User login
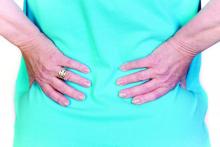
Telehealth for heart failure during pandemic shown effective, safe
The rapid transition to and reliance on telehealth to manage patients with heart failure during the COVID-19 pandemic does not appear to impact clinical outcomes, according to real-world data.
HF outpatients managed with telehealth visits did not show a significantly higher adjusted risk for subsequent ED visits, hospital admissions, intensive care use, or death at 30 and 90 days, the investigators reported in JACC: Heart Failure.
“Telehealth is safe and effective in probably some of our highest-risk patients who traditionally have needed hands-on, in-person assessment and evaluation – those patients who have heart failure – so we shouldn’t be afraid to use it all the time, not when needed as a minimum,” senior author Brett W. Sperry, MD, said in an interview.
Heart failure is a perfect case example to examine telehealth because the chronic condition not only requires continual assessment and medication adjustments, but HF patients are also particularly vulnerable to complications related to COVID-19 infection, he noted. A small, single-center report on telehealth early in Italy’s outbreak showed fewer HF hospitalizations and similar mortality, compared with in-person visits in 2019 but, overall, few data exist.
The current analysis took a wider sweep, comparing HF patients seen from March 15 to June 15, 2020 with those seen during the same time period in 2018 and 2019 at 16 cardiology clinics in Saint Luke’s Health System, which serves the Kansas City metro area and surrounding suburbs in Missouri and Kansas.
Among 8,263 unique patients and 15,421 visits identified, telehealth was not used in 2018 or 2019 but accounted for 88.5% of visits during the study period in 2020, 70% of which were by telephone and 30% of which were by video.
“We had zero telehealth before March 2020 and basically built an entire telehealth apparatus in a week or 2,” explained Dr. Sperry. “Initially it was a lot of telephone visits while we were getting the video stuff figured out, which is reflected in the paper, and then went to mostly video visits.”
Despite the pandemic, however, more outpatients were seen in 2020 than in 2018 and 2019 (4,063 vs. 3675 and 3,619 patients, respectively). This likely reflects the shift of personnel and resources from hospital duties to outpatient virtual visits, which were strongly recommended by the Heart Failure Society of America and other professional societies to manage patients during the pandemic, he said.
Unadjusted analyses demonstrated fewer ED visits and hospital admissions and more ICU admissions and all-cause mortality in 2020 than in previous years.
A propensity-matched analysis involving 4541 pairs of patients, however, showed admissions to the ED or hospital were lower after the telehealth visits than after in-person visits at 30 days (6.8% vs 10.4%; P < .001) and 90 days (17.9% vs. 23.3%; P < .001).
Among hospitalized patients, there was no difference between telehealth and in-patient visits in ICU admissions at 30 or 90 days. Mortality was also similar at 30 days (0.8% vs. 0.7%; P = .465) and 90 days (2.9% vs. 2.4%; P = .133).
Dr. Sperry said the pendulum has swung since 2020 and that the team is back to seeing most people in person, with about 15% of his clinic visits that day done via video. Standardized quality of life assessments prior to outpatient visits can help triage patients to telehealth in-patient visits, but in-person visits will still be needed for cases with greater acuity, older patients, and those with limited or no access to quality telephone videos or the internet.
“It isn’t for everyone,” Dr. Sperry said. “You’re going to need some kind of hybrid model with both in-person and video visits available and be able to offer both for patients and be able to titrate that as the pandemic changes in the future.”
Ankit Bhatia, MD, an advanced HF cardiologist at Christ Hospital in Cincinnati, who was not part of the study, said in an interview the use of telehealth in 85% of patients may be higher than the norm at most centers but that the study provides much-needed data.
“I’m really appreciative of a study like this because we were all in such a rush last year to get patients seen that very few people thought how could we design a study to really ensure we’re treating our patients within an equipoise with prior practices,” he said.
“The fact that they were able to do that [85%] and demonstrate in a propensity-matched analysis that outcomes were similar really just shows that telehealth is a strategy that we can use well in patients with heart failure to extend our ability to take care of them,” said Dr. Bhatia, a member of the American College of Cardiology Health Care Innovation Council.
Even beyond the pandemic, he said, the trend in health care is for patients to want health care delivered closer to home and for health care systems to become more patient centric. “This accelerated that but what I think this study showed me was that it’s okay to have this be part of my care model and I’m not sacrificing on my patient care if I choose to intersperse telehealth with inpatient visits.”
Besides the inherent limitations of retrospective studies, the authors noted that diagnoses in the study were based on ICD-10 codes and that subsequent ED visits or hospitalizations outside the single system may have been underreported. A further limitation is that they could not identify the cause of death or reasons for hospital encounters.
“Further data are needed to confirm the relative safety of a telehealth strategy in the HF population over a more sustained period of time, although we hypothesize that greater risks would be observed early after telehealth visits, where patients’ acuity might be misjudged,” they wrote.
Dr. Sperry is a consultant to Pfizer and Alnylam. Coauthor John A. Spertus is the principal investigator of grants from National Institutes of Health, Abbott Vascular, and the American College of Cardiology Foundation; is a consultant to Janssen, Novartis, Amgen, Myokardia, AstraZeneca, Bayer, and Merck; serves on the scientific advisory board of United Healthcare and the board of directors for Blue Cross Blue Shield of Kansas City; owns the copyright to the Kansas City Cardiomyopathy Questionnaire, Seattle Angina Questionnaire, and Peripheral Artery Questionnaire; and has an equity interest in Health Outcomes Sciences. All other authors and Dr. Bhatia reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
The rapid transition to and reliance on telehealth to manage patients with heart failure during the COVID-19 pandemic does not appear to impact clinical outcomes, according to real-world data.
HF outpatients managed with telehealth visits did not show a significantly higher adjusted risk for subsequent ED visits, hospital admissions, intensive care use, or death at 30 and 90 days, the investigators reported in JACC: Heart Failure.
“Telehealth is safe and effective in probably some of our highest-risk patients who traditionally have needed hands-on, in-person assessment and evaluation – those patients who have heart failure – so we shouldn’t be afraid to use it all the time, not when needed as a minimum,” senior author Brett W. Sperry, MD, said in an interview.
Heart failure is a perfect case example to examine telehealth because the chronic condition not only requires continual assessment and medication adjustments, but HF patients are also particularly vulnerable to complications related to COVID-19 infection, he noted. A small, single-center report on telehealth early in Italy’s outbreak showed fewer HF hospitalizations and similar mortality, compared with in-person visits in 2019 but, overall, few data exist.
The current analysis took a wider sweep, comparing HF patients seen from March 15 to June 15, 2020 with those seen during the same time period in 2018 and 2019 at 16 cardiology clinics in Saint Luke’s Health System, which serves the Kansas City metro area and surrounding suburbs in Missouri and Kansas.
Among 8,263 unique patients and 15,421 visits identified, telehealth was not used in 2018 or 2019 but accounted for 88.5% of visits during the study period in 2020, 70% of which were by telephone and 30% of which were by video.
“We had zero telehealth before March 2020 and basically built an entire telehealth apparatus in a week or 2,” explained Dr. Sperry. “Initially it was a lot of telephone visits while we were getting the video stuff figured out, which is reflected in the paper, and then went to mostly video visits.”
Despite the pandemic, however, more outpatients were seen in 2020 than in 2018 and 2019 (4,063 vs. 3675 and 3,619 patients, respectively). This likely reflects the shift of personnel and resources from hospital duties to outpatient virtual visits, which were strongly recommended by the Heart Failure Society of America and other professional societies to manage patients during the pandemic, he said.
Unadjusted analyses demonstrated fewer ED visits and hospital admissions and more ICU admissions and all-cause mortality in 2020 than in previous years.
A propensity-matched analysis involving 4541 pairs of patients, however, showed admissions to the ED or hospital were lower after the telehealth visits than after in-person visits at 30 days (6.8% vs 10.4%; P < .001) and 90 days (17.9% vs. 23.3%; P < .001).
Among hospitalized patients, there was no difference between telehealth and in-patient visits in ICU admissions at 30 or 90 days. Mortality was also similar at 30 days (0.8% vs. 0.7%; P = .465) and 90 days (2.9% vs. 2.4%; P = .133).
Dr. Sperry said the pendulum has swung since 2020 and that the team is back to seeing most people in person, with about 15% of his clinic visits that day done via video. Standardized quality of life assessments prior to outpatient visits can help triage patients to telehealth in-patient visits, but in-person visits will still be needed for cases with greater acuity, older patients, and those with limited or no access to quality telephone videos or the internet.
“It isn’t for everyone,” Dr. Sperry said. “You’re going to need some kind of hybrid model with both in-person and video visits available and be able to offer both for patients and be able to titrate that as the pandemic changes in the future.”
Ankit Bhatia, MD, an advanced HF cardiologist at Christ Hospital in Cincinnati, who was not part of the study, said in an interview the use of telehealth in 85% of patients may be higher than the norm at most centers but that the study provides much-needed data.
“I’m really appreciative of a study like this because we were all in such a rush last year to get patients seen that very few people thought how could we design a study to really ensure we’re treating our patients within an equipoise with prior practices,” he said.
“The fact that they were able to do that [85%] and demonstrate in a propensity-matched analysis that outcomes were similar really just shows that telehealth is a strategy that we can use well in patients with heart failure to extend our ability to take care of them,” said Dr. Bhatia, a member of the American College of Cardiology Health Care Innovation Council.
Even beyond the pandemic, he said, the trend in health care is for patients to want health care delivered closer to home and for health care systems to become more patient centric. “This accelerated that but what I think this study showed me was that it’s okay to have this be part of my care model and I’m not sacrificing on my patient care if I choose to intersperse telehealth with inpatient visits.”
Besides the inherent limitations of retrospective studies, the authors noted that diagnoses in the study were based on ICD-10 codes and that subsequent ED visits or hospitalizations outside the single system may have been underreported. A further limitation is that they could not identify the cause of death or reasons for hospital encounters.
“Further data are needed to confirm the relative safety of a telehealth strategy in the HF population over a more sustained period of time, although we hypothesize that greater risks would be observed early after telehealth visits, where patients’ acuity might be misjudged,” they wrote.
Dr. Sperry is a consultant to Pfizer and Alnylam. Coauthor John A. Spertus is the principal investigator of grants from National Institutes of Health, Abbott Vascular, and the American College of Cardiology Foundation; is a consultant to Janssen, Novartis, Amgen, Myokardia, AstraZeneca, Bayer, and Merck; serves on the scientific advisory board of United Healthcare and the board of directors for Blue Cross Blue Shield of Kansas City; owns the copyright to the Kansas City Cardiomyopathy Questionnaire, Seattle Angina Questionnaire, and Peripheral Artery Questionnaire; and has an equity interest in Health Outcomes Sciences. All other authors and Dr. Bhatia reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
The rapid transition to and reliance on telehealth to manage patients with heart failure during the COVID-19 pandemic does not appear to impact clinical outcomes, according to real-world data.
HF outpatients managed with telehealth visits did not show a significantly higher adjusted risk for subsequent ED visits, hospital admissions, intensive care use, or death at 30 and 90 days, the investigators reported in JACC: Heart Failure.
“Telehealth is safe and effective in probably some of our highest-risk patients who traditionally have needed hands-on, in-person assessment and evaluation – those patients who have heart failure – so we shouldn’t be afraid to use it all the time, not when needed as a minimum,” senior author Brett W. Sperry, MD, said in an interview.
Heart failure is a perfect case example to examine telehealth because the chronic condition not only requires continual assessment and medication adjustments, but HF patients are also particularly vulnerable to complications related to COVID-19 infection, he noted. A small, single-center report on telehealth early in Italy’s outbreak showed fewer HF hospitalizations and similar mortality, compared with in-person visits in 2019 but, overall, few data exist.
The current analysis took a wider sweep, comparing HF patients seen from March 15 to June 15, 2020 with those seen during the same time period in 2018 and 2019 at 16 cardiology clinics in Saint Luke’s Health System, which serves the Kansas City metro area and surrounding suburbs in Missouri and Kansas.
Among 8,263 unique patients and 15,421 visits identified, telehealth was not used in 2018 or 2019 but accounted for 88.5% of visits during the study period in 2020, 70% of which were by telephone and 30% of which were by video.
“We had zero telehealth before March 2020 and basically built an entire telehealth apparatus in a week or 2,” explained Dr. Sperry. “Initially it was a lot of telephone visits while we were getting the video stuff figured out, which is reflected in the paper, and then went to mostly video visits.”
Despite the pandemic, however, more outpatients were seen in 2020 than in 2018 and 2019 (4,063 vs. 3675 and 3,619 patients, respectively). This likely reflects the shift of personnel and resources from hospital duties to outpatient virtual visits, which were strongly recommended by the Heart Failure Society of America and other professional societies to manage patients during the pandemic, he said.
Unadjusted analyses demonstrated fewer ED visits and hospital admissions and more ICU admissions and all-cause mortality in 2020 than in previous years.
A propensity-matched analysis involving 4541 pairs of patients, however, showed admissions to the ED or hospital were lower after the telehealth visits than after in-person visits at 30 days (6.8% vs 10.4%; P < .001) and 90 days (17.9% vs. 23.3%; P < .001).
Among hospitalized patients, there was no difference between telehealth and in-patient visits in ICU admissions at 30 or 90 days. Mortality was also similar at 30 days (0.8% vs. 0.7%; P = .465) and 90 days (2.9% vs. 2.4%; P = .133).
Dr. Sperry said the pendulum has swung since 2020 and that the team is back to seeing most people in person, with about 15% of his clinic visits that day done via video. Standardized quality of life assessments prior to outpatient visits can help triage patients to telehealth in-patient visits, but in-person visits will still be needed for cases with greater acuity, older patients, and those with limited or no access to quality telephone videos or the internet.
“It isn’t for everyone,” Dr. Sperry said. “You’re going to need some kind of hybrid model with both in-person and video visits available and be able to offer both for patients and be able to titrate that as the pandemic changes in the future.”
Ankit Bhatia, MD, an advanced HF cardiologist at Christ Hospital in Cincinnati, who was not part of the study, said in an interview the use of telehealth in 85% of patients may be higher than the norm at most centers but that the study provides much-needed data.
“I’m really appreciative of a study like this because we were all in such a rush last year to get patients seen that very few people thought how could we design a study to really ensure we’re treating our patients within an equipoise with prior practices,” he said.
“The fact that they were able to do that [85%] and demonstrate in a propensity-matched analysis that outcomes were similar really just shows that telehealth is a strategy that we can use well in patients with heart failure to extend our ability to take care of them,” said Dr. Bhatia, a member of the American College of Cardiology Health Care Innovation Council.
Even beyond the pandemic, he said, the trend in health care is for patients to want health care delivered closer to home and for health care systems to become more patient centric. “This accelerated that but what I think this study showed me was that it’s okay to have this be part of my care model and I’m not sacrificing on my patient care if I choose to intersperse telehealth with inpatient visits.”
Besides the inherent limitations of retrospective studies, the authors noted that diagnoses in the study were based on ICD-10 codes and that subsequent ED visits or hospitalizations outside the single system may have been underreported. A further limitation is that they could not identify the cause of death or reasons for hospital encounters.
“Further data are needed to confirm the relative safety of a telehealth strategy in the HF population over a more sustained period of time, although we hypothesize that greater risks would be observed early after telehealth visits, where patients’ acuity might be misjudged,” they wrote.
Dr. Sperry is a consultant to Pfizer and Alnylam. Coauthor John A. Spertus is the principal investigator of grants from National Institutes of Health, Abbott Vascular, and the American College of Cardiology Foundation; is a consultant to Janssen, Novartis, Amgen, Myokardia, AstraZeneca, Bayer, and Merck; serves on the scientific advisory board of United Healthcare and the board of directors for Blue Cross Blue Shield of Kansas City; owns the copyright to the Kansas City Cardiomyopathy Questionnaire, Seattle Angina Questionnaire, and Peripheral Artery Questionnaire; and has an equity interest in Health Outcomes Sciences. All other authors and Dr. Bhatia reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
Steroid a promising short-term treatment option for major depression?
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
FROM ECNP 2021
Early-Stage NSCLC Highlights From ESMO 2021
Benjamin Cooper, MD, director of Proton Therapy services at NYU Langone Health, shares key findings from early-stage non-small cell lung cancer (NSCLC) trials presented at the 2021 ESMO Congress.
Dr Cooper begins with the LungART trial, which evaluated whether postoperative radiotherapy (PORT) would benefit patients with completely resected NSCLC and mediastinal N2 involvement. Use of PORT reduced the risk of mediastinal relapse but did not show significant impact on disease-free survival (DFS).
Next, he turns to findings from the COAST trial, which compared durvalumab monotherapy, durvalumab plus oleclumab, and durvalumab plus monalizumab in patients with locally advanced, unresectable stage III NSCLC. Both combination regimens increased the objective response rate and significantly improved progression-free survival (PFS) vs durvalumab alone.
Dr Cooper also reviews sites of disease relapse and post-relapse treatment from IMpower010, which evaluated atezolizumab versus best supportive care after adjuvant chemotherapy in patients with resected stage IB-IIIA NSCLC. Similar patterns of relapse were seen across study arms, but patients with PD-L1 levels of 50% or higher experienced greatest DFS benefits.
Lastly, Dr Cooper highlights GEMSTONE-301, which tested the novel anti-PD-L1 drug sugemalimab in patients with unresectable, stage III NSCLC who did not progress after concurrent or sequential radiotherapy. There was a statistically significant and clinically meaningful PFS improvement in patients receiving sugemalimab compared to placebo.
--
Benjamin Cooper, MD, Assistant Professor, Department of Radiation Oncology, Director, Proton Therapy Services, NYU Grossman School of Medicine, New York, New York
Benjamin Cooper, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca.
Benjamin Cooper, MD, director of Proton Therapy services at NYU Langone Health, shares key findings from early-stage non-small cell lung cancer (NSCLC) trials presented at the 2021 ESMO Congress.
Dr Cooper begins with the LungART trial, which evaluated whether postoperative radiotherapy (PORT) would benefit patients with completely resected NSCLC and mediastinal N2 involvement. Use of PORT reduced the risk of mediastinal relapse but did not show significant impact on disease-free survival (DFS).
Next, he turns to findings from the COAST trial, which compared durvalumab monotherapy, durvalumab plus oleclumab, and durvalumab plus monalizumab in patients with locally advanced, unresectable stage III NSCLC. Both combination regimens increased the objective response rate and significantly improved progression-free survival (PFS) vs durvalumab alone.
Dr Cooper also reviews sites of disease relapse and post-relapse treatment from IMpower010, which evaluated atezolizumab versus best supportive care after adjuvant chemotherapy in patients with resected stage IB-IIIA NSCLC. Similar patterns of relapse were seen across study arms, but patients with PD-L1 levels of 50% or higher experienced greatest DFS benefits.
Lastly, Dr Cooper highlights GEMSTONE-301, which tested the novel anti-PD-L1 drug sugemalimab in patients with unresectable, stage III NSCLC who did not progress after concurrent or sequential radiotherapy. There was a statistically significant and clinically meaningful PFS improvement in patients receiving sugemalimab compared to placebo.
--
Benjamin Cooper, MD, Assistant Professor, Department of Radiation Oncology, Director, Proton Therapy Services, NYU Grossman School of Medicine, New York, New York
Benjamin Cooper, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca.
Benjamin Cooper, MD, director of Proton Therapy services at NYU Langone Health, shares key findings from early-stage non-small cell lung cancer (NSCLC) trials presented at the 2021 ESMO Congress.
Dr Cooper begins with the LungART trial, which evaluated whether postoperative radiotherapy (PORT) would benefit patients with completely resected NSCLC and mediastinal N2 involvement. Use of PORT reduced the risk of mediastinal relapse but did not show significant impact on disease-free survival (DFS).
Next, he turns to findings from the COAST trial, which compared durvalumab monotherapy, durvalumab plus oleclumab, and durvalumab plus monalizumab in patients with locally advanced, unresectable stage III NSCLC. Both combination regimens increased the objective response rate and significantly improved progression-free survival (PFS) vs durvalumab alone.
Dr Cooper also reviews sites of disease relapse and post-relapse treatment from IMpower010, which evaluated atezolizumab versus best supportive care after adjuvant chemotherapy in patients with resected stage IB-IIIA NSCLC. Similar patterns of relapse were seen across study arms, but patients with PD-L1 levels of 50% or higher experienced greatest DFS benefits.
Lastly, Dr Cooper highlights GEMSTONE-301, which tested the novel anti-PD-L1 drug sugemalimab in patients with unresectable, stage III NSCLC who did not progress after concurrent or sequential radiotherapy. There was a statistically significant and clinically meaningful PFS improvement in patients receiving sugemalimab compared to placebo.
--
Benjamin Cooper, MD, Assistant Professor, Department of Radiation Oncology, Director, Proton Therapy Services, NYU Grossman School of Medicine, New York, New York
Benjamin Cooper, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca.

FDA approves avacopan for rare ANCA autoimmune disease
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
FDA issues warning about use of dermal fillers with needle-free devices
.
Specifically, the warning advises consumers and health care professionals “not to use needle-free devices such as hyaluron pens for injection of hyaluronic acid (HA) or other lip and facial fillers, collectively and commonly referred to as dermal fillers or fillers.”
According to the statement, the agency “is aware of serious injuries and in some cases, permanent harm to the skin, lips, or eyes with the use of needle-free devices for injection of fillers.”
Needle-free devices and lip and facial fillers for use with these devices are being sold directly to consumers online, and are promoted on social media “to increase lip volume, improve the appearance of wrinkles, change the shape of the nose, and other similar procedures,” according to the FDA warning.
The FDA points out that FDA-approved dermal fillers are for prescription use only, and should be administered only by licensed health care professionals using a syringe with a needle or cannula, and advises consumers not to buy or use lip or facial fillers sold directly to the public.
These products may be contaminated with infectious agents or chemicals. Moreover, “needle-free injection devices for aesthetic purposes do not provide enough control over where the injected product is placed,” the statement adds. In addition to infections, other risks include bleeding and bruising, formation of lumps, allergic reactions, blockage of a blood vessel (which can result in necrosis, blindness, or stroke), and transmission of diseases from sharing devices.
The FDA’s recommendations for health care providers include not using any aesthetic fillers with a needle-free device, and not using approved dermal fillers in such devices.
The American Society for Dermatologic Surgery Association (ASDSA) commended the FDA on the safety communication in a statement issued on October 11. In February, the ASDSA issued an alert about children using hyaluron pens to self-inject hyaluronic filler into the epidermal and upper dermal skin layers.
“I am pleased that the FDA has taken notice of this disturbing new trend, especially that of children using these devices on social media,” ASDSA president Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center, at Massachusetts General Hospital, Boston, said in the statement. “The complexity of facial anatomy requires in-depth knowledge and expertise, and patients should always have medical procedures done by a physician who also has knowledge of adverse events,” he added, urging consumers to see a board-certified dermatologist before undergoing any cosmetic procedure.
In response to a query, an FDA spokesperson did not have an estimate of the number of reports of these adverse events.
People who have problems or are concerned about having had a filler injected with a needle-free device should contact a licensed health care provider. Consumers and health care professionals should report adverse events related to injection of fillers with a needle-free device to the FDA’s MedWatch program. In addition to MedWatch, adverse events can also be reported to the Cutaneous Procedures Adverse Events Reporting (CAPER) Registry, established earlier this year by the ASDSA with the department of dermatology at Northwestern University, Chicago.
*This story was updated on October 12.
.
Specifically, the warning advises consumers and health care professionals “not to use needle-free devices such as hyaluron pens for injection of hyaluronic acid (HA) or other lip and facial fillers, collectively and commonly referred to as dermal fillers or fillers.”
According to the statement, the agency “is aware of serious injuries and in some cases, permanent harm to the skin, lips, or eyes with the use of needle-free devices for injection of fillers.”
Needle-free devices and lip and facial fillers for use with these devices are being sold directly to consumers online, and are promoted on social media “to increase lip volume, improve the appearance of wrinkles, change the shape of the nose, and other similar procedures,” according to the FDA warning.
The FDA points out that FDA-approved dermal fillers are for prescription use only, and should be administered only by licensed health care professionals using a syringe with a needle or cannula, and advises consumers not to buy or use lip or facial fillers sold directly to the public.
These products may be contaminated with infectious agents or chemicals. Moreover, “needle-free injection devices for aesthetic purposes do not provide enough control over where the injected product is placed,” the statement adds. In addition to infections, other risks include bleeding and bruising, formation of lumps, allergic reactions, blockage of a blood vessel (which can result in necrosis, blindness, or stroke), and transmission of diseases from sharing devices.
The FDA’s recommendations for health care providers include not using any aesthetic fillers with a needle-free device, and not using approved dermal fillers in such devices.
The American Society for Dermatologic Surgery Association (ASDSA) commended the FDA on the safety communication in a statement issued on October 11. In February, the ASDSA issued an alert about children using hyaluron pens to self-inject hyaluronic filler into the epidermal and upper dermal skin layers.
“I am pleased that the FDA has taken notice of this disturbing new trend, especially that of children using these devices on social media,” ASDSA president Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center, at Massachusetts General Hospital, Boston, said in the statement. “The complexity of facial anatomy requires in-depth knowledge and expertise, and patients should always have medical procedures done by a physician who also has knowledge of adverse events,” he added, urging consumers to see a board-certified dermatologist before undergoing any cosmetic procedure.
In response to a query, an FDA spokesperson did not have an estimate of the number of reports of these adverse events.
People who have problems or are concerned about having had a filler injected with a needle-free device should contact a licensed health care provider. Consumers and health care professionals should report adverse events related to injection of fillers with a needle-free device to the FDA’s MedWatch program. In addition to MedWatch, adverse events can also be reported to the Cutaneous Procedures Adverse Events Reporting (CAPER) Registry, established earlier this year by the ASDSA with the department of dermatology at Northwestern University, Chicago.
*This story was updated on October 12.
.
Specifically, the warning advises consumers and health care professionals “not to use needle-free devices such as hyaluron pens for injection of hyaluronic acid (HA) or other lip and facial fillers, collectively and commonly referred to as dermal fillers or fillers.”
According to the statement, the agency “is aware of serious injuries and in some cases, permanent harm to the skin, lips, or eyes with the use of needle-free devices for injection of fillers.”
Needle-free devices and lip and facial fillers for use with these devices are being sold directly to consumers online, and are promoted on social media “to increase lip volume, improve the appearance of wrinkles, change the shape of the nose, and other similar procedures,” according to the FDA warning.
The FDA points out that FDA-approved dermal fillers are for prescription use only, and should be administered only by licensed health care professionals using a syringe with a needle or cannula, and advises consumers not to buy or use lip or facial fillers sold directly to the public.
These products may be contaminated with infectious agents or chemicals. Moreover, “needle-free injection devices for aesthetic purposes do not provide enough control over where the injected product is placed,” the statement adds. In addition to infections, other risks include bleeding and bruising, formation of lumps, allergic reactions, blockage of a blood vessel (which can result in necrosis, blindness, or stroke), and transmission of diseases from sharing devices.
The FDA’s recommendations for health care providers include not using any aesthetic fillers with a needle-free device, and not using approved dermal fillers in such devices.
The American Society for Dermatologic Surgery Association (ASDSA) commended the FDA on the safety communication in a statement issued on October 11. In February, the ASDSA issued an alert about children using hyaluron pens to self-inject hyaluronic filler into the epidermal and upper dermal skin layers.
“I am pleased that the FDA has taken notice of this disturbing new trend, especially that of children using these devices on social media,” ASDSA president Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center, at Massachusetts General Hospital, Boston, said in the statement. “The complexity of facial anatomy requires in-depth knowledge and expertise, and patients should always have medical procedures done by a physician who also has knowledge of adverse events,” he added, urging consumers to see a board-certified dermatologist before undergoing any cosmetic procedure.
In response to a query, an FDA spokesperson did not have an estimate of the number of reports of these adverse events.
People who have problems or are concerned about having had a filler injected with a needle-free device should contact a licensed health care provider. Consumers and health care professionals should report adverse events related to injection of fillers with a needle-free device to the FDA’s MedWatch program. In addition to MedWatch, adverse events can also be reported to the Cutaneous Procedures Adverse Events Reporting (CAPER) Registry, established earlier this year by the ASDSA with the department of dermatology at Northwestern University, Chicago.
*This story was updated on October 12.
Psychiatrists shift stance on gender dysphoria, recommend therapy
A new position statement from the Royal Australian and New Zealand College of Psychiatrists (RANZCP) stresses the importance of a mental health evaluation for people with gender dysphoria – in particular for children and adolescents – before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries, often referred to as “gender-affirming care.”
“There is a paucity of quality evidence on the outcomes of those presenting with gender dysphoria. In particular, there is a need for better evidence in relation to outcomes for children and young people,” the guidance states.
Because gender dysphoria “is associated with significant distress ... each case should be assessed by a mental health professional, which will frequently be a psychiatrist, with the person at the center of care. It is important the psychological state and context in which gender dysphoria has arisen is explored to assess the most appropriate treatment,” it adds.
The move by the psychiatry body represents a big shift in the landscape regarding recommendations for the treatment of gender dysphoria in Australia and New Zealand.
Asked to explain the new RANZCP position, Philip Morris, MBBS, FRANZCP, said: “The College acknowledged the complexity of the issues and the legitimacy of different approaches.”
Exploration of a patient’s reasons for identifying as transgender is essential, he said in an interview, especially when it comes to young people.
“There may be other reasons for doing it, and we need to look for those, identify them and treat them. This needs to be done before initiating hormones and changing the whole physical nature of the child,” he said.
“A cautious psychotherapy-first approach makes sense. If we can do that with adolescents, then we will take a big step in the right direction,” stressed Dr. Morris, who is president of the National Association of Practising Psychiatrists in Australia.
Keira Bell case and Scandinavian stance lead to more open discussion
The rapid rise in gender dysphoria among adolescents in the Western world, referred to as “rapid-onset” or “late-onset” gender dysphoria, has seen a huge increase in the number of natal girls presenting and created frenzied debate that has intensified worldwide in the last 12 months about how to best treat youth with gender dysphoria.
Concerns have arisen that some transgender identification is due to social contagion, and there is a growing number of “detransitioners” – people who identified as transgender, transitioned to the opposite gender, but then regretted their decision, changed their minds, and “detransitioned” back to their birth sex. If they have had hormone therapy, and in some cases surgery, they are left with irreversible changes to their bodies.
As a result, Scandinavian countries, most notably Finland, once eager advocates of the gender-affirmative approach, have pulled back and issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
This, along with a landmark High Court decision in the U.K. regarding the use of puberty-blocking drugs for children with gender dysphoria, brought by detransitioner Keira Bell, which was recently overturned by the Appeal Court, but which Ms. Bell now says she will take to the Supreme Court, has led to a considerable shift in the conversation around treating transgender adolescents with hormonal therapy, says Dr. Morris.
“This [has moved from] ... a topic that could previously not be talked about freely to one that we can discuss more openly now. This is a big improvement. Previously, everyone thought it was all settled, but it’s not, certainly not from a medical angle,” he states.
At odds with prior Australian recommendations
The RANZCP had previously endorsed the standard guidelines of the Royal Children’s Hospital (RCH) Melbourne, followed by most gender-identity services in Australia and similar guidance from New Zealand, which both recommend gender-affirming care.
“Increasing evidence demonstrates that with supportive, gender-affirming care during childhood and adolescence, harms can be ameliorated and mental health and well-being outcomes can be significantly improved,” state the RCH guidelines.
But in 2019, RANZCP removed its endorsement of the RCH guidelines and started a consultation, which resulted in the new position statement.
However, Ken Pang, MD, of the Murdoch Children’s Research Institute in Melbourne and an author of the RCH guidelines, says the key recommendations of the new RANZCP position statement are consistent with their own guidelines.
The former note “the need for a skilled mental health clinician in providing comprehensive exploration of a child or adolescent’s biopsychosocial context,” Dr. Pang says.
However, it’s difficult not to see the contrast in stance when the new RANZCP statement maintains: “Research on gender dysphoria is still emerging. There are polarized views and mixed evidence regarding treatment options for people presenting with gender identity concerns, especially children and young people.”
Dr. Pang says the RCH guidelines do, however, recognize the need for further research in the field.
“I look forward to being able to incorporate such research, including from our own Trans20 study, into future revisions of our guidelines,” he told this news organization.
Watch your backs with affirmative therapy: Will there be a compromise?
Dr. Morris says there will obviously be cases where “the child might transition with a medical intervention, but that wouldn’t be the first step.”
And yet, he adds, “There are those who push the pro-trans view that everyone should be allowed to transition, and the doctors are only technicians that provide hormones with no questions asked.”
But from a doctor’s perspective, clinicians will still be held responsible in medical and legal terms for the treatments given, he stressed.
“I don’t think they will ever not be accountable for that. They will always need to determine in their own mind whether their actions have positive value that outweigh any disadvantages,” Dr. Morris continues.
The RANZCP statement does, in fact, stress just this.
All health care professionals need to “be aware of ethical and medicolegal dilemmas” pertaining to affirmative therapy, it indicates. “Psychiatrists should practice within the relevant laws and accepted professional standards in relation to assessing capacity and obtaining consent...”
Dr. Morris hopes there will ultimately be many more checks and balances in place and that courts and clinicians will need to step back and not assume every child who seeks to transition is doing it as a result of pure gender dysphoria.
He predicts that things will end in a compromise.
“In my view, this compromise will treat children with respect and approach them like any other patient that presents with a condition that requires proper assessment and treatment.”
“In the end, some cases will be transitioned, but there will be fewer than [are] transitioned at the moment,” he predicts.
Dr. Morris has reported no relevant financial relationships. Dr. Pang is a member of the Australian Professional Association for Trans Health and its research committee.
A version of this article first appeared on Medscape.com.
A new position statement from the Royal Australian and New Zealand College of Psychiatrists (RANZCP) stresses the importance of a mental health evaluation for people with gender dysphoria – in particular for children and adolescents – before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries, often referred to as “gender-affirming care.”
“There is a paucity of quality evidence on the outcomes of those presenting with gender dysphoria. In particular, there is a need for better evidence in relation to outcomes for children and young people,” the guidance states.
Because gender dysphoria “is associated with significant distress ... each case should be assessed by a mental health professional, which will frequently be a psychiatrist, with the person at the center of care. It is important the psychological state and context in which gender dysphoria has arisen is explored to assess the most appropriate treatment,” it adds.
The move by the psychiatry body represents a big shift in the landscape regarding recommendations for the treatment of gender dysphoria in Australia and New Zealand.
Asked to explain the new RANZCP position, Philip Morris, MBBS, FRANZCP, said: “The College acknowledged the complexity of the issues and the legitimacy of different approaches.”
Exploration of a patient’s reasons for identifying as transgender is essential, he said in an interview, especially when it comes to young people.
“There may be other reasons for doing it, and we need to look for those, identify them and treat them. This needs to be done before initiating hormones and changing the whole physical nature of the child,” he said.
“A cautious psychotherapy-first approach makes sense. If we can do that with adolescents, then we will take a big step in the right direction,” stressed Dr. Morris, who is president of the National Association of Practising Psychiatrists in Australia.
Keira Bell case and Scandinavian stance lead to more open discussion
The rapid rise in gender dysphoria among adolescents in the Western world, referred to as “rapid-onset” or “late-onset” gender dysphoria, has seen a huge increase in the number of natal girls presenting and created frenzied debate that has intensified worldwide in the last 12 months about how to best treat youth with gender dysphoria.
Concerns have arisen that some transgender identification is due to social contagion, and there is a growing number of “detransitioners” – people who identified as transgender, transitioned to the opposite gender, but then regretted their decision, changed their minds, and “detransitioned” back to their birth sex. If they have had hormone therapy, and in some cases surgery, they are left with irreversible changes to their bodies.
As a result, Scandinavian countries, most notably Finland, once eager advocates of the gender-affirmative approach, have pulled back and issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
This, along with a landmark High Court decision in the U.K. regarding the use of puberty-blocking drugs for children with gender dysphoria, brought by detransitioner Keira Bell, which was recently overturned by the Appeal Court, but which Ms. Bell now says she will take to the Supreme Court, has led to a considerable shift in the conversation around treating transgender adolescents with hormonal therapy, says Dr. Morris.
“This [has moved from] ... a topic that could previously not be talked about freely to one that we can discuss more openly now. This is a big improvement. Previously, everyone thought it was all settled, but it’s not, certainly not from a medical angle,” he states.
At odds with prior Australian recommendations
The RANZCP had previously endorsed the standard guidelines of the Royal Children’s Hospital (RCH) Melbourne, followed by most gender-identity services in Australia and similar guidance from New Zealand, which both recommend gender-affirming care.
“Increasing evidence demonstrates that with supportive, gender-affirming care during childhood and adolescence, harms can be ameliorated and mental health and well-being outcomes can be significantly improved,” state the RCH guidelines.
But in 2019, RANZCP removed its endorsement of the RCH guidelines and started a consultation, which resulted in the new position statement.
However, Ken Pang, MD, of the Murdoch Children’s Research Institute in Melbourne and an author of the RCH guidelines, says the key recommendations of the new RANZCP position statement are consistent with their own guidelines.
The former note “the need for a skilled mental health clinician in providing comprehensive exploration of a child or adolescent’s biopsychosocial context,” Dr. Pang says.
However, it’s difficult not to see the contrast in stance when the new RANZCP statement maintains: “Research on gender dysphoria is still emerging. There are polarized views and mixed evidence regarding treatment options for people presenting with gender identity concerns, especially children and young people.”
Dr. Pang says the RCH guidelines do, however, recognize the need for further research in the field.
“I look forward to being able to incorporate such research, including from our own Trans20 study, into future revisions of our guidelines,” he told this news organization.
Watch your backs with affirmative therapy: Will there be a compromise?
Dr. Morris says there will obviously be cases where “the child might transition with a medical intervention, but that wouldn’t be the first step.”
And yet, he adds, “There are those who push the pro-trans view that everyone should be allowed to transition, and the doctors are only technicians that provide hormones with no questions asked.”
But from a doctor’s perspective, clinicians will still be held responsible in medical and legal terms for the treatments given, he stressed.
“I don’t think they will ever not be accountable for that. They will always need to determine in their own mind whether their actions have positive value that outweigh any disadvantages,” Dr. Morris continues.
The RANZCP statement does, in fact, stress just this.
All health care professionals need to “be aware of ethical and medicolegal dilemmas” pertaining to affirmative therapy, it indicates. “Psychiatrists should practice within the relevant laws and accepted professional standards in relation to assessing capacity and obtaining consent...”
Dr. Morris hopes there will ultimately be many more checks and balances in place and that courts and clinicians will need to step back and not assume every child who seeks to transition is doing it as a result of pure gender dysphoria.
He predicts that things will end in a compromise.
“In my view, this compromise will treat children with respect and approach them like any other patient that presents with a condition that requires proper assessment and treatment.”
“In the end, some cases will be transitioned, but there will be fewer than [are] transitioned at the moment,” he predicts.
Dr. Morris has reported no relevant financial relationships. Dr. Pang is a member of the Australian Professional Association for Trans Health and its research committee.
A version of this article first appeared on Medscape.com.
A new position statement from the Royal Australian and New Zealand College of Psychiatrists (RANZCP) stresses the importance of a mental health evaluation for people with gender dysphoria – in particular for children and adolescents – before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries, often referred to as “gender-affirming care.”
“There is a paucity of quality evidence on the outcomes of those presenting with gender dysphoria. In particular, there is a need for better evidence in relation to outcomes for children and young people,” the guidance states.
Because gender dysphoria “is associated with significant distress ... each case should be assessed by a mental health professional, which will frequently be a psychiatrist, with the person at the center of care. It is important the psychological state and context in which gender dysphoria has arisen is explored to assess the most appropriate treatment,” it adds.
The move by the psychiatry body represents a big shift in the landscape regarding recommendations for the treatment of gender dysphoria in Australia and New Zealand.
Asked to explain the new RANZCP position, Philip Morris, MBBS, FRANZCP, said: “The College acknowledged the complexity of the issues and the legitimacy of different approaches.”
Exploration of a patient’s reasons for identifying as transgender is essential, he said in an interview, especially when it comes to young people.
“There may be other reasons for doing it, and we need to look for those, identify them and treat them. This needs to be done before initiating hormones and changing the whole physical nature of the child,” he said.
“A cautious psychotherapy-first approach makes sense. If we can do that with adolescents, then we will take a big step in the right direction,” stressed Dr. Morris, who is president of the National Association of Practising Psychiatrists in Australia.
Keira Bell case and Scandinavian stance lead to more open discussion
The rapid rise in gender dysphoria among adolescents in the Western world, referred to as “rapid-onset” or “late-onset” gender dysphoria, has seen a huge increase in the number of natal girls presenting and created frenzied debate that has intensified worldwide in the last 12 months about how to best treat youth with gender dysphoria.
Concerns have arisen that some transgender identification is due to social contagion, and there is a growing number of “detransitioners” – people who identified as transgender, transitioned to the opposite gender, but then regretted their decision, changed their minds, and “detransitioned” back to their birth sex. If they have had hormone therapy, and in some cases surgery, they are left with irreversible changes to their bodies.
As a result, Scandinavian countries, most notably Finland, once eager advocates of the gender-affirmative approach, have pulled back and issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
This, along with a landmark High Court decision in the U.K. regarding the use of puberty-blocking drugs for children with gender dysphoria, brought by detransitioner Keira Bell, which was recently overturned by the Appeal Court, but which Ms. Bell now says she will take to the Supreme Court, has led to a considerable shift in the conversation around treating transgender adolescents with hormonal therapy, says Dr. Morris.
“This [has moved from] ... a topic that could previously not be talked about freely to one that we can discuss more openly now. This is a big improvement. Previously, everyone thought it was all settled, but it’s not, certainly not from a medical angle,” he states.
At odds with prior Australian recommendations
The RANZCP had previously endorsed the standard guidelines of the Royal Children’s Hospital (RCH) Melbourne, followed by most gender-identity services in Australia and similar guidance from New Zealand, which both recommend gender-affirming care.
“Increasing evidence demonstrates that with supportive, gender-affirming care during childhood and adolescence, harms can be ameliorated and mental health and well-being outcomes can be significantly improved,” state the RCH guidelines.
But in 2019, RANZCP removed its endorsement of the RCH guidelines and started a consultation, which resulted in the new position statement.
However, Ken Pang, MD, of the Murdoch Children’s Research Institute in Melbourne and an author of the RCH guidelines, says the key recommendations of the new RANZCP position statement are consistent with their own guidelines.
The former note “the need for a skilled mental health clinician in providing comprehensive exploration of a child or adolescent’s biopsychosocial context,” Dr. Pang says.
However, it’s difficult not to see the contrast in stance when the new RANZCP statement maintains: “Research on gender dysphoria is still emerging. There are polarized views and mixed evidence regarding treatment options for people presenting with gender identity concerns, especially children and young people.”
Dr. Pang says the RCH guidelines do, however, recognize the need for further research in the field.
“I look forward to being able to incorporate such research, including from our own Trans20 study, into future revisions of our guidelines,” he told this news organization.
Watch your backs with affirmative therapy: Will there be a compromise?
Dr. Morris says there will obviously be cases where “the child might transition with a medical intervention, but that wouldn’t be the first step.”
And yet, he adds, “There are those who push the pro-trans view that everyone should be allowed to transition, and the doctors are only technicians that provide hormones with no questions asked.”
But from a doctor’s perspective, clinicians will still be held responsible in medical and legal terms for the treatments given, he stressed.
“I don’t think they will ever not be accountable for that. They will always need to determine in their own mind whether their actions have positive value that outweigh any disadvantages,” Dr. Morris continues.
The RANZCP statement does, in fact, stress just this.
All health care professionals need to “be aware of ethical and medicolegal dilemmas” pertaining to affirmative therapy, it indicates. “Psychiatrists should practice within the relevant laws and accepted professional standards in relation to assessing capacity and obtaining consent...”
Dr. Morris hopes there will ultimately be many more checks and balances in place and that courts and clinicians will need to step back and not assume every child who seeks to transition is doing it as a result of pure gender dysphoria.
He predicts that things will end in a compromise.
“In my view, this compromise will treat children with respect and approach them like any other patient that presents with a condition that requires proper assessment and treatment.”
“In the end, some cases will be transitioned, but there will be fewer than [are] transitioned at the moment,” he predicts.
Dr. Morris has reported no relevant financial relationships. Dr. Pang is a member of the Australian Professional Association for Trans Health and its research committee.
A version of this article first appeared on Medscape.com.
Lie down for orthostatic hypotension assessment
New research shows that supine orthostatic hypotension is more common and better predicts falls and orthostatic symptoms than seated OH, supporting a supine OH protocol in clinical practice, the researchers say.
“Older adults at risk for falls undergoing assessment for OH should lie supine rather than sitting prior to standing to get the most informative OH assessment,” study author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, said in an interview.
“The findings call for a change in current practice,” Dr. Juraschek said.
He presented the study Sept. 29 at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The seated position for detecting OH is “commonly used for convenience. Since many clinics already perform a seated blood pressure, it saves time for people to stand shortly afterward,” he explained.
“It has also been thought that the two are interchangeable [i.e., the change in blood pressure from seated to standing was just a lower magnitude than the change from supine to standing]. However, we showed that the physiology is on average quite different, questioning prior perspectives on the interchangeability of the two protocols,” he added.
The researchers studied 522 adults (mean age, 76 years; 42% women) at high risk for falls and with vitamin D levels in the insufficient/deficient range participating in the Study to Understand Fall Reduction and Vitamin D (STURDY).
The study showed that vitamin D supplementation was not associated with OH or the main study outcome of falls.
The study used two different OH assessment protocols – seated to standing and supine to standing – and Dr. Juraschek’s team used the data to gauge the impact of supine and seated positions on OH prevalence and its relation with fall risk and orthostatic symptoms.
OH was defined as a drop in systolic BP of at least 20 mm Hg or diastolic BP of at least 10 mm Hg.
At baseline, mean BP was 129/68 mm Hg. Mean BP increased 3.4/2.6 mm Hg after sitting, but decreased 3.7/0.7 mm Hg after lying supine.
Of the 953 OH assessments (supine and seated), OH was detected in 14.8% of the supine measurements but in only 2.2% of the seated measures.
Supine OH better predicted falls (hazard ratio, 1.60; 95% CI, 0.98-2.61; P = .06) than seated OH (HR, 0.70; 95% CI, 0.30-1.60; P = .39).
Although both were nonsignificant, “potentially due to power,” the association with falls was stronger for supine OH than for seated OH, Dr. Juraschek said.
In addition, seated OH was not associated with orthostatic symptoms, whereas supine OH was significantly associated with a greater risk of fainting, blacking out, seeing spots, room spinning, and headache in the previous month (P = .048-.002).
Useful study confirms anecdotal evidence
This is a “useful study” from a “reputable” group, “and the results reveal what I would have expected,” Robert Carey, MD, University of Virginia, Charlottesville, who wasn’t involved in the study, said in an interview.
The findings, Dr. Carey said, show that measuring supine, compared with standing, “actually correlates much better with the untoward effects of orthostatic hypotension which are falls and symptoms such as dizziness and spots before your eyes.”
“Seated BP is mostly used for convenience and a little bit shorter protocol. Most clinical trials do seated orthostatic hypotension measurements. I’ve always taught my medical students and others to use the supine to standing because I’ve just anecdotally felt that this was a much better way of detecting true orthostatic hypotension and that’s how we do it at the University of Virginia Hospital,” Dr. Carey said.
The study had no funding. Dr. Juraschek and Dr. Carey have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research shows that supine orthostatic hypotension is more common and better predicts falls and orthostatic symptoms than seated OH, supporting a supine OH protocol in clinical practice, the researchers say.
“Older adults at risk for falls undergoing assessment for OH should lie supine rather than sitting prior to standing to get the most informative OH assessment,” study author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, said in an interview.
“The findings call for a change in current practice,” Dr. Juraschek said.
He presented the study Sept. 29 at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The seated position for detecting OH is “commonly used for convenience. Since many clinics already perform a seated blood pressure, it saves time for people to stand shortly afterward,” he explained.
“It has also been thought that the two are interchangeable [i.e., the change in blood pressure from seated to standing was just a lower magnitude than the change from supine to standing]. However, we showed that the physiology is on average quite different, questioning prior perspectives on the interchangeability of the two protocols,” he added.
The researchers studied 522 adults (mean age, 76 years; 42% women) at high risk for falls and with vitamin D levels in the insufficient/deficient range participating in the Study to Understand Fall Reduction and Vitamin D (STURDY).
The study showed that vitamin D supplementation was not associated with OH or the main study outcome of falls.
The study used two different OH assessment protocols – seated to standing and supine to standing – and Dr. Juraschek’s team used the data to gauge the impact of supine and seated positions on OH prevalence and its relation with fall risk and orthostatic symptoms.
OH was defined as a drop in systolic BP of at least 20 mm Hg or diastolic BP of at least 10 mm Hg.
At baseline, mean BP was 129/68 mm Hg. Mean BP increased 3.4/2.6 mm Hg after sitting, but decreased 3.7/0.7 mm Hg after lying supine.
Of the 953 OH assessments (supine and seated), OH was detected in 14.8% of the supine measurements but in only 2.2% of the seated measures.
Supine OH better predicted falls (hazard ratio, 1.60; 95% CI, 0.98-2.61; P = .06) than seated OH (HR, 0.70; 95% CI, 0.30-1.60; P = .39).
Although both were nonsignificant, “potentially due to power,” the association with falls was stronger for supine OH than for seated OH, Dr. Juraschek said.
In addition, seated OH was not associated with orthostatic symptoms, whereas supine OH was significantly associated with a greater risk of fainting, blacking out, seeing spots, room spinning, and headache in the previous month (P = .048-.002).
Useful study confirms anecdotal evidence
This is a “useful study” from a “reputable” group, “and the results reveal what I would have expected,” Robert Carey, MD, University of Virginia, Charlottesville, who wasn’t involved in the study, said in an interview.
The findings, Dr. Carey said, show that measuring supine, compared with standing, “actually correlates much better with the untoward effects of orthostatic hypotension which are falls and symptoms such as dizziness and spots before your eyes.”
“Seated BP is mostly used for convenience and a little bit shorter protocol. Most clinical trials do seated orthostatic hypotension measurements. I’ve always taught my medical students and others to use the supine to standing because I’ve just anecdotally felt that this was a much better way of detecting true orthostatic hypotension and that’s how we do it at the University of Virginia Hospital,” Dr. Carey said.
The study had no funding. Dr. Juraschek and Dr. Carey have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research shows that supine orthostatic hypotension is more common and better predicts falls and orthostatic symptoms than seated OH, supporting a supine OH protocol in clinical practice, the researchers say.
“Older adults at risk for falls undergoing assessment for OH should lie supine rather than sitting prior to standing to get the most informative OH assessment,” study author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, said in an interview.
“The findings call for a change in current practice,” Dr. Juraschek said.
He presented the study Sept. 29 at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The seated position for detecting OH is “commonly used for convenience. Since many clinics already perform a seated blood pressure, it saves time for people to stand shortly afterward,” he explained.
“It has also been thought that the two are interchangeable [i.e., the change in blood pressure from seated to standing was just a lower magnitude than the change from supine to standing]. However, we showed that the physiology is on average quite different, questioning prior perspectives on the interchangeability of the two protocols,” he added.
The researchers studied 522 adults (mean age, 76 years; 42% women) at high risk for falls and with vitamin D levels in the insufficient/deficient range participating in the Study to Understand Fall Reduction and Vitamin D (STURDY).
The study showed that vitamin D supplementation was not associated with OH or the main study outcome of falls.
The study used two different OH assessment protocols – seated to standing and supine to standing – and Dr. Juraschek’s team used the data to gauge the impact of supine and seated positions on OH prevalence and its relation with fall risk and orthostatic symptoms.
OH was defined as a drop in systolic BP of at least 20 mm Hg or diastolic BP of at least 10 mm Hg.
At baseline, mean BP was 129/68 mm Hg. Mean BP increased 3.4/2.6 mm Hg after sitting, but decreased 3.7/0.7 mm Hg after lying supine.
Of the 953 OH assessments (supine and seated), OH was detected in 14.8% of the supine measurements but in only 2.2% of the seated measures.
Supine OH better predicted falls (hazard ratio, 1.60; 95% CI, 0.98-2.61; P = .06) than seated OH (HR, 0.70; 95% CI, 0.30-1.60; P = .39).
Although both were nonsignificant, “potentially due to power,” the association with falls was stronger for supine OH than for seated OH, Dr. Juraschek said.
In addition, seated OH was not associated with orthostatic symptoms, whereas supine OH was significantly associated with a greater risk of fainting, blacking out, seeing spots, room spinning, and headache in the previous month (P = .048-.002).
Useful study confirms anecdotal evidence
This is a “useful study” from a “reputable” group, “and the results reveal what I would have expected,” Robert Carey, MD, University of Virginia, Charlottesville, who wasn’t involved in the study, said in an interview.
The findings, Dr. Carey said, show that measuring supine, compared with standing, “actually correlates much better with the untoward effects of orthostatic hypotension which are falls and symptoms such as dizziness and spots before your eyes.”
“Seated BP is mostly used for convenience and a little bit shorter protocol. Most clinical trials do seated orthostatic hypotension measurements. I’ve always taught my medical students and others to use the supine to standing because I’ve just anecdotally felt that this was a much better way of detecting true orthostatic hypotension and that’s how we do it at the University of Virginia Hospital,” Dr. Carey said.
The study had no funding. Dr. Juraschek and Dr. Carey have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Adolescents who exercised after a concussion recovered faster in RCT
After a concussion, resuming aerobic exercise relatively early on – at an intensity that does not worsen symptoms – may help young athletes recover sooner, compared with stretching, a randomized controlled trial (RCT) shows.
The study adds to emerging evidence that clinicians should prescribe exercise, rather than strict rest, to facilitate concussion recovery, researchers said.
Tamara McLeod, PhD, ATC, professor and director of athletic training programs at A.T. Still University in Mesa, Ariz., hopes the findings help clinicians see that “this is an approach that should be taken.”
“Too often with concussion, patients are given a laundry list of things they are NOT allowed to do,” including sports, school, and social activities, said Dr. McLeod, who was not involved in the study.
The research, published in The Lancet Child & Adolescent Health, largely replicates the findings of a prior trial while addressing limitations of the previous study’s design, researchers said.
For the trial, John J. Leddy, MD, with the State University of New York at Buffalo and colleagues recruited 118 male and female adolescent athletes aged 13-18 years who had had a sport-related concussion in the past 10 days. Investigators at three community and hospital-affiliated sports medicine concussion centers in the United States randomly assigned the athletes to individualized subsymptom-threshold aerobic exercise (61 participants) or stretching exercise (57 participants) at least 20 minutes per day for up to 4 weeks. Aerobic exercise included walking, jogging, or stationary cycling at home.
“It is important that the general clinician community appreciates that prolonged rest and avoidance of physical activity until spontaneous symptom resolution is no longer an acceptable approach to caring for adolescents with concussion,” Dr. Leddy and coauthors said.
The investigators improved on the “the scientific rigor of their previous RCT by including intention-to-treat and per-protocol analyses, daily symptom reporting, objective exercise adherence measurements, and greater heterogeneity of concussion severity,” said Carolyn A. Emery, PhD, and Jonathan Smirl, PhD, both with the University of Calgary (Alta.), in a related commentary. The new study is the first to show that early targeted heart rate subsymptom-threshold aerobic exercise, relative to stretching, shortened recovery time within 4 weeks after sport-related concussion (hazard ratio, 0.52) when controlling for sex, study site, and average daily exercise time, Dr. Emery and Dr. Smirl said.
A larger proportion of athletes assigned to stretching did not recover by 4 weeks, compared with those assigned to aerobic exercise (32% vs. 21%). The median time to full recovery was longer for the stretching group than for the aerobic exercise group (19 days vs. 14 days).
Among athletes who adhered to their assigned regimens, the differences were more pronounced: The median recovery time was 21 days for the stretching group, compared with 12 days for the aerobic exercise group. The rate of postconcussion symptoms beyond 28 days was 9% in the aerobic exercise group versus 31% in the stretching group, among adherent participants.
More research is needed to establish the efficacy of postconcussion aerobic exercise in adults and for nonsport injury, the researchers noted. Possible mechanisms underlying aerobic exercise’s benefits could include increased parasympathetic autonomic tone, improved cerebral blood flow regulation, or enhanced neuron repair, they suggested.
The right amount and timing of exercise, and doing so at an intensity that does not exacerbate symptoms, may be key. Other research has suggested that too much exercise, too soon may delay recovery, Dr. Emery said in an interview. “But there is now a lot of evidence to support low and moderate levels of physical activity to expedite recovery,” she said.
The study was funded by the American Medical Society for Sports Medicine. The study and commentary authors and Dr. McLeod had no disclosures.
After a concussion, resuming aerobic exercise relatively early on – at an intensity that does not worsen symptoms – may help young athletes recover sooner, compared with stretching, a randomized controlled trial (RCT) shows.
The study adds to emerging evidence that clinicians should prescribe exercise, rather than strict rest, to facilitate concussion recovery, researchers said.
Tamara McLeod, PhD, ATC, professor and director of athletic training programs at A.T. Still University in Mesa, Ariz., hopes the findings help clinicians see that “this is an approach that should be taken.”
“Too often with concussion, patients are given a laundry list of things they are NOT allowed to do,” including sports, school, and social activities, said Dr. McLeod, who was not involved in the study.
The research, published in The Lancet Child & Adolescent Health, largely replicates the findings of a prior trial while addressing limitations of the previous study’s design, researchers said.
For the trial, John J. Leddy, MD, with the State University of New York at Buffalo and colleagues recruited 118 male and female adolescent athletes aged 13-18 years who had had a sport-related concussion in the past 10 days. Investigators at three community and hospital-affiliated sports medicine concussion centers in the United States randomly assigned the athletes to individualized subsymptom-threshold aerobic exercise (61 participants) or stretching exercise (57 participants) at least 20 minutes per day for up to 4 weeks. Aerobic exercise included walking, jogging, or stationary cycling at home.
“It is important that the general clinician community appreciates that prolonged rest and avoidance of physical activity until spontaneous symptom resolution is no longer an acceptable approach to caring for adolescents with concussion,” Dr. Leddy and coauthors said.
The investigators improved on the “the scientific rigor of their previous RCT by including intention-to-treat and per-protocol analyses, daily symptom reporting, objective exercise adherence measurements, and greater heterogeneity of concussion severity,” said Carolyn A. Emery, PhD, and Jonathan Smirl, PhD, both with the University of Calgary (Alta.), in a related commentary. The new study is the first to show that early targeted heart rate subsymptom-threshold aerobic exercise, relative to stretching, shortened recovery time within 4 weeks after sport-related concussion (hazard ratio, 0.52) when controlling for sex, study site, and average daily exercise time, Dr. Emery and Dr. Smirl said.
A larger proportion of athletes assigned to stretching did not recover by 4 weeks, compared with those assigned to aerobic exercise (32% vs. 21%). The median time to full recovery was longer for the stretching group than for the aerobic exercise group (19 days vs. 14 days).
Among athletes who adhered to their assigned regimens, the differences were more pronounced: The median recovery time was 21 days for the stretching group, compared with 12 days for the aerobic exercise group. The rate of postconcussion symptoms beyond 28 days was 9% in the aerobic exercise group versus 31% in the stretching group, among adherent participants.
More research is needed to establish the efficacy of postconcussion aerobic exercise in adults and for nonsport injury, the researchers noted. Possible mechanisms underlying aerobic exercise’s benefits could include increased parasympathetic autonomic tone, improved cerebral blood flow regulation, or enhanced neuron repair, they suggested.
The right amount and timing of exercise, and doing so at an intensity that does not exacerbate symptoms, may be key. Other research has suggested that too much exercise, too soon may delay recovery, Dr. Emery said in an interview. “But there is now a lot of evidence to support low and moderate levels of physical activity to expedite recovery,” she said.
The study was funded by the American Medical Society for Sports Medicine. The study and commentary authors and Dr. McLeod had no disclosures.
After a concussion, resuming aerobic exercise relatively early on – at an intensity that does not worsen symptoms – may help young athletes recover sooner, compared with stretching, a randomized controlled trial (RCT) shows.
The study adds to emerging evidence that clinicians should prescribe exercise, rather than strict rest, to facilitate concussion recovery, researchers said.
Tamara McLeod, PhD, ATC, professor and director of athletic training programs at A.T. Still University in Mesa, Ariz., hopes the findings help clinicians see that “this is an approach that should be taken.”
“Too often with concussion, patients are given a laundry list of things they are NOT allowed to do,” including sports, school, and social activities, said Dr. McLeod, who was not involved in the study.
The research, published in The Lancet Child & Adolescent Health, largely replicates the findings of a prior trial while addressing limitations of the previous study’s design, researchers said.
For the trial, John J. Leddy, MD, with the State University of New York at Buffalo and colleagues recruited 118 male and female adolescent athletes aged 13-18 years who had had a sport-related concussion in the past 10 days. Investigators at three community and hospital-affiliated sports medicine concussion centers in the United States randomly assigned the athletes to individualized subsymptom-threshold aerobic exercise (61 participants) or stretching exercise (57 participants) at least 20 minutes per day for up to 4 weeks. Aerobic exercise included walking, jogging, or stationary cycling at home.
“It is important that the general clinician community appreciates that prolonged rest and avoidance of physical activity until spontaneous symptom resolution is no longer an acceptable approach to caring for adolescents with concussion,” Dr. Leddy and coauthors said.
The investigators improved on the “the scientific rigor of their previous RCT by including intention-to-treat and per-protocol analyses, daily symptom reporting, objective exercise adherence measurements, and greater heterogeneity of concussion severity,” said Carolyn A. Emery, PhD, and Jonathan Smirl, PhD, both with the University of Calgary (Alta.), in a related commentary. The new study is the first to show that early targeted heart rate subsymptom-threshold aerobic exercise, relative to stretching, shortened recovery time within 4 weeks after sport-related concussion (hazard ratio, 0.52) when controlling for sex, study site, and average daily exercise time, Dr. Emery and Dr. Smirl said.
A larger proportion of athletes assigned to stretching did not recover by 4 weeks, compared with those assigned to aerobic exercise (32% vs. 21%). The median time to full recovery was longer for the stretching group than for the aerobic exercise group (19 days vs. 14 days).
Among athletes who adhered to their assigned regimens, the differences were more pronounced: The median recovery time was 21 days for the stretching group, compared with 12 days for the aerobic exercise group. The rate of postconcussion symptoms beyond 28 days was 9% in the aerobic exercise group versus 31% in the stretching group, among adherent participants.
More research is needed to establish the efficacy of postconcussion aerobic exercise in adults and for nonsport injury, the researchers noted. Possible mechanisms underlying aerobic exercise’s benefits could include increased parasympathetic autonomic tone, improved cerebral blood flow regulation, or enhanced neuron repair, they suggested.
The right amount and timing of exercise, and doing so at an intensity that does not exacerbate symptoms, may be key. Other research has suggested that too much exercise, too soon may delay recovery, Dr. Emery said in an interview. “But there is now a lot of evidence to support low and moderate levels of physical activity to expedite recovery,” she said.
The study was funded by the American Medical Society for Sports Medicine. The study and commentary authors and Dr. McLeod had no disclosures.
FROM THE LANCET CHILD & ADOLESCENT HEALTH
Retraining the brain may eliminate chronic back pain
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Do you use intrapartum warm compresses to the perineum or perineal massage in your practice?
[polldaddy:10937454]
[polldaddy:10937454]
[polldaddy:10937454]