User login
COVID-19 apps for the ObGyn health care provider: An update
More than one year after COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, the disease continues to persist, infecting more than 110 million individuals to date globally.1 As new information emerges about the coronavirus, the literature on diagnosis and management also has grown exponentially over the last year, including specific guidance for obstetric populations. With abundant information available to health care providers, COVID-19 mobile apps have the advantage of summarizing and presenting information in an organized and easily accessible manner.2
This updated review expands on a previous article by Bogaert and Chen at the start of the COVID-19 pandemic.3 Using the same methodology, in March 2021 we searched the Apple iTunes and Google Play stores using the term “COVID.” The search yielded 230 unique applications available for download. We excluded apps that were primarily developed as geographic area-specific case trackers or personal symptom trackers (193), those that provide telemedicine services (7), and nonmedical apps or ones published in a language other than English (20).
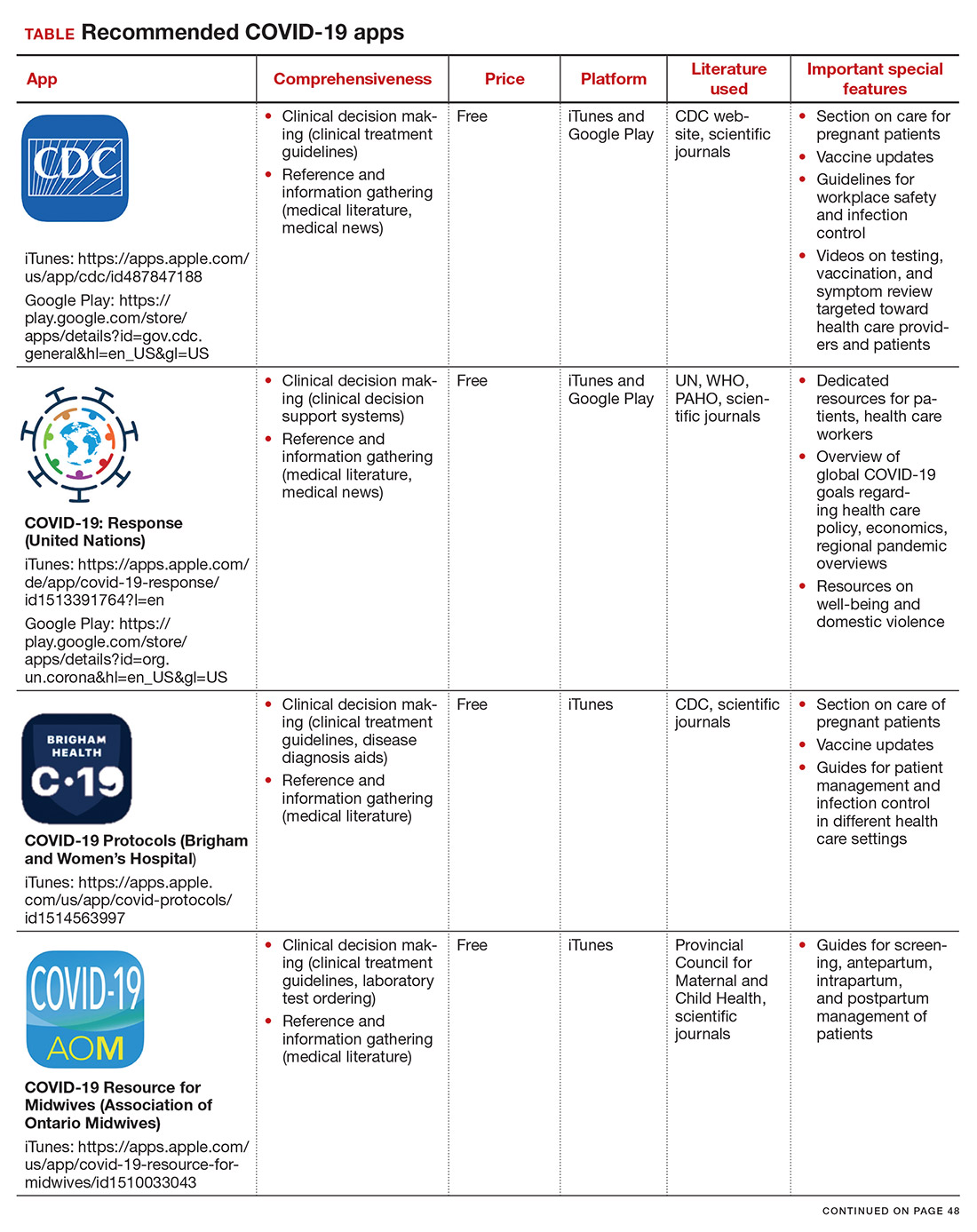
Here, we focus on the 3 mobile apps previously discussed (CDC, My Osler, and Relief Central) and 7 additional apps (TABLE). Most summarize information on the prevention, diagnosis, and treatment of coronavirus, and several also provide information on the COVID-19 vaccine. One app (COVID-19 Resource for Midwives) is specifically designed for obstetric providers, and 4 others (CDC, COVID-19 Protocols, Medscape, and WHO Academy) contain information on specific guidance for obstetric and gynecologic patient populations.
Each app was evaluated based on a condensed version of the APPLICATIONS scoring system, APPLI (comprehensiveness, price, platform, literature used, and special features).4
We hope that these mobile apps will assist the ObGyn health care provider in continuing to care for patients during this pandemic.
- World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed March 12, 2021.
2. Kondylakis H, Katehakis DG, Kouroubali A, et al. COVID-19 mobile apps: a systematic review of the literature. J Med Internet Res. 2020;22:e23170.
3. Bogaert K, Chen KT. COVID-19 apps for the ObGyn health care provider. OBG Manag. 2020; 32(5):44, 46.
4. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
More than one year after COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, the disease continues to persist, infecting more than 110 million individuals to date globally.1 As new information emerges about the coronavirus, the literature on diagnosis and management also has grown exponentially over the last year, including specific guidance for obstetric populations. With abundant information available to health care providers, COVID-19 mobile apps have the advantage of summarizing and presenting information in an organized and easily accessible manner.2
This updated review expands on a previous article by Bogaert and Chen at the start of the COVID-19 pandemic.3 Using the same methodology, in March 2021 we searched the Apple iTunes and Google Play stores using the term “COVID.” The search yielded 230 unique applications available for download. We excluded apps that were primarily developed as geographic area-specific case trackers or personal symptom trackers (193), those that provide telemedicine services (7), and nonmedical apps or ones published in a language other than English (20).
Here, we focus on the 3 mobile apps previously discussed (CDC, My Osler, and Relief Central) and 7 additional apps (TABLE). Most summarize information on the prevention, diagnosis, and treatment of coronavirus, and several also provide information on the COVID-19 vaccine. One app (COVID-19 Resource for Midwives) is specifically designed for obstetric providers, and 4 others (CDC, COVID-19 Protocols, Medscape, and WHO Academy) contain information on specific guidance for obstetric and gynecologic patient populations.
Each app was evaluated based on a condensed version of the APPLICATIONS scoring system, APPLI (comprehensiveness, price, platform, literature used, and special features).4
We hope that these mobile apps will assist the ObGyn health care provider in continuing to care for patients during this pandemic.



More than one year after COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, the disease continues to persist, infecting more than 110 million individuals to date globally.1 As new information emerges about the coronavirus, the literature on diagnosis and management also has grown exponentially over the last year, including specific guidance for obstetric populations. With abundant information available to health care providers, COVID-19 mobile apps have the advantage of summarizing and presenting information in an organized and easily accessible manner.2
This updated review expands on a previous article by Bogaert and Chen at the start of the COVID-19 pandemic.3 Using the same methodology, in March 2021 we searched the Apple iTunes and Google Play stores using the term “COVID.” The search yielded 230 unique applications available for download. We excluded apps that were primarily developed as geographic area-specific case trackers or personal symptom trackers (193), those that provide telemedicine services (7), and nonmedical apps or ones published in a language other than English (20).
Here, we focus on the 3 mobile apps previously discussed (CDC, My Osler, and Relief Central) and 7 additional apps (TABLE). Most summarize information on the prevention, diagnosis, and treatment of coronavirus, and several also provide information on the COVID-19 vaccine. One app (COVID-19 Resource for Midwives) is specifically designed for obstetric providers, and 4 others (CDC, COVID-19 Protocols, Medscape, and WHO Academy) contain information on specific guidance for obstetric and gynecologic patient populations.
Each app was evaluated based on a condensed version of the APPLICATIONS scoring system, APPLI (comprehensiveness, price, platform, literature used, and special features).4
We hope that these mobile apps will assist the ObGyn health care provider in continuing to care for patients during this pandemic.



- World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed March 12, 2021.
2. Kondylakis H, Katehakis DG, Kouroubali A, et al. COVID-19 mobile apps: a systematic review of the literature. J Med Internet Res. 2020;22:e23170.
3. Bogaert K, Chen KT. COVID-19 apps for the ObGyn health care provider. OBG Manag. 2020; 32(5):44, 46.
4. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
- World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed March 12, 2021.
2. Kondylakis H, Katehakis DG, Kouroubali A, et al. COVID-19 mobile apps: a systematic review of the literature. J Med Internet Res. 2020;22:e23170.
3. Bogaert K, Chen KT. COVID-19 apps for the ObGyn health care provider. OBG Manag. 2020; 32(5):44, 46.
4. Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
Study IDs most common lingering symptoms 8 months after mild COVID
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
High-Potency Topical Steroid Treatment of Multiple Keratoacanthomas Associated With Prurigo Nodularis
Practice Gap
Multiple keratoacanthomas (KAs) of the legs often are a challenge to treat, especially when these lesions appear within a field of prurigo nodules. Multiple KAs associated with prurigo nodularis is a rarer finding; more often, the condition is reported on the lower limbs of elderly women with actinically damaged skin.1,2 At times, it can be difficult to distinguish between KA and prurigo nodularis in these patients, who often report notable pruritus and might have associated eczematous dermatitis.2
Keratoacanthomas often are treated with aggressive modalities, such as Mohs micrographic surgery, excision, and electrodesiccation and curettage. Some patients are hesitant to undergo surgical treatment, however, preferring a less invasive approach. Trauma from these aggressive modalities also can be associated with recurrence of existing lesions or development of new KAs, possibly related to stimulation of a local inflammatory response and upregulation of helper T cells.2-4
Acitretin and other systemic retinoids often are considered first-line therapy for multiple KAs. Cyclosporine has been added as adjunctive treatment in cases associated with prurigo nodularis or eczematous dermatitis1,2; however, these treatments have a high rate of discontinuation because of adverse effects, including transaminitis, xerostomia, alopecia (acitretin), and renal toxicity (cyclosporine).2
Another treatment option for patients with coexisting KA and prurigo nodularis is intralesional corticosteroids, often administered in combination with systemic retinoids.3 Topical 5-fluorouracil (5-FU) has been used successfully for KA, but topical treatment options are limited if 5-FU fails. Topical imiquimod and cryotherapy are thought to be of little benefit, and the appearance of new KA within imiquimod and cryotherapy treatment fields has been reported.1,2 Topical corticosteroids have been used as an adjuvant therapy for multiple KAs associated with prurigo nodularis; however, a PubMed search of articles indexed for MEDLINE using the terms keratoacanthoma and steroid and keratoacanthoma and prurigo nodularis yielded no published reports of successful use of topical corticosteroids as monotherapy.2
The Technique
For patients who want to continue topical treatment of coexisting KA and prurigo nodularis after topical 5-FU fails, we have found success applying a high-potency topical corticosteroid to affected areas under occlusion nightly for 6 to 8 weeks. This treatment not only leads to resolution of KA but also simultaneously treats prurigo nodules that might be clinically difficult to distinguish from KA in some presentations. This regimen has been implemented in our practice with remarkable reduction of KA burden and relief of pruritus.
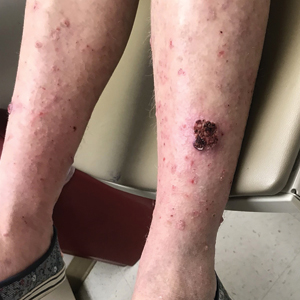
In a 68-year-old woman who was treated with this technique, multiple biopsies had shown KA (or well-differentiated squamous cell carcinoma that appeared clinically as KA) on the shin (Figure, A) arising amid many lesions consistent with prurigo nodules. Topical 5-FU had failed, but the patient did not want to be treated with a more invasive modality, such as excision or injection.
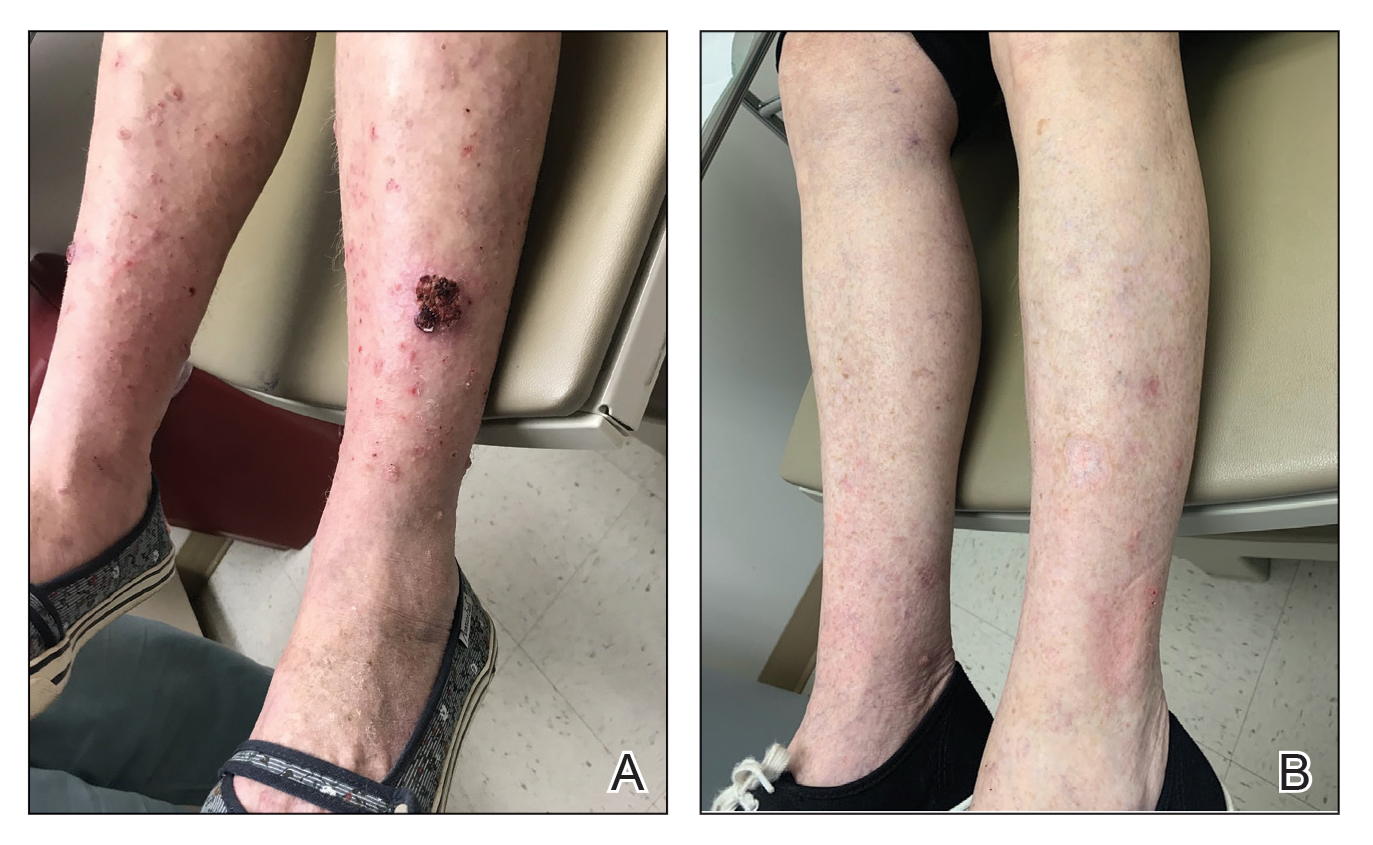
Instead, we treated the patient with clobetasol propionate ointment 0.05% under occlusion nightly for 6 weeks. This strategy produced resolution of both KA and prurigo nodules (Figure, B). When lesions recurred after a few months, they were successfully re-treated with topical clobetasol under occlusion in a second 6-week course.
Practical Implications
Treatment of multiple KAs associated with prurigo nodularis can present a distinct challenge. For the subset of patients who want to pursue topical treatment, options reported in the literature are limited. We have found success treating multiple KAs and associated prurigo nodules with a high-potency topical corticosteroid under occlusion, with minimal or no adverse effects. We believe that a topical corticosteroid can be implemented easily in clinical practice before a more invasive surgical or intralesional modality is considered.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Wu TP, Miller K, Cohen DE, et al. Keratoacanthomas arising in association with prurigo nodules in pruritic, actinically damaged skin. J Am Acad Dermatol. 2013;69:426-430. doi:10.1016/J.JAAD.2013.03.035
- Sanders S, Busam KJ, Halpern AC, et al. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958. doi:10.1046/j.1524-4725.2002.02069.x
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:E117-E119. doi:10.1111/ajd.12501
Practice Gap
Multiple keratoacanthomas (KAs) of the legs often are a challenge to treat, especially when these lesions appear within a field of prurigo nodules. Multiple KAs associated with prurigo nodularis is a rarer finding; more often, the condition is reported on the lower limbs of elderly women with actinically damaged skin.1,2 At times, it can be difficult to distinguish between KA and prurigo nodularis in these patients, who often report notable pruritus and might have associated eczematous dermatitis.2
Keratoacanthomas often are treated with aggressive modalities, such as Mohs micrographic surgery, excision, and electrodesiccation and curettage. Some patients are hesitant to undergo surgical treatment, however, preferring a less invasive approach. Trauma from these aggressive modalities also can be associated with recurrence of existing lesions or development of new KAs, possibly related to stimulation of a local inflammatory response and upregulation of helper T cells.2-4
Acitretin and other systemic retinoids often are considered first-line therapy for multiple KAs. Cyclosporine has been added as adjunctive treatment in cases associated with prurigo nodularis or eczematous dermatitis1,2; however, these treatments have a high rate of discontinuation because of adverse effects, including transaminitis, xerostomia, alopecia (acitretin), and renal toxicity (cyclosporine).2
Another treatment option for patients with coexisting KA and prurigo nodularis is intralesional corticosteroids, often administered in combination with systemic retinoids.3 Topical 5-fluorouracil (5-FU) has been used successfully for KA, but topical treatment options are limited if 5-FU fails. Topical imiquimod and cryotherapy are thought to be of little benefit, and the appearance of new KA within imiquimod and cryotherapy treatment fields has been reported.1,2 Topical corticosteroids have been used as an adjuvant therapy for multiple KAs associated with prurigo nodularis; however, a PubMed search of articles indexed for MEDLINE using the terms keratoacanthoma and steroid and keratoacanthoma and prurigo nodularis yielded no published reports of successful use of topical corticosteroids as monotherapy.2
The Technique
For patients who want to continue topical treatment of coexisting KA and prurigo nodularis after topical 5-FU fails, we have found success applying a high-potency topical corticosteroid to affected areas under occlusion nightly for 6 to 8 weeks. This treatment not only leads to resolution of KA but also simultaneously treats prurigo nodules that might be clinically difficult to distinguish from KA in some presentations. This regimen has been implemented in our practice with remarkable reduction of KA burden and relief of pruritus.
In a 68-year-old woman who was treated with this technique, multiple biopsies had shown KA (or well-differentiated squamous cell carcinoma that appeared clinically as KA) on the shin (Figure, A) arising amid many lesions consistent with prurigo nodules. Topical 5-FU had failed, but the patient did not want to be treated with a more invasive modality, such as excision or injection.

Instead, we treated the patient with clobetasol propionate ointment 0.05% under occlusion nightly for 6 weeks. This strategy produced resolution of both KA and prurigo nodules (Figure, B). When lesions recurred after a few months, they were successfully re-treated with topical clobetasol under occlusion in a second 6-week course.
Practical Implications
Treatment of multiple KAs associated with prurigo nodularis can present a distinct challenge. For the subset of patients who want to pursue topical treatment, options reported in the literature are limited. We have found success treating multiple KAs and associated prurigo nodules with a high-potency topical corticosteroid under occlusion, with minimal or no adverse effects. We believe that a topical corticosteroid can be implemented easily in clinical practice before a more invasive surgical or intralesional modality is considered.
Practice Gap
Multiple keratoacanthomas (KAs) of the legs often are a challenge to treat, especially when these lesions appear within a field of prurigo nodules. Multiple KAs associated with prurigo nodularis is a rarer finding; more often, the condition is reported on the lower limbs of elderly women with actinically damaged skin.1,2 At times, it can be difficult to distinguish between KA and prurigo nodularis in these patients, who often report notable pruritus and might have associated eczematous dermatitis.2
Keratoacanthomas often are treated with aggressive modalities, such as Mohs micrographic surgery, excision, and electrodesiccation and curettage. Some patients are hesitant to undergo surgical treatment, however, preferring a less invasive approach. Trauma from these aggressive modalities also can be associated with recurrence of existing lesions or development of new KAs, possibly related to stimulation of a local inflammatory response and upregulation of helper T cells.2-4
Acitretin and other systemic retinoids often are considered first-line therapy for multiple KAs. Cyclosporine has been added as adjunctive treatment in cases associated with prurigo nodularis or eczematous dermatitis1,2; however, these treatments have a high rate of discontinuation because of adverse effects, including transaminitis, xerostomia, alopecia (acitretin), and renal toxicity (cyclosporine).2
Another treatment option for patients with coexisting KA and prurigo nodularis is intralesional corticosteroids, often administered in combination with systemic retinoids.3 Topical 5-fluorouracil (5-FU) has been used successfully for KA, but topical treatment options are limited if 5-FU fails. Topical imiquimod and cryotherapy are thought to be of little benefit, and the appearance of new KA within imiquimod and cryotherapy treatment fields has been reported.1,2 Topical corticosteroids have been used as an adjuvant therapy for multiple KAs associated with prurigo nodularis; however, a PubMed search of articles indexed for MEDLINE using the terms keratoacanthoma and steroid and keratoacanthoma and prurigo nodularis yielded no published reports of successful use of topical corticosteroids as monotherapy.2
The Technique
For patients who want to continue topical treatment of coexisting KA and prurigo nodularis after topical 5-FU fails, we have found success applying a high-potency topical corticosteroid to affected areas under occlusion nightly for 6 to 8 weeks. This treatment not only leads to resolution of KA but also simultaneously treats prurigo nodules that might be clinically difficult to distinguish from KA in some presentations. This regimen has been implemented in our practice with remarkable reduction of KA burden and relief of pruritus.
In a 68-year-old woman who was treated with this technique, multiple biopsies had shown KA (or well-differentiated squamous cell carcinoma that appeared clinically as KA) on the shin (Figure, A) arising amid many lesions consistent with prurigo nodules. Topical 5-FU had failed, but the patient did not want to be treated with a more invasive modality, such as excision or injection.

Instead, we treated the patient with clobetasol propionate ointment 0.05% under occlusion nightly for 6 weeks. This strategy produced resolution of both KA and prurigo nodules (Figure, B). When lesions recurred after a few months, they were successfully re-treated with topical clobetasol under occlusion in a second 6-week course.
Practical Implications
Treatment of multiple KAs associated with prurigo nodularis can present a distinct challenge. For the subset of patients who want to pursue topical treatment, options reported in the literature are limited. We have found success treating multiple KAs and associated prurigo nodules with a high-potency topical corticosteroid under occlusion, with minimal or no adverse effects. We believe that a topical corticosteroid can be implemented easily in clinical practice before a more invasive surgical or intralesional modality is considered.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Wu TP, Miller K, Cohen DE, et al. Keratoacanthomas arising in association with prurigo nodules in pruritic, actinically damaged skin. J Am Acad Dermatol. 2013;69:426-430. doi:10.1016/J.JAAD.2013.03.035
- Sanders S, Busam KJ, Halpern AC, et al. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958. doi:10.1046/j.1524-4725.2002.02069.x
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:E117-E119. doi:10.1111/ajd.12501
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Wu TP, Miller K, Cohen DE, et al. Keratoacanthomas arising in association with prurigo nodules in pruritic, actinically damaged skin. J Am Acad Dermatol. 2013;69:426-430. doi:10.1016/J.JAAD.2013.03.035
- Sanders S, Busam KJ, Halpern AC, et al. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatol Surg. 2002;28:954-958. doi:10.1046/j.1524-4725.2002.02069.x
- Lee S, Coutts I, Ryan A, et al. Keratoacanthoma formation after skin grafting: a brief report and pathophysiological hypothesis. Australas J Dermatol. 2017;58:E117-E119. doi:10.1111/ajd.12501
Gynecologic and Obstetric Implications of Darier Disease: A Dermatologist’s Perspective
Darier disease (DD)(also known as dyskeratosis follicularis) is a rare, autosomal-dominant genodermatosis characterized by greasy, rough, keratotic papules; typical nail abnormalities; mucosal changes; and characteristic dyskeratotic acantholysis that is called corps ronds and grains on histopathologic analysis. Darier disease is caused by mutations of the ATP2A2 gene on chromosome 12q23-24.1,2
Because of the autosomal-dominant pattern of inheritance in DD, if either parent is affected by DD, approximately 50% of their offspring will have the disorder. Therefore, couples need to be offered genetic counseling at a preconception visit or early in pregnancy. Although penetrance of DD is complete, spontaneous mutations are frequent and expressivity is variable1; prenatal diagnosis, though available since the 1980s, is therefore unreliable in DD, given the considerable variation in phenotypic expressivity. Differing phenotypes underscore the importance of proper counseling by the treating dermatologist or other provider. Females with a mild or nearly undetectable phenotype can give birth to a child with severe disease.
Lack of clear understanding about the variable phenotypic expressivity of DD can cause considerable anger, anxiety, guilt, psychological trauma, and fear in parents, should their child later develop a severe phenotype. They may feel that they were not properly prepared for the outcome. The physician-parent or physician-patient relationship can be negatively impacted if ongoing counseling is inadequate.
Clinically, DD presents in early adolescence (age range, 6–20 years) in most patients, which means that the disease and female reproductive years are contemporaneous. However, gynecologic and obstetric issues and complications of DD rarely have been addressed.3 Oromucosal involvement in DD is reported in 13% to 50% of cases, yet vaginal and cervical mucosal involvement rarely has been described,4,5 likely due to underreporting. Therefore, in this rare disease, it is important to address these aspects so that the patients are provided with appropriate management options.
Implications for Cervical Screening and Papanicolaou Tests
Cytopathologic findings of a Papanicolaou test taken from a patient with DD can lead to erroneous diagnosis of a low-grade squamous intraepithelial lesion due to cervical involvement by the disease process; therefore, correct interpretation of a smear may be inappropriate and erroneous. The cytopathologist needs to be informed of the patient’s diagnosis of DD in advance for appropriate reporting.5,6
Obstetric Implications
Fertility is normal in DD patients, and pregnancy usually has a normal course; however, exacerbation and remission of disease have been reported. de la Rosa Carrillo7 reported a case of vegetating DD during pregnancy. He described it as an exacerbation with concurrent bacterial infection and bilateral external otitis.7 Spouge et al8 reported a case of a 58-year-old woman who was the mother of 4 DD patients. She experienced an exacerbation of DD during all 6 pregnancies but improved immediately postpartum.8 Espy et al9 evaluated 8 cases of women with DD and described spontaneous improvement of the disorder during pregnancy (1 case) or while taking an oral contraceptive (3 cases).
Prenatal Counseling
Women with DD should be encouraged to talk to their dermatologist, obstetrician, or other provider of prenatal care regarding plans for pregnancy, labor, and delivery, as these events might be affected by the disorder. During pregnancy, careful monitoring and self-care remain essential. Simple measures to reduce the impact of irritants on DD during pregnancy include keeping the skin cool, using a soothing moisturizer, applying photoprotection, and using sunscreen. Treatment with systemic retinoids must be avoided if pregnancy is planned.
Warty plaques and papules of DD can involve flexures (groin, vulva, and perineum), with resultant malodor and pruritus10 as well as the potential for (drug resistant) secondary infection (eg, Staphylococcus aureus, group B Streptococcus, viruses [eg, Kaposi varicelliform eruption]). Skin swabs should be taken for culture and susceptibility testing, and infection should be treated at the earliest sign.
Management Concerns During Pregnancy and Delivery
Because the benefits of treating DD might outweigh risk in certain cases, thorough discussion with the patient about options is recommended, including the following concerns:
• Because mucocutaneous elasticity of the birth canal, including the vulva, perineum, and groin, is essential for nontraumatic vaginal delivery, it might be necessary to schedule an elective cesarean delivery in DD patients in whom these regions are involved.11
• In females with lower abdominal lesions, using a Pfannenstiel-Kerr incision for cesarean delivery might be problematic.11
• A single case report has described successful anesthetic management of labor, delivery, and postpartum care in a DD patient.12 Involvement of the skin of the back might preclude safe administration of regional anesthesia; however, because DD lesions are considered noninfectious, the authors operatively administered a subarachnoid block at the L3-L4 interspace through a lesion-free area. Postpartum, the patient was observed in the intensive care unit. She and the baby remained stable; she did not develop infectious complications, including a central nervous system infection.12
•Mucosal involvement is relatively rare in DD and has not been reported to compromise airway management.8
Postnatal Considerations
Breastfeeding might have to be stopped early or withheld altogether if there is widespread involvement of the skin of the breast or the nipple.11 Darier disease has been associated with neuropsychiatric manifestations, including major depression (30%), suicide attempts (13%), suicidal thoughts (31%), cyclothymia, bipolar disorder (4%), and epilepsy (3%).13,14 Therefore, patients should be screened for postpartum psychiatric manifestations at an early follow-up visit.
Final Thoughts
Although the etiology of DD is well known, the gynelogic and obstretric implications of this genodermatosis have rarely been described. This brief commentary is an attempt to provide the important information to a practicing dermatologist for appropriate management of female DD patients.
- Bale SJ, Toro JR. Genetic basis of Darier-White disease: bad pumps cause bumps. J Cutan Med Surg. 2000;4:103-106. doi:10.1177/120347540000400212
- Kansal NK, Hazarika N, Rao S. Familial case of Darier disease with guttate leukoderma: a case series from India. Indian Dermatol Online J. 2018;9:62-63. doi:10.4103/idoj.IDOJ_52_17
- Lynch PJ. Vulvar dermatoses: the eczematous diseases. In: Black M, Ambros-Rudolph CM, Edwards L, Lynch P, eds. Obstetric and Gynecologic Dermatology. 3rd ed. Mosby-Elsevier; 2008:192-194.
- Adam AE. Ectopic Darier’s disease of the cervix: an extraordinary cause of an abnormal smear. Cytopathology. 1996;7:414-421. doi:10.1111/j.1365-2303.1996.tb00547.x
- Suárez-Peñaranda JM, Antúnez JR, Del Rio E, et al. Vaginal involvement in a woman with Darier’s disease: a case report. Acta Cytol. 2005;49:530-532. doi:10.1159/000326200
- Boon ME. Dr. Darier’s lesson: it can be advantageous to the patient to ignore evident cytonuclear changes. Acta Cytol. 2005;49:469-470. doi:10.1159/000326189
- de la Rosa Carrillo D. Vegetating Darier’s disease during pregnancy. Acta Derm Venereol. 2006;86:259-260. doi:10.2340/00015555-0066
- Spouge JD, Trott JR, Chesko G. Darier-White’s disease: a cause of white lesions of the mucosa. report of four cases. Oral Surg Oral Med Oral Pathol. 1966;21:441-457. doi:10.1016/0030-4220(66)90401-4
- Espy PD, Stone S, Jolly HW Jr. Hormonal dependency in Darier disease. Cutis. 1976;17:315-320.
- De D, Kanwar AJ, Saikia UN. Uncommon flexural presentation of Darier disease. J Cutan Med Surg. 2008;12:249-252. doi:10.2310/7750.2008.07035
- Quinlivan JA, O'Halloran LC. Darier’s disease and pregnancy. Dermatol Aspects. 2013;1:1-3. doi:10.7243/2053-5309-1-1
- Sharma R, Singh BP, Das SN. Anesthetic management of cesarean section in a parturient with Darier’s disease. Acta Anaesthesiol Taiwan. 2010;48:158-159. doi:10.1016/S1875-4597(10)60051-3
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Dodiuk-Gad RP, Cohen-Barak E, Khayat M, et al. Darier disease in Israel: combined evaluation of genetic and neuropsychiatric aspects. Br J Dermatol. 2016;174:562-568. doi:10.1111/bjd.14220
Darier disease (DD)(also known as dyskeratosis follicularis) is a rare, autosomal-dominant genodermatosis characterized by greasy, rough, keratotic papules; typical nail abnormalities; mucosal changes; and characteristic dyskeratotic acantholysis that is called corps ronds and grains on histopathologic analysis. Darier disease is caused by mutations of the ATP2A2 gene on chromosome 12q23-24.1,2
Because of the autosomal-dominant pattern of inheritance in DD, if either parent is affected by DD, approximately 50% of their offspring will have the disorder. Therefore, couples need to be offered genetic counseling at a preconception visit or early in pregnancy. Although penetrance of DD is complete, spontaneous mutations are frequent and expressivity is variable1; prenatal diagnosis, though available since the 1980s, is therefore unreliable in DD, given the considerable variation in phenotypic expressivity. Differing phenotypes underscore the importance of proper counseling by the treating dermatologist or other provider. Females with a mild or nearly undetectable phenotype can give birth to a child with severe disease.
Lack of clear understanding about the variable phenotypic expressivity of DD can cause considerable anger, anxiety, guilt, psychological trauma, and fear in parents, should their child later develop a severe phenotype. They may feel that they were not properly prepared for the outcome. The physician-parent or physician-patient relationship can be negatively impacted if ongoing counseling is inadequate.
Clinically, DD presents in early adolescence (age range, 6–20 years) in most patients, which means that the disease and female reproductive years are contemporaneous. However, gynecologic and obstetric issues and complications of DD rarely have been addressed.3 Oromucosal involvement in DD is reported in 13% to 50% of cases, yet vaginal and cervical mucosal involvement rarely has been described,4,5 likely due to underreporting. Therefore, in this rare disease, it is important to address these aspects so that the patients are provided with appropriate management options.
Implications for Cervical Screening and Papanicolaou Tests
Cytopathologic findings of a Papanicolaou test taken from a patient with DD can lead to erroneous diagnosis of a low-grade squamous intraepithelial lesion due to cervical involvement by the disease process; therefore, correct interpretation of a smear may be inappropriate and erroneous. The cytopathologist needs to be informed of the patient’s diagnosis of DD in advance for appropriate reporting.5,6
Obstetric Implications
Fertility is normal in DD patients, and pregnancy usually has a normal course; however, exacerbation and remission of disease have been reported. de la Rosa Carrillo7 reported a case of vegetating DD during pregnancy. He described it as an exacerbation with concurrent bacterial infection and bilateral external otitis.7 Spouge et al8 reported a case of a 58-year-old woman who was the mother of 4 DD patients. She experienced an exacerbation of DD during all 6 pregnancies but improved immediately postpartum.8 Espy et al9 evaluated 8 cases of women with DD and described spontaneous improvement of the disorder during pregnancy (1 case) or while taking an oral contraceptive (3 cases).
Prenatal Counseling
Women with DD should be encouraged to talk to their dermatologist, obstetrician, or other provider of prenatal care regarding plans for pregnancy, labor, and delivery, as these events might be affected by the disorder. During pregnancy, careful monitoring and self-care remain essential. Simple measures to reduce the impact of irritants on DD during pregnancy include keeping the skin cool, using a soothing moisturizer, applying photoprotection, and using sunscreen. Treatment with systemic retinoids must be avoided if pregnancy is planned.
Warty plaques and papules of DD can involve flexures (groin, vulva, and perineum), with resultant malodor and pruritus10 as well as the potential for (drug resistant) secondary infection (eg, Staphylococcus aureus, group B Streptococcus, viruses [eg, Kaposi varicelliform eruption]). Skin swabs should be taken for culture and susceptibility testing, and infection should be treated at the earliest sign.
Management Concerns During Pregnancy and Delivery
Because the benefits of treating DD might outweigh risk in certain cases, thorough discussion with the patient about options is recommended, including the following concerns:
• Because mucocutaneous elasticity of the birth canal, including the vulva, perineum, and groin, is essential for nontraumatic vaginal delivery, it might be necessary to schedule an elective cesarean delivery in DD patients in whom these regions are involved.11
• In females with lower abdominal lesions, using a Pfannenstiel-Kerr incision for cesarean delivery might be problematic.11
• A single case report has described successful anesthetic management of labor, delivery, and postpartum care in a DD patient.12 Involvement of the skin of the back might preclude safe administration of regional anesthesia; however, because DD lesions are considered noninfectious, the authors operatively administered a subarachnoid block at the L3-L4 interspace through a lesion-free area. Postpartum, the patient was observed in the intensive care unit. She and the baby remained stable; she did not develop infectious complications, including a central nervous system infection.12
•Mucosal involvement is relatively rare in DD and has not been reported to compromise airway management.8
Postnatal Considerations
Breastfeeding might have to be stopped early or withheld altogether if there is widespread involvement of the skin of the breast or the nipple.11 Darier disease has been associated with neuropsychiatric manifestations, including major depression (30%), suicide attempts (13%), suicidal thoughts (31%), cyclothymia, bipolar disorder (4%), and epilepsy (3%).13,14 Therefore, patients should be screened for postpartum psychiatric manifestations at an early follow-up visit.
Final Thoughts
Although the etiology of DD is well known, the gynelogic and obstretric implications of this genodermatosis have rarely been described. This brief commentary is an attempt to provide the important information to a practicing dermatologist for appropriate management of female DD patients.
Darier disease (DD)(also known as dyskeratosis follicularis) is a rare, autosomal-dominant genodermatosis characterized by greasy, rough, keratotic papules; typical nail abnormalities; mucosal changes; and characteristic dyskeratotic acantholysis that is called corps ronds and grains on histopathologic analysis. Darier disease is caused by mutations of the ATP2A2 gene on chromosome 12q23-24.1,2
Because of the autosomal-dominant pattern of inheritance in DD, if either parent is affected by DD, approximately 50% of their offspring will have the disorder. Therefore, couples need to be offered genetic counseling at a preconception visit or early in pregnancy. Although penetrance of DD is complete, spontaneous mutations are frequent and expressivity is variable1; prenatal diagnosis, though available since the 1980s, is therefore unreliable in DD, given the considerable variation in phenotypic expressivity. Differing phenotypes underscore the importance of proper counseling by the treating dermatologist or other provider. Females with a mild or nearly undetectable phenotype can give birth to a child with severe disease.
Lack of clear understanding about the variable phenotypic expressivity of DD can cause considerable anger, anxiety, guilt, psychological trauma, and fear in parents, should their child later develop a severe phenotype. They may feel that they were not properly prepared for the outcome. The physician-parent or physician-patient relationship can be negatively impacted if ongoing counseling is inadequate.
Clinically, DD presents in early adolescence (age range, 6–20 years) in most patients, which means that the disease and female reproductive years are contemporaneous. However, gynecologic and obstetric issues and complications of DD rarely have been addressed.3 Oromucosal involvement in DD is reported in 13% to 50% of cases, yet vaginal and cervical mucosal involvement rarely has been described,4,5 likely due to underreporting. Therefore, in this rare disease, it is important to address these aspects so that the patients are provided with appropriate management options.
Implications for Cervical Screening and Papanicolaou Tests
Cytopathologic findings of a Papanicolaou test taken from a patient with DD can lead to erroneous diagnosis of a low-grade squamous intraepithelial lesion due to cervical involvement by the disease process; therefore, correct interpretation of a smear may be inappropriate and erroneous. The cytopathologist needs to be informed of the patient’s diagnosis of DD in advance for appropriate reporting.5,6
Obstetric Implications
Fertility is normal in DD patients, and pregnancy usually has a normal course; however, exacerbation and remission of disease have been reported. de la Rosa Carrillo7 reported a case of vegetating DD during pregnancy. He described it as an exacerbation with concurrent bacterial infection and bilateral external otitis.7 Spouge et al8 reported a case of a 58-year-old woman who was the mother of 4 DD patients. She experienced an exacerbation of DD during all 6 pregnancies but improved immediately postpartum.8 Espy et al9 evaluated 8 cases of women with DD and described spontaneous improvement of the disorder during pregnancy (1 case) or while taking an oral contraceptive (3 cases).
Prenatal Counseling
Women with DD should be encouraged to talk to their dermatologist, obstetrician, or other provider of prenatal care regarding plans for pregnancy, labor, and delivery, as these events might be affected by the disorder. During pregnancy, careful monitoring and self-care remain essential. Simple measures to reduce the impact of irritants on DD during pregnancy include keeping the skin cool, using a soothing moisturizer, applying photoprotection, and using sunscreen. Treatment with systemic retinoids must be avoided if pregnancy is planned.
Warty plaques and papules of DD can involve flexures (groin, vulva, and perineum), with resultant malodor and pruritus10 as well as the potential for (drug resistant) secondary infection (eg, Staphylococcus aureus, group B Streptococcus, viruses [eg, Kaposi varicelliform eruption]). Skin swabs should be taken for culture and susceptibility testing, and infection should be treated at the earliest sign.
Management Concerns During Pregnancy and Delivery
Because the benefits of treating DD might outweigh risk in certain cases, thorough discussion with the patient about options is recommended, including the following concerns:
• Because mucocutaneous elasticity of the birth canal, including the vulva, perineum, and groin, is essential for nontraumatic vaginal delivery, it might be necessary to schedule an elective cesarean delivery in DD patients in whom these regions are involved.11
• In females with lower abdominal lesions, using a Pfannenstiel-Kerr incision for cesarean delivery might be problematic.11
• A single case report has described successful anesthetic management of labor, delivery, and postpartum care in a DD patient.12 Involvement of the skin of the back might preclude safe administration of regional anesthesia; however, because DD lesions are considered noninfectious, the authors operatively administered a subarachnoid block at the L3-L4 interspace through a lesion-free area. Postpartum, the patient was observed in the intensive care unit. She and the baby remained stable; she did not develop infectious complications, including a central nervous system infection.12
•Mucosal involvement is relatively rare in DD and has not been reported to compromise airway management.8
Postnatal Considerations
Breastfeeding might have to be stopped early or withheld altogether if there is widespread involvement of the skin of the breast or the nipple.11 Darier disease has been associated with neuropsychiatric manifestations, including major depression (30%), suicide attempts (13%), suicidal thoughts (31%), cyclothymia, bipolar disorder (4%), and epilepsy (3%).13,14 Therefore, patients should be screened for postpartum psychiatric manifestations at an early follow-up visit.
Final Thoughts
Although the etiology of DD is well known, the gynelogic and obstretric implications of this genodermatosis have rarely been described. This brief commentary is an attempt to provide the important information to a practicing dermatologist for appropriate management of female DD patients.
- Bale SJ, Toro JR. Genetic basis of Darier-White disease: bad pumps cause bumps. J Cutan Med Surg. 2000;4:103-106. doi:10.1177/120347540000400212
- Kansal NK, Hazarika N, Rao S. Familial case of Darier disease with guttate leukoderma: a case series from India. Indian Dermatol Online J. 2018;9:62-63. doi:10.4103/idoj.IDOJ_52_17
- Lynch PJ. Vulvar dermatoses: the eczematous diseases. In: Black M, Ambros-Rudolph CM, Edwards L, Lynch P, eds. Obstetric and Gynecologic Dermatology. 3rd ed. Mosby-Elsevier; 2008:192-194.
- Adam AE. Ectopic Darier’s disease of the cervix: an extraordinary cause of an abnormal smear. Cytopathology. 1996;7:414-421. doi:10.1111/j.1365-2303.1996.tb00547.x
- Suárez-Peñaranda JM, Antúnez JR, Del Rio E, et al. Vaginal involvement in a woman with Darier’s disease: a case report. Acta Cytol. 2005;49:530-532. doi:10.1159/000326200
- Boon ME. Dr. Darier’s lesson: it can be advantageous to the patient to ignore evident cytonuclear changes. Acta Cytol. 2005;49:469-470. doi:10.1159/000326189
- de la Rosa Carrillo D. Vegetating Darier’s disease during pregnancy. Acta Derm Venereol. 2006;86:259-260. doi:10.2340/00015555-0066
- Spouge JD, Trott JR, Chesko G. Darier-White’s disease: a cause of white lesions of the mucosa. report of four cases. Oral Surg Oral Med Oral Pathol. 1966;21:441-457. doi:10.1016/0030-4220(66)90401-4
- Espy PD, Stone S, Jolly HW Jr. Hormonal dependency in Darier disease. Cutis. 1976;17:315-320.
- De D, Kanwar AJ, Saikia UN. Uncommon flexural presentation of Darier disease. J Cutan Med Surg. 2008;12:249-252. doi:10.2310/7750.2008.07035
- Quinlivan JA, O'Halloran LC. Darier’s disease and pregnancy. Dermatol Aspects. 2013;1:1-3. doi:10.7243/2053-5309-1-1
- Sharma R, Singh BP, Das SN. Anesthetic management of cesarean section in a parturient with Darier’s disease. Acta Anaesthesiol Taiwan. 2010;48:158-159. doi:10.1016/S1875-4597(10)60051-3
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Dodiuk-Gad RP, Cohen-Barak E, Khayat M, et al. Darier disease in Israel: combined evaluation of genetic and neuropsychiatric aspects. Br J Dermatol. 2016;174:562-568. doi:10.1111/bjd.14220
- Bale SJ, Toro JR. Genetic basis of Darier-White disease: bad pumps cause bumps. J Cutan Med Surg. 2000;4:103-106. doi:10.1177/120347540000400212
- Kansal NK, Hazarika N, Rao S. Familial case of Darier disease with guttate leukoderma: a case series from India. Indian Dermatol Online J. 2018;9:62-63. doi:10.4103/idoj.IDOJ_52_17
- Lynch PJ. Vulvar dermatoses: the eczematous diseases. In: Black M, Ambros-Rudolph CM, Edwards L, Lynch P, eds. Obstetric and Gynecologic Dermatology. 3rd ed. Mosby-Elsevier; 2008:192-194.
- Adam AE. Ectopic Darier’s disease of the cervix: an extraordinary cause of an abnormal smear. Cytopathology. 1996;7:414-421. doi:10.1111/j.1365-2303.1996.tb00547.x
- Suárez-Peñaranda JM, Antúnez JR, Del Rio E, et al. Vaginal involvement in a woman with Darier’s disease: a case report. Acta Cytol. 2005;49:530-532. doi:10.1159/000326200
- Boon ME. Dr. Darier’s lesson: it can be advantageous to the patient to ignore evident cytonuclear changes. Acta Cytol. 2005;49:469-470. doi:10.1159/000326189
- de la Rosa Carrillo D. Vegetating Darier’s disease during pregnancy. Acta Derm Venereol. 2006;86:259-260. doi:10.2340/00015555-0066
- Spouge JD, Trott JR, Chesko G. Darier-White’s disease: a cause of white lesions of the mucosa. report of four cases. Oral Surg Oral Med Oral Pathol. 1966;21:441-457. doi:10.1016/0030-4220(66)90401-4
- Espy PD, Stone S, Jolly HW Jr. Hormonal dependency in Darier disease. Cutis. 1976;17:315-320.
- De D, Kanwar AJ, Saikia UN. Uncommon flexural presentation of Darier disease. J Cutan Med Surg. 2008;12:249-252. doi:10.2310/7750.2008.07035
- Quinlivan JA, O'Halloran LC. Darier’s disease and pregnancy. Dermatol Aspects. 2013;1:1-3. doi:10.7243/2053-5309-1-1
- Sharma R, Singh BP, Das SN. Anesthetic management of cesarean section in a parturient with Darier’s disease. Acta Anaesthesiol Taiwan. 2010;48:158-159. doi:10.1016/S1875-4597(10)60051-3
- Gordon-Smith K, Jones LA, Burge SM, et al. The neuropsychiatric phenotype in Darier disease. Br J Dermatol. 2010;163:515-522. doi:10.1111/j.1365-2133.2010.09834.x
- Dodiuk-Gad RP, Cohen-Barak E, Khayat M, et al. Darier disease in Israel: combined evaluation of genetic and neuropsychiatric aspects. Br J Dermatol. 2016;174:562-568. doi:10.1111/bjd.14220
Practice Points
- Because Darier disease (DD) manifests during reproductive years, systemic retinoids should be used carefully in female patients.
- For a Papanicolaou test to be properly interpreted in a patient with DD, the cytopathologist must be informed of the DD diagnosis.
- Darier disease may be exacerbated or relieved during pregnancy.
A thoughtful approach to drug screening and addiction
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
Botanical Briefs: Phytophotodermatitis Is an Occupational and Recreational Dermatosis in the Limelight
Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of
Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.

- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of

Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.

Phytophotodermatitis (PPD) is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.1-3 It sometimes is known by colorful names such as margarita photodermatitis, in which a slice of lime in a refreshing summer drink may be etiologic,4,5 or berloque dermatitis, caused by exposure to perfumes containing bergapten (5-methoxypsoralen).6,7 Phytophotodermatitis may develop when phototoxic agents such as furocoumarins, which protect plants from fungal pathogens, and psoralens are applied to the skin followed by exposure to UV light, more specifically in the UVA range of 320 to 400 nm. Thus, these chemicals produce a phototoxic rather than photoallergic reaction, leading to cellular damage. Furocoumarins and psoralens often are found in plants such as celery and figs as well as in citrus fruits such as limes, lemons, and grapefruits. Exposure may be cryptic, as the patient may not consider or mention the eruption as possibly caused by activities such as soaking one’s feet in a folk remedy containing fig leaves.7,8 Once these phototoxic agents come in contact with the skin, the symptoms of PPD may arise within 24 hours of exposure, beginning as an acute dermatitis with erythema, edema, vesicles, or bullae accompanied by pain and itching.
Etiology
Phytophotodermatitis is caused by exposure to several different types of plants, including Ficus carica (common fig), the genus Citrus (eg, lime, lemon), or Pastina sativa (wild parsnip). Each of these contain furocoumarins and psoralens—phototoxic agents that cause cellular damage with epidermal necrosis and resultant pain when the skin is exposed to UVA light.1-4 There are 2 types of photochemical reactions in PPD: type I reactions occur in the absence of oxygen, whereas oxygen is present in type II reactions. Both damage cell membranes and DNA, which then results in DNA interstrand cross-linking between the psoralen furan ring and the thymine or cytosine of DNA, activating arachidonic acid metabolic pathways to produce cell death.1
Epidemiology
The incidence of PPD is unknown due to the high variability of reactions in individuals spanning from children to the elderly. It can be caused by many different wild and domestic plants in many areas of the world and can affect any individual regardless of age, race, gender, or ethnicity. Some individuals may be affected by hyperpigmentation without prominent inflammation.8 Diagnosis of PPD can be challenging, and an occupation and recreational history of exposure or recent travel with possible contact with plants may be required.
Occupational Dermatitis
Recreational Dermatitis
Phytophotodermatitis may be caused by exposure to phototoxic agents during leisure activities. Recreational exposure can occur almost anywhere, including in the kitchen, backyard, park, or woods, as well as at the beach. One notable culprit in recreational PPD is cooking with limes, parsley, or parsnips—plants that often are employed as garnishes in dishes, allowing early exposure of juices on the hands. Individuals who garden recreationally should be aware of ornamental plants such as hogweed and figs, which are notorious for causing PPD.13 Children’s camp counselors should have knowledge of PPD, as children have considerable curiosity and may touch or play with attractive plants such as hogweed. Children enjoying sports in parks can accidentally fall onto or be exposed to wild parsnip or hogweed growing nearby and wake up the next day with erythema and burning.14 Photoprotection is important, but sunscreens containing carrot extract can produce PPD.15 Widespread PPD over 80% of the body surface area due to sunbathing after applying fig leaf tea as a tanning agent has been described.16 Eating figs does not cause photosensitization unless the juice is smeared onto the skin. Margarita dermatitis and “Mexican beer dermatitis” can occur due to limes and other citrus fruits being used as ingredients in summer drinks.5 Similarly, preparing sangria may produce PPD from lime and lemon juices.17 In one report, hiking in Corsica resulted in PPD following incidental contact with the endemic plant Peucedanum paniculatum.18
Perfume (Berloque) Dermatitis
Perfume dermatitis, or berloque dermatitis, is a type of PPD for which the name is derived from the German word berlock or the French word berloque meaning trinket or charm; it was first described in 1925 by Rosenthal7 with regard to pendantlike streaks of pigmentation on the neck, face, arms, or trunk. The dermatitis develops due to bergapten, a component of bergamot oil, which is derived from the rind of Citrus bergamia. Many perfumes contain bergamot oil, but the incidence of this condition has been diminished due to use of artificial bergamot oil.6
Clinical Manifestation
Phytophotodermatitis is first evident as erythematous patches that appear within 24 hours of initial exposure to a phototoxic agent and UVA light, sometimes with a burning sensation. Solar exposure within 48 hours of sufficient plant exposure is required. Perfuse sweating may enhance the reaction.19 Rarely, it first may be seen with the sudden appearance of

Differential Diagnosis
Phytophotodermatitis may resemble other types of dermatitis, particularly other forms of contact dermatitis such poison ivy, and occasionally other environmental simulants such as jellyfish stings.1-6,20,21 Photosensitizing disorders including porphyria cutanea tarda, pseudoporphyria, and lupus erythematosus must be distinguished from PPD.22-24 Photosensitizing medications such tetracyclines, thiazide diuretics, sulfonamides, griseofulvin, and sulfonylureas should be considered. Airborne contact dermatitis may resemble PPD, as when poison ivy is burned and is exposed to the skin in sites of airborne contact.20 Excessive solar exposure is popular, particularly among adolescents, so sunburn and sunburnlike reactions can be noteworthy.25,26
Treatment
Phytophotodermatitis can be treated with topical steroids, sometimes adding an oral antihistamine, and occasionally oral steroids.2-4 Localized pain or a burning sensation should respond to therapy. Alternatively, a cold compress applied to the skin can relieve the pain and pruritus, and the burn can be debrided and dressed daily with silver sulfadiazine plus an oral nonsteroidal anti-inflammatory drug. This eruption should be self-limited as long as it is recognized early and the cause avoided. Management of acute exposure includes prompt application of soap and water and avoidance of UV light exposure for 48 to 72 hours to prevent psoralen photoactivation.
Because PPD is essentially a chemical burn, a burn protocol and possible referral to a burn center may be needed, whether the reaction is acute or widespread.11,12,14,27,28 Surgical debridement and skin grafting rarely may be mandated.14 Postinflammatory hyperpigmentation may ensue as the dermatitis resolves but is not common.
The best approach for PPD is prevention (Figure 2). Individuals who are at risk should be aware of their surroundings and potential plants of concern and employ personal protective equipment to shield the skin from plant sap, which should be promptly removed if it comes in contact with the skin.

- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
- Zhang R, Zhu W. Phytophotodermatitis due to Chinese herbal medicine decoction. Indian J Dermatol. 2011;56:329-331.
- Harshman J, Quan Y, Hsiang D. Phytophotodermatitis: rash with many faces. Can Fam Physician. 2017;63:938-940.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Abramowitz AI, Resnik KS, Cohen KR. Margarita photodermatitis. N Engl J Med. 2013;328:891.
- Quaak MS, Martens H, Hassing RJ, et al. The sunny side of lime. J Travel Med. 2012;19:327-328.
- Rosenthal O. Berloque dermatitis: Berliner Dermatologische Gesellschaft. Dermatol Zeitschrift. 1925;42:295.
- Choi JY, Hwang S, Lee SH, et al. Asymptomatic hyperpigmentation without preceding inflammation as a clinical feature of citrus fruits–induced phytophotodermatitis. Ann Dermatol. 2018;30:75-78.
- Wynn P, Bell S. Phytophotodermatitis in grounds operatives. Occup Med (Lond). 2005;55:393-395.
- Klimaszyk P, Klimaszyk D, Piotrowiak M, et al. Unusual complications after occupational exposure to giant hogweed (Heracleum mantegazzianum): a case report. Int J Occup Med Environ Health. 2014;27:141-144.
- Downs JW, Cumpston KL, Feldman MJ. Giant hogweed phytophotodermatitis. Clin Toxicol (Phila). 2019;57:822-823.
- Maso MJ, Ruszkowski AM, Bauerle J, et al. Celery phytophotodermatitis in a chef. Arch Dermatol. 1991;127:912-913.
- Derraik JG, Rademaker M. Phytophotodermatitis caused by contact with a fig tree (Ficus carica). New Zealand Med J. 2007;120:U2720.
- Chan JC, Sullivan PJ, O’Sullivan MJ, et al. Full thickness burn caused by exposure to giant hogweed: delayed presentation, histological features and surgical management. J Plast Reconstr Aesthet Surg. 2011;64:128-130.
- Bosanac SS, Clark AK, Sivamani RK. Phytophotodermatitis related to carrot extract–containing sunscreen. Dermatol Online J. 2018;24:1-3.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Mioduszewski M, Beecker J. Phytophotodermatitis from making sangria: a phototoxic reaction to lime and lemon juice. CMAJ. 2015;187:756.
- Torrents R, Schmitt C, Domangé B, et al. Phytophotodermatitis with Peucedanum paniculatum: an endemic species to Corsica. Clin Toxicol (Phila). 2019;57:68-69.
- Sarhane KA, Ibrahim A, Fagan SP, et al. Phytophotodermatitis. Eplasty. 2013;13:ic57.
- DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. results of photopatch testing in New York, 1985 to 1990. Arch Dermatol. 1992;128:1513-1518.
- Kimyon RS, Warshaw EM. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-115.
- Miteva L, Broshtilova V, Schwartz RA. Unusual clinical manifestations of chronic discoid lupus erythematosus. Serbian J Dermatol Venereol. 2014;6:69-72.
- Handler NS, Handler MZ, Stephany MP, et al. Porphyria cutanea tarda: an intriguing genetic disease and marker. Int J Dermatol. 2017;56:E106-E117.
- Papadopoulos AJ, Schwartz RA, Fekete Z, et al. Pseudoporphyria: an atypical variant resembling toxic epidermal necrolysis. J Cutan Med Surg. 2001;5:479-485.
- Jasterzbski TJ, Janniger EJ, Schwartz RA. Adolescent tanning practices: understanding the popularity of excessive ultraviolet light exposure. In: Or
anje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:177-185. - Lai YC, Janniger EJ, Schwartz RA. Solar protection policy in school children: proposals for progress. In: Oranje A, Al-Mutairi N, Shwayder T, eds. Practical Pediatric Dermatology. Controversies in Diagnosis and Treatment. Springer Verlag; 2016:165-176.
- Lagey K, Duinslaeger L, Vanderkelen A. Burns induced by plants. Burns. 1995;21:542-543.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:e238745.
Practice Points
- Phytophotodermatitis (PPD) can be both an occupational and recreational dermatosis.
- Phytophotodermatitis is a nonallergic contact dermatitis and thus is independent of the immune system, so prior sensitization is not required.
- Individuals who work with plants should be aware of PPD and methods of prevention.
- Phytophotodermatitis may be evident only as asymptomatic hyperpigmentation.
FDA approves first AI device to detect colon lesions
The GI Genius (Cosmo Artificial Intelligence) identifies areas of the colon where a colorectal polyp or tumor might be located. Clinicians then follow up with a closer examination and possible treatment.
“With the FDA’s authorization of this device today, clinicians now have a tool that could help improve their ability to detect gastrointestinal lesions they may have missed otherwise,” said Courtney H. Lias, PhD, acting director of the FDA’s gastrorenal, ob.gyn., general hospital, and urology devices office, in a media release.
The GI Genius consists of both hardware and software designed to work with an endoscope. It uses machine learning to recognize possible polyps during a colonoscopy. It marks these areas with green squares on the video generated by the endoscope’s camera and emits a short, low-volume sound. Clinicians decide if a lesion is truly present and whether to sample or remove such a lesion.
The device does not diagnose the lesions or recommend treatments and is not intended to take the place of laboratory sampling
The FDA based its approval on a trial in which 700 people aged 40-80 years underwent colonoscopies for colorectal cancer screening, surveillance, follow-up from positive results of a fecal occult blood test, or gastrointestinal symptoms of possible colon cancer.
Of these participants, 263 were being screened or surveilled every 3 years or more. The researchers randomly divided patients into a group of 136 who underwent white-light standard colonoscopy with the GI Genius, and 127 who underwent white-light standard colonoscopy without the GI Genius.
Using the GI Genius, clinicians identified adenomas or carcinomas that were later confirmed through lab results in 55.1% of patients. Without the GI Genius, the clinicians identified such lesions in 42.0% of patients.
The patients examined with the GI Genius received more biopsies, including slightly more that were not adenomas. But the biopsies did not lead to any adverse events such as perforations, infections, bleeding, or further biopsies.
More information on the GI Genius is available on the FDA website.
A version of this article first appeared on Medscape.com .
The GI Genius (Cosmo Artificial Intelligence) identifies areas of the colon where a colorectal polyp or tumor might be located. Clinicians then follow up with a closer examination and possible treatment.
“With the FDA’s authorization of this device today, clinicians now have a tool that could help improve their ability to detect gastrointestinal lesions they may have missed otherwise,” said Courtney H. Lias, PhD, acting director of the FDA’s gastrorenal, ob.gyn., general hospital, and urology devices office, in a media release.
The GI Genius consists of both hardware and software designed to work with an endoscope. It uses machine learning to recognize possible polyps during a colonoscopy. It marks these areas with green squares on the video generated by the endoscope’s camera and emits a short, low-volume sound. Clinicians decide if a lesion is truly present and whether to sample or remove such a lesion.
The device does not diagnose the lesions or recommend treatments and is not intended to take the place of laboratory sampling
The FDA based its approval on a trial in which 700 people aged 40-80 years underwent colonoscopies for colorectal cancer screening, surveillance, follow-up from positive results of a fecal occult blood test, or gastrointestinal symptoms of possible colon cancer.
Of these participants, 263 were being screened or surveilled every 3 years or more. The researchers randomly divided patients into a group of 136 who underwent white-light standard colonoscopy with the GI Genius, and 127 who underwent white-light standard colonoscopy without the GI Genius.
Using the GI Genius, clinicians identified adenomas or carcinomas that were later confirmed through lab results in 55.1% of patients. Without the GI Genius, the clinicians identified such lesions in 42.0% of patients.
The patients examined with the GI Genius received more biopsies, including slightly more that were not adenomas. But the biopsies did not lead to any adverse events such as perforations, infections, bleeding, or further biopsies.
More information on the GI Genius is available on the FDA website.
A version of this article first appeared on Medscape.com .
The GI Genius (Cosmo Artificial Intelligence) identifies areas of the colon where a colorectal polyp or tumor might be located. Clinicians then follow up with a closer examination and possible treatment.
“With the FDA’s authorization of this device today, clinicians now have a tool that could help improve their ability to detect gastrointestinal lesions they may have missed otherwise,” said Courtney H. Lias, PhD, acting director of the FDA’s gastrorenal, ob.gyn., general hospital, and urology devices office, in a media release.
The GI Genius consists of both hardware and software designed to work with an endoscope. It uses machine learning to recognize possible polyps during a colonoscopy. It marks these areas with green squares on the video generated by the endoscope’s camera and emits a short, low-volume sound. Clinicians decide if a lesion is truly present and whether to sample or remove such a lesion.
The device does not diagnose the lesions or recommend treatments and is not intended to take the place of laboratory sampling
The FDA based its approval on a trial in which 700 people aged 40-80 years underwent colonoscopies for colorectal cancer screening, surveillance, follow-up from positive results of a fecal occult blood test, or gastrointestinal symptoms of possible colon cancer.
Of these participants, 263 were being screened or surveilled every 3 years or more. The researchers randomly divided patients into a group of 136 who underwent white-light standard colonoscopy with the GI Genius, and 127 who underwent white-light standard colonoscopy without the GI Genius.
Using the GI Genius, clinicians identified adenomas or carcinomas that were later confirmed through lab results in 55.1% of patients. Without the GI Genius, the clinicians identified such lesions in 42.0% of patients.
The patients examined with the GI Genius received more biopsies, including slightly more that were not adenomas. But the biopsies did not lead to any adverse events such as perforations, infections, bleeding, or further biopsies.
More information on the GI Genius is available on the FDA website.
A version of this article first appeared on Medscape.com .
Endometrial thickness could predict cancer, guide lymph node assessment
In a retrospective study of 378 patients who had hysterectomies for EIN, those with a preoperative endometrial stripe of 20 mm or greater were two times more likely to have endometrial cancer on final pathology, and those with an endometrial thickness of 15 mm or greater were 1.8 times more likely to have cancer.
“This data suggests that increasing endometrial thickness may be a useful preoperative marker to identify who’s at higher risk of concurrent endometrial cancer. It could also be considered a criterion for selectively using a sentinel lymph node algorithm in patients with a preoperative diagnosis of EIN. However, prospective studies are warranted to further establish this association,” said Devon Abt, MD, of Beth Israel Deaconess Medical Center in Boston.
She presented the data at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 11103).
Risk of overtreatment
There are no clear consensus guidelines on lymph node assessment for patients with EIN, Dr. Abt noted. She pointed out that roughly 40% of patients with EIN are diagnosed with endometrial cancer. However, it’s usually low-stage, low-grade disease, and only about 10% of patients will have high-risk features that warrant lymph node evaluation.
“Typically, we identify patients with concurrent endometrial cancer based on intraoperative pathology, or frozen section,” Dr. Abt explained. “We then apply the Mayo criteria, which stratifies patients as high or low risk for lymph node metastasis based on pathologic criteria. ... This information helps guide our intraoperative decisions to perform, or not perform, pelvic and para-aortic lymphadenectomy.”
Dr. Abt noted, however, that “lymphadenectomy is not benign” and increases surgical time as well as the risk of complications.
Taking these factors into account, some centers have implemented routine sentinel lymph node algorithms for staging endometrial cancers, Dr. Abt said.
What she and her colleagues wanted to determine is if there is value in this practice. Should sentinel lymph node mapping and biopsy be offered routinely to all patients with a preoperative diagnosis of EIN?
Study details
Dr. Abt and colleagues conducted a retrospective, single-center study of 378 patients with EIN. Ultimately, 27% (n = 103) of the patients were diagnosed with endometrial cancer – 95% with stage 1a disease and 5% with stage 1b.
Increasing age, White race, and hypertension were significantly associated with the presence of endometrial cancer. Body mass index, parity, hormone therapy exposure, and baseline CA 125 were not.
The median preoperative endometrial thickness was 14 mm among patients with endometrial cancer and 11 mm in patients without cancer (P = .002).
Overall, 31% of the cancer cases were considered high risk for nodal metastases by Mayo criteria, but an endometrial stripe of 15 mm or higher increased the chance of being considered high risk.
The risk of cancer was 47% among patients with an endometrial stripe of at least 20 mm versus 21% among patients with a measurement below 15 mm.
Only 10 patients underwent lymph node evaluation, 5 with sentinel lymph node dissection and 5 with lymphadenectomy. Six of the 10 patients had endometrial cancer on final pathology, but none had positive lymph nodes.
“Given the low-grade and early-stage disease in this cohort, adherence to a routine sentinel lymph node algorithm in all patients with EIN would result in overtreatment,” Dr. Abt said.
Discussant Nicole Fleming, MD, of the University of Texas MD Anderson Cancer Center, Houston, said she would advocate for more selective use of sentinel lymph node biopsies in EIN as well.
Dr. Fleming said, in general, lymph node biopsy may be reasonable in settings where frozen sections are unreliable and the patient seems to be at high risk of invasive cancer. However, at academic centers with dedicated gynecologic pathologists, given the low risk of invasive cancer and the fact that lymph nodes “are probably not going to provide you a lot of useful therapeutic decision-making tools,” potentially eliminating sentinel lymph node biopsy might make sense, Dr. Fleming said.
Dr. Fleming disclosed relationships with Tesaro, Bristol-Myers Squibb, Pfizer, and GlaxoSmithKline. Dr. Abt reported having no relevant disclosures and did not report any study funding.
In a retrospective study of 378 patients who had hysterectomies for EIN, those with a preoperative endometrial stripe of 20 mm or greater were two times more likely to have endometrial cancer on final pathology, and those with an endometrial thickness of 15 mm or greater were 1.8 times more likely to have cancer.
“This data suggests that increasing endometrial thickness may be a useful preoperative marker to identify who’s at higher risk of concurrent endometrial cancer. It could also be considered a criterion for selectively using a sentinel lymph node algorithm in patients with a preoperative diagnosis of EIN. However, prospective studies are warranted to further establish this association,” said Devon Abt, MD, of Beth Israel Deaconess Medical Center in Boston.
She presented the data at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 11103).
Risk of overtreatment
There are no clear consensus guidelines on lymph node assessment for patients with EIN, Dr. Abt noted. She pointed out that roughly 40% of patients with EIN are diagnosed with endometrial cancer. However, it’s usually low-stage, low-grade disease, and only about 10% of patients will have high-risk features that warrant lymph node evaluation.
“Typically, we identify patients with concurrent endometrial cancer based on intraoperative pathology, or frozen section,” Dr. Abt explained. “We then apply the Mayo criteria, which stratifies patients as high or low risk for lymph node metastasis based on pathologic criteria. ... This information helps guide our intraoperative decisions to perform, or not perform, pelvic and para-aortic lymphadenectomy.”
Dr. Abt noted, however, that “lymphadenectomy is not benign” and increases surgical time as well as the risk of complications.
Taking these factors into account, some centers have implemented routine sentinel lymph node algorithms for staging endometrial cancers, Dr. Abt said.
What she and her colleagues wanted to determine is if there is value in this practice. Should sentinel lymph node mapping and biopsy be offered routinely to all patients with a preoperative diagnosis of EIN?
Study details
Dr. Abt and colleagues conducted a retrospective, single-center study of 378 patients with EIN. Ultimately, 27% (n = 103) of the patients were diagnosed with endometrial cancer – 95% with stage 1a disease and 5% with stage 1b.
Increasing age, White race, and hypertension were significantly associated with the presence of endometrial cancer. Body mass index, parity, hormone therapy exposure, and baseline CA 125 were not.
The median preoperative endometrial thickness was 14 mm among patients with endometrial cancer and 11 mm in patients without cancer (P = .002).
Overall, 31% of the cancer cases were considered high risk for nodal metastases by Mayo criteria, but an endometrial stripe of 15 mm or higher increased the chance of being considered high risk.
The risk of cancer was 47% among patients with an endometrial stripe of at least 20 mm versus 21% among patients with a measurement below 15 mm.
Only 10 patients underwent lymph node evaluation, 5 with sentinel lymph node dissection and 5 with lymphadenectomy. Six of the 10 patients had endometrial cancer on final pathology, but none had positive lymph nodes.
“Given the low-grade and early-stage disease in this cohort, adherence to a routine sentinel lymph node algorithm in all patients with EIN would result in overtreatment,” Dr. Abt said.
Discussant Nicole Fleming, MD, of the University of Texas MD Anderson Cancer Center, Houston, said she would advocate for more selective use of sentinel lymph node biopsies in EIN as well.
Dr. Fleming said, in general, lymph node biopsy may be reasonable in settings where frozen sections are unreliable and the patient seems to be at high risk of invasive cancer. However, at academic centers with dedicated gynecologic pathologists, given the low risk of invasive cancer and the fact that lymph nodes “are probably not going to provide you a lot of useful therapeutic decision-making tools,” potentially eliminating sentinel lymph node biopsy might make sense, Dr. Fleming said.
Dr. Fleming disclosed relationships with Tesaro, Bristol-Myers Squibb, Pfizer, and GlaxoSmithKline. Dr. Abt reported having no relevant disclosures and did not report any study funding.
In a retrospective study of 378 patients who had hysterectomies for EIN, those with a preoperative endometrial stripe of 20 mm or greater were two times more likely to have endometrial cancer on final pathology, and those with an endometrial thickness of 15 mm or greater were 1.8 times more likely to have cancer.
“This data suggests that increasing endometrial thickness may be a useful preoperative marker to identify who’s at higher risk of concurrent endometrial cancer. It could also be considered a criterion for selectively using a sentinel lymph node algorithm in patients with a preoperative diagnosis of EIN. However, prospective studies are warranted to further establish this association,” said Devon Abt, MD, of Beth Israel Deaconess Medical Center in Boston.
She presented the data at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 11103).
Risk of overtreatment
There are no clear consensus guidelines on lymph node assessment for patients with EIN, Dr. Abt noted. She pointed out that roughly 40% of patients with EIN are diagnosed with endometrial cancer. However, it’s usually low-stage, low-grade disease, and only about 10% of patients will have high-risk features that warrant lymph node evaluation.
“Typically, we identify patients with concurrent endometrial cancer based on intraoperative pathology, or frozen section,” Dr. Abt explained. “We then apply the Mayo criteria, which stratifies patients as high or low risk for lymph node metastasis based on pathologic criteria. ... This information helps guide our intraoperative decisions to perform, or not perform, pelvic and para-aortic lymphadenectomy.”
Dr. Abt noted, however, that “lymphadenectomy is not benign” and increases surgical time as well as the risk of complications.
Taking these factors into account, some centers have implemented routine sentinel lymph node algorithms for staging endometrial cancers, Dr. Abt said.
What she and her colleagues wanted to determine is if there is value in this practice. Should sentinel lymph node mapping and biopsy be offered routinely to all patients with a preoperative diagnosis of EIN?
Study details
Dr. Abt and colleagues conducted a retrospective, single-center study of 378 patients with EIN. Ultimately, 27% (n = 103) of the patients were diagnosed with endometrial cancer – 95% with stage 1a disease and 5% with stage 1b.
Increasing age, White race, and hypertension were significantly associated with the presence of endometrial cancer. Body mass index, parity, hormone therapy exposure, and baseline CA 125 were not.
The median preoperative endometrial thickness was 14 mm among patients with endometrial cancer and 11 mm in patients without cancer (P = .002).
Overall, 31% of the cancer cases were considered high risk for nodal metastases by Mayo criteria, but an endometrial stripe of 15 mm or higher increased the chance of being considered high risk.
The risk of cancer was 47% among patients with an endometrial stripe of at least 20 mm versus 21% among patients with a measurement below 15 mm.
Only 10 patients underwent lymph node evaluation, 5 with sentinel lymph node dissection and 5 with lymphadenectomy. Six of the 10 patients had endometrial cancer on final pathology, but none had positive lymph nodes.
“Given the low-grade and early-stage disease in this cohort, adherence to a routine sentinel lymph node algorithm in all patients with EIN would result in overtreatment,” Dr. Abt said.
Discussant Nicole Fleming, MD, of the University of Texas MD Anderson Cancer Center, Houston, said she would advocate for more selective use of sentinel lymph node biopsies in EIN as well.
Dr. Fleming said, in general, lymph node biopsy may be reasonable in settings where frozen sections are unreliable and the patient seems to be at high risk of invasive cancer. However, at academic centers with dedicated gynecologic pathologists, given the low risk of invasive cancer and the fact that lymph nodes “are probably not going to provide you a lot of useful therapeutic decision-making tools,” potentially eliminating sentinel lymph node biopsy might make sense, Dr. Fleming said.
Dr. Fleming disclosed relationships with Tesaro, Bristol-Myers Squibb, Pfizer, and GlaxoSmithKline. Dr. Abt reported having no relevant disclosures and did not report any study funding.
FROM SGO 2021
Despite new ichthyosis treatment recommendations, ‘many questions still exist’
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
.
According to a consensus statement published in the February issue of Pediatric Dermatology, adequate data exist in the medical literature to demonstrate an improvement in use of systemic retinoids for select genotypes of congenital ichthyosiform erythroderma, epidermolytic ichthyosis, erythrokeratodermia variabilis, harlequin ichthyosis, IFAP syndrome (ichthyosis with confetti, ichthyosis follicularis, atrichia, and photophobia), KID syndrome (keratitis-ichthyosis-deafness), KLICK syndrome (keratosis linearis with ichthyosis congenita and sclerosing keratoderma), lamellar ichthyosis, loricrin keratoderma, neutral lipid storage disease with ichthyosis, recessive X-linked ichthyosis, and Sjögren-Larsson syndrome.
At the same time, limited or no data exist to support the use of systemic retinoids for CHILD syndrome (congenital hemidysplasia with ichthyosiform erythroderma and limb defects), CHIME syndrome (colobomas, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), Conradi-Hunermann-Happle syndrome, ichthyosis-hypotrichosis, ichthyosis-hypotrichosis-sclerosis cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome (mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma), peeling skin disease, Refsum syndrome, and trichothiodystrophy, according to the statement.
“In particular, we did note that, with any disorder that was associated with atopy, the retinoids were often counterproductive,” one of the consensus statement cochairs, Andrea L. Zaenglein, MD, said during the Society for Pediatric Dermatology pre-AAD meeting. “In Netherton syndrome, for example, retinoids seemed to make the skin fragility a lot worse, so typically, they would be avoided in those patients.”
The statement, which she assembled with cochair pediatric dermatologist Moise L. Levy, MD, professor of pediatrics, University of Texas at Austin, and 21 other multidisciplinary experts, recommends considering use of topical retinoids to help decrease scaling of the skin,“but [they] are particularly helpful for more localized complications of ichthyosis, such as digital contractures and ectropion,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey. “A lot of it has to do with the size and the volume of the tubes and getting enough [product] to be able to apply it over larger areas. We do tend to use them more focally.”
While systemic absorption can occur with widespread use, no specific lab monitoring is required. Dr. Zaenglein and her colleagues also recommend avoiding the use of tazarotene during pregnancy, since it is contraindicated in pregnancy (category X), but monthly pregnancy tests are not recommended.
During an overview of the document at the meeting, she noted that the recommended dosing for both isotretinoin and acitretin is 0.5-1.0 mg/kg per day and the side effects tend to be dose dependent, “except teratogenicity, which can occur with even low doses of systemic retinoid exposure and early on in pregnancy.” The authors also advise patients to consider drug holidays or lower doses “especially during warmer, more humid months, where you might not need the higher doses to achieve cutaneous effects,” she said.
They emphasized the importance of avoiding pregnancy for 3 years after completion of treatment with acitretin. “While the half-life of acitretin is 49 hours, it’s easily converted with any alcohol exposure to etretinate,” Dr. Zaenglein noted. “Then, the half-life is 120 days.”
The statement, which was sponsored by the Pediatric Dermatology Research Alliance (PEDRA), also addresses the clinical considerations and consequences of long-term systemic retinoid use on bone health, such as premature epiphyseal closure in preadolescent children. “In general, this risk is greater with higher doses of therapies – above 1 mg/kg per day – and over prolonged periods of time, typically 4-6 years,” she said. Other potential effects on bone health include calcifications of tendons and ligaments, osteophytes or “bone spurs,” DISH (diffuse idiopathic skeletal hyperostosis), and potential alterations in bone density and growth.
“We also have to worry about concomitant effects of contraception, particularly if you’re using progestin-only formulations that carry a black box warning for osteoporosis,” Dr. Zaenglein said. “It is recommended that you limit their use to 3 years.” Other factors to consider include genetic risk and modifiable factors that affect bone health, such as diet and physical activity, which may impact susceptibility to systemic retinoid bone toxicity and should be discussed with the patient.
Recommended bone monitoring in children starts with a comprehensive family and personal medical history for skeletal toxicity risk factors, followed by an annual growth assessment (height, weight, body mass index, and growth curve), asking regularly about musculoskeletal symptoms, and following up with appropriate imaging. “Inquiring about their diet is recommended as well, so making sure they’re getting sufficient amounts of calcium and vitamin D, and no additional vitamin A sources that may compound the side effects from systemic retinoids,” Dr. Zaenglein said.
The document also advises that a baseline skeletal radiographic survey be performed in patients aged 16-18 years. This may include imaging of the lateral cervical and thoracic spine, lateral view of the calcanei to include Achilles tendon, hips and symptomatic areas, and bone density evaluation.
The statement addressed the psychiatric considerations and consequences of long-term systemic retinoid use. One cross-sectional study of children with ichthyosis found that 30% screened positive for depression and 38% screened positive for anxiety, “but the role of retinoids is unclear,” Dr. Zaenglein said. “It’s a complicated matter, but patients with a personal history of depression, anxiety, and other affective disorders prior to initiation of systemic retinoid treatment should be monitored carefully for exacerbation of symptoms. Comanagement with a mental health provider should be considered.”
As for contraception considerations with long-term systemic retinoid therapy use, the authors recommend that two forms of contraception be used. “Consider long-acting reversible contraception, especially in sexually active adolescents who have a history of noncompliance, or to remove the risk of teratogenicity for them,” she said. “We’re not sure what additive effects progestin/lower estrogen have on long-term cardiovascular health, including lipids and bone density.”
The authors noted that iPLEDGE is not designed for long-term use. “It’s really designed for the on-label use of systemic retinoids in severe acne, where you’re using it for 5-6 months, not for 5-6 years,” Dr. Zaenglein said. “iPLEDGE does impose significant and financial barriers for our patients. More advocacy is needed to adapt that program for our patients.”
She and her coauthors acknowledged practice gaps and unmet needs in patients with disorders of cornification/types of ichthyosis, including the optimal formulation of retinoids based on ichthyosis subtype, whether there is a benefit to intermittent therapy with respect to risk of toxicity and maintenance of efficacy, and how to minimize the bone-related changes that can occur with treatment. “These are some of the things that we can look further into,” she said. “For now, though, retinoids can improve function and quality of life in patients with ichthyosis and disorders of cornification. Many questions still exist, and more data and research are needed.”
Sun Pharmaceuticals and the Foundation for Ichthyosis and Related Skin Types (FIRST) provided an unrestricted grant for development of the recommendations.
Dr. Zaenglein disclosed that she is a consultant for Pfizer. She is also an advisory board member for Dermata, Sol-Gel, Regeneron, Verrica, and Cassiopea, and has conducted contracted research for AbbVie, Incyte, Arcutis, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or had other relationships with various pharmaceutical companies.
FROM THE SPD PRE-AAD MEETING
New-onset hirsutism
A 74-year-old woman presented to the dermatology clinic for follow-up 3 months after the surgical excision of a basal cell carcinoma on her left jawline. During this postop period, the patient developed new-onset hirsutism. She appeared to be in otherwise good health.
Family and personal medical history were unremarkable. Her medication regimen included aspirin 81 mg/d and a daily multivitamin. The patient was postmenopausal and had a body mass index of 28 and a history of acid reflux and osteoarthritis.
Physical examination of the patient’s scalp showed male-pattern alopecia (FIGURE 1A). She also had coarse terminal hairs on her forearms and back, as well as on her chin (FIGURE 1B).
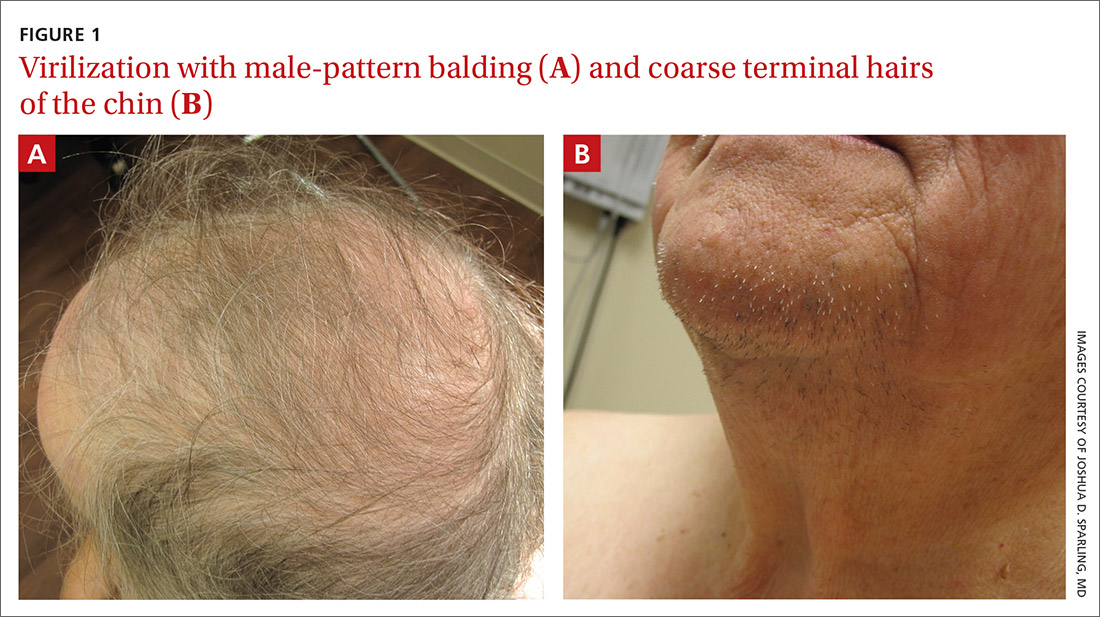
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Androgen-secreting ovarian tumor
Based on the distribution of terminal hairs and marked change over 3 months, as well as the male-pattern alopecia, a diagnosis of androgen excess was suspected. Laboratory work-up, including thyroid-stimulating hormone, dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone, luteinizing hormone, prolactin, complete blood count, and complete metabolic panel, was within normal limits. Pelvic ultrasound of the ovaries and abdominal computed tomography (CT) of the adrenal glands were also normal.
Further testing showed an elevated testosterone level of 464 ng/dL (reference range: 2-45 ng/dL) and an elevated free testosterone level of 66.8 ng/dL (reference range: 0.2-3.7 ng/dL). These levels pointed to an androgen-secreting ovarian tumor; the androgen excess was likely the cause of her hirsutism.
Hirsutism or hypertrichosis?
Hirsutism, a common disorder affecting up to 8% of women, is defined by excess terminal hairs that appear in a male pattern in women due to production of excess androgens.1 This should be distinguished from hypertrichosis, which is generalized excessive hair growth not caused by androgen excess.
Testosterone and DHEAS—produced in the ovaries and adrenal glands, respectively—contribute to the development of hirsutism.1 Hirsutism is more often associated with adrenal or ovarian tumors in postmenopausal patients.2 Generalized hypertrichosis can be associated with porphyria cutanea tarda, severe anorexia nervosa, and rarely, malignancies; it also can be secondary to certain agents, such as cyclosporin, phenytoin, and minoxidil.
While hirsutism is associated with hyperandrogenemia, its degree correlates poorly with serum levels. Notably, about half of women with hirsutism have been found to have normal levels of circulating androgens.1 Severe signs of hyperandrogenemia include rapid onset of symptoms, signs of virilization, and a palpable abdominal or pelvic mass.3
Continue to: Is the patient pre- or postmenopausal?
Is the patient pre- or postmenopausal? Polycystic ovary syndrome (PCOS) accounts for up to three-fourths of premenopausal hirsutism.3 The likelihood of hirsutism is actually decreased in postmenopausal women because estrogen levels can drop abruptly after menopause. That said, conditions linked to hirsutism in postmenopausal women include adrenal hyperplasia, thyroid dysfunction, Cushing syndrome, and least frequently, androgen-secreting tumors (seen in this patient). (Hirsutism can also be idiopathic or iatrogenic [medications].)
Methods for detection
Research suggests that when a female patient is given a diagnosis of hirsutism, it’s important to explore possible underlying ovarian and/or adrenal tumors and adult-onset adrenal hyperplasia.1 The following tests and procedure can be helpful:
Serum testosterone and DHEAS. Levels of total testosterone > 200 ng/dL and/or DHEAS > 700 ng/dL are strongly indicative of androgen-secreting tumors.1
Imaging—including ultrasound, CT, or magnetic resonance imaging—can be used for evaluation of the adrenal glands and ovaries. However, imaging is often unable to identify these small tumors.4
Selective venous catheterization can be useful in the localization and lateralization of an androgen-secreting tumor, although a nondiagnostic result with this technique is not uncommon.4
Continue to: Dynamic hormonal testing
Dynamic hormonal testing may assist in determining the pathology of disease but not laterality.2 For example, testing for gonadotropin-releasing hormone agonists can be helpful because the constant administration of such agonists can lead to ovarian suppression without affecting adrenal androgen secretion.5
Testing with oral dexamethasone may induce adrenal hormonal depression of androgens and subsequent estradiol through aromatase conversion, which can help rule out an ovarian source.6 Exogenous administration of follicle-stimulating hormone or luteinizing hormone can further differentiate the source from ovarian theca or granulosa cell production.4
Treatment varies
The specific etiology of a patient’s hirsutism dictates the most appropriate treatment. For example, medication-induced hirsutism often requires discontinuation of the offending agent, whereas PCOS would necessitate appropriate nonpharmacologic and pharmacologic interventions.
For our patient, the elevated testosterone and free testosterone levels with normal DHEAS strongly suggested the presence of an androgen-secreting ovarian tumor. These findings led to a referral for bilateral salpingo-oophorectomy. The surgical gross appearance of the patient’s ovaries was unremarkable, but gross dissection and pathology of the ovaries (which were not postoperatively identified to determine laterality) showed one was larger (2.7 × 1.5 × 0.8 cm vs 3.2 × 1.4 × 1.2 cm).
The larger ovary contained an area of brown induration measuring 2.3 × 1.1 × 1.1 cm. This area corresponded to abundant eosinophilic cytoplasm with nuclear, rich, round-cell proliferation, consistent with the diagnosis of a benign ovarian Leydig cell tumor (FIGURE 2). Thus, the bilateral salpingo-oophorectomy was both diagnostic and therapeutic.
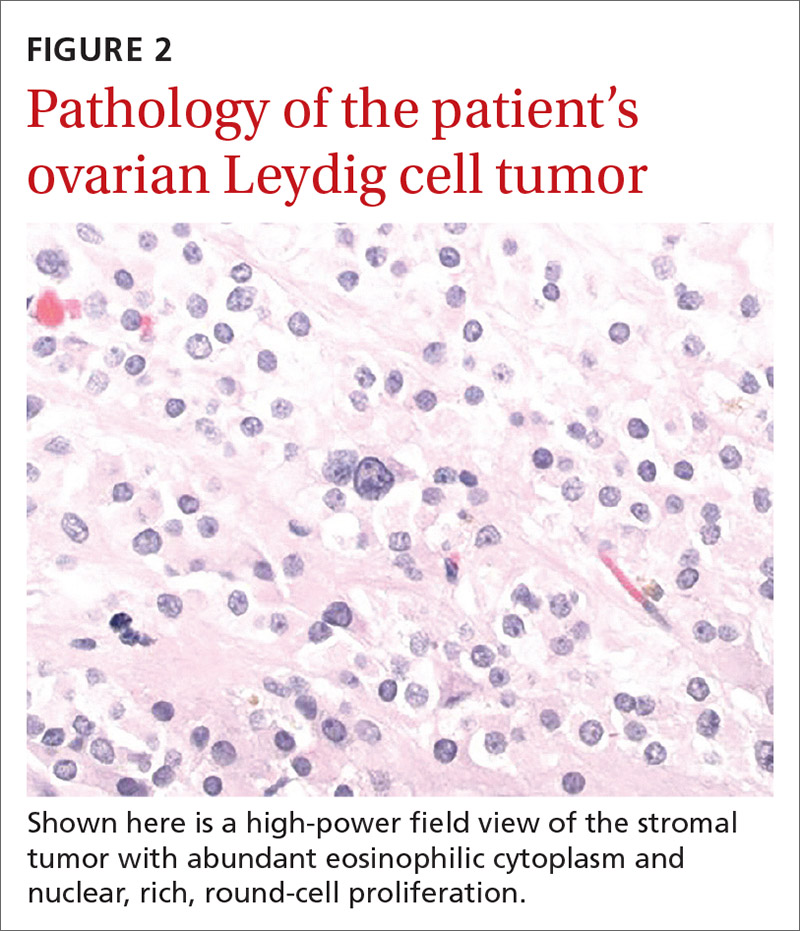
Six weeks after the surgery, blood work showed normalization of testosterone and free testosterone levels. The patient’s hirsutism completely resolved over the course of the next several months.
1. Hunter M, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Physician. 2003;67:2565-2572.
2. Alpañés M, González-Casbas JM, Sánchez J, et al. Management of postmenopausal virilization. J Clin Endocrinol Metab. 2012;97:2584-2588.
3. Bode D, Seehusen DA, Baird D. Hirsutism in women. Am Fam Physician. 2012;85:373-380.
4. Cohen I, Nabriski D, Fishman A. Noninvasive test for the diagnosis of ovarian hormone-secreting-neopolasm in postmenopausal women. Gynecol Oncol Rep. 2016;15:12-15.
5. Gandrapu B, Sundar P, Phillips B. Hyperandrogenism in a postmenaupsal woman secondary to testosterone secreting ovarian stromal tumor with acoustic schwannoma. Case Rep Endocrinol. 2018;2018:8154513.
6. Curran DR, Moore C, Huber T. What is the best approach to the evaluation of hirsutism? J Fam Pract. 2005;54:458-473.
A 74-year-old woman presented to the dermatology clinic for follow-up 3 months after the surgical excision of a basal cell carcinoma on her left jawline. During this postop period, the patient developed new-onset hirsutism. She appeared to be in otherwise good health.
Family and personal medical history were unremarkable. Her medication regimen included aspirin 81 mg/d and a daily multivitamin. The patient was postmenopausal and had a body mass index of 28 and a history of acid reflux and osteoarthritis.
Physical examination of the patient’s scalp showed male-pattern alopecia (FIGURE 1A). She also had coarse terminal hairs on her forearms and back, as well as on her chin (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Androgen-secreting ovarian tumor
Based on the distribution of terminal hairs and marked change over 3 months, as well as the male-pattern alopecia, a diagnosis of androgen excess was suspected. Laboratory work-up, including thyroid-stimulating hormone, dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone, luteinizing hormone, prolactin, complete blood count, and complete metabolic panel, was within normal limits. Pelvic ultrasound of the ovaries and abdominal computed tomography (CT) of the adrenal glands were also normal.
Further testing showed an elevated testosterone level of 464 ng/dL (reference range: 2-45 ng/dL) and an elevated free testosterone level of 66.8 ng/dL (reference range: 0.2-3.7 ng/dL). These levels pointed to an androgen-secreting ovarian tumor; the androgen excess was likely the cause of her hirsutism.
Hirsutism or hypertrichosis?
Hirsutism, a common disorder affecting up to 8% of women, is defined by excess terminal hairs that appear in a male pattern in women due to production of excess androgens.1 This should be distinguished from hypertrichosis, which is generalized excessive hair growth not caused by androgen excess.
Testosterone and DHEAS—produced in the ovaries and adrenal glands, respectively—contribute to the development of hirsutism.1 Hirsutism is more often associated with adrenal or ovarian tumors in postmenopausal patients.2 Generalized hypertrichosis can be associated with porphyria cutanea tarda, severe anorexia nervosa, and rarely, malignancies; it also can be secondary to certain agents, such as cyclosporin, phenytoin, and minoxidil.
While hirsutism is associated with hyperandrogenemia, its degree correlates poorly with serum levels. Notably, about half of women with hirsutism have been found to have normal levels of circulating androgens.1 Severe signs of hyperandrogenemia include rapid onset of symptoms, signs of virilization, and a palpable abdominal or pelvic mass.3
Continue to: Is the patient pre- or postmenopausal?
Is the patient pre- or postmenopausal? Polycystic ovary syndrome (PCOS) accounts for up to three-fourths of premenopausal hirsutism.3 The likelihood of hirsutism is actually decreased in postmenopausal women because estrogen levels can drop abruptly after menopause. That said, conditions linked to hirsutism in postmenopausal women include adrenal hyperplasia, thyroid dysfunction, Cushing syndrome, and least frequently, androgen-secreting tumors (seen in this patient). (Hirsutism can also be idiopathic or iatrogenic [medications].)
Methods for detection
Research suggests that when a female patient is given a diagnosis of hirsutism, it’s important to explore possible underlying ovarian and/or adrenal tumors and adult-onset adrenal hyperplasia.1 The following tests and procedure can be helpful:
Serum testosterone and DHEAS. Levels of total testosterone > 200 ng/dL and/or DHEAS > 700 ng/dL are strongly indicative of androgen-secreting tumors.1
Imaging—including ultrasound, CT, or magnetic resonance imaging—can be used for evaluation of the adrenal glands and ovaries. However, imaging is often unable to identify these small tumors.4
Selective venous catheterization can be useful in the localization and lateralization of an androgen-secreting tumor, although a nondiagnostic result with this technique is not uncommon.4
Continue to: Dynamic hormonal testing
Dynamic hormonal testing may assist in determining the pathology of disease but not laterality.2 For example, testing for gonadotropin-releasing hormone agonists can be helpful because the constant administration of such agonists can lead to ovarian suppression without affecting adrenal androgen secretion.5
Testing with oral dexamethasone may induce adrenal hormonal depression of androgens and subsequent estradiol through aromatase conversion, which can help rule out an ovarian source.6 Exogenous administration of follicle-stimulating hormone or luteinizing hormone can further differentiate the source from ovarian theca or granulosa cell production.4
Treatment varies
The specific etiology of a patient’s hirsutism dictates the most appropriate treatment. For example, medication-induced hirsutism often requires discontinuation of the offending agent, whereas PCOS would necessitate appropriate nonpharmacologic and pharmacologic interventions.
For our patient, the elevated testosterone and free testosterone levels with normal DHEAS strongly suggested the presence of an androgen-secreting ovarian tumor. These findings led to a referral for bilateral salpingo-oophorectomy. The surgical gross appearance of the patient’s ovaries was unremarkable, but gross dissection and pathology of the ovaries (which were not postoperatively identified to determine laterality) showed one was larger (2.7 × 1.5 × 0.8 cm vs 3.2 × 1.4 × 1.2 cm).
The larger ovary contained an area of brown induration measuring 2.3 × 1.1 × 1.1 cm. This area corresponded to abundant eosinophilic cytoplasm with nuclear, rich, round-cell proliferation, consistent with the diagnosis of a benign ovarian Leydig cell tumor (FIGURE 2). Thus, the bilateral salpingo-oophorectomy was both diagnostic and therapeutic.

Six weeks after the surgery, blood work showed normalization of testosterone and free testosterone levels. The patient’s hirsutism completely resolved over the course of the next several months.
A 74-year-old woman presented to the dermatology clinic for follow-up 3 months after the surgical excision of a basal cell carcinoma on her left jawline. During this postop period, the patient developed new-onset hirsutism. She appeared to be in otherwise good health.
Family and personal medical history were unremarkable. Her medication regimen included aspirin 81 mg/d and a daily multivitamin. The patient was postmenopausal and had a body mass index of 28 and a history of acid reflux and osteoarthritis.
Physical examination of the patient’s scalp showed male-pattern alopecia (FIGURE 1A). She also had coarse terminal hairs on her forearms and back, as well as on her chin (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Androgen-secreting ovarian tumor
Based on the distribution of terminal hairs and marked change over 3 months, as well as the male-pattern alopecia, a diagnosis of androgen excess was suspected. Laboratory work-up, including thyroid-stimulating hormone, dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone, luteinizing hormone, prolactin, complete blood count, and complete metabolic panel, was within normal limits. Pelvic ultrasound of the ovaries and abdominal computed tomography (CT) of the adrenal glands were also normal.
Further testing showed an elevated testosterone level of 464 ng/dL (reference range: 2-45 ng/dL) and an elevated free testosterone level of 66.8 ng/dL (reference range: 0.2-3.7 ng/dL). These levels pointed to an androgen-secreting ovarian tumor; the androgen excess was likely the cause of her hirsutism.
Hirsutism or hypertrichosis?
Hirsutism, a common disorder affecting up to 8% of women, is defined by excess terminal hairs that appear in a male pattern in women due to production of excess androgens.1 This should be distinguished from hypertrichosis, which is generalized excessive hair growth not caused by androgen excess.
Testosterone and DHEAS—produced in the ovaries and adrenal glands, respectively—contribute to the development of hirsutism.1 Hirsutism is more often associated with adrenal or ovarian tumors in postmenopausal patients.2 Generalized hypertrichosis can be associated with porphyria cutanea tarda, severe anorexia nervosa, and rarely, malignancies; it also can be secondary to certain agents, such as cyclosporin, phenytoin, and minoxidil.
While hirsutism is associated with hyperandrogenemia, its degree correlates poorly with serum levels. Notably, about half of women with hirsutism have been found to have normal levels of circulating androgens.1 Severe signs of hyperandrogenemia include rapid onset of symptoms, signs of virilization, and a palpable abdominal or pelvic mass.3
Continue to: Is the patient pre- or postmenopausal?
Is the patient pre- or postmenopausal? Polycystic ovary syndrome (PCOS) accounts for up to three-fourths of premenopausal hirsutism.3 The likelihood of hirsutism is actually decreased in postmenopausal women because estrogen levels can drop abruptly after menopause. That said, conditions linked to hirsutism in postmenopausal women include adrenal hyperplasia, thyroid dysfunction, Cushing syndrome, and least frequently, androgen-secreting tumors (seen in this patient). (Hirsutism can also be idiopathic or iatrogenic [medications].)
Methods for detection
Research suggests that when a female patient is given a diagnosis of hirsutism, it’s important to explore possible underlying ovarian and/or adrenal tumors and adult-onset adrenal hyperplasia.1 The following tests and procedure can be helpful:
Serum testosterone and DHEAS. Levels of total testosterone > 200 ng/dL and/or DHEAS > 700 ng/dL are strongly indicative of androgen-secreting tumors.1
Imaging—including ultrasound, CT, or magnetic resonance imaging—can be used for evaluation of the adrenal glands and ovaries. However, imaging is often unable to identify these small tumors.4
Selective venous catheterization can be useful in the localization and lateralization of an androgen-secreting tumor, although a nondiagnostic result with this technique is not uncommon.4
Continue to: Dynamic hormonal testing
Dynamic hormonal testing may assist in determining the pathology of disease but not laterality.2 For example, testing for gonadotropin-releasing hormone agonists can be helpful because the constant administration of such agonists can lead to ovarian suppression without affecting adrenal androgen secretion.5
Testing with oral dexamethasone may induce adrenal hormonal depression of androgens and subsequent estradiol through aromatase conversion, which can help rule out an ovarian source.6 Exogenous administration of follicle-stimulating hormone or luteinizing hormone can further differentiate the source from ovarian theca or granulosa cell production.4
Treatment varies
The specific etiology of a patient’s hirsutism dictates the most appropriate treatment. For example, medication-induced hirsutism often requires discontinuation of the offending agent, whereas PCOS would necessitate appropriate nonpharmacologic and pharmacologic interventions.
For our patient, the elevated testosterone and free testosterone levels with normal DHEAS strongly suggested the presence of an androgen-secreting ovarian tumor. These findings led to a referral for bilateral salpingo-oophorectomy. The surgical gross appearance of the patient’s ovaries was unremarkable, but gross dissection and pathology of the ovaries (which were not postoperatively identified to determine laterality) showed one was larger (2.7 × 1.5 × 0.8 cm vs 3.2 × 1.4 × 1.2 cm).
The larger ovary contained an area of brown induration measuring 2.3 × 1.1 × 1.1 cm. This area corresponded to abundant eosinophilic cytoplasm with nuclear, rich, round-cell proliferation, consistent with the diagnosis of a benign ovarian Leydig cell tumor (FIGURE 2). Thus, the bilateral salpingo-oophorectomy was both diagnostic and therapeutic.

Six weeks after the surgery, blood work showed normalization of testosterone and free testosterone levels. The patient’s hirsutism completely resolved over the course of the next several months.
1. Hunter M, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Physician. 2003;67:2565-2572.
2. Alpañés M, González-Casbas JM, Sánchez J, et al. Management of postmenopausal virilization. J Clin Endocrinol Metab. 2012;97:2584-2588.
3. Bode D, Seehusen DA, Baird D. Hirsutism in women. Am Fam Physician. 2012;85:373-380.
4. Cohen I, Nabriski D, Fishman A. Noninvasive test for the diagnosis of ovarian hormone-secreting-neopolasm in postmenopausal women. Gynecol Oncol Rep. 2016;15:12-15.
5. Gandrapu B, Sundar P, Phillips B. Hyperandrogenism in a postmenaupsal woman secondary to testosterone secreting ovarian stromal tumor with acoustic schwannoma. Case Rep Endocrinol. 2018;2018:8154513.
6. Curran DR, Moore C, Huber T. What is the best approach to the evaluation of hirsutism? J Fam Pract. 2005;54:458-473.
1. Hunter M, Carek PJ. Evaluation and treatment of women with hirsutism. Am Fam Physician. 2003;67:2565-2572.
2. Alpañés M, González-Casbas JM, Sánchez J, et al. Management of postmenopausal virilization. J Clin Endocrinol Metab. 2012;97:2584-2588.
3. Bode D, Seehusen DA, Baird D. Hirsutism in women. Am Fam Physician. 2012;85:373-380.
4. Cohen I, Nabriski D, Fishman A. Noninvasive test for the diagnosis of ovarian hormone-secreting-neopolasm in postmenopausal women. Gynecol Oncol Rep. 2016;15:12-15.
5. Gandrapu B, Sundar P, Phillips B. Hyperandrogenism in a postmenaupsal woman secondary to testosterone secreting ovarian stromal tumor with acoustic schwannoma. Case Rep Endocrinol. 2018;2018:8154513.
6. Curran DR, Moore C, Huber T. What is the best approach to the evaluation of hirsutism? J Fam Pract. 2005;54:458-473.