User login
Contraception for women taking enzyme-inducing antiepileptics
Topiramate, introduced as an antiepileptic drug (AED), is currently most widely used for prevention of migraine headaches.
Because reproductive-aged women represent a population in which migraines are prevalent, clinicians need guidance to help women taking topiramate make sound contraceptive choices.
Several issues are relevant here. First, women who have migraines with aura should avoid estrogen-containing contraceptive pills, patches, and rings. Instead, progestin-only methods, including the contraceptive implant, may be recommended to patients with migraines.
Second, because topiramate, as with a number of other AEDs, is a teratogen, women using this medication need highly effective contraception. This consideration may also lead clinicians to recommend use of the implant in women with migraines.
Finally, topiramate, along with other AEDs (phenytoin, carbamazepine, barbiturates, primidone, and oxcarbazepine) induces hepatic enzymes, which results in reduced serum contraceptive steroid levels.
Because there is uncertainty regarding the degree to which the use of topiramate reduces serum levels of etonogestrel (the progestin released by the implant), investigators performed a prospective study to assess the pharmacokinetic impact of topiramate in women with the implant.
Ongoing users of contraceptive implants who agreed to use additional nonhormonal contraception were recruited to a 6-week study, during which they took topiramate and periodically had blood drawn.
Overall, use of topiramate was found to lower serum etonogestrel levels from baseline on a dose-related basis. At study completion, almost one-third of study participants were found to have serum progestin levels lower than the threshold associated with predictable ovulation suppression.
The results of this carefully conducted study support guidance from the Centers for Disease Control and Prevention that women seeking contraception and using topiramate or other enzyme-inducing AEDs should be encouraged to use intrauterine devices or injectable contraception. The contraceptive efficacy of these latter methods is not diminished by concomitant use of enzyme inducers.
I am Andrew Kaunitz. Please take care of yourself and each other.
Any views expressed above are the author’s own and do not necessarily reflect the views of WebMD or Medscape.
Andrew M. Kaunitz is a professor and Associate Chairman, department of obstetrics and gynecology, University of Florida, Jacksonville.
A version of this article first appeared on Medscape.com.
Topiramate, introduced as an antiepileptic drug (AED), is currently most widely used for prevention of migraine headaches.
Because reproductive-aged women represent a population in which migraines are prevalent, clinicians need guidance to help women taking topiramate make sound contraceptive choices.
Several issues are relevant here. First, women who have migraines with aura should avoid estrogen-containing contraceptive pills, patches, and rings. Instead, progestin-only methods, including the contraceptive implant, may be recommended to patients with migraines.
Second, because topiramate, as with a number of other AEDs, is a teratogen, women using this medication need highly effective contraception. This consideration may also lead clinicians to recommend use of the implant in women with migraines.
Finally, topiramate, along with other AEDs (phenytoin, carbamazepine, barbiturates, primidone, and oxcarbazepine) induces hepatic enzymes, which results in reduced serum contraceptive steroid levels.
Because there is uncertainty regarding the degree to which the use of topiramate reduces serum levels of etonogestrel (the progestin released by the implant), investigators performed a prospective study to assess the pharmacokinetic impact of topiramate in women with the implant.
Ongoing users of contraceptive implants who agreed to use additional nonhormonal contraception were recruited to a 6-week study, during which they took topiramate and periodically had blood drawn.
Overall, use of topiramate was found to lower serum etonogestrel levels from baseline on a dose-related basis. At study completion, almost one-third of study participants were found to have serum progestin levels lower than the threshold associated with predictable ovulation suppression.
The results of this carefully conducted study support guidance from the Centers for Disease Control and Prevention that women seeking contraception and using topiramate or other enzyme-inducing AEDs should be encouraged to use intrauterine devices or injectable contraception. The contraceptive efficacy of these latter methods is not diminished by concomitant use of enzyme inducers.
I am Andrew Kaunitz. Please take care of yourself and each other.
Any views expressed above are the author’s own and do not necessarily reflect the views of WebMD or Medscape.
Andrew M. Kaunitz is a professor and Associate Chairman, department of obstetrics and gynecology, University of Florida, Jacksonville.
A version of this article first appeared on Medscape.com.
Topiramate, introduced as an antiepileptic drug (AED), is currently most widely used for prevention of migraine headaches.
Because reproductive-aged women represent a population in which migraines are prevalent, clinicians need guidance to help women taking topiramate make sound contraceptive choices.
Several issues are relevant here. First, women who have migraines with aura should avoid estrogen-containing contraceptive pills, patches, and rings. Instead, progestin-only methods, including the contraceptive implant, may be recommended to patients with migraines.
Second, because topiramate, as with a number of other AEDs, is a teratogen, women using this medication need highly effective contraception. This consideration may also lead clinicians to recommend use of the implant in women with migraines.
Finally, topiramate, along with other AEDs (phenytoin, carbamazepine, barbiturates, primidone, and oxcarbazepine) induces hepatic enzymes, which results in reduced serum contraceptive steroid levels.
Because there is uncertainty regarding the degree to which the use of topiramate reduces serum levels of etonogestrel (the progestin released by the implant), investigators performed a prospective study to assess the pharmacokinetic impact of topiramate in women with the implant.
Ongoing users of contraceptive implants who agreed to use additional nonhormonal contraception were recruited to a 6-week study, during which they took topiramate and periodically had blood drawn.
Overall, use of topiramate was found to lower serum etonogestrel levels from baseline on a dose-related basis. At study completion, almost one-third of study participants were found to have serum progestin levels lower than the threshold associated with predictable ovulation suppression.
The results of this carefully conducted study support guidance from the Centers for Disease Control and Prevention that women seeking contraception and using topiramate or other enzyme-inducing AEDs should be encouraged to use intrauterine devices or injectable contraception. The contraceptive efficacy of these latter methods is not diminished by concomitant use of enzyme inducers.
I am Andrew Kaunitz. Please take care of yourself and each other.
Any views expressed above are the author’s own and do not necessarily reflect the views of WebMD or Medscape.
Andrew M. Kaunitz is a professor and Associate Chairman, department of obstetrics and gynecology, University of Florida, Jacksonville.
A version of this article first appeared on Medscape.com.
Belmont Stakes to support initiatives focused on improving the patient experience
There is a variety of ways to support the many impactful new programs the CHEST Foundation will launch in 2022, but one of the most anticipated options is the annual Belmont Stakes Dinner and Auction on June 11 in New York City. This fun-filled evening will include a viewing of the 154th running of “The Championship Track,” a cocktail reception and plated dinner, a silent auction, a rooftop party, and much more.
This year, the dinner and auction will support the CHEST Foundation’s work in patient education and CHEST initiatives to improve patient care. Two areas of focus are disparities in care delivery and improving patients’ quality of life through partnerships designed to encourage earlier diagnosis and treatment.
With these goals in mind, new initiatives include an extension of the 2020 Foundation Listening Tour designed to help clinicians increase trust, equity, and access to health care for patients in traditionally marginalized communities.
In addition, CHEST is partnering with the Three Lakes Foundation on a program dedicated to shortening the time to diagnosis for pulmonary fibrosis (PF). This initiative will bring together pulmonologists and primary care physicians to develop a strategy for identifying PF more quickly, ensuring treatment can begin earlier in the disease trajectory. Early detection of PF is associated with better quality of life for patients, so improving clinicians’ understanding of the signs and symptoms of this rare disease and formulating better guidance for diagnosing it could result in drastic improvements for those living with PF.
To highlight the importance of these efforts, the evening also will include speeches from two patient advocates who have turned their own experiences with living with chronic lung disease into incredible action on behalf of patients.
To learn more about the CHEST Foundation’s initiatives in 2022 and how you can attend the Belmont Stakes Dinner and Auction to support these efforts, visit foundation.chestnet.org.
There is a variety of ways to support the many impactful new programs the CHEST Foundation will launch in 2022, but one of the most anticipated options is the annual Belmont Stakes Dinner and Auction on June 11 in New York City. This fun-filled evening will include a viewing of the 154th running of “The Championship Track,” a cocktail reception and plated dinner, a silent auction, a rooftop party, and much more.
This year, the dinner and auction will support the CHEST Foundation’s work in patient education and CHEST initiatives to improve patient care. Two areas of focus are disparities in care delivery and improving patients’ quality of life through partnerships designed to encourage earlier diagnosis and treatment.
With these goals in mind, new initiatives include an extension of the 2020 Foundation Listening Tour designed to help clinicians increase trust, equity, and access to health care for patients in traditionally marginalized communities.
In addition, CHEST is partnering with the Three Lakes Foundation on a program dedicated to shortening the time to diagnosis for pulmonary fibrosis (PF). This initiative will bring together pulmonologists and primary care physicians to develop a strategy for identifying PF more quickly, ensuring treatment can begin earlier in the disease trajectory. Early detection of PF is associated with better quality of life for patients, so improving clinicians’ understanding of the signs and symptoms of this rare disease and formulating better guidance for diagnosing it could result in drastic improvements for those living with PF.
To highlight the importance of these efforts, the evening also will include speeches from two patient advocates who have turned their own experiences with living with chronic lung disease into incredible action on behalf of patients.
To learn more about the CHEST Foundation’s initiatives in 2022 and how you can attend the Belmont Stakes Dinner and Auction to support these efforts, visit foundation.chestnet.org.
There is a variety of ways to support the many impactful new programs the CHEST Foundation will launch in 2022, but one of the most anticipated options is the annual Belmont Stakes Dinner and Auction on June 11 in New York City. This fun-filled evening will include a viewing of the 154th running of “The Championship Track,” a cocktail reception and plated dinner, a silent auction, a rooftop party, and much more.
This year, the dinner and auction will support the CHEST Foundation’s work in patient education and CHEST initiatives to improve patient care. Two areas of focus are disparities in care delivery and improving patients’ quality of life through partnerships designed to encourage earlier diagnosis and treatment.
With these goals in mind, new initiatives include an extension of the 2020 Foundation Listening Tour designed to help clinicians increase trust, equity, and access to health care for patients in traditionally marginalized communities.
In addition, CHEST is partnering with the Three Lakes Foundation on a program dedicated to shortening the time to diagnosis for pulmonary fibrosis (PF). This initiative will bring together pulmonologists and primary care physicians to develop a strategy for identifying PF more quickly, ensuring treatment can begin earlier in the disease trajectory. Early detection of PF is associated with better quality of life for patients, so improving clinicians’ understanding of the signs and symptoms of this rare disease and formulating better guidance for diagnosing it could result in drastic improvements for those living with PF.
To highlight the importance of these efforts, the evening also will include speeches from two patient advocates who have turned their own experiences with living with chronic lung disease into incredible action on behalf of patients.
To learn more about the CHEST Foundation’s initiatives in 2022 and how you can attend the Belmont Stakes Dinner and Auction to support these efforts, visit foundation.chestnet.org.
What COVID-19 taught us: The challenge of maintaining contingency level care to proactively forestall crisis care
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).
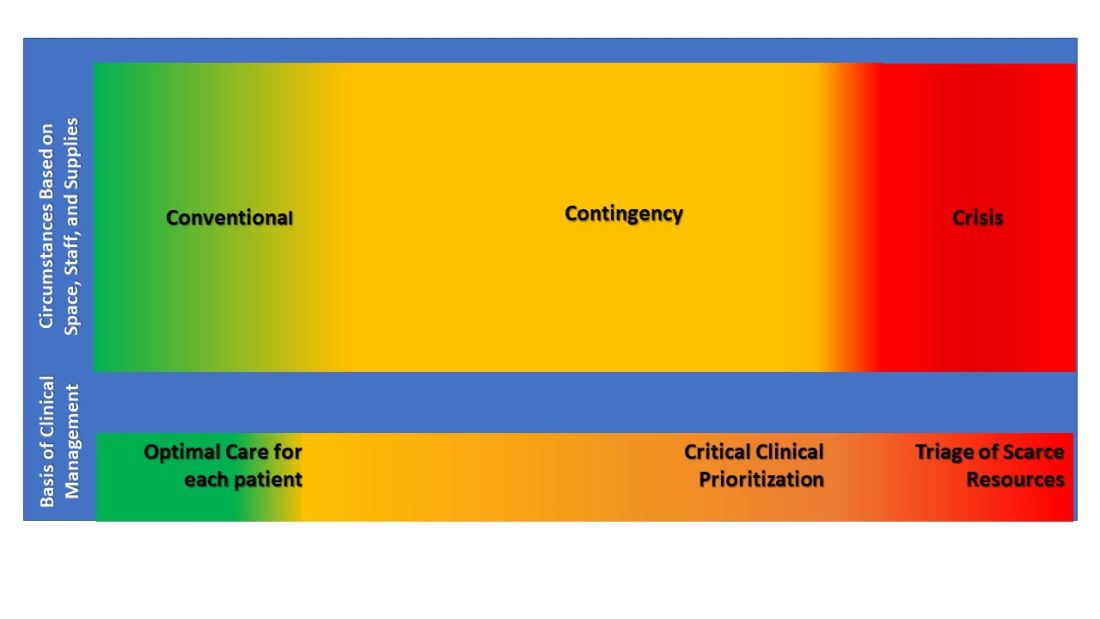
At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).

At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).

At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
Building CHEST 2022: A look into the Scientific Program Committee Meeting
A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2022 is on-track to offer the highest tier of education for those in pulmonary, critical care, and sleep medicine.
Although planning for the meeting started after CHEST 2021 wrapped up, the real magic started to happen a few months ago when the schedule began coming together. In mid-February, members of the Scientific Planning Committee gathered both virtually and in-person at the CHEST headquarters to solidify the schedule for the upcoming CHEST 2022 meeting taking place in Nashville, TN, October 16-19.
The excitement in the room was palpable as committee members gathered for the first time in over a year to plan what will be the first in-person meeting since CHEST 2019 in New Orleans.
Chair of CHEST 2022, Subani Chandra, MD, FCCP, has high expectations for the meeting and is excited for everyone to be together in Nashville. “There is something special about an in-person meeting and my goal for CHEST 2022 is to not only meet the academic needs of the attendees, but also to serve as a chance to recharge after a long haul in managing COVID-19,” says Dr. Chandra. “Many first-time CHEST attendees are fellows and, with the last two meetings being virtual, there are a lot of fellows who have yet to attend a meeting in-person, so that is a big responsibility for us and opportunity for them. We want to make sure they have a fun and productive meeting – learn from the best, understand how to apply the latest research, get to present their work, network, participate, and have fun doing it all!”
With something for everyone in chest medicine, the CHEST 2022 meeting will feature over 200 sessions covering eight curriculum groups:
- Obstructive lung disease
- Sleep
- Chest infections
- Cardiovascular/pulmonary vascular disease
- Pulmonary procedures/lung cancer/cardiothoracic surgery
- Interstitial lung disease/radiology
- Interdisciplinary/practice operations/education
- Critical care
Covering a large breadth of information, the sessions will include the latest trends in COVID-19 care – recommended protocols, surge-planning and best practices; deeper looks into the latest CHEST guidelines – thromboprophylaxis in patients with COVID-19, antithrombotic therapy for VTE disease, and the guidelines for lung cancer screening; and sessions speaking to diversity, inclusion, and equity within medicine, including how lung disease affects populations differently.
Dr. Chandra says diversity was top of mind throughout the planning process. When submitting session ideas, it was noted that “submissions with speakers representing one gender and/or one institution will not be considered,” and that “selection priority will be given to outstanding submissions with proposed speakers who represent diversity of race, ethnicity, and professional status.”
During February’s meeting, as the committee members confirmed each of the sessions, they took the time to ensure every single one had presenters from a variety of backgrounds, including diversity of gender, race, credentialing, and years of experience in medicine.
It was important to the committee that this not be a physician-only meeting, because both CHEST and Pulmonary/Critical Care Medicine feature an array of team members including physicians, advance practice providers, respiratory therapists, nurses and other members of the care team and the sessions will reflect that.
When asked what she hopes attendees will gain from CHEST 2022, Dr. Chandra says, “I want attendees to feel the joy that comes from not only being together, but learning together.”
She continued, “I want this meeting to remind clinicians why they fell in love with medicine and to remember why it is that we do what we do, especially after two grueling years. Attendees should leave feeling reinvigorated and charged with the latest literature and clinical expertise ready to be implemented into practice. Most of all, I want all of the attendees to have fun, because we are there to learn, but CHEST is also about enjoying medicine and those around you. I just cannot wait.”
A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2022 is on-track to offer the highest tier of education for those in pulmonary, critical care, and sleep medicine.
Although planning for the meeting started after CHEST 2021 wrapped up, the real magic started to happen a few months ago when the schedule began coming together. In mid-February, members of the Scientific Planning Committee gathered both virtually and in-person at the CHEST headquarters to solidify the schedule for the upcoming CHEST 2022 meeting taking place in Nashville, TN, October 16-19.
The excitement in the room was palpable as committee members gathered for the first time in over a year to plan what will be the first in-person meeting since CHEST 2019 in New Orleans.
Chair of CHEST 2022, Subani Chandra, MD, FCCP, has high expectations for the meeting and is excited for everyone to be together in Nashville. “There is something special about an in-person meeting and my goal for CHEST 2022 is to not only meet the academic needs of the attendees, but also to serve as a chance to recharge after a long haul in managing COVID-19,” says Dr. Chandra. “Many first-time CHEST attendees are fellows and, with the last two meetings being virtual, there are a lot of fellows who have yet to attend a meeting in-person, so that is a big responsibility for us and opportunity for them. We want to make sure they have a fun and productive meeting – learn from the best, understand how to apply the latest research, get to present their work, network, participate, and have fun doing it all!”
With something for everyone in chest medicine, the CHEST 2022 meeting will feature over 200 sessions covering eight curriculum groups:
- Obstructive lung disease
- Sleep
- Chest infections
- Cardiovascular/pulmonary vascular disease
- Pulmonary procedures/lung cancer/cardiothoracic surgery
- Interstitial lung disease/radiology
- Interdisciplinary/practice operations/education
- Critical care
Covering a large breadth of information, the sessions will include the latest trends in COVID-19 care – recommended protocols, surge-planning and best practices; deeper looks into the latest CHEST guidelines – thromboprophylaxis in patients with COVID-19, antithrombotic therapy for VTE disease, and the guidelines for lung cancer screening; and sessions speaking to diversity, inclusion, and equity within medicine, including how lung disease affects populations differently.
Dr. Chandra says diversity was top of mind throughout the planning process. When submitting session ideas, it was noted that “submissions with speakers representing one gender and/or one institution will not be considered,” and that “selection priority will be given to outstanding submissions with proposed speakers who represent diversity of race, ethnicity, and professional status.”
During February’s meeting, as the committee members confirmed each of the sessions, they took the time to ensure every single one had presenters from a variety of backgrounds, including diversity of gender, race, credentialing, and years of experience in medicine.
It was important to the committee that this not be a physician-only meeting, because both CHEST and Pulmonary/Critical Care Medicine feature an array of team members including physicians, advance practice providers, respiratory therapists, nurses and other members of the care team and the sessions will reflect that.
When asked what she hopes attendees will gain from CHEST 2022, Dr. Chandra says, “I want attendees to feel the joy that comes from not only being together, but learning together.”
She continued, “I want this meeting to remind clinicians why they fell in love with medicine and to remember why it is that we do what we do, especially after two grueling years. Attendees should leave feeling reinvigorated and charged with the latest literature and clinical expertise ready to be implemented into practice. Most of all, I want all of the attendees to have fun, because we are there to learn, but CHEST is also about enjoying medicine and those around you. I just cannot wait.”
A quality educational meeting starts with a great slate of programs tailored to its audience, and CHEST 2022 is on-track to offer the highest tier of education for those in pulmonary, critical care, and sleep medicine.
Although planning for the meeting started after CHEST 2021 wrapped up, the real magic started to happen a few months ago when the schedule began coming together. In mid-February, members of the Scientific Planning Committee gathered both virtually and in-person at the CHEST headquarters to solidify the schedule for the upcoming CHEST 2022 meeting taking place in Nashville, TN, October 16-19.
The excitement in the room was palpable as committee members gathered for the first time in over a year to plan what will be the first in-person meeting since CHEST 2019 in New Orleans.
Chair of CHEST 2022, Subani Chandra, MD, FCCP, has high expectations for the meeting and is excited for everyone to be together in Nashville. “There is something special about an in-person meeting and my goal for CHEST 2022 is to not only meet the academic needs of the attendees, but also to serve as a chance to recharge after a long haul in managing COVID-19,” says Dr. Chandra. “Many first-time CHEST attendees are fellows and, with the last two meetings being virtual, there are a lot of fellows who have yet to attend a meeting in-person, so that is a big responsibility for us and opportunity for them. We want to make sure they have a fun and productive meeting – learn from the best, understand how to apply the latest research, get to present their work, network, participate, and have fun doing it all!”
With something for everyone in chest medicine, the CHEST 2022 meeting will feature over 200 sessions covering eight curriculum groups:
- Obstructive lung disease
- Sleep
- Chest infections
- Cardiovascular/pulmonary vascular disease
- Pulmonary procedures/lung cancer/cardiothoracic surgery
- Interstitial lung disease/radiology
- Interdisciplinary/practice operations/education
- Critical care
Covering a large breadth of information, the sessions will include the latest trends in COVID-19 care – recommended protocols, surge-planning and best practices; deeper looks into the latest CHEST guidelines – thromboprophylaxis in patients with COVID-19, antithrombotic therapy for VTE disease, and the guidelines for lung cancer screening; and sessions speaking to diversity, inclusion, and equity within medicine, including how lung disease affects populations differently.
Dr. Chandra says diversity was top of mind throughout the planning process. When submitting session ideas, it was noted that “submissions with speakers representing one gender and/or one institution will not be considered,” and that “selection priority will be given to outstanding submissions with proposed speakers who represent diversity of race, ethnicity, and professional status.”
During February’s meeting, as the committee members confirmed each of the sessions, they took the time to ensure every single one had presenters from a variety of backgrounds, including diversity of gender, race, credentialing, and years of experience in medicine.
It was important to the committee that this not be a physician-only meeting, because both CHEST and Pulmonary/Critical Care Medicine feature an array of team members including physicians, advance practice providers, respiratory therapists, nurses and other members of the care team and the sessions will reflect that.
When asked what she hopes attendees will gain from CHEST 2022, Dr. Chandra says, “I want attendees to feel the joy that comes from not only being together, but learning together.”
She continued, “I want this meeting to remind clinicians why they fell in love with medicine and to remember why it is that we do what we do, especially after two grueling years. Attendees should leave feeling reinvigorated and charged with the latest literature and clinical expertise ready to be implemented into practice. Most of all, I want all of the attendees to have fun, because we are there to learn, but CHEST is also about enjoying medicine and those around you. I just cannot wait.”
Continuous remote patient monitoring
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
About ABIM’s Longitudinal Knowledge Assessment
Physicians from every specialty have stepped up in extraordinary ways during the pandemic; however, ABIM recognizes that pulmonary disease and critical care physicians, along with hospitalists and infectious disease specialists, have been especially burdened. ABIM has heard from many pulmonary disease and critical care medicine physicians asking for greater flexibility and choice in how they can maintain their board certifications.
For that reason, ABIM has extended deadlines for all Maintenance of Certification (MOC) requirements to 12/31/22 and to 2023 for Critical Care Medicine, Hospital Medicine, Infectious Disease, and Pulmonary Disease.
What assessment options does ABIM offer?
If you haven’t needed to take an MOC exam for a while, you might not be aware of ABIM’s current options and how they might work for you:
- The traditional, 10-year MOC assessment (a point-in-time exam taken at a test center)
- The new Longitudinal Knowledge Assessment (LKATM) (available in 12 specialties including Internal Medicine and Sleep Medicine now, and in Critical Care Medicine and Pulmonary Disease in 2023)
The 2-year Knowledge Check-In was retired at the end of 2021 with the introduction of the LKA.
How the new LKA works
As a longitudinal assessment, the LKA is designed to help you measure your medical knowledge over time and better melds assessment and learning. It consists of a 5-year cycle, during which you’ll be offered 30 questions each quarter, and need to open at least 500 out of 600 questions to meet the LKA Participation Requirement. You can choose not to open up to 100 questions over 5 years, allowing you to take breaks when you need them.
Once enrolled, you can take questions on your laptop, desktop, or smartphone. You’ll also be able to answer questions where and when it’s convenient for you, such as at your home or office – with no need to schedule an appointment or go to a test center. You can use all the same resources you use in practice – journals, apps, and your own personal notes—anything except another person. For most questions, you’ll find out immediately if your answer was correct or not, and you’ll receive a rationale explaining why, along with one or more references.
You’ll have 4 minutes to answer each question and can add extra time if needed by drawing from an annual 30-minute time bank. For each correct answer, you’ll earn 0.2 MOC points, and if you choose to participate in LKA for more than one of your certificates, you’ll have even more opportunities to earn points. In addition, beginning in your second year of participation, interim score reports will give you helpful information to let you know how you’re doing, so you can re-adjust your approach and focus your studies as needed. A pass/fail decision is made at the end of the 5-year cycle.
About eligibility
If you are currently certified in Critical Care Medicine or Pulmonary Disease and had an assessment due in 2020, 2021 or 2022, you don’t need to take an assessment this year and will be eligible to enroll in the LKA in 2023, or you can choose to take the traditional 10-year MOC exam.
Upon enrolling, you will continue to be reported as “Certified” as long as you are meeting the LKA Participation Requirement. If your next assessment isn’t due for a while, you will be able to enroll in the LKA in your assessment due year—not before then.
More information about eligibility can be found in a special section of ABIM’s website.
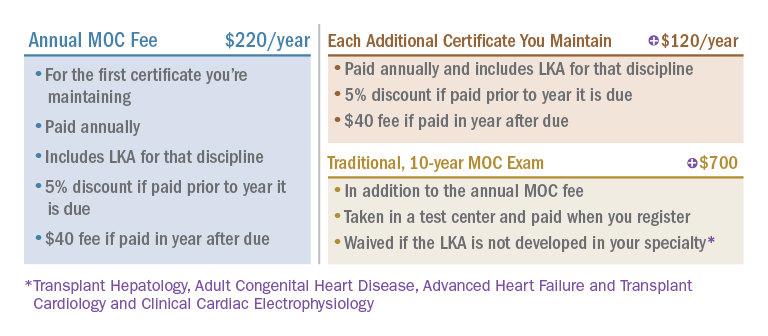
How much does it cost?
ABIM revised its MOC fees in 2022 to provide an option to pay less over time than previously, and the LKA will be included in your annual MOC fee at no additional cost. Here’s how it works:
In closing
Thousands of physicians have already started taking the LKA in 2022 and are reporting positive experiences with it. The ABIM is excited that physicians in additional disciplines, including Critical Care Medicine and Pulmonary Disease, will get to experience it themselves in 2023.
Physicians from every specialty have stepped up in extraordinary ways during the pandemic; however, ABIM recognizes that pulmonary disease and critical care physicians, along with hospitalists and infectious disease specialists, have been especially burdened. ABIM has heard from many pulmonary disease and critical care medicine physicians asking for greater flexibility and choice in how they can maintain their board certifications.
For that reason, ABIM has extended deadlines for all Maintenance of Certification (MOC) requirements to 12/31/22 and to 2023 for Critical Care Medicine, Hospital Medicine, Infectious Disease, and Pulmonary Disease.
What assessment options does ABIM offer?
If you haven’t needed to take an MOC exam for a while, you might not be aware of ABIM’s current options and how they might work for you:
- The traditional, 10-year MOC assessment (a point-in-time exam taken at a test center)
- The new Longitudinal Knowledge Assessment (LKATM) (available in 12 specialties including Internal Medicine and Sleep Medicine now, and in Critical Care Medicine and Pulmonary Disease in 2023)
The 2-year Knowledge Check-In was retired at the end of 2021 with the introduction of the LKA.
How the new LKA works
As a longitudinal assessment, the LKA is designed to help you measure your medical knowledge over time and better melds assessment and learning. It consists of a 5-year cycle, during which you’ll be offered 30 questions each quarter, and need to open at least 500 out of 600 questions to meet the LKA Participation Requirement. You can choose not to open up to 100 questions over 5 years, allowing you to take breaks when you need them.
Once enrolled, you can take questions on your laptop, desktop, or smartphone. You’ll also be able to answer questions where and when it’s convenient for you, such as at your home or office – with no need to schedule an appointment or go to a test center. You can use all the same resources you use in practice – journals, apps, and your own personal notes—anything except another person. For most questions, you’ll find out immediately if your answer was correct or not, and you’ll receive a rationale explaining why, along with one or more references.
You’ll have 4 minutes to answer each question and can add extra time if needed by drawing from an annual 30-minute time bank. For each correct answer, you’ll earn 0.2 MOC points, and if you choose to participate in LKA for more than one of your certificates, you’ll have even more opportunities to earn points. In addition, beginning in your second year of participation, interim score reports will give you helpful information to let you know how you’re doing, so you can re-adjust your approach and focus your studies as needed. A pass/fail decision is made at the end of the 5-year cycle.
About eligibility
If you are currently certified in Critical Care Medicine or Pulmonary Disease and had an assessment due in 2020, 2021 or 2022, you don’t need to take an assessment this year and will be eligible to enroll in the LKA in 2023, or you can choose to take the traditional 10-year MOC exam.
Upon enrolling, you will continue to be reported as “Certified” as long as you are meeting the LKA Participation Requirement. If your next assessment isn’t due for a while, you will be able to enroll in the LKA in your assessment due year—not before then.
More information about eligibility can be found in a special section of ABIM’s website.
How much does it cost?
ABIM revised its MOC fees in 2022 to provide an option to pay less over time than previously, and the LKA will be included in your annual MOC fee at no additional cost. Here’s how it works:

In closing
Thousands of physicians have already started taking the LKA in 2022 and are reporting positive experiences with it. The ABIM is excited that physicians in additional disciplines, including Critical Care Medicine and Pulmonary Disease, will get to experience it themselves in 2023.
Physicians from every specialty have stepped up in extraordinary ways during the pandemic; however, ABIM recognizes that pulmonary disease and critical care physicians, along with hospitalists and infectious disease specialists, have been especially burdened. ABIM has heard from many pulmonary disease and critical care medicine physicians asking for greater flexibility and choice in how they can maintain their board certifications.
For that reason, ABIM has extended deadlines for all Maintenance of Certification (MOC) requirements to 12/31/22 and to 2023 for Critical Care Medicine, Hospital Medicine, Infectious Disease, and Pulmonary Disease.
What assessment options does ABIM offer?
If you haven’t needed to take an MOC exam for a while, you might not be aware of ABIM’s current options and how they might work for you:
- The traditional, 10-year MOC assessment (a point-in-time exam taken at a test center)
- The new Longitudinal Knowledge Assessment (LKATM) (available in 12 specialties including Internal Medicine and Sleep Medicine now, and in Critical Care Medicine and Pulmonary Disease in 2023)
The 2-year Knowledge Check-In was retired at the end of 2021 with the introduction of the LKA.
How the new LKA works
As a longitudinal assessment, the LKA is designed to help you measure your medical knowledge over time and better melds assessment and learning. It consists of a 5-year cycle, during which you’ll be offered 30 questions each quarter, and need to open at least 500 out of 600 questions to meet the LKA Participation Requirement. You can choose not to open up to 100 questions over 5 years, allowing you to take breaks when you need them.
Once enrolled, you can take questions on your laptop, desktop, or smartphone. You’ll also be able to answer questions where and when it’s convenient for you, such as at your home or office – with no need to schedule an appointment or go to a test center. You can use all the same resources you use in practice – journals, apps, and your own personal notes—anything except another person. For most questions, you’ll find out immediately if your answer was correct or not, and you’ll receive a rationale explaining why, along with one or more references.
You’ll have 4 minutes to answer each question and can add extra time if needed by drawing from an annual 30-minute time bank. For each correct answer, you’ll earn 0.2 MOC points, and if you choose to participate in LKA for more than one of your certificates, you’ll have even more opportunities to earn points. In addition, beginning in your second year of participation, interim score reports will give you helpful information to let you know how you’re doing, so you can re-adjust your approach and focus your studies as needed. A pass/fail decision is made at the end of the 5-year cycle.
About eligibility
If you are currently certified in Critical Care Medicine or Pulmonary Disease and had an assessment due in 2020, 2021 or 2022, you don’t need to take an assessment this year and will be eligible to enroll in the LKA in 2023, or you can choose to take the traditional 10-year MOC exam.
Upon enrolling, you will continue to be reported as “Certified” as long as you are meeting the LKA Participation Requirement. If your next assessment isn’t due for a while, you will be able to enroll in the LKA in your assessment due year—not before then.
More information about eligibility can be found in a special section of ABIM’s website.
How much does it cost?
ABIM revised its MOC fees in 2022 to provide an option to pay less over time than previously, and the LKA will be included in your annual MOC fee at no additional cost. Here’s how it works:

In closing
Thousands of physicians have already started taking the LKA in 2022 and are reporting positive experiences with it. The ABIM is excited that physicians in additional disciplines, including Critical Care Medicine and Pulmonary Disease, will get to experience it themselves in 2023.
Patients need Sep-1: Why don’t some doctors like it?
Since its inception, the CMS Sep-1 Core Quality Measure has been unpopular in some circles. It is now under official attack by the American College of Emergency Physicians (ACEP) and the Infectious Diseases Society of America (IDSA), along with a handful of smaller professional societies. These societies appealed the National Quality Forum’s (NQF) 2021 recommendation that the measure be renewed. The NQF is the multidisciplinary and broadly representative group of evaluators who evaluate proposals for CMS-sponsored quality improvement on behalf of the American people and of the Centers for Medicare & Medicaid Services (CMS). Readers of CHEST Physician are likely familiar with core measures, in general, and with Sep-1, in particular. CMS requires hospitals to publicly report their compliance with several Core Quality Measures, and the failure to do so results in across the board reductions in Medicare payments. As of now, no penalties are levied for the degree of compliance but only for failure to report the degree of compliance.
The measure asks, in the main, for hospitals to perform what most physicians can agree should be standard care for patients with sepsis. Depending on whether shock is present, the measure requires:
1. Blood cultures before antibiotics
2. Antibiotics within 3 hours of recognition of sepsis
3. Serum lactate measurement in the first 3 hours and, if increased, a repeat measurement by 6 hours
4. If the patient is hypotensive, 30 mL/kg IV crystalloid within 3 hours, or documentation of why that is not appropriate for the patient
5. If hypotension persists, vasopressors within 6 hours
6. Repeat cardiovascular assessment within 6 hours for patients with shock
If I evaluate these criteria as a patient who has been hospitalized for a serious infection, which I am, they do not seem particularly stringent. In fact, as a patient, I would want my doctors and nurses to act substantially faster than this if I had sepsis or septic shock. If my doctor did not come back in less than 6 hours to check on my shock status, I would be disappointed, to say the least. Nevertheless, some physicians and professional societies see no reason why these should be standards and state that the data underlying them are of low quality. Meanwhile, according to CMS’ own careful evaluation, national compliance with the measures is less than 50%, while being compliant with the measures reduces absolute overall mortality by approximately 4%, from 26.3% to 22.2% (Townsend SR et al. Chest. 2022;161[2]:392-406). This would translate to between 14,000 and 15,000 fewer patients dying from sepsis per year, if all patients received bundled, measure-compliant care. These are patients I don’t care to ignore.
ACEP and IDSA point specifically to the new Surviving Sepsis Campaign Guidelines (SSC) recommendations as evidence that the antibiotic measure is based on low quality evidence (Evans L et al. Crit Care Med. 2021;49[11]:1974-82). In this regard, they are technically correct; the system of evidence review that the SSC panel uses, Grading of Assessment, Recommendations, and Evaluation (GRADE), considers that retrospective analyses, which nearly all of these studies are, can be graded no higher than low quality. Clearly, retrospective studies will never achieve the level of certainty that we achieve with randomized controlled trials, but the NQF, itself, typically views that when a number of well-performed retrospective studies point in the same direction, the level of evidence is at least moderate. After all, just as it would be inappropriate to randomize participants to decades of smoking vs nonsmoking in order prove that smoking causes lung cancer, it is not appropriate to randomize patients with sepsis to receive delayed antibiotics before we accept that such delays are harmful to them.
ACEP and IDSA also assert that the association of early antibiotics with survival is “stronger” for septic shock than for sepsis. In fact, the association is quite strong for both severities of illness. Until it progresses to septic shock, the expected mortality of sepsis is lower, and the percent reduction in mortality is less than for septic shock. However, the opportunity for lives preserved is quite large, because the number of patients with sepsis at presentation is approximately 10 times higher than the number with septic shock at presentation. Antibiotic delays are also associated with progression from infection or sepsis to septic shock (Whiles BB et al. Crit Care Med. 2017;45[4]:623-29; Bisarya R et al. Chest. 2022;161[1]:112-20). Importantly, SSC gave a strong recommendation for all patients with suspected sepsis to receive antibiotics within 3 hours of suspecting sepsis and within 1 hour of suspecting septic shock, a recommendation even stronger than that of Sep-1.
Critics opine that CMS should stop looking at the process measures and focus only on the outcomes of sepsis care. There is a certain attractiveness to this proposition. One could say that it does not matter so much how a hospital achieves lower mortality as long as they do achieve it. However, the question would then become – how low should the mortality rate be? I have a notion that whatever the number, the Sep-1 critics would find it unbearable.
There is a core principle embedded in the Sep-1 process measures, in SSC guidelines, and in the concept of early goal-directed therapy that preceded them: success is not dependent only on what we do but on when we do it. All of you have experienced this. Each of you has attended a professional school, whether medical, nursing, respiratory therapy, etc. None of you showed up unannounced on opening day of the semester and was admitted to that school. All of you garnered the grades, solicited the letters of recommendation, took the entrance exams, and submitted an application. Some of you went to an interview. All of these things were done in a timely fashion; professional schools do not accept incomplete applications or late applications. Doing the right things at the wrong time would have left us all pursuing different careers.
Very early in my career as an attending physician in the ICU, I found myself exasperated by the circumstances of many patients who we received in the ICU with sepsis. I would peruse their medical records and find that they had been septic, ie, had met criteria for severe sepsis, 1 to 2 days before their deterioration to septic shock, yet they had not been diagnosed with sepsis until shock developed. In the ICU, we began resuscitative fluids, ensured appropriate antibiotics, and started vasopressors, but it was often to no avail. The treatments we gave made no difference for many patients, because they were given too late. For me, this was career altering; much of my career since that time has focused on teaching medical personnel how to recognize sepsis, how to give timely and appropriate treatments, and how to keep the data to show when they have done that and when they have not.
Before Sep-1 many, if not most, of the hospitals in the United States had no particular strategy in place to recognize and treat patients with sepsis, even though it was and is the most common cause of death and the costliest condition in American hospitals. Now, most hospitals do have such strategies. Assertions by professional societies that it is difficult to collect the data for Sep-1 reporting are likely true. However, keeping patients safe and alive is a hospital’s primary reason for existing. As long as hospitals are tracking each antibiotic and every liter of fluid so that they can bill for them, my own ears are deaf to hearing that it is too difficult to make sure that we are doing our job. Modifying or eliminating Sep-1 for any reason except data that show we can clearly further improve the outcome for all patients with sepsis is the wrong move to make. So far, other professional societies want to remove elements of Sep-1 without evidence that it would improve our care for patients with sepsis or their outcomes. Thankfully, from the time we proposed the first criteria for diagnosing sepsis, CHEST has promoted what is best for patients, whether it is difficult or not.
Dr. Simpson is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement, including by serving as a senior adviser to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services and an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. Dr. Simpson is the senior medical adviser for Sepsis Alliance, a nationwide patient information and advocacy organization. He is the immediate past president of CHEST.
Since its inception, the CMS Sep-1 Core Quality Measure has been unpopular in some circles. It is now under official attack by the American College of Emergency Physicians (ACEP) and the Infectious Diseases Society of America (IDSA), along with a handful of smaller professional societies. These societies appealed the National Quality Forum’s (NQF) 2021 recommendation that the measure be renewed. The NQF is the multidisciplinary and broadly representative group of evaluators who evaluate proposals for CMS-sponsored quality improvement on behalf of the American people and of the Centers for Medicare & Medicaid Services (CMS). Readers of CHEST Physician are likely familiar with core measures, in general, and with Sep-1, in particular. CMS requires hospitals to publicly report their compliance with several Core Quality Measures, and the failure to do so results in across the board reductions in Medicare payments. As of now, no penalties are levied for the degree of compliance but only for failure to report the degree of compliance.
The measure asks, in the main, for hospitals to perform what most physicians can agree should be standard care for patients with sepsis. Depending on whether shock is present, the measure requires:
1. Blood cultures before antibiotics
2. Antibiotics within 3 hours of recognition of sepsis
3. Serum lactate measurement in the first 3 hours and, if increased, a repeat measurement by 6 hours
4. If the patient is hypotensive, 30 mL/kg IV crystalloid within 3 hours, or documentation of why that is not appropriate for the patient
5. If hypotension persists, vasopressors within 6 hours
6. Repeat cardiovascular assessment within 6 hours for patients with shock
If I evaluate these criteria as a patient who has been hospitalized for a serious infection, which I am, they do not seem particularly stringent. In fact, as a patient, I would want my doctors and nurses to act substantially faster than this if I had sepsis or septic shock. If my doctor did not come back in less than 6 hours to check on my shock status, I would be disappointed, to say the least. Nevertheless, some physicians and professional societies see no reason why these should be standards and state that the data underlying them are of low quality. Meanwhile, according to CMS’ own careful evaluation, national compliance with the measures is less than 50%, while being compliant with the measures reduces absolute overall mortality by approximately 4%, from 26.3% to 22.2% (Townsend SR et al. Chest. 2022;161[2]:392-406). This would translate to between 14,000 and 15,000 fewer patients dying from sepsis per year, if all patients received bundled, measure-compliant care. These are patients I don’t care to ignore.
ACEP and IDSA point specifically to the new Surviving Sepsis Campaign Guidelines (SSC) recommendations as evidence that the antibiotic measure is based on low quality evidence (Evans L et al. Crit Care Med. 2021;49[11]:1974-82). In this regard, they are technically correct; the system of evidence review that the SSC panel uses, Grading of Assessment, Recommendations, and Evaluation (GRADE), considers that retrospective analyses, which nearly all of these studies are, can be graded no higher than low quality. Clearly, retrospective studies will never achieve the level of certainty that we achieve with randomized controlled trials, but the NQF, itself, typically views that when a number of well-performed retrospective studies point in the same direction, the level of evidence is at least moderate. After all, just as it would be inappropriate to randomize participants to decades of smoking vs nonsmoking in order prove that smoking causes lung cancer, it is not appropriate to randomize patients with sepsis to receive delayed antibiotics before we accept that such delays are harmful to them.
ACEP and IDSA also assert that the association of early antibiotics with survival is “stronger” for septic shock than for sepsis. In fact, the association is quite strong for both severities of illness. Until it progresses to septic shock, the expected mortality of sepsis is lower, and the percent reduction in mortality is less than for septic shock. However, the opportunity for lives preserved is quite large, because the number of patients with sepsis at presentation is approximately 10 times higher than the number with septic shock at presentation. Antibiotic delays are also associated with progression from infection or sepsis to septic shock (Whiles BB et al. Crit Care Med. 2017;45[4]:623-29; Bisarya R et al. Chest. 2022;161[1]:112-20). Importantly, SSC gave a strong recommendation for all patients with suspected sepsis to receive antibiotics within 3 hours of suspecting sepsis and within 1 hour of suspecting septic shock, a recommendation even stronger than that of Sep-1.
Critics opine that CMS should stop looking at the process measures and focus only on the outcomes of sepsis care. There is a certain attractiveness to this proposition. One could say that it does not matter so much how a hospital achieves lower mortality as long as they do achieve it. However, the question would then become – how low should the mortality rate be? I have a notion that whatever the number, the Sep-1 critics would find it unbearable.
There is a core principle embedded in the Sep-1 process measures, in SSC guidelines, and in the concept of early goal-directed therapy that preceded them: success is not dependent only on what we do but on when we do it. All of you have experienced this. Each of you has attended a professional school, whether medical, nursing, respiratory therapy, etc. None of you showed up unannounced on opening day of the semester and was admitted to that school. All of you garnered the grades, solicited the letters of recommendation, took the entrance exams, and submitted an application. Some of you went to an interview. All of these things were done in a timely fashion; professional schools do not accept incomplete applications or late applications. Doing the right things at the wrong time would have left us all pursuing different careers.
Very early in my career as an attending physician in the ICU, I found myself exasperated by the circumstances of many patients who we received in the ICU with sepsis. I would peruse their medical records and find that they had been septic, ie, had met criteria for severe sepsis, 1 to 2 days before their deterioration to septic shock, yet they had not been diagnosed with sepsis until shock developed. In the ICU, we began resuscitative fluids, ensured appropriate antibiotics, and started vasopressors, but it was often to no avail. The treatments we gave made no difference for many patients, because they were given too late. For me, this was career altering; much of my career since that time has focused on teaching medical personnel how to recognize sepsis, how to give timely and appropriate treatments, and how to keep the data to show when they have done that and when they have not.
Before Sep-1 many, if not most, of the hospitals in the United States had no particular strategy in place to recognize and treat patients with sepsis, even though it was and is the most common cause of death and the costliest condition in American hospitals. Now, most hospitals do have such strategies. Assertions by professional societies that it is difficult to collect the data for Sep-1 reporting are likely true. However, keeping patients safe and alive is a hospital’s primary reason for existing. As long as hospitals are tracking each antibiotic and every liter of fluid so that they can bill for them, my own ears are deaf to hearing that it is too difficult to make sure that we are doing our job. Modifying or eliminating Sep-1 for any reason except data that show we can clearly further improve the outcome for all patients with sepsis is the wrong move to make. So far, other professional societies want to remove elements of Sep-1 without evidence that it would improve our care for patients with sepsis or their outcomes. Thankfully, from the time we proposed the first criteria for diagnosing sepsis, CHEST has promoted what is best for patients, whether it is difficult or not.
Dr. Simpson is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement, including by serving as a senior adviser to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services and an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. Dr. Simpson is the senior medical adviser for Sepsis Alliance, a nationwide patient information and advocacy organization. He is the immediate past president of CHEST.
Since its inception, the CMS Sep-1 Core Quality Measure has been unpopular in some circles. It is now under official attack by the American College of Emergency Physicians (ACEP) and the Infectious Diseases Society of America (IDSA), along with a handful of smaller professional societies. These societies appealed the National Quality Forum’s (NQF) 2021 recommendation that the measure be renewed. The NQF is the multidisciplinary and broadly representative group of evaluators who evaluate proposals for CMS-sponsored quality improvement on behalf of the American people and of the Centers for Medicare & Medicaid Services (CMS). Readers of CHEST Physician are likely familiar with core measures, in general, and with Sep-1, in particular. CMS requires hospitals to publicly report their compliance with several Core Quality Measures, and the failure to do so results in across the board reductions in Medicare payments. As of now, no penalties are levied for the degree of compliance but only for failure to report the degree of compliance.
The measure asks, in the main, for hospitals to perform what most physicians can agree should be standard care for patients with sepsis. Depending on whether shock is present, the measure requires:
1. Blood cultures before antibiotics
2. Antibiotics within 3 hours of recognition of sepsis
3. Serum lactate measurement in the first 3 hours and, if increased, a repeat measurement by 6 hours
4. If the patient is hypotensive, 30 mL/kg IV crystalloid within 3 hours, or documentation of why that is not appropriate for the patient
5. If hypotension persists, vasopressors within 6 hours
6. Repeat cardiovascular assessment within 6 hours for patients with shock
If I evaluate these criteria as a patient who has been hospitalized for a serious infection, which I am, they do not seem particularly stringent. In fact, as a patient, I would want my doctors and nurses to act substantially faster than this if I had sepsis or septic shock. If my doctor did not come back in less than 6 hours to check on my shock status, I would be disappointed, to say the least. Nevertheless, some physicians and professional societies see no reason why these should be standards and state that the data underlying them are of low quality. Meanwhile, according to CMS’ own careful evaluation, national compliance with the measures is less than 50%, while being compliant with the measures reduces absolute overall mortality by approximately 4%, from 26.3% to 22.2% (Townsend SR et al. Chest. 2022;161[2]:392-406). This would translate to between 14,000 and 15,000 fewer patients dying from sepsis per year, if all patients received bundled, measure-compliant care. These are patients I don’t care to ignore.
ACEP and IDSA point specifically to the new Surviving Sepsis Campaign Guidelines (SSC) recommendations as evidence that the antibiotic measure is based on low quality evidence (Evans L et al. Crit Care Med. 2021;49[11]:1974-82). In this regard, they are technically correct; the system of evidence review that the SSC panel uses, Grading of Assessment, Recommendations, and Evaluation (GRADE), considers that retrospective analyses, which nearly all of these studies are, can be graded no higher than low quality. Clearly, retrospective studies will never achieve the level of certainty that we achieve with randomized controlled trials, but the NQF, itself, typically views that when a number of well-performed retrospective studies point in the same direction, the level of evidence is at least moderate. After all, just as it would be inappropriate to randomize participants to decades of smoking vs nonsmoking in order prove that smoking causes lung cancer, it is not appropriate to randomize patients with sepsis to receive delayed antibiotics before we accept that such delays are harmful to them.
ACEP and IDSA also assert that the association of early antibiotics with survival is “stronger” for septic shock than for sepsis. In fact, the association is quite strong for both severities of illness. Until it progresses to septic shock, the expected mortality of sepsis is lower, and the percent reduction in mortality is less than for septic shock. However, the opportunity for lives preserved is quite large, because the number of patients with sepsis at presentation is approximately 10 times higher than the number with septic shock at presentation. Antibiotic delays are also associated with progression from infection or sepsis to septic shock (Whiles BB et al. Crit Care Med. 2017;45[4]:623-29; Bisarya R et al. Chest. 2022;161[1]:112-20). Importantly, SSC gave a strong recommendation for all patients with suspected sepsis to receive antibiotics within 3 hours of suspecting sepsis and within 1 hour of suspecting septic shock, a recommendation even stronger than that of Sep-1.
Critics opine that CMS should stop looking at the process measures and focus only on the outcomes of sepsis care. There is a certain attractiveness to this proposition. One could say that it does not matter so much how a hospital achieves lower mortality as long as they do achieve it. However, the question would then become – how low should the mortality rate be? I have a notion that whatever the number, the Sep-1 critics would find it unbearable.
There is a core principle embedded in the Sep-1 process measures, in SSC guidelines, and in the concept of early goal-directed therapy that preceded them: success is not dependent only on what we do but on when we do it. All of you have experienced this. Each of you has attended a professional school, whether medical, nursing, respiratory therapy, etc. None of you showed up unannounced on opening day of the semester and was admitted to that school. All of you garnered the grades, solicited the letters of recommendation, took the entrance exams, and submitted an application. Some of you went to an interview. All of these things were done in a timely fashion; professional schools do not accept incomplete applications or late applications. Doing the right things at the wrong time would have left us all pursuing different careers.
Very early in my career as an attending physician in the ICU, I found myself exasperated by the circumstances of many patients who we received in the ICU with sepsis. I would peruse their medical records and find that they had been septic, ie, had met criteria for severe sepsis, 1 to 2 days before their deterioration to septic shock, yet they had not been diagnosed with sepsis until shock developed. In the ICU, we began resuscitative fluids, ensured appropriate antibiotics, and started vasopressors, but it was often to no avail. The treatments we gave made no difference for many patients, because they were given too late. For me, this was career altering; much of my career since that time has focused on teaching medical personnel how to recognize sepsis, how to give timely and appropriate treatments, and how to keep the data to show when they have done that and when they have not.
Before Sep-1 many, if not most, of the hospitals in the United States had no particular strategy in place to recognize and treat patients with sepsis, even though it was and is the most common cause of death and the costliest condition in American hospitals. Now, most hospitals do have such strategies. Assertions by professional societies that it is difficult to collect the data for Sep-1 reporting are likely true. However, keeping patients safe and alive is a hospital’s primary reason for existing. As long as hospitals are tracking each antibiotic and every liter of fluid so that they can bill for them, my own ears are deaf to hearing that it is too difficult to make sure that we are doing our job. Modifying or eliminating Sep-1 for any reason except data that show we can clearly further improve the outcome for all patients with sepsis is the wrong move to make. So far, other professional societies want to remove elements of Sep-1 without evidence that it would improve our care for patients with sepsis or their outcomes. Thankfully, from the time we proposed the first criteria for diagnosing sepsis, CHEST has promoted what is best for patients, whether it is difficult or not.
Dr. Simpson is a pulmonologist and intensivist with an extensive background in sepsis and in critical care quality improvement, including by serving as a senior adviser to the Solving Sepsis initiative of the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services and an author of the 2016 and 2020 updates of the Surviving Sepsis Campaign Guidelines. Dr. Simpson is the senior medical adviser for Sepsis Alliance, a nationwide patient information and advocacy organization. He is the immediate past president of CHEST.
CHEST in the news
CHEST works to provide opportunities for members to serve as expert sources for both mainstream and trade media to create a stronger voice for members in pulmonary, critical care, and sleep medicine.
Below are media coverage highlights from the past few months that work to expand awareness of CHEST and to promote the expertise of CHEST members in the media.
Improving NIV access for patients with COPD
In December, Pulmonology Advisor covered recommendations from the noninvasive ventilation Technical Expert Panel report published in the journal CHEST® by The American College of Chest Physicians, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society.
The article shares that, in the United States, patients with COPD are often prescribed home mechanical ventilators rather than more appropriate devices, due largely to current Centers for Medicare & Medicaid Services (CMS) policies that do not always take into account unique complexities of patients’ conditions.
In addition to the recommendations covered in Optimal NIV Medicare Access Promotion: Patients With COPD, the Technical Expert Panel also published reports on patients with Obstructive Sleep Apnea, patients with Central Sleep Apnea, patients with Hypoventilation Syndromes, and patients with Thoracic Restrictive Disorders in the journal CHEST.
The full article, Expert Panel Guidelines Promote Access to In-Home NIV for Patients With COPD, can be found on the Pulmonology Advisor website.
OSA and cardiovascular mortality
A journal CHEST® article, “A Validation Study of Four Different Cluster Analyses of OSA and the Incidence of Cardiovascular Mortality in a Hispanic Population,” by Gonzalo Labarca, MD, et al. was featured in a Healio Pulmonology article.
The research showed an association between excessive sleepiness and increased risk for cardiovascular mortality in Hispanic adults with moderate to severe Obstructive Sleep Apnea and, in the article, Dr. Labarca says, “The Latino population is underrepresented in the scientific literature. Therefore, validation data regarding novel approaches to better identify a subtype of OSA patients at high risk of CV mortality is strongly needed.”
The full article, Risk for CV Mortality Elevated in Hispanic Adults with OSA, Excessive Sleepiness, can be found on the Healio website.
Member in the news:
Chair of the CHEST COVID-19 Task Force, Ryan Maves, MD , joined New York Times podcast, “The Daily” to discuss how the omicron COVID-19 surge was different than previous surges because unvaccinated deaths are skewing younger. During the podcast, Dr. Maves said, “You know, many more [unvaccinated] people in their 40s and 50s are dying. And it’s a grim feeling, watching people who are your own age and maybe not that much older than you die of an entirely preventable illness.” The full podcast, This COVID Surge Feels Different can be found on the New York Times website.
CHEST news
CHEST regularly issues statements and press releases on a variety of topics, including closing the synthetic nicotine loophole and requests for Congress to extend telehealth services.
For all recent CHEST News, including these statements, visit the CHEST Newsroom at chestnet.org, and follow the hashtag #CHESTNews on Twitter.
If you have been included in a recent news article and would like it to be featured, send the coverage to [email protected].
CHEST works to provide opportunities for members to serve as expert sources for both mainstream and trade media to create a stronger voice for members in pulmonary, critical care, and sleep medicine.
Below are media coverage highlights from the past few months that work to expand awareness of CHEST and to promote the expertise of CHEST members in the media.
Improving NIV access for patients with COPD
In December, Pulmonology Advisor covered recommendations from the noninvasive ventilation Technical Expert Panel report published in the journal CHEST® by The American College of Chest Physicians, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society.
The article shares that, in the United States, patients with COPD are often prescribed home mechanical ventilators rather than more appropriate devices, due largely to current Centers for Medicare & Medicaid Services (CMS) policies that do not always take into account unique complexities of patients’ conditions.
In addition to the recommendations covered in Optimal NIV Medicare Access Promotion: Patients With COPD, the Technical Expert Panel also published reports on patients with Obstructive Sleep Apnea, patients with Central Sleep Apnea, patients with Hypoventilation Syndromes, and patients with Thoracic Restrictive Disorders in the journal CHEST.
The full article, Expert Panel Guidelines Promote Access to In-Home NIV for Patients With COPD, can be found on the Pulmonology Advisor website.
OSA and cardiovascular mortality
A journal CHEST® article, “A Validation Study of Four Different Cluster Analyses of OSA and the Incidence of Cardiovascular Mortality in a Hispanic Population,” by Gonzalo Labarca, MD, et al. was featured in a Healio Pulmonology article.
The research showed an association between excessive sleepiness and increased risk for cardiovascular mortality in Hispanic adults with moderate to severe Obstructive Sleep Apnea and, in the article, Dr. Labarca says, “The Latino population is underrepresented in the scientific literature. Therefore, validation data regarding novel approaches to better identify a subtype of OSA patients at high risk of CV mortality is strongly needed.”
The full article, Risk for CV Mortality Elevated in Hispanic Adults with OSA, Excessive Sleepiness, can be found on the Healio website.
Member in the news:
Chair of the CHEST COVID-19 Task Force, Ryan Maves, MD , joined New York Times podcast, “The Daily” to discuss how the omicron COVID-19 surge was different than previous surges because unvaccinated deaths are skewing younger. During the podcast, Dr. Maves said, “You know, many more [unvaccinated] people in their 40s and 50s are dying. And it’s a grim feeling, watching people who are your own age and maybe not that much older than you die of an entirely preventable illness.” The full podcast, This COVID Surge Feels Different can be found on the New York Times website.
CHEST news
CHEST regularly issues statements and press releases on a variety of topics, including closing the synthetic nicotine loophole and requests for Congress to extend telehealth services.
For all recent CHEST News, including these statements, visit the CHEST Newsroom at chestnet.org, and follow the hashtag #CHESTNews on Twitter.
If you have been included in a recent news article and would like it to be featured, send the coverage to [email protected].
CHEST works to provide opportunities for members to serve as expert sources for both mainstream and trade media to create a stronger voice for members in pulmonary, critical care, and sleep medicine.
Below are media coverage highlights from the past few months that work to expand awareness of CHEST and to promote the expertise of CHEST members in the media.
Improving NIV access for patients with COPD
In December, Pulmonology Advisor covered recommendations from the noninvasive ventilation Technical Expert Panel report published in the journal CHEST® by The American College of Chest Physicians, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society.
The article shares that, in the United States, patients with COPD are often prescribed home mechanical ventilators rather than more appropriate devices, due largely to current Centers for Medicare & Medicaid Services (CMS) policies that do not always take into account unique complexities of patients’ conditions.
In addition to the recommendations covered in Optimal NIV Medicare Access Promotion: Patients With COPD, the Technical Expert Panel also published reports on patients with Obstructive Sleep Apnea, patients with Central Sleep Apnea, patients with Hypoventilation Syndromes, and patients with Thoracic Restrictive Disorders in the journal CHEST.
The full article, Expert Panel Guidelines Promote Access to In-Home NIV for Patients With COPD, can be found on the Pulmonology Advisor website.
OSA and cardiovascular mortality
A journal CHEST® article, “A Validation Study of Four Different Cluster Analyses of OSA and the Incidence of Cardiovascular Mortality in a Hispanic Population,” by Gonzalo Labarca, MD, et al. was featured in a Healio Pulmonology article.
The research showed an association between excessive sleepiness and increased risk for cardiovascular mortality in Hispanic adults with moderate to severe Obstructive Sleep Apnea and, in the article, Dr. Labarca says, “The Latino population is underrepresented in the scientific literature. Therefore, validation data regarding novel approaches to better identify a subtype of OSA patients at high risk of CV mortality is strongly needed.”
The full article, Risk for CV Mortality Elevated in Hispanic Adults with OSA, Excessive Sleepiness, can be found on the Healio website.
Member in the news:
Chair of the CHEST COVID-19 Task Force, Ryan Maves, MD , joined New York Times podcast, “The Daily” to discuss how the omicron COVID-19 surge was different than previous surges because unvaccinated deaths are skewing younger. During the podcast, Dr. Maves said, “You know, many more [unvaccinated] people in their 40s and 50s are dying. And it’s a grim feeling, watching people who are your own age and maybe not that much older than you die of an entirely preventable illness.” The full podcast, This COVID Surge Feels Different can be found on the New York Times website.
CHEST news
CHEST regularly issues statements and press releases on a variety of topics, including closing the synthetic nicotine loophole and requests for Congress to extend telehealth services.
For all recent CHEST News, including these statements, visit the CHEST Newsroom at chestnet.org, and follow the hashtag #CHESTNews on Twitter.
If you have been included in a recent news article and would like it to be featured, send the coverage to [email protected].
Building trust together
During the fall of 2020, the CHEST Foundation launched a Listening Tour in areas of the United States that were experiencing disproportionate incidents and mortality from COVID-19. This program was initiated to gain insights in order toand identify solutions to combat lung health inequities among marginalized communities. The COVID-19 pandemic has exacerbated health disparities in America. Underserved communities, communities with higher rates of poverty, and communities of color have suffered disproportionate rates of illness and mortality due to COVID-19.
Even before the COVID-19 pandemic, underserved communities were impacted disproportionately by four of the most common lung diseases: asthma, chronic obstructive pulmonary diseaseCOPD, interstitial lung disease, and lung cancer. Inequities in care and health outcomes are well documented. Inequities are due to a multitude of factors, including socioeconomic status, environmental issues such as air populationpollution, and issues that impact access to care, such as individuals being uninsured or under insured, and a lack of specialists in underserved communities.
The CHEST Foundation selected Listening Tour cities based on a number of criteria, including documented inequities in lung health and prevalence of the predominant lung diseases. Listening Tour events were held virtually in Jackson, MS; New York, NY; Chicago, IL; South Texas; and the US Southwest. In each location, the CHEST Foundation approached community leaders, clinicians, patients, and families to participate. Individual interviews focused on lung health experiences, positive and negative; needs from clinicians, patients, families, and community leaders; and help actually received (or not) based on these needs.
A theme that emerged centered on the importance trust plays in the patient/clinician relationship. Barriers to the establishment of trust as expressed by patients related to:
- Perceived dismissive attitudes among physicians
- Lack of understanding and/or appreciation about social determinants of health
- Overuse of highly technical/medical terminology that can be intimidating to patients
- General cultural and philosophical differences that may contribute to implicit biases
Gaining trust and building rapport among patients is not only limited to key findings from the Listening Tour but also corroborated through peer- reviewed studies. Many studies have documented that trust is the foundation on which patient/clinician relationshipss are built and without it, patients are less likely to maintain adherence to treatment plans, miss appointments, minimize sharing information about their symptoms, and suffer from poorer health and overall quality of life.
In response, the CHEST Foundation is proposing a project with the aim of broader replication based upon key findings. Building trust and developing rapport with patients areis key in creating an environment where they are active participants in their care. An empathetic care training model will provide clinicians with an understanding of the barriers that exist and the tools needed to establish trust with their patients.
The major components of the project include:
1. Development and standardization of a culturally competent toolkit for use during the first five 5 minutes of clinician/patient encounters
2. Creating Creation of education on the tool and training clinicians that who will pilot the tool in health care clinics/medical institutions and collect data on its impact
3. Implementation of the tool use during clinician/patient visits and data collection
4. Data analysis and synthesis of findings for use in refinement and scalability for broader impact
Future plans include scaling the project to additional sites and health care settings; disseminating the culturally competent tool along with education for its utilization to CHEST’s membership and to a larger audience of health care providers; and sharing results and lessons learned. The CHEST Foundation is hoping to build a national, sustainable program that helps achieve health equity, but in order to achieve this, we need your help. Make a donation, and join the CHEST Foundation as we embark on a bold new initiative to build trust, identify and remove barriers, and promote health care access for all in order to help fight lung disease. Together, we will build trust and understanding within communities, specifically between patients, their families, their caregivers, and their clinicians.
During the fall of 2020, the CHEST Foundation launched a Listening Tour in areas of the United States that were experiencing disproportionate incidents and mortality from COVID-19. This program was initiated to gain insights in order toand identify solutions to combat lung health inequities among marginalized communities. The COVID-19 pandemic has exacerbated health disparities in America. Underserved communities, communities with higher rates of poverty, and communities of color have suffered disproportionate rates of illness and mortality due to COVID-19.
Even before the COVID-19 pandemic, underserved communities were impacted disproportionately by four of the most common lung diseases: asthma, chronic obstructive pulmonary diseaseCOPD, interstitial lung disease, and lung cancer. Inequities in care and health outcomes are well documented. Inequities are due to a multitude of factors, including socioeconomic status, environmental issues such as air populationpollution, and issues that impact access to care, such as individuals being uninsured or under insured, and a lack of specialists in underserved communities.
The CHEST Foundation selected Listening Tour cities based on a number of criteria, including documented inequities in lung health and prevalence of the predominant lung diseases. Listening Tour events were held virtually in Jackson, MS; New York, NY; Chicago, IL; South Texas; and the US Southwest. In each location, the CHEST Foundation approached community leaders, clinicians, patients, and families to participate. Individual interviews focused on lung health experiences, positive and negative; needs from clinicians, patients, families, and community leaders; and help actually received (or not) based on these needs.
A theme that emerged centered on the importance trust plays in the patient/clinician relationship. Barriers to the establishment of trust as expressed by patients related to:
- Perceived dismissive attitudes among physicians
- Lack of understanding and/or appreciation about social determinants of health
- Overuse of highly technical/medical terminology that can be intimidating to patients
- General cultural and philosophical differences that may contribute to implicit biases
Gaining trust and building rapport among patients is not only limited to key findings from the Listening Tour but also corroborated through peer- reviewed studies. Many studies have documented that trust is the foundation on which patient/clinician relationshipss are built and without it, patients are less likely to maintain adherence to treatment plans, miss appointments, minimize sharing information about their symptoms, and suffer from poorer health and overall quality of life.
In response, the CHEST Foundation is proposing a project with the aim of broader replication based upon key findings. Building trust and developing rapport with patients areis key in creating an environment where they are active participants in their care. An empathetic care training model will provide clinicians with an understanding of the barriers that exist and the tools needed to establish trust with their patients.
The major components of the project include:
1. Development and standardization of a culturally competent toolkit for use during the first five 5 minutes of clinician/patient encounters
2. Creating Creation of education on the tool and training clinicians that who will pilot the tool in health care clinics/medical institutions and collect data on its impact
3. Implementation of the tool use during clinician/patient visits and data collection
4. Data analysis and synthesis of findings for use in refinement and scalability for broader impact
Future plans include scaling the project to additional sites and health care settings; disseminating the culturally competent tool along with education for its utilization to CHEST’s membership and to a larger audience of health care providers; and sharing results and lessons learned. The CHEST Foundation is hoping to build a national, sustainable program that helps achieve health equity, but in order to achieve this, we need your help. Make a donation, and join the CHEST Foundation as we embark on a bold new initiative to build trust, identify and remove barriers, and promote health care access for all in order to help fight lung disease. Together, we will build trust and understanding within communities, specifically between patients, their families, their caregivers, and their clinicians.
During the fall of 2020, the CHEST Foundation launched a Listening Tour in areas of the United States that were experiencing disproportionate incidents and mortality from COVID-19. This program was initiated to gain insights in order toand identify solutions to combat lung health inequities among marginalized communities. The COVID-19 pandemic has exacerbated health disparities in America. Underserved communities, communities with higher rates of poverty, and communities of color have suffered disproportionate rates of illness and mortality due to COVID-19.
Even before the COVID-19 pandemic, underserved communities were impacted disproportionately by four of the most common lung diseases: asthma, chronic obstructive pulmonary diseaseCOPD, interstitial lung disease, and lung cancer. Inequities in care and health outcomes are well documented. Inequities are due to a multitude of factors, including socioeconomic status, environmental issues such as air populationpollution, and issues that impact access to care, such as individuals being uninsured or under insured, and a lack of specialists in underserved communities.
The CHEST Foundation selected Listening Tour cities based on a number of criteria, including documented inequities in lung health and prevalence of the predominant lung diseases. Listening Tour events were held virtually in Jackson, MS; New York, NY; Chicago, IL; South Texas; and the US Southwest. In each location, the CHEST Foundation approached community leaders, clinicians, patients, and families to participate. Individual interviews focused on lung health experiences, positive and negative; needs from clinicians, patients, families, and community leaders; and help actually received (or not) based on these needs.
A theme that emerged centered on the importance trust plays in the patient/clinician relationship. Barriers to the establishment of trust as expressed by patients related to:
- Perceived dismissive attitudes among physicians
- Lack of understanding and/or appreciation about social determinants of health
- Overuse of highly technical/medical terminology that can be intimidating to patients
- General cultural and philosophical differences that may contribute to implicit biases
Gaining trust and building rapport among patients is not only limited to key findings from the Listening Tour but also corroborated through peer- reviewed studies. Many studies have documented that trust is the foundation on which patient/clinician relationshipss are built and without it, patients are less likely to maintain adherence to treatment plans, miss appointments, minimize sharing information about their symptoms, and suffer from poorer health and overall quality of life.
In response, the CHEST Foundation is proposing a project with the aim of broader replication based upon key findings. Building trust and developing rapport with patients areis key in creating an environment where they are active participants in their care. An empathetic care training model will provide clinicians with an understanding of the barriers that exist and the tools needed to establish trust with their patients.
The major components of the project include:
1. Development and standardization of a culturally competent toolkit for use during the first five 5 minutes of clinician/patient encounters
2. Creating Creation of education on the tool and training clinicians that who will pilot the tool in health care clinics/medical institutions and collect data on its impact
3. Implementation of the tool use during clinician/patient visits and data collection
4. Data analysis and synthesis of findings for use in refinement and scalability for broader impact
Future plans include scaling the project to additional sites and health care settings; disseminating the culturally competent tool along with education for its utilization to CHEST’s membership and to a larger audience of health care providers; and sharing results and lessons learned. The CHEST Foundation is hoping to build a national, sustainable program that helps achieve health equity, but in order to achieve this, we need your help. Make a donation, and join the CHEST Foundation as we embark on a bold new initiative to build trust, identify and remove barriers, and promote health care access for all in order to help fight lung disease. Together, we will build trust and understanding within communities, specifically between patients, their families, their caregivers, and their clinicians.
Asthma, IPF, mechanical ventilation and more...
Airway disorders network: Asthma and COPD section
Betting on asthma: The over and under of diagnosis
Asthma is one of the major chronic respiratory diseases worldwide (WHO 2020), yet it is a clinical syndrome that lacks a consensus on its definition, is comprised of nonspecific respiratory symptoms, and is without a gold standard diagnostic test or a set guideline on confirmation of bronchial hyperresponsiveness (Sá-Sousa A et al. Clin Transl Allergy. 2014 Aug 4;4:24). In addition, once adequately treated, there is an absence of an algorithm to diagnose disease remission (Aaron SD et al. Am J Respir Crit Care Med. 2018 Oct 15;198[8]:1012-20). It is estimated that 20%-70% of people with asthma worldwide across the spectrum of all ages remain undiagnosed.
Spirometry and bronchoprovocation challenges with fixed cut-off values demonstrate reduced sensitivity with day-to-day, diurnal, and long-term variation in airflow obstruction, inflammation, and bronchial hyperresponsiveness (Wang R et al. Thorax. 2021 Jun;76[6]:624-31). Inflammatory biomarkers like fractional exhaled nitric oxide (FeNO) have higher specificity but are subject to diurnal variation and confounding diagnoses.
Overdiagnosis of asthma can result in lost opportunity to diagnose significant cardiopulmonary diseases, unnecessary escalation of the asthma treatment regimen for poorly controlled respiratory symptoms, potential for medication adverse effects, and, increased cost burden to the patient and to the health care system (Aaron SD et al. JAMA. 2017;317:269-79; Shaw D et al. Prim Care Respir J. 2012;21:283-7). Among the newly physician-diagnosed asthmatics, <50% have spirometry performed within 1 year of diagnosis (Sokol KC et al. Am J Med. 2015 May;128[5]:502-8). Spirometry was further underutilized with limit on aerosol-generating procedures during COVID-19 pandemic (Kankaanranta H et al. J Allergy Clin Immunol Pract. 2021 Dec;9[12]:4252-3); 30%-35% obese and nonobese patients with physician-diagnosed asthma did not have current asthma when objectively assessed for airflow limitation (Aaron SD et al. JAMA. 2017;317:269-79; van Huisstede A, et al. Respir Med. 2013;107:1356-64).
Clinical remission is greater in early-onset asthma as compared with late-onset asthma (De Marco R et al. J Allergy Clin Immunol. 2002;110:228-35). If asthma is well controlled, a stepping down treatment regimen is suggested (Global Initiative for Asthma 2021;Usmani et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5[5]:1378-87.e5; Hagan JB et al. Allergy. 2014 Apr;69[4]:510-6), and although a randomized trial is lacking, it may be feasible to “undiagnose” patients who don’t experience clinical worsening, airflow obstruction, or bronchial hyperresponsiveness after being tapered off all asthma medications with a low relapse rate (Aaron SD et al. JAMA. 2017;317:269-79; J Fam Pract. 2018;67(11):704-7).
Asthma over- and underdiagnosis is prevalent and has clinical and global health consequences. New standardized algorithms with improved biomarkers may help alter this oversight.
Richa Nahar, MD
Network Member-at-Large
Allen J. Blaivas, DO, FCCP
Network Steering Committee Chair
Diffuse lung disease and lung transplant network: Interstitial lung disease section
Future therapies for IPF
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease characterized by progressive fibrosis, respiratory failure, and a mortality rate of 80% at 5 years. Only two drugs are currently FDA-approved for IPF treatment.
The antifibrotics pirfenidone and nintedanib reduce the rate of forced vital capacity (FVC) decline and improve progression free survival (King TE et al. N Engl J Med. 2014;370:2083-92; King TE et al. N Engl J Med. 2014;370:2071-82). While considered revolutionary when introduced, these medications neither reverse disease progression nor improve symptoms. More recently, the Galapagos ISABELA Phase III clinical trial of ziritaxestat in IPF was discontinued due to an unfavorable risk-benefit profile. Despite this, several prospects for IPF therapy exist.
Post hoc analysis of the INCREASE Trial demonstrated a positive effect of inhaled treprostinil on FVC in patients with IPF and group 3 pulmonary hypertension (Waxman A et al. N Engl J Med. 2021;384:325-34). Consequently, a phase 3 randomized trial investigating its safety and efficacy in patients with IPF alone is ongoing. Additional targeted therapies for IPF are also emerging. Recombinant human pentraxin-2, an inhibitor of monocyte differentiation into proinflammatory macrophages, and pamrevlumab, a recombinant human monoclonal antibody against connective tissue growth factor, both demonstrated attenuation of FVC decline compared with placebo in phase 2 trials. Both are currently in phase 3 studies (Raghu G et al. JAMA. 2018 Jun 12;319[22]:2299-307; Sgalla G et al. Expert Opin Investig Drugs. 2020 Aug;29[8]:771-7) Lastly, in February the Food and Drug Administration granted breakthrough therapy designation to BI 1015550 for treatment of IPF based on a 12-week phase 2 randomized, double-blind, placebo-controlled trial. (Data will be presented at ATS). BI 1015550 is an oral, phosphodiesterase 4B (PDE4B) inhibitor with both antifibrotic and anti-inflammatory properties. These advances in drug development provide hope for a future where IPF is transformed from a fatal disease to one manageable over many years.
Adrian Shifren, MD
Network Member-at-Large
Gabriel Schroeder, MD
Network Member-at-Large
Sleep medicine network: Home-based mechanical ventilation and neuromuscular section
Role of airway clearance therapies in neuromuscular disease
Individuals with neuromuscular weakness have an impaired ability to cough and clear secretions from the airway, which can result in atelectasis and pneumonia. Proximal airway clearance therapies (ACT), including manual lung volume recruitment (LVR) and mechanical in-exsufflation (MI-E), mobilize secretions, improve cough efficacy, maintain chest wall compliance, and slow progression of restrictive lung impairment (Chatwin et al. Respir Med. 2018;136:98-110; Sheers et al. Respirology. 2019;24:512-520).
ACT are recommended in international care guidelines for respiratory management of individuals with neuromuscular disease. At a recent Home-based Mechanical Ventilation and Neuromuscular Disease Section “PEEPS Talking PAP” rounds, participants discussed their approach to ACT. Practices varied by country and between adult/pediatric care providers. MI-E is most often used in the United States, but elsewhere in the world, LVR with a self-inflating bag and one-way valve is first-line therapy. Clinical care guidelines suggest initiation of regular ACT when cough peak flow is < 270 L/minute, forced vital capacity < 40%-60% predicted, or with subjectively weak cough (Hull et al. Thorax. 2012;67(7):654-655; Amin et al. Can J Resp Crit Care Sleep Med. 2017;1(1):7-36; McKim et al. Can Resp J. 2011;18(4):197-215; Birnkrant et al. Lancet Neurol. 2018;17(4):347-361; Sheehan et al. Pediatrics. 2018;142(Suppl 2):S62-s71).
Optimal timing for initiation of routine ACT, however, is not clear. A newly published randomized controlled trial of twice daily LVR in boys with Duchenne muscular dystrophy with relatively normal baseline lung function did not demonstrate a significant slowing of decline in forced vital capacity over 2 years. In individuals with preserved lung function, the burden of regular therapy may outweigh benefit (Katz et al. Thorax. 2022; doi: 10.1136/thoraxjnl-2021-218196). (While we are still learning about how best to apply this therapy in less advanced neuromuscular disease, ACT has demonstrated benefits during respiratory exacerbations, and routine use plays a role in preservation of lung function in more advanced disease (Katz et al. Ann Am Thorac Soc. 2016;13(2):217-222; McKim et al. Arch Phys Med Rehab. 2012;93(7):1117-1122; O’Sullivan et al. Arch Phys Med Rehabil. 2021;102(5):976-983; Bach et al. Am J Phys Med Rehab. 2008;87(9):720-725).
Sherri Katz, MD, FCCP
Section Steering Committee Chair
Critical care network: Mechanical ventilation and airways management section
NIV following extubation: Which devices and which patients?
For those of us interested in studying mechanical ventilation, an interesting paradox exists: despite our interest and enthusiasm in studying it, our patients benefit from avoiding it! Patients who require re-intubation are at high risk of in-hospital mortality (Frutos-Vivar et al. J Crit Care. 2011;26:502-9).
Studies in high-risk patients receiving mechanical ventilation have demonstrated that patients treated with immediate noninvasive ventilation (NIV) following extubation had reduced risk of re-intubation. CHEST guidelines focused on ventilator liberation considered these studies in a metanalysis which led to recommendations to employ NIV immediately after extubation in high-risk patients to reduce re-intubation rates (Ouellette D et al. Chest. 2017;151:166-80).
In the years since the publication of the CHEST guidelines, more information has been forthcoming. Evidence has emerged that treatment with high-flow nasal cannula devices following extubation may mitigate against re-intubation. An interesting strategy from the High-Wean Study Group suggested that postextubation combination therapy with both a high-flow cannula and NIV leads to improved outcomes compared with high-flow alone (Thille AW et al. JAMA. 2019;322:1465-75).
Thille and coworkers recently broadened our concept of patients who may benefit from NIV post extubation. They examined a cohort of obese patients requiring mechanical ventilation, finding that when patients were treated with NIV and high-flow nasal cannula post extubation, that they had a reduced risk of re-intubation compared with a group receiving high flow alone (Thille AW, et al. Am J Respir Crit Care Med. 2022;205:440-9).
As the incoming chair of the Mechanical Ventilation and Airways Management Section of the CHEST Critical Care Network, I look forward during the next 2 years to having interesting conversations about topics like this one and working with section members to develop exciting new projects concerning mechanical ventilation.
Daniel Ouellette, MD, MS, FCCP
Section Steering Committee Chair
Thoracic oncology and chest procedures network: Pleural disease section
Management of recurrent transudative pleural effusions (REDUCE trial)
Nonmalignant pleural effusions contribute significantly to health care costs and mortality (Mummadi SR et al. CHEST. 2021 Oct;160[4]:1534-51; Walker SP et al. CHEST. 2017 May;151[5]:1099-105). Management of transudative effusions has traditionally been to treat the underlying etiology. However, despite maximal medical therapies, these recurrent effusions may add to patients’ symptom burden and often create a challenge for the clinician. In 2017, the FDA approved the use of indwelling pleural catheters (IPC) in patients with recurrent transudative effusions, but data are limited.
In a recent prospective multicenter randomized control trial, Walker and colleagues (Eur Respir J. 2022 Feb;59:2101362) aimed to compare IPCs to repeated therapeutic thoracentesis (TT) in the management of transudative effusions. Pleural fluid etiologies included heart (68%), liver (24%), and renal failure (8%). The primary outcome was mean dyspnea score (daily visual analog scales) over 12 weeks, and there was no significant difference noted (39.7 vs. 45.0, mean difference –2.9 mm, 95% confidence interval [CI] –16.1 to 10.3; P = .67). Secondary outcomes demonstrated increased overall drainage in the IPC vs. TT group (17,412 mL vs. 2,901 mL, difference 13,892 mL, 95% CI, 7,669-20,116 mL; P < .001) and fewer invasive procedures required in the IPC group. Adverse events were noted in 59% of the IPC group compared with 37% managed with TT (OR, 3.13, 95% CI, 1.07-9.13, P = .04).
The REDUCE trial offers valuable data, but failure to meet primary outcome, study size, and adverse events highlight limitations to a definitive change in practice. Further study with specific-disease processes (ie, cardiac) may be helpful in the future. As in malignant pleural effusions, the selection of definitive pleural intervention should be tailored for each patient.
Maria Azhar, MD
Network Member-at-Large
Saadia A. Faiz, MD FCCP
Section Steering Committee Chair
Airway disorders network: Asthma and COPD section
Betting on asthma: The over and under of diagnosis
Asthma is one of the major chronic respiratory diseases worldwide (WHO 2020), yet it is a clinical syndrome that lacks a consensus on its definition, is comprised of nonspecific respiratory symptoms, and is without a gold standard diagnostic test or a set guideline on confirmation of bronchial hyperresponsiveness (Sá-Sousa A et al. Clin Transl Allergy. 2014 Aug 4;4:24). In addition, once adequately treated, there is an absence of an algorithm to diagnose disease remission (Aaron SD et al. Am J Respir Crit Care Med. 2018 Oct 15;198[8]:1012-20). It is estimated that 20%-70% of people with asthma worldwide across the spectrum of all ages remain undiagnosed.
Spirometry and bronchoprovocation challenges with fixed cut-off values demonstrate reduced sensitivity with day-to-day, diurnal, and long-term variation in airflow obstruction, inflammation, and bronchial hyperresponsiveness (Wang R et al. Thorax. 2021 Jun;76[6]:624-31). Inflammatory biomarkers like fractional exhaled nitric oxide (FeNO) have higher specificity but are subject to diurnal variation and confounding diagnoses.
Overdiagnosis of asthma can result in lost opportunity to diagnose significant cardiopulmonary diseases, unnecessary escalation of the asthma treatment regimen for poorly controlled respiratory symptoms, potential for medication adverse effects, and, increased cost burden to the patient and to the health care system (Aaron SD et al. JAMA. 2017;317:269-79; Shaw D et al. Prim Care Respir J. 2012;21:283-7). Among the newly physician-diagnosed asthmatics, <50% have spirometry performed within 1 year of diagnosis (Sokol KC et al. Am J Med. 2015 May;128[5]:502-8). Spirometry was further underutilized with limit on aerosol-generating procedures during COVID-19 pandemic (Kankaanranta H et al. J Allergy Clin Immunol Pract. 2021 Dec;9[12]:4252-3); 30%-35% obese and nonobese patients with physician-diagnosed asthma did not have current asthma when objectively assessed for airflow limitation (Aaron SD et al. JAMA. 2017;317:269-79; van Huisstede A, et al. Respir Med. 2013;107:1356-64).
Clinical remission is greater in early-onset asthma as compared with late-onset asthma (De Marco R et al. J Allergy Clin Immunol. 2002;110:228-35). If asthma is well controlled, a stepping down treatment regimen is suggested (Global Initiative for Asthma 2021;Usmani et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5[5]:1378-87.e5; Hagan JB et al. Allergy. 2014 Apr;69[4]:510-6), and although a randomized trial is lacking, it may be feasible to “undiagnose” patients who don’t experience clinical worsening, airflow obstruction, or bronchial hyperresponsiveness after being tapered off all asthma medications with a low relapse rate (Aaron SD et al. JAMA. 2017;317:269-79; J Fam Pract. 2018;67(11):704-7).
Asthma over- and underdiagnosis is prevalent and has clinical and global health consequences. New standardized algorithms with improved biomarkers may help alter this oversight.
Richa Nahar, MD
Network Member-at-Large
Allen J. Blaivas, DO, FCCP
Network Steering Committee Chair
Diffuse lung disease and lung transplant network: Interstitial lung disease section
Future therapies for IPF
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease characterized by progressive fibrosis, respiratory failure, and a mortality rate of 80% at 5 years. Only two drugs are currently FDA-approved for IPF treatment.
The antifibrotics pirfenidone and nintedanib reduce the rate of forced vital capacity (FVC) decline and improve progression free survival (King TE et al. N Engl J Med. 2014;370:2083-92; King TE et al. N Engl J Med. 2014;370:2071-82). While considered revolutionary when introduced, these medications neither reverse disease progression nor improve symptoms. More recently, the Galapagos ISABELA Phase III clinical trial of ziritaxestat in IPF was discontinued due to an unfavorable risk-benefit profile. Despite this, several prospects for IPF therapy exist.
Post hoc analysis of the INCREASE Trial demonstrated a positive effect of inhaled treprostinil on FVC in patients with IPF and group 3 pulmonary hypertension (Waxman A et al. N Engl J Med. 2021;384:325-34). Consequently, a phase 3 randomized trial investigating its safety and efficacy in patients with IPF alone is ongoing. Additional targeted therapies for IPF are also emerging. Recombinant human pentraxin-2, an inhibitor of monocyte differentiation into proinflammatory macrophages, and pamrevlumab, a recombinant human monoclonal antibody against connective tissue growth factor, both demonstrated attenuation of FVC decline compared with placebo in phase 2 trials. Both are currently in phase 3 studies (Raghu G et al. JAMA. 2018 Jun 12;319[22]:2299-307; Sgalla G et al. Expert Opin Investig Drugs. 2020 Aug;29[8]:771-7) Lastly, in February the Food and Drug Administration granted breakthrough therapy designation to BI 1015550 for treatment of IPF based on a 12-week phase 2 randomized, double-blind, placebo-controlled trial. (Data will be presented at ATS). BI 1015550 is an oral, phosphodiesterase 4B (PDE4B) inhibitor with both antifibrotic and anti-inflammatory properties. These advances in drug development provide hope for a future where IPF is transformed from a fatal disease to one manageable over many years.
Adrian Shifren, MD
Network Member-at-Large
Gabriel Schroeder, MD
Network Member-at-Large
Sleep medicine network: Home-based mechanical ventilation and neuromuscular section
Role of airway clearance therapies in neuromuscular disease
Individuals with neuromuscular weakness have an impaired ability to cough and clear secretions from the airway, which can result in atelectasis and pneumonia. Proximal airway clearance therapies (ACT), including manual lung volume recruitment (LVR) and mechanical in-exsufflation (MI-E), mobilize secretions, improve cough efficacy, maintain chest wall compliance, and slow progression of restrictive lung impairment (Chatwin et al. Respir Med. 2018;136:98-110; Sheers et al. Respirology. 2019;24:512-520).
ACT are recommended in international care guidelines for respiratory management of individuals with neuromuscular disease. At a recent Home-based Mechanical Ventilation and Neuromuscular Disease Section “PEEPS Talking PAP” rounds, participants discussed their approach to ACT. Practices varied by country and between adult/pediatric care providers. MI-E is most often used in the United States, but elsewhere in the world, LVR with a self-inflating bag and one-way valve is first-line therapy. Clinical care guidelines suggest initiation of regular ACT when cough peak flow is < 270 L/minute, forced vital capacity < 40%-60% predicted, or with subjectively weak cough (Hull et al. Thorax. 2012;67(7):654-655; Amin et al. Can J Resp Crit Care Sleep Med. 2017;1(1):7-36; McKim et al. Can Resp J. 2011;18(4):197-215; Birnkrant et al. Lancet Neurol. 2018;17(4):347-361; Sheehan et al. Pediatrics. 2018;142(Suppl 2):S62-s71).
Optimal timing for initiation of routine ACT, however, is not clear. A newly published randomized controlled trial of twice daily LVR in boys with Duchenne muscular dystrophy with relatively normal baseline lung function did not demonstrate a significant slowing of decline in forced vital capacity over 2 years. In individuals with preserved lung function, the burden of regular therapy may outweigh benefit (Katz et al. Thorax. 2022; doi: 10.1136/thoraxjnl-2021-218196). (While we are still learning about how best to apply this therapy in less advanced neuromuscular disease, ACT has demonstrated benefits during respiratory exacerbations, and routine use plays a role in preservation of lung function in more advanced disease (Katz et al. Ann Am Thorac Soc. 2016;13(2):217-222; McKim et al. Arch Phys Med Rehab. 2012;93(7):1117-1122; O’Sullivan et al. Arch Phys Med Rehabil. 2021;102(5):976-983; Bach et al. Am J Phys Med Rehab. 2008;87(9):720-725).
Sherri Katz, MD, FCCP
Section Steering Committee Chair
Critical care network: Mechanical ventilation and airways management section
NIV following extubation: Which devices and which patients?
For those of us interested in studying mechanical ventilation, an interesting paradox exists: despite our interest and enthusiasm in studying it, our patients benefit from avoiding it! Patients who require re-intubation are at high risk of in-hospital mortality (Frutos-Vivar et al. J Crit Care. 2011;26:502-9).
Studies in high-risk patients receiving mechanical ventilation have demonstrated that patients treated with immediate noninvasive ventilation (NIV) following extubation had reduced risk of re-intubation. CHEST guidelines focused on ventilator liberation considered these studies in a metanalysis which led to recommendations to employ NIV immediately after extubation in high-risk patients to reduce re-intubation rates (Ouellette D et al. Chest. 2017;151:166-80).
In the years since the publication of the CHEST guidelines, more information has been forthcoming. Evidence has emerged that treatment with high-flow nasal cannula devices following extubation may mitigate against re-intubation. An interesting strategy from the High-Wean Study Group suggested that postextubation combination therapy with both a high-flow cannula and NIV leads to improved outcomes compared with high-flow alone (Thille AW et al. JAMA. 2019;322:1465-75).
Thille and coworkers recently broadened our concept of patients who may benefit from NIV post extubation. They examined a cohort of obese patients requiring mechanical ventilation, finding that when patients were treated with NIV and high-flow nasal cannula post extubation, that they had a reduced risk of re-intubation compared with a group receiving high flow alone (Thille AW, et al. Am J Respir Crit Care Med. 2022;205:440-9).
As the incoming chair of the Mechanical Ventilation and Airways Management Section of the CHEST Critical Care Network, I look forward during the next 2 years to having interesting conversations about topics like this one and working with section members to develop exciting new projects concerning mechanical ventilation.
Daniel Ouellette, MD, MS, FCCP
Section Steering Committee Chair
Thoracic oncology and chest procedures network: Pleural disease section
Management of recurrent transudative pleural effusions (REDUCE trial)
Nonmalignant pleural effusions contribute significantly to health care costs and mortality (Mummadi SR et al. CHEST. 2021 Oct;160[4]:1534-51; Walker SP et al. CHEST. 2017 May;151[5]:1099-105). Management of transudative effusions has traditionally been to treat the underlying etiology. However, despite maximal medical therapies, these recurrent effusions may add to patients’ symptom burden and often create a challenge for the clinician. In 2017, the FDA approved the use of indwelling pleural catheters (IPC) in patients with recurrent transudative effusions, but data are limited.
In a recent prospective multicenter randomized control trial, Walker and colleagues (Eur Respir J. 2022 Feb;59:2101362) aimed to compare IPCs to repeated therapeutic thoracentesis (TT) in the management of transudative effusions. Pleural fluid etiologies included heart (68%), liver (24%), and renal failure (8%). The primary outcome was mean dyspnea score (daily visual analog scales) over 12 weeks, and there was no significant difference noted (39.7 vs. 45.0, mean difference –2.9 mm, 95% confidence interval [CI] –16.1 to 10.3; P = .67). Secondary outcomes demonstrated increased overall drainage in the IPC vs. TT group (17,412 mL vs. 2,901 mL, difference 13,892 mL, 95% CI, 7,669-20,116 mL; P < .001) and fewer invasive procedures required in the IPC group. Adverse events were noted in 59% of the IPC group compared with 37% managed with TT (OR, 3.13, 95% CI, 1.07-9.13, P = .04).
The REDUCE trial offers valuable data, but failure to meet primary outcome, study size, and adverse events highlight limitations to a definitive change in practice. Further study with specific-disease processes (ie, cardiac) may be helpful in the future. As in malignant pleural effusions, the selection of definitive pleural intervention should be tailored for each patient.
Maria Azhar, MD
Network Member-at-Large
Saadia A. Faiz, MD FCCP
Section Steering Committee Chair
Airway disorders network: Asthma and COPD section
Betting on asthma: The over and under of diagnosis
Asthma is one of the major chronic respiratory diseases worldwide (WHO 2020), yet it is a clinical syndrome that lacks a consensus on its definition, is comprised of nonspecific respiratory symptoms, and is without a gold standard diagnostic test or a set guideline on confirmation of bronchial hyperresponsiveness (Sá-Sousa A et al. Clin Transl Allergy. 2014 Aug 4;4:24). In addition, once adequately treated, there is an absence of an algorithm to diagnose disease remission (Aaron SD et al. Am J Respir Crit Care Med. 2018 Oct 15;198[8]:1012-20). It is estimated that 20%-70% of people with asthma worldwide across the spectrum of all ages remain undiagnosed.
Spirometry and bronchoprovocation challenges with fixed cut-off values demonstrate reduced sensitivity with day-to-day, diurnal, and long-term variation in airflow obstruction, inflammation, and bronchial hyperresponsiveness (Wang R et al. Thorax. 2021 Jun;76[6]:624-31). Inflammatory biomarkers like fractional exhaled nitric oxide (FeNO) have higher specificity but are subject to diurnal variation and confounding diagnoses.
Overdiagnosis of asthma can result in lost opportunity to diagnose significant cardiopulmonary diseases, unnecessary escalation of the asthma treatment regimen for poorly controlled respiratory symptoms, potential for medication adverse effects, and, increased cost burden to the patient and to the health care system (Aaron SD et al. JAMA. 2017;317:269-79; Shaw D et al. Prim Care Respir J. 2012;21:283-7). Among the newly physician-diagnosed asthmatics, <50% have spirometry performed within 1 year of diagnosis (Sokol KC et al. Am J Med. 2015 May;128[5]:502-8). Spirometry was further underutilized with limit on aerosol-generating procedures during COVID-19 pandemic (Kankaanranta H et al. J Allergy Clin Immunol Pract. 2021 Dec;9[12]:4252-3); 30%-35% obese and nonobese patients with physician-diagnosed asthma did not have current asthma when objectively assessed for airflow limitation (Aaron SD et al. JAMA. 2017;317:269-79; van Huisstede A, et al. Respir Med. 2013;107:1356-64).
Clinical remission is greater in early-onset asthma as compared with late-onset asthma (De Marco R et al. J Allergy Clin Immunol. 2002;110:228-35). If asthma is well controlled, a stepping down treatment regimen is suggested (Global Initiative for Asthma 2021;Usmani et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5[5]:1378-87.e5; Hagan JB et al. Allergy. 2014 Apr;69[4]:510-6), and although a randomized trial is lacking, it may be feasible to “undiagnose” patients who don’t experience clinical worsening, airflow obstruction, or bronchial hyperresponsiveness after being tapered off all asthma medications with a low relapse rate (Aaron SD et al. JAMA. 2017;317:269-79; J Fam Pract. 2018;67(11):704-7).
Asthma over- and underdiagnosis is prevalent and has clinical and global health consequences. New standardized algorithms with improved biomarkers may help alter this oversight.
Richa Nahar, MD
Network Member-at-Large
Allen J. Blaivas, DO, FCCP
Network Steering Committee Chair
Diffuse lung disease and lung transplant network: Interstitial lung disease section
Future therapies for IPF
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease characterized by progressive fibrosis, respiratory failure, and a mortality rate of 80% at 5 years. Only two drugs are currently FDA-approved for IPF treatment.
The antifibrotics pirfenidone and nintedanib reduce the rate of forced vital capacity (FVC) decline and improve progression free survival (King TE et al. N Engl J Med. 2014;370:2083-92; King TE et al. N Engl J Med. 2014;370:2071-82). While considered revolutionary when introduced, these medications neither reverse disease progression nor improve symptoms. More recently, the Galapagos ISABELA Phase III clinical trial of ziritaxestat in IPF was discontinued due to an unfavorable risk-benefit profile. Despite this, several prospects for IPF therapy exist.
Post hoc analysis of the INCREASE Trial demonstrated a positive effect of inhaled treprostinil on FVC in patients with IPF and group 3 pulmonary hypertension (Waxman A et al. N Engl J Med. 2021;384:325-34). Consequently, a phase 3 randomized trial investigating its safety and efficacy in patients with IPF alone is ongoing. Additional targeted therapies for IPF are also emerging. Recombinant human pentraxin-2, an inhibitor of monocyte differentiation into proinflammatory macrophages, and pamrevlumab, a recombinant human monoclonal antibody against connective tissue growth factor, both demonstrated attenuation of FVC decline compared with placebo in phase 2 trials. Both are currently in phase 3 studies (Raghu G et al. JAMA. 2018 Jun 12;319[22]:2299-307; Sgalla G et al. Expert Opin Investig Drugs. 2020 Aug;29[8]:771-7) Lastly, in February the Food and Drug Administration granted breakthrough therapy designation to BI 1015550 for treatment of IPF based on a 12-week phase 2 randomized, double-blind, placebo-controlled trial. (Data will be presented at ATS). BI 1015550 is an oral, phosphodiesterase 4B (PDE4B) inhibitor with both antifibrotic and anti-inflammatory properties. These advances in drug development provide hope for a future where IPF is transformed from a fatal disease to one manageable over many years.
Adrian Shifren, MD
Network Member-at-Large
Gabriel Schroeder, MD
Network Member-at-Large
Sleep medicine network: Home-based mechanical ventilation and neuromuscular section
Role of airway clearance therapies in neuromuscular disease
Individuals with neuromuscular weakness have an impaired ability to cough and clear secretions from the airway, which can result in atelectasis and pneumonia. Proximal airway clearance therapies (ACT), including manual lung volume recruitment (LVR) and mechanical in-exsufflation (MI-E), mobilize secretions, improve cough efficacy, maintain chest wall compliance, and slow progression of restrictive lung impairment (Chatwin et al. Respir Med. 2018;136:98-110; Sheers et al. Respirology. 2019;24:512-520).
ACT are recommended in international care guidelines for respiratory management of individuals with neuromuscular disease. At a recent Home-based Mechanical Ventilation and Neuromuscular Disease Section “PEEPS Talking PAP” rounds, participants discussed their approach to ACT. Practices varied by country and between adult/pediatric care providers. MI-E is most often used in the United States, but elsewhere in the world, LVR with a self-inflating bag and one-way valve is first-line therapy. Clinical care guidelines suggest initiation of regular ACT when cough peak flow is < 270 L/minute, forced vital capacity < 40%-60% predicted, or with subjectively weak cough (Hull et al. Thorax. 2012;67(7):654-655; Amin et al. Can J Resp Crit Care Sleep Med. 2017;1(1):7-36; McKim et al. Can Resp J. 2011;18(4):197-215; Birnkrant et al. Lancet Neurol. 2018;17(4):347-361; Sheehan et al. Pediatrics. 2018;142(Suppl 2):S62-s71).
Optimal timing for initiation of routine ACT, however, is not clear. A newly published randomized controlled trial of twice daily LVR in boys with Duchenne muscular dystrophy with relatively normal baseline lung function did not demonstrate a significant slowing of decline in forced vital capacity over 2 years. In individuals with preserved lung function, the burden of regular therapy may outweigh benefit (Katz et al. Thorax. 2022; doi: 10.1136/thoraxjnl-2021-218196). (While we are still learning about how best to apply this therapy in less advanced neuromuscular disease, ACT has demonstrated benefits during respiratory exacerbations, and routine use plays a role in preservation of lung function in more advanced disease (Katz et al. Ann Am Thorac Soc. 2016;13(2):217-222; McKim et al. Arch Phys Med Rehab. 2012;93(7):1117-1122; O’Sullivan et al. Arch Phys Med Rehabil. 2021;102(5):976-983; Bach et al. Am J Phys Med Rehab. 2008;87(9):720-725).
Sherri Katz, MD, FCCP
Section Steering Committee Chair
Critical care network: Mechanical ventilation and airways management section
NIV following extubation: Which devices and which patients?
For those of us interested in studying mechanical ventilation, an interesting paradox exists: despite our interest and enthusiasm in studying it, our patients benefit from avoiding it! Patients who require re-intubation are at high risk of in-hospital mortality (Frutos-Vivar et al. J Crit Care. 2011;26:502-9).
Studies in high-risk patients receiving mechanical ventilation have demonstrated that patients treated with immediate noninvasive ventilation (NIV) following extubation had reduced risk of re-intubation. CHEST guidelines focused on ventilator liberation considered these studies in a metanalysis which led to recommendations to employ NIV immediately after extubation in high-risk patients to reduce re-intubation rates (Ouellette D et al. Chest. 2017;151:166-80).
In the years since the publication of the CHEST guidelines, more information has been forthcoming. Evidence has emerged that treatment with high-flow nasal cannula devices following extubation may mitigate against re-intubation. An interesting strategy from the High-Wean Study Group suggested that postextubation combination therapy with both a high-flow cannula and NIV leads to improved outcomes compared with high-flow alone (Thille AW et al. JAMA. 2019;322:1465-75).
Thille and coworkers recently broadened our concept of patients who may benefit from NIV post extubation. They examined a cohort of obese patients requiring mechanical ventilation, finding that when patients were treated with NIV and high-flow nasal cannula post extubation, that they had a reduced risk of re-intubation compared with a group receiving high flow alone (Thille AW, et al. Am J Respir Crit Care Med. 2022;205:440-9).
As the incoming chair of the Mechanical Ventilation and Airways Management Section of the CHEST Critical Care Network, I look forward during the next 2 years to having interesting conversations about topics like this one and working with section members to develop exciting new projects concerning mechanical ventilation.
Daniel Ouellette, MD, MS, FCCP
Section Steering Committee Chair
Thoracic oncology and chest procedures network: Pleural disease section
Management of recurrent transudative pleural effusions (REDUCE trial)
Nonmalignant pleural effusions contribute significantly to health care costs and mortality (Mummadi SR et al. CHEST. 2021 Oct;160[4]:1534-51; Walker SP et al. CHEST. 2017 May;151[5]:1099-105). Management of transudative effusions has traditionally been to treat the underlying etiology. However, despite maximal medical therapies, these recurrent effusions may add to patients’ symptom burden and often create a challenge for the clinician. In 2017, the FDA approved the use of indwelling pleural catheters (IPC) in patients with recurrent transudative effusions, but data are limited.
In a recent prospective multicenter randomized control trial, Walker and colleagues (Eur Respir J. 2022 Feb;59:2101362) aimed to compare IPCs to repeated therapeutic thoracentesis (TT) in the management of transudative effusions. Pleural fluid etiologies included heart (68%), liver (24%), and renal failure (8%). The primary outcome was mean dyspnea score (daily visual analog scales) over 12 weeks, and there was no significant difference noted (39.7 vs. 45.0, mean difference –2.9 mm, 95% confidence interval [CI] –16.1 to 10.3; P = .67). Secondary outcomes demonstrated increased overall drainage in the IPC vs. TT group (17,412 mL vs. 2,901 mL, difference 13,892 mL, 95% CI, 7,669-20,116 mL; P < .001) and fewer invasive procedures required in the IPC group. Adverse events were noted in 59% of the IPC group compared with 37% managed with TT (OR, 3.13, 95% CI, 1.07-9.13, P = .04).
The REDUCE trial offers valuable data, but failure to meet primary outcome, study size, and adverse events highlight limitations to a definitive change in practice. Further study with specific-disease processes (ie, cardiac) may be helpful in the future. As in malignant pleural effusions, the selection of definitive pleural intervention should be tailored for each patient.
Maria Azhar, MD
Network Member-at-Large
Saadia A. Faiz, MD FCCP
Section Steering Committee Chair


















