User login
Hypertensive disorders of pregnancy in SLE contribute to later CV outcomes
Women with systemic lupus erythematosus (SLE) who experience hypertensive disorders of pregnancy may have a higher rate of cardiovascular outcomes after pregnancy, as well as a higher rate of hypertension later in life, than do those without maternal hypertension, according to findings from a Swedish population-based, longitudinal cohort study.
“Premature CVD [cardiovascular disease] is a well-documented complication in women with SLE, which is likely, at least in part, due to renal disease, prothrombotic [antiphospholipid antibodies], and systemic inflammation. Our data confirm that women who experience a hypertensive disorder in pregnancy [HDP] are at greater risk of developing hypertension after pregnancy, and that this association is also evident for women with SLE. Women with SLE and HDP were also at increased risk of CVD, particularly stroke, at young ages and should be monitored closely and consider treatment to attenuate risk,” wrote first author Julia F. Simard, ScD, of Stanford (Calif.) University and colleagues in Arthritis Care & Research.
To reach those conclusions, the researchers identified 3,340 women in the Swedish Medical Birth Register with their first singleton delivery during 1987-2012. They matched each of the 450 women with prevalent SLE from the Medical Birth Register to 5 women without SLE in the National Patient Register based on sex, birth year, calendar time, and county of residence.
During a median follow-up period of nearly 11 years, women with SLE had an unadjusted incidence rate of incident cardiovascular outcomes of 50 cases per 10,000 person-years versus 7.2 for women without SLE. Cardiovascular outcomes included fatal and nonfatal acute MI, fatal and nonfatal stroke, transient ischemic attacks, unstable angina, and heart failure. A history of HDP in women with SLE, including preeclampsia, was linked with about a twofold higher rate of cardiovascular outcomes regardless of multiple sensitivity analyses, both before and after adjusting for maternal age at delivery, county of birth, education, body mass index, and first-trimester smoking.
The researchers found that the hazard ratio for cardiovascular outcomes in women with SLE and HDP was about eight times higher than the hazard ratio for women without SLE but with HDP, but the relative rarity of cardiovascular events seen during the follow-up period, particularly among women without SLE, made it so that they “could not confirm established associations between HDP and CVD, possibly due to the relatively short follow-up time given that premenopausal CVD is rare among women free of SLE.”
HDP was associated with a threefold higher risk for incident hypertension later in life regardless of SLE status, even though the unadjusted incidence rate was 524 cases per 10,000 person-years among women with both SLE and HDP, compared with 177 per 10,000 person-years among women with HDP in the general population, which sensitivity analyses suggested “was not due to misclassification of antihypertensive use for renal disease in women with SLE nor antihypertensive use for possible HDP in subsequent pregnancies,” the researchers wrote.
Several authors reported research grants from the National Institutes of Health, the Karolinska Institute, the Swedish Research Council, Swedish Heart-Lung Foundation, Stockholm County Council, the King Gustaf V 80th Birthday Fund, the Swedish Rheumatism Association, and Ingegerd Johansson’s Foundation that helped to fund the study. All authors reported having no competing interests.
SOURCE: Simard JF et al. Arthritis Care Res. 2020 Jan 31. doi: 10.1002/acr.24160.
Women with systemic lupus erythematosus (SLE) who experience hypertensive disorders of pregnancy may have a higher rate of cardiovascular outcomes after pregnancy, as well as a higher rate of hypertension later in life, than do those without maternal hypertension, according to findings from a Swedish population-based, longitudinal cohort study.
“Premature CVD [cardiovascular disease] is a well-documented complication in women with SLE, which is likely, at least in part, due to renal disease, prothrombotic [antiphospholipid antibodies], and systemic inflammation. Our data confirm that women who experience a hypertensive disorder in pregnancy [HDP] are at greater risk of developing hypertension after pregnancy, and that this association is also evident for women with SLE. Women with SLE and HDP were also at increased risk of CVD, particularly stroke, at young ages and should be monitored closely and consider treatment to attenuate risk,” wrote first author Julia F. Simard, ScD, of Stanford (Calif.) University and colleagues in Arthritis Care & Research.
To reach those conclusions, the researchers identified 3,340 women in the Swedish Medical Birth Register with their first singleton delivery during 1987-2012. They matched each of the 450 women with prevalent SLE from the Medical Birth Register to 5 women without SLE in the National Patient Register based on sex, birth year, calendar time, and county of residence.
During a median follow-up period of nearly 11 years, women with SLE had an unadjusted incidence rate of incident cardiovascular outcomes of 50 cases per 10,000 person-years versus 7.2 for women without SLE. Cardiovascular outcomes included fatal and nonfatal acute MI, fatal and nonfatal stroke, transient ischemic attacks, unstable angina, and heart failure. A history of HDP in women with SLE, including preeclampsia, was linked with about a twofold higher rate of cardiovascular outcomes regardless of multiple sensitivity analyses, both before and after adjusting for maternal age at delivery, county of birth, education, body mass index, and first-trimester smoking.
The researchers found that the hazard ratio for cardiovascular outcomes in women with SLE and HDP was about eight times higher than the hazard ratio for women without SLE but with HDP, but the relative rarity of cardiovascular events seen during the follow-up period, particularly among women without SLE, made it so that they “could not confirm established associations between HDP and CVD, possibly due to the relatively short follow-up time given that premenopausal CVD is rare among women free of SLE.”
HDP was associated with a threefold higher risk for incident hypertension later in life regardless of SLE status, even though the unadjusted incidence rate was 524 cases per 10,000 person-years among women with both SLE and HDP, compared with 177 per 10,000 person-years among women with HDP in the general population, which sensitivity analyses suggested “was not due to misclassification of antihypertensive use for renal disease in women with SLE nor antihypertensive use for possible HDP in subsequent pregnancies,” the researchers wrote.
Several authors reported research grants from the National Institutes of Health, the Karolinska Institute, the Swedish Research Council, Swedish Heart-Lung Foundation, Stockholm County Council, the King Gustaf V 80th Birthday Fund, the Swedish Rheumatism Association, and Ingegerd Johansson’s Foundation that helped to fund the study. All authors reported having no competing interests.
SOURCE: Simard JF et al. Arthritis Care Res. 2020 Jan 31. doi: 10.1002/acr.24160.
Women with systemic lupus erythematosus (SLE) who experience hypertensive disorders of pregnancy may have a higher rate of cardiovascular outcomes after pregnancy, as well as a higher rate of hypertension later in life, than do those without maternal hypertension, according to findings from a Swedish population-based, longitudinal cohort study.
“Premature CVD [cardiovascular disease] is a well-documented complication in women with SLE, which is likely, at least in part, due to renal disease, prothrombotic [antiphospholipid antibodies], and systemic inflammation. Our data confirm that women who experience a hypertensive disorder in pregnancy [HDP] are at greater risk of developing hypertension after pregnancy, and that this association is also evident for women with SLE. Women with SLE and HDP were also at increased risk of CVD, particularly stroke, at young ages and should be monitored closely and consider treatment to attenuate risk,” wrote first author Julia F. Simard, ScD, of Stanford (Calif.) University and colleagues in Arthritis Care & Research.
To reach those conclusions, the researchers identified 3,340 women in the Swedish Medical Birth Register with their first singleton delivery during 1987-2012. They matched each of the 450 women with prevalent SLE from the Medical Birth Register to 5 women without SLE in the National Patient Register based on sex, birth year, calendar time, and county of residence.
During a median follow-up period of nearly 11 years, women with SLE had an unadjusted incidence rate of incident cardiovascular outcomes of 50 cases per 10,000 person-years versus 7.2 for women without SLE. Cardiovascular outcomes included fatal and nonfatal acute MI, fatal and nonfatal stroke, transient ischemic attacks, unstable angina, and heart failure. A history of HDP in women with SLE, including preeclampsia, was linked with about a twofold higher rate of cardiovascular outcomes regardless of multiple sensitivity analyses, both before and after adjusting for maternal age at delivery, county of birth, education, body mass index, and first-trimester smoking.
The researchers found that the hazard ratio for cardiovascular outcomes in women with SLE and HDP was about eight times higher than the hazard ratio for women without SLE but with HDP, but the relative rarity of cardiovascular events seen during the follow-up period, particularly among women without SLE, made it so that they “could not confirm established associations between HDP and CVD, possibly due to the relatively short follow-up time given that premenopausal CVD is rare among women free of SLE.”
HDP was associated with a threefold higher risk for incident hypertension later in life regardless of SLE status, even though the unadjusted incidence rate was 524 cases per 10,000 person-years among women with both SLE and HDP, compared with 177 per 10,000 person-years among women with HDP in the general population, which sensitivity analyses suggested “was not due to misclassification of antihypertensive use for renal disease in women with SLE nor antihypertensive use for possible HDP in subsequent pregnancies,” the researchers wrote.
Several authors reported research grants from the National Institutes of Health, the Karolinska Institute, the Swedish Research Council, Swedish Heart-Lung Foundation, Stockholm County Council, the King Gustaf V 80th Birthday Fund, the Swedish Rheumatism Association, and Ingegerd Johansson’s Foundation that helped to fund the study. All authors reported having no competing interests.
SOURCE: Simard JF et al. Arthritis Care Res. 2020 Jan 31. doi: 10.1002/acr.24160.
FROM ARTHRITIS CARE & RESEARCH
FDA approves novel pandemic influenza vaccine
The Food and Drug Administration has approved the first and only adjuvanted, cell-based pandemic vaccine to provide active immunization against the influenza A virus H5N1 strain.
Influenza A (H5N1) monovalent vaccine, adjuvanted (Audenz, Seqirus) is for use in individuals aged 6 months and older. It’s designed to be rapidly deployed to help protect the U.S. population and can be stockpiled for first responders in the event of a pandemic.
The vaccine and formulated prefilled syringes used in the vaccine are produced in a state-of-the-art production facility built and supported through a multiyear public-private partnership between Seqirus and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health & Human Services.
“Pandemic influenza viruses can be deadly and spread rapidly, making production of safe, effective vaccines essential in saving lives,” BARDA Director Rick Bright, PhD, said in a company news release.
“With this licensure – the latest FDA-approved vaccine to prevent H5N1 influenza — we celebrate a decade-long partnership to achieve health security goals set by the National Strategy for Pandemic Influenza and the 2019 Executive Order to speed the availability of influenza vaccine. Ultimately, this latest licensure means we can protect more people in an influenza pandemic,” said Bright.
“The approval of Audenz represents a key advance in influenza prevention and pandemic preparedness, combining leading-edge, cell-based manufacturing and adjuvant technologies,” Russell Basser, MD, chief scientist and senior vice president of research and development at Seqirus, said in the news release. “This pandemic influenza vaccine exemplifies our commitment to developing innovative technologies that can help provide rapid response during a pandemic emergency.”
Audenz had FDA fast track designation, a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
This article first appeared on Medscape.com.
The Food and Drug Administration has approved the first and only adjuvanted, cell-based pandemic vaccine to provide active immunization against the influenza A virus H5N1 strain.
Influenza A (H5N1) monovalent vaccine, adjuvanted (Audenz, Seqirus) is for use in individuals aged 6 months and older. It’s designed to be rapidly deployed to help protect the U.S. population and can be stockpiled for first responders in the event of a pandemic.
The vaccine and formulated prefilled syringes used in the vaccine are produced in a state-of-the-art production facility built and supported through a multiyear public-private partnership between Seqirus and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health & Human Services.
“Pandemic influenza viruses can be deadly and spread rapidly, making production of safe, effective vaccines essential in saving lives,” BARDA Director Rick Bright, PhD, said in a company news release.
“With this licensure – the latest FDA-approved vaccine to prevent H5N1 influenza — we celebrate a decade-long partnership to achieve health security goals set by the National Strategy for Pandemic Influenza and the 2019 Executive Order to speed the availability of influenza vaccine. Ultimately, this latest licensure means we can protect more people in an influenza pandemic,” said Bright.
“The approval of Audenz represents a key advance in influenza prevention and pandemic preparedness, combining leading-edge, cell-based manufacturing and adjuvant technologies,” Russell Basser, MD, chief scientist and senior vice president of research and development at Seqirus, said in the news release. “This pandemic influenza vaccine exemplifies our commitment to developing innovative technologies that can help provide rapid response during a pandemic emergency.”
Audenz had FDA fast track designation, a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
This article first appeared on Medscape.com.
The Food and Drug Administration has approved the first and only adjuvanted, cell-based pandemic vaccine to provide active immunization against the influenza A virus H5N1 strain.
Influenza A (H5N1) monovalent vaccine, adjuvanted (Audenz, Seqirus) is for use in individuals aged 6 months and older. It’s designed to be rapidly deployed to help protect the U.S. population and can be stockpiled for first responders in the event of a pandemic.
The vaccine and formulated prefilled syringes used in the vaccine are produced in a state-of-the-art production facility built and supported through a multiyear public-private partnership between Seqirus and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health & Human Services.
“Pandemic influenza viruses can be deadly and spread rapidly, making production of safe, effective vaccines essential in saving lives,” BARDA Director Rick Bright, PhD, said in a company news release.
“With this licensure – the latest FDA-approved vaccine to prevent H5N1 influenza — we celebrate a decade-long partnership to achieve health security goals set by the National Strategy for Pandemic Influenza and the 2019 Executive Order to speed the availability of influenza vaccine. Ultimately, this latest licensure means we can protect more people in an influenza pandemic,” said Bright.
“The approval of Audenz represents a key advance in influenza prevention and pandemic preparedness, combining leading-edge, cell-based manufacturing and adjuvant technologies,” Russell Basser, MD, chief scientist and senior vice president of research and development at Seqirus, said in the news release. “This pandemic influenza vaccine exemplifies our commitment to developing innovative technologies that can help provide rapid response during a pandemic emergency.”
Audenz had FDA fast track designation, a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
This article first appeared on Medscape.com.
Preoperative CT shows little value in early vulvar SCC
Preoperative computerized tomography (CT) demonstrated limited value in the clinical management of patients with early-stage vulvar squamous cell carcinoma (VSCC) analyzed in a single-center study.
The findings suggest preoperative CT imaging could be excluded prior to sentinel inguinal lymph node biopsy or staging surgery in patients with early-stage disease.
“In this study, we aimed to investigate if preoperative CT scan influences the overall course of VSCC management in patients without clinical evidence of groin lymphadenopathy,” wrote Rachel Pounds, MD, of the University of Birmingham (England) and colleagues. The study was published in Gynecologic Oncology.
The researchers prospectively studied a cohort of 225 patients with primary or recurrent VSCC who underwent staging surgery at a single institution in the United Kingdom. The patients’ mean age was 67 years (range, 54-79 years), and most had stage 1B disease (57.8%).
The researchers compared preoperative imaging findings with histological results from sentinel inguinal lymph node biopsy. Other clinical information, including surgery type, evidence of groin node involvement, and age at diagnosis was collected from patient files and included in the analysis.
In all, 51.6% of patients underwent preoperative CT imaging. Among these patients, 37.9% had a positive report of radiological groin lymphatic metastases.
“True groin node metastases, confirmed histologically, were observed in 26 patients (22.4%) with a radiologically positive scan report (true positives) and in 18 patients (15.5%) with a radiological negative scan report (false negatives),” the researchers wrote.
The specificity and sensitivity of preoperative CT to detect groin lymphatic metastasis were 77.8% and 59.1%, respectively. The positive and negative predictive values were 61.9% and 75.7%, respectively.
There was no significant difference in overall survival, disease-specific or disease-free survival, or groin node recurrence between patients who underwent preoperative CT and patients who did not.
Groin node recurrence was observed in 10.3% of patients with preoperative CT and 11.5% of patients without it (P = .7768). Disease-specific death occurred in 16.4% of patients with preoperative CT and 13.5% of patients without it (P = .5451).
“Our results highlight the poor reliability of preoperative CT scans in detecting inguinal lymphatic metastasis,” the researchers wrote. “Preoperative CT scan may be omitted in early stage VSCC prior to surgical staging as it does not affect overall management and surgical outcomes.”
No funding sources were reported for this study. The authors reported having no conflicts of interest.
SOURCE: Pounds R et al. Gynecol Oncol. 2020 Jan 24. doi: 10.1016/j.ygyno.2020.01.031.
Preoperative computerized tomography (CT) demonstrated limited value in the clinical management of patients with early-stage vulvar squamous cell carcinoma (VSCC) analyzed in a single-center study.
The findings suggest preoperative CT imaging could be excluded prior to sentinel inguinal lymph node biopsy or staging surgery in patients with early-stage disease.
“In this study, we aimed to investigate if preoperative CT scan influences the overall course of VSCC management in patients without clinical evidence of groin lymphadenopathy,” wrote Rachel Pounds, MD, of the University of Birmingham (England) and colleagues. The study was published in Gynecologic Oncology.
The researchers prospectively studied a cohort of 225 patients with primary or recurrent VSCC who underwent staging surgery at a single institution in the United Kingdom. The patients’ mean age was 67 years (range, 54-79 years), and most had stage 1B disease (57.8%).
The researchers compared preoperative imaging findings with histological results from sentinel inguinal lymph node biopsy. Other clinical information, including surgery type, evidence of groin node involvement, and age at diagnosis was collected from patient files and included in the analysis.
In all, 51.6% of patients underwent preoperative CT imaging. Among these patients, 37.9% had a positive report of radiological groin lymphatic metastases.
“True groin node metastases, confirmed histologically, were observed in 26 patients (22.4%) with a radiologically positive scan report (true positives) and in 18 patients (15.5%) with a radiological negative scan report (false negatives),” the researchers wrote.
The specificity and sensitivity of preoperative CT to detect groin lymphatic metastasis were 77.8% and 59.1%, respectively. The positive and negative predictive values were 61.9% and 75.7%, respectively.
There was no significant difference in overall survival, disease-specific or disease-free survival, or groin node recurrence between patients who underwent preoperative CT and patients who did not.
Groin node recurrence was observed in 10.3% of patients with preoperative CT and 11.5% of patients without it (P = .7768). Disease-specific death occurred in 16.4% of patients with preoperative CT and 13.5% of patients without it (P = .5451).
“Our results highlight the poor reliability of preoperative CT scans in detecting inguinal lymphatic metastasis,” the researchers wrote. “Preoperative CT scan may be omitted in early stage VSCC prior to surgical staging as it does not affect overall management and surgical outcomes.”
No funding sources were reported for this study. The authors reported having no conflicts of interest.
SOURCE: Pounds R et al. Gynecol Oncol. 2020 Jan 24. doi: 10.1016/j.ygyno.2020.01.031.
Preoperative computerized tomography (CT) demonstrated limited value in the clinical management of patients with early-stage vulvar squamous cell carcinoma (VSCC) analyzed in a single-center study.
The findings suggest preoperative CT imaging could be excluded prior to sentinel inguinal lymph node biopsy or staging surgery in patients with early-stage disease.
“In this study, we aimed to investigate if preoperative CT scan influences the overall course of VSCC management in patients without clinical evidence of groin lymphadenopathy,” wrote Rachel Pounds, MD, of the University of Birmingham (England) and colleagues. The study was published in Gynecologic Oncology.
The researchers prospectively studied a cohort of 225 patients with primary or recurrent VSCC who underwent staging surgery at a single institution in the United Kingdom. The patients’ mean age was 67 years (range, 54-79 years), and most had stage 1B disease (57.8%).
The researchers compared preoperative imaging findings with histological results from sentinel inguinal lymph node biopsy. Other clinical information, including surgery type, evidence of groin node involvement, and age at diagnosis was collected from patient files and included in the analysis.
In all, 51.6% of patients underwent preoperative CT imaging. Among these patients, 37.9% had a positive report of radiological groin lymphatic metastases.
“True groin node metastases, confirmed histologically, were observed in 26 patients (22.4%) with a radiologically positive scan report (true positives) and in 18 patients (15.5%) with a radiological negative scan report (false negatives),” the researchers wrote.
The specificity and sensitivity of preoperative CT to detect groin lymphatic metastasis were 77.8% and 59.1%, respectively. The positive and negative predictive values were 61.9% and 75.7%, respectively.
There was no significant difference in overall survival, disease-specific or disease-free survival, or groin node recurrence between patients who underwent preoperative CT and patients who did not.
Groin node recurrence was observed in 10.3% of patients with preoperative CT and 11.5% of patients without it (P = .7768). Disease-specific death occurred in 16.4% of patients with preoperative CT and 13.5% of patients without it (P = .5451).
“Our results highlight the poor reliability of preoperative CT scans in detecting inguinal lymphatic metastasis,” the researchers wrote. “Preoperative CT scan may be omitted in early stage VSCC prior to surgical staging as it does not affect overall management and surgical outcomes.”
No funding sources were reported for this study. The authors reported having no conflicts of interest.
SOURCE: Pounds R et al. Gynecol Oncol. 2020 Jan 24. doi: 10.1016/j.ygyno.2020.01.031.
FROM GYNECOLOGIC ONCOLOGY
Rate of suicide is higher in people with neurologic disorders
The absolute risk difference is small, but statistically significant. “These findings do not necessarily warrant changing the management of treatment for individual patients,” wrote Annette Erlangsen, PhD, a researcher at the Danish Research Institute for Suicide Prevention in Hellerup, and colleagues. “As with all patients, physicians should be aware of the potential for depression, demoralization, and suicide.”
In addition, dementia, Alzheimer’s disease, and intellectual disabilities may be associated with lower suicide rates, according to the study, which was published in JAMA.
“Plausible mechanisms” could underlie the association between neurologic disease and suicide, the authors wrote. A neurologic diagnosis “may constitute a distressing life event,” and the diseases may have psychological, physical, and psychiatric effects. Patients may see themselves as a burden or have less financial security. In addition, the diseases may entail “communication difficulties, poor sleep, and pain.” Neurologic diseases may alter brain circuitry and functioning and influence aggression and impulsivity. “People with neurologic disorders may also have easier access to toxic medication,” they added.
More than a dozen conditions examined
Prior studies have found associations between neurologic conditions and rates of suicide, but data have been inconclusive or inconsistent for some of the disorders. To examine whether people with neurologic disorders have higher suicide rates, relative to people without these disorders, the researchers conducted a retrospective study. They analyzed data from more than 7.3 million people aged 15 years or older who lived in Denmark between 1980 and 2016. The cohort included more than 1.2 million people with neurologic disorders. The investigators identified neurologic disorders using ICD codes for head injury, stroke, epilepsy, polyneuropathy, diseases of the myoneural junction, Parkinson’s disease, multiple sclerosis, CNS infections, meningitis, encephalitis, amyotrophic lateral sclerosis, Huntington’s disease, dementia, intellectual disability, and other brain disorders. They compared incidence rates using a Poisson regression model and adjusted for time period, sex, age, region, socioeconomic status, comorbidity, self-harm or psychiatric hospitalization prior to a neurologic diagnosis, and whether a person lived alone.
In all, 35,483 people in the cohort died by suicide at an average age of about 52 years; 77.4% were male. About 15% of those who died by suicide had a neurologic disorder. The suicide incidence rate among people with a neurologic disorder was 44.0 per 100,000 person-years, whereas the rate among people without a neurologic disorder was 20.1 per 100,000 person-years.
The adjusted incidence rate ratio for people with a neurologic disorder was 1.8. The rate ratio was highest during the 3 months after diagnosis, at 3.1. Huntington’s disease and amyotrophic lateral sclerosis were associated with “the largest excess adjusted [incidence rate ratios] of suicide mortality,” with a rate ratio of 4.9 for each condition, the researchers reported. The adjusted incidence rate ratio was 1.7 for head injury, 1.3 for stroke, 1.7 for epilepsy, 1.4 for intracerebral hemorrhage, 1.3 for cerebral infarction, 1.3 for subarachnoid hemorrhage, 1.7 for polyneuropathy and peripheral neuropathy, 2.2 for Guillain-Barré syndrome, 1.9 for diseases of myoneural junction and muscle, 1.8 for other brain disorders, 1.7 for Parkinson’s disease, 2.2 for multiple sclerosis, and 1.6 for CNS infection.
Compared with people without a neurologic condition, people with dementia, Alzheimer’s disease, and intellectual disabilities had lower suicide rates, with adjusted incidence rate ratios of 0.8, 0.2, and 0.6, respectively. “However, the adjusted [incidence rate ratio] for people with dementia during the first month after diagnosis was 3.0,” the researchers wrote.
In addition, the suicide rate increased with an increasing cumulative number of hospital contacts for neurologic conditions.
Overall incidence rates declined
“Over the study period, the suicide incidence rate for people with neurological disorders decreased from 78.6 per 100,000 person-years during the 1980-1999 years to 27.3 per 100,000 person-years during the 2000-2016 years,” wrote Dr. Erlangsen and colleagues. “The suicide incidence rate for those without a disorder decreased from 26.3 to 12.7 during the same time spans. ... The decline in the overall suicide rate over time did not affect the relative risk pattern.”
The decline in the general suicide rate in Denmark “has largely been attributed to means restriction, such as efforts to limit availability of firearms and particularly toxic medication,” the authors added.
In those time spans, the adjusted incidence rate ratio for suicide among those with dementia decreased from 2.4 to 1.0, and among those with multiple sclerosis from 2.0 to 1.0. “It is possible that the improvements observed for dementia and multiple sclerosis may be related to improvements in treatment and intensified community-based support,” Dr. Erlangsen and coauthors wrote.
When the researchers used people with rheumatoid arthritis as a reference group, those with a neurologic disorder had a higher suicide rate per 100,000 person-years, 30.2 versus 18.4. The adjusted incidence rate ratio for that comparison was 1.4.
In patients with Huntington’s disease, depression mediated by hyperactivity in the hypothalamic-pituitary-adrenal axis may contribute to the risk of suicide. “Witnessing the course of the disease in one’s parent” also may contribute the risk, the researchers wrote.
The analysis may have missed people with neurologic disorders diagnosed before 1977 if they did not have subsequent contact with a hospital, the investigators noted. In addition, diagnoses given in primary care were not included, suicide deaths may be underrecorded, and “adjusting for preexisting mental disorders could be viewed as overadjusting,” they wrote.
The study was supported by a grant from the Psychiatric Research Foundation in Denmark. The authors reported that they had no disclosures.
SOURCE: Erlangsen A et al. JAMA. 2020 Feb 4. doi: 10.1001/jama.2019.21834.
The absolute risk difference is small, but statistically significant. “These findings do not necessarily warrant changing the management of treatment for individual patients,” wrote Annette Erlangsen, PhD, a researcher at the Danish Research Institute for Suicide Prevention in Hellerup, and colleagues. “As with all patients, physicians should be aware of the potential for depression, demoralization, and suicide.”
In addition, dementia, Alzheimer’s disease, and intellectual disabilities may be associated with lower suicide rates, according to the study, which was published in JAMA.
“Plausible mechanisms” could underlie the association between neurologic disease and suicide, the authors wrote. A neurologic diagnosis “may constitute a distressing life event,” and the diseases may have psychological, physical, and psychiatric effects. Patients may see themselves as a burden or have less financial security. In addition, the diseases may entail “communication difficulties, poor sleep, and pain.” Neurologic diseases may alter brain circuitry and functioning and influence aggression and impulsivity. “People with neurologic disorders may also have easier access to toxic medication,” they added.
More than a dozen conditions examined
Prior studies have found associations between neurologic conditions and rates of suicide, but data have been inconclusive or inconsistent for some of the disorders. To examine whether people with neurologic disorders have higher suicide rates, relative to people without these disorders, the researchers conducted a retrospective study. They analyzed data from more than 7.3 million people aged 15 years or older who lived in Denmark between 1980 and 2016. The cohort included more than 1.2 million people with neurologic disorders. The investigators identified neurologic disorders using ICD codes for head injury, stroke, epilepsy, polyneuropathy, diseases of the myoneural junction, Parkinson’s disease, multiple sclerosis, CNS infections, meningitis, encephalitis, amyotrophic lateral sclerosis, Huntington’s disease, dementia, intellectual disability, and other brain disorders. They compared incidence rates using a Poisson regression model and adjusted for time period, sex, age, region, socioeconomic status, comorbidity, self-harm or psychiatric hospitalization prior to a neurologic diagnosis, and whether a person lived alone.
In all, 35,483 people in the cohort died by suicide at an average age of about 52 years; 77.4% were male. About 15% of those who died by suicide had a neurologic disorder. The suicide incidence rate among people with a neurologic disorder was 44.0 per 100,000 person-years, whereas the rate among people without a neurologic disorder was 20.1 per 100,000 person-years.
The adjusted incidence rate ratio for people with a neurologic disorder was 1.8. The rate ratio was highest during the 3 months after diagnosis, at 3.1. Huntington’s disease and amyotrophic lateral sclerosis were associated with “the largest excess adjusted [incidence rate ratios] of suicide mortality,” with a rate ratio of 4.9 for each condition, the researchers reported. The adjusted incidence rate ratio was 1.7 for head injury, 1.3 for stroke, 1.7 for epilepsy, 1.4 for intracerebral hemorrhage, 1.3 for cerebral infarction, 1.3 for subarachnoid hemorrhage, 1.7 for polyneuropathy and peripheral neuropathy, 2.2 for Guillain-Barré syndrome, 1.9 for diseases of myoneural junction and muscle, 1.8 for other brain disorders, 1.7 for Parkinson’s disease, 2.2 for multiple sclerosis, and 1.6 for CNS infection.
Compared with people without a neurologic condition, people with dementia, Alzheimer’s disease, and intellectual disabilities had lower suicide rates, with adjusted incidence rate ratios of 0.8, 0.2, and 0.6, respectively. “However, the adjusted [incidence rate ratio] for people with dementia during the first month after diagnosis was 3.0,” the researchers wrote.
In addition, the suicide rate increased with an increasing cumulative number of hospital contacts for neurologic conditions.
Overall incidence rates declined
“Over the study period, the suicide incidence rate for people with neurological disorders decreased from 78.6 per 100,000 person-years during the 1980-1999 years to 27.3 per 100,000 person-years during the 2000-2016 years,” wrote Dr. Erlangsen and colleagues. “The suicide incidence rate for those without a disorder decreased from 26.3 to 12.7 during the same time spans. ... The decline in the overall suicide rate over time did not affect the relative risk pattern.”
The decline in the general suicide rate in Denmark “has largely been attributed to means restriction, such as efforts to limit availability of firearms and particularly toxic medication,” the authors added.
In those time spans, the adjusted incidence rate ratio for suicide among those with dementia decreased from 2.4 to 1.0, and among those with multiple sclerosis from 2.0 to 1.0. “It is possible that the improvements observed for dementia and multiple sclerosis may be related to improvements in treatment and intensified community-based support,” Dr. Erlangsen and coauthors wrote.
When the researchers used people with rheumatoid arthritis as a reference group, those with a neurologic disorder had a higher suicide rate per 100,000 person-years, 30.2 versus 18.4. The adjusted incidence rate ratio for that comparison was 1.4.
In patients with Huntington’s disease, depression mediated by hyperactivity in the hypothalamic-pituitary-adrenal axis may contribute to the risk of suicide. “Witnessing the course of the disease in one’s parent” also may contribute the risk, the researchers wrote.
The analysis may have missed people with neurologic disorders diagnosed before 1977 if they did not have subsequent contact with a hospital, the investigators noted. In addition, diagnoses given in primary care were not included, suicide deaths may be underrecorded, and “adjusting for preexisting mental disorders could be viewed as overadjusting,” they wrote.
The study was supported by a grant from the Psychiatric Research Foundation in Denmark. The authors reported that they had no disclosures.
SOURCE: Erlangsen A et al. JAMA. 2020 Feb 4. doi: 10.1001/jama.2019.21834.
The absolute risk difference is small, but statistically significant. “These findings do not necessarily warrant changing the management of treatment for individual patients,” wrote Annette Erlangsen, PhD, a researcher at the Danish Research Institute for Suicide Prevention in Hellerup, and colleagues. “As with all patients, physicians should be aware of the potential for depression, demoralization, and suicide.”
In addition, dementia, Alzheimer’s disease, and intellectual disabilities may be associated with lower suicide rates, according to the study, which was published in JAMA.
“Plausible mechanisms” could underlie the association between neurologic disease and suicide, the authors wrote. A neurologic diagnosis “may constitute a distressing life event,” and the diseases may have psychological, physical, and psychiatric effects. Patients may see themselves as a burden or have less financial security. In addition, the diseases may entail “communication difficulties, poor sleep, and pain.” Neurologic diseases may alter brain circuitry and functioning and influence aggression and impulsivity. “People with neurologic disorders may also have easier access to toxic medication,” they added.
More than a dozen conditions examined
Prior studies have found associations between neurologic conditions and rates of suicide, but data have been inconclusive or inconsistent for some of the disorders. To examine whether people with neurologic disorders have higher suicide rates, relative to people without these disorders, the researchers conducted a retrospective study. They analyzed data from more than 7.3 million people aged 15 years or older who lived in Denmark between 1980 and 2016. The cohort included more than 1.2 million people with neurologic disorders. The investigators identified neurologic disorders using ICD codes for head injury, stroke, epilepsy, polyneuropathy, diseases of the myoneural junction, Parkinson’s disease, multiple sclerosis, CNS infections, meningitis, encephalitis, amyotrophic lateral sclerosis, Huntington’s disease, dementia, intellectual disability, and other brain disorders. They compared incidence rates using a Poisson regression model and adjusted for time period, sex, age, region, socioeconomic status, comorbidity, self-harm or psychiatric hospitalization prior to a neurologic diagnosis, and whether a person lived alone.
In all, 35,483 people in the cohort died by suicide at an average age of about 52 years; 77.4% were male. About 15% of those who died by suicide had a neurologic disorder. The suicide incidence rate among people with a neurologic disorder was 44.0 per 100,000 person-years, whereas the rate among people without a neurologic disorder was 20.1 per 100,000 person-years.
The adjusted incidence rate ratio for people with a neurologic disorder was 1.8. The rate ratio was highest during the 3 months after diagnosis, at 3.1. Huntington’s disease and amyotrophic lateral sclerosis were associated with “the largest excess adjusted [incidence rate ratios] of suicide mortality,” with a rate ratio of 4.9 for each condition, the researchers reported. The adjusted incidence rate ratio was 1.7 for head injury, 1.3 for stroke, 1.7 for epilepsy, 1.4 for intracerebral hemorrhage, 1.3 for cerebral infarction, 1.3 for subarachnoid hemorrhage, 1.7 for polyneuropathy and peripheral neuropathy, 2.2 for Guillain-Barré syndrome, 1.9 for diseases of myoneural junction and muscle, 1.8 for other brain disorders, 1.7 for Parkinson’s disease, 2.2 for multiple sclerosis, and 1.6 for CNS infection.
Compared with people without a neurologic condition, people with dementia, Alzheimer’s disease, and intellectual disabilities had lower suicide rates, with adjusted incidence rate ratios of 0.8, 0.2, and 0.6, respectively. “However, the adjusted [incidence rate ratio] for people with dementia during the first month after diagnosis was 3.0,” the researchers wrote.
In addition, the suicide rate increased with an increasing cumulative number of hospital contacts for neurologic conditions.
Overall incidence rates declined
“Over the study period, the suicide incidence rate for people with neurological disorders decreased from 78.6 per 100,000 person-years during the 1980-1999 years to 27.3 per 100,000 person-years during the 2000-2016 years,” wrote Dr. Erlangsen and colleagues. “The suicide incidence rate for those without a disorder decreased from 26.3 to 12.7 during the same time spans. ... The decline in the overall suicide rate over time did not affect the relative risk pattern.”
The decline in the general suicide rate in Denmark “has largely been attributed to means restriction, such as efforts to limit availability of firearms and particularly toxic medication,” the authors added.
In those time spans, the adjusted incidence rate ratio for suicide among those with dementia decreased from 2.4 to 1.0, and among those with multiple sclerosis from 2.0 to 1.0. “It is possible that the improvements observed for dementia and multiple sclerosis may be related to improvements in treatment and intensified community-based support,” Dr. Erlangsen and coauthors wrote.
When the researchers used people with rheumatoid arthritis as a reference group, those with a neurologic disorder had a higher suicide rate per 100,000 person-years, 30.2 versus 18.4. The adjusted incidence rate ratio for that comparison was 1.4.
In patients with Huntington’s disease, depression mediated by hyperactivity in the hypothalamic-pituitary-adrenal axis may contribute to the risk of suicide. “Witnessing the course of the disease in one’s parent” also may contribute the risk, the researchers wrote.
The analysis may have missed people with neurologic disorders diagnosed before 1977 if they did not have subsequent contact with a hospital, the investigators noted. In addition, diagnoses given in primary care were not included, suicide deaths may be underrecorded, and “adjusting for preexisting mental disorders could be viewed as overadjusting,” they wrote.
The study was supported by a grant from the Psychiatric Research Foundation in Denmark. The authors reported that they had no disclosures.
SOURCE: Erlangsen A et al. JAMA. 2020 Feb 4. doi: 10.1001/jama.2019.21834.
FROM JAMA
Losartan showing promise in pediatric epidermolysis bullosa trial
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
LONDON – Treatment with the in an early clinical study.
In the ongoing phase 1/2 REFLECT (Recessive dystrophic EB: Mechanisms of fibrosis and its prevention with Losartan in vivo) trial, involving 29 children, no severe complications have been noted so far, according to one of the study investigators, Dimitra Kiritsi, MD, of the University of Freiburg, Germany. At the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), she presented interim data on 18 patients in the trial, emphasizing that the primary aim of the trial was to evaluate the safety of this treatment approach.
Over the 2 years the trial has been underway, 65 adverse events have been reported, of which 4 have been severe. Two of these were bacterial infections that required hospital treatment and the other two were a reduction in the general health condition of the child.
Losartan is an angiotensin-II receptor blocker (ARB) that has been in clinical use for more than 25 years in adults and 15 years in children over the age of 6 years.
The drug may be used for treating recessive dystrophic EB (RDEB) in the future, Dr. Kiritsi said, because it attenuates tumor necrosis factor–beta (TGF-beta) signaling, which is thought to be involved in the fibrotic process. So while it may not target the genetic defect, it could help ameliorate the effects of the disease.
The precursor to REFLECT was a study performed in a mouse disease model of EB (EMBO Mol Med. 2015;7:1211-28) where a reduction in fibrotic scarring was seen with losartan with “remarkable effects” on “mitten” deformity, Dr. Kiritsi said. The results of that study suggested that the earlier treatment with losartan was started in the course of the disease, the better the effect, she added. (Mitten deformity is the result of fused skin between the fingers or toes, and the subsequent buildup of fibrotic tissue causes the hand or foot to contract.)
REFLECT is an investigator-initiated trial that started in 2017 and is being funded by DEBRA International. It is a dual-center, nonrandomized, single-arm study in which children aged 3-16 years with RDEB are treated with losartan for 10 months, with follow-up at 3 months.
Various secondary endpoints were included to look for the first signs of any efficacy: the Physician’s Global Assessment (PGA), the Birmingham Epidermolysis Bullosa Severity Score (BEBS), the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), the Itch Assessment Scale for the Pediatric Burn Patients, and two quality of life indices: the Quality of Life in EB (QOLEB) questionnaire and the Children’s Dermatology Life Quality Index (CDLQI).
Dr. Kiritsi highlighted a few of the secondary endpoint findings, saying that reduced BEBS scores showed there was “amelioration of the patients’ phenotype” and that EBDASI scores also decreased, with “nearly 60% of the patients having significant improvement of their skin disease.” Importantly, itch improved in most of the patients, she said. Reductions in CDLQI were observed, “meaning that quality of life was significantly better at the end of the trial.” There were also decreases in inflammatory markers, such as C-reactive protein, interleukin-6, and TNF-alpha.
Although there is no validated tool available to assess hand function, Dr. Kiritsi and her team used their own morphometric scoring instrument to measure how far the hand could stretch; their evaluations suggested that this measure improved – or at least did not worsen – with losartan treatment, she noted.
A larger, randomized trial is needed to confirm if there is any benefit of losartan, but first, a new, easy-to-swallow losartan formulation needs to be developed specifically for EB in the pediatric population, Dr. Kiritsi said. Although a pediatric suspension of losartan was previously available, it is no longer on the market, so the next step is to develop a formulation that could be used in a pivotal clinical trial, she noted.
“Losartan faces fewer technical hurdles compared to other novel treatments as it is an established medicine,” Dr. Kiritsi and associates observed in a poster presentation. There are still economic hurdles, however, since “with losartan patents expired, companies cannot expect to recoup an investment into clinical studies” and alternative funding sources are needed.
In 2019, losartan was granted an orphan drug designation for the treatment of EB from both the Food and Drug Administration and the European Medicines Agency, but its use remains off label in children. “We decided to treat children,” Dr. Kiritsi said, “because we wanted to start as early as possible. If you already have mitten deformities, these cannot be reversed.”
DEBRA International funded the study. Dr. Kiritsi received research support from Rheacell GmbH and honoraria or consultation fees from Amryt Pharma and Rheacell GmbH. She has received other support from DEBRA International, EB Research Partnership, Fritz Thyssen Stiftung, German Research Foundation (funding of research projects), and 3R Pharma Consulting and Midas Pharma GmbH (consultation for losartan new drug formulation).
SOURCE: Kiritsi D et al. EB 2020. Poster 47.
REPORTING FROM EB 2020
Distinct Violaceous Plaques in Conjunction With Blisters
The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare autoimmune subepithelial blistering disorder with clinical, pathologic, and immunologic features of lichen planus (LP) and bullous pemphigoid (BP).1 It mainly arises in adults and usually is idiopathic but has been associated with certain infections,2 drugs such as angiotensin-converting enzyme inhibitors,3 phototherapy,4 and malignancy.5 Patients classically present with lichenoid lesions, tense vesiculobullae, and erosions.6 Vesiculobullae formation usually follows the development of lichenoid lesions, occurs on both lichenoid lesions and unaffected skin, and predominantly involves the lower extremities, as in our patient.1,6
The pathogenesis of LPP is not fully understood but likely represents a distinct entity rather than a subtype of BP or the simultaneous occurrence of LP and BP. Lichen planus pemphigoides generally has an earlier onset and better treatment response compared to BP.7 Further, autoantibodies in patients with LPP react to a novel epitope within the C-terminal portion of the BP-180 NC16A domain. Accordingly, it has been postulated that an inflammatory cutaneous process resulting from infection, phototherapy, or LP itself leads to damage of the epidermis and triggers a secondary blistering autoimmune dermatosis mediated by antibody formation against basement membrane (BM) antigens, such as BP-180.7
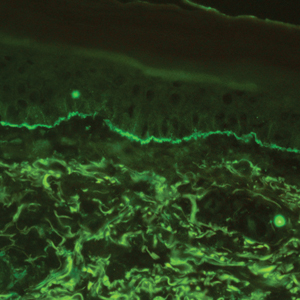
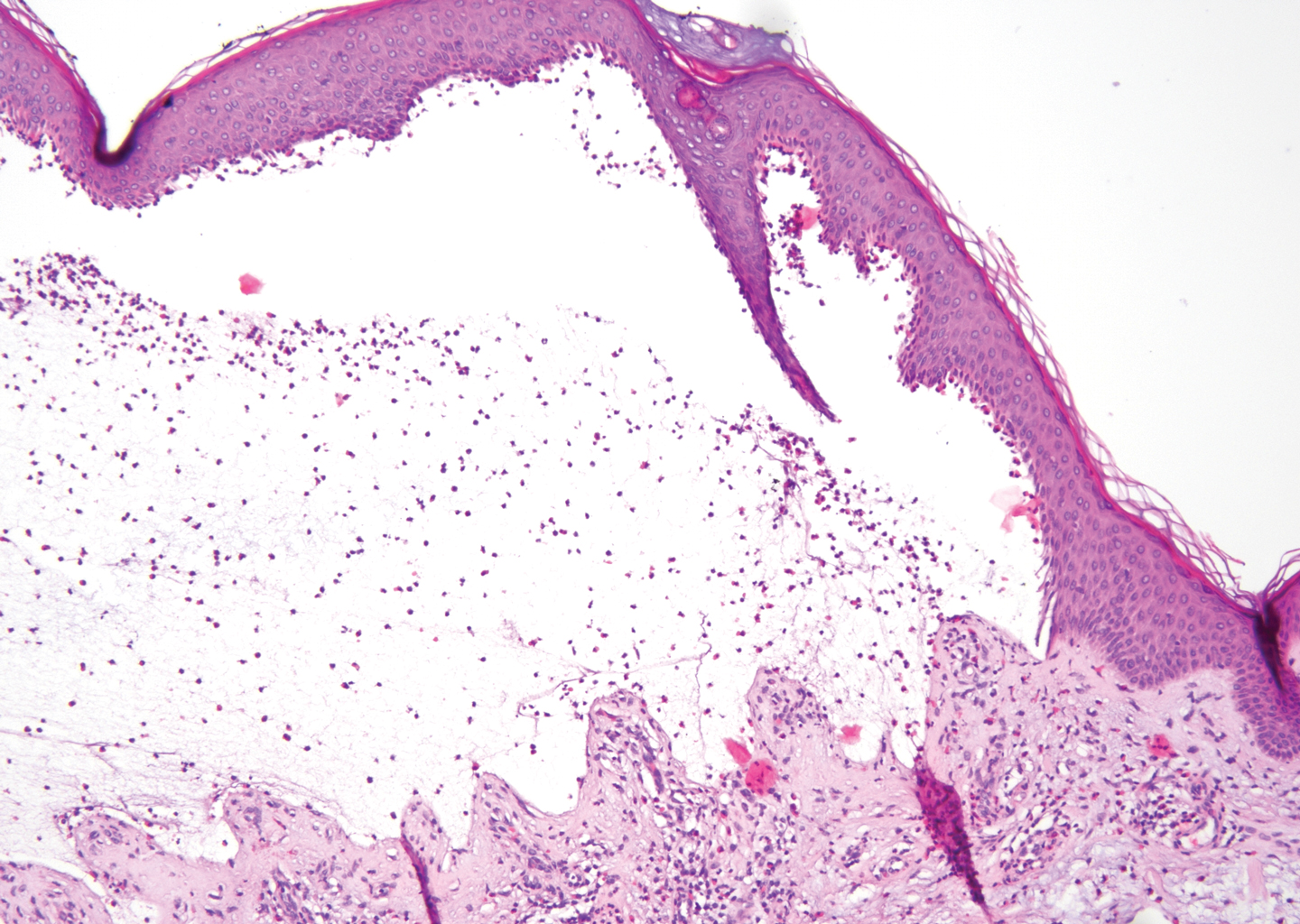
The diagnosis of LPP ultimately is confirmed with immunohistologic analysis. Biopsy of LPP shows findings consistent with both LP and BP (quiz image [top]). In the lichenoid portion, biopsy reveals orthohyperkeratosis, hypergranulosis, and acanthosis of the epidermis; a bandlike infiltrate consisting primarily of lymphocytes in the upper dermis; and apoptotic keratinocytes (colloid bodies) and vacuolar degeneration at the dermoepidermal junction (DEJ).1 Biopsy of bullae reveals eosinophilic spongiosis, a subepithelial blister plane with eosinophils, and a mixed superficial inflammatory cell infiltrate. Direct immunofluorescence from perilesional skin reveals linear deposition of IgG and/or C3 at the DEJ (quiz image [bottom]).1 Measurement of anti-BM antibodies against BP-180 and BP-230 can be useful in suspected cases, as 50% to 60% of patients have circulating antibodies against these antigens.6 Remission usually is achieved with topical and systemic corticosteroids and/or steroid-sparing agents, with rare recurrence following lesion resolution.1 More recently, successful treatment with biologics such as ustekinumab has been reported.8
The predominant differential diagnosis for LPP is bullous LP, a variant of LP in which vesiculobullous disease occurs exclusively on preexisting LP lesions, commonly on the legs due to severe vacuolar degeneration at the DEJ. On histopathology, the characteristic features of LP (eg, orthohyperkeratosis, hypergranulosis, acanthosis, bandlike lymphocytic infiltrate, colloid bodies) along with subepidermal clefting will be seen. However, in bullous LP (Figure 1) there is an absence of linear IgG and/or C3 deposition at the DEJ on direct immunofluorescence. Furthermore, patients lack circulating antibodies against BP-180 and BP-230.9
Lichen planus pemphigoides also can be confused with BP. Bullous pemphigoid is the most common autoimmune blistering disorder; typically arises in older adults; and is caused by autoantibody formation against hemidesmosomal proteins, particularly BP-180 and BP-230. Patients classically present with tense bullae and erosions on an erythematous, urticarial, or normal base. These lesions often are pruritic and concentrated on the trunk, axillary and inguinal folds, and extremity flexures. Histopathologic examination of a bulla edge reveals the classic findings seen in BP (eg, eosinophilic spongiosis, subepithelial blister plane with eosinophils)(Figure 2). Direct immunofluorescence of perilesional skin reveals linear IgG and/or C3 deposition along the DEJ. A large subset of patients also has circulating antibodies against BP-180 and BP-230. In contrast to LPP, however, patients with BP do not develop lichenoid lesions clinically or a lichenoid tissue reaction histopathologically.10
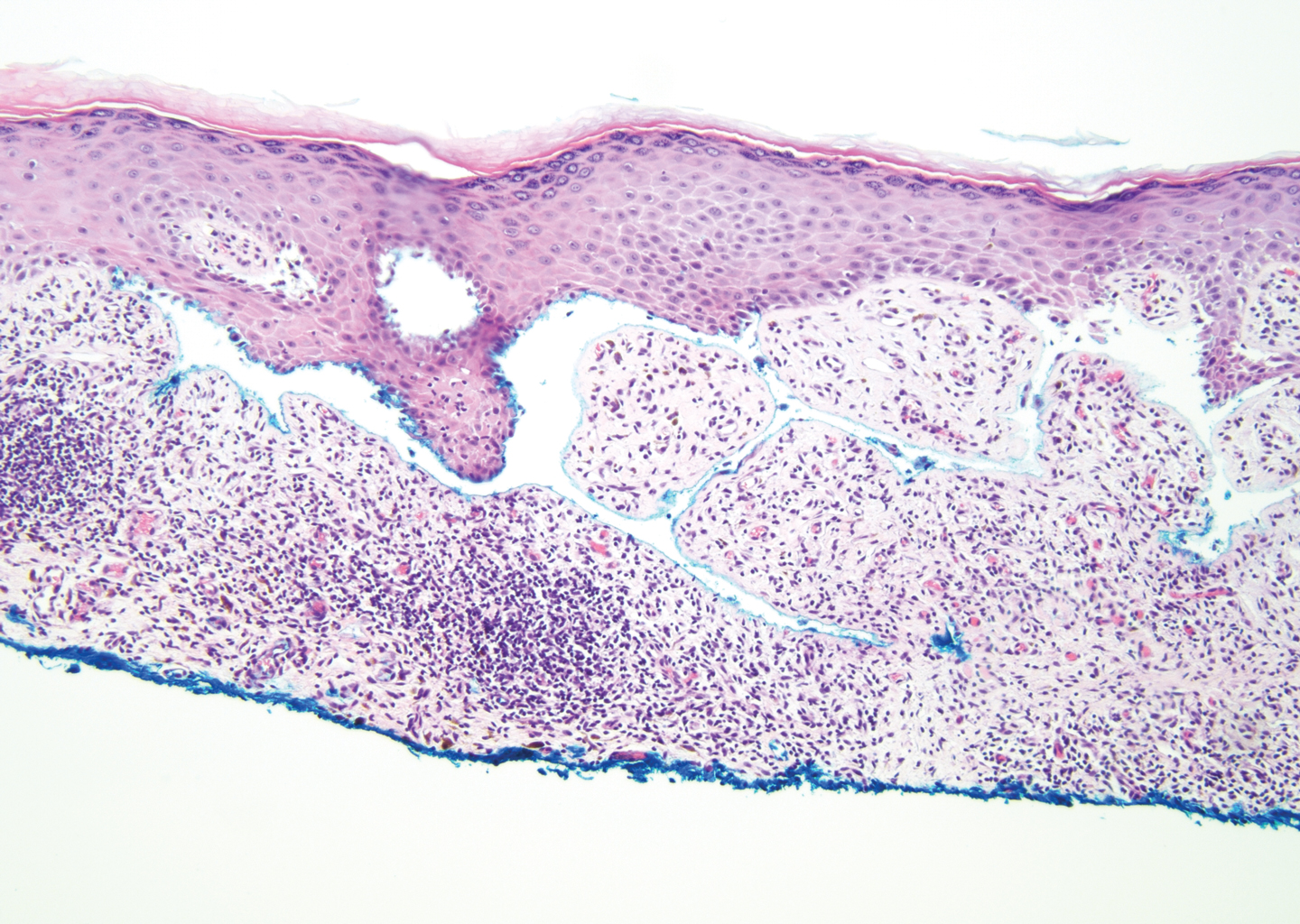
Bullous systemic lupus erythematosus (SLE), a rare cutaneous manifestation of SLE, typically arises in young women of African descent and is due to autoantibody formation against type VII collagen and other BM-zone antigens. Patients generally present with acute onset of tense vesiculobullae on a normal or erythematous base, which often are transient and heal without milia or scarring. Common sites of involvement include the trunk, arms, neck, face, and vermilion border, as well as the oral mucosa. The diagnosis of bullous SLE requires that patients fulfill the criteria for SLE and is confirmed by immunohistologic analysis. Biopsy of a bulla edge reveals a subepidermal blister containing neutrophils and increased mucin within the reticular dermis (Figure 3). Direct immunofluorescence of perilesional skin most commonly reveals linear and/or granular deposition of IgG, IgA, C3, and IgM at the DEJ.11

Bullous tinea is a manifestation of cutaneous dermatophytosis that usually occurs in the setting of tinea pedis. Common causative dermatophytes include Trichophyton mentagrophytes, Trichophyton rubrum, and Epidermophyton floccosum. Diagnosis is made by demonstration of fungal hyphae on potassium hydroxide preparation of the blister roof, biopsy with periodic acid-Schiff stain, or fungal culture. If routine histopathologic analysis is performed, epidermal spongiosis with varying degrees of papillary dermal edema is seen, along with abundant fungal elements in the stratum corneum (Figure 4). Direct immunofluorescence of perilesional skin usually is negative, but C3 deposition in a linear and/or granular pattern along the DEJ has been reported.12

Lichen planus pemphigoides is a rare disease entity and often presents a diagnostic challenge to clinicians. The differential for LPP includes bullous LP as well as other bullous disorders. Ultimately, the diagnosis is confirmed through immunohistologic analysis. Timely diagnosis of LPP is crucial, as most patients can achieve long-term remission with appropriate treatment.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Mohanarao TS, Kumar GA, Chennamsetty K, et al. Childhood lichen planus pemphigoides triggered by chickenpox. Indian Dermatol Online J. 2014;5:S98-S100.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Shimada H, Shono T, Sakai T, et al. Lichen planus pemphigoides concomitant with rectal adenocarcinoma: fortuitous or a true association? Eur J Dermatol. 2015;25:501-503.
- Matos-Pires E, Campos S, Lencastre A, et al. Lichen planus pemphigoides. J Dtsch Dermatol Ges. 2018;16:335-337.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Wagner G, Rose C, Sachse MM. Clinical variants of lichen planus. J Dtsch Dermatol Ges. 2013;11:309-319.
- Bagci IS, Horvath ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare autoimmune subepithelial blistering disorder with clinical, pathologic, and immunologic features of lichen planus (LP) and bullous pemphigoid (BP).1 It mainly arises in adults and usually is idiopathic but has been associated with certain infections,2 drugs such as angiotensin-converting enzyme inhibitors,3 phototherapy,4 and malignancy.5 Patients classically present with lichenoid lesions, tense vesiculobullae, and erosions.6 Vesiculobullae formation usually follows the development of lichenoid lesions, occurs on both lichenoid lesions and unaffected skin, and predominantly involves the lower extremities, as in our patient.1,6
The pathogenesis of LPP is not fully understood but likely represents a distinct entity rather than a subtype of BP or the simultaneous occurrence of LP and BP. Lichen planus pemphigoides generally has an earlier onset and better treatment response compared to BP.7 Further, autoantibodies in patients with LPP react to a novel epitope within the C-terminal portion of the BP-180 NC16A domain. Accordingly, it has been postulated that an inflammatory cutaneous process resulting from infection, phototherapy, or LP itself leads to damage of the epidermis and triggers a secondary blistering autoimmune dermatosis mediated by antibody formation against basement membrane (BM) antigens, such as BP-180.7
The diagnosis of LPP ultimately is confirmed with immunohistologic analysis. Biopsy of LPP shows findings consistent with both LP and BP (quiz image [top]). In the lichenoid portion, biopsy reveals orthohyperkeratosis, hypergranulosis, and acanthosis of the epidermis; a bandlike infiltrate consisting primarily of lymphocytes in the upper dermis; and apoptotic keratinocytes (colloid bodies) and vacuolar degeneration at the dermoepidermal junction (DEJ).1 Biopsy of bullae reveals eosinophilic spongiosis, a subepithelial blister plane with eosinophils, and a mixed superficial inflammatory cell infiltrate. Direct immunofluorescence from perilesional skin reveals linear deposition of IgG and/or C3 at the DEJ (quiz image [bottom]).1 Measurement of anti-BM antibodies against BP-180 and BP-230 can be useful in suspected cases, as 50% to 60% of patients have circulating antibodies against these antigens.6 Remission usually is achieved with topical and systemic corticosteroids and/or steroid-sparing agents, with rare recurrence following lesion resolution.1 More recently, successful treatment with biologics such as ustekinumab has been reported.8
The predominant differential diagnosis for LPP is bullous LP, a variant of LP in which vesiculobullous disease occurs exclusively on preexisting LP lesions, commonly on the legs due to severe vacuolar degeneration at the DEJ. On histopathology, the characteristic features of LP (eg, orthohyperkeratosis, hypergranulosis, acanthosis, bandlike lymphocytic infiltrate, colloid bodies) along with subepidermal clefting will be seen. However, in bullous LP (Figure 1) there is an absence of linear IgG and/or C3 deposition at the DEJ on direct immunofluorescence. Furthermore, patients lack circulating antibodies against BP-180 and BP-230.9

Lichen planus pemphigoides also can be confused with BP. Bullous pemphigoid is the most common autoimmune blistering disorder; typically arises in older adults; and is caused by autoantibody formation against hemidesmosomal proteins, particularly BP-180 and BP-230. Patients classically present with tense bullae and erosions on an erythematous, urticarial, or normal base. These lesions often are pruritic and concentrated on the trunk, axillary and inguinal folds, and extremity flexures. Histopathologic examination of a bulla edge reveals the classic findings seen in BP (eg, eosinophilic spongiosis, subepithelial blister plane with eosinophils)(Figure 2). Direct immunofluorescence of perilesional skin reveals linear IgG and/or C3 deposition along the DEJ. A large subset of patients also has circulating antibodies against BP-180 and BP-230. In contrast to LPP, however, patients with BP do not develop lichenoid lesions clinically or a lichenoid tissue reaction histopathologically.10

Bullous systemic lupus erythematosus (SLE), a rare cutaneous manifestation of SLE, typically arises in young women of African descent and is due to autoantibody formation against type VII collagen and other BM-zone antigens. Patients generally present with acute onset of tense vesiculobullae on a normal or erythematous base, which often are transient and heal without milia or scarring. Common sites of involvement include the trunk, arms, neck, face, and vermilion border, as well as the oral mucosa. The diagnosis of bullous SLE requires that patients fulfill the criteria for SLE and is confirmed by immunohistologic analysis. Biopsy of a bulla edge reveals a subepidermal blister containing neutrophils and increased mucin within the reticular dermis (Figure 3). Direct immunofluorescence of perilesional skin most commonly reveals linear and/or granular deposition of IgG, IgA, C3, and IgM at the DEJ.11

Bullous tinea is a manifestation of cutaneous dermatophytosis that usually occurs in the setting of tinea pedis. Common causative dermatophytes include Trichophyton mentagrophytes, Trichophyton rubrum, and Epidermophyton floccosum. Diagnosis is made by demonstration of fungal hyphae on potassium hydroxide preparation of the blister roof, biopsy with periodic acid-Schiff stain, or fungal culture. If routine histopathologic analysis is performed, epidermal spongiosis with varying degrees of papillary dermal edema is seen, along with abundant fungal elements in the stratum corneum (Figure 4). Direct immunofluorescence of perilesional skin usually is negative, but C3 deposition in a linear and/or granular pattern along the DEJ has been reported.12

Lichen planus pemphigoides is a rare disease entity and often presents a diagnostic challenge to clinicians. The differential for LPP includes bullous LP as well as other bullous disorders. Ultimately, the diagnosis is confirmed through immunohistologic analysis. Timely diagnosis of LPP is crucial, as most patients can achieve long-term remission with appropriate treatment.
The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare autoimmune subepithelial blistering disorder with clinical, pathologic, and immunologic features of lichen planus (LP) and bullous pemphigoid (BP).1 It mainly arises in adults and usually is idiopathic but has been associated with certain infections,2 drugs such as angiotensin-converting enzyme inhibitors,3 phototherapy,4 and malignancy.5 Patients classically present with lichenoid lesions, tense vesiculobullae, and erosions.6 Vesiculobullae formation usually follows the development of lichenoid lesions, occurs on both lichenoid lesions and unaffected skin, and predominantly involves the lower extremities, as in our patient.1,6
The pathogenesis of LPP is not fully understood but likely represents a distinct entity rather than a subtype of BP or the simultaneous occurrence of LP and BP. Lichen planus pemphigoides generally has an earlier onset and better treatment response compared to BP.7 Further, autoantibodies in patients with LPP react to a novel epitope within the C-terminal portion of the BP-180 NC16A domain. Accordingly, it has been postulated that an inflammatory cutaneous process resulting from infection, phototherapy, or LP itself leads to damage of the epidermis and triggers a secondary blistering autoimmune dermatosis mediated by antibody formation against basement membrane (BM) antigens, such as BP-180.7
The diagnosis of LPP ultimately is confirmed with immunohistologic analysis. Biopsy of LPP shows findings consistent with both LP and BP (quiz image [top]). In the lichenoid portion, biopsy reveals orthohyperkeratosis, hypergranulosis, and acanthosis of the epidermis; a bandlike infiltrate consisting primarily of lymphocytes in the upper dermis; and apoptotic keratinocytes (colloid bodies) and vacuolar degeneration at the dermoepidermal junction (DEJ).1 Biopsy of bullae reveals eosinophilic spongiosis, a subepithelial blister plane with eosinophils, and a mixed superficial inflammatory cell infiltrate. Direct immunofluorescence from perilesional skin reveals linear deposition of IgG and/or C3 at the DEJ (quiz image [bottom]).1 Measurement of anti-BM antibodies against BP-180 and BP-230 can be useful in suspected cases, as 50% to 60% of patients have circulating antibodies against these antigens.6 Remission usually is achieved with topical and systemic corticosteroids and/or steroid-sparing agents, with rare recurrence following lesion resolution.1 More recently, successful treatment with biologics such as ustekinumab has been reported.8
The predominant differential diagnosis for LPP is bullous LP, a variant of LP in which vesiculobullous disease occurs exclusively on preexisting LP lesions, commonly on the legs due to severe vacuolar degeneration at the DEJ. On histopathology, the characteristic features of LP (eg, orthohyperkeratosis, hypergranulosis, acanthosis, bandlike lymphocytic infiltrate, colloid bodies) along with subepidermal clefting will be seen. However, in bullous LP (Figure 1) there is an absence of linear IgG and/or C3 deposition at the DEJ on direct immunofluorescence. Furthermore, patients lack circulating antibodies against BP-180 and BP-230.9

Lichen planus pemphigoides also can be confused with BP. Bullous pemphigoid is the most common autoimmune blistering disorder; typically arises in older adults; and is caused by autoantibody formation against hemidesmosomal proteins, particularly BP-180 and BP-230. Patients classically present with tense bullae and erosions on an erythematous, urticarial, or normal base. These lesions often are pruritic and concentrated on the trunk, axillary and inguinal folds, and extremity flexures. Histopathologic examination of a bulla edge reveals the classic findings seen in BP (eg, eosinophilic spongiosis, subepithelial blister plane with eosinophils)(Figure 2). Direct immunofluorescence of perilesional skin reveals linear IgG and/or C3 deposition along the DEJ. A large subset of patients also has circulating antibodies against BP-180 and BP-230. In contrast to LPP, however, patients with BP do not develop lichenoid lesions clinically or a lichenoid tissue reaction histopathologically.10

Bullous systemic lupus erythematosus (SLE), a rare cutaneous manifestation of SLE, typically arises in young women of African descent and is due to autoantibody formation against type VII collagen and other BM-zone antigens. Patients generally present with acute onset of tense vesiculobullae on a normal or erythematous base, which often are transient and heal without milia or scarring. Common sites of involvement include the trunk, arms, neck, face, and vermilion border, as well as the oral mucosa. The diagnosis of bullous SLE requires that patients fulfill the criteria for SLE and is confirmed by immunohistologic analysis. Biopsy of a bulla edge reveals a subepidermal blister containing neutrophils and increased mucin within the reticular dermis (Figure 3). Direct immunofluorescence of perilesional skin most commonly reveals linear and/or granular deposition of IgG, IgA, C3, and IgM at the DEJ.11

Bullous tinea is a manifestation of cutaneous dermatophytosis that usually occurs in the setting of tinea pedis. Common causative dermatophytes include Trichophyton mentagrophytes, Trichophyton rubrum, and Epidermophyton floccosum. Diagnosis is made by demonstration of fungal hyphae on potassium hydroxide preparation of the blister roof, biopsy with periodic acid-Schiff stain, or fungal culture. If routine histopathologic analysis is performed, epidermal spongiosis with varying degrees of papillary dermal edema is seen, along with abundant fungal elements in the stratum corneum (Figure 4). Direct immunofluorescence of perilesional skin usually is negative, but C3 deposition in a linear and/or granular pattern along the DEJ has been reported.12

Lichen planus pemphigoides is a rare disease entity and often presents a diagnostic challenge to clinicians. The differential for LPP includes bullous LP as well as other bullous disorders. Ultimately, the diagnosis is confirmed through immunohistologic analysis. Timely diagnosis of LPP is crucial, as most patients can achieve long-term remission with appropriate treatment.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Mohanarao TS, Kumar GA, Chennamsetty K, et al. Childhood lichen planus pemphigoides triggered by chickenpox. Indian Dermatol Online J. 2014;5:S98-S100.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Shimada H, Shono T, Sakai T, et al. Lichen planus pemphigoides concomitant with rectal adenocarcinoma: fortuitous or a true association? Eur J Dermatol. 2015;25:501-503.
- Matos-Pires E, Campos S, Lencastre A, et al. Lichen planus pemphigoides. J Dtsch Dermatol Ges. 2018;16:335-337.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Wagner G, Rose C, Sachse MM. Clinical variants of lichen planus. J Dtsch Dermatol Ges. 2013;11:309-319.
- Bagci IS, Horvath ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Mohanarao TS, Kumar GA, Chennamsetty K, et al. Childhood lichen planus pemphigoides triggered by chickenpox. Indian Dermatol Online J. 2014;5:S98-S100.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Shimada H, Shono T, Sakai T, et al. Lichen planus pemphigoides concomitant with rectal adenocarcinoma: fortuitous or a true association? Eur J Dermatol. 2015;25:501-503.
- Matos-Pires E, Campos S, Lencastre A, et al. Lichen planus pemphigoides. J Dtsch Dermatol Ges. 2018;16:335-337.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Wagner G, Rose C, Sachse MM. Clinical variants of lichen planus. J Dtsch Dermatol Ges. 2013;11:309-319.
- Bagci IS, Horvath ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Miller DD, Bhawan J. Bullous tinea pedis with direct immunofluorescence positivity: when is a positive result not autoimmune bullous disease? Am J Dermatopathol. 2013;35:587-594.
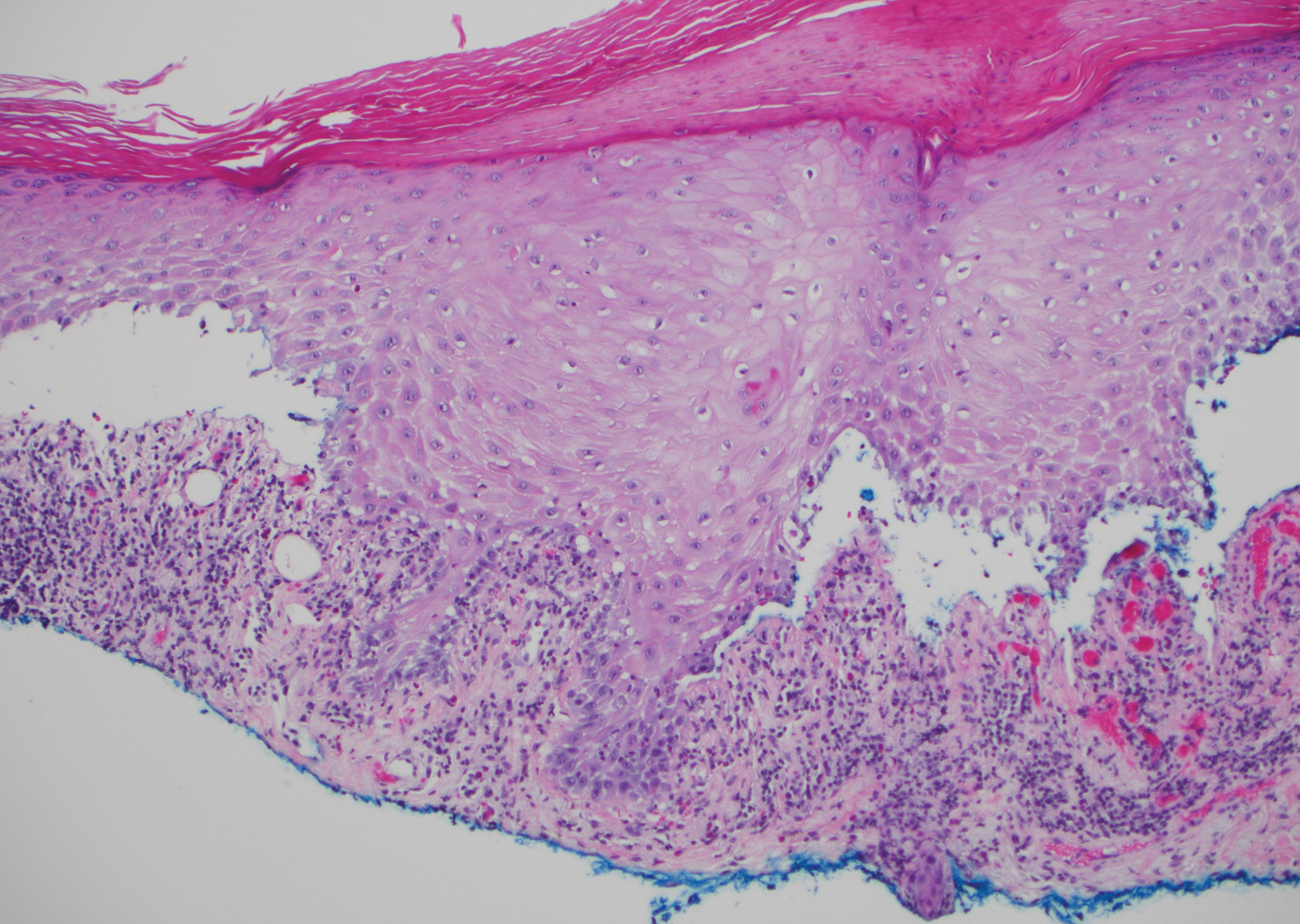
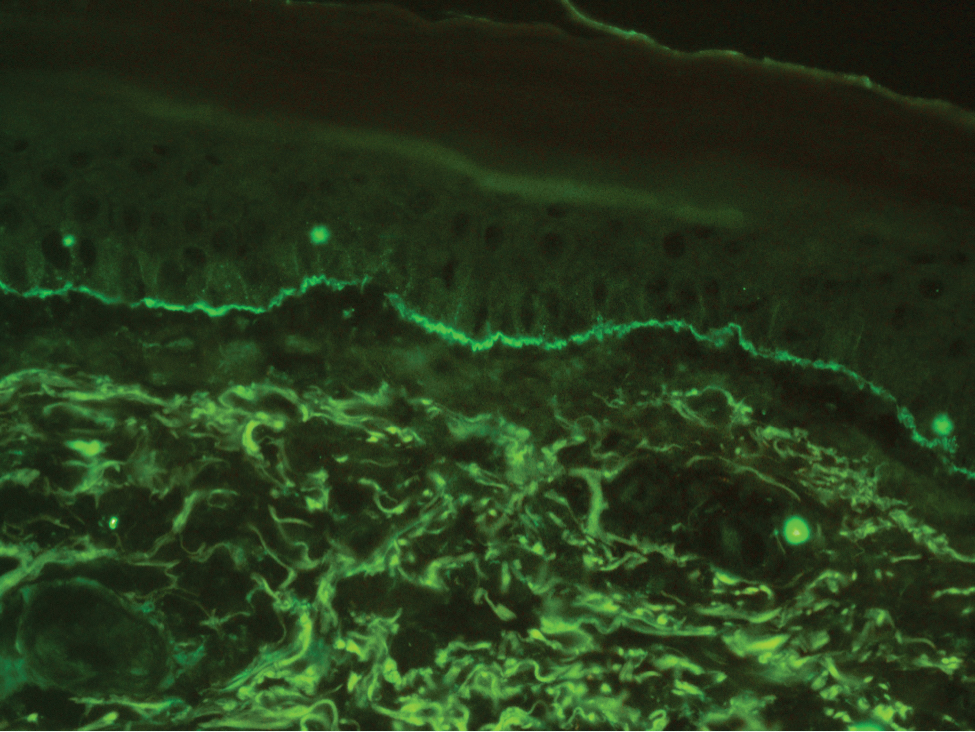
A 72-year-old woman presented to our dermatology clinic with a rash of several months' duration that began as itchy bumps on the wrists and spread to involve the legs. Approximately 2 months prior to presentation, she noted blisters on the feet and legs. She initially went to her primary care physician, who prescribed levofloxacin, cephalexin, and a 5-day course of prednisone. The prednisone initially helped; however the rash worsened on discontinuation. In our clinic, the patient had scattered tense bullae and numerous erosions with crust on the dorsum of the feet and legs, some of which were in conjunction with violaceous papules and plaques. There also was hypertrophic scale on the soles of the feet. A potassium hydroxide preparation of skin scrapings from the feet was negative for fungal elements. Two shave biopsies of a violaceous plaque and bulla as well as a perilesional punch biopsy from the leg were obtained.
Ovarian cancer survival varies between high-income countries
especially for older women with advanced disease, according to a recent study.
The findings suggest additional research is needed to evaluate international differences in both treatment- and patient-specific factors affecting survival.
“This study [evaluated] differences in survival by age and stage at diagnosis within and across seven high-income countries,” wrote Citadel J. Cabasag, PhD, of the International Agency for Research on Cancer in Lyon, France, and colleagues. The results were published in Gynecologic Oncology.
The researchers conducted a retrospective analysis of population-based registry data from 2010 to 2014. Data were collected from 21 cancer registries located in Canada, United Kingdom, New Zealand, Norway, Ireland, Australia, and Denmark.
The cohort included 58,161 women with epithelial and nonepithelial ovarian cancer. The majority of cases were advanced stage, with a median age of 63-67 years at diagnosis, depending on the country.
The researchers compared age- and stage-specific net survival between countries at 1 and 3 years post diagnosis.
The 3-year all-ages net survival was highest for Norway (57%) and Australia (56%), followed by Denmark (54%), Canada (50%), United Kingdom (47%), New Zealand (46%), and Ireland (45%).
Most patients were diagnosed with distant disease (range, 64%-71%), with the greatest proportion of women in the 65- to 74- and 75- to 99-year age categories. The lowest 3-year age-specific survival (range, 20%-34%) was observed in the 75- to 99-year age category.
Marked differences in 3-year net survival between countries were found for women in the 75- to 99-year age group with distant disease (range, 12%-25%).
“International survival differences by age groups were less stark for early-stage disease,” the researchers reported. “Interjurisdictional differences within countries were also observed.”
The researchers acknowledged a key limitation of the analysis was the use of registry data. Variability between, and incomplete data within, registries could have lowered the accuracy of the survival estimates.
“[F]urther research investigating international differences in access to and quality of treatment, and prevalence of comorbid conditions particularly in older women with advanced disease [is needed],” the researchers concluded.
The study was supported by funding provided to the International Cancer Benchmarking Partnership. The authors reported having no conflicts of interest.
SOURCE: Cabasag CJ et al. Gynecol Oncol. 2020 Jan 28. doi: 10.1016/j.ygyno.2019.12.047.
especially for older women with advanced disease, according to a recent study.
The findings suggest additional research is needed to evaluate international differences in both treatment- and patient-specific factors affecting survival.
“This study [evaluated] differences in survival by age and stage at diagnosis within and across seven high-income countries,” wrote Citadel J. Cabasag, PhD, of the International Agency for Research on Cancer in Lyon, France, and colleagues. The results were published in Gynecologic Oncology.
The researchers conducted a retrospective analysis of population-based registry data from 2010 to 2014. Data were collected from 21 cancer registries located in Canada, United Kingdom, New Zealand, Norway, Ireland, Australia, and Denmark.
The cohort included 58,161 women with epithelial and nonepithelial ovarian cancer. The majority of cases were advanced stage, with a median age of 63-67 years at diagnosis, depending on the country.
The researchers compared age- and stage-specific net survival between countries at 1 and 3 years post diagnosis.
The 3-year all-ages net survival was highest for Norway (57%) and Australia (56%), followed by Denmark (54%), Canada (50%), United Kingdom (47%), New Zealand (46%), and Ireland (45%).
Most patients were diagnosed with distant disease (range, 64%-71%), with the greatest proportion of women in the 65- to 74- and 75- to 99-year age categories. The lowest 3-year age-specific survival (range, 20%-34%) was observed in the 75- to 99-year age category.
Marked differences in 3-year net survival between countries were found for women in the 75- to 99-year age group with distant disease (range, 12%-25%).
“International survival differences by age groups were less stark for early-stage disease,” the researchers reported. “Interjurisdictional differences within countries were also observed.”
The researchers acknowledged a key limitation of the analysis was the use of registry data. Variability between, and incomplete data within, registries could have lowered the accuracy of the survival estimates.
“[F]urther research investigating international differences in access to and quality of treatment, and prevalence of comorbid conditions particularly in older women with advanced disease [is needed],” the researchers concluded.
The study was supported by funding provided to the International Cancer Benchmarking Partnership. The authors reported having no conflicts of interest.
SOURCE: Cabasag CJ et al. Gynecol Oncol. 2020 Jan 28. doi: 10.1016/j.ygyno.2019.12.047.
especially for older women with advanced disease, according to a recent study.
The findings suggest additional research is needed to evaluate international differences in both treatment- and patient-specific factors affecting survival.
“This study [evaluated] differences in survival by age and stage at diagnosis within and across seven high-income countries,” wrote Citadel J. Cabasag, PhD, of the International Agency for Research on Cancer in Lyon, France, and colleagues. The results were published in Gynecologic Oncology.
The researchers conducted a retrospective analysis of population-based registry data from 2010 to 2014. Data were collected from 21 cancer registries located in Canada, United Kingdom, New Zealand, Norway, Ireland, Australia, and Denmark.
The cohort included 58,161 women with epithelial and nonepithelial ovarian cancer. The majority of cases were advanced stage, with a median age of 63-67 years at diagnosis, depending on the country.
The researchers compared age- and stage-specific net survival between countries at 1 and 3 years post diagnosis.
The 3-year all-ages net survival was highest for Norway (57%) and Australia (56%), followed by Denmark (54%), Canada (50%), United Kingdom (47%), New Zealand (46%), and Ireland (45%).
Most patients were diagnosed with distant disease (range, 64%-71%), with the greatest proportion of women in the 65- to 74- and 75- to 99-year age categories. The lowest 3-year age-specific survival (range, 20%-34%) was observed in the 75- to 99-year age category.
Marked differences in 3-year net survival between countries were found for women in the 75- to 99-year age group with distant disease (range, 12%-25%).
“International survival differences by age groups were less stark for early-stage disease,” the researchers reported. “Interjurisdictional differences within countries were also observed.”
The researchers acknowledged a key limitation of the analysis was the use of registry data. Variability between, and incomplete data within, registries could have lowered the accuracy of the survival estimates.
“[F]urther research investigating international differences in access to and quality of treatment, and prevalence of comorbid conditions particularly in older women with advanced disease [is needed],” the researchers concluded.
The study was supported by funding provided to the International Cancer Benchmarking Partnership. The authors reported having no conflicts of interest.
SOURCE: Cabasag CJ et al. Gynecol Oncol. 2020 Jan 28. doi: 10.1016/j.ygyno.2019.12.047.
FROM GYNECOLOGIC ONCOLOGY
Bipartisan Bill to Help Reduce Veteran Suicides Readies for Senate Vote
In fiscal year 2010, the VA requested $62 million for suicide prevention outreach; in FY 2020, that leapt to $222 million. Yet despite the dramatic hike in funding, the rate of veteran suicides has remained basically unchanged: An estimated 20 veterans die by suicide every day.
Of those, roughly 14 were not receiving health care from the VA before their death. But a bipartisan bill introduced by US senators Mark Warner (D-VA) and John Boozman (R-AR) brings us “one step closer to making sure veterans get the services and resources they need.”
The senators say the alarming rate of veteran suicides points to “a significant need to empower the VA to work through community partners to expand outreach.” They cite national data indicating that there are > 50,000 organizations that provide suicide prevention services for veterans, yet “they are hard for veterans to find, access, apply for, and use.”
The IMPROVE (Incorporating Measurements and Providing Resources for Outreach to Veterans Everywhere) Well-Being for Veterans Act, introduced in 2019, creates a new grant program to enable the VA to conduct outreach through veteran-serving nonprofits in addition to state and local organizations. The funding would go to organizations with a proven track record of strong mental health and suicide prevention efforts among veterans, Warner says.
The bill supports coordination and planning of veteran mental health and suicide prevention services. Another goal is to provide tools to measure the effectiveness of the programs so the resources can be concentrated where they can do the most good. For example, Warner says, there are no shared tools to measure whether programs help improve mental resiliency and outlook, which can indicate reduced suicide risk.
On January 29, the Senate Veterans Affairs Committee included language from the bill as a provision in a comprehensive bill that expands veterans’ access to mental health services. The legislation unanimously passed the committee and now awaits consideration by the full Senate.
In fiscal year 2010, the VA requested $62 million for suicide prevention outreach; in FY 2020, that leapt to $222 million. Yet despite the dramatic hike in funding, the rate of veteran suicides has remained basically unchanged: An estimated 20 veterans die by suicide every day.
Of those, roughly 14 were not receiving health care from the VA before their death. But a bipartisan bill introduced by US senators Mark Warner (D-VA) and John Boozman (R-AR) brings us “one step closer to making sure veterans get the services and resources they need.”
The senators say the alarming rate of veteran suicides points to “a significant need to empower the VA to work through community partners to expand outreach.” They cite national data indicating that there are > 50,000 organizations that provide suicide prevention services for veterans, yet “they are hard for veterans to find, access, apply for, and use.”
The IMPROVE (Incorporating Measurements and Providing Resources for Outreach to Veterans Everywhere) Well-Being for Veterans Act, introduced in 2019, creates a new grant program to enable the VA to conduct outreach through veteran-serving nonprofits in addition to state and local organizations. The funding would go to organizations with a proven track record of strong mental health and suicide prevention efforts among veterans, Warner says.
The bill supports coordination and planning of veteran mental health and suicide prevention services. Another goal is to provide tools to measure the effectiveness of the programs so the resources can be concentrated where they can do the most good. For example, Warner says, there are no shared tools to measure whether programs help improve mental resiliency and outlook, which can indicate reduced suicide risk.
On January 29, the Senate Veterans Affairs Committee included language from the bill as a provision in a comprehensive bill that expands veterans’ access to mental health services. The legislation unanimously passed the committee and now awaits consideration by the full Senate.
In fiscal year 2010, the VA requested $62 million for suicide prevention outreach; in FY 2020, that leapt to $222 million. Yet despite the dramatic hike in funding, the rate of veteran suicides has remained basically unchanged: An estimated 20 veterans die by suicide every day.
Of those, roughly 14 were not receiving health care from the VA before their death. But a bipartisan bill introduced by US senators Mark Warner (D-VA) and John Boozman (R-AR) brings us “one step closer to making sure veterans get the services and resources they need.”
The senators say the alarming rate of veteran suicides points to “a significant need to empower the VA to work through community partners to expand outreach.” They cite national data indicating that there are > 50,000 organizations that provide suicide prevention services for veterans, yet “they are hard for veterans to find, access, apply for, and use.”
The IMPROVE (Incorporating Measurements and Providing Resources for Outreach to Veterans Everywhere) Well-Being for Veterans Act, introduced in 2019, creates a new grant program to enable the VA to conduct outreach through veteran-serving nonprofits in addition to state and local organizations. The funding would go to organizations with a proven track record of strong mental health and suicide prevention efforts among veterans, Warner says.
The bill supports coordination and planning of veteran mental health and suicide prevention services. Another goal is to provide tools to measure the effectiveness of the programs so the resources can be concentrated where they can do the most good. For example, Warner says, there are no shared tools to measure whether programs help improve mental resiliency and outlook, which can indicate reduced suicide risk.
On January 29, the Senate Veterans Affairs Committee included language from the bill as a provision in a comprehensive bill that expands veterans’ access to mental health services. The legislation unanimously passed the committee and now awaits consideration by the full Senate.
Multiple assessment measures can hone RA treatment
Combining the measures of the Clinical Disease Activity Index and the Disease Activity Score in 28 joints provides an opportunity adjust treatment for patients with RA, based on data from a cross-sectional study of 1,585 adults.
Although the Clinical Disease Activity Index (CDAI) is considered more stringent, comparisons with the Disease Activity Score in 28 joints with erythrocyte sedimentation rate (DAS28-ESR) outside of clinical trials are limited, wrote Satoshi Takanashi, MD, of Keio University School of Medicine in Tokyo, and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from 1,585 consecutive RA patients seen at Keio University Hospital in Tokyo. The average age of the patients was 64 years, 84% were women, and the average duration of disease was 12 years.
Overall, more patients met the CDAI remission criteria but not the DAS28-ESR criteria, with the exception of patients treated with an interleukin-6 inhibitor.
Of the patients in remission based on CDAI, the proportion who were not in DAS28-ESR remission was 19.4% for those treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), 18.2% for tumor necrosis factor inhibitors, 4.2% for IL-6 inhibitors, 27.6% for CTLA4-Ig fusion protein, and 33.3% for Janus kinase inhibitors.
Of the patients in DAS28-ESR remission, those not also in CDAI remission totaled 11.7% with csDMARDs, 15.4% with tumor necrosis factor inhibitors, 29.5% with IL-6 inhibitors, 16.0% with CTLA4-Ig, and 14.3% with Janus kinase inhibitors.
“The fact that many patients fulfilled the CDAI but not DAS28-ESR remission could be explained by several reasons including residual synovitis in joints that are not included in the main 28 joints, which could lead to an increase in acute phase reactants and elevate only DAS28-ESR, extra-articular involvement or other comorbidities that could elevate the C-reactive protein irrelevant to arthritis,” the researchers noted. The prevalence of complications was higher in patients in CDAI remission and DAS28-ESR nonremission independent of rheumatoid or nonrheumatoid comorbid conditions, they added.
The findings were limited by several factors, including the cross-sectional study design that did not evaluate longitudinal radiological and functional progression, the researchers wrote.
“However, patients in both CDAI and DAS28-ESR remission were apparently in better condition than those who met either criteria; therefore, in the management of rheumatoid arthritis, assessing patients with two composite measures can yield important opportunities to consider what causes the discrepancy between the measures and adjust treatment appropriately,” they concluded.
The authors did not report having a specific grant for this research. Two of the paper’s three authors disclosed relationships with multiple companies that market drugs for RA.
SOURCE: Takanashi S et al. Ann Rheum Dis. 2020 Jan 29. doi: 10.1136/annrheumdis-2019-216607.
Combining the measures of the Clinical Disease Activity Index and the Disease Activity Score in 28 joints provides an opportunity adjust treatment for patients with RA, based on data from a cross-sectional study of 1,585 adults.
Although the Clinical Disease Activity Index (CDAI) is considered more stringent, comparisons with the Disease Activity Score in 28 joints with erythrocyte sedimentation rate (DAS28-ESR) outside of clinical trials are limited, wrote Satoshi Takanashi, MD, of Keio University School of Medicine in Tokyo, and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from 1,585 consecutive RA patients seen at Keio University Hospital in Tokyo. The average age of the patients was 64 years, 84% were women, and the average duration of disease was 12 years.
Overall, more patients met the CDAI remission criteria but not the DAS28-ESR criteria, with the exception of patients treated with an interleukin-6 inhibitor.
Of the patients in remission based on CDAI, the proportion who were not in DAS28-ESR remission was 19.4% for those treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), 18.2% for tumor necrosis factor inhibitors, 4.2% for IL-6 inhibitors, 27.6% for CTLA4-Ig fusion protein, and 33.3% for Janus kinase inhibitors.
Of the patients in DAS28-ESR remission, those not also in CDAI remission totaled 11.7% with csDMARDs, 15.4% with tumor necrosis factor inhibitors, 29.5% with IL-6 inhibitors, 16.0% with CTLA4-Ig, and 14.3% with Janus kinase inhibitors.
“The fact that many patients fulfilled the CDAI but not DAS28-ESR remission could be explained by several reasons including residual synovitis in joints that are not included in the main 28 joints, which could lead to an increase in acute phase reactants and elevate only DAS28-ESR, extra-articular involvement or other comorbidities that could elevate the C-reactive protein irrelevant to arthritis,” the researchers noted. The prevalence of complications was higher in patients in CDAI remission and DAS28-ESR nonremission independent of rheumatoid or nonrheumatoid comorbid conditions, they added.
The findings were limited by several factors, including the cross-sectional study design that did not evaluate longitudinal radiological and functional progression, the researchers wrote.
“However, patients in both CDAI and DAS28-ESR remission were apparently in better condition than those who met either criteria; therefore, in the management of rheumatoid arthritis, assessing patients with two composite measures can yield important opportunities to consider what causes the discrepancy between the measures and adjust treatment appropriately,” they concluded.
The authors did not report having a specific grant for this research. Two of the paper’s three authors disclosed relationships with multiple companies that market drugs for RA.
SOURCE: Takanashi S et al. Ann Rheum Dis. 2020 Jan 29. doi: 10.1136/annrheumdis-2019-216607.
Combining the measures of the Clinical Disease Activity Index and the Disease Activity Score in 28 joints provides an opportunity adjust treatment for patients with RA, based on data from a cross-sectional study of 1,585 adults.
Although the Clinical Disease Activity Index (CDAI) is considered more stringent, comparisons with the Disease Activity Score in 28 joints with erythrocyte sedimentation rate (DAS28-ESR) outside of clinical trials are limited, wrote Satoshi Takanashi, MD, of Keio University School of Medicine in Tokyo, and colleagues.
In a study published in Annals of the Rheumatic Diseases, the researchers reviewed data from 1,585 consecutive RA patients seen at Keio University Hospital in Tokyo. The average age of the patients was 64 years, 84% were women, and the average duration of disease was 12 years.
Overall, more patients met the CDAI remission criteria but not the DAS28-ESR criteria, with the exception of patients treated with an interleukin-6 inhibitor.
Of the patients in remission based on CDAI, the proportion who were not in DAS28-ESR remission was 19.4% for those treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), 18.2% for tumor necrosis factor inhibitors, 4.2% for IL-6 inhibitors, 27.6% for CTLA4-Ig fusion protein, and 33.3% for Janus kinase inhibitors.
Of the patients in DAS28-ESR remission, those not also in CDAI remission totaled 11.7% with csDMARDs, 15.4% with tumor necrosis factor inhibitors, 29.5% with IL-6 inhibitors, 16.0% with CTLA4-Ig, and 14.3% with Janus kinase inhibitors.
“The fact that many patients fulfilled the CDAI but not DAS28-ESR remission could be explained by several reasons including residual synovitis in joints that are not included in the main 28 joints, which could lead to an increase in acute phase reactants and elevate only DAS28-ESR, extra-articular involvement or other comorbidities that could elevate the C-reactive protein irrelevant to arthritis,” the researchers noted. The prevalence of complications was higher in patients in CDAI remission and DAS28-ESR nonremission independent of rheumatoid or nonrheumatoid comorbid conditions, they added.
The findings were limited by several factors, including the cross-sectional study design that did not evaluate longitudinal radiological and functional progression, the researchers wrote.
“However, patients in both CDAI and DAS28-ESR remission were apparently in better condition than those who met either criteria; therefore, in the management of rheumatoid arthritis, assessing patients with two composite measures can yield important opportunities to consider what causes the discrepancy between the measures and adjust treatment appropriately,” they concluded.
The authors did not report having a specific grant for this research. Two of the paper’s three authors disclosed relationships with multiple companies that market drugs for RA.
SOURCE: Takanashi S et al. Ann Rheum Dis. 2020 Jan 29. doi: 10.1136/annrheumdis-2019-216607.
FROM ANNALS OF THE RHEUMATIC DISEASES
Multiomics blood test outperforms other tests for colorectal cancer screening
SAN FRANCISCO – A blood-based test that integrates data from multiple molecular “omes,” such as the genome and proteome, performs well at spotting early-stage colorectal cancer, the AI-EMERGE study suggests.
Moreover, the test netted better sensitivity than a fecal immunochemical test (FIT), a circulating tumor DNA (ctDNA) test, and a carcinoembryonic antigen (CEA) test.
These findings were reported in a poster session at the 2020 GI Cancers Symposium, which is sponsored by the American Gastroenterological Association, American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“Today, about a third of age-appropriate adults are not up to date with colorectal cancer screening,” lead study investigator Girish Putcha, MD, PhD, chief medical officer of Freenome in San Francisco, noted at the symposium. “A noninvasive blood-based screening test having high sensitivity and specificity for colorectal cancer generally, but especially for early-stage disease, could help improve adherence and ultimately reduce mortality.”
Dr. Putcha and colleagues evaluated a blood-based multiomics test in 32 patients with colorectal cancer of all stages and 539 colonoscopy-confirmed negative control subjects.
The test uses a multiomics platform to pick up both tumor-derived signal and non–tumor-derived signal from the body’s immune response and other sources. The test uses machine learning, and entails whole-genome sequencing, bisulfite sequencing (for assessment of DNA methylation), and protein quantification methods.
At 94% specificity, the test had a 94% sensitivity for spotting stage I and II colorectal cancer, 91% sensitivity for stage III and IV colorectal cancer, and 91% sensitivity for colorectal cancer of any stage. By location, sensitivity was 92% for distal tumors and 88% for proximal tumors.
The multiomics test outperformed a ctDNA test, a CEA test, and a FIT. At a specificity of 96% for both tests, the multiomics test yielded a higher sensitivity than a commercially available FIT stool test (OC-Auto FIT, Polymedco) for stage I and II disease (100% vs. 70%), stage III and IV disease (100% vs. 50%), and any-stage disease (100% vs. 67%).
When set at 100% specificity, the multiomics test outperformed a commercially available plasma ctDNA test (Avenio, Roche) set at 75% specificity. The multiomics test yielded a higher sensitivity for stage I and II disease (94% vs. 38%), stage III and IV disease (91% vs. 55%), and any-stage disease (90% vs. 47%).
At a specificity of 94% for both tests, the multiomics test yielded a higher sensitivity than plasma CEA level for stage I and II disease (94% vs. 18%), stage III and IV disease (91% vs. 45%), and any-stage disease (91% vs. 31%).
“Although there were many exciting aspects to this study, the test’s ability to detect cancers without loss of sensitivity for early-stage cancers was striking to me,” said Michael J. Hall, MD, of Fox Chase Cancer Center in Philadelphia, who was not involved in this study. “The loss of sensitivity in early tumors has been a limitation of other tests – FOBT [fecal occult blood test], FIT – so if this is replicable, this is exciting.”
Dr. Hall added that the need for only a blood sample would be a plus in screening healthy people. “When we consider the discomfort and inconvenience of colonoscopy, mammogram, and prostate cancer screening, and how they lead to reduced uptake of screening, the attractiveness of a noninvasive blood-based screening only increases further,” he elaborated.
Although the study was small for a colorectal cancer screening assessment, “the preliminary results presented in the poster were certainly compelling enough to support more research,” Dr. Hall said.
Dr. Putcha said that the test will be validated in a prospective, multicenter trial of roughly 10,000 participants at average risk, expected to open later this year. Further research will also help assess the test’s performance among patients with inflammatory bowel disease, for whom false-positive results with some available screening tests have been problematic.
The current study was sponsored by Freenome. Dr. Putcha is employed by Freenome and has a relationship with Palmetto GBA. Dr. Hall disclosed relationships with Ambry Genetics, AstraZeneca, Caris Life Sciences, Foundation Medicine, Invitae, and Myriad Genetics, and he shares a patent with institutional colleagues for a novel method to investigate hereditary colorectal cancer genes.
*This story was updated on Feb. 4, 2020.
SOURCE: Putcha G et al. 2020 GI Cancers Symposium, Abstract 66.
SAN FRANCISCO – A blood-based test that integrates data from multiple molecular “omes,” such as the genome and proteome, performs well at spotting early-stage colorectal cancer, the AI-EMERGE study suggests.
Moreover, the test netted better sensitivity than a fecal immunochemical test (FIT), a circulating tumor DNA (ctDNA) test, and a carcinoembryonic antigen (CEA) test.
These findings were reported in a poster session at the 2020 GI Cancers Symposium, which is sponsored by the American Gastroenterological Association, American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“Today, about a third of age-appropriate adults are not up to date with colorectal cancer screening,” lead study investigator Girish Putcha, MD, PhD, chief medical officer of Freenome in San Francisco, noted at the symposium. “A noninvasive blood-based screening test having high sensitivity and specificity for colorectal cancer generally, but especially for early-stage disease, could help improve adherence and ultimately reduce mortality.”
Dr. Putcha and colleagues evaluated a blood-based multiomics test in 32 patients with colorectal cancer of all stages and 539 colonoscopy-confirmed negative control subjects.
The test uses a multiomics platform to pick up both tumor-derived signal and non–tumor-derived signal from the body’s immune response and other sources. The test uses machine learning, and entails whole-genome sequencing, bisulfite sequencing (for assessment of DNA methylation), and protein quantification methods.
At 94% specificity, the test had a 94% sensitivity for spotting stage I and II colorectal cancer, 91% sensitivity for stage III and IV colorectal cancer, and 91% sensitivity for colorectal cancer of any stage. By location, sensitivity was 92% for distal tumors and 88% for proximal tumors.
The multiomics test outperformed a ctDNA test, a CEA test, and a FIT. At a specificity of 96% for both tests, the multiomics test yielded a higher sensitivity than a commercially available FIT stool test (OC-Auto FIT, Polymedco) for stage I and II disease (100% vs. 70%), stage III and IV disease (100% vs. 50%), and any-stage disease (100% vs. 67%).
When set at 100% specificity, the multiomics test outperformed a commercially available plasma ctDNA test (Avenio, Roche) set at 75% specificity. The multiomics test yielded a higher sensitivity for stage I and II disease (94% vs. 38%), stage III and IV disease (91% vs. 55%), and any-stage disease (90% vs. 47%).
At a specificity of 94% for both tests, the multiomics test yielded a higher sensitivity than plasma CEA level for stage I and II disease (94% vs. 18%), stage III and IV disease (91% vs. 45%), and any-stage disease (91% vs. 31%).
“Although there were many exciting aspects to this study, the test’s ability to detect cancers without loss of sensitivity for early-stage cancers was striking to me,” said Michael J. Hall, MD, of Fox Chase Cancer Center in Philadelphia, who was not involved in this study. “The loss of sensitivity in early tumors has been a limitation of other tests – FOBT [fecal occult blood test], FIT – so if this is replicable, this is exciting.”
Dr. Hall added that the need for only a blood sample would be a plus in screening healthy people. “When we consider the discomfort and inconvenience of colonoscopy, mammogram, and prostate cancer screening, and how they lead to reduced uptake of screening, the attractiveness of a noninvasive blood-based screening only increases further,” he elaborated.
Although the study was small for a colorectal cancer screening assessment, “the preliminary results presented in the poster were certainly compelling enough to support more research,” Dr. Hall said.
Dr. Putcha said that the test will be validated in a prospective, multicenter trial of roughly 10,000 participants at average risk, expected to open later this year. Further research will also help assess the test’s performance among patients with inflammatory bowel disease, for whom false-positive results with some available screening tests have been problematic.
The current study was sponsored by Freenome. Dr. Putcha is employed by Freenome and has a relationship with Palmetto GBA. Dr. Hall disclosed relationships with Ambry Genetics, AstraZeneca, Caris Life Sciences, Foundation Medicine, Invitae, and Myriad Genetics, and he shares a patent with institutional colleagues for a novel method to investigate hereditary colorectal cancer genes.
*This story was updated on Feb. 4, 2020.
SOURCE: Putcha G et al. 2020 GI Cancers Symposium, Abstract 66.
SAN FRANCISCO – A blood-based test that integrates data from multiple molecular “omes,” such as the genome and proteome, performs well at spotting early-stage colorectal cancer, the AI-EMERGE study suggests.
Moreover, the test netted better sensitivity than a fecal immunochemical test (FIT), a circulating tumor DNA (ctDNA) test, and a carcinoembryonic antigen (CEA) test.
These findings were reported in a poster session at the 2020 GI Cancers Symposium, which is sponsored by the American Gastroenterological Association, American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“Today, about a third of age-appropriate adults are not up to date with colorectal cancer screening,” lead study investigator Girish Putcha, MD, PhD, chief medical officer of Freenome in San Francisco, noted at the symposium. “A noninvasive blood-based screening test having high sensitivity and specificity for colorectal cancer generally, but especially for early-stage disease, could help improve adherence and ultimately reduce mortality.”
Dr. Putcha and colleagues evaluated a blood-based multiomics test in 32 patients with colorectal cancer of all stages and 539 colonoscopy-confirmed negative control subjects.
The test uses a multiomics platform to pick up both tumor-derived signal and non–tumor-derived signal from the body’s immune response and other sources. The test uses machine learning, and entails whole-genome sequencing, bisulfite sequencing (for assessment of DNA methylation), and protein quantification methods.
At 94% specificity, the test had a 94% sensitivity for spotting stage I and II colorectal cancer, 91% sensitivity for stage III and IV colorectal cancer, and 91% sensitivity for colorectal cancer of any stage. By location, sensitivity was 92% for distal tumors and 88% for proximal tumors.
The multiomics test outperformed a ctDNA test, a CEA test, and a FIT. At a specificity of 96% for both tests, the multiomics test yielded a higher sensitivity than a commercially available FIT stool test (OC-Auto FIT, Polymedco) for stage I and II disease (100% vs. 70%), stage III and IV disease (100% vs. 50%), and any-stage disease (100% vs. 67%).
When set at 100% specificity, the multiomics test outperformed a commercially available plasma ctDNA test (Avenio, Roche) set at 75% specificity. The multiomics test yielded a higher sensitivity for stage I and II disease (94% vs. 38%), stage III and IV disease (91% vs. 55%), and any-stage disease (90% vs. 47%).
At a specificity of 94% for both tests, the multiomics test yielded a higher sensitivity than plasma CEA level for stage I and II disease (94% vs. 18%), stage III and IV disease (91% vs. 45%), and any-stage disease (91% vs. 31%).
“Although there were many exciting aspects to this study, the test’s ability to detect cancers without loss of sensitivity for early-stage cancers was striking to me,” said Michael J. Hall, MD, of Fox Chase Cancer Center in Philadelphia, who was not involved in this study. “The loss of sensitivity in early tumors has been a limitation of other tests – FOBT [fecal occult blood test], FIT – so if this is replicable, this is exciting.”
Dr. Hall added that the need for only a blood sample would be a plus in screening healthy people. “When we consider the discomfort and inconvenience of colonoscopy, mammogram, and prostate cancer screening, and how they lead to reduced uptake of screening, the attractiveness of a noninvasive blood-based screening only increases further,” he elaborated.
Although the study was small for a colorectal cancer screening assessment, “the preliminary results presented in the poster were certainly compelling enough to support more research,” Dr. Hall said.
Dr. Putcha said that the test will be validated in a prospective, multicenter trial of roughly 10,000 participants at average risk, expected to open later this year. Further research will also help assess the test’s performance among patients with inflammatory bowel disease, for whom false-positive results with some available screening tests have been problematic.
The current study was sponsored by Freenome. Dr. Putcha is employed by Freenome and has a relationship with Palmetto GBA. Dr. Hall disclosed relationships with Ambry Genetics, AstraZeneca, Caris Life Sciences, Foundation Medicine, Invitae, and Myriad Genetics, and he shares a patent with institutional colleagues for a novel method to investigate hereditary colorectal cancer genes.
*This story was updated on Feb. 4, 2020.
SOURCE: Putcha G et al. 2020 GI Cancers Symposium, Abstract 66.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM