User login
A Picture Is Worth a Thousand Words: Unconscious Bias in the Residency Application Process?
Applying for a residency program can be a stressful process for medical students. It is a combination of applying for a job in the “real world” and applying to a college or medical school. In certain fields of medicine or surgery, there may be over 600 residency applications for 40 to 80 interviewee slots. Different specialties, as well as programs within a given specialty, take a different number of residents per year. This can vary from 1 to over 20 available spots, depending on the field of medicine or surgery as well as the specific program. Orthopedic surgery residencies, for example, can match between 2 and 12 residents each year. During the 2013–2014 academic year at our institution, there were over 600 applications received for approximately 50 interview slots for a class of 5 orthopedic surgery residents. Nationally, according to publicly available 2013 National Resident Matching Program (NRMP) data, a total of 1038 applicants (833 US medical school seniors) applied for 693 spots in orthopedic surgery, of which 692 were filled, indicating that orthopedic surgery remains one of the most desired fields among medical school seniors.1 Looking at the statistics provided by the NRMP data, orthopedic applicants remain some of the most competitive, with proportionally higher board scores, publication numbers, and grades, among other factors.1
Each individual program has its own method for sifting through the applications. At some institutions, the individual “in charge” of the selection committee may look through all applications initially, narrow them down, and then distribute them to the other members of the selection committee to determine the final interviewee list. At other institutions, the initial group of applications may be divided and distributed to the committee members so that each member reviews the applications and ultimately decides upon the interview candidates.
The Electronic Residency Application Service (ERAS) application includes the applicant’s name, birth city, current place of residence, education history, standardized test scores, grades achieved during medical school, letters of recommendation, personal statement, extracurricular activities, volunteer activities, research experience, and languages spoken, along with several other pieces of data, all intended to be able to give the committee a better understanding of the applicant. Interestingly, however, the application also includes a photograph of the applicant.
Countless authors have demonstrated that we make assumptions and reach conclusions without even being aware that this is occurring. This is the theory of “unconscious bias.”2-5 Unconscious bias applies to how we perceive other people, and occurs when subconscious beliefs or unrecognized stereotypes about specific characteristics, including gender, ethnicity, religion, socioeconomic status, age, and sexual orientation, result in an automatic and unconscious reaction and/or behavior.6 Unconscious bias has the ability to affect everything from how health care is delivered to how employees are hired.7-12 We are all biased, and becoming aware of our biases will help us mitigate them in the workplace.
Title VII of the Civil Rights Act of 1964 requires that employers rely solely on job-related qualifications, and not physical characteristics, in their interviewing and hiring process. The US Equal Employment Opportunity Commission (EEOC), the federal agency that enforces Title VII, includes asking for photographs during the application stage on its list of prohibited practices for employers.13 It is our belief that including a photograph in the ERAS application, prior to the selection of interview candidates, may produce unconscious bias in the decision for granting (or not granting) an interview, and this component of the application should be eliminated.
Using a wide spectrum of cultural backgrounds in employers, Dion and colleagues14 demonstrated that the “what is beautiful is good” bias is present in all cultures when prospective employees are closely matched in qualification. Attractive individuals are thought to have better professional lives and stable marital relationships and personalities, according to previous studies.14 There has been much research aimed at determining if physical attractiveness is a factor in hiring, and the evidence suggests that the more attractive the applicant is, the greater the chances of being hired.15 Specifically, Watkins and Johnston15 have found that attractive people are thought to have better personalities than less attractive people, and that a photograph can influence the hiring decision process.
Bradley Ruffle at Ben-Gurion University and Ze’ev Shtudiner at Ariel University looked at what happens when job hunters include photographs with their curricula vitae (CV), as is the norm in much of Europe and Asia.16 For over 2500 job postings, they sent 2 identical résumés: one with a photograph and one without a photograph. An equal number of male and female applicants were sent to each posting, as were an equal number of attractive and plain-looking photographs; applications without photographs were also sent as a control group. For men, the results were as expected: CVs of “attractive” men were more likely to elicit a response from the employer (19.7%) compared with those of no-picture men (13.7%) and plain-looking men (9.2%). Interestingly, men who were viewed as “plain-looking” were better off not including a photograph. For the female applicants, however, the results were unexpected: CVs of women without a picture elicited the highest response rate (16.6%), while CVs of “plain-looking” women (13.6%) and of “attractive” women (12.8%) were less likely to receive a response.16
It is an unfortunate reality that personal preference, bias, and, in some cases, discriminatory hiring practices all factor into the selection process.17 This is why, as described above, the EEOC includes asking for photographs during the application stage on its list of prohibited practices for employers.13 The EEOC website also states: “If needed for identification purposes, a photograph may be obtained after an offer of employment is made and accepted.”13 In the residency application scenario, once an applicant has been granted an interview, a photograph can be taken on the day of the interview. With so many interviewees, this may help the interviewers to remember the interviewee. At this point in the process, the applicant has already been granted the interview. The bias associated with merely looking at a photograph is thus eliminated. This is in accordance with Title VII and is clearly different than including a photograph in the initial application, which directly violates Title VII.
Reviewers of applicants may have an unconscious bias due to the applicant’s attractiveness, race, sex, ethnicity, etc. Other, subtler forms of bias may also be present. Without realizing it, people may judge the quality of the photograph, or even what the applicant was wearing in the photograph. In orthopedic surgery, for example, there may be bias in the “size” of the applicant regardless of sex. Reviewers may unconsciously think how is he/she going to hold the leg, cut a rod, reduce a hip, etc. Without even realizing it, this may sway the person reviewing the application to choose one applicant over another. This may occur regardless of the applicant’s actual qualifications as based on the previously described factors, including test scores, grades during medical school, letters of recommendation, personal statement, extracurricular activities, volunteer activities, and research experience.
Unconscious bias is present in everyone. In an ideal world, one would be able to eliminate all sources of unconscious bias in the application process. Bias due to attending an Ivy League school versus a state school, bias due to where the applicant is from, bias due to who wrote the letter of recommendation, along with various other sources of unconscious bias, would be able to be eliminated. Unfortunately, this is not possible. What is possible, however, is to remove the photograph from the application process and to comply with Title VII of the Civil Rights Act of 1964.
1. National Resident Matching Program, Data Release and Research Committee. Results of the 2013 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2013. www.nrmp.org/wp-content/uploads/2013/08/applicantresultsbyspecialty2013.pdf. Accessed July 20, 2015.
2. Santry HP, Wren SM. The role of unconscious bias in surgical safety and outcomes. Surg Clin North Am. 2012;92(1):137–151.
3. Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74(6):1464–1480.
4. Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97(1):17–41.
5. Plessner H, Banse R. Attitude measurement using the Implicit Association Test (IAT). Z Exp Psychol. 2001;48(2):82–84.
6. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504–1510.
7. What you don’t know: the science of unconscious bias and what to do about it in the search and recruitment process [e-learning seminar]. Association of American Medical Colleges website. https://www.aamc.org/members/leadership/catalog/178420/unconscious_bias.html. Accessed July 14, 2015.
8. Haider AH, Schneider EB, Sriram N, et al. Unconscious race and class bias: its association with decision making by trauma and acute care surgeons. J Trauma Acute Care Surg. 2014;77(3):409–416.
9. Blair IV, Steiner JF, Hanratty R, et al. An investigation of associations between clinicians’ ethnic or racial bias and hypertension treatment, medication adherence and blood pressure control. J Gen Intern Med. 2014;29(7):987–995.
10. Ravenell J, Ogedegbe G. Unconscious bias and real-world hypertension outcomes: advancing disparities research. J Gen Intern Med. 2014;29(7):973–975.
11. van Ryn M, Saha S. Exploring unconscious bias in disparities research and medical education. JAMA. 2011;306(9):995–996.
12. Puhl RM, Moss-Racusin CA, Schwartz MB, Brownell KD. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Educ Res. 2008;23(2):347–358.
13. Prohibited employment policies/practices. US Equal Employment Opportunity Commission website. http://www.eeoc.gov/laws/practices/. Accessed July 14, 2015.
14. Dion K, Berscheid E, Walster E. What is beautiful is good. J Pers Soc Psychol. 1972;24(3):285–290.
15. Watkins LM, Johnston L. Screening job applicants: the impact of physical attractiveness and application quality. Int J Selection Assess. 2000;8(2):76–84.
16. Ruffle BJ, Shtudiner Z. Are good-looking people more employable? Manage Sci. http://dx.doi.org/10.1287/mnsc.2014.1927. Published May 29, 2014. Accessed July 14, 2015.
17. Lemay EP Jr, Clark MS, Greenberg A. What is beautiful is good because what is beautiful is desired: physical attractiveness stereotyping as projection of interpersonal goals. Pers Soc Psychol Bull. 2010;36(3):339–353.
Applying for a residency program can be a stressful process for medical students. It is a combination of applying for a job in the “real world” and applying to a college or medical school. In certain fields of medicine or surgery, there may be over 600 residency applications for 40 to 80 interviewee slots. Different specialties, as well as programs within a given specialty, take a different number of residents per year. This can vary from 1 to over 20 available spots, depending on the field of medicine or surgery as well as the specific program. Orthopedic surgery residencies, for example, can match between 2 and 12 residents each year. During the 2013–2014 academic year at our institution, there were over 600 applications received for approximately 50 interview slots for a class of 5 orthopedic surgery residents. Nationally, according to publicly available 2013 National Resident Matching Program (NRMP) data, a total of 1038 applicants (833 US medical school seniors) applied for 693 spots in orthopedic surgery, of which 692 were filled, indicating that orthopedic surgery remains one of the most desired fields among medical school seniors.1 Looking at the statistics provided by the NRMP data, orthopedic applicants remain some of the most competitive, with proportionally higher board scores, publication numbers, and grades, among other factors.1
Each individual program has its own method for sifting through the applications. At some institutions, the individual “in charge” of the selection committee may look through all applications initially, narrow them down, and then distribute them to the other members of the selection committee to determine the final interviewee list. At other institutions, the initial group of applications may be divided and distributed to the committee members so that each member reviews the applications and ultimately decides upon the interview candidates.
The Electronic Residency Application Service (ERAS) application includes the applicant’s name, birth city, current place of residence, education history, standardized test scores, grades achieved during medical school, letters of recommendation, personal statement, extracurricular activities, volunteer activities, research experience, and languages spoken, along with several other pieces of data, all intended to be able to give the committee a better understanding of the applicant. Interestingly, however, the application also includes a photograph of the applicant.
Countless authors have demonstrated that we make assumptions and reach conclusions without even being aware that this is occurring. This is the theory of “unconscious bias.”2-5 Unconscious bias applies to how we perceive other people, and occurs when subconscious beliefs or unrecognized stereotypes about specific characteristics, including gender, ethnicity, religion, socioeconomic status, age, and sexual orientation, result in an automatic and unconscious reaction and/or behavior.6 Unconscious bias has the ability to affect everything from how health care is delivered to how employees are hired.7-12 We are all biased, and becoming aware of our biases will help us mitigate them in the workplace.
Title VII of the Civil Rights Act of 1964 requires that employers rely solely on job-related qualifications, and not physical characteristics, in their interviewing and hiring process. The US Equal Employment Opportunity Commission (EEOC), the federal agency that enforces Title VII, includes asking for photographs during the application stage on its list of prohibited practices for employers.13 It is our belief that including a photograph in the ERAS application, prior to the selection of interview candidates, may produce unconscious bias in the decision for granting (or not granting) an interview, and this component of the application should be eliminated.
Using a wide spectrum of cultural backgrounds in employers, Dion and colleagues14 demonstrated that the “what is beautiful is good” bias is present in all cultures when prospective employees are closely matched in qualification. Attractive individuals are thought to have better professional lives and stable marital relationships and personalities, according to previous studies.14 There has been much research aimed at determining if physical attractiveness is a factor in hiring, and the evidence suggests that the more attractive the applicant is, the greater the chances of being hired.15 Specifically, Watkins and Johnston15 have found that attractive people are thought to have better personalities than less attractive people, and that a photograph can influence the hiring decision process.
Bradley Ruffle at Ben-Gurion University and Ze’ev Shtudiner at Ariel University looked at what happens when job hunters include photographs with their curricula vitae (CV), as is the norm in much of Europe and Asia.16 For over 2500 job postings, they sent 2 identical résumés: one with a photograph and one without a photograph. An equal number of male and female applicants were sent to each posting, as were an equal number of attractive and plain-looking photographs; applications without photographs were also sent as a control group. For men, the results were as expected: CVs of “attractive” men were more likely to elicit a response from the employer (19.7%) compared with those of no-picture men (13.7%) and plain-looking men (9.2%). Interestingly, men who were viewed as “plain-looking” were better off not including a photograph. For the female applicants, however, the results were unexpected: CVs of women without a picture elicited the highest response rate (16.6%), while CVs of “plain-looking” women (13.6%) and of “attractive” women (12.8%) were less likely to receive a response.16
It is an unfortunate reality that personal preference, bias, and, in some cases, discriminatory hiring practices all factor into the selection process.17 This is why, as described above, the EEOC includes asking for photographs during the application stage on its list of prohibited practices for employers.13 The EEOC website also states: “If needed for identification purposes, a photograph may be obtained after an offer of employment is made and accepted.”13 In the residency application scenario, once an applicant has been granted an interview, a photograph can be taken on the day of the interview. With so many interviewees, this may help the interviewers to remember the interviewee. At this point in the process, the applicant has already been granted the interview. The bias associated with merely looking at a photograph is thus eliminated. This is in accordance with Title VII and is clearly different than including a photograph in the initial application, which directly violates Title VII.
Reviewers of applicants may have an unconscious bias due to the applicant’s attractiveness, race, sex, ethnicity, etc. Other, subtler forms of bias may also be present. Without realizing it, people may judge the quality of the photograph, or even what the applicant was wearing in the photograph. In orthopedic surgery, for example, there may be bias in the “size” of the applicant regardless of sex. Reviewers may unconsciously think how is he/she going to hold the leg, cut a rod, reduce a hip, etc. Without even realizing it, this may sway the person reviewing the application to choose one applicant over another. This may occur regardless of the applicant’s actual qualifications as based on the previously described factors, including test scores, grades during medical school, letters of recommendation, personal statement, extracurricular activities, volunteer activities, and research experience.
Unconscious bias is present in everyone. In an ideal world, one would be able to eliminate all sources of unconscious bias in the application process. Bias due to attending an Ivy League school versus a state school, bias due to where the applicant is from, bias due to who wrote the letter of recommendation, along with various other sources of unconscious bias, would be able to be eliminated. Unfortunately, this is not possible. What is possible, however, is to remove the photograph from the application process and to comply with Title VII of the Civil Rights Act of 1964.
Applying for a residency program can be a stressful process for medical students. It is a combination of applying for a job in the “real world” and applying to a college or medical school. In certain fields of medicine or surgery, there may be over 600 residency applications for 40 to 80 interviewee slots. Different specialties, as well as programs within a given specialty, take a different number of residents per year. This can vary from 1 to over 20 available spots, depending on the field of medicine or surgery as well as the specific program. Orthopedic surgery residencies, for example, can match between 2 and 12 residents each year. During the 2013–2014 academic year at our institution, there were over 600 applications received for approximately 50 interview slots for a class of 5 orthopedic surgery residents. Nationally, according to publicly available 2013 National Resident Matching Program (NRMP) data, a total of 1038 applicants (833 US medical school seniors) applied for 693 spots in orthopedic surgery, of which 692 were filled, indicating that orthopedic surgery remains one of the most desired fields among medical school seniors.1 Looking at the statistics provided by the NRMP data, orthopedic applicants remain some of the most competitive, with proportionally higher board scores, publication numbers, and grades, among other factors.1
Each individual program has its own method for sifting through the applications. At some institutions, the individual “in charge” of the selection committee may look through all applications initially, narrow them down, and then distribute them to the other members of the selection committee to determine the final interviewee list. At other institutions, the initial group of applications may be divided and distributed to the committee members so that each member reviews the applications and ultimately decides upon the interview candidates.
The Electronic Residency Application Service (ERAS) application includes the applicant’s name, birth city, current place of residence, education history, standardized test scores, grades achieved during medical school, letters of recommendation, personal statement, extracurricular activities, volunteer activities, research experience, and languages spoken, along with several other pieces of data, all intended to be able to give the committee a better understanding of the applicant. Interestingly, however, the application also includes a photograph of the applicant.
Countless authors have demonstrated that we make assumptions and reach conclusions without even being aware that this is occurring. This is the theory of “unconscious bias.”2-5 Unconscious bias applies to how we perceive other people, and occurs when subconscious beliefs or unrecognized stereotypes about specific characteristics, including gender, ethnicity, religion, socioeconomic status, age, and sexual orientation, result in an automatic and unconscious reaction and/or behavior.6 Unconscious bias has the ability to affect everything from how health care is delivered to how employees are hired.7-12 We are all biased, and becoming aware of our biases will help us mitigate them in the workplace.
Title VII of the Civil Rights Act of 1964 requires that employers rely solely on job-related qualifications, and not physical characteristics, in their interviewing and hiring process. The US Equal Employment Opportunity Commission (EEOC), the federal agency that enforces Title VII, includes asking for photographs during the application stage on its list of prohibited practices for employers.13 It is our belief that including a photograph in the ERAS application, prior to the selection of interview candidates, may produce unconscious bias in the decision for granting (or not granting) an interview, and this component of the application should be eliminated.
Using a wide spectrum of cultural backgrounds in employers, Dion and colleagues14 demonstrated that the “what is beautiful is good” bias is present in all cultures when prospective employees are closely matched in qualification. Attractive individuals are thought to have better professional lives and stable marital relationships and personalities, according to previous studies.14 There has been much research aimed at determining if physical attractiveness is a factor in hiring, and the evidence suggests that the more attractive the applicant is, the greater the chances of being hired.15 Specifically, Watkins and Johnston15 have found that attractive people are thought to have better personalities than less attractive people, and that a photograph can influence the hiring decision process.
Bradley Ruffle at Ben-Gurion University and Ze’ev Shtudiner at Ariel University looked at what happens when job hunters include photographs with their curricula vitae (CV), as is the norm in much of Europe and Asia.16 For over 2500 job postings, they sent 2 identical résumés: one with a photograph and one without a photograph. An equal number of male and female applicants were sent to each posting, as were an equal number of attractive and plain-looking photographs; applications without photographs were also sent as a control group. For men, the results were as expected: CVs of “attractive” men were more likely to elicit a response from the employer (19.7%) compared with those of no-picture men (13.7%) and plain-looking men (9.2%). Interestingly, men who were viewed as “plain-looking” were better off not including a photograph. For the female applicants, however, the results were unexpected: CVs of women without a picture elicited the highest response rate (16.6%), while CVs of “plain-looking” women (13.6%) and of “attractive” women (12.8%) were less likely to receive a response.16
It is an unfortunate reality that personal preference, bias, and, in some cases, discriminatory hiring practices all factor into the selection process.17 This is why, as described above, the EEOC includes asking for photographs during the application stage on its list of prohibited practices for employers.13 The EEOC website also states: “If needed for identification purposes, a photograph may be obtained after an offer of employment is made and accepted.”13 In the residency application scenario, once an applicant has been granted an interview, a photograph can be taken on the day of the interview. With so many interviewees, this may help the interviewers to remember the interviewee. At this point in the process, the applicant has already been granted the interview. The bias associated with merely looking at a photograph is thus eliminated. This is in accordance with Title VII and is clearly different than including a photograph in the initial application, which directly violates Title VII.
Reviewers of applicants may have an unconscious bias due to the applicant’s attractiveness, race, sex, ethnicity, etc. Other, subtler forms of bias may also be present. Without realizing it, people may judge the quality of the photograph, or even what the applicant was wearing in the photograph. In orthopedic surgery, for example, there may be bias in the “size” of the applicant regardless of sex. Reviewers may unconsciously think how is he/she going to hold the leg, cut a rod, reduce a hip, etc. Without even realizing it, this may sway the person reviewing the application to choose one applicant over another. This may occur regardless of the applicant’s actual qualifications as based on the previously described factors, including test scores, grades during medical school, letters of recommendation, personal statement, extracurricular activities, volunteer activities, and research experience.
Unconscious bias is present in everyone. In an ideal world, one would be able to eliminate all sources of unconscious bias in the application process. Bias due to attending an Ivy League school versus a state school, bias due to where the applicant is from, bias due to who wrote the letter of recommendation, along with various other sources of unconscious bias, would be able to be eliminated. Unfortunately, this is not possible. What is possible, however, is to remove the photograph from the application process and to comply with Title VII of the Civil Rights Act of 1964.
1. National Resident Matching Program, Data Release and Research Committee. Results of the 2013 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2013. www.nrmp.org/wp-content/uploads/2013/08/applicantresultsbyspecialty2013.pdf. Accessed July 20, 2015.
2. Santry HP, Wren SM. The role of unconscious bias in surgical safety and outcomes. Surg Clin North Am. 2012;92(1):137–151.
3. Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74(6):1464–1480.
4. Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97(1):17–41.
5. Plessner H, Banse R. Attitude measurement using the Implicit Association Test (IAT). Z Exp Psychol. 2001;48(2):82–84.
6. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504–1510.
7. What you don’t know: the science of unconscious bias and what to do about it in the search and recruitment process [e-learning seminar]. Association of American Medical Colleges website. https://www.aamc.org/members/leadership/catalog/178420/unconscious_bias.html. Accessed July 14, 2015.
8. Haider AH, Schneider EB, Sriram N, et al. Unconscious race and class bias: its association with decision making by trauma and acute care surgeons. J Trauma Acute Care Surg. 2014;77(3):409–416.
9. Blair IV, Steiner JF, Hanratty R, et al. An investigation of associations between clinicians’ ethnic or racial bias and hypertension treatment, medication adherence and blood pressure control. J Gen Intern Med. 2014;29(7):987–995.
10. Ravenell J, Ogedegbe G. Unconscious bias and real-world hypertension outcomes: advancing disparities research. J Gen Intern Med. 2014;29(7):973–975.
11. van Ryn M, Saha S. Exploring unconscious bias in disparities research and medical education. JAMA. 2011;306(9):995–996.
12. Puhl RM, Moss-Racusin CA, Schwartz MB, Brownell KD. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Educ Res. 2008;23(2):347–358.
13. Prohibited employment policies/practices. US Equal Employment Opportunity Commission website. http://www.eeoc.gov/laws/practices/. Accessed July 14, 2015.
14. Dion K, Berscheid E, Walster E. What is beautiful is good. J Pers Soc Psychol. 1972;24(3):285–290.
15. Watkins LM, Johnston L. Screening job applicants: the impact of physical attractiveness and application quality. Int J Selection Assess. 2000;8(2):76–84.
16. Ruffle BJ, Shtudiner Z. Are good-looking people more employable? Manage Sci. http://dx.doi.org/10.1287/mnsc.2014.1927. Published May 29, 2014. Accessed July 14, 2015.
17. Lemay EP Jr, Clark MS, Greenberg A. What is beautiful is good because what is beautiful is desired: physical attractiveness stereotyping as projection of interpersonal goals. Pers Soc Psychol Bull. 2010;36(3):339–353.
1. National Resident Matching Program, Data Release and Research Committee. Results of the 2013 NRMP Applicant Survey by Preferred Specialty and Applicant Type. Washington, DC: National Resident Matching Program; 2013. www.nrmp.org/wp-content/uploads/2013/08/applicantresultsbyspecialty2013.pdf. Accessed July 20, 2015.
2. Santry HP, Wren SM. The role of unconscious bias in surgical safety and outcomes. Surg Clin North Am. 2012;92(1):137–151.
3. Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74(6):1464–1480.
4. Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97(1):17–41.
5. Plessner H, Banse R. Attitude measurement using the Implicit Association Test (IAT). Z Exp Psychol. 2001;48(2):82–84.
6. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504–1510.
7. What you don’t know: the science of unconscious bias and what to do about it in the search and recruitment process [e-learning seminar]. Association of American Medical Colleges website. https://www.aamc.org/members/leadership/catalog/178420/unconscious_bias.html. Accessed July 14, 2015.
8. Haider AH, Schneider EB, Sriram N, et al. Unconscious race and class bias: its association with decision making by trauma and acute care surgeons. J Trauma Acute Care Surg. 2014;77(3):409–416.
9. Blair IV, Steiner JF, Hanratty R, et al. An investigation of associations between clinicians’ ethnic or racial bias and hypertension treatment, medication adherence and blood pressure control. J Gen Intern Med. 2014;29(7):987–995.
10. Ravenell J, Ogedegbe G. Unconscious bias and real-world hypertension outcomes: advancing disparities research. J Gen Intern Med. 2014;29(7):973–975.
11. van Ryn M, Saha S. Exploring unconscious bias in disparities research and medical education. JAMA. 2011;306(9):995–996.
12. Puhl RM, Moss-Racusin CA, Schwartz MB, Brownell KD. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Educ Res. 2008;23(2):347–358.
13. Prohibited employment policies/practices. US Equal Employment Opportunity Commission website. http://www.eeoc.gov/laws/practices/. Accessed July 14, 2015.
14. Dion K, Berscheid E, Walster E. What is beautiful is good. J Pers Soc Psychol. 1972;24(3):285–290.
15. Watkins LM, Johnston L. Screening job applicants: the impact of physical attractiveness and application quality. Int J Selection Assess. 2000;8(2):76–84.
16. Ruffle BJ, Shtudiner Z. Are good-looking people more employable? Manage Sci. http://dx.doi.org/10.1287/mnsc.2014.1927. Published May 29, 2014. Accessed July 14, 2015.
17. Lemay EP Jr, Clark MS, Greenberg A. What is beautiful is good because what is beautiful is desired: physical attractiveness stereotyping as projection of interpersonal goals. Pers Soc Psychol Bull. 2010;36(3):339–353.
Osteochondroma With Contiguous Bronchogenic Cyst of the Scapula
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
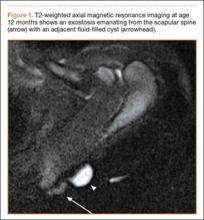
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
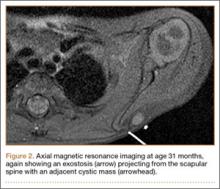
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
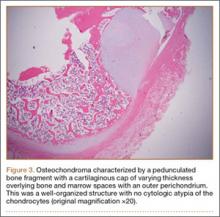
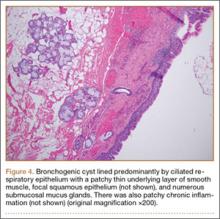
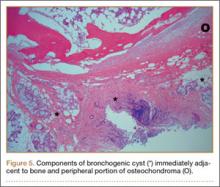
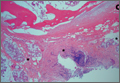
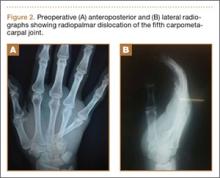
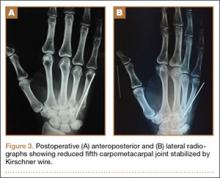
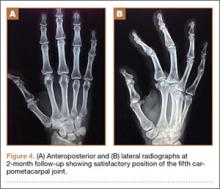
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
Osteochondromas are common benign bone tumors composed of a bony protrusion with an overlying cartilage cap.1 This lesion constitutes 24% to 40% of all benign bone tumors, and the great majority arise from the metaphyseal region of long bones.2 The scapula accounts for only 3% to 5% of all osteochondromas; however, this lesion is the most common benign bone tumor to involve the scapula.3
In contrast, cutaneous bronchogenic cyst of the scapula is an exceedingly rare pathology. The bronchogenic cyst is a congenital cystic mass lined by tracheobronchial structures and respiratory epithelium.4 These are most commonly located in the thorax, although numerous remote locations have also been described, including cutaneous cysts.5 The overall incidence of bronchogenic cysts is thought to be 1 in 42,000 to 1 in 68,000.6 There are only 15 case reports of cutaneous bronchogenic cysts in the scapular region.7
We report the case of a novel dual lesion of both an osteochondroma and a contiguous cutaneous bronchogenic cyst in the scapula. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-month-old boy presented to our clinic with the complaint of a mass over the left scapula. The mass was first noted incidentally several weeks earlier during bathing. Examination revealed a firm, subcutaneous, nontender mass measuring 1×2 cm located over the spine of the scapula. There were no overlying skin changes, and there was normal function of the ipsilateral upper extremity. Anteroposterior and lateral chest radiographs revealed no abnormality. Magnetic resonance imaging (MRI) showed an exostosis projecting from the scapular spine measuring 2×6×7 mm with an adjacent cystic mass measuring 5×8×9 mm that was thought to represent bursitis (Figure 1). The decision was made to observe the mass.
The patient returned to clinic at age 31 months with a new complaint of scant drainage of serous fluid from a pinprick-sized hole located just superolateral to the scapular mass. The child’s mother reported daily manual expression of fluid from the mass via the hole, without which the mass would enlarge. There were no local or systemic signs of infection. A repeat MRI again revealed an exostosis with an adjacent cystic mass with interval enlargement of the cyst (Figure 2). At age 4.5 years, the decision was made to proceed with excision of the osteochondroma and adjacent cystic mass.
The mass was approached via a 2-cm incision designed to excise the tract to the skin. Dissection revealed a sinus tract connecting to a well-defined cystic sac. This sac was attached to the underlying exostosis. The exostosis and attached cyst were excised en bloc. The cyst was opened, revealing foul-smelling, cloudy white fluid that was sent for culture; the specimen was sent for pathology.
The fluid culture grew mixed flora, with no Staphylococcus aureus, group A streptococcus, or Pseudomonas aeruginosa identified. The pathologic examination identified bone with a cartilaginous cap, consistent with osteochondroma (Figure 3), as well as a cyst lined by respiratory epithelium with patchy areas of squamous epithelium and surrounding mucus glands, consistent with bronchogenic cyst (Figure 4). Figure 5 shows the contiguous nature of the 2 lesions.
The postoperative course was uneventful. The patient returned to full use of the left upper extremity and had resolution of all drainage.
Discussion
Osteochondromas are thought to arise from aberrant growth of the epiphyseal growth plate cartilage. A small portion of the physis herniates past the groove of Ranvier and grows parallel to the normal physis with medullary continuity. This can occur idiopathically or, more rarely, secondary to an identified injury to the growth plate.1
The formation of bronchogenic cysts is most often attributed to anomalous budding of the ventral foregut during fetal development,4 hence the alternative designation of these cysts as foregut cysts. An extrathoracic location of the cyst has been postulated to stem from 2 possible events: a preexisting cyst may migrate out of the thorax prior to closure of the sternal plates, or sternal plate closure may itself pinch off the cyst.8,9 An alternative explanation is in situ metaplastic development of respiratory epithelium.10 When located near the skin, these cysts often drain clear fluid.11
Scapular osteochondromas are known to cause various pathologies of the shoulder girdle, including snapping scapula syndrome, chest wall deformity, shoulder impingement, and bursa formation.12-17 This case, however, is the first known finding of a scapular osteochondroma with a contiguous cutaneous bronchogenic cyst. A putative explanation for their co-occurrence is that local disturbances caused by one lesion stimulated the formation of the second. The direct connection between the bronchogenic cyst and the bone, as has been reported in 3 cases,7,9,18 seems to favor this explanation. Definitive conclusions regarding any causal relationship are beyond the scope of this single case report.
Definitive management of bronchogenic cysts is complete excision, although the diagnosis is often not made until histopathologic examination has been completed.19 Osteochondromas are managed with observation unless they are symptomatic.2 Malignant degeneration is a rare but documented occurrence in both lesions.2,20
Conclusion
In approaching the pediatric patient with a cystic mass over the scapula, a cutaneous bronchogenic cyst may be included in the differential diagnosis. This lesion can occur in isolation or can be found with another pathology, such as osteochondroma, as reported here.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
1. Milgram JW. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;174:264-284.
2. Dahlin DC. Osteochondroma (osteocartilaginous exostosis). In: Dahlin DC. Bone Tumors. Springfield, IL: Thomas; 1978: 17-27.
3. Samilson RL, Morris JM, Thompson RW. Tumors of the scapula. A review of the literature and an analysis of 31 cases. Clin Orthop Relat Res. 1968;58:105-115.
4. Rodgers BM, Harman PK, Johnson AM. Bronchopulmonary foregut malformations. The spectrum of anomalies. Ann Surg. 1986;203(5):517-524.
5. Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatr Dermatol. 1998;15(4):277-281.
6. Sanli A, Onen A, Ceylan E, Yilmaz E, Silistreli E, Açikel U. A case of a bronchogenic cyst in a rare location. Ann Thorac Surg. 2004;77(3):1093-1094.
7. Al-Balushi Z, Ehsan MT, Al Sajee D, Al Riyami M. Scapular bronchogenic cyst: a case report and literature review. Oman Med J. 2012;27(2):161-163.
8. Miller OF 3rd, Tyler W. Cutaneous bronchogenic cyst with papilloma and sinus presentation. J Am Acad Dermatol. 1984;11(2 Pt 2):367-371.
9. Fraga S, Helwig EB, Rosen SH. Bronchogenic cyst in the skin and subcutaneous tissue. Am J Clin Pathol. 1971;56(2):230-238.
10. Van der Putte SC, Toonstra J. Cutaneous ‘bronchogenic’ cyst. J Cutan Pathol. 1985;12(5):404-409.
11. Schouten van der Velden AP, Severijnen RS, Wobbes T. A bronchogenic cyst under the scapula with a fistula on the back. Pediatr Surg Int. 2006;22(10):857-860.
12. Lu MT, Abboud JA. Subacromial osteochondroma. Orthopedics. 2011;34(9):581-583.
13. Lazar MA, Kwon YW, Rokito AS. Snapping scapula syndrome. J Bone Joint Surg Am. 2009;91(9):2251-2262.
14. Okada K, Terada K, Sashi R, Hoshi N. Large bursa formation associated with osteochondroma of the scapula: a case report and review of the literature. Jpn J Clin Oncol. 1999;29(7):356-360.
15. Tomo H, Ito Y, Aono M, Takaoka K. Chest wall deformity associated with osteochondroma of the scapula: a case report and review of the literature. J Shoulder Elbow Surg. 2005;14(1):103-106.
16. Jacobi CA, Gellert K, Zieren J. Rapid development of subscapular exostosis bursata. J Shoulder Elbow Surg. 1997;6(2):164-166.
17. Van Riet RP, Van Glabbeek F. Arthroscopic resection of a symptomatic snapping subscapular osteochondroma. Acta Orthop Belg. 2007;73(2):252-254.
18. Das K, Jackson PB, D’Cruz AJ. Periscapular bronchogenic cyst. Indian J Pediatr. 70(2):181-182.
19. Suen HC, Mathisen DJ, Grillo HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993;55(2):476-481.
20. Tanita M, Kikuchi-Numagami K, Ogoshi K, et al. Malignant melanoma arising from cutaneous bronchogenic cyst of the scapular area. J Am Acad Dermatol. 2002;46(2 suppl case reports):S19-S21.
A Rare Cause of Postoperative Abdominal Pain in a Spinal Fusion Patient
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
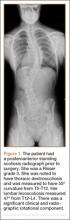
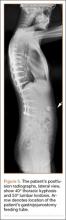
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
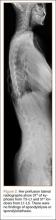
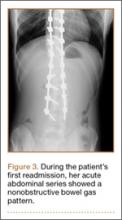
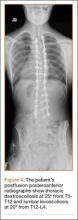
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
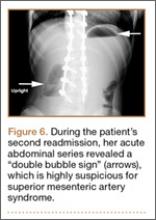
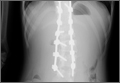
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
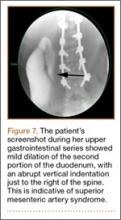
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
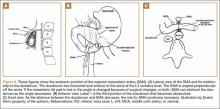
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
Posterior spinal fusion for adolescent idiopathic scoliosis is a relatively common procedure. However, intestinal obstruction is a possible complication in the case of an asthenic adolescent with weight loss after surgery. We present the case of a 12-year-old girl who underwent an uncomplicated posterior spinal fusion with instrumentation for scoliosis and who developed nausea, emesis, and abdominal pain. We also discuss the origins, epidemiology, diagnosis, and treatment of superior mesenteric artery syndrome (SMAS), a rare condition. The patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 12-year-old girl with juvenile idiopathic scoliosis. She was seen by a pediatric orthopedist at age 8 after her primary care physician noticed a curve in her back during her physical examination. Given her age and primary curve of 25º, magnetic resonance imaging was ordered, which was negative for syrinx, tethered cord, or bony abnormalities. An underarm thoracolumbosacral orthosis (Boston Brace) was prescribed to be worn 23 hours/day. There was inconsistent follow-up over the next 4 years, and her curve progressed to 55º (right thoracic) and 47º in the lumbar spine (Figures 1, 2). Given the magnitude of the curves, surgical intervention was recommended, because bracing would no longer be beneficial.
The patient was healthy and appeared vibrant with no medical issues. She weighed 49 kg and her height was 162 cm (body mass index [BMI], 18.6; normal). She underwent segmental posterior spinal instrumentation, and a fusion was performed from T4 to L4 using a cobalt chrome rod. Postoperatively, there were no problems. Her diet was slowly advanced from clear liquids to regular food over 3 days. She was discharged on postoperative day 4. She had no abdominal distention, pain, or nausea. The family was instructed about pain medication (oxycodone liquid, 5 mg every 4 hours as needed) and how to prevent and treat constipation.
Three days after discharge, her mother called to inquire about positioning because the patient was uncomfortable owing to back pain. There were no abdominal complaints, and she was taking her pain medicine every 4 hours. She was instructed to lie in a comfortable position and to ambulate several times daily. The patient took little food or fluids because of a lack of appetite and back pain. On postoperative day 8, she presented to the emergency department with complaints of generalized abdominal pain and 1 day’s emesis. The patient had not had a bowel movement postoperatively. An acute abdominal series (AAS) was obtained (Figure 3), which noted a nonobstructive bowel gas pattern, with some increased colonic fecal retention. The patient was given intravenous (IV) fluids and an IV anti-emetic, and was admitted for observation. The pediatric surgical team evaluated her and concluded her symptoms resulted from constipation. Her symptoms improved over 2 to 3 days, and she had several bowel movements on day 2 after taking polyethylene glycol, sennosides, and bisacodyl suppositories. At discharge, she was noted to be passing gas, and her abdominal examination revealed no tenderness or guarding. She had mild distention, but it had improved from the previous day. She ate breakfast and ambulated several times. She had no complaints of abdominal pain and was released home with her parents. Staff reiterated instructions regarding constipation, diet, and follow-up. Her discharge weight was 48 kg (down 1 kg) and her BMI was 17.2 (down 1.4; underweight). Her height was now 165 cm (up 3 cm). Postoperative radiographs noted stable fixation with corrected curves (Figures 4, 5).
At home, the patient ate little but continued to drink fluids. On postdischarge day 3, she developed nausea, bilious emesis, and generalized abdominal pain. She returned to the emergency department. At this point, the patient weighed 44.5 kg (down 6.6 kg since the initial surgery) and her BMI was 16.1 (down 2.5; underweight). She was admitted, and IV fluids were initiated. She had more than 1300 mL of bilious emesis. A nasogastric (NG) tube was inserted. Initial laboratory findings were unremarkable other than an increase in serum lipase of 261 U/L. Her amylase level was within normal limits. An AAS was again completed and showed a distended stomach and loop of small bowel below the liver with an air fluid level. There were also distended loops of bowel in the pelvis (Figure 6).
A pediatric surgical consultant examined her the next morning. An upper gastrointestinal series (UGI) was obtained and showed air fluid levels in the stomach with prompt gastric emptying into a normal caliber duodenal bulb. However, with supine positioning, there was significant dilatation of the second portion of the duodenum with abrupt vertical cutoff just to the right of the spine, compatible with SMAS (Figure 7). There was reflux of contrast material into the stomach from the duodenum, with no passage of barium into the distal duodenum. After the UGI, a nasojejunal (NJ) feeding tube was placed. The tip was left at the beginning of the fourth part of the duodenum. Repeated attempts to pass the NJ feeding tube beyond the fourth part of the duodenum were unsuccessful because of massive gastric distention. The patient was taken to the operating room for placement of a Stamm gastrostomy feeding tube with insertion of a transgastric jejunal (G-J) feeding tube under fluoroscopy (Figure 5). The patient had the G-J feeding tube in place for 6 weeks to augment her enteral nutrition. As she gained weight, her duodenal emptying improved. She gradually transitioned to normal oral intake. She has done well since the G-J feeding tube was removed.
Discussion
Von Rokitansky first described SMAS in the mid-1800s.1 The exact pathology was further defined 60 years later when vascular involvement was determined to be the definitive mechanism of obstruction.2-4 Superior mesenteric artery syndrome is caused by the superior mesenteric vessels compressing the third portion of the duodenum, resulting in an extrinsic obstruction. This syndrome is also commonly called Wilkie disease, after Dr. David Wilkie, who first published in 1927 results of a comprehensive series of 75 patients.1 The syndrome is also known as arteriomesenteric duodenal compression, aortomesenteric syndrome, chronic duodenal ileus, megaduodenum, and cast syndrome.1,4,5 The term cast syndrome was derived from events in 1878, when Willet applied a body cast to a scoliosis patient who died after what was termed “fatal vomiting.”3
Epidemiology, Incidence, and Prevalence
While not unheard of, SMAS is an uncommon disorder. There have been only 400 documented reports in the English-language literature since 1980.5-8 Studies have stated that the incidence of the affected population is less than 0.4%.5,7,9,10 However, SMAS has been reported to have a mortality rate as high as 33% because of the uncommon nature of the disease and prolonged duration between onset of symptoms and diagnosis.7,9,11,12 The incidence of SMAS is higher after surgical procedures to correct spinal deformities, with rates between 0.5% and 4.7%.10,12,13 Females are affected more frequently than males (3:2 ratio).1,9,14 One large study with 80 patients that spanned 10 years reported that female incidence was 66%, and another study with 75 patients also observed that two-thirds of the patients were women.1,7 This syndrome commonly affects patients who are tall and thin with an asthenic body habitus.1,6,11,12 Superior mesenteric artery syndrome develops more commonly in younger patients. Previous studies noted that two-thirds of patients were between ages 10 and 39 years.1,8 However, given the right set of medical conditions, it can occur in patients of any age.2,9,15,16 In young, thin patients with scoliosis, the risk of developing SMAS after spinal fusion with instrumentation increases, given their already low weight coupled with the surgical intervention at the height of their longitudinal growth spurt.1,11,12
Other patients also at increased risk for developing SMAS include those with anorexia nervosa, psychiatric/emotional disorders, or drug addiction. It can also be found in persons on prolonged bedrest, those who have increased their activity and lost weight volitionally, or patients with illness or injuries, such as burns, trauma, or significant postoperative complications that decrease caloric intake and keep them in a supine position.2,6,17 The syndrome can be acute or chronic in its presentation.
Anatomy and Physiology
The superior mesenteric artery (SMA) comes off the right anterolateral portion of the abdominal aorta, which is just anterior to the L1 vertebra. It passes over the third part of the duodenum, generally at the L2 level (Figure 8A). The duodenum passes across the aorta at the level of the L3 vertebral body and is suspended between the aorta and the SMA by the ligament of Treitz (Figure 8B).3 The angle between the aorta and SMA (aortomesenteric angle) typically ranges from 25º to 60º with an average of 45º (Figure 8A). The distance between the aorta and SMA at the level of the duodenum is called the aortomesenteric distance, and it normally measures from 10 mm to 28 mm. Obstruction is usually observed at 2 mm to 8 mm (Figure 8C).1,3
Compression and outlet obstruction from narrowing of the SMA aortomesenteric angle can be caused by a multitude of problems.3,5,9,17 In chronic conditions, narrowing of the aorto-mesenteric angle could be the result of a shortened ligament, or a low origin of the SMA on the aorta, or a high insertion of the duodenum at the ligament of Treitz. Postoperatively, any change in anatomy caused by adhesions could result in compression as well. Most commonly, however, in those with significant weight loss, such as postoperative spinal fusion patients, there is loss of retroperitoneal fat, which normally acts as a cushion around the duodenum. This allows the SMA to move posteriorly obstructing the duodenum. Lying in a recumbent position along with weight loss also puts patients at risk after surgery.3,5,9,17 SMAS should be distinguished from other conditions that can cause duodenal obstruction, such as duodenal hematomas and congenital webs.
Symptoms and Patient Presentation
Whether SMAS is acute or chronic, most patients with SMAS present in a similar fashion. Almost all patients with acute SMAS complain of abdominal pain, nausea, and emesis (usually bilious) that usually occur after eating. Early satiety is commonly observed, resulting from delayed gastric emptying. Abdominal pain may improve when patients lie prone and are in the knee-chest, or lateral decubitus, position. These patients frequently have upper abdominal distention because of massive retention of gastric contents.4,6,16,18,19 Most spinal fusion patients present with these symptoms 7 to 10 days after surgery.11-13
Diagnosis
Our first diagnostic tool is a comprehensive history and physical examination. Once that is complete, many radiologic tests can be used to confirm the anatomic abnormality. The first test ordered is a simple AAS, which may show a “double bubble sign” (Figure 6), indicative of duodenal obstruction.4 There are several other tests, and each facility and surgeon has a preference as to which is considered the “gold standard.” Upper gastrointestinal (GI) barium studies are the simplest and most reliable. The barium test shows foregut anatomy and, to some extent, function. In SMAS patients, one should see duodenal dilatation and failure of the contrast to flow past the third section of the duodenum, along with an abrupt termination of the barium column as the duodenum crosses the vertebrae. This is the traditional method of diagnosis. There is minimal radiation, and the cost is less than that of many other tests, but it can be uncomfortable for the patient.1-4
At some institutions, an upper GI barium study is combined with angiography, which can be used to measure aortomesenteric angle and distance.1,3 Other practitioners prefer computed tomography (CT) with 3-dimensional reconstruction, which allows for measurement of the aortomesenteric angle and distance. In 1 study, CT was found to have an extremely high sensitivity and specificity for these measurements.10 CT angiography also identifies the obstruction with increased sensitivity, but it is rarely necessary and provides more radiation exposure and increased cost.1,6,14,19 Abdominal ultrasound has been used to measure the angle of the SMA and the aortomesenteric distance. When combined with endoscopy, this offers an alternative way to diagnose SMAS and decreases radiation exposure. However, it may require sedation or anesthesia.7,15,17 Overall, 3 criteria are used to define whether a patient has SMAS: duodenal dilatation, an aortomesenteric angle that is less than 25º, and an SMA that is shown to be compressing the third part of the duodenum.5
Treatment
Conservative treatment of SMAS usually starts by removing any precipitating factors present, such as a splint or cast that was applied for scoliosis, or ending activity associated with significant weight loss. Medical management consists of IV hydration, anti-emetics, oral feeding restriction, posture therapy, and placement of an NG tube for decompression. In most cases, patients will need to have an NJ feeding tube passed distal to the site of obstruction. This provides access for enteral feeding, and patients will gradually gain weight, repleting their retroperitoneal fat stores, which pushes the SMA forward and relieves the pressure on the duodenum. Electrolyte balance should be closely monitored along with weight gain. A nutritionist is often consulted to prevent underfeeding, which can produce a slow return to weight gain, poor wound healing, and loss of lean body muscle mass; or overfeeding, which can result in hyperglycemia and respiratory failure. Once patients are stable on enteral feedings, they can begin a slow return to oral intake.2-4,7,12 Total parental nutrition may be needed in some cases, but the risks associated with IV feeding usually outweigh the benefits.4 Almost all cases of acute SMAS can be successfully treated medically if diagnosed in a timely manner and supportive treatment begins promptly.7
Surgical intervention is rarely necessary for acute SMAS, but when conservative measures fail (after a 4- to 6-week trial), or in the presence of peptic ulcer disease or pancreatitis, this may become an appropriate option. In our patient, multiple attempts at passing an NJ feeding tube were unsuccessful, and she needed an operative procedure for insertion of a G-J feeding tube.
Further surgical intervention is usually reserved for those patients with long-standing SMAS for whom medical management has failed or other issues, such as pancreatitis, colitis, or megaduodenum, have arisen. Many operations are described in the literature. A duodenojejunostomy to bypass the site of the obstruction is one option. Another is duodenal derotation (Strong procedure) to alter the aortomesenteric angle and place the third and fourth duodenal portions to the right of the SMA. Other procedures include a Roux-en-Y duodenojejunostomy and duodenal uncrossing. A lateral duodenojejunostomy between the second portion of the duodenum and the jejunum is considered the simplest surgical technique. It achieves successful outcomes in 90% of cases.2-5,14 With regards to SMAS and scoliosis, it is extremely rare that this kind of surgical intervention would be necessary.
Conclusion
When planning operative spinal correction in scoliosis patients (especially females) who have a low BMI at the time of surgery and who have increased thoracic stiffness, be alert for signs and symptoms of SMAS. This rare complication can develop, and timely diagnosis and medical management will decrease morbidity and shorten the length of time needed for nutritional rehabilitation.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.
1. Lee TH, Lee JS, Jo Y, et al. Superior mesenteric artery syndrome: where do we stand today? J Gastrointest Surg. 2012;16(12):2203-2211.
2. Chan DK, Mak KS, Cheah YL. Successful nutritional therapy for superior mesenteric artery syndrome. Singapore Med J. 2012;53(11):e233-e236.
3. Beltrán OD, Martinez AV, Manrique Mdel C, Rodriguez JS, Febres EL, Peña SR. Superior mesenteric artery syndrome in a patient with Charcot Marie Tooth disease. World J Gastrointest Surg. 2011;3(12):197-200.
4. Verhoef PA, Rampal A. Unique challenges for appropriate management of a 16-year-old girl with superior mesenteric artery syndrome as a result of anorexia nervosa: a case report. J Med Case Rep. 2009;3:127.
5. Kingham TP, Shen R, Ren C. Laparoscopic treatment of superior mesenteric artery syndrome. JSLS. 2004;8(4):376-379.
6. Schauer SG, Thompson AJ, Bebarta VS. Superior mesenteric artery syndrome in a young military basic trainee. Mil Med. 2013;178(3):e398-e399.
7. Karrer FM, Jones SA, Vargas JH. Superior mesenteric artery syndrome. Treatment and management. Medscape. http://emedicine.medscape.com/article/932220. Updated July 27, 2015. Accessed August 3, 2015.
8. Arthurs OJ, Mehta U, Set PA. Nutcracker and SMA syndromes: What is the normal SMA angle in children? Eur J Radiol. 2012;81(8):e854-e861.
9. Capitano S, Donatelli G, Boccoli G. Superior mesenteric artery syndrome--Believe in it! Report of a case. Case Rep Surg. 2012;2012(10):282646.
10. Sabbagh C, Santin E, Potier A, Regimbeau JM. The superior mesenteric artery syndrome: a rare etiology for proximal obstructive syndrome. J Visc Surg. 2012;149(6):428-429.
11. Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665-668.
12. Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2(9):9.
13. Hod-Feins R, Copeliovitch L, Abu-Kishk I, et al. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345-349.
14. Kennedy KV, Yela R, Achalandabaso Mdel M, Martín-Pérez E. Superior mesenteric artery syndrome: diagnostic and therapeutic considerations. Rev Esp Enferm Dig. 2013;105(4):236-238.
15. Agrawal S, Patel H. Superior mesenteric artery syndrome. Surgery. 2013;153(4):601-602.
16. Felton BM, White JM, Racine MA. An uncommon case of abdominal pain: superior mesenteric artery syndrome. West J Emerg Med. 2012;13(6):501-502.
17. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol. 2011;25(11):599-600.
18. Bauer S, Karplus R, Belsky V, Mha HA. Superior mesenteric artery syndrome: a forgotten entity. Isr Med Assoc J. 2013;15(4):189-191.
19. Ricca RL, Kasten J, Javid PJ. Superior mesenteric artery syndrome after minimally invasive correction of pectus excavatum: impact of post-operative weight loss. J Pediatr Surg. 2012;47(11):2137-2139.
Isolated Radiopalmar Dislocation of Fifth Carpometacarpal Joint: A Rare Presentation
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
Nonoperative Management of Multiple Hand Enchondromas in Ollier Disease With Progressive Ossification
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Which interventions can increase breastfeeding duration?
Breastfeeding support, beyond standard care, from lay people or professionals increases both short- and long-term breastfeeding duration (strength of recommendation: B, meta-analyses of randomized controlled trials [RCTs] with demonstrated heterogeneity).
EVIDENCE SUMMARY
A 2012 Cochrane review of 52 studies (44 RCTs and 8 cluster-randomized trials; N=56,451) assessed the overall effectiveness of multiple supportive measures on decreasing cessation of “any” (partial and exclusive) and “exclusive” breastfeeding compared with usual care.1 Participants were healthy breastfeeding mothers of healthy term babies. Support interventions were defined broadly but included individual and group interactions, as well as contact in person or over the phone by professionals or lay volunteers. Patients were approached proactively or reactively upon request, and the interventions occurred one or more times.
The interventions reduced discontinuation rates among both “exclusive” and “any” breastfeeding mothers (TABLE1). The review found lay and professional support to be equally effective at promoting continuation of breastfeeding. Limitations include a moderate to high amount of heterogeneity, as well as the inherent difficulty of blinding subjects in the studies.
Lay support can make a significant difference in the short term
A 2008 systematic review of 38 RCTs (N=29,020) compared any counseling or behavioral intervention initiated from a clinician’s practice (office or hospital) with usual care.2 The review excluded community and peer-initiated interventions. The reviewers defined breastfeeding duration as follows: initiation (up to 2 weeks), short-term (one to 3 months), intermediate-term (4 to 5 months), long-term (6 to 8 months), and prolonged (9 or more months). Investigators also analyzed breastfeeding rates by “exclusive” and “nonexclusive” (formula supplementation) regimens.
For nonexclusive breastfeeding, the review found interventions to promote breastfeeding improved rates only at initiation (18 RCTs, N=7688; relative risk [RR] for cessation of breastfeeding=1.04; 95% confidence interval [CI], 1.0-1.08; number needed to treat [NNT]=38) and in the short term (18 RCTs, N= 19,358; RR=1.10; 95% CI, 1.02-1.19; NNT=7). For exclusive breastfeeding, interventions improved rates only in the short term (17 RCTs, N=20,552; RR=1.72; 95% CI, 1.0-2.97; NNT=3).
The review found that lay support (defined as counseling or social support from peers) but not professional support was significantly associated with improving rates of both “nonexclusive” and “exclusive’ breastfeeding, but only over the short term (5 RCTs, N not provided; RR=1.22; 95% CI, 1.08-1.37; and 4 RCTs, N not provided; RR=1.65; 95% CI, 1.03-2.63; respectively). As with the Cochrane review, the results for all study groups demonstrated moderate to significant heterogeneity.
RECOMMENDATIONS
The Surgeon General, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists all recommend that women be educated about the benefits of breastfeeding and receive supportive interventions before and after delivery.3-6
1. Renfrew MJ, McCormick FM, Wade A, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2012;5:CD001141.
2. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
3. United States Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding. US Department of Health and Human Services, Office of the Surgeon General Web site. Available at: http://www.surgeongeneral.gov/library/calls/breastfeeding/. Accessed January 19, 2015.
4. American Academy of Family Physicians. Breastfeeding, Family Physicians Supporting (Position Paper). American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/breastfeeding-support.html (updated Nov. 4, 2014). Accessed January 19, 2015.
5. Johnson M, Landers S, Noble L, et al. American Academy of Pediatrics, Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841.
6. Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 361: Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 Pt 1):479-480.
Breastfeeding support, beyond standard care, from lay people or professionals increases both short- and long-term breastfeeding duration (strength of recommendation: B, meta-analyses of randomized controlled trials [RCTs] with demonstrated heterogeneity).
EVIDENCE SUMMARY
A 2012 Cochrane review of 52 studies (44 RCTs and 8 cluster-randomized trials; N=56,451) assessed the overall effectiveness of multiple supportive measures on decreasing cessation of “any” (partial and exclusive) and “exclusive” breastfeeding compared with usual care.1 Participants were healthy breastfeeding mothers of healthy term babies. Support interventions were defined broadly but included individual and group interactions, as well as contact in person or over the phone by professionals or lay volunteers. Patients were approached proactively or reactively upon request, and the interventions occurred one or more times.
The interventions reduced discontinuation rates among both “exclusive” and “any” breastfeeding mothers (TABLE1). The review found lay and professional support to be equally effective at promoting continuation of breastfeeding. Limitations include a moderate to high amount of heterogeneity, as well as the inherent difficulty of blinding subjects in the studies.
Lay support can make a significant difference in the short term
A 2008 systematic review of 38 RCTs (N=29,020) compared any counseling or behavioral intervention initiated from a clinician’s practice (office or hospital) with usual care.2 The review excluded community and peer-initiated interventions. The reviewers defined breastfeeding duration as follows: initiation (up to 2 weeks), short-term (one to 3 months), intermediate-term (4 to 5 months), long-term (6 to 8 months), and prolonged (9 or more months). Investigators also analyzed breastfeeding rates by “exclusive” and “nonexclusive” (formula supplementation) regimens.
For nonexclusive breastfeeding, the review found interventions to promote breastfeeding improved rates only at initiation (18 RCTs, N=7688; relative risk [RR] for cessation of breastfeeding=1.04; 95% confidence interval [CI], 1.0-1.08; number needed to treat [NNT]=38) and in the short term (18 RCTs, N= 19,358; RR=1.10; 95% CI, 1.02-1.19; NNT=7). For exclusive breastfeeding, interventions improved rates only in the short term (17 RCTs, N=20,552; RR=1.72; 95% CI, 1.0-2.97; NNT=3).
The review found that lay support (defined as counseling or social support from peers) but not professional support was significantly associated with improving rates of both “nonexclusive” and “exclusive’ breastfeeding, but only over the short term (5 RCTs, N not provided; RR=1.22; 95% CI, 1.08-1.37; and 4 RCTs, N not provided; RR=1.65; 95% CI, 1.03-2.63; respectively). As with the Cochrane review, the results for all study groups demonstrated moderate to significant heterogeneity.

RECOMMENDATIONS
The Surgeon General, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists all recommend that women be educated about the benefits of breastfeeding and receive supportive interventions before and after delivery.3-6
Breastfeeding support, beyond standard care, from lay people or professionals increases both short- and long-term breastfeeding duration (strength of recommendation: B, meta-analyses of randomized controlled trials [RCTs] with demonstrated heterogeneity).
EVIDENCE SUMMARY
A 2012 Cochrane review of 52 studies (44 RCTs and 8 cluster-randomized trials; N=56,451) assessed the overall effectiveness of multiple supportive measures on decreasing cessation of “any” (partial and exclusive) and “exclusive” breastfeeding compared with usual care.1 Participants were healthy breastfeeding mothers of healthy term babies. Support interventions were defined broadly but included individual and group interactions, as well as contact in person or over the phone by professionals or lay volunteers. Patients were approached proactively or reactively upon request, and the interventions occurred one or more times.
The interventions reduced discontinuation rates among both “exclusive” and “any” breastfeeding mothers (TABLE1). The review found lay and professional support to be equally effective at promoting continuation of breastfeeding. Limitations include a moderate to high amount of heterogeneity, as well as the inherent difficulty of blinding subjects in the studies.
Lay support can make a significant difference in the short term
A 2008 systematic review of 38 RCTs (N=29,020) compared any counseling or behavioral intervention initiated from a clinician’s practice (office or hospital) with usual care.2 The review excluded community and peer-initiated interventions. The reviewers defined breastfeeding duration as follows: initiation (up to 2 weeks), short-term (one to 3 months), intermediate-term (4 to 5 months), long-term (6 to 8 months), and prolonged (9 or more months). Investigators also analyzed breastfeeding rates by “exclusive” and “nonexclusive” (formula supplementation) regimens.
For nonexclusive breastfeeding, the review found interventions to promote breastfeeding improved rates only at initiation (18 RCTs, N=7688; relative risk [RR] for cessation of breastfeeding=1.04; 95% confidence interval [CI], 1.0-1.08; number needed to treat [NNT]=38) and in the short term (18 RCTs, N= 19,358; RR=1.10; 95% CI, 1.02-1.19; NNT=7). For exclusive breastfeeding, interventions improved rates only in the short term (17 RCTs, N=20,552; RR=1.72; 95% CI, 1.0-2.97; NNT=3).
The review found that lay support (defined as counseling or social support from peers) but not professional support was significantly associated with improving rates of both “nonexclusive” and “exclusive’ breastfeeding, but only over the short term (5 RCTs, N not provided; RR=1.22; 95% CI, 1.08-1.37; and 4 RCTs, N not provided; RR=1.65; 95% CI, 1.03-2.63; respectively). As with the Cochrane review, the results for all study groups demonstrated moderate to significant heterogeneity.

RECOMMENDATIONS
The Surgeon General, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists all recommend that women be educated about the benefits of breastfeeding and receive supportive interventions before and after delivery.3-6
1. Renfrew MJ, McCormick FM, Wade A, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2012;5:CD001141.
2. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
3. United States Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding. US Department of Health and Human Services, Office of the Surgeon General Web site. Available at: http://www.surgeongeneral.gov/library/calls/breastfeeding/. Accessed January 19, 2015.
4. American Academy of Family Physicians. Breastfeeding, Family Physicians Supporting (Position Paper). American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/breastfeeding-support.html (updated Nov. 4, 2014). Accessed January 19, 2015.
5. Johnson M, Landers S, Noble L, et al. American Academy of Pediatrics, Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841.
6. Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 361: Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 Pt 1):479-480.
1. Renfrew MJ, McCormick FM, Wade A, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev. 2012;5:CD001141.
2. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
3. United States Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding. US Department of Health and Human Services, Office of the Surgeon General Web site. Available at: http://www.surgeongeneral.gov/library/calls/breastfeeding/. Accessed January 19, 2015.
4. American Academy of Family Physicians. Breastfeeding, Family Physicians Supporting (Position Paper). American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/breastfeeding-support.html (updated Nov. 4, 2014). Accessed January 19, 2015.
5. Johnson M, Landers S, Noble L, et al. American Academy of Pediatrics, Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841.
6. Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 361: Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 Pt 1):479-480.
Evidence-based answers from the Family Physicians Inquiries Network
Should these complaints have prompted a colonoscopy? ... Complication of pregnancy goes undetected after delivery
Should these complaints have prompted a colonoscopy?
A 45-YEAR-OLD WOMAN went to her primary care physician due to cramping abdominal pain after eating. She hadn’t seen her physician in 5 years and noted that her bowel movements were somewhat smaller than usual. Her physician suspected an ulcer and treated her with acid-reducing medication.
A month later, the patient returned with similar symptoms and said that her bowel movements were somewhat loose. The physician increased the dosage of the acid-reducing medication. The patient returned again a month later and reported constipation. The stomach issues continued and she was referred to a gynecologist. Ultimately, she went to a gastroenterologist and underwent a colonoscopy 8 months after her first visit. She was diagnosed with stage IV colon cancer with metastasis to the ovaries. The patient passed away 8 years later.
PLAINTIFF’S CLAIM The physician was negligent in failing to suspect colon cancer and perform a colonoscopy, digital rectal exam, or fecal occult blood test.
THE DEFENSE The decedent’s symptoms were inconsistent with cancer and did not indicate the need for a colonoscopy. The cancer was already advanced and the outcome would not have changed.
VERDICT $2.16 million Massachusetts verdict.
COMMENT Wow, this is a tough one! I am not at all sure I would have diagnosed this correctly. Is there a lesson here? Perhaps the history was not sufficiently thorough? Perhaps these were totally new symptoms that should have demanded a more thorough investigation? Or perhaps it would have taken 4 to 6 months for any of us to make this diagnosis in a 45-year-old woman.
Complication of pregnancy goes undetected after delivery
A 31-YEAR-OLD WOMAN went to the emergency department (ED) complaining of tightness in her chest, difficulty breathing, and swelling in her lower legs 4 days after she delivered a child. The ED physician ruled out a pulmonary embolism and discharged her. Three days later, she returned with the same symptoms, but her legs were more swollen and her systolic blood pressure was above 160 mm Hg. She was sent home again. The woman had a seizure 4 days later. In the ambulance on the way to the hospital and following her arrival, she suffered more seizures. A few days later, she was transferred to a different facility and died soon after.
PLAINTIFF’S CLAIM The hospital and 2 ED physicians were negligent in failing to diagnose and treat postpartum preeclampsia during the ED visits. This led to the seizures, brain damage, and death. Antihypertensive and anti-seizure medications would have prevented her death.
THE DEFENSE The actions taken were reasonable, especially because the decedent had no symptoms of preeclampsia during pregnancy or delivery.
VERDICT $6.9 million Illinois settlement.
COMMENT This case speaks for itself. The physicians involved appear to have had a knowledge gap since they apparently did not consider preeclampsia in the differential. Primary care physicians and emergency physicians must be trained to recognize complications of pregnancy.
Should these complaints have prompted a colonoscopy?
A 45-YEAR-OLD WOMAN went to her primary care physician due to cramping abdominal pain after eating. She hadn’t seen her physician in 5 years and noted that her bowel movements were somewhat smaller than usual. Her physician suspected an ulcer and treated her with acid-reducing medication.
A month later, the patient returned with similar symptoms and said that her bowel movements were somewhat loose. The physician increased the dosage of the acid-reducing medication. The patient returned again a month later and reported constipation. The stomach issues continued and she was referred to a gynecologist. Ultimately, she went to a gastroenterologist and underwent a colonoscopy 8 months after her first visit. She was diagnosed with stage IV colon cancer with metastasis to the ovaries. The patient passed away 8 years later.
PLAINTIFF’S CLAIM The physician was negligent in failing to suspect colon cancer and perform a colonoscopy, digital rectal exam, or fecal occult blood test.
THE DEFENSE The decedent’s symptoms were inconsistent with cancer and did not indicate the need for a colonoscopy. The cancer was already advanced and the outcome would not have changed.
VERDICT $2.16 million Massachusetts verdict.
COMMENT Wow, this is a tough one! I am not at all sure I would have diagnosed this correctly. Is there a lesson here? Perhaps the history was not sufficiently thorough? Perhaps these were totally new symptoms that should have demanded a more thorough investigation? Or perhaps it would have taken 4 to 6 months for any of us to make this diagnosis in a 45-year-old woman.
Complication of pregnancy goes undetected after delivery
A 31-YEAR-OLD WOMAN went to the emergency department (ED) complaining of tightness in her chest, difficulty breathing, and swelling in her lower legs 4 days after she delivered a child. The ED physician ruled out a pulmonary embolism and discharged her. Three days later, she returned with the same symptoms, but her legs were more swollen and her systolic blood pressure was above 160 mm Hg. She was sent home again. The woman had a seizure 4 days later. In the ambulance on the way to the hospital and following her arrival, she suffered more seizures. A few days later, she was transferred to a different facility and died soon after.
PLAINTIFF’S CLAIM The hospital and 2 ED physicians were negligent in failing to diagnose and treat postpartum preeclampsia during the ED visits. This led to the seizures, brain damage, and death. Antihypertensive and anti-seizure medications would have prevented her death.
THE DEFENSE The actions taken were reasonable, especially because the decedent had no symptoms of preeclampsia during pregnancy or delivery.
VERDICT $6.9 million Illinois settlement.
COMMENT This case speaks for itself. The physicians involved appear to have had a knowledge gap since they apparently did not consider preeclampsia in the differential. Primary care physicians and emergency physicians must be trained to recognize complications of pregnancy.
Should these complaints have prompted a colonoscopy?
A 45-YEAR-OLD WOMAN went to her primary care physician due to cramping abdominal pain after eating. She hadn’t seen her physician in 5 years and noted that her bowel movements were somewhat smaller than usual. Her physician suspected an ulcer and treated her with acid-reducing medication.
A month later, the patient returned with similar symptoms and said that her bowel movements were somewhat loose. The physician increased the dosage of the acid-reducing medication. The patient returned again a month later and reported constipation. The stomach issues continued and she was referred to a gynecologist. Ultimately, she went to a gastroenterologist and underwent a colonoscopy 8 months after her first visit. She was diagnosed with stage IV colon cancer with metastasis to the ovaries. The patient passed away 8 years later.
PLAINTIFF’S CLAIM The physician was negligent in failing to suspect colon cancer and perform a colonoscopy, digital rectal exam, or fecal occult blood test.
THE DEFENSE The decedent’s symptoms were inconsistent with cancer and did not indicate the need for a colonoscopy. The cancer was already advanced and the outcome would not have changed.
VERDICT $2.16 million Massachusetts verdict.
COMMENT Wow, this is a tough one! I am not at all sure I would have diagnosed this correctly. Is there a lesson here? Perhaps the history was not sufficiently thorough? Perhaps these were totally new symptoms that should have demanded a more thorough investigation? Or perhaps it would have taken 4 to 6 months for any of us to make this diagnosis in a 45-year-old woman.
Complication of pregnancy goes undetected after delivery
A 31-YEAR-OLD WOMAN went to the emergency department (ED) complaining of tightness in her chest, difficulty breathing, and swelling in her lower legs 4 days after she delivered a child. The ED physician ruled out a pulmonary embolism and discharged her. Three days later, she returned with the same symptoms, but her legs were more swollen and her systolic blood pressure was above 160 mm Hg. She was sent home again. The woman had a seizure 4 days later. In the ambulance on the way to the hospital and following her arrival, she suffered more seizures. A few days later, she was transferred to a different facility and died soon after.
PLAINTIFF’S CLAIM The hospital and 2 ED physicians were negligent in failing to diagnose and treat postpartum preeclampsia during the ED visits. This led to the seizures, brain damage, and death. Antihypertensive and anti-seizure medications would have prevented her death.
THE DEFENSE The actions taken were reasonable, especially because the decedent had no symptoms of preeclampsia during pregnancy or delivery.
VERDICT $6.9 million Illinois settlement.
COMMENT This case speaks for itself. The physicians involved appear to have had a knowledge gap since they apparently did not consider preeclampsia in the differential. Primary care physicians and emergency physicians must be trained to recognize complications of pregnancy.
Examine the patient, not just the evidence
Dr. Hickner’s editorial “Let’s talk about the evidence” (J Fam Pract. 2015;64:337) struck a chord with me. I am very supportive of evidence-based medicine (EBM), but am often dismayed by the lack of humility expressed by EBM leaders, including the US Preventive Services Task Force. We have so little evidence about much of what we do in family medicine, and most evidence comes from studies that are narrow by nature (reductionist research).
For example, doing a physical exam is part of “laying on of hands” that is part of the art of medicine. Abraham Verghese, MD, MACP, has written and spoken about the importance of examining the patient and not just depending on data.1 Yet elements of the physical exam, such as the pelvic exam example Dr. Hickner mentioned in his editorial, do not stand up well in EBM due to a lack of diagnostic accuracy. I’ll ask this: Who has studied the harm that may be caused by not examining our patients?
My physical exam “ritual” takes less than 10 minutes, and the value in the relationship I have with patients is more than a diagnostic exercise. Increasingly, I see patients become annoyed and critical of physicians who do not examine them.
Joseph E. Scherger, MD, MPH
Rancho Mirage, Calif
1. TED Talks. Abraham Verghese: A Doctor’s Touch. TED Web site. Available at: http://www.ted.com/talks/abraham_verghese_a_doctor_s_touch. Accessed July 20, 2015.
Author’s response:
Dr. Scherger makes an excellent point about the importance of physical touch for the doctor-patient relationship. The question is: What touching is appropriate? In my own experience, I have noticed that most—but not all—of the women I see are quite relieved that they don’t need yearly pelvic exams, and women I see for pap smears do not seem put off if I do not do a bimanual exam. The data are actually quite strong that routine pelvic exams in asymptomatic women lead to more harm than good. They uncover way too many false positives and almost no true positive findings, leading to unnecessary testing and treatment.1,2
John Hickner, MD, MSc
Chicago, Ill
Dr. Hickner is the editor-in-chief of The Journal of Family Practice
1. Ebell MH, Culp M, Lastinger K, et al. A systematic review of the bimanual examination as a test for ovarian cancer. Am J Prev Med. 2015;48:350–356.
2. Well-woman visit. Committee Opinion No. 534. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:421-424.
Dr. Hickner’s editorial “Let’s talk about the evidence” (J Fam Pract. 2015;64:337) struck a chord with me. I am very supportive of evidence-based medicine (EBM), but am often dismayed by the lack of humility expressed by EBM leaders, including the US Preventive Services Task Force. We have so little evidence about much of what we do in family medicine, and most evidence comes from studies that are narrow by nature (reductionist research).
For example, doing a physical exam is part of “laying on of hands” that is part of the art of medicine. Abraham Verghese, MD, MACP, has written and spoken about the importance of examining the patient and not just depending on data.1 Yet elements of the physical exam, such as the pelvic exam example Dr. Hickner mentioned in his editorial, do not stand up well in EBM due to a lack of diagnostic accuracy. I’ll ask this: Who has studied the harm that may be caused by not examining our patients?
My physical exam “ritual” takes less than 10 minutes, and the value in the relationship I have with patients is more than a diagnostic exercise. Increasingly, I see patients become annoyed and critical of physicians who do not examine them.
Joseph E. Scherger, MD, MPH
Rancho Mirage, Calif
1. TED Talks. Abraham Verghese: A Doctor’s Touch. TED Web site. Available at: http://www.ted.com/talks/abraham_verghese_a_doctor_s_touch. Accessed July 20, 2015.
Author’s response:
Dr. Scherger makes an excellent point about the importance of physical touch for the doctor-patient relationship. The question is: What touching is appropriate? In my own experience, I have noticed that most—but not all—of the women I see are quite relieved that they don’t need yearly pelvic exams, and women I see for pap smears do not seem put off if I do not do a bimanual exam. The data are actually quite strong that routine pelvic exams in asymptomatic women lead to more harm than good. They uncover way too many false positives and almost no true positive findings, leading to unnecessary testing and treatment.1,2
John Hickner, MD, MSc
Chicago, Ill
Dr. Hickner is the editor-in-chief of The Journal of Family Practice
1. Ebell MH, Culp M, Lastinger K, et al. A systematic review of the bimanual examination as a test for ovarian cancer. Am J Prev Med. 2015;48:350–356.
2. Well-woman visit. Committee Opinion No. 534. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:421-424.
Dr. Hickner’s editorial “Let’s talk about the evidence” (J Fam Pract. 2015;64:337) struck a chord with me. I am very supportive of evidence-based medicine (EBM), but am often dismayed by the lack of humility expressed by EBM leaders, including the US Preventive Services Task Force. We have so little evidence about much of what we do in family medicine, and most evidence comes from studies that are narrow by nature (reductionist research).
For example, doing a physical exam is part of “laying on of hands” that is part of the art of medicine. Abraham Verghese, MD, MACP, has written and spoken about the importance of examining the patient and not just depending on data.1 Yet elements of the physical exam, such as the pelvic exam example Dr. Hickner mentioned in his editorial, do not stand up well in EBM due to a lack of diagnostic accuracy. I’ll ask this: Who has studied the harm that may be caused by not examining our patients?
My physical exam “ritual” takes less than 10 minutes, and the value in the relationship I have with patients is more than a diagnostic exercise. Increasingly, I see patients become annoyed and critical of physicians who do not examine them.
Joseph E. Scherger, MD, MPH
Rancho Mirage, Calif
1. TED Talks. Abraham Verghese: A Doctor’s Touch. TED Web site. Available at: http://www.ted.com/talks/abraham_verghese_a_doctor_s_touch. Accessed July 20, 2015.
Author’s response:
Dr. Scherger makes an excellent point about the importance of physical touch for the doctor-patient relationship. The question is: What touching is appropriate? In my own experience, I have noticed that most—but not all—of the women I see are quite relieved that they don’t need yearly pelvic exams, and women I see for pap smears do not seem put off if I do not do a bimanual exam. The data are actually quite strong that routine pelvic exams in asymptomatic women lead to more harm than good. They uncover way too many false positives and almost no true positive findings, leading to unnecessary testing and treatment.1,2
John Hickner, MD, MSc
Chicago, Ill
Dr. Hickner is the editor-in-chief of The Journal of Family Practice
1. Ebell MH, Culp M, Lastinger K, et al. A systematic review of the bimanual examination as a test for ovarian cancer. Am J Prev Med. 2015;48:350–356.
2. Well-woman visit. Committee Opinion No. 534. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2012;120:421-424.
How effective are opioids for chronic low back pain?
Short-term (<4 months) treatment with opioids provides modest relief of chronic low back pain, but only minimal improvement in function compared with placebo (strength of recommendation [SOR]: B, systematic review of lower-quality randomized controlled trials [RCTs]).
Tramadol isn’t superior to nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief (SOR: A, consistent results from RCTs). In addition, oxycodone with titrated morphine isn’t better than naproxen for relieving pain or improving function (SOR: C, a low-quality RCT).
Although no long-term RCTs have been done, cohort studies have shown that 6 to 12 months of opioid use is associated with a small decrease in pain and either very minimal improvement in, or worsening of, disability (SOR: B, prospective cohort trials).
EVIDENCE SUMMARY
A systematic review and meta-analysis of 15 RCTs with a total enrollment of 5540 assessed the efficacy of opioids in adults with chronic low back pain of at least 12 weeks’ duration.1 Five low-quality studies (1378 patients) that compared tramadol with placebo found tramadol to be moderately superior to placebo for relieving pain (standard mean difference [SMD]= -0.55; 95% confidence interval [CI], -0.66 to -0.44) but only modestly better for improving function (SMD= −0.18; 95% CI, -0.29 to -0.07).
Six trials with 1887 patients compared strong opioids (morphine, hydromorphone, oxycodone, oxymorphone, and tapentadol) with placebo. The opioids were better than placebo for improving pain (SMD= -0.43; 95% CI, -0.52 to -0.33) and function (SMD= -0.26; 95% CI, -0.37 to -0.15). The general interpretation of SMD effect size is 0.2=small, 0.5=medium, 0.8=large. In this case, larger negative numbers correlate with greater improvement.
How opioids stack up against NSAIDs
Two separate double-blind, double-dummy studies randomized adults with low back pain of at least 12 weeks’ duration to receive celecoxib 200 mg twice daily (404 and 398 patients, respectively) or tramadol 50 mg 4 times daily (392 and 404 patients, respectively) for 6 weeks.2 The primary outcome measure was at least a 30% improvement in pain using a 0 (no pain) to 10 (worst possible pain) scale. In both studies, more patients taking celecoxib had positive responses than patients taking tramadol (63% vs 50%, P<.001, and 64% vs 55%, P<.008, respectively).
A small RCT (36 patients who had suffered low back pain for more than 6 months) randomized patients to one of 3 treatment groups for 16 weeks: oxycodone as much as 20 mg/d (13 patients); naproxen as much as 1 g/d (12 patients); or oxycodone and sustained-release morphine (titrated up to 200 mg morphine equivalent/d (11 patients).3 After 16 weeks, patients receiving oxycodone or naproxen were treated with oxycodone and sustained-release morphine for another 16 weeks, as were patients already receiving this therapy. Pain was assessed on a 0 (none) to 100 (worst possible pain) scale.
Both opioid groups had significantly less pain on average (59.8 for oxycodone, 54.9 for titrated morphine) than the naproxen group (65.5; F=16.07; P<.001) but no significant difference in activity level. However, an independent analysis of the naproxen group and titrated morphine group found no significant difference in either pain relief (SMD= -0.58; 95% CI, -1.42 to 0.26) or disability (SMD= -0.06; 95% CI, -0.88 to 0.76) between the 2 groups.4
How does long-term opioid use affect pain and function?
Two prospective cohort studies have evaluated long-term opioid use. The first (715 patients) used a Roland-Morris Disability Questionnaire (RMDQ) to assess disability at 6 months in patients taking opioids compared with patients not taking opioids.5 Patients using opioids showed an increase in RMDQ score of 1.18 units (95% CI, 0.17-2.19) on a 0 to 24 scale, with 24 representing greatest disability.
The second study evaluated pain and function in 1843 adults with acute back injuries taking opioids for a year.6 Pain, rated on a 0 to 10 scale, decreased from 7.7 at baseline to 6.8 at one year (no P value). At the end of the first quarter, the RMDQ score decreased from 18.8 at baseline (the end of the first quarter) to 17.5 at one year (no P value). Clinically meaningful improvement in pain and function (30% or more) occurred in 26% (95% CI, 18%-36%) and 16% (95% CI, 10%-25%) of patients, respectively.
RECOMMENDATIONS
The 2007 clinical practice guideline on low back pain from The American College of Physicians and American Pain Society recommends opioids, including tramadol, for patients with severe back pain who don’t get adequate relief from acetaminophen or NSAIDs.7
The 2009 National Institute for Health and Care Excellence (NICE) guidelines for early management of persistent, nonspecific low back pain recommend considering strong opioids (buprenorphine, fentanyl, and oxycodone) for short-term use in severe pain and referral to a specialist for patients requiring prolonged use of strong opioids.8
The 2013 British Pain Society guidelines for low back and radicular pain recommend tight restrictions on the use of strong opioids. They also recommend giving the lowest possible dose of opioids for the shortest time possible.9
1. Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976). 2014;39:556-563.
2. O’Donnell JB, Ekman EF, Spalding WM, et al. The effectiveness of a weak opioid medication versus a cyclo-oxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug in treating flare-up of chronic low-back pain: results from two randomized, double-blind, 6-week studies. J Int Med Res. 2009;37:1789-1802.
3. Jamison RN, Raymond SA, Slawsby EA, et al. Opioid therapy for chronic noncancer back pain. A randomized prospective study. Spine (Phila Pa 1976). 1998;23:2591-2600.
4. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36(21 Suppl):S131-S43.
5. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
6. Franklin GM, Rahman EA, Turner JA, et al. Opioid use for chronic low back pain: A prospective, population-based study among injured workers in Washington state, 2002-2005. Clin J Pain. 2009;25:743-751.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
8. National Institute for Health and Care Excellence. Low back pain: early management of persistent non-specific low back pain. National Institute for Health and Care Excellence Web site. Available at: http://guidance.nice.org.uk/CG88. Accessed April 1, 2015.
9. Lee J, Gupta S, Price C, et al; British Pain Society. Low back and radicular pain: a pathway for care developed by the British Pain Society. Br J Anaesth. 2013;111:112-120.
Short-term (<4 months) treatment with opioids provides modest relief of chronic low back pain, but only minimal improvement in function compared with placebo (strength of recommendation [SOR]: B, systematic review of lower-quality randomized controlled trials [RCTs]).
Tramadol isn’t superior to nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief (SOR: A, consistent results from RCTs). In addition, oxycodone with titrated morphine isn’t better than naproxen for relieving pain or improving function (SOR: C, a low-quality RCT).
Although no long-term RCTs have been done, cohort studies have shown that 6 to 12 months of opioid use is associated with a small decrease in pain and either very minimal improvement in, or worsening of, disability (SOR: B, prospective cohort trials).
EVIDENCE SUMMARY
A systematic review and meta-analysis of 15 RCTs with a total enrollment of 5540 assessed the efficacy of opioids in adults with chronic low back pain of at least 12 weeks’ duration.1 Five low-quality studies (1378 patients) that compared tramadol with placebo found tramadol to be moderately superior to placebo for relieving pain (standard mean difference [SMD]= -0.55; 95% confidence interval [CI], -0.66 to -0.44) but only modestly better for improving function (SMD= −0.18; 95% CI, -0.29 to -0.07).
Six trials with 1887 patients compared strong opioids (morphine, hydromorphone, oxycodone, oxymorphone, and tapentadol) with placebo. The opioids were better than placebo for improving pain (SMD= -0.43; 95% CI, -0.52 to -0.33) and function (SMD= -0.26; 95% CI, -0.37 to -0.15). The general interpretation of SMD effect size is 0.2=small, 0.5=medium, 0.8=large. In this case, larger negative numbers correlate with greater improvement.
How opioids stack up against NSAIDs
Two separate double-blind, double-dummy studies randomized adults with low back pain of at least 12 weeks’ duration to receive celecoxib 200 mg twice daily (404 and 398 patients, respectively) or tramadol 50 mg 4 times daily (392 and 404 patients, respectively) for 6 weeks.2 The primary outcome measure was at least a 30% improvement in pain using a 0 (no pain) to 10 (worst possible pain) scale. In both studies, more patients taking celecoxib had positive responses than patients taking tramadol (63% vs 50%, P<.001, and 64% vs 55%, P<.008, respectively).
A small RCT (36 patients who had suffered low back pain for more than 6 months) randomized patients to one of 3 treatment groups for 16 weeks: oxycodone as much as 20 mg/d (13 patients); naproxen as much as 1 g/d (12 patients); or oxycodone and sustained-release morphine (titrated up to 200 mg morphine equivalent/d (11 patients).3 After 16 weeks, patients receiving oxycodone or naproxen were treated with oxycodone and sustained-release morphine for another 16 weeks, as were patients already receiving this therapy. Pain was assessed on a 0 (none) to 100 (worst possible pain) scale.
Both opioid groups had significantly less pain on average (59.8 for oxycodone, 54.9 for titrated morphine) than the naproxen group (65.5; F=16.07; P<.001) but no significant difference in activity level. However, an independent analysis of the naproxen group and titrated morphine group found no significant difference in either pain relief (SMD= -0.58; 95% CI, -1.42 to 0.26) or disability (SMD= -0.06; 95% CI, -0.88 to 0.76) between the 2 groups.4
How does long-term opioid use affect pain and function?
Two prospective cohort studies have evaluated long-term opioid use. The first (715 patients) used a Roland-Morris Disability Questionnaire (RMDQ) to assess disability at 6 months in patients taking opioids compared with patients not taking opioids.5 Patients using opioids showed an increase in RMDQ score of 1.18 units (95% CI, 0.17-2.19) on a 0 to 24 scale, with 24 representing greatest disability.
The second study evaluated pain and function in 1843 adults with acute back injuries taking opioids for a year.6 Pain, rated on a 0 to 10 scale, decreased from 7.7 at baseline to 6.8 at one year (no P value). At the end of the first quarter, the RMDQ score decreased from 18.8 at baseline (the end of the first quarter) to 17.5 at one year (no P value). Clinically meaningful improvement in pain and function (30% or more) occurred in 26% (95% CI, 18%-36%) and 16% (95% CI, 10%-25%) of patients, respectively.
RECOMMENDATIONS
The 2007 clinical practice guideline on low back pain from The American College of Physicians and American Pain Society recommends opioids, including tramadol, for patients with severe back pain who don’t get adequate relief from acetaminophen or NSAIDs.7
The 2009 National Institute for Health and Care Excellence (NICE) guidelines for early management of persistent, nonspecific low back pain recommend considering strong opioids (buprenorphine, fentanyl, and oxycodone) for short-term use in severe pain and referral to a specialist for patients requiring prolonged use of strong opioids.8
The 2013 British Pain Society guidelines for low back and radicular pain recommend tight restrictions on the use of strong opioids. They also recommend giving the lowest possible dose of opioids for the shortest time possible.9
Short-term (<4 months) treatment with opioids provides modest relief of chronic low back pain, but only minimal improvement in function compared with placebo (strength of recommendation [SOR]: B, systematic review of lower-quality randomized controlled trials [RCTs]).
Tramadol isn’t superior to nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief (SOR: A, consistent results from RCTs). In addition, oxycodone with titrated morphine isn’t better than naproxen for relieving pain or improving function (SOR: C, a low-quality RCT).
Although no long-term RCTs have been done, cohort studies have shown that 6 to 12 months of opioid use is associated with a small decrease in pain and either very minimal improvement in, or worsening of, disability (SOR: B, prospective cohort trials).
EVIDENCE SUMMARY
A systematic review and meta-analysis of 15 RCTs with a total enrollment of 5540 assessed the efficacy of opioids in adults with chronic low back pain of at least 12 weeks’ duration.1 Five low-quality studies (1378 patients) that compared tramadol with placebo found tramadol to be moderately superior to placebo for relieving pain (standard mean difference [SMD]= -0.55; 95% confidence interval [CI], -0.66 to -0.44) but only modestly better for improving function (SMD= −0.18; 95% CI, -0.29 to -0.07).
Six trials with 1887 patients compared strong opioids (morphine, hydromorphone, oxycodone, oxymorphone, and tapentadol) with placebo. The opioids were better than placebo for improving pain (SMD= -0.43; 95% CI, -0.52 to -0.33) and function (SMD= -0.26; 95% CI, -0.37 to -0.15). The general interpretation of SMD effect size is 0.2=small, 0.5=medium, 0.8=large. In this case, larger negative numbers correlate with greater improvement.
How opioids stack up against NSAIDs
Two separate double-blind, double-dummy studies randomized adults with low back pain of at least 12 weeks’ duration to receive celecoxib 200 mg twice daily (404 and 398 patients, respectively) or tramadol 50 mg 4 times daily (392 and 404 patients, respectively) for 6 weeks.2 The primary outcome measure was at least a 30% improvement in pain using a 0 (no pain) to 10 (worst possible pain) scale. In both studies, more patients taking celecoxib had positive responses than patients taking tramadol (63% vs 50%, P<.001, and 64% vs 55%, P<.008, respectively).
A small RCT (36 patients who had suffered low back pain for more than 6 months) randomized patients to one of 3 treatment groups for 16 weeks: oxycodone as much as 20 mg/d (13 patients); naproxen as much as 1 g/d (12 patients); or oxycodone and sustained-release morphine (titrated up to 200 mg morphine equivalent/d (11 patients).3 After 16 weeks, patients receiving oxycodone or naproxen were treated with oxycodone and sustained-release morphine for another 16 weeks, as were patients already receiving this therapy. Pain was assessed on a 0 (none) to 100 (worst possible pain) scale.
Both opioid groups had significantly less pain on average (59.8 for oxycodone, 54.9 for titrated morphine) than the naproxen group (65.5; F=16.07; P<.001) but no significant difference in activity level. However, an independent analysis of the naproxen group and titrated morphine group found no significant difference in either pain relief (SMD= -0.58; 95% CI, -1.42 to 0.26) or disability (SMD= -0.06; 95% CI, -0.88 to 0.76) between the 2 groups.4
How does long-term opioid use affect pain and function?
Two prospective cohort studies have evaluated long-term opioid use. The first (715 patients) used a Roland-Morris Disability Questionnaire (RMDQ) to assess disability at 6 months in patients taking opioids compared with patients not taking opioids.5 Patients using opioids showed an increase in RMDQ score of 1.18 units (95% CI, 0.17-2.19) on a 0 to 24 scale, with 24 representing greatest disability.
The second study evaluated pain and function in 1843 adults with acute back injuries taking opioids for a year.6 Pain, rated on a 0 to 10 scale, decreased from 7.7 at baseline to 6.8 at one year (no P value). At the end of the first quarter, the RMDQ score decreased from 18.8 at baseline (the end of the first quarter) to 17.5 at one year (no P value). Clinically meaningful improvement in pain and function (30% or more) occurred in 26% (95% CI, 18%-36%) and 16% (95% CI, 10%-25%) of patients, respectively.
RECOMMENDATIONS
The 2007 clinical practice guideline on low back pain from The American College of Physicians and American Pain Society recommends opioids, including tramadol, for patients with severe back pain who don’t get adequate relief from acetaminophen or NSAIDs.7
The 2009 National Institute for Health and Care Excellence (NICE) guidelines for early management of persistent, nonspecific low back pain recommend considering strong opioids (buprenorphine, fentanyl, and oxycodone) for short-term use in severe pain and referral to a specialist for patients requiring prolonged use of strong opioids.8
The 2013 British Pain Society guidelines for low back and radicular pain recommend tight restrictions on the use of strong opioids. They also recommend giving the lowest possible dose of opioids for the shortest time possible.9
1. Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976). 2014;39:556-563.
2. O’Donnell JB, Ekman EF, Spalding WM, et al. The effectiveness of a weak opioid medication versus a cyclo-oxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug in treating flare-up of chronic low-back pain: results from two randomized, double-blind, 6-week studies. J Int Med Res. 2009;37:1789-1802.
3. Jamison RN, Raymond SA, Slawsby EA, et al. Opioid therapy for chronic noncancer back pain. A randomized prospective study. Spine (Phila Pa 1976). 1998;23:2591-2600.
4. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36(21 Suppl):S131-S43.
5. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
6. Franklin GM, Rahman EA, Turner JA, et al. Opioid use for chronic low back pain: A prospective, population-based study among injured workers in Washington state, 2002-2005. Clin J Pain. 2009;25:743-751.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
8. National Institute for Health and Care Excellence. Low back pain: early management of persistent non-specific low back pain. National Institute for Health and Care Excellence Web site. Available at: http://guidance.nice.org.uk/CG88. Accessed April 1, 2015.
9. Lee J, Gupta S, Price C, et al; British Pain Society. Low back and radicular pain: a pathway for care developed by the British Pain Society. Br J Anaesth. 2013;111:112-120.
1. Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976). 2014;39:556-563.
2. O’Donnell JB, Ekman EF, Spalding WM, et al. The effectiveness of a weak opioid medication versus a cyclo-oxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug in treating flare-up of chronic low-back pain: results from two randomized, double-blind, 6-week studies. J Int Med Res. 2009;37:1789-1802.
3. Jamison RN, Raymond SA, Slawsby EA, et al. Opioid therapy for chronic noncancer back pain. A randomized prospective study. Spine (Phila Pa 1976). 1998;23:2591-2600.
4. White AP, Arnold PM, Norvell DC, et al. Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine (Phila Pa 1976). 2011;36(21 Suppl):S131-S43.
5. Ashworth J, Green DJ, Dunn KM, et al. Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain. 2013;154:1038-1044.
6. Franklin GM, Rahman EA, Turner JA, et al. Opioid use for chronic low back pain: A prospective, population-based study among injured workers in Washington state, 2002-2005. Clin J Pain. 2009;25:743-751.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
8. National Institute for Health and Care Excellence. Low back pain: early management of persistent non-specific low back pain. National Institute for Health and Care Excellence Web site. Available at: http://guidance.nice.org.uk/CG88. Accessed April 1, 2015.
9. Lee J, Gupta S, Price C, et al; British Pain Society. Low back and radicular pain: a pathway for care developed by the British Pain Society. Br J Anaesth. 2013;111:112-120.
Evidence-based answers from the Family Physicians Inquiries Network
Fingertip Amputation Treatment: A Survey Study
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
Finger injuries are common, representing an estimated 3 million emergency department visits per year in the United States, with 44% of these diagnosed as lacerations.1 Amputations of the finger (partial and complete) in non-work-related accidents alone are estimated at 30,000 per year.1 The fingertip is a highly specialized structure that contributes to precision function of the hand through tactile feedback and fine motor control as well as hand aesthetics. An injury can compromise a variety of fingertip structures, including the distal phalanx, which provides length and structural support; the fingernail, germinal matrix, and sterile matrix, which protect the fingertip and function as tools; and the volar skin pad, which is important for sensation and fine motor activity.
There is considerable debate regarding optimal management of fingertip amputations, and to date there have been no prospective, randomly controlled trials to guide treatment.2 Injury characteristics, amputation levels, and patient priorities all contribute to management decisions. Treatment goals are to maintain length when possible; to provide stable, supple, and sensate skin coverage; to ensure the nail plate regrows without complication; and to maintain normal overall finger shape and cosmesis. In addition, a simple, cost-effective treatment with short recovery time and no donor-site morbidity is desired.
Treatment recommendations are wide-ranging, and evidence-based literature is sparse. About 30 years ago, 2 retrospective comparative studies found no difference in outcomes between simpler treatments (primary closure, secondary wound healing) and various operative strategies.3,4 Since then, most of the scientific studies have been retrospective noncomparative case series, all reporting good to excellent results.5-17 Investigators generally implied superior results of a studied procedure over those of more conservative treatments. Recommended treatments include secondary wound healing, simple flaps, staged flaps, pedicle flaps, allograft and autograft coverage, composite grafting, and replantation, for all levels of fingertip injury.
Given our surgical advances, improved techniques, and accumulating experience, we may have expected better outcomes with newer and more complex reconstructive efforts. Unfortunately, in a recent review of 53 fingertip injuries treated with a reconstructive procedure, bone shortening with closure, or secondary healing, Wang and colleagues18 found no discernible differences in outcomes at 4.5-year follow-up. They questioned whether complex reconstructive procedures are worth the time, expense, and risk. In the absence of prospective, comparative studies, surgeons must rely on anecdotal evidence (including predominantly level IV evidence), training bias, previous experience, and the prevailing common wisdom.
Toward that end, we became interested in identifying treatment preferences for fingertip amputations. We conducted a study to better understand how surgeon and patient factors influence the treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. We hypothesized that hand surgeons’ treatment preferences would be varied and influenced by surgeon and patient demographics.
Materials and Methods
An online multiple-choice survey was created and powered by Constant Contact. The survey consisted of 6 surgeon demographic questions; 5 treatment preference questions regarding patient age, sex, occupation, and germinal matrix management; and 5 clinical scenarios based on Allen levels 2, 3 (with and without exposed distal phalanx), and 4 and volar oblique middle-finger amputations. The Allen classification designates level 2 injuries as those involving only the distal pulp and nail.19 Level 3 injuries also involve the terminal distal phalanx, and level 4 injuries extend to the lunula. The survey questions are listed in the Appendix. For the clinical scenario questions, treatment choices included wound care, skeletal shortening and closure, composite graft, autograft, allograft, V-Y/Kutler flap, advancement flap, thenar flap, cross-finger flap, pedicle and homodigital flap, replantation, and other.
An email invitation was sent to members of the American Association for Hand Surgery (AAHS). The survey was also submitted to personal contacts of international hand societies named on the AAHS website to expand the international response. A reminder email was sent 1 week after the original invitation. The survey was closed 5 weeks later, and the responses were analyzed with all non-US hand surgeons grouped collectively as an international group, compared with the US group. Institutional review board approval was not needed for this survey study.
Statistics
A generalized linear regression model was used to implement logistic regression with random effects for question and respondent. This approach accounts for multiple observations from the same respondent, assuming that both respondent and question are random samples from a larger population. The model estimated the probability that a given surgical approach (eg, skeletal shortening, wound care) would be selected, based on the predictors of the US versus international respondent, time in practice, practice type, and whether the fingertip was available. The model returned adjusted odds ratios (ORs) for each predictor, controlling for all the others. By convention, P < .05 was considered significant. No attempt was made to prune the model of nonsignificant factors. Analyses were performed using the lme4 package on the R statistical platform (R Foundation for Statistical Computing).
Results
One hundred ninety-eight responses were recorded. Of the 1054 AAHS members invited to take the survey, 174 (US, international) responded (17% response rate). One hundred twenty-three responses and 62% of the total were generated from US hand surgeons. Fifty-eight percent of US responses were from the Mid-South, Midwest, or Mid-Atlantic region. Fifty-seven percent of international responses were from Brazil and Europe. Respondents’ demographic data are listed in Tables 1 and 2.
Responses to the 5 clinical scenarios showed a wide variation in treatment preferences. The top 6 preferred treatment selections for an acute, clean long-finger amputation in a healthy 40-year-old office worker are shown in Figures 1 to 5. When surgeons who preferred replant were asked what they would do if the amputated part was not available, they indicated flap coverage more often than less complex treatments, such as skeletal shortening/primary closure or wound care.
There were statistically significant differences in treatment preferences between US and international hand surgeons when controlling for all other demographic variables. Adjusted ORs and their confidence intervals (CIs) for the aggregate clinical scenarios are presented in a forest plot in Figure 6. Figure 4 shows that US surgeons were more likely to choose wound care (OR, 3.6; P < .0004) and less likely to attempt a replant (OR, 0.01; P < .0001). US surgeons were also less likely to use a pedicle or homodigital island flap when the amputated fingertip was both available (OR, 0.04; P = .039) and unavailable (OR, 0.47; Ps = .029).
Among all respondents and across all clinical scenarios, skeletal shortening with closure was favored among hand surgeons in practice less than 5 years compared with those in practice longer (OR, 2.11; 95% CI, 1.36-3.25; P = .0008). Similarly, surgeons with more than 30 years of experience were the least likely to favor wound care (OR, 0.2; 95% CI, 0.09-0.93; P = .037). Compared with orthopedic surgeons, plastic surgeons opted for wound care less often (OR, 0.44; 95% CI, 0.23-0.98; P = .018) and appeared to prefer replantation, but the difference was not statistically significant (OR, 8.86; 95% CI, 0.99-79.61; P = .054).
Replantation was less often chosen by private practice versus full-time academic surgeons (OR, 0.09; 95% CI, 0.01-0.91; P = .041.) Part-time academics were no more or less likely to perform replantation than full-time academics were (OR, 0.52; 95% CI, 0.05-5.41; P = .58). Of the 59 respondents who performed more than 10 microvascular cases a year, 18 (31%) chose replant for Allen level 4 amputations. In comparison, 9 (20%) of the 45 respondents who performed fewer than 3 microvascular cases a year chose replant for amputations at this level. Amount of time working with fellows did not affect treatment preferences.
Patient demographics (age, sex, occupation) also played a role in treatment decisions (Table 3). The most significant factors appeared to be age and occupation. Regarding age, 41% of respondents chose more complex procedures for patients younger than 15, and 62% chose less complex procedures for patients older than 70 years. Regarding occupation, 61% chose more complex procedures for professional musicians, and 60% chose less complex procedures for manual laborers. Sex did not influence clinical decisions for 78% of respondents. There was also substantial variation in both the indications for germinal matrix ablation and the frequency of sterile matrix transplant (Table 3).
Discussion
Although there is a variety of treatment options and published treatment guidelines for distal fingertip amputations, few comparative studies support use of one treatment over another. In our experience, treatment decisions are based mainly on injury parameters, but surgeon preference and patient factors (age, sex, occupation) can also influence care. Our goal in this study was to better understand how surgeon and patient factors influence treatment preferences for distal fingertip amputations among a cross section of US and international hand surgeons. Our survey results showed lack of consensus among hand surgeons and highlighted several trends.
As expected, we found a wide range of treatment preferences for each clinical scenario queried, ranging from more simple treatments (eg, wound care) to more complex ones (eg, replantation). With patient parameters (age, profession, finger, acuity, injury type, tissue preservation, smoking status) standardized in the clinical scenarios, the treatment differences noted should reflect surgeon preference. However, other patient factors (eg, cultural differences, religious beliefs, surgeon setting, practice pattern, resource availability) that were not included in the clinical scenarios could also affect treatment preferences.
One particularly interesting finding was that international hand surgeons were 6.8 times more likely to replant a distal fingertip amputation. One possible explanation for this variation is the influence of cultural differences. For example, in East Asian countries, there can be a cultural stigma associated with loss of a fingertip, and therefore more of a desire on the part of the patient to restore the original finger.20,21 In addition, the international respondents were biased toward academic practices—which could skew the treatment preference toward replantation, as we found that academic surgeons were more inclined to replantation.
Our finding that replantation was more commonly preferred by academic versus private practice surgeons may suggest a training bias, an affinity for more complex or interesting procedures, or access to hospital equipment and staff, including residents and fellows, not usually found at smaller community hospitals, where private practice surgeons are more commonly based. Jazayeri and colleagues22 found that institutions specializing in microsurgery often produced better outcomes than nonspecializing institutions. Therefore, it is not surprising that private practice hand surgeons may less often opt to replant a distal fingertip amputation. It is also not surprising that plastic surgeons are more inclined to perform a replantation or flap coverage, as their training is more microsurgery-intensive and their practice more focused on aesthetics compared with the other specialists.
Distal fingertip replantation is accepted by most as technically demanding, but it seems that the additional effort and resources would be justified if the procedure provided a superior outcome. However, other factors, such as cost of treatment and length of recovery, should also be considered. Average replantation cost has been estimated to range from $7500 to $14,000, compared with $2800 for non-replantation-related care, and median stay is about 4 days longer for replantation-related care.23,24 These estimates do not include indirect costs, such as for postoperative rehabilitation, which is likely longer and more expensive, even in distal fingertip replantation. These disparities may not justify the outcome (of having a complete fingertip) if more conservative treatments yield similar results.17,18 In addition, there is the expected failure rate of limb replantation surgery. In analysis of the overall societal costs and benefits of larger upper extremity limb replantation, the loss of invested resources sustained with failed limb replantation may be outweighed by the benefit of another patient having a successful outcome. In the case of fingertip replantation, however, does the undefined benefit of the successful patient outcome outweigh the investment of resources lost in cases of replantation failure? Understandably, there is a need for more robust clinical outcome and cost-comparative evidence to better inform decisions regarding distal fingertip amputation.
We found that wound care and skeletal shortening with primary closure (particularly with Allen level 3 injuries) were preferred more by surgeons within the first 5 years of practice. This finding seems to imply a lack of experience or confidence on the part of younger surgeons performing more complex procedures, such as flap coverage. Conversely, this finding may indicate a shift in treatment principle based on recent literature suggesting equivalent outcomes with simpler procedures.17,18 Although our survey study did not provide an option for treatment combinations or staged procedures, several respondents wrote in that skeletal shortening supplemented with various types of autografts and allografts would be their preferred treatment.
Patient factors also play a significant role in clinical decisions. Age and profession seem to be important determinants, with more than 50% of respondents, on average, changing their treatment recommendation based on these 2 factors. A majority of respondents would perform a less involved procedure for a manual laborer, suggesting a quicker return to work is prioritized over a perceived improved clinical outcome. Interestingly, for patients younger than 15 years, the preference was divided, with 41% of surgeons opting for a more complex procedure. This suggests the importance of restoring anatomy in a younger patient, or the perceived decreased risk or failure rate with more involved treatment. Twenty percent preferred a less complex procedure in a younger patient, perhaps relying on the patient’s developmental potential for a good outcome or suggesting a concern for patient intolerance or compliance with complex surgery.
Nail plate regrowth can be a problem with fingertip amputations. Nail deformity is highly correlated with injury level, with amputations proximal to the lunula more likely to cause nail plate deformity.25,26 Jebson and colleagues27 recommended germinal matrix ablation for amputations proximal to the lunula. We found respondents often performed ablations for other indications, including injured or minimal remaining sterile matrix and lack of bony support for the sterile matrix. Forty-six percent of respondents had never performed sterile matrix transplant, which could indicate that they were unfamiliar with the technique or had donor-site concerns, or that postinjury nail deformities are uncommon, well tolerated, or treated along with other procedures, such as germinal matrix ablation.
Several weaknesses of this study must be highlighted. First, our response rate was smaller than desired. Although this work incorporated a large number of surgeon responses, nearly 200, the response rate was only 17%. In addition, although number of responses was likely adequate to show the diversity of opinion, the preferences and trends reported might not be representative of all hand surgeons. We could not perform a nonresponder analysis because of a lack of specific demographic data for the AAHS and international hand society members. However, AAHS has an approximate 50/50 mix of plastic and orthopedic surgeons, similar to our responder demographic, suggesting our smaller subset of responses might be representative of the whole. According to AAHS, a majority of its members are “academic” hand surgeons, so our results might not adequately reflect the preferences of community hand surgeons and ultimately might overstate the frequency of more complex treatments. Last, our international response was limited to a few countries. A larger, more broadly distributed response would provide a better understanding of regional preferences, which could shed light on the importance of cultural differences.
Variations in patient insurance status were not queried in this survey but might also affect treatment decisions. More involved, costly, and highly reimbursing procedures might be deemed reasonable options for a small perceived clinical benefit for insured patients.
When multiple digits or the thumb is injured, or there are other concomitant injuries, surgeons may alter their choice of intervention. In mangled extremities, preservation of salvageable functional units takes precedence over aesthetics and likely affects choice of treatment for the amputated fingertips. Similarly, multiple fingertip amputations, even if all at the same level, may be differently regarded than a solitary injury.
Conclusion
For distal fingertip amputations, there is little evidence supporting one approach over another. Without level I comparative data guiding treatment, anecdotal evidence and surgeon personal preferences likely contribute to the large variation noted in this survey. Our study results showed the disparity of fingertip treatment preferences among a cross section of US and international hand surgeons. More important, results underscored the need for a well-designed comparative study to determine the most effective treatments for distal fingertip amputations.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.
1. Conn JM, Annest JL, Ryan GW, Budnitz DS. Non-work-related finger amputations in the United States, 2001-2002. Ann Emerg Med. 2005;45(6):630-635.
2. Bickel KD, Dosanjh A. Fingertip reconstruction. J Hand Surg Am. 2008;33(8):1417-1419.
3. Söderberg T, Nyström Å, Hallmans G, Hultén J. Treatment of fingertip amputations with bone exposure. A comparative study between surgical and conservative treatment methods. Scand J Plast Reconstr Surg. 1983;17(2):147-152.
4. Braun M, Horton RC, Snelling CF. Fingertip amputation: review of 100 digits. Can J Surg. 1985;28(1):72-75.
5. Sammut D. Fingertip injuries. A review of indications and methods of management. Curr Orthop. 2002;16:271-285.
6. Mennen U, Wiese A. Fingertip injuries management with semi-occlusive dressing. J Hand Surg Br. 1993;18(4):416-422.
7. Atasoy E, Ioakimidis E, Kasdan ML, Kutz JE, Kleinert HE. Reconstruction of the amputated fingertip with a triangular volar flap. A new surgical procedure. J Bone Joint Surg Am. 1970;52(5):921-926.
8. Kutler W. A new method for finger tip amputation. J Am Med Assoc. 1947;133(1):29-30.
9. Takeishi M, Shinoda A, Sugiyama A, Ui K. Innervated reverse dorsal digital island flap for fingertip reconstruction. J Hand Surg Am. 2006;31(7):1094-1099.
10. Tuncali D, Barutcu AY, Gokrem S, Terzioglu A, Aslan G. The hatchet flap for reconstruction of fingertip amputations. Plast Reconstr Surg. 2006;117(6):1933-1939.
11. Teoh LC, Tay SC, Yong FC, Tan SH, Khoo DB. Heterodigital arterialized flaps for large finger wounds: results and indications. Plast Reconstr Surg. 2003;111(6):1905-1913.
12. Nishikawa H, Smith PJ. The recovery of sensation and function after cross-finger flaps for fingertip injury. J Hand Surg Br. 1992;17(1):102-107.
13. Rinker B. Fingertip reconstruction with the laterally based thenar flap: indications and long-term functional results. Hand. 2006;1(1):2-8.
14. Jung MS, Lim YK, Hong YT, Kim HN. Treatment of fingertip amputation in adults by palmar pocketing of the amputated part. Arch Plast Surg. 2012;39(4):404-410.
15. Venkatramani H, Sabapathy SR. Fingertip replantation: technical considerations and outcome analysis of 24 consecutive fingertip replantations. Indian J Plast Surg. 2011;44(2):237-245.
16. Chen SY, Wang CH, Fu JP, Chang SC, Chen SG. Composite grafting for traumatic fingertip amputation in adults: technique reinforcement and experience in 31 digits. J Trauma. 2011;70(1):148-153.
17. van den Berg WB, Vergeer RA, van der Sluis CK, Ten Duis HJ, Werker PM. Comparison of three types of treatment modalities on the outcome of fingertip injuries. J Trauma Acute Care Surg. 2012;72(6):1681-1687.
18. Wang K, Sears ED, Shauver MJ, Chung KC. A systematic review of outcomes of revision amputation treatment for fingertip amputations. Hand. 2013;8(2):139-145.
19. Allen MJ. Conservative management of finger tip injuries in adults. Hand. 1980;12(3):257-265.
20. Chen CT, Wei FC, Chen HC, Chuang CC, Chen HT, Hsu WM. Distal phalanx replantation. Microsurgery. 1994;15(1):77-82.
21. Kim WK, Lim JH, Han SK. Fingertip replantations: clinical evaluation of 135 digits. Plast Reconstr Surg. 1996;98(3):470-476.
22. Jazayeri L, Klausner JQ, Chang J. Distal digital replantation. Plast Reconstr Surg. 2013;132(5):1207-1217.
23. Hattori Y, Doi K, Sakamoto S, Yamasaki H, Wahegaonkar A, Addosooki A. Fingertip replantation. J Hand Surg Am. 2007;32(4):548-555.
24. Goldner RD, Stevanovic MV, Nunley JA, Urbaniak JR. Digital replantation at the level of the distal interphalangeal joint and the distal phalanx. J Hand Surg Am. 1989;14(2 pt 1):214-220.
25. Nishi G, Shibata Y, Tago K, Kubota M, Suzuki M. Nail regeneration in digits replanted after amputation through the distal phalanx. J Hand Surg Am. 1996;21(2):229-233.
26. Yamano Y. Replantation of the amputated distal part of the fingers. J Hand Surg Am. 1985;10(2):211-218.
27. Jebson PJ, Louis DS, Bagg M. Amputations. In: Wolfe SW, Pederson WC, Hotchkiss RN, Kozin SH, eds. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2010:1885-1927.