User login
Preadmission antidiabetic drug use and mortality risk in COVID-19 patients with T2D
Key clinical point: The preadmission antidiabetic medications may influence mortality outcomes in patients with COVID-19 and type 2 diabetes (T2D).
Major finding: The risk for in-hospital mortality was significantly lower among patients taking metformin (odd ratio [OR] 0.54; 95% CI 0.47-0.62), glucagon-like peptide-1 receptor agonist (OR 0.51; 95% CI 0.37-0.69), and sodium-glucose transporter-2 inhibitor (OR 0.60; 95% CI 0.40-0.88), but higher among those taking dipeptidyl peptidase-4 inhibitor (OR 1.23; 95% CI 1.07-1.42) and insulin (OR 1.70; 95% CI 1.33-2.19), compared with patients taking none of these medications. Sulfonylurea, thiazolidinedione, and alpha-glucosidase inhibitors showed neutral effects on mortality.
Study details: The data come from a meta-analysis of 61 studies including 3,061,584 patients with COVID-19 and T2D.
Disclosures: This study received no specific grant from any funding agency.
Source: Nguyen NN et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism. 2022;131:155196 (Mar 31). Doi: 10.1016/j.metabol.2022.155196
Key clinical point: The preadmission antidiabetic medications may influence mortality outcomes in patients with COVID-19 and type 2 diabetes (T2D).
Major finding: The risk for in-hospital mortality was significantly lower among patients taking metformin (odd ratio [OR] 0.54; 95% CI 0.47-0.62), glucagon-like peptide-1 receptor agonist (OR 0.51; 95% CI 0.37-0.69), and sodium-glucose transporter-2 inhibitor (OR 0.60; 95% CI 0.40-0.88), but higher among those taking dipeptidyl peptidase-4 inhibitor (OR 1.23; 95% CI 1.07-1.42) and insulin (OR 1.70; 95% CI 1.33-2.19), compared with patients taking none of these medications. Sulfonylurea, thiazolidinedione, and alpha-glucosidase inhibitors showed neutral effects on mortality.
Study details: The data come from a meta-analysis of 61 studies including 3,061,584 patients with COVID-19 and T2D.
Disclosures: This study received no specific grant from any funding agency.
Source: Nguyen NN et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism. 2022;131:155196 (Mar 31). Doi: 10.1016/j.metabol.2022.155196
Key clinical point: The preadmission antidiabetic medications may influence mortality outcomes in patients with COVID-19 and type 2 diabetes (T2D).
Major finding: The risk for in-hospital mortality was significantly lower among patients taking metformin (odd ratio [OR] 0.54; 95% CI 0.47-0.62), glucagon-like peptide-1 receptor agonist (OR 0.51; 95% CI 0.37-0.69), and sodium-glucose transporter-2 inhibitor (OR 0.60; 95% CI 0.40-0.88), but higher among those taking dipeptidyl peptidase-4 inhibitor (OR 1.23; 95% CI 1.07-1.42) and insulin (OR 1.70; 95% CI 1.33-2.19), compared with patients taking none of these medications. Sulfonylurea, thiazolidinedione, and alpha-glucosidase inhibitors showed neutral effects on mortality.
Study details: The data come from a meta-analysis of 61 studies including 3,061,584 patients with COVID-19 and T2D.
Disclosures: This study received no specific grant from any funding agency.
Source: Nguyen NN et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism. 2022;131:155196 (Mar 31). Doi: 10.1016/j.metabol.2022.155196
SGLT2is offers real-world renal protective benefits over DPP4i in T2D
Key clinical point: In patients with type 2 diabetes (T2D), the use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) vs. dipeptidyl peptidase-4 inhibitors (DPP4i) was associated with a lower risk for end-stage renal disease (ESRD) and acute renal failure (ARF) and a slower decline in the estimated glomerular filtration rate (eGFR).
Major finding: Over a median follow-up of 3.8 years, the use of SGLT2i vs. DPP4i was associated with a significantly lower risk for ESRD (hazard ratio [HR] 0.51; P < .001) and ARF (HR 0.59; P < .001) and a significantly slower decline in eGFR (−0.060 vs. −0.625 mL/min/1.73m2 per year; Pinteraction < .001).
Study details: This retrospective cohort study propensity score matched 6333 patients with T2D receiving an SGLT2i with 25,332 of those receiving a DPP4i.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Au PCM et al. Association between SGLT20iInhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022 (Mar 18). Doi: 10.1210/clinem/dgac164
Key clinical point: In patients with type 2 diabetes (T2D), the use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) vs. dipeptidyl peptidase-4 inhibitors (DPP4i) was associated with a lower risk for end-stage renal disease (ESRD) and acute renal failure (ARF) and a slower decline in the estimated glomerular filtration rate (eGFR).
Major finding: Over a median follow-up of 3.8 years, the use of SGLT2i vs. DPP4i was associated with a significantly lower risk for ESRD (hazard ratio [HR] 0.51; P < .001) and ARF (HR 0.59; P < .001) and a significantly slower decline in eGFR (−0.060 vs. −0.625 mL/min/1.73m2 per year; Pinteraction < .001).
Study details: This retrospective cohort study propensity score matched 6333 patients with T2D receiving an SGLT2i with 25,332 of those receiving a DPP4i.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Au PCM et al. Association between SGLT20iInhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022 (Mar 18). Doi: 10.1210/clinem/dgac164
Key clinical point: In patients with type 2 diabetes (T2D), the use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) vs. dipeptidyl peptidase-4 inhibitors (DPP4i) was associated with a lower risk for end-stage renal disease (ESRD) and acute renal failure (ARF) and a slower decline in the estimated glomerular filtration rate (eGFR).
Major finding: Over a median follow-up of 3.8 years, the use of SGLT2i vs. DPP4i was associated with a significantly lower risk for ESRD (hazard ratio [HR] 0.51; P < .001) and ARF (HR 0.59; P < .001) and a significantly slower decline in eGFR (−0.060 vs. −0.625 mL/min/1.73m2 per year; Pinteraction < .001).
Study details: This retrospective cohort study propensity score matched 6333 patients with T2D receiving an SGLT2i with 25,332 of those receiving a DPP4i.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Au PCM et al. Association between SGLT20iInhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022 (Mar 18). Doi: 10.1210/clinem/dgac164
Resistance training reduces HbA1c levels in patients with T2D
Key clinical point: Resistance training (RT) effectively reduces glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2D), with RT interventions triggering a larger vs. medium or smaller improvement in muscular strength leading to a greater reduction in HbA1c.
Major finding: RT intervention vs. control treatment significantly decreased HbA1c (weighted mean difference −0.39; P < .001), with a larger vs. medium or small effect on muscular strength leading to a greater reduction in HbA1c (β −0.99; P = .0470).
Study details: Findings are from a meta-analysis of 20 trials including 1172 patients with T2DM.
Disclosures: The study received no specific funding. The authors declared no competing interests.
Source: Jansson AK et al. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2022;10:e002595 (Mar 10). Doi: 10.1136/bmjdrc-2021-002595
Key clinical point: Resistance training (RT) effectively reduces glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2D), with RT interventions triggering a larger vs. medium or smaller improvement in muscular strength leading to a greater reduction in HbA1c.
Major finding: RT intervention vs. control treatment significantly decreased HbA1c (weighted mean difference −0.39; P < .001), with a larger vs. medium or small effect on muscular strength leading to a greater reduction in HbA1c (β −0.99; P = .0470).
Study details: Findings are from a meta-analysis of 20 trials including 1172 patients with T2DM.
Disclosures: The study received no specific funding. The authors declared no competing interests.
Source: Jansson AK et al. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2022;10:e002595 (Mar 10). Doi: 10.1136/bmjdrc-2021-002595
Key clinical point: Resistance training (RT) effectively reduces glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2D), with RT interventions triggering a larger vs. medium or smaller improvement in muscular strength leading to a greater reduction in HbA1c.
Major finding: RT intervention vs. control treatment significantly decreased HbA1c (weighted mean difference −0.39; P < .001), with a larger vs. medium or small effect on muscular strength leading to a greater reduction in HbA1c (β −0.99; P = .0470).
Study details: Findings are from a meta-analysis of 20 trials including 1172 patients with T2DM.
Disclosures: The study received no specific funding. The authors declared no competing interests.
Source: Jansson AK et al. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2022;10:e002595 (Mar 10). Doi: 10.1136/bmjdrc-2021-002595
Fenofibrate improves heart failure outcomes in patients with T2D treated with simvastatin
Key clinical point: Fenofibrate reduced the composite outcome of heart failure (HF) hospitalizations or cardiovascular death in patients with type 2 diabetes (T2D) treated with simvastatin, predominantly in those who received the standard background glucose-lowering therapy.
Major finding: The composite outcome of HF hospitalization or cardiovascular death was significantly lower with fenofibrate vs. placebo (hazard ratio [HR] 0.82; P = .048), with reduction primarily observed with the standard glucose-lowering strategy (HR 0.64; 95% CI 0.48-0.85), but not with the intensive glucose-lowering strategy (HR 1.02; 95% CI 0.79-1.33; Pinteraction = .017).
Study details: Findings are from the ACCORD Lipid trial including 5518 patients with T2D who were randomly assigned to receive simvastatin plus fenofibrate (n = 2765) or simvastatin plus placebo (n = 2753).
Disclosures: The study was funded by national funds through FCT-Portuguese Foundation for
Science and Technology, under the scope of the Cardiovascular R&D Center-UnIC. Some authors declared being consultants and receiving research support or personal fees from various sources.
Source: Ferreira JP et al. Fenofibrate and heart failure outcomes in patients with type 2 diabetes: analysis from ACCORD. Diabetes Care. 2022 (Mar 23). Doi: 10.2337/dc21-1977
Key clinical point: Fenofibrate reduced the composite outcome of heart failure (HF) hospitalizations or cardiovascular death in patients with type 2 diabetes (T2D) treated with simvastatin, predominantly in those who received the standard background glucose-lowering therapy.
Major finding: The composite outcome of HF hospitalization or cardiovascular death was significantly lower with fenofibrate vs. placebo (hazard ratio [HR] 0.82; P = .048), with reduction primarily observed with the standard glucose-lowering strategy (HR 0.64; 95% CI 0.48-0.85), but not with the intensive glucose-lowering strategy (HR 1.02; 95% CI 0.79-1.33; Pinteraction = .017).
Study details: Findings are from the ACCORD Lipid trial including 5518 patients with T2D who were randomly assigned to receive simvastatin plus fenofibrate (n = 2765) or simvastatin plus placebo (n = 2753).
Disclosures: The study was funded by national funds through FCT-Portuguese Foundation for
Science and Technology, under the scope of the Cardiovascular R&D Center-UnIC. Some authors declared being consultants and receiving research support or personal fees from various sources.
Source: Ferreira JP et al. Fenofibrate and heart failure outcomes in patients with type 2 diabetes: analysis from ACCORD. Diabetes Care. 2022 (Mar 23). Doi: 10.2337/dc21-1977
Key clinical point: Fenofibrate reduced the composite outcome of heart failure (HF) hospitalizations or cardiovascular death in patients with type 2 diabetes (T2D) treated with simvastatin, predominantly in those who received the standard background glucose-lowering therapy.
Major finding: The composite outcome of HF hospitalization or cardiovascular death was significantly lower with fenofibrate vs. placebo (hazard ratio [HR] 0.82; P = .048), with reduction primarily observed with the standard glucose-lowering strategy (HR 0.64; 95% CI 0.48-0.85), but not with the intensive glucose-lowering strategy (HR 1.02; 95% CI 0.79-1.33; Pinteraction = .017).
Study details: Findings are from the ACCORD Lipid trial including 5518 patients with T2D who were randomly assigned to receive simvastatin plus fenofibrate (n = 2765) or simvastatin plus placebo (n = 2753).
Disclosures: The study was funded by national funds through FCT-Portuguese Foundation for
Science and Technology, under the scope of the Cardiovascular R&D Center-UnIC. Some authors declared being consultants and receiving research support or personal fees from various sources.
Source: Ferreira JP et al. Fenofibrate and heart failure outcomes in patients with type 2 diabetes: analysis from ACCORD. Diabetes Care. 2022 (Mar 23). Doi: 10.2337/dc21-1977
T2D: Empagliflozin improves cognitive and physical function in older adults with HFpEF
Key clinical point: Empagliflozin showed a beneficial effect on cognitive and physical impairment in frail older patients with type 2 diabetes (T2D) and heart failure with preserved ejection fraction (HFpEF).
Major finding: The mean Montreal Cognitive Assessment score significantly improved from baseline to 1 month in the empagliflozin group (19.80 vs. 22.25; P < .001) but not in the metformin (P = .26) and insulin (P = .81) groups, with empagliflozin showing a significant effect on amelioration of cognitive impairment (odds ratio 3.609; P = .03). The 5-meter gait speed improved significantly in the empagliflozin and metformin groups (both P < .05), but not in the insulin group.
Study details: This prospective observational study included 162 frail older patients aged >65 years who had T2D and HFpEF and were treated with empagliflozin (n = 52), metformin (n = 56), or insulin (n = 54).
Disclosures: The study was partly supported by the US National Institute of Diabetes and Digestive and Kidney Diseases, US National Heart, Lung, and Blood Institute, and US National Institute on Aging, among others. The authors declared no conflicts of interest.
Source: Mone P et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022 (Mar 21). Doi: 10.2337/dc21-2434
Key clinical point: Empagliflozin showed a beneficial effect on cognitive and physical impairment in frail older patients with type 2 diabetes (T2D) and heart failure with preserved ejection fraction (HFpEF).
Major finding: The mean Montreal Cognitive Assessment score significantly improved from baseline to 1 month in the empagliflozin group (19.80 vs. 22.25; P < .001) but not in the metformin (P = .26) and insulin (P = .81) groups, with empagliflozin showing a significant effect on amelioration of cognitive impairment (odds ratio 3.609; P = .03). The 5-meter gait speed improved significantly in the empagliflozin and metformin groups (both P < .05), but not in the insulin group.
Study details: This prospective observational study included 162 frail older patients aged >65 years who had T2D and HFpEF and were treated with empagliflozin (n = 52), metformin (n = 56), or insulin (n = 54).
Disclosures: The study was partly supported by the US National Institute of Diabetes and Digestive and Kidney Diseases, US National Heart, Lung, and Blood Institute, and US National Institute on Aging, among others. The authors declared no conflicts of interest.
Source: Mone P et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022 (Mar 21). Doi: 10.2337/dc21-2434
Key clinical point: Empagliflozin showed a beneficial effect on cognitive and physical impairment in frail older patients with type 2 diabetes (T2D) and heart failure with preserved ejection fraction (HFpEF).
Major finding: The mean Montreal Cognitive Assessment score significantly improved from baseline to 1 month in the empagliflozin group (19.80 vs. 22.25; P < .001) but not in the metformin (P = .26) and insulin (P = .81) groups, with empagliflozin showing a significant effect on amelioration of cognitive impairment (odds ratio 3.609; P = .03). The 5-meter gait speed improved significantly in the empagliflozin and metformin groups (both P < .05), but not in the insulin group.
Study details: This prospective observational study included 162 frail older patients aged >65 years who had T2D and HFpEF and were treated with empagliflozin (n = 52), metformin (n = 56), or insulin (n = 54).
Disclosures: The study was partly supported by the US National Institute of Diabetes and Digestive and Kidney Diseases, US National Heart, Lung, and Blood Institute, and US National Institute on Aging, among others. The authors declared no conflicts of interest.
Source: Mone P et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022 (Mar 21). Doi: 10.2337/dc21-2434
Dapagliflozin shows promise in young people with T2D in phase 3
Key clinical point: Dapagliflozin in addition to standard-of-care treatment demonstrated a clinically relevant decrease in glycated hemoglobin (HbA1c) and an acceptable safety profile in young people with type 2 diabetes (T2D).
Major finding: At 24 weeks, the adjusted mean change in HbA1c was not significantly different between the dapagliflozin and placebo groups in the intention-to-treat analysis (between-group difference [Δ] −0.75%; P = .10), but was significantly different in the sensitivity analysis in the per-protocol population (Δ −1.13%; P = .012). No new safety signals or episodes of death or diabetic ketoacidosis were recorded.
Study details: The data come from a phase 3 trial including 72 participants aged 10-24 years with T2D and HbA1c concentration of 6.5%-11% who were randomly assigned to receive oral dapagliflozin (10 mg) or placebo in addition to standard-of-care treatment for 24 weeks followed by dapagliflozin for 28 weeks
Disclosures: The study was funded by AstraZeneca. Some authors declared receiving consulting fees or research grants or serving on advisory boards for various sources, including AstraZeneca. Three authors declared being stockholders or employees of AstraZeneca.
Source: Tamborlane WV, Laffel LM et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022 (Apr 1). Doi: 10.1016/S2213-8587(22)00052-3
Key clinical point: Dapagliflozin in addition to standard-of-care treatment demonstrated a clinically relevant decrease in glycated hemoglobin (HbA1c) and an acceptable safety profile in young people with type 2 diabetes (T2D).
Major finding: At 24 weeks, the adjusted mean change in HbA1c was not significantly different between the dapagliflozin and placebo groups in the intention-to-treat analysis (between-group difference [Δ] −0.75%; P = .10), but was significantly different in the sensitivity analysis in the per-protocol population (Δ −1.13%; P = .012). No new safety signals or episodes of death or diabetic ketoacidosis were recorded.
Study details: The data come from a phase 3 trial including 72 participants aged 10-24 years with T2D and HbA1c concentration of 6.5%-11% who were randomly assigned to receive oral dapagliflozin (10 mg) or placebo in addition to standard-of-care treatment for 24 weeks followed by dapagliflozin for 28 weeks
Disclosures: The study was funded by AstraZeneca. Some authors declared receiving consulting fees or research grants or serving on advisory boards for various sources, including AstraZeneca. Three authors declared being stockholders or employees of AstraZeneca.
Source: Tamborlane WV, Laffel LM et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022 (Apr 1). Doi: 10.1016/S2213-8587(22)00052-3
Key clinical point: Dapagliflozin in addition to standard-of-care treatment demonstrated a clinically relevant decrease in glycated hemoglobin (HbA1c) and an acceptable safety profile in young people with type 2 diabetes (T2D).
Major finding: At 24 weeks, the adjusted mean change in HbA1c was not significantly different between the dapagliflozin and placebo groups in the intention-to-treat analysis (between-group difference [Δ] −0.75%; P = .10), but was significantly different in the sensitivity analysis in the per-protocol population (Δ −1.13%; P = .012). No new safety signals or episodes of death or diabetic ketoacidosis were recorded.
Study details: The data come from a phase 3 trial including 72 participants aged 10-24 years with T2D and HbA1c concentration of 6.5%-11% who were randomly assigned to receive oral dapagliflozin (10 mg) or placebo in addition to standard-of-care treatment for 24 weeks followed by dapagliflozin for 28 weeks
Disclosures: The study was funded by AstraZeneca. Some authors declared receiving consulting fees or research grants or serving on advisory boards for various sources, including AstraZeneca. Three authors declared being stockholders or employees of AstraZeneca.
Source: Tamborlane WV, Laffel LM et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022 (Apr 1). Doi: 10.1016/S2213-8587(22)00052-3
Commentary: Meningococcal vaccine shows moderate protective effect against gonorrhea
The data on cross-protection against gonorrhea by outer membrane vesicle (OMV)–based meningococcal B vaccine continue to look encouraging from a recent study in Clinical Infectious Diseases (2022; doi: 10.1093/cid/ciac436). The authors report matched-cohort study data involving over 33,000 teens/young adults followed at Kaiser Permanente Southern California during 2016-2020. Like the studies above, chlamydia-infected patients (n = 26,471) served as negative controls for the 6,641 gonorrhea patients. The researchers compared chances of getting gonorrhea vs. getting chlamydia in light of having previously gotten C4MenB vaccine (OMV-based) or MenACWY vaccine (not OMV-based). The authors reported gonorrhea incidence rates of 2.0/1,000 person-years (95% CI, 1.3–2.8) in 4CMenB vaccinees vs. 5.2 (4.6–5.8) for MenACWY recipients. An adjusted analysis revealed 46% lower gonorrhea rates in 4CMenB vs. MenACWY vaccinees. There was no difference in chlamydia rates.
We await prospective controlled data to validate these observational studies. However, it is intriguing that OMV-based meningococcal vaccine may be a two-fer vaccine with partial cross protection against gonorrhea because of outer membrane protein similarities between the two pathogens. These data seem worth sharing with families who are making decisions about whether to vaccinate their children against B strains of meningococcus whether or not the child has already had conjugate MenACWY.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data on cross-protection against gonorrhea by outer membrane vesicle (OMV)–based meningococcal B vaccine continue to look encouraging from a recent study in Clinical Infectious Diseases (2022; doi: 10.1093/cid/ciac436). The authors report matched-cohort study data involving over 33,000 teens/young adults followed at Kaiser Permanente Southern California during 2016-2020. Like the studies above, chlamydia-infected patients (n = 26,471) served as negative controls for the 6,641 gonorrhea patients. The researchers compared chances of getting gonorrhea vs. getting chlamydia in light of having previously gotten C4MenB vaccine (OMV-based) or MenACWY vaccine (not OMV-based). The authors reported gonorrhea incidence rates of 2.0/1,000 person-years (95% CI, 1.3–2.8) in 4CMenB vaccinees vs. 5.2 (4.6–5.8) for MenACWY recipients. An adjusted analysis revealed 46% lower gonorrhea rates in 4CMenB vs. MenACWY vaccinees. There was no difference in chlamydia rates.
We await prospective controlled data to validate these observational studies. However, it is intriguing that OMV-based meningococcal vaccine may be a two-fer vaccine with partial cross protection against gonorrhea because of outer membrane protein similarities between the two pathogens. These data seem worth sharing with families who are making decisions about whether to vaccinate their children against B strains of meningococcus whether or not the child has already had conjugate MenACWY.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data on cross-protection against gonorrhea by outer membrane vesicle (OMV)–based meningococcal B vaccine continue to look encouraging from a recent study in Clinical Infectious Diseases (2022; doi: 10.1093/cid/ciac436). The authors report matched-cohort study data involving over 33,000 teens/young adults followed at Kaiser Permanente Southern California during 2016-2020. Like the studies above, chlamydia-infected patients (n = 26,471) served as negative controls for the 6,641 gonorrhea patients. The researchers compared chances of getting gonorrhea vs. getting chlamydia in light of having previously gotten C4MenB vaccine (OMV-based) or MenACWY vaccine (not OMV-based). The authors reported gonorrhea incidence rates of 2.0/1,000 person-years (95% CI, 1.3–2.8) in 4CMenB vaccinees vs. 5.2 (4.6–5.8) for MenACWY recipients. An adjusted analysis revealed 46% lower gonorrhea rates in 4CMenB vs. MenACWY vaccinees. There was no difference in chlamydia rates.
We await prospective controlled data to validate these observational studies. However, it is intriguing that OMV-based meningococcal vaccine may be a two-fer vaccine with partial cross protection against gonorrhea because of outer membrane protein similarities between the two pathogens. These data seem worth sharing with families who are making decisions about whether to vaccinate their children against B strains of meningococcus whether or not the child has already had conjugate MenACWY.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Novel combo drug shows promise as first-line Parkinson’s disease treatment
, new research suggests. Results from a phase 3 trial of P2B001, a combination of pramipexole and rasagiline at currently unavailable low doses, showed the drug was more effective than its individual components and as effective as higher-dose pramipexole ER – with far less daytime sleepiness.
The combination drug is taken once per day and does not require titration, which investigators say make it a good option for first-line treatment of Parkinson’s disease.
“I don’t think people, including me, expected intuitively that if you used small doses and combined it with a little rasagiline it would be equal to full doses of pramipexole, but it appears that it is,” said lead investigator Warren Olanow, MD, professor and chair emeritus of neurology and professor emeritus of neuroscience at Icahn School of Medicine at Mount Sinai, New York.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
‘Synergistic effects’
Levodopa is considered to be the most effective treatment for Parkinson’s disease, but long-term use is associated with increased risk for motor complications, such as dyskinesia. Dopamine agonists such as pramipexole have been linked in previous research to excessive daytime sleepiness and impulse control disorders. In addition, monoamine oxidase-B inhibitors such as rasagiline are not as effective at controlling Parkinson’s disease as other treatment options.
“There is no consistent agreement on how to initiate treatment because no one treatment is ideal,” Dr. Olanow said.
P2B001, developed by Pharma Two B, is a combination of 0.6 mg of pramipexole and 0.75 mg of rasagiline. The drugs work by dual mechanisms, which investigators suspected might have “synergistic effects.”
Following promising results from an earlier trial, researches launched a phase 3, 12-week, international, randomized, double-blind trial to study the efficacy, safety, and tolerability of P2B001, compared with its individual components and with a calibration arm of pramipexole ER in 519 patients with early Parkinson’s disease.
Participants received P2B001, 0.6 mg of pramipexole ER, 0.75 mg of rasagiline ER, or pramipexole ER titrated to an optimal dose for each patient (1.5-4.5 mg).
New first-line treatment?
Results showed that the adjusted mean change from baseline in total Unified Parkinson’s Disease Rating Scale (UPDRS) score was -2.66 points for P2B001 versus pramipexole (P = .0018) and -3.30 points for P2B001 versus rasagiline (P = .0001).
There was no significant difference in UPDRS scores between P2B001 and pramipexole ER, but patients who received P2B001 reported significantly less daytime sleepiness.
The adjusted mean change from baseline in Epworth Sleepiness Scale score for P2B001 versus pramipexole ER was -2.66 points (P < .0001).
In addition, fewer dopaminergic adverse events were reported with the combination drug versus pramipexole ER (44.7% vs. 66.2%), including somnolence (14.7% vs. 31.1%) and orthostatic hypotension (2.7% vs. 12.2%).
As a first-line treatment, P2B001 could offer an effective option instead of levodopa, Dr. Olanow said. “It could be really good for patients because it would delay the introduction of levodopa and allow levodopa to be used in lower doses when the time comes and hopefully reduce the risk of complications,” he added.
Questions, cost concerns
Commenting on the study, Alfonso Fasano, MD, PhD, professor of neurology and chair in neuromodulation, University of Toronto, agreed that better therapeutic options are needed for Parkinson’s disease.
Combining available treatments into one pill “might help patients’ adherence, although this can compromise our ability to dose each compound individually,” said Dr. Fasano, who was not involved with the research.
He added that there are also questions about dosage modification as a patient’s disease progresses and whether a higher dose might pose safety problems. There is also the issue of cost. “Conducting large clinical trials like this one is expensive, and I wonder about the cost of the drug when approved,” Dr. Fasano noted. “Do we really need to invest in combination pills containing two already well-known compounds?”
Dr. Olanow, who is not directly involved with Pharma Two B, the developer of P2B001, said he has no information on what the drug might cost or how it might be marketed if approved for use.
“The advantage of the combination is the component doses are not replicable, they are both in an extended-release formulation, it doesn’t require titration, and it has been tested and proven to work,” he said.
The study was funded by Pharma Two B. Dr. Olanow is employed by Clintrex Research Corporation and owns stock in Clintrex Research Corporation. Dr. Fasano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a phase 3 trial of P2B001, a combination of pramipexole and rasagiline at currently unavailable low doses, showed the drug was more effective than its individual components and as effective as higher-dose pramipexole ER – with far less daytime sleepiness.
The combination drug is taken once per day and does not require titration, which investigators say make it a good option for first-line treatment of Parkinson’s disease.
“I don’t think people, including me, expected intuitively that if you used small doses and combined it with a little rasagiline it would be equal to full doses of pramipexole, but it appears that it is,” said lead investigator Warren Olanow, MD, professor and chair emeritus of neurology and professor emeritus of neuroscience at Icahn School of Medicine at Mount Sinai, New York.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
‘Synergistic effects’
Levodopa is considered to be the most effective treatment for Parkinson’s disease, but long-term use is associated with increased risk for motor complications, such as dyskinesia. Dopamine agonists such as pramipexole have been linked in previous research to excessive daytime sleepiness and impulse control disorders. In addition, monoamine oxidase-B inhibitors such as rasagiline are not as effective at controlling Parkinson’s disease as other treatment options.
“There is no consistent agreement on how to initiate treatment because no one treatment is ideal,” Dr. Olanow said.
P2B001, developed by Pharma Two B, is a combination of 0.6 mg of pramipexole and 0.75 mg of rasagiline. The drugs work by dual mechanisms, which investigators suspected might have “synergistic effects.”
Following promising results from an earlier trial, researches launched a phase 3, 12-week, international, randomized, double-blind trial to study the efficacy, safety, and tolerability of P2B001, compared with its individual components and with a calibration arm of pramipexole ER in 519 patients with early Parkinson’s disease.
Participants received P2B001, 0.6 mg of pramipexole ER, 0.75 mg of rasagiline ER, or pramipexole ER titrated to an optimal dose for each patient (1.5-4.5 mg).
New first-line treatment?
Results showed that the adjusted mean change from baseline in total Unified Parkinson’s Disease Rating Scale (UPDRS) score was -2.66 points for P2B001 versus pramipexole (P = .0018) and -3.30 points for P2B001 versus rasagiline (P = .0001).
There was no significant difference in UPDRS scores between P2B001 and pramipexole ER, but patients who received P2B001 reported significantly less daytime sleepiness.
The adjusted mean change from baseline in Epworth Sleepiness Scale score for P2B001 versus pramipexole ER was -2.66 points (P < .0001).
In addition, fewer dopaminergic adverse events were reported with the combination drug versus pramipexole ER (44.7% vs. 66.2%), including somnolence (14.7% vs. 31.1%) and orthostatic hypotension (2.7% vs. 12.2%).
As a first-line treatment, P2B001 could offer an effective option instead of levodopa, Dr. Olanow said. “It could be really good for patients because it would delay the introduction of levodopa and allow levodopa to be used in lower doses when the time comes and hopefully reduce the risk of complications,” he added.
Questions, cost concerns
Commenting on the study, Alfonso Fasano, MD, PhD, professor of neurology and chair in neuromodulation, University of Toronto, agreed that better therapeutic options are needed for Parkinson’s disease.
Combining available treatments into one pill “might help patients’ adherence, although this can compromise our ability to dose each compound individually,” said Dr. Fasano, who was not involved with the research.
He added that there are also questions about dosage modification as a patient’s disease progresses and whether a higher dose might pose safety problems. There is also the issue of cost. “Conducting large clinical trials like this one is expensive, and I wonder about the cost of the drug when approved,” Dr. Fasano noted. “Do we really need to invest in combination pills containing two already well-known compounds?”
Dr. Olanow, who is not directly involved with Pharma Two B, the developer of P2B001, said he has no information on what the drug might cost or how it might be marketed if approved for use.
“The advantage of the combination is the component doses are not replicable, they are both in an extended-release formulation, it doesn’t require titration, and it has been tested and proven to work,” he said.
The study was funded by Pharma Two B. Dr. Olanow is employed by Clintrex Research Corporation and owns stock in Clintrex Research Corporation. Dr. Fasano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from a phase 3 trial of P2B001, a combination of pramipexole and rasagiline at currently unavailable low doses, showed the drug was more effective than its individual components and as effective as higher-dose pramipexole ER – with far less daytime sleepiness.
The combination drug is taken once per day and does not require titration, which investigators say make it a good option for first-line treatment of Parkinson’s disease.
“I don’t think people, including me, expected intuitively that if you used small doses and combined it with a little rasagiline it would be equal to full doses of pramipexole, but it appears that it is,” said lead investigator Warren Olanow, MD, professor and chair emeritus of neurology and professor emeritus of neuroscience at Icahn School of Medicine at Mount Sinai, New York.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
‘Synergistic effects’
Levodopa is considered to be the most effective treatment for Parkinson’s disease, but long-term use is associated with increased risk for motor complications, such as dyskinesia. Dopamine agonists such as pramipexole have been linked in previous research to excessive daytime sleepiness and impulse control disorders. In addition, monoamine oxidase-B inhibitors such as rasagiline are not as effective at controlling Parkinson’s disease as other treatment options.
“There is no consistent agreement on how to initiate treatment because no one treatment is ideal,” Dr. Olanow said.
P2B001, developed by Pharma Two B, is a combination of 0.6 mg of pramipexole and 0.75 mg of rasagiline. The drugs work by dual mechanisms, which investigators suspected might have “synergistic effects.”
Following promising results from an earlier trial, researches launched a phase 3, 12-week, international, randomized, double-blind trial to study the efficacy, safety, and tolerability of P2B001, compared with its individual components and with a calibration arm of pramipexole ER in 519 patients with early Parkinson’s disease.
Participants received P2B001, 0.6 mg of pramipexole ER, 0.75 mg of rasagiline ER, or pramipexole ER titrated to an optimal dose for each patient (1.5-4.5 mg).
New first-line treatment?
Results showed that the adjusted mean change from baseline in total Unified Parkinson’s Disease Rating Scale (UPDRS) score was -2.66 points for P2B001 versus pramipexole (P = .0018) and -3.30 points for P2B001 versus rasagiline (P = .0001).
There was no significant difference in UPDRS scores between P2B001 and pramipexole ER, but patients who received P2B001 reported significantly less daytime sleepiness.
The adjusted mean change from baseline in Epworth Sleepiness Scale score for P2B001 versus pramipexole ER was -2.66 points (P < .0001).
In addition, fewer dopaminergic adverse events were reported with the combination drug versus pramipexole ER (44.7% vs. 66.2%), including somnolence (14.7% vs. 31.1%) and orthostatic hypotension (2.7% vs. 12.2%).
As a first-line treatment, P2B001 could offer an effective option instead of levodopa, Dr. Olanow said. “It could be really good for patients because it would delay the introduction of levodopa and allow levodopa to be used in lower doses when the time comes and hopefully reduce the risk of complications,” he added.
Questions, cost concerns
Commenting on the study, Alfonso Fasano, MD, PhD, professor of neurology and chair in neuromodulation, University of Toronto, agreed that better therapeutic options are needed for Parkinson’s disease.
Combining available treatments into one pill “might help patients’ adherence, although this can compromise our ability to dose each compound individually,” said Dr. Fasano, who was not involved with the research.
He added that there are also questions about dosage modification as a patient’s disease progresses and whether a higher dose might pose safety problems. There is also the issue of cost. “Conducting large clinical trials like this one is expensive, and I wonder about the cost of the drug when approved,” Dr. Fasano noted. “Do we really need to invest in combination pills containing two already well-known compounds?”
Dr. Olanow, who is not directly involved with Pharma Two B, the developer of P2B001, said he has no information on what the drug might cost or how it might be marketed if approved for use.
“The advantage of the combination is the component doses are not replicable, they are both in an extended-release formulation, it doesn’t require titration, and it has been tested and proven to work,” he said.
The study was funded by Pharma Two B. Dr. Olanow is employed by Clintrex Research Corporation and owns stock in Clintrex Research Corporation. Dr. Fasano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
CO2 laser excision therapy for hidradenitis suppurativa shows no keloid risk
BOSTON – The use of , new research shows.
“With keloids disproportionately affecting Black and other skin of color patients, denying treatment on a notion that lacks evidentiary support further potentiates the health disparities experienced by these marginalized groups,” the researchers reported at the Annual Meeting of the Skin of Color Society Scientific Symposium (SOCS) 2022. In their retrospective study of 129 patients with HS treated with CO2 laser, “there were no cases of keloid formation,” they say.
HS, a potentially debilitating chronic inflammatory condition that involves painful nodules, boils, and abscesses, is often refractory to standard treatment. CO2 laser excision therapy has yielded favorable outcomes in some studies.
Although CO2 laser therapy is also used to treat keloids, some clinicians hesitate to use this treatment in these patients because of concerns that its use for treating HS could trigger the development of keloids.
“Many patients come in telling us they were denied [CO2 laser] surgery due to keloids,” senior author Iltefat Hamzavi, MD, a senior staff physician in the Department of Dermatology at the Henry Ford Health System, Detroit, told this news organization.
Although patients with HS are commonly treated with CO2 laser excision in his department, this treatment approach “is underused nationally,” he said.
“Of note, the sinus tunnels of hidradenitis suppurativa can look like keloids, so this might drive surgeons away from treating [those] lesions,” Dr. Hamzavi said.
To further evaluate the risk of developing keloids with the treatment, Dr. Hamzavi and his colleagues conducted a retrospective review of 129 patients with HS treated at Henry Ford who had undergone follicular destruction with CO2 laser between 2014 and 2021; 102 (79%) patients were female. The mean age was about 38 years (range, 15-78 years).
Of the patients, almost half were Black, almost 40% were White, 5% were Asian, and 3% were of unknown ethnicity.
Medical records of nine patients included diagnoses of keloids or hypertrophic scars. Further review indicated that none of the diagnoses were for keloids but were for hypertrophic scars, hypertrophic granulation tissue, an HS nodule, or contracture scar, the authors report.
“While the emergence of hypertrophic scars, hypertrophic granulation tissue, and scar contracture following CO2 laser excision therapy for hidradenitis suppurativa has been documented in the literature, existing evidence does not support postoperative keloid formation,” the authors conclude.
Because healing time with CO2 laser treatment is prolonged and there is an increase in risk of adverse events, Dr. Hamzavi underscored that “safety protocols for CO2 lasers should be followed, and wound prep instructions should be provided along with counseling on healing times.”
Regarding patient selection, he noted that “the disease should be medically stable with reduction in drainage to help control postop bleeding risk.”
The findings of the study are supported by a recent systematic review that compared outcomes and adverse effects of treatment with ablative laser therapies with nonablative lasers for skin resurfacing. The review included 34 studies and involved 1,093 patients. The conditions that were treated ranged from photodamage and acne scars to HS and post-traumatic scarring from basal cell carcinoma excision.
That review found that overall, rates of adverse events were higher with nonablative therapies (12.2%, 31 events), compared with ablative laser therapy, such as with CO2 laser (8.28%, 81 events). In addition, when transient events were excluded, ablative lasers were associated with fewer complications overall, compared with nonablative lasers (2.56% vs. 7.48%).
The authors conclude: “It is our hope that this study will facilitate continued research in this domain in an effort to combat these inequities and improve access to CO2 excision or standardized excisional therapy for hidradenitis suppurativa treatment.”
Dr. Hamzavi and the other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The use of , new research shows.
“With keloids disproportionately affecting Black and other skin of color patients, denying treatment on a notion that lacks evidentiary support further potentiates the health disparities experienced by these marginalized groups,” the researchers reported at the Annual Meeting of the Skin of Color Society Scientific Symposium (SOCS) 2022. In their retrospective study of 129 patients with HS treated with CO2 laser, “there were no cases of keloid formation,” they say.
HS, a potentially debilitating chronic inflammatory condition that involves painful nodules, boils, and abscesses, is often refractory to standard treatment. CO2 laser excision therapy has yielded favorable outcomes in some studies.
Although CO2 laser therapy is also used to treat keloids, some clinicians hesitate to use this treatment in these patients because of concerns that its use for treating HS could trigger the development of keloids.
“Many patients come in telling us they were denied [CO2 laser] surgery due to keloids,” senior author Iltefat Hamzavi, MD, a senior staff physician in the Department of Dermatology at the Henry Ford Health System, Detroit, told this news organization.
Although patients with HS are commonly treated with CO2 laser excision in his department, this treatment approach “is underused nationally,” he said.
“Of note, the sinus tunnels of hidradenitis suppurativa can look like keloids, so this might drive surgeons away from treating [those] lesions,” Dr. Hamzavi said.
To further evaluate the risk of developing keloids with the treatment, Dr. Hamzavi and his colleagues conducted a retrospective review of 129 patients with HS treated at Henry Ford who had undergone follicular destruction with CO2 laser between 2014 and 2021; 102 (79%) patients were female. The mean age was about 38 years (range, 15-78 years).
Of the patients, almost half were Black, almost 40% were White, 5% were Asian, and 3% were of unknown ethnicity.
Medical records of nine patients included diagnoses of keloids or hypertrophic scars. Further review indicated that none of the diagnoses were for keloids but were for hypertrophic scars, hypertrophic granulation tissue, an HS nodule, or contracture scar, the authors report.
“While the emergence of hypertrophic scars, hypertrophic granulation tissue, and scar contracture following CO2 laser excision therapy for hidradenitis suppurativa has been documented in the literature, existing evidence does not support postoperative keloid formation,” the authors conclude.
Because healing time with CO2 laser treatment is prolonged and there is an increase in risk of adverse events, Dr. Hamzavi underscored that “safety protocols for CO2 lasers should be followed, and wound prep instructions should be provided along with counseling on healing times.”
Regarding patient selection, he noted that “the disease should be medically stable with reduction in drainage to help control postop bleeding risk.”
The findings of the study are supported by a recent systematic review that compared outcomes and adverse effects of treatment with ablative laser therapies with nonablative lasers for skin resurfacing. The review included 34 studies and involved 1,093 patients. The conditions that were treated ranged from photodamage and acne scars to HS and post-traumatic scarring from basal cell carcinoma excision.
That review found that overall, rates of adverse events were higher with nonablative therapies (12.2%, 31 events), compared with ablative laser therapy, such as with CO2 laser (8.28%, 81 events). In addition, when transient events were excluded, ablative lasers were associated with fewer complications overall, compared with nonablative lasers (2.56% vs. 7.48%).
The authors conclude: “It is our hope that this study will facilitate continued research in this domain in an effort to combat these inequities and improve access to CO2 excision or standardized excisional therapy for hidradenitis suppurativa treatment.”
Dr. Hamzavi and the other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – The use of , new research shows.
“With keloids disproportionately affecting Black and other skin of color patients, denying treatment on a notion that lacks evidentiary support further potentiates the health disparities experienced by these marginalized groups,” the researchers reported at the Annual Meeting of the Skin of Color Society Scientific Symposium (SOCS) 2022. In their retrospective study of 129 patients with HS treated with CO2 laser, “there were no cases of keloid formation,” they say.
HS, a potentially debilitating chronic inflammatory condition that involves painful nodules, boils, and abscesses, is often refractory to standard treatment. CO2 laser excision therapy has yielded favorable outcomes in some studies.
Although CO2 laser therapy is also used to treat keloids, some clinicians hesitate to use this treatment in these patients because of concerns that its use for treating HS could trigger the development of keloids.
“Many patients come in telling us they were denied [CO2 laser] surgery due to keloids,” senior author Iltefat Hamzavi, MD, a senior staff physician in the Department of Dermatology at the Henry Ford Health System, Detroit, told this news organization.
Although patients with HS are commonly treated with CO2 laser excision in his department, this treatment approach “is underused nationally,” he said.
“Of note, the sinus tunnels of hidradenitis suppurativa can look like keloids, so this might drive surgeons away from treating [those] lesions,” Dr. Hamzavi said.
To further evaluate the risk of developing keloids with the treatment, Dr. Hamzavi and his colleagues conducted a retrospective review of 129 patients with HS treated at Henry Ford who had undergone follicular destruction with CO2 laser between 2014 and 2021; 102 (79%) patients were female. The mean age was about 38 years (range, 15-78 years).
Of the patients, almost half were Black, almost 40% were White, 5% were Asian, and 3% were of unknown ethnicity.
Medical records of nine patients included diagnoses of keloids or hypertrophic scars. Further review indicated that none of the diagnoses were for keloids but were for hypertrophic scars, hypertrophic granulation tissue, an HS nodule, or contracture scar, the authors report.
“While the emergence of hypertrophic scars, hypertrophic granulation tissue, and scar contracture following CO2 laser excision therapy for hidradenitis suppurativa has been documented in the literature, existing evidence does not support postoperative keloid formation,” the authors conclude.
Because healing time with CO2 laser treatment is prolonged and there is an increase in risk of adverse events, Dr. Hamzavi underscored that “safety protocols for CO2 lasers should be followed, and wound prep instructions should be provided along with counseling on healing times.”
Regarding patient selection, he noted that “the disease should be medically stable with reduction in drainage to help control postop bleeding risk.”
The findings of the study are supported by a recent systematic review that compared outcomes and adverse effects of treatment with ablative laser therapies with nonablative lasers for skin resurfacing. The review included 34 studies and involved 1,093 patients. The conditions that were treated ranged from photodamage and acne scars to HS and post-traumatic scarring from basal cell carcinoma excision.
That review found that overall, rates of adverse events were higher with nonablative therapies (12.2%, 31 events), compared with ablative laser therapy, such as with CO2 laser (8.28%, 81 events). In addition, when transient events were excluded, ablative lasers were associated with fewer complications overall, compared with nonablative lasers (2.56% vs. 7.48%).
The authors conclude: “It is our hope that this study will facilitate continued research in this domain in an effort to combat these inequities and improve access to CO2 excision or standardized excisional therapy for hidradenitis suppurativa treatment.”
Dr. Hamzavi and the other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT SOCS 2022
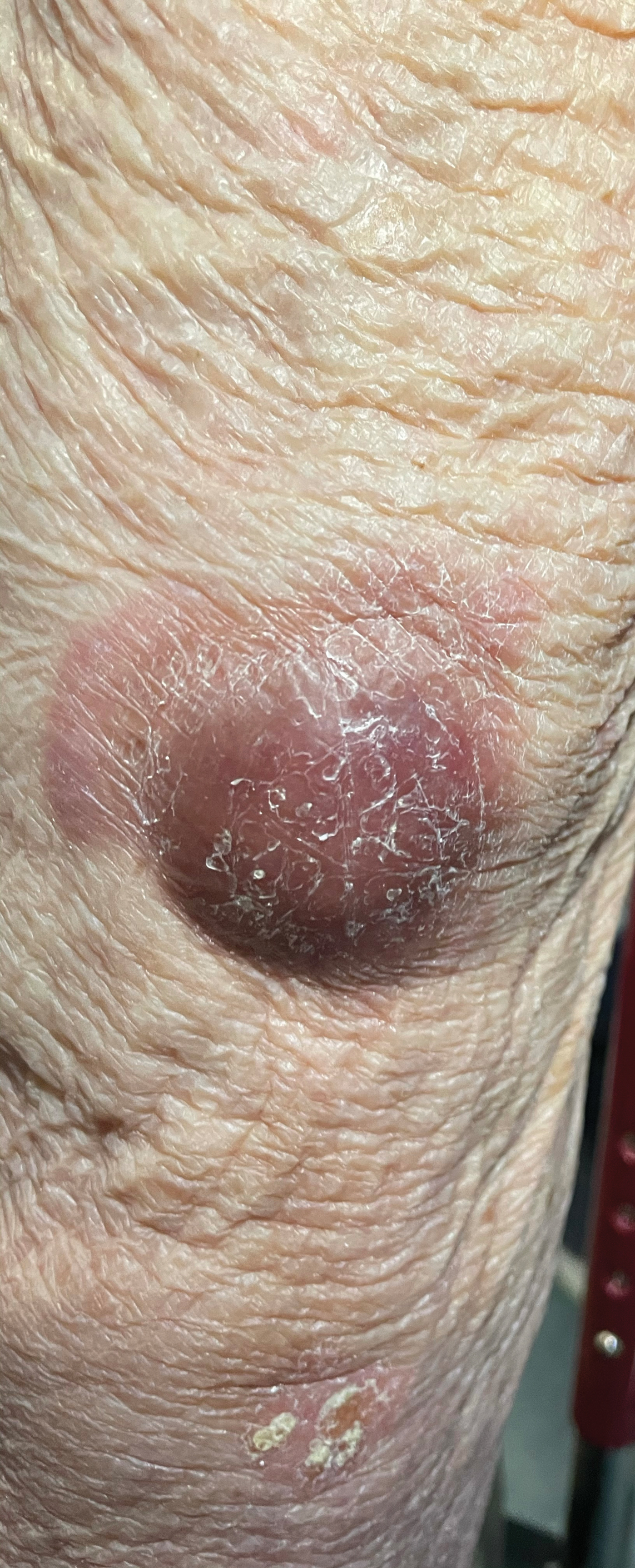
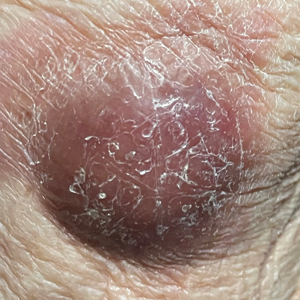
Violaceous Nodules on the Lower Leg
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.
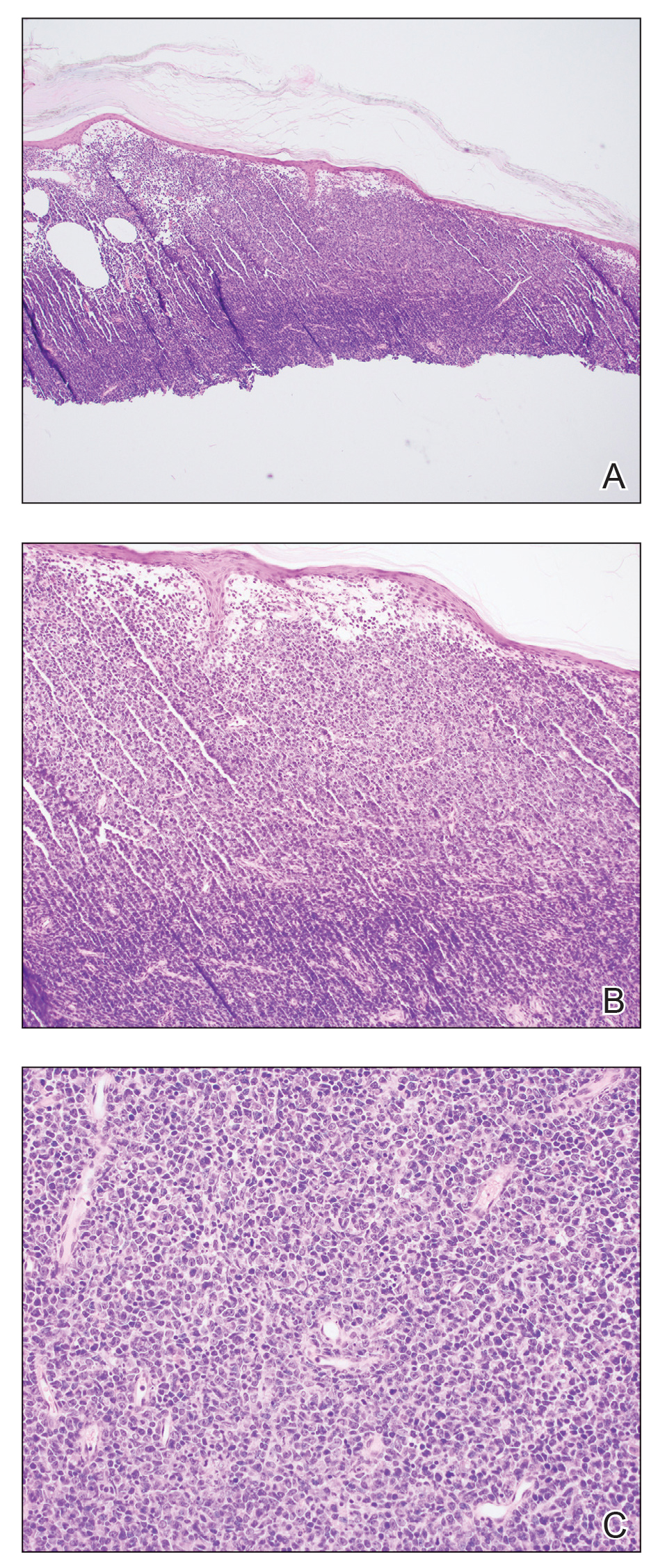
Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
A 79-year-old man presented to the dermatology clinic with 4 enlarging, asymptomatic, violaceous, desquamating nodules on the left pretibial region and calf of 3 months’ duration. He denied any constitutional symptoms such as night sweats or weight loss. His medical history included a malignant melanoma on the left ear that was excised 5 years prior. He also had a history of peripheral edema, hypertension, and rheumatoid arthritis, as well as a 50-pack-year history of smoking. Physical examination revealed 2 large nodules measuring 3.0×3.0 cm each and 2 smaller nodules measuring 1.0×1.0 cm each. There was no appreciable lymphadenopathy.