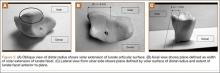
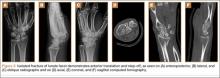
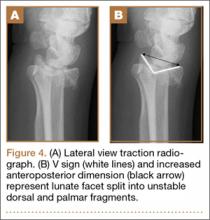
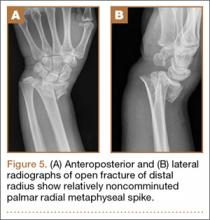
User login
Does qHPV vaccine prevent anal intraepithelial neoplasia and condylomata in men?
Yes. Quadrivalent human papillomavirus (qHPV) vaccine reduces rates of anal intraepithelial neoplasia (AIN) by 50% to 54%, and persistent anal infection by 59%, associated with the 4 types of HPV in the vaccine (6, 11, 16, and 18) in young men who have sex with men (MSM); it also reduces external genital lesions by 66%, and persistent HPV infection associated with the same 4 HPV types by 48 to 59% in all young men, heterosexual men,and MSM (strength of recommendation [SOR]: B, randomized, placebo-controlled trials [RCTs]).
In addition, the vaccine is associated with a 50% to 55% decrease in recurrent high-grade AIN and anogenital condylomatain older MSM (SOR: B, cohort studies).
EVIDENCE SUMMARY
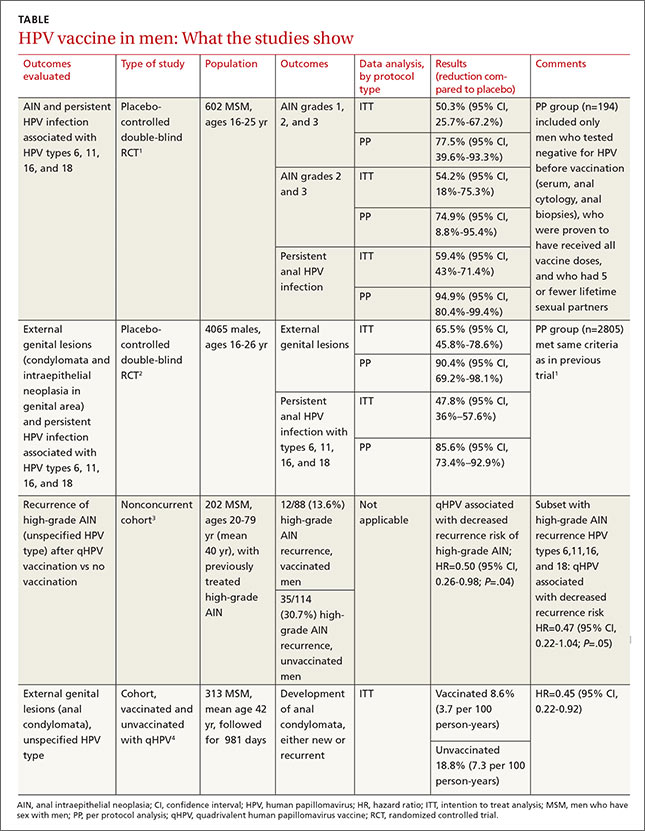
Two RCTs that evaluated qHPV in young men for preventing outcomes associated with the 4 HPV subtypes in the vaccine (6, 11, 16, and 18) found that it reduced them by 50% to 66% using an intention-to-treat protocol (TABLE1-4).
Vaccination reduces AIN and persistent infection in MSM
The first RCT evaluated a subset of 602 MSM from the second, larger RCT for preventing AIN and persistent HPV infection.1 The intention-to-treat population included men with 5 or fewer lifetime sexual partners who had engaged in insertive or receptive anal intercourse or oral sex within the last year, were not necessarily HPV-negative at enrollment, and received at least one dose of vaccine (or placebo).
The vaccine reduced AIN associated with the 4 HPV types (6.3 vs 12.6 events per 100 person-years; relative risk reduction [RRR]=50.3%; 95% confidence interval [CI], 25.7-67.2; number needed to treat [NNT]=16 to prevent one AIN case per year) and with HPV of any type (13 vs 17.5 events per 100 person-years; RRR=25.7%; 95% CI, -1.1 to 45.6). It also reduced the rate of persistent HPV infection with the 4 HPV vaccine subtypes (8.8 vs 21.6 events per 100 person-years; RRR=59.4%; 95% CI, 43%-71%; NNT=8 to prevent one persistent HPV infection per year).
Investigators in the study also evaluated vaccine efficacy in a smaller subset (194 men) using per-protocol analysis and found higher prevention rates (78% for AIN due to HPV types 6, 11, 16, and 18). Investigators followed these subjects every 6 months for 36 months with polymerase chain reaction testing for HPV DNA, high-resolution anoscopy with anal cytology, and anal biopsy and histology if there were atypia.
The vaccine decreases persistent HPV infection and external genital lesions
The second RCT, including both MSM and heterosexual men, found that qHPV vaccine reduced rates of persistent HPV infection by 48%, and external genital lesions (condylomata or intraepithelial neoplasia involving the penis, perineum, or perianal area) by 66% associated with HPV types 6, 11, 16, and 18 using the intention-to-treat protocol.2
Investigators used the same protocols used in the first RCT, and the per-protocol population again had higher prevention rates (84% for any HPV type, 90% against the 4 vaccine types). The only adverse effect of the vaccine was injection site pain (57% vs 51% with placebo; P<.001).
The vaccine also helps older MSM
A nonconcurrent cohort study that evaluated qHPV vaccination among older MSM with previously treated high-grade AIN found a 50% decrease in recurrence rates in the 2 years after vaccination.3 Investigators recruited HIV-negative men, some of whom chose vaccination (not randomized), and followed them for 2 years. Study limitations included using medical records for data collection and the predominance of white, nonsmoking men with private insurance.
A post-hoc analysis of older men without previous anal condylomata (210 men) or with treated condylomata and no recurrence in the year before vaccination (103 men) found that qHPV vaccination was associated with 55% lower rates of anal condylomata.4
RECOMMENDATIONS
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends routine use of qHPV vaccine in males ages 11 through 21 years, and optional use in unvaccinated men as old as 26 years.5
1. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
2. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
3. Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891-898.
4. Swedish KA, Goldstone SE. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One. 2014;9:e93393.
5. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
Yes. Quadrivalent human papillomavirus (qHPV) vaccine reduces rates of anal intraepithelial neoplasia (AIN) by 50% to 54%, and persistent anal infection by 59%, associated with the 4 types of HPV in the vaccine (6, 11, 16, and 18) in young men who have sex with men (MSM); it also reduces external genital lesions by 66%, and persistent HPV infection associated with the same 4 HPV types by 48 to 59% in all young men, heterosexual men,and MSM (strength of recommendation [SOR]: B, randomized, placebo-controlled trials [RCTs]).
In addition, the vaccine is associated with a 50% to 55% decrease in recurrent high-grade AIN and anogenital condylomatain older MSM (SOR: B, cohort studies).
EVIDENCE SUMMARY
Two RCTs that evaluated qHPV in young men for preventing outcomes associated with the 4 HPV subtypes in the vaccine (6, 11, 16, and 18) found that it reduced them by 50% to 66% using an intention-to-treat protocol (TABLE1-4).
Vaccination reduces AIN and persistent infection in MSM
The first RCT evaluated a subset of 602 MSM from the second, larger RCT for preventing AIN and persistent HPV infection.1 The intention-to-treat population included men with 5 or fewer lifetime sexual partners who had engaged in insertive or receptive anal intercourse or oral sex within the last year, were not necessarily HPV-negative at enrollment, and received at least one dose of vaccine (or placebo).
The vaccine reduced AIN associated with the 4 HPV types (6.3 vs 12.6 events per 100 person-years; relative risk reduction [RRR]=50.3%; 95% confidence interval [CI], 25.7-67.2; number needed to treat [NNT]=16 to prevent one AIN case per year) and with HPV of any type (13 vs 17.5 events per 100 person-years; RRR=25.7%; 95% CI, -1.1 to 45.6). It also reduced the rate of persistent HPV infection with the 4 HPV vaccine subtypes (8.8 vs 21.6 events per 100 person-years; RRR=59.4%; 95% CI, 43%-71%; NNT=8 to prevent one persistent HPV infection per year).
Investigators in the study also evaluated vaccine efficacy in a smaller subset (194 men) using per-protocol analysis and found higher prevention rates (78% for AIN due to HPV types 6, 11, 16, and 18). Investigators followed these subjects every 6 months for 36 months with polymerase chain reaction testing for HPV DNA, high-resolution anoscopy with anal cytology, and anal biopsy and histology if there were atypia.
The vaccine decreases persistent HPV infection and external genital lesions
The second RCT, including both MSM and heterosexual men, found that qHPV vaccine reduced rates of persistent HPV infection by 48%, and external genital lesions (condylomata or intraepithelial neoplasia involving the penis, perineum, or perianal area) by 66% associated with HPV types 6, 11, 16, and 18 using the intention-to-treat protocol.2
Investigators used the same protocols used in the first RCT, and the per-protocol population again had higher prevention rates (84% for any HPV type, 90% against the 4 vaccine types). The only adverse effect of the vaccine was injection site pain (57% vs 51% with placebo; P<.001).

The vaccine also helps older MSM
A nonconcurrent cohort study that evaluated qHPV vaccination among older MSM with previously treated high-grade AIN found a 50% decrease in recurrence rates in the 2 years after vaccination.3 Investigators recruited HIV-negative men, some of whom chose vaccination (not randomized), and followed them for 2 years. Study limitations included using medical records for data collection and the predominance of white, nonsmoking men with private insurance.
A post-hoc analysis of older men without previous anal condylomata (210 men) or with treated condylomata and no recurrence in the year before vaccination (103 men) found that qHPV vaccination was associated with 55% lower rates of anal condylomata.4
RECOMMENDATIONS
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends routine use of qHPV vaccine in males ages 11 through 21 years, and optional use in unvaccinated men as old as 26 years.5
Yes. Quadrivalent human papillomavirus (qHPV) vaccine reduces rates of anal intraepithelial neoplasia (AIN) by 50% to 54%, and persistent anal infection by 59%, associated with the 4 types of HPV in the vaccine (6, 11, 16, and 18) in young men who have sex with men (MSM); it also reduces external genital lesions by 66%, and persistent HPV infection associated with the same 4 HPV types by 48 to 59% in all young men, heterosexual men,and MSM (strength of recommendation [SOR]: B, randomized, placebo-controlled trials [RCTs]).
In addition, the vaccine is associated with a 50% to 55% decrease in recurrent high-grade AIN and anogenital condylomatain older MSM (SOR: B, cohort studies).
EVIDENCE SUMMARY
Two RCTs that evaluated qHPV in young men for preventing outcomes associated with the 4 HPV subtypes in the vaccine (6, 11, 16, and 18) found that it reduced them by 50% to 66% using an intention-to-treat protocol (TABLE1-4).
Vaccination reduces AIN and persistent infection in MSM
The first RCT evaluated a subset of 602 MSM from the second, larger RCT for preventing AIN and persistent HPV infection.1 The intention-to-treat population included men with 5 or fewer lifetime sexual partners who had engaged in insertive or receptive anal intercourse or oral sex within the last year, were not necessarily HPV-negative at enrollment, and received at least one dose of vaccine (or placebo).
The vaccine reduced AIN associated with the 4 HPV types (6.3 vs 12.6 events per 100 person-years; relative risk reduction [RRR]=50.3%; 95% confidence interval [CI], 25.7-67.2; number needed to treat [NNT]=16 to prevent one AIN case per year) and with HPV of any type (13 vs 17.5 events per 100 person-years; RRR=25.7%; 95% CI, -1.1 to 45.6). It also reduced the rate of persistent HPV infection with the 4 HPV vaccine subtypes (8.8 vs 21.6 events per 100 person-years; RRR=59.4%; 95% CI, 43%-71%; NNT=8 to prevent one persistent HPV infection per year).
Investigators in the study also evaluated vaccine efficacy in a smaller subset (194 men) using per-protocol analysis and found higher prevention rates (78% for AIN due to HPV types 6, 11, 16, and 18). Investigators followed these subjects every 6 months for 36 months with polymerase chain reaction testing for HPV DNA, high-resolution anoscopy with anal cytology, and anal biopsy and histology if there were atypia.
The vaccine decreases persistent HPV infection and external genital lesions
The second RCT, including both MSM and heterosexual men, found that qHPV vaccine reduced rates of persistent HPV infection by 48%, and external genital lesions (condylomata or intraepithelial neoplasia involving the penis, perineum, or perianal area) by 66% associated with HPV types 6, 11, 16, and 18 using the intention-to-treat protocol.2
Investigators used the same protocols used in the first RCT, and the per-protocol population again had higher prevention rates (84% for any HPV type, 90% against the 4 vaccine types). The only adverse effect of the vaccine was injection site pain (57% vs 51% with placebo; P<.001).

The vaccine also helps older MSM
A nonconcurrent cohort study that evaluated qHPV vaccination among older MSM with previously treated high-grade AIN found a 50% decrease in recurrence rates in the 2 years after vaccination.3 Investigators recruited HIV-negative men, some of whom chose vaccination (not randomized), and followed them for 2 years. Study limitations included using medical records for data collection and the predominance of white, nonsmoking men with private insurance.
A post-hoc analysis of older men without previous anal condylomata (210 men) or with treated condylomata and no recurrence in the year before vaccination (103 men) found that qHPV vaccination was associated with 55% lower rates of anal condylomata.4
RECOMMENDATIONS
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommends routine use of qHPV vaccine in males ages 11 through 21 years, and optional use in unvaccinated men as old as 26 years.5
1. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
2. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
3. Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891-898.
4. Swedish KA, Goldstone SE. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One. 2014;9:e93393.
5. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
1. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
2. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411.
3. Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54:891-898.
4. Swedish KA, Goldstone SE. Prevention of anal condyloma with quadrivalent human papillomavirus vaccination of older men who have sex with men. PLoS One. 2014;9:e93393.
5. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1-30.
Evidence-based answers from the Family Physicians Inquiries Network
Factors Affecting Perceptions of Open, Mini-Open, and Arthroscopic Rotator Cuff Repair Techniques Among Medical Professionals
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
Rotator cuff tears are a common condition affecting the shoulder joint. Initial open repair techniques were associated with several complications, including severe early postoperative pain, deltoid detachment and/or weakness, risk for infection, and arthrofibrosis.1-3 In addition, open procedures cannot address other possible diagnoses, such as labral tears and loose bodies. These disadvantages promoted the development of an arthroscopically assisted mini-open technique.4 Superior long-term results, with more than 90% of patients achieving good to excellent results,5-13 established the mini-open rotator cuff repair (RCR) as the gold standard.3,6,10,12,14-16
Recently, as instrumentation for arthroscopy has improved, enthusiasm for all-arthroscopic techniques (hereafter referred to as arthroscopic repair) has grown. The appeal of arthroscopic repair includes potentially less initial pain, ability to treat intra-articular lesions concurrently, smaller skin incisions with better cosmesis, less soft-tissue dissection, and low risk for deltoid detachment.3,17 The potential advantages of arthroscopic repair can lead to perceptions of quicker healing and shorter recovery, which are not supported by the literature. However, arthroscopic repair is technically more challenging, time-consuming, and expensive than open or mini-open repairs,18,19 and though some investigators have reported a trend toward fewer complications,3 the long-term outcome of arthroscopic RCRs has not been shown to be better than that of other techniques.
Given that no differences have been shown between the emerging arthroscopic repair technique and mini-open repair with respect to range of motion or clinical scores in the short term,3 it is unclear what perceptions influence choice of technique for one’s own personal RCR.
We conducted a study to determine which RCR technique medical professionals (orthopedic attendings and residents, anesthesiologists, internal medicine attendings, main operating room nurses, and physical therapists) preferred for their own surgery and to analyze perceptions shaping those opinions. Orthopedic surgeons have the best concept of rotator cuff surgery, but anesthesiologists and nurses have a “front row seat” and opinions on types of rotator cuff surgery. Physical therapists, who treat patients with rotator cuff tears, also have a working knowledge of rotator cuff surgery. Finally, internists represent a rotator cuff injury referral service and may have patients who have undergone rotator cuff surgery. We hypothesized that most medical professionals, irrespective of specialty or career length, would prefer arthroscopic RCR because of its perceived superior outcome and fast recovery.
Materials and Methods
This cross-sectional, descriptive, survey-based study was approved by our institutional review board (IRB) and offered via 3 emails between April 2011 and June 2011 to attendings (orthopedists, internists, anesthesiologists), residents, and allied health professionals (AHPs; operating room nurses, physical therapists) involved in orthopedic care at our institution. Each email contained a hyperlink to the online survey (Appendix), which took about 10 minutes to complete and explored respondent demographics, exposure to the different techniques, and opinions regarding different aspects of RCR surgery and recovery.
There were 84 respondents. The sexes were equally represented, and age ranged from 25 to 78 years (Table 1). Of the respondents, 41 (49%) were attendings, 20 (24%) were residents, and 23 (27%) were AHPs. Of the attendings, 13 (32%) were orthopedic surgeons, 26 (63%) were primary care physicians, and 2 (5%) did not specify their specialty. Four orthopedic surgeons had fellowship training in sports medicine or shoulder and elbow surgery. The attendings were overall more experienced in their profession than the other groups were, with 68% reporting more than 5 years of experience.
Descriptive statistics, including means and standard errors, were calculated. Fisher exact test was used to compare preferences of RCR type according to type of training and years of experience. Significance was set at P ≤ .05.
Results
Overall Responses (Table 2)
Of the 84 respondents, almost half (46%) preferred deferring their choice of RCR to their surgeon. Most of the other respondents preferred the arthroscopic technique (26%) or the mini-open repair (23%). There was no association between technique preference and medical professional type. Most respondents (63%) had never assisted in or performed rotator cuff surgery.
Seventy-four percent of all respondents indicated they thought arthroscopic, mini-open, and open RCRs are safe, and about half thought these procedures are fast. About half expressed no opinion about the cost-effectiveness of arthroscopic, mini-open, or open RCRs (54%, 52%, and 48%, respectively), and slightly more than half expressed no opinion about whether arthroscopic, mini-open, or open RCR provide the best outcome (58%, 60%, and 62%, respectively). Significantly (P < .05) more respondents thought arthroscopic and mini-open repairs, rather than open repairs, promote quick healing (64% and 45%, respectively, vs 15%), good cosmetic results (81% and 51%, respectively, vs 10%), and patient satisfaction (50% and 48%, respectively, vs 30%). However, a significant (P < .05) number also thought arthroscopic and mini-open repairs are harder to learn/more challenging to perform than open repairs (52% and 38%, respectively, vs 17%).
Of all factors considered, safety of arthroscopic repair garnered the highest consensus: 82%. Respondents were least opinionated about the outcome of the open repair technique, with more than 62% expressing no opinion about the outcome. The responses to the questions on the learning curves for the 3 techniques varied the most.
Responses by Group (Table 2)
Attendings. Of the 41 attendings, 24 (59%) responded they would defer to their surgeon’s technique preference for RCR. Of the other 17 who expressed a preference, most indicated arthroscopic or mini-open repair (17% each). There was a difference (P < .05) between years of experience and RCR preference: of the 13 attendings with less than 5 years of experience, arthroscopic repair was preferred by 31%; in contrast, of the 28 attendings with more than 5 years of experience, only 11% preferred arthroscopic repair.
Of the 11 attendings who performed rotator cuff surgery, 55% used the open technique, but most (8) preferred to have their own rotator cuff fixed arthroscopically or according to their surgeon’s preference. Only 1 surgeon preferred open repair for his own rotator cuff. Of the 4 surgeons who performed arthroscopic RCRs, 3 had less than 5 years of experience. Conversely, all 7 surgeons who performed mini-open or open repairs had more than 5 years of experience.
Of the 30 attendings who did not perform rotator cuff surgery, most (20) responded they would defer to their surgeon’s technique preference for RCR.
The attendings’ opinions on factors affecting rotator cuff surgery were similar to those of the other respondents with respect to safety, cost-effectiveness, recovery, cosmesis, patient satisfaction, outcome, and technical difficulty. Unlike the others, however, attendings considered all 3 repair techniques fast.
Residents. Of the 20 residents, 7 preferred arthroscopic, 5 preferred mini-open, and 1 preferred open repair; the other 7 responded they would defer to their surgeon’s preference. Residents’ opinions on each factor were more polarized and consistent across categories than those of the other groups. Residents overwhelmingly thought all 3 techniques (arthroscopic, mini-open, open) are safe (19, 19, and 18, respectively) and cost-effective (12, 14, and 14, respectively). Although most residents considered the open and mini-open repair techniques fast (19 and 15, respectively), only 8 considered arthroscopic RCR fast, and 4 considered it slow. Residents’ opinions about the technique that produces the best outcome were mixed. As with the other respondents, residents thought arthroscopic RCRs heal fast and produce great cosmetic results, but are challenging to perform and have a steep learning curve. Unlike the other respondents, most residents (12) considered open RCR easy to learn (P = .006), with a learning curve of fewer than 20 procedures.
AHPs. No AHP expressed a preference for open RCR. This group was evenly divided among 3 choices: deferring to their surgeon’s preference, arthroscopic repair, and mini-open repair. The 23 AHPs thought arthroscopic, mini-open, and open repairs are safe (17, 15, and 12, respectively), but most indicated they were “equivocal” about which techniques are cost-effective, challenging to perform, and produce the best outcomes. A significantly (P = .014) larger number of AHPs (7) considered open rotator cuff surgery slow compared with arthroscopic (0) and mini-open (2) repair techniques. As with the overall cohort, AHPs reported arthroscopic and mini-open repairs promote quick healing and good cosmetic results, but are challenging to perform.
Discussion
As our population ages and continues to remain active, the demand for RCR has accelerated. National data show that 272,148 ambulatory RCRs and 20,433 inpatient RCRs were performed in 2006—an overall 141% increase in RCR since 1996.20 In 1996, 41 per 100,000 population underwent RCR.20 By 2006, this number ballooned to 98 per 100,000 population.20 There are 3 predominant techniques for repairing the rotator cuff: open, mini-open, and arthroscopic. As RCR use increases, we should consider the factors that medical professionals consider important when choosing a method for their own RCR.
Of the 84 medical professionals in our cohort, 39 (46%) indicated they would defer to their surgeon’s technique preference for RCR. Of the other 45, about equal numbers preferred arthroscopic and mini-open RCRs; only 2 preferred open RCRs. This finding suggests that the individual opinions of surgeons who perform RCRs have a substantial influence on a large proportion of medical professionals’ ultimate choice of RCR method. Interestingly, of the attendings who performed open RCR, only 1 expressed a preference for the open technique for his own RCR. This finding might suggest a shift in opinion and an emerging perception among surgeons performing RCR about the value of this technique.
Several factors may account for these evolving beliefs. We hypothesized that a biased favorable view of arthroscopic repair outcome might influence opinions. However, our results did not support the hypothesis. Medical professionals in our cohort were equivocal about the best RCR technique. No consensus was evident among attendings, residents, or AHPs. This lack of clinical agreement about rotator cuff surgery has been observed elsewhere—for example, among members of the American Academy of Orthopaedic Surgeons (AAOS)21 and the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy.22 Despite theoretical advantages of arthroscopic repair, there has been no documented significant difference in patient outcomes when compared with other techniques.23 To our knowledge, there have been only a few clinical studies comparing the different RCR techniques. A meta-analysis of 5 clinical studies comparing arthroscopic and mini-open RCR techniques showed no difference in clinical outcomes or complication rates.8 The 2012 AAOS clinical practice guidelines for RCR reflect these observations.24 That consortium of leading shoulder surgeons could not recommend a modality of surgical rotator cuff tear repair given the lack of conclusive evidence.24
At our institution, arthroscopic, mini-open, and open RCRs were performed by 36%, 9%, and 55% of our surgeons, respectively. A survey of AAOS surgeons showed that, of those who perform RCRs, 14.5%, 46.2%, and 36.6% used arthroscopic, mini-open, and open techniques, respectively.21 The greater use of open repairs at our institution might reflect the seniority of our faculty. Dunn and colleagues21 found that surgeons who preferred open RCR had been in practice longer than those who preferred the arthroscopic or mini-open technique. Of our 4 faculty who performed arthroscopic repairs, 3 were less than 5 years from completing their training. In contrast, all faculty who performed mini-open or open repairs were more than 5 years from completing their training. Furthermore, mean age of the surgeons who performed arthroscopic repair was 39.8 years (range, 32-51 years), and these surgeons were significantly younger than those who performed mini-open or open repair (mean age, 56.3 years; range, 41-78 years). Younger surgeon age has been associated with higher rates of arthroscopic repair.25
Attendings unaccustomed to arthroscopy may find it more challenging than the younger generation of surgeons, who are exposed to it early in training. Dunn and colleagues21 noted that the likelihood of performing an arthroscopic repair was influenced by the surgeon’s experience level. Fellowship-trained shoulder and sports medicine surgeons are also more likely to perform arthroscopic repairs than those with training limited to orthopedic residency.25 Arthroscopic RCR demands a high level of technical skill that many acquire in fellowship training.26 Mauro and colleagues26 found that surgeons trained in a sports medicine fellowship performed 82.6% of subacromial decompression and/or RCR procedures arthroscopically, compared with 54.5% to 70.1% for surgeons trained in other fellowships. In our cohort, with the exception of 1 surgeon, all fellowship-trained shoulder and sports medicine surgeons performed arthroscopic RCRs.
Although no conclusive evidence in the literature supports arthroscopic over the other repair types, the demand for arthroscopic RCR has rapidly increased relative to that for the others. Between 1996 and 2006, use of arthroscopic RCR increased 600%, from 8 to 58 per 100,000 population.20 In that same period, use of open RCR increased by only 34%.20 Similarly, Mauro and colleagues26 found that the proportion of subacromial decompression and RCRs performed arthroscopically rose from 58.3% in 2004 to 83.7% in 2009. Using the 2006 New York State Ambulatory Surgery Database, Churchill and Ghorai27 found that 74.5% of RCRs with acromioplasty were performed arthroscopically.
Respondent-indicated factors that may have contributed to the more favorable opinion of arthroscopic and mini-open repair include quick healing, good cosmetic results, and better perceived patient satisfaction. The literature supports these perceptions. Baker and Liu14 found shorter hospital stays and quicker return to activity with arthroscopic repair compared with open repair. Vitale and colleagues25 also noted that, compared with open or mini-open repair techniques, arthroscopic repair resulted in shorter hospitalization and quicker overall recovery.
If these selected health care professionals with some inside information on rotator cuff surgery have biases that affect their selection of rotator cuff procedures, we should acknowledge that nonmedical personnel, in particular our patients, also have biases. The knowledge base of patients may be further influenced by friends or family members who have had rotator cuff surgery, by lay publications, and by the Internet. Satisfaction with any surgical procedure depends not only on the success of the surgery and the rehabilitation but also on patient and provider expectations. Such expectations are influenced, in part, by biases.
Our medical professionals had similar opinions on safety, recovery, cosmesis, and overall outcome of the RCR techniques, but different opinions on procedure durations and associated training requirements. All residents except one indicated open repair was a quick procedure. In contrast, a significant number of AHPs thought open repair was time-consuming. The attendings considered all the methods fast. The residents’ opinions were the most consistent with the true operating times reported. According to the literature, total operating time for mini-open repair ranges from 10 to 16 minutes faster than that for arthroscopic repair.18,20,27 Ultimately, procedure duration did not affect the respondents’ technique preference for RCR.
There was substantial disagreement about the number of procedures needed to become proficient in the different repair techniques. Overall, however, there was consensus that arthroscopic and mini-open repairs had longer learning curves than open repair. Given the lack of agreement among orthopedic department chairmen and sports medicine fellowship directors regarding the minimum exposure needed (during residency) to become proficient in diagnostic shoulder arthroscopy,28 this finding is not surprising. Guttmann and colleagues29 attempted to quantify the learning curve for arthroscopic RCR by tracking operating time as a surrogate measure. They found that RCR operative time decreased rapidly during the initial block of 10 cases to the second block of 10 cases, but thereafter improvement continued at a much lower rate.29 None of our respondents thought the learning curve for arthroscopic RCR was under 10 cases, but no group, not even the attendings who performed RCRs, could agree on the minimum number of cases needed for proficiency. The longer learning curve for arthroscopic RCR did not discourage the respondents who preferred arthroscopic or mini-open RCR.
Cost was not an influential factor in opinions about which RCR method is optimal. Medical professionals were ambivalent about the cost-effectiveness of the different procedures, with most expressing no opinion on cost. Multiple investigators have shown that arthroscopic RCR costs as much as $1144 more than mini-open RCR,18,27 which has many of the advantages of arthroscopic repair but not the costly implants and instruments. As our medical community becomes more cost-conscious, concern about this factor may increase among medical professionals.
Our study had several limitations. Its results must be interpreted carefully, given they represent the viewpoints of a nonrandomized sample of motivated respondents at one institution. A selection bias excluded surgeons who were uncomfortable with RCR and unwilling to report any shortcomings. The conclusions cannot be generalized to other medical professionals or to other institutions. Furthermore, to develop a simple, straightforward survey focused on a specific type of rotator cuff tear, and to avoid confusion, we assumed that the treatment preference for the described tear was generalizable to all encountered tears. However, some surgeons have reported different repair techniques for different types and sizes of rotator cuff tears.25
Conclusion
Most of our surveyed medical professionals were willing to defer to their surgeon’s decision about which technique would be appropriate for their own personal RCR. There is a trend nationally, and at our institution, for increased use of arthroscopic RCR. Although medical professionals readily acknowledge it is unclear which repair method provides the best ultimate outcome, many perceive fast recovery and good cosmetic results with arthroscopic and mini-open repairs. When medical professionals are counseling patients, we need to recognize these personal biases because many patients defer to their surgeon’s counsel. For some medical professionals, cosmesis can be an important factor, but cost, procedure duration, potential technical challenges of arthroscopic repair, and other considerations may make other techniques more desirable for others.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
1. Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(4):380-390.
2. Bennett WF. Arthroscopic repair of full-thickness supraspinatus tears (small-to-medium): a prospective study with 2- to 4-year follow-up. Arthroscopy. 2003;19(3):249-256.
3. Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89(suppl 3):127-136.
4. Duralde XA, Greene RT. Mini-open rotator cuff repair via an anterosuperior approach. J Shoulder Elbow Surg. 2008;17(5):715-721.
5. Blevins FT, Warren RF, Cavo C, et al. Arthroscopic assisted rotator cuff repair: results using a mini-open deltoid splitting approach. Arthroscopy. 1996;12(1):50-59.
6. Levy HJ, Uribe JW, Delaney LG. Arthroscopic assisted rotator cuff repair: preliminary results. Arthroscopy. 1990;6(1):55-60.
7. Liu SH. Arthroscopically-assisted rotator-cuff repair. J Bone Joint Surg Br. 1994;76(4):592-595.
8. Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36(9):1824-1828.
9. Park JY, Levine WN, Marra G, Pollock RG, Flatow EL, Bigliani LU. Portal-extension approach for the repair of small and medium rotator cuff tears. Am J Sports Med. 2000;28(3):312-316.
10. Paulos LE, Kody MH. Arthroscopically enhanced “miniapproach” to rotator cuff repair. Am J Sports Med. 1994;22(1):19-25.
11. Posada A, Uribe JW, Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE. Mini-deltoid splitting rotator cuff repair: do results deteriorate with time? Arthroscopy. 2000;16(2):137-141.
12. Shinners TJ, Noordsij PG, Orwin JF. Arthroscopically assisted mini-open rotator cuff repair. Arthroscopy. 2002;18(1):21-26.
13. Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999;15(2):126-131.
14. Baker CL, Liu SH. Comparison of open and arthroscopically assisted rotator cuff repairs. Am J Sports Med. 1995;23(1):99-104.
15. Liu SH, Baker CL. Arthroscopically assisted rotator cuff repair: correlation of functional results with integrity of the cuff. Arthroscopy. 1994;10(1):54-60.
16. Pollock RG, Flatow EL. The rotator cuff, part II. Full-thickness tears. Mini-open repair. Orthop Clin North Am. 1997;28(2):169-177.
17. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
18. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
19. Kose KC, Tezen E, Cebesoy O, et al. Mini-open versus all-arthroscopic rotator cuff repair: comparison of the operative costs and the clinical outcomes. Adv Ther. 2008;25(3):249-259.
20. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
21. Dunn WR, Schackman BR, Walsh C, et al. Variation in orthopaedic surgeons’ perceptions about the indications for rotator cuff surgery. J Bone Joint Surg Am. 2005;87(9):1978-1984.
22. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):803-815.
23. Aleem AW, Brophy RH. Outcomes of rotator cuff surgery: what does the evidence tell us? Clin Sports Med. 2012;31(4):665-674.
24. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
25. Vitale MA, Kleweno CP, Jacir AM, Levine WN, Bigliani LU, Ahmad CS. Training resources in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2007;89(6):1393-1398.
26. Mauro CS, Jordan SS, Irrgang JJ, Harner CD. Practice patterns for subacromial decompression and rotator cuff repair: an analysis of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2012;94(16):1492-1499.
27. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
28. O’Neill PJ, Cosgarea AJ, Freedman JA, Queale WS, McFarland EG. Arthroscopic proficiency: a survey of orthopaedic sports medicine fellowship directors and orthopaedic surgery department chairs. Arthroscopy. 2002;18(7):795-800.
29. Guttmann D, Graham RD, MacLennan MJ, Lubowitz JH. Arthroscopic rotator cuff repair: the learning curve. Arthroscopy. 2005;21(4):394-400.
Fibromyalgia • anxiety/depression • urinary retention • Dx?
THE CASE
A 72-year-old woman came to our internal medicine department clinic for a follow-up appointment for her fibromyalgia. Thirteen months earlier, she had sought care at our facility not only for fibromyalgia, but for insomnia, anxiety, depression, and urinary incontinence. At the time, we prescribed amitriptyline 10 mg/d—for her pain and depression—as well as clonazepam 10 mg/d and paracetamol 650 mg, as needed.
When she came in for the follow-up, she indicated that for the past 8 months, she’d been experiencing urinary retention that required her to self-catheterize 2 to 3 times a day. She said she hadn’t used other medicines or herbal products during this time.
The patient had visited her family physician several times over the previous few months, and had been referred to a urologist. During an episode of acute urinary retention, she went to the emergency department (ED), where the ED physician performed urinary catheterization and referred her to the hospital’s Urology Department. After 48 hours, she was evaluated by a urologist, who diagnosed chronic urinary retention related to a hypercontractile bladder, without any particular cause. She was advised to continue to catheterize herself when needed. She was also prescribed pyridostigmine bromide, but she stopped taking it because of abdominal pain and bloating.
Two months prior to her visit with us, the patient suffered a second acute urinary retention episode and returned to the ED. Urinary catheterization was performed for 72 hours. At her next visit to her urologist, she was told to continue self-catheterization and was prescribed silodosin 8 mg/d.
THE DIAGNOSIS
Based on the patient’s history, we suspected the urinary retention was secondary to the anticholinergic effects of amitriptyline. We were able to determine that the patient’s urinary retention was likely the result of an adverse drug reaction (ADR) by using the causality algorithm of the Spanish Pharmacovigilance System, which suggests the following criteria:1 a) a positive time sequence (ie, onset of symptoms closely followed administration of the medication), b) the existence of an ADR that is well known and consistent with the mechanism of action of the drug,2 c) symptoms that resolve after suspending the drug; d) no repeat exposure (to the adverse effects of amitriptyline) due to ethical reasons; and e) the absence of an alternative explanation for the symptoms.3
DISCUSSION
Although indicated for depression, amitriptyline is also used for other conditions, including nocturnal enuresis and chronic neuropathic pain.4 Amitriptyline exhibits anticholinergic effects that can cause symptoms related to the nervous system (agitation, disorientation, sleepiness, delirium, cognitive impairment), ocular system (blurred vision, dry eye, accommodation disturbances, increased intraocular pressure), cardiovascular system (tachycardia), gastrointestinal tract (dry mouth, paralytic ileus, constipation), urinary system (urinary retention); and skin and mucosal membranes (dryness).5,6 Anticholinergic effects can also induce hyperthermia or increase the risk of falls.5,6
Anticholinergic medications can cause ADRs in high-risk older patients and thus are usually considered inappropriate for this patient population.6 The Anticholinergic Risk Scale (ARS) can be used to categorize medications based on their potential for anticholinergic adverse effects (TABLE).7 Amitriptyline is included in the group with the highest risk of ADRs. Amitriptyline is also included in the list of drugs that should be avoided in older adults, according to the 2012 American Geriatrics Society Beers Criteria.8
Our patient. We instructed her to stop taking amitriptyline, and her urinary retention disappeared within 48 hours. Two months later, she remained asymptomatic.
THE TAKEAWAY
Although many medications are known to cause adverse events, they can be missed when clinicians fail to pinpoint exactly when a new sign, symptom, or health problem appeared. This often leads to a chain reaction of unnecessary explorations, harmful treatment, patient suffering, and unjustified costs.9-11 Our patient had seen 4 different health care providers (a family physician, urologist, and 2 ED physicians) before we saw her and ultimately made the diagnosis. Family physicians can prevent anticholinergic ADRs by using a scale, such as the ARS, before prescribing a medication.
1. Meyboom RH, Royer RJ. Causality classification at pharmacovigilance centres in the European community. Pharmacoepidemiol Drug Saf. 1992;1:87–97.
2. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Ficha Técnica Tryptizol. Agencia Española de Medicamentosy Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/cima/pdfs/en/ft/51064/FT_51064.pdf. Accessed July 24, 2015.
3. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). ¿Qué es el Sistema Español de Farmacovigilancia de medicamentos de Uso Humano? Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/vigilancia/medicamentosUsoHumano/SEFV-H/home.htm. Accessed July 6, 2015.
4. Parfitt K, ed. Martindale: The Complete Drug Reference. 32nd ed. London, UK: Pharmaceutical Press;1999:273-276.
5. Rang HP, Dale MM, Ritter JM. Farmacología. 4th ed. Barcelona, Spain: Ediciones Harcourt, S.A. Impresión Mateu Cromo, S.A.;2000:123-128,594-600.
6. Ness J, Hoth A, Barnett MJ, et al. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4:42-51.
7. Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508-513.
8. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
9. CSM Update. Br Med J (Clin Res Ed). 1985;291:1638.
10. Palop Larrea V, Sempere i Verdú E, Martínez-Mir I. Anamnesis farmacológica y reacciones adversas a medicamentos. Aten Primaria. 2000;25:666,668.
11. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096-1099.
THE CASE
A 72-year-old woman came to our internal medicine department clinic for a follow-up appointment for her fibromyalgia. Thirteen months earlier, she had sought care at our facility not only for fibromyalgia, but for insomnia, anxiety, depression, and urinary incontinence. At the time, we prescribed amitriptyline 10 mg/d—for her pain and depression—as well as clonazepam 10 mg/d and paracetamol 650 mg, as needed.
When she came in for the follow-up, she indicated that for the past 8 months, she’d been experiencing urinary retention that required her to self-catheterize 2 to 3 times a day. She said she hadn’t used other medicines or herbal products during this time.
The patient had visited her family physician several times over the previous few months, and had been referred to a urologist. During an episode of acute urinary retention, she went to the emergency department (ED), where the ED physician performed urinary catheterization and referred her to the hospital’s Urology Department. After 48 hours, she was evaluated by a urologist, who diagnosed chronic urinary retention related to a hypercontractile bladder, without any particular cause. She was advised to continue to catheterize herself when needed. She was also prescribed pyridostigmine bromide, but she stopped taking it because of abdominal pain and bloating.
Two months prior to her visit with us, the patient suffered a second acute urinary retention episode and returned to the ED. Urinary catheterization was performed for 72 hours. At her next visit to her urologist, she was told to continue self-catheterization and was prescribed silodosin 8 mg/d.
THE DIAGNOSIS
Based on the patient’s history, we suspected the urinary retention was secondary to the anticholinergic effects of amitriptyline. We were able to determine that the patient’s urinary retention was likely the result of an adverse drug reaction (ADR) by using the causality algorithm of the Spanish Pharmacovigilance System, which suggests the following criteria:1 a) a positive time sequence (ie, onset of symptoms closely followed administration of the medication), b) the existence of an ADR that is well known and consistent with the mechanism of action of the drug,2 c) symptoms that resolve after suspending the drug; d) no repeat exposure (to the adverse effects of amitriptyline) due to ethical reasons; and e) the absence of an alternative explanation for the symptoms.3
DISCUSSION
Although indicated for depression, amitriptyline is also used for other conditions, including nocturnal enuresis and chronic neuropathic pain.4 Amitriptyline exhibits anticholinergic effects that can cause symptoms related to the nervous system (agitation, disorientation, sleepiness, delirium, cognitive impairment), ocular system (blurred vision, dry eye, accommodation disturbances, increased intraocular pressure), cardiovascular system (tachycardia), gastrointestinal tract (dry mouth, paralytic ileus, constipation), urinary system (urinary retention); and skin and mucosal membranes (dryness).5,6 Anticholinergic effects can also induce hyperthermia or increase the risk of falls.5,6
Anticholinergic medications can cause ADRs in high-risk older patients and thus are usually considered inappropriate for this patient population.6 The Anticholinergic Risk Scale (ARS) can be used to categorize medications based on their potential for anticholinergic adverse effects (TABLE).7 Amitriptyline is included in the group with the highest risk of ADRs. Amitriptyline is also included in the list of drugs that should be avoided in older adults, according to the 2012 American Geriatrics Society Beers Criteria.8
Our patient. We instructed her to stop taking amitriptyline, and her urinary retention disappeared within 48 hours. Two months later, she remained asymptomatic.
THE TAKEAWAY
Although many medications are known to cause adverse events, they can be missed when clinicians fail to pinpoint exactly when a new sign, symptom, or health problem appeared. This often leads to a chain reaction of unnecessary explorations, harmful treatment, patient suffering, and unjustified costs.9-11 Our patient had seen 4 different health care providers (a family physician, urologist, and 2 ED physicians) before we saw her and ultimately made the diagnosis. Family physicians can prevent anticholinergic ADRs by using a scale, such as the ARS, before prescribing a medication.
THE CASE
A 72-year-old woman came to our internal medicine department clinic for a follow-up appointment for her fibromyalgia. Thirteen months earlier, she had sought care at our facility not only for fibromyalgia, but for insomnia, anxiety, depression, and urinary incontinence. At the time, we prescribed amitriptyline 10 mg/d—for her pain and depression—as well as clonazepam 10 mg/d and paracetamol 650 mg, as needed.
When she came in for the follow-up, she indicated that for the past 8 months, she’d been experiencing urinary retention that required her to self-catheterize 2 to 3 times a day. She said she hadn’t used other medicines or herbal products during this time.
The patient had visited her family physician several times over the previous few months, and had been referred to a urologist. During an episode of acute urinary retention, she went to the emergency department (ED), where the ED physician performed urinary catheterization and referred her to the hospital’s Urology Department. After 48 hours, she was evaluated by a urologist, who diagnosed chronic urinary retention related to a hypercontractile bladder, without any particular cause. She was advised to continue to catheterize herself when needed. She was also prescribed pyridostigmine bromide, but she stopped taking it because of abdominal pain and bloating.
Two months prior to her visit with us, the patient suffered a second acute urinary retention episode and returned to the ED. Urinary catheterization was performed for 72 hours. At her next visit to her urologist, she was told to continue self-catheterization and was prescribed silodosin 8 mg/d.
THE DIAGNOSIS
Based on the patient’s history, we suspected the urinary retention was secondary to the anticholinergic effects of amitriptyline. We were able to determine that the patient’s urinary retention was likely the result of an adverse drug reaction (ADR) by using the causality algorithm of the Spanish Pharmacovigilance System, which suggests the following criteria:1 a) a positive time sequence (ie, onset of symptoms closely followed administration of the medication), b) the existence of an ADR that is well known and consistent with the mechanism of action of the drug,2 c) symptoms that resolve after suspending the drug; d) no repeat exposure (to the adverse effects of amitriptyline) due to ethical reasons; and e) the absence of an alternative explanation for the symptoms.3
DISCUSSION
Although indicated for depression, amitriptyline is also used for other conditions, including nocturnal enuresis and chronic neuropathic pain.4 Amitriptyline exhibits anticholinergic effects that can cause symptoms related to the nervous system (agitation, disorientation, sleepiness, delirium, cognitive impairment), ocular system (blurred vision, dry eye, accommodation disturbances, increased intraocular pressure), cardiovascular system (tachycardia), gastrointestinal tract (dry mouth, paralytic ileus, constipation), urinary system (urinary retention); and skin and mucosal membranes (dryness).5,6 Anticholinergic effects can also induce hyperthermia or increase the risk of falls.5,6
Anticholinergic medications can cause ADRs in high-risk older patients and thus are usually considered inappropriate for this patient population.6 The Anticholinergic Risk Scale (ARS) can be used to categorize medications based on their potential for anticholinergic adverse effects (TABLE).7 Amitriptyline is included in the group with the highest risk of ADRs. Amitriptyline is also included in the list of drugs that should be avoided in older adults, according to the 2012 American Geriatrics Society Beers Criteria.8
Our patient. We instructed her to stop taking amitriptyline, and her urinary retention disappeared within 48 hours. Two months later, she remained asymptomatic.
THE TAKEAWAY
Although many medications are known to cause adverse events, they can be missed when clinicians fail to pinpoint exactly when a new sign, symptom, or health problem appeared. This often leads to a chain reaction of unnecessary explorations, harmful treatment, patient suffering, and unjustified costs.9-11 Our patient had seen 4 different health care providers (a family physician, urologist, and 2 ED physicians) before we saw her and ultimately made the diagnosis. Family physicians can prevent anticholinergic ADRs by using a scale, such as the ARS, before prescribing a medication.
1. Meyboom RH, Royer RJ. Causality classification at pharmacovigilance centres in the European community. Pharmacoepidemiol Drug Saf. 1992;1:87–97.
2. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Ficha Técnica Tryptizol. Agencia Española de Medicamentosy Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/cima/pdfs/en/ft/51064/FT_51064.pdf. Accessed July 24, 2015.
3. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). ¿Qué es el Sistema Español de Farmacovigilancia de medicamentos de Uso Humano? Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/vigilancia/medicamentosUsoHumano/SEFV-H/home.htm. Accessed July 6, 2015.
4. Parfitt K, ed. Martindale: The Complete Drug Reference. 32nd ed. London, UK: Pharmaceutical Press;1999:273-276.
5. Rang HP, Dale MM, Ritter JM. Farmacología. 4th ed. Barcelona, Spain: Ediciones Harcourt, S.A. Impresión Mateu Cromo, S.A.;2000:123-128,594-600.
6. Ness J, Hoth A, Barnett MJ, et al. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4:42-51.
7. Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508-513.
8. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
9. CSM Update. Br Med J (Clin Res Ed). 1985;291:1638.
10. Palop Larrea V, Sempere i Verdú E, Martínez-Mir I. Anamnesis farmacológica y reacciones adversas a medicamentos. Aten Primaria. 2000;25:666,668.
11. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096-1099.
1. Meyboom RH, Royer RJ. Causality classification at pharmacovigilance centres in the European community. Pharmacoepidemiol Drug Saf. 1992;1:87–97.
2. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Ficha Técnica Tryptizol. Agencia Española de Medicamentosy Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/cima/pdfs/en/ft/51064/FT_51064.pdf. Accessed July 24, 2015.
3. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). ¿Qué es el Sistema Español de Farmacovigilancia de medicamentos de Uso Humano? Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/vigilancia/medicamentosUsoHumano/SEFV-H/home.htm. Accessed July 6, 2015.
4. Parfitt K, ed. Martindale: The Complete Drug Reference. 32nd ed. London, UK: Pharmaceutical Press;1999:273-276.
5. Rang HP, Dale MM, Ritter JM. Farmacología. 4th ed. Barcelona, Spain: Ediciones Harcourt, S.A. Impresión Mateu Cromo, S.A.;2000:123-128,594-600.
6. Ness J, Hoth A, Barnett MJ, et al. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4:42-51.
7. Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508-513.
8. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
9. CSM Update. Br Med J (Clin Res Ed). 1985;291:1638.
10. Palop Larrea V, Sempere i Verdú E, Martínez-Mir I. Anamnesis farmacológica y reacciones adversas a medicamentos. Aten Primaria. 2000;25:666,668.
11. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096-1099.
Safety of Tourniquet Use in Total Knee Arthroplasty in Patients With Radiographic Evidence of Vascular Calcifications
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
The value and veracity of psychiatric themes depicted in modern cinema
Perhaps more than any other medical specialty, psychiatry enjoys a longstanding and, at times, complicated relationship with cinema. Recent award-winning films, such as Still Alice, Silver Linings Playbook, and Birdman: Or (The Unexpected Virtue of Ignorance) continue traditions rooted in One Flew Over the Cuckoo’s Nest, Martha Marcy May Marlene, Spellbound, and dozens of other films. Through these films, psychiatry is afforded exposure unavailable to most medical specialties. This exposure has proven to be a double-edged sword, however.
Exposure vs accurate portrayal
Relative benefits and disadvantages of psychiatry’s position in film and popular media are difficult to calculate. A film such as Still Alice can provide a vivid, concrete personal narrative of a patient with Alzheimer’s disease, equipping millions of viewers with knowledge that might otherwise remain esoteric and inaccessible. Martha Marcy May Marlene offers a similar stage for posttraumatic stress disorder (PTSD), as does Spellbound for dissociative amnesia.
Such exposure comes at a cost, inevitably, because information about psychiatry is incorporated into a dramatic storyline assembled by filmmakers who are not medical professionals and who are bound by conflicting pressures. At times, those pressures outweigh the desire to accurately portray psychiatric illness.
‘Magical realism’
Two of last year’s celebrated films, Birdman and Still Alice, have continued the longstanding tradition of portraying mental illness in film. Medicine often is touted as art and science; film likewise sits at this intersection. However, filmmakers are artists, primarily, and the nature of storytelling is to emphasize art over scientific accuracy.
The main character in Birdman, for example, manifests psychosis, but many of his presenting signs and symptoms are incongruent with any diagnosable form of psychosis. To tell its story, the film employs magical realism, a celebrated literary and film technique. Although magical realism might detract from the accuracy of the condition portrayed, it adds cinematic appeal to the film, likely creates more entertainment value, and, in turn, garners appreciation from a broader audience.
Expansion of medical information, accurate and otherwise
As in the 1970’s, we are, today, in the midst of rapidly evolving societal norms. One of the most rapid changes is in how the public acquires information. We are in the midst of the “Googlification” of medical knowledge and the expansion of online medical resources. These resources can, simultaneously, inform and mislead the public.
Popular films behave in much the same way. There is no motion picture-guild requirement that mentally ill characters in films such as Birdman meet any set of psychiatric criteria, from DSM-5 or elsewhere. Similarly, the fact that psychiatrists do not control the information in films that portray mental illness comes as no surprise.
‘One flew East, one flew West…’
The tension between engaging storytelling and medical accuracy certainly is not a new phenomenon, extending not only to representations of disease but to representations of treatment. Consider director Miloš Forman’s seminal 1975 film, One Flew Over the Cuckoo’s Nest, whose chief importance for psychiatry rests not in its individualized representations of patients but in a portrayal of the environment in which they are treated. Louise Fletcher’s Academy Award-winning portrayal of cruel Nurse Ratched has lingered in the public consciousness, remaining a prominent image for many Americans when they think of a psychiatric institution.
When Cuckoo’s Nest was released, it was considered by critics to be an “exploration of society’s enforcement of conformism” that “almost willfully overlooked the realities of mental illness”1 so that it could vivify its protagonist’s struggle against tyrannical Nurse Ratched. The film’s primary intent might not have been to make a statement about the injustices of the time, but it has certainly had a lasting effect on the public’s perception of psychiatric illness and treatment.
Films offer an opportunity for discussion
Films on the theme of psychiatry and mental illness have long held a distinctive position in the canon of Western cinema. In this vein, films from the past year have made timely contributions to the genre. Although Still Alice and Birdman might prove to be ground-breaking in changing societal views over time, we must not expect them to do so.
Nevertheless, psychiatry ought to take advantage of popular films’ wide exposure and ability to destigmatize mental illness—rather than lament medical inaccuracies in these films.
Cinema is, first and foremost, an art. Although patients and the public might pick up misconceptions about psychosis, Alzheimer’s disease, or PTSD because popular films take artistic liberty about mental illness, psychiatrists are available to set the record straight. After all, psychiatry has long been about managing perceptions, and patient education is at the core of our specialty.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Reference
1. Ebert R. “One Flew Over The Cuckoo’s Nest (review). http://www.rogerebert.com/reviews/great-movie-one-flew-overthe-cuckoos-nest-1975. February 2, 2003. Accessed September 9, 2015.
Perhaps more than any other medical specialty, psychiatry enjoys a longstanding and, at times, complicated relationship with cinema. Recent award-winning films, such as Still Alice, Silver Linings Playbook, and Birdman: Or (The Unexpected Virtue of Ignorance) continue traditions rooted in One Flew Over the Cuckoo’s Nest, Martha Marcy May Marlene, Spellbound, and dozens of other films. Through these films, psychiatry is afforded exposure unavailable to most medical specialties. This exposure has proven to be a double-edged sword, however.
Exposure vs accurate portrayal
Relative benefits and disadvantages of psychiatry’s position in film and popular media are difficult to calculate. A film such as Still Alice can provide a vivid, concrete personal narrative of a patient with Alzheimer’s disease, equipping millions of viewers with knowledge that might otherwise remain esoteric and inaccessible. Martha Marcy May Marlene offers a similar stage for posttraumatic stress disorder (PTSD), as does Spellbound for dissociative amnesia.
Such exposure comes at a cost, inevitably, because information about psychiatry is incorporated into a dramatic storyline assembled by filmmakers who are not medical professionals and who are bound by conflicting pressures. At times, those pressures outweigh the desire to accurately portray psychiatric illness.
‘Magical realism’
Two of last year’s celebrated films, Birdman and Still Alice, have continued the longstanding tradition of portraying mental illness in film. Medicine often is touted as art and science; film likewise sits at this intersection. However, filmmakers are artists, primarily, and the nature of storytelling is to emphasize art over scientific accuracy.
The main character in Birdman, for example, manifests psychosis, but many of his presenting signs and symptoms are incongruent with any diagnosable form of psychosis. To tell its story, the film employs magical realism, a celebrated literary and film technique. Although magical realism might detract from the accuracy of the condition portrayed, it adds cinematic appeal to the film, likely creates more entertainment value, and, in turn, garners appreciation from a broader audience.
Expansion of medical information, accurate and otherwise
As in the 1970’s, we are, today, in the midst of rapidly evolving societal norms. One of the most rapid changes is in how the public acquires information. We are in the midst of the “Googlification” of medical knowledge and the expansion of online medical resources. These resources can, simultaneously, inform and mislead the public.
Popular films behave in much the same way. There is no motion picture-guild requirement that mentally ill characters in films such as Birdman meet any set of psychiatric criteria, from DSM-5 or elsewhere. Similarly, the fact that psychiatrists do not control the information in films that portray mental illness comes as no surprise.
‘One flew East, one flew West…’
The tension between engaging storytelling and medical accuracy certainly is not a new phenomenon, extending not only to representations of disease but to representations of treatment. Consider director Miloš Forman’s seminal 1975 film, One Flew Over the Cuckoo’s Nest, whose chief importance for psychiatry rests not in its individualized representations of patients but in a portrayal of the environment in which they are treated. Louise Fletcher’s Academy Award-winning portrayal of cruel Nurse Ratched has lingered in the public consciousness, remaining a prominent image for many Americans when they think of a psychiatric institution.
When Cuckoo’s Nest was released, it was considered by critics to be an “exploration of society’s enforcement of conformism” that “almost willfully overlooked the realities of mental illness”1 so that it could vivify its protagonist’s struggle against tyrannical Nurse Ratched. The film’s primary intent might not have been to make a statement about the injustices of the time, but it has certainly had a lasting effect on the public’s perception of psychiatric illness and treatment.
Films offer an opportunity for discussion
Films on the theme of psychiatry and mental illness have long held a distinctive position in the canon of Western cinema. In this vein, films from the past year have made timely contributions to the genre. Although Still Alice and Birdman might prove to be ground-breaking in changing societal views over time, we must not expect them to do so.
Nevertheless, psychiatry ought to take advantage of popular films’ wide exposure and ability to destigmatize mental illness—rather than lament medical inaccuracies in these films.
Cinema is, first and foremost, an art. Although patients and the public might pick up misconceptions about psychosis, Alzheimer’s disease, or PTSD because popular films take artistic liberty about mental illness, psychiatrists are available to set the record straight. After all, psychiatry has long been about managing perceptions, and patient education is at the core of our specialty.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Perhaps more than any other medical specialty, psychiatry enjoys a longstanding and, at times, complicated relationship with cinema. Recent award-winning films, such as Still Alice, Silver Linings Playbook, and Birdman: Or (The Unexpected Virtue of Ignorance) continue traditions rooted in One Flew Over the Cuckoo’s Nest, Martha Marcy May Marlene, Spellbound, and dozens of other films. Through these films, psychiatry is afforded exposure unavailable to most medical specialties. This exposure has proven to be a double-edged sword, however.
Exposure vs accurate portrayal
Relative benefits and disadvantages of psychiatry’s position in film and popular media are difficult to calculate. A film such as Still Alice can provide a vivid, concrete personal narrative of a patient with Alzheimer’s disease, equipping millions of viewers with knowledge that might otherwise remain esoteric and inaccessible. Martha Marcy May Marlene offers a similar stage for posttraumatic stress disorder (PTSD), as does Spellbound for dissociative amnesia.
Such exposure comes at a cost, inevitably, because information about psychiatry is incorporated into a dramatic storyline assembled by filmmakers who are not medical professionals and who are bound by conflicting pressures. At times, those pressures outweigh the desire to accurately portray psychiatric illness.
‘Magical realism’
Two of last year’s celebrated films, Birdman and Still Alice, have continued the longstanding tradition of portraying mental illness in film. Medicine often is touted as art and science; film likewise sits at this intersection. However, filmmakers are artists, primarily, and the nature of storytelling is to emphasize art over scientific accuracy.
The main character in Birdman, for example, manifests psychosis, but many of his presenting signs and symptoms are incongruent with any diagnosable form of psychosis. To tell its story, the film employs magical realism, a celebrated literary and film technique. Although magical realism might detract from the accuracy of the condition portrayed, it adds cinematic appeal to the film, likely creates more entertainment value, and, in turn, garners appreciation from a broader audience.
Expansion of medical information, accurate and otherwise
As in the 1970’s, we are, today, in the midst of rapidly evolving societal norms. One of the most rapid changes is in how the public acquires information. We are in the midst of the “Googlification” of medical knowledge and the expansion of online medical resources. These resources can, simultaneously, inform and mislead the public.
Popular films behave in much the same way. There is no motion picture-guild requirement that mentally ill characters in films such as Birdman meet any set of psychiatric criteria, from DSM-5 or elsewhere. Similarly, the fact that psychiatrists do not control the information in films that portray mental illness comes as no surprise.
‘One flew East, one flew West…’
The tension between engaging storytelling and medical accuracy certainly is not a new phenomenon, extending not only to representations of disease but to representations of treatment. Consider director Miloš Forman’s seminal 1975 film, One Flew Over the Cuckoo’s Nest, whose chief importance for psychiatry rests not in its individualized representations of patients but in a portrayal of the environment in which they are treated. Louise Fletcher’s Academy Award-winning portrayal of cruel Nurse Ratched has lingered in the public consciousness, remaining a prominent image for many Americans when they think of a psychiatric institution.
When Cuckoo’s Nest was released, it was considered by critics to be an “exploration of society’s enforcement of conformism” that “almost willfully overlooked the realities of mental illness”1 so that it could vivify its protagonist’s struggle against tyrannical Nurse Ratched. The film’s primary intent might not have been to make a statement about the injustices of the time, but it has certainly had a lasting effect on the public’s perception of psychiatric illness and treatment.
Films offer an opportunity for discussion
Films on the theme of psychiatry and mental illness have long held a distinctive position in the canon of Western cinema. In this vein, films from the past year have made timely contributions to the genre. Although Still Alice and Birdman might prove to be ground-breaking in changing societal views over time, we must not expect them to do so.
Nevertheless, psychiatry ought to take advantage of popular films’ wide exposure and ability to destigmatize mental illness—rather than lament medical inaccuracies in these films.
Cinema is, first and foremost, an art. Although patients and the public might pick up misconceptions about psychosis, Alzheimer’s disease, or PTSD because popular films take artistic liberty about mental illness, psychiatrists are available to set the record straight. After all, psychiatry has long been about managing perceptions, and patient education is at the core of our specialty.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Reference
1. Ebert R. “One Flew Over The Cuckoo’s Nest (review). http://www.rogerebert.com/reviews/great-movie-one-flew-overthe-cuckoos-nest-1975. February 2, 2003. Accessed September 9, 2015.
Reference
1. Ebert R. “One Flew Over The Cuckoo’s Nest (review). http://www.rogerebert.com/reviews/great-movie-one-flew-overthe-cuckoos-nest-1975. February 2, 2003. Accessed September 9, 2015.
Hot flashes and night sweats • amenorrhea • positive home pregnancy test • Dx?
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
Failure to reproduce
In my struggle to keep abreast of all things pediatric, I sample a variety of sources.
Of course each month I scan almost all of the abstracts in the journal Pediatrics. But to get a sense of what the nonmedical community is reading, I begin each morning with a cruise through the electronic versions of the New York Times and the Portland (Maine) Press Herald.
By lunch time I usually have hopscotched my way through the Wall Street Journal. And during our evening adult beverage quiet time, I amuse myself with our local daily. If a news story includes a link to an original article, I usually bore down deep enough to at least read the abstract. Keep in mind that this whole process of keeping current takes little more than a half an hour, 45 minutes tops.
It seems that psychology-related topics dominate the science and medicine stories that I encounter. This shouldn’t surprise you because most of us want to know more about why humans behave the way we do. We also wonder if animal behavior may provide some clues.
It may be because I was trained by careful and skeptical “hard” scientists that I have always read psychosocial and behavioral studies with several grains of salt. Despite my skepticism, I am not beneath embracing the odd study that seems to support one of my biases. The studies that don’t sync with my world view I quickly cast on the rubbish heap because the “sample group was too small,” or the “variables were not adequately controlled for,” or simply because I thought the study was poorly done.
It turns out that my skepticism has not only been well founded, but should have been broader in scope. In a recent study published in the journal Science, three young psychologists undertook a heroic and courageous effort to reproduce 100 studies from three leading psychology journals (Science 2015 Aug 28. doi:10.1126/science.aac4716). Chosen from a larger group, these studies were thought to reflect the core knowledge from which psychologists develop their understanding of such basics as learning, memory, and relationships.
The investigators found that in more than half the studies, they were unable to reproduce the results reported in the original studies despite the fact that in many cases, they were assisted by the original investigators in their attempts to replicate the conditions of the initial studies.
The authors quickly assert that their findings do not suggest that the original investigators were attempting to deceive. Nor does the failure to reproduce results necessarily mean that other future studies might confirm the original findings. Their primary point is that evaluating reproducibility is difficult.
However, this new study is troubling for two reasons. First, it casts even more doubt on the decision to expand the MCAT (Medical College Admission Test) by adding several hours of questions based on psychosocial topics in hopes of creating physicians who are more in tune with the emotional needs and social challenges of their future patients. If the results of more than half of the studies that might be considered the underpinnings of modern psychology can’t be reproduced, are we just asking aspiring medical students to learn a larger collection of half truths? And thus have medical students spend less time learning basic science and developing better critical thinking skills? There are better ways to sort for more empathetic and sensitive physicians than by building an unevenly weighted exam.
Second, although this study highlights the core of what makes science such a powerful and effective tool for discovering the truth, the anti-science folks will point to it as just another example of how we shouldn’t trust anything science tells us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” Email him at [email protected].
In my struggle to keep abreast of all things pediatric, I sample a variety of sources.
Of course each month I scan almost all of the abstracts in the journal Pediatrics. But to get a sense of what the nonmedical community is reading, I begin each morning with a cruise through the electronic versions of the New York Times and the Portland (Maine) Press Herald.
By lunch time I usually have hopscotched my way through the Wall Street Journal. And during our evening adult beverage quiet time, I amuse myself with our local daily. If a news story includes a link to an original article, I usually bore down deep enough to at least read the abstract. Keep in mind that this whole process of keeping current takes little more than a half an hour, 45 minutes tops.
It seems that psychology-related topics dominate the science and medicine stories that I encounter. This shouldn’t surprise you because most of us want to know more about why humans behave the way we do. We also wonder if animal behavior may provide some clues.
It may be because I was trained by careful and skeptical “hard” scientists that I have always read psychosocial and behavioral studies with several grains of salt. Despite my skepticism, I am not beneath embracing the odd study that seems to support one of my biases. The studies that don’t sync with my world view I quickly cast on the rubbish heap because the “sample group was too small,” or the “variables were not adequately controlled for,” or simply because I thought the study was poorly done.
It turns out that my skepticism has not only been well founded, but should have been broader in scope. In a recent study published in the journal Science, three young psychologists undertook a heroic and courageous effort to reproduce 100 studies from three leading psychology journals (Science 2015 Aug 28. doi:10.1126/science.aac4716). Chosen from a larger group, these studies were thought to reflect the core knowledge from which psychologists develop their understanding of such basics as learning, memory, and relationships.
The investigators found that in more than half the studies, they were unable to reproduce the results reported in the original studies despite the fact that in many cases, they were assisted by the original investigators in their attempts to replicate the conditions of the initial studies.
The authors quickly assert that their findings do not suggest that the original investigators were attempting to deceive. Nor does the failure to reproduce results necessarily mean that other future studies might confirm the original findings. Their primary point is that evaluating reproducibility is difficult.
However, this new study is troubling for two reasons. First, it casts even more doubt on the decision to expand the MCAT (Medical College Admission Test) by adding several hours of questions based on psychosocial topics in hopes of creating physicians who are more in tune with the emotional needs and social challenges of their future patients. If the results of more than half of the studies that might be considered the underpinnings of modern psychology can’t be reproduced, are we just asking aspiring medical students to learn a larger collection of half truths? And thus have medical students spend less time learning basic science and developing better critical thinking skills? There are better ways to sort for more empathetic and sensitive physicians than by building an unevenly weighted exam.
Second, although this study highlights the core of what makes science such a powerful and effective tool for discovering the truth, the anti-science folks will point to it as just another example of how we shouldn’t trust anything science tells us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” Email him at [email protected].
In my struggle to keep abreast of all things pediatric, I sample a variety of sources.
Of course each month I scan almost all of the abstracts in the journal Pediatrics. But to get a sense of what the nonmedical community is reading, I begin each morning with a cruise through the electronic versions of the New York Times and the Portland (Maine) Press Herald.
By lunch time I usually have hopscotched my way through the Wall Street Journal. And during our evening adult beverage quiet time, I amuse myself with our local daily. If a news story includes a link to an original article, I usually bore down deep enough to at least read the abstract. Keep in mind that this whole process of keeping current takes little more than a half an hour, 45 minutes tops.
It seems that psychology-related topics dominate the science and medicine stories that I encounter. This shouldn’t surprise you because most of us want to know more about why humans behave the way we do. We also wonder if animal behavior may provide some clues.
It may be because I was trained by careful and skeptical “hard” scientists that I have always read psychosocial and behavioral studies with several grains of salt. Despite my skepticism, I am not beneath embracing the odd study that seems to support one of my biases. The studies that don’t sync with my world view I quickly cast on the rubbish heap because the “sample group was too small,” or the “variables were not adequately controlled for,” or simply because I thought the study was poorly done.
It turns out that my skepticism has not only been well founded, but should have been broader in scope. In a recent study published in the journal Science, three young psychologists undertook a heroic and courageous effort to reproduce 100 studies from three leading psychology journals (Science 2015 Aug 28. doi:10.1126/science.aac4716). Chosen from a larger group, these studies were thought to reflect the core knowledge from which psychologists develop their understanding of such basics as learning, memory, and relationships.
The investigators found that in more than half the studies, they were unable to reproduce the results reported in the original studies despite the fact that in many cases, they were assisted by the original investigators in their attempts to replicate the conditions of the initial studies.
The authors quickly assert that their findings do not suggest that the original investigators were attempting to deceive. Nor does the failure to reproduce results necessarily mean that other future studies might confirm the original findings. Their primary point is that evaluating reproducibility is difficult.
However, this new study is troubling for two reasons. First, it casts even more doubt on the decision to expand the MCAT (Medical College Admission Test) by adding several hours of questions based on psychosocial topics in hopes of creating physicians who are more in tune with the emotional needs and social challenges of their future patients. If the results of more than half of the studies that might be considered the underpinnings of modern psychology can’t be reproduced, are we just asking aspiring medical students to learn a larger collection of half truths? And thus have medical students spend less time learning basic science and developing better critical thinking skills? There are better ways to sort for more empathetic and sensitive physicians than by building an unevenly weighted exam.
Second, although this study highlights the core of what makes science such a powerful and effective tool for discovering the truth, the anti-science folks will point to it as just another example of how we shouldn’t trust anything science tells us.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Coping with a Picky Eater.” Email him at [email protected].
Sustentaculum Lunatum: Appreciation of the Palmar Lunate Facet in Management of Complex Intra-Articular Fractures of the Distal Radius
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
Fracture of the distal radius is the wrist injury most often encountered by orthopedic and hand surgeons.1 The number of fractures of the distal radius in the United States was estimated to be 640,000 in 2001, and the incidence is increasing.2,3 Recent evidence has shown a substantial increase in treating these fractures with internal rather than closed fixation, even in the elderly.4
Treatment of complex intra-articular fractures of the distal radius requires an accurate diagnosis of the fracture pattern and a thoughtful approach to fixation. Although a majority of the fractures that meet the operative criteria are now treated with various anterior locked-plating techniques with good results, a subset requires more technically demanding fixation approaches, including fragment-specific approaches, dorsal and palmar plating, and combined internal and external fixation.
The sustentaculum lunatum, as we have named the palmar lunate facet, deserves specific attention because of its importance in load transmission across the radiocarpal joint and its key role in restoring the anatomy of the palmar distal radial metaphysis during internal fixation. This fragment in comminuted fractures was first ascribed special importance by Melone5 in his description of common fracture patterns. In the present article, we describe the anatomical characteristics of the sustentaculum lunatum and the clinical relevance of this fragment to management of fractures of the distal radius.
Classification
A variety of classification systems have been proposed to characterize and guide treatment of fractures of the distal radius. The earliest descriptions of fracture patterns were presented by Castaing6 and Frykman7 in the 1960s. The Frykman classification historically has been popular but is limited in accuracy in its characterization of fragments and their displacement and is limited in its ability to guide treatment. The classification system proposed by Melone and colleagues5,8-10 was the first to truly describe fracture of the distal radius fragments in a relevant manner, including their characteristic “4 parts” (Figure 1). The authors emphasized the importance of the “medial complex” as the cornerstone of the radiocarpal and radioulnar joints.
The classification system developed by Müller and colleagues,11 which was adopted by the AO (Arbeitsgemeinschaft für Osteosynthesefragen), might be the most descriptive and informative system, and it is widely used to conduct research and direct treatment. This system classifies fractures into A (extra-articular), B (partial articular), and C (complete articular) types and subclassifies them according to fracture location and comminution. These classifications, along with a conceptualization of the distal forearm as a 3-column structure involving the radial, ulnar, and intermediate columns (including the lunate facet), as proposed by Peine and colleagues,12 gave us a framework for approaching fixation of fractures of the distal radius.
Etymology and Definition
Sustentaculum, from the Latin sustinere, “to support, check, or put off,” and taculum, “receptacle or holding space,” is a fitting description of the most distal portion of the palmar lunate facet, as it supports and holds the carpus, and specifically the lunate, on the radial articular surface. This portion is analogous to the sustentaculum tali, the named portion of the calcaneus that supports and articulates with the middle calcaneal articular surface of the talus13 and provides a reliable fragment for internal fixation of the calcaneus.
Anatomical and Biomechanical Considerations
The distal radial articular surface is composed of distinct scaphoid and lunate facets that articulate with their respective carpal bones. Several studies have characterized the anatomy of the distal radius.14-17 Linscheid14 found that the lunate and scaphoid facets account for 46% and 43% of the contact area across the radiocarpal joint, respectively; this has been corroborated by others.15 A biomechanical study by Genda and Horii18 showed that the majority of stress across the wrist joint was concentrated at the palmar side of the distal radius in the neutral position. Although it is recognized that the scaphoid facet bears most of the load across the wrist in the neutral wrist position, most activities of daily living place the wrist in a slightly extended and ulnarly deviated position. This position results in a shift of the majority of load to the radiolunar articulation, constituting 53% of total force transmission.18 Subchondral bone density analyses have supported this lunate-predominant loading pattern across the radiocarpal articulation in most people.19 This loading pattern is also supported by the observation that failure of fixation and carpal subluxation generally occurs at the radiolunate articulation.
The palmar lip of the distal radius traditionally has been depicted and conceptualized as a flat extension of the metaphysis, leading to the development of implants that are not ideally designed for capturing this area in the fracture setting. A 3-dimensional (3-D) computed tomography (CT) study of the distal radii of healthy volunteers, conducted by Andermahr and colleagues,20 showed that the contour of the palmar lunate facet projects from the palmar cortex of the radius by 3 mm on average and is about 19 mm in width (radial to ulnar dimension) (Figures 2A-2C). In the axial plane, the anterior cortex of the distal radius slopes in a palmar direction, from radial to ulnar. This presents a challenge in attempts to support the entire surface (scaphoid and lunate facets) with a single palmar implant.20-25
A study conducted by Harness and colleagues24 showed that the majority of palmar shear fractures are composed of multiple fragments of the lunar articular facet. Anatomical studies of the distal radiocarpal articulation have also described the ligamentous attachments to the sustentaculum lunatum.26 The short radiolunate ligament, which originates from this fragment and inserts onto the lunate, provides stability to the carpus and, if not adequately fixed, leads to an incompetent restraint to palmar carpal translation. Isolated injuries of the short radiolunate ligament or fractures of the palmar lunate facet have been shown to result in palmar carpal translation.27,28 In addition, attachments of the palmar radioulnar ligament and other more ulnar radiocarpal ligaments act as deforming forces on the palmar lunate facet.24,26
Fracture Pattern Recognition
Although the AO type B palmar shear fracture pattern, also known as the Barton fracture, has classically been recognized as the fracture involving the palmar lunate facet and requiring special attention, many complete articular fractures feature involvement and fragmentation of this portion of the distal radius (Figures 3A-3F).29 In highly comminuted complete articular and palmar shear fracture patterns, the morphology of the sustentaculum lunatum should be appreciated, and its adequate fixation to the radial metaphysis ensured, to prevent loss of reduction.
Visualization of the palmar lunate facet as a distinct fragment might be difficult in cases of highly comminuted fracture patterns. Standard CT or more recently described 3-D CT techniques with subtraction of the carpus might facilitate appreciation of this fragment for preoperative planning of approach and fixation.29,30 Our institutional protocol involves obtaining preoperative traction radiographs of every fracture of the distal radius. These radiographs have reduced the need for CT in understanding the fracture pattern and aid in decision making.31
Besides appreciating the existence of the sustentaculum lunatum fragment, we should recognize that some injury patterns that split the lunate facet into unstable dorsal and palmar fragments might necessitate a separate dorsal approach to reduce and fix the dorsal lunate fragment. Traction radiographs can be especially useful in recognizing these patterns (a V sign is present) (Figures 4A, 4B).
Open Fractures
Highly comminuted fractures of the distal radius presenting with displaced lunate facet fragments can have high-energy mechanisms of injury. Although open fractures of the distal radius are associated with lower risk for infection (compared with open fractures of other long bones), they deserve special attention because of associated tendon and neurovascular injuries. Few studies have specifically assessed open fractures of the distal radius.32-35 Only the study by Rozental and Blazar34 listed associated injuries at the wrist level. The authors identified 4 patients (out of 18) with concomitant flexor tendon or neurovascular injuries that included radial or ulnar artery injury. In our experience, many open fractures of the distal radius are caused by an inside-out mechanism and present with an open wound either over the ulnar styloid or in the area of the ulnar side of the palmar radial metaphysis corresponding to the metaphyseal spike that mates with the sustentaculum lunatum (Figures 5A, 5B). Given these findings, we approach this intermediate column with particular care in cases of open fracture, paying attention to important structures (flexors, neurovascular) and looking for contamination from the environment into the fracture.
Fixation Techniques
The approach to fixation of partial articular palmar shear fractures is fairly straightforward. Buttress plate fixation has been well described and has had reliably good results.36 However, in very distal fracture patterns and in cases in which the palmar lunate facet is fragmented as part of a complete articular fracture, a fragment-specific approach to fixation with or without spanning external fixation often is necessary.37 The unrecognized sustentaculum lunatum fragment in comminuted complete articular fractures can lead to inadequate fixation constructs, resulting in loss of reduction and carpal subluxation in a palmar direction.24,34,38
Our surgical approach uses the standard anterior interval between the radial artery and the flexor carpi radialis, as described by Henry.39 The flexor pollicis longus is retracted ulnarly, revealing the pronator quadratus. We then reflect the pronator quadratus from the distal radial metaphysis until the most proximal and ulnar extent of the fracture is easily visualized. The palmar ulnar metaphyseal cortex that mates with the displaced sustentaculum lunatum is, in our experience, often the least comminuted portion of the metaphysis, thus providing a cortical key for restoration of height and alignment (Figures 5A, 5B). At our institution, fixation typically is achieved by contouring miniplates (1.3 or 1.5 mm) to capture and buttress the sustentaculum lunatum (Figures 6A, 6B). In our experience, the screw lengths in the most distal fixed-angle constructs at the palmar lip are limited to 6 mm or less to avoid penetration of the articular surface, though this has not been previously reported in the literature. After restoring the length and tilt of this intermediate column of the distal radius, we proceed with “rebuilding” the remainder of the fragments to our stabilized initial construct.
Various authors40-43 have described alternative fixation methods for the palmar lunate facet fragment. Jupiter and Marent-Huber42 described 2.4-mm locked-plate fixation with either a standard palmar plate or T- or L-plates for cases in which the palmar lip fragment is very distal and small. In fact, some newer anatomical distal radius implants include features designed to target these fragments (Figures 7A, 7B). An alternative fixation method involves use of a 26-gauge stainless steel wire passed through drill holes in the metaphysis 1 cm proximal to the fracture and then passed through the palmar capsule just distal to the fragment and secured in figure-8 fashion while the fragment is manually held reduced.41 Still others have recommended limited internal fixation of the sustentaculum lunatum through an ulna-sided palmar approach to the distal radius (between the ulnar neurovascular bundle and the flexor tendons) combined with external fixation to restore length and palmar tilt in highly comminuted fractures.40,43
A method involving arthroscopically assisted reduction and fixation of the lunate facet has also been described, though this procedure is technically demanding and has limited indications.44 It uses a Freer elevator passed through the standard 3-4 portal after initial visualization and evacuation of hematoma. The Freer elevator is used to disimpact the sustentaculum lunatum and to elevate it from its depressed position. With the dorsal lunate facet left displaced to facilitate access to the palmar fragment, a nerve hook retractor is used to reduce the palmar facet to the radial styloid, and Kirschner wires are used to achieve interfragmentary fixation. The dorsal lunate fragment is then pieced back to the articular segment, and the entire construct is fixed to the radial metaphysis with additional Kirschner wires.
Discussion
Given the increasing incidence of fractures of the distal radius, internal fixation of these injuries will continue to be relevant. American Academy of Orthopaedic Surgeons guidelines recommend operative fixation for fractures with postreduction radial shortening of more than 3 mm, dorsal tilt of more than 10°, or intra-articular displacement or step-off of more than 2 mm.45 Dr. Eglseder and Dr. Pensy indicate operative treatment of any incongruity of more than 2 mm in a young, active adult with a fracture of the distal radius. For the multifragmentary distal radius being treated operatively, attempts are made to achieve reduction more accurate than this, but formal dorsal exposure or direct visualization of the joint surface via dorsal capsulotomy is carefully chosen based on age, activity level, and bone quality. Recent high-level evidence46 showed that closed treatment of unstable fractures of the distal radius results in good outcomes in the elderly. However, it is important to note that fractures displaced in a palmar direction and palmar shear patterns were excluded from that work. It is widely accepted that palmar carpal translation should be addressed with internal fixation, and specific attention must therefore be paid to the lunate facet as the cornerstone of the distal radius. Furthermore, high-energy comminuted fractures in young patients still necessitate internal fixation of fragments to restore alignment and articular congruity.
Conclusion
The importance of the palmar lunate facet in providing support and restraint to palmar carpal translation and the key role of this facet in restoring the anatomy of the distal radius have been known. This fragment deserves special attention because failure to adequately stabilize it results in loss of fixation and carpal subluxation. Various approaches and fixation techniques have been recommended, including the method we prefer and have described here. Our newly proposed term, sustentaculum lunatum, our review of its structure and function, and our descriptions of fixation techniques are intended to promote awareness of this fragment in the treatment of fractures of the distal radius.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
1. Jupiter JB. Fractures of the distal end of the radius. J Bone Joint Surg Am. 1991;73(3):461-469.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113-125.
4. Chung KC, Shauver MJ, Birkmeyer JD. Trends in the United States in the treatment of distal radial fractures in the elderly. J Bone Joint Surg Am. 2009;91(8):1868-1873.
5. Melone CP Jr. Articular fractures of the distal radius. Orthop Clin North Am. 1984;15(2):217-236.
6. Castaing J. Recent fractures of the lower extremity of the radius in adults [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1964;50:581-696.
7. Frykman G. Fracture of the distal radius including sequelae—shoulder-hand-finger syndrome, disturbance in the distal radio-ulnar joint and impairment of nerve function. A clinical and experimental study. Acta Orthop Scand. 1967;(suppl 108):3+.
8. Isani A, Melone CP Jr. Classification and management of intra-articular fractures of the distal radius. Hand Clin. 1988;4(3):349-360.
9. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
10. Rettig ME, Dassa GL, Raskin KB, Melone CP Jr. Wrist fractures in the athlete: distal radius and carpal fractures. Clin Sports Med. 1998;17(3):469-489.
11. Müller ME, Koch P, Nazarian S, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin, Germany: Springer-Verlag; 1990.
12. Peine R, Rikli DA, Hoffmann R, Duda G, Regazzoni P. Comparison of three different plating techniques for the dorsum of the distal radius: a biomechanical study. J Hand Surg Am. 2000;25(1):29-33.
13. Williams PL, Warwick R, Dyson M, Bannister LH, eds. Gray’s Anatomy. 37th ed. New York, NY: Churchill Livingstone; 1989.
14. Linscheid RL. Kinematic considerations of the wrist. Clin Orthop Relat Res. 1986;(202):27-39.
15. Mekhail AO, Ebraheim NA, McCreath WA, Jackson WT, Yeasting RA. Anatomic and x-ray film studies of the distal articular surface of the radius. J Hand Surg Am. 1996;21(4):567-573.
16. Schuind FA, Linscheid RL, An KN, Chao EY. A normal data base of posteroanterior roentgenographic measurements of the wrist. J Bone Joint Surg Am. 1992;74(9):1418-1429.
17. Schuind F, Alemzadeh S, Stallenberg B, Burny F. Does the normal contralateral wrist provide the best reference for x-ray film measurements of the pathologic wrist? J Hand Surg Am. 1996;21(1):24-30.
18. Genda E, Horii E. Theoretical stress analysis in wrist joint: neutral position and functional position. J Hand Surg Br. 2000;25(3):292-295.
19. Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck’s disease. J Hand Surg Br. 1997;22(1):16-20.
20. Andermahr J, Lozano-Calderon S, Trafton T, Crisco JJ, Ring D. The volar extension of the lunate facet of the distal radius: a quantitative anatomic study. J Hand Surg Am. 2006;31(6):892-895.
21. Bo WJ, Meschan I, Krueger WA. Basic Atlas of Cross-Sectional Anatomy. Philadelphia, PA: Saunders; 1980.
22. Cahill DR, Orland MJ, Miller GM. Atlas of Human Cross-Sectional Anatomy: With CT and MR Images. 3rd ed. New York, NY: Wiley; 1995.
23. El-Khoury GY, Bergman RA, Montgomery WJ. Sectional Anatomy by MRI. 2nd ed. New York, NY: Churchill Livingstone; 1995.
24. Harness NG, Jupiter JB, Orbay JL, Raskin KB, Fernandez DL. Loss of fixation of the volar lunate facet fragment in fractures of the distal part of the radius. J Bone Joint Surg Am. 2004;86(9):1900-1908.
25. Lewis OJ, Hamshere RJ, Bucknill TM. The anatomy of the wrist joint. J Anat. 1970;106(Pt 3):539-552.
26. Berger RA, Landsmeer JM. The palmar radiocarpal ligaments: a study of adult and fetal human wrist joints. J Hand Surg Am. 1990;15(6):847-854.
27. Apergis E, Darmanis S, Theodoratos G, Maris J. Beware of the ulno-palmar distal radial fragment. J Hand Surg Br. 2002;27(2):139-145.
28. Chang EY, Chen KC, Meunier MJ, Chung CB. Acute short radiolunate ligament rupture in a rock climber. Skeletal Radiol. 2014;43(2):235-238.
29. Souer JS, Wiggers J, Ring D. Quantitative 3-dimensional computed tomography measurement of volar shearing fractures of the distal radius. J Hand Surg Am. 2011;36(4):599-603.
30. Pruitt DL, Gilula LA, Manske PR, Vannier MW. Computed tomography scanning with image reconstruction in evaluation of distal radius fractures. J Hand Surg Am. 1994(5);19:720-727.
31. Goldwyn E, Pensy R, O’Toole RV, et al. Do traction radiographs of distal radial fractures influence fracture characterization and treatment? J Bone Joint Surg Am. 2012;94(22):2055-2062.
32. Glueck DA, Charoglu CP, Lawton JN. Factors associated with infection following open distal radius fractures. Hand. 2009;4(3):330-334.
33. Kurylo JC, Axelrad TW, Tornetta P 3rd, Jawa A. Open fractures of the distal radius: the effects of delayed debridement and immediate internal fixation on infection rates and the need for secondary procedures. J Hand Surg Am. 2011;36(7):1131-1134.
34. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
35. Rozental TD, Beredjiklian PK, Steinberg DR, Bozentka DJ. Open fractures of the distal radius. J Hand Surg Am. 2002;27(1):77-85.
36. Nana AD, Joshi A, Lichtman DM. Plating of the distal radius. J Am Acad Orthop Surg. 2005;13(3):159-171.
37. Bae DS, Koris MJ. Fragment-specific internal fixation of distal radius fractures. Hand Clin. 2005;21(3):355-362.
38. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
39. Henry AK. Extensile Exposure. 2nd ed. New York, NY: Churchill Livingstone; 1973.
40. Axelrod T, Paley D, Green J, McMurtry RY. Limited open reduction of the lunate facet in comminuted intra-articular fractures of the distal radius. J Hand Surg Am. 1988;13(3):372-377.
41. Chin KR, Jupiter JB. Wire-loop fixation of volar displaced osteochondral fractures of the distal radius. J Hand Surg Am. 1999;24(3):525-533.
42. Jupiter JB, Marent-Huber M; LCP Study Group. Operative management of distal radial fractures with 2.4-millimeter locking plates: a multicenter prospective case series. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1, pt 1):96-106.
43. Ruch DS, Yang C, Smith BP. Results of palmar plating of the lunate facet combined with external fixation for the treatment of high-energy compression fractures of the distal radius. J Orthop Trauma. 2004;18(1):28-33.
44. Wiesler ER, Chloros GD, Lucas RM, Kuzma GR. Arthroscopic management of volar lunate facet fractures of the distal radius. Tech Hand Up Extrem Surg. 2006;10(3):139-144.
45. American Academy of Orthopaedic Surgeons. The Treatment of Distal Radius Fractures: Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. http://www.aaos.org/research/guidelines/drfguideline.pdf. Accessed August 4, 2015.
46. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146-2153.
Med students: Look up from your EMRs
“I have acute appendicitis,” I told my wife. “I need to go to the radiologist.”
A computed tomography (CT) scan of my abdomen confirmed my suspicion. After learning that I also had leukocytosis, we headed to the emergency department. The ED doctor was pleasantly surprised that someone had come to his facility completely evaluated. All he had to do was call the surgeon. But first he introduced me to a 4th-year medical student who was participating in a surgical rotation.
Prioritizing the EMR over the patient
The student wheeled his large computer to the side of my gurney and began to question me about my abdominal pain. Within 5 minutes, this unsupervised student had somehow acquired all the information he needed for my admission. He thanked me for my time and told me that he would see me in the operating room.
Unfortunately for him, I was not about to let him leave my cubicle without a redirect. I told him I have type 1 diabetes and several comorbidities. I wear an insulin pump and continuous glucose sensor that alerts me to impending hypoglycemia. I take 11 medications to successfully manage my metabolic disorders.
The student wheeled his machine back to the side of my gurney.
With his eyes fixed squarely on his computer and his finger on a mouse, he asked me to list all of my medications. He had never heard of a rapid-acting insulin analogue, nor was he familiar with my GLP-1 receptor agonist or SGLT2 inhibitor. And the pump and sensor? There were no check boxes for these devices in his electronic medical record (EMR).
He—like several of the doctors I met during my subsequent stay—suggested that I remove the pump and meter so that they could manage my diabetes.
Still in considerable pain, I suggested to the student and anyone else who would listen that my pump and sensor were off limits. As long as I was conscious, I would self-manage my diabetes.
I also told him that his history and physical exam were deficient. Although he did listen to my bowel sounds (or lack thereof) through a blanket and hospital gown, he overlooked examining my heart, lungs, eyes, mouth, and feet.
“You failed to ask me about my medical history or my diabetes," I said. The student searched his EMR for the appropriate questions to ask, but to no avail. Stunned, he appeared to be at a loss of words. I suggested that he ask about the type of diabetes I had, the duration of the disease, how well my glucose levels were controlled, my most recent HbA1c, and if I had developed any long-term microvascular or macrovascular complications. He politely thanked me, moved the mouse around on his computer stand, and began to wheel his computer away.
“Wait!” I thought. “Don’t you think you should examine my eyes, mouth, and feet?” I reminded myself that this student hadn’t evaluated me for peritoneal signs. So why should I insist that he look at non-critical parts of my body?
My physical pain was increasing and I was becoming increasingly distressed. The student was more interested in inputting data into the EMR than learning about acute abdomens and type 1 diabetes.
Postop: From bad to worse
My postoperative course was dreadful. I nearly died from complications that included acute renal failure, dehydration, hypokalemia, and a postoperative ileus that persisted for 8 days. My blood glucose levels, however, were perfect. Still, the Attendings and the students blamed my complications on diabetes.
“Yeah, I see this all the time,” said the hospitalist who was caring for me. “Diabetes causes gastroparesis. What we should do is have you take off that pump and sensor device. We’ll have the pharmacist help you manage your diabetes.” The hospitalist who suggested this course of action was immediately relieved of his duties by my wife as I drifted in and out of consciousness in the intensive care unit (ICU).
Despite the state of my health, I began to provide professional guidance for my own care. I demanded that the nurse give me a 250 cc rider of normal saline and increase my IV flow rate from 50 cc to 150 cc. The nasogastric tube was removed and I began using IV erythromycin, which increases gastric motility. I received oral and IV potassium.
While in the ICU, I was questioned by physicians and medical students, but never examined. I am convinced that had I not been an experienced family physician, I would have suffered a fatal postoperative event. The medical students assigned to my care would not have known that I died, unless they received a notification via Twitter.
Providing care in a digital age
My experience as a patient was in stark contrast to the way I practice medicine.
I use an EMR only to e-prescribe, and have chosen not to participate in submitting meaningful use data to the government. Rather than spending 2 hours a day making eye contact with an EMR, I prefer to use that time to listen to my patients’ concerns about their health. I know how to conduct a review of systems and I touch my patients at each visit. I look at their feet, skin, and eyes, listen to their heart and lungs, and palpate their abdomen. I perform a rectal exam on every patient who presents with abdominal pain.
I have learned to communicate my suspicions and thoughts (both positive and negative) to all of my patients. I take notes on scratch paper, not on a computer, just as my grandfather and father used to do when they were practicing medicine. I only order tests to confirm a suspected diagnosis, not as a primary means of evaluating patients.
Could my hospital experience lead to change?
Upon my discharge from the hospital, I reached out to the director of clinical studies at the local medical school and explained the deficiencies I’d encountered. I explained that the 4th-year medical students were ill-equipped to perform an adequate history or physical exam. They lacked knowledge of basic pharmacology. And they failed to appropriately follow a patient during the perioperative period.
The director appreciated my concern and provided me with the details of a corrective action plan that she had been working on.
“We need to implement our patient simulation computer program designed to teach our students how to appropriately interact with their distressed patients,” she said.
Really?
I suggested that the medical students needed to unplug their smartphones, computers, and iPads. Let them spend a day or 2 with one of us “old-time docs” who still work with our hands—hands that are skilled at evaluating patients, rather than texting and data entry. We’ll show these students how to become caring, intelligent, and dedicated clinicians.
“I have acute appendicitis,” I told my wife. “I need to go to the radiologist.”
A computed tomography (CT) scan of my abdomen confirmed my suspicion. After learning that I also had leukocytosis, we headed to the emergency department. The ED doctor was pleasantly surprised that someone had come to his facility completely evaluated. All he had to do was call the surgeon. But first he introduced me to a 4th-year medical student who was participating in a surgical rotation.
Prioritizing the EMR over the patient
The student wheeled his large computer to the side of my gurney and began to question me about my abdominal pain. Within 5 minutes, this unsupervised student had somehow acquired all the information he needed for my admission. He thanked me for my time and told me that he would see me in the operating room.
Unfortunately for him, I was not about to let him leave my cubicle without a redirect. I told him I have type 1 diabetes and several comorbidities. I wear an insulin pump and continuous glucose sensor that alerts me to impending hypoglycemia. I take 11 medications to successfully manage my metabolic disorders.
The student wheeled his machine back to the side of my gurney.
With his eyes fixed squarely on his computer and his finger on a mouse, he asked me to list all of my medications. He had never heard of a rapid-acting insulin analogue, nor was he familiar with my GLP-1 receptor agonist or SGLT2 inhibitor. And the pump and sensor? There were no check boxes for these devices in his electronic medical record (EMR).
He—like several of the doctors I met during my subsequent stay—suggested that I remove the pump and meter so that they could manage my diabetes.
Still in considerable pain, I suggested to the student and anyone else who would listen that my pump and sensor were off limits. As long as I was conscious, I would self-manage my diabetes.
I also told him that his history and physical exam were deficient. Although he did listen to my bowel sounds (or lack thereof) through a blanket and hospital gown, he overlooked examining my heart, lungs, eyes, mouth, and feet.
“You failed to ask me about my medical history or my diabetes," I said. The student searched his EMR for the appropriate questions to ask, but to no avail. Stunned, he appeared to be at a loss of words. I suggested that he ask about the type of diabetes I had, the duration of the disease, how well my glucose levels were controlled, my most recent HbA1c, and if I had developed any long-term microvascular or macrovascular complications. He politely thanked me, moved the mouse around on his computer stand, and began to wheel his computer away.
“Wait!” I thought. “Don’t you think you should examine my eyes, mouth, and feet?” I reminded myself that this student hadn’t evaluated me for peritoneal signs. So why should I insist that he look at non-critical parts of my body?
My physical pain was increasing and I was becoming increasingly distressed. The student was more interested in inputting data into the EMR than learning about acute abdomens and type 1 diabetes.
Postop: From bad to worse
My postoperative course was dreadful. I nearly died from complications that included acute renal failure, dehydration, hypokalemia, and a postoperative ileus that persisted for 8 days. My blood glucose levels, however, were perfect. Still, the Attendings and the students blamed my complications on diabetes.
“Yeah, I see this all the time,” said the hospitalist who was caring for me. “Diabetes causes gastroparesis. What we should do is have you take off that pump and sensor device. We’ll have the pharmacist help you manage your diabetes.” The hospitalist who suggested this course of action was immediately relieved of his duties by my wife as I drifted in and out of consciousness in the intensive care unit (ICU).
Despite the state of my health, I began to provide professional guidance for my own care. I demanded that the nurse give me a 250 cc rider of normal saline and increase my IV flow rate from 50 cc to 150 cc. The nasogastric tube was removed and I began using IV erythromycin, which increases gastric motility. I received oral and IV potassium.
While in the ICU, I was questioned by physicians and medical students, but never examined. I am convinced that had I not been an experienced family physician, I would have suffered a fatal postoperative event. The medical students assigned to my care would not have known that I died, unless they received a notification via Twitter.
Providing care in a digital age
My experience as a patient was in stark contrast to the way I practice medicine.
I use an EMR only to e-prescribe, and have chosen not to participate in submitting meaningful use data to the government. Rather than spending 2 hours a day making eye contact with an EMR, I prefer to use that time to listen to my patients’ concerns about their health. I know how to conduct a review of systems and I touch my patients at each visit. I look at their feet, skin, and eyes, listen to their heart and lungs, and palpate their abdomen. I perform a rectal exam on every patient who presents with abdominal pain.
I have learned to communicate my suspicions and thoughts (both positive and negative) to all of my patients. I take notes on scratch paper, not on a computer, just as my grandfather and father used to do when they were practicing medicine. I only order tests to confirm a suspected diagnosis, not as a primary means of evaluating patients.
Could my hospital experience lead to change?
Upon my discharge from the hospital, I reached out to the director of clinical studies at the local medical school and explained the deficiencies I’d encountered. I explained that the 4th-year medical students were ill-equipped to perform an adequate history or physical exam. They lacked knowledge of basic pharmacology. And they failed to appropriately follow a patient during the perioperative period.
The director appreciated my concern and provided me with the details of a corrective action plan that she had been working on.
“We need to implement our patient simulation computer program designed to teach our students how to appropriately interact with their distressed patients,” she said.
Really?
I suggested that the medical students needed to unplug their smartphones, computers, and iPads. Let them spend a day or 2 with one of us “old-time docs” who still work with our hands—hands that are skilled at evaluating patients, rather than texting and data entry. We’ll show these students how to become caring, intelligent, and dedicated clinicians.
“I have acute appendicitis,” I told my wife. “I need to go to the radiologist.”
A computed tomography (CT) scan of my abdomen confirmed my suspicion. After learning that I also had leukocytosis, we headed to the emergency department. The ED doctor was pleasantly surprised that someone had come to his facility completely evaluated. All he had to do was call the surgeon. But first he introduced me to a 4th-year medical student who was participating in a surgical rotation.
Prioritizing the EMR over the patient
The student wheeled his large computer to the side of my gurney and began to question me about my abdominal pain. Within 5 minutes, this unsupervised student had somehow acquired all the information he needed for my admission. He thanked me for my time and told me that he would see me in the operating room.
Unfortunately for him, I was not about to let him leave my cubicle without a redirect. I told him I have type 1 diabetes and several comorbidities. I wear an insulin pump and continuous glucose sensor that alerts me to impending hypoglycemia. I take 11 medications to successfully manage my metabolic disorders.
The student wheeled his machine back to the side of my gurney.
With his eyes fixed squarely on his computer and his finger on a mouse, he asked me to list all of my medications. He had never heard of a rapid-acting insulin analogue, nor was he familiar with my GLP-1 receptor agonist or SGLT2 inhibitor. And the pump and sensor? There were no check boxes for these devices in his electronic medical record (EMR).
He—like several of the doctors I met during my subsequent stay—suggested that I remove the pump and meter so that they could manage my diabetes.
Still in considerable pain, I suggested to the student and anyone else who would listen that my pump and sensor were off limits. As long as I was conscious, I would self-manage my diabetes.
I also told him that his history and physical exam were deficient. Although he did listen to my bowel sounds (or lack thereof) through a blanket and hospital gown, he overlooked examining my heart, lungs, eyes, mouth, and feet.
“You failed to ask me about my medical history or my diabetes," I said. The student searched his EMR for the appropriate questions to ask, but to no avail. Stunned, he appeared to be at a loss of words. I suggested that he ask about the type of diabetes I had, the duration of the disease, how well my glucose levels were controlled, my most recent HbA1c, and if I had developed any long-term microvascular or macrovascular complications. He politely thanked me, moved the mouse around on his computer stand, and began to wheel his computer away.
“Wait!” I thought. “Don’t you think you should examine my eyes, mouth, and feet?” I reminded myself that this student hadn’t evaluated me for peritoneal signs. So why should I insist that he look at non-critical parts of my body?
My physical pain was increasing and I was becoming increasingly distressed. The student was more interested in inputting data into the EMR than learning about acute abdomens and type 1 diabetes.
Postop: From bad to worse
My postoperative course was dreadful. I nearly died from complications that included acute renal failure, dehydration, hypokalemia, and a postoperative ileus that persisted for 8 days. My blood glucose levels, however, were perfect. Still, the Attendings and the students blamed my complications on diabetes.
“Yeah, I see this all the time,” said the hospitalist who was caring for me. “Diabetes causes gastroparesis. What we should do is have you take off that pump and sensor device. We’ll have the pharmacist help you manage your diabetes.” The hospitalist who suggested this course of action was immediately relieved of his duties by my wife as I drifted in and out of consciousness in the intensive care unit (ICU).
Despite the state of my health, I began to provide professional guidance for my own care. I demanded that the nurse give me a 250 cc rider of normal saline and increase my IV flow rate from 50 cc to 150 cc. The nasogastric tube was removed and I began using IV erythromycin, which increases gastric motility. I received oral and IV potassium.
While in the ICU, I was questioned by physicians and medical students, but never examined. I am convinced that had I not been an experienced family physician, I would have suffered a fatal postoperative event. The medical students assigned to my care would not have known that I died, unless they received a notification via Twitter.
Providing care in a digital age
My experience as a patient was in stark contrast to the way I practice medicine.
I use an EMR only to e-prescribe, and have chosen not to participate in submitting meaningful use data to the government. Rather than spending 2 hours a day making eye contact with an EMR, I prefer to use that time to listen to my patients’ concerns about their health. I know how to conduct a review of systems and I touch my patients at each visit. I look at their feet, skin, and eyes, listen to their heart and lungs, and palpate their abdomen. I perform a rectal exam on every patient who presents with abdominal pain.
I have learned to communicate my suspicions and thoughts (both positive and negative) to all of my patients. I take notes on scratch paper, not on a computer, just as my grandfather and father used to do when they were practicing medicine. I only order tests to confirm a suspected diagnosis, not as a primary means of evaluating patients.
Could my hospital experience lead to change?
Upon my discharge from the hospital, I reached out to the director of clinical studies at the local medical school and explained the deficiencies I’d encountered. I explained that the 4th-year medical students were ill-equipped to perform an adequate history or physical exam. They lacked knowledge of basic pharmacology. And they failed to appropriately follow a patient during the perioperative period.
The director appreciated my concern and provided me with the details of a corrective action plan that she had been working on.
“We need to implement our patient simulation computer program designed to teach our students how to appropriately interact with their distressed patients,” she said.
Really?
I suggested that the medical students needed to unplug their smartphones, computers, and iPads. Let them spend a day or 2 with one of us “old-time docs” who still work with our hands—hands that are skilled at evaluating patients, rather than texting and data entry. We’ll show these students how to become caring, intelligent, and dedicated clinicians.
Perilunate Injuries
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
Perilunate injuries typically stem from a high-energy insult to the carpus. Because of their relative infrequency and often subtle radiographic and physical examination findings, these injuries are often undetected in the emergency department setting.1 Early anatomic reduction of any carpal malalignment is essential. Even with optimal treatment, complications such as generalized wrist stiffness, diminished grip strength, and posttraumatic arthritis, commonly develop; however, recent studies suggest these issues are often well tolerated.1-5 In this article, the diagnosis, treatment, and outcomes after perilunate injuries are examined.
History and Physical Examination
Perilunate injuries result from high-energy trauma to the carpus. Patients with these injuries often present with vague wrist pain and loss of wrist motion. Their fingers are frequently held in slight flexion. The patient may complain of numbness and tingling in the median nerve distribution. An acute carpal tunnel syndrome can rapidly develop. The general belief is that acute carpal tunnel syndrome occurs more commonly in pure volar lunate dislocations than in dorsal perilunate dislocations. However, no studies compare the incidence of acute carpal tunnel syndrome in lunate versus perilunate dislocations.
Radiographic Evaluation
Standard radiographic evaluation of a potential perilunate injury includes posteroanterior (PA), lateral, and oblique views of the wrist (Figure 1). A scaphoid view (ie, PA view with the wrist in ulnar deviation) may also be helpful. The PA view is particularly helpful because it enables assessment of Gilula lines, which are imaginary lines drawn across the proximal and distal aspects of the proximal carpal row and the proximal aspect of the distal carpal row. These lines should appear as 3 smooth arcs running nearly parallel to each other.6 Any disruption in these lines suggests carpal incongruity. It may be possible to note a triangular-shaped lunate on the PA view, which is a sign of lunate dislocation.7
While the PA view is certainly useful, the lateral view is the most important in diagnosing a perilunate injury. The lateral view allows assessment of the collinearity of radius, lunate, and capitate. Any disruption in this collinearity strongly suggests a perilunate dislocation.7,8
Classification
Mayfield and colleagues9,10 described 4 stages of perilunate instability proceeding from a radial to an ulnar direction around the lunate. Stage I involves disruption of the scapholunate joint, while stage II involves both the scapholunate and capitolunate joints. In stage III, the scapholunate, capitolunate, and lunotriquetral ligaments are disrupted, and the result is a perilunate dislocation, usually dorsal. Finally, in stage IV, all the ligaments surrounding the lunate are disrupted and the lunate dislocates, most often volarly.
Lastly, perilunate injuries can be classified as greater-arc injuries if concomitant fracture of the carpus occurs, lesser-arc injuries if the injury is purely ligamentous, or inferior-arc injuries if there is an associated fracture of the volar rim of the distal radius.8
Treatment
Closed Reduction
All acute perilunate dislocations should be managed initially with an attempted closed reduction.11 If the injury is older than 72 hours, such an attempt may be futile. For any closed reduction performed in the emergency department setting, intravenous sedation is generally advised for muscle relaxation. Gentle traction with finger traps can also be used prior to the reduction attempt. For a dorsal perilunate dislocation, longitudinal traction followed by volar flexion of the wrist with volar pressure on the lunate and dorsal pressure on the capitate (ie, Tavernier’s maneuver) is required. Once reduction is complete, PA and lateral views of the wrist should be obtained to assess carpal alignment. If closed reduction is unsuccessful, an open reduction is required, although the timing of said procedure is an area of debate, which we will discuss later.1,3 Restoration of anatomic carpal alignment is essential to optimizing outcome, although it does not guarantee a good overall result.
Open Reduction
If successful closed reduction is achieved, the patient can be immobilized temporarily in a short-arm plaster splint. However, open reduction and either pinning or internal fixation will be required to maintain this alignment. The exact timing of open reduction and fixation is debatable and often dictated by the absence or presence of median nerve symptoms.1,3 If a patient with no median nerve symptoms undergoes a successful closed reduction, he or she may be stabilized surgically within 3 to 5 days (or longer) with either pins or headless screws. If closed reduction is unsuccessful, an open reduction should be done within 2 to 3 days. However, if the patient has progressive numbness in the median nerve distribution upon presentation that fails to improve or worsens despite a successful closed reduction, an urgent open reduction (within 24 hours) and carpal tunnel release should be performed to prevent permanent damage to the median nerve.
Once open reduction is undertaken, a dorsal, volar, and combined approach can be used.2-4 In most cases the dorsal approach is selected first. A longitudinal incision is made over the dorsum of the wrist, centered on the Lister tubercle. Dissection occurs between the third and fourth dorsal compartments. After the capsule is exposed, reduction of the lunate to the capitate is confirmed. If any fractures are present in the carpus (eg, scaphoid), they are internally fixed. The scapholunate articulation is then addressed. In general, the scapholunate ligament is not disrupted with a transscaphoid perilunate dislocation. However, if the scapholunate ligament is disrupted, the joint should be reduced and pinned. Repair or reconstruction of the scapholunate ligament is performed. Finally, the lunotriquetral articulation is reduced and stabilized with pins. There are no studies that specifically suggest direct repair of the lunotriquetral ligament versus pinning of the lunotriquetral articulation, but the lunotriquetral ligament could be repaired in similar fashion to the scapholunate ligament at the surgeon’s discretion.
As an alternative to percutaneous pinning, intercarpal screw fixation can be used to stabilize the carpus. A 2007 study by Souer and colleagues12 showed no substantial difference in outcome between the 2 methods of fixation. However, a second procedure is required to remove the screws.
The volar approach, if selected, is typically done second and performed via an extended carpal tunnel incision. It allows decompression of the carpal tunnel and enables repair of volar capsular ligaments (ie, long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament), which increases overall carpal stability. Currently, many surgeons favor a combined dorsal-volar approach for its efficacy.2,3 Some use a dorsal approach in all patients and perform a volar procedure only if the patient has median nerve symptoms.4 However, Başar and colleagues13 report use of only the volar approach for treatment of perilunate injuries. The authors repaired the long and short radiolunate ligaments, volar scapholunate ligament, and volar lunotriquetral ligament. They reported reasonably good outcomes, which are equivalent to those reported in similar studies using dorsal or combined dorsal-volar approaches. However, no studies in the literature directly compare any of the different approaches with each other.
Postoperatively, patients are placed in a long-arm thumb-spica cast for 4 weeks, and then in a short-arm cast for 4 to 8 weeks (Figure 2). If present, pins are removed in 3 to 12 weeks, with most authors recommending removal at 8 weeks.2,14
Lastly, carpal tunnel symptoms can develop late and even after a successful reduction and surgical stabilization. One theory is that a significant perilunate injury can create slightly higher baseline carpal tunnel pressures, which can compromise the blood flow to the median nerve and cause carpal tunnel symptoms. Additionally, it is possible that direct median nerve contusion and/or traction injury via a displaced lunate can also cause these symptoms. Whatever the inciting cause of median-nerve irritation, a delayed carpal tunnel release is sometimes required.
Conclusion
Outcomes after either perilunate or lunate dislocation are fair to good at best but can be optimized with prompt, appropriate treatment. Closed reduction and casting as definitive treatment has been abandoned because of frequent loss of reduction.12 Early open reduction (ie, less than 3 days after injury) has been shown to be beneficial.1,2 However, even those treated early and with anatomic restoration of carpal alignment can expect a loss of grip strength and a range of motion of approximately 70% compared with the contralateral side.2-5 A recent study has suggested that lesser-arc injures generally have a poorer overall outcome than their greater-arc counterparts.15
More than half of all patients with perilunate injuries will develop radiographic signs of osteoarthritis, and some will require additional salvage procedures.3-5 Kremer and colleagues4 showed that overall results after perilunate injuries deteriorate with time. However, according to a paper by Forli and colleagues5 in which patients were followed a minimum of 10 years after their injuries, the authors found that, despite radiographic progression of arthritis, most patients maintained reasonable hand function.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.
1. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
2. Sotereanos DG, Mitsionis GJ, Giannakopoulos PN, Tomaino MM, Herndon JH. Perilunate dislocation and fracture dislocation: a critical analysis of the volar-dorsal approach. J Hand Surg Am. 1997;22(1):49-56.
3. Hildebrand KA, Ross DC, Patterson SD, Roth JH, MacDermid JC, King GJ. Dorsal perilunate dislocations and fracture-dislocations: questionnaire, clinical, and radiographic evaluation. J Hand Surg Am. 2000;25(6):1069-1079.
4. Kremer T, Wendt M, Riedel K, Sauerbier M, Germann G, Bickert B. Open reduction for perilunate injuries--clinical outcome and patient satisfaction. J Hand Surg Am. 2010;35(10):1599-1606.
5. Forli A, Courvoisier A, Wimsey S, Corcella D, Moutet F. Perilunate dislocations and transscaphoid perilunate fracture-dislocations: a retrospective study with minimum ten-year follow-up. J Hand Surg Am. 2010;35(1):62-68.
6. Gilula LA. Carpal injuries: analytic approach and case exercises. AJR Am J Roentgenol. 1979;133(3):503-517.
7. Kozin SH. Perilunate injuries: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(2):114-120.
8. Graham TJ. The inferior arc injury: an addition to the family of complex carpal fracture-dislocation patterns. Am J Orthop. 2003;32(9 suppl):10-19.
9. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
10. Mayfield JK. Mechanism of carpal injuries. Clin Orthop Relat Res. 1980;149:45-54.
11. Adkison JW, Chapman MW. Treatment of acute lunate and perilunate dislocations. Clin Orthop Relat Res. 1982;164:199-207.
12. Souer JS, Rutgers M, Andermahr J, Jupiter JB, Ring D. Perilunate fracture-dislocations of the wrist: comparison of temporary screw versus K-wire fixation. J Hand Surg Am. 2007;32(3):318-325.
13. Başar H, Başar B, Erol B, Tetik C. Isolated volar surgical approach for the treatment of perilunate and lunate dislocations. Indian J Orthop. 2014;48(3):301-315.
14. Komurcu M, Kürklü M, Ozturan KE, Mahirogullari M, Basbozkurt M. Early and delayed treatment of dorsal transscaphoid perilunate fracture-dislocations. J Orthop Trauma. 2008;22:535-540.
15. Massoud AH, Naam NH. Functional outcome of open reduction of chronic perilunate injuries. J Hand Surg Am. 2012;37(9):1852-1860.