User login
Patient Adherence to Pharmacological Thromboprophylaxis Improves with Interventions
Clinical question: How can patient adherence to pharmacological thromboprophylaxis be improved?
Background: Prior studies suggest that the hospital-wide prevalence of nonadministration of VTE thromboprophylaxis orders ranges from 5% to 13%, with patient refusal listed as the most common reason for nonadministration.
Study design: Quasi-experimental, pre-post intervention, with intervention and control units.
Setting: Academic medical center in Philadelphia.
Synopsis: Researchers identified 20,208 admissions for the study; 8,293 (41%) admissions occurred prior to the intervention and 11,915 (59%) after. The three-part intervention, which was composed of (1) standardized nurse response to patient refusal, (2) integration of daily assessment of VTE into rounds, and (3) regular audit with feedback, resulted in a decrease in nonadministration rates during the intervention. Rates continued to decline in the 21-month follow-up period.
After the intervention, the rate of missed doses of pharmacological thromboprophylaxis decreased from 24.7% to 14.7% (P<0.01). This was due to a decrease in patient refusal from 18.3% to 9.4% (P<0.01).
Although there was a decrease in the missed doses of thromboprophylaxis, there was no statistically significant change in the rate of hospital-associated VTE.
Bottom line: A multifaceted intervention resulted in a decrease in the proportion of missed and refused doses of pharmacological VTE thromboprophylaxis, but this was not associated with a statistically significant change in VTE rates.
Citation: Baillie CA, Guevara JP, Boston RC, Hecht TE. A unit-based intervention aimed at improving patient adherence to pharmacological thromboprophylaxis [published online ahead of print June 2, 2015]. BMJ Qual Saf. doi:10.1136/bmjqs-2015-003992.
Clinical question: How can patient adherence to pharmacological thromboprophylaxis be improved?
Background: Prior studies suggest that the hospital-wide prevalence of nonadministration of VTE thromboprophylaxis orders ranges from 5% to 13%, with patient refusal listed as the most common reason for nonadministration.
Study design: Quasi-experimental, pre-post intervention, with intervention and control units.
Setting: Academic medical center in Philadelphia.
Synopsis: Researchers identified 20,208 admissions for the study; 8,293 (41%) admissions occurred prior to the intervention and 11,915 (59%) after. The three-part intervention, which was composed of (1) standardized nurse response to patient refusal, (2) integration of daily assessment of VTE into rounds, and (3) regular audit with feedback, resulted in a decrease in nonadministration rates during the intervention. Rates continued to decline in the 21-month follow-up period.
After the intervention, the rate of missed doses of pharmacological thromboprophylaxis decreased from 24.7% to 14.7% (P<0.01). This was due to a decrease in patient refusal from 18.3% to 9.4% (P<0.01).
Although there was a decrease in the missed doses of thromboprophylaxis, there was no statistically significant change in the rate of hospital-associated VTE.
Bottom line: A multifaceted intervention resulted in a decrease in the proportion of missed and refused doses of pharmacological VTE thromboprophylaxis, but this was not associated with a statistically significant change in VTE rates.
Citation: Baillie CA, Guevara JP, Boston RC, Hecht TE. A unit-based intervention aimed at improving patient adherence to pharmacological thromboprophylaxis [published online ahead of print June 2, 2015]. BMJ Qual Saf. doi:10.1136/bmjqs-2015-003992.
Clinical question: How can patient adherence to pharmacological thromboprophylaxis be improved?
Background: Prior studies suggest that the hospital-wide prevalence of nonadministration of VTE thromboprophylaxis orders ranges from 5% to 13%, with patient refusal listed as the most common reason for nonadministration.
Study design: Quasi-experimental, pre-post intervention, with intervention and control units.
Setting: Academic medical center in Philadelphia.
Synopsis: Researchers identified 20,208 admissions for the study; 8,293 (41%) admissions occurred prior to the intervention and 11,915 (59%) after. The three-part intervention, which was composed of (1) standardized nurse response to patient refusal, (2) integration of daily assessment of VTE into rounds, and (3) regular audit with feedback, resulted in a decrease in nonadministration rates during the intervention. Rates continued to decline in the 21-month follow-up period.
After the intervention, the rate of missed doses of pharmacological thromboprophylaxis decreased from 24.7% to 14.7% (P<0.01). This was due to a decrease in patient refusal from 18.3% to 9.4% (P<0.01).
Although there was a decrease in the missed doses of thromboprophylaxis, there was no statistically significant change in the rate of hospital-associated VTE.
Bottom line: A multifaceted intervention resulted in a decrease in the proportion of missed and refused doses of pharmacological VTE thromboprophylaxis, but this was not associated with a statistically significant change in VTE rates.
Citation: Baillie CA, Guevara JP, Boston RC, Hecht TE. A unit-based intervention aimed at improving patient adherence to pharmacological thromboprophylaxis [published online ahead of print June 2, 2015]. BMJ Qual Saf. doi:10.1136/bmjqs-2015-003992.
Mortality Risk in Patients Older than 75 Presenting with Non-ST-Elevation Acute Coronary Syndrome
Clinical question: Is there a score that will predict the mortality rate in elderly patients presenting with a non-ST-elevation myocardial infarction (NSTEMI)?
Background: Although they represent only 9% of patients in clinical trials, patients over the age of 75 make up one third of patients with NSTEMI, accounting for more than half of NSTEMI-related mortality.
Study design: Retrospective cohort analysis for score calculator design, with prospective cohort validation.
Setting: The retrospective cohort was derived from a meta-analysis of 55 papers. The prospective validation arm used a cohort of patients from a randomized multicenter Italian trial.
Synopsis: The authors developed and validated a mortality predictor for patients 75 and older who present with an NSTEMI. The calculator: hemoglobin less than 10 g/dl (two points), elevated troponin levels, ECG ischemic changes, estimated glomerular filtration rate (eGFR) less than 45, previous vascular event (one point each two). The calculator predicted probabilities of death in one year ranging from 2% (score of zero) to 75% (score of six). The calculator allowed stratification into low (score: zero to one), intermediate (score: two), or high (score: three or greater) risk. High-risk patients appeared to benefit from intervention with significantly reduced risk for mortality (odds ratio 0.44).
Bottom line: A simple risk calculator stratifies elderly patients into low, intermediate, or high risk to predict mortality from NSTEMI. High-risk patients appear to achieve a mortality benefit from intervention.
Citation: Angeli F, Cavallini C, Verdecchia P, et al. A risk score for predicting 1-year mortality in patients ≥75 years of age presenting with non-ST-elevation acute coronary syndrome. Am J Cardiol. 2015;116(2):208-213.
Clinical question: Is there a score that will predict the mortality rate in elderly patients presenting with a non-ST-elevation myocardial infarction (NSTEMI)?
Background: Although they represent only 9% of patients in clinical trials, patients over the age of 75 make up one third of patients with NSTEMI, accounting for more than half of NSTEMI-related mortality.
Study design: Retrospective cohort analysis for score calculator design, with prospective cohort validation.
Setting: The retrospective cohort was derived from a meta-analysis of 55 papers. The prospective validation arm used a cohort of patients from a randomized multicenter Italian trial.
Synopsis: The authors developed and validated a mortality predictor for patients 75 and older who present with an NSTEMI. The calculator: hemoglobin less than 10 g/dl (two points), elevated troponin levels, ECG ischemic changes, estimated glomerular filtration rate (eGFR) less than 45, previous vascular event (one point each two). The calculator predicted probabilities of death in one year ranging from 2% (score of zero) to 75% (score of six). The calculator allowed stratification into low (score: zero to one), intermediate (score: two), or high (score: three or greater) risk. High-risk patients appeared to benefit from intervention with significantly reduced risk for mortality (odds ratio 0.44).
Bottom line: A simple risk calculator stratifies elderly patients into low, intermediate, or high risk to predict mortality from NSTEMI. High-risk patients appear to achieve a mortality benefit from intervention.
Citation: Angeli F, Cavallini C, Verdecchia P, et al. A risk score for predicting 1-year mortality in patients ≥75 years of age presenting with non-ST-elevation acute coronary syndrome. Am J Cardiol. 2015;116(2):208-213.
Clinical question: Is there a score that will predict the mortality rate in elderly patients presenting with a non-ST-elevation myocardial infarction (NSTEMI)?
Background: Although they represent only 9% of patients in clinical trials, patients over the age of 75 make up one third of patients with NSTEMI, accounting for more than half of NSTEMI-related mortality.
Study design: Retrospective cohort analysis for score calculator design, with prospective cohort validation.
Setting: The retrospective cohort was derived from a meta-analysis of 55 papers. The prospective validation arm used a cohort of patients from a randomized multicenter Italian trial.
Synopsis: The authors developed and validated a mortality predictor for patients 75 and older who present with an NSTEMI. The calculator: hemoglobin less than 10 g/dl (two points), elevated troponin levels, ECG ischemic changes, estimated glomerular filtration rate (eGFR) less than 45, previous vascular event (one point each two). The calculator predicted probabilities of death in one year ranging from 2% (score of zero) to 75% (score of six). The calculator allowed stratification into low (score: zero to one), intermediate (score: two), or high (score: three or greater) risk. High-risk patients appeared to benefit from intervention with significantly reduced risk for mortality (odds ratio 0.44).
Bottom line: A simple risk calculator stratifies elderly patients into low, intermediate, or high risk to predict mortality from NSTEMI. High-risk patients appear to achieve a mortality benefit from intervention.
Citation: Angeli F, Cavallini C, Verdecchia P, et al. A risk score for predicting 1-year mortality in patients ≥75 years of age presenting with non-ST-elevation acute coronary syndrome. Am J Cardiol. 2015;116(2):208-213.
An Ancient Recipe to Cure a Modern Pathogen

At the Society for General Microbiology Annual Conference (March 30–April 2, 2015), Harrison et al presented a paper describing their experience with a 1000-year-old antimicrobial remedy with antistaphylococcal activity (S19We1006). The unique team of investigators—consisting of an expert in Viking studies and 3 microbiologists from the University of Nottingham, England, and another microbiologist who performs mouse model studies from Texas Tech University (Lubbock, Texas)—pooled their talents to reconstruct and test an ancient recipe for treating eyelash follicle infection. The potion not only killed methicillin-resistant Staphylococcus aureus (MRSA) grown in established biofilms but also performed as good, if not better, than the conventional antibiotics on MRSA-infected skin wounds in mice.
“Take cropleek and garlic, of both equal quantities, pound them well together . . . take wine and bullocks gall, mix with the leek . . . let it stand 9 days in the brass vessel” is an excerpt of the recipe for treatment of a sty from the 9th century Anglo-Saxon text Bald’s Leechbook (translated from Old English). The modern day chefs cooked their potion using: (1) 2 Allium species (equal amounts of garlic and either leek or onion, finely chopped and crushed in a mortar for 2 minutes), (2) wine (add 25 mL [0.87 fl oz] of an organic vintage from a historic English vineyard near Glastonbury), (3) bullock gall (dissolve bile from a cow’s stomach in distilled water), and (4) brass (glass bottles with squares of brass sheets immersed in the mixture were used because a brass vessel would be not only hard to sterilize but also expensive). After brewing (in the “brass vessel”), the solution was purified by straining it and left to chill at 4oC for 9 days before the mixture was used.
What’s the issue?
Advances in technology provide the opportunity to create new medical treatments. However, efficacious therapies may remain hidden in ancient texts, waiting to be discovered. Historic Chinese literature has been the source for modern day drugs such as artemisinin for Plasmodium falciparum malaria. The ancient recipe in Bald’s Leechbook may be the next major advance in the topical management of MRSA. I anticipate, in the future, that physicians may be prescribing “Bald’s potion” to treat impetigo. What do you think?
We want to know your views! Tell us what you think.
Suggested Readings
- AncientBiotics—a medieval remedy for modern day superbugs [news release]? United Kingdom: The University of Nottingham; March 30, 2015. http://www.nottingham.ac.uk/news/pressreleases/2015/march/ancientbiotics---a-medieval-remedy-for-modern-day-superbugs.aspx. Accessed August 12, 2015.
- Feilden T. 1,000-year-old onion and garlic eye remedy kills MRSA. BBC News. March 30, 2015. http://www.bbc.com/news/uk-england-nottinghamshire-32117815. Accessed August 12, 2015.
- Wilson C. Anglo-Saxon remedy kills hospital superbug MRSA. New Scientist. March 30, 2015. http://www.newscientist.com/article/dn27263-anglosaxon-remedy-kills-hospital-superbug-mrsa.html#.VTPpsqazDzl. Accessed August 12, 2015.

At the Society for General Microbiology Annual Conference (March 30–April 2, 2015), Harrison et al presented a paper describing their experience with a 1000-year-old antimicrobial remedy with antistaphylococcal activity (S19We1006). The unique team of investigators—consisting of an expert in Viking studies and 3 microbiologists from the University of Nottingham, England, and another microbiologist who performs mouse model studies from Texas Tech University (Lubbock, Texas)—pooled their talents to reconstruct and test an ancient recipe for treating eyelash follicle infection. The potion not only killed methicillin-resistant Staphylococcus aureus (MRSA) grown in established biofilms but also performed as good, if not better, than the conventional antibiotics on MRSA-infected skin wounds in mice.
“Take cropleek and garlic, of both equal quantities, pound them well together . . . take wine and bullocks gall, mix with the leek . . . let it stand 9 days in the brass vessel” is an excerpt of the recipe for treatment of a sty from the 9th century Anglo-Saxon text Bald’s Leechbook (translated from Old English). The modern day chefs cooked their potion using: (1) 2 Allium species (equal amounts of garlic and either leek or onion, finely chopped and crushed in a mortar for 2 minutes), (2) wine (add 25 mL [0.87 fl oz] of an organic vintage from a historic English vineyard near Glastonbury), (3) bullock gall (dissolve bile from a cow’s stomach in distilled water), and (4) brass (glass bottles with squares of brass sheets immersed in the mixture were used because a brass vessel would be not only hard to sterilize but also expensive). After brewing (in the “brass vessel”), the solution was purified by straining it and left to chill at 4oC for 9 days before the mixture was used.
What’s the issue?
Advances in technology provide the opportunity to create new medical treatments. However, efficacious therapies may remain hidden in ancient texts, waiting to be discovered. Historic Chinese literature has been the source for modern day drugs such as artemisinin for Plasmodium falciparum malaria. The ancient recipe in Bald’s Leechbook may be the next major advance in the topical management of MRSA. I anticipate, in the future, that physicians may be prescribing “Bald’s potion” to treat impetigo. What do you think?
We want to know your views! Tell us what you think.
Suggested Readings
- AncientBiotics—a medieval remedy for modern day superbugs [news release]? United Kingdom: The University of Nottingham; March 30, 2015. http://www.nottingham.ac.uk/news/pressreleases/2015/march/ancientbiotics---a-medieval-remedy-for-modern-day-superbugs.aspx. Accessed August 12, 2015.
- Feilden T. 1,000-year-old onion and garlic eye remedy kills MRSA. BBC News. March 30, 2015. http://www.bbc.com/news/uk-england-nottinghamshire-32117815. Accessed August 12, 2015.
- Wilson C. Anglo-Saxon remedy kills hospital superbug MRSA. New Scientist. March 30, 2015. http://www.newscientist.com/article/dn27263-anglosaxon-remedy-kills-hospital-superbug-mrsa.html#.VTPpsqazDzl. Accessed August 12, 2015.

At the Society for General Microbiology Annual Conference (March 30–April 2, 2015), Harrison et al presented a paper describing their experience with a 1000-year-old antimicrobial remedy with antistaphylococcal activity (S19We1006). The unique team of investigators—consisting of an expert in Viking studies and 3 microbiologists from the University of Nottingham, England, and another microbiologist who performs mouse model studies from Texas Tech University (Lubbock, Texas)—pooled their talents to reconstruct and test an ancient recipe for treating eyelash follicle infection. The potion not only killed methicillin-resistant Staphylococcus aureus (MRSA) grown in established biofilms but also performed as good, if not better, than the conventional antibiotics on MRSA-infected skin wounds in mice.
“Take cropleek and garlic, of both equal quantities, pound them well together . . . take wine and bullocks gall, mix with the leek . . . let it stand 9 days in the brass vessel” is an excerpt of the recipe for treatment of a sty from the 9th century Anglo-Saxon text Bald’s Leechbook (translated from Old English). The modern day chefs cooked their potion using: (1) 2 Allium species (equal amounts of garlic and either leek or onion, finely chopped and crushed in a mortar for 2 minutes), (2) wine (add 25 mL [0.87 fl oz] of an organic vintage from a historic English vineyard near Glastonbury), (3) bullock gall (dissolve bile from a cow’s stomach in distilled water), and (4) brass (glass bottles with squares of brass sheets immersed in the mixture were used because a brass vessel would be not only hard to sterilize but also expensive). After brewing (in the “brass vessel”), the solution was purified by straining it and left to chill at 4oC for 9 days before the mixture was used.
What’s the issue?
Advances in technology provide the opportunity to create new medical treatments. However, efficacious therapies may remain hidden in ancient texts, waiting to be discovered. Historic Chinese literature has been the source for modern day drugs such as artemisinin for Plasmodium falciparum malaria. The ancient recipe in Bald’s Leechbook may be the next major advance in the topical management of MRSA. I anticipate, in the future, that physicians may be prescribing “Bald’s potion” to treat impetigo. What do you think?
We want to know your views! Tell us what you think.
Suggested Readings
- AncientBiotics—a medieval remedy for modern day superbugs [news release]? United Kingdom: The University of Nottingham; March 30, 2015. http://www.nottingham.ac.uk/news/pressreleases/2015/march/ancientbiotics---a-medieval-remedy-for-modern-day-superbugs.aspx. Accessed August 12, 2015.
- Feilden T. 1,000-year-old onion and garlic eye remedy kills MRSA. BBC News. March 30, 2015. http://www.bbc.com/news/uk-england-nottinghamshire-32117815. Accessed August 12, 2015.
- Wilson C. Anglo-Saxon remedy kills hospital superbug MRSA. New Scientist. March 30, 2015. http://www.newscientist.com/article/dn27263-anglosaxon-remedy-kills-hospital-superbug-mrsa.html#.VTPpsqazDzl. Accessed August 12, 2015.
Family Physicians Propose Payment for PCPs’ Hospital Consult Visits
The American Academy of Family Physicians (AAFP) has appealed to some of the nation's largest insurers, asking for coverage of hospital consults conducted by PCPs on behalf of hospitalists for their patients who are in the hospital.
"We believe that there is value in paying primary care physicians to see their patients in a hospital setting and that there is some evidence to suggest that doing so has benefits in terms of both improved outcomes and cost savings to the health system," says AAFP's commercial insurance strategist Brennan Cantrell. Cantrell's letter to the insurers asks them to review their coverage and payment policies.
Some insurers have downplayed the value of a hospital consultation by the outpatient physician, but advocates have emphasized its value in improving communication and enhancing care transitions.
"Payors' responses to our letter have been positive,” Cantrell tells The Hospitalist. The seven companies indicate that they have policies to permit separate payment for consults done by hospitalists and by PCPs brought in for hospital consults.
Hospitalist Claudia K. Geyer, MD, SFHM, chair of SHM's Family Medicine Committee, says AAFP's consultation initiative could help to formalize the social consult visit for primary care physicians, turning it into a true multidisciplinary approach to managing transitions. However, which patients are most appropriate for this type of consult, how it is initiated, and how many PCPs would be willing and able to provide the service—even if it were billable—require further clarification.
"The key would be for the hospitalist and PCP to work together as a team," Dr. Geyer says.
Visit our website for more information on inpatient visits by PCPs.
The American Academy of Family Physicians (AAFP) has appealed to some of the nation's largest insurers, asking for coverage of hospital consults conducted by PCPs on behalf of hospitalists for their patients who are in the hospital.
"We believe that there is value in paying primary care physicians to see their patients in a hospital setting and that there is some evidence to suggest that doing so has benefits in terms of both improved outcomes and cost savings to the health system," says AAFP's commercial insurance strategist Brennan Cantrell. Cantrell's letter to the insurers asks them to review their coverage and payment policies.
Some insurers have downplayed the value of a hospital consultation by the outpatient physician, but advocates have emphasized its value in improving communication and enhancing care transitions.
"Payors' responses to our letter have been positive,” Cantrell tells The Hospitalist. The seven companies indicate that they have policies to permit separate payment for consults done by hospitalists and by PCPs brought in for hospital consults.
Hospitalist Claudia K. Geyer, MD, SFHM, chair of SHM's Family Medicine Committee, says AAFP's consultation initiative could help to formalize the social consult visit for primary care physicians, turning it into a true multidisciplinary approach to managing transitions. However, which patients are most appropriate for this type of consult, how it is initiated, and how many PCPs would be willing and able to provide the service—even if it were billable—require further clarification.
"The key would be for the hospitalist and PCP to work together as a team," Dr. Geyer says.
Visit our website for more information on inpatient visits by PCPs.
The American Academy of Family Physicians (AAFP) has appealed to some of the nation's largest insurers, asking for coverage of hospital consults conducted by PCPs on behalf of hospitalists for their patients who are in the hospital.
"We believe that there is value in paying primary care physicians to see their patients in a hospital setting and that there is some evidence to suggest that doing so has benefits in terms of both improved outcomes and cost savings to the health system," says AAFP's commercial insurance strategist Brennan Cantrell. Cantrell's letter to the insurers asks them to review their coverage and payment policies.
Some insurers have downplayed the value of a hospital consultation by the outpatient physician, but advocates have emphasized its value in improving communication and enhancing care transitions.
"Payors' responses to our letter have been positive,” Cantrell tells The Hospitalist. The seven companies indicate that they have policies to permit separate payment for consults done by hospitalists and by PCPs brought in for hospital consults.
Hospitalist Claudia K. Geyer, MD, SFHM, chair of SHM's Family Medicine Committee, says AAFP's consultation initiative could help to formalize the social consult visit for primary care physicians, turning it into a true multidisciplinary approach to managing transitions. However, which patients are most appropriate for this type of consult, how it is initiated, and how many PCPs would be willing and able to provide the service—even if it were billable—require further clarification.
"The key would be for the hospitalist and PCP to work together as a team," Dr. Geyer says.
Visit our website for more information on inpatient visits by PCPs.
App Helps Hospitalists Prevent Inpatient Falls
Every year, hundreds of thousands of hospitalized patients fall. Now, hospitalists can get help in dramatically reducing those numbers. According to Erin DuPree, MD, FACOG, vice president and chief medical officer at The Joint Commission's Center for Transforming Healthcare, 30% to 50% of inpatients sustain an injury in a fall, incurring hospital costs of roughly $14,000 and adding, on average, 6.3 days to a hospital stay. It's an ongoing challenge.
"Hospitals have been working on preventing falls forever," Dr. DuPree says. "It's complex, and we needed to look at this in a data-driven way."
The center has done just that, and the result is a new web application called the Preventing Falls Targeted Solutions Tool. Anyone at a Joint Commission–accredited organization can gain complimentary access to the app, which guides users through a systematic, data-driven, Lean Six Sigma approach to reducing falls.
"It guides them through data collection and analyzes the data," Dr. DuPree adds. "Then the tool identifies your top contributing factors to falls and the solutions for those factors. We know every hospital has different contributing factors that matter; this is very local and dependent on the data that's entered."
Seven healthcare institutions in Missouri, Texas, Minnesota, California, North Carolina, and New Hampshire assisted the Joint Commission in developing the tool. Altogether, the pilot institutions reduced their rate of falls by an average of 35% and decreased their rate of patients injured in a fall by an average of 62%.
Hospitalists have a crucial role to play in bringing this process to their own workplace. "It's an opportunity for them to assert their leadership in their clinical role by collaborating with other disciplines on a big patient-safety issue," Dr. DuPree says.
It's also an opportunity for hospitalists to learn about quality improvement, she adds. "If they want to learn something about Lean Six Sigma," she says, "they can do a pilot project on their unit. I hope hospitalists gain access to the tool and start a falls project or work with their team to see what things in the tool could be of value to them."
Visit our website for more information on hospitalists and preventing inpatient falls.
Every year, hundreds of thousands of hospitalized patients fall. Now, hospitalists can get help in dramatically reducing those numbers. According to Erin DuPree, MD, FACOG, vice president and chief medical officer at The Joint Commission's Center for Transforming Healthcare, 30% to 50% of inpatients sustain an injury in a fall, incurring hospital costs of roughly $14,000 and adding, on average, 6.3 days to a hospital stay. It's an ongoing challenge.
"Hospitals have been working on preventing falls forever," Dr. DuPree says. "It's complex, and we needed to look at this in a data-driven way."
The center has done just that, and the result is a new web application called the Preventing Falls Targeted Solutions Tool. Anyone at a Joint Commission–accredited organization can gain complimentary access to the app, which guides users through a systematic, data-driven, Lean Six Sigma approach to reducing falls.
"It guides them through data collection and analyzes the data," Dr. DuPree adds. "Then the tool identifies your top contributing factors to falls and the solutions for those factors. We know every hospital has different contributing factors that matter; this is very local and dependent on the data that's entered."
Seven healthcare institutions in Missouri, Texas, Minnesota, California, North Carolina, and New Hampshire assisted the Joint Commission in developing the tool. Altogether, the pilot institutions reduced their rate of falls by an average of 35% and decreased their rate of patients injured in a fall by an average of 62%.
Hospitalists have a crucial role to play in bringing this process to their own workplace. "It's an opportunity for them to assert their leadership in their clinical role by collaborating with other disciplines on a big patient-safety issue," Dr. DuPree says.
It's also an opportunity for hospitalists to learn about quality improvement, she adds. "If they want to learn something about Lean Six Sigma," she says, "they can do a pilot project on their unit. I hope hospitalists gain access to the tool and start a falls project or work with their team to see what things in the tool could be of value to them."
Visit our website for more information on hospitalists and preventing inpatient falls.
Every year, hundreds of thousands of hospitalized patients fall. Now, hospitalists can get help in dramatically reducing those numbers. According to Erin DuPree, MD, FACOG, vice president and chief medical officer at The Joint Commission's Center for Transforming Healthcare, 30% to 50% of inpatients sustain an injury in a fall, incurring hospital costs of roughly $14,000 and adding, on average, 6.3 days to a hospital stay. It's an ongoing challenge.
"Hospitals have been working on preventing falls forever," Dr. DuPree says. "It's complex, and we needed to look at this in a data-driven way."
The center has done just that, and the result is a new web application called the Preventing Falls Targeted Solutions Tool. Anyone at a Joint Commission–accredited organization can gain complimentary access to the app, which guides users through a systematic, data-driven, Lean Six Sigma approach to reducing falls.
"It guides them through data collection and analyzes the data," Dr. DuPree adds. "Then the tool identifies your top contributing factors to falls and the solutions for those factors. We know every hospital has different contributing factors that matter; this is very local and dependent on the data that's entered."
Seven healthcare institutions in Missouri, Texas, Minnesota, California, North Carolina, and New Hampshire assisted the Joint Commission in developing the tool. Altogether, the pilot institutions reduced their rate of falls by an average of 35% and decreased their rate of patients injured in a fall by an average of 62%.
Hospitalists have a crucial role to play in bringing this process to their own workplace. "It's an opportunity for them to assert their leadership in their clinical role by collaborating with other disciplines on a big patient-safety issue," Dr. DuPree says.
It's also an opportunity for hospitalists to learn about quality improvement, she adds. "If they want to learn something about Lean Six Sigma," she says, "they can do a pilot project on their unit. I hope hospitalists gain access to the tool and start a falls project or work with their team to see what things in the tool could be of value to them."
Visit our website for more information on hospitalists and preventing inpatient falls.
David Henry's JCSO podcast, July 2015
In this month’s podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses a Community Translations article on lenvatinib, which was approved earlier this year for the treatment of patients with advanced differentiated thyroid cancer whose disease has progressed after radioactive iodine therapy. Also in the line-up are two Original Reports, one on health care expenditures associated with depression in adults with cancer and another on maximizing accessibility to and the efficacy of a weekly speech and language therapy service for patients with head and neck cancer who are receiving radiotherapy. A Case Report on a patient with inflammatory metastatic breast cancer with gallbladder metastases, a Feature article on new lung cancer treatments, and a summary of key findings from the 2015 annual meeting of the American Society of Clinical Oncology, round off the podcast.
In this month’s podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses a Community Translations article on lenvatinib, which was approved earlier this year for the treatment of patients with advanced differentiated thyroid cancer whose disease has progressed after radioactive iodine therapy. Also in the line-up are two Original Reports, one on health care expenditures associated with depression in adults with cancer and another on maximizing accessibility to and the efficacy of a weekly speech and language therapy service for patients with head and neck cancer who are receiving radiotherapy. A Case Report on a patient with inflammatory metastatic breast cancer with gallbladder metastases, a Feature article on new lung cancer treatments, and a summary of key findings from the 2015 annual meeting of the American Society of Clinical Oncology, round off the podcast.
In this month’s podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses a Community Translations article on lenvatinib, which was approved earlier this year for the treatment of patients with advanced differentiated thyroid cancer whose disease has progressed after radioactive iodine therapy. Also in the line-up are two Original Reports, one on health care expenditures associated with depression in adults with cancer and another on maximizing accessibility to and the efficacy of a weekly speech and language therapy service for patients with head and neck cancer who are receiving radiotherapy. A Case Report on a patient with inflammatory metastatic breast cancer with gallbladder metastases, a Feature article on new lung cancer treatments, and a summary of key findings from the 2015 annual meeting of the American Society of Clinical Oncology, round off the podcast.
Rise of the Chief Patient Experience Officer
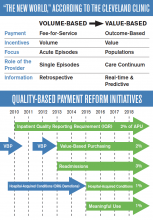
The Cleveland Clinic was the first major academic medical center to make improving patient experience a strategic goal. In 2007, the medical institution hired its first chief patient experience officer (CXO) and established the Office of Patient Experience. These were also firsts.
“When we took a step back to evaluate why we come to work every day, we realized it’s for one reason—the patients,” says Adrienne Boissy, MD, MA, the clinic’s CXO. “Toby [Cosgrove, MD, president and CEO of Cleveland Clinic] had some key early experiences, which prompted us to think about patient experience as an organizational priority. These experiences led him to recognize the importance of caring for the soul of the patient, not just the body.
“The CXO was embedded into our executive team, which effectively wove it into our fabric.”
Since then, more than 60 other medical institutions across the country have followed suit.
But improving patient experience isn’t just “nice to do,” Dr. Boissy says. “The great part of these shifts in healthcare is that the government has created the burning platform for us. Organizations won’t survive in the current market if they don’t make it a strategic priority.”
So what are the key drivers of a positive patient experience?
“You cannot deliver an exceptional patient experience without safe care, high quality, or high value,” says Dr. Boissy, who adds that hospitalists shouldn’t underestimate their role in the patient’s experience.
“Hospitalists are leaders whether they have an official title or not,” she adds. “People watch how they behave and interact with patients and colleagues. Raising your own awareness about your ability to influence is key. Model the skills you hope to embed.”
The Cleveland Clinic was the first major academic medical center to make improving patient experience a strategic goal. In 2007, the medical institution hired its first chief patient experience officer (CXO) and established the Office of Patient Experience. These were also firsts.
“When we took a step back to evaluate why we come to work every day, we realized it’s for one reason—the patients,” says Adrienne Boissy, MD, MA, the clinic’s CXO. “Toby [Cosgrove, MD, president and CEO of Cleveland Clinic] had some key early experiences, which prompted us to think about patient experience as an organizational priority. These experiences led him to recognize the importance of caring for the soul of the patient, not just the body.
“The CXO was embedded into our executive team, which effectively wove it into our fabric.”
Since then, more than 60 other medical institutions across the country have followed suit.
But improving patient experience isn’t just “nice to do,” Dr. Boissy says. “The great part of these shifts in healthcare is that the government has created the burning platform for us. Organizations won’t survive in the current market if they don’t make it a strategic priority.”
So what are the key drivers of a positive patient experience?
“You cannot deliver an exceptional patient experience without safe care, high quality, or high value,” says Dr. Boissy, who adds that hospitalists shouldn’t underestimate their role in the patient’s experience.
“Hospitalists are leaders whether they have an official title or not,” she adds. “People watch how they behave and interact with patients and colleagues. Raising your own awareness about your ability to influence is key. Model the skills you hope to embed.”
The Cleveland Clinic was the first major academic medical center to make improving patient experience a strategic goal. In 2007, the medical institution hired its first chief patient experience officer (CXO) and established the Office of Patient Experience. These were also firsts.
“When we took a step back to evaluate why we come to work every day, we realized it’s for one reason—the patients,” says Adrienne Boissy, MD, MA, the clinic’s CXO. “Toby [Cosgrove, MD, president and CEO of Cleveland Clinic] had some key early experiences, which prompted us to think about patient experience as an organizational priority. These experiences led him to recognize the importance of caring for the soul of the patient, not just the body.
“The CXO was embedded into our executive team, which effectively wove it into our fabric.”
Since then, more than 60 other medical institutions across the country have followed suit.
But improving patient experience isn’t just “nice to do,” Dr. Boissy says. “The great part of these shifts in healthcare is that the government has created the burning platform for us. Organizations won’t survive in the current market if they don’t make it a strategic priority.”
So what are the key drivers of a positive patient experience?
“You cannot deliver an exceptional patient experience without safe care, high quality, or high value,” says Dr. Boissy, who adds that hospitalists shouldn’t underestimate their role in the patient’s experience.
“Hospitalists are leaders whether they have an official title or not,” she adds. “People watch how they behave and interact with patients and colleagues. Raising your own awareness about your ability to influence is key. Model the skills you hope to embed.”
Cognitive, Emotional Memory Disconnect Impacts Patient Satisfaction
There are two types of memory, the cognitive and the emotional, and the latter is more enduring. Maya Angelou characterized the distinction between these two types of memory most eloquently and succinctly when she said, “I’ve learned that people will forget what you said, people will forget what you did, but people will never forget how you made them feel.” She was ahead of her time, because neurocognitive research has objectified with science what Ms. Angelou captured so elegantly in her prose. Emotional events are processed in the sensory systems and then transmitted to the medial-temporal lobe and the amygdale for the formation of an emotional memory. When the memory is cued and retrieved from the amygdale, it triggers an emotional response. Emotional experiences leave strong traces in the brain. Memories about emotional situations are stored in both the conscious and unconscious memory, which is part of the reason emotional memories are so enduring.1 Studies of patients with severe anterograde amnesia following circumscribed bilateral hippocampal brain damage showed enduring memories of emotion despite the absence of conscious memories.2 This has a demonstrably practical application in patients with dementia, who we now know have feelings of happiness and sadness long after they have forgotten what caused the emotion.3
The distinction is important because patients judge the quality of their medical care based on emotions. The patient satisfaction disconnect arises from the fact that physicians live in their cognitive memory, while patients live in their emotional memory. Being cognitive and objective is a critical skill a physician must bring to the bedside every day; the reason we don’t allow physicians to treat family members is that their ability to remain objective will be impaired. I realized that my emotion, my passion, and my empathy for the dying would impair my judgment when I started medical school, and I launched myself on a conscious and systematic discipline to keep those feelings out of my mind during patient care. The effort worked and, for the most part, I have been able to remain objective and unemotional as I care for my patients. Recently, however, I realized that my focus on objectivity negatively impacts patient experience. As a result, I have expanded my view: While I must stay objective and detached with my thinking, I must be emotionally engaged to provide a great patient experience.
I can remain objective and detached in my clinical judgment as I engage and connect emotionally during my patient encounters. This delicate balancing act has taken years of trial and error, however. I recently cared for a woman in her 60s who had fallen and broke her hip. Everyone was pleased that a top orthopedic surgeon was on call and able to give her the first-rate care she needed to begin walking again. The surgery went smoothly, and she was transferred to the medical/surgical ward, where things took a turn for the worse. She had a lot of anxiety in addition to her osteoporosis. Objectively, she was doing great, and we had a big success on our hands; however, she remained anxious, and she peppered the surgeon with fears that, while unfounded, were very real in her mind. The surgeon brushed them off, saying that her fears were not real and that he didn’t need to address them; his response made her emotional state spiral out of control. Her nurse notified me of the situation, and I came to her bedside. She was very agitated. I sat down at a low level and just started listening. She got all of her anxieties out in words. I held her hand, looked her in the eye, and assured her that I would be there for her and that things were going to be alright. Subsequently, she wrote letters of gratitude and proclaimed to any medical staff who would listen what a talented and great doctor I was. I did not have the skill to fix her broken hip; if it had been left to me alone, she would still be bed-bound. But I did have the human skills to connect with her and fix her agitated mind. If we remember the enduring power of the emotional memory, we can create great patient experiences.
The importance of these experiences was illustrated to me at the 2014 Dignity Health Patient Experience Summit, a powerful event featuring motivational speakers and leaders from across the country. The most powerful speakers, however, were patients. These patients had received terrible diagnoses that committed them to a prolonged interaction with the healthcare system. They were scared of what their diagnoses would mean for their future, they were subjected to uncomfortable procedures in which they struggled to maintain their dignity, and they repeatedly met the indifference of healthcare providers and clerical people who were only there to do a job. They related how the lack of caring and empathy made fears and anxiety much worse. But each of them had a story about that one person, that one care provider, who took the time to reassure them, to show that they cared, and to ensure that the patient did not feel alone. In most of these stories, the stand-out care providers took the time to hold their hands and reassure the patients. They took the time to connect with the patient’s emotional memory in a positive way, and that simple gesture of empathy had a powerful and lasting impact on the patient.
Invariably, the care provider at the heart of the patients’ stories was a nurse. Nurses have the reputation for being angels of mercy because they do the simple, empathetic gestures that let a patient know they are being cared for. These feelings endure in the patients’ memories long after the treatment is over. Doctors can, and should, be that type of care provider. It requires us to recognize that patients are scared and anxious, even though they may do their best not to show it. We, as physicians, often don’t see their anxiety, and we are so focused on the cognitive memory that we don’t address the anxiety and fear that is just under the surface. But taking just a few minutes to acknowledge their emotions, to explore them, and to reassure the patient that we are there for them has a lasting impact. In my group, we talk about the “human-business-human” encounter with patients. We begin all interactions with a human interaction (“Hello, I am Dr. McIlraith…”), conduct the business we came to provide (“Now I am going to examine you…”), and end with a human interaction (“What else can I do for you today?”). Patients expect physical contact with us during the “business” part of that interaction. I find that respectful, reassuring, and appropriate physical contact during the final “human” portion of that interaction helps solidify my patients’ experience. It helps make them feel that they have been cared for, particularly if the visit includes bad news.
Much of the recent focus on patient satisfaction has been driven by financial incentives. In 2013, CMS began penalizing hospitals 1.25% for poor HCAHPS scores as a part of the Affordable Care Act. In 2014, the maximum penalty increased to 2%, and to 3% in 2015. Hospitals have notoriously high overhead costs and slim profit margins, so these penalties can have a profound impact on the financial viability of an institution. But, while hospitals across the country have taken notice (see related article in this edition of The Hospitalist), I find doctors are more motivated by the well-being of their patients than are their hospital administrators. Satisfied patients are more compliant with treatment plans and have better outcomes.4,5 Hospitalists spend a lot of effort making sure their heart failure patients are on an ACE inhibitor, and their heart attack patients are discharged on aspirin, beta blockers, and statins so that they will have a good outcome following treatment for their acute illness. The same outcome-driven, evidence-based practice of medicine relates to patient satisfaction, however. Success in HCAHPS is as important as core measures when it comes to patient outcomes. And if I can’t convince you patient satisfaction is important because of the good it does for hospitals and patients, think about yourself for a minute. Satisfied patients are much less likely to sue their physicians.6 Practicing quality, evidence-based medicine will keep you out of peer review; however, satisfied patients will keep you out of the courtroom.
I frequently hear the comment that “we can do great on patient satisfaction, but then it gets busy, and patient satisfaction goes out the window.” My own experience contradicts this maxim, however. It is not how much time you spend with your patient but, rather, what you do with the time you have. One of the most powerful things we can do is listen. I used to make the mistake that I only wanted to hear the information I needed to figure out my patients’ problems so I could start treating them; however, I have come to learn that being heard is, in itself, therapy for my patients. It is often quoted that physicians interrupt their patients within 18 seconds of starting the interview.7 A lot of physicians dispense with attentive listening when they are under time pressure, when they should instead dispense with lengthy discourses on the patient treatment plan. It is important to educate our patients on their illness and treatment, I admit. I find a lot of hospitalists want to impart their knowledge and their treatment rationale to their patients; however, they frequently give patients and families much more information than they can hold in their cognitive memory. And time pressures are not the only anxieties hospitalists carry with them to the bedside. Our increasingly metric-driven profession means that we not only have to worry about morning discharges, interdisciplinary rounds, length of stay, and so on, but we also have to consider patient experience. It is not easy to hide all the stress we are under when we come to the bedside of a patient, but we have to. The easiest way to do that is to take a deep breath, sit next to the patient, ask an open-ended question, and then say nothing until the patient is done speaking. Active listening with good eye contact and encouragement to continue solidifies the patient’s experience of being heard. There are extreme cases when a patient is in a manic phase and won’t ever stop speaking; bend the rules a bit in those circumstances. However, the above rule works very effectively in the majority of physician-patient interactions. Being heard leaves an enduring emotional memory with our patients.
Hospital medicine often looks to other industries for inspiration on how we can improve. The airline industry is often held up as an example of how we can model patient safety, for instance, but these comparisons oversimplify the challenges we face. The same is true with patient satisfaction. In the business world, adages like “The customer is always right” are central to customer satisfaction, yet completely irrelevant to HM practice. Patients and families frequently have inappropriate and unrealistic expectations of their hospitalist physicians. We cannot, and should not, tell the patient addicted to narcotics that they can have as much IV Dilaudid as they would like. We cannot fix the patient with end-stage cancer, heart failure, or dementia. This is where we have to part ways with comparisons to principles that guide other industries if we are going to find a way forward with patient experience in hospital medicine. Because we have to set limits for patients, we often have to give our patients and families bad news, and because we have to tell them things they don’t like to hear, like “You can’t have any salt in your diet,” or “You must quit drinking alcohol,” we must develop our own principles on patient experience and satisfaction. Otherwise our options are either delivering inappropriate medical care or abandoning the pursuit of patient satisfaction all together. This is when we must remember that emotional memories are more enduring. We can’t always give our patients what they want, and we can’t always tell them what they want to hear, but we can always show them that we care. When we show our patients that we care in a palpable way, we leave them with the feeling that they have been cared for regardless of their condition, and the positive memory will endure despite the negative information we may have to convey. Maybe they won’t cut down on their salt or quit drinking alcohol, but they will never forget that their hospitalist physician cared.
And if they remember that the physician cared, it is much more likely that they will cut down on the salt or quit drinking alcohol when they go home. To paraphrase Maya Angelou, “I can’t always tell my patients what they want to hear, I can’t always tell them that their lifestyle is appropriate, but I can always show them that I care.”
Dr. McIlraith is chairman of the department of hospital medicine of Mercy Medical Group in Sacramento, Calif.
References
- LeDoux JE. Emotional memory. Scholarpedia. Accessed August 2, 2015.
- Feinstein JS, Duff MC, D Tranel D. Sustained experience of emotion after loss of memory in patients with amnesia. Proc Natl Acad Sci. 2010:107(17):7674-7679.
- Guzmán-Vélez E, Feinstein JS, Tranel D. Feelings without memory in Alzheimer disease. Cogn Behav Neurol. 2014;27(3):117-129.
- Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. March 2001. Accessed August 2, 2015.
- Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
- Stelfox HT, Gandhi TK, Orav EJ, Gustafson ML. The relation of patient statisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133.
- Beckman HB, Frankel RM. The effect of physician behavior on the collection of data. Ann Intern Med. 1984;101(5):692-696.
There are two types of memory, the cognitive and the emotional, and the latter is more enduring. Maya Angelou characterized the distinction between these two types of memory most eloquently and succinctly when she said, “I’ve learned that people will forget what you said, people will forget what you did, but people will never forget how you made them feel.” She was ahead of her time, because neurocognitive research has objectified with science what Ms. Angelou captured so elegantly in her prose. Emotional events are processed in the sensory systems and then transmitted to the medial-temporal lobe and the amygdale for the formation of an emotional memory. When the memory is cued and retrieved from the amygdale, it triggers an emotional response. Emotional experiences leave strong traces in the brain. Memories about emotional situations are stored in both the conscious and unconscious memory, which is part of the reason emotional memories are so enduring.1 Studies of patients with severe anterograde amnesia following circumscribed bilateral hippocampal brain damage showed enduring memories of emotion despite the absence of conscious memories.2 This has a demonstrably practical application in patients with dementia, who we now know have feelings of happiness and sadness long after they have forgotten what caused the emotion.3
The distinction is important because patients judge the quality of their medical care based on emotions. The patient satisfaction disconnect arises from the fact that physicians live in their cognitive memory, while patients live in their emotional memory. Being cognitive and objective is a critical skill a physician must bring to the bedside every day; the reason we don’t allow physicians to treat family members is that their ability to remain objective will be impaired. I realized that my emotion, my passion, and my empathy for the dying would impair my judgment when I started medical school, and I launched myself on a conscious and systematic discipline to keep those feelings out of my mind during patient care. The effort worked and, for the most part, I have been able to remain objective and unemotional as I care for my patients. Recently, however, I realized that my focus on objectivity negatively impacts patient experience. As a result, I have expanded my view: While I must stay objective and detached with my thinking, I must be emotionally engaged to provide a great patient experience.
I can remain objective and detached in my clinical judgment as I engage and connect emotionally during my patient encounters. This delicate balancing act has taken years of trial and error, however. I recently cared for a woman in her 60s who had fallen and broke her hip. Everyone was pleased that a top orthopedic surgeon was on call and able to give her the first-rate care she needed to begin walking again. The surgery went smoothly, and she was transferred to the medical/surgical ward, where things took a turn for the worse. She had a lot of anxiety in addition to her osteoporosis. Objectively, she was doing great, and we had a big success on our hands; however, she remained anxious, and she peppered the surgeon with fears that, while unfounded, were very real in her mind. The surgeon brushed them off, saying that her fears were not real and that he didn’t need to address them; his response made her emotional state spiral out of control. Her nurse notified me of the situation, and I came to her bedside. She was very agitated. I sat down at a low level and just started listening. She got all of her anxieties out in words. I held her hand, looked her in the eye, and assured her that I would be there for her and that things were going to be alright. Subsequently, she wrote letters of gratitude and proclaimed to any medical staff who would listen what a talented and great doctor I was. I did not have the skill to fix her broken hip; if it had been left to me alone, she would still be bed-bound. But I did have the human skills to connect with her and fix her agitated mind. If we remember the enduring power of the emotional memory, we can create great patient experiences.
The importance of these experiences was illustrated to me at the 2014 Dignity Health Patient Experience Summit, a powerful event featuring motivational speakers and leaders from across the country. The most powerful speakers, however, were patients. These patients had received terrible diagnoses that committed them to a prolonged interaction with the healthcare system. They were scared of what their diagnoses would mean for their future, they were subjected to uncomfortable procedures in which they struggled to maintain their dignity, and they repeatedly met the indifference of healthcare providers and clerical people who were only there to do a job. They related how the lack of caring and empathy made fears and anxiety much worse. But each of them had a story about that one person, that one care provider, who took the time to reassure them, to show that they cared, and to ensure that the patient did not feel alone. In most of these stories, the stand-out care providers took the time to hold their hands and reassure the patients. They took the time to connect with the patient’s emotional memory in a positive way, and that simple gesture of empathy had a powerful and lasting impact on the patient.
Invariably, the care provider at the heart of the patients’ stories was a nurse. Nurses have the reputation for being angels of mercy because they do the simple, empathetic gestures that let a patient know they are being cared for. These feelings endure in the patients’ memories long after the treatment is over. Doctors can, and should, be that type of care provider. It requires us to recognize that patients are scared and anxious, even though they may do their best not to show it. We, as physicians, often don’t see their anxiety, and we are so focused on the cognitive memory that we don’t address the anxiety and fear that is just under the surface. But taking just a few minutes to acknowledge their emotions, to explore them, and to reassure the patient that we are there for them has a lasting impact. In my group, we talk about the “human-business-human” encounter with patients. We begin all interactions with a human interaction (“Hello, I am Dr. McIlraith…”), conduct the business we came to provide (“Now I am going to examine you…”), and end with a human interaction (“What else can I do for you today?”). Patients expect physical contact with us during the “business” part of that interaction. I find that respectful, reassuring, and appropriate physical contact during the final “human” portion of that interaction helps solidify my patients’ experience. It helps make them feel that they have been cared for, particularly if the visit includes bad news.
Much of the recent focus on patient satisfaction has been driven by financial incentives. In 2013, CMS began penalizing hospitals 1.25% for poor HCAHPS scores as a part of the Affordable Care Act. In 2014, the maximum penalty increased to 2%, and to 3% in 2015. Hospitals have notoriously high overhead costs and slim profit margins, so these penalties can have a profound impact on the financial viability of an institution. But, while hospitals across the country have taken notice (see related article in this edition of The Hospitalist), I find doctors are more motivated by the well-being of their patients than are their hospital administrators. Satisfied patients are more compliant with treatment plans and have better outcomes.4,5 Hospitalists spend a lot of effort making sure their heart failure patients are on an ACE inhibitor, and their heart attack patients are discharged on aspirin, beta blockers, and statins so that they will have a good outcome following treatment for their acute illness. The same outcome-driven, evidence-based practice of medicine relates to patient satisfaction, however. Success in HCAHPS is as important as core measures when it comes to patient outcomes. And if I can’t convince you patient satisfaction is important because of the good it does for hospitals and patients, think about yourself for a minute. Satisfied patients are much less likely to sue their physicians.6 Practicing quality, evidence-based medicine will keep you out of peer review; however, satisfied patients will keep you out of the courtroom.
I frequently hear the comment that “we can do great on patient satisfaction, but then it gets busy, and patient satisfaction goes out the window.” My own experience contradicts this maxim, however. It is not how much time you spend with your patient but, rather, what you do with the time you have. One of the most powerful things we can do is listen. I used to make the mistake that I only wanted to hear the information I needed to figure out my patients’ problems so I could start treating them; however, I have come to learn that being heard is, in itself, therapy for my patients. It is often quoted that physicians interrupt their patients within 18 seconds of starting the interview.7 A lot of physicians dispense with attentive listening when they are under time pressure, when they should instead dispense with lengthy discourses on the patient treatment plan. It is important to educate our patients on their illness and treatment, I admit. I find a lot of hospitalists want to impart their knowledge and their treatment rationale to their patients; however, they frequently give patients and families much more information than they can hold in their cognitive memory. And time pressures are not the only anxieties hospitalists carry with them to the bedside. Our increasingly metric-driven profession means that we not only have to worry about morning discharges, interdisciplinary rounds, length of stay, and so on, but we also have to consider patient experience. It is not easy to hide all the stress we are under when we come to the bedside of a patient, but we have to. The easiest way to do that is to take a deep breath, sit next to the patient, ask an open-ended question, and then say nothing until the patient is done speaking. Active listening with good eye contact and encouragement to continue solidifies the patient’s experience of being heard. There are extreme cases when a patient is in a manic phase and won’t ever stop speaking; bend the rules a bit in those circumstances. However, the above rule works very effectively in the majority of physician-patient interactions. Being heard leaves an enduring emotional memory with our patients.
Hospital medicine often looks to other industries for inspiration on how we can improve. The airline industry is often held up as an example of how we can model patient safety, for instance, but these comparisons oversimplify the challenges we face. The same is true with patient satisfaction. In the business world, adages like “The customer is always right” are central to customer satisfaction, yet completely irrelevant to HM practice. Patients and families frequently have inappropriate and unrealistic expectations of their hospitalist physicians. We cannot, and should not, tell the patient addicted to narcotics that they can have as much IV Dilaudid as they would like. We cannot fix the patient with end-stage cancer, heart failure, or dementia. This is where we have to part ways with comparisons to principles that guide other industries if we are going to find a way forward with patient experience in hospital medicine. Because we have to set limits for patients, we often have to give our patients and families bad news, and because we have to tell them things they don’t like to hear, like “You can’t have any salt in your diet,” or “You must quit drinking alcohol,” we must develop our own principles on patient experience and satisfaction. Otherwise our options are either delivering inappropriate medical care or abandoning the pursuit of patient satisfaction all together. This is when we must remember that emotional memories are more enduring. We can’t always give our patients what they want, and we can’t always tell them what they want to hear, but we can always show them that we care. When we show our patients that we care in a palpable way, we leave them with the feeling that they have been cared for regardless of their condition, and the positive memory will endure despite the negative information we may have to convey. Maybe they won’t cut down on their salt or quit drinking alcohol, but they will never forget that their hospitalist physician cared.
And if they remember that the physician cared, it is much more likely that they will cut down on the salt or quit drinking alcohol when they go home. To paraphrase Maya Angelou, “I can’t always tell my patients what they want to hear, I can’t always tell them that their lifestyle is appropriate, but I can always show them that I care.”
Dr. McIlraith is chairman of the department of hospital medicine of Mercy Medical Group in Sacramento, Calif.
References
- LeDoux JE. Emotional memory. Scholarpedia. Accessed August 2, 2015.
- Feinstein JS, Duff MC, D Tranel D. Sustained experience of emotion after loss of memory in patients with amnesia. Proc Natl Acad Sci. 2010:107(17):7674-7679.
- Guzmán-Vélez E, Feinstein JS, Tranel D. Feelings without memory in Alzheimer disease. Cogn Behav Neurol. 2014;27(3):117-129.
- Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. March 2001. Accessed August 2, 2015.
- Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
- Stelfox HT, Gandhi TK, Orav EJ, Gustafson ML. The relation of patient statisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133.
- Beckman HB, Frankel RM. The effect of physician behavior on the collection of data. Ann Intern Med. 1984;101(5):692-696.
There are two types of memory, the cognitive and the emotional, and the latter is more enduring. Maya Angelou characterized the distinction between these two types of memory most eloquently and succinctly when she said, “I’ve learned that people will forget what you said, people will forget what you did, but people will never forget how you made them feel.” She was ahead of her time, because neurocognitive research has objectified with science what Ms. Angelou captured so elegantly in her prose. Emotional events are processed in the sensory systems and then transmitted to the medial-temporal lobe and the amygdale for the formation of an emotional memory. When the memory is cued and retrieved from the amygdale, it triggers an emotional response. Emotional experiences leave strong traces in the brain. Memories about emotional situations are stored in both the conscious and unconscious memory, which is part of the reason emotional memories are so enduring.1 Studies of patients with severe anterograde amnesia following circumscribed bilateral hippocampal brain damage showed enduring memories of emotion despite the absence of conscious memories.2 This has a demonstrably practical application in patients with dementia, who we now know have feelings of happiness and sadness long after they have forgotten what caused the emotion.3
The distinction is important because patients judge the quality of their medical care based on emotions. The patient satisfaction disconnect arises from the fact that physicians live in their cognitive memory, while patients live in their emotional memory. Being cognitive and objective is a critical skill a physician must bring to the bedside every day; the reason we don’t allow physicians to treat family members is that their ability to remain objective will be impaired. I realized that my emotion, my passion, and my empathy for the dying would impair my judgment when I started medical school, and I launched myself on a conscious and systematic discipline to keep those feelings out of my mind during patient care. The effort worked and, for the most part, I have been able to remain objective and unemotional as I care for my patients. Recently, however, I realized that my focus on objectivity negatively impacts patient experience. As a result, I have expanded my view: While I must stay objective and detached with my thinking, I must be emotionally engaged to provide a great patient experience.
I can remain objective and detached in my clinical judgment as I engage and connect emotionally during my patient encounters. This delicate balancing act has taken years of trial and error, however. I recently cared for a woman in her 60s who had fallen and broke her hip. Everyone was pleased that a top orthopedic surgeon was on call and able to give her the first-rate care she needed to begin walking again. The surgery went smoothly, and she was transferred to the medical/surgical ward, where things took a turn for the worse. She had a lot of anxiety in addition to her osteoporosis. Objectively, she was doing great, and we had a big success on our hands; however, she remained anxious, and she peppered the surgeon with fears that, while unfounded, were very real in her mind. The surgeon brushed them off, saying that her fears were not real and that he didn’t need to address them; his response made her emotional state spiral out of control. Her nurse notified me of the situation, and I came to her bedside. She was very agitated. I sat down at a low level and just started listening. She got all of her anxieties out in words. I held her hand, looked her in the eye, and assured her that I would be there for her and that things were going to be alright. Subsequently, she wrote letters of gratitude and proclaimed to any medical staff who would listen what a talented and great doctor I was. I did not have the skill to fix her broken hip; if it had been left to me alone, she would still be bed-bound. But I did have the human skills to connect with her and fix her agitated mind. If we remember the enduring power of the emotional memory, we can create great patient experiences.
The importance of these experiences was illustrated to me at the 2014 Dignity Health Patient Experience Summit, a powerful event featuring motivational speakers and leaders from across the country. The most powerful speakers, however, were patients. These patients had received terrible diagnoses that committed them to a prolonged interaction with the healthcare system. They were scared of what their diagnoses would mean for their future, they were subjected to uncomfortable procedures in which they struggled to maintain their dignity, and they repeatedly met the indifference of healthcare providers and clerical people who were only there to do a job. They related how the lack of caring and empathy made fears and anxiety much worse. But each of them had a story about that one person, that one care provider, who took the time to reassure them, to show that they cared, and to ensure that the patient did not feel alone. In most of these stories, the stand-out care providers took the time to hold their hands and reassure the patients. They took the time to connect with the patient’s emotional memory in a positive way, and that simple gesture of empathy had a powerful and lasting impact on the patient.
Invariably, the care provider at the heart of the patients’ stories was a nurse. Nurses have the reputation for being angels of mercy because they do the simple, empathetic gestures that let a patient know they are being cared for. These feelings endure in the patients’ memories long after the treatment is over. Doctors can, and should, be that type of care provider. It requires us to recognize that patients are scared and anxious, even though they may do their best not to show it. We, as physicians, often don’t see their anxiety, and we are so focused on the cognitive memory that we don’t address the anxiety and fear that is just under the surface. But taking just a few minutes to acknowledge their emotions, to explore them, and to reassure the patient that we are there for them has a lasting impact. In my group, we talk about the “human-business-human” encounter with patients. We begin all interactions with a human interaction (“Hello, I am Dr. McIlraith…”), conduct the business we came to provide (“Now I am going to examine you…”), and end with a human interaction (“What else can I do for you today?”). Patients expect physical contact with us during the “business” part of that interaction. I find that respectful, reassuring, and appropriate physical contact during the final “human” portion of that interaction helps solidify my patients’ experience. It helps make them feel that they have been cared for, particularly if the visit includes bad news.
Much of the recent focus on patient satisfaction has been driven by financial incentives. In 2013, CMS began penalizing hospitals 1.25% for poor HCAHPS scores as a part of the Affordable Care Act. In 2014, the maximum penalty increased to 2%, and to 3% in 2015. Hospitals have notoriously high overhead costs and slim profit margins, so these penalties can have a profound impact on the financial viability of an institution. But, while hospitals across the country have taken notice (see related article in this edition of The Hospitalist), I find doctors are more motivated by the well-being of their patients than are their hospital administrators. Satisfied patients are more compliant with treatment plans and have better outcomes.4,5 Hospitalists spend a lot of effort making sure their heart failure patients are on an ACE inhibitor, and their heart attack patients are discharged on aspirin, beta blockers, and statins so that they will have a good outcome following treatment for their acute illness. The same outcome-driven, evidence-based practice of medicine relates to patient satisfaction, however. Success in HCAHPS is as important as core measures when it comes to patient outcomes. And if I can’t convince you patient satisfaction is important because of the good it does for hospitals and patients, think about yourself for a minute. Satisfied patients are much less likely to sue their physicians.6 Practicing quality, evidence-based medicine will keep you out of peer review; however, satisfied patients will keep you out of the courtroom.
I frequently hear the comment that “we can do great on patient satisfaction, but then it gets busy, and patient satisfaction goes out the window.” My own experience contradicts this maxim, however. It is not how much time you spend with your patient but, rather, what you do with the time you have. One of the most powerful things we can do is listen. I used to make the mistake that I only wanted to hear the information I needed to figure out my patients’ problems so I could start treating them; however, I have come to learn that being heard is, in itself, therapy for my patients. It is often quoted that physicians interrupt their patients within 18 seconds of starting the interview.7 A lot of physicians dispense with attentive listening when they are under time pressure, when they should instead dispense with lengthy discourses on the patient treatment plan. It is important to educate our patients on their illness and treatment, I admit. I find a lot of hospitalists want to impart their knowledge and their treatment rationale to their patients; however, they frequently give patients and families much more information than they can hold in their cognitive memory. And time pressures are not the only anxieties hospitalists carry with them to the bedside. Our increasingly metric-driven profession means that we not only have to worry about morning discharges, interdisciplinary rounds, length of stay, and so on, but we also have to consider patient experience. It is not easy to hide all the stress we are under when we come to the bedside of a patient, but we have to. The easiest way to do that is to take a deep breath, sit next to the patient, ask an open-ended question, and then say nothing until the patient is done speaking. Active listening with good eye contact and encouragement to continue solidifies the patient’s experience of being heard. There are extreme cases when a patient is in a manic phase and won’t ever stop speaking; bend the rules a bit in those circumstances. However, the above rule works very effectively in the majority of physician-patient interactions. Being heard leaves an enduring emotional memory with our patients.
Hospital medicine often looks to other industries for inspiration on how we can improve. The airline industry is often held up as an example of how we can model patient safety, for instance, but these comparisons oversimplify the challenges we face. The same is true with patient satisfaction. In the business world, adages like “The customer is always right” are central to customer satisfaction, yet completely irrelevant to HM practice. Patients and families frequently have inappropriate and unrealistic expectations of their hospitalist physicians. We cannot, and should not, tell the patient addicted to narcotics that they can have as much IV Dilaudid as they would like. We cannot fix the patient with end-stage cancer, heart failure, or dementia. This is where we have to part ways with comparisons to principles that guide other industries if we are going to find a way forward with patient experience in hospital medicine. Because we have to set limits for patients, we often have to give our patients and families bad news, and because we have to tell them things they don’t like to hear, like “You can’t have any salt in your diet,” or “You must quit drinking alcohol,” we must develop our own principles on patient experience and satisfaction. Otherwise our options are either delivering inappropriate medical care or abandoning the pursuit of patient satisfaction all together. This is when we must remember that emotional memories are more enduring. We can’t always give our patients what they want, and we can’t always tell them what they want to hear, but we can always show them that we care. When we show our patients that we care in a palpable way, we leave them with the feeling that they have been cared for regardless of their condition, and the positive memory will endure despite the negative information we may have to convey. Maybe they won’t cut down on their salt or quit drinking alcohol, but they will never forget that their hospitalist physician cared.
And if they remember that the physician cared, it is much more likely that they will cut down on the salt or quit drinking alcohol when they go home. To paraphrase Maya Angelou, “I can’t always tell my patients what they want to hear, I can’t always tell them that their lifestyle is appropriate, but I can always show them that I care.”
Dr. McIlraith is chairman of the department of hospital medicine of Mercy Medical Group in Sacramento, Calif.
References
- LeDoux JE. Emotional memory. Scholarpedia. Accessed August 2, 2015.
- Feinstein JS, Duff MC, D Tranel D. Sustained experience of emotion after loss of memory in patients with amnesia. Proc Natl Acad Sci. 2010:107(17):7674-7679.
- Guzmán-Vélez E, Feinstein JS, Tranel D. Feelings without memory in Alzheimer disease. Cogn Behav Neurol. 2014;27(3):117-129.
- Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. March 2001. Accessed August 2, 2015.
- Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
- Stelfox HT, Gandhi TK, Orav EJ, Gustafson ML. The relation of patient statisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133.
- Beckman HB, Frankel RM. The effect of physician behavior on the collection of data. Ann Intern Med. 1984;101(5):692-696.
Evaluation of Gender as a Clinically Relevant Outcome Variable in the Treatment of Onychomycosis With Efinaconazole Topical Solution 10%
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).
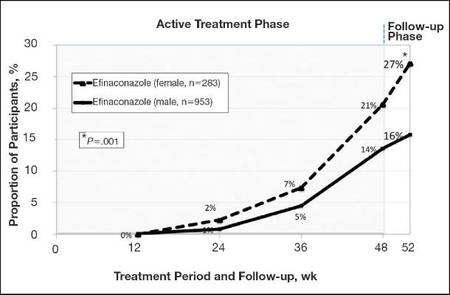
|
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |
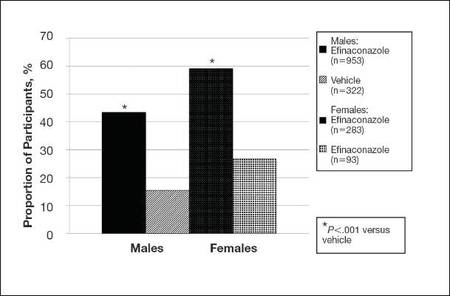
|
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).

|
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |

|
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).

|
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |

|
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
Practice Points
- Men, particularly as they age, are more likely to develop onychomycosis.
- Treatment adherence may be a bigger issue among male patients.
- Onychomycosis in males may be more difficult to treat for a variety of reasons.
Preop chemo feasible for high-risk soft-tissue sarcomas
Preoperative chemotherapy is feasible for high-risk localized soft-tissue sarcomas of the limbs or superficial trunk, even with concurrent radiotherapy and even in selected elderly patients, investigators reported online Sept. 7 in Journal of Clinical Oncology.
Full-dose preoperative chemotherapy now can be offered and an excellent chemotherapeutic dose intensity now can be achieved with concomitant radiotherapy in cases “when surgical reasons suggest that major preoperative shrinkage may help,” said Dr. Elena Palassini of Istituto Nazionale dei Tumori, Bologna (Italy), and her associates in the Italian Sarcoma Group and Spanish Sarcoma Group.
The investigators already reported the efficacy results of their international phase III randomized clinical trial assessing preoperative chemotherapy (with or without radiotherapy, at the treating physician’s discretion). In the current analysis, they focused on the toxicity data for 303 of the trial participants, all of whom had high-grade, deep, large, adult-type soft-tissue sarcomas arising from the extremities or trunk wall.
The median patient age was 48 years (range, 15-79 years), and 13% of the study population was aged 65 years or older. A total of 152 received preoperative radiotherapy along with epirubicin plus ifosfamide.
“The most interesting clinical finding was the tolerability of the combination of preoperative chemotherapy and radiotherapy,” which is particularly remarkable because patients chosen for combination treatment had the largest tumors and the most challenging presentations, the investigators wrote.
Participants who received combination preoperative therapy showed “only limited worsening of toxicities” compared with those who had preoperative chemotherapy alone, Dr. Palassini and her associates said (J Clin Oncol. 2015 Sept. 7. doi: 10.1200/JCO.2015.62.9394).
Grade 3 or 4 thrombocytopenia was more frequent with combined therapy than with chemotherapy alone, but grade 4 leukopenia and grade 3 or 4 anemia were not. There were no cases of fatal toxicity, and the rate of wound complications was not significantly higher when radiotherapy was added to chemotherapy (17.1%) than when it was not (9.9%).
Even though some patients failed to complete all planned cycles of chemotherapy or experienced dose reductions or interruptions because of toxic effects, the overall median chemotherapeutic dose index remained “excellent” at greater than 90%. This was true even in patients aged 65 years and older, which “clearly suggests the possibility of selecting and treating at least a proportion of [older] patients in the adjuvant setting with full-dose regimens.” This finding is especially important because older patients comprise 30% of those newly diagnosed as having soft-tissue sarcoma, the investigators noted.
The authors did not identify a sponsor of this study. Dr. Palassini reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
Preoperative chemotherapy is feasible for high-risk localized soft-tissue sarcomas of the limbs or superficial trunk, even with concurrent radiotherapy and even in selected elderly patients, investigators reported online Sept. 7 in Journal of Clinical Oncology.
Full-dose preoperative chemotherapy now can be offered and an excellent chemotherapeutic dose intensity now can be achieved with concomitant radiotherapy in cases “when surgical reasons suggest that major preoperative shrinkage may help,” said Dr. Elena Palassini of Istituto Nazionale dei Tumori, Bologna (Italy), and her associates in the Italian Sarcoma Group and Spanish Sarcoma Group.
The investigators already reported the efficacy results of their international phase III randomized clinical trial assessing preoperative chemotherapy (with or without radiotherapy, at the treating physician’s discretion). In the current analysis, they focused on the toxicity data for 303 of the trial participants, all of whom had high-grade, deep, large, adult-type soft-tissue sarcomas arising from the extremities or trunk wall.
The median patient age was 48 years (range, 15-79 years), and 13% of the study population was aged 65 years or older. A total of 152 received preoperative radiotherapy along with epirubicin plus ifosfamide.
“The most interesting clinical finding was the tolerability of the combination of preoperative chemotherapy and radiotherapy,” which is particularly remarkable because patients chosen for combination treatment had the largest tumors and the most challenging presentations, the investigators wrote.
Participants who received combination preoperative therapy showed “only limited worsening of toxicities” compared with those who had preoperative chemotherapy alone, Dr. Palassini and her associates said (J Clin Oncol. 2015 Sept. 7. doi: 10.1200/JCO.2015.62.9394).
Grade 3 or 4 thrombocytopenia was more frequent with combined therapy than with chemotherapy alone, but grade 4 leukopenia and grade 3 or 4 anemia were not. There were no cases of fatal toxicity, and the rate of wound complications was not significantly higher when radiotherapy was added to chemotherapy (17.1%) than when it was not (9.9%).
Even though some patients failed to complete all planned cycles of chemotherapy or experienced dose reductions or interruptions because of toxic effects, the overall median chemotherapeutic dose index remained “excellent” at greater than 90%. This was true even in patients aged 65 years and older, which “clearly suggests the possibility of selecting and treating at least a proportion of [older] patients in the adjuvant setting with full-dose regimens.” This finding is especially important because older patients comprise 30% of those newly diagnosed as having soft-tissue sarcoma, the investigators noted.
The authors did not identify a sponsor of this study. Dr. Palassini reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
Preoperative chemotherapy is feasible for high-risk localized soft-tissue sarcomas of the limbs or superficial trunk, even with concurrent radiotherapy and even in selected elderly patients, investigators reported online Sept. 7 in Journal of Clinical Oncology.
Full-dose preoperative chemotherapy now can be offered and an excellent chemotherapeutic dose intensity now can be achieved with concomitant radiotherapy in cases “when surgical reasons suggest that major preoperative shrinkage may help,” said Dr. Elena Palassini of Istituto Nazionale dei Tumori, Bologna (Italy), and her associates in the Italian Sarcoma Group and Spanish Sarcoma Group.
The investigators already reported the efficacy results of their international phase III randomized clinical trial assessing preoperative chemotherapy (with or without radiotherapy, at the treating physician’s discretion). In the current analysis, they focused on the toxicity data for 303 of the trial participants, all of whom had high-grade, deep, large, adult-type soft-tissue sarcomas arising from the extremities or trunk wall.
The median patient age was 48 years (range, 15-79 years), and 13% of the study population was aged 65 years or older. A total of 152 received preoperative radiotherapy along with epirubicin plus ifosfamide.
“The most interesting clinical finding was the tolerability of the combination of preoperative chemotherapy and radiotherapy,” which is particularly remarkable because patients chosen for combination treatment had the largest tumors and the most challenging presentations, the investigators wrote.
Participants who received combination preoperative therapy showed “only limited worsening of toxicities” compared with those who had preoperative chemotherapy alone, Dr. Palassini and her associates said (J Clin Oncol. 2015 Sept. 7. doi: 10.1200/JCO.2015.62.9394).
Grade 3 or 4 thrombocytopenia was more frequent with combined therapy than with chemotherapy alone, but grade 4 leukopenia and grade 3 or 4 anemia were not. There were no cases of fatal toxicity, and the rate of wound complications was not significantly higher when radiotherapy was added to chemotherapy (17.1%) than when it was not (9.9%).
Even though some patients failed to complete all planned cycles of chemotherapy or experienced dose reductions or interruptions because of toxic effects, the overall median chemotherapeutic dose index remained “excellent” at greater than 90%. This was true even in patients aged 65 years and older, which “clearly suggests the possibility of selecting and treating at least a proportion of [older] patients in the adjuvant setting with full-dose regimens.” This finding is especially important because older patients comprise 30% of those newly diagnosed as having soft-tissue sarcoma, the investigators noted.
The authors did not identify a sponsor of this study. Dr. Palassini reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Preoperative chemotherapy is feasible for high-risk localized soft-tissue sarcomas, even with concurrent radiotherapy.
Major finding: The overall median chemotherapeutic dose index was “excellent” at greater than 90%.
Data source: An analysis of toxicity data from a 5-year international phase III randomized trial involving 303 patients.
Disclosures: The authors did not identify a sponsor of this study. Dr. Palassini reported having no relevant financial disclosures, and her associates reported ties to numerous industry sources.