User login
Heme Themes: Transplant timing for myelofibrosis in the era of JAK2 inhibitors
ALEXANDRIA, VA. – How are mutation analysis gene panels affecting risk stratification and decision making regarding stem cell transplants in myelofibrosis patients? How are the results of the Dynamic International Prognostic Scoring System (DIPSS) and performance status improvements seen with JAK2 inhibitors influencing who is a candidate for transplant and the timing of transplants?
Watch the conversation between Dr. Vikas Gupta of the leukemia and bone marrow transplant programs at the Princess Margaret Cancer Centre, Toronto, and associate professor of medicine at the University of Toronto, and Dr. Rami S. Komrokji of the Moffitt Cancer Center, Tampa, Fla., as they discuss their individual approaches that consider patient wishes and goals, type of donor, and disease risk in their decisions to perform stem cell transplants upfront or to delay them until after JAK2 inhibitor therapy.
Have an insight to share? Post a comment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ALEXANDRIA, VA. – How are mutation analysis gene panels affecting risk stratification and decision making regarding stem cell transplants in myelofibrosis patients? How are the results of the Dynamic International Prognostic Scoring System (DIPSS) and performance status improvements seen with JAK2 inhibitors influencing who is a candidate for transplant and the timing of transplants?
Watch the conversation between Dr. Vikas Gupta of the leukemia and bone marrow transplant programs at the Princess Margaret Cancer Centre, Toronto, and associate professor of medicine at the University of Toronto, and Dr. Rami S. Komrokji of the Moffitt Cancer Center, Tampa, Fla., as they discuss their individual approaches that consider patient wishes and goals, type of donor, and disease risk in their decisions to perform stem cell transplants upfront or to delay them until after JAK2 inhibitor therapy.
Have an insight to share? Post a comment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ALEXANDRIA, VA. – How are mutation analysis gene panels affecting risk stratification and decision making regarding stem cell transplants in myelofibrosis patients? How are the results of the Dynamic International Prognostic Scoring System (DIPSS) and performance status improvements seen with JAK2 inhibitors influencing who is a candidate for transplant and the timing of transplants?
Watch the conversation between Dr. Vikas Gupta of the leukemia and bone marrow transplant programs at the Princess Margaret Cancer Centre, Toronto, and associate professor of medicine at the University of Toronto, and Dr. Rami S. Komrokji of the Moffitt Cancer Center, Tampa, Fla., as they discuss their individual approaches that consider patient wishes and goals, type of donor, and disease risk in their decisions to perform stem cell transplants upfront or to delay them until after JAK2 inhibitor therapy.
Have an insight to share? Post a comment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT U.S. FOCUS ON MPN AND MDS
Heme Themes: Challenges in averting the progression of MPN, MDS
ALEXANDRIA, VA. – What are the emerging combination therapies for slowing disease progression and improving therapeutic tolerability in myeloproliferative neoplasms and myelodysplastic syndromes?
Join Dr. Jaroslaw Maciejewski, chairman of the department of translational hematology and oncology research at the Taussig Cancer Institute, Cleveland Clinic, and professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, and Dr. Ruben A. Mesa, chair of the Division of Hematology/Oncology, department of internal medicine, Mayo Clinic, Scottsdale, Arizona, for their one-on-one discussion of emerging treatment approaches. Then join the conversation by posting your comments and recommendations for other Heme Themes.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ALEXANDRIA, VA. – What are the emerging combination therapies for slowing disease progression and improving therapeutic tolerability in myeloproliferative neoplasms and myelodysplastic syndromes?
Join Dr. Jaroslaw Maciejewski, chairman of the department of translational hematology and oncology research at the Taussig Cancer Institute, Cleveland Clinic, and professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, and Dr. Ruben A. Mesa, chair of the Division of Hematology/Oncology, department of internal medicine, Mayo Clinic, Scottsdale, Arizona, for their one-on-one discussion of emerging treatment approaches. Then join the conversation by posting your comments and recommendations for other Heme Themes.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ALEXANDRIA, VA. – What are the emerging combination therapies for slowing disease progression and improving therapeutic tolerability in myeloproliferative neoplasms and myelodysplastic syndromes?
Join Dr. Jaroslaw Maciejewski, chairman of the department of translational hematology and oncology research at the Taussig Cancer Institute, Cleveland Clinic, and professor of medicine at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, and Dr. Ruben A. Mesa, chair of the Division of Hematology/Oncology, department of internal medicine, Mayo Clinic, Scottsdale, Arizona, for their one-on-one discussion of emerging treatment approaches. Then join the conversation by posting your comments and recommendations for other Heme Themes.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM U.S. FOCUS ON MPN & MDS
Kidney and Bladder Stones Do Not Increase Postmenopausal Women’s Risk of Osteoporosis
Postmenopausal women with kidney or bladder stones are not at increased risk for osteoporosis, but they do have about a 15% increased risk of another painful stone, according to a study published online ahead of print May 19 in the Journal of Bone and Mineral Research.
Researchers examined data on approximately 150,000 postmenopausal women and found, despite the 2 conditions being clearly associated in men, the same did not hold true for women. “We know in men that if you have a kidney stone, you are more likely to have osteoporosis,” said Laura D. Carbone, MD, MS, Division Chief Chief of Rheumatology in the Department of Medicine at the Medical College of Georgia at Georgia Regents University in Augusta. What Dr. Carbone and colleagues found was that “unlike what has been reported in men, a woman having a kidney stone is not a risk factor for osteoporosis. However, having one urinary tract stone does put women at increased risk for a second stone.”
Data came from participants in the National Institutes of Health Women’s Health Initiative. Out of more than 150,000 women followed in the Women’s Health Initiative, 9,856 women reported urinary tract stones at the start of or over the course of the study. The women were followed for about 8 years, on average.
Dr. Carbone and colleagues looked at the data several different ways, adjusting for factors that could also influence outcome, such as physical inactivity. Investigators only looked at whether urinary tract stones increased the risk of osteoporosis, not the reverse. In unadjusted models there was a significant association of urinary tract stones with incident total fractures (hazard ratio, 1.10) However, in covariate adjusted analyses, urinary tract stones were not significantly related to changes in bone mineral density at any skeletal site or to incident fractures.
The Osteoporotic Fractures in Men study, which looked at nearly 6,000 men with a mean age of 73.7 to determine risk factors for osteoporosis, identified urinary tract stones are a risk factor.
One link between the seemingly disparate conditions of stones and weak bones is an excess of calcium in the urine, which tends to be more common in men, Dr. Carbone said. Sodium and calcium share a common transport mechanism in the kidney, the researchers pointed out, and sodium affects reabsorption of calcium by that organ. When sodium levels are high, more calcium in eliminated in the urine. “Overactivity of the parathyroid glands, which regulate levels of calcium in the blood, is associated with both urinary tract stones and fractures of the vertebra in the spine,” the researchers said.
Some treatments for osteoporosis, including calcium supplementation, can increase the risk of stones. Conversely, individuals who’ve already experienced a urinary tract stone might avoid calcium to help avoid another a subsequent stone and inadvertently increase their osteoporosis risk, the researchers wrote.
“Women with a stone likely should work with their physician to reduce their increased risk of a subsequent stone,” the physicians said, noting that low water and fluid intake, and a high-salt, high-calorie diet are common stone risks.
Suggested Reading
Carbone LD, Hovey KM, Andrews CA, et al. Urinary tract stones and osteoporosis: findings from the women’s health initiative. J Bone Miner Res. 2015 May 19 [Epub ahead of print].
Postmenopausal women with kidney or bladder stones are not at increased risk for osteoporosis, but they do have about a 15% increased risk of another painful stone, according to a study published online ahead of print May 19 in the Journal of Bone and Mineral Research.
Researchers examined data on approximately 150,000 postmenopausal women and found, despite the 2 conditions being clearly associated in men, the same did not hold true for women. “We know in men that if you have a kidney stone, you are more likely to have osteoporosis,” said Laura D. Carbone, MD, MS, Division Chief Chief of Rheumatology in the Department of Medicine at the Medical College of Georgia at Georgia Regents University in Augusta. What Dr. Carbone and colleagues found was that “unlike what has been reported in men, a woman having a kidney stone is not a risk factor for osteoporosis. However, having one urinary tract stone does put women at increased risk for a second stone.”
Data came from participants in the National Institutes of Health Women’s Health Initiative. Out of more than 150,000 women followed in the Women’s Health Initiative, 9,856 women reported urinary tract stones at the start of or over the course of the study. The women were followed for about 8 years, on average.
Dr. Carbone and colleagues looked at the data several different ways, adjusting for factors that could also influence outcome, such as physical inactivity. Investigators only looked at whether urinary tract stones increased the risk of osteoporosis, not the reverse. In unadjusted models there was a significant association of urinary tract stones with incident total fractures (hazard ratio, 1.10) However, in covariate adjusted analyses, urinary tract stones were not significantly related to changes in bone mineral density at any skeletal site or to incident fractures.
The Osteoporotic Fractures in Men study, which looked at nearly 6,000 men with a mean age of 73.7 to determine risk factors for osteoporosis, identified urinary tract stones are a risk factor.
One link between the seemingly disparate conditions of stones and weak bones is an excess of calcium in the urine, which tends to be more common in men, Dr. Carbone said. Sodium and calcium share a common transport mechanism in the kidney, the researchers pointed out, and sodium affects reabsorption of calcium by that organ. When sodium levels are high, more calcium in eliminated in the urine. “Overactivity of the parathyroid glands, which regulate levels of calcium in the blood, is associated with both urinary tract stones and fractures of the vertebra in the spine,” the researchers said.
Some treatments for osteoporosis, including calcium supplementation, can increase the risk of stones. Conversely, individuals who’ve already experienced a urinary tract stone might avoid calcium to help avoid another a subsequent stone and inadvertently increase their osteoporosis risk, the researchers wrote.
“Women with a stone likely should work with their physician to reduce their increased risk of a subsequent stone,” the physicians said, noting that low water and fluid intake, and a high-salt, high-calorie diet are common stone risks.
Postmenopausal women with kidney or bladder stones are not at increased risk for osteoporosis, but they do have about a 15% increased risk of another painful stone, according to a study published online ahead of print May 19 in the Journal of Bone and Mineral Research.
Researchers examined data on approximately 150,000 postmenopausal women and found, despite the 2 conditions being clearly associated in men, the same did not hold true for women. “We know in men that if you have a kidney stone, you are more likely to have osteoporosis,” said Laura D. Carbone, MD, MS, Division Chief Chief of Rheumatology in the Department of Medicine at the Medical College of Georgia at Georgia Regents University in Augusta. What Dr. Carbone and colleagues found was that “unlike what has been reported in men, a woman having a kidney stone is not a risk factor for osteoporosis. However, having one urinary tract stone does put women at increased risk for a second stone.”
Data came from participants in the National Institutes of Health Women’s Health Initiative. Out of more than 150,000 women followed in the Women’s Health Initiative, 9,856 women reported urinary tract stones at the start of or over the course of the study. The women were followed for about 8 years, on average.
Dr. Carbone and colleagues looked at the data several different ways, adjusting for factors that could also influence outcome, such as physical inactivity. Investigators only looked at whether urinary tract stones increased the risk of osteoporosis, not the reverse. In unadjusted models there was a significant association of urinary tract stones with incident total fractures (hazard ratio, 1.10) However, in covariate adjusted analyses, urinary tract stones were not significantly related to changes in bone mineral density at any skeletal site or to incident fractures.
The Osteoporotic Fractures in Men study, which looked at nearly 6,000 men with a mean age of 73.7 to determine risk factors for osteoporosis, identified urinary tract stones are a risk factor.
One link between the seemingly disparate conditions of stones and weak bones is an excess of calcium in the urine, which tends to be more common in men, Dr. Carbone said. Sodium and calcium share a common transport mechanism in the kidney, the researchers pointed out, and sodium affects reabsorption of calcium by that organ. When sodium levels are high, more calcium in eliminated in the urine. “Overactivity of the parathyroid glands, which regulate levels of calcium in the blood, is associated with both urinary tract stones and fractures of the vertebra in the spine,” the researchers said.
Some treatments for osteoporosis, including calcium supplementation, can increase the risk of stones. Conversely, individuals who’ve already experienced a urinary tract stone might avoid calcium to help avoid another a subsequent stone and inadvertently increase their osteoporosis risk, the researchers wrote.
“Women with a stone likely should work with their physician to reduce their increased risk of a subsequent stone,” the physicians said, noting that low water and fluid intake, and a high-salt, high-calorie diet are common stone risks.
Suggested Reading
Carbone LD, Hovey KM, Andrews CA, et al. Urinary tract stones and osteoporosis: findings from the women’s health initiative. J Bone Miner Res. 2015 May 19 [Epub ahead of print].
Suggested Reading
Carbone LD, Hovey KM, Andrews CA, et al. Urinary tract stones and osteoporosis: findings from the women’s health initiative. J Bone Miner Res. 2015 May 19 [Epub ahead of print].
Obinutuzumab trends better than rituxumab in relapsed indolent lymphoma
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
Patients with relapsed follicular lymphoma who were treated with obinutuzumab experienced higher response rates than did patients given rituximab with an acceptable safety profile, according to new findings.
However, the difference did not translate into an improvement in progression-free survival, so the clinical value of obinutuzumab in this patient population is still unclear.
The quality of remissions was better with obinutuzumab, with an almost twofold higher complete response/unconfirmed complete response rate (41.9% vs. 22.7%; P = .006),” wrote Dr. Laurie Sehn from the Centre for Lymphoid Cancer, British Columbia Cancer Agency and the University of British Columbia, Vancouver, and her colleagues (J Clin Oncol. 2015 Aug 17. doi:10.1200/JCO.2014.59.2139).
On the basis of an independent review, the best overall response was better in the obinutuzumab arm (P = .04), but the complete response/unconfirmed response rate was not different for the two groups.
The study was published online Aug. 17 in the Journal of Clinical Oncology.
A total of 175 patients with relapsed CD20+ indolent lymphoma were randomized 1:1 to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Those without any evidence of disease progression after completing induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
At the end of induction, the investigator assessed overall response rate was 44.6% in the obinutuzumab arm and 33.3% in the rituximab arm (P = .08); nine patients receiving obinutuzumab (12.2%) and four given rituximab (5.3%) achieved complete response or unconfirmed complete response, but the difference was not significant (P = .07).
Independent review also found the overall response rate to be higher with obinutuzumab vs. rituximab (44.6% vs. 26.7%; P = .01), but with no difference in complete response/unconfirmed complete response rate (5.4 vs. 4.0; P = .34).
Adverse events were similar in each group, and most episodes were grade 1 to 2. Higher rates of infusion-related reactions (74% vs. 51%) and cough (24% vs. 9%) were observed in the obinutuzumab vs. the rituximab arm.
Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to, Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Obinutuzumab was associated with a higher overall response rate as compared with rituximab, but obinutuzumab’s clinical benefit in non–Hodgkin lymphoma is still unclear.
Major finding: Among patients with follicular lymphoma (n = 149), overall response rate trended higher for obinutuzumab, compared with rituximab (44.6% vs. 33.3%; P = .08).
Data source: An open-label, multicenter, randomized, phase II study of 175 patients with relapsed CD20+ indolent lymphoma that compared induction with obinutuzumab vs. rituximab.
Disclosures: Dr. Sehn receives research funding and honoraria from, and serves in a consulting or advisory role to Roche/Genentech, the maker of obinutuzumab (Gyzyva) and rituximab (Rituxan). She also receives honoraria from and serves in a consulting or advisory role to Amgen, Janssen, Seattle Genetics, Lundbeck, and Celgene.
Knee Replacement Proves Effective for Degeneration Caused by Blount Disease
Total knee replacements can effectively treat degeneration caused by Blount disease, according to a study published online ahead of print July 11 in the Journal of Arthroplasty.
Middle-aged patients with Blount disease who underwent joint replacements on 1 or both knees were found to have stable knees, excellent range of motion, and no need for pain medications, according to the study conducted at Loyola University Medical Center in Illinois.
“With proper attention paid to technical details, patients with Blount or Blount-like deformity can undergo successful total knee arthroplasty,” said Harold Rees, MD, Assistant Professor of Reconstructive Surgery and Joint Replacement at Loyola University Chicago Stritch School of Medicine in Maywood, Illinois, and colleagues.
For the study, Dr. Rees and colleagues reviewed the records of 5 patients with Blount disease. Three patients had replacements on both knees and 2 patients had replacements on 1 knee. Four patients were African American and 4 were male. All were obese. The average age at the time of the knee replacements was 49.9. Patients were followed-up an average of 75.2 months after their knee replacements.
Mean proximal tibial metaphyseal-diaphyseal angle was 20.75 degrees. Each patient had substantial posteromedial tibial bony defects and 6 knees required extensive medial releases. Two knees required increased constraint at index procedure. One patient underwent bilateral revision surgery with rotating hinge prostheses.
The researchers used a scoring system, devised by the Knee Society, that combines clinical, functional, and satisfaction scores. The mean Knee Society score was 212.5, out of a maximum possible score of 255. Patients also were rated on the Western Ontario and McMaster Universities Osteoarthritis Index.
“The main purpose was to highlight surgical considerations in performing total knee arthroplasty in patients with Blount disease or Blount-like deformity. Despite a challenging patient population in which to perform total knee arthroplasty, we show that it can be done with a low risk of complication and reasonable medium-term results,” said the study authors. “Surgeons should be prepared to address posteromedial tibial bony defects and consider constrained arthroplasty at the index procedure,” they said.
Suggested Reading
Natoli RM, Nypaver CM, Schiff AP, et al. Total knee arthroplasty in patients with blount disease or blount-like deformity. J Arthroplasty. 2015 Jul 11 [Epub ahead of print].
Total knee replacements can effectively treat degeneration caused by Blount disease, according to a study published online ahead of print July 11 in the Journal of Arthroplasty.
Middle-aged patients with Blount disease who underwent joint replacements on 1 or both knees were found to have stable knees, excellent range of motion, and no need for pain medications, according to the study conducted at Loyola University Medical Center in Illinois.
“With proper attention paid to technical details, patients with Blount or Blount-like deformity can undergo successful total knee arthroplasty,” said Harold Rees, MD, Assistant Professor of Reconstructive Surgery and Joint Replacement at Loyola University Chicago Stritch School of Medicine in Maywood, Illinois, and colleagues.
For the study, Dr. Rees and colleagues reviewed the records of 5 patients with Blount disease. Three patients had replacements on both knees and 2 patients had replacements on 1 knee. Four patients were African American and 4 were male. All were obese. The average age at the time of the knee replacements was 49.9. Patients were followed-up an average of 75.2 months after their knee replacements.
Mean proximal tibial metaphyseal-diaphyseal angle was 20.75 degrees. Each patient had substantial posteromedial tibial bony defects and 6 knees required extensive medial releases. Two knees required increased constraint at index procedure. One patient underwent bilateral revision surgery with rotating hinge prostheses.
The researchers used a scoring system, devised by the Knee Society, that combines clinical, functional, and satisfaction scores. The mean Knee Society score was 212.5, out of a maximum possible score of 255. Patients also were rated on the Western Ontario and McMaster Universities Osteoarthritis Index.
“The main purpose was to highlight surgical considerations in performing total knee arthroplasty in patients with Blount disease or Blount-like deformity. Despite a challenging patient population in which to perform total knee arthroplasty, we show that it can be done with a low risk of complication and reasonable medium-term results,” said the study authors. “Surgeons should be prepared to address posteromedial tibial bony defects and consider constrained arthroplasty at the index procedure,” they said.
Total knee replacements can effectively treat degeneration caused by Blount disease, according to a study published online ahead of print July 11 in the Journal of Arthroplasty.
Middle-aged patients with Blount disease who underwent joint replacements on 1 or both knees were found to have stable knees, excellent range of motion, and no need for pain medications, according to the study conducted at Loyola University Medical Center in Illinois.
“With proper attention paid to technical details, patients with Blount or Blount-like deformity can undergo successful total knee arthroplasty,” said Harold Rees, MD, Assistant Professor of Reconstructive Surgery and Joint Replacement at Loyola University Chicago Stritch School of Medicine in Maywood, Illinois, and colleagues.
For the study, Dr. Rees and colleagues reviewed the records of 5 patients with Blount disease. Three patients had replacements on both knees and 2 patients had replacements on 1 knee. Four patients were African American and 4 were male. All were obese. The average age at the time of the knee replacements was 49.9. Patients were followed-up an average of 75.2 months after their knee replacements.
Mean proximal tibial metaphyseal-diaphyseal angle was 20.75 degrees. Each patient had substantial posteromedial tibial bony defects and 6 knees required extensive medial releases. Two knees required increased constraint at index procedure. One patient underwent bilateral revision surgery with rotating hinge prostheses.
The researchers used a scoring system, devised by the Knee Society, that combines clinical, functional, and satisfaction scores. The mean Knee Society score was 212.5, out of a maximum possible score of 255. Patients also were rated on the Western Ontario and McMaster Universities Osteoarthritis Index.
“The main purpose was to highlight surgical considerations in performing total knee arthroplasty in patients with Blount disease or Blount-like deformity. Despite a challenging patient population in which to perform total knee arthroplasty, we show that it can be done with a low risk of complication and reasonable medium-term results,” said the study authors. “Surgeons should be prepared to address posteromedial tibial bony defects and consider constrained arthroplasty at the index procedure,” they said.
Suggested Reading
Natoli RM, Nypaver CM, Schiff AP, et al. Total knee arthroplasty in patients with blount disease or blount-like deformity. J Arthroplasty. 2015 Jul 11 [Epub ahead of print].
Suggested Reading
Natoli RM, Nypaver CM, Schiff AP, et al. Total knee arthroplasty in patients with blount disease or blount-like deformity. J Arthroplasty. 2015 Jul 11 [Epub ahead of print].
Cosmetic Corner: Dermatologists Weigh in on OTC Dandruff Treatments
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC dandruff treatments. Consideration must be given to:
- Head & Shoulders Shampoo
Procter & Gamble
“OTC dandruff products are more for maintenance rather than active treatment, which is why many consumers and patients become frustrated with their use. I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
- Moroccanoil Treatment
Moroccanoil
“I think it’s great to actually put [this product] directly onto the scalp after shampooing to get any remaining scales off.”—Anthony M. Rossi, MD, New York, New York
- Neutrogena T/Gel Therapeutic Hair Care
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Neutrogena T/Sal Therapeutic Shampoo
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Nizoral A-D Ketoconazole Shampoo 1%
McNeil-PPC, Inc
“I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
Cutis invites readers to send us their recommendations. Eye creams, men’s shaving products, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC dandruff treatments. Consideration must be given to:
- Head & Shoulders Shampoo
Procter & Gamble
“OTC dandruff products are more for maintenance rather than active treatment, which is why many consumers and patients become frustrated with their use. I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
- Moroccanoil Treatment
Moroccanoil
“I think it’s great to actually put [this product] directly onto the scalp after shampooing to get any remaining scales off.”—Anthony M. Rossi, MD, New York, New York
- Neutrogena T/Gel Therapeutic Hair Care
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Neutrogena T/Sal Therapeutic Shampoo
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Nizoral A-D Ketoconazole Shampoo 1%
McNeil-PPC, Inc
“I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
Cutis invites readers to send us their recommendations. Eye creams, men’s shaving products, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on the top OTC dandruff treatments. Consideration must be given to:
- Head & Shoulders Shampoo
Procter & Gamble
“OTC dandruff products are more for maintenance rather than active treatment, which is why many consumers and patients become frustrated with their use. I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
- Moroccanoil Treatment
Moroccanoil
“I think it’s great to actually put [this product] directly onto the scalp after shampooing to get any remaining scales off.”—Anthony M. Rossi, MD, New York, New York
- Neutrogena T/Gel Therapeutic Hair Care
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Neutrogena T/Sal Therapeutic Shampoo
Johnson & Johnson Consumer Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Nizoral A-D Ketoconazole Shampoo 1%
McNeil-PPC, Inc
“I recommend to soak [this product] on the scalp skin (not hair) for 5 minutes 2 to 3 times per week.”—Adam Friedman, MD, Washington, DC
Cutis invites readers to send us their recommendations. Eye creams, men’s shaving products, and products for babies will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to [email protected].
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Reducing maternal mortality in the United States—Let’s get organized!
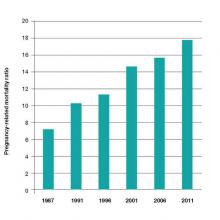
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A mother’s untimely death in childbirth is a grave loss that sends shock waves of grief across generations of her family and community. As obstetricians practicing in the United States, we face a terrible problem. We have a continually rising rate of maternal death in a country with exceptional medical resources (FIGURE).1 Our national decentralized approach to dealing with maternal mortality is a factor contributing to the decades-long increase in the maternal mortality ratio. Let’s get organized to better respond to this public health crisis.
Medical education— Let’s get focused on maternal mortality
The 140-page Council on Resident Education in Obstetrics and Gynecology CREOG Educational Objectives: Core Curriculum in Obstetrics and Gynecology provides a detailed enumeration of the key learning objectives for residents in obstetrics and gynecology.2 Surprisingly, the CREOG objectives do not mention reducing maternal mortality as an important curricular goal. Learning clinical processes and practices that decrease the risk of maternal mortality should be an important educational goal for all residents training in obstetrics and gynecology.
Nationwide action is needed to address the problem
Many countries have organized widespread efforts to reduce maternal mortality. In the United Kingdom and France there are nationwide reviews of maternal deaths with detailed analyses of clinical events and identification of areas for future improvement. These reviews result in the dissemination of countrywide clinical recommendations that change practice and hopefully reduce the risk of future maternal deaths. For example, following the identification of pulmonary embolism as a leading cause of maternal death in the United Kingdom there was a nationwide effort to increase the use of mechanical and pharmacologic prophylaxis to prevent deep venous thrombosis.
In the United States, experts have proposed that a national program of clinical review of severe maternal morbidity cases should be mandatory. (There are many more cases of “near misses” with severe maternal morbidity than there are maternal deaths.) The greater number of cases available for review should help institutions to quickly recognize potential areas for clinical improvement. One group of experts has recommended that all deliveries in which a pregnant woman received 4 or more units of blood or was admitted to an intensive care unit should be thoroughly reviewed to identify opportunities for clinical improvement.3
In the United Kingdom a contemporary clinical problem that is being addressed in an organized and systematic manner is how to respond to the rising rate of severe maternal morbidity caused by placenta accreta. Experts have concluded that women with a suspected placenta accreta should deliver in regional centers with advanced clinical resources—including an emergency surgical response team, interventional radiology, a high capacity blood bank, and an intensive care unit.
A similar approach has been proposed for managing placenta accreta in the United States.4 The American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal Fetal Medicine (SMFM) have proposed a tiered system of obstetric care with more complex cases being referred to regional perinatal centers.5 Regionalization of trauma services has been an important part of the US health care system for decades. Cases of severe trauma are brought to regional centers equipped to emergently treat complex injuries. A similar system of regulation and regionalization could be adapted for optimizing maternity care.
High-risk clinical events: Is your unit prepared?
In the United States the leading causes of maternal mortality, in descending order, are6−8:
- cardiovascular diseases
- infection
- hemorrhage
- cardiomyopathy
- pulmonary embolism
- hypertension
- amniotic fluid embolism
- stroke
- anesthesia complications.
Over the last decade, the Joint Commission has recommended that birthing centers develop standardized protocols and use simulation to improve the institution’s ability to respond in a timely manner to clinical events that may result in maternal morbidity or death.
The quality of published protocols dealing with hemorrhage, hypertension, and thromboembolism is continuously improving, and every birthing center should have written protocols that are updated on a regular timetable for these common high-risk events.9,10 Does your birthing unit have written protocols to deal with cardiac diseases, infection, obstetric hemorrhage, thromboembolism, and severe hypertension? Are simulation exercises used to strengthen familiarity with the protocols?
High-risk patients
An amazing fact of today’s medical care is that sexually active women of reproductive age who have high-risk medical problems often have not been counseled to use a highly effective contraceptive, resulting in an increased risk of unintended pregnancy and maternal death. For example, adult women with a history of congenital heart disease are known to be at increased risk of death if they become pregnant. In a recent study, women with a history of congenital heart disease had 178 maternal deaths per 100,000 deliveries—a rate approximately 10-fold higher than the US maternal mortality ratio.11 Yet, many of these women are not using a highly effective contraceptive, and this results in a high rate of unplanned pregnancy.12
In order to reduce the risk of unintended pregnancy in women with high-risk medical problems, health systems could make contraception an important “vital sign” for women with high-risk medical conditions.
Race and age matter greatly when it comes to maternal mortality risk
There are major racial differences in pregnancy-related mortality, with black women having much higher rates than white women. In the United States in 2011, the pregnancy-related mortality ratio for white, black, and women of other races was 12.5, 42.8, and 17.3 deaths per 100,000 live births, respectively. This represents a major racial disparity in pregnancy outcomes.1
The age of the mother is an important determinant of the risk of maternal death. Women younger than age 35 years have the lowest risk of maternal death. From 2006 to 2010, pregnant women older than age 40 had a risk of death approximately 3 times greater than women aged 34 or younger.2
References
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www .cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125(1):5−12.
Let’s get organized
In a country with a history of embracing the “live free or die” ethic, it is often difficult for physicians to enthusiastically embrace the need for a higher level of organization and a potential reduction in individual freedom in order to improve health outcomes. And with a US maternal mortality ratio of 1 maternal death for every 5,400 births, many obstetricians will never have one of their patients die in childbirth. In fact, most obstetricians will have only 1 maternal death during their entire career. In this reality, when clinical events occur rarely, it is not possible for any single clinician, working alone, to impact the overall outcomes of those rare events. Therefore, teamwork and national efforts, such as the National Partnership for Maternal Safety,13 will be necessary to reverse our alarming trend of increasing maternal mortality. Let’s get organized to stop the rise of maternal deaths in the United States.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
- Chescheir NC. Enough already! Obstet Gynecol. 2015;125(1):2−4.
- Council on Resident Education in Obstetrics and Gynecology (CREOG) Educational Objectives: Core Curriculum in Obstetrics and Gynecology. 10th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2013:140.
- Callaghan WM, Grobman WA, Kilpatrick SJ, Main EK, D’Alton M. Facility-based identification of women with severe maternal morbidity: it is time to start. Obstet Gynecol. 2014;123(5):978−981.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561−568.
- American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine. Obstetric care consensus No 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502−515.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998−2005. Obstet Gynecol. 2010;116(6):1302−1309.
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006−2010. Obstet Gynecol. 2015;125(1):5−12.
- Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed August 20, 2015.
- Shields LE, Wiesner S, Fulton J, Pelletreau B. Comprehensive maternal hemorrhage protocols reduce the use of blood products and improve patient safety. Am J Obstet Gynecol. 2015;212(3):272−280.
- James A. Committee on Practice Bulletins—Obstetrics. Practice bulletin No. 123: thromboembolism in pregnancy. ACOG. Obstet Gynecol. 2011;118(3):718−729.
- Thompson JL, Kuklina EV, Bateman BT, Callaghan WM, James AH, Grotegut CA. Medical and obstetrical outcomes among pregnant women with congenital heart disease. Obstet Gynecol. 2015;126(2):346−354.
- Lindley KJ, Madden T, Cahill AG, Ludbrook PA, Billadello JJ. Contraceptive use and unintended pregnancy in women with congenital heart disease. Obstet Gynecol. 2015;126(2):363−369.
- D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973−977.
HHS: Expand antidiscrimination protections to transgender patients
Transgender patients who receive health care via government programs or funding must receive equal access to treatments and insurance coverage, according to a proposed rule issued Sept. 3 by the Health and Human Services department. The rule would extend antidiscrimination policies under the Affordable Care Act to include gender identity.
The rule would apply to health providers who accept patients covered by Medicare and Medicaid as well as insurance purchased via the health insurance marketplaces.
“The proposed rule clarifies and harmonizes existing well-established federal civil rights laws and clarifies the standards that HHS and in particular, the Office of Civil Rights, will apply in implementing [ACA] Section 1557,” Jocelyn Samuels, OCR director, said in a press conference. “Prior laws enforced by the Office of Civil Rights barred discrimination based only on race, color, national origin, age, or disability. All of the protections against sex discrimination that will be incorporated into the rule are new in this space.”
Section 1557 of the ACA extends civil rights protections to ban sex discrimination in federal health care programs and activities. The new proposed rule establishes that the prohibition on sex discrimination includes discrimination based on gender identity. The rule also includes requirements for effective communication for patients with disabilities and enhanced language assistance for patients with limited English proficiency.
Specifics of the proposed rule include:
• Patients must be treated equally and consistent with their gender identity by health providers. Insurers must provide fair access to coverage regardless of gender identity. For example, some insurers have historically excluded coverage of all care related to gender transition. Such categorical exclusions are prohibited under the proposed rule.
• Women must be treated equally with men in the health care they receive, not only in the health coverage they obtain but in the services they seek from providers.
• For patients with disabilities, the rule contains requirements for the provision of auxiliary aids and services, including alternative formats and sign language interpreters and the accessibility of programs offered through electronic and information technology.
• The rule bolsters language assistance for people with limited English proficiency so that patients are able to more effectively communicate with their providers to describe their symptoms and understand treatment.
During the press conference, Ms. Samuels clarified that the rule does not mean that health insurers must cover any specific treatments or procedures, rather they must apply nondiscriminatory criteria when assessing coverage requests.
The proposed extension of protections is, in part, driven by ongoing cases of sex and identity discrimination by some health providers. In one case, a hospital denied a transgender patient a room assignment consistent with her gender identity. In another, a male domestic violence victim was denied services at a hospital because he did not fit the traditional profile of a domestic violence victim, Ms. Samuels said. In another case, a health provider required that a husband be the guarantor for his wife’s medical bills but did not require the same for male patients and their spouses.
“There continue to be serious problems of discrimination in the health care arena,” she said. “This proposed rule provides very valuable tools for us to be able to appropriately address them.”
HHS is requesting comments on whether Section 1557 should include exemptions for religious organizations and, if so, to what extent. The administration notes that nothing in the proposed rule would affect the application of existing protections for religious beliefs and practices, such as provider conscience laws and regulations under the ACA involving preventive health services.
Comments on the rule will be accepted at www.regulations.gov until Nov. 6.
On Twitter @legal_med
Transgender patients who receive health care via government programs or funding must receive equal access to treatments and insurance coverage, according to a proposed rule issued Sept. 3 by the Health and Human Services department. The rule would extend antidiscrimination policies under the Affordable Care Act to include gender identity.
The rule would apply to health providers who accept patients covered by Medicare and Medicaid as well as insurance purchased via the health insurance marketplaces.
“The proposed rule clarifies and harmonizes existing well-established federal civil rights laws and clarifies the standards that HHS and in particular, the Office of Civil Rights, will apply in implementing [ACA] Section 1557,” Jocelyn Samuels, OCR director, said in a press conference. “Prior laws enforced by the Office of Civil Rights barred discrimination based only on race, color, national origin, age, or disability. All of the protections against sex discrimination that will be incorporated into the rule are new in this space.”
Section 1557 of the ACA extends civil rights protections to ban sex discrimination in federal health care programs and activities. The new proposed rule establishes that the prohibition on sex discrimination includes discrimination based on gender identity. The rule also includes requirements for effective communication for patients with disabilities and enhanced language assistance for patients with limited English proficiency.
Specifics of the proposed rule include:
• Patients must be treated equally and consistent with their gender identity by health providers. Insurers must provide fair access to coverage regardless of gender identity. For example, some insurers have historically excluded coverage of all care related to gender transition. Such categorical exclusions are prohibited under the proposed rule.
• Women must be treated equally with men in the health care they receive, not only in the health coverage they obtain but in the services they seek from providers.
• For patients with disabilities, the rule contains requirements for the provision of auxiliary aids and services, including alternative formats and sign language interpreters and the accessibility of programs offered through electronic and information technology.
• The rule bolsters language assistance for people with limited English proficiency so that patients are able to more effectively communicate with their providers to describe their symptoms and understand treatment.
During the press conference, Ms. Samuels clarified that the rule does not mean that health insurers must cover any specific treatments or procedures, rather they must apply nondiscriminatory criteria when assessing coverage requests.
The proposed extension of protections is, in part, driven by ongoing cases of sex and identity discrimination by some health providers. In one case, a hospital denied a transgender patient a room assignment consistent with her gender identity. In another, a male domestic violence victim was denied services at a hospital because he did not fit the traditional profile of a domestic violence victim, Ms. Samuels said. In another case, a health provider required that a husband be the guarantor for his wife’s medical bills but did not require the same for male patients and their spouses.
“There continue to be serious problems of discrimination in the health care arena,” she said. “This proposed rule provides very valuable tools for us to be able to appropriately address them.”
HHS is requesting comments on whether Section 1557 should include exemptions for religious organizations and, if so, to what extent. The administration notes that nothing in the proposed rule would affect the application of existing protections for religious beliefs and practices, such as provider conscience laws and regulations under the ACA involving preventive health services.
Comments on the rule will be accepted at www.regulations.gov until Nov. 6.
On Twitter @legal_med
Transgender patients who receive health care via government programs or funding must receive equal access to treatments and insurance coverage, according to a proposed rule issued Sept. 3 by the Health and Human Services department. The rule would extend antidiscrimination policies under the Affordable Care Act to include gender identity.
The rule would apply to health providers who accept patients covered by Medicare and Medicaid as well as insurance purchased via the health insurance marketplaces.
“The proposed rule clarifies and harmonizes existing well-established federal civil rights laws and clarifies the standards that HHS and in particular, the Office of Civil Rights, will apply in implementing [ACA] Section 1557,” Jocelyn Samuels, OCR director, said in a press conference. “Prior laws enforced by the Office of Civil Rights barred discrimination based only on race, color, national origin, age, or disability. All of the protections against sex discrimination that will be incorporated into the rule are new in this space.”
Section 1557 of the ACA extends civil rights protections to ban sex discrimination in federal health care programs and activities. The new proposed rule establishes that the prohibition on sex discrimination includes discrimination based on gender identity. The rule also includes requirements for effective communication for patients with disabilities and enhanced language assistance for patients with limited English proficiency.
Specifics of the proposed rule include:
• Patients must be treated equally and consistent with their gender identity by health providers. Insurers must provide fair access to coverage regardless of gender identity. For example, some insurers have historically excluded coverage of all care related to gender transition. Such categorical exclusions are prohibited under the proposed rule.
• Women must be treated equally with men in the health care they receive, not only in the health coverage they obtain but in the services they seek from providers.
• For patients with disabilities, the rule contains requirements for the provision of auxiliary aids and services, including alternative formats and sign language interpreters and the accessibility of programs offered through electronic and information technology.
• The rule bolsters language assistance for people with limited English proficiency so that patients are able to more effectively communicate with their providers to describe their symptoms and understand treatment.
During the press conference, Ms. Samuels clarified that the rule does not mean that health insurers must cover any specific treatments or procedures, rather they must apply nondiscriminatory criteria when assessing coverage requests.
The proposed extension of protections is, in part, driven by ongoing cases of sex and identity discrimination by some health providers. In one case, a hospital denied a transgender patient a room assignment consistent with her gender identity. In another, a male domestic violence victim was denied services at a hospital because he did not fit the traditional profile of a domestic violence victim, Ms. Samuels said. In another case, a health provider required that a husband be the guarantor for his wife’s medical bills but did not require the same for male patients and their spouses.
“There continue to be serious problems of discrimination in the health care arena,” she said. “This proposed rule provides very valuable tools for us to be able to appropriately address them.”
HHS is requesting comments on whether Section 1557 should include exemptions for religious organizations and, if so, to what extent. The administration notes that nothing in the proposed rule would affect the application of existing protections for religious beliefs and practices, such as provider conscience laws and regulations under the ACA involving preventive health services.
Comments on the rule will be accepted at www.regulations.gov until Nov. 6.
On Twitter @legal_med
Medical Roundtable: Practical Management of Chronic Myelogenous Leukemia
Moderator: Matt Kalaycio, MD1
Discussants: Michael Mauro, MD2; Michael Deininger, MD, PhD3
From Cleveland Clinic, Cleveland, OH1; Memorial Sloan Kettering Cancer Center, New York, NY2; Huntsman Cancer Institute, Salt Lake City, UT3
Address for correspondence: Matt Kalaycio, MD, Cleveland Clinic Main Campus, Mail Code R32, 9500 Euclid Avenue, Cleveland, OH 44195
E-mail: [email protected]
Biographical sketch:
Dr. Kalaycio has been published in numerous scientific publications including Bone Marrow Transplantation, Journal of Clinical Oncology, and Leukemia. He also is the editor of a book on leukemia and co-editor of a book on clinical malignant hematology. His research interests focus on testing new treatments for leukemia.
Dr. Kalaycio received his degree from West Virginia University School of Medicine in Morgantown. He completed his residency in internal medicine at Mercy Hospital of Pittsburgh and fellowships in hematology and medical oncology and bone marrow transplantation at Cleveland Clinic.
Michael Deininger, MD, PhD, is Professor and Chief of Hematology and Hematologic Malignancies for the Department of Internal Medicine and for the Huntsman Cancer Institute (HCI) at the University of Utah. He is an HCI investigator and member of the Experimental Therapeutics program. He has extensive experience treating patients with blood cancers, including chronic myeloid leukemia (CML) and myeloproliferative neoplasms, a group of blood cancers related to leukemia.
Dr
Dr. Deininger’s scientific focus is leukemia, specifically myeloproliferative neoplasms including chronic myeloid leukemia (CML). As a clinician-scientist with a translational research focus Dr. Deininger is heading an extramurally funded research laboratory that is dedicated to the study of signaling pathways, drug resistance and new molecular therapies in leukemia. Dr. Deininger’s work describing the selective effects of imatinib on CML cells provided the rationale for clinical trials that led to the approval of Gleevec as the first molecularly-based therapy for leukemia. Current work in his lab is focused on understanding the role of the bone marrow microenvironment in leukemia drug resistance, discovering novel therapeutic targets and developing more specific signal transduction inhibitors. Dr. Deininger’s work encompasses more than 170 articles in the peer-reviewed literature, including journals like Blood, Journal of Clinical Investigation and the New England Journal of Medicine. He has co-authored more than 10 book chapters, with contributions in leading textbooks such as deVita’s Principles of Oncology. He is a regular speaker at major international scientific meetings, such as the American Society of Hematology and the European Hematology Association and a peer reviewer for journals like Nature Genetics and Cancer Cell. His honors include the Alexandra Kefalides Prize for Leukemia Research and membership on the Editorial Board of Blood, the leading journal in Hematology. Dr. Deininger was named among the world's Highly Cited Researchers by Thomson Reuters in 2014.
Dr. Kalaycio: My name is Matt Kalaycio and I'm the Chairman of the Department of Hematology and Medical Oncology at the Cleveland Clinic. Today I’m joined by Drs. Mike Deininger, Division Chief of Hematology and Hematologic Malignancies at the University of Utah Huntsman Cancer Institute and Michael Mauro, Myeloproliferative Neoplasm Program Director at Memorial Sloan Kettering Cancer Center, New York, New York – together we will discuss practical issues surrounding CML diagnosis, management and treatment options including TKIs and investigational therapies.
Initial Presentation: Assessment & Treatment Options
Dr. Kalaycio: Often, the patient with chronic myelogenous leukemia (CML) gets admitted to the hospital, or an emergency consult is called, because the white count is up for a concern of leukemia. The treatment team sees the differential circulating blasts, and they worry about acute leukemia. Then the hematologist comes and needs to make a decision about whether or not to do a bone marrow biopsy.
When the patient presents in such a manner, I often see that bone marrow biopsies are not performed. I would like to start by asking where you both stand with regard to the necessity of a bone marrow biopsy at the time of diagnosis. I'll start with Dr. Deininger.
Dr. Deininger: I would strongly be in favor of a diagnostic biopsy as well as a smear. The reason is that I think this is the one and only chance to get a clear disease classification into chronic phase, accelerated phase, and blast crisis.
The third may be rare. There are occasional patients who have sheets of blasts who would not be seen on just a differential. For these patients, of course, the treatment decisions are going to be very different compared to a patient in chronic phase.
I think this is an opportunity that shouldn't be missed and I would always recommend that.
Dr. Kalaycio: Dr. Mauro.
Dr. Mauro: I couldn't concur more. With so much focus on the change in disease status from presentation to early response, I think understanding the scope of the disease at diagnosis, including the bone marrow, is essential and either revealing or reassuring. Although we focus mostly on early molecular response, when expectations go awry, not having as full a picture of the disease prior to treatment leaves you less informed about best treatment.
Making sense of accelerated phase features is a good example; the difference in outcomes between chronic phase patients and those who have morphologic features of accelerated phase—with or without cytogenetic features (clonal evolution)—compared with those with clonal evolution can only drive initial and long term treatment decisions. Without cytogenetics and morphology together, such key pieces of the puzzle are missing; it is worth it for the practitioner and patient to have all the data in hand at all times.
Dr. Kalaycio: Once the diagnosis has been made and the biopsy was not done and now you're seeing the patient having either been on hydroxyurea for a month or having had tyrosine kinase inhibitor (TKI) for a month, do you bother with the bone marrow biopsy at that point?
Dr. Deininger: Well, that is a very good question. I think we would probably still do it most of the time. I think it's very clear that the information you can gather from that is less valuable. I think one should try to make up for the omissions as much as possible. We would still go for it.
Dr. Kalaycio: Interesting. The other thing that happens a lot is a patient will be started on hydrea while you're waiting for BCR-ABL to return either by fluorescence in situ hybridization or quantitative polymerase chain reaction (PCR).
I wonder if you have your own set of internal guidelines for when hydroxyurea should be employed or not.
Dr. Mauro: If you look back at some of the original imatinib trials—the phase I trials—patients initiated TKI with higher blood counts, a median around 25,000 and up to 200,000, and responded with excellent tolerance.1 I think there's a bit of overapplication of hydroxyurea early in CML, prior to initiation of TKI, which often confounds and complicates the early myelosuppressive toxicity of TKIs. With use of more potent TKIs, there may be greater amounts of myelosuppression as the leukemic clone may clear faster.
I think we may get ourselves into a bit of a bind by overusing hydroxyurea and then needing to hold and lower TKI dosages quickly when that may not have been necessary had we simply deployed the TKI sooner.
My general rule would be to use hydroxyurea for symptoms if necessary or for more extreme counts where leukostasis is a concern. – Michael Mauro, MDMy general rule would be to use hydroxyurea for symptoms if necessary or for more extreme counts where leukostasis is a concern. The other question that often comes up is about tumor lysis and hydration, and how closely these need to be managed. The likelihood of tumor lysis is low in CML treated with TKIs, but more frequent early labs and good hydration are always the right thing to do; better safe than sorry!
Dr. Deininger: I second what Michael just said. To your question about whether we have an algorithm, I have to admit we don't. I think it's up to the discretion of the treating physician to initiate hydroxyurea or not.
Dr. Kalaycio: Sure. When you start hydroxyurea, do you routinely add allopurinol?
Dr. Deininger: We tend to do that. I know that some people think it's unnecessary. It's such a low-risk and low-cost intervention that I think that it's hard to get anything wrong here.
Dr. Mauro: Right, we tend to do the same. Whether we need to or not is a different question, I suppose.
Dr. Kalaycio: One more thing about the initial presentation and assessment of a patient with what you think might be a myeloproliferative disorder, CML, how important do you find it, Dr. Mauro, to measure splenomegaly and calculate a risk score?
Dr. Mauro: I think it's very useful information. The spleen size factors heavily into the risk score and the risk score does forecast response to a degree. We've looked at calculation of risk score in recent large observational studies and it is under-reported and underutilized. It winds up being useful for two reasons. One, it does set expectations for response, and US treatment guidelines (National Comprehensive Cancer Network [NCCN] guidelines) note that treatment choice may be different for low versus high risk Sokal score.2
I think the second and most intriguing reason to assess Sokal risk seems now to be the impact that risk score has on the ability to proceed to a treatment-free remission (TFR). There appear to be differences in outcomes in patients with high-risk versus low-risk disease despite both having what is required to proceed to TFR in trials, namely consistent and deep molecular remission over a number of years.
Given these implications, we really ought to be gathering the initial risk stratification and quantifying spleen size. It's an important part of our initial assessment.
Dr. Kalaycio: Dr. Deininger?
Dr. Deininger: I think there's a lot of agreement today. I absolutely think that measuring the spleen size ascertains that you've got all these diagnostic parameters available. I think that should be part of the initial evaluation and work-up and will allow some prognostication.
Testing, Risk Factors & Considerations of Treatment: Nilotinib & Dasatinib
Dr. Kalaycio: I'm starting with the less controversial questions to begin with. I think the next set have the potential for some more controversy. Before we leave the initial assessment of CML, do either of you have observations regarding referrals that you get about which you would like to either dispel myths or remind practitioners about best practices in patients newly diagnosed with CML?
I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. – Michael Deininger, MD, PhDDr. Mauro: I can mention one thing. I think when thinking about initial molecular diagnostic results, it is important to point out that testing should screen broadly for different fusions, namely P190 and P210, or variant (p230) transcripts. There are rare patients in chronic phase with non-p210 fusion who need to be followed with specific PCR. On this same topic, the measurement of transcripts at presentation (ie, before treatment) has become quite important and whereas formerly had not been emphasized, presently all patients should have ”baseline” transcript levels.
Dr. Deininger: I think one issue that comes up once in a while is that spleen measurements are done by ultrasound. Technically it's more accurate, but all the clinical risk scores and the prognostication is based on the old fashioned—but probably highly inaccurate—technology of palpation. This is what counts, this is the value to document. I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. Getting a good handle of those risk factors at diagnosis is also important.
Dr. Kalaycio: I think that's a very important point. That's where I was going to go next with this conversation. I was going to avoid the conversation about which TKI to choose as initial treatment. I think that's a debate unto itself.
I would like to ask you how you might assess cardiovascular risk before placing a patient on nilotinib. You got to that a little bit, Dr. Deininger. Could you expand on what else you might review as far as whether or not you feel a patient is a good candidate to start on nilotinib?
Dr. Deininger: Specifically, with regard to nilotinib, we would always get a baseline electrocardiogram. We would do a clinical exam. We would not do an echocardiogram just routinely in the absence of a cardiovascular history or any clinical evidence for heart failure or other cardiovascular issues.
We've adopted the practice of doing a lipid panel. Of course we would include fasting glucose as well. Some of these recommendations are probably somewhat on the soft side, because it's not yet clear what to do with the information.
On the other hand, I think for a patient who is being considered for nilotinib one wants to make sure that one really does the best to minimize the cardiovascular risk factors. Of course that would include smoking history and taking blood pressure and making sure that these risk factors are controlled.
If people have a presentation that is really out of whack in terms of their risk factor management, I would send them to an internist or even a cardiologist to help me optimize the cardiovascular prevention strategy.
Dr. Kalaycio: Great. Similarly, Dr. Mauro, how do you assess pulmonary risk before placing a patient on dasatinib?
Dr. Mauro: I think here we are focused on the less frequent and also less well understood potential toxicity of pulmonary hypertension, coupled with the more common risk of pleural and pericardial effusions.
I'm not sure how much we've learned in clinical studies looking at baseline chest X-rays or timing of X-rays during treatment. I think our best tool in the prevention and management of pleural and pericardial effusions is full discussion with patients about risk and what to look for, attention to any and all symptoms, and appropriate deployment of diagnostics as indicated.
It's interesting to consider whether baseline echocardiography for measurement of pulmonary pressures is warranted. I would say now we're on probably somewhat softer ground, first because on routine echocardiogram pulmonary pressure can't be measured readily unless there is some valvular regurgitation. As well, it is stated that pulmonary hypertension is only properly diagnosed by right heart catheterization. While I'm tempted to do routine echocardiogram studies, I think that such a recommendation still may be perhaps the realm of a clinical study. We need to explore that further. I think with dasatinib there may be certain patients at higher risk, although the data are somewhat limited. There seem to be certain conditions potentially associated with more pleural and pericardial toxicity, including cardiovascular disease and autoimmune disease. There may be circumstances during treatment—lymphocytosis, for example—that may be associated with greater risk. I think expectant management may still be the right approach and echocardiography and more aggressive diagnostics be reserved for patients in whom there might be much more clinical consequence.
Dr. Kalaycio: I'd like to pursue that a little bit further because sometimes the patients will come to us having already had an echocardiogram that may actually show some mild pulmonary hypertension and maybe they've got significant cardiovascular risk factors where you would otherwise be thinking about using dasatinib. Here's someone with pulmonary hypertension, at least by echocardiographic criteria, would that be enough to dissuade you from the use of dasatinib?
Dr. Mauro: I think it would certainly require significant consideration, understanding what the basis of the pulmonary hypertension is for that patient, and risk with adding dasatinib. I think the good news is the low incidence and the reversibility for the most part of dasatinib-associated pulmonary hypertension.
Again, I think the mechanism of action and the pathophysiology isn't completely understood, although there is the intriguing notion that imatinib has been reported to potentially mitigate pulmonary hypertension whereas dasatinib triggers it—a ”closed loop” if you will and an area ripe for research.
I would probably think that a patient with preexisting pulmonary hypertension in the new diagnosis setting might be the kind of patient for whom you really might weigh the pluses versus the minuses of a second generation TKI versus imatinib.
After Evaluation & Diagnosis: Following the Patient
Dr. Kalaycio: I agree. We have fully evaluated our patient and we've made the diagnosis. Now, we start therapy with a TKI based on patient risk profile and side effect potentials. It's time to follow the patient and determine next steps.
Current guidelines3,4 suggest monitoring quantitative PCR after 3 months of therapy and to gauge response. Dr. Deininger, how do you interpret and act on those results following the first 3 months of therapy?
Dr. Deininger: I think what you're getting at is the 10% mark that is a highly predictive value in terms of subsequent achievement of major molecular response and also overall progression free and overall survival.
We'll certainly get this data point. Then we'll put it in a clinical context. I think this context needs to take into account the initial BCR-ABL transcript level. I think Dr. Mauro mentioned that they always determine that. I think that is really good advice, so you can make a comparison with the diagnostic value.
One scenario, of course, is the patient is well below 10% and then things are just in the green range and you would wave people through and reassess in 3 months. If people are very high, I think then you have to ask yourself why that's the case.
Some people have a lot of toxicity issues. For example, they may not have been able to take the required amount of medication. It's not always easy to clearly distinguish between resistance and intolerance.
No matter what, I think it's going to be critical to consider a potential change in treatment if people are in the 70%—80% range. In my mind, there's a gray area.
These are the people who are maybe between 10%—20%. Here, I think this initial value can really help. If there's a significant reduction compared to baseline, I would not necessarily change at that point.
If there is literally no change compared to baseline, I would strongly consider a change unless I am concerned about other issues like noncompliance or drug interactions. What I'm trying to say in a rather long-winded way is that the 10% value shouldn't be seen as a dogma.
It still needs to be placed in a clinical context. One should not rush to any conclusions. Ten percent, 11%, and 9% are identical values in the world of PCR testing. One should not over-interpret that.
Dr. Kalaycio: I think that you're making an important point about absolutes in the interpretation of these tests. Dr. Mauro, how do you interpret the 3-month data that comes back?
Dr. Mauro: I agree with my colleague on the approach that it has to be put in a clinical context. I think what we've learned is the importance of the starting value and relative reduction and to not consider response milestones as black and white guides.
I think there are certain scenarios that require some caution. The patient who is on imatinib who is close but has not reached the landmark may be different that a similar patient on dasatinib or nilotinib who has not had significant reduction—that's probably a more pressing situation.
It's ironic that guidelines—maybe because of lack of options—don't encourage us to think about changing therapy early in someone who hasn't met milestones in 3 months when they've been put on a more potent agent.
I also think that careful consideration is needed regarding treatment intensity and the impact of interruptions, in conjunction with all the other facets—the rate of change, the absolute change from the patient's baseline, the starting response level and the timing of the PCR. We need to look at the actual day it's performed. If it's really not 3 months of therapy, we can misjudge.
Dr. Kalaycio: Right. Now, as you're monitoring patients, Dr. Mauro, at what point would you consider testing for kinase domain mutations?
Dr. Mauro: I think we have pretty good guidance from studies to date regarding when mutation testing is indicated and of higher yield. It's generally earlier on into treatment when we have clinical scenarios that are of greater risk, namely failure to achieve cytogenetic responses. In such scenarios if mutation testing informs treatment decision making it is very helpful.
Patients who have defined themselves as not achieving early molecular response—which we discussed earlier—especially someone who's had a second-generation TKI, warrants mutation testing in my view. Patients who don't achieve classical cytogenetic response landmarks at 6 and 12 (or 18) months, who thus have a higher residual volume of disease and perhaps as a function more clonal instability, I think also warrant attention.
I think we run into trouble when we start to, in an overly critical manner, assess patients’ longer term and deeper molecular response trajectories. Mutation testing becomes difficult or impossible for patients who are at or near major molecular remission (MMR). Mutation yield is generally very low for patients who have not lost MMR and also likely those with small volume of change around the MMR threshold. Looking ahead, I think further investigation into early time points and the setting of minimal residual disease may yield data to be able to predict potential resistance earlier than observing it clinically.
Dr. Kalaycio: Right. Dr. Deininger, if you're monitoring a patient and he’s missing milestones and you do obtain a mutation analysis and find a T315I mutation, do you offer that patient an opportunity to see how well they're going to do with ponatinib, or do you refer that patient to your transplant team for consideration of transplant as soon as a donor is available?As a transplanter, even I would not suggest transplant upon the recognition of the T315I. I would wait until all available TKIs have been tried and have either not worked or weren't tolerated. – Matt Kalaycio, MD
Dr. Deininger: I think we do the first and half of the second. We would certainly offer a trial of ponatinib or, if possible, a clinical trial unless there are insurmountable contraindications.
I don't really think there are any such circumstances with optimized cardiovascular management at the same time, and we may involve a cardiologist at that point to help us if there are cardiovascular risk factors.
We would also do a referral to a transplant center unless the patient is as per performance status, just not a transplant candidate or is too old. Otherwise, we would always make a referral, but we would not pursue a transplant as the first modality, provided that the patient is in chronic phase. Progression to accelerated phase or blastic phase would be looked at totally differently. Here we would certainly put people on a transplant course.
Dr. Kalaycio: I agree. As a transplanter, even I would not suggest transplant upon the recognition of the T315I. I would wait until all available TKIs have been tried and have either not worked or weren't tolerated.
As the two of you know, we're doing fewer and fewer transplants for CML these days. The results we're getting in folks who are failing all of these agents are not as good as they used to be when we were transplanting in the first 3 months of a new diagnosis. Bone marrow transplant is something to defer for as long as possible, at least in my mind.
TKI Dosage Considerations
Dr. Kalaycio: Now we touched a little bit on the side effects of nilotinib and dasatinib. We've talked about progressive disease, perhaps with or without mutations. Dr. Mauro, could you comment on where you think bosutinib might play a role in patients with relapsed or progressive CML?
Dr. Mauro: I think bosutinib remains an underutilized TKI. It is potent and thus offers good salvage activity. I think it offers a fairly distinct side effect profile making it a good choice for cases of intolerance. I think we've learned more about how to initiate bosutinib and manage its side effects; one element of this is that current trials start at slightly lower doses of bosutinib to avoid some of the early gastrointestinal toxicity.
Bosutinib continues to seek a place as a first-line therapy. I think this may be worthwhile as we are looking for the right balance of safety and side effect risk to maximize early response. Regarding the salvage setting—intolerance and resistance to other agents—I find myself speaking to patients about bosutinib as a potent and viable alternative. Bosutinib offers a similar spectrum of activity as dasatinib but can allow us to avoid cardiovascular toxicity and/or allow clearance of pleural and pericardial toxicity occurring with dasatinib.
The one place I would say bosutinib may not hold up as strongly to its competition would be as a third-line therapy after failure of a second-generation TKI. I think while there is reasonable activity with bosutinib in this setting of somewhat fairly drug-resistant CML, the performance of ponatinib is better. There is likely often a struggle with the long-term safety question of ponatinib, making some feel that a trial of bosutinib is often a worthwhile, logical, or even necessary step before ponatinib. My concerns about this approach are related to treatment decision-making based on safety and theoretical risk of adverse effects rather than efficacy and risk of progression; these have to be weighed carefully and appropriately against each other.
Dr. Kalaycio: That's an interesting perspective. You mentioned starting with a lower dose. Dr. Deininger, I'd like to ask you your thoughts about dose modifications of the TKIs in general. It used to be anathema to either start with a low dose or to maintain patients on a lower dose.
I'm aware of at least some data suggesting that those patients who tolerate these TKIs poorly particularly with regard to myelosuppression can be treated long term at lower than typically prescribed doses without adverse effect. What are your thoughts surrounding dose modification of the TKIs?
Dr. Deininger: I would stratify according to TKIs. I think imatinib at 400 mg is probably just rightly dosed, maybe not even at the optimal dose. It could be 600 mg. If you go to 300 mg, that is still acceptable.
If you go to 200 mg, I think I would make double sure that I monitor patients very frequently. People should be, in my mind, in a major molecular response to treat them with 200 mg long term. With the second-generation TKIs, it's a little different.
I cannot really speak to bosutinib because I'm not aware of a lot of data on those modifications. What seems to be clear from dasatinib is that the initial recommended dose of 100 mg is quite high, especially in older people.
I believe that's been corroborated by a study that hasn't been published yet. Apparently the drug excretion in the older individuals is quite a bit slower than in younger people. I think in our practice and in other people's practices as well, about 50% of those people end at doses substantially lower than 100 mg, maybe 50 mg, maybe 40 mg, some even 20 mg per day.
I think in the case of dasatinib you have a lot of maneuvering space. You can adjust the dose according to tolerability and molecular response. With nilotinib I think it's kind of similar. We have a few patients who are on maybe 150 mg twice a day because they have issues in terms of side effects at the higher doses.
I should again thoroughly qualify that by saying if you give up through dose reductions the goal of a major molecular response, then I think you really have to think about the strategy in general because at that point, I think you basically say "this patient is not ever going to be reaching a safe haven and is not tolerating inhibitors well enough to get him there."
That's quite a significant statement to make. Myelosuppression is frequently a situation where you cannot deliver enough dose but you also don't get a good response, whereas other side effects like pleural effusions or excessive fluid retention may well be cause for dose reduction and yet responses may be acceptable. Maybe Dr. Mauro could chime in here and share his experience.
Dr. Mauro: I agree. I think some patients, based on their disease status, may not tolerate the dose that is going to—at least in theory—get them to a deep molecular remission. The biggest problem we often face is myelosuppression. Some patients may have indolent disease and still have a good outcome, at least in the short to medium term, with lower doses of drug. In general I try to work through myelosuppression, especially early, as the combination of myelosuppression and suboptimal or response failure is dangerous.
I think treatment shouldn't be too chaotic and change too much, if possible. That was a very nice summary of the way we view the doses. There's still a fair bit of flexibility amongst the TKI doses, although I don't advocate for starting low and titration up.
In general I think, with regard to optimal TKI dose, we might have overshot; for example, with dasatinib. I think we might have overshot with bosutinib. Trials ongoing now initiate bosutinib at 400 mg rather than 500 mg. That's what I was alluding to.
Treatment Free Remission: Approaches
Dr. Kalaycio: Very interesting discussions. As we wind down the conversation, I want to get to the idea of stopping therapy. We're all aware of data that suggest it's at least possible for a proportion of patients to stop treatment.5
However, the guidelines3,4 such as they are, suggest that stopping should not be done outside the context of a clinical trial. I think most of us would agree with that. I wonder in a practical sense, Dr. Mauro, in your practice, do you have your own set of internal criteria for stopping a TKI and observing patients in the absence of any therapy?
Dr. Mauro: I think I try to incorporate all the experience we have to date when considering this question. That being said I'm a little hesitant to agree entirely with the current thinking regarding retreatment during a Treatment Free Remission trial, which is waiting until patients lose MMR in order to resume treatment but agree it might be hard to retreat based on lesser degrees of molecular relapse. I just worry a little bit about that amount of proliferation without treatment.
On the other hand I think we're coming to the realization that TFR may be feasible in patients who are simply nonproliferative and have low volume of disease based on newer data that patients may not need to have consistent "complete molecular response" or disease reduction below 4.5 logs in order to consider TFR. This will mean more patients may be eligible for such a strategy.
With that being said, I strongly advocate for discontinuation occurring in trials still, especially in the United States. I think we still have to ensure regular monitoring and not run the risk of loss of CML remission as a result of this endeavor. I think we have to have a clear message that this is still an investigational approach.
As we apply TFR strategies more broadly and explore it in patients with different circumstances, rather than those already studied in clinical trials, we may see slightly different results. We may need to exercise more caution.
Outside of a trial I don't discontinue TKI therapy unless there's a clear medical indication and there's no way around it, such as pregnancy or other illness that precludes TKI treatment. I would still pursue TFR only in a clinical trial.
Dr. Kalaycio: Dr. Deininger, I'll give you the last word for your thoughts regarding both treatment discontinuation and the future of CML therapies.
Dr. Deininger: As far as TFR is concerned, I agree with Dr. Mauro. Unfortunately, in the United States we're not quite as far advanced as the Europeans at monitoring diligence. There's a bit of a concern that if we elevate that into daily practice, we may see that patients are not monitored frequently enough and then relapse, but that doesn't get caught. You could imagine a scenario where we're actually seeing a decline in outcomes because people are discontinued and then not restarted if they have a recurrence at the molecular level.
All in all, where this is going, I think it will be really interesting to see more confirmatory data for second-generation TKIs. Let me put that differently: whether a similar proportion of patients in deep molecular response can maintain responses and have TFR if they needed a second-generation TKI to get there rather than just imatinib.
If so, this would be very promising. That would mean that second-generation TKIs actually somewhat impact the natural history of the disease and its biology. Then TFR may become a reality for a substantial proportion of our patients.
I don't think we have the data yet, but at least there are some suggestions that this may be the case. If you do the math, you will still see that many patients with CML will never reach a state where they can consider TFR.
They'll never get into a deep molecular response or they will have been diagnosed with accelerated phase. For all these patients who are not candidates for TFR, we still have to think about treatment optimization in order to make them catch up with the rest.
Now I have a couple of interesting developments. One is clearly to see whether ponatinib administered at a lower dose will have a more practical, therapeutic window in terms of toxicity and yet maintain the excellent efficacy that it has in the setting.
These data need to be produced. Another scenario would be that people would get started on intense induction treatment with higher-risk drugs such as ponatinib. Then if they are a good responder, they can switch to something that is lower-risk and that may be better tolerated.
Actually some of these trials are underway in Europe. Of course there's the question, are there still other drugs that will enter the CML space. There's one promising molecule called ABL001, a TKI with a different mode of action, which exploits an allosteric site rather than the catalytic center of the kinase.
It’s really conceptually very interesting. The expectation would be that this molecule has fewer side effects but still may be quite potent. This could be a very interesting development and add something to the armamentarium that we currently don't have.
I also think there will be patients whose diseases are just beyond the reach of a TKI alone for many reasons, maybe additional mutations or things that have to do with the host and metabolism.
In these cases, we'll still have to think about combination treatments and non-TKI treatments. Here, I think an honest answer is the labs have pulled out a lot of interesting leads, but so far, nothing has really made it into a serious clinical context, either because of side effects or because the target may not be as good in humans as it seemed from mouse models.
I think a lot more work is required here to find the best combination therapies and to define those pathways that need to be inhibited together with BCR-ABL. I think there's a field that we'll develop further and that will be the cutting edge.
The Wrap-Up
Dr. Kalaycio: Although an uncommon diagnosis, CML in chronic phase can be treated with an expectation for nearly 100% 5-year survival. For that reason, clinicians need to manage their patients expertly. Steps taken at diagnosis are critical to subsequent decision-making. Once treatment begins, close monitoring is required to screen for side effects as well as ensuring treatment success. With so many effective agents available, patients can be selected for the agent least likely to cause them long-term harm. Perhaps in the future we will learn which patients can stop treatment altogether.
Gentleman, thank you so much for your time. Every time I talk to you I learn something.
1. Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22(5):935–942.
2. National Comprehensive Cancer Network guidelines for treatment of cancer by site. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed August 20, 2015.
3. O'Brien S, Radich JP, Abboud CN, et al. Chronic myelogenous leukemia, version 1.2015. J Natl Compr Canc Netw. 2014;12(11):1590–1610.
4. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884.
5. Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035.
Moderator: Matt Kalaycio, MD1
Discussants: Michael Mauro, MD2; Michael Deininger, MD, PhD3
From Cleveland Clinic, Cleveland, OH1; Memorial Sloan Kettering Cancer Center, New York, NY2; Huntsman Cancer Institute, Salt Lake City, UT3
Address for correspondence: Matt Kalaycio, MD, Cleveland Clinic Main Campus, Mail Code R32, 9500 Euclid Avenue, Cleveland, OH 44195
E-mail: [email protected]
Biographical sketch:
Dr. Kalaycio has been published in numerous scientific publications including Bone Marrow Transplantation, Journal of Clinical Oncology, and Leukemia. He also is the editor of a book on leukemia and co-editor of a book on clinical malignant hematology. His research interests focus on testing new treatments for leukemia.
Dr. Kalaycio received his degree from West Virginia University School of Medicine in Morgantown. He completed his residency in internal medicine at Mercy Hospital of Pittsburgh and fellowships in hematology and medical oncology and bone marrow transplantation at Cleveland Clinic.
Michael Deininger, MD, PhD, is Professor and Chief of Hematology and Hematologic Malignancies for the Department of Internal Medicine and for the Huntsman Cancer Institute (HCI) at the University of Utah. He is an HCI investigator and member of the Experimental Therapeutics program. He has extensive experience treating patients with blood cancers, including chronic myeloid leukemia (CML) and myeloproliferative neoplasms, a group of blood cancers related to leukemia.
Dr
Dr. Deininger’s scientific focus is leukemia, specifically myeloproliferative neoplasms including chronic myeloid leukemia (CML). As a clinician-scientist with a translational research focus Dr. Deininger is heading an extramurally funded research laboratory that is dedicated to the study of signaling pathways, drug resistance and new molecular therapies in leukemia. Dr. Deininger’s work describing the selective effects of imatinib on CML cells provided the rationale for clinical trials that led to the approval of Gleevec as the first molecularly-based therapy for leukemia. Current work in his lab is focused on understanding the role of the bone marrow microenvironment in leukemia drug resistance, discovering novel therapeutic targets and developing more specific signal transduction inhibitors. Dr. Deininger’s work encompasses more than 170 articles in the peer-reviewed literature, including journals like Blood, Journal of Clinical Investigation and the New England Journal of Medicine. He has co-authored more than 10 book chapters, with contributions in leading textbooks such as deVita’s Principles of Oncology. He is a regular speaker at major international scientific meetings, such as the American Society of Hematology and the European Hematology Association and a peer reviewer for journals like Nature Genetics and Cancer Cell. His honors include the Alexandra Kefalides Prize for Leukemia Research and membership on the Editorial Board of Blood, the leading journal in Hematology. Dr. Deininger was named among the world's Highly Cited Researchers by Thomson Reuters in 2014.
Dr. Kalaycio: My name is Matt Kalaycio and I'm the Chairman of the Department of Hematology and Medical Oncology at the Cleveland Clinic. Today I’m joined by Drs. Mike Deininger, Division Chief of Hematology and Hematologic Malignancies at the University of Utah Huntsman Cancer Institute and Michael Mauro, Myeloproliferative Neoplasm Program Director at Memorial Sloan Kettering Cancer Center, New York, New York – together we will discuss practical issues surrounding CML diagnosis, management and treatment options including TKIs and investigational therapies.
Initial Presentation: Assessment & Treatment Options
Dr. Kalaycio: Often, the patient with chronic myelogenous leukemia (CML) gets admitted to the hospital, or an emergency consult is called, because the white count is up for a concern of leukemia. The treatment team sees the differential circulating blasts, and they worry about acute leukemia. Then the hematologist comes and needs to make a decision about whether or not to do a bone marrow biopsy.
When the patient presents in such a manner, I often see that bone marrow biopsies are not performed. I would like to start by asking where you both stand with regard to the necessity of a bone marrow biopsy at the time of diagnosis. I'll start with Dr. Deininger.
Dr. Deininger: I would strongly be in favor of a diagnostic biopsy as well as a smear. The reason is that I think this is the one and only chance to get a clear disease classification into chronic phase, accelerated phase, and blast crisis.
The third may be rare. There are occasional patients who have sheets of blasts who would not be seen on just a differential. For these patients, of course, the treatment decisions are going to be very different compared to a patient in chronic phase.
I think this is an opportunity that shouldn't be missed and I would always recommend that.
Dr. Kalaycio: Dr. Mauro.
Dr. Mauro: I couldn't concur more. With so much focus on the change in disease status from presentation to early response, I think understanding the scope of the disease at diagnosis, including the bone marrow, is essential and either revealing or reassuring. Although we focus mostly on early molecular response, when expectations go awry, not having as full a picture of the disease prior to treatment leaves you less informed about best treatment.
Making sense of accelerated phase features is a good example; the difference in outcomes between chronic phase patients and those who have morphologic features of accelerated phase—with or without cytogenetic features (clonal evolution)—compared with those with clonal evolution can only drive initial and long term treatment decisions. Without cytogenetics and morphology together, such key pieces of the puzzle are missing; it is worth it for the practitioner and patient to have all the data in hand at all times.
Dr. Kalaycio: Once the diagnosis has been made and the biopsy was not done and now you're seeing the patient having either been on hydroxyurea for a month or having had tyrosine kinase inhibitor (TKI) for a month, do you bother with the bone marrow biopsy at that point?
Dr. Deininger: Well, that is a very good question. I think we would probably still do it most of the time. I think it's very clear that the information you can gather from that is less valuable. I think one should try to make up for the omissions as much as possible. We would still go for it.
Dr. Kalaycio: Interesting. The other thing that happens a lot is a patient will be started on hydrea while you're waiting for BCR-ABL to return either by fluorescence in situ hybridization or quantitative polymerase chain reaction (PCR).
I wonder if you have your own set of internal guidelines for when hydroxyurea should be employed or not.
Dr. Mauro: If you look back at some of the original imatinib trials—the phase I trials—patients initiated TKI with higher blood counts, a median around 25,000 and up to 200,000, and responded with excellent tolerance.1 I think there's a bit of overapplication of hydroxyurea early in CML, prior to initiation of TKI, which often confounds and complicates the early myelosuppressive toxicity of TKIs. With use of more potent TKIs, there may be greater amounts of myelosuppression as the leukemic clone may clear faster.
I think we may get ourselves into a bit of a bind by overusing hydroxyurea and then needing to hold and lower TKI dosages quickly when that may not have been necessary had we simply deployed the TKI sooner.
My general rule would be to use hydroxyurea for symptoms if necessary or for more extreme counts where leukostasis is a concern. – Michael Mauro, MDMy general rule would be to use hydroxyurea for symptoms if necessary or for more extreme counts where leukostasis is a concern. The other question that often comes up is about tumor lysis and hydration, and how closely these need to be managed. The likelihood of tumor lysis is low in CML treated with TKIs, but more frequent early labs and good hydration are always the right thing to do; better safe than sorry!
Dr. Deininger: I second what Michael just said. To your question about whether we have an algorithm, I have to admit we don't. I think it's up to the discretion of the treating physician to initiate hydroxyurea or not.
Dr. Kalaycio: Sure. When you start hydroxyurea, do you routinely add allopurinol?
Dr. Deininger: We tend to do that. I know that some people think it's unnecessary. It's such a low-risk and low-cost intervention that I think that it's hard to get anything wrong here.
Dr. Mauro: Right, we tend to do the same. Whether we need to or not is a different question, I suppose.
Dr. Kalaycio: One more thing about the initial presentation and assessment of a patient with what you think might be a myeloproliferative disorder, CML, how important do you find it, Dr. Mauro, to measure splenomegaly and calculate a risk score?
Dr. Mauro: I think it's very useful information. The spleen size factors heavily into the risk score and the risk score does forecast response to a degree. We've looked at calculation of risk score in recent large observational studies and it is under-reported and underutilized. It winds up being useful for two reasons. One, it does set expectations for response, and US treatment guidelines (National Comprehensive Cancer Network [NCCN] guidelines) note that treatment choice may be different for low versus high risk Sokal score.2
I think the second and most intriguing reason to assess Sokal risk seems now to be the impact that risk score has on the ability to proceed to a treatment-free remission (TFR). There appear to be differences in outcomes in patients with high-risk versus low-risk disease despite both having what is required to proceed to TFR in trials, namely consistent and deep molecular remission over a number of years.
Given these implications, we really ought to be gathering the initial risk stratification and quantifying spleen size. It's an important part of our initial assessment.
Dr. Kalaycio: Dr. Deininger?
Dr. Deininger: I think there's a lot of agreement today. I absolutely think that measuring the spleen size ascertains that you've got all these diagnostic parameters available. I think that should be part of the initial evaluation and work-up and will allow some prognostication.
Testing, Risk Factors & Considerations of Treatment: Nilotinib & Dasatinib
Dr. Kalaycio: I'm starting with the less controversial questions to begin with. I think the next set have the potential for some more controversy. Before we leave the initial assessment of CML, do either of you have observations regarding referrals that you get about which you would like to either dispel myths or remind practitioners about best practices in patients newly diagnosed with CML?
I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. – Michael Deininger, MD, PhDDr. Mauro: I can mention one thing. I think when thinking about initial molecular diagnostic results, it is important to point out that testing should screen broadly for different fusions, namely P190 and P210, or variant (p230) transcripts. There are rare patients in chronic phase with non-p210 fusion who need to be followed with specific PCR. On this same topic, the measurement of transcripts at presentation (ie, before treatment) has become quite important and whereas formerly had not been emphasized, presently all patients should have ”baseline” transcript levels.
Dr. Deininger: I think one issue that comes up once in a while is that spleen measurements are done by ultrasound. Technically it's more accurate, but all the clinical risk scores and the prognostication is based on the old fashioned—but probably highly inaccurate—technology of palpation. This is what counts, this is the value to document. I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. Getting a good handle of those risk factors at diagnosis is also important.
Dr. Kalaycio: I think that's a very important point. That's where I was going to go next with this conversation. I was going to avoid the conversation about which TKI to choose as initial treatment. I think that's a debate unto itself.
I would like to ask you how you might assess cardiovascular risk before placing a patient on nilotinib. You got to that a little bit, Dr. Deininger. Could you expand on what else you might review as far as whether or not you feel a patient is a good candidate to start on nilotinib?
Dr. Deininger: Specifically, with regard to nilotinib, we would always get a baseline electrocardiogram. We would do a clinical exam. We would not do an echocardiogram just routinely in the absence of a cardiovascular history or any clinical evidence for heart failure or other cardiovascular issues.
We've adopted the practice of doing a lipid panel. Of course we would include fasting glucose as well. Some of these recommendations are probably somewhat on the soft side, because it's not yet clear what to do with the information.
On the other hand, I think for a patient who is being considered for nilotinib one wants to make sure that one really does the best to minimize the cardiovascular risk factors. Of course that would include smoking history and taking blood pressure and making sure that these risk factors are controlled.
If people have a presentation that is really out of whack in terms of their risk factor management, I would send them to an internist or even a cardiologist to help me optimize the cardiovascular prevention strategy.
Dr. Kalaycio: Great. Similarly, Dr. Mauro, how do you assess pulmonary risk before placing a patient on dasatinib?
Dr. Mauro: I think here we are focused on the less frequent and also less well understood potential toxicity of pulmonary hypertension, coupled with the more common risk of pleural and pericardial effusions.
I'm not sure how much we've learned in clinical studies looking at baseline chest X-rays or timing of X-rays during treatment. I think our best tool in the prevention and management of pleural and pericardial effusions is full discussion with patients about risk and what to look for, attention to any and all symptoms, and appropriate deployment of diagnostics as indicated.
It's interesting to consider whether baseline echocardiography for measurement of pulmonary pressures is warranted. I would say now we're on probably somewhat softer ground, first because on routine echocardiogram pulmonary pressure can't be measured readily unless there is some valvular regurgitation. As well, it is stated that pulmonary hypertension is only properly diagnosed by right heart catheterization. While I'm tempted to do routine echocardiogram studies, I think that such a recommendation still may be perhaps the realm of a clinical study. We need to explore that further. I think with dasatinib there may be certain patients at higher risk, although the data are somewhat limited. There seem to be certain conditions potentially associated with more pleural and pericardial toxicity, including cardiovascular disease and autoimmune disease. There may be circumstances during treatment—lymphocytosis, for example—that may be associated with greater risk. I think expectant management may still be the right approach and echocardiography and more aggressive diagnostics be reserved for patients in whom there might be much more clinical consequence.
Dr. Kalaycio: I'd like to pursue that a little bit further because sometimes the patients will come to us having already had an echocardiogram that may actually show some mild pulmonary hypertension and maybe they've got significant cardiovascular risk factors where you would otherwise be thinking about using dasatinib. Here's someone with pulmonary hypertension, at least by echocardiographic criteria, would that be enough to dissuade you from the use of dasatinib?
Dr. Mauro: I think it would certainly require significant consideration, understanding what the basis of the pulmonary hypertension is for that patient, and risk with adding dasatinib. I think the good news is the low incidence and the reversibility for the most part of dasatinib-associated pulmonary hypertension.
Again, I think the mechanism of action and the pathophysiology isn't completely understood, although there is the intriguing notion that imatinib has been reported to potentially mitigate pulmonary hypertension whereas dasatinib triggers it—a ”closed loop” if you will and an area ripe for research.
I would probably think that a patient with preexisting pulmonary hypertension in the new diagnosis setting might be the kind of patient for whom you really might weigh the pluses versus the minuses of a second generation TKI versus imatinib.
After Evaluation & Diagnosis: Following the Patient
Dr. Kalaycio: I agree. We have fully evaluated our patient and we've made the diagnosis. Now, we start therapy with a TKI based on patient risk profile and side effect potentials. It's time to follow the patient and determine next steps.
Current guidelines3,4 suggest monitoring quantitative PCR after 3 months of therapy and to gauge response. Dr. Deininger, how do you interpret and act on those results following the first 3 months of therapy?
Dr. Deininger: I think what you're getting at is the 10% mark that is a highly predictive value in terms of subsequent achievement of major molecular response and also overall progression free and overall survival.
We'll certainly get this data point. Then we'll put it in a clinical context. I think this context needs to take into account the initial BCR-ABL transcript level. I think Dr. Mauro mentioned that they always determine that. I think that is really good advice, so you can make a comparison with the diagnostic value.
One scenario, of course, is the patient is well below 10% and then things are just in the green range and you would wave people through and reassess in 3 months. If people are very high, I think then you have to ask yourself why that's the case.
Some people have a lot of toxicity issues. For example, they may not have been able to take the required amount of medication. It's not always easy to clearly distinguish between resistance and intolerance.
No matter what, I think it's going to be critical to consider a potential change in treatment if people are in the 70%—80% range. In my mind, there's a gray area.
These are the people who are maybe between 10%—20%. Here, I think this initial value can really help. If there's a significant reduction compared to baseline, I would not necessarily change at that point.
If there is literally no change compared to baseline, I would strongly consider a change unless I am concerned about other issues like noncompliance or drug interactions. What I'm trying to say in a rather long-winded way is that the 10% value shouldn't be seen as a dogma.
It still needs to be placed in a clinical context. One should not rush to any conclusions. Ten percent, 11%, and 9% are identical values in the world of PCR testing. One should not over-interpret that.
Dr. Kalaycio: I think that you're making an important point about absolutes in the interpretation of these tests. Dr. Mauro, how do you interpret the 3-month data that comes back?
Dr. Mauro: I agree with my colleague on the approach that it has to be put in a clinical context. I think what we've learned is the importance of the starting value and relative reduction and to not consider response milestones as black and white guides.
I think there are certain scenarios that require some caution. The patient who is on imatinib who is close but has not reached the landmark may be different that a similar patient on dasatinib or nilotinib who has not had significant reduction—that's probably a more pressing situation.
It's ironic that guidelines—maybe because of lack of options—don't encourage us to think about changing therapy early in someone who hasn't met milestones in 3 months when they've been put on a more potent agent.
I also think that careful consideration is needed regarding treatment intensity and the impact of interruptions, in conjunction with all the other facets—the rate of change, the absolute change from the patient's baseline, the starting response level and the timing of the PCR. We need to look at the actual day it's performed. If it's really not 3 months of therapy, we can misjudge.
Dr. Kalaycio: Right. Now, as you're monitoring patients, Dr. Mauro, at what point would you consider testing for kinase domain mutations?
Dr. Mauro: I think we have pretty good guidance from studies to date regarding when mutation testing is indicated and of higher yield. It's generally earlier on into treatment when we have clinical scenarios that are of greater risk, namely failure to achieve cytogenetic responses. In such scenarios if mutation testing informs treatment decision making it is very helpful.
Patients who have defined themselves as not achieving early molecular response—which we discussed earlier—especially someone who's had a second-generation TKI, warrants mutation testing in my view. Patients who don't achieve classical cytogenetic response landmarks at 6 and 12 (or 18) months, who thus have a higher residual volume of disease and perhaps as a function more clonal instability, I think also warrant attention.
I think we run into trouble when we start to, in an overly critical manner, assess patients’ longer term and deeper molecular response trajectories. Mutation testing becomes difficult or impossible for patients who are at or near major molecular remission (MMR). Mutation yield is generally very low for patients who have not lost MMR and also likely those with small volume of change around the MMR threshold. Looking ahead, I think further investigation into early time points and the setting of minimal residual disease may yield data to be able to predict potential resistance earlier than observing it clinically.
Dr. Kalaycio: Right. Dr. Deininger, if you're monitoring a patient and he’s missing milestones and you do obtain a mutation analysis and find a T315I mutation, do you offer that patient an opportunity to see how well they're going to do with ponatinib, or do you refer that patient to your transplant team for consideration of transplant as soon as a donor is available?As a transplanter, even I would not suggest transplant upon the recognition of the T315I. I would wait until all available TKIs have been tried and have either not worked or weren't tolerated. – Matt Kalaycio, MD
Dr. Deininger: I think we do the first and half of the second. We would certainly offer a trial of ponatinib or, if possible, a clinical trial unless there are insurmountable contraindications.
I don't really think there are any such circumstances with optimized cardiovascular management at the same time, and we may involve a cardiologist at that point to help us if there are cardiovascular risk factors.
We would also do a referral to a transplant center unless the patient is as per performance status, just not a transplant candidate or is too old. Otherwise, we would always make a referral, but we would not pursue a transplant as the first modality, provided that the patient is in chronic phase. Progression to accelerated phase or blastic phase would be looked at totally differently. Here we would certainly put people on a transplant course.
Dr. Kalaycio: I agree. As a transplanter, even I would not suggest transplant upon the recognition of the T315I. I would wait until all available TKIs have been tried and have either not worked or weren't tolerated.
As the two of you know, we're doing fewer and fewer transplants for CML these days. The results we're getting in folks who are failing all of these agents are not as good as they used to be when we were transplanting in the first 3 months of a new diagnosis. Bone marrow transplant is something to defer for as long as possible, at least in my mind.
TKI Dosage Considerations
Dr. Kalaycio: Now we touched a little bit on the side effects of nilotinib and dasatinib. We've talked about progressive disease, perhaps with or without mutations. Dr. Mauro, could you comment on where you think bosutinib might play a role in patients with relapsed or progressive CML?
Dr. Mauro: I think bosutinib remains an underutilized TKI. It is potent and thus offers good salvage activity. I think it offers a fairly distinct side effect profile making it a good choice for cases of intolerance. I think we've learned more about how to initiate bosutinib and manage its side effects; one element of this is that current trials start at slightly lower doses of bosutinib to avoid some of the early gastrointestinal toxicity.
Bosutinib continues to seek a place as a first-line therapy. I think this may be worthwhile as we are looking for the right balance of safety and side effect risk to maximize early response. Regarding the salvage setting—intolerance and resistance to other agents—I find myself speaking to patients about bosutinib as a potent and viable alternative. Bosutinib offers a similar spectrum of activity as dasatinib but can allow us to avoid cardiovascular toxicity and/or allow clearance of pleural and pericardial toxicity occurring with dasatinib.
The one place I would say bosutinib may not hold up as strongly to its competition would be as a third-line therapy after failure of a second-generation TKI. I think while there is reasonable activity with bosutinib in this setting of somewhat fairly drug-resistant CML, the performance of ponatinib is better. There is likely often a struggle with the long-term safety question of ponatinib, making some feel that a trial of bosutinib is often a worthwhile, logical, or even necessary step before ponatinib. My concerns about this approach are related to treatment decision-making based on safety and theoretical risk of adverse effects rather than efficacy and risk of progression; these have to be weighed carefully and appropriately against each other.
Dr. Kalaycio: That's an interesting perspective. You mentioned starting with a lower dose. Dr. Deininger, I'd like to ask you your thoughts about dose modifications of the TKIs in general. It used to be anathema to either start with a low dose or to maintain patients on a lower dose.
I'm aware of at least some data suggesting that those patients who tolerate these TKIs poorly particularly with regard to myelosuppression can be treated long term at lower than typically prescribed doses without adverse effect. What are your thoughts surrounding dose modification of the TKIs?
Dr. Deininger: I would stratify according to TKIs. I think imatinib at 400 mg is probably just rightly dosed, maybe not even at the optimal dose. It could be 600 mg. If you go to 300 mg, that is still acceptable.
If you go to 200 mg, I think I would make double sure that I monitor patients very frequently. People should be, in my mind, in a major molecular response to treat them with 200 mg long term. With the second-generation TKIs, it's a little different.
I cannot really speak to bosutinib because I'm not aware of a lot of data on those modifications. What seems to be clear from dasatinib is that the initial recommended dose of 100 mg is quite high, especially in older people.
I believe that's been corroborated by a study that hasn't been published yet. Apparently the drug excretion in the older individuals is quite a bit slower than in younger people. I think in our practice and in other people's practices as well, about 50% of those people end at doses substantially lower than 100 mg, maybe 50 mg, maybe 40 mg, some even 20 mg per day.
I think in the case of dasatinib you have a lot of maneuvering space. You can adjust the dose according to tolerability and molecular response. With nilotinib I think it's kind of similar. We have a few patients who are on maybe 150 mg twice a day because they have issues in terms of side effects at the higher doses.
I should again thoroughly qualify that by saying if you give up through dose reductions the goal of a major molecular response, then I think you really have to think about the strategy in general because at that point, I think you basically say "this patient is not ever going to be reaching a safe haven and is not tolerating inhibitors well enough to get him there."
That's quite a significant statement to make. Myelosuppression is frequently a situation where you cannot deliver enough dose but you also don't get a good response, whereas other side effects like pleural effusions or excessive fluid retention may well be cause for dose reduction and yet responses may be acceptable. Maybe Dr. Mauro could chime in here and share his experience.
Dr. Mauro: I agree. I think some patients, based on their disease status, may not tolerate the dose that is going to—at least in theory—get them to a deep molecular remission. The biggest problem we often face is myelosuppression. Some patients may have indolent disease and still have a good outcome, at least in the short to medium term, with lower doses of drug. In general I try to work through myelosuppression, especially early, as the combination of myelosuppression and suboptimal or response failure is dangerous.
I think treatment shouldn't be too chaotic and change too much, if possible. That was a very nice summary of the way we view the doses. There's still a fair bit of flexibility amongst the TKI doses, although I don't advocate for starting low and titration up.
In general I think, with regard to optimal TKI dose, we might have overshot; for example, with dasatinib. I think we might have overshot with bosutinib. Trials ongoing now initiate bosutinib at 400 mg rather than 500 mg. That's what I was alluding to.
Treatment Free Remission: Approaches
Dr. Kalaycio: Very interesting discussions. As we wind down the conversation, I want to get to the idea of stopping therapy. We're all aware of data that suggest it's at least possible for a proportion of patients to stop treatment.5
However, the guidelines3,4 such as they are, suggest that stopping should not be done outside the context of a clinical trial. I think most of us would agree with that. I wonder in a practical sense, Dr. Mauro, in your practice, do you have your own set of internal criteria for stopping a TKI and observing patients in the absence of any therapy?
Dr. Mauro: I think I try to incorporate all the experience we have to date when considering this question. That being said I'm a little hesitant to agree entirely with the current thinking regarding retreatment during a Treatment Free Remission trial, which is waiting until patients lose MMR in order to resume treatment but agree it might be hard to retreat based on lesser degrees of molecular relapse. I just worry a little bit about that amount of proliferation without treatment.
On the other hand I think we're coming to the realization that TFR may be feasible in patients who are simply nonproliferative and have low volume of disease based on newer data that patients may not need to have consistent "complete molecular response" or disease reduction below 4.5 logs in order to consider TFR. This will mean more patients may be eligible for such a strategy.
With that being said, I strongly advocate for discontinuation occurring in trials still, especially in the United States. I think we still have to ensure regular monitoring and not run the risk of loss of CML remission as a result of this endeavor. I think we have to have a clear message that this is still an investigational approach.
As we apply TFR strategies more broadly and explore it in patients with different circumstances, rather than those already studied in clinical trials, we may see slightly different results. We may need to exercise more caution.
Outside of a trial I don't discontinue TKI therapy unless there's a clear medical indication and there's no way around it, such as pregnancy or other illness that precludes TKI treatment. I would still pursue TFR only in a clinical trial.
Dr. Kalaycio: Dr. Deininger, I'll give you the last word for your thoughts regarding both treatment discontinuation and the future of CML therapies.
Dr. Deininger: As far as TFR is concerned, I agree with Dr. Mauro. Unfortunately, in the United States we're not quite as far advanced as the Europeans at monitoring diligence. There's a bit of a concern that if we elevate that into daily practice, we may see that patients are not monitored frequently enough and then relapse, but that doesn't get caught. You could imagine a scenario where we're actually seeing a decline in outcomes because people are discontinued and then not restarted if they have a recurrence at the molecular level.
All in all, where this is going, I think it will be really interesting to see more confirmatory data for second-generation TKIs. Let me put that differently: whether a similar proportion of patients in deep molecular response can maintain responses and have TFR if they needed a second-generation TKI to get there rather than just imatinib.
If so, this would be very promising. That would mean that second-generation TKIs actually somewhat impact the natural history of the disease and its biology. Then TFR may become a reality for a substantial proportion of our patients.
I don't think we have the data yet, but at least there are some suggestions that this may be the case. If you do the math, you will still see that many patients with CML will never reach a state where they can consider TFR.
They'll never get into a deep molecular response or they will have been diagnosed with accelerated phase. For all these patients who are not candidates for TFR, we still have to think about treatment optimization in order to make them catch up with the rest.
Now I have a couple of interesting developments. One is clearly to see whether ponatinib administered at a lower dose will have a more practical, therapeutic window in terms of toxicity and yet maintain the excellent efficacy that it has in the setting.
These data need to be produced. Another scenario would be that people would get started on intense induction treatment with higher-risk drugs such as ponatinib. Then if they are a good responder, they can switch to something that is lower-risk and that may be better tolerated.
Actually some of these trials are underway in Europe. Of course there's the question, are there still other drugs that will enter the CML space. There's one promising molecule called ABL001, a TKI with a different mode of action, which exploits an allosteric site rather than the catalytic center of the kinase.
It’s really conceptually very interesting. The expectation would be that this molecule has fewer side effects but still may be quite potent. This could be a very interesting development and add something to the armamentarium that we currently don't have.
I also think there will be patients whose diseases are just beyond the reach of a TKI alone for many reasons, maybe additional mutations or things that have to do with the host and metabolism.
In these cases, we'll still have to think about combination treatments and non-TKI treatments. Here, I think an honest answer is the labs have pulled out a lot of interesting leads, but so far, nothing has really made it into a serious clinical context, either because of side effects or because the target may not be as good in humans as it seemed from mouse models.
I think a lot more work is required here to find the best combination therapies and to define those pathways that need to be inhibited together with BCR-ABL. I think there's a field that we'll develop further and that will be the cutting edge.
The Wrap-Up
Dr. Kalaycio: Although an uncommon diagnosis, CML in chronic phase can be treated with an expectation for nearly 100% 5-year survival. For that reason, clinicians need to manage their patients expertly. Steps taken at diagnosis are critical to subsequent decision-making. Once treatment begins, close monitoring is required to screen for side effects as well as ensuring treatment success. With so many effective agents available, patients can be selected for the agent least likely to cause them long-term harm. Perhaps in the future we will learn which patients can stop treatment altogether.
Gentleman, thank you so much for your time. Every time I talk to you I learn something.
Moderator: Matt Kalaycio, MD1
Discussants: Michael Mauro, MD2; Michael Deininger, MD, PhD3
From Cleveland Clinic, Cleveland, OH1; Memorial Sloan Kettering Cancer Center, New York, NY2; Huntsman Cancer Institute, Salt Lake City, UT3
Address for correspondence: Matt Kalaycio, MD, Cleveland Clinic Main Campus, Mail Code R32, 9500 Euclid Avenue, Cleveland, OH 44195
E-mail: [email protected]
Biographical sketch:
Dr. Kalaycio has been published in numerous scientific publications including Bone Marrow Transplantation, Journal of Clinical Oncology, and Leukemia. He also is the editor of a book on leukemia and co-editor of a book on clinical malignant hematology. His research interests focus on testing new treatments for leukemia.
Dr. Kalaycio received his degree from West Virginia University School of Medicine in Morgantown. He completed his residency in internal medicine at Mercy Hospital of Pittsburgh and fellowships in hematology and medical oncology and bone marrow transplantation at Cleveland Clinic.
Michael Deininger, MD, PhD, is Professor and Chief of Hematology and Hematologic Malignancies for the Department of Internal Medicine and for the Huntsman Cancer Institute (HCI) at the University of Utah. He is an HCI investigator and member of the Experimental Therapeutics program. He has extensive experience treating patients with blood cancers, including chronic myeloid leukemia (CML) and myeloproliferative neoplasms, a group of blood cancers related to leukemia.
Dr
Dr. Deininger’s scientific focus is leukemia, specifically myeloproliferative neoplasms including chronic myeloid leukemia (CML). As a clinician-scientist with a translational research focus Dr. Deininger is heading an extramurally funded research laboratory that is dedicated to the study of signaling pathways, drug resistance and new molecular therapies in leukemia. Dr. Deininger’s work describing the selective effects of imatinib on CML cells provided the rationale for clinical trials that led to the approval of Gleevec as the first molecularly-based therapy for leukemia. Current work in his lab is focused on understanding the role of the bone marrow microenvironment in leukemia drug resistance, discovering novel therapeutic targets and developing more specific signal transduction inhibitors. Dr. Deininger’s work encompasses more than 170 articles in the peer-reviewed literature, including journals like Blood, Journal of Clinical Investigation and the New England Journal of Medicine. He has co-authored more than 10 book chapters, with contributions in leading textbooks such as deVita’s Principles of Oncology. He is a regular speaker at major international scientific meetings, such as the American Society of Hematology and the European Hematology Association and a peer reviewer for journals like Nature Genetics and Cancer Cell. His honors include the Alexandra Kefalides Prize for Leukemia Research and membership on the Editorial Board of Blood, the leading journal in Hematology. Dr. Deininger was named among the world's Highly Cited Researchers by Thomson Reuters in 2014.
Dr. Kalaycio: My name is Matt Kalaycio and I'm the Chairman of the Department of Hematology and Medical Oncology at the Cleveland Clinic. Today I’m joined by Drs. Mike Deininger, Division Chief of Hematology and Hematologic Malignancies at the University of Utah Huntsman Cancer Institute and Michael Mauro, Myeloproliferative Neoplasm Program Director at Memorial Sloan Kettering Cancer Center, New York, New York – together we will discuss practical issues surrounding CML diagnosis, management and treatment options including TKIs and investigational therapies.
Initial Presentation: Assessment & Treatment Options
Dr. Kalaycio: Often, the patient with chronic myelogenous leukemia (CML) gets admitted to the hospital, or an emergency consult is called, because the white count is up for a concern of leukemia. The treatment team sees the differential circulating blasts, and they worry about acute leukemia. Then the hematologist comes and needs to make a decision about whether or not to do a bone marrow biopsy.
When the patient presents in such a manner, I often see that bone marrow biopsies are not performed. I would like to start by asking where you both stand with regard to the necessity of a bone marrow biopsy at the time of diagnosis. I'll start with Dr. Deininger.
Dr. Deininger: I would strongly be in favor of a diagnostic biopsy as well as a smear. The reason is that I think this is the one and only chance to get a clear disease classification into chronic phase, accelerated phase, and blast crisis.
The third may be rare. There are occasional patients who have sheets of blasts who would not be seen on just a differential. For these patients, of course, the treatment decisions are going to be very different compared to a patient in chronic phase.
I think this is an opportunity that shouldn't be missed and I would always recommend that.
Dr. Kalaycio: Dr. Mauro.
Dr. Mauro: I couldn't concur more. With so much focus on the change in disease status from presentation to early response, I think understanding the scope of the disease at diagnosis, including the bone marrow, is essential and either revealing or reassuring. Although we focus mostly on early molecular response, when expectations go awry, not having as full a picture of the disease prior to treatment leaves you less informed about best treatment.
Making sense of accelerated phase features is a good example; the difference in outcomes between chronic phase patients and those who have morphologic features of accelerated phase—with or without cytogenetic features (clonal evolution)—compared with those with clonal evolution can only drive initial and long term treatment decisions. Without cytogenetics and morphology together, such key pieces of the puzzle are missing; it is worth it for the practitioner and patient to have all the data in hand at all times.
Dr. Kalaycio: Once the diagnosis has been made and the biopsy was not done and now you're seeing the patient having either been on hydroxyurea for a month or having had tyrosine kinase inhibitor (TKI) for a month, do you bother with the bone marrow biopsy at that point?
Dr. Deininger: Well, that is a very good question. I think we would probably still do it most of the time. I think it's very clear that the information you can gather from that is less valuable. I think one should try to make up for the omissions as much as possible. We would still go for it.
Dr. Kalaycio: Interesting. The other thing that happens a lot is a patient will be started on hydrea while you're waiting for BCR-ABL to return either by fluorescence in situ hybridization or quantitative polymerase chain reaction (PCR).
I wonder if you have your own set of internal guidelines for when hydroxyurea should be employed or not.
Dr. Mauro: If you look back at some of the original imatinib trials—the phase I trials—patients initiated TKI with higher blood counts, a median around 25,000 and up to 200,000, and responded with excellent tolerance.1 I think there's a bit of overapplication of hydroxyurea early in CML, prior to initiation of TKI, which often confounds and complicates the early myelosuppressive toxicity of TKIs. With use of more potent TKIs, there may be greater amounts of myelosuppression as the leukemic clone may clear faster.
I think we may get ourselves into a bit of a bind by overusing hydroxyurea and then needing to hold and lower TKI dosages quickly when that may not have been necessary had we simply deployed the TKI sooner.
My general rule would be to use hydroxyurea for symptoms if necessary or for more extreme counts where leukostasis is a concern. – Michael Mauro, MDMy general rule would be to use hydroxyurea for symptoms if necessary or for more extreme counts where leukostasis is a concern. The other question that often comes up is about tumor lysis and hydration, and how closely these need to be managed. The likelihood of tumor lysis is low in CML treated with TKIs, but more frequent early labs and good hydration are always the right thing to do; better safe than sorry!
Dr. Deininger: I second what Michael just said. To your question about whether we have an algorithm, I have to admit we don't. I think it's up to the discretion of the treating physician to initiate hydroxyurea or not.
Dr. Kalaycio: Sure. When you start hydroxyurea, do you routinely add allopurinol?
Dr. Deininger: We tend to do that. I know that some people think it's unnecessary. It's such a low-risk and low-cost intervention that I think that it's hard to get anything wrong here.
Dr. Mauro: Right, we tend to do the same. Whether we need to or not is a different question, I suppose.
Dr. Kalaycio: One more thing about the initial presentation and assessment of a patient with what you think might be a myeloproliferative disorder, CML, how important do you find it, Dr. Mauro, to measure splenomegaly and calculate a risk score?
Dr. Mauro: I think it's very useful information. The spleen size factors heavily into the risk score and the risk score does forecast response to a degree. We've looked at calculation of risk score in recent large observational studies and it is under-reported and underutilized. It winds up being useful for two reasons. One, it does set expectations for response, and US treatment guidelines (National Comprehensive Cancer Network [NCCN] guidelines) note that treatment choice may be different for low versus high risk Sokal score.2
I think the second and most intriguing reason to assess Sokal risk seems now to be the impact that risk score has on the ability to proceed to a treatment-free remission (TFR). There appear to be differences in outcomes in patients with high-risk versus low-risk disease despite both having what is required to proceed to TFR in trials, namely consistent and deep molecular remission over a number of years.
Given these implications, we really ought to be gathering the initial risk stratification and quantifying spleen size. It's an important part of our initial assessment.
Dr. Kalaycio: Dr. Deininger?
Dr. Deininger: I think there's a lot of agreement today. I absolutely think that measuring the spleen size ascertains that you've got all these diagnostic parameters available. I think that should be part of the initial evaluation and work-up and will allow some prognostication.
Testing, Risk Factors & Considerations of Treatment: Nilotinib & Dasatinib
Dr. Kalaycio: I'm starting with the less controversial questions to begin with. I think the next set have the potential for some more controversy. Before we leave the initial assessment of CML, do either of you have observations regarding referrals that you get about which you would like to either dispel myths or remind practitioners about best practices in patients newly diagnosed with CML?
I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. – Michael Deininger, MD, PhDDr. Mauro: I can mention one thing. I think when thinking about initial molecular diagnostic results, it is important to point out that testing should screen broadly for different fusions, namely P190 and P210, or variant (p230) transcripts. There are rare patients in chronic phase with non-p210 fusion who need to be followed with specific PCR. On this same topic, the measurement of transcripts at presentation (ie, before treatment) has become quite important and whereas formerly had not been emphasized, presently all patients should have ”baseline” transcript levels.
Dr. Deininger: I think one issue that comes up once in a while is that spleen measurements are done by ultrasound. Technically it's more accurate, but all the clinical risk scores and the prognostication is based on the old fashioned—but probably highly inaccurate—technology of palpation. This is what counts, this is the value to document. I think, moving forward it would be really important to have documentation of concomitant risk factors such as smoking and so on that are essentially driving outcomes more than the TKI treatment or the CML itself. Getting a good handle of those risk factors at diagnosis is also important.
Dr. Kalaycio: I think that's a very important point. That's where I was going to go next with this conversation. I was going to avoid the conversation about which TKI to choose as initial treatment. I think that's a debate unto itself.
I would like to ask you how you might assess cardiovascular risk before placing a patient on nilotinib. You got to that a little bit, Dr. Deininger. Could you expand on what else you might review as far as whether or not you feel a patient is a good candidate to start on nilotinib?
Dr. Deininger: Specifically, with regard to nilotinib, we would always get a baseline electrocardiogram. We would do a clinical exam. We would not do an echocardiogram just routinely in the absence of a cardiovascular history or any clinical evidence for heart failure or other cardiovascular issues.
We've adopted the practice of doing a lipid panel. Of course we would include fasting glucose as well. Some of these recommendations are probably somewhat on the soft side, because it's not yet clear what to do with the information.
On the other hand, I think for a patient who is being considered for nilotinib one wants to make sure that one really does the best to minimize the cardiovascular risk factors. Of course that would include smoking history and taking blood pressure and making sure that these risk factors are controlled.
If people have a presentation that is really out of whack in terms of their risk factor management, I would send them to an internist or even a cardiologist to help me optimize the cardiovascular prevention strategy.
Dr. Kalaycio: Great. Similarly, Dr. Mauro, how do you assess pulmonary risk before placing a patient on dasatinib?
Dr. Mauro: I think here we are focused on the less frequent and also less well understood potential toxicity of pulmonary hypertension, coupled with the more common risk of pleural and pericardial effusions.
I'm not sure how much we've learned in clinical studies looking at baseline chest X-rays or timing of X-rays during treatment. I think our best tool in the prevention and management of pleural and pericardial effusions is full discussion with patients about risk and what to look for, attention to any and all symptoms, and appropriate deployment of diagnostics as indicated.
It's interesting to consider whether baseline echocardiography for measurement of pulmonary pressures is warranted. I would say now we're on probably somewhat softer ground, first because on routine echocardiogram pulmonary pressure can't be measured readily unless there is some valvular regurgitation. As well, it is stated that pulmonary hypertension is only properly diagnosed by right heart catheterization. While I'm tempted to do routine echocardiogram studies, I think that such a recommendation still may be perhaps the realm of a clinical study. We need to explore that further. I think with dasatinib there may be certain patients at higher risk, although the data are somewhat limited. There seem to be certain conditions potentially associated with more pleural and pericardial toxicity, including cardiovascular disease and autoimmune disease. There may be circumstances during treatment—lymphocytosis, for example—that may be associated with greater risk. I think expectant management may still be the right approach and echocardiography and more aggressive diagnostics be reserved for patients in whom there might be much more clinical consequence.
Dr. Kalaycio: I'd like to pursue that a little bit further because sometimes the patients will come to us having already had an echocardiogram that may actually show some mild pulmonary hypertension and maybe they've got significant cardiovascular risk factors where you would otherwise be thinking about using dasatinib. Here's someone with pulmonary hypertension, at least by echocardiographic criteria, would that be enough to dissuade you from the use of dasatinib?
Dr. Mauro: I think it would certainly require significant consideration, understanding what the basis of the pulmonary hypertension is for that patient, and risk with adding dasatinib. I think the good news is the low incidence and the reversibility for the most part of dasatinib-associated pulmonary hypertension.
Again, I think the mechanism of action and the pathophysiology isn't completely understood, although there is the intriguing notion that imatinib has been reported to potentially mitigate pulmonary hypertension whereas dasatinib triggers it—a ”closed loop” if you will and an area ripe for research.
I would probably think that a patient with preexisting pulmonary hypertension in the new diagnosis setting might be the kind of patient for whom you really might weigh the pluses versus the minuses of a second generation TKI versus imatinib.
After Evaluation & Diagnosis: Following the Patient
Dr. Kalaycio: I agree. We have fully evaluated our patient and we've made the diagnosis. Now, we start therapy with a TKI based on patient risk profile and side effect potentials. It's time to follow the patient and determine next steps.
Current guidelines3,4 suggest monitoring quantitative PCR after 3 months of therapy and to gauge response. Dr. Deininger, how do you interpret and act on those results following the first 3 months of therapy?
Dr. Deininger: I think what you're getting at is the 10% mark that is a highly predictive value in terms of subsequent achievement of major molecular response and also overall progression free and overall survival.
We'll certainly get this data point. Then we'll put it in a clinical context. I think this context needs to take into account the initial BCR-ABL transcript level. I think Dr. Mauro mentioned that they always determine that. I think that is really good advice, so you can make a comparison with the diagnostic value.
One scenario, of course, is the patient is well below 10% and then things are just in the green range and you would wave people through and reassess in 3 months. If people are very high, I think then you have to ask yourself why that's the case.
Some people have a lot of toxicity issues. For example, they may not have been able to take the required amount of medication. It's not always easy to clearly distinguish between resistance and intolerance.
No matter what, I think it's going to be critical to consider a potential change in treatment if people are in the 70%—80% range. In my mind, there's a gray area.
These are the people who are maybe between 10%—20%. Here, I think this initial value can really help. If there's a significant reduction compared to baseline, I would not necessarily change at that point.
If there is literally no change compared to baseline, I would strongly consider a change unless I am concerned about other issues like noncompliance or drug interactions. What I'm trying to say in a rather long-winded way is that the 10% value shouldn't be seen as a dogma.
It still needs to be placed in a clinical context. One should not rush to any conclusions. Ten percent, 11%, and 9% are identical values in the world of PCR testing. One should not over-interpret that.
Dr. Kalaycio: I think that you're making an important point about absolutes in the interpretation of these tests. Dr. Mauro, how do you interpret the 3-month data that comes back?
Dr. Mauro: I agree with my colleague on the approach that it has to be put in a clinical context. I think what we've learned is the importance of the starting value and relative reduction and to not consider response milestones as black and white guides.
I think there are certain scenarios that require some caution. The patient who is on imatinib who is close but has not reached the landmark may be different that a similar patient on dasatinib or nilotinib who has not had significant reduction—that's probably a more pressing situation.
It's ironic that guidelines—maybe because of lack of options—don't encourage us to think about changing therapy early in someone who hasn't met milestones in 3 months when they've been put on a more potent agent.
I also think that careful consideration is needed regarding treatment intensity and the impact of interruptions, in conjunction with all the other facets—the rate of change, the absolute change from the patient's baseline, the starting response level and the timing of the PCR. We need to look at the actual day it's performed. If it's really not 3 months of therapy, we can misjudge.
Dr. Kalaycio: Right. Now, as you're monitoring patients, Dr. Mauro, at what point would you consider testing for kinase domain mutations?
Dr. Mauro: I think we have pretty good guidance from studies to date regarding when mutation testing is indicated and of higher yield. It's generally earlier on into treatment when we have clinical scenarios that are of greater risk, namely failure to achieve cytogenetic responses. In such scenarios if mutation testing informs treatment decision making it is very helpful.
Patients who have defined themselves as not achieving early molecular response—which we discussed earlier—especially someone who's had a second-generation TKI, warrants mutation testing in my view. Patients who don't achieve classical cytogenetic response landmarks at 6 and 12 (or 18) months, who thus have a higher residual volume of disease and perhaps as a function more clonal instability, I think also warrant attention.
I think we run into trouble when we start to, in an overly critical manner, assess patients’ longer term and deeper molecular response trajectories. Mutation testing becomes difficult or impossible for patients who are at or near major molecular remission (MMR). Mutation yield is generally very low for patients who have not lost MMR and also likely those with small volume of change around the MMR threshold. Looking ahead, I think further investigation into early time points and the setting of minimal residual disease may yield data to be able to predict potential resistance earlier than observing it clinically.
Dr. Kalaycio: Right. Dr. Deininger, if you're monitoring a patient and he’s missing milestones and you do obtain a mutation analysis and find a T315I mutation, do you offer that patient an opportunity to see how well they're going to do with ponatinib, or do you refer that patient to your transplant team for consideration of transplant as soon as a donor is available?As a transplanter, even I would not suggest transplant upon the recognition of the T315I. I would wait until all available TKIs have been tried and have either not worked or weren't tolerated. – Matt Kalaycio, MD
Dr. Deininger: I think we do the first and half of the second. We would certainly offer a trial of ponatinib or, if possible, a clinical trial unless there are insurmountable contraindications.
I don't really think there are any such circumstances with optimized cardiovascular management at the same time, and we may involve a cardiologist at that point to help us if there are cardiovascular risk factors.
We would also do a referral to a transplant center unless the patient is as per performance status, just not a transplant candidate or is too old. Otherwise, we would always make a referral, but we would not pursue a transplant as the first modality, provided that the patient is in chronic phase. Progression to accelerated phase or blastic phase would be looked at totally differently. Here we would certainly put people on a transplant course.
Dr. Kalaycio: I agree. As a transplanter, even I would not suggest transplant upon the recognition of the T315I. I would wait until all available TKIs have been tried and have either not worked or weren't tolerated.
As the two of you know, we're doing fewer and fewer transplants for CML these days. The results we're getting in folks who are failing all of these agents are not as good as they used to be when we were transplanting in the first 3 months of a new diagnosis. Bone marrow transplant is something to defer for as long as possible, at least in my mind.
TKI Dosage Considerations
Dr. Kalaycio: Now we touched a little bit on the side effects of nilotinib and dasatinib. We've talked about progressive disease, perhaps with or without mutations. Dr. Mauro, could you comment on where you think bosutinib might play a role in patients with relapsed or progressive CML?
Dr. Mauro: I think bosutinib remains an underutilized TKI. It is potent and thus offers good salvage activity. I think it offers a fairly distinct side effect profile making it a good choice for cases of intolerance. I think we've learned more about how to initiate bosutinib and manage its side effects; one element of this is that current trials start at slightly lower doses of bosutinib to avoid some of the early gastrointestinal toxicity.
Bosutinib continues to seek a place as a first-line therapy. I think this may be worthwhile as we are looking for the right balance of safety and side effect risk to maximize early response. Regarding the salvage setting—intolerance and resistance to other agents—I find myself speaking to patients about bosutinib as a potent and viable alternative. Bosutinib offers a similar spectrum of activity as dasatinib but can allow us to avoid cardiovascular toxicity and/or allow clearance of pleural and pericardial toxicity occurring with dasatinib.
The one place I would say bosutinib may not hold up as strongly to its competition would be as a third-line therapy after failure of a second-generation TKI. I think while there is reasonable activity with bosutinib in this setting of somewhat fairly drug-resistant CML, the performance of ponatinib is better. There is likely often a struggle with the long-term safety question of ponatinib, making some feel that a trial of bosutinib is often a worthwhile, logical, or even necessary step before ponatinib. My concerns about this approach are related to treatment decision-making based on safety and theoretical risk of adverse effects rather than efficacy and risk of progression; these have to be weighed carefully and appropriately against each other.
Dr. Kalaycio: That's an interesting perspective. You mentioned starting with a lower dose. Dr. Deininger, I'd like to ask you your thoughts about dose modifications of the TKIs in general. It used to be anathema to either start with a low dose or to maintain patients on a lower dose.
I'm aware of at least some data suggesting that those patients who tolerate these TKIs poorly particularly with regard to myelosuppression can be treated long term at lower than typically prescribed doses without adverse effect. What are your thoughts surrounding dose modification of the TKIs?
Dr. Deininger: I would stratify according to TKIs. I think imatinib at 400 mg is probably just rightly dosed, maybe not even at the optimal dose. It could be 600 mg. If you go to 300 mg, that is still acceptable.
If you go to 200 mg, I think I would make double sure that I monitor patients very frequently. People should be, in my mind, in a major molecular response to treat them with 200 mg long term. With the second-generation TKIs, it's a little different.
I cannot really speak to bosutinib because I'm not aware of a lot of data on those modifications. What seems to be clear from dasatinib is that the initial recommended dose of 100 mg is quite high, especially in older people.
I believe that's been corroborated by a study that hasn't been published yet. Apparently the drug excretion in the older individuals is quite a bit slower than in younger people. I think in our practice and in other people's practices as well, about 50% of those people end at doses substantially lower than 100 mg, maybe 50 mg, maybe 40 mg, some even 20 mg per day.
I think in the case of dasatinib you have a lot of maneuvering space. You can adjust the dose according to tolerability and molecular response. With nilotinib I think it's kind of similar. We have a few patients who are on maybe 150 mg twice a day because they have issues in terms of side effects at the higher doses.
I should again thoroughly qualify that by saying if you give up through dose reductions the goal of a major molecular response, then I think you really have to think about the strategy in general because at that point, I think you basically say "this patient is not ever going to be reaching a safe haven and is not tolerating inhibitors well enough to get him there."
That's quite a significant statement to make. Myelosuppression is frequently a situation where you cannot deliver enough dose but you also don't get a good response, whereas other side effects like pleural effusions or excessive fluid retention may well be cause for dose reduction and yet responses may be acceptable. Maybe Dr. Mauro could chime in here and share his experience.
Dr. Mauro: I agree. I think some patients, based on their disease status, may not tolerate the dose that is going to—at least in theory—get them to a deep molecular remission. The biggest problem we often face is myelosuppression. Some patients may have indolent disease and still have a good outcome, at least in the short to medium term, with lower doses of drug. In general I try to work through myelosuppression, especially early, as the combination of myelosuppression and suboptimal or response failure is dangerous.
I think treatment shouldn't be too chaotic and change too much, if possible. That was a very nice summary of the way we view the doses. There's still a fair bit of flexibility amongst the TKI doses, although I don't advocate for starting low and titration up.
In general I think, with regard to optimal TKI dose, we might have overshot; for example, with dasatinib. I think we might have overshot with bosutinib. Trials ongoing now initiate bosutinib at 400 mg rather than 500 mg. That's what I was alluding to.
Treatment Free Remission: Approaches
Dr. Kalaycio: Very interesting discussions. As we wind down the conversation, I want to get to the idea of stopping therapy. We're all aware of data that suggest it's at least possible for a proportion of patients to stop treatment.5
However, the guidelines3,4 such as they are, suggest that stopping should not be done outside the context of a clinical trial. I think most of us would agree with that. I wonder in a practical sense, Dr. Mauro, in your practice, do you have your own set of internal criteria for stopping a TKI and observing patients in the absence of any therapy?
Dr. Mauro: I think I try to incorporate all the experience we have to date when considering this question. That being said I'm a little hesitant to agree entirely with the current thinking regarding retreatment during a Treatment Free Remission trial, which is waiting until patients lose MMR in order to resume treatment but agree it might be hard to retreat based on lesser degrees of molecular relapse. I just worry a little bit about that amount of proliferation without treatment.
On the other hand I think we're coming to the realization that TFR may be feasible in patients who are simply nonproliferative and have low volume of disease based on newer data that patients may not need to have consistent "complete molecular response" or disease reduction below 4.5 logs in order to consider TFR. This will mean more patients may be eligible for such a strategy.
With that being said, I strongly advocate for discontinuation occurring in trials still, especially in the United States. I think we still have to ensure regular monitoring and not run the risk of loss of CML remission as a result of this endeavor. I think we have to have a clear message that this is still an investigational approach.
As we apply TFR strategies more broadly and explore it in patients with different circumstances, rather than those already studied in clinical trials, we may see slightly different results. We may need to exercise more caution.
Outside of a trial I don't discontinue TKI therapy unless there's a clear medical indication and there's no way around it, such as pregnancy or other illness that precludes TKI treatment. I would still pursue TFR only in a clinical trial.
Dr. Kalaycio: Dr. Deininger, I'll give you the last word for your thoughts regarding both treatment discontinuation and the future of CML therapies.
Dr. Deininger: As far as TFR is concerned, I agree with Dr. Mauro. Unfortunately, in the United States we're not quite as far advanced as the Europeans at monitoring diligence. There's a bit of a concern that if we elevate that into daily practice, we may see that patients are not monitored frequently enough and then relapse, but that doesn't get caught. You could imagine a scenario where we're actually seeing a decline in outcomes because people are discontinued and then not restarted if they have a recurrence at the molecular level.
All in all, where this is going, I think it will be really interesting to see more confirmatory data for second-generation TKIs. Let me put that differently: whether a similar proportion of patients in deep molecular response can maintain responses and have TFR if they needed a second-generation TKI to get there rather than just imatinib.
If so, this would be very promising. That would mean that second-generation TKIs actually somewhat impact the natural history of the disease and its biology. Then TFR may become a reality for a substantial proportion of our patients.
I don't think we have the data yet, but at least there are some suggestions that this may be the case. If you do the math, you will still see that many patients with CML will never reach a state where they can consider TFR.
They'll never get into a deep molecular response or they will have been diagnosed with accelerated phase. For all these patients who are not candidates for TFR, we still have to think about treatment optimization in order to make them catch up with the rest.
Now I have a couple of interesting developments. One is clearly to see whether ponatinib administered at a lower dose will have a more practical, therapeutic window in terms of toxicity and yet maintain the excellent efficacy that it has in the setting.
These data need to be produced. Another scenario would be that people would get started on intense induction treatment with higher-risk drugs such as ponatinib. Then if they are a good responder, they can switch to something that is lower-risk and that may be better tolerated.
Actually some of these trials are underway in Europe. Of course there's the question, are there still other drugs that will enter the CML space. There's one promising molecule called ABL001, a TKI with a different mode of action, which exploits an allosteric site rather than the catalytic center of the kinase.
It’s really conceptually very interesting. The expectation would be that this molecule has fewer side effects but still may be quite potent. This could be a very interesting development and add something to the armamentarium that we currently don't have.
I also think there will be patients whose diseases are just beyond the reach of a TKI alone for many reasons, maybe additional mutations or things that have to do with the host and metabolism.
In these cases, we'll still have to think about combination treatments and non-TKI treatments. Here, I think an honest answer is the labs have pulled out a lot of interesting leads, but so far, nothing has really made it into a serious clinical context, either because of side effects or because the target may not be as good in humans as it seemed from mouse models.
I think a lot more work is required here to find the best combination therapies and to define those pathways that need to be inhibited together with BCR-ABL. I think there's a field that we'll develop further and that will be the cutting edge.
The Wrap-Up
Dr. Kalaycio: Although an uncommon diagnosis, CML in chronic phase can be treated with an expectation for nearly 100% 5-year survival. For that reason, clinicians need to manage their patients expertly. Steps taken at diagnosis are critical to subsequent decision-making. Once treatment begins, close monitoring is required to screen for side effects as well as ensuring treatment success. With so many effective agents available, patients can be selected for the agent least likely to cause them long-term harm. Perhaps in the future we will learn which patients can stop treatment altogether.
Gentleman, thank you so much for your time. Every time I talk to you I learn something.
1. Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22(5):935–942.
2. National Comprehensive Cancer Network guidelines for treatment of cancer by site. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed August 20, 2015.
3. O'Brien S, Radich JP, Abboud CN, et al. Chronic myelogenous leukemia, version 1.2015. J Natl Compr Canc Netw. 2014;12(11):1590–1610.
4. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884.
5. Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035.
1. Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22(5):935–942.
2. National Comprehensive Cancer Network guidelines for treatment of cancer by site. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed August 20, 2015.
3. O'Brien S, Radich JP, Abboud CN, et al. Chronic myelogenous leukemia, version 1.2015. J Natl Compr Canc Netw. 2014;12(11):1590–1610.
4. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884.
5. Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035.
Team reports latest results of CTL019 in CLL

Photo from Penn Medicine
The chimeric antigen receptor (CAR) T-cell therapy CTL019 can produce durable responses in patients with relapsed/refractory chronic lymphocytic leukemia (CLL), according to research published in Science Translational Medicine.
Eight of 14 patients responded to CTL019—4 complete responses (CRs) and 4 partial responses (PRs).
Three of the patients with CRs were still alive and in remission at last follow-up. The longest remission has lasted 53 months.
Only 1 patient with a PR was still alive at last follow-up, and that patient progressed.
Nine patients developed cytokine release syndrome (CRS), some requiring intensive care. And there were 2 cases of tumor lysis syndrome.
These results are the most mature data from this trial. Results from this study were previously presented at ASH 2013 and ASH 2012, and they were published in NEJM and Science Translational Medicine in August 2011.
This study was supported by grants from Novartis, the Leukemia and Lymphoma Society, and the National Institutes of Health. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis.
Treatment and outcomes
The trial enrolled 23 CLL patients, but only 14 received CTL019. The 14 patients had a median age of 66 (range, 51 to 78), and most (n=14) were male.
They had received a median of 5 prior therapies (range, 1 to 11), and 8 patients had 17p deletion. All patients had active disease at the time of CTL019 infusion.
Patients received CTL019 at doses of 0.14 × 108 to 11 × 108 cells (median, 1.6 × 108 cells). Eight patients responded to the treatment, for an overall response rate of 57%.
Four patients (29%) achieved a CR. One of these patients died while in remission at 21 months due to infectious complications that occurred after removal of a basal cell carcinoma on his leg.
The other 3 CR patients remained alive at the time of analysis, with no evidence of leukemia at 28 months, 52 months, and 53 months after receiving their infusions. They did not receive additional therapy after CTL019.
“The durability of the remissions we have observed in this study are remarkable and have given us great hope that personalized cell therapies are going to be important options for patients whose cancers are no longer treatable with standard approaches,” said study author David L. Porter, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia.
Four patients (29%) achieved a PR after receiving CTL019, with responses lasting a median of 7 months. Two of these patients died of disease progression 10 months and 27 months after receiving CTL019.
One PR patient died after suffering a pulmonary embolism 6 months after CTL019 infusion. The last PR patient experienced disease progression at 13 months, but the patient remained alive on other therapies 36 months after receiving CTL019.
Six patients (43%) did not respond to CTL019 and progressed within 1 month to 9 months. Tests revealed that the modified T cells did not expand as robustly in these patients as in those who experienced remissions.
Two of these patients later died from their disease or complications of other therapies, and 4 are receiving other types of treatment.
CRS and other toxicity
The investigators said infusional toxicities were infrequent and mild (less than grade 2). They were primarily low-grade fevers and chills.
The most frequent related adverse events were associated with complications of neutropenia (including fevers) and delayed CRS, which was correlated with in vivo CTL019 expansion. Nine patients (including all 8 responders) developed CRS.
Five patients with CRS required anti-cytokine-directed therapy—tocilizumab (n=4) and/or steroids (n=3). Four patients required intensive care for complications related to CRS, such as hypotension and hypoxia. They remained in the intensive care unit for a median of 6 days (range, 1-9).
Concurrent with CRS were 6 neurologic events in 5 patients—grade 1/2 hallucinations, confusion, or delirium typically associated with high fevers, intensive care, or medication use. There was 1 case of grade 4 confusion that lasted 2 days and was attributed, at least partly, to CTL019.
There were 2 cases of tumor lysis syndrome. And 1 patient died in remission 21 months after CTL019 infusion, having developed overwhelming ecthyma gangrenosum from a pseudomonas wound infection from a skin biopsy site.
CTL019 durability
“Importantly, our tests of patients who experienced complete remissions showed that the modified cells remain in patients’ bodies for years after their infusions, with no sign of cancerous or normal B cells,” said study author Carl H. June, MD, of the University of Pennsylvania Perelman School of Medicine.
“This suggests that at least some of the CTL019 cells retain their ability to hunt for cancerous cells for long periods of time.”
A lab experiment using CAR T cells isolated from one of the first patients to receive CTL019 confirmed the potential for long-term function of these cells. At nearly 3 years after infusion, the patient’s CTL019 cells demonstrated immediate and specific reactivity against cells expressing CD19.
CTL019 development
The investigators did not identify demographic or disease-related factors, such as age or types of prior therapies, that could be used to predict response to CTL019. And there was no association between T-cell dose and patient response.
An ongoing dose-optimization study is exploring this relationship in greater detail. Further future areas of study may include strategies to combine CTL019 with immune checkpoint inhibitors or other therapies to stimulate T-cell recognition of tumor cells.
In addition to CLL, CTL019 is under investigation in patients with acute lymphoblastic leukemia, non-Hodgkin lymphoma, and myeloma. The product has breakthrough designation from the US Food and Drug Administration for acute lymphoblastic leukemia. ![]()

Photo from Penn Medicine
The chimeric antigen receptor (CAR) T-cell therapy CTL019 can produce durable responses in patients with relapsed/refractory chronic lymphocytic leukemia (CLL), according to research published in Science Translational Medicine.
Eight of 14 patients responded to CTL019—4 complete responses (CRs) and 4 partial responses (PRs).
Three of the patients with CRs were still alive and in remission at last follow-up. The longest remission has lasted 53 months.
Only 1 patient with a PR was still alive at last follow-up, and that patient progressed.
Nine patients developed cytokine release syndrome (CRS), some requiring intensive care. And there were 2 cases of tumor lysis syndrome.
These results are the most mature data from this trial. Results from this study were previously presented at ASH 2013 and ASH 2012, and they were published in NEJM and Science Translational Medicine in August 2011.
This study was supported by grants from Novartis, the Leukemia and Lymphoma Society, and the National Institutes of Health. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis.
Treatment and outcomes
The trial enrolled 23 CLL patients, but only 14 received CTL019. The 14 patients had a median age of 66 (range, 51 to 78), and most (n=14) were male.
They had received a median of 5 prior therapies (range, 1 to 11), and 8 patients had 17p deletion. All patients had active disease at the time of CTL019 infusion.
Patients received CTL019 at doses of 0.14 × 108 to 11 × 108 cells (median, 1.6 × 108 cells). Eight patients responded to the treatment, for an overall response rate of 57%.
Four patients (29%) achieved a CR. One of these patients died while in remission at 21 months due to infectious complications that occurred after removal of a basal cell carcinoma on his leg.
The other 3 CR patients remained alive at the time of analysis, with no evidence of leukemia at 28 months, 52 months, and 53 months after receiving their infusions. They did not receive additional therapy after CTL019.
“The durability of the remissions we have observed in this study are remarkable and have given us great hope that personalized cell therapies are going to be important options for patients whose cancers are no longer treatable with standard approaches,” said study author David L. Porter, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia.
Four patients (29%) achieved a PR after receiving CTL019, with responses lasting a median of 7 months. Two of these patients died of disease progression 10 months and 27 months after receiving CTL019.
One PR patient died after suffering a pulmonary embolism 6 months after CTL019 infusion. The last PR patient experienced disease progression at 13 months, but the patient remained alive on other therapies 36 months after receiving CTL019.
Six patients (43%) did not respond to CTL019 and progressed within 1 month to 9 months. Tests revealed that the modified T cells did not expand as robustly in these patients as in those who experienced remissions.
Two of these patients later died from their disease or complications of other therapies, and 4 are receiving other types of treatment.
CRS and other toxicity
The investigators said infusional toxicities were infrequent and mild (less than grade 2). They were primarily low-grade fevers and chills.
The most frequent related adverse events were associated with complications of neutropenia (including fevers) and delayed CRS, which was correlated with in vivo CTL019 expansion. Nine patients (including all 8 responders) developed CRS.
Five patients with CRS required anti-cytokine-directed therapy—tocilizumab (n=4) and/or steroids (n=3). Four patients required intensive care for complications related to CRS, such as hypotension and hypoxia. They remained in the intensive care unit for a median of 6 days (range, 1-9).
Concurrent with CRS were 6 neurologic events in 5 patients—grade 1/2 hallucinations, confusion, or delirium typically associated with high fevers, intensive care, or medication use. There was 1 case of grade 4 confusion that lasted 2 days and was attributed, at least partly, to CTL019.
There were 2 cases of tumor lysis syndrome. And 1 patient died in remission 21 months after CTL019 infusion, having developed overwhelming ecthyma gangrenosum from a pseudomonas wound infection from a skin biopsy site.
CTL019 durability
“Importantly, our tests of patients who experienced complete remissions showed that the modified cells remain in patients’ bodies for years after their infusions, with no sign of cancerous or normal B cells,” said study author Carl H. June, MD, of the University of Pennsylvania Perelman School of Medicine.
“This suggests that at least some of the CTL019 cells retain their ability to hunt for cancerous cells for long periods of time.”
A lab experiment using CAR T cells isolated from one of the first patients to receive CTL019 confirmed the potential for long-term function of these cells. At nearly 3 years after infusion, the patient’s CTL019 cells demonstrated immediate and specific reactivity against cells expressing CD19.
CTL019 development
The investigators did not identify demographic or disease-related factors, such as age or types of prior therapies, that could be used to predict response to CTL019. And there was no association between T-cell dose and patient response.
An ongoing dose-optimization study is exploring this relationship in greater detail. Further future areas of study may include strategies to combine CTL019 with immune checkpoint inhibitors or other therapies to stimulate T-cell recognition of tumor cells.
In addition to CLL, CTL019 is under investigation in patients with acute lymphoblastic leukemia, non-Hodgkin lymphoma, and myeloma. The product has breakthrough designation from the US Food and Drug Administration for acute lymphoblastic leukemia. ![]()

Photo from Penn Medicine
The chimeric antigen receptor (CAR) T-cell therapy CTL019 can produce durable responses in patients with relapsed/refractory chronic lymphocytic leukemia (CLL), according to research published in Science Translational Medicine.
Eight of 14 patients responded to CTL019—4 complete responses (CRs) and 4 partial responses (PRs).
Three of the patients with CRs were still alive and in remission at last follow-up. The longest remission has lasted 53 months.
Only 1 patient with a PR was still alive at last follow-up, and that patient progressed.
Nine patients developed cytokine release syndrome (CRS), some requiring intensive care. And there were 2 cases of tumor lysis syndrome.
These results are the most mature data from this trial. Results from this study were previously presented at ASH 2013 and ASH 2012, and they were published in NEJM and Science Translational Medicine in August 2011.
This study was supported by grants from Novartis, the Leukemia and Lymphoma Society, and the National Institutes of Health. CTL019 was originally developed at the University of Pennsylvania, but the university licensed the technology to Novartis.
Treatment and outcomes
The trial enrolled 23 CLL patients, but only 14 received CTL019. The 14 patients had a median age of 66 (range, 51 to 78), and most (n=14) were male.
They had received a median of 5 prior therapies (range, 1 to 11), and 8 patients had 17p deletion. All patients had active disease at the time of CTL019 infusion.
Patients received CTL019 at doses of 0.14 × 108 to 11 × 108 cells (median, 1.6 × 108 cells). Eight patients responded to the treatment, for an overall response rate of 57%.
Four patients (29%) achieved a CR. One of these patients died while in remission at 21 months due to infectious complications that occurred after removal of a basal cell carcinoma on his leg.
The other 3 CR patients remained alive at the time of analysis, with no evidence of leukemia at 28 months, 52 months, and 53 months after receiving their infusions. They did not receive additional therapy after CTL019.
“The durability of the remissions we have observed in this study are remarkable and have given us great hope that personalized cell therapies are going to be important options for patients whose cancers are no longer treatable with standard approaches,” said study author David L. Porter, MD, of the University of Pennsylvania Perelman School of Medicine in Philadelphia.
Four patients (29%) achieved a PR after receiving CTL019, with responses lasting a median of 7 months. Two of these patients died of disease progression 10 months and 27 months after receiving CTL019.
One PR patient died after suffering a pulmonary embolism 6 months after CTL019 infusion. The last PR patient experienced disease progression at 13 months, but the patient remained alive on other therapies 36 months after receiving CTL019.
Six patients (43%) did not respond to CTL019 and progressed within 1 month to 9 months. Tests revealed that the modified T cells did not expand as robustly in these patients as in those who experienced remissions.
Two of these patients later died from their disease or complications of other therapies, and 4 are receiving other types of treatment.
CRS and other toxicity
The investigators said infusional toxicities were infrequent and mild (less than grade 2). They were primarily low-grade fevers and chills.
The most frequent related adverse events were associated with complications of neutropenia (including fevers) and delayed CRS, which was correlated with in vivo CTL019 expansion. Nine patients (including all 8 responders) developed CRS.
Five patients with CRS required anti-cytokine-directed therapy—tocilizumab (n=4) and/or steroids (n=3). Four patients required intensive care for complications related to CRS, such as hypotension and hypoxia. They remained in the intensive care unit for a median of 6 days (range, 1-9).
Concurrent with CRS were 6 neurologic events in 5 patients—grade 1/2 hallucinations, confusion, or delirium typically associated with high fevers, intensive care, or medication use. There was 1 case of grade 4 confusion that lasted 2 days and was attributed, at least partly, to CTL019.
There were 2 cases of tumor lysis syndrome. And 1 patient died in remission 21 months after CTL019 infusion, having developed overwhelming ecthyma gangrenosum from a pseudomonas wound infection from a skin biopsy site.
CTL019 durability
“Importantly, our tests of patients who experienced complete remissions showed that the modified cells remain in patients’ bodies for years after their infusions, with no sign of cancerous or normal B cells,” said study author Carl H. June, MD, of the University of Pennsylvania Perelman School of Medicine.
“This suggests that at least some of the CTL019 cells retain their ability to hunt for cancerous cells for long periods of time.”
A lab experiment using CAR T cells isolated from one of the first patients to receive CTL019 confirmed the potential for long-term function of these cells. At nearly 3 years after infusion, the patient’s CTL019 cells demonstrated immediate and specific reactivity against cells expressing CD19.
CTL019 development
The investigators did not identify demographic or disease-related factors, such as age or types of prior therapies, that could be used to predict response to CTL019. And there was no association between T-cell dose and patient response.
An ongoing dose-optimization study is exploring this relationship in greater detail. Further future areas of study may include strategies to combine CTL019 with immune checkpoint inhibitors or other therapies to stimulate T-cell recognition of tumor cells.
In addition to CLL, CTL019 is under investigation in patients with acute lymphoblastic leukemia, non-Hodgkin lymphoma, and myeloma. The product has breakthrough designation from the US Food and Drug Administration for acute lymphoblastic leukemia. ![]()