User login
Treatment outcomes in stage IIIA non–small-cell lung cancer in a community cancer center
Objective To analyze demographics and treatment outcomes in patients with stage IIIA NSCLC at a community cancer center.
Methods We reviewed charts of 226 patients diagnosed with stage IIIA NSCLC from January 2003 to December 2008 treated at our community cancer center. Results Median overall survival for all patients and sequentially and concurrently treated chemoradiation patients were 18 months, and 18 months, and 20 months, respectively. Median overall survival for women and men was 24 months and 16 months, respectively.
Limitations Study design was retrospective and some medical records were not available. However, this population is likely representative of patients treated in similar settings.
Conclusions In our population, advanced age and male gender were associated with lower median survival. Responses to concurrent and sequential chemoradiation seemed to differ based on age group, which may be useful as a prognostic guideline for similar populations.
Funding Helen F Graham Cancer Center and Research Institute
Click on the PDF icon at the top of this introduction to read the full article.
Objective To analyze demographics and treatment outcomes in patients with stage IIIA NSCLC at a community cancer center.
Methods We reviewed charts of 226 patients diagnosed with stage IIIA NSCLC from January 2003 to December 2008 treated at our community cancer center. Results Median overall survival for all patients and sequentially and concurrently treated chemoradiation patients were 18 months, and 18 months, and 20 months, respectively. Median overall survival for women and men was 24 months and 16 months, respectively.
Limitations Study design was retrospective and some medical records were not available. However, this population is likely representative of patients treated in similar settings.
Conclusions In our population, advanced age and male gender were associated with lower median survival. Responses to concurrent and sequential chemoradiation seemed to differ based on age group, which may be useful as a prognostic guideline for similar populations.
Funding Helen F Graham Cancer Center and Research Institute
Click on the PDF icon at the top of this introduction to read the full article.
Objective To analyze demographics and treatment outcomes in patients with stage IIIA NSCLC at a community cancer center.
Methods We reviewed charts of 226 patients diagnosed with stage IIIA NSCLC from January 2003 to December 2008 treated at our community cancer center. Results Median overall survival for all patients and sequentially and concurrently treated chemoradiation patients were 18 months, and 18 months, and 20 months, respectively. Median overall survival for women and men was 24 months and 16 months, respectively.
Limitations Study design was retrospective and some medical records were not available. However, this population is likely representative of patients treated in similar settings.
Conclusions In our population, advanced age and male gender were associated with lower median survival. Responses to concurrent and sequential chemoradiation seemed to differ based on age group, which may be useful as a prognostic guideline for similar populations.
Funding Helen F Graham Cancer Center and Research Institute
Click on the PDF icon at the top of this introduction to read the full article.
Migraine May Increase Smokers’ Risk of Stroke
Among current smokers, migraine may increase the risk of stroke and combined vascular events, according to research published online ahead of print July 22 in Neurology. Migraine may not be associated with these outcomes among nonsmokers, however.
Teshamae S. Monteith, MD, Assistant Professor of Clinical Neurology at University of Miami School of Medicine, and colleagues found that study participants with migraine had twice the risk of silent brain infarctions, but they considered the findings to be consistent with previous data that suggest that migraine is not a significant risk factor for stroke among older subjects. “We thought that factors associated with a greater migraine burden, such as obesity, might put migraineurs more at risk of vascular events, but this was not the case,” said Dr. Monteith.
Cohort Was Ethnically Diverse
Data have suggested that migraine with aura is an independent risk factor for ischemic stroke in women younger than 45. Migraine also has been associated with an unfavorable cardiovascular risk profile. Dr. Monteith and colleagues initiated their study to assess the association between migraine with and without aura and stroke. They examined data from the prospective Northern Manhattan Study, which enrolled an ethnically diverse, older, community-based cohort.
Eligible participants were stroke-free, older than 40, and had lived in northern Manhattan for three months or longer. Dr. Monteith’s group excluded participants with a history of meningitis, head trauma, or radiation to rule out individuals with the potential for secondary headache. They also excluded people with a myocardial infarction before baseline.
Bilingual research assistants collected data through interviews in English or Spanish. Baseline data included demographics, socioeconomic factors, medical history and medication use, vascular risk factors, family history, and migraine history. The investigators adapted standard questions regarding hypertension, diabetes, cigarette smoking, and cardiac conditions using the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System. Self-reported migraine was assessed with a questionnaire, and additional questions closely adhered to the International Classification of Headache Disorders, second edition criteria for migraine.
Study participants were screened annually by phone for changes in clinical status, and patients who screened positive were invited for an interview and examination by a neurologist. The primary outcome was adjudicated stroke. The secondary outcomes were confirmed combined vascular events (ie, stroke, myocardial infarction, or vascular death), myocardial infarction, and vascular death.
Migraine Tripled Smokers’ Stroke Risk
The researchers had information on migraine status for 1,292 participants. Among this population, 262 participants had migraine (75 with aura, 187 without aura). People with migraine were younger and more likely to be women and have Medicaid or no insurance and several vascular risk factors. Over a mean follow-up of 11 years, the researchers observed 294 combined vascular events, including 114 strokes, 94 myocardial infarctions, and 178 vascular deaths.
Migraine was not associated with risk of combined vascular events including stroke or stroke-only outcomes. When they examined migraine with aura and migraine without aura separately, the investigators found no associations in relation to combined vascular events including stroke or to stroke alone. Age at baseline, sex, race or ethnicity, smoking, moderate alcohol use, moderate to heavy physical activity, BMI, hypertension, hypercholesterolemia, or diabetes did not modify the effect.
The researchers did, however, observe an interaction between current smoking and stroke. They also found an interaction between current smoking and combined vascular events. A stratified analysis yielded a hazard ratio of stroke for migraine versus no migraine among current smokers of 3.17. Among former smokers, the hazard ratio was 0.87, and among participants who had never smoked, the hazard ratio was 0.49 when controlling for socioeconomic and vascular risk factors.
Mechanism of Increased Risk Is Unclear
Previous research has indicated that migraine with aura is an independent risk factor of recurrent ischemic stroke and other vascular events in young patients with ischemic stroke. In addition, stroke risk associated with migraine with aura was greater in younger than in older women in the Women’s Health Study. “Perhaps our participants were too old to display such a relationship between migraine with aura and stroke in both men and women of postmenopausal age,” said Dr. Monteith.
Oxidative stress may be the mechanism by which migraine increases stroke risk among smokers. Oxidative stress may have a role in migraine and may increase susceptibility to vascular events among active smokers. Furthermore, prothrombotic states, decreased platelet hemostasis time, and endothelial dysfunction, which are associated with migraine, are plausible mechanisms that may enhance stroke risk in active smokers. “We suspect that a synergic action may occur between vascular changes of migraine and smoking as an effect modifier, although further work is necessary to elucidate this association,” said Dr. Monteith.
The data appear to suggest that vascular changes in migraine are an important subclinical vascular marker for stroke and combined vascular events among active smokers. Because smoking may be common among migraineurs, the authors recommended that smoking cessation counseling be encouraged as a part of routine migraine care throughout the patient’s lifetime. “The identification of modifiable vascular risk factors and treatments may have beneficial outcomes for stroke reduction in the elderly population with migraine,” they concluded.
—Erik Greb
Suggested Reading
Monteith TS, Gardener H, Rundek T, et al. Migraine and risk of stroke in older adults: Northern Manhattan Study. Neurology. 2015 Jul 22 [Epub ahead of print].
Among current smokers, migraine may increase the risk of stroke and combined vascular events, according to research published online ahead of print July 22 in Neurology. Migraine may not be associated with these outcomes among nonsmokers, however.
Teshamae S. Monteith, MD, Assistant Professor of Clinical Neurology at University of Miami School of Medicine, and colleagues found that study participants with migraine had twice the risk of silent brain infarctions, but they considered the findings to be consistent with previous data that suggest that migraine is not a significant risk factor for stroke among older subjects. “We thought that factors associated with a greater migraine burden, such as obesity, might put migraineurs more at risk of vascular events, but this was not the case,” said Dr. Monteith.
Cohort Was Ethnically Diverse
Data have suggested that migraine with aura is an independent risk factor for ischemic stroke in women younger than 45. Migraine also has been associated with an unfavorable cardiovascular risk profile. Dr. Monteith and colleagues initiated their study to assess the association between migraine with and without aura and stroke. They examined data from the prospective Northern Manhattan Study, which enrolled an ethnically diverse, older, community-based cohort.
Eligible participants were stroke-free, older than 40, and had lived in northern Manhattan for three months or longer. Dr. Monteith’s group excluded participants with a history of meningitis, head trauma, or radiation to rule out individuals with the potential for secondary headache. They also excluded people with a myocardial infarction before baseline.
Bilingual research assistants collected data through interviews in English or Spanish. Baseline data included demographics, socioeconomic factors, medical history and medication use, vascular risk factors, family history, and migraine history. The investigators adapted standard questions regarding hypertension, diabetes, cigarette smoking, and cardiac conditions using the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System. Self-reported migraine was assessed with a questionnaire, and additional questions closely adhered to the International Classification of Headache Disorders, second edition criteria for migraine.
Study participants were screened annually by phone for changes in clinical status, and patients who screened positive were invited for an interview and examination by a neurologist. The primary outcome was adjudicated stroke. The secondary outcomes were confirmed combined vascular events (ie, stroke, myocardial infarction, or vascular death), myocardial infarction, and vascular death.
Migraine Tripled Smokers’ Stroke Risk
The researchers had information on migraine status for 1,292 participants. Among this population, 262 participants had migraine (75 with aura, 187 without aura). People with migraine were younger and more likely to be women and have Medicaid or no insurance and several vascular risk factors. Over a mean follow-up of 11 years, the researchers observed 294 combined vascular events, including 114 strokes, 94 myocardial infarctions, and 178 vascular deaths.
Migraine was not associated with risk of combined vascular events including stroke or stroke-only outcomes. When they examined migraine with aura and migraine without aura separately, the investigators found no associations in relation to combined vascular events including stroke or to stroke alone. Age at baseline, sex, race or ethnicity, smoking, moderate alcohol use, moderate to heavy physical activity, BMI, hypertension, hypercholesterolemia, or diabetes did not modify the effect.
The researchers did, however, observe an interaction between current smoking and stroke. They also found an interaction between current smoking and combined vascular events. A stratified analysis yielded a hazard ratio of stroke for migraine versus no migraine among current smokers of 3.17. Among former smokers, the hazard ratio was 0.87, and among participants who had never smoked, the hazard ratio was 0.49 when controlling for socioeconomic and vascular risk factors.
Mechanism of Increased Risk Is Unclear
Previous research has indicated that migraine with aura is an independent risk factor of recurrent ischemic stroke and other vascular events in young patients with ischemic stroke. In addition, stroke risk associated with migraine with aura was greater in younger than in older women in the Women’s Health Study. “Perhaps our participants were too old to display such a relationship between migraine with aura and stroke in both men and women of postmenopausal age,” said Dr. Monteith.
Oxidative stress may be the mechanism by which migraine increases stroke risk among smokers. Oxidative stress may have a role in migraine and may increase susceptibility to vascular events among active smokers. Furthermore, prothrombotic states, decreased platelet hemostasis time, and endothelial dysfunction, which are associated with migraine, are plausible mechanisms that may enhance stroke risk in active smokers. “We suspect that a synergic action may occur between vascular changes of migraine and smoking as an effect modifier, although further work is necessary to elucidate this association,” said Dr. Monteith.
The data appear to suggest that vascular changes in migraine are an important subclinical vascular marker for stroke and combined vascular events among active smokers. Because smoking may be common among migraineurs, the authors recommended that smoking cessation counseling be encouraged as a part of routine migraine care throughout the patient’s lifetime. “The identification of modifiable vascular risk factors and treatments may have beneficial outcomes for stroke reduction in the elderly population with migraine,” they concluded.
—Erik Greb
Among current smokers, migraine may increase the risk of stroke and combined vascular events, according to research published online ahead of print July 22 in Neurology. Migraine may not be associated with these outcomes among nonsmokers, however.
Teshamae S. Monteith, MD, Assistant Professor of Clinical Neurology at University of Miami School of Medicine, and colleagues found that study participants with migraine had twice the risk of silent brain infarctions, but they considered the findings to be consistent with previous data that suggest that migraine is not a significant risk factor for stroke among older subjects. “We thought that factors associated with a greater migraine burden, such as obesity, might put migraineurs more at risk of vascular events, but this was not the case,” said Dr. Monteith.
Cohort Was Ethnically Diverse
Data have suggested that migraine with aura is an independent risk factor for ischemic stroke in women younger than 45. Migraine also has been associated with an unfavorable cardiovascular risk profile. Dr. Monteith and colleagues initiated their study to assess the association between migraine with and without aura and stroke. They examined data from the prospective Northern Manhattan Study, which enrolled an ethnically diverse, older, community-based cohort.
Eligible participants were stroke-free, older than 40, and had lived in northern Manhattan for three months or longer. Dr. Monteith’s group excluded participants with a history of meningitis, head trauma, or radiation to rule out individuals with the potential for secondary headache. They also excluded people with a myocardial infarction before baseline.
Bilingual research assistants collected data through interviews in English or Spanish. Baseline data included demographics, socioeconomic factors, medical history and medication use, vascular risk factors, family history, and migraine history. The investigators adapted standard questions regarding hypertension, diabetes, cigarette smoking, and cardiac conditions using the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System. Self-reported migraine was assessed with a questionnaire, and additional questions closely adhered to the International Classification of Headache Disorders, second edition criteria for migraine.
Study participants were screened annually by phone for changes in clinical status, and patients who screened positive were invited for an interview and examination by a neurologist. The primary outcome was adjudicated stroke. The secondary outcomes were confirmed combined vascular events (ie, stroke, myocardial infarction, or vascular death), myocardial infarction, and vascular death.
Migraine Tripled Smokers’ Stroke Risk
The researchers had information on migraine status for 1,292 participants. Among this population, 262 participants had migraine (75 with aura, 187 without aura). People with migraine were younger and more likely to be women and have Medicaid or no insurance and several vascular risk factors. Over a mean follow-up of 11 years, the researchers observed 294 combined vascular events, including 114 strokes, 94 myocardial infarctions, and 178 vascular deaths.
Migraine was not associated with risk of combined vascular events including stroke or stroke-only outcomes. When they examined migraine with aura and migraine without aura separately, the investigators found no associations in relation to combined vascular events including stroke or to stroke alone. Age at baseline, sex, race or ethnicity, smoking, moderate alcohol use, moderate to heavy physical activity, BMI, hypertension, hypercholesterolemia, or diabetes did not modify the effect.
The researchers did, however, observe an interaction between current smoking and stroke. They also found an interaction between current smoking and combined vascular events. A stratified analysis yielded a hazard ratio of stroke for migraine versus no migraine among current smokers of 3.17. Among former smokers, the hazard ratio was 0.87, and among participants who had never smoked, the hazard ratio was 0.49 when controlling for socioeconomic and vascular risk factors.
Mechanism of Increased Risk Is Unclear
Previous research has indicated that migraine with aura is an independent risk factor of recurrent ischemic stroke and other vascular events in young patients with ischemic stroke. In addition, stroke risk associated with migraine with aura was greater in younger than in older women in the Women’s Health Study. “Perhaps our participants were too old to display such a relationship between migraine with aura and stroke in both men and women of postmenopausal age,” said Dr. Monteith.
Oxidative stress may be the mechanism by which migraine increases stroke risk among smokers. Oxidative stress may have a role in migraine and may increase susceptibility to vascular events among active smokers. Furthermore, prothrombotic states, decreased platelet hemostasis time, and endothelial dysfunction, which are associated with migraine, are plausible mechanisms that may enhance stroke risk in active smokers. “We suspect that a synergic action may occur between vascular changes of migraine and smoking as an effect modifier, although further work is necessary to elucidate this association,” said Dr. Monteith.
The data appear to suggest that vascular changes in migraine are an important subclinical vascular marker for stroke and combined vascular events among active smokers. Because smoking may be common among migraineurs, the authors recommended that smoking cessation counseling be encouraged as a part of routine migraine care throughout the patient’s lifetime. “The identification of modifiable vascular risk factors and treatments may have beneficial outcomes for stroke reduction in the elderly population with migraine,” they concluded.
—Erik Greb
Suggested Reading
Monteith TS, Gardener H, Rundek T, et al. Migraine and risk of stroke in older adults: Northern Manhattan Study. Neurology. 2015 Jul 22 [Epub ahead of print].
Suggested Reading
Monteith TS, Gardener H, Rundek T, et al. Migraine and risk of stroke in older adults: Northern Manhattan Study. Neurology. 2015 Jul 22 [Epub ahead of print].
Impact of bladder volume on radiation dose to the rectum in the definitive treatment of prostate cancer
Background and objective Our group created and routinely reviewed a dedicated prostate intensity-modulated radiation therapy (IMRT) delivery program. Previously, a retrospective review of our experience demonstrated that a larger bladder volume reduced radiation dose to the rectum. We conducted an observational study to confirm this relationship.
Methods Men receiving definitive radiation for prostate cancer were eligible for the study. Eligible patients received 2 computed axial tomography (CT) scans on the day of their planning CT scan: 1 with a full bladder and 1 with an empty bladder. On each CT data set, the prostate, rectum, bladder, penile bulb, and femoral heads were contoured. 2 IMRT plans were completed on each dataset: 1 by a medical dosimetrist and 1 by a medical physicist. The study plans targeted the prostate to 79.2 Gray (Gy) while respecting predefined dose tolerances to the other contoured structures. Rectal doses were compared on empty and full bladder CT data sets.
Results From June 29, 2010 to December 14, 2011, 17 full bladder data sets and 15 empty bladder data sets were available for analysis. Median change in bladder volume was 63 ml. Full vs empty bladder set-up was associated with a statistically significant reduction in the mean rectal dose of 25.41 Gy vs 27.6 Gy (P = .031).
Limitations Small sample size and small variations in bladder volumes.
Conclusions A greater bladder volume resulted in a reduced mean dose to the rectum irrespective of planning method.
Funding/sponsorship None
Click on the PDF icon at the top of this introduction to read the full article.
Background and objective Our group created and routinely reviewed a dedicated prostate intensity-modulated radiation therapy (IMRT) delivery program. Previously, a retrospective review of our experience demonstrated that a larger bladder volume reduced radiation dose to the rectum. We conducted an observational study to confirm this relationship.
Methods Men receiving definitive radiation for prostate cancer were eligible for the study. Eligible patients received 2 computed axial tomography (CT) scans on the day of their planning CT scan: 1 with a full bladder and 1 with an empty bladder. On each CT data set, the prostate, rectum, bladder, penile bulb, and femoral heads were contoured. 2 IMRT plans were completed on each dataset: 1 by a medical dosimetrist and 1 by a medical physicist. The study plans targeted the prostate to 79.2 Gray (Gy) while respecting predefined dose tolerances to the other contoured structures. Rectal doses were compared on empty and full bladder CT data sets.
Results From June 29, 2010 to December 14, 2011, 17 full bladder data sets and 15 empty bladder data sets were available for analysis. Median change in bladder volume was 63 ml. Full vs empty bladder set-up was associated with a statistically significant reduction in the mean rectal dose of 25.41 Gy vs 27.6 Gy (P = .031).
Limitations Small sample size and small variations in bladder volumes.
Conclusions A greater bladder volume resulted in a reduced mean dose to the rectum irrespective of planning method.
Funding/sponsorship None
Click on the PDF icon at the top of this introduction to read the full article.
Background and objective Our group created and routinely reviewed a dedicated prostate intensity-modulated radiation therapy (IMRT) delivery program. Previously, a retrospective review of our experience demonstrated that a larger bladder volume reduced radiation dose to the rectum. We conducted an observational study to confirm this relationship.
Methods Men receiving definitive radiation for prostate cancer were eligible for the study. Eligible patients received 2 computed axial tomography (CT) scans on the day of their planning CT scan: 1 with a full bladder and 1 with an empty bladder. On each CT data set, the prostate, rectum, bladder, penile bulb, and femoral heads were contoured. 2 IMRT plans were completed on each dataset: 1 by a medical dosimetrist and 1 by a medical physicist. The study plans targeted the prostate to 79.2 Gray (Gy) while respecting predefined dose tolerances to the other contoured structures. Rectal doses were compared on empty and full bladder CT data sets.
Results From June 29, 2010 to December 14, 2011, 17 full bladder data sets and 15 empty bladder data sets were available for analysis. Median change in bladder volume was 63 ml. Full vs empty bladder set-up was associated with a statistically significant reduction in the mean rectal dose of 25.41 Gy vs 27.6 Gy (P = .031).
Limitations Small sample size and small variations in bladder volumes.
Conclusions A greater bladder volume resulted in a reduced mean dose to the rectum irrespective of planning method.
Funding/sponsorship None
Click on the PDF icon at the top of this introduction to read the full article.
Lung cancer in HIV-infected patients and the role of targeted therapy
Lung cancer is one of the most common malignancies in HIV-infected patients. Prevalence and mortality outcomes are higher in HIV-infected populations than in noninfected patients. There are several oral agents available for patients who harbor specific mutations, but little is known about mutations and affected pathways in HIV-infected patients with lung cancer. Recent trials have facilitated the inclusion of HIV-infected patients in clinical trials, but the population is remains underrepresented in oncology trials. Here, we review the literature on lung cancer in HIV-infected patients, and discuss common mutations in lung cancer and HIV-infected patients, the role of mutational analysis, and the potential role of targeted therapy in the treatment of lung cancer in HIV-infected populations.
Click on the PDF icon at the top of this introduction to read the full article.
Lung cancer is one of the most common malignancies in HIV-infected patients. Prevalence and mortality outcomes are higher in HIV-infected populations than in noninfected patients. There are several oral agents available for patients who harbor specific mutations, but little is known about mutations and affected pathways in HIV-infected patients with lung cancer. Recent trials have facilitated the inclusion of HIV-infected patients in clinical trials, but the population is remains underrepresented in oncology trials. Here, we review the literature on lung cancer in HIV-infected patients, and discuss common mutations in lung cancer and HIV-infected patients, the role of mutational analysis, and the potential role of targeted therapy in the treatment of lung cancer in HIV-infected populations.
Click on the PDF icon at the top of this introduction to read the full article.
Lung cancer is one of the most common malignancies in HIV-infected patients. Prevalence and mortality outcomes are higher in HIV-infected populations than in noninfected patients. There are several oral agents available for patients who harbor specific mutations, but little is known about mutations and affected pathways in HIV-infected patients with lung cancer. Recent trials have facilitated the inclusion of HIV-infected patients in clinical trials, but the population is remains underrepresented in oncology trials. Here, we review the literature on lung cancer in HIV-infected patients, and discuss common mutations in lung cancer and HIV-infected patients, the role of mutational analysis, and the potential role of targeted therapy in the treatment of lung cancer in HIV-infected populations.
Click on the PDF icon at the top of this introduction to read the full article.
FDA expands use of eltrombopag

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved an expanded use for eltrombopag (Promacta) to include children 1 year of age and older with chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
The updated label also includes a new oral suspension formulation of eltrombopag designed for younger children who may not be able to swallow tablets.
Eltrombopag was previously approved by the FDA in a tablet formulation in June 2015 for ITP patients ages 6 and older and in 2008 for use in adults with ITP.
The label expansion of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT or AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient (1%).
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study.
Prescribing information
The recommended dose and schedule of eltrombopag for pediatric patients age 6 and older is 50 mg daily or 25 mg daily of the tablet formulation for patients with East Asian ancestry. The recommended dose for all patients age 1 to 5 years is 25 mg daily of the powder for oral suspension formulation.
Eltrombopag is marketed as Promacta in the US and Revolade in most other countries. For more information on the drug, see the full prescribing information. ![]()

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved an expanded use for eltrombopag (Promacta) to include children 1 year of age and older with chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
The updated label also includes a new oral suspension formulation of eltrombopag designed for younger children who may not be able to swallow tablets.
Eltrombopag was previously approved by the FDA in a tablet formulation in June 2015 for ITP patients ages 6 and older and in 2008 for use in adults with ITP.
The label expansion of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT or AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient (1%).
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study.
Prescribing information
The recommended dose and schedule of eltrombopag for pediatric patients age 6 and older is 50 mg daily or 25 mg daily of the tablet formulation for patients with East Asian ancestry. The recommended dose for all patients age 1 to 5 years is 25 mg daily of the powder for oral suspension formulation.
Eltrombopag is marketed as Promacta in the US and Revolade in most other countries. For more information on the drug, see the full prescribing information. ![]()

Photo courtesy of GSK
The US Food and Drug Administration (FDA) has approved an expanded use for eltrombopag (Promacta) to include children 1 year of age and older with chronic immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
The updated label also includes a new oral suspension formulation of eltrombopag designed for younger children who may not be able to swallow tablets.
Eltrombopag was previously approved by the FDA in a tablet formulation in June 2015 for ITP patients ages 6 and older and in 2008 for use in adults with ITP.
The label expansion of eltrombopag was based on data from 2 double-blind, placebo-controlled trials—the phase 2 PETIT trial and the phase 3 PETIT2 trial.
PETIT trials: Efficacy
The PETIT trial included 67 ITP patients stratified by age cohort (12-17 years, 6-11 years, and 1-5 years). They were randomized (2:1) to receive eltrombopag or placebo for 7 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects achieving platelet counts of 50 x 109/L or higher at least once between days 8 and 43 of the randomized period of the study.
Significantly more patients in the eltrombopag arm met this endpoint—62.2%—compared to 31.8% in the placebo arm (P=0.011).
The PETIT2 trial enrolled 92 patients with chronic ITP who were randomized (2:1) to receive eltrombopag or placebo for 13 weeks. The eltrombopag dose was titrated to a target platelet count of 50-200 x 109/L.
The primary efficacy endpoint was the proportion of subjects who achieved platelet counts of 50 x 109/L or higher for at least 6 out of 8 weeks, between weeks 5 and 12 of the randomized period.
Significantly more patients in the eltrombopag arm met this endpoint—41.3%—compared to 3.4% of patients in the placebo arm (P<0.001).
PETIT trials: Safety
For both trials, there were 107 eltrombopag-treated patients evaluable for safety.
The most common adverse events that occurred more frequently in the eltrombopag arms than the placebo arms were upper respiratory tract infection, nasopharyngitis, cough, diarrhea, pyrexia, rhinitis, abdominal pain, oropharyngeal pain, toothache, increased ALT or AST, rash, and rhinorrhea.
Serious adverse events were reported in 8% of patients during the randomized part of both trials, although no serious adverse event occurred in more than 1 patient (1%).
An ALT elevation of at least 3 times the upper limit of normal occurred in 5% of eltrombopag-treated patients. Of those patients, 2% had ALT increases of at least 5 times the upper limit of normal.
There were no deaths or thromboembolic events during either study.
Prescribing information
The recommended dose and schedule of eltrombopag for pediatric patients age 6 and older is 50 mg daily or 25 mg daily of the tablet formulation for patients with East Asian ancestry. The recommended dose for all patients age 1 to 5 years is 25 mg daily of the powder for oral suspension formulation.
Eltrombopag is marketed as Promacta in the US and Revolade in most other countries. For more information on the drug, see the full prescribing information. ![]()
Enzyme may be target for malaria, toxoplasmosis
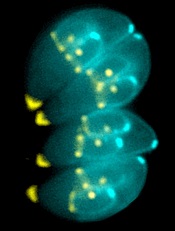
Image by Ke Hu & John Murray
Researchers say they have determined the structure of an enzyme that is vital to the infectious behavior of the parasites that cause toxoplasmosis and malaria.
And this has revealed a potentially druggable target that could prevent the parasites from entering and exiting host cells.
Sebastian Lourido, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues described this work in PNAS.
The researchers noted that the toxoplasmosis-causing parasite Toxoplasma gondii is closely related to the malaria-causing Plasmodium parasites. So research on T gondii can provide insights into Plasmodium’s inner workings.
For this study, Dr Lourido and his colleagues wanted to learn more about calcium-dependent protein kinases (CDPKs), enzymes that are needed for T gondii and related parasites to invade and exit host cells, move, and reproduce.
To investigate CDPKs, the team used single-domain antibody fragments derived from alpacas. Unlike humans, whose antibodies have a heavy chain and a light chain, alpacas create heavy-chain-only antibodies, which can be engineered into even smaller antibody fragments known as nanobodies.
Alpaca nanobodies have a unique shape that allows them to reach into a protein’s nooks and crannies, which are inaccessible to conventional antibodies.
The researchers identified a nanobody against the T gondii enzyme CDPK1 that binds the kinase’s regulatory domain and revealed a previously unappreciated feature of its activation.
The nanobody, called 1B7, stabilizes CDPK1 in a conformation that allowed the researchers to determine the kinase’s structure and describe the nanobody’s interaction with the molecule.
With the structure in hand, the team created long-timescale molecular dynamics simulations of the enzyme, to model the events leading to kinase inactivation.
Structural homology between CDPKs and the calmodulin-dependent kinases (CaMKs) found in humans led to earlier assumptions that both types of enzymes are activated in a similar fashion. But this new work shows otherwise.
A CaMK is activated when a wedge holding it in an inactive state is knocked away. In contrast, Dr Lourido likened a CDPK’s active conformation to a broken arm that must be splinted in two places to maintain its integrity.
When the rigid splint is removed, the kinase loses its structural ability to function. By blocking CDPK1’s regulatory domain, the 1B7 nanobody inhibits the kinase by preventing the enzyme’s “splint” from attaching.
“This work reveals something interesting about this class of enzymes,” Dr Lourido said. “It’s the first time a calcium-regulated kinase has been shown to be activated in this manner. The principle that we identify is really important. We’ve found a new vulnerability within an enzyme that we know is extremely important to this class of parasites, including Plasmodium . . . , and is absent from humans.”
Because humans lack similar kinases, drugs that target CDPKs would not affect host cells.
“The location where 1B7 binds to CDPK1 is a new drug target that people had not considered before,” said study author Jessica Ingram, PhD, also of the Whitehead Institute for Biomedical Research.
“We’d like to do some drug screens in the presence of the nanobody to see if we can find small molecules that bind in the same way. We could also look at other nanobodies against other kinases to see if this is applicable to other parasites and systems.” ![]()

Image by Ke Hu & John Murray
Researchers say they have determined the structure of an enzyme that is vital to the infectious behavior of the parasites that cause toxoplasmosis and malaria.
And this has revealed a potentially druggable target that could prevent the parasites from entering and exiting host cells.
Sebastian Lourido, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues described this work in PNAS.
The researchers noted that the toxoplasmosis-causing parasite Toxoplasma gondii is closely related to the malaria-causing Plasmodium parasites. So research on T gondii can provide insights into Plasmodium’s inner workings.
For this study, Dr Lourido and his colleagues wanted to learn more about calcium-dependent protein kinases (CDPKs), enzymes that are needed for T gondii and related parasites to invade and exit host cells, move, and reproduce.
To investigate CDPKs, the team used single-domain antibody fragments derived from alpacas. Unlike humans, whose antibodies have a heavy chain and a light chain, alpacas create heavy-chain-only antibodies, which can be engineered into even smaller antibody fragments known as nanobodies.
Alpaca nanobodies have a unique shape that allows them to reach into a protein’s nooks and crannies, which are inaccessible to conventional antibodies.
The researchers identified a nanobody against the T gondii enzyme CDPK1 that binds the kinase’s regulatory domain and revealed a previously unappreciated feature of its activation.
The nanobody, called 1B7, stabilizes CDPK1 in a conformation that allowed the researchers to determine the kinase’s structure and describe the nanobody’s interaction with the molecule.
With the structure in hand, the team created long-timescale molecular dynamics simulations of the enzyme, to model the events leading to kinase inactivation.
Structural homology between CDPKs and the calmodulin-dependent kinases (CaMKs) found in humans led to earlier assumptions that both types of enzymes are activated in a similar fashion. But this new work shows otherwise.
A CaMK is activated when a wedge holding it in an inactive state is knocked away. In contrast, Dr Lourido likened a CDPK’s active conformation to a broken arm that must be splinted in two places to maintain its integrity.
When the rigid splint is removed, the kinase loses its structural ability to function. By blocking CDPK1’s regulatory domain, the 1B7 nanobody inhibits the kinase by preventing the enzyme’s “splint” from attaching.
“This work reveals something interesting about this class of enzymes,” Dr Lourido said. “It’s the first time a calcium-regulated kinase has been shown to be activated in this manner. The principle that we identify is really important. We’ve found a new vulnerability within an enzyme that we know is extremely important to this class of parasites, including Plasmodium . . . , and is absent from humans.”
Because humans lack similar kinases, drugs that target CDPKs would not affect host cells.
“The location where 1B7 binds to CDPK1 is a new drug target that people had not considered before,” said study author Jessica Ingram, PhD, also of the Whitehead Institute for Biomedical Research.
“We’d like to do some drug screens in the presence of the nanobody to see if we can find small molecules that bind in the same way. We could also look at other nanobodies against other kinases to see if this is applicable to other parasites and systems.” ![]()

Image by Ke Hu & John Murray
Researchers say they have determined the structure of an enzyme that is vital to the infectious behavior of the parasites that cause toxoplasmosis and malaria.
And this has revealed a potentially druggable target that could prevent the parasites from entering and exiting host cells.
Sebastian Lourido, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues described this work in PNAS.
The researchers noted that the toxoplasmosis-causing parasite Toxoplasma gondii is closely related to the malaria-causing Plasmodium parasites. So research on T gondii can provide insights into Plasmodium’s inner workings.
For this study, Dr Lourido and his colleagues wanted to learn more about calcium-dependent protein kinases (CDPKs), enzymes that are needed for T gondii and related parasites to invade and exit host cells, move, and reproduce.
To investigate CDPKs, the team used single-domain antibody fragments derived from alpacas. Unlike humans, whose antibodies have a heavy chain and a light chain, alpacas create heavy-chain-only antibodies, which can be engineered into even smaller antibody fragments known as nanobodies.
Alpaca nanobodies have a unique shape that allows them to reach into a protein’s nooks and crannies, which are inaccessible to conventional antibodies.
The researchers identified a nanobody against the T gondii enzyme CDPK1 that binds the kinase’s regulatory domain and revealed a previously unappreciated feature of its activation.
The nanobody, called 1B7, stabilizes CDPK1 in a conformation that allowed the researchers to determine the kinase’s structure and describe the nanobody’s interaction with the molecule.
With the structure in hand, the team created long-timescale molecular dynamics simulations of the enzyme, to model the events leading to kinase inactivation.
Structural homology between CDPKs and the calmodulin-dependent kinases (CaMKs) found in humans led to earlier assumptions that both types of enzymes are activated in a similar fashion. But this new work shows otherwise.
A CaMK is activated when a wedge holding it in an inactive state is knocked away. In contrast, Dr Lourido likened a CDPK’s active conformation to a broken arm that must be splinted in two places to maintain its integrity.
When the rigid splint is removed, the kinase loses its structural ability to function. By blocking CDPK1’s regulatory domain, the 1B7 nanobody inhibits the kinase by preventing the enzyme’s “splint” from attaching.
“This work reveals something interesting about this class of enzymes,” Dr Lourido said. “It’s the first time a calcium-regulated kinase has been shown to be activated in this manner. The principle that we identify is really important. We’ve found a new vulnerability within an enzyme that we know is extremely important to this class of parasites, including Plasmodium . . . , and is absent from humans.”
Because humans lack similar kinases, drugs that target CDPKs would not affect host cells.
“The location where 1B7 binds to CDPK1 is a new drug target that people had not considered before,” said study author Jessica Ingram, PhD, also of the Whitehead Institute for Biomedical Research.
“We’d like to do some drug screens in the presence of the nanobody to see if we can find small molecules that bind in the same way. We could also look at other nanobodies against other kinases to see if this is applicable to other parasites and systems.” ![]()
Drug gets orphan designation for CDI
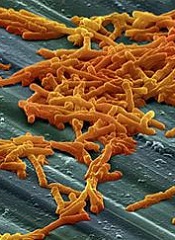
The US Food and Drug Administration (FDA) has granted orphan designation to SER-109 for the prevention of recurrent Clostridium difficile infection (CDI) in adults.
SER-109 is a microbiome therapeutic designed to treat recurrent CDI by correcting dysbiosis of the human microbiome.
In a single dose of 4 capsules, SER-109 re-introduces an ecology of purified bacterial spores that should restore the microbiome to a healthy state, allowing it to carry out key biological functions, including resisting Clostridium difficile.
“SER-109 is intended to re-introduce essential bacteria that restore the body’s natural resistance to CDI by re-establishing the ecology of the colonic microbiome,” explained Roger Pomerantz, MD, of Seres Therapeutics, Inc., the company developing SER-109.
“Because we’re focused on treating the underlying cause of the disease, we believe we have the potential to break the cycle of recurrent CDI and have a significant impact for patients.”
SER-109 is currently being investigated in a phase 2 trial. In addition to orphan designation, SER-109 has breakthrough designation from the FDA.
Trials of SER-109
Researchers reported phase 1/2 results with SER-109 at the 2014 Interscience Conference on Antimicrobial Agents and Chemotherapy.
The study had 2 cohorts containing 15 patients each. Patients were between 18 and 90 years old, had 3 or more laboratory-confirmed CDI episodes over 1 year, had a life expectancy greater than 3 months, and were able to give informed consent.
Patients in cohort 1 received a mean SER-109 dose of 1.5 x 109 spores, and those in cohort 2 received a mean dose of 1 x 108 spores. SER-109 was deemed effective if patients did not have a CDI recurrence in the 8-week period after they received SER-109.
In cohort 1, 87% of patients (13/15) achieved the efficacy endpoint. Two patients had transient, self-limited diarrhea with a positive C difficile test, but both reached the week 8 endpoint without needing antibiotic therapy for CDI. Thus, in cohort 1, the clinical cure rate was 100%.
In cohort 2, 93% of patients (14/15) reached the 8-week endpoint CDI-free. One patient failed per protocol.
The researchers said there were no drug-related serious adverse events in this trial.
Seres Therapeutics is currently conducting a multicenter, randomized, placebo-controlled, phase 2 study (ECOSPOR) to assess the efficacy and safety of SER-109 in preventing recurrent CDI. The company expects results from this study to be available mid-2016.
About orphan and breakthrough designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
The benefits of breakthrough designation include the same benefits as fast track designation—priority review of a new drug application, rolling review, etc.—plus an organizational commitment involving the FDA’s senior managers with more intensive guidance from the FDA. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation to SER-109 for the prevention of recurrent Clostridium difficile infection (CDI) in adults.
SER-109 is a microbiome therapeutic designed to treat recurrent CDI by correcting dysbiosis of the human microbiome.
In a single dose of 4 capsules, SER-109 re-introduces an ecology of purified bacterial spores that should restore the microbiome to a healthy state, allowing it to carry out key biological functions, including resisting Clostridium difficile.
“SER-109 is intended to re-introduce essential bacteria that restore the body’s natural resistance to CDI by re-establishing the ecology of the colonic microbiome,” explained Roger Pomerantz, MD, of Seres Therapeutics, Inc., the company developing SER-109.
“Because we’re focused on treating the underlying cause of the disease, we believe we have the potential to break the cycle of recurrent CDI and have a significant impact for patients.”
SER-109 is currently being investigated in a phase 2 trial. In addition to orphan designation, SER-109 has breakthrough designation from the FDA.
Trials of SER-109
Researchers reported phase 1/2 results with SER-109 at the 2014 Interscience Conference on Antimicrobial Agents and Chemotherapy.
The study had 2 cohorts containing 15 patients each. Patients were between 18 and 90 years old, had 3 or more laboratory-confirmed CDI episodes over 1 year, had a life expectancy greater than 3 months, and were able to give informed consent.
Patients in cohort 1 received a mean SER-109 dose of 1.5 x 109 spores, and those in cohort 2 received a mean dose of 1 x 108 spores. SER-109 was deemed effective if patients did not have a CDI recurrence in the 8-week period after they received SER-109.
In cohort 1, 87% of patients (13/15) achieved the efficacy endpoint. Two patients had transient, self-limited diarrhea with a positive C difficile test, but both reached the week 8 endpoint without needing antibiotic therapy for CDI. Thus, in cohort 1, the clinical cure rate was 100%.
In cohort 2, 93% of patients (14/15) reached the 8-week endpoint CDI-free. One patient failed per protocol.
The researchers said there were no drug-related serious adverse events in this trial.
Seres Therapeutics is currently conducting a multicenter, randomized, placebo-controlled, phase 2 study (ECOSPOR) to assess the efficacy and safety of SER-109 in preventing recurrent CDI. The company expects results from this study to be available mid-2016.
About orphan and breakthrough designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
The benefits of breakthrough designation include the same benefits as fast track designation—priority review of a new drug application, rolling review, etc.—plus an organizational commitment involving the FDA’s senior managers with more intensive guidance from the FDA. ![]()

The US Food and Drug Administration (FDA) has granted orphan designation to SER-109 for the prevention of recurrent Clostridium difficile infection (CDI) in adults.
SER-109 is a microbiome therapeutic designed to treat recurrent CDI by correcting dysbiosis of the human microbiome.
In a single dose of 4 capsules, SER-109 re-introduces an ecology of purified bacterial spores that should restore the microbiome to a healthy state, allowing it to carry out key biological functions, including resisting Clostridium difficile.
“SER-109 is intended to re-introduce essential bacteria that restore the body’s natural resistance to CDI by re-establishing the ecology of the colonic microbiome,” explained Roger Pomerantz, MD, of Seres Therapeutics, Inc., the company developing SER-109.
“Because we’re focused on treating the underlying cause of the disease, we believe we have the potential to break the cycle of recurrent CDI and have a significant impact for patients.”
SER-109 is currently being investigated in a phase 2 trial. In addition to orphan designation, SER-109 has breakthrough designation from the FDA.
Trials of SER-109
Researchers reported phase 1/2 results with SER-109 at the 2014 Interscience Conference on Antimicrobial Agents and Chemotherapy.
The study had 2 cohorts containing 15 patients each. Patients were between 18 and 90 years old, had 3 or more laboratory-confirmed CDI episodes over 1 year, had a life expectancy greater than 3 months, and were able to give informed consent.
Patients in cohort 1 received a mean SER-109 dose of 1.5 x 109 spores, and those in cohort 2 received a mean dose of 1 x 108 spores. SER-109 was deemed effective if patients did not have a CDI recurrence in the 8-week period after they received SER-109.
In cohort 1, 87% of patients (13/15) achieved the efficacy endpoint. Two patients had transient, self-limited diarrhea with a positive C difficile test, but both reached the week 8 endpoint without needing antibiotic therapy for CDI. Thus, in cohort 1, the clinical cure rate was 100%.
In cohort 2, 93% of patients (14/15) reached the 8-week endpoint CDI-free. One patient failed per protocol.
The researchers said there were no drug-related serious adverse events in this trial.
Seres Therapeutics is currently conducting a multicenter, randomized, placebo-controlled, phase 2 study (ECOSPOR) to assess the efficacy and safety of SER-109 in preventing recurrent CDI. The company expects results from this study to be available mid-2016.
About orphan and breakthrough designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US.
Orphan designation provides the sponsor of a drug with various development incentives, including opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, and 7 years of US marketing exclusivity if the drug is approved.
The FDA’s breakthrough therapy designation is intended to expedite the development and review of a drug candidate intended to treat a serious or life-threatening condition.
The benefits of breakthrough designation include the same benefits as fast track designation—priority review of a new drug application, rolling review, etc.—plus an organizational commitment involving the FDA’s senior managers with more intensive guidance from the FDA. ![]()
BET inhibitor appears to cause memory loss in mice

Photo by Aaron Logan
New research suggests the BET inhibitor JQ1 causes molecular changes in mouse neurons and can lead to memory loss in mice.
Investigators believe this discovery, published in Nature Neuroscience, will fuel more research into the neurological effects of BET inhibitors, which are currently under development as potential treatments for a range of hematologic and solid tumor malignancies.
The researchers noted that, although JQ1 has the ability to cross the blood-brain barrier, this may not be the case for other BET inhibitors.
Several companies are testing the inhibitors using unique formulations they’ve optimized in proprietary ways—for example, by adding chemical groups to make a compound more targeted or effective—which might make it more difficult for the drug to cross the blood-brain barrier.
Still, the investigators said their findings suggests more research is needed to determine whether other BET inhibitors can enter the brain, since that could potentially cause unwanted side effects.
“We found that if a drug blocks a BET protein throughout the body, and that drug can get into the brain, you could very well produce neurological side effects,” said study author Erica Korb, PhD, of The Rockefeller University in New York, New York.
Experiments with JQ1
To assess the effects of BET inhibitors on the brain, the researchers used a compound that was designed to thwart the activity of a specific BET protein, Brd4. They used the drug JQ1, which they knew could cross the blood-brain barrier.
The investigators added the drug to mouse neurons grown in the lab, then stimulated the cells in a way that mimicked the process of memory formation. Normally, when neurons receive this type of signal, they begin transcribing genes into proteins, resulting in the formation of new memories—a process that is partly regulated by Brd4.
“To turn a recent experience into a long-term memory, you need to have gene transcription in response to these extracellular signals,” Dr Korb said.
Indeed, when the researchers stimulated mouse neurons with signals that mimicked those they would normally receive in the brain, there were “massive changes” in gene transcription. But when the team performed this experiment after adding JQ1, they saw much less activity.
“After administering a Brd4 inhibitor, we no longer saw those changes in transcription after stimuli,” Dr Korb said.
To test how the drug affected the animals’ memories, the investigators placed the mice in a box with two objects they had never seen before, such as pieces of Lego or tiny figurines. Mice typically explore anything unfamiliar, climbing and sniffing around.
After a few minutes, the researchers took the mice out of the box. One day later, the team put the mice back in, this time with one of the objects from the day before and another, unfamiliar one.
Mice that received a placebo were much more interested in the new object, presumably because the one from the day before was familiar. But mice treated with JQ1 were equally interested in both objects, suggesting they didn’t remember the previous day’s experience.
Next, the investigators took their findings a step further. If JQ1 reduces molecular activity in the brain, they wondered if it could help in conditions marked by too much brain activity, such as epilepsy.
Brd4 regulates a receptor protein present at the synapse, a structure where two neurons connect and transmit signals. When the researchers administered the Brd4 inhibitor, they saw decreased levels of that receptor, and neurons fired much less frequently.
Next, the team gave the drug to mice for a week, then added a chemical that induces seizures. Mice that received JQ1 had a much lower rate of seizures than mice given a placebo.
“In the case of the epileptic brain, when there’s too much activity and neurons talking to each other, this drug could be potentially be beneficial,” Dr Korb concluded. ![]()

Photo by Aaron Logan
New research suggests the BET inhibitor JQ1 causes molecular changes in mouse neurons and can lead to memory loss in mice.
Investigators believe this discovery, published in Nature Neuroscience, will fuel more research into the neurological effects of BET inhibitors, which are currently under development as potential treatments for a range of hematologic and solid tumor malignancies.
The researchers noted that, although JQ1 has the ability to cross the blood-brain barrier, this may not be the case for other BET inhibitors.
Several companies are testing the inhibitors using unique formulations they’ve optimized in proprietary ways—for example, by adding chemical groups to make a compound more targeted or effective—which might make it more difficult for the drug to cross the blood-brain barrier.
Still, the investigators said their findings suggests more research is needed to determine whether other BET inhibitors can enter the brain, since that could potentially cause unwanted side effects.
“We found that if a drug blocks a BET protein throughout the body, and that drug can get into the brain, you could very well produce neurological side effects,” said study author Erica Korb, PhD, of The Rockefeller University in New York, New York.
Experiments with JQ1
To assess the effects of BET inhibitors on the brain, the researchers used a compound that was designed to thwart the activity of a specific BET protein, Brd4. They used the drug JQ1, which they knew could cross the blood-brain barrier.
The investigators added the drug to mouse neurons grown in the lab, then stimulated the cells in a way that mimicked the process of memory formation. Normally, when neurons receive this type of signal, they begin transcribing genes into proteins, resulting in the formation of new memories—a process that is partly regulated by Brd4.
“To turn a recent experience into a long-term memory, you need to have gene transcription in response to these extracellular signals,” Dr Korb said.
Indeed, when the researchers stimulated mouse neurons with signals that mimicked those they would normally receive in the brain, there were “massive changes” in gene transcription. But when the team performed this experiment after adding JQ1, they saw much less activity.
“After administering a Brd4 inhibitor, we no longer saw those changes in transcription after stimuli,” Dr Korb said.
To test how the drug affected the animals’ memories, the investigators placed the mice in a box with two objects they had never seen before, such as pieces of Lego or tiny figurines. Mice typically explore anything unfamiliar, climbing and sniffing around.
After a few minutes, the researchers took the mice out of the box. One day later, the team put the mice back in, this time with one of the objects from the day before and another, unfamiliar one.
Mice that received a placebo were much more interested in the new object, presumably because the one from the day before was familiar. But mice treated with JQ1 were equally interested in both objects, suggesting they didn’t remember the previous day’s experience.
Next, the investigators took their findings a step further. If JQ1 reduces molecular activity in the brain, they wondered if it could help in conditions marked by too much brain activity, such as epilepsy.
Brd4 regulates a receptor protein present at the synapse, a structure where two neurons connect and transmit signals. When the researchers administered the Brd4 inhibitor, they saw decreased levels of that receptor, and neurons fired much less frequently.
Next, the team gave the drug to mice for a week, then added a chemical that induces seizures. Mice that received JQ1 had a much lower rate of seizures than mice given a placebo.
“In the case of the epileptic brain, when there’s too much activity and neurons talking to each other, this drug could be potentially be beneficial,” Dr Korb concluded. ![]()

Photo by Aaron Logan
New research suggests the BET inhibitor JQ1 causes molecular changes in mouse neurons and can lead to memory loss in mice.
Investigators believe this discovery, published in Nature Neuroscience, will fuel more research into the neurological effects of BET inhibitors, which are currently under development as potential treatments for a range of hematologic and solid tumor malignancies.
The researchers noted that, although JQ1 has the ability to cross the blood-brain barrier, this may not be the case for other BET inhibitors.
Several companies are testing the inhibitors using unique formulations they’ve optimized in proprietary ways—for example, by adding chemical groups to make a compound more targeted or effective—which might make it more difficult for the drug to cross the blood-brain barrier.
Still, the investigators said their findings suggests more research is needed to determine whether other BET inhibitors can enter the brain, since that could potentially cause unwanted side effects.
“We found that if a drug blocks a BET protein throughout the body, and that drug can get into the brain, you could very well produce neurological side effects,” said study author Erica Korb, PhD, of The Rockefeller University in New York, New York.
Experiments with JQ1
To assess the effects of BET inhibitors on the brain, the researchers used a compound that was designed to thwart the activity of a specific BET protein, Brd4. They used the drug JQ1, which they knew could cross the blood-brain barrier.
The investigators added the drug to mouse neurons grown in the lab, then stimulated the cells in a way that mimicked the process of memory formation. Normally, when neurons receive this type of signal, they begin transcribing genes into proteins, resulting in the formation of new memories—a process that is partly regulated by Brd4.
“To turn a recent experience into a long-term memory, you need to have gene transcription in response to these extracellular signals,” Dr Korb said.
Indeed, when the researchers stimulated mouse neurons with signals that mimicked those they would normally receive in the brain, there were “massive changes” in gene transcription. But when the team performed this experiment after adding JQ1, they saw much less activity.
“After administering a Brd4 inhibitor, we no longer saw those changes in transcription after stimuli,” Dr Korb said.
To test how the drug affected the animals’ memories, the investigators placed the mice in a box with two objects they had never seen before, such as pieces of Lego or tiny figurines. Mice typically explore anything unfamiliar, climbing and sniffing around.
After a few minutes, the researchers took the mice out of the box. One day later, the team put the mice back in, this time with one of the objects from the day before and another, unfamiliar one.
Mice that received a placebo were much more interested in the new object, presumably because the one from the day before was familiar. But mice treated with JQ1 were equally interested in both objects, suggesting they didn’t remember the previous day’s experience.
Next, the investigators took their findings a step further. If JQ1 reduces molecular activity in the brain, they wondered if it could help in conditions marked by too much brain activity, such as epilepsy.
Brd4 regulates a receptor protein present at the synapse, a structure where two neurons connect and transmit signals. When the researchers administered the Brd4 inhibitor, they saw decreased levels of that receptor, and neurons fired much less frequently.
Next, the team gave the drug to mice for a week, then added a chemical that induces seizures. Mice that received JQ1 had a much lower rate of seizures than mice given a placebo.
“In the case of the epileptic brain, when there’s too much activity and neurons talking to each other, this drug could be potentially be beneficial,” Dr Korb concluded. ![]()
FDA expands Promacta approval to include pediatric patients
The Food and Drug Administration has extended its approval of Promacta (eltrombopag) to include pediatric patients with chronic immune thrombocytopenic purpura, the agency announced Aug. 24.
Approved in 2008 for adults with immune thrombocytopenic purpura (ITP), eltrombopag is now approved for the treatment of the rare blood disorder in patients aged 1 year and older. The drug may be used in children who have not responded to other ITP medications or spleen surgery, the FDA said in a statement.
Eltrombopag’s efficacy and safety in children aged 1-17 years was established in two placebo-controlled trials comprising 159 patients. Findings from the first trial found that over 7 weeks, 62% of patients given eltrombopag had improved platelet counts without rescue therapy between weeks 1 and 6, compared with 32% in the placebo group.
In the second trial, which lasted 13 weeks, 41% of patients taking eltrombopag experienced increased platelet counts for at least 6 out of 8 weeks between weeks 5 and 12, compared with 3% of patients in the placebo group, the FDA reported.
“In both trials, patients taking Promacta also had less need for other treatments to increase their platelet counts, such as corticosteroids or platelet transfusions,” the FDA said. “Among patients taking one or more ITP medications at the start of the trials, about half were able to reduce or discontinue their use of these medications, primarily corticosteroids.”
Eltrombopag may be taken once daily in tablet form, or as a powder mixed with liquid for children aged 1-5 years. It should be used only in ITP patients with an increased risk of bleeding.
The most common side effects in children were infections of the upper respiratory tract or nose and throat, diarrhea, abdominal pain, rash, and increase in liver enzymes.
Promacta is manufactured by Novartis in East Hanover, N.J.
The Food and Drug Administration has extended its approval of Promacta (eltrombopag) to include pediatric patients with chronic immune thrombocytopenic purpura, the agency announced Aug. 24.
Approved in 2008 for adults with immune thrombocytopenic purpura (ITP), eltrombopag is now approved for the treatment of the rare blood disorder in patients aged 1 year and older. The drug may be used in children who have not responded to other ITP medications or spleen surgery, the FDA said in a statement.
Eltrombopag’s efficacy and safety in children aged 1-17 years was established in two placebo-controlled trials comprising 159 patients. Findings from the first trial found that over 7 weeks, 62% of patients given eltrombopag had improved platelet counts without rescue therapy between weeks 1 and 6, compared with 32% in the placebo group.
In the second trial, which lasted 13 weeks, 41% of patients taking eltrombopag experienced increased platelet counts for at least 6 out of 8 weeks between weeks 5 and 12, compared with 3% of patients in the placebo group, the FDA reported.
“In both trials, patients taking Promacta also had less need for other treatments to increase their platelet counts, such as corticosteroids or platelet transfusions,” the FDA said. “Among patients taking one or more ITP medications at the start of the trials, about half were able to reduce or discontinue their use of these medications, primarily corticosteroids.”
Eltrombopag may be taken once daily in tablet form, or as a powder mixed with liquid for children aged 1-5 years. It should be used only in ITP patients with an increased risk of bleeding.
The most common side effects in children were infections of the upper respiratory tract or nose and throat, diarrhea, abdominal pain, rash, and increase in liver enzymes.
Promacta is manufactured by Novartis in East Hanover, N.J.
The Food and Drug Administration has extended its approval of Promacta (eltrombopag) to include pediatric patients with chronic immune thrombocytopenic purpura, the agency announced Aug. 24.
Approved in 2008 for adults with immune thrombocytopenic purpura (ITP), eltrombopag is now approved for the treatment of the rare blood disorder in patients aged 1 year and older. The drug may be used in children who have not responded to other ITP medications or spleen surgery, the FDA said in a statement.
Eltrombopag’s efficacy and safety in children aged 1-17 years was established in two placebo-controlled trials comprising 159 patients. Findings from the first trial found that over 7 weeks, 62% of patients given eltrombopag had improved platelet counts without rescue therapy between weeks 1 and 6, compared with 32% in the placebo group.
In the second trial, which lasted 13 weeks, 41% of patients taking eltrombopag experienced increased platelet counts for at least 6 out of 8 weeks between weeks 5 and 12, compared with 3% of patients in the placebo group, the FDA reported.
“In both trials, patients taking Promacta also had less need for other treatments to increase their platelet counts, such as corticosteroids or platelet transfusions,” the FDA said. “Among patients taking one or more ITP medications at the start of the trials, about half were able to reduce or discontinue their use of these medications, primarily corticosteroids.”
Eltrombopag may be taken once daily in tablet form, or as a powder mixed with liquid for children aged 1-5 years. It should be used only in ITP patients with an increased risk of bleeding.
The most common side effects in children were infections of the upper respiratory tract or nose and throat, diarrhea, abdominal pain, rash, and increase in liver enzymes.
Promacta is manufactured by Novartis in East Hanover, N.J.
Plasma Tau Level Is Chronically Elevated in TBI
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury (TBI) and appear to correlate with the severity of postconcussive symptoms, according to a report published online ahead of print August 3 in JAMA Neurology.
If these findings are confirmed, tau may be the first biomarker that is sensitive and specific to persistent TBI-related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood–brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” said Anlys Olivera, PhD, of the National Institute of Nursing Research in Bethesda, Maryland, and her associates.
Tau stabilizes the structure of the axonal cytoskeleton. It is elevated in the CSF and the peripheral blood (albeit in extremely low concentrations) of patients with severe TBI, professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a two-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 military control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all participants in the TBI group had been injured at least 18 months previously. The most common causes of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing number of TBIs, and with increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of post-traumatic stress disorder and depression, which were prevalent in the TBI group, the investigators said.
Tau is not only a marker of brain injury, it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the study limitations cited by the investigators are lack of neuroimaging and neuropsychologic data.
—Mary Ann Moon
Suggested Reading
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015 Aug 3 [Epub ahead of print].
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury (TBI) and appear to correlate with the severity of postconcussive symptoms, according to a report published online ahead of print August 3 in JAMA Neurology.
If these findings are confirmed, tau may be the first biomarker that is sensitive and specific to persistent TBI-related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood–brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” said Anlys Olivera, PhD, of the National Institute of Nursing Research in Bethesda, Maryland, and her associates.
Tau stabilizes the structure of the axonal cytoskeleton. It is elevated in the CSF and the peripheral blood (albeit in extremely low concentrations) of patients with severe TBI, professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a two-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 military control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all participants in the TBI group had been injured at least 18 months previously. The most common causes of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing number of TBIs, and with increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of post-traumatic stress disorder and depression, which were prevalent in the TBI group, the investigators said.
Tau is not only a marker of brain injury, it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the study limitations cited by the investigators are lack of neuroimaging and neuropsychologic data.
—Mary Ann Moon
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury (TBI) and appear to correlate with the severity of postconcussive symptoms, according to a report published online ahead of print August 3 in JAMA Neurology.
If these findings are confirmed, tau may be the first biomarker that is sensitive and specific to persistent TBI-related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood–brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” said Anlys Olivera, PhD, of the National Institute of Nursing Research in Bethesda, Maryland, and her associates.
Tau stabilizes the structure of the axonal cytoskeleton. It is elevated in the CSF and the peripheral blood (albeit in extremely low concentrations) of patients with severe TBI, professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a two-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 military control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all participants in the TBI group had been injured at least 18 months previously. The most common causes of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing number of TBIs, and with increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of post-traumatic stress disorder and depression, which were prevalent in the TBI group, the investigators said.
Tau is not only a marker of brain injury, it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the study limitations cited by the investigators are lack of neuroimaging and neuropsychologic data.
—Mary Ann Moon
Suggested Reading
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015 Aug 3 [Epub ahead of print].
Suggested Reading
Olivera A, Lejbman N, Jeromin A, et al. Peripheral total tau in military personnel who sustain traumatic brain injuries during deployment. JAMA Neurol. 2015 Aug 3 [Epub ahead of print].