User login
Acyclovir-Resistant Cutaneous Herpes Simplex Virus in DOCK8 Deficiency
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.
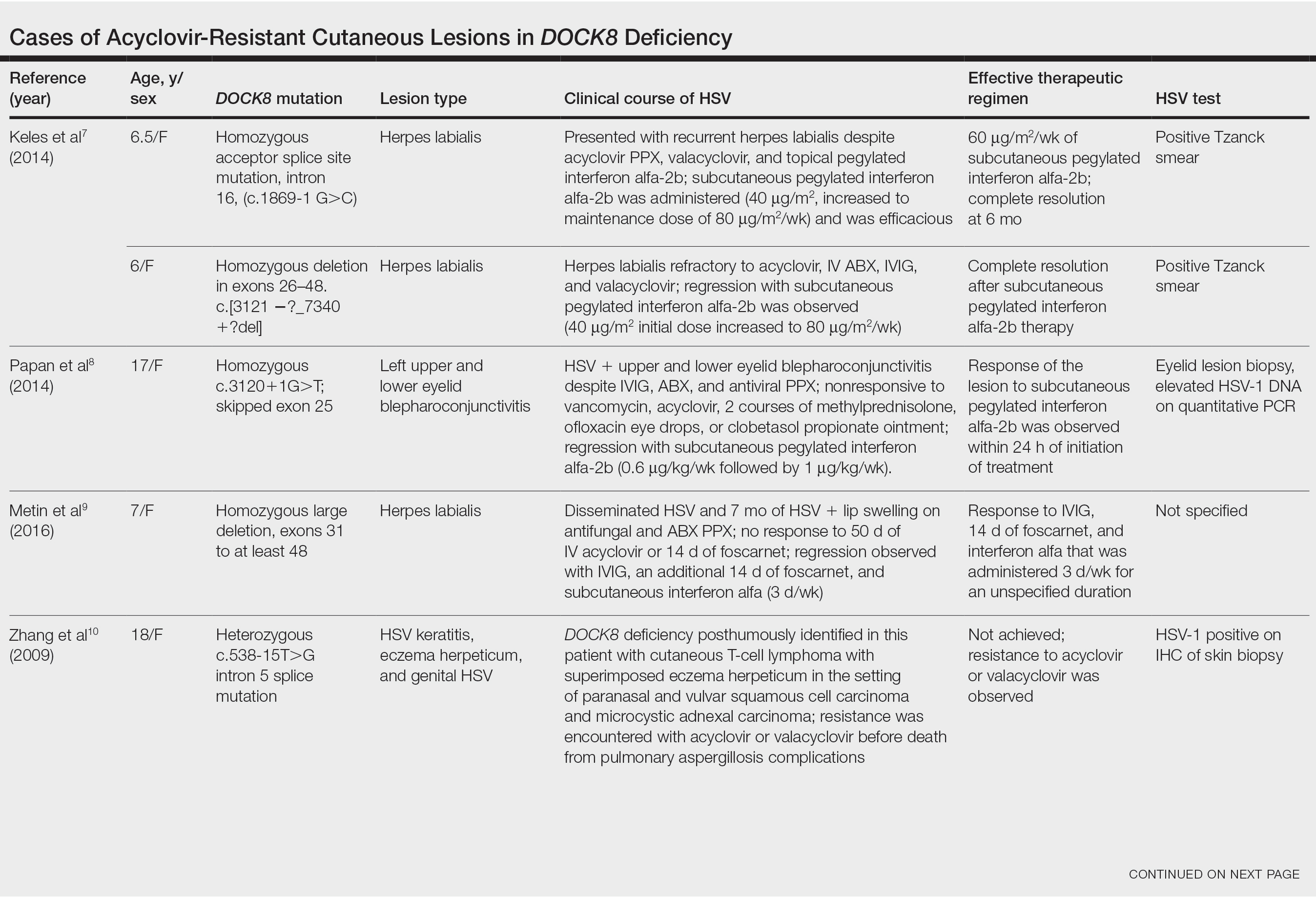
Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.
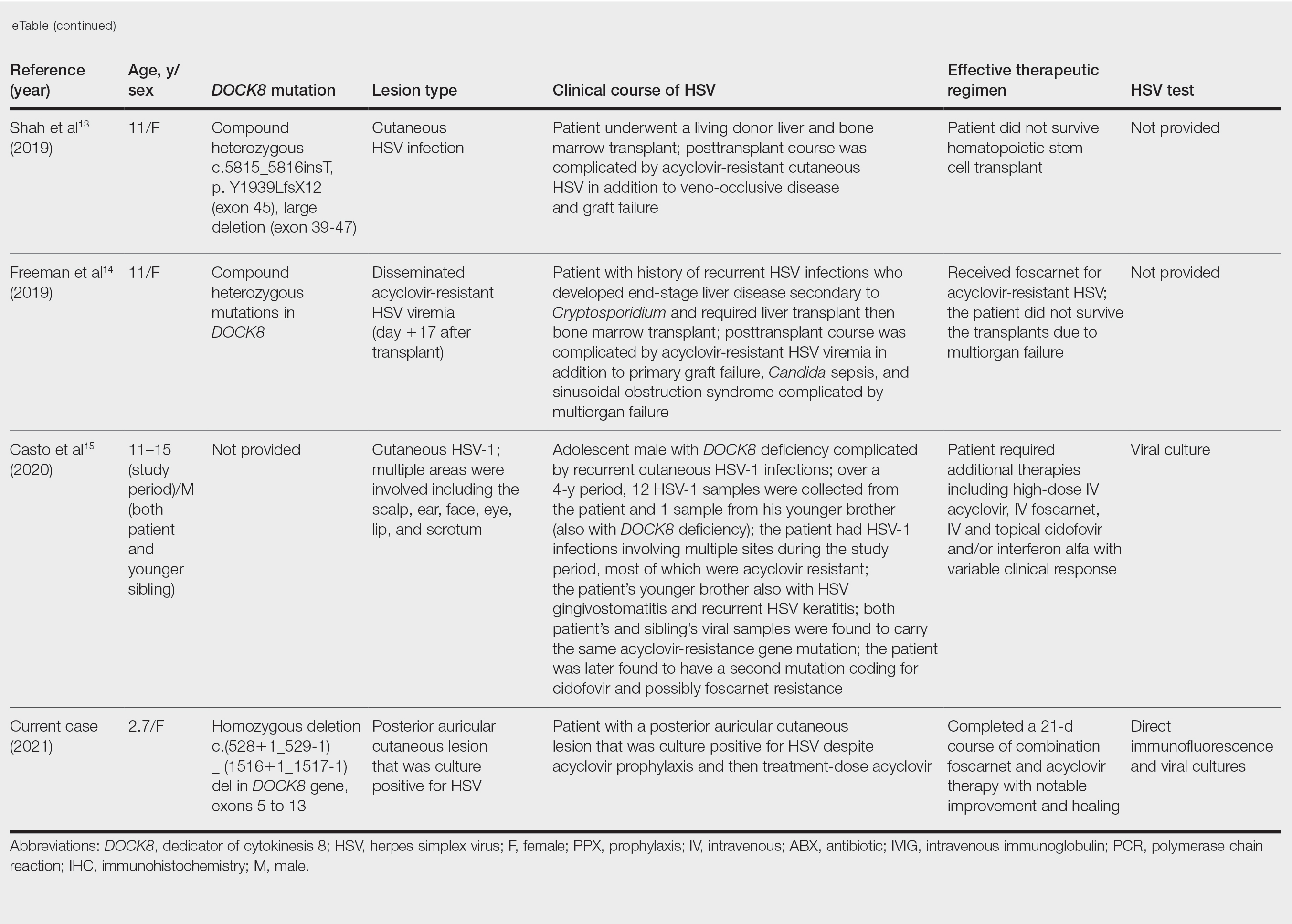
Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.

Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.

Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.

Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.

Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
Practice Points
- Patients with dedicator of cytokinesis 8 ( DOCK 8 ) deficiency are susceptible to development of severe recalcitrant viral cutaneous infections, including herpes simplex virus (HSV).
- Dermatologists should be aware that prophylactic acyclovir may not be sufficient for HSV suppression in the setting of severe immunodeficiency.
- Acyclovir-resistant cutaneous HSV lesions require escalation of therapy, which may include addition of foscarnet, cidofovir, or subcutaneous pegylated interferon alfa-2b to the therapeutic regimen.
- Viral culture should be performed on suspicious lesions in DOCK 8 -deficient patients despite acyclovir prophylaxis, and the threshold for HSV resistance testing should be low.
Developing a career in medical pancreatology: An emerging postfellowship career path
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
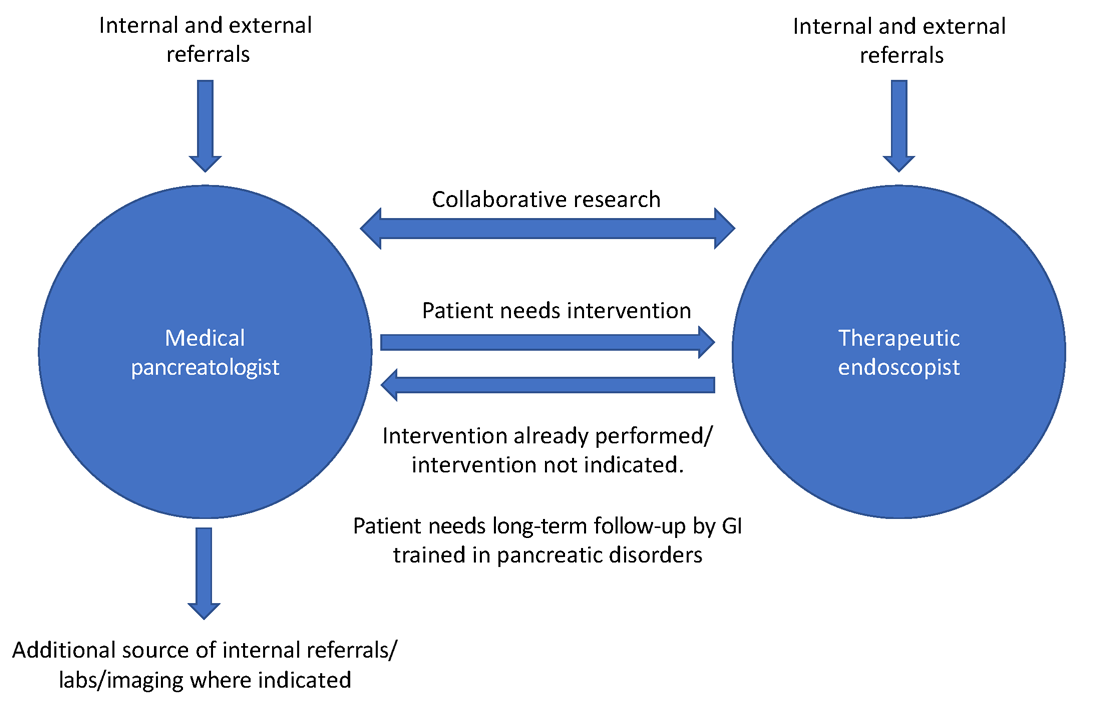
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.
Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
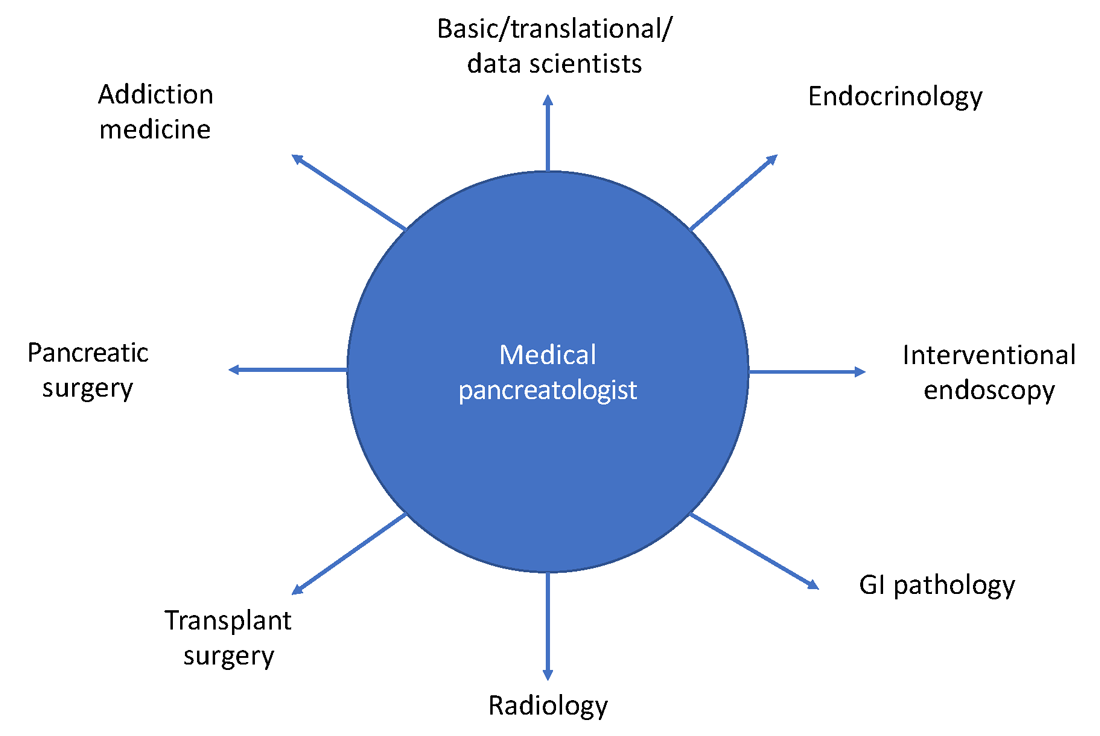
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.
Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.

Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.

Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
Although described by the Greek physician Herophilos around 300 B.C., it was not until the 19th century that enzymes began to be isolated from pancreatic secretions and their digestive action described, and not until early in the 20th century that Banting, Macleod, and Best received the Nobel prize for purifying insulin from the pancreata of dogs. For centuries in between, the pancreas was considered to be just a ‘beautiful piece of flesh’ (kallikreas), the main role of which was to protect the blood vessels in the abdomen and to serve as a cushion to the stomach.1 Certainly, the pancreas has come a long way since then but, like most other organs in the body, is oft ignored until it develops issues.
Like many other disorders in gastroenterology, pancreatic disorders were historically approached as mechanical or “plumbing” issues. As modern technology and innovation percolated through the world of endoscopy, a wide array of state-of-the-art tools were devised. Availability of newer “toys” and development of newer techniques also means that an ever-increasing curriculum has been squeezed into a generally single year of therapeutic endoscopy training, such that trainees can no longer limit themselves to learning only endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) or intervening on pancreatic disease alone. Modern, subspecialized approaches to disease and economic considerations often dictate that the therapeutic endoscopist of today must perform a wide range of procedures besides ERCP and EUS, such as advanced resection using endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), per-oral endoscopic myotomy (POEM), endoscopic bariatric procedures, and newer techniques and acronyms that continue to evolve on a regular basis. This leaves the therapeutic endoscopist with little time for outpatient management of many patients that don’t need interventional procedures but are often very complex and need ongoing, long-term follow-up. In addition, any clinic slots available for interventional endoscopists may be utilized by patients coming in to discuss complex procedures or for postprocedure follow-up. Endoscopic management is not the definitive treatment for most pancreatic disorders. In fact, as our knowledge of pancreatic disease has continued to evolve, endoscopic intervention is now required in a minority of cases.
Role of the medical pancreatologist
Patient Care
As part of a comprehensive, multidisciplinary team that also includes an interventional gastroenterologist, pancreatic surgeon, transplant surgeon (in centers offering islet autotransplantation with total pancreatectomy), radiology, endocrinology, and GI pathologist, the medical pancreatologist helps lead the care of patients with pancreatic disorders, such as pancreatic cysts, acute and chronic pancreatitis (especially in cases where there is no role for active endoscopic intervention), autoimmune pancreatitis, indeterminate pancreatic masses, as well as screens high-risk patients for pancreatic cancer in conjunction with a genetic counselor. The medical pancreatologist often also serves as a bridge between various members of a large multidisciplinary team that, formally in the form of conferences or informally, discusses the management of complex patients, with each member available to help the other based on the patient’s most immediate clinical need at that time. A schematic showing how the medical pancreatologist collaborates with the therapeutic endoscopist is provided in Figure 1.

Uzma Siddiqui, MD, director for the Center for Endoscopic Research and Technology (CERT) at the University of Chicago said, “The management of pancreatic diseases is often challenging. Surgeons and endoscopists can offer some treatments that focus on one aspect or symptom, but the medical pancreatologist brings focus to the patient as a whole and helps organize care. It is only with everyone’s combined efforts and the added perspective of the medical pancreatologist that we can provide the best care for our shared patients.”
David Xin, MD, MPH, a medical pancreatologist at Brigham and Women’s Hospital, Boston, added, “I am often asked what it means to be a medical pancreatologist. What do I do if not EUS and ERCP? I provide longitudinal care, coordinate multidisciplinary management, assess nutritional status, optimize quality of life, and manage pain. But perhaps most importantly, I make myself available for patients who seek understanding and sympathy regarding their complex disease. I became a medical pancreatologist because my mentors during training helped me recognize how rewarding this career would be.”
Insights from other medical pancreatologists and therapeutic endoscopists are provided in Figure 2.

Education
Having a dedicated medical pancreatology clinic has the potential to add a unique element to the training of gastroenterology fellows. In my own experience, besides fellows interested in medical pancreatology, even those interested in therapeutic endoscopy find it useful to rotate through the pancreas clinic and follow patients after or leading to their procedures, becoming comfortable with noninterventional pain management of patients with pancreatic disorders and risk stratification of pancreatic cystic lesions, and learning about the management of rare disorders such as autoimmune pancreatitis. Most importantly, this allows trainees to identify cases where endoscopic intervention may not offer definitive treatment for complex conditions such as pancreatic pain. Trainee-centered organizations such as the Collaborative Alliance for Pancreatic Education and Research (CAPER) enable trainees and young investigators to network with other physicians who are passionate about the pancreas and establish early research collaborations for current and future research endeavors that will help advance this field.
Research
Having a trained medical pancreatologist adds the possibility of adding a unique angle to ongoing research within a gastroenterology division, especially in collaboration with others. For example, during my fellowship training I was able to focus on histological changes in pancreatic islets of patients with pancreatic cancer that develop diabetes, compared with those that do not, in collaboration with a pathologist who focused on studying islet pathology and under the guidance of my mentor, Dr. Suresh Chari, a medical pancreatologist.2 I was also part of other studies within the GI division with other medical pancreatologists, such as Dr. Santhi Vege and Dr. Shounak Majumder, who have continued to serve as career and research mentors.3 Collaborative, multicenter studies on pancreatic disease are also conducted by CAPER, the organization mentioned above. A list of potential collaborations for the fellow interested
in medical pancreatology is provided in Figure 3.

Marketing considerations for the gastroenterology division
Having a medical pancreatologist in the team is not only attractive for referring physicians within an institution but is often a great asset from a marketing standpoint, especially for tertiary care academic centers and large community practices with a broad referral base. Given that there are a limited number of medical pancreatologists in the country, having one as part of the faculty can certainly provide a competitive edge to that center within the area, especially with an ever-increasing preference of patients for hyperspecialized care.
How to develop a career in medical pancreatology
Gastroenterology fellows often start their fellowships “undifferentiated” and try to get exposed to a wide variety of GI pathology, either through general GI clinics or as part of subspecialized clinics, as they attempt to decide how they want their careers to look down the line. Similar to other subspecialities, if a trainee has already decided to pursue medical pancreatology (as happened in my case), they should strongly consider ranking programs with available opportunities for research/clinic in medical pancreatology and ideally undergo an additional year of training. Fellows who decide during the course of their fellowship that they want to pursue a career in medical pancreatology should consider applying for a 4th year in the subject to not only obtain further training in the field but to also conduct research in the area and become more “marketable” as a person that could start a medical pancreatology program at their future academic or community position. Trainees interested in medical pancreatology should try to focus their time on long-term, clinical management of patients with pancreatic disorders, engaging a multidisciplinary team composed of interventional endoscopists, pancreatic surgeons, transplant surgeons (if total pancreatectomy and islet autotransplantation is available), radiology, addiction medicine (if available), endocrinology, and pathology. The list of places that offer a 4th year in medical pancreatology is increasing every year, and as of the writing of this article there are six programs that have this opportunity, which include:
- Mayo Clinic, Rochester, Minn.
- Beth Israel Deaconess Medical Center, Boston
- Brigham and Women’s Hospital, Boston
- Johns Hopkins Hospital, Baltimore
- University of Pittsburgh Medical Center, Pittsburgh, Penn.
The CAPER website is also a great resource for education as well as for identifying potential medical pancreatology programs.
In summary, medical pancreatology is an evolving and rapidly growing career path for gastroenterology fellows interested in providing care to patients with pancreatic disease in close collaboration with multiple other subspecialties, especially therapeutic endoscopy and pancreatic surgery. The field is also ripe for fellows interested in clinical, translational, and basic science research related to pancreatic disorders.
Dr. Nagpal is assistant professor of medicine, director, pancreas clinic, University of Chicago. He had no conflicts to disclose.
References
1. Feldman M et al. “Sleisenger and Fordtran’s Gastrointestinal and Liver Disease,” 11th ed. (Philadelphia: Elsevier, 2021).
2. Nagpal SJS et al. Pancreatology. 2020 Jul;20(5):929-35.
3. Nagpal SJS et al. Pancreatology. 2019 Mar;19(2):290-5.
The importance of education and screening for nonalcoholic fatty liver disease
For the past 18 months, we’ve all been focused on defeating the COVID-19 pandemic and preparing for the effects of cancer screenings that were delayed or put off entirely. But COVID isn’t the only epidemic we’re facing in the United States. Obesity is the second leading cause of preventable death in the United States. and its related diseases account for $480.7 billion in direct health care costs, with an additional $1.24 trillion in indirect costs from lost economic productivity.
More than two in five Americans are obese and that number is predicted to grow to more than half of the U.S. population by 2030. Obesity is a risk factor for nonalcoholic fatty liver disease (NAFLD), a buildup of fat in the liver with little or no inflammation or cell damage that affects one in three (30%-37%) of adults in the U.S.
NAFLD can progress to nonalcoholic steatohepatitis (NASH), which affects about 1 in 10 (8%-12%) of adults in the U.S. NASH is fat in the liver with inflammation and cell damage, and it can lead to fibrosis and liver failure. The number of patients we see with NALFD and NASH continues to rise and it’s taking its toll. One in five people who have NASH will have the disease progress to liver cirrhosis. NASH is expected to be the leading cause of liver transplant in the U.S. for the next 5 years.
Stemming the tide of NAFLD and NASH
In terms of diet, limiting sugar and eating a diet rich in vegetables, whole grains, and healthy fats can prevent the factors that lead to liver disease.
If this were easy, we wouldn’t be facing the obesity epidemic that is plaguing the United States. One of the issues is that medicine has only recognized obesity as a disease for less than 10 years. We aren’t trained in medical school, residencies, or fellowships in managing obesity, beyond advising people to exercise and eat right. We know this doesn’t work.
That’s why many independent GI groups are exploring comprehensive weight management programs that take a holistic approach to weight management involving a team of health care providers and educators helping patients gradually exercise more and eat healthy while providing a social support system to lose weight and keep it off.
The best way to educate is to listen first
As gastroenterologists, we see many obesity-related issues and have an opportunity to intervene before other more serious issues show up – like cancer, hypertension, and stroke. And educating the public and primary care physicians is key to ensuring that patients who are high risk are screened for liver disease.
Some GI practices leverage awareness events such as International NASH Day in June, or National Liver Cancer Awareness Month in October, to provide primary care physicians and patients with educational materials about making healthier choices and what options are available to screen for NAFLD and NASH.
While the awareness events offer a ready-made context for outreach, the physicians in my practice work year-round to provide information on liver disease. When patients are brought in for issues that may indicate future problems, we look for signs of chronic liver disease and educate them and their family members about liver disease and cirrhosis.
Discussions of weight are very personal, and it’s important to approach the conversation with sensitivity. It’s also good to understand as best as possible any cultural implications of discussing a person’s weight to ensure that the patient or their family members are not embarrassed by the discussion. I find that oftentimes the best approach is to listen to the patient and hear what factors are influencing their ability to exercise and eat healthy foods so that you can work together to find the best solution.
It’s also important to recognize that racial disparities exist in many aspects of NAFLD, including prevalence, severity, genetic predisposition, and overall chance of recovery. For instance, Hispanics and Asian Americans have a higher prevalence of NAFLD, compared with other ethnic and racial groups.
Early detection is key
Screenings have become a lot simpler and more convenient. There are alternatives to the painful, expensive liver biopsy. There are blood biomarker tests designed to assess liver fibrosis in patients. Specialized vibration-controlled transient elastography, such as Fibroscan, can measure scarring and fat buildup in the liver. And because it’s noninvasive, it doesn’t come with the same risks as a traditional liver biopsy. It also costs about four or five times less, which is important in this era of value-based care.
These simple tests can be reassuring, or they can lead down another path of treating the disease, but not being screened at all can come at a steep price. Severe fibrosis can lead to cirrhosis, a dangerous condition where the liver can no longer function correctly. NAFLD and NASH can also lead to liver cancer.
There are some medications that are in phase 2 and some in phase 3 clinical trials that aim to reduce fatty liver by cutting down fibrosis and steatosis, and there are other medications that can be used to help with weight loss. But the reality is that lifestyle changes are currently the best way to reverse NAFLD or stop it from progressing to NASH or cirrhosis.
Join an innovative practice
For the next 20 years, the obesity epidemic will be the biggest issue facing our society and a major focus of our cancer prevention efforts. Early-career physicians who are looking to join an independent GI practice should ask questions to determine whether the partners in the practice are taking a comprehensive approach to treating issues of obesity, NAFLD, NASH, and liver disease. Discuss what steps the practice takes to educate primary care physicians and their patients about the dangers of NAFLD and NASH.
We’re looking for early-career physicians who are entrepreneurial, not just for the sake of the practice, but because the future is in digital technologies and chronic care management, such as Chronwell, that help people maintain health through remote care and coaching. We want people who are thinking about fixing the problems of today and tomorrow with new technologies and scalable solutions. Through education and new screening and treatment options, we can ensure that fewer people develop serious liver disease or cancer.
Dr. Sanjay Sandhir is a practicing gastroenterologist at Dayton Gastroenterology, One GI in Ohio and is an executive committee member of the Digestive Health Physicians Association. He has no conflicts to declare.
For the past 18 months, we’ve all been focused on defeating the COVID-19 pandemic and preparing for the effects of cancer screenings that were delayed or put off entirely. But COVID isn’t the only epidemic we’re facing in the United States. Obesity is the second leading cause of preventable death in the United States. and its related diseases account for $480.7 billion in direct health care costs, with an additional $1.24 trillion in indirect costs from lost economic productivity.
More than two in five Americans are obese and that number is predicted to grow to more than half of the U.S. population by 2030. Obesity is a risk factor for nonalcoholic fatty liver disease (NAFLD), a buildup of fat in the liver with little or no inflammation or cell damage that affects one in three (30%-37%) of adults in the U.S.
NAFLD can progress to nonalcoholic steatohepatitis (NASH), which affects about 1 in 10 (8%-12%) of adults in the U.S. NASH is fat in the liver with inflammation and cell damage, and it can lead to fibrosis and liver failure. The number of patients we see with NALFD and NASH continues to rise and it’s taking its toll. One in five people who have NASH will have the disease progress to liver cirrhosis. NASH is expected to be the leading cause of liver transplant in the U.S. for the next 5 years.
Stemming the tide of NAFLD and NASH
In terms of diet, limiting sugar and eating a diet rich in vegetables, whole grains, and healthy fats can prevent the factors that lead to liver disease.
If this were easy, we wouldn’t be facing the obesity epidemic that is plaguing the United States. One of the issues is that medicine has only recognized obesity as a disease for less than 10 years. We aren’t trained in medical school, residencies, or fellowships in managing obesity, beyond advising people to exercise and eat right. We know this doesn’t work.
That’s why many independent GI groups are exploring comprehensive weight management programs that take a holistic approach to weight management involving a team of health care providers and educators helping patients gradually exercise more and eat healthy while providing a social support system to lose weight and keep it off.
The best way to educate is to listen first
As gastroenterologists, we see many obesity-related issues and have an opportunity to intervene before other more serious issues show up – like cancer, hypertension, and stroke. And educating the public and primary care physicians is key to ensuring that patients who are high risk are screened for liver disease.
Some GI practices leverage awareness events such as International NASH Day in June, or National Liver Cancer Awareness Month in October, to provide primary care physicians and patients with educational materials about making healthier choices and what options are available to screen for NAFLD and NASH.
While the awareness events offer a ready-made context for outreach, the physicians in my practice work year-round to provide information on liver disease. When patients are brought in for issues that may indicate future problems, we look for signs of chronic liver disease and educate them and their family members about liver disease and cirrhosis.
Discussions of weight are very personal, and it’s important to approach the conversation with sensitivity. It’s also good to understand as best as possible any cultural implications of discussing a person’s weight to ensure that the patient or their family members are not embarrassed by the discussion. I find that oftentimes the best approach is to listen to the patient and hear what factors are influencing their ability to exercise and eat healthy foods so that you can work together to find the best solution.
It’s also important to recognize that racial disparities exist in many aspects of NAFLD, including prevalence, severity, genetic predisposition, and overall chance of recovery. For instance, Hispanics and Asian Americans have a higher prevalence of NAFLD, compared with other ethnic and racial groups.
Early detection is key
Screenings have become a lot simpler and more convenient. There are alternatives to the painful, expensive liver biopsy. There are blood biomarker tests designed to assess liver fibrosis in patients. Specialized vibration-controlled transient elastography, such as Fibroscan, can measure scarring and fat buildup in the liver. And because it’s noninvasive, it doesn’t come with the same risks as a traditional liver biopsy. It also costs about four or five times less, which is important in this era of value-based care.
These simple tests can be reassuring, or they can lead down another path of treating the disease, but not being screened at all can come at a steep price. Severe fibrosis can lead to cirrhosis, a dangerous condition where the liver can no longer function correctly. NAFLD and NASH can also lead to liver cancer.
There are some medications that are in phase 2 and some in phase 3 clinical trials that aim to reduce fatty liver by cutting down fibrosis and steatosis, and there are other medications that can be used to help with weight loss. But the reality is that lifestyle changes are currently the best way to reverse NAFLD or stop it from progressing to NASH or cirrhosis.
Join an innovative practice
For the next 20 years, the obesity epidemic will be the biggest issue facing our society and a major focus of our cancer prevention efforts. Early-career physicians who are looking to join an independent GI practice should ask questions to determine whether the partners in the practice are taking a comprehensive approach to treating issues of obesity, NAFLD, NASH, and liver disease. Discuss what steps the practice takes to educate primary care physicians and their patients about the dangers of NAFLD and NASH.
We’re looking for early-career physicians who are entrepreneurial, not just for the sake of the practice, but because the future is in digital technologies and chronic care management, such as Chronwell, that help people maintain health through remote care and coaching. We want people who are thinking about fixing the problems of today and tomorrow with new technologies and scalable solutions. Through education and new screening and treatment options, we can ensure that fewer people develop serious liver disease or cancer.
Dr. Sanjay Sandhir is a practicing gastroenterologist at Dayton Gastroenterology, One GI in Ohio and is an executive committee member of the Digestive Health Physicians Association. He has no conflicts to declare.
For the past 18 months, we’ve all been focused on defeating the COVID-19 pandemic and preparing for the effects of cancer screenings that were delayed or put off entirely. But COVID isn’t the only epidemic we’re facing in the United States. Obesity is the second leading cause of preventable death in the United States. and its related diseases account for $480.7 billion in direct health care costs, with an additional $1.24 trillion in indirect costs from lost economic productivity.
More than two in five Americans are obese and that number is predicted to grow to more than half of the U.S. population by 2030. Obesity is a risk factor for nonalcoholic fatty liver disease (NAFLD), a buildup of fat in the liver with little or no inflammation or cell damage that affects one in three (30%-37%) of adults in the U.S.
NAFLD can progress to nonalcoholic steatohepatitis (NASH), which affects about 1 in 10 (8%-12%) of adults in the U.S. NASH is fat in the liver with inflammation and cell damage, and it can lead to fibrosis and liver failure. The number of patients we see with NALFD and NASH continues to rise and it’s taking its toll. One in five people who have NASH will have the disease progress to liver cirrhosis. NASH is expected to be the leading cause of liver transplant in the U.S. for the next 5 years.
Stemming the tide of NAFLD and NASH
In terms of diet, limiting sugar and eating a diet rich in vegetables, whole grains, and healthy fats can prevent the factors that lead to liver disease.
If this were easy, we wouldn’t be facing the obesity epidemic that is plaguing the United States. One of the issues is that medicine has only recognized obesity as a disease for less than 10 years. We aren’t trained in medical school, residencies, or fellowships in managing obesity, beyond advising people to exercise and eat right. We know this doesn’t work.
That’s why many independent GI groups are exploring comprehensive weight management programs that take a holistic approach to weight management involving a team of health care providers and educators helping patients gradually exercise more and eat healthy while providing a social support system to lose weight and keep it off.
The best way to educate is to listen first
As gastroenterologists, we see many obesity-related issues and have an opportunity to intervene before other more serious issues show up – like cancer, hypertension, and stroke. And educating the public and primary care physicians is key to ensuring that patients who are high risk are screened for liver disease.
Some GI practices leverage awareness events such as International NASH Day in June, or National Liver Cancer Awareness Month in October, to provide primary care physicians and patients with educational materials about making healthier choices and what options are available to screen for NAFLD and NASH.
While the awareness events offer a ready-made context for outreach, the physicians in my practice work year-round to provide information on liver disease. When patients are brought in for issues that may indicate future problems, we look for signs of chronic liver disease and educate them and their family members about liver disease and cirrhosis.
Discussions of weight are very personal, and it’s important to approach the conversation with sensitivity. It’s also good to understand as best as possible any cultural implications of discussing a person’s weight to ensure that the patient or their family members are not embarrassed by the discussion. I find that oftentimes the best approach is to listen to the patient and hear what factors are influencing their ability to exercise and eat healthy foods so that you can work together to find the best solution.
It’s also important to recognize that racial disparities exist in many aspects of NAFLD, including prevalence, severity, genetic predisposition, and overall chance of recovery. For instance, Hispanics and Asian Americans have a higher prevalence of NAFLD, compared with other ethnic and racial groups.
Early detection is key
Screenings have become a lot simpler and more convenient. There are alternatives to the painful, expensive liver biopsy. There are blood biomarker tests designed to assess liver fibrosis in patients. Specialized vibration-controlled transient elastography, such as Fibroscan, can measure scarring and fat buildup in the liver. And because it’s noninvasive, it doesn’t come with the same risks as a traditional liver biopsy. It also costs about four or five times less, which is important in this era of value-based care.
These simple tests can be reassuring, or they can lead down another path of treating the disease, but not being screened at all can come at a steep price. Severe fibrosis can lead to cirrhosis, a dangerous condition where the liver can no longer function correctly. NAFLD and NASH can also lead to liver cancer.
There are some medications that are in phase 2 and some in phase 3 clinical trials that aim to reduce fatty liver by cutting down fibrosis and steatosis, and there are other medications that can be used to help with weight loss. But the reality is that lifestyle changes are currently the best way to reverse NAFLD or stop it from progressing to NASH or cirrhosis.
Join an innovative practice
For the next 20 years, the obesity epidemic will be the biggest issue facing our society and a major focus of our cancer prevention efforts. Early-career physicians who are looking to join an independent GI practice should ask questions to determine whether the partners in the practice are taking a comprehensive approach to treating issues of obesity, NAFLD, NASH, and liver disease. Discuss what steps the practice takes to educate primary care physicians and their patients about the dangers of NAFLD and NASH.
We’re looking for early-career physicians who are entrepreneurial, not just for the sake of the practice, but because the future is in digital technologies and chronic care management, such as Chronwell, that help people maintain health through remote care and coaching. We want people who are thinking about fixing the problems of today and tomorrow with new technologies and scalable solutions. Through education and new screening and treatment options, we can ensure that fewer people develop serious liver disease or cancer.
Dr. Sanjay Sandhir is a practicing gastroenterologist at Dayton Gastroenterology, One GI in Ohio and is an executive committee member of the Digestive Health Physicians Association. He has no conflicts to declare.
Bystander actions can reduce children’s risk of drowning
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
The likelihood that a child will survive a near-drowning without long-term damage is substantially greater if a bystander attempts a rescue, even if that person doesn’t perform cardiopulmonary resuscitation (CPR), according to new research presented October 10 at the American Academy of Pediatrics (AAP) 2021 National Conference.
“The extent to which bystander rescue is associated with reduced odds of unfavorable drowning outcomes was surprising,” said lead investigator Rohit P. Shenoi, MD, professor of pediatrics at Baylor College of Medicine and attending physician at Texas Children’s Hospital, Houston.
“While we do know that early rescue and resuscitation is helpful in preventing severe drowning injury, the degree of benefit from bystander rescue in all cases of pediatric drowning has not been described so far,” he told this news organization.
The fact that a bystander’s rescue attempt improves a child’s odds of a good outcome is not surprising on its own, but the magnitude of the finding really affirms the importance of bystander intervention, said Benjamin Hoffman, MD, professor of pediatrics at the Oregon Health & Science University School of Medicine and medical director of the Tom Sargent Safety Center at the Doernbecher Children’s Hospital, Portland.
“If an adult finds a child in the water, even if they don’t administer formal CPR, they’re going to be doing things” to try to help, Dr. Hoffman, who was not involved in this research but who specializes in child injury prevention, said in an interview. The act of intervening – whether it’s formal CPR or a CPR attempt or even just calling appropriate first responders – “likely impacts the duration of the submersion” and “clearly makes a difference.”
Drowning is the leading cause of death for children younger than 4 years, Dr. Hoffman noted, adding that the AAP recommends swimming lessons for children older than 1 year to reduce that risk.
In their cross-sectional study, Dr. Shenoi and his colleagues analyzed data on drownings and near-drownings in children and adolescents younger than 18 years using hospital, emergency medical services, and child fatality records from Harris County, Texas.
They analyzed 237 incidents from 2010 to 2013 in which the young person was submerged. Median age of the victims was 3.2 years, 60% were male, 64% were Black, Hispanic, or Native American, and 78% occurred in a swimming pool.
Unfavorable outcomes – defined as death or severe impairment after hospital discharge – were experienced by 38 victims (16%) and were significantly associated with being submerged for longer than 5 minutes (P < .001).
The odds of an unfavorable outcome dropped by 80% if a bystander attempted a rescue, whether or not they performed CPR (adjusted odds ratio, 0.2; P = .004). If the bystander performed CPR, the odds of an unfavorable outcome dropped by a similar amount, but the difference was not statistically significant (aOR, 0.22; P = .07).
However, previous research has shown a significant reduction in poor outcomes when CPR is administered to children who have been submerged, Dr. Hoffman explained.
The most important thing a bystander can do is simply get a submerged child out of the water. “Early rescue in drowning terminates what is initially a respiratory arrest from progressing to a full cardiopulmonary arrest with severe hypoxic brain injury and death,” Dr. Shenoi said.
“CPR is also very important, and rescue and resuscitation go hand in hand. We encourage all laypersons to be trained in CPR so that they can administer correct CPR techniques,” he added.
Both Dr. Shenoi and Dr. Hoffman emphasized the value of CPR training for adults, as the AAP recommends, and the importance of other precautions that reduce the risk of drowning.
“Drowning prevention should consist of multiple layers of prevention,” Dr. Shenoi said. These consist of “close, constant, and attentive supervision; isolation fencing for swimming pools; and water competency, including water-safety knowledge, basic swim skills, and the ability to recognize and respond to a swimmer in trouble, use of life jackets, and early bystander CPR.”
The relative importance of each of those layers depends on geography and circumstances, Dr. Hoffman said. Pools are the most common drowning sites in the United States overall, but they’re much more common in warmer states, such as California, Florida, and Texas, which have more pools. In contrast, drownings in Oregon are more likely to occur in rivers, so prevention is more about access to life jackets and increasing access to swim lessons.
The findings from this study drive home how important it is for physicians to provide anticipatory guidance to families on reducing the risk of drowning. Pediatricians should convey to families the need for different layers of protection, he added.
“If your family spends a lot of time around water, whether open water or swimming pools, the more layers you can provide, the better off you’re going to be,” Dr. Hoffman said.
Dr. Shenoi echoed this sentiment.
“The take-home message is to be observant if you are entrusted with the care of a child around water,” Dr. Shenoi said. “If you notice the child to be drowning, either attempt rescue yourself if it is safe to do so or enlist the help of others to save the victim as soon as possible. However, the rescuer should not place himself or herself in danger when attempting rescue.”
The five steps in the “drowning chain of survival” – preventing drowning, recognizing distress, providing flotation, removing the victim from the water, and providing care and CPR as needed – are key to reducing drowning deaths and injury, Dr. Shenoi emphasized.
Dr. Shenoi has disclosed no relevant financial relationships. Dr. Hoffman is a paid consultant on child drowning prevention for the nonprofit Anonymous Philanthropy.
A version of this article first appeared on Medscape.com.
Alleged on-the-job violence, racism, prompts psych workers to head to D.C.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
A dozen workers from a psychiatric hospital near Seattle flew to Washington, D.C. to picket the National Association for Behavioral Healthcare’s annual meeting in an effort to get their employer to meet demands for a safer work environment, better staffing, and the hiring of security professionals.
They are also demanding that their employer, Cascade Behavioral Health Hospital, a private psychiatric facility owned by Acadia Healthcare and located in Tukwila, Washington, address what they call “racist harassment” by managers who have allegedly told many workers, who are primarily people of color, that they are going to be “filtered out,” Alazar Yirgu, a mental health technician at the facility, told this news organization.
The workers have been conducting a “safety strike” to protest working conditions at Cascade since early August. The protest in Tukwila began after a dozen or more workers were hurt in an August 1 incident during which they had attempted to restrain a violent patient.
“We’ve been out there for 2 months, and we will continue until our voice is heard,” said Mr. Yirgu, who was hospitalized as a result of the August patient outburst that he said has left him unable to work since the incident.
On Oct. 7, Mr. Yirgu and coworkers brought the protest to Washington, D.C., in a continued effort to voice their need for adequate personal protective equipment, increased staffing, and the hiring of security personnel.
“Any health care professional should not be fearful to do their job, because once they are in that state of mind, once they are fearful for themselves, then they are not doing their jobs; they are preoccupied with their fears,” said Mr. Yirgu, who has worked as a technician for 6 years.
Unsafe patient load
The workers reacted quickly after the August 1 patient outburst because there have been multiple previous incidents, Mr. Yirgu said.
In a 2019 news story by the Seattle Times, the newspaper reported there had been 65 assaults on patients or staff at Cascade from 2016 to 2018, resulting in concussions and broken bones in some instances.
Mr. Yirgu said that more recently, a patient broke a second story window, jumped to the ground, and ran off.
At the facility, workers are often assigned to as many as a dozen or more patients, he said, noting that at other psychiatric institutions, he’s cared for a maximum of five patients at once.
The Tukwila police have pushed back against the workers’ description of the incident in which Mr. Yirgu was injured, and Cascade Behavioral Health has aggressively defended its facility.
According to Mr. Yirgu, the expletive-spewing patient was clearly a danger to himself and others – especially after he stole a key card that would give him access to the entire facility, including the kitchen where knives were stored.
When more than a dozen staff answered the unit’s “Code Gray,” they were unable to subdue or restrain him. Mr. Yirgu ended up on the floor underneath the patient after the patient had jumped off a table.
As the incident unfolded, several workers called the police, who initially refused to go to the facility, saying that a new law prevented them from assisting with the restraint if there was no assault.
The Tukwila Police Department report shows that officers finally did go to the facility and determined that “a crime had not been committed based on the information presented to them, that there was no imminent threat of bodily harm, and that there was no legal grounds or authority for them to assist medical staff with physically restraining a patient.”
Cascade pushes back
A Service Employees International Union (SEIU) report shows about 70 workers refused to come in to work after the incident and began picketing outside the facility.
Cascade called it an illegal strike because the protesters had not given 10-days’ notice, as required by federal law, and moved to terminate those who participated. The local SEIU chapter, 1199NW, suggested the workers call their walkout a “safety strike,” because it was organized primarily to protest working conditions.
Meanwhile Cascade, which has erected a large fence so that no one in the facility can see the protesters, has said the strike is primarily about ongoing contract negotiations with the facility’s nurses and its union.
“The Union has been trying to apply unfair – and in some cases we believe unlawful – external pressures to this process, including picketing, work stoppages, smear campaigns, and false accusations,” Cascade CEO Christopher West wrote on the company’s website in mid-August.
He said the facility had “ample personal protective equipment” and that the “well-being and safety of our patients and staff always have been and will be our key priorities.”
In response to a request for comment, Cascade said in an emailed statement that physical confrontations had decreased by almost 50% and elopements (unauthorized leaving of the facility) by 80% from 2018 to 2021.
Cascade spokesperson Gretchen Hommrich said in the statement that the workers it has terminated “were let go for cause in violation to their employment agreement” and said the company still aimed to negotiate a new agreement with the union.
The “efforts outside of the bargaining process serve no productive purpose and have only brought harm to the residents they claim to serve,” said Ms. Hommrich.
‘Safety is the sole purpose’
Mr. Yirgu said it was outrageous to suggest workers were picketing over contract negotiations. “Safety is the sole purpose of this strike,” he said.
He noted that his patient care goal is to have a lot of one-on-one time with his patients, helping them navigate back to the outside world. The facility is supposed to be a safe place, Mr. Yirgu added. Violence inside the facility traumatizes the patients and may worsen their condition and delay their progress, he said.
“If I can’t keep them safe, there’s no way I’m going to be able to see them eye-to-eye when I told them I’d keep them safe and then they’re not anymore,” said Mr. Yirgu.
So far, 22 workers have been “terminated,” meaning they received a termination notice, have been taken off the work schedule by the employer, or otherwise been informed that the employer has deemed them to be separated, the SEIU reports. The organization has filed unfair labor practice (ULPs) for all 22.
A version of this article first appeared on Medscape.com.
Community mourns nurse knocked over, killed in Times Square
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
In Crohn’s disease, no decline in stomas despite increasing use of anti-TNF
Despite increasing use of anti–tumor necrosis factor (TNF) medications in recent years and a lower rate of proctectomy, there has been no decline in stoma incidence among Crohn’s disease (CD) patients, according to a new retrospective analysis of a Swedish population database.
The overall 5-year stoma incidence was 2.5%, and there was no significant difference between calendar periods, wrote Åsa H Everhov, MD, PhD, of the Karolinska Institutet, Stockholm, and colleagues. Their report is in Inflammatory Bowel Diseases. Previous population studies looking at temporal trends have found mixed results with respect to stoma formation. However, many previous studies analyzed cohorts from referral centers that included patients with more severe disease. Others had small sample sizes.
“This is somewhat surprising as the rate of overall surgery for Crohn’s has decreased with the advent of biologic therapies,” said Miguel Regueiro, MD, who was asked to comment on the study. He is chair of the Digestive Disease and Surgery Institute, and professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University.
Dr. Regueiro pointed out that the rate of stoma creation was quite low, and characteristics of the disease may explain the lack of a trend. For example, anorectal disease, especially when accompanied by fistula and strictures, is often medication refractory. “Although the study could not delineate this, it is my clinical practice experience that some patients present with destructive anorectal disease early, and that despite medications the ‘damage is too far gone’ to reverse,” said Dr. Regueiro. Also, the anorectum does not allow for an anastomosis since there is no region to connect to that isn’t damaged, he added.
The findings shouldn’t affect patient management or counseling, according to Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, who was not involved in the study. “The indications for surgery in Crohn’s disease have not changed with the advent of newer therapies. Complications such as strictures and abscesses are still treated with similar surgeries. It is hopeful that the future course after surgery can be modified by effective biologic therapies,” he said.
The study authors noted that the findings are consistent with previous studies suggesting that the rate of abdominal surgery had begun to decline before anti-TNF drugs were introduced. The Swedish National Patient Register used in the current work includes only inpatient data before 2001, preventing a broader analysis prior to 1999, when infliximab gained approval in Sweden. But the researchers looked at time from first surgery to stoma, and found a decline in stoma formation between 1994 and 1997. A similar analysis found no decrease in the 2000s.
The researchers pointed out that anti-TNF inhibitor use would be expected to reduce the rate of stomas by inducing remission, although temporary stomas may be created to relieve symptoms while waiting for the medication to take effect. But anti-TNF agents could also encourage patients and physicians to postpone surgery. The delay could raise the chance of surgical complications, which in turn could lead to creation of a temporary stoma. Permanent stomas are typically created in cases of severe perianal CD.
The study could come as a disappointment to some patients and physicians, according to the authors. “The more active and early use of anti-TNF (therapeutics), in combination with the decreasing incidence of abdominal surgery in general for CD, has raised hopes of decreased incidence of stoma formation, but this was not observed in the present study,” the authors wrote.
But the news wasn’t all bad. The study found a lower cumulative stoma incidence than had previous studies, and the incidence of permanent stoma was just 0.8% at 5 years, “which should be reassuring for patients,” according to the authors.
CD patients often fear that a stoma will become necessary, but a stoma can be quite beneficial, allowing patients to regain some control over their lives. That can lead to more work participation and social activity. Qualitative studies showed that both patients and clinicians described outcomes of stoma placement as exceeding expectations, with a key factor being support from other inflammatory bowel disease patients with stomas. The authors encouraged clinicians to discuss the possibility of a stoma early on in the treatment process, and to avoid referring to it as a “last resort.”
The study also suggests that patients with anal and anorectal Crohn’s disease should receive biologics early, and a multidisciplinary approach involving both the gastroenterologist and surgeon is important. And patients should be counseled on the impact of disease on diet and psychological health, according to Dr. Regueiro.
The new study analyzed data between 2003 and 2014, from 18,815 Crohn’s disease patients who had not undergone previous surgery. The median age was 39 years, 53% were women, and 12% were pediatric patients.
After a median follow-up of 9.6 years, 9.5% of patients had perianal disease. Overall, 36% of patients had been treated with immunomodulators, and 17% with anti-TNF agents; 3.5% had stoma surgery, and just 0.05% underwent proctectomy.
Among those who had a stoma placed, the median age at diagnosis was 47 years, and 53% were men. In all, 12.6% had perianal disease at diagnosis, and 24.5% had perianal disease by the end of follow-up; 43% had received immunomodulators, and 26% anti-TNF agents. Among stomas, 64% were ileostomies, and 44% were temporary, with 88% of removals performed within 2 years of surgery.
The calendar periods of CD onset (2003-2006, 2007-2010, and 2011-2014) had similar rates of cumulative stoma placement (log rank test, P = .61). Overall, the 1-year cumulative incidence of stoma was 1.3%, the 3-year incidence was 1.9%, and the 5-year incidence was 2.5%.
The cumulative incidence of ever-use of anti-TNF agents increased with each successive calendar period (P < .001), but there was no significant difference in cumulative stoma incidence (P = .07).
One limitation of the study is the lack of detailed patient characteristics, and another is the short follow-up for patients diagnosed during 2011-2014. Another is that the results may not be generalizable to a U.S. population.
The study authors have received funding and consulted for various pharmaceutical companies. Dr. Regueiro has received grants from and has consulted or been on a scientific advisory board for Abbvie, UCB, and Janssen. Dr. Hanauer has no relevant financial disclosures.
This article was updated Oct. 13, 2021.
Despite increasing use of anti–tumor necrosis factor (TNF) medications in recent years and a lower rate of proctectomy, there has been no decline in stoma incidence among Crohn’s disease (CD) patients, according to a new retrospective analysis of a Swedish population database.
The overall 5-year stoma incidence was 2.5%, and there was no significant difference between calendar periods, wrote Åsa H Everhov, MD, PhD, of the Karolinska Institutet, Stockholm, and colleagues. Their report is in Inflammatory Bowel Diseases. Previous population studies looking at temporal trends have found mixed results with respect to stoma formation. However, many previous studies analyzed cohorts from referral centers that included patients with more severe disease. Others had small sample sizes.
“This is somewhat surprising as the rate of overall surgery for Crohn’s has decreased with the advent of biologic therapies,” said Miguel Regueiro, MD, who was asked to comment on the study. He is chair of the Digestive Disease and Surgery Institute, and professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University.
Dr. Regueiro pointed out that the rate of stoma creation was quite low, and characteristics of the disease may explain the lack of a trend. For example, anorectal disease, especially when accompanied by fistula and strictures, is often medication refractory. “Although the study could not delineate this, it is my clinical practice experience that some patients present with destructive anorectal disease early, and that despite medications the ‘damage is too far gone’ to reverse,” said Dr. Regueiro. Also, the anorectum does not allow for an anastomosis since there is no region to connect to that isn’t damaged, he added.
The findings shouldn’t affect patient management or counseling, according to Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, who was not involved in the study. “The indications for surgery in Crohn’s disease have not changed with the advent of newer therapies. Complications such as strictures and abscesses are still treated with similar surgeries. It is hopeful that the future course after surgery can be modified by effective biologic therapies,” he said.
The study authors noted that the findings are consistent with previous studies suggesting that the rate of abdominal surgery had begun to decline before anti-TNF drugs were introduced. The Swedish National Patient Register used in the current work includes only inpatient data before 2001, preventing a broader analysis prior to 1999, when infliximab gained approval in Sweden. But the researchers looked at time from first surgery to stoma, and found a decline in stoma formation between 1994 and 1997. A similar analysis found no decrease in the 2000s.
The researchers pointed out that anti-TNF inhibitor use would be expected to reduce the rate of stomas by inducing remission, although temporary stomas may be created to relieve symptoms while waiting for the medication to take effect. But anti-TNF agents could also encourage patients and physicians to postpone surgery. The delay could raise the chance of surgical complications, which in turn could lead to creation of a temporary stoma. Permanent stomas are typically created in cases of severe perianal CD.
The study could come as a disappointment to some patients and physicians, according to the authors. “The more active and early use of anti-TNF (therapeutics), in combination with the decreasing incidence of abdominal surgery in general for CD, has raised hopes of decreased incidence of stoma formation, but this was not observed in the present study,” the authors wrote.
But the news wasn’t all bad. The study found a lower cumulative stoma incidence than had previous studies, and the incidence of permanent stoma was just 0.8% at 5 years, “which should be reassuring for patients,” according to the authors.
CD patients often fear that a stoma will become necessary, but a stoma can be quite beneficial, allowing patients to regain some control over their lives. That can lead to more work participation and social activity. Qualitative studies showed that both patients and clinicians described outcomes of stoma placement as exceeding expectations, with a key factor being support from other inflammatory bowel disease patients with stomas. The authors encouraged clinicians to discuss the possibility of a stoma early on in the treatment process, and to avoid referring to it as a “last resort.”
The study also suggests that patients with anal and anorectal Crohn’s disease should receive biologics early, and a multidisciplinary approach involving both the gastroenterologist and surgeon is important. And patients should be counseled on the impact of disease on diet and psychological health, according to Dr. Regueiro.
The new study analyzed data between 2003 and 2014, from 18,815 Crohn’s disease patients who had not undergone previous surgery. The median age was 39 years, 53% were women, and 12% were pediatric patients.
After a median follow-up of 9.6 years, 9.5% of patients had perianal disease. Overall, 36% of patients had been treated with immunomodulators, and 17% with anti-TNF agents; 3.5% had stoma surgery, and just 0.05% underwent proctectomy.
Among those who had a stoma placed, the median age at diagnosis was 47 years, and 53% were men. In all, 12.6% had perianal disease at diagnosis, and 24.5% had perianal disease by the end of follow-up; 43% had received immunomodulators, and 26% anti-TNF agents. Among stomas, 64% were ileostomies, and 44% were temporary, with 88% of removals performed within 2 years of surgery.
The calendar periods of CD onset (2003-2006, 2007-2010, and 2011-2014) had similar rates of cumulative stoma placement (log rank test, P = .61). Overall, the 1-year cumulative incidence of stoma was 1.3%, the 3-year incidence was 1.9%, and the 5-year incidence was 2.5%.
The cumulative incidence of ever-use of anti-TNF agents increased with each successive calendar period (P < .001), but there was no significant difference in cumulative stoma incidence (P = .07).
One limitation of the study is the lack of detailed patient characteristics, and another is the short follow-up for patients diagnosed during 2011-2014. Another is that the results may not be generalizable to a U.S. population.
The study authors have received funding and consulted for various pharmaceutical companies. Dr. Regueiro has received grants from and has consulted or been on a scientific advisory board for Abbvie, UCB, and Janssen. Dr. Hanauer has no relevant financial disclosures.
This article was updated Oct. 13, 2021.
Despite increasing use of anti–tumor necrosis factor (TNF) medications in recent years and a lower rate of proctectomy, there has been no decline in stoma incidence among Crohn’s disease (CD) patients, according to a new retrospective analysis of a Swedish population database.
The overall 5-year stoma incidence was 2.5%, and there was no significant difference between calendar periods, wrote Åsa H Everhov, MD, PhD, of the Karolinska Institutet, Stockholm, and colleagues. Their report is in Inflammatory Bowel Diseases. Previous population studies looking at temporal trends have found mixed results with respect to stoma formation. However, many previous studies analyzed cohorts from referral centers that included patients with more severe disease. Others had small sample sizes.
“This is somewhat surprising as the rate of overall surgery for Crohn’s has decreased with the advent of biologic therapies,” said Miguel Regueiro, MD, who was asked to comment on the study. He is chair of the Digestive Disease and Surgery Institute, and professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University.
Dr. Regueiro pointed out that the rate of stoma creation was quite low, and characteristics of the disease may explain the lack of a trend. For example, anorectal disease, especially when accompanied by fistula and strictures, is often medication refractory. “Although the study could not delineate this, it is my clinical practice experience that some patients present with destructive anorectal disease early, and that despite medications the ‘damage is too far gone’ to reverse,” said Dr. Regueiro. Also, the anorectum does not allow for an anastomosis since there is no region to connect to that isn’t damaged, he added.
The findings shouldn’t affect patient management or counseling, according to Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, who was not involved in the study. “The indications for surgery in Crohn’s disease have not changed with the advent of newer therapies. Complications such as strictures and abscesses are still treated with similar surgeries. It is hopeful that the future course after surgery can be modified by effective biologic therapies,” he said.
The study authors noted that the findings are consistent with previous studies suggesting that the rate of abdominal surgery had begun to decline before anti-TNF drugs were introduced. The Swedish National Patient Register used in the current work includes only inpatient data before 2001, preventing a broader analysis prior to 1999, when infliximab gained approval in Sweden. But the researchers looked at time from first surgery to stoma, and found a decline in stoma formation between 1994 and 1997. A similar analysis found no decrease in the 2000s.
The researchers pointed out that anti-TNF inhibitor use would be expected to reduce the rate of stomas by inducing remission, although temporary stomas may be created to relieve symptoms while waiting for the medication to take effect. But anti-TNF agents could also encourage patients and physicians to postpone surgery. The delay could raise the chance of surgical complications, which in turn could lead to creation of a temporary stoma. Permanent stomas are typically created in cases of severe perianal CD.
The study could come as a disappointment to some patients and physicians, according to the authors. “The more active and early use of anti-TNF (therapeutics), in combination with the decreasing incidence of abdominal surgery in general for CD, has raised hopes of decreased incidence of stoma formation, but this was not observed in the present study,” the authors wrote.
But the news wasn’t all bad. The study found a lower cumulative stoma incidence than had previous studies, and the incidence of permanent stoma was just 0.8% at 5 years, “which should be reassuring for patients,” according to the authors.
CD patients often fear that a stoma will become necessary, but a stoma can be quite beneficial, allowing patients to regain some control over their lives. That can lead to more work participation and social activity. Qualitative studies showed that both patients and clinicians described outcomes of stoma placement as exceeding expectations, with a key factor being support from other inflammatory bowel disease patients with stomas. The authors encouraged clinicians to discuss the possibility of a stoma early on in the treatment process, and to avoid referring to it as a “last resort.”
The study also suggests that patients with anal and anorectal Crohn’s disease should receive biologics early, and a multidisciplinary approach involving both the gastroenterologist and surgeon is important. And patients should be counseled on the impact of disease on diet and psychological health, according to Dr. Regueiro.
The new study analyzed data between 2003 and 2014, from 18,815 Crohn’s disease patients who had not undergone previous surgery. The median age was 39 years, 53% were women, and 12% were pediatric patients.
After a median follow-up of 9.6 years, 9.5% of patients had perianal disease. Overall, 36% of patients had been treated with immunomodulators, and 17% with anti-TNF agents; 3.5% had stoma surgery, and just 0.05% underwent proctectomy.
Among those who had a stoma placed, the median age at diagnosis was 47 years, and 53% were men. In all, 12.6% had perianal disease at diagnosis, and 24.5% had perianal disease by the end of follow-up; 43% had received immunomodulators, and 26% anti-TNF agents. Among stomas, 64% were ileostomies, and 44% were temporary, with 88% of removals performed within 2 years of surgery.
The calendar periods of CD onset (2003-2006, 2007-2010, and 2011-2014) had similar rates of cumulative stoma placement (log rank test, P = .61). Overall, the 1-year cumulative incidence of stoma was 1.3%, the 3-year incidence was 1.9%, and the 5-year incidence was 2.5%.
The cumulative incidence of ever-use of anti-TNF agents increased with each successive calendar period (P < .001), but there was no significant difference in cumulative stoma incidence (P = .07).
One limitation of the study is the lack of detailed patient characteristics, and another is the short follow-up for patients diagnosed during 2011-2014. Another is that the results may not be generalizable to a U.S. population.
The study authors have received funding and consulted for various pharmaceutical companies. Dr. Regueiro has received grants from and has consulted or been on a scientific advisory board for Abbvie, UCB, and Janssen. Dr. Hanauer has no relevant financial disclosures.
This article was updated Oct. 13, 2021.
FROM INFLAMMATORY BOWEL DISEASES
Morphology drives optical evaluation’s accuracy for predicting SMIC
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” Michael Bourke, MBBS, of the University of Sydney and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Bourke and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of submucosal invasive cancer was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
This article was updated Oct. 13, 2021.
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” Michael Bourke, MBBS, of the University of Sydney and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Bourke and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of submucosal invasive cancer was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
This article was updated Oct. 13, 2021.
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” Michael Bourke, MBBS, of the University of Sydney and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Bourke and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of submucosal invasive cancer was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
This article was updated Oct. 13, 2021.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
GERD: Composite pH impedance monitoring better identifies treatment escalation need
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
Combinations of abnormal pH-impedance metrics better predicted nonresponse to proton pump inhibitor therapy, as well as benefit of treatment escalation, than individual metrics in patients with gastroesophageal reflux disease (GERD) on twice-daily PPI.
The researchers found a higher proportion of nonresponders to PPI in a group of patients that had combinations of abnormal reflux burden, characterized as acid exposure time greater than 4%, more than 80 reflux episodes, and/or mean nocturnal baseline impedance (MNBI) less than 1,500 ohms, with 85% of these patients improving following initiation of invasive GERD management such as antireflux surgery or magnetic sphincter augmentation.
Not only does the combination of metrics offer more value in identifying responders to PPI than individual metrics, but the combination also offer greater value in “subsequently predicting response to escalation of antireflux management,” study authors C. Prakash Gyawali, MD, of Washington University, St. Louis, and colleagues wrote in Gastroenterology.
Currently in question is the applicability of thresholds for metrics from pH impedance monitoring for studies performed on PPI. According to Dr. Gyawali and colleagues, thresholds from the Lyon Consensus may be too high and likewise lack optimal sensitivity for detecting refractory acid burden in patients on PPI, while thresholds based on pH-metry alone, as reported in other publications, may also lack specificity.
To determine which metrics from “on PPI” pH impedance studies predict escalation therapy needs, the researchers analyzed deidentified pH impedance studies performed in healthy volunteers (n=66; median age, 37.5 years) and patients with GERD (n = 43; median age, 57.0 years); both groups were on twice-daily PPI. The investigators compared median values for pH impedance metrics between healthy volunteers and patients with proven GERD using validated measures.
Data were included from a total of three groups: tracings from European and North American healthy volunteers who received twice-daily PPI for 5-7 days; tracings from European patients with heartburn-predominant proven GERD with prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI; and tracings from a cohort of patients with regurgitation-predominant, proven GERD and prior abnormal reflux monitoring off PPI who subsequently received twice-daily PPI.
A improvement in heartburn of at least 50%, as recorded on 4-point Likert-type scales, defined PPI responders and improvements following antireflux surgery in the European comparison group. Additionally, an improvement of at least 50% on the GERD Health-Related Quality of Life scale also characterized PPI responders and improvements following magnetic sphincter augmentation in the North American comparison group.
There was no significant difference between PPI responders and nonresponders in terms of individual conventional and novel reflux metrics. The combinations of metrics associated with abnormal reflux burden and abnormal mucosal integrity (acid exposure time >4%, >80 reflux episodes, and MNBI <1,500 ohms) were observed in 32.6% of patients with heartburn and 40.5% of patients with regurgitation-predominant GERD, but no healthy volunteers. The combinations were also observed in 57.1% and 82.4% of nonresponders, respectively.
The authors defined a borderline category (acid exposure time, >0.5% but <4%; >40 but <80 reflux episodes), which accounted for 32.6% of patients with heartburn-predominant GERD and 50% of those regurgitation-predominant GERD. Nonresponse among these borderline cases was identified in 28.6% and 81%, respectively.
“Performance characteristics of the presence of abnormal reflux burden and/or abnormal mucosal integrity in predicting PPI nonresponse consisted of sensitivity, 0.50; specificity, 0.71; and AUC, 0.59 (P = .15),” the authors explained. “Performance characteristics of abnormal and borderline reflux burden categories together in predicting PPI nonresponse consisted of sensitivity, 0.86; specificity, 0.36; and AUC, 0.62 (P = .07).”
Limitations of this study included its retrospective nature, small sample sizes for the healthy volunteer and GERD populations, and the lack of data on relevant clinical information, including body mass index, dietary patterns, and PPI types and doses. Additionally, the findings may lack generalizability because of the inclusion of only patients with GERD who underwent surgical management.
Despite these limitations, the researchers wrote that the findings and identified “thresholds will be useful in planning prospective outcome studies to conclusively determine when to escalate antireflux therapy when GERD symptoms persist despite bid PPI therapy.”
The study researchers reported conflicts of interest with several pharmaceutical companies. No funding was reported for the study.
FROM GASTROENTEROLOGY
Autopsy findings reveal venous thromboembolism in patients with COVID-19
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: Despite the increased mortality rate of the novel coronavirus compared with influenza, little is understood about its pathogenicity. Prior studies have identified D-dimer levels, high Sequential Organ Failure Assessment score, and older age as markers for more severe disease and mortality. The specific cause of death of COVID-19 remains largely unknown.
Study design: Prospective cohort study.
Setting: Single academic center in Germany.
Synopsis: A complete autopsy was performed on 12 consecutive COVID-19 patient deaths at a single center. Seven had evidence of venous thromboembolism (VTE): three with bilateral lower extremity deep venous thrombosis (DVT) and four with massive pulmonary embolism/associated lower-extremity DVT. Prior to death, VTE was suspected clinically in only a single patient.
This small case series piques interest in the potential underrecognized thromboembolic pathology of COVID-19. While not practice changing, this study highlights the importance of hospitalists staying attuned to further studies regarding VTE prophylaxis in COVID-19.
Bottom line: Autopsies of COVID-19 patients revealed a high incidence of thromboembolic events; COVID-19–induced coagulopathy may play an underrecognized role in pathogenesis.
Citation: Wichmann D et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268-77.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.





