User login
CLL patients achieve remission with CAR-modified T-cells
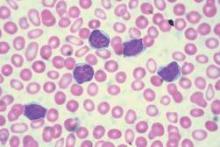
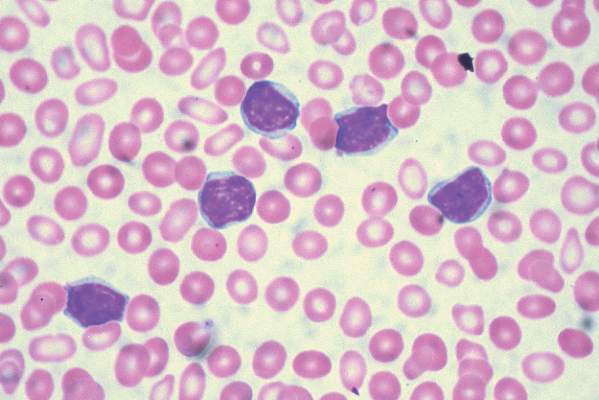
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: CAR-modified T cell therapy lacks the toxicities and limitations of allogeneic stem cell transplantation and may be an effective treatment for chronic lymphocytic leukemia.
Major finding: CAR-modified T cell therapy elicited a response in 8 of 14 patients (57%) with relapsed and refractory chronic lymphocytic leukemia, and 4 patients (29%) achieved a complete remission.
Data source: Mature results from a pilot clinical trial.
Disclosures: Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Society of Hospital Medicine Builds Awareness of HM Career Benefits
It’s that time of year again. Across the country, thousands of students are starting medical school. And in just a short time—it goes faster than you think—they’ll be faced with the decision of what specialty they want to pursue.
To make that decision, these students will have to consider a variety of factors beyond their personal interests, including the training, compensation, lifestyle, and career path associated with each specialty.
When I was in medical school, hospital medicine wasn’t a career option—our field didn’t even exist. I remember going through the decision process like it was yesterday. The first two years, as you know, provide little exposure to the true day-to-day challenges and lifestyle of any of the potential choices. That time comes in the third and fourth years, and many students are forced to make a decision after experiencing only a few of the myriad possibilities.
My personal experience with this process was quite a wandering path. I began medical school with tunnel vision around becoming an orthopedic surgeon. I struggled through the first two years of physiology and embryology and the like, eager to get my hands on the “tools” of a real surgeon.
Gradually, I moved away from that plan as I witnessed firsthand how grueling the residency training was and how it personally impacted those residents I knew and their families. I was briefly tempted by a vascular surgery attending who offered to take me under his wing, but I soon came to my senses.
I then gravitated toward specialties that were less technical and more intellectual. After a very enjoyable third-year pediatrics rotation, I could clearly see myself taking care of “little people.” Internal medicine challenged me with the most interesting of clinical conundrums, and I began to see myself solving great mysteries. Family medicine gave me a nice mix of both, and that is where I eventually settled. Having the option of hospital medicine would certainly have made the choice more difficult for me. The attraction would have been, and frankly, still is, the combination of caring for the patient and the system simultaneously.
A Career Choice
Today, hospital medicine is medicine’s fastest growing field. Unfortunately, many young physicians don’t think about hospital medicine when planning their careers. It’s often considered a brief stop between residency and a fellowship—a way to make some money and pay back medical school loans before continuing toward a career in a specialty like cardiology, gastroenterology, or hematology.
That’s why SHM has been making a concerted effort to increase awareness among medical school students about the benefits of a career in hospital medicine. We have launched several programs geared specifically to medical students and residents.
For example, SHM has built a “Future of Hospital Medicine” website for students and residents, filled with information about what it’s like to have a career in hospital medicine. Plus, we now offer free membership dues and electronic access to our publications for medical students who want to join SHM.
SHM launched the “Future of Hospital Medicine” (FOHM) campaign in 2013 under then-President Dr. Eric Howell’s leadership. Since then:
- Student membership has increased 217%;
- Resident/fellow membership has increased by 97%;
- We’ve conducted five “FOHM” live events in Philadelphia, New York, Baltimore, and Chicago (twice) and will be hosting another event in Los Angeles this fall; and
- We offer more focused student and resident content at HM15, as well as the largest attendance to date for both groups.
We also recently developed the Society of Hospital Medicine Student Hospitalist Scholar Grant program. Through the program, eligible students can receive a $5,000 summer stipend for scholarly work on a project related to patient safety/quality improvement or other areas relevant to the field of hospital medicine. The program also provides up to $1,500 in travel-related reimbursement for the student attending the SHM annual meeting.
This year, the grant program’s first, three students are participating in summer research on topics that include post-hospital syndrome and physiologic alarm responses. You can follow their progress on the SHM blog. Eventually, we expect the program to grow to 10 grantees every summer.
Awareness Is Key
We hope efforts like these will help raise awareness and interest in hospital medicine and SHM among medical students and young physicians. Although our field is relatively young, it’s full of opportunities for building a rewarding, lasting career.
Hospital medicine is so much more than a medical pit stop. As we know at SHM, it’s a specialty in which young physicians can launch and build a sustainable career.
If you are a career hospitalist, I ask you to help spread the word. And, if you are a student contemplating your future, I invite you to visit us and learn more. I know you will not be disappointed.

It’s that time of year again. Across the country, thousands of students are starting medical school. And in just a short time—it goes faster than you think—they’ll be faced with the decision of what specialty they want to pursue.
To make that decision, these students will have to consider a variety of factors beyond their personal interests, including the training, compensation, lifestyle, and career path associated with each specialty.
When I was in medical school, hospital medicine wasn’t a career option—our field didn’t even exist. I remember going through the decision process like it was yesterday. The first two years, as you know, provide little exposure to the true day-to-day challenges and lifestyle of any of the potential choices. That time comes in the third and fourth years, and many students are forced to make a decision after experiencing only a few of the myriad possibilities.
My personal experience with this process was quite a wandering path. I began medical school with tunnel vision around becoming an orthopedic surgeon. I struggled through the first two years of physiology and embryology and the like, eager to get my hands on the “tools” of a real surgeon.
Gradually, I moved away from that plan as I witnessed firsthand how grueling the residency training was and how it personally impacted those residents I knew and their families. I was briefly tempted by a vascular surgery attending who offered to take me under his wing, but I soon came to my senses.
I then gravitated toward specialties that were less technical and more intellectual. After a very enjoyable third-year pediatrics rotation, I could clearly see myself taking care of “little people.” Internal medicine challenged me with the most interesting of clinical conundrums, and I began to see myself solving great mysteries. Family medicine gave me a nice mix of both, and that is where I eventually settled. Having the option of hospital medicine would certainly have made the choice more difficult for me. The attraction would have been, and frankly, still is, the combination of caring for the patient and the system simultaneously.
A Career Choice
Today, hospital medicine is medicine’s fastest growing field. Unfortunately, many young physicians don’t think about hospital medicine when planning their careers. It’s often considered a brief stop between residency and a fellowship—a way to make some money and pay back medical school loans before continuing toward a career in a specialty like cardiology, gastroenterology, or hematology.
That’s why SHM has been making a concerted effort to increase awareness among medical school students about the benefits of a career in hospital medicine. We have launched several programs geared specifically to medical students and residents.
For example, SHM has built a “Future of Hospital Medicine” website for students and residents, filled with information about what it’s like to have a career in hospital medicine. Plus, we now offer free membership dues and electronic access to our publications for medical students who want to join SHM.
SHM launched the “Future of Hospital Medicine” (FOHM) campaign in 2013 under then-President Dr. Eric Howell’s leadership. Since then:
- Student membership has increased 217%;
- Resident/fellow membership has increased by 97%;
- We’ve conducted five “FOHM” live events in Philadelphia, New York, Baltimore, and Chicago (twice) and will be hosting another event in Los Angeles this fall; and
- We offer more focused student and resident content at HM15, as well as the largest attendance to date for both groups.
We also recently developed the Society of Hospital Medicine Student Hospitalist Scholar Grant program. Through the program, eligible students can receive a $5,000 summer stipend for scholarly work on a project related to patient safety/quality improvement or other areas relevant to the field of hospital medicine. The program also provides up to $1,500 in travel-related reimbursement for the student attending the SHM annual meeting.
This year, the grant program’s first, three students are participating in summer research on topics that include post-hospital syndrome and physiologic alarm responses. You can follow their progress on the SHM blog. Eventually, we expect the program to grow to 10 grantees every summer.
Awareness Is Key
We hope efforts like these will help raise awareness and interest in hospital medicine and SHM among medical students and young physicians. Although our field is relatively young, it’s full of opportunities for building a rewarding, lasting career.
Hospital medicine is so much more than a medical pit stop. As we know at SHM, it’s a specialty in which young physicians can launch and build a sustainable career.
If you are a career hospitalist, I ask you to help spread the word. And, if you are a student contemplating your future, I invite you to visit us and learn more. I know you will not be disappointed.

It’s that time of year again. Across the country, thousands of students are starting medical school. And in just a short time—it goes faster than you think—they’ll be faced with the decision of what specialty they want to pursue.
To make that decision, these students will have to consider a variety of factors beyond their personal interests, including the training, compensation, lifestyle, and career path associated with each specialty.
When I was in medical school, hospital medicine wasn’t a career option—our field didn’t even exist. I remember going through the decision process like it was yesterday. The first two years, as you know, provide little exposure to the true day-to-day challenges and lifestyle of any of the potential choices. That time comes in the third and fourth years, and many students are forced to make a decision after experiencing only a few of the myriad possibilities.
My personal experience with this process was quite a wandering path. I began medical school with tunnel vision around becoming an orthopedic surgeon. I struggled through the first two years of physiology and embryology and the like, eager to get my hands on the “tools” of a real surgeon.
Gradually, I moved away from that plan as I witnessed firsthand how grueling the residency training was and how it personally impacted those residents I knew and their families. I was briefly tempted by a vascular surgery attending who offered to take me under his wing, but I soon came to my senses.
I then gravitated toward specialties that were less technical and more intellectual. After a very enjoyable third-year pediatrics rotation, I could clearly see myself taking care of “little people.” Internal medicine challenged me with the most interesting of clinical conundrums, and I began to see myself solving great mysteries. Family medicine gave me a nice mix of both, and that is where I eventually settled. Having the option of hospital medicine would certainly have made the choice more difficult for me. The attraction would have been, and frankly, still is, the combination of caring for the patient and the system simultaneously.
A Career Choice
Today, hospital medicine is medicine’s fastest growing field. Unfortunately, many young physicians don’t think about hospital medicine when planning their careers. It’s often considered a brief stop between residency and a fellowship—a way to make some money and pay back medical school loans before continuing toward a career in a specialty like cardiology, gastroenterology, or hematology.
That’s why SHM has been making a concerted effort to increase awareness among medical school students about the benefits of a career in hospital medicine. We have launched several programs geared specifically to medical students and residents.
For example, SHM has built a “Future of Hospital Medicine” website for students and residents, filled with information about what it’s like to have a career in hospital medicine. Plus, we now offer free membership dues and electronic access to our publications for medical students who want to join SHM.
SHM launched the “Future of Hospital Medicine” (FOHM) campaign in 2013 under then-President Dr. Eric Howell’s leadership. Since then:
- Student membership has increased 217%;
- Resident/fellow membership has increased by 97%;
- We’ve conducted five “FOHM” live events in Philadelphia, New York, Baltimore, and Chicago (twice) and will be hosting another event in Los Angeles this fall; and
- We offer more focused student and resident content at HM15, as well as the largest attendance to date for both groups.
We also recently developed the Society of Hospital Medicine Student Hospitalist Scholar Grant program. Through the program, eligible students can receive a $5,000 summer stipend for scholarly work on a project related to patient safety/quality improvement or other areas relevant to the field of hospital medicine. The program also provides up to $1,500 in travel-related reimbursement for the student attending the SHM annual meeting.
This year, the grant program’s first, three students are participating in summer research on topics that include post-hospital syndrome and physiologic alarm responses. You can follow their progress on the SHM blog. Eventually, we expect the program to grow to 10 grantees every summer.
Awareness Is Key
We hope efforts like these will help raise awareness and interest in hospital medicine and SHM among medical students and young physicians. Although our field is relatively young, it’s full of opportunities for building a rewarding, lasting career.
Hospital medicine is so much more than a medical pit stop. As we know at SHM, it’s a specialty in which young physicians can launch and build a sustainable career.
If you are a career hospitalist, I ask you to help spread the word. And, if you are a student contemplating your future, I invite you to visit us and learn more. I know you will not be disappointed.

Flu vaccine and heart attacks
As we head into influenza season, we are likely steeling our nerves for the inevitable debate with some of our patients about receiving the influenza vaccination.
We are used to hearing patients say, “I have never had it, and I have never gotten the flu,” or (my favorite), “Last time I got the shot, I got the flu.” Arguments that – while defying chance, logic, and science in general – keep us rooted in the daily joys of clinical practice.
Some handy influenza facts:
1. In well-matched years, the number needed to treat (NNT) to prevent one flu-like illness is 33.
2. In unmatched years, the NNT is 100.
We should be adding to this discussion some information about the observed association between the influenza vaccine and acute myocardial infarction (AMI). If compelling arguments about preventing flu-like symptoms don’t carry the day, maybe preventing heart attack will.
Dr. Michelle Barnes and her colleagues at UNSW Australia, Sydney, published the results of a systematic review of case-control studies evaluating the association between the influenza vaccine and AMI (Heart, 2015 Aug. 26. doi:10.1136/heartjnl-2015-307691). In this study, the investigators identified 16 studies on AMI and influenza vaccination or influenza infection.
The odds of influenza infection, influenza-like illness, or respiratory infection were significantly greater in patients with AMI (odds ratio, 2.01; 95% confidence interval: 1.47-2.76). Influenza vaccine was associated with a lower risk of AMI (OR, 0.71; 95% CI: 0.56-0.91).
This is the first meta-analysis compiling all case-control data on the relationship between AMI and the influenza vaccine. Overall, cases had double the risk of influenza or respiratory tract infection, compared with controls.
Influenza has been hypothesized to cause coronary artery occlusion through stenosis of subcritical atherosclerotic plaque, and it has been shown to promote atherogenesis in animal models. The connection between AMI and influenza was first observed in the 1930s during the flu season.
But medicine has a short memory, and our patients sometimes do as well. So, it is time we remind them about this link and encourage them to get their flu shots.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
As we head into influenza season, we are likely steeling our nerves for the inevitable debate with some of our patients about receiving the influenza vaccination.
We are used to hearing patients say, “I have never had it, and I have never gotten the flu,” or (my favorite), “Last time I got the shot, I got the flu.” Arguments that – while defying chance, logic, and science in general – keep us rooted in the daily joys of clinical practice.
Some handy influenza facts:
1. In well-matched years, the number needed to treat (NNT) to prevent one flu-like illness is 33.
2. In unmatched years, the NNT is 100.
We should be adding to this discussion some information about the observed association between the influenza vaccine and acute myocardial infarction (AMI). If compelling arguments about preventing flu-like symptoms don’t carry the day, maybe preventing heart attack will.
Dr. Michelle Barnes and her colleagues at UNSW Australia, Sydney, published the results of a systematic review of case-control studies evaluating the association between the influenza vaccine and AMI (Heart, 2015 Aug. 26. doi:10.1136/heartjnl-2015-307691). In this study, the investigators identified 16 studies on AMI and influenza vaccination or influenza infection.
The odds of influenza infection, influenza-like illness, or respiratory infection were significantly greater in patients with AMI (odds ratio, 2.01; 95% confidence interval: 1.47-2.76). Influenza vaccine was associated with a lower risk of AMI (OR, 0.71; 95% CI: 0.56-0.91).
This is the first meta-analysis compiling all case-control data on the relationship between AMI and the influenza vaccine. Overall, cases had double the risk of influenza or respiratory tract infection, compared with controls.
Influenza has been hypothesized to cause coronary artery occlusion through stenosis of subcritical atherosclerotic plaque, and it has been shown to promote atherogenesis in animal models. The connection between AMI and influenza was first observed in the 1930s during the flu season.
But medicine has a short memory, and our patients sometimes do as well. So, it is time we remind them about this link and encourage them to get their flu shots.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
As we head into influenza season, we are likely steeling our nerves for the inevitable debate with some of our patients about receiving the influenza vaccination.
We are used to hearing patients say, “I have never had it, and I have never gotten the flu,” or (my favorite), “Last time I got the shot, I got the flu.” Arguments that – while defying chance, logic, and science in general – keep us rooted in the daily joys of clinical practice.
Some handy influenza facts:
1. In well-matched years, the number needed to treat (NNT) to prevent one flu-like illness is 33.
2. In unmatched years, the NNT is 100.
We should be adding to this discussion some information about the observed association between the influenza vaccine and acute myocardial infarction (AMI). If compelling arguments about preventing flu-like symptoms don’t carry the day, maybe preventing heart attack will.
Dr. Michelle Barnes and her colleagues at UNSW Australia, Sydney, published the results of a systematic review of case-control studies evaluating the association between the influenza vaccine and AMI (Heart, 2015 Aug. 26. doi:10.1136/heartjnl-2015-307691). In this study, the investigators identified 16 studies on AMI and influenza vaccination or influenza infection.
The odds of influenza infection, influenza-like illness, or respiratory infection were significantly greater in patients with AMI (odds ratio, 2.01; 95% confidence interval: 1.47-2.76). Influenza vaccine was associated with a lower risk of AMI (OR, 0.71; 95% CI: 0.56-0.91).
This is the first meta-analysis compiling all case-control data on the relationship between AMI and the influenza vaccine. Overall, cases had double the risk of influenza or respiratory tract infection, compared with controls.
Influenza has been hypothesized to cause coronary artery occlusion through stenosis of subcritical atherosclerotic plaque, and it has been shown to promote atherogenesis in animal models. The connection between AMI and influenza was first observed in the 1930s during the flu season.
But medicine has a short memory, and our patients sometimes do as well. So, it is time we remind them about this link and encourage them to get their flu shots.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Why Hospitalist Morale is Declining and Ways to Improve It
Using quotes to ensure that the results were only those that include the two words adjacent to one another, rather than separated, I entered the following phrases into my Google search engine:
- “hospitalist burnout” = 1,580 results
- “hospitalist morale” = 208 results
- “hospitalist well-being” = 0 results
I think the number of results suggests the level of interest in each topic and, if that is the case, clearly thinking about how hospitalists are doing in their careers is more commonly done through the paradigm of burnout than the other two terms. (Of course, there may be other terms that I didn’t consider.) In fact, there have been a handful of published studies of hospitalist burnout and job satisfaction.1,2
Those studies generally have shown both reasonably high levels of job satisfaction and troubling levels of burnout.
But I’ve been thinking about hospitalist morale for a while. I think morale is reasonably distinct from both burnout and job satisfaction.
Causes of a National Decline in Hospitalist Morale
I think hospitalist morale has declined some over the past two or three years across the country. This observation is meaningful because it comes from my experience working with a lot of hospitalist groups coast to coast. But I’m the first to admit it is just anecdotal and is subject to my own biases.
I can think of several things contributing to a decline in morale.
EHR adoption. Near the top of the list is the adoption of EHRs in many hospitals, which typically leads doctors in other specialties to seek hospitalist assistance with EHR-related tasks (e.g. medicine reconciliation and order writing) even in cases where there is little or no clinical reason for hospitalist involvement. Lots of hospitalists complain about this. To be clear, in many hospitals the hospitalists are reasonably content with using the EHR, but they experience ongoing frustration and low morale resulting from nonclinical work other doctors pressure them to take over.
Observation status. Many hospitals began classifying a larger portion of patients as observation status over the last few years; at the same time, patients and families have become more aware of how much of a disadvantage this is. In many cases, it is the hospitalist who takes the brunt of patient and family frustration. This can get awfully stressful and frustrating, and I think it is a contributor to allegations of malpractice.
Budgetary stress. Ever since SHM began collecting survey data in the late 1990s, the financial support hospitals have been providing to hospitalists has increased dramatically. The most recent State of Hospital Medicine report, published in 2104, showed median support provided by hospitals of $156,063 per FTE hospitalist, per year. Some hospitals have begun to resist providing more support, and this translates into stress and lower morale for hospitalists. This is far from a universal issue, but it does lead to lower morale for hospitalists who face it.
Many other factors may be contributing to a national decline in morale, but I think these are some of the most important.
What Can Be Done?
Some hospitalist groups have great morale now and don’t need to do much of anything right now, but some groups should think about a deliberate strategy to improve it.
Sadly, there isn’t a prescription that is sure to work. But there are some things you can try.
Self-care. The field of palliative care has thought a lot about caring for caregivers, and hospitalist groups might want to adopt some of their practices. Search the Internet on “self-care” + “palliative care,” and you’ll find a lot of interesting things. The group I’m part of launched a deliberate program of professionally led and facilitated hospitalist self-care, with high hopes that included mindful meditation, among other things. As soon as we had designed our program, the Mayo Clinic published their favorable experience with a program that was very similar to what we had planned, and I thought we would see similar benefits.3
But, while all who attended the sessions thought they were valuable, attendance was so poor that we ended up cancelling the program. The hospitalists were interested in attending but were either on service and busy seeing patients, or were off and didn’t want to drive in to work solely for the purpose of reducing work stress.
I’m convinced a self-care program is valuable but very tricky to schedule effectively. Maybe others have come up with effective ways of overcoming this problem.
Social connections. Some hospitalist groups seem to have little social and personal connection to other physicians and hospital leaders. I think this results in lower hospitalist morale and tends to be self-reinforcing. If you’re in such a group, you and your hospitalist colleagues should deliberately seek better relationships with other doctors and hospital administrative leaders. Ensure that you visit with others at lunch, talk with them at committee meetings, ask about their vacation and personal activities, and pursue activities with them outside of work.
When these sorts of social connections are strong, work is far more satisfying and you’re much more likely to be treated as a peer by other doctors. I think this is really important and shouldn’t be overlooked if your group is suffering from low morale.
Adaptive work. Lastly, you might want to approach changes to your work and morale as “adaptive work,” rather than “technical work.” Space doesn’t permit a description of these, but it is worth reading about how they differ. Many groups will find value in reframing their approach to aspects of work they don’t like as adaptive work.

References
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
- Hoff TH, Whitcomb WF, Williams K, Nelson JR, Cheesman RA. Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851-858.
- West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
Using quotes to ensure that the results were only those that include the two words adjacent to one another, rather than separated, I entered the following phrases into my Google search engine:
- “hospitalist burnout” = 1,580 results
- “hospitalist morale” = 208 results
- “hospitalist well-being” = 0 results
I think the number of results suggests the level of interest in each topic and, if that is the case, clearly thinking about how hospitalists are doing in their careers is more commonly done through the paradigm of burnout than the other two terms. (Of course, there may be other terms that I didn’t consider.) In fact, there have been a handful of published studies of hospitalist burnout and job satisfaction.1,2
Those studies generally have shown both reasonably high levels of job satisfaction and troubling levels of burnout.
But I’ve been thinking about hospitalist morale for a while. I think morale is reasonably distinct from both burnout and job satisfaction.
Causes of a National Decline in Hospitalist Morale
I think hospitalist morale has declined some over the past two or three years across the country. This observation is meaningful because it comes from my experience working with a lot of hospitalist groups coast to coast. But I’m the first to admit it is just anecdotal and is subject to my own biases.
I can think of several things contributing to a decline in morale.
EHR adoption. Near the top of the list is the adoption of EHRs in many hospitals, which typically leads doctors in other specialties to seek hospitalist assistance with EHR-related tasks (e.g. medicine reconciliation and order writing) even in cases where there is little or no clinical reason for hospitalist involvement. Lots of hospitalists complain about this. To be clear, in many hospitals the hospitalists are reasonably content with using the EHR, but they experience ongoing frustration and low morale resulting from nonclinical work other doctors pressure them to take over.
Observation status. Many hospitals began classifying a larger portion of patients as observation status over the last few years; at the same time, patients and families have become more aware of how much of a disadvantage this is. In many cases, it is the hospitalist who takes the brunt of patient and family frustration. This can get awfully stressful and frustrating, and I think it is a contributor to allegations of malpractice.
Budgetary stress. Ever since SHM began collecting survey data in the late 1990s, the financial support hospitals have been providing to hospitalists has increased dramatically. The most recent State of Hospital Medicine report, published in 2104, showed median support provided by hospitals of $156,063 per FTE hospitalist, per year. Some hospitals have begun to resist providing more support, and this translates into stress and lower morale for hospitalists. This is far from a universal issue, but it does lead to lower morale for hospitalists who face it.
Many other factors may be contributing to a national decline in morale, but I think these are some of the most important.
What Can Be Done?
Some hospitalist groups have great morale now and don’t need to do much of anything right now, but some groups should think about a deliberate strategy to improve it.
Sadly, there isn’t a prescription that is sure to work. But there are some things you can try.
Self-care. The field of palliative care has thought a lot about caring for caregivers, and hospitalist groups might want to adopt some of their practices. Search the Internet on “self-care” + “palliative care,” and you’ll find a lot of interesting things. The group I’m part of launched a deliberate program of professionally led and facilitated hospitalist self-care, with high hopes that included mindful meditation, among other things. As soon as we had designed our program, the Mayo Clinic published their favorable experience with a program that was very similar to what we had planned, and I thought we would see similar benefits.3
But, while all who attended the sessions thought they were valuable, attendance was so poor that we ended up cancelling the program. The hospitalists were interested in attending but were either on service and busy seeing patients, or were off and didn’t want to drive in to work solely for the purpose of reducing work stress.
I’m convinced a self-care program is valuable but very tricky to schedule effectively. Maybe others have come up with effective ways of overcoming this problem.
Social connections. Some hospitalist groups seem to have little social and personal connection to other physicians and hospital leaders. I think this results in lower hospitalist morale and tends to be self-reinforcing. If you’re in such a group, you and your hospitalist colleagues should deliberately seek better relationships with other doctors and hospital administrative leaders. Ensure that you visit with others at lunch, talk with them at committee meetings, ask about their vacation and personal activities, and pursue activities with them outside of work.
When these sorts of social connections are strong, work is far more satisfying and you’re much more likely to be treated as a peer by other doctors. I think this is really important and shouldn’t be overlooked if your group is suffering from low morale.
Adaptive work. Lastly, you might want to approach changes to your work and morale as “adaptive work,” rather than “technical work.” Space doesn’t permit a description of these, but it is worth reading about how they differ. Many groups will find value in reframing their approach to aspects of work they don’t like as adaptive work.

References
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
- Hoff TH, Whitcomb WF, Williams K, Nelson JR, Cheesman RA. Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851-858.
- West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
Using quotes to ensure that the results were only those that include the two words adjacent to one another, rather than separated, I entered the following phrases into my Google search engine:
- “hospitalist burnout” = 1,580 results
- “hospitalist morale” = 208 results
- “hospitalist well-being” = 0 results
I think the number of results suggests the level of interest in each topic and, if that is the case, clearly thinking about how hospitalists are doing in their careers is more commonly done through the paradigm of burnout than the other two terms. (Of course, there may be other terms that I didn’t consider.) In fact, there have been a handful of published studies of hospitalist burnout and job satisfaction.1,2
Those studies generally have shown both reasonably high levels of job satisfaction and troubling levels of burnout.
But I’ve been thinking about hospitalist morale for a while. I think morale is reasonably distinct from both burnout and job satisfaction.
Causes of a National Decline in Hospitalist Morale
I think hospitalist morale has declined some over the past two or three years across the country. This observation is meaningful because it comes from my experience working with a lot of hospitalist groups coast to coast. But I’m the first to admit it is just anecdotal and is subject to my own biases.
I can think of several things contributing to a decline in morale.
EHR adoption. Near the top of the list is the adoption of EHRs in many hospitals, which typically leads doctors in other specialties to seek hospitalist assistance with EHR-related tasks (e.g. medicine reconciliation and order writing) even in cases where there is little or no clinical reason for hospitalist involvement. Lots of hospitalists complain about this. To be clear, in many hospitals the hospitalists are reasonably content with using the EHR, but they experience ongoing frustration and low morale resulting from nonclinical work other doctors pressure them to take over.
Observation status. Many hospitals began classifying a larger portion of patients as observation status over the last few years; at the same time, patients and families have become more aware of how much of a disadvantage this is. In many cases, it is the hospitalist who takes the brunt of patient and family frustration. This can get awfully stressful and frustrating, and I think it is a contributor to allegations of malpractice.
Budgetary stress. Ever since SHM began collecting survey data in the late 1990s, the financial support hospitals have been providing to hospitalists has increased dramatically. The most recent State of Hospital Medicine report, published in 2104, showed median support provided by hospitals of $156,063 per FTE hospitalist, per year. Some hospitals have begun to resist providing more support, and this translates into stress and lower morale for hospitalists. This is far from a universal issue, but it does lead to lower morale for hospitalists who face it.
Many other factors may be contributing to a national decline in morale, but I think these are some of the most important.
What Can Be Done?
Some hospitalist groups have great morale now and don’t need to do much of anything right now, but some groups should think about a deliberate strategy to improve it.
Sadly, there isn’t a prescription that is sure to work. But there are some things you can try.
Self-care. The field of palliative care has thought a lot about caring for caregivers, and hospitalist groups might want to adopt some of their practices. Search the Internet on “self-care” + “palliative care,” and you’ll find a lot of interesting things. The group I’m part of launched a deliberate program of professionally led and facilitated hospitalist self-care, with high hopes that included mindful meditation, among other things. As soon as we had designed our program, the Mayo Clinic published their favorable experience with a program that was very similar to what we had planned, and I thought we would see similar benefits.3
But, while all who attended the sessions thought they were valuable, attendance was so poor that we ended up cancelling the program. The hospitalists were interested in attending but were either on service and busy seeing patients, or were off and didn’t want to drive in to work solely for the purpose of reducing work stress.
I’m convinced a self-care program is valuable but very tricky to schedule effectively. Maybe others have come up with effective ways of overcoming this problem.
Social connections. Some hospitalist groups seem to have little social and personal connection to other physicians and hospital leaders. I think this results in lower hospitalist morale and tends to be self-reinforcing. If you’re in such a group, you and your hospitalist colleagues should deliberately seek better relationships with other doctors and hospital administrative leaders. Ensure that you visit with others at lunch, talk with them at committee meetings, ask about their vacation and personal activities, and pursue activities with them outside of work.
When these sorts of social connections are strong, work is far more satisfying and you’re much more likely to be treated as a peer by other doctors. I think this is really important and shouldn’t be overlooked if your group is suffering from low morale.
Adaptive work. Lastly, you might want to approach changes to your work and morale as “adaptive work,” rather than “technical work.” Space doesn’t permit a description of these, but it is worth reading about how they differ. Many groups will find value in reframing their approach to aspects of work they don’t like as adaptive work.

References
- Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
- Hoff TH, Whitcomb WF, Williams K, Nelson JR, Cheesman RA. Characteristics and work experiences of hospitalists in the United States. Arch Intern Med. 2001;161(6):851-858.
- West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
Turn down the androgens to treat female pattern hair loss
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Rivaroxaban appears safe, effective in the real world

Photo courtesy of the CDC
LONDON—Patients with atrial fibrillation (AF) who receive the anticoagulant rivaroxaban as stroke prophylaxis have low rates of major bleeding and stroke, according to real-world data from the XANTUS trial.
Investigators said this finding is similar to clinical trial results with rivaroxaban and suggest the drug is safe and effective for stroke prevention in patients with AF who have a high or low risk of thromboembolic events.
The team reported results of the XANTUS trial in the European Heart Journal and at the ESC Congress 2015 (abstract 5072). The study was sponsored by Bayer, the company developing rivaroxaban in cooperation with Janssen Pharmaceuticals, Inc.
“The findings reaffirm the positive benefit-risk profile of rivaroxaban established in the phase 3 clinical trial ROCKET AF, in which rivaroxaban was shown to provide effective stroke prevention with a similar overall bleeding profile and significantly lower rates of the most feared intracranial and fatal bleeds compared with vitamin K antagonists,” said John A. Camm, MD, of St. George’s University of London in the UK.
“The patients included in ROCKET AF were at moderate to high risk of stroke, with a mean CHADS2 score of 3.5, and the incidence of major bleeding in those taking rivaroxaban was 3.6 per 100 person-years. In XANTUS, patients seen in daily clinical practice had a lower risk of stroke, with a mean CHADS2 score of 2.0, and the incidence rate of major bleeding was lower, at 2.1 per 100 person-years.”
Patients and treatment
For the XANTUS trial, Dr Camm and his colleagues evaluated the outcomes of rivaroxaban use in 6784 patients with non-valvular AF who were treated at 311 centers across Europe and Canada in routine clinical practice.
Patients had newly diagnosed AF (18.5%), paroxysmal AF (40.6%), persistent AF (13.6%), and permanent AF (27%). For 0.2% of patients, data on their exact diagnosis was missing.
Comorbidities included hypertension (74.7%), diabetes mellitus (19.6%), prior stroke/non-central nervous system systemic embolism/transient ischemic attack (19%), congestive heart failure (18.6%), and prior myocardial infarction (10.1%).
The patients’ mean age was 71.5, and 37.2% were older than 75. Their mean CHADS2 score was 2, and their mean CHA2DS2VASc score was 3.4. A little more than half (54.5%) of patients were vitamin K agonist-naïve.
All treatment and dosing decisions for rivaroxaban were at the discretion of the treating physicians. Patients received the drug at 15 mg (n=1410), 20 mg (n=5336), or other doses (n=35).
They were followed for 1 year or until 30 days after premature treatment discontinuation. Bleeding events and major thromboembolic events were centrally adjudicated by an independent committee.
Results
By the end of the observation period, most patients (96.1%) had not experienced treatment-emergent major bleeding, all-cause death, or stroke/systemic embolism. And the majority of patients (80%) continued taking rivaroxaban throughout the 1-year study period.
The rate of stroke was 0.7% per year, and the annual rate of stroke/systemic embolism was 0.8%.
Overall, 2.1% of patients per year experienced treatment-emergent major bleeding. Non-major bleeding events occurred in 15.4% of patients per year.
The yearly rate of fatal bleeding was 0.2%, critical organ bleeding was 0.7%, intracranial hemorrhage was 0.4%, mucosal bleeding was 1.0%, and gastrointestinal bleeding was 0.9%. In addition, 0.9% of patients required transfusions of 2 or more units of packed red blood cells or whole blood per year.
The rate of on-treatment, all-cause mortality was 1.9% per year. There were 118 deaths in all, and some patients had multiple reasons reported as the cause of death. Causes of death were cardiovascular events (n=49), cancer (n=23), bleeding (n=12), infectious disease (n=10), other (n=16), and unexplained (n=9).
“These real-world insights from XANTUS complement and expand on what we already know from clinical trials and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with AF seen in their everyday clinical practice,” Dr Camm concluded. ![]()

Photo courtesy of the CDC
LONDON—Patients with atrial fibrillation (AF) who receive the anticoagulant rivaroxaban as stroke prophylaxis have low rates of major bleeding and stroke, according to real-world data from the XANTUS trial.
Investigators said this finding is similar to clinical trial results with rivaroxaban and suggest the drug is safe and effective for stroke prevention in patients with AF who have a high or low risk of thromboembolic events.
The team reported results of the XANTUS trial in the European Heart Journal and at the ESC Congress 2015 (abstract 5072). The study was sponsored by Bayer, the company developing rivaroxaban in cooperation with Janssen Pharmaceuticals, Inc.
“The findings reaffirm the positive benefit-risk profile of rivaroxaban established in the phase 3 clinical trial ROCKET AF, in which rivaroxaban was shown to provide effective stroke prevention with a similar overall bleeding profile and significantly lower rates of the most feared intracranial and fatal bleeds compared with vitamin K antagonists,” said John A. Camm, MD, of St. George’s University of London in the UK.
“The patients included in ROCKET AF were at moderate to high risk of stroke, with a mean CHADS2 score of 3.5, and the incidence of major bleeding in those taking rivaroxaban was 3.6 per 100 person-years. In XANTUS, patients seen in daily clinical practice had a lower risk of stroke, with a mean CHADS2 score of 2.0, and the incidence rate of major bleeding was lower, at 2.1 per 100 person-years.”
Patients and treatment
For the XANTUS trial, Dr Camm and his colleagues evaluated the outcomes of rivaroxaban use in 6784 patients with non-valvular AF who were treated at 311 centers across Europe and Canada in routine clinical practice.
Patients had newly diagnosed AF (18.5%), paroxysmal AF (40.6%), persistent AF (13.6%), and permanent AF (27%). For 0.2% of patients, data on their exact diagnosis was missing.
Comorbidities included hypertension (74.7%), diabetes mellitus (19.6%), prior stroke/non-central nervous system systemic embolism/transient ischemic attack (19%), congestive heart failure (18.6%), and prior myocardial infarction (10.1%).
The patients’ mean age was 71.5, and 37.2% were older than 75. Their mean CHADS2 score was 2, and their mean CHA2DS2VASc score was 3.4. A little more than half (54.5%) of patients were vitamin K agonist-naïve.
All treatment and dosing decisions for rivaroxaban were at the discretion of the treating physicians. Patients received the drug at 15 mg (n=1410), 20 mg (n=5336), or other doses (n=35).
They were followed for 1 year or until 30 days after premature treatment discontinuation. Bleeding events and major thromboembolic events were centrally adjudicated by an independent committee.
Results
By the end of the observation period, most patients (96.1%) had not experienced treatment-emergent major bleeding, all-cause death, or stroke/systemic embolism. And the majority of patients (80%) continued taking rivaroxaban throughout the 1-year study period.
The rate of stroke was 0.7% per year, and the annual rate of stroke/systemic embolism was 0.8%.
Overall, 2.1% of patients per year experienced treatment-emergent major bleeding. Non-major bleeding events occurred in 15.4% of patients per year.
The yearly rate of fatal bleeding was 0.2%, critical organ bleeding was 0.7%, intracranial hemorrhage was 0.4%, mucosal bleeding was 1.0%, and gastrointestinal bleeding was 0.9%. In addition, 0.9% of patients required transfusions of 2 or more units of packed red blood cells or whole blood per year.
The rate of on-treatment, all-cause mortality was 1.9% per year. There were 118 deaths in all, and some patients had multiple reasons reported as the cause of death. Causes of death were cardiovascular events (n=49), cancer (n=23), bleeding (n=12), infectious disease (n=10), other (n=16), and unexplained (n=9).
“These real-world insights from XANTUS complement and expand on what we already know from clinical trials and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with AF seen in their everyday clinical practice,” Dr Camm concluded. ![]()

Photo courtesy of the CDC
LONDON—Patients with atrial fibrillation (AF) who receive the anticoagulant rivaroxaban as stroke prophylaxis have low rates of major bleeding and stroke, according to real-world data from the XANTUS trial.
Investigators said this finding is similar to clinical trial results with rivaroxaban and suggest the drug is safe and effective for stroke prevention in patients with AF who have a high or low risk of thromboembolic events.
The team reported results of the XANTUS trial in the European Heart Journal and at the ESC Congress 2015 (abstract 5072). The study was sponsored by Bayer, the company developing rivaroxaban in cooperation with Janssen Pharmaceuticals, Inc.
“The findings reaffirm the positive benefit-risk profile of rivaroxaban established in the phase 3 clinical trial ROCKET AF, in which rivaroxaban was shown to provide effective stroke prevention with a similar overall bleeding profile and significantly lower rates of the most feared intracranial and fatal bleeds compared with vitamin K antagonists,” said John A. Camm, MD, of St. George’s University of London in the UK.
“The patients included in ROCKET AF were at moderate to high risk of stroke, with a mean CHADS2 score of 3.5, and the incidence of major bleeding in those taking rivaroxaban was 3.6 per 100 person-years. In XANTUS, patients seen in daily clinical practice had a lower risk of stroke, with a mean CHADS2 score of 2.0, and the incidence rate of major bleeding was lower, at 2.1 per 100 person-years.”
Patients and treatment
For the XANTUS trial, Dr Camm and his colleagues evaluated the outcomes of rivaroxaban use in 6784 patients with non-valvular AF who were treated at 311 centers across Europe and Canada in routine clinical practice.
Patients had newly diagnosed AF (18.5%), paroxysmal AF (40.6%), persistent AF (13.6%), and permanent AF (27%). For 0.2% of patients, data on their exact diagnosis was missing.
Comorbidities included hypertension (74.7%), diabetes mellitus (19.6%), prior stroke/non-central nervous system systemic embolism/transient ischemic attack (19%), congestive heart failure (18.6%), and prior myocardial infarction (10.1%).
The patients’ mean age was 71.5, and 37.2% were older than 75. Their mean CHADS2 score was 2, and their mean CHA2DS2VASc score was 3.4. A little more than half (54.5%) of patients were vitamin K agonist-naïve.
All treatment and dosing decisions for rivaroxaban were at the discretion of the treating physicians. Patients received the drug at 15 mg (n=1410), 20 mg (n=5336), or other doses (n=35).
They were followed for 1 year or until 30 days after premature treatment discontinuation. Bleeding events and major thromboembolic events were centrally adjudicated by an independent committee.
Results
By the end of the observation period, most patients (96.1%) had not experienced treatment-emergent major bleeding, all-cause death, or stroke/systemic embolism. And the majority of patients (80%) continued taking rivaroxaban throughout the 1-year study period.
The rate of stroke was 0.7% per year, and the annual rate of stroke/systemic embolism was 0.8%.
Overall, 2.1% of patients per year experienced treatment-emergent major bleeding. Non-major bleeding events occurred in 15.4% of patients per year.
The yearly rate of fatal bleeding was 0.2%, critical organ bleeding was 0.7%, intracranial hemorrhage was 0.4%, mucosal bleeding was 1.0%, and gastrointestinal bleeding was 0.9%. In addition, 0.9% of patients required transfusions of 2 or more units of packed red blood cells or whole blood per year.
The rate of on-treatment, all-cause mortality was 1.9% per year. There were 118 deaths in all, and some patients had multiple reasons reported as the cause of death. Causes of death were cardiovascular events (n=49), cancer (n=23), bleeding (n=12), infectious disease (n=10), other (n=16), and unexplained (n=9).
“These real-world insights from XANTUS complement and expand on what we already know from clinical trials and provide physicians with reassurance to prescribe rivaroxaban as an effective and well-tolerated treatment option for the broad range of patients with AF seen in their everyday clinical practice,” Dr Camm concluded. ![]()
Extending DAPT may provide benefit for some, speaker says
LONDON—Extending dual antiplatelet therapy (DAPT) beyond the recommended 12 months after coronary stenting “should be considered” in patients with a drug-eluting stent (DES) who are at low risk for bleeding, according to investigators from the OPTIDUAL trial.
The study showed that receiving DAPT—aspirin and clopidogrel—for an additional 36 months did not decrease the rate of major adverse cardiovascular and cerebrovascular events (MACCE).
However, there was a suggestion that it might reduce ischemic outcomes without excess bleeding.
“Given the lack of harm and the signal for benefit of prolonged DAPT in the OPTIDUAL trial, and the results from prior randomized trials testing long durations of DAPT, prolongation of DAPT beyond 12 months should be considered in patients without high-risk bleeding who have received a drug-eluting coronary stent and are event-free at 12 months,” said study investigator Gérard Helft, MD, PhD, of Hôpital Universitaire Pitié-Salpétrière in Paris, France.
He presented results of the trial at the ESC Congress 2015 (abstract 3159).
The study included 1385 patients from 58 French sites who had undergone percutaneous coronary intervention with placement of at least 1 DES for either stable coronary artery disease or acute coronary syndrome.
All patients had been on DAPT for 1 year and were randomly assigned to continue or to remain on aspirin alone for an additional 36 months.
There was no significant difference between the groups with regard to MACCE, a composite of all-cause death, myocardial infarction, stroke, and major bleeding. These events occurred in 5.8% of patients in the extended-DAPT group and 7.5% in the aspirin-only group (hazard ratio (HR=0.75, P=0.17).
Likewise, there was no significant difference between the treatment groups for any of the components of MACCE—stroke (HR=0.69, P=0.53), myocardial infarction (HR=0.67, P=0.31), major bleeding (HR=0.98, P=0.95), or death (HR=0.65, P=0.18).
There was a non-significant (but borderline) reduction in ischemic outcomes—a post-hoc outcome composite rate of death, myocardial infarction, or stroke—with extended DAPT. These events occurred in 4.2% of patients in the extended-DAPT group and 6.4% in the aspirin-only group (HR=0.64, P=0.06).
“The results are consistent with the recent findings on ischemic outcomes from the DAPT trial regarding the value of prolonging DAPT after DES placement,” Dr Helft said.
“There was no apparent harm, and the post-hoc efficacy signal on MACCE is consistent with the benefit seen in the DAPT trial. Thus, OPTIDUAL adds to the evidence suggesting benefit to extended DAPT after DES in patients who are event-free at 12 months.”
This study was funded by Assistance Publique - Hôpitaux de Paris and the Fédération Française de Cardiologie, with unrestricted grants from Cordis, Boston, Medtronic, Terumo, and Biotronik. ![]()

LONDON—Extending dual antiplatelet therapy (DAPT) beyond the recommended 12 months after coronary stenting “should be considered” in patients with a drug-eluting stent (DES) who are at low risk for bleeding, according to investigators from the OPTIDUAL trial.
The study showed that receiving DAPT—aspirin and clopidogrel—for an additional 36 months did not decrease the rate of major adverse cardiovascular and cerebrovascular events (MACCE).
However, there was a suggestion that it might reduce ischemic outcomes without excess bleeding.
“Given the lack of harm and the signal for benefit of prolonged DAPT in the OPTIDUAL trial, and the results from prior randomized trials testing long durations of DAPT, prolongation of DAPT beyond 12 months should be considered in patients without high-risk bleeding who have received a drug-eluting coronary stent and are event-free at 12 months,” said study investigator Gérard Helft, MD, PhD, of Hôpital Universitaire Pitié-Salpétrière in Paris, France.
He presented results of the trial at the ESC Congress 2015 (abstract 3159).
The study included 1385 patients from 58 French sites who had undergone percutaneous coronary intervention with placement of at least 1 DES for either stable coronary artery disease or acute coronary syndrome.
All patients had been on DAPT for 1 year and were randomly assigned to continue or to remain on aspirin alone for an additional 36 months.
There was no significant difference between the groups with regard to MACCE, a composite of all-cause death, myocardial infarction, stroke, and major bleeding. These events occurred in 5.8% of patients in the extended-DAPT group and 7.5% in the aspirin-only group (hazard ratio (HR=0.75, P=0.17).
Likewise, there was no significant difference between the treatment groups for any of the components of MACCE—stroke (HR=0.69, P=0.53), myocardial infarction (HR=0.67, P=0.31), major bleeding (HR=0.98, P=0.95), or death (HR=0.65, P=0.18).
There was a non-significant (but borderline) reduction in ischemic outcomes—a post-hoc outcome composite rate of death, myocardial infarction, or stroke—with extended DAPT. These events occurred in 4.2% of patients in the extended-DAPT group and 6.4% in the aspirin-only group (HR=0.64, P=0.06).
“The results are consistent with the recent findings on ischemic outcomes from the DAPT trial regarding the value of prolonging DAPT after DES placement,” Dr Helft said.
“There was no apparent harm, and the post-hoc efficacy signal on MACCE is consistent with the benefit seen in the DAPT trial. Thus, OPTIDUAL adds to the evidence suggesting benefit to extended DAPT after DES in patients who are event-free at 12 months.”
This study was funded by Assistance Publique - Hôpitaux de Paris and the Fédération Française de Cardiologie, with unrestricted grants from Cordis, Boston, Medtronic, Terumo, and Biotronik. ![]()

LONDON—Extending dual antiplatelet therapy (DAPT) beyond the recommended 12 months after coronary stenting “should be considered” in patients with a drug-eluting stent (DES) who are at low risk for bleeding, according to investigators from the OPTIDUAL trial.
The study showed that receiving DAPT—aspirin and clopidogrel—for an additional 36 months did not decrease the rate of major adverse cardiovascular and cerebrovascular events (MACCE).
However, there was a suggestion that it might reduce ischemic outcomes without excess bleeding.
“Given the lack of harm and the signal for benefit of prolonged DAPT in the OPTIDUAL trial, and the results from prior randomized trials testing long durations of DAPT, prolongation of DAPT beyond 12 months should be considered in patients without high-risk bleeding who have received a drug-eluting coronary stent and are event-free at 12 months,” said study investigator Gérard Helft, MD, PhD, of Hôpital Universitaire Pitié-Salpétrière in Paris, France.
He presented results of the trial at the ESC Congress 2015 (abstract 3159).
The study included 1385 patients from 58 French sites who had undergone percutaneous coronary intervention with placement of at least 1 DES for either stable coronary artery disease or acute coronary syndrome.
All patients had been on DAPT for 1 year and were randomly assigned to continue or to remain on aspirin alone for an additional 36 months.
There was no significant difference between the groups with regard to MACCE, a composite of all-cause death, myocardial infarction, stroke, and major bleeding. These events occurred in 5.8% of patients in the extended-DAPT group and 7.5% in the aspirin-only group (hazard ratio (HR=0.75, P=0.17).
Likewise, there was no significant difference between the treatment groups for any of the components of MACCE—stroke (HR=0.69, P=0.53), myocardial infarction (HR=0.67, P=0.31), major bleeding (HR=0.98, P=0.95), or death (HR=0.65, P=0.18).
There was a non-significant (but borderline) reduction in ischemic outcomes—a post-hoc outcome composite rate of death, myocardial infarction, or stroke—with extended DAPT. These events occurred in 4.2% of patients in the extended-DAPT group and 6.4% in the aspirin-only group (HR=0.64, P=0.06).
“The results are consistent with the recent findings on ischemic outcomes from the DAPT trial regarding the value of prolonging DAPT after DES placement,” Dr Helft said.
“There was no apparent harm, and the post-hoc efficacy signal on MACCE is consistent with the benefit seen in the DAPT trial. Thus, OPTIDUAL adds to the evidence suggesting benefit to extended DAPT after DES in patients who are event-free at 12 months.”
This study was funded by Assistance Publique - Hôpitaux de Paris and the Fédération Française de Cardiologie, with unrestricted grants from Cordis, Boston, Medtronic, Terumo, and Biotronik. ![]()
Sequencing aids management of young cancer patients

patient and her father
Photo by Rhoda Baer
Exome and transcriptome sequencing results can inform the management of young patients with relapsed, refractory, and rare malignancies, a new study suggests.
In a consecutive case series, sequencing data revealed potentially actionable findings for 46% of patients.
As a result, 15% of patients changed treatment, and 10% underwent genetic counseling.
Investigators described this research in JAMA.
“We found that, for some children with rare, difficult-to-treat, and aggressive cancers, this technology can dramatically change the course of their treatment,” said study author Rajen Mody, MBBS, of the University of Michigan in Ann Arbor.
Dr Mody and his colleagues evaluated 102 patients with relapsed, refractory, or rare cancers. Their median age was 11.5 (range, 0-22).
The patients underwent integrative clinical exome (tumor and germline DNA) and transcriptome (tumor RNA) sequencing. Ninety-one patients (89%) had adequate tumor tissue to complete sequencing, including 28 patients (31%) with hematologic malignancies and 63 (69%) with solid tumors.
All sequencing results were discussed at a precision medicine tumor board, which included pediatric and adult oncologists, pathologists, genetics specialists, and other professionals. This group discussed the results and assessed the feasibility of pursuing treatment options based on the findings.
Actionable findings
Forty-two patients (46%) had potentially actionable findings, 15 (54%) with hematologic malignancies and 27 (43%) with solid tumors.
Actionable findings included a change in diagnosis (n=2), the presence of a genetic anomaly that could be targeted by an approved or experimental drug (n=31), and the need for genetic counseling for inherited cancer risk that could affect the patient or the whole family (n=9).
“We were excited to see an actionable finding in such a substantial percentage of patients, and we think it could potentially be higher over time,” said study author Arul Chinnaiyan, MD, PhD, also of the University of Michigan.
“These are patients who had exhausted all proven therapeutic options or who had an extremely rare diagnosis. If we can find a clinically actionable event and have a chance to act upon it, we show in this study that it can have a big impact on that patient.”
Actions were taken in 23 of the 42 patients. Fourteen patients (15%) had their treatment changed, and 9 of these patients (10%) had durable partial or complete remissions (CRs) as a result.
Nine patients (10%) underwent genetic counseling because of sequencing results. The researchers noted that 4 of these patients had no notable family history to suggest an inherited risk, and they would not otherwise have been referred for genetic counseling.
Hematologic malignancies
Fifteen patients with hematologic malignancies had potentially actionable findings, and 4 underwent treatment changes as a result. (None of the patients required genetic counseling.)
For a patient with pre-B acute lymphoblastic leukemia (ALL), sequencing revealed a homozygous CDKN2A deletion and an ETV6-ABL1 fusion. So the patient was placed on imatinib and had a sustained CR for 21 months.
A patient with early T-cell precursor ALL had a FLT3-ITD mutation, Chr16p gain, Chr16q loss, and FLT3 overexpression. The patient achieved a CR after transplant, was placed on the FLT3 inhibitor sorafenib, and remained in CR for 15 months.
Another patient with pre-B ALL had a FLT3 nonframeshift deletion and BLK and FLT3 overexpression. The patient was in CR for 9 months after a transplant and received sorafenib for 6 months.
A patient with biphenotypic leukemia had mutations in NRAS and PHF6; SPI1, ASXL1, and CBLC frameshift insertions; a JAK3-activating mutation; and JAK3 overexpression. The patient received the JAK3 inhibitor tofacitinib but could not tolerate the full dose and died of progressive disease.
Cost and turn-around time
The cost for sequencing was approximately $6000 per patient and was covered under the research protocol.
It took the researchers about 7 to 8 weeks to report the sequencing results back to treating physicians and families.
“These are early days, and the full promise of precision medicine is yet to be fully realized,” Dr Mody said. “We need better targeted therapies designed for children, and turnaround time for sequencing needs to be less than 2 weeks for it to be a regular part of a patient’s treatment plan.” ![]()

patient and her father
Photo by Rhoda Baer
Exome and transcriptome sequencing results can inform the management of young patients with relapsed, refractory, and rare malignancies, a new study suggests.
In a consecutive case series, sequencing data revealed potentially actionable findings for 46% of patients.
As a result, 15% of patients changed treatment, and 10% underwent genetic counseling.
Investigators described this research in JAMA.
“We found that, for some children with rare, difficult-to-treat, and aggressive cancers, this technology can dramatically change the course of their treatment,” said study author Rajen Mody, MBBS, of the University of Michigan in Ann Arbor.
Dr Mody and his colleagues evaluated 102 patients with relapsed, refractory, or rare cancers. Their median age was 11.5 (range, 0-22).
The patients underwent integrative clinical exome (tumor and germline DNA) and transcriptome (tumor RNA) sequencing. Ninety-one patients (89%) had adequate tumor tissue to complete sequencing, including 28 patients (31%) with hematologic malignancies and 63 (69%) with solid tumors.
All sequencing results were discussed at a precision medicine tumor board, which included pediatric and adult oncologists, pathologists, genetics specialists, and other professionals. This group discussed the results and assessed the feasibility of pursuing treatment options based on the findings.
Actionable findings
Forty-two patients (46%) had potentially actionable findings, 15 (54%) with hematologic malignancies and 27 (43%) with solid tumors.
Actionable findings included a change in diagnosis (n=2), the presence of a genetic anomaly that could be targeted by an approved or experimental drug (n=31), and the need for genetic counseling for inherited cancer risk that could affect the patient or the whole family (n=9).
“We were excited to see an actionable finding in such a substantial percentage of patients, and we think it could potentially be higher over time,” said study author Arul Chinnaiyan, MD, PhD, also of the University of Michigan.
“These are patients who had exhausted all proven therapeutic options or who had an extremely rare diagnosis. If we can find a clinically actionable event and have a chance to act upon it, we show in this study that it can have a big impact on that patient.”
Actions were taken in 23 of the 42 patients. Fourteen patients (15%) had their treatment changed, and 9 of these patients (10%) had durable partial or complete remissions (CRs) as a result.
Nine patients (10%) underwent genetic counseling because of sequencing results. The researchers noted that 4 of these patients had no notable family history to suggest an inherited risk, and they would not otherwise have been referred for genetic counseling.
Hematologic malignancies
Fifteen patients with hematologic malignancies had potentially actionable findings, and 4 underwent treatment changes as a result. (None of the patients required genetic counseling.)
For a patient with pre-B acute lymphoblastic leukemia (ALL), sequencing revealed a homozygous CDKN2A deletion and an ETV6-ABL1 fusion. So the patient was placed on imatinib and had a sustained CR for 21 months.
A patient with early T-cell precursor ALL had a FLT3-ITD mutation, Chr16p gain, Chr16q loss, and FLT3 overexpression. The patient achieved a CR after transplant, was placed on the FLT3 inhibitor sorafenib, and remained in CR for 15 months.
Another patient with pre-B ALL had a FLT3 nonframeshift deletion and BLK and FLT3 overexpression. The patient was in CR for 9 months after a transplant and received sorafenib for 6 months.
A patient with biphenotypic leukemia had mutations in NRAS and PHF6; SPI1, ASXL1, and CBLC frameshift insertions; a JAK3-activating mutation; and JAK3 overexpression. The patient received the JAK3 inhibitor tofacitinib but could not tolerate the full dose and died of progressive disease.
Cost and turn-around time
The cost for sequencing was approximately $6000 per patient and was covered under the research protocol.
It took the researchers about 7 to 8 weeks to report the sequencing results back to treating physicians and families.
“These are early days, and the full promise of precision medicine is yet to be fully realized,” Dr Mody said. “We need better targeted therapies designed for children, and turnaround time for sequencing needs to be less than 2 weeks for it to be a regular part of a patient’s treatment plan.” ![]()

patient and her father
Photo by Rhoda Baer
Exome and transcriptome sequencing results can inform the management of young patients with relapsed, refractory, and rare malignancies, a new study suggests.
In a consecutive case series, sequencing data revealed potentially actionable findings for 46% of patients.
As a result, 15% of patients changed treatment, and 10% underwent genetic counseling.
Investigators described this research in JAMA.
“We found that, for some children with rare, difficult-to-treat, and aggressive cancers, this technology can dramatically change the course of their treatment,” said study author Rajen Mody, MBBS, of the University of Michigan in Ann Arbor.
Dr Mody and his colleagues evaluated 102 patients with relapsed, refractory, or rare cancers. Their median age was 11.5 (range, 0-22).
The patients underwent integrative clinical exome (tumor and germline DNA) and transcriptome (tumor RNA) sequencing. Ninety-one patients (89%) had adequate tumor tissue to complete sequencing, including 28 patients (31%) with hematologic malignancies and 63 (69%) with solid tumors.
All sequencing results were discussed at a precision medicine tumor board, which included pediatric and adult oncologists, pathologists, genetics specialists, and other professionals. This group discussed the results and assessed the feasibility of pursuing treatment options based on the findings.
Actionable findings
Forty-two patients (46%) had potentially actionable findings, 15 (54%) with hematologic malignancies and 27 (43%) with solid tumors.
Actionable findings included a change in diagnosis (n=2), the presence of a genetic anomaly that could be targeted by an approved or experimental drug (n=31), and the need for genetic counseling for inherited cancer risk that could affect the patient or the whole family (n=9).
“We were excited to see an actionable finding in such a substantial percentage of patients, and we think it could potentially be higher over time,” said study author Arul Chinnaiyan, MD, PhD, also of the University of Michigan.
“These are patients who had exhausted all proven therapeutic options or who had an extremely rare diagnosis. If we can find a clinically actionable event and have a chance to act upon it, we show in this study that it can have a big impact on that patient.”
Actions were taken in 23 of the 42 patients. Fourteen patients (15%) had their treatment changed, and 9 of these patients (10%) had durable partial or complete remissions (CRs) as a result.
Nine patients (10%) underwent genetic counseling because of sequencing results. The researchers noted that 4 of these patients had no notable family history to suggest an inherited risk, and they would not otherwise have been referred for genetic counseling.
Hematologic malignancies
Fifteen patients with hematologic malignancies had potentially actionable findings, and 4 underwent treatment changes as a result. (None of the patients required genetic counseling.)
For a patient with pre-B acute lymphoblastic leukemia (ALL), sequencing revealed a homozygous CDKN2A deletion and an ETV6-ABL1 fusion. So the patient was placed on imatinib and had a sustained CR for 21 months.
A patient with early T-cell precursor ALL had a FLT3-ITD mutation, Chr16p gain, Chr16q loss, and FLT3 overexpression. The patient achieved a CR after transplant, was placed on the FLT3 inhibitor sorafenib, and remained in CR for 15 months.
Another patient with pre-B ALL had a FLT3 nonframeshift deletion and BLK and FLT3 overexpression. The patient was in CR for 9 months after a transplant and received sorafenib for 6 months.
A patient with biphenotypic leukemia had mutations in NRAS and PHF6; SPI1, ASXL1, and CBLC frameshift insertions; a JAK3-activating mutation; and JAK3 overexpression. The patient received the JAK3 inhibitor tofacitinib but could not tolerate the full dose and died of progressive disease.
Cost and turn-around time
The cost for sequencing was approximately $6000 per patient and was covered under the research protocol.
It took the researchers about 7 to 8 weeks to report the sequencing results back to treating physicians and families.
“These are early days, and the full promise of precision medicine is yet to be fully realized,” Dr Mody said. “We need better targeted therapies designed for children, and turnaround time for sequencing needs to be less than 2 weeks for it to be a regular part of a patient’s treatment plan.” ![]()
How a toxin in wasp venom kills cancer cells

Polybia paulista
Photo by Mario Palma/
São Paulo State University
The wasp Polybia paulista protects itself from predators by producing venom known to contain a cancer-fighting toxin.
A study published in Biophysical Journal helps explain how the venom’s toxin, MP1 (Polybia-MP1), selectively kills cancer cells without harming normal cells.
MP1 interacts with lipids that are abnormally distributed on the surface of cancer cells, creating holes that facilitate the escape of molecules crucial for cell function.
“Cancer therapies that attack the lipid composition of the cell membrane would be an entirely new class of anticancer drugs,” said study author Paul Beales, PhD, of the University of Leeds in the UK.
MP1 is known to act against microbial pathogens by disrupting the bacterial cell membrane. The peptide has shown promise for treating cancers, as it can inhibit the growth of prostate and bladder cancer cells, as well as multi-drug resistant leukemic cells.
However, it has not been clear how MP1 selectively destroys cancer cells without harming normal cells. Dr Beales and his colleagues thought an explanation might lie in the unique properties of cancer cell membranes.
In healthy cell membranes, the phospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) are located in the inner membrane leaflet facing the inside of the cell. But in cancer cells, PS and PE are embedded in the outer membrane leaflet facing the cell surroundings.
The researchers tested their theory by creating model membranes, some of which contained PE and/or PS, and exposing them to MP1. They used a wide range of imaging and biophysical techniques to characterize MP1’s destructive effects on the membranes.
The team found that PS increased the binding of MP1 to the membrane by a factor of 7 to 8. On the other hand, PE enhanced MP1’s ability to quickly disrupt the membrane, increasing the size of holes by a factor of 20 to 30.
“Formed in only seconds, these large pores are big enough to allow critical molecules such as RNA and proteins to easily escape cells,” said study author João Ruggiero Neto, of São Paulo State University in Brazil.
“The dramatic enhancement of the permeabilization induced by the peptide in the presence of PE and the dimensions of the pores in these membranes was surprising.”
In future studies, the researchers plan to alter MP1’s amino acid sequence to examine how the peptide’s structure relates to its function and further improve the peptide’s selectivity and potency for clinical purposes.
“Understanding the mechanism of action of this peptide will help in translational studies to further assess the potential for this peptide to be used in medicine,” Dr Beales said.
“As it has been shown to be selective to cancer cells and non-toxic to normal cells in the lab, this peptide has the potential to be safe, but further work would be required to prove that.” ![]()

Polybia paulista
Photo by Mario Palma/
São Paulo State University
The wasp Polybia paulista protects itself from predators by producing venom known to contain a cancer-fighting toxin.
A study published in Biophysical Journal helps explain how the venom’s toxin, MP1 (Polybia-MP1), selectively kills cancer cells without harming normal cells.
MP1 interacts with lipids that are abnormally distributed on the surface of cancer cells, creating holes that facilitate the escape of molecules crucial for cell function.
“Cancer therapies that attack the lipid composition of the cell membrane would be an entirely new class of anticancer drugs,” said study author Paul Beales, PhD, of the University of Leeds in the UK.
MP1 is known to act against microbial pathogens by disrupting the bacterial cell membrane. The peptide has shown promise for treating cancers, as it can inhibit the growth of prostate and bladder cancer cells, as well as multi-drug resistant leukemic cells.
However, it has not been clear how MP1 selectively destroys cancer cells without harming normal cells. Dr Beales and his colleagues thought an explanation might lie in the unique properties of cancer cell membranes.
In healthy cell membranes, the phospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) are located in the inner membrane leaflet facing the inside of the cell. But in cancer cells, PS and PE are embedded in the outer membrane leaflet facing the cell surroundings.
The researchers tested their theory by creating model membranes, some of which contained PE and/or PS, and exposing them to MP1. They used a wide range of imaging and biophysical techniques to characterize MP1’s destructive effects on the membranes.
The team found that PS increased the binding of MP1 to the membrane by a factor of 7 to 8. On the other hand, PE enhanced MP1’s ability to quickly disrupt the membrane, increasing the size of holes by a factor of 20 to 30.
“Formed in only seconds, these large pores are big enough to allow critical molecules such as RNA and proteins to easily escape cells,” said study author João Ruggiero Neto, of São Paulo State University in Brazil.
“The dramatic enhancement of the permeabilization induced by the peptide in the presence of PE and the dimensions of the pores in these membranes was surprising.”
In future studies, the researchers plan to alter MP1’s amino acid sequence to examine how the peptide’s structure relates to its function and further improve the peptide’s selectivity and potency for clinical purposes.
“Understanding the mechanism of action of this peptide will help in translational studies to further assess the potential for this peptide to be used in medicine,” Dr Beales said.
“As it has been shown to be selective to cancer cells and non-toxic to normal cells in the lab, this peptide has the potential to be safe, but further work would be required to prove that.” ![]()

Polybia paulista
Photo by Mario Palma/
São Paulo State University
The wasp Polybia paulista protects itself from predators by producing venom known to contain a cancer-fighting toxin.
A study published in Biophysical Journal helps explain how the venom’s toxin, MP1 (Polybia-MP1), selectively kills cancer cells without harming normal cells.
MP1 interacts with lipids that are abnormally distributed on the surface of cancer cells, creating holes that facilitate the escape of molecules crucial for cell function.
“Cancer therapies that attack the lipid composition of the cell membrane would be an entirely new class of anticancer drugs,” said study author Paul Beales, PhD, of the University of Leeds in the UK.
MP1 is known to act against microbial pathogens by disrupting the bacterial cell membrane. The peptide has shown promise for treating cancers, as it can inhibit the growth of prostate and bladder cancer cells, as well as multi-drug resistant leukemic cells.
However, it has not been clear how MP1 selectively destroys cancer cells without harming normal cells. Dr Beales and his colleagues thought an explanation might lie in the unique properties of cancer cell membranes.
In healthy cell membranes, the phospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) are located in the inner membrane leaflet facing the inside of the cell. But in cancer cells, PS and PE are embedded in the outer membrane leaflet facing the cell surroundings.
The researchers tested their theory by creating model membranes, some of which contained PE and/or PS, and exposing them to MP1. They used a wide range of imaging and biophysical techniques to characterize MP1’s destructive effects on the membranes.
The team found that PS increased the binding of MP1 to the membrane by a factor of 7 to 8. On the other hand, PE enhanced MP1’s ability to quickly disrupt the membrane, increasing the size of holes by a factor of 20 to 30.
“Formed in only seconds, these large pores are big enough to allow critical molecules such as RNA and proteins to easily escape cells,” said study author João Ruggiero Neto, of São Paulo State University in Brazil.
“The dramatic enhancement of the permeabilization induced by the peptide in the presence of PE and the dimensions of the pores in these membranes was surprising.”
In future studies, the researchers plan to alter MP1’s amino acid sequence to examine how the peptide’s structure relates to its function and further improve the peptide’s selectivity and potency for clinical purposes.
“Understanding the mechanism of action of this peptide will help in translational studies to further assess the potential for this peptide to be used in medicine,” Dr Beales said.
“As it has been shown to be selective to cancer cells and non-toxic to normal cells in the lab, this peptide has the potential to be safe, but further work would be required to prove that.” ![]()
Influenza vaccination: What’s new this season
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.