User login
Azithromycin, a macrolide antibiotic, received US Food and Drug Administration (FDA) approval in 1991 and is 1 of the most prescribed antibiotics used for a variety of infections, including community‐acquired pneumonia, bacterial sinusitis, urethritis, and cervicitis. In 2011, it was estimated that 40.3 million outpatients received a prescription for azithromycin.[1] In addition to treating acute bacterial infections, recent literature has pointed to using azithromycin for its unlabeled immunomodulatory and anti‐inflammatory effects, particularly in cystic fibrosis, chronic obstructive pulmonary disease (COPD), and lung transplant recipients.[2, 3, 4] Azithromycin decreases bacterial load and virulence, thus reducing airway secretion, as well as decreasing airway neutrophil accumulation through a reduction in proinflammatory cytokine expression.[4]
Cardiac toxicity can occur with macrolide antibiotics, and prolongation of the QT interval with subsequent Torsades de pointes has been documented with azithromycin.[1, 5, 6] In 2012, Ray et al. published data on a cohort of outpatients receiving azithromycin compared to amoxicillin, ciprofloxacin, or no antibiotics, and showed a small but absolute increase in cardiovascular deaths.[7, 8] Subsequent data, however, have not illustrated increased risk of death from cardiovascular causes. Mortensen et al. showed a lower risk of 90‐day mortality in older patients treated for community acquired pneumonia with azithromycin and ceftriaxone, although there was a nonstatistically significant increased risk of myocardial infarction in this group.[8, 9, 10] In March 2013, the FDA released an official statement regarding increased cardiovascular risk with azithromycin, stating that healthcare professionals should consider the risk of fatal heart rhythms with azithromycin when considering treatment options for patients who are at risk for cardiovascular events.[11]
In recent years, the potential for corrected QT (QTc) prolongation and Torsades de pointes has received increased attention due to its catastrophic nature, and it is thought that hospitalized patients are at a greater risk of drug‐induced Torsades de pointes due to the likelihood of having more risk factors.[12, 13] The American Heart Association released a statement in 2010 to raise awareness among healthcare professionals about risk, electrocardiogram (ECG) monitoring, and management of drug‐induced QT interval prolongation in hospitalized patients, although little data exist regarding quantification of risk in this patient population.[13, 14]
Prescribers currently have no standardized practice guidelines related to cardiovascular safety when prescribing QTc prolonging medications. Given the dramatic increase in azithromycin prescriptions and ongoing concern for cardiovascular risk and QTc prolongation, we investigated the prescribing practices with azithromycin within our institution. Our primary aims were 3‐fold. First, we aimed to describe the frequency azithromycin was prescribed with additional QTc prolonging medications. Second, we assessed the relationship between the number of arrhythmogenic drugs prescribed in addition to azithromycin with ordering telemetry. Finally, we assessed the relationship between baseline ECG abnormalities and telemetry monitoring in patients prescribed azithromycin. The purpose of these objectives was to better understand physician prescribing practices and to determine if patients have a potential risk of developing fatal cardiac arrhythmias
METHODS
Data
For this retrospective review, we utilized data from the University of Alabama at Birmingham Health Care system, a 1157licensed bed hospital. The institutional review board approved this study with a waiver of informed consent. Patients were eligible to be included in this study if they were 19 years of age with an inpatient hospital length of stay 3 days. Patients were considered to be receiving azithromycin and were included only when they were dispensed 1 dose of azithromycin by the pharmacy. Between October 1, 2012 and April 30, 2013, 1610 encounters were identified, of which 100 patient encounters were randomly selected for evaluation via a Microsoft Excel (Microsoft Corp., Redmond, WA) function. One patient was randomly included twice in this study, but had 2 separate admissions in which he received azithromycin.
QTc prolonging medications in our hospital formulary were identified via Micromedex and package inserts (see Supporting Information, Appendix, in the online version of this article for the full list).
Measures
The primary study measures were number of medications associated with QTc prolongation, baseline ECG findings, and telemetry monitoring. Secondary study measures include indication, dose, duration of use, formulation, length of stay, and admitting service (Table 1). Indications, dosage, and duration were defined by the FDA package insert for azithromycin (see Supporting Information, Appendix, in the online version of this article). Indication for use was defined as (1) empiric for a specific infection; (2) anti‐inflammatory for patients with COPD, lung transplant recipients, or cystic fibrosis patients; and (3) culture proven if evidence of a particular pathogen grown on culture. Indications were defined by prescriber notes. Dosage is defined as appropriate if FDA guidelines were followed for the defined indication. If patients were given azithromycin for anti‐inflammatory purposes, dosing was considered appropriate if it followed previous literature dosing of 250mg daily.
| |
| Age, y | |
| Average | 5519.5 |
| Range | 2197 |
| Gender | |
| Female | 61% |
| Male | 39% |
| Length of stay, d | |
| Average | 9.713.1 |
| Range | 3115 |
| Admitting service | |
| Hospitalist | 37% |
| Pulmonary | 23% |
| Obstetrics | 9% |
| General medicine | 8% |
| Hematology/oncology | 6% |
| Othera | 17% |
| Days of therapy | |
| Average | 4.53.9 |
| Range | 128 |
| Median | 4 |
| Indication for use | |
| Empiric | 79% |
| Anti‐inflammatory | 20% |
| Culture proven | 1% |
| Dosage | |
| Appropriate | 67% |
| Inappropriate | 14% |
| Unknown | 19% |
| Duration | |
| Appropriate | 63% |
| Inappropriate | 19% |
| Unknown | 18% |
| Formulation | |
| Intravenous only | 21% |
| Intravenous followed by tablet | 13% |
| Suspension | 2% |
| Tablet | 64% |
| Diagnosis‐related group | |
| Simple pneumonia with pleurisy | 14% |
| Septicemia with sepsis | 8% |
| Respiratory infection with inflammation | 8% |
| Chronic obstructive pulmonary disease | 8% |
| Pulmonary edema with respiratory failure | 6% |
| Vaginal delivery with complications | 6% |
| Respiratory diagnosis with ventilator support | 4% |
| Otherb | 46% |
Patients were divided into drug interaction risk levels based on the number of medications prescribed with the potential for QT prolongation (Table 2). Patients were considered low risk if they received azithromycin alone, medium risk if they received 2 to 3 QT‐prolonging medications including azithromycin, and high risk if they received 4 or more QT‐prolonging medications including azithromycin.
| Medication | % of Patients Receiving Interacting Medication With Azithromycin |
|---|---|
| |
| Ondansetron | 48 |
| Trazodone | 23 |
| Moxifloxacin | 17 |
| Promethazine, haloperidol | 10 |
| Ciprofloxacin, citalopram, fluconazole | 7 |
| Amiodarone, amitriptyline | 5 |
| Quetiapine, methadone | 4 |
| Clarithromycin, octreotide, voriconazole | 2 |
| Erythromycin, granisetron, salmeterol, sotalol, ziprasidone | 1 |
The QT interval was measured from the beginning of the QRS complex to the end of the T wave as it returns to baseline. QTc has been defined by the most universally adopted method known as Bazett's formula ( , where QT is the measured QT interval and RR is the interval in seconds).[15]
Baseline QTc was evaluated through the use of most recent ECG within the past 6 months of admission. Borderline QTc was defined as 431 to 450 ms in males and 451 to 470 ms in females. Abnormal QTc was defined as >450 ms in males and >470 ms in females.[16]
Following admission, inpatient charges for telemetry during hospitalization were included. Telemetry was documented based on telemetry charges at any point in the hospital.
Statistical Analysis
Patient data were initially collected via Excel and analyzed with SAS version 9.4 software (SAS Institute, Cary, NC). Univariate analysis including central tendency and dispersion were utilized for aim 1. P values were calculated using 2 analysis and Fisher exact test for probability if cells with numerical values were <5 for aims 2 and 3.
RESULTS
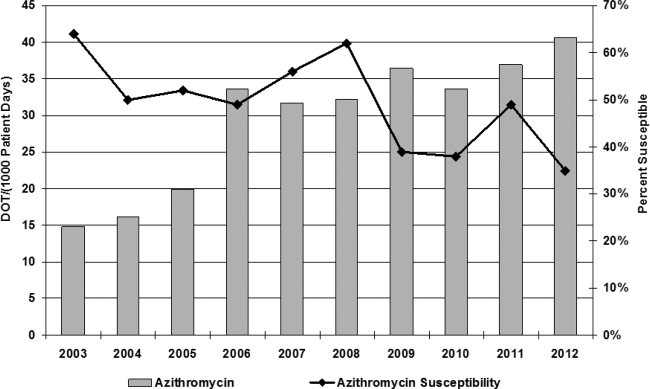
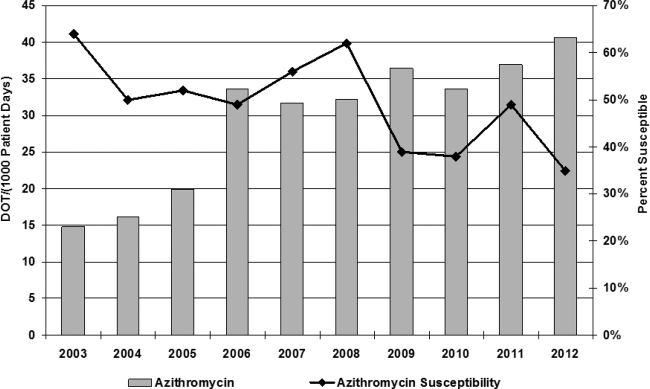
Azithromycin use within our hospital system has increased from 15 days of therapy per 1000 patient days in 2002 to 40 days of therapy per 1000 patient days in 2013 (Figure 1). At the same time, azithromycin susceptibility in Streptococcus pneumoniae isolates has decreased over the past decade from 65% to 35% in our hospital.
The baseline characteristics of patients included in this study are noted in Table 1. The mean age of patients was 55 years, with a range of 21 to 97 years, and 61% were female. Forty‐five percent of patients were admitted to either the general medicine teaching service or hospitalist service, and 23% were admitted to the pulmonary service, which includes intensive care unit admission. The average length of patient stay was 9.7 days (range, 3115 days; median 6 days).
Seventy‐nine percent of azithromycin use was empiric for the treatment of suspected infection. The second most common use was for anti‐inflammatory effects (20%), as documented by prescribers in the medical record for patients with cystic fibrosis, lung transplant, and chronic obstructive pulmonary disease. Azithromycin was dosed appropriately according to the documented indication in 67% of patients, with the most discrepancy in dosing noted for anti‐inflammatory use. The average duration of azithromycin therapy was 4.5 days (range, 128 days). Duration was appropriate in 63% of patients. Twenty‐one percent of patients received intravenous formulation of azithromycin, 13% received intravenous followed by oral formulation, and 64% of patient received tablet formulation alone.
Thirty‐five medications have been identified in our formulary as having a potential major drug‐drug interaction when prescribed with azithromycin (see Supporting Information, Appendix, in the online version of this article), and of these medications, 20 were prescribed with azithromycin, with an average overlap of therapy of 4.5 days (Table 2). Seventy‐six percent of patients were concomitantly prescribed a QT‐prolonging drug in addition to azithromycin. The most commonly prescribed agents were ondansetron (48%), trazodone (22%), and moxifloxacin (17%).
Telemetry monitoring was assessed for each patient based on inpatient charges during their hospitalization (Table 3). Forty‐three percent of patients were placed on telemetry. Twenty‐four (24%) of the patients were prescribed azithromycin alone, of whom 45.8% were placed on telemetry. Fifty‐seven percent of patients were prescribed azithromycin with 1 to 2 additional QT‐prolonging medications (medium‐risk arm); 38.5% of patients in this group were placed on telemetry. In the high‐risk arm, 19% of patients were prescribed at least 3 QT‐prolonging medications in addition to azithromycin, of which only 52.6% of patients were monitored with telemetry. No statistically significant association was observed between risk level and telemetry placement (P=0.07).
| Telemetry (%) | No Telemetry (%) | Total | P Valueb | |
|---|---|---|---|---|
| ||||
| Drug interaction risk levela | ||||
| Low | 11 (45.8) | 13 (54.2) | 24 | |
| Medium | 22 (38.5) | 35 (61.4) | 57 | |
| High | 10 (52.6) | 9 (47.4) | 19 | |
| Total | 43 | 57 | 100 | 0.07 |
| QTc | ||||
| Normal | 14 (50) | 14 (50) | 28 | |
| Borderline | 6 (66.7) | 3 (33.3) | 9 | |
| Abnormal | 15 (51.7) | 14 (48.3) | 29 | |
| Total | 35 | 31 | 66 | 0.22 |
Telemetry charges were further examined by analyzing baseline ECG evaluation within the past 6 months of their hospitalization (Table 3). Sixty‐six patients received baseline ECGs prior to initiation of azithromycin. Telemetry placement was not statistically correlated to abnormal QTc at baseline (P=0.22). Of those who underwent baseline ECG evaluation, 8.3% were noted to have borderline QTc, and 12.5% had abnormal QTc on admission prior to receiving azithromycin in the low‐risk level (Table 4). Within the medium‐risk level, 63.2% had baseline ECG evaluation, with 5.3% with borderline QTc and 35.7% with abnormal QTc. In the high‐risk level, 73.6% received a baseline ECG, with 21% with borderline QTc and 31.6% with abnormal QTc. No statistically significant association was observed between risk level and obtainment of baseline ECG (P=0.7). In 17 out of 66 patients, average repeat ECGs were obtained on day 3 (range, 27 days). Ten of the 17 ECGs showed increase in QTc (range, 397ms; average 27 ms), whereas the other 7 had a decrease in their QTc interval (range, 618 ms; average 13 ms; P=0.17).
| QTcb | Low, n=24 (%) | Medium, n=57 (%) | High, n=19 (%) | Total |
|---|---|---|---|---|
| ||||
| Normal | 11 (45.8%) | 13 (22.8%) | 4 (21.0%) | 28 |
| Borderline | 2 (8.3%) | 3 (5.3%) | 4 (21.0%) | 9 |
| Abnormal | 3 (12.5%) | 20 (35.7%) | 6 (31.6%) | 29 |
| Total | 16 (66.7%) | 36 (63.2%) | 14 (73.6%) | 66 |
| P valuec | 0.03 | 0.11 | ||
As risk level increased, having an abnormal QTc at baseline was statistically different between low‐ and medium‐risk levels (P=0.03), but this association was lost when comparing the low‐risk arm to the high‐risk arm (P=0.11). When the medium‐ and high‐risk categories were combined, there was a noted statistical significance of having an abnormal ECG at baseline (P=0.03).
Of the 9 patients prescribed azithromycin chronically, 3 patients were in the low‐risk category, 4 in the medium‐risk category, and 2 in the high‐risk category. Only 2 had baseline ECGs obtained, 1 of which was noted to have abnormal QTc and was in the high‐risk category. Only 1 patient was placed on telemetry, but was considered low risk based on medications prescribed.
DISCUSSION
In this study, 76% of patients were prescribed azithromycin with 1 or more medications known to affect QT prolongation; 19% received 3 or more QT‐prolonging medications in addition to azithromycin. Of patients who received a baseline ECG, 43% were documented to have borderline or prolonged QTc on admission. Telemetry monitoring was ordered 43% of the time, but there was no significant association between telemetry placement and risk level (P=0.07), suggesting that telemetry was ordered based on symptoms more than risk. Despite more drug‐druginteracting medications prescribed, there was no association to either telemetry orders or baseline ECG evaluation. Furthermore, if an abnormal QTc was documented on admission, there was no relationship to ordering telemetry as an inpatient (P=0.215), suggesting that healthcare providers are not considering risk of QTc medication accumulation. Given increased warnings issued by the FDA for azithromycin, further prospective studies are indicated to fully assess risk of QTc prolongation and arrhythmias in the setting of multiple drug interactions. This study elucidates the potential for drug‐drug interactions and need for increased vigilance and education of providers in the healthcare setting for QTc prolongation and subsequent arrhythmias.
Forty‐eight percent of patients receiving other QTc prolonging medications were prescribed ondansetron, followed by 23% of patients prescribed trazodone. Both of these medications are included on the admission order set in our institution and can be easily ordered for patients. Despite ordering multiple medications that have potential for QTc prolongation, there are no current alerts set up in our electronic medical record. When patients are separated into drug interaction risk levels, there is a trend of having an abnormal QTc on admission, but this is driven by the large number of patients in the medium‐risk category, and the rate does not increase (and is not significant) when comparing high risk to low risk. However, patients who receive any QTc‐prolonging medication are more likely to have an abnormal QTc when compared to azithromycin prescription alone (P=0.03). The small sample size limits the power and generalizability of this study, and further larger studies are indicated to assess if risk of QTc prolongation is additive.
In the 9 patients prescribed azithromycin chronically, dosing was not consistent, and a vast majority of patients were not placed on telemetry nor had baseline ECGs on admission. This further correlates with the idea that risk of arrhythmia is not fully considered in this patient population, as patients prescribed more than 1 QTc‐prolonging medication were not included in prior studies that examined azithromycin for its anti‐inflammatory effects.[2]
Azithromycin was added to our hospital formulary in 1998, and prescription of this agent remained relatively low until 2006, when azithromycin use increased dramatically from 15 days of therapy (DOT) per 1000 patient days to 40 DOT per 1000 patient days. Although numerous factors may have led to this increase, literature was published in 2006 and 2011 citing benefit from the anti‐inflammatory effects of azithromycin.[2, 17] At the same time, azithromycin susceptibility among Streptococcus pneumoniae in patients within our hospital has decreased over the past decade; studies have found a correlation between increasing use of macrolides and the development of resistance in Streptococcus species.[18, 19, 20] In this study, 79% of patients were prescribed azithromycin empirically for treatment of bacterial infections, whereas 20% were given azithromycin for its anti‐inflammatory effects; both dose and frequency varied among patients, raising the concern for development of resistance. Published studies have shown improvement in quality of life and decreased frequency of exacerbation and infection when azithromycin is used as an anti‐inflammatory agent; however, no QTc monitoring was noted.[2] Drug‐induced QTc prolongation>10 ms above baseline suggests the potential for clinical significance, whereas a QTc prolongation >20 milliseconds above baseline has a substantially increased likelihood of being proarrhythmic.[1] Unfortunately, drug‐induced QT prolongation is unpredictable, and additional risk factors play a role in facilitating Torsades de pointes, including female sex, advanced age, electrolyte disturbances, intravenous formulation, and concurrent use of more than 1 drug that can prolong the QT interval.[15] Azithromycin has recently been added to the growing list of medications that can prolong the QT interval, with 12 cases of Torsades de pointes reported in the literature. In March 2013, the FDA released a warning regarding prescribing azithromycin, but there is a lack of guidance for clinicians in identifying risk of cardiovascular events in susceptible patients.
There are some limitations to this study. Given data were acquired retrospectively and telemetry sheets were unable to be reviewed. Some patients were noted to have arrhythmias, but these data were obtained through physician notes and not examined directly from telemetry sheets. Seventeen patients had repeat ECGs, but most were performed serially for chest pain and not QTc monitoring. Four patients died in this study, but cause of death could not be determined through electronic medical records provided for all 4 patients; families pursued withdrawal of care.
Despite the published FDA warning, there are no national guidelines for clinicians in prescribing QTc‐prolonging medications. The American Heart Association published recommendations in 2010 for prescribing these drugs in the inpatient setting, but because hospitals differ in cardiac monitoring, there is no one‐size‐fits‐all strategy in reducing risk of cardiac events.[14] If the benefit of azithromycin outweighs the risk, QTc prolongation should not limit therapy; however, institutional awareness is necessary, whether it be through automatic stop dates on azithromycin, electronic alerts regarding drug‐drug interaction, enhanced prescriber education, or a combination of all of the above.
Disclosure: Nothing to report.
- , , . Azithromycin and the risk of cardiovascular complications. J Pharm Pract. 2014;27(5):496–500.
- , , , et al., Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698.
- , , , . Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;11:CD002203.
- , , . Long‐term macrolide treatment for chronic respiratory disease. Eur Respir J. 2013;42(1):239–251.
- , . Antimicrobial‐associated QT interval prolongation: pointes of interest. Clin Infect Dis. 2006;43(12):1603–1611.
- . Azithromycin‐induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47(11):1547–1551.
- , , , , . Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890.
- , , , et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121–127.
- , , , et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014;311(21):2199–2208.
- , , . Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368(18):1704–1712.
- U.S. Food and Drug Administration Drug Information. FDA drug safety communication: azithromycin (zithromax or zmax) and the risk of potentially fatal heart rhythms. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm341822.htm. Accessed December 1, 2014.
- , , , , . QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29(12):1719–1726.
- , , , et al., Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–487.
- , , , et al.; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular Nursing; American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(9):934–947.
- , , . Drug‐induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3(5):241–253.
- , , . QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17(3):333–336.
- , , , , . Anti‐inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2006;350(4):977–982.
- , , , , , ; Finnish Study Group for Antimicrobial Resistance (FiRe‐Network). Macrolide‐resistant Streptococcus pneumoniae and use of antimicrobial agents. Clin Infect Dis. 2001;33(4):483–488.
- , , , , , . Community prescribing and resistant Streptococcus pneumoniae. Emerg Infect Dis. 2005;11(6):829–837.
- , , , , ; Finnish Study Group for Antimicrobial Resistance (FiRe Network). Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2006;50(11):3646–3650.
Azithromycin, a macrolide antibiotic, received US Food and Drug Administration (FDA) approval in 1991 and is 1 of the most prescribed antibiotics used for a variety of infections, including community‐acquired pneumonia, bacterial sinusitis, urethritis, and cervicitis. In 2011, it was estimated that 40.3 million outpatients received a prescription for azithromycin.[1] In addition to treating acute bacterial infections, recent literature has pointed to using azithromycin for its unlabeled immunomodulatory and anti‐inflammatory effects, particularly in cystic fibrosis, chronic obstructive pulmonary disease (COPD), and lung transplant recipients.[2, 3, 4] Azithromycin decreases bacterial load and virulence, thus reducing airway secretion, as well as decreasing airway neutrophil accumulation through a reduction in proinflammatory cytokine expression.[4]
Cardiac toxicity can occur with macrolide antibiotics, and prolongation of the QT interval with subsequent Torsades de pointes has been documented with azithromycin.[1, 5, 6] In 2012, Ray et al. published data on a cohort of outpatients receiving azithromycin compared to amoxicillin, ciprofloxacin, or no antibiotics, and showed a small but absolute increase in cardiovascular deaths.[7, 8] Subsequent data, however, have not illustrated increased risk of death from cardiovascular causes. Mortensen et al. showed a lower risk of 90‐day mortality in older patients treated for community acquired pneumonia with azithromycin and ceftriaxone, although there was a nonstatistically significant increased risk of myocardial infarction in this group.[8, 9, 10] In March 2013, the FDA released an official statement regarding increased cardiovascular risk with azithromycin, stating that healthcare professionals should consider the risk of fatal heart rhythms with azithromycin when considering treatment options for patients who are at risk for cardiovascular events.[11]
In recent years, the potential for corrected QT (QTc) prolongation and Torsades de pointes has received increased attention due to its catastrophic nature, and it is thought that hospitalized patients are at a greater risk of drug‐induced Torsades de pointes due to the likelihood of having more risk factors.[12, 13] The American Heart Association released a statement in 2010 to raise awareness among healthcare professionals about risk, electrocardiogram (ECG) monitoring, and management of drug‐induced QT interval prolongation in hospitalized patients, although little data exist regarding quantification of risk in this patient population.[13, 14]
Prescribers currently have no standardized practice guidelines related to cardiovascular safety when prescribing QTc prolonging medications. Given the dramatic increase in azithromycin prescriptions and ongoing concern for cardiovascular risk and QTc prolongation, we investigated the prescribing practices with azithromycin within our institution. Our primary aims were 3‐fold. First, we aimed to describe the frequency azithromycin was prescribed with additional QTc prolonging medications. Second, we assessed the relationship between the number of arrhythmogenic drugs prescribed in addition to azithromycin with ordering telemetry. Finally, we assessed the relationship between baseline ECG abnormalities and telemetry monitoring in patients prescribed azithromycin. The purpose of these objectives was to better understand physician prescribing practices and to determine if patients have a potential risk of developing fatal cardiac arrhythmias
METHODS
Data
For this retrospective review, we utilized data from the University of Alabama at Birmingham Health Care system, a 1157licensed bed hospital. The institutional review board approved this study with a waiver of informed consent. Patients were eligible to be included in this study if they were 19 years of age with an inpatient hospital length of stay 3 days. Patients were considered to be receiving azithromycin and were included only when they were dispensed 1 dose of azithromycin by the pharmacy. Between October 1, 2012 and April 30, 2013, 1610 encounters were identified, of which 100 patient encounters were randomly selected for evaluation via a Microsoft Excel (Microsoft Corp., Redmond, WA) function. One patient was randomly included twice in this study, but had 2 separate admissions in which he received azithromycin.
QTc prolonging medications in our hospital formulary were identified via Micromedex and package inserts (see Supporting Information, Appendix, in the online version of this article for the full list).
Measures
The primary study measures were number of medications associated with QTc prolongation, baseline ECG findings, and telemetry monitoring. Secondary study measures include indication, dose, duration of use, formulation, length of stay, and admitting service (Table 1). Indications, dosage, and duration were defined by the FDA package insert for azithromycin (see Supporting Information, Appendix, in the online version of this article). Indication for use was defined as (1) empiric for a specific infection; (2) anti‐inflammatory for patients with COPD, lung transplant recipients, or cystic fibrosis patients; and (3) culture proven if evidence of a particular pathogen grown on culture. Indications were defined by prescriber notes. Dosage is defined as appropriate if FDA guidelines were followed for the defined indication. If patients were given azithromycin for anti‐inflammatory purposes, dosing was considered appropriate if it followed previous literature dosing of 250mg daily.
| |
| Age, y | |
| Average | 5519.5 |
| Range | 2197 |
| Gender | |
| Female | 61% |
| Male | 39% |
| Length of stay, d | |
| Average | 9.713.1 |
| Range | 3115 |
| Admitting service | |
| Hospitalist | 37% |
| Pulmonary | 23% |
| Obstetrics | 9% |
| General medicine | 8% |
| Hematology/oncology | 6% |
| Othera | 17% |
| Days of therapy | |
| Average | 4.53.9 |
| Range | 128 |
| Median | 4 |
| Indication for use | |
| Empiric | 79% |
| Anti‐inflammatory | 20% |
| Culture proven | 1% |
| Dosage | |
| Appropriate | 67% |
| Inappropriate | 14% |
| Unknown | 19% |
| Duration | |
| Appropriate | 63% |
| Inappropriate | 19% |
| Unknown | 18% |
| Formulation | |
| Intravenous only | 21% |
| Intravenous followed by tablet | 13% |
| Suspension | 2% |
| Tablet | 64% |
| Diagnosis‐related group | |
| Simple pneumonia with pleurisy | 14% |
| Septicemia with sepsis | 8% |
| Respiratory infection with inflammation | 8% |
| Chronic obstructive pulmonary disease | 8% |
| Pulmonary edema with respiratory failure | 6% |
| Vaginal delivery with complications | 6% |
| Respiratory diagnosis with ventilator support | 4% |
| Otherb | 46% |
Patients were divided into drug interaction risk levels based on the number of medications prescribed with the potential for QT prolongation (Table 2). Patients were considered low risk if they received azithromycin alone, medium risk if they received 2 to 3 QT‐prolonging medications including azithromycin, and high risk if they received 4 or more QT‐prolonging medications including azithromycin.
| Medication | % of Patients Receiving Interacting Medication With Azithromycin |
|---|---|
| |
| Ondansetron | 48 |
| Trazodone | 23 |
| Moxifloxacin | 17 |
| Promethazine, haloperidol | 10 |
| Ciprofloxacin, citalopram, fluconazole | 7 |
| Amiodarone, amitriptyline | 5 |
| Quetiapine, methadone | 4 |
| Clarithromycin, octreotide, voriconazole | 2 |
| Erythromycin, granisetron, salmeterol, sotalol, ziprasidone | 1 |
The QT interval was measured from the beginning of the QRS complex to the end of the T wave as it returns to baseline. QTc has been defined by the most universally adopted method known as Bazett's formula ( , where QT is the measured QT interval and RR is the interval in seconds).[15]
Baseline QTc was evaluated through the use of most recent ECG within the past 6 months of admission. Borderline QTc was defined as 431 to 450 ms in males and 451 to 470 ms in females. Abnormal QTc was defined as >450 ms in males and >470 ms in females.[16]
Following admission, inpatient charges for telemetry during hospitalization were included. Telemetry was documented based on telemetry charges at any point in the hospital.
Statistical Analysis
Patient data were initially collected via Excel and analyzed with SAS version 9.4 software (SAS Institute, Cary, NC). Univariate analysis including central tendency and dispersion were utilized for aim 1. P values were calculated using 2 analysis and Fisher exact test for probability if cells with numerical values were <5 for aims 2 and 3.
RESULTS
Azithromycin use within our hospital system has increased from 15 days of therapy per 1000 patient days in 2002 to 40 days of therapy per 1000 patient days in 2013 (Figure 1). At the same time, azithromycin susceptibility in Streptococcus pneumoniae isolates has decreased over the past decade from 65% to 35% in our hospital.

The baseline characteristics of patients included in this study are noted in Table 1. The mean age of patients was 55 years, with a range of 21 to 97 years, and 61% were female. Forty‐five percent of patients were admitted to either the general medicine teaching service or hospitalist service, and 23% were admitted to the pulmonary service, which includes intensive care unit admission. The average length of patient stay was 9.7 days (range, 3115 days; median 6 days).
Seventy‐nine percent of azithromycin use was empiric for the treatment of suspected infection. The second most common use was for anti‐inflammatory effects (20%), as documented by prescribers in the medical record for patients with cystic fibrosis, lung transplant, and chronic obstructive pulmonary disease. Azithromycin was dosed appropriately according to the documented indication in 67% of patients, with the most discrepancy in dosing noted for anti‐inflammatory use. The average duration of azithromycin therapy was 4.5 days (range, 128 days). Duration was appropriate in 63% of patients. Twenty‐one percent of patients received intravenous formulation of azithromycin, 13% received intravenous followed by oral formulation, and 64% of patient received tablet formulation alone.
Thirty‐five medications have been identified in our formulary as having a potential major drug‐drug interaction when prescribed with azithromycin (see Supporting Information, Appendix, in the online version of this article), and of these medications, 20 were prescribed with azithromycin, with an average overlap of therapy of 4.5 days (Table 2). Seventy‐six percent of patients were concomitantly prescribed a QT‐prolonging drug in addition to azithromycin. The most commonly prescribed agents were ondansetron (48%), trazodone (22%), and moxifloxacin (17%).
Telemetry monitoring was assessed for each patient based on inpatient charges during their hospitalization (Table 3). Forty‐three percent of patients were placed on telemetry. Twenty‐four (24%) of the patients were prescribed azithromycin alone, of whom 45.8% were placed on telemetry. Fifty‐seven percent of patients were prescribed azithromycin with 1 to 2 additional QT‐prolonging medications (medium‐risk arm); 38.5% of patients in this group were placed on telemetry. In the high‐risk arm, 19% of patients were prescribed at least 3 QT‐prolonging medications in addition to azithromycin, of which only 52.6% of patients were monitored with telemetry. No statistically significant association was observed between risk level and telemetry placement (P=0.07).
| Telemetry (%) | No Telemetry (%) | Total | P Valueb | |
|---|---|---|---|---|
| ||||
| Drug interaction risk levela | ||||
| Low | 11 (45.8) | 13 (54.2) | 24 | |
| Medium | 22 (38.5) | 35 (61.4) | 57 | |
| High | 10 (52.6) | 9 (47.4) | 19 | |
| Total | 43 | 57 | 100 | 0.07 |
| QTc | ||||
| Normal | 14 (50) | 14 (50) | 28 | |
| Borderline | 6 (66.7) | 3 (33.3) | 9 | |
| Abnormal | 15 (51.7) | 14 (48.3) | 29 | |
| Total | 35 | 31 | 66 | 0.22 |
Telemetry charges were further examined by analyzing baseline ECG evaluation within the past 6 months of their hospitalization (Table 3). Sixty‐six patients received baseline ECGs prior to initiation of azithromycin. Telemetry placement was not statistically correlated to abnormal QTc at baseline (P=0.22). Of those who underwent baseline ECG evaluation, 8.3% were noted to have borderline QTc, and 12.5% had abnormal QTc on admission prior to receiving azithromycin in the low‐risk level (Table 4). Within the medium‐risk level, 63.2% had baseline ECG evaluation, with 5.3% with borderline QTc and 35.7% with abnormal QTc. In the high‐risk level, 73.6% received a baseline ECG, with 21% with borderline QTc and 31.6% with abnormal QTc. No statistically significant association was observed between risk level and obtainment of baseline ECG (P=0.7). In 17 out of 66 patients, average repeat ECGs were obtained on day 3 (range, 27 days). Ten of the 17 ECGs showed increase in QTc (range, 397ms; average 27 ms), whereas the other 7 had a decrease in their QTc interval (range, 618 ms; average 13 ms; P=0.17).
| QTcb | Low, n=24 (%) | Medium, n=57 (%) | High, n=19 (%) | Total |
|---|---|---|---|---|
| ||||
| Normal | 11 (45.8%) | 13 (22.8%) | 4 (21.0%) | 28 |
| Borderline | 2 (8.3%) | 3 (5.3%) | 4 (21.0%) | 9 |
| Abnormal | 3 (12.5%) | 20 (35.7%) | 6 (31.6%) | 29 |
| Total | 16 (66.7%) | 36 (63.2%) | 14 (73.6%) | 66 |
| P valuec | 0.03 | 0.11 | ||
As risk level increased, having an abnormal QTc at baseline was statistically different between low‐ and medium‐risk levels (P=0.03), but this association was lost when comparing the low‐risk arm to the high‐risk arm (P=0.11). When the medium‐ and high‐risk categories were combined, there was a noted statistical significance of having an abnormal ECG at baseline (P=0.03).
Of the 9 patients prescribed azithromycin chronically, 3 patients were in the low‐risk category, 4 in the medium‐risk category, and 2 in the high‐risk category. Only 2 had baseline ECGs obtained, 1 of which was noted to have abnormal QTc and was in the high‐risk category. Only 1 patient was placed on telemetry, but was considered low risk based on medications prescribed.
DISCUSSION
In this study, 76% of patients were prescribed azithromycin with 1 or more medications known to affect QT prolongation; 19% received 3 or more QT‐prolonging medications in addition to azithromycin. Of patients who received a baseline ECG, 43% were documented to have borderline or prolonged QTc on admission. Telemetry monitoring was ordered 43% of the time, but there was no significant association between telemetry placement and risk level (P=0.07), suggesting that telemetry was ordered based on symptoms more than risk. Despite more drug‐druginteracting medications prescribed, there was no association to either telemetry orders or baseline ECG evaluation. Furthermore, if an abnormal QTc was documented on admission, there was no relationship to ordering telemetry as an inpatient (P=0.215), suggesting that healthcare providers are not considering risk of QTc medication accumulation. Given increased warnings issued by the FDA for azithromycin, further prospective studies are indicated to fully assess risk of QTc prolongation and arrhythmias in the setting of multiple drug interactions. This study elucidates the potential for drug‐drug interactions and need for increased vigilance and education of providers in the healthcare setting for QTc prolongation and subsequent arrhythmias.
Forty‐eight percent of patients receiving other QTc prolonging medications were prescribed ondansetron, followed by 23% of patients prescribed trazodone. Both of these medications are included on the admission order set in our institution and can be easily ordered for patients. Despite ordering multiple medications that have potential for QTc prolongation, there are no current alerts set up in our electronic medical record. When patients are separated into drug interaction risk levels, there is a trend of having an abnormal QTc on admission, but this is driven by the large number of patients in the medium‐risk category, and the rate does not increase (and is not significant) when comparing high risk to low risk. However, patients who receive any QTc‐prolonging medication are more likely to have an abnormal QTc when compared to azithromycin prescription alone (P=0.03). The small sample size limits the power and generalizability of this study, and further larger studies are indicated to assess if risk of QTc prolongation is additive.
In the 9 patients prescribed azithromycin chronically, dosing was not consistent, and a vast majority of patients were not placed on telemetry nor had baseline ECGs on admission. This further correlates with the idea that risk of arrhythmia is not fully considered in this patient population, as patients prescribed more than 1 QTc‐prolonging medication were not included in prior studies that examined azithromycin for its anti‐inflammatory effects.[2]
Azithromycin was added to our hospital formulary in 1998, and prescription of this agent remained relatively low until 2006, when azithromycin use increased dramatically from 15 days of therapy (DOT) per 1000 patient days to 40 DOT per 1000 patient days. Although numerous factors may have led to this increase, literature was published in 2006 and 2011 citing benefit from the anti‐inflammatory effects of azithromycin.[2, 17] At the same time, azithromycin susceptibility among Streptococcus pneumoniae in patients within our hospital has decreased over the past decade; studies have found a correlation between increasing use of macrolides and the development of resistance in Streptococcus species.[18, 19, 20] In this study, 79% of patients were prescribed azithromycin empirically for treatment of bacterial infections, whereas 20% were given azithromycin for its anti‐inflammatory effects; both dose and frequency varied among patients, raising the concern for development of resistance. Published studies have shown improvement in quality of life and decreased frequency of exacerbation and infection when azithromycin is used as an anti‐inflammatory agent; however, no QTc monitoring was noted.[2] Drug‐induced QTc prolongation>10 ms above baseline suggests the potential for clinical significance, whereas a QTc prolongation >20 milliseconds above baseline has a substantially increased likelihood of being proarrhythmic.[1] Unfortunately, drug‐induced QT prolongation is unpredictable, and additional risk factors play a role in facilitating Torsades de pointes, including female sex, advanced age, electrolyte disturbances, intravenous formulation, and concurrent use of more than 1 drug that can prolong the QT interval.[15] Azithromycin has recently been added to the growing list of medications that can prolong the QT interval, with 12 cases of Torsades de pointes reported in the literature. In March 2013, the FDA released a warning regarding prescribing azithromycin, but there is a lack of guidance for clinicians in identifying risk of cardiovascular events in susceptible patients.
There are some limitations to this study. Given data were acquired retrospectively and telemetry sheets were unable to be reviewed. Some patients were noted to have arrhythmias, but these data were obtained through physician notes and not examined directly from telemetry sheets. Seventeen patients had repeat ECGs, but most were performed serially for chest pain and not QTc monitoring. Four patients died in this study, but cause of death could not be determined through electronic medical records provided for all 4 patients; families pursued withdrawal of care.
Despite the published FDA warning, there are no national guidelines for clinicians in prescribing QTc‐prolonging medications. The American Heart Association published recommendations in 2010 for prescribing these drugs in the inpatient setting, but because hospitals differ in cardiac monitoring, there is no one‐size‐fits‐all strategy in reducing risk of cardiac events.[14] If the benefit of azithromycin outweighs the risk, QTc prolongation should not limit therapy; however, institutional awareness is necessary, whether it be through automatic stop dates on azithromycin, electronic alerts regarding drug‐drug interaction, enhanced prescriber education, or a combination of all of the above.
Disclosure: Nothing to report.
Azithromycin, a macrolide antibiotic, received US Food and Drug Administration (FDA) approval in 1991 and is 1 of the most prescribed antibiotics used for a variety of infections, including community‐acquired pneumonia, bacterial sinusitis, urethritis, and cervicitis. In 2011, it was estimated that 40.3 million outpatients received a prescription for azithromycin.[1] In addition to treating acute bacterial infections, recent literature has pointed to using azithromycin for its unlabeled immunomodulatory and anti‐inflammatory effects, particularly in cystic fibrosis, chronic obstructive pulmonary disease (COPD), and lung transplant recipients.[2, 3, 4] Azithromycin decreases bacterial load and virulence, thus reducing airway secretion, as well as decreasing airway neutrophil accumulation through a reduction in proinflammatory cytokine expression.[4]
Cardiac toxicity can occur with macrolide antibiotics, and prolongation of the QT interval with subsequent Torsades de pointes has been documented with azithromycin.[1, 5, 6] In 2012, Ray et al. published data on a cohort of outpatients receiving azithromycin compared to amoxicillin, ciprofloxacin, or no antibiotics, and showed a small but absolute increase in cardiovascular deaths.[7, 8] Subsequent data, however, have not illustrated increased risk of death from cardiovascular causes. Mortensen et al. showed a lower risk of 90‐day mortality in older patients treated for community acquired pneumonia with azithromycin and ceftriaxone, although there was a nonstatistically significant increased risk of myocardial infarction in this group.[8, 9, 10] In March 2013, the FDA released an official statement regarding increased cardiovascular risk with azithromycin, stating that healthcare professionals should consider the risk of fatal heart rhythms with azithromycin when considering treatment options for patients who are at risk for cardiovascular events.[11]
In recent years, the potential for corrected QT (QTc) prolongation and Torsades de pointes has received increased attention due to its catastrophic nature, and it is thought that hospitalized patients are at a greater risk of drug‐induced Torsades de pointes due to the likelihood of having more risk factors.[12, 13] The American Heart Association released a statement in 2010 to raise awareness among healthcare professionals about risk, electrocardiogram (ECG) monitoring, and management of drug‐induced QT interval prolongation in hospitalized patients, although little data exist regarding quantification of risk in this patient population.[13, 14]
Prescribers currently have no standardized practice guidelines related to cardiovascular safety when prescribing QTc prolonging medications. Given the dramatic increase in azithromycin prescriptions and ongoing concern for cardiovascular risk and QTc prolongation, we investigated the prescribing practices with azithromycin within our institution. Our primary aims were 3‐fold. First, we aimed to describe the frequency azithromycin was prescribed with additional QTc prolonging medications. Second, we assessed the relationship between the number of arrhythmogenic drugs prescribed in addition to azithromycin with ordering telemetry. Finally, we assessed the relationship between baseline ECG abnormalities and telemetry monitoring in patients prescribed azithromycin. The purpose of these objectives was to better understand physician prescribing practices and to determine if patients have a potential risk of developing fatal cardiac arrhythmias
METHODS
Data
For this retrospective review, we utilized data from the University of Alabama at Birmingham Health Care system, a 1157licensed bed hospital. The institutional review board approved this study with a waiver of informed consent. Patients were eligible to be included in this study if they were 19 years of age with an inpatient hospital length of stay 3 days. Patients were considered to be receiving azithromycin and were included only when they were dispensed 1 dose of azithromycin by the pharmacy. Between October 1, 2012 and April 30, 2013, 1610 encounters were identified, of which 100 patient encounters were randomly selected for evaluation via a Microsoft Excel (Microsoft Corp., Redmond, WA) function. One patient was randomly included twice in this study, but had 2 separate admissions in which he received azithromycin.
QTc prolonging medications in our hospital formulary were identified via Micromedex and package inserts (see Supporting Information, Appendix, in the online version of this article for the full list).
Measures
The primary study measures were number of medications associated with QTc prolongation, baseline ECG findings, and telemetry monitoring. Secondary study measures include indication, dose, duration of use, formulation, length of stay, and admitting service (Table 1). Indications, dosage, and duration were defined by the FDA package insert for azithromycin (see Supporting Information, Appendix, in the online version of this article). Indication for use was defined as (1) empiric for a specific infection; (2) anti‐inflammatory for patients with COPD, lung transplant recipients, or cystic fibrosis patients; and (3) culture proven if evidence of a particular pathogen grown on culture. Indications were defined by prescriber notes. Dosage is defined as appropriate if FDA guidelines were followed for the defined indication. If patients were given azithromycin for anti‐inflammatory purposes, dosing was considered appropriate if it followed previous literature dosing of 250mg daily.
| |
| Age, y | |
| Average | 5519.5 |
| Range | 2197 |
| Gender | |
| Female | 61% |
| Male | 39% |
| Length of stay, d | |
| Average | 9.713.1 |
| Range | 3115 |
| Admitting service | |
| Hospitalist | 37% |
| Pulmonary | 23% |
| Obstetrics | 9% |
| General medicine | 8% |
| Hematology/oncology | 6% |
| Othera | 17% |
| Days of therapy | |
| Average | 4.53.9 |
| Range | 128 |
| Median | 4 |
| Indication for use | |
| Empiric | 79% |
| Anti‐inflammatory | 20% |
| Culture proven | 1% |
| Dosage | |
| Appropriate | 67% |
| Inappropriate | 14% |
| Unknown | 19% |
| Duration | |
| Appropriate | 63% |
| Inappropriate | 19% |
| Unknown | 18% |
| Formulation | |
| Intravenous only | 21% |
| Intravenous followed by tablet | 13% |
| Suspension | 2% |
| Tablet | 64% |
| Diagnosis‐related group | |
| Simple pneumonia with pleurisy | 14% |
| Septicemia with sepsis | 8% |
| Respiratory infection with inflammation | 8% |
| Chronic obstructive pulmonary disease | 8% |
| Pulmonary edema with respiratory failure | 6% |
| Vaginal delivery with complications | 6% |
| Respiratory diagnosis with ventilator support | 4% |
| Otherb | 46% |
Patients were divided into drug interaction risk levels based on the number of medications prescribed with the potential for QT prolongation (Table 2). Patients were considered low risk if they received azithromycin alone, medium risk if they received 2 to 3 QT‐prolonging medications including azithromycin, and high risk if they received 4 or more QT‐prolonging medications including azithromycin.
| Medication | % of Patients Receiving Interacting Medication With Azithromycin |
|---|---|
| |
| Ondansetron | 48 |
| Trazodone | 23 |
| Moxifloxacin | 17 |
| Promethazine, haloperidol | 10 |
| Ciprofloxacin, citalopram, fluconazole | 7 |
| Amiodarone, amitriptyline | 5 |
| Quetiapine, methadone | 4 |
| Clarithromycin, octreotide, voriconazole | 2 |
| Erythromycin, granisetron, salmeterol, sotalol, ziprasidone | 1 |
The QT interval was measured from the beginning of the QRS complex to the end of the T wave as it returns to baseline. QTc has been defined by the most universally adopted method known as Bazett's formula ( , where QT is the measured QT interval and RR is the interval in seconds).[15]
Baseline QTc was evaluated through the use of most recent ECG within the past 6 months of admission. Borderline QTc was defined as 431 to 450 ms in males and 451 to 470 ms in females. Abnormal QTc was defined as >450 ms in males and >470 ms in females.[16]
Following admission, inpatient charges for telemetry during hospitalization were included. Telemetry was documented based on telemetry charges at any point in the hospital.
Statistical Analysis
Patient data were initially collected via Excel and analyzed with SAS version 9.4 software (SAS Institute, Cary, NC). Univariate analysis including central tendency and dispersion were utilized for aim 1. P values were calculated using 2 analysis and Fisher exact test for probability if cells with numerical values were <5 for aims 2 and 3.
RESULTS
Azithromycin use within our hospital system has increased from 15 days of therapy per 1000 patient days in 2002 to 40 days of therapy per 1000 patient days in 2013 (Figure 1). At the same time, azithromycin susceptibility in Streptococcus pneumoniae isolates has decreased over the past decade from 65% to 35% in our hospital.

The baseline characteristics of patients included in this study are noted in Table 1. The mean age of patients was 55 years, with a range of 21 to 97 years, and 61% were female. Forty‐five percent of patients were admitted to either the general medicine teaching service or hospitalist service, and 23% were admitted to the pulmonary service, which includes intensive care unit admission. The average length of patient stay was 9.7 days (range, 3115 days; median 6 days).
Seventy‐nine percent of azithromycin use was empiric for the treatment of suspected infection. The second most common use was for anti‐inflammatory effects (20%), as documented by prescribers in the medical record for patients with cystic fibrosis, lung transplant, and chronic obstructive pulmonary disease. Azithromycin was dosed appropriately according to the documented indication in 67% of patients, with the most discrepancy in dosing noted for anti‐inflammatory use. The average duration of azithromycin therapy was 4.5 days (range, 128 days). Duration was appropriate in 63% of patients. Twenty‐one percent of patients received intravenous formulation of azithromycin, 13% received intravenous followed by oral formulation, and 64% of patient received tablet formulation alone.
Thirty‐five medications have been identified in our formulary as having a potential major drug‐drug interaction when prescribed with azithromycin (see Supporting Information, Appendix, in the online version of this article), and of these medications, 20 were prescribed with azithromycin, with an average overlap of therapy of 4.5 days (Table 2). Seventy‐six percent of patients were concomitantly prescribed a QT‐prolonging drug in addition to azithromycin. The most commonly prescribed agents were ondansetron (48%), trazodone (22%), and moxifloxacin (17%).
Telemetry monitoring was assessed for each patient based on inpatient charges during their hospitalization (Table 3). Forty‐three percent of patients were placed on telemetry. Twenty‐four (24%) of the patients were prescribed azithromycin alone, of whom 45.8% were placed on telemetry. Fifty‐seven percent of patients were prescribed azithromycin with 1 to 2 additional QT‐prolonging medications (medium‐risk arm); 38.5% of patients in this group were placed on telemetry. In the high‐risk arm, 19% of patients were prescribed at least 3 QT‐prolonging medications in addition to azithromycin, of which only 52.6% of patients were monitored with telemetry. No statistically significant association was observed between risk level and telemetry placement (P=0.07).
| Telemetry (%) | No Telemetry (%) | Total | P Valueb | |
|---|---|---|---|---|
| ||||
| Drug interaction risk levela | ||||
| Low | 11 (45.8) | 13 (54.2) | 24 | |
| Medium | 22 (38.5) | 35 (61.4) | 57 | |
| High | 10 (52.6) | 9 (47.4) | 19 | |
| Total | 43 | 57 | 100 | 0.07 |
| QTc | ||||
| Normal | 14 (50) | 14 (50) | 28 | |
| Borderline | 6 (66.7) | 3 (33.3) | 9 | |
| Abnormal | 15 (51.7) | 14 (48.3) | 29 | |
| Total | 35 | 31 | 66 | 0.22 |
Telemetry charges were further examined by analyzing baseline ECG evaluation within the past 6 months of their hospitalization (Table 3). Sixty‐six patients received baseline ECGs prior to initiation of azithromycin. Telemetry placement was not statistically correlated to abnormal QTc at baseline (P=0.22). Of those who underwent baseline ECG evaluation, 8.3% were noted to have borderline QTc, and 12.5% had abnormal QTc on admission prior to receiving azithromycin in the low‐risk level (Table 4). Within the medium‐risk level, 63.2% had baseline ECG evaluation, with 5.3% with borderline QTc and 35.7% with abnormal QTc. In the high‐risk level, 73.6% received a baseline ECG, with 21% with borderline QTc and 31.6% with abnormal QTc. No statistically significant association was observed between risk level and obtainment of baseline ECG (P=0.7). In 17 out of 66 patients, average repeat ECGs were obtained on day 3 (range, 27 days). Ten of the 17 ECGs showed increase in QTc (range, 397ms; average 27 ms), whereas the other 7 had a decrease in their QTc interval (range, 618 ms; average 13 ms; P=0.17).
| QTcb | Low, n=24 (%) | Medium, n=57 (%) | High, n=19 (%) | Total |
|---|---|---|---|---|
| ||||
| Normal | 11 (45.8%) | 13 (22.8%) | 4 (21.0%) | 28 |
| Borderline | 2 (8.3%) | 3 (5.3%) | 4 (21.0%) | 9 |
| Abnormal | 3 (12.5%) | 20 (35.7%) | 6 (31.6%) | 29 |
| Total | 16 (66.7%) | 36 (63.2%) | 14 (73.6%) | 66 |
| P valuec | 0.03 | 0.11 | ||
As risk level increased, having an abnormal QTc at baseline was statistically different between low‐ and medium‐risk levels (P=0.03), but this association was lost when comparing the low‐risk arm to the high‐risk arm (P=0.11). When the medium‐ and high‐risk categories were combined, there was a noted statistical significance of having an abnormal ECG at baseline (P=0.03).
Of the 9 patients prescribed azithromycin chronically, 3 patients were in the low‐risk category, 4 in the medium‐risk category, and 2 in the high‐risk category. Only 2 had baseline ECGs obtained, 1 of which was noted to have abnormal QTc and was in the high‐risk category. Only 1 patient was placed on telemetry, but was considered low risk based on medications prescribed.
DISCUSSION
In this study, 76% of patients were prescribed azithromycin with 1 or more medications known to affect QT prolongation; 19% received 3 or more QT‐prolonging medications in addition to azithromycin. Of patients who received a baseline ECG, 43% were documented to have borderline or prolonged QTc on admission. Telemetry monitoring was ordered 43% of the time, but there was no significant association between telemetry placement and risk level (P=0.07), suggesting that telemetry was ordered based on symptoms more than risk. Despite more drug‐druginteracting medications prescribed, there was no association to either telemetry orders or baseline ECG evaluation. Furthermore, if an abnormal QTc was documented on admission, there was no relationship to ordering telemetry as an inpatient (P=0.215), suggesting that healthcare providers are not considering risk of QTc medication accumulation. Given increased warnings issued by the FDA for azithromycin, further prospective studies are indicated to fully assess risk of QTc prolongation and arrhythmias in the setting of multiple drug interactions. This study elucidates the potential for drug‐drug interactions and need for increased vigilance and education of providers in the healthcare setting for QTc prolongation and subsequent arrhythmias.
Forty‐eight percent of patients receiving other QTc prolonging medications were prescribed ondansetron, followed by 23% of patients prescribed trazodone. Both of these medications are included on the admission order set in our institution and can be easily ordered for patients. Despite ordering multiple medications that have potential for QTc prolongation, there are no current alerts set up in our electronic medical record. When patients are separated into drug interaction risk levels, there is a trend of having an abnormal QTc on admission, but this is driven by the large number of patients in the medium‐risk category, and the rate does not increase (and is not significant) when comparing high risk to low risk. However, patients who receive any QTc‐prolonging medication are more likely to have an abnormal QTc when compared to azithromycin prescription alone (P=0.03). The small sample size limits the power and generalizability of this study, and further larger studies are indicated to assess if risk of QTc prolongation is additive.
In the 9 patients prescribed azithromycin chronically, dosing was not consistent, and a vast majority of patients were not placed on telemetry nor had baseline ECGs on admission. This further correlates with the idea that risk of arrhythmia is not fully considered in this patient population, as patients prescribed more than 1 QTc‐prolonging medication were not included in prior studies that examined azithromycin for its anti‐inflammatory effects.[2]
Azithromycin was added to our hospital formulary in 1998, and prescription of this agent remained relatively low until 2006, when azithromycin use increased dramatically from 15 days of therapy (DOT) per 1000 patient days to 40 DOT per 1000 patient days. Although numerous factors may have led to this increase, literature was published in 2006 and 2011 citing benefit from the anti‐inflammatory effects of azithromycin.[2, 17] At the same time, azithromycin susceptibility among Streptococcus pneumoniae in patients within our hospital has decreased over the past decade; studies have found a correlation between increasing use of macrolides and the development of resistance in Streptococcus species.[18, 19, 20] In this study, 79% of patients were prescribed azithromycin empirically for treatment of bacterial infections, whereas 20% were given azithromycin for its anti‐inflammatory effects; both dose and frequency varied among patients, raising the concern for development of resistance. Published studies have shown improvement in quality of life and decreased frequency of exacerbation and infection when azithromycin is used as an anti‐inflammatory agent; however, no QTc monitoring was noted.[2] Drug‐induced QTc prolongation>10 ms above baseline suggests the potential for clinical significance, whereas a QTc prolongation >20 milliseconds above baseline has a substantially increased likelihood of being proarrhythmic.[1] Unfortunately, drug‐induced QT prolongation is unpredictable, and additional risk factors play a role in facilitating Torsades de pointes, including female sex, advanced age, electrolyte disturbances, intravenous formulation, and concurrent use of more than 1 drug that can prolong the QT interval.[15] Azithromycin has recently been added to the growing list of medications that can prolong the QT interval, with 12 cases of Torsades de pointes reported in the literature. In March 2013, the FDA released a warning regarding prescribing azithromycin, but there is a lack of guidance for clinicians in identifying risk of cardiovascular events in susceptible patients.
There are some limitations to this study. Given data were acquired retrospectively and telemetry sheets were unable to be reviewed. Some patients were noted to have arrhythmias, but these data were obtained through physician notes and not examined directly from telemetry sheets. Seventeen patients had repeat ECGs, but most were performed serially for chest pain and not QTc monitoring. Four patients died in this study, but cause of death could not be determined through electronic medical records provided for all 4 patients; families pursued withdrawal of care.
Despite the published FDA warning, there are no national guidelines for clinicians in prescribing QTc‐prolonging medications. The American Heart Association published recommendations in 2010 for prescribing these drugs in the inpatient setting, but because hospitals differ in cardiac monitoring, there is no one‐size‐fits‐all strategy in reducing risk of cardiac events.[14] If the benefit of azithromycin outweighs the risk, QTc prolongation should not limit therapy; however, institutional awareness is necessary, whether it be through automatic stop dates on azithromycin, electronic alerts regarding drug‐drug interaction, enhanced prescriber education, or a combination of all of the above.
Disclosure: Nothing to report.
- , , . Azithromycin and the risk of cardiovascular complications. J Pharm Pract. 2014;27(5):496–500.
- , , , et al., Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698.
- , , , . Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;11:CD002203.
- , , . Long‐term macrolide treatment for chronic respiratory disease. Eur Respir J. 2013;42(1):239–251.
- , . Antimicrobial‐associated QT interval prolongation: pointes of interest. Clin Infect Dis. 2006;43(12):1603–1611.
- . Azithromycin‐induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47(11):1547–1551.
- , , , , . Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890.
- , , , et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121–127.
- , , , et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014;311(21):2199–2208.
- , , . Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368(18):1704–1712.
- U.S. Food and Drug Administration Drug Information. FDA drug safety communication: azithromycin (zithromax or zmax) and the risk of potentially fatal heart rhythms. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm341822.htm. Accessed December 1, 2014.
- , , , , . QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29(12):1719–1726.
- , , , et al., Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–487.
- , , , et al.; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular Nursing; American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(9):934–947.
- , , . Drug‐induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3(5):241–253.
- , , . QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17(3):333–336.
- , , , , . Anti‐inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2006;350(4):977–982.
- , , , , , ; Finnish Study Group for Antimicrobial Resistance (FiRe‐Network). Macrolide‐resistant Streptococcus pneumoniae and use of antimicrobial agents. Clin Infect Dis. 2001;33(4):483–488.
- , , , , , . Community prescribing and resistant Streptococcus pneumoniae. Emerg Infect Dis. 2005;11(6):829–837.
- , , , , ; Finnish Study Group for Antimicrobial Resistance (FiRe Network). Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2006;50(11):3646–3650.
- , , . Azithromycin and the risk of cardiovascular complications. J Pharm Pract. 2014;27(5):496–500.
- , , , et al., Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698.
- , , , . Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;11:CD002203.
- , , . Long‐term macrolide treatment for chronic respiratory disease. Eur Respir J. 2013;42(1):239–251.
- , . Antimicrobial‐associated QT interval prolongation: pointes of interest. Clin Infect Dis. 2006;43(12):1603–1611.
- . Azithromycin‐induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47(11):1547–1551.
- , , , , . Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890.
- , , , et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121–127.
- , , , et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014;311(21):2199–2208.
- , , . Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368(18):1704–1712.
- U.S. Food and Drug Administration Drug Information. FDA drug safety communication: azithromycin (zithromax or zmax) and the risk of potentially fatal heart rhythms. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm341822.htm. Accessed December 1, 2014.
- , , , , . QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;29(12):1719–1726.
- , , , et al., Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–487.
- , , , et al.; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular Nursing; American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55(9):934–947.
- , , . Drug‐induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3(5):241–253.
- , , . QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17(3):333–336.
- , , , , . Anti‐inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2006;350(4):977–982.
- , , , , , ; Finnish Study Group for Antimicrobial Resistance (FiRe‐Network). Macrolide‐resistant Streptococcus pneumoniae and use of antimicrobial agents. Clin Infect Dis. 2001;33(4):483–488.
- , , , , , . Community prescribing and resistant Streptococcus pneumoniae. Emerg Infect Dis. 2005;11(6):829–837.
- , , , , ; Finnish Study Group for Antimicrobial Resistance (FiRe Network). Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2006;50(11):3646–3650.
© 2015 Society of Hospital Medicine