User login
MATRIX trial update: No clear winner

LONDON—The latest results* of the MATRIX trial haven’t provided any cut-and-dried answers when it comes to anticoagulation in the context of percutaneous coronary intervention (PCI), according to articles published in NEJM and data presented at the ESC Congress 2015.
The trial suggested that, overall, bivalirudin and heparin produce comparable results in patients undergoing PCI.
The drugs produced similar rates of major adverse cardiovascular events (MACE) and net adverse clinical events (NACE).
In addition, among patients in the bivalirudin arm, there was no significant difference in a composite primary outcome between patients who received an additional infusion of bivalirudin after PCI and those who did not.
Results from this trial were published in NEJM alongside a related editorial. At the ESC Congress 2015, the bivalirudin dosing comparison was reported in abstract 6004.
MATRIX was sponsored by the Societa Italiana di Cardiologia Invasiva (GISE), with funding from The Medicines Company and Terumo.
The trial enrolled 7213 patients with acute coronary syndrome who were set to undergo PCI. Patients were randomized to receive bivalirudin (n=3610) or unfractionated heparin (n=3603). Patients in the bivalirudin arm were then randomized to either receive a post-PCI infusion of bivalirudin (n=1799) or not (n=1811).
Primary outcomes for the comparison between bivalirudin and heparin were the occurrence of MACE (a composite of death, myocardial infarction, or stroke) and NACE (a composite of major bleeding or a major adverse cardiovascular event).
The primary outcome for the comparison of post-PCI bivalirudin infusion with no infusion was a composite of urgent target-vessel revascularization, definite stent thrombosis, or NACE.
Heparin vs bivalirudin
There was no significant difference between the bivalirudin and heparin groups with regard to MACE—10.3% and 10.9%, respectively (P=0.44). And the same was true for NACE—11.2% and 12.4%, respectively (P=0.12).
Likewise, there was no significant difference between the bivalirudin arm and the heparin arm with regard to myocardial infarction—8.6% and 8.5%, respectively (P=0.93)—or stroke—0.4% and 0.5%, respectively (P=0.57).
However, bivalirudin was associated with a significantly lower rate of all-cause mortality than heparin—1.7% and 2.3%, respectively (P=0.04)—and a significantly lower rate of cardiac-related death—1.5% and 2.2%, respectively (P=0.03).
The rate of definite stent thrombosis was significantly higher in the bivalirudin group than the heparin group—1.0% and 0.6%, respectively (P=0.048). But there was no significant difference in the rate of definite or probable stent thrombosis—1.3% and 1.0% (P=0.27).
The rate of any bleeding was significantly lower with bivalirudin than with heparin—11.0% and 13.6%, respectively (P=0.001). And the same was true for major bleeding (BARC 3 or 5)—1.4% and 2.5%, respectively (P<0.001).
Bivalirudin duration
“Post-PCI bivalirudin did not reduce the composite outcome of ischemic and bleeding outcomes, including stent thrombosis risk, as compared to no post-PCI bivalirudin infusion,” said study investigator Marco Valgimigli, MD, PhD, of the Swiss Cardiovascular Center in Bern, Switzerland.
The proportion of patients who met the primary endpoint was 11.0% in the post-PCI infusion group and 11.9% in the no-infusion group (P=0.34).
Similarly, there was no significant difference between the infusion and no-infusion groups in the risk of definite stent thrombosis—1.3% and 0.7%, respectively (P=0.09)—or definite/probable stent thrombosis—1.5% and 1.1%, respectively (P=0.29).
And there was no significant difference in the rate of any bleeding—11.3% and 10.7%, respectively (P=0.62)—but the rate of BARC 3 or 5 bleeding was lower in the group that received the post-PCI infusion—1.0% vs 1.8% (P=0.03).
“Both treatment options are allowed per current European label and observational studies,” Dr Valgimigli said.
“Hence, I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients, as well as perhaps based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors. This is in keeping with the current labeling of the drug in EU and USA.”
Cost concerns
In the NEJM editorial, Peter Berger, MD, of North Shore–Long Island Jewish Health System in Great Neck, New York, noted that bivalirudin costs more than 400 times the price of heparin.
“Many studies have shown that bivalirudin is safer than heparin—that it causes fewer bleeding complications,” Dr Berger said. “But other studies have shown that heparin is every bit as safe, especially when used in lower doses. And one recent, large study actually suggested that heparin is more effective than bivalirudin.”
Despite these conflicting results, Dr Berger said “nearly everyone” agrees that if the more expensive drug is not superior in some way, the less expensive one should be used.
“Many studies raise more questions than they answer,” he added. “It will be interesting to see whether doctors accept the results of the MATRIX trial or wait for more studies before deciding which blood thinner they prefer.” ![]()
*MATRIX investigators previously compared vascular access sites and found that radial access outperformed femoral access. These results were published earlier this year in The Lancet.

LONDON—The latest results* of the MATRIX trial haven’t provided any cut-and-dried answers when it comes to anticoagulation in the context of percutaneous coronary intervention (PCI), according to articles published in NEJM and data presented at the ESC Congress 2015.
The trial suggested that, overall, bivalirudin and heparin produce comparable results in patients undergoing PCI.
The drugs produced similar rates of major adverse cardiovascular events (MACE) and net adverse clinical events (NACE).
In addition, among patients in the bivalirudin arm, there was no significant difference in a composite primary outcome between patients who received an additional infusion of bivalirudin after PCI and those who did not.
Results from this trial were published in NEJM alongside a related editorial. At the ESC Congress 2015, the bivalirudin dosing comparison was reported in abstract 6004.
MATRIX was sponsored by the Societa Italiana di Cardiologia Invasiva (GISE), with funding from The Medicines Company and Terumo.
The trial enrolled 7213 patients with acute coronary syndrome who were set to undergo PCI. Patients were randomized to receive bivalirudin (n=3610) or unfractionated heparin (n=3603). Patients in the bivalirudin arm were then randomized to either receive a post-PCI infusion of bivalirudin (n=1799) or not (n=1811).
Primary outcomes for the comparison between bivalirudin and heparin were the occurrence of MACE (a composite of death, myocardial infarction, or stroke) and NACE (a composite of major bleeding or a major adverse cardiovascular event).
The primary outcome for the comparison of post-PCI bivalirudin infusion with no infusion was a composite of urgent target-vessel revascularization, definite stent thrombosis, or NACE.
Heparin vs bivalirudin
There was no significant difference between the bivalirudin and heparin groups with regard to MACE—10.3% and 10.9%, respectively (P=0.44). And the same was true for NACE—11.2% and 12.4%, respectively (P=0.12).
Likewise, there was no significant difference between the bivalirudin arm and the heparin arm with regard to myocardial infarction—8.6% and 8.5%, respectively (P=0.93)—or stroke—0.4% and 0.5%, respectively (P=0.57).
However, bivalirudin was associated with a significantly lower rate of all-cause mortality than heparin—1.7% and 2.3%, respectively (P=0.04)—and a significantly lower rate of cardiac-related death—1.5% and 2.2%, respectively (P=0.03).
The rate of definite stent thrombosis was significantly higher in the bivalirudin group than the heparin group—1.0% and 0.6%, respectively (P=0.048). But there was no significant difference in the rate of definite or probable stent thrombosis—1.3% and 1.0% (P=0.27).
The rate of any bleeding was significantly lower with bivalirudin than with heparin—11.0% and 13.6%, respectively (P=0.001). And the same was true for major bleeding (BARC 3 or 5)—1.4% and 2.5%, respectively (P<0.001).
Bivalirudin duration
“Post-PCI bivalirudin did not reduce the composite outcome of ischemic and bleeding outcomes, including stent thrombosis risk, as compared to no post-PCI bivalirudin infusion,” said study investigator Marco Valgimigli, MD, PhD, of the Swiss Cardiovascular Center in Bern, Switzerland.
The proportion of patients who met the primary endpoint was 11.0% in the post-PCI infusion group and 11.9% in the no-infusion group (P=0.34).
Similarly, there was no significant difference between the infusion and no-infusion groups in the risk of definite stent thrombosis—1.3% and 0.7%, respectively (P=0.09)—or definite/probable stent thrombosis—1.5% and 1.1%, respectively (P=0.29).
And there was no significant difference in the rate of any bleeding—11.3% and 10.7%, respectively (P=0.62)—but the rate of BARC 3 or 5 bleeding was lower in the group that received the post-PCI infusion—1.0% vs 1.8% (P=0.03).
“Both treatment options are allowed per current European label and observational studies,” Dr Valgimigli said.
“Hence, I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients, as well as perhaps based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors. This is in keeping with the current labeling of the drug in EU and USA.”
Cost concerns
In the NEJM editorial, Peter Berger, MD, of North Shore–Long Island Jewish Health System in Great Neck, New York, noted that bivalirudin costs more than 400 times the price of heparin.
“Many studies have shown that bivalirudin is safer than heparin—that it causes fewer bleeding complications,” Dr Berger said. “But other studies have shown that heparin is every bit as safe, especially when used in lower doses. And one recent, large study actually suggested that heparin is more effective than bivalirudin.”
Despite these conflicting results, Dr Berger said “nearly everyone” agrees that if the more expensive drug is not superior in some way, the less expensive one should be used.
“Many studies raise more questions than they answer,” he added. “It will be interesting to see whether doctors accept the results of the MATRIX trial or wait for more studies before deciding which blood thinner they prefer.” ![]()
*MATRIX investigators previously compared vascular access sites and found that radial access outperformed femoral access. These results were published earlier this year in The Lancet.

LONDON—The latest results* of the MATRIX trial haven’t provided any cut-and-dried answers when it comes to anticoagulation in the context of percutaneous coronary intervention (PCI), according to articles published in NEJM and data presented at the ESC Congress 2015.
The trial suggested that, overall, bivalirudin and heparin produce comparable results in patients undergoing PCI.
The drugs produced similar rates of major adverse cardiovascular events (MACE) and net adverse clinical events (NACE).
In addition, among patients in the bivalirudin arm, there was no significant difference in a composite primary outcome between patients who received an additional infusion of bivalirudin after PCI and those who did not.
Results from this trial were published in NEJM alongside a related editorial. At the ESC Congress 2015, the bivalirudin dosing comparison was reported in abstract 6004.
MATRIX was sponsored by the Societa Italiana di Cardiologia Invasiva (GISE), with funding from The Medicines Company and Terumo.
The trial enrolled 7213 patients with acute coronary syndrome who were set to undergo PCI. Patients were randomized to receive bivalirudin (n=3610) or unfractionated heparin (n=3603). Patients in the bivalirudin arm were then randomized to either receive a post-PCI infusion of bivalirudin (n=1799) or not (n=1811).
Primary outcomes for the comparison between bivalirudin and heparin were the occurrence of MACE (a composite of death, myocardial infarction, or stroke) and NACE (a composite of major bleeding or a major adverse cardiovascular event).
The primary outcome for the comparison of post-PCI bivalirudin infusion with no infusion was a composite of urgent target-vessel revascularization, definite stent thrombosis, or NACE.
Heparin vs bivalirudin
There was no significant difference between the bivalirudin and heparin groups with regard to MACE—10.3% and 10.9%, respectively (P=0.44). And the same was true for NACE—11.2% and 12.4%, respectively (P=0.12).
Likewise, there was no significant difference between the bivalirudin arm and the heparin arm with regard to myocardial infarction—8.6% and 8.5%, respectively (P=0.93)—or stroke—0.4% and 0.5%, respectively (P=0.57).
However, bivalirudin was associated with a significantly lower rate of all-cause mortality than heparin—1.7% and 2.3%, respectively (P=0.04)—and a significantly lower rate of cardiac-related death—1.5% and 2.2%, respectively (P=0.03).
The rate of definite stent thrombosis was significantly higher in the bivalirudin group than the heparin group—1.0% and 0.6%, respectively (P=0.048). But there was no significant difference in the rate of definite or probable stent thrombosis—1.3% and 1.0% (P=0.27).
The rate of any bleeding was significantly lower with bivalirudin than with heparin—11.0% and 13.6%, respectively (P=0.001). And the same was true for major bleeding (BARC 3 or 5)—1.4% and 2.5%, respectively (P<0.001).
Bivalirudin duration
“Post-PCI bivalirudin did not reduce the composite outcome of ischemic and bleeding outcomes, including stent thrombosis risk, as compared to no post-PCI bivalirudin infusion,” said study investigator Marco Valgimigli, MD, PhD, of the Swiss Cardiovascular Center in Bern, Switzerland.
The proportion of patients who met the primary endpoint was 11.0% in the post-PCI infusion group and 11.9% in the no-infusion group (P=0.34).
Similarly, there was no significant difference between the infusion and no-infusion groups in the risk of definite stent thrombosis—1.3% and 0.7%, respectively (P=0.09)—or definite/probable stent thrombosis—1.5% and 1.1%, respectively (P=0.29).
And there was no significant difference in the rate of any bleeding—11.3% and 10.7%, respectively (P=0.62)—but the rate of BARC 3 or 5 bleeding was lower in the group that received the post-PCI infusion—1.0% vs 1.8% (P=0.03).
“Both treatment options are allowed per current European label and observational studies,” Dr Valgimigli said.
“Hence, I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients, as well as perhaps based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors. This is in keeping with the current labeling of the drug in EU and USA.”
Cost concerns
In the NEJM editorial, Peter Berger, MD, of North Shore–Long Island Jewish Health System in Great Neck, New York, noted that bivalirudin costs more than 400 times the price of heparin.
“Many studies have shown that bivalirudin is safer than heparin—that it causes fewer bleeding complications,” Dr Berger said. “But other studies have shown that heparin is every bit as safe, especially when used in lower doses. And one recent, large study actually suggested that heparin is more effective than bivalirudin.”
Despite these conflicting results, Dr Berger said “nearly everyone” agrees that if the more expensive drug is not superior in some way, the less expensive one should be used.
“Many studies raise more questions than they answer,” he added. “It will be interesting to see whether doctors accept the results of the MATRIX trial or wait for more studies before deciding which blood thinner they prefer.” ![]()
*MATRIX investigators previously compared vascular access sites and found that radial access outperformed femoral access. These results were published earlier this year in The Lancet.
FDA approves drug to prevent delayed CINV in adults

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved rolapitant (Varubi) for use in adult cancer patients receiving initial and repeat courses of emetogenic chemotherapy.
Rolapitant is to be used in combination with other antiemetic agents to prevent delayed chemotherapy-induced nausea and vomiting (CINV).
Tesaro, Inc., the company developing rolapitant, plans to launch the drug in the fourth quarter of this year.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 (NK-1) receptors, with a plasma half-life of approximately 7 days. Activation of NK-1 receptors plays a central role in CINV, particularly in the delayed phase (the 25- to 120-hour period after chemotherapy administration).
Rolapitant comes in tablet form. The recommended dose is 180 mg, given approximately 1 to 2 hours prior to chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone. No dosage adjustment is required for dexamethasone when administering rolapitant.
Rolapitant inhibits the CYP2D6 enzyme, so it is contraindicated with the use of thioridazine, a drug metabolized by the CYP2D6 enzyme. Use of these drugs together may increase the amount of thioridazine in the blood and cause an abnormal heart rhythm that can be serious.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were recently published in The Lancet Oncology.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (≥3%) were neutropenia (9% rolapitant and 8% control), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy regimens. Results from this trial were recently published in The Lancet Oncology.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (≥3%) were decreased appetite (9% rolapitant and 7% control), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%).
The full prescribing information for rolapitant is available at www.varubirx.com. ![]()

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved rolapitant (Varubi) for use in adult cancer patients receiving initial and repeat courses of emetogenic chemotherapy.
Rolapitant is to be used in combination with other antiemetic agents to prevent delayed chemotherapy-induced nausea and vomiting (CINV).
Tesaro, Inc., the company developing rolapitant, plans to launch the drug in the fourth quarter of this year.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 (NK-1) receptors, with a plasma half-life of approximately 7 days. Activation of NK-1 receptors plays a central role in CINV, particularly in the delayed phase (the 25- to 120-hour period after chemotherapy administration).
Rolapitant comes in tablet form. The recommended dose is 180 mg, given approximately 1 to 2 hours prior to chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone. No dosage adjustment is required for dexamethasone when administering rolapitant.
Rolapitant inhibits the CYP2D6 enzyme, so it is contraindicated with the use of thioridazine, a drug metabolized by the CYP2D6 enzyme. Use of these drugs together may increase the amount of thioridazine in the blood and cause an abnormal heart rhythm that can be serious.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were recently published in The Lancet Oncology.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (≥3%) were neutropenia (9% rolapitant and 8% control), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy regimens. Results from this trial were recently published in The Lancet Oncology.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (≥3%) were decreased appetite (9% rolapitant and 7% control), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%).
The full prescribing information for rolapitant is available at www.varubirx.com. ![]()

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved rolapitant (Varubi) for use in adult cancer patients receiving initial and repeat courses of emetogenic chemotherapy.
Rolapitant is to be used in combination with other antiemetic agents to prevent delayed chemotherapy-induced nausea and vomiting (CINV).
Tesaro, Inc., the company developing rolapitant, plans to launch the drug in the fourth quarter of this year.
Rolapitant is a selective and competitive antagonist of human substance P/neurokinin 1 (NK-1) receptors, with a plasma half-life of approximately 7 days. Activation of NK-1 receptors plays a central role in CINV, particularly in the delayed phase (the 25- to 120-hour period after chemotherapy administration).
Rolapitant comes in tablet form. The recommended dose is 180 mg, given approximately 1 to 2 hours prior to chemotherapy administration in combination with a 5-HT3 receptor antagonist and dexamethasone. No dosage adjustment is required for dexamethasone when administering rolapitant.
Rolapitant inhibits the CYP2D6 enzyme, so it is contraindicated with the use of thioridazine, a drug metabolized by the CYP2D6 enzyme. Use of these drugs together may increase the amount of thioridazine in the blood and cause an abnormal heart rhythm that can be serious.
Rolapitant clinical trials
Results from three phase 3 trials suggested that rolapitant (at 180 mg) in combination with a 5-HT3 receptor antagonist and dexamethasone was more effective than the 5-HT3 receptor antagonist and dexamethasone on their own (active control).
The 3-drug combination demonstrated a significant reduction in episodes of vomiting or use of rescue medication during the 25- to 120-hour period following administration of highly emetogenic and moderately emetogenic chemotherapy regimens.
In addition, patients who received rolapitant reported experiencing less nausea that interfered with normal daily life and fewer episodes of vomiting or retching over multiple cycles of chemotherapy.
Highly emetogenic chemotherapy
The clinical profile of rolapitant in cisplatin-based, highly emetogenic chemotherapy (HEC) was confirmed in two phase 3 studies: HEC1 and HEC2. Results from these trials were recently published in The Lancet Oncology.
Both trials met their primary endpoint of complete response (CR) and demonstrated statistical superiority of the rolapitant combination compared to active control.
In HEC1, 264 patients received the rolapitant combination, and 262 received active control. The proportion of patients achieving a CR was 72.7% and 58.4%, respectively (P<0.001).
In HEC2, 271 patients received the rolapitant combination, and 273 received active control. The proportion of patients achieving a CR was 70.1% and 61.9%, respectively (P=0.043).
The most common adverse events (≥3%) were neutropenia (9% rolapitant and 8% control), hiccups (5% and 4%), and abdominal pain (3% and 2%).
Moderately emetogenic chemotherapy
Researchers conducted another phase 3 trial to compare the rolapitant combination with active control in 1332 patients receiving moderately emetogenic chemotherapy regimens. Results from this trial were recently published in The Lancet Oncology.
This trial met its primary endpoint of CR and demonstrated statistical superiority of the rolapitant combination compared to active control. The proportion of patients achieving a CR was 71.3% and 61.6%, respectively (P<0.001).
The most common adverse events (≥3%) were decreased appetite (9% rolapitant and 7% control), neutropenia (7% and 6%), dizziness (6% and 4%), dyspepsia (4% and 2%), urinary tract infection (4% and 3%), stomatitis (4% and 2%), and anemia (3% and 2%).
The full prescribing information for rolapitant is available at www.varubirx.com. ![]()
Eltrombopag approved to treat SAA in EU

The European Commission has approved eltrombopag (Revolade) for the treatment of adults with severe aplastic anemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplant.
Eltrombopag is the first therapy approved in the European Union (EU) for this patient population.
The approval applies to all 28 EU member states plus Iceland, Norway, and Liechtenstein.
Trials of eltrombopag in SAA
The European Commission’s approval is based primarily on results of a phase 2 pilot study (NCT00922883) conducted by the National Heart, Lung and Blood Institute at the National Institutes of Health.
Results from the ongoing study were published in NEJM in 2012 and Blood in 2013. The trial has enrolled 43 patients with SAA who had an insufficient response to at least 1 prior immunosuppressive therapy and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) had received at least 2 prior immunosuppressive therapies.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least 1 lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow-up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 1 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments.
The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()

The European Commission has approved eltrombopag (Revolade) for the treatment of adults with severe aplastic anemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplant.
Eltrombopag is the first therapy approved in the European Union (EU) for this patient population.
The approval applies to all 28 EU member states plus Iceland, Norway, and Liechtenstein.
Trials of eltrombopag in SAA
The European Commission’s approval is based primarily on results of a phase 2 pilot study (NCT00922883) conducted by the National Heart, Lung and Blood Institute at the National Institutes of Health.
Results from the ongoing study were published in NEJM in 2012 and Blood in 2013. The trial has enrolled 43 patients with SAA who had an insufficient response to at least 1 prior immunosuppressive therapy and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) had received at least 2 prior immunosuppressive therapies.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least 1 lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow-up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 1 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments.
The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()

The European Commission has approved eltrombopag (Revolade) for the treatment of adults with severe aplastic anemia (SAA) who were either refractory to prior immunosuppressive therapy or heavily pretreated and are unsuitable for hematopoietic stem cell transplant.
Eltrombopag is the first therapy approved in the European Union (EU) for this patient population.
The approval applies to all 28 EU member states plus Iceland, Norway, and Liechtenstein.
Trials of eltrombopag in SAA
The European Commission’s approval is based primarily on results of a phase 2 pilot study (NCT00922883) conducted by the National Heart, Lung and Blood Institute at the National Institutes of Health.
Results from the ongoing study were published in NEJM in 2012 and Blood in 2013. The trial has enrolled 43 patients with SAA who had an insufficient response to at least 1 prior immunosuppressive therapy and who had a platelet count of 30 x 109/L or less.
At baseline, the median platelet count was 20 x 109/L, hemoglobin was 8.4 g/dL, the absolute neutrophil count was 0.58 x 109/L, and absolute reticulocyte count was 24.3 x 109/L.
Patients had a median age of 45 (range, 17 to 77), and 56% were male. The majority of patients (84%) had received at least 2 prior immunosuppressive therapies.
Patients received eltrombopag at an initial dose of 50 mg once daily for 2 weeks. The dose increased over 2-week periods to a maximum of 150 mg once daily.
The study’s primary endpoint was hematologic response, which was initially assessed after 12 weeks of treatment. Treatment was discontinued after 16 weeks in patients who did not exhibit a hematologic response.
Forty percent of patients (n=17) experienced a hematologic response in at least 1 lineage—platelets, red blood cells (RBCs), or white blood cells—after week 12.
In the extension phase of the study, 8 patients achieved a multilineage response. Four of these patients subsequently tapered off treatment and maintained the response. The median follow-up was 8.1 months (range, 7.2 to 10.6 months).
Ninety-one percent of patients were platelet-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require platelet transfusions for a median of 200 days (range, 8 to 1096 days).
Eighty-six percent of patients were RBC-transfusion-dependent at baseline. Patients who responded to eltrombopag did not require RBC transfusions for a median of 208 days (range, 15 to 1082 days).
The most common adverse events (≥20%) associated with eltrombopag were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%).
Patients were also evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality after treatment, including 5 patients who had complex changes in chromosome 7.
Patients who develop new cytogenetic abnormalities while on eltrombopag may need to be taken off treatment.
About eltrombopag
Eltrombopag is already approved to treat SAA in the US and Canada. The drug recently gained approval in the US to treat children age 1 and older who have chronic immune thrombocytopenia and have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Eltrombopag is approved in more than 100 countries to treat adults with chronic immune thrombocytopenia who have had an inadequate response to or are intolerant of other treatments.
The drug is approved in more than 45 countries for the treatment of thrombocytopenia in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy.
Eltrombopag is marketed under the brand name Promacta in the US and Revolade in most other countries. For more details on the drug, see the European Medicines Agency’s Summary of Product Characteristics. ![]()
Liraglutide Produces Clinically Significant Weight Loss in Nondiabetic Patients, But At What Cost?
Study Overview
Objective. To evaluate the efficacy of liraglutide for weight loss in a group of nondiabetic patients with obesity.
Design. Randomized double-blind placebo-controlled trial.
Setting and participants. This trial took place across 27 countries in Europe, North America, South America, Asia, Africa and Australia. It was funded by NovoNordisk, the pharmaceutical company that manufactures liraglutide. Participants were 18 years or older, with a BMI of 30 kg/m2 (or 27 kg/m2 with hypertension or dyslipidemia). Patients with diabetes, those on medications known to induce weight gain (or loss), those with history of bariatric surgery, and those with psychiatric illness were excluded from participating. Patients with prediabetes were not excluded.
Intervention. Participants were randomized (2:1 in favor of study drug) to liraglutide or placebo, stratified according to BMI category and pre-diabetes status. They were started at a 0.6–mg dose of medication and up-titrated as tolerated to a dose of 3.0 mg over several weeks. All received counseling on behavioral changes to promote weight loss. Participants were then followed for 56 weeks. A small subgroup in the liraglutide arm was randomly assigned to switch to placebo after 12 weeks on medication to examine for durability of effect of medication, and to evaluate for safety issues that might occur on drug discontinuation.
Main outcome measures. This study focused on 3 primary outcomes: individual-level weight change from baseline, group-level percentage of participants achieving at least 5% weight loss, and percentage of participants with at least 10% weight loss, all assessed at 56 weeks.
Secondary outcomes included change in BMI, waist circumference, markers of glycemia (hemoglobin A1c, insulin level), markers of cardiometabolic health (blood pressure, lipids, CRP), and health-related quality of life (using several validated survey measures). Adverse events were also assessed.
The investigators used an intention-to-treat analysis, comparing outcomes among all patients who were randomized and received at least 1 dose of liraglutide or placebo. For patients with missing values (eg, due to dropout), outcome values were imputed using the last-observation-carried-forward method. A multivariable analysis of covariance model was used to analyze changes in the primary outcomes and included a covariate for the baseline measure of the outcome in question. Sensitivity analyses were conducted in which the investigators used different imputation techniques (multiple imputation, repeated measures) to account for missing data.
Results. The trial enrolled 3731 participants, 2487 of whom were randomized to receive liraglutide and 1244 of whom received placebo. The groups were similar on measured baseline characteristics, with a mean age of 45 years, mostly female participants (78.7% in liraglutide arm, 78.1% in placebo), and the vast majority of participants identified as “white” race/ethnicity (84.7% in liraglutide, 85.3% in placebo). Mean baseline BMI was 38.3 kg/m2 in both groups. Although overweight patients with BMI 27 kg/m2 or greater were included, they represented a small fraction of all participants (2.7% in liraglutide group and 3.5% in placebo group). Furthermore, although patients with overt diabetes were excluded from participating, over half of the participants qualified as having prediabetes (61.4% in liraglutide group, 60.9% in placebo group). Just over one-third (34.2% of liraglutide group, 35.9% placebo) had hypertension diagnosed at baseline. Study withdrawal was relatively substantial in both groups – 71.9% remained enrolled at 56 weeks in the liraglutide group, and 64.4% remained in the placebo arm. The investigators note that withdrawal due to adverse events was more common in the liraglutide group (9.9% of withdrawals vs. 3.8% in placebo), while other reasons for withdrawing (ineffective therapy, withdrawal of consent) were more common among placebo participants.
Liraglutide participants lost significantly more weight than placebo participants at 56 weeks (mean [SD] 8.0 [6.7] kg vs. 2.6 [5.7] kg). Similarly, more patients in the liraglutide group achieved at least 5% weight loss (63% vs. 27%), and 10% weight loss (33.1% vs. 10.6%) than those taking placebo. When subgroups of patients were examined according to baseline BMI, the investigators suggested that liraglutide appeared to be more effective at promoting weight loss among patients starting below 40 kg/m2.
Hemoglobin A1c dropped significantly more (–0.23 points, P < 0.001) among liraglutide participants than among placebo participants. Similarly, fasting insulin levels dropped by 8% more (P < 0.001) in the liraglutide group at 56 weeks. In keeping with the greater weight loss, markers of cardiometabolic health also improved to a greater extent among liraglutide participants, with larger decreases in blood pressure (SBP –2.8 mm Hg lower in liraglutide, P < 0.001), and LDL (–2.4% difference, P = 0.002), and a larger increase in HDL (1.9% difference, P = 0.001). By week 56, 14% of prediabetic patients in the placebo arm had received a new diagnosis of diabetes, compared to just 4% in the liraglutide group (P < 0.001).
Quality of life scores were higher for liraglutide participants on all included measures except those related to side effects of treatment, where placebo participants reported lower levels of side effects. The most common side effects reported by liraglutide participants related to GI upset, including nausea (40%), diarrhea (21%), and vomiting (16%). More serious events, including cholelithiasis (0.8%), cholecystitis (0.5%), and pancreatitis (0.2%), were also reported. Somewhat surprisingly, although liraglutide is also used to improve glycemic control in diabetics, rates of reported spontaneous hypoglycemia were fairly low in the liraglutide group (1.3% vs. 1.0% in placebo).
Conclusion. Liraglutide given at a dose of 3.0 mg daily, along with lifestyle advice, produces clinically significant weight loss and improvement in glycemic and cardiometabolic parameters that is sustained after 1 full year of treatment.
Commentary
Over the past few years, the FDA has approved a growing list of medications for the treatment of obesity [1,2]. Unlike the prior mainstay for prescription weight management, phentermine, which can only be used for a few months at a time due to concerns about abuse, many of these newer medications are approved for long-term use, aligning well with the growing recognition of obesity as a chronic illness. Interestingly, most of the drugs that have emerged onto the market do not represent novel compounds, but rather are existing drugs that have been repurposed and repackaged for the indication of weight management. These “recycled” medications include Qsymia (a mix of phentermine and topiramate) [1], Contrave (naltrexone and buproprion) [2], and now, Saxenda (liraglutide, also marketed as Victoza for treatment of type 2 diabetes). Liraglutide is a glucagon-like-peptide 1 (GLP-1) analogue, meaning it has an effect similar to that of GLP-1, a gut hormone that stimulates insulin secretion, inhibits pancreatic beta cell apoptosis, inhibits gastric emptying, and decreases appetite by acting on the brain’s satiety centers [3]. For several years, endocrinologists and some internists have been using liraglutide (Victoza) to help with glycemic control in diabetics, with the known benefit that, unlike some other diabetes medications, it tends to promote modest weight loss [4].
In this large multicenter trial, Pi-Sunyer et al evaluated the efficacy of liraglutide at a 3.0 mg daily dose (almost twice the dose used for diabetes) for weight management. The trial utilized a strong study design, with double blinding, randomization of a subgroup for early discontinuation (to evaluate for weight regain and stopping-related side effects), and, importantly, the intervention for both groups also included a behavior change component (albeit one of relatively low intensity, based on the limited description). Patients were followed for 56 weeks on the medication, making the “intervention” phase of the study longer than what has been done in many diet trials. Testing for a long-lasting impact on weight, and at the same time attempting to quantify risks associated with longer-term use of a medication, was an important contribution for this study given that liraglutide is being marketed for long-term use.
After a year on liraglutide, participants in that group had lost around 12 lb more, on average, than those using placebo, and had achieved greater improvements cardiometabolic risk markers, with a much lower risk of developing diabetes. While these findings are promising from a clinical standpoint, it is not clear whether the moderate health impacts of this drug will be sufficient to outweigh several issues that may impede its widespread use in practice. The rate of GI side effects (nausea, vomiting, diarrhea) in liraglutide participants was fairly high, and it is worth considering whether the side effects themselves could have been driving some of the weight loss observed in that group. Furthermore, the out of pocket cost of this medication, when used for weight loss in nondiabetics, is likely to be around $1000 per month. For most patients, this high price will prohibit longer-term use of liraglutide. Even in the setting of a trial where participants faced no out of pocket costs, almost one-third in the liraglutide arm did not complete a year of treatment. On a related note, the primary analysis for this trial used a “last observation carried forward” approach—somewhat concerning given that patients are likely to regain weight after stopping any weight loss intervention, pharmaceutical or otherwise. The authors do report that a range of sensitivity analyses with varying imputation techniques were conducted and did not change the main conclusions of the trial.
Despite the promising findings from this trial, several important clinical questions remain. What is the durability of health effects for patients who discontinue the medication after a year? What safety concerns may arise in those who can afford to continue using liraglutide at this higher dose for several years? A 2-year follow-up study on participants from the current trial has been completed and those results are expected soon, which may help to shed light on some of these issues [5]. Cost-effectiveness evaluations, and head-to-head comparisons of liraglutide with lower cost weight management options would also be very helpful for clinicians presenting a range of treatment options to patients with obesity.
Applications for Clinical Practice
Liraglutide at a daily dose of 3.0 mg represents a new option for treatment of patients with obesity. It should be used in conjunction with behavioral interventions that promote a more healthful diet and increased physical activity, and may result in clinically meaningful weight loss and decreased risk of diabetes. On the other hand, the medication is costly and associated with some unpleasant GI side effects, both important factors that may limit patients’ ability to use it in the long-term. More studies are needed to establish durability of effects and safety beyond a year and that offer direct comparisons with other evidence-based weight loss tools, pharmaceutical and otherwise.
—Kristina Lewis, MD, MPH
1. Bray GA, Ryan DH. Update on obesity pharmacotherapy. Ann N Y Acad Sci 2014;1311:1–13.
2. Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA 2015;313:1213–4.
3. de Mello AH, Pra M, Cardoso LC, de Bona Schraiber R, Rezin GT. Incretin-based therapies for obesity treatment. Metabolism 23 May 2015.
4. Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 2015;4:212283.
5. Siraj ES, Williams KJ. Another agent for obesity—will this time be different? N Engl J Med 2015;373:82–3.
Study Overview
Objective. To evaluate the efficacy of liraglutide for weight loss in a group of nondiabetic patients with obesity.
Design. Randomized double-blind placebo-controlled trial.
Setting and participants. This trial took place across 27 countries in Europe, North America, South America, Asia, Africa and Australia. It was funded by NovoNordisk, the pharmaceutical company that manufactures liraglutide. Participants were 18 years or older, with a BMI of 30 kg/m2 (or 27 kg/m2 with hypertension or dyslipidemia). Patients with diabetes, those on medications known to induce weight gain (or loss), those with history of bariatric surgery, and those with psychiatric illness were excluded from participating. Patients with prediabetes were not excluded.
Intervention. Participants were randomized (2:1 in favor of study drug) to liraglutide or placebo, stratified according to BMI category and pre-diabetes status. They were started at a 0.6–mg dose of medication and up-titrated as tolerated to a dose of 3.0 mg over several weeks. All received counseling on behavioral changes to promote weight loss. Participants were then followed for 56 weeks. A small subgroup in the liraglutide arm was randomly assigned to switch to placebo after 12 weeks on medication to examine for durability of effect of medication, and to evaluate for safety issues that might occur on drug discontinuation.
Main outcome measures. This study focused on 3 primary outcomes: individual-level weight change from baseline, group-level percentage of participants achieving at least 5% weight loss, and percentage of participants with at least 10% weight loss, all assessed at 56 weeks.
Secondary outcomes included change in BMI, waist circumference, markers of glycemia (hemoglobin A1c, insulin level), markers of cardiometabolic health (blood pressure, lipids, CRP), and health-related quality of life (using several validated survey measures). Adverse events were also assessed.
The investigators used an intention-to-treat analysis, comparing outcomes among all patients who were randomized and received at least 1 dose of liraglutide or placebo. For patients with missing values (eg, due to dropout), outcome values were imputed using the last-observation-carried-forward method. A multivariable analysis of covariance model was used to analyze changes in the primary outcomes and included a covariate for the baseline measure of the outcome in question. Sensitivity analyses were conducted in which the investigators used different imputation techniques (multiple imputation, repeated measures) to account for missing data.
Results. The trial enrolled 3731 participants, 2487 of whom were randomized to receive liraglutide and 1244 of whom received placebo. The groups were similar on measured baseline characteristics, with a mean age of 45 years, mostly female participants (78.7% in liraglutide arm, 78.1% in placebo), and the vast majority of participants identified as “white” race/ethnicity (84.7% in liraglutide, 85.3% in placebo). Mean baseline BMI was 38.3 kg/m2 in both groups. Although overweight patients with BMI 27 kg/m2 or greater were included, they represented a small fraction of all participants (2.7% in liraglutide group and 3.5% in placebo group). Furthermore, although patients with overt diabetes were excluded from participating, over half of the participants qualified as having prediabetes (61.4% in liraglutide group, 60.9% in placebo group). Just over one-third (34.2% of liraglutide group, 35.9% placebo) had hypertension diagnosed at baseline. Study withdrawal was relatively substantial in both groups – 71.9% remained enrolled at 56 weeks in the liraglutide group, and 64.4% remained in the placebo arm. The investigators note that withdrawal due to adverse events was more common in the liraglutide group (9.9% of withdrawals vs. 3.8% in placebo), while other reasons for withdrawing (ineffective therapy, withdrawal of consent) were more common among placebo participants.
Liraglutide participants lost significantly more weight than placebo participants at 56 weeks (mean [SD] 8.0 [6.7] kg vs. 2.6 [5.7] kg). Similarly, more patients in the liraglutide group achieved at least 5% weight loss (63% vs. 27%), and 10% weight loss (33.1% vs. 10.6%) than those taking placebo. When subgroups of patients were examined according to baseline BMI, the investigators suggested that liraglutide appeared to be more effective at promoting weight loss among patients starting below 40 kg/m2.
Hemoglobin A1c dropped significantly more (–0.23 points, P < 0.001) among liraglutide participants than among placebo participants. Similarly, fasting insulin levels dropped by 8% more (P < 0.001) in the liraglutide group at 56 weeks. In keeping with the greater weight loss, markers of cardiometabolic health also improved to a greater extent among liraglutide participants, with larger decreases in blood pressure (SBP –2.8 mm Hg lower in liraglutide, P < 0.001), and LDL (–2.4% difference, P = 0.002), and a larger increase in HDL (1.9% difference, P = 0.001). By week 56, 14% of prediabetic patients in the placebo arm had received a new diagnosis of diabetes, compared to just 4% in the liraglutide group (P < 0.001).
Quality of life scores were higher for liraglutide participants on all included measures except those related to side effects of treatment, where placebo participants reported lower levels of side effects. The most common side effects reported by liraglutide participants related to GI upset, including nausea (40%), diarrhea (21%), and vomiting (16%). More serious events, including cholelithiasis (0.8%), cholecystitis (0.5%), and pancreatitis (0.2%), were also reported. Somewhat surprisingly, although liraglutide is also used to improve glycemic control in diabetics, rates of reported spontaneous hypoglycemia were fairly low in the liraglutide group (1.3% vs. 1.0% in placebo).
Conclusion. Liraglutide given at a dose of 3.0 mg daily, along with lifestyle advice, produces clinically significant weight loss and improvement in glycemic and cardiometabolic parameters that is sustained after 1 full year of treatment.
Commentary
Over the past few years, the FDA has approved a growing list of medications for the treatment of obesity [1,2]. Unlike the prior mainstay for prescription weight management, phentermine, which can only be used for a few months at a time due to concerns about abuse, many of these newer medications are approved for long-term use, aligning well with the growing recognition of obesity as a chronic illness. Interestingly, most of the drugs that have emerged onto the market do not represent novel compounds, but rather are existing drugs that have been repurposed and repackaged for the indication of weight management. These “recycled” medications include Qsymia (a mix of phentermine and topiramate) [1], Contrave (naltrexone and buproprion) [2], and now, Saxenda (liraglutide, also marketed as Victoza for treatment of type 2 diabetes). Liraglutide is a glucagon-like-peptide 1 (GLP-1) analogue, meaning it has an effect similar to that of GLP-1, a gut hormone that stimulates insulin secretion, inhibits pancreatic beta cell apoptosis, inhibits gastric emptying, and decreases appetite by acting on the brain’s satiety centers [3]. For several years, endocrinologists and some internists have been using liraglutide (Victoza) to help with glycemic control in diabetics, with the known benefit that, unlike some other diabetes medications, it tends to promote modest weight loss [4].
In this large multicenter trial, Pi-Sunyer et al evaluated the efficacy of liraglutide at a 3.0 mg daily dose (almost twice the dose used for diabetes) for weight management. The trial utilized a strong study design, with double blinding, randomization of a subgroup for early discontinuation (to evaluate for weight regain and stopping-related side effects), and, importantly, the intervention for both groups also included a behavior change component (albeit one of relatively low intensity, based on the limited description). Patients were followed for 56 weeks on the medication, making the “intervention” phase of the study longer than what has been done in many diet trials. Testing for a long-lasting impact on weight, and at the same time attempting to quantify risks associated with longer-term use of a medication, was an important contribution for this study given that liraglutide is being marketed for long-term use.
After a year on liraglutide, participants in that group had lost around 12 lb more, on average, than those using placebo, and had achieved greater improvements cardiometabolic risk markers, with a much lower risk of developing diabetes. While these findings are promising from a clinical standpoint, it is not clear whether the moderate health impacts of this drug will be sufficient to outweigh several issues that may impede its widespread use in practice. The rate of GI side effects (nausea, vomiting, diarrhea) in liraglutide participants was fairly high, and it is worth considering whether the side effects themselves could have been driving some of the weight loss observed in that group. Furthermore, the out of pocket cost of this medication, when used for weight loss in nondiabetics, is likely to be around $1000 per month. For most patients, this high price will prohibit longer-term use of liraglutide. Even in the setting of a trial where participants faced no out of pocket costs, almost one-third in the liraglutide arm did not complete a year of treatment. On a related note, the primary analysis for this trial used a “last observation carried forward” approach—somewhat concerning given that patients are likely to regain weight after stopping any weight loss intervention, pharmaceutical or otherwise. The authors do report that a range of sensitivity analyses with varying imputation techniques were conducted and did not change the main conclusions of the trial.
Despite the promising findings from this trial, several important clinical questions remain. What is the durability of health effects for patients who discontinue the medication after a year? What safety concerns may arise in those who can afford to continue using liraglutide at this higher dose for several years? A 2-year follow-up study on participants from the current trial has been completed and those results are expected soon, which may help to shed light on some of these issues [5]. Cost-effectiveness evaluations, and head-to-head comparisons of liraglutide with lower cost weight management options would also be very helpful for clinicians presenting a range of treatment options to patients with obesity.
Applications for Clinical Practice
Liraglutide at a daily dose of 3.0 mg represents a new option for treatment of patients with obesity. It should be used in conjunction with behavioral interventions that promote a more healthful diet and increased physical activity, and may result in clinically meaningful weight loss and decreased risk of diabetes. On the other hand, the medication is costly and associated with some unpleasant GI side effects, both important factors that may limit patients’ ability to use it in the long-term. More studies are needed to establish durability of effects and safety beyond a year and that offer direct comparisons with other evidence-based weight loss tools, pharmaceutical and otherwise.
—Kristina Lewis, MD, MPH
Study Overview
Objective. To evaluate the efficacy of liraglutide for weight loss in a group of nondiabetic patients with obesity.
Design. Randomized double-blind placebo-controlled trial.
Setting and participants. This trial took place across 27 countries in Europe, North America, South America, Asia, Africa and Australia. It was funded by NovoNordisk, the pharmaceutical company that manufactures liraglutide. Participants were 18 years or older, with a BMI of 30 kg/m2 (or 27 kg/m2 with hypertension or dyslipidemia). Patients with diabetes, those on medications known to induce weight gain (or loss), those with history of bariatric surgery, and those with psychiatric illness were excluded from participating. Patients with prediabetes were not excluded.
Intervention. Participants were randomized (2:1 in favor of study drug) to liraglutide or placebo, stratified according to BMI category and pre-diabetes status. They were started at a 0.6–mg dose of medication and up-titrated as tolerated to a dose of 3.0 mg over several weeks. All received counseling on behavioral changes to promote weight loss. Participants were then followed for 56 weeks. A small subgroup in the liraglutide arm was randomly assigned to switch to placebo after 12 weeks on medication to examine for durability of effect of medication, and to evaluate for safety issues that might occur on drug discontinuation.
Main outcome measures. This study focused on 3 primary outcomes: individual-level weight change from baseline, group-level percentage of participants achieving at least 5% weight loss, and percentage of participants with at least 10% weight loss, all assessed at 56 weeks.
Secondary outcomes included change in BMI, waist circumference, markers of glycemia (hemoglobin A1c, insulin level), markers of cardiometabolic health (blood pressure, lipids, CRP), and health-related quality of life (using several validated survey measures). Adverse events were also assessed.
The investigators used an intention-to-treat analysis, comparing outcomes among all patients who were randomized and received at least 1 dose of liraglutide or placebo. For patients with missing values (eg, due to dropout), outcome values were imputed using the last-observation-carried-forward method. A multivariable analysis of covariance model was used to analyze changes in the primary outcomes and included a covariate for the baseline measure of the outcome in question. Sensitivity analyses were conducted in which the investigators used different imputation techniques (multiple imputation, repeated measures) to account for missing data.
Results. The trial enrolled 3731 participants, 2487 of whom were randomized to receive liraglutide and 1244 of whom received placebo. The groups were similar on measured baseline characteristics, with a mean age of 45 years, mostly female participants (78.7% in liraglutide arm, 78.1% in placebo), and the vast majority of participants identified as “white” race/ethnicity (84.7% in liraglutide, 85.3% in placebo). Mean baseline BMI was 38.3 kg/m2 in both groups. Although overweight patients with BMI 27 kg/m2 or greater were included, they represented a small fraction of all participants (2.7% in liraglutide group and 3.5% in placebo group). Furthermore, although patients with overt diabetes were excluded from participating, over half of the participants qualified as having prediabetes (61.4% in liraglutide group, 60.9% in placebo group). Just over one-third (34.2% of liraglutide group, 35.9% placebo) had hypertension diagnosed at baseline. Study withdrawal was relatively substantial in both groups – 71.9% remained enrolled at 56 weeks in the liraglutide group, and 64.4% remained in the placebo arm. The investigators note that withdrawal due to adverse events was more common in the liraglutide group (9.9% of withdrawals vs. 3.8% in placebo), while other reasons for withdrawing (ineffective therapy, withdrawal of consent) were more common among placebo participants.
Liraglutide participants lost significantly more weight than placebo participants at 56 weeks (mean [SD] 8.0 [6.7] kg vs. 2.6 [5.7] kg). Similarly, more patients in the liraglutide group achieved at least 5% weight loss (63% vs. 27%), and 10% weight loss (33.1% vs. 10.6%) than those taking placebo. When subgroups of patients were examined according to baseline BMI, the investigators suggested that liraglutide appeared to be more effective at promoting weight loss among patients starting below 40 kg/m2.
Hemoglobin A1c dropped significantly more (–0.23 points, P < 0.001) among liraglutide participants than among placebo participants. Similarly, fasting insulin levels dropped by 8% more (P < 0.001) in the liraglutide group at 56 weeks. In keeping with the greater weight loss, markers of cardiometabolic health also improved to a greater extent among liraglutide participants, with larger decreases in blood pressure (SBP –2.8 mm Hg lower in liraglutide, P < 0.001), and LDL (–2.4% difference, P = 0.002), and a larger increase in HDL (1.9% difference, P = 0.001). By week 56, 14% of prediabetic patients in the placebo arm had received a new diagnosis of diabetes, compared to just 4% in the liraglutide group (P < 0.001).
Quality of life scores were higher for liraglutide participants on all included measures except those related to side effects of treatment, where placebo participants reported lower levels of side effects. The most common side effects reported by liraglutide participants related to GI upset, including nausea (40%), diarrhea (21%), and vomiting (16%). More serious events, including cholelithiasis (0.8%), cholecystitis (0.5%), and pancreatitis (0.2%), were also reported. Somewhat surprisingly, although liraglutide is also used to improve glycemic control in diabetics, rates of reported spontaneous hypoglycemia were fairly low in the liraglutide group (1.3% vs. 1.0% in placebo).
Conclusion. Liraglutide given at a dose of 3.0 mg daily, along with lifestyle advice, produces clinically significant weight loss and improvement in glycemic and cardiometabolic parameters that is sustained after 1 full year of treatment.
Commentary
Over the past few years, the FDA has approved a growing list of medications for the treatment of obesity [1,2]. Unlike the prior mainstay for prescription weight management, phentermine, which can only be used for a few months at a time due to concerns about abuse, many of these newer medications are approved for long-term use, aligning well with the growing recognition of obesity as a chronic illness. Interestingly, most of the drugs that have emerged onto the market do not represent novel compounds, but rather are existing drugs that have been repurposed and repackaged for the indication of weight management. These “recycled” medications include Qsymia (a mix of phentermine and topiramate) [1], Contrave (naltrexone and buproprion) [2], and now, Saxenda (liraglutide, also marketed as Victoza for treatment of type 2 diabetes). Liraglutide is a glucagon-like-peptide 1 (GLP-1) analogue, meaning it has an effect similar to that of GLP-1, a gut hormone that stimulates insulin secretion, inhibits pancreatic beta cell apoptosis, inhibits gastric emptying, and decreases appetite by acting on the brain’s satiety centers [3]. For several years, endocrinologists and some internists have been using liraglutide (Victoza) to help with glycemic control in diabetics, with the known benefit that, unlike some other diabetes medications, it tends to promote modest weight loss [4].
In this large multicenter trial, Pi-Sunyer et al evaluated the efficacy of liraglutide at a 3.0 mg daily dose (almost twice the dose used for diabetes) for weight management. The trial utilized a strong study design, with double blinding, randomization of a subgroup for early discontinuation (to evaluate for weight regain and stopping-related side effects), and, importantly, the intervention for both groups also included a behavior change component (albeit one of relatively low intensity, based on the limited description). Patients were followed for 56 weeks on the medication, making the “intervention” phase of the study longer than what has been done in many diet trials. Testing for a long-lasting impact on weight, and at the same time attempting to quantify risks associated with longer-term use of a medication, was an important contribution for this study given that liraglutide is being marketed for long-term use.
After a year on liraglutide, participants in that group had lost around 12 lb more, on average, than those using placebo, and had achieved greater improvements cardiometabolic risk markers, with a much lower risk of developing diabetes. While these findings are promising from a clinical standpoint, it is not clear whether the moderate health impacts of this drug will be sufficient to outweigh several issues that may impede its widespread use in practice. The rate of GI side effects (nausea, vomiting, diarrhea) in liraglutide participants was fairly high, and it is worth considering whether the side effects themselves could have been driving some of the weight loss observed in that group. Furthermore, the out of pocket cost of this medication, when used for weight loss in nondiabetics, is likely to be around $1000 per month. For most patients, this high price will prohibit longer-term use of liraglutide. Even in the setting of a trial where participants faced no out of pocket costs, almost one-third in the liraglutide arm did not complete a year of treatment. On a related note, the primary analysis for this trial used a “last observation carried forward” approach—somewhat concerning given that patients are likely to regain weight after stopping any weight loss intervention, pharmaceutical or otherwise. The authors do report that a range of sensitivity analyses with varying imputation techniques were conducted and did not change the main conclusions of the trial.
Despite the promising findings from this trial, several important clinical questions remain. What is the durability of health effects for patients who discontinue the medication after a year? What safety concerns may arise in those who can afford to continue using liraglutide at this higher dose for several years? A 2-year follow-up study on participants from the current trial has been completed and those results are expected soon, which may help to shed light on some of these issues [5]. Cost-effectiveness evaluations, and head-to-head comparisons of liraglutide with lower cost weight management options would also be very helpful for clinicians presenting a range of treatment options to patients with obesity.
Applications for Clinical Practice
Liraglutide at a daily dose of 3.0 mg represents a new option for treatment of patients with obesity. It should be used in conjunction with behavioral interventions that promote a more healthful diet and increased physical activity, and may result in clinically meaningful weight loss and decreased risk of diabetes. On the other hand, the medication is costly and associated with some unpleasant GI side effects, both important factors that may limit patients’ ability to use it in the long-term. More studies are needed to establish durability of effects and safety beyond a year and that offer direct comparisons with other evidence-based weight loss tools, pharmaceutical and otherwise.
—Kristina Lewis, MD, MPH
1. Bray GA, Ryan DH. Update on obesity pharmacotherapy. Ann N Y Acad Sci 2014;1311:1–13.
2. Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA 2015;313:1213–4.
3. de Mello AH, Pra M, Cardoso LC, de Bona Schraiber R, Rezin GT. Incretin-based therapies for obesity treatment. Metabolism 23 May 2015.
4. Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 2015;4:212283.
5. Siraj ES, Williams KJ. Another agent for obesity—will this time be different? N Engl J Med 2015;373:82–3.
1. Bray GA, Ryan DH. Update on obesity pharmacotherapy. Ann N Y Acad Sci 2014;1311:1–13.
2. Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA 2015;313:1213–4.
3. de Mello AH, Pra M, Cardoso LC, de Bona Schraiber R, Rezin GT. Incretin-based therapies for obesity treatment. Metabolism 23 May 2015.
4. Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 2015;4:212283.
5. Siraj ES, Williams KJ. Another agent for obesity—will this time be different? N Engl J Med 2015;373:82–3.
A Multipronged Approach to Decrease the Risk of Clostridium difficile Infection at a Community Hospital and Long-Term Care Facility
From Sharp HealthCare, San Diego, CA.
Abstract
- Objective: To examine the relationship between the rate of Clostridium difficile infections (CDI) and implementation of 3 interventions aimed at preserving the fecal microbiome: (1) reduction of antimicrobial pressure; (2) reduction in intensity of gastrointestinal prophylaxis with proton-pump inhibitors (PPIs); and (3) expansion of probiotic therapy.
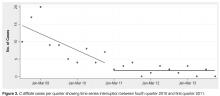
- Methods: We conducted a retrospective analysis of all inpatients with CDI between January 2009 and December 2013 receiving care at our community hospital and associated long-term care (LTC) facility. We used interrupted time series analysis to assess CDI rates during the implementation phase (2008–2010) and the postimplementation phase (2011–2013).
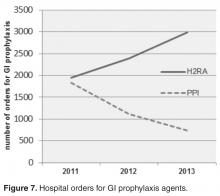
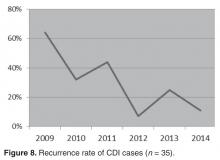
- Results: A reduction in the rate of health care facility–associated CDIs was seen. The mean number of cases per 10,000 patient days fell from 11.9 to 3.6 in acute care and 6.1 to 1.1 in LTC. Recurrence rates decreased from 64% in 2009 to 16% by 2014. The likelihood of CDI recurring was 3 times higher in those exposed to PPI and 0.35 times less likely in those who received probiotics with their initial CDI therapy.
- Conclusion: The risk of CDI incidence and recurrence was significantly reduced in our inpatients, with recurrent CDI associated with PPI use, multiple antibiotic courses, and lack of probiotics. We attribute our success to the combined effect of intensified antibiotic stewardship, reduced PPI use, and expanded probiotic use.
Clostridium difficile is classified as an urgent public health threat by the Centers for Disease Control and Prevention [1]. A recent study by the CDC found that it caused more than 400,000 infections in the United States in 2011, leading to over 29,000 deaths [2]. The costs of treating CDI are substantial and recurrences are common. While rates for many health care–associated infections are declining, C. difficile infection (CDI) rates remain at historically high levels [1] with the elderly at greatest risk for infection and mortality from the illness [3].
CDIs can be prevented. A principal recommendation for preventing CDIs is improving antibiotic use. Antibiotic use increases the risk for developing CDI by disrupting the colonic microbiome. Hospitalized and long-term care (LTC) patients are frequently prescribed antibiotics, but studies indicate that much of this use is inappropriate [4]. Antimicrobial stewardship has been shown to be effective in reducing CDI rates. Other infection prevention measures commonly employed to decrease the risk of hospital-onset CDI include monitoring of hand hygiene compliance using soap and water, terminal cleaning with bleach products of rooms occupied by patients with CDI, and daily cleaning of highly touched areas. At our institution, patients identified with CDI are placed on contact precautions until they have been adequately treated and have had resolution of diarrhea for 48 hours.
In addition to preventing CDI transmission through antimicrobial stewardship, attention is being paid to the possibility that restricting PPI use may help in preventing CDI. The increasing utilization of proton-pump inhibitors (PPIs) in recent years has coincided with the trend of increasing CDI rates. Although C. difficile spores are acid-resistant, vegetative forms are easily affected by acidity. Several studies have shown the association of acid suppression and greater susceptibility of acquiring CDI or recurrences [5–7]. Elevated gastric pH by PPIs facilitates the growth of potentially pathogenic upper and lower gastrointestinal (GI) tract flora, including the conversion of C. difficile from spore to vegetative form in the upper GI tract [5,8].
A growing body of evidence indicates that probiotics are both safe and effective for preventing CDIs [9]. Probiotics may counteract disturbances in intestinal flora, thereby reducing the risk for colonization by pathogenic bacteria. Probiotics can inhibit pathogen adhesion, colonization, and invasion of the gastrointestinal mucosa [10].
We hypothesized that preservation and/or restoration of the diversity of the fecal microbiome would prevent CDI and disease recurrence in our facility. Prior to 2009, we had strict infection prevention measures in place to prevent disease transmission, similar to many other institutions. In 2009, we implemented 3 additional interventions to reduce the rising incidence of CDI: (1) an antibiotic stewardship program, (2) lowering the intensity of acid suppression, and (3) expanding the use of probiotic therapy. The 3 interventions were initiated over the 19-month period January 2009 through July 2010. This study addresses the effects of these interventions.
Methods
Patients and Data Collection
The study was conducted at a community hospital (59 beds) that has an associated LTC facility (122 beds). We conducted a retrospective analysis of hospital and LTC data from all documented cases of CDI between January 2009 and December 2013. Study subjects included all patients with stools positive for C. difficile antigen and toxin with associated symptoms of infection (n = 123). Institutional review board approval was obtained prior to data collection.
The following information was collected: admission diagnosis, number of days from admission until confirmed CDI, residence prior to admission, duration and type of antibiotics received prior to or during symptoms of CDI, type of GI prophylaxis received within 14 days prior to and during CDI treatment, probiotic received and duration, and the type and duration of antibiotic treatment given for the CDI. The data collected was used to determine the likely origin of each C. difficile case, dates of recurrences, and the possible effects of the interventions. Antibiotic use was categorized as: (1) recent antibiotic course (antibiotics received within the preceding 4 weeks), (2) antibiotic courses greater than 10 days, and (3) multiple antibiotic courses (more than 1 antibiotic course received sequentially or concurrently).
Positive C. difficile infections were detected using a 2-step algorithm, starting in 2009. The samples were first screened with a rapid membrane enzyme immunoassay for glutamate dehydrogenase (GDH) antigen and toxin A and B in stool (C. Diff Quik Chek Complete, Techlab, Blacksburg, VA). Discrepant samples (GDH positive and toxin A and B negative) were reflexed to DNA-based PCR testing. The PCR assay was changed to the Verigene C. difficile test (Nanosphere, Northbrook, IL) in 2012. Up to 30 days after discharge from our facility, positive results were considered as acquired from our facility and positive results within 2 days of admission with symptoms of CDI were considered positive on admission and were not attributed to our facility. A primary episode of CDI was defined to be the first identified episode or event in each patient. Recurrent CDI was defined as a repeated case of CDI within 180 days of the original CDI event.
Interventions to Reduce CDI
Reduction of Antibiotic Pressure
Other actions taken to improve antimicrobial prescribing as part of the stewardship program included medication usage evaluations (MUEs) for levofloxacin and carbapenems, implementing an automatic dosing/duration protocol for levofloxacin, and carbapenem restriction to prevent inappropriate use. Nursing and pharmacy staffs were educated on vancomycin appropriateness, benefits of MRSA screening for de-escalation, procalcitonin, and treatment of sepsis. Emergency department staff was educated on (1) empiric antimicrobial treatment recommendations for urinary and skin and soft tissue infections based on outpatient antibiogram data, (2) renal adjustment of antimicrobials, (3) fluoroquinolones: resistance formation, higher CDI risk and higher dosing recommendations, (4) GI prophylaxis recommendations, and (5) probiotics.
Reduction in the Intensity of Acid Suppression for GI Prophylaxis
PPIs were substituted with histamine-2 receptor antagonists (H2RA) whenever acid suppression for GI prophylaxis was warranted. If GI symptoms persisted, sucralfate was added. In May 2010, all eligible LTC patients were converted from PPIs to H2RA.
Expanding the Use of Probiotics
We expanded the use of probiotics as an adjunctive treatment for CDI with metronidazole ± vancomycin oral therapies. Probiotics were included concurrently with any broad-spectrum antibiotic administration, longer antibiotic courses (≥ 7 days), and/or multiple courses of antibiotics. The combination of Saccromyces boulardii plus Lactobacillus acidophilus and L. bulgaricus was given with twice daily dosing until the end of 2011. In January 2012, our facility switched over to daily administration of a probiotic with the active ingredients of Lactobacillus acidophilus and Lactobacillus casei, 50 billion colony-forming units. Probiotics were given during the antibiotic course plus for 1 additional week after course completion. Probiotics were not administered to selected groups of patients: (1) immunocompromised patients, (2) patients who were NPO, or (3) patients excluded by their physicians.
There was no change or enhanced targeting of infection prevention or environmental hygiene strategies during the study period.
Data Analysis and Statistical Methods
All data were collected on data collection sheets and transcribed into Microsoft Office Excel 2007 Service Pack 3. No data were excluded from analysis. Continuous variables, eg, number of cases of CDI, are reported as mean ± standard deviation. Categorical variables, eg, number of recurrent CDI cases, are reported as the count and percentage. Comparison of populations was done with the Wilcoxon rank sum test. Segments of the interrupted time series were assessed using linear regression. Associations were tested using χ2. Statistical tests were deemed significant when the α probability was < 0.05. No adjustments were made for multiplicity. Data descriptive statistics (including frequency histograms for visual examination of distributions) and statistical analyses were performed using Stata 11.1 (StataCorp, College Station, TX).
Results
CDIs
Within the population of patients having a CDI or recurrence, we found that those patients in the later time period (2011–2013) were significantly less likely to have a recurrence than those in the earlier time period (pre- Jan 2011) (chi square = 5.975, df = 1, P = 0.015). The odds ratio (OR) was 0.35 (95% CI 0.15 to 0.83).
Patients in the earlier (2009–2010) vs. the later post-intervention group (2011–2013) had more likely received multiple antibiotic courses (chi square = 5.32, df = 1, P = 0.021, OR 2.56), a PPI (chi square = 8.86, df = 1, P = 0.003, OR 3.38), and had a health care facility–associated infection originating from our institution as opposed to outside facility transfers or community-acquired cases (chi square = 7.09, df = 1, P = 0.008, OR 2.94).
Antibiotic Pressure
Acid Suppression
In evaluating the effects of limiting the use of PPIs, patients who received an H2RA or no antacid prophylaxis were significantly less likely to have a recurrence of CDI than those who received a PPI (chi square = 6.35, df = 1, P = 0.012). The OR for recurrence with PPIs was 3.05 (95% CI 1.25 to 7.44). Of patients exposed to PPIs, those exposed in the later time period (2011 through 2013) were significantly less likely to have a recurrence than those exposed in the early time period (third quarter 2008 through 2010; chi square = 15.14, df = 1, P < 0.001). The OR was 0.23 (95% CI, 0.11 to 0.49).
Probiotics
During 2009–2011, only 15% of the CDI patients had received probiotics with an antibiotic course. Probiotic therapy as part of CDI treatment increased from 60% in 2009 to 91% in 2011. Among patients that contracted CDI in 2012–2013, only 2 patients received probiotics with their antibiotic courses.
Recurrences
With regard to the effect of probiotics within this population, those who received
One patient with significant initial antibiotic pressure was continued on her PPI during CDI treatment and continued to have recurrences, despite probiotic use. After her fourth recurrence, her PPI was changed to an H2RA, and she had no further recurrences. She continues off PPI therapy and is CDI-free 2 years later. Another patient who remained on his PPI had 3 recurrences, until finally a probiotic was added and the recurrences abated.
Discussion
CDI is common in hospitalized patients, and its incidence has increased due to multiple factors, which include the widespread use of broad-spectrum antimicrobials and increased use of PPIs. Our observational study showed a statistically significant reduction in the number of health care–associated CDI cases during our implementation period (mid–2008 through 2010). From 2011 on, all initiatives were maintained. As the lower rates of CDI continued, physician confidence in antimicrobial stewardship recommendations increased. During this latter portion of the study period, hospitalists uniformly switched patients to H2RA for GI prophylaxis, added prophylactic probiotics to antibiotic courses as well as CDI therapy, and were more receptive to streamlining and limiting durations of antibiotic therapy. Although the study was completed in 2013, follow-up data have shown that the low CDI incidence has continued through 2014.
The average age of the patients in our study was 69 years. In 2009, there were 41 C. difficile cases originating from our institution; however, by the end of 2011, only 9 cases had been reported, a 75% reduction. The majority of our cases of C. difficile in 2009–2010 originated from our facility’s LTC units (Figure 2). Risk factors in the LTC population included older age (72% are > 65 years) with multiple comorbidities, exposure to frequent multiple courses of broad-spectrum antibiotics, and use of PPIs as the standard for GI prophylaxis therapy. Multiple antibiotic courses had a strong association with PPI administration in the patients who contracted CDI, while recent antibiotics and antibiotics greater than 10 days did not. Implications may include an increased risk of CDI in patients requiring multiple antibiotic courses concurrent with PPI exposure.
Infection prevention strategies were promulgated among the health care team during the study period but were not specifically targeted for quality improvement efforts. Therefore, in contrast to other studies where infection prevention measures and environmental hygiene were prominent components of a CDI prevention “bundle,” our focus was on antimicrobial stewardship and PPI and probiotic use, not enhancement of standard infection prevention and environmental hygiene measures.
The antibiotics used prior to the development of CDI in our study were similar to findings from other studies that have associated broad-spectrum antibiotics with increased susceptibility to CDI [11]. Antimicrobials disrupt the normal GI flora, which is essential for eradicating many C. difficile spores [12]. The utilization of high-risk antibiotics and prolonged antimicrobial therapy were reduced with implementation of our antimicrobial stewardship program. In 2012, the antimicrobial stewardship program developed a LTC fever protocol, providing education to LTC nurses, physicians, and pharmacists using the modified McGeer criteria [13] for infection in LTC units and empiric antibiotic recommendations from our epidemiologist. A formal recommendation for a LTC 7-day stop date for urinary, respiratory, and skin and soft tissue infections was initiated, which included are-assessment at day 6–7 for resolution of symptoms.
With regard to PPI therapy, our study revealed that patients who had received a PPI at some point were 3.05 times more likely to have a recurrence of CDI than those who had not. These findings are consistent with the literature. Linsky et al [5] found a 42% increased risk of CDI recurrence in patients receiving PPIs concurrent with CDI treatment while considering covariates that may influence the risk of recurrent CDI or exposure to PPIs. A meta-analysis of 16 observational studies involving more than 1.2 million hospitalized patients by Janarthanan et al [14] explored the association between CDI and PPIs and showed a 65% increase in the incidence of CDI among PPI users. Those receiving PPI for GI prophylaxis in the earlier time period (before 2011) were 77% more likely to have a recurrence than those who received PPI in the later period. This finding might be associated with the more appropriate antimicrobial use and the more consistent use of consistent prophylactic probiotics in the later study period.
Our results showed that those who received probiotics with the initial CDI treatment were significantly less likely to have a recurrence than those who did not. Patients receiving probiotics in the later period (2011–2013) were 74% less likely to have a recurrence than patients in the earlier group (2009–2010). Despite the standard use of probiotics for primary CDI prevention at our institution, we could not show direct significance to the lack of probiotic use found in the identified CDI patients with this observational study design. The higher benefit in more recent years could possibly be attributed to the fact that these patients were much less likely to have received a PPI, that most had likely received probiotics concurrently plus 1 week after their antibiotic courses, and their antibiotic therapy was likely more focused and streamlined to prevent C. difficile infection. A meta-analysis of probiotic efficacy in primary CDI prevention suggested that probiotics can lead to a 64% reduction in the incidence of CDI, in addition to reducing GI-associated symptoms related to infection or antibiotic use [9]. A dose-response study of the efficacy of a probiotic formula showed a lower incidence of CDI, 1.2% for higher dose vs. 9.4% for lower dose vs. 23.8% for placebo [15]. Maziade et al [16] added prophylactic probiotics to a bundle of standard preventative measures for C. difficile infections, and were able to show an enhanced and sustained decrease in CDI rates (73%) and recurrences (39%). However, many of the probiotic studies which have studied the relationship to CDI have been criticized for reporting abnormally high rates of infection [9,16] missing data, a lack of controls or excessive patient exclusion criteria [17,18] The more recent PLACIDE study by Allen et al [19] was a large multicenter randomized controlled trial that did not show any benefit to CDI prevention with probiotics; however, with 83% of screened patients excluded, the patients were low risk, with the resulting CDI incidence (0.99%) too low to show a benefit. Acid suppression was also not revealed in the specific CDI cases, and others have found this to be a significant risk factor [5–7].
Limitations of this study include the study design (an observational, retrospective analysis), the small size of our facility, and the difficulty in obtaining probiotic history prior to admission in some cases. Due to a change in computer systems, hospital orders for GI prophylaxis agents could not be obtained for 2009–2010. Due to the fact that we instituted our interventions somewhat concurrently, it is difficult to analyze their individual impact. Randomized controlled trials evaluating the combined role of probiotics, GI prophylaxis, and antibiotic pressure in CDI are needed to further define the importance of this approach.
Corresponding author: Bridget Olson, RPh, Sharp Coronado Hospital & Villa Coronado Long-Term Care Facility, 250
Prospect Pl., Coronado CA 92118, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BO, TH, KW, RO; analysis and interpretation of data, RAF; drafting of article, BO, RAF; critical revision of the article, RAF, JH, TH; provision of study materials or patients, BO; statistical expertise, RAF; administrative or technical support, KW, RO; collection and assembly of data, BO.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html.
2. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–34.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005;173:1037–42.
4. Warren JW, Palumbo FB, Fitterman L, Speedie SM. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc 1991;39:963–72.
5. Linsky A, Gupta K, Lawler E, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010;170:772–8.
6. Dial S, Delaney JA, Barkun AN, Sulssa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989–95.
7. Howell M, Novack V, Grgurich P, et.al. Iatrogenic gastric acid suppression and the risk if nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784–90.
8. Radulovic Z, Petrovic T, Bulajic S. Antibiotic susceptibility of probiotic bacteria. In Pana M, editor. Antibiotic resistant bacteria: a continuous challenge in the new millennium. Rijeka, Croatia: InTech; 2012.
9. Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD006095.
10. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea. Ann Intern Med 2012;157:878–88.
11. Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009;63:203–37.
12. Elliott B, Chang BJ, Golledge CL et al. Clostridium difficile-associated diarrhoea. Intern Med J 2007;37:561–8.
13. Stone, ND, Ashraf, MS et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol 2012;33:965–77.
14. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–10.
15. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010;105:1636-41.
16. Maziade PJ, Andriessen JA, Pereira P, et.al. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr Med Res Opin 2013;29:1341–7.
17. Islam, J, Cohen J, Rajkumar C, Llewelyn M. Probiotics for the prevention and treatment of Clostridium difficile in older patients. Age Ageing 2012;41:706–11.
18. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007;335:80.
19. Allen S J, Wareham K, Wang, D, et.al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomized, double-blind, placebo-controlled, multi-centre trial. Lancet 2013;382:1249–57.
From Sharp HealthCare, San Diego, CA.
Abstract
- Objective: To examine the relationship between the rate of Clostridium difficile infections (CDI) and implementation of 3 interventions aimed at preserving the fecal microbiome: (1) reduction of antimicrobial pressure; (2) reduction in intensity of gastrointestinal prophylaxis with proton-pump inhibitors (PPIs); and (3) expansion of probiotic therapy.
- Methods: We conducted a retrospective analysis of all inpatients with CDI between January 2009 and December 2013 receiving care at our community hospital and associated long-term care (LTC) facility. We used interrupted time series analysis to assess CDI rates during the implementation phase (2008–2010) and the postimplementation phase (2011–2013).
- Results: A reduction in the rate of health care facility–associated CDIs was seen. The mean number of cases per 10,000 patient days fell from 11.9 to 3.6 in acute care and 6.1 to 1.1 in LTC. Recurrence rates decreased from 64% in 2009 to 16% by 2014. The likelihood of CDI recurring was 3 times higher in those exposed to PPI and 0.35 times less likely in those who received probiotics with their initial CDI therapy.
- Conclusion: The risk of CDI incidence and recurrence was significantly reduced in our inpatients, with recurrent CDI associated with PPI use, multiple antibiotic courses, and lack of probiotics. We attribute our success to the combined effect of intensified antibiotic stewardship, reduced PPI use, and expanded probiotic use.
Clostridium difficile is classified as an urgent public health threat by the Centers for Disease Control and Prevention [1]. A recent study by the CDC found that it caused more than 400,000 infections in the United States in 2011, leading to over 29,000 deaths [2]. The costs of treating CDI are substantial and recurrences are common. While rates for many health care–associated infections are declining, C. difficile infection (CDI) rates remain at historically high levels [1] with the elderly at greatest risk for infection and mortality from the illness [3].
CDIs can be prevented. A principal recommendation for preventing CDIs is improving antibiotic use. Antibiotic use increases the risk for developing CDI by disrupting the colonic microbiome. Hospitalized and long-term care (LTC) patients are frequently prescribed antibiotics, but studies indicate that much of this use is inappropriate [4]. Antimicrobial stewardship has been shown to be effective in reducing CDI rates. Other infection prevention measures commonly employed to decrease the risk of hospital-onset CDI include monitoring of hand hygiene compliance using soap and water, terminal cleaning with bleach products of rooms occupied by patients with CDI, and daily cleaning of highly touched areas. At our institution, patients identified with CDI are placed on contact precautions until they have been adequately treated and have had resolution of diarrhea for 48 hours.
In addition to preventing CDI transmission through antimicrobial stewardship, attention is being paid to the possibility that restricting PPI use may help in preventing CDI. The increasing utilization of proton-pump inhibitors (PPIs) in recent years has coincided with the trend of increasing CDI rates. Although C. difficile spores are acid-resistant, vegetative forms are easily affected by acidity. Several studies have shown the association of acid suppression and greater susceptibility of acquiring CDI or recurrences [5–7]. Elevated gastric pH by PPIs facilitates the growth of potentially pathogenic upper and lower gastrointestinal (GI) tract flora, including the conversion of C. difficile from spore to vegetative form in the upper GI tract [5,8].
A growing body of evidence indicates that probiotics are both safe and effective for preventing CDIs [9]. Probiotics may counteract disturbances in intestinal flora, thereby reducing the risk for colonization by pathogenic bacteria. Probiotics can inhibit pathogen adhesion, colonization, and invasion of the gastrointestinal mucosa [10].
We hypothesized that preservation and/or restoration of the diversity of the fecal microbiome would prevent CDI and disease recurrence in our facility. Prior to 2009, we had strict infection prevention measures in place to prevent disease transmission, similar to many other institutions. In 2009, we implemented 3 additional interventions to reduce the rising incidence of CDI: (1) an antibiotic stewardship program, (2) lowering the intensity of acid suppression, and (3) expanding the use of probiotic therapy. The 3 interventions were initiated over the 19-month period January 2009 through July 2010. This study addresses the effects of these interventions.
Methods
Patients and Data Collection
The study was conducted at a community hospital (59 beds) that has an associated LTC facility (122 beds). We conducted a retrospective analysis of hospital and LTC data from all documented cases of CDI between January 2009 and December 2013. Study subjects included all patients with stools positive for C. difficile antigen and toxin with associated symptoms of infection (n = 123). Institutional review board approval was obtained prior to data collection.
The following information was collected: admission diagnosis, number of days from admission until confirmed CDI, residence prior to admission, duration and type of antibiotics received prior to or during symptoms of CDI, type of GI prophylaxis received within 14 days prior to and during CDI treatment, probiotic received and duration, and the type and duration of antibiotic treatment given for the CDI. The data collected was used to determine the likely origin of each C. difficile case, dates of recurrences, and the possible effects of the interventions. Antibiotic use was categorized as: (1) recent antibiotic course (antibiotics received within the preceding 4 weeks), (2) antibiotic courses greater than 10 days, and (3) multiple antibiotic courses (more than 1 antibiotic course received sequentially or concurrently).
Positive C. difficile infections were detected using a 2-step algorithm, starting in 2009. The samples were first screened with a rapid membrane enzyme immunoassay for glutamate dehydrogenase (GDH) antigen and toxin A and B in stool (C. Diff Quik Chek Complete, Techlab, Blacksburg, VA). Discrepant samples (GDH positive and toxin A and B negative) were reflexed to DNA-based PCR testing. The PCR assay was changed to the Verigene C. difficile test (Nanosphere, Northbrook, IL) in 2012. Up to 30 days after discharge from our facility, positive results were considered as acquired from our facility and positive results within 2 days of admission with symptoms of CDI were considered positive on admission and were not attributed to our facility. A primary episode of CDI was defined to be the first identified episode or event in each patient. Recurrent CDI was defined as a repeated case of CDI within 180 days of the original CDI event.
Interventions to Reduce CDI
Reduction of Antibiotic Pressure
Other actions taken to improve antimicrobial prescribing as part of the stewardship program included medication usage evaluations (MUEs) for levofloxacin and carbapenems, implementing an automatic dosing/duration protocol for levofloxacin, and carbapenem restriction to prevent inappropriate use. Nursing and pharmacy staffs were educated on vancomycin appropriateness, benefits of MRSA screening for de-escalation, procalcitonin, and treatment of sepsis. Emergency department staff was educated on (1) empiric antimicrobial treatment recommendations for urinary and skin and soft tissue infections based on outpatient antibiogram data, (2) renal adjustment of antimicrobials, (3) fluoroquinolones: resistance formation, higher CDI risk and higher dosing recommendations, (4) GI prophylaxis recommendations, and (5) probiotics.
Reduction in the Intensity of Acid Suppression for GI Prophylaxis
PPIs were substituted with histamine-2 receptor antagonists (H2RA) whenever acid suppression for GI prophylaxis was warranted. If GI symptoms persisted, sucralfate was added. In May 2010, all eligible LTC patients were converted from PPIs to H2RA.
Expanding the Use of Probiotics
We expanded the use of probiotics as an adjunctive treatment for CDI with metronidazole ± vancomycin oral therapies. Probiotics were included concurrently with any broad-spectrum antibiotic administration, longer antibiotic courses (≥ 7 days), and/or multiple courses of antibiotics. The combination of Saccromyces boulardii plus Lactobacillus acidophilus and L. bulgaricus was given with twice daily dosing until the end of 2011. In January 2012, our facility switched over to daily administration of a probiotic with the active ingredients of Lactobacillus acidophilus and Lactobacillus casei, 50 billion colony-forming units. Probiotics were given during the antibiotic course plus for 1 additional week after course completion. Probiotics were not administered to selected groups of patients: (1) immunocompromised patients, (2) patients who were NPO, or (3) patients excluded by their physicians.
There was no change or enhanced targeting of infection prevention or environmental hygiene strategies during the study period.
Data Analysis and Statistical Methods
All data were collected on data collection sheets and transcribed into Microsoft Office Excel 2007 Service Pack 3. No data were excluded from analysis. Continuous variables, eg, number of cases of CDI, are reported as mean ± standard deviation. Categorical variables, eg, number of recurrent CDI cases, are reported as the count and percentage. Comparison of populations was done with the Wilcoxon rank sum test. Segments of the interrupted time series were assessed using linear regression. Associations were tested using χ2. Statistical tests were deemed significant when the α probability was < 0.05. No adjustments were made for multiplicity. Data descriptive statistics (including frequency histograms for visual examination of distributions) and statistical analyses were performed using Stata 11.1 (StataCorp, College Station, TX).
Results
CDIs
Within the population of patients having a CDI or recurrence, we found that those patients in the later time period (2011–2013) were significantly less likely to have a recurrence than those in the earlier time period (pre- Jan 2011) (chi square = 5.975, df = 1, P = 0.015). The odds ratio (OR) was 0.35 (95% CI 0.15 to 0.83).
Patients in the earlier (2009–2010) vs. the later post-intervention group (2011–2013) had more likely received multiple antibiotic courses (chi square = 5.32, df = 1, P = 0.021, OR 2.56), a PPI (chi square = 8.86, df = 1, P = 0.003, OR 3.38), and had a health care facility–associated infection originating from our institution as opposed to outside facility transfers or community-acquired cases (chi square = 7.09, df = 1, P = 0.008, OR 2.94).
Antibiotic Pressure
Acid Suppression
In evaluating the effects of limiting the use of PPIs, patients who received an H2RA or no antacid prophylaxis were significantly less likely to have a recurrence of CDI than those who received a PPI (chi square = 6.35, df = 1, P = 0.012). The OR for recurrence with PPIs was 3.05 (95% CI 1.25 to 7.44). Of patients exposed to PPIs, those exposed in the later time period (2011 through 2013) were significantly less likely to have a recurrence than those exposed in the early time period (third quarter 2008 through 2010; chi square = 15.14, df = 1, P < 0.001). The OR was 0.23 (95% CI, 0.11 to 0.49).
Probiotics
During 2009–2011, only 15% of the CDI patients had received probiotics with an antibiotic course. Probiotic therapy as part of CDI treatment increased from 60% in 2009 to 91% in 2011. Among patients that contracted CDI in 2012–2013, only 2 patients received probiotics with their antibiotic courses.
Recurrences
With regard to the effect of probiotics within this population, those who received
One patient with significant initial antibiotic pressure was continued on her PPI during CDI treatment and continued to have recurrences, despite probiotic use. After her fourth recurrence, her PPI was changed to an H2RA, and she had no further recurrences. She continues off PPI therapy and is CDI-free 2 years later. Another patient who remained on his PPI had 3 recurrences, until finally a probiotic was added and the recurrences abated.
Discussion
CDI is common in hospitalized patients, and its incidence has increased due to multiple factors, which include the widespread use of broad-spectrum antimicrobials and increased use of PPIs. Our observational study showed a statistically significant reduction in the number of health care–associated CDI cases during our implementation period (mid–2008 through 2010). From 2011 on, all initiatives were maintained. As the lower rates of CDI continued, physician confidence in antimicrobial stewardship recommendations increased. During this latter portion of the study period, hospitalists uniformly switched patients to H2RA for GI prophylaxis, added prophylactic probiotics to antibiotic courses as well as CDI therapy, and were more receptive to streamlining and limiting durations of antibiotic therapy. Although the study was completed in 2013, follow-up data have shown that the low CDI incidence has continued through 2014.
The average age of the patients in our study was 69 years. In 2009, there were 41 C. difficile cases originating from our institution; however, by the end of 2011, only 9 cases had been reported, a 75% reduction. The majority of our cases of C. difficile in 2009–2010 originated from our facility’s LTC units (Figure 2). Risk factors in the LTC population included older age (72% are > 65 years) with multiple comorbidities, exposure to frequent multiple courses of broad-spectrum antibiotics, and use of PPIs as the standard for GI prophylaxis therapy. Multiple antibiotic courses had a strong association with PPI administration in the patients who contracted CDI, while recent antibiotics and antibiotics greater than 10 days did not. Implications may include an increased risk of CDI in patients requiring multiple antibiotic courses concurrent with PPI exposure.
Infection prevention strategies were promulgated among the health care team during the study period but were not specifically targeted for quality improvement efforts. Therefore, in contrast to other studies where infection prevention measures and environmental hygiene were prominent components of a CDI prevention “bundle,” our focus was on antimicrobial stewardship and PPI and probiotic use, not enhancement of standard infection prevention and environmental hygiene measures.
The antibiotics used prior to the development of CDI in our study were similar to findings from other studies that have associated broad-spectrum antibiotics with increased susceptibility to CDI [11]. Antimicrobials disrupt the normal GI flora, which is essential for eradicating many C. difficile spores [12]. The utilization of high-risk antibiotics and prolonged antimicrobial therapy were reduced with implementation of our antimicrobial stewardship program. In 2012, the antimicrobial stewardship program developed a LTC fever protocol, providing education to LTC nurses, physicians, and pharmacists using the modified McGeer criteria [13] for infection in LTC units and empiric antibiotic recommendations from our epidemiologist. A formal recommendation for a LTC 7-day stop date for urinary, respiratory, and skin and soft tissue infections was initiated, which included are-assessment at day 6–7 for resolution of symptoms.
With regard to PPI therapy, our study revealed that patients who had received a PPI at some point were 3.05 times more likely to have a recurrence of CDI than those who had not. These findings are consistent with the literature. Linsky et al [5] found a 42% increased risk of CDI recurrence in patients receiving PPIs concurrent with CDI treatment while considering covariates that may influence the risk of recurrent CDI or exposure to PPIs. A meta-analysis of 16 observational studies involving more than 1.2 million hospitalized patients by Janarthanan et al [14] explored the association between CDI and PPIs and showed a 65% increase in the incidence of CDI among PPI users. Those receiving PPI for GI prophylaxis in the earlier time period (before 2011) were 77% more likely to have a recurrence than those who received PPI in the later period. This finding might be associated with the more appropriate antimicrobial use and the more consistent use of consistent prophylactic probiotics in the later study period.
Our results showed that those who received probiotics with the initial CDI treatment were significantly less likely to have a recurrence than those who did not. Patients receiving probiotics in the later period (2011–2013) were 74% less likely to have a recurrence than patients in the earlier group (2009–2010). Despite the standard use of probiotics for primary CDI prevention at our institution, we could not show direct significance to the lack of probiotic use found in the identified CDI patients with this observational study design. The higher benefit in more recent years could possibly be attributed to the fact that these patients were much less likely to have received a PPI, that most had likely received probiotics concurrently plus 1 week after their antibiotic courses, and their antibiotic therapy was likely more focused and streamlined to prevent C. difficile infection. A meta-analysis of probiotic efficacy in primary CDI prevention suggested that probiotics can lead to a 64% reduction in the incidence of CDI, in addition to reducing GI-associated symptoms related to infection or antibiotic use [9]. A dose-response study of the efficacy of a probiotic formula showed a lower incidence of CDI, 1.2% for higher dose vs. 9.4% for lower dose vs. 23.8% for placebo [15]. Maziade et al [16] added prophylactic probiotics to a bundle of standard preventative measures for C. difficile infections, and were able to show an enhanced and sustained decrease in CDI rates (73%) and recurrences (39%). However, many of the probiotic studies which have studied the relationship to CDI have been criticized for reporting abnormally high rates of infection [9,16] missing data, a lack of controls or excessive patient exclusion criteria [17,18] The more recent PLACIDE study by Allen et al [19] was a large multicenter randomized controlled trial that did not show any benefit to CDI prevention with probiotics; however, with 83% of screened patients excluded, the patients were low risk, with the resulting CDI incidence (0.99%) too low to show a benefit. Acid suppression was also not revealed in the specific CDI cases, and others have found this to be a significant risk factor [5–7].
Limitations of this study include the study design (an observational, retrospective analysis), the small size of our facility, and the difficulty in obtaining probiotic history prior to admission in some cases. Due to a change in computer systems, hospital orders for GI prophylaxis agents could not be obtained for 2009–2010. Due to the fact that we instituted our interventions somewhat concurrently, it is difficult to analyze their individual impact. Randomized controlled trials evaluating the combined role of probiotics, GI prophylaxis, and antibiotic pressure in CDI are needed to further define the importance of this approach.
Corresponding author: Bridget Olson, RPh, Sharp Coronado Hospital & Villa Coronado Long-Term Care Facility, 250
Prospect Pl., Coronado CA 92118, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BO, TH, KW, RO; analysis and interpretation of data, RAF; drafting of article, BO, RAF; critical revision of the article, RAF, JH, TH; provision of study materials or patients, BO; statistical expertise, RAF; administrative or technical support, KW, RO; collection and assembly of data, BO.
From Sharp HealthCare, San Diego, CA.
Abstract
- Objective: To examine the relationship between the rate of Clostridium difficile infections (CDI) and implementation of 3 interventions aimed at preserving the fecal microbiome: (1) reduction of antimicrobial pressure; (2) reduction in intensity of gastrointestinal prophylaxis with proton-pump inhibitors (PPIs); and (3) expansion of probiotic therapy.
- Methods: We conducted a retrospective analysis of all inpatients with CDI between January 2009 and December 2013 receiving care at our community hospital and associated long-term care (LTC) facility. We used interrupted time series analysis to assess CDI rates during the implementation phase (2008–2010) and the postimplementation phase (2011–2013).
- Results: A reduction in the rate of health care facility–associated CDIs was seen. The mean number of cases per 10,000 patient days fell from 11.9 to 3.6 in acute care and 6.1 to 1.1 in LTC. Recurrence rates decreased from 64% in 2009 to 16% by 2014. The likelihood of CDI recurring was 3 times higher in those exposed to PPI and 0.35 times less likely in those who received probiotics with their initial CDI therapy.
- Conclusion: The risk of CDI incidence and recurrence was significantly reduced in our inpatients, with recurrent CDI associated with PPI use, multiple antibiotic courses, and lack of probiotics. We attribute our success to the combined effect of intensified antibiotic stewardship, reduced PPI use, and expanded probiotic use.
Clostridium difficile is classified as an urgent public health threat by the Centers for Disease Control and Prevention [1]. A recent study by the CDC found that it caused more than 400,000 infections in the United States in 2011, leading to over 29,000 deaths [2]. The costs of treating CDI are substantial and recurrences are common. While rates for many health care–associated infections are declining, C. difficile infection (CDI) rates remain at historically high levels [1] with the elderly at greatest risk for infection and mortality from the illness [3].
CDIs can be prevented. A principal recommendation for preventing CDIs is improving antibiotic use. Antibiotic use increases the risk for developing CDI by disrupting the colonic microbiome. Hospitalized and long-term care (LTC) patients are frequently prescribed antibiotics, but studies indicate that much of this use is inappropriate [4]. Antimicrobial stewardship has been shown to be effective in reducing CDI rates. Other infection prevention measures commonly employed to decrease the risk of hospital-onset CDI include monitoring of hand hygiene compliance using soap and water, terminal cleaning with bleach products of rooms occupied by patients with CDI, and daily cleaning of highly touched areas. At our institution, patients identified with CDI are placed on contact precautions until they have been adequately treated and have had resolution of diarrhea for 48 hours.
In addition to preventing CDI transmission through antimicrobial stewardship, attention is being paid to the possibility that restricting PPI use may help in preventing CDI. The increasing utilization of proton-pump inhibitors (PPIs) in recent years has coincided with the trend of increasing CDI rates. Although C. difficile spores are acid-resistant, vegetative forms are easily affected by acidity. Several studies have shown the association of acid suppression and greater susceptibility of acquiring CDI or recurrences [5–7]. Elevated gastric pH by PPIs facilitates the growth of potentially pathogenic upper and lower gastrointestinal (GI) tract flora, including the conversion of C. difficile from spore to vegetative form in the upper GI tract [5,8].
A growing body of evidence indicates that probiotics are both safe and effective for preventing CDIs [9]. Probiotics may counteract disturbances in intestinal flora, thereby reducing the risk for colonization by pathogenic bacteria. Probiotics can inhibit pathogen adhesion, colonization, and invasion of the gastrointestinal mucosa [10].
We hypothesized that preservation and/or restoration of the diversity of the fecal microbiome would prevent CDI and disease recurrence in our facility. Prior to 2009, we had strict infection prevention measures in place to prevent disease transmission, similar to many other institutions. In 2009, we implemented 3 additional interventions to reduce the rising incidence of CDI: (1) an antibiotic stewardship program, (2) lowering the intensity of acid suppression, and (3) expanding the use of probiotic therapy. The 3 interventions were initiated over the 19-month period January 2009 through July 2010. This study addresses the effects of these interventions.
Methods
Patients and Data Collection
The study was conducted at a community hospital (59 beds) that has an associated LTC facility (122 beds). We conducted a retrospective analysis of hospital and LTC data from all documented cases of CDI between January 2009 and December 2013. Study subjects included all patients with stools positive for C. difficile antigen and toxin with associated symptoms of infection (n = 123). Institutional review board approval was obtained prior to data collection.
The following information was collected: admission diagnosis, number of days from admission until confirmed CDI, residence prior to admission, duration and type of antibiotics received prior to or during symptoms of CDI, type of GI prophylaxis received within 14 days prior to and during CDI treatment, probiotic received and duration, and the type and duration of antibiotic treatment given for the CDI. The data collected was used to determine the likely origin of each C. difficile case, dates of recurrences, and the possible effects of the interventions. Antibiotic use was categorized as: (1) recent antibiotic course (antibiotics received within the preceding 4 weeks), (2) antibiotic courses greater than 10 days, and (3) multiple antibiotic courses (more than 1 antibiotic course received sequentially or concurrently).
Positive C. difficile infections were detected using a 2-step algorithm, starting in 2009. The samples were first screened with a rapid membrane enzyme immunoassay for glutamate dehydrogenase (GDH) antigen and toxin A and B in stool (C. Diff Quik Chek Complete, Techlab, Blacksburg, VA). Discrepant samples (GDH positive and toxin A and B negative) were reflexed to DNA-based PCR testing. The PCR assay was changed to the Verigene C. difficile test (Nanosphere, Northbrook, IL) in 2012. Up to 30 days after discharge from our facility, positive results were considered as acquired from our facility and positive results within 2 days of admission with symptoms of CDI were considered positive on admission and were not attributed to our facility. A primary episode of CDI was defined to be the first identified episode or event in each patient. Recurrent CDI was defined as a repeated case of CDI within 180 days of the original CDI event.
Interventions to Reduce CDI
Reduction of Antibiotic Pressure
Other actions taken to improve antimicrobial prescribing as part of the stewardship program included medication usage evaluations (MUEs) for levofloxacin and carbapenems, implementing an automatic dosing/duration protocol for levofloxacin, and carbapenem restriction to prevent inappropriate use. Nursing and pharmacy staffs were educated on vancomycin appropriateness, benefits of MRSA screening for de-escalation, procalcitonin, and treatment of sepsis. Emergency department staff was educated on (1) empiric antimicrobial treatment recommendations for urinary and skin and soft tissue infections based on outpatient antibiogram data, (2) renal adjustment of antimicrobials, (3) fluoroquinolones: resistance formation, higher CDI risk and higher dosing recommendations, (4) GI prophylaxis recommendations, and (5) probiotics.
Reduction in the Intensity of Acid Suppression for GI Prophylaxis
PPIs were substituted with histamine-2 receptor antagonists (H2RA) whenever acid suppression for GI prophylaxis was warranted. If GI symptoms persisted, sucralfate was added. In May 2010, all eligible LTC patients were converted from PPIs to H2RA.
Expanding the Use of Probiotics
We expanded the use of probiotics as an adjunctive treatment for CDI with metronidazole ± vancomycin oral therapies. Probiotics were included concurrently with any broad-spectrum antibiotic administration, longer antibiotic courses (≥ 7 days), and/or multiple courses of antibiotics. The combination of Saccromyces boulardii plus Lactobacillus acidophilus and L. bulgaricus was given with twice daily dosing until the end of 2011. In January 2012, our facility switched over to daily administration of a probiotic with the active ingredients of Lactobacillus acidophilus and Lactobacillus casei, 50 billion colony-forming units. Probiotics were given during the antibiotic course plus for 1 additional week after course completion. Probiotics were not administered to selected groups of patients: (1) immunocompromised patients, (2) patients who were NPO, or (3) patients excluded by their physicians.
There was no change or enhanced targeting of infection prevention or environmental hygiene strategies during the study period.
Data Analysis and Statistical Methods
All data were collected on data collection sheets and transcribed into Microsoft Office Excel 2007 Service Pack 3. No data were excluded from analysis. Continuous variables, eg, number of cases of CDI, are reported as mean ± standard deviation. Categorical variables, eg, number of recurrent CDI cases, are reported as the count and percentage. Comparison of populations was done with the Wilcoxon rank sum test. Segments of the interrupted time series were assessed using linear regression. Associations were tested using χ2. Statistical tests were deemed significant when the α probability was < 0.05. No adjustments were made for multiplicity. Data descriptive statistics (including frequency histograms for visual examination of distributions) and statistical analyses were performed using Stata 11.1 (StataCorp, College Station, TX).
Results
CDIs
Within the population of patients having a CDI or recurrence, we found that those patients in the later time period (2011–2013) were significantly less likely to have a recurrence than those in the earlier time period (pre- Jan 2011) (chi square = 5.975, df = 1, P = 0.015). The odds ratio (OR) was 0.35 (95% CI 0.15 to 0.83).
Patients in the earlier (2009–2010) vs. the later post-intervention group (2011–2013) had more likely received multiple antibiotic courses (chi square = 5.32, df = 1, P = 0.021, OR 2.56), a PPI (chi square = 8.86, df = 1, P = 0.003, OR 3.38), and had a health care facility–associated infection originating from our institution as opposed to outside facility transfers or community-acquired cases (chi square = 7.09, df = 1, P = 0.008, OR 2.94).
Antibiotic Pressure
Acid Suppression
In evaluating the effects of limiting the use of PPIs, patients who received an H2RA or no antacid prophylaxis were significantly less likely to have a recurrence of CDI than those who received a PPI (chi square = 6.35, df = 1, P = 0.012). The OR for recurrence with PPIs was 3.05 (95% CI 1.25 to 7.44). Of patients exposed to PPIs, those exposed in the later time period (2011 through 2013) were significantly less likely to have a recurrence than those exposed in the early time period (third quarter 2008 through 2010; chi square = 15.14, df = 1, P < 0.001). The OR was 0.23 (95% CI, 0.11 to 0.49).
Probiotics
During 2009–2011, only 15% of the CDI patients had received probiotics with an antibiotic course. Probiotic therapy as part of CDI treatment increased from 60% in 2009 to 91% in 2011. Among patients that contracted CDI in 2012–2013, only 2 patients received probiotics with their antibiotic courses.
Recurrences
With regard to the effect of probiotics within this population, those who received
One patient with significant initial antibiotic pressure was continued on her PPI during CDI treatment and continued to have recurrences, despite probiotic use. After her fourth recurrence, her PPI was changed to an H2RA, and she had no further recurrences. She continues off PPI therapy and is CDI-free 2 years later. Another patient who remained on his PPI had 3 recurrences, until finally a probiotic was added and the recurrences abated.
Discussion
CDI is common in hospitalized patients, and its incidence has increased due to multiple factors, which include the widespread use of broad-spectrum antimicrobials and increased use of PPIs. Our observational study showed a statistically significant reduction in the number of health care–associated CDI cases during our implementation period (mid–2008 through 2010). From 2011 on, all initiatives were maintained. As the lower rates of CDI continued, physician confidence in antimicrobial stewardship recommendations increased. During this latter portion of the study period, hospitalists uniformly switched patients to H2RA for GI prophylaxis, added prophylactic probiotics to antibiotic courses as well as CDI therapy, and were more receptive to streamlining and limiting durations of antibiotic therapy. Although the study was completed in 2013, follow-up data have shown that the low CDI incidence has continued through 2014.
The average age of the patients in our study was 69 years. In 2009, there were 41 C. difficile cases originating from our institution; however, by the end of 2011, only 9 cases had been reported, a 75% reduction. The majority of our cases of C. difficile in 2009–2010 originated from our facility’s LTC units (Figure 2). Risk factors in the LTC population included older age (72% are > 65 years) with multiple comorbidities, exposure to frequent multiple courses of broad-spectrum antibiotics, and use of PPIs as the standard for GI prophylaxis therapy. Multiple antibiotic courses had a strong association with PPI administration in the patients who contracted CDI, while recent antibiotics and antibiotics greater than 10 days did not. Implications may include an increased risk of CDI in patients requiring multiple antibiotic courses concurrent with PPI exposure.
Infection prevention strategies were promulgated among the health care team during the study period but were not specifically targeted for quality improvement efforts. Therefore, in contrast to other studies where infection prevention measures and environmental hygiene were prominent components of a CDI prevention “bundle,” our focus was on antimicrobial stewardship and PPI and probiotic use, not enhancement of standard infection prevention and environmental hygiene measures.
The antibiotics used prior to the development of CDI in our study were similar to findings from other studies that have associated broad-spectrum antibiotics with increased susceptibility to CDI [11]. Antimicrobials disrupt the normal GI flora, which is essential for eradicating many C. difficile spores [12]. The utilization of high-risk antibiotics and prolonged antimicrobial therapy were reduced with implementation of our antimicrobial stewardship program. In 2012, the antimicrobial stewardship program developed a LTC fever protocol, providing education to LTC nurses, physicians, and pharmacists using the modified McGeer criteria [13] for infection in LTC units and empiric antibiotic recommendations from our epidemiologist. A formal recommendation for a LTC 7-day stop date for urinary, respiratory, and skin and soft tissue infections was initiated, which included are-assessment at day 6–7 for resolution of symptoms.
With regard to PPI therapy, our study revealed that patients who had received a PPI at some point were 3.05 times more likely to have a recurrence of CDI than those who had not. These findings are consistent with the literature. Linsky et al [5] found a 42% increased risk of CDI recurrence in patients receiving PPIs concurrent with CDI treatment while considering covariates that may influence the risk of recurrent CDI or exposure to PPIs. A meta-analysis of 16 observational studies involving more than 1.2 million hospitalized patients by Janarthanan et al [14] explored the association between CDI and PPIs and showed a 65% increase in the incidence of CDI among PPI users. Those receiving PPI for GI prophylaxis in the earlier time period (before 2011) were 77% more likely to have a recurrence than those who received PPI in the later period. This finding might be associated with the more appropriate antimicrobial use and the more consistent use of consistent prophylactic probiotics in the later study period.
Our results showed that those who received probiotics with the initial CDI treatment were significantly less likely to have a recurrence than those who did not. Patients receiving probiotics in the later period (2011–2013) were 74% less likely to have a recurrence than patients in the earlier group (2009–2010). Despite the standard use of probiotics for primary CDI prevention at our institution, we could not show direct significance to the lack of probiotic use found in the identified CDI patients with this observational study design. The higher benefit in more recent years could possibly be attributed to the fact that these patients were much less likely to have received a PPI, that most had likely received probiotics concurrently plus 1 week after their antibiotic courses, and their antibiotic therapy was likely more focused and streamlined to prevent C. difficile infection. A meta-analysis of probiotic efficacy in primary CDI prevention suggested that probiotics can lead to a 64% reduction in the incidence of CDI, in addition to reducing GI-associated symptoms related to infection or antibiotic use [9]. A dose-response study of the efficacy of a probiotic formula showed a lower incidence of CDI, 1.2% for higher dose vs. 9.4% for lower dose vs. 23.8% for placebo [15]. Maziade et al [16] added prophylactic probiotics to a bundle of standard preventative measures for C. difficile infections, and were able to show an enhanced and sustained decrease in CDI rates (73%) and recurrences (39%). However, many of the probiotic studies which have studied the relationship to CDI have been criticized for reporting abnormally high rates of infection [9,16] missing data, a lack of controls or excessive patient exclusion criteria [17,18] The more recent PLACIDE study by Allen et al [19] was a large multicenter randomized controlled trial that did not show any benefit to CDI prevention with probiotics; however, with 83% of screened patients excluded, the patients were low risk, with the resulting CDI incidence (0.99%) too low to show a benefit. Acid suppression was also not revealed in the specific CDI cases, and others have found this to be a significant risk factor [5–7].
Limitations of this study include the study design (an observational, retrospective analysis), the small size of our facility, and the difficulty in obtaining probiotic history prior to admission in some cases. Due to a change in computer systems, hospital orders for GI prophylaxis agents could not be obtained for 2009–2010. Due to the fact that we instituted our interventions somewhat concurrently, it is difficult to analyze their individual impact. Randomized controlled trials evaluating the combined role of probiotics, GI prophylaxis, and antibiotic pressure in CDI are needed to further define the importance of this approach.
Corresponding author: Bridget Olson, RPh, Sharp Coronado Hospital & Villa Coronado Long-Term Care Facility, 250
Prospect Pl., Coronado CA 92118, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BO, TH, KW, RO; analysis and interpretation of data, RAF; drafting of article, BO, RAF; critical revision of the article, RAF, JH, TH; provision of study materials or patients, BO; statistical expertise, RAF; administrative or technical support, KW, RO; collection and assembly of data, BO.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html.
2. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–34.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005;173:1037–42.
4. Warren JW, Palumbo FB, Fitterman L, Speedie SM. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc 1991;39:963–72.
5. Linsky A, Gupta K, Lawler E, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010;170:772–8.
6. Dial S, Delaney JA, Barkun AN, Sulssa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989–95.
7. Howell M, Novack V, Grgurich P, et.al. Iatrogenic gastric acid suppression and the risk if nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784–90.
8. Radulovic Z, Petrovic T, Bulajic S. Antibiotic susceptibility of probiotic bacteria. In Pana M, editor. Antibiotic resistant bacteria: a continuous challenge in the new millennium. Rijeka, Croatia: InTech; 2012.
9. Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD006095.
10. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea. Ann Intern Med 2012;157:878–88.
11. Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009;63:203–37.
12. Elliott B, Chang BJ, Golledge CL et al. Clostridium difficile-associated diarrhoea. Intern Med J 2007;37:561–8.
13. Stone, ND, Ashraf, MS et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol 2012;33:965–77.
14. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–10.
15. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010;105:1636-41.
16. Maziade PJ, Andriessen JA, Pereira P, et.al. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr Med Res Opin 2013;29:1341–7.
17. Islam, J, Cohen J, Rajkumar C, Llewelyn M. Probiotics for the prevention and treatment of Clostridium difficile in older patients. Age Ageing 2012;41:706–11.
18. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007;335:80.
19. Allen S J, Wareham K, Wang, D, et.al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomized, double-blind, placebo-controlled, multi-centre trial. Lancet 2013;382:1249–57.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html.
2. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–34.
3. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ 2005;173:1037–42.
4. Warren JW, Palumbo FB, Fitterman L, Speedie SM. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc 1991;39:963–72.
5. Linsky A, Gupta K, Lawler E, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med 2010;170:772–8.
6. Dial S, Delaney JA, Barkun AN, Sulssa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989–95.
7. Howell M, Novack V, Grgurich P, et.al. Iatrogenic gastric acid suppression and the risk if nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784–90.
8. Radulovic Z, Petrovic T, Bulajic S. Antibiotic susceptibility of probiotic bacteria. In Pana M, editor. Antibiotic resistant bacteria: a continuous challenge in the new millennium. Rijeka, Croatia: InTech; 2012.
9. Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2013;5:CD006095.
10. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea. Ann Intern Med 2012;157:878–88.
11. Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009;63:203–37.
12. Elliott B, Chang BJ, Golledge CL et al. Clostridium difficile-associated diarrhoea. Intern Med J 2007;37:561–8.
13. Stone, ND, Ashraf, MS et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol 2012;33:965–77.
14. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001–10.
15. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010;105:1636-41.
16. Maziade PJ, Andriessen JA, Pereira P, et.al. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr Med Res Opin 2013;29:1341–7.
17. Islam, J, Cohen J, Rajkumar C, Llewelyn M. Probiotics for the prevention and treatment of Clostridium difficile in older patients. Age Ageing 2012;41:706–11.
18. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007;335:80.
19. Allen S J, Wareham K, Wang, D, et.al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomized, double-blind, placebo-controlled, multi-centre trial. Lancet 2013;382:1249–57.
The Value of Routine Transthoracic Echocardiography in Defining the Source of Stroke in a Community Hospital
From Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Background: Acute stroke or cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating consequences, particularly in the setting of recurrence. Cardiac sources are potentially remediable; thus, a transthoracic echocardiogram (TTE) is frequently ordered to evaluate for a cardiac source of embolism.
- Objective: To evaluate the utility of performing TTE on patients experiencing a CVA or transient ischemic attack (TIA) to evaluate for a cardiac source of embolism.
- Methods: Retrospective review of TTE reports and patient electronic medical records at Anne Arundel Medical Center, a 385-bed community hospital. Medical charts for all CVA patients receiving a TTE between February 2012 to April 2013 were reviewed for TTEs showing unequivocal cardiac sources of embolism as evaluated by the reviewing cardiologist. Patient information and clinical morbidities were also noted to construct a composite demographic of CVA patients.
- Results: One TTE of 371 (0.270%) identified a clear cardiac embolus. Risk factors for stroke included hyper-tension (n = 302), cardiovascular disease (n = 204), cardiomyopathy (n = 131), and diabetes (n = 146).
- Conclusion: In the setting of stroke, TTE is of limited value when determining the etiology of stroke and should be used provisionally rather than routinely in evaluating patients experiencing CVA or TIA.
Acute cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating clinical consequences, particularly in the setting of recurrence. Defining the etiology of CVA and transient ischemic attacks (TIA) when they occur is important so that appropriate therapy can be initiated. Transthoracic echocardiograms (TTEs) are frequently ordered to evaluate for a cardiac source of embolism. No consensus exists about the use of imaging strategies to identify potential cardiovascular sources of emboli in patients who have had strokes.
A few published studies have investigated the yield of TTE in identifying cardiac sources of CVA. The yield has been reported to be between < 1% and as high as 37% [1–3]. However, some of the reported sources of CVA included mitral valve prolapse and patent foramen ovale [4,5], conditions for which the association with stroke has been questioned [6–8]. In addition, many of these studies were performed using clinical data from tertiary referral centers, which may have increased the yield of cardiac sources [9].
The purpose of this study was to evaluate the yield of TTE in evaluating for a clear cardiac source of embolism in a consecutive series of patients diagnosed with a CVA or TIA in a community hospital.
Methods
Setting
All data was collected from the echocardiography lab at Anne Arundel Medical Center, a 384-bed community hospital in Annapolis, MD. The medical center sees about 250 patients a day in its emergency department and admits about 30,000 patients annually. All echocardiograms are performed by a centralized laboratory accredited by the Intersocietal Commission for the Accreditation of Echocardiography Laboratories, which performs approximately 6000 echocardiograms annually.
All TTEs done for the diagnosis of CVA or TIA between 1 February 2013 and 1 May 2013 were evaluated in consecutive fashion by report review. Reports were searched for any cardiac source of embolism to include thrombus, tumor, vegetation, shunt, aortic atheroma, or any other finding that was felt to be a clear source of embolism by the interpreting cardiologist. We did not include entities such as mitral valve prolapse, patent foramen ovale, and isolated atrial septal aneurysms since their association with CVA/TIA has been questioned. Also not included was cardiomyopathy without aneurysm, apical wall motion abnormality, or intra-cavitary thrombus solely because the ejection fraction was less than 35%, as the literature does not support these conditions as clear causes of TIA or CVA.
In addition to reviewing echocardiogram reports, all patient records were evaluated for clinical variables including age, gender, presence of atrial fibrillation, hypertension, diabetes, past CVA, left atrial dilation by calculation of indexed direct left atrial volume, recent myocardial infarction, and known cardiovascular disease.
All echocardiograms were performed on Hewlet-Packard Vivid 7 or Vivid 9 (GE Healthcare, Wauwatosa, WI) by technicians who held registered diagnostic cardiac sonographer status. All TTEs contained the “standard” views in accordance with published guidelines [10].Saline contrast to look for shunts was not standard on these studies. Echocardiogram images were stored digitally and read from an EchoPac (GE Healthcare, Wauwatosa, WI) reading station. All echocardiograms were interpreted by one of 16 American Board of Internal Medicine–certified cardiologists, 5 of whom were testamurs (physicians who have passed one of the examinations of special competence in echocardiography). These 5 interpreted 20% of the studies.
Results
Discussion
Our data are in keeping with those of others, though our yield was even lower than that reported in previous studies [1–3]. The low yield may be explained by a number of factors. First, we did not include patent foramen ovale or atrial septal aneurysms (which account for a high percentage of embolic sources in other publications) since there is not a clear consensus that any of those entities are associated with an increased risk of embolic events. The exclusion of cardiomyopathy as a cause of CVA or TIA is arguable, but its link to CVA or TIA is also unproven. One study did associate cardiomyopathy with CVA [12]; however, the mechanism is not clear, as the incidence of CVA in cardiomyopathy has been described as similar regardless of the severity of left ventricular dysfunction [13].Many past reports have come from tertiary care centers, where there may be referral bias whereas our data come from consecutive patients at a single community hospital.
TTE is relatively quick to perform and interpret and carries no physical risk to a patient. However, our data suggest that ordering TTE routinely in the setting of CVA offers little value. With health care organizations turning their attention to reducing low-value care, which potentially wastes limited resources, considerations of value and effectiveness continue to be a priority. Our findings suggest TTE use in this setting conflicts with the current trajectory of value-based medical practice. As well, a prior Markov model decision analysis found that TTE is not cost-effective when used routinely to identify source of emboli in stroke [14].
Despite the low yield of TTE in evaluating for a cardiac source of CVA, TTEs continue to be frequently ordered. In our own institution, 48% of patients with a CVA or TIA underwent a TTE based on preferences and habits of individual admitting physicians and without any structured criteria. Order sets for CVA admissions do not include this test; physicians are adding it but not for any particular patient characteristic or exam finding.
There are a number of reasons that echocardiograms may be ordered more frequently by some. A documented decline in ordering echocardiograms was seen following education at one center [15], suggesting that lack of knowledge about the limitations of TTE may be a factor. A second potential factor is fear of medicolegal consequences. Indeed, the current American Heart Association/American Stroke Association guidelines for the early management of adults with ischemic stroke [16] offers no formal recommendations or clear indications.
Computerized decision support (CDS) that links the medical record to appropriateness criteria could potentially reduce the inappropriate use of TTE. CDS has been shown to be effective in reducing unnecessary ordering of tests in other settings [17–19].
Among the limitations in our analysis is the heterogeneity in echocardiogram readers. However, this heterogeneity may makes the study more relevant as it reflects the reality in most community hospitals. Another potential limitation is that saline contrast studies were not used routinely; however, this too is typical at community hospitals. Also, while all echocardiograms were interpreted by “board-certified” cardiologists, only 5 had passed the “examination of special competence” to be certified as a testamur of the National Board of Echocardiography, raising the question as to whether subtle findings could have been missed. However, there were no relevant findings in the 20% of studies interpreted by the testamurs, suggesting that the other echocardiographers were not missing diagnoses. Finally, we had only 10 patients younger than age 45 and so the study conclusions are less definitive for that age-group.
Conclusion
TTE was of limited utility in uncovering a cardiac source of embolism in a typical population with CVA or TIA.Based upon the data, we believe that TTE should not be used routinely in the setting of CVA; however, we do recognize that TTE may be of value in patients who have other comorbidities that would place them at increased risk of embolic CVA such as a recent anterior MI, those at risk for endocarditis, or those with brain imaging findings suggestive of embolic CVA [20]. Ordering a low-value test such as a TTE in the setting of TIA or CVA adds cost and does not often yield a clinically meaningful results. In addition, a “negative” TTE can be misinterpreted as a normal heart and forestall additional workup such as transesophageal echocardiography and long-term rhythm analysis, which may be of higher value. We suggest that in a community hospital setting the determination of need for TTE be made based on the clinical nuances of the case rather than by habit or as part of standardized order sets.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BM, WCM; analysis and interpretation of data, BM, WCM; drafting of article, RHB, BM, WCM; critical revision of the article, BM, WCM; administrative or technical support, JC; collection and assembly of data, RHB, JC.
1. Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996;27:691.
2. Khan MA, Khealanj B, Kamal, A. Diagnostic yield of transthoracic echocardiography for stroke patients in a developing country. J Pak Med Assoc 2008;58:375–7.
3. de Abreu T, Mateus S, José Correia J. Therapy implications of transthoracic echocardiography in acute ischemic stroke patients. Stroke 2005;36:1565–6.
4. de Bruijn SFTM, Agema WRP, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531–4.
5. Putaala J, Metso AJ, MD, Metso T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke. Stroke 2009;40:1195–203.
6. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent fora-men ovale in patients with stroke. N Engl J Med 1988;318:1148–52.
7. Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
8. Orencia AJ, Petty GW, Khandheria BK, et al. Risk of stroke with mitral valve prolapse in population-based cohort study; Stroke 1995;26:7–13.
9. Holmes M, Rathbone J, Littlewood C. Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess 2014;18:1–176.
10. Ryan T, Armstrong W. Feigenbaum’s echocardiography. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
11. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263–72.
12. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–76.
13. Hays AG, Sacco RL, Rundek T. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke 2006;37:1715–9.
14. McNamara RL, Lima JA, Whelton PK, Powe NR. Echocardiographic identification of cardiovascular sources of emboli to guide clinical management of stroke: a cost-effectiveness analysis. Ann Intern Med 1997;127:775–87.
15. Alberts MJ, Bennett CA, Rutledge VR. Hospital charges for stroke patients. Stroke 1996;27:1825–8.
16. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947.
17. Levick DL, Stern G, Meyerhoefer CD, et al. Reducing unnecessary testing in a CPOE system through implementation of a targeted CDS intervention. BMC Med Inform Decis 2013;13:43.
18. Chen P, Tanasijevic MJ, Schoenenberger RA, et al. A computer-based intervention for improving the appropriateness of antiepileptic drug level monitoring. Am J Clin Pathol 2003;119:432–8.
19. Solberg LI, Wei F, Butler JC, et al. Effects of electronic decision support on high-tech diagnostic imaging orders and patients. Am J Manag Care 2010;16:102–6.
20. Menon BK, Coulter JI, Simerpret B, et al. Acute ischaemic stroke or transient ischaemic attack and the need for inpatient echocardiography. Postgrad Med J 2014;90:434–8.
From Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Background: Acute stroke or cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating consequences, particularly in the setting of recurrence. Cardiac sources are potentially remediable; thus, a transthoracic echocardiogram (TTE) is frequently ordered to evaluate for a cardiac source of embolism.
- Objective: To evaluate the utility of performing TTE on patients experiencing a CVA or transient ischemic attack (TIA) to evaluate for a cardiac source of embolism.
- Methods: Retrospective review of TTE reports and patient electronic medical records at Anne Arundel Medical Center, a 385-bed community hospital. Medical charts for all CVA patients receiving a TTE between February 2012 to April 2013 were reviewed for TTEs showing unequivocal cardiac sources of embolism as evaluated by the reviewing cardiologist. Patient information and clinical morbidities were also noted to construct a composite demographic of CVA patients.
- Results: One TTE of 371 (0.270%) identified a clear cardiac embolus. Risk factors for stroke included hyper-tension (n = 302), cardiovascular disease (n = 204), cardiomyopathy (n = 131), and diabetes (n = 146).
- Conclusion: In the setting of stroke, TTE is of limited value when determining the etiology of stroke and should be used provisionally rather than routinely in evaluating patients experiencing CVA or TIA.
Acute cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating clinical consequences, particularly in the setting of recurrence. Defining the etiology of CVA and transient ischemic attacks (TIA) when they occur is important so that appropriate therapy can be initiated. Transthoracic echocardiograms (TTEs) are frequently ordered to evaluate for a cardiac source of embolism. No consensus exists about the use of imaging strategies to identify potential cardiovascular sources of emboli in patients who have had strokes.
A few published studies have investigated the yield of TTE in identifying cardiac sources of CVA. The yield has been reported to be between < 1% and as high as 37% [1–3]. However, some of the reported sources of CVA included mitral valve prolapse and patent foramen ovale [4,5], conditions for which the association with stroke has been questioned [6–8]. In addition, many of these studies were performed using clinical data from tertiary referral centers, which may have increased the yield of cardiac sources [9].
The purpose of this study was to evaluate the yield of TTE in evaluating for a clear cardiac source of embolism in a consecutive series of patients diagnosed with a CVA or TIA in a community hospital.
Methods
Setting
All data was collected from the echocardiography lab at Anne Arundel Medical Center, a 384-bed community hospital in Annapolis, MD. The medical center sees about 250 patients a day in its emergency department and admits about 30,000 patients annually. All echocardiograms are performed by a centralized laboratory accredited by the Intersocietal Commission for the Accreditation of Echocardiography Laboratories, which performs approximately 6000 echocardiograms annually.
All TTEs done for the diagnosis of CVA or TIA between 1 February 2013 and 1 May 2013 were evaluated in consecutive fashion by report review. Reports were searched for any cardiac source of embolism to include thrombus, tumor, vegetation, shunt, aortic atheroma, or any other finding that was felt to be a clear source of embolism by the interpreting cardiologist. We did not include entities such as mitral valve prolapse, patent foramen ovale, and isolated atrial septal aneurysms since their association with CVA/TIA has been questioned. Also not included was cardiomyopathy without aneurysm, apical wall motion abnormality, or intra-cavitary thrombus solely because the ejection fraction was less than 35%, as the literature does not support these conditions as clear causes of TIA or CVA.
In addition to reviewing echocardiogram reports, all patient records were evaluated for clinical variables including age, gender, presence of atrial fibrillation, hypertension, diabetes, past CVA, left atrial dilation by calculation of indexed direct left atrial volume, recent myocardial infarction, and known cardiovascular disease.
All echocardiograms were performed on Hewlet-Packard Vivid 7 or Vivid 9 (GE Healthcare, Wauwatosa, WI) by technicians who held registered diagnostic cardiac sonographer status. All TTEs contained the “standard” views in accordance with published guidelines [10].Saline contrast to look for shunts was not standard on these studies. Echocardiogram images were stored digitally and read from an EchoPac (GE Healthcare, Wauwatosa, WI) reading station. All echocardiograms were interpreted by one of 16 American Board of Internal Medicine–certified cardiologists, 5 of whom were testamurs (physicians who have passed one of the examinations of special competence in echocardiography). These 5 interpreted 20% of the studies.
Results
Discussion
Our data are in keeping with those of others, though our yield was even lower than that reported in previous studies [1–3]. The low yield may be explained by a number of factors. First, we did not include patent foramen ovale or atrial septal aneurysms (which account for a high percentage of embolic sources in other publications) since there is not a clear consensus that any of those entities are associated with an increased risk of embolic events. The exclusion of cardiomyopathy as a cause of CVA or TIA is arguable, but its link to CVA or TIA is also unproven. One study did associate cardiomyopathy with CVA [12]; however, the mechanism is not clear, as the incidence of CVA in cardiomyopathy has been described as similar regardless of the severity of left ventricular dysfunction [13].Many past reports have come from tertiary care centers, where there may be referral bias whereas our data come from consecutive patients at a single community hospital.
TTE is relatively quick to perform and interpret and carries no physical risk to a patient. However, our data suggest that ordering TTE routinely in the setting of CVA offers little value. With health care organizations turning their attention to reducing low-value care, which potentially wastes limited resources, considerations of value and effectiveness continue to be a priority. Our findings suggest TTE use in this setting conflicts with the current trajectory of value-based medical practice. As well, a prior Markov model decision analysis found that TTE is not cost-effective when used routinely to identify source of emboli in stroke [14].
Despite the low yield of TTE in evaluating for a cardiac source of CVA, TTEs continue to be frequently ordered. In our own institution, 48% of patients with a CVA or TIA underwent a TTE based on preferences and habits of individual admitting physicians and without any structured criteria. Order sets for CVA admissions do not include this test; physicians are adding it but not for any particular patient characteristic or exam finding.
There are a number of reasons that echocardiograms may be ordered more frequently by some. A documented decline in ordering echocardiograms was seen following education at one center [15], suggesting that lack of knowledge about the limitations of TTE may be a factor. A second potential factor is fear of medicolegal consequences. Indeed, the current American Heart Association/American Stroke Association guidelines for the early management of adults with ischemic stroke [16] offers no formal recommendations or clear indications.
Computerized decision support (CDS) that links the medical record to appropriateness criteria could potentially reduce the inappropriate use of TTE. CDS has been shown to be effective in reducing unnecessary ordering of tests in other settings [17–19].
Among the limitations in our analysis is the heterogeneity in echocardiogram readers. However, this heterogeneity may makes the study more relevant as it reflects the reality in most community hospitals. Another potential limitation is that saline contrast studies were not used routinely; however, this too is typical at community hospitals. Also, while all echocardiograms were interpreted by “board-certified” cardiologists, only 5 had passed the “examination of special competence” to be certified as a testamur of the National Board of Echocardiography, raising the question as to whether subtle findings could have been missed. However, there were no relevant findings in the 20% of studies interpreted by the testamurs, suggesting that the other echocardiographers were not missing diagnoses. Finally, we had only 10 patients younger than age 45 and so the study conclusions are less definitive for that age-group.
Conclusion
TTE was of limited utility in uncovering a cardiac source of embolism in a typical population with CVA or TIA.Based upon the data, we believe that TTE should not be used routinely in the setting of CVA; however, we do recognize that TTE may be of value in patients who have other comorbidities that would place them at increased risk of embolic CVA such as a recent anterior MI, those at risk for endocarditis, or those with brain imaging findings suggestive of embolic CVA [20]. Ordering a low-value test such as a TTE in the setting of TIA or CVA adds cost and does not often yield a clinically meaningful results. In addition, a “negative” TTE can be misinterpreted as a normal heart and forestall additional workup such as transesophageal echocardiography and long-term rhythm analysis, which may be of higher value. We suggest that in a community hospital setting the determination of need for TTE be made based on the clinical nuances of the case rather than by habit or as part of standardized order sets.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BM, WCM; analysis and interpretation of data, BM, WCM; drafting of article, RHB, BM, WCM; critical revision of the article, BM, WCM; administrative or technical support, JC; collection and assembly of data, RHB, JC.
From Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Background: Acute stroke or cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating consequences, particularly in the setting of recurrence. Cardiac sources are potentially remediable; thus, a transthoracic echocardiogram (TTE) is frequently ordered to evaluate for a cardiac source of embolism.
- Objective: To evaluate the utility of performing TTE on patients experiencing a CVA or transient ischemic attack (TIA) to evaluate for a cardiac source of embolism.
- Methods: Retrospective review of TTE reports and patient electronic medical records at Anne Arundel Medical Center, a 385-bed community hospital. Medical charts for all CVA patients receiving a TTE between February 2012 to April 2013 were reviewed for TTEs showing unequivocal cardiac sources of embolism as evaluated by the reviewing cardiologist. Patient information and clinical morbidities were also noted to construct a composite demographic of CVA patients.
- Results: One TTE of 371 (0.270%) identified a clear cardiac embolus. Risk factors for stroke included hyper-tension (n = 302), cardiovascular disease (n = 204), cardiomyopathy (n = 131), and diabetes (n = 146).
- Conclusion: In the setting of stroke, TTE is of limited value when determining the etiology of stroke and should be used provisionally rather than routinely in evaluating patients experiencing CVA or TIA.
Acute cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating clinical consequences, particularly in the setting of recurrence. Defining the etiology of CVA and transient ischemic attacks (TIA) when they occur is important so that appropriate therapy can be initiated. Transthoracic echocardiograms (TTEs) are frequently ordered to evaluate for a cardiac source of embolism. No consensus exists about the use of imaging strategies to identify potential cardiovascular sources of emboli in patients who have had strokes.
A few published studies have investigated the yield of TTE in identifying cardiac sources of CVA. The yield has been reported to be between < 1% and as high as 37% [1–3]. However, some of the reported sources of CVA included mitral valve prolapse and patent foramen ovale [4,5], conditions for which the association with stroke has been questioned [6–8]. In addition, many of these studies were performed using clinical data from tertiary referral centers, which may have increased the yield of cardiac sources [9].
The purpose of this study was to evaluate the yield of TTE in evaluating for a clear cardiac source of embolism in a consecutive series of patients diagnosed with a CVA or TIA in a community hospital.
Methods
Setting
All data was collected from the echocardiography lab at Anne Arundel Medical Center, a 384-bed community hospital in Annapolis, MD. The medical center sees about 250 patients a day in its emergency department and admits about 30,000 patients annually. All echocardiograms are performed by a centralized laboratory accredited by the Intersocietal Commission for the Accreditation of Echocardiography Laboratories, which performs approximately 6000 echocardiograms annually.
All TTEs done for the diagnosis of CVA or TIA between 1 February 2013 and 1 May 2013 were evaluated in consecutive fashion by report review. Reports were searched for any cardiac source of embolism to include thrombus, tumor, vegetation, shunt, aortic atheroma, or any other finding that was felt to be a clear source of embolism by the interpreting cardiologist. We did not include entities such as mitral valve prolapse, patent foramen ovale, and isolated atrial septal aneurysms since their association with CVA/TIA has been questioned. Also not included was cardiomyopathy without aneurysm, apical wall motion abnormality, or intra-cavitary thrombus solely because the ejection fraction was less than 35%, as the literature does not support these conditions as clear causes of TIA or CVA.
In addition to reviewing echocardiogram reports, all patient records were evaluated for clinical variables including age, gender, presence of atrial fibrillation, hypertension, diabetes, past CVA, left atrial dilation by calculation of indexed direct left atrial volume, recent myocardial infarction, and known cardiovascular disease.
All echocardiograms were performed on Hewlet-Packard Vivid 7 or Vivid 9 (GE Healthcare, Wauwatosa, WI) by technicians who held registered diagnostic cardiac sonographer status. All TTEs contained the “standard” views in accordance with published guidelines [10].Saline contrast to look for shunts was not standard on these studies. Echocardiogram images were stored digitally and read from an EchoPac (GE Healthcare, Wauwatosa, WI) reading station. All echocardiograms were interpreted by one of 16 American Board of Internal Medicine–certified cardiologists, 5 of whom were testamurs (physicians who have passed one of the examinations of special competence in echocardiography). These 5 interpreted 20% of the studies.
Results
Discussion
Our data are in keeping with those of others, though our yield was even lower than that reported in previous studies [1–3]. The low yield may be explained by a number of factors. First, we did not include patent foramen ovale or atrial septal aneurysms (which account for a high percentage of embolic sources in other publications) since there is not a clear consensus that any of those entities are associated with an increased risk of embolic events. The exclusion of cardiomyopathy as a cause of CVA or TIA is arguable, but its link to CVA or TIA is also unproven. One study did associate cardiomyopathy with CVA [12]; however, the mechanism is not clear, as the incidence of CVA in cardiomyopathy has been described as similar regardless of the severity of left ventricular dysfunction [13].Many past reports have come from tertiary care centers, where there may be referral bias whereas our data come from consecutive patients at a single community hospital.
TTE is relatively quick to perform and interpret and carries no physical risk to a patient. However, our data suggest that ordering TTE routinely in the setting of CVA offers little value. With health care organizations turning their attention to reducing low-value care, which potentially wastes limited resources, considerations of value and effectiveness continue to be a priority. Our findings suggest TTE use in this setting conflicts with the current trajectory of value-based medical practice. As well, a prior Markov model decision analysis found that TTE is not cost-effective when used routinely to identify source of emboli in stroke [14].
Despite the low yield of TTE in evaluating for a cardiac source of CVA, TTEs continue to be frequently ordered. In our own institution, 48% of patients with a CVA or TIA underwent a TTE based on preferences and habits of individual admitting physicians and without any structured criteria. Order sets for CVA admissions do not include this test; physicians are adding it but not for any particular patient characteristic or exam finding.
There are a number of reasons that echocardiograms may be ordered more frequently by some. A documented decline in ordering echocardiograms was seen following education at one center [15], suggesting that lack of knowledge about the limitations of TTE may be a factor. A second potential factor is fear of medicolegal consequences. Indeed, the current American Heart Association/American Stroke Association guidelines for the early management of adults with ischemic stroke [16] offers no formal recommendations or clear indications.
Computerized decision support (CDS) that links the medical record to appropriateness criteria could potentially reduce the inappropriate use of TTE. CDS has been shown to be effective in reducing unnecessary ordering of tests in other settings [17–19].
Among the limitations in our analysis is the heterogeneity in echocardiogram readers. However, this heterogeneity may makes the study more relevant as it reflects the reality in most community hospitals. Another potential limitation is that saline contrast studies were not used routinely; however, this too is typical at community hospitals. Also, while all echocardiograms were interpreted by “board-certified” cardiologists, only 5 had passed the “examination of special competence” to be certified as a testamur of the National Board of Echocardiography, raising the question as to whether subtle findings could have been missed. However, there were no relevant findings in the 20% of studies interpreted by the testamurs, suggesting that the other echocardiographers were not missing diagnoses. Finally, we had only 10 patients younger than age 45 and so the study conclusions are less definitive for that age-group.
Conclusion
TTE was of limited utility in uncovering a cardiac source of embolism in a typical population with CVA or TIA.Based upon the data, we believe that TTE should not be used routinely in the setting of CVA; however, we do recognize that TTE may be of value in patients who have other comorbidities that would place them at increased risk of embolic CVA such as a recent anterior MI, those at risk for endocarditis, or those with brain imaging findings suggestive of embolic CVA [20]. Ordering a low-value test such as a TTE in the setting of TIA or CVA adds cost and does not often yield a clinically meaningful results. In addition, a “negative” TTE can be misinterpreted as a normal heart and forestall additional workup such as transesophageal echocardiography and long-term rhythm analysis, which may be of higher value. We suggest that in a community hospital setting the determination of need for TTE be made based on the clinical nuances of the case rather than by habit or as part of standardized order sets.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
Author contributions: conception and design, BM, WCM; analysis and interpretation of data, BM, WCM; drafting of article, RHB, BM, WCM; critical revision of the article, BM, WCM; administrative or technical support, JC; collection and assembly of data, RHB, JC.
1. Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996;27:691.
2. Khan MA, Khealanj B, Kamal, A. Diagnostic yield of transthoracic echocardiography for stroke patients in a developing country. J Pak Med Assoc 2008;58:375–7.
3. de Abreu T, Mateus S, José Correia J. Therapy implications of transthoracic echocardiography in acute ischemic stroke patients. Stroke 2005;36:1565–6.
4. de Bruijn SFTM, Agema WRP, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531–4.
5. Putaala J, Metso AJ, MD, Metso T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke. Stroke 2009;40:1195–203.
6. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent fora-men ovale in patients with stroke. N Engl J Med 1988;318:1148–52.
7. Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
8. Orencia AJ, Petty GW, Khandheria BK, et al. Risk of stroke with mitral valve prolapse in population-based cohort study; Stroke 1995;26:7–13.
9. Holmes M, Rathbone J, Littlewood C. Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess 2014;18:1–176.
10. Ryan T, Armstrong W. Feigenbaum’s echocardiography. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
11. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263–72.
12. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–76.
13. Hays AG, Sacco RL, Rundek T. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke 2006;37:1715–9.
14. McNamara RL, Lima JA, Whelton PK, Powe NR. Echocardiographic identification of cardiovascular sources of emboli to guide clinical management of stroke: a cost-effectiveness analysis. Ann Intern Med 1997;127:775–87.
15. Alberts MJ, Bennett CA, Rutledge VR. Hospital charges for stroke patients. Stroke 1996;27:1825–8.
16. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947.
17. Levick DL, Stern G, Meyerhoefer CD, et al. Reducing unnecessary testing in a CPOE system through implementation of a targeted CDS intervention. BMC Med Inform Decis 2013;13:43.
18. Chen P, Tanasijevic MJ, Schoenenberger RA, et al. A computer-based intervention for improving the appropriateness of antiepileptic drug level monitoring. Am J Clin Pathol 2003;119:432–8.
19. Solberg LI, Wei F, Butler JC, et al. Effects of electronic decision support on high-tech diagnostic imaging orders and patients. Am J Manag Care 2010;16:102–6.
20. Menon BK, Coulter JI, Simerpret B, et al. Acute ischaemic stroke or transient ischaemic attack and the need for inpatient echocardiography. Postgrad Med J 2014;90:434–8.
1. Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996;27:691.
2. Khan MA, Khealanj B, Kamal, A. Diagnostic yield of transthoracic echocardiography for stroke patients in a developing country. J Pak Med Assoc 2008;58:375–7.
3. de Abreu T, Mateus S, José Correia J. Therapy implications of transthoracic echocardiography in acute ischemic stroke patients. Stroke 2005;36:1565–6.
4. de Bruijn SFTM, Agema WRP, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531–4.
5. Putaala J, Metso AJ, MD, Metso T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke. Stroke 2009;40:1195–203.
6. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent fora-men ovale in patients with stroke. N Engl J Med 1988;318:1148–52.
7. Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
8. Orencia AJ, Petty GW, Khandheria BK, et al. Risk of stroke with mitral valve prolapse in population-based cohort study; Stroke 1995;26:7–13.
9. Holmes M, Rathbone J, Littlewood C. Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess 2014;18:1–176.
10. Ryan T, Armstrong W. Feigenbaum’s echocardiography. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
11. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263–72.
12. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–76.
13. Hays AG, Sacco RL, Rundek T. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke 2006;37:1715–9.
14. McNamara RL, Lima JA, Whelton PK, Powe NR. Echocardiographic identification of cardiovascular sources of emboli to guide clinical management of stroke: a cost-effectiveness analysis. Ann Intern Med 1997;127:775–87.
15. Alberts MJ, Bennett CA, Rutledge VR. Hospital charges for stroke patients. Stroke 1996;27:1825–8.
16. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947.
17. Levick DL, Stern G, Meyerhoefer CD, et al. Reducing unnecessary testing in a CPOE system through implementation of a targeted CDS intervention. BMC Med Inform Decis 2013;13:43.
18. Chen P, Tanasijevic MJ, Schoenenberger RA, et al. A computer-based intervention for improving the appropriateness of antiepileptic drug level monitoring. Am J Clin Pathol 2003;119:432–8.
19. Solberg LI, Wei F, Butler JC, et al. Effects of electronic decision support on high-tech diagnostic imaging orders and patients. Am J Manag Care 2010;16:102–6.
20. Menon BK, Coulter JI, Simerpret B, et al. Acute ischaemic stroke or transient ischaemic attack and the need for inpatient echocardiography. Postgrad Med J 2014;90:434–8.
Lessons Learned from a Quality Improvement Project to Reduce Missed Opportunities to Vaccinate Adults
From Abington Health, Abington, PA (Ms. Walter) and Duquesne University School of Nursing, Pittsburgh, PA (Dr. Guimond).
Abstract
- Background: National coverage rates for many recommended adult vaccines are low. Tetanus toxoid, diphtheria, and acellular pertussis (Tdap) and pneumococcal vaccination rates among adults are 20% and 16%, respectively. To address these low rates in our practice, we identified missed opportunities for vaccination as a target for improvement.
- Objective: To examine the effectiveness of a vaccine reminder checklist at the point of care and assess providers’ perceived vaccine practices.
- Methods: The quick sample method was used to assess pre- and post-intervention pneumococcal polysaccharide (PPSV) and Tdap vaccination rates among the target population (adults 18-64 for Tdap; high-risk adults 18-64 for PPSV). A post-intervention survey was used to assess providers’ adult vaccination practices and their opinion of the reminder tool.
- Results: The Tdap vaccination rate did not change and was constant at 47%. PPSV vaccination rates decreased from 50% to 40%. Among the providers, 47% reported ordering immunizations at sick visits, as compared to 76% at follow-up visits. The providers reported the reminder checklist was useful for determining a patient’s eligibility for a vaccine.
- Conclusion: No improvement in vaccination rates was detected for this project, which may be partially explained by challenges originating at patient check-in. In the future, buy-in from all staff in our practice setting will be sought. Results indicate that providers may hesitate to administer immunizations at sick visits and may need education on vaccination contraindications.
Vaccines are an important public health tool that offer safe and effective protection against certain diseases and reduce the health care burden [1,2]. Missed opportunities to vaccinate, defined as any primary care encounter in which a patient eligible for a vaccine is not administered a vaccine, lead to suboptimal immunization coverage among adults. Providers have been urged to review patients’ vaccine status at every patient encounter [3]. Rates of vaccinations recommended in 2012 by the Advisory Committee on Immunization Practices (ACIP) remain low [4], particularly coverage rates for tetanus toxoid, diphtheria, and acelluar pertussis vaccine (Tdap) vaccine among adults, and for pneumococcal polysaccharide vaccine (PPSV) among high-risk adults [2]. Nationally, uptake rates are approximately 16% for Tdap and 20% for pneumococcal vaccines among eligible adults aged 18 to 64 years [5]. These low uptake rates suggest that programs are needed to reduce missed opportunities to vaccinate and improve vaccination rates among adults.
There is a strong case for improving Tdap and pneumococcal vaccination uptake among high-risk adults. Since the 1970s, the incidence of pertussis in the United States has increased substantially, with numbers of reported cases reaching as high as 48,277 and 28,639 in 2012 and 2013, respectively [2]. Some states experienced epidemic levels of pertussis [2,6]. Pertussis is often fatal among infected infants, and infection in adolescents and adults may cost upwards of $800 per case [7,8]. In 2005, high-risk adults for whom the PPSV was indicated accounted for half of the 40,000 pneumococcal infections in the United States [9]. PPSV boasts a 50% to 80% effectiveness rate in preventing pneumococcal disease among high-risk patients [9]. In a CDC cost-effectiveness analysis, immunization of immunocompromised patients with the pneumococcal conjugate vaccine (PCV-13) at the time of diagnosis followed with PPSV vaccinations starting 1 year later led to savings of $7.6 million, added 1360 quality-adjusted life years, and prevented 57 cases of invasive pneumococcal disease [10].
Recognizing and overcoming practice-specific barriers to vaccinating adults are needed to improve uptake. A lack of patient- and provider-focused reminders may lead to missed opportunities to vaccinate [11,12]. Provider and patient-focused reminder tools can be effective in increasing vaccine uptake [1,13,14], but interventions that combine reminder tools with patient outreach may be more effective [15]. Furthermore, involving an interdisciplinary team to coordinate the administration of vaccines among adults may improve vaccine uptake rates [16]. These studies suggested the need to determine a standard, effective reminder tool and incorporate multilevel interventions to increase uptake of adult vaccines.
An informal electronic query at a large, suburban family practice revealed approximately 30% Tdap and PPSV coverage rates among eligible adults served by the practice, suggesting that providers fail to assess patients’ vaccine status at every opportunity. Electronic medical records provide no alerts for vaccines that may be due. PPSV and Tdap uptake rates were chosen for this quality improvement project to address low baseline coverage rates among adults. The objective of this project was to increase adult Tdap and 23-valent PPSV uptake rates using a reminder checklist at the point of care. A secondary objective was to assess providers’ vaccination practices during various types of visits, appraise their perceived vaccination practices and barriers to vaccinating adults, and to determine providers’ perceived effectiveness of the reminder checklist.
Methods
This quality improvement project was implemented in a large family practice that is home to a family medicine residency program. Approximately two-thirds of the patients served are adults, over half of whom are minorities, and nearly half are on Medicaid or underinsured. Providers in the practice included 21 resident physicians, 8 attending physicians, and 1 nurse practitioner who served as the primary investigator. Institutional review board approval for this study was obtained.
Measures included pre- and post-intervention vaccination rates for Tdap and PPSV, the providers’ perceived vaccination practices during various types of visits, the providers’ perceptions of practice-specific barriers to vaccinating adults, and the providers’ perceived usefulness of a vaccine checklist. To evaluate the effect of the reminder tool on immunization rates, we conducted a chart review. A random sample of 30 charts was derived separately for each vaccine, pre- and post-intervention, by selecting every 5th chart via the electronic health record after filtering for vaccine eligibility. Eligibility for the vaccines was based on age, vaccine history, and diagnoses noted in the medical history and problem list.
After the 3-month intervention period, an 18-item survey was distributed to participating providers to assess their vaccination practices, their perception of practice-related barriers to vaccinating, and their perception of the use of the checklist at the point of care. The survey included 5 demographic items, 5 Yes/No questions asking about the providers’ vaccination practices (adapted from [18]), and 8 questions asking about the providers’ perceptions of practice-specific vaccination barriers and the usefulness of the checklist. For these 8 questions providers were asked to choose a response along a 5-point Likert scale ranging from “strongly agree” to “strongly disagree.”
Results
All providers responded to the vaccination practice-related questions. These questions and the frequency of responses are presented in Table 3. At follow-up visits, such as those for blood pressure checks, 82% of the providers stated they checked the patient’s immunization status and 76% of providers stated they ordered an immunization. In contrast, although 76% of providers indicated they checked the immunization status, only 47% noted they routinely ordered a vaccine at a sick visit. A Fisher’s exact test of independence to examine the relationship between providers’ years of experience and decision to vaccinate at sick visits revealed a result that was not significant (P = 1).
Discussion and Lessons Learned
Introduction of the reminder checklist at the point of care did not improve the administration or uptake of Tdap or PPSV during the intervention period. Limitations to our analysis include the small sample size. Also, this project was conducted in the fall, when influenza vaccines are usually given and providers may be more attuned to checking for vaccine eligibility. Future iterations of the project may be conducted to allow for samples over several months and use a process control chart to retrieve a more representative sample of participants from each vaccine-eligible group in the practice.
Usage of a paper reminder system after implementation of an electronic health record may have affected the results. Providers who are focused on the computer documentation may have overlooked paper reminders unless patients asked about vaccination. Although the checklist was printed on bright green paper as a visual cue, patients’ failure to present the reminder to providers undermined effectiveness of the paper system. In another study that used pre-visit paper reminders to improve physician performance on measures of chronic disease and preventive care, no benefit was found [19]. Developers of future vaccination programs should consider integrating reminder systems into the current system to mitigate this potential obstacle.
Staff and practice-related barriers may have also contributed to the limited success of the reminder checklist. Staff informally cited a paperwork burden as a challenge for patients. Informal feedback indicated that patients did not fully understand the questionnaire and often did not complete the form, even after being requested and instructed to do so. A systematic review of barriers to the use of reminders for immunizations showed that reminders can be perceived as disruptive to workflow and therefore not implemented or maintained [20]. These findings were congruent with behavior demonstrated by the office staff in the practice, whose buy-in to using the intervention waned over the course of the project. The front office staff needed reinforcement to continue the intervention as time passed. Informal interviews with staff suggested that patients who could have been given a checklist at the front desk did not receive it. These possibilities underline limitations in the study. Future projects may include collecting data such as perceptions of the office staff involved with vaccine interventions and proportion of patients who receive and complete the reminder checklist. A regression analysis is recommended to identify barriers that are more likely to decrease the likelihood of vaccination.
Providers’ responses to survey questions yielded insights into surveyed providers’ perceptions re the importance of immunizing adults and the use of the reminder checklist as an intervention. The majority of the providers (n = 16, 94%) acknowledged that the office had a procedure in place for immunization of adults. Review of the protocol and more vaccine education may be needed to increase providers’ knowledge of adult vaccine indications. Responses to survey questions related to vaccination barriers suggested that providers believed there was adequate time to assess for and order vaccines at routine visits. The possibility exists that an additional barrier may be present that was not uncovered by this project. Further investigation is needed to determine practice-barriers to administering vaccinations to adults at all types of visits for health care.
The findings of this review suggest that missed opportunities to vaccinate continue to exist. This project revealed that sick and problem visits may be an area warranting further exploration for opportunities to vaccinate adults. Survey findings that 76% of providers in the practice routinely check the immunization status of adult patients at sick visits and only 47% routinely order an immunization at sick visits point to the need in future vaccination programs to target sick visits as opportunities to increase adult vaccine administration. In other studies, years of experience has not been well correlated with performance of evidence based practice [21]. However, in our study, no relationship was identified between years of practice and decision to vaccinate during a sick visit. Follow-up visits, for which 76% of the surveyed providers reported ordering immunization, are another opportunity for improvement. A survey of pediatricians and family physicians regarding their adolescent patient vaccination practices revealed similar low rates for both checking the immunization status and administering vaccinations at sick and follow-up visits [18]. Based on these findings, a larger scale review is warranted that focuses on sick and follow-up visits to determine the rate of vaccination at these visits, barriers to vaccinating at sick and follow-up visits, and successful interventions to increase vaccination rates during these encounters.
Providers’ perceived vaccination behaviors at sick and follow-up visits may be related to time restrictions resulting from shorter appointments as well as providers’ varying degrees of comfort with offering vaccines during those visits. In addition, misunderstanding of vaccination contraindications has led to missed opportunities to vaccinate women and children [22] and this may apply to adults as well. Providers need to be aware of the true contraindications to vaccines. Mild acute illness is neither a contraindication nor a precaution to administering a vaccine [23]. Future projects may also focus on educating providers (at all levels of experience) regarding the safety and efficacy of administering vaccinations during illness-related visits and actual contraindications.
Also, to address time constraints during sick and follow-up visits, making the entire practice responsible for vaccination assessment and administration should be more widely employed to reduce the burden on the primary care provider. A successful model for increasing uptake involves using teamwork [16] among clinical and non-clinical staff. Successful implementation of an adult vaccination program may be improved by using a similar approach that will increase staff buy-in and accountability.
Conclusion
While care providers in this project generally perceived the reminder checklist at the point of care as helpful as a provider reminder, a patient engager, and a tool to determine vaccine eligibility, it was not effective in increasing Tdap or PPSV coverage among adult patients in the practice. Practice and workflow-related barriers to success of the intervention imply the need for careful consideration of the type of reminder system put in place in various practices. Hesitation to vaccinate during illness-related and follow-up visits denotes the need for further education of providers regarding true contraindications to particular vaccinations and further investigation of ways to make immunizing a collective responsibility shared by the patient, the office staff, and the primary and ancillary providers.
Corresponding author: Dyllan Walter, DNP, CRNP, North Hills Health Center, 212 Girard Ave., Glenside, PA 19038, [email protected].
Financial disclosures: None.
1. Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med 2002;136:641–51.
2. Centers for Disease Control and Prevention. Pertussis outbreak trends. 2015. Available at www.cdc.gov/pertussis/outbreaks/trends.html.
3. Centers for Disease Control and Prevention. Standards for adult immunization practice. 2014. Available at www.cdc.gov/vaccines/hcp/patient-ed/adults/for-practice/standards.html.
4. Bridges CB. Adult immunization in the United States: 2012 update. Available at www.womeningovernment.org/files/file/CarolynBridges.pdf
5. Williams WW, Lu P-J, O’Halloran A, et al. Noninfluenza vaccination coverage among adults—United States, 2012. MMWR 2014;63:95–102.
6. Winter K, Glaser C, Watt J, Harriman K; Centers for Disease Control and Prevention (CDC). Pertussis epidemic--California, 2014. MMWR Morb Mortal Wkly Rep 2014;63:1129–32.
7. Grizas AP, Camenga D, Vázquez M. Cocooning: A concept to protect young children from infectious diseases. Curr Opin Pediatr 2012;24:92–7.
8. Gidengil CA, Sandora TJ, Lee GM. Tetanus-diphtheria-acellular pertussis vaccination of adults in the USA. Expert Rev Vaccines 2008;7:621–34.
9. Wolfe RM. Update on adult immunizations. J Am Board Fam Med 2012;25:496–510.
10. Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2012;61:816–19.
11. Head KJ, Vanderpool RC, Mills LA. Health care providers’ perspectives on low HPV vaccine uptake and adherence in Appalachian Kentucky. Public Health Nurs 2013;30:351–60.
12. Perkins RB, Clark JA. What affects human papilloma virus vaccination rates? A qualitative analysis of providers’ perceptions. Womens Health Issues 2012;22:e379–86.
13. Thomas RE, Russell ML, Lorenzetti DL. Systematic review of interventions to increase influenza vaccination rates of those 60 years and older. Vaccine 2010;28:1684–70.
14. Briss PA, Rodewald LE, Hinman AR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med 2000;18:97–140.
15. Humiston SG, Bennett NM, Long C, et al. Increasing inner-city adult influenza vaccination rates: A randomized controlled trial. Public Health Rep 2011;126:39–47.
16. Gannon M, Qaseem A, Snooks Q, Snow V. Improving adult immunization practices using a team approach in the primary setting. Am J Public Health 2012;102:e46–e52.
17. Immunization Action Coalition. Do I need any vaccinations today? 2014. Available at www.immunize.org/catg.d/p4036.pdf.
18. Schaffer SJ, Humiston SG, Shone LP, et al. Adolescent immunization practices: A national survey of US physicians. Arch Pediat Adol Med 2001;155:566–71.
19. Baker DW, Persell SD, Kho AN, et al. The marginal value of pre-visit paper reminders when added to a multifaceted electronic health record based quality improvement system. J Am Med Informat Assoc 2011;18:805–11.
20. Pereira JA, Quach S, Heidebrecht CL, et al. Barriers to the use of reminder/recall interventions for immunizations: a systematic review. BMC Med Inform Decis Mak 2012;12:145.
21. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: The relationship between clinical experience and quality of health care. Ann Intern Med 2005;142:260–73.
22. Hutchins SS, Jansen HAFM, Robertson SE, et al. Missed opportunities for immunization: review of studies from developing and industrialized countries. Bull World Health Org 1993;71:549–60.
23. Immunization Action Coalition. Precautions and contraindications. 2015. Available at www.immunize.org/askexperts/precautions-contraindications.asp.
From Abington Health, Abington, PA (Ms. Walter) and Duquesne University School of Nursing, Pittsburgh, PA (Dr. Guimond).
Abstract
- Background: National coverage rates for many recommended adult vaccines are low. Tetanus toxoid, diphtheria, and acellular pertussis (Tdap) and pneumococcal vaccination rates among adults are 20% and 16%, respectively. To address these low rates in our practice, we identified missed opportunities for vaccination as a target for improvement.
- Objective: To examine the effectiveness of a vaccine reminder checklist at the point of care and assess providers’ perceived vaccine practices.
- Methods: The quick sample method was used to assess pre- and post-intervention pneumococcal polysaccharide (PPSV) and Tdap vaccination rates among the target population (adults 18-64 for Tdap; high-risk adults 18-64 for PPSV). A post-intervention survey was used to assess providers’ adult vaccination practices and their opinion of the reminder tool.
- Results: The Tdap vaccination rate did not change and was constant at 47%. PPSV vaccination rates decreased from 50% to 40%. Among the providers, 47% reported ordering immunizations at sick visits, as compared to 76% at follow-up visits. The providers reported the reminder checklist was useful for determining a patient’s eligibility for a vaccine.
- Conclusion: No improvement in vaccination rates was detected for this project, which may be partially explained by challenges originating at patient check-in. In the future, buy-in from all staff in our practice setting will be sought. Results indicate that providers may hesitate to administer immunizations at sick visits and may need education on vaccination contraindications.
Vaccines are an important public health tool that offer safe and effective protection against certain diseases and reduce the health care burden [1,2]. Missed opportunities to vaccinate, defined as any primary care encounter in which a patient eligible for a vaccine is not administered a vaccine, lead to suboptimal immunization coverage among adults. Providers have been urged to review patients’ vaccine status at every patient encounter [3]. Rates of vaccinations recommended in 2012 by the Advisory Committee on Immunization Practices (ACIP) remain low [4], particularly coverage rates for tetanus toxoid, diphtheria, and acelluar pertussis vaccine (Tdap) vaccine among adults, and for pneumococcal polysaccharide vaccine (PPSV) among high-risk adults [2]. Nationally, uptake rates are approximately 16% for Tdap and 20% for pneumococcal vaccines among eligible adults aged 18 to 64 years [5]. These low uptake rates suggest that programs are needed to reduce missed opportunities to vaccinate and improve vaccination rates among adults.
There is a strong case for improving Tdap and pneumococcal vaccination uptake among high-risk adults. Since the 1970s, the incidence of pertussis in the United States has increased substantially, with numbers of reported cases reaching as high as 48,277 and 28,639 in 2012 and 2013, respectively [2]. Some states experienced epidemic levels of pertussis [2,6]. Pertussis is often fatal among infected infants, and infection in adolescents and adults may cost upwards of $800 per case [7,8]. In 2005, high-risk adults for whom the PPSV was indicated accounted for half of the 40,000 pneumococcal infections in the United States [9]. PPSV boasts a 50% to 80% effectiveness rate in preventing pneumococcal disease among high-risk patients [9]. In a CDC cost-effectiveness analysis, immunization of immunocompromised patients with the pneumococcal conjugate vaccine (PCV-13) at the time of diagnosis followed with PPSV vaccinations starting 1 year later led to savings of $7.6 million, added 1360 quality-adjusted life years, and prevented 57 cases of invasive pneumococcal disease [10].
Recognizing and overcoming practice-specific barriers to vaccinating adults are needed to improve uptake. A lack of patient- and provider-focused reminders may lead to missed opportunities to vaccinate [11,12]. Provider and patient-focused reminder tools can be effective in increasing vaccine uptake [1,13,14], but interventions that combine reminder tools with patient outreach may be more effective [15]. Furthermore, involving an interdisciplinary team to coordinate the administration of vaccines among adults may improve vaccine uptake rates [16]. These studies suggested the need to determine a standard, effective reminder tool and incorporate multilevel interventions to increase uptake of adult vaccines.
An informal electronic query at a large, suburban family practice revealed approximately 30% Tdap and PPSV coverage rates among eligible adults served by the practice, suggesting that providers fail to assess patients’ vaccine status at every opportunity. Electronic medical records provide no alerts for vaccines that may be due. PPSV and Tdap uptake rates were chosen for this quality improvement project to address low baseline coverage rates among adults. The objective of this project was to increase adult Tdap and 23-valent PPSV uptake rates using a reminder checklist at the point of care. A secondary objective was to assess providers’ vaccination practices during various types of visits, appraise their perceived vaccination practices and barriers to vaccinating adults, and to determine providers’ perceived effectiveness of the reminder checklist.
Methods
This quality improvement project was implemented in a large family practice that is home to a family medicine residency program. Approximately two-thirds of the patients served are adults, over half of whom are minorities, and nearly half are on Medicaid or underinsured. Providers in the practice included 21 resident physicians, 8 attending physicians, and 1 nurse practitioner who served as the primary investigator. Institutional review board approval for this study was obtained.
Measures included pre- and post-intervention vaccination rates for Tdap and PPSV, the providers’ perceived vaccination practices during various types of visits, the providers’ perceptions of practice-specific barriers to vaccinating adults, and the providers’ perceived usefulness of a vaccine checklist. To evaluate the effect of the reminder tool on immunization rates, we conducted a chart review. A random sample of 30 charts was derived separately for each vaccine, pre- and post-intervention, by selecting every 5th chart via the electronic health record after filtering for vaccine eligibility. Eligibility for the vaccines was based on age, vaccine history, and diagnoses noted in the medical history and problem list.
After the 3-month intervention period, an 18-item survey was distributed to participating providers to assess their vaccination practices, their perception of practice-related barriers to vaccinating, and their perception of the use of the checklist at the point of care. The survey included 5 demographic items, 5 Yes/No questions asking about the providers’ vaccination practices (adapted from [18]), and 8 questions asking about the providers’ perceptions of practice-specific vaccination barriers and the usefulness of the checklist. For these 8 questions providers were asked to choose a response along a 5-point Likert scale ranging from “strongly agree” to “strongly disagree.”
Results
All providers responded to the vaccination practice-related questions. These questions and the frequency of responses are presented in Table 3. At follow-up visits, such as those for blood pressure checks, 82% of the providers stated they checked the patient’s immunization status and 76% of providers stated they ordered an immunization. In contrast, although 76% of providers indicated they checked the immunization status, only 47% noted they routinely ordered a vaccine at a sick visit. A Fisher’s exact test of independence to examine the relationship between providers’ years of experience and decision to vaccinate at sick visits revealed a result that was not significant (P = 1).
Discussion and Lessons Learned
Introduction of the reminder checklist at the point of care did not improve the administration or uptake of Tdap or PPSV during the intervention period. Limitations to our analysis include the small sample size. Also, this project was conducted in the fall, when influenza vaccines are usually given and providers may be more attuned to checking for vaccine eligibility. Future iterations of the project may be conducted to allow for samples over several months and use a process control chart to retrieve a more representative sample of participants from each vaccine-eligible group in the practice.
Usage of a paper reminder system after implementation of an electronic health record may have affected the results. Providers who are focused on the computer documentation may have overlooked paper reminders unless patients asked about vaccination. Although the checklist was printed on bright green paper as a visual cue, patients’ failure to present the reminder to providers undermined effectiveness of the paper system. In another study that used pre-visit paper reminders to improve physician performance on measures of chronic disease and preventive care, no benefit was found [19]. Developers of future vaccination programs should consider integrating reminder systems into the current system to mitigate this potential obstacle.
Staff and practice-related barriers may have also contributed to the limited success of the reminder checklist. Staff informally cited a paperwork burden as a challenge for patients. Informal feedback indicated that patients did not fully understand the questionnaire and often did not complete the form, even after being requested and instructed to do so. A systematic review of barriers to the use of reminders for immunizations showed that reminders can be perceived as disruptive to workflow and therefore not implemented or maintained [20]. These findings were congruent with behavior demonstrated by the office staff in the practice, whose buy-in to using the intervention waned over the course of the project. The front office staff needed reinforcement to continue the intervention as time passed. Informal interviews with staff suggested that patients who could have been given a checklist at the front desk did not receive it. These possibilities underline limitations in the study. Future projects may include collecting data such as perceptions of the office staff involved with vaccine interventions and proportion of patients who receive and complete the reminder checklist. A regression analysis is recommended to identify barriers that are more likely to decrease the likelihood of vaccination.
Providers’ responses to survey questions yielded insights into surveyed providers’ perceptions re the importance of immunizing adults and the use of the reminder checklist as an intervention. The majority of the providers (n = 16, 94%) acknowledged that the office had a procedure in place for immunization of adults. Review of the protocol and more vaccine education may be needed to increase providers’ knowledge of adult vaccine indications. Responses to survey questions related to vaccination barriers suggested that providers believed there was adequate time to assess for and order vaccines at routine visits. The possibility exists that an additional barrier may be present that was not uncovered by this project. Further investigation is needed to determine practice-barriers to administering vaccinations to adults at all types of visits for health care.
The findings of this review suggest that missed opportunities to vaccinate continue to exist. This project revealed that sick and problem visits may be an area warranting further exploration for opportunities to vaccinate adults. Survey findings that 76% of providers in the practice routinely check the immunization status of adult patients at sick visits and only 47% routinely order an immunization at sick visits point to the need in future vaccination programs to target sick visits as opportunities to increase adult vaccine administration. In other studies, years of experience has not been well correlated with performance of evidence based practice [21]. However, in our study, no relationship was identified between years of practice and decision to vaccinate during a sick visit. Follow-up visits, for which 76% of the surveyed providers reported ordering immunization, are another opportunity for improvement. A survey of pediatricians and family physicians regarding their adolescent patient vaccination practices revealed similar low rates for both checking the immunization status and administering vaccinations at sick and follow-up visits [18]. Based on these findings, a larger scale review is warranted that focuses on sick and follow-up visits to determine the rate of vaccination at these visits, barriers to vaccinating at sick and follow-up visits, and successful interventions to increase vaccination rates during these encounters.
Providers’ perceived vaccination behaviors at sick and follow-up visits may be related to time restrictions resulting from shorter appointments as well as providers’ varying degrees of comfort with offering vaccines during those visits. In addition, misunderstanding of vaccination contraindications has led to missed opportunities to vaccinate women and children [22] and this may apply to adults as well. Providers need to be aware of the true contraindications to vaccines. Mild acute illness is neither a contraindication nor a precaution to administering a vaccine [23]. Future projects may also focus on educating providers (at all levels of experience) regarding the safety and efficacy of administering vaccinations during illness-related visits and actual contraindications.
Also, to address time constraints during sick and follow-up visits, making the entire practice responsible for vaccination assessment and administration should be more widely employed to reduce the burden on the primary care provider. A successful model for increasing uptake involves using teamwork [16] among clinical and non-clinical staff. Successful implementation of an adult vaccination program may be improved by using a similar approach that will increase staff buy-in and accountability.
Conclusion
While care providers in this project generally perceived the reminder checklist at the point of care as helpful as a provider reminder, a patient engager, and a tool to determine vaccine eligibility, it was not effective in increasing Tdap or PPSV coverage among adult patients in the practice. Practice and workflow-related barriers to success of the intervention imply the need for careful consideration of the type of reminder system put in place in various practices. Hesitation to vaccinate during illness-related and follow-up visits denotes the need for further education of providers regarding true contraindications to particular vaccinations and further investigation of ways to make immunizing a collective responsibility shared by the patient, the office staff, and the primary and ancillary providers.
Corresponding author: Dyllan Walter, DNP, CRNP, North Hills Health Center, 212 Girard Ave., Glenside, PA 19038, [email protected].
Financial disclosures: None.
From Abington Health, Abington, PA (Ms. Walter) and Duquesne University School of Nursing, Pittsburgh, PA (Dr. Guimond).
Abstract
- Background: National coverage rates for many recommended adult vaccines are low. Tetanus toxoid, diphtheria, and acellular pertussis (Tdap) and pneumococcal vaccination rates among adults are 20% and 16%, respectively. To address these low rates in our practice, we identified missed opportunities for vaccination as a target for improvement.
- Objective: To examine the effectiveness of a vaccine reminder checklist at the point of care and assess providers’ perceived vaccine practices.
- Methods: The quick sample method was used to assess pre- and post-intervention pneumococcal polysaccharide (PPSV) and Tdap vaccination rates among the target population (adults 18-64 for Tdap; high-risk adults 18-64 for PPSV). A post-intervention survey was used to assess providers’ adult vaccination practices and their opinion of the reminder tool.
- Results: The Tdap vaccination rate did not change and was constant at 47%. PPSV vaccination rates decreased from 50% to 40%. Among the providers, 47% reported ordering immunizations at sick visits, as compared to 76% at follow-up visits. The providers reported the reminder checklist was useful for determining a patient’s eligibility for a vaccine.
- Conclusion: No improvement in vaccination rates was detected for this project, which may be partially explained by challenges originating at patient check-in. In the future, buy-in from all staff in our practice setting will be sought. Results indicate that providers may hesitate to administer immunizations at sick visits and may need education on vaccination contraindications.
Vaccines are an important public health tool that offer safe and effective protection against certain diseases and reduce the health care burden [1,2]. Missed opportunities to vaccinate, defined as any primary care encounter in which a patient eligible for a vaccine is not administered a vaccine, lead to suboptimal immunization coverage among adults. Providers have been urged to review patients’ vaccine status at every patient encounter [3]. Rates of vaccinations recommended in 2012 by the Advisory Committee on Immunization Practices (ACIP) remain low [4], particularly coverage rates for tetanus toxoid, diphtheria, and acelluar pertussis vaccine (Tdap) vaccine among adults, and for pneumococcal polysaccharide vaccine (PPSV) among high-risk adults [2]. Nationally, uptake rates are approximately 16% for Tdap and 20% for pneumococcal vaccines among eligible adults aged 18 to 64 years [5]. These low uptake rates suggest that programs are needed to reduce missed opportunities to vaccinate and improve vaccination rates among adults.
There is a strong case for improving Tdap and pneumococcal vaccination uptake among high-risk adults. Since the 1970s, the incidence of pertussis in the United States has increased substantially, with numbers of reported cases reaching as high as 48,277 and 28,639 in 2012 and 2013, respectively [2]. Some states experienced epidemic levels of pertussis [2,6]. Pertussis is often fatal among infected infants, and infection in adolescents and adults may cost upwards of $800 per case [7,8]. In 2005, high-risk adults for whom the PPSV was indicated accounted for half of the 40,000 pneumococcal infections in the United States [9]. PPSV boasts a 50% to 80% effectiveness rate in preventing pneumococcal disease among high-risk patients [9]. In a CDC cost-effectiveness analysis, immunization of immunocompromised patients with the pneumococcal conjugate vaccine (PCV-13) at the time of diagnosis followed with PPSV vaccinations starting 1 year later led to savings of $7.6 million, added 1360 quality-adjusted life years, and prevented 57 cases of invasive pneumococcal disease [10].
Recognizing and overcoming practice-specific barriers to vaccinating adults are needed to improve uptake. A lack of patient- and provider-focused reminders may lead to missed opportunities to vaccinate [11,12]. Provider and patient-focused reminder tools can be effective in increasing vaccine uptake [1,13,14], but interventions that combine reminder tools with patient outreach may be more effective [15]. Furthermore, involving an interdisciplinary team to coordinate the administration of vaccines among adults may improve vaccine uptake rates [16]. These studies suggested the need to determine a standard, effective reminder tool and incorporate multilevel interventions to increase uptake of adult vaccines.
An informal electronic query at a large, suburban family practice revealed approximately 30% Tdap and PPSV coverage rates among eligible adults served by the practice, suggesting that providers fail to assess patients’ vaccine status at every opportunity. Electronic medical records provide no alerts for vaccines that may be due. PPSV and Tdap uptake rates were chosen for this quality improvement project to address low baseline coverage rates among adults. The objective of this project was to increase adult Tdap and 23-valent PPSV uptake rates using a reminder checklist at the point of care. A secondary objective was to assess providers’ vaccination practices during various types of visits, appraise their perceived vaccination practices and barriers to vaccinating adults, and to determine providers’ perceived effectiveness of the reminder checklist.
Methods
This quality improvement project was implemented in a large family practice that is home to a family medicine residency program. Approximately two-thirds of the patients served are adults, over half of whom are minorities, and nearly half are on Medicaid or underinsured. Providers in the practice included 21 resident physicians, 8 attending physicians, and 1 nurse practitioner who served as the primary investigator. Institutional review board approval for this study was obtained.
Measures included pre- and post-intervention vaccination rates for Tdap and PPSV, the providers’ perceived vaccination practices during various types of visits, the providers’ perceptions of practice-specific barriers to vaccinating adults, and the providers’ perceived usefulness of a vaccine checklist. To evaluate the effect of the reminder tool on immunization rates, we conducted a chart review. A random sample of 30 charts was derived separately for each vaccine, pre- and post-intervention, by selecting every 5th chart via the electronic health record after filtering for vaccine eligibility. Eligibility for the vaccines was based on age, vaccine history, and diagnoses noted in the medical history and problem list.
After the 3-month intervention period, an 18-item survey was distributed to participating providers to assess their vaccination practices, their perception of practice-related barriers to vaccinating, and their perception of the use of the checklist at the point of care. The survey included 5 demographic items, 5 Yes/No questions asking about the providers’ vaccination practices (adapted from [18]), and 8 questions asking about the providers’ perceptions of practice-specific vaccination barriers and the usefulness of the checklist. For these 8 questions providers were asked to choose a response along a 5-point Likert scale ranging from “strongly agree” to “strongly disagree.”
Results
All providers responded to the vaccination practice-related questions. These questions and the frequency of responses are presented in Table 3. At follow-up visits, such as those for blood pressure checks, 82% of the providers stated they checked the patient’s immunization status and 76% of providers stated they ordered an immunization. In contrast, although 76% of providers indicated they checked the immunization status, only 47% noted they routinely ordered a vaccine at a sick visit. A Fisher’s exact test of independence to examine the relationship between providers’ years of experience and decision to vaccinate at sick visits revealed a result that was not significant (P = 1).
Discussion and Lessons Learned
Introduction of the reminder checklist at the point of care did not improve the administration or uptake of Tdap or PPSV during the intervention period. Limitations to our analysis include the small sample size. Also, this project was conducted in the fall, when influenza vaccines are usually given and providers may be more attuned to checking for vaccine eligibility. Future iterations of the project may be conducted to allow for samples over several months and use a process control chart to retrieve a more representative sample of participants from each vaccine-eligible group in the practice.
Usage of a paper reminder system after implementation of an electronic health record may have affected the results. Providers who are focused on the computer documentation may have overlooked paper reminders unless patients asked about vaccination. Although the checklist was printed on bright green paper as a visual cue, patients’ failure to present the reminder to providers undermined effectiveness of the paper system. In another study that used pre-visit paper reminders to improve physician performance on measures of chronic disease and preventive care, no benefit was found [19]. Developers of future vaccination programs should consider integrating reminder systems into the current system to mitigate this potential obstacle.
Staff and practice-related barriers may have also contributed to the limited success of the reminder checklist. Staff informally cited a paperwork burden as a challenge for patients. Informal feedback indicated that patients did not fully understand the questionnaire and often did not complete the form, even after being requested and instructed to do so. A systematic review of barriers to the use of reminders for immunizations showed that reminders can be perceived as disruptive to workflow and therefore not implemented or maintained [20]. These findings were congruent with behavior demonstrated by the office staff in the practice, whose buy-in to using the intervention waned over the course of the project. The front office staff needed reinforcement to continue the intervention as time passed. Informal interviews with staff suggested that patients who could have been given a checklist at the front desk did not receive it. These possibilities underline limitations in the study. Future projects may include collecting data such as perceptions of the office staff involved with vaccine interventions and proportion of patients who receive and complete the reminder checklist. A regression analysis is recommended to identify barriers that are more likely to decrease the likelihood of vaccination.
Providers’ responses to survey questions yielded insights into surveyed providers’ perceptions re the importance of immunizing adults and the use of the reminder checklist as an intervention. The majority of the providers (n = 16, 94%) acknowledged that the office had a procedure in place for immunization of adults. Review of the protocol and more vaccine education may be needed to increase providers’ knowledge of adult vaccine indications. Responses to survey questions related to vaccination barriers suggested that providers believed there was adequate time to assess for and order vaccines at routine visits. The possibility exists that an additional barrier may be present that was not uncovered by this project. Further investigation is needed to determine practice-barriers to administering vaccinations to adults at all types of visits for health care.
The findings of this review suggest that missed opportunities to vaccinate continue to exist. This project revealed that sick and problem visits may be an area warranting further exploration for opportunities to vaccinate adults. Survey findings that 76% of providers in the practice routinely check the immunization status of adult patients at sick visits and only 47% routinely order an immunization at sick visits point to the need in future vaccination programs to target sick visits as opportunities to increase adult vaccine administration. In other studies, years of experience has not been well correlated with performance of evidence based practice [21]. However, in our study, no relationship was identified between years of practice and decision to vaccinate during a sick visit. Follow-up visits, for which 76% of the surveyed providers reported ordering immunization, are another opportunity for improvement. A survey of pediatricians and family physicians regarding their adolescent patient vaccination practices revealed similar low rates for both checking the immunization status and administering vaccinations at sick and follow-up visits [18]. Based on these findings, a larger scale review is warranted that focuses on sick and follow-up visits to determine the rate of vaccination at these visits, barriers to vaccinating at sick and follow-up visits, and successful interventions to increase vaccination rates during these encounters.
Providers’ perceived vaccination behaviors at sick and follow-up visits may be related to time restrictions resulting from shorter appointments as well as providers’ varying degrees of comfort with offering vaccines during those visits. In addition, misunderstanding of vaccination contraindications has led to missed opportunities to vaccinate women and children [22] and this may apply to adults as well. Providers need to be aware of the true contraindications to vaccines. Mild acute illness is neither a contraindication nor a precaution to administering a vaccine [23]. Future projects may also focus on educating providers (at all levels of experience) regarding the safety and efficacy of administering vaccinations during illness-related visits and actual contraindications.
Also, to address time constraints during sick and follow-up visits, making the entire practice responsible for vaccination assessment and administration should be more widely employed to reduce the burden on the primary care provider. A successful model for increasing uptake involves using teamwork [16] among clinical and non-clinical staff. Successful implementation of an adult vaccination program may be improved by using a similar approach that will increase staff buy-in and accountability.
Conclusion
While care providers in this project generally perceived the reminder checklist at the point of care as helpful as a provider reminder, a patient engager, and a tool to determine vaccine eligibility, it was not effective in increasing Tdap or PPSV coverage among adult patients in the practice. Practice and workflow-related barriers to success of the intervention imply the need for careful consideration of the type of reminder system put in place in various practices. Hesitation to vaccinate during illness-related and follow-up visits denotes the need for further education of providers regarding true contraindications to particular vaccinations and further investigation of ways to make immunizing a collective responsibility shared by the patient, the office staff, and the primary and ancillary providers.
Corresponding author: Dyllan Walter, DNP, CRNP, North Hills Health Center, 212 Girard Ave., Glenside, PA 19038, [email protected].
Financial disclosures: None.
1. Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med 2002;136:641–51.
2. Centers for Disease Control and Prevention. Pertussis outbreak trends. 2015. Available at www.cdc.gov/pertussis/outbreaks/trends.html.
3. Centers for Disease Control and Prevention. Standards for adult immunization practice. 2014. Available at www.cdc.gov/vaccines/hcp/patient-ed/adults/for-practice/standards.html.
4. Bridges CB. Adult immunization in the United States: 2012 update. Available at www.womeningovernment.org/files/file/CarolynBridges.pdf
5. Williams WW, Lu P-J, O’Halloran A, et al. Noninfluenza vaccination coverage among adults—United States, 2012. MMWR 2014;63:95–102.
6. Winter K, Glaser C, Watt J, Harriman K; Centers for Disease Control and Prevention (CDC). Pertussis epidemic--California, 2014. MMWR Morb Mortal Wkly Rep 2014;63:1129–32.
7. Grizas AP, Camenga D, Vázquez M. Cocooning: A concept to protect young children from infectious diseases. Curr Opin Pediatr 2012;24:92–7.
8. Gidengil CA, Sandora TJ, Lee GM. Tetanus-diphtheria-acellular pertussis vaccination of adults in the USA. Expert Rev Vaccines 2008;7:621–34.
9. Wolfe RM. Update on adult immunizations. J Am Board Fam Med 2012;25:496–510.
10. Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2012;61:816–19.
11. Head KJ, Vanderpool RC, Mills LA. Health care providers’ perspectives on low HPV vaccine uptake and adherence in Appalachian Kentucky. Public Health Nurs 2013;30:351–60.
12. Perkins RB, Clark JA. What affects human papilloma virus vaccination rates? A qualitative analysis of providers’ perceptions. Womens Health Issues 2012;22:e379–86.
13. Thomas RE, Russell ML, Lorenzetti DL. Systematic review of interventions to increase influenza vaccination rates of those 60 years and older. Vaccine 2010;28:1684–70.
14. Briss PA, Rodewald LE, Hinman AR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med 2000;18:97–140.
15. Humiston SG, Bennett NM, Long C, et al. Increasing inner-city adult influenza vaccination rates: A randomized controlled trial. Public Health Rep 2011;126:39–47.
16. Gannon M, Qaseem A, Snooks Q, Snow V. Improving adult immunization practices using a team approach in the primary setting. Am J Public Health 2012;102:e46–e52.
17. Immunization Action Coalition. Do I need any vaccinations today? 2014. Available at www.immunize.org/catg.d/p4036.pdf.
18. Schaffer SJ, Humiston SG, Shone LP, et al. Adolescent immunization practices: A national survey of US physicians. Arch Pediat Adol Med 2001;155:566–71.
19. Baker DW, Persell SD, Kho AN, et al. The marginal value of pre-visit paper reminders when added to a multifaceted electronic health record based quality improvement system. J Am Med Informat Assoc 2011;18:805–11.
20. Pereira JA, Quach S, Heidebrecht CL, et al. Barriers to the use of reminder/recall interventions for immunizations: a systematic review. BMC Med Inform Decis Mak 2012;12:145.
21. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: The relationship between clinical experience and quality of health care. Ann Intern Med 2005;142:260–73.
22. Hutchins SS, Jansen HAFM, Robertson SE, et al. Missed opportunities for immunization: review of studies from developing and industrialized countries. Bull World Health Org 1993;71:549–60.
23. Immunization Action Coalition. Precautions and contraindications. 2015. Available at www.immunize.org/askexperts/precautions-contraindications.asp.
1. Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med 2002;136:641–51.
2. Centers for Disease Control and Prevention. Pertussis outbreak trends. 2015. Available at www.cdc.gov/pertussis/outbreaks/trends.html.
3. Centers for Disease Control and Prevention. Standards for adult immunization practice. 2014. Available at www.cdc.gov/vaccines/hcp/patient-ed/adults/for-practice/standards.html.
4. Bridges CB. Adult immunization in the United States: 2012 update. Available at www.womeningovernment.org/files/file/CarolynBridges.pdf
5. Williams WW, Lu P-J, O’Halloran A, et al. Noninfluenza vaccination coverage among adults—United States, 2012. MMWR 2014;63:95–102.
6. Winter K, Glaser C, Watt J, Harriman K; Centers for Disease Control and Prevention (CDC). Pertussis epidemic--California, 2014. MMWR Morb Mortal Wkly Rep 2014;63:1129–32.
7. Grizas AP, Camenga D, Vázquez M. Cocooning: A concept to protect young children from infectious diseases. Curr Opin Pediatr 2012;24:92–7.
8. Gidengil CA, Sandora TJ, Lee GM. Tetanus-diphtheria-acellular pertussis vaccination of adults in the USA. Expert Rev Vaccines 2008;7:621–34.
9. Wolfe RM. Update on adult immunizations. J Am Board Fam Med 2012;25:496–510.
10. Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2012;61:816–19.
11. Head KJ, Vanderpool RC, Mills LA. Health care providers’ perspectives on low HPV vaccine uptake and adherence in Appalachian Kentucky. Public Health Nurs 2013;30:351–60.
12. Perkins RB, Clark JA. What affects human papilloma virus vaccination rates? A qualitative analysis of providers’ perceptions. Womens Health Issues 2012;22:e379–86.
13. Thomas RE, Russell ML, Lorenzetti DL. Systematic review of interventions to increase influenza vaccination rates of those 60 years and older. Vaccine 2010;28:1684–70.
14. Briss PA, Rodewald LE, Hinman AR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med 2000;18:97–140.
15. Humiston SG, Bennett NM, Long C, et al. Increasing inner-city adult influenza vaccination rates: A randomized controlled trial. Public Health Rep 2011;126:39–47.
16. Gannon M, Qaseem A, Snooks Q, Snow V. Improving adult immunization practices using a team approach in the primary setting. Am J Public Health 2012;102:e46–e52.
17. Immunization Action Coalition. Do I need any vaccinations today? 2014. Available at www.immunize.org/catg.d/p4036.pdf.
18. Schaffer SJ, Humiston SG, Shone LP, et al. Adolescent immunization practices: A national survey of US physicians. Arch Pediat Adol Med 2001;155:566–71.
19. Baker DW, Persell SD, Kho AN, et al. The marginal value of pre-visit paper reminders when added to a multifaceted electronic health record based quality improvement system. J Am Med Informat Assoc 2011;18:805–11.
20. Pereira JA, Quach S, Heidebrecht CL, et al. Barriers to the use of reminder/recall interventions for immunizations: a systematic review. BMC Med Inform Decis Mak 2012;12:145.
21. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: The relationship between clinical experience and quality of health care. Ann Intern Med 2005;142:260–73.
22. Hutchins SS, Jansen HAFM, Robertson SE, et al. Missed opportunities for immunization: review of studies from developing and industrialized countries. Bull World Health Org 1993;71:549–60.
23. Immunization Action Coalition. Precautions and contraindications. 2015. Available at www.immunize.org/askexperts/precautions-contraindications.asp.
Imetelstat elicits response in myelofibrosis, thrombocythemia
The telomerase inhibitor imetelstat showed promise against advanced myelofibrosis and essential thrombocythemia in two industry-funded preliminary studies, according to separate reports published online Sept. 3 in the New England Journal of Medicine.
In previous in vitro and animal studies, imetelstat inhibited the proliferation of various types of malignant cells but was not active in normal somatic tissue. Researchers assessed the agent for advanced myelofibrosis in part because, at present, only one available treatment – allogeneic stem-cell transplantation (ASCT) – sometimes induces long-term remission. ASCT carries a relatively high rate of treatment-related death and complications, and is contraindicated in many older patients.
In the first report, researchers conducted a small, single-center cohort study to collect preliminary data on the agent’s efficacy and safety in 33 patients with primary myelofibrosis (18 participants), myelofibrosis that was related to polycythemia (10 participants), or myelofibrosis associated with essential thrombocytopenia (10 participants). Imetelstat was administered in 2-hour intravenous infusions given in 3-week cycles, said Dr. Ayalew Tefferi of the division of hematology, Mayo Clinic, Rochester Minn.
The median duration of treatment was 8.6 months (range, 1.4-21.7 months). Seven patients (21%) had either a complete or partial response; the 4 patients with a complete response had documented complete reversal of bone marrow fibrosis. The time to onset of response was 3.5 months (range, 1.4-7.2 months), and the median duration of response was 18 months (range, 13-20 months).
These remissions “confirm selective anticlonal activity, which has not previously been documented in drug treatment of myelofibrosis,” noted Dr. Tefferi and his associates (N Engl J Med 2015 Sep 3. doi:10.1056/NEJMoa1310523). Three of the seven patients who responded to imetelstat “had been heavily dependent on red-cell transfusions at study entry and became transfusion-independent and sustained a hemoglobin level of more than 10 g/dL for a minimum of 3 months during therapy,” they noted.
In addition, 8 of 10 patients who had marked leukocytosis at baseline had either a complete resolution (3 patients) or a reduction of at least 50% in white-cell counts (5 patients). All 11 participants who had thrombocytosis at baseline had either complete resolution (10 patients) or a reduction in platelet count of at least 50% (1 patient). Of the 27 participants who had leukoerythroblastosis at baseline, 22 showed either complete resolution (13 patients) or a reduction of at least 50% in the percentage of immature myeloid cells and nucleated red cells (9 patients). Also, 17 of the 21 participants who had at least 1% circulating blasts at baseline had either a complete disappearance of circulating blasts (14 patients) or a reduction of at least 50% (3 patients).
The most clinically significant adverse effect of imetelstat, myelosuppression, occurred in 22 patients (67%) and often necessitated dose reductions. Low-grade elevations in liver enzymes also were a concern. One patient died from an intracranial hemorrhage that the treating physician attributed to drug-induced grade 4 thrombocytopenia. Other adverse events that may or may not have been treatment related included fever, epistaxis, bruising, hematoma, lung infection, skin infection, and upper-GI hemorrhage.
These findings not only identify imetelstat as a possible treatment for myelofibrosis, they also suggest that other telomerase-targeting strategies may be beneficial in this disease, Dr. Tefferi and his associates added.
In the second report, researchers performing a phase-II study at seven medical centers in the United States, Germany, and Switzerland found that imetelstat produced rapid and durable hematologic and molecular responses in all 18 patients in their study of essential thrombocythemia refractory to other treatments. This result is particularly encouraging because current standard therapies “induce nonspecific reductions in platelet counts but do not typically eliminate or alter the biologic characteristics of the disease,” said Dr. Gabriela M. Baerlocher of the department of hematology and the Stem Cell Molecular Diagnostics Laboratory, University of Bern, Switzerland.
These study participants had either failed to respond to hydroxyurea, anagrelide, and interferon therapy or were forced to discontinue these agents because of adverse effects. After weekly treatment with imetelstat at one of two doses, 100% of the patients achieved a hematologic response, attaining platelet counts of 250,000-300,000 per cc. Sixteen participants (89%) achieved a complete hematologic response. The median time to complete response was 6.1 weeks, Dr. Baerlocher and her associates said (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMoa1503479).
After a median follow-up of 17 months on a maintenance dose of imetelstat, 10 patients were still receiving treatment. The median duration of response had not been reached as of press time (range, 5-30 months).
The most important adverse events were neutropenia (15 patients) and abnormal results on liver-function tests (14 patients). The treating physicians attributed 18 adverse events of grade 3 or higher to the study drug, including headache, anemia, and one syncopal episode. Other adverse events included fatigue, nausea, diarrhea, infections, and rash.
The results of both of these studies are compelling and certainly warrant further research, given the limited treatment options for myeloproliferative disorders.
Although imetelstat’s mechanism of action remains to be elucidated, both studies hint at the possibility that the agent may actually change the natural history of these debilitating disorders.
More important, assessing imetelstat’s long-term safety profile is a vital next step for researchers.
Dr. Mary Armanios and Carol W. Greider, Ph.D., are at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore. Dr. Armanios reported having no relevant disclosures; Dr. Greider reported patents related to an RNA component of telomerase and telomerase-associated proteins. Dr. Armanios and Dr. Greider made these remarks in an editorial accompanying the two reports on imetelstat (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMe1508740).
The results of both of these studies are compelling and certainly warrant further research, given the limited treatment options for myeloproliferative disorders.
Although imetelstat’s mechanism of action remains to be elucidated, both studies hint at the possibility that the agent may actually change the natural history of these debilitating disorders.
More important, assessing imetelstat’s long-term safety profile is a vital next step for researchers.
Dr. Mary Armanios and Carol W. Greider, Ph.D., are at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore. Dr. Armanios reported having no relevant disclosures; Dr. Greider reported patents related to an RNA component of telomerase and telomerase-associated proteins. Dr. Armanios and Dr. Greider made these remarks in an editorial accompanying the two reports on imetelstat (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMe1508740).
The results of both of these studies are compelling and certainly warrant further research, given the limited treatment options for myeloproliferative disorders.
Although imetelstat’s mechanism of action remains to be elucidated, both studies hint at the possibility that the agent may actually change the natural history of these debilitating disorders.
More important, assessing imetelstat’s long-term safety profile is a vital next step for researchers.
Dr. Mary Armanios and Carol W. Greider, Ph.D., are at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore. Dr. Armanios reported having no relevant disclosures; Dr. Greider reported patents related to an RNA component of telomerase and telomerase-associated proteins. Dr. Armanios and Dr. Greider made these remarks in an editorial accompanying the two reports on imetelstat (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMe1508740).
The telomerase inhibitor imetelstat showed promise against advanced myelofibrosis and essential thrombocythemia in two industry-funded preliminary studies, according to separate reports published online Sept. 3 in the New England Journal of Medicine.
In previous in vitro and animal studies, imetelstat inhibited the proliferation of various types of malignant cells but was not active in normal somatic tissue. Researchers assessed the agent for advanced myelofibrosis in part because, at present, only one available treatment – allogeneic stem-cell transplantation (ASCT) – sometimes induces long-term remission. ASCT carries a relatively high rate of treatment-related death and complications, and is contraindicated in many older patients.
In the first report, researchers conducted a small, single-center cohort study to collect preliminary data on the agent’s efficacy and safety in 33 patients with primary myelofibrosis (18 participants), myelofibrosis that was related to polycythemia (10 participants), or myelofibrosis associated with essential thrombocytopenia (10 participants). Imetelstat was administered in 2-hour intravenous infusions given in 3-week cycles, said Dr. Ayalew Tefferi of the division of hematology, Mayo Clinic, Rochester Minn.
The median duration of treatment was 8.6 months (range, 1.4-21.7 months). Seven patients (21%) had either a complete or partial response; the 4 patients with a complete response had documented complete reversal of bone marrow fibrosis. The time to onset of response was 3.5 months (range, 1.4-7.2 months), and the median duration of response was 18 months (range, 13-20 months).
These remissions “confirm selective anticlonal activity, which has not previously been documented in drug treatment of myelofibrosis,” noted Dr. Tefferi and his associates (N Engl J Med 2015 Sep 3. doi:10.1056/NEJMoa1310523). Three of the seven patients who responded to imetelstat “had been heavily dependent on red-cell transfusions at study entry and became transfusion-independent and sustained a hemoglobin level of more than 10 g/dL for a minimum of 3 months during therapy,” they noted.
In addition, 8 of 10 patients who had marked leukocytosis at baseline had either a complete resolution (3 patients) or a reduction of at least 50% in white-cell counts (5 patients). All 11 participants who had thrombocytosis at baseline had either complete resolution (10 patients) or a reduction in platelet count of at least 50% (1 patient). Of the 27 participants who had leukoerythroblastosis at baseline, 22 showed either complete resolution (13 patients) or a reduction of at least 50% in the percentage of immature myeloid cells and nucleated red cells (9 patients). Also, 17 of the 21 participants who had at least 1% circulating blasts at baseline had either a complete disappearance of circulating blasts (14 patients) or a reduction of at least 50% (3 patients).
The most clinically significant adverse effect of imetelstat, myelosuppression, occurred in 22 patients (67%) and often necessitated dose reductions. Low-grade elevations in liver enzymes also were a concern. One patient died from an intracranial hemorrhage that the treating physician attributed to drug-induced grade 4 thrombocytopenia. Other adverse events that may or may not have been treatment related included fever, epistaxis, bruising, hematoma, lung infection, skin infection, and upper-GI hemorrhage.
These findings not only identify imetelstat as a possible treatment for myelofibrosis, they also suggest that other telomerase-targeting strategies may be beneficial in this disease, Dr. Tefferi and his associates added.
In the second report, researchers performing a phase-II study at seven medical centers in the United States, Germany, and Switzerland found that imetelstat produced rapid and durable hematologic and molecular responses in all 18 patients in their study of essential thrombocythemia refractory to other treatments. This result is particularly encouraging because current standard therapies “induce nonspecific reductions in platelet counts but do not typically eliminate or alter the biologic characteristics of the disease,” said Dr. Gabriela M. Baerlocher of the department of hematology and the Stem Cell Molecular Diagnostics Laboratory, University of Bern, Switzerland.
These study participants had either failed to respond to hydroxyurea, anagrelide, and interferon therapy or were forced to discontinue these agents because of adverse effects. After weekly treatment with imetelstat at one of two doses, 100% of the patients achieved a hematologic response, attaining platelet counts of 250,000-300,000 per cc. Sixteen participants (89%) achieved a complete hematologic response. The median time to complete response was 6.1 weeks, Dr. Baerlocher and her associates said (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMoa1503479).
After a median follow-up of 17 months on a maintenance dose of imetelstat, 10 patients were still receiving treatment. The median duration of response had not been reached as of press time (range, 5-30 months).
The most important adverse events were neutropenia (15 patients) and abnormal results on liver-function tests (14 patients). The treating physicians attributed 18 adverse events of grade 3 or higher to the study drug, including headache, anemia, and one syncopal episode. Other adverse events included fatigue, nausea, diarrhea, infections, and rash.
The telomerase inhibitor imetelstat showed promise against advanced myelofibrosis and essential thrombocythemia in two industry-funded preliminary studies, according to separate reports published online Sept. 3 in the New England Journal of Medicine.
In previous in vitro and animal studies, imetelstat inhibited the proliferation of various types of malignant cells but was not active in normal somatic tissue. Researchers assessed the agent for advanced myelofibrosis in part because, at present, only one available treatment – allogeneic stem-cell transplantation (ASCT) – sometimes induces long-term remission. ASCT carries a relatively high rate of treatment-related death and complications, and is contraindicated in many older patients.
In the first report, researchers conducted a small, single-center cohort study to collect preliminary data on the agent’s efficacy and safety in 33 patients with primary myelofibrosis (18 participants), myelofibrosis that was related to polycythemia (10 participants), or myelofibrosis associated with essential thrombocytopenia (10 participants). Imetelstat was administered in 2-hour intravenous infusions given in 3-week cycles, said Dr. Ayalew Tefferi of the division of hematology, Mayo Clinic, Rochester Minn.
The median duration of treatment was 8.6 months (range, 1.4-21.7 months). Seven patients (21%) had either a complete or partial response; the 4 patients with a complete response had documented complete reversal of bone marrow fibrosis. The time to onset of response was 3.5 months (range, 1.4-7.2 months), and the median duration of response was 18 months (range, 13-20 months).
These remissions “confirm selective anticlonal activity, which has not previously been documented in drug treatment of myelofibrosis,” noted Dr. Tefferi and his associates (N Engl J Med 2015 Sep 3. doi:10.1056/NEJMoa1310523). Three of the seven patients who responded to imetelstat “had been heavily dependent on red-cell transfusions at study entry and became transfusion-independent and sustained a hemoglobin level of more than 10 g/dL for a minimum of 3 months during therapy,” they noted.
In addition, 8 of 10 patients who had marked leukocytosis at baseline had either a complete resolution (3 patients) or a reduction of at least 50% in white-cell counts (5 patients). All 11 participants who had thrombocytosis at baseline had either complete resolution (10 patients) or a reduction in platelet count of at least 50% (1 patient). Of the 27 participants who had leukoerythroblastosis at baseline, 22 showed either complete resolution (13 patients) or a reduction of at least 50% in the percentage of immature myeloid cells and nucleated red cells (9 patients). Also, 17 of the 21 participants who had at least 1% circulating blasts at baseline had either a complete disappearance of circulating blasts (14 patients) or a reduction of at least 50% (3 patients).
The most clinically significant adverse effect of imetelstat, myelosuppression, occurred in 22 patients (67%) and often necessitated dose reductions. Low-grade elevations in liver enzymes also were a concern. One patient died from an intracranial hemorrhage that the treating physician attributed to drug-induced grade 4 thrombocytopenia. Other adverse events that may or may not have been treatment related included fever, epistaxis, bruising, hematoma, lung infection, skin infection, and upper-GI hemorrhage.
These findings not only identify imetelstat as a possible treatment for myelofibrosis, they also suggest that other telomerase-targeting strategies may be beneficial in this disease, Dr. Tefferi and his associates added.
In the second report, researchers performing a phase-II study at seven medical centers in the United States, Germany, and Switzerland found that imetelstat produced rapid and durable hematologic and molecular responses in all 18 patients in their study of essential thrombocythemia refractory to other treatments. This result is particularly encouraging because current standard therapies “induce nonspecific reductions in platelet counts but do not typically eliminate or alter the biologic characteristics of the disease,” said Dr. Gabriela M. Baerlocher of the department of hematology and the Stem Cell Molecular Diagnostics Laboratory, University of Bern, Switzerland.
These study participants had either failed to respond to hydroxyurea, anagrelide, and interferon therapy or were forced to discontinue these agents because of adverse effects. After weekly treatment with imetelstat at one of two doses, 100% of the patients achieved a hematologic response, attaining platelet counts of 250,000-300,000 per cc. Sixteen participants (89%) achieved a complete hematologic response. The median time to complete response was 6.1 weeks, Dr. Baerlocher and her associates said (N Engl J Med. 2015 Sep 3. doi:10.1056/NEJMoa1503479).
After a median follow-up of 17 months on a maintenance dose of imetelstat, 10 patients were still receiving treatment. The median duration of response had not been reached as of press time (range, 5-30 months).
The most important adverse events were neutropenia (15 patients) and abnormal results on liver-function tests (14 patients). The treating physicians attributed 18 adverse events of grade 3 or higher to the study drug, including headache, anemia, and one syncopal episode. Other adverse events included fatigue, nausea, diarrhea, infections, and rash.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The telomerase inhibitor imetelstat showed promise in separate preliminary studies for treatment of myelofibrosis and thrombocythemia.
Major finding: A complete or partial response to imetelstat was seen in 7 of 33 patients with advanced myelofibrosis and 18 of 18 with thrombocythemia.
Data source: An international phase-II open-label study involving 18 patients with essential thrombocythemia and a single-center observational cohort study involving 33 patients with myelofibrosis.
Disclosures: Both studies were funded by Geron.
Psychological Distress and Cardiovascular Disease
From the California State University, Long Beach, School of Nursing, Long Beach, CA (Dr. McGuire, Ms. Ahearn), and the University of California, Los Angeles, School of Nursing, Los Angeles, CA (Dr. Doering).
Abstract
- Objective: To review the current literature regarding psychological distress in patients with cardiovascular disease (CVD).
- Methods: Relevant and current (2005–2015) studies were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases along with manual extraction from the reference lists of pertinent studies. Narrative and tabular summaries of the findings are reported.
- Results: There is a vast literature on psychological distress and CVD. Depression is the most common disorder studied followed by anxiety and post-traumatic stress disorder. Physiologic mechanisms linking psychological distress to CVD are well theorized. Screening for psychological distress in CVD is recommended. Referral and treatment issues need further exploration. Pharmacologic treatment of psychological distress in CVD remains equivocal; however, promising data exists for other therapies such as cognitive behavioral therapy and social support strategies.
- Conclusion: Psychological distress has a significant negative impact on patients with CVD and is underrecognized by health care providers. Primary care providers and cardiovascular specialty providers are called upon to improve their recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment.
The association between the heart and the mind has been proposed by scientists since the 17th century. However, it was not until the 1970s that the relationship between cardiovascular disease (CVD) and psychological states came into scientific focus. The study of heart-psyche interactions began with investigations of cardiovascular risk and “type A” personality behaviors (aggressiveness, impatience, a sense of time-urgency, intense achievement drive, seeking recognition) [1,2]. Hundreds of studies generated over the last 10 years have yielded an extensive body of literature regarding this complex interaction.
CVD continues to be the leading cause of death globally. Worldwide and in the United States, CVD accounts for 30% of deaths and more than 2000 deaths per day, respectively [3,4]. Psychological distress (specifically depression) has been reported by the World Health Organization (WHO) as the leading cause of disability in the world [4]. Taken together, CVD and depression constitute an immense health burden and result in poor health status, increased care giver burden [5], increased readmission rates to hospitals, increased utilization of primary care services, poor health compliance [6], decreased health related quality of life [7], and a greater than 2 times increase in mortality [8,9].
Despite its devastating consequences, comorbid CVD and psychological distress remains poorly recognized and treated. In this paper, we present a review of the evidence related to key aspects of psychological distress and CVD (for the purposes of this paper, defined as ischemic heart disease and stroke), and provide information to help improve identification among health care providers. Relevant and current (2005-2015) studies for this review were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases, along with manual extraction from the reference lists of pertinent studies. Due to the breadth and extent of the literature, a comprehensive review of the literature is beyond the scope of this article. However, the reader will be directed to current systematic reviews, meta-analyses, and recent select research studies sourced for this summary and presented in tabular form.
Mechanisms of Psychological Distress
Psychological Distress Disorders Related to CVD
Depression, anxiety, and post-traumatic stress disorder (PTSD) are the 3 most common psychological distress disorders related to CVD [20]. Cardiac disease and depression has been most commonly studied. In stroke, the science is not as well evolved due to greater heterogeneity of study samples and outcome measures.
Depression
Dysphoria (feeling blue), anhedonia (inability to experience joy in otherwise enjoyable activities), insomnia or hypersomnia, fatigue or loss of energy, increased guilt or worthlessness, decreased concentration, appetite change with significant weight loss or gain, psychomotor retardation or agitation, and suicidal ideation are the symptoms of depression [21]. These symptoms exist on a continuum, ranging from mild symptoms with short duration and limited functional impairment to major depression. Importantly, among otherwise healthy individuals, even minor depressive symptoms have been significantly associated with increased incidence of coronary disease [22].
Screening Issues
In recognition of the high prevalence of depression in patients with CHD, an American Heart Association (AHA) science advisory in 2008 recommended routine screening for depression in patients with CHD, with follow-up evaluation for diagnosis and treatment of depression by qualified professionals for positive cases [26]. In 2014, an AHA scientific statement recommended elevating depression to the level of a risk factor in ACS patients [27]. The recommendation for screening was initially met with some concern as being premature [28], when past supporters spoke out against the routine screening of depression in cardiac patients [29]. The dissenting authors claimed that there was a lack of scientific evidence supporting the efficacy of treatment for depression in cardiac patients, and that potential negative effects of routine screening and follow-up treatment were unknown. They argued the following: limited data from randomized controlled trials and/or evidenced-based reviews exist demonstrating improved outcomes in cardiac patients based on screening and referral [30]; antidepressants are not yet recognized to be effective in cardiac populations and there is a lack of evidence related to potential harms [28]; concerns exist about the potential for mass screening to increase health care resource use at the expense of other health care needs [29]; and routine screening may cause unnecessary negative social stigma related to false-positive findings [31].
Although clinical trials of depression treatment in cardiac patients have not demonstrated an increase in survival, treatment has been shown to be effective in reducing depression symptoms, improving patient satisfaction with depression care and improving health related quality of life [32–34]. Further, recent studies described the AHA recommendation as well accepted by cardiac unit staff, not heavily resource intensive, feasible, and accurate [35,36]. Bigger and Glassman [37] published a recent analytical review of the AHA advisory and concluded that the advisory is supported by the literature. A salient point regarding the depression screening debate is that screening without proper follow-up for further diagnosis and potential treatment may be harmful [28,29,31]. Despite concerns of the potential negative impact of depression screening in cardiac patients raised in the literature, the preponderance of the literature indicates that its benefits are likely to outweigh its risks [32,34,36,38–40].
Outcomes of Depression Treatment
Answers to questions about improvement in cardiovascular and all-cause mortality outcomes with depression treatment remain elusive in the literature. However, data show an improvement in depressive symptoms and quality of life for depressed patients receiving some types of treatment [33,41–45]. The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study was a landmark study of MI patients with a 6-month treatment intervention of cognitive behavioral therapy (CBT) plus pharmacologic intervention if indicated for depression [33]. Patients were followed for an average of 29 months post-MI. A significant improvement was seen in depressive symptoms and social isolation in the treatment group; however, there was no improvement in event-free survival [33]. When outcome measures are restricted to mortality alone, subsequent trials of antidepressant medications for treatment of depression in cardiac patients have shown them to be ineffective [46]. However, CBT and other supportive stress management strategies are effective in decreasing depressive symptoms and improving the quality of life in patients suffering with depression and CVD [46].
Promising results are emerging in the literature as researchers refocus their analysis on subgroups of depressed cardiac patients. In one large study of 442 depressed and 325 non-depressed patients, the number of depressive symptoms after an MI irrespective of the pre-MI depression status was associated with worse cardiac outcomes [47]. For every 1 additional depressive symptom reported 1 year post-MI, patients had a 15% increased risk for a new cardiac event in the next 2.5 years [47]. Another study demonstrated an improvement in depressive symptoms by 75.3% in in post-cardiac surgery patients with low ejection fraction (< 40%) after 8 weeks of nurse-guided CBT and worsening in depressive symptoms by 26.8% in usual care patients. More moderate findings were seen in the those with higher ejection fraction receiving the same CBT intervention for depression [45]. A treatment-resistant depression subgroup analyzed in a recent secondary analysis of the ENRICHD trial showed a twofold increase in mortality when compared to those in the non–treatment-resistant depression group [48]. Since treatment does not work for all patients with depression, including depressed post-MI patients, further evaluation with a focus on those who respond to treatment is needed.
Depression in Stroke
Issues related to treatment of stroke patients parallel those of depressed patients with cardiac disease, as the effect on mortality and survival is unknown. Depression has been reported to go untreated in up to 67.9% of depressed post-stroke patients [54]. In addition, mismatches between antidepressant prescription and those with depression suggested that some patients without depression were being treated for depression while some patients with depression were not being treated [54].
Anxiety
Anxiety disorders create behavioral disturbances of fear and avoidance related to an individual’s propensity to overestimate dangers [21]. Though a number of anxiety disorders are described in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), anxiety as a general constellation of symptoms (afraid, inability to relax, worry about everyday problems, feelings of panic) is described in most of the literature related to CVD [55–57]. To a lesser extent, generalized anxiety disorder (GAD), defined as anxiety symptoms on more days than not and lasting more than 6 months [58,59] has also been studied. Because anxiety can be a component of depression, the 2 are often discussed together. Taken together, in the context of CHD, individuals with both anxiety and depression are at significantly greater risk for death (odds ratio 2.35, 95% confidence interval 1.23-4.47, P =0.01), compared with those without symptoms [60].
Post-Traumatic Stress Disorder
PTSD presents with a heterogeneous cluster of symptoms that are generally described as avoidance, re-experiencing, arousal, and negative cognitions and mood [21]. Previously classified as a anxiety disorder in DSM-IV, PTSD is in a new category of trauma and stress or related disorders in the revised DSM-V [21]. In addition, the temporal component of the symptoms has been changed to a disturbance lasting more than 1 month, without reference to acuity or chronicity.
Implications for Clinical Practice
Despite the extensive body of literature regarding the negative association of psychological distress to health outcomes in CVD patients, there remains a significant practice gap related to screening, referral, and treatment of psychological distress in CVD patients [30,36,66]. Busy clinical practices focused on physical symptoms (which may not be recognized as mental health–related), along with a health system that has historically regarded mental health issues as the sole domain of mental health professionals creates barriers that need to be overcome. Many health care providers are not proactive in screening patients for psychological distress [11]. Additionally, psychological distress is often perceived as a metaphysical, inexact phenomenon, and is not regarded with the same import as physiological indices, such blood pressure and lipid levels [13]. Another significant barrier may be that the importance of psychological distress, especially depression, has been minimized by some investigators and clinicians because of the lack of data that show improvement with treatment of “hard” outcomes, such as mortality. Lastly, no studies have conclusively demonstrated that treating depression in the general population would lower subsequent cardiovascular clinical events, adding to the minimization of the importance of screening, referral, and treatment of psychological distress among clinicians.
The challenges associated with psychological distress and CVD are centered on the perceived role of the health care provider and role of the patient [11,67]. To date, identification of patients with psychological distress in CVD populations has not been considered a part of routine practice, and only a small percent of those identified with psychological distress are treated. In one study, 17.6% of 1181 patients had moderate to severe depression and of those, only 24.5% were recognized as depressed by their health care providers [68]. In a smaller study of 35 patients with depression after an acute MI, only 10% received treatment with antidepressants [66]. Similarly poor treatment rates were seen in a recent study of antidepressant use after stroke and transient ischemic attack (TIA), with 67.9% of stroke patients and 70.0% of TIA patients with persistent depression going untreated [54].
Stress, depression, anxiety, and PTSD are often not self-contained or experienced in isolation. Rather, some or all of these conditions may present as an interconnected phenomenon [69,70]. There are several reasons for this. First, symptom identification relies on self-report and/or observation of the symptoms, which may confound validity and reliability of diagnosis [70]. Second, the conditions share some symptoms, which may complicate diagnosis of a primary condition. In addition, the overlap of somatic, cognitive, and affective symptoms [71] may deter health care providers from using screening tools that have physical symptoms as part of the screening process. In a recent study of depressed cardiac patients, investigators clustered symptoms and demonstrated that cognitive affective symptoms of depression predict depression in patients with heart disease [72]. Certain events, such as stroke, may make screening especially difficult due to the presence of neurological changes that may complicate the screening process [73]. These issues highlight the need for formalized depression screening tools such as the Patient Health Questionnaire 2-item screening tool (PHQ-2) and/or Patient Health Questionnaire nine-item screening tool (PHQ-9) (Appendix), as recommended by the AHA. The PHQs have been validated in stroke patients and cardiac patients and have been found to be accurate, easy to use, and feasible [35,36,73].
Patients look to their providers for information and security during vulnerable times in their life. In a 2014 study regarding the perceptions of psychosocial consequences and access to support after MI, patients reported a high sense of security regarding being able to contact their providers [74]. Providers in both the primary and acute care settings should use these opportunities to assess for psychological distress. Many inpatient health care providers assume that it is the primary care providers’ responsibility to screen for depression [75]; however, screening should be done in all practice settings caring for patients with CVD. Clinical practice environments should develop policies and procedures to define who, when, and how screening for psychological distress is accomplished using currently available brief screening tools.
A plan for insuring that proper referrals are made following screening to ensure accurate diagnosis, effective treatment, and follow-up should be in place. To date, there is ample evidence that patients benefit from screening in the context of an interdisciplinary treatment approach [34]. Collaborative care, utilizing a team of health professionals (physician, case manager trained in working with patients with psychological distress, and mental health specialist) working with the patient, is an ideal model to improve outcomes [44]. A Cochrane review of 79 RCTs with over 24,000 participants compared utilization of a collaborative team with routine care and found decreased depression and anxiety symptoms in patients receiving team care for up 2 years, along with improved medication adherence, improved quality of life, and improved patient satisfaction with care [39].
Conclusion
Psychological distress remains underrecognized in CVD patients. Brief screening tools such as the PHQ-2 and PHQ-9 are available, easy to use, and reliable for use by clinicians to improve case finding. Primary care providers and cardiovascular specialty providers are called upon to improve the recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment of mental health issues. Longitudinal studies focused on the impact of primary/secondary/tertiary psychological distress prevention strategies in the general population, as well as those with CVD, are needed to bring the state of the science forward and provide evidence to enhance the care of those with psychological distress and CVD.
Corresponding author: Anthony McGuire, RN, PhD, ACNP-BC, CSULB, School of Nursing, #2 1250 Bellflower Blvd., Long Beach, CA 90804, [email protected].
Financial disclosures: None
Author contributions: conception and design, AWM, EA, LVD; drafting of article, AWM, EA, LVD; critical revision of the article, AWM, EA, LVD; collection and assembly of data, AWM, EA, LVD.
1. Dembroski TM, MacDougall JM, Shields JL, et al. Components of the type A coronary-prone behavior pattern and cardiovascular responses to psychomotor performance challenge. J Behav Med 1978;1:159–76.
2. Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation 2005;111:250–3.
3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292.
4. Mathers C, Stevens G, Mascarenhas M. Global health risks: Mortality and burden of diesease attributable to select major risks. Geneva, Switzerland: World Health Organization, 2009.
5. Randall G, Molloy GJ, Steptoe A. The impact of an acute cardiac event on the partners of patients: A systematic review. Health Psychol Rev 2009;3:1–84.
6. Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006;21:1178–83.
7. Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. J Psychosom Res 2007;62:401–10.
8. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802–13.
9. van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814–22.
10. Doyle F, McGee HM, Conroy RM, Delaney M. What predicts depression in cardiac patients: sociodemographic factors, disease severity or theoretical vulnerabilities? Psychol Health 2011;26:619–34.
11. Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med 2009;122:704–12.
12. Matthews KA. Matters of the heart: advancing psychological perspectives on cardiovascular diseases. Persp Psychol Sci 2013;8:676–8.
13. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol 2013;9:327–54.
14. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation 2011;124:346–54.
15. Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol 2008;52:2156–62.
16. Richardson S, Shaffer JA, Falzon L, et al. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol 2012;110:1711–6.
17. Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70.
18. Brumby S, Chandrasekara A, McCoombe S, et al. Cardiovascular risk factors and psychological distress in Australian farming communities. Aust J Rural Health 2012;20:131–7.
19. Arnold SV, Smolderen KG, Buchanan DM, et al. Perceived stress in myocardial infarction long-term mortality and health status outcomes. J Am Coll Cardiol 2012;60:1756–63.
20. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 2015.
21. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
22. Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006;68:645–50.
23. Niranjan A, Corujo A, Ziegelstein RC, Nwulia E. Depression and heart disease in US adults. Gen Hosp Psychiatry 2012;34:254–61.
24. Seldenrijk A, Vogelzangs N, Batelaan NM, et al. Depression, anxiety and 6-year risk of cardiovascular disease. J Psychosom Res 2015;78:123–9.
25. Ayerbe L, Ayis S, Crichton S, et al. The natural history of depression up to 15 years after stroke: The South London Stroke Register. Stroke 2013.
26. Lichtman JH, Bigger JT Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008;118:1768–75.
27. Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69.
28. Hasnain M, Vieweg WV, Lesnefsky EJ, Pandurangi AK. Depression screening in patients with coronary heart disease: a critical evaluation of the AHA guidelines. J Psychosom Res 2011;71:6–12.
29. Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol 2009;54:886–90.
30. Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005;67:393–7.
31. Whooley MA. To screen or not to screen? Depression in patients with cardiovascular disease. J Am Coll Cardiol 2009;54:891–3.
32. Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med 2010;170:600–8.
33. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–16.
34. Whooley M, Unutzer J. Interdisciplinary stepped care for depression after acute coronary syndrome. Arch Intern Med 2010;170:585–6.
35. McGuire AW, Eastwood JA, Macabasco-O’Connell A, et al. Depression screening: utility of the patient health questionnaire in patients with acute coronary syndrome. Am J Crit Care 2013;22:12–9.
36. Sowden G, Mastromauro CA, Januzzi JL, et al. Detection of depression in cardiac inpatients: feasibility and results of systematic screening. Am Heart J 2010;159:780–7.
37. Bigger JT, Glassman AH. The American Heart Association science advisory on depression and coronary heart disease: an exploration of the issues raised. Cleve Clin J Med 2010;77 Suppl 3:S12–9.
38. Page KN, Davidson P, Edward KL, et al. Recovering from an acute cardiac event--the relationship between depression and life satisfaction. J Clin Nurs 2010;19:736–43.
39. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525.
40. Blumenthal JA, O’Connor C. No laughing matter. J Am Coll Cardiol 2010;55:836.
41. Davidson KW, Korin MR. Depression and cardiovascular disease: selected findings, controversies, and clinical implications from 2009. Cleve Clin J Med 2010;77 Suppl 3:S20–6.
42. Doering LV, McGuire A, Eastwood JA, et al. Cognitive behavioral therapy for depression improves pain and perceived control in cardiac surgery patients. Eur J Cardiovasc Nurs 2015.
43. Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry 2009;66:387–96.
44. Huffman JC, Mastromauro CA, Sowden GL, et al. collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics 2011;52:26–33.
45. Hwang B, Eastwood JA, McGuire A, et al. Cognitive behavioral therapy in depressed cardiac surgery patients: role of ejection fraction. J Cardiovasc Nurs 2015;30:319–24.
46. Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depression Anxiety 2013;30:328–41.
47. Zuidersma M, Ormel J, Conradi HJ, de Jonge P. An increase in depressive symptoms after myocardial infarction predicts new cardiac events irrespective of depressive symptoms before myocardial infarction. Psychol Med 2012;42:683–93.
48. Banankhah SK, Friedmann E, Thomas S. Effective treatment of depression improves post-myocardial infarction survival. World J Cardiol 2015;7:215–23.
49. Ayerbe L, Ayis S, Crichton S, et al. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry 2014;85:514–21.
50. Hama S, Yamashita H, Yamawaki S, Kurisu K. Post-stroke depression and apathy: Interactions between functional recovery, lesion location, and emotional response. Psychogeriatrics 2011;11:68–76.
51. Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2013;35:23–39.
52. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag 2013;9:483–9.
53.Karamchandani R, Vahidy F, Bajgur S, et al. Early Depression Screening is Feasible in Hospitalized Stroke Patients. Neurology 2014;82(10 Supplement):S62.005.
54. El Husseini N, Goldstein LB, Peterson ED, et al. Depression and antidepressant use after stroke and transient ischemic attack. Stroke 2012;43:1609–16.
55. D’Aniello GE, Scarpina F, Mauro A, Mori I, et al. Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J Neurol Sci 2014;338:191–6.
56. Huffman JC, Smith FA, Blais MA, et al. Anxiety, independent of depressive symptoms, is associated with in-hospital cardiac complications after acute myocardial infarction. J Psychosom Res 2008;65:557–63.
57. Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men: the unique contribution of anxiety among psychologic factors. J Am Coll Cardiol 2008;51:113–9.
58. Butnoriene J, Bunevicius A, Saudargiene A, et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol 2015;190:360–6.
59. Roest AM, Zuidersma M, de Jonge P. Myocardial infarction and generalised anxiety disorder: 10-year follow-up. Br J Psychiatry 2012;200:324–9.
60. Doering LV, Moser DK, Riegel B, et al. Persistent comorbid symptoms of depression and anxiety predict mortality in heart disease. Int J Cardiol 2010;145:188–92.
61. Edmondson D, Kronish IM, Shaffer JA, et al. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 2013;166:806–14.
62. Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 2011;108:29–33.
63. Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol 2012;31:194–201.
64. Chung MC, Dennis I, Berger Z, et al. Posttraumatic stress disorder following myocardial infarction: personality, coping, and trauma exposure characteristics. Int J Psychiatry Med 2011;42:393–419.
65. Bluvstein I, Moravchick L, Sheps D, et al. Posttraumatic growth, posttraumatic stress symptoms and mental health among coronary heart disease survivors. J Clin Psychol Med Settings 2013;20:164–72.
66. Huffman JC, Smith FA, Blais MA, et al. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol 2006;98:319–24.
67. Crosson JC, Heisler M, Subramanian U, et al. Physicians’ perceptions of barriers to cardiovascular disease risk factor control among patients with diabetes: results from the translating research into action for diabetes (TRIAD) study. J Am Board Fam Med 2010;23:171–8.
68. Amin AA, Jones AM, Nugent K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006;152:928–34.
69. Chung MC, Berger Z, Jones R, Rudd H. Posttraumatic stress and co-morbidity following myocardial infarction among older patients: the role of coping. Aging Ment Health 2008;12:124–33.
70. Neylon A, Canniffe C, Anand S, et al. A global perspective on psychosocial risk factors for cardiovascular disease. Prog Cardiovasc Dis 2013;55:574–81.
71. Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med 2012;74:33–8.
72. McGuire AW, Eastwood JA, Hays RD, Macabasco-O’Connell A, et al. Depressed or not depressed: untangling symptoms of depression in patients hospitalized with coronary heart disease. Am J Crit Care 2014;23:106–16.
73. Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005;36:635–8.
74. Junehag L, Asplund K, Svedlund M. A qualitative study: perceptions of the psychosocial consequences and access to support after an acute myocardial infarction. Intensive Crit Care Nurs 2014;30:22–30.
75. Lea P. Factors affecting nurses’ intent to assess for depression in heart failure patients. Dimens Crit Care Nurs 2014;33:320–6.
From the California State University, Long Beach, School of Nursing, Long Beach, CA (Dr. McGuire, Ms. Ahearn), and the University of California, Los Angeles, School of Nursing, Los Angeles, CA (Dr. Doering).
Abstract
- Objective: To review the current literature regarding psychological distress in patients with cardiovascular disease (CVD).
- Methods: Relevant and current (2005–2015) studies were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases along with manual extraction from the reference lists of pertinent studies. Narrative and tabular summaries of the findings are reported.
- Results: There is a vast literature on psychological distress and CVD. Depression is the most common disorder studied followed by anxiety and post-traumatic stress disorder. Physiologic mechanisms linking psychological distress to CVD are well theorized. Screening for psychological distress in CVD is recommended. Referral and treatment issues need further exploration. Pharmacologic treatment of psychological distress in CVD remains equivocal; however, promising data exists for other therapies such as cognitive behavioral therapy and social support strategies.
- Conclusion: Psychological distress has a significant negative impact on patients with CVD and is underrecognized by health care providers. Primary care providers and cardiovascular specialty providers are called upon to improve their recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment.
The association between the heart and the mind has been proposed by scientists since the 17th century. However, it was not until the 1970s that the relationship between cardiovascular disease (CVD) and psychological states came into scientific focus. The study of heart-psyche interactions began with investigations of cardiovascular risk and “type A” personality behaviors (aggressiveness, impatience, a sense of time-urgency, intense achievement drive, seeking recognition) [1,2]. Hundreds of studies generated over the last 10 years have yielded an extensive body of literature regarding this complex interaction.
CVD continues to be the leading cause of death globally. Worldwide and in the United States, CVD accounts for 30% of deaths and more than 2000 deaths per day, respectively [3,4]. Psychological distress (specifically depression) has been reported by the World Health Organization (WHO) as the leading cause of disability in the world [4]. Taken together, CVD and depression constitute an immense health burden and result in poor health status, increased care giver burden [5], increased readmission rates to hospitals, increased utilization of primary care services, poor health compliance [6], decreased health related quality of life [7], and a greater than 2 times increase in mortality [8,9].
Despite its devastating consequences, comorbid CVD and psychological distress remains poorly recognized and treated. In this paper, we present a review of the evidence related to key aspects of psychological distress and CVD (for the purposes of this paper, defined as ischemic heart disease and stroke), and provide information to help improve identification among health care providers. Relevant and current (2005-2015) studies for this review were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases, along with manual extraction from the reference lists of pertinent studies. Due to the breadth and extent of the literature, a comprehensive review of the literature is beyond the scope of this article. However, the reader will be directed to current systematic reviews, meta-analyses, and recent select research studies sourced for this summary and presented in tabular form.
Mechanisms of Psychological Distress
Psychological Distress Disorders Related to CVD
Depression, anxiety, and post-traumatic stress disorder (PTSD) are the 3 most common psychological distress disorders related to CVD [20]. Cardiac disease and depression has been most commonly studied. In stroke, the science is not as well evolved due to greater heterogeneity of study samples and outcome measures.
Depression
Dysphoria (feeling blue), anhedonia (inability to experience joy in otherwise enjoyable activities), insomnia or hypersomnia, fatigue or loss of energy, increased guilt or worthlessness, decreased concentration, appetite change with significant weight loss or gain, psychomotor retardation or agitation, and suicidal ideation are the symptoms of depression [21]. These symptoms exist on a continuum, ranging from mild symptoms with short duration and limited functional impairment to major depression. Importantly, among otherwise healthy individuals, even minor depressive symptoms have been significantly associated with increased incidence of coronary disease [22].
Screening Issues
In recognition of the high prevalence of depression in patients with CHD, an American Heart Association (AHA) science advisory in 2008 recommended routine screening for depression in patients with CHD, with follow-up evaluation for diagnosis and treatment of depression by qualified professionals for positive cases [26]. In 2014, an AHA scientific statement recommended elevating depression to the level of a risk factor in ACS patients [27]. The recommendation for screening was initially met with some concern as being premature [28], when past supporters spoke out against the routine screening of depression in cardiac patients [29]. The dissenting authors claimed that there was a lack of scientific evidence supporting the efficacy of treatment for depression in cardiac patients, and that potential negative effects of routine screening and follow-up treatment were unknown. They argued the following: limited data from randomized controlled trials and/or evidenced-based reviews exist demonstrating improved outcomes in cardiac patients based on screening and referral [30]; antidepressants are not yet recognized to be effective in cardiac populations and there is a lack of evidence related to potential harms [28]; concerns exist about the potential for mass screening to increase health care resource use at the expense of other health care needs [29]; and routine screening may cause unnecessary negative social stigma related to false-positive findings [31].
Although clinical trials of depression treatment in cardiac patients have not demonstrated an increase in survival, treatment has been shown to be effective in reducing depression symptoms, improving patient satisfaction with depression care and improving health related quality of life [32–34]. Further, recent studies described the AHA recommendation as well accepted by cardiac unit staff, not heavily resource intensive, feasible, and accurate [35,36]. Bigger and Glassman [37] published a recent analytical review of the AHA advisory and concluded that the advisory is supported by the literature. A salient point regarding the depression screening debate is that screening without proper follow-up for further diagnosis and potential treatment may be harmful [28,29,31]. Despite concerns of the potential negative impact of depression screening in cardiac patients raised in the literature, the preponderance of the literature indicates that its benefits are likely to outweigh its risks [32,34,36,38–40].
Outcomes of Depression Treatment
Answers to questions about improvement in cardiovascular and all-cause mortality outcomes with depression treatment remain elusive in the literature. However, data show an improvement in depressive symptoms and quality of life for depressed patients receiving some types of treatment [33,41–45]. The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study was a landmark study of MI patients with a 6-month treatment intervention of cognitive behavioral therapy (CBT) plus pharmacologic intervention if indicated for depression [33]. Patients were followed for an average of 29 months post-MI. A significant improvement was seen in depressive symptoms and social isolation in the treatment group; however, there was no improvement in event-free survival [33]. When outcome measures are restricted to mortality alone, subsequent trials of antidepressant medications for treatment of depression in cardiac patients have shown them to be ineffective [46]. However, CBT and other supportive stress management strategies are effective in decreasing depressive symptoms and improving the quality of life in patients suffering with depression and CVD [46].
Promising results are emerging in the literature as researchers refocus their analysis on subgroups of depressed cardiac patients. In one large study of 442 depressed and 325 non-depressed patients, the number of depressive symptoms after an MI irrespective of the pre-MI depression status was associated with worse cardiac outcomes [47]. For every 1 additional depressive symptom reported 1 year post-MI, patients had a 15% increased risk for a new cardiac event in the next 2.5 years [47]. Another study demonstrated an improvement in depressive symptoms by 75.3% in in post-cardiac surgery patients with low ejection fraction (< 40%) after 8 weeks of nurse-guided CBT and worsening in depressive symptoms by 26.8% in usual care patients. More moderate findings were seen in the those with higher ejection fraction receiving the same CBT intervention for depression [45]. A treatment-resistant depression subgroup analyzed in a recent secondary analysis of the ENRICHD trial showed a twofold increase in mortality when compared to those in the non–treatment-resistant depression group [48]. Since treatment does not work for all patients with depression, including depressed post-MI patients, further evaluation with a focus on those who respond to treatment is needed.
Depression in Stroke
Issues related to treatment of stroke patients parallel those of depressed patients with cardiac disease, as the effect on mortality and survival is unknown. Depression has been reported to go untreated in up to 67.9% of depressed post-stroke patients [54]. In addition, mismatches between antidepressant prescription and those with depression suggested that some patients without depression were being treated for depression while some patients with depression were not being treated [54].
Anxiety
Anxiety disorders create behavioral disturbances of fear and avoidance related to an individual’s propensity to overestimate dangers [21]. Though a number of anxiety disorders are described in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), anxiety as a general constellation of symptoms (afraid, inability to relax, worry about everyday problems, feelings of panic) is described in most of the literature related to CVD [55–57]. To a lesser extent, generalized anxiety disorder (GAD), defined as anxiety symptoms on more days than not and lasting more than 6 months [58,59] has also been studied. Because anxiety can be a component of depression, the 2 are often discussed together. Taken together, in the context of CHD, individuals with both anxiety and depression are at significantly greater risk for death (odds ratio 2.35, 95% confidence interval 1.23-4.47, P =0.01), compared with those without symptoms [60].
Post-Traumatic Stress Disorder
PTSD presents with a heterogeneous cluster of symptoms that are generally described as avoidance, re-experiencing, arousal, and negative cognitions and mood [21]. Previously classified as a anxiety disorder in DSM-IV, PTSD is in a new category of trauma and stress or related disorders in the revised DSM-V [21]. In addition, the temporal component of the symptoms has been changed to a disturbance lasting more than 1 month, without reference to acuity or chronicity.
Implications for Clinical Practice
Despite the extensive body of literature regarding the negative association of psychological distress to health outcomes in CVD patients, there remains a significant practice gap related to screening, referral, and treatment of psychological distress in CVD patients [30,36,66]. Busy clinical practices focused on physical symptoms (which may not be recognized as mental health–related), along with a health system that has historically regarded mental health issues as the sole domain of mental health professionals creates barriers that need to be overcome. Many health care providers are not proactive in screening patients for psychological distress [11]. Additionally, psychological distress is often perceived as a metaphysical, inexact phenomenon, and is not regarded with the same import as physiological indices, such blood pressure and lipid levels [13]. Another significant barrier may be that the importance of psychological distress, especially depression, has been minimized by some investigators and clinicians because of the lack of data that show improvement with treatment of “hard” outcomes, such as mortality. Lastly, no studies have conclusively demonstrated that treating depression in the general population would lower subsequent cardiovascular clinical events, adding to the minimization of the importance of screening, referral, and treatment of psychological distress among clinicians.
The challenges associated with psychological distress and CVD are centered on the perceived role of the health care provider and role of the patient [11,67]. To date, identification of patients with psychological distress in CVD populations has not been considered a part of routine practice, and only a small percent of those identified with psychological distress are treated. In one study, 17.6% of 1181 patients had moderate to severe depression and of those, only 24.5% were recognized as depressed by their health care providers [68]. In a smaller study of 35 patients with depression after an acute MI, only 10% received treatment with antidepressants [66]. Similarly poor treatment rates were seen in a recent study of antidepressant use after stroke and transient ischemic attack (TIA), with 67.9% of stroke patients and 70.0% of TIA patients with persistent depression going untreated [54].
Stress, depression, anxiety, and PTSD are often not self-contained or experienced in isolation. Rather, some or all of these conditions may present as an interconnected phenomenon [69,70]. There are several reasons for this. First, symptom identification relies on self-report and/or observation of the symptoms, which may confound validity and reliability of diagnosis [70]. Second, the conditions share some symptoms, which may complicate diagnosis of a primary condition. In addition, the overlap of somatic, cognitive, and affective symptoms [71] may deter health care providers from using screening tools that have physical symptoms as part of the screening process. In a recent study of depressed cardiac patients, investigators clustered symptoms and demonstrated that cognitive affective symptoms of depression predict depression in patients with heart disease [72]. Certain events, such as stroke, may make screening especially difficult due to the presence of neurological changes that may complicate the screening process [73]. These issues highlight the need for formalized depression screening tools such as the Patient Health Questionnaire 2-item screening tool (PHQ-2) and/or Patient Health Questionnaire nine-item screening tool (PHQ-9) (Appendix), as recommended by the AHA. The PHQs have been validated in stroke patients and cardiac patients and have been found to be accurate, easy to use, and feasible [35,36,73].
Patients look to their providers for information and security during vulnerable times in their life. In a 2014 study regarding the perceptions of psychosocial consequences and access to support after MI, patients reported a high sense of security regarding being able to contact their providers [74]. Providers in both the primary and acute care settings should use these opportunities to assess for psychological distress. Many inpatient health care providers assume that it is the primary care providers’ responsibility to screen for depression [75]; however, screening should be done in all practice settings caring for patients with CVD. Clinical practice environments should develop policies and procedures to define who, when, and how screening for psychological distress is accomplished using currently available brief screening tools.
A plan for insuring that proper referrals are made following screening to ensure accurate diagnosis, effective treatment, and follow-up should be in place. To date, there is ample evidence that patients benefit from screening in the context of an interdisciplinary treatment approach [34]. Collaborative care, utilizing a team of health professionals (physician, case manager trained in working with patients with psychological distress, and mental health specialist) working with the patient, is an ideal model to improve outcomes [44]. A Cochrane review of 79 RCTs with over 24,000 participants compared utilization of a collaborative team with routine care and found decreased depression and anxiety symptoms in patients receiving team care for up 2 years, along with improved medication adherence, improved quality of life, and improved patient satisfaction with care [39].
Conclusion
Psychological distress remains underrecognized in CVD patients. Brief screening tools such as the PHQ-2 and PHQ-9 are available, easy to use, and reliable for use by clinicians to improve case finding. Primary care providers and cardiovascular specialty providers are called upon to improve the recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment of mental health issues. Longitudinal studies focused on the impact of primary/secondary/tertiary psychological distress prevention strategies in the general population, as well as those with CVD, are needed to bring the state of the science forward and provide evidence to enhance the care of those with psychological distress and CVD.
Corresponding author: Anthony McGuire, RN, PhD, ACNP-BC, CSULB, School of Nursing, #2 1250 Bellflower Blvd., Long Beach, CA 90804, [email protected].
Financial disclosures: None
Author contributions: conception and design, AWM, EA, LVD; drafting of article, AWM, EA, LVD; critical revision of the article, AWM, EA, LVD; collection and assembly of data, AWM, EA, LVD.
From the California State University, Long Beach, School of Nursing, Long Beach, CA (Dr. McGuire, Ms. Ahearn), and the University of California, Los Angeles, School of Nursing, Los Angeles, CA (Dr. Doering).
Abstract
- Objective: To review the current literature regarding psychological distress in patients with cardiovascular disease (CVD).
- Methods: Relevant and current (2005–2015) studies were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases along with manual extraction from the reference lists of pertinent studies. Narrative and tabular summaries of the findings are reported.
- Results: There is a vast literature on psychological distress and CVD. Depression is the most common disorder studied followed by anxiety and post-traumatic stress disorder. Physiologic mechanisms linking psychological distress to CVD are well theorized. Screening for psychological distress in CVD is recommended. Referral and treatment issues need further exploration. Pharmacologic treatment of psychological distress in CVD remains equivocal; however, promising data exists for other therapies such as cognitive behavioral therapy and social support strategies.
- Conclusion: Psychological distress has a significant negative impact on patients with CVD and is underrecognized by health care providers. Primary care providers and cardiovascular specialty providers are called upon to improve their recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment.
The association between the heart and the mind has been proposed by scientists since the 17th century. However, it was not until the 1970s that the relationship between cardiovascular disease (CVD) and psychological states came into scientific focus. The study of heart-psyche interactions began with investigations of cardiovascular risk and “type A” personality behaviors (aggressiveness, impatience, a sense of time-urgency, intense achievement drive, seeking recognition) [1,2]. Hundreds of studies generated over the last 10 years have yielded an extensive body of literature regarding this complex interaction.
CVD continues to be the leading cause of death globally. Worldwide and in the United States, CVD accounts for 30% of deaths and more than 2000 deaths per day, respectively [3,4]. Psychological distress (specifically depression) has been reported by the World Health Organization (WHO) as the leading cause of disability in the world [4]. Taken together, CVD and depression constitute an immense health burden and result in poor health status, increased care giver burden [5], increased readmission rates to hospitals, increased utilization of primary care services, poor health compliance [6], decreased health related quality of life [7], and a greater than 2 times increase in mortality [8,9].
Despite its devastating consequences, comorbid CVD and psychological distress remains poorly recognized and treated. In this paper, we present a review of the evidence related to key aspects of psychological distress and CVD (for the purposes of this paper, defined as ischemic heart disease and stroke), and provide information to help improve identification among health care providers. Relevant and current (2005-2015) studies for this review were retrieved by a series of searches conducted in the PubMed and PsychINFO databases using Boolean terms/phrases, along with manual extraction from the reference lists of pertinent studies. Due to the breadth and extent of the literature, a comprehensive review of the literature is beyond the scope of this article. However, the reader will be directed to current systematic reviews, meta-analyses, and recent select research studies sourced for this summary and presented in tabular form.
Mechanisms of Psychological Distress
Psychological Distress Disorders Related to CVD
Depression, anxiety, and post-traumatic stress disorder (PTSD) are the 3 most common psychological distress disorders related to CVD [20]. Cardiac disease and depression has been most commonly studied. In stroke, the science is not as well evolved due to greater heterogeneity of study samples and outcome measures.
Depression
Dysphoria (feeling blue), anhedonia (inability to experience joy in otherwise enjoyable activities), insomnia or hypersomnia, fatigue or loss of energy, increased guilt or worthlessness, decreased concentration, appetite change with significant weight loss or gain, psychomotor retardation or agitation, and suicidal ideation are the symptoms of depression [21]. These symptoms exist on a continuum, ranging from mild symptoms with short duration and limited functional impairment to major depression. Importantly, among otherwise healthy individuals, even minor depressive symptoms have been significantly associated with increased incidence of coronary disease [22].
Screening Issues
In recognition of the high prevalence of depression in patients with CHD, an American Heart Association (AHA) science advisory in 2008 recommended routine screening for depression in patients with CHD, with follow-up evaluation for diagnosis and treatment of depression by qualified professionals for positive cases [26]. In 2014, an AHA scientific statement recommended elevating depression to the level of a risk factor in ACS patients [27]. The recommendation for screening was initially met with some concern as being premature [28], when past supporters spoke out against the routine screening of depression in cardiac patients [29]. The dissenting authors claimed that there was a lack of scientific evidence supporting the efficacy of treatment for depression in cardiac patients, and that potential negative effects of routine screening and follow-up treatment were unknown. They argued the following: limited data from randomized controlled trials and/or evidenced-based reviews exist demonstrating improved outcomes in cardiac patients based on screening and referral [30]; antidepressants are not yet recognized to be effective in cardiac populations and there is a lack of evidence related to potential harms [28]; concerns exist about the potential for mass screening to increase health care resource use at the expense of other health care needs [29]; and routine screening may cause unnecessary negative social stigma related to false-positive findings [31].
Although clinical trials of depression treatment in cardiac patients have not demonstrated an increase in survival, treatment has been shown to be effective in reducing depression symptoms, improving patient satisfaction with depression care and improving health related quality of life [32–34]. Further, recent studies described the AHA recommendation as well accepted by cardiac unit staff, not heavily resource intensive, feasible, and accurate [35,36]. Bigger and Glassman [37] published a recent analytical review of the AHA advisory and concluded that the advisory is supported by the literature. A salient point regarding the depression screening debate is that screening without proper follow-up for further diagnosis and potential treatment may be harmful [28,29,31]. Despite concerns of the potential negative impact of depression screening in cardiac patients raised in the literature, the preponderance of the literature indicates that its benefits are likely to outweigh its risks [32,34,36,38–40].
Outcomes of Depression Treatment
Answers to questions about improvement in cardiovascular and all-cause mortality outcomes with depression treatment remain elusive in the literature. However, data show an improvement in depressive symptoms and quality of life for depressed patients receiving some types of treatment [33,41–45]. The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study was a landmark study of MI patients with a 6-month treatment intervention of cognitive behavioral therapy (CBT) plus pharmacologic intervention if indicated for depression [33]. Patients were followed for an average of 29 months post-MI. A significant improvement was seen in depressive symptoms and social isolation in the treatment group; however, there was no improvement in event-free survival [33]. When outcome measures are restricted to mortality alone, subsequent trials of antidepressant medications for treatment of depression in cardiac patients have shown them to be ineffective [46]. However, CBT and other supportive stress management strategies are effective in decreasing depressive symptoms and improving the quality of life in patients suffering with depression and CVD [46].
Promising results are emerging in the literature as researchers refocus their analysis on subgroups of depressed cardiac patients. In one large study of 442 depressed and 325 non-depressed patients, the number of depressive symptoms after an MI irrespective of the pre-MI depression status was associated with worse cardiac outcomes [47]. For every 1 additional depressive symptom reported 1 year post-MI, patients had a 15% increased risk for a new cardiac event in the next 2.5 years [47]. Another study demonstrated an improvement in depressive symptoms by 75.3% in in post-cardiac surgery patients with low ejection fraction (< 40%) after 8 weeks of nurse-guided CBT and worsening in depressive symptoms by 26.8% in usual care patients. More moderate findings were seen in the those with higher ejection fraction receiving the same CBT intervention for depression [45]. A treatment-resistant depression subgroup analyzed in a recent secondary analysis of the ENRICHD trial showed a twofold increase in mortality when compared to those in the non–treatment-resistant depression group [48]. Since treatment does not work for all patients with depression, including depressed post-MI patients, further evaluation with a focus on those who respond to treatment is needed.
Depression in Stroke
Issues related to treatment of stroke patients parallel those of depressed patients with cardiac disease, as the effect on mortality and survival is unknown. Depression has been reported to go untreated in up to 67.9% of depressed post-stroke patients [54]. In addition, mismatches between antidepressant prescription and those with depression suggested that some patients without depression were being treated for depression while some patients with depression were not being treated [54].
Anxiety
Anxiety disorders create behavioral disturbances of fear and avoidance related to an individual’s propensity to overestimate dangers [21]. Though a number of anxiety disorders are described in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), anxiety as a general constellation of symptoms (afraid, inability to relax, worry about everyday problems, feelings of panic) is described in most of the literature related to CVD [55–57]. To a lesser extent, generalized anxiety disorder (GAD), defined as anxiety symptoms on more days than not and lasting more than 6 months [58,59] has also been studied. Because anxiety can be a component of depression, the 2 are often discussed together. Taken together, in the context of CHD, individuals with both anxiety and depression are at significantly greater risk for death (odds ratio 2.35, 95% confidence interval 1.23-4.47, P =0.01), compared with those without symptoms [60].
Post-Traumatic Stress Disorder
PTSD presents with a heterogeneous cluster of symptoms that are generally described as avoidance, re-experiencing, arousal, and negative cognitions and mood [21]. Previously classified as a anxiety disorder in DSM-IV, PTSD is in a new category of trauma and stress or related disorders in the revised DSM-V [21]. In addition, the temporal component of the symptoms has been changed to a disturbance lasting more than 1 month, without reference to acuity or chronicity.
Implications for Clinical Practice
Despite the extensive body of literature regarding the negative association of psychological distress to health outcomes in CVD patients, there remains a significant practice gap related to screening, referral, and treatment of psychological distress in CVD patients [30,36,66]. Busy clinical practices focused on physical symptoms (which may not be recognized as mental health–related), along with a health system that has historically regarded mental health issues as the sole domain of mental health professionals creates barriers that need to be overcome. Many health care providers are not proactive in screening patients for psychological distress [11]. Additionally, psychological distress is often perceived as a metaphysical, inexact phenomenon, and is not regarded with the same import as physiological indices, such blood pressure and lipid levels [13]. Another significant barrier may be that the importance of psychological distress, especially depression, has been minimized by some investigators and clinicians because of the lack of data that show improvement with treatment of “hard” outcomes, such as mortality. Lastly, no studies have conclusively demonstrated that treating depression in the general population would lower subsequent cardiovascular clinical events, adding to the minimization of the importance of screening, referral, and treatment of psychological distress among clinicians.
The challenges associated with psychological distress and CVD are centered on the perceived role of the health care provider and role of the patient [11,67]. To date, identification of patients with psychological distress in CVD populations has not been considered a part of routine practice, and only a small percent of those identified with psychological distress are treated. In one study, 17.6% of 1181 patients had moderate to severe depression and of those, only 24.5% were recognized as depressed by their health care providers [68]. In a smaller study of 35 patients with depression after an acute MI, only 10% received treatment with antidepressants [66]. Similarly poor treatment rates were seen in a recent study of antidepressant use after stroke and transient ischemic attack (TIA), with 67.9% of stroke patients and 70.0% of TIA patients with persistent depression going untreated [54].
Stress, depression, anxiety, and PTSD are often not self-contained or experienced in isolation. Rather, some or all of these conditions may present as an interconnected phenomenon [69,70]. There are several reasons for this. First, symptom identification relies on self-report and/or observation of the symptoms, which may confound validity and reliability of diagnosis [70]. Second, the conditions share some symptoms, which may complicate diagnosis of a primary condition. In addition, the overlap of somatic, cognitive, and affective symptoms [71] may deter health care providers from using screening tools that have physical symptoms as part of the screening process. In a recent study of depressed cardiac patients, investigators clustered symptoms and demonstrated that cognitive affective symptoms of depression predict depression in patients with heart disease [72]. Certain events, such as stroke, may make screening especially difficult due to the presence of neurological changes that may complicate the screening process [73]. These issues highlight the need for formalized depression screening tools such as the Patient Health Questionnaire 2-item screening tool (PHQ-2) and/or Patient Health Questionnaire nine-item screening tool (PHQ-9) (Appendix), as recommended by the AHA. The PHQs have been validated in stroke patients and cardiac patients and have been found to be accurate, easy to use, and feasible [35,36,73].
Patients look to their providers for information and security during vulnerable times in their life. In a 2014 study regarding the perceptions of psychosocial consequences and access to support after MI, patients reported a high sense of security regarding being able to contact their providers [74]. Providers in both the primary and acute care settings should use these opportunities to assess for psychological distress. Many inpatient health care providers assume that it is the primary care providers’ responsibility to screen for depression [75]; however, screening should be done in all practice settings caring for patients with CVD. Clinical practice environments should develop policies and procedures to define who, when, and how screening for psychological distress is accomplished using currently available brief screening tools.
A plan for insuring that proper referrals are made following screening to ensure accurate diagnosis, effective treatment, and follow-up should be in place. To date, there is ample evidence that patients benefit from screening in the context of an interdisciplinary treatment approach [34]. Collaborative care, utilizing a team of health professionals (physician, case manager trained in working with patients with psychological distress, and mental health specialist) working with the patient, is an ideal model to improve outcomes [44]. A Cochrane review of 79 RCTs with over 24,000 participants compared utilization of a collaborative team with routine care and found decreased depression and anxiety symptoms in patients receiving team care for up 2 years, along with improved medication adherence, improved quality of life, and improved patient satisfaction with care [39].
Conclusion
Psychological distress remains underrecognized in CVD patients. Brief screening tools such as the PHQ-2 and PHQ-9 are available, easy to use, and reliable for use by clinicians to improve case finding. Primary care providers and cardiovascular specialty providers are called upon to improve the recognition of psychological distress in their patients and assure referrals are made to collaborative care teams for proper diagnosis and treatment of mental health issues. Longitudinal studies focused on the impact of primary/secondary/tertiary psychological distress prevention strategies in the general population, as well as those with CVD, are needed to bring the state of the science forward and provide evidence to enhance the care of those with psychological distress and CVD.
Corresponding author: Anthony McGuire, RN, PhD, ACNP-BC, CSULB, School of Nursing, #2 1250 Bellflower Blvd., Long Beach, CA 90804, [email protected].
Financial disclosures: None
Author contributions: conception and design, AWM, EA, LVD; drafting of article, AWM, EA, LVD; critical revision of the article, AWM, EA, LVD; collection and assembly of data, AWM, EA, LVD.
1. Dembroski TM, MacDougall JM, Shields JL, et al. Components of the type A coronary-prone behavior pattern and cardiovascular responses to psychomotor performance challenge. J Behav Med 1978;1:159–76.
2. Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation 2005;111:250–3.
3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292.
4. Mathers C, Stevens G, Mascarenhas M. Global health risks: Mortality and burden of diesease attributable to select major risks. Geneva, Switzerland: World Health Organization, 2009.
5. Randall G, Molloy GJ, Steptoe A. The impact of an acute cardiac event on the partners of patients: A systematic review. Health Psychol Rev 2009;3:1–84.
6. Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006;21:1178–83.
7. Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. J Psychosom Res 2007;62:401–10.
8. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802–13.
9. van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814–22.
10. Doyle F, McGee HM, Conroy RM, Delaney M. What predicts depression in cardiac patients: sociodemographic factors, disease severity or theoretical vulnerabilities? Psychol Health 2011;26:619–34.
11. Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med 2009;122:704–12.
12. Matthews KA. Matters of the heart: advancing psychological perspectives on cardiovascular diseases. Persp Psychol Sci 2013;8:676–8.
13. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol 2013;9:327–54.
14. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation 2011;124:346–54.
15. Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol 2008;52:2156–62.
16. Richardson S, Shaffer JA, Falzon L, et al. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol 2012;110:1711–6.
17. Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70.
18. Brumby S, Chandrasekara A, McCoombe S, et al. Cardiovascular risk factors and psychological distress in Australian farming communities. Aust J Rural Health 2012;20:131–7.
19. Arnold SV, Smolderen KG, Buchanan DM, et al. Perceived stress in myocardial infarction long-term mortality and health status outcomes. J Am Coll Cardiol 2012;60:1756–63.
20. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 2015.
21. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
22. Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006;68:645–50.
23. Niranjan A, Corujo A, Ziegelstein RC, Nwulia E. Depression and heart disease in US adults. Gen Hosp Psychiatry 2012;34:254–61.
24. Seldenrijk A, Vogelzangs N, Batelaan NM, et al. Depression, anxiety and 6-year risk of cardiovascular disease. J Psychosom Res 2015;78:123–9.
25. Ayerbe L, Ayis S, Crichton S, et al. The natural history of depression up to 15 years after stroke: The South London Stroke Register. Stroke 2013.
26. Lichtman JH, Bigger JT Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008;118:1768–75.
27. Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69.
28. Hasnain M, Vieweg WV, Lesnefsky EJ, Pandurangi AK. Depression screening in patients with coronary heart disease: a critical evaluation of the AHA guidelines. J Psychosom Res 2011;71:6–12.
29. Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol 2009;54:886–90.
30. Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005;67:393–7.
31. Whooley MA. To screen or not to screen? Depression in patients with cardiovascular disease. J Am Coll Cardiol 2009;54:891–3.
32. Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med 2010;170:600–8.
33. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–16.
34. Whooley M, Unutzer J. Interdisciplinary stepped care for depression after acute coronary syndrome. Arch Intern Med 2010;170:585–6.
35. McGuire AW, Eastwood JA, Macabasco-O’Connell A, et al. Depression screening: utility of the patient health questionnaire in patients with acute coronary syndrome. Am J Crit Care 2013;22:12–9.
36. Sowden G, Mastromauro CA, Januzzi JL, et al. Detection of depression in cardiac inpatients: feasibility and results of systematic screening. Am Heart J 2010;159:780–7.
37. Bigger JT, Glassman AH. The American Heart Association science advisory on depression and coronary heart disease: an exploration of the issues raised. Cleve Clin J Med 2010;77 Suppl 3:S12–9.
38. Page KN, Davidson P, Edward KL, et al. Recovering from an acute cardiac event--the relationship between depression and life satisfaction. J Clin Nurs 2010;19:736–43.
39. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525.
40. Blumenthal JA, O’Connor C. No laughing matter. J Am Coll Cardiol 2010;55:836.
41. Davidson KW, Korin MR. Depression and cardiovascular disease: selected findings, controversies, and clinical implications from 2009. Cleve Clin J Med 2010;77 Suppl 3:S20–6.
42. Doering LV, McGuire A, Eastwood JA, et al. Cognitive behavioral therapy for depression improves pain and perceived control in cardiac surgery patients. Eur J Cardiovasc Nurs 2015.
43. Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry 2009;66:387–96.
44. Huffman JC, Mastromauro CA, Sowden GL, et al. collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics 2011;52:26–33.
45. Hwang B, Eastwood JA, McGuire A, et al. Cognitive behavioral therapy in depressed cardiac surgery patients: role of ejection fraction. J Cardiovasc Nurs 2015;30:319–24.
46. Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depression Anxiety 2013;30:328–41.
47. Zuidersma M, Ormel J, Conradi HJ, de Jonge P. An increase in depressive symptoms after myocardial infarction predicts new cardiac events irrespective of depressive symptoms before myocardial infarction. Psychol Med 2012;42:683–93.
48. Banankhah SK, Friedmann E, Thomas S. Effective treatment of depression improves post-myocardial infarction survival. World J Cardiol 2015;7:215–23.
49. Ayerbe L, Ayis S, Crichton S, et al. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry 2014;85:514–21.
50. Hama S, Yamashita H, Yamawaki S, Kurisu K. Post-stroke depression and apathy: Interactions between functional recovery, lesion location, and emotional response. Psychogeriatrics 2011;11:68–76.
51. Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2013;35:23–39.
52. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag 2013;9:483–9.
53.Karamchandani R, Vahidy F, Bajgur S, et al. Early Depression Screening is Feasible in Hospitalized Stroke Patients. Neurology 2014;82(10 Supplement):S62.005.
54. El Husseini N, Goldstein LB, Peterson ED, et al. Depression and antidepressant use after stroke and transient ischemic attack. Stroke 2012;43:1609–16.
55. D’Aniello GE, Scarpina F, Mauro A, Mori I, et al. Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J Neurol Sci 2014;338:191–6.
56. Huffman JC, Smith FA, Blais MA, et al. Anxiety, independent of depressive symptoms, is associated with in-hospital cardiac complications after acute myocardial infarction. J Psychosom Res 2008;65:557–63.
57. Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men: the unique contribution of anxiety among psychologic factors. J Am Coll Cardiol 2008;51:113–9.
58. Butnoriene J, Bunevicius A, Saudargiene A, et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol 2015;190:360–6.
59. Roest AM, Zuidersma M, de Jonge P. Myocardial infarction and generalised anxiety disorder: 10-year follow-up. Br J Psychiatry 2012;200:324–9.
60. Doering LV, Moser DK, Riegel B, et al. Persistent comorbid symptoms of depression and anxiety predict mortality in heart disease. Int J Cardiol 2010;145:188–92.
61. Edmondson D, Kronish IM, Shaffer JA, et al. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 2013;166:806–14.
62. Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 2011;108:29–33.
63. Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol 2012;31:194–201.
64. Chung MC, Dennis I, Berger Z, et al. Posttraumatic stress disorder following myocardial infarction: personality, coping, and trauma exposure characteristics. Int J Psychiatry Med 2011;42:393–419.
65. Bluvstein I, Moravchick L, Sheps D, et al. Posttraumatic growth, posttraumatic stress symptoms and mental health among coronary heart disease survivors. J Clin Psychol Med Settings 2013;20:164–72.
66. Huffman JC, Smith FA, Blais MA, et al. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol 2006;98:319–24.
67. Crosson JC, Heisler M, Subramanian U, et al. Physicians’ perceptions of barriers to cardiovascular disease risk factor control among patients with diabetes: results from the translating research into action for diabetes (TRIAD) study. J Am Board Fam Med 2010;23:171–8.
68. Amin AA, Jones AM, Nugent K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006;152:928–34.
69. Chung MC, Berger Z, Jones R, Rudd H. Posttraumatic stress and co-morbidity following myocardial infarction among older patients: the role of coping. Aging Ment Health 2008;12:124–33.
70. Neylon A, Canniffe C, Anand S, et al. A global perspective on psychosocial risk factors for cardiovascular disease. Prog Cardiovasc Dis 2013;55:574–81.
71. Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med 2012;74:33–8.
72. McGuire AW, Eastwood JA, Hays RD, Macabasco-O’Connell A, et al. Depressed or not depressed: untangling symptoms of depression in patients hospitalized with coronary heart disease. Am J Crit Care 2014;23:106–16.
73. Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005;36:635–8.
74. Junehag L, Asplund K, Svedlund M. A qualitative study: perceptions of the psychosocial consequences and access to support after an acute myocardial infarction. Intensive Crit Care Nurs 2014;30:22–30.
75. Lea P. Factors affecting nurses’ intent to assess for depression in heart failure patients. Dimens Crit Care Nurs 2014;33:320–6.
1. Dembroski TM, MacDougall JM, Shields JL, et al. Components of the type A coronary-prone behavior pattern and cardiovascular responses to psychomotor performance challenge. J Behav Med 1978;1:159–76.
2. Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation 2005;111:250–3.
3. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292.
4. Mathers C, Stevens G, Mascarenhas M. Global health risks: Mortality and burden of diesease attributable to select major risks. Geneva, Switzerland: World Health Organization, 2009.
5. Randall G, Molloy GJ, Steptoe A. The impact of an acute cardiac event on the partners of patients: A systematic review. Health Psychol Rev 2009;3:1–84.
6. Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006;21:1178–83.
7. Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. J Psychosom Res 2007;62:401–10.
8. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802–13.
9. van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814–22.
10. Doyle F, McGee HM, Conroy RM, Delaney M. What predicts depression in cardiac patients: sociodemographic factors, disease severity or theoretical vulnerabilities? Psychol Health 2011;26:619–34.
11. Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med 2009;122:704–12.
12. Matthews KA. Matters of the heart: advancing psychological perspectives on cardiovascular diseases. Persp Psychol Sci 2013;8:676–8.
13. Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol 2013;9:327–54.
14. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation 2011;124:346–54.
15. Hamer M, Molloy GJ, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol 2008;52:2156–62.
16. Richardson S, Shaffer JA, Falzon L, et al. Meta-analysis of perceived stress and its association with incident coronary heart disease. Am J Cardiol 2012;110:1711–6.
17. Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol 2012;9:360–70.
18. Brumby S, Chandrasekara A, McCoombe S, et al. Cardiovascular risk factors and psychological distress in Australian farming communities. Aust J Rural Health 2012;20:131–7.
19. Arnold SV, Smolderen KG, Buchanan DM, et al. Perceived stress in myocardial infarction long-term mortality and health status outcomes. J Am Coll Cardiol 2012;60:1756–63.
20. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens 2015.
21. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
22. Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006;68:645–50.
23. Niranjan A, Corujo A, Ziegelstein RC, Nwulia E. Depression and heart disease in US adults. Gen Hosp Psychiatry 2012;34:254–61.
24. Seldenrijk A, Vogelzangs N, Batelaan NM, et al. Depression, anxiety and 6-year risk of cardiovascular disease. J Psychosom Res 2015;78:123–9.
25. Ayerbe L, Ayis S, Crichton S, et al. The natural history of depression up to 15 years after stroke: The South London Stroke Register. Stroke 2013.
26. Lichtman JH, Bigger JT Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008;118:1768–75.
27. Lichtman JH, Froelicher ES, Blumenthal JA, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350–69.
28. Hasnain M, Vieweg WV, Lesnefsky EJ, Pandurangi AK. Depression screening in patients with coronary heart disease: a critical evaluation of the AHA guidelines. J Psychosom Res 2011;71:6–12.
29. Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol 2009;54:886–90.
30. Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005;67:393–7.
31. Whooley MA. To screen or not to screen? Depression in patients with cardiovascular disease. J Am Coll Cardiol 2009;54:891–3.
32. Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med 2010;170:600–8.
33. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106–16.
34. Whooley M, Unutzer J. Interdisciplinary stepped care for depression after acute coronary syndrome. Arch Intern Med 2010;170:585–6.
35. McGuire AW, Eastwood JA, Macabasco-O’Connell A, et al. Depression screening: utility of the patient health questionnaire in patients with acute coronary syndrome. Am J Crit Care 2013;22:12–9.
36. Sowden G, Mastromauro CA, Januzzi JL, et al. Detection of depression in cardiac inpatients: feasibility and results of systematic screening. Am Heart J 2010;159:780–7.
37. Bigger JT, Glassman AH. The American Heart Association science advisory on depression and coronary heart disease: an exploration of the issues raised. Cleve Clin J Med 2010;77 Suppl 3:S12–9.
38. Page KN, Davidson P, Edward KL, et al. Recovering from an acute cardiac event--the relationship between depression and life satisfaction. J Clin Nurs 2010;19:736–43.
39. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10:CD006525.
40. Blumenthal JA, O’Connor C. No laughing matter. J Am Coll Cardiol 2010;55:836.
41. Davidson KW, Korin MR. Depression and cardiovascular disease: selected findings, controversies, and clinical implications from 2009. Cleve Clin J Med 2010;77 Suppl 3:S20–6.
42. Doering LV, McGuire A, Eastwood JA, et al. Cognitive behavioral therapy for depression improves pain and perceived control in cardiac surgery patients. Eur J Cardiovasc Nurs 2015.
43. Freedland KE, Skala JA, Carney RM, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Arch Gen Psychiatry 2009;66:387–96.
44. Huffman JC, Mastromauro CA, Sowden GL, et al. collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics 2011;52:26–33.
45. Hwang B, Eastwood JA, McGuire A, et al. Cognitive behavioral therapy in depressed cardiac surgery patients: role of ejection fraction. J Cardiovasc Nurs 2015;30:319–24.
46. Mavrides N, Nemeroff C. Treatment of depression in cardiovascular disease. Depression Anxiety 2013;30:328–41.
47. Zuidersma M, Ormel J, Conradi HJ, de Jonge P. An increase in depressive symptoms after myocardial infarction predicts new cardiac events irrespective of depressive symptoms before myocardial infarction. Psychol Med 2012;42:683–93.
48. Banankhah SK, Friedmann E, Thomas S. Effective treatment of depression improves post-myocardial infarction survival. World J Cardiol 2015;7:215–23.
49. Ayerbe L, Ayis S, Crichton S, et al. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry 2014;85:514–21.
50. Hama S, Yamashita H, Yamawaki S, Kurisu K. Post-stroke depression and apathy: Interactions between functional recovery, lesion location, and emotional response. Psychogeriatrics 2011;11:68–76.
51. Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2013;35:23–39.
52. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag 2013;9:483–9.
53.Karamchandani R, Vahidy F, Bajgur S, et al. Early Depression Screening is Feasible in Hospitalized Stroke Patients. Neurology 2014;82(10 Supplement):S62.005.
54. El Husseini N, Goldstein LB, Peterson ED, et al. Depression and antidepressant use after stroke and transient ischemic attack. Stroke 2012;43:1609–16.
55. D’Aniello GE, Scarpina F, Mauro A, Mori I, et al. Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J Neurol Sci 2014;338:191–6.
56. Huffman JC, Smith FA, Blais MA, et al. Anxiety, independent of depressive symptoms, is associated with in-hospital cardiac complications after acute myocardial infarction. J Psychosom Res 2008;65:557–63.
57. Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men: the unique contribution of anxiety among psychologic factors. J Am Coll Cardiol 2008;51:113–9.
58. Butnoriene J, Bunevicius A, Saudargiene A, et al. Metabolic syndrome, major depression, generalized anxiety disorder, and ten-year all-cause and cardiovascular mortality in middle aged and elderly patients. Int J Cardiol 2015;190:360–6.
59. Roest AM, Zuidersma M, de Jonge P. Myocardial infarction and generalised anxiety disorder: 10-year follow-up. Br J Psychiatry 2012;200:324–9.
60. Doering LV, Moser DK, Riegel B, et al. Persistent comorbid symptoms of depression and anxiety predict mortality in heart disease. Int J Cardiol 2010;145:188–92.
61. Edmondson D, Kronish IM, Shaffer JA, et al. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 2013;166:806–14.
62. Ahmadi N, Hajsadeghi F, Mirshkarlo HB, et al. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 2011;108:29–33.
63. Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol 2012;31:194–201.
64. Chung MC, Dennis I, Berger Z, et al. Posttraumatic stress disorder following myocardial infarction: personality, coping, and trauma exposure characteristics. Int J Psychiatry Med 2011;42:393–419.
65. Bluvstein I, Moravchick L, Sheps D, et al. Posttraumatic growth, posttraumatic stress symptoms and mental health among coronary heart disease survivors. J Clin Psychol Med Settings 2013;20:164–72.
66. Huffman JC, Smith FA, Blais MA, et al. Recognition and treatment of depression and anxiety in patients with acute myocardial infarction. Am J Cardiol 2006;98:319–24.
67. Crosson JC, Heisler M, Subramanian U, et al. Physicians’ perceptions of barriers to cardiovascular disease risk factor control among patients with diabetes: results from the translating research into action for diabetes (TRIAD) study. J Am Board Fam Med 2010;23:171–8.
68. Amin AA, Jones AM, Nugent K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006;152:928–34.
69. Chung MC, Berger Z, Jones R, Rudd H. Posttraumatic stress and co-morbidity following myocardial infarction among older patients: the role of coping. Aging Ment Health 2008;12:124–33.
70. Neylon A, Canniffe C, Anand S, et al. A global perspective on psychosocial risk factors for cardiovascular disease. Prog Cardiovasc Dis 2013;55:574–81.
71. Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med 2012;74:33–8.
72. McGuire AW, Eastwood JA, Hays RD, Macabasco-O’Connell A, et al. Depressed or not depressed: untangling symptoms of depression in patients hospitalized with coronary heart disease. Am J Crit Care 2014;23:106–16.
73. Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005;36:635–8.
74. Junehag L, Asplund K, Svedlund M. A qualitative study: perceptions of the psychosocial consequences and access to support after an acute myocardial infarction. Intensive Crit Care Nurs 2014;30:22–30.
75. Lea P. Factors affecting nurses’ intent to assess for depression in heart failure patients. Dimens Crit Care Nurs 2014;33:320–6.
ESC: Celecoxib safety study may soothe cardio concerns
LONDON – Celecoxib was associated with very low cardiovascular event rates, and its use posed no more risk than other painkillers commonly used to treat elderly individuals with arthritic conditions but no heart disease in a large, pragmatic, family practice–based study.
Results of the Standard Care Versus Celecoxib Outcome Trial (SCOT) reported at the annual congress of the European Society of Cardiology also showed that celecoxib was no more likely than nonselective nonsteroidal anti-inflammatory drugs (nsNSAIDs) to cause ulcer-related upper gastrointestinal (GI) tract complications.
In fact, the rates of both cardiovascular and GI events were so low overall that it made the trial difficult to complete, said study investigator Dr. Tom MacDonald, professor of clinical pharmacology and pharmacoepidemiology at the University of Dundee (Scotland), which sponsored the study.
The on-treatment and intention-to-treat (ITT) cardiovascular event rates were 0.9% and 1.1% per 100 patient-years, he observed, adding that he would have expected the event rate to be around 2%-3% in the population studied. GI complication rates were even lower, with just 12 on-treatment and 15 ITT events reported during the entire follow-up period, which was a maximum of 6.3 years and mean of about 3 years.
“You may remember the brouhaha surrounding the use of rofecoxib and other [cyclo-oxygenase-2 inhibitors],” said Dr. MacDonald. Both coxibs and nsNSAIDs have been associated with adverse cardiovascular outcomes such as myocardial infarction (BMJ 2005;330:1366), and rofecoxib was voluntarily withdrawn in 2004 by its manufacturer from the U.S. market. A recent meta-analysis (Lancet 2013;382:769-79) has suggested that coxibs increase the risk of major cardiovascular events by about 37%.
The SCOT study (BMJ Open 2013;3:e002295) was designed to assess if celecoxib was better, worse, or the same as the other available NSAIDs in terms of its cardiovascular and gastrointestinal safety. It was originally set up because of a requirement by the European Medicines Agency, Dr. McDonald explained.
More than 9,400 patients aged 60 years or older with osteoarthritis or rheumatoid arthritis who were prescribed chronic NSAID therapy and had no existing cardiovascular disease were screened at 706 family practices in Scotland, England, Denmark, and the Netherlands. A total of 7,297 patients were included in the prospective study and were randomized to switch to treatment with celecoxib or to continue their current nsNSAID.
General practice records were linked to hospital and mortality databases to derive the primary composite endpoint of the first occurrence of hospitalization for nonfatal MI, nonfatal stroke, or cardiovascular death, as well as secondary endpoints such as time to first hospitalization or death from upper GI complications and all-cause mortality.
Randomized patients were about 68 years old, and about 40% of patients were male. Dr. MacDonald noted that, although there was no known existing cardiovascular disease at enrollment, the baseline characteristics showed that around 44% of patients had high blood pressure; a third of patients had high cholesterol; and 20%, 12%, and 38% were taking a statin, aspirin, or ulcer-healing treatments, respectively. The most common nsNSAIDs being used were diclofenac (38.7%) and ibuprofen (31%).
There was no significant difference between celecoxib or nsNSAIDs for any of the cardiovascular endpoints studied, with hazard ratios (HR) for the primary composite cardiovascular endpoints of 1.12 (95% confidence interval, 0.81-1.55; P = .5) while on celecoxib treatment and 1.04 (95% CI, 0.81-1.33; P = .75) in the ITT analysis. Similar results were obtained for all-cause mortality (HR, 1.2 and 0.92, respectively).
Dr. MacDonald reported that 50% of patients randomized to celecoxib and 30% randomized to continue nsNSAIDs withdrew from the study. The main reasons for stopping celecoxib were a lack of efficacy (11.2% vs. 2% for nsNSAIDs), adverse events (8.3% vs. 4.4%), patient request (6% vs. 2.3%), not tolerated (3.9% vs. 1.2%), or a serious adverse event (2.6% vs. 1.9%). There was, however, a lot of adverse publicity about the coxibs, he noted, and patients who had been happy on an nsNSAID might not have been happy with the switch.
The rates of serious cardiovascular adverse events (31.7% vs. 32.4%) or reactions (5.2% vs. 5.8%) were similar with celecoxib and nsNSAIDs, but there were significantly fewer serious GI adverse reactions with celecoxib than with nsNSAIDs (38 vs. 66; P = .007). Overall, the adverse reaction rate was 22% vs. 16.1%, respectively (P <.001).
“In the study population, nsNSAIDs and celecoxib both appeared acceptably safe,” Dr. MacDonald concluded. “In patients who get significant symptomatic relief from these medicines, the benefit/risk balance appears positive.”
Although the findings are perhaps reassuring, they are unlikely to change clinical practice, observed Dr. José López-Sendon, who was invited to comment on the study results after their presentation at the conference.
The study findings suggest that celecoxib may continue to be safe to use in patients without existing cardiac disease, noted Dr. López-Sendon of Hospital Universitario La Paz in Madrid, but he would not modify the guidelines that advise that the lowest effective dose be used for the shortest duration of time in low-risk patients.
The study was sponsored by the University of Dundee and funded by an investigator-initiated research grant from Pfizer. The university’s Medicines Monitoring Unit also holds research grants from Amgen, Menarini, and Novartis. Dr. MacDonald has consulted on the use of NSAIDs for AstraZeneca, NiCox, Novartis, and Pfizer. Dr. López-Sendon did not have any disclosures relevant to his comments.
LONDON – Celecoxib was associated with very low cardiovascular event rates, and its use posed no more risk than other painkillers commonly used to treat elderly individuals with arthritic conditions but no heart disease in a large, pragmatic, family practice–based study.
Results of the Standard Care Versus Celecoxib Outcome Trial (SCOT) reported at the annual congress of the European Society of Cardiology also showed that celecoxib was no more likely than nonselective nonsteroidal anti-inflammatory drugs (nsNSAIDs) to cause ulcer-related upper gastrointestinal (GI) tract complications.
In fact, the rates of both cardiovascular and GI events were so low overall that it made the trial difficult to complete, said study investigator Dr. Tom MacDonald, professor of clinical pharmacology and pharmacoepidemiology at the University of Dundee (Scotland), which sponsored the study.
The on-treatment and intention-to-treat (ITT) cardiovascular event rates were 0.9% and 1.1% per 100 patient-years, he observed, adding that he would have expected the event rate to be around 2%-3% in the population studied. GI complication rates were even lower, with just 12 on-treatment and 15 ITT events reported during the entire follow-up period, which was a maximum of 6.3 years and mean of about 3 years.
“You may remember the brouhaha surrounding the use of rofecoxib and other [cyclo-oxygenase-2 inhibitors],” said Dr. MacDonald. Both coxibs and nsNSAIDs have been associated with adverse cardiovascular outcomes such as myocardial infarction (BMJ 2005;330:1366), and rofecoxib was voluntarily withdrawn in 2004 by its manufacturer from the U.S. market. A recent meta-analysis (Lancet 2013;382:769-79) has suggested that coxibs increase the risk of major cardiovascular events by about 37%.
The SCOT study (BMJ Open 2013;3:e002295) was designed to assess if celecoxib was better, worse, or the same as the other available NSAIDs in terms of its cardiovascular and gastrointestinal safety. It was originally set up because of a requirement by the European Medicines Agency, Dr. McDonald explained.
More than 9,400 patients aged 60 years or older with osteoarthritis or rheumatoid arthritis who were prescribed chronic NSAID therapy and had no existing cardiovascular disease were screened at 706 family practices in Scotland, England, Denmark, and the Netherlands. A total of 7,297 patients were included in the prospective study and were randomized to switch to treatment with celecoxib or to continue their current nsNSAID.
General practice records were linked to hospital and mortality databases to derive the primary composite endpoint of the first occurrence of hospitalization for nonfatal MI, nonfatal stroke, or cardiovascular death, as well as secondary endpoints such as time to first hospitalization or death from upper GI complications and all-cause mortality.
Randomized patients were about 68 years old, and about 40% of patients were male. Dr. MacDonald noted that, although there was no known existing cardiovascular disease at enrollment, the baseline characteristics showed that around 44% of patients had high blood pressure; a third of patients had high cholesterol; and 20%, 12%, and 38% were taking a statin, aspirin, or ulcer-healing treatments, respectively. The most common nsNSAIDs being used were diclofenac (38.7%) and ibuprofen (31%).
There was no significant difference between celecoxib or nsNSAIDs for any of the cardiovascular endpoints studied, with hazard ratios (HR) for the primary composite cardiovascular endpoints of 1.12 (95% confidence interval, 0.81-1.55; P = .5) while on celecoxib treatment and 1.04 (95% CI, 0.81-1.33; P = .75) in the ITT analysis. Similar results were obtained for all-cause mortality (HR, 1.2 and 0.92, respectively).
Dr. MacDonald reported that 50% of patients randomized to celecoxib and 30% randomized to continue nsNSAIDs withdrew from the study. The main reasons for stopping celecoxib were a lack of efficacy (11.2% vs. 2% for nsNSAIDs), adverse events (8.3% vs. 4.4%), patient request (6% vs. 2.3%), not tolerated (3.9% vs. 1.2%), or a serious adverse event (2.6% vs. 1.9%). There was, however, a lot of adverse publicity about the coxibs, he noted, and patients who had been happy on an nsNSAID might not have been happy with the switch.
The rates of serious cardiovascular adverse events (31.7% vs. 32.4%) or reactions (5.2% vs. 5.8%) were similar with celecoxib and nsNSAIDs, but there were significantly fewer serious GI adverse reactions with celecoxib than with nsNSAIDs (38 vs. 66; P = .007). Overall, the adverse reaction rate was 22% vs. 16.1%, respectively (P <.001).
“In the study population, nsNSAIDs and celecoxib both appeared acceptably safe,” Dr. MacDonald concluded. “In patients who get significant symptomatic relief from these medicines, the benefit/risk balance appears positive.”
Although the findings are perhaps reassuring, they are unlikely to change clinical practice, observed Dr. José López-Sendon, who was invited to comment on the study results after their presentation at the conference.
The study findings suggest that celecoxib may continue to be safe to use in patients without existing cardiac disease, noted Dr. López-Sendon of Hospital Universitario La Paz in Madrid, but he would not modify the guidelines that advise that the lowest effective dose be used for the shortest duration of time in low-risk patients.
The study was sponsored by the University of Dundee and funded by an investigator-initiated research grant from Pfizer. The university’s Medicines Monitoring Unit also holds research grants from Amgen, Menarini, and Novartis. Dr. MacDonald has consulted on the use of NSAIDs for AstraZeneca, NiCox, Novartis, and Pfizer. Dr. López-Sendon did not have any disclosures relevant to his comments.
LONDON – Celecoxib was associated with very low cardiovascular event rates, and its use posed no more risk than other painkillers commonly used to treat elderly individuals with arthritic conditions but no heart disease in a large, pragmatic, family practice–based study.
Results of the Standard Care Versus Celecoxib Outcome Trial (SCOT) reported at the annual congress of the European Society of Cardiology also showed that celecoxib was no more likely than nonselective nonsteroidal anti-inflammatory drugs (nsNSAIDs) to cause ulcer-related upper gastrointestinal (GI) tract complications.
In fact, the rates of both cardiovascular and GI events were so low overall that it made the trial difficult to complete, said study investigator Dr. Tom MacDonald, professor of clinical pharmacology and pharmacoepidemiology at the University of Dundee (Scotland), which sponsored the study.
The on-treatment and intention-to-treat (ITT) cardiovascular event rates were 0.9% and 1.1% per 100 patient-years, he observed, adding that he would have expected the event rate to be around 2%-3% in the population studied. GI complication rates were even lower, with just 12 on-treatment and 15 ITT events reported during the entire follow-up period, which was a maximum of 6.3 years and mean of about 3 years.
“You may remember the brouhaha surrounding the use of rofecoxib and other [cyclo-oxygenase-2 inhibitors],” said Dr. MacDonald. Both coxibs and nsNSAIDs have been associated with adverse cardiovascular outcomes such as myocardial infarction (BMJ 2005;330:1366), and rofecoxib was voluntarily withdrawn in 2004 by its manufacturer from the U.S. market. A recent meta-analysis (Lancet 2013;382:769-79) has suggested that coxibs increase the risk of major cardiovascular events by about 37%.
The SCOT study (BMJ Open 2013;3:e002295) was designed to assess if celecoxib was better, worse, or the same as the other available NSAIDs in terms of its cardiovascular and gastrointestinal safety. It was originally set up because of a requirement by the European Medicines Agency, Dr. McDonald explained.
More than 9,400 patients aged 60 years or older with osteoarthritis or rheumatoid arthritis who were prescribed chronic NSAID therapy and had no existing cardiovascular disease were screened at 706 family practices in Scotland, England, Denmark, and the Netherlands. A total of 7,297 patients were included in the prospective study and were randomized to switch to treatment with celecoxib or to continue their current nsNSAID.
General practice records were linked to hospital and mortality databases to derive the primary composite endpoint of the first occurrence of hospitalization for nonfatal MI, nonfatal stroke, or cardiovascular death, as well as secondary endpoints such as time to first hospitalization or death from upper GI complications and all-cause mortality.
Randomized patients were about 68 years old, and about 40% of patients were male. Dr. MacDonald noted that, although there was no known existing cardiovascular disease at enrollment, the baseline characteristics showed that around 44% of patients had high blood pressure; a third of patients had high cholesterol; and 20%, 12%, and 38% were taking a statin, aspirin, or ulcer-healing treatments, respectively. The most common nsNSAIDs being used were diclofenac (38.7%) and ibuprofen (31%).
There was no significant difference between celecoxib or nsNSAIDs for any of the cardiovascular endpoints studied, with hazard ratios (HR) for the primary composite cardiovascular endpoints of 1.12 (95% confidence interval, 0.81-1.55; P = .5) while on celecoxib treatment and 1.04 (95% CI, 0.81-1.33; P = .75) in the ITT analysis. Similar results were obtained for all-cause mortality (HR, 1.2 and 0.92, respectively).
Dr. MacDonald reported that 50% of patients randomized to celecoxib and 30% randomized to continue nsNSAIDs withdrew from the study. The main reasons for stopping celecoxib were a lack of efficacy (11.2% vs. 2% for nsNSAIDs), adverse events (8.3% vs. 4.4%), patient request (6% vs. 2.3%), not tolerated (3.9% vs. 1.2%), or a serious adverse event (2.6% vs. 1.9%). There was, however, a lot of adverse publicity about the coxibs, he noted, and patients who had been happy on an nsNSAID might not have been happy with the switch.
The rates of serious cardiovascular adverse events (31.7% vs. 32.4%) or reactions (5.2% vs. 5.8%) were similar with celecoxib and nsNSAIDs, but there were significantly fewer serious GI adverse reactions with celecoxib than with nsNSAIDs (38 vs. 66; P = .007). Overall, the adverse reaction rate was 22% vs. 16.1%, respectively (P <.001).
“In the study population, nsNSAIDs and celecoxib both appeared acceptably safe,” Dr. MacDonald concluded. “In patients who get significant symptomatic relief from these medicines, the benefit/risk balance appears positive.”
Although the findings are perhaps reassuring, they are unlikely to change clinical practice, observed Dr. José López-Sendon, who was invited to comment on the study results after their presentation at the conference.
The study findings suggest that celecoxib may continue to be safe to use in patients without existing cardiac disease, noted Dr. López-Sendon of Hospital Universitario La Paz in Madrid, but he would not modify the guidelines that advise that the lowest effective dose be used for the shortest duration of time in low-risk patients.
The study was sponsored by the University of Dundee and funded by an investigator-initiated research grant from Pfizer. The university’s Medicines Monitoring Unit also holds research grants from Amgen, Menarini, and Novartis. Dr. MacDonald has consulted on the use of NSAIDs for AstraZeneca, NiCox, Novartis, and Pfizer. Dr. López-Sendon did not have any disclosures relevant to his comments.
AT THE ESC CONGRESS 2015
Key clinical point: Celecoxib and nonselective NSAIDs were “acceptably safe” in a population without confirmed cardiovascular disease.
Major finding: The hazard ratio for the primary composite cardiovascular endpoint with celecoxib use was 1.12 (95% confidence interval, 0.81-1.55; P = .5), compared with NSAIDs.
Data source: The Standard Care Versus Celecoxib Outcome Trial (SCOT) of more than 7,200 elderly patients with osteoarthritis or rheumatoid arthritis and no confirmed cardiovascular disease.
Disclosures: The study was sponsored by the University of Dundee and funded by an investigator-initiated research grant from Pfizer. The university’s Medicines Monitoring Unit also holds research grants from Amgen, Menarini, and Novartis. Dr. MacDonald has consulted on the use of NSAIDs for AstraZeneca, NiCox, Novartis, and Pfizer. Dr. López-Sendon did not have any disclosures relevant to his comments.