User login
Pediatricians can effectively promote gun safety
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.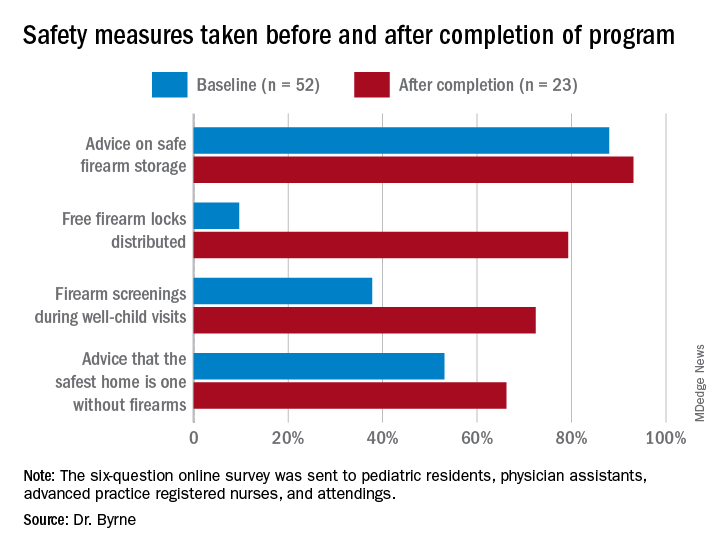
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When pediatricians and other pediatric providers are given training and resource materials, levels of firearm screenings and anticipatory guidance about firearm safety increase significantly, according to two new studies presented at the annual meeting of the American Academy of Pediatrics.
“With the rise in firearm sales and injuries during the COVID-19 pandemic, it is more important than ever that pediatricians address the firearm epidemic,” said Alexandra Byrne, MD, a pediatric resident at the University of Florida in Gainesville, who presented one of the studies.
There were 4.3 million more firearms purchased from March through July 2020 than expected, a recent study estimates, and 4,075 more firearm injuries than expected from April through July 2020.
In states with more excess purchases, firearm injuries related to domestic violence increased in April (rate ratio, 2.60; 95% CI, 1.32-5.93) and May (RR, 1.79; 95% CI, 1.19-2.91) 2020. However, excess gun purchases had no effect on rates of firearm violence outside the home.
In addition to the link between firearms in the home and domestic violence, they are also linked to a three- to fourfold greater risk for teen suicide, and both depression and suicidal thoughts have risen in teens during the pandemic.
“The data are pretty clear that if you have an unlocked, loaded weapon in your home, and you have a kid who’s depressed or anxious or dysregulated or doing maladaptive things for the pandemic, they’re much more likely to inadvertently take their own or someone else’s life by grabbing [a gun],” said Cora Breuner, MD, MPH, professor of pediatrics at Seattle Children’s Hospital.
However, there is no difference in gun ownership or gun-safety measures between homes with and without at-risk children, previous research shows.
Training, guidance, and locks
Previous research has also shown that there has been a reluctance by pediatricians to conduct firearm screenings and counsel parents about gun safety in the home.
For their two-step program, Dr. Byrne’s team used a plan-do-study-act approach. They started by providing training on firearm safety, evidence-based recommendations for firearm screening, and anticipatory guidance regarding safe firearm storage to members of the general pediatrics division at the University of Florida. And they supplied clinics with free firearm locks.
Next they supplied clinics with posters and educational cards from the Be SMART campaign, an initiative of the Everytown for Gun Safety Support Fund, which provides materials for anyone, including physicians, to use.
During their study, the researchers sent three anonymous six-question online surveys – at baseline and 3 to 4 months after each of the two steps – to pediatric residents, physician assistants, advanced practice registered nurses, and attendings to assess the project. There were 52 responses to the first survey, for a response rate of 58.4%, 42 responses to the second survey, for a response rate of 47.2%, and 23 responses to the third survey, for a rate of response 25.8%.
The program nearly doubled screenings during well-child visits and dramatically increased the proportion of families who received a firearm lock when they told providers they had a firearm at home.
Previous research has shown “a significant increase in safe firearm storage when firearm locks were provided to families in clinic compared to verbal counseling alone,” Dr. Byrne said. “We know that safe firearm storage reduces injuries. Roughly one in three children in the United States lives in a home with a firearm. Individuals with a firearm are at two times the risk of homicide and three to four times the risk of suicide, so it is essential we further study how pediatricians can be most effective when it comes to firearm counseling.”
The difference in lock distribution as a result of the program is a “tremendous increase,” said Christopher S. Greeley, MD, MS, chief of the division of public health pediatrics at Texas Children’s Hospital and professor of pediatrics at Baylor College of Medicine in Houston, who was not involved in the research.
“Locks could go a long way to minimizing the risk,” he said in an interview, adding that nearly half of all teen suicide deaths that occurred over a decade in Houston involved a firearm.
Adding a social-history component
A program to increase firearm screening was also presented at the AAP conference.
After random review of medical records from 30 patients admitted to the hospital documented zero firearm screenings, Marjorie Farrington, MD, and Samantha Gunkelman, MD, from Akron Children’s Hospital in Ohio, implemented a program that they hope will increase firearm screenings during inpatient admissions to at least 50%.
They started their ongoing program in April 2020 by adding a social-history component to the history and physical (H&P) exam template and educating residents on how to screen and included guidance on safe firearm storage.
They also had physicians with firearm expertise give gun-safety lectures, and they plan to involve the Family Resource Center at their hospital in the creation of resources that can be incorporated into discharge instructions.
From April 2020 to June 2021, after the addition to the H&P template, 63% of the 5196 patients admitted to the hospital underwent a firearm screening. Of the 25% of patients who reported guns at home, 3% were not storing their firearms safely.
The pair used the “Store It Safe” Physician Handout provided by the Ohio chapter of the AAP.
Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic.
The BulletPoints Project — developed by the Violence Prevention Research Program at the University of California, Davis — can also help physicians talk to patients about guns.
“Many pediatricians and pediatric trainees are not comfortable counseling on firearm safety, often a result of inadequate training on the topic,” Dr. Byrne said in an interview. “Additionally, it is a challenging topic that can often be met with resistance from patients and families. Lack of time during visits is also a huge barrier.”
Lack of training is an obstacle to greater firearm screenings, Dr. Greeley agreed, as are the feeling that guidance simply won’t make a difference and concerns about political pressure and divineness. The lack of research on firearm injuries and the impact of firearm screenings and anticipatory guidance is a challenge, he added, although that is starting to change.
Pediatricians need education on how to make a difference when it comes to firearm safety, and should follow AAP guidelines, Dr. Greeley said.
Counseling on firearm safety is in the same category as immunizations, seatbelts, substance use, helmets, and other public-health issues that are important to address at visits, regardless of how difficult it might be, Dr. Breuner told this news organization.
“It is our mission, as pediatricians, to provide every ounce of prevention in our well-child and anticipatory guidance visits,” she said. “It’s our job, so we shouldn’t shy away from it even though it’s hard.”
Doctors are more comfortable discussing firearm safety if they are firearm owners, previous research has shown, so she advises pediatricians who feel unqualified to discuss firearms to seek guidance from their peers on how to approach screenings and anticipatory guidance, she noted.
The firearm study being done in an academic center gives me great pause. The populations are often very different than private practice.
Both of these studies were conducted at single institutions and might not reflect what would work in private clinics.
“The firearm study being done in an academic center gives me great pause,” Dr. Greeley said. “The populations are often very different than private practice. I think that there is still a lot that remains unknown about decreasing household firearm injury and death.”
And the degree to which findings from these two gun-safety programs can be generalized to other academic centers or children’s hospitals is unclear.
“There are states where, I suspect, firearm screening is much more common. Some states have very pro-firearm cultures and others are anti-firearm,” Dr. Greeley said. “There are also likely differences within states,” particularly between urban and rural regions.
“Firearms are often a very personal issue for families, and pediatricians in ‘pro-firearm’ communities may have greater resistance to working on this,” he pointed out.
Nevertheless, Dr. Greeley said, “this is a promising strategy that could be part of a broad injury prevention initiative.”
Neither study noted any external funding. Dr. Byrne is a member of the Moms Demand Action Gainesville Chapter, which donated the firearm locks for the project. Dr. Breuner, Dr. Greeley, and Dr. Farrington have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAP 2021
Homicide remains a top cause of maternal mortality
The prevalence of homicide was 16% higher in pregnant women or postpartum women than nonpregnant or nonpostpartum women in the United States, according to 2018 and 2019 mortality data from the National Center for Health Statistics.
Homicide has long been identified as a leading cause of death during pregnancy, but homicide is not counted in estimates of maternal mortality, nor is it emphasized as a target for prevention and intervention, wrote Maeve Wallace, PhD, of Tulane University, New Orleans, and colleagues.
Data on maternal mortality (defined as “death while pregnant or within 42 days of the end of pregnancy from causes related to or aggravated by pregnancy”) were limited until the addition of pregnancy to the U.S. Standard Certificate of Death in 2003; all 50 states had adopted it by 2018, the researchers noted.
In a study published in Obstetrics & Gynecology, the researchers analyzed the first 2 years of nationally available data to identify pregnancy-associated mortality and characterize other risk factors such as age and race.
The researchers identified 4,705 female homicides in 2018 and 2019. Of these, 273 (5.8%) occurred in women who were pregnant or within a year of the end of pregnancy. Approximately half (50.2%) of the pregnant or postpartum victims were non-Hispanic Black, 30% were non-Hispanic white, 9.5% were Hispanic, and 10.3% were other races; approximately one-third (35.5%) were in the 20- to 24-year age group.
Overall, the ratio was 3.62 homicides per 100,000 live births among females who were either pregnant or within 1 year post partum, compared to 3.12 homicides per 100,000 live births in nonpregnant, nonpostpartum females aged 10-44 years (P = .05).
“Patterns were similar in further stratification by both race and age such that pregnancy was associated with more than a doubled risk of homicide among girls and women aged 10–24 in both the non-Hispanic White and non-Hispanic Black populations,” the researchers wrote.
The findings are consistent with previous studies, which “implicates health and social system failures. Although we are unable to directly evaluate the involvement of intimate partner violence (IPV) in this report, we did find that a majority of pregnancy-associated homicides occurred in the home, implicating the likelihood of involvement by persons known to the victim,” they noted. In addition, the data showed that approximately 70% of the incidents of homicide in pregnant and postpartum women involved a firearm, an increase over previous estimates.
The study findings were limited by several factors including the lack of circumstantial information and incomplete data on victim characteristics, the researchers noted. Other key limitations included the potential for false-positives and false-negatives when recording pregnancy status, which could lead to underestimates of pregnancy-associated homicides, and the lack of data on pregnancy outcomes for women who experienced live birth, abortion, or miscarriage within a year of death.
However, the results highlight the need for increased awareness and training of physicians in completing the pregnancy checkbox on death certificates, and the need for action on recommendations and interventions to prevent maternal deaths from homicide, they emphasized.
“Although encouraging, a commitment to the actual implementation of policies and investments known to be effective at protecting and the promoting the health and safety of girls and women must follow,” they concluded.
Data highlight disparities
“This study could not be done effectively prior to now, as the adoption of the pregnancy checkbox on the U.S. Standard Certificate of Death was only available in all 50 states as of 2018,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview.
“This study also demonstrates what was already known, which is that pregnancy is a high-risk time period for intimate partner violence, including homicide. The differences in homicide rates based on race and ethnicity also highlight the clear disparities in maternal mortality in the U.S. that are attributable to racism. There is more attention being paid to maternal mortality and the differential experience based on race, and this demonstrates that simply addressing medical management during pregnancy is not enough – we need to address root causes of racism if we truly want to reduce maternal mortality,” Dr. Prager said.
“The primary take-home message for clinicians is to ascertain safety from every patient, and to try to reduce the impacts of racism on health care for patients, especially during pregnancy,” she said.
Although more detailed records would help with elucidating causes versus associations, “more research is not the answer,” Dr. Prager stated. “The real solution here is to have better gun safety laws, and to put significant resources toward reducing the impacts of racism on health care and our society.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
The prevalence of homicide was 16% higher in pregnant women or postpartum women than nonpregnant or nonpostpartum women in the United States, according to 2018 and 2019 mortality data from the National Center for Health Statistics.
Homicide has long been identified as a leading cause of death during pregnancy, but homicide is not counted in estimates of maternal mortality, nor is it emphasized as a target for prevention and intervention, wrote Maeve Wallace, PhD, of Tulane University, New Orleans, and colleagues.
Data on maternal mortality (defined as “death while pregnant or within 42 days of the end of pregnancy from causes related to or aggravated by pregnancy”) were limited until the addition of pregnancy to the U.S. Standard Certificate of Death in 2003; all 50 states had adopted it by 2018, the researchers noted.
In a study published in Obstetrics & Gynecology, the researchers analyzed the first 2 years of nationally available data to identify pregnancy-associated mortality and characterize other risk factors such as age and race.
The researchers identified 4,705 female homicides in 2018 and 2019. Of these, 273 (5.8%) occurred in women who were pregnant or within a year of the end of pregnancy. Approximately half (50.2%) of the pregnant or postpartum victims were non-Hispanic Black, 30% were non-Hispanic white, 9.5% were Hispanic, and 10.3% were other races; approximately one-third (35.5%) were in the 20- to 24-year age group.
Overall, the ratio was 3.62 homicides per 100,000 live births among females who were either pregnant or within 1 year post partum, compared to 3.12 homicides per 100,000 live births in nonpregnant, nonpostpartum females aged 10-44 years (P = .05).
“Patterns were similar in further stratification by both race and age such that pregnancy was associated with more than a doubled risk of homicide among girls and women aged 10–24 in both the non-Hispanic White and non-Hispanic Black populations,” the researchers wrote.
The findings are consistent with previous studies, which “implicates health and social system failures. Although we are unable to directly evaluate the involvement of intimate partner violence (IPV) in this report, we did find that a majority of pregnancy-associated homicides occurred in the home, implicating the likelihood of involvement by persons known to the victim,” they noted. In addition, the data showed that approximately 70% of the incidents of homicide in pregnant and postpartum women involved a firearm, an increase over previous estimates.
The study findings were limited by several factors including the lack of circumstantial information and incomplete data on victim characteristics, the researchers noted. Other key limitations included the potential for false-positives and false-negatives when recording pregnancy status, which could lead to underestimates of pregnancy-associated homicides, and the lack of data on pregnancy outcomes for women who experienced live birth, abortion, or miscarriage within a year of death.
However, the results highlight the need for increased awareness and training of physicians in completing the pregnancy checkbox on death certificates, and the need for action on recommendations and interventions to prevent maternal deaths from homicide, they emphasized.
“Although encouraging, a commitment to the actual implementation of policies and investments known to be effective at protecting and the promoting the health and safety of girls and women must follow,” they concluded.
Data highlight disparities
“This study could not be done effectively prior to now, as the adoption of the pregnancy checkbox on the U.S. Standard Certificate of Death was only available in all 50 states as of 2018,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview.
“This study also demonstrates what was already known, which is that pregnancy is a high-risk time period for intimate partner violence, including homicide. The differences in homicide rates based on race and ethnicity also highlight the clear disparities in maternal mortality in the U.S. that are attributable to racism. There is more attention being paid to maternal mortality and the differential experience based on race, and this demonstrates that simply addressing medical management during pregnancy is not enough – we need to address root causes of racism if we truly want to reduce maternal mortality,” Dr. Prager said.
“The primary take-home message for clinicians is to ascertain safety from every patient, and to try to reduce the impacts of racism on health care for patients, especially during pregnancy,” she said.
Although more detailed records would help with elucidating causes versus associations, “more research is not the answer,” Dr. Prager stated. “The real solution here is to have better gun safety laws, and to put significant resources toward reducing the impacts of racism on health care and our society.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
The prevalence of homicide was 16% higher in pregnant women or postpartum women than nonpregnant or nonpostpartum women in the United States, according to 2018 and 2019 mortality data from the National Center for Health Statistics.
Homicide has long been identified as a leading cause of death during pregnancy, but homicide is not counted in estimates of maternal mortality, nor is it emphasized as a target for prevention and intervention, wrote Maeve Wallace, PhD, of Tulane University, New Orleans, and colleagues.
Data on maternal mortality (defined as “death while pregnant or within 42 days of the end of pregnancy from causes related to or aggravated by pregnancy”) were limited until the addition of pregnancy to the U.S. Standard Certificate of Death in 2003; all 50 states had adopted it by 2018, the researchers noted.
In a study published in Obstetrics & Gynecology, the researchers analyzed the first 2 years of nationally available data to identify pregnancy-associated mortality and characterize other risk factors such as age and race.
The researchers identified 4,705 female homicides in 2018 and 2019. Of these, 273 (5.8%) occurred in women who were pregnant or within a year of the end of pregnancy. Approximately half (50.2%) of the pregnant or postpartum victims were non-Hispanic Black, 30% were non-Hispanic white, 9.5% were Hispanic, and 10.3% were other races; approximately one-third (35.5%) were in the 20- to 24-year age group.
Overall, the ratio was 3.62 homicides per 100,000 live births among females who were either pregnant or within 1 year post partum, compared to 3.12 homicides per 100,000 live births in nonpregnant, nonpostpartum females aged 10-44 years (P = .05).
“Patterns were similar in further stratification by both race and age such that pregnancy was associated with more than a doubled risk of homicide among girls and women aged 10–24 in both the non-Hispanic White and non-Hispanic Black populations,” the researchers wrote.
The findings are consistent with previous studies, which “implicates health and social system failures. Although we are unable to directly evaluate the involvement of intimate partner violence (IPV) in this report, we did find that a majority of pregnancy-associated homicides occurred in the home, implicating the likelihood of involvement by persons known to the victim,” they noted. In addition, the data showed that approximately 70% of the incidents of homicide in pregnant and postpartum women involved a firearm, an increase over previous estimates.
The study findings were limited by several factors including the lack of circumstantial information and incomplete data on victim characteristics, the researchers noted. Other key limitations included the potential for false-positives and false-negatives when recording pregnancy status, which could lead to underestimates of pregnancy-associated homicides, and the lack of data on pregnancy outcomes for women who experienced live birth, abortion, or miscarriage within a year of death.
However, the results highlight the need for increased awareness and training of physicians in completing the pregnancy checkbox on death certificates, and the need for action on recommendations and interventions to prevent maternal deaths from homicide, they emphasized.
“Although encouraging, a commitment to the actual implementation of policies and investments known to be effective at protecting and the promoting the health and safety of girls and women must follow,” they concluded.
Data highlight disparities
“This study could not be done effectively prior to now, as the adoption of the pregnancy checkbox on the U.S. Standard Certificate of Death was only available in all 50 states as of 2018,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview.
“This study also demonstrates what was already known, which is that pregnancy is a high-risk time period for intimate partner violence, including homicide. The differences in homicide rates based on race and ethnicity also highlight the clear disparities in maternal mortality in the U.S. that are attributable to racism. There is more attention being paid to maternal mortality and the differential experience based on race, and this demonstrates that simply addressing medical management during pregnancy is not enough – we need to address root causes of racism if we truly want to reduce maternal mortality,” Dr. Prager said.
“The primary take-home message for clinicians is to ascertain safety from every patient, and to try to reduce the impacts of racism on health care for patients, especially during pregnancy,” she said.
Although more detailed records would help with elucidating causes versus associations, “more research is not the answer,” Dr. Prager stated. “The real solution here is to have better gun safety laws, and to put significant resources toward reducing the impacts of racism on health care and our society.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
FROM OBSTETRICS & GYNECOLOGY
You’ve been uneasy about the mother’s boyfriend: This may be why
The first patient of the afternoon is a 4-month-old in for his health maintenance visit. You’ve known his 20-year-old mother since she was a toddler. This infant has a 2-year-old sister. Also in the exam room is a young man you don’t recognize whom the mother introduces as Jason, her new boyfriend. He never makes eye contact and despite your best efforts you can’t get him to engage.
At the child’s next visit you are relieved to see the 6-month-old is alive and well and learn that your former patient and her two children have moved back in with her parents and Jason is no longer in the picture.
You don’t have to have been doing pediatrics very long to have learned that a “family” that includes an infant and a young adult male who is probably not the father is an environment in which the infant’s health and well-being is at significant risk. It is a situation in which child abuse even to the point of infanticide should be waving a red flag in your face.
Infanticide occurs in many animal species including our own. As abhorrent we may find the act, it occurs often enough to be, if not normal, at least not unexpected in certain circumstances. Theories abound as to what advantage the act of infanticide might convey to the success of a species. However, little if anything is known about any possible mechanisms that would allow it to occur.
Recently, a professor of molecular and cellular biology at Harvard University discovered a specific set of neurons in the mouse brain that controls aggressive behavior toward infants (Biological triggers for infant abuse, by Juan Siliezar, The Harvard Gazette, Sept 27, 2021). This same set of neurons also appears to trigger avoidance and neglect behaviors as well.
Research in other animal species has found that these antiparental behaviors occur in both virgins and sexually mature males who are strangers to the group. Interestingly, the behaviors switch off once individuals have their own offspring or have had the opportunity to familiarize themselves with infants. Not surprisingly, other studies have found that in some species environmental stress such as food shortage or threats of predation have triggered females to attack or ignore their offspring.
I think it is safe to assume a similar collection of neurons controlling aggressive behavior also exists in humans. One can imagine some well-read defense attorney dredging up this study and claiming that because his client had not yet fathered a child of his own that it was his nervous system’s normal response that made him toss his girlfriend’s baby against the wall.
The lead author of the study intends to study this collection of neurons in more depth to discover more about the process. It is conceivable that with more information her initial findings may help in the development of treatment and specific prevention strategies. Until that happens, we must rely on our intuition and keep our antennae tuned and alert for high-risk scenarios like the one I described at the opening of this letter.
We are left with leaning heavily on our community social work networks to keep close tabs on these high-risk families, offering both financial and emotional support. Parenting classes may be helpful, but some of this research leads me to suspect that immersing these young parents-to-be in hands-on child care situations might provide the best protection we can offer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The first patient of the afternoon is a 4-month-old in for his health maintenance visit. You’ve known his 20-year-old mother since she was a toddler. This infant has a 2-year-old sister. Also in the exam room is a young man you don’t recognize whom the mother introduces as Jason, her new boyfriend. He never makes eye contact and despite your best efforts you can’t get him to engage.
At the child’s next visit you are relieved to see the 6-month-old is alive and well and learn that your former patient and her two children have moved back in with her parents and Jason is no longer in the picture.
You don’t have to have been doing pediatrics very long to have learned that a “family” that includes an infant and a young adult male who is probably not the father is an environment in which the infant’s health and well-being is at significant risk. It is a situation in which child abuse even to the point of infanticide should be waving a red flag in your face.
Infanticide occurs in many animal species including our own. As abhorrent we may find the act, it occurs often enough to be, if not normal, at least not unexpected in certain circumstances. Theories abound as to what advantage the act of infanticide might convey to the success of a species. However, little if anything is known about any possible mechanisms that would allow it to occur.
Recently, a professor of molecular and cellular biology at Harvard University discovered a specific set of neurons in the mouse brain that controls aggressive behavior toward infants (Biological triggers for infant abuse, by Juan Siliezar, The Harvard Gazette, Sept 27, 2021). This same set of neurons also appears to trigger avoidance and neglect behaviors as well.
Research in other animal species has found that these antiparental behaviors occur in both virgins and sexually mature males who are strangers to the group. Interestingly, the behaviors switch off once individuals have their own offspring or have had the opportunity to familiarize themselves with infants. Not surprisingly, other studies have found that in some species environmental stress such as food shortage or threats of predation have triggered females to attack or ignore their offspring.
I think it is safe to assume a similar collection of neurons controlling aggressive behavior also exists in humans. One can imagine some well-read defense attorney dredging up this study and claiming that because his client had not yet fathered a child of his own that it was his nervous system’s normal response that made him toss his girlfriend’s baby against the wall.
The lead author of the study intends to study this collection of neurons in more depth to discover more about the process. It is conceivable that with more information her initial findings may help in the development of treatment and specific prevention strategies. Until that happens, we must rely on our intuition and keep our antennae tuned and alert for high-risk scenarios like the one I described at the opening of this letter.
We are left with leaning heavily on our community social work networks to keep close tabs on these high-risk families, offering both financial and emotional support. Parenting classes may be helpful, but some of this research leads me to suspect that immersing these young parents-to-be in hands-on child care situations might provide the best protection we can offer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The first patient of the afternoon is a 4-month-old in for his health maintenance visit. You’ve known his 20-year-old mother since she was a toddler. This infant has a 2-year-old sister. Also in the exam room is a young man you don’t recognize whom the mother introduces as Jason, her new boyfriend. He never makes eye contact and despite your best efforts you can’t get him to engage.
At the child’s next visit you are relieved to see the 6-month-old is alive and well and learn that your former patient and her two children have moved back in with her parents and Jason is no longer in the picture.
You don’t have to have been doing pediatrics very long to have learned that a “family” that includes an infant and a young adult male who is probably not the father is an environment in which the infant’s health and well-being is at significant risk. It is a situation in which child abuse even to the point of infanticide should be waving a red flag in your face.
Infanticide occurs in many animal species including our own. As abhorrent we may find the act, it occurs often enough to be, if not normal, at least not unexpected in certain circumstances. Theories abound as to what advantage the act of infanticide might convey to the success of a species. However, little if anything is known about any possible mechanisms that would allow it to occur.
Recently, a professor of molecular and cellular biology at Harvard University discovered a specific set of neurons in the mouse brain that controls aggressive behavior toward infants (Biological triggers for infant abuse, by Juan Siliezar, The Harvard Gazette, Sept 27, 2021). This same set of neurons also appears to trigger avoidance and neglect behaviors as well.
Research in other animal species has found that these antiparental behaviors occur in both virgins and sexually mature males who are strangers to the group. Interestingly, the behaviors switch off once individuals have their own offspring or have had the opportunity to familiarize themselves with infants. Not surprisingly, other studies have found that in some species environmental stress such as food shortage or threats of predation have triggered females to attack or ignore their offspring.
I think it is safe to assume a similar collection of neurons controlling aggressive behavior also exists in humans. One can imagine some well-read defense attorney dredging up this study and claiming that because his client had not yet fathered a child of his own that it was his nervous system’s normal response that made him toss his girlfriend’s baby against the wall.
The lead author of the study intends to study this collection of neurons in more depth to discover more about the process. It is conceivable that with more information her initial findings may help in the development of treatment and specific prevention strategies. Until that happens, we must rely on our intuition and keep our antennae tuned and alert for high-risk scenarios like the one I described at the opening of this letter.
We are left with leaning heavily on our community social work networks to keep close tabs on these high-risk families, offering both financial and emotional support. Parenting classes may be helpful, but some of this research leads me to suspect that immersing these young parents-to-be in hands-on child care situations might provide the best protection we can offer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Synthetic chemical in consumer products linked to early death, study says
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Children and COVID-19: U.S. adds latest million cases in record time
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
The United States just passed the 6-million mark in COVID-19 cases among children, with the last million cases taking less time to record than any of the first five, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The five-millionth case was reported during the week of Aug. 27 to Sept. 2, and case number 6 million came during the week of Oct. 1-7, just 5 weeks later, compared with the 6 weeks it took to go from 1 million to 2 million last November and December, the AAP and CHA said in their weekly COVID-19 report.
New cases continued to drop, however, and that weekly count was down by 14.6% from the previous week and by 41.1% from the peak of almost 252,000 reached in early September, the two groups said while also noting limitations to the data, such as three states (Alabama, Nebraska, and Texas) that are no longer updating their COVID-19 dashboards.
Other metrics show similar drops in recent weeks. Among children aged 0-11 years, emergency department visits involving a COVID-19 diagnosis dropped from 4.1% of all ED visits in late August to 1.4% of ED visits on Oct. 6. ED visits with a COVID-19 diagnosis fell from a peak of 8.5% on Aug. 22 to 1.5% on Oct. 6 for 12- to 15-year-olds and from 8.5% to 1.5% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
The rate of new hospital admissions for children aged 0-17 years was down to 0.26 per 100,000 population on Oct. 9 after reaching 0.51 per 100,000 on Sept. 4. Hospitalizations in children totaled just over 64,000 from Aug. 1, 2020, to Oct. 9, 2021, which is just over 2% of all COVID-19–related admissions over that time period, the CDC said on its COVID Data Tracker.
That pattern, unfortunately, also applies to vaccinations. “The number of children receiving their first COVID-19 vaccine this week [Sept. 30 to Oct. 6], about 156,000, was the lowest number since vaccines were available,” the AAP said in a separate report on vaccination trends, adding that “the number of children receiving their first dose has steadily declined from 8 weeks ago when 586,000 children received their initial dose the week ending Aug. 11.”
Underrepresented Minority Students Applying to Dermatology Residency in the COVID-19 Era: Challenges and Considerations
The COVID-19 pandemic has markedly changed the dermatology residency application process. As medical students head into this application cycle, the impacts of systemic racism and deeply rooted structural barriers continue to be exacerbated for students who identify as an underrepresented minority (URM) in medicine—historically defined as those who self-identify as Hispanic or Latinx; Black or African American; American Indian or Alaska Native; or Native Hawaiian or Pacific Islander. The Association of American Medical Colleges (AAMC) defines URMs as racial and ethnic populations that are underrepresented in medicine relative to their numbers in the general population.1 Although these groups account for approximately 34% of the population of the United States, they constitute only 11% of the country’s physician workforce.2,3
Of the total physician workforce in the United States, Black and African American physicians account for 5% of practicing physicians; Hispanic physicians, 5.8%; American Indian and Alaska Native physicians, 0.3%; and Native Hawaiian and Pacific Islander physicians, 0.1%.2 In competitive medical specialties, the disproportionality of these numbers compared to our current demographics in the United States as shown above is even more staggering. In 2018, for example, 10% of practicing dermatologists identified as female URM physicians; 6%, as male URM physicians.2 In this article, we discuss some of the challenges and considerations for URM students applying to dermatology residency in the era of the COVID-19 pandemic.
Barriers for URM Students in Dermatology
Multiple studies have attempted to identify some of the barriers faced by URM students in medicine that might explain the lack of diversity in competitive specialties. Vasquez and colleagues4 identified 4 major factors that play a role in dermatology: lack of equitable resources, lack of support, financial limitations, and the lack of group identity. More than half of URM students surveyed (1) identified lack of support as a barrier and (2) reported having been encouraged to seek a specialty more reflective of their community.4
Soliman et al5 reported that URM barriers in dermatology extend to include lack of diversity in the field, socioeconomic factors, lack of mentorship, and a negative perception of minority students by residency programs. Dermatology is the second least diverse specialty in medicine after orthopedic surgery, which, in and of itself, might further discourage URM students from applying to dermatology.5
With the minimal exposure that URM students have to the field of dermatology, the lack of pipeline programs, and reports that URMs often are encouraged to pursue primary care, the current diversity deficiency in dermatology comes as no surprise. In addition, the substantial disadvantage for URM students is perpetuated by the traditional highly selective process that favors grades, board scores, and honor society status over holistic assessment of the individual student and their unique experiences and potential for contribution.
Looking Beyond Test Scores
The US Medical Licensing Examination (USMLE) traditionally has been used to select dermatology residency applicants, with high cutoff scores often excluding outstanding URM students. Research has suggested that the use of USMLE examination test scores for residency recruitment lacks validity because it has poor predictability of residency performance.6 Although the USMLE Step 1 examination is transitioning to pass/fail scoring, applicants for the next cycle will still have a 3-digit numerical score.
We strongly recommend that dermatology programs transition from emphasizing scores of residency candidates to reviewing each candidate holistically. The AAMC defines “holistic review” as a “flexible, individualized way of assessing an applicant’s capabilities, by which balanced consideration is given to experiences, attributes, competencies, and academic or scholarly metrics and, when considered in combination, how the individual might contribute value to the institution’s mission.”7 Furthermore, we recommend that dermatology residency programs have multiple faculty members review each application, including a representative of the diversity, inclusion, and equity committee.
Applying to Residency in the COVID-19 Virtual Environment
In the COVID-19 era, dermatology externship opportunities that would have allowed URM students to work directly with potential residency programs, showcase their abilities, and network have been limited. Virtual residency interviews could make it more challenging to evaluate candidates, especially URM students from less prestigious programs or unusual socioeconomic backgrounds, or with lower board scores. In addition, virtual interviews can more easily become one-dimensional, depriving URM students of the opportunity to gauge their personal fit in a specific dermatology residency program and its community. Questions and concerns of URM students might include: Will I be appropriately supported and mentored? Will my cultural preferences, religion, sexual preference, hairstyle, and beliefs be accepted? Can I advocate for minorities and support antiracism and diversity and inclusion initiatives? To that end, we recommend that dermatology programs continue to host virtual meet-and-greet events for potential students to meet faculty and learn more about the program. In addition, programs should consider having current residents interact virtually with candidates to allow students to better understand the culture of the department and residents’ experiences as trainees in such an environment. For URM students, this is highly important because diversity, inclusion, and antiracism policies and initiatives might not be explicitly available on the institution’s website or residency information page.
Organizations Championing Diversity
Recently, multiple dermatology societies and organizations have been emphasizing the need for diversity and inclusion as well as promoting holistic application review. The American Academy of Dermatology pioneered the Diversity Champion Workshop in 2019 and continues to offer the Diversity Mentorship program, connecting URM students to mentors nationally. The Skin of Color Society offers yearly grants and awards to medical students to develop mentorship and research, and recently hosted webinars to guide medical students and residency programs on diversity and inclusion, residency application and review, and COVID-19 virtual interviews. Other national societies, such as the Student National Medical Association and Latino Medical Student Association, have been promoting workshops and interview mentoring for URM students, including dermatology-specific events. Although it is estimated that more than 90% of medical schools in the United States already perform holistic application review and that such review has been adopted by many dermatology programs nationwide, data regarding dermatology residency programs’ implementation of holistic application review are lacking.8
In addition, we encourage continuation of the proposed coordinated interview invite release from the Association of Professors of Dermatology, which was implemented in the 2020-2021 cycle. In light of the recent AAMC letter9 on the maldistribution of interview invitations to highest-tier applicants, coordination of interview release dates and other similar initiatives to prevent programs from offering more invites than their available slots and improve transparency about interview days are needed. Furthermore, continuing to offer optional virtual interviews for applicants in future cycles could make the process less cost-prohibitive for many URM students.4,5
Final Thoughts
Dermatology residency programs must intentionally guard against falling back to traditional standards of assessment as the only means of student evaluation, especially in this virtual era. It is our responsibility to remove artificial barriers that continue to stall progress in diversity, inclusion, equity, and belonging in dermatology.
- Underrepresented in medicine definition. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
- Diversity in medicine: facts and figures 2019. table 13. practice specialty, males by race/ethnicity, 2018. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/data-reports/workforce/data/table-13-practice-specialty-males-race/ethnicity-2018 1B
- US Census Bureau. Quick facts: United States. Updated July 1, 2019. Accessed September 20, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Williams C, Kwan B, Pereira A, et al. A call to improve conditions for conducting holistic review in graduate medical education recruitment. MedEdPublish. 2019;8:6. https://doi.org/10.15694/mep.2019.000076.1
- Holistic principles in resident selection: an introduction. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/system/files/2020-08/aa-member-capacity-building-holistic-review-transcript-activities-GME-081420.pdf
- Luke J, Cornelius L, Lim H. Dermatology resident selection: shifting toward holistic review? J Am Acad Dermatol. 2020;84:1208-1209. doi:10.1016/j.jaad.2020.11.025
- Open letter on residency interviews from Alison Whelan, MD, AAMC Chief Medical Education Officer. Association of American Medical Colleges website. Published December 18, 2020. Accessed September 27, 2021. https://www.aamc.org/media/50291/download
The COVID-19 pandemic has markedly changed the dermatology residency application process. As medical students head into this application cycle, the impacts of systemic racism and deeply rooted structural barriers continue to be exacerbated for students who identify as an underrepresented minority (URM) in medicine—historically defined as those who self-identify as Hispanic or Latinx; Black or African American; American Indian or Alaska Native; or Native Hawaiian or Pacific Islander. The Association of American Medical Colleges (AAMC) defines URMs as racial and ethnic populations that are underrepresented in medicine relative to their numbers in the general population.1 Although these groups account for approximately 34% of the population of the United States, they constitute only 11% of the country’s physician workforce.2,3
Of the total physician workforce in the United States, Black and African American physicians account for 5% of practicing physicians; Hispanic physicians, 5.8%; American Indian and Alaska Native physicians, 0.3%; and Native Hawaiian and Pacific Islander physicians, 0.1%.2 In competitive medical specialties, the disproportionality of these numbers compared to our current demographics in the United States as shown above is even more staggering. In 2018, for example, 10% of practicing dermatologists identified as female URM physicians; 6%, as male URM physicians.2 In this article, we discuss some of the challenges and considerations for URM students applying to dermatology residency in the era of the COVID-19 pandemic.
Barriers for URM Students in Dermatology
Multiple studies have attempted to identify some of the barriers faced by URM students in medicine that might explain the lack of diversity in competitive specialties. Vasquez and colleagues4 identified 4 major factors that play a role in dermatology: lack of equitable resources, lack of support, financial limitations, and the lack of group identity. More than half of URM students surveyed (1) identified lack of support as a barrier and (2) reported having been encouraged to seek a specialty more reflective of their community.4
Soliman et al5 reported that URM barriers in dermatology extend to include lack of diversity in the field, socioeconomic factors, lack of mentorship, and a negative perception of minority students by residency programs. Dermatology is the second least diverse specialty in medicine after orthopedic surgery, which, in and of itself, might further discourage URM students from applying to dermatology.5
With the minimal exposure that URM students have to the field of dermatology, the lack of pipeline programs, and reports that URMs often are encouraged to pursue primary care, the current diversity deficiency in dermatology comes as no surprise. In addition, the substantial disadvantage for URM students is perpetuated by the traditional highly selective process that favors grades, board scores, and honor society status over holistic assessment of the individual student and their unique experiences and potential for contribution.
Looking Beyond Test Scores
The US Medical Licensing Examination (USMLE) traditionally has been used to select dermatology residency applicants, with high cutoff scores often excluding outstanding URM students. Research has suggested that the use of USMLE examination test scores for residency recruitment lacks validity because it has poor predictability of residency performance.6 Although the USMLE Step 1 examination is transitioning to pass/fail scoring, applicants for the next cycle will still have a 3-digit numerical score.
We strongly recommend that dermatology programs transition from emphasizing scores of residency candidates to reviewing each candidate holistically. The AAMC defines “holistic review” as a “flexible, individualized way of assessing an applicant’s capabilities, by which balanced consideration is given to experiences, attributes, competencies, and academic or scholarly metrics and, when considered in combination, how the individual might contribute value to the institution’s mission.”7 Furthermore, we recommend that dermatology residency programs have multiple faculty members review each application, including a representative of the diversity, inclusion, and equity committee.
Applying to Residency in the COVID-19 Virtual Environment
In the COVID-19 era, dermatology externship opportunities that would have allowed URM students to work directly with potential residency programs, showcase their abilities, and network have been limited. Virtual residency interviews could make it more challenging to evaluate candidates, especially URM students from less prestigious programs or unusual socioeconomic backgrounds, or with lower board scores. In addition, virtual interviews can more easily become one-dimensional, depriving URM students of the opportunity to gauge their personal fit in a specific dermatology residency program and its community. Questions and concerns of URM students might include: Will I be appropriately supported and mentored? Will my cultural preferences, religion, sexual preference, hairstyle, and beliefs be accepted? Can I advocate for minorities and support antiracism and diversity and inclusion initiatives? To that end, we recommend that dermatology programs continue to host virtual meet-and-greet events for potential students to meet faculty and learn more about the program. In addition, programs should consider having current residents interact virtually with candidates to allow students to better understand the culture of the department and residents’ experiences as trainees in such an environment. For URM students, this is highly important because diversity, inclusion, and antiracism policies and initiatives might not be explicitly available on the institution’s website or residency information page.
Organizations Championing Diversity
Recently, multiple dermatology societies and organizations have been emphasizing the need for diversity and inclusion as well as promoting holistic application review. The American Academy of Dermatology pioneered the Diversity Champion Workshop in 2019 and continues to offer the Diversity Mentorship program, connecting URM students to mentors nationally. The Skin of Color Society offers yearly grants and awards to medical students to develop mentorship and research, and recently hosted webinars to guide medical students and residency programs on diversity and inclusion, residency application and review, and COVID-19 virtual interviews. Other national societies, such as the Student National Medical Association and Latino Medical Student Association, have been promoting workshops and interview mentoring for URM students, including dermatology-specific events. Although it is estimated that more than 90% of medical schools in the United States already perform holistic application review and that such review has been adopted by many dermatology programs nationwide, data regarding dermatology residency programs’ implementation of holistic application review are lacking.8
In addition, we encourage continuation of the proposed coordinated interview invite release from the Association of Professors of Dermatology, which was implemented in the 2020-2021 cycle. In light of the recent AAMC letter9 on the maldistribution of interview invitations to highest-tier applicants, coordination of interview release dates and other similar initiatives to prevent programs from offering more invites than their available slots and improve transparency about interview days are needed. Furthermore, continuing to offer optional virtual interviews for applicants in future cycles could make the process less cost-prohibitive for many URM students.4,5
Final Thoughts
Dermatology residency programs must intentionally guard against falling back to traditional standards of assessment as the only means of student evaluation, especially in this virtual era. It is our responsibility to remove artificial barriers that continue to stall progress in diversity, inclusion, equity, and belonging in dermatology.
The COVID-19 pandemic has markedly changed the dermatology residency application process. As medical students head into this application cycle, the impacts of systemic racism and deeply rooted structural barriers continue to be exacerbated for students who identify as an underrepresented minority (URM) in medicine—historically defined as those who self-identify as Hispanic or Latinx; Black or African American; American Indian or Alaska Native; or Native Hawaiian or Pacific Islander. The Association of American Medical Colleges (AAMC) defines URMs as racial and ethnic populations that are underrepresented in medicine relative to their numbers in the general population.1 Although these groups account for approximately 34% of the population of the United States, they constitute only 11% of the country’s physician workforce.2,3
Of the total physician workforce in the United States, Black and African American physicians account for 5% of practicing physicians; Hispanic physicians, 5.8%; American Indian and Alaska Native physicians, 0.3%; and Native Hawaiian and Pacific Islander physicians, 0.1%.2 In competitive medical specialties, the disproportionality of these numbers compared to our current demographics in the United States as shown above is even more staggering. In 2018, for example, 10% of practicing dermatologists identified as female URM physicians; 6%, as male URM physicians.2 In this article, we discuss some of the challenges and considerations for URM students applying to dermatology residency in the era of the COVID-19 pandemic.
Barriers for URM Students in Dermatology
Multiple studies have attempted to identify some of the barriers faced by URM students in medicine that might explain the lack of diversity in competitive specialties. Vasquez and colleagues4 identified 4 major factors that play a role in dermatology: lack of equitable resources, lack of support, financial limitations, and the lack of group identity. More than half of URM students surveyed (1) identified lack of support as a barrier and (2) reported having been encouraged to seek a specialty more reflective of their community.4
Soliman et al5 reported that URM barriers in dermatology extend to include lack of diversity in the field, socioeconomic factors, lack of mentorship, and a negative perception of minority students by residency programs. Dermatology is the second least diverse specialty in medicine after orthopedic surgery, which, in and of itself, might further discourage URM students from applying to dermatology.5
With the minimal exposure that URM students have to the field of dermatology, the lack of pipeline programs, and reports that URMs often are encouraged to pursue primary care, the current diversity deficiency in dermatology comes as no surprise. In addition, the substantial disadvantage for URM students is perpetuated by the traditional highly selective process that favors grades, board scores, and honor society status over holistic assessment of the individual student and their unique experiences and potential for contribution.
Looking Beyond Test Scores
The US Medical Licensing Examination (USMLE) traditionally has been used to select dermatology residency applicants, with high cutoff scores often excluding outstanding URM students. Research has suggested that the use of USMLE examination test scores for residency recruitment lacks validity because it has poor predictability of residency performance.6 Although the USMLE Step 1 examination is transitioning to pass/fail scoring, applicants for the next cycle will still have a 3-digit numerical score.
We strongly recommend that dermatology programs transition from emphasizing scores of residency candidates to reviewing each candidate holistically. The AAMC defines “holistic review” as a “flexible, individualized way of assessing an applicant’s capabilities, by which balanced consideration is given to experiences, attributes, competencies, and academic or scholarly metrics and, when considered in combination, how the individual might contribute value to the institution’s mission.”7 Furthermore, we recommend that dermatology residency programs have multiple faculty members review each application, including a representative of the diversity, inclusion, and equity committee.
Applying to Residency in the COVID-19 Virtual Environment
In the COVID-19 era, dermatology externship opportunities that would have allowed URM students to work directly with potential residency programs, showcase their abilities, and network have been limited. Virtual residency interviews could make it more challenging to evaluate candidates, especially URM students from less prestigious programs or unusual socioeconomic backgrounds, or with lower board scores. In addition, virtual interviews can more easily become one-dimensional, depriving URM students of the opportunity to gauge their personal fit in a specific dermatology residency program and its community. Questions and concerns of URM students might include: Will I be appropriately supported and mentored? Will my cultural preferences, religion, sexual preference, hairstyle, and beliefs be accepted? Can I advocate for minorities and support antiracism and diversity and inclusion initiatives? To that end, we recommend that dermatology programs continue to host virtual meet-and-greet events for potential students to meet faculty and learn more about the program. In addition, programs should consider having current residents interact virtually with candidates to allow students to better understand the culture of the department and residents’ experiences as trainees in such an environment. For URM students, this is highly important because diversity, inclusion, and antiracism policies and initiatives might not be explicitly available on the institution’s website or residency information page.
Organizations Championing Diversity
Recently, multiple dermatology societies and organizations have been emphasizing the need for diversity and inclusion as well as promoting holistic application review. The American Academy of Dermatology pioneered the Diversity Champion Workshop in 2019 and continues to offer the Diversity Mentorship program, connecting URM students to mentors nationally. The Skin of Color Society offers yearly grants and awards to medical students to develop mentorship and research, and recently hosted webinars to guide medical students and residency programs on diversity and inclusion, residency application and review, and COVID-19 virtual interviews. Other national societies, such as the Student National Medical Association and Latino Medical Student Association, have been promoting workshops and interview mentoring for URM students, including dermatology-specific events. Although it is estimated that more than 90% of medical schools in the United States already perform holistic application review and that such review has been adopted by many dermatology programs nationwide, data regarding dermatology residency programs’ implementation of holistic application review are lacking.8
In addition, we encourage continuation of the proposed coordinated interview invite release from the Association of Professors of Dermatology, which was implemented in the 2020-2021 cycle. In light of the recent AAMC letter9 on the maldistribution of interview invitations to highest-tier applicants, coordination of interview release dates and other similar initiatives to prevent programs from offering more invites than their available slots and improve transparency about interview days are needed. Furthermore, continuing to offer optional virtual interviews for applicants in future cycles could make the process less cost-prohibitive for many URM students.4,5
Final Thoughts
Dermatology residency programs must intentionally guard against falling back to traditional standards of assessment as the only means of student evaluation, especially in this virtual era. It is our responsibility to remove artificial barriers that continue to stall progress in diversity, inclusion, equity, and belonging in dermatology.
- Underrepresented in medicine definition. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
- Diversity in medicine: facts and figures 2019. table 13. practice specialty, males by race/ethnicity, 2018. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/data-reports/workforce/data/table-13-practice-specialty-males-race/ethnicity-2018 1B
- US Census Bureau. Quick facts: United States. Updated July 1, 2019. Accessed September 20, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Williams C, Kwan B, Pereira A, et al. A call to improve conditions for conducting holistic review in graduate medical education recruitment. MedEdPublish. 2019;8:6. https://doi.org/10.15694/mep.2019.000076.1
- Holistic principles in resident selection: an introduction. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/system/files/2020-08/aa-member-capacity-building-holistic-review-transcript-activities-GME-081420.pdf
- Luke J, Cornelius L, Lim H. Dermatology resident selection: shifting toward holistic review? J Am Acad Dermatol. 2020;84:1208-1209. doi:10.1016/j.jaad.2020.11.025
- Open letter on residency interviews from Alison Whelan, MD, AAMC Chief Medical Education Officer. Association of American Medical Colleges website. Published December 18, 2020. Accessed September 27, 2021. https://www.aamc.org/media/50291/download
- Underrepresented in medicine definition. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/what-we-do/mission-areas/diversity-inclusion/underrepresented-in-medicine
- Diversity in medicine: facts and figures 2019. table 13. practice specialty, males by race/ethnicity, 2018. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/data-reports/workforce/data/table-13-practice-specialty-males-race/ethnicity-2018 1B
- US Census Bureau. Quick facts: United States. Updated July 1, 2019. Accessed September 20, 2021. https://www.census.gov/quickfacts/fact/table/US/PST045219
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by underrepresented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Williams C, Kwan B, Pereira A, et al. A call to improve conditions for conducting holistic review in graduate medical education recruitment. MedEdPublish. 2019;8:6. https://doi.org/10.15694/mep.2019.000076.1
- Holistic principles in resident selection: an introduction. Association of American Medical Colleges website. Accessed September 27, 2021. https://www.aamc.org/system/files/2020-08/aa-member-capacity-building-holistic-review-transcript-activities-GME-081420.pdf
- Luke J, Cornelius L, Lim H. Dermatology resident selection: shifting toward holistic review? J Am Acad Dermatol. 2020;84:1208-1209. doi:10.1016/j.jaad.2020.11.025
- Open letter on residency interviews from Alison Whelan, MD, AAMC Chief Medical Education Officer. Association of American Medical Colleges website. Published December 18, 2020. Accessed September 27, 2021. https://www.aamc.org/media/50291/download
Practice Points
- Dermatology remains one of the least diverse medical specialties.
- Underrepresented minority (URM) in medicine residency applicants might be negatively affected by the COVID-19 pandemic.
- The implementation of holistic review, diversity and inclusion initiatives, and virtual opportunities might mitigate some of the barriers faced by URM applicants.
Anxiety, depression symptoms rose and fell with new COVID cases
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.
“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.

“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
Anxiety and depression symptoms increased in adults last winter as COVID-19 surged in the United States but declined in the spring as COVID activity approached its nadir, according to an analysis from the Centers for Disease Control and Prevention.

“The relative increases and decreases in frequency of reported symptoms of anxiety and depression at both the national and state levels mirrored the national weekly number of new COVID-19 cases during the same period,” Haomiao Jia, PhD, and associates wrote in the Morbidity and Mortality Weekly Report.
In a national survey conducted Aug. 19-31, 2020, the average anxiety severity score was 2.0 and the average depression score was 1.6 among adults in all 50 states. Those scores rose to 2.3 (+13.0%) and 2.0 (+14.8%), respectively, by Dec. 9-21, but then fell to 1.7 (–26.8%) and 1.4 (–24.8%) during the survey conducted from May 26 to June 7, 2021, the investigators reported.
Despite that decrease in the spring, however, “the frequency of symptoms ... in June 2021 remained elevated compared with estimates from” 2019, said Dr. Jia of Columbia University, New York, and associates. Data from the National Health Interview Survey put the prepandemic severity scores at 0.63 for anxiety and 0.51 for depression.
Weekly symptom frequency in the Household Pulse Survey, which began in April 2020, was assessed with the four-item Patient Health Questionnaire, which includes two questions on anxiety and two on depression. Each answer scored on a scale from 0 (no symptoms at all) to 3 (symptoms nearly every day), making a total of 6 possible for each severity score, they explained. Sample sizes for the biweekly surveys ranged from 58,729 to 110,019.
Among the states, there was something of a pattern involving the drop in scores during the fall and the rise over the winter and spring months. “States with larger increases in severity scores during August–December 2020 also tended to have larger decreases during January–June 2021,” the researchers noted.
That group includes Minnesota, Mississippi, South Dakota, and Utah for anxiety and Idaho, Michigan, Minnesota, and Wisconsin for depression, the survey data show.
Florida and New York had the smallest increases in depression and anxiety scores, respectively, from August to December, and New York had the smallest decrease in both anxiety and depression from January to June, Dr. Jia and associates said.
“ during national emergencies. The observed differences in severity score magnitude and peaks across states in this study indicate that these efforts are important at both the national and state levels,” they wrote.
FROM THE MMWR
Duration of Adalimumab Therapy in Hidradenitis Suppurativa With and Without Oral Immunosuppressants
To the Editor:
The tumor necrosis factor α inhibitor adalimumab is the only US Food and Drug Administration–approved treatment of hidradenitis suppurativa (HS). Although 50.6% of patients fulfilled Hidradenitis Suppurativa Clinical Response criteria with adalimumab at 12 weeks, many responders were not satisfied with their disease control, and secondary loss of Hidradenitis Suppurativa Clinical Response fulfillment occurred in 15.9% of patients within approximately 3 years.1 Without other US Food and Drug Administration–approved HS treatments, some dermatologists have combined adalimumab with methotrexate (MTX) and/or mycophenolate mofetil (MMF) to attempt to increase the duration of satisfactory disease control while on adalimumab. Combining tumor necrosis factor α inhibitors with oral immunosuppressants is a well-established approach in psoriasis, psoriatic arthritis, and inflammatory bowel disease; however, to the best of our knowledge, this approach has not been studied for HS.2,3
To assess whether there is a role for combining adalimumab with MTX and/or MMF in the treatment of HS, we performed a single-institution retrospective chart review at the University of Connecticut Department of Dermatology to determine whether patients receiving combination therapy stayed on adalimumab longer than those who received adalimumab monotherapy. All patients receiving adalimumab for the treatment of HS with at least 1 follow-up visit 3 or more months after treatment initiation were included. Duration of treatment with adalimumab was defined as the length of time between initiation and termination of adalimumab, regardless of flares, adverse events, or addition of adjuvant therapy that occurred during this time span. Because standardized rating scales measuring the severity of HS at this time are not recorded routinely at our institution, treatment duration with adalimumab was used as a surrogate for measuring therapeutic success. Additionally, treatment duration is a meaningful end point, as patients with HS may require indefinite treatment. Patients were eligible for inclusion if they were receiving adalimumab for the treatment of HS. Patients were excluded if they were lost to follow-up or had received adalimumab for less than 6 months, as data suggest that biologics do not reach peak effect for up to 6 months in HS.4
We identified 116 eligible patients with HS, 32 of whom received combination therapy. Five patients received 40 mg of adalimumab every other week, and 111 patients received 40 mg of adalimumab each week. Patients receiving oral immunosuppressants were more likely to be male and as likely to be biologic naïve compared to patients on monotherapy (Table). The average weekly dose of MTX was 14.63 mg, and the average daily dose of MMF was 1000 mg. The average number of days between starting adalimumab and starting an oral immunosuppressant was 114.5 (SD, 217; median, 0) days. Reasons for discontinuation of adalimumab included insufficient response, noncompliance, dislike of injections, adverse events, fear of adverse events, other medical issues unrelated to HS, and insurance coverage issues. Patients who ended treatment with adalimumab owing to insurance coverage issues were still included in our study because insurance coverage remains a major determinant of treatment choice in HS and is relevant to the dynamics of medical decision-making.
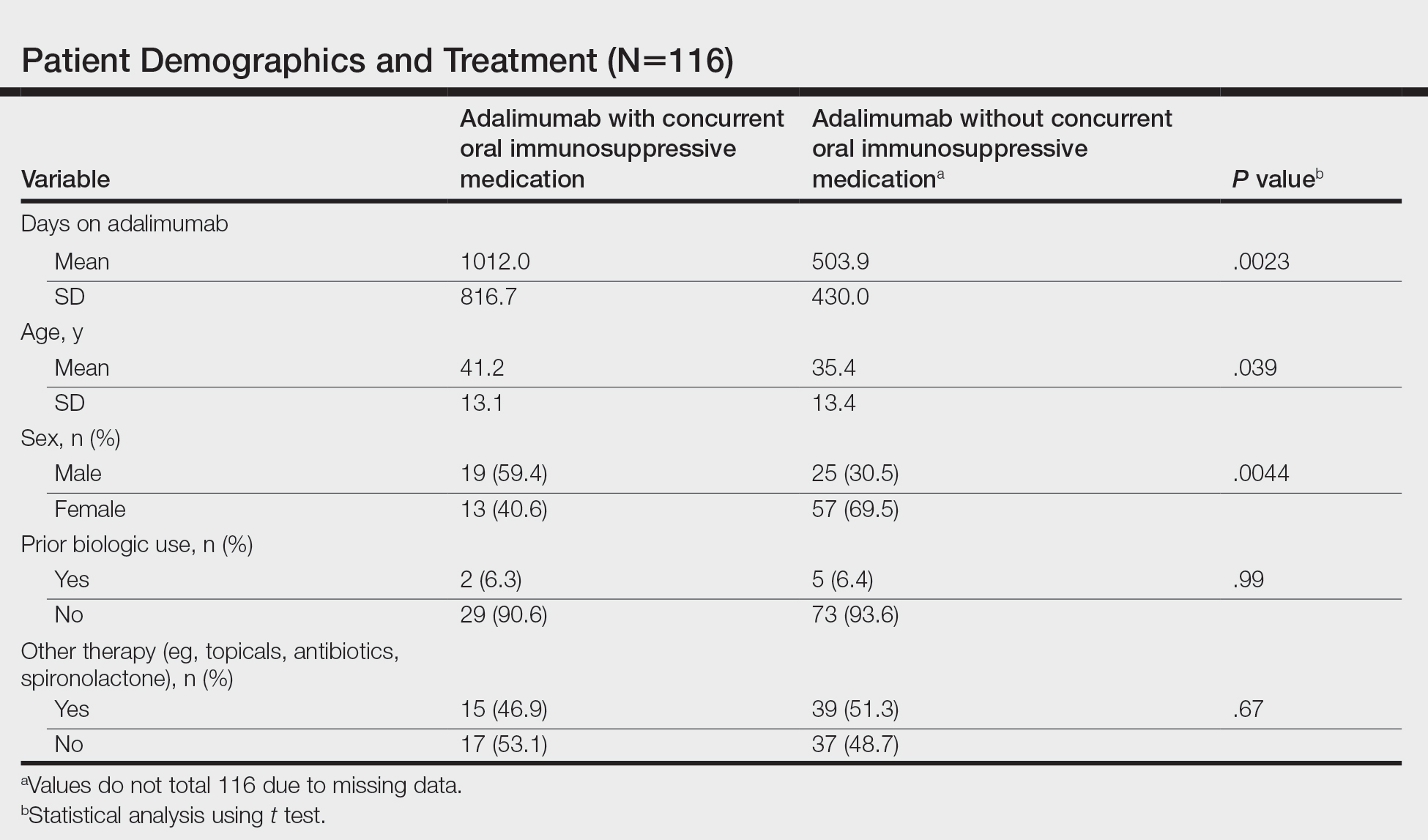
Statistical analysis was conducted on all patients inclusive of any reason for discontinuation to avoid bias in the calculation of treatment duration. Cox regression analysis was conducted for all independent variables and was noncontributory. Kaplan-Meier methodology was used to assess the duration of treatment of adalimumab with and without concomitant oral immunosuppressants, and quartile survival times were calculated. Quartile survival time is the time point after adalimumab initiation at which 25% of patients have discontinued adalimumab. We chose quartile survival time instead of average treatment duration to adequately power this study, given our small patient pool.
Although patients receiving adalimumab with oral immunosuppressants had a longer quartile treatment duration (450 days; 95% CI, 185-1800) than the group without oral immunosuppressants (360 days; 95% CI, 200-700), neither MTX nor MMF was shown to significantly prolong duration of therapy with adalimumab (log-rank test: P=.12). Additionally, patients receiving combination therapy were just as likely to discontinue adalimumab as those on monotherapy (χ2 test: P=.93). Patients who took both MTX and MMF at different times did show a statistically significant increase in adalimumab quartile treatment duration (1710 days; 95% CI, 1620 [upper limit not calculable]), but this is likely because these patients were kept on adalimumab while trialing adjunctive medications.
The results of our study indicate that MTX and MMF do not prolong duration of adalimumab therapy, which suggests that adalimumab combination therapy with MTX and MMF may not improve HS more than adalimumab alone, and/or partial responders to adalimumab monotherapy are unlikely to be converted to satisfactory responders with the addition of oral immunosuppressants. Limitations of our study include that it was retrospective, used treatment duration as a surrogate for objective efficacy measures, and relied on a single-institution data source. Additionally, owing to our small sample size, we were unable to account for certain potential confounders, including patient weight and insurance status. Future controlled prospective studies using objective end points are needed to further elucidate whether oral immunosuppressants have a role as an adjunct in the treatment of HS.
- Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi:10.1016/j.jaad.2018.05.040
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Sultan KS, Berkowitz JC, Khan S. Combination therapy for inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2017;8:103-113. doi:10.4292/wjgpt.v8.i2.103
- Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181:609-611.
To the Editor:
The tumor necrosis factor α inhibitor adalimumab is the only US Food and Drug Administration–approved treatment of hidradenitis suppurativa (HS). Although 50.6% of patients fulfilled Hidradenitis Suppurativa Clinical Response criteria with adalimumab at 12 weeks, many responders were not satisfied with their disease control, and secondary loss of Hidradenitis Suppurativa Clinical Response fulfillment occurred in 15.9% of patients within approximately 3 years.1 Without other US Food and Drug Administration–approved HS treatments, some dermatologists have combined adalimumab with methotrexate (MTX) and/or mycophenolate mofetil (MMF) to attempt to increase the duration of satisfactory disease control while on adalimumab. Combining tumor necrosis factor α inhibitors with oral immunosuppressants is a well-established approach in psoriasis, psoriatic arthritis, and inflammatory bowel disease; however, to the best of our knowledge, this approach has not been studied for HS.2,3
To assess whether there is a role for combining adalimumab with MTX and/or MMF in the treatment of HS, we performed a single-institution retrospective chart review at the University of Connecticut Department of Dermatology to determine whether patients receiving combination therapy stayed on adalimumab longer than those who received adalimumab monotherapy. All patients receiving adalimumab for the treatment of HS with at least 1 follow-up visit 3 or more months after treatment initiation were included. Duration of treatment with adalimumab was defined as the length of time between initiation and termination of adalimumab, regardless of flares, adverse events, or addition of adjuvant therapy that occurred during this time span. Because standardized rating scales measuring the severity of HS at this time are not recorded routinely at our institution, treatment duration with adalimumab was used as a surrogate for measuring therapeutic success. Additionally, treatment duration is a meaningful end point, as patients with HS may require indefinite treatment. Patients were eligible for inclusion if they were receiving adalimumab for the treatment of HS. Patients were excluded if they were lost to follow-up or had received adalimumab for less than 6 months, as data suggest that biologics do not reach peak effect for up to 6 months in HS.4
We identified 116 eligible patients with HS, 32 of whom received combination therapy. Five patients received 40 mg of adalimumab every other week, and 111 patients received 40 mg of adalimumab each week. Patients receiving oral immunosuppressants were more likely to be male and as likely to be biologic naïve compared to patients on monotherapy (Table). The average weekly dose of MTX was 14.63 mg, and the average daily dose of MMF was 1000 mg. The average number of days between starting adalimumab and starting an oral immunosuppressant was 114.5 (SD, 217; median, 0) days. Reasons for discontinuation of adalimumab included insufficient response, noncompliance, dislike of injections, adverse events, fear of adverse events, other medical issues unrelated to HS, and insurance coverage issues. Patients who ended treatment with adalimumab owing to insurance coverage issues were still included in our study because insurance coverage remains a major determinant of treatment choice in HS and is relevant to the dynamics of medical decision-making.

Statistical analysis was conducted on all patients inclusive of any reason for discontinuation to avoid bias in the calculation of treatment duration. Cox regression analysis was conducted for all independent variables and was noncontributory. Kaplan-Meier methodology was used to assess the duration of treatment of adalimumab with and without concomitant oral immunosuppressants, and quartile survival times were calculated. Quartile survival time is the time point after adalimumab initiation at which 25% of patients have discontinued adalimumab. We chose quartile survival time instead of average treatment duration to adequately power this study, given our small patient pool.
Although patients receiving adalimumab with oral immunosuppressants had a longer quartile treatment duration (450 days; 95% CI, 185-1800) than the group without oral immunosuppressants (360 days; 95% CI, 200-700), neither MTX nor MMF was shown to significantly prolong duration of therapy with adalimumab (log-rank test: P=.12). Additionally, patients receiving combination therapy were just as likely to discontinue adalimumab as those on monotherapy (χ2 test: P=.93). Patients who took both MTX and MMF at different times did show a statistically significant increase in adalimumab quartile treatment duration (1710 days; 95% CI, 1620 [upper limit not calculable]), but this is likely because these patients were kept on adalimumab while trialing adjunctive medications.
The results of our study indicate that MTX and MMF do not prolong duration of adalimumab therapy, which suggests that adalimumab combination therapy with MTX and MMF may not improve HS more than adalimumab alone, and/or partial responders to adalimumab monotherapy are unlikely to be converted to satisfactory responders with the addition of oral immunosuppressants. Limitations of our study include that it was retrospective, used treatment duration as a surrogate for objective efficacy measures, and relied on a single-institution data source. Additionally, owing to our small sample size, we were unable to account for certain potential confounders, including patient weight and insurance status. Future controlled prospective studies using objective end points are needed to further elucidate whether oral immunosuppressants have a role as an adjunct in the treatment of HS.
To the Editor:
The tumor necrosis factor α inhibitor adalimumab is the only US Food and Drug Administration–approved treatment of hidradenitis suppurativa (HS). Although 50.6% of patients fulfilled Hidradenitis Suppurativa Clinical Response criteria with adalimumab at 12 weeks, many responders were not satisfied with their disease control, and secondary loss of Hidradenitis Suppurativa Clinical Response fulfillment occurred in 15.9% of patients within approximately 3 years.1 Without other US Food and Drug Administration–approved HS treatments, some dermatologists have combined adalimumab with methotrexate (MTX) and/or mycophenolate mofetil (MMF) to attempt to increase the duration of satisfactory disease control while on adalimumab. Combining tumor necrosis factor α inhibitors with oral immunosuppressants is a well-established approach in psoriasis, psoriatic arthritis, and inflammatory bowel disease; however, to the best of our knowledge, this approach has not been studied for HS.2,3
To assess whether there is a role for combining adalimumab with MTX and/or MMF in the treatment of HS, we performed a single-institution retrospective chart review at the University of Connecticut Department of Dermatology to determine whether patients receiving combination therapy stayed on adalimumab longer than those who received adalimumab monotherapy. All patients receiving adalimumab for the treatment of HS with at least 1 follow-up visit 3 or more months after treatment initiation were included. Duration of treatment with adalimumab was defined as the length of time between initiation and termination of adalimumab, regardless of flares, adverse events, or addition of adjuvant therapy that occurred during this time span. Because standardized rating scales measuring the severity of HS at this time are not recorded routinely at our institution, treatment duration with adalimumab was used as a surrogate for measuring therapeutic success. Additionally, treatment duration is a meaningful end point, as patients with HS may require indefinite treatment. Patients were eligible for inclusion if they were receiving adalimumab for the treatment of HS. Patients were excluded if they were lost to follow-up or had received adalimumab for less than 6 months, as data suggest that biologics do not reach peak effect for up to 6 months in HS.4
We identified 116 eligible patients with HS, 32 of whom received combination therapy. Five patients received 40 mg of adalimumab every other week, and 111 patients received 40 mg of adalimumab each week. Patients receiving oral immunosuppressants were more likely to be male and as likely to be biologic naïve compared to patients on monotherapy (Table). The average weekly dose of MTX was 14.63 mg, and the average daily dose of MMF was 1000 mg. The average number of days between starting adalimumab and starting an oral immunosuppressant was 114.5 (SD, 217; median, 0) days. Reasons for discontinuation of adalimumab included insufficient response, noncompliance, dislike of injections, adverse events, fear of adverse events, other medical issues unrelated to HS, and insurance coverage issues. Patients who ended treatment with adalimumab owing to insurance coverage issues were still included in our study because insurance coverage remains a major determinant of treatment choice in HS and is relevant to the dynamics of medical decision-making.

Statistical analysis was conducted on all patients inclusive of any reason for discontinuation to avoid bias in the calculation of treatment duration. Cox regression analysis was conducted for all independent variables and was noncontributory. Kaplan-Meier methodology was used to assess the duration of treatment of adalimumab with and without concomitant oral immunosuppressants, and quartile survival times were calculated. Quartile survival time is the time point after adalimumab initiation at which 25% of patients have discontinued adalimumab. We chose quartile survival time instead of average treatment duration to adequately power this study, given our small patient pool.
Although patients receiving adalimumab with oral immunosuppressants had a longer quartile treatment duration (450 days; 95% CI, 185-1800) than the group without oral immunosuppressants (360 days; 95% CI, 200-700), neither MTX nor MMF was shown to significantly prolong duration of therapy with adalimumab (log-rank test: P=.12). Additionally, patients receiving combination therapy were just as likely to discontinue adalimumab as those on monotherapy (χ2 test: P=.93). Patients who took both MTX and MMF at different times did show a statistically significant increase in adalimumab quartile treatment duration (1710 days; 95% CI, 1620 [upper limit not calculable]), but this is likely because these patients were kept on adalimumab while trialing adjunctive medications.
The results of our study indicate that MTX and MMF do not prolong duration of adalimumab therapy, which suggests that adalimumab combination therapy with MTX and MMF may not improve HS more than adalimumab alone, and/or partial responders to adalimumab monotherapy are unlikely to be converted to satisfactory responders with the addition of oral immunosuppressants. Limitations of our study include that it was retrospective, used treatment duration as a surrogate for objective efficacy measures, and relied on a single-institution data source. Additionally, owing to our small sample size, we were unable to account for certain potential confounders, including patient weight and insurance status. Future controlled prospective studies using objective end points are needed to further elucidate whether oral immunosuppressants have a role as an adjunct in the treatment of HS.
- Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi:10.1016/j.jaad.2018.05.040
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Sultan KS, Berkowitz JC, Khan S. Combination therapy for inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2017;8:103-113. doi:10.4292/wjgpt.v8.i2.103
- Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181:609-611.
- Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi:10.1016/j.jaad.2018.05.040
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Sultan KS, Berkowitz JC, Khan S. Combination therapy for inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2017;8:103-113. doi:10.4292/wjgpt.v8.i2.103
- Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181:609-611.
Practice Points
- Adalimumab is the only medication approved by the US Food and Drug Administration for treatment of hidradenitis suppurativa (HS), yet many patients on adalimumab do not achieve satisfactory results. New treatment options are in demand for patients affected by HS.
- Although combining tumor necrosis factor α inhibitors with oral immunosuppressants such as methotrexate and mycophenolate mofetil appears to be beneficial in treating other conditions such as psoriasis, these treatments may not have as great a benefit for patients with HS.
Mineral Oil Scabies Preparation From Under the Fingernail
Practice Gap
The Sarcoptes scabiei mite is a microscopic organism that causes scabies in the human host. The scabies mite is highly transmissible, making scabies a common disease in heavily populated areas. The mite survives by burrowing into the epidermis, where it feeds, lays eggs, and defecates.1
The rash in the host represents an allergic reaction to the body of the scabies mite, producing symptoms such as intense itching, rash, and erosions of the skin. The scabies rash tends to occur in warm and occluded areas of the body such as the hands, axillae, groin, buttocks, and feet.1,2
Delaying treatment of scabies can be hazardous because of the risk of rapid spread from one person to another. This rapid spread can be debilitating in specific populations, such as the immunocompromised, elderly, and disabled.
Mineral oil preparation is the classic method used to identify scabies (Figure 1). This method relies on obtaining mites by applying mineral oil to the skin and using a 15-mm blade to scrape off layers of the affected skin. The scraped material is spread onto a microscope slide with mineral oil, a coverslip is applied, and the specimen is analyzed by direct microscopy. This method proves only as effective as knowing where the few mites are located.
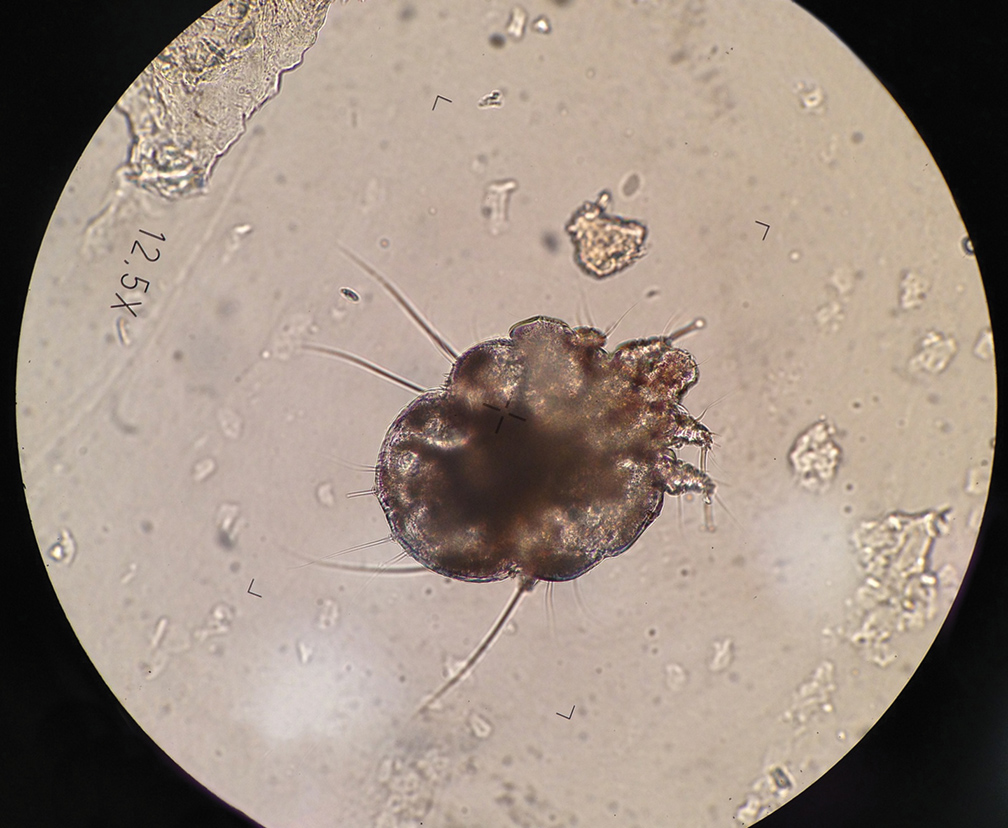
At any time, only 10 to 12 mites live on a human host.3 Therefore, it can be challenging to obtain a mite for diagnosis because the location of the skin mites may be unknown. Dermoscopy can be used to locate burrows and other signs of S scabiei. With a dermatoscope, the scabies mite can be identified by the so-called delta-wing jet sign.4
However, dermoscopy is not always successful because extensive hemorrhagic crusting and erosions of the skin secondary to constant scratching can obscure the appearance of burrows and mites. Because patients are constantly scratching areas of irritation, it is possible that S scabiei can be located under the fingernail of the dominant hand.
The Technique
To address this practice gap, a mineral oil scabies preparation can be performed by scraping under the fingernail plate at the level of the hyponychium. Mites might accumulate underneath the fingernails of the dominant hand when patients scratch the area of the skin where S scabiei mites are burrowing and reproducing.
A convenient and painless way to obtain a mineral oil scabies preparation from under the fingernail is to use the tip of a disposable hyfrecator, readily available in most dermatology practices for use in electrosurgery (Figure 2). Using the blunt end of the hyfrecator tip for the mineral oil preparation would be done without attachment to the full apparatus.
The hyponychium of the fingernail is prepared with mineral oil, which aids in collecting and suspending the material obtained from under the nail plate. Using the blunt end of the hyfrecator tip, material from underneath the fingernail is removed using a gentle sweeping motion (Figure 3). The specimen is then analyzed under the microscope similar to a routine mineral oil scabies preparation. This method can be utilized by health care providers for easy and painless diagnosis of scabies.

Practice Implications
Use of a blunt hyfrecator tip to extract S scabiei from underneath the fingernail plate can be used for efficient diagnosis of scabies. This technique can be implemented in any clinic where blunt-tip hyfrecator electrodes are available. Using a gentle sweeping motion, the blunt-tip hyfrecator allows the provider to extract material from under the fingernail for diagnosis. The material obtained is used to prepare a mineral oil scabies preparation for direct microscopic analysis.
This technique can diagnose scabies efficiently, and treatment can be initiated promptly. Use of a disposable blunt-tip hyfrecator for scabies extraction is a novel technique that can be added to the armamentarium of tools to diagnose scabies, which includes traditional mineral oil preparation and dermoscopy.
- Banerji A; Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee. Scabies. Paediatr Child Health. 2015;20:395-402. doi:10.1093/pch/20.7.395
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35:197-206. doi:10.1017/S0031182000021612
- Fox G. Diagnosis of scabies by dermoscopy [published online February 2, 2009]. BMJ Case Rep. 2009;2009:bcr06.2008.0279. doi:10.1136/bcr.06.2008.0279
Practice Gap
The Sarcoptes scabiei mite is a microscopic organism that causes scabies in the human host. The scabies mite is highly transmissible, making scabies a common disease in heavily populated areas. The mite survives by burrowing into the epidermis, where it feeds, lays eggs, and defecates.1
The rash in the host represents an allergic reaction to the body of the scabies mite, producing symptoms such as intense itching, rash, and erosions of the skin. The scabies rash tends to occur in warm and occluded areas of the body such as the hands, axillae, groin, buttocks, and feet.1,2
Delaying treatment of scabies can be hazardous because of the risk of rapid spread from one person to another. This rapid spread can be debilitating in specific populations, such as the immunocompromised, elderly, and disabled.
Mineral oil preparation is the classic method used to identify scabies (Figure 1). This method relies on obtaining mites by applying mineral oil to the skin and using a 15-mm blade to scrape off layers of the affected skin. The scraped material is spread onto a microscope slide with mineral oil, a coverslip is applied, and the specimen is analyzed by direct microscopy. This method proves only as effective as knowing where the few mites are located.

At any time, only 10 to 12 mites live on a human host.3 Therefore, it can be challenging to obtain a mite for diagnosis because the location of the skin mites may be unknown. Dermoscopy can be used to locate burrows and other signs of S scabiei. With a dermatoscope, the scabies mite can be identified by the so-called delta-wing jet sign.4
However, dermoscopy is not always successful because extensive hemorrhagic crusting and erosions of the skin secondary to constant scratching can obscure the appearance of burrows and mites. Because patients are constantly scratching areas of irritation, it is possible that S scabiei can be located under the fingernail of the dominant hand.
The Technique
To address this practice gap, a mineral oil scabies preparation can be performed by scraping under the fingernail plate at the level of the hyponychium. Mites might accumulate underneath the fingernails of the dominant hand when patients scratch the area of the skin where S scabiei mites are burrowing and reproducing.
A convenient and painless way to obtain a mineral oil scabies preparation from under the fingernail is to use the tip of a disposable hyfrecator, readily available in most dermatology practices for use in electrosurgery (Figure 2). Using the blunt end of the hyfrecator tip for the mineral oil preparation would be done without attachment to the full apparatus.

The hyponychium of the fingernail is prepared with mineral oil, which aids in collecting and suspending the material obtained from under the nail plate. Using the blunt end of the hyfrecator tip, material from underneath the fingernail is removed using a gentle sweeping motion (Figure 3). The specimen is then analyzed under the microscope similar to a routine mineral oil scabies preparation. This method can be utilized by health care providers for easy and painless diagnosis of scabies.

Practice Implications
Use of a blunt hyfrecator tip to extract S scabiei from underneath the fingernail plate can be used for efficient diagnosis of scabies. This technique can be implemented in any clinic where blunt-tip hyfrecator electrodes are available. Using a gentle sweeping motion, the blunt-tip hyfrecator allows the provider to extract material from under the fingernail for diagnosis. The material obtained is used to prepare a mineral oil scabies preparation for direct microscopic analysis.
This technique can diagnose scabies efficiently, and treatment can be initiated promptly. Use of a disposable blunt-tip hyfrecator for scabies extraction is a novel technique that can be added to the armamentarium of tools to diagnose scabies, which includes traditional mineral oil preparation and dermoscopy.
Practice Gap
The Sarcoptes scabiei mite is a microscopic organism that causes scabies in the human host. The scabies mite is highly transmissible, making scabies a common disease in heavily populated areas. The mite survives by burrowing into the epidermis, where it feeds, lays eggs, and defecates.1
The rash in the host represents an allergic reaction to the body of the scabies mite, producing symptoms such as intense itching, rash, and erosions of the skin. The scabies rash tends to occur in warm and occluded areas of the body such as the hands, axillae, groin, buttocks, and feet.1,2
Delaying treatment of scabies can be hazardous because of the risk of rapid spread from one person to another. This rapid spread can be debilitating in specific populations, such as the immunocompromised, elderly, and disabled.
Mineral oil preparation is the classic method used to identify scabies (Figure 1). This method relies on obtaining mites by applying mineral oil to the skin and using a 15-mm blade to scrape off layers of the affected skin. The scraped material is spread onto a microscope slide with mineral oil, a coverslip is applied, and the specimen is analyzed by direct microscopy. This method proves only as effective as knowing where the few mites are located.

At any time, only 10 to 12 mites live on a human host.3 Therefore, it can be challenging to obtain a mite for diagnosis because the location of the skin mites may be unknown. Dermoscopy can be used to locate burrows and other signs of S scabiei. With a dermatoscope, the scabies mite can be identified by the so-called delta-wing jet sign.4
However, dermoscopy is not always successful because extensive hemorrhagic crusting and erosions of the skin secondary to constant scratching can obscure the appearance of burrows and mites. Because patients are constantly scratching areas of irritation, it is possible that S scabiei can be located under the fingernail of the dominant hand.
The Technique
To address this practice gap, a mineral oil scabies preparation can be performed by scraping under the fingernail plate at the level of the hyponychium. Mites might accumulate underneath the fingernails of the dominant hand when patients scratch the area of the skin where S scabiei mites are burrowing and reproducing.
A convenient and painless way to obtain a mineral oil scabies preparation from under the fingernail is to use the tip of a disposable hyfrecator, readily available in most dermatology practices for use in electrosurgery (Figure 2). Using the blunt end of the hyfrecator tip for the mineral oil preparation would be done without attachment to the full apparatus.

The hyponychium of the fingernail is prepared with mineral oil, which aids in collecting and suspending the material obtained from under the nail plate. Using the blunt end of the hyfrecator tip, material from underneath the fingernail is removed using a gentle sweeping motion (Figure 3). The specimen is then analyzed under the microscope similar to a routine mineral oil scabies preparation. This method can be utilized by health care providers for easy and painless diagnosis of scabies.

Practice Implications
Use of a blunt hyfrecator tip to extract S scabiei from underneath the fingernail plate can be used for efficient diagnosis of scabies. This technique can be implemented in any clinic where blunt-tip hyfrecator electrodes are available. Using a gentle sweeping motion, the blunt-tip hyfrecator allows the provider to extract material from under the fingernail for diagnosis. The material obtained is used to prepare a mineral oil scabies preparation for direct microscopic analysis.
This technique can diagnose scabies efficiently, and treatment can be initiated promptly. Use of a disposable blunt-tip hyfrecator for scabies extraction is a novel technique that can be added to the armamentarium of tools to diagnose scabies, which includes traditional mineral oil preparation and dermoscopy.
- Banerji A; Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee. Scabies. Paediatr Child Health. 2015;20:395-402. doi:10.1093/pch/20.7.395
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35:197-206. doi:10.1017/S0031182000021612
- Fox G. Diagnosis of scabies by dermoscopy [published online February 2, 2009]. BMJ Case Rep. 2009;2009:bcr06.2008.0279. doi:10.1136/bcr.06.2008.0279
- Banerji A; Canadian Paediatric Society, First Nations, Inuit and Métis Health Committee. Scabies. Paediatr Child Health. 2015;20:395-402. doi:10.1093/pch/20.7.395
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Mellanby K. The development of symptoms, parasitic infection and immunity in human scabies. Parasitology. 1944;35:197-206. doi:10.1017/S0031182000021612
- Fox G. Diagnosis of scabies by dermoscopy [published online February 2, 2009]. BMJ Case Rep. 2009;2009:bcr06.2008.0279. doi:10.1136/bcr.06.2008.0279
Cutaneous Manifestations and Clinical Disparities in Patients Without Housing
More than half a million individuals are without housing (NWH) on any given night in the United States, as estimated by the US Department of Housing and Urban Development. 1 Lack of hygiene, increased risk of infection and infestation due to living conditions, and barriers to health care put these individuals at increased risk for disease. 2 Skin disease, including fungal infection and acne, are within the top 10 most prevalent diseases worldwide and can cause major psychologic impairment, yet dermatologic concerns and clinical outcomes in NWH patients have not been well characterized. 2-5 Further, because this vulnerable demographic tends to be underinsured, they frequently present to the emergency department (ED) for management of disease. 1,6 Survey of common concerns in NWH patients is of utility to consulting dermatologists and nondermatologist providers in the ED, who can familiarize themselves with management of diseases they are more likely to encounter. Few studies examine dermatologic conditions in the ED, and a thorough literature review indicates none have included homelessness as a variable. 6,7 Additionally, comparison with a matched control group of patients with housing (WH) is limited. 5,8 We present one of the largest comparisons of cutaneous disease in NWH vs WH patients in a single hospital system to elucidate the types of cutaneous disease that motivate patients to seek care, the location of skin disease, and differences in clinical care.
Methods
A retrospective medical record review of patients seen for an inclusive list of dermatologic diagnoses in the ED or while admitted at University Medical Center New Orleans, Louisiana (UMC), between January 1, 2018, and April 21, 2020, was conducted. This study was qualified as exempt from the institutional review board by Louisiana State University because it proposed zero risk to the patients and remained completely anonymous. Eight hundred forty-two total medical records were reviewed (NWH, 421; WH, 421)(Table 1). Patients with housing were matched based on self-identified race and ethnicity, sex, and age. Disease categories were constructed based on fundamental pathophysiology adapted from Dermatology9: infectious, noninfectious inflammatory, neoplasm, trauma and wounds, drug-related eruptions, vascular, pruritic, pigmented, bullous, neuropsychiatric, and other. Other included unspecified eruptions as well as miscellaneous lesions such as calluses. The current chief concern, anatomic location, and configuration were recorded, as well as biopsied lesions and outpatient referrals or inpatient consultations to dermatology or other specialties, including wound care, infectious disease, podiatry, and surgery. χ2 analysis was used to analyze significance of cutaneous categories, body location, and referrals. Groups smaller than 5 defaulted to the Fisher exact test.

Results
The total diagnoses (including both chief concerns and secondary diagnoses) are shown in Table 2. Chief concerns were more frequently cutaneous or dermatologic for WH (NWH, 209; WH, 307; P<.001). In both groups, cutaneous infectious etiologies were more likely to be a patient’s presenting chief concern (58% NWH, P=.002; 42% WH, P<.001). Noninfectious inflammatory etiologies and pigmented lesions were more likely to be secondary diagnoses with an unrelated noncutaneous concern; noninfectious inflammatory etiologies were only 16% of the total cutaneous chief concerns (11% NWH, P=.04; 20% WH, P=.03), and no pigmented lesions were chief concerns.
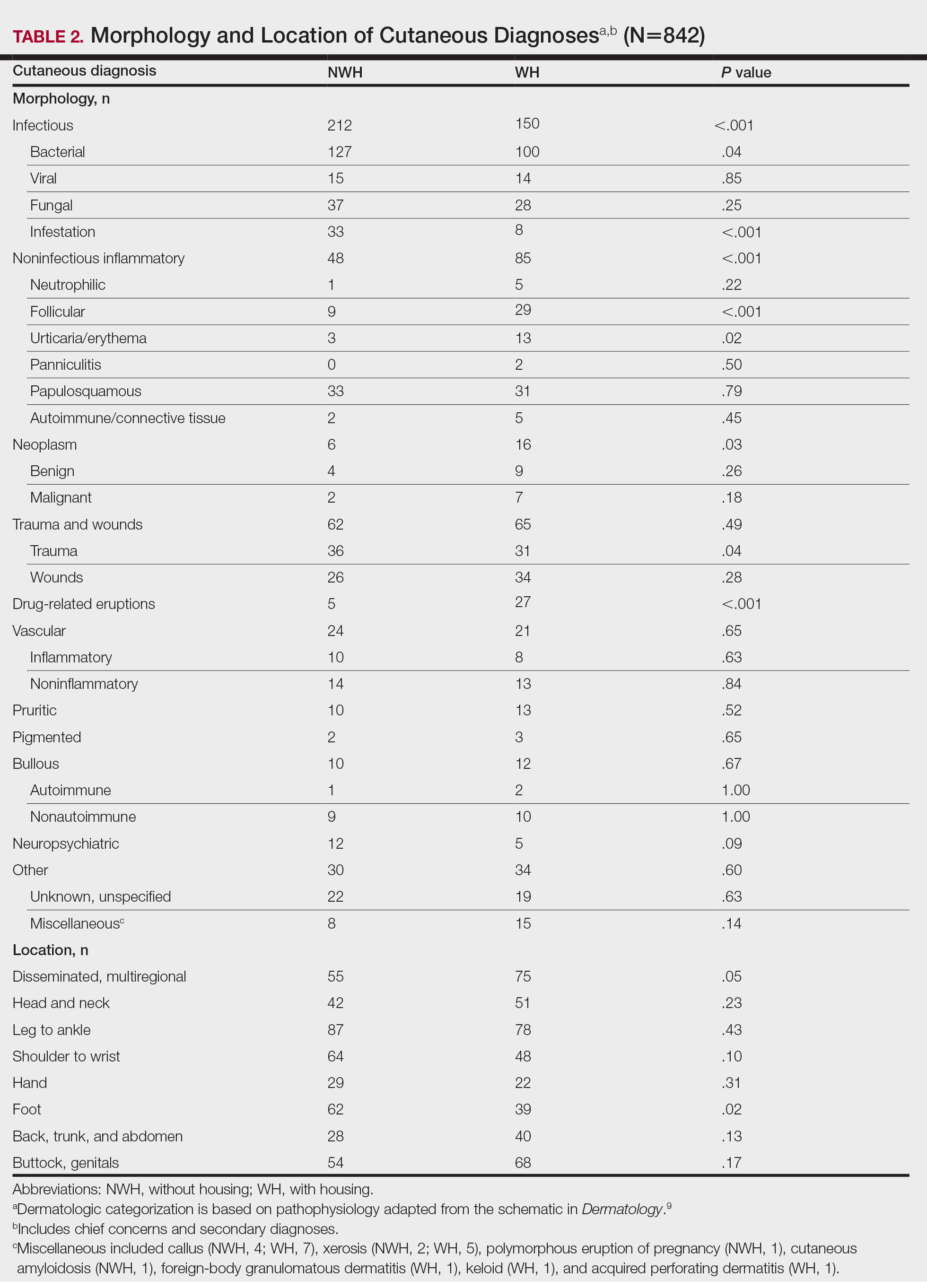
Infection was the most common chief concern, though NWH patients presented with significantly more infectious concerns (NWH, 212; WH, 150; P<.001), particularly infestations (NWH, 33; WH, 8; P<.001) and bacterial etiologies (NWH, 127; WH, 100; P=.04). The majority of bacterial etiologies were either an abscess or cellulitis (NWH, 106; WH, 83), though infected chronic wounds were categorized as bacterial infection when treated definitively as such (eg, in the case of sacral ulcers causing osteomyelitis)(NWH, 21; WH, 17). Of note, infectious etiology was associated with intravenous drug use (IVDU) in both NWH and WH patients. Of 184 NWH who reported IVDU, 127 had an infectious diagnosis (P<.001). Similarly, 43 of 56 total WH patients who reported IVDU had an infectious diagnosis (P<.001). Infestation (within the infectious category) included scabies (NWH, 20; WH, 3) and insect or arthropod bites (NWH, 12; WH, 5). Two NWH patients also presented with swelling of the lower extremities and were subsequently diagnosed with maggot infestations. Fungal and viral etiologies were not significantly increased in either group; however, NWH did have a higher incidence of tinea pedis (NWH, 14; WH, 4; P=.03).
More neoplasms (NWH, 6; WH, 16; P=.03), noninfectious inflammatory eruptions (NWH, 48; WH, 85; P<.001), and cutaneous drug eruptions (NWH, 5; WH, 27; P<.001) were reported in WH patients. There was no significant difference in benign vs malignant neoplastic processes between groups. More noninfectious inflammatory eruptions in WH were specifically driven by a markedly increased incidence of follicular (NWH, 9; WH, 29; P<.001) and urticarial/erythematous (NWH, 3; WH, 13; P=.02) lesions. Follicular etiologies included acne (NWH, 1; WH, 6; P=.12), folliculitis (NWH, 5; WH, 2; P=.45), hidradenitis suppurativa (NWH, 2; WH, 11; P=.02), and pilonidal and sebaceous cysts (NWH, 1; WH, 10; P=.01). Allergic urticaria dominated the urticarial/erythematous category (NWH, 3; WH, 11; P=.06), though there were 2 WH presentations of diffuse erythema and skin peeling.
Another substantial proportion of cutaneous etiologies were due to trauma or chronic wounds. Significantly more traumatic injuries presented in NWH patients vs WH patients (36 vs 31; P=.04). Trauma included human or dog bites (NWH, 5; WH, 4), sunburns (NWH, 3; WH, 0), other burns (NWH, 11; WH, 13), abrasions and lacerations (NWH, 16; WH, 3; P=.004), and foreign bodies (NWH, 1; WH, 1). Wounds consisted of chronic wounds such as those due to diabetes mellitus (foot ulcers) or immobility (sacral ulcers); numbers were similar between groups.
Looking at location, NWH patients had more pathology on the feet (NWH, 62; WH, 39; P=.02), whereas WH patients had more disseminated multiregional concerns (NWH, 55; WH, 75; P=.05). No one body location was notably more likely to warrant a chief concern.
For clinical outcomes, more WH patients received a consultation of any kind (NWH, 171; WH, 217; P<.001), consultation to dermatology (NWH, 49; WH, 87; P<.001), and consultation to surgery (NWH, 64; WH, 110; P<.001)(Table 3 and Figure). More outpatient referrals to dermatology were made for WH patients (NWH, 61; WH, 82; P=.05). Notably, NWH patients presented for 80% fewer hospital follow-up appointments (NWH, 11; WH, 55; P<.001). It is essential to note that these findings were not affected by self-reported race or ethnicity. Results remained significant when broken into cohorts consisting of patients with and without skin of color.
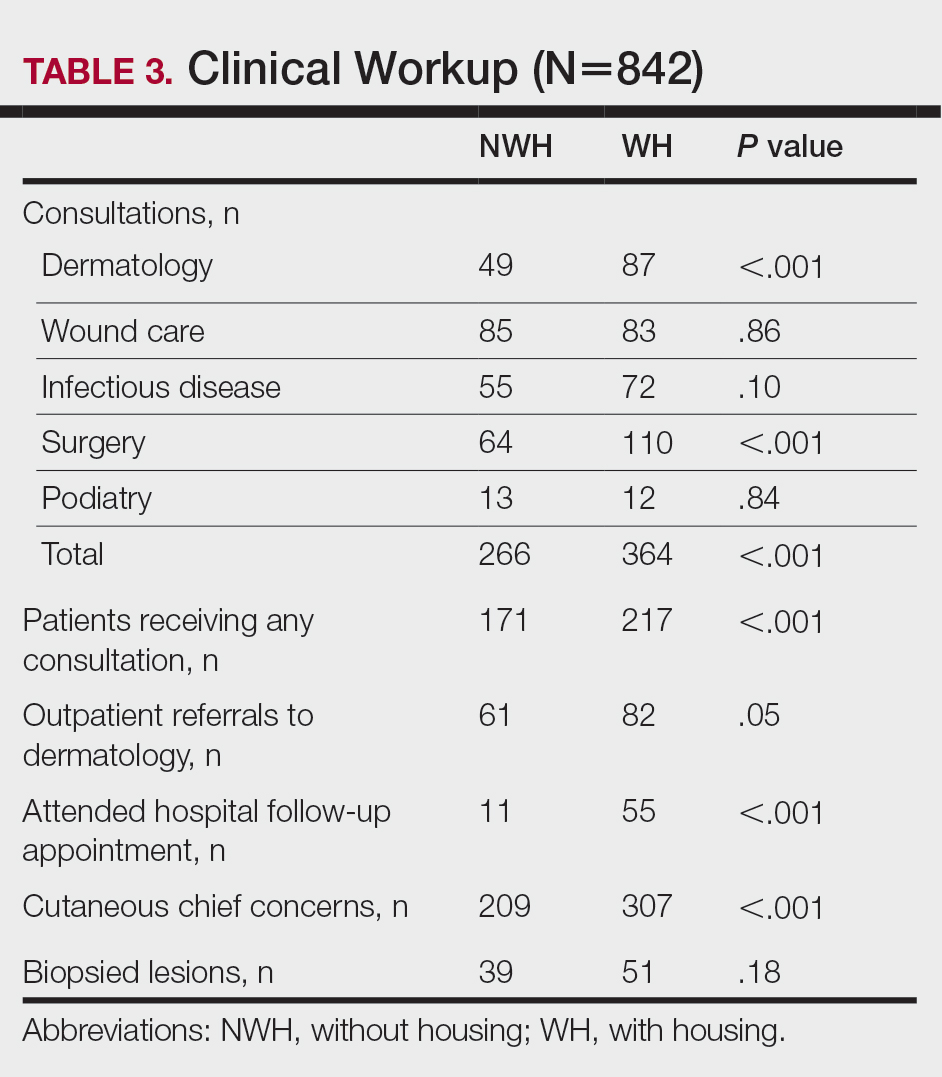
Comment
Cutaneous Concerns in NWH Patients—Although cutaneous disease has been reported to disproportionately affect NWH patients,10 in our cohort, NWH patients had fewer cutaneous chief concerns than WH patients. However, without comparing with all patients entering the ED at UMC, we cannot make a statement on this claim. We do present a few reasons why NWH patients do not have more cutaneous concerns. First, they may wait to present with cutaneous disease until it becomes more severe (eg, until chronic wounds have progressed to infections). Second, as discussed in depth by Hollestein and Nijsten,3 dermatologic disease may be a major contributor to the overall count of disability-adjusted life years but may play a minor role in individual disability. Therefore, skin disease often is considered less important on an individual basis, despite substantial psychosocial burden, leading to further stigmatization of this vulnerable population and discouraged care-seeking behavior, particularly for noninfectious inflammatory eruptions, which were notably more present in WH individuals. Third, fewer dermatologic lesions were reported on NWH patients, which may explain why all 3 WH pigmented lesions were diagnosed after presentation with a noncutaneous concern (eg, headache, anemia, nausea).
Infectious Cutaneous Diagnoses—The increased presentation of infectious etiologies, especially bacterial, is linked to the increased numbers of IVDUs reported in NWH individuals as well as increased exposure and decreased access to basic hygienic supplies. Intravenous drug use acted as an effect modifier of infectious etiology diagnoses, playing a major role in both NWH and WH cohorts. Although Black and Hispanic individuals as well as individuals with low socioeconomic status have increased proportions of skin cancer, there are inadequate data on the prevalence in NWH individuals.4 We found no increase in malignant dermatologic processes in NWH individuals; however, this may be secondary to inadequate screening with a total body skin examination.
Clinical Workup of NWH Patients—Because most NWH individuals present to the ED to receive care, their care compared with WH patients should be considered. In this cohort, WH patients received a less extensive clinical workup. They received almost half as many dermatologic consultations and fewer outpatient referrals to dermatology. Major communication barriers may affect NWH presentation to follow-up, which was drastically lower than WH individuals, as scheduling typically occurs well after discharge from the ED or inpatient unit. We suggest a few alterations to improve dermatologic care for NWH individuals:
• Consider inpatient consultation for serious dermatologic conditions—even if chronic—to improve disease control, considering that many barriers inhibit follow-up in clinic.
• Involve outreach teams, such as the Assertive Community Treatment teams, that assist individuals by delivering medicine for psychiatric disorders, conducting total-body skin examinations, assisting with wound care, providing basic skin barrier creams or medicaments, and carrying information regarding outpatient follow-up.
• Educate ED providers on the most common skin concerns, especially those that fall within the noninfectious inflammatory category, such as hidradenitis suppurativa, which could easily be misdiagnosed as an abscess.
Future Directions—Owing to limitations of a retrospective cohort study, we present several opportunities for further research on this vulnerable population. The severity of disease, especially infectious etiologies, should be graded to determine if NWH patients truly present later in the disease course. The duration and quality of housing for NWH patients could be categorized based on living conditions (eg, on the street vs in a shelter). Although the findings of our NWH cohort presenting to the ED at UMC provide helpful insight into dermatologic disease, these findings may be disparate from those conducted at other locations in the United States. University Medical Center provides care to mostly subsidized insurance plans in a racially diverse community. Improved outcomes for the NWH individuals living in New Orleans start with obtaining a greater understanding of their diseases and where disparities exist that can be bridged with better care.
Acknowledgment—The dataset generated during this study and used for analysis is not publicly available to protect public health information but is available from the corresponding author on reasonable request.
- Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529-1540. doi:10.1016/S0140-6736(14)61132-6
- Contag C, Lowenstein SE, Jain S, et al. Survey of symptomatic dermatologic disease in homeless patients at a shelter-based clinic. Our Dermatol Online. 2017;8:133-137. doi:10.7241/ourd.20172.37
- Hollestein LM, Nijsten T. An insight into the global burden of skin diseases. J Invest Dermatol. 2014;134:1499-1501. doi:10.1038/jid.2013.513
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Mackelprang JL, Graves JM, Rivara FP. Homeless in America: injuries treated in US emergency departments, 2007-2011. Int J Inj Contr Saf Promot. 2014;21:289-297. doi:10.1038/jid.2014.371
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313. doi:10.1016/j.jaad.2014.03.013
- Stratigos AJ, Stern R, Gonzalez E, et al. Prevalence of skin disease in a cohort of shelter-based homeless men. J Am Acad Dermatol. 1999;41:197-202. doi:10.1016/S0190-9622(99)70048-4
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012.
- Badiaga S, Menard A, Tissot Dupont H, et al. Prevalence of skin infections in sheltered homeless. Eur J Dermatol. 2005;15:382-386.
More than half a million individuals are without housing (NWH) on any given night in the United States, as estimated by the US Department of Housing and Urban Development. 1 Lack of hygiene, increased risk of infection and infestation due to living conditions, and barriers to health care put these individuals at increased risk for disease. 2 Skin disease, including fungal infection and acne, are within the top 10 most prevalent diseases worldwide and can cause major psychologic impairment, yet dermatologic concerns and clinical outcomes in NWH patients have not been well characterized. 2-5 Further, because this vulnerable demographic tends to be underinsured, they frequently present to the emergency department (ED) for management of disease. 1,6 Survey of common concerns in NWH patients is of utility to consulting dermatologists and nondermatologist providers in the ED, who can familiarize themselves with management of diseases they are more likely to encounter. Few studies examine dermatologic conditions in the ED, and a thorough literature review indicates none have included homelessness as a variable. 6,7 Additionally, comparison with a matched control group of patients with housing (WH) is limited. 5,8 We present one of the largest comparisons of cutaneous disease in NWH vs WH patients in a single hospital system to elucidate the types of cutaneous disease that motivate patients to seek care, the location of skin disease, and differences in clinical care.
Methods
A retrospective medical record review of patients seen for an inclusive list of dermatologic diagnoses in the ED or while admitted at University Medical Center New Orleans, Louisiana (UMC), between January 1, 2018, and April 21, 2020, was conducted. This study was qualified as exempt from the institutional review board by Louisiana State University because it proposed zero risk to the patients and remained completely anonymous. Eight hundred forty-two total medical records were reviewed (NWH, 421; WH, 421)(Table 1). Patients with housing were matched based on self-identified race and ethnicity, sex, and age. Disease categories were constructed based on fundamental pathophysiology adapted from Dermatology9: infectious, noninfectious inflammatory, neoplasm, trauma and wounds, drug-related eruptions, vascular, pruritic, pigmented, bullous, neuropsychiatric, and other. Other included unspecified eruptions as well as miscellaneous lesions such as calluses. The current chief concern, anatomic location, and configuration were recorded, as well as biopsied lesions and outpatient referrals or inpatient consultations to dermatology or other specialties, including wound care, infectious disease, podiatry, and surgery. χ2 analysis was used to analyze significance of cutaneous categories, body location, and referrals. Groups smaller than 5 defaulted to the Fisher exact test.

Results
The total diagnoses (including both chief concerns and secondary diagnoses) are shown in Table 2. Chief concerns were more frequently cutaneous or dermatologic for WH (NWH, 209; WH, 307; P<.001). In both groups, cutaneous infectious etiologies were more likely to be a patient’s presenting chief concern (58% NWH, P=.002; 42% WH, P<.001). Noninfectious inflammatory etiologies and pigmented lesions were more likely to be secondary diagnoses with an unrelated noncutaneous concern; noninfectious inflammatory etiologies were only 16% of the total cutaneous chief concerns (11% NWH, P=.04; 20% WH, P=.03), and no pigmented lesions were chief concerns.

Infection was the most common chief concern, though NWH patients presented with significantly more infectious concerns (NWH, 212; WH, 150; P<.001), particularly infestations (NWH, 33; WH, 8; P<.001) and bacterial etiologies (NWH, 127; WH, 100; P=.04). The majority of bacterial etiologies were either an abscess or cellulitis (NWH, 106; WH, 83), though infected chronic wounds were categorized as bacterial infection when treated definitively as such (eg, in the case of sacral ulcers causing osteomyelitis)(NWH, 21; WH, 17). Of note, infectious etiology was associated with intravenous drug use (IVDU) in both NWH and WH patients. Of 184 NWH who reported IVDU, 127 had an infectious diagnosis (P<.001). Similarly, 43 of 56 total WH patients who reported IVDU had an infectious diagnosis (P<.001). Infestation (within the infectious category) included scabies (NWH, 20; WH, 3) and insect or arthropod bites (NWH, 12; WH, 5). Two NWH patients also presented with swelling of the lower extremities and were subsequently diagnosed with maggot infestations. Fungal and viral etiologies were not significantly increased in either group; however, NWH did have a higher incidence of tinea pedis (NWH, 14; WH, 4; P=.03).
More neoplasms (NWH, 6; WH, 16; P=.03), noninfectious inflammatory eruptions (NWH, 48; WH, 85; P<.001), and cutaneous drug eruptions (NWH, 5; WH, 27; P<.001) were reported in WH patients. There was no significant difference in benign vs malignant neoplastic processes between groups. More noninfectious inflammatory eruptions in WH were specifically driven by a markedly increased incidence of follicular (NWH, 9; WH, 29; P<.001) and urticarial/erythematous (NWH, 3; WH, 13; P=.02) lesions. Follicular etiologies included acne (NWH, 1; WH, 6; P=.12), folliculitis (NWH, 5; WH, 2; P=.45), hidradenitis suppurativa (NWH, 2; WH, 11; P=.02), and pilonidal and sebaceous cysts (NWH, 1; WH, 10; P=.01). Allergic urticaria dominated the urticarial/erythematous category (NWH, 3; WH, 11; P=.06), though there were 2 WH presentations of diffuse erythema and skin peeling.
Another substantial proportion of cutaneous etiologies were due to trauma or chronic wounds. Significantly more traumatic injuries presented in NWH patients vs WH patients (36 vs 31; P=.04). Trauma included human or dog bites (NWH, 5; WH, 4), sunburns (NWH, 3; WH, 0), other burns (NWH, 11; WH, 13), abrasions and lacerations (NWH, 16; WH, 3; P=.004), and foreign bodies (NWH, 1; WH, 1). Wounds consisted of chronic wounds such as those due to diabetes mellitus (foot ulcers) or immobility (sacral ulcers); numbers were similar between groups.
Looking at location, NWH patients had more pathology on the feet (NWH, 62; WH, 39; P=.02), whereas WH patients had more disseminated multiregional concerns (NWH, 55; WH, 75; P=.05). No one body location was notably more likely to warrant a chief concern.
For clinical outcomes, more WH patients received a consultation of any kind (NWH, 171; WH, 217; P<.001), consultation to dermatology (NWH, 49; WH, 87; P<.001), and consultation to surgery (NWH, 64; WH, 110; P<.001)(Table 3 and Figure). More outpatient referrals to dermatology were made for WH patients (NWH, 61; WH, 82; P=.05). Notably, NWH patients presented for 80% fewer hospital follow-up appointments (NWH, 11; WH, 55; P<.001). It is essential to note that these findings were not affected by self-reported race or ethnicity. Results remained significant when broken into cohorts consisting of patients with and without skin of color.

Comment
Cutaneous Concerns in NWH Patients—Although cutaneous disease has been reported to disproportionately affect NWH patients,10 in our cohort, NWH patients had fewer cutaneous chief concerns than WH patients. However, without comparing with all patients entering the ED at UMC, we cannot make a statement on this claim. We do present a few reasons why NWH patients do not have more cutaneous concerns. First, they may wait to present with cutaneous disease until it becomes more severe (eg, until chronic wounds have progressed to infections). Second, as discussed in depth by Hollestein and Nijsten,3 dermatologic disease may be a major contributor to the overall count of disability-adjusted life years but may play a minor role in individual disability. Therefore, skin disease often is considered less important on an individual basis, despite substantial psychosocial burden, leading to further stigmatization of this vulnerable population and discouraged care-seeking behavior, particularly for noninfectious inflammatory eruptions, which were notably more present in WH individuals. Third, fewer dermatologic lesions were reported on NWH patients, which may explain why all 3 WH pigmented lesions were diagnosed after presentation with a noncutaneous concern (eg, headache, anemia, nausea).

Infectious Cutaneous Diagnoses—The increased presentation of infectious etiologies, especially bacterial, is linked to the increased numbers of IVDUs reported in NWH individuals as well as increased exposure and decreased access to basic hygienic supplies. Intravenous drug use acted as an effect modifier of infectious etiology diagnoses, playing a major role in both NWH and WH cohorts. Although Black and Hispanic individuals as well as individuals with low socioeconomic status have increased proportions of skin cancer, there are inadequate data on the prevalence in NWH individuals.4 We found no increase in malignant dermatologic processes in NWH individuals; however, this may be secondary to inadequate screening with a total body skin examination.
Clinical Workup of NWH Patients—Because most NWH individuals present to the ED to receive care, their care compared with WH patients should be considered. In this cohort, WH patients received a less extensive clinical workup. They received almost half as many dermatologic consultations and fewer outpatient referrals to dermatology. Major communication barriers may affect NWH presentation to follow-up, which was drastically lower than WH individuals, as scheduling typically occurs well after discharge from the ED or inpatient unit. We suggest a few alterations to improve dermatologic care for NWH individuals:
• Consider inpatient consultation for serious dermatologic conditions—even if chronic—to improve disease control, considering that many barriers inhibit follow-up in clinic.
• Involve outreach teams, such as the Assertive Community Treatment teams, that assist individuals by delivering medicine for psychiatric disorders, conducting total-body skin examinations, assisting with wound care, providing basic skin barrier creams or medicaments, and carrying information regarding outpatient follow-up.
• Educate ED providers on the most common skin concerns, especially those that fall within the noninfectious inflammatory category, such as hidradenitis suppurativa, which could easily be misdiagnosed as an abscess.
Future Directions—Owing to limitations of a retrospective cohort study, we present several opportunities for further research on this vulnerable population. The severity of disease, especially infectious etiologies, should be graded to determine if NWH patients truly present later in the disease course. The duration and quality of housing for NWH patients could be categorized based on living conditions (eg, on the street vs in a shelter). Although the findings of our NWH cohort presenting to the ED at UMC provide helpful insight into dermatologic disease, these findings may be disparate from those conducted at other locations in the United States. University Medical Center provides care to mostly subsidized insurance plans in a racially diverse community. Improved outcomes for the NWH individuals living in New Orleans start with obtaining a greater understanding of their diseases and where disparities exist that can be bridged with better care.
Acknowledgment—The dataset generated during this study and used for analysis is not publicly available to protect public health information but is available from the corresponding author on reasonable request.
More than half a million individuals are without housing (NWH) on any given night in the United States, as estimated by the US Department of Housing and Urban Development. 1 Lack of hygiene, increased risk of infection and infestation due to living conditions, and barriers to health care put these individuals at increased risk for disease. 2 Skin disease, including fungal infection and acne, are within the top 10 most prevalent diseases worldwide and can cause major psychologic impairment, yet dermatologic concerns and clinical outcomes in NWH patients have not been well characterized. 2-5 Further, because this vulnerable demographic tends to be underinsured, they frequently present to the emergency department (ED) for management of disease. 1,6 Survey of common concerns in NWH patients is of utility to consulting dermatologists and nondermatologist providers in the ED, who can familiarize themselves with management of diseases they are more likely to encounter. Few studies examine dermatologic conditions in the ED, and a thorough literature review indicates none have included homelessness as a variable. 6,7 Additionally, comparison with a matched control group of patients with housing (WH) is limited. 5,8 We present one of the largest comparisons of cutaneous disease in NWH vs WH patients in a single hospital system to elucidate the types of cutaneous disease that motivate patients to seek care, the location of skin disease, and differences in clinical care.
Methods
A retrospective medical record review of patients seen for an inclusive list of dermatologic diagnoses in the ED or while admitted at University Medical Center New Orleans, Louisiana (UMC), between January 1, 2018, and April 21, 2020, was conducted. This study was qualified as exempt from the institutional review board by Louisiana State University because it proposed zero risk to the patients and remained completely anonymous. Eight hundred forty-two total medical records were reviewed (NWH, 421; WH, 421)(Table 1). Patients with housing were matched based on self-identified race and ethnicity, sex, and age. Disease categories were constructed based on fundamental pathophysiology adapted from Dermatology9: infectious, noninfectious inflammatory, neoplasm, trauma and wounds, drug-related eruptions, vascular, pruritic, pigmented, bullous, neuropsychiatric, and other. Other included unspecified eruptions as well as miscellaneous lesions such as calluses. The current chief concern, anatomic location, and configuration were recorded, as well as biopsied lesions and outpatient referrals or inpatient consultations to dermatology or other specialties, including wound care, infectious disease, podiatry, and surgery. χ2 analysis was used to analyze significance of cutaneous categories, body location, and referrals. Groups smaller than 5 defaulted to the Fisher exact test.

Results
The total diagnoses (including both chief concerns and secondary diagnoses) are shown in Table 2. Chief concerns were more frequently cutaneous or dermatologic for WH (NWH, 209; WH, 307; P<.001). In both groups, cutaneous infectious etiologies were more likely to be a patient’s presenting chief concern (58% NWH, P=.002; 42% WH, P<.001). Noninfectious inflammatory etiologies and pigmented lesions were more likely to be secondary diagnoses with an unrelated noncutaneous concern; noninfectious inflammatory etiologies were only 16% of the total cutaneous chief concerns (11% NWH, P=.04; 20% WH, P=.03), and no pigmented lesions were chief concerns.

Infection was the most common chief concern, though NWH patients presented with significantly more infectious concerns (NWH, 212; WH, 150; P<.001), particularly infestations (NWH, 33; WH, 8; P<.001) and bacterial etiologies (NWH, 127; WH, 100; P=.04). The majority of bacterial etiologies were either an abscess or cellulitis (NWH, 106; WH, 83), though infected chronic wounds were categorized as bacterial infection when treated definitively as such (eg, in the case of sacral ulcers causing osteomyelitis)(NWH, 21; WH, 17). Of note, infectious etiology was associated with intravenous drug use (IVDU) in both NWH and WH patients. Of 184 NWH who reported IVDU, 127 had an infectious diagnosis (P<.001). Similarly, 43 of 56 total WH patients who reported IVDU had an infectious diagnosis (P<.001). Infestation (within the infectious category) included scabies (NWH, 20; WH, 3) and insect or arthropod bites (NWH, 12; WH, 5). Two NWH patients also presented with swelling of the lower extremities and were subsequently diagnosed with maggot infestations. Fungal and viral etiologies were not significantly increased in either group; however, NWH did have a higher incidence of tinea pedis (NWH, 14; WH, 4; P=.03).
More neoplasms (NWH, 6; WH, 16; P=.03), noninfectious inflammatory eruptions (NWH, 48; WH, 85; P<.001), and cutaneous drug eruptions (NWH, 5; WH, 27; P<.001) were reported in WH patients. There was no significant difference in benign vs malignant neoplastic processes between groups. More noninfectious inflammatory eruptions in WH were specifically driven by a markedly increased incidence of follicular (NWH, 9; WH, 29; P<.001) and urticarial/erythematous (NWH, 3; WH, 13; P=.02) lesions. Follicular etiologies included acne (NWH, 1; WH, 6; P=.12), folliculitis (NWH, 5; WH, 2; P=.45), hidradenitis suppurativa (NWH, 2; WH, 11; P=.02), and pilonidal and sebaceous cysts (NWH, 1; WH, 10; P=.01). Allergic urticaria dominated the urticarial/erythematous category (NWH, 3; WH, 11; P=.06), though there were 2 WH presentations of diffuse erythema and skin peeling.
Another substantial proportion of cutaneous etiologies were due to trauma or chronic wounds. Significantly more traumatic injuries presented in NWH patients vs WH patients (36 vs 31; P=.04). Trauma included human or dog bites (NWH, 5; WH, 4), sunburns (NWH, 3; WH, 0), other burns (NWH, 11; WH, 13), abrasions and lacerations (NWH, 16; WH, 3; P=.004), and foreign bodies (NWH, 1; WH, 1). Wounds consisted of chronic wounds such as those due to diabetes mellitus (foot ulcers) or immobility (sacral ulcers); numbers were similar between groups.
Looking at location, NWH patients had more pathology on the feet (NWH, 62; WH, 39; P=.02), whereas WH patients had more disseminated multiregional concerns (NWH, 55; WH, 75; P=.05). No one body location was notably more likely to warrant a chief concern.
For clinical outcomes, more WH patients received a consultation of any kind (NWH, 171; WH, 217; P<.001), consultation to dermatology (NWH, 49; WH, 87; P<.001), and consultation to surgery (NWH, 64; WH, 110; P<.001)(Table 3 and Figure). More outpatient referrals to dermatology were made for WH patients (NWH, 61; WH, 82; P=.05). Notably, NWH patients presented for 80% fewer hospital follow-up appointments (NWH, 11; WH, 55; P<.001). It is essential to note that these findings were not affected by self-reported race or ethnicity. Results remained significant when broken into cohorts consisting of patients with and without skin of color.

Comment
Cutaneous Concerns in NWH Patients—Although cutaneous disease has been reported to disproportionately affect NWH patients,10 in our cohort, NWH patients had fewer cutaneous chief concerns than WH patients. However, without comparing with all patients entering the ED at UMC, we cannot make a statement on this claim. We do present a few reasons why NWH patients do not have more cutaneous concerns. First, they may wait to present with cutaneous disease until it becomes more severe (eg, until chronic wounds have progressed to infections). Second, as discussed in depth by Hollestein and Nijsten,3 dermatologic disease may be a major contributor to the overall count of disability-adjusted life years but may play a minor role in individual disability. Therefore, skin disease often is considered less important on an individual basis, despite substantial psychosocial burden, leading to further stigmatization of this vulnerable population and discouraged care-seeking behavior, particularly for noninfectious inflammatory eruptions, which were notably more present in WH individuals. Third, fewer dermatologic lesions were reported on NWH patients, which may explain why all 3 WH pigmented lesions were diagnosed after presentation with a noncutaneous concern (eg, headache, anemia, nausea).

Infectious Cutaneous Diagnoses—The increased presentation of infectious etiologies, especially bacterial, is linked to the increased numbers of IVDUs reported in NWH individuals as well as increased exposure and decreased access to basic hygienic supplies. Intravenous drug use acted as an effect modifier of infectious etiology diagnoses, playing a major role in both NWH and WH cohorts. Although Black and Hispanic individuals as well as individuals with low socioeconomic status have increased proportions of skin cancer, there are inadequate data on the prevalence in NWH individuals.4 We found no increase in malignant dermatologic processes in NWH individuals; however, this may be secondary to inadequate screening with a total body skin examination.
Clinical Workup of NWH Patients—Because most NWH individuals present to the ED to receive care, their care compared with WH patients should be considered. In this cohort, WH patients received a less extensive clinical workup. They received almost half as many dermatologic consultations and fewer outpatient referrals to dermatology. Major communication barriers may affect NWH presentation to follow-up, which was drastically lower than WH individuals, as scheduling typically occurs well after discharge from the ED or inpatient unit. We suggest a few alterations to improve dermatologic care for NWH individuals:
• Consider inpatient consultation for serious dermatologic conditions—even if chronic—to improve disease control, considering that many barriers inhibit follow-up in clinic.
• Involve outreach teams, such as the Assertive Community Treatment teams, that assist individuals by delivering medicine for psychiatric disorders, conducting total-body skin examinations, assisting with wound care, providing basic skin barrier creams or medicaments, and carrying information regarding outpatient follow-up.
• Educate ED providers on the most common skin concerns, especially those that fall within the noninfectious inflammatory category, such as hidradenitis suppurativa, which could easily be misdiagnosed as an abscess.
Future Directions—Owing to limitations of a retrospective cohort study, we present several opportunities for further research on this vulnerable population. The severity of disease, especially infectious etiologies, should be graded to determine if NWH patients truly present later in the disease course. The duration and quality of housing for NWH patients could be categorized based on living conditions (eg, on the street vs in a shelter). Although the findings of our NWH cohort presenting to the ED at UMC provide helpful insight into dermatologic disease, these findings may be disparate from those conducted at other locations in the United States. University Medical Center provides care to mostly subsidized insurance plans in a racially diverse community. Improved outcomes for the NWH individuals living in New Orleans start with obtaining a greater understanding of their diseases and where disparities exist that can be bridged with better care.
Acknowledgment—The dataset generated during this study and used for analysis is not publicly available to protect public health information but is available from the corresponding author on reasonable request.
- Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529-1540. doi:10.1016/S0140-6736(14)61132-6
- Contag C, Lowenstein SE, Jain S, et al. Survey of symptomatic dermatologic disease in homeless patients at a shelter-based clinic. Our Dermatol Online. 2017;8:133-137. doi:10.7241/ourd.20172.37
- Hollestein LM, Nijsten T. An insight into the global burden of skin diseases. J Invest Dermatol. 2014;134:1499-1501. doi:10.1038/jid.2013.513
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Mackelprang JL, Graves JM, Rivara FP. Homeless in America: injuries treated in US emergency departments, 2007-2011. Int J Inj Contr Saf Promot. 2014;21:289-297. doi:10.1038/jid.2014.371
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313. doi:10.1016/j.jaad.2014.03.013
- Stratigos AJ, Stern R, Gonzalez E, et al. Prevalence of skin disease in a cohort of shelter-based homeless men. J Am Acad Dermatol. 1999;41:197-202. doi:10.1016/S0190-9622(99)70048-4
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012.
- Badiaga S, Menard A, Tissot Dupont H, et al. Prevalence of skin infections in sheltered homeless. Eur J Dermatol. 2005;15:382-386.
- Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529-1540. doi:10.1016/S0140-6736(14)61132-6
- Contag C, Lowenstein SE, Jain S, et al. Survey of symptomatic dermatologic disease in homeless patients at a shelter-based clinic. Our Dermatol Online. 2017;8:133-137. doi:10.7241/ourd.20172.37
- Hollestein LM, Nijsten T. An insight into the global burden of skin diseases. J Invest Dermatol. 2014;134:1499-1501. doi:10.1038/jid.2013.513
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59. doi:10.1016/j.det.2011.08.002
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Mackelprang JL, Graves JM, Rivara FP. Homeless in America: injuries treated in US emergency departments, 2007-2011. Int J Inj Contr Saf Promot. 2014;21:289-297. doi:10.1038/jid.2014.371
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313. doi:10.1016/j.jaad.2014.03.013
- Stratigos AJ, Stern R, Gonzalez E, et al. Prevalence of skin disease in a cohort of shelter-based homeless men. J Am Acad Dermatol. 1999;41:197-202. doi:10.1016/S0190-9622(99)70048-4
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012.
- Badiaga S, Menard A, Tissot Dupont H, et al. Prevalence of skin infections in sheltered homeless. Eur J Dermatol. 2005;15:382-386.
Practice Points
- Dermatologic disease in patients without housing (NWH) is characterized by more infectious concerns and fewer follicular and urticarial noninfectious inflammatory eruptions compared with matched controls of those with housing.
- Patients with housing more frequently presented with cutaneous chief concerns and received more consultations while in the hospital.
- This study uncovered notable pathological and clinical differences in treating dermatologic conditions in NWH patients.