User login
Managing herpes simplex virus genital infection in pregnancy
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
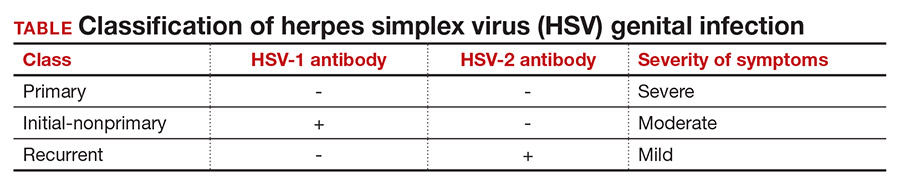
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.
Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1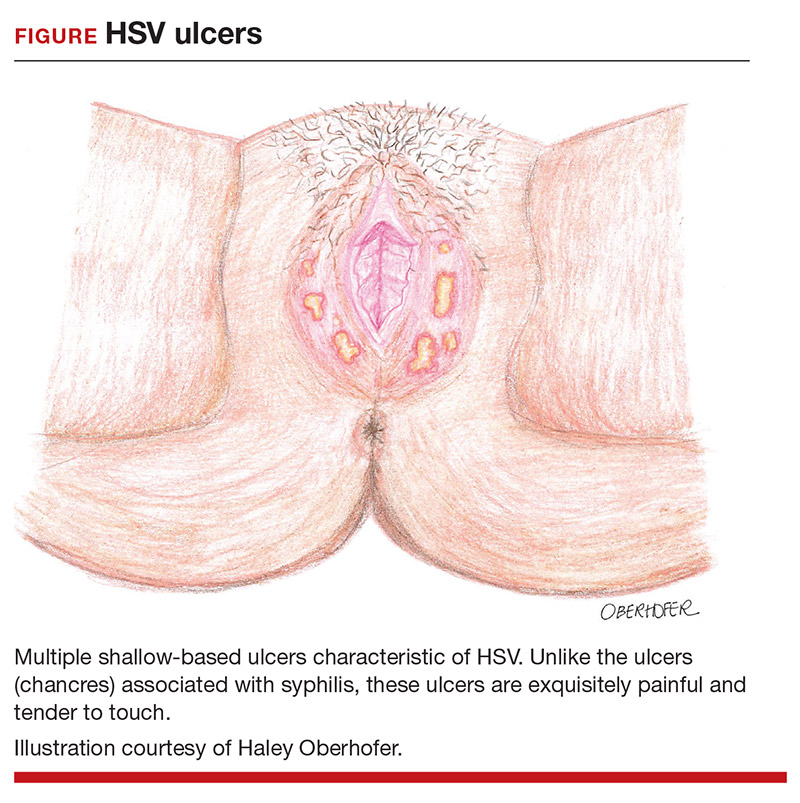
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
CASE Pregnant woman with herpes simplex virus
A 26-year-old primigravid woman at 12 weeks of gestation indicates that she had an initial episode of herpes simplex virus (HSV) 6 years prior to presentation. Subsequently, she has had 1 to 2 recurrent episodes each year. She asks about the implications of HSV infection in pregnancy, particularly if anything can be done to prevent a recurrent outbreak near her due date and reduce the need for a cesarean delivery.
How would you counsel this patient?
Meet our perpetrator
Herpes simplex virus (HSV), the most prevalent sexually transmitted infection, is a DNA virus that has 2 major strains: HSV-1 and HSV-2. HSV-1 frequently is acquired in early childhood through nonsexual contact and typically causes orolabial and, less commonly, genital outbreaks. HSV-2 is almost always acquired through sexual contact and causes mainly genital outbreaks.1
There are 3 classifications of HSV infection: primary, initial-nonprimary, and recurrent (TABLE).
Primary infection refers to infection in a person without antibodies to either type of HSV.
Initial-nonprimary infection refers to acquisition of HSV-2 in a patient with preexisting antibodies to HSV-1 or vice versa. Patients tend to have more severe symptoms with primary as opposed to initial-nonprimary infection because, with the latter condition, preexisting antibodies provide partial protection against the opposing HSV type.1 According to the Centers for Disease Control and Prevention, the seroprevalence of HSV-1 has decreased by approximately 23% in adolescents aged 14 to 19 years, with a resultant increase in the number of primary HSV-1 genital infections through oral-sexual contact in adulthood.2
Recurrent infection refers to reactivation of the same HSV type corresponding to the serum antibodies.

Clinical presentation
After an incubation period of 4 to 7 days, symptomatic patients with primary and initial-nonprimary genital HSV infections typically present with multiple, bilateral genital lesions at various stages of development. These lesions begin as small erythematous macules and then progress to papules, vesicles, pustules, ulcers, and crusted scabs over a period of 3 to 6 weeks1 (FIGURE). Patients also may present with fever, headache, fatigue, dysuria, and painful inguinal lymphadenopathy. Patients with recurrent infections usually experience prodromal itching or tingling for 2 to 5 days prior to the appearance of unilateral lesions, which persist for only 5 to 10 days. Systemic symptoms rarely are present. HSV-1 genital infection has a symptomatic recurrence rate of 20% to 50% within the first year, while HSV-2 has a recurrence rate of 70% to 90%.1
The majority of primary and initial-nonprimary infections are subclinical. One study showed that 74% of HSV-1 and 63% of HSV-2 initial genital herpes infections were asymptomatic.3 The relevance of this observation is that patients may not present for evaluation unless they experience a symptomatic recurrent infection. Meanwhile, they are asymptomatically shedding the virus and unknowingly transmitting HSV to their sexual partners. Asymptomatic viral shedding is more common with HSV-2 and is the most common source of transmission.4 The rate of asymptomatic shedding is unpredictable and has been shown to occur on 10% to 20% of days.1
Diagnosis and treatment
The gold standard for diagnosing HSV infection is viral culture; however, polymerase chain reaction (PCR) assays are faster to result and more sensitive.4,5 Both culture and PCR studies can distinguish the HSV type, allowing physicians to counsel patients regarding the expected clinical course, rate of recurrence, and implications for future pregnancies. After an initial infection, it may take up to 12 weeks for patients to develop detectable antibodies. Therefore, serology can be quite useful in determining the timing and classification of the infection. For example, a patient with HSV-2 isolated on viral culture or PCR and HSV-1 antibodies identified on serology is classified as having an initial-nonprimary infection.4
HSV treatment is dependent on the classification of infection. Treatment of primary and initial-nonprimary infection includes:
- acyclovir 400 mg orally 3 times daily
- valacyclovir 1,000 mg orally twice daily, or
- famciclovir 250 mg orally 3 times daily for 7 to 10 days.
Ideally, treatment should be initiated within 72 hours of symptom onset.
Recurrent infections may be treated with:
- acyclovir 400 mg orally three times daily for 5 days
- valacyclovir 1,000 mg orally once daily for 5 days, or
- famciclovir 1,000 mg orally every 12 hours for 2 doses.
Ideally, treatment should begin within 24 hours of symptom onset.4,6
Patients with immunocompromising conditions, severe/frequent outbreaks (>6 per year), or who desire to reduce the risk of transmission to HSV-uninfected partners are candidates for chronic suppressive therapy. Suppressive options include acyclovir 400 mg orally twice daily, valacyclovir 500 mg orally once daily, and famciclovir 250 mg orally twice daily. Of note, there are many regimens available for acyclovir, valacyclovir, and famciclovir; all have similar efficacy in decreasing symptom severity, time to lesion healing, and duration of viral shedding.6 Acyclovir generally is the least expensive option.4
Continue to: Pregnancy and prevention...
Pregnancy and prevention
During pregnancy, 2% of women will acquire HSV, and 70% of these women will be asymptomatic.4,7 Approximately one-third to one-half of neonatal infections are caused by HSV-1.8 The most devastating complication of HSV infection in pregnancy is transmission to the newborn. Neonatal herpes is defined as the diagnosis of an HSV infection in a neonate within the first 28 days of life. The disease spectrum varies widely, and early recognition and treatment can substantially reduce the degree of morbidity and mortality associated with systemic infections.
HSV infection limited to the skin, eyes, and mucosal surfaces accounts for 45% of neonatal infections. When this condition is promptly recognized, neonates typically respond well to intravenous acyclovir, with prevention of systemic progression and overall good clinical outcomes. Infections of the central nervous system account for 30% of infections and are more difficult to diagnose due to the nonspecific symptomatology, including lethargy, poor feeding, seizures, and possible absence of lesions. The risk for death decreases from 50% to 6% with treatment; however, most neonates will still require close long-term surveillance for achievement of neurodevelopmental milestones and frequent ophthalmologic and hearing assessments.8,9 Disseminated HSV accounts for 25% of infections and can cause multiorgan failure, with a 31% risk for death despite treatment.5 Therefore, the cornerstone of managing HSV infection in pregnancy is focusing clinical efforts on prevention of transmission to the neonate.
More than 90% of neonatal herpes infections are acquired intrapartum,4 with 60% to 80% of cases occurring in women who developed HSV in the third trimester near the time of delivery.5 Neonates delivered vaginally to these women have a 30% to 50% risk of infection, compared to a <1% risk in neonates born to women with recurrent HSV.1,5,10 The discrepancy in infection risk is thought to be secondary to higher HSV viral loads after an initial infection as opposed to a recurrent infection. Furthermore, acquisition of HSV near term does not allow for the 6 to 12 weeks necessary to develop antibodies that can cross the placenta and provide neonatal protection. The risk of vertical transmission is approximately 25% with an initial-nonprimary episode, reflecting the partial protection afforded by antibody against the other viral serotype.11
Prophylactic therapy has been shown to reduce the rate of asymptomatic viral shedding and recurrent infections near term.7 To reduce the risk of intrapartum transmission, women with a history of HSV prior to or during pregnancy should be treated with acyclovir 400 mg orally 3 times daily starting at 36 weeks of gestation. When patients present with rupture of membranes or labor, they should be asked about prodromal symptoms and thoroughly examined. If prodromal symptoms are present or genital lesions identified, patients should undergo cesarean delivery.12 Some experts also recommend cesarean delivery for women who acquire primary or initial-nonprimary HSV infection in the third trimester due to higher viral loads and potential lack of antibodies at the time of delivery.8,12 However, this recommendation has not been validated by a rigorous prospective randomized clinical trial. When clinically feasible, avoidance of invasive fetal monitoring during labor also has been shown to decrease the risk of HSV transmission by approximately 84% in women with asymptomatic viral shedding.12 This concept may be extrapolated to include assisted delivery with vacuum or forceps.
Universal screening for HSV infection in pregnancy is controversial and widely debated. Most HSV seropositive patients are asymptomatic and will not report a history of HSV infection at the initial prenatal visit. Universal screening, therefore, may increase the rate of unnecessary cesarean deliveries and medical interventions. HSV serology may be beneficial, however, in identifying seronegative pregnant women who have seropositive partners. Two recent studies have shown that 15% to 25% of couples have discordant HSV serologies and consequently are at risk of acquiring primary or initial-nonprimary HSV near term.4,5 These couples should be counseled concerning the use of condoms in the first and second trimester (50% reduction in HSV transmission) and abstinence in the third trimester.5 The seropositive partner also can be offered suppressive therapy, which provides a 48% reduction in the risk of HSV transmission.4 Ultimately, the difficulty lies in balancing the clinical benefits and cost of asymptomatic screening.11
CASE Resolved
The patient should be counseled that HSV infection rarely affects the fetus in utero, and transmission almost always occurs during the delivery process. This patient should receive prophylactic treatment with acyclovir beginning at 36 weeks of gestation to reduce the risk of an outbreak near the time of delivery. ●
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
- Gnann JW, Whitley RJ. Genital herpes. N Engl J Med. 2016;375:666-674.
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2 — United States, 1999–2010. J Infect Dis. 2014;209:325-333.
- Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infec Dis. 2012;56:344-351.
- Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2006;107:426-437.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376-1385.
- Albrecht MA. Treatment of genital herpes simplex virus infection. UpToDate website. Updated June 4, 2019. Accessed March 21, 2021. https://www.uptodate.com/contents/treatment-of-genital-herpes-simplex-virus-infection?search=hsv+treatment
- Sheffield J, Wendel G Jr, Stuart G, et al. Acyclovir prophylaxis to prevent herpes simplex virus recurrence at delivery: a systematic review. Obstet Gynecol. 2003;102:1396-1403.
- American College of Obstetricians and Gynecologists. Management of genital herpes in pregnancy: ACOG practice bulletin summary, number 220. Obstet Gynecol. 2020;135:1236-1238.
- Kimberlin DW. Oral acyclovir suppression after neonatal herpes. N Engl J Med. 2011;365:1284-1292.
- Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247-1252.
- Chatroux IC, Hersh AR, Caughey AB. Herpes simplex virus serotyping in pregnant women with a history of genital herpes and an outbreak in the third trimester. a cost effectiveness analysis. Obstet Gynecol. 2021;137:63-71.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203-209.
Patient-centered contraceptive care for medically complex patients
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
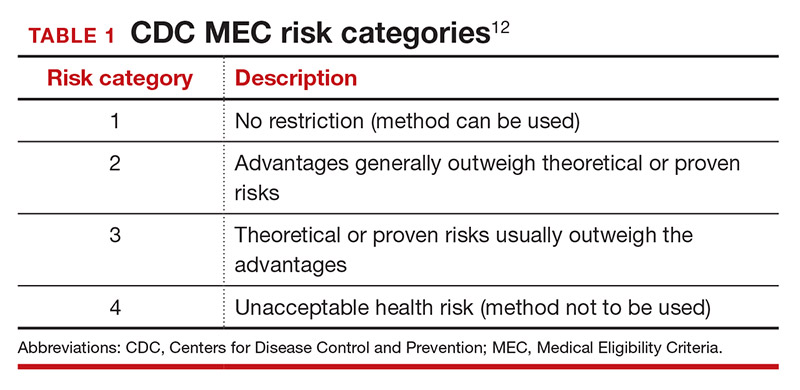
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).
In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
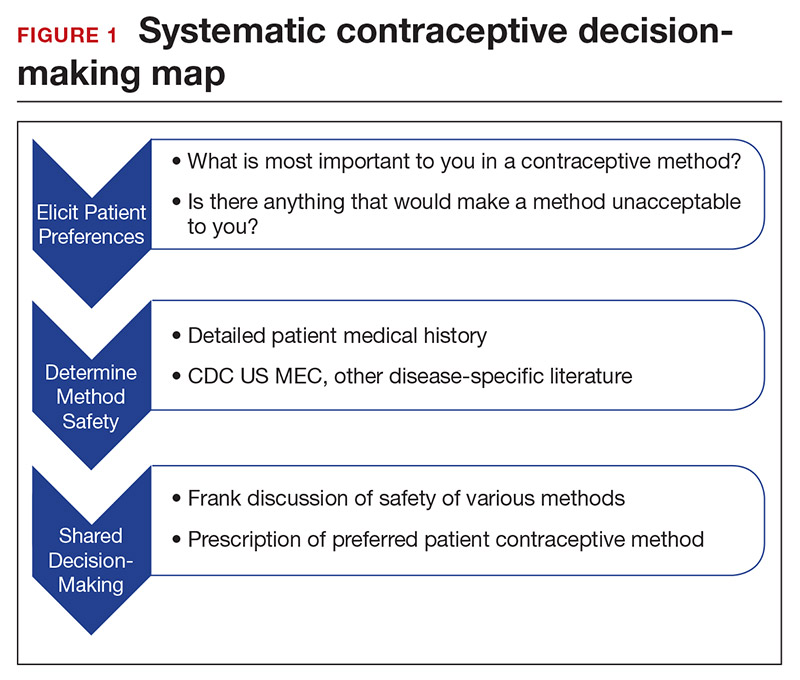
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.
CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
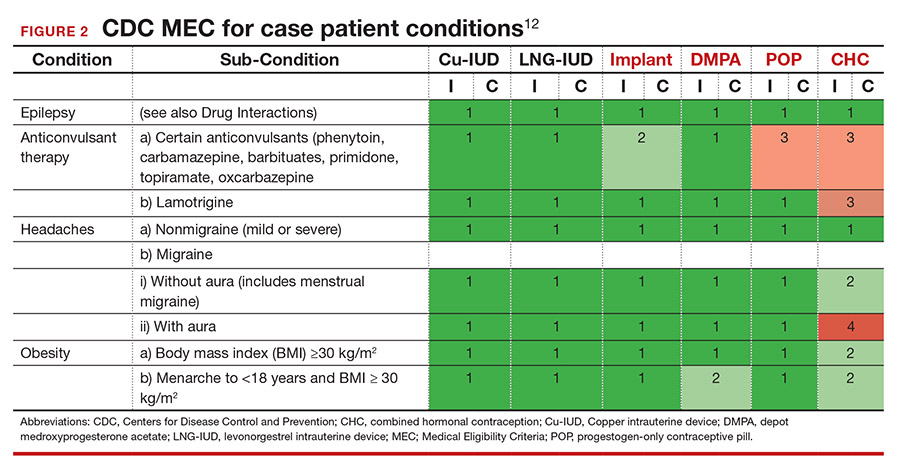
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12
Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).


In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.

CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12

Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).


In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.

CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12

Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
Can a once-daily oral formulation treat symptoms of uterine fibroids without causing hot flashes or bone loss?
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Bedtime soon after meals raises reflux risk in pregnancy
A shorter period between eating and going to sleep increased the risk of GERD during pregnancy by approximately 12%, according to data from 400 women.
Gastroesophageal reflux disease (GERD) is a common condition in pregnancy because of changes in gastrointestinal motility caused by hormonal changes, and a short meal-to-bed time (MTBT) also has been associated with increased GERD symptoms, but data on the impact of MTBT on GERD in pregnant women in particular are lacking, wrote Duc T. Quach, MD, of the University of Medicine and Pharmacy in Ho Chi Minh City, Vietnam, and colleagues.
In a cross-sectional study published in the Journal of Clinical Gastroenterology, the researchers identified 400 pregnant women aged 18 years and older in various stages of pregnancy who were seen at a single hospital in Vietnam. A short MTBT was defined as going to bed 2 hours or less after eating. Primary outcomes were GERD, defined as troublesome heartburn and/or regurgitation at least once a week, and reflux-related insomnia, defined as trouble initiating or maintaining nighttime sleep. Participants also reported the number of days of troublesome reflux symptoms and frequency of reflux-related insomnia over the last 7 days.
A total of 154 participants had a diagnosis of GERD, for an overall prevalence of 38.5%, similar to that seen in GERD studies of GERD and pregnancy, the researchers noted, and of those with GERD, 20 participants (13.0%) reported reflux-related insomnia.
The overall prevalence of heartburn, regurgitation, nausea with or without vomiting, and epigastric pain were 11.8%, 35.8%, 30.0%, and 5.5%, respectively. A total of 139 women reported reflux symptoms on at least 2 of the past 7 days, and 40 women reported both daytime and nighttime reflux symptoms.
Short meal-to-bed time shows strongest association
A short MTBT was the strongest predictor of GERD in multivariate analysis (odds ratio, 12.73; 95% confidence interval, 2.92-55.45; P = .001); previous history of reflux symptoms (OR, 9.05; 95% CI, 5.29-15.50; P < 001) and being in the third trimester versus first or second of pregnancy (OR, 1.66, 95% CI, 1.03-2.69; P = .039) also remained significant predictors in a multivariate analysis. In addition, nighttime short MTBT (but not daytime short MTBT) was the strongest risk factor for reflux-related insomnia (OR, 4.60), although alcohol consumption and a history of reflux-related symptoms also remained significant in multivariate analysis.
“Interestingly, the number of days during which reflux symptoms were experienced during the last 7 days sequentially increased across subgroups of participants with no short MTBT, either daytime or nighttime short MTBT, and with both daytime and nighttime MTBT,” the researchers wrote. At 4-7 days, none of the patients with no short MTBT reported reflux symptoms, compared with 7.5% of those with either daytime or nighttime MTBT and 20.9% of those with both daytime and nighttime MTBT.
The study findings were limited by several factors, including the inability to accurately record participants’ diets and the potential for overestimating the odds ratio of risk factors in patients with reflux-related insomnia because of the small numbers. However, the results support findings from previous studies and suggest that dietary modifications could provide a nonpharmacological treatment target for managing GERD in pregnant women, they concluded.
Behavioral intervention may benefit pregnant women
The study is important because heartburn and regurgitation are common challenges during pregnancy, Ziad F. Gellad, MD, of Duke University, Durham, N.C., said in an interview. “Understanding risk factors for these conditions can be helpful in designing behavioral and pharmaceutical therapeutic interventions.”
The link between short MTBT and increased risk for GERD is well-known, said Dr. Gellad. “Lengthening the time to laying supine after a meal is a common recommendation given to patients with GERD and is included in published GERD guidelines.” Although pregnant woman may have been excluded from trials on which the guidelines and recommendations are based, “it is reasonable to expect that findings would translate to this population that is generally higher risk for reflux,” he noted.
Dr. Gellad was interested to see the dose response between MTBT and reflux, with those patients having both daytime and nighttime short MTBT experiencing reflux more often than those with short MTBT in only one of those time periods (4-7 days vs. 1-3 days).
The key message for clinicians is that, for all individuals, pregnant or not, “avoiding late night meals and short meal-to-bed time is an appropriate behavioral intervention to recommend for patients with troublesome heartburn or regurgitation,” Dr. Gellad emphasized. However, more research is needed in some areas, “implementation studies would be helpful to understand how best to educate patients on behavioral modifications known to decrease reflux symptoms.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gellad had no relevant financial disclosures, but serves as a member of the GI & Hepatology News board of editors.
A shorter period between eating and going to sleep increased the risk of GERD during pregnancy by approximately 12%, according to data from 400 women.
Gastroesophageal reflux disease (GERD) is a common condition in pregnancy because of changes in gastrointestinal motility caused by hormonal changes, and a short meal-to-bed time (MTBT) also has been associated with increased GERD symptoms, but data on the impact of MTBT on GERD in pregnant women in particular are lacking, wrote Duc T. Quach, MD, of the University of Medicine and Pharmacy in Ho Chi Minh City, Vietnam, and colleagues.
In a cross-sectional study published in the Journal of Clinical Gastroenterology, the researchers identified 400 pregnant women aged 18 years and older in various stages of pregnancy who were seen at a single hospital in Vietnam. A short MTBT was defined as going to bed 2 hours or less after eating. Primary outcomes were GERD, defined as troublesome heartburn and/or regurgitation at least once a week, and reflux-related insomnia, defined as trouble initiating or maintaining nighttime sleep. Participants also reported the number of days of troublesome reflux symptoms and frequency of reflux-related insomnia over the last 7 days.
A total of 154 participants had a diagnosis of GERD, for an overall prevalence of 38.5%, similar to that seen in GERD studies of GERD and pregnancy, the researchers noted, and of those with GERD, 20 participants (13.0%) reported reflux-related insomnia.
The overall prevalence of heartburn, regurgitation, nausea with or without vomiting, and epigastric pain were 11.8%, 35.8%, 30.0%, and 5.5%, respectively. A total of 139 women reported reflux symptoms on at least 2 of the past 7 days, and 40 women reported both daytime and nighttime reflux symptoms.
Short meal-to-bed time shows strongest association
A short MTBT was the strongest predictor of GERD in multivariate analysis (odds ratio, 12.73; 95% confidence interval, 2.92-55.45; P = .001); previous history of reflux symptoms (OR, 9.05; 95% CI, 5.29-15.50; P < 001) and being in the third trimester versus first or second of pregnancy (OR, 1.66, 95% CI, 1.03-2.69; P = .039) also remained significant predictors in a multivariate analysis. In addition, nighttime short MTBT (but not daytime short MTBT) was the strongest risk factor for reflux-related insomnia (OR, 4.60), although alcohol consumption and a history of reflux-related symptoms also remained significant in multivariate analysis.
“Interestingly, the number of days during which reflux symptoms were experienced during the last 7 days sequentially increased across subgroups of participants with no short MTBT, either daytime or nighttime short MTBT, and with both daytime and nighttime MTBT,” the researchers wrote. At 4-7 days, none of the patients with no short MTBT reported reflux symptoms, compared with 7.5% of those with either daytime or nighttime MTBT and 20.9% of those with both daytime and nighttime MTBT.
The study findings were limited by several factors, including the inability to accurately record participants’ diets and the potential for overestimating the odds ratio of risk factors in patients with reflux-related insomnia because of the small numbers. However, the results support findings from previous studies and suggest that dietary modifications could provide a nonpharmacological treatment target for managing GERD in pregnant women, they concluded.
Behavioral intervention may benefit pregnant women
The study is important because heartburn and regurgitation are common challenges during pregnancy, Ziad F. Gellad, MD, of Duke University, Durham, N.C., said in an interview. “Understanding risk factors for these conditions can be helpful in designing behavioral and pharmaceutical therapeutic interventions.”
The link between short MTBT and increased risk for GERD is well-known, said Dr. Gellad. “Lengthening the time to laying supine after a meal is a common recommendation given to patients with GERD and is included in published GERD guidelines.” Although pregnant woman may have been excluded from trials on which the guidelines and recommendations are based, “it is reasonable to expect that findings would translate to this population that is generally higher risk for reflux,” he noted.
Dr. Gellad was interested to see the dose response between MTBT and reflux, with those patients having both daytime and nighttime short MTBT experiencing reflux more often than those with short MTBT in only one of those time periods (4-7 days vs. 1-3 days).
The key message for clinicians is that, for all individuals, pregnant or not, “avoiding late night meals and short meal-to-bed time is an appropriate behavioral intervention to recommend for patients with troublesome heartburn or regurgitation,” Dr. Gellad emphasized. However, more research is needed in some areas, “implementation studies would be helpful to understand how best to educate patients on behavioral modifications known to decrease reflux symptoms.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gellad had no relevant financial disclosures, but serves as a member of the GI & Hepatology News board of editors.
A shorter period between eating and going to sleep increased the risk of GERD during pregnancy by approximately 12%, according to data from 400 women.
Gastroesophageal reflux disease (GERD) is a common condition in pregnancy because of changes in gastrointestinal motility caused by hormonal changes, and a short meal-to-bed time (MTBT) also has been associated with increased GERD symptoms, but data on the impact of MTBT on GERD in pregnant women in particular are lacking, wrote Duc T. Quach, MD, of the University of Medicine and Pharmacy in Ho Chi Minh City, Vietnam, and colleagues.
In a cross-sectional study published in the Journal of Clinical Gastroenterology, the researchers identified 400 pregnant women aged 18 years and older in various stages of pregnancy who were seen at a single hospital in Vietnam. A short MTBT was defined as going to bed 2 hours or less after eating. Primary outcomes were GERD, defined as troublesome heartburn and/or regurgitation at least once a week, and reflux-related insomnia, defined as trouble initiating or maintaining nighttime sleep. Participants also reported the number of days of troublesome reflux symptoms and frequency of reflux-related insomnia over the last 7 days.
A total of 154 participants had a diagnosis of GERD, for an overall prevalence of 38.5%, similar to that seen in GERD studies of GERD and pregnancy, the researchers noted, and of those with GERD, 20 participants (13.0%) reported reflux-related insomnia.
The overall prevalence of heartburn, regurgitation, nausea with or without vomiting, and epigastric pain were 11.8%, 35.8%, 30.0%, and 5.5%, respectively. A total of 139 women reported reflux symptoms on at least 2 of the past 7 days, and 40 women reported both daytime and nighttime reflux symptoms.
Short meal-to-bed time shows strongest association
A short MTBT was the strongest predictor of GERD in multivariate analysis (odds ratio, 12.73; 95% confidence interval, 2.92-55.45; P = .001); previous history of reflux symptoms (OR, 9.05; 95% CI, 5.29-15.50; P < 001) and being in the third trimester versus first or second of pregnancy (OR, 1.66, 95% CI, 1.03-2.69; P = .039) also remained significant predictors in a multivariate analysis. In addition, nighttime short MTBT (but not daytime short MTBT) was the strongest risk factor for reflux-related insomnia (OR, 4.60), although alcohol consumption and a history of reflux-related symptoms also remained significant in multivariate analysis.
“Interestingly, the number of days during which reflux symptoms were experienced during the last 7 days sequentially increased across subgroups of participants with no short MTBT, either daytime or nighttime short MTBT, and with both daytime and nighttime MTBT,” the researchers wrote. At 4-7 days, none of the patients with no short MTBT reported reflux symptoms, compared with 7.5% of those with either daytime or nighttime MTBT and 20.9% of those with both daytime and nighttime MTBT.
The study findings were limited by several factors, including the inability to accurately record participants’ diets and the potential for overestimating the odds ratio of risk factors in patients with reflux-related insomnia because of the small numbers. However, the results support findings from previous studies and suggest that dietary modifications could provide a nonpharmacological treatment target for managing GERD in pregnant women, they concluded.
Behavioral intervention may benefit pregnant women
The study is important because heartburn and regurgitation are common challenges during pregnancy, Ziad F. Gellad, MD, of Duke University, Durham, N.C., said in an interview. “Understanding risk factors for these conditions can be helpful in designing behavioral and pharmaceutical therapeutic interventions.”
The link between short MTBT and increased risk for GERD is well-known, said Dr. Gellad. “Lengthening the time to laying supine after a meal is a common recommendation given to patients with GERD and is included in published GERD guidelines.” Although pregnant woman may have been excluded from trials on which the guidelines and recommendations are based, “it is reasonable to expect that findings would translate to this population that is generally higher risk for reflux,” he noted.
Dr. Gellad was interested to see the dose response between MTBT and reflux, with those patients having both daytime and nighttime short MTBT experiencing reflux more often than those with short MTBT in only one of those time periods (4-7 days vs. 1-3 days).
The key message for clinicians is that, for all individuals, pregnant or not, “avoiding late night meals and short meal-to-bed time is an appropriate behavioral intervention to recommend for patients with troublesome heartburn or regurgitation,” Dr. Gellad emphasized. However, more research is needed in some areas, “implementation studies would be helpful to understand how best to educate patients on behavioral modifications known to decrease reflux symptoms.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gellad had no relevant financial disclosures, but serves as a member of the GI & Hepatology News board of editors.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Adult separation anxiety raises suicidality risk
Separation anxiety plays a substantial role in suicidality in patients with mood and anxiety disorders, new research suggests.
Results of a study that included 500 outpatients with mood or anxiety disorders showed adult separation anxiety disorder (ASAD) was more frequent in patients with suicidal thoughts versus those who did not have the disorder. In addition, depression and separation anxiety also significantly predicted lifetime suicide risk.
“This study indicates a substantial role of separation anxiety in predicting suicidal thoughts, both as state-related symptoms ... and as longitudinal dimension symptoms,” say the investigators, led by Stefano Pini, MD, of the department of clinical and experimental medicine, section of psychiatry, University of Pisa (Italy).
“ for reducing suicide risk,” they add.
The study was published in the March/April issue of the Journal of Clinical Psychiatry.
Frequently underdiagnosed
The authors describe a “close link between suicidal behaviors and interpersonal difficulties extending beyond the traditional approach of comprehending suicide as a phenomenon mainly related to depression.”
Previous research indicates that insecure adult attachment style might be associated with a greater likelihood of suicidal thoughts and attempts, and there might be an association between individual abnormal attachment sensitivity and suicide.
“Suicidal ideation or suicide attempts may be associated with disturbances in attachment, which may lead not only to a devastating experience of losing the feeling of interdependence and closeness but also to a rejection of life itself,” the authors suggest.
ASAD may be a “key factor” in understanding the relationship between individual attachment sensitivity to separation and suicidality.
An ASAD diagnosis was traditionally reserved for children and adolescents, but DSM-5 expanded the diagnosis to include adults over 18 years of age because research had “found a later onset to be common,” spanning the life course, even in the absence of a history of separation anxiety in childhood.
“Separation anxiety is an important clinical dimension, often with roots in childhood, but likely to manifest across the lifespan,” the authors note, adding that it is “frequently underdiagnosed.”
The relationship between ASAD and suicidality has not been explored extensively, so the researchers set out to examine the association.
The study included 509 consecutively recruited adult psychiatric outpatients with mood or anxiety disorders as a principle diagnosis.
Participants completed an array of scales, including item 3 on the Hamilton Depression Rating Scale (HDRS), which measures suicidality, as well as the Mood Spectrum Self-Report (MOODS-SR), a questionnaire evaluating lifetime suicidal symptoms.
Three scales were used to measure separation anxiety disorder: The Structured Interview for Separation Anxiety Symptoms in Adulthood/Childhood (SCI-SAS-A/C); the Separation Anxiety Symptom Inventory (SASI); and the Adult Separation Anxiety Scale (ASA-27).
Waxing and waning
Of the total sample, 215 patients were diagnosed with separation anxiety disorder (mean age at onset 15 years). Of the total sample, 19.9% scored ≥ 1 on the HDRS item 3, indicating the presence of suicidality.
Patients with suicidal thoughts more frequently experienced ASAD, compared with those without suicidal thoughts (53.6% vs. 39.6%, respectively, P = .01).
“All measures of adult as well as childhood separation anxiety were significantly elevated in the group of patients with current suicidality, based on HDRS item 3,” the authors report.
Logistic regression found that ASAD, major depression, bipolar I, and bipolar II disorders all predicted suicidal thoughts.
A linear regression model found that depression (P = .001) and ASA-27 separation anxiety (P = .001) significantly predicted lifetime suicide risk, based on the MOODS-SR scale.
In addition, “mediation analysis showed that, besides a direct effect, there is also an indirect effect of depression severity on the MOODS-SR suicidality score through the ASA-27 score, indicating that separation anxiety may act as an important mediating factor in the relationship between depression and suicidality,” the authors state.
The authors observe that separation anxiety “is an important clinical dimension, often with roots in childhood, but likely to wax and wane across the lifespan and even to manifest for the first time during adulthood.”
Treatment target?
Commenting on the study for this news organization, Megan Rogers, PhD, postdoctoral research fellow, Mount Sinai Beth Israel, New York, said the findings “point to symptoms of separation anxiety as a potential indicator of suicidal ideation, and should these findings be replicated and extended through longitudinal research, it suggests that symptoms of separation anxiety may be a relevant treatment target in certain populations to mitigate suicide risk.”
Dr. Rogers, who is the student division director at the American Association of Suicidology and was not involved with the study, said she thinks that studies of suicide have focused more on “individual symptoms of separation anxiety, such as excessive worry about loved ones or distress when anticipating separation from loved ones, rather than on separation anxiety as a categorical diagnosis.”
However, the study has an important take-home message for practicing clinicians, Dr. Rogers said. “In individuals with separation anxiety disorders, particularly those with comorbid mood conditions, it may be worth conducting a more thorough assessment of suicide risk, given the possibility of elevated suicidality in these patients.”
The study was supported in part by the German Research Foundation and the Fondazione Cassa di Risparmio di la Spezia. The authors and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Separation anxiety plays a substantial role in suicidality in patients with mood and anxiety disorders, new research suggests.
Results of a study that included 500 outpatients with mood or anxiety disorders showed adult separation anxiety disorder (ASAD) was more frequent in patients with suicidal thoughts versus those who did not have the disorder. In addition, depression and separation anxiety also significantly predicted lifetime suicide risk.
“This study indicates a substantial role of separation anxiety in predicting suicidal thoughts, both as state-related symptoms ... and as longitudinal dimension symptoms,” say the investigators, led by Stefano Pini, MD, of the department of clinical and experimental medicine, section of psychiatry, University of Pisa (Italy).
“ for reducing suicide risk,” they add.
The study was published in the March/April issue of the Journal of Clinical Psychiatry.
Frequently underdiagnosed
The authors describe a “close link between suicidal behaviors and interpersonal difficulties extending beyond the traditional approach of comprehending suicide as a phenomenon mainly related to depression.”
Previous research indicates that insecure adult attachment style might be associated with a greater likelihood of suicidal thoughts and attempts, and there might be an association between individual abnormal attachment sensitivity and suicide.
“Suicidal ideation or suicide attempts may be associated with disturbances in attachment, which may lead not only to a devastating experience of losing the feeling of interdependence and closeness but also to a rejection of life itself,” the authors suggest.
ASAD may be a “key factor” in understanding the relationship between individual attachment sensitivity to separation and suicidality.
An ASAD diagnosis was traditionally reserved for children and adolescents, but DSM-5 expanded the diagnosis to include adults over 18 years of age because research had “found a later onset to be common,” spanning the life course, even in the absence of a history of separation anxiety in childhood.
“Separation anxiety is an important clinical dimension, often with roots in childhood, but likely to manifest across the lifespan,” the authors note, adding that it is “frequently underdiagnosed.”
The relationship between ASAD and suicidality has not been explored extensively, so the researchers set out to examine the association.
The study included 509 consecutively recruited adult psychiatric outpatients with mood or anxiety disorders as a principle diagnosis.
Participants completed an array of scales, including item 3 on the Hamilton Depression Rating Scale (HDRS), which measures suicidality, as well as the Mood Spectrum Self-Report (MOODS-SR), a questionnaire evaluating lifetime suicidal symptoms.
Three scales were used to measure separation anxiety disorder: The Structured Interview for Separation Anxiety Symptoms in Adulthood/Childhood (SCI-SAS-A/C); the Separation Anxiety Symptom Inventory (SASI); and the Adult Separation Anxiety Scale (ASA-27).
Waxing and waning
Of the total sample, 215 patients were diagnosed with separation anxiety disorder (mean age at onset 15 years). Of the total sample, 19.9% scored ≥ 1 on the HDRS item 3, indicating the presence of suicidality.
Patients with suicidal thoughts more frequently experienced ASAD, compared with those without suicidal thoughts (53.6% vs. 39.6%, respectively, P = .01).
“All measures of adult as well as childhood separation anxiety were significantly elevated in the group of patients with current suicidality, based on HDRS item 3,” the authors report.
Logistic regression found that ASAD, major depression, bipolar I, and bipolar II disorders all predicted suicidal thoughts.
A linear regression model found that depression (P = .001) and ASA-27 separation anxiety (P = .001) significantly predicted lifetime suicide risk, based on the MOODS-SR scale.
In addition, “mediation analysis showed that, besides a direct effect, there is also an indirect effect of depression severity on the MOODS-SR suicidality score through the ASA-27 score, indicating that separation anxiety may act as an important mediating factor in the relationship between depression and suicidality,” the authors state.
The authors observe that separation anxiety “is an important clinical dimension, often with roots in childhood, but likely to wax and wane across the lifespan and even to manifest for the first time during adulthood.”
Treatment target?
Commenting on the study for this news organization, Megan Rogers, PhD, postdoctoral research fellow, Mount Sinai Beth Israel, New York, said the findings “point to symptoms of separation anxiety as a potential indicator of suicidal ideation, and should these findings be replicated and extended through longitudinal research, it suggests that symptoms of separation anxiety may be a relevant treatment target in certain populations to mitigate suicide risk.”
Dr. Rogers, who is the student division director at the American Association of Suicidology and was not involved with the study, said she thinks that studies of suicide have focused more on “individual symptoms of separation anxiety, such as excessive worry about loved ones or distress when anticipating separation from loved ones, rather than on separation anxiety as a categorical diagnosis.”
However, the study has an important take-home message for practicing clinicians, Dr. Rogers said. “In individuals with separation anxiety disorders, particularly those with comorbid mood conditions, it may be worth conducting a more thorough assessment of suicide risk, given the possibility of elevated suicidality in these patients.”
The study was supported in part by the German Research Foundation and the Fondazione Cassa di Risparmio di la Spezia. The authors and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Separation anxiety plays a substantial role in suicidality in patients with mood and anxiety disorders, new research suggests.
Results of a study that included 500 outpatients with mood or anxiety disorders showed adult separation anxiety disorder (ASAD) was more frequent in patients with suicidal thoughts versus those who did not have the disorder. In addition, depression and separation anxiety also significantly predicted lifetime suicide risk.
“This study indicates a substantial role of separation anxiety in predicting suicidal thoughts, both as state-related symptoms ... and as longitudinal dimension symptoms,” say the investigators, led by Stefano Pini, MD, of the department of clinical and experimental medicine, section of psychiatry, University of Pisa (Italy).
“ for reducing suicide risk,” they add.
The study was published in the March/April issue of the Journal of Clinical Psychiatry.
Frequently underdiagnosed
The authors describe a “close link between suicidal behaviors and interpersonal difficulties extending beyond the traditional approach of comprehending suicide as a phenomenon mainly related to depression.”
Previous research indicates that insecure adult attachment style might be associated with a greater likelihood of suicidal thoughts and attempts, and there might be an association between individual abnormal attachment sensitivity and suicide.
“Suicidal ideation or suicide attempts may be associated with disturbances in attachment, which may lead not only to a devastating experience of losing the feeling of interdependence and closeness but also to a rejection of life itself,” the authors suggest.
ASAD may be a “key factor” in understanding the relationship between individual attachment sensitivity to separation and suicidality.
An ASAD diagnosis was traditionally reserved for children and adolescents, but DSM-5 expanded the diagnosis to include adults over 18 years of age because research had “found a later onset to be common,” spanning the life course, even in the absence of a history of separation anxiety in childhood.
“Separation anxiety is an important clinical dimension, often with roots in childhood, but likely to manifest across the lifespan,” the authors note, adding that it is “frequently underdiagnosed.”
The relationship between ASAD and suicidality has not been explored extensively, so the researchers set out to examine the association.
The study included 509 consecutively recruited adult psychiatric outpatients with mood or anxiety disorders as a principle diagnosis.
Participants completed an array of scales, including item 3 on the Hamilton Depression Rating Scale (HDRS), which measures suicidality, as well as the Mood Spectrum Self-Report (MOODS-SR), a questionnaire evaluating lifetime suicidal symptoms.
Three scales were used to measure separation anxiety disorder: The Structured Interview for Separation Anxiety Symptoms in Adulthood/Childhood (SCI-SAS-A/C); the Separation Anxiety Symptom Inventory (SASI); and the Adult Separation Anxiety Scale (ASA-27).
Waxing and waning
Of the total sample, 215 patients were diagnosed with separation anxiety disorder (mean age at onset 15 years). Of the total sample, 19.9% scored ≥ 1 on the HDRS item 3, indicating the presence of suicidality.
Patients with suicidal thoughts more frequently experienced ASAD, compared with those without suicidal thoughts (53.6% vs. 39.6%, respectively, P = .01).
“All measures of adult as well as childhood separation anxiety were significantly elevated in the group of patients with current suicidality, based on HDRS item 3,” the authors report.
Logistic regression found that ASAD, major depression, bipolar I, and bipolar II disorders all predicted suicidal thoughts.
A linear regression model found that depression (P = .001) and ASA-27 separation anxiety (P = .001) significantly predicted lifetime suicide risk, based on the MOODS-SR scale.
In addition, “mediation analysis showed that, besides a direct effect, there is also an indirect effect of depression severity on the MOODS-SR suicidality score through the ASA-27 score, indicating that separation anxiety may act as an important mediating factor in the relationship between depression and suicidality,” the authors state.
The authors observe that separation anxiety “is an important clinical dimension, often with roots in childhood, but likely to wax and wane across the lifespan and even to manifest for the first time during adulthood.”
Treatment target?
Commenting on the study for this news organization, Megan Rogers, PhD, postdoctoral research fellow, Mount Sinai Beth Israel, New York, said the findings “point to symptoms of separation anxiety as a potential indicator of suicidal ideation, and should these findings be replicated and extended through longitudinal research, it suggests that symptoms of separation anxiety may be a relevant treatment target in certain populations to mitigate suicide risk.”
Dr. Rogers, who is the student division director at the American Association of Suicidology and was not involved with the study, said she thinks that studies of suicide have focused more on “individual symptoms of separation anxiety, such as excessive worry about loved ones or distress when anticipating separation from loved ones, rather than on separation anxiety as a categorical diagnosis.”
However, the study has an important take-home message for practicing clinicians, Dr. Rogers said. “In individuals with separation anxiety disorders, particularly those with comorbid mood conditions, it may be worth conducting a more thorough assessment of suicide risk, given the possibility of elevated suicidality in these patients.”
The study was supported in part by the German Research Foundation and the Fondazione Cassa di Risparmio di la Spezia. The authors and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Novel antiplatelet drug: Hope for efficacy without bleeding?
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
The interplay between staffing and scheduling
Top five findings from the 2020 SoHM
The biennial State of Hospital Medicine (SoHM) Report was released in fall 2020, reflecting surveys collected just as the pandemic was ramping up. Thus, a COVID Addendum of results was collected and published a few months later. What did these reports tell us about existing and developing trends in staffing and scheduling?
Here is a top five list of findings for hospital medicine programs (HMGs) serving adults only. These are just highlights; for further detail, visit the SHM website to learn more and purchase your copy. The information in the SoHM is extraordinarily helpful in planning for your group’s future staffing and scheduling needs.
5. Average group size has increased
Andrew White, MD, SFHM, associate professor of medicine at the University of Washington, Seattle, provided a deep-dive discussion on the increase of group sizes in the March 2021 issue of The Hospitalist. Group size has impacted the way a hospitalist group schedules, when reviewing correlating scheduling survey responses.
Group size can have a direct correlation to scheduling methodology. The number of employed/contracted physician hospitalists in individual groups is up about 25%. Alongside the increase in physician hospitalists is an increase in both nurse practitioners (NPs) and physician assistants (PAs) in adult hospitalist groups, with the largest growth in PAs. In fact, in 2020, the average number of NPs and PAs per hospitalist group is approaching similar numbers.
In 2020, more than half of all programs reported not having a backup call system. One could speculate that the larger group size has allowed adult hospitalist groups better ability to staff upper fluctuations in daily volume. This could have resulted in day-to-day scheduling ease and flexibility.
4. Shift-type is shifting
In scheduling adult hospitalist groups, fewer groups reported dedicated nocturnists and more groups reported dedicated day admitters.
Just above a third of all adult hospitalist programs report having dedicated day admitter shifts, and the presence of nocturnists is at a 6-year low. One speculation that could be made is that hospitalists are making more of an effort to ensure that more admissions are done during the day and not held over for nighttime. This would be consistent with the strong pressure for hospitals to decrease door-to-floor admission times. However, the presence of nocturnists increases steadily with group size. Ninety-four percent of HMGs with 30-49 physician FTEs and 98% of groups with 50 or more physician FTEs reported using dedicated nocturnists.
3. COVID-19 impacts hospitalist workflows
It’s not hard to imagine that COVID-19 has affected all hospitalist groups: adult, pediatric, and adult/pediatric groups. COVID-19 has affected all lives – at home and at work – across the world. More than 80% of all adult hospitalist groups report having implemented changes (beyond dedicated COVID-19 teams) in workflows and/or how work is allocated among its providers. And nearly 20% report that this is likely a permanent change.
2. Schedules have been disrupted by COVID-19
More than half of adult hospitalist groups report having their schedules disrupted by COVID-19. The top two disruptors are loss of staff time due to exposure quarantine, and lost provider time due to COVID-19 illness. All the while, adult hospitalist groups have been taking care of more and more hospitalized patients.
While some groups have not made any changes in scheduling due to COVID-19, many have. Nearly 60% of all groups have increased scheduling flexibility or changed their scheduling model. For about 11% of all groups, this change is likely to be permanent.
1. COVID-19 has changed scheduling methodologies – perhaps for the long-term
Three out of four adult hospitalist groups have created new COVID-19 dedicated teams each day. This has likely had one of the most impacts.
Unit-based assignment reported in the 2020 SoHM was already up from the 2018 SoHM Report (42.7% vs 36%). Now, in addition to nocturnists, day admitter, rounder roles, and unit-based assignments, hospital medicine groups must also incorporate COVID-19 teams into daily scheduling considerations. One in five groups report this change may likely be a permanent addition to the hospitalist schedule. Wow.
As we think forward to the 2022 SoHM, staffing and scheduling of adult hospital medicine group will be a key topic of the survey. How does COVID-19 change hospital medicine groups in the medium and long term? One thing is for sure – hospital medicine groups are resilient and have proven to be creative in ensuring our hospitalized patients are well cared for.
Post your thoughts and questions for your peer network on the SHM Online Community: HMX. Let’s keep the conversation going on how we can help each other create sustainable staffing and scheduling models that are continuously adapting to our peripandemic environment.
Ms. Trask is national vice president of the Hospital Medicine Service Line at Catholic Health Initiatives in Englewood, Colo.
Top five findings from the 2020 SoHM
Top five findings from the 2020 SoHM
The biennial State of Hospital Medicine (SoHM) Report was released in fall 2020, reflecting surveys collected just as the pandemic was ramping up. Thus, a COVID Addendum of results was collected and published a few months later. What did these reports tell us about existing and developing trends in staffing and scheduling?
Here is a top five list of findings for hospital medicine programs (HMGs) serving adults only. These are just highlights; for further detail, visit the SHM website to learn more and purchase your copy. The information in the SoHM is extraordinarily helpful in planning for your group’s future staffing and scheduling needs.
5. Average group size has increased
Andrew White, MD, SFHM, associate professor of medicine at the University of Washington, Seattle, provided a deep-dive discussion on the increase of group sizes in the March 2021 issue of The Hospitalist. Group size has impacted the way a hospitalist group schedules, when reviewing correlating scheduling survey responses.
Group size can have a direct correlation to scheduling methodology. The number of employed/contracted physician hospitalists in individual groups is up about 25%. Alongside the increase in physician hospitalists is an increase in both nurse practitioners (NPs) and physician assistants (PAs) in adult hospitalist groups, with the largest growth in PAs. In fact, in 2020, the average number of NPs and PAs per hospitalist group is approaching similar numbers.
In 2020, more than half of all programs reported not having a backup call system. One could speculate that the larger group size has allowed adult hospitalist groups better ability to staff upper fluctuations in daily volume. This could have resulted in day-to-day scheduling ease and flexibility.
4. Shift-type is shifting
In scheduling adult hospitalist groups, fewer groups reported dedicated nocturnists and more groups reported dedicated day admitters.
Just above a third of all adult hospitalist programs report having dedicated day admitter shifts, and the presence of nocturnists is at a 6-year low. One speculation that could be made is that hospitalists are making more of an effort to ensure that more admissions are done during the day and not held over for nighttime. This would be consistent with the strong pressure for hospitals to decrease door-to-floor admission times. However, the presence of nocturnists increases steadily with group size. Ninety-four percent of HMGs with 30-49 physician FTEs and 98% of groups with 50 or more physician FTEs reported using dedicated nocturnists.
3. COVID-19 impacts hospitalist workflows
It’s not hard to imagine that COVID-19 has affected all hospitalist groups: adult, pediatric, and adult/pediatric groups. COVID-19 has affected all lives – at home and at work – across the world. More than 80% of all adult hospitalist groups report having implemented changes (beyond dedicated COVID-19 teams) in workflows and/or how work is allocated among its providers. And nearly 20% report that this is likely a permanent change.
2. Schedules have been disrupted by COVID-19
More than half of adult hospitalist groups report having their schedules disrupted by COVID-19. The top two disruptors are loss of staff time due to exposure quarantine, and lost provider time due to COVID-19 illness. All the while, adult hospitalist groups have been taking care of more and more hospitalized patients.
While some groups have not made any changes in scheduling due to COVID-19, many have. Nearly 60% of all groups have increased scheduling flexibility or changed their scheduling model. For about 11% of all groups, this change is likely to be permanent.
1. COVID-19 has changed scheduling methodologies – perhaps for the long-term
Three out of four adult hospitalist groups have created new COVID-19 dedicated teams each day. This has likely had one of the most impacts.
Unit-based assignment reported in the 2020 SoHM was already up from the 2018 SoHM Report (42.7% vs 36%). Now, in addition to nocturnists, day admitter, rounder roles, and unit-based assignments, hospital medicine groups must also incorporate COVID-19 teams into daily scheduling considerations. One in five groups report this change may likely be a permanent addition to the hospitalist schedule. Wow.
As we think forward to the 2022 SoHM, staffing and scheduling of adult hospital medicine group will be a key topic of the survey. How does COVID-19 change hospital medicine groups in the medium and long term? One thing is for sure – hospital medicine groups are resilient and have proven to be creative in ensuring our hospitalized patients are well cared for.
Post your thoughts and questions for your peer network on the SHM Online Community: HMX. Let’s keep the conversation going on how we can help each other create sustainable staffing and scheduling models that are continuously adapting to our peripandemic environment.
Ms. Trask is national vice president of the Hospital Medicine Service Line at Catholic Health Initiatives in Englewood, Colo.
The biennial State of Hospital Medicine (SoHM) Report was released in fall 2020, reflecting surveys collected just as the pandemic was ramping up. Thus, a COVID Addendum of results was collected and published a few months later. What did these reports tell us about existing and developing trends in staffing and scheduling?
Here is a top five list of findings for hospital medicine programs (HMGs) serving adults only. These are just highlights; for further detail, visit the SHM website to learn more and purchase your copy. The information in the SoHM is extraordinarily helpful in planning for your group’s future staffing and scheduling needs.
5. Average group size has increased
Andrew White, MD, SFHM, associate professor of medicine at the University of Washington, Seattle, provided a deep-dive discussion on the increase of group sizes in the March 2021 issue of The Hospitalist. Group size has impacted the way a hospitalist group schedules, when reviewing correlating scheduling survey responses.
Group size can have a direct correlation to scheduling methodology. The number of employed/contracted physician hospitalists in individual groups is up about 25%. Alongside the increase in physician hospitalists is an increase in both nurse practitioners (NPs) and physician assistants (PAs) in adult hospitalist groups, with the largest growth in PAs. In fact, in 2020, the average number of NPs and PAs per hospitalist group is approaching similar numbers.
In 2020, more than half of all programs reported not having a backup call system. One could speculate that the larger group size has allowed adult hospitalist groups better ability to staff upper fluctuations in daily volume. This could have resulted in day-to-day scheduling ease and flexibility.
4. Shift-type is shifting
In scheduling adult hospitalist groups, fewer groups reported dedicated nocturnists and more groups reported dedicated day admitters.
Just above a third of all adult hospitalist programs report having dedicated day admitter shifts, and the presence of nocturnists is at a 6-year low. One speculation that could be made is that hospitalists are making more of an effort to ensure that more admissions are done during the day and not held over for nighttime. This would be consistent with the strong pressure for hospitals to decrease door-to-floor admission times. However, the presence of nocturnists increases steadily with group size. Ninety-four percent of HMGs with 30-49 physician FTEs and 98% of groups with 50 or more physician FTEs reported using dedicated nocturnists.
3. COVID-19 impacts hospitalist workflows
It’s not hard to imagine that COVID-19 has affected all hospitalist groups: adult, pediatric, and adult/pediatric groups. COVID-19 has affected all lives – at home and at work – across the world. More than 80% of all adult hospitalist groups report having implemented changes (beyond dedicated COVID-19 teams) in workflows and/or how work is allocated among its providers. And nearly 20% report that this is likely a permanent change.
2. Schedules have been disrupted by COVID-19
More than half of adult hospitalist groups report having their schedules disrupted by COVID-19. The top two disruptors are loss of staff time due to exposure quarantine, and lost provider time due to COVID-19 illness. All the while, adult hospitalist groups have been taking care of more and more hospitalized patients.
While some groups have not made any changes in scheduling due to COVID-19, many have. Nearly 60% of all groups have increased scheduling flexibility or changed their scheduling model. For about 11% of all groups, this change is likely to be permanent.
1. COVID-19 has changed scheduling methodologies – perhaps for the long-term
Three out of four adult hospitalist groups have created new COVID-19 dedicated teams each day. This has likely had one of the most impacts.
Unit-based assignment reported in the 2020 SoHM was already up from the 2018 SoHM Report (42.7% vs 36%). Now, in addition to nocturnists, day admitter, rounder roles, and unit-based assignments, hospital medicine groups must also incorporate COVID-19 teams into daily scheduling considerations. One in five groups report this change may likely be a permanent addition to the hospitalist schedule. Wow.
As we think forward to the 2022 SoHM, staffing and scheduling of adult hospital medicine group will be a key topic of the survey. How does COVID-19 change hospital medicine groups in the medium and long term? One thing is for sure – hospital medicine groups are resilient and have proven to be creative in ensuring our hospitalized patients are well cared for.
Post your thoughts and questions for your peer network on the SHM Online Community: HMX. Let’s keep the conversation going on how we can help each other create sustainable staffing and scheduling models that are continuously adapting to our peripandemic environment.
Ms. Trask is national vice president of the Hospital Medicine Service Line at Catholic Health Initiatives in Englewood, Colo.
Verification bias casts doubt on IgA tTG in celiac disease
Immunoglobulin A tissue transglutaminase offers a noninvasive way to detect celiac disease, but new research suggests that its sensitivity may be overestimated and that it may not be an effective screening test, at least in asymptomatic individuals. The reason comes down to verification bias, wherein a technique appears to have higher sensitivity and lower specificity because individuals who screen positive are more likely to have their disease confirmed by a follow-up small-bowel biopsy while those who screen negative are unlikely to have a follow-up biopsy that could reveal missed celiac disease.
“The issue with verification bias is that only the patients that screen positive on that index test are going to be getting the reference test, so there’s probably a good chance that if they screen positive when they go to that reference test they’ll also be positive. What you’re missing from when you’re calculating sensitivity is, what about the ones that are negative on the index test? Would they have been positive on that reference test? That’s not even coming into your calculation because they’re not getting that reference test,” said Marisa Stahl, MD, a physician and researcher at the Children’s Hospital Colorado Center of Celiac Disease in Aurora. Dr. Stahl was not involved in the meta-analysis, but commented on it in an interview.
The only way to fully correct for this bias is to conduct both IgA tissue transglutaminase (tTG) testing and small bowel biopsy on a complete or random sample of patients and compare the sensitivity and specificity of IgA tTG with the preferred method small-bowel biopsy. However, this is rarely done. Instead, when the U.S. Preventive Services Task Force concluded that evidence was insufficient for IgA tTG testing for celiac disease, it relied on a 2016 comparative effectiveness review of nine studies that estimated sensitivity at 92.6% and specificity at 97.6%. USPSTF remained noncommittal because of inadequate evidence surrounding the balance of benefit and harms of screening for celiac disease in asymptomatic individuals.
In the current meta-analysis, Isabel Hujoel, MD, of the Mayo Clinic, Rochester, Minn., and colleagues tested whether the studies used by USPSTF may have overestimated sensitivity because of verification bias. In a report in the Journal of Clinical Gastroenterology, they reviewed those same nine studies to see the potential impact of verification bias. They rated each individual study as being at high, low, or unclear risk of verification bias and found five they considered to be high risk.
To reveal the impact of small-bowel biopsy referral rates on sensitivity and specificity, the researchers reviewed a separate set of nine retrospective and prospective studies to determine the frequency of referral for both IgA tTG–positive patients (positive referral rate) and IgA tTG–negative patients (negative referral rate), which were 79.2% and 3.6%, respectively.
The researchers then used these values to recalculate the sensitivities and specificities in the five original studies considered high risk for verification bias, then pooled those adjusted values with the remaining, unadjusted values from the studies considered low or unclear risk of bias. The new overall values were 57.1% sensitivity (95% confidence interval, 35.4%-76.4%) and 99.6% specificity (95% CI, 98.4%-99.9%).
“The reported sensitivity and specificity of IgA tTG ... are substantially biased due to a lack of adjustment for verification bias. Specifically, adjusting for verification bias decreases the sensitivity of IgA tTG from 92.5% to 57.1%, with a drop in the lower limit of the 95% CI to 35.4%, and an increase in the specificity from 97.9% to 99.6%, The low estimated sensitivity of IgA tTG raises concern on the accuracy of this test and supports performing a systematic review that accounts for verification bias. ... After adjusting for verification bias, the estimated sensitivity of IgA tTG falls to the point where the serologic marker may no longer be clinically useful as a screening test,” the authors wrote.
The numbers came as a bit of a shock to Dr. Stahl because the sensitivity was so much lower than has been traditionally accepted. “But the more important concept from the paper is that the sensitivity is probably lower than what we oftentimes reference, and we should think more about the population of patients that could potentially screen negative and still have celiac disease,” she said. Although there is no literature to back this up at this time, Dr. Stahl also believes that this may be more common in adults, who have a higher incidence of seronegative Celiac disease.
The issue isn’t restricted to celiac disease. Verification bias can also affect the sensitivity and specificity values from other index screens that are followed by invasive reference tests, like occult blood and colonoscopy or hepatitis C serology and liver biopsy. “A lot of times you ethically cannot put everyone through the [more invasive] reference test, so it definitely applies to other tests we screen for in GI. When we’re quoting numbers and doing systematic reviews and meta-analyses, we should be accounting for those biases,” said Dr. Stahl.
No source of funding was disclosed. The authors declared that they have nothing to disclose. Dr. Stahl consults for Evo-Endo.
Immunoglobulin A tissue transglutaminase offers a noninvasive way to detect celiac disease, but new research suggests that its sensitivity may be overestimated and that it may not be an effective screening test, at least in asymptomatic individuals. The reason comes down to verification bias, wherein a technique appears to have higher sensitivity and lower specificity because individuals who screen positive are more likely to have their disease confirmed by a follow-up small-bowel biopsy while those who screen negative are unlikely to have a follow-up biopsy that could reveal missed celiac disease.
“The issue with verification bias is that only the patients that screen positive on that index test are going to be getting the reference test, so there’s probably a good chance that if they screen positive when they go to that reference test they’ll also be positive. What you’re missing from when you’re calculating sensitivity is, what about the ones that are negative on the index test? Would they have been positive on that reference test? That’s not even coming into your calculation because they’re not getting that reference test,” said Marisa Stahl, MD, a physician and researcher at the Children’s Hospital Colorado Center of Celiac Disease in Aurora. Dr. Stahl was not involved in the meta-analysis, but commented on it in an interview.
The only way to fully correct for this bias is to conduct both IgA tissue transglutaminase (tTG) testing and small bowel biopsy on a complete or random sample of patients and compare the sensitivity and specificity of IgA tTG with the preferred method small-bowel biopsy. However, this is rarely done. Instead, when the U.S. Preventive Services Task Force concluded that evidence was insufficient for IgA tTG testing for celiac disease, it relied on a 2016 comparative effectiveness review of nine studies that estimated sensitivity at 92.6% and specificity at 97.6%. USPSTF remained noncommittal because of inadequate evidence surrounding the balance of benefit and harms of screening for celiac disease in asymptomatic individuals.
In the current meta-analysis, Isabel Hujoel, MD, of the Mayo Clinic, Rochester, Minn., and colleagues tested whether the studies used by USPSTF may have overestimated sensitivity because of verification bias. In a report in the Journal of Clinical Gastroenterology, they reviewed those same nine studies to see the potential impact of verification bias. They rated each individual study as being at high, low, or unclear risk of verification bias and found five they considered to be high risk.
To reveal the impact of small-bowel biopsy referral rates on sensitivity and specificity, the researchers reviewed a separate set of nine retrospective and prospective studies to determine the frequency of referral for both IgA tTG–positive patients (positive referral rate) and IgA tTG–negative patients (negative referral rate), which were 79.2% and 3.6%, respectively.
The researchers then used these values to recalculate the sensitivities and specificities in the five original studies considered high risk for verification bias, then pooled those adjusted values with the remaining, unadjusted values from the studies considered low or unclear risk of bias. The new overall values were 57.1% sensitivity (95% confidence interval, 35.4%-76.4%) and 99.6% specificity (95% CI, 98.4%-99.9%).
“The reported sensitivity and specificity of IgA tTG ... are substantially biased due to a lack of adjustment for verification bias. Specifically, adjusting for verification bias decreases the sensitivity of IgA tTG from 92.5% to 57.1%, with a drop in the lower limit of the 95% CI to 35.4%, and an increase in the specificity from 97.9% to 99.6%, The low estimated sensitivity of IgA tTG raises concern on the accuracy of this test and supports performing a systematic review that accounts for verification bias. ... After adjusting for verification bias, the estimated sensitivity of IgA tTG falls to the point where the serologic marker may no longer be clinically useful as a screening test,” the authors wrote.
The numbers came as a bit of a shock to Dr. Stahl because the sensitivity was so much lower than has been traditionally accepted. “But the more important concept from the paper is that the sensitivity is probably lower than what we oftentimes reference, and we should think more about the population of patients that could potentially screen negative and still have celiac disease,” she said. Although there is no literature to back this up at this time, Dr. Stahl also believes that this may be more common in adults, who have a higher incidence of seronegative Celiac disease.
The issue isn’t restricted to celiac disease. Verification bias can also affect the sensitivity and specificity values from other index screens that are followed by invasive reference tests, like occult blood and colonoscopy or hepatitis C serology and liver biopsy. “A lot of times you ethically cannot put everyone through the [more invasive] reference test, so it definitely applies to other tests we screen for in GI. When we’re quoting numbers and doing systematic reviews and meta-analyses, we should be accounting for those biases,” said Dr. Stahl.
No source of funding was disclosed. The authors declared that they have nothing to disclose. Dr. Stahl consults for Evo-Endo.
Immunoglobulin A tissue transglutaminase offers a noninvasive way to detect celiac disease, but new research suggests that its sensitivity may be overestimated and that it may not be an effective screening test, at least in asymptomatic individuals. The reason comes down to verification bias, wherein a technique appears to have higher sensitivity and lower specificity because individuals who screen positive are more likely to have their disease confirmed by a follow-up small-bowel biopsy while those who screen negative are unlikely to have a follow-up biopsy that could reveal missed celiac disease.
“The issue with verification bias is that only the patients that screen positive on that index test are going to be getting the reference test, so there’s probably a good chance that if they screen positive when they go to that reference test they’ll also be positive. What you’re missing from when you’re calculating sensitivity is, what about the ones that are negative on the index test? Would they have been positive on that reference test? That’s not even coming into your calculation because they’re not getting that reference test,” said Marisa Stahl, MD, a physician and researcher at the Children’s Hospital Colorado Center of Celiac Disease in Aurora. Dr. Stahl was not involved in the meta-analysis, but commented on it in an interview.
The only way to fully correct for this bias is to conduct both IgA tissue transglutaminase (tTG) testing and small bowel biopsy on a complete or random sample of patients and compare the sensitivity and specificity of IgA tTG with the preferred method small-bowel biopsy. However, this is rarely done. Instead, when the U.S. Preventive Services Task Force concluded that evidence was insufficient for IgA tTG testing for celiac disease, it relied on a 2016 comparative effectiveness review of nine studies that estimated sensitivity at 92.6% and specificity at 97.6%. USPSTF remained noncommittal because of inadequate evidence surrounding the balance of benefit and harms of screening for celiac disease in asymptomatic individuals.
In the current meta-analysis, Isabel Hujoel, MD, of the Mayo Clinic, Rochester, Minn., and colleagues tested whether the studies used by USPSTF may have overestimated sensitivity because of verification bias. In a report in the Journal of Clinical Gastroenterology, they reviewed those same nine studies to see the potential impact of verification bias. They rated each individual study as being at high, low, or unclear risk of verification bias and found five they considered to be high risk.
To reveal the impact of small-bowel biopsy referral rates on sensitivity and specificity, the researchers reviewed a separate set of nine retrospective and prospective studies to determine the frequency of referral for both IgA tTG–positive patients (positive referral rate) and IgA tTG–negative patients (negative referral rate), which were 79.2% and 3.6%, respectively.
The researchers then used these values to recalculate the sensitivities and specificities in the five original studies considered high risk for verification bias, then pooled those adjusted values with the remaining, unadjusted values from the studies considered low or unclear risk of bias. The new overall values were 57.1% sensitivity (95% confidence interval, 35.4%-76.4%) and 99.6% specificity (95% CI, 98.4%-99.9%).
“The reported sensitivity and specificity of IgA tTG ... are substantially biased due to a lack of adjustment for verification bias. Specifically, adjusting for verification bias decreases the sensitivity of IgA tTG from 92.5% to 57.1%, with a drop in the lower limit of the 95% CI to 35.4%, and an increase in the specificity from 97.9% to 99.6%, The low estimated sensitivity of IgA tTG raises concern on the accuracy of this test and supports performing a systematic review that accounts for verification bias. ... After adjusting for verification bias, the estimated sensitivity of IgA tTG falls to the point where the serologic marker may no longer be clinically useful as a screening test,” the authors wrote.
The numbers came as a bit of a shock to Dr. Stahl because the sensitivity was so much lower than has been traditionally accepted. “But the more important concept from the paper is that the sensitivity is probably lower than what we oftentimes reference, and we should think more about the population of patients that could potentially screen negative and still have celiac disease,” she said. Although there is no literature to back this up at this time, Dr. Stahl also believes that this may be more common in adults, who have a higher incidence of seronegative Celiac disease.
The issue isn’t restricted to celiac disease. Verification bias can also affect the sensitivity and specificity values from other index screens that are followed by invasive reference tests, like occult blood and colonoscopy or hepatitis C serology and liver biopsy. “A lot of times you ethically cannot put everyone through the [more invasive] reference test, so it definitely applies to other tests we screen for in GI. When we’re quoting numbers and doing systematic reviews and meta-analyses, we should be accounting for those biases,” said Dr. Stahl.
No source of funding was disclosed. The authors declared that they have nothing to disclose. Dr. Stahl consults for Evo-Endo.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Optimize your treatment of endometriosis by using an FDA-approved hormonal medication
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Managing the second stage of labor: An evidence-based approach

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●

CASE Woman in second stage with prolonged pushing
Ms. J. is an 18-year-old woman (G1P0) at 39 weeks’ gestation whose cervix is completely dilated; she has been actively pushing for 60 minutes. The estimated fetal weight is 8 lb, and electronic fetal monitoring shows a Category I fetal heart rate (FHR) tracing. The presenting part remains at 0 station and occiput transverse despite great pushing effort.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. During the third hour of the second stage, Ms. J. is diagnosed with chorioamnionitis and the fetus remains at 0 station. She undergoes a primary cesarean delivery (CD) complicated by bilateral lower uterine extensions and postpartum hemorrhage. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 4 and 8, respectively. The umbilical cord arterial pH was 7.03.
Ms. J. and her baby were discharged home on postoperative day 4.
In 2014, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine jointly released a document, “Safe prevention of the primary cesarean delivery,” in response to the sharp rise in cesarean births from 1996 to 2011.1 It described management strategies to safely reduce the most common indications for a primary CD in nulliparous women. Specifically, it recommended that the second stage of labor—defined as the interval from complete cervical dilation through delivery of the neonate—may be prolonged, as “longer durations may be appropriate on an individualized basis (eg, with the use of epidural analgesia or with fetal malposition) as long as progress is being documented.”1
A prolonged second stage was defined as 3 hours of pushing in nulliparous women and 2 hours in multiparous women, with 1 additional hour (or longer) in those receiving epidural analgesia. Indeed, the primary CD rate decreased slightly to 21.7% in 2018, down from 21.9% in 2017.2 More recent evidence, however, has shown an increase in maternal and neonatal morbidity with prolonged second stage.3-8
Efforts to manage the second stage from an evidence-based perspective are critical to balance the desired outcome of a safe vaginal delivery against the risks of prolonged second stage and operative vaginal delivery or CD.
Perspectives on the “ideal” labor duration
It is important to consider the historical context that led to the 2014 change in recommendations for duration of the second stage.9 In 1955, Dr. Emanuel Friedman published a prospective observational study of 622 consecutive primigravid parturients at term, of which 500 were included in the analysis that led to the graphicostatistical labor curve, or the well-known “Friedman’s curve.”10 The mean duration of the second stage was 0.95 hour. The statistical maximum for “ideal labor” for the second stage was set at 2 hours, with an additional hour allotted for patients receiving epidural analgesia.
In 2010, Zhang and colleagues published contemporary labor curves using data from the Consortium on Safe Labor, a multicenter retrospective observational study of 62,415 parturients.11 Among more than 25,000 nulliparous women, the median duration (95th percentile) of the second stage in hours was 1.1 (3.6), respectively. Notably, this analysis included only women with a spontaneous vaginal delivery and normal neonatal outcome.
Prior to the publication of the “Safe prevention of primary cesarean delivery,” multiple investigations examined the relationship between the duration of the second stage and adverse maternal and neonatal outcomes, and the findings have been inconsistent.12-15
For example, Cheng and colleagues noted increased maternal complications that included postpartum hemorrhage, third- and fourth-degree perineal lacerations, and chorioamnionitis, but not neonatal morbidity, with each increasing hour within the second stage.12 By contrast, a large, population-based cohort study among low-risk women showed an increase in low 5-minute Apgar scores, admission to the neonatal intensive care unit (NICU), and composite perinatal morbidity with prolonged second stage.15 Furthermore, a secondary analysis of the Pushing Early or Pushing Late with Epidural (PEOPLE) trial showed that the chances of a vaginal delivery with a newborn without signs of asphyxia decreased significantly every hour after the first hour, and the risk of postpartum hemorrhage and intrapartum fever increased significantly after 2 hours of pushing.14
While these findings may represent the risks inherent with the intervention of operative delivery and not the duration of second stage of labor per se, one could posit that if the intervention were initiated earlier, could it prevent or at least reduce maternal and neonatal morbidity?
Continue to: Factors to assess and monitor in the second stage...
Factors to assess and monitor in the second stage
When assessing progress in the second stage of labor, consider:
- maternal factors
- fetal/neonatal factors, and
- modifiable factors.
Maternal factors that influence the second stage of labor include parity, body mass index (BMI), age, and clinical pelvimetry.11,16-19 Fetal/neonatal factors that impact the second stage include the estimated fetal weight, fetal presentation (cephalic, face, and so on), position, and station, as well as the FHR Category.20, 21 Factors that can be modified in the second stage include the effect of epidural analgesia (turning it down to reduce motor blockade while maintaining sensory pain relief so that patients feel the “urge” to push), maternal pushing position and technique, the presence of maternal support person(s), manual rotation for a fetal position that is not optimal, immediate versus delayed pushing, and prevention of perineal tears.22-32 Interestingly, epidural analgesia, parity, birth weight, and station at complete dilation predicted second stage duration but accounted for only 25% of the variability in second stage length, leaving 75% of the variance unexplained.16
A specific absolute maximum length of time spent in the second stage of labor beyond which all women should undergo operative delivery has not been identified.1 Therefore, maternal, fetal/neonatal, and modifiable factors need to be critically assessed and continually monitored to determine whether a prolonged second stage or an operative delivery is warranted to prevent or minimize adverse maternal and neonatal outcomes.
Maternal factors
Maternal age correlates directly with the length of the second stage. That is, the length of the second stage increases with increasing age.17
Multiparous women have a shorter length of the second stage, regardless of epidural analgesia, compared with nulliparous women.11 In the Consortium for Safe Labor, multiparous women had a significantly shorter median second stage compared with nulliparous women.11
In adjusted analyses, maternal obesity was associated with an increased risk for CD, with the risk of CD more than 3 times greater in women with a BMI higher than 40 kg/m2 compared with those who had a BMI less than 25 kg/m2.18 There were no significant differences in the length of the second stage of labor by BMI catgeories.19
Fetal factors
Birth weight greater than 4,000 g was associated with an increased risk for arrest of descent during the second stage.33
Persistent fetal occiput posterior or transverse position may impact the duration of the second stage. A retrospective cohort study in women who underwent a trial of manual rotation compared with expectant management during the second stage of labor with the fetus in occiput posterior or occiput transverse position found that women with manual rotation were less likely to have a CD, severe perineal laceration, postpartum hemorrhage, and chorioamnionitis. However, an increased risk of cervical laceration was associated with manual rotation.20
Regarding FHR status, FHR abnormalities occurred in 91% of second stage labor patterns, with Category II being the most common.21 The fetal status should remain reassuring to allow for continuation of the second stage.
Continue to: Epidural analgesia...
Epidural analgesia
About 60% of women receive neuraxial analgesia in the United States,22 although rates vary widely across different populations. A Cochrane review showed no difference in the duration of the second stage among women who had early versus late initiation of epidural analgesia in labor.23 Epidural analgesia has no impact on the risk of CD; however, women with epidural analgesia experienced more hypotension, motor blockade, fever, and urinary retention.24
One management practice has been to discontinue epidural analgesia to allow resumption of sensory and motor nerve function. Another Cochrane systematic review found no difference in mode of delivery or neonatal outcomes.25 Rather than discontinuing epidural analgesia, which results in a profound increase in inadequate pain relief, one may consider titrating the dose with joint patient decision-making to allow for greater motor capability while maintaining adequate analgesia.34
Immediate vs delayed pushing
The 2 most common approaches to managing the second stage were either to initiate pushing with contractions once complete dilation occurred (immediate pushing) or to allow for a rest period in which the fetus passively rotated and descended while conserving a woman’s energy for pushing efforts (delayed pushing, laboring down, or passive descent). Since the publication of “Safe prevention of primary cesarean delivery,” however, studies have shown a concerning association between maternal and neonatal complications and prolonged second stage (which may occur with delayed pushing).3-8,35 An observational study of nearly 44,000 nulliparous women without epidural analgesia found that prolonged second stage was associated with increased chorioamnionitis, third- and fourth-degree lacerations, neonatal sepsis, neonatal asphyxia, and perinatal mortality.35
A pragmatic multicenter randomized clinical trial on the optimal management of second stage of labor across the United States recently was conducted.7 More than 2,000 nulliparous women at term in spontaneous or induced labor with epidural analgesia were randomly assigned at complete dilation to immediate pushing or delayed pushing (1 hour after complete dilation). There was no difference in the rate of vaginal delivery. The rate of postpartum hemorrhage was significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (2.3% vs 4.0%, respectively; relative risk [RR], 0.6; 95% confidence interval [CI], 0.3–0.9; P = .03). Furthermore, rates of chorioamnionitis were significantly lower among women in the immediate-pushing group compared with the delayed-pushing group (6.7% vs 9.1%, respectively; RR, 0.70; 95% CI, 0.66–0.90; P = .005). No significant difference occurred in the composite outcome of neonatal morbidity between the groups. However neonatal acidemia (umbilical cord arterial pH <7.1) and confirmed or suspected sepsis were significantly increased in the delayed-pushing group.
The evidence supports active pushing at the start of the second stage. Women who consider delayed pushing should be informed that delayed pushing has not been shown to increase the likelihood of vaginal birth and that it is associated with increased risks of infection, hemorrhage, and neonatal acidemia.36
Maternal pushing position and technique
Spontaneous pushing (in which women are free to follow their instincts and generally push 3 to 5 times per contraction) versus directed pushing (women are encouraged to take a deep breath at the beginning of a contraction then hold it and bear down throughout the contraction) demonstrated no clear difference in duration of the second stage, perineal laceration, episiotomy, time spent pushing, or number of women with spontaneous vaginal birth. There was no difference in 5-minute Apgar score less than 7 or admission to the NICU.26
With regard to maternal positioning during the second stage, a Cochrane systematic review found benefits for upright posture, including a very small reduction in the duration of the second stage, reduction in episiotomy rates, and reduction in assisted deliveries.37 There was an increased risk of blood loss greater than 500 mL and possibly an increased risk in second-degree tears.37 Compared with women allocated to lying down, women in the upright position during the second stage with epidural analgesia had significantly fewer spontaneous vaginal births. There was no difference in operative vaginal delivery, obstetric anal sphincter injury (OASI), infant Apgar score of less than 4 at 5 minutes, and maternal fecal incontinence at 1 year.28
Continue to: Maternal support person...
Maternal support person
Continuous support during labor may improve outcomes for women and infants, including increased spontaneous vaginal birth, shorter duration of labor, and decreased cesarean birth.29 In a randomized trial of 412 healthy nulliparous women, women in labor were assigned to either a support group that received continuous support from a doula or an observed group that was monitored by an inconspicuous observer. Continuous labor support significantly reduced the rate of CDs and forceps deliveries.30,36 Indeed, during the COVID-19 pandemic, doulas have found innovative ways to continue to provide this essential support through virtual health.38
Prevention of perineal tears
Evidence suggests that warm compresses, and massage, may reduce third- and fourth-degree perineal tears.31 A meta-analysis of observational studies showed a significant reduction in the risk of OASI.32
Second stage steps: Recap
Throughout the second stage of labor, the decision to continue with expectant management or intervene with either an operative vaginal delivery or a CD is complex and requires consistent assessment and integration of multiple factors. An evidence-based approach to second stage labor management includes active pushing that is either Valsalva pushing or spontaneous, coached or uncoached, but most importantly, at the start of the second stage when a patient reaches complete dilation. Reassessment should occur at regular intervals to determine progress, after ensuring maternal and fetal well-being.
If there has been no advancement in station, an attempt at manual rotation or titration of epidural analgesia should be considered. Importantly, fetal descent with adequate pushing should be demonstrated throughout the second stage.
Additional considerations that improve outcomes include warm compresses or perineal massage to prevent third- and fourth-degree tears and the presence of a continuous support person to reduce the risk for an operative delivery.
Delivery should be expected within 2 hours for multiparous women and 3 hours for nulliparous women in the second stage. Prolonging the second stage beyond these thresholds should be individualized and occur only in the setting of assured maternal and fetal well-being.

CASE An alternative management strategy
Despite Ms. J.’s great active pushing effort for 60 minutes, the presenting part remains at 0 station and occiput transverse. Ms. J. is counseled regarding the risks and benefits of an attempt at manual rotation of the fetal head, and she wishes to proceed. The fetal position remains occiput transverse.
After another hour of active pushing, the FHR becomes Category II with repetitive variable decelerations. At this time, Ms. J. is informed that there has been no descent, and she is counseled on the risks and benefits of continued pushing versus CD. Through shared decision-making, she consents to a CD. She undergoes a primary CD without complication. The birth weight was 4,100 g, and 5- and 10-minute Apgar scores were 8 and 9, respectively. The umbilical cord arterial pH was 7.13.
Ms. J. and her baby were discharged home on postoperative day 4. ●
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine; Caughey AB, Cahill AG, Guise JM, et al. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210:179-193. doi:10.1016/j.ajog.2014.01.026.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68:1-47.
- Grobman WA, Bailit J, Lai Y, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol. 2016;127:667-673. doi:10.1097/aog.0000000000001354.
- Gimovsky AC, Berghella V. Randomized controlled trial of prolonged second stage: extending the time limit vs usual guidelines. Am J Obstet Gynecol. 2016;214:361.e1-6. doi:10.1016/j.ajog.2015.12.042.
- Rosenbloom JI, Stout MJ, Tuuli MG, et al. New labor management guidelines and changes in cesarean delivery patterns. Am J Obstet Gynecol. 2017;217:689.e1-689.e8. doi:10.1016/j.ajog.2017.10.007.
- Grantz KL, Sundaram R, Ma L, et al. Reassessing the duration of the second stage of labor in relation to maternal and neonatal morbidity. Obstet Gynecol. 2018;131:345-353. doi:10.1097/aog.0000000000002431.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454. doi:10.1001/jama.2018.13986.
- Zipori Y, Grunwald O, Ginsberg Y, et al. The impact of extending the second stage of labor to prevent primary cesarean delivery on maternal and neonatal outcomes. Am J Obstet Gynecol. 2019;220:191.e1-191.e7. doi:10.1016/j.ajog.2018.10.028.
- Leveno KJ, Nelson DB, McIntire DD. Second-stage labor: how long is too long? Am J Obstet Gynecol. 2016;214:484-489. doi:10.1016/j.ajog.2015.10.926.
- Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567-589. doi:10.1097/00006250-195512000-00001.
- Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281-1287. doi:10.1097/AOG.0b013e3181fdef6e.
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol. 2004;191:933-938. doi:10.1016/j.ajog.2004.05.044.
- Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201:357.e1-7. doi:10.1016/j.ajog.2009.08.003.
- Le Ray C, Audibert F, Goffinet F, et al. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol. 2009;201:361.e1-7. doi:10.1016/j.ajog.2009.08.002.
- Allen VM, Baskett TF, O’Connell CM, et al. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol. 2009;113:1248-1258. doi:10.1097/AOG.0b013e3181a722d6.
- Piper JM, Bolling DR, Newton ER. The second stage of labor: factors influencing duration. Am J Obstet Gynecol. 1991;165(4 pt 1):976-979. doi:10.1016/0002-9378(91)90452-w.
- Zaki MN, Hibbard JU, Kominiarek MA. Contemporary labor patterns and maternal age. Obstet Gynecol. 2013;122:1018-1024. doi:10.1097/AOG.0b013e3182a9c92c.
- Kominiarek MA, Vanveldhuisen P, Hibbard J, et al; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203:264.e1-7. doi:10.1016/j.ajog.2010.06.024.
- Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244.e1-8. doi:10.1016/j.ajog.2011.06.014.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72. doi:10.3109/14767051003710276.
- Krebs HB, Petres RE, Dunn LJ. Intrapartum fetal heart rate monitoring. V. Fetal heart rate patterns in the second stage of labor. Am J Obstet Gynecol. 1981;140:435-439. doi:10.1016/0002-9378(81)90041-7.
- Grant EN, Tao W, Craig M, et al. Neuraxial analgesia effects on labour progression: facts, fallacies, uncertainties and the future. BJOG. 2015;122:288-293. doi:10.1111/1471-0528.12966.
- Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation of epidural analgesia for labour. Cochrane Database Syst Rev. 2014;(10):CD007238. doi:10.1002/14651858.CD007238.pub2.
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi:10.1002/14651858.CD000331.pub4.
- Torvaldsen S, Roberts CL, Bell JC, et al. Discontinuation of epidural analgesia late in labour for reducing the adverse delivery outcomes associated with epidural analgesia. Cochrane Database Syst Rev. 2004;(4):CD004457. doi:10.1002/14651858.CD004457.pub2.
- Lemos A, Amorim MM, Dornelas de Andrade A, et al. Pushing/bearing down methods for the second stage of labour. Cochrane Database Syst Rev. 2017;3(3):CD009124. doi:10.1002/14651858.CD009124.pub3.
- Prins M, Boxem J, Lucas C, et al. Effect of spontaneous pushing versus Valsalva pushing in the second stage of labour on mother and fetus: a systematic review of randomised trials. BJOG. 2011;118:662-670. doi:10.1111/j.1471-0528.2011 .02910.x.
- Epidural and Position Trial Collaborative Group. Upright versus lying down position in second stage of labour in nulliparous women with low dose epidural: BUMPES randomised controlled trial. BMJ. 2017;359:j4471. doi:10.1136/bmj.j4471.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7(7):CD003766. doi:10.1002/14651858.CD003766.pub6.
- Kennell J, Klaus M, McGrath S, et al. Continuous emotional support during labor in a US hospital. A randomized controlled trial. JAMA. 1991;265:2197-2201.
- Aasheim V, Nilsen AB, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;6(6):CD006672. doi:10.1002/14651858.CD006672.pub3.
- Bulchandani S, Watts E, Sucharitha A, et al. Manual perineal support at the time of childbirth: a systematic review and meta-analysis. BJOG. 2015;122:1157-1165. doi:10.1111/1471-0528.13431.
- Feinstein U, Sheiner E, Levy A, et al. Risk factors for arrest of descent during the second stage of labor. Int J Gynaecol Obstet. 2002;77:7-14. doi:10.1016/s0020-7292(02)00007-3.
- Cheng YW, Caughey AB. Defining and managing normal and abnormal second stage of labor. Obstet Gynecol Clin North Am. 2017;44:547-566. doi:10.1016/j.ogc.2017.08.009.
- Laughon SK, Berghella V, Reddy UM, et al. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol. 2014;124:57-67. doi:10.1097/aog.0000000000000278.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 766: approaches to limit intervention during labor and birth. Obstet Gynecol. 2019;133:e164-e173. doi:10.1097/aog.0000000000003074.
- Gupta JK, Sood A, Hofmeyr GJ, et al. Position in the second stage of labour for women without epidural anaesthesia. Cochrane Database Syst Rev. 2017;5(5):CD002006. doi:10.1002/14651858.CD002006.pub4.
- Castaneda AN, Searcy JJ. Practising intimate labour: birth doulas respond during COVID-19. Anthropol Action. 2021;28:21-24. https://www.berghahnjournals.com/view/ journals/aia/28/1/aia280104.xml. Accessed February 8, 2021.