User login
Statins tied to lower risk for parkinsonism
, new research suggests. An observational study showed older adults taking statins had a lower risk for parkinsonism than their counterparts not taking statins – an effect that may be partially mediated by less severe intracranial atherosclerosis in statin users.
“These findings further support the idea that cerebrovascular disease pathologies accumulating in older brains may be an unrecognized contributor to the common occurrence of parkinsonism in old age,” the investigators wrote. “More importantly, these findings suggest that statins may have a potential therapeutic role in decreasing the magnitude of parkinsonism in older adults,” they added.
The study was published online in Neurology.
No clinical recommendations ... yet
The findings are based on 2,841 older adults enrolled in one of three ongoing clinical pathological studies at Rush Alzheimer’s Disease Center, Chicago.
Participants’ average age at baseline was 76 years, and 75% were women. None had parkinsonism at the start of the study. One-third of participants (n = 936) were taking statins. During an average follow-up of 6 years, 1,432 (50%) participants developed parkinsonism.
After controlling for demographics, vascular risk factors, and diseases, use of a statin at baseline was associated with a 16% lower risk for parkinsonism (hazard ratio, 0.84; 95% confidence interval, 0.74-0.96; P = .008). Compared with low-intensity statin therapy, moderate- or high-intensity statin therapy was associated with a 7% lower risk for parkinsonism (HR, 0.93; 95% CI, 0.87-1.00; P = .043).
The researchers also examined the brains of 1,044 people who died during the study at a mean age of 89 years. They found statin use prior to death was associated with a 37% lower odds of cerebral atherosclerosis, compared with no statin use prior to death (odds ratio, 0.63; 95% CI, 0.50-0.79; P < .001).
In a mediation analysis, both a direct (OR, 0.73; 95% CI, 0.54-0.93; P = .008) and an indirect (OR, 0.92; 95% CI, 0.88-0.97; P = .002) pathway via less severe cerebrovascular disease linked statins to parkinsonism, indicating that cerebral atherosclerosis mediated 17% of the association between statins and parkinsonism.
In line with other studies, there was no association between statins and other neurodegenerative pathologies, including Parkinson’s disease pathology. However, even older adults with a clinical diagnosis of Parkinson’s disease often show mixed brain pathologies, including cerebrovascular disease pathologies.
“Therefore, we think that statins may be beneficial against parkinsonism in patients with Parkinson’s disease, dependent on how much cerebrovascular disease pathologies they have, including atherosclerosis,” said study investigator Shahram Oveisgharan, MD, with Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago.
However, since the results stem from an observational study, “we do not yet recommend using statins in large scale for older adults at risk for parkinsonism,” Dr. Oveisgharan said.
A mixed picture
Reached for comment, Shaheen Lakhan, MD, neurologist in Newton, Massachusetts, noted that since statins were first discovered in the fermented broth of a common soil fungus in the late 1970s, they have proven to reduce cholesterol, heart disease, and stroke.
“The jury is out, however, on [their] effects on diseases such as dementia, autoimmune/inflammatory conditions, bacterial/viral infections, cancer, and parkinsonism,” he said.
“Also, the question often remains whether any benefit gained from statins is from cholesterol-lowering or through another mechanism. When there is such a mixed picture, it generally means that the drug has an effect, but not for everyone,” Dr. Lakhan said. “Much work must now be done to stratify for which patients are statins effective, ineffective, or even harmful in these conditions,” he added.
The study was supported by the National Institutes of Health. Dr. Oveisgharan and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. An observational study showed older adults taking statins had a lower risk for parkinsonism than their counterparts not taking statins – an effect that may be partially mediated by less severe intracranial atherosclerosis in statin users.
“These findings further support the idea that cerebrovascular disease pathologies accumulating in older brains may be an unrecognized contributor to the common occurrence of parkinsonism in old age,” the investigators wrote. “More importantly, these findings suggest that statins may have a potential therapeutic role in decreasing the magnitude of parkinsonism in older adults,” they added.
The study was published online in Neurology.
No clinical recommendations ... yet
The findings are based on 2,841 older adults enrolled in one of three ongoing clinical pathological studies at Rush Alzheimer’s Disease Center, Chicago.
Participants’ average age at baseline was 76 years, and 75% were women. None had parkinsonism at the start of the study. One-third of participants (n = 936) were taking statins. During an average follow-up of 6 years, 1,432 (50%) participants developed parkinsonism.
After controlling for demographics, vascular risk factors, and diseases, use of a statin at baseline was associated with a 16% lower risk for parkinsonism (hazard ratio, 0.84; 95% confidence interval, 0.74-0.96; P = .008). Compared with low-intensity statin therapy, moderate- or high-intensity statin therapy was associated with a 7% lower risk for parkinsonism (HR, 0.93; 95% CI, 0.87-1.00; P = .043).
The researchers also examined the brains of 1,044 people who died during the study at a mean age of 89 years. They found statin use prior to death was associated with a 37% lower odds of cerebral atherosclerosis, compared with no statin use prior to death (odds ratio, 0.63; 95% CI, 0.50-0.79; P < .001).
In a mediation analysis, both a direct (OR, 0.73; 95% CI, 0.54-0.93; P = .008) and an indirect (OR, 0.92; 95% CI, 0.88-0.97; P = .002) pathway via less severe cerebrovascular disease linked statins to parkinsonism, indicating that cerebral atherosclerosis mediated 17% of the association between statins and parkinsonism.
In line with other studies, there was no association between statins and other neurodegenerative pathologies, including Parkinson’s disease pathology. However, even older adults with a clinical diagnosis of Parkinson’s disease often show mixed brain pathologies, including cerebrovascular disease pathologies.
“Therefore, we think that statins may be beneficial against parkinsonism in patients with Parkinson’s disease, dependent on how much cerebrovascular disease pathologies they have, including atherosclerosis,” said study investigator Shahram Oveisgharan, MD, with Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago.
However, since the results stem from an observational study, “we do not yet recommend using statins in large scale for older adults at risk for parkinsonism,” Dr. Oveisgharan said.
A mixed picture
Reached for comment, Shaheen Lakhan, MD, neurologist in Newton, Massachusetts, noted that since statins were first discovered in the fermented broth of a common soil fungus in the late 1970s, they have proven to reduce cholesterol, heart disease, and stroke.
“The jury is out, however, on [their] effects on diseases such as dementia, autoimmune/inflammatory conditions, bacterial/viral infections, cancer, and parkinsonism,” he said.
“Also, the question often remains whether any benefit gained from statins is from cholesterol-lowering or through another mechanism. When there is such a mixed picture, it generally means that the drug has an effect, but not for everyone,” Dr. Lakhan said. “Much work must now be done to stratify for which patients are statins effective, ineffective, or even harmful in these conditions,” he added.
The study was supported by the National Institutes of Health. Dr. Oveisgharan and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. An observational study showed older adults taking statins had a lower risk for parkinsonism than their counterparts not taking statins – an effect that may be partially mediated by less severe intracranial atherosclerosis in statin users.
“These findings further support the idea that cerebrovascular disease pathologies accumulating in older brains may be an unrecognized contributor to the common occurrence of parkinsonism in old age,” the investigators wrote. “More importantly, these findings suggest that statins may have a potential therapeutic role in decreasing the magnitude of parkinsonism in older adults,” they added.
The study was published online in Neurology.
No clinical recommendations ... yet
The findings are based on 2,841 older adults enrolled in one of three ongoing clinical pathological studies at Rush Alzheimer’s Disease Center, Chicago.
Participants’ average age at baseline was 76 years, and 75% were women. None had parkinsonism at the start of the study. One-third of participants (n = 936) were taking statins. During an average follow-up of 6 years, 1,432 (50%) participants developed parkinsonism.
After controlling for demographics, vascular risk factors, and diseases, use of a statin at baseline was associated with a 16% lower risk for parkinsonism (hazard ratio, 0.84; 95% confidence interval, 0.74-0.96; P = .008). Compared with low-intensity statin therapy, moderate- or high-intensity statin therapy was associated with a 7% lower risk for parkinsonism (HR, 0.93; 95% CI, 0.87-1.00; P = .043).
The researchers also examined the brains of 1,044 people who died during the study at a mean age of 89 years. They found statin use prior to death was associated with a 37% lower odds of cerebral atherosclerosis, compared with no statin use prior to death (odds ratio, 0.63; 95% CI, 0.50-0.79; P < .001).
In a mediation analysis, both a direct (OR, 0.73; 95% CI, 0.54-0.93; P = .008) and an indirect (OR, 0.92; 95% CI, 0.88-0.97; P = .002) pathway via less severe cerebrovascular disease linked statins to parkinsonism, indicating that cerebral atherosclerosis mediated 17% of the association between statins and parkinsonism.
In line with other studies, there was no association between statins and other neurodegenerative pathologies, including Parkinson’s disease pathology. However, even older adults with a clinical diagnosis of Parkinson’s disease often show mixed brain pathologies, including cerebrovascular disease pathologies.
“Therefore, we think that statins may be beneficial against parkinsonism in patients with Parkinson’s disease, dependent on how much cerebrovascular disease pathologies they have, including atherosclerosis,” said study investigator Shahram Oveisgharan, MD, with Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago.
However, since the results stem from an observational study, “we do not yet recommend using statins in large scale for older adults at risk for parkinsonism,” Dr. Oveisgharan said.
A mixed picture
Reached for comment, Shaheen Lakhan, MD, neurologist in Newton, Massachusetts, noted that since statins were first discovered in the fermented broth of a common soil fungus in the late 1970s, they have proven to reduce cholesterol, heart disease, and stroke.
“The jury is out, however, on [their] effects on diseases such as dementia, autoimmune/inflammatory conditions, bacterial/viral infections, cancer, and parkinsonism,” he said.
“Also, the question often remains whether any benefit gained from statins is from cholesterol-lowering or through another mechanism. When there is such a mixed picture, it generally means that the drug has an effect, but not for everyone,” Dr. Lakhan said. “Much work must now be done to stratify for which patients are statins effective, ineffective, or even harmful in these conditions,” he added.
The study was supported by the National Institutes of Health. Dr. Oveisgharan and Dr. Lakhan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anticipation key to tackling perioperative anemia
MANCHESTER, ENGLAND –
Anemia management may include dietary changes, iron supplementation, blood transfusion, perioperative physiological optimization, delay or review of the surgical plan, medication reviews, and greater intraoperative care.
It is quite clear that patients have a better experience if management covers the whole pathway, said lead author of the guidelines, Scarlett McNally, MD, PhD, East Sussex Healthcare NHS Trust, Eastbourne, England.
It’s much better for the patient “if every individual member of staff knows what’s supposed to happen, not just for their bit, but the whole way along,” she said. “Otherwise things go wrong, and people don’t anticipate things early enough.”
The new guidelines, to be published in full later this year by the Centre for Perioperative Care, cover emergency and elective surgery for all ages.
It follows the 2021 publication of a guideline for perioperative diabetes management, and a previous document that covered frailty.
Dr. McNally was presenting the new guidelines on perioperative anemia at the British Society for Haematology 62nd Annual Scientific Meeting.
Although perioperative anemia is a “big issue” in clinical management, “some health care professionals know a lot about one area,” but tend to work in “silos,” Dr. McNally said.
The result is clinicians believe that all other areas are “complex” and opaque, and they “don’t make the simple decisions” that could have a big impact on patient care.
As an example, she said there are already some excellent guidelines out there, but they are not widely read.
One example of a comprehensive guideline, Dr. McNally said, is that issued by the British Society of Gastroenterology. This guideline notes that in cases where a man or a postmenopausal woman has anemia of unknown cause, about 30% of those cases end up having a gastrointestinal cause, and so gastroenterologists are happy to have those patients referred to them.
But Dr. McNally said that she personally, as an orthopedic surgeon, wouldn’t have known what to do with such a patient, and may have referred that person back to primary care to be investigated.
The new guidelines contain algorithms to help staff plan care. Without those, she said, “a lot is resting on the preassessment nurses, but they are having to think about everything else.”
The guidance suggests proactive measures to identify and manage anemia. These include testing for anemia while assessing renal function ahead of a CT scan, or asking patients about their nutrition.
For low-risk patients, it may be enough to give general advice about a good diet and exercise to try to get them through the operation.
However, patients who are high risk (defined as likely to lose > 500 mL or > 10% of blood volume during surgery) need to be identified as such early on, so that early measures can be put in place, as well as a senior review of their care plan.
The guidelines also recommend that operating room staff consider tranexamic acid and other bloodless minimization strategies, and that senior staff give clinical input in cases of functional iron deficiency, a marker of ill health.
To maximize postoperative outcomes, it is suggested that staff work with prehabilitation services and mobilize patients, as symptoms allow.
More importantly, they emphasize the need for shared decision-making about potential surgery, ensuring that the patients understand “Benefits, Risks, Alternatives, and what if we do Nothing (BRAN).”
No funding was declared. One study author declared relationships with the National Institute for Health Research and Pfizer.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND –
Anemia management may include dietary changes, iron supplementation, blood transfusion, perioperative physiological optimization, delay or review of the surgical plan, medication reviews, and greater intraoperative care.
It is quite clear that patients have a better experience if management covers the whole pathway, said lead author of the guidelines, Scarlett McNally, MD, PhD, East Sussex Healthcare NHS Trust, Eastbourne, England.
It’s much better for the patient “if every individual member of staff knows what’s supposed to happen, not just for their bit, but the whole way along,” she said. “Otherwise things go wrong, and people don’t anticipate things early enough.”
The new guidelines, to be published in full later this year by the Centre for Perioperative Care, cover emergency and elective surgery for all ages.
It follows the 2021 publication of a guideline for perioperative diabetes management, and a previous document that covered frailty.
Dr. McNally was presenting the new guidelines on perioperative anemia at the British Society for Haematology 62nd Annual Scientific Meeting.
Although perioperative anemia is a “big issue” in clinical management, “some health care professionals know a lot about one area,” but tend to work in “silos,” Dr. McNally said.
The result is clinicians believe that all other areas are “complex” and opaque, and they “don’t make the simple decisions” that could have a big impact on patient care.
As an example, she said there are already some excellent guidelines out there, but they are not widely read.
One example of a comprehensive guideline, Dr. McNally said, is that issued by the British Society of Gastroenterology. This guideline notes that in cases where a man or a postmenopausal woman has anemia of unknown cause, about 30% of those cases end up having a gastrointestinal cause, and so gastroenterologists are happy to have those patients referred to them.
But Dr. McNally said that she personally, as an orthopedic surgeon, wouldn’t have known what to do with such a patient, and may have referred that person back to primary care to be investigated.
The new guidelines contain algorithms to help staff plan care. Without those, she said, “a lot is resting on the preassessment nurses, but they are having to think about everything else.”
The guidance suggests proactive measures to identify and manage anemia. These include testing for anemia while assessing renal function ahead of a CT scan, or asking patients about their nutrition.
For low-risk patients, it may be enough to give general advice about a good diet and exercise to try to get them through the operation.
However, patients who are high risk (defined as likely to lose > 500 mL or > 10% of blood volume during surgery) need to be identified as such early on, so that early measures can be put in place, as well as a senior review of their care plan.
The guidelines also recommend that operating room staff consider tranexamic acid and other bloodless minimization strategies, and that senior staff give clinical input in cases of functional iron deficiency, a marker of ill health.
To maximize postoperative outcomes, it is suggested that staff work with prehabilitation services and mobilize patients, as symptoms allow.
More importantly, they emphasize the need for shared decision-making about potential surgery, ensuring that the patients understand “Benefits, Risks, Alternatives, and what if we do Nothing (BRAN).”
No funding was declared. One study author declared relationships with the National Institute for Health Research and Pfizer.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND –
Anemia management may include dietary changes, iron supplementation, blood transfusion, perioperative physiological optimization, delay or review of the surgical plan, medication reviews, and greater intraoperative care.
It is quite clear that patients have a better experience if management covers the whole pathway, said lead author of the guidelines, Scarlett McNally, MD, PhD, East Sussex Healthcare NHS Trust, Eastbourne, England.
It’s much better for the patient “if every individual member of staff knows what’s supposed to happen, not just for their bit, but the whole way along,” she said. “Otherwise things go wrong, and people don’t anticipate things early enough.”
The new guidelines, to be published in full later this year by the Centre for Perioperative Care, cover emergency and elective surgery for all ages.
It follows the 2021 publication of a guideline for perioperative diabetes management, and a previous document that covered frailty.
Dr. McNally was presenting the new guidelines on perioperative anemia at the British Society for Haematology 62nd Annual Scientific Meeting.
Although perioperative anemia is a “big issue” in clinical management, “some health care professionals know a lot about one area,” but tend to work in “silos,” Dr. McNally said.
The result is clinicians believe that all other areas are “complex” and opaque, and they “don’t make the simple decisions” that could have a big impact on patient care.
As an example, she said there are already some excellent guidelines out there, but they are not widely read.
One example of a comprehensive guideline, Dr. McNally said, is that issued by the British Society of Gastroenterology. This guideline notes that in cases where a man or a postmenopausal woman has anemia of unknown cause, about 30% of those cases end up having a gastrointestinal cause, and so gastroenterologists are happy to have those patients referred to them.
But Dr. McNally said that she personally, as an orthopedic surgeon, wouldn’t have known what to do with such a patient, and may have referred that person back to primary care to be investigated.
The new guidelines contain algorithms to help staff plan care. Without those, she said, “a lot is resting on the preassessment nurses, but they are having to think about everything else.”
The guidance suggests proactive measures to identify and manage anemia. These include testing for anemia while assessing renal function ahead of a CT scan, or asking patients about their nutrition.
For low-risk patients, it may be enough to give general advice about a good diet and exercise to try to get them through the operation.
However, patients who are high risk (defined as likely to lose > 500 mL or > 10% of blood volume during surgery) need to be identified as such early on, so that early measures can be put in place, as well as a senior review of their care plan.
The guidelines also recommend that operating room staff consider tranexamic acid and other bloodless minimization strategies, and that senior staff give clinical input in cases of functional iron deficiency, a marker of ill health.
To maximize postoperative outcomes, it is suggested that staff work with prehabilitation services and mobilize patients, as symptoms allow.
More importantly, they emphasize the need for shared decision-making about potential surgery, ensuring that the patients understand “Benefits, Risks, Alternatives, and what if we do Nothing (BRAN).”
No funding was declared. One study author declared relationships with the National Institute for Health Research and Pfizer.
A version of this article first appeared on Medscape.com.
Psilocybin ‘rewires’ the brain to alleviate depression
Led by investigators from the University of California, San Francisco, and Imperial College London’s Centre for Psychedelic Research, the findings come from a new analysis of brain scans of almost 60 patients with resistant depression treated with psilocybin.
“Not much is known about the changes in brain function after psychedelic experience. There has been much more research done on the acute brain action of psychedelics, but there is very little on the postacute or subacute changes in brain function,” study investigator Robin Carhart-Harris, PhD, former head of the Imperial Centre for Psychedelic Research and now director of the Neuroscape psychedelics division at UCSF, and senior author of the study, told this news organization.
“This research is a major advance because it is showing replication across two datasets with different designs. One in which the scanning is done 1 day after intervention and the other one when the posttreatment scanning is done 3 weeks after the second of two psilocybin therapy sessions,” Dr. Carhart-Harris added.
The study was published online in Nature Medicine.
A disruptor?
Psilocybin is one of a number of psychedelics under investigation as a potential therapy for psychiatric disorders. In the last 15 years, at least six separate clinical trials have reported impressive improvements in depressive symptoms with psilocybin therapy. Several studies have tested a synthesized a form of the drug to treat patients with depression and anxiety – with promising results.
However, the therapeutic action of psilocybin and other serotonergic psychedelics is still not completely understood, although it is known that they affect 5-HT2A receptors and are hypothesized to briefly disrupt these connections, allowing them to reform in new ways in the days and weeks following treatment.
This research assessed the subacute impact of psilocybin on brain function in two clinical trials of depression:
The first trial was an open-label trial of oral psilocybin in patients with treatment-resistant depression.
Patients had baseline clinical assessment and resting-state functional MRI, followed by fixed-order “low” (10 mg) and “high” (25 mg) psilocybin therapy dosing days separated by 1 week. Of the 19 patients recruited, 3 were excluded as a result of excessive fMRI head motion. The team confirmed an antidepressant effect of psilocybin in 16 patients via reduced questionnaire scores from baseline.
Brain network modularity was significantly reduced 1 day after psilocybin therapy in 10 of 16 participants (mean difference, –0.29; t15, 2.87; 95% confidence interval, 0.07-0.50; P = .012; d = 0.72). This result implies an increase in functional connectivity between the brain’s main intrinsic networks.
Pre- vs posttreatment change in modularity significantly correlated with change in Beck Depression Inventory (BDI) score at 6 months, relative to baseline (r14 = 0.54, 95% CI, 0.14-0.78, P = .033). Results imply that decreased brain modularity 1 day after psilocybin therapy relates to long-term improvements in symptom severity.
Effective antidepressant alternative?
The second trial was a double-blind, phase 2, randomized, controlled trial comparing psilocybin with escitalopram (Lexapro). Twenty-one patients were included in the escitalopram imaging sample and 22 patients were included in the psilocybin imaging sample.
Patients received either 2 x 25 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily placebo (psilocybin arm) or 2 x 1 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily escitalopram (10-20 mg) (escitalopram arm). Functional MRI was recorded at baseline and 3 weeks after the second psilocybin dose.
On average, BDI-measured reductions in depressive symptom severity were significantly greater under psilocybin than escitalopram, indicating superior efficacy of psilocybin therapy versus escitalopram.
Evidence indicated that the reduction in network modularity and its relationship to depression severity was specific to the psilocybin group. In the escitalopram group, network modularity did not change from baseline and there was no significant correlation between changes in modularity and changes in BDI scores.
Post–psilocybin therapy changes in network flexibility were correlated with changes in BDI score. After false discovery rate correction, increased executive network dynamic flexibility strongly correlated with greater symptom improvement at the 6-week primary endpoint for the psilocybin arm (r20, –0.76, 95% CI, −0.90 to –0.50, P = .001).
There were no significant correlations between changes in BDI scores and changes in dynamic flexibility in the escitalopram arm.
“These findings are important because for the first time we find that psilocybin works differently from conventional antidepressants, making the brain more flexible and fluid and less entrenched in the negative thinking patterns associated with depression. This supports our initial predictions and confirms psilocybin could be a real alternative approach to depression treatments,” study investigator David Nutt, DM, head of the Imperial Centre for Psychedelic Research, London, said in a release.
“In previous studies we had seen a similar effect in the brain when people were scanned whilst on a psychedelic, but here we’re seeing it weeks after treatment for depression, which suggests a carryover of the acute drug action,” said Dr. Carhart-Harris.
Durable effect?
“We don’t yet know how long the changes in brain activity seen with psilocybin therapy last, and we need to do more research to understand this,” said Dr. Carhart-Harris, who is a member of the UCSF Weill Institute for Neurosciences. “If the changes don’t last, then is it related to relapse into a depressive episode? We need to do follow-up scans to see where people’s brains are at 3 months or even 6 months after treatment.
“We do know that some people relapse, and it may be that after a while their brains revert to the rigid patterns of activity we see in depression.
“One exciting implication of our findings is that we have discovered a fundamental mechanism via which psychedelic therapy works not just for depression but other mental illnesses, such as anorexia or addiction. We now need to test if this is the case, and if it is, then we have found something important,” added Dr. Carhart-Harris.
Successful phase 3, double-blind randomized, controlled trials will be required to achieve licensing for psilocybin therapy, but pragmatic trials may better address questions regarding treatment practicability, specificity, and optimization. Given the emerging research into psychedelic therapy, it is important for large-scale trials to establish the generalizability, reliability, and specificity of the drug’s antidepressant response.
So how close are we to full federal approval for psilocybin in the treatment of depression? Dr. Carhart-Harris estimated that within 4-5 years is realistic at the federal level. At the state level, in Oregon psilocybin therapy is on track for approval in 2023, including for patients currently undergoing treatment for depressive disorders. In addition, things are opening up in Canada, with some special-access opportunities.
The researchers cautioned that, while these findings are encouraging, trials assessing psilocybin for depression have taken place under controlled, clinical conditions, using a regulated dose formulated in a laboratory, and involved extensive psychological support by a mental health professional – before, during, and after dosing. Taking psychedelics in the absence of these combined safeguards may not have a positive outcome.
The research was supported by funding from the Alex Mosley Charitable Trust and founding donors of the Imperial Centre for Psychedelic Research. One coauthor was supported by the Imperial College London EPSRC Centre London for doctoral training in neurotechnology.
A version of this article first appeared on Medscape.com.
Led by investigators from the University of California, San Francisco, and Imperial College London’s Centre for Psychedelic Research, the findings come from a new analysis of brain scans of almost 60 patients with resistant depression treated with psilocybin.
“Not much is known about the changes in brain function after psychedelic experience. There has been much more research done on the acute brain action of psychedelics, but there is very little on the postacute or subacute changes in brain function,” study investigator Robin Carhart-Harris, PhD, former head of the Imperial Centre for Psychedelic Research and now director of the Neuroscape psychedelics division at UCSF, and senior author of the study, told this news organization.
“This research is a major advance because it is showing replication across two datasets with different designs. One in which the scanning is done 1 day after intervention and the other one when the posttreatment scanning is done 3 weeks after the second of two psilocybin therapy sessions,” Dr. Carhart-Harris added.
The study was published online in Nature Medicine.
A disruptor?
Psilocybin is one of a number of psychedelics under investigation as a potential therapy for psychiatric disorders. In the last 15 years, at least six separate clinical trials have reported impressive improvements in depressive symptoms with psilocybin therapy. Several studies have tested a synthesized a form of the drug to treat patients with depression and anxiety – with promising results.
However, the therapeutic action of psilocybin and other serotonergic psychedelics is still not completely understood, although it is known that they affect 5-HT2A receptors and are hypothesized to briefly disrupt these connections, allowing them to reform in new ways in the days and weeks following treatment.
This research assessed the subacute impact of psilocybin on brain function in two clinical trials of depression:
The first trial was an open-label trial of oral psilocybin in patients with treatment-resistant depression.
Patients had baseline clinical assessment and resting-state functional MRI, followed by fixed-order “low” (10 mg) and “high” (25 mg) psilocybin therapy dosing days separated by 1 week. Of the 19 patients recruited, 3 were excluded as a result of excessive fMRI head motion. The team confirmed an antidepressant effect of psilocybin in 16 patients via reduced questionnaire scores from baseline.
Brain network modularity was significantly reduced 1 day after psilocybin therapy in 10 of 16 participants (mean difference, –0.29; t15, 2.87; 95% confidence interval, 0.07-0.50; P = .012; d = 0.72). This result implies an increase in functional connectivity between the brain’s main intrinsic networks.
Pre- vs posttreatment change in modularity significantly correlated with change in Beck Depression Inventory (BDI) score at 6 months, relative to baseline (r14 = 0.54, 95% CI, 0.14-0.78, P = .033). Results imply that decreased brain modularity 1 day after psilocybin therapy relates to long-term improvements in symptom severity.
Effective antidepressant alternative?
The second trial was a double-blind, phase 2, randomized, controlled trial comparing psilocybin with escitalopram (Lexapro). Twenty-one patients were included in the escitalopram imaging sample and 22 patients were included in the psilocybin imaging sample.
Patients received either 2 x 25 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily placebo (psilocybin arm) or 2 x 1 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily escitalopram (10-20 mg) (escitalopram arm). Functional MRI was recorded at baseline and 3 weeks after the second psilocybin dose.
On average, BDI-measured reductions in depressive symptom severity were significantly greater under psilocybin than escitalopram, indicating superior efficacy of psilocybin therapy versus escitalopram.
Evidence indicated that the reduction in network modularity and its relationship to depression severity was specific to the psilocybin group. In the escitalopram group, network modularity did not change from baseline and there was no significant correlation between changes in modularity and changes in BDI scores.
Post–psilocybin therapy changes in network flexibility were correlated with changes in BDI score. After false discovery rate correction, increased executive network dynamic flexibility strongly correlated with greater symptom improvement at the 6-week primary endpoint for the psilocybin arm (r20, –0.76, 95% CI, −0.90 to –0.50, P = .001).
There were no significant correlations between changes in BDI scores and changes in dynamic flexibility in the escitalopram arm.
“These findings are important because for the first time we find that psilocybin works differently from conventional antidepressants, making the brain more flexible and fluid and less entrenched in the negative thinking patterns associated with depression. This supports our initial predictions and confirms psilocybin could be a real alternative approach to depression treatments,” study investigator David Nutt, DM, head of the Imperial Centre for Psychedelic Research, London, said in a release.
“In previous studies we had seen a similar effect in the brain when people were scanned whilst on a psychedelic, but here we’re seeing it weeks after treatment for depression, which suggests a carryover of the acute drug action,” said Dr. Carhart-Harris.
Durable effect?
“We don’t yet know how long the changes in brain activity seen with psilocybin therapy last, and we need to do more research to understand this,” said Dr. Carhart-Harris, who is a member of the UCSF Weill Institute for Neurosciences. “If the changes don’t last, then is it related to relapse into a depressive episode? We need to do follow-up scans to see where people’s brains are at 3 months or even 6 months after treatment.
“We do know that some people relapse, and it may be that after a while their brains revert to the rigid patterns of activity we see in depression.
“One exciting implication of our findings is that we have discovered a fundamental mechanism via which psychedelic therapy works not just for depression but other mental illnesses, such as anorexia or addiction. We now need to test if this is the case, and if it is, then we have found something important,” added Dr. Carhart-Harris.
Successful phase 3, double-blind randomized, controlled trials will be required to achieve licensing for psilocybin therapy, but pragmatic trials may better address questions regarding treatment practicability, specificity, and optimization. Given the emerging research into psychedelic therapy, it is important for large-scale trials to establish the generalizability, reliability, and specificity of the drug’s antidepressant response.
So how close are we to full federal approval for psilocybin in the treatment of depression? Dr. Carhart-Harris estimated that within 4-5 years is realistic at the federal level. At the state level, in Oregon psilocybin therapy is on track for approval in 2023, including for patients currently undergoing treatment for depressive disorders. In addition, things are opening up in Canada, with some special-access opportunities.
The researchers cautioned that, while these findings are encouraging, trials assessing psilocybin for depression have taken place under controlled, clinical conditions, using a regulated dose formulated in a laboratory, and involved extensive psychological support by a mental health professional – before, during, and after dosing. Taking psychedelics in the absence of these combined safeguards may not have a positive outcome.
The research was supported by funding from the Alex Mosley Charitable Trust and founding donors of the Imperial Centre for Psychedelic Research. One coauthor was supported by the Imperial College London EPSRC Centre London for doctoral training in neurotechnology.
A version of this article first appeared on Medscape.com.
Led by investigators from the University of California, San Francisco, and Imperial College London’s Centre for Psychedelic Research, the findings come from a new analysis of brain scans of almost 60 patients with resistant depression treated with psilocybin.
“Not much is known about the changes in brain function after psychedelic experience. There has been much more research done on the acute brain action of psychedelics, but there is very little on the postacute or subacute changes in brain function,” study investigator Robin Carhart-Harris, PhD, former head of the Imperial Centre for Psychedelic Research and now director of the Neuroscape psychedelics division at UCSF, and senior author of the study, told this news organization.
“This research is a major advance because it is showing replication across two datasets with different designs. One in which the scanning is done 1 day after intervention and the other one when the posttreatment scanning is done 3 weeks after the second of two psilocybin therapy sessions,” Dr. Carhart-Harris added.
The study was published online in Nature Medicine.
A disruptor?
Psilocybin is one of a number of psychedelics under investigation as a potential therapy for psychiatric disorders. In the last 15 years, at least six separate clinical trials have reported impressive improvements in depressive symptoms with psilocybin therapy. Several studies have tested a synthesized a form of the drug to treat patients with depression and anxiety – with promising results.
However, the therapeutic action of psilocybin and other serotonergic psychedelics is still not completely understood, although it is known that they affect 5-HT2A receptors and are hypothesized to briefly disrupt these connections, allowing them to reform in new ways in the days and weeks following treatment.
This research assessed the subacute impact of psilocybin on brain function in two clinical trials of depression:
The first trial was an open-label trial of oral psilocybin in patients with treatment-resistant depression.
Patients had baseline clinical assessment and resting-state functional MRI, followed by fixed-order “low” (10 mg) and “high” (25 mg) psilocybin therapy dosing days separated by 1 week. Of the 19 patients recruited, 3 were excluded as a result of excessive fMRI head motion. The team confirmed an antidepressant effect of psilocybin in 16 patients via reduced questionnaire scores from baseline.
Brain network modularity was significantly reduced 1 day after psilocybin therapy in 10 of 16 participants (mean difference, –0.29; t15, 2.87; 95% confidence interval, 0.07-0.50; P = .012; d = 0.72). This result implies an increase in functional connectivity between the brain’s main intrinsic networks.
Pre- vs posttreatment change in modularity significantly correlated with change in Beck Depression Inventory (BDI) score at 6 months, relative to baseline (r14 = 0.54, 95% CI, 0.14-0.78, P = .033). Results imply that decreased brain modularity 1 day after psilocybin therapy relates to long-term improvements in symptom severity.
Effective antidepressant alternative?
The second trial was a double-blind, phase 2, randomized, controlled trial comparing psilocybin with escitalopram (Lexapro). Twenty-one patients were included in the escitalopram imaging sample and 22 patients were included in the psilocybin imaging sample.
Patients received either 2 x 25 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily placebo (psilocybin arm) or 2 x 1 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily escitalopram (10-20 mg) (escitalopram arm). Functional MRI was recorded at baseline and 3 weeks after the second psilocybin dose.
On average, BDI-measured reductions in depressive symptom severity were significantly greater under psilocybin than escitalopram, indicating superior efficacy of psilocybin therapy versus escitalopram.
Evidence indicated that the reduction in network modularity and its relationship to depression severity was specific to the psilocybin group. In the escitalopram group, network modularity did not change from baseline and there was no significant correlation between changes in modularity and changes in BDI scores.
Post–psilocybin therapy changes in network flexibility were correlated with changes in BDI score. After false discovery rate correction, increased executive network dynamic flexibility strongly correlated with greater symptom improvement at the 6-week primary endpoint for the psilocybin arm (r20, –0.76, 95% CI, −0.90 to –0.50, P = .001).
There were no significant correlations between changes in BDI scores and changes in dynamic flexibility in the escitalopram arm.
“These findings are important because for the first time we find that psilocybin works differently from conventional antidepressants, making the brain more flexible and fluid and less entrenched in the negative thinking patterns associated with depression. This supports our initial predictions and confirms psilocybin could be a real alternative approach to depression treatments,” study investigator David Nutt, DM, head of the Imperial Centre for Psychedelic Research, London, said in a release.
“In previous studies we had seen a similar effect in the brain when people were scanned whilst on a psychedelic, but here we’re seeing it weeks after treatment for depression, which suggests a carryover of the acute drug action,” said Dr. Carhart-Harris.
Durable effect?
“We don’t yet know how long the changes in brain activity seen with psilocybin therapy last, and we need to do more research to understand this,” said Dr. Carhart-Harris, who is a member of the UCSF Weill Institute for Neurosciences. “If the changes don’t last, then is it related to relapse into a depressive episode? We need to do follow-up scans to see where people’s brains are at 3 months or even 6 months after treatment.
“We do know that some people relapse, and it may be that after a while their brains revert to the rigid patterns of activity we see in depression.
“One exciting implication of our findings is that we have discovered a fundamental mechanism via which psychedelic therapy works not just for depression but other mental illnesses, such as anorexia or addiction. We now need to test if this is the case, and if it is, then we have found something important,” added Dr. Carhart-Harris.
Successful phase 3, double-blind randomized, controlled trials will be required to achieve licensing for psilocybin therapy, but pragmatic trials may better address questions regarding treatment practicability, specificity, and optimization. Given the emerging research into psychedelic therapy, it is important for large-scale trials to establish the generalizability, reliability, and specificity of the drug’s antidepressant response.
So how close are we to full federal approval for psilocybin in the treatment of depression? Dr. Carhart-Harris estimated that within 4-5 years is realistic at the federal level. At the state level, in Oregon psilocybin therapy is on track for approval in 2023, including for patients currently undergoing treatment for depressive disorders. In addition, things are opening up in Canada, with some special-access opportunities.
The researchers cautioned that, while these findings are encouraging, trials assessing psilocybin for depression have taken place under controlled, clinical conditions, using a regulated dose formulated in a laboratory, and involved extensive psychological support by a mental health professional – before, during, and after dosing. Taking psychedelics in the absence of these combined safeguards may not have a positive outcome.
The research was supported by funding from the Alex Mosley Charitable Trust and founding donors of the Imperial Centre for Psychedelic Research. One coauthor was supported by the Imperial College London EPSRC Centre London for doctoral training in neurotechnology.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Erectile dysfunction drugs linked to ocular conditions
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).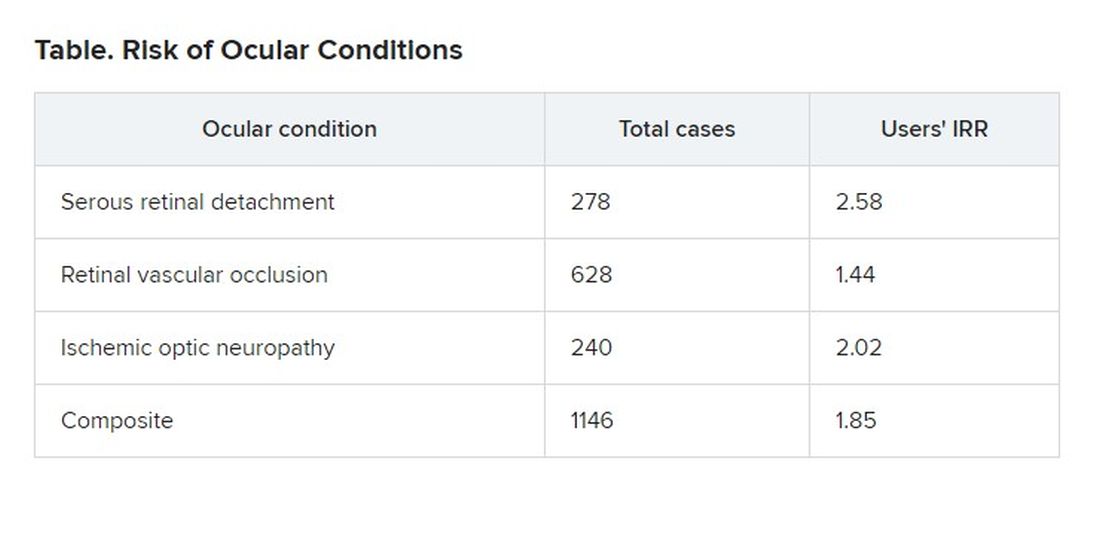
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
Breakthrough COVID dangerous for vaccinated cancer patients
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
FROM JAMA ONCOLOGY
Nontuberculous mycobacterial lung disease can be challenging to treat
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Long-term smell loss in COVID-19 tied to damage in the brain’s olfactory bulb
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Meningococcal vaccine shows moderate protective effect against gonorrhea
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
FROM THE LANCET INFECTIOUS DISEASES
Children with RMDs not at high risk for severe COVID-19, study finds
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Locoregional therapy lowers wait-list dropout in HCC
The use of bridging locoregional therapy (LRT) before liver transplantation in patients with hepatocellular carcinoma (HCC) has significantly increased in the United States within the past 15 years, a recent analysis suggests. Data show that liver transplant candidates with HCC who have elevated tumor burden and patients with more compensated liver disease have received a greater number of treatments while awaiting transplant.
According to the researchers, led by Allison Kwong, MD, of Stanford (Calif.) University, liver transplant remains a curative option for individuals with unresectable HCC who meet prespecified size criteria. In the United States, a mandated waiting period of 6 months prior “to gaining exception points has been implemented” in an effort “to allow for consideration of tumor biology and reduce the disparities in wait-list dropout between HCC and non-HCC patients,” the researchers wrote.
Several forms of LRT are now available for HCC, including chemoembolization, external beam radiation, radioembolization, and radiofrequency or microwave ablation. In the liver transplant setting, these LRT options enable management of intrahepatic disease in patients who are waiting for liver transplant, Dr. Kwong and colleagues explained.
The researchers, who published their study findings in the May issue of Clinical Gastroenterology and Hepatology, sought to examine the national temporal trends and wait-list outcomes of LRT in 31,609 patients eligible for liver transplant with greater than or equal to one approved HCC exception application in the United States.
Patient data were obtained from the Organ Procurement and Transplantation Network database and comprised primary adult LT candidates who were listed from the years 2003 to 2018. The investigators assessed explant histology and performed multivariable competing risk analysis to examine the relationship between the type of first LRT and time to wait-list dropout.
The wait-list dropout variable was defined by list removal because of death or excessive illness. The researchers noted that list removal likely represents disease progression “beyond transplantable criteria and beyond which patients were unlikely to benefit from or be eligible for further LRT.”
In the study population, the median age was 59 years, and approximately 77% of patients were male. More than half (53.1%) of the cohort had hepatitis C as the predominant liver disease etiology. Patients had a median follow-up period of 214 days on the waiting list.
Most patients (79%) received deceased or living-donor transplants, and 18.6% of patients were removed from the waiting list. Between the 2003 and 2006 period, the median wait-list time was 123 days, but this median wait-list duration increased to 257 days for patients listed between 2015 and 2018.
A total of 34,610 LRTs were performed among 24,145 liver transplant candidates during the study period. From 2003 to 2018, the proportion of patients with greater than or equal to 1 LRT recorded in the database rose from 42.3% to 92.4%, respectively. Most patients (67.8%) who received liver-directed therapy had a single LRT, while 23.8% of patients had two LRTs, 6.2% had three LRTs, and 2.2% had greater than or equal to four LRTs.
The most frequent type of LRT performed was chemoembolization, followed by thermal ablation. Radioembolization increased from less than 5% in 2013 to 19% in 2018. Moreover, in 2018, chemoembolization accounted for 50% of LRTs, while thermal ablation accounted for 22% of LRTs.
The incidence rates of LRT per 100 wait-list days was above average in patients who had an initial tumor burden beyond the Milan criteria (0.188), an alpha-fetoprotein level of 21-40 (0.171) or 41-500 ng/mL (0.179), Child-Pugh class A (0.160), patients in short (0.151) and medium (0.154) wait-time regions, as well as patients who were listed following implementation of cap-and-delay in October 2015 (0.192).
In the multivariable competing-risk analysis for wait-list dropout, adjusting for initial tumor burden and AFP, Child-Pugh class, wait region, and listing era, no locoregional therapy was associated with an increased risk of wait-list dropout versus chemoembolization as the first LRT in a multivariable competing-risk analysis (subhazard ratio, 1.37; 95% CI, 1.28-1.47). The inverse probability of treatment weighting–adjusted analysis found an association between radioembolization, when compared with chemoembolization, and a reduced risk of wait-list dropout (sHR, 0.85; 95% CI, 0.81-0.89). Thermal ablation was also associated with a reduced risk of wait-list dropout, compared with chemoembolization (sHR, 0.95; 95% CI, 0.91-0.99). “Radioembolization and thermal ablation may be superior to chemoembolization and prove to be more cost-effective options, depending on the clinical context,” the researchers wrote.
The researchers noted that they were unable to distinguish patients who were removed from the waiting list between those with disease progression versus liver failure.
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
In 1996, Mazzaferro and colleagues reported the results of a cohort of 48 patients with cirrhosis who had small, unresectable hepatocellular carcinoma (HCC). The actuarial survival rate was 75% at 4 years, and 83% of these patients had no recurrence, so, orthotopic liver transplantation became one of the standard options with curative intent for the treatment HCC. Because of HCC biology, some of these tumors grow or, worst-case scenario, are outside the Milan criteria. Locoregional therapies (LRT) were applied to arrest or downsize the tumor(s) to be within the liver transplantation criteria.
Kwong and colleagues, using the data of the Organ Procurement and Transplantation Network database, showed an exponential increase of LRT over 15 years: from 32.5% in 2003 to 92.4% in 2018. The Barcelona Clinic Liver Cancer staging system classifies chemoembolization, the most common LRT modality used in this cohort, as a palliative treatment rather than curative. Not surprisingly, the authors found that radioembolization was independently associated with a 15% reduction in the wait-list dropout rate, compared with chemoembolization. Further, listing in longer wait-time regions and more recent years was independently associated with a higher likelihood of wait-list dropout.
These data may be worrisome for patients listed for HCC. The median Model for End-Stage Liver Disease at Transplant Minus 3 National Policy, introduced in May 2019, decreases the transplantation rates in patients with HCC. Consequently, longer wait-list time leads to increase utilization of LRT to keep these patients within criteria. Radioembolization could become the preferred LRT therapy to stop tumor growth than chemoembolization and, probably, will be more cost effective. Future work should address explant outcomes and outcome on downstaging with external radiation therapy and adjuvant use of immunotherapy.
Ruben Hernaez, MD, MPH, PhD, is an assistant professor at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston. He has no relevant conflicts to disclose.
In 1996, Mazzaferro and colleagues reported the results of a cohort of 48 patients with cirrhosis who had small, unresectable hepatocellular carcinoma (HCC). The actuarial survival rate was 75% at 4 years, and 83% of these patients had no recurrence, so, orthotopic liver transplantation became one of the standard options with curative intent for the treatment HCC. Because of HCC biology, some of these tumors grow or, worst-case scenario, are outside the Milan criteria. Locoregional therapies (LRT) were applied to arrest or downsize the tumor(s) to be within the liver transplantation criteria.
Kwong and colleagues, using the data of the Organ Procurement and Transplantation Network database, showed an exponential increase of LRT over 15 years: from 32.5% in 2003 to 92.4% in 2018. The Barcelona Clinic Liver Cancer staging system classifies chemoembolization, the most common LRT modality used in this cohort, as a palliative treatment rather than curative. Not surprisingly, the authors found that radioembolization was independently associated with a 15% reduction in the wait-list dropout rate, compared with chemoembolization. Further, listing in longer wait-time regions and more recent years was independently associated with a higher likelihood of wait-list dropout.
These data may be worrisome for patients listed for HCC. The median Model for End-Stage Liver Disease at Transplant Minus 3 National Policy, introduced in May 2019, decreases the transplantation rates in patients with HCC. Consequently, longer wait-list time leads to increase utilization of LRT to keep these patients within criteria. Radioembolization could become the preferred LRT therapy to stop tumor growth than chemoembolization and, probably, will be more cost effective. Future work should address explant outcomes and outcome on downstaging with external radiation therapy and adjuvant use of immunotherapy.
Ruben Hernaez, MD, MPH, PhD, is an assistant professor at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston. He has no relevant conflicts to disclose.
In 1996, Mazzaferro and colleagues reported the results of a cohort of 48 patients with cirrhosis who had small, unresectable hepatocellular carcinoma (HCC). The actuarial survival rate was 75% at 4 years, and 83% of these patients had no recurrence, so, orthotopic liver transplantation became one of the standard options with curative intent for the treatment HCC. Because of HCC biology, some of these tumors grow or, worst-case scenario, are outside the Milan criteria. Locoregional therapies (LRT) were applied to arrest or downsize the tumor(s) to be within the liver transplantation criteria.
Kwong and colleagues, using the data of the Organ Procurement and Transplantation Network database, showed an exponential increase of LRT over 15 years: from 32.5% in 2003 to 92.4% in 2018. The Barcelona Clinic Liver Cancer staging system classifies chemoembolization, the most common LRT modality used in this cohort, as a palliative treatment rather than curative. Not surprisingly, the authors found that radioembolization was independently associated with a 15% reduction in the wait-list dropout rate, compared with chemoembolization. Further, listing in longer wait-time regions and more recent years was independently associated with a higher likelihood of wait-list dropout.
These data may be worrisome for patients listed for HCC. The median Model for End-Stage Liver Disease at Transplant Minus 3 National Policy, introduced in May 2019, decreases the transplantation rates in patients with HCC. Consequently, longer wait-list time leads to increase utilization of LRT to keep these patients within criteria. Radioembolization could become the preferred LRT therapy to stop tumor growth than chemoembolization and, probably, will be more cost effective. Future work should address explant outcomes and outcome on downstaging with external radiation therapy and adjuvant use of immunotherapy.
Ruben Hernaez, MD, MPH, PhD, is an assistant professor at the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston. He has no relevant conflicts to disclose.
The use of bridging locoregional therapy (LRT) before liver transplantation in patients with hepatocellular carcinoma (HCC) has significantly increased in the United States within the past 15 years, a recent analysis suggests. Data show that liver transplant candidates with HCC who have elevated tumor burden and patients with more compensated liver disease have received a greater number of treatments while awaiting transplant.
According to the researchers, led by Allison Kwong, MD, of Stanford (Calif.) University, liver transplant remains a curative option for individuals with unresectable HCC who meet prespecified size criteria. In the United States, a mandated waiting period of 6 months prior “to gaining exception points has been implemented” in an effort “to allow for consideration of tumor biology and reduce the disparities in wait-list dropout between HCC and non-HCC patients,” the researchers wrote.
Several forms of LRT are now available for HCC, including chemoembolization, external beam radiation, radioembolization, and radiofrequency or microwave ablation. In the liver transplant setting, these LRT options enable management of intrahepatic disease in patients who are waiting for liver transplant, Dr. Kwong and colleagues explained.
The researchers, who published their study findings in the May issue of Clinical Gastroenterology and Hepatology, sought to examine the national temporal trends and wait-list outcomes of LRT in 31,609 patients eligible for liver transplant with greater than or equal to one approved HCC exception application in the United States.
Patient data were obtained from the Organ Procurement and Transplantation Network database and comprised primary adult LT candidates who were listed from the years 2003 to 2018. The investigators assessed explant histology and performed multivariable competing risk analysis to examine the relationship between the type of first LRT and time to wait-list dropout.
The wait-list dropout variable was defined by list removal because of death or excessive illness. The researchers noted that list removal likely represents disease progression “beyond transplantable criteria and beyond which patients were unlikely to benefit from or be eligible for further LRT.”
In the study population, the median age was 59 years, and approximately 77% of patients were male. More than half (53.1%) of the cohort had hepatitis C as the predominant liver disease etiology. Patients had a median follow-up period of 214 days on the waiting list.
Most patients (79%) received deceased or living-donor transplants, and 18.6% of patients were removed from the waiting list. Between the 2003 and 2006 period, the median wait-list time was 123 days, but this median wait-list duration increased to 257 days for patients listed between 2015 and 2018.
A total of 34,610 LRTs were performed among 24,145 liver transplant candidates during the study period. From 2003 to 2018, the proportion of patients with greater than or equal to 1 LRT recorded in the database rose from 42.3% to 92.4%, respectively. Most patients (67.8%) who received liver-directed therapy had a single LRT, while 23.8% of patients had two LRTs, 6.2% had three LRTs, and 2.2% had greater than or equal to four LRTs.
The most frequent type of LRT performed was chemoembolization, followed by thermal ablation. Radioembolization increased from less than 5% in 2013 to 19% in 2018. Moreover, in 2018, chemoembolization accounted for 50% of LRTs, while thermal ablation accounted for 22% of LRTs.
The incidence rates of LRT per 100 wait-list days was above average in patients who had an initial tumor burden beyond the Milan criteria (0.188), an alpha-fetoprotein level of 21-40 (0.171) or 41-500 ng/mL (0.179), Child-Pugh class A (0.160), patients in short (0.151) and medium (0.154) wait-time regions, as well as patients who were listed following implementation of cap-and-delay in October 2015 (0.192).
In the multivariable competing-risk analysis for wait-list dropout, adjusting for initial tumor burden and AFP, Child-Pugh class, wait region, and listing era, no locoregional therapy was associated with an increased risk of wait-list dropout versus chemoembolization as the first LRT in a multivariable competing-risk analysis (subhazard ratio, 1.37; 95% CI, 1.28-1.47). The inverse probability of treatment weighting–adjusted analysis found an association between radioembolization, when compared with chemoembolization, and a reduced risk of wait-list dropout (sHR, 0.85; 95% CI, 0.81-0.89). Thermal ablation was also associated with a reduced risk of wait-list dropout, compared with chemoembolization (sHR, 0.95; 95% CI, 0.91-0.99). “Radioembolization and thermal ablation may be superior to chemoembolization and prove to be more cost-effective options, depending on the clinical context,” the researchers wrote.
The researchers noted that they were unable to distinguish patients who were removed from the waiting list between those with disease progression versus liver failure.
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
The use of bridging locoregional therapy (LRT) before liver transplantation in patients with hepatocellular carcinoma (HCC) has significantly increased in the United States within the past 15 years, a recent analysis suggests. Data show that liver transplant candidates with HCC who have elevated tumor burden and patients with more compensated liver disease have received a greater number of treatments while awaiting transplant.
According to the researchers, led by Allison Kwong, MD, of Stanford (Calif.) University, liver transplant remains a curative option for individuals with unresectable HCC who meet prespecified size criteria. In the United States, a mandated waiting period of 6 months prior “to gaining exception points has been implemented” in an effort “to allow for consideration of tumor biology and reduce the disparities in wait-list dropout between HCC and non-HCC patients,” the researchers wrote.
Several forms of LRT are now available for HCC, including chemoembolization, external beam radiation, radioembolization, and radiofrequency or microwave ablation. In the liver transplant setting, these LRT options enable management of intrahepatic disease in patients who are waiting for liver transplant, Dr. Kwong and colleagues explained.
The researchers, who published their study findings in the May issue of Clinical Gastroenterology and Hepatology, sought to examine the national temporal trends and wait-list outcomes of LRT in 31,609 patients eligible for liver transplant with greater than or equal to one approved HCC exception application in the United States.
Patient data were obtained from the Organ Procurement and Transplantation Network database and comprised primary adult LT candidates who were listed from the years 2003 to 2018. The investigators assessed explant histology and performed multivariable competing risk analysis to examine the relationship between the type of first LRT and time to wait-list dropout.
The wait-list dropout variable was defined by list removal because of death or excessive illness. The researchers noted that list removal likely represents disease progression “beyond transplantable criteria and beyond which patients were unlikely to benefit from or be eligible for further LRT.”
In the study population, the median age was 59 years, and approximately 77% of patients were male. More than half (53.1%) of the cohort had hepatitis C as the predominant liver disease etiology. Patients had a median follow-up period of 214 days on the waiting list.
Most patients (79%) received deceased or living-donor transplants, and 18.6% of patients were removed from the waiting list. Between the 2003 and 2006 period, the median wait-list time was 123 days, but this median wait-list duration increased to 257 days for patients listed between 2015 and 2018.
A total of 34,610 LRTs were performed among 24,145 liver transplant candidates during the study period. From 2003 to 2018, the proportion of patients with greater than or equal to 1 LRT recorded in the database rose from 42.3% to 92.4%, respectively. Most patients (67.8%) who received liver-directed therapy had a single LRT, while 23.8% of patients had two LRTs, 6.2% had three LRTs, and 2.2% had greater than or equal to four LRTs.
The most frequent type of LRT performed was chemoembolization, followed by thermal ablation. Radioembolization increased from less than 5% in 2013 to 19% in 2018. Moreover, in 2018, chemoembolization accounted for 50% of LRTs, while thermal ablation accounted for 22% of LRTs.
The incidence rates of LRT per 100 wait-list days was above average in patients who had an initial tumor burden beyond the Milan criteria (0.188), an alpha-fetoprotein level of 21-40 (0.171) or 41-500 ng/mL (0.179), Child-Pugh class A (0.160), patients in short (0.151) and medium (0.154) wait-time regions, as well as patients who were listed following implementation of cap-and-delay in October 2015 (0.192).
In the multivariable competing-risk analysis for wait-list dropout, adjusting for initial tumor burden and AFP, Child-Pugh class, wait region, and listing era, no locoregional therapy was associated with an increased risk of wait-list dropout versus chemoembolization as the first LRT in a multivariable competing-risk analysis (subhazard ratio, 1.37; 95% CI, 1.28-1.47). The inverse probability of treatment weighting–adjusted analysis found an association between radioembolization, when compared with chemoembolization, and a reduced risk of wait-list dropout (sHR, 0.85; 95% CI, 0.81-0.89). Thermal ablation was also associated with a reduced risk of wait-list dropout, compared with chemoembolization (sHR, 0.95; 95% CI, 0.91-0.99). “Radioembolization and thermal ablation may be superior to chemoembolization and prove to be more cost-effective options, depending on the clinical context,” the researchers wrote.
The researchers noted that they were unable to distinguish patients who were removed from the waiting list between those with disease progression versus liver failure.
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY




