User login
Younger men and women show similar rates of osteopenia
according to findings from a cross-sectional study.
The high prevalence of osteopenia – once viewed as restricted largely to older women – in the study’s younger, cross-sex population should spur physicians to ask all patients about calcium intake and exercise as well as to screen for osteoporosis in all patients, Martha A. Bass, PhD,`wrote in the Journal of the American Osteopathic Association.
“It is important that early detection of the precursors for osteoporosis become part of the annual physical for people in this age range, as well as in older patients,” noted Dr Bass of the University of Mississippi School of Applied Sciences in Oxford, and coauthors. “Primary care physicians should begin educating patients as early as adolescence or young adulthood so the consequences of osteoporosis can be prevented. The result would be the prevention of future bone fractures and the morbidity and mortality associated with bone fractures, thus leading to improved quality of life.”
The researchers set out to examine the likelihood of low bone mineral density (BMD) and related risk factors in 173 adults aged 35-50 years. All of the participants completed a questionnaire assessing calcium intake, weekly exercise, smoking, and body mass index, and all underwent screening for BMD. The study’s primary outcome was BMD at the femoral neck, trochanter, intertrochanteric crest, total femur, and lumbar spine.
Among the 81 men in the sample, 25 (30%) had a normal body mass index, and the remainder were either overweight (47.5%) or obese (22.5%). One of the women was underweight, 48.9% were normal weight, 28.3% were overweight, and 21.7% were obese.
Most of the sample, regardless of gender, reported consuming fewer than three dairy items per day. Exercise frequency was better, with 68% of men and 56.4% of women saying they exercised at least 20 times per month.
There were no total femur osteoporosis findings in either sex. However, osteopenia at the femoral neck was present in 28.4% of the men and 26.1% of the women. Osteopenia at the lumbar spine occurred in 21% of men and 15.2% of women, with 6.2% of men and 2.2% of women showing osteoporosis at this site.
An adjusted analysis determined that exercise correlated significantly and negatively with femoral neck BMD in men. But in women, there was a significant and positive correlation with BMD at the lumbar spine and at all femoral measurements.
Body mass index also played into the risk picture. Among men, almost all BMD measurements (trochanter, intertrochanteric crest, total femur, and lumbar spine) were positively associated with higher BMI. For women, higher BMI was associated with better BMD at the all the femoral sites, but not at the lumbar spine.
The negative correlation between femoral neck BMD and exercise in men seemed to contradict findings from previous studies. The authors said that could be a result of reporting bias, with men overestimating their amount of exercise, and could suggest that higher BMI confers some protection against bone loss in men.
The study found no significant correlations between dairy intake and BMD at any site in either sex. The finding suggests that both sexes need to improve both vitamin D and calcium intake.
None of the authors reported any financial disclosures.
SOURCE: Bass MA et al. J Am Osteopath Assoc. 2019;119(6):357-63.
according to findings from a cross-sectional study.
The high prevalence of osteopenia – once viewed as restricted largely to older women – in the study’s younger, cross-sex population should spur physicians to ask all patients about calcium intake and exercise as well as to screen for osteoporosis in all patients, Martha A. Bass, PhD,`wrote in the Journal of the American Osteopathic Association.
“It is important that early detection of the precursors for osteoporosis become part of the annual physical for people in this age range, as well as in older patients,” noted Dr Bass of the University of Mississippi School of Applied Sciences in Oxford, and coauthors. “Primary care physicians should begin educating patients as early as adolescence or young adulthood so the consequences of osteoporosis can be prevented. The result would be the prevention of future bone fractures and the morbidity and mortality associated with bone fractures, thus leading to improved quality of life.”
The researchers set out to examine the likelihood of low bone mineral density (BMD) and related risk factors in 173 adults aged 35-50 years. All of the participants completed a questionnaire assessing calcium intake, weekly exercise, smoking, and body mass index, and all underwent screening for BMD. The study’s primary outcome was BMD at the femoral neck, trochanter, intertrochanteric crest, total femur, and lumbar spine.
Among the 81 men in the sample, 25 (30%) had a normal body mass index, and the remainder were either overweight (47.5%) or obese (22.5%). One of the women was underweight, 48.9% were normal weight, 28.3% were overweight, and 21.7% were obese.
Most of the sample, regardless of gender, reported consuming fewer than three dairy items per day. Exercise frequency was better, with 68% of men and 56.4% of women saying they exercised at least 20 times per month.
There were no total femur osteoporosis findings in either sex. However, osteopenia at the femoral neck was present in 28.4% of the men and 26.1% of the women. Osteopenia at the lumbar spine occurred in 21% of men and 15.2% of women, with 6.2% of men and 2.2% of women showing osteoporosis at this site.
An adjusted analysis determined that exercise correlated significantly and negatively with femoral neck BMD in men. But in women, there was a significant and positive correlation with BMD at the lumbar spine and at all femoral measurements.
Body mass index also played into the risk picture. Among men, almost all BMD measurements (trochanter, intertrochanteric crest, total femur, and lumbar spine) were positively associated with higher BMI. For women, higher BMI was associated with better BMD at the all the femoral sites, but not at the lumbar spine.
The negative correlation between femoral neck BMD and exercise in men seemed to contradict findings from previous studies. The authors said that could be a result of reporting bias, with men overestimating their amount of exercise, and could suggest that higher BMI confers some protection against bone loss in men.
The study found no significant correlations between dairy intake and BMD at any site in either sex. The finding suggests that both sexes need to improve both vitamin D and calcium intake.
None of the authors reported any financial disclosures.
SOURCE: Bass MA et al. J Am Osteopath Assoc. 2019;119(6):357-63.
according to findings from a cross-sectional study.
The high prevalence of osteopenia – once viewed as restricted largely to older women – in the study’s younger, cross-sex population should spur physicians to ask all patients about calcium intake and exercise as well as to screen for osteoporosis in all patients, Martha A. Bass, PhD,`wrote in the Journal of the American Osteopathic Association.
“It is important that early detection of the precursors for osteoporosis become part of the annual physical for people in this age range, as well as in older patients,” noted Dr Bass of the University of Mississippi School of Applied Sciences in Oxford, and coauthors. “Primary care physicians should begin educating patients as early as adolescence or young adulthood so the consequences of osteoporosis can be prevented. The result would be the prevention of future bone fractures and the morbidity and mortality associated with bone fractures, thus leading to improved quality of life.”
The researchers set out to examine the likelihood of low bone mineral density (BMD) and related risk factors in 173 adults aged 35-50 years. All of the participants completed a questionnaire assessing calcium intake, weekly exercise, smoking, and body mass index, and all underwent screening for BMD. The study’s primary outcome was BMD at the femoral neck, trochanter, intertrochanteric crest, total femur, and lumbar spine.
Among the 81 men in the sample, 25 (30%) had a normal body mass index, and the remainder were either overweight (47.5%) or obese (22.5%). One of the women was underweight, 48.9% were normal weight, 28.3% were overweight, and 21.7% were obese.
Most of the sample, regardless of gender, reported consuming fewer than three dairy items per day. Exercise frequency was better, with 68% of men and 56.4% of women saying they exercised at least 20 times per month.
There were no total femur osteoporosis findings in either sex. However, osteopenia at the femoral neck was present in 28.4% of the men and 26.1% of the women. Osteopenia at the lumbar spine occurred in 21% of men and 15.2% of women, with 6.2% of men and 2.2% of women showing osteoporosis at this site.
An adjusted analysis determined that exercise correlated significantly and negatively with femoral neck BMD in men. But in women, there was a significant and positive correlation with BMD at the lumbar spine and at all femoral measurements.
Body mass index also played into the risk picture. Among men, almost all BMD measurements (trochanter, intertrochanteric crest, total femur, and lumbar spine) were positively associated with higher BMI. For women, higher BMI was associated with better BMD at the all the femoral sites, but not at the lumbar spine.
The negative correlation between femoral neck BMD and exercise in men seemed to contradict findings from previous studies. The authors said that could be a result of reporting bias, with men overestimating their amount of exercise, and could suggest that higher BMI confers some protection against bone loss in men.
The study found no significant correlations between dairy intake and BMD at any site in either sex. The finding suggests that both sexes need to improve both vitamin D and calcium intake.
None of the authors reported any financial disclosures.
SOURCE: Bass MA et al. J Am Osteopath Assoc. 2019;119(6):357-63.
FROM THE JOURNAL OF THE AMERICAN OSTEOPATHIC ASSOCIATION
June 2019 - Question 2
Q2. Correct Answer: C
Rationale
This patient has a main duct IPMN, which has a high potential for malignant transformation and should be resected if possible. Resection is also indicated for branch-duct IPMN's, which are symptomatic (e.g. pancreatitis), associated with obstructive jaundice or main duct involvement, have a solid component within the cyst, or have concerning features on EUS-FNA.
Reference
1. Elta GH, et al, ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-79.
[email protected]
Q2. Correct Answer: C
Rationale
This patient has a main duct IPMN, which has a high potential for malignant transformation and should be resected if possible. Resection is also indicated for branch-duct IPMN's, which are symptomatic (e.g. pancreatitis), associated with obstructive jaundice or main duct involvement, have a solid component within the cyst, or have concerning features on EUS-FNA.
Reference
1. Elta GH, et al, ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-79.
[email protected]
Q2. Correct Answer: C
Rationale
This patient has a main duct IPMN, which has a high potential for malignant transformation and should be resected if possible. Resection is also indicated for branch-duct IPMN's, which are symptomatic (e.g. pancreatitis), associated with obstructive jaundice or main duct involvement, have a solid component within the cyst, or have concerning features on EUS-FNA.
Reference
1. Elta GH, et al, ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-79.
[email protected]
Q2. A 64-year-old male with a recent history of acute pancreatitis has a dilated main pancreatic duct with prominent side branch lesions seen on CT scan. Endoscopic evaluation reveals mucus extruding from a dilated ampulla.
June 2019 - Question 1
Q1. Correct Answer: D
Rationale
The severe reflux may be due to the hiatal hernia and worsened by the obesity. This patient has medically complicated obesity and thus bariatric surgery is an option. A gastric bypass in this situation offers the best anti-reflux procedure for this patient. A fundoplication in the setting of obesity has a higher rate of recurrence of symptoms (Answers A, B). While a gastric sleeve is an option for the obesity, a gastric sleeve (Answer E) may cause de novo reflux or worsen pre-existing symptoms. Magnetic sphincter augmentation (Answer C) has demonstrated promising results in patients with a BMI less than 35 and hiatal hernia less than 3 cm. Data are not available for patients with higher BMIs.
References
1. Abdelrahman T, Latif A, Chan DS, et al. Outcomes after laparoscopic anti-reflux surgery related to obesity: A systematic review and meta-analysis. Int J Surg. 2018 Mar;51:76-82.
2. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015 Sep 28;21(36):10348-57.
Q1. Correct Answer: D
Rationale
The severe reflux may be due to the hiatal hernia and worsened by the obesity. This patient has medically complicated obesity and thus bariatric surgery is an option. A gastric bypass in this situation offers the best anti-reflux procedure for this patient. A fundoplication in the setting of obesity has a higher rate of recurrence of symptoms (Answers A, B). While a gastric sleeve is an option for the obesity, a gastric sleeve (Answer E) may cause de novo reflux or worsen pre-existing symptoms. Magnetic sphincter augmentation (Answer C) has demonstrated promising results in patients with a BMI less than 35 and hiatal hernia less than 3 cm. Data are not available for patients with higher BMIs.
References
1. Abdelrahman T, Latif A, Chan DS, et al. Outcomes after laparoscopic anti-reflux surgery related to obesity: A systematic review and meta-analysis. Int J Surg. 2018 Mar;51:76-82.
2. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015 Sep 28;21(36):10348-57.
Q1. Correct Answer: D
Rationale
The severe reflux may be due to the hiatal hernia and worsened by the obesity. This patient has medically complicated obesity and thus bariatric surgery is an option. A gastric bypass in this situation offers the best anti-reflux procedure for this patient. A fundoplication in the setting of obesity has a higher rate of recurrence of symptoms (Answers A, B). While a gastric sleeve is an option for the obesity, a gastric sleeve (Answer E) may cause de novo reflux or worsen pre-existing symptoms. Magnetic sphincter augmentation (Answer C) has demonstrated promising results in patients with a BMI less than 35 and hiatal hernia less than 3 cm. Data are not available for patients with higher BMIs.
References
1. Abdelrahman T, Latif A, Chan DS, et al. Outcomes after laparoscopic anti-reflux surgery related to obesity: A systematic review and meta-analysis. Int J Surg. 2018 Mar;51:76-82.
2. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015 Sep 28;21(36):10348-57.
Q1. A 56-year-old female with a BMI of 42 (kg/m2), diabetes, and hyperlipidemia presents with a 5-cm hiatal hernia. She has symptoms of heartburn during the day and significant nocturnal regurgitation such that she is sleeping in a recliner at night.
Steady advances made since recognition of neuromyelitis optica 20 years ago
SEATTLE – At the annual meeting of the Consortium of Multiple Sclerosis Centers, Brian Weinshenker, MD, professor of neurology at the Mayo Clinic in Rochester, Minn., summarized some of the milestones in the timeline of NMO research.
These milestones include the 2004 identification of NMO-IgG, an autoantibody marker of NMO that distinguishes it from multiple sclerosis; the 2005 discovery that the antibody was reactive to aquaporin 4, the dominant CNS water channel and an astrocyte protein; further characterizations of NMO manifestations; the revised international panel diagnostic criteria in 2015; and the current phase 3 trials of three potential treatments for NMO – eculizumab, inebilizumab, and satralizumab.
Dr. Weinshenker reported the following disclosures: receiving royalties from the RSR Group, Oxford University, Hospices Civils de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen for a patent of NMO-IgG as a diagnostic test for NMO and related disorders; serving as an adjudication committee member for clinical trials in NMO being conducted by MedImmune and Alexion; and consulting for Chugai regarding a clinical trial for NMO.
SEATTLE – At the annual meeting of the Consortium of Multiple Sclerosis Centers, Brian Weinshenker, MD, professor of neurology at the Mayo Clinic in Rochester, Minn., summarized some of the milestones in the timeline of NMO research.
These milestones include the 2004 identification of NMO-IgG, an autoantibody marker of NMO that distinguishes it from multiple sclerosis; the 2005 discovery that the antibody was reactive to aquaporin 4, the dominant CNS water channel and an astrocyte protein; further characterizations of NMO manifestations; the revised international panel diagnostic criteria in 2015; and the current phase 3 trials of three potential treatments for NMO – eculizumab, inebilizumab, and satralizumab.
Dr. Weinshenker reported the following disclosures: receiving royalties from the RSR Group, Oxford University, Hospices Civils de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen for a patent of NMO-IgG as a diagnostic test for NMO and related disorders; serving as an adjudication committee member for clinical trials in NMO being conducted by MedImmune and Alexion; and consulting for Chugai regarding a clinical trial for NMO.
SEATTLE – At the annual meeting of the Consortium of Multiple Sclerosis Centers, Brian Weinshenker, MD, professor of neurology at the Mayo Clinic in Rochester, Minn., summarized some of the milestones in the timeline of NMO research.
These milestones include the 2004 identification of NMO-IgG, an autoantibody marker of NMO that distinguishes it from multiple sclerosis; the 2005 discovery that the antibody was reactive to aquaporin 4, the dominant CNS water channel and an astrocyte protein; further characterizations of NMO manifestations; the revised international panel diagnostic criteria in 2015; and the current phase 3 trials of three potential treatments for NMO – eculizumab, inebilizumab, and satralizumab.
Dr. Weinshenker reported the following disclosures: receiving royalties from the RSR Group, Oxford University, Hospices Civils de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen for a patent of NMO-IgG as a diagnostic test for NMO and related disorders; serving as an adjudication committee member for clinical trials in NMO being conducted by MedImmune and Alexion; and consulting for Chugai regarding a clinical trial for NMO.
EXPERT ANALYSIS FROM CMSC 2019
Persistent fatigue plagues many IBD patients
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
REPORTING FROM DDW 2019
Tick-borne disease has become a national issue
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.
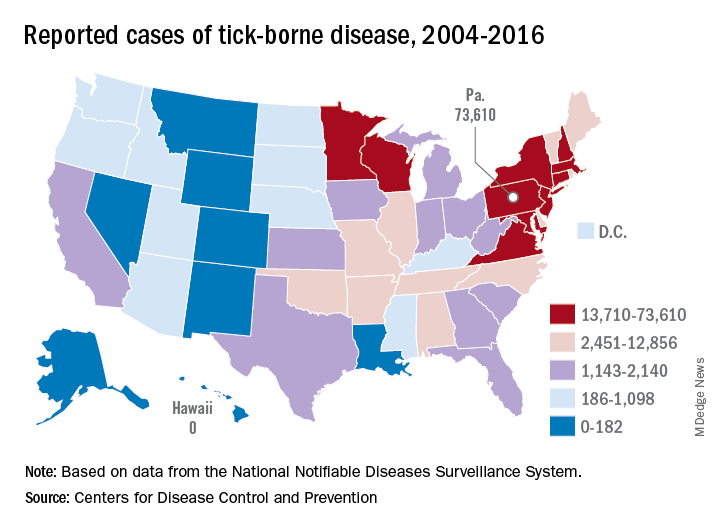
There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
FDA: Vinpocetine associated with fetal harms, miscarriage
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
2019 Update on menopause
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
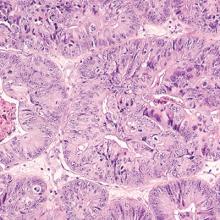
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
What’s in store for ObGyn reimbursement in the EHR age and beyond
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
In an effort to reduce burden on physicians and qualified health care professionals, the Centers for Medicare and Medicaid Services ( CMS) has made changes to Evaluation and Management (E/M) documentation requirements and payment policies. Get ready for fairly extensive changes planned for CY 2021. Here we outline already-implemented and future changes and describe the commitment of the American College of Obstetricians and Gynecologists (ACOG) to ObGyn payment in its collaborations with CMS and the American Medical Association (AMA).

E/M services: CMS reduced documentation
Effective January 2019, the CMS made changes to the documentation requirements for E/M services to provide some common-sense relief for physicians facing excessive documentation requirements in their practices. Most physicians agree that modern medical practice, with the use of electronic health records (EHRs), is different now than in the mid-1990s, when the current E/M structures were developed and implemented. Streamlining documentation requirements reduces paperwork burden and some of the time-consuming duplicative work involved in medical practice today.
For instance, when relevant information is already contained in the medical record, it is not necessary to re-document a full medical history. Physicians will now be able to focus their documentation on the interval since the previous visit. Physicians should still review prior data, update as necessary, and indicate in the medical record that they have done so.
Also, for E/M office and outpatient visits for both new and established patients, physicians are no longer required to re-document information that has already been entered in the patient’s record by practice staff or by the patient. If the patient’s chief complaint and history already has been entered by ancillary staff or the beneficiary, the physician should simply indicate in the medical record that the information has been reviewed and verified.
For E/M visits furnished by teaching physicians, CMS has removed the requirement for potentially duplicative notations that may have been made previously in the medical records by residents or other members of the medical team.
Finally, CMS eliminated the requirement to document the medical necessity of a home visit in lieu of an office visit.
Continue to: Outpatient coding changes for 2021...
Outpatient coding changes for 2021
Outpatient coding for E/M will continue in its current form for the remainder of 2019 and 2020. However, in 2021, expect substantial changes to take effect. If the CMS rule is instituted, payment for E/M office and outpatient visits will be drastically “simplified.” The current CMS plan for 2021 is to collapse payment for existing E/M Levels 2 through 4 to one payment level for new patients and one payment level for established patients, with optional add-on codes. Level 5 visits will continue at a separate payment level and with continuation of current documentation requirements.
In addition to collapsing the payment in E/M Levels 2, 3, and 4, CMS also will allow flexibility in how those E/M office and outpatient visits are documented. Specifically, documentation may be based on any of the following:
- current framework (1995 or 1997 guidelines)
- medical decision making (MDM)
- time.
When using MDM or the current 1995/1997 framework to document an office visit, Medicare will only require documentation to support a Level 2 E/M outpatient visit code for history, exam, and/or MDM. When time is used as the basis for coding the visit, physicians will document the medical necessity of the visit and that the billing practitioner personally spent the required amount of time face-to-face with the beneficiary.
CMS also has finalized the creation of new add-on codes that describe the additional resources inherent in visits for primary care and particular kinds of nonprocedural specialized medical care (and will not be restricted by physician specialty). These codes would only be reportable with E/M office and outpatient level 2 through 4 visits, and their use generally would not impose new documentation requirements. It is not clear which types of visits would support the use of these add-on codes at this time.
Finally, a new “extended visit” add-on code will be available for use only with E/M Level 2 through 4 visits to account for the additional resources required when spending extended time with a patient.
CMS believes these policies will allow physicians, and all who bill E/M codes, greater flexibility to exercise clinical judgment in their documentation, so that they can focus on what is clinically relevant and medically necessary for the beneficiary.
ACOG’s voice in the process
ACOG strongly opposed several proposals that CMS made during the rule-making process that the agency decided not to finalize. These aspects of the proposal would have:
1. reduced payment by 50% for the least expensive procedure or visit when an E/M office or outpatient visit is furnished on the same day as a procedure by the same physician. These are separately identifiable E/M visits that normally would be reported with a modifier 25.
2. established separate coding and payment for podiatric E/M visits, or
3. standardized the allocation of practice expense relative value units (RVUs) for the codes that describe these services.
CMS has stated that they intend to engage in further discussions with the public and stakeholders to potentially further refine the policies for CY 2021.
Continue to: AMA-CPT and RUC initiative...
AMA-CPT and RUC initiative
Although the AMA, ACOG, and physicians in general applauded the CMS initiative to reduce the administrative and documentation burden on providers, there was concern about the unintended consequences of the payment changes that are currently scheduled to take effect in 2021. To address these concerns, the AMA convened a work group of physician experts who are knowledgeable in the Current Procedural Terminology (CPT) code development and valuation processes. The charge to the E/M work group is to collaborate across the provider, payer, and coding communities to establish or revise the coding structure and guidelines for outpatient E/M services. The members formed a multispecialty work group representing primary care and surgical specialties and have experience in developing, defining, and valuing codes.
Dr. Barbara Levy, ACOG’s Vice President of Health Policy, co-chaired this expert panel with geriatrician Dr. Peter Hollmann to develop comprehensive consensus-led changes to revise and modernize E/M codes. The work group followed 4 guiding principles to inform their E/M work:
- to decrease the administrative burden of documentation and coding
- to decrease the need for audits
- to decrease unnecessary and redundant documentation in the medical record that is not needed for patient care
- to ensure that payment for E/M services is resource based. There is no direct goal for payment redistribution among specialties.
A primary concern expressed by physicians about the CMS proposal was that the collapse of payment for E/M visit across levels 2–4 might lead to a lack of appropriate care for more complex patients since the CMS rule does not provide payment based on the resources required to perform the work of the visit. No one believes that the work needed to care for someone with a sore throat or pink eye is equivalent to the work involved in diagnosing and managing depression, for example.
Beginning in August 2018, the work group met regularly to build consensus. The work group worked at an accelerated pace to develop and value codes that better fit the current medical workflows and meet patient needs.
The work group submitted a code change proposal for E/M codes to the CPT Editorial Panel for consideration during the February 2019 meeting. The next step was the code valuation process through the AMA/Specialty Society RVS Update Committee (RUC) process.
CMS has stated that the 2-year delay to 2021 in implementation of their original proposed changes is to allow time for the E/M code change proposals to move through the development and valuation process and subsequent review by the agency. To date, commercial payers and coders have been supportive of the AMA E/M work group proposals. Dr. Levy, Dr. Hollmann, and AMA staff are meeting with CMS and Department of Health and Human Services staff to provide clarity as they review the CPT proposals. ACOG supports the changes, which would simplify documentation for outpatient E/M codes while retaining differential payments. CMS is closely following the progress of the code changes through the CPT process and RUC code valuation process. We await further rulemaking by CMS in defining and valuing this important code set.
- CPT code 99201 to be deleted
- Revision of codes 99202-99215 as follows:
- removing history and examination as key components
(A) for selecting the level of service but requiring a medically appropriate history and or examination be performed in order to report codes 99202-99215
(B) making the basis for code selection on either the level of medical decision making (MDM) performed or the total time spent performing the service on the day of the encounter
(C) changing the definition of the time element associated with codes 99202-99215 from typical face-to-face time to total time spent on the day of the encounter and changing the amount of time associated with each code.
- Revision of the MDM elements associated with codes 99202-99215 as follows:
(i) revising "Number of Diagnoses or Management Options" to "Number and Complexity of Problems Addressed";
(ii) revising "Amount and/or Complexity of Data to be Reviewed" to "Amount and/or Complexity of Data to be Reviewed and Analyzed"; and
(iii) revising "Risk of Complications and/or Morbidity or Mortality" to "Risk of Complications and/or Morbidity or Mortality of Patient Management."
- Revision of the E/M guidelines by:
(A) restructuring the guidelines into three sections: "Guidelines Common to All E/M Services," "Guidelines for Hospital Observation, Hospital Inpatient, Consultations, Emergency Department, Nursing Facility, Domiciliary, Rest Home or Custodial Care and Home E/M Services," and "Guidelines for Office or Other Outpatient E/M Services" to distinguish the new reporting guidelines for the Office or Other Outpatient Services codes 99202-99215
(B) adding new guidelines that are applicable only to Office or Other Outpatient codes (99202-99215); adding a Summary of Guideline Differences table of the differences between the sets of guidelines
(C) revised existing E/M guidelines to ensure there is no conflicting information between the different sets of guidelines
(D) adding definitions of terms associated with the elements of MDM applicable to codes 99202-99215
(E) adding an MDM table that is applicable to codes 99202-99215
(F) defining total time associated with codes 99202-99215
(G) adding guidelines for reporting time when more than one individual performs distinct parts of an E/M service; revision of the MDM table in the Amount and/or Complexity of Data to be Reviewed and Analyzed column:
(1) inserted a dash (-) after the asterisk in the asterisk definition, "* - Each unique test, order, or document may be summed if multiple," to clarify this is the meaning of the asterisk and not an asterisked item itself
(2) for limited amount of data to be reviewed and analyzed (codes 99203/99213), the parenthetical regarding the number of categories for which requirements must be met was revised to state, "¬categories of tests and documents, or independent historian(s)" rather than "categories within tests, documents, or independent historian(s)"
(3) removing the word "or" after each of the bulleted items for limited, moderate (codes 99202/99214), and high (99205/99215) amount and/or complexity of data to be reviewed and analyzed.
Continue to: ACOG is at the helm, with a watchful eye...
ACOG is at the helm, with a watchful eye
This is a challenging undertaking because E/M codes are used across specialties for office visits and outpatient care. The potential for unintended consequences for all services that include E/M, such as the global obstetrical services or 90-day global surgical services, is substantial. ACOG is intimately involved in this undertaking, watching the developments carefully to ensure that the interests of ObGyns and their patients are protected.
VIDEO: Did You Know? Psoriasis and mental health
The American Academy of Dermatology and the National Psoriasis Foundation recently issued a joint guideline on the management and treatment of psoriasis, with a focus on comorbidities. The guideline offers information and recommendations on mental health in patients with psoriasis.

The American Academy of Dermatology and the National Psoriasis Foundation recently issued a joint guideline on the management and treatment of psoriasis, with a focus on comorbidities. The guideline offers information and recommendations on mental health in patients with psoriasis.

The American Academy of Dermatology and the National Psoriasis Foundation recently issued a joint guideline on the management and treatment of psoriasis, with a focus on comorbidities. The guideline offers information and recommendations on mental health in patients with psoriasis.