User login
Nontuberculous Mycobacterial Pulmonary Disease
Nontuberculous mycobacterial pulmonary disease is a broad term for a group of pulmonary disorders caused and characterized by exposure to environmental mycobacteria other than those belonging to the Mycobacterium tuberculosis complex and Mycobacterium leprae. Mycobacteria are aerobic, nonmotile organisms that appear positive with acid-fast alcohol stains. Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and have been recovered from domestic and natural water sources, soil, and food products, and from around livestock, cattle, and wildlife.1-3 To date, no evidence exists of human-to-human or animal-to-human transmission of NTM in the general population. Infections in humans are usually acquired from environmental exposures, although the specific source of infection cannot always be identified. Similarly, the mode of infection with NTM has not been established with certainty, but it is highly likely that the organism is implanted, ingested, aspirated, or inhaled. Aerosolization of droplets associated with use of bathroom showerheads and municipal water sources and soil contamination are some of the factors associated with the transmission of infection. Proven routes of transmission include showerheads and potting soil dust.2,3
NTM pulmonary disease occurs in individuals with or without comorbid conditions such as bronchiectasis, chronic obstructive pulmonary disease, pulmonary fibrosis, or structural lung diseases. Slender, middle-aged or elderly white females with marfanoid body habitus, with or without apparent immune or genetic disorders, showing impaired airway and mucus clearance present with this infection as a form of underlying bronchiectasis (Lady Windermere syndrome). It is unclear why NTM infections and escalation to clinical disease occur in certain individuals. Many risk factors, including inherited and acquired defects of host immune response (eg, cystic fibrosis trait and α1 antitrypsin deficiency), have been associated with increased susceptibility to NTM infections.4
NTM infection can lead to chronic symptoms, frequent exacerbations, progressive functional and structural lung destruction, and impaired quality of life, and is associated with an increased risk of hospitalization and higher 5-year all-cause mortality. As such, NTM disease is drawing increasing attention at the clinical, academic, and research levels.5 This case-based review outlines the clinical features of NTM infection, with a focus on the challenges in diagnosis, treatment, and management of NTM pulmonary disease. The cases use Mycobacterium avium complex (MAC), a slow-growing mycobacteria (SGM), and Mycobacterium abscessus, a rapidly growing mycobacteria (RGM), as prototypes in a non–cystic fibrosis, non-HIV clinical setting.
Epidemiology
Of the almost 200 isolated species of NTM, the most prevalent pathogens for respiratory disease in the United States are MAC, Mycobacterium kansasii, and M. abscessus. MAC accounts for more than 80% of cases of NTM respiratory disease in the United States.6 The prevalence of NTM disease is increasing at a rate of about 8% each year, with 75,000 to 105,000 patients diagnosed with NTM lung disease in the United States annually. NTM infections in the United States are increasing among patients aged 65 years and older, a population that is expected to nearly double by 2030.7,8
Isolation and prevalence of many NTM species are higher in certain geographic areas of the United States, especially in the southeast. The US coastal regions have a higher prevalence of NTM pulmonary disease, and account for 70% of NTM cases in the United States each year. Half of patients diagnosed with NTM lung disease reside in 7 states: Florida, New York, Texas, California, Pennsylvania, New Jersey, and Ohio, with 1 in 7 residing in Florida. Three parishes in Louisiana are among the top 10 counties with the highest prevalence in United States. The prevalence of NTM infection–associated hospitalizations is increasing worldwide as well. Co-infection with tuberculosis and multiple NTMs in individual patients has been observed clinically and documented in patients with and without HIV.9,10
It is not clear why the prevalence of NTM pulmonary disease is increasing, but there may be several contributing factors: (1) an increased awareness and identification of NTM infection sources in the environment; (2) an expanding cohort of immunocompromised individuals with exogenous or endogenous immune deficiencies; (3) availability of improved diagnostic techniques, such as use of high-performance liquid chromatography (HPLC), DNA probes, and gene sequencing; and (4) an increased awareness of the morbidity and mortality associated with NTM pulmonary disease. However, it is important to recognize that to best understand the clinical relevance of epidemiologic studies based on laboratory diagnosis and identification, the findings must be evaluated by correlating them with the microbiological and other clinical criteria established by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines.11
Continue to: Mycobacterium avium Complex
Mycobacterium avium Complex
Case Patient 1
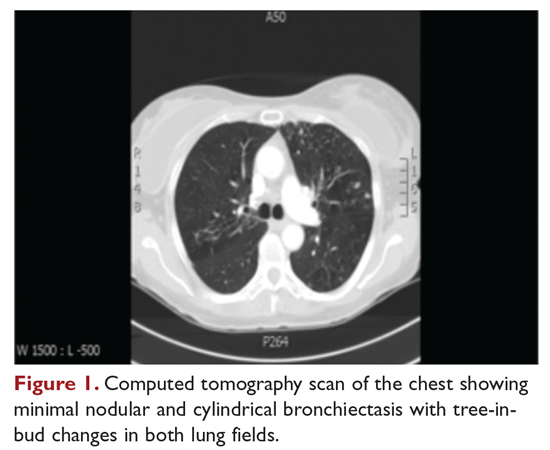
A 48-year-old woman who has never smoked and has no past medical problems, except seasonal allergic rhinitis and “colds and flu-like illness” once or twice a year, is evaluated for a chronic lingering cough with occasional sputum production. The patient denies any other chronic symptoms and is otherwise active. Physical examination reveals no specific findings except mild pectus excavatum and mild scoliosis. Body mass index is 22 kg/m2. Chest radiograph shows nonspecific increased markings in the lower zones. Computed tomography (CT) scan of the chest reveals minimal nodular and cylindrical bronchiectasis in both lungs (Figure 1). No previous radiographs are available for comparison. The patient is HIV-negative. Sputum tests reveal normal flora, and both fungus and acid-fast bacilli smear are negative. Culture for mycobacteria shows scanty growth of MAC in 1 specimen.
What is the clinical presentation of MAC pulmonary disease?
Among NTM, MAC is the most common cause of pulmonary disease worldwide.6 MAC primarily includes 2 species: M. avium and Mycobacterium intracellulare. M. avium is the more important pathogen in disseminated disease, whereas M. intracellulare is the more common respiratory pathogen.11 These organisms are genetically similar and generally not differentiated in the clinical microbiology laboratory, although there are isolated reports in the literature suggesting differences in prevalence, presentation, and prognosis in M. avium infection versus M. intracellulare infection.12
Three major disease syndromes are produced by MAC in humans: pulmonary disease, usually in adults whose systemic immunity is intact; disseminated disease, usually in patients with advanced HIV infection; and cervical lymphadenitis.13 Pulmonary disease caused by MAC may take on 1 of several clinically different forms, including asymptomatic “colonization” or persistent minimal infection without obvious clinical significance; endobronchial involvement; progressive pulmonary disease with radiographic and clinical deterioration and nodular bronchiectasis or cavitary lung disease; hypersensitivity pneumonitis; or persistent, overwhelming mycobacterial growth with symptomatic manifestations, often in a lung with underlying damage due to either chronic obstructive lung disease or pulmonary fibrosis (Table 1).14

Cavitary Disease
The traditionally recognized presentation of MAC lung disease has been apical cavitary lung disease in men in their late 40s and early 50s who have a history of cigarette smoking, and frequently, excessive alcohol use. If left untreated, or in the case of erratic treatment or macrolide drug resistance, this form of disease is generally progressive within a relatively short time and can result in extensive cavitary lung destruction and progressive respiratory failure.15
Nodular Bronchiectasis
The more common presentation of MAC lung disease, which is outlined in the case described here, is interstitial nodular infiltrates, frequently involving the right middle lobe or lingula and predominantly occurring in postmenopausal, nonsmoking white women. This is sometimes labelled “Lady Windermere syndrome.” These patients with M. avium infection appear to have similar clinical characteristics and body types, including lean build, scoliosis, pectus excavatum, and mitral valve prolapse.16,17 The mechanism by which this body morphotype predisposes to pulmonary mycobacterial infection is not defined, but ineffective mucociliary clearance is a possible explanation. Evidence suggests that some patients may be predisposed to NTM lung disease because of preexisting bronchiectasis. Some potential etiologies of bronchiectasis in this population include chronic sinusitis, gastroesophageal reflux with chronic aspiration, α1 antitrypsin deficiency, and cystic fibrosis genetic traits and mutations.18 Risk factors for increased morbidity and mortality include the development of cavitary disease, age, weight loss, lower body mass index, and other comorbid conditions.
This form of disease, termed nodular bronchiectasis, tends to have a much slower progression than cavitary disease, such that long-term follow-up (months to years) may be necessary to demonstrate clinical or radiographic changes.11 The radiographic term “tree-in-bud” has been used to describe what may reflect inflammatory changes, including bronchiolitis. High-resolution CT scans of the chest are especially helpful for diagnosing this pattern of MAC lung disease, as bronchiectasis and small nodules may not be easily discernible on plain chest radiograph. The nodular/bronchiectasis radiographic pattern can also be seen with other NTM pathogens, including M. abscessus, Mycobacterium simiae, and M. kansasii. Solitary nodules and dense consolidation have also been described. Pleural effusions are uncommon, but reactive pleural thickening is frequently seen. Co-pathogens may be isolated from culture, including Pseudomonas aeruginosa, Staphylococcus aureus, and, occasionally, other NTM such as M. abscessus or Mycobacterium chelonae.19-21
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis, initially described in patients who were exposed to hot tubs, mimics allergic hypersensitivity pneumonitis, with respiratory symptoms and culture/tissue identification of MAC or sometimes other NTM. It is unclear whether hypersensitivity pneumonitis is an inflammatory process, an infection, or both, and opinion regarding the need for specific antibiotic treatment is divided.11,22 However, avoidance of exposure is prudent and recommended.
Disseminated Disease
Disseminated NTM disease is associated with very low CD4+ lymphocyte counts and is seen in approximately 5% of patients with HIV infection.23-25 Although disseminated NTM disease is rarely seen in immunosuppressed patients without HIV infection, it has been reported in patients who have undergone renal or cardiac transplant, patients on long-term corticosteroid therapy, and those with leukemia or lymphoma. More than 90% of infections are caused by MAC; other potential pathogens include M. kansasii, M. chelonae, M. abscessus, and Mycobacterium haemophilum. Although seen less frequently since the advent of highly active antiretroviral therapy, disseminated infection can develop progressively from an apparently indolent or localized infection or a respiratory or gastrointestinal source. Signs and symptoms of disseminated infection (specifically MAC-associated disease) are nonspecific and include fever, night sweats, weight loss, and abdominal tenderness. Disseminated MAC disease occurs primarily in patients with more advanced HIV disease (CD4+ count typically < 50 cells/μL). Clinically, disseminated MAC manifests as intermittent or persistent fever, constitutional symptoms with organomegaly and organ-specific abnormalities (eg, anemia, neutropenia from bone marrow involvement, adenopathy, hepatosplenomegaly), and elevations of liver enzymes or lung infiltrates from pulmonary involvement.
Continue to: What are the criteria for diagnosing NTM pulmonary disease?
What are the criteria for diagnosing NTM pulmonary disease?
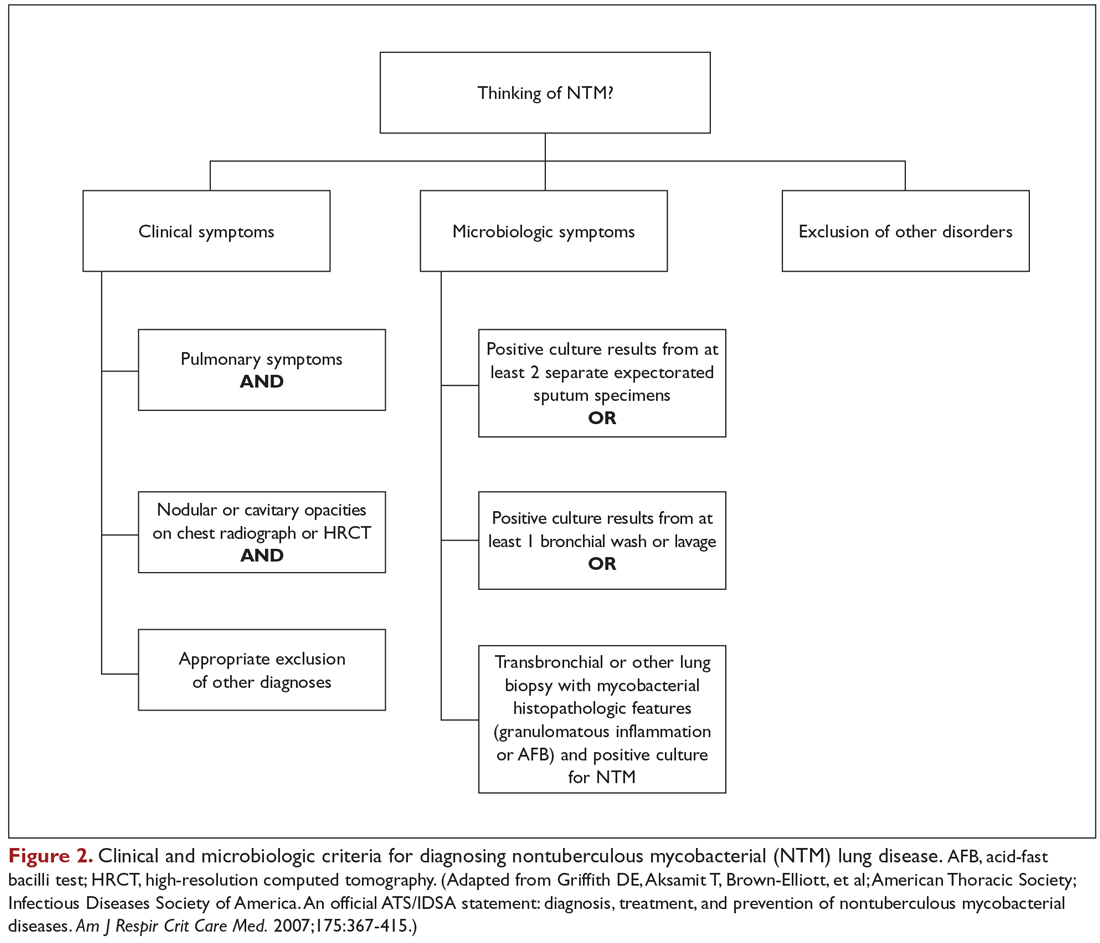
The diagnosis of NTM disease is based on clinical, radiologic, and mycobacterial correlation with good communication between the experts in this field. The ATS/IDSA criteria for diagnosing NTM lung disease are shown in Figure 2. These criteria best apply to MAC, M. kansasii, and M. abscessus, but are also clinically applied to other NTM respiratory pathogens. The diagnosis of MAC infection is most readily established by culture of blood, bone marrow, respiratory secretions/fluid, or tissue specimens from suspected sites of involvement. Due to erratic shedding of MAC into the respiratory secretions in patients with nodular bronchiectasis, as compared to those with the cavitary form of the disease, sputum may be intermittently positive, with variable colony counts and polyclonal infections.12 Prior to the advent of high-resolution CT, isolation of MAC organisms from the sputum of such patients was frequently dismissed as colonization.
Mycobacterial Testing
Because of the nonspecific symptoms and lack of diagnostic specificity of chest imaging, the diagnosis of NTM lung disease requires microbiologic confirmation. Specimens sent to the laboratory for identification of NTM must be handled with care to prevent contamination and false-positive results. Transport media and preservatives should be avoided, and transportation of the specimens should be prompt. These measures will prevent bacterial overgrowth. Furthermore, the yield of NTM may be affected if the patient has used antibiotics, such as macrolides and fluoroquinolones, prior to obtaining the specimen.
NTM should be identified at the species and subspecies level, although this is not practical in community practice settings. The preferred staining procedure in the laboratory is the fluorochrome method. Some species require special growth conditions and/or lower incubation temperatures, and other identification methods may have to be employed, such as DNA probes, polymerase chain reaction genotyping, nucleic acid sequence determination, and high-performance liquid chromatography. As a gold standard, clinical specimens for mycobacterial cultures should be inoculated onto 1 or more solid media (eg, Middlebrook 7H11 media and/or Lowenstein-Jensen media, the former of which is the preferred medium for NTM) and into a liquid medium (eg, BACTEC 12B broth or Mycobacteria growth indicator tube broth). Growth of visible colonies on solid media typically requires 2 to 4 weeks, but liquid media (eg, the radiometric BACTEC system), used as a supplementary and not as an exclusive test, usually produce results within 10 to 14 days. Furthermore, even after initial growth, identification of specific isolates based on the growth characteristics on solid media requires additional time. Use of specific nucleic acid probes for MAC and M. kansasii and HPLC testing of mycolic acid patterns in acid-fast bacilli smear–positive specimens can reduce the turnaround time of specific identification of a primary culture–positive sample. However, HPLC is not sufficient for definitive identification of many NTM species, including the RGM. Other newer techniques, including 16S ribosomal DNA sequencing and polymerase chain reaction-restriction fragment length polymorphism analysis, also allow NTM to be identified and speciated more reliably and rapidly from clinical specimens.
Cost and other practical considerations limit widespread adoption of these techniques. However, the recognition that M. abscessus can be separated into more than 1 subspecies, and that there are important prognostic implications of that separation, lends urgency to the broader adoption of newer molecular techniques in the mycobacteriology laboratory. Susceptibility testing is based on the broth microdilution method; RGM usually grow within 7 days of subculture, and the laboratory time to culture is a helpful hint, although not necessarily specific. Recognizing the morphology of mycobacterial colony growth may also be helpful in identification.
Are skin tests helpful in diagnosing NTM infection?
Tuberculin skin testing remains a nonspecific marker of mycobacterial infection and does not help in further elucidating NTM infection. However, epidemiologic and laboratory studies with well-characterized antigens have shown that dual skin testing with tuberculosis- versus NTM-derived tuberculin can discriminate between prior NTM and prior tuberculosis disease. Species-specific skin test antigens are not commercially available and are not helpful in the diagnosis of NTM disease because of cross-reactivity of M. tuberculosis and some NTM. However, increased prevalence of NTM sensitization based on purified protein derivative testing has been noted in a recent survey, which is consistent with an observed increase in the rates of NTM infections, specifically MAC, in the United States.26,27
Interferon-gamma release assays (IGRAs) are now being used as an alternative to tuberculin skin testing to diagnose M. tuberculosis infection. Certain NTM species also contain gene sequences that encode for ESAT-6 or CFP-10 antigens used in the IGRAs, and hence, yield a positive IGRA test. These include M. marinum, M. szulgai, and M. kansasii.28,29 However, MAC organisms do not produce positive results on assays that use these antigens.
Continue to: What is the approach to management of NTM pulmonary disease?
What is the approach to management of NTM pulmonary disease?
The correlation of symptoms with radiographic and microbiologic evidence is essential to categorize the disease and determine the need for therapy. Making the diagnosis of NTM lung disease does not necessitate the institution of therapy. The decision to treat should be weighed against potential risks and benefits to the individual patient based on symptomatic, radiographic, and microbiologic criteria, as well as underlying systemic or pulmonary immune status. In the absence of evidence of clinical, radiologic, or mycobacterial progression of disease, pursuing airway clearance therapy and clinical surveillance without initiating specific anti-MAC therapy is a reasonable option.11 Identifying the sustained presence of NTM infection, especially MAC, in a patient with underlying clinical and radiographic evidence of bronchiectasis is of value in determining comprehensive treatment and management strategies. Close observation is indicated if the decision not to treat is made. If treatment is initiated, comprehensive management includes long-term follow-up with periodic bacteriologic surveillance, watching for drug toxicity and drug-drug interactions, ensuring adherence and compliance to treatment, and managing comorbidity.
The Bronchiectasis Severity Index is a useful clinical predictive tool that identifies patients at risk of future mortality, hospitalization, and exacerbations and can be used to evaluate the need for specific treatment.30 The index is based on dyspnea score, lung function tests, colonization of pathogens, and extent of disease.
Case 1 Continued
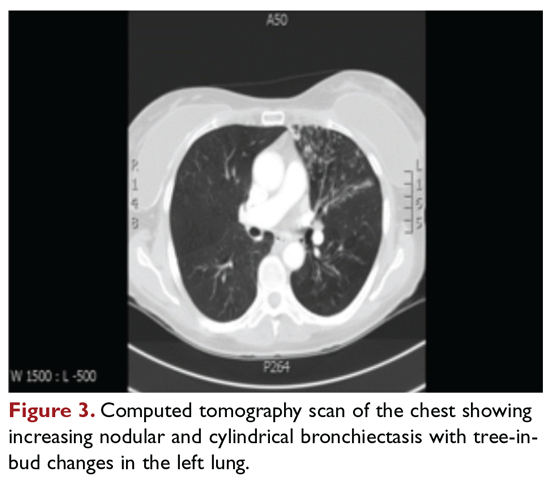
After approximately 2 months of observation and symptomatic treatment, without specific antibiotic therapy, the patient’s symptoms continue. She now develops intermittent hemoptysis. Repeat sputum studies reveal moderate growth of M. avium. A follow-up CT scan shows progression of disease, with an increase in the tree-in-bud pattern (Figure 3).
What treatment protocols are recommended for MAC pulmonary disease?
As per the ATS/IDSA statement, macrolides are the mainstay of treatment for pulmonary MAC disease.11 Macrolides achieve an increased concentration in the lung, and when used for treatment of pulmonary MAC disease, there is a strong correlation between in vitro susceptibility, in vivo (clinical) response, and the immunomodulating effects of macrolides.31,32 Macrolide-containing regimens have demonstrated efficacy in patients with MAC pulmonary disease33,34; however, macrolide monotherapy should be avoided to prevent the development of resistance.
At the outset, it is critical to establish the objective criteria for determining response and to ensure that the patient understands the goals of the treatment and expectations of the treatment plan. Moreover, experts suggest that due to the possibility of drug intolerance, side effects, and the need for prolonged therapy, a “step ladder” ramping up approach to treatment could be adopted, with gradual introduction of therapy within a short time period; this approach may improve compliance and adherence to treatment.11 If this approach is used, the doses may have to be divided. Patients who are unable to tolerate daily medications, even with dosage adjustment, should be tried on an intermittent treatment regimen. Older female patients frequently require gradual introduction of medications (ie, 1 medication added to the regimen every 1 to 2 weeks) to evaluate tolerance to each medication and medication dose.11 Commonly encountered adverse effects of NTM treatment include intolerance to clarithromycin due to gastrointestinal problems, low body mass index, or age older than 70 years.
After determining that the patient requires therapy, the standard recommended treatment for MAC pulmonary disease includes the following: for most patients with nodular/bronchiectasis disease, a thrice-weekly regimen of clarithromycin (1000 mg) or azithromycin (500 mg), rifampin (600 mg), and ethambutol (25 mg/kg) is recommended. For patients with cavitary MAC pulmonary disease or severe nodular/bronchiectasis disease, the guidelines recommend a daily regimen of clarithromycin (500-1000 mg) or azithromycin (250 mg), rifampin (600 mg) or rifabutin (150–300 mg), and ethambutol (15 mg/kg), with consideration of intravenous (IV) amikacin 3 times/week early in therapy (Table 2).11
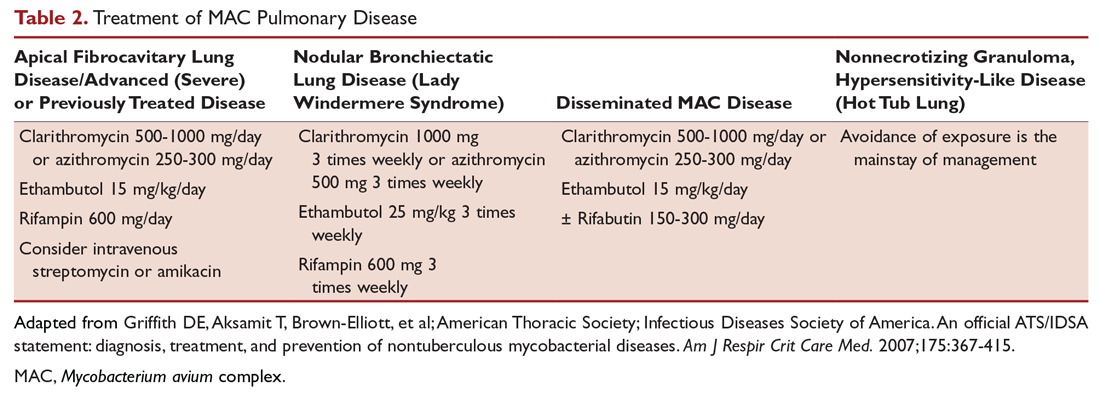
The treatment of MAC hypersensitivity-like disease speaks to the controversy of whether this is an inflammatory process, infectious process, or a combination of inflammation and infection. Avoidance of exposure is the mainstay of management. In some cases, steroids are used with or without a short course of anti-MAC therapy (ie, clarithromycin or azithromycin with rifampin and ethambutol).
Prophylaxis for disseminated MAC disease should be given to adults with HIV infection who have a CD4+ count less than 50 cells/μL. Azithromycin 1200 mg/week or clarithromycin 1000 mg/day has proven efficacy, and rifabutin 300 mg/day is also effective but less well tolerated. Rifabutin is more active in vitro against MAC than rifampin, and is used in HIV-positive patients because of drug-drug interaction between antiretroviral drugs and rifampin.
Continue to: Case 1 Continued
Case 1 Continued
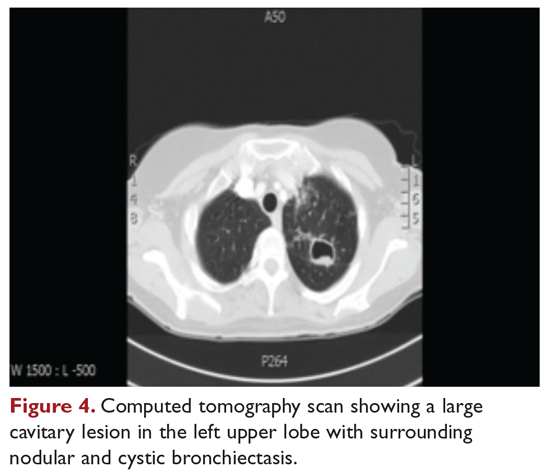
The patient is treated with clarithromycin, rifampin, and ethambutol for 1 year, with sputum conversion after 9 months. In the latter part of her treatment, she experiences decreased visual acuity. Treatment is discontinued prematurely after 1 year due to drug toxicity and continued intolerance to drug therapy. The patient remains asymptomatic for 8 months, and then begins to experience mild to moderate hemoptysis, with increasing cough and sputum production associated with postural changes during exercise. Physical examination overall remains unchanged. Three sputum results reveal heavy growth of MAC, and a CT scan of the chest shows a cavitary lesion in the left upper lobe along with the nodular bronchiectasis (Figure 4).
What are the management options at this stage?
Based on this patient’s continued symptoms, progression of radiologic abnormalities, and current culture growth, she requires re-treatment. With the adverse effects associated with ethambutol during the first round of therapy, the drug regimen needs to be modified. Several considerations are relevant at this stage. Relapse rates range from 20% to 30% after treatment with a macrolide-based therapy.11,34 Obtaining a culture-sensitivity profile is imperative in these cases. Of note, treatment should not be discontinued altogether, but instead the toxic agent should be removed from the treatment regimen. Continuing treatment with a 2-drug regimen of clarithromycin and rifampin may be considered in this patient. Re-infection with multiple genotypes may also occur after successful drug therapy, but this is primarily seen in MAC patients with nodular bronchiectasis.34,35 Patients in whom previous therapy has failed, even those with macrolide-susceptible MAC isolates, are less likely to respond to subsequent therapy. Data suggest that intermittent medication dosing is not effective for patients with severe or cavitary disease or in those in whom previous therapy has failed.36 In this case, treatment should include a daily 3-drug therapy, with an injectable thrice-weekly aminoglycoside. Other agents such as linezolid and clofazimine may have to be tried. Cycloserine, ethionamide, and other agents are sometimes used, but their efficacy is unproven and doubtful. Pyrazinamide and isoniazid have no activity against MAC.
Treatment Failure and Drug Resistance
Treatment failure is considered to have occurred if patients have not had a response (microbiologic, clinical, or radiographic) after 6 months of appropriate therapy or had not achieved conversion of sputum to culture-negative after 12 months of appropriate therapy.11 This occurs in about 40% of patients. Multiple factors can interfere with the successful treatment of MAC pulmonary disease, including medication nonadherence, medication side effects or intolerance, lack of response to a medication regimen, or the emergence of a macrolide-resistant or multidrug-resistant strain. Inducible macrolide resistance remains a potential factor.34-36 A number of characteristics of NTM contribute to the poor response to currently used antibiotics: the organisms have a lipid outer membrane and prefer to adhere to surfaces and form biofilms, which makes them relatively impermeable to antibiotics.37 Also, NTM replicate in phagocytic cells, allowing them to subvert normal cellular defense mechanisms. Furthermore, NTM can display colony variants, whereby single colony isolates switch between antibiotic-susceptible and -resistant variants. These factors have also impeded in development of new antibiotics for NTM infection.37
Recent limited approval of amikacin liposomal inhalation suspension (ALIS) for treatment failure and refractory MAC infection in combination with guideline-based antimicrobial therapy (GBT) is a promising addition to the available treatment armamentarium. In a multinational trial, the addition of ALIS to GBT for treatment-refractory MAC lung disease achieved significantly greater culture conversion rates by month 6 than GBT alone, with comparable rates of serious adverse events.38
Is therapeutic drug monitoring recommended during treatment of MAC pulmonary disease?
Treatment failure may also be drug-related, including poor drug penetration into the damaged lung tissue or drug-drug interactions leading to suboptimal drug levels. Peak serum concentrations have been found to be below target ranges in approximately 50% of patients using a macrolide and ethambutol. Concurrent use of rifampin decreases the peak serum concentration of macrolides and quinolones, with acceptable target levels seen in only 18% to 57% of cases. Whether this alters patient outcomes is not clear.39-42 Factors identified as contributing to the poor response to therapy include poor compliance, cavitary disease, previous treatment for MAC pulmonary disease, and a history of chronic obstructive lung disease. Studies by Koh and colleagues40 and van Ingen and colleagues41 with pharmacokinetic and pharmacodynamics data showed that, in patients on MAC treatment with both clarithromycin and rifampicin, plasma levels of clarithromycin were lower than the recommended minimal inhibitory concentrations (MIC) against MAC for that drug. The studies also showed that rifampicin lowered clarithromycin concentrations more than did rifabutin, with the AUC/MIC ratio being suboptimal in nearly half the cases. However, low plasma clarithromycin concentrations did not have any correlation with treatment outcomes, as the peak plasma drug concentrations and the peak plasma drug concentration/MIC ratios did not differ between patients with unfavorable treatment outcomes and those with favorable outcomes. This is further compounded by the fact that macrolides achieve higher levels in lung tissue than in plasma, and hence the significance of low plasma levels is unclear; however, it is postulated that achieving higher drug levels could, in fact, lead to better clinical outcomes. Pending specific well-designed, prospective randomized controlled trials, routine therapeutic drug monitoring is not currently recommended, although some referral centers do this as their practice pattern.
Is surgery an option in this case?
The overall 5-year mortality for MAC pulmonary disease was approximately 28% in a retrospective analysis, with patients with cavitary disease at increased risk for death at 5 years.42 As such, surgery is an option in selected cases as part of adjunctive therapy along with anti-MAC therapy based on mycobacterial sensitivity. Surgery is used as either a curative approach or a “debulking” measure.11 When present, clearly localized disease, especially in the upper lobe, lends itself best to surgical intervention. Surgical resection of a solitary pulmonary nodule due to MAC, in addition to concomitant medical treatment, is recommended. Surgical intervention should be considered early in the course of the disease because it may provide a cure without prolonged treatment and its associated problems, and this approach may lead to early sputum conversion. Surgery should also be considered in patients with macrolide-resistant or multidrug-resistant MAC infection or in those who cannot tolerate the side effects of therapy, provided that the disease is focal and limited. Patients with poor preoperative lung function have poorer outcomes than those with good lung function, and postoperative complications arising from treatment, especially with a right-sided pneumonectomy, tend to occur more frequently in these patients. Thoracic surgery for NTM pulmonary disease must be considered cautiously, as this is associated with significant morbidity and mortality and is best performed at specialized centers that have expertise and experience in this field.43
Continue to: Mycobacterium abscessus Complex
Mycobacterium abscessus Complex
Case Patient 2
A 64-year-old man who is an ex-smoker presents with chronic cough, mild shortness of breath on exertion, low-grade fever, and unintentional weight loss of 10 lb. Physical exam is unremarkable. The patient was diagnosed with immunoglobulin deficiency (low IgM and low IgG4) in 2002, and has been on replacement therapy since then. He also has had multiple episodes of NTM infection, with MAC and M. kansasii infections diagnosed in 2012-2014, which required 18 months of multi-drug antibiotic treatment that resulted in sputum conversion. Pulmonary function testing done on this visit in 2017 shows mild obstructive impairment.
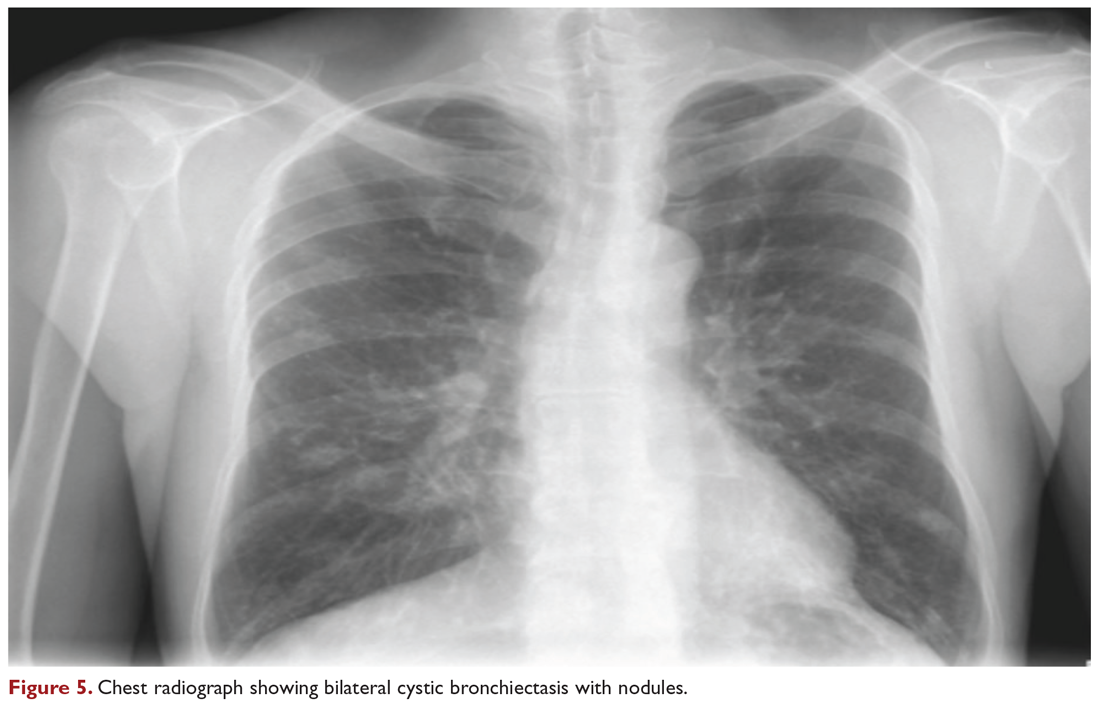
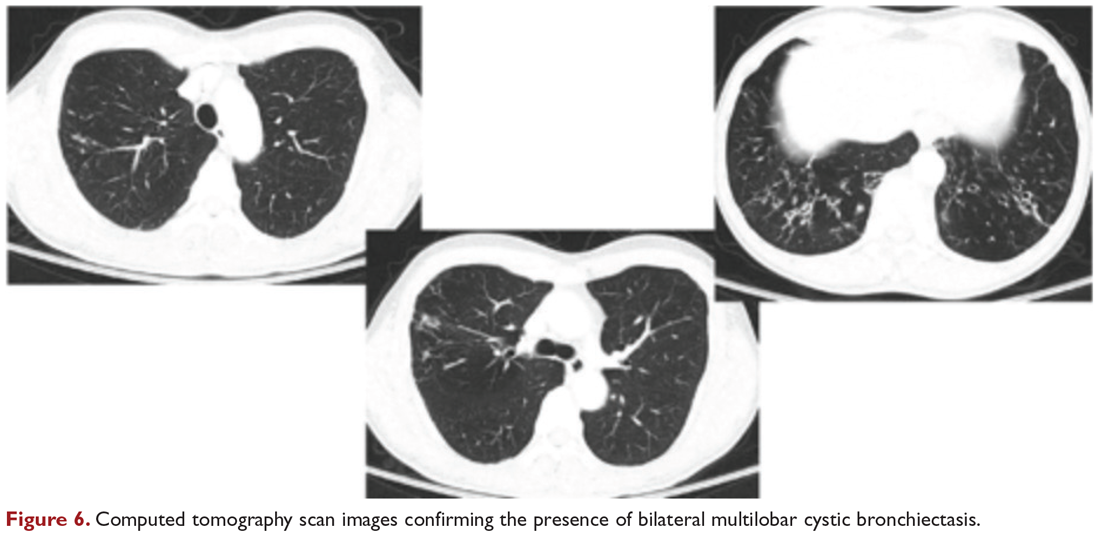
Chest radiograph and CT scan show bilateral bronchiectasis (Figure 5 and Figure 6).
The results of serial sputum microbiology testing performed over the course of 6 months are outlined below:
- 5/2017 (bronchoalveolar lavage): 2+; M. abscessus
- 9/2017 × 2: smear (–); group IV RGM
- 11/2017: smear (–); M. abscessus (> 50 CFU)
- 12/2017: smear (–); M. abscessus (> 50 CFU)
What are the clinical considerations in this patient with multiple NTM infections?
M. abscessus complex was originally described in soft tissue abscesses and skin infections possibly resulting from soil or water contamination. Subspeciation of M. abscessus complex during laboratory testing is critical to facilitate selection of a specific therapeutic approach; treatment decisions are impacted by the presence of an active erm gene and in vitro macrolide sensitivity, which differ between subspecies. The most acceptable classification outlines 3 species in the M. abscessus complex: Mycobacterium abscessus subsp abscessus, Mycobacterium abscessus subsp bolletii (both with an active erm gene responsible for macrolide resistance), and Mycobacterium abscessus subsp massiliense (with an inactive erm gene and therefore susceptible to macrolides).44
RGM typically manifest in skin, soft tissue, and bone, and can cause soft tissue, surgical wound, and catheter-related infections. Although the role of RGM as pulmonary pathogens is unclear, underlying diseases associated with RGM include previously treated mycobacterial disease, coexistent pulmonary diseases with or without MAC, cystic fibrosis, malignancies, and gastroesophageal disorders. M. abscessus is the third most commonly identified respiratory NTM and accounts for the majority (80%) of RGM respiratory isolates. Other NTM reported to cause both lung disease and skin, bone, and joint infections include Mycobacterium simiae, Mycobacterium xenopi, and Mycobacterium malmoense. Ocular granulomatous diseases, such as chorioretinitis and keratitis, have been reported with both RGM and Runyon group III SGM, such as MAC or M. szulgai, following trauma or refractive surgery. These can mimic fungal, herpetic, or amebic keratitis. The pulmonary syndromes associated with multiple culture positivity are seen in elderly women with bronchiectasis or cavitary lung disease and/or associated with gastrointestinal symptoms of acid reflux, with or without achalasia and concomitant lipoid interstitial pneumonia.45
Generally, pulmonary disease progresses slowly, but lung disease attributed to RGM can result in respiratory failure. Thus, RGM should be recognized as a possible cause of chronic mycobacterial lung disease, especially in immunocompromised patients, and respiratory isolates should be assessed carefully. Identification and drug susceptibility testing are essential before initiation of treatment for RGM.
What is the approach to management of M. abscessus pulmonary disease in a patient without cystic fibrosis?
The management of M. abscessus pulmonary infection as a subset of RGM requires a considered step-wise approach. The criteria for diagnosis and threshold for starting treatment are the same as those used in the management of MAC pulmonary disease,11 but the treatment of M. abscessus pulmonary infection is more complex and has lower rates of success and cure. Also, antibiotic treatment presents challenges related to rapid identification of the causative organism, nomenclature, resistance patterns, and tolerance of treatment and side effects. If a source such as catheter, access port, or any surgical site is identified, prompt removal and clearance of the infected site are strongly advised
In the absence of any controlled clinical trials, treatment of RGM is based on in vitro susceptibility testing and expert opinion. As in MAC pulmonary disease, macrolides are the mainstay of treatment, with an induction phase of intravenous antibiotics. Treatment may include a combination of injectable aminoglycosides, imipenem, or cefoxitin and oral drugs such as a macrolide (eg, clarithromycin, azithromycin), doxycycline, fluoroquinolones, trimethoprim/sulfamethoxazole, or linezolid. While antibiotic treatment of M. abscessus pulmonary disease is based on in vitro sensitivity pattern to a greater degree than is treatment of MAC pulmonary disease, this approach has significant practical limitations and hence variable applicability. The final choice of antibiotics is best based on the extended susceptibility results, if available. The presence of an active erm gene on a prolonged growth specimen in M. abscessus subsp abscessus and M. abscessus subsp bolletii precludes the use of a macrolide. In such cases, amikacin, especially in an intravenous form, is the mainstay of treatment based on MIC. Recently, there has been a resurgence in interest in the use of clofazimine in combination with amikacin when treatment is not successful in patients with M. abscessus subsp abscessus or M. bolletii with an active erm gene.45,46 When localized abscess formation is noted, surgery may be the best option, with emphasis on removal of implants and catheters if implicated in RGM infection.
Attention must also be given to confounding pulmonary and associated comorbidities. This includes management of bronchiectasis with appropriately aggressive airway clearance techniques; anti-reflux measures for prevention of micro-aspiration; and management of other comorbid pulmonary conditions, such as chronic obstructive pulmonary disease, pulmonary fibrosis, and sarcoidosis, if applicable. These interventions play a critical role in clearing the M. abscessus infection, preventing progression of disease, and reducing morbidity. The role of immunomodulatory therapy needs to be considered on a regular, ongoing basis. Identification of genetic factors and correction of immune deficiencies may help in managing the infection.
Case Patient 2 Conclusion
The treatment regimen adopted in this case includes a 3-month course of daily intravenous amikacin and imipenem with oral azithromycin, followed by a continuation phase of azithromycin with clofazimine and linezolid. Airway clearance techniques such as Vest/Acapella/CPT are intensified and monthly intravenous immunoglobulin therapy is continued. The patient responds to treatment, with resolution of his clinical symptoms and reduction in the colony count of M. abscessus in the sputum.
Summary
NTM are ubiquitous in the environment, and NTM infection has variable manifestations, especially in patients with no recognizable immune impairments. Underlying comorbid conditions with bronchiectasis complicate its management. Treatment strategies must be individualized based on degree of involvement, associated comorbidities, immune deficiencies, goals of therapy, outcome-based risk-benefit ratio assessment, and patient engagement and expectations. In diffuse pulmonary disease, drug treatment remains difficult due to poor match of in vitro and in vivo culture sensitivity, side effects of medications, and high failure rates. When a localized resectable foci of infection is identified, especially in RGM disease, surgical treatment may be the best approach in selected patients, but it must be performed in centers with expertise and experience in this field.
1. Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210-220.
2. Falkinham JO III. Environmental sources of NTM. Clin Chest Med. 2015;36:35-41.
3. Falkinham JO III, Current epidemiological trends in NTM. Curr Environ Health Rep. 2016;3:161-167.
4. Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med. 2015;36:1-11.
5. Marras TK, Mirsaeidi M, Chou E, et al. Health care utilization and expenditures following diagnosis of nontuberculous mycobacterial lung disease in the United States. Manag Care Spec Pharm. 2018;24:964-974.
6. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med. 2010;182:970-976.
7. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977-982.
8. Adjemian, Olivier KN, Seitz AE, J et al. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185;881-886.
9. Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalizations associated with pulmonary nontuberculous mycobacterial infections in Germany, 2005-2011. BMC Infect Dis. 2013;13:231.
10. Aliyu G, El-Kamary SS, Abimiku A, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170.
11. Griffith DE, Aksamit T, Brown-Elliott, et al; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-415.
12. Wallace RJ Jr, Zhang Y, Brown BA, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158:1235-1244.
13. Gordin FM, Horsburgh CR Jr. Mycobacterium avium complex. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Elsevier; 2015.
14. Chitty S, Ali J. Mycobacterium avium complex pulmonary disease in immune competent patients. South Med J. 2005;98:646-52.
15. Ramirez J, Mason C, Ali J, Lopez FA. MAC pulmonary disease: management options in HIV-negative patients. J La State Med Soc. 2008;160:248-254.
16. Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914-916.
17. Kim RD, Greenburg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066-1074.
18. Ziedalski TM, Kao PN, Henig NR, et al. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995-1002.
19. Koh WJ, Lee KS, Kwon OJ, et al. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005;235:282-288.
20. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994;105:49-52.
21. Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115:1033-1040.
22. Cappelluti E, Fraire AE, Schaefer OP. A case of “hot tub lung” due to Mycobacterium avium complex in an immunocompetent host. Arch Intern Med. 2003;163:845-848.
23. Nightingale SD, Byrd LT, Southern PM, et al. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082-1085.
24. Horsburgh CR Jr, Selik RM. The epidemiology of disseminated tuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989;139:4-7.
25. Chin DP, Hopewell PC, Yajko DM, et al. Mycobacterium avium complex in the respiratory or gastrointestinal tract and the risk of M. avium complex bacteremia in patients with human immunodeficiency virus infection. J Infect Dis. 1994;169:289-295.
26. Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176:306-313.
27. Lillo M, Orengo S, Cernoch P, Harris RL. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis. 1990;12:760-767.
28. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099-1104.
29. Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797-1807.
30. James D, Chalmers JD, Goeminne P, et al. The Bronchiectasis Severity Index: an international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576-585.
31. Heifets L. MIC as a quantitative measurement of the susceptibility of Mycobacterium avium strains to seven antituberculosis drugs. Antimicrob Agents Chemother. 1988;32:1131-1136.
32. Horsburgh CR Jr, Mason UG 3rd, Heifits LB, et al. Response to therapy of pulmonary Mycobacterium avium intracellulare infection correlates with results of in vitro susceptibility testing. Am Rev Respir Dis. 1987;135:418-421.
33. Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S-78S.
34. Wallace RJ Jr, Brown BA, Griffith DE, et al. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153:1766-1772.
35. Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928-934.
36. Lam PK, Griffith DE, Aksamit TR, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173:1283-1289.
37. Falkinham J III. Challenges of NTM drug development. Front Microbiol. 2018;9:1613.
38. Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198:1559-1569.
39. Schluger NW. Treatment of pulmonary Mycobacterium avium complex infections: do drug levels matter? Am J Respir Crit Care Med. 2012;186:710-711.
40. Van Ingen J, Egelund EF, Levin A, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186:559-565.
41. Koh WJ, Jeong BH, Jeon K, et al. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186:797-802.
42. Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary MAC disease. Int J Tuberc Lung Dis. 2012;16:408-414.
43. Yuji S, Yutsuki N, Keiichiso T, et al. Surgery for Mycobacterium avium lung disease in the clarithromycin era. Eur J Cardiothor Surg. 2002;21:314-318.
44. Tortoli E, Kohl TA, Brown-Elliott BA, et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. Int J Syst Evol Microbiol. 2016; 66:4471-4479.
45. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271-1278.
46. Koh WJ, Jeong BH, Kim SY, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309-316.
Nontuberculous mycobacterial pulmonary disease is a broad term for a group of pulmonary disorders caused and characterized by exposure to environmental mycobacteria other than those belonging to the Mycobacterium tuberculosis complex and Mycobacterium leprae. Mycobacteria are aerobic, nonmotile organisms that appear positive with acid-fast alcohol stains. Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and have been recovered from domestic and natural water sources, soil, and food products, and from around livestock, cattle, and wildlife.1-3 To date, no evidence exists of human-to-human or animal-to-human transmission of NTM in the general population. Infections in humans are usually acquired from environmental exposures, although the specific source of infection cannot always be identified. Similarly, the mode of infection with NTM has not been established with certainty, but it is highly likely that the organism is implanted, ingested, aspirated, or inhaled. Aerosolization of droplets associated with use of bathroom showerheads and municipal water sources and soil contamination are some of the factors associated with the transmission of infection. Proven routes of transmission include showerheads and potting soil dust.2,3
NTM pulmonary disease occurs in individuals with or without comorbid conditions such as bronchiectasis, chronic obstructive pulmonary disease, pulmonary fibrosis, or structural lung diseases. Slender, middle-aged or elderly white females with marfanoid body habitus, with or without apparent immune or genetic disorders, showing impaired airway and mucus clearance present with this infection as a form of underlying bronchiectasis (Lady Windermere syndrome). It is unclear why NTM infections and escalation to clinical disease occur in certain individuals. Many risk factors, including inherited and acquired defects of host immune response (eg, cystic fibrosis trait and α1 antitrypsin deficiency), have been associated with increased susceptibility to NTM infections.4
NTM infection can lead to chronic symptoms, frequent exacerbations, progressive functional and structural lung destruction, and impaired quality of life, and is associated with an increased risk of hospitalization and higher 5-year all-cause mortality. As such, NTM disease is drawing increasing attention at the clinical, academic, and research levels.5 This case-based review outlines the clinical features of NTM infection, with a focus on the challenges in diagnosis, treatment, and management of NTM pulmonary disease. The cases use Mycobacterium avium complex (MAC), a slow-growing mycobacteria (SGM), and Mycobacterium abscessus, a rapidly growing mycobacteria (RGM), as prototypes in a non–cystic fibrosis, non-HIV clinical setting.
Epidemiology
Of the almost 200 isolated species of NTM, the most prevalent pathogens for respiratory disease in the United States are MAC, Mycobacterium kansasii, and M. abscessus. MAC accounts for more than 80% of cases of NTM respiratory disease in the United States.6 The prevalence of NTM disease is increasing at a rate of about 8% each year, with 75,000 to 105,000 patients diagnosed with NTM lung disease in the United States annually. NTM infections in the United States are increasing among patients aged 65 years and older, a population that is expected to nearly double by 2030.7,8
Isolation and prevalence of many NTM species are higher in certain geographic areas of the United States, especially in the southeast. The US coastal regions have a higher prevalence of NTM pulmonary disease, and account for 70% of NTM cases in the United States each year. Half of patients diagnosed with NTM lung disease reside in 7 states: Florida, New York, Texas, California, Pennsylvania, New Jersey, and Ohio, with 1 in 7 residing in Florida. Three parishes in Louisiana are among the top 10 counties with the highest prevalence in United States. The prevalence of NTM infection–associated hospitalizations is increasing worldwide as well. Co-infection with tuberculosis and multiple NTMs in individual patients has been observed clinically and documented in patients with and without HIV.9,10
It is not clear why the prevalence of NTM pulmonary disease is increasing, but there may be several contributing factors: (1) an increased awareness and identification of NTM infection sources in the environment; (2) an expanding cohort of immunocompromised individuals with exogenous or endogenous immune deficiencies; (3) availability of improved diagnostic techniques, such as use of high-performance liquid chromatography (HPLC), DNA probes, and gene sequencing; and (4) an increased awareness of the morbidity and mortality associated with NTM pulmonary disease. However, it is important to recognize that to best understand the clinical relevance of epidemiologic studies based on laboratory diagnosis and identification, the findings must be evaluated by correlating them with the microbiological and other clinical criteria established by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines.11
Continue to: Mycobacterium avium Complex
Mycobacterium avium Complex
Case Patient 1
A 48-year-old woman who has never smoked and has no past medical problems, except seasonal allergic rhinitis and “colds and flu-like illness” once or twice a year, is evaluated for a chronic lingering cough with occasional sputum production. The patient denies any other chronic symptoms and is otherwise active. Physical examination reveals no specific findings except mild pectus excavatum and mild scoliosis. Body mass index is 22 kg/m2. Chest radiograph shows nonspecific increased markings in the lower zones. Computed tomography (CT) scan of the chest reveals minimal nodular and cylindrical bronchiectasis in both lungs (Figure 1). No previous radiographs are available for comparison. The patient is HIV-negative. Sputum tests reveal normal flora, and both fungus and acid-fast bacilli smear are negative. Culture for mycobacteria shows scanty growth of MAC in 1 specimen.

What is the clinical presentation of MAC pulmonary disease?
Among NTM, MAC is the most common cause of pulmonary disease worldwide.6 MAC primarily includes 2 species: M. avium and Mycobacterium intracellulare. M. avium is the more important pathogen in disseminated disease, whereas M. intracellulare is the more common respiratory pathogen.11 These organisms are genetically similar and generally not differentiated in the clinical microbiology laboratory, although there are isolated reports in the literature suggesting differences in prevalence, presentation, and prognosis in M. avium infection versus M. intracellulare infection.12
Three major disease syndromes are produced by MAC in humans: pulmonary disease, usually in adults whose systemic immunity is intact; disseminated disease, usually in patients with advanced HIV infection; and cervical lymphadenitis.13 Pulmonary disease caused by MAC may take on 1 of several clinically different forms, including asymptomatic “colonization” or persistent minimal infection without obvious clinical significance; endobronchial involvement; progressive pulmonary disease with radiographic and clinical deterioration and nodular bronchiectasis or cavitary lung disease; hypersensitivity pneumonitis; or persistent, overwhelming mycobacterial growth with symptomatic manifestations, often in a lung with underlying damage due to either chronic obstructive lung disease or pulmonary fibrosis (Table 1).14

Cavitary Disease
The traditionally recognized presentation of MAC lung disease has been apical cavitary lung disease in men in their late 40s and early 50s who have a history of cigarette smoking, and frequently, excessive alcohol use. If left untreated, or in the case of erratic treatment or macrolide drug resistance, this form of disease is generally progressive within a relatively short time and can result in extensive cavitary lung destruction and progressive respiratory failure.15
Nodular Bronchiectasis
The more common presentation of MAC lung disease, which is outlined in the case described here, is interstitial nodular infiltrates, frequently involving the right middle lobe or lingula and predominantly occurring in postmenopausal, nonsmoking white women. This is sometimes labelled “Lady Windermere syndrome.” These patients with M. avium infection appear to have similar clinical characteristics and body types, including lean build, scoliosis, pectus excavatum, and mitral valve prolapse.16,17 The mechanism by which this body morphotype predisposes to pulmonary mycobacterial infection is not defined, but ineffective mucociliary clearance is a possible explanation. Evidence suggests that some patients may be predisposed to NTM lung disease because of preexisting bronchiectasis. Some potential etiologies of bronchiectasis in this population include chronic sinusitis, gastroesophageal reflux with chronic aspiration, α1 antitrypsin deficiency, and cystic fibrosis genetic traits and mutations.18 Risk factors for increased morbidity and mortality include the development of cavitary disease, age, weight loss, lower body mass index, and other comorbid conditions.
This form of disease, termed nodular bronchiectasis, tends to have a much slower progression than cavitary disease, such that long-term follow-up (months to years) may be necessary to demonstrate clinical or radiographic changes.11 The radiographic term “tree-in-bud” has been used to describe what may reflect inflammatory changes, including bronchiolitis. High-resolution CT scans of the chest are especially helpful for diagnosing this pattern of MAC lung disease, as bronchiectasis and small nodules may not be easily discernible on plain chest radiograph. The nodular/bronchiectasis radiographic pattern can also be seen with other NTM pathogens, including M. abscessus, Mycobacterium simiae, and M. kansasii. Solitary nodules and dense consolidation have also been described. Pleural effusions are uncommon, but reactive pleural thickening is frequently seen. Co-pathogens may be isolated from culture, including Pseudomonas aeruginosa, Staphylococcus aureus, and, occasionally, other NTM such as M. abscessus or Mycobacterium chelonae.19-21
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis, initially described in patients who were exposed to hot tubs, mimics allergic hypersensitivity pneumonitis, with respiratory symptoms and culture/tissue identification of MAC or sometimes other NTM. It is unclear whether hypersensitivity pneumonitis is an inflammatory process, an infection, or both, and opinion regarding the need for specific antibiotic treatment is divided.11,22 However, avoidance of exposure is prudent and recommended.
Disseminated Disease
Disseminated NTM disease is associated with very low CD4+ lymphocyte counts and is seen in approximately 5% of patients with HIV infection.23-25 Although disseminated NTM disease is rarely seen in immunosuppressed patients without HIV infection, it has been reported in patients who have undergone renal or cardiac transplant, patients on long-term corticosteroid therapy, and those with leukemia or lymphoma. More than 90% of infections are caused by MAC; other potential pathogens include M. kansasii, M. chelonae, M. abscessus, and Mycobacterium haemophilum. Although seen less frequently since the advent of highly active antiretroviral therapy, disseminated infection can develop progressively from an apparently indolent or localized infection or a respiratory or gastrointestinal source. Signs and symptoms of disseminated infection (specifically MAC-associated disease) are nonspecific and include fever, night sweats, weight loss, and abdominal tenderness. Disseminated MAC disease occurs primarily in patients with more advanced HIV disease (CD4+ count typically < 50 cells/μL). Clinically, disseminated MAC manifests as intermittent or persistent fever, constitutional symptoms with organomegaly and organ-specific abnormalities (eg, anemia, neutropenia from bone marrow involvement, adenopathy, hepatosplenomegaly), and elevations of liver enzymes or lung infiltrates from pulmonary involvement.
Continue to: What are the criteria for diagnosing NTM pulmonary disease?
What are the criteria for diagnosing NTM pulmonary disease?
The diagnosis of NTM disease is based on clinical, radiologic, and mycobacterial correlation with good communication between the experts in this field. The ATS/IDSA criteria for diagnosing NTM lung disease are shown in Figure 2. These criteria best apply to MAC, M. kansasii, and M. abscessus, but are also clinically applied to other NTM respiratory pathogens. The diagnosis of MAC infection is most readily established by culture of blood, bone marrow, respiratory secretions/fluid, or tissue specimens from suspected sites of involvement. Due to erratic shedding of MAC into the respiratory secretions in patients with nodular bronchiectasis, as compared to those with the cavitary form of the disease, sputum may be intermittently positive, with variable colony counts and polyclonal infections.12 Prior to the advent of high-resolution CT, isolation of MAC organisms from the sputum of such patients was frequently dismissed as colonization.

Mycobacterial Testing
Because of the nonspecific symptoms and lack of diagnostic specificity of chest imaging, the diagnosis of NTM lung disease requires microbiologic confirmation. Specimens sent to the laboratory for identification of NTM must be handled with care to prevent contamination and false-positive results. Transport media and preservatives should be avoided, and transportation of the specimens should be prompt. These measures will prevent bacterial overgrowth. Furthermore, the yield of NTM may be affected if the patient has used antibiotics, such as macrolides and fluoroquinolones, prior to obtaining the specimen.
NTM should be identified at the species and subspecies level, although this is not practical in community practice settings. The preferred staining procedure in the laboratory is the fluorochrome method. Some species require special growth conditions and/or lower incubation temperatures, and other identification methods may have to be employed, such as DNA probes, polymerase chain reaction genotyping, nucleic acid sequence determination, and high-performance liquid chromatography. As a gold standard, clinical specimens for mycobacterial cultures should be inoculated onto 1 or more solid media (eg, Middlebrook 7H11 media and/or Lowenstein-Jensen media, the former of which is the preferred medium for NTM) and into a liquid medium (eg, BACTEC 12B broth or Mycobacteria growth indicator tube broth). Growth of visible colonies on solid media typically requires 2 to 4 weeks, but liquid media (eg, the radiometric BACTEC system), used as a supplementary and not as an exclusive test, usually produce results within 10 to 14 days. Furthermore, even after initial growth, identification of specific isolates based on the growth characteristics on solid media requires additional time. Use of specific nucleic acid probes for MAC and M. kansasii and HPLC testing of mycolic acid patterns in acid-fast bacilli smear–positive specimens can reduce the turnaround time of specific identification of a primary culture–positive sample. However, HPLC is not sufficient for definitive identification of many NTM species, including the RGM. Other newer techniques, including 16S ribosomal DNA sequencing and polymerase chain reaction-restriction fragment length polymorphism analysis, also allow NTM to be identified and speciated more reliably and rapidly from clinical specimens.
Cost and other practical considerations limit widespread adoption of these techniques. However, the recognition that M. abscessus can be separated into more than 1 subspecies, and that there are important prognostic implications of that separation, lends urgency to the broader adoption of newer molecular techniques in the mycobacteriology laboratory. Susceptibility testing is based on the broth microdilution method; RGM usually grow within 7 days of subculture, and the laboratory time to culture is a helpful hint, although not necessarily specific. Recognizing the morphology of mycobacterial colony growth may also be helpful in identification.
Are skin tests helpful in diagnosing NTM infection?
Tuberculin skin testing remains a nonspecific marker of mycobacterial infection and does not help in further elucidating NTM infection. However, epidemiologic and laboratory studies with well-characterized antigens have shown that dual skin testing with tuberculosis- versus NTM-derived tuberculin can discriminate between prior NTM and prior tuberculosis disease. Species-specific skin test antigens are not commercially available and are not helpful in the diagnosis of NTM disease because of cross-reactivity of M. tuberculosis and some NTM. However, increased prevalence of NTM sensitization based on purified protein derivative testing has been noted in a recent survey, which is consistent with an observed increase in the rates of NTM infections, specifically MAC, in the United States.26,27
Interferon-gamma release assays (IGRAs) are now being used as an alternative to tuberculin skin testing to diagnose M. tuberculosis infection. Certain NTM species also contain gene sequences that encode for ESAT-6 or CFP-10 antigens used in the IGRAs, and hence, yield a positive IGRA test. These include M. marinum, M. szulgai, and M. kansasii.28,29 However, MAC organisms do not produce positive results on assays that use these antigens.
Continue to: What is the approach to management of NTM pulmonary disease?
What is the approach to management of NTM pulmonary disease?
The correlation of symptoms with radiographic and microbiologic evidence is essential to categorize the disease and determine the need for therapy. Making the diagnosis of NTM lung disease does not necessitate the institution of therapy. The decision to treat should be weighed against potential risks and benefits to the individual patient based on symptomatic, radiographic, and microbiologic criteria, as well as underlying systemic or pulmonary immune status. In the absence of evidence of clinical, radiologic, or mycobacterial progression of disease, pursuing airway clearance therapy and clinical surveillance without initiating specific anti-MAC therapy is a reasonable option.11 Identifying the sustained presence of NTM infection, especially MAC, in a patient with underlying clinical and radiographic evidence of bronchiectasis is of value in determining comprehensive treatment and management strategies. Close observation is indicated if the decision not to treat is made. If treatment is initiated, comprehensive management includes long-term follow-up with periodic bacteriologic surveillance, watching for drug toxicity and drug-drug interactions, ensuring adherence and compliance to treatment, and managing comorbidity.
The Bronchiectasis Severity Index is a useful clinical predictive tool that identifies patients at risk of future mortality, hospitalization, and exacerbations and can be used to evaluate the need for specific treatment.30 The index is based on dyspnea score, lung function tests, colonization of pathogens, and extent of disease.
Case 1 Continued
After approximately 2 months of observation and symptomatic treatment, without specific antibiotic therapy, the patient’s symptoms continue. She now develops intermittent hemoptysis. Repeat sputum studies reveal moderate growth of M. avium. A follow-up CT scan shows progression of disease, with an increase in the tree-in-bud pattern (Figure 3).

What treatment protocols are recommended for MAC pulmonary disease?
As per the ATS/IDSA statement, macrolides are the mainstay of treatment for pulmonary MAC disease.11 Macrolides achieve an increased concentration in the lung, and when used for treatment of pulmonary MAC disease, there is a strong correlation between in vitro susceptibility, in vivo (clinical) response, and the immunomodulating effects of macrolides.31,32 Macrolide-containing regimens have demonstrated efficacy in patients with MAC pulmonary disease33,34; however, macrolide monotherapy should be avoided to prevent the development of resistance.
At the outset, it is critical to establish the objective criteria for determining response and to ensure that the patient understands the goals of the treatment and expectations of the treatment plan. Moreover, experts suggest that due to the possibility of drug intolerance, side effects, and the need for prolonged therapy, a “step ladder” ramping up approach to treatment could be adopted, with gradual introduction of therapy within a short time period; this approach may improve compliance and adherence to treatment.11 If this approach is used, the doses may have to be divided. Patients who are unable to tolerate daily medications, even with dosage adjustment, should be tried on an intermittent treatment regimen. Older female patients frequently require gradual introduction of medications (ie, 1 medication added to the regimen every 1 to 2 weeks) to evaluate tolerance to each medication and medication dose.11 Commonly encountered adverse effects of NTM treatment include intolerance to clarithromycin due to gastrointestinal problems, low body mass index, or age older than 70 years.
After determining that the patient requires therapy, the standard recommended treatment for MAC pulmonary disease includes the following: for most patients with nodular/bronchiectasis disease, a thrice-weekly regimen of clarithromycin (1000 mg) or azithromycin (500 mg), rifampin (600 mg), and ethambutol (25 mg/kg) is recommended. For patients with cavitary MAC pulmonary disease or severe nodular/bronchiectasis disease, the guidelines recommend a daily regimen of clarithromycin (500-1000 mg) or azithromycin (250 mg), rifampin (600 mg) or rifabutin (150–300 mg), and ethambutol (15 mg/kg), with consideration of intravenous (IV) amikacin 3 times/week early in therapy (Table 2).11

The treatment of MAC hypersensitivity-like disease speaks to the controversy of whether this is an inflammatory process, infectious process, or a combination of inflammation and infection. Avoidance of exposure is the mainstay of management. In some cases, steroids are used with or without a short course of anti-MAC therapy (ie, clarithromycin or azithromycin with rifampin and ethambutol).
Prophylaxis for disseminated MAC disease should be given to adults with HIV infection who have a CD4+ count less than 50 cells/μL. Azithromycin 1200 mg/week or clarithromycin 1000 mg/day has proven efficacy, and rifabutin 300 mg/day is also effective but less well tolerated. Rifabutin is more active in vitro against MAC than rifampin, and is used in HIV-positive patients because of drug-drug interaction between antiretroviral drugs and rifampin.
Continue to: Case 1 Continued
Case 1 Continued
The patient is treated with clarithromycin, rifampin, and ethambutol for 1 year, with sputum conversion after 9 months. In the latter part of her treatment, she experiences decreased visual acuity. Treatment is discontinued prematurely after 1 year due to drug toxicity and continued intolerance to drug therapy. The patient remains asymptomatic for 8 months, and then begins to experience mild to moderate hemoptysis, with increasing cough and sputum production associated with postural changes during exercise. Physical examination overall remains unchanged. Three sputum results reveal heavy growth of MAC, and a CT scan of the chest shows a cavitary lesion in the left upper lobe along with the nodular bronchiectasis (Figure 4).

What are the management options at this stage?
Based on this patient’s continued symptoms, progression of radiologic abnormalities, and current culture growth, she requires re-treatment. With the adverse effects associated with ethambutol during the first round of therapy, the drug regimen needs to be modified. Several considerations are relevant at this stage. Relapse rates range from 20% to 30% after treatment with a macrolide-based therapy.11,34 Obtaining a culture-sensitivity profile is imperative in these cases. Of note, treatment should not be discontinued altogether, but instead the toxic agent should be removed from the treatment regimen. Continuing treatment with a 2-drug regimen of clarithromycin and rifampin may be considered in this patient. Re-infection with multiple genotypes may also occur after successful drug therapy, but this is primarily seen in MAC patients with nodular bronchiectasis.34,35 Patients in whom previous therapy has failed, even those with macrolide-susceptible MAC isolates, are less likely to respond to subsequent therapy. Data suggest that intermittent medication dosing is not effective for patients with severe or cavitary disease or in those in whom previous therapy has failed.36 In this case, treatment should include a daily 3-drug therapy, with an injectable thrice-weekly aminoglycoside. Other agents such as linezolid and clofazimine may have to be tried. Cycloserine, ethionamide, and other agents are sometimes used, but their efficacy is unproven and doubtful. Pyrazinamide and isoniazid have no activity against MAC.
Treatment Failure and Drug Resistance
Treatment failure is considered to have occurred if patients have not had a response (microbiologic, clinical, or radiographic) after 6 months of appropriate therapy or had not achieved conversion of sputum to culture-negative after 12 months of appropriate therapy.11 This occurs in about 40% of patients. Multiple factors can interfere with the successful treatment of MAC pulmonary disease, including medication nonadherence, medication side effects or intolerance, lack of response to a medication regimen, or the emergence of a macrolide-resistant or multidrug-resistant strain. Inducible macrolide resistance remains a potential factor.34-36 A number of characteristics of NTM contribute to the poor response to currently used antibiotics: the organisms have a lipid outer membrane and prefer to adhere to surfaces and form biofilms, which makes them relatively impermeable to antibiotics.37 Also, NTM replicate in phagocytic cells, allowing them to subvert normal cellular defense mechanisms. Furthermore, NTM can display colony variants, whereby single colony isolates switch between antibiotic-susceptible and -resistant variants. These factors have also impeded in development of new antibiotics for NTM infection.37
Recent limited approval of amikacin liposomal inhalation suspension (ALIS) for treatment failure and refractory MAC infection in combination with guideline-based antimicrobial therapy (GBT) is a promising addition to the available treatment armamentarium. In a multinational trial, the addition of ALIS to GBT for treatment-refractory MAC lung disease achieved significantly greater culture conversion rates by month 6 than GBT alone, with comparable rates of serious adverse events.38
Is therapeutic drug monitoring recommended during treatment of MAC pulmonary disease?
Treatment failure may also be drug-related, including poor drug penetration into the damaged lung tissue or drug-drug interactions leading to suboptimal drug levels. Peak serum concentrations have been found to be below target ranges in approximately 50% of patients using a macrolide and ethambutol. Concurrent use of rifampin decreases the peak serum concentration of macrolides and quinolones, with acceptable target levels seen in only 18% to 57% of cases. Whether this alters patient outcomes is not clear.39-42 Factors identified as contributing to the poor response to therapy include poor compliance, cavitary disease, previous treatment for MAC pulmonary disease, and a history of chronic obstructive lung disease. Studies by Koh and colleagues40 and van Ingen and colleagues41 with pharmacokinetic and pharmacodynamics data showed that, in patients on MAC treatment with both clarithromycin and rifampicin, plasma levels of clarithromycin were lower than the recommended minimal inhibitory concentrations (MIC) against MAC for that drug. The studies also showed that rifampicin lowered clarithromycin concentrations more than did rifabutin, with the AUC/MIC ratio being suboptimal in nearly half the cases. However, low plasma clarithromycin concentrations did not have any correlation with treatment outcomes, as the peak plasma drug concentrations and the peak plasma drug concentration/MIC ratios did not differ between patients with unfavorable treatment outcomes and those with favorable outcomes. This is further compounded by the fact that macrolides achieve higher levels in lung tissue than in plasma, and hence the significance of low plasma levels is unclear; however, it is postulated that achieving higher drug levels could, in fact, lead to better clinical outcomes. Pending specific well-designed, prospective randomized controlled trials, routine therapeutic drug monitoring is not currently recommended, although some referral centers do this as their practice pattern.
Is surgery an option in this case?
The overall 5-year mortality for MAC pulmonary disease was approximately 28% in a retrospective analysis, with patients with cavitary disease at increased risk for death at 5 years.42 As such, surgery is an option in selected cases as part of adjunctive therapy along with anti-MAC therapy based on mycobacterial sensitivity. Surgery is used as either a curative approach or a “debulking” measure.11 When present, clearly localized disease, especially in the upper lobe, lends itself best to surgical intervention. Surgical resection of a solitary pulmonary nodule due to MAC, in addition to concomitant medical treatment, is recommended. Surgical intervention should be considered early in the course of the disease because it may provide a cure without prolonged treatment and its associated problems, and this approach may lead to early sputum conversion. Surgery should also be considered in patients with macrolide-resistant or multidrug-resistant MAC infection or in those who cannot tolerate the side effects of therapy, provided that the disease is focal and limited. Patients with poor preoperative lung function have poorer outcomes than those with good lung function, and postoperative complications arising from treatment, especially with a right-sided pneumonectomy, tend to occur more frequently in these patients. Thoracic surgery for NTM pulmonary disease must be considered cautiously, as this is associated with significant morbidity and mortality and is best performed at specialized centers that have expertise and experience in this field.43
Continue to: Mycobacterium abscessus Complex
Mycobacterium abscessus Complex
Case Patient 2
A 64-year-old man who is an ex-smoker presents with chronic cough, mild shortness of breath on exertion, low-grade fever, and unintentional weight loss of 10 lb. Physical exam is unremarkable. The patient was diagnosed with immunoglobulin deficiency (low IgM and low IgG4) in 2002, and has been on replacement therapy since then. He also has had multiple episodes of NTM infection, with MAC and M. kansasii infections diagnosed in 2012-2014, which required 18 months of multi-drug antibiotic treatment that resulted in sputum conversion. Pulmonary function testing done on this visit in 2017 shows mild obstructive impairment.
Chest radiograph and CT scan show bilateral bronchiectasis (Figure 5 and Figure 6).

The results of serial sputum microbiology testing performed over the course of 6 months are outlined below:
- 5/2017 (bronchoalveolar lavage): 2+; M. abscessus
- 9/2017 × 2: smear (–); group IV RGM
- 11/2017: smear (–); M. abscessus (> 50 CFU)
- 12/2017: smear (–); M. abscessus (> 50 CFU)

What are the clinical considerations in this patient with multiple NTM infections?
M. abscessus complex was originally described in soft tissue abscesses and skin infections possibly resulting from soil or water contamination. Subspeciation of M. abscessus complex during laboratory testing is critical to facilitate selection of a specific therapeutic approach; treatment decisions are impacted by the presence of an active erm gene and in vitro macrolide sensitivity, which differ between subspecies. The most acceptable classification outlines 3 species in the M. abscessus complex: Mycobacterium abscessus subsp abscessus, Mycobacterium abscessus subsp bolletii (both with an active erm gene responsible for macrolide resistance), and Mycobacterium abscessus subsp massiliense (with an inactive erm gene and therefore susceptible to macrolides).44
RGM typically manifest in skin, soft tissue, and bone, and can cause soft tissue, surgical wound, and catheter-related infections. Although the role of RGM as pulmonary pathogens is unclear, underlying diseases associated with RGM include previously treated mycobacterial disease, coexistent pulmonary diseases with or without MAC, cystic fibrosis, malignancies, and gastroesophageal disorders. M. abscessus is the third most commonly identified respiratory NTM and accounts for the majority (80%) of RGM respiratory isolates. Other NTM reported to cause both lung disease and skin, bone, and joint infections include Mycobacterium simiae, Mycobacterium xenopi, and Mycobacterium malmoense. Ocular granulomatous diseases, such as chorioretinitis and keratitis, have been reported with both RGM and Runyon group III SGM, such as MAC or M. szulgai, following trauma or refractive surgery. These can mimic fungal, herpetic, or amebic keratitis. The pulmonary syndromes associated with multiple culture positivity are seen in elderly women with bronchiectasis or cavitary lung disease and/or associated with gastrointestinal symptoms of acid reflux, with or without achalasia and concomitant lipoid interstitial pneumonia.45
Generally, pulmonary disease progresses slowly, but lung disease attributed to RGM can result in respiratory failure. Thus, RGM should be recognized as a possible cause of chronic mycobacterial lung disease, especially in immunocompromised patients, and respiratory isolates should be assessed carefully. Identification and drug susceptibility testing are essential before initiation of treatment for RGM.
What is the approach to management of M. abscessus pulmonary disease in a patient without cystic fibrosis?
The management of M. abscessus pulmonary infection as a subset of RGM requires a considered step-wise approach. The criteria for diagnosis and threshold for starting treatment are the same as those used in the management of MAC pulmonary disease,11 but the treatment of M. abscessus pulmonary infection is more complex and has lower rates of success and cure. Also, antibiotic treatment presents challenges related to rapid identification of the causative organism, nomenclature, resistance patterns, and tolerance of treatment and side effects. If a source such as catheter, access port, or any surgical site is identified, prompt removal and clearance of the infected site are strongly advised
In the absence of any controlled clinical trials, treatment of RGM is based on in vitro susceptibility testing and expert opinion. As in MAC pulmonary disease, macrolides are the mainstay of treatment, with an induction phase of intravenous antibiotics. Treatment may include a combination of injectable aminoglycosides, imipenem, or cefoxitin and oral drugs such as a macrolide (eg, clarithromycin, azithromycin), doxycycline, fluoroquinolones, trimethoprim/sulfamethoxazole, or linezolid. While antibiotic treatment of M. abscessus pulmonary disease is based on in vitro sensitivity pattern to a greater degree than is treatment of MAC pulmonary disease, this approach has significant practical limitations and hence variable applicability. The final choice of antibiotics is best based on the extended susceptibility results, if available. The presence of an active erm gene on a prolonged growth specimen in M. abscessus subsp abscessus and M. abscessus subsp bolletii precludes the use of a macrolide. In such cases, amikacin, especially in an intravenous form, is the mainstay of treatment based on MIC. Recently, there has been a resurgence in interest in the use of clofazimine in combination with amikacin when treatment is not successful in patients with M. abscessus subsp abscessus or M. bolletii with an active erm gene.45,46 When localized abscess formation is noted, surgery may be the best option, with emphasis on removal of implants and catheters if implicated in RGM infection.
Attention must also be given to confounding pulmonary and associated comorbidities. This includes management of bronchiectasis with appropriately aggressive airway clearance techniques; anti-reflux measures for prevention of micro-aspiration; and management of other comorbid pulmonary conditions, such as chronic obstructive pulmonary disease, pulmonary fibrosis, and sarcoidosis, if applicable. These interventions play a critical role in clearing the M. abscessus infection, preventing progression of disease, and reducing morbidity. The role of immunomodulatory therapy needs to be considered on a regular, ongoing basis. Identification of genetic factors and correction of immune deficiencies may help in managing the infection.
Case Patient 2 Conclusion
The treatment regimen adopted in this case includes a 3-month course of daily intravenous amikacin and imipenem with oral azithromycin, followed by a continuation phase of azithromycin with clofazimine and linezolid. Airway clearance techniques such as Vest/Acapella/CPT are intensified and monthly intravenous immunoglobulin therapy is continued. The patient responds to treatment, with resolution of his clinical symptoms and reduction in the colony count of M. abscessus in the sputum.
Summary
NTM are ubiquitous in the environment, and NTM infection has variable manifestations, especially in patients with no recognizable immune impairments. Underlying comorbid conditions with bronchiectasis complicate its management. Treatment strategies must be individualized based on degree of involvement, associated comorbidities, immune deficiencies, goals of therapy, outcome-based risk-benefit ratio assessment, and patient engagement and expectations. In diffuse pulmonary disease, drug treatment remains difficult due to poor match of in vitro and in vivo culture sensitivity, side effects of medications, and high failure rates. When a localized resectable foci of infection is identified, especially in RGM disease, surgical treatment may be the best approach in selected patients, but it must be performed in centers with expertise and experience in this field.
Nontuberculous mycobacterial pulmonary disease is a broad term for a group of pulmonary disorders caused and characterized by exposure to environmental mycobacteria other than those belonging to the Mycobacterium tuberculosis complex and Mycobacterium leprae. Mycobacteria are aerobic, nonmotile organisms that appear positive with acid-fast alcohol stains. Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and have been recovered from domestic and natural water sources, soil, and food products, and from around livestock, cattle, and wildlife.1-3 To date, no evidence exists of human-to-human or animal-to-human transmission of NTM in the general population. Infections in humans are usually acquired from environmental exposures, although the specific source of infection cannot always be identified. Similarly, the mode of infection with NTM has not been established with certainty, but it is highly likely that the organism is implanted, ingested, aspirated, or inhaled. Aerosolization of droplets associated with use of bathroom showerheads and municipal water sources and soil contamination are some of the factors associated with the transmission of infection. Proven routes of transmission include showerheads and potting soil dust.2,3
NTM pulmonary disease occurs in individuals with or without comorbid conditions such as bronchiectasis, chronic obstructive pulmonary disease, pulmonary fibrosis, or structural lung diseases. Slender, middle-aged or elderly white females with marfanoid body habitus, with or without apparent immune or genetic disorders, showing impaired airway and mucus clearance present with this infection as a form of underlying bronchiectasis (Lady Windermere syndrome). It is unclear why NTM infections and escalation to clinical disease occur in certain individuals. Many risk factors, including inherited and acquired defects of host immune response (eg, cystic fibrosis trait and α1 antitrypsin deficiency), have been associated with increased susceptibility to NTM infections.4
NTM infection can lead to chronic symptoms, frequent exacerbations, progressive functional and structural lung destruction, and impaired quality of life, and is associated with an increased risk of hospitalization and higher 5-year all-cause mortality. As such, NTM disease is drawing increasing attention at the clinical, academic, and research levels.5 This case-based review outlines the clinical features of NTM infection, with a focus on the challenges in diagnosis, treatment, and management of NTM pulmonary disease. The cases use Mycobacterium avium complex (MAC), a slow-growing mycobacteria (SGM), and Mycobacterium abscessus, a rapidly growing mycobacteria (RGM), as prototypes in a non–cystic fibrosis, non-HIV clinical setting.
Epidemiology
Of the almost 200 isolated species of NTM, the most prevalent pathogens for respiratory disease in the United States are MAC, Mycobacterium kansasii, and M. abscessus. MAC accounts for more than 80% of cases of NTM respiratory disease in the United States.6 The prevalence of NTM disease is increasing at a rate of about 8% each year, with 75,000 to 105,000 patients diagnosed with NTM lung disease in the United States annually. NTM infections in the United States are increasing among patients aged 65 years and older, a population that is expected to nearly double by 2030.7,8
Isolation and prevalence of many NTM species are higher in certain geographic areas of the United States, especially in the southeast. The US coastal regions have a higher prevalence of NTM pulmonary disease, and account for 70% of NTM cases in the United States each year. Half of patients diagnosed with NTM lung disease reside in 7 states: Florida, New York, Texas, California, Pennsylvania, New Jersey, and Ohio, with 1 in 7 residing in Florida. Three parishes in Louisiana are among the top 10 counties with the highest prevalence in United States. The prevalence of NTM infection–associated hospitalizations is increasing worldwide as well. Co-infection with tuberculosis and multiple NTMs in individual patients has been observed clinically and documented in patients with and without HIV.9,10
It is not clear why the prevalence of NTM pulmonary disease is increasing, but there may be several contributing factors: (1) an increased awareness and identification of NTM infection sources in the environment; (2) an expanding cohort of immunocompromised individuals with exogenous or endogenous immune deficiencies; (3) availability of improved diagnostic techniques, such as use of high-performance liquid chromatography (HPLC), DNA probes, and gene sequencing; and (4) an increased awareness of the morbidity and mortality associated with NTM pulmonary disease. However, it is important to recognize that to best understand the clinical relevance of epidemiologic studies based on laboratory diagnosis and identification, the findings must be evaluated by correlating them with the microbiological and other clinical criteria established by the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines.11
Continue to: Mycobacterium avium Complex
Mycobacterium avium Complex
Case Patient 1
A 48-year-old woman who has never smoked and has no past medical problems, except seasonal allergic rhinitis and “colds and flu-like illness” once or twice a year, is evaluated for a chronic lingering cough with occasional sputum production. The patient denies any other chronic symptoms and is otherwise active. Physical examination reveals no specific findings except mild pectus excavatum and mild scoliosis. Body mass index is 22 kg/m2. Chest radiograph shows nonspecific increased markings in the lower zones. Computed tomography (CT) scan of the chest reveals minimal nodular and cylindrical bronchiectasis in both lungs (Figure 1). No previous radiographs are available for comparison. The patient is HIV-negative. Sputum tests reveal normal flora, and both fungus and acid-fast bacilli smear are negative. Culture for mycobacteria shows scanty growth of MAC in 1 specimen.

What is the clinical presentation of MAC pulmonary disease?
Among NTM, MAC is the most common cause of pulmonary disease worldwide.6 MAC primarily includes 2 species: M. avium and Mycobacterium intracellulare. M. avium is the more important pathogen in disseminated disease, whereas M. intracellulare is the more common respiratory pathogen.11 These organisms are genetically similar and generally not differentiated in the clinical microbiology laboratory, although there are isolated reports in the literature suggesting differences in prevalence, presentation, and prognosis in M. avium infection versus M. intracellulare infection.12
Three major disease syndromes are produced by MAC in humans: pulmonary disease, usually in adults whose systemic immunity is intact; disseminated disease, usually in patients with advanced HIV infection; and cervical lymphadenitis.13 Pulmonary disease caused by MAC may take on 1 of several clinically different forms, including asymptomatic “colonization” or persistent minimal infection without obvious clinical significance; endobronchial involvement; progressive pulmonary disease with radiographic and clinical deterioration and nodular bronchiectasis or cavitary lung disease; hypersensitivity pneumonitis; or persistent, overwhelming mycobacterial growth with symptomatic manifestations, often in a lung with underlying damage due to either chronic obstructive lung disease or pulmonary fibrosis (Table 1).14

Cavitary Disease
The traditionally recognized presentation of MAC lung disease has been apical cavitary lung disease in men in their late 40s and early 50s who have a history of cigarette smoking, and frequently, excessive alcohol use. If left untreated, or in the case of erratic treatment or macrolide drug resistance, this form of disease is generally progressive within a relatively short time and can result in extensive cavitary lung destruction and progressive respiratory failure.15
Nodular Bronchiectasis
The more common presentation of MAC lung disease, which is outlined in the case described here, is interstitial nodular infiltrates, frequently involving the right middle lobe or lingula and predominantly occurring in postmenopausal, nonsmoking white women. This is sometimes labelled “Lady Windermere syndrome.” These patients with M. avium infection appear to have similar clinical characteristics and body types, including lean build, scoliosis, pectus excavatum, and mitral valve prolapse.16,17 The mechanism by which this body morphotype predisposes to pulmonary mycobacterial infection is not defined, but ineffective mucociliary clearance is a possible explanation. Evidence suggests that some patients may be predisposed to NTM lung disease because of preexisting bronchiectasis. Some potential etiologies of bronchiectasis in this population include chronic sinusitis, gastroesophageal reflux with chronic aspiration, α1 antitrypsin deficiency, and cystic fibrosis genetic traits and mutations.18 Risk factors for increased morbidity and mortality include the development of cavitary disease, age, weight loss, lower body mass index, and other comorbid conditions.
This form of disease, termed nodular bronchiectasis, tends to have a much slower progression than cavitary disease, such that long-term follow-up (months to years) may be necessary to demonstrate clinical or radiographic changes.11 The radiographic term “tree-in-bud” has been used to describe what may reflect inflammatory changes, including bronchiolitis. High-resolution CT scans of the chest are especially helpful for diagnosing this pattern of MAC lung disease, as bronchiectasis and small nodules may not be easily discernible on plain chest radiograph. The nodular/bronchiectasis radiographic pattern can also be seen with other NTM pathogens, including M. abscessus, Mycobacterium simiae, and M. kansasii. Solitary nodules and dense consolidation have also been described. Pleural effusions are uncommon, but reactive pleural thickening is frequently seen. Co-pathogens may be isolated from culture, including Pseudomonas aeruginosa, Staphylococcus aureus, and, occasionally, other NTM such as M. abscessus or Mycobacterium chelonae.19-21
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis, initially described in patients who were exposed to hot tubs, mimics allergic hypersensitivity pneumonitis, with respiratory symptoms and culture/tissue identification of MAC or sometimes other NTM. It is unclear whether hypersensitivity pneumonitis is an inflammatory process, an infection, or both, and opinion regarding the need for specific antibiotic treatment is divided.11,22 However, avoidance of exposure is prudent and recommended.
Disseminated Disease
Disseminated NTM disease is associated with very low CD4+ lymphocyte counts and is seen in approximately 5% of patients with HIV infection.23-25 Although disseminated NTM disease is rarely seen in immunosuppressed patients without HIV infection, it has been reported in patients who have undergone renal or cardiac transplant, patients on long-term corticosteroid therapy, and those with leukemia or lymphoma. More than 90% of infections are caused by MAC; other potential pathogens include M. kansasii, M. chelonae, M. abscessus, and Mycobacterium haemophilum. Although seen less frequently since the advent of highly active antiretroviral therapy, disseminated infection can develop progressively from an apparently indolent or localized infection or a respiratory or gastrointestinal source. Signs and symptoms of disseminated infection (specifically MAC-associated disease) are nonspecific and include fever, night sweats, weight loss, and abdominal tenderness. Disseminated MAC disease occurs primarily in patients with more advanced HIV disease (CD4+ count typically < 50 cells/μL). Clinically, disseminated MAC manifests as intermittent or persistent fever, constitutional symptoms with organomegaly and organ-specific abnormalities (eg, anemia, neutropenia from bone marrow involvement, adenopathy, hepatosplenomegaly), and elevations of liver enzymes or lung infiltrates from pulmonary involvement.
Continue to: What are the criteria for diagnosing NTM pulmonary disease?
What are the criteria for diagnosing NTM pulmonary disease?
The diagnosis of NTM disease is based on clinical, radiologic, and mycobacterial correlation with good communication between the experts in this field. The ATS/IDSA criteria for diagnosing NTM lung disease are shown in Figure 2. These criteria best apply to MAC, M. kansasii, and M. abscessus, but are also clinically applied to other NTM respiratory pathogens. The diagnosis of MAC infection is most readily established by culture of blood, bone marrow, respiratory secretions/fluid, or tissue specimens from suspected sites of involvement. Due to erratic shedding of MAC into the respiratory secretions in patients with nodular bronchiectasis, as compared to those with the cavitary form of the disease, sputum may be intermittently positive, with variable colony counts and polyclonal infections.12 Prior to the advent of high-resolution CT, isolation of MAC organisms from the sputum of such patients was frequently dismissed as colonization.

Mycobacterial Testing
Because of the nonspecific symptoms and lack of diagnostic specificity of chest imaging, the diagnosis of NTM lung disease requires microbiologic confirmation. Specimens sent to the laboratory for identification of NTM must be handled with care to prevent contamination and false-positive results. Transport media and preservatives should be avoided, and transportation of the specimens should be prompt. These measures will prevent bacterial overgrowth. Furthermore, the yield of NTM may be affected if the patient has used antibiotics, such as macrolides and fluoroquinolones, prior to obtaining the specimen.
NTM should be identified at the species and subspecies level, although this is not practical in community practice settings. The preferred staining procedure in the laboratory is the fluorochrome method. Some species require special growth conditions and/or lower incubation temperatures, and other identification methods may have to be employed, such as DNA probes, polymerase chain reaction genotyping, nucleic acid sequence determination, and high-performance liquid chromatography. As a gold standard, clinical specimens for mycobacterial cultures should be inoculated onto 1 or more solid media (eg, Middlebrook 7H11 media and/or Lowenstein-Jensen media, the former of which is the preferred medium for NTM) and into a liquid medium (eg, BACTEC 12B broth or Mycobacteria growth indicator tube broth). Growth of visible colonies on solid media typically requires 2 to 4 weeks, but liquid media (eg, the radiometric BACTEC system), used as a supplementary and not as an exclusive test, usually produce results within 10 to 14 days. Furthermore, even after initial growth, identification of specific isolates based on the growth characteristics on solid media requires additional time. Use of specific nucleic acid probes for MAC and M. kansasii and HPLC testing of mycolic acid patterns in acid-fast bacilli smear–positive specimens can reduce the turnaround time of specific identification of a primary culture–positive sample. However, HPLC is not sufficient for definitive identification of many NTM species, including the RGM. Other newer techniques, including 16S ribosomal DNA sequencing and polymerase chain reaction-restriction fragment length polymorphism analysis, also allow NTM to be identified and speciated more reliably and rapidly from clinical specimens.
Cost and other practical considerations limit widespread adoption of these techniques. However, the recognition that M. abscessus can be separated into more than 1 subspecies, and that there are important prognostic implications of that separation, lends urgency to the broader adoption of newer molecular techniques in the mycobacteriology laboratory. Susceptibility testing is based on the broth microdilution method; RGM usually grow within 7 days of subculture, and the laboratory time to culture is a helpful hint, although not necessarily specific. Recognizing the morphology of mycobacterial colony growth may also be helpful in identification.
Are skin tests helpful in diagnosing NTM infection?
Tuberculin skin testing remains a nonspecific marker of mycobacterial infection and does not help in further elucidating NTM infection. However, epidemiologic and laboratory studies with well-characterized antigens have shown that dual skin testing with tuberculosis- versus NTM-derived tuberculin can discriminate between prior NTM and prior tuberculosis disease. Species-specific skin test antigens are not commercially available and are not helpful in the diagnosis of NTM disease because of cross-reactivity of M. tuberculosis and some NTM. However, increased prevalence of NTM sensitization based on purified protein derivative testing has been noted in a recent survey, which is consistent with an observed increase in the rates of NTM infections, specifically MAC, in the United States.26,27
Interferon-gamma release assays (IGRAs) are now being used as an alternative to tuberculin skin testing to diagnose M. tuberculosis infection. Certain NTM species also contain gene sequences that encode for ESAT-6 or CFP-10 antigens used in the IGRAs, and hence, yield a positive IGRA test. These include M. marinum, M. szulgai, and M. kansasii.28,29 However, MAC organisms do not produce positive results on assays that use these antigens.
Continue to: What is the approach to management of NTM pulmonary disease?
What is the approach to management of NTM pulmonary disease?
The correlation of symptoms with radiographic and microbiologic evidence is essential to categorize the disease and determine the need for therapy. Making the diagnosis of NTM lung disease does not necessitate the institution of therapy. The decision to treat should be weighed against potential risks and benefits to the individual patient based on symptomatic, radiographic, and microbiologic criteria, as well as underlying systemic or pulmonary immune status. In the absence of evidence of clinical, radiologic, or mycobacterial progression of disease, pursuing airway clearance therapy and clinical surveillance without initiating specific anti-MAC therapy is a reasonable option.11 Identifying the sustained presence of NTM infection, especially MAC, in a patient with underlying clinical and radiographic evidence of bronchiectasis is of value in determining comprehensive treatment and management strategies. Close observation is indicated if the decision not to treat is made. If treatment is initiated, comprehensive management includes long-term follow-up with periodic bacteriologic surveillance, watching for drug toxicity and drug-drug interactions, ensuring adherence and compliance to treatment, and managing comorbidity.
The Bronchiectasis Severity Index is a useful clinical predictive tool that identifies patients at risk of future mortality, hospitalization, and exacerbations and can be used to evaluate the need for specific treatment.30 The index is based on dyspnea score, lung function tests, colonization of pathogens, and extent of disease.
Case 1 Continued
After approximately 2 months of observation and symptomatic treatment, without specific antibiotic therapy, the patient’s symptoms continue. She now develops intermittent hemoptysis. Repeat sputum studies reveal moderate growth of M. avium. A follow-up CT scan shows progression of disease, with an increase in the tree-in-bud pattern (Figure 3).

What treatment protocols are recommended for MAC pulmonary disease?
As per the ATS/IDSA statement, macrolides are the mainstay of treatment for pulmonary MAC disease.11 Macrolides achieve an increased concentration in the lung, and when used for treatment of pulmonary MAC disease, there is a strong correlation between in vitro susceptibility, in vivo (clinical) response, and the immunomodulating effects of macrolides.31,32 Macrolide-containing regimens have demonstrated efficacy in patients with MAC pulmonary disease33,34; however, macrolide monotherapy should be avoided to prevent the development of resistance.
At the outset, it is critical to establish the objective criteria for determining response and to ensure that the patient understands the goals of the treatment and expectations of the treatment plan. Moreover, experts suggest that due to the possibility of drug intolerance, side effects, and the need for prolonged therapy, a “step ladder” ramping up approach to treatment could be adopted, with gradual introduction of therapy within a short time period; this approach may improve compliance and adherence to treatment.11 If this approach is used, the doses may have to be divided. Patients who are unable to tolerate daily medications, even with dosage adjustment, should be tried on an intermittent treatment regimen. Older female patients frequently require gradual introduction of medications (ie, 1 medication added to the regimen every 1 to 2 weeks) to evaluate tolerance to each medication and medication dose.11 Commonly encountered adverse effects of NTM treatment include intolerance to clarithromycin due to gastrointestinal problems, low body mass index, or age older than 70 years.
After determining that the patient requires therapy, the standard recommended treatment for MAC pulmonary disease includes the following: for most patients with nodular/bronchiectasis disease, a thrice-weekly regimen of clarithromycin (1000 mg) or azithromycin (500 mg), rifampin (600 mg), and ethambutol (25 mg/kg) is recommended. For patients with cavitary MAC pulmonary disease or severe nodular/bronchiectasis disease, the guidelines recommend a daily regimen of clarithromycin (500-1000 mg) or azithromycin (250 mg), rifampin (600 mg) or rifabutin (150–300 mg), and ethambutol (15 mg/kg), with consideration of intravenous (IV) amikacin 3 times/week early in therapy (Table 2).11

The treatment of MAC hypersensitivity-like disease speaks to the controversy of whether this is an inflammatory process, infectious process, or a combination of inflammation and infection. Avoidance of exposure is the mainstay of management. In some cases, steroids are used with or without a short course of anti-MAC therapy (ie, clarithromycin or azithromycin with rifampin and ethambutol).
Prophylaxis for disseminated MAC disease should be given to adults with HIV infection who have a CD4+ count less than 50 cells/μL. Azithromycin 1200 mg/week or clarithromycin 1000 mg/day has proven efficacy, and rifabutin 300 mg/day is also effective but less well tolerated. Rifabutin is more active in vitro against MAC than rifampin, and is used in HIV-positive patients because of drug-drug interaction between antiretroviral drugs and rifampin.
Continue to: Case 1 Continued
Case 1 Continued
The patient is treated with clarithromycin, rifampin, and ethambutol for 1 year, with sputum conversion after 9 months. In the latter part of her treatment, she experiences decreased visual acuity. Treatment is discontinued prematurely after 1 year due to drug toxicity and continued intolerance to drug therapy. The patient remains asymptomatic for 8 months, and then begins to experience mild to moderate hemoptysis, with increasing cough and sputum production associated with postural changes during exercise. Physical examination overall remains unchanged. Three sputum results reveal heavy growth of MAC, and a CT scan of the chest shows a cavitary lesion in the left upper lobe along with the nodular bronchiectasis (Figure 4).

What are the management options at this stage?
Based on this patient’s continued symptoms, progression of radiologic abnormalities, and current culture growth, she requires re-treatment. With the adverse effects associated with ethambutol during the first round of therapy, the drug regimen needs to be modified. Several considerations are relevant at this stage. Relapse rates range from 20% to 30% after treatment with a macrolide-based therapy.11,34 Obtaining a culture-sensitivity profile is imperative in these cases. Of note, treatment should not be discontinued altogether, but instead the toxic agent should be removed from the treatment regimen. Continuing treatment with a 2-drug regimen of clarithromycin and rifampin may be considered in this patient. Re-infection with multiple genotypes may also occur after successful drug therapy, but this is primarily seen in MAC patients with nodular bronchiectasis.34,35 Patients in whom previous therapy has failed, even those with macrolide-susceptible MAC isolates, are less likely to respond to subsequent therapy. Data suggest that intermittent medication dosing is not effective for patients with severe or cavitary disease or in those in whom previous therapy has failed.36 In this case, treatment should include a daily 3-drug therapy, with an injectable thrice-weekly aminoglycoside. Other agents such as linezolid and clofazimine may have to be tried. Cycloserine, ethionamide, and other agents are sometimes used, but their efficacy is unproven and doubtful. Pyrazinamide and isoniazid have no activity against MAC.
Treatment Failure and Drug Resistance
Treatment failure is considered to have occurred if patients have not had a response (microbiologic, clinical, or radiographic) after 6 months of appropriate therapy or had not achieved conversion of sputum to culture-negative after 12 months of appropriate therapy.11 This occurs in about 40% of patients. Multiple factors can interfere with the successful treatment of MAC pulmonary disease, including medication nonadherence, medication side effects or intolerance, lack of response to a medication regimen, or the emergence of a macrolide-resistant or multidrug-resistant strain. Inducible macrolide resistance remains a potential factor.34-36 A number of characteristics of NTM contribute to the poor response to currently used antibiotics: the organisms have a lipid outer membrane and prefer to adhere to surfaces and form biofilms, which makes them relatively impermeable to antibiotics.37 Also, NTM replicate in phagocytic cells, allowing them to subvert normal cellular defense mechanisms. Furthermore, NTM can display colony variants, whereby single colony isolates switch between antibiotic-susceptible and -resistant variants. These factors have also impeded in development of new antibiotics for NTM infection.37
Recent limited approval of amikacin liposomal inhalation suspension (ALIS) for treatment failure and refractory MAC infection in combination with guideline-based antimicrobial therapy (GBT) is a promising addition to the available treatment armamentarium. In a multinational trial, the addition of ALIS to GBT for treatment-refractory MAC lung disease achieved significantly greater culture conversion rates by month 6 than GBT alone, with comparable rates of serious adverse events.38
Is therapeutic drug monitoring recommended during treatment of MAC pulmonary disease?
Treatment failure may also be drug-related, including poor drug penetration into the damaged lung tissue or drug-drug interactions leading to suboptimal drug levels. Peak serum concentrations have been found to be below target ranges in approximately 50% of patients using a macrolide and ethambutol. Concurrent use of rifampin decreases the peak serum concentration of macrolides and quinolones, with acceptable target levels seen in only 18% to 57% of cases. Whether this alters patient outcomes is not clear.39-42 Factors identified as contributing to the poor response to therapy include poor compliance, cavitary disease, previous treatment for MAC pulmonary disease, and a history of chronic obstructive lung disease. Studies by Koh and colleagues40 and van Ingen and colleagues41 with pharmacokinetic and pharmacodynamics data showed that, in patients on MAC treatment with both clarithromycin and rifampicin, plasma levels of clarithromycin were lower than the recommended minimal inhibitory concentrations (MIC) against MAC for that drug. The studies also showed that rifampicin lowered clarithromycin concentrations more than did rifabutin, with the AUC/MIC ratio being suboptimal in nearly half the cases. However, low plasma clarithromycin concentrations did not have any correlation with treatment outcomes, as the peak plasma drug concentrations and the peak plasma drug concentration/MIC ratios did not differ between patients with unfavorable treatment outcomes and those with favorable outcomes. This is further compounded by the fact that macrolides achieve higher levels in lung tissue than in plasma, and hence the significance of low plasma levels is unclear; however, it is postulated that achieving higher drug levels could, in fact, lead to better clinical outcomes. Pending specific well-designed, prospective randomized controlled trials, routine therapeutic drug monitoring is not currently recommended, although some referral centers do this as their practice pattern.
Is surgery an option in this case?
The overall 5-year mortality for MAC pulmonary disease was approximately 28% in a retrospective analysis, with patients with cavitary disease at increased risk for death at 5 years.42 As such, surgery is an option in selected cases as part of adjunctive therapy along with anti-MAC therapy based on mycobacterial sensitivity. Surgery is used as either a curative approach or a “debulking” measure.11 When present, clearly localized disease, especially in the upper lobe, lends itself best to surgical intervention. Surgical resection of a solitary pulmonary nodule due to MAC, in addition to concomitant medical treatment, is recommended. Surgical intervention should be considered early in the course of the disease because it may provide a cure without prolonged treatment and its associated problems, and this approach may lead to early sputum conversion. Surgery should also be considered in patients with macrolide-resistant or multidrug-resistant MAC infection or in those who cannot tolerate the side effects of therapy, provided that the disease is focal and limited. Patients with poor preoperative lung function have poorer outcomes than those with good lung function, and postoperative complications arising from treatment, especially with a right-sided pneumonectomy, tend to occur more frequently in these patients. Thoracic surgery for NTM pulmonary disease must be considered cautiously, as this is associated with significant morbidity and mortality and is best performed at specialized centers that have expertise and experience in this field.43
Continue to: Mycobacterium abscessus Complex
Mycobacterium abscessus Complex
Case Patient 2
A 64-year-old man who is an ex-smoker presents with chronic cough, mild shortness of breath on exertion, low-grade fever, and unintentional weight loss of 10 lb. Physical exam is unremarkable. The patient was diagnosed with immunoglobulin deficiency (low IgM and low IgG4) in 2002, and has been on replacement therapy since then. He also has had multiple episodes of NTM infection, with MAC and M. kansasii infections diagnosed in 2012-2014, which required 18 months of multi-drug antibiotic treatment that resulted in sputum conversion. Pulmonary function testing done on this visit in 2017 shows mild obstructive impairment.
Chest radiograph and CT scan show bilateral bronchiectasis (Figure 5 and Figure 6).

The results of serial sputum microbiology testing performed over the course of 6 months are outlined below:
- 5/2017 (bronchoalveolar lavage): 2+; M. abscessus
- 9/2017 × 2: smear (–); group IV RGM
- 11/2017: smear (–); M. abscessus (> 50 CFU)
- 12/2017: smear (–); M. abscessus (> 50 CFU)

What are the clinical considerations in this patient with multiple NTM infections?
M. abscessus complex was originally described in soft tissue abscesses and skin infections possibly resulting from soil or water contamination. Subspeciation of M. abscessus complex during laboratory testing is critical to facilitate selection of a specific therapeutic approach; treatment decisions are impacted by the presence of an active erm gene and in vitro macrolide sensitivity, which differ between subspecies. The most acceptable classification outlines 3 species in the M. abscessus complex: Mycobacterium abscessus subsp abscessus, Mycobacterium abscessus subsp bolletii (both with an active erm gene responsible for macrolide resistance), and Mycobacterium abscessus subsp massiliense (with an inactive erm gene and therefore susceptible to macrolides).44
RGM typically manifest in skin, soft tissue, and bone, and can cause soft tissue, surgical wound, and catheter-related infections. Although the role of RGM as pulmonary pathogens is unclear, underlying diseases associated with RGM include previously treated mycobacterial disease, coexistent pulmonary diseases with or without MAC, cystic fibrosis, malignancies, and gastroesophageal disorders. M. abscessus is the third most commonly identified respiratory NTM and accounts for the majority (80%) of RGM respiratory isolates. Other NTM reported to cause both lung disease and skin, bone, and joint infections include Mycobacterium simiae, Mycobacterium xenopi, and Mycobacterium malmoense. Ocular granulomatous diseases, such as chorioretinitis and keratitis, have been reported with both RGM and Runyon group III SGM, such as MAC or M. szulgai, following trauma or refractive surgery. These can mimic fungal, herpetic, or amebic keratitis. The pulmonary syndromes associated with multiple culture positivity are seen in elderly women with bronchiectasis or cavitary lung disease and/or associated with gastrointestinal symptoms of acid reflux, with or without achalasia and concomitant lipoid interstitial pneumonia.45
Generally, pulmonary disease progresses slowly, but lung disease attributed to RGM can result in respiratory failure. Thus, RGM should be recognized as a possible cause of chronic mycobacterial lung disease, especially in immunocompromised patients, and respiratory isolates should be assessed carefully. Identification and drug susceptibility testing are essential before initiation of treatment for RGM.
What is the approach to management of M. abscessus pulmonary disease in a patient without cystic fibrosis?
The management of M. abscessus pulmonary infection as a subset of RGM requires a considered step-wise approach. The criteria for diagnosis and threshold for starting treatment are the same as those used in the management of MAC pulmonary disease,11 but the treatment of M. abscessus pulmonary infection is more complex and has lower rates of success and cure. Also, antibiotic treatment presents challenges related to rapid identification of the causative organism, nomenclature, resistance patterns, and tolerance of treatment and side effects. If a source such as catheter, access port, or any surgical site is identified, prompt removal and clearance of the infected site are strongly advised
In the absence of any controlled clinical trials, treatment of RGM is based on in vitro susceptibility testing and expert opinion. As in MAC pulmonary disease, macrolides are the mainstay of treatment, with an induction phase of intravenous antibiotics. Treatment may include a combination of injectable aminoglycosides, imipenem, or cefoxitin and oral drugs such as a macrolide (eg, clarithromycin, azithromycin), doxycycline, fluoroquinolones, trimethoprim/sulfamethoxazole, or linezolid. While antibiotic treatment of M. abscessus pulmonary disease is based on in vitro sensitivity pattern to a greater degree than is treatment of MAC pulmonary disease, this approach has significant practical limitations and hence variable applicability. The final choice of antibiotics is best based on the extended susceptibility results, if available. The presence of an active erm gene on a prolonged growth specimen in M. abscessus subsp abscessus and M. abscessus subsp bolletii precludes the use of a macrolide. In such cases, amikacin, especially in an intravenous form, is the mainstay of treatment based on MIC. Recently, there has been a resurgence in interest in the use of clofazimine in combination with amikacin when treatment is not successful in patients with M. abscessus subsp abscessus or M. bolletii with an active erm gene.45,46 When localized abscess formation is noted, surgery may be the best option, with emphasis on removal of implants and catheters if implicated in RGM infection.
Attention must also be given to confounding pulmonary and associated comorbidities. This includes management of bronchiectasis with appropriately aggressive airway clearance techniques; anti-reflux measures for prevention of micro-aspiration; and management of other comorbid pulmonary conditions, such as chronic obstructive pulmonary disease, pulmonary fibrosis, and sarcoidosis, if applicable. These interventions play a critical role in clearing the M. abscessus infection, preventing progression of disease, and reducing morbidity. The role of immunomodulatory therapy needs to be considered on a regular, ongoing basis. Identification of genetic factors and correction of immune deficiencies may help in managing the infection.
Case Patient 2 Conclusion
The treatment regimen adopted in this case includes a 3-month course of daily intravenous amikacin and imipenem with oral azithromycin, followed by a continuation phase of azithromycin with clofazimine and linezolid. Airway clearance techniques such as Vest/Acapella/CPT are intensified and monthly intravenous immunoglobulin therapy is continued. The patient responds to treatment, with resolution of his clinical symptoms and reduction in the colony count of M. abscessus in the sputum.
Summary
NTM are ubiquitous in the environment, and NTM infection has variable manifestations, especially in patients with no recognizable immune impairments. Underlying comorbid conditions with bronchiectasis complicate its management. Treatment strategies must be individualized based on degree of involvement, associated comorbidities, immune deficiencies, goals of therapy, outcome-based risk-benefit ratio assessment, and patient engagement and expectations. In diffuse pulmonary disease, drug treatment remains difficult due to poor match of in vitro and in vivo culture sensitivity, side effects of medications, and high failure rates. When a localized resectable foci of infection is identified, especially in RGM disease, surgical treatment may be the best approach in selected patients, but it must be performed in centers with expertise and experience in this field.
1. Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210-220.
2. Falkinham JO III. Environmental sources of NTM. Clin Chest Med. 2015;36:35-41.
3. Falkinham JO III, Current epidemiological trends in NTM. Curr Environ Health Rep. 2016;3:161-167.
4. Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med. 2015;36:1-11.
5. Marras TK, Mirsaeidi M, Chou E, et al. Health care utilization and expenditures following diagnosis of nontuberculous mycobacterial lung disease in the United States. Manag Care Spec Pharm. 2018;24:964-974.
6. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med. 2010;182:970-976.
7. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977-982.
8. Adjemian, Olivier KN, Seitz AE, J et al. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185;881-886.
9. Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalizations associated with pulmonary nontuberculous mycobacterial infections in Germany, 2005-2011. BMC Infect Dis. 2013;13:231.
10. Aliyu G, El-Kamary SS, Abimiku A, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170.
11. Griffith DE, Aksamit T, Brown-Elliott, et al; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-415.
12. Wallace RJ Jr, Zhang Y, Brown BA, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158:1235-1244.
13. Gordin FM, Horsburgh CR Jr. Mycobacterium avium complex. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Elsevier; 2015.
14. Chitty S, Ali J. Mycobacterium avium complex pulmonary disease in immune competent patients. South Med J. 2005;98:646-52.
15. Ramirez J, Mason C, Ali J, Lopez FA. MAC pulmonary disease: management options in HIV-negative patients. J La State Med Soc. 2008;160:248-254.
16. Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914-916.
17. Kim RD, Greenburg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066-1074.
18. Ziedalski TM, Kao PN, Henig NR, et al. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995-1002.
19. Koh WJ, Lee KS, Kwon OJ, et al. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005;235:282-288.
20. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994;105:49-52.
21. Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115:1033-1040.
22. Cappelluti E, Fraire AE, Schaefer OP. A case of “hot tub lung” due to Mycobacterium avium complex in an immunocompetent host. Arch Intern Med. 2003;163:845-848.
23. Nightingale SD, Byrd LT, Southern PM, et al. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082-1085.
24. Horsburgh CR Jr, Selik RM. The epidemiology of disseminated tuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989;139:4-7.
25. Chin DP, Hopewell PC, Yajko DM, et al. Mycobacterium avium complex in the respiratory or gastrointestinal tract and the risk of M. avium complex bacteremia in patients with human immunodeficiency virus infection. J Infect Dis. 1994;169:289-295.
26. Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176:306-313.
27. Lillo M, Orengo S, Cernoch P, Harris RL. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis. 1990;12:760-767.
28. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099-1104.
29. Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797-1807.
30. James D, Chalmers JD, Goeminne P, et al. The Bronchiectasis Severity Index: an international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576-585.
31. Heifets L. MIC as a quantitative measurement of the susceptibility of Mycobacterium avium strains to seven antituberculosis drugs. Antimicrob Agents Chemother. 1988;32:1131-1136.
32. Horsburgh CR Jr, Mason UG 3rd, Heifits LB, et al. Response to therapy of pulmonary Mycobacterium avium intracellulare infection correlates with results of in vitro susceptibility testing. Am Rev Respir Dis. 1987;135:418-421.
33. Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S-78S.
34. Wallace RJ Jr, Brown BA, Griffith DE, et al. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153:1766-1772.
35. Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928-934.
36. Lam PK, Griffith DE, Aksamit TR, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173:1283-1289.
37. Falkinham J III. Challenges of NTM drug development. Front Microbiol. 2018;9:1613.
38. Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198:1559-1569.
39. Schluger NW. Treatment of pulmonary Mycobacterium avium complex infections: do drug levels matter? Am J Respir Crit Care Med. 2012;186:710-711.
40. Van Ingen J, Egelund EF, Levin A, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186:559-565.
41. Koh WJ, Jeong BH, Jeon K, et al. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186:797-802.
42. Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary MAC disease. Int J Tuberc Lung Dis. 2012;16:408-414.
43. Yuji S, Yutsuki N, Keiichiso T, et al. Surgery for Mycobacterium avium lung disease in the clarithromycin era. Eur J Cardiothor Surg. 2002;21:314-318.
44. Tortoli E, Kohl TA, Brown-Elliott BA, et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. Int J Syst Evol Microbiol. 2016; 66:4471-4479.
45. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271-1278.
46. Koh WJ, Jeong BH, Kim SY, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309-316.
1. Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210-220.
2. Falkinham JO III. Environmental sources of NTM. Clin Chest Med. 2015;36:35-41.
3. Falkinham JO III, Current epidemiological trends in NTM. Curr Environ Health Rep. 2016;3:161-167.
4. Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med. 2015;36:1-11.
5. Marras TK, Mirsaeidi M, Chou E, et al. Health care utilization and expenditures following diagnosis of nontuberculous mycobacterial lung disease in the United States. Manag Care Spec Pharm. 2018;24:964-974.
6. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated healthcare delivery systems. Am J Respir Crit Care Med. 2010;182:970-976.
7. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977-982.
8. Adjemian, Olivier KN, Seitz AE, J et al. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185;881-886.
9. Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalizations associated with pulmonary nontuberculous mycobacterial infections in Germany, 2005-2011. BMC Infect Dis. 2013;13:231.
10. Aliyu G, El-Kamary SS, Abimiku A, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170.
11. Griffith DE, Aksamit T, Brown-Elliott, et al; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-415.
12. Wallace RJ Jr, Zhang Y, Brown BA, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158:1235-1244.
13. Gordin FM, Horsburgh CR Jr. Mycobacterium avium complex. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Elsevier; 2015.
14. Chitty S, Ali J. Mycobacterium avium complex pulmonary disease in immune competent patients. South Med J. 2005;98:646-52.
15. Ramirez J, Mason C, Ali J, Lopez FA. MAC pulmonary disease: management options in HIV-negative patients. J La State Med Soc. 2008;160:248-254.
16. Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914-916.
17. Kim RD, Greenburg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066-1074.
18. Ziedalski TM, Kao PN, Henig NR, et al. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995-1002.
19. Koh WJ, Lee KS, Kwon OJ, et al. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005;235:282-288.
20. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994;105:49-52.
21. Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115:1033-1040.
22. Cappelluti E, Fraire AE, Schaefer OP. A case of “hot tub lung” due to Mycobacterium avium complex in an immunocompetent host. Arch Intern Med. 2003;163:845-848.
23. Nightingale SD, Byrd LT, Southern PM, et al. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992;165:1082-1085.
24. Horsburgh CR Jr, Selik RM. The epidemiology of disseminated tuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989;139:4-7.
25. Chin DP, Hopewell PC, Yajko DM, et al. Mycobacterium avium complex in the respiratory or gastrointestinal tract and the risk of M. avium complex bacteremia in patients with human immunodeficiency virus infection. J Infect Dis. 1994;169:289-295.
26. Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med. 2007;176:306-313.
27. Lillo M, Orengo S, Cernoch P, Harris RL. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis. 1990;12:760-767.
28. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099-1104.
29. Arend SM, van Meijgaarden KE, de Boer K, et al. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J Infect Dis. 2002;186:1797-1807.
30. James D, Chalmers JD, Goeminne P, et al. The Bronchiectasis Severity Index: an international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576-585.
31. Heifets L. MIC as a quantitative measurement of the susceptibility of Mycobacterium avium strains to seven antituberculosis drugs. Antimicrob Agents Chemother. 1988;32:1131-1136.
32. Horsburgh CR Jr, Mason UG 3rd, Heifits LB, et al. Response to therapy of pulmonary Mycobacterium avium intracellulare infection correlates with results of in vitro susceptibility testing. Am Rev Respir Dis. 1987;135:418-421.
33. Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S-78S.
34. Wallace RJ Jr, Brown BA, Griffith DE, et al. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153:1766-1772.
35. Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928-934.
36. Lam PK, Griffith DE, Aksamit TR, et al. Factors related to response to intermittent treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;173:1283-1289.
37. Falkinham J III. Challenges of NTM drug development. Front Microbiol. 2018;9:1613.
38. Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198:1559-1569.
39. Schluger NW. Treatment of pulmonary Mycobacterium avium complex infections: do drug levels matter? Am J Respir Crit Care Med. 2012;186:710-711.
40. Van Ingen J, Egelund EF, Levin A, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186:559-565.
41. Koh WJ, Jeong BH, Jeon K, et al. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;186:797-802.
42. Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary MAC disease. Int J Tuberc Lung Dis. 2012;16:408-414.
43. Yuji S, Yutsuki N, Keiichiso T, et al. Surgery for Mycobacterium avium lung disease in the clarithromycin era. Eur J Cardiothor Surg. 2002;21:314-318.
44. Tortoli E, Kohl TA, Brown-Elliott BA, et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. Int J Syst Evol Microbiol. 2016; 66:4471-4479.
45. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271-1278.
46. Koh WJ, Jeong BH, Kim SY, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309-316.
Measles, scarlet fever among infectious diseases to watch for in 2020
ORLANDO – – leading into 2020, Justin Finch, MD, said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
While group A streptococcus has declined over the past century, there has been “an unprecedented” resurgence in severe, invasive group A streptococcal infections and severe epidemics of scarlet fever worldwide, including in industrialized regions like the United Kingdom. Shedding some light on why this may be occurring, Dr. Finch referred to a recently published population-based molecular epidemiologic study identified a new dominant emm1UK lineage of Streptococcus pyogenes associated with such cases in England (Lancet Infect Dis. 2019 Nov;19(11):1209-18). This new lineage of S. pyogenes was genotypically distinct from other emm1 isolates and had greatly increased expression of the streptococcal pyrogenic exotoxin A, one of the exotoxins responsible for the clinical features of scarlet fever.
“We have not, to my knowledge, seen the strain yet in the United States,” said Dr. Finch, of Central Connecticut Dermatology in Cromwell. “Have it on your radar. With all of the worldwide travel patterns, I expect that you will see this in the United States at some point in the not-too-distant future.”
Also in 2019, promising data on the safety and effectiveness of the recombinant herpes zoster vaccine in immunocompromised patients became available for the first time. A randomized clinical trial published in JAMA of 1,846 patients who were immunosuppressed after autologous hematopoietic stem cell transplantation and received two doses of a recombinant zoster vaccine found that the patients had a reduced incidence of herpes zoster after a median follow-up of 21 months (JAMA. 2019 Jul 9;322[2]:123-33). The study found that the recombinant vaccine was both safe and effective in these immunocompromised patients, “so we can easily generalize this to our dermatology population as well,” Dr. Finch said. In comparing the live attenuated and recombinant vaccines, he noted the recombinant vaccine requires two doses but appears to be slightly more effective. “The number needed to treat to prevent [one case] of zoster is about half as high as that for the live vaccine, and most importantly for us is, it’s safe in immunocompromised patients.”
2019 also saw a record high in the number of measles cases in the United States, the highest since 1993, Dr. Finch pointed out. Most cases were seen in the area in and around New York City, but the percentage of people across the United States who are vaccinated against measles is below the threshold for herd immunity to protect immunocompromised patients. Measles requires a population vaccination rate of 94%, and less than half of U.S. counties in 2014 and 2015 reached that vaccination rate.
“Furthermore, if we look at that over the last 20 years, comparing the domestic measles cases to imported measles cases, we are increasingly breeding these measles epidemics right here at home, whereas they used to be imported from throughout the world,” said Dr. Finch. Patients with measles can be treated with vitamin A, he added, referring to a Cochrane review showing that 200,000 units of vitamin A given daily for 2 days decreased the mortality rate of measles by about 80%. Measles is on the Centers for Disease Control and Prevention’s list of reportable diseases, so should be reported to local health authorities, and will be followed up with confirmatory testing.
In 2019, a study examining herd protection of oral human papillomavirus infection in men and women compared the prevalence of oral HPV infection based on the 4 HPV types present in the quadrivalent HPV vaccine with 33 nonvaccine types from 2009 to 2016. There was no change in the prevalence of nonvaccine type oral HPV infections among men who were unvaccinated, but the prevalence of oral HPV infections because of the four strains in the quadrivalent HPV vaccine declined from 2.7% in 2009-2010 to 1.6% in 2015-2016 (JAMA. 2019 Sep 10;322[10]:977-9). Among unvaccinated women, the prevalence of nonvaccine- and vaccine-type oral HPV infections did not change between the two time periods.
“Notably, this only occurred in men,” Dr. Finch said. Herd immunity is being achieved in men “because we’re vaccinating all women, [but] we’re not seeing that herd immunity in women. Which begs the question: Why are we still vaccinating only half of our population?”
One study published in 2019 (Br J Dermatol. 2019 Nov;181[5]:1093-5) described a patient with CARD9 mutations, which predispose individuals to deep invasive infections – a disseminated Microsporum infection in this case, Dr. Finch said. “You shouldn’t see that,” he added, noting that these mutations are known to predispose individuals to severe Trichophyton infections and familial candidiasis.
“What I think is interesting about this is that, as we look forward to 2020, we’re going to increasingly see studies like this that are identifying specific mutations in our community that underlie a lot of these weird infections,” he added. “I wouldn’t be surprised if within the span of our careers, we find that a lot of those severe treatment-refractory reports that so commonly plague your everyday clinic have some underlying, specific immunity.”
Dr. Finch reported no relevant conflicts of interest.
ORLANDO – – leading into 2020, Justin Finch, MD, said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
While group A streptococcus has declined over the past century, there has been “an unprecedented” resurgence in severe, invasive group A streptococcal infections and severe epidemics of scarlet fever worldwide, including in industrialized regions like the United Kingdom. Shedding some light on why this may be occurring, Dr. Finch referred to a recently published population-based molecular epidemiologic study identified a new dominant emm1UK lineage of Streptococcus pyogenes associated with such cases in England (Lancet Infect Dis. 2019 Nov;19(11):1209-18). This new lineage of S. pyogenes was genotypically distinct from other emm1 isolates and had greatly increased expression of the streptococcal pyrogenic exotoxin A, one of the exotoxins responsible for the clinical features of scarlet fever.
“We have not, to my knowledge, seen the strain yet in the United States,” said Dr. Finch, of Central Connecticut Dermatology in Cromwell. “Have it on your radar. With all of the worldwide travel patterns, I expect that you will see this in the United States at some point in the not-too-distant future.”
Also in 2019, promising data on the safety and effectiveness of the recombinant herpes zoster vaccine in immunocompromised patients became available for the first time. A randomized clinical trial published in JAMA of 1,846 patients who were immunosuppressed after autologous hematopoietic stem cell transplantation and received two doses of a recombinant zoster vaccine found that the patients had a reduced incidence of herpes zoster after a median follow-up of 21 months (JAMA. 2019 Jul 9;322[2]:123-33). The study found that the recombinant vaccine was both safe and effective in these immunocompromised patients, “so we can easily generalize this to our dermatology population as well,” Dr. Finch said. In comparing the live attenuated and recombinant vaccines, he noted the recombinant vaccine requires two doses but appears to be slightly more effective. “The number needed to treat to prevent [one case] of zoster is about half as high as that for the live vaccine, and most importantly for us is, it’s safe in immunocompromised patients.”
2019 also saw a record high in the number of measles cases in the United States, the highest since 1993, Dr. Finch pointed out. Most cases were seen in the area in and around New York City, but the percentage of people across the United States who are vaccinated against measles is below the threshold for herd immunity to protect immunocompromised patients. Measles requires a population vaccination rate of 94%, and less than half of U.S. counties in 2014 and 2015 reached that vaccination rate.
“Furthermore, if we look at that over the last 20 years, comparing the domestic measles cases to imported measles cases, we are increasingly breeding these measles epidemics right here at home, whereas they used to be imported from throughout the world,” said Dr. Finch. Patients with measles can be treated with vitamin A, he added, referring to a Cochrane review showing that 200,000 units of vitamin A given daily for 2 days decreased the mortality rate of measles by about 80%. Measles is on the Centers for Disease Control and Prevention’s list of reportable diseases, so should be reported to local health authorities, and will be followed up with confirmatory testing.
In 2019, a study examining herd protection of oral human papillomavirus infection in men and women compared the prevalence of oral HPV infection based on the 4 HPV types present in the quadrivalent HPV vaccine with 33 nonvaccine types from 2009 to 2016. There was no change in the prevalence of nonvaccine type oral HPV infections among men who were unvaccinated, but the prevalence of oral HPV infections because of the four strains in the quadrivalent HPV vaccine declined from 2.7% in 2009-2010 to 1.6% in 2015-2016 (JAMA. 2019 Sep 10;322[10]:977-9). Among unvaccinated women, the prevalence of nonvaccine- and vaccine-type oral HPV infections did not change between the two time periods.
“Notably, this only occurred in men,” Dr. Finch said. Herd immunity is being achieved in men “because we’re vaccinating all women, [but] we’re not seeing that herd immunity in women. Which begs the question: Why are we still vaccinating only half of our population?”
One study published in 2019 (Br J Dermatol. 2019 Nov;181[5]:1093-5) described a patient with CARD9 mutations, which predispose individuals to deep invasive infections – a disseminated Microsporum infection in this case, Dr. Finch said. “You shouldn’t see that,” he added, noting that these mutations are known to predispose individuals to severe Trichophyton infections and familial candidiasis.
“What I think is interesting about this is that, as we look forward to 2020, we’re going to increasingly see studies like this that are identifying specific mutations in our community that underlie a lot of these weird infections,” he added. “I wouldn’t be surprised if within the span of our careers, we find that a lot of those severe treatment-refractory reports that so commonly plague your everyday clinic have some underlying, specific immunity.”
Dr. Finch reported no relevant conflicts of interest.
ORLANDO – – leading into 2020, Justin Finch, MD, said at the ODAC Dermatology, Aesthetic, & Surgical Conference.
While group A streptococcus has declined over the past century, there has been “an unprecedented” resurgence in severe, invasive group A streptococcal infections and severe epidemics of scarlet fever worldwide, including in industrialized regions like the United Kingdom. Shedding some light on why this may be occurring, Dr. Finch referred to a recently published population-based molecular epidemiologic study identified a new dominant emm1UK lineage of Streptococcus pyogenes associated with such cases in England (Lancet Infect Dis. 2019 Nov;19(11):1209-18). This new lineage of S. pyogenes was genotypically distinct from other emm1 isolates and had greatly increased expression of the streptococcal pyrogenic exotoxin A, one of the exotoxins responsible for the clinical features of scarlet fever.
“We have not, to my knowledge, seen the strain yet in the United States,” said Dr. Finch, of Central Connecticut Dermatology in Cromwell. “Have it on your radar. With all of the worldwide travel patterns, I expect that you will see this in the United States at some point in the not-too-distant future.”
Also in 2019, promising data on the safety and effectiveness of the recombinant herpes zoster vaccine in immunocompromised patients became available for the first time. A randomized clinical trial published in JAMA of 1,846 patients who were immunosuppressed after autologous hematopoietic stem cell transplantation and received two doses of a recombinant zoster vaccine found that the patients had a reduced incidence of herpes zoster after a median follow-up of 21 months (JAMA. 2019 Jul 9;322[2]:123-33). The study found that the recombinant vaccine was both safe and effective in these immunocompromised patients, “so we can easily generalize this to our dermatology population as well,” Dr. Finch said. In comparing the live attenuated and recombinant vaccines, he noted the recombinant vaccine requires two doses but appears to be slightly more effective. “The number needed to treat to prevent [one case] of zoster is about half as high as that for the live vaccine, and most importantly for us is, it’s safe in immunocompromised patients.”
2019 also saw a record high in the number of measles cases in the United States, the highest since 1993, Dr. Finch pointed out. Most cases were seen in the area in and around New York City, but the percentage of people across the United States who are vaccinated against measles is below the threshold for herd immunity to protect immunocompromised patients. Measles requires a population vaccination rate of 94%, and less than half of U.S. counties in 2014 and 2015 reached that vaccination rate.
“Furthermore, if we look at that over the last 20 years, comparing the domestic measles cases to imported measles cases, we are increasingly breeding these measles epidemics right here at home, whereas they used to be imported from throughout the world,” said Dr. Finch. Patients with measles can be treated with vitamin A, he added, referring to a Cochrane review showing that 200,000 units of vitamin A given daily for 2 days decreased the mortality rate of measles by about 80%. Measles is on the Centers for Disease Control and Prevention’s list of reportable diseases, so should be reported to local health authorities, and will be followed up with confirmatory testing.
In 2019, a study examining herd protection of oral human papillomavirus infection in men and women compared the prevalence of oral HPV infection based on the 4 HPV types present in the quadrivalent HPV vaccine with 33 nonvaccine types from 2009 to 2016. There was no change in the prevalence of nonvaccine type oral HPV infections among men who were unvaccinated, but the prevalence of oral HPV infections because of the four strains in the quadrivalent HPV vaccine declined from 2.7% in 2009-2010 to 1.6% in 2015-2016 (JAMA. 2019 Sep 10;322[10]:977-9). Among unvaccinated women, the prevalence of nonvaccine- and vaccine-type oral HPV infections did not change between the two time periods.
“Notably, this only occurred in men,” Dr. Finch said. Herd immunity is being achieved in men “because we’re vaccinating all women, [but] we’re not seeing that herd immunity in women. Which begs the question: Why are we still vaccinating only half of our population?”
One study published in 2019 (Br J Dermatol. 2019 Nov;181[5]:1093-5) described a patient with CARD9 mutations, which predispose individuals to deep invasive infections – a disseminated Microsporum infection in this case, Dr. Finch said. “You shouldn’t see that,” he added, noting that these mutations are known to predispose individuals to severe Trichophyton infections and familial candidiasis.
“What I think is interesting about this is that, as we look forward to 2020, we’re going to increasingly see studies like this that are identifying specific mutations in our community that underlie a lot of these weird infections,” he added. “I wouldn’t be surprised if within the span of our careers, we find that a lot of those severe treatment-refractory reports that so commonly plague your everyday clinic have some underlying, specific immunity.”
Dr. Finch reported no relevant conflicts of interest.
REPORTING FROM ODAC 2020
Trump takes on multiple health topics in State of the Union
President Donald J. Trump took on multiple health care issues in his State of the Union address, imploring Congress to avoid the “socialism” of Medicare-for-all, to pass legislation banning late-term abortions, and to protect insurance coverage for preexisting conditions while joining together to reduce rising drug prices.
Mr. Trump said his administration has already been “taking on the big pharmaceutical companies,” claiming that, in 2019, “for the first time in 51 years, the cost of prescription drugs actually went down.”
That statement was called “misleading” by the New York Times because such efforts have excluded some high-cost drugs, and prices had risen by the end of the year, the publication noted in a fact-check of the president’s speech.
A survey issued in December 2019 found that the United States pays the highest prices in the world for pharmaceuticals, as reported by Medscape Medical News.
But the president did throw down a gauntlet for Congress. “Working together, the Congress can reduce drug prices substantially from current levels,” he said, stating that he had been “speaking to Sen. Chuck Grassley of Iowa and others in the Congress in order to get something on drug pricing done, and done properly.
“Get a bill to my desk, and I will sign it into law without delay,” Mr. Trump said.
A group of House Democrats then stood up in the chamber and loudly chanted, “HR3, HR3,” referring to the Lower Drug Costs Now Act, which the House passed in December 2019.
The bill would give the Department of Health & Human Services the power to negotiate directly with drug companies on up to 250 drugs per year, in particular, the highest-costing and most-utilized drugs.
The Senate has not taken up the legislation, but Sen. Grassley (R) and Sen. Ron Wyden (D-Ore.) introduced a similar bill, the Prescription Drug Pricing Reduction Act. It has been approved by the Senate Finance Committee but has not been moved to the Senate floor.
“I appreciate President Trump recognizing the work we’re doing to lower prescription drug prices,” Sen. Grassley said in a statement after the State of the Union. “Iowans and Americans across the country are demanding reforms that lower sky-high drug costs. A recent poll showed 70% of Americans want Congress to make lowering drug prices its top priority.”
Rep. Greg Walden (R-Ore.), the ranking Republican on the House Energy and Commerce Committee, said he believed Trump was committed to lowering drug costs. “I’ve never seen a president lean in further than President Donald Trump on lowering health care costs,” said Rep. Walden in a statement after the speech.
Trump touted his price transparency rule, which he said would go into effect next January, as a key way to cut health care costs.
Preexisting conditions
The president said that since he’d taken office, insurance had become more affordable and that the quality of health care had improved. He also said that he was making what he called an “iron-clad pledge” to American families.
“We will always protect patients with preexisting conditions – that is a guarantee,” Mr. Trump said.
In a press conference before the speech, Speaker of the House Nancy Pelosi (D-Calif.) took issue with that pledge. “The president swears that he supports protections for people with preexisting conditions, but right now, he is fighting in federal court to eliminate these lifesaving protections and every last protection and benefit of the Affordable Care Act,” she said.
During the speech, Rep. G. K. Butterfield (D-N.C.) tweeted “#FactCheck: Claiming to protect Americans with preexisting conditions, Trump and his administration have repeatedly sought to undermine protections offered by the ACA through executive orders and the courts. He is seeking to strike down the law and its protections entirely.”
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, pointed out in a tweet that insurance plans that Trump touted as “affordable alternatives” are in fact missing those protections.
“Ironically, the cheaper health insurance plans that President Trump has expanded are short-term plans that don’t cover preexisting conditions,” Mr. Levitt said.
Socialist takeover
Mr. Trump condemned the Medicare-for-all proposals that have been introduced in Congress and that are being backed in whole or in part by all of the Democratic candidates for president.
“As we work to improve Americans’ health care, there are those who want to take away your health care, take away your doctor, and abolish private insurance entirely,” said Mr. Trump.
He said that 132 members of Congress “have endorsed legislation to impose a socialist takeover of our health care system, wiping out the private health insurance plans of 180 million Americans.”
Added Mr. Trump: “We will never let socialism destroy American health care!”
Medicare-for-all has waxed and waned in popularity among voters, with generally more Democrats than Republicans favoring a single-payer system, with or without a public option.
Preliminary exit polls in Iowa that were conducted during Monday’s caucus found that 57% of Iowa Democratic caucus-goers supported a single-payer plan; 38% opposed such a plan, according to the Washington Post.
Opioids, the coronavirus, and abortion
In some of his final remarks on health care, Mr. Trump cited progress in the opioid crisis, noting that, in 2019, drug overdose deaths declined for the first time in 30 years.
He said that his administration was coordinating with the Chinese government regarding the coronavirus outbreak and noted the launch of initiatives to improve care for people with kidney disease, Alzheimer’s, and mental health problems.
Mr. Trump repeated his 2019 State of the Union claim that the government would help end AIDS in America by the end of the decade.
The president also announced that he was asking Congress for “an additional $50 million” to fund neonatal research. He followed that up with a plea about abortion.
“I am calling upon the members of Congress here tonight to pass legislation finally banning the late-term abortion of babies,” he said.
Insulin costs?
In the days before the speech, some news outlets had reported that Mr. Trump and the HHS were working on a plan to lower insulin prices for Medicare beneficiaries, and there were suggestions it would come up in the speech.
At least 13 members of Congress invited people advocating for lower insulin costs as their guests for the State of the Union, Stat reported. Rep. Pelosi invited twins from San Francisco with type 1 diabetes as her guests.
But Mr. Trump never mentioned insulin in his speech.
This article first appeared on Medscape.com.
President Donald J. Trump took on multiple health care issues in his State of the Union address, imploring Congress to avoid the “socialism” of Medicare-for-all, to pass legislation banning late-term abortions, and to protect insurance coverage for preexisting conditions while joining together to reduce rising drug prices.
Mr. Trump said his administration has already been “taking on the big pharmaceutical companies,” claiming that, in 2019, “for the first time in 51 years, the cost of prescription drugs actually went down.”
That statement was called “misleading” by the New York Times because such efforts have excluded some high-cost drugs, and prices had risen by the end of the year, the publication noted in a fact-check of the president’s speech.
A survey issued in December 2019 found that the United States pays the highest prices in the world for pharmaceuticals, as reported by Medscape Medical News.
But the president did throw down a gauntlet for Congress. “Working together, the Congress can reduce drug prices substantially from current levels,” he said, stating that he had been “speaking to Sen. Chuck Grassley of Iowa and others in the Congress in order to get something on drug pricing done, and done properly.
“Get a bill to my desk, and I will sign it into law without delay,” Mr. Trump said.
A group of House Democrats then stood up in the chamber and loudly chanted, “HR3, HR3,” referring to the Lower Drug Costs Now Act, which the House passed in December 2019.
The bill would give the Department of Health & Human Services the power to negotiate directly with drug companies on up to 250 drugs per year, in particular, the highest-costing and most-utilized drugs.
The Senate has not taken up the legislation, but Sen. Grassley (R) and Sen. Ron Wyden (D-Ore.) introduced a similar bill, the Prescription Drug Pricing Reduction Act. It has been approved by the Senate Finance Committee but has not been moved to the Senate floor.
“I appreciate President Trump recognizing the work we’re doing to lower prescription drug prices,” Sen. Grassley said in a statement after the State of the Union. “Iowans and Americans across the country are demanding reforms that lower sky-high drug costs. A recent poll showed 70% of Americans want Congress to make lowering drug prices its top priority.”
Rep. Greg Walden (R-Ore.), the ranking Republican on the House Energy and Commerce Committee, said he believed Trump was committed to lowering drug costs. “I’ve never seen a president lean in further than President Donald Trump on lowering health care costs,” said Rep. Walden in a statement after the speech.
Trump touted his price transparency rule, which he said would go into effect next January, as a key way to cut health care costs.
Preexisting conditions
The president said that since he’d taken office, insurance had become more affordable and that the quality of health care had improved. He also said that he was making what he called an “iron-clad pledge” to American families.
“We will always protect patients with preexisting conditions – that is a guarantee,” Mr. Trump said.
In a press conference before the speech, Speaker of the House Nancy Pelosi (D-Calif.) took issue with that pledge. “The president swears that he supports protections for people with preexisting conditions, but right now, he is fighting in federal court to eliminate these lifesaving protections and every last protection and benefit of the Affordable Care Act,” she said.
During the speech, Rep. G. K. Butterfield (D-N.C.) tweeted “#FactCheck: Claiming to protect Americans with preexisting conditions, Trump and his administration have repeatedly sought to undermine protections offered by the ACA through executive orders and the courts. He is seeking to strike down the law and its protections entirely.”
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, pointed out in a tweet that insurance plans that Trump touted as “affordable alternatives” are in fact missing those protections.
“Ironically, the cheaper health insurance plans that President Trump has expanded are short-term plans that don’t cover preexisting conditions,” Mr. Levitt said.
Socialist takeover
Mr. Trump condemned the Medicare-for-all proposals that have been introduced in Congress and that are being backed in whole or in part by all of the Democratic candidates for president.
“As we work to improve Americans’ health care, there are those who want to take away your health care, take away your doctor, and abolish private insurance entirely,” said Mr. Trump.
He said that 132 members of Congress “have endorsed legislation to impose a socialist takeover of our health care system, wiping out the private health insurance plans of 180 million Americans.”
Added Mr. Trump: “We will never let socialism destroy American health care!”
Medicare-for-all has waxed and waned in popularity among voters, with generally more Democrats than Republicans favoring a single-payer system, with or without a public option.
Preliminary exit polls in Iowa that were conducted during Monday’s caucus found that 57% of Iowa Democratic caucus-goers supported a single-payer plan; 38% opposed such a plan, according to the Washington Post.
Opioids, the coronavirus, and abortion
In some of his final remarks on health care, Mr. Trump cited progress in the opioid crisis, noting that, in 2019, drug overdose deaths declined for the first time in 30 years.
He said that his administration was coordinating with the Chinese government regarding the coronavirus outbreak and noted the launch of initiatives to improve care for people with kidney disease, Alzheimer’s, and mental health problems.
Mr. Trump repeated his 2019 State of the Union claim that the government would help end AIDS in America by the end of the decade.
The president also announced that he was asking Congress for “an additional $50 million” to fund neonatal research. He followed that up with a plea about abortion.
“I am calling upon the members of Congress here tonight to pass legislation finally banning the late-term abortion of babies,” he said.
Insulin costs?
In the days before the speech, some news outlets had reported that Mr. Trump and the HHS were working on a plan to lower insulin prices for Medicare beneficiaries, and there were suggestions it would come up in the speech.
At least 13 members of Congress invited people advocating for lower insulin costs as their guests for the State of the Union, Stat reported. Rep. Pelosi invited twins from San Francisco with type 1 diabetes as her guests.
But Mr. Trump never mentioned insulin in his speech.
This article first appeared on Medscape.com.
President Donald J. Trump took on multiple health care issues in his State of the Union address, imploring Congress to avoid the “socialism” of Medicare-for-all, to pass legislation banning late-term abortions, and to protect insurance coverage for preexisting conditions while joining together to reduce rising drug prices.
Mr. Trump said his administration has already been “taking on the big pharmaceutical companies,” claiming that, in 2019, “for the first time in 51 years, the cost of prescription drugs actually went down.”
That statement was called “misleading” by the New York Times because such efforts have excluded some high-cost drugs, and prices had risen by the end of the year, the publication noted in a fact-check of the president’s speech.
A survey issued in December 2019 found that the United States pays the highest prices in the world for pharmaceuticals, as reported by Medscape Medical News.
But the president did throw down a gauntlet for Congress. “Working together, the Congress can reduce drug prices substantially from current levels,” he said, stating that he had been “speaking to Sen. Chuck Grassley of Iowa and others in the Congress in order to get something on drug pricing done, and done properly.
“Get a bill to my desk, and I will sign it into law without delay,” Mr. Trump said.
A group of House Democrats then stood up in the chamber and loudly chanted, “HR3, HR3,” referring to the Lower Drug Costs Now Act, which the House passed in December 2019.
The bill would give the Department of Health & Human Services the power to negotiate directly with drug companies on up to 250 drugs per year, in particular, the highest-costing and most-utilized drugs.
The Senate has not taken up the legislation, but Sen. Grassley (R) and Sen. Ron Wyden (D-Ore.) introduced a similar bill, the Prescription Drug Pricing Reduction Act. It has been approved by the Senate Finance Committee but has not been moved to the Senate floor.
“I appreciate President Trump recognizing the work we’re doing to lower prescription drug prices,” Sen. Grassley said in a statement after the State of the Union. “Iowans and Americans across the country are demanding reforms that lower sky-high drug costs. A recent poll showed 70% of Americans want Congress to make lowering drug prices its top priority.”
Rep. Greg Walden (R-Ore.), the ranking Republican on the House Energy and Commerce Committee, said he believed Trump was committed to lowering drug costs. “I’ve never seen a president lean in further than President Donald Trump on lowering health care costs,” said Rep. Walden in a statement after the speech.
Trump touted his price transparency rule, which he said would go into effect next January, as a key way to cut health care costs.
Preexisting conditions
The president said that since he’d taken office, insurance had become more affordable and that the quality of health care had improved. He also said that he was making what he called an “iron-clad pledge” to American families.
“We will always protect patients with preexisting conditions – that is a guarantee,” Mr. Trump said.
In a press conference before the speech, Speaker of the House Nancy Pelosi (D-Calif.) took issue with that pledge. “The president swears that he supports protections for people with preexisting conditions, but right now, he is fighting in federal court to eliminate these lifesaving protections and every last protection and benefit of the Affordable Care Act,” she said.
During the speech, Rep. G. K. Butterfield (D-N.C.) tweeted “#FactCheck: Claiming to protect Americans with preexisting conditions, Trump and his administration have repeatedly sought to undermine protections offered by the ACA through executive orders and the courts. He is seeking to strike down the law and its protections entirely.”
Larry Levitt, executive vice president for health policy at the Kaiser Family Foundation, pointed out in a tweet that insurance plans that Trump touted as “affordable alternatives” are in fact missing those protections.
“Ironically, the cheaper health insurance plans that President Trump has expanded are short-term plans that don’t cover preexisting conditions,” Mr. Levitt said.
Socialist takeover
Mr. Trump condemned the Medicare-for-all proposals that have been introduced in Congress and that are being backed in whole or in part by all of the Democratic candidates for president.
“As we work to improve Americans’ health care, there are those who want to take away your health care, take away your doctor, and abolish private insurance entirely,” said Mr. Trump.
He said that 132 members of Congress “have endorsed legislation to impose a socialist takeover of our health care system, wiping out the private health insurance plans of 180 million Americans.”
Added Mr. Trump: “We will never let socialism destroy American health care!”
Medicare-for-all has waxed and waned in popularity among voters, with generally more Democrats than Republicans favoring a single-payer system, with or without a public option.
Preliminary exit polls in Iowa that were conducted during Monday’s caucus found that 57% of Iowa Democratic caucus-goers supported a single-payer plan; 38% opposed such a plan, according to the Washington Post.
Opioids, the coronavirus, and abortion
In some of his final remarks on health care, Mr. Trump cited progress in the opioid crisis, noting that, in 2019, drug overdose deaths declined for the first time in 30 years.
He said that his administration was coordinating with the Chinese government regarding the coronavirus outbreak and noted the launch of initiatives to improve care for people with kidney disease, Alzheimer’s, and mental health problems.
Mr. Trump repeated his 2019 State of the Union claim that the government would help end AIDS in America by the end of the decade.
The president also announced that he was asking Congress for “an additional $50 million” to fund neonatal research. He followed that up with a plea about abortion.
“I am calling upon the members of Congress here tonight to pass legislation finally banning the late-term abortion of babies,” he said.
Insulin costs?
In the days before the speech, some news outlets had reported that Mr. Trump and the HHS were working on a plan to lower insulin prices for Medicare beneficiaries, and there were suggestions it would come up in the speech.
At least 13 members of Congress invited people advocating for lower insulin costs as their guests for the State of the Union, Stat reported. Rep. Pelosi invited twins from San Francisco with type 1 diabetes as her guests.
But Mr. Trump never mentioned insulin in his speech.
This article first appeared on Medscape.com.
Home BP now a class Ia recommendation, with good reason
SNOWMASS, COLO. – The redefinition of hypertension as 130/80 mm Hg or higher introduced in the current American College of Cardiology/American Heart Association hypertension management guidelines has generated considerable controversy. Often overlooked, however, has been another major innovation included in the 2017 guidelines: the rise in the status of out-of-office 24-hour ambulatory blood pressure monitoring and home blood pressure self-measurement to a class I, level of evidence A recommendation, Andrew M. Kates, MD, observed at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
It’s a guideline he strongly endorses.
“We do a lot of this. It can be a challenge to get 24-hour ambulatory blood pressure monitoring covered by payers, so said Dr. Kates, professor of medicine and director of the cardiology fellowship program at Washington University, St. Louis.
He explained that one of the four key questions the guideline committee was tasked with answering at the outset of deliberations was this: What’s the evidence base for self-directed out-of-office blood pressure monitoring? Based on the panel’s systematic review of the literature, this practice wound up receiving the strongest possible class Ia recommendation, specifically for confirming the diagnosis of hypertension and for titration of antihypertensive medications. Moreover, the guidelines also endorsed home blood pressure monitoring for the detection of white-coat hypertension, this time as a Class IIa recommendation, as well as for identification of patients with masked hypertension, with class IIb status (Circulation. 2018 Oct 23;138[17]:e484-594).
The 2017 ACC/AHA guidelines include a detailed checklist for obtaining accurate measurements of office blood pressure. The suggestions include having the patient sit relaxed in a chair with both feet on the floor for at least 5 minutes before taking the measurement, no coffee or exercise for 30 minutes beforehand, empty the bladder, no talking, no clothing over the arm, and other recommendations. Many busy clinicians roll their eyes at the impracticality of doing all this on a routine basis.
“I don’t want to take an audience survey, but I’ll say that even in our office we are not successful in doing this. Patients run up the stairs to the office after dealing with traffic and the parking garage, they’re late for their appointment, in winter they’re wearing a sweater and don’t want to take it off. These are things we don’t do well, and they’re low-hanging fruit where we could do better,” Dr. Kates commented.
The challenges inherent in performing by-the-book office blood pressure measurement reinforce the importance of home self-monitoring of blood pressure in what is hopefully a more stress-free environment.
“We can give patients specific guidance about checking their blood pressure an hour after taking their medications, sitting for 5 minutes, and checking the pressures on a bare arm and not with the sleeve rolled up,” he noted.
The guidelines recommend using home blood pressure monitoring or ambulatory monitoring to detect white-coat hypertension in patients with an office blood pressure of 130/80 mm Hg or more, but less than 160/100 mm Hg, after a 3-month trial of lifestyle modification. If the home blood pressure is less than 130/80 mm Hg, that’s evidence of white-coat hypertension, for which the recommended treatment consists of continued lifestyle modification plus periodic monitoring of out-of-office blood pressures in order to promptly detect progression to hypertension. If, however, the out-of-office blood pressure is not less than 130/80 mm Hg, that’s hypertension, and the guidelines recommend starting dual-agent antihypertensive drug therapy while continuing lifestyle modification.
A confusing array of definitions of hypertension are now in use by various medical societies. While the 2017 ACC/AHA hypertension guidelines define hypertension as office blood pressure of 130/80 mm Hg or more, the 2018 European Society of Cardiology/European Society of Hypertension guidelines use a threshold of 140/90 mm Hg or more. Joint American Academy of Family Physicians/American College of Physicians guidelines recommend a treatment target of less than 150 mm Hg in hypertensive patients aged 60 years or older. And at the other end of the spectrum, the SPRINT trial showed a significant cardiovascular benefit for intensive treatment of hypertension to a target systolic blood pressure below 120 mm Hg, rather than less than 140 mm Hg (N Engl J Med. 2015 Nov 26;373[22]:2103-16).
Dr. Kates believes the debate over the “right” treatment target misses the central point, which is that hypertension is staggeringly undertreated. Indeed, the Centers for Disease Control and Prevention estimates only one in four adults with hypertension have their disease under control. That’s a disconcerting statistic given that hypertension accounts for more cardiovascular deaths than any other modifiable cardiovascular risk factor.
“There’s been some concern raised that maybe too much weight has been put on the SPRINT trial in making the ACC/AHA recommendations, but I think it’s helpful to understand that we vastly undertreat patients with hypertension. So I think that, rather than being so concerned that we’re going to be treating people to too low a target or we’re being overly aggressive, it should give us some pause to think about the fact that we’re ordinarily not being aggressive enough with many of our patients as it is,” the cardiologist said.
Dr. Kates reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – The redefinition of hypertension as 130/80 mm Hg or higher introduced in the current American College of Cardiology/American Heart Association hypertension management guidelines has generated considerable controversy. Often overlooked, however, has been another major innovation included in the 2017 guidelines: the rise in the status of out-of-office 24-hour ambulatory blood pressure monitoring and home blood pressure self-measurement to a class I, level of evidence A recommendation, Andrew M. Kates, MD, observed at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
It’s a guideline he strongly endorses.
“We do a lot of this. It can be a challenge to get 24-hour ambulatory blood pressure monitoring covered by payers, so said Dr. Kates, professor of medicine and director of the cardiology fellowship program at Washington University, St. Louis.
He explained that one of the four key questions the guideline committee was tasked with answering at the outset of deliberations was this: What’s the evidence base for self-directed out-of-office blood pressure monitoring? Based on the panel’s systematic review of the literature, this practice wound up receiving the strongest possible class Ia recommendation, specifically for confirming the diagnosis of hypertension and for titration of antihypertensive medications. Moreover, the guidelines also endorsed home blood pressure monitoring for the detection of white-coat hypertension, this time as a Class IIa recommendation, as well as for identification of patients with masked hypertension, with class IIb status (Circulation. 2018 Oct 23;138[17]:e484-594).
The 2017 ACC/AHA guidelines include a detailed checklist for obtaining accurate measurements of office blood pressure. The suggestions include having the patient sit relaxed in a chair with both feet on the floor for at least 5 minutes before taking the measurement, no coffee or exercise for 30 minutes beforehand, empty the bladder, no talking, no clothing over the arm, and other recommendations. Many busy clinicians roll their eyes at the impracticality of doing all this on a routine basis.
“I don’t want to take an audience survey, but I’ll say that even in our office we are not successful in doing this. Patients run up the stairs to the office after dealing with traffic and the parking garage, they’re late for their appointment, in winter they’re wearing a sweater and don’t want to take it off. These are things we don’t do well, and they’re low-hanging fruit where we could do better,” Dr. Kates commented.
The challenges inherent in performing by-the-book office blood pressure measurement reinforce the importance of home self-monitoring of blood pressure in what is hopefully a more stress-free environment.
“We can give patients specific guidance about checking their blood pressure an hour after taking their medications, sitting for 5 minutes, and checking the pressures on a bare arm and not with the sleeve rolled up,” he noted.
The guidelines recommend using home blood pressure monitoring or ambulatory monitoring to detect white-coat hypertension in patients with an office blood pressure of 130/80 mm Hg or more, but less than 160/100 mm Hg, after a 3-month trial of lifestyle modification. If the home blood pressure is less than 130/80 mm Hg, that’s evidence of white-coat hypertension, for which the recommended treatment consists of continued lifestyle modification plus periodic monitoring of out-of-office blood pressures in order to promptly detect progression to hypertension. If, however, the out-of-office blood pressure is not less than 130/80 mm Hg, that’s hypertension, and the guidelines recommend starting dual-agent antihypertensive drug therapy while continuing lifestyle modification.
A confusing array of definitions of hypertension are now in use by various medical societies. While the 2017 ACC/AHA hypertension guidelines define hypertension as office blood pressure of 130/80 mm Hg or more, the 2018 European Society of Cardiology/European Society of Hypertension guidelines use a threshold of 140/90 mm Hg or more. Joint American Academy of Family Physicians/American College of Physicians guidelines recommend a treatment target of less than 150 mm Hg in hypertensive patients aged 60 years or older. And at the other end of the spectrum, the SPRINT trial showed a significant cardiovascular benefit for intensive treatment of hypertension to a target systolic blood pressure below 120 mm Hg, rather than less than 140 mm Hg (N Engl J Med. 2015 Nov 26;373[22]:2103-16).
Dr. Kates believes the debate over the “right” treatment target misses the central point, which is that hypertension is staggeringly undertreated. Indeed, the Centers for Disease Control and Prevention estimates only one in four adults with hypertension have their disease under control. That’s a disconcerting statistic given that hypertension accounts for more cardiovascular deaths than any other modifiable cardiovascular risk factor.
“There’s been some concern raised that maybe too much weight has been put on the SPRINT trial in making the ACC/AHA recommendations, but I think it’s helpful to understand that we vastly undertreat patients with hypertension. So I think that, rather than being so concerned that we’re going to be treating people to too low a target or we’re being overly aggressive, it should give us some pause to think about the fact that we’re ordinarily not being aggressive enough with many of our patients as it is,” the cardiologist said.
Dr. Kates reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – The redefinition of hypertension as 130/80 mm Hg or higher introduced in the current American College of Cardiology/American Heart Association hypertension management guidelines has generated considerable controversy. Often overlooked, however, has been another major innovation included in the 2017 guidelines: the rise in the status of out-of-office 24-hour ambulatory blood pressure monitoring and home blood pressure self-measurement to a class I, level of evidence A recommendation, Andrew M. Kates, MD, observed at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
It’s a guideline he strongly endorses.
“We do a lot of this. It can be a challenge to get 24-hour ambulatory blood pressure monitoring covered by payers, so said Dr. Kates, professor of medicine and director of the cardiology fellowship program at Washington University, St. Louis.
He explained that one of the four key questions the guideline committee was tasked with answering at the outset of deliberations was this: What’s the evidence base for self-directed out-of-office blood pressure monitoring? Based on the panel’s systematic review of the literature, this practice wound up receiving the strongest possible class Ia recommendation, specifically for confirming the diagnosis of hypertension and for titration of antihypertensive medications. Moreover, the guidelines also endorsed home blood pressure monitoring for the detection of white-coat hypertension, this time as a Class IIa recommendation, as well as for identification of patients with masked hypertension, with class IIb status (Circulation. 2018 Oct 23;138[17]:e484-594).
The 2017 ACC/AHA guidelines include a detailed checklist for obtaining accurate measurements of office blood pressure. The suggestions include having the patient sit relaxed in a chair with both feet on the floor for at least 5 minutes before taking the measurement, no coffee or exercise for 30 minutes beforehand, empty the bladder, no talking, no clothing over the arm, and other recommendations. Many busy clinicians roll their eyes at the impracticality of doing all this on a routine basis.
“I don’t want to take an audience survey, but I’ll say that even in our office we are not successful in doing this. Patients run up the stairs to the office after dealing with traffic and the parking garage, they’re late for their appointment, in winter they’re wearing a sweater and don’t want to take it off. These are things we don’t do well, and they’re low-hanging fruit where we could do better,” Dr. Kates commented.
The challenges inherent in performing by-the-book office blood pressure measurement reinforce the importance of home self-monitoring of blood pressure in what is hopefully a more stress-free environment.
“We can give patients specific guidance about checking their blood pressure an hour after taking their medications, sitting for 5 minutes, and checking the pressures on a bare arm and not with the sleeve rolled up,” he noted.
The guidelines recommend using home blood pressure monitoring or ambulatory monitoring to detect white-coat hypertension in patients with an office blood pressure of 130/80 mm Hg or more, but less than 160/100 mm Hg, after a 3-month trial of lifestyle modification. If the home blood pressure is less than 130/80 mm Hg, that’s evidence of white-coat hypertension, for which the recommended treatment consists of continued lifestyle modification plus periodic monitoring of out-of-office blood pressures in order to promptly detect progression to hypertension. If, however, the out-of-office blood pressure is not less than 130/80 mm Hg, that’s hypertension, and the guidelines recommend starting dual-agent antihypertensive drug therapy while continuing lifestyle modification.
A confusing array of definitions of hypertension are now in use by various medical societies. While the 2017 ACC/AHA hypertension guidelines define hypertension as office blood pressure of 130/80 mm Hg or more, the 2018 European Society of Cardiology/European Society of Hypertension guidelines use a threshold of 140/90 mm Hg or more. Joint American Academy of Family Physicians/American College of Physicians guidelines recommend a treatment target of less than 150 mm Hg in hypertensive patients aged 60 years or older. And at the other end of the spectrum, the SPRINT trial showed a significant cardiovascular benefit for intensive treatment of hypertension to a target systolic blood pressure below 120 mm Hg, rather than less than 140 mm Hg (N Engl J Med. 2015 Nov 26;373[22]:2103-16).
Dr. Kates believes the debate over the “right” treatment target misses the central point, which is that hypertension is staggeringly undertreated. Indeed, the Centers for Disease Control and Prevention estimates only one in four adults with hypertension have their disease under control. That’s a disconcerting statistic given that hypertension accounts for more cardiovascular deaths than any other modifiable cardiovascular risk factor.
“There’s been some concern raised that maybe too much weight has been put on the SPRINT trial in making the ACC/AHA recommendations, but I think it’s helpful to understand that we vastly undertreat patients with hypertension. So I think that, rather than being so concerned that we’re going to be treating people to too low a target or we’re being overly aggressive, it should give us some pause to think about the fact that we’re ordinarily not being aggressive enough with many of our patients as it is,” the cardiologist said.
Dr. Kates reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
Pembrolizumab-Induced Lobular Panniculitis in the Setting of Metastatic Melanoma
To the Editor:
Pembrolizumab is an anti–programmed death receptor 1 humanized monoclonal antibody used for treating advanced or metastatic melanoma.1 It is associated with several immune-related adverse events because it blocks a T-cell receptor checkpoint.2 The most common dermatologic immune-related adverse event seen with anti–programmed death receptor 1 medications is a nonspecific morbilliform rash, usually seen after the second treatment cycle; however, pruritus, vitiligo, bullous disorders, and lichenoid reactions also have been reported.3 We report a case of pembrolizumab-induced, self-limited lobular panniculitis in a patient with metastatic melanoma.
A 37-year-old woman with malignant melanoma presented with tender, erythematous, subcutaneous nodules on the hips and legs of 2 weeks’ duration (Figure 1). Twelve years prior to the current presentation, she was diagnosed with metastases to the cecum, lung, and brain. A review of systems was otherwise negative. She had been receiving pembrolizumab infusions (2 mg/kg every 3 weeks) for the last 2.7 years as second-line therapy after previously undergoing chemotherapy, radiation, and resection. She was not taking oral contraceptives or other hormone-based medications and did not report any new medications.
Laboratory testing was negative for infectious processes including Lyme disease, tuberculosis, and Streptococcus due to recent upper respiratory infection. Punch biopsy of a left shin lesion revealed a lobular panniculitis with lymphohistiocytic inflammation, a focal lymphocytic vasculitis, and small granulomas (Figure 2). Periodic acid–Schiff, Gram, and acid-fast bacilli stains were negative. After ruling out alternative causes, the etiology of the panniculitis was deemed to be a pembrolizumab side effect. The patient was treated conservatively with ibuprofen; pembrolizumab was not discontinued. Two weeks later, the panniculitis had resolved without additional treatment. She remains on pembrolizumab and is doing well.
Panniculitis is known to be associated with certain BRAF inhibitors used for the treatment of melanoma positive for the BRAF V600E mutation, including vemurafenib and dabrafenib.4,5 Reports of panniculitis in the setting of pembrolizumab are limited and are seen within the larger context of sarcoidosis. One patient on pembrolizumab for metastatic melanoma developed granulomatous lobular panniculitis with oligoarthritis, high fever, and hilar/mediastinal adenopathy, consistent with pembrolizumab-induced sarcoidosis. It developed after her second pembrolizumab infusion and resolved with prednisone and temporary pembrolizumab cessation.6 In another case, pembrolizumab triggered a flare of sarcoidosis with similar granulomatous subcutaneous nodules in a patient with stage IV lymphoma who was previously diagnosed with sarcoidosis but lacked cutaneous manifestations. The lesions resolved with prednisone therapy.7
Chest computed tomography was normal in our patient, and she reported no systemic symptoms. Additional laboratory studies to evaluate for sarcoidosis were not obtained. Furthermore, the lesions quickly resolved despite continued use of pembrolizumab. We report this case to highlight that pembrolizumab may induce an isolated, self-limited lobular panniculitis years after medication initiation.
- Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973-1981.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Ramani NS, Curry JL, Kapil J, et al. Panniculitis with necrotizing granulomata in a patient on BRAF inhibitor (dabrafenib) therapy for metastatic melanoma. Am J Dermatopathol. 2015;37:E96-E99.
- Burillo-Martinez S, Morales-Raya C, Prieto-Barrios M, et al. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721-722.
- Cotliar J, Querfeld C, Boswell WJ, et al. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2:290-293.
To the Editor:
Pembrolizumab is an anti–programmed death receptor 1 humanized monoclonal antibody used for treating advanced or metastatic melanoma.1 It is associated with several immune-related adverse events because it blocks a T-cell receptor checkpoint.2 The most common dermatologic immune-related adverse event seen with anti–programmed death receptor 1 medications is a nonspecific morbilliform rash, usually seen after the second treatment cycle; however, pruritus, vitiligo, bullous disorders, and lichenoid reactions also have been reported.3 We report a case of pembrolizumab-induced, self-limited lobular panniculitis in a patient with metastatic melanoma.
A 37-year-old woman with malignant melanoma presented with tender, erythematous, subcutaneous nodules on the hips and legs of 2 weeks’ duration (Figure 1). Twelve years prior to the current presentation, she was diagnosed with metastases to the cecum, lung, and brain. A review of systems was otherwise negative. She had been receiving pembrolizumab infusions (2 mg/kg every 3 weeks) for the last 2.7 years as second-line therapy after previously undergoing chemotherapy, radiation, and resection. She was not taking oral contraceptives or other hormone-based medications and did not report any new medications.

Laboratory testing was negative for infectious processes including Lyme disease, tuberculosis, and Streptococcus due to recent upper respiratory infection. Punch biopsy of a left shin lesion revealed a lobular panniculitis with lymphohistiocytic inflammation, a focal lymphocytic vasculitis, and small granulomas (Figure 2). Periodic acid–Schiff, Gram, and acid-fast bacilli stains were negative. After ruling out alternative causes, the etiology of the panniculitis was deemed to be a pembrolizumab side effect. The patient was treated conservatively with ibuprofen; pembrolizumab was not discontinued. Two weeks later, the panniculitis had resolved without additional treatment. She remains on pembrolizumab and is doing well.

Panniculitis is known to be associated with certain BRAF inhibitors used for the treatment of melanoma positive for the BRAF V600E mutation, including vemurafenib and dabrafenib.4,5 Reports of panniculitis in the setting of pembrolizumab are limited and are seen within the larger context of sarcoidosis. One patient on pembrolizumab for metastatic melanoma developed granulomatous lobular panniculitis with oligoarthritis, high fever, and hilar/mediastinal adenopathy, consistent with pembrolizumab-induced sarcoidosis. It developed after her second pembrolizumab infusion and resolved with prednisone and temporary pembrolizumab cessation.6 In another case, pembrolizumab triggered a flare of sarcoidosis with similar granulomatous subcutaneous nodules in a patient with stage IV lymphoma who was previously diagnosed with sarcoidosis but lacked cutaneous manifestations. The lesions resolved with prednisone therapy.7
Chest computed tomography was normal in our patient, and she reported no systemic symptoms. Additional laboratory studies to evaluate for sarcoidosis were not obtained. Furthermore, the lesions quickly resolved despite continued use of pembrolizumab. We report this case to highlight that pembrolizumab may induce an isolated, self-limited lobular panniculitis years after medication initiation.
To the Editor:
Pembrolizumab is an anti–programmed death receptor 1 humanized monoclonal antibody used for treating advanced or metastatic melanoma.1 It is associated with several immune-related adverse events because it blocks a T-cell receptor checkpoint.2 The most common dermatologic immune-related adverse event seen with anti–programmed death receptor 1 medications is a nonspecific morbilliform rash, usually seen after the second treatment cycle; however, pruritus, vitiligo, bullous disorders, and lichenoid reactions also have been reported.3 We report a case of pembrolizumab-induced, self-limited lobular panniculitis in a patient with metastatic melanoma.
A 37-year-old woman with malignant melanoma presented with tender, erythematous, subcutaneous nodules on the hips and legs of 2 weeks’ duration (Figure 1). Twelve years prior to the current presentation, she was diagnosed with metastases to the cecum, lung, and brain. A review of systems was otherwise negative. She had been receiving pembrolizumab infusions (2 mg/kg every 3 weeks) for the last 2.7 years as second-line therapy after previously undergoing chemotherapy, radiation, and resection. She was not taking oral contraceptives or other hormone-based medications and did not report any new medications.

Laboratory testing was negative for infectious processes including Lyme disease, tuberculosis, and Streptococcus due to recent upper respiratory infection. Punch biopsy of a left shin lesion revealed a lobular panniculitis with lymphohistiocytic inflammation, a focal lymphocytic vasculitis, and small granulomas (Figure 2). Periodic acid–Schiff, Gram, and acid-fast bacilli stains were negative. After ruling out alternative causes, the etiology of the panniculitis was deemed to be a pembrolizumab side effect. The patient was treated conservatively with ibuprofen; pembrolizumab was not discontinued. Two weeks later, the panniculitis had resolved without additional treatment. She remains on pembrolizumab and is doing well.

Panniculitis is known to be associated with certain BRAF inhibitors used for the treatment of melanoma positive for the BRAF V600E mutation, including vemurafenib and dabrafenib.4,5 Reports of panniculitis in the setting of pembrolizumab are limited and are seen within the larger context of sarcoidosis. One patient on pembrolizumab for metastatic melanoma developed granulomatous lobular panniculitis with oligoarthritis, high fever, and hilar/mediastinal adenopathy, consistent with pembrolizumab-induced sarcoidosis. It developed after her second pembrolizumab infusion and resolved with prednisone and temporary pembrolizumab cessation.6 In another case, pembrolizumab triggered a flare of sarcoidosis with similar granulomatous subcutaneous nodules in a patient with stage IV lymphoma who was previously diagnosed with sarcoidosis but lacked cutaneous manifestations. The lesions resolved with prednisone therapy.7
Chest computed tomography was normal in our patient, and she reported no systemic symptoms. Additional laboratory studies to evaluate for sarcoidosis were not obtained. Furthermore, the lesions quickly resolved despite continued use of pembrolizumab. We report this case to highlight that pembrolizumab may induce an isolated, self-limited lobular panniculitis years after medication initiation.
- Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973-1981.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Ramani NS, Curry JL, Kapil J, et al. Panniculitis with necrotizing granulomata in a patient on BRAF inhibitor (dabrafenib) therapy for metastatic melanoma. Am J Dermatopathol. 2015;37:E96-E99.
- Burillo-Martinez S, Morales-Raya C, Prieto-Barrios M, et al. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721-722.
- Cotliar J, Querfeld C, Boswell WJ, et al. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2:290-293.
- Poole RM. Pembrolizumab: first global approval. Drugs. 2014;74:1973-1981.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148.
- Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Ramani NS, Curry JL, Kapil J, et al. Panniculitis with necrotizing granulomata in a patient on BRAF inhibitor (dabrafenib) therapy for metastatic melanoma. Am J Dermatopathol. 2015;37:E96-E99.
- Burillo-Martinez S, Morales-Raya C, Prieto-Barrios M, et al. Pembrolizumab-induced extensive panniculitis and nevus regression: two novel cutaneous manifestations of the post-immunotherapy granulomatous reactions spectrum. JAMA Dermatol. 2017;153:721-722.
- Cotliar J, Querfeld C, Boswell WJ, et al. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2:290-293.
Practice Points
- Pembrolizumab may cause lobular panniculitis years after treatment initiation.
- Pembrolizumab-induced lobular panniculitis may self-resolve without discontinuing the medication.
Metastatic Melanoma Mimicking Eruptive Keratoacanthomas
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.
A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).
The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.

A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).

The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
To the Editor:
Melanoma is the third most common skin cancer. It is estimated that 18% of melanoma patients will develop skin metastases, with skin being the first site of involvement in 56% of cases.1 Of all cancers, it is estimated that 5% will develop skin metastases. It is the presenting sign in nearly 1% of visceral cancers.2 Melanoma and nonmelanoma metastases can have sundry presentations. We present a case of metastatic melanoma with multiple keratoacanthoma (KA)–like skin lesions in a patient with a known history of nonmelanoma skin cancer (NMSC) as well as melanoma.
A 76-year-old man with a history of pT2aNXMX melanoma on the left upper back presented for a routine 3-month follow-up and reported several new asymptomatic bumps on the chest, back, and right upper extremity within the last 2 weeks. The melanoma was removed via wide local excision 2 years prior at an outside facility with a Breslow depth of 1.05 mm and a negative sentinel lymph node biopsy. The mitotic rate or ulceration status was unknown. He also had a history of several NMSCs, as well as a medical history of coronary artery disease, myocardial infarction, and ventricular tachycardia with cardiac defibrillator placement. Physical examination revealed 5 pink, volcano-shaped nodules with central keratotic plugs on the upper back (Figure 1), chest, and right upper extremity, in addition to 1 pink pearly nodule on the right side of the chest. The history and appearance of the lesions were suspicious for eruptive KAs. There was no evidence of cancer recurrence at the prior melanoma and NMSC sites.

A deep shave skin biopsy was performed at all 6 sites. Histopathology showed a diffuse dermal infiltrate of elongated nests of melanocytes and nonnested melanocytes. Marked cytologic atypia and ulceration were present. Minimal connection to the overlying epidermis and a lack of junctional nests was noted. Immunohistochemical studies revealed scattered positivity for Melan-A and negative staining for AE1, AE3, cytokeratin 5, and cytokeratin 6 at all 6 sites (Figure 2). A subsequent metastatic workup showed widespread metastatic disease in the liver, bone, lung, and inferior vena cava. Computed tomography of the head was unremarkable. Magnetic resonance imaging of the brain was not performed due to the cardiac defibrillator. The patient’s lactate dehydrogenase level showed a mild increase compared to 2 months prior to the metastatic melanoma diagnosis (144 U/L vs 207 U/L [reference range, 100–200 U/L]).

The patient had no systemic symptoms at follow-up 5 weeks later. He was already evaluated by an oncologist and received his first dose of ipilimumab. He was BRAF-mutation negative. He had developed 2 new skin metastases. Five of 6 initially biopsied metastases returned and were growing; they were tender and friable with intermittent bleeding. He was subsequently referred to surgical oncology for excision of symptomatic nodules as palliative care.
Although melanoma is well known to metastasize years and even decades later, KA-like lesions have not been reported as manifestations of metastatic melanoma.4,5 Our patient likely had a primary amelanotic melanoma, as the medical records from the outside facility stated that basal cell carcinoma was ruled out via biopsy. The amelanotic nature of the primary melanoma may have influenced the amelanotic appearance of the metastases. Our patient had no signs of immunosuppression that could have contributed to the sudden skin metastases.
Depending on the subtype of cutaneous metastases (eg, satellitosis, in-transit disease, distant cutaneous metastases), the location prevalence of the primary melanoma varies. In a study of 4865 melanoma patients who were diagnosed and followed prospectively over a 30-year period, skin metastases were mostly locoregional and presentation on the leg and foot were disproportionate.1 In contrast, the trunk was overrepresented for distant metastases. Distant metastases also were more associated with concurrent metastases to the viscera.1 Accordingly, a patient’s prognosis and management will differ depending on the subtype of cutaneous metastases.
Eruptive or multiple KAs classically have been associated with the Grzybowski variant, the Ferguson-Smith familial variant, and Muir-Torre syndrome. It was reported as a paraneoplastic syndrome associated with colon cancer, ovarian cancer, and once with myelodysplastic syndrome.3 Keratoacanthomas are being classified as well-differentiated squamous cell carcinomas and have metastatic potential. A biopsy is recommended to diagnose KAs as opposed to historically being monitored for resolution. A skin biopsy is the standard of care in management of KAs.
In addition to being associated with Muir-Torre syndrome and classified as a paraneoplastic syndrome,3 eruptive KAs can occur following skin resurfacing for actinic damage, fractional photothermolysis, cryotherapy, Jessner peels, and trichloroacetic acid peels.6 A couple other uncommon settings include a case report of an arc welder with job-associated radiation and multiple reports of tattoo-induced KAs.7,8 There is the new increasingly common association of squamous cell carcinomas with BRAF inhibitors, such as vemurafenib, for metastatic melanoma.9
In a 2012 review article on cutaneous metastases, Riahi and Cohen10 found 8 cases of cutaneous metastases presenting as KA-like lesions; none were metastatic melanoma. All were solitary lesions, not multiple lesions, as in our patient. The sources were lung (3 cases), breast, esophagus, chondrosarcoma, bronchial, and mesothelioma. The most common location was the upper lip. Additionally, similar to our patient, they behaved clinically as KAs with rapid growth and keratotic plugs and were asymptomatic.10
Metastatic melanoma may mimic many other cutaneous processes that may make the diagnosis more difficult. Our case indicates that cutaneous metastases may mimic KAs. Although multiple KA-like lesions can spontaneously occur, a paraneoplastic syndrome and other underlying etiologies should be considered.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
- Savoia P, Fava P, Nardò T, et al. Skin metastases of malignant melanoma: a clinical and prognostic study. Melanoma Res. 2009;19:321-326.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. J Am Acad Dermatol. 1990;22:19-26.
- Behzad M, Michl C, Pfützner W. Multiple eruptive keratoacanthomas associated with myelodysplastic syndrome. J Dtsch Dermatol Ges. 2012;10:359-360.
- Cheung WL, Patel RR, Leonard A, et al. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012;39:33-39.
- Ferrari A, Piccolo D, Fargnoli MC, et al. Cutaneous amelanotic melanoma metastasis and dermatofibromas showing a dotted vascular pattern. Acta Dermato Venereologica. 2004;84:164-165.
- Mohr B, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthoma after Jessner’s and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331-333.
- Wolfe CM, Green WH, Cognetta AB, et al. Multiple squamous cell carcinomas and eruptive keratoacanthomas in an arc welder. Dermatol Surg. 2013;39:328-330.
- Kluger N, Phan A, Debarbieux S, et al. Skin cancers arising in tattoos: coincidental or not? Dermatology. 2008;217:219-221.
- Mays R, Curry J, Kim K, et al. Eruptive squamous cell carcinomas after vemurafenib therapy. J Cutan Med Surg. 2013;17:419-422.
- Riahi RR, Cohen PR. Clinical manifestations of cutaneous metastases: a review with special emphasis on cutaneous metastases mimicking keratoacanthoma. Am J Clin Dermatol. 2012;13:103-112.
Practice Points
- Cutaneous metastatic melanoma can have variable clinical presentations.
- Patients with a history of melanoma should be monitored closely with a low threshold for biopsy of new skin lesions.
Statin, antihypertensive treatment don’t guarantee healthier lifestyles
When people learn they have enough cardiovascular disease risk to start treatment with a statin or antihypertensive drug, the impact on their healthy-lifestyle choices seems to often be a wash, based on findings from more than 40,000 Finland residents followed for at least 4 years after starting their primary-prevention regimen.
“Patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” wrote Maarit J. Korhonen, PhD, and associates in a report published in the Journal of the American Heart Association.
“Initiation of antihypertensive or statin therapy appears to be associated with lifestyle changes, some positive and others negative,” wrote Dr. Korhonen, a pharmacoepidemiologist at the University of Turku (Finland), and associates. This was the first reported study to assess a large-scale and prospectively followed cohort to look for associations between the use of medicines that prevent cardiovascular disease (CVD) and lifestyle changes. Most previous studies of these associations “have been cross sectional and provide no information on potential lifestyle changes during the time window around the initiation of medication use,” they added.
The new study specifically found that, on average, people who began treatment with at least one CVD-prevention medication for the first time were more likely to gain weight and more likely to become less active during the years following their treatment onset. But at the same time, these patients were also more likely to either quit or cut down on their smoking and alcohol consumption, the researchers found.
Their analysis used data from 41,225 people enrolled in the Finnish Public Sector Study, which prospectively began collecting data on a large number of Finland residents in the 1990s. They specifically focused on 81,772 completed questionnaires – collected at 4-year intervals – from people who completed at least two consecutive rounds of the survey during 2000-2013, and who were also at least 40 years old and free of prevalent CVD at the time of their first survey. The participants averaged nearly 53 years of age at their first survey, and 84% were women.
The researchers subdivided the survey responses into 8,837 (11%) people who began a statin, antihypertensive drug, or both during their participation; 26,914 (33%) already on a statin or antihypertensive drug when they completed their first questionnaire; and 46,021 response sets (56%) from people who never began treatment with either drug class. People who initiated a relevant drug began a median of 1.7 years following completion of their first survey, and a median of 2.4 years before their next survey. During follow-up, about 2% of all participants became newly diagnosed with some form of CVD.
The results showed that, after full adjustment for possible confounders, the mean increase in body mass index was larger among those who initiated a CVD-prevention drug, compared with those who did not. Among participants who were obese at entry, those who started a CVD drug had a statistically significant 37% increased rate of remaining obese, compared with those not starting these drugs. Among those who were not obese at baseline, those who began a CVD prevention drug had a statistically significant 82%% higher rate of becoming obese, compared with those not on a CVD-prevention drug. In addition, average daily energy expenditure, a measure of physical activity, showed a statistically significant decline among those who started a CVD drug, compared with those who did not. In contrast, CVD drug initiators had an average 1.85 gram/week decline in alcohol intake, compared with noninitiators, and those who were current smokers at the first survey and then started a CVD drug had a 26% relative drop in their smoking prevalence, compared with those who did not start a CVD drug, both statistically significant differences.
The findings suggest that “patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” the authors concluded. “This means that expansion of pharmacologic interventions toward populations at low CVD risk may not necessarily lead to expected benefits at the population level.”
The study received no commercial funding. Dr. Korhonen had no disclosures.
SOURCE: Korhonen MJ et al. J Am Heart Assoc. 2020 Feb 5. doi: 10.1161/JAHA.119.014.168.
When people learn they have enough cardiovascular disease risk to start treatment with a statin or antihypertensive drug, the impact on their healthy-lifestyle choices seems to often be a wash, based on findings from more than 40,000 Finland residents followed for at least 4 years after starting their primary-prevention regimen.
“Patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” wrote Maarit J. Korhonen, PhD, and associates in a report published in the Journal of the American Heart Association.
“Initiation of antihypertensive or statin therapy appears to be associated with lifestyle changes, some positive and others negative,” wrote Dr. Korhonen, a pharmacoepidemiologist at the University of Turku (Finland), and associates. This was the first reported study to assess a large-scale and prospectively followed cohort to look for associations between the use of medicines that prevent cardiovascular disease (CVD) and lifestyle changes. Most previous studies of these associations “have been cross sectional and provide no information on potential lifestyle changes during the time window around the initiation of medication use,” they added.
The new study specifically found that, on average, people who began treatment with at least one CVD-prevention medication for the first time were more likely to gain weight and more likely to become less active during the years following their treatment onset. But at the same time, these patients were also more likely to either quit or cut down on their smoking and alcohol consumption, the researchers found.
Their analysis used data from 41,225 people enrolled in the Finnish Public Sector Study, which prospectively began collecting data on a large number of Finland residents in the 1990s. They specifically focused on 81,772 completed questionnaires – collected at 4-year intervals – from people who completed at least two consecutive rounds of the survey during 2000-2013, and who were also at least 40 years old and free of prevalent CVD at the time of their first survey. The participants averaged nearly 53 years of age at their first survey, and 84% were women.
The researchers subdivided the survey responses into 8,837 (11%) people who began a statin, antihypertensive drug, or both during their participation; 26,914 (33%) already on a statin or antihypertensive drug when they completed their first questionnaire; and 46,021 response sets (56%) from people who never began treatment with either drug class. People who initiated a relevant drug began a median of 1.7 years following completion of their first survey, and a median of 2.4 years before their next survey. During follow-up, about 2% of all participants became newly diagnosed with some form of CVD.
The results showed that, after full adjustment for possible confounders, the mean increase in body mass index was larger among those who initiated a CVD-prevention drug, compared with those who did not. Among participants who were obese at entry, those who started a CVD drug had a statistically significant 37% increased rate of remaining obese, compared with those not starting these drugs. Among those who were not obese at baseline, those who began a CVD prevention drug had a statistically significant 82%% higher rate of becoming obese, compared with those not on a CVD-prevention drug. In addition, average daily energy expenditure, a measure of physical activity, showed a statistically significant decline among those who started a CVD drug, compared with those who did not. In contrast, CVD drug initiators had an average 1.85 gram/week decline in alcohol intake, compared with noninitiators, and those who were current smokers at the first survey and then started a CVD drug had a 26% relative drop in their smoking prevalence, compared with those who did not start a CVD drug, both statistically significant differences.
The findings suggest that “patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” the authors concluded. “This means that expansion of pharmacologic interventions toward populations at low CVD risk may not necessarily lead to expected benefits at the population level.”
The study received no commercial funding. Dr. Korhonen had no disclosures.
SOURCE: Korhonen MJ et al. J Am Heart Assoc. 2020 Feb 5. doi: 10.1161/JAHA.119.014.168.
When people learn they have enough cardiovascular disease risk to start treatment with a statin or antihypertensive drug, the impact on their healthy-lifestyle choices seems to often be a wash, based on findings from more than 40,000 Finland residents followed for at least 4 years after starting their primary-prevention regimen.
“Patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” wrote Maarit J. Korhonen, PhD, and associates in a report published in the Journal of the American Heart Association.
“Initiation of antihypertensive or statin therapy appears to be associated with lifestyle changes, some positive and others negative,” wrote Dr. Korhonen, a pharmacoepidemiologist at the University of Turku (Finland), and associates. This was the first reported study to assess a large-scale and prospectively followed cohort to look for associations between the use of medicines that prevent cardiovascular disease (CVD) and lifestyle changes. Most previous studies of these associations “have been cross sectional and provide no information on potential lifestyle changes during the time window around the initiation of medication use,” they added.
The new study specifically found that, on average, people who began treatment with at least one CVD-prevention medication for the first time were more likely to gain weight and more likely to become less active during the years following their treatment onset. But at the same time, these patients were also more likely to either quit or cut down on their smoking and alcohol consumption, the researchers found.
Their analysis used data from 41,225 people enrolled in the Finnish Public Sector Study, which prospectively began collecting data on a large number of Finland residents in the 1990s. They specifically focused on 81,772 completed questionnaires – collected at 4-year intervals – from people who completed at least two consecutive rounds of the survey during 2000-2013, and who were also at least 40 years old and free of prevalent CVD at the time of their first survey. The participants averaged nearly 53 years of age at their first survey, and 84% were women.
The researchers subdivided the survey responses into 8,837 (11%) people who began a statin, antihypertensive drug, or both during their participation; 26,914 (33%) already on a statin or antihypertensive drug when they completed their first questionnaire; and 46,021 response sets (56%) from people who never began treatment with either drug class. People who initiated a relevant drug began a median of 1.7 years following completion of their first survey, and a median of 2.4 years before their next survey. During follow-up, about 2% of all participants became newly diagnosed with some form of CVD.
The results showed that, after full adjustment for possible confounders, the mean increase in body mass index was larger among those who initiated a CVD-prevention drug, compared with those who did not. Among participants who were obese at entry, those who started a CVD drug had a statistically significant 37% increased rate of remaining obese, compared with those not starting these drugs. Among those who were not obese at baseline, those who began a CVD prevention drug had a statistically significant 82%% higher rate of becoming obese, compared with those not on a CVD-prevention drug. In addition, average daily energy expenditure, a measure of physical activity, showed a statistically significant decline among those who started a CVD drug, compared with those who did not. In contrast, CVD drug initiators had an average 1.85 gram/week decline in alcohol intake, compared with noninitiators, and those who were current smokers at the first survey and then started a CVD drug had a 26% relative drop in their smoking prevalence, compared with those who did not start a CVD drug, both statistically significant differences.
The findings suggest that “patients’ awareness of their risk factors alone seems not to be effective in improving health behaviors,” the authors concluded. “This means that expansion of pharmacologic interventions toward populations at low CVD risk may not necessarily lead to expected benefits at the population level.”
The study received no commercial funding. Dr. Korhonen had no disclosures.
SOURCE: Korhonen MJ et al. J Am Heart Assoc. 2020 Feb 5. doi: 10.1161/JAHA.119.014.168.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
New diet linked to reduced IBD symptoms
AUSTIN, TEX. – A customized diet developed to relieve inflammatory bowel disease (IBD) symptoms without compromising nutrition has uncovered a novel molecular mechanism of the diet-microbiome immune interaction that may allow gastroenterologists to tailor patient diets to enhance the gut microbiome, according to a poster presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
The study found that P-glycoprotein (P-gp) expression, associated with healthy gut, increased after adoption of the IBD-Anti-Inflammatory Diet (IBD-AID), said poster presenter and study leader Ana Luisa Maldonado-Contreras, PhD, of the University of Massachusetts Medical School, Worcester. The study involved 19 IBD patients placed on the IBD-AID. This is reportedly the first evidence of a whole-dietary recommendation that may help patients with IBD to reduce their symptoms.
“The IBD-AID has been rationally designed to feed a health-promoting, anti-inflammatory microbiome aiming at reducing chronic inflammation” Dr. Maldonado-Contreras said in an interview. The UMass researchers, led by Barbara Olendzki, RD, MPH, director of the Center for Applied Nutrition, derived the IBD-AID diet from a specific carbohydrate diet and modified it based on their research to increase the diversity of bacteria that produce short-chain fatty acids (SCFAs) and modulate the local immune response.
“SCFAs, such as acetate, propionate, and butyrate, are crucial in maintaining intestinal homeostasis by fueling colonocytes, strengthening the gut barrier function, and controlling local mucosal inflammation,” Dr. Maldonado-Contreras said. SCFAs regulate the production of proinflammatory mediators such as cytokines (tumor necrosis factor–alpha and interleukin 2, 6, and 10), eicosanoids, and chemokines, such as MCP-1 and CINC-2, by acting on macrophages and endothelial cells. High levels of SCFAs down-regulate those proinflammatory mediators.
The study found IBD-AID favored a beneficial gut microbiota. Prebiotic foods such as oats, barley, beans, and tempeh correlated with beneficial counts of Bacteroides and Parabacteroides, both capable of producing SCFAs. Probiotic foods like yogurt, fermented cabbage, and kefir correlated with high levels of Clostridium bolteae, a bacterium that plays a critical role in regulatory T-cell induction. Vegetables and nuts correlated with an abundance of Roseburia hominis, Eubacterium rectale, and Faecalibacterium prausnitzii, which tend to be reduced in IBD patients and are potent butyrate-producing Clostridia with known anti-inflammatory activity. Declines in putative pathogenic strains, such as Escherichia, Alistipes, and Eggerthella accompanied the increase of SCFA-producing bacteria.
Among the study patients treated for at least 8 weeks, the 61.3% who achieved at least 50% dietary compliance reported a dramatic decrease of symptoms and disease severity.
Dr. Maldonado-Contreras explained the role P-gp has as a biomarker of gut microbiota. “P-gp is an ABC-transporter located in the apical side of intestinal epithelial cells and is responsible for suppressing neutrophil migration in healthy individuals,” she said. “Loss of P-gp expression, or a reduction in its function, correlates with inflammation in the gastrointestinal tract in both mice and humans.” The study compared P-gp expression before and after patients went on the IBD-AID diet.
Dr. Maldonado-Contreras credited the study’s reported diet compliance of 76% to adoption of the patient-centered counseling model (J Am Diet Assoc. 2001;101:332-41). “With the patient-centered counseling model, we aimed to build self-efficacy, self-management strategies and to provide cooking-skill abilities to promote long-term behavioral habits related to the IBD-AID,” she said. The IBD-AID recipes, menus, and tips are available online (https://www.umassmed.edu/nutrition/).
The Dr. Maldonado-Contreras along with researchers at Icahn School of Medicine at Mount Sinai in New York are further evaluating an adapted version of the IBD-AID diet in pregnancy in the MELODY trial. “We are evaluating whether adherence to the modified IBD-AID during pregnancy in women with Crohn’s disease could beneficially shift the microbiome of mom and their babies, thereby promoting a healthier immune system during a critical time of the baby’s immune system development,” Dr. Maldonado-Contreras said. The trial has recruited 50 patients with Crohn’s disease and healthy controls so far.
Dr. Maldonado-Contreras has no financial relationships to disclose.
AUSTIN, TEX. – A customized diet developed to relieve inflammatory bowel disease (IBD) symptoms without compromising nutrition has uncovered a novel molecular mechanism of the diet-microbiome immune interaction that may allow gastroenterologists to tailor patient diets to enhance the gut microbiome, according to a poster presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
The study found that P-glycoprotein (P-gp) expression, associated with healthy gut, increased after adoption of the IBD-Anti-Inflammatory Diet (IBD-AID), said poster presenter and study leader Ana Luisa Maldonado-Contreras, PhD, of the University of Massachusetts Medical School, Worcester. The study involved 19 IBD patients placed on the IBD-AID. This is reportedly the first evidence of a whole-dietary recommendation that may help patients with IBD to reduce their symptoms.
“The IBD-AID has been rationally designed to feed a health-promoting, anti-inflammatory microbiome aiming at reducing chronic inflammation” Dr. Maldonado-Contreras said in an interview. The UMass researchers, led by Barbara Olendzki, RD, MPH, director of the Center for Applied Nutrition, derived the IBD-AID diet from a specific carbohydrate diet and modified it based on their research to increase the diversity of bacteria that produce short-chain fatty acids (SCFAs) and modulate the local immune response.
“SCFAs, such as acetate, propionate, and butyrate, are crucial in maintaining intestinal homeostasis by fueling colonocytes, strengthening the gut barrier function, and controlling local mucosal inflammation,” Dr. Maldonado-Contreras said. SCFAs regulate the production of proinflammatory mediators such as cytokines (tumor necrosis factor–alpha and interleukin 2, 6, and 10), eicosanoids, and chemokines, such as MCP-1 and CINC-2, by acting on macrophages and endothelial cells. High levels of SCFAs down-regulate those proinflammatory mediators.
The study found IBD-AID favored a beneficial gut microbiota. Prebiotic foods such as oats, barley, beans, and tempeh correlated with beneficial counts of Bacteroides and Parabacteroides, both capable of producing SCFAs. Probiotic foods like yogurt, fermented cabbage, and kefir correlated with high levels of Clostridium bolteae, a bacterium that plays a critical role in regulatory T-cell induction. Vegetables and nuts correlated with an abundance of Roseburia hominis, Eubacterium rectale, and Faecalibacterium prausnitzii, which tend to be reduced in IBD patients and are potent butyrate-producing Clostridia with known anti-inflammatory activity. Declines in putative pathogenic strains, such as Escherichia, Alistipes, and Eggerthella accompanied the increase of SCFA-producing bacteria.
Among the study patients treated for at least 8 weeks, the 61.3% who achieved at least 50% dietary compliance reported a dramatic decrease of symptoms and disease severity.
Dr. Maldonado-Contreras explained the role P-gp has as a biomarker of gut microbiota. “P-gp is an ABC-transporter located in the apical side of intestinal epithelial cells and is responsible for suppressing neutrophil migration in healthy individuals,” she said. “Loss of P-gp expression, or a reduction in its function, correlates with inflammation in the gastrointestinal tract in both mice and humans.” The study compared P-gp expression before and after patients went on the IBD-AID diet.
Dr. Maldonado-Contreras credited the study’s reported diet compliance of 76% to adoption of the patient-centered counseling model (J Am Diet Assoc. 2001;101:332-41). “With the patient-centered counseling model, we aimed to build self-efficacy, self-management strategies and to provide cooking-skill abilities to promote long-term behavioral habits related to the IBD-AID,” she said. The IBD-AID recipes, menus, and tips are available online (https://www.umassmed.edu/nutrition/).
The Dr. Maldonado-Contreras along with researchers at Icahn School of Medicine at Mount Sinai in New York are further evaluating an adapted version of the IBD-AID diet in pregnancy in the MELODY trial. “We are evaluating whether adherence to the modified IBD-AID during pregnancy in women with Crohn’s disease could beneficially shift the microbiome of mom and their babies, thereby promoting a healthier immune system during a critical time of the baby’s immune system development,” Dr. Maldonado-Contreras said. The trial has recruited 50 patients with Crohn’s disease and healthy controls so far.
Dr. Maldonado-Contreras has no financial relationships to disclose.
AUSTIN, TEX. – A customized diet developed to relieve inflammatory bowel disease (IBD) symptoms without compromising nutrition has uncovered a novel molecular mechanism of the diet-microbiome immune interaction that may allow gastroenterologists to tailor patient diets to enhance the gut microbiome, according to a poster presented at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
The study found that P-glycoprotein (P-gp) expression, associated with healthy gut, increased after adoption of the IBD-Anti-Inflammatory Diet (IBD-AID), said poster presenter and study leader Ana Luisa Maldonado-Contreras, PhD, of the University of Massachusetts Medical School, Worcester. The study involved 19 IBD patients placed on the IBD-AID. This is reportedly the first evidence of a whole-dietary recommendation that may help patients with IBD to reduce their symptoms.
“The IBD-AID has been rationally designed to feed a health-promoting, anti-inflammatory microbiome aiming at reducing chronic inflammation” Dr. Maldonado-Contreras said in an interview. The UMass researchers, led by Barbara Olendzki, RD, MPH, director of the Center for Applied Nutrition, derived the IBD-AID diet from a specific carbohydrate diet and modified it based on their research to increase the diversity of bacteria that produce short-chain fatty acids (SCFAs) and modulate the local immune response.
“SCFAs, such as acetate, propionate, and butyrate, are crucial in maintaining intestinal homeostasis by fueling colonocytes, strengthening the gut barrier function, and controlling local mucosal inflammation,” Dr. Maldonado-Contreras said. SCFAs regulate the production of proinflammatory mediators such as cytokines (tumor necrosis factor–alpha and interleukin 2, 6, and 10), eicosanoids, and chemokines, such as MCP-1 and CINC-2, by acting on macrophages and endothelial cells. High levels of SCFAs down-regulate those proinflammatory mediators.
The study found IBD-AID favored a beneficial gut microbiota. Prebiotic foods such as oats, barley, beans, and tempeh correlated with beneficial counts of Bacteroides and Parabacteroides, both capable of producing SCFAs. Probiotic foods like yogurt, fermented cabbage, and kefir correlated with high levels of Clostridium bolteae, a bacterium that plays a critical role in regulatory T-cell induction. Vegetables and nuts correlated with an abundance of Roseburia hominis, Eubacterium rectale, and Faecalibacterium prausnitzii, which tend to be reduced in IBD patients and are potent butyrate-producing Clostridia with known anti-inflammatory activity. Declines in putative pathogenic strains, such as Escherichia, Alistipes, and Eggerthella accompanied the increase of SCFA-producing bacteria.
Among the study patients treated for at least 8 weeks, the 61.3% who achieved at least 50% dietary compliance reported a dramatic decrease of symptoms and disease severity.
Dr. Maldonado-Contreras explained the role P-gp has as a biomarker of gut microbiota. “P-gp is an ABC-transporter located in the apical side of intestinal epithelial cells and is responsible for suppressing neutrophil migration in healthy individuals,” she said. “Loss of P-gp expression, or a reduction in its function, correlates with inflammation in the gastrointestinal tract in both mice and humans.” The study compared P-gp expression before and after patients went on the IBD-AID diet.
Dr. Maldonado-Contreras credited the study’s reported diet compliance of 76% to adoption of the patient-centered counseling model (J Am Diet Assoc. 2001;101:332-41). “With the patient-centered counseling model, we aimed to build self-efficacy, self-management strategies and to provide cooking-skill abilities to promote long-term behavioral habits related to the IBD-AID,” she said. The IBD-AID recipes, menus, and tips are available online (https://www.umassmed.edu/nutrition/).
The Dr. Maldonado-Contreras along with researchers at Icahn School of Medicine at Mount Sinai in New York are further evaluating an adapted version of the IBD-AID diet in pregnancy in the MELODY trial. “We are evaluating whether adherence to the modified IBD-AID during pregnancy in women with Crohn’s disease could beneficially shift the microbiome of mom and their babies, thereby promoting a healthier immune system during a critical time of the baby’s immune system development,” Dr. Maldonado-Contreras said. The trial has recruited 50 patients with Crohn’s disease and healthy controls so far.
Dr. Maldonado-Contreras has no financial relationships to disclose.
REPORTING FROM CROHN’S & COLITIS CONGRESS
U.S. cancer centers embroiled in Chinese research thefts
Academic cancer centers around the United States continue to get caught up in an ever-evolving investigation into researchers – American and Chinese – who did not disclose payments from or the work they did for Chinese institutions while simultaneously accepting taxpayer money through U.S. government grants.
The U.S. Federal Bureau of Investigation has been ferreting out researchers it says have acted illegally.
On Jan. 28, the agency arrested Charles Lieber, a chemist from Harvard University, Cambridge, Mass., and also unveiled charges against Zheng Zaosong, a cancer researcher who is in the United States on a Harvard-sponsored visa.
The FBI said Mr. Zheng, who worked at the Harvard-affiliated Beth Israel Deaconess Medical Center, Boston, tried to smuggle 21 vials of biological material and research to China. Mr. Zheng was arrested in December at Boston’s Logan Airport. He admitted he planned to conduct and publish research in China using the stolen samples, said the FBI.
“All of the individuals charged today were either directly or indirectly working for the Chinese government, at our country’s expense,” said the agent in charge of the FBI’s Boston office, Joseph R. Bonavolonta.
Sen. Charles Grassley (R-IA), who has been pushing for more government action against foreign theft of U.S. research, said in a statement, “I’m glad the FBI appears to be taking foreign threats to taxpayer-funded research seriously, but I fear that this case is only the tip of the iceberg.”
The FBI said it is investigating China-related cases in all 50 states.
Ross McKinney, MD, the chief scientific officer at the Association of American Medical Colleges (AAMC), said he is aware of some 200 investigations, not all of which are cancer related, at 70-75 institutions.
“It’s a very ubiquitous problem,” Dr. McKinney said in an interview.
He also pointed out that some 6,000 National Institutes of Health–funded principal investigators are of Asian background. “So that 200 is a pretty small proportion,” said Dr. McKinney.
The NIH warned some 10,000 institutions in August 2018 that it had uncovered Chinese manipulation of peer review and a lack of disclosure of work for Chinese institutions. It urged the institutions to report irregularities.
For universities, “the trouble is sorting out who is the violator from who is not,” said Dr. McKinney. He noted that they are not set up to investigate whether someone has a laboratory in China.
“The fact that the Chinese government exploited the fact that universities are typically fairly trusting is extremely disappointing,” he said.
Moffitt story still unfolding
The most serious allegations have been leveled against six former employees of the Moffitt Cancer Center and Research Institute in Tampa, Florida.
In December 2019, Moffitt announced that the six – including President and CEO Alan List, MD, and the center director, Thomas Sellers, PhD – had left Moffitt as a result of “violations of conflict of interest rules through their work in China.”
New details have emerged, thanks to a new investigative report from a committee of the Florida House of Representatives.
The report said that Sheng Wei, a naturalized U.S. citizen who had worked at Moffitt since 2008 – when Moffitt began its affiliation with the Tianjin Medical University Cancer Institute and Hospital – was instrumental in recruiting top executives into the Thousand Talents program, which Wei had joined in 2010, according to the report. These executives included Dr. List, Dr. Sellers, and also Daniel Sullivan, head of Moffitt’s clinical science program, and cancer biologist Pearlie Epling-Burnette, it noted.
Begun in 2008, China’s Thousand Talents Plan gave salaries, funding, laboratory space, and other incentives to researchers who promised to bring U.S.-gained knowledge and research to China.
All information about this program has been removed from the Internet, but the program may still be active, Dr. McKinney commented.
According to the report, Dr. List pledged to work for the Tianjin cancer center 9 months a year for $71,000 annually. He was appointed head of the hematology department ($85,300 a year) in 2016. He opened a bank account in China to receive that salary and other Thousand Talents payments, the report found. The report notes that the exact amount Dr. List was paid is still not known.
Initially, Dr. Sellers, who was the principal investigator for Moffitt’s National Cancer Institute core grant, said he had not been involved in the Thousand Talents program. He later admitted that he had pledged to work in China 2 months a year for the program and that he’d opened a Chinese bank account and had deposited at least $35,000 into the account, the report notes.
The others pledged to work for the Thousand Talents program and also opened bank accounts in China and received money in those accounts.
Another Moffitt employee, Howard McLeod, MD, had worked for Thousand Talents before he joined Moffitt but did not disclose his China work. Dr. McLeod also supervised and had a close relationship with another researcher, Yijing (Bob) He, MD, who was employed by Moffitt but who lived in China, unbeknownst to Moffitt. “Dr. He appears to have functioned as an agent of Dr. McLeod in China,” said the report.
The report concluded that “none of the Moffitt faculty who were Talents program participants properly or timely disclosed their Talents program involvement to Moffitt, and none disclosed the full extent of their Talents program activities prior to Moffitt’s internal investigation.”
No charges have been filed against any of the former Moffitt employees.
However, the Cancer Letter has reported that Dr. Sellers is claiming he was not involved in the program and that he is preparing to sue Moffitt.
AAMC’s Dr. McKinney notes that it is illegal for researchers to take U.S. government grant money and pledge a certain amount of time but not deliver on that commitment because they are working for someone else – in this case, China. They also lied about not having any other research support, which is also illegal, he said.
The researchers received Chinese money and deposited it in Chinese accounts, which was never reported to the U.S. Internal Revenue Service.
“One of the hallmarks of the Chinese recruitment program was that people were instructed to not tell their normal U.S. host institution and not tell any U.S. government agency about their relationship with China,” Dr. McKinney said. “It was creating a culture where dishonesty in this situation was norm,” he added.
The lack of honesty brings up bigger questions for the field, he said. “Once you start lying about one thing, do you lie about your science, too?”
Lack of oversight?
Dr. McKinney said the NIH, as well as universities and hospitals, had a long and trusting relationship with China and should not be blamed for falling prey to the Chinese government’s concerted effort to steal intellectual property.
But some government watchdog groups have chided the NIH for lax oversight. In February 2019, the federal Health & Human Services’ Office of Inspector General found that “NIH has not assessed the risks to national security when permitting data access to foreign [principal investigators].”
Federal investigators have said that Thousand Talents has been one of the biggest threats.
The U.S. Senate Permanent Subcommittee on Investigations reported in November 2019 that “the federal government’s grant-making agencies did little to prevent this from happening, nor did the FBI and other federal agencies develop a coordinated response to mitigate the threat.”
The NIH invests $31 billion a year in medical research through 50,000 competitive grants to more than 300,000 researchers, according to that report. Even after uncovering grant fraud and peer-review manipulation that benefited China, “significant gaps in NIH’s grant integrity process remain,” the report states. Site visits by the NIH’s Division of Grants Compliance and Oversight dropped from 28 in 2012 to just 3 in 2018, the report noted.
Widening dragnet
In April 2019, Science reported that the NIH identified five researchers at MD Anderson Cancer Center in Houston who had failed to disclose their ties to Chinese enterprises and who had failed to keep peer review confidential.
Two resigned before they could be fired, one was fired, another eventually left the institution, and the fifth was found to have not willfully engaged in subterfuge.
Just a month later, Emory University in Atlanta announced that it had fired a husband and wife research team. The neuroscientists were known for their studies of Huntington disease. Both were U.S. citizens and had worked at Emory for more than 2 decades, according to the Science report.
The Moffitt situation led to the Florida legislature’s investigation, and also prompted some soul searching. The Tampa Bay Times reported that U.S. Senator Rick Scott (R-FL) asked state universities to provide information on what they are doing to stop foreign influence. The University of Florida then acknowledged that four faculty members resigned or were terminated because of ties to a foreign recruitment program.
This article first appeared on Medscape.com.
Academic cancer centers around the United States continue to get caught up in an ever-evolving investigation into researchers – American and Chinese – who did not disclose payments from or the work they did for Chinese institutions while simultaneously accepting taxpayer money through U.S. government grants.
The U.S. Federal Bureau of Investigation has been ferreting out researchers it says have acted illegally.
On Jan. 28, the agency arrested Charles Lieber, a chemist from Harvard University, Cambridge, Mass., and also unveiled charges against Zheng Zaosong, a cancer researcher who is in the United States on a Harvard-sponsored visa.
The FBI said Mr. Zheng, who worked at the Harvard-affiliated Beth Israel Deaconess Medical Center, Boston, tried to smuggle 21 vials of biological material and research to China. Mr. Zheng was arrested in December at Boston’s Logan Airport. He admitted he planned to conduct and publish research in China using the stolen samples, said the FBI.
“All of the individuals charged today were either directly or indirectly working for the Chinese government, at our country’s expense,” said the agent in charge of the FBI’s Boston office, Joseph R. Bonavolonta.
Sen. Charles Grassley (R-IA), who has been pushing for more government action against foreign theft of U.S. research, said in a statement, “I’m glad the FBI appears to be taking foreign threats to taxpayer-funded research seriously, but I fear that this case is only the tip of the iceberg.”
The FBI said it is investigating China-related cases in all 50 states.
Ross McKinney, MD, the chief scientific officer at the Association of American Medical Colleges (AAMC), said he is aware of some 200 investigations, not all of which are cancer related, at 70-75 institutions.
“It’s a very ubiquitous problem,” Dr. McKinney said in an interview.
He also pointed out that some 6,000 National Institutes of Health–funded principal investigators are of Asian background. “So that 200 is a pretty small proportion,” said Dr. McKinney.
The NIH warned some 10,000 institutions in August 2018 that it had uncovered Chinese manipulation of peer review and a lack of disclosure of work for Chinese institutions. It urged the institutions to report irregularities.
For universities, “the trouble is sorting out who is the violator from who is not,” said Dr. McKinney. He noted that they are not set up to investigate whether someone has a laboratory in China.
“The fact that the Chinese government exploited the fact that universities are typically fairly trusting is extremely disappointing,” he said.
Moffitt story still unfolding
The most serious allegations have been leveled against six former employees of the Moffitt Cancer Center and Research Institute in Tampa, Florida.
In December 2019, Moffitt announced that the six – including President and CEO Alan List, MD, and the center director, Thomas Sellers, PhD – had left Moffitt as a result of “violations of conflict of interest rules through their work in China.”
New details have emerged, thanks to a new investigative report from a committee of the Florida House of Representatives.
The report said that Sheng Wei, a naturalized U.S. citizen who had worked at Moffitt since 2008 – when Moffitt began its affiliation with the Tianjin Medical University Cancer Institute and Hospital – was instrumental in recruiting top executives into the Thousand Talents program, which Wei had joined in 2010, according to the report. These executives included Dr. List, Dr. Sellers, and also Daniel Sullivan, head of Moffitt’s clinical science program, and cancer biologist Pearlie Epling-Burnette, it noted.
Begun in 2008, China’s Thousand Talents Plan gave salaries, funding, laboratory space, and other incentives to researchers who promised to bring U.S.-gained knowledge and research to China.
All information about this program has been removed from the Internet, but the program may still be active, Dr. McKinney commented.
According to the report, Dr. List pledged to work for the Tianjin cancer center 9 months a year for $71,000 annually. He was appointed head of the hematology department ($85,300 a year) in 2016. He opened a bank account in China to receive that salary and other Thousand Talents payments, the report found. The report notes that the exact amount Dr. List was paid is still not known.
Initially, Dr. Sellers, who was the principal investigator for Moffitt’s National Cancer Institute core grant, said he had not been involved in the Thousand Talents program. He later admitted that he had pledged to work in China 2 months a year for the program and that he’d opened a Chinese bank account and had deposited at least $35,000 into the account, the report notes.
The others pledged to work for the Thousand Talents program and also opened bank accounts in China and received money in those accounts.
Another Moffitt employee, Howard McLeod, MD, had worked for Thousand Talents before he joined Moffitt but did not disclose his China work. Dr. McLeod also supervised and had a close relationship with another researcher, Yijing (Bob) He, MD, who was employed by Moffitt but who lived in China, unbeknownst to Moffitt. “Dr. He appears to have functioned as an agent of Dr. McLeod in China,” said the report.
The report concluded that “none of the Moffitt faculty who were Talents program participants properly or timely disclosed their Talents program involvement to Moffitt, and none disclosed the full extent of their Talents program activities prior to Moffitt’s internal investigation.”
No charges have been filed against any of the former Moffitt employees.
However, the Cancer Letter has reported that Dr. Sellers is claiming he was not involved in the program and that he is preparing to sue Moffitt.
AAMC’s Dr. McKinney notes that it is illegal for researchers to take U.S. government grant money and pledge a certain amount of time but not deliver on that commitment because they are working for someone else – in this case, China. They also lied about not having any other research support, which is also illegal, he said.
The researchers received Chinese money and deposited it in Chinese accounts, which was never reported to the U.S. Internal Revenue Service.
“One of the hallmarks of the Chinese recruitment program was that people were instructed to not tell their normal U.S. host institution and not tell any U.S. government agency about their relationship with China,” Dr. McKinney said. “It was creating a culture where dishonesty in this situation was norm,” he added.
The lack of honesty brings up bigger questions for the field, he said. “Once you start lying about one thing, do you lie about your science, too?”
Lack of oversight?
Dr. McKinney said the NIH, as well as universities and hospitals, had a long and trusting relationship with China and should not be blamed for falling prey to the Chinese government’s concerted effort to steal intellectual property.
But some government watchdog groups have chided the NIH for lax oversight. In February 2019, the federal Health & Human Services’ Office of Inspector General found that “NIH has not assessed the risks to national security when permitting data access to foreign [principal investigators].”
Federal investigators have said that Thousand Talents has been one of the biggest threats.
The U.S. Senate Permanent Subcommittee on Investigations reported in November 2019 that “the federal government’s grant-making agencies did little to prevent this from happening, nor did the FBI and other federal agencies develop a coordinated response to mitigate the threat.”
The NIH invests $31 billion a year in medical research through 50,000 competitive grants to more than 300,000 researchers, according to that report. Even after uncovering grant fraud and peer-review manipulation that benefited China, “significant gaps in NIH’s grant integrity process remain,” the report states. Site visits by the NIH’s Division of Grants Compliance and Oversight dropped from 28 in 2012 to just 3 in 2018, the report noted.
Widening dragnet
In April 2019, Science reported that the NIH identified five researchers at MD Anderson Cancer Center in Houston who had failed to disclose their ties to Chinese enterprises and who had failed to keep peer review confidential.
Two resigned before they could be fired, one was fired, another eventually left the institution, and the fifth was found to have not willfully engaged in subterfuge.
Just a month later, Emory University in Atlanta announced that it had fired a husband and wife research team. The neuroscientists were known for their studies of Huntington disease. Both were U.S. citizens and had worked at Emory for more than 2 decades, according to the Science report.
The Moffitt situation led to the Florida legislature’s investigation, and also prompted some soul searching. The Tampa Bay Times reported that U.S. Senator Rick Scott (R-FL) asked state universities to provide information on what they are doing to stop foreign influence. The University of Florida then acknowledged that four faculty members resigned or were terminated because of ties to a foreign recruitment program.
This article first appeared on Medscape.com.
Academic cancer centers around the United States continue to get caught up in an ever-evolving investigation into researchers – American and Chinese – who did not disclose payments from or the work they did for Chinese institutions while simultaneously accepting taxpayer money through U.S. government grants.
The U.S. Federal Bureau of Investigation has been ferreting out researchers it says have acted illegally.
On Jan. 28, the agency arrested Charles Lieber, a chemist from Harvard University, Cambridge, Mass., and also unveiled charges against Zheng Zaosong, a cancer researcher who is in the United States on a Harvard-sponsored visa.
The FBI said Mr. Zheng, who worked at the Harvard-affiliated Beth Israel Deaconess Medical Center, Boston, tried to smuggle 21 vials of biological material and research to China. Mr. Zheng was arrested in December at Boston’s Logan Airport. He admitted he planned to conduct and publish research in China using the stolen samples, said the FBI.
“All of the individuals charged today were either directly or indirectly working for the Chinese government, at our country’s expense,” said the agent in charge of the FBI’s Boston office, Joseph R. Bonavolonta.
Sen. Charles Grassley (R-IA), who has been pushing for more government action against foreign theft of U.S. research, said in a statement, “I’m glad the FBI appears to be taking foreign threats to taxpayer-funded research seriously, but I fear that this case is only the tip of the iceberg.”
The FBI said it is investigating China-related cases in all 50 states.
Ross McKinney, MD, the chief scientific officer at the Association of American Medical Colleges (AAMC), said he is aware of some 200 investigations, not all of which are cancer related, at 70-75 institutions.
“It’s a very ubiquitous problem,” Dr. McKinney said in an interview.
He also pointed out that some 6,000 National Institutes of Health–funded principal investigators are of Asian background. “So that 200 is a pretty small proportion,” said Dr. McKinney.
The NIH warned some 10,000 institutions in August 2018 that it had uncovered Chinese manipulation of peer review and a lack of disclosure of work for Chinese institutions. It urged the institutions to report irregularities.
For universities, “the trouble is sorting out who is the violator from who is not,” said Dr. McKinney. He noted that they are not set up to investigate whether someone has a laboratory in China.
“The fact that the Chinese government exploited the fact that universities are typically fairly trusting is extremely disappointing,” he said.
Moffitt story still unfolding
The most serious allegations have been leveled against six former employees of the Moffitt Cancer Center and Research Institute in Tampa, Florida.
In December 2019, Moffitt announced that the six – including President and CEO Alan List, MD, and the center director, Thomas Sellers, PhD – had left Moffitt as a result of “violations of conflict of interest rules through their work in China.”
New details have emerged, thanks to a new investigative report from a committee of the Florida House of Representatives.
The report said that Sheng Wei, a naturalized U.S. citizen who had worked at Moffitt since 2008 – when Moffitt began its affiliation with the Tianjin Medical University Cancer Institute and Hospital – was instrumental in recruiting top executives into the Thousand Talents program, which Wei had joined in 2010, according to the report. These executives included Dr. List, Dr. Sellers, and also Daniel Sullivan, head of Moffitt’s clinical science program, and cancer biologist Pearlie Epling-Burnette, it noted.
Begun in 2008, China’s Thousand Talents Plan gave salaries, funding, laboratory space, and other incentives to researchers who promised to bring U.S.-gained knowledge and research to China.
All information about this program has been removed from the Internet, but the program may still be active, Dr. McKinney commented.
According to the report, Dr. List pledged to work for the Tianjin cancer center 9 months a year for $71,000 annually. He was appointed head of the hematology department ($85,300 a year) in 2016. He opened a bank account in China to receive that salary and other Thousand Talents payments, the report found. The report notes that the exact amount Dr. List was paid is still not known.
Initially, Dr. Sellers, who was the principal investigator for Moffitt’s National Cancer Institute core grant, said he had not been involved in the Thousand Talents program. He later admitted that he had pledged to work in China 2 months a year for the program and that he’d opened a Chinese bank account and had deposited at least $35,000 into the account, the report notes.
The others pledged to work for the Thousand Talents program and also opened bank accounts in China and received money in those accounts.
Another Moffitt employee, Howard McLeod, MD, had worked for Thousand Talents before he joined Moffitt but did not disclose his China work. Dr. McLeod also supervised and had a close relationship with another researcher, Yijing (Bob) He, MD, who was employed by Moffitt but who lived in China, unbeknownst to Moffitt. “Dr. He appears to have functioned as an agent of Dr. McLeod in China,” said the report.
The report concluded that “none of the Moffitt faculty who were Talents program participants properly or timely disclosed their Talents program involvement to Moffitt, and none disclosed the full extent of their Talents program activities prior to Moffitt’s internal investigation.”
No charges have been filed against any of the former Moffitt employees.
However, the Cancer Letter has reported that Dr. Sellers is claiming he was not involved in the program and that he is preparing to sue Moffitt.
AAMC’s Dr. McKinney notes that it is illegal for researchers to take U.S. government grant money and pledge a certain amount of time but not deliver on that commitment because they are working for someone else – in this case, China. They also lied about not having any other research support, which is also illegal, he said.
The researchers received Chinese money and deposited it in Chinese accounts, which was never reported to the U.S. Internal Revenue Service.
“One of the hallmarks of the Chinese recruitment program was that people were instructed to not tell their normal U.S. host institution and not tell any U.S. government agency about their relationship with China,” Dr. McKinney said. “It was creating a culture where dishonesty in this situation was norm,” he added.
The lack of honesty brings up bigger questions for the field, he said. “Once you start lying about one thing, do you lie about your science, too?”
Lack of oversight?
Dr. McKinney said the NIH, as well as universities and hospitals, had a long and trusting relationship with China and should not be blamed for falling prey to the Chinese government’s concerted effort to steal intellectual property.
But some government watchdog groups have chided the NIH for lax oversight. In February 2019, the federal Health & Human Services’ Office of Inspector General found that “NIH has not assessed the risks to national security when permitting data access to foreign [principal investigators].”
Federal investigators have said that Thousand Talents has been one of the biggest threats.
The U.S. Senate Permanent Subcommittee on Investigations reported in November 2019 that “the federal government’s grant-making agencies did little to prevent this from happening, nor did the FBI and other federal agencies develop a coordinated response to mitigate the threat.”
The NIH invests $31 billion a year in medical research through 50,000 competitive grants to more than 300,000 researchers, according to that report. Even after uncovering grant fraud and peer-review manipulation that benefited China, “significant gaps in NIH’s grant integrity process remain,” the report states. Site visits by the NIH’s Division of Grants Compliance and Oversight dropped from 28 in 2012 to just 3 in 2018, the report noted.
Widening dragnet
In April 2019, Science reported that the NIH identified five researchers at MD Anderson Cancer Center in Houston who had failed to disclose their ties to Chinese enterprises and who had failed to keep peer review confidential.
Two resigned before they could be fired, one was fired, another eventually left the institution, and the fifth was found to have not willfully engaged in subterfuge.
Just a month later, Emory University in Atlanta announced that it had fired a husband and wife research team. The neuroscientists were known for their studies of Huntington disease. Both were U.S. citizens and had worked at Emory for more than 2 decades, according to the Science report.
The Moffitt situation led to the Florida legislature’s investigation, and also prompted some soul searching. The Tampa Bay Times reported that U.S. Senator Rick Scott (R-FL) asked state universities to provide information on what they are doing to stop foreign influence. The University of Florida then acknowledged that four faculty members resigned or were terminated because of ties to a foreign recruitment program.
This article first appeared on Medscape.com.
Halobetasol Propionate for the Management of Psoriasis
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).
This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).

This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).


Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).

Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20

HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21

Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).

This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).


Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).

Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20

HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21

Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
Practice Points
- The widespread use of superpotent topical corticosteroids in treating psoriasis is limited by labelling that restricts short-term use, concerns about side effects, and a paucity of clinical data with longer-term use.
- Long-term management and treatment options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life.
- A novel formulation of halobetasol propionate lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets.