User login
Rare Neurological Disease Special Report
Read the issue by clicking on the cover image or HERE
--
This 2021 issue is our seventh annual Rare Neurological Disease Special Report. It comes at a time when the global pandemic is still very much a part of our everyday lives. While the COVID-19 crisis is the polar opposite of a rare disease, it may offer some insights into the rare disease community. Collectively, we faced a disease no one knew much about—an untreatable condition that sent us into isolation, changed our world view, and altered our daily lives in ways we never imagined. We experienced, many of us for the first time, the fear and helplessness of a medical situation that was potentially fatal. Isn’t that what patients with rare diseases and their families face every day? Fear of the unknown; the desperate search for answers; isolation from a world and, all too often, a medical community that does not understand your situation; the terror of suspecting that time may not be on your side, and the valiant effort to press on despite desperate odds—these are things the rare disease community know all too well.
In publishing the Rare Neurological Disease Special Report, our goal has always been to educate the medical community on conditions they may rarely see and offer some hope to those who battle a rare neurological disease. I hope you enjoy reading our seventh annual issue.
—Glenn S. Williams, vice president, group editor, Neurology Reviews and MDedge Neurology
Read the issue by clicking on the cover image or HERE
--
This 2021 issue is our seventh annual Rare Neurological Disease Special Report. It comes at a time when the global pandemic is still very much a part of our everyday lives. While the COVID-19 crisis is the polar opposite of a rare disease, it may offer some insights into the rare disease community. Collectively, we faced a disease no one knew much about—an untreatable condition that sent us into isolation, changed our world view, and altered our daily lives in ways we never imagined. We experienced, many of us for the first time, the fear and helplessness of a medical situation that was potentially fatal. Isn’t that what patients with rare diseases and their families face every day? Fear of the unknown; the desperate search for answers; isolation from a world and, all too often, a medical community that does not understand your situation; the terror of suspecting that time may not be on your side, and the valiant effort to press on despite desperate odds—these are things the rare disease community know all too well.
In publishing the Rare Neurological Disease Special Report, our goal has always been to educate the medical community on conditions they may rarely see and offer some hope to those who battle a rare neurological disease. I hope you enjoy reading our seventh annual issue.
—Glenn S. Williams, vice president, group editor, Neurology Reviews and MDedge Neurology
Read the issue by clicking on the cover image or HERE
--
This 2021 issue is our seventh annual Rare Neurological Disease Special Report. It comes at a time when the global pandemic is still very much a part of our everyday lives. While the COVID-19 crisis is the polar opposite of a rare disease, it may offer some insights into the rare disease community. Collectively, we faced a disease no one knew much about—an untreatable condition that sent us into isolation, changed our world view, and altered our daily lives in ways we never imagined. We experienced, many of us for the first time, the fear and helplessness of a medical situation that was potentially fatal. Isn’t that what patients with rare diseases and their families face every day? Fear of the unknown; the desperate search for answers; isolation from a world and, all too often, a medical community that does not understand your situation; the terror of suspecting that time may not be on your side, and the valiant effort to press on despite desperate odds—these are things the rare disease community know all too well.
In publishing the Rare Neurological Disease Special Report, our goal has always been to educate the medical community on conditions they may rarely see and offer some hope to those who battle a rare neurological disease. I hope you enjoy reading our seventh annual issue.
—Glenn S. Williams, vice president, group editor, Neurology Reviews and MDedge Neurology
Empowering Residents to Address Socioeconomic Disparities in Dermatology
Studding almost every inch of skin except the face are gray lichenified plaques coating a patient’s body like worn leather. Raking his nails across his arm, the patient reminds me how long he had waited to receive this referral and how early he had awoken for this appointment. He was well acquainted with the value of promptness; in his world, it might make the difference between sleeping on a cot and a night spent on concrete.
Over the last year, the patient had cycled through the few safety-net clinics scattered throughout the city. He had accumulated numerous different diagnoses from atopic dermatitis to disseminated tinea corporis. A few minutes, one #15 scalpel, and mineral oil were all it took for us to unravel the mystery. As the attending and I peered through the microscope at the scabies ovum, I couldn’t help but wonder about the alternative outcomes to his case. Left untreated, scabies compromises the skin barrier, paving the way for secondary infections such as cellulitis. Depending on the pathogen, this infection may in turn evolve into acute postinfectious glomerulonephritis.1-4 An elusive diagnosis can quietly escalate into considerable morbidity for patients. This case highlights the dire consequences of dermatologic health disparities and places medicine’s primordial function into sharp focus: the alleviation of suffering.
The Dermatologic Burden of Disease
As a major contributor to global disease burden, dermatologic disease is the fourth greatest cause of disability worldwide when mortality is factored out.5,6 Among global rural populations, dermatologic disease constitutes one of the leading causes of death and/or loss of professional capabilities.7 In the United States alone, nearly 27% of the population saw a physician for at least 1 dermatologic disease in 2013.5 The tremendous prevalence of skin disease magnifies discrepancies in access to dermatologic care, which has been observed to be influenced by age, socioeconomic background, rurality, and sex.8
There has been growing focus on the national shortage of dermatologists over the last 2 decades.9,10 With an aging population and rising incidence of skin cancer, this undersupply is projected to increase and disproportionately impact ethnic minorities as well as those from socioeconomically disadvantaged backgrounds.8,9,11-14 These trends are of particular importance to residents and medical trainees. Multiple studies have demonstrated that the patient demographic of hospital-based resident clinics includes primarily minority and disenfranchised populations with poorer overall health.15-17 In contrast to faculty clinics, residents treat patients who are more likely to be nonwhite and more likely to be reimbursed by Medicaid.18 The unique demographic makeup of hospital-based resident clinics raises questions about the preparedness and comfort of resident physicians in managing the nuances of health care delivery in these settings.10
Providing equitable care to marginalized populations within the constraints of 15- to 30-minute visits can be challenging to physicians and trainees. Even clinicians with the best of intentions may be impeded by a lack of familiarity with the daily realities of impoverished living conditions, implicit prejudice against people living in poverty, and adapting recommendations to varying levels of health literacy among patients.19,20 Contending with these daunting obstacles can be discouraging. Given how entrenched certain institutional barriers are, questioning them may seem an exercise in futility, yet history demonstrates that residents can and have been empowered to improve tangible outcomes for vulnerable populations. In reflecting on approaches of the general medical education system, The Josiah Macy Jr. Foundation President George E. Thibault, MD, observed that, “When appropriately trained, deployed and incented, [residents] can help achieve institutional goals to improve quality, safety and efficiency.”21
Start Small But Dream Big
Action begins with awareness. Medical school and teaching hospital curricula are increasingly integrating educational exercises regarding the social determinants of health and populations with unmet needs. Medical training presents an exclusive opportunity to gain exposure to and familiarity with patient populations that one might not otherwise encounter. Immersion programs provide invaluable experience in tailoring health care delivery to the needs of vulnerable communities. Although opportunities for international rotations abound, domestic rotations among underserved populations can be just as transformative, including correctional medicine, homeless clinics, the Indian Health Service, and rural communities.
Create Partnerships to Broaden Impact of Service
Affecting the largest and most visible organ, skin disease often presents a substantial concern for patients and can herald systemic disease. The nature of dermatologic disease engenders close collaboration between general practitioners and specialists. For example, while resident-run or safety-net clinics characteristically center on providing holistic care for patients through internal medicine or primary care, these overworked and understaffed clinics often are in need of evaluation by specialists for specific concerns. Some clinic models feature dermatology faculty who volunteer routinely (ie, every 2 weeks, every month) to examine all the clinic’s patients presenting with concerns pertinent to the specialty. Drawing on their respective areas of expertise, general practitioners and dermatologists therefore can collaborate to connect disadvantaged patients with the specialized care they need.
Challenges Present Opportunities for Innovation
Adhering to the social distancing requirements of the COVID-19 pandemic protocol has driven clinicians to utilize innovative approaches to patient care. The rural-urban misdistribution of the dermatologist workforce has long been established, with rural patients often experiencing lengthy wait times to see a specialist.9 Both synchronous and asynchronous teledermatology modalities provide an ideal platform for triaging patients with dermatologic concerns who otherwise have meager access to a dermatologist.
Final Thoughts
Residency training is a prime opportunity to gain exposure to the broad spectrum of disease within dermatology as well as the diverse range of affected patients. Drawing on the aforementioned strategies, residents can leverage this knowledge in the service of underserved patients.
- McCarthy JS, Kemp DJ, Walton SF, et al. Scabies: more than just an irritation. Postgrad Med J. 2004;80:382-387.
- Svartman M, Finklea JF, Earle DP, et al. Epidemic scabies and acute glomerulonephritis in Trinidad. Lancet. 1972;1:249-251.
- Hersch C. Acute glomerulonephritis due to skin disease, with special reference to scabies. S Afr Med J. 1967;41:29-34.
- Carapetis JR, Connors C, Yarmirr D, et al. Success of a scabies control program in an Australian aboriginal community. Pediatr Infect Dis J. 1997;16:494-499.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States [published online March 1, 2017]. J Am Acad Dermatol. 2017;76:958-972.e2.
- Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017;153:406-412.
- Morrone A. Poverty, dignity, and forgotten skin care: dermatology in the stream of human mobile population. Dermatol Clin. 2008;26:245-256, vi-vii.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Kosmadaki MG, Gilchrest BA. The demographics of aging in the United States: implications for dermatology. Arch Dermatol. 2002;138:1427-1428.
- Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg. 2011;30:3-5.
- Dall TM, Gallo PD, Chakrabarti R, et al. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff (Millwood). 2013;32:2013-2020.
- Sauaia A, Dellavalle RP. Health care inequities: an introduction for dermatology providers. Dermatol Clin. 2009;27:103-107.
- Brook RH, Fink A, Kosecoff J, et al. Educating physicians and treating patients in the ambulatory setting. where are we going and how will we know when we arrive? Ann Intern Med. 1987;107:392-398.
- Yancy WS Jr, Macpherson DS, Hanusa BH, et al. Patient satisfaction in resident and attending ambulatory care clinics. J Gen Intern Med. 2001;16:755-762. 17. Fiebach NH,
- Wong JG. Taking care of patients in resident clinics: where do we stand? J Gen Intern Med. 2001;16:787-789.
- Loignon C, Boudreault-Fournier A, Truchon K, et al. Medical residents reflect on their prejudices toward poverty: a photovoice training project. BMC Med Educ. 2014;14:1050.
- Scheid D, Logue E, Gilchrist VJ, et al. Do we practice what we preach? comparing the patients of faculty and residents. Fam Med. 1995;27:519-524.
- Loignon C, Gottin T, Dupéré S, et al. General practitioners’ perspective on poverty: a qualitative study in Montreal, Canada. Fam Pract. 2018;35:105-110.
- Parks T. Empowered residents can help transform medical care. American Medical Association website. Published November 30, 2016. Accessed March 18, 2021. www.ama-assn.org/education/improve-gme/empowered-residents-can-help-transform-medical-care
Studding almost every inch of skin except the face are gray lichenified plaques coating a patient’s body like worn leather. Raking his nails across his arm, the patient reminds me how long he had waited to receive this referral and how early he had awoken for this appointment. He was well acquainted with the value of promptness; in his world, it might make the difference between sleeping on a cot and a night spent on concrete.
Over the last year, the patient had cycled through the few safety-net clinics scattered throughout the city. He had accumulated numerous different diagnoses from atopic dermatitis to disseminated tinea corporis. A few minutes, one #15 scalpel, and mineral oil were all it took for us to unravel the mystery. As the attending and I peered through the microscope at the scabies ovum, I couldn’t help but wonder about the alternative outcomes to his case. Left untreated, scabies compromises the skin barrier, paving the way for secondary infections such as cellulitis. Depending on the pathogen, this infection may in turn evolve into acute postinfectious glomerulonephritis.1-4 An elusive diagnosis can quietly escalate into considerable morbidity for patients. This case highlights the dire consequences of dermatologic health disparities and places medicine’s primordial function into sharp focus: the alleviation of suffering.
The Dermatologic Burden of Disease
As a major contributor to global disease burden, dermatologic disease is the fourth greatest cause of disability worldwide when mortality is factored out.5,6 Among global rural populations, dermatologic disease constitutes one of the leading causes of death and/or loss of professional capabilities.7 In the United States alone, nearly 27% of the population saw a physician for at least 1 dermatologic disease in 2013.5 The tremendous prevalence of skin disease magnifies discrepancies in access to dermatologic care, which has been observed to be influenced by age, socioeconomic background, rurality, and sex.8
There has been growing focus on the national shortage of dermatologists over the last 2 decades.9,10 With an aging population and rising incidence of skin cancer, this undersupply is projected to increase and disproportionately impact ethnic minorities as well as those from socioeconomically disadvantaged backgrounds.8,9,11-14 These trends are of particular importance to residents and medical trainees. Multiple studies have demonstrated that the patient demographic of hospital-based resident clinics includes primarily minority and disenfranchised populations with poorer overall health.15-17 In contrast to faculty clinics, residents treat patients who are more likely to be nonwhite and more likely to be reimbursed by Medicaid.18 The unique demographic makeup of hospital-based resident clinics raises questions about the preparedness and comfort of resident physicians in managing the nuances of health care delivery in these settings.10
Providing equitable care to marginalized populations within the constraints of 15- to 30-minute visits can be challenging to physicians and trainees. Even clinicians with the best of intentions may be impeded by a lack of familiarity with the daily realities of impoverished living conditions, implicit prejudice against people living in poverty, and adapting recommendations to varying levels of health literacy among patients.19,20 Contending with these daunting obstacles can be discouraging. Given how entrenched certain institutional barriers are, questioning them may seem an exercise in futility, yet history demonstrates that residents can and have been empowered to improve tangible outcomes for vulnerable populations. In reflecting on approaches of the general medical education system, The Josiah Macy Jr. Foundation President George E. Thibault, MD, observed that, “When appropriately trained, deployed and incented, [residents] can help achieve institutional goals to improve quality, safety and efficiency.”21
Start Small But Dream Big
Action begins with awareness. Medical school and teaching hospital curricula are increasingly integrating educational exercises regarding the social determinants of health and populations with unmet needs. Medical training presents an exclusive opportunity to gain exposure to and familiarity with patient populations that one might not otherwise encounter. Immersion programs provide invaluable experience in tailoring health care delivery to the needs of vulnerable communities. Although opportunities for international rotations abound, domestic rotations among underserved populations can be just as transformative, including correctional medicine, homeless clinics, the Indian Health Service, and rural communities.
Create Partnerships to Broaden Impact of Service
Affecting the largest and most visible organ, skin disease often presents a substantial concern for patients and can herald systemic disease. The nature of dermatologic disease engenders close collaboration between general practitioners and specialists. For example, while resident-run or safety-net clinics characteristically center on providing holistic care for patients through internal medicine or primary care, these overworked and understaffed clinics often are in need of evaluation by specialists for specific concerns. Some clinic models feature dermatology faculty who volunteer routinely (ie, every 2 weeks, every month) to examine all the clinic’s patients presenting with concerns pertinent to the specialty. Drawing on their respective areas of expertise, general practitioners and dermatologists therefore can collaborate to connect disadvantaged patients with the specialized care they need.
Challenges Present Opportunities for Innovation
Adhering to the social distancing requirements of the COVID-19 pandemic protocol has driven clinicians to utilize innovative approaches to patient care. The rural-urban misdistribution of the dermatologist workforce has long been established, with rural patients often experiencing lengthy wait times to see a specialist.9 Both synchronous and asynchronous teledermatology modalities provide an ideal platform for triaging patients with dermatologic concerns who otherwise have meager access to a dermatologist.
Final Thoughts
Residency training is a prime opportunity to gain exposure to the broad spectrum of disease within dermatology as well as the diverse range of affected patients. Drawing on the aforementioned strategies, residents can leverage this knowledge in the service of underserved patients.
Studding almost every inch of skin except the face are gray lichenified plaques coating a patient’s body like worn leather. Raking his nails across his arm, the patient reminds me how long he had waited to receive this referral and how early he had awoken for this appointment. He was well acquainted with the value of promptness; in his world, it might make the difference between sleeping on a cot and a night spent on concrete.
Over the last year, the patient had cycled through the few safety-net clinics scattered throughout the city. He had accumulated numerous different diagnoses from atopic dermatitis to disseminated tinea corporis. A few minutes, one #15 scalpel, and mineral oil were all it took for us to unravel the mystery. As the attending and I peered through the microscope at the scabies ovum, I couldn’t help but wonder about the alternative outcomes to his case. Left untreated, scabies compromises the skin barrier, paving the way for secondary infections such as cellulitis. Depending on the pathogen, this infection may in turn evolve into acute postinfectious glomerulonephritis.1-4 An elusive diagnosis can quietly escalate into considerable morbidity for patients. This case highlights the dire consequences of dermatologic health disparities and places medicine’s primordial function into sharp focus: the alleviation of suffering.
The Dermatologic Burden of Disease
As a major contributor to global disease burden, dermatologic disease is the fourth greatest cause of disability worldwide when mortality is factored out.5,6 Among global rural populations, dermatologic disease constitutes one of the leading causes of death and/or loss of professional capabilities.7 In the United States alone, nearly 27% of the population saw a physician for at least 1 dermatologic disease in 2013.5 The tremendous prevalence of skin disease magnifies discrepancies in access to dermatologic care, which has been observed to be influenced by age, socioeconomic background, rurality, and sex.8
There has been growing focus on the national shortage of dermatologists over the last 2 decades.9,10 With an aging population and rising incidence of skin cancer, this undersupply is projected to increase and disproportionately impact ethnic minorities as well as those from socioeconomically disadvantaged backgrounds.8,9,11-14 These trends are of particular importance to residents and medical trainees. Multiple studies have demonstrated that the patient demographic of hospital-based resident clinics includes primarily minority and disenfranchised populations with poorer overall health.15-17 In contrast to faculty clinics, residents treat patients who are more likely to be nonwhite and more likely to be reimbursed by Medicaid.18 The unique demographic makeup of hospital-based resident clinics raises questions about the preparedness and comfort of resident physicians in managing the nuances of health care delivery in these settings.10
Providing equitable care to marginalized populations within the constraints of 15- to 30-minute visits can be challenging to physicians and trainees. Even clinicians with the best of intentions may be impeded by a lack of familiarity with the daily realities of impoverished living conditions, implicit prejudice against people living in poverty, and adapting recommendations to varying levels of health literacy among patients.19,20 Contending with these daunting obstacles can be discouraging. Given how entrenched certain institutional barriers are, questioning them may seem an exercise in futility, yet history demonstrates that residents can and have been empowered to improve tangible outcomes for vulnerable populations. In reflecting on approaches of the general medical education system, The Josiah Macy Jr. Foundation President George E. Thibault, MD, observed that, “When appropriately trained, deployed and incented, [residents] can help achieve institutional goals to improve quality, safety and efficiency.”21
Start Small But Dream Big
Action begins with awareness. Medical school and teaching hospital curricula are increasingly integrating educational exercises regarding the social determinants of health and populations with unmet needs. Medical training presents an exclusive opportunity to gain exposure to and familiarity with patient populations that one might not otherwise encounter. Immersion programs provide invaluable experience in tailoring health care delivery to the needs of vulnerable communities. Although opportunities for international rotations abound, domestic rotations among underserved populations can be just as transformative, including correctional medicine, homeless clinics, the Indian Health Service, and rural communities.
Create Partnerships to Broaden Impact of Service
Affecting the largest and most visible organ, skin disease often presents a substantial concern for patients and can herald systemic disease. The nature of dermatologic disease engenders close collaboration between general practitioners and specialists. For example, while resident-run or safety-net clinics characteristically center on providing holistic care for patients through internal medicine or primary care, these overworked and understaffed clinics often are in need of evaluation by specialists for specific concerns. Some clinic models feature dermatology faculty who volunteer routinely (ie, every 2 weeks, every month) to examine all the clinic’s patients presenting with concerns pertinent to the specialty. Drawing on their respective areas of expertise, general practitioners and dermatologists therefore can collaborate to connect disadvantaged patients with the specialized care they need.
Challenges Present Opportunities for Innovation
Adhering to the social distancing requirements of the COVID-19 pandemic protocol has driven clinicians to utilize innovative approaches to patient care. The rural-urban misdistribution of the dermatologist workforce has long been established, with rural patients often experiencing lengthy wait times to see a specialist.9 Both synchronous and asynchronous teledermatology modalities provide an ideal platform for triaging patients with dermatologic concerns who otherwise have meager access to a dermatologist.
Final Thoughts
Residency training is a prime opportunity to gain exposure to the broad spectrum of disease within dermatology as well as the diverse range of affected patients. Drawing on the aforementioned strategies, residents can leverage this knowledge in the service of underserved patients.
- McCarthy JS, Kemp DJ, Walton SF, et al. Scabies: more than just an irritation. Postgrad Med J. 2004;80:382-387.
- Svartman M, Finklea JF, Earle DP, et al. Epidemic scabies and acute glomerulonephritis in Trinidad. Lancet. 1972;1:249-251.
- Hersch C. Acute glomerulonephritis due to skin disease, with special reference to scabies. S Afr Med J. 1967;41:29-34.
- Carapetis JR, Connors C, Yarmirr D, et al. Success of a scabies control program in an Australian aboriginal community. Pediatr Infect Dis J. 1997;16:494-499.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States [published online March 1, 2017]. J Am Acad Dermatol. 2017;76:958-972.e2.
- Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017;153:406-412.
- Morrone A. Poverty, dignity, and forgotten skin care: dermatology in the stream of human mobile population. Dermatol Clin. 2008;26:245-256, vi-vii.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Kosmadaki MG, Gilchrest BA. The demographics of aging in the United States: implications for dermatology. Arch Dermatol. 2002;138:1427-1428.
- Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg. 2011;30:3-5.
- Dall TM, Gallo PD, Chakrabarti R, et al. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff (Millwood). 2013;32:2013-2020.
- Sauaia A, Dellavalle RP. Health care inequities: an introduction for dermatology providers. Dermatol Clin. 2009;27:103-107.
- Brook RH, Fink A, Kosecoff J, et al. Educating physicians and treating patients in the ambulatory setting. where are we going and how will we know when we arrive? Ann Intern Med. 1987;107:392-398.
- Yancy WS Jr, Macpherson DS, Hanusa BH, et al. Patient satisfaction in resident and attending ambulatory care clinics. J Gen Intern Med. 2001;16:755-762. 17. Fiebach NH,
- Wong JG. Taking care of patients in resident clinics: where do we stand? J Gen Intern Med. 2001;16:787-789.
- Loignon C, Boudreault-Fournier A, Truchon K, et al. Medical residents reflect on their prejudices toward poverty: a photovoice training project. BMC Med Educ. 2014;14:1050.
- Scheid D, Logue E, Gilchrist VJ, et al. Do we practice what we preach? comparing the patients of faculty and residents. Fam Med. 1995;27:519-524.
- Loignon C, Gottin T, Dupéré S, et al. General practitioners’ perspective on poverty: a qualitative study in Montreal, Canada. Fam Pract. 2018;35:105-110.
- Parks T. Empowered residents can help transform medical care. American Medical Association website. Published November 30, 2016. Accessed March 18, 2021. www.ama-assn.org/education/improve-gme/empowered-residents-can-help-transform-medical-care
- McCarthy JS, Kemp DJ, Walton SF, et al. Scabies: more than just an irritation. Postgrad Med J. 2004;80:382-387.
- Svartman M, Finklea JF, Earle DP, et al. Epidemic scabies and acute glomerulonephritis in Trinidad. Lancet. 1972;1:249-251.
- Hersch C. Acute glomerulonephritis due to skin disease, with special reference to scabies. S Afr Med J. 1967;41:29-34.
- Carapetis JR, Connors C, Yarmirr D, et al. Success of a scabies control program in an Australian aboriginal community. Pediatr Infect Dis J. 1997;16:494-499.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States [published online March 1, 2017]. J Am Acad Dermatol. 2017;76:958-972.e2.
- Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017;153:406-412.
- Morrone A. Poverty, dignity, and forgotten skin care: dermatology in the stream of human mobile population. Dermatol Clin. 2008;26:245-256, vi-vii.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Blanco G, Vasquez R, Nezafati K, et al. How residency programs can foster practice for the underserved. J Am Acad Dermatol. 2012;67:158-159.
- Kosmadaki MG, Gilchrest BA. The demographics of aging in the United States: implications for dermatology. Arch Dermatol. 2002;138:1427-1428.
- Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg. 2011;30:3-5.
- Dall TM, Gallo PD, Chakrabarti R, et al. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health Aff (Millwood). 2013;32:2013-2020.
- Sauaia A, Dellavalle RP. Health care inequities: an introduction for dermatology providers. Dermatol Clin. 2009;27:103-107.
- Brook RH, Fink A, Kosecoff J, et al. Educating physicians and treating patients in the ambulatory setting. where are we going and how will we know when we arrive? Ann Intern Med. 1987;107:392-398.
- Yancy WS Jr, Macpherson DS, Hanusa BH, et al. Patient satisfaction in resident and attending ambulatory care clinics. J Gen Intern Med. 2001;16:755-762. 17. Fiebach NH,
- Wong JG. Taking care of patients in resident clinics: where do we stand? J Gen Intern Med. 2001;16:787-789.
- Loignon C, Boudreault-Fournier A, Truchon K, et al. Medical residents reflect on their prejudices toward poverty: a photovoice training project. BMC Med Educ. 2014;14:1050.
- Scheid D, Logue E, Gilchrist VJ, et al. Do we practice what we preach? comparing the patients of faculty and residents. Fam Med. 1995;27:519-524.
- Loignon C, Gottin T, Dupéré S, et al. General practitioners’ perspective on poverty: a qualitative study in Montreal, Canada. Fam Pract. 2018;35:105-110.
- Parks T. Empowered residents can help transform medical care. American Medical Association website. Published November 30, 2016. Accessed March 18, 2021. www.ama-assn.org/education/improve-gme/empowered-residents-can-help-transform-medical-care
Resident Pearl
- Even while in training, dermatology residents have the agency to impact their communities by connecting their expertise to the patients in greatest need.
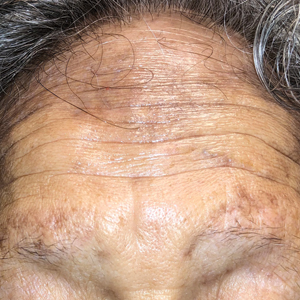
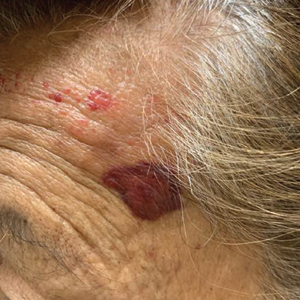
Hyperpigmentation on the Head and Neck
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
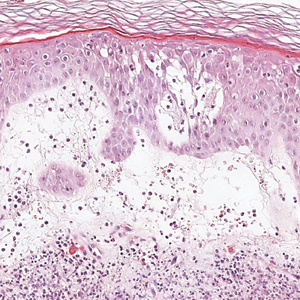
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
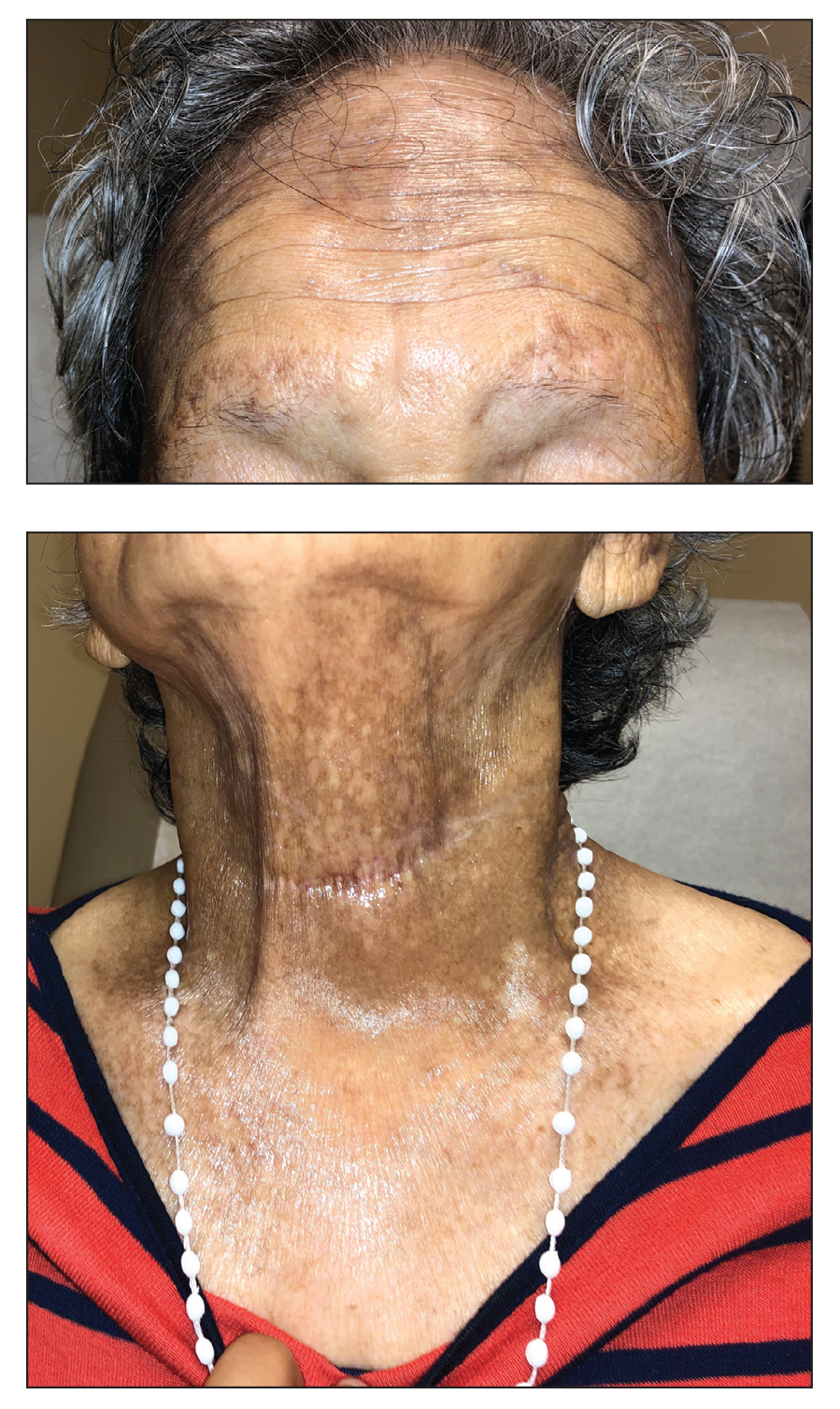
A 78-year-old Asian woman presented to the dermatology clinic with progressively worsening dark spots on the forehead and neck of 3 months’ duration. She noted mild pruritis and hair loss involving the eyebrows and anterior scalp. Her medical history was notable for type 2 diabetes mellitus. She denied any new medical conditions or medications and had no prior history of similar symptoms. Physical examination showed hyperpigmented brown macules and patches on the forehead (top) and anterior neck (bottom) with sparing of the posterior neck and lower face. Alopecia with areas of perifollicular erythema and hyperpigmentation with reduced follicular openings were present on the eyebrows and anterior forehead. Two punch biopsies of head and neck lesions were performed.
Remote cardio visits expand access for underserved during COVID
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Remote cardiology clinic visits during COVID-19 were used more often by certain traditionally underserved patient groups, but were also associated with less frequent testing and prescribing, new research shows.
“The COVID-19 pandemic has led to an unprecedented shift in ambulatory cardiovascular care from in-person to remote visits,” lead author Neal Yuan, MD, a cardiology fellow at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Their findings were published online April 5 in JAMA Network Open.
“We wanted to explore whether the transition to remote visits was associated with disparities in how patients accessed care, and also how this transition affected diagnostic test ordering and medication prescribing,” Dr. Yuan said.
The researchers used electronic health records data for all ambulatory cardiology visits at an urban, multisite health system in Los Angeles County during two periods: April 1 to Dec. 31, 2019, the pre-COVID era; and April 1 to Dec. 31, 2020, the COVID era.
The investigators compared patient characteristics and frequencies of medication ordering and cardiology-specific testing across four visit types: pre-COVID in person, used as reference; COVID-era in person; COVID-era video; and COVID-era telephone.
The study looked at 176,781 ambulatory cardiology visits. Of these visits, 87,182 were conducted in person in the pre-COVID period; 74,498 were conducted in person in the COVID era; 4,720 were COVID-era video visits; and 10,381 were COVID-era telephone visits.
In the study cohort, 79,572 patients (45.0%) were female, 127,080 patients (71.9%) were non-Hispanic White, and the mean age was 68.1 years (standard deviation, 17.0).
Patients accessing COVID-era remote visits were more likely to be Asian, Black, or Hispanic, to have private insurance, and to have cardiovascular comorbidities, such as hypertension and heart failure.
Also, patients whose visits were conducted by video were significantly younger than patients whose visits were conducted in person or by telephone (P < .001).
In addition, the study found that clinicians ordered fewer diagnostic tests, such as electrocardiograms and echocardiograms, and were less likely to order any medication, in the pre-COVID era than during the COVID era.
“If you don’t have a patient in front of you, it’s much more difficult to get a physical exam or obtain reliable vital signs,” said Dr. Yuan. Communication can sometimes be difficult, often because of technical issues, like a bad connection. “You might be more reticent to get testing or to prescribe medications if you don’t feel confident knowing what the patient’s vital signs are.”
In addition, he added, “a lot of medications used in the cardiology setting require monitoring patients’ kidney function and electrolytes, and if you can’t do that reliably, you might be more cautious about prescribing those types of medications.”
An eye-opening study
Cardiologist Nieca Goldberg, MD, medical director of the New York University Langone womens’ heart program and spokesperson for the American Heart Association, recounted her experience with telemedicine at the height of the pandemic in New York, when everything, including medical outpatient offices, had to close.
“We were experienced with telemedicine because we had started a virtual urgent care program well ahead of the pandemic,” she said. “We started using that to screen people with potential COVID symptoms so that they wouldn’t have to come into the hospital, the medical center, or to the offices and expose people. We learned that it was great to have the telemedicine option from the infectious disease standpoint, and I did visits like that for my own patient population.”
An equally if not more important finding from the study is the fact that telemedicine increased access to care among traditionally underserved demographics, she said.
“This is eye-opening, that you can actually improve access to care by doing telemedicine visits. It was really important to see that telemedicine has added benefit to the way we can see people in the health care system.”
Telemedicine visits had a positive impact at a time when people were isolated at home, Dr. Goldberg said.
“It was a way for them to connect with their doctor and in some ways it was more personal,” she added. “I actually got to meet some of my patients’ family members. It was like making a remote house call.”
Stable cardiology patients can take their blood pressure at home, weigh themselves, and take their own pulse to give an excellent set of vital signs that will indicate how they are doing, said Dr. Goldberg.
“During a remote visit, we can talk to the patient and notice whether or not they are short of breath or coughing, but we can’t listen to their heart or do an EKG or any of the traditional cardiac testing. Still, for someone who is not having symptoms and is able to reliably monitor their blood pressure and weight, a remote visit is sufficient to give you a good sense of how that patient is doing,” she said. “We can talk to them about their medications, any potential side effects, and we can use their blood pressure information to adjust their medications.”
Many patients are becoming more savvy about using tech gadgets and devices to monitor their health.
“Some of my patients were using Apple watches and the Kardia app to address their heart rate. Many had purchased inexpensive pulse oximeters to check their oxygen during the pandemic, and that also reads the pulse,” Dr. Goldberg said.
In-person visits were reserved for symptomatic cardiac patients, she explained.
“Initially during the pandemic, we did mostly telemedicine visits and we organized the office so that each cardiologist would come in 1 day a week to take care of symptomatic cardiac patients. In that way, we were able to socially distance – they provided us with [personal protective equipment]; at NYU there was no problem with that – and nobody waited in the waiting room. To this day, office issues are more efficient and people are not waiting in the waiting room,” she added. “Telemedicine improves access to health care in populations where such access is limited.”
Dr. Yuan’s research is supported by a grant from the National Institutes of Health. Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
23-year-old woman • syncopal episode • sinus bradycardia • history of bipolar disorder • Dx?
THE CASE
A 23-year-old woman with past medical history of bipolar II disorder and a REM-specific seizure disorder that resolved at age 9 presented after a syncopal episode. The patient reported an initial sensation of lightheadedness while at work, which was followed by a syncopal episode with brief (1-2 min) loss of consciousness and a minor head injury.
She denied other prodromal symptoms including chest pain, shortness of breath, palpitations, and nausea. She also did not experience convulsions, urinary/bowel incontinence, or confusion upon regaining consciousness.
She denied previous syncopal episodes. However, she reported that, 2 weeks prior, there had been an event similar to that of her presenting complaint. During that episode, she experienced lightheadedness and a fall without loss of consciousness.
The patient had been prescribed a regimen of sertraline 100 mg/d and aripiprazole 10 mg/d to maintain mood stability. She had self-discontinued these medications about 8 months prior to presentation. A recent return of her depressive features had prompted a restart of this regimen 1 week before her first fall, without an initial taper upward.
While in the emergency department, she became bradycardic (heart rate, 38 beats/min) and hypotensive (blood pressure, 70/40 mm Hg). She subsequently became increasingly somnolent and had 1 episode of emesis. An electrocardiogram (EKG) revealed sinus bradycardia without other acute abnormalities (FIGURE).
Blood work including a basic metabolic panel, complete blood count, and cardiac enzymes were all within normal limits. Computed tomography of the head revealed no intracranial pathology. Her vitals were initially unresponsive to a fluid bolus but improved and stabilized after administration of intravenous atropine 0.5 mg.
Aripiprazole was held and sertraline was decreased to 75 mg on hospital Day 1, with close monitoring of her mood. Cardiology was consulted and followed the patient during her stay. The patient was monitored on telemetry for 3 days, exhibiting only sinus bradycardia with a stable heart rate of 45-55 beats/min. Systolic blood pressures were stable within 120 to 130 mm Hg. Transthoracic echocardiogram performed on hospital Day 2 was unremarkable, revealing a normal left ventricular ejection fraction of 65% and no wall motion abnormalities. She had no recurrence of the syncope or emesis.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
Given her benign cardiac work-up and symptom onset coinciding with the abrupt resumption of high doses of aripiprazole after an 8-month abstinence, the patient’s presentation was attributed to a rather uncommon adverse drug reaction to aripiprazole. This has only been described in a few case reports.
DISCUSSION
Aripiprazole (Abilify) is an atypical antipsychotic frequently used in the treatment of psychiatric conditions, including bipolar disorder and schizophrenia. While the specific therapeutic mechanism is unknown, it is believed that drug efficacy is related to partial agonism at dopamine D2, serotonin 5-HT1A, and serotonin 5-HT2A.1 As aripiprazole works on a variety of receptors involved in other physiologic processes, clinical adverse effects have been reported, most of which are associated with the adrenergic alpha1 receptors.1 These include cognitive impairment and seizures. Cardiovascular adverse effects of aripiprazole include orthostatic hypotension, cardiac arrhythmia, prolonged QT interval, and syncope.1-5
Selective serotonin reuptake inhibitors (SSRIs) such as sertraline (Zoloft) have also been shown to cause cardiac arrhythmia and syncope.6 Although sertraline may have contributed to the patient’s cardiac symptoms, it is more likely that the aripiprazole was the direct cause, as she remained asymptomatic while on a therapeutic dose of sertraline. Furthermore, aripiprazole is primarily metabolized though hepatic CYP2D6, which sertraline has been shown to inhibit.1,7 Therefore, the concomitant use of sertraline with no initial taper of either medication likely led to an increased effective dose of aripiprazole in our patient and subsequently to her presentation.
Few prior cases have identified aripiprazole as a cause of antipsychotic-associated bradycardic response.8 Based on the Adverse Drug Reaction Probability Scale, often referred to as the Naranjo Scale, we believe this to be a probable adverse response in our patient.9 Bradycardia followed a reasonable temporal sequence after aripiprazole use with a response previously described in the literature. Symptoms also improved after discontinuation of the drug and other etiologies of the bradycardia were ruled out.
Our patient was discharged with a 30-day cardiac event monitor and a scheduled appointment with Cardiology.
Continue to: THE TAKEAWAY
THE TAKEAWAY
As this case suggests, there may be an association between aripiprazole and symptomatic bradycardia. Therefore, family physicians should inquire about aripiprazole use in patients who present with cardiac symptoms and consider tapering this medication if other causes cannot be identified. Additionally, given the potential cardiac adverse effects of atypical antipsychotics, physicians may consider ordering baseline and follow-up EKGs to monitor for arrhythmias in patients prescribed aripiprazole. This may be especially prudent when an atypical antipsychotic is combined with an SSRI, as potential cardiac adverse effects may occur more frequently.
CORRESPONDENCE
Kyle Fletke, MD, Department of Family and Community Medicine, University of Maryland School of Medicine, 29 South Paca Street, Baltimore, MD 21201; [email protected]
1. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
2. Belemonte C, Ochoa D, Román M, et al. Evaluation of the relationship between pharmacokinetics and the safety of aripiprazole and its cardiovascular side effects in health volunteers. J Clin Psychopharmacol. 2016;36:608-614.
3. Torgovnic J, Sethi NK, Arsura E. Aripiprazole-induced orthostatic hypotension and cardiac arrhythmia. Psychiatry Clin Neurosci. 2008:62:485.
4. Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463-2475.
5. Russo L, Rizzo A, Di Vincenzo A, et al. Aripiprazole overdose and transient 2:1 second degree atrioventricular block: only a coincidence? Curr Drug Saf. 2019;14:155-157.
6. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-90.
7. Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drub Metab. 2002;3:13-37.
8. Snarr BS, Phan SV, Garner A, et al. Symptomatic bradycardia with oral aripiprazole and oral ziprasidone. Ann Pharmacother. 2010;44:760-763.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
THE CASE
A 23-year-old woman with past medical history of bipolar II disorder and a REM-specific seizure disorder that resolved at age 9 presented after a syncopal episode. The patient reported an initial sensation of lightheadedness while at work, which was followed by a syncopal episode with brief (1-2 min) loss of consciousness and a minor head injury.
She denied other prodromal symptoms including chest pain, shortness of breath, palpitations, and nausea. She also did not experience convulsions, urinary/bowel incontinence, or confusion upon regaining consciousness.
She denied previous syncopal episodes. However, she reported that, 2 weeks prior, there had been an event similar to that of her presenting complaint. During that episode, she experienced lightheadedness and a fall without loss of consciousness.
The patient had been prescribed a regimen of sertraline 100 mg/d and aripiprazole 10 mg/d to maintain mood stability. She had self-discontinued these medications about 8 months prior to presentation. A recent return of her depressive features had prompted a restart of this regimen 1 week before her first fall, without an initial taper upward.
While in the emergency department, she became bradycardic (heart rate, 38 beats/min) and hypotensive (blood pressure, 70/40 mm Hg). She subsequently became increasingly somnolent and had 1 episode of emesis. An electrocardiogram (EKG) revealed sinus bradycardia without other acute abnormalities (FIGURE).

Blood work including a basic metabolic panel, complete blood count, and cardiac enzymes were all within normal limits. Computed tomography of the head revealed no intracranial pathology. Her vitals were initially unresponsive to a fluid bolus but improved and stabilized after administration of intravenous atropine 0.5 mg.
Aripiprazole was held and sertraline was decreased to 75 mg on hospital Day 1, with close monitoring of her mood. Cardiology was consulted and followed the patient during her stay. The patient was monitored on telemetry for 3 days, exhibiting only sinus bradycardia with a stable heart rate of 45-55 beats/min. Systolic blood pressures were stable within 120 to 130 mm Hg. Transthoracic echocardiogram performed on hospital Day 2 was unremarkable, revealing a normal left ventricular ejection fraction of 65% and no wall motion abnormalities. She had no recurrence of the syncope or emesis.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
Given her benign cardiac work-up and symptom onset coinciding with the abrupt resumption of high doses of aripiprazole after an 8-month abstinence, the patient’s presentation was attributed to a rather uncommon adverse drug reaction to aripiprazole. This has only been described in a few case reports.
DISCUSSION
Aripiprazole (Abilify) is an atypical antipsychotic frequently used in the treatment of psychiatric conditions, including bipolar disorder and schizophrenia. While the specific therapeutic mechanism is unknown, it is believed that drug efficacy is related to partial agonism at dopamine D2, serotonin 5-HT1A, and serotonin 5-HT2A.1 As aripiprazole works on a variety of receptors involved in other physiologic processes, clinical adverse effects have been reported, most of which are associated with the adrenergic alpha1 receptors.1 These include cognitive impairment and seizures. Cardiovascular adverse effects of aripiprazole include orthostatic hypotension, cardiac arrhythmia, prolonged QT interval, and syncope.1-5
Selective serotonin reuptake inhibitors (SSRIs) such as sertraline (Zoloft) have also been shown to cause cardiac arrhythmia and syncope.6 Although sertraline may have contributed to the patient’s cardiac symptoms, it is more likely that the aripiprazole was the direct cause, as she remained asymptomatic while on a therapeutic dose of sertraline. Furthermore, aripiprazole is primarily metabolized though hepatic CYP2D6, which sertraline has been shown to inhibit.1,7 Therefore, the concomitant use of sertraline with no initial taper of either medication likely led to an increased effective dose of aripiprazole in our patient and subsequently to her presentation.
Few prior cases have identified aripiprazole as a cause of antipsychotic-associated bradycardic response.8 Based on the Adverse Drug Reaction Probability Scale, often referred to as the Naranjo Scale, we believe this to be a probable adverse response in our patient.9 Bradycardia followed a reasonable temporal sequence after aripiprazole use with a response previously described in the literature. Symptoms also improved after discontinuation of the drug and other etiologies of the bradycardia were ruled out.
Our patient was discharged with a 30-day cardiac event monitor and a scheduled appointment with Cardiology.
Continue to: THE TAKEAWAY
THE TAKEAWAY
As this case suggests, there may be an association between aripiprazole and symptomatic bradycardia. Therefore, family physicians should inquire about aripiprazole use in patients who present with cardiac symptoms and consider tapering this medication if other causes cannot be identified. Additionally, given the potential cardiac adverse effects of atypical antipsychotics, physicians may consider ordering baseline and follow-up EKGs to monitor for arrhythmias in patients prescribed aripiprazole. This may be especially prudent when an atypical antipsychotic is combined with an SSRI, as potential cardiac adverse effects may occur more frequently.
CORRESPONDENCE
Kyle Fletke, MD, Department of Family and Community Medicine, University of Maryland School of Medicine, 29 South Paca Street, Baltimore, MD 21201; [email protected]
THE CASE
A 23-year-old woman with past medical history of bipolar II disorder and a REM-specific seizure disorder that resolved at age 9 presented after a syncopal episode. The patient reported an initial sensation of lightheadedness while at work, which was followed by a syncopal episode with brief (1-2 min) loss of consciousness and a minor head injury.
She denied other prodromal symptoms including chest pain, shortness of breath, palpitations, and nausea. She also did not experience convulsions, urinary/bowel incontinence, or confusion upon regaining consciousness.
She denied previous syncopal episodes. However, she reported that, 2 weeks prior, there had been an event similar to that of her presenting complaint. During that episode, she experienced lightheadedness and a fall without loss of consciousness.
The patient had been prescribed a regimen of sertraline 100 mg/d and aripiprazole 10 mg/d to maintain mood stability. She had self-discontinued these medications about 8 months prior to presentation. A recent return of her depressive features had prompted a restart of this regimen 1 week before her first fall, without an initial taper upward.
While in the emergency department, she became bradycardic (heart rate, 38 beats/min) and hypotensive (blood pressure, 70/40 mm Hg). She subsequently became increasingly somnolent and had 1 episode of emesis. An electrocardiogram (EKG) revealed sinus bradycardia without other acute abnormalities (FIGURE).

Blood work including a basic metabolic panel, complete blood count, and cardiac enzymes were all within normal limits. Computed tomography of the head revealed no intracranial pathology. Her vitals were initially unresponsive to a fluid bolus but improved and stabilized after administration of intravenous atropine 0.5 mg.
Aripiprazole was held and sertraline was decreased to 75 mg on hospital Day 1, with close monitoring of her mood. Cardiology was consulted and followed the patient during her stay. The patient was monitored on telemetry for 3 days, exhibiting only sinus bradycardia with a stable heart rate of 45-55 beats/min. Systolic blood pressures were stable within 120 to 130 mm Hg. Transthoracic echocardiogram performed on hospital Day 2 was unremarkable, revealing a normal left ventricular ejection fraction of 65% and no wall motion abnormalities. She had no recurrence of the syncope or emesis.
Continue to: THE DIAGNOSIS
THE DIAGNOSIS
Given her benign cardiac work-up and symptom onset coinciding with the abrupt resumption of high doses of aripiprazole after an 8-month abstinence, the patient’s presentation was attributed to a rather uncommon adverse drug reaction to aripiprazole. This has only been described in a few case reports.
DISCUSSION
Aripiprazole (Abilify) is an atypical antipsychotic frequently used in the treatment of psychiatric conditions, including bipolar disorder and schizophrenia. While the specific therapeutic mechanism is unknown, it is believed that drug efficacy is related to partial agonism at dopamine D2, serotonin 5-HT1A, and serotonin 5-HT2A.1 As aripiprazole works on a variety of receptors involved in other physiologic processes, clinical adverse effects have been reported, most of which are associated with the adrenergic alpha1 receptors.1 These include cognitive impairment and seizures. Cardiovascular adverse effects of aripiprazole include orthostatic hypotension, cardiac arrhythmia, prolonged QT interval, and syncope.1-5
Selective serotonin reuptake inhibitors (SSRIs) such as sertraline (Zoloft) have also been shown to cause cardiac arrhythmia and syncope.6 Although sertraline may have contributed to the patient’s cardiac symptoms, it is more likely that the aripiprazole was the direct cause, as she remained asymptomatic while on a therapeutic dose of sertraline. Furthermore, aripiprazole is primarily metabolized though hepatic CYP2D6, which sertraline has been shown to inhibit.1,7 Therefore, the concomitant use of sertraline with no initial taper of either medication likely led to an increased effective dose of aripiprazole in our patient and subsequently to her presentation.
Few prior cases have identified aripiprazole as a cause of antipsychotic-associated bradycardic response.8 Based on the Adverse Drug Reaction Probability Scale, often referred to as the Naranjo Scale, we believe this to be a probable adverse response in our patient.9 Bradycardia followed a reasonable temporal sequence after aripiprazole use with a response previously described in the literature. Symptoms also improved after discontinuation of the drug and other etiologies of the bradycardia were ruled out.
Our patient was discharged with a 30-day cardiac event monitor and a scheduled appointment with Cardiology.
Continue to: THE TAKEAWAY
THE TAKEAWAY
As this case suggests, there may be an association between aripiprazole and symptomatic bradycardia. Therefore, family physicians should inquire about aripiprazole use in patients who present with cardiac symptoms and consider tapering this medication if other causes cannot be identified. Additionally, given the potential cardiac adverse effects of atypical antipsychotics, physicians may consider ordering baseline and follow-up EKGs to monitor for arrhythmias in patients prescribed aripiprazole. This may be especially prudent when an atypical antipsychotic is combined with an SSRI, as potential cardiac adverse effects may occur more frequently.
CORRESPONDENCE
Kyle Fletke, MD, Department of Family and Community Medicine, University of Maryland School of Medicine, 29 South Paca Street, Baltimore, MD 21201; [email protected]
1. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
2. Belemonte C, Ochoa D, Román M, et al. Evaluation of the relationship between pharmacokinetics and the safety of aripiprazole and its cardiovascular side effects in health volunteers. J Clin Psychopharmacol. 2016;36:608-614.
3. Torgovnic J, Sethi NK, Arsura E. Aripiprazole-induced orthostatic hypotension and cardiac arrhythmia. Psychiatry Clin Neurosci. 2008:62:485.
4. Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463-2475.
5. Russo L, Rizzo A, Di Vincenzo A, et al. Aripiprazole overdose and transient 2:1 second degree atrioventricular block: only a coincidence? Curr Drug Saf. 2019;14:155-157.
6. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-90.
7. Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drub Metab. 2002;3:13-37.
8. Snarr BS, Phan SV, Garner A, et al. Symptomatic bradycardia with oral aripiprazole and oral ziprasidone. Ann Pharmacother. 2010;44:760-763.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
1. Abilify [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc; 2014.
2. Belemonte C, Ochoa D, Román M, et al. Evaluation of the relationship between pharmacokinetics and the safety of aripiprazole and its cardiovascular side effects in health volunteers. J Clin Psychopharmacol. 2016;36:608-614.
3. Torgovnic J, Sethi NK, Arsura E. Aripiprazole-induced orthostatic hypotension and cardiac arrhythmia. Psychiatry Clin Neurosci. 2008:62:485.
4. Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463-2475.
5. Russo L, Rizzo A, Di Vincenzo A, et al. Aripiprazole overdose and transient 2:1 second degree atrioventricular block: only a coincidence? Curr Drug Saf. 2019;14:155-157.
6. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-90.
7. Hemeryck A, Belpaire FM. Selective serotonin reuptake inhibitors and cytochrome P-450 mediated drug-drug interactions: an update. Curr Drub Metab. 2002;3:13-37.
8. Snarr BS, Phan SV, Garner A, et al. Symptomatic bradycardia with oral aripiprazole and oral ziprasidone. Ann Pharmacother. 2010;44:760-763.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
Tender, Diffuse, Edematous, and Erythematous Papules on the Face, Neck, Chest, and Extremities
The Diagnosis: Sweet Syndrome
Sweet syndrome, alternatively known as acute febrile neutrophilic dermatosis, typically presents with variably tender, erythematous papules, plaques, or nodules in middle-aged adults.1 Systemic symptoms such as fever, fatigue, and arthralgia often accompany these cutaneous findings.1,2 Although the pathophysiology has not been fully elucidated, this syndrome frequently is associated with infections, especially upper respiratory illnesses; medications; and malignancies. Among cases of malignancy-associated Sweet syndrome, hematologic malignancies, particularly acute myeloid leukemia and myelodysplastic syndrome, are more common than solid organ malignancies.1,2 Sweet syndrome may precede the associated malignancy by several months; thus, patients without an identifiable trigger for Sweet syndrome should be closely followed.2 Treatment with systemic steroids typically is effective.1,3 Typical histologic features include papillary dermal edema and a brisk neutrophilic infiltrate in the superficial to mid dermis (quiz image).4 Overlying epidermal spongiosis with or without vesiculation also can be seen.4 Leukocytoclasia and endothelial swelling without fibrinoid necrosis are typical, though full-blown leukocytoclastic vasculitis can be seen.3,4 A histiocytoid variant also has been described in which the dermal infiltrate is composed of mononuclear cells reminiscent of histiocytes that are thought to be immature cells of myeloid origin. This variant histologically can simulate leukemia cutis.5
Perniosis, also known as chilblains, typically presents with red to violaceous macules or papules on acral sites, particularly the distal fingers and toes.6,7 It tends to affect young women more frequently than other demographic groups. Although the pathophysiology is not fully understood, perniosis is thought to represent an abnormal inflammatory response to cold environmental conditions. It can occur as an idiopathic disorder or in association with various systemic illnesses including lupus erythematosus.6,7 The typical histologic findings include papillary dermal edema and a lymphocytic infiltrate in the superficial to deep dermis, often with perivascular and perieccrine accentuation (Figure 1).3,6 Other less common microscopic findings include sparse keratinocyte necrosis, basal layer vacuolar change, swelling of endothelial cells, and lymphocytic vasculitis.6 The lesions typically resolve spontaneously within a few weeks, but in some cases they may be chronic.3
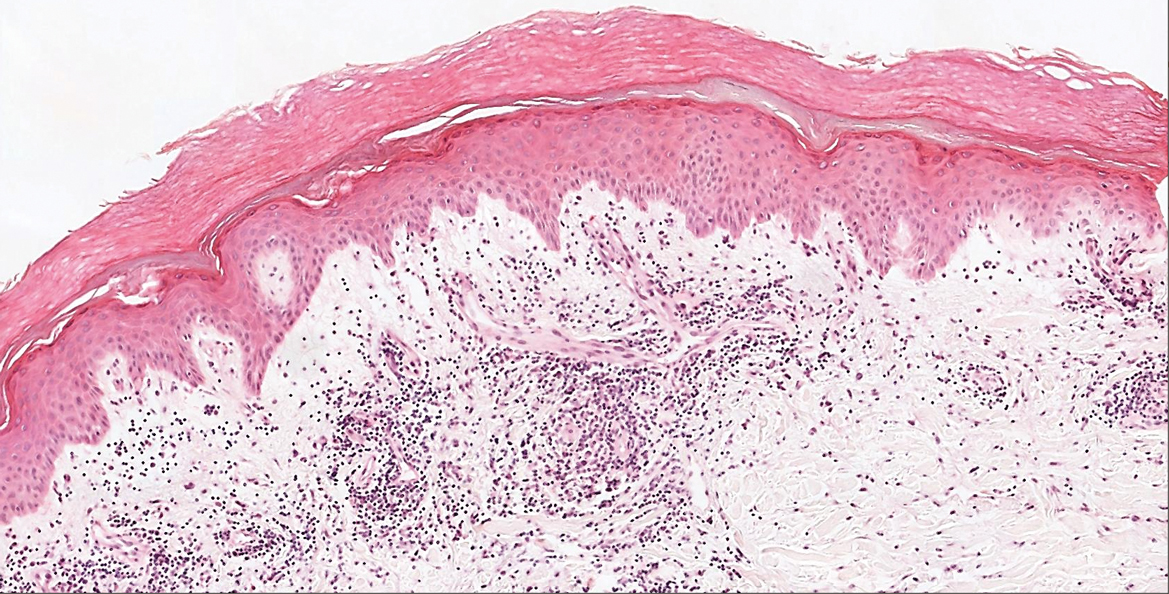
Polymorphous light eruption, a common photodermatosis induced by UV light exposure, typically presents in adolescence or early adulthood with a female predominance. Patients usually develop this pruritic rash on sun-exposed skin other than the face and dorsal aspects of the hands in the spring or early summer upon increased sun exposure after the winter season.3,8 Consistent sunlight exposure throughout the summer months results in decreased flares. Various cutaneous morphologies including papules, vesicles, and plaques can be seen.3,8 Histologic findings include papillary dermal edema and a perivascular lymphocytic infiltrate in the superficial to deep dermis (Figure 2).4
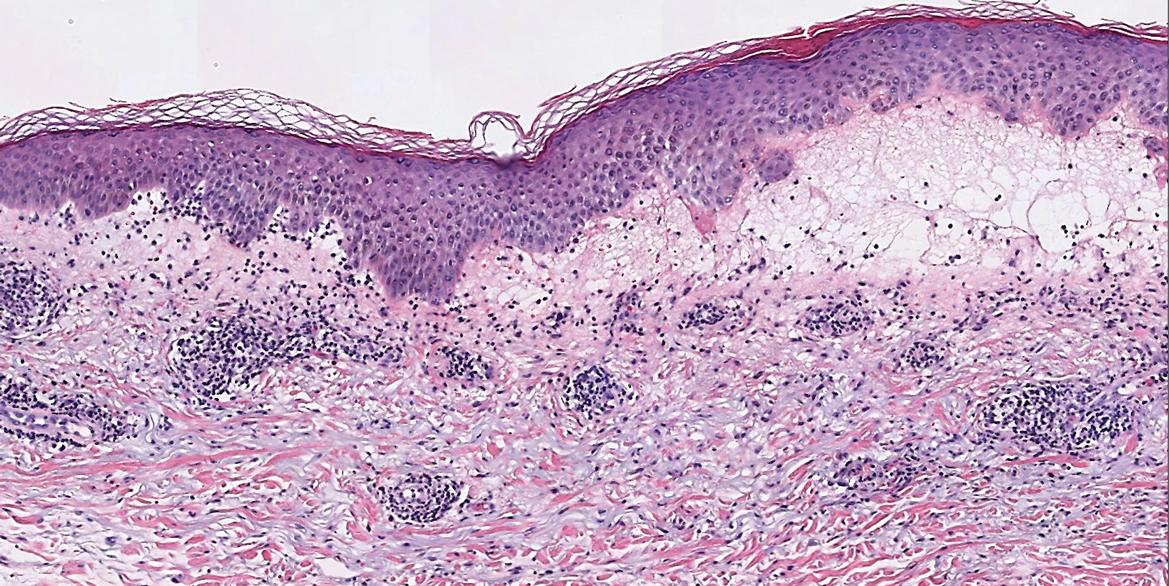
Tinea corporis, a superficial cutaneous dermatophyte infection, typically presents as annular scaly plaques with central clearing. Vesicles and pustules also can be seen.3 The diagnosis can be confirmed via fungal culture, identification of hyphae on microscopic examination of skin scrapings using potassium hydroxide, or cutaneous biopsy. Histologic clues to diagnosis include a "compact stratum corneum (either uniform or forming a layer beneath a basket weave stratum corneum), parakeratosis, mild spongiosis, and neutrophils in the stratum corneum" (Figure 3).9 Papillary dermal edema also may be present, though this finding less commonly is reported.9,10 Because fungal hyphae can be difficult to identify on hematoxylin and eosin-stained slides, special stains such as periodic acid-Schiff or Grocott-Gomori methenamine-silver may be helpful.9 These infections are managed with topical or oral antifungal medications.

Wells syndrome, also known as eosinophilic cellulitis, presents with an acute eruption that can clinically resemble bacterial cellulitis.3 It has been described in children and adults with various clinical morphologies including plaques, bullae, papulovesicles, and papulonodules. Peripheral eosinophilia may be present.11 The clinical lesions usually resolve spontaneously in a few weeks to months, but recurrences are typical.3,11 Histologic findings include papillary dermal edema with or without subepidermal bulla formation and epidermal spongiosis as well as a mixed inflammatory infiltrate with a predominance of eosinophils and flame figures (Figure 4).4 Flame figures are collagen fibers coated with major basic protein and other constituents of degranulated eosinophils.3 Although flame figures often are present in Wells syndrome, they are not specific to this condition.3,4 Some consider Wells syndrome an exaggerated reaction pattern rather than a specific entity.3
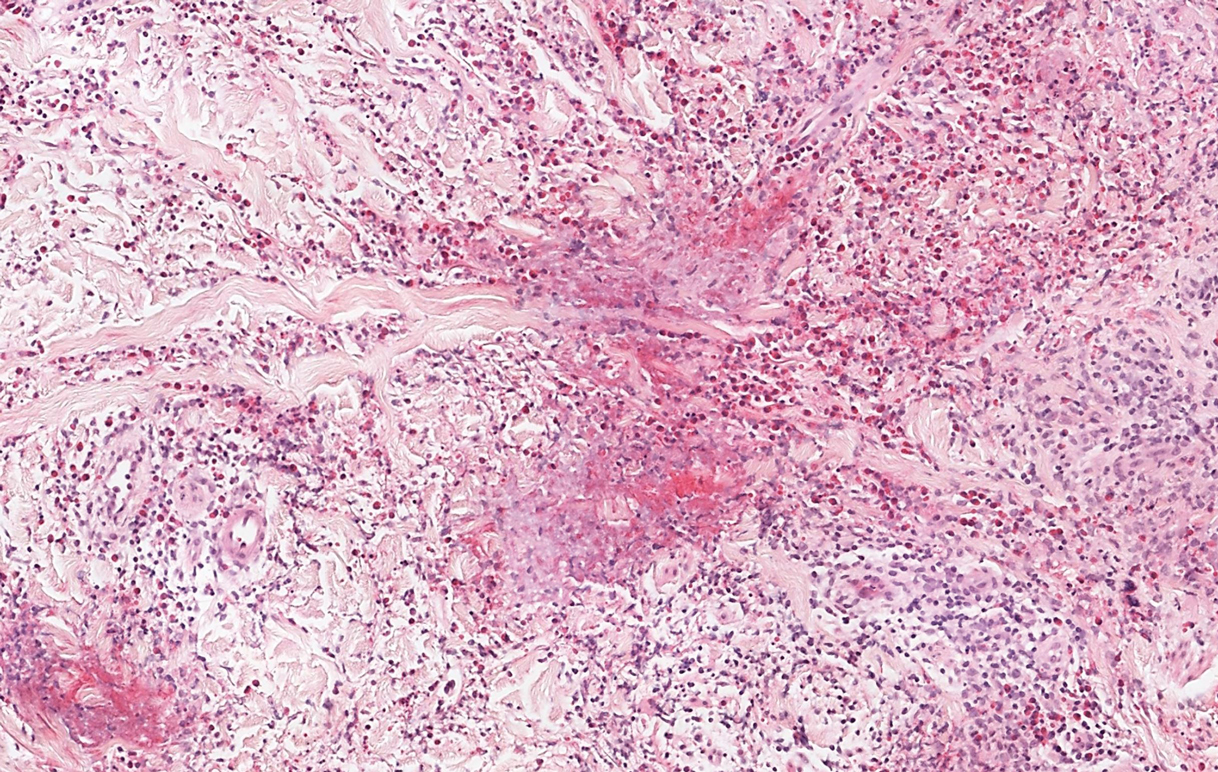
- Rochet N, Chavan R, Cappel M, et al. Sweet syndrome: clinical presentation, associations, and response to treatment in 77 patients. J Am Acad Dermatol. 2013;69:557-564.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Elsevier; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. Elsevier Saunders; 2012.
- Alegría-Landa V, Rodríguez-Pinilla S, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Boada A, Bielsa I, Fernández-Figueras M, et al. Perniosis: clinical and histopathological analysis. Am J Dermatopathol. 2010;32:19-23.
- Takci Z, Vahaboglu G, Eksioglu H. Epidemiological patterns of perniosis, and its association with systemic disorder. Clin Exp Dermatol. 2012;37:844-849.
- Gruber-Wackernagel A, Byrne S, Wolf P. Polymorphous light eruption: clinic aspects and pathogenesis. Dermatol Clin. 2014;32:315-334.
- Elbendary A, Valdebran M, Gad A, et al. When to suspect tinea; a histopathologic study of 103 cases of PAS-positive tinea. J Cutan Pathol. 2016;46:852-857.
- Hoss D, Berke A, Kerr P, et al. Prominent papillary dermal edema in dermatophytosis (tinea corporis). J Cutan Pathol. 2010;37:237-242.
- Caputo R, Marzano A, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
The Diagnosis: Sweet Syndrome
Sweet syndrome, alternatively known as acute febrile neutrophilic dermatosis, typically presents with variably tender, erythematous papules, plaques, or nodules in middle-aged adults.1 Systemic symptoms such as fever, fatigue, and arthralgia often accompany these cutaneous findings.1,2 Although the pathophysiology has not been fully elucidated, this syndrome frequently is associated with infections, especially upper respiratory illnesses; medications; and malignancies. Among cases of malignancy-associated Sweet syndrome, hematologic malignancies, particularly acute myeloid leukemia and myelodysplastic syndrome, are more common than solid organ malignancies.1,2 Sweet syndrome may precede the associated malignancy by several months; thus, patients without an identifiable trigger for Sweet syndrome should be closely followed.2 Treatment with systemic steroids typically is effective.1,3 Typical histologic features include papillary dermal edema and a brisk neutrophilic infiltrate in the superficial to mid dermis (quiz image).4 Overlying epidermal spongiosis with or without vesiculation also can be seen.4 Leukocytoclasia and endothelial swelling without fibrinoid necrosis are typical, though full-blown leukocytoclastic vasculitis can be seen.3,4 A histiocytoid variant also has been described in which the dermal infiltrate is composed of mononuclear cells reminiscent of histiocytes that are thought to be immature cells of myeloid origin. This variant histologically can simulate leukemia cutis.5
Perniosis, also known as chilblains, typically presents with red to violaceous macules or papules on acral sites, particularly the distal fingers and toes.6,7 It tends to affect young women more frequently than other demographic groups. Although the pathophysiology is not fully understood, perniosis is thought to represent an abnormal inflammatory response to cold environmental conditions. It can occur as an idiopathic disorder or in association with various systemic illnesses including lupus erythematosus.6,7 The typical histologic findings include papillary dermal edema and a lymphocytic infiltrate in the superficial to deep dermis, often with perivascular and perieccrine accentuation (Figure 1).3,6 Other less common microscopic findings include sparse keratinocyte necrosis, basal layer vacuolar change, swelling of endothelial cells, and lymphocytic vasculitis.6 The lesions typically resolve spontaneously within a few weeks, but in some cases they may be chronic.3

Polymorphous light eruption, a common photodermatosis induced by UV light exposure, typically presents in adolescence or early adulthood with a female predominance. Patients usually develop this pruritic rash on sun-exposed skin other than the face and dorsal aspects of the hands in the spring or early summer upon increased sun exposure after the winter season.3,8 Consistent sunlight exposure throughout the summer months results in decreased flares. Various cutaneous morphologies including papules, vesicles, and plaques can be seen.3,8 Histologic findings include papillary dermal edema and a perivascular lymphocytic infiltrate in the superficial to deep dermis (Figure 2).4

Tinea corporis, a superficial cutaneous dermatophyte infection, typically presents as annular scaly plaques with central clearing. Vesicles and pustules also can be seen.3 The diagnosis can be confirmed via fungal culture, identification of hyphae on microscopic examination of skin scrapings using potassium hydroxide, or cutaneous biopsy. Histologic clues to diagnosis include a "compact stratum corneum (either uniform or forming a layer beneath a basket weave stratum corneum), parakeratosis, mild spongiosis, and neutrophils in the stratum corneum" (Figure 3).9 Papillary dermal edema also may be present, though this finding less commonly is reported.9,10 Because fungal hyphae can be difficult to identify on hematoxylin and eosin-stained slides, special stains such as periodic acid-Schiff or Grocott-Gomori methenamine-silver may be helpful.9 These infections are managed with topical or oral antifungal medications.

Wells syndrome, also known as eosinophilic cellulitis, presents with an acute eruption that can clinically resemble bacterial cellulitis.3 It has been described in children and adults with various clinical morphologies including plaques, bullae, papulovesicles, and papulonodules. Peripheral eosinophilia may be present.11 The clinical lesions usually resolve spontaneously in a few weeks to months, but recurrences are typical.3,11 Histologic findings include papillary dermal edema with or without subepidermal bulla formation and epidermal spongiosis as well as a mixed inflammatory infiltrate with a predominance of eosinophils and flame figures (Figure 4).4 Flame figures are collagen fibers coated with major basic protein and other constituents of degranulated eosinophils.3 Although flame figures often are present in Wells syndrome, they are not specific to this condition.3,4 Some consider Wells syndrome an exaggerated reaction pattern rather than a specific entity.3

The Diagnosis: Sweet Syndrome
Sweet syndrome, alternatively known as acute febrile neutrophilic dermatosis, typically presents with variably tender, erythematous papules, plaques, or nodules in middle-aged adults.1 Systemic symptoms such as fever, fatigue, and arthralgia often accompany these cutaneous findings.1,2 Although the pathophysiology has not been fully elucidated, this syndrome frequently is associated with infections, especially upper respiratory illnesses; medications; and malignancies. Among cases of malignancy-associated Sweet syndrome, hematologic malignancies, particularly acute myeloid leukemia and myelodysplastic syndrome, are more common than solid organ malignancies.1,2 Sweet syndrome may precede the associated malignancy by several months; thus, patients without an identifiable trigger for Sweet syndrome should be closely followed.2 Treatment with systemic steroids typically is effective.1,3 Typical histologic features include papillary dermal edema and a brisk neutrophilic infiltrate in the superficial to mid dermis (quiz image).4 Overlying epidermal spongiosis with or without vesiculation also can be seen.4 Leukocytoclasia and endothelial swelling without fibrinoid necrosis are typical, though full-blown leukocytoclastic vasculitis can be seen.3,4 A histiocytoid variant also has been described in which the dermal infiltrate is composed of mononuclear cells reminiscent of histiocytes that are thought to be immature cells of myeloid origin. This variant histologically can simulate leukemia cutis.5
Perniosis, also known as chilblains, typically presents with red to violaceous macules or papules on acral sites, particularly the distal fingers and toes.6,7 It tends to affect young women more frequently than other demographic groups. Although the pathophysiology is not fully understood, perniosis is thought to represent an abnormal inflammatory response to cold environmental conditions. It can occur as an idiopathic disorder or in association with various systemic illnesses including lupus erythematosus.6,7 The typical histologic findings include papillary dermal edema and a lymphocytic infiltrate in the superficial to deep dermis, often with perivascular and perieccrine accentuation (Figure 1).3,6 Other less common microscopic findings include sparse keratinocyte necrosis, basal layer vacuolar change, swelling of endothelial cells, and lymphocytic vasculitis.6 The lesions typically resolve spontaneously within a few weeks, but in some cases they may be chronic.3

Polymorphous light eruption, a common photodermatosis induced by UV light exposure, typically presents in adolescence or early adulthood with a female predominance. Patients usually develop this pruritic rash on sun-exposed skin other than the face and dorsal aspects of the hands in the spring or early summer upon increased sun exposure after the winter season.3,8 Consistent sunlight exposure throughout the summer months results in decreased flares. Various cutaneous morphologies including papules, vesicles, and plaques can be seen.3,8 Histologic findings include papillary dermal edema and a perivascular lymphocytic infiltrate in the superficial to deep dermis (Figure 2).4

Tinea corporis, a superficial cutaneous dermatophyte infection, typically presents as annular scaly plaques with central clearing. Vesicles and pustules also can be seen.3 The diagnosis can be confirmed via fungal culture, identification of hyphae on microscopic examination of skin scrapings using potassium hydroxide, or cutaneous biopsy. Histologic clues to diagnosis include a "compact stratum corneum (either uniform or forming a layer beneath a basket weave stratum corneum), parakeratosis, mild spongiosis, and neutrophils in the stratum corneum" (Figure 3).9 Papillary dermal edema also may be present, though this finding less commonly is reported.9,10 Because fungal hyphae can be difficult to identify on hematoxylin and eosin-stained slides, special stains such as periodic acid-Schiff or Grocott-Gomori methenamine-silver may be helpful.9 These infections are managed with topical or oral antifungal medications.

Wells syndrome, also known as eosinophilic cellulitis, presents with an acute eruption that can clinically resemble bacterial cellulitis.3 It has been described in children and adults with various clinical morphologies including plaques, bullae, papulovesicles, and papulonodules. Peripheral eosinophilia may be present.11 The clinical lesions usually resolve spontaneously in a few weeks to months, but recurrences are typical.3,11 Histologic findings include papillary dermal edema with or without subepidermal bulla formation and epidermal spongiosis as well as a mixed inflammatory infiltrate with a predominance of eosinophils and flame figures (Figure 4).4 Flame figures are collagen fibers coated with major basic protein and other constituents of degranulated eosinophils.3 Although flame figures often are present in Wells syndrome, they are not specific to this condition.3,4 Some consider Wells syndrome an exaggerated reaction pattern rather than a specific entity.3

- Rochet N, Chavan R, Cappel M, et al. Sweet syndrome: clinical presentation, associations, and response to treatment in 77 patients. J Am Acad Dermatol. 2013;69:557-564.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Elsevier; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. Elsevier Saunders; 2012.
- Alegría-Landa V, Rodríguez-Pinilla S, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Boada A, Bielsa I, Fernández-Figueras M, et al. Perniosis: clinical and histopathological analysis. Am J Dermatopathol. 2010;32:19-23.
- Takci Z, Vahaboglu G, Eksioglu H. Epidemiological patterns of perniosis, and its association with systemic disorder. Clin Exp Dermatol. 2012;37:844-849.
- Gruber-Wackernagel A, Byrne S, Wolf P. Polymorphous light eruption: clinic aspects and pathogenesis. Dermatol Clin. 2014;32:315-334.
- Elbendary A, Valdebran M, Gad A, et al. When to suspect tinea; a histopathologic study of 103 cases of PAS-positive tinea. J Cutan Pathol. 2016;46:852-857.
- Hoss D, Berke A, Kerr P, et al. Prominent papillary dermal edema in dermatophytosis (tinea corporis). J Cutan Pathol. 2010;37:237-242.
- Caputo R, Marzano A, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
- Rochet N, Chavan R, Cappel M, et al. Sweet syndrome: clinical presentation, associations, and response to treatment in 77 patients. J Am Acad Dermatol. 2013;69:557-564.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Elsevier; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. Elsevier Saunders; 2012.
- Alegría-Landa V, Rodríguez-Pinilla S, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Boada A, Bielsa I, Fernández-Figueras M, et al. Perniosis: clinical and histopathological analysis. Am J Dermatopathol. 2010;32:19-23.
- Takci Z, Vahaboglu G, Eksioglu H. Epidemiological patterns of perniosis, and its association with systemic disorder. Clin Exp Dermatol. 2012;37:844-849.
- Gruber-Wackernagel A, Byrne S, Wolf P. Polymorphous light eruption: clinic aspects and pathogenesis. Dermatol Clin. 2014;32:315-334.
- Elbendary A, Valdebran M, Gad A, et al. When to suspect tinea; a histopathologic study of 103 cases of PAS-positive tinea. J Cutan Pathol. 2016;46:852-857.
- Hoss D, Berke A, Kerr P, et al. Prominent papillary dermal edema in dermatophytosis (tinea corporis). J Cutan Pathol. 2010;37:237-242.
- Caputo R, Marzano A, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
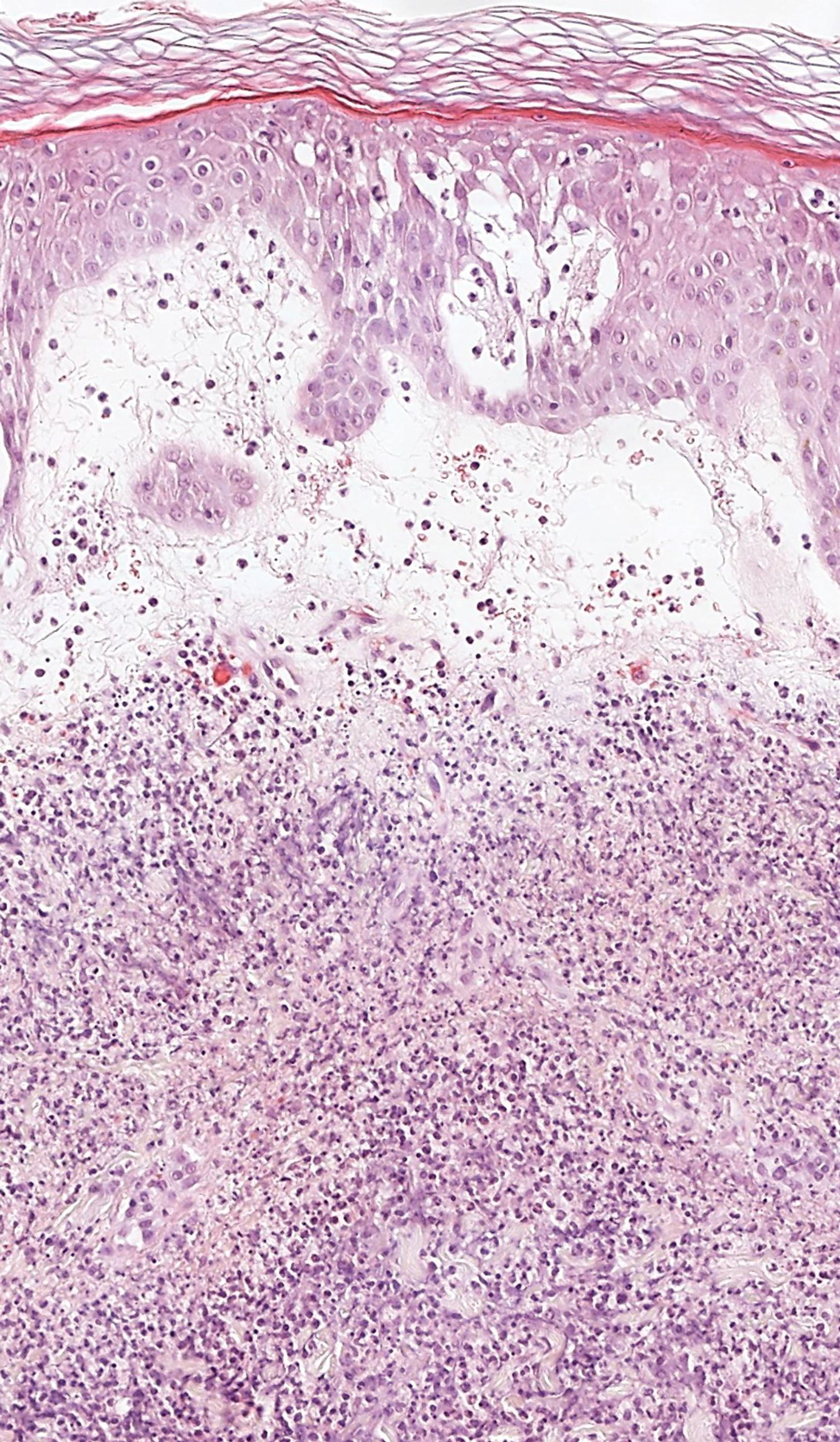
A 62-year-old woman presented with a tender diffuse eruption of erythematous and edematous papules and plaques on the face, neck, chest, and extremities, some appearing vesiculopustular.
The waiting room: Then and now
Recently my wife had surgery to remove some old hardware from her knee.
Although it was an outpatient procedure, it was done at the main hospital. I was told it would be about 5 hours total, so I set up shop in the waiting room with my laptop to get some work done.
There were a few other people waiting there and one volunteer at the desk. The whole time went fairly uneventfully. Others busied themselves with iPads, phones, books, etc. It was, overall, a pleasantly quiet atmosphere. There were the occasional hushed tones of someone on the phone or talking to a doctor, the sound of someone crying in the private discussion room, the voice of a volunteer answering questions, and the intermittent whirring of the Keurig machine.
I sat there and thought about how different it was from times in the past. On weekends when I’d take call I’d come through this same room. It was often packed – standing room only. Almost always there were children running amok because their parents were too distracted or tired to control them. There were food wrappers and dirty cafeteria trays sitting on tables. The Keurig machine was often empty from frequent use – the volunteer too overwhelmed to resupply it.
Now, in the COVID-19 era, it’s a whole different world with visitor restrictions, and I found myself wondering: “Why go back to that?”
Seriously. Isn’t a calm, quiet, atmosphere supposed to be what a hospital (or doctor’s) waiting room should be? Is it really critical that large numbers of an extended family be in the waiting room for every case?
Granted, there should be exceptions. Critical and terminal illness, withdrawal of care, maybe a few others. But
Limiting it to one, maybe two family members for most circumstances isn’t a bad idea. A hospital isn’t an airport, and shouldn’t be run the same way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently my wife had surgery to remove some old hardware from her knee.
Although it was an outpatient procedure, it was done at the main hospital. I was told it would be about 5 hours total, so I set up shop in the waiting room with my laptop to get some work done.
There were a few other people waiting there and one volunteer at the desk. The whole time went fairly uneventfully. Others busied themselves with iPads, phones, books, etc. It was, overall, a pleasantly quiet atmosphere. There were the occasional hushed tones of someone on the phone or talking to a doctor, the sound of someone crying in the private discussion room, the voice of a volunteer answering questions, and the intermittent whirring of the Keurig machine.
I sat there and thought about how different it was from times in the past. On weekends when I’d take call I’d come through this same room. It was often packed – standing room only. Almost always there were children running amok because their parents were too distracted or tired to control them. There were food wrappers and dirty cafeteria trays sitting on tables. The Keurig machine was often empty from frequent use – the volunteer too overwhelmed to resupply it.
Now, in the COVID-19 era, it’s a whole different world with visitor restrictions, and I found myself wondering: “Why go back to that?”
Seriously. Isn’t a calm, quiet, atmosphere supposed to be what a hospital (or doctor’s) waiting room should be? Is it really critical that large numbers of an extended family be in the waiting room for every case?
Granted, there should be exceptions. Critical and terminal illness, withdrawal of care, maybe a few others. But
Limiting it to one, maybe two family members for most circumstances isn’t a bad idea. A hospital isn’t an airport, and shouldn’t be run the same way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently my wife had surgery to remove some old hardware from her knee.
Although it was an outpatient procedure, it was done at the main hospital. I was told it would be about 5 hours total, so I set up shop in the waiting room with my laptop to get some work done.
There were a few other people waiting there and one volunteer at the desk. The whole time went fairly uneventfully. Others busied themselves with iPads, phones, books, etc. It was, overall, a pleasantly quiet atmosphere. There were the occasional hushed tones of someone on the phone or talking to a doctor, the sound of someone crying in the private discussion room, the voice of a volunteer answering questions, and the intermittent whirring of the Keurig machine.
I sat there and thought about how different it was from times in the past. On weekends when I’d take call I’d come through this same room. It was often packed – standing room only. Almost always there were children running amok because their parents were too distracted or tired to control them. There were food wrappers and dirty cafeteria trays sitting on tables. The Keurig machine was often empty from frequent use – the volunteer too overwhelmed to resupply it.
Now, in the COVID-19 era, it’s a whole different world with visitor restrictions, and I found myself wondering: “Why go back to that?”
Seriously. Isn’t a calm, quiet, atmosphere supposed to be what a hospital (or doctor’s) waiting room should be? Is it really critical that large numbers of an extended family be in the waiting room for every case?
Granted, there should be exceptions. Critical and terminal illness, withdrawal of care, maybe a few others. But
Limiting it to one, maybe two family members for most circumstances isn’t a bad idea. A hospital isn’t an airport, and shouldn’t be run the same way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Asymptomatic Hemorrhagic Lesions in an Anemic Woman
The Diagnosis: Bullous Amyloidosis
A punch biopsy from the left temple showed deposits of amorphous eosinophilic material at the tips of dermal papillae and in the papillary dermis with hemorrhage present (Figure 1). A diagnosis of amyloidosis was confirmed on the biopsy of the skin bulla. The low κ/λ light chain ratio and M-spike with notably elevated free λ light chains in both serum and urine were consistent with a λ light chain primary systemic amyloidosis. The patient was seen by hematology and oncology. A bone marrow biopsy demonstrated that 15% to 20% of the clonal-cell population was λ light chain restricted. Eosinophilic extracellular deposits found in the adjacent soft tissue and bone marrow space were confirmed as amyloid with apple green birefringence under polarized light on Congo red stain and metachromatic staining with crystal violet. The patient ultimately was diagnosed with λ light chain multiple myeloma and primary systemic amyloidosis.
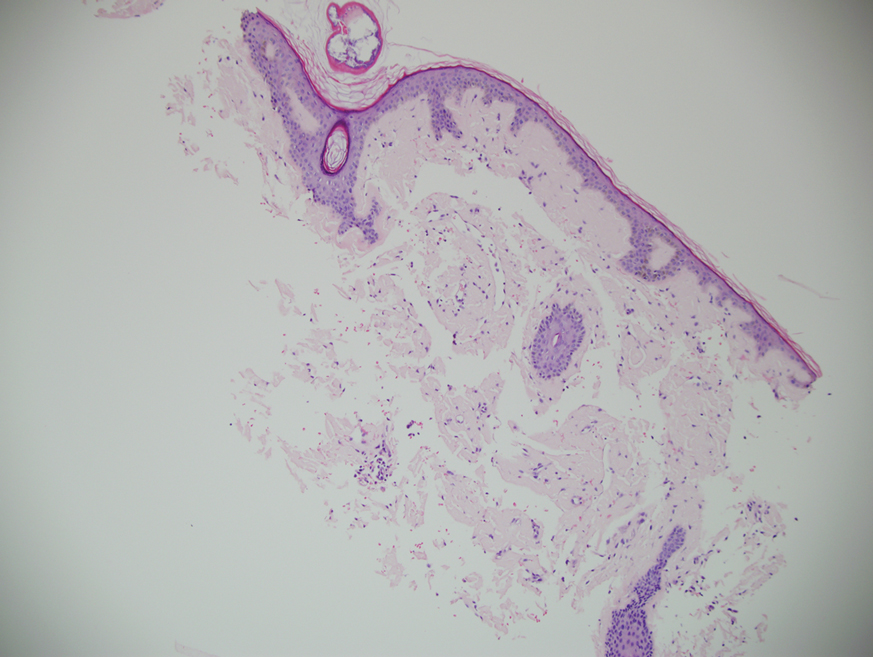
Our patient was treated with a combination therapy of bortezomib, cyclophosphamide, and dexamethasone on 21-day cycles, with bortezomib on days 1, 4, 8, and 11. She had received 3 cycles of chemotherapy before developing diarrhea, hypotension, acute on chronic heart failure, and acute renal failure requiring hospitalization. She had several related complications due to amyloid light chain (AL) amyloidosis and subsequently died 16 days after her initial hospitalization from complications of methicillin-resistant Staphylococcus aureus bacteremia and septic shock.
Amyloidosis is the pathologic deposition of abnormal protein in the extracellular space of any tissue. Various soluble precursor proteins can make up amyloid, and these proteins polymerize into insoluble fibrils that damage the surrounding parenchyma. The clinical presentation of amyloidosis varies depending on the affected tissue as well as the constituent protein. The amyloidoses are divided into localized cutaneous, primary systemic, and secondary systemic variants. The initial distinction in amyloidosis is determining whether it is skin limited or systemic. Localized cutaneous amyloidosis comprises 30% to 40% of all amyloidosis cases and is further divided into 3 main subtypes: macular, lichen, and nodular amyloidosis.1 Macular and lichen amyloidosis are composed of keratin derivatives and typically are induced by patients when rubbing or scratching the skin. Histologically, macular and lichen amyloidosis are restricted to the superficial papillary dermis.1 Nodular amyloidosis is composed of λ or κ light chain immunoglobulins, which are produced by cutaneous infiltrates of monoclonal plasma cells. Histologically, nodular amyloidosis is characterized by a diffuse dermal infiltrate of amorphous eosinophilic material.1 Primary systemic amyloidosis is associated with an underlying plasma cell dyscrasia, and unlike secondary keratinocyte-derived amyloid, it can involve internal organs. Similar to nodular amyloidosis, primary systemic amyloidosis is composed of AL proteins, and it is histologically similar to nodular amyloidosis.1
Primary systemic AL amyloidosis commonly affects individuals aged 50 to 60 years. Males and females are equally affected. Macroglossia and periorbital purpura are some of the pathognomonic presentations in AL amyloidosis. The major cause of death in these patients is cardiac and renal involvement. Renal involvement commonly presents as nephrotic syndrome, and cardiac involvement can present as a restrictive cardiomyopathy with dyspnea. Other symptoms include edema, hepatosplenomegaly, bleeding diathesis, and carpal tunnel syndrome.2 An evaluation for AL amyloidosis should include a complete review of systems and physical examination with studies such as complete blood cell count, comprehensive metabolic panel, serum and urine protein electrophoresis and immunofixation, and electrocardiogram.
Cutaneous involvement in AL amyloidosis most commonly includes yellowish waxy papules, nodules, and plaques but also can include purpura and petechiae.2 Bullous amyloidosis, as seen in our patient, is a rare cutaneous presentation of AL amyloidosis that usually is negative for the Nikolsky sign (Figure 2). Bullae form due to weakness in amyloid-laden dermal connective tissue.3 Eighty-eight percent of cases of bullous amyloidosis have systemic involvement.1 Some cases have reported a familial linkage, suggesting there might be a genetic component to the disease.4 A PubMed search of articles indexed for MEDLINE using the terms bullous amyloidosis, bullous, amyloidosis, and amyloid revealed fewer than 35 cases of bullous amyloidosis in the English-language literature.5 Bullae can be located intradermally or subepidermally and commonly are hemorrhagic but also can be translucent, tender, and tense.
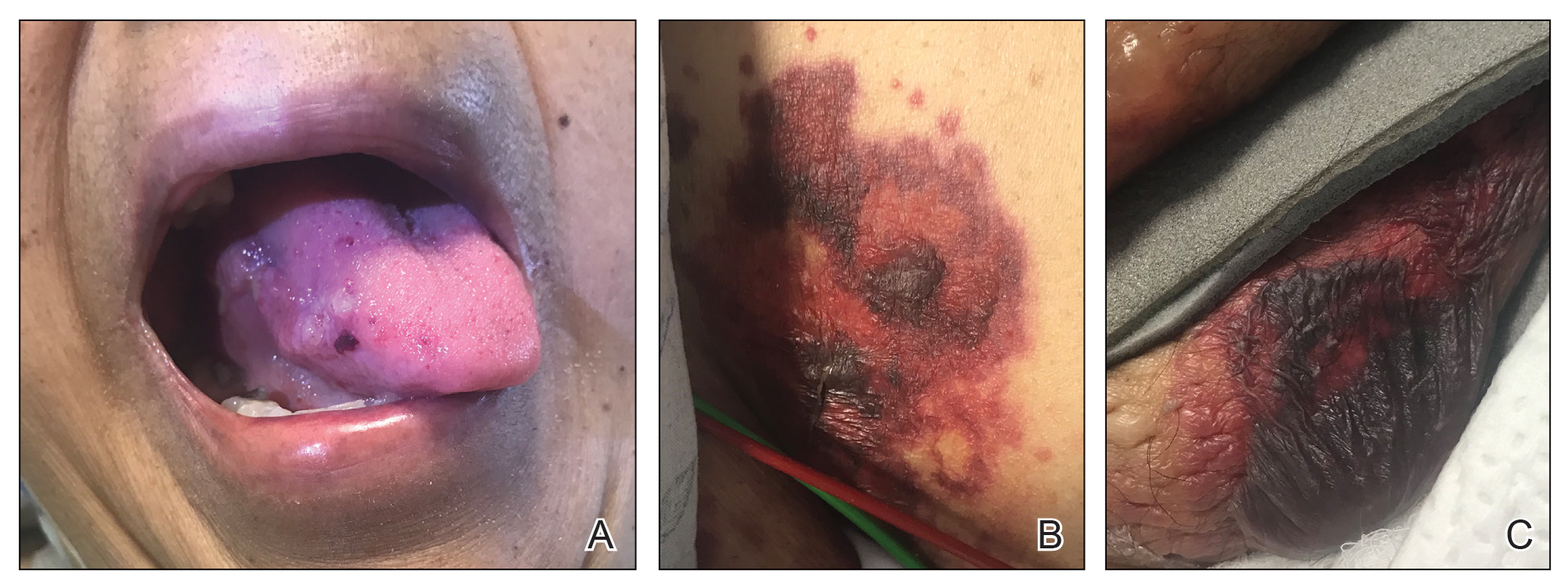
A study of electron microscopy in a patient with systemic bullous amyloidosis demonstrated amyloid and keratinocyte protrusions that perforated the dermis through the spaces in the lamina densa. The study concluded that the disintegration of the lamina densa and expansion of the intercellular spaces between keratinocytes were the causes of skin fragility as well as fluid exudation.5 Trauma or friction to the skin are local precipitating factors for blister formation in bullous amyloidosis.
Bullae can become apparent at any stage of AL amyloidosis, but they generally increase in size and number over time and are most common in intertriginous areas. Bullous amyloid lesions, especially those located in intertriginous areas, can have secondary impetiginization.6 In many cases, patients who present with bullous amyloidosis ultimately will be diagnosed with multiple myeloma or another plasma cell dyscrasia. In AL amyloidosis, only 10% to 15% of cases meet criteria for multiple myeloma, whereas 80% or more patients have a monoclonal gammopathy of undetermined significance.7
The prognosis of cutaneous amyloidosis depends on the extent of organ involvement and response to treatment. Treatment is aimed at eliminating clonal plasma cell populations to decrease the production of light chains, thereby decreasing protein burden and amyloid progression. Historically, treatment options included cytotoxic chemotherapy such as oral melphalan and dexamethasone, followed by hematopoietic stem cell transplant. More recent treatment options include bortezomib, thalidomide, pomalidomide, and lenalidomide.8 Our patient received a regimen of bortezomib, cyclophosphamide, and dexamethasone that is used for patients with extensive multiple myeloma.
The differential diagnosis in our patient included bullous drug eruption, which should be considered if the bullae are reoccurring at the same location and in association with the administration of a culprit drug. Bullous pemphigoid is preceded by pruritus, and biopsy demonstrates subepidermal bullae with associated eosinophilic infiltrate. Epidermolysis bullosa acquisita can present with milia and a linear pattern along the basement membrane zone with direct immunofluorescence. Traumatic purpura usually present with the classic shape and hue of an ecchymosis, and the patient will have a history of trauma.
Cutaneous involvement of amyloidosis can be an early clue to the diagnosis of plasma cell dyscrasia. Early diagnosis and treatment can portend a better prognosis and prevent progression to renal or cardiac disease.
- Heaton J, Steinhoff N, Wanner B, et al. A review of primary cutaneous amyloidosis. J Am Osteopath Coll Dermatol. doi:10.1007/springerreference_42272
- Ventarola DJ, Schuster MW, Cohen JA, et al. JAAD grand rounds quiz. bullae and nodules on the legs of a 57-year-old woman. J Am Acad Dermatol. 2014;71:1035-1037.
- Chang SL, Lai PC, Cheng CJ, et al. Bullous amyloidosis in a hemodialysis patient is myeloma-associated rather than hemodialysis-associated amyloidosis. Amyloid. 2007;14:153-156.
- Suranagi VV, Siddramappa B, Bannur HB, et al. Bullous variant of familial biphasic lichen amyloidosis: a unique combination of three rare presentations. Indian J Dermatol. 2015;60:105.
- Antúnez-Lay A, Jaque A, González S. Hemorrhagic bullous skin lesions. Int J Dermatol. 2017;56:145-147.
- Reddy K, Hoda S, Penstein A, et al. Bullous amyloidosis complicated by cellulitis and sepsis: a case report. Arch Dermatol. 2011;147:126-127.
- Chu CH, Chan JY, Hsieh SW, et al. Diffuse ecchymoses and blisters on a yellowish waxy base: a case of bullous amyloidosis. J Dermatol. 2016;43:713-714.
- Gonzalez-Ramos J, Garrido-Gutiérrez C, González-Silva Y, et al. Relapsing bullous amyloidosis of the oral mucosa and acquired cutis laxa in a patient with multiple myeloma: a rare triple association. Clin Exp Dermatol. 2017;42:410-412.
The Diagnosis: Bullous Amyloidosis
A punch biopsy from the left temple showed deposits of amorphous eosinophilic material at the tips of dermal papillae and in the papillary dermis with hemorrhage present (Figure 1). A diagnosis of amyloidosis was confirmed on the biopsy of the skin bulla. The low κ/λ light chain ratio and M-spike with notably elevated free λ light chains in both serum and urine were consistent with a λ light chain primary systemic amyloidosis. The patient was seen by hematology and oncology. A bone marrow biopsy demonstrated that 15% to 20% of the clonal-cell population was λ light chain restricted. Eosinophilic extracellular deposits found in the adjacent soft tissue and bone marrow space were confirmed as amyloid with apple green birefringence under polarized light on Congo red stain and metachromatic staining with crystal violet. The patient ultimately was diagnosed with λ light chain multiple myeloma and primary systemic amyloidosis.

Our patient was treated with a combination therapy of bortezomib, cyclophosphamide, and dexamethasone on 21-day cycles, with bortezomib on days 1, 4, 8, and 11. She had received 3 cycles of chemotherapy before developing diarrhea, hypotension, acute on chronic heart failure, and acute renal failure requiring hospitalization. She had several related complications due to amyloid light chain (AL) amyloidosis and subsequently died 16 days after her initial hospitalization from complications of methicillin-resistant Staphylococcus aureus bacteremia and septic shock.
Amyloidosis is the pathologic deposition of abnormal protein in the extracellular space of any tissue. Various soluble precursor proteins can make up amyloid, and these proteins polymerize into insoluble fibrils that damage the surrounding parenchyma. The clinical presentation of amyloidosis varies depending on the affected tissue as well as the constituent protein. The amyloidoses are divided into localized cutaneous, primary systemic, and secondary systemic variants. The initial distinction in amyloidosis is determining whether it is skin limited or systemic. Localized cutaneous amyloidosis comprises 30% to 40% of all amyloidosis cases and is further divided into 3 main subtypes: macular, lichen, and nodular amyloidosis.1 Macular and lichen amyloidosis are composed of keratin derivatives and typically are induced by patients when rubbing or scratching the skin. Histologically, macular and lichen amyloidosis are restricted to the superficial papillary dermis.1 Nodular amyloidosis is composed of λ or κ light chain immunoglobulins, which are produced by cutaneous infiltrates of monoclonal plasma cells. Histologically, nodular amyloidosis is characterized by a diffuse dermal infiltrate of amorphous eosinophilic material.1 Primary systemic amyloidosis is associated with an underlying plasma cell dyscrasia, and unlike secondary keratinocyte-derived amyloid, it can involve internal organs. Similar to nodular amyloidosis, primary systemic amyloidosis is composed of AL proteins, and it is histologically similar to nodular amyloidosis.1
Primary systemic AL amyloidosis commonly affects individuals aged 50 to 60 years. Males and females are equally affected. Macroglossia and periorbital purpura are some of the pathognomonic presentations in AL amyloidosis. The major cause of death in these patients is cardiac and renal involvement. Renal involvement commonly presents as nephrotic syndrome, and cardiac involvement can present as a restrictive cardiomyopathy with dyspnea. Other symptoms include edema, hepatosplenomegaly, bleeding diathesis, and carpal tunnel syndrome.2 An evaluation for AL amyloidosis should include a complete review of systems and physical examination with studies such as complete blood cell count, comprehensive metabolic panel, serum and urine protein electrophoresis and immunofixation, and electrocardiogram.
Cutaneous involvement in AL amyloidosis most commonly includes yellowish waxy papules, nodules, and plaques but also can include purpura and petechiae.2 Bullous amyloidosis, as seen in our patient, is a rare cutaneous presentation of AL amyloidosis that usually is negative for the Nikolsky sign (Figure 2). Bullae form due to weakness in amyloid-laden dermal connective tissue.3 Eighty-eight percent of cases of bullous amyloidosis have systemic involvement.1 Some cases have reported a familial linkage, suggesting there might be a genetic component to the disease.4 A PubMed search of articles indexed for MEDLINE using the terms bullous amyloidosis, bullous, amyloidosis, and amyloid revealed fewer than 35 cases of bullous amyloidosis in the English-language literature.5 Bullae can be located intradermally or subepidermally and commonly are hemorrhagic but also can be translucent, tender, and tense.

A study of electron microscopy in a patient with systemic bullous amyloidosis demonstrated amyloid and keratinocyte protrusions that perforated the dermis through the spaces in the lamina densa. The study concluded that the disintegration of the lamina densa and expansion of the intercellular spaces between keratinocytes were the causes of skin fragility as well as fluid exudation.5 Trauma or friction to the skin are local precipitating factors for blister formation in bullous amyloidosis.
Bullae can become apparent at any stage of AL amyloidosis, but they generally increase in size and number over time and are most common in intertriginous areas. Bullous amyloid lesions, especially those located in intertriginous areas, can have secondary impetiginization.6 In many cases, patients who present with bullous amyloidosis ultimately will be diagnosed with multiple myeloma or another plasma cell dyscrasia. In AL amyloidosis, only 10% to 15% of cases meet criteria for multiple myeloma, whereas 80% or more patients have a monoclonal gammopathy of undetermined significance.7
The prognosis of cutaneous amyloidosis depends on the extent of organ involvement and response to treatment. Treatment is aimed at eliminating clonal plasma cell populations to decrease the production of light chains, thereby decreasing protein burden and amyloid progression. Historically, treatment options included cytotoxic chemotherapy such as oral melphalan and dexamethasone, followed by hematopoietic stem cell transplant. More recent treatment options include bortezomib, thalidomide, pomalidomide, and lenalidomide.8 Our patient received a regimen of bortezomib, cyclophosphamide, and dexamethasone that is used for patients with extensive multiple myeloma.
The differential diagnosis in our patient included bullous drug eruption, which should be considered if the bullae are reoccurring at the same location and in association with the administration of a culprit drug. Bullous pemphigoid is preceded by pruritus, and biopsy demonstrates subepidermal bullae with associated eosinophilic infiltrate. Epidermolysis bullosa acquisita can present with milia and a linear pattern along the basement membrane zone with direct immunofluorescence. Traumatic purpura usually present with the classic shape and hue of an ecchymosis, and the patient will have a history of trauma.
Cutaneous involvement of amyloidosis can be an early clue to the diagnosis of plasma cell dyscrasia. Early diagnosis and treatment can portend a better prognosis and prevent progression to renal or cardiac disease.
The Diagnosis: Bullous Amyloidosis
A punch biopsy from the left temple showed deposits of amorphous eosinophilic material at the tips of dermal papillae and in the papillary dermis with hemorrhage present (Figure 1). A diagnosis of amyloidosis was confirmed on the biopsy of the skin bulla. The low κ/λ light chain ratio and M-spike with notably elevated free λ light chains in both serum and urine were consistent with a λ light chain primary systemic amyloidosis. The patient was seen by hematology and oncology. A bone marrow biopsy demonstrated that 15% to 20% of the clonal-cell population was λ light chain restricted. Eosinophilic extracellular deposits found in the adjacent soft tissue and bone marrow space were confirmed as amyloid with apple green birefringence under polarized light on Congo red stain and metachromatic staining with crystal violet. The patient ultimately was diagnosed with λ light chain multiple myeloma and primary systemic amyloidosis.

Our patient was treated with a combination therapy of bortezomib, cyclophosphamide, and dexamethasone on 21-day cycles, with bortezomib on days 1, 4, 8, and 11. She had received 3 cycles of chemotherapy before developing diarrhea, hypotension, acute on chronic heart failure, and acute renal failure requiring hospitalization. She had several related complications due to amyloid light chain (AL) amyloidosis and subsequently died 16 days after her initial hospitalization from complications of methicillin-resistant Staphylococcus aureus bacteremia and septic shock.
Amyloidosis is the pathologic deposition of abnormal protein in the extracellular space of any tissue. Various soluble precursor proteins can make up amyloid, and these proteins polymerize into insoluble fibrils that damage the surrounding parenchyma. The clinical presentation of amyloidosis varies depending on the affected tissue as well as the constituent protein. The amyloidoses are divided into localized cutaneous, primary systemic, and secondary systemic variants. The initial distinction in amyloidosis is determining whether it is skin limited or systemic. Localized cutaneous amyloidosis comprises 30% to 40% of all amyloidosis cases and is further divided into 3 main subtypes: macular, lichen, and nodular amyloidosis.1 Macular and lichen amyloidosis are composed of keratin derivatives and typically are induced by patients when rubbing or scratching the skin. Histologically, macular and lichen amyloidosis are restricted to the superficial papillary dermis.1 Nodular amyloidosis is composed of λ or κ light chain immunoglobulins, which are produced by cutaneous infiltrates of monoclonal plasma cells. Histologically, nodular amyloidosis is characterized by a diffuse dermal infiltrate of amorphous eosinophilic material.1 Primary systemic amyloidosis is associated with an underlying plasma cell dyscrasia, and unlike secondary keratinocyte-derived amyloid, it can involve internal organs. Similar to nodular amyloidosis, primary systemic amyloidosis is composed of AL proteins, and it is histologically similar to nodular amyloidosis.1
Primary systemic AL amyloidosis commonly affects individuals aged 50 to 60 years. Males and females are equally affected. Macroglossia and periorbital purpura are some of the pathognomonic presentations in AL amyloidosis. The major cause of death in these patients is cardiac and renal involvement. Renal involvement commonly presents as nephrotic syndrome, and cardiac involvement can present as a restrictive cardiomyopathy with dyspnea. Other symptoms include edema, hepatosplenomegaly, bleeding diathesis, and carpal tunnel syndrome.2 An evaluation for AL amyloidosis should include a complete review of systems and physical examination with studies such as complete blood cell count, comprehensive metabolic panel, serum and urine protein electrophoresis and immunofixation, and electrocardiogram.
Cutaneous involvement in AL amyloidosis most commonly includes yellowish waxy papules, nodules, and plaques but also can include purpura and petechiae.2 Bullous amyloidosis, as seen in our patient, is a rare cutaneous presentation of AL amyloidosis that usually is negative for the Nikolsky sign (Figure 2). Bullae form due to weakness in amyloid-laden dermal connective tissue.3 Eighty-eight percent of cases of bullous amyloidosis have systemic involvement.1 Some cases have reported a familial linkage, suggesting there might be a genetic component to the disease.4 A PubMed search of articles indexed for MEDLINE using the terms bullous amyloidosis, bullous, amyloidosis, and amyloid revealed fewer than 35 cases of bullous amyloidosis in the English-language literature.5 Bullae can be located intradermally or subepidermally and commonly are hemorrhagic but also can be translucent, tender, and tense.

A study of electron microscopy in a patient with systemic bullous amyloidosis demonstrated amyloid and keratinocyte protrusions that perforated the dermis through the spaces in the lamina densa. The study concluded that the disintegration of the lamina densa and expansion of the intercellular spaces between keratinocytes were the causes of skin fragility as well as fluid exudation.5 Trauma or friction to the skin are local precipitating factors for blister formation in bullous amyloidosis.
Bullae can become apparent at any stage of AL amyloidosis, but they generally increase in size and number over time and are most common in intertriginous areas. Bullous amyloid lesions, especially those located in intertriginous areas, can have secondary impetiginization.6 In many cases, patients who present with bullous amyloidosis ultimately will be diagnosed with multiple myeloma or another plasma cell dyscrasia. In AL amyloidosis, only 10% to 15% of cases meet criteria for multiple myeloma, whereas 80% or more patients have a monoclonal gammopathy of undetermined significance.7
The prognosis of cutaneous amyloidosis depends on the extent of organ involvement and response to treatment. Treatment is aimed at eliminating clonal plasma cell populations to decrease the production of light chains, thereby decreasing protein burden and amyloid progression. Historically, treatment options included cytotoxic chemotherapy such as oral melphalan and dexamethasone, followed by hematopoietic stem cell transplant. More recent treatment options include bortezomib, thalidomide, pomalidomide, and lenalidomide.8 Our patient received a regimen of bortezomib, cyclophosphamide, and dexamethasone that is used for patients with extensive multiple myeloma.
The differential diagnosis in our patient included bullous drug eruption, which should be considered if the bullae are reoccurring at the same location and in association with the administration of a culprit drug. Bullous pemphigoid is preceded by pruritus, and biopsy demonstrates subepidermal bullae with associated eosinophilic infiltrate. Epidermolysis bullosa acquisita can present with milia and a linear pattern along the basement membrane zone with direct immunofluorescence. Traumatic purpura usually present with the classic shape and hue of an ecchymosis, and the patient will have a history of trauma.
Cutaneous involvement of amyloidosis can be an early clue to the diagnosis of plasma cell dyscrasia. Early diagnosis and treatment can portend a better prognosis and prevent progression to renal or cardiac disease.
- Heaton J, Steinhoff N, Wanner B, et al. A review of primary cutaneous amyloidosis. J Am Osteopath Coll Dermatol. doi:10.1007/springerreference_42272
- Ventarola DJ, Schuster MW, Cohen JA, et al. JAAD grand rounds quiz. bullae and nodules on the legs of a 57-year-old woman. J Am Acad Dermatol. 2014;71:1035-1037.
- Chang SL, Lai PC, Cheng CJ, et al. Bullous amyloidosis in a hemodialysis patient is myeloma-associated rather than hemodialysis-associated amyloidosis. Amyloid. 2007;14:153-156.
- Suranagi VV, Siddramappa B, Bannur HB, et al. Bullous variant of familial biphasic lichen amyloidosis: a unique combination of three rare presentations. Indian J Dermatol. 2015;60:105.
- Antúnez-Lay A, Jaque A, González S. Hemorrhagic bullous skin lesions. Int J Dermatol. 2017;56:145-147.
- Reddy K, Hoda S, Penstein A, et al. Bullous amyloidosis complicated by cellulitis and sepsis: a case report. Arch Dermatol. 2011;147:126-127.
- Chu CH, Chan JY, Hsieh SW, et al. Diffuse ecchymoses and blisters on a yellowish waxy base: a case of bullous amyloidosis. J Dermatol. 2016;43:713-714.
- Gonzalez-Ramos J, Garrido-Gutiérrez C, González-Silva Y, et al. Relapsing bullous amyloidosis of the oral mucosa and acquired cutis laxa in a patient with multiple myeloma: a rare triple association. Clin Exp Dermatol. 2017;42:410-412.
- Heaton J, Steinhoff N, Wanner B, et al. A review of primary cutaneous amyloidosis. J Am Osteopath Coll Dermatol. doi:10.1007/springerreference_42272
- Ventarola DJ, Schuster MW, Cohen JA, et al. JAAD grand rounds quiz. bullae and nodules on the legs of a 57-year-old woman. J Am Acad Dermatol. 2014;71:1035-1037.
- Chang SL, Lai PC, Cheng CJ, et al. Bullous amyloidosis in a hemodialysis patient is myeloma-associated rather than hemodialysis-associated amyloidosis. Amyloid. 2007;14:153-156.
- Suranagi VV, Siddramappa B, Bannur HB, et al. Bullous variant of familial biphasic lichen amyloidosis: a unique combination of three rare presentations. Indian J Dermatol. 2015;60:105.
- Antúnez-Lay A, Jaque A, González S. Hemorrhagic bullous skin lesions. Int J Dermatol. 2017;56:145-147.
- Reddy K, Hoda S, Penstein A, et al. Bullous amyloidosis complicated by cellulitis and sepsis: a case report. Arch Dermatol. 2011;147:126-127.
- Chu CH, Chan JY, Hsieh SW, et al. Diffuse ecchymoses and blisters on a yellowish waxy base: a case of bullous amyloidosis. J Dermatol. 2016;43:713-714.
- Gonzalez-Ramos J, Garrido-Gutiérrez C, González-Silva Y, et al. Relapsing bullous amyloidosis of the oral mucosa and acquired cutis laxa in a patient with multiple myeloma: a rare triple association. Clin Exp Dermatol. 2017;42:410-412.
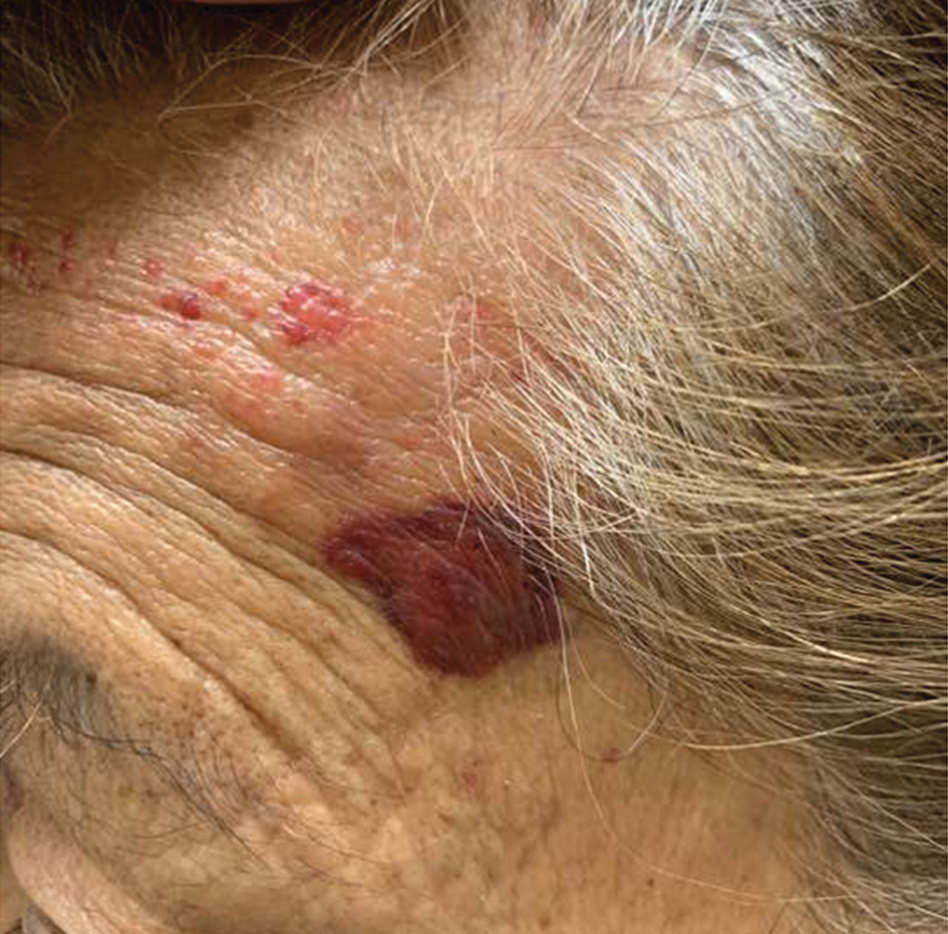
A 67-year-old woman with a medical history of type 2 diabetes mellitus, unspecified leukocytosis, and anemia presented to the dermatology clinic with asymptomatic hemorrhagic bullae on the face, chest, and tongue, as well as a large, tender, tense, hemorrhagic bulla on the groin of 3 to 4 months’ duration. A review of systems was negative for fever, chills, night sweats, malaise, shortness of breath, and dyspnea on exertion. A complete blood cell count showed mild leukocytosis, anemia, and thrombocytopenia. Her creatinine level was slightly elevated. Chest computed tomography showed early pulmonary fibrosis and coronary artery calcification. An echocardiogram showed diastolic dysfunction with moderate left ventricle thickening. A serum and urine electrophoresis demonstrated elevated free λ light chains with an M-spike. A punch biopsy was performed.
Microbiota-directed therapy may improve growth rate in malnourished children
according to new research.
Moderate acute malnutrition affects more than 30 million children worldwide, according to the Food and Nutrition Bulletin. The World Health Organization defines the condition by a weight-for-height measurement that is 2 or 3 standard deviations below the international standard.
A 2014 study published in Nature has shown that malnourishment is associated with defects in children’s gut microbiota, including having microbial communities that are immature and younger than those of their healthy counterparts. Microbiota immaturity also correlates with stunted growth.
The authors of new study, which was published in the New England Journal of Medicine on April 7, 2021, wrote that nutritional interventions and treatments, such as therapeutic calorie-dense foods, have limited effectiveness because they don’t restore growth or fully address repairing the gut microbiome.
“This work supports the notion that healthy growth of children is linked to healthy development of their gut microbiota,” study author Jeffrey Gordon, MD, director of the Edison Family Center for Genome Sciences & Systems Biology at Washington University, St. Louis, said in an interview. “This, in turn, indicates that we need to have a more encompassing view of human developmental biology – one that considers both our ‘human’ and ‘microbial’ parts.”
The study establishes the impact of microbiota repair on a child’s growth rate, which may have implications on policies related to complementary feeding practices, Dr. Gorden noted.
Better outcomes seen with microbiota-directed complementary food prototype
For the research, 123 children with moderate acute malnutrition aged between 12 and 18 months were randomly assigned to receive a microbiota-directed complementary food prototype (MDCF-2) or ready-to-use supplementary food (RUSF). The supplementation was given to the kids twice daily for 3 months, followed by 1 month of monitoring. They looked at the weekly rate of change in the weight-for-length z score, weight-for-age z score, mid-upper-arm circumference, length-for-age z score, medical complication, gut microbiota, and blood samples in the group to determine the effectiveness of each food intervention therapy.
They found that, of the 118 children who completed the study, those in the MDCF-2 group had better outcomes than those in the RUSF group based on greater weekly growth in z scores, indicating faster growth rates. For those in the MDCF-2 group, the mean weekly change in weight-for-length z score was 0.021, compared to the RUSF group’s 0.010. When it came to weight-for-age z score, the mean weekly change was 0.017 in the MDCF-2 group and 0.010 in the RUSF group. The mean weekly changes in the mid-upper-arm circumference and length-for-age z scores were similar in both groups.
When examining blood samples of the cohort, researchers noted that 714 proteins were significantly altered after 3-month MDCF-2 supplementation, compared with 82 proteins having shown significant alterations in the RUSF group.
Overall, the findings show that repairing gut microbiota was accompanied by improved weight gain and marked changes in circulating levels of protein biomarkers and mediators of numerous aspects of healthy growth.
Results need to be verified on a larger scale
Tim Joos, MD, who was not part of the study, said it is surprising that MDCF-2 was better at promoting growth than existing nutritional supplements.
“The study suggests that remedying malnutrition requires more than just ensuring adequate calorie and nutrient intake,” Dr. Joos, a pediatrician at NeighborCare Health in Seattle, noted in an interview. “It is a small study and needs to be verified on a larger scale and in more diverse locations and pediatric ages (outside of the 12- to 18-month-old cohort studied).”
Wendy S. Garrett, MD, PhD, who also didn’t participate in the study, wrote in an accompanying editorial that overarching questions remain concerning the long-lasting effects of the intervention on children’s growth trajectory and cognitive development. She also said that the study “provides an abundance of fascinating microbiome profile data, plasma protein correlates, and metadata to sift through.”
Dr. Gordon and colleagues said the findings underscore the broad effects gut microbiota has on human biology and they hope this will open the door to better definitions of wellness for infants/children.
Dr. Garrett is the Irene Heinz Given Professor of Immunology and Infectious Diseases in the departments of immunology and infectious diseases and of molecular metabolism at the Harvard School of Public Health, Boston, and she has no disclosures. Dr. Gordon is the recipient of a Thought Leader award from Agilent Technologies. Dr. Joos disclosed no relevant financial relationships.
according to new research.
Moderate acute malnutrition affects more than 30 million children worldwide, according to the Food and Nutrition Bulletin. The World Health Organization defines the condition by a weight-for-height measurement that is 2 or 3 standard deviations below the international standard.
A 2014 study published in Nature has shown that malnourishment is associated with defects in children’s gut microbiota, including having microbial communities that are immature and younger than those of their healthy counterparts. Microbiota immaturity also correlates with stunted growth.
The authors of new study, which was published in the New England Journal of Medicine on April 7, 2021, wrote that nutritional interventions and treatments, such as therapeutic calorie-dense foods, have limited effectiveness because they don’t restore growth or fully address repairing the gut microbiome.
“This work supports the notion that healthy growth of children is linked to healthy development of their gut microbiota,” study author Jeffrey Gordon, MD, director of the Edison Family Center for Genome Sciences & Systems Biology at Washington University, St. Louis, said in an interview. “This, in turn, indicates that we need to have a more encompassing view of human developmental biology – one that considers both our ‘human’ and ‘microbial’ parts.”
The study establishes the impact of microbiota repair on a child’s growth rate, which may have implications on policies related to complementary feeding practices, Dr. Gorden noted.
Better outcomes seen with microbiota-directed complementary food prototype
For the research, 123 children with moderate acute malnutrition aged between 12 and 18 months were randomly assigned to receive a microbiota-directed complementary food prototype (MDCF-2) or ready-to-use supplementary food (RUSF). The supplementation was given to the kids twice daily for 3 months, followed by 1 month of monitoring. They looked at the weekly rate of change in the weight-for-length z score, weight-for-age z score, mid-upper-arm circumference, length-for-age z score, medical complication, gut microbiota, and blood samples in the group to determine the effectiveness of each food intervention therapy.
They found that, of the 118 children who completed the study, those in the MDCF-2 group had better outcomes than those in the RUSF group based on greater weekly growth in z scores, indicating faster growth rates. For those in the MDCF-2 group, the mean weekly change in weight-for-length z score was 0.021, compared to the RUSF group’s 0.010. When it came to weight-for-age z score, the mean weekly change was 0.017 in the MDCF-2 group and 0.010 in the RUSF group. The mean weekly changes in the mid-upper-arm circumference and length-for-age z scores were similar in both groups.
When examining blood samples of the cohort, researchers noted that 714 proteins were significantly altered after 3-month MDCF-2 supplementation, compared with 82 proteins having shown significant alterations in the RUSF group.
Overall, the findings show that repairing gut microbiota was accompanied by improved weight gain and marked changes in circulating levels of protein biomarkers and mediators of numerous aspects of healthy growth.
Results need to be verified on a larger scale
Tim Joos, MD, who was not part of the study, said it is surprising that MDCF-2 was better at promoting growth than existing nutritional supplements.
“The study suggests that remedying malnutrition requires more than just ensuring adequate calorie and nutrient intake,” Dr. Joos, a pediatrician at NeighborCare Health in Seattle, noted in an interview. “It is a small study and needs to be verified on a larger scale and in more diverse locations and pediatric ages (outside of the 12- to 18-month-old cohort studied).”
Wendy S. Garrett, MD, PhD, who also didn’t participate in the study, wrote in an accompanying editorial that overarching questions remain concerning the long-lasting effects of the intervention on children’s growth trajectory and cognitive development. She also said that the study “provides an abundance of fascinating microbiome profile data, plasma protein correlates, and metadata to sift through.”
Dr. Gordon and colleagues said the findings underscore the broad effects gut microbiota has on human biology and they hope this will open the door to better definitions of wellness for infants/children.
Dr. Garrett is the Irene Heinz Given Professor of Immunology and Infectious Diseases in the departments of immunology and infectious diseases and of molecular metabolism at the Harvard School of Public Health, Boston, and she has no disclosures. Dr. Gordon is the recipient of a Thought Leader award from Agilent Technologies. Dr. Joos disclosed no relevant financial relationships.
according to new research.
Moderate acute malnutrition affects more than 30 million children worldwide, according to the Food and Nutrition Bulletin. The World Health Organization defines the condition by a weight-for-height measurement that is 2 or 3 standard deviations below the international standard.
A 2014 study published in Nature has shown that malnourishment is associated with defects in children’s gut microbiota, including having microbial communities that are immature and younger than those of their healthy counterparts. Microbiota immaturity also correlates with stunted growth.
The authors of new study, which was published in the New England Journal of Medicine on April 7, 2021, wrote that nutritional interventions and treatments, such as therapeutic calorie-dense foods, have limited effectiveness because they don’t restore growth or fully address repairing the gut microbiome.
“This work supports the notion that healthy growth of children is linked to healthy development of their gut microbiota,” study author Jeffrey Gordon, MD, director of the Edison Family Center for Genome Sciences & Systems Biology at Washington University, St. Louis, said in an interview. “This, in turn, indicates that we need to have a more encompassing view of human developmental biology – one that considers both our ‘human’ and ‘microbial’ parts.”
The study establishes the impact of microbiota repair on a child’s growth rate, which may have implications on policies related to complementary feeding practices, Dr. Gorden noted.
Better outcomes seen with microbiota-directed complementary food prototype
For the research, 123 children with moderate acute malnutrition aged between 12 and 18 months were randomly assigned to receive a microbiota-directed complementary food prototype (MDCF-2) or ready-to-use supplementary food (RUSF). The supplementation was given to the kids twice daily for 3 months, followed by 1 month of monitoring. They looked at the weekly rate of change in the weight-for-length z score, weight-for-age z score, mid-upper-arm circumference, length-for-age z score, medical complication, gut microbiota, and blood samples in the group to determine the effectiveness of each food intervention therapy.
They found that, of the 118 children who completed the study, those in the MDCF-2 group had better outcomes than those in the RUSF group based on greater weekly growth in z scores, indicating faster growth rates. For those in the MDCF-2 group, the mean weekly change in weight-for-length z score was 0.021, compared to the RUSF group’s 0.010. When it came to weight-for-age z score, the mean weekly change was 0.017 in the MDCF-2 group and 0.010 in the RUSF group. The mean weekly changes in the mid-upper-arm circumference and length-for-age z scores were similar in both groups.
When examining blood samples of the cohort, researchers noted that 714 proteins were significantly altered after 3-month MDCF-2 supplementation, compared with 82 proteins having shown significant alterations in the RUSF group.
Overall, the findings show that repairing gut microbiota was accompanied by improved weight gain and marked changes in circulating levels of protein biomarkers and mediators of numerous aspects of healthy growth.
Results need to be verified on a larger scale
Tim Joos, MD, who was not part of the study, said it is surprising that MDCF-2 was better at promoting growth than existing nutritional supplements.
“The study suggests that remedying malnutrition requires more than just ensuring adequate calorie and nutrient intake,” Dr. Joos, a pediatrician at NeighborCare Health in Seattle, noted in an interview. “It is a small study and needs to be verified on a larger scale and in more diverse locations and pediatric ages (outside of the 12- to 18-month-old cohort studied).”
Wendy S. Garrett, MD, PhD, who also didn’t participate in the study, wrote in an accompanying editorial that overarching questions remain concerning the long-lasting effects of the intervention on children’s growth trajectory and cognitive development. She also said that the study “provides an abundance of fascinating microbiome profile data, plasma protein correlates, and metadata to sift through.”
Dr. Gordon and colleagues said the findings underscore the broad effects gut microbiota has on human biology and they hope this will open the door to better definitions of wellness for infants/children.
Dr. Garrett is the Irene Heinz Given Professor of Immunology and Infectious Diseases in the departments of immunology and infectious diseases and of molecular metabolism at the Harvard School of Public Health, Boston, and she has no disclosures. Dr. Gordon is the recipient of a Thought Leader award from Agilent Technologies. Dr. Joos disclosed no relevant financial relationships.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Pregnancy after pioneering treatment for early menopause
A novel therapy combining platelet-rich plasma (PRP) with follicle-stimulating hormone that is injected directly into the ovaries has the potential to restore ovarian function for women who experience early menopause, possibly allowing for pregnancy without the need for donor eggs.
“The resumption of ovarian function in our participants means women with early menopause could have the opportunity to pursue pregnancy through IVF [in vitro fertilization] using their own eggs,” the authors of the groundbreaking pilot study report.
In the small study, published online March 29 in Menopause, menstruation resumed within a mean of about 5 weeks for 11 of 12 patients with early menopause who were treated with the technique. One patient achieved a clinical pregnancy.
In commenting on the study, Stephanie S. Faubion, MD, medical director of the North American Menopause Society, was cautious in her interpretation, noting the need for more research in larger samples.
“Any pregnancy that results from a regenerative therapy is novel,” she told this news organization. “Still, we are a long way away from this being a standard therapy for women with premature ovarian insufficiency.”
Pilot study: Platelet-rich plasma combination with FSH
Early menopause is the cessation of ovarian function at or before the age of 45 years. It is estimated that 12.2% of women experience early menopause. For these women, currently, the only chance of becoming pregnant is with donor eggs.
PRP, an autologous plasma preparation containing more than 10 times the concentration of growth factors and active metabolites than normal plasma, has recently been shown to have the potential to restore the menstrual cycles in perimenopausal women, allowing IVF. It has also been shown to benefit women with premature ovarian insufficiency (POI). However, there have been few reports of pregnancies or live births.
Chao Chin Hsu, MD, PhD, of the National Taiwan University Hospital, Taipei, and colleagues investigated whether the combination of the activated PRP treatment with gondatrophins such as FSH could provide a more robust effect so as to sufficiently stimulate follicles. They used the intraovarian injection of the combination to treat a 38-year-old woman with POI.
The effort was successful, and the woman gave birth to healthy twins.
To further evaluate the approach, the authors conducted a pilot study involving 12 women with early menopause (mean age, 44.4 years) between November 2018 and November 2019.
The women received intraovarian injection with PRP prepared from 40 mL of autologous peripheral blood combined with recombinant FSH.
Following the treatment, 11 of the 12 women experienced resumption of menstruation within a mean of 37 days. For seven patients, menstruation resumed within a month; for three, it resumed within about 2 months; and for one, it resumed after approximately 3 months.
Of note, the menstrual cycles were mostly irregular, with an interval of about 45.6 days.
The women’s average serum FSH level dropped significantly from 70.5 IU/L at baseline to 26.2 IU/L within days of treatment, as did the average luteinizing hormone level (34.8 before and 14.3 IU/L after treatment), indicative of improved ovary function.
For six participants, 10 oocyte retrieval procedures were performed after a mean of about 2 months. Thirteen mature eggs were retrieved, and fertilization via intracytoplasmic sperm injection was attempted, resulting in 10 fertilized oocytes.
Cleavage-stage embryos were transferred into two of the participants. One achieved a clinical pregnancy, defined as a pregnancy that was confirmed by ultrasound and by the presence of a fetal heartbeat. The pregnancy ended in miscarriage at 7 weeks’ gestation.
The length of controlled ovarian stimulation necessary for follicle growth ranged from 8 to 14 days, which the authors note is similar to that seen with women of normal reproductive age.
“Although the use of PRP in reproductive medicine is considered experimental, we demonstrated the restoration of ovarian function in early menopausal women who adopted whole dimension subcortical ovarian injection of PRP/gonadotropin,” the authors write.
“Most remarkably, an early menopausal woman achieved pregnancy after the treatment followed by IVF with her mature ovulating follicle,” they report.
Mechanisms, caveats
The mechanisms thought to underlie the success of the approach include increases in ovarian vascularization and stromal cell proliferation and reductions in oxidative stress and cell death in ovaries, the authors explain.
Key caveats with the treatment include the fact that anesthesia and laparoscopy are required, and precise administration is required at 15 injection sites in 1-2 mm of the ovarian subcortical area, which can be difficult to achieve, Dr. Hsu said in an interview.
“If a new instrument could be developed in which physicians can carry out this treatment through a vaginal approach, like the transvaginal retrieval of eggs in IVF treatments,” the approach could become more acceptable, Dr. Hsu added.
The authors call for studies with larger sample sizes and say it will also be interesting to determine effects in different groups: For example, women with cancer who have undergone chemotherapy.
Dr. Faubion, who is director of the Mayo Clinic Women’s Health, Rochester, Minn., says the causes of early menopause could be important in determining the treatment’s efficacy.
“[The therapy’s] success may depend on the reason the woman experienced early menopause: For instance, due to chemotherapy, radiation, virus, autoimmune disease, genetic mutation, or other cause,” she said.
She also noted that cost could be an important factor.
“I don’t see a cost estimate, but it will be substantial,” she said. “So, even if the success rate improves as this technique is further studied, cost and the invasive nature of the treatment may prove to be substantial barriers to this therapy becoming mainstream,” she said.
The authors and Dr. Faubion have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel therapy combining platelet-rich plasma (PRP) with follicle-stimulating hormone that is injected directly into the ovaries has the potential to restore ovarian function for women who experience early menopause, possibly allowing for pregnancy without the need for donor eggs.
“The resumption of ovarian function in our participants means women with early menopause could have the opportunity to pursue pregnancy through IVF [in vitro fertilization] using their own eggs,” the authors of the groundbreaking pilot study report.
In the small study, published online March 29 in Menopause, menstruation resumed within a mean of about 5 weeks for 11 of 12 patients with early menopause who were treated with the technique. One patient achieved a clinical pregnancy.
In commenting on the study, Stephanie S. Faubion, MD, medical director of the North American Menopause Society, was cautious in her interpretation, noting the need for more research in larger samples.
“Any pregnancy that results from a regenerative therapy is novel,” she told this news organization. “Still, we are a long way away from this being a standard therapy for women with premature ovarian insufficiency.”
Pilot study: Platelet-rich plasma combination with FSH
Early menopause is the cessation of ovarian function at or before the age of 45 years. It is estimated that 12.2% of women experience early menopause. For these women, currently, the only chance of becoming pregnant is with donor eggs.
PRP, an autologous plasma preparation containing more than 10 times the concentration of growth factors and active metabolites than normal plasma, has recently been shown to have the potential to restore the menstrual cycles in perimenopausal women, allowing IVF. It has also been shown to benefit women with premature ovarian insufficiency (POI). However, there have been few reports of pregnancies or live births.
Chao Chin Hsu, MD, PhD, of the National Taiwan University Hospital, Taipei, and colleagues investigated whether the combination of the activated PRP treatment with gondatrophins such as FSH could provide a more robust effect so as to sufficiently stimulate follicles. They used the intraovarian injection of the combination to treat a 38-year-old woman with POI.
The effort was successful, and the woman gave birth to healthy twins.
To further evaluate the approach, the authors conducted a pilot study involving 12 women with early menopause (mean age, 44.4 years) between November 2018 and November 2019.
The women received intraovarian injection with PRP prepared from 40 mL of autologous peripheral blood combined with recombinant FSH.
Following the treatment, 11 of the 12 women experienced resumption of menstruation within a mean of 37 days. For seven patients, menstruation resumed within a month; for three, it resumed within about 2 months; and for one, it resumed after approximately 3 months.
Of note, the menstrual cycles were mostly irregular, with an interval of about 45.6 days.
The women’s average serum FSH level dropped significantly from 70.5 IU/L at baseline to 26.2 IU/L within days of treatment, as did the average luteinizing hormone level (34.8 before and 14.3 IU/L after treatment), indicative of improved ovary function.
For six participants, 10 oocyte retrieval procedures were performed after a mean of about 2 months. Thirteen mature eggs were retrieved, and fertilization via intracytoplasmic sperm injection was attempted, resulting in 10 fertilized oocytes.
Cleavage-stage embryos were transferred into two of the participants. One achieved a clinical pregnancy, defined as a pregnancy that was confirmed by ultrasound and by the presence of a fetal heartbeat. The pregnancy ended in miscarriage at 7 weeks’ gestation.
The length of controlled ovarian stimulation necessary for follicle growth ranged from 8 to 14 days, which the authors note is similar to that seen with women of normal reproductive age.
“Although the use of PRP in reproductive medicine is considered experimental, we demonstrated the restoration of ovarian function in early menopausal women who adopted whole dimension subcortical ovarian injection of PRP/gonadotropin,” the authors write.
“Most remarkably, an early menopausal woman achieved pregnancy after the treatment followed by IVF with her mature ovulating follicle,” they report.
Mechanisms, caveats
The mechanisms thought to underlie the success of the approach include increases in ovarian vascularization and stromal cell proliferation and reductions in oxidative stress and cell death in ovaries, the authors explain.
Key caveats with the treatment include the fact that anesthesia and laparoscopy are required, and precise administration is required at 15 injection sites in 1-2 mm of the ovarian subcortical area, which can be difficult to achieve, Dr. Hsu said in an interview.
“If a new instrument could be developed in which physicians can carry out this treatment through a vaginal approach, like the transvaginal retrieval of eggs in IVF treatments,” the approach could become more acceptable, Dr. Hsu added.
The authors call for studies with larger sample sizes and say it will also be interesting to determine effects in different groups: For example, women with cancer who have undergone chemotherapy.
Dr. Faubion, who is director of the Mayo Clinic Women’s Health, Rochester, Minn., says the causes of early menopause could be important in determining the treatment’s efficacy.
“[The therapy’s] success may depend on the reason the woman experienced early menopause: For instance, due to chemotherapy, radiation, virus, autoimmune disease, genetic mutation, or other cause,” she said.
She also noted that cost could be an important factor.
“I don’t see a cost estimate, but it will be substantial,” she said. “So, even if the success rate improves as this technique is further studied, cost and the invasive nature of the treatment may prove to be substantial barriers to this therapy becoming mainstream,” she said.
The authors and Dr. Faubion have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel therapy combining platelet-rich plasma (PRP) with follicle-stimulating hormone that is injected directly into the ovaries has the potential to restore ovarian function for women who experience early menopause, possibly allowing for pregnancy without the need for donor eggs.
“The resumption of ovarian function in our participants means women with early menopause could have the opportunity to pursue pregnancy through IVF [in vitro fertilization] using their own eggs,” the authors of the groundbreaking pilot study report.
In the small study, published online March 29 in Menopause, menstruation resumed within a mean of about 5 weeks for 11 of 12 patients with early menopause who were treated with the technique. One patient achieved a clinical pregnancy.
In commenting on the study, Stephanie S. Faubion, MD, medical director of the North American Menopause Society, was cautious in her interpretation, noting the need for more research in larger samples.
“Any pregnancy that results from a regenerative therapy is novel,” she told this news organization. “Still, we are a long way away from this being a standard therapy for women with premature ovarian insufficiency.”
Pilot study: Platelet-rich plasma combination with FSH
Early menopause is the cessation of ovarian function at or before the age of 45 years. It is estimated that 12.2% of women experience early menopause. For these women, currently, the only chance of becoming pregnant is with donor eggs.
PRP, an autologous plasma preparation containing more than 10 times the concentration of growth factors and active metabolites than normal plasma, has recently been shown to have the potential to restore the menstrual cycles in perimenopausal women, allowing IVF. It has also been shown to benefit women with premature ovarian insufficiency (POI). However, there have been few reports of pregnancies or live births.
Chao Chin Hsu, MD, PhD, of the National Taiwan University Hospital, Taipei, and colleagues investigated whether the combination of the activated PRP treatment with gondatrophins such as FSH could provide a more robust effect so as to sufficiently stimulate follicles. They used the intraovarian injection of the combination to treat a 38-year-old woman with POI.
The effort was successful, and the woman gave birth to healthy twins.
To further evaluate the approach, the authors conducted a pilot study involving 12 women with early menopause (mean age, 44.4 years) between November 2018 and November 2019.
The women received intraovarian injection with PRP prepared from 40 mL of autologous peripheral blood combined with recombinant FSH.
Following the treatment, 11 of the 12 women experienced resumption of menstruation within a mean of 37 days. For seven patients, menstruation resumed within a month; for three, it resumed within about 2 months; and for one, it resumed after approximately 3 months.
Of note, the menstrual cycles were mostly irregular, with an interval of about 45.6 days.
The women’s average serum FSH level dropped significantly from 70.5 IU/L at baseline to 26.2 IU/L within days of treatment, as did the average luteinizing hormone level (34.8 before and 14.3 IU/L after treatment), indicative of improved ovary function.
For six participants, 10 oocyte retrieval procedures were performed after a mean of about 2 months. Thirteen mature eggs were retrieved, and fertilization via intracytoplasmic sperm injection was attempted, resulting in 10 fertilized oocytes.
Cleavage-stage embryos were transferred into two of the participants. One achieved a clinical pregnancy, defined as a pregnancy that was confirmed by ultrasound and by the presence of a fetal heartbeat. The pregnancy ended in miscarriage at 7 weeks’ gestation.
The length of controlled ovarian stimulation necessary for follicle growth ranged from 8 to 14 days, which the authors note is similar to that seen with women of normal reproductive age.
“Although the use of PRP in reproductive medicine is considered experimental, we demonstrated the restoration of ovarian function in early menopausal women who adopted whole dimension subcortical ovarian injection of PRP/gonadotropin,” the authors write.
“Most remarkably, an early menopausal woman achieved pregnancy after the treatment followed by IVF with her mature ovulating follicle,” they report.
Mechanisms, caveats
The mechanisms thought to underlie the success of the approach include increases in ovarian vascularization and stromal cell proliferation and reductions in oxidative stress and cell death in ovaries, the authors explain.
Key caveats with the treatment include the fact that anesthesia and laparoscopy are required, and precise administration is required at 15 injection sites in 1-2 mm of the ovarian subcortical area, which can be difficult to achieve, Dr. Hsu said in an interview.
“If a new instrument could be developed in which physicians can carry out this treatment through a vaginal approach, like the transvaginal retrieval of eggs in IVF treatments,” the approach could become more acceptable, Dr. Hsu added.
The authors call for studies with larger sample sizes and say it will also be interesting to determine effects in different groups: For example, women with cancer who have undergone chemotherapy.
Dr. Faubion, who is director of the Mayo Clinic Women’s Health, Rochester, Minn., says the causes of early menopause could be important in determining the treatment’s efficacy.
“[The therapy’s] success may depend on the reason the woman experienced early menopause: For instance, due to chemotherapy, radiation, virus, autoimmune disease, genetic mutation, or other cause,” she said.
She also noted that cost could be an important factor.
“I don’t see a cost estimate, but it will be substantial,” she said. “So, even if the success rate improves as this technique is further studied, cost and the invasive nature of the treatment may prove to be substantial barriers to this therapy becoming mainstream,” she said.
The authors and Dr. Faubion have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.