User login
Survey reveals inconsistency in infection prevention practices
in the intensive care unit
Results of an anonymous survey suggest healthcare professionals in the US may not consistently follow recommendations for preventing bloodstream infections in patients with arterial catheters.
Of the roughly 1000 critical care clinicians surveyed, fewer than half said they comply with the current Centers for Disease Control and Prevention (CDC) guidelines, which recommend the use of limited barrier precautions during arterial catheter insertion.
This includes sterile gloves, a surgical cap, a surgical mask, and a small sterile drape.
“Barrier precautions are employed inconsistently by critical care clinicians across the nation, and such individuals underestimate the infection risks posed by arterial catheters,” said Leonard A. Mermel, DO, of Rhode Island Hospital in Providence.
He and his colleagues reported these findings in Critical Care Medicine.
The researchers sent an anonymous, web-based survey to 11,361 physicians, nurse practitioners, physician assistants, respiratory therapists, and registered nurses who receive emails from the Society of Critical Care Medicine.
There were 1265 responses (an 11% response rate) and 1029 eligible participants after exclusions.
Of the eligible respondents, 44% said they used CDC-recommended barrier precautions during arterial catheter insertion, and 15% said they use full barrier precautions.
However, 39% of respondents said they would support mandatory use of full barrier precautions during arterial catheter insertion.
“There appears to be a significant deviation from clinical guidelines regarding a very commonly performed procedure in critically ill patients,” said study author Andrew Levinson, MD, also of Rhode Island Hospital.
“Bloodstream infections are largely preventable, and if the survey results mirror the clinical practice in the US, there’s work to be done in reducing risk of such infections.”
The survey also indicated that respondents underestimate the risk of bloodstream infections associated with arterial catheters. The respondents’ mean estimate of infection incidence was 0.3 per 1000 catheter-days, and the median estimate was 0.1 per 1000 catheter-days.
However, Dr Mermel and his colleagues said recent studies have suggested the incidence of bloodstream infections associated with arterial catheters in the US is 0.9 to 3.4 per 1000 catheter-days. ![]()

in the intensive care unit
Results of an anonymous survey suggest healthcare professionals in the US may not consistently follow recommendations for preventing bloodstream infections in patients with arterial catheters.
Of the roughly 1000 critical care clinicians surveyed, fewer than half said they comply with the current Centers for Disease Control and Prevention (CDC) guidelines, which recommend the use of limited barrier precautions during arterial catheter insertion.
This includes sterile gloves, a surgical cap, a surgical mask, and a small sterile drape.
“Barrier precautions are employed inconsistently by critical care clinicians across the nation, and such individuals underestimate the infection risks posed by arterial catheters,” said Leonard A. Mermel, DO, of Rhode Island Hospital in Providence.
He and his colleagues reported these findings in Critical Care Medicine.
The researchers sent an anonymous, web-based survey to 11,361 physicians, nurse practitioners, physician assistants, respiratory therapists, and registered nurses who receive emails from the Society of Critical Care Medicine.
There were 1265 responses (an 11% response rate) and 1029 eligible participants after exclusions.
Of the eligible respondents, 44% said they used CDC-recommended barrier precautions during arterial catheter insertion, and 15% said they use full barrier precautions.
However, 39% of respondents said they would support mandatory use of full barrier precautions during arterial catheter insertion.
“There appears to be a significant deviation from clinical guidelines regarding a very commonly performed procedure in critically ill patients,” said study author Andrew Levinson, MD, also of Rhode Island Hospital.
“Bloodstream infections are largely preventable, and if the survey results mirror the clinical practice in the US, there’s work to be done in reducing risk of such infections.”
The survey also indicated that respondents underestimate the risk of bloodstream infections associated with arterial catheters. The respondents’ mean estimate of infection incidence was 0.3 per 1000 catheter-days, and the median estimate was 0.1 per 1000 catheter-days.
However, Dr Mermel and his colleagues said recent studies have suggested the incidence of bloodstream infections associated with arterial catheters in the US is 0.9 to 3.4 per 1000 catheter-days. ![]()

in the intensive care unit
Results of an anonymous survey suggest healthcare professionals in the US may not consistently follow recommendations for preventing bloodstream infections in patients with arterial catheters.
Of the roughly 1000 critical care clinicians surveyed, fewer than half said they comply with the current Centers for Disease Control and Prevention (CDC) guidelines, which recommend the use of limited barrier precautions during arterial catheter insertion.
This includes sterile gloves, a surgical cap, a surgical mask, and a small sterile drape.
“Barrier precautions are employed inconsistently by critical care clinicians across the nation, and such individuals underestimate the infection risks posed by arterial catheters,” said Leonard A. Mermel, DO, of Rhode Island Hospital in Providence.
He and his colleagues reported these findings in Critical Care Medicine.
The researchers sent an anonymous, web-based survey to 11,361 physicians, nurse practitioners, physician assistants, respiratory therapists, and registered nurses who receive emails from the Society of Critical Care Medicine.
There were 1265 responses (an 11% response rate) and 1029 eligible participants after exclusions.
Of the eligible respondents, 44% said they used CDC-recommended barrier precautions during arterial catheter insertion, and 15% said they use full barrier precautions.
However, 39% of respondents said they would support mandatory use of full barrier precautions during arterial catheter insertion.
“There appears to be a significant deviation from clinical guidelines regarding a very commonly performed procedure in critically ill patients,” said study author Andrew Levinson, MD, also of Rhode Island Hospital.
“Bloodstream infections are largely preventable, and if the survey results mirror the clinical practice in the US, there’s work to be done in reducing risk of such infections.”
The survey also indicated that respondents underestimate the risk of bloodstream infections associated with arterial catheters. The respondents’ mean estimate of infection incidence was 0.3 per 1000 catheter-days, and the median estimate was 0.1 per 1000 catheter-days.
However, Dr Mermel and his colleagues said recent studies have suggested the incidence of bloodstream infections associated with arterial catheters in the US is 0.9 to 3.4 per 1000 catheter-days. ![]()
Daylight photodynamic therapy best for disseminated actinic keratoses
NEW YORK – For treating actinic keratoses appearing over large or disseminated surface areas, daylight is often a better strategy than artificial light is for activating photodynamic therapy (PDT), according to an update on strategies at the summer meeting of the American Academy of Dermatology.
The evidence that daylight is at least as effective as artificial light for grade 1 and 2 actinic keratoses has created a “really exciting new opportunity” for those with disseminated disease, according to Dr. Emily J. Fisher, director of Mohs surgery at Mercy Health Physicians, Cincinnati, Ohio. This approach is not only a more efficient way to treat large or multiple areas of skin, but it is better tolerated, further facilitating treatment of bigger surface areas.
Moreover, for physicians not currently equipped with blue or red artificial light, daylight activation “is a great way to incorporate PDT into your practice,” Dr. Fisher added. She suggested that PDT, which has been associated with clearance rates exceeding 70% with a single treatment in many studies, appears to be at least as effective as the topical treatments that are employed more often, such as 5-fluorouracil (5-FU) or imiquimod.
When comparing efficacy across studies, “PDT seems to have a higher response than [does] our topical treatments,” but Dr. Fisher cautioned that there are no high-quality, head-to-head comparisons, so there is no definitive evidence of the superiority of one over the other.
However, in patients with multiple actinic keratoses spread over a substantial area of the skin or who have lesions in more than one anatomical site, daylight PDT is a practical approach now widely used in Europe and several other parts of the world, according to Dr. Fisher. She reported that five randomized trials have demonstrated that daylight PDT is effective.
Most aspects of daylight PDT are the same as conventional PDT, according to Dr. Fisher. The skin is first prepared by removing scales and crusts to improve penetration of the photodynamic agent, whether aminolevulinate acid (ALA) or methyl aminolevulinate (MAL). Prior to light exposure, the occlusion time with the photodynamic agent is the same 3 hours. Also, light exposure in both cases should begin within 30 minutes, which may be a consideration for those depending on daylight.
“Patients really should not go indoors or into shade for more than 5 minutes at a time,” Dr. Fisher reported. The problem is that the activity of the intracellular photosensitizing chemicals can build up without light exposure, producing pain and reducing the tolerability of the treatment.
Consensus guidelines have been published for daylight PDT in Australia (J Eur Acad Dermatol Venereol. 2012 Jun;26[6]:673-9.). According to Dr. Fisher, the guidelines recommend a light intensity of greater than 130 W/m2, which is a dose provided by sunlight within the continental United States but not at distant points from the equator, such as Alaska. The guidelines also specify that sun exposure take place with a minimum temperature of 10° C. Dr. Fisher said that activity is diminished at lower temperatures.
“After 2 hours of exposure, patients should wash off the ALA and avoid further exposure for about 48 hours,” said Dr. Fisher, who recommended chemical sunscreens on areas of the skin not being treated.
“I think that over the next few years, this is going to have a big place in patient treatment. It is more convenient and better tolerated,” Dr. Fisher reported. She said that several modifications with the potential to enhance efficacy, such as pretreatment with retinoids or employing 5-FU after PDT, are strategies that have shown promise in small studies and may prove to be helpful through expanded clinical investigation.
NEW YORK – For treating actinic keratoses appearing over large or disseminated surface areas, daylight is often a better strategy than artificial light is for activating photodynamic therapy (PDT), according to an update on strategies at the summer meeting of the American Academy of Dermatology.
The evidence that daylight is at least as effective as artificial light for grade 1 and 2 actinic keratoses has created a “really exciting new opportunity” for those with disseminated disease, according to Dr. Emily J. Fisher, director of Mohs surgery at Mercy Health Physicians, Cincinnati, Ohio. This approach is not only a more efficient way to treat large or multiple areas of skin, but it is better tolerated, further facilitating treatment of bigger surface areas.
Moreover, for physicians not currently equipped with blue or red artificial light, daylight activation “is a great way to incorporate PDT into your practice,” Dr. Fisher added. She suggested that PDT, which has been associated with clearance rates exceeding 70% with a single treatment in many studies, appears to be at least as effective as the topical treatments that are employed more often, such as 5-fluorouracil (5-FU) or imiquimod.
When comparing efficacy across studies, “PDT seems to have a higher response than [does] our topical treatments,” but Dr. Fisher cautioned that there are no high-quality, head-to-head comparisons, so there is no definitive evidence of the superiority of one over the other.
However, in patients with multiple actinic keratoses spread over a substantial area of the skin or who have lesions in more than one anatomical site, daylight PDT is a practical approach now widely used in Europe and several other parts of the world, according to Dr. Fisher. She reported that five randomized trials have demonstrated that daylight PDT is effective.
Most aspects of daylight PDT are the same as conventional PDT, according to Dr. Fisher. The skin is first prepared by removing scales and crusts to improve penetration of the photodynamic agent, whether aminolevulinate acid (ALA) or methyl aminolevulinate (MAL). Prior to light exposure, the occlusion time with the photodynamic agent is the same 3 hours. Also, light exposure in both cases should begin within 30 minutes, which may be a consideration for those depending on daylight.
“Patients really should not go indoors or into shade for more than 5 minutes at a time,” Dr. Fisher reported. The problem is that the activity of the intracellular photosensitizing chemicals can build up without light exposure, producing pain and reducing the tolerability of the treatment.
Consensus guidelines have been published for daylight PDT in Australia (J Eur Acad Dermatol Venereol. 2012 Jun;26[6]:673-9.). According to Dr. Fisher, the guidelines recommend a light intensity of greater than 130 W/m2, which is a dose provided by sunlight within the continental United States but not at distant points from the equator, such as Alaska. The guidelines also specify that sun exposure take place with a minimum temperature of 10° C. Dr. Fisher said that activity is diminished at lower temperatures.
“After 2 hours of exposure, patients should wash off the ALA and avoid further exposure for about 48 hours,” said Dr. Fisher, who recommended chemical sunscreens on areas of the skin not being treated.
“I think that over the next few years, this is going to have a big place in patient treatment. It is more convenient and better tolerated,” Dr. Fisher reported. She said that several modifications with the potential to enhance efficacy, such as pretreatment with retinoids or employing 5-FU after PDT, are strategies that have shown promise in small studies and may prove to be helpful through expanded clinical investigation.
NEW YORK – For treating actinic keratoses appearing over large or disseminated surface areas, daylight is often a better strategy than artificial light is for activating photodynamic therapy (PDT), according to an update on strategies at the summer meeting of the American Academy of Dermatology.
The evidence that daylight is at least as effective as artificial light for grade 1 and 2 actinic keratoses has created a “really exciting new opportunity” for those with disseminated disease, according to Dr. Emily J. Fisher, director of Mohs surgery at Mercy Health Physicians, Cincinnati, Ohio. This approach is not only a more efficient way to treat large or multiple areas of skin, but it is better tolerated, further facilitating treatment of bigger surface areas.
Moreover, for physicians not currently equipped with blue or red artificial light, daylight activation “is a great way to incorporate PDT into your practice,” Dr. Fisher added. She suggested that PDT, which has been associated with clearance rates exceeding 70% with a single treatment in many studies, appears to be at least as effective as the topical treatments that are employed more often, such as 5-fluorouracil (5-FU) or imiquimod.
When comparing efficacy across studies, “PDT seems to have a higher response than [does] our topical treatments,” but Dr. Fisher cautioned that there are no high-quality, head-to-head comparisons, so there is no definitive evidence of the superiority of one over the other.
However, in patients with multiple actinic keratoses spread over a substantial area of the skin or who have lesions in more than one anatomical site, daylight PDT is a practical approach now widely used in Europe and several other parts of the world, according to Dr. Fisher. She reported that five randomized trials have demonstrated that daylight PDT is effective.
Most aspects of daylight PDT are the same as conventional PDT, according to Dr. Fisher. The skin is first prepared by removing scales and crusts to improve penetration of the photodynamic agent, whether aminolevulinate acid (ALA) or methyl aminolevulinate (MAL). Prior to light exposure, the occlusion time with the photodynamic agent is the same 3 hours. Also, light exposure in both cases should begin within 30 minutes, which may be a consideration for those depending on daylight.
“Patients really should not go indoors or into shade for more than 5 minutes at a time,” Dr. Fisher reported. The problem is that the activity of the intracellular photosensitizing chemicals can build up without light exposure, producing pain and reducing the tolerability of the treatment.
Consensus guidelines have been published for daylight PDT in Australia (J Eur Acad Dermatol Venereol. 2012 Jun;26[6]:673-9.). According to Dr. Fisher, the guidelines recommend a light intensity of greater than 130 W/m2, which is a dose provided by sunlight within the continental United States but not at distant points from the equator, such as Alaska. The guidelines also specify that sun exposure take place with a minimum temperature of 10° C. Dr. Fisher said that activity is diminished at lower temperatures.
“After 2 hours of exposure, patients should wash off the ALA and avoid further exposure for about 48 hours,” said Dr. Fisher, who recommended chemical sunscreens on areas of the skin not being treated.
“I think that over the next few years, this is going to have a big place in patient treatment. It is more convenient and better tolerated,” Dr. Fisher reported. She said that several modifications with the potential to enhance efficacy, such as pretreatment with retinoids or employing 5-FU after PDT, are strategies that have shown promise in small studies and may prove to be helpful through expanded clinical investigation.
AT THE SUMMER MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: For disseminated actinic keratoses, daylight is often the best way to activate photodynamic therapy.
Major finding: As effective as artificial light in five randomized trials, daylight photodynamic therapy for actinic keratoses is better tolerated and allows treatment of larger surface areas.
Data source: Review of data from randomized trials.
Disclosures: Dr. Fisher reported having no financial disclosures.
Separating heart disease myths from facts
There’s a lot of information out there about heart disease and how to prevent it, but there’s a lot of misinformation out there as well, and it’s important to get the facts straight when it comes to your heart, according to a Harvard Heart Letter report.
While it may seem logical to limit physical activity if you have a heart problem, in nearly all cases, a person with heart disease can benefit from regular, moderate amounts of exercise. And while it may also make sense to eat a very-low-fat diet, it is really only saturated fat that is harmful to the heart. A diet rich in unsaturated fat from foods such as fish, olive oil, and low-fat dairy products actually reduces the risk of heart disease.
Cholesterol-lowering drugs reduce cholesterol produced by the liver; they do not reduce cholesterol you get from food, so taking such a drug is not a free pass to continue eating fatty food. Along those lines, even if you take a drug to manage diabetes, the disease still can cause heart disease.
While it is true that women under the age of 60 years are significantly less likely to get heart disease than are men, this disparity disappears over the age of 60, and by age 80 years, women are slightly more likely to have heart disease. It is also true that quitting smoking has immediate benefits, even if you’ve been smoking for years. Heart attack risk drops by 50% after 1 year being tobacco free, and the increased risk disappears entirely after 10 years.
A small heart attack may not do much damage – it may be barely noticeable. But any heart attack is indicative of a big problem. While surgical procedures such as stenting or bypasses do a lot for managing symptoms, they do not fix the problem, and life changes are still recommended.
For more information about heart disease myths, visit the Harvard Medical School website.
There’s a lot of information out there about heart disease and how to prevent it, but there’s a lot of misinformation out there as well, and it’s important to get the facts straight when it comes to your heart, according to a Harvard Heart Letter report.
While it may seem logical to limit physical activity if you have a heart problem, in nearly all cases, a person with heart disease can benefit from regular, moderate amounts of exercise. And while it may also make sense to eat a very-low-fat diet, it is really only saturated fat that is harmful to the heart. A diet rich in unsaturated fat from foods such as fish, olive oil, and low-fat dairy products actually reduces the risk of heart disease.
Cholesterol-lowering drugs reduce cholesterol produced by the liver; they do not reduce cholesterol you get from food, so taking such a drug is not a free pass to continue eating fatty food. Along those lines, even if you take a drug to manage diabetes, the disease still can cause heart disease.
While it is true that women under the age of 60 years are significantly less likely to get heart disease than are men, this disparity disappears over the age of 60, and by age 80 years, women are slightly more likely to have heart disease. It is also true that quitting smoking has immediate benefits, even if you’ve been smoking for years. Heart attack risk drops by 50% after 1 year being tobacco free, and the increased risk disappears entirely after 10 years.
A small heart attack may not do much damage – it may be barely noticeable. But any heart attack is indicative of a big problem. While surgical procedures such as stenting or bypasses do a lot for managing symptoms, they do not fix the problem, and life changes are still recommended.
For more information about heart disease myths, visit the Harvard Medical School website.
There’s a lot of information out there about heart disease and how to prevent it, but there’s a lot of misinformation out there as well, and it’s important to get the facts straight when it comes to your heart, according to a Harvard Heart Letter report.
While it may seem logical to limit physical activity if you have a heart problem, in nearly all cases, a person with heart disease can benefit from regular, moderate amounts of exercise. And while it may also make sense to eat a very-low-fat diet, it is really only saturated fat that is harmful to the heart. A diet rich in unsaturated fat from foods such as fish, olive oil, and low-fat dairy products actually reduces the risk of heart disease.
Cholesterol-lowering drugs reduce cholesterol produced by the liver; they do not reduce cholesterol you get from food, so taking such a drug is not a free pass to continue eating fatty food. Along those lines, even if you take a drug to manage diabetes, the disease still can cause heart disease.
While it is true that women under the age of 60 years are significantly less likely to get heart disease than are men, this disparity disappears over the age of 60, and by age 80 years, women are slightly more likely to have heart disease. It is also true that quitting smoking has immediate benefits, even if you’ve been smoking for years. Heart attack risk drops by 50% after 1 year being tobacco free, and the increased risk disappears entirely after 10 years.
A small heart attack may not do much damage – it may be barely noticeable. But any heart attack is indicative of a big problem. While surgical procedures such as stenting or bypasses do a lot for managing symptoms, they do not fix the problem, and life changes are still recommended.
For more information about heart disease myths, visit the Harvard Medical School website.
CUT DOWNTIME: The Lean way for a busy practitioner to improve efficiency
The mnemonic CUT DOWNTIME, which I have adapted and modified from the book Lean Healthcare Deployment and Sustainability,1 breaks down waste in health care—an activity that adds no value to a service—into 11 major categories (Table). This mnemonic provides the busy practitioner a simple framework for improving quality and efficiency of services by identifying and eliminating wastes the Lean way.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Reference
1. Dean ML. Lean healthcare deployment and sustainability. New York, NY: McGraw-Hill; 2013.
The mnemonic CUT DOWNTIME, which I have adapted and modified from the book Lean Healthcare Deployment and Sustainability,1 breaks down waste in health care—an activity that adds no value to a service—into 11 major categories (Table). This mnemonic provides the busy practitioner a simple framework for improving quality and efficiency of services by identifying and eliminating wastes the Lean way.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
The mnemonic CUT DOWNTIME, which I have adapted and modified from the book Lean Healthcare Deployment and Sustainability,1 breaks down waste in health care—an activity that adds no value to a service—into 11 major categories (Table). This mnemonic provides the busy practitioner a simple framework for improving quality and efficiency of services by identifying and eliminating wastes the Lean way.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Reference
1. Dean ML. Lean healthcare deployment and sustainability. New York, NY: McGraw-Hill; 2013.
Reference
1. Dean ML. Lean healthcare deployment and sustainability. New York, NY: McGraw-Hill; 2013.
‘It’s my money, and I want it now!’ Clinical variables related to payeeship under Social Security
The Social Security Administration (SSA) does not provide much guidance on the contentious issue of determining payeeship for disability beneficiaries. The only description available is stated on the “Physician/medical officer’s statement of patient’s capability to manage benefits” (form SSA-787): “By capable we mean that the patient: Is able to understand and act on the ordinary affairs of life, such as providing for own adequate food, housing, etc., and is able, in spite of physical impairments, to manage funds or direct others how to manage them.”
Physicians will be asked to make a capability statement if they are performing a consultative examination for SSA or if their patient:
• is applying for benefits
• needs to have a payee.
Regrettably, the published literature on capability is scant.1,2 Based on decades of personal experience, here is the approach I have adopted to determine capability.
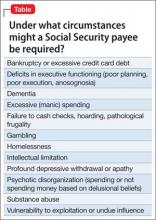
Diagnoses, circumstances, and clinical syndromes that strongly suggest the need for a payee include those listed in the Table.
The psychiatric rehabilitation agency I work at adheres to a recovery model. I consult with caseworkers on the issue of capability, but generally endorse a “team” recommendation for initiating or terminating payeeship. A number of factors are involved:
Adherence to recovery means that we encourage autonomy; we do not attempt to prevent every bad decision.
Demands for money from the patient and demands to terminate payeeship can be strident and potentially violent.
Confrontations over payeeship can be a safety risk for family or staff who have been acting as the payee.
Guardianship (or conservatorship) is a judicially determined restriction of financial decision-making.
Payeeship is an extrajudicial restriction of financial decision-making. Treating physicians, understandably, may feel uneasy restricting the rights of a patient. Additionally, there is ethical stress when a physician does anything that might compromise the primacy of the treatment relationship.
If all parties agree that payeeship should be terminated, I recommend the payee (whether the family or an institutional payee) begin a 3-month trial, during which the payee does not pay bills or keep a budget. The patient receives his (her) money in a lump sum at the beginning of the month, which begins a naturalistic trial of the patient’s capability to pay rent and budget adequately for all other necessities. If the patient demonstrates capability, I sign the SSA-787 form.
Offering a structured plan for restoring a patient’s benefits could defuse hostile demands.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Marson DC, Savage R, Phillips J. Financial capacity in persons with schizophrenia and serious mental illness: clinical and research ethics aspects. Schizophr Bull. 2006; 32(1):81-91.
2. Rosen MI. The ‘check effect’ reconsidered. Addiction. 2011;106(6):1071-1077.
The Social Security Administration (SSA) does not provide much guidance on the contentious issue of determining payeeship for disability beneficiaries. The only description available is stated on the “Physician/medical officer’s statement of patient’s capability to manage benefits” (form SSA-787): “By capable we mean that the patient: Is able to understand and act on the ordinary affairs of life, such as providing for own adequate food, housing, etc., and is able, in spite of physical impairments, to manage funds or direct others how to manage them.”
Physicians will be asked to make a capability statement if they are performing a consultative examination for SSA or if their patient:
• is applying for benefits
• needs to have a payee.
Regrettably, the published literature on capability is scant.1,2 Based on decades of personal experience, here is the approach I have adopted to determine capability.
Diagnoses, circumstances, and clinical syndromes that strongly suggest the need for a payee include those listed in the Table.
The psychiatric rehabilitation agency I work at adheres to a recovery model. I consult with caseworkers on the issue of capability, but generally endorse a “team” recommendation for initiating or terminating payeeship. A number of factors are involved:
Adherence to recovery means that we encourage autonomy; we do not attempt to prevent every bad decision.
Demands for money from the patient and demands to terminate payeeship can be strident and potentially violent.
Confrontations over payeeship can be a safety risk for family or staff who have been acting as the payee.
Guardianship (or conservatorship) is a judicially determined restriction of financial decision-making.
Payeeship is an extrajudicial restriction of financial decision-making. Treating physicians, understandably, may feel uneasy restricting the rights of a patient. Additionally, there is ethical stress when a physician does anything that might compromise the primacy of the treatment relationship.
If all parties agree that payeeship should be terminated, I recommend the payee (whether the family or an institutional payee) begin a 3-month trial, during which the payee does not pay bills or keep a budget. The patient receives his (her) money in a lump sum at the beginning of the month, which begins a naturalistic trial of the patient’s capability to pay rent and budget adequately for all other necessities. If the patient demonstrates capability, I sign the SSA-787 form.
Offering a structured plan for restoring a patient’s benefits could defuse hostile demands.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
The Social Security Administration (SSA) does not provide much guidance on the contentious issue of determining payeeship for disability beneficiaries. The only description available is stated on the “Physician/medical officer’s statement of patient’s capability to manage benefits” (form SSA-787): “By capable we mean that the patient: Is able to understand and act on the ordinary affairs of life, such as providing for own adequate food, housing, etc., and is able, in spite of physical impairments, to manage funds or direct others how to manage them.”
Physicians will be asked to make a capability statement if they are performing a consultative examination for SSA or if their patient:
• is applying for benefits
• needs to have a payee.
Regrettably, the published literature on capability is scant.1,2 Based on decades of personal experience, here is the approach I have adopted to determine capability.
Diagnoses, circumstances, and clinical syndromes that strongly suggest the need for a payee include those listed in the Table.
The psychiatric rehabilitation agency I work at adheres to a recovery model. I consult with caseworkers on the issue of capability, but generally endorse a “team” recommendation for initiating or terminating payeeship. A number of factors are involved:
Adherence to recovery means that we encourage autonomy; we do not attempt to prevent every bad decision.
Demands for money from the patient and demands to terminate payeeship can be strident and potentially violent.
Confrontations over payeeship can be a safety risk for family or staff who have been acting as the payee.
Guardianship (or conservatorship) is a judicially determined restriction of financial decision-making.
Payeeship is an extrajudicial restriction of financial decision-making. Treating physicians, understandably, may feel uneasy restricting the rights of a patient. Additionally, there is ethical stress when a physician does anything that might compromise the primacy of the treatment relationship.
If all parties agree that payeeship should be terminated, I recommend the payee (whether the family or an institutional payee) begin a 3-month trial, during which the payee does not pay bills or keep a budget. The patient receives his (her) money in a lump sum at the beginning of the month, which begins a naturalistic trial of the patient’s capability to pay rent and budget adequately for all other necessities. If the patient demonstrates capability, I sign the SSA-787 form.
Offering a structured plan for restoring a patient’s benefits could defuse hostile demands.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Marson DC, Savage R, Phillips J. Financial capacity in persons with schizophrenia and serious mental illness: clinical and research ethics aspects. Schizophr Bull. 2006; 32(1):81-91.
2. Rosen MI. The ‘check effect’ reconsidered. Addiction. 2011;106(6):1071-1077.
1. Marson DC, Savage R, Phillips J. Financial capacity in persons with schizophrenia and serious mental illness: clinical and research ethics aspects. Schizophr Bull. 2006; 32(1):81-91.
2. Rosen MI. The ‘check effect’ reconsidered. Addiction. 2011;106(6):1071-1077.
Peaceful feeling, or up in smoke? Medical marijuana in medicolegal context
Dear Dr. Mossman,
I practice in a state that allows medical marijuana use. A few of my patients have asked me to help them obtain marijuana for their conditions. How risky would it be to oblige?
Submitted by “Dr. J”
In recent years, public debate about marijuana has acquired 2 new dimensions: (1) the wishes and medical needs of people who seek marijuana for its purported health benefits, and (2) the role of physicians who practice where “medical marijuana” is legal. This article, the authors’ joint effort to address Dr. J’s concerns, hits 3 topics:
• the intersection of marijuana policy and health care in the United States
• the risks and possible benefits of marijuana use
• the medicolegal problems faced by physicians who might advise patients to use marijuana.
Legal haze
Two cannabinoids—dronabinol and nabilone—have received FDA approval as appetite enhancers and anti-nausea agents. Third-party payors usually cover these types of medications, but no insurer pays for medical marijuana.1 The Controlled Substances Act of 19702 classified marijuana as a Schedule I drug because of its abuse potential, lack of accepted medical applications, and uncertain safety. The FDA has not approved marijuana use for any medical condition.
Although people commonly speak of “prescribing” marijuana, physicians cannot legally do this in the United States. What physicians may do, in the 23 states that allow medical marijuana, is recommend or certify a patient’s marijuana use—an action that has constitutional protection under the First Amendment’s freedom of speech clause.3,4
A physician may complete documentation that a patient has one of the qualifying medical conditions for which the jurisdiction has legalized medical marijuana. Either the patient or the physician then submits that documentation to the appropriate government agency (eg, the state’s department of health).
If the documentation receives approval, the agency will issue the patient a registration card that allows possession of medical marijuana, with which the patient can obtain or grow a small amount of marijuana. The cannabinoid content of marijuana products varies considerably,5 and physicians who certify marijuana typically defer dosage recommendations to the patient or the dispensary.1
In states that allow medical marijuana, users may assert an affirmative defense of medical necessity if they face criminal prosecution.3,6 Possession of marijuana remains illegal under federal law, however, regardless of one’s reason for having it.7,8 Since October 2009, the Attorney General’s office has discouraged federal prosecutions of persons “whose actions are in clear and unambiguous compliance with existing state laws providing for the medical use of marijuana.”9 But given the remaining conflicts between state and federal laws, “the legal implications of certifying patients for medical marijuana remain unclear.”10
Physicians have few resources to instruct them on the legal risks of certifying medical marijuana. When Canada legalized medical marijuana, the organization that provides malpractice insurance to Canadian physicians told its members that “prescribing medical marijuana cannot be compared to prescribing prescription drugs” and recommended that physicians obtain signed release forms documenting that they have discussed the risks of medical marijuana with patients.11 For some risky approved drugs, the FDA has established a risk evaluation and mitigation strategy, but no such guidance is available for marijuana.
Highlighting the benefits and risks
Proponents of medical marijuana claim that Cannabis can help patients, and dispassionate experts acknowledge that at least modest evidence supports the benefits of using “marijuana for nausea and vomiting related to chemotherapy, specific pain syndromes, and spasticity from multiple sclerosis.”10 For several other conditions— HIV/AIDS, depression, anxiety disorders, sleep disorders, psychosis, Tourette syndrome—evidence of benefit is poor.12 Rigorous evaluation of medical marijuana is difficult because the plant contains hundreds of active chemical compounds. The chemical content of marijuana is highly variable, depending on its preparation and administration,10,13—one reason why only a few good randomized controlled trials of marijuana have been conducted.
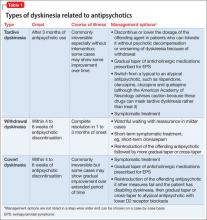
Marijuana has several side effects and carries many health risks (Table 1).4,14-20
On the highway: Marijuana and driving
Marijuana use impairs driving ability.14 Following enactment of more lenient marijuana laws, several states have reported higher numbers of fatally injured drivers who tested positive for Cannabis21-23 and had a positive screen of tetrahydrocannabinol (THC) in driving under the influence cases.24,25 One study showed that a blood THC concentration >5 ng/mL (comparable to a blood alcohol concentration of 0.15%) increased the crash odds ratio to 6.6.25,26
Marijuana impairs reaction time, information processing, motor performance, attention, and visual processing.14,16,27,28 Drivers who are under the influence of marijuana make more driving errors, despite being cautious about how they react to traffic.29 Even after weeks of abstinence, previous daily users of marijuana display some cognitive processing and driving-related impairments.30,31
Courts have found physicians negligent if their patients’ treatment-induced driving impairments injured others when the risk of driving-related injury was foreseeable.32 The Massachusetts case of Coombes v Florio33 likened the physician’s duty to that of a liquor store that sells alcohol to a minor who subsequently crashes, or to a father who did not lock his firearms away from his violent adult son.
Three variables influence a court’s judgment about whether risk is “foreseeable”: “the relative knowledge of the risk as between lay persons and physicians, whether the patient has previously used the medication and/or experienced the adverse effect, and whether a warning would otherwise have been futile.”34 A physician who certified a patient to use marijuana without adequately explaining the risks of driving might be vulnerable to a lawsuit if the patient’s driving accident occurred while the patient was under the influence of the drug. Recommending marijuana as a treatment also could lead to a malpractice action if a patient experienced and was harmed by the drug’s adverse effects.
Other drags
Another malpractice risk stems from marijuana’s addiction potential. Although many people think Cannabis isn’t addictive, nearly 10% of all marijuana users develop dependence.10,17 Regular Cannabis users are more likely to use alcohol, tobacco, and “recreational” drugs,17,35 and using alcohol and marijuana together greatly heightens the risk of driving accidents.14,15 Although we know of no case that relates directly to marijuana, physicians have faced lawsuits for injuries stemming from a patient’s addiction to prescription drugs,36 particularly when the patient’s behavior should have led the physician to suspect abuse or overuse.37
When certifying marijuana use, physicians have the same obligations that apply to more conventional medical treatment:
• establishing a proper physician–patient relationship
• taking an appropriate history
• conducting a proper examination
• reviewing records
• developing a comprehensive treatment plan
• weighing risks and alternatives
• providing follow-up care.
Neglecting these steps could lead to medical board sanctions and suspension or revocation of a medical license.13
The blunt reality
We advise against recommending marijuana for your patients. But if you have exhausted the alternatives, see marijuana as the last resort, and believe that taking the risk is worth the potential benefit, you can take some steps to reduce your legal risk (Table 2,1,32,37,38 and Table 313).
Bottom LinE
Medical marijuana is a controversial topic that demands more rigorous research and regulatory consideration. In the present climate, cautious physicians will avoid recommending marijuana to their patients. If you think that a patient has a medical indication, with no treatment option better than medical marijuana, be sure to understand the medical and legal ramifications before you authorize its use.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474-2483.
2. Controlled Substances Act title 21, §801.
3. Frezza C. Medical marijuana: a drug without a medical model. Georgetown Law J. 2013;101:1117-1145.
4. Conant v Walters, 309 F3d 629, 637 (9th Cir 2002).
5. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2493.
6. Thompson AE. JAMA patient page. Medical marijuana. JAMA. 2015;313(24):2508.
7. United States v Oakland Cannabis Buyers’ Cooperative, 532 U.S. 483 (2001).
8. Gonzales v Raich, 545 U.S. 1 (2005).
9. Ogden DW. Memorandum for selected United States Attorneys on investigations and prosecutions in states authorizing the medical use of marijuana. http://www. justice.gov/opa/blog/memorandum-selected-united-state-attorneys-investigations-and-prosecutions-states. Published October 19, 2009. Accessed July 11, 2015.
10. D’Souza DC, Ranganathan M. Medical marijuana: is the cart before the horse? JAMA. 2015;313(24):2431-2432.
11. Picard A. Pot-prescribing doctors warned. The Globe and Mail. http://www.theglobeandmail.com/news/national/ pot-prescribing-doctors-warned/article22506373. Published October 19, 2005. Accessed July 21, 2015.
12. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
13. Barthwell AG, Baxter LE, Cermak T, et al. The role of the physician in “medical” marijuana: American Society of Addiction Medicine. http://www.aoaam.org/usr/ ASAM_Med_Marijuana_White_Paper_Final.pdf. Published September 2010. Accessed July 11, 2015.
14. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73(2):109-119.
15. Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59(3):478-492.
16. Kondrad E, Reid A. Colorado family physicians’ attitudes toward medical marijuana. J Am Board Fam Med. 2013;26(1):52-60.
17. Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19-35.
18. Huang YH, Zhang ZF, Tashkin DP, et al. An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol Biomarkers Prev. 2015;24(1):15-31.
19. Delforterie MJ, Lynskey MT, Huizink AC, et al. The relationship between cannabis involvement and suicidal thoughts and behaviors. Drug Alcohol Depend. 2015;150:98-104.
20. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot-a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
21. Masten SV, Guenzburger GV. Changes in driver cannabinoid prevalence in 12 U.S. states after implementing medical marijuana laws. J Safety Res. 2014;50:35-52.
22. Pollini RA, Romano E, Johnson MB, et al. The impact of marijuana decriminalization on California drivers. Drug Alcohol Depend. 2015;150:135-140.
23. Salomonsen-Sautel S, Min SJ, Sakai JT, et al. Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend. 2014;140:137-144.
24. Urfer S, Morton J, Beall V, et al. Analysis of Δ9- tetrahydrocannabinol driving under the influence of drug cases in Colorado from January 2011 to February 2014. J Anal Toxicol. 2014;38(8):575-581.
25. Couper FJ, Peterson BL. The prevalence of marijuana in suspected impaired driving cases in Washington state. J Anal Toxicol. 2014;38(8):569-574.
26. Drummer OH, Gerostamoulos J, Batziris H, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36(2):239-248.
27. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101-106.
28. Schwitzer T, Schwan R, Angioi-Duprez K, et al. The cannabinoid system and visual processing: a review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol. 2015;25(1):100-112.
29. Neavyn MJ, Blohm E, Babu KM, et al. Medical marijuana and driving: a review. J Med Toxicol. 2014;10(3):269-279.
30. Bosker WM, Karschner EL, Lee D, et al. Sustained abstinence improves psychomotor function in chronic daily cannabis smokers. Paper presented at: SOFT 2012: Society of Forensic Toxicologists 2012 Annual Meeting; July 1-6, 2012; Boston, MA.
31. Fabritius M, Augsburger M, Chtioui H, et al. Fitness to drive and cannabis: validation of two blood THCCOOH thresholds to distinguish occasional users from heavy users. Forensic Sci Int. 2014;242:1-8.
32. Annas GJ. Doctors, drugs, and driving—tort liability for patient-caused accidents. New Engl J Med. 2008;359(5):521-525.
33. Coombes v Florio, 877 NE2d 567 (Mass 2007).
34. McKenzie v Hawaii Permanente Medical Group, Inc. 47 P3d 209 (Haw 2002).
35. Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend. 2013;132(3):654-659.
36. Osborne v United States, 166 F Supp 2d 479 (SDW Va 2001).
37. Conrad-Hutsell v Colturi, 2002 Ohio App. LEXIS 2740 (2002).
38. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.
Dear Dr. Mossman,
I practice in a state that allows medical marijuana use. A few of my patients have asked me to help them obtain marijuana for their conditions. How risky would it be to oblige?
Submitted by “Dr. J”
In recent years, public debate about marijuana has acquired 2 new dimensions: (1) the wishes and medical needs of people who seek marijuana for its purported health benefits, and (2) the role of physicians who practice where “medical marijuana” is legal. This article, the authors’ joint effort to address Dr. J’s concerns, hits 3 topics:
• the intersection of marijuana policy and health care in the United States
• the risks and possible benefits of marijuana use
• the medicolegal problems faced by physicians who might advise patients to use marijuana.
Legal haze
Two cannabinoids—dronabinol and nabilone—have received FDA approval as appetite enhancers and anti-nausea agents. Third-party payors usually cover these types of medications, but no insurer pays for medical marijuana.1 The Controlled Substances Act of 19702 classified marijuana as a Schedule I drug because of its abuse potential, lack of accepted medical applications, and uncertain safety. The FDA has not approved marijuana use for any medical condition.
Although people commonly speak of “prescribing” marijuana, physicians cannot legally do this in the United States. What physicians may do, in the 23 states that allow medical marijuana, is recommend or certify a patient’s marijuana use—an action that has constitutional protection under the First Amendment’s freedom of speech clause.3,4
A physician may complete documentation that a patient has one of the qualifying medical conditions for which the jurisdiction has legalized medical marijuana. Either the patient or the physician then submits that documentation to the appropriate government agency (eg, the state’s department of health).
If the documentation receives approval, the agency will issue the patient a registration card that allows possession of medical marijuana, with which the patient can obtain or grow a small amount of marijuana. The cannabinoid content of marijuana products varies considerably,5 and physicians who certify marijuana typically defer dosage recommendations to the patient or the dispensary.1
In states that allow medical marijuana, users may assert an affirmative defense of medical necessity if they face criminal prosecution.3,6 Possession of marijuana remains illegal under federal law, however, regardless of one’s reason for having it.7,8 Since October 2009, the Attorney General’s office has discouraged federal prosecutions of persons “whose actions are in clear and unambiguous compliance with existing state laws providing for the medical use of marijuana.”9 But given the remaining conflicts between state and federal laws, “the legal implications of certifying patients for medical marijuana remain unclear.”10
Physicians have few resources to instruct them on the legal risks of certifying medical marijuana. When Canada legalized medical marijuana, the organization that provides malpractice insurance to Canadian physicians told its members that “prescribing medical marijuana cannot be compared to prescribing prescription drugs” and recommended that physicians obtain signed release forms documenting that they have discussed the risks of medical marijuana with patients.11 For some risky approved drugs, the FDA has established a risk evaluation and mitigation strategy, but no such guidance is available for marijuana.
Highlighting the benefits and risks
Proponents of medical marijuana claim that Cannabis can help patients, and dispassionate experts acknowledge that at least modest evidence supports the benefits of using “marijuana for nausea and vomiting related to chemotherapy, specific pain syndromes, and spasticity from multiple sclerosis.”10 For several other conditions— HIV/AIDS, depression, anxiety disorders, sleep disorders, psychosis, Tourette syndrome—evidence of benefit is poor.12 Rigorous evaluation of medical marijuana is difficult because the plant contains hundreds of active chemical compounds. The chemical content of marijuana is highly variable, depending on its preparation and administration,10,13—one reason why only a few good randomized controlled trials of marijuana have been conducted.
Marijuana has several side effects and carries many health risks (Table 1).4,14-20
On the highway: Marijuana and driving
Marijuana use impairs driving ability.14 Following enactment of more lenient marijuana laws, several states have reported higher numbers of fatally injured drivers who tested positive for Cannabis21-23 and had a positive screen of tetrahydrocannabinol (THC) in driving under the influence cases.24,25 One study showed that a blood THC concentration >5 ng/mL (comparable to a blood alcohol concentration of 0.15%) increased the crash odds ratio to 6.6.25,26
Marijuana impairs reaction time, information processing, motor performance, attention, and visual processing.14,16,27,28 Drivers who are under the influence of marijuana make more driving errors, despite being cautious about how they react to traffic.29 Even after weeks of abstinence, previous daily users of marijuana display some cognitive processing and driving-related impairments.30,31
Courts have found physicians negligent if their patients’ treatment-induced driving impairments injured others when the risk of driving-related injury was foreseeable.32 The Massachusetts case of Coombes v Florio33 likened the physician’s duty to that of a liquor store that sells alcohol to a minor who subsequently crashes, or to a father who did not lock his firearms away from his violent adult son.
Three variables influence a court’s judgment about whether risk is “foreseeable”: “the relative knowledge of the risk as between lay persons and physicians, whether the patient has previously used the medication and/or experienced the adverse effect, and whether a warning would otherwise have been futile.”34 A physician who certified a patient to use marijuana without adequately explaining the risks of driving might be vulnerable to a lawsuit if the patient’s driving accident occurred while the patient was under the influence of the drug. Recommending marijuana as a treatment also could lead to a malpractice action if a patient experienced and was harmed by the drug’s adverse effects.
Other drags
Another malpractice risk stems from marijuana’s addiction potential. Although many people think Cannabis isn’t addictive, nearly 10% of all marijuana users develop dependence.10,17 Regular Cannabis users are more likely to use alcohol, tobacco, and “recreational” drugs,17,35 and using alcohol and marijuana together greatly heightens the risk of driving accidents.14,15 Although we know of no case that relates directly to marijuana, physicians have faced lawsuits for injuries stemming from a patient’s addiction to prescription drugs,36 particularly when the patient’s behavior should have led the physician to suspect abuse or overuse.37
When certifying marijuana use, physicians have the same obligations that apply to more conventional medical treatment:
• establishing a proper physician–patient relationship
• taking an appropriate history
• conducting a proper examination
• reviewing records
• developing a comprehensive treatment plan
• weighing risks and alternatives
• providing follow-up care.
Neglecting these steps could lead to medical board sanctions and suspension or revocation of a medical license.13
The blunt reality
We advise against recommending marijuana for your patients. But if you have exhausted the alternatives, see marijuana as the last resort, and believe that taking the risk is worth the potential benefit, you can take some steps to reduce your legal risk (Table 2,1,32,37,38 and Table 313).
Bottom LinE
Medical marijuana is a controversial topic that demands more rigorous research and regulatory consideration. In the present climate, cautious physicians will avoid recommending marijuana to their patients. If you think that a patient has a medical indication, with no treatment option better than medical marijuana, be sure to understand the medical and legal ramifications before you authorize its use.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dear Dr. Mossman,
I practice in a state that allows medical marijuana use. A few of my patients have asked me to help them obtain marijuana for their conditions. How risky would it be to oblige?
Submitted by “Dr. J”
In recent years, public debate about marijuana has acquired 2 new dimensions: (1) the wishes and medical needs of people who seek marijuana for its purported health benefits, and (2) the role of physicians who practice where “medical marijuana” is legal. This article, the authors’ joint effort to address Dr. J’s concerns, hits 3 topics:
• the intersection of marijuana policy and health care in the United States
• the risks and possible benefits of marijuana use
• the medicolegal problems faced by physicians who might advise patients to use marijuana.
Legal haze
Two cannabinoids—dronabinol and nabilone—have received FDA approval as appetite enhancers and anti-nausea agents. Third-party payors usually cover these types of medications, but no insurer pays for medical marijuana.1 The Controlled Substances Act of 19702 classified marijuana as a Schedule I drug because of its abuse potential, lack of accepted medical applications, and uncertain safety. The FDA has not approved marijuana use for any medical condition.
Although people commonly speak of “prescribing” marijuana, physicians cannot legally do this in the United States. What physicians may do, in the 23 states that allow medical marijuana, is recommend or certify a patient’s marijuana use—an action that has constitutional protection under the First Amendment’s freedom of speech clause.3,4
A physician may complete documentation that a patient has one of the qualifying medical conditions for which the jurisdiction has legalized medical marijuana. Either the patient or the physician then submits that documentation to the appropriate government agency (eg, the state’s department of health).
If the documentation receives approval, the agency will issue the patient a registration card that allows possession of medical marijuana, with which the patient can obtain or grow a small amount of marijuana. The cannabinoid content of marijuana products varies considerably,5 and physicians who certify marijuana typically defer dosage recommendations to the patient or the dispensary.1
In states that allow medical marijuana, users may assert an affirmative defense of medical necessity if they face criminal prosecution.3,6 Possession of marijuana remains illegal under federal law, however, regardless of one’s reason for having it.7,8 Since October 2009, the Attorney General’s office has discouraged federal prosecutions of persons “whose actions are in clear and unambiguous compliance with existing state laws providing for the medical use of marijuana.”9 But given the remaining conflicts between state and federal laws, “the legal implications of certifying patients for medical marijuana remain unclear.”10
Physicians have few resources to instruct them on the legal risks of certifying medical marijuana. When Canada legalized medical marijuana, the organization that provides malpractice insurance to Canadian physicians told its members that “prescribing medical marijuana cannot be compared to prescribing prescription drugs” and recommended that physicians obtain signed release forms documenting that they have discussed the risks of medical marijuana with patients.11 For some risky approved drugs, the FDA has established a risk evaluation and mitigation strategy, but no such guidance is available for marijuana.
Highlighting the benefits and risks
Proponents of medical marijuana claim that Cannabis can help patients, and dispassionate experts acknowledge that at least modest evidence supports the benefits of using “marijuana for nausea and vomiting related to chemotherapy, specific pain syndromes, and spasticity from multiple sclerosis.”10 For several other conditions— HIV/AIDS, depression, anxiety disorders, sleep disorders, psychosis, Tourette syndrome—evidence of benefit is poor.12 Rigorous evaluation of medical marijuana is difficult because the plant contains hundreds of active chemical compounds. The chemical content of marijuana is highly variable, depending on its preparation and administration,10,13—one reason why only a few good randomized controlled trials of marijuana have been conducted.
Marijuana has several side effects and carries many health risks (Table 1).4,14-20
On the highway: Marijuana and driving
Marijuana use impairs driving ability.14 Following enactment of more lenient marijuana laws, several states have reported higher numbers of fatally injured drivers who tested positive for Cannabis21-23 and had a positive screen of tetrahydrocannabinol (THC) in driving under the influence cases.24,25 One study showed that a blood THC concentration >5 ng/mL (comparable to a blood alcohol concentration of 0.15%) increased the crash odds ratio to 6.6.25,26
Marijuana impairs reaction time, information processing, motor performance, attention, and visual processing.14,16,27,28 Drivers who are under the influence of marijuana make more driving errors, despite being cautious about how they react to traffic.29 Even after weeks of abstinence, previous daily users of marijuana display some cognitive processing and driving-related impairments.30,31
Courts have found physicians negligent if their patients’ treatment-induced driving impairments injured others when the risk of driving-related injury was foreseeable.32 The Massachusetts case of Coombes v Florio33 likened the physician’s duty to that of a liquor store that sells alcohol to a minor who subsequently crashes, or to a father who did not lock his firearms away from his violent adult son.
Three variables influence a court’s judgment about whether risk is “foreseeable”: “the relative knowledge of the risk as between lay persons and physicians, whether the patient has previously used the medication and/or experienced the adverse effect, and whether a warning would otherwise have been futile.”34 A physician who certified a patient to use marijuana without adequately explaining the risks of driving might be vulnerable to a lawsuit if the patient’s driving accident occurred while the patient was under the influence of the drug. Recommending marijuana as a treatment also could lead to a malpractice action if a patient experienced and was harmed by the drug’s adverse effects.
Other drags
Another malpractice risk stems from marijuana’s addiction potential. Although many people think Cannabis isn’t addictive, nearly 10% of all marijuana users develop dependence.10,17 Regular Cannabis users are more likely to use alcohol, tobacco, and “recreational” drugs,17,35 and using alcohol and marijuana together greatly heightens the risk of driving accidents.14,15 Although we know of no case that relates directly to marijuana, physicians have faced lawsuits for injuries stemming from a patient’s addiction to prescription drugs,36 particularly when the patient’s behavior should have led the physician to suspect abuse or overuse.37
When certifying marijuana use, physicians have the same obligations that apply to more conventional medical treatment:
• establishing a proper physician–patient relationship
• taking an appropriate history
• conducting a proper examination
• reviewing records
• developing a comprehensive treatment plan
• weighing risks and alternatives
• providing follow-up care.
Neglecting these steps could lead to medical board sanctions and suspension or revocation of a medical license.13
The blunt reality
We advise against recommending marijuana for your patients. But if you have exhausted the alternatives, see marijuana as the last resort, and believe that taking the risk is worth the potential benefit, you can take some steps to reduce your legal risk (Table 2,1,32,37,38 and Table 313).
Bottom LinE
Medical marijuana is a controversial topic that demands more rigorous research and regulatory consideration. In the present climate, cautious physicians will avoid recommending marijuana to their patients. If you think that a patient has a medical indication, with no treatment option better than medical marijuana, be sure to understand the medical and legal ramifications before you authorize its use.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474-2483.
2. Controlled Substances Act title 21, §801.
3. Frezza C. Medical marijuana: a drug without a medical model. Georgetown Law J. 2013;101:1117-1145.
4. Conant v Walters, 309 F3d 629, 637 (9th Cir 2002).
5. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2493.
6. Thompson AE. JAMA patient page. Medical marijuana. JAMA. 2015;313(24):2508.
7. United States v Oakland Cannabis Buyers’ Cooperative, 532 U.S. 483 (2001).
8. Gonzales v Raich, 545 U.S. 1 (2005).
9. Ogden DW. Memorandum for selected United States Attorneys on investigations and prosecutions in states authorizing the medical use of marijuana. http://www. justice.gov/opa/blog/memorandum-selected-united-state-attorneys-investigations-and-prosecutions-states. Published October 19, 2009. Accessed July 11, 2015.
10. D’Souza DC, Ranganathan M. Medical marijuana: is the cart before the horse? JAMA. 2015;313(24):2431-2432.
11. Picard A. Pot-prescribing doctors warned. The Globe and Mail. http://www.theglobeandmail.com/news/national/ pot-prescribing-doctors-warned/article22506373. Published October 19, 2005. Accessed July 21, 2015.
12. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
13. Barthwell AG, Baxter LE, Cermak T, et al. The role of the physician in “medical” marijuana: American Society of Addiction Medicine. http://www.aoaam.org/usr/ ASAM_Med_Marijuana_White_Paper_Final.pdf. Published September 2010. Accessed July 11, 2015.
14. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73(2):109-119.
15. Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59(3):478-492.
16. Kondrad E, Reid A. Colorado family physicians’ attitudes toward medical marijuana. J Am Board Fam Med. 2013;26(1):52-60.
17. Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19-35.
18. Huang YH, Zhang ZF, Tashkin DP, et al. An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol Biomarkers Prev. 2015;24(1):15-31.
19. Delforterie MJ, Lynskey MT, Huizink AC, et al. The relationship between cannabis involvement and suicidal thoughts and behaviors. Drug Alcohol Depend. 2015;150:98-104.
20. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot-a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
21. Masten SV, Guenzburger GV. Changes in driver cannabinoid prevalence in 12 U.S. states after implementing medical marijuana laws. J Safety Res. 2014;50:35-52.
22. Pollini RA, Romano E, Johnson MB, et al. The impact of marijuana decriminalization on California drivers. Drug Alcohol Depend. 2015;150:135-140.
23. Salomonsen-Sautel S, Min SJ, Sakai JT, et al. Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend. 2014;140:137-144.
24. Urfer S, Morton J, Beall V, et al. Analysis of Δ9- tetrahydrocannabinol driving under the influence of drug cases in Colorado from January 2011 to February 2014. J Anal Toxicol. 2014;38(8):575-581.
25. Couper FJ, Peterson BL. The prevalence of marijuana in suspected impaired driving cases in Washington state. J Anal Toxicol. 2014;38(8):569-574.
26. Drummer OH, Gerostamoulos J, Batziris H, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36(2):239-248.
27. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101-106.
28. Schwitzer T, Schwan R, Angioi-Duprez K, et al. The cannabinoid system and visual processing: a review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol. 2015;25(1):100-112.
29. Neavyn MJ, Blohm E, Babu KM, et al. Medical marijuana and driving: a review. J Med Toxicol. 2014;10(3):269-279.
30. Bosker WM, Karschner EL, Lee D, et al. Sustained abstinence improves psychomotor function in chronic daily cannabis smokers. Paper presented at: SOFT 2012: Society of Forensic Toxicologists 2012 Annual Meeting; July 1-6, 2012; Boston, MA.
31. Fabritius M, Augsburger M, Chtioui H, et al. Fitness to drive and cannabis: validation of two blood THCCOOH thresholds to distinguish occasional users from heavy users. Forensic Sci Int. 2014;242:1-8.
32. Annas GJ. Doctors, drugs, and driving—tort liability for patient-caused accidents. New Engl J Med. 2008;359(5):521-525.
33. Coombes v Florio, 877 NE2d 567 (Mass 2007).
34. McKenzie v Hawaii Permanente Medical Group, Inc. 47 P3d 209 (Haw 2002).
35. Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend. 2013;132(3):654-659.
36. Osborne v United States, 166 F Supp 2d 479 (SDW Va 2001).
37. Conrad-Hutsell v Colturi, 2002 Ohio App. LEXIS 2740 (2002).
38. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.
1. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474-2483.
2. Controlled Substances Act title 21, §801.
3. Frezza C. Medical marijuana: a drug without a medical model. Georgetown Law J. 2013;101:1117-1145.
4. Conant v Walters, 309 F3d 629, 637 (9th Cir 2002).
5. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2493.
6. Thompson AE. JAMA patient page. Medical marijuana. JAMA. 2015;313(24):2508.
7. United States v Oakland Cannabis Buyers’ Cooperative, 532 U.S. 483 (2001).
8. Gonzales v Raich, 545 U.S. 1 (2005).
9. Ogden DW. Memorandum for selected United States Attorneys on investigations and prosecutions in states authorizing the medical use of marijuana. http://www. justice.gov/opa/blog/memorandum-selected-united-state-attorneys-investigations-and-prosecutions-states. Published October 19, 2009. Accessed July 11, 2015.
10. D’Souza DC, Ranganathan M. Medical marijuana: is the cart before the horse? JAMA. 2015;313(24):2431-2432.
11. Picard A. Pot-prescribing doctors warned. The Globe and Mail. http://www.theglobeandmail.com/news/national/ pot-prescribing-doctors-warned/article22506373. Published October 19, 2005. Accessed July 21, 2015.
12. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473.
13. Barthwell AG, Baxter LE, Cermak T, et al. The role of the physician in “medical” marijuana: American Society of Addiction Medicine. http://www.aoaam.org/usr/ ASAM_Med_Marijuana_White_Paper_Final.pdf. Published September 2010. Accessed July 11, 2015.
14. Ramaekers JG, Berghaus G, van Laar M, et al. Dose related risk of motor vehicle crashes after cannabis use. Drug Alcohol Depend. 2004;73(2):109-119.
15. Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59(3):478-492.
16. Kondrad E, Reid A. Colorado family physicians’ attitudes toward medical marijuana. J Am Board Fam Med. 2013;26(1):52-60.
17. Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19-35.
18. Huang YH, Zhang ZF, Tashkin DP, et al. An epidemiologic review of marijuana and cancer: an update. Cancer Epidemiol Biomarkers Prev. 2015;24(1):15-31.
19. Delforterie MJ, Lynskey MT, Huizink AC, et al. The relationship between cannabis involvement and suicidal thoughts and behaviors. Drug Alcohol Depend. 2015;150:98-104.
20. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to pot-a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
21. Masten SV, Guenzburger GV. Changes in driver cannabinoid prevalence in 12 U.S. states after implementing medical marijuana laws. J Safety Res. 2014;50:35-52.
22. Pollini RA, Romano E, Johnson MB, et al. The impact of marijuana decriminalization on California drivers. Drug Alcohol Depend. 2015;150:135-140.
23. Salomonsen-Sautel S, Min SJ, Sakai JT, et al. Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend. 2014;140:137-144.
24. Urfer S, Morton J, Beall V, et al. Analysis of Δ9- tetrahydrocannabinol driving under the influence of drug cases in Colorado from January 2011 to February 2014. J Anal Toxicol. 2014;38(8):575-581.
25. Couper FJ, Peterson BL. The prevalence of marijuana in suspected impaired driving cases in Washington state. J Anal Toxicol. 2014;38(8):569-574.
26. Drummer OH, Gerostamoulos J, Batziris H, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36(2):239-248.
27. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101-106.
28. Schwitzer T, Schwan R, Angioi-Duprez K, et al. The cannabinoid system and visual processing: a review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol. 2015;25(1):100-112.
29. Neavyn MJ, Blohm E, Babu KM, et al. Medical marijuana and driving: a review. J Med Toxicol. 2014;10(3):269-279.
30. Bosker WM, Karschner EL, Lee D, et al. Sustained abstinence improves psychomotor function in chronic daily cannabis smokers. Paper presented at: SOFT 2012: Society of Forensic Toxicologists 2012 Annual Meeting; July 1-6, 2012; Boston, MA.
31. Fabritius M, Augsburger M, Chtioui H, et al. Fitness to drive and cannabis: validation of two blood THCCOOH thresholds to distinguish occasional users from heavy users. Forensic Sci Int. 2014;242:1-8.
32. Annas GJ. Doctors, drugs, and driving—tort liability for patient-caused accidents. New Engl J Med. 2008;359(5):521-525.
33. Coombes v Florio, 877 NE2d 567 (Mass 2007).
34. McKenzie v Hawaii Permanente Medical Group, Inc. 47 P3d 209 (Haw 2002).
35. Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend. 2013;132(3):654-659.
36. Osborne v United States, 166 F Supp 2d 479 (SDW Va 2001).
37. Conrad-Hutsell v Colturi, 2002 Ohio App. LEXIS 2740 (2002).
38. Edersheim JG, Stern TA. Liability associated with prescribing medications. Prim Care Companion J Clin Psychiatry. 2009;11(3):115-119.
Keeping laparoscopy safe for the obese patient
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
As I was writing my introduction to this current edition of the Master Class in Gynecologic Surgery, focusing on minimally invasive surgery for the obese female patient, I was listening to Chuck Todd, host of “Meet the Press.” Instantaneously, television and my thoughts became one; in the last segment of the program, Mr. Todd discussed what he was able to consume for $50 at the Iowa State Fair. I learned that his diet that day consisted of a pork chop on a stick, mac and cheese, a bacon-wrapped corn dog, cheese on a stick with jalapeños, a deep-fried Twinkie, and even fried apple pie with bacon. While Mr. Todd is thin and healthy, the array of foods at the fair reflects our nation’s penchant toward fast food that is fat laden and fried. Though our county is not alone in the world, obesity has reached epidemic proportion in the United States.
According to a May 2015 Department of Health & Human Services report on the health status of the nation, 69% of adults in the United States are overweight and 35% are obese. As a result, the minimally invasive gynecologic surgeon is dealing with an increasing population of women with comorbidities related to their obesity that can confound surgery outcomes. Moreover, anatomic landmarks that the young medical student learns in his or her first anatomy classes are modified due to the size of panniculus and the migration of the umbilicus relative to the bifurcation of the aorta.
I asked Dr. Amina Ahmed to join me in discussing the management of the obese patient undergoing minimally invasive gynecologic surgery. After completing her fellowship in gynecologic oncology, Dr. Ahmed has been on staff at both the University of Iowa Hospitals and Clinics, Iowa City, and Advocate Lutheran General Hospital, Park Ridge, Ill. She will soon join the gynecologic oncology faculty at Rush University Medical Center, Chicago. Given the increased rate of obesity in both Chicago and Iowa, Dr. Ahmed has become an expert in this area in a short period of time.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago, immediate past president of the International Society for Gynecologic Endoscopy (ISGE), and a past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville, Ill., and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill; and the medical editor of this column, Master Class. Dr. Miller disclosed that he is a consultant and on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien.
Positioning obese patients for minimally invasive gynecologic surgery
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at [email protected].
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at [email protected].
The current epidemic of obesity presents gynecologic surgeons with the challenge of safely and successfully performing minimally invasive surgery in women who are morbidly or superobese.
In 2004, the prevalence of a body mass index greater than 40 kg/m2 was almost 7.0% in females in the United States (JAMA. 2006 Apr 5;295[13]:1549-55.). Most recently, 8.3% of women were reported to have a BMI greater than 40 (JAMA. 2014 Feb 26;311[8]:806-14.). This is a value that the World Health Organization defines as Class III obesity and that, according to further stratification reported in the surgical literature, includes the categories of morbid obesity (40-44.9), superobesity (greater than 45), and super-superobesity (greater than 60).
As a gynecologic oncologist, I see firsthand the impact of obesity on the risk of multiple gynecologic conditions and female cancers, including endometrial cancer, as well as the benefits of a minimally invasive approach. I frequently perform hysterectomies via the minimally invasive approach to treat precancer and cancer of the uterus in morbidly and superobese women who have significant central adiposity.
MIGS benefits in the obese
In the past 15 years, and particularly in the past decade, evidence that obese patients benefit from laparoscopic surgery compared with traditional laparotomy has increased. I consider minimally invasive surgery the standard of care for women with endometrial cancer, regardless of the BMI.
As Dr. Stacey A. Scheib and her colleagues wrote in a recent review on laparoscopy in the morbidly obese, most of the gynecologic literature comparing laparoscopic surgery with laparotomy in this population is focused on gynecologic oncology because obesity is so strongly associated with endometrial and other cancers in women (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:182-95.). In one prospective study of women with clinical stage I endometrial cancer and BMIs between 28 and 60, those who underwent laparoscopic surgery – 40 of 42 women over 2 years – had significantly longer operative times but less operative morbidity, shorter hospital stays, faster recovery and better postsurgical quality of life, compared with women who had undergone laparotomy in the previous 2 years. The control patients also had clinical stage I endometrial cancer and similar BMIs (Gynecol Oncol. 2000 Sep;78[3 Pt 1]:329-35.).
Research comparing robotics and conventional laparoscopy in obese gynecologic surgery patients is limited, and findings are inconsistent. It will remain difficult to compare the two approaches because few surgeons are equally skilled in both approaches and because the learning curve for conventional laparoscopy is so much steeper than for robotics.
I favor the robotic approach for morbidly and superobese patients for its superior visualization and ergonomics.
Patient positioning
It is important to use an operative bed that will accommodate the weight and width of obese patients and enable Trendelenburg positioning of up to 45 degrees. We use a bariatric bed with a 1,000-pound weight limit.
Obese patients are at greater risk for neuromuscular injuries and pressure sores, so careful patient positioning and padding of pressure points is critically important. We have found a surgical bean bag to be much more effective in preventing slippage for the morbidly or superobese patient than is egg-crate foam. The bean bag conforms nicely to the shape of the patient’s back, neck, and arms when it is appropriately desufflated. After desufflation, the bean bag must be well taped onto the operative bed.
I sometimes use shoulder blocks for extra assurance. When used, these braces must be attached to the bean bag and not to the patient.
We typically pad the arms completely with gel pads or foam before the bean bag is desufflated. We also often pad the knees and calves before the legs are placed and secured in stirrups made for the morbidly obese, with the buttocks slightly off the table.
In a review of literature on obesity and laparoscopy outcomes, Dr. Georgine Lamvu and her associates recommended that the arms be tucked in the “military” position, along the length of the body (Am J Obstet Gynecol. 2004 Aug;191[2]:669-74.). To ensure that both arms are properly tucked against the length of the body, we use bed extenders or sleds to widen the bed as necessary.
Abdominal access
I use the open Hasson technique in my obese patients and enter the peritoneum under direct visualization. In patients with high levels of morbid obesity, I have found it helpful to retract the adipose tissue using thin Breisky vaginal retractors. These retractors can hold the adipose tissue away from the fascia to facilitate entry into the abdominal cavity via the open technique.
Utilizing the umbilicus as the initial entry point – often desirable in minimally invasive surgery – is frequently not possible in morbidly obese patients because as BMI increases, the umbilicus migrates toward the pubic bone and away from the aortic bifurcation. In patients who were overweight (BMI greater than 25), Dr. W.W. Hurd and his associates noted a repositioning of the umbilicus below the aortic bifurcation of 2 cm or greater (Obstet Gynecol. 1992 Jul;80[1]:48-51.).
Instead, a supraumbilical or left upper quadrant site for initial entry enables optimal triangulation of trocars and visualization of disease. The trocars must then be placed more lateral and cephalad than in thinner women. In doing so, risk to the inferior epigastric is mitigated. Moreover, longer trocar lengths (150 mm) may be required.
To utilize an umbilical entry, it is imperative that the panniculus be placed cephalad to a position between the two anterior iliac spines (Obstet Gynecol. 1998 Nov;92[5]:869-72.). By doing this, the umbilicus is now repositioned relative to the bifurcation of the aorta similar to the thinner patient. This can either be accomplished using assistants to move the panniculus cephalad or taping the panniculus.
Alternatively, if the Hasson technique is not utilized, a Veress needle (50 mm in length) may be used. Based on MRI and CT visualization, Dr. Hurd has long recommended using a 90-degree angle in the obese population, compared with a 45-degree angle in nonobese women (J Reprod Med. 1991;36[7]:473-6.).
I usually place the patient into a moderate Trendelenburg position before docking the robot and observe the patient’s cardiac and respiratory responses to the induction of anesthesia. Adjustments in the degree of Trendelenburg positioning, the insufflation pressure level, and the ventilation settings can then be made if necessary. Occasionally I will decrease the insufflation pressure from 15 to 12 mm Hg, for instance, to accommodate ventilation needs.
A note from Dr. Charles E. Miller, Master Class Medical Editor
It must be recognized that not all physicians agree with the use of shoulder braces. In a review of literature on brachial plexus injuries in gynecologic surgery during 1980-2012, Dr. Nigel Pereira and his associates identified eight case reports, all of which involved Trendelenburg positioning and seven of which utilized shoulder braces. In their evaluation of the literature, the authors concluded that “the force of the shoulder braces on the clavicle and scapula opposes the force of gravity on the humerus, thereby stretching the brachial plexus and leading to nerve injury. This is particularly exaggerated when the arm is hyperabducted (less than 90 degrees), the head is laterally flexed to the opposite side, or the abducted arm is sagging.”
The authors also point out that longer times spent under general anesthesia (commensurate with increased operating times) increase the risk of brachial plexus injury “by increasing joint mobility (particularly when muscle relaxants are used) because the neighboring bony structure is more likely to compress or impinge on the brachial plexus” (CRSLS e2014.00077. [doi:10.4293/CRSLS.2014.00077]).
More pearls from Dr. Miller
Preoperative care. Prior to surgery it is important to examine a patient’s panniculus closely for evidence of infection. As the area underneath the panniculus receives little oxygen, it is at greater risk for both bacterial and fungal infections. If infection is noted, treatment prior to surgery is strongly recommended. Moreover, as the skin under the panniculus is often times “broken down,” which can compromise healing, lateral incisions should not be made in this area.
Since obese women have more severe comorbidities (such as metabolic syndrome, obstructed sleep apnea, coronary artery disease, poorly controlled hypertension, and a difficult airway) and a greater risk of perioperative complications than women who are not obese, they generally require a more-extensive preoperative work-up and additional perioperative considerations. If the minimally invasive gynecologic surgeon is uncomfortable with evaluation of cardiac and pulmonary status, medical clearance and perioperative consultation with an anesthesiologist prior to surgery is strongly recommended.
Perioperative care. There are no studies in the literature supporting the use of antibiotic prophylaxis prior to surgery despite the increased risk of postoperative wound infection in morbidly obese patients. Increased risk of surgical site infection post abdominal hysterectomy has been noted in women with a BMI greater than 35. Therefore, consideration should be given to the use of prophylactic antibiotics. For patients weighing more than 80 kg, I advise using 2 gm prophylactic cefazolin; increase this to 3 gm in patients that weigh more than 120 kg.
The morbidly obese patient is also at greater risk of deep venous thrombosis, especially when the procedure is lengthy. Sequential compression devices are essential. Moreover, use of such antithrombotic agents as Lovenox [enoxaparin] and heparin should be considered until the patient is ambulating.
Postoperative care. It is imperative to stress the need for extensive pulmonary toilet or hygiene (i.e., coughing and breathing deeply to clear mucus and secretions from the airways) as well as early ambulation. The patient should also be counseled to use pain medication judiciously. And until the patient is mobile, the use of antithrombotic agents, such as Lovenox and heparin, should be continued.
Dr. Ahmed reports that she has no disclosures related to this Master Class. Dr. Miller disclosed that he is a consultant and is on the speakers bureau for Ethicon and Intuitive Surgical, and is a consultant for Covidien. Email Dr. Ahmed and Dr. Miller at [email protected].
A medication change, then involuntary lip smacking and tongue rolling
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.
Ingenol mebutate helped clear actinic keratoses
Cryosurgery followed by topical ingenol mebutate cleared extensive regions of actinic keratosis, which helped reveal residual squamous cell carcinomas, according to a report in the August issue of the Journal of Drugs in Dermatology.
The findings show that ingenol mebutate can clear multiple AKs and reduce the number of scarring biopsies required to identify SCCs, said Dr. Miriam S. Bettencourt, a dermatologist in group practice in Henderson, Nev. “In our dermatology clinic, many of the patients with a long history of AK who were treated with ingenol mebutate used sequentially after cryosurgery have achieved complete or partial clearance of AKs.”
Ingenol mebutate gel after cryosurgery cleared AKs more effectively than cryosurgery alone in a recent phase III trial (J Drugs Dermatol. 2014 Jun;13[6]741-7), Dr. Bettencourt noted. She described six men and one woman who each had at least 10 recurrent or hyperkeratotic AKs and previously had undergone cryosurgery. She treated all patients with cryosurgery, followed 2 weeks later by two or three once-daily applications of ingenol mebutate gel at strengths of 0.05% or 0.015%, respectively (J Drugs Dermatol. 2015 Aug;14[8];813-8). One course of ingenol mebutate gel cleared 50%-100% of AKs, Dr. Bettencourt said. She treated residual AKs with cryosurgery, and five patients also received at least one more course of ingenol mebutate to re-treat a partially cleared area or to treat a separate area. Shave biopsies of 10 residual suspicious lesions taken 3-8 months later all revealed invasive SCCs, which were treated with Mohs micrographic surgery (MMS). “These lesions may have been preexisting at the time of topical treatment but not readily recognized as suspicious in the heavily actinically damaged skin, in which suspected or small SCCs may be adjacent to or obscured by AKs,” she said. “Alternatively, these tumors may have been spontaneous new SCCs. In either case, we suggest that effective clearance of AKs from the palette of sun-damaged skin with ingenol mebutate permitted prompt recognition of these lesions as suspicious, and led to further diagnosis and treatment with MMS.”
All patients developed mild to moderate localized redness, flaking, and crusting starting on the second day of ingenol mebutate treatment and resolving within a week of finishing the course, Dr. Bettencourt said.
She reported that she had no relevant financial conflicts.
Cryosurgery followed by topical ingenol mebutate cleared extensive regions of actinic keratosis, which helped reveal residual squamous cell carcinomas, according to a report in the August issue of the Journal of Drugs in Dermatology.
The findings show that ingenol mebutate can clear multiple AKs and reduce the number of scarring biopsies required to identify SCCs, said Dr. Miriam S. Bettencourt, a dermatologist in group practice in Henderson, Nev. “In our dermatology clinic, many of the patients with a long history of AK who were treated with ingenol mebutate used sequentially after cryosurgery have achieved complete or partial clearance of AKs.”
Ingenol mebutate gel after cryosurgery cleared AKs more effectively than cryosurgery alone in a recent phase III trial (J Drugs Dermatol. 2014 Jun;13[6]741-7), Dr. Bettencourt noted. She described six men and one woman who each had at least 10 recurrent or hyperkeratotic AKs and previously had undergone cryosurgery. She treated all patients with cryosurgery, followed 2 weeks later by two or three once-daily applications of ingenol mebutate gel at strengths of 0.05% or 0.015%, respectively (J Drugs Dermatol. 2015 Aug;14[8];813-8). One course of ingenol mebutate gel cleared 50%-100% of AKs, Dr. Bettencourt said. She treated residual AKs with cryosurgery, and five patients also received at least one more course of ingenol mebutate to re-treat a partially cleared area or to treat a separate area. Shave biopsies of 10 residual suspicious lesions taken 3-8 months later all revealed invasive SCCs, which were treated with Mohs micrographic surgery (MMS). “These lesions may have been preexisting at the time of topical treatment but not readily recognized as suspicious in the heavily actinically damaged skin, in which suspected or small SCCs may be adjacent to or obscured by AKs,” she said. “Alternatively, these tumors may have been spontaneous new SCCs. In either case, we suggest that effective clearance of AKs from the palette of sun-damaged skin with ingenol mebutate permitted prompt recognition of these lesions as suspicious, and led to further diagnosis and treatment with MMS.”
All patients developed mild to moderate localized redness, flaking, and crusting starting on the second day of ingenol mebutate treatment and resolving within a week of finishing the course, Dr. Bettencourt said.
She reported that she had no relevant financial conflicts.
Cryosurgery followed by topical ingenol mebutate cleared extensive regions of actinic keratosis, which helped reveal residual squamous cell carcinomas, according to a report in the August issue of the Journal of Drugs in Dermatology.
The findings show that ingenol mebutate can clear multiple AKs and reduce the number of scarring biopsies required to identify SCCs, said Dr. Miriam S. Bettencourt, a dermatologist in group practice in Henderson, Nev. “In our dermatology clinic, many of the patients with a long history of AK who were treated with ingenol mebutate used sequentially after cryosurgery have achieved complete or partial clearance of AKs.”
Ingenol mebutate gel after cryosurgery cleared AKs more effectively than cryosurgery alone in a recent phase III trial (J Drugs Dermatol. 2014 Jun;13[6]741-7), Dr. Bettencourt noted. She described six men and one woman who each had at least 10 recurrent or hyperkeratotic AKs and previously had undergone cryosurgery. She treated all patients with cryosurgery, followed 2 weeks later by two or three once-daily applications of ingenol mebutate gel at strengths of 0.05% or 0.015%, respectively (J Drugs Dermatol. 2015 Aug;14[8];813-8). One course of ingenol mebutate gel cleared 50%-100% of AKs, Dr. Bettencourt said. She treated residual AKs with cryosurgery, and five patients also received at least one more course of ingenol mebutate to re-treat a partially cleared area or to treat a separate area. Shave biopsies of 10 residual suspicious lesions taken 3-8 months later all revealed invasive SCCs, which were treated with Mohs micrographic surgery (MMS). “These lesions may have been preexisting at the time of topical treatment but not readily recognized as suspicious in the heavily actinically damaged skin, in which suspected or small SCCs may be adjacent to or obscured by AKs,” she said. “Alternatively, these tumors may have been spontaneous new SCCs. In either case, we suggest that effective clearance of AKs from the palette of sun-damaged skin with ingenol mebutate permitted prompt recognition of these lesions as suspicious, and led to further diagnosis and treatment with MMS.”
All patients developed mild to moderate localized redness, flaking, and crusting starting on the second day of ingenol mebutate treatment and resolving within a week of finishing the course, Dr. Bettencourt said.
She reported that she had no relevant financial conflicts.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
Key clinical point: Several courses of cryosurgery and ingenol mebutate helped clear actinic keratoses, helping a clinician identify residual squamous cell carcinomas.
Major finding: Lesion counts dropped by 50%-100% after cryosurgery followed by one to three courses of ingenol mebutate gel.
Data source: A case series of seven patients who had multiple AKs and 10 SCCs.
Disclosures: Dr. Bettencourt reported that she had no relevant financial conflicts.