User login
Hysterectomy in patients with history of prior cesarean delivery: A reverse dissection technique for vesicouterine adhesions
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
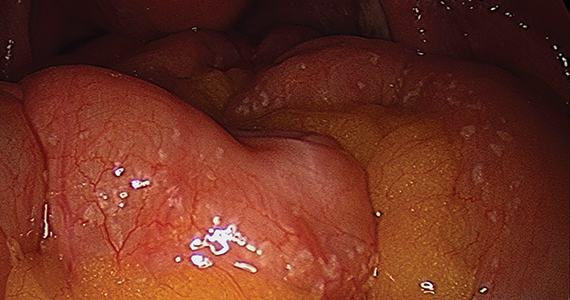
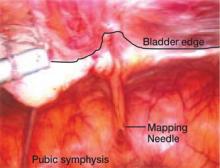
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).
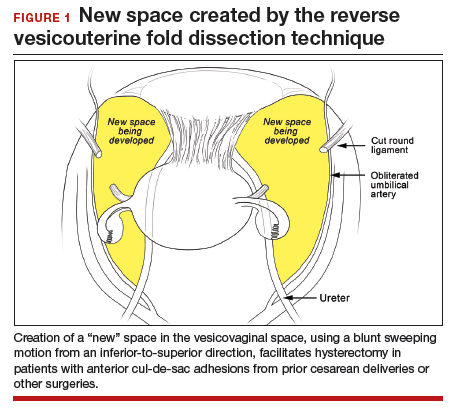
Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
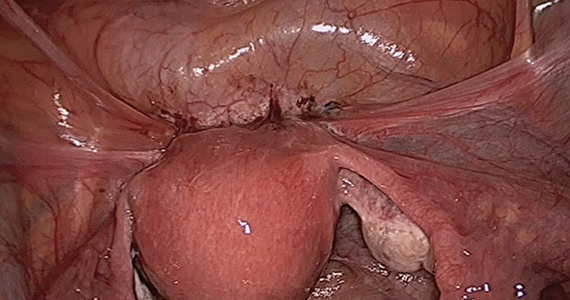
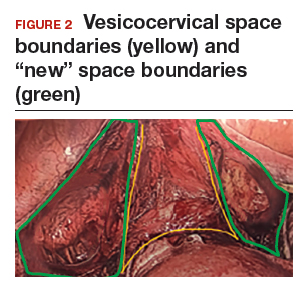
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).
Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
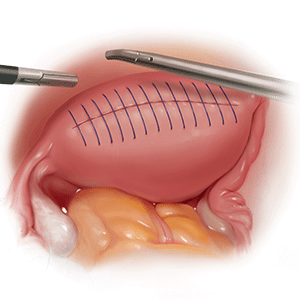
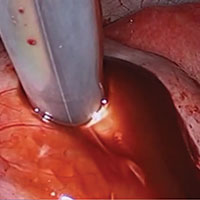
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).
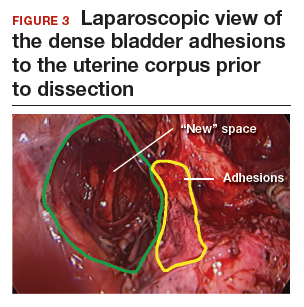
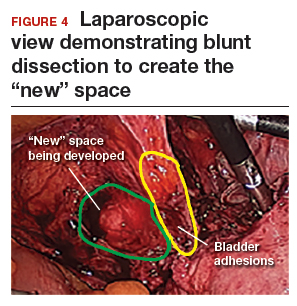
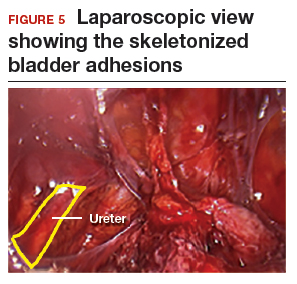
Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).

Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).

Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).



Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).

Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).

Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).



Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
Modern surgical techniques for gastrointestinal endometriosis
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
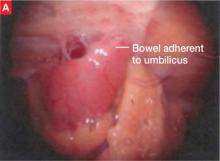
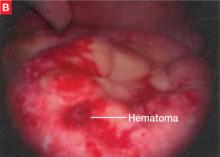
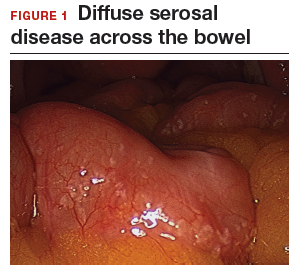
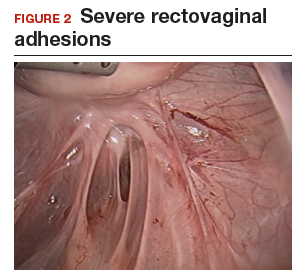
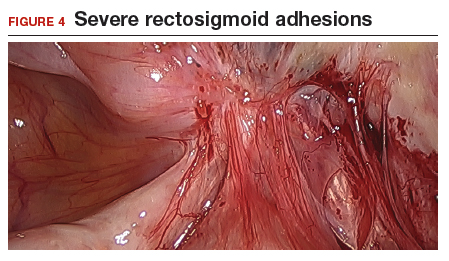
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.


Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
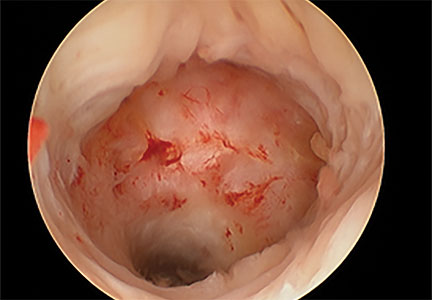
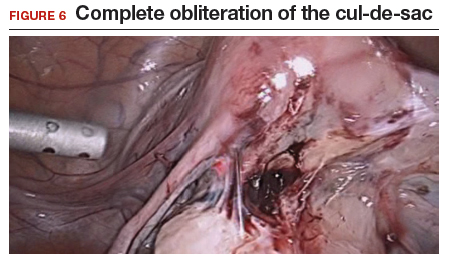
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.
Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
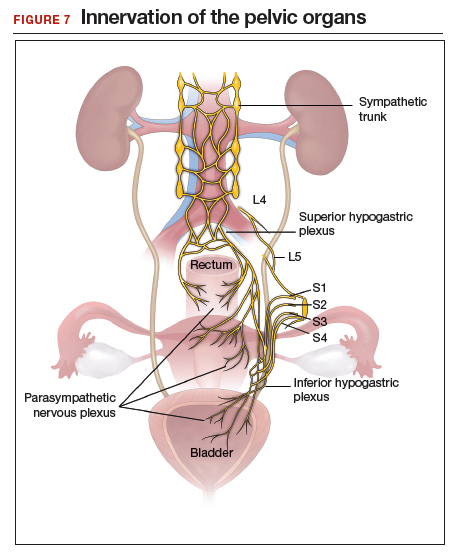
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2
Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.





Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.

Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2

Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.





Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.

Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2

Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
Genitourinary endometriosis: Diagnosis and management
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18
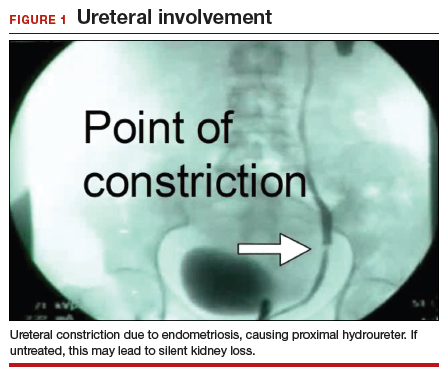
Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36
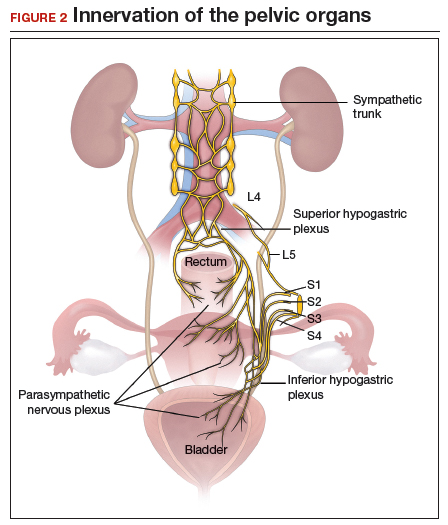
Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43
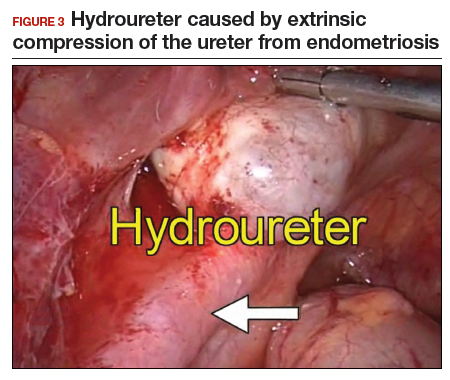
Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18

Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36

Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43

Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
Endometriosis is a benign disease characterized by endometrial glands and stroma outside of the uterine cavity. It is commonly associated with pelvic pain and infertility. Ectopic endometrial tissue is predominantly located in the pelvis, but it can appear anywhere in the body, where it is referred to as extragenital endometriosis. The bowel and urinary tract are the most common sites of extragenital endometriosis.1
Laparoscopic management of extragenital endometriosis has been described since the 1980s.2 However, laparoscopic management of genitourinary endometriosis is still not widespread.3,4 Physicians are often unfamiliar with the signs and symptoms of genitourinary endometriosis and fail to consider it when a patient presents with bladder pain or hematuria, which may or may not be cyclic. Furthermore, many gynecologists do not have the experience to correctly identify the various forms of endometriosis that may appear on the pelvic organ, including the serosa and peritoneum, as variable colored spots, blebs, lesions, or adhesions. Many surgeons are also not adequately trained in the advanced laparoscopic techniques required to treat genitourinary endometriosis.4
In this article, we describe the clinical presentation and diagnosis of genitourinary endometriosis and discuss laparoscopic management strategies with and without robotic assistance.
Clinical presentation and diagnosis of genitourinary endometriosis
While ureteral and bladder endometriosis are both diseases of the urinary tract, they are not always found together in the same patient. The bladder is the most commonly affected organ, followed by the ureter and kidney.3,5,6 Endometriosis of the bladder usually presents with significant lower urinary tract symptoms. In contrast, ureteral endometriosis is usually silent with no apparent urinary symptoms.
Ureteral endometriosis. Cyclic hematuria is present in less than 15% of patients with ureteral endometriosis. Some patients experience cyclic, nonspecific colicky flank pain.7-9 Otherwise, most patients present with the usual symptoms of endometriosis, such as pelvic pain and dysmenorrhea. In a systematic review, Cavaco-Gomes and colleagues described 700 patients with ureteral endometriosis; 81% reported dysmenorrhea, 70% had pelvic pain, and 66% had dyspareunia.10 Rarely, ureteral endometriosis can result in silent kidney loss if the ureter becomes severely obstructed without treatment.11,12
Continue to: The lack of symptoms makes...
The lack of symptoms makes the early diagnosis of ureteral endometriosis difficult. As with all types of endometriosis, histologic evaluation of a biopsy sample is diagnostic. Multiple imaging modalities have been used to preoperatively diagnose ureteral involvement, including computed tomography,13 magnetic resonance imaging (MRI),14 intravenous pyelogram (IVP), and pelvic ultrasonography. However, each of these imaging modalities is limited in both sensitivity and the ability to characterize depth of tissue invasion.
In a study of 245 women undergoing pelvic ultrasonography, Pateman and colleagues reported that an experienced sonographer was able to visualize the bilateral ureters in 93% of cases.15 Renal ultrasonography is indicated in any woman suspected of having genitourinary tract involvement with the degree of hydroureter and level of obstruction noted during the exam.16
In our group, we perform renography to assess kidney function when hydroureter is noted preoperatively. Studies suggest that if greater than 10% of normal glomerular filtration rate remains, the kidney is considered salvageable, and near-normal function often returns following resection of disease. If preoperative kidney function is noted to be less than 10%, consultation with a nephrologist for possible nephrectomy is warranted.
We find that IVP is often helpful for preoperatively identifying the level and degree of ureteral involvement, and it also can be used postoperatively to evaluate for ureteral continuity (FIGURE 1). Sillou and colleagues showed MRI to be adequately sensitive for the detection of intrinsic ureteral endometriosis, but they reported that MRI often overestimates the frequency of disease.17 Authors of a 2016 Cochrane review of imaging modalities for endometriosis, including 4,807 women in 49 studies, reported that no imaging test was superior to surgery for diagnosing endometriosis.18 However, the review notably excluded genitourinary tract endometriosis, as surgery is not an acceptable reference standard, given that many surgeons cannot reliably identify such lesions.18

Bladder endometriosis. Unlike patients with ureteral endometriosis, those with bladder endometriosis are typically symptomatic and experience dysuria, hematuria, urinary frequency, and suprapubic tenderness.7,19 Urinary tract infection, interstitial cystitis, and cancer must be considered in the differential diagnosis. Urinalysis and urine culture should be performed, and other diagnostic procedures such as ultrasonography, MRI, and cystoscopy should be considered to evaluate for endometriosis of the bladder.
Ultrasound and MRI of the bladder both demonstrate a high specificity for detecting bladder endometriosis, but they lack sensitivity for lesions less than 3 cm.20 Deep infiltrating endometriosis of the bladder can be identified at the time of cystoscopy, which can assist in determining the need for ureteroneocystostomy if lesions are within 2 cm of the urethral opening.20 Cystoscopy also allows for biopsy to be performed if underlying malignancy is of concern.19
In our group, when bladder endometriosis is suspected, we routinely perform preoperative bladder ultrasonography to identify the lesion and plan to perform intraoperative cystoscopy at the time of laparoscopic resection.19,21
Continue to: Treatment...
Treatment
Medical management
Empiric medical therapies for endometriosis are centered around the idea that ectopic endometrial tissue responds to treatment in a similar manner as normal eutopic endometrium. The goals of treatment are to reduce or eliminate cyclic menstruation, thereby decreasing peritoneal seeding and suppressing the growth and activity of established ectopic implants. Medical therapy commonly involves the use of gonadotropin-releasing hormone agonists or antagonists, danazol, combined oral contraceptives, progestins, and aromatase inhibitors.
Medical therapy is commonly employed for patients with mild or early-stage disease and in those who are poor surgical candidates or decline surgery. Medical management alone clearly is contraindicated in the setting of ureteral obstruction and—in our opinion—may not be a good option for those with endometriosis of the ureter, given the potential for recurrence and potential serious sequelae of reduced renal function.22 Therefore, surgery has become the standard approach to therapy for mild to moderate cases of ureteral endometriosis.3
Medical therapy for patients with endometriosis of the bladder is generally considered a temporary solution as hormonal suppression, with its associated adverse effects, must be maintained throughout menopause. However, if endometriosis lesions lie within close proximity to the trigone, medical management is preferred, as surgical excision in the area of the trigone may predispose patients to neurogenic bladder and retrograde bladder reflux.23,24
Surgical management
The objectives of surgical treatment for genitourinary tract endometriosis are to excise all visible disease, to prevent or delay recurrence of the disease to the extent possible, and to avoid any further morbidity—in particular, to preserve renal function in cases of ureteral endometriosis—and to avoid iatrogenic injury to surrounding pelvic nervous structures25-27 (FIGURE 2). The surgical approach depends on the technical expertise of the surgeon and the availability of necessary instrumentation. In our experience, laparoscopy with or without robotic assistance is the preferred surgical approach.3,4,6,11,28-32
Others have reported on the benefits of laparoscopy over laparotomy for the surgical management of genitourinary endometriosis. In a review of 61 patients who underwent either robot-assisted laparoscopic (n = 25) or open (n = 41) ureteroneocystostomy (n = 41), Isac and colleagues reported the procedure was longer in the laparoscopic group (279 min vs 200 min, P<.001), but the laparoscopic group had a shorter hospital stay (3 days vs 5 days, P<.001), used fewer narcotics postoperatively (P<.001), and had lower intraoperative blood loss (100 mL vs 150 mL, P<.001).32 No differences in long-term outcomes were observed in either cohort.
In a systematic review of 700 patients undergoing laparoscopic surgery for ureteral endometriosis, Cavaco-Gomes and colleagues reported that conversion to laparotomy occurred in only 3% to 7% of cases.10 In instances of ureteral endometriosis, laparoscopy provides greater visualization of the intraperitoneal contents over laparotomy, enabling better evaluation and treatment of lesions.3,29,33,34 Robot-assisted laparoscopy provides the additional advantages of 3D visualization, potential for an accelerated learning curve over traditional laparoscopy, improvement in dissection technique, and ease of suturing technique.6,35,36

Continue to: Extrinsic disease...
Extrinsic disease. In our group, we perform ureterolysis for extrinsic disease.25 The peritoneal incision is made in an area unaffected by endometriosis. Using the suction irrigator, a potential space is developed under the serosa of the ureter by injecting normal saline or lactated Ringer’s solution. By creating a fluid barrier between the serosa and underlying tissues, the depth of surgical incision and lateral thermal spread are minimized. Grasping forceps are used to peel the peritoneum away.25,37,38
Intrinsic disease. Unlike extrinsic disease, intrinsic disease can infiltrate the muscularis, lamina propria, and ureteral lumen, resulting in proximal dilation of the ureter with strictures.8 In this situation, ureteral compromise is likely and resection of the ureter is indicated3,28 (FIGURE 3). Intrinsic disease can be suggested by preoperative imaging or when there is evidence of deep infiltrating disease on physical exam, such as rectovaginal nodularity.30,39 When intrinsic ureteral disease is known, consultation with a urologist to plan a joint procedure is advisable. The procedure chosen to re-establish a functional ureter following resection depends on the location and extent of the involved ureter. Resection in close proximity to the bladder may be repaired by ureteroneocystostomy with or without psoas hitch,30,39,40 whereas resection of more proximal ureter may be repaired using Boari flap, ileal interposition, or autotransplantation. Lesions in the upper third or middle ureter may be repaired using ureterouretral anastomosis.6,7,30,41-43

Continue to: Bladder endometriosis...
Bladder endometriosis. Surgical treatment for bladder endometriosis depends on the depth of invasion and the location of the lesion (FIGURE 4). Lesions of the superficial aspect of the bladder identified at the time of laparoscopy can be treated with either excision or fulguration28,35,44 In our group, we perform excision over fulguration to remove the entire lesion and obtain a pathologic diagnosis. Deeper lesions involving the detrusor muscle are likely to be an endometrioma of the bladder. In these cases, laparoscopic excision is recommended.7 Rarely, lesions close to the interureteric ridge may require ureteroneocystostomy.19,45
In our experience, laparoscopic resection of bladder endometriomas is associated with better results in terms of symptom relief, progression of disease, and recurrence risk compared with other approaches. When performing laparoscopic excision of bladder lesions, we concomitantly evaluate the bladder lesion via cystoscopy to ensure adequate margins are obtained. Double-J stent placement is advised when lesions are within 2 cm of the urethral meatus to ensure ureteral patency during the postoperative period.45 A postoperative cystogram routinely is performed 7 to 14 days after surgery to ensure adequate repair prior to removing the urinary catheter.9,25,46,47

Postsurgical follow-up
Follow-up after treatment of genitourinary tract endometriosis should include monitoring the patient for symptoms of recurrence. Regular history and physical examination, renal function testing, and, in some instances, pelvic ultrasonography, all have a role in surveillance for recurrent ureteric disease. IVP or MRI may be warranted if a recurrence is suspected. A high index of suspicion should be maintained on the part of the clinician to avoid the devastating consequences of silent kidney loss. Patients should be counseled about the risk of disease recurrence, especially in those not undergoing postoperative hormonal suppression.
In conclusion
While endometriosis of the genitourinary tract is rare, patients can experience significant morbidity. Medical management of the disease is often limited by substantial adverse effects that limit treatment duration and is best used postoperatively after excision. An adequate physical exam and preoperative diagnostic imaging can be employed to characterize the extent of disease. When extensive disease involving the ureter is suspected, preoperative consultation with a urologist is encouraged to plan a multidisciplinary approach to surgical resection.
The ideal approach to surgery is laparoscopic resection with or without robotic assistance. Treatment of ureteral disease most commonly involves ureterolysis for cases of extrinsic disease but may require total resection of the ureter with concurrent ureteral reconstruction when disease is intrinsic to the ureter. Surgery for bladder endometriosis depends on the depth of invasion and location of the lesion. Superficial bladder lesions can be treated with fulguration or excision, while deeper lesions involving the detrusor muscle require excision. Lesions in close proximity to the interureteric ridge may require ureteroneocystostomy. Follow-up after excisional procedures involves monitoring the patient for signs and symptoms of disease recurrence, especially in cases of ureteral involvement, to avoid the devastating consequences of silent kidney loss.
The definitive cause of endometriosis remains unknown, but several prominent theories have been proposed.
Sampson's theory of retrograde menstruation through the fallopian tubes is the most well-known theory,1 although Schron had acknowledged a similar thought 3 centuries before.2 This theory posits that refluxed endometrial cells enter the abdominal cavity and invade the peritoneum, developing a blood supply necessary for survival and growth. Early reports supported this theory by suggesting that women with genital tract obstruction have a higher incidence of endometriosis.3,4 However, it was later confirmed that women without genital tract obstruction have a similar incidence of retrograde menstruation. In fact, up to 90% of women are found to have retrograde menstruation, but only 10% develop endometriosis. This suggests that once endometrial cells have escaped the uterine cavity, other events are necessary for endometrial cells to implant and survive.3,5 Other evidence to support the theory of retrograde menstruation is the observation that endometriosis is most commonly observed in the dependent portions of the pelvis, on the ovaries, in the anterior and posterior cul-de-sacs, and on the uterosacral ligament.6
The coelomic metaplasia theory holds that endometriosis results from spontaneous metaplastic change to mesothelial cells derived from the coelomic epithelium (located in the peritoneum and the pleura) upon exposure to menstrual effluent or other stimuli.7 Evidence for this theory is supported by the observation that intact endometrial cells have no access to the thoracic cavity in the absence of anatomical defect; therefore, the implantation theory cannot explain cases of pleural or pulmonary endometriosis.
Immune dysregulation also has been invoked to explain endometriosis implants both inside and outside the genitourinary tract. Studies have shown a higher incidence of endometriosis in women with other autoimmune disorders, including hypothyroidism, chronic fatigue syndrome, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, and multiple sclerosis as well as in women with allergies, asthma, and eczema.8 In such women, dysregulation of the innate and adaptive immune system might promote the disease by inhibiting apoptosis of ectopic endometrial cells and by promoting their attachment, invasion, and proliferation into healthy peritoneum through the secretion of various growth factors and cytokines.9,10
Other possible theories that might explain the pathogenesis of endometriosis exist.11-13 While each theory has documented supporting evidence, no single theory currently accounts for all cases of endometriosis. It is likely, then, that endometriosis is a multifactorial disease with a combination of immune dysregulation, molecular abnormalities, genetic and epigenetic factors, and environmental exposures involved in its pathogenesis.
References
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422-469.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Halme J, Hammond MG, Hulka JF, et al. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151-154.
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39-43.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511-519.
- Vercellini P, Chapron C, Fedele L, et al. Evidence for asymmetric distribution of lower intestinal tract endometriosis. BJOG. 2004;111:1213-1217.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715-2724.
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1-10.
- Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955: 159-173; discussion 199-200, 396-406.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;252-258.
- Buka NJ. Vesical endometriosis after cesarean section. Am J Obstet Gynecol. 1988;158:1117-1118.
- Price DT, Maloney KE, Ibrahim GK, et al. Vesical endometriosis: report of two cases and review of the literature. Urology. 1996;48:639-643.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Nezhat C, Crowgey SR, Garrison GP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Bosev D, Nicoll LM, Bhagan L, et al. Laparoscopic management of ureteral endometriosis: the Stanford University hospital experience with 96 consecutive cases. J Urol. 2009;182:2748-2752.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1-6.
- Nezhat C, Modest AM, King LP. The role of the robot in treating urinary tract endometriosis. Curr Opin Obstet Gynecol. 2013;25:308-311.
- Comiter CV. Endometriosis of the urinary tract. Urol Clin North Am. 2002;29:625-635.
- Gustilo-Ashby AM, Paraiso MF. Treatment of urinary tract endometriosis. J Minim Invasive Gynecol. 2006;13:559-565.
- Berlanda N, Somigliana E, Frattaruolo MP, et al. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gyneocol Reprod Biol. 2017;209:67-71.
- Cavaco-Gomes J, Martinho M, Gilabert-Aguilar J, et al. Laparoscopic management of ureteral endometriosis: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;210:94-101.
- Nezhat C, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992;148:865-868.
- Nezhat C, Paka C, Gomaa M, et al. Silent loss of kidney secondary to ureteral endometriosis. JSLS. 2012;16:451-455.
- Iosca S, Lumia D, Bracchi E, et al. Multislice computed tomography with colon water distention (MSCT-c) in the study of intestinal and ureteral endometriosis. Clin Imaging. 2013;37(6):1061-1068.
- Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611-621.
- Pateman K, Mavrelos D, Hoo WL, et al. Visualization of ureters on standard gynecological transvaginal scan: a feasibility study. Ultrasound Obstet Gynecol. 2013;41:696-701.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318-332.
- Sillou S, Poirée S, Millischer AE, et al. Urinary endometriosis: MR imaging appearance with surgical and histological correlations. Diagn Interv Imaging. 2015;96:373-381.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Nezhat CH, Malik S, Osias J, et al. Laparoscopic management of 15 patients with infiltrating endometriosis of the bladder and a case of primary intravesical endometrioid adenosarcoma. Fertil Steril. 2002;78:872-875.
- Kolodziej A, Krajewski W, Dolowy L, et al. Urinary tract endometriosis. Urol J. 2015;12:2213-2217.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013;265.
- Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164-166.
- Hastings JC, Van Winkle W, Barker E, et al. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet. 1975;140:933-937.
- Cornell KK. Cystotomy, partial cystectomy, and tube cystostomy. Clin Tech Small Anim Pract. 2000;15:11-16.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013.
- Uccella S, Cromi A, Casarin J, et al. Laparoscopy for ureteral endometriosis: surgical details, long-term follow-up, and fertility outcomes. Fertil Steril. 2014;102:160-166.e2.
- Knabben L, Imboden S, Fellmann B, et al. Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril. 2015;103:147-152.
- Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
- Nezhat CH, Nezhat F, Seidman D, et al. Laparoscopic ureteroureterostomy: a prospective follow-up of 9 patients. Prim Care Update Ob Gyns. 1998;5:200.
- Nezhat CH, Bracale U, Scala A, et al. Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3-7.
- Nezhat C, Lewis M, Kotikela S, et al. Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril. 2010;94:2758-2760.
- Isac W, Kaouk J, Altunrende F, et al. Robotic-assisted ureteroneocytostomy: techniques and comparative outcomes. J Endourol. 2013;27:318-323.
- Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80(3 pt 2):543-544.
- De Cicco C, Ussia A, Koninckx PR. Laparoscopic ureteral repair in gynaecological surgery. Curr Opin Obstet Gynecol. 2011;23:296-300.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Fadhlaoui A, Gillon T, Lebbi I, et al. Endometriosis and vesico-sphincteral disorders. Front Surg. 2015;2:23.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Nezhat C, Winer W, Nezhat FA. Comparison of the CO2, argon, and KTP/532 lasers in the videolaseroscopic treatment of endometriosis. J Gynecol Surg. 2009;41-47.
- Azioni G, Bracale U, Scala A, et al. Laparoscopic ureteroneocytostomy and vesicopsoas hitch for infiltrative ureteral endometriosis. Minim Invasive Ther Allied Technol. 2010;19:292-297.
- Stepniewska A, Grosso G, Molon A, et al. Ureteral endometriosis: clinical and radiological follow-up after laparoscopic ureterocystoneostomy. Hum Reprod. 2011;26:112-116.
- Nezhat CH, Nezhat FR, Freiha F, et al. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
- Scioscia M, Molon A, Grosso G, et al. Laparoscopic management of ureteral endometriosis. Curr Opin Obstet Gynecol. 2009;21:325-328.
- Antonelli A. Urinary tract endometriosis. Urologia. 2012;79:167-170.
- Camanni M, Bonino L, Delpiano EM, et al. Laparoscopic conservative management of ureteral endometriosis: a survey of eighty patients submitted to ureterolysis. Reprod Biol Endocrinol. 2009;7:109.
- Chapron C, Bourret A, Chopin N, et al. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod. 2010;25:884-889.
- Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis: a report of two cases. Obstet Gynecol. 1993;81(5 pt 2):882-884.
- Bourdel N, Cognet S, Canis M, et al. Laparoscopic ureteroneocystostomy: be prepared! J Minim Invasive Gynecol. 2015;22:827-833.
- Page B. Camran Nezhat and the Advent of Advanced Operative Video-laparoscopy. In: Nezhat C, ed. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-187.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Published by American College of Surgeons 2012. Tampa, FL: Faircount Media Group; 2013.
- Kelley WE. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS: J Soc Laparoendoscopic Surgeons. 2008;12:351-357.
A patient with severe adenomyosis requests uterine-sparing surgery
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.
Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25
Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9
References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.
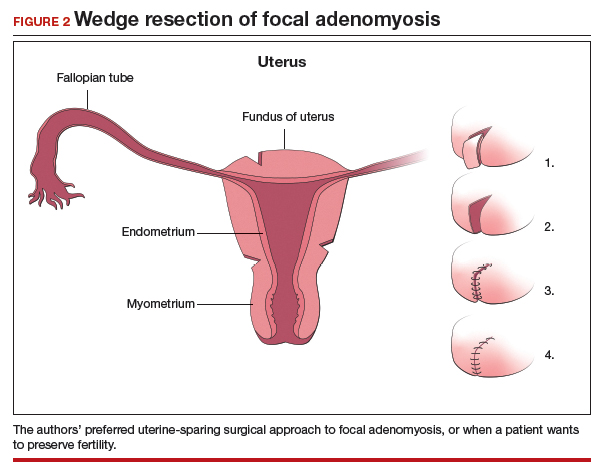
CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.

Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25

Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9

References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.

CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
CASE
A 28-year-old patient presents for evaluation and management of her chronic pelvic pain, dysmenorrhea, and menorrhagia. She previously tried ibuprofen with no pain relief. She also tried oral and long-acting reversible contraceptives but continued to be symptomatic. She underwent pelvic sonography, which demonstrated a large globular uterus with myometrial thickening and myometrial cysts with increased hypervascularity. Subsequent magnetic resonance imaging indicated a thickened junctional zone. Feeling she had exhausted medical manegement options with no significant improvement, she desired surgical treatment, but wanted to retain her future fertility. As a newlywed, she and her husband were planning on building a family so she desired to retain her uterus for potential future pregnancy.
How would you address this patient’s disruptive symptoms, while affirming her long-term plans by choosing the proper intervention?
Adenomyosis is characterized by endometrial-like glands and stroma deep within the myometrium of the uterus and generally is classified as diffuse or focal. This common, benign gynecologic condition is known to cause enlargement of the uterus secondary to stimulation of ectopic endometrial-like cells.1-3 Although the true incidence of adenomyosis is unknown because of the difficulty of making the diagnosis, prevalence has been variously reported at 6% to 70% among reproductive-aged women.4,5
In this review, we first examine the clinical presentation and diagnosis of adenomyosis. We then discuss clinical indications for, and surgical techniques of, adenomyomectomy, including our preferred uterine-sparing approach for focal disease or when the patient wants to preserve fertility: video laparoscopic resection with or without robotic assistance, aided by minilaparotomy when indicated.

Treatment evolved in a century and a half
Adenomyosis was first described more than 150 years ago; historically, hysterectomy was the mainstay of treatment.2,6 Conservative surgical treatment for adenomyosis has been reported since the early 1950s.6-8 Surgical treatment initially became more widespread following the introduction of wedge resection, which allowed for partial excision of adenomyotic nodules.9
More recent developments in diagnostic technologies and capabilities have allowed for the emergence of additional uterine-sparing and minimally invasive surgical treatment options for adenomyosis.3,10 Although the use of laparoscopic approaches is limited because a high level of technical skill is required to undertake these procedures, such approaches are becoming increasingly important as more and more patients seek fertility conservation.11-13
How does adenomyosis present?
Adenomyosis symptoms commonly consist of abnormal uterine bleeding and dysmenorrhea, affecting approximately 40% to 60% and 15% to 30% of patients with the condition, respectively.14 These symptoms are considered nonspecific because they are also associated with other uterine abnormalities.15 Although menorrhagia is not associated with extent of disease, dysmenorrhea is associated with both the number and depth of adenomyotic foci.14
Other symptoms reported with adenomyosis include chronic pelvic pain, dyspareunia, as well as infertility. Note, however, that a large percentage of patients are asymptomatic.16,17
On physical examination, patients commonly exhibit a diffusely enlarged, globular uterus. This finding is secondary to uniform hyperplasia and hypertrophy of the myometrium, caused by stimulation of ectopic endometrial cells.2 A subset of patients experience significant uterine tenderness.18 Other common findings associated with adenomyosis include uterine abnormalities, such as leiomyomata, endometriosis, and endometrial polyps.
Continue to: Two-pronged route to diagnosis and a differential...
Two-pronged route to diagnosis and a differential
Histology
Adenomyosis is definitively diagnosed based on histologic findings of endometrial-like tissue within the myometrium. Historically, histologic analysis was performed on specimens following hysterectomy but, more recently, has utilized specimens obtained from hysteroscopic and laparoscopic myometrial biopsies.19 Importantly, although hysteroscopic and laparoscopic biopsies are taken under direct visualization, there are no pathognomonic signs for adenomyosis; a diagnosis can therefore be missed if adenomyosis is not present at biopsied sites.1 The sensitivity of random biopsy at laparoscopy has been found to be as low as 2% to as high as 56%.20
Imaging
Imaging can be helpful in clinical decision making and to guide the differential diagnosis. Transvaginal ultrasonography (TVUS) is often the first mode of imaging used for the investigation of abnormal uterine bleeding or pelvic pain. Diagnosis by TVUS is difficult because the modality is operator dependent and standard diagnostic criteria are lacking.5
The most commonly reported ultrasonographic features of adenomyosis are21,22:
- a globally enlarged uterus
- asymmetry
- myometrial thickening with heterogeneity
- poorly defined foci of hyperechoic regions, surrounded by hypoechoic areas that correspond to smooth-muscle hyperplasia
- myometrial cysts.
Doppler ultrasound examination in patients with adenomyosis reveals increased flow to the myometrium without evidence of large blood vessels.
3-dimensional (3-D) ultrasonography. Integration of 3-D ultrasonography has allowed for identification of the thicker junctional zone that suggests adenomyosis. In a systematic review of the accuracy of TVUS, investigators reported a pooled sensitivity and specificity for 2-dimensional ultrasonography of 83.8% and 63.9%, respectively, and a pooled sensitivity and specificity for 3-dimensional ultrasonography of 88.9% and 56.0%, respectively.22
Magnetic resonance imaging (MRI) is also used in the evaluation of adenomyosis. Although MRI is considered a more accurate diagnostic modality because it is not operator dependent, expense often prohibits its use in the work-up of abnormal uterine bleeding and chronic pelvic pain.2,23
The most commonly reported MRI findings in adenomyosis include a globular or asymmetric uterus, heterogeneity of myometrial signal intensity, and thickening of the junctional zone24 (FIGURE 1). In a systematic review, researchers reported a pooled sensitivity and specificity of 77% and 89%, respectively, for the diagnosis of adenomyosis using MRI.25

Approaches to treatment
Medical management
No medical therapies or guidelines specific to the treatment of adenomyosis exist.9 Often, nonsteroidal anti-inflammatory drugs (NSAIDs) are employed to combat cramping and pain associated with increased prostaglandin levels.26 A systematic review found that NSAIDs are significantly better at treating dysmenorrhea than placebo alone.26
Moreover, adenomyosis is an estrogen-dependent disease; consequently, many medical treatments are targeted at suppressing the hypothalamic–pituitary–ovarian axis and inducing endometrial atrophy. Medications commonly used (off-label) for this effect include combined or progestin-only oral contraceptive pills, gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine devices, danazol, and aromatase inhibitors.
Use of a GnRH agonist, such as leuprolide, is limited to a short course (<6 months) because menopausal-like symptoms, such as hot flashes, vaginal atrophy, and loss of bone-mineral density, can develop.16 Symptoms of adenomyosis often return upon cessation of hormonal treatment.1
Novel therapies are under investigation, including GnRH antagonists, selective progesterone-receptor modulators, and antiplatelet therapy.27
Although there are few data showing the effectiveness of medical therapy on adenomyosis-specific outcomes, medications are particularly useful in patients who are poor surgical candidates or who may prefer not to undergo surgery. Furthermore, medical therapy has considerable use in conjunction with surgical intervention; a prospective observational study showed that women who underwent GnRH agonist treatment following surgery had significantly greater improvement of their dysmenorrhea and menorrhagia, compared with those who underwent surgery only.28 In addition, preoperative administration of a GnRH agonist or danazol several months prior to surgery has been shown to reduce uterine vascularity and, thus, blood loss at surgery.29,30
- Adenomyosis is common and benign, but remains underdiagnosed because of a nonspecific clinical presentation and lack of standardized diagnostic criteria.
- Adenomyosis can cause significant associated morbidity: dysmenorrhea, heavy menstrual bleeding, chronic pelvic pain, and infertility.
- High clinical suspicion warrants evaluation by imaging.
- Medical management is largely aimed at ameliorating symptoms.
- A patient who does not respond to medical treatment or does not desire pregnancy has a variety of surgical options; the extent of disease and the patient’s wish for uterine preservation guide the selection of surgical technique.
- Hysterectomy is the definitive treatment but, in patients who want to avoid radical resection, techniques developed for laparotomy are available, to allow conservative resection using laparoscopy.
- Ideally, surgery is performed using a combined laparoscopy and minilaparotomy approach, after appropriate imaging.
Continue to: Surgery
Surgery
The objective of surgical management is to ameliorate symptoms in a conservative manner, by excision or cytoreduction of adenomyotic lesions, while preserving, even improving, fertility.3,11,31 The choice of procedure depends, ultimately, on the location and extent of disease, the patient’s desire for uterine preservation and fertility, and surgical skill.3
Historically, hysterectomy was used to treat adenomyosis; for patients declining fertility preservation, hysterectomy remains the definitive treatment. Since the early 1950s, several techniques for laparotomic reduction have been developed. Surgeries that achieve partial reduction include:
Wedge resection of the uterine wall entails removal of the seromuscular layer at the identified location of adenomyotic tissue, with subsequent repair of the remaining muscular and serosal layers surrounding the wound.3,32 Because adenomyotic tissue can remain on either side of the incision in wedge resection, clinical improvement in symptoms of dysmenorrhea and menorrhagia are modest, and recurrence is possible.7
Modified reduction surgery. Modifications of reduction surgery include slicing adenomyotic tissue using microsurgery and partial excision.33
Transverse-H incision of the uterine wall involves a transverse incision on the uterine fundus, separating serosa and myometrium, followed by removal of diseased tissue using an electrosurgical scalpel or scissors. Tensionless suturing is used to close the myometrial layers in 1 or 2 layers to establish hemostasis and close the defect; serosal flaps are closed with subserosal interrupted sutures.34 Data show that, following surgery with this technique, 21.4% to 38.7% of patients who attempt conception achieve clinical pregnancy.7
Complete, conservative resection in cases of diffuse and focal adenomyosis is possible using the triple-flap method, in which total resection is achieved by removing diseased myometrium until healthy, soft tissue—with normal texture, color, and vascularity—is reached.2 Repair with this technique reduces the risk of uterine rupture by reconstructing the uterine wall using a muscle flap prepared by metroplasty.7 In a study of 64 women who underwent triple-flap resection, a clinical pregnancy rate of 74% and a live birth rate of 52% were reported.7
Minimally invasive approaches. Although several techniques have been developed for focal excision of adenomyosis by laparotomy,7 the trend has been toward minimally invasive surgery, which reduces estimated blood loss, decreases length of stay, and reduces adhesion formation—all without a statistically significant difference in long-term clinical outcomes, compared to other techniques.35-39 Furthermore, enhanced visualization of pelvic organs provided by laparoscopy is vital in the case of adenomyosis.3,31
How our group approaches surgical management. A challenge in laparoscopic surgery of adenomyosis is extraction of an extensive amount of diseased tissue. In 1994, our group described the use of simultaneous operative laparoscopy and minilaparotomy technique as an effective and safe alternative to laparotomy in the treatment of myomectomy6; the surgical principles of that approach are applied to adenomyomectomy. The technique involves treatment of pelvic pathology with laparoscopy, removal of tissue through the minilaparotomy incision, and repair of the uterine wall defect in layers.
How adenomyosis originates is not fully understood. Several theories have been proposed, however (including, more prominently, the first 2 below):
Invasion theory. The endometrial basalis layer invaginates and invades the myometrium1,2 (FIGURE); the etiology of invagination remains unknown.
Reaction theory. Myometrial weakness or dysfunction, brought on by trauma from previous uterine surgery or pregnancy, could predispose uterine musculature to deep invasion.3
Metaplasia theory. Adenomyosis is a result of metaplasia of pluripotent Müllerian rests.
Müllerian remnant theory. Related to the Müllerian metaplasia theory, adenomyosis is formed de novo from 1) adult stem cells located in the endometrial basalis that is involved in the cyclic regeneration of the endometrium4-6 or 2) adult stem cells displaced from bone marrow.7,8
Once adenomyosis is established, it is thought to progress by epithelial–mesenchymal transition,2 a process by which epithelial cells become highly motile mesenchymal cells that are capable of migration and invasion, due to loss of cell–cell adhesion properties.9

References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol.2016; 23:164-185.
- García-Solares J, Donnez J, Donnez O, et al. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril.2018;109:371-379.
- Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312-322.
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87-101.
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738-1750.
- Schwab KE, Chan RWS, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124-1130.
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106-115.
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16:126-139.
- Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438-1449.
Continue to: In 57 women who underwent…
In 57 women who underwent this procedure, the mean operative time was 127 minutes; average estimated blood loss was 267 mL.40 Overall, laparoscopy with minilaparotomy was found to be a less technically difficult technique for laparoscopic myomectomy; allowed better closure of the uterine defect; and might have required less time to perform.3
We therefore advocate video laparoscopic wedge resection with or without robotic assistance, aided by minilaparotomy when necessary for safe removal of larger adenomyomas, as the preferred uterine-sparing surgical approach for focal adenomyosis or when the patient wants to preserve fertility (FIGURE 2). We think that this technique allows focal adenomyosis to be treated by wedge resection of the diseased myometrium, with subsequent closure of the remaining myometrial defect using a barbed V-Loc (Medtronic, Minneapolis, Minnesota) delayed absorbable suture in layers (FIGURE 3). Minilaparotomy can be utilized when indicated to aid removal of the resected myometrial specimen.
In our extensive experience, we have found that this technique provides significant relief of symptoms and improvements in fertility outcomes while minimizing surgical morbidity.

CASE Resolved
The patient underwent successful wedge resection of her adenomyosis by laparoscopy. She experienced nearly complete resolution of her symptoms of dysmenorrhea, menorrhagia, and pelvic pain. She retained good uterine integrity. Three years later, she and her husband became parents when she delivered their first child by cesarean delivery at full term. After she completed childbearing, she ultimately opted for minimally invasive hysterectomy.

The authors would like to acknowledge Mailinh Vu, MD, Fellow at Camran Nezhat Institute, for reviewing and editing this article.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428-437.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. Cambridge, UK: Cambridge University Press; 2013.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Azziz R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221-235.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
- Rokitansky C. Ueber Uterusdrsen-Neubildung in Uterus- und Ovarial-Sarcomen. Gesellschaft der Ärzte in Wien. 1860;16:1-4.
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109:406-417.
- Van Praagh I. Conservative surgical treatment for adenomyosis uteri in young women: local excision and metroplasty. Can Med Assoc J. 1965;93:1174-1175.
- Donnez J, Donnez O, Dolmans MM. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil Steril. 2018;109:369-370.
- Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715-719.
- Abbott JA. Adenomyosis and abnormal uterine bleeding (AUB-A)--Pathogenesis, diagnosis, and management. Best Pract Res Clin Obstet Gynaecol. 2017;40:68-81.
- Matalliotakis IM, Katsikis IK, Panidis DK. Adenomyosis: what is the impact on fertility? Curr Opin Obstet Gynecol. 2005;17:261-264.
- Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update. 2003;9:139-147.
- Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol. 2000;95:688-691.
- Weiss G, Maseelall P, Schott LL, et al. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women's Health Across the Nation (SWAN). Fertil Steril. 2009;91:201-206.
- Huang F, Kung FT, Chang SY, et al. Effects of short-course buserelin therapy on adenomyosis. A report of two cases. J Reprod Med. 1999;44:741-744.
- Benson RC, Sneeden VD. Adenomyosis: a reappraisal of symptomatology. Am J Obstet Gynecol. 1958;76:1044-1061.
- Shrestha A, Sedai LB. Understanding clinical features of adenomyosis: a case control study. Nepal Med Coll J. 2012;14:176-179.
- Fernández C, Ricci P, Fernández E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 2007;14:555-556.
- Brosens JJ, Barker FG. The role of myometrial needle biopsies in the diagnosis of adenomyosis. Fertil Steril. 1995;63:1347-1349.
- Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16-24.
- Andres MP, Borrelli GM, Ribeiro J, et al. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol. 2018;25:257-264.
- Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427-2433.
- Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception. 2007;76:195-199.
- Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010; 89:1374-1384.
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;(1):CD001751.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109:398-405.
- Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876-885.
- Wood C, Maher P, Woods R. Laparoscopic surgical techniques for endometriosis and adenomyosis. Diagn Ther Endosc. 2000;6:153-168.
- Wang CJ, Yuen LT, Chang SD, et al. Use of laparoscopic cytoreductive surgery to treat infertile women with localized adenomyosis. Fertil Steril. 2006;86:462.e5-e8.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Sun A, Luo M, Wang W, et al. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J. 2011;124:1322-1326.
- Fedele L, Bianchi S, Zanotti F, et al. Surgery: Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708-1710.
- Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132-138.
- Operative Laparoscopy Study Group. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Fertil Steril. 1991;55:700-704.
- Luciano AA, Maier DB, Koch EI, et al. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989;74:220-224.
- Lundorff P, Hahlin M, Källfelt B, et al. Adhesion formation after laparoscopic surgery in tubal pregnancy: a randomized trial versus laparotomy. Fertil Steril. 1991;55:911-915.
- Kwack JY, Kwon YS. Laparoscopic surgery for focal adenomyosis. JSLS. 2017;21. pii:e2017.00014.
- Podratz K. Degrees of Freedom: Advances in Gynecological and Obstetrical Surgery. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years 1913-2012. Chicago, IL: American College of Surgeons; 2012.
- Nezhat C, Nezhat F, Bess O, et al. Laparoscopically assisted myomectomy: a report of a new technique in 57 cases. Int J Fertil Menopausal Stud. 1994;39:39-44.
Laparoscopic excision of type I and type II endometriomas

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cesarean scar defect: What is it and how should it be treated?
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).
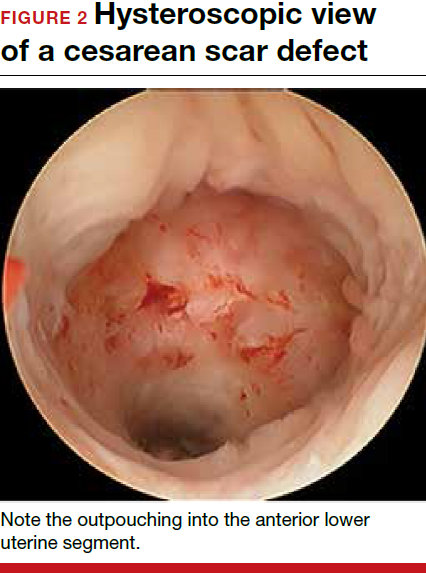
Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.
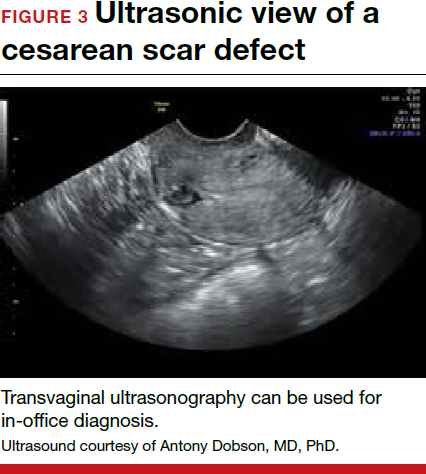
Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9
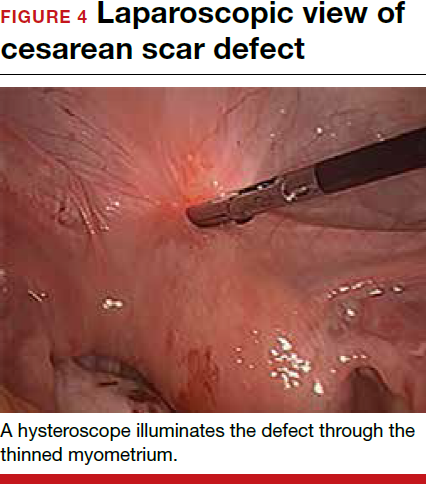
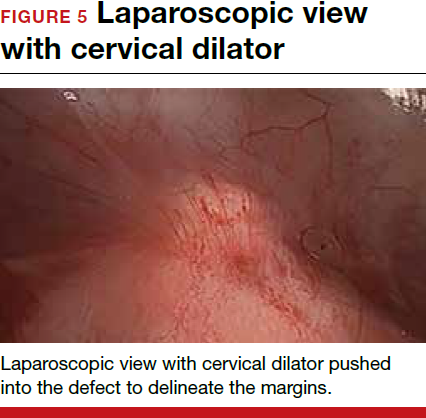
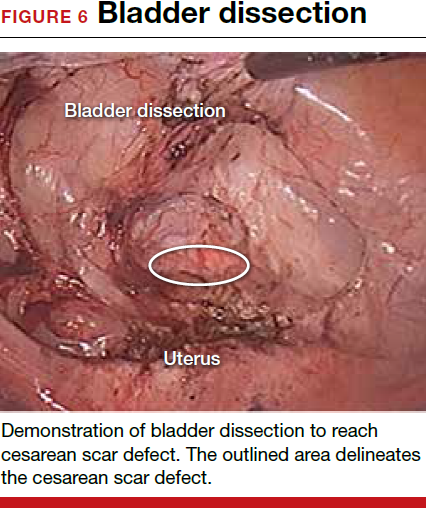
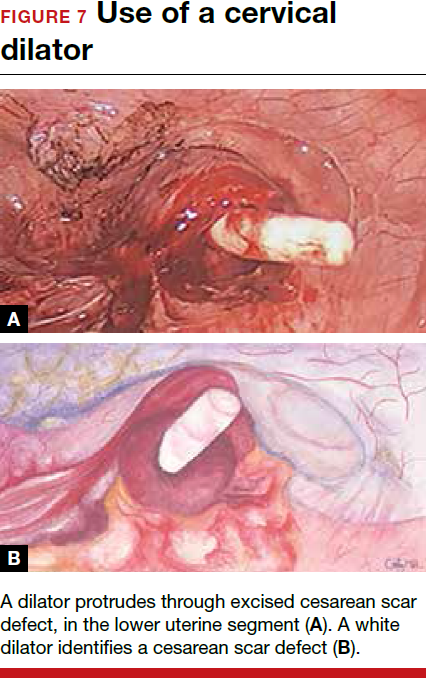
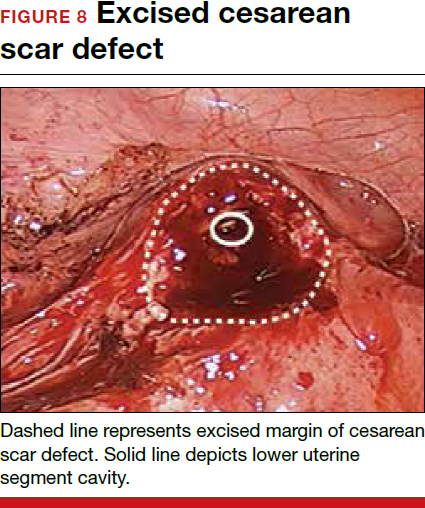
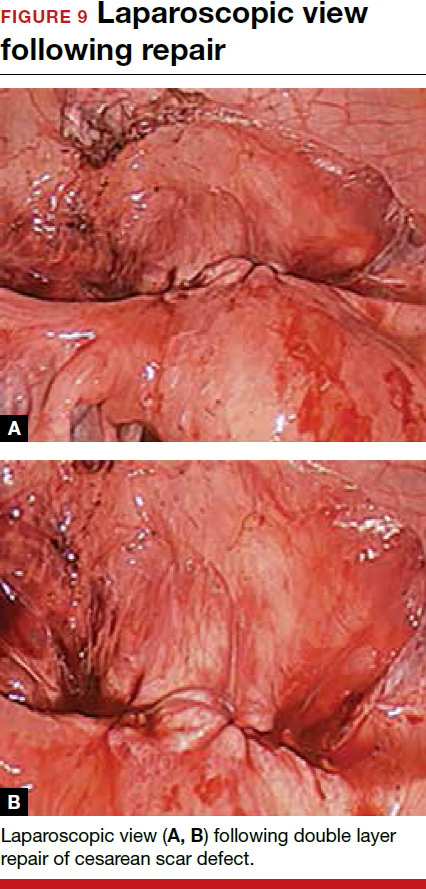
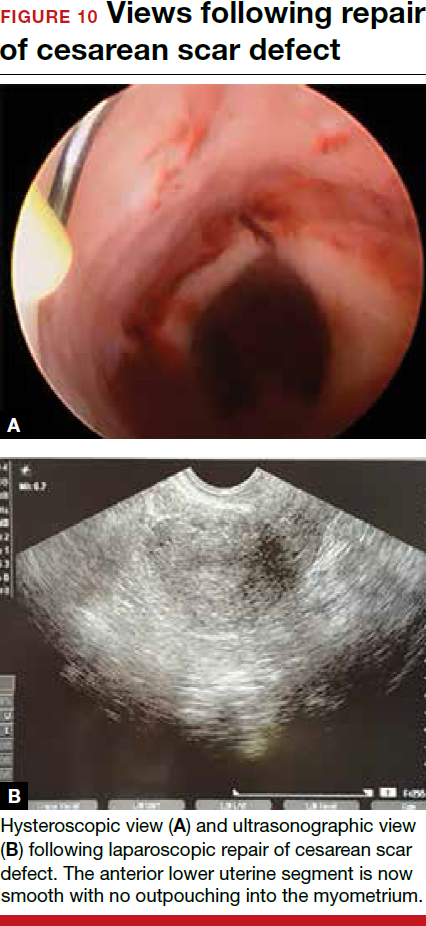
Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).

Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.

Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9







Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).

Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.

Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9







Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Article
Averting complications of laparoscopy: Pearls from 5 patients
To view three clips of surgical pearls for laparoscopy, visit the Video Library.
<huc>Q.</huc>What is the only surgical procedure that is completely safe?
<huc>A.</huc>The surgical procedure that is not performed.
The unfortunate truth is that complications can occur during any operative procedure, despite our best efforts—and laparoscopy is no exception. Being vigilant for iatrogenic injuries, both during and after surgery, and ensuring that repairs are both thorough and timely, are two of our best weapons against major complications, along with meticulous technique and adequate experience.
This article features five cases that illustrate some of the most serious complications of laparoscopy—and how to prevent and manage them.
CASE 1: Surgical patient returns with signs of ureteral injury
A 42-year-old woman with a history of endometriosis undergoes laparoscopic hysterectomy and bilateral salpingo-oophorectomy. She is discharged 2 days later. Two days after that, she returns to the hospital complaining of fluid leaking from the vagina. She has no fever or any other significant complaint or physical findings other than abdominal tenderness, which is to be expected after surgery. A computed tomography (CT) scan with intravenous (IV) contrast reveals left ureteral obstruction near the bladder, with extravasation of contrast media into the abdominal cavity. Further investigation reveals a left ureteral transection.
Could this injury have been avoided? How should it be managed?
Postoperative diagnosis of ureteral injury can be challenging, in part because up to 50% of unilateral cases are asymptomatic. Be on the lookout for this complication in women who have undergone pelvic sidewall dissection or laparoscopic hysterectomy, such as the patient in the case just described. As the number of laparoscopic hysterectomies and retroperitoneal procedures has risen in recent years, so has the rate of ureteral injury, with an incidence of 0.3% to 2%.1,2
Ureteral injury can be caused by ligation, ischemia, resection, transection, crushing, or angulation. Three sites are particularly troublesome: the infundibulopelvic ligament, ovarian fossa, and ureteral tunnel.3,4 In Case 1, injury to the ureter was proximal to the bladder and probably occurred during transection of the uterosacral cardinal ligament complex.
What’s the best preventive strategy?
Meticulous technique is imperative to protect the ureters. This includes adequate visualization, intraperitoneal or retroperitoneal dissection, and early identification of the ureter. In a high-risk patient likely to have distorted anatomy due to severe endometriosis and fibrosis, retroperitoneal dissection of any adhesions or tumor and identification of the ureter are the best ways to avoid injury.
Intraperitoneal identification and dissection of the ureters can be enhanced by hydrodissection and resection of the affected peritoneum.3,4 To create a safe operating plane, make a small opening in the peritoneum below the ureter and inject 50 to 100 mL of lactated Ringer’s solution along the course of the ureter, which will displace it laterally.5
Although neither IV indigo carmine nor ureteral catheterization has been shown to reduce the risk of ureteral injury or identify ligation or thermal injury,3,6 both can help the surgeon identify intraoperative perforation of the ureter. Liberal use of cystoscopy with indigo carmine administration for identification of ureteral flow and ureteral catheterization can be used in potentially high-risk patients. If there is suspicion for devascularization or thermal injury, use prophylactic ureteral stents postoperatively for 2 to 4 weeks.
Don’t hesitate to consult a urologist
In Case 1, the surgeon sought immediate urologic consultation and the patient underwent laparotomy with ureteroneocystotomy without sequelae.
In general, management of ureteral injury depends on its severity and location, as well as the comfort level of the surgeon. Minor injuries are sometimes managed with cystoscopic stent placement, but more severe cases may require operative ureteral repair.
In cases like this one, where ureteral injury occurred in close proximity to the bladder, a ureteroneocystotomy is possible. However, in more cephalad injuries, there may be insufficient residual ureter to allow such a repair. In these cases, a Boari flap may be attempted to use bladder tissue to bridge the gap to the ureteral edge. Rarely, in high ureteral injuries, trans-ureteroureterostomy may be appropriate. This procedure carries the greatest risk, given that both kidneys are reliant on one ureter.
Is laparoscopic repair reasonable?
When surgical intervention is necessary, the choice between laparoscopy and laparotomy depends on the skill and comfort level of the surgeon and the availability of instruments and support team.6,7 That said, ureteral injury is usually treated via laparotomy.1 As operative laparoscopy becomes even more commonplace, reconstruction of the urinary system will increasingly be managed laparoscopically.
Depending on the size and location of the injury, reconstruction may involve ureteral reimplantation with or without a psoas hitch, Boari flap, or primary endtoend anastomosis.8-10
CASE 2: Postoperative symptoms lead to rehospitalization
A 35-year-old patient undergoes laparoscopic ovarian cystectomy and returns home the same day. She is readmitted 72 hours later because of lower abdominal tenderness, worsening nausea and vomiting, and urine-like drainage from her midline suprapubic trocar site. Analysis of the leaking fluid shows high creatinine levels consistent with urine. The patient has no fever and is hemodynamically stable. Examination reveals a moderately distended abdomen with decreased bowel sounds. Hematuria is evident on urine analysis.
Urologic consultation is obtained, and the patient undergoes simultaneous laparoscopy and cystoscopy, during which perforation of the bladder dome is discovered, apparently caused by the mid suprapubic trocar. The bladder is mobilized anteriorly, and both anterior and posterior aspects of the perforation are repaired in one layer laparoscopically.
After continuous drainage with a transurethral Foley catheter for 7 days, cystography shows complete healing of the bladder, and the Foley catheter is removed. The patient recovers completely.
Vesical injury sometimes occurs in patients who have a history of laparotomy, a full bladder at the time of surgery, or displaced anatomy due to pelvic adhesions.11 Although bladder injury is rare, laparoscopy increases the risk. Trocars, uterine manipulators, and blunt instruments can perforate or lacerate the bladder, and energy devices can cause thermal injury. The risk of bladder injury increases during laparoscopic hysterectomy.
Be vigilant about trocar placement and dissection techniques
Accessory trocars can injure a full bladder. Injury can also occur when distorted anatomy from a previous pelvic operation obscures bladder boundaries, making insertion of the midline trocar potentially perilous (FIGURE 1). The Veress needle and Rubin’s cannula can perforate the bladder.11-13 And in the anterior cul-de-sac, adhesiolysis, deep coagulation, laser ablation, or sharp excision of endometriosis implants can predispose a patient to bladder injury.
In women with severe endometriosis, lower-segment myoma, or a history of cesarean section, the bladder is vulnerable to laceration when blunt dissection is used during laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy (LAVH). A vesical injury also can occur at the time of laparoscopic bladder-neck suspension upon entry into, and dissection of, the space of Retzius.
FIGURE 1 A bladder at risk
In this patient with a previous cesarean section, the bladder is adherent to the anterior abdominal wall. Needle mapping in the conventional midline trocar position indicates that the trocar must be relocated to avoid bladder injury.
Intraoperative findings that suggest bladder injury include air in the urinary catheter, hematuria, trocar site drainage of urine, or indigo carmine leakage. Postoperative signs and symptoms include leaking from incisional sites, a mass in the abdominal wall, and abdominal swelling.
Liberal use of cystoscopy or distension of the bladder with 300 to 500 mL of normal saline is recommended whenever there is a suspicion of bladder injury, especially during laparoscopic hysterectomy or LAVH. When a trocar causes the injury, look for both entry and exit punctures, both of which should be treated.
No matter how much care is taken, some bladder injuries, such as vesicovaginal fistulae, become apparent only postoperatively. More rarely, peritonitis or pseudoascites herald the injury. Retrograde cystography may aid identification.
Treatment of bladder injuries
Small perforations recognized intraoperatively may be conservatively managed by postoperative bladder drainage for 5 to 7 days. Most other bladder injuries require prompt intervention. For example, trocar injury to the bladder dome requires one- or two-layer closure followed by 5 to 7 days of urinary drainage. (Both closing and healing are promoted by drainage.)
Laparoscopy or laparotomy? Laparoscopic repair has become increasingly common, and bladder injury is a common complication of LAVH.13,14
CASE 3: Postop pain, tachycardia
A 41-year-old obese woman undergoes laparoscopic cystectomy for an 8-cm left ovarian mass. The abdomen is entered on the second attempt with a long Veress needle. The umbilical trocar is reinserted “several” times because of difficulty opening the peritoneum with the tip of the trocar sheath. The surgical procedure is completed within 2 hours, and the patient is discharged 23 hours later.
The next day, she experiences increasing abdominal pain and presents to the emergency room. Upon admission she reports intermittent chills, but denies nausea and vomiting. She is in mild distress, pale and tachycardic, with a temperature of 96.4°, pulse of 117, respiration rate of 20, blood pressure of 106/64 mm Hg, and oxygen saturation of 92%. She also has a diffusely tender abdomen but normal blood work. Abdominal and chest x-rays show a large right subphrenic air-fluid level that is consistent with free intraperitoneal air, unsurprising given her recent surgery. Bibasilar atelectasis and consolidation are noted on the initial chest x-ray.
During observation over the next 2 days, she remains afebrile and tachycardic, but her shortness of breath becomes progressively worse. Neither spiral CT nor lower-extremity Doppler suggests pulmonary embolism or deep venous thrombosis. Supplemental oxygen, aggressive pain management, albuterol, ipratropium, and acetylcysteine are initiated after pulmonary consultation.
The patient tolerates a regular diet on postoperative day 3 and has a bowel movement on day 5. However, the same day she begins vomiting and reports worsening abdominal pain. CT imaging of the abdomen and pelvis reveals free air in the abdomen and loculated fluid with air bubbles suspicious for intra-abdominal infection and perforated bowel.
Exploratory laparotomy reveals diffuse feculent peritonitis, as well as food particles and contrast media. There is a perforation in the antimesenteric side of the ileum approximately 1.5 feet proximal to the ileocecal valve. This perforation measures approximately 1 cm in diameter and is freely spilling intestinal contents. Small bowel resection is performed to treat the perforation.
Following the surgery, the patient recovers slowly.
Could the bowel perforation have been detected sooner?
Intestinal tract injury is a serious complication, particularly with postoperative diagnosis.15 Damage can occur during insertion of the Veress needle or trocar when the bowel is immobilized by adhesions, or during enterolysis.16 Unrecognized thermal injury can cause delayed bowel injury.
Small-bowel damage often occurs during uncontrolled insertion of the Veress needle or primary umbilical trocar. It also may result from sharp dissection or thermal injury.17,18 Abrasions and lacerations can occur if traction is exerted on the bowel using serrated graspers. When adhesions are dense and tissue planes poorly defined, the risk of laceration due to energy sources or sharp dissection increases.
Be cautious during bowel manipulation. Avoid blunt dissection. Be especially careful when the small bowel is adherent to the anterior abdominal wall (FIGURE 2A), particularly during evaluation of patients with a history of bowel resection, exploratory laparotomy for trauma-related peritonitis, or tumor debulking.
Remove the primary and ancillary cannulas under direct visualization with the laparoscope to prevent formation of a vacuum that can draw bowel into the incision and cause herniation.19
FIGURE 2 Adherent bowel, minor bleeding
A: Veress needle pressure measurements are persistently elevated before primary trocar insertion in this patient, raising the suspicion of adhesive disease from earlier surgery. As a result, the primary trocar is relocated to the left upper quadrant. Inspection confirms that small bowel is adherent to the anterior abdominal wall.
FIGURE 2 Adherent bowel, minor bleeding
B: After the small-bowel adhesions are dissected off the anterior abdominal wall via laparoscopy, a small hematoma is discovered, likely caused by the Veress needle. The patient is managed conservatively and recovers.
The value of open laparoscopy
In open laparoscopy, an abdominal incision is made into the peritoneal cavity so that the trocar can be placed under direct vision, after which the abdomen is insufflated. This approach can prevent bowel injury only when the adhesions and attachments are to the anterior abdominal wall and away from the entry site. When the attachment lies directly beneath the umbilicus, however, open laparoscopy is no guarantee against injury.
When bowel adhesions are severe, use alternative trocar sites such as the left upper quadrant (Palmer’s point) for the Veress needle and primary trocars.5,20,21
The likelihood of perforation can be reduced with preoperative bowel prep when there is a risk of bowel adhesions.
Identifying bowel injury
We recommend routine inspection of the structures beneath the primary trocar upon insertion of the laparoscope to look for injury to the bowel, mesentery, or vascular structures. If adhesions are found, evaluate the area carefully to rule out injury to the bowel or omentum. It may be necessary to change the position of the laparoscope to assess the patient.
Trauma to the intestinal tract can be mechanical or electrical in nature, and each type of trauma creates a distinctive, characteristic pattern. Thermal injury can be subtle and present as simple blanching or a distinct burn and charring. A small hole or obvious tear in the bowel wall can be the result of mechanical injury.22
Benign-appearing, superficial thermal bowel injuries may be managed conservatively.22 Minimal serosal burns (smaller than 5 mm in diameter) can be managed expectantly. Immediate surgical intervention is needed if the area of blanching on the intestinal serosa exceeds 5 mm in diameter or if the burn appears to involve more than the serosa.23
Small-bowel injuries that escape notice intraoperatively generally become apparent 2 to 4 days later, when the patient develops fever, nausea, lower abdominal pain, and anorexia. On postoperative day 5 or 6, the white blood cell (WBC) count rises and earlier symptoms may become worse. Radiography may reveal multiple air and fluid levels—another sign of bowel injury. Be aware that if the patient has clinical symptoms of gastrointestinal injury, even if the WBC count is normal, exploratory laparoscopy or laparotomy is necessary for accurate diagnosis.
Intraoperatively discovered injury
Careful inspection may reveal no leakage or bleeding in the affected area. Small punctures or superficial lacerations seal readily and may not require further treatment (FIGURE 2B), but larger perforations require repair. Straightforward repair is not always possible when the injury is extensive and considerable time has elapsed before it is discovered.
Inspect the intestine thoroughly at the conclusion of a procedure; obvious leakage requires intervention. Repair the small intestine in one or two layers, using the initial row of interrupted sutures to approximate the mucosa and muscularis.24 To lessen the risk of stenosis, close all lacerations transversely when they are smaller than one half the diameter of the bowel. If the laceration exceeds that size, segmental resection and anastomosis are necessary. Resection is prudent if the mesenteric blood supply is compromised.25
When performing one-layer repair of the small bowel, delayed absorbable suture (eg, Vicryl or PDS) or nonabsorbable suture (eg, silk) is recommended.26
At the conclusion of a repair, copiously irrigate the entire abdomen. Place a nasogastric tube only if ileus is anticipated; the tube can be removed when drainage diminishes and active bowel sounds and flatus appear. Do not give anything by mouth until the patient has return of bowel function and active peristalsis. Prescribe prophylactic antibiotics.
Note that peritonitis sometimes develops after repair of the bowel.25 This can be managed with prolonged bowel rest and peripheral or total parenteral nutrition.
Conservative management may be possible
Patients whose symptoms of bowel laceration become apparent after discharge can sometimes be managed conservatively. More than 50% of patients treated conservatively require no surgery.23 Inpatient management consists of monitoring the WBC count, providing hydration and IV antibiotics, and examining the patient every 6 hours, giving nothing by mouth.
When injury is discovered later
If conservative management with observation and bowel rest fails, or the patient complains of severe abdominal pain, vomiting, nausea, obstipation, or signs and symptoms of peritonitis, such as the patient in Case 3, immediate surgical intervention is necessary. When an injury is not detected until some time after initial surgery, resection of all necrotic tissue is mandatory. In most cases, the perforation is managed by segmental resection and reanastomosis. Evaluate the entire small and large bowel to rule out any other injury, and irrigate generously. Bowel rest, parenteral nutrition, and IV antibiotics also are indicated.
Of 36,928 procedures reported by members of the American Association of Gynecologic Laparoscopists, there were two deaths—both caused by unrecognized bowel injury.15
CASE 4: Large-bowel injury precipitates lengthy recovery
A surgeon performs a left laparoscopic salpingo-oophorectomy to remove an 8-cm ovarian endometrioma that is adherent to the rectosigmoid colon of a 40-year-old diabetic woman. Sharp and electrosurgical scissors are used to separate the adnexa from the rectosigmoid colon. No injury is observed, and she is discharged the same day. Four days later, she returns with severe abdominal pain, nausea, vomiting, and fatigue. Lab tests reveal a WBC count of 17,000; a CT scan shows pockets of air beneath the diaphragm, as well as fluid collection suggestive of a pelvic abscess.
Immediate laparotomy is performed, during which the surgeon discovers contamination of the abdominal viscera by bowel contents, as well as a 0.5-cm perforation of the rectosigmoid colon. The perforation is repaired in two layers after its edges are trimmed, and a diverting colostomy is performed. The patient is admitted to the ICU and requires antibiotic treatment, total parenteral nutrition, and bowel rest due to severe peritonitis. She gradually recovers and is discharged 3 weeks later. The diverting colostomy is reversed 3 months later.
Even small perforations in the large bowel can cause infection and abscess due to the high bacterial content of the colon. The most common cause of injury to the rectosigmoid colon is pelvic adhesiolysis during cul-de-sac dissection, treatment of pelvic endometriosis, and resection of adherent pelvic masses.
Sharp dissection with scissors or high-powered lasers is relatively safe near the bowel. When dissecting the cul-de-sac, identify the vagina and rectum by placing a probe or finger in each area. Begin dissection from the unaffected pararectal space, and proceed toward the obliterated cul-de-sac.27,28
Bowel prep is indicated before extensive pelvic surgery and when the history suggests endometriosis or significant pelvic adhesions. Some general surgeons base their decision to perform colostomy (or not) on whether the bowel was prepped preoperatively.29
If the large bowel is perforated by the Veress needle, the saline aspiration test will yield brownish fluid. When significant pelvic adhesiolysis or pelvic or endometriotic tumor resection is performed, inject air into the rectum afterward via a sigmoidoscope or bulb syringe and assess the submerged rectum and rectosigmoid colon for bubbling. The rectal wall may be weakened during these types of procedures, so instruct the patient to use oral stool softeners and avoid enemas.30
Delay in detection can have serious ramifications
When a large-bowel injury goes undetected at the time of operation, the patient generally presents on the third or fourth postoperative day with mild fever, occasionally sudden sharp epigastric pain, lower abdominal pain, slight nausea, and anorexia. By the fifth or sixth day, these symptoms have become more severe and are accompanied by peritonitis and an elevated WBC count.
Whenever a patient complains of abdominal pain and a deteriorating condition, assume that bowel injury is the cause until it is proved otherwise.
Intraoperative management
Repair small trocar wounds using primary suture closure. Copious lavage of the peritoneal cavity, drainage, and a broad-spectrum antibiotic minimize the risk of infection. Manage deep electrical injury to the right colon by resecting the injured segment and performing primary anastomosis. Primary closure or resection and reanastomosis may not be adequate when the vascular supply of the descending colon or rectum is compromised. In that case, perform a diverting colostomy or ileostomy, which can be reversed 6 to 12 weeks later.25,26
CASE 5: Vascular injury
A tall, thin, athletic 19-year-old undergoes diagnostic laparoscopy to rule out pelvic pathology after she complains of severe, monthly abdominal pain. Upon insertion of the laparoscope, the surgeon observes a large hematoma forming at the right pelvic sidewall. At the same time, the anesthesiologist reports a significant drop in blood pressure, and vascular injury is diagnosed. The surgeon attempts to control the bleeding using bipolar coagulation, but the problem only becomes worse. He decides to switch to laparotomy.
A vascular surgeon is called in, and injury to the right common iliac artery and vein—apparently caused during insertion of the primary umbilical trocar—is repaired. The patient is given 5 U of red blood cells. She goes home 10 days later, but returns with thrombophlebitis and rejection of the graft. After several surgeries, she finally recovers, with some sequelae, such as unilateral leg edema.
Management of vascular injury depends on the source and type of injury. On major vessels, electrocoagulation is contraindicated. After immediate atraumatic compression with tamponade to control bleeding, vascular repair, in consultation with a vascular surgeon, is indicated. At times, a vascular graft may be required.
Smaller vessels, such as the infundibulopelvic ligament or uterine vessels, can be managed by clips, suture, or loop ligatures. If thermal energy is used in the repair, be careful to avoid injury to surrounding structures.
Most emergency laparotomies are performed for uncontrolled bleeding.30,31 Lack of control or a wrong angle at insertion of the Veress needle and trocars is a major cause of large-vessel injury. Sharp dissection of adhesions, uterosacral ablation, transection of vascular pedicles without adequate dessication, and rough handling of tissues can all cause bleeding. Distorted anatomy is a main cause of vascular injury and can compound injury in areas more prone to bleeding, such as the oviduct, infundibulopelvic ligament, mesosalpinx, and pelvic sidewall vessels.
The return of pressure gradients to normal levels at the end of a procedure can be accompanied by bleeding into the retroperitoneal space, so evaluate the patient in a supine position after intra-abdominal pressure is reduced.
A vascular surgeon may be required
Depending on the type of vessel, size and location of the injury, and degree of bleeding, you may use unipolar or bipolar electrocoagulation, suture, clips, vasopressin, or loop ligatures to control bleeding. Although diluted vasopressin (10 U in 60 mL of lactated Ringer’s saline) can decrease oozing from raw peritoneal areas, injury to a major vessel, such as the iliac vessels, vena cava, or aorta, needs immediate control and proper repair. The decision to perform laparoscopy or laparotomy depends on your preference and experience. In any case, a vascular surgeon may be consulted for major vascular injuries.32
If a major vessel is injured, do not crush-clamp it. If possible (and if your laparoscopic skills are advanced), insert a sponge via a 10-mm trocar and apply pressure to the vessel to minimize bleeding and enhance visualization. The decision to repair the injury laparoscopically or by laparotomy should be made judiciously and promptly. n
The authors acknowledge the editorial contributions of Kristina Petrasek and Barbara Page, of the University of California, Berkeley, to the manuscript of this article.
1. Ostrzenski A, Radolinski B, Ostrzenska K. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794-799.
2. Kabalin J. Chapter 1—Surgical anatomy of the retroperitoneum, kidneys, and ureters. In: Walsh P, Retik A, Wein A, eds. Campbell’s Urology. 8th ed. Philadelphia: Saunders; 2002 36-40.
3. Chan J, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
4. Grainger DA, Soderstrom RM, Schiff SF, Glickman MG, DeCherney AH, Diamond MP. Ureteral injuries at laparoscopy: insights into diagnosis, management, and prevention. Obstet Gynecol. 1990;75:839-843.
5. Nezhat C. Chapter 20. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York: McGraw–Hill; 2000.
6. Ou CS, Huang IA, Rowbotham R. Laparoscopic ureteroureteral anastomosis for repair of ureteral injury involving stricture. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:155-157.
7. Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystostomy for distal ureteral injuries. Urology. 2005;66:751-753.
8. Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
9. Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80:543-544.
10. Nezhat CH, Nezhat FR, Freiha F, Nezhat CR. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
11. Georgy FM, Fettman HH, Chefetz MD. Complications of laparoscopy: two cases of perforated urinary bladder. Am J Obstet Gynecol. 1974;120:1121-1122.
12. Sherer DM. Inadvertent transvaginal cystotomy during laparoscopy. Int J Gynaecol Obstet. 1990;32:77-79.
13. Nezhat CH, Seidman DS, Nezhat F, et al. Laparoscopic management of internal and unintentional cystotomy. J Urol. 1996;156:1400-1402.
14. Lee CL, Lai YM, Soong YK. Management of urinary bladder injuries in laparoscopic assisted vaginal hysterectomy. Acta Obstet Gynecol Scand. 1996;75:174-177.
15. Peterson HB, et al. American Association of Gynecologic Laparoscopists’ 1988 membership survey on operative laparoscopy. J Reprod Med. 1990;35:587-589.
16. Phillips JM, Hulka JF, Hulka B, et al. 1978 AAGL membership survey. J Reprod Med. 1981;26:529-533.
17. Chapron C, Pierre F, et al. Gastrointestinal injuries during laparoscopy. Hum Reprod. 1999;14:333-337.
18. Schrenk P, Woisetschlager R, Rieger R, et al. Mechanism, management, and prevention of laparoscopic bowel injuries. Gastrointest Endosc. 1996;43:572-574.
19. Sauer M, Jarrett JC. Small bowel obstruction following diagnostic laparoscopy. Fertil Steril. 1984;42:653-654.
20. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
21. Brill A, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparatomy: a laparoscopic appraisal. Obstet Gynecol. 1995;85:269-272.
22. Levy BS, Soderstrom RM, Dail DH. Bowel injuries during laparoscopy: gross anatomy and histology. J Reprod Med. 1985;30:168-172.
23. Wheeless CR. Gastrointestinal injuries associated with laparoscopy. In: Phillips JM, ed. Endoscopy in Gynecology. Santa Fe Springs, Calif: American Association of Gynecologic Laparoscopists; 1978.
24. Borton M. Laparoscopic Complications: Prevention and Management. Philadelphia: Decker; 1986.
25. DeCherney AH. Laparoscopy with unexpected viscus penetration. In: Nichols DH, ed. Clinical Problems, Injuries, and Complications of Gynecologic Surgery. Baltimore: Williams & Wilkins; 1988.
26. Nezhat C, Nezhat F, Ambroze W, Pennington E. Laparoscopic repair of small bowel and colon: a report of 26 cases. Surg Endosc. 1993;7:88-89.
27. Redwine D. Laparoscopic en bloc resection for treatment of the obliterated cul-de-sac in endometriosis. J Reprod Med. 1992;37:696-698.
28. Nezhat C, Nezhat F, et al. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaseroscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
29. Nezhat C, Seidman D, Nezhat F, et al. The role of intraoperative proctosigmoidoscopy in laparoscopic pelvic surgery. J Am Assoc Gynecol Laparosc. 2004;11:47-49.
30. Chapron CM, et al. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
31. Geers J, Holden C. Major vascular injury as a complication of laparoscopic surgery: a report of three cases and review of the literature. Am Surg. 1996;62:377-379.
32. Nezhat C, Childers J, Nezhat F, et al. Major retroperitoneal vascular injury during laparoscopic surgery. Hum Reprod. 1997;12:480-483.
Dr. Farr Nezhat reports no relevant financial relationships. Dr. Ceana Nezhat is a speaker and consultant for Karl Storz, Johnson & Johnson, Valleylab, US Surgical, and Viking. Dr. Camran Nezhat is a speaker for or receives educational support from Karl Storz, Stryker, Johnson & Johnson, Valleylab, and Baxter.
To view three clips of surgical pearls for laparoscopy, visit the Video Library.
<huc>Q.</huc>What is the only surgical procedure that is completely safe?
<huc>A.</huc>The surgical procedure that is not performed.
The unfortunate truth is that complications can occur during any operative procedure, despite our best efforts—and laparoscopy is no exception. Being vigilant for iatrogenic injuries, both during and after surgery, and ensuring that repairs are both thorough and timely, are two of our best weapons against major complications, along with meticulous technique and adequate experience.
This article features five cases that illustrate some of the most serious complications of laparoscopy—and how to prevent and manage them.
CASE 1: Surgical patient returns with signs of ureteral injury
A 42-year-old woman with a history of endometriosis undergoes laparoscopic hysterectomy and bilateral salpingo-oophorectomy. She is discharged 2 days later. Two days after that, she returns to the hospital complaining of fluid leaking from the vagina. She has no fever or any other significant complaint or physical findings other than abdominal tenderness, which is to be expected after surgery. A computed tomography (CT) scan with intravenous (IV) contrast reveals left ureteral obstruction near the bladder, with extravasation of contrast media into the abdominal cavity. Further investigation reveals a left ureteral transection.
Could this injury have been avoided? How should it be managed?
Postoperative diagnosis of ureteral injury can be challenging, in part because up to 50% of unilateral cases are asymptomatic. Be on the lookout for this complication in women who have undergone pelvic sidewall dissection or laparoscopic hysterectomy, such as the patient in the case just described. As the number of laparoscopic hysterectomies and retroperitoneal procedures has risen in recent years, so has the rate of ureteral injury, with an incidence of 0.3% to 2%.1,2
Ureteral injury can be caused by ligation, ischemia, resection, transection, crushing, or angulation. Three sites are particularly troublesome: the infundibulopelvic ligament, ovarian fossa, and ureteral tunnel.3,4 In Case 1, injury to the ureter was proximal to the bladder and probably occurred during transection of the uterosacral cardinal ligament complex.
What’s the best preventive strategy?
Meticulous technique is imperative to protect the ureters. This includes adequate visualization, intraperitoneal or retroperitoneal dissection, and early identification of the ureter. In a high-risk patient likely to have distorted anatomy due to severe endometriosis and fibrosis, retroperitoneal dissection of any adhesions or tumor and identification of the ureter are the best ways to avoid injury.
Intraperitoneal identification and dissection of the ureters can be enhanced by hydrodissection and resection of the affected peritoneum.3,4 To create a safe operating plane, make a small opening in the peritoneum below the ureter and inject 50 to 100 mL of lactated Ringer’s solution along the course of the ureter, which will displace it laterally.5
Although neither IV indigo carmine nor ureteral catheterization has been shown to reduce the risk of ureteral injury or identify ligation or thermal injury,3,6 both can help the surgeon identify intraoperative perforation of the ureter. Liberal use of cystoscopy with indigo carmine administration for identification of ureteral flow and ureteral catheterization can be used in potentially high-risk patients. If there is suspicion for devascularization or thermal injury, use prophylactic ureteral stents postoperatively for 2 to 4 weeks.
Don’t hesitate to consult a urologist
In Case 1, the surgeon sought immediate urologic consultation and the patient underwent laparotomy with ureteroneocystotomy without sequelae.
In general, management of ureteral injury depends on its severity and location, as well as the comfort level of the surgeon. Minor injuries are sometimes managed with cystoscopic stent placement, but more severe cases may require operative ureteral repair.
In cases like this one, where ureteral injury occurred in close proximity to the bladder, a ureteroneocystotomy is possible. However, in more cephalad injuries, there may be insufficient residual ureter to allow such a repair. In these cases, a Boari flap may be attempted to use bladder tissue to bridge the gap to the ureteral edge. Rarely, in high ureteral injuries, trans-ureteroureterostomy may be appropriate. This procedure carries the greatest risk, given that both kidneys are reliant on one ureter.
Is laparoscopic repair reasonable?
When surgical intervention is necessary, the choice between laparoscopy and laparotomy depends on the skill and comfort level of the surgeon and the availability of instruments and support team.6,7 That said, ureteral injury is usually treated via laparotomy.1 As operative laparoscopy becomes even more commonplace, reconstruction of the urinary system will increasingly be managed laparoscopically.
Depending on the size and location of the injury, reconstruction may involve ureteral reimplantation with or without a psoas hitch, Boari flap, or primary endtoend anastomosis.8-10
CASE 2: Postoperative symptoms lead to rehospitalization
A 35-year-old patient undergoes laparoscopic ovarian cystectomy and returns home the same day. She is readmitted 72 hours later because of lower abdominal tenderness, worsening nausea and vomiting, and urine-like drainage from her midline suprapubic trocar site. Analysis of the leaking fluid shows high creatinine levels consistent with urine. The patient has no fever and is hemodynamically stable. Examination reveals a moderately distended abdomen with decreased bowel sounds. Hematuria is evident on urine analysis.
Urologic consultation is obtained, and the patient undergoes simultaneous laparoscopy and cystoscopy, during which perforation of the bladder dome is discovered, apparently caused by the mid suprapubic trocar. The bladder is mobilized anteriorly, and both anterior and posterior aspects of the perforation are repaired in one layer laparoscopically.
After continuous drainage with a transurethral Foley catheter for 7 days, cystography shows complete healing of the bladder, and the Foley catheter is removed. The patient recovers completely.
Vesical injury sometimes occurs in patients who have a history of laparotomy, a full bladder at the time of surgery, or displaced anatomy due to pelvic adhesions.11 Although bladder injury is rare, laparoscopy increases the risk. Trocars, uterine manipulators, and blunt instruments can perforate or lacerate the bladder, and energy devices can cause thermal injury. The risk of bladder injury increases during laparoscopic hysterectomy.
Be vigilant about trocar placement and dissection techniques
Accessory trocars can injure a full bladder. Injury can also occur when distorted anatomy from a previous pelvic operation obscures bladder boundaries, making insertion of the midline trocar potentially perilous (FIGURE 1). The Veress needle and Rubin’s cannula can perforate the bladder.11-13 And in the anterior cul-de-sac, adhesiolysis, deep coagulation, laser ablation, or sharp excision of endometriosis implants can predispose a patient to bladder injury.
In women with severe endometriosis, lower-segment myoma, or a history of cesarean section, the bladder is vulnerable to laceration when blunt dissection is used during laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy (LAVH). A vesical injury also can occur at the time of laparoscopic bladder-neck suspension upon entry into, and dissection of, the space of Retzius.
FIGURE 1 A bladder at risk
In this patient with a previous cesarean section, the bladder is adherent to the anterior abdominal wall. Needle mapping in the conventional midline trocar position indicates that the trocar must be relocated to avoid bladder injury.
Intraoperative findings that suggest bladder injury include air in the urinary catheter, hematuria, trocar site drainage of urine, or indigo carmine leakage. Postoperative signs and symptoms include leaking from incisional sites, a mass in the abdominal wall, and abdominal swelling.
Liberal use of cystoscopy or distension of the bladder with 300 to 500 mL of normal saline is recommended whenever there is a suspicion of bladder injury, especially during laparoscopic hysterectomy or LAVH. When a trocar causes the injury, look for both entry and exit punctures, both of which should be treated.
No matter how much care is taken, some bladder injuries, such as vesicovaginal fistulae, become apparent only postoperatively. More rarely, peritonitis or pseudoascites herald the injury. Retrograde cystography may aid identification.
Treatment of bladder injuries
Small perforations recognized intraoperatively may be conservatively managed by postoperative bladder drainage for 5 to 7 days. Most other bladder injuries require prompt intervention. For example, trocar injury to the bladder dome requires one- or two-layer closure followed by 5 to 7 days of urinary drainage. (Both closing and healing are promoted by drainage.)
Laparoscopy or laparotomy? Laparoscopic repair has become increasingly common, and bladder injury is a common complication of LAVH.13,14
CASE 3: Postop pain, tachycardia
A 41-year-old obese woman undergoes laparoscopic cystectomy for an 8-cm left ovarian mass. The abdomen is entered on the second attempt with a long Veress needle. The umbilical trocar is reinserted “several” times because of difficulty opening the peritoneum with the tip of the trocar sheath. The surgical procedure is completed within 2 hours, and the patient is discharged 23 hours later.
The next day, she experiences increasing abdominal pain and presents to the emergency room. Upon admission she reports intermittent chills, but denies nausea and vomiting. She is in mild distress, pale and tachycardic, with a temperature of 96.4°, pulse of 117, respiration rate of 20, blood pressure of 106/64 mm Hg, and oxygen saturation of 92%. She also has a diffusely tender abdomen but normal blood work. Abdominal and chest x-rays show a large right subphrenic air-fluid level that is consistent with free intraperitoneal air, unsurprising given her recent surgery. Bibasilar atelectasis and consolidation are noted on the initial chest x-ray.
During observation over the next 2 days, she remains afebrile and tachycardic, but her shortness of breath becomes progressively worse. Neither spiral CT nor lower-extremity Doppler suggests pulmonary embolism or deep venous thrombosis. Supplemental oxygen, aggressive pain management, albuterol, ipratropium, and acetylcysteine are initiated after pulmonary consultation.
The patient tolerates a regular diet on postoperative day 3 and has a bowel movement on day 5. However, the same day she begins vomiting and reports worsening abdominal pain. CT imaging of the abdomen and pelvis reveals free air in the abdomen and loculated fluid with air bubbles suspicious for intra-abdominal infection and perforated bowel.
Exploratory laparotomy reveals diffuse feculent peritonitis, as well as food particles and contrast media. There is a perforation in the antimesenteric side of the ileum approximately 1.5 feet proximal to the ileocecal valve. This perforation measures approximately 1 cm in diameter and is freely spilling intestinal contents. Small bowel resection is performed to treat the perforation.
Following the surgery, the patient recovers slowly.
Could the bowel perforation have been detected sooner?
Intestinal tract injury is a serious complication, particularly with postoperative diagnosis.15 Damage can occur during insertion of the Veress needle or trocar when the bowel is immobilized by adhesions, or during enterolysis.16 Unrecognized thermal injury can cause delayed bowel injury.
Small-bowel damage often occurs during uncontrolled insertion of the Veress needle or primary umbilical trocar. It also may result from sharp dissection or thermal injury.17,18 Abrasions and lacerations can occur if traction is exerted on the bowel using serrated graspers. When adhesions are dense and tissue planes poorly defined, the risk of laceration due to energy sources or sharp dissection increases.
Be cautious during bowel manipulation. Avoid blunt dissection. Be especially careful when the small bowel is adherent to the anterior abdominal wall (FIGURE 2A), particularly during evaluation of patients with a history of bowel resection, exploratory laparotomy for trauma-related peritonitis, or tumor debulking.
Remove the primary and ancillary cannulas under direct visualization with the laparoscope to prevent formation of a vacuum that can draw bowel into the incision and cause herniation.19
FIGURE 2 Adherent bowel, minor bleeding
A: Veress needle pressure measurements are persistently elevated before primary trocar insertion in this patient, raising the suspicion of adhesive disease from earlier surgery. As a result, the primary trocar is relocated to the left upper quadrant. Inspection confirms that small bowel is adherent to the anterior abdominal wall.
FIGURE 2 Adherent bowel, minor bleeding
B: After the small-bowel adhesions are dissected off the anterior abdominal wall via laparoscopy, a small hematoma is discovered, likely caused by the Veress needle. The patient is managed conservatively and recovers.
The value of open laparoscopy
In open laparoscopy, an abdominal incision is made into the peritoneal cavity so that the trocar can be placed under direct vision, after which the abdomen is insufflated. This approach can prevent bowel injury only when the adhesions and attachments are to the anterior abdominal wall and away from the entry site. When the attachment lies directly beneath the umbilicus, however, open laparoscopy is no guarantee against injury.
When bowel adhesions are severe, use alternative trocar sites such as the left upper quadrant (Palmer’s point) for the Veress needle and primary trocars.5,20,21
The likelihood of perforation can be reduced with preoperative bowel prep when there is a risk of bowel adhesions.
Identifying bowel injury
We recommend routine inspection of the structures beneath the primary trocar upon insertion of the laparoscope to look for injury to the bowel, mesentery, or vascular structures. If adhesions are found, evaluate the area carefully to rule out injury to the bowel or omentum. It may be necessary to change the position of the laparoscope to assess the patient.
Trauma to the intestinal tract can be mechanical or electrical in nature, and each type of trauma creates a distinctive, characteristic pattern. Thermal injury can be subtle and present as simple blanching or a distinct burn and charring. A small hole or obvious tear in the bowel wall can be the result of mechanical injury.22
Benign-appearing, superficial thermal bowel injuries may be managed conservatively.22 Minimal serosal burns (smaller than 5 mm in diameter) can be managed expectantly. Immediate surgical intervention is needed if the area of blanching on the intestinal serosa exceeds 5 mm in diameter or if the burn appears to involve more than the serosa.23
Small-bowel injuries that escape notice intraoperatively generally become apparent 2 to 4 days later, when the patient develops fever, nausea, lower abdominal pain, and anorexia. On postoperative day 5 or 6, the white blood cell (WBC) count rises and earlier symptoms may become worse. Radiography may reveal multiple air and fluid levels—another sign of bowel injury. Be aware that if the patient has clinical symptoms of gastrointestinal injury, even if the WBC count is normal, exploratory laparoscopy or laparotomy is necessary for accurate diagnosis.
Intraoperatively discovered injury
Careful inspection may reveal no leakage or bleeding in the affected area. Small punctures or superficial lacerations seal readily and may not require further treatment (FIGURE 2B), but larger perforations require repair. Straightforward repair is not always possible when the injury is extensive and considerable time has elapsed before it is discovered.
Inspect the intestine thoroughly at the conclusion of a procedure; obvious leakage requires intervention. Repair the small intestine in one or two layers, using the initial row of interrupted sutures to approximate the mucosa and muscularis.24 To lessen the risk of stenosis, close all lacerations transversely when they are smaller than one half the diameter of the bowel. If the laceration exceeds that size, segmental resection and anastomosis are necessary. Resection is prudent if the mesenteric blood supply is compromised.25
When performing one-layer repair of the small bowel, delayed absorbable suture (eg, Vicryl or PDS) or nonabsorbable suture (eg, silk) is recommended.26
At the conclusion of a repair, copiously irrigate the entire abdomen. Place a nasogastric tube only if ileus is anticipated; the tube can be removed when drainage diminishes and active bowel sounds and flatus appear. Do not give anything by mouth until the patient has return of bowel function and active peristalsis. Prescribe prophylactic antibiotics.
Note that peritonitis sometimes develops after repair of the bowel.25 This can be managed with prolonged bowel rest and peripheral or total parenteral nutrition.
Conservative management may be possible
Patients whose symptoms of bowel laceration become apparent after discharge can sometimes be managed conservatively. More than 50% of patients treated conservatively require no surgery.23 Inpatient management consists of monitoring the WBC count, providing hydration and IV antibiotics, and examining the patient every 6 hours, giving nothing by mouth.
When injury is discovered later
If conservative management with observation and bowel rest fails, or the patient complains of severe abdominal pain, vomiting, nausea, obstipation, or signs and symptoms of peritonitis, such as the patient in Case 3, immediate surgical intervention is necessary. When an injury is not detected until some time after initial surgery, resection of all necrotic tissue is mandatory. In most cases, the perforation is managed by segmental resection and reanastomosis. Evaluate the entire small and large bowel to rule out any other injury, and irrigate generously. Bowel rest, parenteral nutrition, and IV antibiotics also are indicated.
Of 36,928 procedures reported by members of the American Association of Gynecologic Laparoscopists, there were two deaths—both caused by unrecognized bowel injury.15
CASE 4: Large-bowel injury precipitates lengthy recovery
A surgeon performs a left laparoscopic salpingo-oophorectomy to remove an 8-cm ovarian endometrioma that is adherent to the rectosigmoid colon of a 40-year-old diabetic woman. Sharp and electrosurgical scissors are used to separate the adnexa from the rectosigmoid colon. No injury is observed, and she is discharged the same day. Four days later, she returns with severe abdominal pain, nausea, vomiting, and fatigue. Lab tests reveal a WBC count of 17,000; a CT scan shows pockets of air beneath the diaphragm, as well as fluid collection suggestive of a pelvic abscess.
Immediate laparotomy is performed, during which the surgeon discovers contamination of the abdominal viscera by bowel contents, as well as a 0.5-cm perforation of the rectosigmoid colon. The perforation is repaired in two layers after its edges are trimmed, and a diverting colostomy is performed. The patient is admitted to the ICU and requires antibiotic treatment, total parenteral nutrition, and bowel rest due to severe peritonitis. She gradually recovers and is discharged 3 weeks later. The diverting colostomy is reversed 3 months later.
Even small perforations in the large bowel can cause infection and abscess due to the high bacterial content of the colon. The most common cause of injury to the rectosigmoid colon is pelvic adhesiolysis during cul-de-sac dissection, treatment of pelvic endometriosis, and resection of adherent pelvic masses.
Sharp dissection with scissors or high-powered lasers is relatively safe near the bowel. When dissecting the cul-de-sac, identify the vagina and rectum by placing a probe or finger in each area. Begin dissection from the unaffected pararectal space, and proceed toward the obliterated cul-de-sac.27,28
Bowel prep is indicated before extensive pelvic surgery and when the history suggests endometriosis or significant pelvic adhesions. Some general surgeons base their decision to perform colostomy (or not) on whether the bowel was prepped preoperatively.29
If the large bowel is perforated by the Veress needle, the saline aspiration test will yield brownish fluid. When significant pelvic adhesiolysis or pelvic or endometriotic tumor resection is performed, inject air into the rectum afterward via a sigmoidoscope or bulb syringe and assess the submerged rectum and rectosigmoid colon for bubbling. The rectal wall may be weakened during these types of procedures, so instruct the patient to use oral stool softeners and avoid enemas.30
Delay in detection can have serious ramifications
When a large-bowel injury goes undetected at the time of operation, the patient generally presents on the third or fourth postoperative day with mild fever, occasionally sudden sharp epigastric pain, lower abdominal pain, slight nausea, and anorexia. By the fifth or sixth day, these symptoms have become more severe and are accompanied by peritonitis and an elevated WBC count.
Whenever a patient complains of abdominal pain and a deteriorating condition, assume that bowel injury is the cause until it is proved otherwise.
Intraoperative management
Repair small trocar wounds using primary suture closure. Copious lavage of the peritoneal cavity, drainage, and a broad-spectrum antibiotic minimize the risk of infection. Manage deep electrical injury to the right colon by resecting the injured segment and performing primary anastomosis. Primary closure or resection and reanastomosis may not be adequate when the vascular supply of the descending colon or rectum is compromised. In that case, perform a diverting colostomy or ileostomy, which can be reversed 6 to 12 weeks later.25,26
CASE 5: Vascular injury
A tall, thin, athletic 19-year-old undergoes diagnostic laparoscopy to rule out pelvic pathology after she complains of severe, monthly abdominal pain. Upon insertion of the laparoscope, the surgeon observes a large hematoma forming at the right pelvic sidewall. At the same time, the anesthesiologist reports a significant drop in blood pressure, and vascular injury is diagnosed. The surgeon attempts to control the bleeding using bipolar coagulation, but the problem only becomes worse. He decides to switch to laparotomy.
A vascular surgeon is called in, and injury to the right common iliac artery and vein—apparently caused during insertion of the primary umbilical trocar—is repaired. The patient is given 5 U of red blood cells. She goes home 10 days later, but returns with thrombophlebitis and rejection of the graft. After several surgeries, she finally recovers, with some sequelae, such as unilateral leg edema.
Management of vascular injury depends on the source and type of injury. On major vessels, electrocoagulation is contraindicated. After immediate atraumatic compression with tamponade to control bleeding, vascular repair, in consultation with a vascular surgeon, is indicated. At times, a vascular graft may be required.
Smaller vessels, such as the infundibulopelvic ligament or uterine vessels, can be managed by clips, suture, or loop ligatures. If thermal energy is used in the repair, be careful to avoid injury to surrounding structures.
Most emergency laparotomies are performed for uncontrolled bleeding.30,31 Lack of control or a wrong angle at insertion of the Veress needle and trocars is a major cause of large-vessel injury. Sharp dissection of adhesions, uterosacral ablation, transection of vascular pedicles without adequate dessication, and rough handling of tissues can all cause bleeding. Distorted anatomy is a main cause of vascular injury and can compound injury in areas more prone to bleeding, such as the oviduct, infundibulopelvic ligament, mesosalpinx, and pelvic sidewall vessels.
The return of pressure gradients to normal levels at the end of a procedure can be accompanied by bleeding into the retroperitoneal space, so evaluate the patient in a supine position after intra-abdominal pressure is reduced.
A vascular surgeon may be required
Depending on the type of vessel, size and location of the injury, and degree of bleeding, you may use unipolar or bipolar electrocoagulation, suture, clips, vasopressin, or loop ligatures to control bleeding. Although diluted vasopressin (10 U in 60 mL of lactated Ringer’s saline) can decrease oozing from raw peritoneal areas, injury to a major vessel, such as the iliac vessels, vena cava, or aorta, needs immediate control and proper repair. The decision to perform laparoscopy or laparotomy depends on your preference and experience. In any case, a vascular surgeon may be consulted for major vascular injuries.32
If a major vessel is injured, do not crush-clamp it. If possible (and if your laparoscopic skills are advanced), insert a sponge via a 10-mm trocar and apply pressure to the vessel to minimize bleeding and enhance visualization. The decision to repair the injury laparoscopically or by laparotomy should be made judiciously and promptly. n
The authors acknowledge the editorial contributions of Kristina Petrasek and Barbara Page, of the University of California, Berkeley, to the manuscript of this article.
To view three clips of surgical pearls for laparoscopy, visit the Video Library.
<huc>Q.</huc>What is the only surgical procedure that is completely safe?
<huc>A.</huc>The surgical procedure that is not performed.
The unfortunate truth is that complications can occur during any operative procedure, despite our best efforts—and laparoscopy is no exception. Being vigilant for iatrogenic injuries, both during and after surgery, and ensuring that repairs are both thorough and timely, are two of our best weapons against major complications, along with meticulous technique and adequate experience.
This article features five cases that illustrate some of the most serious complications of laparoscopy—and how to prevent and manage them.
CASE 1: Surgical patient returns with signs of ureteral injury
A 42-year-old woman with a history of endometriosis undergoes laparoscopic hysterectomy and bilateral salpingo-oophorectomy. She is discharged 2 days later. Two days after that, she returns to the hospital complaining of fluid leaking from the vagina. She has no fever or any other significant complaint or physical findings other than abdominal tenderness, which is to be expected after surgery. A computed tomography (CT) scan with intravenous (IV) contrast reveals left ureteral obstruction near the bladder, with extravasation of contrast media into the abdominal cavity. Further investigation reveals a left ureteral transection.
Could this injury have been avoided? How should it be managed?
Postoperative diagnosis of ureteral injury can be challenging, in part because up to 50% of unilateral cases are asymptomatic. Be on the lookout for this complication in women who have undergone pelvic sidewall dissection or laparoscopic hysterectomy, such as the patient in the case just described. As the number of laparoscopic hysterectomies and retroperitoneal procedures has risen in recent years, so has the rate of ureteral injury, with an incidence of 0.3% to 2%.1,2
Ureteral injury can be caused by ligation, ischemia, resection, transection, crushing, or angulation. Three sites are particularly troublesome: the infundibulopelvic ligament, ovarian fossa, and ureteral tunnel.3,4 In Case 1, injury to the ureter was proximal to the bladder and probably occurred during transection of the uterosacral cardinal ligament complex.
What’s the best preventive strategy?
Meticulous technique is imperative to protect the ureters. This includes adequate visualization, intraperitoneal or retroperitoneal dissection, and early identification of the ureter. In a high-risk patient likely to have distorted anatomy due to severe endometriosis and fibrosis, retroperitoneal dissection of any adhesions or tumor and identification of the ureter are the best ways to avoid injury.
Intraperitoneal identification and dissection of the ureters can be enhanced by hydrodissection and resection of the affected peritoneum.3,4 To create a safe operating plane, make a small opening in the peritoneum below the ureter and inject 50 to 100 mL of lactated Ringer’s solution along the course of the ureter, which will displace it laterally.5
Although neither IV indigo carmine nor ureteral catheterization has been shown to reduce the risk of ureteral injury or identify ligation or thermal injury,3,6 both can help the surgeon identify intraoperative perforation of the ureter. Liberal use of cystoscopy with indigo carmine administration for identification of ureteral flow and ureteral catheterization can be used in potentially high-risk patients. If there is suspicion for devascularization or thermal injury, use prophylactic ureteral stents postoperatively for 2 to 4 weeks.
Don’t hesitate to consult a urologist
In Case 1, the surgeon sought immediate urologic consultation and the patient underwent laparotomy with ureteroneocystotomy without sequelae.
In general, management of ureteral injury depends on its severity and location, as well as the comfort level of the surgeon. Minor injuries are sometimes managed with cystoscopic stent placement, but more severe cases may require operative ureteral repair.
In cases like this one, where ureteral injury occurred in close proximity to the bladder, a ureteroneocystotomy is possible. However, in more cephalad injuries, there may be insufficient residual ureter to allow such a repair. In these cases, a Boari flap may be attempted to use bladder tissue to bridge the gap to the ureteral edge. Rarely, in high ureteral injuries, trans-ureteroureterostomy may be appropriate. This procedure carries the greatest risk, given that both kidneys are reliant on one ureter.
Is laparoscopic repair reasonable?
When surgical intervention is necessary, the choice between laparoscopy and laparotomy depends on the skill and comfort level of the surgeon and the availability of instruments and support team.6,7 That said, ureteral injury is usually treated via laparotomy.1 As operative laparoscopy becomes even more commonplace, reconstruction of the urinary system will increasingly be managed laparoscopically.
Depending on the size and location of the injury, reconstruction may involve ureteral reimplantation with or without a psoas hitch, Boari flap, or primary endtoend anastomosis.8-10
CASE 2: Postoperative symptoms lead to rehospitalization
A 35-year-old patient undergoes laparoscopic ovarian cystectomy and returns home the same day. She is readmitted 72 hours later because of lower abdominal tenderness, worsening nausea and vomiting, and urine-like drainage from her midline suprapubic trocar site. Analysis of the leaking fluid shows high creatinine levels consistent with urine. The patient has no fever and is hemodynamically stable. Examination reveals a moderately distended abdomen with decreased bowel sounds. Hematuria is evident on urine analysis.
Urologic consultation is obtained, and the patient undergoes simultaneous laparoscopy and cystoscopy, during which perforation of the bladder dome is discovered, apparently caused by the mid suprapubic trocar. The bladder is mobilized anteriorly, and both anterior and posterior aspects of the perforation are repaired in one layer laparoscopically.
After continuous drainage with a transurethral Foley catheter for 7 days, cystography shows complete healing of the bladder, and the Foley catheter is removed. The patient recovers completely.
Vesical injury sometimes occurs in patients who have a history of laparotomy, a full bladder at the time of surgery, or displaced anatomy due to pelvic adhesions.11 Although bladder injury is rare, laparoscopy increases the risk. Trocars, uterine manipulators, and blunt instruments can perforate or lacerate the bladder, and energy devices can cause thermal injury. The risk of bladder injury increases during laparoscopic hysterectomy.
Be vigilant about trocar placement and dissection techniques
Accessory trocars can injure a full bladder. Injury can also occur when distorted anatomy from a previous pelvic operation obscures bladder boundaries, making insertion of the midline trocar potentially perilous (FIGURE 1). The Veress needle and Rubin’s cannula can perforate the bladder.11-13 And in the anterior cul-de-sac, adhesiolysis, deep coagulation, laser ablation, or sharp excision of endometriosis implants can predispose a patient to bladder injury.
In women with severe endometriosis, lower-segment myoma, or a history of cesarean section, the bladder is vulnerable to laceration when blunt dissection is used during laparoscopic hysterectomy or laparoscopically assisted vaginal hysterectomy (LAVH). A vesical injury also can occur at the time of laparoscopic bladder-neck suspension upon entry into, and dissection of, the space of Retzius.
FIGURE 1 A bladder at risk
In this patient with a previous cesarean section, the bladder is adherent to the anterior abdominal wall. Needle mapping in the conventional midline trocar position indicates that the trocar must be relocated to avoid bladder injury.
Intraoperative findings that suggest bladder injury include air in the urinary catheter, hematuria, trocar site drainage of urine, or indigo carmine leakage. Postoperative signs and symptoms include leaking from incisional sites, a mass in the abdominal wall, and abdominal swelling.
Liberal use of cystoscopy or distension of the bladder with 300 to 500 mL of normal saline is recommended whenever there is a suspicion of bladder injury, especially during laparoscopic hysterectomy or LAVH. When a trocar causes the injury, look for both entry and exit punctures, both of which should be treated.
No matter how much care is taken, some bladder injuries, such as vesicovaginal fistulae, become apparent only postoperatively. More rarely, peritonitis or pseudoascites herald the injury. Retrograde cystography may aid identification.
Treatment of bladder injuries
Small perforations recognized intraoperatively may be conservatively managed by postoperative bladder drainage for 5 to 7 days. Most other bladder injuries require prompt intervention. For example, trocar injury to the bladder dome requires one- or two-layer closure followed by 5 to 7 days of urinary drainage. (Both closing and healing are promoted by drainage.)
Laparoscopy or laparotomy? Laparoscopic repair has become increasingly common, and bladder injury is a common complication of LAVH.13,14
CASE 3: Postop pain, tachycardia
A 41-year-old obese woman undergoes laparoscopic cystectomy for an 8-cm left ovarian mass. The abdomen is entered on the second attempt with a long Veress needle. The umbilical trocar is reinserted “several” times because of difficulty opening the peritoneum with the tip of the trocar sheath. The surgical procedure is completed within 2 hours, and the patient is discharged 23 hours later.
The next day, she experiences increasing abdominal pain and presents to the emergency room. Upon admission she reports intermittent chills, but denies nausea and vomiting. She is in mild distress, pale and tachycardic, with a temperature of 96.4°, pulse of 117, respiration rate of 20, blood pressure of 106/64 mm Hg, and oxygen saturation of 92%. She also has a diffusely tender abdomen but normal blood work. Abdominal and chest x-rays show a large right subphrenic air-fluid level that is consistent with free intraperitoneal air, unsurprising given her recent surgery. Bibasilar atelectasis and consolidation are noted on the initial chest x-ray.
During observation over the next 2 days, she remains afebrile and tachycardic, but her shortness of breath becomes progressively worse. Neither spiral CT nor lower-extremity Doppler suggests pulmonary embolism or deep venous thrombosis. Supplemental oxygen, aggressive pain management, albuterol, ipratropium, and acetylcysteine are initiated after pulmonary consultation.
The patient tolerates a regular diet on postoperative day 3 and has a bowel movement on day 5. However, the same day she begins vomiting and reports worsening abdominal pain. CT imaging of the abdomen and pelvis reveals free air in the abdomen and loculated fluid with air bubbles suspicious for intra-abdominal infection and perforated bowel.
Exploratory laparotomy reveals diffuse feculent peritonitis, as well as food particles and contrast media. There is a perforation in the antimesenteric side of the ileum approximately 1.5 feet proximal to the ileocecal valve. This perforation measures approximately 1 cm in diameter and is freely spilling intestinal contents. Small bowel resection is performed to treat the perforation.
Following the surgery, the patient recovers slowly.
Could the bowel perforation have been detected sooner?
Intestinal tract injury is a serious complication, particularly with postoperative diagnosis.15 Damage can occur during insertion of the Veress needle or trocar when the bowel is immobilized by adhesions, or during enterolysis.16 Unrecognized thermal injury can cause delayed bowel injury.
Small-bowel damage often occurs during uncontrolled insertion of the Veress needle or primary umbilical trocar. It also may result from sharp dissection or thermal injury.17,18 Abrasions and lacerations can occur if traction is exerted on the bowel using serrated graspers. When adhesions are dense and tissue planes poorly defined, the risk of laceration due to energy sources or sharp dissection increases.
Be cautious during bowel manipulation. Avoid blunt dissection. Be especially careful when the small bowel is adherent to the anterior abdominal wall (FIGURE 2A), particularly during evaluation of patients with a history of bowel resection, exploratory laparotomy for trauma-related peritonitis, or tumor debulking.
Remove the primary and ancillary cannulas under direct visualization with the laparoscope to prevent formation of a vacuum that can draw bowel into the incision and cause herniation.19
FIGURE 2 Adherent bowel, minor bleeding
A: Veress needle pressure measurements are persistently elevated before primary trocar insertion in this patient, raising the suspicion of adhesive disease from earlier surgery. As a result, the primary trocar is relocated to the left upper quadrant. Inspection confirms that small bowel is adherent to the anterior abdominal wall.
FIGURE 2 Adherent bowel, minor bleeding
B: After the small-bowel adhesions are dissected off the anterior abdominal wall via laparoscopy, a small hematoma is discovered, likely caused by the Veress needle. The patient is managed conservatively and recovers.
The value of open laparoscopy
In open laparoscopy, an abdominal incision is made into the peritoneal cavity so that the trocar can be placed under direct vision, after which the abdomen is insufflated. This approach can prevent bowel injury only when the adhesions and attachments are to the anterior abdominal wall and away from the entry site. When the attachment lies directly beneath the umbilicus, however, open laparoscopy is no guarantee against injury.
When bowel adhesions are severe, use alternative trocar sites such as the left upper quadrant (Palmer’s point) for the Veress needle and primary trocars.5,20,21
The likelihood of perforation can be reduced with preoperative bowel prep when there is a risk of bowel adhesions.
Identifying bowel injury
We recommend routine inspection of the structures beneath the primary trocar upon insertion of the laparoscope to look for injury to the bowel, mesentery, or vascular structures. If adhesions are found, evaluate the area carefully to rule out injury to the bowel or omentum. It may be necessary to change the position of the laparoscope to assess the patient.
Trauma to the intestinal tract can be mechanical or electrical in nature, and each type of trauma creates a distinctive, characteristic pattern. Thermal injury can be subtle and present as simple blanching or a distinct burn and charring. A small hole or obvious tear in the bowel wall can be the result of mechanical injury.22
Benign-appearing, superficial thermal bowel injuries may be managed conservatively.22 Minimal serosal burns (smaller than 5 mm in diameter) can be managed expectantly. Immediate surgical intervention is needed if the area of blanching on the intestinal serosa exceeds 5 mm in diameter or if the burn appears to involve more than the serosa.23
Small-bowel injuries that escape notice intraoperatively generally become apparent 2 to 4 days later, when the patient develops fever, nausea, lower abdominal pain, and anorexia. On postoperative day 5 or 6, the white blood cell (WBC) count rises and earlier symptoms may become worse. Radiography may reveal multiple air and fluid levels—another sign of bowel injury. Be aware that if the patient has clinical symptoms of gastrointestinal injury, even if the WBC count is normal, exploratory laparoscopy or laparotomy is necessary for accurate diagnosis.
Intraoperatively discovered injury
Careful inspection may reveal no leakage or bleeding in the affected area. Small punctures or superficial lacerations seal readily and may not require further treatment (FIGURE 2B), but larger perforations require repair. Straightforward repair is not always possible when the injury is extensive and considerable time has elapsed before it is discovered.
Inspect the intestine thoroughly at the conclusion of a procedure; obvious leakage requires intervention. Repair the small intestine in one or two layers, using the initial row of interrupted sutures to approximate the mucosa and muscularis.24 To lessen the risk of stenosis, close all lacerations transversely when they are smaller than one half the diameter of the bowel. If the laceration exceeds that size, segmental resection and anastomosis are necessary. Resection is prudent if the mesenteric blood supply is compromised.25
When performing one-layer repair of the small bowel, delayed absorbable suture (eg, Vicryl or PDS) or nonabsorbable suture (eg, silk) is recommended.26
At the conclusion of a repair, copiously irrigate the entire abdomen. Place a nasogastric tube only if ileus is anticipated; the tube can be removed when drainage diminishes and active bowel sounds and flatus appear. Do not give anything by mouth until the patient has return of bowel function and active peristalsis. Prescribe prophylactic antibiotics.
Note that peritonitis sometimes develops after repair of the bowel.25 This can be managed with prolonged bowel rest and peripheral or total parenteral nutrition.
Conservative management may be possible
Patients whose symptoms of bowel laceration become apparent after discharge can sometimes be managed conservatively. More than 50% of patients treated conservatively require no surgery.23 Inpatient management consists of monitoring the WBC count, providing hydration and IV antibiotics, and examining the patient every 6 hours, giving nothing by mouth.
When injury is discovered later
If conservative management with observation and bowel rest fails, or the patient complains of severe abdominal pain, vomiting, nausea, obstipation, or signs and symptoms of peritonitis, such as the patient in Case 3, immediate surgical intervention is necessary. When an injury is not detected until some time after initial surgery, resection of all necrotic tissue is mandatory. In most cases, the perforation is managed by segmental resection and reanastomosis. Evaluate the entire small and large bowel to rule out any other injury, and irrigate generously. Bowel rest, parenteral nutrition, and IV antibiotics also are indicated.
Of 36,928 procedures reported by members of the American Association of Gynecologic Laparoscopists, there were two deaths—both caused by unrecognized bowel injury.15
CASE 4: Large-bowel injury precipitates lengthy recovery
A surgeon performs a left laparoscopic salpingo-oophorectomy to remove an 8-cm ovarian endometrioma that is adherent to the rectosigmoid colon of a 40-year-old diabetic woman. Sharp and electrosurgical scissors are used to separate the adnexa from the rectosigmoid colon. No injury is observed, and she is discharged the same day. Four days later, she returns with severe abdominal pain, nausea, vomiting, and fatigue. Lab tests reveal a WBC count of 17,000; a CT scan shows pockets of air beneath the diaphragm, as well as fluid collection suggestive of a pelvic abscess.
Immediate laparotomy is performed, during which the surgeon discovers contamination of the abdominal viscera by bowel contents, as well as a 0.5-cm perforation of the rectosigmoid colon. The perforation is repaired in two layers after its edges are trimmed, and a diverting colostomy is performed. The patient is admitted to the ICU and requires antibiotic treatment, total parenteral nutrition, and bowel rest due to severe peritonitis. She gradually recovers and is discharged 3 weeks later. The diverting colostomy is reversed 3 months later.
Even small perforations in the large bowel can cause infection and abscess due to the high bacterial content of the colon. The most common cause of injury to the rectosigmoid colon is pelvic adhesiolysis during cul-de-sac dissection, treatment of pelvic endometriosis, and resection of adherent pelvic masses.
Sharp dissection with scissors or high-powered lasers is relatively safe near the bowel. When dissecting the cul-de-sac, identify the vagina and rectum by placing a probe or finger in each area. Begin dissection from the unaffected pararectal space, and proceed toward the obliterated cul-de-sac.27,28
Bowel prep is indicated before extensive pelvic surgery and when the history suggests endometriosis or significant pelvic adhesions. Some general surgeons base their decision to perform colostomy (or not) on whether the bowel was prepped preoperatively.29
If the large bowel is perforated by the Veress needle, the saline aspiration test will yield brownish fluid. When significant pelvic adhesiolysis or pelvic or endometriotic tumor resection is performed, inject air into the rectum afterward via a sigmoidoscope or bulb syringe and assess the submerged rectum and rectosigmoid colon for bubbling. The rectal wall may be weakened during these types of procedures, so instruct the patient to use oral stool softeners and avoid enemas.30
Delay in detection can have serious ramifications
When a large-bowel injury goes undetected at the time of operation, the patient generally presents on the third or fourth postoperative day with mild fever, occasionally sudden sharp epigastric pain, lower abdominal pain, slight nausea, and anorexia. By the fifth or sixth day, these symptoms have become more severe and are accompanied by peritonitis and an elevated WBC count.
Whenever a patient complains of abdominal pain and a deteriorating condition, assume that bowel injury is the cause until it is proved otherwise.
Intraoperative management
Repair small trocar wounds using primary suture closure. Copious lavage of the peritoneal cavity, drainage, and a broad-spectrum antibiotic minimize the risk of infection. Manage deep electrical injury to the right colon by resecting the injured segment and performing primary anastomosis. Primary closure or resection and reanastomosis may not be adequate when the vascular supply of the descending colon or rectum is compromised. In that case, perform a diverting colostomy or ileostomy, which can be reversed 6 to 12 weeks later.25,26
CASE 5: Vascular injury
A tall, thin, athletic 19-year-old undergoes diagnostic laparoscopy to rule out pelvic pathology after she complains of severe, monthly abdominal pain. Upon insertion of the laparoscope, the surgeon observes a large hematoma forming at the right pelvic sidewall. At the same time, the anesthesiologist reports a significant drop in blood pressure, and vascular injury is diagnosed. The surgeon attempts to control the bleeding using bipolar coagulation, but the problem only becomes worse. He decides to switch to laparotomy.
A vascular surgeon is called in, and injury to the right common iliac artery and vein—apparently caused during insertion of the primary umbilical trocar—is repaired. The patient is given 5 U of red blood cells. She goes home 10 days later, but returns with thrombophlebitis and rejection of the graft. After several surgeries, she finally recovers, with some sequelae, such as unilateral leg edema.
Management of vascular injury depends on the source and type of injury. On major vessels, electrocoagulation is contraindicated. After immediate atraumatic compression with tamponade to control bleeding, vascular repair, in consultation with a vascular surgeon, is indicated. At times, a vascular graft may be required.
Smaller vessels, such as the infundibulopelvic ligament or uterine vessels, can be managed by clips, suture, or loop ligatures. If thermal energy is used in the repair, be careful to avoid injury to surrounding structures.
Most emergency laparotomies are performed for uncontrolled bleeding.30,31 Lack of control or a wrong angle at insertion of the Veress needle and trocars is a major cause of large-vessel injury. Sharp dissection of adhesions, uterosacral ablation, transection of vascular pedicles without adequate dessication, and rough handling of tissues can all cause bleeding. Distorted anatomy is a main cause of vascular injury and can compound injury in areas more prone to bleeding, such as the oviduct, infundibulopelvic ligament, mesosalpinx, and pelvic sidewall vessels.
The return of pressure gradients to normal levels at the end of a procedure can be accompanied by bleeding into the retroperitoneal space, so evaluate the patient in a supine position after intra-abdominal pressure is reduced.
A vascular surgeon may be required
Depending on the type of vessel, size and location of the injury, and degree of bleeding, you may use unipolar or bipolar electrocoagulation, suture, clips, vasopressin, or loop ligatures to control bleeding. Although diluted vasopressin (10 U in 60 mL of lactated Ringer’s saline) can decrease oozing from raw peritoneal areas, injury to a major vessel, such as the iliac vessels, vena cava, or aorta, needs immediate control and proper repair. The decision to perform laparoscopy or laparotomy depends on your preference and experience. In any case, a vascular surgeon may be consulted for major vascular injuries.32
If a major vessel is injured, do not crush-clamp it. If possible (and if your laparoscopic skills are advanced), insert a sponge via a 10-mm trocar and apply pressure to the vessel to minimize bleeding and enhance visualization. The decision to repair the injury laparoscopically or by laparotomy should be made judiciously and promptly. n
The authors acknowledge the editorial contributions of Kristina Petrasek and Barbara Page, of the University of California, Berkeley, to the manuscript of this article.
1. Ostrzenski A, Radolinski B, Ostrzenska K. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794-799.
2. Kabalin J. Chapter 1—Surgical anatomy of the retroperitoneum, kidneys, and ureters. In: Walsh P, Retik A, Wein A, eds. Campbell’s Urology. 8th ed. Philadelphia: Saunders; 2002 36-40.
3. Chan J, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
4. Grainger DA, Soderstrom RM, Schiff SF, Glickman MG, DeCherney AH, Diamond MP. Ureteral injuries at laparoscopy: insights into diagnosis, management, and prevention. Obstet Gynecol. 1990;75:839-843.
5. Nezhat C. Chapter 20. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York: McGraw–Hill; 2000.
6. Ou CS, Huang IA, Rowbotham R. Laparoscopic ureteroureteral anastomosis for repair of ureteral injury involving stricture. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:155-157.
7. Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystostomy for distal ureteral injuries. Urology. 2005;66:751-753.
8. Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
9. Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80:543-544.
10. Nezhat CH, Nezhat FR, Freiha F, Nezhat CR. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
11. Georgy FM, Fettman HH, Chefetz MD. Complications of laparoscopy: two cases of perforated urinary bladder. Am J Obstet Gynecol. 1974;120:1121-1122.
12. Sherer DM. Inadvertent transvaginal cystotomy during laparoscopy. Int J Gynaecol Obstet. 1990;32:77-79.
13. Nezhat CH, Seidman DS, Nezhat F, et al. Laparoscopic management of internal and unintentional cystotomy. J Urol. 1996;156:1400-1402.
14. Lee CL, Lai YM, Soong YK. Management of urinary bladder injuries in laparoscopic assisted vaginal hysterectomy. Acta Obstet Gynecol Scand. 1996;75:174-177.
15. Peterson HB, et al. American Association of Gynecologic Laparoscopists’ 1988 membership survey on operative laparoscopy. J Reprod Med. 1990;35:587-589.
16. Phillips JM, Hulka JF, Hulka B, et al. 1978 AAGL membership survey. J Reprod Med. 1981;26:529-533.
17. Chapron C, Pierre F, et al. Gastrointestinal injuries during laparoscopy. Hum Reprod. 1999;14:333-337.
18. Schrenk P, Woisetschlager R, Rieger R, et al. Mechanism, management, and prevention of laparoscopic bowel injuries. Gastrointest Endosc. 1996;43:572-574.
19. Sauer M, Jarrett JC. Small bowel obstruction following diagnostic laparoscopy. Fertil Steril. 1984;42:653-654.
20. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
21. Brill A, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparatomy: a laparoscopic appraisal. Obstet Gynecol. 1995;85:269-272.
22. Levy BS, Soderstrom RM, Dail DH. Bowel injuries during laparoscopy: gross anatomy and histology. J Reprod Med. 1985;30:168-172.
23. Wheeless CR. Gastrointestinal injuries associated with laparoscopy. In: Phillips JM, ed. Endoscopy in Gynecology. Santa Fe Springs, Calif: American Association of Gynecologic Laparoscopists; 1978.
24. Borton M. Laparoscopic Complications: Prevention and Management. Philadelphia: Decker; 1986.
25. DeCherney AH. Laparoscopy with unexpected viscus penetration. In: Nichols DH, ed. Clinical Problems, Injuries, and Complications of Gynecologic Surgery. Baltimore: Williams & Wilkins; 1988.
26. Nezhat C, Nezhat F, Ambroze W, Pennington E. Laparoscopic repair of small bowel and colon: a report of 26 cases. Surg Endosc. 1993;7:88-89.
27. Redwine D. Laparoscopic en bloc resection for treatment of the obliterated cul-de-sac in endometriosis. J Reprod Med. 1992;37:696-698.
28. Nezhat C, Nezhat F, et al. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaseroscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
29. Nezhat C, Seidman D, Nezhat F, et al. The role of intraoperative proctosigmoidoscopy in laparoscopic pelvic surgery. J Am Assoc Gynecol Laparosc. 2004;11:47-49.
30. Chapron CM, et al. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
31. Geers J, Holden C. Major vascular injury as a complication of laparoscopic surgery: a report of three cases and review of the literature. Am Surg. 1996;62:377-379.
32. Nezhat C, Childers J, Nezhat F, et al. Major retroperitoneal vascular injury during laparoscopic surgery. Hum Reprod. 1997;12:480-483.
Dr. Farr Nezhat reports no relevant financial relationships. Dr. Ceana Nezhat is a speaker and consultant for Karl Storz, Johnson & Johnson, Valleylab, US Surgical, and Viking. Dr. Camran Nezhat is a speaker for or receives educational support from Karl Storz, Stryker, Johnson & Johnson, Valleylab, and Baxter.
1. Ostrzenski A, Radolinski B, Ostrzenska K. A review of laparoscopic ureteral injury in pelvic surgery. Obstet Gynecol Surv. 2003;58:794-799.
2. Kabalin J. Chapter 1—Surgical anatomy of the retroperitoneum, kidneys, and ureters. In: Walsh P, Retik A, Wein A, eds. Campbell’s Urology. 8th ed. Philadelphia: Saunders; 2002 36-40.
3. Chan J, Morrow J, Manetta A. Prevention of ureteral injuries in gynecologic surgery. Am J Obstet Gynecol. 2003;188:1273-1277.
4. Grainger DA, Soderstrom RM, Schiff SF, Glickman MG, DeCherney AH, Diamond MP. Ureteral injuries at laparoscopy: insights into diagnosis, management, and prevention. Obstet Gynecol. 1990;75:839-843.
5. Nezhat C. Chapter 20. Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York: McGraw–Hill; 2000.
6. Ou CS, Huang IA, Rowbotham R. Laparoscopic ureteroureteral anastomosis for repair of ureteral injury involving stricture. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:155-157.
7. Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystostomy for distal ureteral injuries. Urology. 2005;66:751-753.
8. Nezhat C, Nezhat F, Nezhat CH, et al. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920-924.
9. Nezhat C, Nezhat F. Laparoscopic repair of ureter resected during operative laparoscopy. Obstet Gynecol. 1992;80:543-544.
10. Nezhat CH, Nezhat FR, Freiha F, Nezhat CR. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999;71:376-379.
11. Georgy FM, Fettman HH, Chefetz MD. Complications of laparoscopy: two cases of perforated urinary bladder. Am J Obstet Gynecol. 1974;120:1121-1122.
12. Sherer DM. Inadvertent transvaginal cystotomy during laparoscopy. Int J Gynaecol Obstet. 1990;32:77-79.
13. Nezhat CH, Seidman DS, Nezhat F, et al. Laparoscopic management of internal and unintentional cystotomy. J Urol. 1996;156:1400-1402.
14. Lee CL, Lai YM, Soong YK. Management of urinary bladder injuries in laparoscopic assisted vaginal hysterectomy. Acta Obstet Gynecol Scand. 1996;75:174-177.
15. Peterson HB, et al. American Association of Gynecologic Laparoscopists’ 1988 membership survey on operative laparoscopy. J Reprod Med. 1990;35:587-589.
16. Phillips JM, Hulka JF, Hulka B, et al. 1978 AAGL membership survey. J Reprod Med. 1981;26:529-533.
17. Chapron C, Pierre F, et al. Gastrointestinal injuries during laparoscopy. Hum Reprod. 1999;14:333-337.
18. Schrenk P, Woisetschlager R, Rieger R, et al. Mechanism, management, and prevention of laparoscopic bowel injuries. Gastrointest Endosc. 1996;43:572-574.
19. Sauer M, Jarrett JC. Small bowel obstruction following diagnostic laparoscopy. Fertil Steril. 1984;42:653-654.
20. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
21. Brill A, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparatomy: a laparoscopic appraisal. Obstet Gynecol. 1995;85:269-272.
22. Levy BS, Soderstrom RM, Dail DH. Bowel injuries during laparoscopy: gross anatomy and histology. J Reprod Med. 1985;30:168-172.
23. Wheeless CR. Gastrointestinal injuries associated with laparoscopy. In: Phillips JM, ed. Endoscopy in Gynecology. Santa Fe Springs, Calif: American Association of Gynecologic Laparoscopists; 1978.
24. Borton M. Laparoscopic Complications: Prevention and Management. Philadelphia: Decker; 1986.
25. DeCherney AH. Laparoscopy with unexpected viscus penetration. In: Nichols DH, ed. Clinical Problems, Injuries, and Complications of Gynecologic Surgery. Baltimore: Williams & Wilkins; 1988.
26. Nezhat C, Nezhat F, Ambroze W, Pennington E. Laparoscopic repair of small bowel and colon: a report of 26 cases. Surg Endosc. 1993;7:88-89.
27. Redwine D. Laparoscopic en bloc resection for treatment of the obliterated cul-de-sac in endometriosis. J Reprod Med. 1992;37:696-698.
28. Nezhat C, Nezhat F, et al. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaseroscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
29. Nezhat C, Seidman D, Nezhat F, et al. The role of intraoperative proctosigmoidoscopy in laparoscopic pelvic surgery. J Am Assoc Gynecol Laparosc. 2004;11:47-49.
30. Chapron CM, et al. Major vascular injuries during gynecologic laparoscopy. J Am Coll Surg. 1997;185:461-465.
31. Geers J, Holden C. Major vascular injury as a complication of laparoscopic surgery: a report of three cases and review of the literature. Am Surg. 1996;62:377-379.
32. Nezhat C, Childers J, Nezhat F, et al. Major retroperitoneal vascular injury during laparoscopic surgery. Hum Reprod. 1997;12:480-483.
Dr. Farr Nezhat reports no relevant financial relationships. Dr. Ceana Nezhat is a speaker and consultant for Karl Storz, Johnson & Johnson, Valleylab, US Surgical, and Viking. Dr. Camran Nezhat is a speaker for or receives educational support from Karl Storz, Stryker, Johnson & Johnson, Valleylab, and Baxter.