User login
Bullous Amyloidosis Masquerading as Pseudoporphyria
Cutaneous amyloidosis encompasses a variety of clinical presentations. Primary localized cutaneous amyloidosis comprises lichen amyloidosis, macular amyloidosis, and nodular amyloidosis.1 Macular and lichen amyloidosis result from keratin deposits, while nodular amyloidosis results from cutaneous infiltration of plasma cells.2 Primary systemic amyloidosis is due to a plasma cell dyscrasia, particularly multiple myeloma, while secondary systemic amyloidosis occurs in the setting of restrictive cardiomyopathy, congestive heart failure, renal dysfunction, or chronic inflammation, as seen with rheumatoid arthritis, tuberculosis, and various autoinflammatory disorders.2 Plasma cell proliferative disorders are associated with various skin disorders, which may result from aggregated misfolded monoclonal immunoglobulins, indicating light chain–related systemic amyloidosis. Mucocutaneous lesions can occur in 30% to 40% of cases of primary systemic amyloidosis and may present as purpura, ecchymoses, waxy thickening, plaques, subcutaneous nodules, and/or bullae.3,4 When blistering is present, the differential diagnosis is broad and includes autoimmune bullous disease, drug eruptions, enoxaparin-induced bullous hemorrhagic dermatosis, deposition diseases, allergic contact dermatitis, bullous cellulitis, bullous bite reactions, neutrophilic dermatosis, and bullous lichen sclerosus.5 Herein, we present a case of a woman with a bullous skin eruption who eventually was diagnosed with bullous amyloidosis subsequent to a diagnosis of multiple myeloma.
Case Report
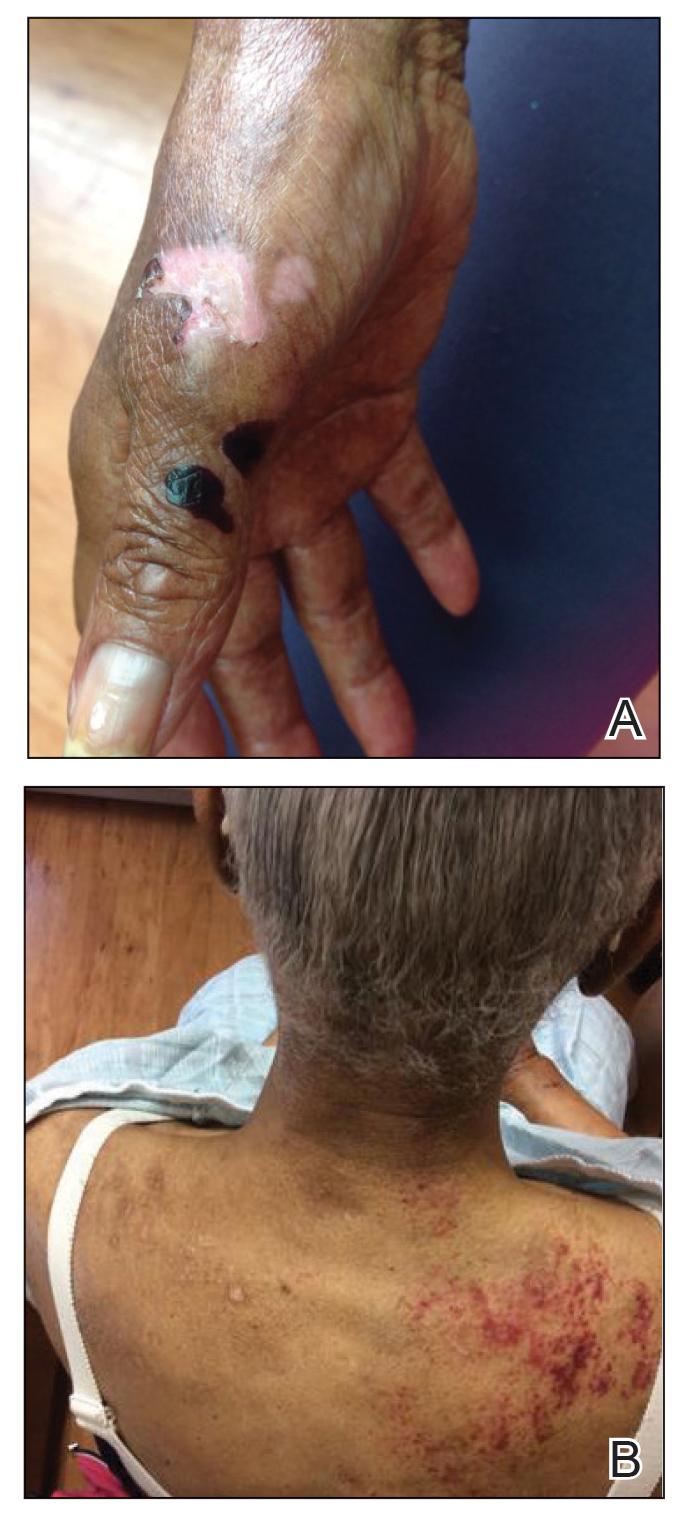
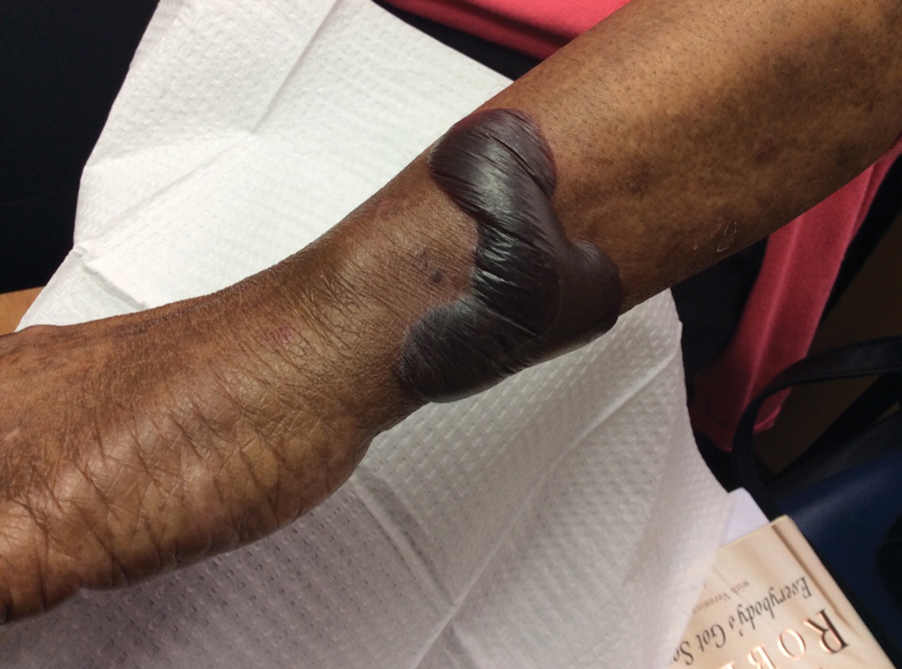
A 70-year-old woman presented to our dermatology clinic for evaluation of well-demarcated, hemorrhagic, flaccid vesicles and focal erosions with a rim of erythema on the distal forearms and hands. A shave biopsy from the right forearm showed cell-poor subepidermal vesicular dermatitis. Enzyme-linked immunosorbent assays for bullous pemphigoid antigens 1 and 2 as well as urinary porphyrins were negative. Direct immunofluorescence showed granular IgM at the basement membrane zone around vessels and cytoid bodies. At this time, a preliminary diagnosis of pseudoporphyria was suspected, though no classic medications (eg, nonsteroidal anti-inflammatory drugs, furosemide, antibiotics) or exogenous trigger factors (eg, UV light exposure, dialysis) were temporally related. Three months later, the patient presented with a large hemorrhagic bulla on the distal left forearm (Figure 1) and healing erosions on the dorsal fingers and upper back. Clobetasol ointment was initiated, as an autoimmune bullous dermatosis was suspected.
Approximately 1 year after she was first seen in our outpatient clinic, the patient was hospitalized for induction of chemotherapy—cyclophosphamide, bortezomib, and dexamethasone—for a new diagnosis of stage III multiple myeloma. A workup for back pain revealed multiple compression fractures and a plasma cell neoplasm with elevated λ light chains, which was confirmed with a bone marrow biopsy. During an inpatient dermatology consultation, we noted the development of intraoral hemorrhagic vesicles and worsening generalization of the hemorrhagic bullae, with healing erosions and intact hemorrhagic bullae on the dorsal hands, fingers (Figure 2), and upper back.
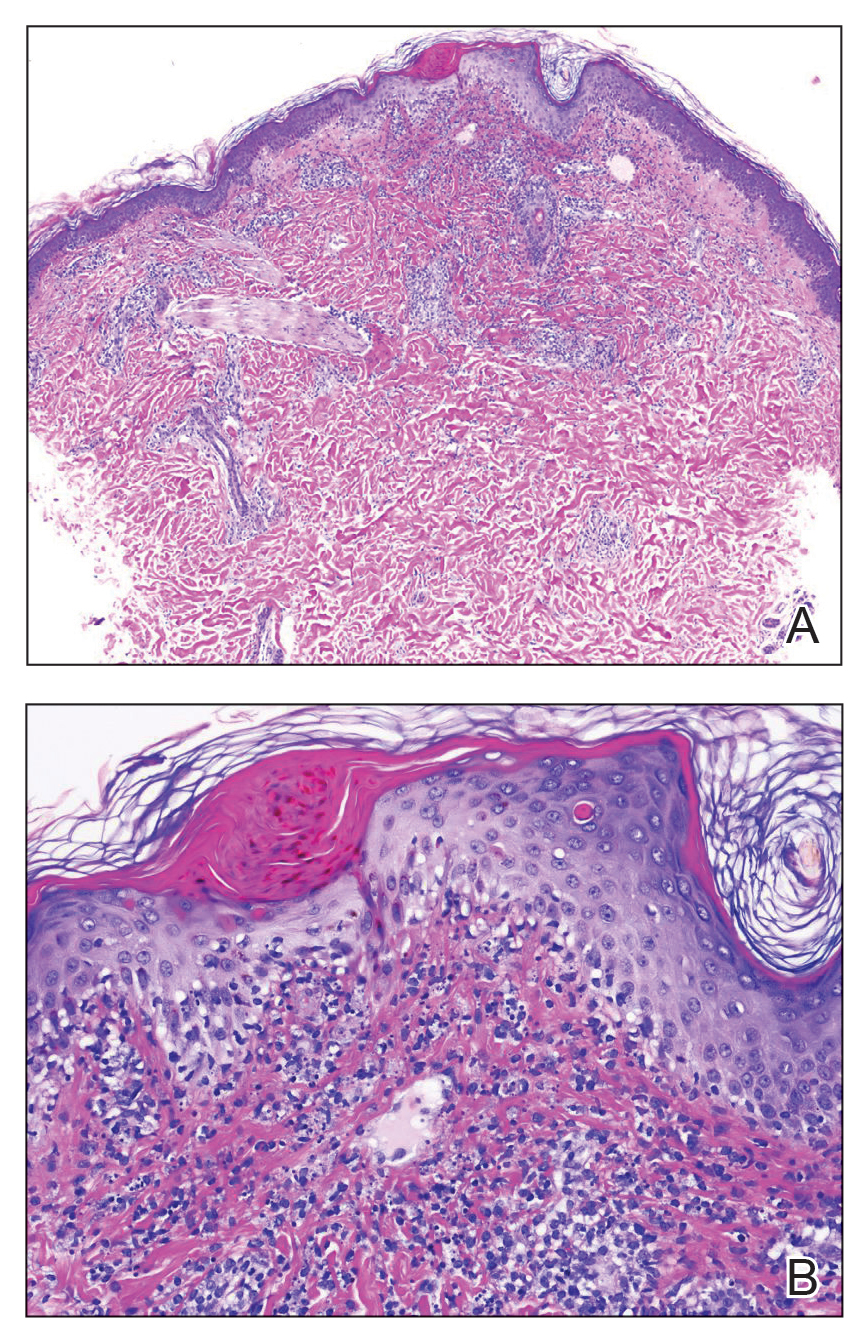
A repeat biopsy displayed bullous amyloidosis. Histopathologic examination revealed an ulcerated subepidermal blister with fibrin deposition at the ulcer base. A periadnexal, scant, eosinophilic deposition with extravasated red blood cells was appreciated. Amorphous eosinophilic deposits were found within the detached fragment of the epidermis and inflammatory infiltrate. A Congo red stain highlighted these areas with a salmon pink–colored material. Congo red staining showed a moderate amount of pale, apple green, birefringent deposit within these areas on polarized light examination.
A few months later, the patient was re-admitted, and the amount of skin detachment prompted the primary team to ask for another consultation. Although the extensive skin sloughing resembled toxic epidermal necrolysis, a repeat biopsy confirmed bullous amyloidosis.
Comment
Amyloidosis Histopathology—Amyloidoses represent a wide array of disorders with deposition of β-pleated sheets or amyloid fibrils, often with cutaneous manifestations.2,3 Primary systemic amyloidosis has been associated with underlying dyscrasia or multiple myeloma.6 In such cases, the skin lesions of multiple myeloma may result from a collection of misfolded monoclonal immunoglobulins or their fragments, as in light chain–related systemic amyloidosis.3 Histopathologically, both systemic and cutaneous amyloidosis appear similar and display deposition of amorphous, eosinophilic, fissured amyloid material in the dermis. Congo red stains the material orange-red and will display a characteristic apple green birefringence under polarized light.4 Although bullous amyloid lesions are rare, the cutaneous forms of these lesions can be an important sign of plasma cell dyscrasia.7
Presentation of Bullous Amyloidosis—Bullous manifestations rarely have been noted in the primary cutaneous forms of amyloidosis.5,8,9 Importantly, cutaneous blistering more often is linked to systemic forms of amyloidosis with multiorgan involvement, including primary systemic and myeloma-associated amyloidosis.5,10 However, patients with localized bullous cutaneous amyloidosis without systemic involvement also have been seen.10,11 Bullae may occur at any time, with contents that frequently are hemorrhagic due to capillary fragility.12,13 Bullous manifestations raise the differential diagnoses of bullous pemphigoid, epidermolysis bullosa acquisita, linear IgA disease, porphyria cutanea tarda, pseudoporphyria, bullous drug eruption, bullous eruption of renal dialysis, or bullous lupus erythematosus.5,13-17
In our patient, the acral distribution of bullae, presence of hemorrhage, chronicity of symptoms, and negative enzyme-linked immunosorbent assay initially suggested a diagnosis of pseudoporphyria. However, the presence of intraoral hemorrhagic vesicles and subsequent confirmatory pathology aided in differentiating bullous amyloidosis from pseudoporphyria. Nodular localized primary cutaneous amyloidosis, a rare form of skin-restricted amyloidoses, can coexist with bullous lesions. Of note, reported cases of nodular localized primary cutaneous amyloidosis did not result in development of multiple myeloma.5,10
Bullae are located either subepidermally or intradermally, and bullous lesions of cutaneous amyloidosis typically demonstrate subepidermal or superficial intradermal clefting on light microscopy.5,10,12 Histopathology of bullous amyloidosis shows intradermal or subepidermal blister formation and amorphous eosinophilic material showing apple green birefringence with Congo red staining deposited in the dermis and/or around the adipocytes and blood vessel walls.12,18-20 In prior cases, direct immunofluorescence of bullous amyloidosis revealed absent immunoglobulin (IgG, IgA, IgM) or complement (C3 and C9) deposits in the basement membrane zone or dermis.13,21,22 In these cases, electron microscopy was useful in diagnosis, as it showed the presence of amyloid deposits.21,22
Cause of Bullae—Various mechanisms are thought to trigger the blister formation in amyloidosis. Bullae created from trauma or friction often present as tense painful blisters that commonly are hemorrhagic.10,23 Amyloid deposits in the walls of blood vessels and the affinity of dermal amyloid in blood vessel walls to surrounding collagen likely leads to increased fragility of capillaries and the dermal matrix, hemorrhagic tendency, and infrapapillary blisters, thus creating hemorrhagic bullous eruptions.24,25 Specifically, close proximity of immunoglobulin-derived amyloid oligomers to epidermal keratinocytes may be toxic and therefore could trigger subepidermal bullous change.5 Additionally, alteration in the physicochemical properties of the amyloidal protein might explain bullous eruption.9 Trauma or rubbing of the hands and feet may precipitate the acral blister formation in bullous amyloidosis.5,11
Due to deposition of these amyloid fibrils, skin bleeding in these patients is called amyloid or pinch purpura. Vessel wall fragility and damage by amyloid are the principal causes of periorbital and gastrointestinal tract bleeding.26 Destruction of the lamina densa and widening of the intercellular space between keratinocytes by amyloid globules induce skin fragility.11
Although uncommon, various cases of bullous amyloidosis have been reported in the literature. Multiple myeloma patients represent the majority of those reported to have bullous amyloidosis.6,7,13,24,27-30 Plasmacytoma-associated bullous amyloid purpura and paraproteinemia also have been noted.25 Multiple myeloma with secondary AL amyloidosis has been seen with amyloid purpura and atraumatic ecchymoses of the face, highlighting the hemorrhage noted in these patients.26
Management of Amyloidosis—Various treatment options have been attempted for primary cutaneous amyloidosis, including oral retinoids, corticosteroids, cyclophosphamide, cyclosporine, amitriptyline, colchicine, cepharanthin, tacrolimus, dimethyl sulfoxide, vitamin D3 analogs, capsaicin, menthol, hydrocolloid dressings, surgical modalities, laser treatment, and phototherapy.1 There is no clear consensus for therapeutic modalities except for treating the underlying plasma cell dyscrasia in primary systemic amyloidosis.
Conclusion
We report the case of a patient displaying signs of pseudoporphyria that ultimately proved to be bullous amyloidosis, or what we termed pseudopseudoporphyria. Bullous amyloidosis should be considered in the differential diagnoses of hemorrhagic bullous skin eruptions. Particular attention should be given to a systemic workup for multiple myeloma when hemorrhagic vesicles/bullae are chronic and coexist with purpura, angina bullosa hemorrhagica, fatigue/weight loss, and/or macroglossia.
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Amyloidosis. Dermatology Essentials. Elsevier Saunders; 2014:341-345.
- Bhutani M, Shahid Z, Schnebelen A, et al. Cutaneous manifestations of multiple myeloma and other plasma cell proliferative disorders. Semin Oncol. 2016;43:395-400.
- Terushkin V, Boyd KP, Patel RR, et al. Primary localized cutaneous amyloidosis. Dermatol Online J. 2013;19:20711.
- LaChance A, Phelps A, Finch J, et al. Nodular localized primary cutaneous amyloidosis: a bullous variant. Clin Exp Dermatol. 2014;39:344-347.
- Gonzalez-Ramos J, Garrido-Gutiérrez C, González-Silva Y, et al. Relapsing bullous amyloidosis of the oral mucosa and acquired cutis laxa in a patient with multiple myeloma: a rare triple association. Clin Exp Dermatol. 2017;42:410-412.
- Kanoh T. Bullous amyloidosis [in Japanese]. Rinsho Ketsueki. 1993;34:1050-1052.
- Johnson TM, Rapini RP, Hebert AA, et al. Bullous amyloidosis. Cutis. 1989;43:346-352.
- Houman MH, Smiti KM, Ben Ghorbel I, et al. Bullous amyloidosis. Ann Dermatol Venereol. 2002;129:299-302.
- Sanusi T, Li Y, Qian Y, et al. Primary localized cutaneous nodular amyloidosis with bullous lesions. Indian J Dermatol Venereol Leprol. 2015;81:400-402.
- Ochiai T, Morishima T, Hao T, et al. Bullous amyloidosis: the mechanism of blister formation revealed by electron microscopy. J Cutan Pathol. 2001;28:407-411.
- Chu CH, Chan JY, Hsieh SW, et al. Diffuse ecchymoses and blisters on a yellowish waxy base: a case of bullous amyloidosis. J Dermatol. 2016;43:713-714.
- Wang XD, Shen H, Liu ZH. Diffuse haemorrhagic bullous amyloidosis with multiple myeloma. Clin Exp Dermatol. 2008;33:94-96.
- Biswas P, Aggarwal I, Sen D, et al. Bullous pemphigoid clinically presenting as lichen amyloidosis. Indian J Dermatol Venereol Leprol. 2014;80:544-546.
- Bluhm JF 3rd. Bullous dermatosis vs amyloidosis. Arch Dermatol. 1981;117:252.
- Bluhm JF 3rd. Bullous amyloidosis vs epidermolysis bullosa acquisita. JAMA. 1981;245:32.
- Murphy GM, Wright J, Nicholls DS, et al. Sunbed-induced pseudoporphyria. Br J Dermatol. 1989;120:555-562.
- Pramatarov K, Lazarova A, Mateev G, et al. Bullous hemorrhagic primary systemic amyloidosis. Int J Dermatol. 1990;29:211-213.
- Bieber T, Ruzicka T, Linke RP, et al. Hemorrhagic bullous amyloidosis. a histologic, immunocytochemical, and ultrastructural study of two patients. Arch Dermatol. 1988;124:1683-1686.
- Khoo BP, Tay YK. Lichen amyloidosis: a bullous variant. Ann Acad Med Singapore. 2000;29:105-107.
- Asahina A, Hasegawa K, Ishiyama M, et al. Bullous amyloidosis mimicking bullous pemphigoid: usefulness of electron microscopic examination. Acta Derm Venereol. 2010;90:427-428.
- Schmutz JL, Barbaud A, Cuny JF, et al. Bullous amyloidosis [in French]. Ann Dermatol Venereol. 1988;115:295-301.
- Lachmann HJ, Hawkins PN. Amyloidosis of the skin. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. McGraw-Hill; 2012:1574-1583.
- Grundmann JU, Bonnekoh B, Gollnick H. Extensive haemorrhagic-bullous skin manifestation of systemic AA-amyloidosis associated with IgG lambda-myeloma. Eur J Dermatol. 2000;10:139-142.
- Hödl S, Turek TD, Kerl H. Plasmocytoma-associated bullous hemorrhagic amyloidosis of the skin [in German]. Hautarzt. 1982;33:556-558.
- Colucci G, Alberio L, Demarmels Biasiutti F, et al. Bilateral periorbital ecchymoses. an often missed sign of amyloid purpura. Hamostaseologie. 2014;34:249-252.
- Behera B, Pattnaik M, Sahu B, et al. Cutaneous manifestations of multiple myeloma. Indian J Dermatol. 2016;61:668-671.
- Fujita Y, Tsuji-Abe Y, Sato-Matsumura KC, et al. Nail dystrophy and blisters as sole manifestations in myeloma-associated amyloidosis. J Am Acad Dermatol. 2006;54:712-714.
- Chang SL, Lai PC, Cheng CJ, et al. Bullous amyloidosis in a hemodialysis patient is myeloma-associated rather than hemodialysis-associated amyloidosis. Amyloid. 2007;14:153-156.
- Winzer M, Ruppert M, Baretton G, et al. Bullous poikilodermatitic amyloidosis of the skin with junctional bulla development in IgG light chain plasmacytoma of the lambda type. histology, immunohistology and electron microscopy [in German]. Hautarzt. 1992;43:199-204.
Cutaneous amyloidosis encompasses a variety of clinical presentations. Primary localized cutaneous amyloidosis comprises lichen amyloidosis, macular amyloidosis, and nodular amyloidosis.1 Macular and lichen amyloidosis result from keratin deposits, while nodular amyloidosis results from cutaneous infiltration of plasma cells.2 Primary systemic amyloidosis is due to a plasma cell dyscrasia, particularly multiple myeloma, while secondary systemic amyloidosis occurs in the setting of restrictive cardiomyopathy, congestive heart failure, renal dysfunction, or chronic inflammation, as seen with rheumatoid arthritis, tuberculosis, and various autoinflammatory disorders.2 Plasma cell proliferative disorders are associated with various skin disorders, which may result from aggregated misfolded monoclonal immunoglobulins, indicating light chain–related systemic amyloidosis. Mucocutaneous lesions can occur in 30% to 40% of cases of primary systemic amyloidosis and may present as purpura, ecchymoses, waxy thickening, plaques, subcutaneous nodules, and/or bullae.3,4 When blistering is present, the differential diagnosis is broad and includes autoimmune bullous disease, drug eruptions, enoxaparin-induced bullous hemorrhagic dermatosis, deposition diseases, allergic contact dermatitis, bullous cellulitis, bullous bite reactions, neutrophilic dermatosis, and bullous lichen sclerosus.5 Herein, we present a case of a woman with a bullous skin eruption who eventually was diagnosed with bullous amyloidosis subsequent to a diagnosis of multiple myeloma.
Case Report
A 70-year-old woman presented to our dermatology clinic for evaluation of well-demarcated, hemorrhagic, flaccid vesicles and focal erosions with a rim of erythema on the distal forearms and hands. A shave biopsy from the right forearm showed cell-poor subepidermal vesicular dermatitis. Enzyme-linked immunosorbent assays for bullous pemphigoid antigens 1 and 2 as well as urinary porphyrins were negative. Direct immunofluorescence showed granular IgM at the basement membrane zone around vessels and cytoid bodies. At this time, a preliminary diagnosis of pseudoporphyria was suspected, though no classic medications (eg, nonsteroidal anti-inflammatory drugs, furosemide, antibiotics) or exogenous trigger factors (eg, UV light exposure, dialysis) were temporally related. Three months later, the patient presented with a large hemorrhagic bulla on the distal left forearm (Figure 1) and healing erosions on the dorsal fingers and upper back. Clobetasol ointment was initiated, as an autoimmune bullous dermatosis was suspected.

Approximately 1 year after she was first seen in our outpatient clinic, the patient was hospitalized for induction of chemotherapy—cyclophosphamide, bortezomib, and dexamethasone—for a new diagnosis of stage III multiple myeloma. A workup for back pain revealed multiple compression fractures and a plasma cell neoplasm with elevated λ light chains, which was confirmed with a bone marrow biopsy. During an inpatient dermatology consultation, we noted the development of intraoral hemorrhagic vesicles and worsening generalization of the hemorrhagic bullae, with healing erosions and intact hemorrhagic bullae on the dorsal hands, fingers (Figure 2), and upper back.
A repeat biopsy displayed bullous amyloidosis. Histopathologic examination revealed an ulcerated subepidermal blister with fibrin deposition at the ulcer base. A periadnexal, scant, eosinophilic deposition with extravasated red blood cells was appreciated. Amorphous eosinophilic deposits were found within the detached fragment of the epidermis and inflammatory infiltrate. A Congo red stain highlighted these areas with a salmon pink–colored material. Congo red staining showed a moderate amount of pale, apple green, birefringent deposit within these areas on polarized light examination.
A few months later, the patient was re-admitted, and the amount of skin detachment prompted the primary team to ask for another consultation. Although the extensive skin sloughing resembled toxic epidermal necrolysis, a repeat biopsy confirmed bullous amyloidosis.
Comment
Amyloidosis Histopathology—Amyloidoses represent a wide array of disorders with deposition of β-pleated sheets or amyloid fibrils, often with cutaneous manifestations.2,3 Primary systemic amyloidosis has been associated with underlying dyscrasia or multiple myeloma.6 In such cases, the skin lesions of multiple myeloma may result from a collection of misfolded monoclonal immunoglobulins or their fragments, as in light chain–related systemic amyloidosis.3 Histopathologically, both systemic and cutaneous amyloidosis appear similar and display deposition of amorphous, eosinophilic, fissured amyloid material in the dermis. Congo red stains the material orange-red and will display a characteristic apple green birefringence under polarized light.4 Although bullous amyloid lesions are rare, the cutaneous forms of these lesions can be an important sign of plasma cell dyscrasia.7
Presentation of Bullous Amyloidosis—Bullous manifestations rarely have been noted in the primary cutaneous forms of amyloidosis.5,8,9 Importantly, cutaneous blistering more often is linked to systemic forms of amyloidosis with multiorgan involvement, including primary systemic and myeloma-associated amyloidosis.5,10 However, patients with localized bullous cutaneous amyloidosis without systemic involvement also have been seen.10,11 Bullae may occur at any time, with contents that frequently are hemorrhagic due to capillary fragility.12,13 Bullous manifestations raise the differential diagnoses of bullous pemphigoid, epidermolysis bullosa acquisita, linear IgA disease, porphyria cutanea tarda, pseudoporphyria, bullous drug eruption, bullous eruption of renal dialysis, or bullous lupus erythematosus.5,13-17
In our patient, the acral distribution of bullae, presence of hemorrhage, chronicity of symptoms, and negative enzyme-linked immunosorbent assay initially suggested a diagnosis of pseudoporphyria. However, the presence of intraoral hemorrhagic vesicles and subsequent confirmatory pathology aided in differentiating bullous amyloidosis from pseudoporphyria. Nodular localized primary cutaneous amyloidosis, a rare form of skin-restricted amyloidoses, can coexist with bullous lesions. Of note, reported cases of nodular localized primary cutaneous amyloidosis did not result in development of multiple myeloma.5,10
Bullae are located either subepidermally or intradermally, and bullous lesions of cutaneous amyloidosis typically demonstrate subepidermal or superficial intradermal clefting on light microscopy.5,10,12 Histopathology of bullous amyloidosis shows intradermal or subepidermal blister formation and amorphous eosinophilic material showing apple green birefringence with Congo red staining deposited in the dermis and/or around the adipocytes and blood vessel walls.12,18-20 In prior cases, direct immunofluorescence of bullous amyloidosis revealed absent immunoglobulin (IgG, IgA, IgM) or complement (C3 and C9) deposits in the basement membrane zone or dermis.13,21,22 In these cases, electron microscopy was useful in diagnosis, as it showed the presence of amyloid deposits.21,22
Cause of Bullae—Various mechanisms are thought to trigger the blister formation in amyloidosis. Bullae created from trauma or friction often present as tense painful blisters that commonly are hemorrhagic.10,23 Amyloid deposits in the walls of blood vessels and the affinity of dermal amyloid in blood vessel walls to surrounding collagen likely leads to increased fragility of capillaries and the dermal matrix, hemorrhagic tendency, and infrapapillary blisters, thus creating hemorrhagic bullous eruptions.24,25 Specifically, close proximity of immunoglobulin-derived amyloid oligomers to epidermal keratinocytes may be toxic and therefore could trigger subepidermal bullous change.5 Additionally, alteration in the physicochemical properties of the amyloidal protein might explain bullous eruption.9 Trauma or rubbing of the hands and feet may precipitate the acral blister formation in bullous amyloidosis.5,11
Due to deposition of these amyloid fibrils, skin bleeding in these patients is called amyloid or pinch purpura. Vessel wall fragility and damage by amyloid are the principal causes of periorbital and gastrointestinal tract bleeding.26 Destruction of the lamina densa and widening of the intercellular space between keratinocytes by amyloid globules induce skin fragility.11
Although uncommon, various cases of bullous amyloidosis have been reported in the literature. Multiple myeloma patients represent the majority of those reported to have bullous amyloidosis.6,7,13,24,27-30 Plasmacytoma-associated bullous amyloid purpura and paraproteinemia also have been noted.25 Multiple myeloma with secondary AL amyloidosis has been seen with amyloid purpura and atraumatic ecchymoses of the face, highlighting the hemorrhage noted in these patients.26
Management of Amyloidosis—Various treatment options have been attempted for primary cutaneous amyloidosis, including oral retinoids, corticosteroids, cyclophosphamide, cyclosporine, amitriptyline, colchicine, cepharanthin, tacrolimus, dimethyl sulfoxide, vitamin D3 analogs, capsaicin, menthol, hydrocolloid dressings, surgical modalities, laser treatment, and phototherapy.1 There is no clear consensus for therapeutic modalities except for treating the underlying plasma cell dyscrasia in primary systemic amyloidosis.
Conclusion
We report the case of a patient displaying signs of pseudoporphyria that ultimately proved to be bullous amyloidosis, or what we termed pseudopseudoporphyria. Bullous amyloidosis should be considered in the differential diagnoses of hemorrhagic bullous skin eruptions. Particular attention should be given to a systemic workup for multiple myeloma when hemorrhagic vesicles/bullae are chronic and coexist with purpura, angina bullosa hemorrhagica, fatigue/weight loss, and/or macroglossia.
Cutaneous amyloidosis encompasses a variety of clinical presentations. Primary localized cutaneous amyloidosis comprises lichen amyloidosis, macular amyloidosis, and nodular amyloidosis.1 Macular and lichen amyloidosis result from keratin deposits, while nodular amyloidosis results from cutaneous infiltration of plasma cells.2 Primary systemic amyloidosis is due to a plasma cell dyscrasia, particularly multiple myeloma, while secondary systemic amyloidosis occurs in the setting of restrictive cardiomyopathy, congestive heart failure, renal dysfunction, or chronic inflammation, as seen with rheumatoid arthritis, tuberculosis, and various autoinflammatory disorders.2 Plasma cell proliferative disorders are associated with various skin disorders, which may result from aggregated misfolded monoclonal immunoglobulins, indicating light chain–related systemic amyloidosis. Mucocutaneous lesions can occur in 30% to 40% of cases of primary systemic amyloidosis and may present as purpura, ecchymoses, waxy thickening, plaques, subcutaneous nodules, and/or bullae.3,4 When blistering is present, the differential diagnosis is broad and includes autoimmune bullous disease, drug eruptions, enoxaparin-induced bullous hemorrhagic dermatosis, deposition diseases, allergic contact dermatitis, bullous cellulitis, bullous bite reactions, neutrophilic dermatosis, and bullous lichen sclerosus.5 Herein, we present a case of a woman with a bullous skin eruption who eventually was diagnosed with bullous amyloidosis subsequent to a diagnosis of multiple myeloma.
Case Report
A 70-year-old woman presented to our dermatology clinic for evaluation of well-demarcated, hemorrhagic, flaccid vesicles and focal erosions with a rim of erythema on the distal forearms and hands. A shave biopsy from the right forearm showed cell-poor subepidermal vesicular dermatitis. Enzyme-linked immunosorbent assays for bullous pemphigoid antigens 1 and 2 as well as urinary porphyrins were negative. Direct immunofluorescence showed granular IgM at the basement membrane zone around vessels and cytoid bodies. At this time, a preliminary diagnosis of pseudoporphyria was suspected, though no classic medications (eg, nonsteroidal anti-inflammatory drugs, furosemide, antibiotics) or exogenous trigger factors (eg, UV light exposure, dialysis) were temporally related. Three months later, the patient presented with a large hemorrhagic bulla on the distal left forearm (Figure 1) and healing erosions on the dorsal fingers and upper back. Clobetasol ointment was initiated, as an autoimmune bullous dermatosis was suspected.

Approximately 1 year after she was first seen in our outpatient clinic, the patient was hospitalized for induction of chemotherapy—cyclophosphamide, bortezomib, and dexamethasone—for a new diagnosis of stage III multiple myeloma. A workup for back pain revealed multiple compression fractures and a plasma cell neoplasm with elevated λ light chains, which was confirmed with a bone marrow biopsy. During an inpatient dermatology consultation, we noted the development of intraoral hemorrhagic vesicles and worsening generalization of the hemorrhagic bullae, with healing erosions and intact hemorrhagic bullae on the dorsal hands, fingers (Figure 2), and upper back.
A repeat biopsy displayed bullous amyloidosis. Histopathologic examination revealed an ulcerated subepidermal blister with fibrin deposition at the ulcer base. A periadnexal, scant, eosinophilic deposition with extravasated red blood cells was appreciated. Amorphous eosinophilic deposits were found within the detached fragment of the epidermis and inflammatory infiltrate. A Congo red stain highlighted these areas with a salmon pink–colored material. Congo red staining showed a moderate amount of pale, apple green, birefringent deposit within these areas on polarized light examination.
A few months later, the patient was re-admitted, and the amount of skin detachment prompted the primary team to ask for another consultation. Although the extensive skin sloughing resembled toxic epidermal necrolysis, a repeat biopsy confirmed bullous amyloidosis.
Comment
Amyloidosis Histopathology—Amyloidoses represent a wide array of disorders with deposition of β-pleated sheets or amyloid fibrils, often with cutaneous manifestations.2,3 Primary systemic amyloidosis has been associated with underlying dyscrasia or multiple myeloma.6 In such cases, the skin lesions of multiple myeloma may result from a collection of misfolded monoclonal immunoglobulins or their fragments, as in light chain–related systemic amyloidosis.3 Histopathologically, both systemic and cutaneous amyloidosis appear similar and display deposition of amorphous, eosinophilic, fissured amyloid material in the dermis. Congo red stains the material orange-red and will display a characteristic apple green birefringence under polarized light.4 Although bullous amyloid lesions are rare, the cutaneous forms of these lesions can be an important sign of plasma cell dyscrasia.7
Presentation of Bullous Amyloidosis—Bullous manifestations rarely have been noted in the primary cutaneous forms of amyloidosis.5,8,9 Importantly, cutaneous blistering more often is linked to systemic forms of amyloidosis with multiorgan involvement, including primary systemic and myeloma-associated amyloidosis.5,10 However, patients with localized bullous cutaneous amyloidosis without systemic involvement also have been seen.10,11 Bullae may occur at any time, with contents that frequently are hemorrhagic due to capillary fragility.12,13 Bullous manifestations raise the differential diagnoses of bullous pemphigoid, epidermolysis bullosa acquisita, linear IgA disease, porphyria cutanea tarda, pseudoporphyria, bullous drug eruption, bullous eruption of renal dialysis, or bullous lupus erythematosus.5,13-17
In our patient, the acral distribution of bullae, presence of hemorrhage, chronicity of symptoms, and negative enzyme-linked immunosorbent assay initially suggested a diagnosis of pseudoporphyria. However, the presence of intraoral hemorrhagic vesicles and subsequent confirmatory pathology aided in differentiating bullous amyloidosis from pseudoporphyria. Nodular localized primary cutaneous amyloidosis, a rare form of skin-restricted amyloidoses, can coexist with bullous lesions. Of note, reported cases of nodular localized primary cutaneous amyloidosis did not result in development of multiple myeloma.5,10
Bullae are located either subepidermally or intradermally, and bullous lesions of cutaneous amyloidosis typically demonstrate subepidermal or superficial intradermal clefting on light microscopy.5,10,12 Histopathology of bullous amyloidosis shows intradermal or subepidermal blister formation and amorphous eosinophilic material showing apple green birefringence with Congo red staining deposited in the dermis and/or around the adipocytes and blood vessel walls.12,18-20 In prior cases, direct immunofluorescence of bullous amyloidosis revealed absent immunoglobulin (IgG, IgA, IgM) or complement (C3 and C9) deposits in the basement membrane zone or dermis.13,21,22 In these cases, electron microscopy was useful in diagnosis, as it showed the presence of amyloid deposits.21,22
Cause of Bullae—Various mechanisms are thought to trigger the blister formation in amyloidosis. Bullae created from trauma or friction often present as tense painful blisters that commonly are hemorrhagic.10,23 Amyloid deposits in the walls of blood vessels and the affinity of dermal amyloid in blood vessel walls to surrounding collagen likely leads to increased fragility of capillaries and the dermal matrix, hemorrhagic tendency, and infrapapillary blisters, thus creating hemorrhagic bullous eruptions.24,25 Specifically, close proximity of immunoglobulin-derived amyloid oligomers to epidermal keratinocytes may be toxic and therefore could trigger subepidermal bullous change.5 Additionally, alteration in the physicochemical properties of the amyloidal protein might explain bullous eruption.9 Trauma or rubbing of the hands and feet may precipitate the acral blister formation in bullous amyloidosis.5,11
Due to deposition of these amyloid fibrils, skin bleeding in these patients is called amyloid or pinch purpura. Vessel wall fragility and damage by amyloid are the principal causes of periorbital and gastrointestinal tract bleeding.26 Destruction of the lamina densa and widening of the intercellular space between keratinocytes by amyloid globules induce skin fragility.11
Although uncommon, various cases of bullous amyloidosis have been reported in the literature. Multiple myeloma patients represent the majority of those reported to have bullous amyloidosis.6,7,13,24,27-30 Plasmacytoma-associated bullous amyloid purpura and paraproteinemia also have been noted.25 Multiple myeloma with secondary AL amyloidosis has been seen with amyloid purpura and atraumatic ecchymoses of the face, highlighting the hemorrhage noted in these patients.26
Management of Amyloidosis—Various treatment options have been attempted for primary cutaneous amyloidosis, including oral retinoids, corticosteroids, cyclophosphamide, cyclosporine, amitriptyline, colchicine, cepharanthin, tacrolimus, dimethyl sulfoxide, vitamin D3 analogs, capsaicin, menthol, hydrocolloid dressings, surgical modalities, laser treatment, and phototherapy.1 There is no clear consensus for therapeutic modalities except for treating the underlying plasma cell dyscrasia in primary systemic amyloidosis.
Conclusion
We report the case of a patient displaying signs of pseudoporphyria that ultimately proved to be bullous amyloidosis, or what we termed pseudopseudoporphyria. Bullous amyloidosis should be considered in the differential diagnoses of hemorrhagic bullous skin eruptions. Particular attention should be given to a systemic workup for multiple myeloma when hemorrhagic vesicles/bullae are chronic and coexist with purpura, angina bullosa hemorrhagica, fatigue/weight loss, and/or macroglossia.
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Amyloidosis. Dermatology Essentials. Elsevier Saunders; 2014:341-345.
- Bhutani M, Shahid Z, Schnebelen A, et al. Cutaneous manifestations of multiple myeloma and other plasma cell proliferative disorders. Semin Oncol. 2016;43:395-400.
- Terushkin V, Boyd KP, Patel RR, et al. Primary localized cutaneous amyloidosis. Dermatol Online J. 2013;19:20711.
- LaChance A, Phelps A, Finch J, et al. Nodular localized primary cutaneous amyloidosis: a bullous variant. Clin Exp Dermatol. 2014;39:344-347.
- Gonzalez-Ramos J, Garrido-Gutiérrez C, González-Silva Y, et al. Relapsing bullous amyloidosis of the oral mucosa and acquired cutis laxa in a patient with multiple myeloma: a rare triple association. Clin Exp Dermatol. 2017;42:410-412.
- Kanoh T. Bullous amyloidosis [in Japanese]. Rinsho Ketsueki. 1993;34:1050-1052.
- Johnson TM, Rapini RP, Hebert AA, et al. Bullous amyloidosis. Cutis. 1989;43:346-352.
- Houman MH, Smiti KM, Ben Ghorbel I, et al. Bullous amyloidosis. Ann Dermatol Venereol. 2002;129:299-302.
- Sanusi T, Li Y, Qian Y, et al. Primary localized cutaneous nodular amyloidosis with bullous lesions. Indian J Dermatol Venereol Leprol. 2015;81:400-402.
- Ochiai T, Morishima T, Hao T, et al. Bullous amyloidosis: the mechanism of blister formation revealed by electron microscopy. J Cutan Pathol. 2001;28:407-411.
- Chu CH, Chan JY, Hsieh SW, et al. Diffuse ecchymoses and blisters on a yellowish waxy base: a case of bullous amyloidosis. J Dermatol. 2016;43:713-714.
- Wang XD, Shen H, Liu ZH. Diffuse haemorrhagic bullous amyloidosis with multiple myeloma. Clin Exp Dermatol. 2008;33:94-96.
- Biswas P, Aggarwal I, Sen D, et al. Bullous pemphigoid clinically presenting as lichen amyloidosis. Indian J Dermatol Venereol Leprol. 2014;80:544-546.
- Bluhm JF 3rd. Bullous dermatosis vs amyloidosis. Arch Dermatol. 1981;117:252.
- Bluhm JF 3rd. Bullous amyloidosis vs epidermolysis bullosa acquisita. JAMA. 1981;245:32.
- Murphy GM, Wright J, Nicholls DS, et al. Sunbed-induced pseudoporphyria. Br J Dermatol. 1989;120:555-562.
- Pramatarov K, Lazarova A, Mateev G, et al. Bullous hemorrhagic primary systemic amyloidosis. Int J Dermatol. 1990;29:211-213.
- Bieber T, Ruzicka T, Linke RP, et al. Hemorrhagic bullous amyloidosis. a histologic, immunocytochemical, and ultrastructural study of two patients. Arch Dermatol. 1988;124:1683-1686.
- Khoo BP, Tay YK. Lichen amyloidosis: a bullous variant. Ann Acad Med Singapore. 2000;29:105-107.
- Asahina A, Hasegawa K, Ishiyama M, et al. Bullous amyloidosis mimicking bullous pemphigoid: usefulness of electron microscopic examination. Acta Derm Venereol. 2010;90:427-428.
- Schmutz JL, Barbaud A, Cuny JF, et al. Bullous amyloidosis [in French]. Ann Dermatol Venereol. 1988;115:295-301.
- Lachmann HJ, Hawkins PN. Amyloidosis of the skin. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. McGraw-Hill; 2012:1574-1583.
- Grundmann JU, Bonnekoh B, Gollnick H. Extensive haemorrhagic-bullous skin manifestation of systemic AA-amyloidosis associated with IgG lambda-myeloma. Eur J Dermatol. 2000;10:139-142.
- Hödl S, Turek TD, Kerl H. Plasmocytoma-associated bullous hemorrhagic amyloidosis of the skin [in German]. Hautarzt. 1982;33:556-558.
- Colucci G, Alberio L, Demarmels Biasiutti F, et al. Bilateral periorbital ecchymoses. an often missed sign of amyloid purpura. Hamostaseologie. 2014;34:249-252.
- Behera B, Pattnaik M, Sahu B, et al. Cutaneous manifestations of multiple myeloma. Indian J Dermatol. 2016;61:668-671.
- Fujita Y, Tsuji-Abe Y, Sato-Matsumura KC, et al. Nail dystrophy and blisters as sole manifestations in myeloma-associated amyloidosis. J Am Acad Dermatol. 2006;54:712-714.
- Chang SL, Lai PC, Cheng CJ, et al. Bullous amyloidosis in a hemodialysis patient is myeloma-associated rather than hemodialysis-associated amyloidosis. Amyloid. 2007;14:153-156.
- Winzer M, Ruppert M, Baretton G, et al. Bullous poikilodermatitic amyloidosis of the skin with junctional bulla development in IgG light chain plasmacytoma of the lambda type. histology, immunohistology and electron microscopy [in German]. Hautarzt. 1992;43:199-204.
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Amyloidosis. Dermatology Essentials. Elsevier Saunders; 2014:341-345.
- Bhutani M, Shahid Z, Schnebelen A, et al. Cutaneous manifestations of multiple myeloma and other plasma cell proliferative disorders. Semin Oncol. 2016;43:395-400.
- Terushkin V, Boyd KP, Patel RR, et al. Primary localized cutaneous amyloidosis. Dermatol Online J. 2013;19:20711.
- LaChance A, Phelps A, Finch J, et al. Nodular localized primary cutaneous amyloidosis: a bullous variant. Clin Exp Dermatol. 2014;39:344-347.
- Gonzalez-Ramos J, Garrido-Gutiérrez C, González-Silva Y, et al. Relapsing bullous amyloidosis of the oral mucosa and acquired cutis laxa in a patient with multiple myeloma: a rare triple association. Clin Exp Dermatol. 2017;42:410-412.
- Kanoh T. Bullous amyloidosis [in Japanese]. Rinsho Ketsueki. 1993;34:1050-1052.
- Johnson TM, Rapini RP, Hebert AA, et al. Bullous amyloidosis. Cutis. 1989;43:346-352.
- Houman MH, Smiti KM, Ben Ghorbel I, et al. Bullous amyloidosis. Ann Dermatol Venereol. 2002;129:299-302.
- Sanusi T, Li Y, Qian Y, et al. Primary localized cutaneous nodular amyloidosis with bullous lesions. Indian J Dermatol Venereol Leprol. 2015;81:400-402.
- Ochiai T, Morishima T, Hao T, et al. Bullous amyloidosis: the mechanism of blister formation revealed by electron microscopy. J Cutan Pathol. 2001;28:407-411.
- Chu CH, Chan JY, Hsieh SW, et al. Diffuse ecchymoses and blisters on a yellowish waxy base: a case of bullous amyloidosis. J Dermatol. 2016;43:713-714.
- Wang XD, Shen H, Liu ZH. Diffuse haemorrhagic bullous amyloidosis with multiple myeloma. Clin Exp Dermatol. 2008;33:94-96.
- Biswas P, Aggarwal I, Sen D, et al. Bullous pemphigoid clinically presenting as lichen amyloidosis. Indian J Dermatol Venereol Leprol. 2014;80:544-546.
- Bluhm JF 3rd. Bullous dermatosis vs amyloidosis. Arch Dermatol. 1981;117:252.
- Bluhm JF 3rd. Bullous amyloidosis vs epidermolysis bullosa acquisita. JAMA. 1981;245:32.
- Murphy GM, Wright J, Nicholls DS, et al. Sunbed-induced pseudoporphyria. Br J Dermatol. 1989;120:555-562.
- Pramatarov K, Lazarova A, Mateev G, et al. Bullous hemorrhagic primary systemic amyloidosis. Int J Dermatol. 1990;29:211-213.
- Bieber T, Ruzicka T, Linke RP, et al. Hemorrhagic bullous amyloidosis. a histologic, immunocytochemical, and ultrastructural study of two patients. Arch Dermatol. 1988;124:1683-1686.
- Khoo BP, Tay YK. Lichen amyloidosis: a bullous variant. Ann Acad Med Singapore. 2000;29:105-107.
- Asahina A, Hasegawa K, Ishiyama M, et al. Bullous amyloidosis mimicking bullous pemphigoid: usefulness of electron microscopic examination. Acta Derm Venereol. 2010;90:427-428.
- Schmutz JL, Barbaud A, Cuny JF, et al. Bullous amyloidosis [in French]. Ann Dermatol Venereol. 1988;115:295-301.
- Lachmann HJ, Hawkins PN. Amyloidosis of the skin. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. McGraw-Hill; 2012:1574-1583.
- Grundmann JU, Bonnekoh B, Gollnick H. Extensive haemorrhagic-bullous skin manifestation of systemic AA-amyloidosis associated with IgG lambda-myeloma. Eur J Dermatol. 2000;10:139-142.
- Hödl S, Turek TD, Kerl H. Plasmocytoma-associated bullous hemorrhagic amyloidosis of the skin [in German]. Hautarzt. 1982;33:556-558.
- Colucci G, Alberio L, Demarmels Biasiutti F, et al. Bilateral periorbital ecchymoses. an often missed sign of amyloid purpura. Hamostaseologie. 2014;34:249-252.
- Behera B, Pattnaik M, Sahu B, et al. Cutaneous manifestations of multiple myeloma. Indian J Dermatol. 2016;61:668-671.
- Fujita Y, Tsuji-Abe Y, Sato-Matsumura KC, et al. Nail dystrophy and blisters as sole manifestations in myeloma-associated amyloidosis. J Am Acad Dermatol. 2006;54:712-714.
- Chang SL, Lai PC, Cheng CJ, et al. Bullous amyloidosis in a hemodialysis patient is myeloma-associated rather than hemodialysis-associated amyloidosis. Amyloid. 2007;14:153-156.
- Winzer M, Ruppert M, Baretton G, et al. Bullous poikilodermatitic amyloidosis of the skin with junctional bulla development in IgG light chain plasmacytoma of the lambda type. histology, immunohistology and electron microscopy [in German]. Hautarzt. 1992;43:199-204.
Practice Points
- Primary systemic amyloidosis, including the rare cutaneous bullous amyloidosis, often is difficult to diagnose and has been associated with underlying plasma cell dyscrasia or multiple myeloma.
- When evaluating patients with initially convincing signs of pseudoporphyria, it is imperative to consider the diagnosis of bullous amyloidosis, which additionally can present with intraoral hemorrhagic vesicles and have confirmatory histopathologic features.
- Further investigation for multiple myeloma is warranted when patients with a chronic hemorrhagic bullous condition also present with symptoms of purpura, angina bullosa hemorrhagica, fatigue, weight loss, and/or macroglossia. Accurate diagnosis of bullous amyloidosis and timely treatment of its underlying cause will contribute to better, more proactive patient care.
Kikuchi-Fujimoto Disease in an Adolescent Boy
To the Editor:
Kikuchi-Fujimoto Disease, also called histiocytic necrotizing lymphadenitis, was described in 1972 by both Kikuchi1 and Fujimoto et al.2 Most cases are reported in Asia, with limited reports in the United States.3-5 Kikuchi-Fujimoto disease is a rare, self-limiting condition consisting of benign lymphadenopathy and oftentimes fever and systemic symptoms. Lymph node involvement may mimic non-Hodgkin lymphoma or other reactive lymphadenopathy, rendering diagnostic accuracy challenging.5 Cutaneous manifestations are reported in only 16% to 40% of patients.6,7 Herein, we describe the clinical and pathologic features of a case of Kikuchi-Fujimoto disease with cutaneous involvement in an adolescent boy.
A 13-year-old adolescent boy with no notable medical history presented to the pediatric emergency department with cervical lymphadenopathy, weight loss, intermittent fever, and an evolving rash on the face, ears, arms, and thighs of 6 weeks’ duration. The illness began with enlarged lymph nodes and erythematous macules on the face and was diagnosed by his primary care physician as lymphadenitis that was unresponsive to clindamycin. Over the subsequent weeks, the rash worsened, and he developed intermittent fevers, night sweats, abdominal pain, and nausea with a 20-pound weight loss. He presented to the emergency department 3 weeks prior to the current admission and was noted to have elevated cytomegalovirus (CMV) IgM and IgG in addition to lymphopenia and anemia. He was discharged with outpatient follow-up. The rash progressed to involve the face, ears, arms, and thighs. One day prior to the current admission, the patient’s abdominal pain worsened acutely, and he experienced several episodes of emesis. He presented to the pediatric emergency department for further evaluation, and a dermatology consultation was requested at that time.
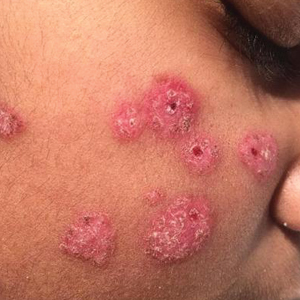
The patient’s rash was asymptomatic. In addition to the above symptoms, he also noted frequent nosebleeds, gingival bleeding, and diffuse myalgia that was most prominent on the hands and feet; he denied diarrhea, sick contacts, recent travel, or insect bites. His vital signs were normal, and he remained afebrile throughout the hospitalization. Physical examination revealed an ill-appearing patient with sunken eyes and dry lips. He had pink, oval, scaly plaques on the cheeks, ears, and arms (Figure 1). The thighs exhibited folliculocentric erythematous papules. The ocular conjunctivae were clear, but white exudative plaques were noted on the tongue. Tender, bilateral, cervical lymphadenopathy and diffuse abdominal tenderness with guarding and hepatosplenomegaly also were present. The fingers and toes were tender upon palpation.
Laboratory workup at admission revealed the following: low white blood cell count, 2700/μL (reference range, 4500–11,000/μL); low hemoglobin, 9.6 g/dL (reference range, 14.0–17.5 g/dL); elevated aspartate aminotransferase, 91 U/L (reference range, 10–30 U/L); and elevated alanine aminotransferase, 118 U/L (reference range, 10–40 U/L). Lactate dehydrogenase (582 U/L [reference range, 100–200 U/L]), ferritin (1681 ng/mL [reference range, 15–200 ng/mL]), and C-reactive protein (6.0 mg/L [reference range, 0.08–3.1 mg/L]) also were elevated. A respiratory viral panel was unremarkable. Blood cultures were negative, and an HIV 1/2 assay was nonreactive. A chest radiograph demonstrated clear lung fields. Computed tomography of the abdomen and pelvis showed prominent mesenteric, ileocolic, and retroperitoneal lymph nodes.
The differential diagnoses at this time included acute connective tissue disease, a paraneoplastic phenomenon, cutaneous lymphoma, or an infectious etiology. A punch biopsy of the skin as well as tissue cultures were performed from a lesion on the right arm. Quantitative immunoglobulin (IgA, IgG, IgM) levels were checked, all of which were within reference range. An antinuclear antibody (ANA) assay and rheumatoid factor were normal.
The tissue cultures were negative for bacteria, fungi, and mycobacteria. Microscopic examination of the skin biopsy revealed a moderate perivascular and interstitial infiltrate of predominantly histiocytes and lymphocytes with prominent karyorrhectic debris (nuclear dust) in the upper dermis as well as focal vacuolar interface changes with scattered necrotic keratinocytes in the epidermis (Figure 2). Based on these histopathologic findings, a diagnosis of Kikuchi-Fujimoto disease was considered. To confirm the diagnosis and to rule out the possibility of lymphoma, an excisional biopsy of the cervical lymph node was performed, which showed typical histopathologic features of histiocytic necrotizing lymphadenitis.
Given the patient’s clinical presentation with arthralgia, anorexia, lymphadenitis, and hepatosplenomegaly along with histopathologic findings from both the skin and lymph node biopsies, a diagnosis of Kikuchi-Fujimoto disease was made. The patient was conservatively managed with acetaminophen and was discharged with improvement in his appetite and systemic symptoms.
He was seen for follow-up 3 months later in the outpatient clinic. He denied any recurrence of systemic symptoms but endorsed a recent shedding of hair consistent with telogen effluvium. The rash had substantially improved, though residual asymptomatic erythematous plaques remained on the right forehead and right cheek (Figure 3). He was prescribed triamcinolone acetonide cream 0.1% to apply to the active area twice daily for the following 2 to 3 weeks.
Kikuchi-Fujimoto disease presents with a wide clinical spectrum, classically with benign lymphadenopathy and fever of unknown etiology.5,6 Lymphadenopathy most often is cervical (55%–99%)8 and unilateral,4,7 but patients can present with polyadenopathy (52%).7,8 Constitutional signs commonly include fever (35%–76%), weight loss, arthritis (5%–34%), and leukopenia (25%–74%).4,8,9
Cutaneous findings have been described in up to 40% of cases, of which clinical presentation is variable.6 Lesions may include blanchable, erythematous, painful, and/or indurated plaques, nodules, or maculopapules with confluence into patches, urticaria, morbilliform lesions, erythema multiforme, eyelid edema, leukocytoclastic vasculitis, papulopustules, ulcerated gingivae, and mucositis.6,7,10-13 Patients with skin lesions may be at an increased risk for developing systemic lupus erythematosus (SLE).8 Our patient presented with erythematous scaly plaques with a predominance of lesions in photodistributed locations, which clinically mimicked an underlying connective tissue disease process such as SLE.
Infectious agents such as CMV, parvovirus B19, human herpesvirus 6, human herpesvirus 8 and human T-cell lymphotropic virus 1, HIV, Yersinia enterocolitica, and Toxoplasma have all been implicated as possible causes of Kikuchi-Fujimoto disease, but studies have failed to provide convincing causal evidence.9,14,15 Our patient had positive IgM and IgG for CMV, which may have incited his disease.
Definitive diagnosis of Kikuchi-Fujimoto disease is made by lymph node excisional biopsy, which histologically exhibits a histiocytic cell proliferation with paracortical foci of necrosis and abundant karyorrhectic debris.5 Cutaneous histologic findings that support the diagnosis are variable and may include a dermal histiocytic infiltrate, epidermal change with necrotic keratinocytes, non-neutrophilic karyorrhectic debris, basal vacuolar change, papillary dermal edema, a nonspecific superficial and deep perivascular infiltrate, and a patchy infiltration of histiocytes and lymphocytes.6,13
Clinical and histopathological features of this disease can mimic other diseases, specifically SLE or lymphoma.7 An association with SLE has been suspected, though it is not well defined and more frequently is associated with cases from Asia than from Europe (28% and 9%, respectively).9 Patients presenting concomitantly with positive ANA, weight loss, arthralgia, and skin lesions are more likely to develop SLE.8 Furthermore, the cutaneous histologic finding of interface change suggests a link between the two diseases. As such, recommendations have been made for ANA screenings and follow-up of patients diagnosed with Kikuchi-Fujimoto disease for clinical evidence of autoimmune disease, particularly SLE.6 Although our patient did not have a positive ANA, his biopsy did demonstrate interface change, and he should be monitored for possible progression of disease in the future.
Kikuchi-Fujimoto disease differs from lymphoma, as it initially presents with rapid lymph node enlargement as opposed to the gradual enlargement seen in lymphoma. The lymph nodes in Kikuchi-Fujimoto disease often are firm and moveable compared to hard and immobile in lymphoma.3 Excisional lymph node biopsy is necessary for both confirming the diagnosis of Kikuchi-Fujimoto disease and ruling out lymphoma.5
Spontaneous resolution usually occurs in 1 to 4 months.3,6 As such, observation is the most common approach to management. When patients have symptoms that limit activities or cause undue distress such as fevers, joint pains, or abdominal pain, systemic treatment options may be desired. Symptomatic treatment can be managed with a short duration of oral corticosteroids,10,11 nonsteroidal anti-inflammatory drugs, antimalarials, and/or antipyretics.8-15 There are no guidelines regarding systemic steroid regimens, and various treatment schedules have been successful. Systemic therapy was considered for our patient for his weight loss and abdominal pain; however, by the time of discharge the patient was tolerating oral intake and his abdominal pain had improved.
- Kikuchi M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytosis. Nippon Ketsueki Gakkai Zasshi. 1972;35:379-380.
- Fujimoto Y, Kojima Y, Yamaguchi K. Cervical subacute necrotizing lymphadenitis: a new clinicopathological entity. Naika. 1972;30:920-927.
- Feder Jr HM, Liu J, Rezuke WN. Kikuchi disease in Connecticut. J Pediatr. 2014;164:196-200.
- Kang HM, Kim JY, Choi EH, et al. Clinical characteristics of severe histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) in children. J Pediatr. 2016;171:208-212.
- Hutchinson CB, Wang E. Kikuchi-Fujimoto disease. Arch Pathol Lab Med. 2010;134:289-293.
- Atwater AR, Longly BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yen H-R, Lin P-Y, Chuang W-Y, et al. Skin manifestations of Kikuchi-Fujimoto disease: case report and review. Eur J Pediatr. 2004;163:210-213.
- Dumas G, Prendki V, Haroche J, et al. Kikuchi-Fujimoto disease: retrospective study of 91 cases and review of literature. Medicine. 2014;93:372-382.
- Kuc ukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Yasukawa K, Matsumura T, Sato-Matsumura KC, et al. Kikuchi’s disease and the skin: case report and review of the literature. Br J Dermatol. 2001;144:885-889.
- Kaur S, Thami GP, Mohan H, et al. Kikuchi disease with facial rash and erythema multiforme. Pediatr Dermatol. 2001;18:403-405.
- Mauleón C, Valdivielso-Ramos M, Cabeza R, et al. Kikuchi disease with skin lesions mimicking lupus erythematosus. J Dermatol Case Rep. 2012;3:82-85.
- Obara K, Amoh Y. A case of Kikuchi’s disease (histiocytic necrotizing lymphoadenitis) with histiocytic cutaneous involvement. Rheumatol Int. 2015;35:1111-1113.
- Rosado FGN, Tang Y-W, Hasserjian RP, et al. Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr virus, human herpesvirus 6, and human herpesvirus 8. Hum Pathol. 2013;44:255-259.
- Chiu CF, Chow KC, Lin TY, et al. Virus infection in patients with histiocytic necrotizing lymphadenitis in Taiwan. detection of Epstein-Barr virus, type I human T-cell lymphotropic virus, and parvovirus B19. Am J Clin Pathol. 2000;113:774-781.
To the Editor:
Kikuchi-Fujimoto Disease, also called histiocytic necrotizing lymphadenitis, was described in 1972 by both Kikuchi1 and Fujimoto et al.2 Most cases are reported in Asia, with limited reports in the United States.3-5 Kikuchi-Fujimoto disease is a rare, self-limiting condition consisting of benign lymphadenopathy and oftentimes fever and systemic symptoms. Lymph node involvement may mimic non-Hodgkin lymphoma or other reactive lymphadenopathy, rendering diagnostic accuracy challenging.5 Cutaneous manifestations are reported in only 16% to 40% of patients.6,7 Herein, we describe the clinical and pathologic features of a case of Kikuchi-Fujimoto disease with cutaneous involvement in an adolescent boy.
A 13-year-old adolescent boy with no notable medical history presented to the pediatric emergency department with cervical lymphadenopathy, weight loss, intermittent fever, and an evolving rash on the face, ears, arms, and thighs of 6 weeks’ duration. The illness began with enlarged lymph nodes and erythematous macules on the face and was diagnosed by his primary care physician as lymphadenitis that was unresponsive to clindamycin. Over the subsequent weeks, the rash worsened, and he developed intermittent fevers, night sweats, abdominal pain, and nausea with a 20-pound weight loss. He presented to the emergency department 3 weeks prior to the current admission and was noted to have elevated cytomegalovirus (CMV) IgM and IgG in addition to lymphopenia and anemia. He was discharged with outpatient follow-up. The rash progressed to involve the face, ears, arms, and thighs. One day prior to the current admission, the patient’s abdominal pain worsened acutely, and he experienced several episodes of emesis. He presented to the pediatric emergency department for further evaluation, and a dermatology consultation was requested at that time.
The patient’s rash was asymptomatic. In addition to the above symptoms, he also noted frequent nosebleeds, gingival bleeding, and diffuse myalgia that was most prominent on the hands and feet; he denied diarrhea, sick contacts, recent travel, or insect bites. His vital signs were normal, and he remained afebrile throughout the hospitalization. Physical examination revealed an ill-appearing patient with sunken eyes and dry lips. He had pink, oval, scaly plaques on the cheeks, ears, and arms (Figure 1). The thighs exhibited folliculocentric erythematous papules. The ocular conjunctivae were clear, but white exudative plaques were noted on the tongue. Tender, bilateral, cervical lymphadenopathy and diffuse abdominal tenderness with guarding and hepatosplenomegaly also were present. The fingers and toes were tender upon palpation.
Laboratory workup at admission revealed the following: low white blood cell count, 2700/μL (reference range, 4500–11,000/μL); low hemoglobin, 9.6 g/dL (reference range, 14.0–17.5 g/dL); elevated aspartate aminotransferase, 91 U/L (reference range, 10–30 U/L); and elevated alanine aminotransferase, 118 U/L (reference range, 10–40 U/L). Lactate dehydrogenase (582 U/L [reference range, 100–200 U/L]), ferritin (1681 ng/mL [reference range, 15–200 ng/mL]), and C-reactive protein (6.0 mg/L [reference range, 0.08–3.1 mg/L]) also were elevated. A respiratory viral panel was unremarkable. Blood cultures were negative, and an HIV 1/2 assay was nonreactive. A chest radiograph demonstrated clear lung fields. Computed tomography of the abdomen and pelvis showed prominent mesenteric, ileocolic, and retroperitoneal lymph nodes.
The differential diagnoses at this time included acute connective tissue disease, a paraneoplastic phenomenon, cutaneous lymphoma, or an infectious etiology. A punch biopsy of the skin as well as tissue cultures were performed from a lesion on the right arm. Quantitative immunoglobulin (IgA, IgG, IgM) levels were checked, all of which were within reference range. An antinuclear antibody (ANA) assay and rheumatoid factor were normal.
The tissue cultures were negative for bacteria, fungi, and mycobacteria. Microscopic examination of the skin biopsy revealed a moderate perivascular and interstitial infiltrate of predominantly histiocytes and lymphocytes with prominent karyorrhectic debris (nuclear dust) in the upper dermis as well as focal vacuolar interface changes with scattered necrotic keratinocytes in the epidermis (Figure 2). Based on these histopathologic findings, a diagnosis of Kikuchi-Fujimoto disease was considered. To confirm the diagnosis and to rule out the possibility of lymphoma, an excisional biopsy of the cervical lymph node was performed, which showed typical histopathologic features of histiocytic necrotizing lymphadenitis.
Given the patient’s clinical presentation with arthralgia, anorexia, lymphadenitis, and hepatosplenomegaly along with histopathologic findings from both the skin and lymph node biopsies, a diagnosis of Kikuchi-Fujimoto disease was made. The patient was conservatively managed with acetaminophen and was discharged with improvement in his appetite and systemic symptoms.
He was seen for follow-up 3 months later in the outpatient clinic. He denied any recurrence of systemic symptoms but endorsed a recent shedding of hair consistent with telogen effluvium. The rash had substantially improved, though residual asymptomatic erythematous plaques remained on the right forehead and right cheek (Figure 3). He was prescribed triamcinolone acetonide cream 0.1% to apply to the active area twice daily for the following 2 to 3 weeks.
Kikuchi-Fujimoto disease presents with a wide clinical spectrum, classically with benign lymphadenopathy and fever of unknown etiology.5,6 Lymphadenopathy most often is cervical (55%–99%)8 and unilateral,4,7 but patients can present with polyadenopathy (52%).7,8 Constitutional signs commonly include fever (35%–76%), weight loss, arthritis (5%–34%), and leukopenia (25%–74%).4,8,9
Cutaneous findings have been described in up to 40% of cases, of which clinical presentation is variable.6 Lesions may include blanchable, erythematous, painful, and/or indurated plaques, nodules, or maculopapules with confluence into patches, urticaria, morbilliform lesions, erythema multiforme, eyelid edema, leukocytoclastic vasculitis, papulopustules, ulcerated gingivae, and mucositis.6,7,10-13 Patients with skin lesions may be at an increased risk for developing systemic lupus erythematosus (SLE).8 Our patient presented with erythematous scaly plaques with a predominance of lesions in photodistributed locations, which clinically mimicked an underlying connective tissue disease process such as SLE.
Infectious agents such as CMV, parvovirus B19, human herpesvirus 6, human herpesvirus 8 and human T-cell lymphotropic virus 1, HIV, Yersinia enterocolitica, and Toxoplasma have all been implicated as possible causes of Kikuchi-Fujimoto disease, but studies have failed to provide convincing causal evidence.9,14,15 Our patient had positive IgM and IgG for CMV, which may have incited his disease.
Definitive diagnosis of Kikuchi-Fujimoto disease is made by lymph node excisional biopsy, which histologically exhibits a histiocytic cell proliferation with paracortical foci of necrosis and abundant karyorrhectic debris.5 Cutaneous histologic findings that support the diagnosis are variable and may include a dermal histiocytic infiltrate, epidermal change with necrotic keratinocytes, non-neutrophilic karyorrhectic debris, basal vacuolar change, papillary dermal edema, a nonspecific superficial and deep perivascular infiltrate, and a patchy infiltration of histiocytes and lymphocytes.6,13
Clinical and histopathological features of this disease can mimic other diseases, specifically SLE or lymphoma.7 An association with SLE has been suspected, though it is not well defined and more frequently is associated with cases from Asia than from Europe (28% and 9%, respectively).9 Patients presenting concomitantly with positive ANA, weight loss, arthralgia, and skin lesions are more likely to develop SLE.8 Furthermore, the cutaneous histologic finding of interface change suggests a link between the two diseases. As such, recommendations have been made for ANA screenings and follow-up of patients diagnosed with Kikuchi-Fujimoto disease for clinical evidence of autoimmune disease, particularly SLE.6 Although our patient did not have a positive ANA, his biopsy did demonstrate interface change, and he should be monitored for possible progression of disease in the future.
Kikuchi-Fujimoto disease differs from lymphoma, as it initially presents with rapid lymph node enlargement as opposed to the gradual enlargement seen in lymphoma. The lymph nodes in Kikuchi-Fujimoto disease often are firm and moveable compared to hard and immobile in lymphoma.3 Excisional lymph node biopsy is necessary for both confirming the diagnosis of Kikuchi-Fujimoto disease and ruling out lymphoma.5
Spontaneous resolution usually occurs in 1 to 4 months.3,6 As such, observation is the most common approach to management. When patients have symptoms that limit activities or cause undue distress such as fevers, joint pains, or abdominal pain, systemic treatment options may be desired. Symptomatic treatment can be managed with a short duration of oral corticosteroids,10,11 nonsteroidal anti-inflammatory drugs, antimalarials, and/or antipyretics.8-15 There are no guidelines regarding systemic steroid regimens, and various treatment schedules have been successful. Systemic therapy was considered for our patient for his weight loss and abdominal pain; however, by the time of discharge the patient was tolerating oral intake and his abdominal pain had improved.
To the Editor:
Kikuchi-Fujimoto Disease, also called histiocytic necrotizing lymphadenitis, was described in 1972 by both Kikuchi1 and Fujimoto et al.2 Most cases are reported in Asia, with limited reports in the United States.3-5 Kikuchi-Fujimoto disease is a rare, self-limiting condition consisting of benign lymphadenopathy and oftentimes fever and systemic symptoms. Lymph node involvement may mimic non-Hodgkin lymphoma or other reactive lymphadenopathy, rendering diagnostic accuracy challenging.5 Cutaneous manifestations are reported in only 16% to 40% of patients.6,7 Herein, we describe the clinical and pathologic features of a case of Kikuchi-Fujimoto disease with cutaneous involvement in an adolescent boy.
A 13-year-old adolescent boy with no notable medical history presented to the pediatric emergency department with cervical lymphadenopathy, weight loss, intermittent fever, and an evolving rash on the face, ears, arms, and thighs of 6 weeks’ duration. The illness began with enlarged lymph nodes and erythematous macules on the face and was diagnosed by his primary care physician as lymphadenitis that was unresponsive to clindamycin. Over the subsequent weeks, the rash worsened, and he developed intermittent fevers, night sweats, abdominal pain, and nausea with a 20-pound weight loss. He presented to the emergency department 3 weeks prior to the current admission and was noted to have elevated cytomegalovirus (CMV) IgM and IgG in addition to lymphopenia and anemia. He was discharged with outpatient follow-up. The rash progressed to involve the face, ears, arms, and thighs. One day prior to the current admission, the patient’s abdominal pain worsened acutely, and he experienced several episodes of emesis. He presented to the pediatric emergency department for further evaluation, and a dermatology consultation was requested at that time.
The patient’s rash was asymptomatic. In addition to the above symptoms, he also noted frequent nosebleeds, gingival bleeding, and diffuse myalgia that was most prominent on the hands and feet; he denied diarrhea, sick contacts, recent travel, or insect bites. His vital signs were normal, and he remained afebrile throughout the hospitalization. Physical examination revealed an ill-appearing patient with sunken eyes and dry lips. He had pink, oval, scaly plaques on the cheeks, ears, and arms (Figure 1). The thighs exhibited folliculocentric erythematous papules. The ocular conjunctivae were clear, but white exudative plaques were noted on the tongue. Tender, bilateral, cervical lymphadenopathy and diffuse abdominal tenderness with guarding and hepatosplenomegaly also were present. The fingers and toes were tender upon palpation.
Laboratory workup at admission revealed the following: low white blood cell count, 2700/μL (reference range, 4500–11,000/μL); low hemoglobin, 9.6 g/dL (reference range, 14.0–17.5 g/dL); elevated aspartate aminotransferase, 91 U/L (reference range, 10–30 U/L); and elevated alanine aminotransferase, 118 U/L (reference range, 10–40 U/L). Lactate dehydrogenase (582 U/L [reference range, 100–200 U/L]), ferritin (1681 ng/mL [reference range, 15–200 ng/mL]), and C-reactive protein (6.0 mg/L [reference range, 0.08–3.1 mg/L]) also were elevated. A respiratory viral panel was unremarkable. Blood cultures were negative, and an HIV 1/2 assay was nonreactive. A chest radiograph demonstrated clear lung fields. Computed tomography of the abdomen and pelvis showed prominent mesenteric, ileocolic, and retroperitoneal lymph nodes.
The differential diagnoses at this time included acute connective tissue disease, a paraneoplastic phenomenon, cutaneous lymphoma, or an infectious etiology. A punch biopsy of the skin as well as tissue cultures were performed from a lesion on the right arm. Quantitative immunoglobulin (IgA, IgG, IgM) levels were checked, all of which were within reference range. An antinuclear antibody (ANA) assay and rheumatoid factor were normal.
The tissue cultures were negative for bacteria, fungi, and mycobacteria. Microscopic examination of the skin biopsy revealed a moderate perivascular and interstitial infiltrate of predominantly histiocytes and lymphocytes with prominent karyorrhectic debris (nuclear dust) in the upper dermis as well as focal vacuolar interface changes with scattered necrotic keratinocytes in the epidermis (Figure 2). Based on these histopathologic findings, a diagnosis of Kikuchi-Fujimoto disease was considered. To confirm the diagnosis and to rule out the possibility of lymphoma, an excisional biopsy of the cervical lymph node was performed, which showed typical histopathologic features of histiocytic necrotizing lymphadenitis.
Given the patient’s clinical presentation with arthralgia, anorexia, lymphadenitis, and hepatosplenomegaly along with histopathologic findings from both the skin and lymph node biopsies, a diagnosis of Kikuchi-Fujimoto disease was made. The patient was conservatively managed with acetaminophen and was discharged with improvement in his appetite and systemic symptoms.
He was seen for follow-up 3 months later in the outpatient clinic. He denied any recurrence of systemic symptoms but endorsed a recent shedding of hair consistent with telogen effluvium. The rash had substantially improved, though residual asymptomatic erythematous plaques remained on the right forehead and right cheek (Figure 3). He was prescribed triamcinolone acetonide cream 0.1% to apply to the active area twice daily for the following 2 to 3 weeks.
Kikuchi-Fujimoto disease presents with a wide clinical spectrum, classically with benign lymphadenopathy and fever of unknown etiology.5,6 Lymphadenopathy most often is cervical (55%–99%)8 and unilateral,4,7 but patients can present with polyadenopathy (52%).7,8 Constitutional signs commonly include fever (35%–76%), weight loss, arthritis (5%–34%), and leukopenia (25%–74%).4,8,9
Cutaneous findings have been described in up to 40% of cases, of which clinical presentation is variable.6 Lesions may include blanchable, erythematous, painful, and/or indurated plaques, nodules, or maculopapules with confluence into patches, urticaria, morbilliform lesions, erythema multiforme, eyelid edema, leukocytoclastic vasculitis, papulopustules, ulcerated gingivae, and mucositis.6,7,10-13 Patients with skin lesions may be at an increased risk for developing systemic lupus erythematosus (SLE).8 Our patient presented with erythematous scaly plaques with a predominance of lesions in photodistributed locations, which clinically mimicked an underlying connective tissue disease process such as SLE.
Infectious agents such as CMV, parvovirus B19, human herpesvirus 6, human herpesvirus 8 and human T-cell lymphotropic virus 1, HIV, Yersinia enterocolitica, and Toxoplasma have all been implicated as possible causes of Kikuchi-Fujimoto disease, but studies have failed to provide convincing causal evidence.9,14,15 Our patient had positive IgM and IgG for CMV, which may have incited his disease.
Definitive diagnosis of Kikuchi-Fujimoto disease is made by lymph node excisional biopsy, which histologically exhibits a histiocytic cell proliferation with paracortical foci of necrosis and abundant karyorrhectic debris.5 Cutaneous histologic findings that support the diagnosis are variable and may include a dermal histiocytic infiltrate, epidermal change with necrotic keratinocytes, non-neutrophilic karyorrhectic debris, basal vacuolar change, papillary dermal edema, a nonspecific superficial and deep perivascular infiltrate, and a patchy infiltration of histiocytes and lymphocytes.6,13
Clinical and histopathological features of this disease can mimic other diseases, specifically SLE or lymphoma.7 An association with SLE has been suspected, though it is not well defined and more frequently is associated with cases from Asia than from Europe (28% and 9%, respectively).9 Patients presenting concomitantly with positive ANA, weight loss, arthralgia, and skin lesions are more likely to develop SLE.8 Furthermore, the cutaneous histologic finding of interface change suggests a link between the two diseases. As such, recommendations have been made for ANA screenings and follow-up of patients diagnosed with Kikuchi-Fujimoto disease for clinical evidence of autoimmune disease, particularly SLE.6 Although our patient did not have a positive ANA, his biopsy did demonstrate interface change, and he should be monitored for possible progression of disease in the future.
Kikuchi-Fujimoto disease differs from lymphoma, as it initially presents with rapid lymph node enlargement as opposed to the gradual enlargement seen in lymphoma. The lymph nodes in Kikuchi-Fujimoto disease often are firm and moveable compared to hard and immobile in lymphoma.3 Excisional lymph node biopsy is necessary for both confirming the diagnosis of Kikuchi-Fujimoto disease and ruling out lymphoma.5
Spontaneous resolution usually occurs in 1 to 4 months.3,6 As such, observation is the most common approach to management. When patients have symptoms that limit activities or cause undue distress such as fevers, joint pains, or abdominal pain, systemic treatment options may be desired. Symptomatic treatment can be managed with a short duration of oral corticosteroids,10,11 nonsteroidal anti-inflammatory drugs, antimalarials, and/or antipyretics.8-15 There are no guidelines regarding systemic steroid regimens, and various treatment schedules have been successful. Systemic therapy was considered for our patient for his weight loss and abdominal pain; however, by the time of discharge the patient was tolerating oral intake and his abdominal pain had improved.
- Kikuchi M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytosis. Nippon Ketsueki Gakkai Zasshi. 1972;35:379-380.
- Fujimoto Y, Kojima Y, Yamaguchi K. Cervical subacute necrotizing lymphadenitis: a new clinicopathological entity. Naika. 1972;30:920-927.
- Feder Jr HM, Liu J, Rezuke WN. Kikuchi disease in Connecticut. J Pediatr. 2014;164:196-200.
- Kang HM, Kim JY, Choi EH, et al. Clinical characteristics of severe histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) in children. J Pediatr. 2016;171:208-212.
- Hutchinson CB, Wang E. Kikuchi-Fujimoto disease. Arch Pathol Lab Med. 2010;134:289-293.
- Atwater AR, Longly BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yen H-R, Lin P-Y, Chuang W-Y, et al. Skin manifestations of Kikuchi-Fujimoto disease: case report and review. Eur J Pediatr. 2004;163:210-213.
- Dumas G, Prendki V, Haroche J, et al. Kikuchi-Fujimoto disease: retrospective study of 91 cases and review of literature. Medicine. 2014;93:372-382.
- Kuc ukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Yasukawa K, Matsumura T, Sato-Matsumura KC, et al. Kikuchi’s disease and the skin: case report and review of the literature. Br J Dermatol. 2001;144:885-889.
- Kaur S, Thami GP, Mohan H, et al. Kikuchi disease with facial rash and erythema multiforme. Pediatr Dermatol. 2001;18:403-405.
- Mauleón C, Valdivielso-Ramos M, Cabeza R, et al. Kikuchi disease with skin lesions mimicking lupus erythematosus. J Dermatol Case Rep. 2012;3:82-85.
- Obara K, Amoh Y. A case of Kikuchi’s disease (histiocytic necrotizing lymphoadenitis) with histiocytic cutaneous involvement. Rheumatol Int. 2015;35:1111-1113.
- Rosado FGN, Tang Y-W, Hasserjian RP, et al. Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr virus, human herpesvirus 6, and human herpesvirus 8. Hum Pathol. 2013;44:255-259.
- Chiu CF, Chow KC, Lin TY, et al. Virus infection in patients with histiocytic necrotizing lymphadenitis in Taiwan. detection of Epstein-Barr virus, type I human T-cell lymphotropic virus, and parvovirus B19. Am J Clin Pathol. 2000;113:774-781.
- Kikuchi M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytosis. Nippon Ketsueki Gakkai Zasshi. 1972;35:379-380.
- Fujimoto Y, Kojima Y, Yamaguchi K. Cervical subacute necrotizing lymphadenitis: a new clinicopathological entity. Naika. 1972;30:920-927.
- Feder Jr HM, Liu J, Rezuke WN. Kikuchi disease in Connecticut. J Pediatr. 2014;164:196-200.
- Kang HM, Kim JY, Choi EH, et al. Clinical characteristics of severe histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) in children. J Pediatr. 2016;171:208-212.
- Hutchinson CB, Wang E. Kikuchi-Fujimoto disease. Arch Pathol Lab Med. 2010;134:289-293.
- Atwater AR, Longly BJ, Aughenbaugh WD. Kikuchi’s disease: case report and systematic review of cutaneous and histopathologic presentations. J Am Acad Dermatol. 2008;59:130-136.
- Yen H-R, Lin P-Y, Chuang W-Y, et al. Skin manifestations of Kikuchi-Fujimoto disease: case report and review. Eur J Pediatr. 2004;163:210-213.
- Dumas G, Prendki V, Haroche J, et al. Kikuchi-Fujimoto disease: retrospective study of 91 cases and review of literature. Medicine. 2014;93:372-382.
- Kuc ukardali Y, Solmazgul E, Kunter E, et al. Kikuchi-Fujimoto disease: analysis of 244 cases. Clin Rheumatol. 2007;26:50-54.
- Yasukawa K, Matsumura T, Sato-Matsumura KC, et al. Kikuchi’s disease and the skin: case report and review of the literature. Br J Dermatol. 2001;144:885-889.
- Kaur S, Thami GP, Mohan H, et al. Kikuchi disease with facial rash and erythema multiforme. Pediatr Dermatol. 2001;18:403-405.
- Mauleón C, Valdivielso-Ramos M, Cabeza R, et al. Kikuchi disease with skin lesions mimicking lupus erythematosus. J Dermatol Case Rep. 2012;3:82-85.
- Obara K, Amoh Y. A case of Kikuchi’s disease (histiocytic necrotizing lymphoadenitis) with histiocytic cutaneous involvement. Rheumatol Int. 2015;35:1111-1113.
- Rosado FGN, Tang Y-W, Hasserjian RP, et al. Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr virus, human herpesvirus 6, and human herpesvirus 8. Hum Pathol. 2013;44:255-259.
- Chiu CF, Chow KC, Lin TY, et al. Virus infection in patients with histiocytic necrotizing lymphadenitis in Taiwan. detection of Epstein-Barr virus, type I human T-cell lymphotropic virus, and parvovirus B19. Am J Clin Pathol. 2000;113:774-781.
Practice Points
- Kikuchi-Fujimoto disease is an uncommon, self-limited condition characterized by benign lymphadenopathy and variable systemic symptoms.
- Definitive diagnosis is made by excisional lymph node biopsy.
- Treatment options include oral corticosteroids, nonsteroidal anti-inflammatory drugs, antimalarials, and/or antipyretics.
New York’s largest health care provider fires 1,400 unvaccinated employees
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
Unexpected thrombocytosis could flag occult cancer
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
Biomarker testing in metastatic breast cancer management: ‘Essential’
Identifying biomarkers in metastatic breast cancer (MBC) has become an integral part of choosing treatments and understanding disease progression. The American Society of Clinical Oncology Clinical Practice Guideline, published in 2015, recommends an initial biopsy to confirm estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) status as well as repeat biopsies to watch for receptor status changes over time.
“Decisions concerning the initiation of systemic therapy or selection of systemic therapy for metastatic breast cancer should be guided by ER, PR, and HER2 status in conjunction with clinical evaluation, judgment, and the patient’s goals for care,” according to the guideline authors.This news organization reached out to Kelly McCann, MD, PhD, a hematologist and oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles, to explore the role biomarker testing plays in managing MBC.
Question: How important is biomarker testing in guiding MBC treatments? Is there a standard or recommended process?
Dr. McCann: Biomarker testing is essential to breast cancer treatment and the development of targeted therapies. Oncologists typically identify a tumor’s canonical biomarkers — ER, PR, and HER2 — using immunohistochemistry or fluorescence in situ hybridization (FISH) testing and then try to match the tumor biology to drugs that target that subtype.
For tumors that lack canonical biomarkers — for example, triple-negative breast cancer (TNBC) — I send the tumor tissue for next-generation sequencing at the time of metastatic diagnosis to identify a wider range of potential targets or oncogenic drivers, such as somatic or germline mutations in homologous recombination repair genes ( BRCA1, BRCA2, and PALB2 ) or mutations in the PI3K/AKT/mTOR pathway.
In our attempts to define tumor biology and design a treatment strategy, two additional issues quickly arise. First, tumors are heterogeneous from the start. Second, tumors evolve.
Let’s start with how we define or subtype a tumor. Would you walk us through this process?
Defining a breast tumor can be tricky because these cancers often don’t fit neatly into predefined categories. Let’s take the estrogen receptor. In clinical trials, we need to define the cutoff for what constitutes ER-positive MBC or TNBC. Some trials define ER-positive as 1% or greater, others define it as 10% or greater.
But is a PR- and HER2-negative tumor with 1% or even 5% ER expression really ER-positive in the biological or prognostic sense? Probably not. A tumor with less than 10% ER expression, for instance, will actually behave like a triple-negative tumor. Instead of choosing a regimen targeting the ER-positive cells, I’ll lean more toward cytotoxic chemotherapy, the standard treatment for TNBC.
Tumors may have multiple drivers as well. What are some aberrations in addition to the main subtypes?
Tumors also often harbor more than one targetable driver. For instance, PIK3CA gene mutations are present in about 40% of hormone receptor–positive, HER2-negative tumors. Activating mutations in ESR1 develop in anywhere from 10% to 50% of MBCs as a resistance mechanism to estrogen deprivation therapy, conferring estrogen independence to the cells. Activating mutations in ERBB2, which essentially turns HER2 into an active receptor, are found in 2%-4% of breast cancers, including ER-positive, HER2-mutant breast cancers, and are enriched in lobular breast cancers, which are typically ER positive, HER2 negative.
What about tumor evolution, given the growing body of evidence that biomarker status in MBC can change over time?
Patients with MBC often have several active areas of cancer, and these areas will evolve differently. During each line of treatment, some metastases will develop resistance and others won’t. For instance, if my patient’s liver metastases start to grow, I will change therapy immediately. If, however, a single bone metastasis begins to grow and the liver metastases have responded well, I might consider local therapy — such as radiation — to target that bone metastasis, though this particular approach hasn’t been formally studied.
Ultimately, we can expect tumors to change over time as they become more biologically aggressive or resistant to current therapy. The most common biomarker change is probably loss of ER or PR expression, but the frequency of ER, PR, or HER2 biomarker changes is still not well understood.
Resistance mutations can also happen. When, for instance, activating mutations in ESR1 occur, the estrogen receptor becomes independent of estrogen and tumors then develop resistance to endocrine therapies. We see a similar problem arise in metastatic prostate cancer. With chronic testosterone deprivation, eventually the androgen receptor evolves to become independent of testosterone in a stage known as castrate-resistant prostate cancer.
Which biomarkers or combinations of biomarkers can be paired with an approved treatment?
We have a range of treatments targeting ER-positive and HER2-positive MBC in particular. For tumors harboring additional targetable mutations, preliminary data suggest that HER2-targeted tyrosine kinase inhibitors (TKIs), such as tucatinib and neratinib, are effective against activating mutations in ERBB2.
The PI3K inhibitor alpelisib in combination with fulvestrant has been approved for patients with ER-positive, HER2-negative MBC and mutations in PIK3CA. The mTOR inhibitor everolimus plus exemestane is an option for patients with ER-positive, HER2-negative. And for those with activating mutations in ESR1, I switch patients to a selective estrogen receptor degrader, such as fulvestrant.
PARP inhibitors, including olaparib or talazoparib, target metastatic HR-positive disease or TNBC with deleterious germline BRCA1 or BRCA2 mutations. Sacituzumab govitecan has been approved for treating metastatic TNBC and targets the cell surface protein TROP2, expressed in almost 90% of TNBC tumors.
What targets, on the other hand, are less informative for treatment choice?
When we order next-generation sequencing, we also will get a list of possible targets for which there are currently no therapeutic options, but there may be in the future. I find this knowledge is helpful. For example, an activating mutation in KRAS tells me that the cancer has a very strong oncogenic driver that I won›t be able to target. I know that activating KRAS mutations in lung cancer and colon cancer portend a poorer prognosis, which helps me to prepare the patient and family.
Atezolizumab in combination with paclitaxel has been FDA-approved for PD-L1 TNBC in the first-line setting, though data show that immune checkpoint inhibitors may be effective even without PD-L1 expression. Although cell surface protein TROP2 has emerged as a target in recent years, its expression is so common in TNBC that confirmatory testing for TROP2 expression is not required to prescribe sacituzumab govitecan.
What factors do you weigh when selecting among the large number of tests available for tumor testing?
We have many biomarker tests available, but the National Comprehensive Cancer Network does not have guidelines for tumor genetics testing in breast cancer. That means insurance does not have to cover the cost, and many companies don’t. Ultimately, though, drug companies and some testing companies have an incentive to cover the cost themselves because a companion diagnostic might be linked to their drug — therascreen PIK3CA RGQ PCR kit for alpelisib, for instance.
I tend not to use a companion diagnostic test because I want more information with a wider panel. The tumor tests I often use are FoundationOne CDx, Caris Molecular Intelligence, and Tempus. I use Tempus because their financial aid is very generous and almost all of my patients qualify to be tested for less than $100. For germline genetic testing, Invitae, Myriad, and Color are also options. Invitae and Color are about $250 out of pocket without insurance. Many academic centers have their own gene panels as well.
How far have we come in identifying biomarkers in MBC?
Targeted treatment for breast cancer has advanced significantly since doing my PhD research in cancer biology about 15 years ago. Of course, targeted therapies for ER-positive and HER2-amplified cancers were available at that point, but many more have been developed. The most significant advance has been the development of efficient and affordable genome sequencing, which has led to these large panels and identification of therapeutic targets. We’ve also expanded our knowledge of genetic predispositions for breast cancer beyond BRCA1 and BRCA2, which not only allows us to preemptively advise patients and their families about cancer risks and recommendations for cancer screening, but also to select a therapy to target a cancer’s DNA repair deficits.
I feel that we are in an exciting discovery phase in oncology. We currently rely on biomarkers to manage MBC and will continue to refine our strategies and develop more effective drug therapies as we identify more oncogenic drivers, tumor-specific proteins, and cancer cell vulnerabilities.
Identifying biomarkers in metastatic breast cancer (MBC) has become an integral part of choosing treatments and understanding disease progression. The American Society of Clinical Oncology Clinical Practice Guideline, published in 2015, recommends an initial biopsy to confirm estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) status as well as repeat biopsies to watch for receptor status changes over time.
“Decisions concerning the initiation of systemic therapy or selection of systemic therapy for metastatic breast cancer should be guided by ER, PR, and HER2 status in conjunction with clinical evaluation, judgment, and the patient’s goals for care,” according to the guideline authors.This news organization reached out to Kelly McCann, MD, PhD, a hematologist and oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles, to explore the role biomarker testing plays in managing MBC.
Question: How important is biomarker testing in guiding MBC treatments? Is there a standard or recommended process?
Dr. McCann: Biomarker testing is essential to breast cancer treatment and the development of targeted therapies. Oncologists typically identify a tumor’s canonical biomarkers — ER, PR, and HER2 — using immunohistochemistry or fluorescence in situ hybridization (FISH) testing and then try to match the tumor biology to drugs that target that subtype.
For tumors that lack canonical biomarkers — for example, triple-negative breast cancer (TNBC) — I send the tumor tissue for next-generation sequencing at the time of metastatic diagnosis to identify a wider range of potential targets or oncogenic drivers, such as somatic or germline mutations in homologous recombination repair genes ( BRCA1, BRCA2, and PALB2 ) or mutations in the PI3K/AKT/mTOR pathway.
In our attempts to define tumor biology and design a treatment strategy, two additional issues quickly arise. First, tumors are heterogeneous from the start. Second, tumors evolve.
Let’s start with how we define or subtype a tumor. Would you walk us through this process?
Defining a breast tumor can be tricky because these cancers often don’t fit neatly into predefined categories. Let’s take the estrogen receptor. In clinical trials, we need to define the cutoff for what constitutes ER-positive MBC or TNBC. Some trials define ER-positive as 1% or greater, others define it as 10% or greater.
But is a PR- and HER2-negative tumor with 1% or even 5% ER expression really ER-positive in the biological or prognostic sense? Probably not. A tumor with less than 10% ER expression, for instance, will actually behave like a triple-negative tumor. Instead of choosing a regimen targeting the ER-positive cells, I’ll lean more toward cytotoxic chemotherapy, the standard treatment for TNBC.
Tumors may have multiple drivers as well. What are some aberrations in addition to the main subtypes?
Tumors also often harbor more than one targetable driver. For instance, PIK3CA gene mutations are present in about 40% of hormone receptor–positive, HER2-negative tumors. Activating mutations in ESR1 develop in anywhere from 10% to 50% of MBCs as a resistance mechanism to estrogen deprivation therapy, conferring estrogen independence to the cells. Activating mutations in ERBB2, which essentially turns HER2 into an active receptor, are found in 2%-4% of breast cancers, including ER-positive, HER2-mutant breast cancers, and are enriched in lobular breast cancers, which are typically ER positive, HER2 negative.
What about tumor evolution, given the growing body of evidence that biomarker status in MBC can change over time?
Patients with MBC often have several active areas of cancer, and these areas will evolve differently. During each line of treatment, some metastases will develop resistance and others won’t. For instance, if my patient’s liver metastases start to grow, I will change therapy immediately. If, however, a single bone metastasis begins to grow and the liver metastases have responded well, I might consider local therapy — such as radiation — to target that bone metastasis, though this particular approach hasn’t been formally studied.
Ultimately, we can expect tumors to change over time as they become more biologically aggressive or resistant to current therapy. The most common biomarker change is probably loss of ER or PR expression, but the frequency of ER, PR, or HER2 biomarker changes is still not well understood.
Resistance mutations can also happen. When, for instance, activating mutations in ESR1 occur, the estrogen receptor becomes independent of estrogen and tumors then develop resistance to endocrine therapies. We see a similar problem arise in metastatic prostate cancer. With chronic testosterone deprivation, eventually the androgen receptor evolves to become independent of testosterone in a stage known as castrate-resistant prostate cancer.
Which biomarkers or combinations of biomarkers can be paired with an approved treatment?
We have a range of treatments targeting ER-positive and HER2-positive MBC in particular. For tumors harboring additional targetable mutations, preliminary data suggest that HER2-targeted tyrosine kinase inhibitors (TKIs), such as tucatinib and neratinib, are effective against activating mutations in ERBB2.
The PI3K inhibitor alpelisib in combination with fulvestrant has been approved for patients with ER-positive, HER2-negative MBC and mutations in PIK3CA. The mTOR inhibitor everolimus plus exemestane is an option for patients with ER-positive, HER2-negative. And for those with activating mutations in ESR1, I switch patients to a selective estrogen receptor degrader, such as fulvestrant.
PARP inhibitors, including olaparib or talazoparib, target metastatic HR-positive disease or TNBC with deleterious germline BRCA1 or BRCA2 mutations. Sacituzumab govitecan has been approved for treating metastatic TNBC and targets the cell surface protein TROP2, expressed in almost 90% of TNBC tumors.
What targets, on the other hand, are less informative for treatment choice?
When we order next-generation sequencing, we also will get a list of possible targets for which there are currently no therapeutic options, but there may be in the future. I find this knowledge is helpful. For example, an activating mutation in KRAS tells me that the cancer has a very strong oncogenic driver that I won›t be able to target. I know that activating KRAS mutations in lung cancer and colon cancer portend a poorer prognosis, which helps me to prepare the patient and family.
Atezolizumab in combination with paclitaxel has been FDA-approved for PD-L1 TNBC in the first-line setting, though data show that immune checkpoint inhibitors may be effective even without PD-L1 expression. Although cell surface protein TROP2 has emerged as a target in recent years, its expression is so common in TNBC that confirmatory testing for TROP2 expression is not required to prescribe sacituzumab govitecan.
What factors do you weigh when selecting among the large number of tests available for tumor testing?
We have many biomarker tests available, but the National Comprehensive Cancer Network does not have guidelines for tumor genetics testing in breast cancer. That means insurance does not have to cover the cost, and many companies don’t. Ultimately, though, drug companies and some testing companies have an incentive to cover the cost themselves because a companion diagnostic might be linked to their drug — therascreen PIK3CA RGQ PCR kit for alpelisib, for instance.
I tend not to use a companion diagnostic test because I want more information with a wider panel. The tumor tests I often use are FoundationOne CDx, Caris Molecular Intelligence, and Tempus. I use Tempus because their financial aid is very generous and almost all of my patients qualify to be tested for less than $100. For germline genetic testing, Invitae, Myriad, and Color are also options. Invitae and Color are about $250 out of pocket without insurance. Many academic centers have their own gene panels as well.
How far have we come in identifying biomarkers in MBC?
Targeted treatment for breast cancer has advanced significantly since doing my PhD research in cancer biology about 15 years ago. Of course, targeted therapies for ER-positive and HER2-amplified cancers were available at that point, but many more have been developed. The most significant advance has been the development of efficient and affordable genome sequencing, which has led to these large panels and identification of therapeutic targets. We’ve also expanded our knowledge of genetic predispositions for breast cancer beyond BRCA1 and BRCA2, which not only allows us to preemptively advise patients and their families about cancer risks and recommendations for cancer screening, but also to select a therapy to target a cancer’s DNA repair deficits.
I feel that we are in an exciting discovery phase in oncology. We currently rely on biomarkers to manage MBC and will continue to refine our strategies and develop more effective drug therapies as we identify more oncogenic drivers, tumor-specific proteins, and cancer cell vulnerabilities.
Identifying biomarkers in metastatic breast cancer (MBC) has become an integral part of choosing treatments and understanding disease progression. The American Society of Clinical Oncology Clinical Practice Guideline, published in 2015, recommends an initial biopsy to confirm estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) status as well as repeat biopsies to watch for receptor status changes over time.
“Decisions concerning the initiation of systemic therapy or selection of systemic therapy for metastatic breast cancer should be guided by ER, PR, and HER2 status in conjunction with clinical evaluation, judgment, and the patient’s goals for care,” according to the guideline authors.This news organization reached out to Kelly McCann, MD, PhD, a hematologist and oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles, to explore the role biomarker testing plays in managing MBC.
Question: How important is biomarker testing in guiding MBC treatments? Is there a standard or recommended process?
Dr. McCann: Biomarker testing is essential to breast cancer treatment and the development of targeted therapies. Oncologists typically identify a tumor’s canonical biomarkers — ER, PR, and HER2 — using immunohistochemistry or fluorescence in situ hybridization (FISH) testing and then try to match the tumor biology to drugs that target that subtype.
For tumors that lack canonical biomarkers — for example, triple-negative breast cancer (TNBC) — I send the tumor tissue for next-generation sequencing at the time of metastatic diagnosis to identify a wider range of potential targets or oncogenic drivers, such as somatic or germline mutations in homologous recombination repair genes ( BRCA1, BRCA2, and PALB2 ) or mutations in the PI3K/AKT/mTOR pathway.
In our attempts to define tumor biology and design a treatment strategy, two additional issues quickly arise. First, tumors are heterogeneous from the start. Second, tumors evolve.
Let’s start with how we define or subtype a tumor. Would you walk us through this process?
Defining a breast tumor can be tricky because these cancers often don’t fit neatly into predefined categories. Let’s take the estrogen receptor. In clinical trials, we need to define the cutoff for what constitutes ER-positive MBC or TNBC. Some trials define ER-positive as 1% or greater, others define it as 10% or greater.
But is a PR- and HER2-negative tumor with 1% or even 5% ER expression really ER-positive in the biological or prognostic sense? Probably not. A tumor with less than 10% ER expression, for instance, will actually behave like a triple-negative tumor. Instead of choosing a regimen targeting the ER-positive cells, I’ll lean more toward cytotoxic chemotherapy, the standard treatment for TNBC.
Tumors may have multiple drivers as well. What are some aberrations in addition to the main subtypes?
Tumors also often harbor more than one targetable driver. For instance, PIK3CA gene mutations are present in about 40% of hormone receptor–positive, HER2-negative tumors. Activating mutations in ESR1 develop in anywhere from 10% to 50% of MBCs as a resistance mechanism to estrogen deprivation therapy, conferring estrogen independence to the cells. Activating mutations in ERBB2, which essentially turns HER2 into an active receptor, are found in 2%-4% of breast cancers, including ER-positive, HER2-mutant breast cancers, and are enriched in lobular breast cancers, which are typically ER positive, HER2 negative.
What about tumor evolution, given the growing body of evidence that biomarker status in MBC can change over time?
Patients with MBC often have several active areas of cancer, and these areas will evolve differently. During each line of treatment, some metastases will develop resistance and others won’t. For instance, if my patient’s liver metastases start to grow, I will change therapy immediately. If, however, a single bone metastasis begins to grow and the liver metastases have responded well, I might consider local therapy — such as radiation — to target that bone metastasis, though this particular approach hasn’t been formally studied.
Ultimately, we can expect tumors to change over time as they become more biologically aggressive or resistant to current therapy. The most common biomarker change is probably loss of ER or PR expression, but the frequency of ER, PR, or HER2 biomarker changes is still not well understood.
Resistance mutations can also happen. When, for instance, activating mutations in ESR1 occur, the estrogen receptor becomes independent of estrogen and tumors then develop resistance to endocrine therapies. We see a similar problem arise in metastatic prostate cancer. With chronic testosterone deprivation, eventually the androgen receptor evolves to become independent of testosterone in a stage known as castrate-resistant prostate cancer.
Which biomarkers or combinations of biomarkers can be paired with an approved treatment?
We have a range of treatments targeting ER-positive and HER2-positive MBC in particular. For tumors harboring additional targetable mutations, preliminary data suggest that HER2-targeted tyrosine kinase inhibitors (TKIs), such as tucatinib and neratinib, are effective against activating mutations in ERBB2.
The PI3K inhibitor alpelisib in combination with fulvestrant has been approved for patients with ER-positive, HER2-negative MBC and mutations in PIK3CA. The mTOR inhibitor everolimus plus exemestane is an option for patients with ER-positive, HER2-negative. And for those with activating mutations in ESR1, I switch patients to a selective estrogen receptor degrader, such as fulvestrant.
PARP inhibitors, including olaparib or talazoparib, target metastatic HR-positive disease or TNBC with deleterious germline BRCA1 or BRCA2 mutations. Sacituzumab govitecan has been approved for treating metastatic TNBC and targets the cell surface protein TROP2, expressed in almost 90% of TNBC tumors.
What targets, on the other hand, are less informative for treatment choice?
When we order next-generation sequencing, we also will get a list of possible targets for which there are currently no therapeutic options, but there may be in the future. I find this knowledge is helpful. For example, an activating mutation in KRAS tells me that the cancer has a very strong oncogenic driver that I won›t be able to target. I know that activating KRAS mutations in lung cancer and colon cancer portend a poorer prognosis, which helps me to prepare the patient and family.
Atezolizumab in combination with paclitaxel has been FDA-approved for PD-L1 TNBC in the first-line setting, though data show that immune checkpoint inhibitors may be effective even without PD-L1 expression. Although cell surface protein TROP2 has emerged as a target in recent years, its expression is so common in TNBC that confirmatory testing for TROP2 expression is not required to prescribe sacituzumab govitecan.
What factors do you weigh when selecting among the large number of tests available for tumor testing?
We have many biomarker tests available, but the National Comprehensive Cancer Network does not have guidelines for tumor genetics testing in breast cancer. That means insurance does not have to cover the cost, and many companies don’t. Ultimately, though, drug companies and some testing companies have an incentive to cover the cost themselves because a companion diagnostic might be linked to their drug — therascreen PIK3CA RGQ PCR kit for alpelisib, for instance.
I tend not to use a companion diagnostic test because I want more information with a wider panel. The tumor tests I often use are FoundationOne CDx, Caris Molecular Intelligence, and Tempus. I use Tempus because their financial aid is very generous and almost all of my patients qualify to be tested for less than $100. For germline genetic testing, Invitae, Myriad, and Color are also options. Invitae and Color are about $250 out of pocket without insurance. Many academic centers have their own gene panels as well.
How far have we come in identifying biomarkers in MBC?
Targeted treatment for breast cancer has advanced significantly since doing my PhD research in cancer biology about 15 years ago. Of course, targeted therapies for ER-positive and HER2-amplified cancers were available at that point, but many more have been developed. The most significant advance has been the development of efficient and affordable genome sequencing, which has led to these large panels and identification of therapeutic targets. We’ve also expanded our knowledge of genetic predispositions for breast cancer beyond BRCA1 and BRCA2, which not only allows us to preemptively advise patients and their families about cancer risks and recommendations for cancer screening, but also to select a therapy to target a cancer’s DNA repair deficits.
I feel that we are in an exciting discovery phase in oncology. We currently rely on biomarkers to manage MBC and will continue to refine our strategies and develop more effective drug therapies as we identify more oncogenic drivers, tumor-specific proteins, and cancer cell vulnerabilities.
Statins tied to diabetes progression
Statin use is associated with increased likelihood of diabetes progression, according to a new matched cohort analysis of data from the Department of Veteran Affairs.
Patients with diabetes who were on statins were more likely to begin taking insulin, become hyperglycemic, and to develop acute glycemic complications, and they were also more likely to be prescribed medications from more glucose-lowering drug classes.
Although previous observational and randomized, controlled trials suggested a link between statin use and diabetes progression, they typically relied on measures like insulin resistance, hemoglobin A1c, or fasting blood glucose levels. The new work, however, outlines changes in glycemic control.
The differences between fasting glucose levels and A1c levels were generally smaller than the differences in insulin sensitivity. But A1c and fasting glucose may underestimate a potential effect of statins, since physicians may escalate antidiabetes therapy in response to changes.
Insulin sensitivity is also rarely measured in real-world settings. “This study translated findings reported on academic studies of increased insulin resistance associated with statin use in research papers into everyday language of patient care. That is, patients on statins may need to escalate their antidiabetes therapy and there may have higher occurrences of uncontrolled diabetes events,” lead author Ishak Mansi, MD, said in an interview.
The study was published online in JAMA Internal Medicine.
Dr. Mansi, who is staff internist at the VA North Texas Health System and a professor of medicine and data and population science at the University of Texas, both in Dallas, cautioned about overinterpretation of the findings. “This is an observational study; therefore, it can establish association, but not causation.”
No reason to turn down statins
Dr. Mansi noted that it’s important to distinguish between those being prescribed statins as a primary preventive measurement against cardiovascular disease, and those starting statins with preexisting cardiovascular disease for secondary prevention. Statins are a key therapeutic class for secondary prevention. “Their benefits are tremendous, and we should emphasize that no patient should stop taking their statins based on our study – rather, they should talk to their doctors,” said Dr. Mansi.
The study is one of few to look at statin use and diabetes progression in patients who already have diabetes, and the first with a propensity-matched design, according to Om Ganda, MD, who was asked for comment. The results should not deter physicians from prescribing and patients from accepting statins. “Statins should not be withheld in people with high risk of cardiovascular disease, even for primary prevention, as the risk of progression of glucose levels is relatively much smaller and manageable, rather than risking cardiovascular events by stopping or not initiating when indicated by current guidelines,” said Dr. Ganda, who is the medical director of the Lipid Clinic at the Joslin Diabetes Center and an associate professor of medicine at Harvard Medical School, both in Boston.
It’s possible that statins could increase risk of diabetes progression through promoting insulin resistance, and they may also reduce beta-cell function, which could in turn reduce insulin secretion, according to Dr. Ganda.
The study group included 83,022 pairs of statin users and matched controls, of whom 95% were men; 68.2% were White; 22% were Black; 2.1% were Native American, Pacific Islander, or Alaska Native; and 0.8% were Asian. The mean age was 60 years.
Some 56% of statin users experienced diabetes progression, compared with 48% of control patients (odds ratio, 1.37; P < .001). Progression was defined as intensification of diabetes therapy through new use of insulin or increase in the number of medication classes, new onset chronic hyperglycemia, or acute complications from hyperglycemia.
The association was seen in the component measures, including an increased number of glucose-lowering medication classes (OR, 1.41; P < .001), the frequency of new insulin use (OR, 1.16; P < .001), persistent glycemia (OR, 1.13; P < .001), and a new diagnosis of ketoacidosis or uncontrolled diabetes (OR, 1.24; P < .001).
There was also a dose-response relationship between the intensity of LDL cholesterol–lowering medication and diabetes progression.
More research needed
The findings don’t necessarily have a strong clinical impact, but the researchers hope it pushes toward greater personalization of statin treatment. The benefits of statins have been well studied, but their potential harms have not received the same attention. Dr. Mansi hopes to learn more about which populations stand to gain the most for primary cardiovascular disease prevention, such as older versus younger populations, healthier or sicker patients, and those with well-controlled versus uncontrolled diabetes. “Answering these questions [would] impact hundreds of millions of patients and cannot be postponed,” said Dr. Mansi. He also called for dedicated funding for research into the adverse events of frequently used medications.
Dr. Mansi and Dr. Ganda have no relevant financial disclosures.
Statin use is associated with increased likelihood of diabetes progression, according to a new matched cohort analysis of data from the Department of Veteran Affairs.
Patients with diabetes who were on statins were more likely to begin taking insulin, become hyperglycemic, and to develop acute glycemic complications, and they were also more likely to be prescribed medications from more glucose-lowering drug classes.
Although previous observational and randomized, controlled trials suggested a link between statin use and diabetes progression, they typically relied on measures like insulin resistance, hemoglobin A1c, or fasting blood glucose levels. The new work, however, outlines changes in glycemic control.
The differences between fasting glucose levels and A1c levels were generally smaller than the differences in insulin sensitivity. But A1c and fasting glucose may underestimate a potential effect of statins, since physicians may escalate antidiabetes therapy in response to changes.
Insulin sensitivity is also rarely measured in real-world settings. “This study translated findings reported on academic studies of increased insulin resistance associated with statin use in research papers into everyday language of patient care. That is, patients on statins may need to escalate their antidiabetes therapy and there may have higher occurrences of uncontrolled diabetes events,” lead author Ishak Mansi, MD, said in an interview.
The study was published online in JAMA Internal Medicine.
Dr. Mansi, who is staff internist at the VA North Texas Health System and a professor of medicine and data and population science at the University of Texas, both in Dallas, cautioned about overinterpretation of the findings. “This is an observational study; therefore, it can establish association, but not causation.”
No reason to turn down statins
Dr. Mansi noted that it’s important to distinguish between those being prescribed statins as a primary preventive measurement against cardiovascular disease, and those starting statins with preexisting cardiovascular disease for secondary prevention. Statins are a key therapeutic class for secondary prevention. “Their benefits are tremendous, and we should emphasize that no patient should stop taking their statins based on our study – rather, they should talk to their doctors,” said Dr. Mansi.
The study is one of few to look at statin use and diabetes progression in patients who already have diabetes, and the first with a propensity-matched design, according to Om Ganda, MD, who was asked for comment. The results should not deter physicians from prescribing and patients from accepting statins. “Statins should not be withheld in people with high risk of cardiovascular disease, even for primary prevention, as the risk of progression of glucose levels is relatively much smaller and manageable, rather than risking cardiovascular events by stopping or not initiating when indicated by current guidelines,” said Dr. Ganda, who is the medical director of the Lipid Clinic at the Joslin Diabetes Center and an associate professor of medicine at Harvard Medical School, both in Boston.
It’s possible that statins could increase risk of diabetes progression through promoting insulin resistance, and they may also reduce beta-cell function, which could in turn reduce insulin secretion, according to Dr. Ganda.
The study group included 83,022 pairs of statin users and matched controls, of whom 95% were men; 68.2% were White; 22% were Black; 2.1% were Native American, Pacific Islander, or Alaska Native; and 0.8% were Asian. The mean age was 60 years.
Some 56% of statin users experienced diabetes progression, compared with 48% of control patients (odds ratio, 1.37; P < .001). Progression was defined as intensification of diabetes therapy through new use of insulin or increase in the number of medication classes, new onset chronic hyperglycemia, or acute complications from hyperglycemia.
The association was seen in the component measures, including an increased number of glucose-lowering medication classes (OR, 1.41; P < .001), the frequency of new insulin use (OR, 1.16; P < .001), persistent glycemia (OR, 1.13; P < .001), and a new diagnosis of ketoacidosis or uncontrolled diabetes (OR, 1.24; P < .001).
There was also a dose-response relationship between the intensity of LDL cholesterol–lowering medication and diabetes progression.
More research needed
The findings don’t necessarily have a strong clinical impact, but the researchers hope it pushes toward greater personalization of statin treatment. The benefits of statins have been well studied, but their potential harms have not received the same attention. Dr. Mansi hopes to learn more about which populations stand to gain the most for primary cardiovascular disease prevention, such as older versus younger populations, healthier or sicker patients, and those with well-controlled versus uncontrolled diabetes. “Answering these questions [would] impact hundreds of millions of patients and cannot be postponed,” said Dr. Mansi. He also called for dedicated funding for research into the adverse events of frequently used medications.
Dr. Mansi and Dr. Ganda have no relevant financial disclosures.
Statin use is associated with increased likelihood of diabetes progression, according to a new matched cohort analysis of data from the Department of Veteran Affairs.
Patients with diabetes who were on statins were more likely to begin taking insulin, become hyperglycemic, and to develop acute glycemic complications, and they were also more likely to be prescribed medications from more glucose-lowering drug classes.
Although previous observational and randomized, controlled trials suggested a link between statin use and diabetes progression, they typically relied on measures like insulin resistance, hemoglobin A1c, or fasting blood glucose levels. The new work, however, outlines changes in glycemic control.
The differences between fasting glucose levels and A1c levels were generally smaller than the differences in insulin sensitivity. But A1c and fasting glucose may underestimate a potential effect of statins, since physicians may escalate antidiabetes therapy in response to changes.
Insulin sensitivity is also rarely measured in real-world settings. “This study translated findings reported on academic studies of increased insulin resistance associated with statin use in research papers into everyday language of patient care. That is, patients on statins may need to escalate their antidiabetes therapy and there may have higher occurrences of uncontrolled diabetes events,” lead author Ishak Mansi, MD, said in an interview.
The study was published online in JAMA Internal Medicine.
Dr. Mansi, who is staff internist at the VA North Texas Health System and a professor of medicine and data and population science at the University of Texas, both in Dallas, cautioned about overinterpretation of the findings. “This is an observational study; therefore, it can establish association, but not causation.”
No reason to turn down statins
Dr. Mansi noted that it’s important to distinguish between those being prescribed statins as a primary preventive measurement against cardiovascular disease, and those starting statins with preexisting cardiovascular disease for secondary prevention. Statins are a key therapeutic class for secondary prevention. “Their benefits are tremendous, and we should emphasize that no patient should stop taking their statins based on our study – rather, they should talk to their doctors,” said Dr. Mansi.
The study is one of few to look at statin use and diabetes progression in patients who already have diabetes, and the first with a propensity-matched design, according to Om Ganda, MD, who was asked for comment. The results should not deter physicians from prescribing and patients from accepting statins. “Statins should not be withheld in people with high risk of cardiovascular disease, even for primary prevention, as the risk of progression of glucose levels is relatively much smaller and manageable, rather than risking cardiovascular events by stopping or not initiating when indicated by current guidelines,” said Dr. Ganda, who is the medical director of the Lipid Clinic at the Joslin Diabetes Center and an associate professor of medicine at Harvard Medical School, both in Boston.
It’s possible that statins could increase risk of diabetes progression through promoting insulin resistance, and they may also reduce beta-cell function, which could in turn reduce insulin secretion, according to Dr. Ganda.
The study group included 83,022 pairs of statin users and matched controls, of whom 95% were men; 68.2% were White; 22% were Black; 2.1% were Native American, Pacific Islander, or Alaska Native; and 0.8% were Asian. The mean age was 60 years.
Some 56% of statin users experienced diabetes progression, compared with 48% of control patients (odds ratio, 1.37; P < .001). Progression was defined as intensification of diabetes therapy through new use of insulin or increase in the number of medication classes, new onset chronic hyperglycemia, or acute complications from hyperglycemia.
The association was seen in the component measures, including an increased number of glucose-lowering medication classes (OR, 1.41; P < .001), the frequency of new insulin use (OR, 1.16; P < .001), persistent glycemia (OR, 1.13; P < .001), and a new diagnosis of ketoacidosis or uncontrolled diabetes (OR, 1.24; P < .001).
There was also a dose-response relationship between the intensity of LDL cholesterol–lowering medication and diabetes progression.
More research needed
The findings don’t necessarily have a strong clinical impact, but the researchers hope it pushes toward greater personalization of statin treatment. The benefits of statins have been well studied, but their potential harms have not received the same attention. Dr. Mansi hopes to learn more about which populations stand to gain the most for primary cardiovascular disease prevention, such as older versus younger populations, healthier or sicker patients, and those with well-controlled versus uncontrolled diabetes. “Answering these questions [would] impact hundreds of millions of patients and cannot be postponed,” said Dr. Mansi. He also called for dedicated funding for research into the adverse events of frequently used medications.
Dr. Mansi and Dr. Ganda have no relevant financial disclosures.
FROM JAMA INTERNAL MEDICINE
Circulating post-STEMI ketones elevated, hints at treatment role
Circulating ketone bodies (KBs) are substantially elevated at presentation and 24 hours after ST-segment elevation myocardial infarction (STEMI), according to new research.
The study also showed that greater KB levels measured after 24 hours of reperfusion were associated with larger infarct size and reduced left ventricular ejection fraction (LVEF).
The findings suggest a potential role for ketone metabolism in response to myocardial ischemia, conclude researchers in their report, published in the October 5 issue of the Journal of the American College of Cardiology.
“Ketones serve as an alternative source of energy for the heart,” lead author Marie-Sophie L.Y. de Koning, MD, University Medical Center Groningen, the Netherlands, told this news organization.
“These results might suggest that ketone bodies may be an important fuel for the heart after myocardial ischemia.” The role of KBs in heart failure has been previously studied, but their role in myocardial infarction has not, Dr. De Koning said.
“In heart failure, metabolic changes occur that cause the heart to increasingly rely on ketone bodies as an important energy source. Accordingly, concentrations of circulating ketone bodies are elevated and higher concentrations have been linked with more severe heart failure,” she said.
”This might suggest that upregulation of ketone metabolism is a universal cardiac response to stress,” Dr. De Koning added. “But the role of ketone bodies in myocardial infarction remained largely unknown, and this triggered us to investigate circulating ketone bodies in patients presenting with STEMI.”
She and her team measured circulating KBs in archived plasma samples from 369 participants in the randomized GIPS-III trial. The study had primarily looked at the effect of 4 months of metformin therapy, compared with placebo, on LVEF in nondiabetic patients with a first STEMI.
Blood samples had been taken at baseline before percutaneous coronary intervention (PCI), at 24 hours after reperfusion, and at 4 months.
The current study investigated longitudinal post-STEMI changes in the circulating KBs beta-hydroxybutyrate, acetoacetate, and acetone. It also looked at associations of KBs with infarct size and LVEF, both of which were measured with cardiac magnetic resonance (CMR) imaging 4 months after STEMI.
Circulating KB levels were three times higher at STEMI presentation than at 4 months. At presentation, the median total KB level was 520 μmol/L. It was still higher 24 hours after reperfusion than at 4 months (206 vs. 166 μmol/L; P < .001).
The 24-hour KB elevations were independently and positively associated with larger infarct size (P = .016) and lower LVEF (P = .012), the group reports.
“Our results indicate a possible role for ketone bodies during myocardial infarction,” Dr. De Koning said.
The KB elevations were probably followed by “an increase in cardiac ketone body metabolism, in order to fuel the heart that is energetically depleted.”
But the study didn’t explore cardiac KB consumption, Dr. De Koning cautioned, adding that the next steps in this research should be to investigate post-STEMI cardiac ketone metabolism and its pathophysiologic mechanisms. “This may facilitate future trials to study therapeutic effects of ketone body supplementation during or after STEMI.”
The current findings “form an essential basis for our understanding of the role of KBs in ischemia/reperfusion,” write Salva R. Yurista, MD, PhD, and colleagues, Massachusetts General Hospital and Harvard Medical School, Boston, in an accompanying editorial.
“Although the appeal of enhancing KBs as a therapeutic approach is understandable, additional rigorous preclinical and clinical studies will be required to test this therapeutic hypothesis and determine the mechanisms contributing to any benefits observed,” they note.
”Exposure to cardiac stress, such as ischemia, infarction, or heart failure, will stimulate the release of neurohormones, pro-inflammatory cytokines, and natriuretic peptides, which may play roles in stimulating ketogenesis or the production of ketone bodies,” Dr. Yurista told this news organization.
The increased circulating ketone concentrations and myocardial ketone oxidation that were associated with poor functional outcomes have been reported in other clinical contexts, including heart failure with reduced ejection fraction, heart failure with preserved cardiac function, diabetic cardiomyopathy, and arrhythmogenic cardiomyopathy, he said.
Dr. Yurista agrees that KBs could have therapeutic merit.
“Circulating ketone concentrations determine the contribution of ketones to the cardiac diet,” he said. “Thus, increasing cardiac delivery of ketone bodies through supplementation or other means to the heart undergoing stress, including STEMI and heart failure, could have therapeutic potential.”
The GIPS-III trial was supported by the Netherlands Organization for Health Research and Development (ZonMw). Neither Dr. De Koning nor the other authors report relevant financial relationships. Dr. Yurista and the other editorialists report no relevant relationships.
A version of this article first appeared on Medscape.com.
Circulating ketone bodies (KBs) are substantially elevated at presentation and 24 hours after ST-segment elevation myocardial infarction (STEMI), according to new research.
The study also showed that greater KB levels measured after 24 hours of reperfusion were associated with larger infarct size and reduced left ventricular ejection fraction (LVEF).
The findings suggest a potential role for ketone metabolism in response to myocardial ischemia, conclude researchers in their report, published in the October 5 issue of the Journal of the American College of Cardiology.
“Ketones serve as an alternative source of energy for the heart,” lead author Marie-Sophie L.Y. de Koning, MD, University Medical Center Groningen, the Netherlands, told this news organization.
“These results might suggest that ketone bodies may be an important fuel for the heart after myocardial ischemia.” The role of KBs in heart failure has been previously studied, but their role in myocardial infarction has not, Dr. De Koning said.
“In heart failure, metabolic changes occur that cause the heart to increasingly rely on ketone bodies as an important energy source. Accordingly, concentrations of circulating ketone bodies are elevated and higher concentrations have been linked with more severe heart failure,” she said.
”This might suggest that upregulation of ketone metabolism is a universal cardiac response to stress,” Dr. De Koning added. “But the role of ketone bodies in myocardial infarction remained largely unknown, and this triggered us to investigate circulating ketone bodies in patients presenting with STEMI.”
She and her team measured circulating KBs in archived plasma samples from 369 participants in the randomized GIPS-III trial. The study had primarily looked at the effect of 4 months of metformin therapy, compared with placebo, on LVEF in nondiabetic patients with a first STEMI.
Blood samples had been taken at baseline before percutaneous coronary intervention (PCI), at 24 hours after reperfusion, and at 4 months.
The current study investigated longitudinal post-STEMI changes in the circulating KBs beta-hydroxybutyrate, acetoacetate, and acetone. It also looked at associations of KBs with infarct size and LVEF, both of which were measured with cardiac magnetic resonance (CMR) imaging 4 months after STEMI.
Circulating KB levels were three times higher at STEMI presentation than at 4 months. At presentation, the median total KB level was 520 μmol/L. It was still higher 24 hours after reperfusion than at 4 months (206 vs. 166 μmol/L; P < .001).
The 24-hour KB elevations were independently and positively associated with larger infarct size (P = .016) and lower LVEF (P = .012), the group reports.
“Our results indicate a possible role for ketone bodies during myocardial infarction,” Dr. De Koning said.
The KB elevations were probably followed by “an increase in cardiac ketone body metabolism, in order to fuel the heart that is energetically depleted.”
But the study didn’t explore cardiac KB consumption, Dr. De Koning cautioned, adding that the next steps in this research should be to investigate post-STEMI cardiac ketone metabolism and its pathophysiologic mechanisms. “This may facilitate future trials to study therapeutic effects of ketone body supplementation during or after STEMI.”
The current findings “form an essential basis for our understanding of the role of KBs in ischemia/reperfusion,” write Salva R. Yurista, MD, PhD, and colleagues, Massachusetts General Hospital and Harvard Medical School, Boston, in an accompanying editorial.
“Although the appeal of enhancing KBs as a therapeutic approach is understandable, additional rigorous preclinical and clinical studies will be required to test this therapeutic hypothesis and determine the mechanisms contributing to any benefits observed,” they note.
”Exposure to cardiac stress, such as ischemia, infarction, or heart failure, will stimulate the release of neurohormones, pro-inflammatory cytokines, and natriuretic peptides, which may play roles in stimulating ketogenesis or the production of ketone bodies,” Dr. Yurista told this news organization.
The increased circulating ketone concentrations and myocardial ketone oxidation that were associated with poor functional outcomes have been reported in other clinical contexts, including heart failure with reduced ejection fraction, heart failure with preserved cardiac function, diabetic cardiomyopathy, and arrhythmogenic cardiomyopathy, he said.
Dr. Yurista agrees that KBs could have therapeutic merit.
“Circulating ketone concentrations determine the contribution of ketones to the cardiac diet,” he said. “Thus, increasing cardiac delivery of ketone bodies through supplementation or other means to the heart undergoing stress, including STEMI and heart failure, could have therapeutic potential.”
The GIPS-III trial was supported by the Netherlands Organization for Health Research and Development (ZonMw). Neither Dr. De Koning nor the other authors report relevant financial relationships. Dr. Yurista and the other editorialists report no relevant relationships.
A version of this article first appeared on Medscape.com.
Circulating ketone bodies (KBs) are substantially elevated at presentation and 24 hours after ST-segment elevation myocardial infarction (STEMI), according to new research.
The study also showed that greater KB levels measured after 24 hours of reperfusion were associated with larger infarct size and reduced left ventricular ejection fraction (LVEF).
The findings suggest a potential role for ketone metabolism in response to myocardial ischemia, conclude researchers in their report, published in the October 5 issue of the Journal of the American College of Cardiology.
“Ketones serve as an alternative source of energy for the heart,” lead author Marie-Sophie L.Y. de Koning, MD, University Medical Center Groningen, the Netherlands, told this news organization.
“These results might suggest that ketone bodies may be an important fuel for the heart after myocardial ischemia.” The role of KBs in heart failure has been previously studied, but their role in myocardial infarction has not, Dr. De Koning said.
“In heart failure, metabolic changes occur that cause the heart to increasingly rely on ketone bodies as an important energy source. Accordingly, concentrations of circulating ketone bodies are elevated and higher concentrations have been linked with more severe heart failure,” she said.
”This might suggest that upregulation of ketone metabolism is a universal cardiac response to stress,” Dr. De Koning added. “But the role of ketone bodies in myocardial infarction remained largely unknown, and this triggered us to investigate circulating ketone bodies in patients presenting with STEMI.”
She and her team measured circulating KBs in archived plasma samples from 369 participants in the randomized GIPS-III trial. The study had primarily looked at the effect of 4 months of metformin therapy, compared with placebo, on LVEF in nondiabetic patients with a first STEMI.
Blood samples had been taken at baseline before percutaneous coronary intervention (PCI), at 24 hours after reperfusion, and at 4 months.
The current study investigated longitudinal post-STEMI changes in the circulating KBs beta-hydroxybutyrate, acetoacetate, and acetone. It also looked at associations of KBs with infarct size and LVEF, both of which were measured with cardiac magnetic resonance (CMR) imaging 4 months after STEMI.
Circulating KB levels were three times higher at STEMI presentation than at 4 months. At presentation, the median total KB level was 520 μmol/L. It was still higher 24 hours after reperfusion than at 4 months (206 vs. 166 μmol/L; P < .001).
The 24-hour KB elevations were independently and positively associated with larger infarct size (P = .016) and lower LVEF (P = .012), the group reports.
“Our results indicate a possible role for ketone bodies during myocardial infarction,” Dr. De Koning said.
The KB elevations were probably followed by “an increase in cardiac ketone body metabolism, in order to fuel the heart that is energetically depleted.”
But the study didn’t explore cardiac KB consumption, Dr. De Koning cautioned, adding that the next steps in this research should be to investigate post-STEMI cardiac ketone metabolism and its pathophysiologic mechanisms. “This may facilitate future trials to study therapeutic effects of ketone body supplementation during or after STEMI.”
The current findings “form an essential basis for our understanding of the role of KBs in ischemia/reperfusion,” write Salva R. Yurista, MD, PhD, and colleagues, Massachusetts General Hospital and Harvard Medical School, Boston, in an accompanying editorial.
“Although the appeal of enhancing KBs as a therapeutic approach is understandable, additional rigorous preclinical and clinical studies will be required to test this therapeutic hypothesis and determine the mechanisms contributing to any benefits observed,” they note.
”Exposure to cardiac stress, such as ischemia, infarction, or heart failure, will stimulate the release of neurohormones, pro-inflammatory cytokines, and natriuretic peptides, which may play roles in stimulating ketogenesis or the production of ketone bodies,” Dr. Yurista told this news organization.
The increased circulating ketone concentrations and myocardial ketone oxidation that were associated with poor functional outcomes have been reported in other clinical contexts, including heart failure with reduced ejection fraction, heart failure with preserved cardiac function, diabetic cardiomyopathy, and arrhythmogenic cardiomyopathy, he said.
Dr. Yurista agrees that KBs could have therapeutic merit.
“Circulating ketone concentrations determine the contribution of ketones to the cardiac diet,” he said. “Thus, increasing cardiac delivery of ketone bodies through supplementation or other means to the heart undergoing stress, including STEMI and heart failure, could have therapeutic potential.”
The GIPS-III trial was supported by the Netherlands Organization for Health Research and Development (ZonMw). Neither Dr. De Koning nor the other authors report relevant financial relationships. Dr. Yurista and the other editorialists report no relevant relationships.
A version of this article first appeared on Medscape.com.
Handheld device highly sensitive in detecting amblyopia; can be used in children as young as 2 years of age
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handheld vision screening device to test for amblyopia and strabismus has been found to have a sensitivity of 100%, a specificity of 85%, and a median acquisition time of 28 seconds, according to a study published in the Journal of American Association for Pediatric Ophthalmology and Strabismus.
The prospective study involved 300 children recruited from two Kaiser Permanente Southern California pediatric clinics. The patients, aged 24-72 months, were first screened by trained research staff for amblyopia and strabismus using the device, called the Pediatric Vision Scanner (PVS). They were subsequently screened by a pediatric ophthalmologist who was masked to the previous screening results and who then performed a comprehensive eye examination.
With the gold-standard ophthalmologist examination, six children (2%) were identified as having amblyopia and/or strabismus. Using the PVS, all six children with amblyopia and/or strabismus were identified, yielding 100% sensitivity. PVS findings were normal for 45 children (15%), yielding a specificity rate of 85%. The positive predictive value was 26.0% (95% confidence interval, 12.4%-32.4%), and the negative predictive value was 100% (95% CI, 97.1%-100%).
The findings suggest that the device could be used to screen for amblyopia, according to Shaival S. Shah, MD, the study’s first author, who is a pediatric ophthalmologist and regional section lead of pediatric ophthalmology, Southern California Permanente Medical Group.
“A strength of this device is that it is user friendly and easy to use and very quick, which is essential when working with young children,” said Dr. Shah in an interview. He noted that the device could be used for children as young as 2 years.
Dr. Shah pointed out that the children were recruited from a pediatrician’s office and reflect more of a “real-world setting” than had they been recruited from a pediatric ophthalmology clinic.
Dr. Shah added that, with a negative predictive value of 100%, the device is highly reliable at informing the clinician that amblyopia is not present. “It did have a positive predictive value of 26%, which needs to be considered when deciding one’s vision screening strategy,” he said.
A limitation of the study is that there was no head-to-head comparison with another screening device, noted Dr. Shah. “While it may have been more useful to include another vision screening device to have a head-to-head comparison, we did not do this to limit complexity and cost.”
Michael J. Wan, MD, FRCSC, pediatric ophthalmologist, Sick Kids Hospital, Toronto, and assistant professor at the University of Toronto, told this news organization that the device has multiple strengths, including quick acquisition time and excellent detection rate of amblyopia and strabismus in children as young as 2 years.
“It is highly reliable at informing the clinician that amblyopia is not present,” said Dr. Wan, who was not involved in the study. “The PVS uses an elegant mechanism to test for amblyopia directly (as opposed to other screening devices, which only detect risk factors). This study demonstrates the impressive diagnostic accuracy of this approach. With a study population of 300 children, the PVS had a sensitivity of 100% and specificity of 85% (over 90% in cooperative children). This means that the PVS would detect essentially all cases of amblyopia and strabismus while minimizing the number of unnecessary referrals and examinations.”
He added that, although the study included children as young as 2 years, only 2.5% of the children were unable to complete the PVS test. “Detecting amblyopia in children at an age when treatment is still effective has been a longstanding goal in pediatric ophthalmology,” said Dr. Wan, who described the technology as user friendly. “Based on this study, the search for an accurate and practical pediatric vision screening device appears to be over.”
Dr. Wan said it would be useful to replicate this study with a different population to confirm the findings.
Dr. Shah and Dr. Wan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA clears first mobile rapid test for concussion
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
, the company has announced.
Eye-Sync is a virtual reality eye-tracking platform that provides objective measurements to aid in the assessment of concussion. It’s the first mobile, rapid test for concussion that has been cleared by the FDA, the company said.
As reported by this news organization, Eye-Sync received breakthrough designation from the FDA for this indication in March 2019.
The FDA initially cleared the Eye-Sync platform for recording, viewing, and analyzing eye movements to help clinicians identify visual tracking impairment.
The Eye-Sync technology uses a series of 60-second eye tracking assessments, neurocognitive batteries, symptom inventories, and standardized patient inventories to identify the type and severity of impairment after concussion.
“The platform generates customizable and interpretive reports that support clinical decision making and offers visual and vestibular therapies to remedy deficits and monitor improvement over time,” the company said.
In support of the application for use in concussion, SyncThink enrolled 1,655 children and adults into a clinical study that collected comprehensive patient and concussion-related data for over 12 months.
The company used these data to develop proprietary algorithms and deep learning models to identify a positive or negative indication of concussion.
The study showed that Eye-Sinc had sensitivity greater than 82% and specificity greater than 93%, “thereby providing clinicians with significant and actionable data when evaluating individuals with concussion,” the company said in a news release.
“The outcome of this study very clearly shows the effectiveness of our technology at detecting concussion and definitively demonstrates the clinical utility of Eye-Sinc,” SyncThink Chief Clinical Officer Scott Anderson said in the release.
“It also shows that the future of concussion diagnosis is no longer purely symptom-based but that of a technology driven multi-modal approach,” Mr. Anderson said.
A version of this article first appeared on Medscape.com.
Racism a strong factor in Black women’s high rate of premature births, study finds
Dr. Paula Braveman, director of the Center on Social Disparities in Health at the University of California, San Francisco, says her latest research revealed an “astounding” level of evidence that racism is a decisive “upstream” cause of higher rates of preterm birth among Black women.
The tipping point for Dr. Paula Braveman came when a longtime patient of hers at a community clinic in San Francisco’s Mission District slipped past the front desk and knocked on her office door to say goodbye. He wouldn’t be coming to the clinic anymore, he told her, because he could no longer afford it.
It was a decisive moment for Dr. Braveman, who decided she wanted not only to heal ailing patients but also to advocate for policies that would help them be healthier when they arrived at her clinic. In the nearly four decades since, Dr. Braveman has dedicated herself to studying the “social determinants of health” – how the spaces where we live, work, play and learn, and the relationships we have in those places influence how healthy we are.
As director of the Center on Social Disparities in Health at the University of California, San Francisco, Dr. Braveman has studied the link between neighborhood wealth and children’s health, and how access to insurance influences prenatal care. A longtime advocate of translating research into policy, she has collaborated on major health initiatives with the health department in San Francisco, the federal Centers for Disease Control and Prevention, and the World Health Organization.
Dr. Braveman has a particular interest in maternal and infant health. Her latest research reviews what’s known about the persistent gap in preterm birth rates between Black and White women in the United States. Black women are about 1.6 times as likely as White women to give birth more than three weeks before the due date. That statistic bears alarming and costly health consequences, as infants born prematurely are at higher risk for breathing, heart, and brain abnormalities, among other complications.
Dr. Braveman coauthored the review with a group of experts convened by the March of Dimes that included geneticists, clinicians, epidemiologists, biomedical experts, and neurologists. They examined more than two dozen suspected causes of preterm births – including quality of prenatal care, environmental toxics, chronic stress, poverty and obesity – and determined that racism, directly or indirectly, best explained the racial disparities in preterm birth rates.
(Note: In the review, the authors make extensive use of the terms “upstream” and “downstream” to describe what determines people’s health. A downstream risk is the condition or factor most directly responsible for a health outcome, while an upstream factor is what causes or fuels the downstream risk – and often what needs to change to prevent someone from becoming sick. For example, a person living near drinking water polluted with toxic chemicals might get sick from drinking the water. The downstream fix would be telling individuals to use filters. The upstream solution would be to stop the dumping of toxic chemicals.)
KHN spoke with Dr. Braveman about the study and its findings. The excerpts have been edited for length and style.
Q: You have been studying the issue of preterm birth and racial disparities for so long. Were there any findings from this review that surprised you?
The process of systematically going through all of the risk factors that are written about in the literature and then seeing how the story of racism was an upstream determinant for virtually all of them. That was kind of astounding.
The other thing that was very impressive: When we looked at the idea that genetic factors could be the cause of the Black-White disparity in preterm birth. The genetics experts in the group, and there were three or four of them, concluded from the evidence that genetic factors might influence the disparity in preterm birth, but at most the effect would be very small, very small indeed. This could not account for the greater rate of preterm birth among Black women compared to White women.
Q: You were looking to identify not just what causes preterm birth but also to explain racial differences in rates of preterm birth. Are there examples of factors that can influence preterm birth that don’t explain racial disparities?
It does look like there are genetic components to preterm birth, but they don’t explain the Black-White disparity in preterm birth. Another example is having an early elective C-section. That’s one of the problems contributing to avoidable preterm birth, but it doesn’t look like that’s really contributing to the Black-White disparity in preterm birth.
Q: You and your colleagues listed exactly one upstream cause of preterm birth: racism. How would you characterize the certainty that racism is a decisive upstream cause of higher rates of preterm birth among Black women?
It makes me think of this saying: A randomized clinical trial wouldn’t be necessary to give certainty about the importance of having a parachute on if you jump from a plane. To me, at this point, it is close to that.
Going through that paper – and we worked on that paper over a three- or four-year period, so there was a lot of time to think about it – I don’t see how the evidence that we have could be explained otherwise.
Q: What did you learn about how a mother’s broader lifetime experience of racism might affect birth outcomes versus what she experienced within the medical establishment during pregnancy?
There were many ways that experiencing racial discrimination would affect a woman’s pregnancy, but one major way would be through pathways and biological mechanisms involved in stress and stress physiology. In neuroscience, what’s been clear is that a chronic stressor seems to be more damaging to health than an acute stressor.
So it doesn’t make much sense to be looking only during pregnancy. But that’s where most of that research has been done: stress during pregnancy and racial discrimination, and its role in birth outcomes. Very few studies have looked at experiences of racial discrimination across the life course.
My colleagues and I have published a paper where we asked African American women about their experiences of racism, and we didn’t even define what we meant. Women did not talk a lot about the experiences of racism during pregnancy from their medical providers; they talked about the lifetime experience and particularly experiences going back to childhood. And they talked about having to worry, and constant vigilance, so that even if they’re not experiencing an incident, their antennae have to be out to be prepared in case an incident does occur.
Putting all of it together with what we know about stress physiology, I would put my money on the lifetime experiences being so much more important than experiences during pregnancy. There isn’t enough known about preterm birth, but from what is known, inflammation is involved, immune dysfunction, and that’s what stress leads to. The neuroscientists have shown us that chronic stress produces inflammation and immune system dysfunction.
Q: What policies do you think are most important at this stage for reducing preterm birth for Black women?
I wish I could just say one policy or two policies, but I think it does get back to the need to dismantle racism in our society. In all of its manifestations. That’s unfortunate, not to be able to say, “Oh, here, I have this magic bullet, and if you just go with that, that will solve the problem.”
If you take the conclusions of this study seriously, you say, well, policies to just go after these downstream factors are not going to work. It’s up to the upstream investment in trying to achieve a more equitable and less racist society. Ultimately, I think that’s the take-home, and it’s a tall, tall order.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Dr. Paula Braveman, director of the Center on Social Disparities in Health at the University of California, San Francisco, says her latest research revealed an “astounding” level of evidence that racism is a decisive “upstream” cause of higher rates of preterm birth among Black women.
The tipping point for Dr. Paula Braveman came when a longtime patient of hers at a community clinic in San Francisco’s Mission District slipped past the front desk and knocked on her office door to say goodbye. He wouldn’t be coming to the clinic anymore, he told her, because he could no longer afford it.
It was a decisive moment for Dr. Braveman, who decided she wanted not only to heal ailing patients but also to advocate for policies that would help them be healthier when they arrived at her clinic. In the nearly four decades since, Dr. Braveman has dedicated herself to studying the “social determinants of health” – how the spaces where we live, work, play and learn, and the relationships we have in those places influence how healthy we are.
As director of the Center on Social Disparities in Health at the University of California, San Francisco, Dr. Braveman has studied the link between neighborhood wealth and children’s health, and how access to insurance influences prenatal care. A longtime advocate of translating research into policy, she has collaborated on major health initiatives with the health department in San Francisco, the federal Centers for Disease Control and Prevention, and the World Health Organization.
Dr. Braveman has a particular interest in maternal and infant health. Her latest research reviews what’s known about the persistent gap in preterm birth rates between Black and White women in the United States. Black women are about 1.6 times as likely as White women to give birth more than three weeks before the due date. That statistic bears alarming and costly health consequences, as infants born prematurely are at higher risk for breathing, heart, and brain abnormalities, among other complications.
Dr. Braveman coauthored the review with a group of experts convened by the March of Dimes that included geneticists, clinicians, epidemiologists, biomedical experts, and neurologists. They examined more than two dozen suspected causes of preterm births – including quality of prenatal care, environmental toxics, chronic stress, poverty and obesity – and determined that racism, directly or indirectly, best explained the racial disparities in preterm birth rates.
(Note: In the review, the authors make extensive use of the terms “upstream” and “downstream” to describe what determines people’s health. A downstream risk is the condition or factor most directly responsible for a health outcome, while an upstream factor is what causes or fuels the downstream risk – and often what needs to change to prevent someone from becoming sick. For example, a person living near drinking water polluted with toxic chemicals might get sick from drinking the water. The downstream fix would be telling individuals to use filters. The upstream solution would be to stop the dumping of toxic chemicals.)
KHN spoke with Dr. Braveman about the study and its findings. The excerpts have been edited for length and style.
Q: You have been studying the issue of preterm birth and racial disparities for so long. Were there any findings from this review that surprised you?
The process of systematically going through all of the risk factors that are written about in the literature and then seeing how the story of racism was an upstream determinant for virtually all of them. That was kind of astounding.
The other thing that was very impressive: When we looked at the idea that genetic factors could be the cause of the Black-White disparity in preterm birth. The genetics experts in the group, and there were three or four of them, concluded from the evidence that genetic factors might influence the disparity in preterm birth, but at most the effect would be very small, very small indeed. This could not account for the greater rate of preterm birth among Black women compared to White women.
Q: You were looking to identify not just what causes preterm birth but also to explain racial differences in rates of preterm birth. Are there examples of factors that can influence preterm birth that don’t explain racial disparities?
It does look like there are genetic components to preterm birth, but they don’t explain the Black-White disparity in preterm birth. Another example is having an early elective C-section. That’s one of the problems contributing to avoidable preterm birth, but it doesn’t look like that’s really contributing to the Black-White disparity in preterm birth.
Q: You and your colleagues listed exactly one upstream cause of preterm birth: racism. How would you characterize the certainty that racism is a decisive upstream cause of higher rates of preterm birth among Black women?
It makes me think of this saying: A randomized clinical trial wouldn’t be necessary to give certainty about the importance of having a parachute on if you jump from a plane. To me, at this point, it is close to that.
Going through that paper – and we worked on that paper over a three- or four-year period, so there was a lot of time to think about it – I don’t see how the evidence that we have could be explained otherwise.
Q: What did you learn about how a mother’s broader lifetime experience of racism might affect birth outcomes versus what she experienced within the medical establishment during pregnancy?
There were many ways that experiencing racial discrimination would affect a woman’s pregnancy, but one major way would be through pathways and biological mechanisms involved in stress and stress physiology. In neuroscience, what’s been clear is that a chronic stressor seems to be more damaging to health than an acute stressor.
So it doesn’t make much sense to be looking only during pregnancy. But that’s where most of that research has been done: stress during pregnancy and racial discrimination, and its role in birth outcomes. Very few studies have looked at experiences of racial discrimination across the life course.
My colleagues and I have published a paper where we asked African American women about their experiences of racism, and we didn’t even define what we meant. Women did not talk a lot about the experiences of racism during pregnancy from their medical providers; they talked about the lifetime experience and particularly experiences going back to childhood. And they talked about having to worry, and constant vigilance, so that even if they’re not experiencing an incident, their antennae have to be out to be prepared in case an incident does occur.
Putting all of it together with what we know about stress physiology, I would put my money on the lifetime experiences being so much more important than experiences during pregnancy. There isn’t enough known about preterm birth, but from what is known, inflammation is involved, immune dysfunction, and that’s what stress leads to. The neuroscientists have shown us that chronic stress produces inflammation and immune system dysfunction.
Q: What policies do you think are most important at this stage for reducing preterm birth for Black women?
I wish I could just say one policy or two policies, but I think it does get back to the need to dismantle racism in our society. In all of its manifestations. That’s unfortunate, not to be able to say, “Oh, here, I have this magic bullet, and if you just go with that, that will solve the problem.”
If you take the conclusions of this study seriously, you say, well, policies to just go after these downstream factors are not going to work. It’s up to the upstream investment in trying to achieve a more equitable and less racist society. Ultimately, I think that’s the take-home, and it’s a tall, tall order.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Dr. Paula Braveman, director of the Center on Social Disparities in Health at the University of California, San Francisco, says her latest research revealed an “astounding” level of evidence that racism is a decisive “upstream” cause of higher rates of preterm birth among Black women.
The tipping point for Dr. Paula Braveman came when a longtime patient of hers at a community clinic in San Francisco’s Mission District slipped past the front desk and knocked on her office door to say goodbye. He wouldn’t be coming to the clinic anymore, he told her, because he could no longer afford it.
It was a decisive moment for Dr. Braveman, who decided she wanted not only to heal ailing patients but also to advocate for policies that would help them be healthier when they arrived at her clinic. In the nearly four decades since, Dr. Braveman has dedicated herself to studying the “social determinants of health” – how the spaces where we live, work, play and learn, and the relationships we have in those places influence how healthy we are.
As director of the Center on Social Disparities in Health at the University of California, San Francisco, Dr. Braveman has studied the link between neighborhood wealth and children’s health, and how access to insurance influences prenatal care. A longtime advocate of translating research into policy, she has collaborated on major health initiatives with the health department in San Francisco, the federal Centers for Disease Control and Prevention, and the World Health Organization.
Dr. Braveman has a particular interest in maternal and infant health. Her latest research reviews what’s known about the persistent gap in preterm birth rates between Black and White women in the United States. Black women are about 1.6 times as likely as White women to give birth more than three weeks before the due date. That statistic bears alarming and costly health consequences, as infants born prematurely are at higher risk for breathing, heart, and brain abnormalities, among other complications.
Dr. Braveman coauthored the review with a group of experts convened by the March of Dimes that included geneticists, clinicians, epidemiologists, biomedical experts, and neurologists. They examined more than two dozen suspected causes of preterm births – including quality of prenatal care, environmental toxics, chronic stress, poverty and obesity – and determined that racism, directly or indirectly, best explained the racial disparities in preterm birth rates.
(Note: In the review, the authors make extensive use of the terms “upstream” and “downstream” to describe what determines people’s health. A downstream risk is the condition or factor most directly responsible for a health outcome, while an upstream factor is what causes or fuels the downstream risk – and often what needs to change to prevent someone from becoming sick. For example, a person living near drinking water polluted with toxic chemicals might get sick from drinking the water. The downstream fix would be telling individuals to use filters. The upstream solution would be to stop the dumping of toxic chemicals.)
KHN spoke with Dr. Braveman about the study and its findings. The excerpts have been edited for length and style.
Q: You have been studying the issue of preterm birth and racial disparities for so long. Were there any findings from this review that surprised you?
The process of systematically going through all of the risk factors that are written about in the literature and then seeing how the story of racism was an upstream determinant for virtually all of them. That was kind of astounding.
The other thing that was very impressive: When we looked at the idea that genetic factors could be the cause of the Black-White disparity in preterm birth. The genetics experts in the group, and there were three or four of them, concluded from the evidence that genetic factors might influence the disparity in preterm birth, but at most the effect would be very small, very small indeed. This could not account for the greater rate of preterm birth among Black women compared to White women.
Q: You were looking to identify not just what causes preterm birth but also to explain racial differences in rates of preterm birth. Are there examples of factors that can influence preterm birth that don’t explain racial disparities?
It does look like there are genetic components to preterm birth, but they don’t explain the Black-White disparity in preterm birth. Another example is having an early elective C-section. That’s one of the problems contributing to avoidable preterm birth, but it doesn’t look like that’s really contributing to the Black-White disparity in preterm birth.
Q: You and your colleagues listed exactly one upstream cause of preterm birth: racism. How would you characterize the certainty that racism is a decisive upstream cause of higher rates of preterm birth among Black women?
It makes me think of this saying: A randomized clinical trial wouldn’t be necessary to give certainty about the importance of having a parachute on if you jump from a plane. To me, at this point, it is close to that.
Going through that paper – and we worked on that paper over a three- or four-year period, so there was a lot of time to think about it – I don’t see how the evidence that we have could be explained otherwise.
Q: What did you learn about how a mother’s broader lifetime experience of racism might affect birth outcomes versus what she experienced within the medical establishment during pregnancy?
There were many ways that experiencing racial discrimination would affect a woman’s pregnancy, but one major way would be through pathways and biological mechanisms involved in stress and stress physiology. In neuroscience, what’s been clear is that a chronic stressor seems to be more damaging to health than an acute stressor.
So it doesn’t make much sense to be looking only during pregnancy. But that’s where most of that research has been done: stress during pregnancy and racial discrimination, and its role in birth outcomes. Very few studies have looked at experiences of racial discrimination across the life course.
My colleagues and I have published a paper where we asked African American women about their experiences of racism, and we didn’t even define what we meant. Women did not talk a lot about the experiences of racism during pregnancy from their medical providers; they talked about the lifetime experience and particularly experiences going back to childhood. And they talked about having to worry, and constant vigilance, so that even if they’re not experiencing an incident, their antennae have to be out to be prepared in case an incident does occur.
Putting all of it together with what we know about stress physiology, I would put my money on the lifetime experiences being so much more important than experiences during pregnancy. There isn’t enough known about preterm birth, but from what is known, inflammation is involved, immune dysfunction, and that’s what stress leads to. The neuroscientists have shown us that chronic stress produces inflammation and immune system dysfunction.
Q: What policies do you think are most important at this stage for reducing preterm birth for Black women?
I wish I could just say one policy or two policies, but I think it does get back to the need to dismantle racism in our society. In all of its manifestations. That’s unfortunate, not to be able to say, “Oh, here, I have this magic bullet, and if you just go with that, that will solve the problem.”
If you take the conclusions of this study seriously, you say, well, policies to just go after these downstream factors are not going to work. It’s up to the upstream investment in trying to achieve a more equitable and less racist society. Ultimately, I think that’s the take-home, and it’s a tall, tall order.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.