User login
Lenalidomide + rituximab combo effective in recurrent follicular lymphoma
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
The combination of lenalidomide and rituximab was more active in patients with recurrent follicular lymphoma, compared with lenalidomide alone, and significantly increased the overall response rate, according to new data published online Aug. 24 in the Journal of Clinical Oncology.
Although both lenalidomide and rituximab are active agents in follicular lymphoma, their combined use in recurrent follicular lymphoma has not been previously evaluated in randomized clinical trials, said Dr. John P. Leonard of Cornell University, New York, and his colleagues.
The overall response rate of patients receiving the combination regimen was significantly higher than that of patients who received lenalidomide alone (P = .029). In the cohort receiving lenalidomide alone, 24 patients (53%) achieved an objective response (9 complete responses [20%]), while 35 patients (76%) in the lenalidomide/rituximab group were responders (18 complete responses [39%]).
At a median follow-up of 2.5 years (range, 0.1-4.8 years), the addition of rituximab to lenalidomide in this population also significantly increased the median time to progression: 1.1 year for lenalidomide alone versus 2 years for the combined therapy (P = .002).
Overall survival was 4.5 years for lenalidomide alone and has not yet been reached for the combination arm (P = .149.
This trial helps to establish the safety profile of single-agent lenalidomide in follicular lymphoma, while its “randomized nature also allows a direct assessment of potential toxicity resulting from the addition of rituximab to lenalidomide,” wrote Dr. Leonard and his associates (J Clin Oncol. 2015 Aug 24. doi: 10.1200/JCO.2014.59.9258).
“There was no evidence of increased toxicity from the lenalidomide/rituximab combination compared with lenalidomide alone,” they pointed out.
Both lenalidomide alone and lenalidomide/rituximab were well tolerated, with grade 3-4 adverse events occurring in 58% and 53% of patients, respectively, with 9% and 11% of patients experiencing grade 4 toxicity, respectively. The most common grade 3-4 adverse events included neutropenia (16% vs. 20%), fatigue (9% vs. 13%), and rash (4% vs. 4%).
The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Lenalidomide combined with rituximab is more active in recurrent follicular lymphoma, compared with lenalidomide monotherapy.
Major finding: Overall response rate was 53% (20% complete response) for lenalidomide alone versus 76% (39% complete response) for lenalidomide and rituximab (P = .029).
Data source: Randomized phase II clinical trial of 91 patients with follicular lymphoma who were assigned to receive lenalidomide alone or lenalidomide combined with rituximab.
Disclosures: The study was supported in part by grants from the National Cancer Institute to the Alliance for Clinical Trials in Oncology. Dr. Leonard reported financial relationships with Celgene and Genentech, and several coauthors also reported relationships with industry.
Outcomes worse with secondary and therapy-related AML compared with de novo AML
Clinical outcomes were significantly worse among younger patients with secondary and therapy-related acute myeloid leukemia (sAML and tAML, respectively) compared with de novo disease, suggesting the presence of distinct AML subtypes, in a study published online Aug. 24 in Journal of Clinical Oncology.
In the Danish population-based study, patients under age 60 with de novo AML had a 3-year survival rate of 52%, compared with 35% for AML secondary to myelodysplastic syndrome (MDS-sAML), 19% for AML secondary to chronic myelomonocytic leukemia or myeloproliferative neoplasia (non-MDS-sAML), and 27% for tAML.
Non-MDS-sAML was associated with poorer outcomes at any age, but patients older than 60 years with MDS-sAML and tAML had outcomes similar to those of de novo AML, Dr. Lene Sofie Granfeldt Ostgard and associates reported (J Clin Oncol. 2015 Aug 24. doi:10.1200/JCO.2014.60.0890).
The cohort study evaluated records of 3,055 patients diagnosed with AML from 2000 to 2013. Overall, 73.6% had de novo AML, 19.8% had sAML (11.5% MDS, 8.3% non-MDS), and 6.6% had tAML. The researchers focused on the 1,567 patients (51.3%) who underwent curative intent therapy. Patients with sAML were less likely to receive intensive therapy than were patients with tAML or de novo AML. Patients who underwent intensive treatment had superior survival rates compared with patients within the same subgroup who did not undergo treatment.
Contrary to previous reports, the investigators did not observe an increase in the incidence of AML, sAML, or tAML over the 14-year study period. They did, however, find that clinical trial accrual rates for patients with sAML and tAML increased significantly over that period, which may account for reports of a temporal increase in tAML.
Adverse-risk cytogenetics were most prevalent in patients with tAML, likely because of clonal selection of chemotherapy-resistant p53-mutated cells. Patients with non-MDS-sAML had a higher frequency of aberrant karyotypes, though not classified as adverse, which the authors speculate may explain the worse outcomes observed in this group.
“Given the extremely poor prognosis associated with an antecedent diagnosis of CMML [chronic myelomonocytic leukemia] or MPN [myeloproliferative neoplasia], these entities should be considered separately from prior MDS when investigating outcomes and new treatment strategies in patients with sAML,” wrote Dr. Ostgard of Aarhus University Hospital, Denmark, and colleagues.
The presence of MDS-sAML and tAML was associated with worse survival, but the association was weaker in older patients and those with adverse cytogenetics, suggesting the relative effect of MDS or prior chemotherapy is small in patients with an already poor prognosis.
Dr. Ostgard reported consulting or advisory roles with Bristol-Myers Squibb and Abbvie. Several of her coauthors reported ties to industry sources.
Clinical outcomes were significantly worse among younger patients with secondary and therapy-related acute myeloid leukemia (sAML and tAML, respectively) compared with de novo disease, suggesting the presence of distinct AML subtypes, in a study published online Aug. 24 in Journal of Clinical Oncology.
In the Danish population-based study, patients under age 60 with de novo AML had a 3-year survival rate of 52%, compared with 35% for AML secondary to myelodysplastic syndrome (MDS-sAML), 19% for AML secondary to chronic myelomonocytic leukemia or myeloproliferative neoplasia (non-MDS-sAML), and 27% for tAML.
Non-MDS-sAML was associated with poorer outcomes at any age, but patients older than 60 years with MDS-sAML and tAML had outcomes similar to those of de novo AML, Dr. Lene Sofie Granfeldt Ostgard and associates reported (J Clin Oncol. 2015 Aug 24. doi:10.1200/JCO.2014.60.0890).
The cohort study evaluated records of 3,055 patients diagnosed with AML from 2000 to 2013. Overall, 73.6% had de novo AML, 19.8% had sAML (11.5% MDS, 8.3% non-MDS), and 6.6% had tAML. The researchers focused on the 1,567 patients (51.3%) who underwent curative intent therapy. Patients with sAML were less likely to receive intensive therapy than were patients with tAML or de novo AML. Patients who underwent intensive treatment had superior survival rates compared with patients within the same subgroup who did not undergo treatment.
Contrary to previous reports, the investigators did not observe an increase in the incidence of AML, sAML, or tAML over the 14-year study period. They did, however, find that clinical trial accrual rates for patients with sAML and tAML increased significantly over that period, which may account for reports of a temporal increase in tAML.
Adverse-risk cytogenetics were most prevalent in patients with tAML, likely because of clonal selection of chemotherapy-resistant p53-mutated cells. Patients with non-MDS-sAML had a higher frequency of aberrant karyotypes, though not classified as adverse, which the authors speculate may explain the worse outcomes observed in this group.
“Given the extremely poor prognosis associated with an antecedent diagnosis of CMML [chronic myelomonocytic leukemia] or MPN [myeloproliferative neoplasia], these entities should be considered separately from prior MDS when investigating outcomes and new treatment strategies in patients with sAML,” wrote Dr. Ostgard of Aarhus University Hospital, Denmark, and colleagues.
The presence of MDS-sAML and tAML was associated with worse survival, but the association was weaker in older patients and those with adverse cytogenetics, suggesting the relative effect of MDS or prior chemotherapy is small in patients with an already poor prognosis.
Dr. Ostgard reported consulting or advisory roles with Bristol-Myers Squibb and Abbvie. Several of her coauthors reported ties to industry sources.
Clinical outcomes were significantly worse among younger patients with secondary and therapy-related acute myeloid leukemia (sAML and tAML, respectively) compared with de novo disease, suggesting the presence of distinct AML subtypes, in a study published online Aug. 24 in Journal of Clinical Oncology.
In the Danish population-based study, patients under age 60 with de novo AML had a 3-year survival rate of 52%, compared with 35% for AML secondary to myelodysplastic syndrome (MDS-sAML), 19% for AML secondary to chronic myelomonocytic leukemia or myeloproliferative neoplasia (non-MDS-sAML), and 27% for tAML.
Non-MDS-sAML was associated with poorer outcomes at any age, but patients older than 60 years with MDS-sAML and tAML had outcomes similar to those of de novo AML, Dr. Lene Sofie Granfeldt Ostgard and associates reported (J Clin Oncol. 2015 Aug 24. doi:10.1200/JCO.2014.60.0890).
The cohort study evaluated records of 3,055 patients diagnosed with AML from 2000 to 2013. Overall, 73.6% had de novo AML, 19.8% had sAML (11.5% MDS, 8.3% non-MDS), and 6.6% had tAML. The researchers focused on the 1,567 patients (51.3%) who underwent curative intent therapy. Patients with sAML were less likely to receive intensive therapy than were patients with tAML or de novo AML. Patients who underwent intensive treatment had superior survival rates compared with patients within the same subgroup who did not undergo treatment.
Contrary to previous reports, the investigators did not observe an increase in the incidence of AML, sAML, or tAML over the 14-year study period. They did, however, find that clinical trial accrual rates for patients with sAML and tAML increased significantly over that period, which may account for reports of a temporal increase in tAML.
Adverse-risk cytogenetics were most prevalent in patients with tAML, likely because of clonal selection of chemotherapy-resistant p53-mutated cells. Patients with non-MDS-sAML had a higher frequency of aberrant karyotypes, though not classified as adverse, which the authors speculate may explain the worse outcomes observed in this group.
“Given the extremely poor prognosis associated with an antecedent diagnosis of CMML [chronic myelomonocytic leukemia] or MPN [myeloproliferative neoplasia], these entities should be considered separately from prior MDS when investigating outcomes and new treatment strategies in patients with sAML,” wrote Dr. Ostgard of Aarhus University Hospital, Denmark, and colleagues.
The presence of MDS-sAML and tAML was associated with worse survival, but the association was weaker in older patients and those with adverse cytogenetics, suggesting the relative effect of MDS or prior chemotherapy is small in patients with an already poor prognosis.
Dr. Ostgard reported consulting or advisory roles with Bristol-Myers Squibb and Abbvie. Several of her coauthors reported ties to industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Younger patients with secondary and therapy-related acute myeloid leukemia (sAML and tAML, respectively) have worse outcomes than do patients with de novo AML.
Major finding: Three-year survival for patients under 60 years of age with de novo AML was 52%, compared with 35% for sAML (myelodysplastic syndrome), 19% for sAML (nonmyelodysplastic syndrome), and 27% for tAML.
Data source: A population-based Danish cohort study of 3,055 patients with AML diagnosed from 2000 to 2013.
Disclosures: Dr. Ostgard reported consulting or advisory roles with Bristol-Myers Squibb and Abbvie. Several coauthors reported ties to industry sources.
Dry Scaly Rash
The Diagnosis: Phrynoderma
Phrynoderma, meaning “toad skin,” was first described by Nicholls1 in 1933 as a condition due to vitamin A deficiency and characterized by follicular hyperkeratosis. Since then, there has been debate in the literature over the etiology of phrynoderma, as studies have demonstrated different causes for this condition,2-5 which suggests that it is prudent to check several markers of nutritional status including vitamin A, vitamin B, vitamin E, and essential fatty acid studies.
Phrynoderma is most commonly seen in South and East Asia with relatively rare occurrences in the United States. Although it characteristically occurs in children and adolescents aged 5 to 15 years, almost all of the cases reported in the United States have occurred in adults.6,7 A clear gender predilection has not been established.7
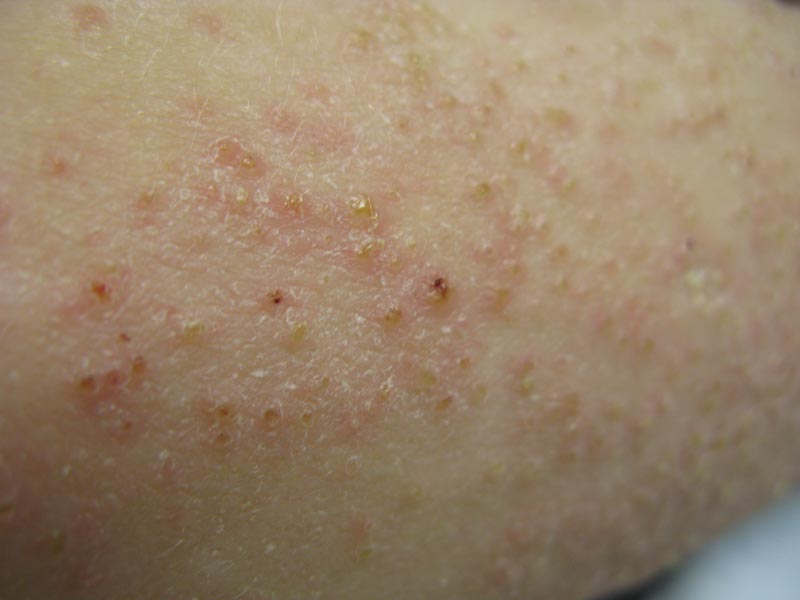
Phrynoderma tends to favor the extensor surfaces. In particular, the elbows, thighs, and buttocks are the most commonly affected locations. The face is typically the last site to become involved. On physical examination of a patient with phrynoderma, there are various-sized, discrete, red-brown to flesh-colored papules with a conical keratotic follicular plug (Figure).3 The eruption is often asymptomatic, but mild pruritus may be reported.7 Additional signs of vitamin A deficiency may be present, including night blindness, hyperpigmentation, or xerosis. Patients with phrynoderma also may have evidence of other nutritional deficiencies, such as angular stomatitis, cheilitis, or glossitis. The differential diagnosis for a patient with this type of papular eruption may include pityriasis rubra pilaris, keratosis pilaris, lichen spinulosus, crusted scabies, and follicular lichen planus.7 History and clinical presentation can generally help to distinguish these diagnoses.
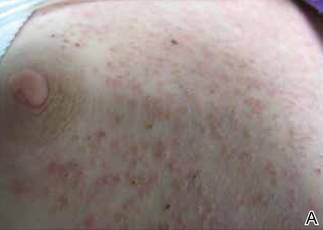
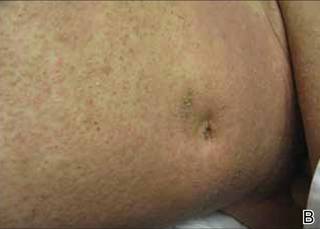
Generalized follicular, erythematous, and scaly papular eruption on the chest (A) and abdomen (B). |
Histopathology typically shows moderate hyperkeratosis with distension of the upper portion of the follicle by large keratotic plugs. Sebaceous glands are greatly reduced in size and may exhibit epithelial atrophy.8,9
A reduced serum vitamin A level can confirm the diagnosis of phrynoderma. Our patient’s laboratory results revealed a vitamin A value of 26 mg/100 dL (reference range, 38–106 mg/100 dL), consistent with his clinical presentation of phrynoderma. Other markers of nutritional status, including vitamins D, E, and K1, were all within reference range. Treatment consists of replacing the nutritional deficit. High doses of vitamin A, such as 200,000 to 500,000 IU daily for 3 to 5 days or daily doses of 2000 to 50,000 IU, can lead to resolution of the rash in 1 to 4 months.8,9 Our patient was started on 10,000 IU of oral vitamin A daily at discharge but unfortunately died a few months later.
Vitamin A has been shown to promote maturation and differentiation of the basal keratinocytes as they move up through the epidermis, which may in part explain why this treatment is effective.10 It is important to be aware of this condition when seeing a patient with follicular hyperkeratosis because even patients in developed countries with malnutrition due to diet or gastrointestinal disease may develop phrynoderma.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gazette. 1933;68:681-687.
- Bagchi K, Halder K, Chowdhury SR. The etiology of phrynoderma; histologic evidence. Am J Clin Nutr. 1959;7:251-258.
- Bleasel NR, Stapleton KM, Lee MS, et al. Vitamin A deficiency phrynoderma: due to malabsorption and inadequate diet. J Am Acad Dermatol. 1999;41(2, pt 2):322-324.
- Ghafoorunissa, Vidyasagar R, Krishnaswamy K. Phrynoderma: is it an EFA deficiency disease? Euro J Clin Nutr. 1988;42:29-39.
- Shrank AB. Phrynoderma. Br Med J. 1966;1:29-30.
- Maronn M, Allen DM, Esterly NB. Phrynoderma: a manifestation of vitamin A deficiency?…the rest of the story. Pediatr Dermatol. 2005;22:60-63.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Neill SM, Pembroke AC, du-Vivier AWP, et al. Phrynoderma and perforating folliculitis due to vitamin A deficiency in a diabetic. J R Soc Med. 1988;81:171-172.
- Wechsler HL. Vitamin A deficiency following small-bowel bypass surgery for obesity. Arch Dermatol. 1979;115:73-75.
- Logan WS. Vitamin A and keratinization. Arch Dermatol. 1972;105:748-753.
The Diagnosis: Phrynoderma
Phrynoderma, meaning “toad skin,” was first described by Nicholls1 in 1933 as a condition due to vitamin A deficiency and characterized by follicular hyperkeratosis. Since then, there has been debate in the literature over the etiology of phrynoderma, as studies have demonstrated different causes for this condition,2-5 which suggests that it is prudent to check several markers of nutritional status including vitamin A, vitamin B, vitamin E, and essential fatty acid studies.
Phrynoderma is most commonly seen in South and East Asia with relatively rare occurrences in the United States. Although it characteristically occurs in children and adolescents aged 5 to 15 years, almost all of the cases reported in the United States have occurred in adults.6,7 A clear gender predilection has not been established.7
Phrynoderma tends to favor the extensor surfaces. In particular, the elbows, thighs, and buttocks are the most commonly affected locations. The face is typically the last site to become involved. On physical examination of a patient with phrynoderma, there are various-sized, discrete, red-brown to flesh-colored papules with a conical keratotic follicular plug (Figure).3 The eruption is often asymptomatic, but mild pruritus may be reported.7 Additional signs of vitamin A deficiency may be present, including night blindness, hyperpigmentation, or xerosis. Patients with phrynoderma also may have evidence of other nutritional deficiencies, such as angular stomatitis, cheilitis, or glossitis. The differential diagnosis for a patient with this type of papular eruption may include pityriasis rubra pilaris, keratosis pilaris, lichen spinulosus, crusted scabies, and follicular lichen planus.7 History and clinical presentation can generally help to distinguish these diagnoses.


Generalized follicular, erythematous, and scaly papular eruption on the chest (A) and abdomen (B). |
Histopathology typically shows moderate hyperkeratosis with distension of the upper portion of the follicle by large keratotic plugs. Sebaceous glands are greatly reduced in size and may exhibit epithelial atrophy.8,9
A reduced serum vitamin A level can confirm the diagnosis of phrynoderma. Our patient’s laboratory results revealed a vitamin A value of 26 mg/100 dL (reference range, 38–106 mg/100 dL), consistent with his clinical presentation of phrynoderma. Other markers of nutritional status, including vitamins D, E, and K1, were all within reference range. Treatment consists of replacing the nutritional deficit. High doses of vitamin A, such as 200,000 to 500,000 IU daily for 3 to 5 days or daily doses of 2000 to 50,000 IU, can lead to resolution of the rash in 1 to 4 months.8,9 Our patient was started on 10,000 IU of oral vitamin A daily at discharge but unfortunately died a few months later.
Vitamin A has been shown to promote maturation and differentiation of the basal keratinocytes as they move up through the epidermis, which may in part explain why this treatment is effective.10 It is important to be aware of this condition when seeing a patient with follicular hyperkeratosis because even patients in developed countries with malnutrition due to diet or gastrointestinal disease may develop phrynoderma.
The Diagnosis: Phrynoderma
Phrynoderma, meaning “toad skin,” was first described by Nicholls1 in 1933 as a condition due to vitamin A deficiency and characterized by follicular hyperkeratosis. Since then, there has been debate in the literature over the etiology of phrynoderma, as studies have demonstrated different causes for this condition,2-5 which suggests that it is prudent to check several markers of nutritional status including vitamin A, vitamin B, vitamin E, and essential fatty acid studies.
Phrynoderma is most commonly seen in South and East Asia with relatively rare occurrences in the United States. Although it characteristically occurs in children and adolescents aged 5 to 15 years, almost all of the cases reported in the United States have occurred in adults.6,7 A clear gender predilection has not been established.7
Phrynoderma tends to favor the extensor surfaces. In particular, the elbows, thighs, and buttocks are the most commonly affected locations. The face is typically the last site to become involved. On physical examination of a patient with phrynoderma, there are various-sized, discrete, red-brown to flesh-colored papules with a conical keratotic follicular plug (Figure).3 The eruption is often asymptomatic, but mild pruritus may be reported.7 Additional signs of vitamin A deficiency may be present, including night blindness, hyperpigmentation, or xerosis. Patients with phrynoderma also may have evidence of other nutritional deficiencies, such as angular stomatitis, cheilitis, or glossitis. The differential diagnosis for a patient with this type of papular eruption may include pityriasis rubra pilaris, keratosis pilaris, lichen spinulosus, crusted scabies, and follicular lichen planus.7 History and clinical presentation can generally help to distinguish these diagnoses.


Generalized follicular, erythematous, and scaly papular eruption on the chest (A) and abdomen (B). |
Histopathology typically shows moderate hyperkeratosis with distension of the upper portion of the follicle by large keratotic plugs. Sebaceous glands are greatly reduced in size and may exhibit epithelial atrophy.8,9
A reduced serum vitamin A level can confirm the diagnosis of phrynoderma. Our patient’s laboratory results revealed a vitamin A value of 26 mg/100 dL (reference range, 38–106 mg/100 dL), consistent with his clinical presentation of phrynoderma. Other markers of nutritional status, including vitamins D, E, and K1, were all within reference range. Treatment consists of replacing the nutritional deficit. High doses of vitamin A, such as 200,000 to 500,000 IU daily for 3 to 5 days or daily doses of 2000 to 50,000 IU, can lead to resolution of the rash in 1 to 4 months.8,9 Our patient was started on 10,000 IU of oral vitamin A daily at discharge but unfortunately died a few months later.
Vitamin A has been shown to promote maturation and differentiation of the basal keratinocytes as they move up through the epidermis, which may in part explain why this treatment is effective.10 It is important to be aware of this condition when seeing a patient with follicular hyperkeratosis because even patients in developed countries with malnutrition due to diet or gastrointestinal disease may develop phrynoderma.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gazette. 1933;68:681-687.
- Bagchi K, Halder K, Chowdhury SR. The etiology of phrynoderma; histologic evidence. Am J Clin Nutr. 1959;7:251-258.
- Bleasel NR, Stapleton KM, Lee MS, et al. Vitamin A deficiency phrynoderma: due to malabsorption and inadequate diet. J Am Acad Dermatol. 1999;41(2, pt 2):322-324.
- Ghafoorunissa, Vidyasagar R, Krishnaswamy K. Phrynoderma: is it an EFA deficiency disease? Euro J Clin Nutr. 1988;42:29-39.
- Shrank AB. Phrynoderma. Br Med J. 1966;1:29-30.
- Maronn M, Allen DM, Esterly NB. Phrynoderma: a manifestation of vitamin A deficiency?…the rest of the story. Pediatr Dermatol. 2005;22:60-63.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Neill SM, Pembroke AC, du-Vivier AWP, et al. Phrynoderma and perforating folliculitis due to vitamin A deficiency in a diabetic. J R Soc Med. 1988;81:171-172.
- Wechsler HL. Vitamin A deficiency following small-bowel bypass surgery for obesity. Arch Dermatol. 1979;115:73-75.
- Logan WS. Vitamin A and keratinization. Arch Dermatol. 1972;105:748-753.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gazette. 1933;68:681-687.
- Bagchi K, Halder K, Chowdhury SR. The etiology of phrynoderma; histologic evidence. Am J Clin Nutr. 1959;7:251-258.
- Bleasel NR, Stapleton KM, Lee MS, et al. Vitamin A deficiency phrynoderma: due to malabsorption and inadequate diet. J Am Acad Dermatol. 1999;41(2, pt 2):322-324.
- Ghafoorunissa, Vidyasagar R, Krishnaswamy K. Phrynoderma: is it an EFA deficiency disease? Euro J Clin Nutr. 1988;42:29-39.
- Shrank AB. Phrynoderma. Br Med J. 1966;1:29-30.
- Maronn M, Allen DM, Esterly NB. Phrynoderma: a manifestation of vitamin A deficiency?…the rest of the story. Pediatr Dermatol. 2005;22:60-63.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56:389-392.
- Neill SM, Pembroke AC, du-Vivier AWP, et al. Phrynoderma and perforating folliculitis due to vitamin A deficiency in a diabetic. J R Soc Med. 1988;81:171-172.
- Wechsler HL. Vitamin A deficiency following small-bowel bypass surgery for obesity. Arch Dermatol. 1979;115:73-75.
- Logan WS. Vitamin A and keratinization. Arch Dermatol. 1972;105:748-753.
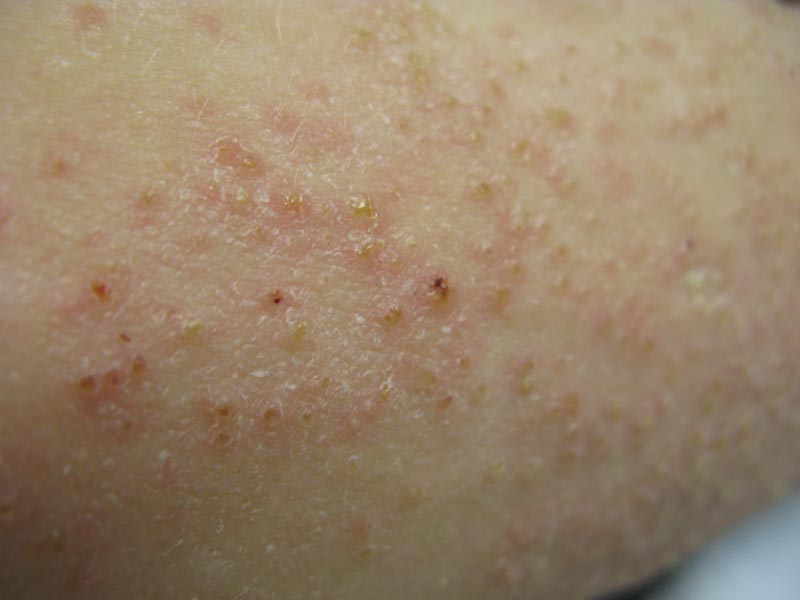
A 22-year-old man with a history of severe mental retardation, a questionable diagnosis of cystic fibrosis, chronic abdominal pain, and a recent diagnosis of primary sclerosing cholangitis was transferred from an outside hospital for worsening abdominal pain, lethargy, anasarca, anorexia, and constipation. An abdominal paracentesis and computed tomography scan of the abdomen were both negative for any acute processes. Blood cultures and paracentesis fluid cultures were negative. The dermatology section was consulted for a dry scaly rash of unknown duration. The eruption had not been treated, and the patient’s dermatologic history was otherwise unremarkable. On physical examination, the patient had a generalized follicular, erythematous, and scaly papular eruption with some areas of yellow-colored hyperkeratotic papules and plaques as well as scattered hemorrhagic crusts on the extensor surface of the right arm. He had normal oral mucosa and nails. The team was unable to gain consent for biopsy, but a blood test was obtained.
In Utero Valproate Exposure May Increase Risk of Autism Symptoms
An elevated rate of autism traits was seen among a cohort of children exposed to antiepileptic drugs (AEDs) in utero. Study findings were reported in the July Epilepsia. “The most important determinant of association with autistic traits was higher doses of sodium valproate exposure,” said Amanda G. Wood, PhD, MPsych, a Senior Lecturer in the School of Psychology at the University of Birmingham in the United Kingdom.
While the use of valproate in women who may become pregnant is generally avoided, there are insufficient data regarding the risk of autism spectrum disorders with low-dose valproate. “If this risk is no greater than with other AEDs, it may enable women with genetic generalized epilepsy to retain optimal seizure control as well as minimize harm to their unborn child,” Dr. Wood said.
Dr. Wood and colleagues conducted a prospective cohort study in children exposed to anticonvulsants during pregnancy, with all assessments conducted by examiners who were blinded to drug-exposure status. Participants were 105 Australian children ages 6 to 8 who were recruited through the Australian Pregnancy Register for Women on Antiepileptic Medication. Maternal epilepsy, pregnancy, and medical history data were obtained prospectively. Autism traits were assessed using the Childhood Autism Rating Scale (CARS).
Among the cohort, 11 children (10.5%) had elevated CARS scores, and this proportion was substantially higher than the estimated prevalence of autism spectrum disorders in age-matched children nationally or internationally. Linear regression analysis showed that the mean valproate dose during pregnancy was a significant predictor of CARS scores after controlling for polytherapy, mean carbamazepine dose, folic acid use, seizures during pregnancy, tobacco and marijuana use, maternal IQ, and socioeconomic status.
Children who had in utero exposure to valproate were most likely to have elevated CARS scores, with 7.7% of the valproate monotherapy group and 46.7% of the valproate polytherapy group displaying autism spectrum disorder symptoms. The dose of valproate taken during pregnancy was found to be an independent risk factor for elevated CARS scores, whereas polytherapy was not. “CARS scores were not elevated in children exposed to polytherapy without valproate, suggesting that valproate, or valproate dose, rather than polytherapy per se, is the critical determinant of the relationship,” the researchers said.
—Glenn S. Williams
Suggested Reading
Wood AG, Nadebaum C, Anderson V, et al. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia. 2015;56(7):1047-1055.
An elevated rate of autism traits was seen among a cohort of children exposed to antiepileptic drugs (AEDs) in utero. Study findings were reported in the July Epilepsia. “The most important determinant of association with autistic traits was higher doses of sodium valproate exposure,” said Amanda G. Wood, PhD, MPsych, a Senior Lecturer in the School of Psychology at the University of Birmingham in the United Kingdom.
While the use of valproate in women who may become pregnant is generally avoided, there are insufficient data regarding the risk of autism spectrum disorders with low-dose valproate. “If this risk is no greater than with other AEDs, it may enable women with genetic generalized epilepsy to retain optimal seizure control as well as minimize harm to their unborn child,” Dr. Wood said.
Dr. Wood and colleagues conducted a prospective cohort study in children exposed to anticonvulsants during pregnancy, with all assessments conducted by examiners who were blinded to drug-exposure status. Participants were 105 Australian children ages 6 to 8 who were recruited through the Australian Pregnancy Register for Women on Antiepileptic Medication. Maternal epilepsy, pregnancy, and medical history data were obtained prospectively. Autism traits were assessed using the Childhood Autism Rating Scale (CARS).
Among the cohort, 11 children (10.5%) had elevated CARS scores, and this proportion was substantially higher than the estimated prevalence of autism spectrum disorders in age-matched children nationally or internationally. Linear regression analysis showed that the mean valproate dose during pregnancy was a significant predictor of CARS scores after controlling for polytherapy, mean carbamazepine dose, folic acid use, seizures during pregnancy, tobacco and marijuana use, maternal IQ, and socioeconomic status.
Children who had in utero exposure to valproate were most likely to have elevated CARS scores, with 7.7% of the valproate monotherapy group and 46.7% of the valproate polytherapy group displaying autism spectrum disorder symptoms. The dose of valproate taken during pregnancy was found to be an independent risk factor for elevated CARS scores, whereas polytherapy was not. “CARS scores were not elevated in children exposed to polytherapy without valproate, suggesting that valproate, or valproate dose, rather than polytherapy per se, is the critical determinant of the relationship,” the researchers said.
—Glenn S. Williams
An elevated rate of autism traits was seen among a cohort of children exposed to antiepileptic drugs (AEDs) in utero. Study findings were reported in the July Epilepsia. “The most important determinant of association with autistic traits was higher doses of sodium valproate exposure,” said Amanda G. Wood, PhD, MPsych, a Senior Lecturer in the School of Psychology at the University of Birmingham in the United Kingdom.
While the use of valproate in women who may become pregnant is generally avoided, there are insufficient data regarding the risk of autism spectrum disorders with low-dose valproate. “If this risk is no greater than with other AEDs, it may enable women with genetic generalized epilepsy to retain optimal seizure control as well as minimize harm to their unborn child,” Dr. Wood said.
Dr. Wood and colleagues conducted a prospective cohort study in children exposed to anticonvulsants during pregnancy, with all assessments conducted by examiners who were blinded to drug-exposure status. Participants were 105 Australian children ages 6 to 8 who were recruited through the Australian Pregnancy Register for Women on Antiepileptic Medication. Maternal epilepsy, pregnancy, and medical history data were obtained prospectively. Autism traits were assessed using the Childhood Autism Rating Scale (CARS).
Among the cohort, 11 children (10.5%) had elevated CARS scores, and this proportion was substantially higher than the estimated prevalence of autism spectrum disorders in age-matched children nationally or internationally. Linear regression analysis showed that the mean valproate dose during pregnancy was a significant predictor of CARS scores after controlling for polytherapy, mean carbamazepine dose, folic acid use, seizures during pregnancy, tobacco and marijuana use, maternal IQ, and socioeconomic status.
Children who had in utero exposure to valproate were most likely to have elevated CARS scores, with 7.7% of the valproate monotherapy group and 46.7% of the valproate polytherapy group displaying autism spectrum disorder symptoms. The dose of valproate taken during pregnancy was found to be an independent risk factor for elevated CARS scores, whereas polytherapy was not. “CARS scores were not elevated in children exposed to polytherapy without valproate, suggesting that valproate, or valproate dose, rather than polytherapy per se, is the critical determinant of the relationship,” the researchers said.
—Glenn S. Williams
Suggested Reading
Wood AG, Nadebaum C, Anderson V, et al. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia. 2015;56(7):1047-1055.
Suggested Reading
Wood AG, Nadebaum C, Anderson V, et al. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia. 2015;56(7):1047-1055.
‘Emergency backup system’ replenishes platelets
Image by Andre E.X. Brown
Preclinical research appears to explain how the body can quickly replenish platelets that are lost during infection.
Investigators discovered an “emergency backup system” in mice that bypasses the known pathway of hematopoietic stem cell (HSC) differentiation so that platelet numbers can be restored rapidly.
Marieke Essers, PhD, of The German Cancer Research Center (Deutsches Krebsforschungszentrum) in Heidelberg, Germany, and her colleagues described this phenomenon in Cell Stem Cell.
The team discovered a small cell population within the HSC compartment that induces differentiation in megakaryocytes.
These quiescent stem cells—dubbed stem-like megakaryocyte-committed progenitors (SL-MkPs)—do not provide the normal supply of platelets but serve as a backup in case of emergency.
When quiescent, SL-MkPs express few proteins. In the event of an acute infection, SL-MkPs are aroused from their quiescent state by interferon α, express the typical megakaryocyte proteins, and are rapidly differentiated into advanced precursor cells.
This quickly replaces the platelets that were lost as a result of the infection.
This emergency backup system bypasses the lengthy process of normal HSC differentiation, thereby ensuring that any life-threatening loss of platelets is compensated for quickly.
However, the investigators found that repeat infections can result in the reservoir of SL-MkPs being depleted. ![]()

Image by Andre E.X. Brown
Preclinical research appears to explain how the body can quickly replenish platelets that are lost during infection.
Investigators discovered an “emergency backup system” in mice that bypasses the known pathway of hematopoietic stem cell (HSC) differentiation so that platelet numbers can be restored rapidly.
Marieke Essers, PhD, of The German Cancer Research Center (Deutsches Krebsforschungszentrum) in Heidelberg, Germany, and her colleagues described this phenomenon in Cell Stem Cell.
The team discovered a small cell population within the HSC compartment that induces differentiation in megakaryocytes.
These quiescent stem cells—dubbed stem-like megakaryocyte-committed progenitors (SL-MkPs)—do not provide the normal supply of platelets but serve as a backup in case of emergency.
When quiescent, SL-MkPs express few proteins. In the event of an acute infection, SL-MkPs are aroused from their quiescent state by interferon α, express the typical megakaryocyte proteins, and are rapidly differentiated into advanced precursor cells.
This quickly replaces the platelets that were lost as a result of the infection.
This emergency backup system bypasses the lengthy process of normal HSC differentiation, thereby ensuring that any life-threatening loss of platelets is compensated for quickly.
However, the investigators found that repeat infections can result in the reservoir of SL-MkPs being depleted. ![]()

Image by Andre E.X. Brown
Preclinical research appears to explain how the body can quickly replenish platelets that are lost during infection.
Investigators discovered an “emergency backup system” in mice that bypasses the known pathway of hematopoietic stem cell (HSC) differentiation so that platelet numbers can be restored rapidly.
Marieke Essers, PhD, of The German Cancer Research Center (Deutsches Krebsforschungszentrum) in Heidelberg, Germany, and her colleagues described this phenomenon in Cell Stem Cell.
The team discovered a small cell population within the HSC compartment that induces differentiation in megakaryocytes.
These quiescent stem cells—dubbed stem-like megakaryocyte-committed progenitors (SL-MkPs)—do not provide the normal supply of platelets but serve as a backup in case of emergency.
When quiescent, SL-MkPs express few proteins. In the event of an acute infection, SL-MkPs are aroused from their quiescent state by interferon α, express the typical megakaryocyte proteins, and are rapidly differentiated into advanced precursor cells.
This quickly replaces the platelets that were lost as a result of the infection.
This emergency backup system bypasses the lengthy process of normal HSC differentiation, thereby ensuring that any life-threatening loss of platelets is compensated for quickly.
However, the investigators found that repeat infections can result in the reservoir of SL-MkPs being depleted. ![]()
Pathway appears key to fighting adenovirus

Using an animal model they developed, researchers have identified a pathway that inhibits replication of the adenovirus.
The team generated a new strain of Syrian hamster, a model in which human adenovirus replicates and causes illness similar to that observed in humans.
Experiments with this model suggested the Type I interferon pathway plays a key role in inhibiting adenovirus replication.
“[L]ike many other viruses, adenovirus can replicate at will when a patient’s immune system is suppressed,” said William Wold, PhD, of Saint Louis University in Missouri.
“Adenovirus can become very dangerous, such as for a child who is undergoing a bone marrow transplant to treat leukemia.”
Previously, Dr Wold led a research team that identified the Syrian hamster as an appropriate animal model to study adenovirus because species C human adenoviruses replicate in these animals.
For the current study, which was published in PLOS Pathogens, Dr Wold and his colleagues conducted experiments with a new Syrian hamster strain. In these animals, the STAT2 gene was functionally knocked out by site-specific gene targeting.
The researchers found that STAT2-knockout hamsters were extremely sensitive to infection with type 5 human adenovirus (Ad5).
The team infected both STAT2-knockout hamsters and wild-type controls with Ad5. Knockout hamsters had 100 to 1000 times the viral load of controls.
The knockout hamsters also had pathology characteristic of advanced adenovirus infection—yellow, mottled livers and enlarged gall bladders—whereas controls did not.
The adaptive immune response to Ad5 remained intact in the STAT2-knockout hamsters, as surviving animals were able to clear the virus.
However, the Type 1 interferon response was hampered in these animals. Knocking out STAT2 disrupted the Type 1 interferon pathway by interrupting the cascade of cell signaling.
The researchers said their findings suggest the disrupted Type I interferon pathway contributed to the increased Ad5 replication in the STAT2-knockout hamsters.
“Besides providing an insight into adenovirus infection in humans, our results are also interesting from the perspective of the animal model,” Dr Wold said. “The STAT2-knockout Syrian hamster may also be an important animal model for studying other viral infections, including Ebola, hanta, and dengue viruses.”
The model was created by Zhongde Wang, PhD, and his colleagues at Utah State University in Logan, Utah. Dr Wang’s lab is the first to develop gene-targeting technologies in the Syrian hamster.
“The success we achieved in conducting gene-targeting in the Syrian hamster has provided the opportunity to create models for many of the human diseases for which there are either no existent animal models or severe limitations in the available animal models,” Dr Wang said. ![]()

Using an animal model they developed, researchers have identified a pathway that inhibits replication of the adenovirus.
The team generated a new strain of Syrian hamster, a model in which human adenovirus replicates and causes illness similar to that observed in humans.
Experiments with this model suggested the Type I interferon pathway plays a key role in inhibiting adenovirus replication.
“[L]ike many other viruses, adenovirus can replicate at will when a patient’s immune system is suppressed,” said William Wold, PhD, of Saint Louis University in Missouri.
“Adenovirus can become very dangerous, such as for a child who is undergoing a bone marrow transplant to treat leukemia.”
Previously, Dr Wold led a research team that identified the Syrian hamster as an appropriate animal model to study adenovirus because species C human adenoviruses replicate in these animals.
For the current study, which was published in PLOS Pathogens, Dr Wold and his colleagues conducted experiments with a new Syrian hamster strain. In these animals, the STAT2 gene was functionally knocked out by site-specific gene targeting.
The researchers found that STAT2-knockout hamsters were extremely sensitive to infection with type 5 human adenovirus (Ad5).
The team infected both STAT2-knockout hamsters and wild-type controls with Ad5. Knockout hamsters had 100 to 1000 times the viral load of controls.
The knockout hamsters also had pathology characteristic of advanced adenovirus infection—yellow, mottled livers and enlarged gall bladders—whereas controls did not.
The adaptive immune response to Ad5 remained intact in the STAT2-knockout hamsters, as surviving animals were able to clear the virus.
However, the Type 1 interferon response was hampered in these animals. Knocking out STAT2 disrupted the Type 1 interferon pathway by interrupting the cascade of cell signaling.
The researchers said their findings suggest the disrupted Type I interferon pathway contributed to the increased Ad5 replication in the STAT2-knockout hamsters.
“Besides providing an insight into adenovirus infection in humans, our results are also interesting from the perspective of the animal model,” Dr Wold said. “The STAT2-knockout Syrian hamster may also be an important animal model for studying other viral infections, including Ebola, hanta, and dengue viruses.”
The model was created by Zhongde Wang, PhD, and his colleagues at Utah State University in Logan, Utah. Dr Wang’s lab is the first to develop gene-targeting technologies in the Syrian hamster.
“The success we achieved in conducting gene-targeting in the Syrian hamster has provided the opportunity to create models for many of the human diseases for which there are either no existent animal models or severe limitations in the available animal models,” Dr Wang said. ![]()

Using an animal model they developed, researchers have identified a pathway that inhibits replication of the adenovirus.
The team generated a new strain of Syrian hamster, a model in which human adenovirus replicates and causes illness similar to that observed in humans.
Experiments with this model suggested the Type I interferon pathway plays a key role in inhibiting adenovirus replication.
“[L]ike many other viruses, adenovirus can replicate at will when a patient’s immune system is suppressed,” said William Wold, PhD, of Saint Louis University in Missouri.
“Adenovirus can become very dangerous, such as for a child who is undergoing a bone marrow transplant to treat leukemia.”
Previously, Dr Wold led a research team that identified the Syrian hamster as an appropriate animal model to study adenovirus because species C human adenoviruses replicate in these animals.
For the current study, which was published in PLOS Pathogens, Dr Wold and his colleagues conducted experiments with a new Syrian hamster strain. In these animals, the STAT2 gene was functionally knocked out by site-specific gene targeting.
The researchers found that STAT2-knockout hamsters were extremely sensitive to infection with type 5 human adenovirus (Ad5).
The team infected both STAT2-knockout hamsters and wild-type controls with Ad5. Knockout hamsters had 100 to 1000 times the viral load of controls.
The knockout hamsters also had pathology characteristic of advanced adenovirus infection—yellow, mottled livers and enlarged gall bladders—whereas controls did not.
The adaptive immune response to Ad5 remained intact in the STAT2-knockout hamsters, as surviving animals were able to clear the virus.
However, the Type 1 interferon response was hampered in these animals. Knocking out STAT2 disrupted the Type 1 interferon pathway by interrupting the cascade of cell signaling.
The researchers said their findings suggest the disrupted Type I interferon pathway contributed to the increased Ad5 replication in the STAT2-knockout hamsters.
“Besides providing an insight into adenovirus infection in humans, our results are also interesting from the perspective of the animal model,” Dr Wold said. “The STAT2-knockout Syrian hamster may also be an important animal model for studying other viral infections, including Ebola, hanta, and dengue viruses.”
The model was created by Zhongde Wang, PhD, and his colleagues at Utah State University in Logan, Utah. Dr Wang’s lab is the first to develop gene-targeting technologies in the Syrian hamster.
“The success we achieved in conducting gene-targeting in the Syrian hamster has provided the opportunity to create models for many of the human diseases for which there are either no existent animal models or severe limitations in the available animal models,” Dr Wang said. ![]()
Secondary CNS lymphoma regimen linked to 41% survival at 5 years
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
The findings of Ferreri et al highlight the significant progress that has been made toward finding a cure for aggressive B-cell lymphoma with concomitant or subsequent CNS involvement.
Just a few years ago, this condition was fatal in nearly all cases, but in light of these and other recent findings, needed improvements in treatment seem possible.
Among other strategies, complete elimination of non-[blood-brain barrier]-crossing agents and administration of more than two cycles of induction chemotherapy might prove to be of value. In addition, oral targeted therapies with small molecules, most of which easily cross the BBB, hold promise.
Dr. Norbert Schmitz and Huei-Shan Wuthey are with Asklepios Hospital St. Georg, Hamburg, Germany. They made their remarks in an editorial(J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.63.1143) that accompanied the study. Dr. Schmitz reported serving in a consulting or advisory role for Roche and receiving research funding from Roche. Huei-Shan Wu reported having no disclosures.
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
Treatment with high doses of antimetabolites followed by rituximab plus high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation was feasible and effective in a multicenter phase II study of 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
The patients, aged 18 to 70 years with Eastern Cooperative Oncology Group performance status of 3 or less at enrollment, were treated with high doses of methotrexate and cytarabine, followed by rituximab plus high-dose sequential chemoimmunotherapy (R-HDS) consisting of cylcophosphamide, cytarabine, and etoposide supported by autologous stem-cell transplantation in eligible patients.
Toxicity was typically manageable, but 30 treatment courses were complicated by grade 3 or 4 febrile neutropenia and/or infections; 4 patients died because of toxicity, Dr. Andres J. M. Ferreri of San Raffaele Scientific Institute, Milan, Italy and colleagues reported online Aug. 17 in the Journal of Clinical Oncology.
The complete response rate was 63% (24 patients), and 17 patients remained relapse free at a median follow-up of 48 months, with a 2-year event-free survival rate of 50%, and a 5-year survival rate of 41%, the investigators said (J Clin Oncol. 2015 Aug 17. doi: 10.1200/JCO.2015.61.1236).
This novel radiotherapy-free regimen, developed based on encouraging outcomes with antimetabolites in patients with primary CNS lymphoma and with R-HDS in relapsed aggressive lymphoma, appears safe and effective in the setting of secondary CNS lymphoma (SCNSL).
“We propose this strategy as the standard of care for patients with SCNSL and as a comparison control regimen for future trials,” they concluded.
Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: High doses of antimetabolites followed by R-HDS and autologous stem-cell transplantation was feasible and effective in 38 patients with aggressive B-cell lymphoma and secondary central nervous system involvement.
Major finding: The 2-year event-free survival rate was 50%, and the 5-year survival rate was 41%
Data source: A multicenter phase II study of 38 adults.
Disclosures: Dr. Ferreri reported having no disclosures. One co-author, Dr. Federico Caligaris-Cappio, reported serving in a consulting or advisory role for Janssen and Pharmacyclics.
Clotting tests fail to predict internal bleeding

Photo by Juan D. Alfonso
A new study indicates that coagulation tests are an unreliable means for measuring the risk of internal bleeding in patients taking the factor Xa inhibitors rivaroxaban and apixaban.
For most patients who experienced internal bleeding in this study, results of coagulation tests were normal.
Prothrombin time (PT), partial thromboplastin time (PTT), and international normalized ratios (INRs) were elevated in a minority of patients treated with rivaroxaban and none of the patients treated with apixaban.
Henry Spiller, of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus, Ohio, and his colleagues conducted this retrospective study and reported the results in Annals of Emergency Medicine.
The study included data on 223 patients from more than 800 hospitals and 8 regional poison centers covering 9 states. The patients’ mean age was 60, and 56% were female. Nine percent of patients (n=20) were children younger than 12.
Eighty-nine percent of patients had taken rivaroxaban (n=198), and 11% had taken apixaban (n=25). The mean dose of rivaroxaban (reported in 182 patients) was 64.5 mg (range, 15 mg to 1200 mg). And the mean dose of apixaban (reported in 21 patients) was 9.6 mg (range, 2.5 mg to 20 mg).
For rivaroxaban, PT was measured in 49 patients (25%) and elevated in 7. PTT was measured in 49 patients (25%) and elevated in 5. And INR was measured in 61 patients (31%) and elevated in 13.
For apixaban, PT and PTT were both measured in 6 patients (24%) and elevated in none. INR was measured in 5 patients (20%) and elevated in none.
Cases of bleeding
Bleeding was reported in 15 patients (7%)—11 on rivaroxaban and 4 on apixaban. The sites of bleeding were gastrointestinal (n=8), oral (n=2), nose (n=1), bruising (n=1), urine (n=1), and subdural (n=1).
All bleeds occurred in adults who were taking the anticoagulants long-term. Twelve bleeds were considered adverse drug reactions, 2 were therapeutic error, and the reason was unknown in 1 case.
Coagulation test results were normal in most patients with bleeding. PT and PTT were both normal in 5 of 6 patients tested (83%), and INR was normal in 5 of 9 patients tested (55%).
PT and PTT were elevated in 1 of 4 patients treated with rivaroxaban and none of the patients treated with apixaban. The INR was elevated in 5 of 8 patients treated with rivaroxaban and none of the patients treated with apixaban.
The researchers said these results suggest that, without specific clarification of methodology and reagent use, PT, PTT, and INR may not reliably predict the risk of bleeding after rivaroxaban or apixaban ingestion.
“One way to overcome the variation in these tests is to use anti-factor Xa chromogenic assays to measure Xa plasma concentrations,” Spiller said.
“However, these are not widely available, and a potential drawback with measuring anti-factor Xa concentrations and plasma rivaroxaban and apixaban concentrations is that the turnaround time for results may be too long to guide a treatment plan.” ![]()

Photo by Juan D. Alfonso
A new study indicates that coagulation tests are an unreliable means for measuring the risk of internal bleeding in patients taking the factor Xa inhibitors rivaroxaban and apixaban.
For most patients who experienced internal bleeding in this study, results of coagulation tests were normal.
Prothrombin time (PT), partial thromboplastin time (PTT), and international normalized ratios (INRs) were elevated in a minority of patients treated with rivaroxaban and none of the patients treated with apixaban.
Henry Spiller, of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus, Ohio, and his colleagues conducted this retrospective study and reported the results in Annals of Emergency Medicine.
The study included data on 223 patients from more than 800 hospitals and 8 regional poison centers covering 9 states. The patients’ mean age was 60, and 56% were female. Nine percent of patients (n=20) were children younger than 12.
Eighty-nine percent of patients had taken rivaroxaban (n=198), and 11% had taken apixaban (n=25). The mean dose of rivaroxaban (reported in 182 patients) was 64.5 mg (range, 15 mg to 1200 mg). And the mean dose of apixaban (reported in 21 patients) was 9.6 mg (range, 2.5 mg to 20 mg).
For rivaroxaban, PT was measured in 49 patients (25%) and elevated in 7. PTT was measured in 49 patients (25%) and elevated in 5. And INR was measured in 61 patients (31%) and elevated in 13.
For apixaban, PT and PTT were both measured in 6 patients (24%) and elevated in none. INR was measured in 5 patients (20%) and elevated in none.
Cases of bleeding
Bleeding was reported in 15 patients (7%)—11 on rivaroxaban and 4 on apixaban. The sites of bleeding were gastrointestinal (n=8), oral (n=2), nose (n=1), bruising (n=1), urine (n=1), and subdural (n=1).
All bleeds occurred in adults who were taking the anticoagulants long-term. Twelve bleeds were considered adverse drug reactions, 2 were therapeutic error, and the reason was unknown in 1 case.
Coagulation test results were normal in most patients with bleeding. PT and PTT were both normal in 5 of 6 patients tested (83%), and INR was normal in 5 of 9 patients tested (55%).
PT and PTT were elevated in 1 of 4 patients treated with rivaroxaban and none of the patients treated with apixaban. The INR was elevated in 5 of 8 patients treated with rivaroxaban and none of the patients treated with apixaban.
The researchers said these results suggest that, without specific clarification of methodology and reagent use, PT, PTT, and INR may not reliably predict the risk of bleeding after rivaroxaban or apixaban ingestion.
“One way to overcome the variation in these tests is to use anti-factor Xa chromogenic assays to measure Xa plasma concentrations,” Spiller said.
“However, these are not widely available, and a potential drawback with measuring anti-factor Xa concentrations and plasma rivaroxaban and apixaban concentrations is that the turnaround time for results may be too long to guide a treatment plan.” ![]()

Photo by Juan D. Alfonso
A new study indicates that coagulation tests are an unreliable means for measuring the risk of internal bleeding in patients taking the factor Xa inhibitors rivaroxaban and apixaban.
For most patients who experienced internal bleeding in this study, results of coagulation tests were normal.
Prothrombin time (PT), partial thromboplastin time (PTT), and international normalized ratios (INRs) were elevated in a minority of patients treated with rivaroxaban and none of the patients treated with apixaban.
Henry Spiller, of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus, Ohio, and his colleagues conducted this retrospective study and reported the results in Annals of Emergency Medicine.
The study included data on 223 patients from more than 800 hospitals and 8 regional poison centers covering 9 states. The patients’ mean age was 60, and 56% were female. Nine percent of patients (n=20) were children younger than 12.
Eighty-nine percent of patients had taken rivaroxaban (n=198), and 11% had taken apixaban (n=25). The mean dose of rivaroxaban (reported in 182 patients) was 64.5 mg (range, 15 mg to 1200 mg). And the mean dose of apixaban (reported in 21 patients) was 9.6 mg (range, 2.5 mg to 20 mg).
For rivaroxaban, PT was measured in 49 patients (25%) and elevated in 7. PTT was measured in 49 patients (25%) and elevated in 5. And INR was measured in 61 patients (31%) and elevated in 13.
For apixaban, PT and PTT were both measured in 6 patients (24%) and elevated in none. INR was measured in 5 patients (20%) and elevated in none.
Cases of bleeding
Bleeding was reported in 15 patients (7%)—11 on rivaroxaban and 4 on apixaban. The sites of bleeding were gastrointestinal (n=8), oral (n=2), nose (n=1), bruising (n=1), urine (n=1), and subdural (n=1).
All bleeds occurred in adults who were taking the anticoagulants long-term. Twelve bleeds were considered adverse drug reactions, 2 were therapeutic error, and the reason was unknown in 1 case.
Coagulation test results were normal in most patients with bleeding. PT and PTT were both normal in 5 of 6 patients tested (83%), and INR was normal in 5 of 9 patients tested (55%).
PT and PTT were elevated in 1 of 4 patients treated with rivaroxaban and none of the patients treated with apixaban. The INR was elevated in 5 of 8 patients treated with rivaroxaban and none of the patients treated with apixaban.
The researchers said these results suggest that, without specific clarification of methodology and reagent use, PT, PTT, and INR may not reliably predict the risk of bleeding after rivaroxaban or apixaban ingestion.
“One way to overcome the variation in these tests is to use anti-factor Xa chromogenic assays to measure Xa plasma concentrations,” Spiller said.
“However, these are not widely available, and a potential drawback with measuring anti-factor Xa concentrations and plasma rivaroxaban and apixaban concentrations is that the turnaround time for results may be too long to guide a treatment plan.” ![]()
Picato adverse events prompt FDA warning
The Food and Drug Administration has issued a Drug Safety Communication warning for the potential for severe allergic reactions, shingles, and severe eye injuries from incorrect application of Picato (ingenol mebutate), a topical gel used to treat actinic keratosis.
Picato’s manufacurer, Leo Pharma Inc., will be required to change the drug’s labeling to reflect the risk for these adverse events and provide more information about safe application of Picato gel.
In the data summary accompanying the announcement, the FDA noted that some of the incorrect use of Picato gel was related either to inaccurate prescribing or dispensing. Adverse events reported were associated with incorrect application of Picato gel, which is to be used on no more than 25 cm2 of skin at a time, and for no more than 3 consecutive days.
Some of the adverse events reports describe mixing Picato with other products, occluding the skin after applying Picato gel, washing it off before the recommended 6 hours, or applying at bedtime.
Additionally, some adverse events occurred when the stronger 0.05% formulation, intended for use on the extremities and trunk, was applied to the face. Facial actinic keratoses are to be treated with the 0.015% formulation.
Adverse events described included severe allergic reactions ranging from significant contact dermatitis to anaphylaxis. Reactivation of herpes zoster was also reported; some of these cases were associated with applying Picato gel to a larger-than-recommended area, or with using an incorrect dose strength.
Another class of adverse events involved accidental transfer of Picato gel, often to the eyes. This occurred even after handwashing. In addition to eyelid swelling and irritation, cases of chemical conjunctivitis and corneal ulceration were reported. Lips, tongue, and rectum were other areas affected by accidental transfer of Picato gel.
On Twitter @karioakes
The Food and Drug Administration has issued a Drug Safety Communication warning for the potential for severe allergic reactions, shingles, and severe eye injuries from incorrect application of Picato (ingenol mebutate), a topical gel used to treat actinic keratosis.
Picato’s manufacurer, Leo Pharma Inc., will be required to change the drug’s labeling to reflect the risk for these adverse events and provide more information about safe application of Picato gel.
In the data summary accompanying the announcement, the FDA noted that some of the incorrect use of Picato gel was related either to inaccurate prescribing or dispensing. Adverse events reported were associated with incorrect application of Picato gel, which is to be used on no more than 25 cm2 of skin at a time, and for no more than 3 consecutive days.
Some of the adverse events reports describe mixing Picato with other products, occluding the skin after applying Picato gel, washing it off before the recommended 6 hours, or applying at bedtime.
Additionally, some adverse events occurred when the stronger 0.05% formulation, intended for use on the extremities and trunk, was applied to the face. Facial actinic keratoses are to be treated with the 0.015% formulation.
Adverse events described included severe allergic reactions ranging from significant contact dermatitis to anaphylaxis. Reactivation of herpes zoster was also reported; some of these cases were associated with applying Picato gel to a larger-than-recommended area, or with using an incorrect dose strength.
Another class of adverse events involved accidental transfer of Picato gel, often to the eyes. This occurred even after handwashing. In addition to eyelid swelling and irritation, cases of chemical conjunctivitis and corneal ulceration were reported. Lips, tongue, and rectum were other areas affected by accidental transfer of Picato gel.
On Twitter @karioakes
The Food and Drug Administration has issued a Drug Safety Communication warning for the potential for severe allergic reactions, shingles, and severe eye injuries from incorrect application of Picato (ingenol mebutate), a topical gel used to treat actinic keratosis.
Picato’s manufacurer, Leo Pharma Inc., will be required to change the drug’s labeling to reflect the risk for these adverse events and provide more information about safe application of Picato gel.
In the data summary accompanying the announcement, the FDA noted that some of the incorrect use of Picato gel was related either to inaccurate prescribing or dispensing. Adverse events reported were associated with incorrect application of Picato gel, which is to be used on no more than 25 cm2 of skin at a time, and for no more than 3 consecutive days.
Some of the adverse events reports describe mixing Picato with other products, occluding the skin after applying Picato gel, washing it off before the recommended 6 hours, or applying at bedtime.
Additionally, some adverse events occurred when the stronger 0.05% formulation, intended for use on the extremities and trunk, was applied to the face. Facial actinic keratoses are to be treated with the 0.015% formulation.
Adverse events described included severe allergic reactions ranging from significant contact dermatitis to anaphylaxis. Reactivation of herpes zoster was also reported; some of these cases were associated with applying Picato gel to a larger-than-recommended area, or with using an incorrect dose strength.
Another class of adverse events involved accidental transfer of Picato gel, often to the eyes. This occurred even after handwashing. In addition to eyelid swelling and irritation, cases of chemical conjunctivitis and corneal ulceration were reported. Lips, tongue, and rectum were other areas affected by accidental transfer of Picato gel.
On Twitter @karioakes
FROM AN FDA MEDWATCH ALERT
Teaching residents can be more rewarding than you think
“Teach residents…really? You want to add yet another time-consuming responsibility to my already hectic schedule?” Residency. The mere mention of the word conjures up chilling memories of 3 am codes, 20-30 hour zombie shifts, and anxiety and stress levels that we never knew before and, fortunately, have not known since. That was a time in life many of us want to put in the deepest recesses of our minds, never to emerge again.
But, on the other hand, there were a lot of good things about our residency training that we should probably never forget, such as the humility with which we approached patient care. At that time in our lives we gladly acknowledged we did not know everything and we were eager to research each and every condition to get a firm handle on what we could and should do to help our patients get better.
Fast forward a decade or two. Now many of us have spouses, children, aging parents, mortgages, and retirement accounts we are feverishly trying to fund. There never seems to be enough time to finish even the most fundamental responsibilities. Not to mention now there are national initiatives, mandatory rules, and sometimes frightening regulations in place that dramatically impact how we practice medicine and sometimes make us feel more like automatons than the physicians we dreamed of becoming when we first applied to medical school years ago.
With all of our current and future responsibilities, how can the average hospitalist embrace young physicians and pour himself into their lives? Or, perhaps the question is better asked, how can we not? None of us morphed from a green medical school graduate to a knowledgeable, well-respected physician without a great deal of hand holding (and sometimes hand wringing), encouragement and investment of time from our teaching attendings. But even if you are hesitant to invest the time and energy to teach resident physicians should you have the opportunity. Keep in mind, in 2015 we are not only teaching them, they are teaching us too!
Young physicians are overflowing with technological knowledge that many of us have never been exposed to, knowledge that can help escalate our own skill sets. They bring fresh ideas, novel approaches to patient care, and frequently, cutting edge medical innovations from the universities from which come.
So, if you are ever asked to teach our future colleagues, remember: you may very well find that the time you invest benefits you as much, if not more, than it does them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
“Teach residents…really? You want to add yet another time-consuming responsibility to my already hectic schedule?” Residency. The mere mention of the word conjures up chilling memories of 3 am codes, 20-30 hour zombie shifts, and anxiety and stress levels that we never knew before and, fortunately, have not known since. That was a time in life many of us want to put in the deepest recesses of our minds, never to emerge again.
But, on the other hand, there were a lot of good things about our residency training that we should probably never forget, such as the humility with which we approached patient care. At that time in our lives we gladly acknowledged we did not know everything and we were eager to research each and every condition to get a firm handle on what we could and should do to help our patients get better.
Fast forward a decade or two. Now many of us have spouses, children, aging parents, mortgages, and retirement accounts we are feverishly trying to fund. There never seems to be enough time to finish even the most fundamental responsibilities. Not to mention now there are national initiatives, mandatory rules, and sometimes frightening regulations in place that dramatically impact how we practice medicine and sometimes make us feel more like automatons than the physicians we dreamed of becoming when we first applied to medical school years ago.
With all of our current and future responsibilities, how can the average hospitalist embrace young physicians and pour himself into their lives? Or, perhaps the question is better asked, how can we not? None of us morphed from a green medical school graduate to a knowledgeable, well-respected physician without a great deal of hand holding (and sometimes hand wringing), encouragement and investment of time from our teaching attendings. But even if you are hesitant to invest the time and energy to teach resident physicians should you have the opportunity. Keep in mind, in 2015 we are not only teaching them, they are teaching us too!
Young physicians are overflowing with technological knowledge that many of us have never been exposed to, knowledge that can help escalate our own skill sets. They bring fresh ideas, novel approaches to patient care, and frequently, cutting edge medical innovations from the universities from which come.
So, if you are ever asked to teach our future colleagues, remember: you may very well find that the time you invest benefits you as much, if not more, than it does them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
“Teach residents…really? You want to add yet another time-consuming responsibility to my already hectic schedule?” Residency. The mere mention of the word conjures up chilling memories of 3 am codes, 20-30 hour zombie shifts, and anxiety and stress levels that we never knew before and, fortunately, have not known since. That was a time in life many of us want to put in the deepest recesses of our minds, never to emerge again.
But, on the other hand, there were a lot of good things about our residency training that we should probably never forget, such as the humility with which we approached patient care. At that time in our lives we gladly acknowledged we did not know everything and we were eager to research each and every condition to get a firm handle on what we could and should do to help our patients get better.
Fast forward a decade or two. Now many of us have spouses, children, aging parents, mortgages, and retirement accounts we are feverishly trying to fund. There never seems to be enough time to finish even the most fundamental responsibilities. Not to mention now there are national initiatives, mandatory rules, and sometimes frightening regulations in place that dramatically impact how we practice medicine and sometimes make us feel more like automatons than the physicians we dreamed of becoming when we first applied to medical school years ago.
With all of our current and future responsibilities, how can the average hospitalist embrace young physicians and pour himself into their lives? Or, perhaps the question is better asked, how can we not? None of us morphed from a green medical school graduate to a knowledgeable, well-respected physician without a great deal of hand holding (and sometimes hand wringing), encouragement and investment of time from our teaching attendings. But even if you are hesitant to invest the time and energy to teach resident physicians should you have the opportunity. Keep in mind, in 2015 we are not only teaching them, they are teaching us too!
Young physicians are overflowing with technological knowledge that many of us have never been exposed to, knowledge that can help escalate our own skill sets. They bring fresh ideas, novel approaches to patient care, and frequently, cutting edge medical innovations from the universities from which come.
So, if you are ever asked to teach our future colleagues, remember: you may very well find that the time you invest benefits you as much, if not more, than it does them.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].