User login
Renal, cardiovascular damage may develop in mild SLE despite treatment
Patients with mild to moderate systemic lupus erythematosus (SLE) disease activity without any past history of organ damage may still progress to develop damage, particularly renal and cardiovascular disease, or death, in a relatively short amount of follow-up time, new research suggests.
The study, published in Lupus Science & Medicine, also showed that use of hydroxychloroquine lowered the risk of death and renal damage, whereas use of NSAIDs or any antihypertensives increased risk for cardiovascular damage.
“The impact of irreversible organ system damage in the prognosis of SLE remains a major concern because patients who develop damage are more likely to accrue additional damage and die,” wrote Deanna Hill, PhD, of GlaxoSmithKline, Collegeville, Pa., and coauthors, including Michelle Petri, MD, of Johns Hopkins University, Baltimore.
The researchers followed 1,168 adult patients with SLE from the Johns Hopkins Lupus Cohort, most of whom were women, 55% of whom were White and 39% of whom were Black. They divided the follow-up period into three parts: first year after enrollment into the cohort as background, second year as observation period, and the remainder of follow-up time until damage occurred, death, or end of available data.
At baseline, 55% of patients had mild to moderate disease, defined as an adjusted mean SELENA-SLEDAI (Safety of Estrogens in Lupus Erythematosus National Assessment SLE Disease Activity Index) score of less than 3. Patients had a median adjusted mean SELENA-SLEDAI score of 3 in the first year, which dropped to 2 in the observation period and remained there during the rest of follow-up.
Eight percent of patients died during the follow-up period. Each one-unit mean increase in SELENA-SLEDAI score during the 1-year observation period was associated with a significant 22% increase in the subsequent risk of death during the subsequent follow-up period (95% confidence interval, 1.13-1.32; P < .001).
Three-quarters of patients (n = 888) had no history of damage at the start of the follow-up period, but 39% of these patients had developed damage by the end of follow-up. Among patients without prior damage, a single-unit increase in disease activity score was also associated with a 9% increase in the risk of accruing organ damage (95% CI, 1.04-1.15; P < .001) after adjustment for confounding factors.
While only 3% of patients – most of whom were women – developed renal damage during the follow-up period, a one-unit increase in disease activity score was associated with a 24% increase in the risk of renal damage (95% CI, 1.08-1.42, P = .003).
The researchers found that 7% of patients developed cardiovascular damage during the follow-up period, and each one-unit increase in disease activity score was associated with a 17% increase in the risk of cardiovascular damage (95% CI, 1.07-1.29; P < .001).
“The findings in this analysis corroborate the influence of disease activity for renal and cardiovascular damage accrual and death and also extend the findings to patients with SLE and mild to moderate disease activity,” the authors wrote.
Impact of treatment
Researchers also examined the effect of treatments, and found that patients treated with hydroxychloroquine during the 1-year observation period had a 54% lower risk of subsequent death (95% CI, 0.29-0.72; P < .05) and a 70% lower risk of renal damage (95% CI, 0.13-0.68, P < .05). However, patients prescribed NSAIDs had a 66% higher risk of cardiovascular damage, while those who used any antihypertensive had an 81% higher risk of cardiovascular damage.
“This may suggest that the known cardiovascular risk of NSAIDs in the general population is also applicable to patients with SLE and highlights the importance of assessing cardiovascular risk in this patient population,” the authors wrote.
Smoking affected the risk of death: Smokers were 74% more likely to die during the follow-up period than were nonsmokers.
There were no significant differences between different ethnicities in the study. While White patients generally had lower disease activity overall, there was no significant differences in the risk of death or organ damage with ethnicity.
The Hopkins Lupus Cohort is supported by the National Institutes of Health, and the study was funded by GlaxoSmithKline. Three authors were paid employees of GlaxoSmithKline and two were paid consultants or contractors.
Patients with mild to moderate systemic lupus erythematosus (SLE) disease activity without any past history of organ damage may still progress to develop damage, particularly renal and cardiovascular disease, or death, in a relatively short amount of follow-up time, new research suggests.
The study, published in Lupus Science & Medicine, also showed that use of hydroxychloroquine lowered the risk of death and renal damage, whereas use of NSAIDs or any antihypertensives increased risk for cardiovascular damage.
“The impact of irreversible organ system damage in the prognosis of SLE remains a major concern because patients who develop damage are more likely to accrue additional damage and die,” wrote Deanna Hill, PhD, of GlaxoSmithKline, Collegeville, Pa., and coauthors, including Michelle Petri, MD, of Johns Hopkins University, Baltimore.
The researchers followed 1,168 adult patients with SLE from the Johns Hopkins Lupus Cohort, most of whom were women, 55% of whom were White and 39% of whom were Black. They divided the follow-up period into three parts: first year after enrollment into the cohort as background, second year as observation period, and the remainder of follow-up time until damage occurred, death, or end of available data.
At baseline, 55% of patients had mild to moderate disease, defined as an adjusted mean SELENA-SLEDAI (Safety of Estrogens in Lupus Erythematosus National Assessment SLE Disease Activity Index) score of less than 3. Patients had a median adjusted mean SELENA-SLEDAI score of 3 in the first year, which dropped to 2 in the observation period and remained there during the rest of follow-up.
Eight percent of patients died during the follow-up period. Each one-unit mean increase in SELENA-SLEDAI score during the 1-year observation period was associated with a significant 22% increase in the subsequent risk of death during the subsequent follow-up period (95% confidence interval, 1.13-1.32; P < .001).
Three-quarters of patients (n = 888) had no history of damage at the start of the follow-up period, but 39% of these patients had developed damage by the end of follow-up. Among patients without prior damage, a single-unit increase in disease activity score was also associated with a 9% increase in the risk of accruing organ damage (95% CI, 1.04-1.15; P < .001) after adjustment for confounding factors.
While only 3% of patients – most of whom were women – developed renal damage during the follow-up period, a one-unit increase in disease activity score was associated with a 24% increase in the risk of renal damage (95% CI, 1.08-1.42, P = .003).
The researchers found that 7% of patients developed cardiovascular damage during the follow-up period, and each one-unit increase in disease activity score was associated with a 17% increase in the risk of cardiovascular damage (95% CI, 1.07-1.29; P < .001).
“The findings in this analysis corroborate the influence of disease activity for renal and cardiovascular damage accrual and death and also extend the findings to patients with SLE and mild to moderate disease activity,” the authors wrote.
Impact of treatment
Researchers also examined the effect of treatments, and found that patients treated with hydroxychloroquine during the 1-year observation period had a 54% lower risk of subsequent death (95% CI, 0.29-0.72; P < .05) and a 70% lower risk of renal damage (95% CI, 0.13-0.68, P < .05). However, patients prescribed NSAIDs had a 66% higher risk of cardiovascular damage, while those who used any antihypertensive had an 81% higher risk of cardiovascular damage.
“This may suggest that the known cardiovascular risk of NSAIDs in the general population is also applicable to patients with SLE and highlights the importance of assessing cardiovascular risk in this patient population,” the authors wrote.
Smoking affected the risk of death: Smokers were 74% more likely to die during the follow-up period than were nonsmokers.
There were no significant differences between different ethnicities in the study. While White patients generally had lower disease activity overall, there was no significant differences in the risk of death or organ damage with ethnicity.
The Hopkins Lupus Cohort is supported by the National Institutes of Health, and the study was funded by GlaxoSmithKline. Three authors were paid employees of GlaxoSmithKline and two were paid consultants or contractors.
Patients with mild to moderate systemic lupus erythematosus (SLE) disease activity without any past history of organ damage may still progress to develop damage, particularly renal and cardiovascular disease, or death, in a relatively short amount of follow-up time, new research suggests.
The study, published in Lupus Science & Medicine, also showed that use of hydroxychloroquine lowered the risk of death and renal damage, whereas use of NSAIDs or any antihypertensives increased risk for cardiovascular damage.
“The impact of irreversible organ system damage in the prognosis of SLE remains a major concern because patients who develop damage are more likely to accrue additional damage and die,” wrote Deanna Hill, PhD, of GlaxoSmithKline, Collegeville, Pa., and coauthors, including Michelle Petri, MD, of Johns Hopkins University, Baltimore.
The researchers followed 1,168 adult patients with SLE from the Johns Hopkins Lupus Cohort, most of whom were women, 55% of whom were White and 39% of whom were Black. They divided the follow-up period into three parts: first year after enrollment into the cohort as background, second year as observation period, and the remainder of follow-up time until damage occurred, death, or end of available data.
At baseline, 55% of patients had mild to moderate disease, defined as an adjusted mean SELENA-SLEDAI (Safety of Estrogens in Lupus Erythematosus National Assessment SLE Disease Activity Index) score of less than 3. Patients had a median adjusted mean SELENA-SLEDAI score of 3 in the first year, which dropped to 2 in the observation period and remained there during the rest of follow-up.
Eight percent of patients died during the follow-up period. Each one-unit mean increase in SELENA-SLEDAI score during the 1-year observation period was associated with a significant 22% increase in the subsequent risk of death during the subsequent follow-up period (95% confidence interval, 1.13-1.32; P < .001).
Three-quarters of patients (n = 888) had no history of damage at the start of the follow-up period, but 39% of these patients had developed damage by the end of follow-up. Among patients without prior damage, a single-unit increase in disease activity score was also associated with a 9% increase in the risk of accruing organ damage (95% CI, 1.04-1.15; P < .001) after adjustment for confounding factors.
While only 3% of patients – most of whom were women – developed renal damage during the follow-up period, a one-unit increase in disease activity score was associated with a 24% increase in the risk of renal damage (95% CI, 1.08-1.42, P = .003).
The researchers found that 7% of patients developed cardiovascular damage during the follow-up period, and each one-unit increase in disease activity score was associated with a 17% increase in the risk of cardiovascular damage (95% CI, 1.07-1.29; P < .001).
“The findings in this analysis corroborate the influence of disease activity for renal and cardiovascular damage accrual and death and also extend the findings to patients with SLE and mild to moderate disease activity,” the authors wrote.
Impact of treatment
Researchers also examined the effect of treatments, and found that patients treated with hydroxychloroquine during the 1-year observation period had a 54% lower risk of subsequent death (95% CI, 0.29-0.72; P < .05) and a 70% lower risk of renal damage (95% CI, 0.13-0.68, P < .05). However, patients prescribed NSAIDs had a 66% higher risk of cardiovascular damage, while those who used any antihypertensive had an 81% higher risk of cardiovascular damage.
“This may suggest that the known cardiovascular risk of NSAIDs in the general population is also applicable to patients with SLE and highlights the importance of assessing cardiovascular risk in this patient population,” the authors wrote.
Smoking affected the risk of death: Smokers were 74% more likely to die during the follow-up period than were nonsmokers.
There were no significant differences between different ethnicities in the study. While White patients generally had lower disease activity overall, there was no significant differences in the risk of death or organ damage with ethnicity.
The Hopkins Lupus Cohort is supported by the National Institutes of Health, and the study was funded by GlaxoSmithKline. Three authors were paid employees of GlaxoSmithKline and two were paid consultants or contractors.
FROM LUPUS SCIENCE & MEDICINE
ZUMA-2, TRANSCEND data pique interest in earlier CAR T for R/R MCL
The “remarkable” efficacy of chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory mantle cell lymphoma as observed in recent trials supports its evaluation earlier in the course of treatment, according to Roch Houot, MD, PhD.
Patients with relapsed or refractory mantle cell lymphoma (MCL) who progress after treatment with a Bruton’s tyrosine kinase inhibitor (BTKi) have poor clinical outcomes, Dr. Houot, professor of hematology at Rennes (France) University Hospital, explained at the 3rd European CAR T-cell meeting.
Objective response rates in patients who relapse after BTKi therapy range from 25% to 42%, and median overall survival (OS) is less than 10 months with standard therapies, he said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
The recent ZUMA-2 and TRANSCEND NHL 001 trials evaluating the CD19 CAR T-cell products brexucabtagene autoleucel (brexu-cel; Tecartus) and lisocabtagene maraleucel (liso-cel; Breyanzi), respectively, in patients with relapsed or refractory MCL after BTKi therapy, showed dramatically improved outcomes, compared with outcomes seen previously with standard salvage therapies.
The ORR in 68 patients treated with brexu-cel in ZUMA-2 was 92%, including complete response (CR) in 40 patients (67%) and partial response (PR) in 15 patients (25%) with the rare, aggressive subtype of B-cell lymphoma.
“Interestingly, among patients who achieved a CR, 70% remained in remission after a median follow-up of 17.5 months,” he said.
Median duration of response, progression-free survival, and overall survival were not reached at that time, and ongoing responses were consistent across prognostic subgroups, he added.
The ZUMA-2 findings led to accelerated approval of brexu-cel by the Food and Drug Administration in July 2020, as well as priority medicine designation by the European Medicines Agency in December 2020, for the treatment of MCL after two or more prior lines of systemic therapy including a BTKi.
The TRANSCEND study also included patients with MCL who were relapsed or refractory after two or more lines of therapy.
The ORR was 84% in 32 patients who completed treatment – including CRs in 66% and PRs in 19%. An additional 3% had stable disease and 9% of patients progressed, Dr. Houot said.
“The follow-up of the TRANSCEND study is still very short – the median is 5.9 months – so we don’t have survival data yet for these patients,” he noted.
Still, the efficacy in these studies is excellent, particularly considering the challenges of treating MCL patients who relapse or are refractory after BTKi treatment, he said, noting that most patients in both studies had poor prognostic factors.
Toxicities in both studies were similar to those seen in studies of patients with aggressive B-cell lymphomas who were treated with these drugs, he added.
“Longer follow-up is needed to better evaluate long-term efficacy,” he said, concluding that the results nonetheless “support evaluation of CAR T-cell therapy earlier in the therapeutic strategy of mantle cell lymphoma.”
Dr. Houot reported having no disclosures.
The “remarkable” efficacy of chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory mantle cell lymphoma as observed in recent trials supports its evaluation earlier in the course of treatment, according to Roch Houot, MD, PhD.
Patients with relapsed or refractory mantle cell lymphoma (MCL) who progress after treatment with a Bruton’s tyrosine kinase inhibitor (BTKi) have poor clinical outcomes, Dr. Houot, professor of hematology at Rennes (France) University Hospital, explained at the 3rd European CAR T-cell meeting.
Objective response rates in patients who relapse after BTKi therapy range from 25% to 42%, and median overall survival (OS) is less than 10 months with standard therapies, he said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
The recent ZUMA-2 and TRANSCEND NHL 001 trials evaluating the CD19 CAR T-cell products brexucabtagene autoleucel (brexu-cel; Tecartus) and lisocabtagene maraleucel (liso-cel; Breyanzi), respectively, in patients with relapsed or refractory MCL after BTKi therapy, showed dramatically improved outcomes, compared with outcomes seen previously with standard salvage therapies.
The ORR in 68 patients treated with brexu-cel in ZUMA-2 was 92%, including complete response (CR) in 40 patients (67%) and partial response (PR) in 15 patients (25%) with the rare, aggressive subtype of B-cell lymphoma.
“Interestingly, among patients who achieved a CR, 70% remained in remission after a median follow-up of 17.5 months,” he said.
Median duration of response, progression-free survival, and overall survival were not reached at that time, and ongoing responses were consistent across prognostic subgroups, he added.
The ZUMA-2 findings led to accelerated approval of brexu-cel by the Food and Drug Administration in July 2020, as well as priority medicine designation by the European Medicines Agency in December 2020, for the treatment of MCL after two or more prior lines of systemic therapy including a BTKi.
The TRANSCEND study also included patients with MCL who were relapsed or refractory after two or more lines of therapy.
The ORR was 84% in 32 patients who completed treatment – including CRs in 66% and PRs in 19%. An additional 3% had stable disease and 9% of patients progressed, Dr. Houot said.
“The follow-up of the TRANSCEND study is still very short – the median is 5.9 months – so we don’t have survival data yet for these patients,” he noted.
Still, the efficacy in these studies is excellent, particularly considering the challenges of treating MCL patients who relapse or are refractory after BTKi treatment, he said, noting that most patients in both studies had poor prognostic factors.
Toxicities in both studies were similar to those seen in studies of patients with aggressive B-cell lymphomas who were treated with these drugs, he added.
“Longer follow-up is needed to better evaluate long-term efficacy,” he said, concluding that the results nonetheless “support evaluation of CAR T-cell therapy earlier in the therapeutic strategy of mantle cell lymphoma.”
Dr. Houot reported having no disclosures.
The “remarkable” efficacy of chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory mantle cell lymphoma as observed in recent trials supports its evaluation earlier in the course of treatment, according to Roch Houot, MD, PhD.
Patients with relapsed or refractory mantle cell lymphoma (MCL) who progress after treatment with a Bruton’s tyrosine kinase inhibitor (BTKi) have poor clinical outcomes, Dr. Houot, professor of hematology at Rennes (France) University Hospital, explained at the 3rd European CAR T-cell meeting.
Objective response rates in patients who relapse after BTKi therapy range from 25% to 42%, and median overall survival (OS) is less than 10 months with standard therapies, he said at the meeting, which is jointly sponsored by the European Society for Blood and Marrow Transplantation and the European Hematology Association.
The recent ZUMA-2 and TRANSCEND NHL 001 trials evaluating the CD19 CAR T-cell products brexucabtagene autoleucel (brexu-cel; Tecartus) and lisocabtagene maraleucel (liso-cel; Breyanzi), respectively, in patients with relapsed or refractory MCL after BTKi therapy, showed dramatically improved outcomes, compared with outcomes seen previously with standard salvage therapies.
The ORR in 68 patients treated with brexu-cel in ZUMA-2 was 92%, including complete response (CR) in 40 patients (67%) and partial response (PR) in 15 patients (25%) with the rare, aggressive subtype of B-cell lymphoma.
“Interestingly, among patients who achieved a CR, 70% remained in remission after a median follow-up of 17.5 months,” he said.
Median duration of response, progression-free survival, and overall survival were not reached at that time, and ongoing responses were consistent across prognostic subgroups, he added.
The ZUMA-2 findings led to accelerated approval of brexu-cel by the Food and Drug Administration in July 2020, as well as priority medicine designation by the European Medicines Agency in December 2020, for the treatment of MCL after two or more prior lines of systemic therapy including a BTKi.
The TRANSCEND study also included patients with MCL who were relapsed or refractory after two or more lines of therapy.
The ORR was 84% in 32 patients who completed treatment – including CRs in 66% and PRs in 19%. An additional 3% had stable disease and 9% of patients progressed, Dr. Houot said.
“The follow-up of the TRANSCEND study is still very short – the median is 5.9 months – so we don’t have survival data yet for these patients,” he noted.
Still, the efficacy in these studies is excellent, particularly considering the challenges of treating MCL patients who relapse or are refractory after BTKi treatment, he said, noting that most patients in both studies had poor prognostic factors.
Toxicities in both studies were similar to those seen in studies of patients with aggressive B-cell lymphomas who were treated with these drugs, he added.
“Longer follow-up is needed to better evaluate long-term efficacy,” he said, concluding that the results nonetheless “support evaluation of CAR T-cell therapy earlier in the therapeutic strategy of mantle cell lymphoma.”
Dr. Houot reported having no disclosures.
FROM CART21
CDC panel: Pause of J&J COVID-19 vaccine to remain for now
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
I sent my suicidal teen patient to the ED: Whew?
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
You read “thoughts of being better off dead” on your next patient’s PHQ-9 screen results and break into a sweat. After eliciting the teen’s realistic suicide plan and intent you send him to the ED with his parent for crisis mental health evaluation. When you call the family that evening to follow-up you hear that he was discharged with a “mental health counseling” appointment next week.
Have you done enough to prevent this child from dying at his own hand? I imagine that this haunts you as it does me. It is terrifying to know that, of youth with suicidal ideation, over one-third attempt suicide, most within 1-2 years, and 20%-40% do so without having had a plan.
We now know that certain kinds of psychotherapy have evidence for preventing subsequent suicide in teens at high risk due to suicidal ideation and past attempts. Cognitive behavioral therapy (CBT) has the best evidence including its subtypes for youth with relevant histories: for both suicide and substance use (integrated, or I-CBT), trauma focused (TF-CBT), traumatic grief (CTG-CBT), and CBT-I, for the potent risk factor of insomnia. The other treatment shown to reduce risk is dialectical behavioral therapy–adolescent (DBT-A) focused on strengthening skills in interpersonal effectiveness, mindfulness, distress tolerance, and emotion regulation adapted to youth by adding family therapy and multifamily skills training. Interpersonal psychotherapy (IPT) adapted for suicidal and self-harming adolescents (IPT-SA) also has evidence.
Some school programs have shown moderate efficacy, for example (IPT-A-IN) addresses the social and interpersonal context, and Youth Aware of Mental Health, a school curriculum to increase knowledge, help-seeking, and ways of coping with depression and suicidal behavior, that cut suicide attempts by half.
You may be able to recommend, refer to, or check to see if a youth can be provided one of the above therapies with best evidence but getting any counseling at all can be hard and some, especially minority families may decline formal interventions. Any therapy – CBT, DBT, or IPT – acceptable to the youth and family can be helpful. You can often determine if the key components are being provided by asking the teen what they are working on in therapy.
It is clear that checking in regularly with teens who have been through a suicide crisis is crucial to ensure that they continue in therapy long and consistently enough, that the family is involved in treatment, and that they are taught emotion regulation, distress tolerance, and safety planning. Warm, consistent parenting, good parent-child communication, and monitoring are protective factors but also skills that can be boosted to reduce future risk of suicide. When there is family dysfunction, conflict, or weak relationships, getting help for family relationships such as through attachment-based family therapy (ABFT) or family cognitive behavioral therapy is a priority. When bereavement or parental depression is contributing to youth suicidal thoughts, addressing these specifically can reduce suicide risk.
Sometimes family members, even with counseling, are not the best supporters for a teen in pain. When youths nominated their own support team to be informed about risk factors, diagnosis, and treatment plans and to stay in contact weekly there was a 6.6-fold lower risk of death than for nonsupported youth.
But how much of this evidence-based intervention can you ensure from your position in primary care? Refer if you can but regular supportive contacts alone reduce risk so you, trusted staff, school counselors, or even the now more available teletherapists may help. You can work with your patient to fill out a written commitment-to-safety plan (e.g. U. Colorado, CHADIS) of strategies they can use when having suicidal thoughts such as self-distractions, problem-solving, listing things they are looking forward to, things to do to get their mind off suicidal thoughts, and selecting support people to understand their situation with whom to be in regular contact. Any plan needs to take into account how understanding, supportive, and available the family is, factors you are most likely to be able to judge from your ongoing relationship, but that immediate risk may change. Contact within 48 hours, check-in within 1-2 weeks, and provision of crisis hotline information are essential actions.
Recommending home safety is part of routine anticipatory guidance but reduction of lethal means is essential in these cases. Guns are the most lethal method of suicide but discussing safe gun storage has been shown to be more effective than arguing in vain for gun removal. Medication overdose, a common means, can be reduced by not prescribing tricyclics (ineffective and more lethal), and advising parents to lock up all household medications.
You can ask about and coach teens on how to avoid the hazards of participating in online discussion groups, bullying, and cyberbullying (with risk for both perpetrator and victim), all risk factors for suicide. Managing insomnia can improve depression and is within your skills. While pediatricians can’t treat the suicide risk factors of family poverty, unemployment, or loss of culture/identity, we can refer affected families to community resources.
Repeated suicide screens can help but are imperfect, so listen to the child or parent for risk signs such as the youth having self-reported worthlessness, low self-esteem, speaking negatively about self, anhedonia, or poor emotion regulation. Children with impulsive aggression, often familial, are at special risk of suicide. This trait, while more common in ADHD, is not confined to that condition. You can help by optimizing medical management of impulsivity, when appropriate.
Most youth who attempt suicide have one or more mental health diagnoses, particularly major depressive disorder (MDD), eating disorder, ADHD, conduct, or intermittent explosive disorder. When MDD is comorbid with anxiety, suicides increase 9.5-fold. Children on the autism spectrum are more likely to have been bullied and eight times more likely to commit suicide. LGBTQ youth are five times more often bullied and are at high risk for suicide. The more common issues of school failure or substance use also confer risk. While we do our best caring for children with these conditions we may not be thinking about, screening, or monitoring for their suicide risk. It may be important for us to explain that, despite black-box warnings, rates of SSRI prescribing for depression are inversely related to suicides.
Child maltreatment is the highest risk factor for suicide (population attributed risk, or PAR, 9.6%-14.5%), particularly sexual misuse. All together, adverse childhood experiences have a PAR for suicide of 80%. Continuity allows you to monitor for developmental times when distress from past experiences often reemerges, e.g., puberty, dating onset, or divorce. Getting consent and sharing these highly sensitive but potentially triggering factors as well as prior diagnoses with a newly assigned therapist can be helpful to prioritize treatments to prevent a suicide attempt, because they may be difficult to elicit and timeliness is essential.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
References
Brent DA. J Am Acad Child Adolesc Psychiatry. 2019;58(1):25-35.
Cha CB et al. J Child Psychol Psychiatry. 2018;59(4):460-82.
Seeing is bleeding, and smelling is perceiving
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
Chronic breast rash
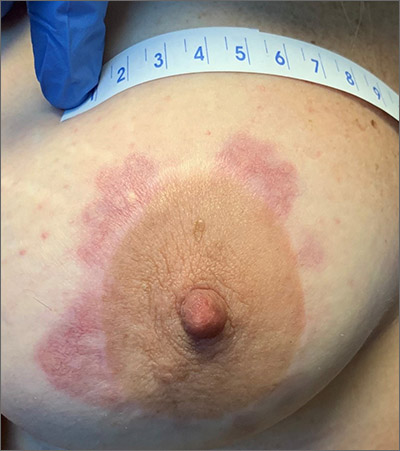
A punch biopsy revealed that the patient had granuloma annulare (GA).
GA is usually a self-limiting disorder that manifests as a single or, less commonly, multiple nonscaly, red, annular lesions that are typically found on the extremities. It frequently starts as a papule or cluster of papules before coalescing into its classic annular pattern. Biopsy is not usually needed to make the diagnosis when annular lesions are present. In this case, the lesions displayed the Koebner phenomenon, occurring along her areolar scar, making diagnosis more difficult and necessitating the biopsy. While the cause of GA is unknown, it has been found more often in women than men, but has no predilection for race, ethnicity, or geographic areas.1
GA is typically asymptomatic and can resolve spontaneously. Treatment is often performed for cosmetic reasons. First-line therapies include topical corticosteroids, topical tacrolimus, imiquimod cream, intralesional injections into the elevated border with 2.5 to 5 mg/mL triamcinolone acetonide, or destructive methods such as cryosurgery or pulsed dye laser therapy.1
After a discussion of treatment options, this patient chose watchful waiting.
Image courtesy of Kamini Geer, MD, and text courtesy of Kamini Geer, MD, AdventHealth East Orlando Osteopathic Family Medicine Residency and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Saunders; 2015.

A punch biopsy revealed that the patient had granuloma annulare (GA).
GA is usually a self-limiting disorder that manifests as a single or, less commonly, multiple nonscaly, red, annular lesions that are typically found on the extremities. It frequently starts as a papule or cluster of papules before coalescing into its classic annular pattern. Biopsy is not usually needed to make the diagnosis when annular lesions are present. In this case, the lesions displayed the Koebner phenomenon, occurring along her areolar scar, making diagnosis more difficult and necessitating the biopsy. While the cause of GA is unknown, it has been found more often in women than men, but has no predilection for race, ethnicity, or geographic areas.1
GA is typically asymptomatic and can resolve spontaneously. Treatment is often performed for cosmetic reasons. First-line therapies include topical corticosteroids, topical tacrolimus, imiquimod cream, intralesional injections into the elevated border with 2.5 to 5 mg/mL triamcinolone acetonide, or destructive methods such as cryosurgery or pulsed dye laser therapy.1
After a discussion of treatment options, this patient chose watchful waiting.
Image courtesy of Kamini Geer, MD, and text courtesy of Kamini Geer, MD, AdventHealth East Orlando Osteopathic Family Medicine Residency and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A punch biopsy revealed that the patient had granuloma annulare (GA).
GA is usually a self-limiting disorder that manifests as a single or, less commonly, multiple nonscaly, red, annular lesions that are typically found on the extremities. It frequently starts as a papule or cluster of papules before coalescing into its classic annular pattern. Biopsy is not usually needed to make the diagnosis when annular lesions are present. In this case, the lesions displayed the Koebner phenomenon, occurring along her areolar scar, making diagnosis more difficult and necessitating the biopsy. While the cause of GA is unknown, it has been found more often in women than men, but has no predilection for race, ethnicity, or geographic areas.1
GA is typically asymptomatic and can resolve spontaneously. Treatment is often performed for cosmetic reasons. First-line therapies include topical corticosteroids, topical tacrolimus, imiquimod cream, intralesional injections into the elevated border with 2.5 to 5 mg/mL triamcinolone acetonide, or destructive methods such as cryosurgery or pulsed dye laser therapy.1
After a discussion of treatment options, this patient chose watchful waiting.
Image courtesy of Kamini Geer, MD, and text courtesy of Kamini Geer, MD, AdventHealth East Orlando Osteopathic Family Medicine Residency and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Saunders; 2015.
1. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Saunders; 2015.
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
New data dim hopes for ‘triumph of drug discovery’
Hopes for a new category of agents recently hailed as “a triumph of drug discovery” have been dimmed somewhat by new data showing many types of acquired resistance.
KRAS is one of the most frequently mutated oncogenes in human cancer and has long been thought “undruggable” – but novel drugs acting specifically on the KRAS G12C mutation have shown promise in clinical trials.
Early results with the experimental KRAS inhibitors sotorasib and adagrasib were deemed promising, but new data presented at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB002) have splashed cold water on that enthusiasm.
The efficacy of these new drugs looks to be threatened by the development of resistance caused by a wide range of genomic and histologic mechanisms.
Mark M. Awad, MD, PhD, from the Dana-Farber Cancer Institute in Boston, reported data from 30 patients with non–small cell lung cancer (NSCLC) or colorectal cancer (CRC) bearing the KRAS G12C mutation who had disease progression while being treated with adagrasib in clinical trials. Investigators found multiple on-target KRAS alterations and off-target bypass mechanisms of acquired resistance to adagrasib in these patients.
“Diverse mechanisms confer resistance to the KRAS G12C inhibitors, including secondary KRAS mutations, MAP [mitogen-activated protein] kinase pathway alterations, acquired genomic rearrangements, and histologic transformation,” Dr. Awad said in a mini-symposium presentation.
“Several cases displayed multiple resistance mechanisms, and novel combinatorial strategies will be necessary to delay or overcome resistance in KRAS G12C-mutant cancers,” he said.
Inactivating KRAS
The KRAS G12C mutation is a glycine-to-cysteine substitution that results in the oncogene being switched on in its active form.
But the mutation has been considered too tough to target because of the KRAS gene’s strong binding affinity for guanosine triphosphate, an essential building block of RNA synthesis, and by a lack of accessible drug-binding sites.
Sotorasib and adagrasib are small-molecule, specific, and irreversible inhibitors of KRAS that interact with a “pocket” on the gene’s surface that is present only in an inactive conformation of KRAS. The drugs inhibit oncogenic signaling and tumorigenesis by preventing cycling of the oncogene into its active form.
Multiple mutations, histologic transformations
Dr. Awad and colleagues studied biopsy samples and circulating tumor DNA (ctDNA) from 30 patients both at baseline and after administration of adagrasib monotherapy. The patients all had initial responses to the drug but then experienced disease progression.
The investigators used mutagenesis screens to identify mechanisms of resistance to KRAS G12C inhibitors.
The 30 patients included 23 with NSCLC and 7 with CRC. Eighteen had unknown mechanisms of resistance, and putative resistance mechanisms were identified in the other 12 patients. Of this latter group, seven appeared to have single resistance mechanisms, and five had multiple mechanisms of resistance.
One patient with NSCLC who had radiographic evidence of response followed by progression was found to have had a novel KRAS Y96C mutation, and three had novel KRAS mutations in other gene regions, with multiple concurrent alterations in genes implicated in other forms of cancer, such as PTEN, BRAF, and MAP2K1.
The investigators also identified amplifications of the KRAS G12C allele, and MET.
In two patients, NSCLC underwent histologic transformation from adenocarcinoma at baseline to squamous cell carcinoma at the time of acquired resistance to the drug. No genomic resistance mechanisms were detected in either of these patients, Dr. Awad said.
“In several cases, we see multiple mechanisms or co-occurring alterations in each individual patient, with the suggestion that perhaps the multiple mutations or resistance mechanism may be more common in the colorectal population, particularly with acquired gene fusions, than in the lung cancer population, although larger datasets will be needed to confirm this observation,” he said.
Does duration of response matter?
In the question-and-answer session following his presentation, Dr. Awad was asked about clinical responses in the patients who developed resistance.
“In this initial reporting of resistance mechanisms we did not overlay or report out the clinical outcomes, including the durations of response or the time to disease progression, in part because this is an ongoing clinical trial, and those data will be reported in full at a later time,” Dr. Awad replied. “But I think it will be really important to identify whether patients are more likely to develop resistance earlier versus later or have different resistance mechanisms.”
Dr. Awad commented further that the resistance to adagrasib appeared to be acquired. “These resistance mutations were not detected to the level of detection at the baseline samples. So presumably they may be present at some low levels at the time of initial diagnosis, or they emerge over the course of therapy,” he said.
“Many of the resistance mechanisms appear to be more subclonal, occurring at an allele fraction lower than the original KRAS G12C mutation, which we know is the clonal event in the entire population of the cancer, and I think when we’re seeing these multiple resistance mechanisms emerging, they are potentially each representing different subclones that can develop simultaneously,” he added.
Adagrasib trials are supported by Mirati Therapeutics. Dr. Awad disclosed consulting for Mirati and others, and institutional research support from several different companies.
A version of this article first appeared on Medscape.com.
Hopes for a new category of agents recently hailed as “a triumph of drug discovery” have been dimmed somewhat by new data showing many types of acquired resistance.
KRAS is one of the most frequently mutated oncogenes in human cancer and has long been thought “undruggable” – but novel drugs acting specifically on the KRAS G12C mutation have shown promise in clinical trials.
Early results with the experimental KRAS inhibitors sotorasib and adagrasib were deemed promising, but new data presented at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB002) have splashed cold water on that enthusiasm.
The efficacy of these new drugs looks to be threatened by the development of resistance caused by a wide range of genomic and histologic mechanisms.
Mark M. Awad, MD, PhD, from the Dana-Farber Cancer Institute in Boston, reported data from 30 patients with non–small cell lung cancer (NSCLC) or colorectal cancer (CRC) bearing the KRAS G12C mutation who had disease progression while being treated with adagrasib in clinical trials. Investigators found multiple on-target KRAS alterations and off-target bypass mechanisms of acquired resistance to adagrasib in these patients.
“Diverse mechanisms confer resistance to the KRAS G12C inhibitors, including secondary KRAS mutations, MAP [mitogen-activated protein] kinase pathway alterations, acquired genomic rearrangements, and histologic transformation,” Dr. Awad said in a mini-symposium presentation.
“Several cases displayed multiple resistance mechanisms, and novel combinatorial strategies will be necessary to delay or overcome resistance in KRAS G12C-mutant cancers,” he said.
Inactivating KRAS
The KRAS G12C mutation is a glycine-to-cysteine substitution that results in the oncogene being switched on in its active form.
But the mutation has been considered too tough to target because of the KRAS gene’s strong binding affinity for guanosine triphosphate, an essential building block of RNA synthesis, and by a lack of accessible drug-binding sites.
Sotorasib and adagrasib are small-molecule, specific, and irreversible inhibitors of KRAS that interact with a “pocket” on the gene’s surface that is present only in an inactive conformation of KRAS. The drugs inhibit oncogenic signaling and tumorigenesis by preventing cycling of the oncogene into its active form.
Multiple mutations, histologic transformations
Dr. Awad and colleagues studied biopsy samples and circulating tumor DNA (ctDNA) from 30 patients both at baseline and after administration of adagrasib monotherapy. The patients all had initial responses to the drug but then experienced disease progression.
The investigators used mutagenesis screens to identify mechanisms of resistance to KRAS G12C inhibitors.
The 30 patients included 23 with NSCLC and 7 with CRC. Eighteen had unknown mechanisms of resistance, and putative resistance mechanisms were identified in the other 12 patients. Of this latter group, seven appeared to have single resistance mechanisms, and five had multiple mechanisms of resistance.
One patient with NSCLC who had radiographic evidence of response followed by progression was found to have had a novel KRAS Y96C mutation, and three had novel KRAS mutations in other gene regions, with multiple concurrent alterations in genes implicated in other forms of cancer, such as PTEN, BRAF, and MAP2K1.
The investigators also identified amplifications of the KRAS G12C allele, and MET.
In two patients, NSCLC underwent histologic transformation from adenocarcinoma at baseline to squamous cell carcinoma at the time of acquired resistance to the drug. No genomic resistance mechanisms were detected in either of these patients, Dr. Awad said.
“In several cases, we see multiple mechanisms or co-occurring alterations in each individual patient, with the suggestion that perhaps the multiple mutations or resistance mechanism may be more common in the colorectal population, particularly with acquired gene fusions, than in the lung cancer population, although larger datasets will be needed to confirm this observation,” he said.
Does duration of response matter?
In the question-and-answer session following his presentation, Dr. Awad was asked about clinical responses in the patients who developed resistance.
“In this initial reporting of resistance mechanisms we did not overlay or report out the clinical outcomes, including the durations of response or the time to disease progression, in part because this is an ongoing clinical trial, and those data will be reported in full at a later time,” Dr. Awad replied. “But I think it will be really important to identify whether patients are more likely to develop resistance earlier versus later or have different resistance mechanisms.”
Dr. Awad commented further that the resistance to adagrasib appeared to be acquired. “These resistance mutations were not detected to the level of detection at the baseline samples. So presumably they may be present at some low levels at the time of initial diagnosis, or they emerge over the course of therapy,” he said.
“Many of the resistance mechanisms appear to be more subclonal, occurring at an allele fraction lower than the original KRAS G12C mutation, which we know is the clonal event in the entire population of the cancer, and I think when we’re seeing these multiple resistance mechanisms emerging, they are potentially each representing different subclones that can develop simultaneously,” he added.
Adagrasib trials are supported by Mirati Therapeutics. Dr. Awad disclosed consulting for Mirati and others, and institutional research support from several different companies.
A version of this article first appeared on Medscape.com.
Hopes for a new category of agents recently hailed as “a triumph of drug discovery” have been dimmed somewhat by new data showing many types of acquired resistance.
KRAS is one of the most frequently mutated oncogenes in human cancer and has long been thought “undruggable” – but novel drugs acting specifically on the KRAS G12C mutation have shown promise in clinical trials.
Early results with the experimental KRAS inhibitors sotorasib and adagrasib were deemed promising, but new data presented at the American Association for Cancer Research Annual Meeting 2021: Week 1 (Abstract LB002) have splashed cold water on that enthusiasm.
The efficacy of these new drugs looks to be threatened by the development of resistance caused by a wide range of genomic and histologic mechanisms.
Mark M. Awad, MD, PhD, from the Dana-Farber Cancer Institute in Boston, reported data from 30 patients with non–small cell lung cancer (NSCLC) or colorectal cancer (CRC) bearing the KRAS G12C mutation who had disease progression while being treated with adagrasib in clinical trials. Investigators found multiple on-target KRAS alterations and off-target bypass mechanisms of acquired resistance to adagrasib in these patients.
“Diverse mechanisms confer resistance to the KRAS G12C inhibitors, including secondary KRAS mutations, MAP [mitogen-activated protein] kinase pathway alterations, acquired genomic rearrangements, and histologic transformation,” Dr. Awad said in a mini-symposium presentation.
“Several cases displayed multiple resistance mechanisms, and novel combinatorial strategies will be necessary to delay or overcome resistance in KRAS G12C-mutant cancers,” he said.
Inactivating KRAS
The KRAS G12C mutation is a glycine-to-cysteine substitution that results in the oncogene being switched on in its active form.
But the mutation has been considered too tough to target because of the KRAS gene’s strong binding affinity for guanosine triphosphate, an essential building block of RNA synthesis, and by a lack of accessible drug-binding sites.
Sotorasib and adagrasib are small-molecule, specific, and irreversible inhibitors of KRAS that interact with a “pocket” on the gene’s surface that is present only in an inactive conformation of KRAS. The drugs inhibit oncogenic signaling and tumorigenesis by preventing cycling of the oncogene into its active form.
Multiple mutations, histologic transformations
Dr. Awad and colleagues studied biopsy samples and circulating tumor DNA (ctDNA) from 30 patients both at baseline and after administration of adagrasib monotherapy. The patients all had initial responses to the drug but then experienced disease progression.
The investigators used mutagenesis screens to identify mechanisms of resistance to KRAS G12C inhibitors.
The 30 patients included 23 with NSCLC and 7 with CRC. Eighteen had unknown mechanisms of resistance, and putative resistance mechanisms were identified in the other 12 patients. Of this latter group, seven appeared to have single resistance mechanisms, and five had multiple mechanisms of resistance.
One patient with NSCLC who had radiographic evidence of response followed by progression was found to have had a novel KRAS Y96C mutation, and three had novel KRAS mutations in other gene regions, with multiple concurrent alterations in genes implicated in other forms of cancer, such as PTEN, BRAF, and MAP2K1.
The investigators also identified amplifications of the KRAS G12C allele, and MET.
In two patients, NSCLC underwent histologic transformation from adenocarcinoma at baseline to squamous cell carcinoma at the time of acquired resistance to the drug. No genomic resistance mechanisms were detected in either of these patients, Dr. Awad said.
“In several cases, we see multiple mechanisms or co-occurring alterations in each individual patient, with the suggestion that perhaps the multiple mutations or resistance mechanism may be more common in the colorectal population, particularly with acquired gene fusions, than in the lung cancer population, although larger datasets will be needed to confirm this observation,” he said.
Does duration of response matter?
In the question-and-answer session following his presentation, Dr. Awad was asked about clinical responses in the patients who developed resistance.
“In this initial reporting of resistance mechanisms we did not overlay or report out the clinical outcomes, including the durations of response or the time to disease progression, in part because this is an ongoing clinical trial, and those data will be reported in full at a later time,” Dr. Awad replied. “But I think it will be really important to identify whether patients are more likely to develop resistance earlier versus later or have different resistance mechanisms.”
Dr. Awad commented further that the resistance to adagrasib appeared to be acquired. “These resistance mutations were not detected to the level of detection at the baseline samples. So presumably they may be present at some low levels at the time of initial diagnosis, or they emerge over the course of therapy,” he said.
“Many of the resistance mechanisms appear to be more subclonal, occurring at an allele fraction lower than the original KRAS G12C mutation, which we know is the clonal event in the entire population of the cancer, and I think when we’re seeing these multiple resistance mechanisms emerging, they are potentially each representing different subclones that can develop simultaneously,” he added.
Adagrasib trials are supported by Mirati Therapeutics. Dr. Awad disclosed consulting for Mirati and others, and institutional research support from several different companies.
A version of this article first appeared on Medscape.com.
FROM AACR 2021
Adjunctive MDMA safe, effective for severe PTSD
Adding 3,4-methylenedioxymethamphetamine (MDMA) to integrative psychotherapy may significantly improve symptoms and well-being for patients with severe posttraumatic stress disorder, including those with the dissociative subtype, new research suggests.
MAPP1 is the first phase 3 randomized controlled trial of MDMA-assisted therapy in this population. Participants who received the active treatment showed greater improvement in PTSD symptoms, mood, and empathy in comparison with participants who received placebo.
MDMA was “extremely effective, particularly for a subpopulation that ordinarily does not respond well to conventional treatment,” study coinvestigator Bessel van der Kolk, MD, professor of psychiatry at Boston University School of Medicine, told delegates attending the virtual European Psychiatric Association (EPA) 2021 Congress.
Growing interest
, particularly because failure rates with most available evidence-based treatments have been relatively high.
As previously reported by this news organization, in 2017, the U.S. Food and Drug Administration approved the trial design of Dr. van der Kolk’s and colleagues’ MAPP1 study after granting MDMA breakthrough designation.
The MAPP1 investigators assessed 90 patients with PTSD (mean age, 41 years; 77% White; 66% women) from 50 sites. For the majority of patients (84%), trauma history was developmental. “In other words, trauma [occurred] very early in life, usually at the hands of their own caregivers,” Dr. van der Kolk noted.
In addition, 18% of the patients were veterans, and 12% had combat exposure. The average duration of PTSD before enrollment was 18 years. All patients underwent screening and three preparatory psychotherapy sessions at enrollment.
Participants were randomly assigned to receive MDMA 80 mg or 120 mg (n = 46) or placebo (n = 44) followed by three integrative psychotherapy sessions lasting a total of 8 hours. A supplemental dose of 40 or 60 mg of MDMA could be administered from 1.5 to 2 hours after the first dose.
The patients stayed in the laboratory on the evening of the treatment session and attended a debriefing the next morning. The session was repeated a month later and again a month after that. In between, patients had telephone contact with the raters, who were blinded to the treatment received.
Follow-up assessments were conducted 2 months after the third treatment session and again at 12 months. The primary outcome measure was change in Clinician Administered PTSD Scale for DSM 5 (CAPS-5) score from baseline.
‘Dramatic improvement’
Results showed that both the MDMA and placebo groups experienced a statistically significant improvement in PTSD symptoms, “but MDMA had a dramatically significant improvement, with an effect size of over 0.9,” Dr. van der Kolk said.
The MDMA group also reported enhanced mood and well-being, increased responsiveness to emotional and sensory stimuli, a greater sense of closeness to other people, and a greater feeling of empathy.
Patients also reported having heightened openness, “and clearly the issue of empathy for themselves and others was a very large part of the process,” said Dr. van der Kolk.
“But for me, the most interesting part of the study is that the Adverse Childhood Experiences scale had no effect,” he noted. In other words, “the amount of childhood adverse experiences did not predict outcomes, which was very surprising because usually those patients are very treatment resistant.”
Dr. van der Kolk added that the dissociative subtype of PTSD was first described in the DSM-5 and that patients are “notoriously unresponsive to most unconventional treatments.”
In the current study, 13 patients met the criteria for the subtype, and investigators found they “did better than people with classical PTSD,” Dr. van der Kolk said. He added that this is a “very, very important finding.”
Carefully controlled
Overall, 82% of patients reported a significant improvement by the end of the study; 56% reported that they no longer had PTSD.
In addition, 67% of patients no longer met diagnostic criteria for PTSD. These included patients who had crossed over to active treatment from the placebo group.
Eleven patients (12%) experienced relapse by 12 months; in nine of the cases, this was due to the presence of additional stressors.
There were “very few adverse side effects” during the study, Dr. van der Kolk noted. In addition, “there were really no serious mental side effects,” despite the patients’ “opening up so much very painful material,” he added.
The most common adverse events among the MDMA group were muscle tightness (63%), decreased appetite (52%), nausea (30%), hyperhidrosis (20%), and feeling cold (20%). These effects were “quite small [and] the sort of side effects you would expect in response to an amphetamine substance like MDMA,” said Dr. van der Kolk.
“An important reason why we think the side effect profile is so good is because the study was extremely carefully done, very carefully controlled,” he added. “There was a great deal of support, [and] we paid an enormous amount of attention to creating a very safe context in which this drug was being used.”
However, he expressed concern that “as people see the very good results, they may skimp a little bit on the creation of the context and not have as careful a psychotherapy protocol as we had here.”
‘On the right track’
Commenting on the findings for this news organization, David Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, said the results are proof that the investigators’ “earlier smaller trials of MDMA were on the right track.”
“This larger and multicenter trial shows that MDMA therapy can be broadened into newer research groups, which augurs well for the much larger rollout that will be required once it gets a license,” said Dr. Nutt, who was not involved with the research.
He added, “the prior evidence of the safety of MDMA has [now] been confirmed.”
The study represents an “important step in the path to the clinical use of MDMA for PTSD,” Dr. Nutt said.
The study was sponsored by the Multidisciplinary Association for Psychedelic Studies. The investigators and Dr. Nutt have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adding 3,4-methylenedioxymethamphetamine (MDMA) to integrative psychotherapy may significantly improve symptoms and well-being for patients with severe posttraumatic stress disorder, including those with the dissociative subtype, new research suggests.
MAPP1 is the first phase 3 randomized controlled trial of MDMA-assisted therapy in this population. Participants who received the active treatment showed greater improvement in PTSD symptoms, mood, and empathy in comparison with participants who received placebo.
MDMA was “extremely effective, particularly for a subpopulation that ordinarily does not respond well to conventional treatment,” study coinvestigator Bessel van der Kolk, MD, professor of psychiatry at Boston University School of Medicine, told delegates attending the virtual European Psychiatric Association (EPA) 2021 Congress.
Growing interest
, particularly because failure rates with most available evidence-based treatments have been relatively high.
As previously reported by this news organization, in 2017, the U.S. Food and Drug Administration approved the trial design of Dr. van der Kolk’s and colleagues’ MAPP1 study after granting MDMA breakthrough designation.
The MAPP1 investigators assessed 90 patients with PTSD (mean age, 41 years; 77% White; 66% women) from 50 sites. For the majority of patients (84%), trauma history was developmental. “In other words, trauma [occurred] very early in life, usually at the hands of their own caregivers,” Dr. van der Kolk noted.
In addition, 18% of the patients were veterans, and 12% had combat exposure. The average duration of PTSD before enrollment was 18 years. All patients underwent screening and three preparatory psychotherapy sessions at enrollment.
Participants were randomly assigned to receive MDMA 80 mg or 120 mg (n = 46) or placebo (n = 44) followed by three integrative psychotherapy sessions lasting a total of 8 hours. A supplemental dose of 40 or 60 mg of MDMA could be administered from 1.5 to 2 hours after the first dose.
The patients stayed in the laboratory on the evening of the treatment session and attended a debriefing the next morning. The session was repeated a month later and again a month after that. In between, patients had telephone contact with the raters, who were blinded to the treatment received.
Follow-up assessments were conducted 2 months after the third treatment session and again at 12 months. The primary outcome measure was change in Clinician Administered PTSD Scale for DSM 5 (CAPS-5) score from baseline.
‘Dramatic improvement’
Results showed that both the MDMA and placebo groups experienced a statistically significant improvement in PTSD symptoms, “but MDMA had a dramatically significant improvement, with an effect size of over 0.9,” Dr. van der Kolk said.
The MDMA group also reported enhanced mood and well-being, increased responsiveness to emotional and sensory stimuli, a greater sense of closeness to other people, and a greater feeling of empathy.
Patients also reported having heightened openness, “and clearly the issue of empathy for themselves and others was a very large part of the process,” said Dr. van der Kolk.
“But for me, the most interesting part of the study is that the Adverse Childhood Experiences scale had no effect,” he noted. In other words, “the amount of childhood adverse experiences did not predict outcomes, which was very surprising because usually those patients are very treatment resistant.”
Dr. van der Kolk added that the dissociative subtype of PTSD was first described in the DSM-5 and that patients are “notoriously unresponsive to most unconventional treatments.”
In the current study, 13 patients met the criteria for the subtype, and investigators found they “did better than people with classical PTSD,” Dr. van der Kolk said. He added that this is a “very, very important finding.”
Carefully controlled
Overall, 82% of patients reported a significant improvement by the end of the study; 56% reported that they no longer had PTSD.
In addition, 67% of patients no longer met diagnostic criteria for PTSD. These included patients who had crossed over to active treatment from the placebo group.
Eleven patients (12%) experienced relapse by 12 months; in nine of the cases, this was due to the presence of additional stressors.
There were “very few adverse side effects” during the study, Dr. van der Kolk noted. In addition, “there were really no serious mental side effects,” despite the patients’ “opening up so much very painful material,” he added.
The most common adverse events among the MDMA group were muscle tightness (63%), decreased appetite (52%), nausea (30%), hyperhidrosis (20%), and feeling cold (20%). These effects were “quite small [and] the sort of side effects you would expect in response to an amphetamine substance like MDMA,” said Dr. van der Kolk.
“An important reason why we think the side effect profile is so good is because the study was extremely carefully done, very carefully controlled,” he added. “There was a great deal of support, [and] we paid an enormous amount of attention to creating a very safe context in which this drug was being used.”
However, he expressed concern that “as people see the very good results, they may skimp a little bit on the creation of the context and not have as careful a psychotherapy protocol as we had here.”
‘On the right track’
Commenting on the findings for this news organization, David Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, said the results are proof that the investigators’ “earlier smaller trials of MDMA were on the right track.”
“This larger and multicenter trial shows that MDMA therapy can be broadened into newer research groups, which augurs well for the much larger rollout that will be required once it gets a license,” said Dr. Nutt, who was not involved with the research.
He added, “the prior evidence of the safety of MDMA has [now] been confirmed.”
The study represents an “important step in the path to the clinical use of MDMA for PTSD,” Dr. Nutt said.
The study was sponsored by the Multidisciplinary Association for Psychedelic Studies. The investigators and Dr. Nutt have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adding 3,4-methylenedioxymethamphetamine (MDMA) to integrative psychotherapy may significantly improve symptoms and well-being for patients with severe posttraumatic stress disorder, including those with the dissociative subtype, new research suggests.
MAPP1 is the first phase 3 randomized controlled trial of MDMA-assisted therapy in this population. Participants who received the active treatment showed greater improvement in PTSD symptoms, mood, and empathy in comparison with participants who received placebo.
MDMA was “extremely effective, particularly for a subpopulation that ordinarily does not respond well to conventional treatment,” study coinvestigator Bessel van der Kolk, MD, professor of psychiatry at Boston University School of Medicine, told delegates attending the virtual European Psychiatric Association (EPA) 2021 Congress.
Growing interest
, particularly because failure rates with most available evidence-based treatments have been relatively high.
As previously reported by this news organization, in 2017, the U.S. Food and Drug Administration approved the trial design of Dr. van der Kolk’s and colleagues’ MAPP1 study after granting MDMA breakthrough designation.
The MAPP1 investigators assessed 90 patients with PTSD (mean age, 41 years; 77% White; 66% women) from 50 sites. For the majority of patients (84%), trauma history was developmental. “In other words, trauma [occurred] very early in life, usually at the hands of their own caregivers,” Dr. van der Kolk noted.
In addition, 18% of the patients were veterans, and 12% had combat exposure. The average duration of PTSD before enrollment was 18 years. All patients underwent screening and three preparatory psychotherapy sessions at enrollment.
Participants were randomly assigned to receive MDMA 80 mg or 120 mg (n = 46) or placebo (n = 44) followed by three integrative psychotherapy sessions lasting a total of 8 hours. A supplemental dose of 40 or 60 mg of MDMA could be administered from 1.5 to 2 hours after the first dose.
The patients stayed in the laboratory on the evening of the treatment session and attended a debriefing the next morning. The session was repeated a month later and again a month after that. In between, patients had telephone contact with the raters, who were blinded to the treatment received.
Follow-up assessments were conducted 2 months after the third treatment session and again at 12 months. The primary outcome measure was change in Clinician Administered PTSD Scale for DSM 5 (CAPS-5) score from baseline.
‘Dramatic improvement’
Results showed that both the MDMA and placebo groups experienced a statistically significant improvement in PTSD symptoms, “but MDMA had a dramatically significant improvement, with an effect size of over 0.9,” Dr. van der Kolk said.
The MDMA group also reported enhanced mood and well-being, increased responsiveness to emotional and sensory stimuli, a greater sense of closeness to other people, and a greater feeling of empathy.
Patients also reported having heightened openness, “and clearly the issue of empathy for themselves and others was a very large part of the process,” said Dr. van der Kolk.
“But for me, the most interesting part of the study is that the Adverse Childhood Experiences scale had no effect,” he noted. In other words, “the amount of childhood adverse experiences did not predict outcomes, which was very surprising because usually those patients are very treatment resistant.”
Dr. van der Kolk added that the dissociative subtype of PTSD was first described in the DSM-5 and that patients are “notoriously unresponsive to most unconventional treatments.”
In the current study, 13 patients met the criteria for the subtype, and investigators found they “did better than people with classical PTSD,” Dr. van der Kolk said. He added that this is a “very, very important finding.”
Carefully controlled
Overall, 82% of patients reported a significant improvement by the end of the study; 56% reported that they no longer had PTSD.
In addition, 67% of patients no longer met diagnostic criteria for PTSD. These included patients who had crossed over to active treatment from the placebo group.
Eleven patients (12%) experienced relapse by 12 months; in nine of the cases, this was due to the presence of additional stressors.
There were “very few adverse side effects” during the study, Dr. van der Kolk noted. In addition, “there were really no serious mental side effects,” despite the patients’ “opening up so much very painful material,” he added.
The most common adverse events among the MDMA group were muscle tightness (63%), decreased appetite (52%), nausea (30%), hyperhidrosis (20%), and feeling cold (20%). These effects were “quite small [and] the sort of side effects you would expect in response to an amphetamine substance like MDMA,” said Dr. van der Kolk.
“An important reason why we think the side effect profile is so good is because the study was extremely carefully done, very carefully controlled,” he added. “There was a great deal of support, [and] we paid an enormous amount of attention to creating a very safe context in which this drug was being used.”
However, he expressed concern that “as people see the very good results, they may skimp a little bit on the creation of the context and not have as careful a psychotherapy protocol as we had here.”
‘On the right track’
Commenting on the findings for this news organization, David Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, said the results are proof that the investigators’ “earlier smaller trials of MDMA were on the right track.”
“This larger and multicenter trial shows that MDMA therapy can be broadened into newer research groups, which augurs well for the much larger rollout that will be required once it gets a license,” said Dr. Nutt, who was not involved with the research.
He added, “the prior evidence of the safety of MDMA has [now] been confirmed.”
The study represents an “important step in the path to the clinical use of MDMA for PTSD,” Dr. Nutt said.
The study was sponsored by the Multidisciplinary Association for Psychedelic Studies. The investigators and Dr. Nutt have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ubrogepant safety and efficacy not affected by triptan therapy
according to a study published in Headache.
“The goal is to get migraine attacks under control as quickly as you can with as few adverse events as possible,” said lead author Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad, California. “Ubrogepant is very efficacious and well tolerated because it has few adverse events.”
Migraine disorder is the third most prevalent disease, and at least one person living in 25% of all U.S. households has the condition.
Clinicians have a wide range of medications at their disposal to treat migraines. These drug classes include triptans, ditans, NSAIDs, dihydroergotamine, and combination analgesics. Although numerous pharmacologic options are available to manage this patient population, an estimated 95% of patients who take oral medications to alleviate their migraine symptoms still fail to achieve relief with at least one acute episode.
Triptans remain a common option and first-line choice for acute migraine relief, but poor tolerability, among other factors, continue to limit their effectiveness. Moreover, their vasoconstrictive properties preclude their use in specific patient populations, such as those who have hypertension, peripheral vascular disease, and cerebral vascular accident. These circumstances, combined with other unmet clinical needs in migraine, have prompted researchers to explore new options, including a newer class of drugs – CGRP receptor antagonists. An endogenous protein, CGRP, has inflammatory and pronociceptive properties that play an active role in contributing to migraine pathogenesis.
Efficacy analyzed by triptan response
To investigate the effects of anti-CGRP treatment in patients with migraine who have a previous history of triptan use, researchers conducted two phase 3, randomized, double-blind, multicenter, single-attack trials, known as ACHIEVE I and ACHIEVE II.
Trial participants ranged in age from 18 to 75 years with a documented history of migraines with or without aura. In ACHIEVE I, investigators randomized 1,327 participants 1:1:1 to receive placebo, ubrogepant 50 mg, or ubrogepant 100 mg and placebo. Randomized patients in ACHIEVE II (n = 1,355) received placebo, ubrogepant 25 mg, or ubrogepant 50 mg to treat a single episode. During the screening process, researchers further placed patients in one of three groups based on their previous triptan use – triptan responder, triptan-insufficient responder, and triptan naive. Patients were further randomized based on their previous experience with triptans and whether they currently used them for migraine prevention. Patients participating in the study had up to 60 days to treat one qualifying migraine of moderate or severe nature at home.
The studies had two primary endpoints. The first was freedom from pain at the 2-hour mark following the initial dose, defined as decreased headache severity from moderate or severe at baseline to no pain. The other primary endpoint was the absence of most bothersome migraine-associated symptom (MBS) – photophobia, phonophobia, or nausea – 2 hours after the initial ubrogepant dose.
The pooled analysis collated data from 1,799 patients in both studies (placebo, n = 912; ubrogepant 50 mg, n = 887). Patients fell into the following categories: 682 triptan responders (placebo, n = 350; ubrogepant, n = 332); 451 triptan-insufficient responders (placebo, n = 223; ubrogepant, n = 228), and 666 triptan naive (placebo, n = 339; ubrogepant, n = 337).
Based on the data, approximately 25% of the patients enrolled in the study fell into the triptan-insufficient category. Of this subpopulation, about 80% of the patients in each treatment group experienced insufficient efficacy when using triptans. In each treatment group of insufficient responders, approximately 17% of patients cited tolerability issues, and 3% had contraindications that precluded them from triptan therapy.
The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs did not differ appreciably across historical triptan experience subgroups. The highest percentage of participants experiencing a treatment-related TEAE in the pooled ubrogepant 50-mg treatment group was found in the triptan-insufficient responders (10.4%), whereas the highest percentage in the placebo group was found in the triptan-naive subgroup (9.7%). No serious AEs (SAEs) were reported in any subgroup.
The researchers concluded that “ubrogepant efficacy and tolerability did not differ for the acute treatment of migraine in participants classified as triptan responders, triptan-insufficient responders, and triptan naive based on their historical experience with triptans.”
Payers limit use
Despite the promising data, payer hurdles limit ubrogepant’s use, said Stewart Tepper, MD, who was asked to comment on the study. Dr. Tepper, a professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., was not involved in the study. “The study shows that ubrogepant will work as well as those who have not responded well to triptans, which is key,” Dr. Tepper said. “However, payers have set up step edits in which patients aren’t allowed to get ubrogepant unless they fail therapy with at least two triptans.”
Dr. Blumenfeld discounts the rationale behind requiring patients to try a second triptan after failing initial triptan therapy. “There are plenty of studies showing that if you fail one triptan, you’re likely to fail another,” he said. “Why would you put the patient on a different triptan when you could switch them to another drug with a different mechanism of action?”
The results showed that more patients in the ubrogepant 50-mg arm achieved pain freedom than those in the placebo arm within 2 hours after the initial dose in each group.
“Migraine is a very disabling condition, so you want to get the attack under control as quickly as possible while limiting the risk for potential side effects,” said Dr. Blumenfeld. “Ubrogepant is very efficacious and with very few adverse effects, but its use is limited because insurance companies require the failure of several triptans.”
One limitation of the study is that it is a subanalysis.
Dr. Blumenfeld has disclosed advisory board service, consulting, speaking, and authorship for AbbVie, Alder, Amgen, Biohaven, Lilly, Novartis, Teva, Theranica, and Zoscano.
according to a study published in Headache.
“The goal is to get migraine attacks under control as quickly as you can with as few adverse events as possible,” said lead author Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad, California. “Ubrogepant is very efficacious and well tolerated because it has few adverse events.”
Migraine disorder is the third most prevalent disease, and at least one person living in 25% of all U.S. households has the condition.
Clinicians have a wide range of medications at their disposal to treat migraines. These drug classes include triptans, ditans, NSAIDs, dihydroergotamine, and combination analgesics. Although numerous pharmacologic options are available to manage this patient population, an estimated 95% of patients who take oral medications to alleviate their migraine symptoms still fail to achieve relief with at least one acute episode.
Triptans remain a common option and first-line choice for acute migraine relief, but poor tolerability, among other factors, continue to limit their effectiveness. Moreover, their vasoconstrictive properties preclude their use in specific patient populations, such as those who have hypertension, peripheral vascular disease, and cerebral vascular accident. These circumstances, combined with other unmet clinical needs in migraine, have prompted researchers to explore new options, including a newer class of drugs – CGRP receptor antagonists. An endogenous protein, CGRP, has inflammatory and pronociceptive properties that play an active role in contributing to migraine pathogenesis.
Efficacy analyzed by triptan response
To investigate the effects of anti-CGRP treatment in patients with migraine who have a previous history of triptan use, researchers conducted two phase 3, randomized, double-blind, multicenter, single-attack trials, known as ACHIEVE I and ACHIEVE II.
Trial participants ranged in age from 18 to 75 years with a documented history of migraines with or without aura. In ACHIEVE I, investigators randomized 1,327 participants 1:1:1 to receive placebo, ubrogepant 50 mg, or ubrogepant 100 mg and placebo. Randomized patients in ACHIEVE II (n = 1,355) received placebo, ubrogepant 25 mg, or ubrogepant 50 mg to treat a single episode. During the screening process, researchers further placed patients in one of three groups based on their previous triptan use – triptan responder, triptan-insufficient responder, and triptan naive. Patients were further randomized based on their previous experience with triptans and whether they currently used them for migraine prevention. Patients participating in the study had up to 60 days to treat one qualifying migraine of moderate or severe nature at home.
The studies had two primary endpoints. The first was freedom from pain at the 2-hour mark following the initial dose, defined as decreased headache severity from moderate or severe at baseline to no pain. The other primary endpoint was the absence of most bothersome migraine-associated symptom (MBS) – photophobia, phonophobia, or nausea – 2 hours after the initial ubrogepant dose.
The pooled analysis collated data from 1,799 patients in both studies (placebo, n = 912; ubrogepant 50 mg, n = 887). Patients fell into the following categories: 682 triptan responders (placebo, n = 350; ubrogepant, n = 332); 451 triptan-insufficient responders (placebo, n = 223; ubrogepant, n = 228), and 666 triptan naive (placebo, n = 339; ubrogepant, n = 337).
Based on the data, approximately 25% of the patients enrolled in the study fell into the triptan-insufficient category. Of this subpopulation, about 80% of the patients in each treatment group experienced insufficient efficacy when using triptans. In each treatment group of insufficient responders, approximately 17% of patients cited tolerability issues, and 3% had contraindications that precluded them from triptan therapy.
The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs did not differ appreciably across historical triptan experience subgroups. The highest percentage of participants experiencing a treatment-related TEAE in the pooled ubrogepant 50-mg treatment group was found in the triptan-insufficient responders (10.4%), whereas the highest percentage in the placebo group was found in the triptan-naive subgroup (9.7%). No serious AEs (SAEs) were reported in any subgroup.
The researchers concluded that “ubrogepant efficacy and tolerability did not differ for the acute treatment of migraine in participants classified as triptan responders, triptan-insufficient responders, and triptan naive based on their historical experience with triptans.”
Payers limit use
Despite the promising data, payer hurdles limit ubrogepant’s use, said Stewart Tepper, MD, who was asked to comment on the study. Dr. Tepper, a professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., was not involved in the study. “The study shows that ubrogepant will work as well as those who have not responded well to triptans, which is key,” Dr. Tepper said. “However, payers have set up step edits in which patients aren’t allowed to get ubrogepant unless they fail therapy with at least two triptans.”
Dr. Blumenfeld discounts the rationale behind requiring patients to try a second triptan after failing initial triptan therapy. “There are plenty of studies showing that if you fail one triptan, you’re likely to fail another,” he said. “Why would you put the patient on a different triptan when you could switch them to another drug with a different mechanism of action?”
The results showed that more patients in the ubrogepant 50-mg arm achieved pain freedom than those in the placebo arm within 2 hours after the initial dose in each group.
“Migraine is a very disabling condition, so you want to get the attack under control as quickly as possible while limiting the risk for potential side effects,” said Dr. Blumenfeld. “Ubrogepant is very efficacious and with very few adverse effects, but its use is limited because insurance companies require the failure of several triptans.”
One limitation of the study is that it is a subanalysis.
Dr. Blumenfeld has disclosed advisory board service, consulting, speaking, and authorship for AbbVie, Alder, Amgen, Biohaven, Lilly, Novartis, Teva, Theranica, and Zoscano.
according to a study published in Headache.
“The goal is to get migraine attacks under control as quickly as you can with as few adverse events as possible,” said lead author Andrew Blumenfeld, MD, director of the Headache Center of Southern California in Carlsbad, California. “Ubrogepant is very efficacious and well tolerated because it has few adverse events.”
Migraine disorder is the third most prevalent disease, and at least one person living in 25% of all U.S. households has the condition.
Clinicians have a wide range of medications at their disposal to treat migraines. These drug classes include triptans, ditans, NSAIDs, dihydroergotamine, and combination analgesics. Although numerous pharmacologic options are available to manage this patient population, an estimated 95% of patients who take oral medications to alleviate their migraine symptoms still fail to achieve relief with at least one acute episode.
Triptans remain a common option and first-line choice for acute migraine relief, but poor tolerability, among other factors, continue to limit their effectiveness. Moreover, their vasoconstrictive properties preclude their use in specific patient populations, such as those who have hypertension, peripheral vascular disease, and cerebral vascular accident. These circumstances, combined with other unmet clinical needs in migraine, have prompted researchers to explore new options, including a newer class of drugs – CGRP receptor antagonists. An endogenous protein, CGRP, has inflammatory and pronociceptive properties that play an active role in contributing to migraine pathogenesis.
Efficacy analyzed by triptan response
To investigate the effects of anti-CGRP treatment in patients with migraine who have a previous history of triptan use, researchers conducted two phase 3, randomized, double-blind, multicenter, single-attack trials, known as ACHIEVE I and ACHIEVE II.
Trial participants ranged in age from 18 to 75 years with a documented history of migraines with or without aura. In ACHIEVE I, investigators randomized 1,327 participants 1:1:1 to receive placebo, ubrogepant 50 mg, or ubrogepant 100 mg and placebo. Randomized patients in ACHIEVE II (n = 1,355) received placebo, ubrogepant 25 mg, or ubrogepant 50 mg to treat a single episode. During the screening process, researchers further placed patients in one of three groups based on their previous triptan use – triptan responder, triptan-insufficient responder, and triptan naive. Patients were further randomized based on their previous experience with triptans and whether they currently used them for migraine prevention. Patients participating in the study had up to 60 days to treat one qualifying migraine of moderate or severe nature at home.
The studies had two primary endpoints. The first was freedom from pain at the 2-hour mark following the initial dose, defined as decreased headache severity from moderate or severe at baseline to no pain. The other primary endpoint was the absence of most bothersome migraine-associated symptom (MBS) – photophobia, phonophobia, or nausea – 2 hours after the initial ubrogepant dose.
The pooled analysis collated data from 1,799 patients in both studies (placebo, n = 912; ubrogepant 50 mg, n = 887). Patients fell into the following categories: 682 triptan responders (placebo, n = 350; ubrogepant, n = 332); 451 triptan-insufficient responders (placebo, n = 223; ubrogepant, n = 228), and 666 triptan naive (placebo, n = 339; ubrogepant, n = 337).
Based on the data, approximately 25% of the patients enrolled in the study fell into the triptan-insufficient category. Of this subpopulation, about 80% of the patients in each treatment group experienced insufficient efficacy when using triptans. In each treatment group of insufficient responders, approximately 17% of patients cited tolerability issues, and 3% had contraindications that precluded them from triptan therapy.
The incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs did not differ appreciably across historical triptan experience subgroups. The highest percentage of participants experiencing a treatment-related TEAE in the pooled ubrogepant 50-mg treatment group was found in the triptan-insufficient responders (10.4%), whereas the highest percentage in the placebo group was found in the triptan-naive subgroup (9.7%). No serious AEs (SAEs) were reported in any subgroup.
The researchers concluded that “ubrogepant efficacy and tolerability did not differ for the acute treatment of migraine in participants classified as triptan responders, triptan-insufficient responders, and triptan naive based on their historical experience with triptans.”
Payers limit use
Despite the promising data, payer hurdles limit ubrogepant’s use, said Stewart Tepper, MD, who was asked to comment on the study. Dr. Tepper, a professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., was not involved in the study. “The study shows that ubrogepant will work as well as those who have not responded well to triptans, which is key,” Dr. Tepper said. “However, payers have set up step edits in which patients aren’t allowed to get ubrogepant unless they fail therapy with at least two triptans.”
Dr. Blumenfeld discounts the rationale behind requiring patients to try a second triptan after failing initial triptan therapy. “There are plenty of studies showing that if you fail one triptan, you’re likely to fail another,” he said. “Why would you put the patient on a different triptan when you could switch them to another drug with a different mechanism of action?”
The results showed that more patients in the ubrogepant 50-mg arm achieved pain freedom than those in the placebo arm within 2 hours after the initial dose in each group.
“Migraine is a very disabling condition, so you want to get the attack under control as quickly as possible while limiting the risk for potential side effects,” said Dr. Blumenfeld. “Ubrogepant is very efficacious and with very few adverse effects, but its use is limited because insurance companies require the failure of several triptans.”
One limitation of the study is that it is a subanalysis.
Dr. Blumenfeld has disclosed advisory board service, consulting, speaking, and authorship for AbbVie, Alder, Amgen, Biohaven, Lilly, Novartis, Teva, Theranica, and Zoscano.
FROM HEADACHE