User login
Do progesterone-only contraceptives lead to more mood changes than other types?
Lower, or comparable depression scores compared with other methods
A retrospective cohort trial compared 298 women on progesterone-only contraception with 6356 women on other or no contraception to examine the association between contraception use and depressive symptoms.1
When surveyed with the Center of Epidemiological Studies Depression Scale (using both a 10-question, 30-point questionnaire and a 20-question, 60-point questionnaire), women on progesterone-only contraception demonstrated significantly lower levels of depressive symptoms compared with women using low-efficacy contraception (early withdrawal, spermicides, contraceptive films) or no contraception (mean deviation [MD]=-1.3; 95% confidence interval [CI], -2.4 to -0.2). No significant difference was seen in depression scores when compared with women on other forms of hormonal contraception (MD=-0.3; 95% CI, -1.2 to 0.6).
No significant difference in depression and less anhedonia for nonusers
A cross-sectional, population-based trial conducted by survey in Finland in 1997, 2002, and 2007 investigated the link between contraception and mood symptoms. It included 759 women using the progesterone-only levonorgestrel-releasing intrauterine system (LNG-IUS) and 7036 women on other forms of contraception or none.2
Current LNG-IUS users vs nonusers had no significant difference in diagnosis of depression, as assessed by asking patients if they had been diagnosed with or treated for depression in the previous year of contraception (8.0% vs 7.3%; P>.05); depressive symptoms in the previous year (24% vs 26%; P>.05), or psychological illness (1.9% vs 2.5%; P>.05).
LNG-IUS users reported significantly less anhedonia than nonusers in the previous year (19% vs 22%; P<.05). Moreover, in a partial correlation analysis, LNG-IUS was negatively correlated with anhedonia (r=0.024; P<.05) and symptoms of depression over the previous month (r=0.098; P<.05).
Did relationship satisfaction, rather than contraceptive, influence depression?
A multicenter prospective cohort trial analyzed the effect of the levonorgestrel implant on mood in 267 women followed for 2 years by evaluating depressive symptoms reported from the Mental Health Inventory, a 6-item questionnaire scored 0 to 24.3
The women demonstrated a significant increase in depressive symptom scores from 7.9 at baseline to 8.8 (P=.01). However, the study authors suggested that relationship satisfaction, not method of birth control, was the cause of depressive symptoms. The 62 women who experienced a decrease in relationship satisfaction exhibited a significant increase in depressive symptoms (6.7-10; P=.001) compared with the 156 women who reported an improvement or no change in relationship satisfaction (7.8-8.2; P=.30).
1. Keyes K, Cheslack-Postava K, Westhoff C, et al. Association of hormonal contraception use with reduced levels of depressive symptoms: a national study of sexually active women in the United States. Am J Epidemiol. 2013;178:1378-1388.
2. Toffol E, Heikinheimo O, Koponen P, et al. Further evidence for lack of negative associations between hormonal contraception and mental health. Contraception. 2012;86:470-480.
3. Westhoff C, Truman C, Kalmuss D, et al. Depressive symptoms and Norplant contraceptive implants. Contraception. 1998;57:241-245.
Lower, or comparable depression scores compared with other methods
A retrospective cohort trial compared 298 women on progesterone-only contraception with 6356 women on other or no contraception to examine the association between contraception use and depressive symptoms.1
When surveyed with the Center of Epidemiological Studies Depression Scale (using both a 10-question, 30-point questionnaire and a 20-question, 60-point questionnaire), women on progesterone-only contraception demonstrated significantly lower levels of depressive symptoms compared with women using low-efficacy contraception (early withdrawal, spermicides, contraceptive films) or no contraception (mean deviation [MD]=-1.3; 95% confidence interval [CI], -2.4 to -0.2). No significant difference was seen in depression scores when compared with women on other forms of hormonal contraception (MD=-0.3; 95% CI, -1.2 to 0.6).
No significant difference in depression and less anhedonia for nonusers
A cross-sectional, population-based trial conducted by survey in Finland in 1997, 2002, and 2007 investigated the link between contraception and mood symptoms. It included 759 women using the progesterone-only levonorgestrel-releasing intrauterine system (LNG-IUS) and 7036 women on other forms of contraception or none.2
Current LNG-IUS users vs nonusers had no significant difference in diagnosis of depression, as assessed by asking patients if they had been diagnosed with or treated for depression in the previous year of contraception (8.0% vs 7.3%; P>.05); depressive symptoms in the previous year (24% vs 26%; P>.05), or psychological illness (1.9% vs 2.5%; P>.05).
LNG-IUS users reported significantly less anhedonia than nonusers in the previous year (19% vs 22%; P<.05). Moreover, in a partial correlation analysis, LNG-IUS was negatively correlated with anhedonia (r=0.024; P<.05) and symptoms of depression over the previous month (r=0.098; P<.05).
Did relationship satisfaction, rather than contraceptive, influence depression?
A multicenter prospective cohort trial analyzed the effect of the levonorgestrel implant on mood in 267 women followed for 2 years by evaluating depressive symptoms reported from the Mental Health Inventory, a 6-item questionnaire scored 0 to 24.3
The women demonstrated a significant increase in depressive symptom scores from 7.9 at baseline to 8.8 (P=.01). However, the study authors suggested that relationship satisfaction, not method of birth control, was the cause of depressive symptoms. The 62 women who experienced a decrease in relationship satisfaction exhibited a significant increase in depressive symptoms (6.7-10; P=.001) compared with the 156 women who reported an improvement or no change in relationship satisfaction (7.8-8.2; P=.30).
Lower, or comparable depression scores compared with other methods
A retrospective cohort trial compared 298 women on progesterone-only contraception with 6356 women on other or no contraception to examine the association between contraception use and depressive symptoms.1
When surveyed with the Center of Epidemiological Studies Depression Scale (using both a 10-question, 30-point questionnaire and a 20-question, 60-point questionnaire), women on progesterone-only contraception demonstrated significantly lower levels of depressive symptoms compared with women using low-efficacy contraception (early withdrawal, spermicides, contraceptive films) or no contraception (mean deviation [MD]=-1.3; 95% confidence interval [CI], -2.4 to -0.2). No significant difference was seen in depression scores when compared with women on other forms of hormonal contraception (MD=-0.3; 95% CI, -1.2 to 0.6).
No significant difference in depression and less anhedonia for nonusers
A cross-sectional, population-based trial conducted by survey in Finland in 1997, 2002, and 2007 investigated the link between contraception and mood symptoms. It included 759 women using the progesterone-only levonorgestrel-releasing intrauterine system (LNG-IUS) and 7036 women on other forms of contraception or none.2
Current LNG-IUS users vs nonusers had no significant difference in diagnosis of depression, as assessed by asking patients if they had been diagnosed with or treated for depression in the previous year of contraception (8.0% vs 7.3%; P>.05); depressive symptoms in the previous year (24% vs 26%; P>.05), or psychological illness (1.9% vs 2.5%; P>.05).
LNG-IUS users reported significantly less anhedonia than nonusers in the previous year (19% vs 22%; P<.05). Moreover, in a partial correlation analysis, LNG-IUS was negatively correlated with anhedonia (r=0.024; P<.05) and symptoms of depression over the previous month (r=0.098; P<.05).
Did relationship satisfaction, rather than contraceptive, influence depression?
A multicenter prospective cohort trial analyzed the effect of the levonorgestrel implant on mood in 267 women followed for 2 years by evaluating depressive symptoms reported from the Mental Health Inventory, a 6-item questionnaire scored 0 to 24.3
The women demonstrated a significant increase in depressive symptom scores from 7.9 at baseline to 8.8 (P=.01). However, the study authors suggested that relationship satisfaction, not method of birth control, was the cause of depressive symptoms. The 62 women who experienced a decrease in relationship satisfaction exhibited a significant increase in depressive symptoms (6.7-10; P=.001) compared with the 156 women who reported an improvement or no change in relationship satisfaction (7.8-8.2; P=.30).
1. Keyes K, Cheslack-Postava K, Westhoff C, et al. Association of hormonal contraception use with reduced levels of depressive symptoms: a national study of sexually active women in the United States. Am J Epidemiol. 2013;178:1378-1388.
2. Toffol E, Heikinheimo O, Koponen P, et al. Further evidence for lack of negative associations between hormonal contraception and mental health. Contraception. 2012;86:470-480.
3. Westhoff C, Truman C, Kalmuss D, et al. Depressive symptoms and Norplant contraceptive implants. Contraception. 1998;57:241-245.
1. Keyes K, Cheslack-Postava K, Westhoff C, et al. Association of hormonal contraception use with reduced levels of depressive symptoms: a national study of sexually active women in the United States. Am J Epidemiol. 2013;178:1378-1388.
2. Toffol E, Heikinheimo O, Koponen P, et al. Further evidence for lack of negative associations between hormonal contraception and mental health. Contraception. 2012;86:470-480.
3. Westhoff C, Truman C, Kalmuss D, et al. Depressive symptoms and Norplant contraceptive implants. Contraception. 1998;57:241-245.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
No. Women taking progesterone-only contraceptives don’t appear to experience more depressive symptoms or mood changes than women on other hormonal contraceptives, and they may experience slightly less depression than women using no contraception (strength of recommendation: B, multiple homogeneous cohorts).
Is an intestinal biopsy necessary when the blood work suggests celiac disease?
EVIDENCE SUMMARY
A 2013 Belgian prospective study of 104 non-IgA–deficient adults and children diagnosed with celiac disease and 537 adults and children without celiac disease evaluated the accuracy of 4 manufacturers’ serologic tests for IgA anti-tTG.1 All patients underwent serologic testing followed by a diagnostic biopsy. A Marsh type 3 or greater lesion on duodenal biopsy was considered diagnostic for celiac disease.
Anti-tTG levels greater than 10 times the manufacturer-recommended level for a positive test (cut-off) were associated with a likelihood ratio of 111 to 294 (depending on the manufacturer) of positive biopsy. Post-test probabilities were calculated based on various pretest probabilities using an anti-tTG level of greater than 10 times the cut-off (TABLE1).
Investigators also obtained IgG anti-DGP levels from 2 of the manufacturers.1 Likelihood ratios increased along with antibody levels. Ratios of 80 and 400, depending on the manufacturer, were found at IgG anti-DGP levels 10-fold greater than the cut-off. Pre- and post-test probabilities weren’t calculated.
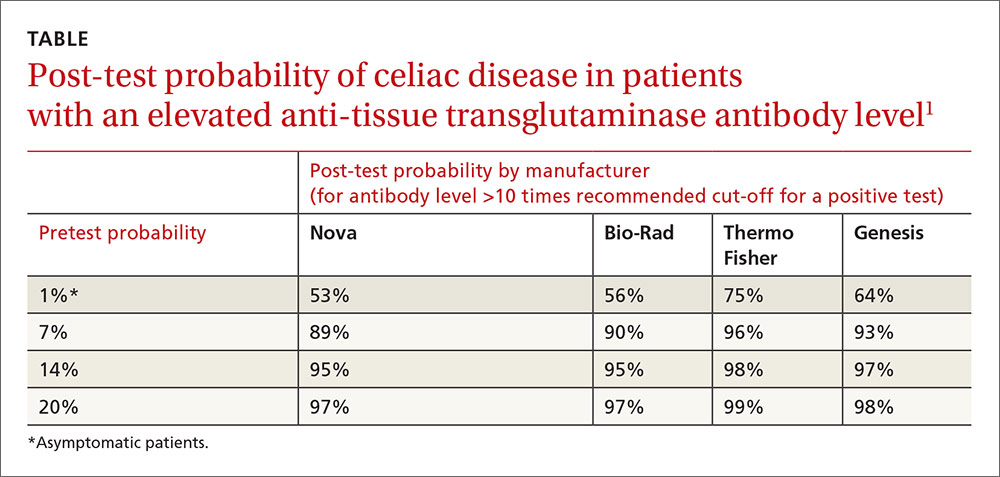
Positive predictive value rises with antibody levels
A 2013 retrospective study evaluated the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition’s recommendation to forego intestinal biopsy in non-IgA–deficient, symptomatic children and adolescents with positive IgA anti-tTG levels greater than 10 times the cut-off value, positive EMA, and positive HLA-DQ2 or HLA-DQ8.2
Overall, 153 symptomatic patients referred to the gastroenterology unit met these criteria. The age range was 9 months to 14.6 years (mean 4 years). All but 3 of the patients had Marsh 2 or greater lesions with biopsy-confirmed diagnoses of celiac disease. The remaining 3 developed biopsy-positive celiac disease on follow-up. The positive predictive value of combined serologic testing in this small selected patient population was 100%.
A 2013 retrospective study of 2477 symptomatic adults (older than 18 years) who received diagnostic testing for celiac disease at 2 academic institutions in Cleveland, Ohio, evaluated the predictive value of IgA anti-tTG and EMA. Of the patients, 610 (25%) had abnormal serologic tests, and 240 (39%) underwent endoscopy with biopsy.
A total of 50 patients (21%) had biopsy results consistent with celiac disease, defined as a Marsh 3 lesion or greater.3 An IgA anti-tTG level of 118 U/mL (5.9-fold the upper limit of normal on the test) had a positive predictive value of 86.4% with a false-positive value of 2%. An EMA titer greater than 1:160 when IgA anti-tTG was between 21 and 118 U/mL had a positive predictive value of 83%.
Antibody levels 10 times normal show 100% positive predictive value
A 2008 retrospective study of one manufacturer’s IgA anti-tTG serologic test sought to establish the serologic antibody level at which the positive predictive value was 100%.4 Overall, 148 people, 15 years and older, with a positive IgA anti-tTG before biopsy or within 21 days of biopsy were included.
Of the patients biopsied, 139 (93%) had positive biopsies of Marsh 2 or greater and were diagnosed with celiac disease. Using a cut-off of 3.3 and 6.7 times the upper limit of normal, investigators calculated a positive predictive value of 95% and 98%, respectively.
A cut-off of 10 times the upper limit of normal or greater had a positive predictive value of 100%. The highest level of IgA anti-tTG in a patient who didn’t have celiac disease on biopsy was 7.3 times the upper limit of normal.
1. Vermeersch P, Geboes K, Mariën G, et al. Defining thresholds of antibody levels improves diagnosis of celiac disease. Clin Gastroenterol Hepatol. 2013;11:398-403;quiz e32.
2. Klapp G, Masip E, Bolonio M, et al. Celiac disease: The new proposed ESPGHAN diagnostic criteria do work well in a selected population. J Pediatr Gastroenterol Nutr. 2013;56:251-256.
3. Wakim-Fleming J, Pagadala MR, Lemyre MS, et al. Diagnosis of celiac disease in adults based on serology test results, without small-bowel biopsy. Clin Gastroenterol Hepatol. 2013;11:511-516.
4. Hill PG, Holmes GK. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008;27:572-577.
EVIDENCE SUMMARY
A 2013 Belgian prospective study of 104 non-IgA–deficient adults and children diagnosed with celiac disease and 537 adults and children without celiac disease evaluated the accuracy of 4 manufacturers’ serologic tests for IgA anti-tTG.1 All patients underwent serologic testing followed by a diagnostic biopsy. A Marsh type 3 or greater lesion on duodenal biopsy was considered diagnostic for celiac disease.
Anti-tTG levels greater than 10 times the manufacturer-recommended level for a positive test (cut-off) were associated with a likelihood ratio of 111 to 294 (depending on the manufacturer) of positive biopsy. Post-test probabilities were calculated based on various pretest probabilities using an anti-tTG level of greater than 10 times the cut-off (TABLE1).
Investigators also obtained IgG anti-DGP levels from 2 of the manufacturers.1 Likelihood ratios increased along with antibody levels. Ratios of 80 and 400, depending on the manufacturer, were found at IgG anti-DGP levels 10-fold greater than the cut-off. Pre- and post-test probabilities weren’t calculated.

Positive predictive value rises with antibody levels
A 2013 retrospective study evaluated the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition’s recommendation to forego intestinal biopsy in non-IgA–deficient, symptomatic children and adolescents with positive IgA anti-tTG levels greater than 10 times the cut-off value, positive EMA, and positive HLA-DQ2 or HLA-DQ8.2
Overall, 153 symptomatic patients referred to the gastroenterology unit met these criteria. The age range was 9 months to 14.6 years (mean 4 years). All but 3 of the patients had Marsh 2 or greater lesions with biopsy-confirmed diagnoses of celiac disease. The remaining 3 developed biopsy-positive celiac disease on follow-up. The positive predictive value of combined serologic testing in this small selected patient population was 100%.
A 2013 retrospective study of 2477 symptomatic adults (older than 18 years) who received diagnostic testing for celiac disease at 2 academic institutions in Cleveland, Ohio, evaluated the predictive value of IgA anti-tTG and EMA. Of the patients, 610 (25%) had abnormal serologic tests, and 240 (39%) underwent endoscopy with biopsy.
A total of 50 patients (21%) had biopsy results consistent with celiac disease, defined as a Marsh 3 lesion or greater.3 An IgA anti-tTG level of 118 U/mL (5.9-fold the upper limit of normal on the test) had a positive predictive value of 86.4% with a false-positive value of 2%. An EMA titer greater than 1:160 when IgA anti-tTG was between 21 and 118 U/mL had a positive predictive value of 83%.
Antibody levels 10 times normal show 100% positive predictive value
A 2008 retrospective study of one manufacturer’s IgA anti-tTG serologic test sought to establish the serologic antibody level at which the positive predictive value was 100%.4 Overall, 148 people, 15 years and older, with a positive IgA anti-tTG before biopsy or within 21 days of biopsy were included.
Of the patients biopsied, 139 (93%) had positive biopsies of Marsh 2 or greater and were diagnosed with celiac disease. Using a cut-off of 3.3 and 6.7 times the upper limit of normal, investigators calculated a positive predictive value of 95% and 98%, respectively.
A cut-off of 10 times the upper limit of normal or greater had a positive predictive value of 100%. The highest level of IgA anti-tTG in a patient who didn’t have celiac disease on biopsy was 7.3 times the upper limit of normal.
EVIDENCE SUMMARY
A 2013 Belgian prospective study of 104 non-IgA–deficient adults and children diagnosed with celiac disease and 537 adults and children without celiac disease evaluated the accuracy of 4 manufacturers’ serologic tests for IgA anti-tTG.1 All patients underwent serologic testing followed by a diagnostic biopsy. A Marsh type 3 or greater lesion on duodenal biopsy was considered diagnostic for celiac disease.
Anti-tTG levels greater than 10 times the manufacturer-recommended level for a positive test (cut-off) were associated with a likelihood ratio of 111 to 294 (depending on the manufacturer) of positive biopsy. Post-test probabilities were calculated based on various pretest probabilities using an anti-tTG level of greater than 10 times the cut-off (TABLE1).
Investigators also obtained IgG anti-DGP levels from 2 of the manufacturers.1 Likelihood ratios increased along with antibody levels. Ratios of 80 and 400, depending on the manufacturer, were found at IgG anti-DGP levels 10-fold greater than the cut-off. Pre- and post-test probabilities weren’t calculated.

Positive predictive value rises with antibody levels
A 2013 retrospective study evaluated the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition’s recommendation to forego intestinal biopsy in non-IgA–deficient, symptomatic children and adolescents with positive IgA anti-tTG levels greater than 10 times the cut-off value, positive EMA, and positive HLA-DQ2 or HLA-DQ8.2
Overall, 153 symptomatic patients referred to the gastroenterology unit met these criteria. The age range was 9 months to 14.6 years (mean 4 years). All but 3 of the patients had Marsh 2 or greater lesions with biopsy-confirmed diagnoses of celiac disease. The remaining 3 developed biopsy-positive celiac disease on follow-up. The positive predictive value of combined serologic testing in this small selected patient population was 100%.
A 2013 retrospective study of 2477 symptomatic adults (older than 18 years) who received diagnostic testing for celiac disease at 2 academic institutions in Cleveland, Ohio, evaluated the predictive value of IgA anti-tTG and EMA. Of the patients, 610 (25%) had abnormal serologic tests, and 240 (39%) underwent endoscopy with biopsy.
A total of 50 patients (21%) had biopsy results consistent with celiac disease, defined as a Marsh 3 lesion or greater.3 An IgA anti-tTG level of 118 U/mL (5.9-fold the upper limit of normal on the test) had a positive predictive value of 86.4% with a false-positive value of 2%. An EMA titer greater than 1:160 when IgA anti-tTG was between 21 and 118 U/mL had a positive predictive value of 83%.
Antibody levels 10 times normal show 100% positive predictive value
A 2008 retrospective study of one manufacturer’s IgA anti-tTG serologic test sought to establish the serologic antibody level at which the positive predictive value was 100%.4 Overall, 148 people, 15 years and older, with a positive IgA anti-tTG before biopsy or within 21 days of biopsy were included.
Of the patients biopsied, 139 (93%) had positive biopsies of Marsh 2 or greater and were diagnosed with celiac disease. Using a cut-off of 3.3 and 6.7 times the upper limit of normal, investigators calculated a positive predictive value of 95% and 98%, respectively.
A cut-off of 10 times the upper limit of normal or greater had a positive predictive value of 100%. The highest level of IgA anti-tTG in a patient who didn’t have celiac disease on biopsy was 7.3 times the upper limit of normal.
1. Vermeersch P, Geboes K, Mariën G, et al. Defining thresholds of antibody levels improves diagnosis of celiac disease. Clin Gastroenterol Hepatol. 2013;11:398-403;quiz e32.
2. Klapp G, Masip E, Bolonio M, et al. Celiac disease: The new proposed ESPGHAN diagnostic criteria do work well in a selected population. J Pediatr Gastroenterol Nutr. 2013;56:251-256.
3. Wakim-Fleming J, Pagadala MR, Lemyre MS, et al. Diagnosis of celiac disease in adults based on serology test results, without small-bowel biopsy. Clin Gastroenterol Hepatol. 2013;11:511-516.
4. Hill PG, Holmes GK. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008;27:572-577.
1. Vermeersch P, Geboes K, Mariën G, et al. Defining thresholds of antibody levels improves diagnosis of celiac disease. Clin Gastroenterol Hepatol. 2013;11:398-403;quiz e32.
2. Klapp G, Masip E, Bolonio M, et al. Celiac disease: The new proposed ESPGHAN diagnostic criteria do work well in a selected population. J Pediatr Gastroenterol Nutr. 2013;56:251-256.
3. Wakim-Fleming J, Pagadala MR, Lemyre MS, et al. Diagnosis of celiac disease in adults based on serology test results, without small-bowel biopsy. Clin Gastroenterol Hepatol. 2013;11:511-516.
4. Hill PG, Holmes GK. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008;27:572-577.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
It depends on the antibody levels in the blood work. Symptomatic patients with serologic levels of immunoglobulin A anti-tissue transglutaminase (IgA anti-tTG) or immunoglobulin G anti-deamidated gliadin peptide antibody (IgG anti-DGP) greater than 10 times the upper limits of normal—especially if they also are positive for endomysial antibodies (EMA) and human leukocyte antigen DQ2 (HLA-DQ2 or HLA-DQ8)—may not need an intestinal biopsy to confirm the diagnosis of celiac disease (strength of recommendation [SOR]: B, inconsistent or limited-quality cohort studies).
Patients with antibody levels lower than 10 times the upper limits of normal or who are asymptomatic most likely need an intestinal biopsy to confirm the diagnosis (SOR: B, inconsistent or limited-quality cohort studies).
Does vitamin D without calcium reduce fracture risk?
EVIDENCE SUMMARY
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.
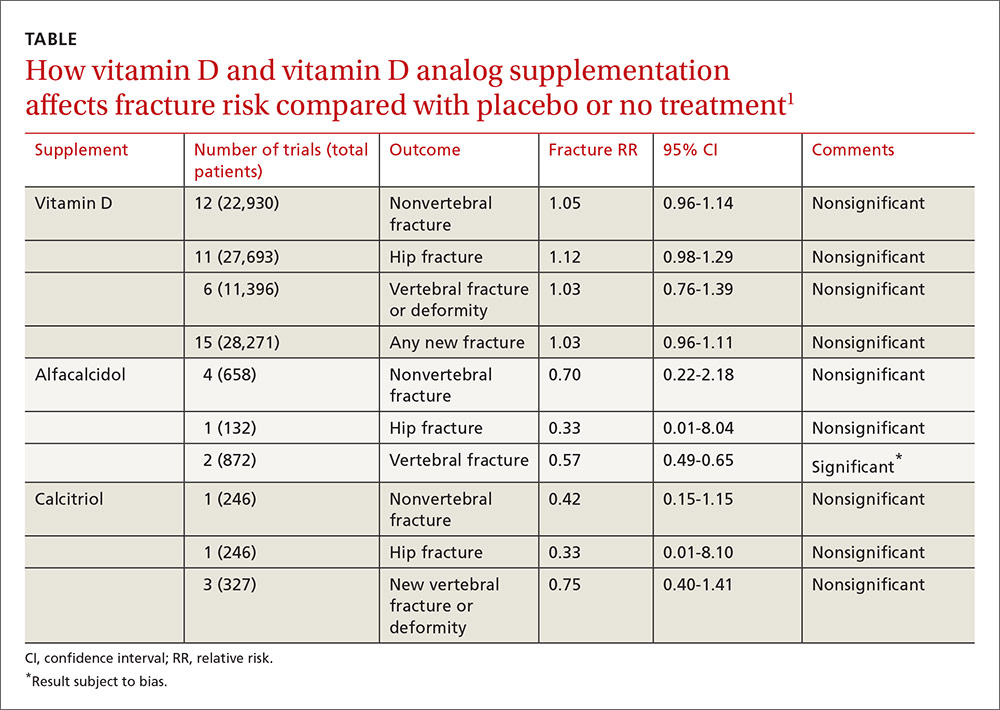
Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
EVIDENCE SUMMARY
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.

Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
EVIDENCE SUMMARY
A 2014 meta-analysis of 15 trials (quasi-random and RCT) with a total of 28,271 patients that compared the effect of vitamin D on fracture risk with placebo or no treatment, found no benefit for vitamin D supplementation (TABLE).1 Patients lived in community and nursing home settings and ranged in age from 50 to 85 years; 24% to 100% were female.
Only 3 trials required patients to have had a previous fracture. Exclusions included: diseases affecting bone metabolism, cognitive impairment, drugs affecting bone metabolism (bisphosphonates, selective estrogen receptor modulators, and corticosteroids), renal failure, hypercalcemia, nephrolithiasis, and decreased mobility (recent stroke recovery and Parkinson’s disease).
Formulations of vitamin D included cholecalciferol (D3) 400 to 2000 IU/d for 4 months to 5 years or 100,000 to 500,000 IU every 3 to 12 months for 1 to 5 years; calcifediol (25(OH)D3) 600 IU/d for 4 years; and ergocalciferol (D2) 400 IU/d for 2 years or 3000 to 300,000 IU every 3 to 12 months for 10 months to 3 years.

Vitamin D analogs generally have no benefit either
The same meta-analysis compared vitamin D analogs to placebo or no treatment (8 trials, quasi-random and RCT, 1743 patients) on the risk of fracture, again finding no benefit in all but one case. Included patients were mostly by referral to tertiary or university hospitals and outpatient community settings.
Most of the studies included only a small number of patients (about 200), with the largest study having 740 patients. The age range was 50 to 77 years, and 50% to 100% were female. Most of the trials required patients to have osteoporosis or vitamin D deficiency with a previous vertebral deformity on imaging. Study exclusions included osteomalacia, malabsorption, hyperparathyroidism, active kidney stones, history of hypercalciuria, cancer, incurable disease, dementia, severe chronic illness (renal or liver failure), recent stroke or fracture, and drugs that affect bone metabolism.
Vitamin D analogs were given as alfacalcidol (1-alphahydroxyvitamin D3) 0.5 mcg twice daily or 1 mcg/d for 36 weeks to 2 years or calcitriol (1,25-dihydroxyvitamin D3) 0.25 to 1 mcg once or twice daily for one to 3 years. Researchers found a significant reduction in vertebral (but not nonvertebral or hip) fractures with alfacalcidol, but the finding occurred in a single trial that was assessed by the authors of the meta-analysis as subject to bias.
Supplementation doesn’t affect mortality, but does have some side effects
Patients taking vitamin D or an analog with or without calcium showed no difference in risk of death compared with patients taking placebo (29 trials, 71,032 patients; relative risk [RR]=0.97; 95% confidence interval [CI], 0.93-1.01).
Patients taking vitamin D or an analog were more likely than controls to have mild hypercalcemia, with an average increase of 2.7 mmol/L (21 trials, 17,124 patients; RR=2.28; 95% CI, 1.57-3.31). Patients taking calcitriol had the highest risk (4 trials, 988 patients; RR=4.41; 95% CI, 2.14-9.09).
Gastrointestinal adverse effects (4% increase) and renal calculi or mild renal insufficiency (16% increase) were more common with vitamin D and analogs than placebo (GI adverse effects: 15 trials, 47,761 patients; RR=1.04; 95% CI, 1.00-1.08; renal calculi or mild renal insufficiency: 11 trials, 46,548 patients; RR=1.16; 95% CI, 1.02-1.33).
RECOMMENDATIONS
There are no guidelines recommending vitamin D supplementation without calcium to prevent fracture.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
1. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
No. Supplemental vitamin D without calcium—in doses averaging as much as 800 IU per day—doesn’t reduce the risk of hip, vertebral, or nonvertebral fractures in postmenopausal women and older men (strength of recommendation [SOR]: A, large, high-quality meta-analysis of randomized or quasi-randomized placebo-controlled trials).
The vitamin D analogs alfacalcidol and calcitriol also don’t reduce hip or nonvertebral fractures (SOR: A, multiple randomized, controlled trials [RCTs]), although alfacalcidol (but not calcitriol) does reduce vertebral fractures by 43% (SOR: B, one RCT and one quasi-randomized trial with potential for bias)
Vitamin D supplementation, with or without calcium, doesn’t affect mortality. It does double the risk of mild hypercalcemia (about 2.7 mmol/L increase), raise the risk of renal calculi or mild renal insufficiency by 16%, and slightly increase (4%) gastrointestinal adverse effects (SOR: A, meta-analysis of RCTs or quasi-randomized trials).
Does breastfeeding affect the risk of childhood obesity?
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. Ever having breastfed during the first year of life is associated with a 15% lower risk of overweight or obesity over the next 2 to 14 years compared with never having breastfed. Breastfeeding exclusively for 6 months is associated with a 30% to 50% reduction in risk (strength of recommendation [SOR]: B, meta-analysis of cohort studies and subsequent cohort studies). However, interventions that increase breastfeeding rates during the first 3 to 6 months of life don’t appear to alter body mass index (BMI) at 11 to 12 years of age (SOR: B, randomized clinical trial [RCT]).
Introducing complementary (solid) foods before 3 months is associated with a 30% greater risk of childhood obesity than later introduction; starting solid foods after 4 months isn’t linked to increased obesity. High caloric density of complementary feedings may be associated with greater childhood obesity (SOR: C, systematic reviews of heterogeneous cohort studies).
Scheduled feeding doubles the risk of rapid infant weight gain compared with on-demand feeding, although it’s unclear whether a direct relationship exists between rapid infant weight gain and childhood obesity (SOR: B, cohort study).
Worsening of longstanding headaches, dizziness, visual symptoms • Dx?
THE CASE
A 59-year-old woman from the Democratic Republic of the Congo presented to our family medicine clinic with acute worsening of longstanding headaches. Using a Swahili interpreter, the patient reported a 15-year history of recurrent, intermittent headaches that had been previously diagnosed as migraines. Over the prior 2 months, the headaches had intensified with new symptoms of dizziness, ocular pain, and blurred vision with red flashes. She had no hemiplegia, dysarthria, respiratory symptoms, night sweats, or weight loss. A neurologic exam was negative.
Before immigrating to the United States 14 years earlier, the patient lived for 6 months in a refugee camp in the Congo. At the time of her immigration, she was negative for human immunodeficiency virus (HIV), and a tuberculosis (TB) skin test was positive. A chest x-ray was normal and she had no respiratory symptoms. Shortly after her immigration, she completed 6 months of isoniazid treatment for latent TB.
THE DIAGNOSIS
A computed tomography (CT) scan of the patient’s head demonstrated a large right frontal mass. The differential diagnosis included neoplasm, sarcoidosis, or, less likely, an infectious etiology. A contrast-enhanced magnetic resonance image (MRI) of the brain showed multiple heterogeneous enhancing lesions, with the largest measuring 4.4 cm x 4.6 cm x 3 cm (FIGURE 1). Significant surrounding edema caused a 1.6-cm midline shift, subfalcine herniation, and impending uncal herniation. A CT of the abdomen and chest showed no pulmonary masses or metastatic disease, but did reveal a single 1-cm lymph node in the mediastinum and a 1.2-cm right axillary node.
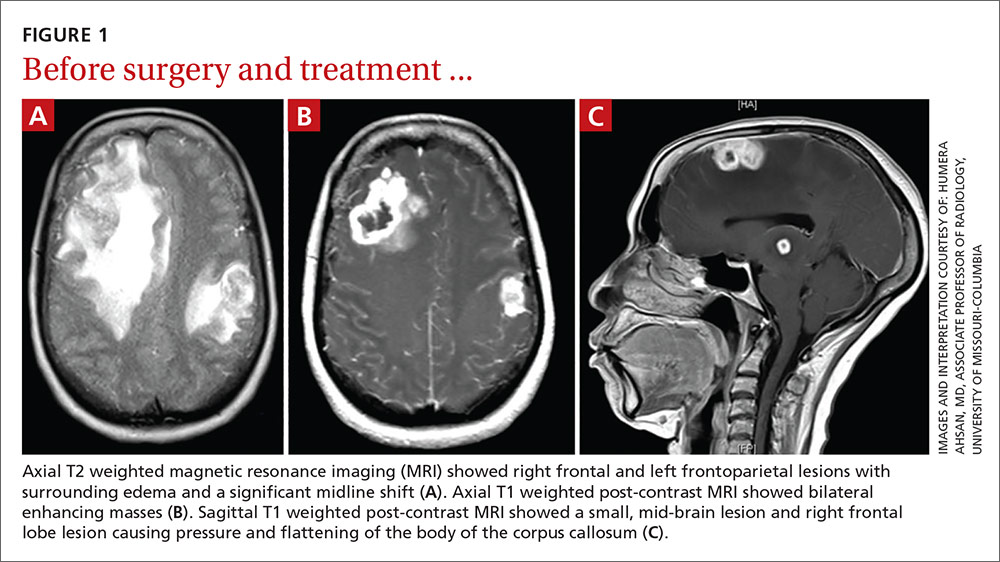
A craniotomy was performed, which confirmed a large mass adhered to the dura. Surgeons removed the mass en bloc; pathology was consistent with a necrotizing granuloma. Acid-fast bacilli (AFB) staining of 3 specimens was negative. Because the tissue was preserved in formalin, mycobacterial cultures could not be obtained. A cerebrospinal fluid analysis showed lymphocytosis and elevated protein, consistent with neurotuberculosis. Blood testing for Mycobacterium tuberculosis with interferon gamma release assay (IGRA) was negative, as was testing for HIV 1 and 2. In addition, induced sputum was AFB-smear negative, as was an M tuberculosis polymerase chain reaction test.
Despite the negative AFB stain and negative IGRA, the patient’s findings were suspicious for TB, so we began to treat her empirically for neurotuberculosis with a 4-drug regimen (isoniazid, rifampin, pyrazinamide, and ethambutol).
In an attempt to confirm the diagnosis of TB and determine sensitivities, we performed a right axillary lymph node biopsy and sent it to the Centers for Disease Control and Prevention (CDC), along with the preserved neural tissue. Using a newly developed technique, the CDC amplified and sequenced mycobacterial DNA from both the central nervous system (CNS) mass and the axillary node, confirming M tuberculosis complex species. Cultures from the axillary node grew pan-sensitive M tuberculosis.
DISCUSSION
About one-third of the world’s population has either active or latent TB.1 In areas where TB is endemic, tuberculomas have accounted for up to 20% of intracranial masses.2 In non-endemic regions, however, they are relatively uncommon. The 3 manifestations of active CNS TB are meningitis, tuberculoma, and abscess.3 The clinical presentation and imaging studies of CNS TB are often indistinguishable from those of patients with malignant neoplasms or metastatic disease. Biopsies may be necessary to distinguish tuberculomas from other intracranial lesions such as pyogenic abscesses or necrotic tumors.4 Mycobacterial cultures were not done on the brain biopsies of our patient because of the high clinical suspicion for neoplasm. Axillary lymph node tissue ultimately confirmed the diagnosis and provided sensitivities.
A diagnosis of CNS tuberculoma without meningitis can be challenging because the clinical presentation is often vague, mild, or even asymptomatic. Constitutional symptoms may include headache, fever, and anorexia.5
In our patient, IGRA testing was also negative. For latent TB, IGRAs are considered to be at least as sensitive as, and considerably more specific than TB skin testing, but their use in CNS TB is less well understood. Studies evaluating IGRA sensitivity for TB meningitis show variable results. In one study, IGRAs were positive in only 50% of culture-confirmed cases of TB meningitis in an HIV-negative population.6
Obtain sputum samples for all patients with extrapulmonary TB
The CDC recommends sputum sampling for all patients with extrapulmonary TB, even in the absence of pulmonary symptoms or radiographic findings, to determine the level of infectivity and potential need for a contact investigation.7
Due to low sensitivity of currently available rapid diagnostic tests and high mortality associated with delayed treatment, initiation of empiric treatment is recommended when the probability of CNS TB is high.5
Treatment duration for CNS tuberculomas is based on one randomized controlled trial,8 a small number of observational studies, a prospective cohort study looking at radiographic resolution,9 and expert opinion. Treatment recommendations often do not distinguish CNS tuberculomas from TB meningitis.10 CNS tuberculomas are commonly treated with a minimum of 12 months of therapy, generally using the same medications and dosages used in the treatment of pulmonary TB, starting with 4 first-line agents: isoniazid, rifampin, pyrazinamide, and ethambutol. Modification of the treatment regimen may be made once sensitivities are available.10
Our patient. After cultures were determined to be pan-sensitive, our patient’s treatment regimen was simplified to rifampin and isoniazid, which she continued for the remainder of her treatment course. Her treatment was discontinued after 18 months when quarterly MRIs showed stabilization of the tuberculomas (FIGURE 2).
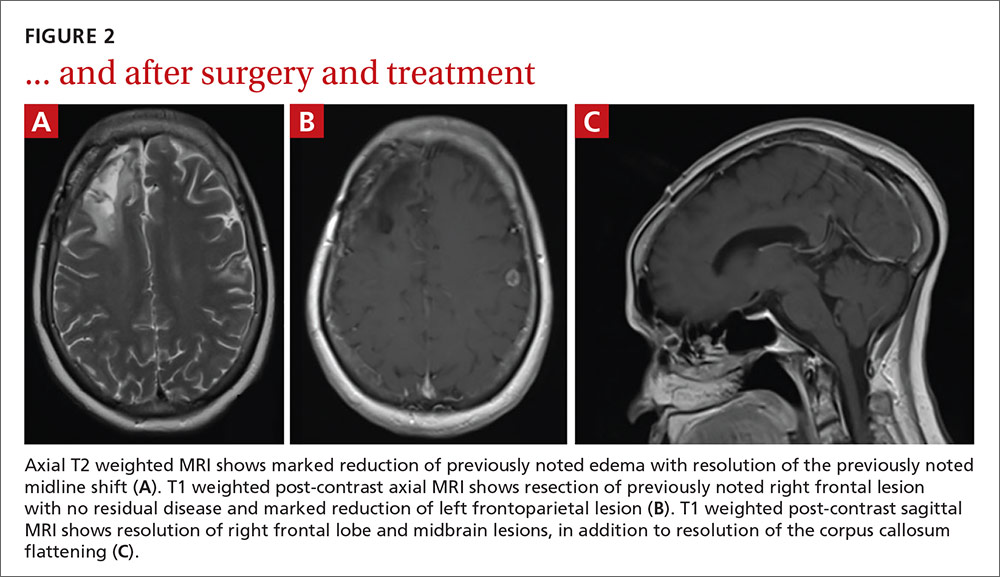
Following her surgery, she was started on levetiractam for seizure prophylaxis. She subsequently had a seizure on 2 occasions when the medication was discontinued or decreased, so we chose to continue it. The patient is asymptomatic from her disease with no residual deficits.
THE TAKEAWAY
A change in headache patterns in a patient over the age of 50 is a red flag that warrants imaging. In patients from countries where TB is endemic,11 consider neurotuberculosis in the differential diagnosis of worsening headaches and progressive neurologic symptoms.
A diagnosis of CNS TB can be difficult and requires a high level of clinical suspicion, but early diagnosis and treatment of neurotuberculosis can minimize the high risk of morbidity and mortality. Treatment for TB shouldn’t be withheld in cases in which there’s a strong clinical suspicion for TB, but for which a definitive diagnosis is still pending.
1. World Health Organization. 10 facts on tuberculosis. Available at: http://www.who.int/features/factfiles/tuberculosis/en/. Accessed September 19, 2014.
2. Dastur DK, Iyer CG. Pathological analysis of 450 intracranial space-occupying lesions. Ind J Cancer. 1966;3:105-115.
3. Chin JH, Mateen FJ. Central nervous system tuberculosis: Challenges and advances in diagnosis and treatment. Curr Infect Dis Rep. 2013;15:631-635.
4. Bayindir C, Mete O, Bilgic B. Retrospective study of 23 pathologically proven cases of central nervous system tuberculomas. Clin Neurol Neurosurg. 2006;108:353-357.
5. Thwaites G, Fisher M, Hemingway C, et al; British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59:167-187.
6. Simmons CP, Thwaites GE, Quyen NT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol. 2006;176:2007-2014.
7. Centers for Disease Control and Prevention (CDC). Core curriculum on tuberculosis: What the clinician should know. 6th ed. Centers for Disease Control and Prevention, Atlanta, GA; 2013.
8. Rajeswari R, Sivasubramanian S, Balambal R, et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis. 1995;76:311-317.
9. Poonnoose SI, Rajshekhar V. Rate of resolution of histologically verified intracranial tuberculomas. Neurosurgery. 2003;53:873-878.
10. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77. Erratum in: MMWR Recomm Rep. 2005;53:1203.
11. Stop TB Partnership. High burden countries. Available at: http://www.stoptb.org/countries/tbdata.asp. Accessed November 7, 2016.
THE CASE
A 59-year-old woman from the Democratic Republic of the Congo presented to our family medicine clinic with acute worsening of longstanding headaches. Using a Swahili interpreter, the patient reported a 15-year history of recurrent, intermittent headaches that had been previously diagnosed as migraines. Over the prior 2 months, the headaches had intensified with new symptoms of dizziness, ocular pain, and blurred vision with red flashes. She had no hemiplegia, dysarthria, respiratory symptoms, night sweats, or weight loss. A neurologic exam was negative.
Before immigrating to the United States 14 years earlier, the patient lived for 6 months in a refugee camp in the Congo. At the time of her immigration, she was negative for human immunodeficiency virus (HIV), and a tuberculosis (TB) skin test was positive. A chest x-ray was normal and she had no respiratory symptoms. Shortly after her immigration, she completed 6 months of isoniazid treatment for latent TB.
THE DIAGNOSIS
A computed tomography (CT) scan of the patient’s head demonstrated a large right frontal mass. The differential diagnosis included neoplasm, sarcoidosis, or, less likely, an infectious etiology. A contrast-enhanced magnetic resonance image (MRI) of the brain showed multiple heterogeneous enhancing lesions, with the largest measuring 4.4 cm x 4.6 cm x 3 cm (FIGURE 1). Significant surrounding edema caused a 1.6-cm midline shift, subfalcine herniation, and impending uncal herniation. A CT of the abdomen and chest showed no pulmonary masses or metastatic disease, but did reveal a single 1-cm lymph node in the mediastinum and a 1.2-cm right axillary node.

A craniotomy was performed, which confirmed a large mass adhered to the dura. Surgeons removed the mass en bloc; pathology was consistent with a necrotizing granuloma. Acid-fast bacilli (AFB) staining of 3 specimens was negative. Because the tissue was preserved in formalin, mycobacterial cultures could not be obtained. A cerebrospinal fluid analysis showed lymphocytosis and elevated protein, consistent with neurotuberculosis. Blood testing for Mycobacterium tuberculosis with interferon gamma release assay (IGRA) was negative, as was testing for HIV 1 and 2. In addition, induced sputum was AFB-smear negative, as was an M tuberculosis polymerase chain reaction test.
Despite the negative AFB stain and negative IGRA, the patient’s findings were suspicious for TB, so we began to treat her empirically for neurotuberculosis with a 4-drug regimen (isoniazid, rifampin, pyrazinamide, and ethambutol).
In an attempt to confirm the diagnosis of TB and determine sensitivities, we performed a right axillary lymph node biopsy and sent it to the Centers for Disease Control and Prevention (CDC), along with the preserved neural tissue. Using a newly developed technique, the CDC amplified and sequenced mycobacterial DNA from both the central nervous system (CNS) mass and the axillary node, confirming M tuberculosis complex species. Cultures from the axillary node grew pan-sensitive M tuberculosis.
DISCUSSION
About one-third of the world’s population has either active or latent TB.1 In areas where TB is endemic, tuberculomas have accounted for up to 20% of intracranial masses.2 In non-endemic regions, however, they are relatively uncommon. The 3 manifestations of active CNS TB are meningitis, tuberculoma, and abscess.3 The clinical presentation and imaging studies of CNS TB are often indistinguishable from those of patients with malignant neoplasms or metastatic disease. Biopsies may be necessary to distinguish tuberculomas from other intracranial lesions such as pyogenic abscesses or necrotic tumors.4 Mycobacterial cultures were not done on the brain biopsies of our patient because of the high clinical suspicion for neoplasm. Axillary lymph node tissue ultimately confirmed the diagnosis and provided sensitivities.
A diagnosis of CNS tuberculoma without meningitis can be challenging because the clinical presentation is often vague, mild, or even asymptomatic. Constitutional symptoms may include headache, fever, and anorexia.5
In our patient, IGRA testing was also negative. For latent TB, IGRAs are considered to be at least as sensitive as, and considerably more specific than TB skin testing, but their use in CNS TB is less well understood. Studies evaluating IGRA sensitivity for TB meningitis show variable results. In one study, IGRAs were positive in only 50% of culture-confirmed cases of TB meningitis in an HIV-negative population.6
Obtain sputum samples for all patients with extrapulmonary TB
The CDC recommends sputum sampling for all patients with extrapulmonary TB, even in the absence of pulmonary symptoms or radiographic findings, to determine the level of infectivity and potential need for a contact investigation.7
Due to low sensitivity of currently available rapid diagnostic tests and high mortality associated with delayed treatment, initiation of empiric treatment is recommended when the probability of CNS TB is high.5
Treatment duration for CNS tuberculomas is based on one randomized controlled trial,8 a small number of observational studies, a prospective cohort study looking at radiographic resolution,9 and expert opinion. Treatment recommendations often do not distinguish CNS tuberculomas from TB meningitis.10 CNS tuberculomas are commonly treated with a minimum of 12 months of therapy, generally using the same medications and dosages used in the treatment of pulmonary TB, starting with 4 first-line agents: isoniazid, rifampin, pyrazinamide, and ethambutol. Modification of the treatment regimen may be made once sensitivities are available.10
Our patient. After cultures were determined to be pan-sensitive, our patient’s treatment regimen was simplified to rifampin and isoniazid, which she continued for the remainder of her treatment course. Her treatment was discontinued after 18 months when quarterly MRIs showed stabilization of the tuberculomas (FIGURE 2).

Following her surgery, she was started on levetiractam for seizure prophylaxis. She subsequently had a seizure on 2 occasions when the medication was discontinued or decreased, so we chose to continue it. The patient is asymptomatic from her disease with no residual deficits.
THE TAKEAWAY
A change in headache patterns in a patient over the age of 50 is a red flag that warrants imaging. In patients from countries where TB is endemic,11 consider neurotuberculosis in the differential diagnosis of worsening headaches and progressive neurologic symptoms.
A diagnosis of CNS TB can be difficult and requires a high level of clinical suspicion, but early diagnosis and treatment of neurotuberculosis can minimize the high risk of morbidity and mortality. Treatment for TB shouldn’t be withheld in cases in which there’s a strong clinical suspicion for TB, but for which a definitive diagnosis is still pending.
THE CASE
A 59-year-old woman from the Democratic Republic of the Congo presented to our family medicine clinic with acute worsening of longstanding headaches. Using a Swahili interpreter, the patient reported a 15-year history of recurrent, intermittent headaches that had been previously diagnosed as migraines. Over the prior 2 months, the headaches had intensified with new symptoms of dizziness, ocular pain, and blurred vision with red flashes. She had no hemiplegia, dysarthria, respiratory symptoms, night sweats, or weight loss. A neurologic exam was negative.
Before immigrating to the United States 14 years earlier, the patient lived for 6 months in a refugee camp in the Congo. At the time of her immigration, she was negative for human immunodeficiency virus (HIV), and a tuberculosis (TB) skin test was positive. A chest x-ray was normal and she had no respiratory symptoms. Shortly after her immigration, she completed 6 months of isoniazid treatment for latent TB.
THE DIAGNOSIS
A computed tomography (CT) scan of the patient’s head demonstrated a large right frontal mass. The differential diagnosis included neoplasm, sarcoidosis, or, less likely, an infectious etiology. A contrast-enhanced magnetic resonance image (MRI) of the brain showed multiple heterogeneous enhancing lesions, with the largest measuring 4.4 cm x 4.6 cm x 3 cm (FIGURE 1). Significant surrounding edema caused a 1.6-cm midline shift, subfalcine herniation, and impending uncal herniation. A CT of the abdomen and chest showed no pulmonary masses or metastatic disease, but did reveal a single 1-cm lymph node in the mediastinum and a 1.2-cm right axillary node.

A craniotomy was performed, which confirmed a large mass adhered to the dura. Surgeons removed the mass en bloc; pathology was consistent with a necrotizing granuloma. Acid-fast bacilli (AFB) staining of 3 specimens was negative. Because the tissue was preserved in formalin, mycobacterial cultures could not be obtained. A cerebrospinal fluid analysis showed lymphocytosis and elevated protein, consistent with neurotuberculosis. Blood testing for Mycobacterium tuberculosis with interferon gamma release assay (IGRA) was negative, as was testing for HIV 1 and 2. In addition, induced sputum was AFB-smear negative, as was an M tuberculosis polymerase chain reaction test.
Despite the negative AFB stain and negative IGRA, the patient’s findings were suspicious for TB, so we began to treat her empirically for neurotuberculosis with a 4-drug regimen (isoniazid, rifampin, pyrazinamide, and ethambutol).
In an attempt to confirm the diagnosis of TB and determine sensitivities, we performed a right axillary lymph node biopsy and sent it to the Centers for Disease Control and Prevention (CDC), along with the preserved neural tissue. Using a newly developed technique, the CDC amplified and sequenced mycobacterial DNA from both the central nervous system (CNS) mass and the axillary node, confirming M tuberculosis complex species. Cultures from the axillary node grew pan-sensitive M tuberculosis.
DISCUSSION
About one-third of the world’s population has either active or latent TB.1 In areas where TB is endemic, tuberculomas have accounted for up to 20% of intracranial masses.2 In non-endemic regions, however, they are relatively uncommon. The 3 manifestations of active CNS TB are meningitis, tuberculoma, and abscess.3 The clinical presentation and imaging studies of CNS TB are often indistinguishable from those of patients with malignant neoplasms or metastatic disease. Biopsies may be necessary to distinguish tuberculomas from other intracranial lesions such as pyogenic abscesses or necrotic tumors.4 Mycobacterial cultures were not done on the brain biopsies of our patient because of the high clinical suspicion for neoplasm. Axillary lymph node tissue ultimately confirmed the diagnosis and provided sensitivities.
A diagnosis of CNS tuberculoma without meningitis can be challenging because the clinical presentation is often vague, mild, or even asymptomatic. Constitutional symptoms may include headache, fever, and anorexia.5
In our patient, IGRA testing was also negative. For latent TB, IGRAs are considered to be at least as sensitive as, and considerably more specific than TB skin testing, but their use in CNS TB is less well understood. Studies evaluating IGRA sensitivity for TB meningitis show variable results. In one study, IGRAs were positive in only 50% of culture-confirmed cases of TB meningitis in an HIV-negative population.6
Obtain sputum samples for all patients with extrapulmonary TB
The CDC recommends sputum sampling for all patients with extrapulmonary TB, even in the absence of pulmonary symptoms or radiographic findings, to determine the level of infectivity and potential need for a contact investigation.7
Due to low sensitivity of currently available rapid diagnostic tests and high mortality associated with delayed treatment, initiation of empiric treatment is recommended when the probability of CNS TB is high.5
Treatment duration for CNS tuberculomas is based on one randomized controlled trial,8 a small number of observational studies, a prospective cohort study looking at radiographic resolution,9 and expert opinion. Treatment recommendations often do not distinguish CNS tuberculomas from TB meningitis.10 CNS tuberculomas are commonly treated with a minimum of 12 months of therapy, generally using the same medications and dosages used in the treatment of pulmonary TB, starting with 4 first-line agents: isoniazid, rifampin, pyrazinamide, and ethambutol. Modification of the treatment regimen may be made once sensitivities are available.10
Our patient. After cultures were determined to be pan-sensitive, our patient’s treatment regimen was simplified to rifampin and isoniazid, which she continued for the remainder of her treatment course. Her treatment was discontinued after 18 months when quarterly MRIs showed stabilization of the tuberculomas (FIGURE 2).

Following her surgery, she was started on levetiractam for seizure prophylaxis. She subsequently had a seizure on 2 occasions when the medication was discontinued or decreased, so we chose to continue it. The patient is asymptomatic from her disease with no residual deficits.
THE TAKEAWAY
A change in headache patterns in a patient over the age of 50 is a red flag that warrants imaging. In patients from countries where TB is endemic,11 consider neurotuberculosis in the differential diagnosis of worsening headaches and progressive neurologic symptoms.
A diagnosis of CNS TB can be difficult and requires a high level of clinical suspicion, but early diagnosis and treatment of neurotuberculosis can minimize the high risk of morbidity and mortality. Treatment for TB shouldn’t be withheld in cases in which there’s a strong clinical suspicion for TB, but for which a definitive diagnosis is still pending.
1. World Health Organization. 10 facts on tuberculosis. Available at: http://www.who.int/features/factfiles/tuberculosis/en/. Accessed September 19, 2014.
2. Dastur DK, Iyer CG. Pathological analysis of 450 intracranial space-occupying lesions. Ind J Cancer. 1966;3:105-115.
3. Chin JH, Mateen FJ. Central nervous system tuberculosis: Challenges and advances in diagnosis and treatment. Curr Infect Dis Rep. 2013;15:631-635.
4. Bayindir C, Mete O, Bilgic B. Retrospective study of 23 pathologically proven cases of central nervous system tuberculomas. Clin Neurol Neurosurg. 2006;108:353-357.
5. Thwaites G, Fisher M, Hemingway C, et al; British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59:167-187.
6. Simmons CP, Thwaites GE, Quyen NT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol. 2006;176:2007-2014.
7. Centers for Disease Control and Prevention (CDC). Core curriculum on tuberculosis: What the clinician should know. 6th ed. Centers for Disease Control and Prevention, Atlanta, GA; 2013.
8. Rajeswari R, Sivasubramanian S, Balambal R, et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis. 1995;76:311-317.
9. Poonnoose SI, Rajshekhar V. Rate of resolution of histologically verified intracranial tuberculomas. Neurosurgery. 2003;53:873-878.
10. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77. Erratum in: MMWR Recomm Rep. 2005;53:1203.
11. Stop TB Partnership. High burden countries. Available at: http://www.stoptb.org/countries/tbdata.asp. Accessed November 7, 2016.
1. World Health Organization. 10 facts on tuberculosis. Available at: http://www.who.int/features/factfiles/tuberculosis/en/. Accessed September 19, 2014.
2. Dastur DK, Iyer CG. Pathological analysis of 450 intracranial space-occupying lesions. Ind J Cancer. 1966;3:105-115.
3. Chin JH, Mateen FJ. Central nervous system tuberculosis: Challenges and advances in diagnosis and treatment. Curr Infect Dis Rep. 2013;15:631-635.
4. Bayindir C, Mete O, Bilgic B. Retrospective study of 23 pathologically proven cases of central nervous system tuberculomas. Clin Neurol Neurosurg. 2006;108:353-357.
5. Thwaites G, Fisher M, Hemingway C, et al; British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59:167-187.
6. Simmons CP, Thwaites GE, Quyen NT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol. 2006;176:2007-2014.
7. Centers for Disease Control and Prevention (CDC). Core curriculum on tuberculosis: What the clinician should know. 6th ed. Centers for Disease Control and Prevention, Atlanta, GA; 2013.
8. Rajeswari R, Sivasubramanian S, Balambal R, et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis. 1995;76:311-317.
9. Poonnoose SI, Rajshekhar V. Rate of resolution of histologically verified intracranial tuberculomas. Neurosurgery. 2003;53:873-878.
10. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77. Erratum in: MMWR Recomm Rep. 2005;53:1203.
11. Stop TB Partnership. High burden countries. Available at: http://www.stoptb.org/countries/tbdata.asp. Accessed November 7, 2016.
Two men with dyspnea, enlarged lymph nodes • Dx?
CASE 1
A 50-year-old man sought care for progressive dyspnea on exertion, abdominal bloating, and bilateral leg edema. He had hypertension that was being treated with atenolol, nifedipine, and enalapril. On examination, his blood pressure was 157/80 mm Hg and his heart rate was 50 beats/min. Jugular venous pressure was grossly elevated with occasional cannon A waves. The patient also had decreased breath sounds in both lower lung zones and moderate pitting edema up to the knees. A chest x-ray showed a small bilateral pleural effusion and no cardiomegaly. An electrocardiogram revealed complete atrioventricular (AV) block with a ventricular response of 50 beats/min. Computed tomography (CT) angiography revealed no evidence of a pulmonary embolus, but did show several enlarged (up to 3.5 cm in diameter) lymph nodes in the upper and middle mediastinum (FIGURE 1). We performed an echocardiogram.
CASE 2
A 79-year-old man with hypertension and diabetes presented to our medical center with acute dyspnea. During the physical examination, we noted bilateral diminished breath sounds with expiratory wheezes and an irregular pulse. Chest x-ray showed mild pulmonary congestion. A chest CT demonstrated bilateral small pleural effusions and multiple enlarged mediastinal lymph nodes with a maximal diameter of 2.4 cm (FIGURE 2A). One week later, the patient’s shortness of breath increased and he was hospitalized. A chest x-ray at that time showed moderate pulmonary congestion, so we performed an echocardiogram.
THE DIAGNOSIS
The echocardiogram for the 50-year-old patient in Case 1 revealed a mildly dilated left ventricle with normal systolic function, diastolic left ventricular (LV) dysfunction, moderate tricuspid regurgitation, and mild pulmonary hypertension. Extensive testing for malignancy and tuberculosis was negative.
For the 79-year-old patient in Case 2, echocardiography demonstrated concentric LV hypertrophy, mild dilatation of the left ventricle, normal LV systolic function, LV diastolic dysfunction with elevated LV diastolic filling pressure, and mild-to-moderate pulmonary hypertension.
Based on these results, we diagnosed both patients with diastolic heart failure. The patient in the second case had features of cardiac asthma, as well. Both patients had also developed reversible mediastinal lymphadenopathy (MLN), of which the diastolic heart failure was the only apparent cause. In both cases, radiologists did not note any suspicious findings for malignancy beyond the MLN.
DISCUSSION
Systolic heart failure has been previously recognized as a cause of MLN.1,2 Other causes of MLN include sarcoidosis, various malignancies, pulmonary infections, and occupational lung diseases. There are, however, no reports of MLN in patients with diastolic heart failure.
Heart failure and MLN. Slanetz et al reported one series of 46 patients who had undergone CT of the chest during periods of congestive heart failure (CHF).1 There was mediastinal lymph node enlargement in 55% of these patients. In a subset of 17 patients who had elevated capillary wedge pressure, 82% had some degree of lymphadenopathy.
Erly et al2 retrospectively studied 44 patients who had a thoracic CT performed before cardiac transplantation. Twenty-nine (66%) had at least one enlarged mediastinal lymph node (>1 cm). Eighty-one percent of patients with an ejection fraction <35% had lymphadenopathy, while none of the patients with an ejection fraction >35% had lymphadenopathy. Most enlarged lymph nodes were pretracheal, with a mean short axis diameter of 1.3 cm.
However, Storto et al reported that an association between CHF and MLN was not found in 7 patients undergoing high-resolution CT imaging.3 There are also cases of MLN in patients with pulmonary hypertension without systolic dysfunction.4
Chabbert et al studied 31 consecutive patients with subacute left heart failure (mean ejection fraction, 39%).5 Enlarged mediastinal lymph nodes were present in 13 patients (42%). Other radiographic features included blurred contour of the lymph nodes in 5 patients (16%) and hazy mediastinal fat in one patient (3%). Follow-up CT showed a significant decrease in the size of the lymph nodes in 8 of 13 patients (62%) following initiation of treatment.
Heart failure and malignancy. A PubMed search with the keywords “diastolic dysfunction” and “lymphoma” found 7 references in the English language. There is a report of 125 survivors of childhood lymphomas treated with mediastinal radiotherapy and anthracyclines,6 another of 44 children treated for acute lymphoblastic leukemia and Hodgkin’s lymphoma7, a report of 294 patients who had received mediastinal irradiation for the treatment of Hodgkin’s disease,8 and another of 106 survivors of non-Hodgkin’s and Hodgkin’s lymphomas.9 None of these reports, however, made any mention of mediastinal lymphadenopathy.
What caused the lymphadenopathy in our patients?
Our 2 patients had volume overload due to diastolic dysfunction with elevated LV end diastolic pressure. Our first patient also had a loss of AV synchronization—which was reversible upon pacemaker insertion—that probably exacerbated the heart failure.
The mechanism for the lymphadenopathy is not clear, but may be due to cardiogenic pulmonary edema causing distension of the pulmonary lymphatic vessels and pulmonary hypertension. In a study of patients with severe systolic dysfunction undergoing evaluation for cardiac transplant, there was a relationship (albeit weak), between MLN and mitral regurgitation, tricuspid regurgitation, elevated mean pulmonary artery pressure, elevated pulmonary capillary wedge pressure, and elevated right atrial pressure.10
How to accurately detect and treat MLN
MLN may be detected by chest x-ray, CT, magnetic resonance imaging, or endoscopic ultrasound examinations. The clinical situation will dictate the imaging modality used. Keep in mind that it is difficult to make a comparison between a finding of lymphadenopathy on one modality and another, especially if one is looking for a change in size.
If clinically appropriate, a trial of diuretics, such as intravenous (IV) furosemide 80 mg,
Our patients. The 50-year-old man in Case 1 responded well to 80 mg of IV furosemide after one hour and improved further upon receipt of a pacemaker the next day. A repeat thoracic CT one month later showed complete resolution of the MLN.
The 79-year-old man in Case 2 also received 80 mg of IV furosemide and improved within 3 hours. A month later, a repeat thoracic CT showed a significant reduction in the size of all the enlarged lymph nodes (FIGURE 2B).
THE TAKEAWAY
The importance of these 2 cases is that they show that heart failure—even diastolic alone—can produce enlarged mediastinal lymph nodes. In patients with heart failure in whom unexpected MLN is detected, consideration should be given to performing a repeat imaging examination after the administration of diuretics.
1. Slanetz PJ, Truong M, Shepard JA, et al. Mediastinal lymphadenopathy and hazy mediastinal fat: new CT findings of congestive heart failure. AJR Am J Roentgenol. 1998;171:1307-1309.
2. Erly WK, Borders RJ, Outwater EK, et al. Location, size, and distribution of mediastinal lymph node enlargement in chronic congestive heart failure. J Comput Assist Tomogr. 2003;27:485-489.
3. Storto ML, Kee ST, Golden JA, et al. Hydrostatic pulmonary edema: high-resolution CT findings. AJR Am J Roentgenol. 1995;165:817-820.
4. Moua T, Levin DL, Carmona EM, et al. Frequency of mediastinal lymphadenopathy in patients with idiopathic pulmonary arterial hypertension. Chest. 2013;143:344-348.
5. Chabbert V, Canevet G, Baixas C, et al. Mediastinal lymphadenopathy in congestive heart failure: a sequential CT evaluation with clinical and echocardiographic correlations. Eur Radiol. 2004;14:881-889.
6. Christiansen JR, Hamre H, Massey R, et al. Left ventricular function in long-term survivors of childhood lymphoma. Am J Cardiol. 2014;114:483-490.
7. Krawczuk-Rybak M, Dakowicz L, Hryniewicz A, et al. Cardiac function in survivors of acute lymphoblastic leukaemia and Hodgkin’s lymphoma. J Paediatr Child Health. 2011;47:455-459.
8. Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977-982.
9. Elbl L, Vasova I, Tomaskova I, et al. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47:843-851.
10. Pastis NJ Jr, Van Bakel AB, Brand TM, et al. Mediastinal lymphadenopathy in patients undergoing cardiac transplant evaluation. Chest. 2011;139:1451-1457.
CASE 1
A 50-year-old man sought care for progressive dyspnea on exertion, abdominal bloating, and bilateral leg edema. He had hypertension that was being treated with atenolol, nifedipine, and enalapril. On examination, his blood pressure was 157/80 mm Hg and his heart rate was 50 beats/min. Jugular venous pressure was grossly elevated with occasional cannon A waves. The patient also had decreased breath sounds in both lower lung zones and moderate pitting edema up to the knees. A chest x-ray showed a small bilateral pleural effusion and no cardiomegaly. An electrocardiogram revealed complete atrioventricular (AV) block with a ventricular response of 50 beats/min. Computed tomography (CT) angiography revealed no evidence of a pulmonary embolus, but did show several enlarged (up to 3.5 cm in diameter) lymph nodes in the upper and middle mediastinum (FIGURE 1). We performed an echocardiogram.

CASE 2
A 79-year-old man with hypertension and diabetes presented to our medical center with acute dyspnea. During the physical examination, we noted bilateral diminished breath sounds with expiratory wheezes and an irregular pulse. Chest x-ray showed mild pulmonary congestion. A chest CT demonstrated bilateral small pleural effusions and multiple enlarged mediastinal lymph nodes with a maximal diameter of 2.4 cm (FIGURE 2A). One week later, the patient’s shortness of breath increased and he was hospitalized. A chest x-ray at that time showed moderate pulmonary congestion, so we performed an echocardiogram.

THE DIAGNOSIS
The echocardiogram for the 50-year-old patient in Case 1 revealed a mildly dilated left ventricle with normal systolic function, diastolic left ventricular (LV) dysfunction, moderate tricuspid regurgitation, and mild pulmonary hypertension. Extensive testing for malignancy and tuberculosis was negative.
For the 79-year-old patient in Case 2, echocardiography demonstrated concentric LV hypertrophy, mild dilatation of the left ventricle, normal LV systolic function, LV diastolic dysfunction with elevated LV diastolic filling pressure, and mild-to-moderate pulmonary hypertension.
Based on these results, we diagnosed both patients with diastolic heart failure. The patient in the second case had features of cardiac asthma, as well. Both patients had also developed reversible mediastinal lymphadenopathy (MLN), of which the diastolic heart failure was the only apparent cause. In both cases, radiologists did not note any suspicious findings for malignancy beyond the MLN.
DISCUSSION
Systolic heart failure has been previously recognized as a cause of MLN.1,2 Other causes of MLN include sarcoidosis, various malignancies, pulmonary infections, and occupational lung diseases. There are, however, no reports of MLN in patients with diastolic heart failure.
Heart failure and MLN. Slanetz et al reported one series of 46 patients who had undergone CT of the chest during periods of congestive heart failure (CHF).1 There was mediastinal lymph node enlargement in 55% of these patients. In a subset of 17 patients who had elevated capillary wedge pressure, 82% had some degree of lymphadenopathy.
Erly et al2 retrospectively studied 44 patients who had a thoracic CT performed before cardiac transplantation. Twenty-nine (66%) had at least one enlarged mediastinal lymph node (>1 cm). Eighty-one percent of patients with an ejection fraction <35% had lymphadenopathy, while none of the patients with an ejection fraction >35% had lymphadenopathy. Most enlarged lymph nodes were pretracheal, with a mean short axis diameter of 1.3 cm.
However, Storto et al reported that an association between CHF and MLN was not found in 7 patients undergoing high-resolution CT imaging.3 There are also cases of MLN in patients with pulmonary hypertension without systolic dysfunction.4
Chabbert et al studied 31 consecutive patients with subacute left heart failure (mean ejection fraction, 39%).5 Enlarged mediastinal lymph nodes were present in 13 patients (42%). Other radiographic features included blurred contour of the lymph nodes in 5 patients (16%) and hazy mediastinal fat in one patient (3%). Follow-up CT showed a significant decrease in the size of the lymph nodes in 8 of 13 patients (62%) following initiation of treatment.
Heart failure and malignancy. A PubMed search with the keywords “diastolic dysfunction” and “lymphoma” found 7 references in the English language. There is a report of 125 survivors of childhood lymphomas treated with mediastinal radiotherapy and anthracyclines,6 another of 44 children treated for acute lymphoblastic leukemia and Hodgkin’s lymphoma7, a report of 294 patients who had received mediastinal irradiation for the treatment of Hodgkin’s disease,8 and another of 106 survivors of non-Hodgkin’s and Hodgkin’s lymphomas.9 None of these reports, however, made any mention of mediastinal lymphadenopathy.
What caused the lymphadenopathy in our patients?
Our 2 patients had volume overload due to diastolic dysfunction with elevated LV end diastolic pressure. Our first patient also had a loss of AV synchronization—which was reversible upon pacemaker insertion—that probably exacerbated the heart failure.
The mechanism for the lymphadenopathy is not clear, but may be due to cardiogenic pulmonary edema causing distension of the pulmonary lymphatic vessels and pulmonary hypertension. In a study of patients with severe systolic dysfunction undergoing evaluation for cardiac transplant, there was a relationship (albeit weak), between MLN and mitral regurgitation, tricuspid regurgitation, elevated mean pulmonary artery pressure, elevated pulmonary capillary wedge pressure, and elevated right atrial pressure.10
How to accurately detect and treat MLN
MLN may be detected by chest x-ray, CT, magnetic resonance imaging, or endoscopic ultrasound examinations. The clinical situation will dictate the imaging modality used. Keep in mind that it is difficult to make a comparison between a finding of lymphadenopathy on one modality and another, especially if one is looking for a change in size.
If clinically appropriate, a trial of diuretics, such as intravenous (IV) furosemide 80 mg,
Our patients. The 50-year-old man in Case 1 responded well to 80 mg of IV furosemide after one hour and improved further upon receipt of a pacemaker the next day. A repeat thoracic CT one month later showed complete resolution of the MLN.
The 79-year-old man in Case 2 also received 80 mg of IV furosemide and improved within 3 hours. A month later, a repeat thoracic CT showed a significant reduction in the size of all the enlarged lymph nodes (FIGURE 2B).
THE TAKEAWAY
The importance of these 2 cases is that they show that heart failure—even diastolic alone—can produce enlarged mediastinal lymph nodes. In patients with heart failure in whom unexpected MLN is detected, consideration should be given to performing a repeat imaging examination after the administration of diuretics.
CASE 1
A 50-year-old man sought care for progressive dyspnea on exertion, abdominal bloating, and bilateral leg edema. He had hypertension that was being treated with atenolol, nifedipine, and enalapril. On examination, his blood pressure was 157/80 mm Hg and his heart rate was 50 beats/min. Jugular venous pressure was grossly elevated with occasional cannon A waves. The patient also had decreased breath sounds in both lower lung zones and moderate pitting edema up to the knees. A chest x-ray showed a small bilateral pleural effusion and no cardiomegaly. An electrocardiogram revealed complete atrioventricular (AV) block with a ventricular response of 50 beats/min. Computed tomography (CT) angiography revealed no evidence of a pulmonary embolus, but did show several enlarged (up to 3.5 cm in diameter) lymph nodes in the upper and middle mediastinum (FIGURE 1). We performed an echocardiogram.

CASE 2
A 79-year-old man with hypertension and diabetes presented to our medical center with acute dyspnea. During the physical examination, we noted bilateral diminished breath sounds with expiratory wheezes and an irregular pulse. Chest x-ray showed mild pulmonary congestion. A chest CT demonstrated bilateral small pleural effusions and multiple enlarged mediastinal lymph nodes with a maximal diameter of 2.4 cm (FIGURE 2A). One week later, the patient’s shortness of breath increased and he was hospitalized. A chest x-ray at that time showed moderate pulmonary congestion, so we performed an echocardiogram.

THE DIAGNOSIS
The echocardiogram for the 50-year-old patient in Case 1 revealed a mildly dilated left ventricle with normal systolic function, diastolic left ventricular (LV) dysfunction, moderate tricuspid regurgitation, and mild pulmonary hypertension. Extensive testing for malignancy and tuberculosis was negative.
For the 79-year-old patient in Case 2, echocardiography demonstrated concentric LV hypertrophy, mild dilatation of the left ventricle, normal LV systolic function, LV diastolic dysfunction with elevated LV diastolic filling pressure, and mild-to-moderate pulmonary hypertension.
Based on these results, we diagnosed both patients with diastolic heart failure. The patient in the second case had features of cardiac asthma, as well. Both patients had also developed reversible mediastinal lymphadenopathy (MLN), of which the diastolic heart failure was the only apparent cause. In both cases, radiologists did not note any suspicious findings for malignancy beyond the MLN.
DISCUSSION
Systolic heart failure has been previously recognized as a cause of MLN.1,2 Other causes of MLN include sarcoidosis, various malignancies, pulmonary infections, and occupational lung diseases. There are, however, no reports of MLN in patients with diastolic heart failure.
Heart failure and MLN. Slanetz et al reported one series of 46 patients who had undergone CT of the chest during periods of congestive heart failure (CHF).1 There was mediastinal lymph node enlargement in 55% of these patients. In a subset of 17 patients who had elevated capillary wedge pressure, 82% had some degree of lymphadenopathy.
Erly et al2 retrospectively studied 44 patients who had a thoracic CT performed before cardiac transplantation. Twenty-nine (66%) had at least one enlarged mediastinal lymph node (>1 cm). Eighty-one percent of patients with an ejection fraction <35% had lymphadenopathy, while none of the patients with an ejection fraction >35% had lymphadenopathy. Most enlarged lymph nodes were pretracheal, with a mean short axis diameter of 1.3 cm.
However, Storto et al reported that an association between CHF and MLN was not found in 7 patients undergoing high-resolution CT imaging.3 There are also cases of MLN in patients with pulmonary hypertension without systolic dysfunction.4
Chabbert et al studied 31 consecutive patients with subacute left heart failure (mean ejection fraction, 39%).5 Enlarged mediastinal lymph nodes were present in 13 patients (42%). Other radiographic features included blurred contour of the lymph nodes in 5 patients (16%) and hazy mediastinal fat in one patient (3%). Follow-up CT showed a significant decrease in the size of the lymph nodes in 8 of 13 patients (62%) following initiation of treatment.
Heart failure and malignancy. A PubMed search with the keywords “diastolic dysfunction” and “lymphoma” found 7 references in the English language. There is a report of 125 survivors of childhood lymphomas treated with mediastinal radiotherapy and anthracyclines,6 another of 44 children treated for acute lymphoblastic leukemia and Hodgkin’s lymphoma7, a report of 294 patients who had received mediastinal irradiation for the treatment of Hodgkin’s disease,8 and another of 106 survivors of non-Hodgkin’s and Hodgkin’s lymphomas.9 None of these reports, however, made any mention of mediastinal lymphadenopathy.
What caused the lymphadenopathy in our patients?
Our 2 patients had volume overload due to diastolic dysfunction with elevated LV end diastolic pressure. Our first patient also had a loss of AV synchronization—which was reversible upon pacemaker insertion—that probably exacerbated the heart failure.
The mechanism for the lymphadenopathy is not clear, but may be due to cardiogenic pulmonary edema causing distension of the pulmonary lymphatic vessels and pulmonary hypertension. In a study of patients with severe systolic dysfunction undergoing evaluation for cardiac transplant, there was a relationship (albeit weak), between MLN and mitral regurgitation, tricuspid regurgitation, elevated mean pulmonary artery pressure, elevated pulmonary capillary wedge pressure, and elevated right atrial pressure.10
How to accurately detect and treat MLN
MLN may be detected by chest x-ray, CT, magnetic resonance imaging, or endoscopic ultrasound examinations. The clinical situation will dictate the imaging modality used. Keep in mind that it is difficult to make a comparison between a finding of lymphadenopathy on one modality and another, especially if one is looking for a change in size.
If clinically appropriate, a trial of diuretics, such as intravenous (IV) furosemide 80 mg,
Our patients. The 50-year-old man in Case 1 responded well to 80 mg of IV furosemide after one hour and improved further upon receipt of a pacemaker the next day. A repeat thoracic CT one month later showed complete resolution of the MLN.
The 79-year-old man in Case 2 also received 80 mg of IV furosemide and improved within 3 hours. A month later, a repeat thoracic CT showed a significant reduction in the size of all the enlarged lymph nodes (FIGURE 2B).
THE TAKEAWAY
The importance of these 2 cases is that they show that heart failure—even diastolic alone—can produce enlarged mediastinal lymph nodes. In patients with heart failure in whom unexpected MLN is detected, consideration should be given to performing a repeat imaging examination after the administration of diuretics.
1. Slanetz PJ, Truong M, Shepard JA, et al. Mediastinal lymphadenopathy and hazy mediastinal fat: new CT findings of congestive heart failure. AJR Am J Roentgenol. 1998;171:1307-1309.
2. Erly WK, Borders RJ, Outwater EK, et al. Location, size, and distribution of mediastinal lymph node enlargement in chronic congestive heart failure. J Comput Assist Tomogr. 2003;27:485-489.
3. Storto ML, Kee ST, Golden JA, et al. Hydrostatic pulmonary edema: high-resolution CT findings. AJR Am J Roentgenol. 1995;165:817-820.
4. Moua T, Levin DL, Carmona EM, et al. Frequency of mediastinal lymphadenopathy in patients with idiopathic pulmonary arterial hypertension. Chest. 2013;143:344-348.
5. Chabbert V, Canevet G, Baixas C, et al. Mediastinal lymphadenopathy in congestive heart failure: a sequential CT evaluation with clinical and echocardiographic correlations. Eur Radiol. 2004;14:881-889.
6. Christiansen JR, Hamre H, Massey R, et al. Left ventricular function in long-term survivors of childhood lymphoma. Am J Cardiol. 2014;114:483-490.
7. Krawczuk-Rybak M, Dakowicz L, Hryniewicz A, et al. Cardiac function in survivors of acute lymphoblastic leukaemia and Hodgkin’s lymphoma. J Paediatr Child Health. 2011;47:455-459.
8. Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977-982.
9. Elbl L, Vasova I, Tomaskova I, et al. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47:843-851.
10. Pastis NJ Jr, Van Bakel AB, Brand TM, et al. Mediastinal lymphadenopathy in patients undergoing cardiac transplant evaluation. Chest. 2011;139:1451-1457.
1. Slanetz PJ, Truong M, Shepard JA, et al. Mediastinal lymphadenopathy and hazy mediastinal fat: new CT findings of congestive heart failure. AJR Am J Roentgenol. 1998;171:1307-1309.
2. Erly WK, Borders RJ, Outwater EK, et al. Location, size, and distribution of mediastinal lymph node enlargement in chronic congestive heart failure. J Comput Assist Tomogr. 2003;27:485-489.
3. Storto ML, Kee ST, Golden JA, et al. Hydrostatic pulmonary edema: high-resolution CT findings. AJR Am J Roentgenol. 1995;165:817-820.
4. Moua T, Levin DL, Carmona EM, et al. Frequency of mediastinal lymphadenopathy in patients with idiopathic pulmonary arterial hypertension. Chest. 2013;143:344-348.
5. Chabbert V, Canevet G, Baixas C, et al. Mediastinal lymphadenopathy in congestive heart failure: a sequential CT evaluation with clinical and echocardiographic correlations. Eur Radiol. 2004;14:881-889.
6. Christiansen JR, Hamre H, Massey R, et al. Left ventricular function in long-term survivors of childhood lymphoma. Am J Cardiol. 2014;114:483-490.
7. Krawczuk-Rybak M, Dakowicz L, Hryniewicz A, et al. Cardiac function in survivors of acute lymphoblastic leukaemia and Hodgkin’s lymphoma. J Paediatr Child Health. 2011;47:455-459.
8. Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977-982.
9. Elbl L, Vasova I, Tomaskova I, et al. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47:843-851.
10. Pastis NJ Jr, Van Bakel AB, Brand TM, et al. Mediastinal lymphadenopathy in patients undergoing cardiac transplant evaluation. Chest. 2011;139:1451-1457.
Perifollicular petechiae and easy bruising
A 45-year-old woman presented to our hospital with recurrent cirrhosis and sepsis secondary to a hepatic abscess from Pseudomonas aeromonas and Candida albicans. Ten years earlier, she had received a liver transplant due to sclerosing cholangitis. The patient had a history of nausea from her liver disease and malnutrition due to a diet that consisted predominantly of cereal with minimal fresh fruits and vegetables. Her family reported that she bruised easily and had worsening dry, flaky skin.
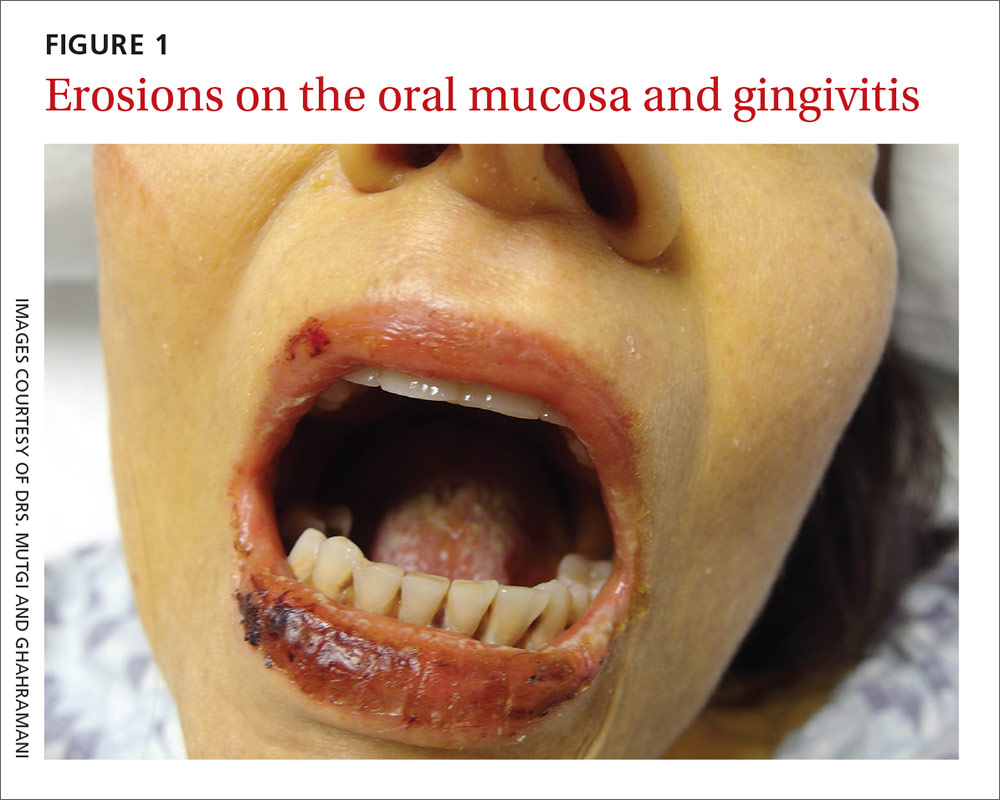
The physical examination revealed jaundice, scleral icterus, purpuric macules, superficial desquamation, gingivitis (FIGURE 1), and perifollicular hemorrhages with corkscrew hairs (FIGURES 2 and 3). The patient also had acute kidney injury, delirium, and pancytopenia. She was admitted to the hospital and was started on piperacillin-tazobactam and fluconazole for sepsis, as well as rifaximin and lactulose for hepatic encephalopathy.
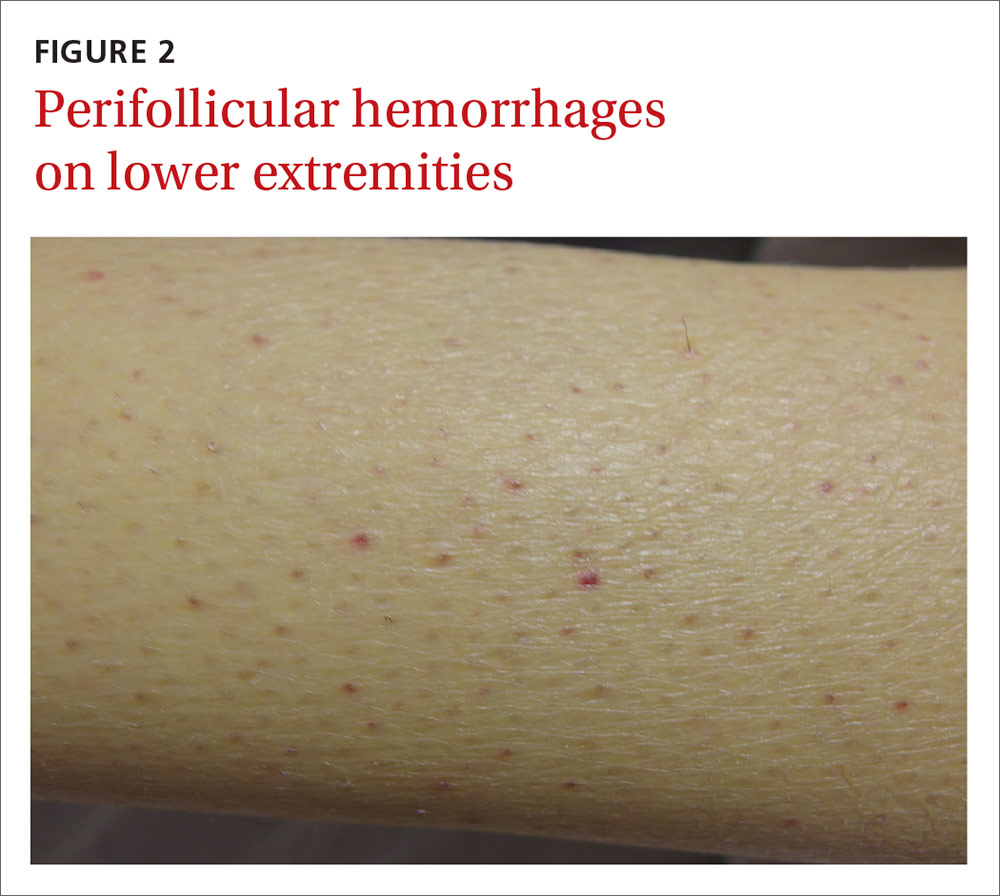
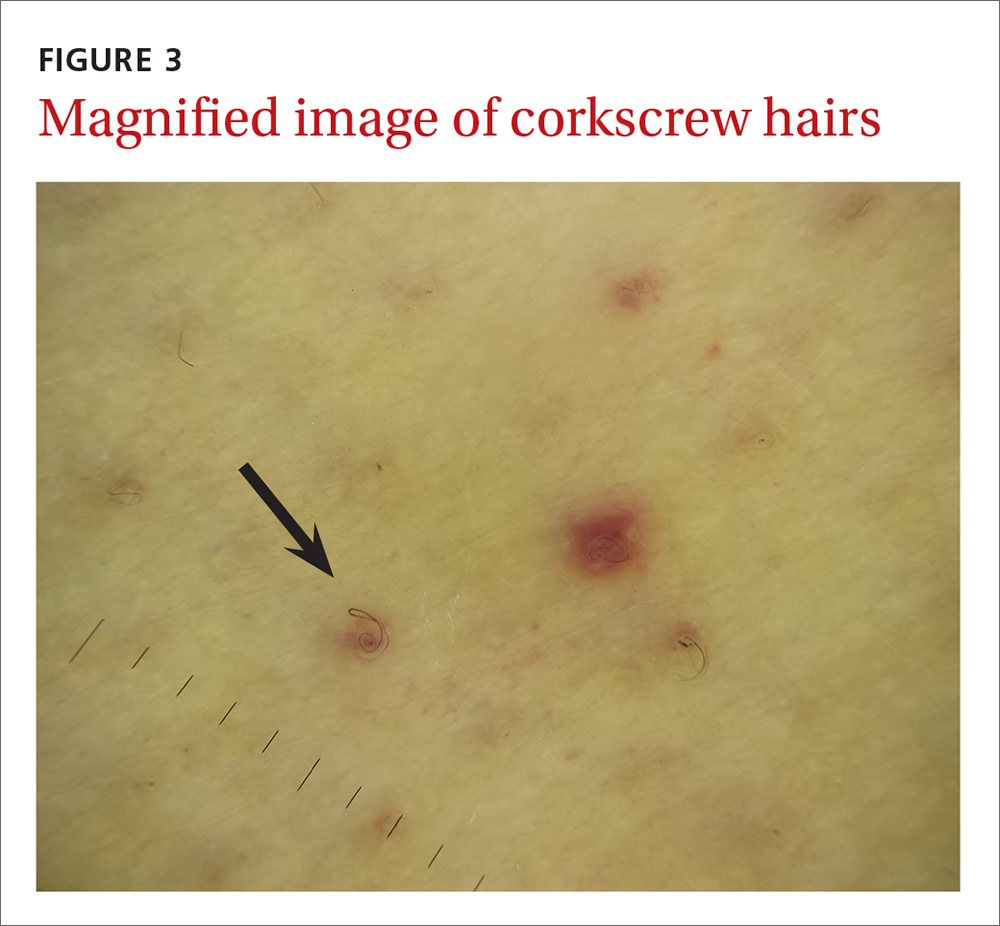
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Vitamin C deficiency
We diagnosed this patient with vitamin C deficiency (scurvy) based on the fact that she had perifollicular hemorrhages with corkscrew hairs, which is a pathognomonic feature of the deficiency. The diagnosis was confirmed with a blood vitamin C level of 10 mcmol/L (normal range: 11-114 mcmol/L). It’s important to note that a vitamin C level drawn after hospitalization may be falsely normal due to an improved diet during hospitalization, so a normal vitamin C level doesn’t always rule out a deficiency.
Vitamin C is absorbed from the small intestine and excreted renally. A low vitamin C level occurs in the presence of several conditions—specifically alcoholism and critical illnesses such as sepsis, trauma, major surgery, or stroke—possibly due to decreased absorption and increased oxidative stress. Vitamin C is necessary for multiple hydroxylation reactions, including hydroxylation of proline and lysine in collagen synthesis, beta-hydroxybutyric acid in carnitine synthesis, and dopamine-beta-hydroxylase in catecholamine synthesis.1
Early vitamin C deficiency presents with nonspecific symptoms, such as malaise, fatigue, and lethargy, followed by the development of anemia, myalgia, bone pain, easy bruising, edema, petechiae, perifollicular hemorrhages, corkscrew hairs, gingivitis, poor wound healing, and mood changes. If untreated, jaundice, neuropathy, hemolysis, seizures, and death can occur.2-4
Perifollicular hemorrhages with corkscrew hairs are characteristic of the Dx
The differential diagnosis of vitamin C deficiency includes vasculitis, thrombocytopenia, multiple myeloma, and folliculitis.
Vasculitis typically presents with palpable purpura and is not exclusively perifollicular.5 Thrombocytopenia may present with petechiae, but the petechiae are generally not perifollicular and hair shaft abnormalities are generally absent. Multiple myeloma may present with purpura with minimal trauma and larger ecchymosis that isn’t petechial in appearance. Folliculitis typically presents with folliculocentric pustules that have surrounding erythema, but no hemorrhaging.
Suspect vitamin C deficiency in patients with alcoholism, a poor diet, and those who are critically ill.6 During the physical exam, look for ecchymosis, edema, gingivitis, impaired wound healing, jaundice, and the tell-tale sign of perifollicular hemorrhages with corkscrew hairs.7
If lab values and/or circumstances leave doubt as to whether a true vitamin C deficiency exists, a punch biopsy of an area of perifollicular hemorrhage may be performed. The punch biopsy should demonstrate follicular hyperkeratosis overlying multiple fragmented hair shafts that are surrounded by a perifollicular lymphohistiocytic infiltrate with extravasated red blood cells.4
Treat with vitamin C
Vitamin C can be replaced via the oral or intravenous (IV) route. There is currently no consensus on a single best replacement regimen, but several effective regimens have been reported in the literature.8,9
We started our patient on IV vitamin C 1 g/d, which improved her cutaneous lesions. Unfortunately, though, she succumbed to complications of her liver disease shortly after starting the therapy.
CORRESPONDENCE
Krishna AJ Mutgi, MD, Nationwide Children’s Hospital, 555 S. 18th Street, Columbus, OH 43205; [email protected].
1. Chambial S, Dwivedi S, Shukla KK, et al. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013;28:314-328.
2. Touyz LZ. Oral scurvy and periodontal disease. J Can Dent Assoc. 1997;63:837-845.
3. Singh S, Richards SJ, Lykins M, et al. An underdiagnosed ailment: scurvy in a tertiary care academic center. Am J Med Sci. 2015;349:372-373.
4. Al-Dabagh A, Milliron BJ, Strowd L, et al. A disease of the present: scurvy in “well-nourished” patients. J Am Acad Dermatol. 2013;69:e246-e247.
5. Francescone MA, Levitt J. Scurvy masquerading as leukocytoclastic vasculitis: a case report and review of the literature. Cutis. 2005;76:261-266.
6. Holley AD, Osland E, Barnes J, et al. Scurvy: historically a plague of the sailor that remains a consideration in the modern intensive care unit. Intern Med J. 2011;41:283-285.
7. Cinotti E, Perrot JL, Labeille B, et al. A dermoscopic clue for scurvy. J Am Acad Dermatol. 2015;72:S37-S38.
8. De Luna RH, Colley BJ 3rd, Smith K, et al. Scurvy: an often forgotten cause of bleeding. Am J Hematol. 2003;74:85-87.
9. Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
A 45-year-old woman presented to our hospital with recurrent cirrhosis and sepsis secondary to a hepatic abscess from Pseudomonas aeromonas and Candida albicans. Ten years earlier, she had received a liver transplant due to sclerosing cholangitis. The patient had a history of nausea from her liver disease and malnutrition due to a diet that consisted predominantly of cereal with minimal fresh fruits and vegetables. Her family reported that she bruised easily and had worsening dry, flaky skin.

The physical examination revealed jaundice, scleral icterus, purpuric macules, superficial desquamation, gingivitis (FIGURE 1), and perifollicular hemorrhages with corkscrew hairs (FIGURES 2 and 3). The patient also had acute kidney injury, delirium, and pancytopenia. She was admitted to the hospital and was started on piperacillin-tazobactam and fluconazole for sepsis, as well as rifaximin and lactulose for hepatic encephalopathy.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Vitamin C deficiency
We diagnosed this patient with vitamin C deficiency (scurvy) based on the fact that she had perifollicular hemorrhages with corkscrew hairs, which is a pathognomonic feature of the deficiency. The diagnosis was confirmed with a blood vitamin C level of 10 mcmol/L (normal range: 11-114 mcmol/L). It’s important to note that a vitamin C level drawn after hospitalization may be falsely normal due to an improved diet during hospitalization, so a normal vitamin C level doesn’t always rule out a deficiency.
Vitamin C is absorbed from the small intestine and excreted renally. A low vitamin C level occurs in the presence of several conditions—specifically alcoholism and critical illnesses such as sepsis, trauma, major surgery, or stroke—possibly due to decreased absorption and increased oxidative stress. Vitamin C is necessary for multiple hydroxylation reactions, including hydroxylation of proline and lysine in collagen synthesis, beta-hydroxybutyric acid in carnitine synthesis, and dopamine-beta-hydroxylase in catecholamine synthesis.1
Early vitamin C deficiency presents with nonspecific symptoms, such as malaise, fatigue, and lethargy, followed by the development of anemia, myalgia, bone pain, easy bruising, edema, petechiae, perifollicular hemorrhages, corkscrew hairs, gingivitis, poor wound healing, and mood changes. If untreated, jaundice, neuropathy, hemolysis, seizures, and death can occur.2-4
Perifollicular hemorrhages with corkscrew hairs are characteristic of the Dx
The differential diagnosis of vitamin C deficiency includes vasculitis, thrombocytopenia, multiple myeloma, and folliculitis.
Vasculitis typically presents with palpable purpura and is not exclusively perifollicular.5 Thrombocytopenia may present with petechiae, but the petechiae are generally not perifollicular and hair shaft abnormalities are generally absent. Multiple myeloma may present with purpura with minimal trauma and larger ecchymosis that isn’t petechial in appearance. Folliculitis typically presents with folliculocentric pustules that have surrounding erythema, but no hemorrhaging.
Suspect vitamin C deficiency in patients with alcoholism, a poor diet, and those who are critically ill.6 During the physical exam, look for ecchymosis, edema, gingivitis, impaired wound healing, jaundice, and the tell-tale sign of perifollicular hemorrhages with corkscrew hairs.7
If lab values and/or circumstances leave doubt as to whether a true vitamin C deficiency exists, a punch biopsy of an area of perifollicular hemorrhage may be performed. The punch biopsy should demonstrate follicular hyperkeratosis overlying multiple fragmented hair shafts that are surrounded by a perifollicular lymphohistiocytic infiltrate with extravasated red blood cells.4
Treat with vitamin C
Vitamin C can be replaced via the oral or intravenous (IV) route. There is currently no consensus on a single best replacement regimen, but several effective regimens have been reported in the literature.8,9
We started our patient on IV vitamin C 1 g/d, which improved her cutaneous lesions. Unfortunately, though, she succumbed to complications of her liver disease shortly after starting the therapy.
CORRESPONDENCE
Krishna AJ Mutgi, MD, Nationwide Children’s Hospital, 555 S. 18th Street, Columbus, OH 43205; [email protected].
A 45-year-old woman presented to our hospital with recurrent cirrhosis and sepsis secondary to a hepatic abscess from Pseudomonas aeromonas and Candida albicans. Ten years earlier, she had received a liver transplant due to sclerosing cholangitis. The patient had a history of nausea from her liver disease and malnutrition due to a diet that consisted predominantly of cereal with minimal fresh fruits and vegetables. Her family reported that she bruised easily and had worsening dry, flaky skin.

The physical examination revealed jaundice, scleral icterus, purpuric macules, superficial desquamation, gingivitis (FIGURE 1), and perifollicular hemorrhages with corkscrew hairs (FIGURES 2 and 3). The patient also had acute kidney injury, delirium, and pancytopenia. She was admitted to the hospital and was started on piperacillin-tazobactam and fluconazole for sepsis, as well as rifaximin and lactulose for hepatic encephalopathy.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Vitamin C deficiency
We diagnosed this patient with vitamin C deficiency (scurvy) based on the fact that she had perifollicular hemorrhages with corkscrew hairs, which is a pathognomonic feature of the deficiency. The diagnosis was confirmed with a blood vitamin C level of 10 mcmol/L (normal range: 11-114 mcmol/L). It’s important to note that a vitamin C level drawn after hospitalization may be falsely normal due to an improved diet during hospitalization, so a normal vitamin C level doesn’t always rule out a deficiency.
Vitamin C is absorbed from the small intestine and excreted renally. A low vitamin C level occurs in the presence of several conditions—specifically alcoholism and critical illnesses such as sepsis, trauma, major surgery, or stroke—possibly due to decreased absorption and increased oxidative stress. Vitamin C is necessary for multiple hydroxylation reactions, including hydroxylation of proline and lysine in collagen synthesis, beta-hydroxybutyric acid in carnitine synthesis, and dopamine-beta-hydroxylase in catecholamine synthesis.1
Early vitamin C deficiency presents with nonspecific symptoms, such as malaise, fatigue, and lethargy, followed by the development of anemia, myalgia, bone pain, easy bruising, edema, petechiae, perifollicular hemorrhages, corkscrew hairs, gingivitis, poor wound healing, and mood changes. If untreated, jaundice, neuropathy, hemolysis, seizures, and death can occur.2-4
Perifollicular hemorrhages with corkscrew hairs are characteristic of the Dx
The differential diagnosis of vitamin C deficiency includes vasculitis, thrombocytopenia, multiple myeloma, and folliculitis.
Vasculitis typically presents with palpable purpura and is not exclusively perifollicular.5 Thrombocytopenia may present with petechiae, but the petechiae are generally not perifollicular and hair shaft abnormalities are generally absent. Multiple myeloma may present with purpura with minimal trauma and larger ecchymosis that isn’t petechial in appearance. Folliculitis typically presents with folliculocentric pustules that have surrounding erythema, but no hemorrhaging.
Suspect vitamin C deficiency in patients with alcoholism, a poor diet, and those who are critically ill.6 During the physical exam, look for ecchymosis, edema, gingivitis, impaired wound healing, jaundice, and the tell-tale sign of perifollicular hemorrhages with corkscrew hairs.7
If lab values and/or circumstances leave doubt as to whether a true vitamin C deficiency exists, a punch biopsy of an area of perifollicular hemorrhage may be performed. The punch biopsy should demonstrate follicular hyperkeratosis overlying multiple fragmented hair shafts that are surrounded by a perifollicular lymphohistiocytic infiltrate with extravasated red blood cells.4
Treat with vitamin C
Vitamin C can be replaced via the oral or intravenous (IV) route. There is currently no consensus on a single best replacement regimen, but several effective regimens have been reported in the literature.8,9
We started our patient on IV vitamin C 1 g/d, which improved her cutaneous lesions. Unfortunately, though, she succumbed to complications of her liver disease shortly after starting the therapy.
CORRESPONDENCE
Krishna AJ Mutgi, MD, Nationwide Children’s Hospital, 555 S. 18th Street, Columbus, OH 43205; [email protected].
1. Chambial S, Dwivedi S, Shukla KK, et al. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013;28:314-328.
2. Touyz LZ. Oral scurvy and periodontal disease. J Can Dent Assoc. 1997;63:837-845.
3. Singh S, Richards SJ, Lykins M, et al. An underdiagnosed ailment: scurvy in a tertiary care academic center. Am J Med Sci. 2015;349:372-373.
4. Al-Dabagh A, Milliron BJ, Strowd L, et al. A disease of the present: scurvy in “well-nourished” patients. J Am Acad Dermatol. 2013;69:e246-e247.
5. Francescone MA, Levitt J. Scurvy masquerading as leukocytoclastic vasculitis: a case report and review of the literature. Cutis. 2005;76:261-266.
6. Holley AD, Osland E, Barnes J, et al. Scurvy: historically a plague of the sailor that remains a consideration in the modern intensive care unit. Intern Med J. 2011;41:283-285.
7. Cinotti E, Perrot JL, Labeille B, et al. A dermoscopic clue for scurvy. J Am Acad Dermatol. 2015;72:S37-S38.
8. De Luna RH, Colley BJ 3rd, Smith K, et al. Scurvy: an often forgotten cause of bleeding. Am J Hematol. 2003;74:85-87.
9. Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
1. Chambial S, Dwivedi S, Shukla KK, et al. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013;28:314-328.
2. Touyz LZ. Oral scurvy and periodontal disease. J Can Dent Assoc. 1997;63:837-845.
3. Singh S, Richards SJ, Lykins M, et al. An underdiagnosed ailment: scurvy in a tertiary care academic center. Am J Med Sci. 2015;349:372-373.
4. Al-Dabagh A, Milliron BJ, Strowd L, et al. A disease of the present: scurvy in “well-nourished” patients. J Am Acad Dermatol. 2013;69:e246-e247.
5. Francescone MA, Levitt J. Scurvy masquerading as leukocytoclastic vasculitis: a case report and review of the literature. Cutis. 2005;76:261-266.
6. Holley AD, Osland E, Barnes J, et al. Scurvy: historically a plague of the sailor that remains a consideration in the modern intensive care unit. Intern Med J. 2011;41:283-285.
7. Cinotti E, Perrot JL, Labeille B, et al. A dermoscopic clue for scurvy. J Am Acad Dermatol. 2015;72:S37-S38.
8. De Luna RH, Colley BJ 3rd, Smith K, et al. Scurvy: an often forgotten cause of bleeding. Am J Hematol. 2003;74:85-87.
9. Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
A more palatable alternative to oral rehydration therapy for kids
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.
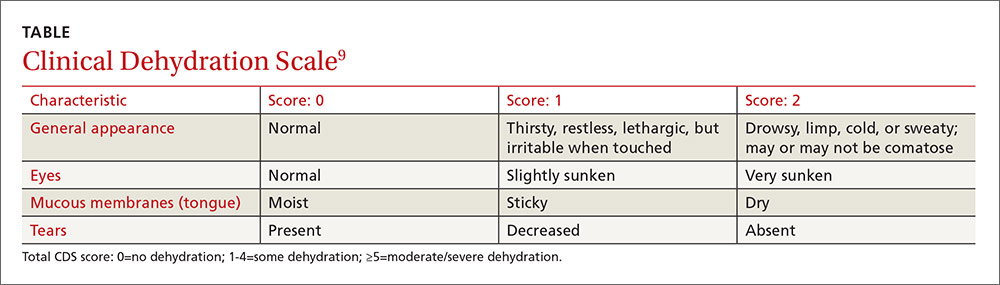
Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.

Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 3-year-old boy is brought by his mother to the office for vomiting and diarrhea that started in the middle of the night. On examination he is slightly dehydrated, but does not have an acute abdomen or other source of infection. He is drinking out of a sippy cup. What fluids should you recommend?
Acute gastroenteritis is a common cause of vomiting and/or diarrhea in children, leading to 1.5 million outpatient visits and 200,000 hospital admissions annually in the United States.2 Children with gastroenteritis are at risk for dehydration, and the recommended treatment for anything less than severe dehydration is oral rehydration therapy (ORT) and early resumption of feeding upon rehydration.2
In 2002, the World Health Organization recommended an ORT with an osmolarity of 245 mOsm/L.3 However, cultural preferences, cost,4 taste,5 availability, and caregiver and professional preference for intravenous hydration6-8 have all been barriers to the use of recommended ORT.2 In fact, a study of ORT preferences in 66 children ages 5 to 10 years found that fewer than half of the children would voluntarily drink the ORT again.5 This study evaluated the use of diluted apple juice as a more palatable alternative to ORT in children with vomiting and/or diarrhea.
STUDY SUMMARY
In kids older than 2, apple juice will do
This study was a single-center, single-blind, non-inferiority randomized controlled trial conducted in the emergency department (ED) of a tertiary care pediatric hospital in Canada. The researchers compared the use of half-strength apple juice to a standard ORT for rehydration in simple gastroenteritis.1 Participants were 6 months to 5 years of age, weighed more than 8 kg (17.7 lbs), and had vomiting and/or diarrhea for less than 96 hours (with ≥3 episodes over the last 24 hours). They also had a Clinical Dehydration Scale (CDS) Score9 <5 and a capillary refill of <2 seconds (TABLE). Of the total, 68% of the children had a CDS score of 0, 25.5% scored 1 to 2; and 6.4% scored 3 to 4. Children with chronic gastrointestinal disease or other significant comorbidities that could affect the clinical state (eg, diabetes mellitus) and potential acute abdominal pathology were excluded.

Children were randomized to receive half-strength apple juice (AJ) (intervention group, n=323) or apple-flavored sucralose-sweetened Pediatric Electrolyte (Pharmascience) (control group, n=324), a common electrolyte maintenance solution (EMS). Immediately on triage, each child received 2 L of their assigned solution, to be used while in the ED and then at home. The children received 5 mL of fluid every 2 to 5 minutes. If a child vomited after starting the fluid, he or she was given oral ondansetron.
At discharge, caregivers were encouraged to replace 2 mL/kg of fluid for a vomiting episode and 10 mL/kg of fluid for a diarrhea episode. At home, children in the AJ group could also drink any other preferred fluid, including sports beverages. The EMS group was instructed to drink only the solution provided or a comparable ORT. Caregivers were contacted daily by phone until the child had no symptoms for 24 hours. They were also asked to keep a daily log of vomiting and diarrhea frequency and any subsequent health care visits. At least one follow-up contact occurred with 99.5% of the children.
The primary outcome was treatment failure defined as a composite measure of any of the following occurring within 7 days of the ED visit: hospitalization, intravenous rehydration, further health care visits for diarrhea/vomiting in any setting, protracted symptoms (ie, ≥3 episodes of vomiting or diarrhea within a 24-hour period occurring >7 days after enrollment), 3% or greater weight loss, or CDS score ≥5 at follow-up.
Treatment failure occurred in 16.7% of the AJ group compared to 25% of the EMS group (difference, -8.3%; 97.5% confidence interval [CI], -∞ to -2; number needed to treat [NNT]=12), consistent with non-inferior effectiveness. The benefit was seen primarily in children ≥24 months of age. In children <24 months, the treatment failure for AJ was 23.9% compared to 24.1% in the EMS group (P=not significant). In older children (≥24 months to 5 years), the treatment failure with AJ was 9.8% compared to 25.9% in the EMS group (difference, -16.2%; 95% CI, -24.2% to -8%; NNT=6.2). Intravenous rehydration in the ED or within 7 days of the initial visit was needed in 2.5% of the AJ group and in 9% of the EMS group (difference, -6.5%; 99% CI, -11.6% to -1.8%; NNT=15.4). There were no differences in hospitalization rate or in diarrhea or vomiting frequency between the 2 groups.
WHAT’S NEW
Kids drink more of what they like
This study, in a developed country, found rehydration with diluted apple juice worked just as well as ORT. In children ≥24 months of age, there were fewer treatment failures.
CAVEATS
Infants may not benefit,and ondansetron played a role
In this study, children were only mildly dehydrated. Also, the study did not include infants younger than 6 months of age, and the greatest benefit was in children ≥24 months of age.
Also noteworthy was the role that oral ondansetron played. Most (67.4%) of the children received an oral dose of ondansetron (0.1 mg/kg). Although expensive, if one dose prevents a hospitalization, it is cost-effective. Previous studies of oral ondansetron show it reduces vomiting (NNT=5); lowers the rate of intravenous hydration in the ED (NNT=5); and reduces the hospitalization rate from the ED (NNT=17).10
Lastly, there are a variety of fluid replacement guidelines. In this study, fluid replacement was 2 mL/kg for a vomiting episode and 10 mL/kg for a diarrhea episode.
CHALLENGES TO IMPLEMENTATION
Given the ease of swapping diluted apple juice for oral rehydration therapy, we see no barriers to implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
1. Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.
2. King CK, Glass R, Bresee JS, et al. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1-16.
3. Essential medicines and health products information portal. A World Health Organization resource. WHO Drug Information. 2002;16(2). Available at: http://apps.who.int/medicinedocs/en/d/Js4950e/2.4.html. Accessed October 20, 2016.
4. Cohen MB, Hardin J. Medicaid coverage of oral rehydration solutions. N Engl J Med. 1993;329:211.
5. Freedman SB, Cho D, Boutis K, et al. Assessing the palatability of oral rehydration solutions in school-aged children: a randomized crossover trial. Arch Pediatr Adolesc Med. 2010;164:696-702.
6. Reis EC, Goepp JG, Katz S, et al. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708-711.
7. Karpas A, Finkelstein M, Reid S. Parental preference for rehydration method for children in the emergency department. Pediatr Emerg Care. 2009;25:301-306.
8. Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics. 2002;109:259-261.
9. Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122:545-549.
10. Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;CD005506.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Recommend that parents give half-strength apple juice to children ≥24 months of age who are minimally dehydrated following a case of simple viral gastroenteritis. The juice reduces the need for further interventions better than oral hydration therapy.
Freedman SB, Willan AR, Boutis K, et al. Effect of dilute apple juice and preferred fluids vs electrolyte maintenance solution on treatment failure among children with mild gastroenteritis: a randomized clinical trial. JAMA. 2016;315:1966-1974.1
STRENGTH OF RECOMMENDATION
B: Based on a single, good quality randomized controlled trial.
6 steps to take when a patient insists on that antibiotic
In this issue of JFP, Wiskirchen and colleagues discuss the appropriate use of antibiotics in outpatient settings, providing stewardship advice for several conditions we frequently see in primary care practice.
One of the symptoms for which we most frequently battle requests for antibiotics is acute cough. Despite the fact that more than 90% of cases of acute cough illness (aka acute bronchitis) are caused by viruses, the prescribing rate for it in the United States remains about 70%.1
Over the years, I’ve honed a “spiel” that I use with patients with acute cough illness to help keep my antibiotic prescribing to a minimum. It must be working; my prescribing rate is less than 20%. What follows are some of my catch phrases and techniques.
1. Acknowledge the patient’s misery. “Sounds like you have a really bad bug."
2. Tell the patient what he or she doesn’t have. “Your lungs sound good, and your throat does not look too bad, so that means you don’t have strep throat or pneumonia. That’s good news.”
3. Explain what viruses are “making the rounds.” If you have surveillance data, that’s even better. “I have seen several other patients with symptoms just like yours this week.” Over 25 years ago, Jon Temte, an FP from Wisconsin, drove down prescribing rates for acute bronchitis below 20% in family medicine residencies by providing feedback to physicians and patients about the viruses circulating in their communities.2
4. Set realistic expectations. Tell patients how long their cough is likely to last. The duration of the typical cough is (unfortunately) about 17 days.3 Most patients (and even some doctors) think a bad cold should be gone in 7 days.3
5. Choose your terms carefully. Don’t use the term “acute bronchitis.” It sounds bad and worthy of an antibiotic. “Chest cold” sounds much more benign; patients are less likely to think they need an antibiotic for a chest cold.4
6. When all else fails, consider a delayed prescription. I reserve this strategy for patients who are insistent on getting an antibiotic even though their illness is clearly viral. Randomized trials of the delayed strategy show that fewer than 50% of patients actually fill the prescription.5
Develop your own spiel to reduce unnecessary antibiotic prescribing. You’ll find that it works a good deal of the time.
1. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311:2020-2022.
2. Temte JL, Shult PA, Kirk CJ, et al. Effects of viral respiratory disease education and surveillance on antibiotic prescribing. Fam Med. 1999;31:101-106.
3. Ebell MH, Lundgren J, Youngpairoj S. How long does a cough last? Comparing patients’ expectations with data from a systematic review of the literature. Ann Fam Med. 2013;11:5-13.
4. Phillips TG, Hickner J. Calling acute bronchitis a chest cold may improve patient satisfaction with appropriate antibiotic use. J Am Board Fam Pract. 2005;18:459-463.
5. Spurling GK, Del Mar CB, Dooley L, et al. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev. 2013:CD004417.
In this issue of JFP, Wiskirchen and colleagues discuss the appropriate use of antibiotics in outpatient settings, providing stewardship advice for several conditions we frequently see in primary care practice.
One of the symptoms for which we most frequently battle requests for antibiotics is acute cough. Despite the fact that more than 90% of cases of acute cough illness (aka acute bronchitis) are caused by viruses, the prescribing rate for it in the United States remains about 70%.1
Over the years, I’ve honed a “spiel” that I use with patients with acute cough illness to help keep my antibiotic prescribing to a minimum. It must be working; my prescribing rate is less than 20%. What follows are some of my catch phrases and techniques.
1. Acknowledge the patient’s misery. “Sounds like you have a really bad bug."
2. Tell the patient what he or she doesn’t have. “Your lungs sound good, and your throat does not look too bad, so that means you don’t have strep throat or pneumonia. That’s good news.”
3. Explain what viruses are “making the rounds.” If you have surveillance data, that’s even better. “I have seen several other patients with symptoms just like yours this week.” Over 25 years ago, Jon Temte, an FP from Wisconsin, drove down prescribing rates for acute bronchitis below 20% in family medicine residencies by providing feedback to physicians and patients about the viruses circulating in their communities.2
4. Set realistic expectations. Tell patients how long their cough is likely to last. The duration of the typical cough is (unfortunately) about 17 days.3 Most patients (and even some doctors) think a bad cold should be gone in 7 days.3
5. Choose your terms carefully. Don’t use the term “acute bronchitis.” It sounds bad and worthy of an antibiotic. “Chest cold” sounds much more benign; patients are less likely to think they need an antibiotic for a chest cold.4
6. When all else fails, consider a delayed prescription. I reserve this strategy for patients who are insistent on getting an antibiotic even though their illness is clearly viral. Randomized trials of the delayed strategy show that fewer than 50% of patients actually fill the prescription.5
Develop your own spiel to reduce unnecessary antibiotic prescribing. You’ll find that it works a good deal of the time.
In this issue of JFP, Wiskirchen and colleagues discuss the appropriate use of antibiotics in outpatient settings, providing stewardship advice for several conditions we frequently see in primary care practice.
One of the symptoms for which we most frequently battle requests for antibiotics is acute cough. Despite the fact that more than 90% of cases of acute cough illness (aka acute bronchitis) are caused by viruses, the prescribing rate for it in the United States remains about 70%.1
Over the years, I’ve honed a “spiel” that I use with patients with acute cough illness to help keep my antibiotic prescribing to a minimum. It must be working; my prescribing rate is less than 20%. What follows are some of my catch phrases and techniques.
1. Acknowledge the patient’s misery. “Sounds like you have a really bad bug."
2. Tell the patient what he or she doesn’t have. “Your lungs sound good, and your throat does not look too bad, so that means you don’t have strep throat or pneumonia. That’s good news.”
3. Explain what viruses are “making the rounds.” If you have surveillance data, that’s even better. “I have seen several other patients with symptoms just like yours this week.” Over 25 years ago, Jon Temte, an FP from Wisconsin, drove down prescribing rates for acute bronchitis below 20% in family medicine residencies by providing feedback to physicians and patients about the viruses circulating in their communities.2
4. Set realistic expectations. Tell patients how long their cough is likely to last. The duration of the typical cough is (unfortunately) about 17 days.3 Most patients (and even some doctors) think a bad cold should be gone in 7 days.3
5. Choose your terms carefully. Don’t use the term “acute bronchitis.” It sounds bad and worthy of an antibiotic. “Chest cold” sounds much more benign; patients are less likely to think they need an antibiotic for a chest cold.4
6. When all else fails, consider a delayed prescription. I reserve this strategy for patients who are insistent on getting an antibiotic even though their illness is clearly viral. Randomized trials of the delayed strategy show that fewer than 50% of patients actually fill the prescription.5
Develop your own spiel to reduce unnecessary antibiotic prescribing. You’ll find that it works a good deal of the time.
1. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311:2020-2022.
2. Temte JL, Shult PA, Kirk CJ, et al. Effects of viral respiratory disease education and surveillance on antibiotic prescribing. Fam Med. 1999;31:101-106.
3. Ebell MH, Lundgren J, Youngpairoj S. How long does a cough last? Comparing patients’ expectations with data from a systematic review of the literature. Ann Fam Med. 2013;11:5-13.
4. Phillips TG, Hickner J. Calling acute bronchitis a chest cold may improve patient satisfaction with appropriate antibiotic use. J Am Board Fam Pract. 2005;18:459-463.
5. Spurling GK, Del Mar CB, Dooley L, et al. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev. 2013:CD004417.
1. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996-2010. JAMA. 2014;311:2020-2022.
2. Temte JL, Shult PA, Kirk CJ, et al. Effects of viral respiratory disease education and surveillance on antibiotic prescribing. Fam Med. 1999;31:101-106.
3. Ebell MH, Lundgren J, Youngpairoj S. How long does a cough last? Comparing patients’ expectations with data from a systematic review of the literature. Ann Fam Med. 2013;11:5-13.
4. Phillips TG, Hickner J. Calling acute bronchitis a chest cold may improve patient satisfaction with appropriate antibiotic use. J Am Board Fam Pract. 2005;18:459-463.
5. Spurling GK, Del Mar CB, Dooley L, et al. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev. 2013:CD004417.
Opioids for chronic pain: The CDC’s 12 recommendations
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1
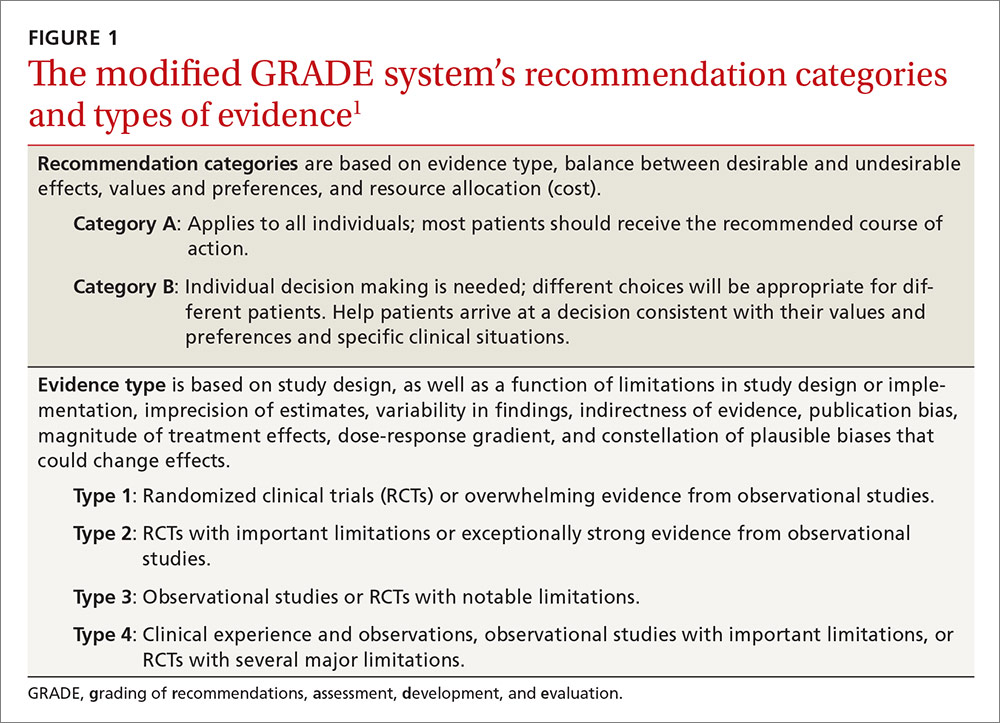
The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7
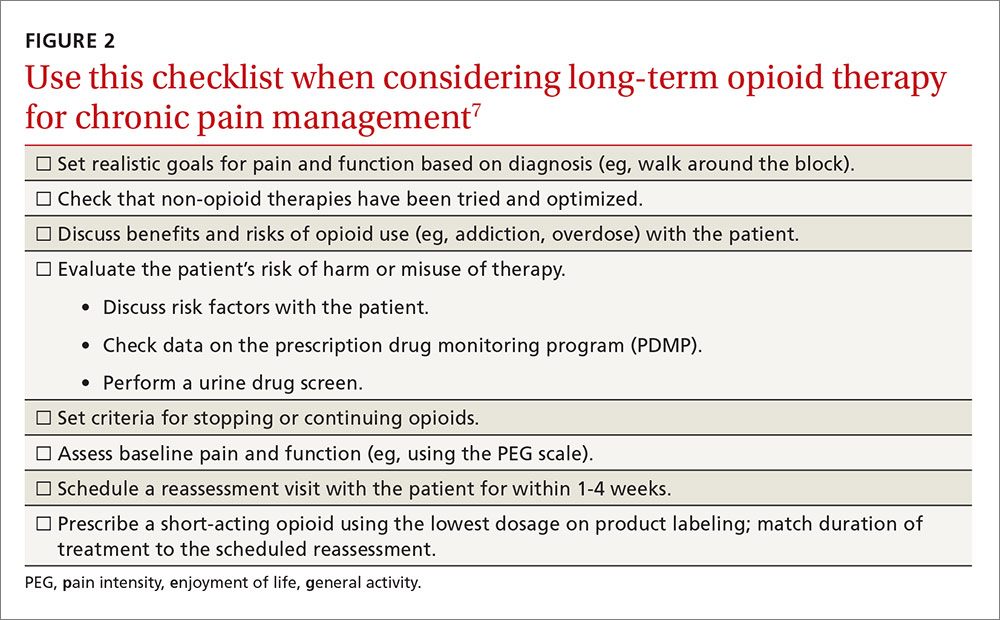
The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1

The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7

The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
Earlier this year, the Centers for Disease Control and Prevention (CDC) published a clinical practice guideline aimed at decreasing opioid use in the treatment of chronic pain.1 It developed this guideline in response to the increasing problem of opioid abuse and opioid-related mortality in the United States.
The CDC notes that an estimated 1.9 million people abused or were dependent on prescription opioid pain medication in 2013.1 Between 1999 and 2014, more than 165,000 people in the United States died from an overdose of opioid pain medication, with that rate increasing markedly in the past decade.1 In 2011, an estimated 420,000 emergency department visits were related to the abuse of narcotic pain relievers.2
While the problem of increasing opioid-related abuse and deaths has been apparent for some time, effective interventions have been elusive. Evidence remains sparse on the benefits and harms of long-term opioid therapy for chronic pain, except for those at the end of life. Evidence has been insufficient to determine long-term benefits of opioid therapy vs no opioid therapy, although the potential for harms from high doses of opioids are documented. There is not much evidence comparing nonpharmacologic and non-opioid pharmacologic treatments with long-term opioid therapy.
This lack of an evidence base is reflected in the CDC guideline. Of the guideline’s 12 recommendations, not one has high-level supporting evidence and only one has even moderate-level evidence behind it. Four recommendations are supported by low-level evidence, and 7 by very-low-level evidence. Yet 11 of the 12 are given an A recommendation, meaning that the guideline panel feels that most patients should receive this course of action.
Methodology used to create the guideline
The guideline committee used a modified GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) to develop the guideline. It is the same system the Advisory Committee on Immunization Practices adopted to assess and make recommendations on vaccines.3 The system’s classification of levels of evidence and recommendation categories are described in FIGURE 1.1

The committee started by assessing evidence with a report on the long-term effectiveness of opioids for chronic pain, produced by the Agency for Health Care Research and Quality in 2014;4 it then augmented that report by performing an updated search for new evidence published since the report came out.5 The committee then conducted a “contextual evidence review”6 on the following 4 areas:
- the effectiveness of nonpharmacologic (cognitive behavioral therapy, exercise therapy, interventional treatments, multimodal pain treatment) and non-opioid pharmacologic treatments (acetaminophen, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants)
- the benefits and harms of opioid therapy
- clinician and patient values and preferences related to opioids and medication risks, benefits, and use
- resource allocation, including costs and economic analyses.
The guideline wording indicates that, for this contextual analysis, the committee used a rapid systematic review methodology, in part because of time constraints given the imperative to produce a guideline to address a pressing problem, and because of a recognition that evidence on the questions would be scant and not of high quality.1 The 12 recommendations are categorized under 3 main headings.
Determining when to initiate or continue opioids for chronic pain
1. Nonpharmacologic therapy and non-opioid pharmacologic therapy are preferred for chronic pain. Consider opioid therapy only if you anticipate that benefits for both pain and function will outweigh risks to the patient. If opioids are used, combine them as appropriate with nonpharmacologic therapy and non-opioid pharmacologic therapy. (Recommendation category: A; evidence type: 3)
2. Before starting opioid therapy for chronic pain, establish treatment goals with the patient, including realistic goals for pain and function, and consider how therapy will be discontinued if the benefits do not outweigh the risks. Continue opioid therapy only if there is clinically meaningful improvement in pain and function that outweighs risks to patient safety. (Recommendation category: A; evidence type: 4)
3. Before starting opioid therapy, and periodically during its course, discuss with patients known risks and realistic benefits of opioid therapy and patient and clinician responsibilities for managing therapy. (Recommendation category: A; evidence type: 3)
Opioid selection, dosage, duration, follow-up, and discontinuation
4. When starting opioid therapy for chronic pain, prescribe immediate-release opioids instead of extended-release/long-acting (ER/LA) agents. (Recommendation category: A; evidence type: 4)
5. When starting opioids, prescribe the lowest effective dosage. Use caution when prescribing opioids at any dosage; carefully reassess the evidence for individual benefits and risks when increasing the dosage to ≥50 morphine milligram equivalents (MME)/d; and avoid increasing the dosage to ≥90 MME/d (or carefully justify such a decision, if made). (Recommendation category: A; evidence type: 3)
6. Long-term opioid use often begins with treatment of acute pain. When opioids are used for acute pain, prescribe the lowest effective dose of immediate-release opioids at a quantity no greater than is needed for the expected duration of pain severe enough to require opioids. Three days or less will often be sufficient; more than 7 days will rarely be needed. (Recommendation category: A; evidence type: 4)
7. In monitoring opioid therapy for chronic pain, reevaluate benefits and harms with patients within one to 4 weeks of starting opioid therapy or escalating the dose. Also, evaluate the benefits and harms of continued therapy with patients every 3 months or more frequently. If the benefits of continued opioid therapy do not outweigh the harms, optimize other therapies and work with patients to taper opioids to lower dosages or to taper and discontinue them. (Recommendation category: A; evidence type: 4)
Assessing risk and addressing harms of opioid use
8. Before starting opioid therapy, and periodically during its continuation, evaluate risk factors for opioid-related harms. Incorporate strategies into the management plan to mitigate risk; consider offering naloxone when factors are present that increase the risk for opioid overdose—eg, a history of overdose, history of substance use disorder, higher opioid dosages (≥50 MME/d), or concurrent benzodiazepine use. (Recommendation category: A; evidence type: 4)
9. Review the patient’s history of controlled substance prescriptions. Use data from the state prescription drug monitoring program (PDMP) to determine whether the patient is receiving opioid dosages or dangerous combinations that put him or her at high risk for overdose. (State Web sites are available at http://www.pdmpassist.org/content/state-pdmp-websites.) Review PDMP data when starting opioid therapy for chronic pain and periodically during its continuation, at least every 3 months and with each new prescription. (Recommendation category: A; evidence type: 4)
10. Before prescribing opioids for chronic pain, use urine drug testing to assess for prescribed medications, as well as other controlled prescription drugs and illicit drugs, and consider urine drug testing at least annually. (Recommendation category: B; evidence type: 4)
11. Avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible. (Recommendation category: A; evidence type: 3)
12. For patients with opioid use disorder, offer or arrange for evidence-based treatment (usually medication-assisted treatment with buprenorphine or methadone in combination with behavioral therapies). (Recommendation category: A; evidence type: 2)
Aids for guideline implementation
The CDC has produced materials to assist physicians in implementing this guideline, including checklists for prescribing or continuing opioids. The checklist for initiation of opioids is reproduced in FIGURE 2.7

The CDC is addressing a severe public health problem and doing so by using contemporary evidence-based methodology and guideline development processes. The lack of high-quality evidence on the topic and the use of a less-than-optimal evidence review process for some key questions may hamper this effort. However, given the prominence of the CDC, this clinical guideline will likely be considered the standard of care for family physicians.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.
1. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1–49. Available at: https://www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed October 17, 20
2. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. 20
3. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
4. Chou R, Deyo R, Devine B, et al. The effectiveness and risks of long-term opioid treatment of chronic pain. Evidence Report/Technology Assessment No. 218. AHRQ Publication No. 14-E005-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2014. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/557/1971/chronic-pain-opioid-treatment-report-141205.pdf. Accessed October 17, 2016.
5. Centers for Disease Control and Prevention. Clinical evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38026. Accessed October 17, 2016.
6. Centers for Disease Control and Prevention. Contextual evidence review for the CDC Guideline for Prescribing Opioids for Chronic Pain – United States, 2016. Available at: https://stacks.cdc.gov/view/cdc/38027. Accessed October 17, 2016.
7. Centers for Disease Control and Prevention. Checklist for prescribing opioids for chronic pain. Available at: https://stacks.cdc.gov/view/cdc/38025. Accessed October 17, 2016.