User login
Major Depressive Disorder in the Primary Care Setting: Strategies to Achieve Remission and Recovery
Family physicians are on the frontline for depression care, often being the first line of defense for diagnosis and management. This 1.25-hour, CME-certified activity will provide clinicians with a comprehensive review on the following:
- Clinical factors in the primary care setting that influence treatment outcomes in major depressive disorder
- Identification and management of residual symptoms
- Tools for effective monitoring of treatment outcomes
- Nonpharmacologic and pharmacologic therapies to treat patients to goal: remission and recovery
- Strategies to promote patient-focused, recovery-oriented care.
Family physicians are on the frontline for depression care, often being the first line of defense for diagnosis and management. This 1.25-hour, CME-certified activity will provide clinicians with a comprehensive review on the following:
- Clinical factors in the primary care setting that influence treatment outcomes in major depressive disorder
- Identification and management of residual symptoms
- Tools for effective monitoring of treatment outcomes
- Nonpharmacologic and pharmacologic therapies to treat patients to goal: remission and recovery
- Strategies to promote patient-focused, recovery-oriented care.
Family physicians are on the frontline for depression care, often being the first line of defense for diagnosis and management. This 1.25-hour, CME-certified activity will provide clinicians with a comprehensive review on the following:
- Clinical factors in the primary care setting that influence treatment outcomes in major depressive disorder
- Identification and management of residual symptoms
- Tools for effective monitoring of treatment outcomes
- Nonpharmacologic and pharmacologic therapies to treat patients to goal: remission and recovery
- Strategies to promote patient-focused, recovery-oriented care.
Tuberculosis testing: Which patients, which test?
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
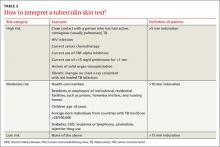
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
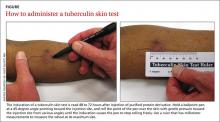
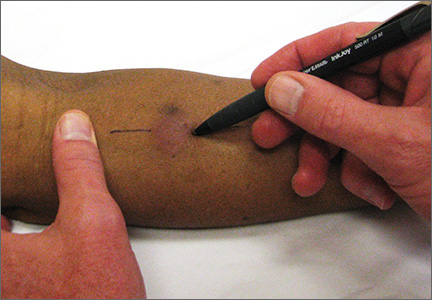
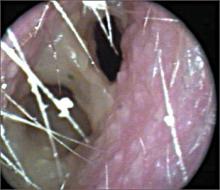
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
› Test for latent tuberculosis (TB) infection by using a tuberculin skin test (TST) or interferon gamma release assay (IGRA) in all patients at risk for developing active TB. B
› Consider patient characteristics such as age, previous vaccination with bacille Calmette-Guérin (BCG), and whether the patient will need serial testing to decide whether TST or IGRA is most appropriate for a specific patient. C
› Don’t use TST or IGRA to make or exclude a diagnosis of active TB; use cultures instead. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Judy C is a newly employed 40-year-old health care worker who was born in China and received the bacille Calmette-Guérin (BCG) vaccination as a child. Her new employer requires her to undergo testing for tuberculosis (TB). Her initial tuberculin skin test (TST) is 0 mm, but on a second TST 2 weeks later, it is 8 mm. She is otherwise healthy, negative for human immunodeficiency virus (HIV), and has no constitutional symptoms. Does she have latent tuberculosis infection (LTBI)?
CASE 2 › A mom brings in her 3-year-old son, Patrick. She reports that a staff member at his day care center traveled outside the country for 3 months and was diagnosed with LTBI upon her return. She wants to know if her son should be tested.
More than 2 billion people—nearly one-third of the world’s population—are infected with Mycobacterium tuberculosis.1 Most harbor the bacilli as LTBI, which means that while they have living TB bacilli within their bodies, these mycobacteria are kept dormant by an intact immune system. These individuals are not contagious, nor are they likely to become ill from active TB unless something adversely affects their immune system and increases the likelihood that LTBI will progress to active TB.
Two tests are available for diagnosing LTBI: the TST and the newer interferon gamma release assay (IGRA). Each test has advantages and disadvantages, and the best test to use depends on various patient-specific factors. This article describes whom you should test for LTBI, which test to use, and how to diagnose active TB.
Why test for LTBI?
LTBI is an asymptomatic infection; patients with LTBI have a 5% to 10% lifetime risk of developing active TB.2 The risk of developing active TB is approximately 5% within the first 18 months of infection, and the remaining risk is spread out over the rest of the patient’s life.2 Screening for LTBI is desirable because early diagnosis and treatment can reduce the activation risk to 1% to 2%,3 and treatment for LTBI is simpler, less costly, and less toxic than treatment for active TB.
Whom to test. Screening for LTBI should target patients for whom the benefits of treatment outweigh the cost and risks of treatment.4 A decision to screen for LTBI implies that the patient will be treated if he or she tests positive.3
The benefit of treatment increases in people who have a significant risk of progression to active TB—primarily those with recently acquired LTBI, or with co-existing conditions that increase their likelihood of progression (TABLE 1).5
All household contacts of patients with active TB and recent immigrants from countries with a high TB prevalence should be tested for LTBI.6 Those with a negative test and recent exposure should be retested in 8 to 12 weeks to allow for the delay in conversion to a positive test after recent infection.7 Health care workers and others who are potentially exposed to active TB on an ongoing basis should be tested at the time of employment, with repeat testing done periodically based on their risk of infection.8,9
Individuals with coexisting conditions should be tested for LTBI as long as the benefit of treatment outweighs the risk of drug-induced hepatitis. Because the risk of drug-induced hepatitis increases with age, the decision to test/treat is affected by age as well as the individual’s risk of progression. Patients with the highest risk conditions would benefit from testing/treating regardless of age, while treatment may not be justified in those with lower-risk conditions. A reasonable strategy is as follows:10
• high-risk conditions: test regardless of age
• moderate-risk conditions: test those <65 years
• low-risk conditions: test those <50 years.
Children with LTBI are at particularly high risk of progression to active TB.5 The American Academy of Pediatrics (AAP) recommends assessing a child’s risk for TB at first contact with the child and once every 6 months for the first year of life. After one year, annual assessment is recommended, but specific TB testing is not required for children who don’t have risk factors.11 The AAP suggests using a TB risk assessment questionnaire that consists of 4 screening questions with follow-up questions if any of the screening questions are positive (TABLE 2).11
Use of TST is well established
To perform a TST, inject 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) intradermally into the inner surface of the forearm using a 27- to 30-gauge needle. (In the United States, PPD is available as Aplisol or Tubersol.) Avoid the former practice of “control” or anergy testing with mumps or Candida antigens because this is rarely helpful in making TB treatment decisions, even in HIV-positive patients.12
To facilitate intradermal injection, gently stretch the skin taut during injection. Raising a wheal confirms correct placement. The test should be read 48 to 72 hours after it is administered by measuring the greatest diameter of induration at the administration site. (Erythema is irrelevant to how the test is interpreted.) Induration is best read by using a ballpoint pen held at a 45-degree angle pointing toward the injection site. Roll the point of the pen over the skin with gentle pressure toward the injection site until induration causes the pen to stop rolling freely (FIGURE). The induration should be measured with a rule that has millimeter measurements and interpreted as positive or negative based on the individual’s risk factors (TABLE 3).3
Watch for these 2 factors that can affect TST results
Bacille Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bovis, is (or has been) used as a routine childhood immunization in many parts of the world, although not in the United States.13 It is ordinarily given as a single dose shortly after birth, and has some utility in preventing serious childhood TB infection. The antigens in PPD and those in BCG are not identical, but they do overlap.
BCG administered after an individual’s first birthday resulted in false positive TSTs >10 mm in 21% of those tested more than 10 years after BCG was administered.14 However, a single BCG vaccine in infancy causes little if any change in the TST result in individuals who are older than age 10 years. When a TST is performed for appropriate reasons, a positive TST in people previously vaccinated with BCG is generally more likely to be the result of LTBI than of BCG.15 Current guidelines from the Centers for Disease Control and Prevention (CDC) recommend that previous BCG status not change the cutoffs used for interpreting TST results.16
Booster phenomenon. In many adults who have undiagnosed LTBI that they acquired in the distant past, or who received BCG vaccination as a child, immunity wanes after several decades. This can result in an initial TST being negative, but because the antigens in the PPD itself stimulate antigenic memory, the next time a TST is performed, it may be positive.
In people who will have annual TST screenings, such as health care workers or nursing home residents, a 2-step PPD can help discriminate this “booster” phenomenon from a new LTBI acquired during the first year of annual TST testing. A second TST is placed 1 to 2 weeks after the initial test, a time interval during which acquisition of LTBI would be unlikely. The result of the second test should be considered the person’s baseline for evaluation of subsequent TSTs. A subsequent TST would be considered positive if the induration is >10 mm and has increased by ≥6 mm since the previous baseline.17
IGRA offers certain benefits
IGRA uses antigens that are more specific for Mycobacterium tuberculosis than the TST, and as a result, this test is not influenced by previous BCG vaccination. It requires only one blood draw, and interpretation does not depend on the patient’s risk category or interpretation of skin induration. The primary disadvantage of IGRAs is high cost (currently $200 to $300 per test), and the need for a laboratory with adequate equipment and personnel trained in performing the test. IGRAs must be collected in special blood tubes, and the samples must be processed within 8 to 16 hours of collection, depending on the test used.5
Currently, 2 IGRAs are approved for use in the United States—the QuantiFERON-TB Gold In-Tube (QFT-GIT) and the T-SPOT.TB assay. Both tests may produce false positives in patients infected with Mycobacterium marinum or Mycobacterium kansasii, but otherwise are highly specific for Mycobacterium tuberculosis. IGRA results may be “boosted” by recent TST (ie, a TST given within the previous 3 months may cause a false positive IGRA result), and this effect may begin as early as 3 days after a TST is administered.18 Therefore, if an IGRA is needed to clarify a TST result, it should be drawn on the day the TST is read.19
CDC guidelines (2010) recommend that IGRAs may be used in place of—but not routinely in addition to—TSTs in all cases in which TST is otherwise indicated.20 There are a few situations where one test may be preferred over the other.21
IGRA may be preferred over TST in individuals in one of 2 categories:
• those who have received BCG immunization. If a patient is unsure of their BCG status, the World Atlas of BCG Policies and Practices, available at www.bcgatlas.org,22 can aid clinicians in determining which patients likely received BCG as part of their routine childhood immunizations.
• those in groups that historically have poor rates of return for TST reading, such as individuals who are homeless or suffer from alcoholism or a substance use disorder.
Individuals in whom TST is preferred over IGRA include:
• children age <5 years, because data guiding use of IGRAs in this age group are limited.23 Both TST and IGRA may be falsely negative in children under the age of 3 months.24
• patients who require serial testing, because individuals with positive IGRAs have been shown to commonly test negative on subsequent tests, and there are limited data on interpretation and prognosis of positive IGRAs in people who require serial testing.25
Individuals in whom performing both tests simultaneously could be helpful include:
• those with an initial negative test, but with a high risk for progression to active TB or a poor outcome if the first result is falsely negative (eg, patients with HIV infection or children ages <5 years who have been exposed to a person with active TB)
• those with an initial positive test who don’t believe the test result and are reluctant to be treated for LTBI.
TST and IGRA have comparable sensitivities—around 80% to 90%, respectively—for diagnosing LTBI. IGRAs have a specificity >95% for diagnosing LTBI. While TST specificity is approximately 97% in patients not vaccinated with BCG, it can be as low as 60% in people previously vaccinated with BCG.26 IGRAs have been shown to have higher positive and negative predictive values than TSTs in high-risk patients.27 A recent study suggested that the IGRAs might have a higher rate of false-positive results compared to TSTs in a low-risk population of health care workers.28
Both the TST and IGRA have lag times of 3 to 8 weeks from the time of a new infection until the test becomes positive. It is therefore best to defer testing for LTBI infection until at least 8 weeks after a known TB exposure to decrease the likelihood of a false-negative test.3
Diagnose active TB based on symptoms, culture
The CDC reported 9412 new cases of active TB in the United States in 2014, for a rate of 3 new cases per 100,000 people.29 This is the lowest rate reported since national reporting began in 1953, when the incidence in the United States was 53 cases per 100,000.
Who should you test for active TB? The risk factors for active TB are the same as those for LTBI: recent exposure to an individual with active TB, and other disease processes or medications that compromise the immune system. Consider active TB when a patient with one of these risk factors presents with:2
• persistent fever
• weight loss
• night sweats
• cough, especially if there is any blood.
Routine laboratory and radiographic studies that should prompt you to consider TB include:2
• upper lobe infiltrates on chest x-ray
• sterile pyuria on urinalysis with a negative culture for routine pathogens
• elevated levels of C-reactive protein or an elevated erythrocyte sedimentation rate without another obvious cause.
Active TB typically presents as pulmonary TB, but it can also affect nearly every other body system. Other common presentations include:30
• vertebral destruction and collapse (“Pott's disease”)
• subacute meningitis
• peritonitis
• lymphadenopathy (especially in children).
Culture is the gold standard. Neither TST or IGRA should ever be relied upon to make or exclude the diagnosis of active TB, as these tests are neither sensitive nor specific for diagnosing active TB.31,32 Instead, the gold standard for the diagnosis of active TB remains a positive culture from infected tissue—commonly sputum, pleura or pleural fluid, cerebrospinal fluid, urine, or peritoneal fluid. Cultures are crucial not only to confirm the diagnosis, but to guide therapy, because of the rapidly increasing resistance to firstline antibiotics used to treat TB.33
Culture results and drug sensitivities are ordinarily not available until 2 to 6 weeks after the culture was obtained. A smear for acid-fast bacilli as well as newer rapid diagnostic tests such as nucleic acid amplification (NAA) tests are generally performed on the tissue sample submitted for culture, and these results, while less trustworthy, are generally available within 24 to 48 hours. The CDC recommends that an NAA test be performed in addition to microscopy and culture for specimens submitted for TB diagnosis.34
Since 2011, the World Health Organization has endorsed the use of a new molecular diagnostic test called Xpert MTB/RIF in settings with high prevalence of HIV infection or multidrug-resistant TB (MDR-TB).35 This test is able to detect M. tuberculosis as well as rifampin resistance, a surrogate for MDR-TB, within 2 hours, with sensitivity and specificity approaching that of culture.36
“Culture-negative” TB? A small but not insignificant proportion of patients will present with risk factors for, and clinical signs and symptoms of, active TB; their cultures, however, will be negative. In such cases, consultation with an infectious disease or pulmonary specialist may be warranted. If no alternative diagnosis is found, such patients are said to have “culture-negative active TB” and should be continued on anti-TB drug therapy, although the course may be shortened.37 This highlights the fact that while cultures are key to diagnosing and treating active TB, the condition is—practically speaking—a clinical diagnosis; treatment should not be withheld or stopped simply because of a negative culture or rapid diagnostic test.
CASE 1 › Based on her risk factors (being a health care worker, born in a country with a high prevalence of TB), Ms. C’s cutoff for a positive test is >10 mm, so her TST result is negative and she is not considered to have LTBI. The increase to 8 mm seen on the second TST probably represents either childhood BCG vaccination or previous infection with nontuberculous Mycobacterium.
CASE 2 › Strictly speaking, 3-year-old Patrick does not need testing, because he was exposed only to LTBI, which is not infectious. However, because children under age 5 are at particularly high risk for progressing to active TB and poor outcomes, it would be best to confirm the mother’s story with the day care center and/or health department. If it turns out that Patrick had, in fact, been exposed to active TB, much more aggressive management would be required.
CORRESPONDENCE
Jeff Hall, MD, Family Medicine Center, 3209 Colonial Drive Columbia, SC 29203; [email protected]
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
1. World Health Organization. Tuberculosis. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/. Accessed July 7, 2015.
2. Zumla A, Raviglione M, Hafner R, et al. Current concepts: tuberculosis. N Engl J Med. 2013;368:745-755.
3. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-51.
4. Hauck FR, Neese BH, Panchal AS, et al. Identification and management of latent tuberculosis infection. Am Fam Physician. 2009;79:879-886.
5. Getahun H, Matteelli A, Chaisson RE, et al. Latent Mycobacterium tuberculosis infection. N Engl J Med. 2015;372:2127-2135.
6. Arshad S, Bavan L, Gajari K, et al. Active screening at entry for tuberculosis among new immigrants: a systematic review and meta-analysis. Eur Respir J. 2010;35:1336-1345.
7. Greenaway C, Sandoe A, Vissandjee B, et al; Canadian Collaboration for Immigrant and Refugee Health. Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ. 2011;183:E939-E951.
8. Jensen PA, Lambert LA, Iademarco MF, et al; CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1-141.
9. Taylor Z, Nolan CM, Blumberg HM; American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1-81.
10. Pai M, Menzies D. Diagnosis of latent tuberculosis infection (tuberculosis screening) in HIV-negative adults. UpToDate Web site. Available at: http://www.uptodate.com/contents/diagnosisof-latent-tuberculosis-infection-tuberculosis-screening-in-hivnegative-adults. Accessed July 7, 2015.
11. Pediatric Tuberculosis Collaborative Group. Targeted tuberculin skin testing and treatment of latent tuberculosis infection in children and adolescents. Pediatrics. 2004;114:1175-1201.
12. Centers for Disease Control and Prevention. Anergy skin testing and tuberculosis [corrected] preventive therapy for HIV-infected persons: revised recommendations. MMWR Recomm Rep. 1997;46:1-10.
13. The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 1996;45:1-18.
14. Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192-1204.
15. Wang L, Turner MO, Elwood RK, et al. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax. 2002;57:804-809.
16. Centers for Disease Control and Prevention (CDC). Fact sheets: BCG vaccine. CDC Web site. Available at: http://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm. Accessed July 16, 2015.
17. Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159:15-21.
18. van Zyl-Smit RN, Zwerling A, Dheda K, et al. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One. 2009;4:e8517.
19. Mazurek GH, Jereb J, Lobue P, et al; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49-55.
20. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1-25.
21. Muñoz L, Santin M. Interferon- release assays versus tuberculin skin test for targeting people for tuberculosis preventive treatment: an evidence-based review. J Infect. 2013;66:381-387.
22. Zwerling A, Behr MA, Verma A, et al. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8:e1001012.
23. Mandalakas AM, Detjen AK, Hesseling AC, et al. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15:1018-1032.
24. American Academy of Pediatrics Committee on Infectious Diseases, Pickering L, ed. Red Book. Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012:741.
25. Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62-70. 26. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177-184.
27. Diel R, Loddenkemper R, Nienhaus A. Predictive value of interferon- release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest. 2012;142:63-75.
28. Dorman SE, Belknap R, Graviss EA, et al; Tuberculosis Epidemiologic Studies Consortium. Interferon-release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med. 2014;189:77-87.
29. Scott C, Kirking HL, Jeffries C, et al; Centers for Disease Control and Prevention (CDC). Tuberculosis trends—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:265-269.
30. Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72:1761-1768.
31. Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45-55.
32. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and metaanalysis. J Infect Dis. 2011;204:S1120-S1129.
33. Keshavjee S, Farmer PE. Tuberculosis, drug resistance, and the history of modern medicine. N Engl J Med. 2012;367:931-936.
34. Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7-10.
35. World Health Organization. Global tuberculosis report 2014. World Health Organization Web site. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed July 17, 2015.
36. Steingart KR, Schiller I, Horne DJ, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593.
37. Hall J, Elliott C. Tuberculosis: Which drug regimen and when. J Fam Practice. 2015;64:27-33.
A Prescription for Trouble
ANSWER
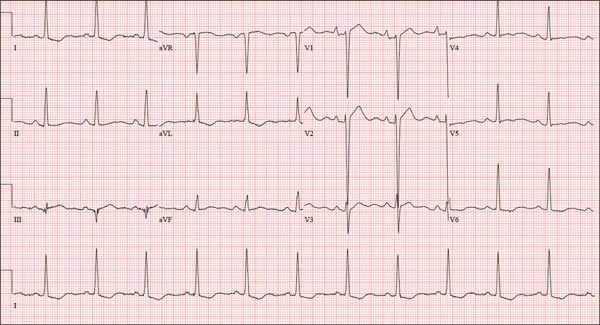
The correct interpretation includes normal sinus rhythm, right atrial enlargement, left ventricular hypertrophy, and a prolonged QT interval. Normal sinus rhythm is indicated by a P for every QRS and a QRS for every P, with a constant PR interval (see rhythm strip of lead I).
Right atrial enlargement is evidenced by the tall P waves in leads II, III, aVF, and V1. Note that there is no biphasic P wave in lead V1, so there is no evidence of accompanying left atrial enlargement.
High-voltage limb leads (sum of R in lead I and S in lead III ≥ 25 mm) or precordial leads (sum of S in V1 and R in V5 or V6 ≥ 35 mm) are indicative of left ventricular hypertrophy.
The QTc interval of 653 ms with a normal sinus rate is worrisome for prolonged QT syndrome. A review of the history shows the patient to be taking two drugs (lithium, azithromycin) known to prolong the QT interval. Although it is not known whether this patient has inherent QT prolongation, use of these types of agents should be avoided.
ANSWER
The correct interpretation includes normal sinus rhythm, right atrial enlargement, left ventricular hypertrophy, and a prolonged QT interval. Normal sinus rhythm is indicated by a P for every QRS and a QRS for every P, with a constant PR interval (see rhythm strip of lead I).
Right atrial enlargement is evidenced by the tall P waves in leads II, III, aVF, and V1. Note that there is no biphasic P wave in lead V1, so there is no evidence of accompanying left atrial enlargement.
High-voltage limb leads (sum of R in lead I and S in lead III ≥ 25 mm) or precordial leads (sum of S in V1 and R in V5 or V6 ≥ 35 mm) are indicative of left ventricular hypertrophy.
The QTc interval of 653 ms with a normal sinus rate is worrisome for prolonged QT syndrome. A review of the history shows the patient to be taking two drugs (lithium, azithromycin) known to prolong the QT interval. Although it is not known whether this patient has inherent QT prolongation, use of these types of agents should be avoided.
ANSWER
The correct interpretation includes normal sinus rhythm, right atrial enlargement, left ventricular hypertrophy, and a prolonged QT interval. Normal sinus rhythm is indicated by a P for every QRS and a QRS for every P, with a constant PR interval (see rhythm strip of lead I).
Right atrial enlargement is evidenced by the tall P waves in leads II, III, aVF, and V1. Note that there is no biphasic P wave in lead V1, so there is no evidence of accompanying left atrial enlargement.
High-voltage limb leads (sum of R in lead I and S in lead III ≥ 25 mm) or precordial leads (sum of S in V1 and R in V5 or V6 ≥ 35 mm) are indicative of left ventricular hypertrophy.
The QTc interval of 653 ms with a normal sinus rate is worrisome for prolonged QT syndrome. A review of the history shows the patient to be taking two drugs (lithium, azithromycin) known to prolong the QT interval. Although it is not known whether this patient has inherent QT prolongation, use of these types of agents should be avoided.
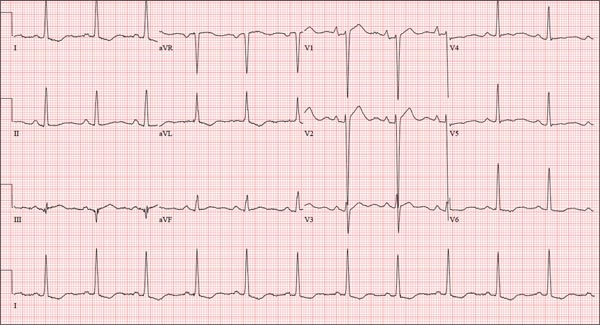
A 74-year-old man is admitted to your service with gastrointestinal bleeding. He has a history of diverticulitis and has had multiple episodes in which he passed bright red blood per rectum, sufficient to warrant blood transfusion. The last episode occurred about 14 months ago. The current one started 12 hours ago; he presents to the emergency department per your instructions. Medical history is remarkable for hypertension, hypothyroidism, and prostatic hypertrophy. He has no prior cardiac history. Surgical history is remarkable for an appendectomy, cholecystectomy, and left rotator cuff repair. He has a positive psychiatric history of bipolar disorder that has been treated with lithium for more than 40 years. The patient retired after working as a welder for 50 years. He is currently married to his second spouse. He has a 60-pack-year history of cigarette smoking and drinks one glass of bourbon per day. He denies using recreational drugs. Family history reveals that his mother died of a stroke at age 97, and his father died of natural causes at 102. The patient has three brothers, all of whom are alive and well. One brother had an MI followed by coronary artery bypass grafting at age 71; the other two brothers’ medical histories are unknown. Current medications include furosemide, metoprolol, l-thyroxine, tamsulosin, and a daily baby aspirin. Three days ago, he started a prescription of azithromycin for an upper respiratory infection (URI) diagnosed at a local urgent care center. Review of systems is positive for a URI manifest by fever, productive cough, and end-expiratory wheezing. The patient says this has improved considerably since initiation of antibiotic therapy. He also says that lithium has held his manic episodes in check for years, and he has refused several attempts to wean him from it. He still experiences urinary hesitancy and frequency despite starting tamsulosin; he has an appointment with a urologist in four weeks to discuss other options. The remainder of the review of systems is unremarkable. Laboratory data upon admission include a hematocrit of 38.2% and a white blood cell count of 11.0 cells/dL. All other lab values are within normal limits. The admission ECG reveals a ventricular rate of 69 beats/min; PR interval, 188 ms; QRS duration, 100 ms; QT/QTc interval, 610/653 ms; P axis, 55°; R axis, 21°; and T axis, 103°. What is your interpretation of this ECG?
Oh, Deer! Accident Leaves Man in Pain
A 50-year-old man is brought to your facility by EMS personnel for evaluation after a motor vehicle crash. He was an unrestrained driver who swerved suddenly to avoid hitting a deer that jumped in front of him. He lost control of his vehicle, which rolled over several times and eventually landed in a ditch. His airbag deployed. The patient’s primary complaint is neck and right leg pain. His medical history is essentially unremarkable. He is awake, alert, and oriented, with stable vital signs. Primary survey shows a large laceration of his right leg over the tibia, with extensive soft-tissue injury and loss through the muscle. He has good range of motion in his knee, with no evident pain or swelling. His ankle and foot also show no injury and appear to be neurovascularly intact. You obtain a radiograph of the right tibia. What is your impression?
Alarming Lesion Speaks for Itself
ANSWER
The correct answer is “all of the above” (choice “d”), for reasons discussed in the next section.
DISCUSSION
Cutaneous horn is the term given to this type of keratotic lesion, for obvious reasons. They range in size from a pinpoint to the larger lesion seen on this patient (and sometimes, even larger). The pathology report in this case confirmed the clinical impression of well-differentiated squamous cell carcinoma (SCC; choice “c”); sun exposure is the most likely causative factor, given the location and the patient’s history of sun damage.
The lesion might have been a wart (choice “a”) caused by a human papillomavirus, some of which can trigger the formation of a type of SCC. Evidence of HPV involvement is often noted in the pathology report.
When skin lesions transition from normal to sun-damaged to cancerous, they often go through an actinic keratosis (choice “b”) stage, usually as a tiny hyperkeratotic papule on the forehead, ears, nose, or other directly sun-exposed area. Some consider actinic keratoses to be a form of early SCC; more prevalent is the view that they are merely “precancerous” with the potential to develop into either a frank SCC or, less often, a basal cell carcinoma. Some actinic keratoses, left completely unmolested, can develop into tag-like lesions and then horny outward projections.
Even when cutaneous horns are found to represent SCC, they are termed well-differentiated, a descriptor meant to denote a relatively benign and nonaggressive prognosis. This is the opposite of a poorly differentiated SCC, which would be expected to behave in a more aggressive, less predictable manner.
For well-differentiated lesions, a deep shave biopsy is probably an adequate method of removal. As such, the case patient did not require re-excision. He was, however, scheduled for a return visit to check the site for the (albeit unlikely) possibility of recurrence.
ANSWER
The correct answer is “all of the above” (choice “d”), for reasons discussed in the next section.
DISCUSSION
Cutaneous horn is the term given to this type of keratotic lesion, for obvious reasons. They range in size from a pinpoint to the larger lesion seen on this patient (and sometimes, even larger). The pathology report in this case confirmed the clinical impression of well-differentiated squamous cell carcinoma (SCC; choice “c”); sun exposure is the most likely causative factor, given the location and the patient’s history of sun damage.
The lesion might have been a wart (choice “a”) caused by a human papillomavirus, some of which can trigger the formation of a type of SCC. Evidence of HPV involvement is often noted in the pathology report.
When skin lesions transition from normal to sun-damaged to cancerous, they often go through an actinic keratosis (choice “b”) stage, usually as a tiny hyperkeratotic papule on the forehead, ears, nose, or other directly sun-exposed area. Some consider actinic keratoses to be a form of early SCC; more prevalent is the view that they are merely “precancerous” with the potential to develop into either a frank SCC or, less often, a basal cell carcinoma. Some actinic keratoses, left completely unmolested, can develop into tag-like lesions and then horny outward projections.
Even when cutaneous horns are found to represent SCC, they are termed well-differentiated, a descriptor meant to denote a relatively benign and nonaggressive prognosis. This is the opposite of a poorly differentiated SCC, which would be expected to behave in a more aggressive, less predictable manner.
For well-differentiated lesions, a deep shave biopsy is probably an adequate method of removal. As such, the case patient did not require re-excision. He was, however, scheduled for a return visit to check the site for the (albeit unlikely) possibility of recurrence.
ANSWER
The correct answer is “all of the above” (choice “d”), for reasons discussed in the next section.
DISCUSSION
Cutaneous horn is the term given to this type of keratotic lesion, for obvious reasons. They range in size from a pinpoint to the larger lesion seen on this patient (and sometimes, even larger). The pathology report in this case confirmed the clinical impression of well-differentiated squamous cell carcinoma (SCC; choice “c”); sun exposure is the most likely causative factor, given the location and the patient’s history of sun damage.
The lesion might have been a wart (choice “a”) caused by a human papillomavirus, some of which can trigger the formation of a type of SCC. Evidence of HPV involvement is often noted in the pathology report.
When skin lesions transition from normal to sun-damaged to cancerous, they often go through an actinic keratosis (choice “b”) stage, usually as a tiny hyperkeratotic papule on the forehead, ears, nose, or other directly sun-exposed area. Some consider actinic keratoses to be a form of early SCC; more prevalent is the view that they are merely “precancerous” with the potential to develop into either a frank SCC or, less often, a basal cell carcinoma. Some actinic keratoses, left completely unmolested, can develop into tag-like lesions and then horny outward projections.
Even when cutaneous horns are found to represent SCC, they are termed well-differentiated, a descriptor meant to denote a relatively benign and nonaggressive prognosis. This is the opposite of a poorly differentiated SCC, which would be expected to behave in a more aggressive, less predictable manner.
For well-differentiated lesions, a deep shave biopsy is probably an adequate method of removal. As such, the case patient did not require re-excision. He was, however, scheduled for a return visit to check the site for the (albeit unlikely) possibility of recurrence.
Two years ago, this 82-year-old man developed a lesion on his forehead that has since grown large enough to cause pain with trauma. Furthermore, he recently reunited with some estranged family members, who upon seeing the lesion for the first time expressed alarm at its appearance. As a result, he requests a referral to dermatology for evaluation. The patient’s history includes several instances of skin cancer; these began when he was in his 40s and have all occurred on his face and scalp. Examination of those areas reveals heavy chronic sun damage, including solar elastosis, solar lentigines, and multiple relatively minor actinic keratoses. The patient has type II skin. An impressive 3 x 2.8–cm hornlike keratotic lesion projects prominently from his left forehead. The distal two-thirds is horny and firm, while the proximal base is pink, fleshy, and telangiectatic. The lesion is removed by saucerization under local anesthesia and submitted to pathology.
ESC: Bivalirudin no better than unfractionated heparin in PCI
LONDON – Bivalirudin did not prove superior to unfractionated heparin in reducing the rate of major adverse cardiovascular events in two nested, open-label, randomized clinical trials involving patients presenting with acute coronary syndrome who were expected to undergo percutaneous coronary intervention, Dr. Marco Valgimigli reported.
In addition, post-PCI infusions of bivalirudin for 4 hours or longer did not reduce the rate of adverse bleeding events, compared with no infusion.
These findings add important data to the understanding of antithrombotic therapy in ACS patients undergoing invasive treatment, but they do not resolve the persistent question of which method is best for preventing thrombotic complications while limiting the risk of bleeding during and after such procedures, said Dr. Valgimigli of Erasmus University in Rotterdam.
Previous studies comparing bivalirudin, a direct thrombin inhibitor, against unfractionated heparin, an indirect thrombin inhibitor, have yielded conflicting results regarding ischemic and bleeding outcomes, so Dr. Valgimigli and his fellow investigators in the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial conducted two industry-sponsored superiority trials to try to settle the question.
The findings of one of these trials were reported by Dr. Valgimigli at the annual congress of the European Society of Cardiology on Sept. 1, when the results of both were simultaneously published online (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMoa1507854).
The MATRIX studies were conducted at 78 medical centers in Italy, the Netherlands, Spain, and Sweden. They involved 7,213 patients who presented with either ST-elevation MI or non-STEMI ACS and were expected to undergo PCI. The first trial, MATRIX Antithrombin, assessed outcomes in 3,610 of these participants who were randomly assigned to receive bivalirudin and 3,603 assigned to receive unfractionated heparin. In the second trial, MATRIX Treatment Duration, the bivalirudin group was further randomized to receive either a post-PCI bivalirudin infusion (1,799 patients) or no post-PCI infusion (1,811 patients).
MATRIX Antithrombin
At 1-month follow-up, the rate of major adverse cardiovascular events (MACEs) – a composite of death from any cause, myocardial infarction, or stroke – was no lower in the bivalirudin group (10.3%) than in the heparin group (10.9%), for a rate ratio of 0.94. Similarly, the rate of net adverse clinical events was not significantly lower with bivalirudin (11.2%) than with heparin (12.4%), for a rate ratio of 0.89.
MATRIX Treatment Duration
The primary outcome in the MATRIX Treatment Duration study – a composite of urgent target-vessel revascularization, definite stent thrombosis, or net adverse clinical events at 30 days – occurred in 11.0% of patients who received post-PCI bivalirudin infusions and 11.9% of those who did not, a nonsignificant difference (rate ratio, 0.91). However, the rate of subacute definite stent thrombosis was significantly higher in the post-PCI infusion group, at 0.7%, compared with 0.2% in the group that didn’t receive post-PCI infusions (RR, 4.37).
“I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients as well as, perhaps, based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors,” Dr. Valgimigli said, noting that this is in keeping with the current labeling of the drug in Europe and the United States.
The MATRIX study was sponsored by the nonprofit Italian Society of Invasive Cardiology and financially supported by the Medicines Company and Terumo Medical. Dr. Valgimigli reported ties to AstraZeneca, the Medicines Company, Terumo Medical, St. Jude Vascular, Alvimedica, Abbott Vascular, and Correvio; his associates reported ties to numerous industry sources.
Mary Ann Moon contributed to this report.
The MATRIX investigators properly conclude that their studies did not produce a clear winner, either in the comparison of bivalirudin vs. heparin or in the comparison of post-PCI bivalirudin infusion vs. no infusion. But this should not diminish the credit due to Dr. Valgimigli and his associates for conducting two trials to address important and complex issues.
The second trial provides the best evidence to date on whether it is beneficial to prolong the infusion of bivalirudin after PCI is completed. The agent did not reduce rates of urgent target-vessel revascularization, definite stent thrombosis, and net adverse clinical events – either as a composite outcome or as individual components.
Dr. Peter B. Berger is with North Shore-Long Island Jewish Health System in Great Neck, N.Y. He reported receiving grants and personal fees from Boehringer Ingelheim, Medicure, Bristol-Myers Squibb/Sanofi, Novartis, Tethys, Thrombovision, Helena, Accumetrics, AstraAeneca, Haemoscope, the Medicines Company, and Corgenix/Aspirinworks. Dr. Berger made these remarks in an editorial accompanying the MATRIX report (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMe1509637).
The MATRIX investigators properly conclude that their studies did not produce a clear winner, either in the comparison of bivalirudin vs. heparin or in the comparison of post-PCI bivalirudin infusion vs. no infusion. But this should not diminish the credit due to Dr. Valgimigli and his associates for conducting two trials to address important and complex issues.
The second trial provides the best evidence to date on whether it is beneficial to prolong the infusion of bivalirudin after PCI is completed. The agent did not reduce rates of urgent target-vessel revascularization, definite stent thrombosis, and net adverse clinical events – either as a composite outcome or as individual components.
Dr. Peter B. Berger is with North Shore-Long Island Jewish Health System in Great Neck, N.Y. He reported receiving grants and personal fees from Boehringer Ingelheim, Medicure, Bristol-Myers Squibb/Sanofi, Novartis, Tethys, Thrombovision, Helena, Accumetrics, AstraAeneca, Haemoscope, the Medicines Company, and Corgenix/Aspirinworks. Dr. Berger made these remarks in an editorial accompanying the MATRIX report (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMe1509637).
The MATRIX investigators properly conclude that their studies did not produce a clear winner, either in the comparison of bivalirudin vs. heparin or in the comparison of post-PCI bivalirudin infusion vs. no infusion. But this should not diminish the credit due to Dr. Valgimigli and his associates for conducting two trials to address important and complex issues.
The second trial provides the best evidence to date on whether it is beneficial to prolong the infusion of bivalirudin after PCI is completed. The agent did not reduce rates of urgent target-vessel revascularization, definite stent thrombosis, and net adverse clinical events – either as a composite outcome or as individual components.
Dr. Peter B. Berger is with North Shore-Long Island Jewish Health System in Great Neck, N.Y. He reported receiving grants and personal fees from Boehringer Ingelheim, Medicure, Bristol-Myers Squibb/Sanofi, Novartis, Tethys, Thrombovision, Helena, Accumetrics, AstraAeneca, Haemoscope, the Medicines Company, and Corgenix/Aspirinworks. Dr. Berger made these remarks in an editorial accompanying the MATRIX report (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMe1509637).
LONDON – Bivalirudin did not prove superior to unfractionated heparin in reducing the rate of major adverse cardiovascular events in two nested, open-label, randomized clinical trials involving patients presenting with acute coronary syndrome who were expected to undergo percutaneous coronary intervention, Dr. Marco Valgimigli reported.
In addition, post-PCI infusions of bivalirudin for 4 hours or longer did not reduce the rate of adverse bleeding events, compared with no infusion.
These findings add important data to the understanding of antithrombotic therapy in ACS patients undergoing invasive treatment, but they do not resolve the persistent question of which method is best for preventing thrombotic complications while limiting the risk of bleeding during and after such procedures, said Dr. Valgimigli of Erasmus University in Rotterdam.
Previous studies comparing bivalirudin, a direct thrombin inhibitor, against unfractionated heparin, an indirect thrombin inhibitor, have yielded conflicting results regarding ischemic and bleeding outcomes, so Dr. Valgimigli and his fellow investigators in the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial conducted two industry-sponsored superiority trials to try to settle the question.
The findings of one of these trials were reported by Dr. Valgimigli at the annual congress of the European Society of Cardiology on Sept. 1, when the results of both were simultaneously published online (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMoa1507854).
The MATRIX studies were conducted at 78 medical centers in Italy, the Netherlands, Spain, and Sweden. They involved 7,213 patients who presented with either ST-elevation MI or non-STEMI ACS and were expected to undergo PCI. The first trial, MATRIX Antithrombin, assessed outcomes in 3,610 of these participants who were randomly assigned to receive bivalirudin and 3,603 assigned to receive unfractionated heparin. In the second trial, MATRIX Treatment Duration, the bivalirudin group was further randomized to receive either a post-PCI bivalirudin infusion (1,799 patients) or no post-PCI infusion (1,811 patients).
MATRIX Antithrombin
At 1-month follow-up, the rate of major adverse cardiovascular events (MACEs) – a composite of death from any cause, myocardial infarction, or stroke – was no lower in the bivalirudin group (10.3%) than in the heparin group (10.9%), for a rate ratio of 0.94. Similarly, the rate of net adverse clinical events was not significantly lower with bivalirudin (11.2%) than with heparin (12.4%), for a rate ratio of 0.89.
MATRIX Treatment Duration
The primary outcome in the MATRIX Treatment Duration study – a composite of urgent target-vessel revascularization, definite stent thrombosis, or net adverse clinical events at 30 days – occurred in 11.0% of patients who received post-PCI bivalirudin infusions and 11.9% of those who did not, a nonsignificant difference (rate ratio, 0.91). However, the rate of subacute definite stent thrombosis was significantly higher in the post-PCI infusion group, at 0.7%, compared with 0.2% in the group that didn’t receive post-PCI infusions (RR, 4.37).
“I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients as well as, perhaps, based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors,” Dr. Valgimigli said, noting that this is in keeping with the current labeling of the drug in Europe and the United States.
The MATRIX study was sponsored by the nonprofit Italian Society of Invasive Cardiology and financially supported by the Medicines Company and Terumo Medical. Dr. Valgimigli reported ties to AstraZeneca, the Medicines Company, Terumo Medical, St. Jude Vascular, Alvimedica, Abbott Vascular, and Correvio; his associates reported ties to numerous industry sources.
Mary Ann Moon contributed to this report.
LONDON – Bivalirudin did not prove superior to unfractionated heparin in reducing the rate of major adverse cardiovascular events in two nested, open-label, randomized clinical trials involving patients presenting with acute coronary syndrome who were expected to undergo percutaneous coronary intervention, Dr. Marco Valgimigli reported.
In addition, post-PCI infusions of bivalirudin for 4 hours or longer did not reduce the rate of adverse bleeding events, compared with no infusion.
These findings add important data to the understanding of antithrombotic therapy in ACS patients undergoing invasive treatment, but they do not resolve the persistent question of which method is best for preventing thrombotic complications while limiting the risk of bleeding during and after such procedures, said Dr. Valgimigli of Erasmus University in Rotterdam.
Previous studies comparing bivalirudin, a direct thrombin inhibitor, against unfractionated heparin, an indirect thrombin inhibitor, have yielded conflicting results regarding ischemic and bleeding outcomes, so Dr. Valgimigli and his fellow investigators in the MATRIX (Minimizing Adverse Hemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox) trial conducted two industry-sponsored superiority trials to try to settle the question.
The findings of one of these trials were reported by Dr. Valgimigli at the annual congress of the European Society of Cardiology on Sept. 1, when the results of both were simultaneously published online (N Engl J Med. 2015 Sept 1. doi: 10.1056/NEJMoa1507854).
The MATRIX studies were conducted at 78 medical centers in Italy, the Netherlands, Spain, and Sweden. They involved 7,213 patients who presented with either ST-elevation MI or non-STEMI ACS and were expected to undergo PCI. The first trial, MATRIX Antithrombin, assessed outcomes in 3,610 of these participants who were randomly assigned to receive bivalirudin and 3,603 assigned to receive unfractionated heparin. In the second trial, MATRIX Treatment Duration, the bivalirudin group was further randomized to receive either a post-PCI bivalirudin infusion (1,799 patients) or no post-PCI infusion (1,811 patients).
MATRIX Antithrombin
At 1-month follow-up, the rate of major adverse cardiovascular events (MACEs) – a composite of death from any cause, myocardial infarction, or stroke – was no lower in the bivalirudin group (10.3%) than in the heparin group (10.9%), for a rate ratio of 0.94. Similarly, the rate of net adverse clinical events was not significantly lower with bivalirudin (11.2%) than with heparin (12.4%), for a rate ratio of 0.89.
MATRIX Treatment Duration
The primary outcome in the MATRIX Treatment Duration study – a composite of urgent target-vessel revascularization, definite stent thrombosis, or net adverse clinical events at 30 days – occurred in 11.0% of patients who received post-PCI bivalirudin infusions and 11.9% of those who did not, a nonsignificant difference (rate ratio, 0.91). However, the rate of subacute definite stent thrombosis was significantly higher in the post-PCI infusion group, at 0.7%, compared with 0.2% in the group that didn’t receive post-PCI infusions (RR, 4.37).
“I believe the option to prolong or stop bivalirudin infusion after PCI remains open for clinicians, who will have to decide based on the ischemic and bleeding risk of individual patients as well as, perhaps, based on type of acute coronary syndrome, timing of loading dose, and type of oral P2Y12 inhibitors,” Dr. Valgimigli said, noting that this is in keeping with the current labeling of the drug in Europe and the United States.
The MATRIX study was sponsored by the nonprofit Italian Society of Invasive Cardiology and financially supported by the Medicines Company and Terumo Medical. Dr. Valgimigli reported ties to AstraZeneca, the Medicines Company, Terumo Medical, St. Jude Vascular, Alvimedica, Abbott Vascular, and Correvio; his associates reported ties to numerous industry sources.
Mary Ann Moon contributed to this report.
AT THE ESC CONGRESS 2015
Key clinical point: Compared with unfractionated heparin, bivalirudin did not reduce the MACE rate in patients with ACS who were candidates for PCI.
Major finding: At the 1-month follow-up, the MACE rate was no lower in the bivalirudin group (10.3%) than in the heparin group (10.9%), for a rate ratio of 0.94.
Data source: A randomized, multicenter, open-label superiority trial involving 7,213 ACS patients expected to undergo PCI.
Disclosures: The MATRIX study was sponsored by the nonprofit Italian Society of Invasive Cardiology and financially supported by the Medicines Company and Terumo Medical. Dr. Valgimigli reported ties to AstraZeneca, the Medicines Company, Terumo Medical, St. Jude Vascular, Alvimedica, Abbott Vascular, and Correvio; his associates reported ties to numerous industry sources.
Earaches Visualized
1. A 30-year-old woman complained of drainage from her ear for the past three months. She admitted that her hearing was diminished in that ear. She had a history of recurrent ear infections since childhood.
Photo courtesy of Vladimir Zlinsky, MD, in Roy F. Sullivan, PhD. Audiology Forum: video otoscopy, www.rcsullivan.com. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Cholesteatoma, similar to an epidermal inclusion cyst in the skin, produces keratinaceous material that fills the middle ear, causing hearing loss and otorrhea.
For more information, see “Diminished hearing.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
2. A 2-year-old child was brought for a well-child exam two months after an episode of acute otitis media. He appeared healthy and was meeting all of his developmental milestones. An otoscopic examination revealed air-fluid levels in the right ear.
Photo courtesy of Frank Miller, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Otitis media with effusion, which is a characterized by fluid in the middle ear in a patient without signs or symptoms of an acute ear infection. The most common problem, present in more than half of patients, is mild hearing loss. This is usually identified when parents express concern regarding their child’s behavior, performance at school, or language development. The absence of signs and symptoms of acute illness assists in differentiating OME from AOM.
For more information, see “Air-fluid levels in ear.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
3. A 15-month-old boy was brought in with a two-day history of fever, irritability, and frequent tugging on his left ear. The week before, he had nasal congestion, cough, and rhinorrhea. On otoscopy, his left tympanic membrane (TM) appeared erythematous, cloudy, and bulging. The TM failed to move on pneumatic otoscopy.
Photo courtesy of William Clark, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Acute otitis media, which is characterized by middle-ear effusion in a patient with signs and symptoms of acute illness (eg, fever, irritability, otalgia).
For more information, see “Ear pain in baby.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
4. A 72-year-old man sought treatment for an earache in his left ear. He said that the pain began when he got a new “in the canal” hearing aid for his left ear a month earlier.
Photo courtesy of Dr. Roy F. Sullivan. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Acute otitis externa, secondary to ear canal damage done by using the new hearing aid, caused the viscous purulent discharge and narrowing of the ear canal.
For more information, see “Earache.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
5. Parents brought their 3-year-old daughter to an urgent care facility because she had been crying all day. The child was irritable, had scant otorrhea, and had been pulling on her right ear.
Photo courtesy of William Clark, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Otoscopy revealed an erythematous, swollen external auditory canal and a foreign object. The parents reported that their child had been playing with a toy beaded necklace when she started crying. The patient was referred to an otolaryngologist, who removed the bead using an operating microscope for visualization. She evaluated the child for a co-existing otitis externa and decided that the external canal was markedly inflamed and probably infected.
For more information, see “Object in ear.” J Fam Pract. 2013.
1. A 30-year-old woman complained of drainage from her ear for the past three months. She admitted that her hearing was diminished in that ear. She had a history of recurrent ear infections since childhood.
Photo courtesy of Vladimir Zlinsky, MD, in Roy F. Sullivan, PhD. Audiology Forum: video otoscopy, www.rcsullivan.com. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Cholesteatoma, similar to an epidermal inclusion cyst in the skin, produces keratinaceous material that fills the middle ear, causing hearing loss and otorrhea.
For more information, see “Diminished hearing.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
2. A 2-year-old child was brought for a well-child exam two months after an episode of acute otitis media. He appeared healthy and was meeting all of his developmental milestones. An otoscopic examination revealed air-fluid levels in the right ear.
Photo courtesy of Frank Miller, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Otitis media with effusion, which is a characterized by fluid in the middle ear in a patient without signs or symptoms of an acute ear infection. The most common problem, present in more than half of patients, is mild hearing loss. This is usually identified when parents express concern regarding their child’s behavior, performance at school, or language development. The absence of signs and symptoms of acute illness assists in differentiating OME from AOM.
For more information, see “Air-fluid levels in ear.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
3. A 15-month-old boy was brought in with a two-day history of fever, irritability, and frequent tugging on his left ear. The week before, he had nasal congestion, cough, and rhinorrhea. On otoscopy, his left tympanic membrane (TM) appeared erythematous, cloudy, and bulging. The TM failed to move on pneumatic otoscopy.
Photo courtesy of William Clark, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Acute otitis media, which is characterized by middle-ear effusion in a patient with signs and symptoms of acute illness (eg, fever, irritability, otalgia).
For more information, see “Ear pain in baby.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
4. A 72-year-old man sought treatment for an earache in his left ear. He said that the pain began when he got a new “in the canal” hearing aid for his left ear a month earlier.
Photo courtesy of Dr. Roy F. Sullivan. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Acute otitis externa, secondary to ear canal damage done by using the new hearing aid, caused the viscous purulent discharge and narrowing of the ear canal.
For more information, see “Earache.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
5. Parents brought their 3-year-old daughter to an urgent care facility because she had been crying all day. The child was irritable, had scant otorrhea, and had been pulling on her right ear.
Photo courtesy of William Clark, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Otoscopy revealed an erythematous, swollen external auditory canal and a foreign object. The parents reported that their child had been playing with a toy beaded necklace when she started crying. The patient was referred to an otolaryngologist, who removed the bead using an operating microscope for visualization. She evaluated the child for a co-existing otitis externa and decided that the external canal was markedly inflamed and probably infected.
For more information, see “Object in ear.” J Fam Pract. 2013.
1. A 30-year-old woman complained of drainage from her ear for the past three months. She admitted that her hearing was diminished in that ear. She had a history of recurrent ear infections since childhood.
Photo courtesy of Vladimir Zlinsky, MD, in Roy F. Sullivan, PhD. Audiology Forum: video otoscopy, www.rcsullivan.com. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Cholesteatoma, similar to an epidermal inclusion cyst in the skin, produces keratinaceous material that fills the middle ear, causing hearing loss and otorrhea.
For more information, see “Diminished hearing.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
2. A 2-year-old child was brought for a well-child exam two months after an episode of acute otitis media. He appeared healthy and was meeting all of his developmental milestones. An otoscopic examination revealed air-fluid levels in the right ear.
Photo courtesy of Frank Miller, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Otitis media with effusion, which is a characterized by fluid in the middle ear in a patient without signs or symptoms of an acute ear infection. The most common problem, present in more than half of patients, is mild hearing loss. This is usually identified when parents express concern regarding their child’s behavior, performance at school, or language development. The absence of signs and symptoms of acute illness assists in differentiating OME from AOM.
For more information, see “Air-fluid levels in ear.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
3. A 15-month-old boy was brought in with a two-day history of fever, irritability, and frequent tugging on his left ear. The week before, he had nasal congestion, cough, and rhinorrhea. On otoscopy, his left tympanic membrane (TM) appeared erythematous, cloudy, and bulging. The TM failed to move on pneumatic otoscopy.
Photo courtesy of William Clark, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Acute otitis media, which is characterized by middle-ear effusion in a patient with signs and symptoms of acute illness (eg, fever, irritability, otalgia).
For more information, see “Ear pain in baby.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
4. A 72-year-old man sought treatment for an earache in his left ear. He said that the pain began when he got a new “in the canal” hearing aid for his left ear a month earlier.
Photo courtesy of Dr. Roy F. Sullivan. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Acute otitis externa, secondary to ear canal damage done by using the new hearing aid, caused the viscous purulent discharge and narrowing of the ear canal.
For more information, see “Earache.” J Fam Pract. 2013.
For the next photograph, proceed to the next page >>
5. Parents brought their 3-year-old daughter to an urgent care facility because she had been crying all day. The child was irritable, had scant otorrhea, and had been pulling on her right ear.
Photo courtesy of William Clark, MD. Reprinted from The Color Atlas of Family Medicine. 2nd ed.
Diagnosis: Otoscopy revealed an erythematous, swollen external auditory canal and a foreign object. The parents reported that their child had been playing with a toy beaded necklace when she started crying. The patient was referred to an otolaryngologist, who removed the bead using an operating microscope for visualization. She evaluated the child for a co-existing otitis externa and decided that the external canal was markedly inflamed and probably infected.
For more information, see “Object in ear.” J Fam Pract. 2013.
Using light to manage sleep-wake issues in patients with dementia
There’s a high incidence of sleep-wake disturbances among patients with dementia, which can lead to institutionalization. Although research has yet to provide a definitive answer about whether circadian-active light can benefit patients with dementia, a Veterans Affairs pilot study shows promising results. To read the full article, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/sleep-disorders/article/using-light-to-manage-sleep-wake-issues-in-patients-with-dementia/0bfe5c444b4ef312595c55c2585d8e60.html.
There’s a high incidence of sleep-wake disturbances among patients with dementia, which can lead to institutionalization. Although research has yet to provide a definitive answer about whether circadian-active light can benefit patients with dementia, a Veterans Affairs pilot study shows promising results. To read the full article, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/sleep-disorders/article/using-light-to-manage-sleep-wake-issues-in-patients-with-dementia/0bfe5c444b4ef312595c55c2585d8e60.html.
There’s a high incidence of sleep-wake disturbances among patients with dementia, which can lead to institutionalization. Although research has yet to provide a definitive answer about whether circadian-active light can benefit patients with dementia, a Veterans Affairs pilot study shows promising results. To read the full article, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/sleep-disorders/article/using-light-to-manage-sleep-wake-issues-in-patients-with-dementia/0bfe5c444b4ef312595c55c2585d8e60.html.
Accelerated hepatitis A and B immunization program may help high-risk patients
Immunization against hepatitis A and B is of great importance for patients with hepatitis C because concomitant infections are damaging to the liver. Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations, such as homeless individuals and intravenous drug users. A retrospective study of the medical records of 284 veterans who were receiving treatment for addictive disorders found that most patients (88%) who began an accelerated dosing program for hepatitis A and B vaccination received at least the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B. To read the full article, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/vaccines/article/accelerated-hepatitis-a-and-b-immunization-in-a-substance-abuse-treatment-program/4beb502484ad80699be3a086fa2e2017.html.
Immunization against hepatitis A and B is of great importance for patients with hepatitis C because concomitant infections are damaging to the liver. Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations, such as homeless individuals and intravenous drug users. A retrospective study of the medical records of 284 veterans who were receiving treatment for addictive disorders found that most patients (88%) who began an accelerated dosing program for hepatitis A and B vaccination received at least the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B. To read the full article, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/vaccines/article/accelerated-hepatitis-a-and-b-immunization-in-a-substance-abuse-treatment-program/4beb502484ad80699be3a086fa2e2017.html.
Immunization against hepatitis A and B is of great importance for patients with hepatitis C because concomitant infections are damaging to the liver. Vaccination offers the best protection against hepatitis A and B, particularly among high-risk populations, such as homeless individuals and intravenous drug users. A retrospective study of the medical records of 284 veterans who were receiving treatment for addictive disorders found that most patients (88%) who began an accelerated dosing program for hepatitis A and B vaccination received at least the first 3 injections of the series, thus possibly conferring substantial immunity to hepatitis A and B. To read the full article, go to Federal Practitioner: http://www.fedprac.com/specialty-focus/vaccines/article/accelerated-hepatitis-a-and-b-immunization-in-a-substance-abuse-treatment-program/4beb502484ad80699be3a086fa2e2017.html.
Problematic Medications: "Stomach Medicine"
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.