User login
Making a difference
Hospitalists engaging in advocacy efforts
Hospitalists around the country are devoting large portions of their spare time to a wide range of advocacy efforts. From health policy to caring for the unhoused population to diversity and equity to advocating for fellow hospitalists, these physicians are passionate about their causes and determined to make a difference.
Championing the unhoused
Sarah Stella, MD, FHM, a hospitalist at Denver Health, was initially drawn there because of the population the hospital serves, which includes a high concentration of people experiencing homelessness. As she cared for her patients, Dr. Stella, who is also associate professor of hospital medicine at the University of Colorado, increasingly felt the desire to help prevent the negative downstream outcomes the hospital sees.
To understand the experiences of the unhoused outside the hospital, Dr. Stella started talking to her patients and people in community-based organizations that serve this population. “I learned a ton,” she said. “Homelessness feels like such an intractable, hopeless thing, but the more I talked to people, the more opportunities I saw to work toward something better.”
This led to a pilot grant to work with the Colorado Coalition for the Homeless to set up a community advisory panel. “My goal was to better understand their experiences and to develop a shared vision for how we collectively can do better,” said Dr. Stella. Eventually, she also received a grant from the University of Colorado, and multiple opportunities have sprung up ever since.
For the past several years, Dr. Stella has worked with Denver Health leadership to improve care for the homeless. “Right now, I’m working with a community team on developing an idea to provide peer support from people with a shared lived experience for people who are experiencing homelessness when they’re hospitalized. That’s really where my passion has been in working on the partnership,” she said.
Her advocacy role has been beneficial in her work as a hospitalist, particularly when COVID began. Dr. Stella again partnered with the Colorado Coalition for the Homeless to start a joint task force. “Everyone on our task force is motivated by this powerful desire to improve the health and lives of this community and that’s one of the silver linings in this pandemic for me,” said Dr. Stella.
Advocacy work has also increased Dr. Stella’s knowledge of what community support options are available for the unhoused. This allows her to educate her patients about their options and how to access them.
While she has colleagues who are able to compartmentalize their work, “I absolutely could not be a hospitalist without being an advocate,” Dr. Stella said. “For me, it has been a protective strategy in terms of burnout because I have to feel like I’m working to advocate for better policies and more appropriate resources to address the gaps that I’m seeing.”
Dr. Stella believes that physicians have a special credibility to advocate, tell stories, and use data to back their stories up. “We have to realize that we have this power, and we have it so we can empower others,” she said. “The people I’ve seen in my community who are working so hard to help people who are experiencing homelessness are the heroes. Understanding that and giving power to those people through our voice and our well-respected place in society drives me.”
Strengthening diversity, equity, and inclusion
In September 2020, Michael Bryant, MD, became the inaugural vice chair of Diversity, Equity, and Inclusion for the department of pediatrics at Children’s Hospital Los Angeles, where he is also the division head of pediatric hospital medicine. “I was motivated to apply for this position because I wanted to be an agent for change to eliminate the institutional racism, social injustice, and marginalization that continues to threaten the lives and well-beings of so many Americans,” Dr. Bryant said.
Between the pandemic, the economic decline it has created, and the divisive political landscape, people of color have been especially affected. “These are poignant examples of the ever-widening divide and disenfranchisement many Americans feel,” said Dr. Bryant. “Gandhi said, ‘Be the change that you want to see,’ and that is what I want to model.”
At work, advocacy for diversity, equality, and inclusion is an innate part of everything he does. From the new physicians he recruits to the candidates he considers for leadership positions, Dr. Bryant strives “to have a workforce that mirrors the diversity of the patients we humbly care for and serve.”
Advocacy is intrinsic to Dr. Bryant’s worldview, in his quest to understand and accept each individual’s uniqueness, his desire “to embrace cultural humility,” his recognition that “our differences enhance us instead of diminishing us,” and his willingness to engage in difficult conversations.
“Advocacy means that I acknowledge that intent does not equal impact and that I must accept that what I do and what I say may have unintended consequences,” he said. “When that happens, I must resist becoming defensive and instead be willing to listen and learn.”
Dr. Bryant is proud of his accomplishments and enjoys his advocacy work. In his workplace, there are few African Americans in leadership roles. This means that he is in high demand when it comes to making sure there’s representation during various processes such as hiring and vetting, a disparity known as the “minority tax.”
“I am thankful for the opportunities, but it does take a toll at times,” Dr. Bryant said, which is yet another reason why he is a proponent of increasing diversity and inclusion. “This allows us to build the resource pool as these needs arise and minimizes the toll of the ‘minority tax’ on any single person or small group of individuals.”
This summer, physicians from Dr. Bryant’s hospital participated in the national “White Coats for Black Lives” effort. He found it to be “an incredibly moving event” that hundreds of his colleagues participated in.
Dr. Bryant’s advice for hospitalists who want to get involved in advocacy efforts is to check out the movie “John Lewis: Good Trouble.” “He was a champion of human rights and fought for these rights until his death,” Dr. Bryant said. “He is a true American hero and a wonderful example.”
Bolstering health care change
Since his residency, Joshua Lenchus, DO, FACP, SFHM, has developed an ever-increasing interest in legislative advocacy, particularly health policy. Getting involved in this arena requires an understanding of civics and government that goes beyond just the basics. “My desire to affect change in my own profession really served as the catalyst to get involved,” said Dr. Lenchus, the regional chief medical officer at Broward Health Medical Center in Fort Lauderdale, Fla. “What better way to do that than by combining what we do on a daily basis in the practice of medicine with this new understanding of how laws are passed and promulgated?”
Dr. Lenchus has been involved with both state and national medical organizations and has served on public policy committees as a member and as a chair. “The charge of these committees is to monitor and navigate position statements and policies that will drive the entire organization,” he said. This means becoming knowledgeable enough about a topic to be able to talk about it eloquently and adding supporting personal or professional illustrations that reinforce the position to lawmakers.
He finds his advocacy efforts “incredibly rewarding” because they contribute to his endeavors “to help my colleagues practice medicine in a safe, efficient, and productive manner.” For instance, some of the organizations Dr. Lenchus was involved with helped make changes to the Affordable Care Act that ended up in its final version, as well as changes after it passed. “There are tangible things that advocacy enables us to do in our daily practice,” he said.
When something his organizations have advocated for does not pass, they know they need to try a different outlet. “You can’t win every fight,” he said. “Every time you go and comment on an issue, you have to understand that you’re there to do your best, and to the extent that the people you’re talking to are willing to listen to what you have to say, that’s where I think you can make the most impact.” When changes he has helped fight for do pass, “it really is amazing that you can tell your colleagues about your role in achieving meaningful change in the profession.”
Dr. Lenchus acknowledges that advocacy “can be all-consuming at times. We have to understand our limits.” That said, he thinks not engaging in advocacy could increase stress and potential burnout. “I think being involved in advocacy efforts really helps people conduct meaningful work and educates them about what it means not just to them, but to the rest of the medical profession and the patients that we serve,” he said.
For hospitalists who are interested in health policy advocacy, there are many ways to get involved, Dr. Lenchus said. You could join an organization (many organized medical societies have public policy committees), participate in advocacy activities, work on a political campaign, or even run for office yourself. “Ultimately, education and some level of involvement really will make the difference in who navigates our future as hospitalists,” he said.
Questioning co-management practices
Though he says he’s in the minority, Hardik Vora, MD, SFHM, medical director for hospital medicine at Riverside Regional Medical Center in Newport News, Va., believes that co-management is going to “make or break hospital medicine. It’s going to have a huge impact on our specialty.”
In the roughly 25-year history of hospital medicine, it has evolved from admitting and caring for patients of primary care physicians to patients of specialists and, more recently, surgical patients. “Now there are (hospital medicine) programs across the country that are pretty much admitting everything,” said Dr. Vora.
As a recruiter for the Riverside Health System for the past eight years, “I have not met a single resident who is trained to do what we’re doing in hospital medicine, because you’re admitting surgical patients all the time and you have primary attending responsibility,” Dr. Vora said. “I see that as a cause of a significant amount of stress because now you’re responsible for something that you don’t have adequate training for.”
In the co-management discussion, Dr. Vora notes that people often bring up the research that shows that the practice has improved surgeon satisfaction. “What bothers me is that…you need to add one more question – how does it affect your hospitalists? And I bet the answer to that question is ‘it has a terrible effect.’”
The expectations surrounding hospitalists these days is a big concern in terms of burnout, Dr. Vora said. “We talk a lot about the drivers of burnout, whether it’s schedule or COVID,” he said. The biggest issue when it comes to burnout, as he sees it, is not COVID; it’s when hospitalists are performing tasks that make them feel they aren’t adding value. “I think that’s a huge topic in hospital medicine right now.”
Dr. Vora believes there should be more discussion and awareness of the potential pitfalls. “Hospitalists should get involved in co-management where they are adding value and certainly not take up the attending responsibility where they’re not adding value and it’s out of the scope of their training and expertise,” he said. “Preventing scope creep and burnout from co-management are some of the key issues I’m really passionate about.”
Dr. Vora said it is important to set realistic goals and remember that it takes time to make change when it comes to advocacy. “You still have to operate within whatever environment is given to you and then you can make change from within,” he said.
His enthusiasm for co-management awareness has led to creating a co-management forum through SHM in his local Hampton Roads chapter. He was also a panelist for an SHM webinar in February 2021 in which the panelists debated co-management.
“I think we really need to look at this as a specialty. Are we going in the right direction?” Dr. Vora asked. “We need to come together as a specialty and make a decision, which is going to be hard because there are competing financial interests and various practice models.”
Improving patient care
Working as a hospitalist at University Medical Center, a safety net hospital in New Orleans, Celeste Newby, MD, PhD, sees plenty of patients who are underinsured or not insured at all. “A lot of my interest in health policy stems from that,” she said.
During her residency, which she finished in 2015, Louisiana became a Medicaid expansion state. This impressed upon Dr. Newby how much Medicaid improved the lives of patients who had previously been uninsured. “We saw procedures getting done that had been put on hold because of financial concerns or medicines that were now affordable that weren’t before,” she said. “It really did make a difference.”
When repeated attempts to repeal the Affordable Care Act began, “it was a call to do health policy work for me personally that just hadn’t come up in the past,” said Dr. Newby, who is also assistant professor of medicine at Tulane University in New Orleans. “I personally found that the best way to do (advocacy work) was to go through medical societies because there is a much stronger voice when you have more people saying the same thing,” she said.
Dr. Newby sits on the Council of Legislation for the Louisiana State Medical Society and participates in the Leadership and Health Policy (LEAHP) Program through the Society of General Internal Medicine.
The LEAHP Program has been instrumental in expanding Dr. Newby’s knowledge of how health policy is made and the mechanisms behind it. It has also taught her “how we can either advise, guide, leverage, or advocate for things that we think would be important for change and moving the country in the right direction in terms of health care.”
Another reason involvement in medical societies is helpful is because, as a busy clinician, it is impossible to keep up with everything. “Working with medical societies, you have people who are more directly involved in the legislature and can give you quicker notice about things that are coming up that are going to be important to you or your co-workers or your patients,” Dr. Newby said.
Dr. Newby feels her advocacy work is an outlet for stress and “a way to work at more of a macro level on problems that I see with my individual patients. It’s a nice compliment.” At the hospital, she can only help one person at a time, but with her advocacy efforts, there’s potential to make changes for many.
“Advocacy now is such a large umbrella that encompasses so many different projects at all kinds of levels,” Dr. Newby said. She suggests looking around your community to see where the needs lie. If you’re passionate about a certain topic or population, see what you can do to help advocate for change there.
Hospitalists engaging in advocacy efforts
Hospitalists engaging in advocacy efforts
Hospitalists around the country are devoting large portions of their spare time to a wide range of advocacy efforts. From health policy to caring for the unhoused population to diversity and equity to advocating for fellow hospitalists, these physicians are passionate about their causes and determined to make a difference.
Championing the unhoused
Sarah Stella, MD, FHM, a hospitalist at Denver Health, was initially drawn there because of the population the hospital serves, which includes a high concentration of people experiencing homelessness. As she cared for her patients, Dr. Stella, who is also associate professor of hospital medicine at the University of Colorado, increasingly felt the desire to help prevent the negative downstream outcomes the hospital sees.
To understand the experiences of the unhoused outside the hospital, Dr. Stella started talking to her patients and people in community-based organizations that serve this population. “I learned a ton,” she said. “Homelessness feels like such an intractable, hopeless thing, but the more I talked to people, the more opportunities I saw to work toward something better.”
This led to a pilot grant to work with the Colorado Coalition for the Homeless to set up a community advisory panel. “My goal was to better understand their experiences and to develop a shared vision for how we collectively can do better,” said Dr. Stella. Eventually, she also received a grant from the University of Colorado, and multiple opportunities have sprung up ever since.
For the past several years, Dr. Stella has worked with Denver Health leadership to improve care for the homeless. “Right now, I’m working with a community team on developing an idea to provide peer support from people with a shared lived experience for people who are experiencing homelessness when they’re hospitalized. That’s really where my passion has been in working on the partnership,” she said.
Her advocacy role has been beneficial in her work as a hospitalist, particularly when COVID began. Dr. Stella again partnered with the Colorado Coalition for the Homeless to start a joint task force. “Everyone on our task force is motivated by this powerful desire to improve the health and lives of this community and that’s one of the silver linings in this pandemic for me,” said Dr. Stella.
Advocacy work has also increased Dr. Stella’s knowledge of what community support options are available for the unhoused. This allows her to educate her patients about their options and how to access them.
While she has colleagues who are able to compartmentalize their work, “I absolutely could not be a hospitalist without being an advocate,” Dr. Stella said. “For me, it has been a protective strategy in terms of burnout because I have to feel like I’m working to advocate for better policies and more appropriate resources to address the gaps that I’m seeing.”
Dr. Stella believes that physicians have a special credibility to advocate, tell stories, and use data to back their stories up. “We have to realize that we have this power, and we have it so we can empower others,” she said. “The people I’ve seen in my community who are working so hard to help people who are experiencing homelessness are the heroes. Understanding that and giving power to those people through our voice and our well-respected place in society drives me.”
Strengthening diversity, equity, and inclusion
In September 2020, Michael Bryant, MD, became the inaugural vice chair of Diversity, Equity, and Inclusion for the department of pediatrics at Children’s Hospital Los Angeles, where he is also the division head of pediatric hospital medicine. “I was motivated to apply for this position because I wanted to be an agent for change to eliminate the institutional racism, social injustice, and marginalization that continues to threaten the lives and well-beings of so many Americans,” Dr. Bryant said.
Between the pandemic, the economic decline it has created, and the divisive political landscape, people of color have been especially affected. “These are poignant examples of the ever-widening divide and disenfranchisement many Americans feel,” said Dr. Bryant. “Gandhi said, ‘Be the change that you want to see,’ and that is what I want to model.”
At work, advocacy for diversity, equality, and inclusion is an innate part of everything he does. From the new physicians he recruits to the candidates he considers for leadership positions, Dr. Bryant strives “to have a workforce that mirrors the diversity of the patients we humbly care for and serve.”
Advocacy is intrinsic to Dr. Bryant’s worldview, in his quest to understand and accept each individual’s uniqueness, his desire “to embrace cultural humility,” his recognition that “our differences enhance us instead of diminishing us,” and his willingness to engage in difficult conversations.
“Advocacy means that I acknowledge that intent does not equal impact and that I must accept that what I do and what I say may have unintended consequences,” he said. “When that happens, I must resist becoming defensive and instead be willing to listen and learn.”
Dr. Bryant is proud of his accomplishments and enjoys his advocacy work. In his workplace, there are few African Americans in leadership roles. This means that he is in high demand when it comes to making sure there’s representation during various processes such as hiring and vetting, a disparity known as the “minority tax.”
“I am thankful for the opportunities, but it does take a toll at times,” Dr. Bryant said, which is yet another reason why he is a proponent of increasing diversity and inclusion. “This allows us to build the resource pool as these needs arise and minimizes the toll of the ‘minority tax’ on any single person or small group of individuals.”
This summer, physicians from Dr. Bryant’s hospital participated in the national “White Coats for Black Lives” effort. He found it to be “an incredibly moving event” that hundreds of his colleagues participated in.
Dr. Bryant’s advice for hospitalists who want to get involved in advocacy efforts is to check out the movie “John Lewis: Good Trouble.” “He was a champion of human rights and fought for these rights until his death,” Dr. Bryant said. “He is a true American hero and a wonderful example.”
Bolstering health care change
Since his residency, Joshua Lenchus, DO, FACP, SFHM, has developed an ever-increasing interest in legislative advocacy, particularly health policy. Getting involved in this arena requires an understanding of civics and government that goes beyond just the basics. “My desire to affect change in my own profession really served as the catalyst to get involved,” said Dr. Lenchus, the regional chief medical officer at Broward Health Medical Center in Fort Lauderdale, Fla. “What better way to do that than by combining what we do on a daily basis in the practice of medicine with this new understanding of how laws are passed and promulgated?”
Dr. Lenchus has been involved with both state and national medical organizations and has served on public policy committees as a member and as a chair. “The charge of these committees is to monitor and navigate position statements and policies that will drive the entire organization,” he said. This means becoming knowledgeable enough about a topic to be able to talk about it eloquently and adding supporting personal or professional illustrations that reinforce the position to lawmakers.
He finds his advocacy efforts “incredibly rewarding” because they contribute to his endeavors “to help my colleagues practice medicine in a safe, efficient, and productive manner.” For instance, some of the organizations Dr. Lenchus was involved with helped make changes to the Affordable Care Act that ended up in its final version, as well as changes after it passed. “There are tangible things that advocacy enables us to do in our daily practice,” he said.
When something his organizations have advocated for does not pass, they know they need to try a different outlet. “You can’t win every fight,” he said. “Every time you go and comment on an issue, you have to understand that you’re there to do your best, and to the extent that the people you’re talking to are willing to listen to what you have to say, that’s where I think you can make the most impact.” When changes he has helped fight for do pass, “it really is amazing that you can tell your colleagues about your role in achieving meaningful change in the profession.”
Dr. Lenchus acknowledges that advocacy “can be all-consuming at times. We have to understand our limits.” That said, he thinks not engaging in advocacy could increase stress and potential burnout. “I think being involved in advocacy efforts really helps people conduct meaningful work and educates them about what it means not just to them, but to the rest of the medical profession and the patients that we serve,” he said.
For hospitalists who are interested in health policy advocacy, there are many ways to get involved, Dr. Lenchus said. You could join an organization (many organized medical societies have public policy committees), participate in advocacy activities, work on a political campaign, or even run for office yourself. “Ultimately, education and some level of involvement really will make the difference in who navigates our future as hospitalists,” he said.
Questioning co-management practices
Though he says he’s in the minority, Hardik Vora, MD, SFHM, medical director for hospital medicine at Riverside Regional Medical Center in Newport News, Va., believes that co-management is going to “make or break hospital medicine. It’s going to have a huge impact on our specialty.”
In the roughly 25-year history of hospital medicine, it has evolved from admitting and caring for patients of primary care physicians to patients of specialists and, more recently, surgical patients. “Now there are (hospital medicine) programs across the country that are pretty much admitting everything,” said Dr. Vora.
As a recruiter for the Riverside Health System for the past eight years, “I have not met a single resident who is trained to do what we’re doing in hospital medicine, because you’re admitting surgical patients all the time and you have primary attending responsibility,” Dr. Vora said. “I see that as a cause of a significant amount of stress because now you’re responsible for something that you don’t have adequate training for.”
In the co-management discussion, Dr. Vora notes that people often bring up the research that shows that the practice has improved surgeon satisfaction. “What bothers me is that…you need to add one more question – how does it affect your hospitalists? And I bet the answer to that question is ‘it has a terrible effect.’”
The expectations surrounding hospitalists these days is a big concern in terms of burnout, Dr. Vora said. “We talk a lot about the drivers of burnout, whether it’s schedule or COVID,” he said. The biggest issue when it comes to burnout, as he sees it, is not COVID; it’s when hospitalists are performing tasks that make them feel they aren’t adding value. “I think that’s a huge topic in hospital medicine right now.”
Dr. Vora believes there should be more discussion and awareness of the potential pitfalls. “Hospitalists should get involved in co-management where they are adding value and certainly not take up the attending responsibility where they’re not adding value and it’s out of the scope of their training and expertise,” he said. “Preventing scope creep and burnout from co-management are some of the key issues I’m really passionate about.”
Dr. Vora said it is important to set realistic goals and remember that it takes time to make change when it comes to advocacy. “You still have to operate within whatever environment is given to you and then you can make change from within,” he said.
His enthusiasm for co-management awareness has led to creating a co-management forum through SHM in his local Hampton Roads chapter. He was also a panelist for an SHM webinar in February 2021 in which the panelists debated co-management.
“I think we really need to look at this as a specialty. Are we going in the right direction?” Dr. Vora asked. “We need to come together as a specialty and make a decision, which is going to be hard because there are competing financial interests and various practice models.”
Improving patient care
Working as a hospitalist at University Medical Center, a safety net hospital in New Orleans, Celeste Newby, MD, PhD, sees plenty of patients who are underinsured or not insured at all. “A lot of my interest in health policy stems from that,” she said.
During her residency, which she finished in 2015, Louisiana became a Medicaid expansion state. This impressed upon Dr. Newby how much Medicaid improved the lives of patients who had previously been uninsured. “We saw procedures getting done that had been put on hold because of financial concerns or medicines that were now affordable that weren’t before,” she said. “It really did make a difference.”
When repeated attempts to repeal the Affordable Care Act began, “it was a call to do health policy work for me personally that just hadn’t come up in the past,” said Dr. Newby, who is also assistant professor of medicine at Tulane University in New Orleans. “I personally found that the best way to do (advocacy work) was to go through medical societies because there is a much stronger voice when you have more people saying the same thing,” she said.
Dr. Newby sits on the Council of Legislation for the Louisiana State Medical Society and participates in the Leadership and Health Policy (LEAHP) Program through the Society of General Internal Medicine.
The LEAHP Program has been instrumental in expanding Dr. Newby’s knowledge of how health policy is made and the mechanisms behind it. It has also taught her “how we can either advise, guide, leverage, or advocate for things that we think would be important for change and moving the country in the right direction in terms of health care.”
Another reason involvement in medical societies is helpful is because, as a busy clinician, it is impossible to keep up with everything. “Working with medical societies, you have people who are more directly involved in the legislature and can give you quicker notice about things that are coming up that are going to be important to you or your co-workers or your patients,” Dr. Newby said.
Dr. Newby feels her advocacy work is an outlet for stress and “a way to work at more of a macro level on problems that I see with my individual patients. It’s a nice compliment.” At the hospital, she can only help one person at a time, but with her advocacy efforts, there’s potential to make changes for many.
“Advocacy now is such a large umbrella that encompasses so many different projects at all kinds of levels,” Dr. Newby said. She suggests looking around your community to see where the needs lie. If you’re passionate about a certain topic or population, see what you can do to help advocate for change there.
Hospitalists around the country are devoting large portions of their spare time to a wide range of advocacy efforts. From health policy to caring for the unhoused population to diversity and equity to advocating for fellow hospitalists, these physicians are passionate about their causes and determined to make a difference.
Championing the unhoused
Sarah Stella, MD, FHM, a hospitalist at Denver Health, was initially drawn there because of the population the hospital serves, which includes a high concentration of people experiencing homelessness. As she cared for her patients, Dr. Stella, who is also associate professor of hospital medicine at the University of Colorado, increasingly felt the desire to help prevent the negative downstream outcomes the hospital sees.
To understand the experiences of the unhoused outside the hospital, Dr. Stella started talking to her patients and people in community-based organizations that serve this population. “I learned a ton,” she said. “Homelessness feels like such an intractable, hopeless thing, but the more I talked to people, the more opportunities I saw to work toward something better.”
This led to a pilot grant to work with the Colorado Coalition for the Homeless to set up a community advisory panel. “My goal was to better understand their experiences and to develop a shared vision for how we collectively can do better,” said Dr. Stella. Eventually, she also received a grant from the University of Colorado, and multiple opportunities have sprung up ever since.
For the past several years, Dr. Stella has worked with Denver Health leadership to improve care for the homeless. “Right now, I’m working with a community team on developing an idea to provide peer support from people with a shared lived experience for people who are experiencing homelessness when they’re hospitalized. That’s really where my passion has been in working on the partnership,” she said.
Her advocacy role has been beneficial in her work as a hospitalist, particularly when COVID began. Dr. Stella again partnered with the Colorado Coalition for the Homeless to start a joint task force. “Everyone on our task force is motivated by this powerful desire to improve the health and lives of this community and that’s one of the silver linings in this pandemic for me,” said Dr. Stella.
Advocacy work has also increased Dr. Stella’s knowledge of what community support options are available for the unhoused. This allows her to educate her patients about their options and how to access them.
While she has colleagues who are able to compartmentalize their work, “I absolutely could not be a hospitalist without being an advocate,” Dr. Stella said. “For me, it has been a protective strategy in terms of burnout because I have to feel like I’m working to advocate for better policies and more appropriate resources to address the gaps that I’m seeing.”
Dr. Stella believes that physicians have a special credibility to advocate, tell stories, and use data to back their stories up. “We have to realize that we have this power, and we have it so we can empower others,” she said. “The people I’ve seen in my community who are working so hard to help people who are experiencing homelessness are the heroes. Understanding that and giving power to those people through our voice and our well-respected place in society drives me.”
Strengthening diversity, equity, and inclusion
In September 2020, Michael Bryant, MD, became the inaugural vice chair of Diversity, Equity, and Inclusion for the department of pediatrics at Children’s Hospital Los Angeles, where he is also the division head of pediatric hospital medicine. “I was motivated to apply for this position because I wanted to be an agent for change to eliminate the institutional racism, social injustice, and marginalization that continues to threaten the lives and well-beings of so many Americans,” Dr. Bryant said.
Between the pandemic, the economic decline it has created, and the divisive political landscape, people of color have been especially affected. “These are poignant examples of the ever-widening divide and disenfranchisement many Americans feel,” said Dr. Bryant. “Gandhi said, ‘Be the change that you want to see,’ and that is what I want to model.”
At work, advocacy for diversity, equality, and inclusion is an innate part of everything he does. From the new physicians he recruits to the candidates he considers for leadership positions, Dr. Bryant strives “to have a workforce that mirrors the diversity of the patients we humbly care for and serve.”
Advocacy is intrinsic to Dr. Bryant’s worldview, in his quest to understand and accept each individual’s uniqueness, his desire “to embrace cultural humility,” his recognition that “our differences enhance us instead of diminishing us,” and his willingness to engage in difficult conversations.
“Advocacy means that I acknowledge that intent does not equal impact and that I must accept that what I do and what I say may have unintended consequences,” he said. “When that happens, I must resist becoming defensive and instead be willing to listen and learn.”
Dr. Bryant is proud of his accomplishments and enjoys his advocacy work. In his workplace, there are few African Americans in leadership roles. This means that he is in high demand when it comes to making sure there’s representation during various processes such as hiring and vetting, a disparity known as the “minority tax.”
“I am thankful for the opportunities, but it does take a toll at times,” Dr. Bryant said, which is yet another reason why he is a proponent of increasing diversity and inclusion. “This allows us to build the resource pool as these needs arise and minimizes the toll of the ‘minority tax’ on any single person or small group of individuals.”
This summer, physicians from Dr. Bryant’s hospital participated in the national “White Coats for Black Lives” effort. He found it to be “an incredibly moving event” that hundreds of his colleagues participated in.
Dr. Bryant’s advice for hospitalists who want to get involved in advocacy efforts is to check out the movie “John Lewis: Good Trouble.” “He was a champion of human rights and fought for these rights until his death,” Dr. Bryant said. “He is a true American hero and a wonderful example.”
Bolstering health care change
Since his residency, Joshua Lenchus, DO, FACP, SFHM, has developed an ever-increasing interest in legislative advocacy, particularly health policy. Getting involved in this arena requires an understanding of civics and government that goes beyond just the basics. “My desire to affect change in my own profession really served as the catalyst to get involved,” said Dr. Lenchus, the regional chief medical officer at Broward Health Medical Center in Fort Lauderdale, Fla. “What better way to do that than by combining what we do on a daily basis in the practice of medicine with this new understanding of how laws are passed and promulgated?”
Dr. Lenchus has been involved with both state and national medical organizations and has served on public policy committees as a member and as a chair. “The charge of these committees is to monitor and navigate position statements and policies that will drive the entire organization,” he said. This means becoming knowledgeable enough about a topic to be able to talk about it eloquently and adding supporting personal or professional illustrations that reinforce the position to lawmakers.
He finds his advocacy efforts “incredibly rewarding” because they contribute to his endeavors “to help my colleagues practice medicine in a safe, efficient, and productive manner.” For instance, some of the organizations Dr. Lenchus was involved with helped make changes to the Affordable Care Act that ended up in its final version, as well as changes after it passed. “There are tangible things that advocacy enables us to do in our daily practice,” he said.
When something his organizations have advocated for does not pass, they know they need to try a different outlet. “You can’t win every fight,” he said. “Every time you go and comment on an issue, you have to understand that you’re there to do your best, and to the extent that the people you’re talking to are willing to listen to what you have to say, that’s where I think you can make the most impact.” When changes he has helped fight for do pass, “it really is amazing that you can tell your colleagues about your role in achieving meaningful change in the profession.”
Dr. Lenchus acknowledges that advocacy “can be all-consuming at times. We have to understand our limits.” That said, he thinks not engaging in advocacy could increase stress and potential burnout. “I think being involved in advocacy efforts really helps people conduct meaningful work and educates them about what it means not just to them, but to the rest of the medical profession and the patients that we serve,” he said.
For hospitalists who are interested in health policy advocacy, there are many ways to get involved, Dr. Lenchus said. You could join an organization (many organized medical societies have public policy committees), participate in advocacy activities, work on a political campaign, or even run for office yourself. “Ultimately, education and some level of involvement really will make the difference in who navigates our future as hospitalists,” he said.
Questioning co-management practices
Though he says he’s in the minority, Hardik Vora, MD, SFHM, medical director for hospital medicine at Riverside Regional Medical Center in Newport News, Va., believes that co-management is going to “make or break hospital medicine. It’s going to have a huge impact on our specialty.”
In the roughly 25-year history of hospital medicine, it has evolved from admitting and caring for patients of primary care physicians to patients of specialists and, more recently, surgical patients. “Now there are (hospital medicine) programs across the country that are pretty much admitting everything,” said Dr. Vora.
As a recruiter for the Riverside Health System for the past eight years, “I have not met a single resident who is trained to do what we’re doing in hospital medicine, because you’re admitting surgical patients all the time and you have primary attending responsibility,” Dr. Vora said. “I see that as a cause of a significant amount of stress because now you’re responsible for something that you don’t have adequate training for.”
In the co-management discussion, Dr. Vora notes that people often bring up the research that shows that the practice has improved surgeon satisfaction. “What bothers me is that…you need to add one more question – how does it affect your hospitalists? And I bet the answer to that question is ‘it has a terrible effect.’”
The expectations surrounding hospitalists these days is a big concern in terms of burnout, Dr. Vora said. “We talk a lot about the drivers of burnout, whether it’s schedule or COVID,” he said. The biggest issue when it comes to burnout, as he sees it, is not COVID; it’s when hospitalists are performing tasks that make them feel they aren’t adding value. “I think that’s a huge topic in hospital medicine right now.”
Dr. Vora believes there should be more discussion and awareness of the potential pitfalls. “Hospitalists should get involved in co-management where they are adding value and certainly not take up the attending responsibility where they’re not adding value and it’s out of the scope of their training and expertise,” he said. “Preventing scope creep and burnout from co-management are some of the key issues I’m really passionate about.”
Dr. Vora said it is important to set realistic goals and remember that it takes time to make change when it comes to advocacy. “You still have to operate within whatever environment is given to you and then you can make change from within,” he said.
His enthusiasm for co-management awareness has led to creating a co-management forum through SHM in his local Hampton Roads chapter. He was also a panelist for an SHM webinar in February 2021 in which the panelists debated co-management.
“I think we really need to look at this as a specialty. Are we going in the right direction?” Dr. Vora asked. “We need to come together as a specialty and make a decision, which is going to be hard because there are competing financial interests and various practice models.”
Improving patient care
Working as a hospitalist at University Medical Center, a safety net hospital in New Orleans, Celeste Newby, MD, PhD, sees plenty of patients who are underinsured or not insured at all. “A lot of my interest in health policy stems from that,” she said.
During her residency, which she finished in 2015, Louisiana became a Medicaid expansion state. This impressed upon Dr. Newby how much Medicaid improved the lives of patients who had previously been uninsured. “We saw procedures getting done that had been put on hold because of financial concerns or medicines that were now affordable that weren’t before,” she said. “It really did make a difference.”
When repeated attempts to repeal the Affordable Care Act began, “it was a call to do health policy work for me personally that just hadn’t come up in the past,” said Dr. Newby, who is also assistant professor of medicine at Tulane University in New Orleans. “I personally found that the best way to do (advocacy work) was to go through medical societies because there is a much stronger voice when you have more people saying the same thing,” she said.
Dr. Newby sits on the Council of Legislation for the Louisiana State Medical Society and participates in the Leadership and Health Policy (LEAHP) Program through the Society of General Internal Medicine.
The LEAHP Program has been instrumental in expanding Dr. Newby’s knowledge of how health policy is made and the mechanisms behind it. It has also taught her “how we can either advise, guide, leverage, or advocate for things that we think would be important for change and moving the country in the right direction in terms of health care.”
Another reason involvement in medical societies is helpful is because, as a busy clinician, it is impossible to keep up with everything. “Working with medical societies, you have people who are more directly involved in the legislature and can give you quicker notice about things that are coming up that are going to be important to you or your co-workers or your patients,” Dr. Newby said.
Dr. Newby feels her advocacy work is an outlet for stress and “a way to work at more of a macro level on problems that I see with my individual patients. It’s a nice compliment.” At the hospital, she can only help one person at a time, but with her advocacy efforts, there’s potential to make changes for many.
“Advocacy now is such a large umbrella that encompasses so many different projects at all kinds of levels,” Dr. Newby said. She suggests looking around your community to see where the needs lie. If you’re passionate about a certain topic or population, see what you can do to help advocate for change there.
Low-calorie diet linked to improved chemo response in leukemia
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and adolescents with leukemia who were placed on a restrictive diet and exercise regimen concurrent with starting chemotherapy showed responses to treatment that were better than those historically seen in such patients.
This apparently improved response suggests it is possible to boost treatment efficacy without raising the dose – or toxicity – of chemotherapy.
“To our knowledge, this is the first study in any hematologic malignancy to demonstrate potential benefit from caloric restriction via diet and exercise to augment chemotherapy efficacy and improve disease response, the authors reported.
The findings come from the IDEAL pilot trial, conducted in 40 young patients (mean age, 15 years; range, 10-21 years) diagnosed with high-risk B-cell acute lymphoblastic leukemia (B-ALL).
The study was published online April 1 in Blood Advances.
The diet and exercise regimen is a departure from current recommendations for patients with leukemia.
“This was a major paradigm shift – until now, many oncologists encouraged ‘comfort foods’ and increased calories to get through the rigor of chemotherapy,” first author Etan Orgel, MD, of Children’s Hospital Los Angeles and the University of Southern California, also in Los Angeles.
The results from this pilot trial suggest that “the era of encouraging comfort food should be in the past; over-nutrition is likely harmful, and diet and exercise are important tools to harness during chemotherapy,” he said.
Dr. Orgel added that childhood ALL was selected because it is the most common cancer of childhood, but the findings could have potential relevance in other cancer types in children as well as adults.
Commenting on the study, Patrick Brown, MD, director of the pediatric leukemia program at Johns Hopkins University, Baltimore, said the findings are important, albeit preliminary.
“I think the most important contribution of this pilot study is to show that it is possible to change the nutrition and exercise habits of children and adolescents during the initial month of treatment for ALL,” he said in an interview.
“We have to be cautious about the preliminary finding that these changes resulted in deeper remissions – this will need to be confirmed in a larger study,” added Dr. Brown, who was not involved with the research.
Dr. Orgel noted that a prospective, randomized trial, IDEAL-2, is launching later this year to further evaluate the intervention.
Obesity linked to poorer chemotherapy response
Among children and adolescents who start treatment for B-ALL, as many as 40% are overweight or obese, noted the study authors.
Those who are obese have more than a twofold greater risk of having persistent minimal residual disease (MRD) at the end of chemotherapy, considered the strongest patient-level predictor of poor outcome and a common guide for therapy intensification.
The problem is compounded by weight gain that is common during treatment as a result of prolonged chemotherapy and sedentary behavior, they commented.
With studies of obese mice linking calorie and fat restriction to improved survival after chemotherapy, the authors theorized that a calorie- and fat-restrictive diet and exercise could help improve outcomes after chemotherapy in humans.
Participants were enrolled at Children’s Hospital Los Angeles and City of Hope National Medical Center in nearby Duarte. After they were started on chemotherapy, they were placed on a low-carb, low-fat, and low-sugar diet tailored to patient needs and preferences, as well as a moderate daily exercise regimen, and continued on this regimen throughout the 4-week induction phase.
Following the intervention, there were no significant reductions observed in median gain of fat mass at the end of the intervention, compared with baseline (P = .13). However, in the subgroup of patients who were overweight or obese at baseline, the reduction in fat mass was indeed significant versus baseline (+1.5% vs. +9.7% at baseline; P = .02).
Importantly, after adjustment for prognostic factors, adherence to the intervention was associated with a significant reduction in the risk of MRD, compared with recent historical controls who received the same induction therapy at the same institution, but no intervention (odds ratio, 0.30; P = .02).
The intervention was also associated with a lower detectable MRD, compared with the historical controls (OR, 0.16; one-sided P = .002).
“Most importantly, the IDEAL intervention reduced risk of MRD at the end of induction in all patients, irrespective of starting [body mass index] and after accounting for prognostic features,” the authors noted.
Adherence to diet high, exercise low
As many as 82% of study participants achieved the goal of 20% or more caloric deficit throughout the chemotherapy.
“Adherence to the diet was excellent, with caloric deficits and macronutrient goals achieved in nearly all patients, including in the lean group,” the authors reported.
Dr. Orgel added that families embraced the chance to play an active role in the cancer therapy. “In our view, they couldn’t control their disease or their chemotherapy, but this, they could,” he said.
Conversely, adherence to the prescribed exercise was low – just 31.2%, with the inactivity during the first month likely contributed to the similar loss of muscle mass that occurred in both cohorts, Dr. Orgel noted.
“The [low exercise adherence] unfortunately was not a surprise, as it is often difficult to exercise and be active during chemotherapy,” he said.
Key aspects of physical activity will be refined in further studies, Dr. Orgel added.
Insulin sensitivity, adiponectin key factors?
Patients receiving the intervention showed improved insulin sensitivity and reductions in circulating insulin, which are notable in that insulin has been linked to mechanisms that counter chemoresistance, the authors noted.
Furthermore, the decreases in insulin were accompanied by notable elevations in circulating adiponectin, a protein hormone produced and secreted by fat cells.
“Adiponectin was certainly a surprise, as until now it did not appear to play a major role in cancer cell resistance to chemotherapy,” Dr. Orgel said.
“It is too soon to say they are central to the mechanism of the intervention, but the large differences in adiponectin and insulin sensitivity found in children in the trial have definitely highlighted these as important for future study,” he added.
Dr. Orgel, the study coauthors, and Dr. Brown disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Helping psychiatric patients heal holistically
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.
When I was asked to write a regular “Holistic Mental Health” column, I decided to write about the Herculean forces that must come together to create a holistic psychiatrist – someone who specializes in helping patients off their medications rather than on.
My journey began when I told a training psychiatrist that I wanted to stop being a psychiatrist. It was a year after my daughter was born, and I had started my third year of adult psychiatry residency at the University of Maryland in Baltimore. I was stressed and exhausted from working on inpatient psychiatric wards for 2 years, countless unpleasant nights on call, and additional sleepless nights caring for an infant.
I told the training psychiatrist that life wasn’t worth living. Was I suicidal, he asked? I laughed bitterly: “All the time!” Once he heard the S-word, he wanted me to take an antidepressant. I finally gave in and began taking Zoloft 25 mg every morning. Within a week, my angst disappeared; but 5 years, another child, and a fellowship later, I was still taking Zoloft. Why? Without much thought, I stopped it. A month later, I found myself brooding on the sofa, numb with depression, and feeling astonishingly suicidal. This “depression” led me to restart my Zoloft. In a week, my mood normalized. I did this on and off for about a year until a light bulb went off: This can’t be depression. It’s withdrawal. I’ve become dependent on Zoloft! Once I realized this, I began taking some St. John’s wort, an herbal alternative that was supposed to help with depression. I used cheaper brands and discovered that brands do matter, because the cheaper ones didn’t work. Through my haphazard exploration of natural alternatives, I came off Zoloft completely. During this time, I developed greater empathy for my patients, openness to natural alternatives, appreciation for supplement quality, and learned about psychotropic withdrawal. Most importantly, I came to understand a patient’s need to be free.
Five years later, in 2002, I had a thriving, but conventional, private practice. Instead of being content, however, I once again wanted to quit psychiatry. Medicating patients felt unrewarding, but I didn’t have another approach. Simultaneously, my practice was filling up with chronically ill, heavily medicated, bipolar patients. Their intense suffering combined with my discontent with psychiatry made me desperate for something better. In this ripe setting, the mother of a patient with bipolar disorder casually mentioned a supplement called EMPower by Truehope that lessened bipolar symptoms. Though my withdrawal from Zoloft allowed me to be more open to holistic approaches, I waited 3 months before calling. I used the supplement for the first time to help a heavily medicated bipolar patient in her 30’s, whose Depakote side effects caused her to wear a diaper, lack any emotions, and suffer severe tremors. Once I made this decision to walk down this new path, I never went back. With guidance from the company, I used this supplement to help many patients lower their medications. At the time, I wondered whether EMPower would be the solution for all my patients. The simplicity and ease of one supplement approach for all mental illnesses appealed to my laziness, so I continued down the holistic path.
Hundreds of supplements, glandulars, essential oils, and homeopathic remedies later, I learned that every patient requires their own unique approach. A year into using the supplement, I discovered that, if patients took too much of it, their old symptoms would reappear. Eventually, I moved out of my comfort zone and tried other supplements. Subsequently, the universe orchestrated two people to tell me about the miraculous outcomes from “thought-field therapy,” an energy-medicine technique. I began exploring “energy medicine” through the support and instruction of a holistic psychotherapist, Mark Bottinick, LCSW-C. Soon, I was connecting the dots between emotional freedom technique and immediate positive changes. Energy medicine allowed me to heal problems without using a pill! I felt as if I had arrived at Solla Sollew by the banks of the Beautiful River Wah-Hoo.
As I discovered and attended conferences in holistic medicine, I got certified in integrative medicine and became a Reiki master. Even as a novice in holistic medicine, I began to experience patients crying with joy, rather than sadness. One psychotic patient got better on some supplements and got a new job in just 2 weeks.
On Feb. 17, 2021, I launched a podcast called “The Holistic Psychiatrist,” with interviews of patients, conversations with practitioners, and insights from me. Of the initial interviews, two of the three patients had bipolar disorder, and were able to safely and successfully withdraw from many medications. They are no longer patients and are free to move on with their lives. A patient who smoothly and successfully lowered six psychiatric medications will be sharing her wisdom and healing journey soon. A naturopathic doctor will also be sharing his insights and successes. He once was a suicidal high school student failing his classes, depressed and anxious, and dependent on marijuana. His recovery occurred more than a decade ago in my holistic practice.
These patients are living proof that holistic approaches can be very powerful and effective. They demonstrate that chronicity may reflect inadequate treatment and not a definition of disease. Over the course of this Holistic Mental Health column, I want to share many incredible healing journeys and insights on holistic psychiatry. I hope that you will be open to this new paradigm and begin your own holistic journey.
Dr. Lee is a psychiatrist with a solo private practice in Lehi, Utah. She integrates functional/orthomolecular medicine and mind/body/energy medicine in her work with patients. Contact her at holisticpsychiatrist.com. She has no conflicts of interest.
Don’t screen for vitamin D in general population, says USPSTF
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 in children: New cases on the rise again
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
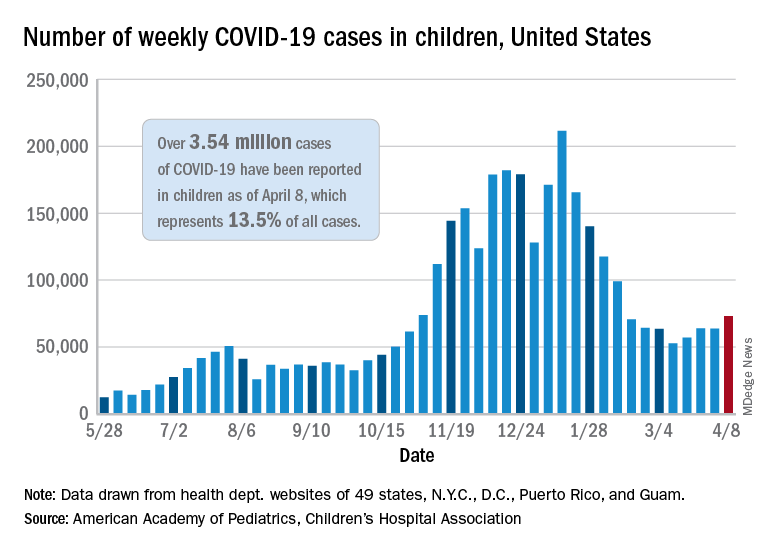
Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
Evidence favors lower-dose R-CHOP for fit, very elderly DLBCL patients
A dose-adjusted R-CHOP may be the best treatment for elderly patients with diffuse large beta-cell lymphoma (DLBCL), according to a review of 38 studies that examined this aged population.
In addition, treatment choices based on new tools such as the Comprehensive Geriatric Assessment appeared to provide useful guidance based on the comorbidities and frailty index of this group of patients, according to Alda Tavares, MD, of Hospital Pedro Hispano, Matosinhos (Portugal) Local Health Unit, and Ilídia Moreira, MD, of the Portuguese Institute of Oncology of Porto.
Study characteristics
Of the 38 studies assessed, 13 were retrospective and 25 were phase II/III clinical trials. Most of these studies investigated the efficacy of dose-adjusted R-CHOP regimen, according to the review published online in Critical Reviews in Oncology/Hematology.
Alternative therapeutic drugs as well as the use of geriatric assessment were also investigated.
In terms of the elderly populations assessed, 11 out of 38 studies included at least 30 patients over age 80 years, although 11 other studies did not specify the number of patients older than 80 years. Eight of the studies included exclusively patients aged 80 years and over. Three of these studies were phase II trials.
Only six of the clinical trials required a geriatric assessment tool for inclusion criteria or therapeutic regime choice, using the Cumulative Illness Rating Scale–Geriatric (CIRS-G), the performance in activities of daily living (ADL) and/or instrumental activities of daily living (IADL) tools.
Most of the studies investigated the efficacy of R-CHOP regimen at different doses and variations, with 11 studies using alternative anthracycline in place of doxorubicin.
MiniCHOP mattered
Elderly patients over 80 years achieved complete response (CR) rates from 37.2% to 66.7% and 2-year overall survival (OS) from 31.9% to 64.7% across the studies reviewed. Overall, for fit patients aged 80 and over, the strongest evidence favored the use of an R-miniCHOP regimen, according to the authors.
In the 25 studies with treatment based on R-CHOP/modified R-CHOP or immunochemotherapy with an alternative anthracycline, the CR rate was below 50% in three studies and over 60% in the majority. Higher CR rates of 71%-88.9% were achieved in eight studies.
For patients over 80 years, the strongest evidence favored rituximab/ofatumumab-miniCHOP, based on two studies. In both studies, patients over 80 years old, without significant comorbidities, received CHOP regime with a dose reduction of about 50% (miniCHOP: cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, and 1 mg vincristine on day 1 of each cycle, and prednisone 40 mg/m2 on days 1-5) plus an anti–CD-20 antibody (rituximab 375 mg/m2 or ofatumumab 1,000 mg). The first of these studies obtained CR rate of 62% and 2-year OS of 59% with low toxicities. The second study achieved slightly better results, according to the reviewers, who suggested the difference was possibly because of a prephase treatment and/or the use of ofatumumab.
One study group developed a simple prognostic model based on multivariate analysis of 108 patients aged 80 years and older treated in their study with R-CHOP at full (48%) or reduced dose (51%). Patients with at least two out of three risk factors (age > 85 years, revised International Prognostic Index score 3-5 and CIRS > 5) had worse survival than did those with 0-1 risk factors, with a median OS of 12 months vs. 45 months, P = .001, respectively).
“All these studies results favor the tailored treatment approach,” the reviewers stated. “More prospective studies are still needed to demonstrate and validate the adequate tools for the selection of patients and their optimal treatment. They would provide the grounds for clinical therapeutic decision, aiming for tailored treatment and fulfilling best individual expectations and outcome,” they concluded.
The authors reported that they received no research funds for the study and that they had no disclosures.
A dose-adjusted R-CHOP may be the best treatment for elderly patients with diffuse large beta-cell lymphoma (DLBCL), according to a review of 38 studies that examined this aged population.
In addition, treatment choices based on new tools such as the Comprehensive Geriatric Assessment appeared to provide useful guidance based on the comorbidities and frailty index of this group of patients, according to Alda Tavares, MD, of Hospital Pedro Hispano, Matosinhos (Portugal) Local Health Unit, and Ilídia Moreira, MD, of the Portuguese Institute of Oncology of Porto.
Study characteristics
Of the 38 studies assessed, 13 were retrospective and 25 were phase II/III clinical trials. Most of these studies investigated the efficacy of dose-adjusted R-CHOP regimen, according to the review published online in Critical Reviews in Oncology/Hematology.
Alternative therapeutic drugs as well as the use of geriatric assessment were also investigated.
In terms of the elderly populations assessed, 11 out of 38 studies included at least 30 patients over age 80 years, although 11 other studies did not specify the number of patients older than 80 years. Eight of the studies included exclusively patients aged 80 years and over. Three of these studies were phase II trials.
Only six of the clinical trials required a geriatric assessment tool for inclusion criteria or therapeutic regime choice, using the Cumulative Illness Rating Scale–Geriatric (CIRS-G), the performance in activities of daily living (ADL) and/or instrumental activities of daily living (IADL) tools.
Most of the studies investigated the efficacy of R-CHOP regimen at different doses and variations, with 11 studies using alternative anthracycline in place of doxorubicin.
MiniCHOP mattered
Elderly patients over 80 years achieved complete response (CR) rates from 37.2% to 66.7% and 2-year overall survival (OS) from 31.9% to 64.7% across the studies reviewed. Overall, for fit patients aged 80 and over, the strongest evidence favored the use of an R-miniCHOP regimen, according to the authors.
In the 25 studies with treatment based on R-CHOP/modified R-CHOP or immunochemotherapy with an alternative anthracycline, the CR rate was below 50% in three studies and over 60% in the majority. Higher CR rates of 71%-88.9% were achieved in eight studies.
For patients over 80 years, the strongest evidence favored rituximab/ofatumumab-miniCHOP, based on two studies. In both studies, patients over 80 years old, without significant comorbidities, received CHOP regime with a dose reduction of about 50% (miniCHOP: cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, and 1 mg vincristine on day 1 of each cycle, and prednisone 40 mg/m2 on days 1-5) plus an anti–CD-20 antibody (rituximab 375 mg/m2 or ofatumumab 1,000 mg). The first of these studies obtained CR rate of 62% and 2-year OS of 59% with low toxicities. The second study achieved slightly better results, according to the reviewers, who suggested the difference was possibly because of a prephase treatment and/or the use of ofatumumab.
One study group developed a simple prognostic model based on multivariate analysis of 108 patients aged 80 years and older treated in their study with R-CHOP at full (48%) or reduced dose (51%). Patients with at least two out of three risk factors (age > 85 years, revised International Prognostic Index score 3-5 and CIRS > 5) had worse survival than did those with 0-1 risk factors, with a median OS of 12 months vs. 45 months, P = .001, respectively).
“All these studies results favor the tailored treatment approach,” the reviewers stated. “More prospective studies are still needed to demonstrate and validate the adequate tools for the selection of patients and their optimal treatment. They would provide the grounds for clinical therapeutic decision, aiming for tailored treatment and fulfilling best individual expectations and outcome,” they concluded.
The authors reported that they received no research funds for the study and that they had no disclosures.
A dose-adjusted R-CHOP may be the best treatment for elderly patients with diffuse large beta-cell lymphoma (DLBCL), according to a review of 38 studies that examined this aged population.
In addition, treatment choices based on new tools such as the Comprehensive Geriatric Assessment appeared to provide useful guidance based on the comorbidities and frailty index of this group of patients, according to Alda Tavares, MD, of Hospital Pedro Hispano, Matosinhos (Portugal) Local Health Unit, and Ilídia Moreira, MD, of the Portuguese Institute of Oncology of Porto.
Study characteristics
Of the 38 studies assessed, 13 were retrospective and 25 were phase II/III clinical trials. Most of these studies investigated the efficacy of dose-adjusted R-CHOP regimen, according to the review published online in Critical Reviews in Oncology/Hematology.
Alternative therapeutic drugs as well as the use of geriatric assessment were also investigated.
In terms of the elderly populations assessed, 11 out of 38 studies included at least 30 patients over age 80 years, although 11 other studies did not specify the number of patients older than 80 years. Eight of the studies included exclusively patients aged 80 years and over. Three of these studies were phase II trials.
Only six of the clinical trials required a geriatric assessment tool for inclusion criteria or therapeutic regime choice, using the Cumulative Illness Rating Scale–Geriatric (CIRS-G), the performance in activities of daily living (ADL) and/or instrumental activities of daily living (IADL) tools.
Most of the studies investigated the efficacy of R-CHOP regimen at different doses and variations, with 11 studies using alternative anthracycline in place of doxorubicin.
MiniCHOP mattered
Elderly patients over 80 years achieved complete response (CR) rates from 37.2% to 66.7% and 2-year overall survival (OS) from 31.9% to 64.7% across the studies reviewed. Overall, for fit patients aged 80 and over, the strongest evidence favored the use of an R-miniCHOP regimen, according to the authors.
In the 25 studies with treatment based on R-CHOP/modified R-CHOP or immunochemotherapy with an alternative anthracycline, the CR rate was below 50% in three studies and over 60% in the majority. Higher CR rates of 71%-88.9% were achieved in eight studies.
For patients over 80 years, the strongest evidence favored rituximab/ofatumumab-miniCHOP, based on two studies. In both studies, patients over 80 years old, without significant comorbidities, received CHOP regime with a dose reduction of about 50% (miniCHOP: cyclophosphamide 400 mg/m2, doxorubicin 25 mg/m2, and 1 mg vincristine on day 1 of each cycle, and prednisone 40 mg/m2 on days 1-5) plus an anti–CD-20 antibody (rituximab 375 mg/m2 or ofatumumab 1,000 mg). The first of these studies obtained CR rate of 62% and 2-year OS of 59% with low toxicities. The second study achieved slightly better results, according to the reviewers, who suggested the difference was possibly because of a prephase treatment and/or the use of ofatumumab.
One study group developed a simple prognostic model based on multivariate analysis of 108 patients aged 80 years and older treated in their study with R-CHOP at full (48%) or reduced dose (51%). Patients with at least two out of three risk factors (age > 85 years, revised International Prognostic Index score 3-5 and CIRS > 5) had worse survival than did those with 0-1 risk factors, with a median OS of 12 months vs. 45 months, P = .001, respectively).
“All these studies results favor the tailored treatment approach,” the reviewers stated. “More prospective studies are still needed to demonstrate and validate the adequate tools for the selection of patients and their optimal treatment. They would provide the grounds for clinical therapeutic decision, aiming for tailored treatment and fulfilling best individual expectations and outcome,” they concluded.
The authors reported that they received no research funds for the study and that they had no disclosures.
FROM CRITICAL REVIEWS IN ONCOLOGY/HEMATOLOGY
Enhancing Diabetes Self-Management Education and Psychological Services for Veterans With Comorbid Chronic Health and Mental Health Conditions
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
Arthritis drug may curb myocardial damage in acute STEMI
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.
The obesity risk everyone forgets
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Clinicians in pediatrics have noticed a troubling pattern emerge during the pandemic, something that is darkly referred to as “the COVID 19,” or the 19 or more pounds that many of our patients have gained in the past year. This phenomenon has underscored many maxims in pediatric weight management: Mainly that frequent snacking, decreased physical activity, and less parental supervision lead to increased weight gain. But could we be missing another lesson this trend is teaching us? What about the relationship between catastrophe and childhood obesity?
Beyond the increased weight gain with lockdowns, I have observed other evidence in my own practice that childhood trauma or adverse experiences increase obesity. Our electronic medical record system gives an alert when a chart with sensitive information is accessed. One example might be if the patient had been seen at a clinic for children who have been abused. I am heartbroken at how often this happens. Academically, I understand the dire statistics about the incidence of child abuse, but the frequency at which I see this pattern is jarring.
Over the years, one striking correlation became clear among my patient population: Children with obesity were more likely to have been seen in the child abuse clinic than normal-weight peers.
I am far from the only one to have observed this relationship. Television shows focusing on severe obesity, such as “My 600-Pound Life,” often show trauma as both a cause and effect of severe obesity. This theme also became apparent on the show “The Biggest Loser,” which highlighted the difficulty of achieving and maintaining substantial weight loss. If even Hollywood has noticed this association, shouldn’t we be much farther ahead?
Pathways to obesity
Adverse childhood experiences (ACE) encompass various causes of child trauma, including abuse or neglect; poverty; household or neighborhood violence; and death, illness, or incarceration of a parent. A pivotal report in 1998 formalized the suspicion that many of us could plainly see: People who suffered ACE have higher incidence of heart disease, COPD, liver disease, incarceration, and drug abuse. For those with six or more ACE, life expectancy averaged 20 years less than those who had none. More recently, a meta-analysis found an odds ratio of 1.46 for adult obesity with known history of childhood trauma.
As a pediatric endocrinologist living in the poorest state of the country, I have clearly observed the correlation between childhood obesity and poverty. While prior generations may have associated child poverty with malnutrition and starvation, we are seeing in modern times that obesity has become a disease of lack. Calorie-dense and processed foods tend to be less expensive, more shelf-stable, and more accessible to people living in both urban and rural food deserts.
I am also a foster mother and have received extensive training in parenting children who have lived through trauma and neglect. For children who have endured food scarcity and deprivation, hoarding food and overeating are expected responses.
But the pathways to abnormal weight gain are myriad and expand beyond binge eating or numbing with food. ACE are particularly troubling because they affect developing brains and the neuroendocrine system; they alter epigenetics and cause heritable changes. Structural brain differences have been evident in the frontopolar cortex, which is linked to centers in the hypothalamus that control appetite. And increased stress raises cortisol release, increases insulin resistance, and alters satiety.
Shifting our approach to treatment
The significant cost of ACE is enormous and affects us all. Health professionals in pediatrics must understand these connections to effectively counsel children and their families dealing with obesity. Handing someone a diet plan and lecturing them about weight loss is never effective, but this common tactic is especially cruel if we do not assess for and address underlying pain. Obviously, blame and shame are ineffective motivators for lifestyle change in any circumstance, but these tactics may be especially harmful in the light of childhood trauma.
Screening for ACE is important in every aspect of pediatric care. The presence of obesity, however, should remind us to be more sensitive to the possibility of causative trauma. Clinicians for adults are not off the hook either. Fully 60% of adults suffered ACE and are dealing with the aftermath.
To improve health outcomes across the board, we must screen for trauma and become educated on trauma-informed care. Perhaps the most important first referral for a child suffering ACE and obesity is to a trained counselor or a social worker. Shepherding children through trauma will be more effective for attaining healthy weight than any remedy I can prescribe as an endocrinologist. Furthermore, this is our necessary role as healers. More than ever, we need to approach chronic diseases, including obesity, with the utmost compassion.
A version of this article first appeared on Medscape.com.
Melanoma presents at later stages, but at an earlier age in Asian Americans
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
, according to a secondary analysis of data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
The findings are consistent with previous studies indicating delayed detection of melanoma in Asians, compared with non-Hispanic Whites, and provide a window into Asian American communities specifically, Erica M. Lin, a medical student at Brown University, Providence, R.I., said at the annual Skin of Color Society Symposium. The majority of studies on melanoma in Asians have originated in Asia, noted Ms. Lin, whose coauthor was Eunyoung Cho, ScD, an associate professor in the department of dermatology and director of the clinical and translational research program at Brown University. Their analysis covered registries from 10 geographic areas representing 54% of the U.S. Asian American population over a 25-year period, from 1990 to 2014.
Asian Americans with melanoma were more likely to present at an invasive stage than non-Hispanic Whites (82.9% vs. 72.2%, P < .001), and they were significantly more likely to present when the disease had progressed to a distant stage (9.39% vs. 2.51%, P < .001), even though they were of younger ages at the time of those diagnoses, Ms. Lin reported at the meeting. (The numbers do not account for unknown or unstaged melanoma cases.)
Significantly fewer Asian Americans presented at the “in situ” stage, compared with non-Hispanic Whites (17.11% vs. 27.78%). The lower extremities were the most common site in Asian Americans, compared with the trunk in Non-Hispanic Whites.
The SEER registries covered the eight largest Asian American groups: Asian Indians/Pakistanis, Chinese, Filipinos, Japanese, Kampucheans (Cambodians), Koreans, Laotians, and Vietnamese. Melanoma was more common in females across the groups (53% of females vs. 47% of males), with the exception of Asian Indians/Pakistanis.
While melanoma increased significantly over time among non-Hispanic Whites – a mean 24% increase per 5-year period – there was “no significant change in melanoma rates in Asians,” Ms. Lin said.
The lack of increase in Asian American communities combined with the other findings is “potentially concerning” and suggests “that there may be cases that are not being identified,” she said in an interview after the meeting. In their abstract, she and Dr. Cho noted that their findings underscore the need for further prevention, screening, and surveillance measures.
The NCI’s SEER program is a coordinated system of cancer registries across the United States that collects data on every case of cancer reported in 19 geographic areas.
FROM SOC SOCIETY 2021