User login
Analysis: Why Alexa’s bedside manner is bad for health care
Amazon has opened a new health care frontier: Now Alexa can be used to transmit patient data. Using this new feature – which Amazon labeled as a “skill” – a company named Livongo will allow diabetes patients – which it calls “members” – to use the device to “query their last blood sugar reading, blood sugar measurement trends, and receive insights and Health Nudges that are personalized to them.”
Private equity and venture capital firms are in love with a legion of companies and startups touting the benefits of virtual doctors’ visits and telemedicine to revolutionize health care, investing almost $10 billion in 2018, a record for the sector. Without stepping into a gym or a clinic, a startup called Kinetxx will provide patients with virtual physical therapy, along with messaging and exercise logging. And Maven Clinic (which is not actually a physical place) offers online medical guidance and personal advice focusing on women’s health needs.
In April, at Fortune’s Brainstorm Health conference in San Diego, Bruce Broussard, CEO of health insurer Humana, said he believes technology will help patients receive help during medical crises, citing the benefits of home monitoring and the ability of doctors’ visits to be conducted by video conference.
But when I returned from Brainstorm Health, I was confronted by an alternative reality of virtual medicine: a $235 medical bill for a telehealth visit that resulted from one of my kids calling a longtime doctor’s office. It was for a five-minute phone call answering a question about a possible infection.
Virtual communications have streamlined life and transformed many of our relationships for the better. There is little need anymore to sit across the desk from a tax accountant or travel agent or to stand in a queue for a bank teller. And there is certainly room for disruptive digital innovation in our confusing and overpriced health care system.
But it remains an open question whether virtual medicine will prove a valuable, convenient adjunct to health care. Or, instead, will it be a way for the U.S. profit-driven health care system to make big bucks by outsourcing core duties – while providing a paler version of actual medical treatment?
After all, my doctors have long answered my questions and dispensed phone and email advice for free – as part of our doctor-patient relationship – though it didn’t have a cool branding moniker like telehealth. And my obstetrician’s office offered great support and advice through two difficult pregnancies – maybe they should have been paid for that valuable service. But $235 for a phone call (which works out to over $2,000 per hour)? Not even a corporate lawyer bills that.
Logic holds that some digital health tools have tremendous potential: A neurologist can view a patient by video to see if lopsided facial movements suggest a stroke. A patient with an irregular heart rhythm could send in digital tracings to see if a new prescription drug is working. But the tangible benefit of many other virtual services offered is less certain. Some people may like receiving feedback about their sleep from an Apple Watch, but I’m not sure that’s medicine.
And if virtual medicine is pursued in the name of business efficiency or just profit, it has enormous potential to make health care worse.
My doctor’s nurse is far better equipped to answer a question about my ongoing health problem than someone at a call center reading from a script. And, however thorough a virtual visit may be, it forsakes some of the diagnostic information that comes when you see and touch the patient.
A study published recently in Pediatrics found that children who had a telemedicine visit for an upper respiratory infection were far more likely to get an antibiotic than those who physically saw a doctor, suggesting overprescribing is at work. It makes sense: A doctor can’t use a stethoscope to listen to lungs or wiggle an otoscope into a kid’s ear by video. Similarly, a virtual physical therapist can’t feel the knots in muscle or notice a fleeting wince on a patient’s face via camera.
More important, perhaps, virtual medicine means losing the support that has long been a crucial part of the profession. There are programs to provide iPads to people in home hospice for resources about grief and chatbots that purport to treat depression. Maybe people at such challenging moments need – and deserve – human contact.
Of course, companies like those mentioned are expecting to be reimbursed for the remote monitoring and virtual advice they provide. Investors, in turn, get generous payback without having to employ so many actual doctors or other health professionals. Livongo, for instance, has raised a total of $235 million in funding over six rounds. And, as of 2018, Medicare announced it would allow such digital monitoring tools to “qualify for reimbursement,” if they are “clinically endorsed.” But, ultimately, will the well-being of patients or investors decide which tools are clinically endorsed?
So far, with its new so-called skill, Alexa will be able to perform a half-dozen health-related services. In addition to diabetes coaching, it can find the earliest urgent care appointment in a given area and check the status of a prescription drug delivery.
But it will not provide many things patients desperately want, which technology should be able to readily deliver, such as a reliable price estimate for an upcoming surgery, the infection rates at the local hospital, the location of the cheapest cholesterol test nearby. And if we’re trying to bring health care into the tech-enabled 21st century, how about starting with low-hanging fruit: Does any other sector still use paper bills and faxes?
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Amazon has opened a new health care frontier: Now Alexa can be used to transmit patient data. Using this new feature – which Amazon labeled as a “skill” – a company named Livongo will allow diabetes patients – which it calls “members” – to use the device to “query their last blood sugar reading, blood sugar measurement trends, and receive insights and Health Nudges that are personalized to them.”
Private equity and venture capital firms are in love with a legion of companies and startups touting the benefits of virtual doctors’ visits and telemedicine to revolutionize health care, investing almost $10 billion in 2018, a record for the sector. Without stepping into a gym or a clinic, a startup called Kinetxx will provide patients with virtual physical therapy, along with messaging and exercise logging. And Maven Clinic (which is not actually a physical place) offers online medical guidance and personal advice focusing on women’s health needs.
In April, at Fortune’s Brainstorm Health conference in San Diego, Bruce Broussard, CEO of health insurer Humana, said he believes technology will help patients receive help during medical crises, citing the benefits of home monitoring and the ability of doctors’ visits to be conducted by video conference.
But when I returned from Brainstorm Health, I was confronted by an alternative reality of virtual medicine: a $235 medical bill for a telehealth visit that resulted from one of my kids calling a longtime doctor’s office. It was for a five-minute phone call answering a question about a possible infection.
Virtual communications have streamlined life and transformed many of our relationships for the better. There is little need anymore to sit across the desk from a tax accountant or travel agent or to stand in a queue for a bank teller. And there is certainly room for disruptive digital innovation in our confusing and overpriced health care system.
But it remains an open question whether virtual medicine will prove a valuable, convenient adjunct to health care. Or, instead, will it be a way for the U.S. profit-driven health care system to make big bucks by outsourcing core duties – while providing a paler version of actual medical treatment?
After all, my doctors have long answered my questions and dispensed phone and email advice for free – as part of our doctor-patient relationship – though it didn’t have a cool branding moniker like telehealth. And my obstetrician’s office offered great support and advice through two difficult pregnancies – maybe they should have been paid for that valuable service. But $235 for a phone call (which works out to over $2,000 per hour)? Not even a corporate lawyer bills that.
Logic holds that some digital health tools have tremendous potential: A neurologist can view a patient by video to see if lopsided facial movements suggest a stroke. A patient with an irregular heart rhythm could send in digital tracings to see if a new prescription drug is working. But the tangible benefit of many other virtual services offered is less certain. Some people may like receiving feedback about their sleep from an Apple Watch, but I’m not sure that’s medicine.
And if virtual medicine is pursued in the name of business efficiency or just profit, it has enormous potential to make health care worse.
My doctor’s nurse is far better equipped to answer a question about my ongoing health problem than someone at a call center reading from a script. And, however thorough a virtual visit may be, it forsakes some of the diagnostic information that comes when you see and touch the patient.
A study published recently in Pediatrics found that children who had a telemedicine visit for an upper respiratory infection were far more likely to get an antibiotic than those who physically saw a doctor, suggesting overprescribing is at work. It makes sense: A doctor can’t use a stethoscope to listen to lungs or wiggle an otoscope into a kid’s ear by video. Similarly, a virtual physical therapist can’t feel the knots in muscle or notice a fleeting wince on a patient’s face via camera.
More important, perhaps, virtual medicine means losing the support that has long been a crucial part of the profession. There are programs to provide iPads to people in home hospice for resources about grief and chatbots that purport to treat depression. Maybe people at such challenging moments need – and deserve – human contact.
Of course, companies like those mentioned are expecting to be reimbursed for the remote monitoring and virtual advice they provide. Investors, in turn, get generous payback without having to employ so many actual doctors or other health professionals. Livongo, for instance, has raised a total of $235 million in funding over six rounds. And, as of 2018, Medicare announced it would allow such digital monitoring tools to “qualify for reimbursement,” if they are “clinically endorsed.” But, ultimately, will the well-being of patients or investors decide which tools are clinically endorsed?
So far, with its new so-called skill, Alexa will be able to perform a half-dozen health-related services. In addition to diabetes coaching, it can find the earliest urgent care appointment in a given area and check the status of a prescription drug delivery.
But it will not provide many things patients desperately want, which technology should be able to readily deliver, such as a reliable price estimate for an upcoming surgery, the infection rates at the local hospital, the location of the cheapest cholesterol test nearby. And if we’re trying to bring health care into the tech-enabled 21st century, how about starting with low-hanging fruit: Does any other sector still use paper bills and faxes?
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Amazon has opened a new health care frontier: Now Alexa can be used to transmit patient data. Using this new feature – which Amazon labeled as a “skill” – a company named Livongo will allow diabetes patients – which it calls “members” – to use the device to “query their last blood sugar reading, blood sugar measurement trends, and receive insights and Health Nudges that are personalized to them.”
Private equity and venture capital firms are in love with a legion of companies and startups touting the benefits of virtual doctors’ visits and telemedicine to revolutionize health care, investing almost $10 billion in 2018, a record for the sector. Without stepping into a gym or a clinic, a startup called Kinetxx will provide patients with virtual physical therapy, along with messaging and exercise logging. And Maven Clinic (which is not actually a physical place) offers online medical guidance and personal advice focusing on women’s health needs.
In April, at Fortune’s Brainstorm Health conference in San Diego, Bruce Broussard, CEO of health insurer Humana, said he believes technology will help patients receive help during medical crises, citing the benefits of home monitoring and the ability of doctors’ visits to be conducted by video conference.
But when I returned from Brainstorm Health, I was confronted by an alternative reality of virtual medicine: a $235 medical bill for a telehealth visit that resulted from one of my kids calling a longtime doctor’s office. It was for a five-minute phone call answering a question about a possible infection.
Virtual communications have streamlined life and transformed many of our relationships for the better. There is little need anymore to sit across the desk from a tax accountant or travel agent or to stand in a queue for a bank teller. And there is certainly room for disruptive digital innovation in our confusing and overpriced health care system.
But it remains an open question whether virtual medicine will prove a valuable, convenient adjunct to health care. Or, instead, will it be a way for the U.S. profit-driven health care system to make big bucks by outsourcing core duties – while providing a paler version of actual medical treatment?
After all, my doctors have long answered my questions and dispensed phone and email advice for free – as part of our doctor-patient relationship – though it didn’t have a cool branding moniker like telehealth. And my obstetrician’s office offered great support and advice through two difficult pregnancies – maybe they should have been paid for that valuable service. But $235 for a phone call (which works out to over $2,000 per hour)? Not even a corporate lawyer bills that.
Logic holds that some digital health tools have tremendous potential: A neurologist can view a patient by video to see if lopsided facial movements suggest a stroke. A patient with an irregular heart rhythm could send in digital tracings to see if a new prescription drug is working. But the tangible benefit of many other virtual services offered is less certain. Some people may like receiving feedback about their sleep from an Apple Watch, but I’m not sure that’s medicine.
And if virtual medicine is pursued in the name of business efficiency or just profit, it has enormous potential to make health care worse.
My doctor’s nurse is far better equipped to answer a question about my ongoing health problem than someone at a call center reading from a script. And, however thorough a virtual visit may be, it forsakes some of the diagnostic information that comes when you see and touch the patient.
A study published recently in Pediatrics found that children who had a telemedicine visit for an upper respiratory infection were far more likely to get an antibiotic than those who physically saw a doctor, suggesting overprescribing is at work. It makes sense: A doctor can’t use a stethoscope to listen to lungs or wiggle an otoscope into a kid’s ear by video. Similarly, a virtual physical therapist can’t feel the knots in muscle or notice a fleeting wince on a patient’s face via camera.
More important, perhaps, virtual medicine means losing the support that has long been a crucial part of the profession. There are programs to provide iPads to people in home hospice for resources about grief and chatbots that purport to treat depression. Maybe people at such challenging moments need – and deserve – human contact.
Of course, companies like those mentioned are expecting to be reimbursed for the remote monitoring and virtual advice they provide. Investors, in turn, get generous payback without having to employ so many actual doctors or other health professionals. Livongo, for instance, has raised a total of $235 million in funding over six rounds. And, as of 2018, Medicare announced it would allow such digital monitoring tools to “qualify for reimbursement,” if they are “clinically endorsed.” But, ultimately, will the well-being of patients or investors decide which tools are clinically endorsed?
So far, with its new so-called skill, Alexa will be able to perform a half-dozen health-related services. In addition to diabetes coaching, it can find the earliest urgent care appointment in a given area and check the status of a prescription drug delivery.
But it will not provide many things patients desperately want, which technology should be able to readily deliver, such as a reliable price estimate for an upcoming surgery, the infection rates at the local hospital, the location of the cheapest cholesterol test nearby. And if we’re trying to bring health care into the tech-enabled 21st century, how about starting with low-hanging fruit: Does any other sector still use paper bills and faxes?
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Modern surgical techniques for gastrointestinal endometriosis
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
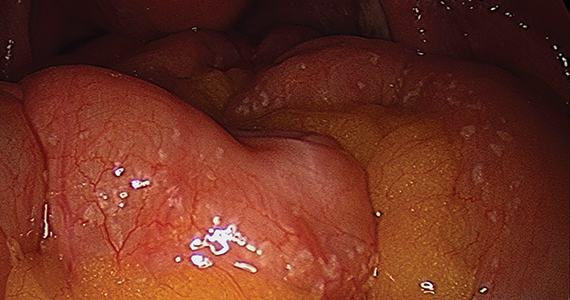
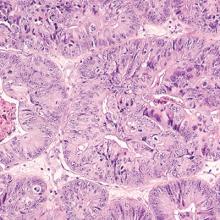
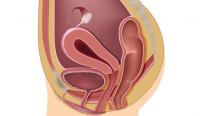
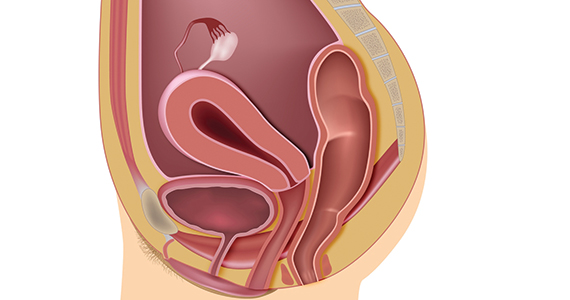
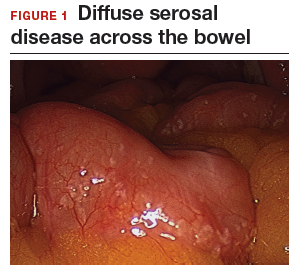
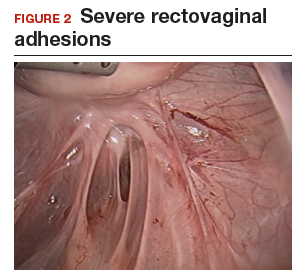
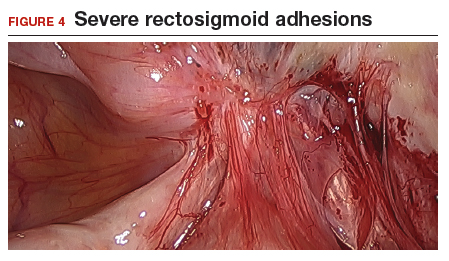
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.


Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
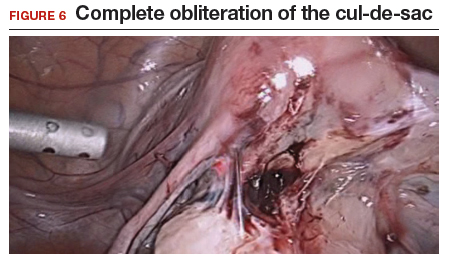
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.
Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
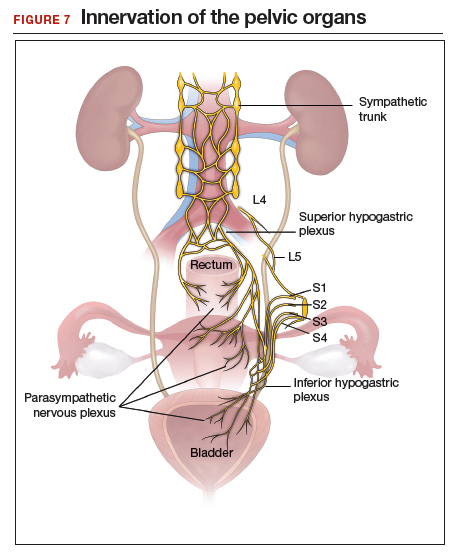
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2
Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.





Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.

Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2

Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.





Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.

Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2

Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
Younger men and women show similar rates of osteopenia
according to findings from a cross-sectional study.
The high prevalence of osteopenia – once viewed as restricted largely to older women – in the study’s younger, cross-sex population should spur physicians to ask all patients about calcium intake and exercise as well as to screen for osteoporosis in all patients, Martha A. Bass, PhD,`wrote in the Journal of the American Osteopathic Association.
“It is important that early detection of the precursors for osteoporosis become part of the annual physical for people in this age range, as well as in older patients,” noted Dr Bass of the University of Mississippi School of Applied Sciences in Oxford, and coauthors. “Primary care physicians should begin educating patients as early as adolescence or young adulthood so the consequences of osteoporosis can be prevented. The result would be the prevention of future bone fractures and the morbidity and mortality associated with bone fractures, thus leading to improved quality of life.”
The researchers set out to examine the likelihood of low bone mineral density (BMD) and related risk factors in 173 adults aged 35-50 years. All of the participants completed a questionnaire assessing calcium intake, weekly exercise, smoking, and body mass index, and all underwent screening for BMD. The study’s primary outcome was BMD at the femoral neck, trochanter, intertrochanteric crest, total femur, and lumbar spine.
Among the 81 men in the sample, 25 (30%) had a normal body mass index, and the remainder were either overweight (47.5%) or obese (22.5%). One of the women was underweight, 48.9% were normal weight, 28.3% were overweight, and 21.7% were obese.
Most of the sample, regardless of gender, reported consuming fewer than three dairy items per day. Exercise frequency was better, with 68% of men and 56.4% of women saying they exercised at least 20 times per month.
There were no total femur osteoporosis findings in either sex. However, osteopenia at the femoral neck was present in 28.4% of the men and 26.1% of the women. Osteopenia at the lumbar spine occurred in 21% of men and 15.2% of women, with 6.2% of men and 2.2% of women showing osteoporosis at this site.
An adjusted analysis determined that exercise correlated significantly and negatively with femoral neck BMD in men. But in women, there was a significant and positive correlation with BMD at the lumbar spine and at all femoral measurements.
Body mass index also played into the risk picture. Among men, almost all BMD measurements (trochanter, intertrochanteric crest, total femur, and lumbar spine) were positively associated with higher BMI. For women, higher BMI was associated with better BMD at the all the femoral sites, but not at the lumbar spine.
The negative correlation between femoral neck BMD and exercise in men seemed to contradict findings from previous studies. The authors said that could be a result of reporting bias, with men overestimating their amount of exercise, and could suggest that higher BMI confers some protection against bone loss in men.
The study found no significant correlations between dairy intake and BMD at any site in either sex. The finding suggests that both sexes need to improve both vitamin D and calcium intake.
None of the authors reported any financial disclosures.
SOURCE: Bass MA et al. J Am Osteopath Assoc. 2019;119(6):357-63.
according to findings from a cross-sectional study.
The high prevalence of osteopenia – once viewed as restricted largely to older women – in the study’s younger, cross-sex population should spur physicians to ask all patients about calcium intake and exercise as well as to screen for osteoporosis in all patients, Martha A. Bass, PhD,`wrote in the Journal of the American Osteopathic Association.
“It is important that early detection of the precursors for osteoporosis become part of the annual physical for people in this age range, as well as in older patients,” noted Dr Bass of the University of Mississippi School of Applied Sciences in Oxford, and coauthors. “Primary care physicians should begin educating patients as early as adolescence or young adulthood so the consequences of osteoporosis can be prevented. The result would be the prevention of future bone fractures and the morbidity and mortality associated with bone fractures, thus leading to improved quality of life.”
The researchers set out to examine the likelihood of low bone mineral density (BMD) and related risk factors in 173 adults aged 35-50 years. All of the participants completed a questionnaire assessing calcium intake, weekly exercise, smoking, and body mass index, and all underwent screening for BMD. The study’s primary outcome was BMD at the femoral neck, trochanter, intertrochanteric crest, total femur, and lumbar spine.
Among the 81 men in the sample, 25 (30%) had a normal body mass index, and the remainder were either overweight (47.5%) or obese (22.5%). One of the women was underweight, 48.9% were normal weight, 28.3% were overweight, and 21.7% were obese.
Most of the sample, regardless of gender, reported consuming fewer than three dairy items per day. Exercise frequency was better, with 68% of men and 56.4% of women saying they exercised at least 20 times per month.
There were no total femur osteoporosis findings in either sex. However, osteopenia at the femoral neck was present in 28.4% of the men and 26.1% of the women. Osteopenia at the lumbar spine occurred in 21% of men and 15.2% of women, with 6.2% of men and 2.2% of women showing osteoporosis at this site.
An adjusted analysis determined that exercise correlated significantly and negatively with femoral neck BMD in men. But in women, there was a significant and positive correlation with BMD at the lumbar spine and at all femoral measurements.
Body mass index also played into the risk picture. Among men, almost all BMD measurements (trochanter, intertrochanteric crest, total femur, and lumbar spine) were positively associated with higher BMI. For women, higher BMI was associated with better BMD at the all the femoral sites, but not at the lumbar spine.
The negative correlation between femoral neck BMD and exercise in men seemed to contradict findings from previous studies. The authors said that could be a result of reporting bias, with men overestimating their amount of exercise, and could suggest that higher BMI confers some protection against bone loss in men.
The study found no significant correlations between dairy intake and BMD at any site in either sex. The finding suggests that both sexes need to improve both vitamin D and calcium intake.
None of the authors reported any financial disclosures.
SOURCE: Bass MA et al. J Am Osteopath Assoc. 2019;119(6):357-63.
according to findings from a cross-sectional study.
The high prevalence of osteopenia – once viewed as restricted largely to older women – in the study’s younger, cross-sex population should spur physicians to ask all patients about calcium intake and exercise as well as to screen for osteoporosis in all patients, Martha A. Bass, PhD,`wrote in the Journal of the American Osteopathic Association.
“It is important that early detection of the precursors for osteoporosis become part of the annual physical for people in this age range, as well as in older patients,” noted Dr Bass of the University of Mississippi School of Applied Sciences in Oxford, and coauthors. “Primary care physicians should begin educating patients as early as adolescence or young adulthood so the consequences of osteoporosis can be prevented. The result would be the prevention of future bone fractures and the morbidity and mortality associated with bone fractures, thus leading to improved quality of life.”
The researchers set out to examine the likelihood of low bone mineral density (BMD) and related risk factors in 173 adults aged 35-50 years. All of the participants completed a questionnaire assessing calcium intake, weekly exercise, smoking, and body mass index, and all underwent screening for BMD. The study’s primary outcome was BMD at the femoral neck, trochanter, intertrochanteric crest, total femur, and lumbar spine.
Among the 81 men in the sample, 25 (30%) had a normal body mass index, and the remainder were either overweight (47.5%) or obese (22.5%). One of the women was underweight, 48.9% were normal weight, 28.3% were overweight, and 21.7% were obese.
Most of the sample, regardless of gender, reported consuming fewer than three dairy items per day. Exercise frequency was better, with 68% of men and 56.4% of women saying they exercised at least 20 times per month.
There were no total femur osteoporosis findings in either sex. However, osteopenia at the femoral neck was present in 28.4% of the men and 26.1% of the women. Osteopenia at the lumbar spine occurred in 21% of men and 15.2% of women, with 6.2% of men and 2.2% of women showing osteoporosis at this site.
An adjusted analysis determined that exercise correlated significantly and negatively with femoral neck BMD in men. But in women, there was a significant and positive correlation with BMD at the lumbar spine and at all femoral measurements.
Body mass index also played into the risk picture. Among men, almost all BMD measurements (trochanter, intertrochanteric crest, total femur, and lumbar spine) were positively associated with higher BMI. For women, higher BMI was associated with better BMD at the all the femoral sites, but not at the lumbar spine.
The negative correlation between femoral neck BMD and exercise in men seemed to contradict findings from previous studies. The authors said that could be a result of reporting bias, with men overestimating their amount of exercise, and could suggest that higher BMI confers some protection against bone loss in men.
The study found no significant correlations between dairy intake and BMD at any site in either sex. The finding suggests that both sexes need to improve both vitamin D and calcium intake.
None of the authors reported any financial disclosures.
SOURCE: Bass MA et al. J Am Osteopath Assoc. 2019;119(6):357-63.
FROM THE JOURNAL OF THE AMERICAN OSTEOPATHIC ASSOCIATION
June 2019 - Question 2
Q2. Correct Answer: C
Rationale
This patient has a main duct IPMN, which has a high potential for malignant transformation and should be resected if possible. Resection is also indicated for branch-duct IPMN's, which are symptomatic (e.g. pancreatitis), associated with obstructive jaundice or main duct involvement, have a solid component within the cyst, or have concerning features on EUS-FNA.
Reference
1. Elta GH, et al, ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-79.
[email protected]
Q2. Correct Answer: C
Rationale
This patient has a main duct IPMN, which has a high potential for malignant transformation and should be resected if possible. Resection is also indicated for branch-duct IPMN's, which are symptomatic (e.g. pancreatitis), associated with obstructive jaundice or main duct involvement, have a solid component within the cyst, or have concerning features on EUS-FNA.
Reference
1. Elta GH, et al, ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-79.
[email protected]
Q2. Correct Answer: C
Rationale
This patient has a main duct IPMN, which has a high potential for malignant transformation and should be resected if possible. Resection is also indicated for branch-duct IPMN's, which are symptomatic (e.g. pancreatitis), associated with obstructive jaundice or main duct involvement, have a solid component within the cyst, or have concerning features on EUS-FNA.
Reference
1. Elta GH, et al, ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-79.
[email protected]
Q2. A 64-year-old male with a recent history of acute pancreatitis has a dilated main pancreatic duct with prominent side branch lesions seen on CT scan. Endoscopic evaluation reveals mucus extruding from a dilated ampulla.
June 2019 - Question 1
Q1. Correct Answer: D
Rationale
The severe reflux may be due to the hiatal hernia and worsened by the obesity. This patient has medically complicated obesity and thus bariatric surgery is an option. A gastric bypass in this situation offers the best anti-reflux procedure for this patient. A fundoplication in the setting of obesity has a higher rate of recurrence of symptoms (Answers A, B). While a gastric sleeve is an option for the obesity, a gastric sleeve (Answer E) may cause de novo reflux or worsen pre-existing symptoms. Magnetic sphincter augmentation (Answer C) has demonstrated promising results in patients with a BMI less than 35 and hiatal hernia less than 3 cm. Data are not available for patients with higher BMIs.
References
1. Abdelrahman T, Latif A, Chan DS, et al. Outcomes after laparoscopic anti-reflux surgery related to obesity: A systematic review and meta-analysis. Int J Surg. 2018 Mar;51:76-82.
2. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015 Sep 28;21(36):10348-57.
Q1. Correct Answer: D
Rationale
The severe reflux may be due to the hiatal hernia and worsened by the obesity. This patient has medically complicated obesity and thus bariatric surgery is an option. A gastric bypass in this situation offers the best anti-reflux procedure for this patient. A fundoplication in the setting of obesity has a higher rate of recurrence of symptoms (Answers A, B). While a gastric sleeve is an option for the obesity, a gastric sleeve (Answer E) may cause de novo reflux or worsen pre-existing symptoms. Magnetic sphincter augmentation (Answer C) has demonstrated promising results in patients with a BMI less than 35 and hiatal hernia less than 3 cm. Data are not available for patients with higher BMIs.
References
1. Abdelrahman T, Latif A, Chan DS, et al. Outcomes after laparoscopic anti-reflux surgery related to obesity: A systematic review and meta-analysis. Int J Surg. 2018 Mar;51:76-82.
2. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015 Sep 28;21(36):10348-57.
Q1. Correct Answer: D
Rationale
The severe reflux may be due to the hiatal hernia and worsened by the obesity. This patient has medically complicated obesity and thus bariatric surgery is an option. A gastric bypass in this situation offers the best anti-reflux procedure for this patient. A fundoplication in the setting of obesity has a higher rate of recurrence of symptoms (Answers A, B). While a gastric sleeve is an option for the obesity, a gastric sleeve (Answer E) may cause de novo reflux or worsen pre-existing symptoms. Magnetic sphincter augmentation (Answer C) has demonstrated promising results in patients with a BMI less than 35 and hiatal hernia less than 3 cm. Data are not available for patients with higher BMIs.
References
1. Abdelrahman T, Latif A, Chan DS, et al. Outcomes after laparoscopic anti-reflux surgery related to obesity: A systematic review and meta-analysis. Int J Surg. 2018 Mar;51:76-82.
2. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J Gastroenterol. 2015 Sep 28;21(36):10348-57.
Q1. A 56-year-old female with a BMI of 42 (kg/m2), diabetes, and hyperlipidemia presents with a 5-cm hiatal hernia. She has symptoms of heartburn during the day and significant nocturnal regurgitation such that she is sleeping in a recliner at night.
Steady advances made since recognition of neuromyelitis optica 20 years ago
SEATTLE – At the annual meeting of the Consortium of Multiple Sclerosis Centers, Brian Weinshenker, MD, professor of neurology at the Mayo Clinic in Rochester, Minn., summarized some of the milestones in the timeline of NMO research.
These milestones include the 2004 identification of NMO-IgG, an autoantibody marker of NMO that distinguishes it from multiple sclerosis; the 2005 discovery that the antibody was reactive to aquaporin 4, the dominant CNS water channel and an astrocyte protein; further characterizations of NMO manifestations; the revised international panel diagnostic criteria in 2015; and the current phase 3 trials of three potential treatments for NMO – eculizumab, inebilizumab, and satralizumab.
Dr. Weinshenker reported the following disclosures: receiving royalties from the RSR Group, Oxford University, Hospices Civils de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen for a patent of NMO-IgG as a diagnostic test for NMO and related disorders; serving as an adjudication committee member for clinical trials in NMO being conducted by MedImmune and Alexion; and consulting for Chugai regarding a clinical trial for NMO.
SEATTLE – At the annual meeting of the Consortium of Multiple Sclerosis Centers, Brian Weinshenker, MD, professor of neurology at the Mayo Clinic in Rochester, Minn., summarized some of the milestones in the timeline of NMO research.
These milestones include the 2004 identification of NMO-IgG, an autoantibody marker of NMO that distinguishes it from multiple sclerosis; the 2005 discovery that the antibody was reactive to aquaporin 4, the dominant CNS water channel and an astrocyte protein; further characterizations of NMO manifestations; the revised international panel diagnostic criteria in 2015; and the current phase 3 trials of three potential treatments for NMO – eculizumab, inebilizumab, and satralizumab.
Dr. Weinshenker reported the following disclosures: receiving royalties from the RSR Group, Oxford University, Hospices Civils de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen for a patent of NMO-IgG as a diagnostic test for NMO and related disorders; serving as an adjudication committee member for clinical trials in NMO being conducted by MedImmune and Alexion; and consulting for Chugai regarding a clinical trial for NMO.
SEATTLE – At the annual meeting of the Consortium of Multiple Sclerosis Centers, Brian Weinshenker, MD, professor of neurology at the Mayo Clinic in Rochester, Minn., summarized some of the milestones in the timeline of NMO research.
These milestones include the 2004 identification of NMO-IgG, an autoantibody marker of NMO that distinguishes it from multiple sclerosis; the 2005 discovery that the antibody was reactive to aquaporin 4, the dominant CNS water channel and an astrocyte protein; further characterizations of NMO manifestations; the revised international panel diagnostic criteria in 2015; and the current phase 3 trials of three potential treatments for NMO – eculizumab, inebilizumab, and satralizumab.
Dr. Weinshenker reported the following disclosures: receiving royalties from the RSR Group, Oxford University, Hospices Civils de Lyon, and MVZ Labor PD Dr. Volkmann und Kollegen for a patent of NMO-IgG as a diagnostic test for NMO and related disorders; serving as an adjudication committee member for clinical trials in NMO being conducted by MedImmune and Alexion; and consulting for Chugai regarding a clinical trial for NMO.
EXPERT ANALYSIS FROM CMSC 2019
Persistent fatigue plagues many IBD patients
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
REPORTING FROM DDW 2019
Tick-borne disease has become a national issue
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.
There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
FDA: Vinpocetine associated with fetal harms, miscarriage
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
according to a statement from the agency.
This warning is based on data reviewed by the FDA, including a report from the National Institutes of Health’s National Toxicology Program, that show associations between vinpocetine use and decreased fetal weight and increased risk of miscarriage in animals. The agency is particularly concerned because products containing this ingredient, including those marketed as improving energy and memory, are widely available to women of childbearing age. As a result, the agency has recommended these women not take vinpocetine.
Vinpocetine is a synthetically produced compound used in dietary supplements either on its own or in combination and may be referred to as Vinca minor extract, lesser periwinkle extract, or common periwinkle extract on product labels. Although vinpocetine is regulated in some countries as a prescription drug, when it’s sold in dietary supplements in the United States, the FDA does not usually review those products or their labeling before they become available to consumers under the same safety and effectiveness standards used to evaluate drug products.
“Today’s safety warning is just one of many steps the FDA is taking to adapt to the realities of the evolving dietary supplement industry,” according to the agency’s statement. “Protecting the public from unsafe dietary supplements remains a top priority for the FDA.”
The full statement regarding vinpocetine and its risks can be found on the FDA website.
2019 Update on menopause
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.