipates that medRvix will house papers on qualitative studies and observational research that face more arduous publication paths. He said he expects to see research on medical education and hopes to see papers on “quality improvement work, which typically involve small interventions at a single institution, and have important insights but are hard to publish because of generalizability and controls.”
And while there has been a “positive shift” in the past 10 years in the publication of negative results in peer-reviewed literature, medRvix may well capture studies that have negative results “because they have challenges with recruitment or other [elements of study design],” Dr. Ross said. “There is still a lot that can be learned by the scientific community from these negative studies, but they’re very difficult to publish in a peer-reviewed journal.”
Road to preprints
BMJ has a history with preprints. The publisher established a preprint server for biomedical research in the late 1990s, but it never took off and was shut down in the early 2000s. “It just didn’t get the uptake,” said Dr. Bloom. “It’s hard to know exactly why.”
What is clear, she said, is what has changed in the past 20 years: Copious use of the Internet overall, a growing desire to stake out one’s research turf online, requests from funders to have preprints listed on grant applications, and disease outbreaks involving the Zika virus and Ebola that have highlighted the advantages of faster dissemination of research findings.
BMJ had begun discussions with John Inglis, PhD, of Cold Spring Harbor Laboratory about launching a preprint server for the medical sciences (building on the experience of bioRxiv) when they heard Harlan Krumholz, MD, professor of medicine at Yale and head of the YODA project, speak at the 2017 meeting of the International Congress on Peer Review & Scientific Publication. In his keynote address, Dr. Krumholz described Yale’s plans to launch a preprint server.
“We all felt it would be better working together than apart,” Dr. Bloom said.
Getting published
Each preprint on medRxiv will get a permanent DOI link and a disclaimer stating that preprints are not peer reviewed, should not be relied on to guide clinical practice, and should not be reported in the news media as established information.
Authors will be required to meet various standards and requirements common in the clinical and medical sciences, such as including details on ethics approvals, patient consent, funding sources and conflicts of interest, and trial registration numbers. They will have the option of adding a revision(s) of their preprint (each preprint will have a “history”), as well as the option of having their preprint marked as “withdrawn” if they can no longer stand by the findings or conclusions. Preprints will automatically be linked to final published papers.
Journals have wrestled with how to handle preprints. A look at several major peer-reviewed journals shows that they’ll consider articles that have appeared in early form as preprints (including the New England Journal of Medicine, according to media relations manager Jennifer Zeis), but there are caveats. JAMA, for instance, will look at whether submitted manuscripts add “meaningfully new” information above what the preprint disseminated.
Similarly, the American Society of Clinical Oncology (ASCO) will consider how preprints affect the “novelty” of the manuscript’s findings for its ASCO journal readers. Editors of the journal Blood will consider “public comments or coverage about [the] preprint” in its evaluation of the manuscript’s impact. Several of the major journals specify that preprints cannot be updated while manuscripts are under review.
A recent review of bioRxiv preprints shows that approximately two-thirds went on to peer-reviewed publication.
And according to the BMJ’s Dr. Bloom, “there is definitely evidence that preprints [overall] are getting cited [in the scientific literature] before peer-reviewed articles appear.”
The server medRvix began accepting manuscripts on June 6 and will go live on June 25. It will accept only research papers – not commentaries or case reports, Dr. Ross emphasized.
For now, Dr. Bloom said, the most immediate and “real question for us is, will clinical researchers embrace preprints? And if they do, can we continue to provide a light touch but rapid way to screen papers while ensuring the safety of what we’re posting?”
For his part, Dr. Viny is bracing for “public consumption” of medRxiv content, especially in the oncology community in which patients are often extraordinarily well educated about their disease and determined to learn about all possible treatment options. “My job as a clinician,” he said, “will be to contextualize the patient’s reference information.”
A new preprint server for the medical and health sciences – medRxiv – has launched, along with safeguards designed to mitigate the risk of non–peer-reviewed findings prematurely guiding clinical practice or misinforming the public.
The new repository of preprints is intended for researcher-to-researcher communication – and mainly to facilitate faster sharing of research findings before publication in peer-reviewed journals. Papers will not be scrutinized for study design or the strength of the science, but they will be screened by an external clinical scientist and – at least for now – by an editor funded by BMJ, the London-based publisher and one of the three cofounding organizations of medRxiv (pronounced “med archive”).
The server’s six-person leadership team – comprising leaders from BMJ and cofounders, Cold Spring Harbor Laboratory in New York, and Yale University in New Haven, Conn. – will make final decisions about whether to post papers that generate concerns.
“We’ve put in place more stringent screening procedures than existed for bioRxiv, [a biological preprint server launched in 2013 by Cold Spring Harbor Laboratory],”said Theodora Bloom, PhD, executive editor of BMJ. “We’ll specifically ask the question, is there a risk to public health or health-related behaviors if this preprint is posted and [turns out to be] wrong?”
Concerns that poor information will be disseminated to the public or that the public will misinterpret information published, were heard by the medRxiv founders as they “work-shopped the idea and talked with the community,” said Joseph Ross, MD, an associate professor of medicine and public health at Yale and codirector of the Yale Open Data Access (YODA) Project
“We’re taking a cautious approach, particularly in the early days as we learn from the process,” he said. “How a paper [could potentially influence clinical practice] will be a guiding question.”
The cofounders had several conversations, Dr. Ross said, with Howard Bauchner, MD, editor in chief of JAMA, who took a strong stance against preprints and shortcutting the peer review process in a 2017 editorial titled “The rush to publication: An editorial and scientific mistake.” (Dr. Ross is an associate editor at JAMA Internal Medicine. Dr. Bauchner was unavailable for comment on the safeguards built into medRvix.)
Aaron D. Viny, MD, a hematologist-oncologist at Memorial Sloan Kettering in New York, said he has mixed feelings about preprints and believes the stakes are higher with medRvix, given that it will house clinical content – including, he anticipates, single-institution, nonprospective outcome studies of off-label drug uses. “These aren’t bona fide clinical trials and may not have the best data,” he said.
Still, there are advantages for investigators – and for the progress of research – with earlier dissemination of findings, Dr. Viny said. He recently had a paper posted on bioRvix for the first time. The paper was undergoing revision for a peer-reviewed journal and was being presented at a national meeting at the time it was posted.
“We timed it [as such], so that not only were we presenting it at a national meeting, but it also got more Twitter buzz,” he said. “I thought it was a good body of work, and I was excited to discuss it online with the scientific community.”
Dr. Viny’s decision is common among preprint authors and reflects the values of the preprint server, Dr. Ross said. “When people are reading or hearing about [new findings] at a meeting, they can go to the papers to get more complete information.” And, he said, the investigators themselves can get more feedback than they otherwise would.
In addition to papers that are well on their way to publication in peer-reviewed journals, Dr. Ross anticipates that medRvix will house papers on qualitative studies and observational research that face more arduous publication paths. He said he expects to see research on medical education and hopes to see papers on “quality improvement work, which typically involve small interventions at a single institution, and have important insights but are hard to publish because of generalizability and controls.”
And while there has been a “positive shift” in the past 10 years in the publication of negative results in peer-reviewed literature, medRvix may well capture studies that have negative results “because they have challenges with recruitment or other [elements of study design],” Dr. Ross said. “There is still a lot that can be learned by the scientific community from these negative studies, but they’re very difficult to publish in a peer-reviewed journal.”
Road to preprints
BMJ has a history with preprints. The publisher established a preprint server for biomedical research in the late 1990s, but it never took off and was shut down in the early 2000s. “It just didn’t get the uptake,” said Dr. Bloom. “It’s hard to know exactly why.”
What is clear, she said, is what has changed in the past 20 years: Copious use of the Internet overall, a growing desire to stake out one’s research turf online, requests from funders to have preprints listed on grant applications, and disease outbreaks involving the Zika virus and Ebola that have highlighted the advantages of faster dissemination of research findings.
BMJ had begun discussions with John Inglis, PhD, of Cold Spring Harbor Laboratory about launching a preprint server for the medical sciences (building on the experience of bioRxiv) when they heard Harlan Krumholz, MD, professor of medicine at Yale and head of the YODA project, speak at the 2017 meeting of the International Congress on Peer Review & Scientific Publication. In his keynote address, Dr. Krumholz described Yale’s plans to launch a preprint server.
“We all felt it would be better working together than apart,” Dr. Bloom said.
Getting published
Each preprint on medRxiv will get a permanent DOI link and a disclaimer stating that preprints are not peer reviewed, should not be relied on to guide clinical practice, and should not be reported in the news media as established information.
Authors will be required to meet various standards and requirements common in the clinical and medical sciences, such as including details on ethics approvals, patient consent, funding sources and conflicts of interest, and trial registration numbers. They will have the option of adding a revision(s) of their preprint (each preprint will have a “history”), as well as the option of having their preprint marked as “withdrawn” if they can no longer stand by the findings or conclusions. Preprints will automatically be linked to final published papers.
Journals have wrestled with how to handle preprints. A look at several major peer-reviewed journals shows that they’ll consider articles that have appeared in early form as preprints (including the New England Journal of Medicine, according to media relations manager Jennifer Zeis), but there are caveats. JAMA, for instance, will look at whether submitted manuscripts add “meaningfully new” information above what the preprint disseminated.
Similarly, the American Society of Clinical Oncology (ASCO) will consider how preprints affect the “novelty” of the manuscript’s findings for its ASCO journal readers. Editors of the journal Blood will consider “public comments or coverage about [the] preprint” in its evaluation of the manuscript’s impact. Several of the major journals specify that preprints cannot be updated while manuscripts are under review.
A recent review of bioRxiv preprints shows that approximately two-thirds went on to peer-reviewed publication.
And according to the BMJ’s Dr. Bloom, “there is definitely evidence that preprints [overall] are getting cited [in the scientific literature] before peer-reviewed articles appear.”
The server medRvix began accepting manuscripts on June 6 and will go live on June 25. It will accept only research papers – not commentaries or case reports, Dr. Ross emphasized.
For now, Dr. Bloom said, the most immediate and “real question for us is, will clinical researchers embrace preprints? And if they do, can we continue to provide a light touch but rapid way to screen papers while ensuring the safety of what we’re posting?”
For his part, Dr. Viny is bracing for “public consumption” of medRxiv content, especially in the oncology community in which patients are often extraordinarily well educated about their disease and determined to learn about all possible treatment options. “My job as a clinician,” he said, “will be to contextualize the patient’s reference information.”
Pregnancy deemed safe in BRCA-mutated breast cancer survivors
CHICAGO – Pregnancy after breast cancer is safe in BRCA-mutated patients, according to a retrospective study.
Pregnancy did not affect disease-free or overall survival in a cohort of BRCA-mutated breast cancer patients. Additionally, fetal and pregnancy complications in this cohort were similar to complications observed in the general population.
“We believe that our findings provide reassurance for counseling young BRCA-mutated breast cancer patients inquiring about the feasibility and safety of future conception,” said Matteo Lambertini, MD, PhD, of Policlinico San Martino Hospital in Genova, Italy.
Dr. Lambertini presented the findings at the annual meeting of the American Society of Clinical Oncology.
He and his colleagues conducted an international, multicenter, retrospective cohort study of 1,252 patients. The patients had been diagnosed with stage I-III breast cancer between January 2000 and December 2012 at age 40 years or younger. All patients had BRCA mutations – 811 with BRCA1 alone, 430 with BRCA2 alone, and 11 with both.
Pregnant versus nonpregnant patients
At a median of 4.5 years after diagnosis, 195 patients (16%) had experienced a pregnancy.
Compared with the nonpregnant women, pregnant patients were younger (P less than .001), more likely to have a BRCA1 mutation (P = .01), have smaller tumors (P = .04), have node-negative disease (P = .003), and have hormone receptor–negative tumors (P = .002). Roughly 95% of patients in both cohorts had received chemotherapy, and the most common regimens were anthracycline or taxane based.
Compared with patients in the nonpregnancy cohort, those in the pregnancy cohort were less likely to receive tamoxifen alone as endocrine therapy (P = .002), were more likely to have a shorter duration of endocrine therapy (P less than .001), and were less likely to undergo salpingo-oophorectomy (P less than .001).
Pregnancy outcomes
“In terms of pregnancy, fetal, and obstetrical outcomes, no alarming signals were observed,” Dr. Lambertini said.
Most pregnant patients had a spontaneous pregnancy (82.1%), completed the pregnancy (76.9%), delivered at term (90.8%), and had no complications (86.6%). However, 10.3% of patients had a spontaneous abortion, 9.2% of pregnancies were pre term, and 1.8% of babies had congenital abnormalities.
“All these rates were highly comparable to rates that are expected in the general healthy population,” Dr. Lambertini said.
Survival analyses
The researchers performed two survival analyses. The first was a case-control approach in which they matched each pregnant patient with three controls (patients without pregnancy) according to the following:
- Disease-free interval from breast cancer diagnosis (equal to or longer than that of pregnant patients).
- Year at diagnosis (plus or minus 2.5 years).
- Nodal status (negative vs. positive).
- Hormone receptor status (positive vs. negative).
- Type of BRCA mutation (BRCA1 vs. BRCA2).
The second survival analysis was an extended Cox model with pregnancy as a time-varying covariate.
Survival outcomes
At a median follow-up of 8.3 years, pregnant patients had better disease-free survival than nonpregnant patients in the case-control analysis, with a hazard ratio of 0.71 (P = .045). With the extended Cox model, the adjusted HR was 0.87 (P = .41). The analysis was adjusted for age, tumor size, nodal status, type of endocrine therapy, hormone receptor status, breast surgery, and BRCA mutation.
There was a significant interaction between type of BRCA mutation and pregnancy, with better disease-free survival observed in the BRCA1-mutated cohort. The HR was 0.53 in the BRCA1 cohort and 1.60 in the BRCA2 cohort (P less than .01). However, as Dr. Lambertini pointed out, only 44 pregnant patients had a BRCA1 mutation.
There was no significant interaction between hormone receptor status and pregnancy (P = .28).
Furthermore, there was no significant difference in overall survival between the pregnant and nonpregnant cohorts. In the case-control analysis, the HR was 0.86 (P = .65). In the extended Cox model, the adjusted HR was 0.88 (P = .66).
Dr. Lambertini disclosed a relationship with Teva.
SOURCE: Lambertini M et al. ASCO 2019, Abstract 11506.
CHICAGO – Pregnancy after breast cancer is safe in BRCA-mutated patients, according to a retrospective study.
Pregnancy did not affect disease-free or overall survival in a cohort of BRCA-mutated breast cancer patients. Additionally, fetal and pregnancy complications in this cohort were similar to complications observed in the general population.
“We believe that our findings provide reassurance for counseling young BRCA-mutated breast cancer patients inquiring about the feasibility and safety of future conception,” said Matteo Lambertini, MD, PhD, of Policlinico San Martino Hospital in Genova, Italy.
Dr. Lambertini presented the findings at the annual meeting of the American Society of Clinical Oncology.
He and his colleagues conducted an international, multicenter, retrospective cohort study of 1,252 patients. The patients had been diagnosed with stage I-III breast cancer between January 2000 and December 2012 at age 40 years or younger. All patients had BRCA mutations – 811 with BRCA1 alone, 430 with BRCA2 alone, and 11 with both.
Pregnant versus nonpregnant patients
At a median of 4.5 years after diagnosis, 195 patients (16%) had experienced a pregnancy.
Compared with the nonpregnant women, pregnant patients were younger (P less than .001), more likely to have a BRCA1 mutation (P = .01), have smaller tumors (P = .04), have node-negative disease (P = .003), and have hormone receptor–negative tumors (P = .002). Roughly 95% of patients in both cohorts had received chemotherapy, and the most common regimens were anthracycline or taxane based.
Compared with patients in the nonpregnancy cohort, those in the pregnancy cohort were less likely to receive tamoxifen alone as endocrine therapy (P = .002), were more likely to have a shorter duration of endocrine therapy (P less than .001), and were less likely to undergo salpingo-oophorectomy (P less than .001).
Pregnancy outcomes
“In terms of pregnancy, fetal, and obstetrical outcomes, no alarming signals were observed,” Dr. Lambertini said.
Most pregnant patients had a spontaneous pregnancy (82.1%), completed the pregnancy (76.9%), delivered at term (90.8%), and had no complications (86.6%). However, 10.3% of patients had a spontaneous abortion, 9.2% of pregnancies were pre term, and 1.8% of babies had congenital abnormalities.
“All these rates were highly comparable to rates that are expected in the general healthy population,” Dr. Lambertini said.
Survival analyses
The researchers performed two survival analyses. The first was a case-control approach in which they matched each pregnant patient with three controls (patients without pregnancy) according to the following:
- Disease-free interval from breast cancer diagnosis (equal to or longer than that of pregnant patients).
- Year at diagnosis (plus or minus 2.5 years).
- Nodal status (negative vs. positive).
- Hormone receptor status (positive vs. negative).
- Type of BRCA mutation (BRCA1 vs. BRCA2).
The second survival analysis was an extended Cox model with pregnancy as a time-varying covariate.
Survival outcomes
At a median follow-up of 8.3 years, pregnant patients had better disease-free survival than nonpregnant patients in the case-control analysis, with a hazard ratio of 0.71 (P = .045). With the extended Cox model, the adjusted HR was 0.87 (P = .41). The analysis was adjusted for age, tumor size, nodal status, type of endocrine therapy, hormone receptor status, breast surgery, and BRCA mutation.
There was a significant interaction between type of BRCA mutation and pregnancy, with better disease-free survival observed in the BRCA1-mutated cohort. The HR was 0.53 in the BRCA1 cohort and 1.60 in the BRCA2 cohort (P less than .01). However, as Dr. Lambertini pointed out, only 44 pregnant patients had a BRCA1 mutation.
There was no significant interaction between hormone receptor status and pregnancy (P = .28).
Furthermore, there was no significant difference in overall survival between the pregnant and nonpregnant cohorts. In the case-control analysis, the HR was 0.86 (P = .65). In the extended Cox model, the adjusted HR was 0.88 (P = .66).
Dr. Lambertini disclosed a relationship with Teva.
SOURCE: Lambertini M et al. ASCO 2019, Abstract 11506.
CHICAGO – Pregnancy after breast cancer is safe in BRCA-mutated patients, according to a retrospective study.
Pregnancy did not affect disease-free or overall survival in a cohort of BRCA-mutated breast cancer patients. Additionally, fetal and pregnancy complications in this cohort were similar to complications observed in the general population.
“We believe that our findings provide reassurance for counseling young BRCA-mutated breast cancer patients inquiring about the feasibility and safety of future conception,” said Matteo Lambertini, MD, PhD, of Policlinico San Martino Hospital in Genova, Italy.
Dr. Lambertini presented the findings at the annual meeting of the American Society of Clinical Oncology.
He and his colleagues conducted an international, multicenter, retrospective cohort study of 1,252 patients. The patients had been diagnosed with stage I-III breast cancer between January 2000 and December 2012 at age 40 years or younger. All patients had BRCA mutations – 811 with BRCA1 alone, 430 with BRCA2 alone, and 11 with both.
Pregnant versus nonpregnant patients
At a median of 4.5 years after diagnosis, 195 patients (16%) had experienced a pregnancy.
Compared with the nonpregnant women, pregnant patients were younger (P less than .001), more likely to have a BRCA1 mutation (P = .01), have smaller tumors (P = .04), have node-negative disease (P = .003), and have hormone receptor–negative tumors (P = .002). Roughly 95% of patients in both cohorts had received chemotherapy, and the most common regimens were anthracycline or taxane based.
Compared with patients in the nonpregnancy cohort, those in the pregnancy cohort were less likely to receive tamoxifen alone as endocrine therapy (P = .002), were more likely to have a shorter duration of endocrine therapy (P less than .001), and were less likely to undergo salpingo-oophorectomy (P less than .001).
Pregnancy outcomes
“In terms of pregnancy, fetal, and obstetrical outcomes, no alarming signals were observed,” Dr. Lambertini said.
Most pregnant patients had a spontaneous pregnancy (82.1%), completed the pregnancy (76.9%), delivered at term (90.8%), and had no complications (86.6%). However, 10.3% of patients had a spontaneous abortion, 9.2% of pregnancies were pre term, and 1.8% of babies had congenital abnormalities.
“All these rates were highly comparable to rates that are expected in the general healthy population,” Dr. Lambertini said.
Survival analyses
The researchers performed two survival analyses. The first was a case-control approach in which they matched each pregnant patient with three controls (patients without pregnancy) according to the following:
- Disease-free interval from breast cancer diagnosis (equal to or longer than that of pregnant patients).
- Year at diagnosis (plus or minus 2.5 years).
- Nodal status (negative vs. positive).
- Hormone receptor status (positive vs. negative).
- Type of BRCA mutation (BRCA1 vs. BRCA2).
The second survival analysis was an extended Cox model with pregnancy as a time-varying covariate.
Survival outcomes
At a median follow-up of 8.3 years, pregnant patients had better disease-free survival than nonpregnant patients in the case-control analysis, with a hazard ratio of 0.71 (P = .045). With the extended Cox model, the adjusted HR was 0.87 (P = .41). The analysis was adjusted for age, tumor size, nodal status, type of endocrine therapy, hormone receptor status, breast surgery, and BRCA mutation.
There was a significant interaction between type of BRCA mutation and pregnancy, with better disease-free survival observed in the BRCA1-mutated cohort. The HR was 0.53 in the BRCA1 cohort and 1.60 in the BRCA2 cohort (P less than .01). However, as Dr. Lambertini pointed out, only 44 pregnant patients had a BRCA1 mutation.
There was no significant interaction between hormone receptor status and pregnancy (P = .28).
Furthermore, there was no significant difference in overall survival between the pregnant and nonpregnant cohorts. In the case-control analysis, the HR was 0.86 (P = .65). In the extended Cox model, the adjusted HR was 0.88 (P = .66).
Dr. Lambertini disclosed a relationship with Teva.
SOURCE: Lambertini M et al. ASCO 2019, Abstract 11506.
REPORTING FROM ASCO 2019
Adjuvant immunotherapy results ‘encouraging’ in early NSCLC
CHICAGO – Neoadjuvant monotherapy with the immune checkpoint inhibitor atezolizumab is associated with “encouraging” responses with no new safety signals for patients with non–small cell lung cancer (NSCLC), an interim analysis of a multicenter phase 2 trial suggests.
Among 77 of a planned 180 patients with resectable NSCLC enrolled in the LCMC3 (Lung Cancer Mutation Consortium 3) trial, the pathological complete response (pCR) rate following two cycles of neoadjuvant atezolizumab (Tecentriq) and surgery was 5%, and the major pathological response (MPR) rate was 19%, reported David J. Kwiatkowski, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“Pathological regression moderately correlated with target lesions’ measurements by RECIST [Response Evaluation Criteria in Solid Tumors] and MPR was observed irrespective of PD-L1 expression, although there was some correlation,” he said at the annual meeting of the American Society of Clinical Oncology.
The study was designed to test whether preoperative immunotherapy with an immune checkpoint inhibitor could have additional clinical benefits for patients with early-stage NSCLC.
Investigators are enrolling patients with stage IB, II, IIIA, or selected IIIB resectable, previously untreated NSCLC. Patients receive 1,200 mg atezolizumab on days 1 and 22 (two cycles), followed by surgery on or about day 40.
The primary endpoint, MPR, “means that at the time of surgical resection, all of the samples of the tumor that are cut into sections are reviewed by a pathologist, and an aggregate score of a percent of viable tumor cells is determined based on a comparison of viable tumor cells and necrotic tumor cells and stroma,” Dr. Kwiatkowski said.
The threshold for MPR was 10% or fewer viable tumor cells at the time of resection.
Following surgery, patients received standard-of-care adjuvant chemotherapy and could receive optional continued atezolizumab for an additional 12 months.
At the time of this interim analysis, with a data cutoff of Sept. 5, 2018, 101 patients had been enrolled and were included in the interim safety analysis. Of this group, 11 did not undergo surgery, because of progressive disease, withdrawal of consent, failed echocardiogram (1 patient), or pulmonary artery involvement (1) patient.
Of the 10 patients with either progressive disease and no surgery or unresectable disease at surgery, 8 had stage IIIA tumors and 2 had stage IIIB tumors. All patients with stage I or II disease underwent resection.
Dr. Kwiatkowski presented interim data on 90 patients intended for surgery, of whom 84 had assessment of the primary endpoint, including 7 positive for EGFR and/or ALK, and 77 whose tumors were either EGFR/ALK negative or had unknown status. These 77 patients were the primary efficacy population.
As noted before, among the 77 in the primary efficacy population, 15 (19%) had a MPR, and 4 patients (5%) had a pCR. In addition, 38 patients (49%) had pathological regression of tumor of 50% or greater. Pathological regression correlated significantly with change in tumor lesion size (P less than .001).
Tumor mutational burden, however, was not significantly correlated with MPR or pathological regression.
Among the 101 patients in the safety population, there were two deaths deemed not related to study treatment: one cardiac death post surgical resection, and one from disease progression. Treatment-related adverse events occurred in 57% of patients, including 6% that were grade 3 or greater. Adverse events leading to treatment withdrawal occurred in 5% of patients.
The efficacy interim analysis passed the prespecified futility boundary, and investigators are continuing to enroll patients.
Invited discussant Maximilian Diehn, MD, PhD, of Stanford (Calif.) University commented that neoadjuvant immunotherapy for NSCLC is promising, but added that the MPR endpoint still needs validation.
“Currently, it is not considered a validated surrogate endpoint for survival and therefore is not currently used for drug approvals. Secondly, the optimal cut point may differ by histology, such as being different for adenocarcinoma and squamous cell carcinoma. And this has potential implications for using this in trials that enroll patients of both histologies. And, third, there are some emerging data that MPR may need to measured somewhat differently after immunotherapy than after chemotherapy,” he said.
The study is supported by Genentech. Dr. Kwiatkowski disclosed research funding and a consulting or advisory role for the company. Dr. Diehn reported stock ownership, consulting, research funding, and travel expenses from various companies.
SOURCE: Kwiatkowski DJ et al. ASCO 2019, Abstract 8503.
CHICAGO – Neoadjuvant monotherapy with the immune checkpoint inhibitor atezolizumab is associated with “encouraging” responses with no new safety signals for patients with non–small cell lung cancer (NSCLC), an interim analysis of a multicenter phase 2 trial suggests.
Among 77 of a planned 180 patients with resectable NSCLC enrolled in the LCMC3 (Lung Cancer Mutation Consortium 3) trial, the pathological complete response (pCR) rate following two cycles of neoadjuvant atezolizumab (Tecentriq) and surgery was 5%, and the major pathological response (MPR) rate was 19%, reported David J. Kwiatkowski, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“Pathological regression moderately correlated with target lesions’ measurements by RECIST [Response Evaluation Criteria in Solid Tumors] and MPR was observed irrespective of PD-L1 expression, although there was some correlation,” he said at the annual meeting of the American Society of Clinical Oncology.
The study was designed to test whether preoperative immunotherapy with an immune checkpoint inhibitor could have additional clinical benefits for patients with early-stage NSCLC.
Investigators are enrolling patients with stage IB, II, IIIA, or selected IIIB resectable, previously untreated NSCLC. Patients receive 1,200 mg atezolizumab on days 1 and 22 (two cycles), followed by surgery on or about day 40.
The primary endpoint, MPR, “means that at the time of surgical resection, all of the samples of the tumor that are cut into sections are reviewed by a pathologist, and an aggregate score of a percent of viable tumor cells is determined based on a comparison of viable tumor cells and necrotic tumor cells and stroma,” Dr. Kwiatkowski said.
The threshold for MPR was 10% or fewer viable tumor cells at the time of resection.
Following surgery, patients received standard-of-care adjuvant chemotherapy and could receive optional continued atezolizumab for an additional 12 months.
At the time of this interim analysis, with a data cutoff of Sept. 5, 2018, 101 patients had been enrolled and were included in the interim safety analysis. Of this group, 11 did not undergo surgery, because of progressive disease, withdrawal of consent, failed echocardiogram (1 patient), or pulmonary artery involvement (1) patient.
Of the 10 patients with either progressive disease and no surgery or unresectable disease at surgery, 8 had stage IIIA tumors and 2 had stage IIIB tumors. All patients with stage I or II disease underwent resection.
Dr. Kwiatkowski presented interim data on 90 patients intended for surgery, of whom 84 had assessment of the primary endpoint, including 7 positive for EGFR and/or ALK, and 77 whose tumors were either EGFR/ALK negative or had unknown status. These 77 patients were the primary efficacy population.
As noted before, among the 77 in the primary efficacy population, 15 (19%) had a MPR, and 4 patients (5%) had a pCR. In addition, 38 patients (49%) had pathological regression of tumor of 50% or greater. Pathological regression correlated significantly with change in tumor lesion size (P less than .001).
Tumor mutational burden, however, was not significantly correlated with MPR or pathological regression.
Among the 101 patients in the safety population, there were two deaths deemed not related to study treatment: one cardiac death post surgical resection, and one from disease progression. Treatment-related adverse events occurred in 57% of patients, including 6% that were grade 3 or greater. Adverse events leading to treatment withdrawal occurred in 5% of patients.
The efficacy interim analysis passed the prespecified futility boundary, and investigators are continuing to enroll patients.
Invited discussant Maximilian Diehn, MD, PhD, of Stanford (Calif.) University commented that neoadjuvant immunotherapy for NSCLC is promising, but added that the MPR endpoint still needs validation.
“Currently, it is not considered a validated surrogate endpoint for survival and therefore is not currently used for drug approvals. Secondly, the optimal cut point may differ by histology, such as being different for adenocarcinoma and squamous cell carcinoma. And this has potential implications for using this in trials that enroll patients of both histologies. And, third, there are some emerging data that MPR may need to measured somewhat differently after immunotherapy than after chemotherapy,” he said.
The study is supported by Genentech. Dr. Kwiatkowski disclosed research funding and a consulting or advisory role for the company. Dr. Diehn reported stock ownership, consulting, research funding, and travel expenses from various companies.
SOURCE: Kwiatkowski DJ et al. ASCO 2019, Abstract 8503.
CHICAGO – Neoadjuvant monotherapy with the immune checkpoint inhibitor atezolizumab is associated with “encouraging” responses with no new safety signals for patients with non–small cell lung cancer (NSCLC), an interim analysis of a multicenter phase 2 trial suggests.
Among 77 of a planned 180 patients with resectable NSCLC enrolled in the LCMC3 (Lung Cancer Mutation Consortium 3) trial, the pathological complete response (pCR) rate following two cycles of neoadjuvant atezolizumab (Tecentriq) and surgery was 5%, and the major pathological response (MPR) rate was 19%, reported David J. Kwiatkowski, MD, PhD, of the Dana-Farber Cancer Institute in Boston.
“Pathological regression moderately correlated with target lesions’ measurements by RECIST [Response Evaluation Criteria in Solid Tumors] and MPR was observed irrespective of PD-L1 expression, although there was some correlation,” he said at the annual meeting of the American Society of Clinical Oncology.
The study was designed to test whether preoperative immunotherapy with an immune checkpoint inhibitor could have additional clinical benefits for patients with early-stage NSCLC.
Investigators are enrolling patients with stage IB, II, IIIA, or selected IIIB resectable, previously untreated NSCLC. Patients receive 1,200 mg atezolizumab on days 1 and 22 (two cycles), followed by surgery on or about day 40.
The primary endpoint, MPR, “means that at the time of surgical resection, all of the samples of the tumor that are cut into sections are reviewed by a pathologist, and an aggregate score of a percent of viable tumor cells is determined based on a comparison of viable tumor cells and necrotic tumor cells and stroma,” Dr. Kwiatkowski said.
The threshold for MPR was 10% or fewer viable tumor cells at the time of resection.
Following surgery, patients received standard-of-care adjuvant chemotherapy and could receive optional continued atezolizumab for an additional 12 months.
At the time of this interim analysis, with a data cutoff of Sept. 5, 2018, 101 patients had been enrolled and were included in the interim safety analysis. Of this group, 11 did not undergo surgery, because of progressive disease, withdrawal of consent, failed echocardiogram (1 patient), or pulmonary artery involvement (1) patient.
Of the 10 patients with either progressive disease and no surgery or unresectable disease at surgery, 8 had stage IIIA tumors and 2 had stage IIIB tumors. All patients with stage I or II disease underwent resection.
Dr. Kwiatkowski presented interim data on 90 patients intended for surgery, of whom 84 had assessment of the primary endpoint, including 7 positive for EGFR and/or ALK, and 77 whose tumors were either EGFR/ALK negative or had unknown status. These 77 patients were the primary efficacy population.
As noted before, among the 77 in the primary efficacy population, 15 (19%) had a MPR, and 4 patients (5%) had a pCR. In addition, 38 patients (49%) had pathological regression of tumor of 50% or greater. Pathological regression correlated significantly with change in tumor lesion size (P less than .001).
Tumor mutational burden, however, was not significantly correlated with MPR or pathological regression.
Among the 101 patients in the safety population, there were two deaths deemed not related to study treatment: one cardiac death post surgical resection, and one from disease progression. Treatment-related adverse events occurred in 57% of patients, including 6% that were grade 3 or greater. Adverse events leading to treatment withdrawal occurred in 5% of patients.
The efficacy interim analysis passed the prespecified futility boundary, and investigators are continuing to enroll patients.
Invited discussant Maximilian Diehn, MD, PhD, of Stanford (Calif.) University commented that neoadjuvant immunotherapy for NSCLC is promising, but added that the MPR endpoint still needs validation.
“Currently, it is not considered a validated surrogate endpoint for survival and therefore is not currently used for drug approvals. Secondly, the optimal cut point may differ by histology, such as being different for adenocarcinoma and squamous cell carcinoma. And this has potential implications for using this in trials that enroll patients of both histologies. And, third, there are some emerging data that MPR may need to measured somewhat differently after immunotherapy than after chemotherapy,” he said.
The study is supported by Genentech. Dr. Kwiatkowski disclosed research funding and a consulting or advisory role for the company. Dr. Diehn reported stock ownership, consulting, research funding, and travel expenses from various companies.
SOURCE: Kwiatkowski DJ et al. ASCO 2019, Abstract 8503.
REPORTING FROM ASCO 2019
Past donor pregnancy, sex do not affect transfusion-related mortality
Red blood cell (RBC) transfusions from either previously pregnant, sex-discordant, or female donors were not significantly associated with higher mortality among transfusion recipients, according to a retrospective analysis of more than 1 million donors.
“This study used data from 3 large cohorts in the United States and Scandinavia to investigate whether blood donor sex and pregnancy history were associated with mortality of transfusion recipients,” wrote Gustaf Edgren, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues. The findings were published in JAMA.
The researchers analyzed data from three separate cohorts that included a combined 1,047,382 red blood cell transfusion recipients. Data collected included donor-related information, such as sex and pregnancy history, as well as survival data of transfusion recipients. The primary outcome measured was in-hospital mortality, and the secondary outcome was long-term mortality. Data were collected until Dec. 31, 2016.
The researchers found no statistically significant associations between either sex-discordant donors (male donor to female recipient or female donor to male recipient), female donors, or previously pregnant donors and in-hospital mortality of transfusion recipients.
The hazard ratio estimates for each unit transfused from a previously pregnant donor ranged from 1.00-1.01 in the three cohorts. Similarly, the HR estimates ranged from 0.99-1.00 for female donors in the three cohorts and 0.99-1.02 for sex discordant donors.
The only significant association found was observed in the smallest cohort of 34,662 recipients. Researchers found an increased risk of death in patients who received one to two sex discordant transfusions (HR, 1.08; 95% confidence interval, 1.03-1.14) or five to six transfusions (HR, 1.14; 95%CI, 1.01-1.29), compared with recipients who received no sex-discordant transfusions.
“The results are reassuring in that the survival of patients who got transfused with red blood cells does not appear to be associated with whether the blood they received was donated by a man, by a woman who had been pregnant — or by one who had not. That’s important to know,” Simone Glynn, MD, chief of the Blood Epidemiology and Clinical Therapeutics Branch at the National Heart, Lung, and Blood Institute, as well as a study author, said in a statement.
The study was funded by the National Heart, Lung, and Blood Institute. The authors reported financial disclosures related to the National Institutes of Health, RTI International, Cerus, AABB, Creative Testing Solutions, and the Nordic Cancer Union.
SOURCE: Edgren G et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.7084.
Red blood cell (RBC) transfusions from either previously pregnant, sex-discordant, or female donors were not significantly associated with higher mortality among transfusion recipients, according to a retrospective analysis of more than 1 million donors.
“This study used data from 3 large cohorts in the United States and Scandinavia to investigate whether blood donor sex and pregnancy history were associated with mortality of transfusion recipients,” wrote Gustaf Edgren, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues. The findings were published in JAMA.
The researchers analyzed data from three separate cohorts that included a combined 1,047,382 red blood cell transfusion recipients. Data collected included donor-related information, such as sex and pregnancy history, as well as survival data of transfusion recipients. The primary outcome measured was in-hospital mortality, and the secondary outcome was long-term mortality. Data were collected until Dec. 31, 2016.
The researchers found no statistically significant associations between either sex-discordant donors (male donor to female recipient or female donor to male recipient), female donors, or previously pregnant donors and in-hospital mortality of transfusion recipients.
The hazard ratio estimates for each unit transfused from a previously pregnant donor ranged from 1.00-1.01 in the three cohorts. Similarly, the HR estimates ranged from 0.99-1.00 for female donors in the three cohorts and 0.99-1.02 for sex discordant donors.
The only significant association found was observed in the smallest cohort of 34,662 recipients. Researchers found an increased risk of death in patients who received one to two sex discordant transfusions (HR, 1.08; 95% confidence interval, 1.03-1.14) or five to six transfusions (HR, 1.14; 95%CI, 1.01-1.29), compared with recipients who received no sex-discordant transfusions.
“The results are reassuring in that the survival of patients who got transfused with red blood cells does not appear to be associated with whether the blood they received was donated by a man, by a woman who had been pregnant — or by one who had not. That’s important to know,” Simone Glynn, MD, chief of the Blood Epidemiology and Clinical Therapeutics Branch at the National Heart, Lung, and Blood Institute, as well as a study author, said in a statement.
The study was funded by the National Heart, Lung, and Blood Institute. The authors reported financial disclosures related to the National Institutes of Health, RTI International, Cerus, AABB, Creative Testing Solutions, and the Nordic Cancer Union.
SOURCE: Edgren G et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.7084.
Red blood cell (RBC) transfusions from either previously pregnant, sex-discordant, or female donors were not significantly associated with higher mortality among transfusion recipients, according to a retrospective analysis of more than 1 million donors.
“This study used data from 3 large cohorts in the United States and Scandinavia to investigate whether blood donor sex and pregnancy history were associated with mortality of transfusion recipients,” wrote Gustaf Edgren, MD, PhD, of Karolinska University Hospital, Stockholm, and colleagues. The findings were published in JAMA.
The researchers analyzed data from three separate cohorts that included a combined 1,047,382 red blood cell transfusion recipients. Data collected included donor-related information, such as sex and pregnancy history, as well as survival data of transfusion recipients. The primary outcome measured was in-hospital mortality, and the secondary outcome was long-term mortality. Data were collected until Dec. 31, 2016.
The researchers found no statistically significant associations between either sex-discordant donors (male donor to female recipient or female donor to male recipient), female donors, or previously pregnant donors and in-hospital mortality of transfusion recipients.
The hazard ratio estimates for each unit transfused from a previously pregnant donor ranged from 1.00-1.01 in the three cohorts. Similarly, the HR estimates ranged from 0.99-1.00 for female donors in the three cohorts and 0.99-1.02 for sex discordant donors.
The only significant association found was observed in the smallest cohort of 34,662 recipients. Researchers found an increased risk of death in patients who received one to two sex discordant transfusions (HR, 1.08; 95% confidence interval, 1.03-1.14) or five to six transfusions (HR, 1.14; 95%CI, 1.01-1.29), compared with recipients who received no sex-discordant transfusions.
“The results are reassuring in that the survival of patients who got transfused with red blood cells does not appear to be associated with whether the blood they received was donated by a man, by a woman who had been pregnant — or by one who had not. That’s important to know,” Simone Glynn, MD, chief of the Blood Epidemiology and Clinical Therapeutics Branch at the National Heart, Lung, and Blood Institute, as well as a study author, said in a statement.
The study was funded by the National Heart, Lung, and Blood Institute. The authors reported financial disclosures related to the National Institutes of Health, RTI International, Cerus, AABB, Creative Testing Solutions, and the Nordic Cancer Union.
SOURCE: Edgren G et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.7084.
FROM JAMA













