User login
‘Push the bar higher’: New statement on type 1 diabetes in adults
A newly published consensus statement on the management of type 1 diabetes in adults addresses the unique clinical needs of the population compared with those of children with type 1 diabetes or adults with type 2 diabetes.
“The focus on adults is kind of new and it is important. ... I do think it’s a bit of a forgotten population. Whenever we talk about diabetes in adults it’s assumed to be about type 2,” document coauthor M. Sue Kirkman, MD, said in an interview.
The document covers diagnosis of type 1 diabetes, goals and targets, schedule of care, self-management education and lifestyle, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis (DKA), pancreas transplant/islet cell transplantation, adjunctive therapies, special populations (pregnant, older, hospitalized), and emergent and future perspectives.
Initially presented in draft form in June at the American Diabetes Association (ADA) 81st scientific sessions, the final version of the joint ADA/EASD statement was presented Oct. 1 at the annual meeting of the European Association for the Study of Diabetes and simultaneously published in Diabetologia and Diabetes Care.
“We are aware of the many and rapid advances in the diagnosis and treatment of type 1 diabetes ... However, despite these advances, there is also a growing recognition of the psychosocial burden of living with type 1 diabetes,” writing group cochair Richard I.G. Holt, MB BChir, PhD, professor of diabetes and endocrinology at the University of Southampton, England, said when introducing the 90-minute EASD session.
“Although there is guidance for the management of type 1 diabetes, the aim of this report is to highlight the major areas that health care professionals should consider when managing adults with type 1 diabetes,” he added.
Noting that the joint EASD/ADA consensus report on type 2 diabetes has been “highly influential,” Dr. Holt said, “EASD and ADA recognized the need to develop a comparable consensus report specifically addressing type 1 diabetes.”
The overriding goals, Dr. Holt said, are to “support people with type 1 diabetes to live a long and healthy life” with four specific strategies: delivery of insulin to keep glucose levels as close to target as possible to prevent complications while minimizing hypoglycemia and preventing DKA; managing cardiovascular risk factors; minimizing psychosocial burden; and promoting psychological well-being.
Diagnostic algorithm
Another coauthor, J. Hans de Vries, MD, PhD, professor of internal medicine at the University of Amsterdam, explained the recommended approach to distinguishing type 1 diabetes from type 2 diabetes or monogenic diabetes in adults, which is often a clinical challenge.
This also was the topic prompting the most questions during the EASD session.
“Especially in adults, misdiagnosis of type of diabetes is common, occurring in up to 40% of patients diagnosed after the age of 30 years,” Dr. de Vries said.
Among the many reasons for the confusion are that C-peptide levels, a reflection of endogenous insulin secretion, can still be relatively high at the time of clinical onset of type 1 diabetes, but islet antibodies don’t have 100% positive predictive value.
Obesity and type 2 diabetes are increasingly seen at younger ages, and DKA can occur in type 2 diabetes (“ketosis-prone”). In addition, monogenic forms of diabetes can be disguised as type 1 diabetes.
“So, we thought there was a need for a diagnostic algorithm,” Dr. de Vries said, adding that the algorithm – displayed as a graphic in the statement – is only for adults in whom type 1 diabetes is suspected, not other types. Also, it’s based on data from White European populations.
The first step is to test islet autoantibodies. If positive, the diagnosis of type 1 diabetes can be made. If negative and the person is younger than 35 years and without signs of type 2 diabetes, testing C-peptide is advised. If that’s below 200 pmol/L, type 1 diabetes is the diagnosis. If above 200 pmol/L, genetic testing for monogenic diabetes is advised. If there are signs of type 2 diabetes and/or the person is over age 35, type 2 diabetes is the most likely diagnosis.
And if uncertainty remains, the recommendation is to try noninsulin therapy and retest C-peptide again in 3 years, as by that time it will be below 200 pmol/L in a person with type 1 diabetes.
Dr. Kirkman commented regarding the algorithm: “It’s very much from a European population perspective. In some ways that’s a limitation, especially in the U.S. where the population is diverse, but I do think it’s still useful to help guide people through how to think about somebody who presents as an adult where it’s not obviously type 2 or type 1 ... There is a lot of in-between stuff.”
Psychosocial support: Essential but often overlooked
Frank J. Snoek, PhD, professor of psychology at Amsterdam University Medical Centers, Vrije Universiteit, presented the section on behavioral and psychosocial care. He pointed out that diabetes-related emotional distress is reported by 20%-40% of adults with type 1 diabetes, and that the risk of such distress is especially high at the time of diagnosis and when complications develop.
About 15% of people with type 1 diabetes have depression, which is linked to elevated A1c levels, increased complication risk, and mortality. Anxiety also is very common and linked with diabetes-specific fears including hypoglycemia. Eating disorders are more prevalent among people with type 1 diabetes than in the general population and can further complicate diabetes management.
Recommendations include periodic evaluation of psychological health and social barriers to self-management and having clear referral pathways and access to psychological or psychiatric care for individuals in need. “All members of the diabetes care team have a responsibility when it comes to offering psychosocial support as part of ongoing diabetes care and education.”
Dr. Kirkman had identified this section as noteworthy: “I think the focus on psychosocial care and making that an ongoing part of diabetes care and assessment is important.”
More data needed on diets, many other areas
During the discussion, several attendees asked about low-carbohydrate diets, embraced by many individuals with type 1 diabetes.
The document states: “While low-carbohydrate and very low-carbohydrate eating patterns have become increasingly popular and reduce A1c levels in the short term, it is important to incorporate these in conjunction with healthy eating guidelines. Additional components of the meal, including high fat and/or high protein, may contribute to delayed hyperglycemia and the need for insulin dose adjustments. Since this is highly variable between individuals, postprandial glucose measurements for up to 3 hours or more may be needed to determine initial dose adjustments.”
Beyond that, Tomasz Klupa, MD, PhD, of the department of metabolic diseases, Jagiellonian University, Krakow, Poland, responded: “We don’t have much data on low-carb diets in type 1 diabetes. ... Compliance to those diets is pretty poor. We don’t have long-term follow-up and the studies are simply not conclusive. Initial results do show reductions in body weight and A1c, but with time the compliance goes down dramatically.”
“Certainly, when we think of low-carb diets, we have to meet our patients where they are,” said Amy Hess-Fischl, a nutritionist and certified diabetes care and education specialist at the University of Chicago. “We don’t have enough data to really say there’s positive long-term evidence. But we can find a happy medium to find some benefits in glycemic and weight control. ... It’s really that collaboration with that person to identify what’s going to work for them in a healthy way.”
The EASD session concluded with writing group cochair Anne L. Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, summing up the many other knowledge gaps, including personalizing use of diabetes technology, the problems of health disparities and lack of access to care, and the feasibility of prevention and/or cure.
She observed: “There is no one-size-fits-all approach to diabetes care, and the more we can individualize our approaches, the more successful we are likely to be. ... Hopefully this consensus statement has pushed the bar a bit higher, telling the powers that be that people with type 1 diabetes need and deserve the best.
“We have a very long way to go before all of our patients reach their goals and health equity is achieved. ... We need to provide each and every person the access to the care we describe in this consensus statement, so that all can prosper and thrive and look forward to a long and healthy life lived with type 1 diabetes.”
Dr. Holt has financial relationships with Novo Nordisk, Abbott, Eli Lilly, Otsuka, and Roche. Dr. de Vries has financial relationships with Afon, Eli Lilly, Novo Nordisk, Adocia, and Zealand Pharma. Ms. Hess-Fischl has financial relationships with Abbott Diabetes Care and Xeris. Dr. Klupa has financial relationships with numerous drug and device companies. Dr. Snoek has financial relationships with Abbott, Eli Lilly, Sanofi, and Novo Nordisk. Dr. Peters has financial relationships with Abbott Diabetes Care, Dexcom, Eli Lilly, Insulet, Novo Nordisk, Medscape, and Zealand Pharmaceuticals. She holds stock options in Omada Health and Livongo and is a special government employee of the Food and Drug Administration.
A version of this article first appeared on Medscape.com.
A newly published consensus statement on the management of type 1 diabetes in adults addresses the unique clinical needs of the population compared with those of children with type 1 diabetes or adults with type 2 diabetes.
“The focus on adults is kind of new and it is important. ... I do think it’s a bit of a forgotten population. Whenever we talk about diabetes in adults it’s assumed to be about type 2,” document coauthor M. Sue Kirkman, MD, said in an interview.
The document covers diagnosis of type 1 diabetes, goals and targets, schedule of care, self-management education and lifestyle, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis (DKA), pancreas transplant/islet cell transplantation, adjunctive therapies, special populations (pregnant, older, hospitalized), and emergent and future perspectives.
Initially presented in draft form in June at the American Diabetes Association (ADA) 81st scientific sessions, the final version of the joint ADA/EASD statement was presented Oct. 1 at the annual meeting of the European Association for the Study of Diabetes and simultaneously published in Diabetologia and Diabetes Care.
“We are aware of the many and rapid advances in the diagnosis and treatment of type 1 diabetes ... However, despite these advances, there is also a growing recognition of the psychosocial burden of living with type 1 diabetes,” writing group cochair Richard I.G. Holt, MB BChir, PhD, professor of diabetes and endocrinology at the University of Southampton, England, said when introducing the 90-minute EASD session.
“Although there is guidance for the management of type 1 diabetes, the aim of this report is to highlight the major areas that health care professionals should consider when managing adults with type 1 diabetes,” he added.
Noting that the joint EASD/ADA consensus report on type 2 diabetes has been “highly influential,” Dr. Holt said, “EASD and ADA recognized the need to develop a comparable consensus report specifically addressing type 1 diabetes.”
The overriding goals, Dr. Holt said, are to “support people with type 1 diabetes to live a long and healthy life” with four specific strategies: delivery of insulin to keep glucose levels as close to target as possible to prevent complications while minimizing hypoglycemia and preventing DKA; managing cardiovascular risk factors; minimizing psychosocial burden; and promoting psychological well-being.
Diagnostic algorithm
Another coauthor, J. Hans de Vries, MD, PhD, professor of internal medicine at the University of Amsterdam, explained the recommended approach to distinguishing type 1 diabetes from type 2 diabetes or monogenic diabetes in adults, which is often a clinical challenge.
This also was the topic prompting the most questions during the EASD session.
“Especially in adults, misdiagnosis of type of diabetes is common, occurring in up to 40% of patients diagnosed after the age of 30 years,” Dr. de Vries said.
Among the many reasons for the confusion are that C-peptide levels, a reflection of endogenous insulin secretion, can still be relatively high at the time of clinical onset of type 1 diabetes, but islet antibodies don’t have 100% positive predictive value.
Obesity and type 2 diabetes are increasingly seen at younger ages, and DKA can occur in type 2 diabetes (“ketosis-prone”). In addition, monogenic forms of diabetes can be disguised as type 1 diabetes.
“So, we thought there was a need for a diagnostic algorithm,” Dr. de Vries said, adding that the algorithm – displayed as a graphic in the statement – is only for adults in whom type 1 diabetes is suspected, not other types. Also, it’s based on data from White European populations.
The first step is to test islet autoantibodies. If positive, the diagnosis of type 1 diabetes can be made. If negative and the person is younger than 35 years and without signs of type 2 diabetes, testing C-peptide is advised. If that’s below 200 pmol/L, type 1 diabetes is the diagnosis. If above 200 pmol/L, genetic testing for monogenic diabetes is advised. If there are signs of type 2 diabetes and/or the person is over age 35, type 2 diabetes is the most likely diagnosis.
And if uncertainty remains, the recommendation is to try noninsulin therapy and retest C-peptide again in 3 years, as by that time it will be below 200 pmol/L in a person with type 1 diabetes.
Dr. Kirkman commented regarding the algorithm: “It’s very much from a European population perspective. In some ways that’s a limitation, especially in the U.S. where the population is diverse, but I do think it’s still useful to help guide people through how to think about somebody who presents as an adult where it’s not obviously type 2 or type 1 ... There is a lot of in-between stuff.”
Psychosocial support: Essential but often overlooked
Frank J. Snoek, PhD, professor of psychology at Amsterdam University Medical Centers, Vrije Universiteit, presented the section on behavioral and psychosocial care. He pointed out that diabetes-related emotional distress is reported by 20%-40% of adults with type 1 diabetes, and that the risk of such distress is especially high at the time of diagnosis and when complications develop.
About 15% of people with type 1 diabetes have depression, which is linked to elevated A1c levels, increased complication risk, and mortality. Anxiety also is very common and linked with diabetes-specific fears including hypoglycemia. Eating disorders are more prevalent among people with type 1 diabetes than in the general population and can further complicate diabetes management.
Recommendations include periodic evaluation of psychological health and social barriers to self-management and having clear referral pathways and access to psychological or psychiatric care for individuals in need. “All members of the diabetes care team have a responsibility when it comes to offering psychosocial support as part of ongoing diabetes care and education.”
Dr. Kirkman had identified this section as noteworthy: “I think the focus on psychosocial care and making that an ongoing part of diabetes care and assessment is important.”
More data needed on diets, many other areas
During the discussion, several attendees asked about low-carbohydrate diets, embraced by many individuals with type 1 diabetes.
The document states: “While low-carbohydrate and very low-carbohydrate eating patterns have become increasingly popular and reduce A1c levels in the short term, it is important to incorporate these in conjunction with healthy eating guidelines. Additional components of the meal, including high fat and/or high protein, may contribute to delayed hyperglycemia and the need for insulin dose adjustments. Since this is highly variable between individuals, postprandial glucose measurements for up to 3 hours or more may be needed to determine initial dose adjustments.”
Beyond that, Tomasz Klupa, MD, PhD, of the department of metabolic diseases, Jagiellonian University, Krakow, Poland, responded: “We don’t have much data on low-carb diets in type 1 diabetes. ... Compliance to those diets is pretty poor. We don’t have long-term follow-up and the studies are simply not conclusive. Initial results do show reductions in body weight and A1c, but with time the compliance goes down dramatically.”
“Certainly, when we think of low-carb diets, we have to meet our patients where they are,” said Amy Hess-Fischl, a nutritionist and certified diabetes care and education specialist at the University of Chicago. “We don’t have enough data to really say there’s positive long-term evidence. But we can find a happy medium to find some benefits in glycemic and weight control. ... It’s really that collaboration with that person to identify what’s going to work for them in a healthy way.”
The EASD session concluded with writing group cochair Anne L. Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, summing up the many other knowledge gaps, including personalizing use of diabetes technology, the problems of health disparities and lack of access to care, and the feasibility of prevention and/or cure.
She observed: “There is no one-size-fits-all approach to diabetes care, and the more we can individualize our approaches, the more successful we are likely to be. ... Hopefully this consensus statement has pushed the bar a bit higher, telling the powers that be that people with type 1 diabetes need and deserve the best.
“We have a very long way to go before all of our patients reach their goals and health equity is achieved. ... We need to provide each and every person the access to the care we describe in this consensus statement, so that all can prosper and thrive and look forward to a long and healthy life lived with type 1 diabetes.”
Dr. Holt has financial relationships with Novo Nordisk, Abbott, Eli Lilly, Otsuka, and Roche. Dr. de Vries has financial relationships with Afon, Eli Lilly, Novo Nordisk, Adocia, and Zealand Pharma. Ms. Hess-Fischl has financial relationships with Abbott Diabetes Care and Xeris. Dr. Klupa has financial relationships with numerous drug and device companies. Dr. Snoek has financial relationships with Abbott, Eli Lilly, Sanofi, and Novo Nordisk. Dr. Peters has financial relationships with Abbott Diabetes Care, Dexcom, Eli Lilly, Insulet, Novo Nordisk, Medscape, and Zealand Pharmaceuticals. She holds stock options in Omada Health and Livongo and is a special government employee of the Food and Drug Administration.
A version of this article first appeared on Medscape.com.
A newly published consensus statement on the management of type 1 diabetes in adults addresses the unique clinical needs of the population compared with those of children with type 1 diabetes or adults with type 2 diabetes.
“The focus on adults is kind of new and it is important. ... I do think it’s a bit of a forgotten population. Whenever we talk about diabetes in adults it’s assumed to be about type 2,” document coauthor M. Sue Kirkman, MD, said in an interview.
The document covers diagnosis of type 1 diabetes, goals and targets, schedule of care, self-management education and lifestyle, glucose monitoring, insulin therapy, hypoglycemia, psychosocial care, diabetic ketoacidosis (DKA), pancreas transplant/islet cell transplantation, adjunctive therapies, special populations (pregnant, older, hospitalized), and emergent and future perspectives.
Initially presented in draft form in June at the American Diabetes Association (ADA) 81st scientific sessions, the final version of the joint ADA/EASD statement was presented Oct. 1 at the annual meeting of the European Association for the Study of Diabetes and simultaneously published in Diabetologia and Diabetes Care.
“We are aware of the many and rapid advances in the diagnosis and treatment of type 1 diabetes ... However, despite these advances, there is also a growing recognition of the psychosocial burden of living with type 1 diabetes,” writing group cochair Richard I.G. Holt, MB BChir, PhD, professor of diabetes and endocrinology at the University of Southampton, England, said when introducing the 90-minute EASD session.
“Although there is guidance for the management of type 1 diabetes, the aim of this report is to highlight the major areas that health care professionals should consider when managing adults with type 1 diabetes,” he added.
Noting that the joint EASD/ADA consensus report on type 2 diabetes has been “highly influential,” Dr. Holt said, “EASD and ADA recognized the need to develop a comparable consensus report specifically addressing type 1 diabetes.”
The overriding goals, Dr. Holt said, are to “support people with type 1 diabetes to live a long and healthy life” with four specific strategies: delivery of insulin to keep glucose levels as close to target as possible to prevent complications while minimizing hypoglycemia and preventing DKA; managing cardiovascular risk factors; minimizing psychosocial burden; and promoting psychological well-being.
Diagnostic algorithm
Another coauthor, J. Hans de Vries, MD, PhD, professor of internal medicine at the University of Amsterdam, explained the recommended approach to distinguishing type 1 diabetes from type 2 diabetes or monogenic diabetes in adults, which is often a clinical challenge.
This also was the topic prompting the most questions during the EASD session.
“Especially in adults, misdiagnosis of type of diabetes is common, occurring in up to 40% of patients diagnosed after the age of 30 years,” Dr. de Vries said.
Among the many reasons for the confusion are that C-peptide levels, a reflection of endogenous insulin secretion, can still be relatively high at the time of clinical onset of type 1 diabetes, but islet antibodies don’t have 100% positive predictive value.
Obesity and type 2 diabetes are increasingly seen at younger ages, and DKA can occur in type 2 diabetes (“ketosis-prone”). In addition, monogenic forms of diabetes can be disguised as type 1 diabetes.
“So, we thought there was a need for a diagnostic algorithm,” Dr. de Vries said, adding that the algorithm – displayed as a graphic in the statement – is only for adults in whom type 1 diabetes is suspected, not other types. Also, it’s based on data from White European populations.
The first step is to test islet autoantibodies. If positive, the diagnosis of type 1 diabetes can be made. If negative and the person is younger than 35 years and without signs of type 2 diabetes, testing C-peptide is advised. If that’s below 200 pmol/L, type 1 diabetes is the diagnosis. If above 200 pmol/L, genetic testing for monogenic diabetes is advised. If there are signs of type 2 diabetes and/or the person is over age 35, type 2 diabetes is the most likely diagnosis.
And if uncertainty remains, the recommendation is to try noninsulin therapy and retest C-peptide again in 3 years, as by that time it will be below 200 pmol/L in a person with type 1 diabetes.
Dr. Kirkman commented regarding the algorithm: “It’s very much from a European population perspective. In some ways that’s a limitation, especially in the U.S. where the population is diverse, but I do think it’s still useful to help guide people through how to think about somebody who presents as an adult where it’s not obviously type 2 or type 1 ... There is a lot of in-between stuff.”
Psychosocial support: Essential but often overlooked
Frank J. Snoek, PhD, professor of psychology at Amsterdam University Medical Centers, Vrije Universiteit, presented the section on behavioral and psychosocial care. He pointed out that diabetes-related emotional distress is reported by 20%-40% of adults with type 1 diabetes, and that the risk of such distress is especially high at the time of diagnosis and when complications develop.
About 15% of people with type 1 diabetes have depression, which is linked to elevated A1c levels, increased complication risk, and mortality. Anxiety also is very common and linked with diabetes-specific fears including hypoglycemia. Eating disorders are more prevalent among people with type 1 diabetes than in the general population and can further complicate diabetes management.
Recommendations include periodic evaluation of psychological health and social barriers to self-management and having clear referral pathways and access to psychological or psychiatric care for individuals in need. “All members of the diabetes care team have a responsibility when it comes to offering psychosocial support as part of ongoing diabetes care and education.”
Dr. Kirkman had identified this section as noteworthy: “I think the focus on psychosocial care and making that an ongoing part of diabetes care and assessment is important.”
More data needed on diets, many other areas
During the discussion, several attendees asked about low-carbohydrate diets, embraced by many individuals with type 1 diabetes.
The document states: “While low-carbohydrate and very low-carbohydrate eating patterns have become increasingly popular and reduce A1c levels in the short term, it is important to incorporate these in conjunction with healthy eating guidelines. Additional components of the meal, including high fat and/or high protein, may contribute to delayed hyperglycemia and the need for insulin dose adjustments. Since this is highly variable between individuals, postprandial glucose measurements for up to 3 hours or more may be needed to determine initial dose adjustments.”
Beyond that, Tomasz Klupa, MD, PhD, of the department of metabolic diseases, Jagiellonian University, Krakow, Poland, responded: “We don’t have much data on low-carb diets in type 1 diabetes. ... Compliance to those diets is pretty poor. We don’t have long-term follow-up and the studies are simply not conclusive. Initial results do show reductions in body weight and A1c, but with time the compliance goes down dramatically.”
“Certainly, when we think of low-carb diets, we have to meet our patients where they are,” said Amy Hess-Fischl, a nutritionist and certified diabetes care and education specialist at the University of Chicago. “We don’t have enough data to really say there’s positive long-term evidence. But we can find a happy medium to find some benefits in glycemic and weight control. ... It’s really that collaboration with that person to identify what’s going to work for them in a healthy way.”
The EASD session concluded with writing group cochair Anne L. Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, summing up the many other knowledge gaps, including personalizing use of diabetes technology, the problems of health disparities and lack of access to care, and the feasibility of prevention and/or cure.
She observed: “There is no one-size-fits-all approach to diabetes care, and the more we can individualize our approaches, the more successful we are likely to be. ... Hopefully this consensus statement has pushed the bar a bit higher, telling the powers that be that people with type 1 diabetes need and deserve the best.
“We have a very long way to go before all of our patients reach their goals and health equity is achieved. ... We need to provide each and every person the access to the care we describe in this consensus statement, so that all can prosper and thrive and look forward to a long and healthy life lived with type 1 diabetes.”
Dr. Holt has financial relationships with Novo Nordisk, Abbott, Eli Lilly, Otsuka, and Roche. Dr. de Vries has financial relationships with Afon, Eli Lilly, Novo Nordisk, Adocia, and Zealand Pharma. Ms. Hess-Fischl has financial relationships with Abbott Diabetes Care and Xeris. Dr. Klupa has financial relationships with numerous drug and device companies. Dr. Snoek has financial relationships with Abbott, Eli Lilly, Sanofi, and Novo Nordisk. Dr. Peters has financial relationships with Abbott Diabetes Care, Dexcom, Eli Lilly, Insulet, Novo Nordisk, Medscape, and Zealand Pharmaceuticals. She holds stock options in Omada Health and Livongo and is a special government employee of the Food and Drug Administration.
A version of this article first appeared on Medscape.com.
FROM EASD 2021
Paraneoplastic Signs in Bladder Transitional Cell Carcinoma: An Unusual Presentation
To the Editor:
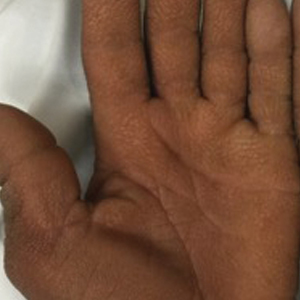
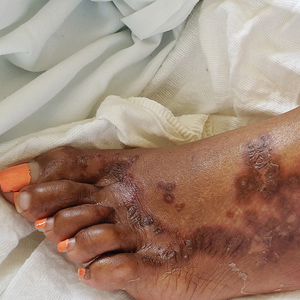
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.
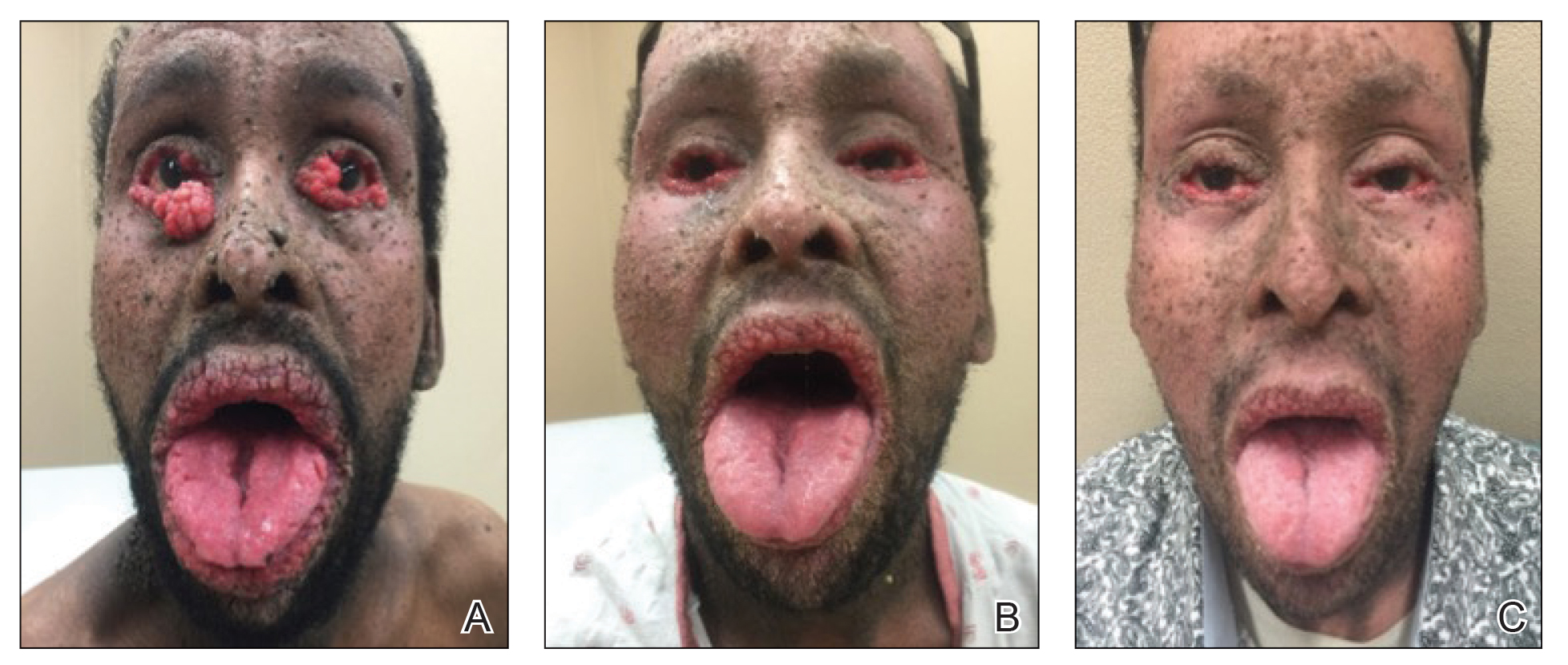
Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.
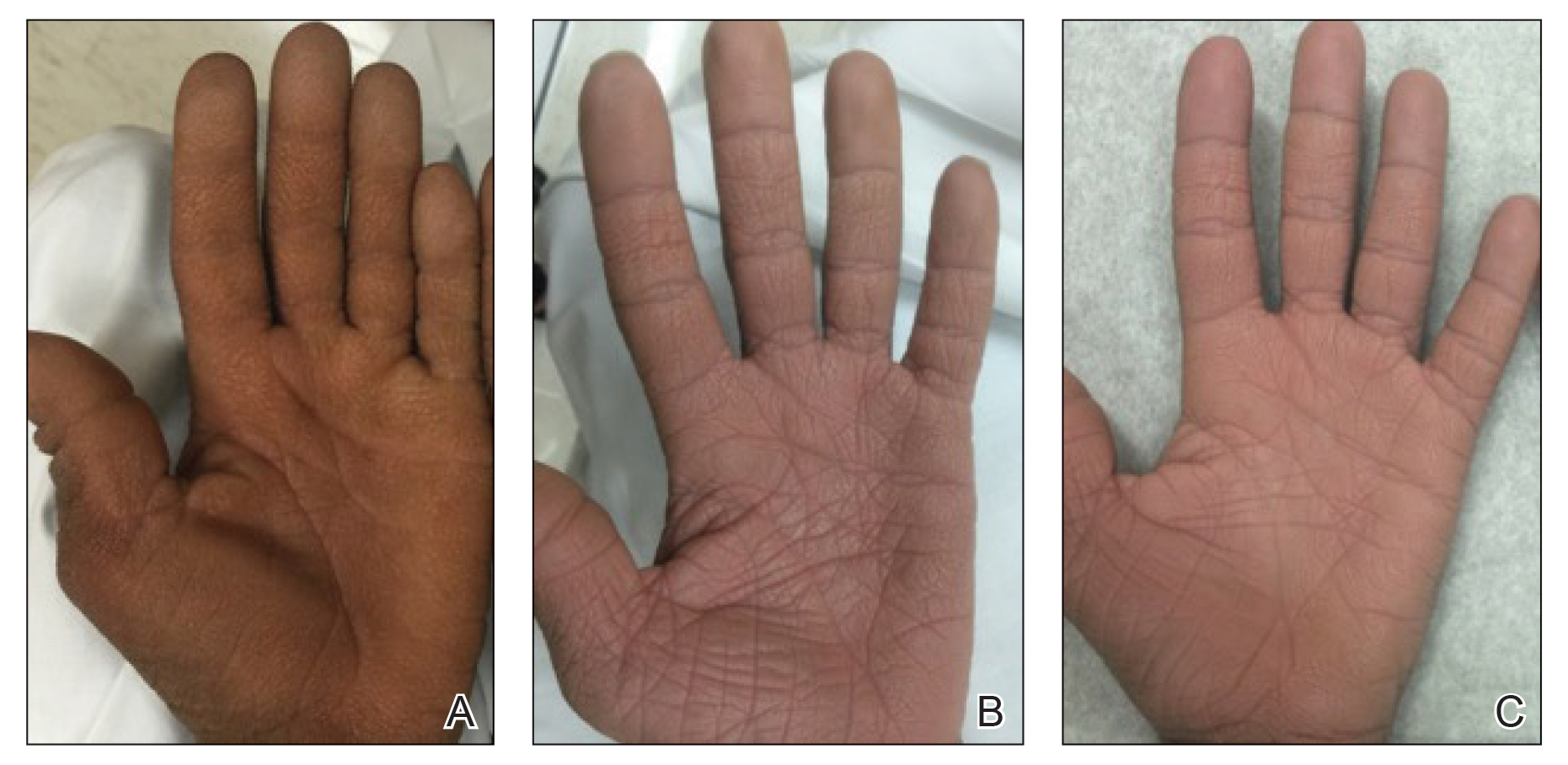
This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
To the Editor:
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.

Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.

This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
To the Editor:
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.

Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.

This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
Practice Points
- Paraneoplastic conditions may present secondary to urologic malignancy. Providers should perform thorough malignancy screening, including urologic cystoscopy, in patients presenting with paraneoplastic signs and no identified malignancy.
- Oral retinoids, such as acitretin, may be used as an adjuvant treatment to treat paraneoplastic cutaneous symptoms. The definitive treatment is malignancy management.
Cold viruses thrived in kids as other viruses faded in 2020
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The common-cold viruses rhinovirus (RV) and enterovirus (EV) continued to circulate among children during the COVID-19 pandemic while there were sharp declines in influenza, respiratory syncytial virus (RSV), and other respiratory viruses, new data indicate.
Researchers used data from the Centers for Disease Control and Prevention’s New Vaccine Surveillance Network. The cases involved 37,676 children in seven geographically diverse U.S. medical centers between December 2016 and January 2021. Patients presented to emergency departments or were hospitalized with RV, EV, and other acute respiratory viruses.
The investigators found that the percentage of children in whom RV/EV was detected from March 2020 to January 2021 was similar to the percentage during the same months in 2017-2018 and 2019-2020. However, the proportion of children infected with influenza, RSV, and other respiratory viruses combined dropped significantly in comparison to the three prior seasons.

Danielle Rankin, MPH, lead author of the study and a doctoral candidate in pediatric infectious disease at Vanderbilt University, in Nashville, Tenn., presented the study on Sept. 30 during a press conference at IDWeek 2021, an annual scientific meeting on infectious diseases.
“Reasoning for rhinovirus and enterovirus circulation is unknown but may be attributed to a number of factors, such as different transmission routes or the prolonged survival of the virus on surfaces,” Ms. Rankin said. “Improved understanding of these persistent factors of RV/EV and the role of nonpharmaceutical interventions on transmission dynamics can further guide future prevention recommendations and guidelines.”
Coauthor Claire Midgley, PhD, an epidemiologist in the Division of Viral Diseases at the CDC, told reporters that further studies will assess why RV and EV remained during the pandemic and which virus types within the RV/EV group persisted.
“We do know that the virus can spread through secretions on people’s hands,” she said. “Washing kids’ hands regularly and trying not to touch your face where possible is a really effective way to prevent transmission,” Dr. Midgley said.
“The more we understand about all of these factors, the better we can inform prevention measures.”
Andrew T. Pavia, MD, chief, division of pediatric infectious diseases, University of Utah, Salt Lake City, who was not involved in the study, told this news organization that rhinoviruses can persist in the nose for a very long time, especially in younger children, which increases the opportunities for transmission.
“Very young children who are unable to wear masks or are unlikely to wear them well may be acting as the reservoir, allowing transmission in households,” he said. “There is also an enormous pool of diverse rhinoviruses, so past colds provide limited immunity, as everyone has found out from experience.”
Martha Perry, MD, associate professor at the University of North Carolina at Chapel Hill and chief of adolescent medicine, told this news organization that some of the differences in the prevalence of viruses may be because of their seasonality.
“Times when there were more mask mandates were times when RSV and influenza are more prevalent,” said Dr. Perry, who was not involved with the study. “We were masking more intently during those times, and there was loosening of restrictions when we see more enterovirus, particularly because that tends to be more of a summer/fall virus.”
She agreed that the differences may result from the way the viruses are transmitted.
“Perhaps masks were helping with RSV and influenza, but perhaps there was not as much hand washing or cleansing as needed to prevent the spread of rhinovirus and enterovirus, because those are viruses that require a bit more hand washing,” Dr. Perry said. “They are less aerosolized and better spread with hand-to-hand contact.”
Dr. Perry added that on the flip side, “it’s really exciting that there are ways we can prevent RSV and influenza, which tend to cause more severe infection.”
Ms. Rankin said limitations of the study include the fact that from March 2020 to January 2021, health care–seeking behaviors may have changed because of the pandemic and that the study does not include the frequency of respiratory viruses in the outpatient setting.
The sharp 2020-2021 decline in RSV reported in the study may have reversed after many of the COVID-19 restrictions were lifted this summer.
This news organization reported in June of this year that the CDC has issued a health advisory to notify clinicians and caregivers about an increase in cases of interseasonal RSV in parts of the southern United States.
The CDC has urged broader testing for RSV among patients presenting with acute respiratory illness who test negative for SARS-CoV-2.
The study’s authors, Ms. Pavia, and Dr. Perry have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lessons from an ethnic skin center: Awareness and respect for diversity
With the strong likelihood that , according to a dermatologist with expertise in these types of cases who spoke at the Skin of Color Update 2021.
“Instead of avoiding the discussion of cultural practices, we should discuss them and be open about them. It fosters a comfortable environment, trust, and better compliance,” reported Neelam Ajit Vashi, MD, founding director of the Boston University Center for Ethnic Skin.
Out of fear of causing offense, a desire to be discreet, or of personal discomfort with foreign cultural practices, some clinicians might elect to limit themselves to the information that the patient volunteers, which is a mistake, according to Dr. Vashi.
“The avoidance of topics around culture actually limits the ability to have a successful relationship,” she maintained.
Successful encounters are not just based on a willingness to listen, Dr. Vashi said. Clinicians should be seeking a base of knowledge. With growing globalization and widespread immigration, “it is increasingly important for dermatologists in the U.S. to understand the role of cultural practices [in creating skin problems] and recognize the sequelae,” Dr. Vashi said.
Taking some common examples of dermatologic complaints created by cosmetic practices originating elsewhere, Dr. Vashi described key clinical points in addressing complications related to henna, hair removal through threading, and placement of decorative adornments on the forehead, called bindi. In addition, she pointed out common issues with facial and body marking created with kumkum powder, hair oils, and skin lightening agents.
Black henna
For cosmetic enhancement, henna is relatively benign. It is also no longer confined to the south Asian communities where it originated. However, Dr. Vashi pointed out that patients of south Asian origin or descent might be more likely to use black henna, a variety with more risks.
Black henna contains additives, such as diaminobenzenes and p-phenylenediamine (PPD), to darken the tone of the product as well as provide other desired characteristics, such as an accelerated drying time. While some patients do develop reactions to conventional henna, the risks of black henna are greater.
“The acute contact dermatitis reactions can include dyspigmentation, leukoderma, and keloids,” Dr. Vashi said. Other complications include erythema multiforme, temporary hypertrichosis, and systemic allergic reactions, such as angioedema.
While those who have had a reaction to henna should avoid further contact, Dr. Vashi warned that sequelae can include cross reactions with latex and rubber as well as some pharmaceutical agents, such as sulfonamides. When taking a patient history, she noted, be aware that risks of henna extend to the hairdressers and cosmeticians who sometimes apply these products on others.
Hair threading, bindi, and kumkum
Hair threading, another practice popularized in south Asia and now growing in popularity globally, involves capturing hairs between cotton threads for removal of both the hair and its follicle. It is a relatively rapid and efficient method of permanent depilation. In addition to pain and erythema, Dr. Vashi reported that the complications associated with hair threading include pigmentary changes, infections such as bullous impetigo, and lesions of koebnerization – such as vitiligo and lichen planus.
Bindi, a Hindi tradition that involves placing adornments between the eyebrows, and kumkum, a powder typically made from turmeric to be employed for decorative markings, have also spread to use outside of their cultural context, according to Dr. Vashi. She said that the complications of these two cosmetic practices are shared, and stem largely from contact dermatitis.
In the case of bindi, para-tertiary-butylphenol in adhesives is one source of reactions, whereas kumkum itself can be an irritant. As these are typically local to the site of application, the diagnosis is not difficult, but treatment can be more challenging for patients unwilling to abandon the practice.
Hair oils, skin-lightening agents
Culturally-linked hair oils among patients from south Asia or Africa – or descendants from these areas – can damage hair in a variety of ways as well as cause contact dermatitis. The oils can also exacerbate existing skin diseases.
“Oils with high oleic acid, such as coconut or olive oils or shea butter, can worsen seborrheic dermatitis,” Dr. Vashi cautioned.
Of this list of dermatologic issues induced by culturally linked cosmetic practices, skin lightening agents might pose the most risk for permanent and irreversible complications. Dr. Vashi said that up to 70% of patients using lighteners develop complications, and there is a relationship between the severity of side effects as duration of use increases.
“The problem is that ingredients of many of these products, which are imported illegally and sold on the black market, are often not disclosed,” Dr. Vashi said. Some contain a high content of metals such as lead, copper, and iron, whether they are added intentionally or end up in the product because of poor quality control. For those developing adverse events associated with the products, the obvious treatment is discontinuation.
When patients are unwilling to discontinue any of the products that have led to dermatologic issues, Dr. Vashi encouraged physicians “to take a middle ground.” Simple avoidance can be challenging for practices that are culturally meaningful. In respecting cultural differences, she encouraged tolerance and compromise.
“Often these patients will be doing an alternative medication or intervention, but this does not mean that they are not accepting what we have to offer,” she said. She indicated that mutual respect will lead to better solutions.
The awareness of common cultural practices that can have a harmful impact on the skin is an area of practice that deserves more attention, Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology at Weill Cornell Medical Center, New York, said in an interview.
He said that he agreed with Dr. Vashi that understanding the role of cultural practices leading to dermatoses is not enough.
“Advising patients to alter or discontinue a specific cultural practice due to a dermatologic complication should be done with respect, humility, and understanding that may be challenging,” said Dr. Alexis.
While being aware of the specific cultural practices that might be causing or exacerbating dermatoses is important for accurate diagnosis, he said he believes that “partnering with the patient to modify the cultural practices in question” is important for a clinical outcome acceptable to the patient.
“Educational resources to inform clinicians of dermatoses associated with cultural practices are available and can be helpful for dermatologists in any practice setting,” he said.
Dr. Vashi reports that she has no relevant financial relationships to disclose. Dr. Alexis reports financial relationships with Abbvie, Allergan, Almirall, Amgen, Arcutis, AstraZeneca, Bristol-Myers Squibb, Cara, Galderma, Genzyme, Janssen, Leo, Menlo, Novartis, Regeneron, Sanofi, and Valeant.
With the strong likelihood that , according to a dermatologist with expertise in these types of cases who spoke at the Skin of Color Update 2021.
“Instead of avoiding the discussion of cultural practices, we should discuss them and be open about them. It fosters a comfortable environment, trust, and better compliance,” reported Neelam Ajit Vashi, MD, founding director of the Boston University Center for Ethnic Skin.
Out of fear of causing offense, a desire to be discreet, or of personal discomfort with foreign cultural practices, some clinicians might elect to limit themselves to the information that the patient volunteers, which is a mistake, according to Dr. Vashi.
“The avoidance of topics around culture actually limits the ability to have a successful relationship,” she maintained.
Successful encounters are not just based on a willingness to listen, Dr. Vashi said. Clinicians should be seeking a base of knowledge. With growing globalization and widespread immigration, “it is increasingly important for dermatologists in the U.S. to understand the role of cultural practices [in creating skin problems] and recognize the sequelae,” Dr. Vashi said.
Taking some common examples of dermatologic complaints created by cosmetic practices originating elsewhere, Dr. Vashi described key clinical points in addressing complications related to henna, hair removal through threading, and placement of decorative adornments on the forehead, called bindi. In addition, she pointed out common issues with facial and body marking created with kumkum powder, hair oils, and skin lightening agents.
Black henna
For cosmetic enhancement, henna is relatively benign. It is also no longer confined to the south Asian communities where it originated. However, Dr. Vashi pointed out that patients of south Asian origin or descent might be more likely to use black henna, a variety with more risks.
Black henna contains additives, such as diaminobenzenes and p-phenylenediamine (PPD), to darken the tone of the product as well as provide other desired characteristics, such as an accelerated drying time. While some patients do develop reactions to conventional henna, the risks of black henna are greater.
“The acute contact dermatitis reactions can include dyspigmentation, leukoderma, and keloids,” Dr. Vashi said. Other complications include erythema multiforme, temporary hypertrichosis, and systemic allergic reactions, such as angioedema.
While those who have had a reaction to henna should avoid further contact, Dr. Vashi warned that sequelae can include cross reactions with latex and rubber as well as some pharmaceutical agents, such as sulfonamides. When taking a patient history, she noted, be aware that risks of henna extend to the hairdressers and cosmeticians who sometimes apply these products on others.
Hair threading, bindi, and kumkum
Hair threading, another practice popularized in south Asia and now growing in popularity globally, involves capturing hairs between cotton threads for removal of both the hair and its follicle. It is a relatively rapid and efficient method of permanent depilation. In addition to pain and erythema, Dr. Vashi reported that the complications associated with hair threading include pigmentary changes, infections such as bullous impetigo, and lesions of koebnerization – such as vitiligo and lichen planus.
Bindi, a Hindi tradition that involves placing adornments between the eyebrows, and kumkum, a powder typically made from turmeric to be employed for decorative markings, have also spread to use outside of their cultural context, according to Dr. Vashi. She said that the complications of these two cosmetic practices are shared, and stem largely from contact dermatitis.
In the case of bindi, para-tertiary-butylphenol in adhesives is one source of reactions, whereas kumkum itself can be an irritant. As these are typically local to the site of application, the diagnosis is not difficult, but treatment can be more challenging for patients unwilling to abandon the practice.
Hair oils, skin-lightening agents
Culturally-linked hair oils among patients from south Asia or Africa – or descendants from these areas – can damage hair in a variety of ways as well as cause contact dermatitis. The oils can also exacerbate existing skin diseases.
“Oils with high oleic acid, such as coconut or olive oils or shea butter, can worsen seborrheic dermatitis,” Dr. Vashi cautioned.
Of this list of dermatologic issues induced by culturally linked cosmetic practices, skin lightening agents might pose the most risk for permanent and irreversible complications. Dr. Vashi said that up to 70% of patients using lighteners develop complications, and there is a relationship between the severity of side effects as duration of use increases.
“The problem is that ingredients of many of these products, which are imported illegally and sold on the black market, are often not disclosed,” Dr. Vashi said. Some contain a high content of metals such as lead, copper, and iron, whether they are added intentionally or end up in the product because of poor quality control. For those developing adverse events associated with the products, the obvious treatment is discontinuation.
When patients are unwilling to discontinue any of the products that have led to dermatologic issues, Dr. Vashi encouraged physicians “to take a middle ground.” Simple avoidance can be challenging for practices that are culturally meaningful. In respecting cultural differences, she encouraged tolerance and compromise.
“Often these patients will be doing an alternative medication or intervention, but this does not mean that they are not accepting what we have to offer,” she said. She indicated that mutual respect will lead to better solutions.
The awareness of common cultural practices that can have a harmful impact on the skin is an area of practice that deserves more attention, Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology at Weill Cornell Medical Center, New York, said in an interview.
He said that he agreed with Dr. Vashi that understanding the role of cultural practices leading to dermatoses is not enough.
“Advising patients to alter or discontinue a specific cultural practice due to a dermatologic complication should be done with respect, humility, and understanding that may be challenging,” said Dr. Alexis.
While being aware of the specific cultural practices that might be causing or exacerbating dermatoses is important for accurate diagnosis, he said he believes that “partnering with the patient to modify the cultural practices in question” is important for a clinical outcome acceptable to the patient.
“Educational resources to inform clinicians of dermatoses associated with cultural practices are available and can be helpful for dermatologists in any practice setting,” he said.
Dr. Vashi reports that she has no relevant financial relationships to disclose. Dr. Alexis reports financial relationships with Abbvie, Allergan, Almirall, Amgen, Arcutis, AstraZeneca, Bristol-Myers Squibb, Cara, Galderma, Genzyme, Janssen, Leo, Menlo, Novartis, Regeneron, Sanofi, and Valeant.
With the strong likelihood that , according to a dermatologist with expertise in these types of cases who spoke at the Skin of Color Update 2021.
“Instead of avoiding the discussion of cultural practices, we should discuss them and be open about them. It fosters a comfortable environment, trust, and better compliance,” reported Neelam Ajit Vashi, MD, founding director of the Boston University Center for Ethnic Skin.
Out of fear of causing offense, a desire to be discreet, or of personal discomfort with foreign cultural practices, some clinicians might elect to limit themselves to the information that the patient volunteers, which is a mistake, according to Dr. Vashi.
“The avoidance of topics around culture actually limits the ability to have a successful relationship,” she maintained.
Successful encounters are not just based on a willingness to listen, Dr. Vashi said. Clinicians should be seeking a base of knowledge. With growing globalization and widespread immigration, “it is increasingly important for dermatologists in the U.S. to understand the role of cultural practices [in creating skin problems] and recognize the sequelae,” Dr. Vashi said.
Taking some common examples of dermatologic complaints created by cosmetic practices originating elsewhere, Dr. Vashi described key clinical points in addressing complications related to henna, hair removal through threading, and placement of decorative adornments on the forehead, called bindi. In addition, she pointed out common issues with facial and body marking created with kumkum powder, hair oils, and skin lightening agents.
Black henna
For cosmetic enhancement, henna is relatively benign. It is also no longer confined to the south Asian communities where it originated. However, Dr. Vashi pointed out that patients of south Asian origin or descent might be more likely to use black henna, a variety with more risks.
Black henna contains additives, such as diaminobenzenes and p-phenylenediamine (PPD), to darken the tone of the product as well as provide other desired characteristics, such as an accelerated drying time. While some patients do develop reactions to conventional henna, the risks of black henna are greater.
“The acute contact dermatitis reactions can include dyspigmentation, leukoderma, and keloids,” Dr. Vashi said. Other complications include erythema multiforme, temporary hypertrichosis, and systemic allergic reactions, such as angioedema.
While those who have had a reaction to henna should avoid further contact, Dr. Vashi warned that sequelae can include cross reactions with latex and rubber as well as some pharmaceutical agents, such as sulfonamides. When taking a patient history, she noted, be aware that risks of henna extend to the hairdressers and cosmeticians who sometimes apply these products on others.
Hair threading, bindi, and kumkum
Hair threading, another practice popularized in south Asia and now growing in popularity globally, involves capturing hairs between cotton threads for removal of both the hair and its follicle. It is a relatively rapid and efficient method of permanent depilation. In addition to pain and erythema, Dr. Vashi reported that the complications associated with hair threading include pigmentary changes, infections such as bullous impetigo, and lesions of koebnerization – such as vitiligo and lichen planus.
Bindi, a Hindi tradition that involves placing adornments between the eyebrows, and kumkum, a powder typically made from turmeric to be employed for decorative markings, have also spread to use outside of their cultural context, according to Dr. Vashi. She said that the complications of these two cosmetic practices are shared, and stem largely from contact dermatitis.
In the case of bindi, para-tertiary-butylphenol in adhesives is one source of reactions, whereas kumkum itself can be an irritant. As these are typically local to the site of application, the diagnosis is not difficult, but treatment can be more challenging for patients unwilling to abandon the practice.
Hair oils, skin-lightening agents
Culturally-linked hair oils among patients from south Asia or Africa – or descendants from these areas – can damage hair in a variety of ways as well as cause contact dermatitis. The oils can also exacerbate existing skin diseases.
“Oils with high oleic acid, such as coconut or olive oils or shea butter, can worsen seborrheic dermatitis,” Dr. Vashi cautioned.
Of this list of dermatologic issues induced by culturally linked cosmetic practices, skin lightening agents might pose the most risk for permanent and irreversible complications. Dr. Vashi said that up to 70% of patients using lighteners develop complications, and there is a relationship between the severity of side effects as duration of use increases.
“The problem is that ingredients of many of these products, which are imported illegally and sold on the black market, are often not disclosed,” Dr. Vashi said. Some contain a high content of metals such as lead, copper, and iron, whether they are added intentionally or end up in the product because of poor quality control. For those developing adverse events associated with the products, the obvious treatment is discontinuation.
When patients are unwilling to discontinue any of the products that have led to dermatologic issues, Dr. Vashi encouraged physicians “to take a middle ground.” Simple avoidance can be challenging for practices that are culturally meaningful. In respecting cultural differences, she encouraged tolerance and compromise.
“Often these patients will be doing an alternative medication or intervention, but this does not mean that they are not accepting what we have to offer,” she said. She indicated that mutual respect will lead to better solutions.
The awareness of common cultural practices that can have a harmful impact on the skin is an area of practice that deserves more attention, Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology at Weill Cornell Medical Center, New York, said in an interview.
He said that he agreed with Dr. Vashi that understanding the role of cultural practices leading to dermatoses is not enough.
“Advising patients to alter or discontinue a specific cultural practice due to a dermatologic complication should be done with respect, humility, and understanding that may be challenging,” said Dr. Alexis.
While being aware of the specific cultural practices that might be causing or exacerbating dermatoses is important for accurate diagnosis, he said he believes that “partnering with the patient to modify the cultural practices in question” is important for a clinical outcome acceptable to the patient.
“Educational resources to inform clinicians of dermatoses associated with cultural practices are available and can be helpful for dermatologists in any practice setting,” he said.
Dr. Vashi reports that she has no relevant financial relationships to disclose. Dr. Alexis reports financial relationships with Abbvie, Allergan, Almirall, Amgen, Arcutis, AstraZeneca, Bristol-Myers Squibb, Cara, Galderma, Genzyme, Janssen, Leo, Menlo, Novartis, Regeneron, Sanofi, and Valeant.
FROM SOC 2021
Infant with edematous, erythematous toe
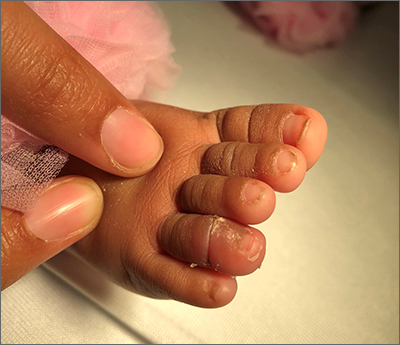
History, presentation, and clinical suspicion led to the diagnosis of hair tourniquet syndrome.
Hair tourniquet syndrome was first described in 1612 by French surgeon Jacques Guillemeau.1 It typically occurs in infants when a long hair gets tightly wrapped around tissue. It most commonly affects the digits, but the penis, labia, or clitoris may also be involved. If left untreated, this condition may lead to serious complications including ischemia and necrosis of the site, and more rarely, bone erosion.
Clinicians who work with children should be aware of this condition, as early diagnosis and treatment can prevent adverse outcomes. Diagnosis requires a high-level of clinical suspicion. Use of ultrasound guidance to confirm the presence of a foreign body may aid in prompt diagnosis.2
Treatment involves release of the constricting hair(s). Hair removal cream may be used if the skin barrier is not compromised. If the hair is visible, clinicians may also attempt to remove it with tweezers. If the hair is deeply embedded within the skin, as in this case, surgical dissection may be necessary.
For this patient, the physician used local anesthesia and surgical loupes to remove 3 strands of hair from beneath newly epithelialized tissue. The digit immediately turned warm and pink. Two minutes later, capillary-refill time was normal. The mother was counseled that women often lose more hair than usual during the postpartum period, and that as a result, it’s important to watch for strands of hair that may get wrapped around the baby’s fingers or toes. Follow-up, 1 month later, showed a healed lesion on a well-perfused and nontender toe.
Image courtesy of Omar Osmani, MD, Spine and Orthopedic Center of New Mexico, Roswell. Text courtesy of Sabah Osmani, BA, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Zimmerman LN, Wagner AJ. Clitoral hair tourniquet: a case report and review of the literature. Int J Pediatr Res. 2015;1:1-2. doi: 10.23937/2469-5769/1510007
2. Sebaratnam DF, Hernández‐Martín Á. Utility of ultrasonography in hair-thread tourniquet syndrome. Pediatr Dermatolo. 2018;35:e138–e139. doi: 10.1111/pde.13400

History, presentation, and clinical suspicion led to the diagnosis of hair tourniquet syndrome.
Hair tourniquet syndrome was first described in 1612 by French surgeon Jacques Guillemeau.1 It typically occurs in infants when a long hair gets tightly wrapped around tissue. It most commonly affects the digits, but the penis, labia, or clitoris may also be involved. If left untreated, this condition may lead to serious complications including ischemia and necrosis of the site, and more rarely, bone erosion.
Clinicians who work with children should be aware of this condition, as early diagnosis and treatment can prevent adverse outcomes. Diagnosis requires a high-level of clinical suspicion. Use of ultrasound guidance to confirm the presence of a foreign body may aid in prompt diagnosis.2
Treatment involves release of the constricting hair(s). Hair removal cream may be used if the skin barrier is not compromised. If the hair is visible, clinicians may also attempt to remove it with tweezers. If the hair is deeply embedded within the skin, as in this case, surgical dissection may be necessary.
For this patient, the physician used local anesthesia and surgical loupes to remove 3 strands of hair from beneath newly epithelialized tissue. The digit immediately turned warm and pink. Two minutes later, capillary-refill time was normal. The mother was counseled that women often lose more hair than usual during the postpartum period, and that as a result, it’s important to watch for strands of hair that may get wrapped around the baby’s fingers or toes. Follow-up, 1 month later, showed a healed lesion on a well-perfused and nontender toe.
Image courtesy of Omar Osmani, MD, Spine and Orthopedic Center of New Mexico, Roswell. Text courtesy of Sabah Osmani, BA, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

History, presentation, and clinical suspicion led to the diagnosis of hair tourniquet syndrome.
Hair tourniquet syndrome was first described in 1612 by French surgeon Jacques Guillemeau.1 It typically occurs in infants when a long hair gets tightly wrapped around tissue. It most commonly affects the digits, but the penis, labia, or clitoris may also be involved. If left untreated, this condition may lead to serious complications including ischemia and necrosis of the site, and more rarely, bone erosion.
Clinicians who work with children should be aware of this condition, as early diagnosis and treatment can prevent adverse outcomes. Diagnosis requires a high-level of clinical suspicion. Use of ultrasound guidance to confirm the presence of a foreign body may aid in prompt diagnosis.2
Treatment involves release of the constricting hair(s). Hair removal cream may be used if the skin barrier is not compromised. If the hair is visible, clinicians may also attempt to remove it with tweezers. If the hair is deeply embedded within the skin, as in this case, surgical dissection may be necessary.
For this patient, the physician used local anesthesia and surgical loupes to remove 3 strands of hair from beneath newly epithelialized tissue. The digit immediately turned warm and pink. Two minutes later, capillary-refill time was normal. The mother was counseled that women often lose more hair than usual during the postpartum period, and that as a result, it’s important to watch for strands of hair that may get wrapped around the baby’s fingers or toes. Follow-up, 1 month later, showed a healed lesion on a well-perfused and nontender toe.
Image courtesy of Omar Osmani, MD, Spine and Orthopedic Center of New Mexico, Roswell. Text courtesy of Sabah Osmani, BA, University of New Mexico School of Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Zimmerman LN, Wagner AJ. Clitoral hair tourniquet: a case report and review of the literature. Int J Pediatr Res. 2015;1:1-2. doi: 10.23937/2469-5769/1510007
2. Sebaratnam DF, Hernández‐Martín Á. Utility of ultrasonography in hair-thread tourniquet syndrome. Pediatr Dermatolo. 2018;35:e138–e139. doi: 10.1111/pde.13400
1. Zimmerman LN, Wagner AJ. Clitoral hair tourniquet: a case report and review of the literature. Int J Pediatr Res. 2015;1:1-2. doi: 10.23937/2469-5769/1510007
2. Sebaratnam DF, Hernández‐Martín Á. Utility of ultrasonography in hair-thread tourniquet syndrome. Pediatr Dermatolo. 2018;35:e138–e139. doi: 10.1111/pde.13400
Mentoring is key to growing women’s leadership in medicine
Men may think they are supportive of women in the workplace, but if you ask women, they say there is a discrepancy, according to W. Brad Johnson, PhD, a clinical psychologist and professor at the United States Naval Academy in Annapolis, Md.
“We may think we are acting as allies to women because we believe in it, but it may not be showing up in the execution,” he said in a presentation at the virtual Advance PHM Gender Equity Conference.
Although women currently account for the majority of medical school students, they make up only 16% of the population of medical school deans, 18% of department chairs, and 25% of full professors, according to 2019 data from the Association of American Medical Colleges, Dr. Johnson said.
The “missing ingredient” in increasing the number of women in medical faculty positions is that women are less mentored. Some barriers to mentorship include men’s concerns that women will take offers of mentorship the wrong way, but “it is incredibly rare for women to make a false accusation” of harassment in a mentorship situation, said Dr. Johnson.
Dr. Johnson offered some guidance for how men can become better allies for women in the workplace through interpersonal allyship, public allyship, and systemic allyship.
Interpersonal allyship and opportunities for mentoring women in medicine start by building trust, friendship, and collegiality between men and women colleagues, Dr. Johnson explained.
He provided some guidance for men to “sharpen their gender intelligence,” which starts with listening. Surveys of women show that they would like male colleagues to be a sounding board, rather than simply offering to jump in with a fix for a problem. “Show humility,” he said, don’t be afraid to ask questions, and don’t assume that a colleague wants something in particular because she is a woman.
“A lot of men get stuck on breaking the ice and getting started with a mentoring conversation,” Dr. Johnson said. One way to is by telling a female colleague who gave an outstanding presentation, or has conducted outstanding research, that you want to keep her in your organization and that she is welcome to talk about her goals. Women appreciate mentoring as “a constellation” and a way to build support, and have one person introduce them to others who can build a network and promote opportunities for leadership. Also, he encouraged men to be open to feedback from female colleagues on how they can be more supportive in the workplace. Sincerity and genuine effort go a long way towards improving gender equity.
Public allyship can take many forms, including putting women center stage to share their own ideas, Dr. Johnson said. Surveys of women show that they often feel dismissed or slighted and not given credit for an idea that was ultimately presented by a male colleague, he noted. Instead, be a female colleague’s biggest fan, and put her in the spotlight if she is truly the expert on the topic at hand.
Women also may be hamstrung in acceding to leadership positions by the use of subjective evaluations, said Dr. Johnson. He cited a 2018 analysis of 81,000 performance evaluations by the Harvard Business Review in which the top positive term used to describe men was analytical, while the top positive term used to describe women was compassionate. “All these things go with pay and promotions, and they tend to disadvantage women,” he said.
Dr. Johnson provided two avenues for how men can effectively show up as allies for women in the workplace.
First, start at the top. CEOs and senior men in an organization have a unique opportunity to set an example and talk publicly about supporting and promoting women, said Dr. Johnson.
Second, work at the grassroots level. He encouraged men to educate themselves with gender equity workshops, and act as collaborators. “Don’t tell women how to do gender equity,” he said, but show up, be present, be mindful, and be patient if someone seems not to respond immediately to opportunities for mentoring or sponsorship.
“Claiming ally or mentor status with someone from a nondominant group may invoke power, privilege, or even ownership” without intention, he said. Instead, “Always let others label you and the nature of the relationship [such as ally or mentor].”
For more information about allyship, visit Dr. Johnson’s website, workplaceallies.com.
Men may think they are supportive of women in the workplace, but if you ask women, they say there is a discrepancy, according to W. Brad Johnson, PhD, a clinical psychologist and professor at the United States Naval Academy in Annapolis, Md.
“We may think we are acting as allies to women because we believe in it, but it may not be showing up in the execution,” he said in a presentation at the virtual Advance PHM Gender Equity Conference.
Although women currently account for the majority of medical school students, they make up only 16% of the population of medical school deans, 18% of department chairs, and 25% of full professors, according to 2019 data from the Association of American Medical Colleges, Dr. Johnson said.
The “missing ingredient” in increasing the number of women in medical faculty positions is that women are less mentored. Some barriers to mentorship include men’s concerns that women will take offers of mentorship the wrong way, but “it is incredibly rare for women to make a false accusation” of harassment in a mentorship situation, said Dr. Johnson.
Dr. Johnson offered some guidance for how men can become better allies for women in the workplace through interpersonal allyship, public allyship, and systemic allyship.
Interpersonal allyship and opportunities for mentoring women in medicine start by building trust, friendship, and collegiality between men and women colleagues, Dr. Johnson explained.
He provided some guidance for men to “sharpen their gender intelligence,” which starts with listening. Surveys of women show that they would like male colleagues to be a sounding board, rather than simply offering to jump in with a fix for a problem. “Show humility,” he said, don’t be afraid to ask questions, and don’t assume that a colleague wants something in particular because she is a woman.
“A lot of men get stuck on breaking the ice and getting started with a mentoring conversation,” Dr. Johnson said. One way to is by telling a female colleague who gave an outstanding presentation, or has conducted outstanding research, that you want to keep her in your organization and that she is welcome to talk about her goals. Women appreciate mentoring as “a constellation” and a way to build support, and have one person introduce them to others who can build a network and promote opportunities for leadership. Also, he encouraged men to be open to feedback from female colleagues on how they can be more supportive in the workplace. Sincerity and genuine effort go a long way towards improving gender equity.
Public allyship can take many forms, including putting women center stage to share their own ideas, Dr. Johnson said. Surveys of women show that they often feel dismissed or slighted and not given credit for an idea that was ultimately presented by a male colleague, he noted. Instead, be a female colleague’s biggest fan, and put her in the spotlight if she is truly the expert on the topic at hand.
Women also may be hamstrung in acceding to leadership positions by the use of subjective evaluations, said Dr. Johnson. He cited a 2018 analysis of 81,000 performance evaluations by the Harvard Business Review in which the top positive term used to describe men was analytical, while the top positive term used to describe women was compassionate. “All these things go with pay and promotions, and they tend to disadvantage women,” he said.
Dr. Johnson provided two avenues for how men can effectively show up as allies for women in the workplace.
First, start at the top. CEOs and senior men in an organization have a unique opportunity to set an example and talk publicly about supporting and promoting women, said Dr. Johnson.
Second, work at the grassroots level. He encouraged men to educate themselves with gender equity workshops, and act as collaborators. “Don’t tell women how to do gender equity,” he said, but show up, be present, be mindful, and be patient if someone seems not to respond immediately to opportunities for mentoring or sponsorship.
“Claiming ally or mentor status with someone from a nondominant group may invoke power, privilege, or even ownership” without intention, he said. Instead, “Always let others label you and the nature of the relationship [such as ally or mentor].”
For more information about allyship, visit Dr. Johnson’s website, workplaceallies.com.
Men may think they are supportive of women in the workplace, but if you ask women, they say there is a discrepancy, according to W. Brad Johnson, PhD, a clinical psychologist and professor at the United States Naval Academy in Annapolis, Md.
“We may think we are acting as allies to women because we believe in it, but it may not be showing up in the execution,” he said in a presentation at the virtual Advance PHM Gender Equity Conference.
Although women currently account for the majority of medical school students, they make up only 16% of the population of medical school deans, 18% of department chairs, and 25% of full professors, according to 2019 data from the Association of American Medical Colleges, Dr. Johnson said.
The “missing ingredient” in increasing the number of women in medical faculty positions is that women are less mentored. Some barriers to mentorship include men’s concerns that women will take offers of mentorship the wrong way, but “it is incredibly rare for women to make a false accusation” of harassment in a mentorship situation, said Dr. Johnson.
Dr. Johnson offered some guidance for how men can become better allies for women in the workplace through interpersonal allyship, public allyship, and systemic allyship.
Interpersonal allyship and opportunities for mentoring women in medicine start by building trust, friendship, and collegiality between men and women colleagues, Dr. Johnson explained.
He provided some guidance for men to “sharpen their gender intelligence,” which starts with listening. Surveys of women show that they would like male colleagues to be a sounding board, rather than simply offering to jump in with a fix for a problem. “Show humility,” he said, don’t be afraid to ask questions, and don’t assume that a colleague wants something in particular because she is a woman.
“A lot of men get stuck on breaking the ice and getting started with a mentoring conversation,” Dr. Johnson said. One way to is by telling a female colleague who gave an outstanding presentation, or has conducted outstanding research, that you want to keep her in your organization and that she is welcome to talk about her goals. Women appreciate mentoring as “a constellation” and a way to build support, and have one person introduce them to others who can build a network and promote opportunities for leadership. Also, he encouraged men to be open to feedback from female colleagues on how they can be more supportive in the workplace. Sincerity and genuine effort go a long way towards improving gender equity.
Public allyship can take many forms, including putting women center stage to share their own ideas, Dr. Johnson said. Surveys of women show that they often feel dismissed or slighted and not given credit for an idea that was ultimately presented by a male colleague, he noted. Instead, be a female colleague’s biggest fan, and put her in the spotlight if she is truly the expert on the topic at hand.
Women also may be hamstrung in acceding to leadership positions by the use of subjective evaluations, said Dr. Johnson. He cited a 2018 analysis of 81,000 performance evaluations by the Harvard Business Review in which the top positive term used to describe men was analytical, while the top positive term used to describe women was compassionate. “All these things go with pay and promotions, and they tend to disadvantage women,” he said.
Dr. Johnson provided two avenues for how men can effectively show up as allies for women in the workplace.
First, start at the top. CEOs and senior men in an organization have a unique opportunity to set an example and talk publicly about supporting and promoting women, said Dr. Johnson.
Second, work at the grassroots level. He encouraged men to educate themselves with gender equity workshops, and act as collaborators. “Don’t tell women how to do gender equity,” he said, but show up, be present, be mindful, and be patient if someone seems not to respond immediately to opportunities for mentoring or sponsorship.
“Claiming ally or mentor status with someone from a nondominant group may invoke power, privilege, or even ownership” without intention, he said. Instead, “Always let others label you and the nature of the relationship [such as ally or mentor].”
For more information about allyship, visit Dr. Johnson’s website, workplaceallies.com.
FROM THE ADVANCE PHM GENDER EQUITY CONFERENCE
Painful Psoriasiform Plaques
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).
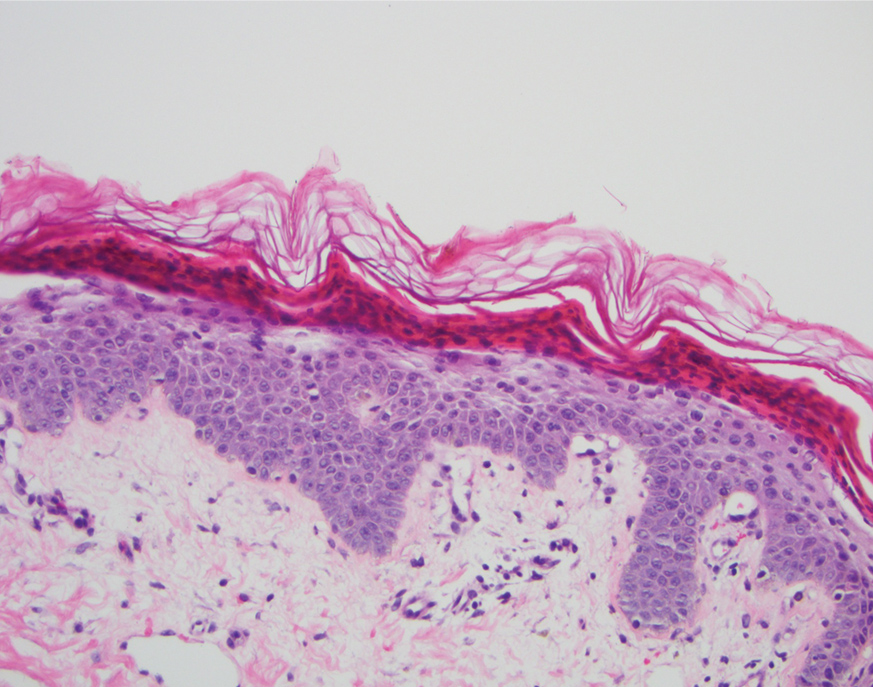
Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).

Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).

Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
A 45-year-old woman presented to the emergency department with a painful skin eruption and malaise of 5 weeks’ duration. She had an orthotopic liver transplant 5 years prior for end-stage liver disease due to mixed nonalcoholic and alcoholic steatohepatitis and was on mycophenolate mofetil and tacrolimus for graft rejection prophylaxis. Her medical history also included Roux-en-Y gastric bypass 15 years prior, alcohol use disorder, hypothyroidism, and depression.
The exanthem began on the legs as pruritic, red, raised, exudative lesions that gradually crusted. Over the 2 weeks prior to the current presentation, the rash became tender as it spread to the feet, thighs, perianal skin, buttocks, and elbows. Triamcinolone ointment prescribed for a presumed nummular dermatitis effected marginal benefit. A review of systems was notable for a 15-pound weight loss over several weeks; lowgrade fever of 3 days’ duration; epigastric abdominal pain; and long-standing, frequent defecation of oily, foul-smelling feces.
Physical examination revealed a combination of flat-topped, violaceous papules and serpiginous, polycyclic, annular plaques coalescing to form larger psoriasiform plaques with hyperkeratotic rims and dusky borders on the dorsal aspect of the feet (top), lateral ankles, legs (bottom), lateral thighs, buttocks, perianal skin, and elbows. Bilateral angular cheilitis, a smooth and fissured tongue, and pitting of all fingernails were noted.
Web of antimicrobials doesn’t hold water
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Cut risedronate drug holiday to under 2 years in older patients
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2021
Customized brain stimulation: New hope for severe depression
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.