User login
Scientists find microplastics in human lung tissue
U.K. scientists said microplastics may pose even more of a threat than previously thought after confirming their presence in lung tissue taken from living people.
Microplastics were identified in all lung regions, but significantly higher levels were found in the lower lung.
The results supported inhalation as an exposure risk, according to the team from the University of Hull and Hull York Medical School (England), who said their findings could support further investigations into the effects of airborne microplastics on respiratory health.
The study, published in Science of the Total Environment, used lung tissue collected from surgical procedures on patients during routine medical care at Castle Hill Hospital in East Yorkshire.
Polypropylene and polyethylene
It found 39 microplastics in 11 of the 13 lung tissue samples tested using micro-Fourier-transform infrared (μFTIR) analysis, which the scientists said was considerably higher than results from previous laboratory tests.
Of microplastics detected, 12 polymer types were identified, of which the most common were polypropylene, (23%) polyethylene terephthalate (18%), and resin (15%). The fibers are commonly found in packaging, bottles, clothing, rope and twine manufacture, and other industries, the scientists said.
Microplastics with dimensions as small as 4 μm were found, but the scientists said they were surprised to discover samples as large as greater than 2 mm within all lung region samples, with the majority being fibrous and fragmented.
The study identified 11 microplastics in the upper part of the lung, seven in the mid part, and 21 in the lower part of the lung.
Laura Sadofsky, the study’s lead author, said: “Microplastics have previously been found in human cadaver autopsy samples. This is the first robust study to show microplastics in lungs from live people. It also shows that they are in the lower parts of the lung. Lung airways are very narrow, so no one thought they could possibly get there, but they clearly have.”
There were also considerably higher levels of microplastics found in male patients, compared with female patients.
Future investigations into health implications
“The characterization of types and levels of microplastics we have found can now inform realistic conditions for laboratory exposure experiments with the aim of determining health impacts,” said Laura Sadofsky, who is a senior lecturer in respiratory medicine in the Centre for Atherothrombotic and Metabolic Research at Hull York Medical School.
The latest investigation followed previous research by the medical school and the University of Hull, which found high levels of atmospheric microplastics within the Humber region.
That study, published in Atmosphere, identified resins, which could have originated from degraded roads, paint marking, or tire rubber, as well as polyethylene fibers.
A version of this article first appeared on Medscape UK.
U.K. scientists said microplastics may pose even more of a threat than previously thought after confirming their presence in lung tissue taken from living people.
Microplastics were identified in all lung regions, but significantly higher levels were found in the lower lung.
The results supported inhalation as an exposure risk, according to the team from the University of Hull and Hull York Medical School (England), who said their findings could support further investigations into the effects of airborne microplastics on respiratory health.
The study, published in Science of the Total Environment, used lung tissue collected from surgical procedures on patients during routine medical care at Castle Hill Hospital in East Yorkshire.
Polypropylene and polyethylene
It found 39 microplastics in 11 of the 13 lung tissue samples tested using micro-Fourier-transform infrared (μFTIR) analysis, which the scientists said was considerably higher than results from previous laboratory tests.
Of microplastics detected, 12 polymer types were identified, of which the most common were polypropylene, (23%) polyethylene terephthalate (18%), and resin (15%). The fibers are commonly found in packaging, bottles, clothing, rope and twine manufacture, and other industries, the scientists said.
Microplastics with dimensions as small as 4 μm were found, but the scientists said they were surprised to discover samples as large as greater than 2 mm within all lung region samples, with the majority being fibrous and fragmented.
The study identified 11 microplastics in the upper part of the lung, seven in the mid part, and 21 in the lower part of the lung.
Laura Sadofsky, the study’s lead author, said: “Microplastics have previously been found in human cadaver autopsy samples. This is the first robust study to show microplastics in lungs from live people. It also shows that they are in the lower parts of the lung. Lung airways are very narrow, so no one thought they could possibly get there, but they clearly have.”
There were also considerably higher levels of microplastics found in male patients, compared with female patients.
Future investigations into health implications
“The characterization of types and levels of microplastics we have found can now inform realistic conditions for laboratory exposure experiments with the aim of determining health impacts,” said Laura Sadofsky, who is a senior lecturer in respiratory medicine in the Centre for Atherothrombotic and Metabolic Research at Hull York Medical School.
The latest investigation followed previous research by the medical school and the University of Hull, which found high levels of atmospheric microplastics within the Humber region.
That study, published in Atmosphere, identified resins, which could have originated from degraded roads, paint marking, or tire rubber, as well as polyethylene fibers.
A version of this article first appeared on Medscape UK.
U.K. scientists said microplastics may pose even more of a threat than previously thought after confirming their presence in lung tissue taken from living people.
Microplastics were identified in all lung regions, but significantly higher levels were found in the lower lung.
The results supported inhalation as an exposure risk, according to the team from the University of Hull and Hull York Medical School (England), who said their findings could support further investigations into the effects of airborne microplastics on respiratory health.
The study, published in Science of the Total Environment, used lung tissue collected from surgical procedures on patients during routine medical care at Castle Hill Hospital in East Yorkshire.
Polypropylene and polyethylene
It found 39 microplastics in 11 of the 13 lung tissue samples tested using micro-Fourier-transform infrared (μFTIR) analysis, which the scientists said was considerably higher than results from previous laboratory tests.
Of microplastics detected, 12 polymer types were identified, of which the most common were polypropylene, (23%) polyethylene terephthalate (18%), and resin (15%). The fibers are commonly found in packaging, bottles, clothing, rope and twine manufacture, and other industries, the scientists said.
Microplastics with dimensions as small as 4 μm were found, but the scientists said they were surprised to discover samples as large as greater than 2 mm within all lung region samples, with the majority being fibrous and fragmented.
The study identified 11 microplastics in the upper part of the lung, seven in the mid part, and 21 in the lower part of the lung.
Laura Sadofsky, the study’s lead author, said: “Microplastics have previously been found in human cadaver autopsy samples. This is the first robust study to show microplastics in lungs from live people. It also shows that they are in the lower parts of the lung. Lung airways are very narrow, so no one thought they could possibly get there, but they clearly have.”
There were also considerably higher levels of microplastics found in male patients, compared with female patients.
Future investigations into health implications
“The characterization of types and levels of microplastics we have found can now inform realistic conditions for laboratory exposure experiments with the aim of determining health impacts,” said Laura Sadofsky, who is a senior lecturer in respiratory medicine in the Centre for Atherothrombotic and Metabolic Research at Hull York Medical School.
The latest investigation followed previous research by the medical school and the University of Hull, which found high levels of atmospheric microplastics within the Humber region.
That study, published in Atmosphere, identified resins, which could have originated from degraded roads, paint marking, or tire rubber, as well as polyethylene fibers.
A version of this article first appeared on Medscape UK.
Sex differences in COPD slow to be recognized, treated
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
Children and COVID: Cases drop again, admission rate up slightly
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.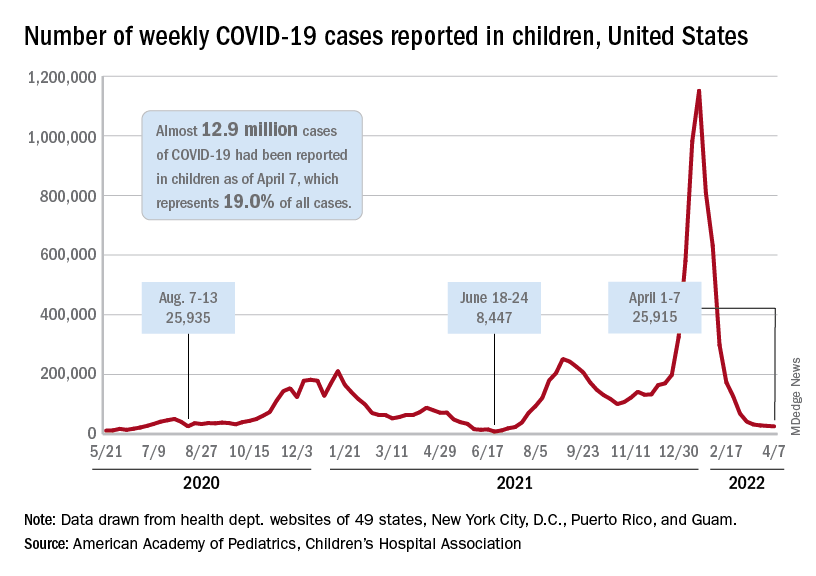
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
Has the anti-benzodiazepine backlash gone too far?
When benzodiazepines were first introduced, they were greeted with enthusiasm. Librium came first, in 1960, followed by Valium in 1962, and they were seen as an improvement over barbiturates for the treatment of anxiety, insomnia, and seizures. From 1968 to 1982, Valium (diazepam) was the No. 1–selling U.S. pharmaceutical: 2.3 billion tablets of Valium were sold in 1978 alone. Valium was even the subject of a 1966 Rolling Stones hit, “Mother’s Little Helper.”
By the 1980s, it became apparent that there was a downside to these medications: patients became tolerant, dependent, and some became addicted to the medications. In older patients an association was noted with falls and cognitive impairment. And while safe in overdoses when they are the only agent, combined with alcohol or opioids, benzodiazepines can be lethal and have played a significant role in the current overdose crisis.
Because of the problems that are associated with their use, benzodiazepines and their relatives, the Z-drugs used for sleep, have become stigmatized, as have the patients who use them and perhaps even the doctors who prescribe them. Still, there are circumstances where patients find these medications to be helpful, where other medications don’t work, or don’t work quickly enough. They provide fast relief in conditions where there are not always good alternatives.
In the Facebook group, “Psychiatry for All Physicians,” it’s not uncommon for physicians to ask what to do with older patients who are transferred to them on therapeutic doses of benzodiazepines or zolpidem. These are outpatients coming for routine care, and they find the medications helpful and don’t want to discontinue them. They have tried other medications that were not helpful. I’ve been surprised at how often the respondents insist the patient should be told he must taper off the medication. “Just say no,” is often the advice, and perhaps it’s more about the doctor’s discomfort than it is about the individual patient. For sleep issues, cognitive-behavioral therapy is given as the gold-standard treatment, while in my practice I have found it difficult to motivate patients to engage in it, and of those who do, it is sometimes helpful, but not a panacea. Severe anxiety and sleepless nights, however, are not benign conditions.
This “just say no, hold the line” sentiment has me wondering if our pendulum has swung too far with respect to prescribing benzodiazepines. Is this just one more issue that has become strongly polarized? Certainly the literature would support that idea, with some physicians writing about how benzodiazepines are underused, and others urging avoidance.
I posted a poll on Twitter: Has the anti-benzo movement gone too far? In addition, I started a Twitter thread of my own thoughts about prescribing and deprescribing these medications and will give a synopsis of those ideas here.
Clearly, benzodiazepines are harmful to some patients, they have side effects, can be difficult to stop because of withdrawal symptoms, and they carry the risk of addiction. That’s not in question. Many medications, however, have the potential to do more harm than good, for example ibuprofen can cause bleeding or renal problems, and Fosamax, used to treat osteoporosis, can cause osteonecrosis of the jaw and femur, to name just two.
It would be so much easier if we could know in advance who benzodiazepines will harm, just as it would be good if we could know in advance who will get tardive dyskinesia or dyslipidemia from antipsychotic medications, or who will have life-threatening adverse reactions from cancer chemotherapy with no tumor response. There are risks to both starting and stopping sedatives, and if we insist a patient stop a medication because of potential risk, then we are cutting them off from being a partner in their own care. It also creates an adversarial relationship that can be draining for the doctor and upsetting for the patient.
By definition, if someone needs hospitalization for a psychiatric condition, their outpatient benzodiazepine is not keeping them stable and stopping it may be a good idea. If someone is seen in an ED for a fall, it’s common to blame the benzodiazepine, but older people who are not on these medications also fall and have memory problems. In his book, “Being Mortal: Illness, Medicine, and What Matters Most in the End” (New York: Picador, 2014), Atul Gawande, MD, makes the point that taking more than four prescriptions medications increases the risk for falls in the elderly. Still, no one is suggesting patients be taken off their antidepressants, antihypertensives, or blood thinners.
Finally, the question is not should we be giving benzodiazepines out without careful consideration – the answer is clearly no. Physicians don’t pass out benzodiazepines “like candy” for all the above reasons. They are initiated because the patient is suffering and sometimes desperate. Anxiety, panic, intractable insomnia, and severe agitation are all miserable, and alternative treatments may take weeks to work, or not work at all. Yet these subjective symptoms may be dismissed by physicians.
So what do I do in my own practice? I don’t encourage patients to take potentially addictive medications, but I do sometimes use them. I give ‘as needed’ benzodiazepines to people in distress who don’t have a history of misusing them. I never plan to start them as a permanent standing medication, though once in a while that ends up happening. As with other medications, it is best to use the minimally effective dose.
There is some controversy as to whether it is best to use anxiety medications on an “as-needed” basis or as a standing dosage. Psychiatrists who prescribe benzodiazepines more liberally often feel it’s better to give standing doses and prevent breakthrough anxiety. Patients may appear to be ‘medication seeking’ not because they are addicted, but because the doses used are too low to adequately treat their anxiety.
My hope is that there is less risk of tolerance, dependence, or addiction with less-frequent dosing, and I prescribe as-needed benzodiazepines for panic attacks, agitated major depression while we wait for the antidepressant to “kick in,” insomnia during manic episodes, and to people who get very anxious in specific situations such as flying or for medical procedures. I sometimes prescribe them for people with insomnia that does not respond to other treatments, or for disabling generalized anxiety.
For patients who have taken benzodiazepines for many years, I continue to discuss the risks, but often they are not looking to fix something that isn’t broken, or to live a risk-free life. A few of the patients who have come to me on low standing doses of sedatives are now in their 80’s, yet they remain active, live independently, drive, travel, and have busy social lives. One could argue either that the medications are working, or that the patient has become dependent on them and needs them to prevent withdrawal.
These medications present a quandary: by denying patients treatment with benzodiazepines, we are sparing some people addictions (this is good, we should be careful), but we are leaving some people to suffer. There is no perfect answer.
What I do know is that doctors should think carefully and consider the patient in front of them. “No Benzos Ever For Anyone” or “you must come off because there is risk and people will think I am a bad doctor for prescribing them to you” can be done by a robot.
So, yes, I think the pendulum has swung a bit too far; there is a place for these medications in acute treatment for those at low risk of addiction, and there are people who benefit from them over the long run. At times, they provide immense relief to someone who is really struggling.
So what was the result of my Twitter poll? Of the 219 voters, 34.2% voted: “No, the pendulum has not swung too far, and these medications are harmful”; 65.8% voted: “Yes, these medications are helpful.” There were many comments expressing a wide variety of sentiments. Of those who had taken prescription benzodiazepines, some felt they had been harmed and wished they had never been started on them, and others continue to find them helpful. Psychiatrists, it seems, see them from the vantage point of the populations they treat.
People who are uncomfortable search for answers, and those answers may come in the form of meditation or exercise, medicines, or illicit drugs. It’s interesting that these same patients can now easily obtain “medical” marijuana, and the Rolling Stones’ “Mother’s Little Helper” is often replaced by a gin and tonic.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
When benzodiazepines were first introduced, they were greeted with enthusiasm. Librium came first, in 1960, followed by Valium in 1962, and they were seen as an improvement over barbiturates for the treatment of anxiety, insomnia, and seizures. From 1968 to 1982, Valium (diazepam) was the No. 1–selling U.S. pharmaceutical: 2.3 billion tablets of Valium were sold in 1978 alone. Valium was even the subject of a 1966 Rolling Stones hit, “Mother’s Little Helper.”
By the 1980s, it became apparent that there was a downside to these medications: patients became tolerant, dependent, and some became addicted to the medications. In older patients an association was noted with falls and cognitive impairment. And while safe in overdoses when they are the only agent, combined with alcohol or opioids, benzodiazepines can be lethal and have played a significant role in the current overdose crisis.
Because of the problems that are associated with their use, benzodiazepines and their relatives, the Z-drugs used for sleep, have become stigmatized, as have the patients who use them and perhaps even the doctors who prescribe them. Still, there are circumstances where patients find these medications to be helpful, where other medications don’t work, or don’t work quickly enough. They provide fast relief in conditions where there are not always good alternatives.
In the Facebook group, “Psychiatry for All Physicians,” it’s not uncommon for physicians to ask what to do with older patients who are transferred to them on therapeutic doses of benzodiazepines or zolpidem. These are outpatients coming for routine care, and they find the medications helpful and don’t want to discontinue them. They have tried other medications that were not helpful. I’ve been surprised at how often the respondents insist the patient should be told he must taper off the medication. “Just say no,” is often the advice, and perhaps it’s more about the doctor’s discomfort than it is about the individual patient. For sleep issues, cognitive-behavioral therapy is given as the gold-standard treatment, while in my practice I have found it difficult to motivate patients to engage in it, and of those who do, it is sometimes helpful, but not a panacea. Severe anxiety and sleepless nights, however, are not benign conditions.
This “just say no, hold the line” sentiment has me wondering if our pendulum has swung too far with respect to prescribing benzodiazepines. Is this just one more issue that has become strongly polarized? Certainly the literature would support that idea, with some physicians writing about how benzodiazepines are underused, and others urging avoidance.
I posted a poll on Twitter: Has the anti-benzo movement gone too far? In addition, I started a Twitter thread of my own thoughts about prescribing and deprescribing these medications and will give a synopsis of those ideas here.
Clearly, benzodiazepines are harmful to some patients, they have side effects, can be difficult to stop because of withdrawal symptoms, and they carry the risk of addiction. That’s not in question. Many medications, however, have the potential to do more harm than good, for example ibuprofen can cause bleeding or renal problems, and Fosamax, used to treat osteoporosis, can cause osteonecrosis of the jaw and femur, to name just two.
It would be so much easier if we could know in advance who benzodiazepines will harm, just as it would be good if we could know in advance who will get tardive dyskinesia or dyslipidemia from antipsychotic medications, or who will have life-threatening adverse reactions from cancer chemotherapy with no tumor response. There are risks to both starting and stopping sedatives, and if we insist a patient stop a medication because of potential risk, then we are cutting them off from being a partner in their own care. It also creates an adversarial relationship that can be draining for the doctor and upsetting for the patient.
By definition, if someone needs hospitalization for a psychiatric condition, their outpatient benzodiazepine is not keeping them stable and stopping it may be a good idea. If someone is seen in an ED for a fall, it’s common to blame the benzodiazepine, but older people who are not on these medications also fall and have memory problems. In his book, “Being Mortal: Illness, Medicine, and What Matters Most in the End” (New York: Picador, 2014), Atul Gawande, MD, makes the point that taking more than four prescriptions medications increases the risk for falls in the elderly. Still, no one is suggesting patients be taken off their antidepressants, antihypertensives, or blood thinners.
Finally, the question is not should we be giving benzodiazepines out without careful consideration – the answer is clearly no. Physicians don’t pass out benzodiazepines “like candy” for all the above reasons. They are initiated because the patient is suffering and sometimes desperate. Anxiety, panic, intractable insomnia, and severe agitation are all miserable, and alternative treatments may take weeks to work, or not work at all. Yet these subjective symptoms may be dismissed by physicians.
So what do I do in my own practice? I don’t encourage patients to take potentially addictive medications, but I do sometimes use them. I give ‘as needed’ benzodiazepines to people in distress who don’t have a history of misusing them. I never plan to start them as a permanent standing medication, though once in a while that ends up happening. As with other medications, it is best to use the minimally effective dose.
There is some controversy as to whether it is best to use anxiety medications on an “as-needed” basis or as a standing dosage. Psychiatrists who prescribe benzodiazepines more liberally often feel it’s better to give standing doses and prevent breakthrough anxiety. Patients may appear to be ‘medication seeking’ not because they are addicted, but because the doses used are too low to adequately treat their anxiety.
My hope is that there is less risk of tolerance, dependence, or addiction with less-frequent dosing, and I prescribe as-needed benzodiazepines for panic attacks, agitated major depression while we wait for the antidepressant to “kick in,” insomnia during manic episodes, and to people who get very anxious in specific situations such as flying or for medical procedures. I sometimes prescribe them for people with insomnia that does not respond to other treatments, or for disabling generalized anxiety.
For patients who have taken benzodiazepines for many years, I continue to discuss the risks, but often they are not looking to fix something that isn’t broken, or to live a risk-free life. A few of the patients who have come to me on low standing doses of sedatives are now in their 80’s, yet they remain active, live independently, drive, travel, and have busy social lives. One could argue either that the medications are working, or that the patient has become dependent on them and needs them to prevent withdrawal.
These medications present a quandary: by denying patients treatment with benzodiazepines, we are sparing some people addictions (this is good, we should be careful), but we are leaving some people to suffer. There is no perfect answer.
What I do know is that doctors should think carefully and consider the patient in front of them. “No Benzos Ever For Anyone” or “you must come off because there is risk and people will think I am a bad doctor for prescribing them to you” can be done by a robot.
So, yes, I think the pendulum has swung a bit too far; there is a place for these medications in acute treatment for those at low risk of addiction, and there are people who benefit from them over the long run. At times, they provide immense relief to someone who is really struggling.
So what was the result of my Twitter poll? Of the 219 voters, 34.2% voted: “No, the pendulum has not swung too far, and these medications are harmful”; 65.8% voted: “Yes, these medications are helpful.” There were many comments expressing a wide variety of sentiments. Of those who had taken prescription benzodiazepines, some felt they had been harmed and wished they had never been started on them, and others continue to find them helpful. Psychiatrists, it seems, see them from the vantage point of the populations they treat.
People who are uncomfortable search for answers, and those answers may come in the form of meditation or exercise, medicines, or illicit drugs. It’s interesting that these same patients can now easily obtain “medical” marijuana, and the Rolling Stones’ “Mother’s Little Helper” is often replaced by a gin and tonic.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
When benzodiazepines were first introduced, they were greeted with enthusiasm. Librium came first, in 1960, followed by Valium in 1962, and they were seen as an improvement over barbiturates for the treatment of anxiety, insomnia, and seizures. From 1968 to 1982, Valium (diazepam) was the No. 1–selling U.S. pharmaceutical: 2.3 billion tablets of Valium were sold in 1978 alone. Valium was even the subject of a 1966 Rolling Stones hit, “Mother’s Little Helper.”
By the 1980s, it became apparent that there was a downside to these medications: patients became tolerant, dependent, and some became addicted to the medications. In older patients an association was noted with falls and cognitive impairment. And while safe in overdoses when they are the only agent, combined with alcohol or opioids, benzodiazepines can be lethal and have played a significant role in the current overdose crisis.
Because of the problems that are associated with their use, benzodiazepines and their relatives, the Z-drugs used for sleep, have become stigmatized, as have the patients who use them and perhaps even the doctors who prescribe them. Still, there are circumstances where patients find these medications to be helpful, where other medications don’t work, or don’t work quickly enough. They provide fast relief in conditions where there are not always good alternatives.
In the Facebook group, “Psychiatry for All Physicians,” it’s not uncommon for physicians to ask what to do with older patients who are transferred to them on therapeutic doses of benzodiazepines or zolpidem. These are outpatients coming for routine care, and they find the medications helpful and don’t want to discontinue them. They have tried other medications that were not helpful. I’ve been surprised at how often the respondents insist the patient should be told he must taper off the medication. “Just say no,” is often the advice, and perhaps it’s more about the doctor’s discomfort than it is about the individual patient. For sleep issues, cognitive-behavioral therapy is given as the gold-standard treatment, while in my practice I have found it difficult to motivate patients to engage in it, and of those who do, it is sometimes helpful, but not a panacea. Severe anxiety and sleepless nights, however, are not benign conditions.
This “just say no, hold the line” sentiment has me wondering if our pendulum has swung too far with respect to prescribing benzodiazepines. Is this just one more issue that has become strongly polarized? Certainly the literature would support that idea, with some physicians writing about how benzodiazepines are underused, and others urging avoidance.
I posted a poll on Twitter: Has the anti-benzo movement gone too far? In addition, I started a Twitter thread of my own thoughts about prescribing and deprescribing these medications and will give a synopsis of those ideas here.
Clearly, benzodiazepines are harmful to some patients, they have side effects, can be difficult to stop because of withdrawal symptoms, and they carry the risk of addiction. That’s not in question. Many medications, however, have the potential to do more harm than good, for example ibuprofen can cause bleeding or renal problems, and Fosamax, used to treat osteoporosis, can cause osteonecrosis of the jaw and femur, to name just two.
It would be so much easier if we could know in advance who benzodiazepines will harm, just as it would be good if we could know in advance who will get tardive dyskinesia or dyslipidemia from antipsychotic medications, or who will have life-threatening adverse reactions from cancer chemotherapy with no tumor response. There are risks to both starting and stopping sedatives, and if we insist a patient stop a medication because of potential risk, then we are cutting them off from being a partner in their own care. It also creates an adversarial relationship that can be draining for the doctor and upsetting for the patient.
By definition, if someone needs hospitalization for a psychiatric condition, their outpatient benzodiazepine is not keeping them stable and stopping it may be a good idea. If someone is seen in an ED for a fall, it’s common to blame the benzodiazepine, but older people who are not on these medications also fall and have memory problems. In his book, “Being Mortal: Illness, Medicine, and What Matters Most in the End” (New York: Picador, 2014), Atul Gawande, MD, makes the point that taking more than four prescriptions medications increases the risk for falls in the elderly. Still, no one is suggesting patients be taken off their antidepressants, antihypertensives, or blood thinners.
Finally, the question is not should we be giving benzodiazepines out without careful consideration – the answer is clearly no. Physicians don’t pass out benzodiazepines “like candy” for all the above reasons. They are initiated because the patient is suffering and sometimes desperate. Anxiety, panic, intractable insomnia, and severe agitation are all miserable, and alternative treatments may take weeks to work, or not work at all. Yet these subjective symptoms may be dismissed by physicians.
So what do I do in my own practice? I don’t encourage patients to take potentially addictive medications, but I do sometimes use them. I give ‘as needed’ benzodiazepines to people in distress who don’t have a history of misusing them. I never plan to start them as a permanent standing medication, though once in a while that ends up happening. As with other medications, it is best to use the minimally effective dose.
There is some controversy as to whether it is best to use anxiety medications on an “as-needed” basis or as a standing dosage. Psychiatrists who prescribe benzodiazepines more liberally often feel it’s better to give standing doses and prevent breakthrough anxiety. Patients may appear to be ‘medication seeking’ not because they are addicted, but because the doses used are too low to adequately treat their anxiety.
My hope is that there is less risk of tolerance, dependence, or addiction with less-frequent dosing, and I prescribe as-needed benzodiazepines for panic attacks, agitated major depression while we wait for the antidepressant to “kick in,” insomnia during manic episodes, and to people who get very anxious in specific situations such as flying or for medical procedures. I sometimes prescribe them for people with insomnia that does not respond to other treatments, or for disabling generalized anxiety.
For patients who have taken benzodiazepines for many years, I continue to discuss the risks, but often they are not looking to fix something that isn’t broken, or to live a risk-free life. A few of the patients who have come to me on low standing doses of sedatives are now in their 80’s, yet they remain active, live independently, drive, travel, and have busy social lives. One could argue either that the medications are working, or that the patient has become dependent on them and needs them to prevent withdrawal.
These medications present a quandary: by denying patients treatment with benzodiazepines, we are sparing some people addictions (this is good, we should be careful), but we are leaving some people to suffer. There is no perfect answer.
What I do know is that doctors should think carefully and consider the patient in front of them. “No Benzos Ever For Anyone” or “you must come off because there is risk and people will think I am a bad doctor for prescribing them to you” can be done by a robot.
So, yes, I think the pendulum has swung a bit too far; there is a place for these medications in acute treatment for those at low risk of addiction, and there are people who benefit from them over the long run. At times, they provide immense relief to someone who is really struggling.
So what was the result of my Twitter poll? Of the 219 voters, 34.2% voted: “No, the pendulum has not swung too far, and these medications are harmful”; 65.8% voted: “Yes, these medications are helpful.” There were many comments expressing a wide variety of sentiments. Of those who had taken prescription benzodiazepines, some felt they had been harmed and wished they had never been started on them, and others continue to find them helpful. Psychiatrists, it seems, see them from the vantage point of the populations they treat.
People who are uncomfortable search for answers, and those answers may come in the form of meditation or exercise, medicines, or illicit drugs. It’s interesting that these same patients can now easily obtain “medical” marijuana, and the Rolling Stones’ “Mother’s Little Helper” is often replaced by a gin and tonic.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. Dr. Miller has no conflicts of interest.
Counterfeit HIV drugs: Justice Department opens investigation
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves Fitbit’s AFib-detection software
A popular fitness tracker company has received approval from the Food and Drug Administration for a new software algorithm to detect atrial fibrillation (AFib), Fitbit announced on April 11.
The algorithm will be the basis of an upcoming Fitbit feature called Irregular Heart Rhythm Notifications, the company said in a press release.
The approval was based on data from the Fitbit Heart Study, which was conducted entirely virtually in more than 455,000 U.S. adults. Participants who had an irregular heart rhythm detected by the software algorithm were notified and invited to meet with a telehealth doctor. They then received a 1-week ECG patch monitor to wear along with the smartwatch or fitness tracker.
Results, presented at the annual scientific sessions of the American Heart Association in November 2021, showed that the positive predictive value of the Fitbit algorithm for detecting undiagnosed AFib with a range of wearable devices was 98%. Notably, irregular heart rhythm detection occurred in 1% of participants overall and 4% of those older than 65 years.
The algorithm works by using an optical measurement method called photoplethysmography (PPG), along with heart rate input from the Fitbit’s photodetector device.
It operates only when the user is still or at rest, so overnight use is important for detection, the company noted.
The upcoming Irregular Heart Rhythm Notifications feature will complement the existing ECG app, providing two ways to detect AFib. The ECG app provides a “spot-check approach” in which the users can screen themselves, and the PPG-based feature will allow for long-term heart rhythm assessment, the statement explained.
“Undiagnosed atrial fibrillation can lead to strokes, and early detection of atrial fibrillation may allow doctors to prescribe medications that are effective at preventing strokes,” said Steven A. Lubitz, MD, MPH, a cardiologist at Harvard University and Massachusetts General Hospital, both in Boston, at the AHA meeting.
A popular fitness tracker company has received approval from the Food and Drug Administration for a new software algorithm to detect atrial fibrillation (AFib), Fitbit announced on April 11.
The algorithm will be the basis of an upcoming Fitbit feature called Irregular Heart Rhythm Notifications, the company said in a press release.
The approval was based on data from the Fitbit Heart Study, which was conducted entirely virtually in more than 455,000 U.S. adults. Participants who had an irregular heart rhythm detected by the software algorithm were notified and invited to meet with a telehealth doctor. They then received a 1-week ECG patch monitor to wear along with the smartwatch or fitness tracker.
Results, presented at the annual scientific sessions of the American Heart Association in November 2021, showed that the positive predictive value of the Fitbit algorithm for detecting undiagnosed AFib with a range of wearable devices was 98%. Notably, irregular heart rhythm detection occurred in 1% of participants overall and 4% of those older than 65 years.
The algorithm works by using an optical measurement method called photoplethysmography (PPG), along with heart rate input from the Fitbit’s photodetector device.
It operates only when the user is still or at rest, so overnight use is important for detection, the company noted.
The upcoming Irregular Heart Rhythm Notifications feature will complement the existing ECG app, providing two ways to detect AFib. The ECG app provides a “spot-check approach” in which the users can screen themselves, and the PPG-based feature will allow for long-term heart rhythm assessment, the statement explained.
“Undiagnosed atrial fibrillation can lead to strokes, and early detection of atrial fibrillation may allow doctors to prescribe medications that are effective at preventing strokes,” said Steven A. Lubitz, MD, MPH, a cardiologist at Harvard University and Massachusetts General Hospital, both in Boston, at the AHA meeting.
A popular fitness tracker company has received approval from the Food and Drug Administration for a new software algorithm to detect atrial fibrillation (AFib), Fitbit announced on April 11.
The algorithm will be the basis of an upcoming Fitbit feature called Irregular Heart Rhythm Notifications, the company said in a press release.
The approval was based on data from the Fitbit Heart Study, which was conducted entirely virtually in more than 455,000 U.S. adults. Participants who had an irregular heart rhythm detected by the software algorithm were notified and invited to meet with a telehealth doctor. They then received a 1-week ECG patch monitor to wear along with the smartwatch or fitness tracker.
Results, presented at the annual scientific sessions of the American Heart Association in November 2021, showed that the positive predictive value of the Fitbit algorithm for detecting undiagnosed AFib with a range of wearable devices was 98%. Notably, irregular heart rhythm detection occurred in 1% of participants overall and 4% of those older than 65 years.
The algorithm works by using an optical measurement method called photoplethysmography (PPG), along with heart rate input from the Fitbit’s photodetector device.
It operates only when the user is still or at rest, so overnight use is important for detection, the company noted.
The upcoming Irregular Heart Rhythm Notifications feature will complement the existing ECG app, providing two ways to detect AFib. The ECG app provides a “spot-check approach” in which the users can screen themselves, and the PPG-based feature will allow for long-term heart rhythm assessment, the statement explained.
“Undiagnosed atrial fibrillation can lead to strokes, and early detection of atrial fibrillation may allow doctors to prescribe medications that are effective at preventing strokes,” said Steven A. Lubitz, MD, MPH, a cardiologist at Harvard University and Massachusetts General Hospital, both in Boston, at the AHA meeting.
New insight into how psychedelics work
What causes the dramatic alterations in subjective awareness experienced during a psychedelic “trip?” A new study maps anatomical changes in specific neurotransmitter systems and brain regions that may be responsible for these effects.
Investigators gathered more than 6,800 accounts from individuals who had taken one of 27 different psychedelic compounds. Using a machine learning strategy, they extracted commonly used words from these testimonials, linking them with 40 different neurotransmitter subtypes that had likely induced these experiences.
The investigators then linked these subjective experiences with specific brain regions where the receptor combinations are most commonly found and, using gene transcription probes, created a 3D whole-brain map of the brain receptors and the subjective experiences linked to them.
“Hallucinogenic drugs may very well turn out to be the next big thing to improve clinical care of major mental health conditions,” senior author Danilo Bzdok, MD, PhD, associate professor, McGill University, Montreal, said in a press release.
“Our study provides a first step, a proof of principle, that we may be able to build machine-learning systems in the future that can accurately predict which neurotransmitter receptor combinations need to be stimulated to induce a specific state of conscious experience in a given person,” said Dr. Bzdok, who is also the Canada CIFAR AI Chair at Mila-Quebec Artificial Intelligence Institute.
The study was published online in Science Advances.
‘Unique window’
Psychedelic drugs “show promise” as treatments for various psychiatric disorders, but subjective alterations of reality are “highly variable across individuals” and this “poses a key challenge as we venture to bring hallucinogenic substances into medical practice,” the investigators note.
Although the 5-HT2A receptor has been regarded as a “putative essential mechanism” of hallucinogenic experiences, it is unclear whether the experiential differences are explained by functional selectivity at the 5-HT2A receptor itself or “orchestrated by the vast array of neurotransmitter receptor subclasses on which these drugs act,” they add.
Lead author Galen Ballentine, MD, psychiatry resident, SUNY Downstate Medical Center, Brooklyn, told this news organization that he was “personally eager to find novel ways to identify the neurobiological underpinnings of different states of conscious awareness.”
Psychedelics, he said, offer a “unique window into a vast array of unusual states of consciousness and are particularly useful because they can point toward underlying mechanistic processes that are initiated in specific areas of receptor expression.”
The investigators wanted to understand “how these drugs work in order to help guide their use in clinical practice,” Dr. Ballentine said.
To explore the issue, they undertook the “largest investigation to date into the neuroscience of psychedelic drug experiences,” Dr. Ballentine said. “While most studies are limited to a single drug on a handful of subjects, this project integrates thousands of experiences induced by dozens of different hallucinogenic compounds, viewing them through the prism of 40 receptor subtypes.”
Unique neurotransmitter fingerprint
The researchers analyzed 6,850 experience reports of people who had taken 1 of 27 psychedelic compounds. The reports were drawn from a database hosted by the Erowid Center, an organization that collects first-hand accounts of experiences elicited by psychoactive drugs.
The researchers constructed a “bag-of-words” encoding of the text descriptions in each testimonial. Using linguistic calculation methods, they derived a final vocabulary of 14,410 words that they analyzed for descriptive experiential terms.
To shed light on the spatial distribution of these compounds that modulate neuronal activity during subjective “trips,” they compared normalized measurements of their relative binding strengths in 40 sites.
- 5-HT (5-HT2A, 5-HT2C, 5-HT2B, 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT5A, 5-HT6, 5-HT7)
- Dopamine (D1, D2, D3, D4, D5)
- Adrenergic (a-1A, a-1B, a-2A, a-2B, a-2C, b-1, b-2)
- Serotonin transporter (SERT)
- Dopamine transporter (DAT)
- Norepinephrine transporter (NET)
- Imidazoline-1 receptor (I1)
- Sigma receptors (s-1, s-2)
- d-opioid receptor (DOR)
- k-opioid receptor (KOR)
- m-opioid receptor (MOR)
- Muscarinic receptors (M1, M2, M3, M4, M5)
- Histamine receptors (H1, H2)
- Calcium ion channel (CA+)
- NMDA glutamate receptor
To map receptor-experience factors to regional levels of receptor gene transcription, they utilized human gene expression data drawn from the Allen Human Brain Atlas, as well as the Shafer-Yeo brain atlas.
Via a machine-learning algorithm, they dissected the “phenomenologically rich anecdotes” into a ranking of constituent brain-behavior factors, each of which was characterized by a “unique neurotransmitter fingerprint of action and a unique experiential context” and ultimately created a dimensional map of these neurotransmitter systems.
Data-driven framework
Cortex-wide distribution of receptor-experience factors was found in both deep and shallow anatomical brain regions. Regions involved in genetic factor expressions were also wide-ranging, spanning from higher association cortices to unimodal sensory cortices.
The dominant factor “elucidated mystical experience in general and the dissolution of self-world boundaries (ego dissolution) in particular,” the authors report, while the second- and third-most explanatory factors “evoked auditory, visual, and emotional themes of mental expansion.”
Ego dissolution was found to be most associated with the 5-HT2A receptor, as well as other serotonin receptors (5-HT2C, 5-HT1A, 5-HT2B), adrenergic receptors a-2A and b-2, and the D2 receptor.
Alterations in sensory perception were associated with expression of the 5-HT2A receptor in the visual cortex, while modulation of the salience network by dopamine and opioid receptors were implicated in the experience transcendence of space, time, and the structure of self. Auditory hallucinations were linked to a weighted blend of receptors expressed throughout the auditory cortex.
“This data-driven framework identifies patterns that undergird diverse psychedelic experiences such as mystical bliss, existential terror, and complex hallucinations,” Dr. Ballentine commented.
“Simultaneously subjective and neurobiological, these patterns align with the leading hypothesis that psychedelics temporarily diminish top-down control of the most evolutionarily advanced regions of the brain, while at the same time amplifying bottom-up sensory processing from primary sensory cortices,” he added.
Forging a new path
Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics and director of the Centre of Excellence at Sheppard Pratt, Towson, Md., said, “As we try to get our arms around understanding the implications of a psychedelic exposure, forward-thinking researchers like Dr. Bzdok et al. are offering interesting ways to capture and understand the experience.”
Dr. Aaronson, an adjunct professor at the University of Maryland School of Medicine who was not involved with the study, continued: “Using the rapidly developing field of natural language processing (NLP), which looks at how language is used for a deeper understanding of human experiences, and combining it with effects of psychedelic compounds on neuronal pathways and neurochemical receptor sites, the authors are forging a new path for further inquiry.”
In an accompanying editorial, Daniel Barron, MD, PhD, medical director, Interventional Pain Psychiatry Program, Brigham and Women’s Hospital, Boston, and Richard Friedman, MD, professor of clinical psychiatry, Weill Cornell Medical College, New York, call the work “impressive” and “clever.”
“Psychedelics paired with new applications of computational tools might help bypass the imprecision of psychiatric diagnosis and connect measures of behavior to specific physiologic targets,” they write.
The research was supported by the Brain Canada Foundation, through the Canada Brain Research Fund, a grant from the NIH grant, and the Canadian Institutes of Health Research. Dr. Bzdok was also supported by the Healthy Brains Healthy Lives initiative (Canada First Research Excellence fund) and the CIFAR Artificial Intelligence Chairs program (Canada Institute for Advanced Research), as well as Research Award and Teaching Award by Google. The other authors’ disclosures are listed on the original paper. No disclosures were listed for Dr. Barron and Dr. Friedman. Dr. Aaronson’s research is supported by Compass Pathways.
A version of this article first appeared on Medscape.com.
What causes the dramatic alterations in subjective awareness experienced during a psychedelic “trip?” A new study maps anatomical changes in specific neurotransmitter systems and brain regions that may be responsible for these effects.
Investigators gathered more than 6,800 accounts from individuals who had taken one of 27 different psychedelic compounds. Using a machine learning strategy, they extracted commonly used words from these testimonials, linking them with 40 different neurotransmitter subtypes that had likely induced these experiences.
The investigators then linked these subjective experiences with specific brain regions where the receptor combinations are most commonly found and, using gene transcription probes, created a 3D whole-brain map of the brain receptors and the subjective experiences linked to them.
“Hallucinogenic drugs may very well turn out to be the next big thing to improve clinical care of major mental health conditions,” senior author Danilo Bzdok, MD, PhD, associate professor, McGill University, Montreal, said in a press release.
“Our study provides a first step, a proof of principle, that we may be able to build machine-learning systems in the future that can accurately predict which neurotransmitter receptor combinations need to be stimulated to induce a specific state of conscious experience in a given person,” said Dr. Bzdok, who is also the Canada CIFAR AI Chair at Mila-Quebec Artificial Intelligence Institute.
The study was published online in Science Advances.
‘Unique window’
Psychedelic drugs “show promise” as treatments for various psychiatric disorders, but subjective alterations of reality are “highly variable across individuals” and this “poses a key challenge as we venture to bring hallucinogenic substances into medical practice,” the investigators note.
Although the 5-HT2A receptor has been regarded as a “putative essential mechanism” of hallucinogenic experiences, it is unclear whether the experiential differences are explained by functional selectivity at the 5-HT2A receptor itself or “orchestrated by the vast array of neurotransmitter receptor subclasses on which these drugs act,” they add.
Lead author Galen Ballentine, MD, psychiatry resident, SUNY Downstate Medical Center, Brooklyn, told this news organization that he was “personally eager to find novel ways to identify the neurobiological underpinnings of different states of conscious awareness.”
Psychedelics, he said, offer a “unique window into a vast array of unusual states of consciousness and are particularly useful because they can point toward underlying mechanistic processes that are initiated in specific areas of receptor expression.”
The investigators wanted to understand “how these drugs work in order to help guide their use in clinical practice,” Dr. Ballentine said.
To explore the issue, they undertook the “largest investigation to date into the neuroscience of psychedelic drug experiences,” Dr. Ballentine said. “While most studies are limited to a single drug on a handful of subjects, this project integrates thousands of experiences induced by dozens of different hallucinogenic compounds, viewing them through the prism of 40 receptor subtypes.”
Unique neurotransmitter fingerprint
The researchers analyzed 6,850 experience reports of people who had taken 1 of 27 psychedelic compounds. The reports were drawn from a database hosted by the Erowid Center, an organization that collects first-hand accounts of experiences elicited by psychoactive drugs.
The researchers constructed a “bag-of-words” encoding of the text descriptions in each testimonial. Using linguistic calculation methods, they derived a final vocabulary of 14,410 words that they analyzed for descriptive experiential terms.
To shed light on the spatial distribution of these compounds that modulate neuronal activity during subjective “trips,” they compared normalized measurements of their relative binding strengths in 40 sites.
- 5-HT (5-HT2A, 5-HT2C, 5-HT2B, 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT5A, 5-HT6, 5-HT7)
- Dopamine (D1, D2, D3, D4, D5)
- Adrenergic (a-1A, a-1B, a-2A, a-2B, a-2C, b-1, b-2)
- Serotonin transporter (SERT)
- Dopamine transporter (DAT)
- Norepinephrine transporter (NET)
- Imidazoline-1 receptor (I1)
- Sigma receptors (s-1, s-2)
- d-opioid receptor (DOR)
- k-opioid receptor (KOR)
- m-opioid receptor (MOR)
- Muscarinic receptors (M1, M2, M3, M4, M5)
- Histamine receptors (H1, H2)
- Calcium ion channel (CA+)
- NMDA glutamate receptor
To map receptor-experience factors to regional levels of receptor gene transcription, they utilized human gene expression data drawn from the Allen Human Brain Atlas, as well as the Shafer-Yeo brain atlas.
Via a machine-learning algorithm, they dissected the “phenomenologically rich anecdotes” into a ranking of constituent brain-behavior factors, each of which was characterized by a “unique neurotransmitter fingerprint of action and a unique experiential context” and ultimately created a dimensional map of these neurotransmitter systems.
Data-driven framework
Cortex-wide distribution of receptor-experience factors was found in both deep and shallow anatomical brain regions. Regions involved in genetic factor expressions were also wide-ranging, spanning from higher association cortices to unimodal sensory cortices.
The dominant factor “elucidated mystical experience in general and the dissolution of self-world boundaries (ego dissolution) in particular,” the authors report, while the second- and third-most explanatory factors “evoked auditory, visual, and emotional themes of mental expansion.”
Ego dissolution was found to be most associated with the 5-HT2A receptor, as well as other serotonin receptors (5-HT2C, 5-HT1A, 5-HT2B), adrenergic receptors a-2A and b-2, and the D2 receptor.
Alterations in sensory perception were associated with expression of the 5-HT2A receptor in the visual cortex, while modulation of the salience network by dopamine and opioid receptors were implicated in the experience transcendence of space, time, and the structure of self. Auditory hallucinations were linked to a weighted blend of receptors expressed throughout the auditory cortex.
“This data-driven framework identifies patterns that undergird diverse psychedelic experiences such as mystical bliss, existential terror, and complex hallucinations,” Dr. Ballentine commented.
“Simultaneously subjective and neurobiological, these patterns align with the leading hypothesis that psychedelics temporarily diminish top-down control of the most evolutionarily advanced regions of the brain, while at the same time amplifying bottom-up sensory processing from primary sensory cortices,” he added.
Forging a new path
Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics and director of the Centre of Excellence at Sheppard Pratt, Towson, Md., said, “As we try to get our arms around understanding the implications of a psychedelic exposure, forward-thinking researchers like Dr. Bzdok et al. are offering interesting ways to capture and understand the experience.”
Dr. Aaronson, an adjunct professor at the University of Maryland School of Medicine who was not involved with the study, continued: “Using the rapidly developing field of natural language processing (NLP), which looks at how language is used for a deeper understanding of human experiences, and combining it with effects of psychedelic compounds on neuronal pathways and neurochemical receptor sites, the authors are forging a new path for further inquiry.”
In an accompanying editorial, Daniel Barron, MD, PhD, medical director, Interventional Pain Psychiatry Program, Brigham and Women’s Hospital, Boston, and Richard Friedman, MD, professor of clinical psychiatry, Weill Cornell Medical College, New York, call the work “impressive” and “clever.”
“Psychedelics paired with new applications of computational tools might help bypass the imprecision of psychiatric diagnosis and connect measures of behavior to specific physiologic targets,” they write.
The research was supported by the Brain Canada Foundation, through the Canada Brain Research Fund, a grant from the NIH grant, and the Canadian Institutes of Health Research. Dr. Bzdok was also supported by the Healthy Brains Healthy Lives initiative (Canada First Research Excellence fund) and the CIFAR Artificial Intelligence Chairs program (Canada Institute for Advanced Research), as well as Research Award and Teaching Award by Google. The other authors’ disclosures are listed on the original paper. No disclosures were listed for Dr. Barron and Dr. Friedman. Dr. Aaronson’s research is supported by Compass Pathways.
A version of this article first appeared on Medscape.com.
What causes the dramatic alterations in subjective awareness experienced during a psychedelic “trip?” A new study maps anatomical changes in specific neurotransmitter systems and brain regions that may be responsible for these effects.
Investigators gathered more than 6,800 accounts from individuals who had taken one of 27 different psychedelic compounds. Using a machine learning strategy, they extracted commonly used words from these testimonials, linking them with 40 different neurotransmitter subtypes that had likely induced these experiences.
The investigators then linked these subjective experiences with specific brain regions where the receptor combinations are most commonly found and, using gene transcription probes, created a 3D whole-brain map of the brain receptors and the subjective experiences linked to them.
“Hallucinogenic drugs may very well turn out to be the next big thing to improve clinical care of major mental health conditions,” senior author Danilo Bzdok, MD, PhD, associate professor, McGill University, Montreal, said in a press release.
“Our study provides a first step, a proof of principle, that we may be able to build machine-learning systems in the future that can accurately predict which neurotransmitter receptor combinations need to be stimulated to induce a specific state of conscious experience in a given person,” said Dr. Bzdok, who is also the Canada CIFAR AI Chair at Mila-Quebec Artificial Intelligence Institute.
The study was published online in Science Advances.
‘Unique window’
Psychedelic drugs “show promise” as treatments for various psychiatric disorders, but subjective alterations of reality are “highly variable across individuals” and this “poses a key challenge as we venture to bring hallucinogenic substances into medical practice,” the investigators note.
Although the 5-HT2A receptor has been regarded as a “putative essential mechanism” of hallucinogenic experiences, it is unclear whether the experiential differences are explained by functional selectivity at the 5-HT2A receptor itself or “orchestrated by the vast array of neurotransmitter receptor subclasses on which these drugs act,” they add.
Lead author Galen Ballentine, MD, psychiatry resident, SUNY Downstate Medical Center, Brooklyn, told this news organization that he was “personally eager to find novel ways to identify the neurobiological underpinnings of different states of conscious awareness.”
Psychedelics, he said, offer a “unique window into a vast array of unusual states of consciousness and are particularly useful because they can point toward underlying mechanistic processes that are initiated in specific areas of receptor expression.”
The investigators wanted to understand “how these drugs work in order to help guide their use in clinical practice,” Dr. Ballentine said.
To explore the issue, they undertook the “largest investigation to date into the neuroscience of psychedelic drug experiences,” Dr. Ballentine said. “While most studies are limited to a single drug on a handful of subjects, this project integrates thousands of experiences induced by dozens of different hallucinogenic compounds, viewing them through the prism of 40 receptor subtypes.”
Unique neurotransmitter fingerprint
The researchers analyzed 6,850 experience reports of people who had taken 1 of 27 psychedelic compounds. The reports were drawn from a database hosted by the Erowid Center, an organization that collects first-hand accounts of experiences elicited by psychoactive drugs.
The researchers constructed a “bag-of-words” encoding of the text descriptions in each testimonial. Using linguistic calculation methods, they derived a final vocabulary of 14,410 words that they analyzed for descriptive experiential terms.
To shed light on the spatial distribution of these compounds that modulate neuronal activity during subjective “trips,” they compared normalized measurements of their relative binding strengths in 40 sites.
- 5-HT (5-HT2A, 5-HT2C, 5-HT2B, 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT5A, 5-HT6, 5-HT7)
- Dopamine (D1, D2, D3, D4, D5)
- Adrenergic (a-1A, a-1B, a-2A, a-2B, a-2C, b-1, b-2)
- Serotonin transporter (SERT)
- Dopamine transporter (DAT)
- Norepinephrine transporter (NET)
- Imidazoline-1 receptor (I1)
- Sigma receptors (s-1, s-2)
- d-opioid receptor (DOR)
- k-opioid receptor (KOR)
- m-opioid receptor (MOR)
- Muscarinic receptors (M1, M2, M3, M4, M5)
- Histamine receptors (H1, H2)
- Calcium ion channel (CA+)
- NMDA glutamate receptor
To map receptor-experience factors to regional levels of receptor gene transcription, they utilized human gene expression data drawn from the Allen Human Brain Atlas, as well as the Shafer-Yeo brain atlas.
Via a machine-learning algorithm, they dissected the “phenomenologically rich anecdotes” into a ranking of constituent brain-behavior factors, each of which was characterized by a “unique neurotransmitter fingerprint of action and a unique experiential context” and ultimately created a dimensional map of these neurotransmitter systems.
Data-driven framework
Cortex-wide distribution of receptor-experience factors was found in both deep and shallow anatomical brain regions. Regions involved in genetic factor expressions were also wide-ranging, spanning from higher association cortices to unimodal sensory cortices.
The dominant factor “elucidated mystical experience in general and the dissolution of self-world boundaries (ego dissolution) in particular,” the authors report, while the second- and third-most explanatory factors “evoked auditory, visual, and emotional themes of mental expansion.”
Ego dissolution was found to be most associated with the 5-HT2A receptor, as well as other serotonin receptors (5-HT2C, 5-HT1A, 5-HT2B), adrenergic receptors a-2A and b-2, and the D2 receptor.
Alterations in sensory perception were associated with expression of the 5-HT2A receptor in the visual cortex, while modulation of the salience network by dopamine and opioid receptors were implicated in the experience transcendence of space, time, and the structure of self. Auditory hallucinations were linked to a weighted blend of receptors expressed throughout the auditory cortex.
“This data-driven framework identifies patterns that undergird diverse psychedelic experiences such as mystical bliss, existential terror, and complex hallucinations,” Dr. Ballentine commented.
“Simultaneously subjective and neurobiological, these patterns align with the leading hypothesis that psychedelics temporarily diminish top-down control of the most evolutionarily advanced regions of the brain, while at the same time amplifying bottom-up sensory processing from primary sensory cortices,” he added.
Forging a new path
Scott Aaronson, MD, chief science officer, Institute for Advanced Diagnostics and Therapeutics and director of the Centre of Excellence at Sheppard Pratt, Towson, Md., said, “As we try to get our arms around understanding the implications of a psychedelic exposure, forward-thinking researchers like Dr. Bzdok et al. are offering interesting ways to capture and understand the experience.”
Dr. Aaronson, an adjunct professor at the University of Maryland School of Medicine who was not involved with the study, continued: “Using the rapidly developing field of natural language processing (NLP), which looks at how language is used for a deeper understanding of human experiences, and combining it with effects of psychedelic compounds on neuronal pathways and neurochemical receptor sites, the authors are forging a new path for further inquiry.”
In an accompanying editorial, Daniel Barron, MD, PhD, medical director, Interventional Pain Psychiatry Program, Brigham and Women’s Hospital, Boston, and Richard Friedman, MD, professor of clinical psychiatry, Weill Cornell Medical College, New York, call the work “impressive” and “clever.”
“Psychedelics paired with new applications of computational tools might help bypass the imprecision of psychiatric diagnosis and connect measures of behavior to specific physiologic targets,” they write.
The research was supported by the Brain Canada Foundation, through the Canada Brain Research Fund, a grant from the NIH grant, and the Canadian Institutes of Health Research. Dr. Bzdok was also supported by the Healthy Brains Healthy Lives initiative (Canada First Research Excellence fund) and the CIFAR Artificial Intelligence Chairs program (Canada Institute for Advanced Research), as well as Research Award and Teaching Award by Google. The other authors’ disclosures are listed on the original paper. No disclosures were listed for Dr. Barron and Dr. Friedman. Dr. Aaronson’s research is supported by Compass Pathways.
A version of this article first appeared on Medscape.com.
Adolescent overdose deaths nearly doubled in 2020 and spiked again in 2021
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
The number of overdose deaths in adolescents nearly doubled in 2020 from the year before and increased substantially again in 2021 after nearly a decade of fairly stable rates, according to data published in a JAMA research letter.
Most of the deaths involved fentanyl, the researchers found.
Joseph Friedman, MPH, of the Center for Social Medicine and Humanities at the University of California, Los Angeles, led the study, which analyzed adolescent (14-18 years old) overdose deaths in the United States from 2010 to June 2021 in light of increasing contamination in the supply of illicit drugs.
The researchers found there were 518 deaths among adolescents (2.40 per 100,000 population) in 2010, and the rates remained stable through 2019 with 492 deaths (2.36 per 100,000).
In 2020, however, deaths spiked to 954 (4.57 per 100 000), increasing by 94.3%, compared with 2019. In 2021, they increased another 20%.
The rise in fentanyl-involved deaths was particularly striking. Fentanyl-involved deaths increased from 253 (1.21 per 100,000) in 2019 to 680 (3.26 per 100,000) in 2020. The numbers through June 2021 were annualized for 2021 and calculations predicted 884 deaths (4.23 per 100,000) for the year.
Numbers point to fentanyl potency
In 2021, more than three-fourths (77.14%) of adolescent overdose deaths involved fentanyl, compared with 13.26% for benzodiazepines, 9.77% for methamphetamine, 7.33% for cocaine, 5.76% for prescription opioids, and 2.27% for heroin.
American Indian and Alaska Native adolescents had the highest overdose rate in 2021 (n = 24; 11.79 per 100,000), followed by Latinx adolescents (n = 354; 6.98 per 100,000).
“These adolescent trends fit a wider pattern of increasing racial and ethnic inequalities in overdose that deserve further investigation and intervention efforts,” the authors wrote.
Pandemic’s role unclear
The spikes in adolescent overdoses overlap the COVID-19 pandemic, but Dr. Friedman said in an interview the pandemic “may or may not have been a big factor. “
The authors wrote that drug use had generally been stable among adolescents between 2010 and 2020. The number of 10th graders reporting any illicit drug use was 30.2% in 2010 and 30.4% in 2020.
“So it’s not that more teens are using drugs. It’s just that drug use is becoming more dangerous due to the spread of counterfeit pills containing fentanyls,” Dr. Friedman said.
The authors noted that “the illicit drug supply has increasingly become contaminated with illicitly manufactured fentanyls and other synthetic opioid and benzodiazepine analogues.”
Mr. Friedman said the pandemic may have accelerated the spread of more dangerous forms of drugs as supply chains were disrupted.
Benjamin Brady, DrPH, an assistant professor at the University of Arizona, Tucson, who also has an appointment in the university’s Comprehensive Pain and Addiction Center, said in an interview the numbers that Dr. Friedman and colleagues present represent “worst fears coming true.”
He said he and his colleagues in the field “were anticipating a rise in overdose deaths for the next 5-10 years because of the way the supply-and-demand environment exists in the U.S.”
Dr. Brady explained that restricting access to prescription opioids has had an unfortunate side effect in decreasing access to a safer supply of drugs.
“Without having solutions that would reduce demand at the same rate, supply of the safer form of the drug has been reduced; that has pushed people toward heroin and street drugs and from 2016 on those have been adulterated with fentanyl,” he said.
He said the United States, compared with other developed nations, has been slower to embrace longer-term harm-reduction strategies and to improve access to treatment and care.
COVID likely also has exacerbated the problem in terms of isolation and reduction in quality of life that has adolescents seeking to fill that void with drugs, Dr. Brady said. They may be completely unaware that the drugs they are seeking are commonly cut with counterfeit fentanyl.
“Fentanyl can be up to 50 times stronger than heroin,” he noted. “Even just a little bit of fentanyl dramatically changes the risk profile on an overdose.”
Increasing rates of mental health concerns among adolescents over decades also contribute to drug-seeking trends, Dr. Brady noted.
Overdose increases in the overall population were smaller
In the overall population, the percentage increases were not nearly as large in 2020 and 2021 as they were for adolescents.
Rates of overdose deaths in the overall population increased steadily from 2010 and reached 70,630 in 2019. In 2020, the deaths increased to 91,799 (an increase of 29.48% from 2019) and increased 11.48% in 2021.
The researchers analyzed numbers from the Centers for Disease Control and Prevention WONDER (Wide-Ranging Online Data for Epidemiologic Research) database, which has records of all U.S. deaths for which drug overdose was listed as the underlying cause.
The authors and Dr. Brady report no relevant financial relationships.
FROM JAMA
Can gram stains guide antibiotics for pneumonia in critical care?
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAVI device shows less deterioration than surgery 5 years out
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
FROM ACC 2022




