User login
Dapivirine vaginal ring cuts new HIV-1 infections
The dapivirine vaginal ring reduced the rate of new HIV-1 infection by 31% in a phase III clinical trial involving 1,959 high-risk women in sub-Saharan Africa, according to a report published in the New England Journal of Medicine.
The dapivirine-containing ring is replaced every month and was used in this study for up to 2 years. It was not associated with any safety concerns, said Annalene Nel, MD, PhD, of the International Partnership for Microbicides, Silver Spring, Md., and her associates.
The International Partnership for Microbicides is a nonprofit group that developed the ring, which can be self-inserted and removed and which provides a sustained release of the antiretroviral drug for at least 4 weeks. QPharma of Malmö, Sweden, manufactures the rings and donated the ones used in this study, but was not otherwise involved in the trial.
The study participants were sexually active women aged 18-45 years (mean age, 26) treated at seven research centers in South Africa and Uganda. Almost all of them (98%) had only one male sexual partner. For the purposes of this trial, the women were asked to attend the participating clinics every 4 weeks to have the rings replaced and to provide a plasma sample. This allowed the researchers to measure the residual amount of dapivirine in the used rings and to measure plasma levels of the drug, both of which were indicators of compliance.
A total of 1,307 women were randomly assigned to receive dapivirine rings and 652 to receive placebo rings. At the data cutoff point, 615 women (31.4%) were still in the trial and 761 (38.8%) had completed it; 583 women (29.8%) had discontinued early. All the participants at one medical center were withdrawn by the sponsor because of high rates of protocol violation at that facility and corresponding high rates of patient nonadherence with the device.
The primary efficacy endpoint – the rate of HIV-1 seroconversion during follow-up – was 4.1 per 100 person-years with the dapivirine ring, compared with 6.1 per 100 person-years with the placebo ring. This represents a 31% lower infection rate with the active treatment (hazard ratio, 0.69). A subgroup analysis that excluded participants at the protocol-violating facility showed a seroconversion rate of 3.6 per 100 person-years with the active treatment vs. 5.4 per 100 person-years with the placebo, a 30% reduction in the infection rate, the investigators said (N Engl J Med. 2016 Dec 1. doi: 10.1056/NEJMoa1602046).
The findings were consistent across other subgroup analyses, including those that categorized the participants according to their adherence levels, as measured by plasma levels and residual ring levels of dapivirine.
The cumulative incidence of adverse events was similar between the two study groups (87.4% and 85.7%, respectively), as was the incidence of grade 3 or 4 adverse events. None of the more serious adverse events were judged to be related to the devices. Mild product-related adverse events occurred in 5 women (0.4%) in the dapivirine group and 3 (0.5%) in the placebo group. There also were no significant differences between the two study groups in the incidence of laboratory abnormalities, rates of sexually transmitted and other genital infections, or pregnancy rates.
The study was funded by the International Partnership for Microbicides, which is supported by the Bill and Melinda Gates Foundation, Irish Aid, the Ministry of Foreign Affairs of Denmark, the Ministry of Foreign Affairs of the Netherlands, the Norwegian Agency for Development Cooperation, the U.K. Department for International Development, the U.S. Agency for International Development, and the President’s Emergency Plan for AIDS Relief.
Dr. Nel reported having no relevant financial disclosures; one of her associates reported ties to Janssen and Johnson & Johnson.
The dapivirine vaginal ring reduced the rate of new HIV-1 infection by 31% in a phase III clinical trial involving 1,959 high-risk women in sub-Saharan Africa, according to a report published in the New England Journal of Medicine.
The dapivirine-containing ring is replaced every month and was used in this study for up to 2 years. It was not associated with any safety concerns, said Annalene Nel, MD, PhD, of the International Partnership for Microbicides, Silver Spring, Md., and her associates.
The International Partnership for Microbicides is a nonprofit group that developed the ring, which can be self-inserted and removed and which provides a sustained release of the antiretroviral drug for at least 4 weeks. QPharma of Malmö, Sweden, manufactures the rings and donated the ones used in this study, but was not otherwise involved in the trial.
The study participants were sexually active women aged 18-45 years (mean age, 26) treated at seven research centers in South Africa and Uganda. Almost all of them (98%) had only one male sexual partner. For the purposes of this trial, the women were asked to attend the participating clinics every 4 weeks to have the rings replaced and to provide a plasma sample. This allowed the researchers to measure the residual amount of dapivirine in the used rings and to measure plasma levels of the drug, both of which were indicators of compliance.
A total of 1,307 women were randomly assigned to receive dapivirine rings and 652 to receive placebo rings. At the data cutoff point, 615 women (31.4%) were still in the trial and 761 (38.8%) had completed it; 583 women (29.8%) had discontinued early. All the participants at one medical center were withdrawn by the sponsor because of high rates of protocol violation at that facility and corresponding high rates of patient nonadherence with the device.
The primary efficacy endpoint – the rate of HIV-1 seroconversion during follow-up – was 4.1 per 100 person-years with the dapivirine ring, compared with 6.1 per 100 person-years with the placebo ring. This represents a 31% lower infection rate with the active treatment (hazard ratio, 0.69). A subgroup analysis that excluded participants at the protocol-violating facility showed a seroconversion rate of 3.6 per 100 person-years with the active treatment vs. 5.4 per 100 person-years with the placebo, a 30% reduction in the infection rate, the investigators said (N Engl J Med. 2016 Dec 1. doi: 10.1056/NEJMoa1602046).
The findings were consistent across other subgroup analyses, including those that categorized the participants according to their adherence levels, as measured by plasma levels and residual ring levels of dapivirine.
The cumulative incidence of adverse events was similar between the two study groups (87.4% and 85.7%, respectively), as was the incidence of grade 3 or 4 adverse events. None of the more serious adverse events were judged to be related to the devices. Mild product-related adverse events occurred in 5 women (0.4%) in the dapivirine group and 3 (0.5%) in the placebo group. There also were no significant differences between the two study groups in the incidence of laboratory abnormalities, rates of sexually transmitted and other genital infections, or pregnancy rates.
The study was funded by the International Partnership for Microbicides, which is supported by the Bill and Melinda Gates Foundation, Irish Aid, the Ministry of Foreign Affairs of Denmark, the Ministry of Foreign Affairs of the Netherlands, the Norwegian Agency for Development Cooperation, the U.K. Department for International Development, the U.S. Agency for International Development, and the President’s Emergency Plan for AIDS Relief.
Dr. Nel reported having no relevant financial disclosures; one of her associates reported ties to Janssen and Johnson & Johnson.
The dapivirine vaginal ring reduced the rate of new HIV-1 infection by 31% in a phase III clinical trial involving 1,959 high-risk women in sub-Saharan Africa, according to a report published in the New England Journal of Medicine.
The dapivirine-containing ring is replaced every month and was used in this study for up to 2 years. It was not associated with any safety concerns, said Annalene Nel, MD, PhD, of the International Partnership for Microbicides, Silver Spring, Md., and her associates.
The International Partnership for Microbicides is a nonprofit group that developed the ring, which can be self-inserted and removed and which provides a sustained release of the antiretroviral drug for at least 4 weeks. QPharma of Malmö, Sweden, manufactures the rings and donated the ones used in this study, but was not otherwise involved in the trial.
The study participants were sexually active women aged 18-45 years (mean age, 26) treated at seven research centers in South Africa and Uganda. Almost all of them (98%) had only one male sexual partner. For the purposes of this trial, the women were asked to attend the participating clinics every 4 weeks to have the rings replaced and to provide a plasma sample. This allowed the researchers to measure the residual amount of dapivirine in the used rings and to measure plasma levels of the drug, both of which were indicators of compliance.
A total of 1,307 women were randomly assigned to receive dapivirine rings and 652 to receive placebo rings. At the data cutoff point, 615 women (31.4%) were still in the trial and 761 (38.8%) had completed it; 583 women (29.8%) had discontinued early. All the participants at one medical center were withdrawn by the sponsor because of high rates of protocol violation at that facility and corresponding high rates of patient nonadherence with the device.
The primary efficacy endpoint – the rate of HIV-1 seroconversion during follow-up – was 4.1 per 100 person-years with the dapivirine ring, compared with 6.1 per 100 person-years with the placebo ring. This represents a 31% lower infection rate with the active treatment (hazard ratio, 0.69). A subgroup analysis that excluded participants at the protocol-violating facility showed a seroconversion rate of 3.6 per 100 person-years with the active treatment vs. 5.4 per 100 person-years with the placebo, a 30% reduction in the infection rate, the investigators said (N Engl J Med. 2016 Dec 1. doi: 10.1056/NEJMoa1602046).
The findings were consistent across other subgroup analyses, including those that categorized the participants according to their adherence levels, as measured by plasma levels and residual ring levels of dapivirine.
The cumulative incidence of adverse events was similar between the two study groups (87.4% and 85.7%, respectively), as was the incidence of grade 3 or 4 adverse events. None of the more serious adverse events were judged to be related to the devices. Mild product-related adverse events occurred in 5 women (0.4%) in the dapivirine group and 3 (0.5%) in the placebo group. There also were no significant differences between the two study groups in the incidence of laboratory abnormalities, rates of sexually transmitted and other genital infections, or pregnancy rates.
The study was funded by the International Partnership for Microbicides, which is supported by the Bill and Melinda Gates Foundation, Irish Aid, the Ministry of Foreign Affairs of Denmark, the Ministry of Foreign Affairs of the Netherlands, the Norwegian Agency for Development Cooperation, the U.K. Department for International Development, the U.S. Agency for International Development, and the President’s Emergency Plan for AIDS Relief.
Dr. Nel reported having no relevant financial disclosures; one of her associates reported ties to Janssen and Johnson & Johnson.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The dapivirine vaginal ring reduced the rate of new HIV-1 infection by 31% in a phase III trial involving 1,959 high-risk women in sub-Saharan Africa.
Major finding: The primary efficacy endpoint – the rate of HIV-1 seroconversion during follow-up – was 4.1 per 100 person-years with the dapivirine ring, compared with 6.1 per 100 person-years with the placebo ring.
Data source: A multicenter randomized double-blind placebo-controlled phase III trial involving 1,959 high-risk women in South Africa and Uganda.
Disclosures: This study was supported by the International Partnership for Microbicides, a nonprofit group that developed the dapivirine vaginal ring and is supported by the Bill and Melinda Gates Foundation, Irish Aid, the Ministry of Foreign Affairs of Denmark, the Ministry of Foreign Affairs of the Netherlands, the Norwegian Agency for Development Cooperation, the U.K. Department for International Development, the U.S. Agency for International Development, and the President’s Emergency Plan for AIDS Relief. Dr. Nel reported having no relevant financial disclosures; one of her associates reported ties to Janssen and Johnson & Johnson.
Can bioprosthetics work for large airway defects?
Large and complex airway defects that primary repair cannot fully close require alternative surgical approaches and techniques that are far more difficult to perform, but bioprosthetic materials may be an option to repair large tracheal and bronchial defects that has achieved good results, without postoperative death or defect recurrence, in a small cohort of patients at Massachusetts General Hospital, in Boston.
Brooks Udelsman, MD, and his coauthors reported their results of bioprosthetic repair of central airway defects in eight patients in the Journal of Thoracic and Cardiovascular Surgery (2016 Nov;152:1388-97). “Although our results are derived from a limited number of heterogeneous patients, they suggest that closure of noncircumferential large airway defects with bioprosthetic materials is feasible, safe and reliable,” Dr. Udelsman said. He previously reported the results at the annual meeting of the American Association for Thoracic Surgery, May 14-18, 2016, in Baltimore.
These complex defects typically exceed 5 cm and can involve communication with the esophagus. For repair of smaller defects, surgeons can use a more conventional approach that involves neck flexion, laryngeal release, airway mobilization, and hilar release, but in larger defects, these techniques increase the risk of too much tension on the anastomosis and dehiscence along with airway failure. Large and complex defects occur in patients who have had a previous airway operation or radiation exposure, requiring alternative strategies, the researchers wrote. “Patients in this rare category should be referred to a high-volume center for careful evaluation by a surgeon experienced in complex airway reconstruction before the decision to abandon primary repair is made,” he said. Among the advantages that bioprosthetic materials have over synthetic materials for airway defect repair are easier handling, minimal immunogenic response, and potential for tissue ingrowth, Dr. Udelsman and his coauthors said.
All eight patients in this study, who underwent repair from 2008 to 2015, had significant comorbidities, including previous surgery of the trachea, esophagus, or thyroid. The etiology of the airway defect included HIV-AIDS–associated esophagitis, malignancy, mesh erosion, and complications from extended intubation. Three patients had previous radiation therapy to the neck or chest. Five patients had defects localized to the membranous tracheal wall, two had defects of the mainstem bronchus or bronchus intermedius, and one patient had a defect of the anterior wall of the trachea.
Dr. Udelsman and his coauthors used both aortic homograft and acellular dermal matrix to repair large defects. Their experience confirmed previous reports of the formation of granulation tissue with aortic autografts, underscoring the importance of frequent bronchoscopy and debridement when necessary. And while previous reports have claimed human acellular dermis resists granulation formation, that wasn’t the case in this study. “The exact histologic basis of bioprosthetic incorporation and reepithelialization in these patients is still elusive and will require further study,” the researchers said.
This study also employed the controversial muscle buttress repair in six patients, which helped, at least theoretically, to secure the repairs when leaks occur, to separate suture lines when both the airway and esophagus were repaired and to support the bioprosthetic material to prevent tissue softening, Dr. Udelsman and his coauthors said.
Postoperative examinations confirmed that the operations successfully closed the airway defects in all eight patients. Long term, most resumed oral intake, but three did not for various reasons: one had a pharyngostomy; another had neurocognitive issues preoperatively; and a third with a tracheoesophageal fistula repair and cervical esophagostomy could resume oral intake but depended on tube feeds to meet caloric needs.
All patients developed granulation at the repair site, two of whom required further debridement and one who underwent balloon dilation. Pneumonia was the most common complication within 30 days of surgery, occurring in two patients. Three patients died within 120 days from metastatic disease, and a fourth patient progressed to end-stage AIDS 6 years after the operation and eventually died.
Dr. Udelsman and his coauthors reported having no financial disclosures.
In his invited commentary, Raja Flores, MD, of Mount Sinai Health System in New York said this study demonstrated “modest success” with bioprosthetic materials for repair of large airway deficits – the same level of success he ascribes to human studies of other surgical approaches to large airway deficits (J Thorac Cardiovasc Surg. 2016 Nov;152:1233-4).
But progress has been slow and animal studies of large airway deficit repair have been “wastefully repetitive” without any advances. “We must build on what these human studies have taught us and not continue unsuccessfully to reinvent the same malfunctioning [airways],” Dr. Flores said.
When surgeons encounter such patients, surgery isn’t necessary for their survival, Dr. Flores said. “T-tubes work just fine.” The goal is to improve their quality of life. “Unless we can provide a reliable, long-lasting solution, an unpredictable life-threatening experimental surgical intervention is not justified to treat a stable, functional patient,” he said.
And while Dr. Udelsman and his colleagues have shown “some progress” in their study, he cautioned surgeons to heed the words of a tracheal surgery pioneer Hermes Grillo, MD, at Boston’s Massachusetts General Hospital and Harvard Medical School: “Success has been announced episodically over the decades in each of these categories, but thus far no one replacement method has held for the long term in any safe and practicable manner.” Dr. Flores added: “This still holds true today.”
Dr. Flores reported having no financial disclosures.
In his invited commentary, Raja Flores, MD, of Mount Sinai Health System in New York said this study demonstrated “modest success” with bioprosthetic materials for repair of large airway deficits – the same level of success he ascribes to human studies of other surgical approaches to large airway deficits (J Thorac Cardiovasc Surg. 2016 Nov;152:1233-4).
But progress has been slow and animal studies of large airway deficit repair have been “wastefully repetitive” without any advances. “We must build on what these human studies have taught us and not continue unsuccessfully to reinvent the same malfunctioning [airways],” Dr. Flores said.
When surgeons encounter such patients, surgery isn’t necessary for their survival, Dr. Flores said. “T-tubes work just fine.” The goal is to improve their quality of life. “Unless we can provide a reliable, long-lasting solution, an unpredictable life-threatening experimental surgical intervention is not justified to treat a stable, functional patient,” he said.
And while Dr. Udelsman and his colleagues have shown “some progress” in their study, he cautioned surgeons to heed the words of a tracheal surgery pioneer Hermes Grillo, MD, at Boston’s Massachusetts General Hospital and Harvard Medical School: “Success has been announced episodically over the decades in each of these categories, but thus far no one replacement method has held for the long term in any safe and practicable manner.” Dr. Flores added: “This still holds true today.”
Dr. Flores reported having no financial disclosures.
In his invited commentary, Raja Flores, MD, of Mount Sinai Health System in New York said this study demonstrated “modest success” with bioprosthetic materials for repair of large airway deficits – the same level of success he ascribes to human studies of other surgical approaches to large airway deficits (J Thorac Cardiovasc Surg. 2016 Nov;152:1233-4).
But progress has been slow and animal studies of large airway deficit repair have been “wastefully repetitive” without any advances. “We must build on what these human studies have taught us and not continue unsuccessfully to reinvent the same malfunctioning [airways],” Dr. Flores said.
When surgeons encounter such patients, surgery isn’t necessary for their survival, Dr. Flores said. “T-tubes work just fine.” The goal is to improve their quality of life. “Unless we can provide a reliable, long-lasting solution, an unpredictable life-threatening experimental surgical intervention is not justified to treat a stable, functional patient,” he said.
And while Dr. Udelsman and his colleagues have shown “some progress” in their study, he cautioned surgeons to heed the words of a tracheal surgery pioneer Hermes Grillo, MD, at Boston’s Massachusetts General Hospital and Harvard Medical School: “Success has been announced episodically over the decades in each of these categories, but thus far no one replacement method has held for the long term in any safe and practicable manner.” Dr. Flores added: “This still holds true today.”
Dr. Flores reported having no financial disclosures.
Large and complex airway defects that primary repair cannot fully close require alternative surgical approaches and techniques that are far more difficult to perform, but bioprosthetic materials may be an option to repair large tracheal and bronchial defects that has achieved good results, without postoperative death or defect recurrence, in a small cohort of patients at Massachusetts General Hospital, in Boston.
Brooks Udelsman, MD, and his coauthors reported their results of bioprosthetic repair of central airway defects in eight patients in the Journal of Thoracic and Cardiovascular Surgery (2016 Nov;152:1388-97). “Although our results are derived from a limited number of heterogeneous patients, they suggest that closure of noncircumferential large airway defects with bioprosthetic materials is feasible, safe and reliable,” Dr. Udelsman said. He previously reported the results at the annual meeting of the American Association for Thoracic Surgery, May 14-18, 2016, in Baltimore.
These complex defects typically exceed 5 cm and can involve communication with the esophagus. For repair of smaller defects, surgeons can use a more conventional approach that involves neck flexion, laryngeal release, airway mobilization, and hilar release, but in larger defects, these techniques increase the risk of too much tension on the anastomosis and dehiscence along with airway failure. Large and complex defects occur in patients who have had a previous airway operation or radiation exposure, requiring alternative strategies, the researchers wrote. “Patients in this rare category should be referred to a high-volume center for careful evaluation by a surgeon experienced in complex airway reconstruction before the decision to abandon primary repair is made,” he said. Among the advantages that bioprosthetic materials have over synthetic materials for airway defect repair are easier handling, minimal immunogenic response, and potential for tissue ingrowth, Dr. Udelsman and his coauthors said.
All eight patients in this study, who underwent repair from 2008 to 2015, had significant comorbidities, including previous surgery of the trachea, esophagus, or thyroid. The etiology of the airway defect included HIV-AIDS–associated esophagitis, malignancy, mesh erosion, and complications from extended intubation. Three patients had previous radiation therapy to the neck or chest. Five patients had defects localized to the membranous tracheal wall, two had defects of the mainstem bronchus or bronchus intermedius, and one patient had a defect of the anterior wall of the trachea.
Dr. Udelsman and his coauthors used both aortic homograft and acellular dermal matrix to repair large defects. Their experience confirmed previous reports of the formation of granulation tissue with aortic autografts, underscoring the importance of frequent bronchoscopy and debridement when necessary. And while previous reports have claimed human acellular dermis resists granulation formation, that wasn’t the case in this study. “The exact histologic basis of bioprosthetic incorporation and reepithelialization in these patients is still elusive and will require further study,” the researchers said.
This study also employed the controversial muscle buttress repair in six patients, which helped, at least theoretically, to secure the repairs when leaks occur, to separate suture lines when both the airway and esophagus were repaired and to support the bioprosthetic material to prevent tissue softening, Dr. Udelsman and his coauthors said.
Postoperative examinations confirmed that the operations successfully closed the airway defects in all eight patients. Long term, most resumed oral intake, but three did not for various reasons: one had a pharyngostomy; another had neurocognitive issues preoperatively; and a third with a tracheoesophageal fistula repair and cervical esophagostomy could resume oral intake but depended on tube feeds to meet caloric needs.
All patients developed granulation at the repair site, two of whom required further debridement and one who underwent balloon dilation. Pneumonia was the most common complication within 30 days of surgery, occurring in two patients. Three patients died within 120 days from metastatic disease, and a fourth patient progressed to end-stage AIDS 6 years after the operation and eventually died.
Dr. Udelsman and his coauthors reported having no financial disclosures.
Large and complex airway defects that primary repair cannot fully close require alternative surgical approaches and techniques that are far more difficult to perform, but bioprosthetic materials may be an option to repair large tracheal and bronchial defects that has achieved good results, without postoperative death or defect recurrence, in a small cohort of patients at Massachusetts General Hospital, in Boston.
Brooks Udelsman, MD, and his coauthors reported their results of bioprosthetic repair of central airway defects in eight patients in the Journal of Thoracic and Cardiovascular Surgery (2016 Nov;152:1388-97). “Although our results are derived from a limited number of heterogeneous patients, they suggest that closure of noncircumferential large airway defects with bioprosthetic materials is feasible, safe and reliable,” Dr. Udelsman said. He previously reported the results at the annual meeting of the American Association for Thoracic Surgery, May 14-18, 2016, in Baltimore.
These complex defects typically exceed 5 cm and can involve communication with the esophagus. For repair of smaller defects, surgeons can use a more conventional approach that involves neck flexion, laryngeal release, airway mobilization, and hilar release, but in larger defects, these techniques increase the risk of too much tension on the anastomosis and dehiscence along with airway failure. Large and complex defects occur in patients who have had a previous airway operation or radiation exposure, requiring alternative strategies, the researchers wrote. “Patients in this rare category should be referred to a high-volume center for careful evaluation by a surgeon experienced in complex airway reconstruction before the decision to abandon primary repair is made,” he said. Among the advantages that bioprosthetic materials have over synthetic materials for airway defect repair are easier handling, minimal immunogenic response, and potential for tissue ingrowth, Dr. Udelsman and his coauthors said.
All eight patients in this study, who underwent repair from 2008 to 2015, had significant comorbidities, including previous surgery of the trachea, esophagus, or thyroid. The etiology of the airway defect included HIV-AIDS–associated esophagitis, malignancy, mesh erosion, and complications from extended intubation. Three patients had previous radiation therapy to the neck or chest. Five patients had defects localized to the membranous tracheal wall, two had defects of the mainstem bronchus or bronchus intermedius, and one patient had a defect of the anterior wall of the trachea.
Dr. Udelsman and his coauthors used both aortic homograft and acellular dermal matrix to repair large defects. Their experience confirmed previous reports of the formation of granulation tissue with aortic autografts, underscoring the importance of frequent bronchoscopy and debridement when necessary. And while previous reports have claimed human acellular dermis resists granulation formation, that wasn’t the case in this study. “The exact histologic basis of bioprosthetic incorporation and reepithelialization in these patients is still elusive and will require further study,” the researchers said.
This study also employed the controversial muscle buttress repair in six patients, which helped, at least theoretically, to secure the repairs when leaks occur, to separate suture lines when both the airway and esophagus were repaired and to support the bioprosthetic material to prevent tissue softening, Dr. Udelsman and his coauthors said.
Postoperative examinations confirmed that the operations successfully closed the airway defects in all eight patients. Long term, most resumed oral intake, but three did not for various reasons: one had a pharyngostomy; another had neurocognitive issues preoperatively; and a third with a tracheoesophageal fistula repair and cervical esophagostomy could resume oral intake but depended on tube feeds to meet caloric needs.
All patients developed granulation at the repair site, two of whom required further debridement and one who underwent balloon dilation. Pneumonia was the most common complication within 30 days of surgery, occurring in two patients. Three patients died within 120 days from metastatic disease, and a fourth patient progressed to end-stage AIDS 6 years after the operation and eventually died.
Dr. Udelsman and his coauthors reported having no financial disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Bioprosthetic materials show progress for reconstruction of large airway defects.
Major finding: Airway defects were successfully closed in all patients, with no postoperative deaths or recurrence of airway defect.
Data source: Eight patients who underwent closure of complex central airway defects with bioprosthetic materials between 2008 and 2015.
Disclosures: Dr. Udelsman and coauthors reported having no relevant financial disclosures.
Oral Fixed Drug Eruption Due to Tinidazole
To the Editor:
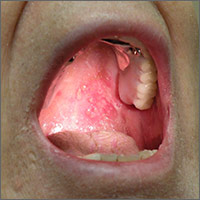
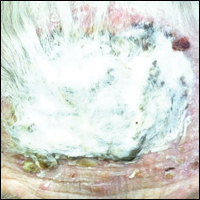
A 50-year-old man presented with a painful ulcer and a burning sensation on the tongue of 2 days’ duration (Figure, A). The ulcer had a yellowish white appearance with erythematous borders. The patient started taking tinidazole 500 mg twice daily 2 days prior, which was prescribed by his primary care physician for an episode of gastroenteritis. He was not taking any other medications and did not smoke or drink. Routine laboratory test results did not reveal any abnormalities. Based on the physical examination as well as the patient’s medical and medication history, a provisional diagnosis of fixed drug eruption (FDE) due to tinidazole was made. Tinidazole was immediately withdrawn and the patient was prescribed beclomethasone dipropionate ointment twice daily to relieve the burning sensation. A punch biopsy of the lesion was recommended; however, the patient opted to wait a week after discontinuing the drug. At follow-up 1 week later, complete healing of the ulcer was observed with no scarring and the burning sensation had resolved (Figure, B). After obtaining informed consent from the patient, an oral challenge test was conducted in the office with 50 mg of tinidazole. Four hours after taking the drug orally, the patient felt a burning sensation and a small ulcerative lesion was observed on the tongue at the same site the next day. The patient was informed of the fixed drug reaction to tinidazole, a drug belonging to the nitroimidazole group, and this information also was conveyed to the patient’s primary care physician.
Tinidazole is a synthetic antiprotozoal and antibacterial agent used primarily in infections such as amebiasis, giardiasis, and trichomoniasis.1 Tinidazole may be a therapeutic alternative to metronidazole. Fixed drug eruption is a distinctive variant of drug eruption with characteristic recurrence at the same site of skin or mucous membranes. It is characterized by onset of round/oval, erythematous, well-defined macules on the skin and/or mucosa associated with itching and burning.1 Fixed drug eruption generally is restricted to the mucous membrane and skin, with the lips, palms, soles, glans penis, and groin area being the most common sites. Intraoral involvement, excluding the lips, of FDE is rare. The tongue is a rare site of an FDE.2 Fixed drug eruption on the tongue has been reported with clarithromycin.3 Dental clinicians have to be aware of the possibility of FDE due to commonly used drugs such tinidazole, which would help in prompt diagnosis of these lesions.
- Prieto A, De Barrio M, Infante S, et al. Recurrent fixed drug eruption due to metronidazole elicited by patch test with tinidazole. Contact Dermatitis. 2005;53:169-170.
- Dhar S, Kanwar AJ. Fixed drug eruption on the tongue of a 4-year-old boy. Pediatr Dermatol. 1995;12:51-52.
- Alonso JC, Melgosa AC, Gonzalo MJ, et al. Fixed drug eruption on the tongue due to clarithromycin. Contact Dermatitis. 2005;53:121-122.
To the Editor:
A 50-year-old man presented with a painful ulcer and a burning sensation on the tongue of 2 days’ duration (Figure, A). The ulcer had a yellowish white appearance with erythematous borders. The patient started taking tinidazole 500 mg twice daily 2 days prior, which was prescribed by his primary care physician for an episode of gastroenteritis. He was not taking any other medications and did not smoke or drink. Routine laboratory test results did not reveal any abnormalities. Based on the physical examination as well as the patient’s medical and medication history, a provisional diagnosis of fixed drug eruption (FDE) due to tinidazole was made. Tinidazole was immediately withdrawn and the patient was prescribed beclomethasone dipropionate ointment twice daily to relieve the burning sensation. A punch biopsy of the lesion was recommended; however, the patient opted to wait a week after discontinuing the drug. At follow-up 1 week later, complete healing of the ulcer was observed with no scarring and the burning sensation had resolved (Figure, B). After obtaining informed consent from the patient, an oral challenge test was conducted in the office with 50 mg of tinidazole. Four hours after taking the drug orally, the patient felt a burning sensation and a small ulcerative lesion was observed on the tongue at the same site the next day. The patient was informed of the fixed drug reaction to tinidazole, a drug belonging to the nitroimidazole group, and this information also was conveyed to the patient’s primary care physician.

Tinidazole is a synthetic antiprotozoal and antibacterial agent used primarily in infections such as amebiasis, giardiasis, and trichomoniasis.1 Tinidazole may be a therapeutic alternative to metronidazole. Fixed drug eruption is a distinctive variant of drug eruption with characteristic recurrence at the same site of skin or mucous membranes. It is characterized by onset of round/oval, erythematous, well-defined macules on the skin and/or mucosa associated with itching and burning.1 Fixed drug eruption generally is restricted to the mucous membrane and skin, with the lips, palms, soles, glans penis, and groin area being the most common sites. Intraoral involvement, excluding the lips, of FDE is rare. The tongue is a rare site of an FDE.2 Fixed drug eruption on the tongue has been reported with clarithromycin.3 Dental clinicians have to be aware of the possibility of FDE due to commonly used drugs such tinidazole, which would help in prompt diagnosis of these lesions.
To the Editor:
A 50-year-old man presented with a painful ulcer and a burning sensation on the tongue of 2 days’ duration (Figure, A). The ulcer had a yellowish white appearance with erythematous borders. The patient started taking tinidazole 500 mg twice daily 2 days prior, which was prescribed by his primary care physician for an episode of gastroenteritis. He was not taking any other medications and did not smoke or drink. Routine laboratory test results did not reveal any abnormalities. Based on the physical examination as well as the patient’s medical and medication history, a provisional diagnosis of fixed drug eruption (FDE) due to tinidazole was made. Tinidazole was immediately withdrawn and the patient was prescribed beclomethasone dipropionate ointment twice daily to relieve the burning sensation. A punch biopsy of the lesion was recommended; however, the patient opted to wait a week after discontinuing the drug. At follow-up 1 week later, complete healing of the ulcer was observed with no scarring and the burning sensation had resolved (Figure, B). After obtaining informed consent from the patient, an oral challenge test was conducted in the office with 50 mg of tinidazole. Four hours after taking the drug orally, the patient felt a burning sensation and a small ulcerative lesion was observed on the tongue at the same site the next day. The patient was informed of the fixed drug reaction to tinidazole, a drug belonging to the nitroimidazole group, and this information also was conveyed to the patient’s primary care physician.

Tinidazole is a synthetic antiprotozoal and antibacterial agent used primarily in infections such as amebiasis, giardiasis, and trichomoniasis.1 Tinidazole may be a therapeutic alternative to metronidazole. Fixed drug eruption is a distinctive variant of drug eruption with characteristic recurrence at the same site of skin or mucous membranes. It is characterized by onset of round/oval, erythematous, well-defined macules on the skin and/or mucosa associated with itching and burning.1 Fixed drug eruption generally is restricted to the mucous membrane and skin, with the lips, palms, soles, glans penis, and groin area being the most common sites. Intraoral involvement, excluding the lips, of FDE is rare. The tongue is a rare site of an FDE.2 Fixed drug eruption on the tongue has been reported with clarithromycin.3 Dental clinicians have to be aware of the possibility of FDE due to commonly used drugs such tinidazole, which would help in prompt diagnosis of these lesions.
- Prieto A, De Barrio M, Infante S, et al. Recurrent fixed drug eruption due to metronidazole elicited by patch test with tinidazole. Contact Dermatitis. 2005;53:169-170.
- Dhar S, Kanwar AJ. Fixed drug eruption on the tongue of a 4-year-old boy. Pediatr Dermatol. 1995;12:51-52.
- Alonso JC, Melgosa AC, Gonzalo MJ, et al. Fixed drug eruption on the tongue due to clarithromycin. Contact Dermatitis. 2005;53:121-122.
- Prieto A, De Barrio M, Infante S, et al. Recurrent fixed drug eruption due to metronidazole elicited by patch test with tinidazole. Contact Dermatitis. 2005;53:169-170.
- Dhar S, Kanwar AJ. Fixed drug eruption on the tongue of a 4-year-old boy. Pediatr Dermatol. 1995;12:51-52.
- Alonso JC, Melgosa AC, Gonzalo MJ, et al. Fixed drug eruption on the tongue due to clarithromycin. Contact Dermatitis. 2005;53:121-122.
Practice Points
- Fixed drug eruption (FDE) is characterized by onset of round/oval, erythematous, well-defined macules on the skin and/or mucosa associated with itching and burning.
- Intraoral involvement of FDE is rare.
- Tinidazole may cause FDE and should be suspected in patients with a spontaneous eruption of macules on mucous membranes.
Sore throat and ear pain
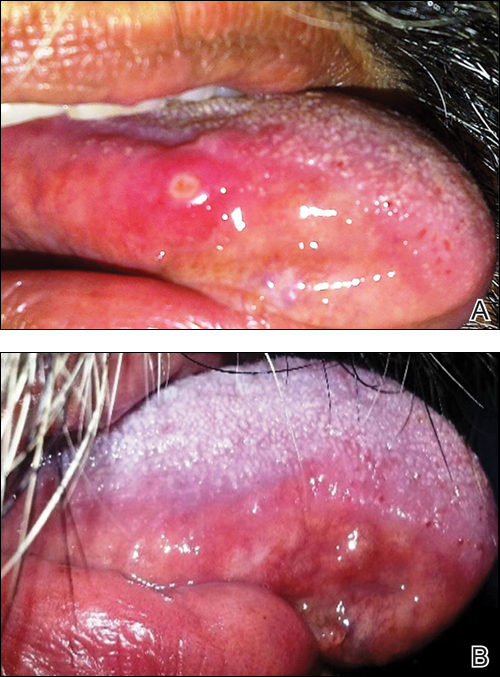
Based on the patient’s clinical presentation, he was given a diagnosis of herpes zoster oticus—also known as Ramsay Hunt syndrome. Patients with Ramsay Hunt syndrome typically develop unilateral facial paralysis and erythematous vesicles that appear ipsilaterally on the ear and/or in the mouth. This syndrome is a rare complication of herpes zoster that occurs when latent varicella zoster virus (VZV) infection reactivates and spreads to affect the geniculate ganglion.
An estimated 5 out of every 100,000 people develop Ramsay Hunt syndrome each year in the United States; men and women are equally affected. Any patient who’s had VZV infection runs the risk of developing Ramsay Hunt syndrome, but it most often develops in individuals older than age 60.
Vesicles in the mouth usually develop on the tongue or hard palate. Other symptoms may include tinnitus, hearing loss, nausea, vomiting, vertigo, and nystagmus. Because the symptoms of Ramsay Hunt syndrome suggest a possible infection, the differential diagnosis should include herpes simplex virus type 1, Epstein-Barr virus, group A Streptococcus, and measles.
A diagnosis of Ramsay Hunt syndrome is typically made clinically, but can be confirmed with direct fluorescent antibody (DFA) analysis, polymerase chain reaction (PCR) testing, or viral culture of vesicular exudates. DFA for VZV has an 87% sensitivity. PCR has a higher sensitivity (92%), is widely available, and is the diagnostic test of choice, according to the Centers for Disease Control and Prevention.
Treatment with an oral steroid, such as prednisone, in addition to an antiviral such as acyclovir or valacyclovir, may reduce the likelihood of postherpetic neuralgia and improve facial motor function. However, these benefits have not been demonstrated in randomized controlled trials.
In this case, the FP prescribed oral valacyclovir 1 g 3 times a day for 7 days and oral prednisone 50 mg/d for 5 days for the patient. After one week of treatment, the patient’s symptoms resolved and the vesicles in his mouth crusted over. He did not experience postherpetic neuralgia or have a recurrence.
Adapted from: Moss DA, Crawford P. Sore throat and left ear pain. J Fam Pract. 2015;64:117-119.

Based on the patient’s clinical presentation, he was given a diagnosis of herpes zoster oticus—also known as Ramsay Hunt syndrome. Patients with Ramsay Hunt syndrome typically develop unilateral facial paralysis and erythematous vesicles that appear ipsilaterally on the ear and/or in the mouth. This syndrome is a rare complication of herpes zoster that occurs when latent varicella zoster virus (VZV) infection reactivates and spreads to affect the geniculate ganglion.
An estimated 5 out of every 100,000 people develop Ramsay Hunt syndrome each year in the United States; men and women are equally affected. Any patient who’s had VZV infection runs the risk of developing Ramsay Hunt syndrome, but it most often develops in individuals older than age 60.
Vesicles in the mouth usually develop on the tongue or hard palate. Other symptoms may include tinnitus, hearing loss, nausea, vomiting, vertigo, and nystagmus. Because the symptoms of Ramsay Hunt syndrome suggest a possible infection, the differential diagnosis should include herpes simplex virus type 1, Epstein-Barr virus, group A Streptococcus, and measles.
A diagnosis of Ramsay Hunt syndrome is typically made clinically, but can be confirmed with direct fluorescent antibody (DFA) analysis, polymerase chain reaction (PCR) testing, or viral culture of vesicular exudates. DFA for VZV has an 87% sensitivity. PCR has a higher sensitivity (92%), is widely available, and is the diagnostic test of choice, according to the Centers for Disease Control and Prevention.
Treatment with an oral steroid, such as prednisone, in addition to an antiviral such as acyclovir or valacyclovir, may reduce the likelihood of postherpetic neuralgia and improve facial motor function. However, these benefits have not been demonstrated in randomized controlled trials.
In this case, the FP prescribed oral valacyclovir 1 g 3 times a day for 7 days and oral prednisone 50 mg/d for 5 days for the patient. After one week of treatment, the patient’s symptoms resolved and the vesicles in his mouth crusted over. He did not experience postherpetic neuralgia or have a recurrence.
Adapted from: Moss DA, Crawford P. Sore throat and left ear pain. J Fam Pract. 2015;64:117-119.

Based on the patient’s clinical presentation, he was given a diagnosis of herpes zoster oticus—also known as Ramsay Hunt syndrome. Patients with Ramsay Hunt syndrome typically develop unilateral facial paralysis and erythematous vesicles that appear ipsilaterally on the ear and/or in the mouth. This syndrome is a rare complication of herpes zoster that occurs when latent varicella zoster virus (VZV) infection reactivates and spreads to affect the geniculate ganglion.
An estimated 5 out of every 100,000 people develop Ramsay Hunt syndrome each year in the United States; men and women are equally affected. Any patient who’s had VZV infection runs the risk of developing Ramsay Hunt syndrome, but it most often develops in individuals older than age 60.
Vesicles in the mouth usually develop on the tongue or hard palate. Other symptoms may include tinnitus, hearing loss, nausea, vomiting, vertigo, and nystagmus. Because the symptoms of Ramsay Hunt syndrome suggest a possible infection, the differential diagnosis should include herpes simplex virus type 1, Epstein-Barr virus, group A Streptococcus, and measles.
A diagnosis of Ramsay Hunt syndrome is typically made clinically, but can be confirmed with direct fluorescent antibody (DFA) analysis, polymerase chain reaction (PCR) testing, or viral culture of vesicular exudates. DFA for VZV has an 87% sensitivity. PCR has a higher sensitivity (92%), is widely available, and is the diagnostic test of choice, according to the Centers for Disease Control and Prevention.
Treatment with an oral steroid, such as prednisone, in addition to an antiviral such as acyclovir or valacyclovir, may reduce the likelihood of postherpetic neuralgia and improve facial motor function. However, these benefits have not been demonstrated in randomized controlled trials.
In this case, the FP prescribed oral valacyclovir 1 g 3 times a day for 7 days and oral prednisone 50 mg/d for 5 days for the patient. After one week of treatment, the patient’s symptoms resolved and the vesicles in his mouth crusted over. He did not experience postherpetic neuralgia or have a recurrence.
Adapted from: Moss DA, Crawford P. Sore throat and left ear pain. J Fam Pract. 2015;64:117-119.
Sarcoidosis and Squamous Cell Carcinoma: A Connection Documented in a Case Series of 3 Patients
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
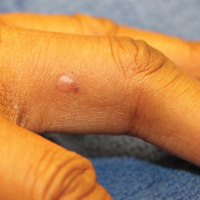
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
Practice Points
- There may be an increased risk of skin cancer in patients with sarcoidosis.
- Sarcoidosis may present with multiple morphologies, including verrucous or hyperkeratotic lesions; superficial biopsy of this type of lesion may be mistaken for a squamous cell carcinoma.
- A biopsy diagnosis of squamous cell carcinoma in a black patient with sarcoidosis should be carefully reviewed for evidence of deeper granulomatous inflammation.
Vitiligo Patients Experience Barriers in Accessing Care
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
Practice Points
- Patients with vitiligo may experience difficulty receiving the care prescribed to them.
- It is best to identify barriers such as work schedule or distance before recommending a treatment plan.
Managing stress in children, parents can reduce obesity risk
SAN FRANCISCO – Obesity is a multifactorial problem, influenced by factors ranging from genetics to lifestyle to the environment. Yet stress can play an outsize role in obesity as well, Elizabeth Prout Parks, MD, said at the annual meeting of the American Academy of Pediatrics.
Although the calorie-in/calorie-out model of energy balance has driven much of the thought about obesity, it’s not that simple, suggested Dr. Parks, the medical director of the Healthy Weight Adolescent Bariatrics Program at the Children’s Hospital of Philadelphia. Physical activity accounts for an estimated 15%-30% of energy expenditure, and thermogenesis accounts for an estimated 10%. But the energy expenditure required for basal metabolism can range from 60% to 75%, a sufficiently wide range for significant variation across different individuals.
The psychosocial effects can lead to anxiety, depression, disordered eating behaviors such as emotional eating, a sedentary lifestyle, poor sleep, and low maintenance with self-care activities. Further, poor sleep on its own is additionally associated with childhood obesity. The combination of these physiologic and psychosocial effects can increase the risk of metabolic syndrome, type 2 diabetes, and cardiovascular disease or events. While acute stress and chronic stress follow similar pathways in the brain, it’s chronic stress that carries the greater risk of behavioral and physical conditions.
Measuring and understanding child and parental stress
Several clinical assessments can measure stress in children, including the Daily Hassles Scale, which looks at everyday interactions in the environment and factors such as children’s school, family, neighborhood, peers, and lack of resources. The Multidimensional Life Events Rating Questionnaire and Adolescent Stress Questionnaire both are more appropriate for middle school and older adolescents.
In children, the primary biologic indicators of stress are cortisol levels, heart rate, and blood pressure, but it is perceived stress that has been most clearly linked to emotional eating and other disordered eating behaviors, Dr. Parks said. One 2008 study found perceived stress to be associated with emotional eating among middle school students both with and without obesity. A high level of perceived stress in adolescents was associated with a greater waist circumference and body mass index in a 2009 study.
The findings are somewhat more mixed, however, when it comes to parental stress and child weight. A 2012 study identified a link between parents’ perception of their stress and increased fast food consumption in their children, and a 2008 study identified a link between parenting stress and both overweight and underweight children. Yet a different study in 2008 found no association between child obesity and parenting stress. Research in 2011 found a relationship between children’s consumption of fruits and vegetables and their family’s overall functioning, as well as parental psychological stress and child behavior. Within a family, stress can come from financial strain (such as poverty or changes in employment or insurance), the family’s structure, and changes in physical or mental health of one or more family members.
Addressing the effects of stress on diet
Clinicians can help families manage the ways stress can lead to obesity by helping them with ideas for increasing fruit and vegetable intake, and planning ahead for on-the-go eating. For example, to ensure children get in their recommended five servings of fruit and vegetables each day, parents can serve fruit with breakfast every day and offer vegetables and/or fruit as a snack. Including side salads and a frozen vegetable with dinner will add two more servings, and children can munch on chopped veggies while parents prepare dinner. Offering fruit as a dessert provides another opportunity to bump up kids’ fruit and veggie intake, Dr. Parks said.
To manage the risk of unhealthy eating when out and about, Dr. Parks recommends planning ahead by packing a snack such as yogurt, fruit and vegetables, a sandwich or wrap, and water.
She described the “apple test” for determining whether someone is eating because of boredom or stress or because of actual hunger.“The next time you are thinking about a mini meal or second helpings at a meal, ask yourself, ‘Would I eat an apple instead?’ ” Dr. Parks said. “If the answer is no, then you probably are not really hungry and just need to get away from food.”
Other things people can consider when about to eat something are whether they are actually hungry and whether a distraction such as the television is contributing to distracted eating. “People may eat when they’re happy, sad, or bored,” Dr. Parks said, noting that outside messages such as commercials, advertisements, and passing restaurants may make someone feel like eating even if they don’t need sustenance at that moment. “Consider whether you really are hungry before you eat,” she said.
Avoiding emotional eating and using mindfulness
Additionally, parents and children can avoid emotional eating by skipping the food when they feel angry, tired, nervous, bored, or sad, instead choosing activities such as journaling, taking a walk, listening to music, reading a book, or taking deep breaths while thinking pleasant thoughts. It’s only time to eat if you physically feel hungry, your stomach is rumbling, you are not craving some specific sweet or salty food, or it’s a meal or snack time (or at least 2.5-4 hours since the last time you ate).
Dr. Parks also reviewed ways that mindfulness may help reduce the risk of obesity by reducing stress, enhancing a person’s ability to regulate their everyday behaviors, and teaching individuals to accept discomfort. Another stress reduction strategy is repeated use of “4-7-8 breathing,” which begins with exhalation while the mouth is closed. Then, inhale through the nose for 4 seconds, hold the breath for 7 seconds and slowly exhale out the mouth for 8 seconds.
Reducing the risk of obesity from stress comes from learning to manage stress. Clinicians can play a role in helping both parents and children learn strategies to manage and cope with stress in the short term while developing resilience over the longer term and reducing the likelihood of poor eating and emotional eating.
Dr. Parks reported no disclosures.
SAN FRANCISCO – Obesity is a multifactorial problem, influenced by factors ranging from genetics to lifestyle to the environment. Yet stress can play an outsize role in obesity as well, Elizabeth Prout Parks, MD, said at the annual meeting of the American Academy of Pediatrics.
Although the calorie-in/calorie-out model of energy balance has driven much of the thought about obesity, it’s not that simple, suggested Dr. Parks, the medical director of the Healthy Weight Adolescent Bariatrics Program at the Children’s Hospital of Philadelphia. Physical activity accounts for an estimated 15%-30% of energy expenditure, and thermogenesis accounts for an estimated 10%. But the energy expenditure required for basal metabolism can range from 60% to 75%, a sufficiently wide range for significant variation across different individuals.
The psychosocial effects can lead to anxiety, depression, disordered eating behaviors such as emotional eating, a sedentary lifestyle, poor sleep, and low maintenance with self-care activities. Further, poor sleep on its own is additionally associated with childhood obesity. The combination of these physiologic and psychosocial effects can increase the risk of metabolic syndrome, type 2 diabetes, and cardiovascular disease or events. While acute stress and chronic stress follow similar pathways in the brain, it’s chronic stress that carries the greater risk of behavioral and physical conditions.
Measuring and understanding child and parental stress
Several clinical assessments can measure stress in children, including the Daily Hassles Scale, which looks at everyday interactions in the environment and factors such as children’s school, family, neighborhood, peers, and lack of resources. The Multidimensional Life Events Rating Questionnaire and Adolescent Stress Questionnaire both are more appropriate for middle school and older adolescents.
In children, the primary biologic indicators of stress are cortisol levels, heart rate, and blood pressure, but it is perceived stress that has been most clearly linked to emotional eating and other disordered eating behaviors, Dr. Parks said. One 2008 study found perceived stress to be associated with emotional eating among middle school students both with and without obesity. A high level of perceived stress in adolescents was associated with a greater waist circumference and body mass index in a 2009 study.
The findings are somewhat more mixed, however, when it comes to parental stress and child weight. A 2012 study identified a link between parents’ perception of their stress and increased fast food consumption in their children, and a 2008 study identified a link between parenting stress and both overweight and underweight children. Yet a different study in 2008 found no association between child obesity and parenting stress. Research in 2011 found a relationship between children’s consumption of fruits and vegetables and their family’s overall functioning, as well as parental psychological stress and child behavior. Within a family, stress can come from financial strain (such as poverty or changes in employment or insurance), the family’s structure, and changes in physical or mental health of one or more family members.
Addressing the effects of stress on diet
Clinicians can help families manage the ways stress can lead to obesity by helping them with ideas for increasing fruit and vegetable intake, and planning ahead for on-the-go eating. For example, to ensure children get in their recommended five servings of fruit and vegetables each day, parents can serve fruit with breakfast every day and offer vegetables and/or fruit as a snack. Including side salads and a frozen vegetable with dinner will add two more servings, and children can munch on chopped veggies while parents prepare dinner. Offering fruit as a dessert provides another opportunity to bump up kids’ fruit and veggie intake, Dr. Parks said.
To manage the risk of unhealthy eating when out and about, Dr. Parks recommends planning ahead by packing a snack such as yogurt, fruit and vegetables, a sandwich or wrap, and water.
She described the “apple test” for determining whether someone is eating because of boredom or stress or because of actual hunger.“The next time you are thinking about a mini meal or second helpings at a meal, ask yourself, ‘Would I eat an apple instead?’ ” Dr. Parks said. “If the answer is no, then you probably are not really hungry and just need to get away from food.”
Other things people can consider when about to eat something are whether they are actually hungry and whether a distraction such as the television is contributing to distracted eating. “People may eat when they’re happy, sad, or bored,” Dr. Parks said, noting that outside messages such as commercials, advertisements, and passing restaurants may make someone feel like eating even if they don’t need sustenance at that moment. “Consider whether you really are hungry before you eat,” she said.
Avoiding emotional eating and using mindfulness
Additionally, parents and children can avoid emotional eating by skipping the food when they feel angry, tired, nervous, bored, or sad, instead choosing activities such as journaling, taking a walk, listening to music, reading a book, or taking deep breaths while thinking pleasant thoughts. It’s only time to eat if you physically feel hungry, your stomach is rumbling, you are not craving some specific sweet or salty food, or it’s a meal or snack time (or at least 2.5-4 hours since the last time you ate).
Dr. Parks also reviewed ways that mindfulness may help reduce the risk of obesity by reducing stress, enhancing a person’s ability to regulate their everyday behaviors, and teaching individuals to accept discomfort. Another stress reduction strategy is repeated use of “4-7-8 breathing,” which begins with exhalation while the mouth is closed. Then, inhale through the nose for 4 seconds, hold the breath for 7 seconds and slowly exhale out the mouth for 8 seconds.
Reducing the risk of obesity from stress comes from learning to manage stress. Clinicians can play a role in helping both parents and children learn strategies to manage and cope with stress in the short term while developing resilience over the longer term and reducing the likelihood of poor eating and emotional eating.
Dr. Parks reported no disclosures.
SAN FRANCISCO – Obesity is a multifactorial problem, influenced by factors ranging from genetics to lifestyle to the environment. Yet stress can play an outsize role in obesity as well, Elizabeth Prout Parks, MD, said at the annual meeting of the American Academy of Pediatrics.
Although the calorie-in/calorie-out model of energy balance has driven much of the thought about obesity, it’s not that simple, suggested Dr. Parks, the medical director of the Healthy Weight Adolescent Bariatrics Program at the Children’s Hospital of Philadelphia. Physical activity accounts for an estimated 15%-30% of energy expenditure, and thermogenesis accounts for an estimated 10%. But the energy expenditure required for basal metabolism can range from 60% to 75%, a sufficiently wide range for significant variation across different individuals.
The psychosocial effects can lead to anxiety, depression, disordered eating behaviors such as emotional eating, a sedentary lifestyle, poor sleep, and low maintenance with self-care activities. Further, poor sleep on its own is additionally associated with childhood obesity. The combination of these physiologic and psychosocial effects can increase the risk of metabolic syndrome, type 2 diabetes, and cardiovascular disease or events. While acute stress and chronic stress follow similar pathways in the brain, it’s chronic stress that carries the greater risk of behavioral and physical conditions.
Measuring and understanding child and parental stress
Several clinical assessments can measure stress in children, including the Daily Hassles Scale, which looks at everyday interactions in the environment and factors such as children’s school, family, neighborhood, peers, and lack of resources. The Multidimensional Life Events Rating Questionnaire and Adolescent Stress Questionnaire both are more appropriate for middle school and older adolescents.
In children, the primary biologic indicators of stress are cortisol levels, heart rate, and blood pressure, but it is perceived stress that has been most clearly linked to emotional eating and other disordered eating behaviors, Dr. Parks said. One 2008 study found perceived stress to be associated with emotional eating among middle school students both with and without obesity. A high level of perceived stress in adolescents was associated with a greater waist circumference and body mass index in a 2009 study.
The findings are somewhat more mixed, however, when it comes to parental stress and child weight. A 2012 study identified a link between parents’ perception of their stress and increased fast food consumption in their children, and a 2008 study identified a link between parenting stress and both overweight and underweight children. Yet a different study in 2008 found no association between child obesity and parenting stress. Research in 2011 found a relationship between children’s consumption of fruits and vegetables and their family’s overall functioning, as well as parental psychological stress and child behavior. Within a family, stress can come from financial strain (such as poverty or changes in employment or insurance), the family’s structure, and changes in physical or mental health of one or more family members.
Addressing the effects of stress on diet
Clinicians can help families manage the ways stress can lead to obesity by helping them with ideas for increasing fruit and vegetable intake, and planning ahead for on-the-go eating. For example, to ensure children get in their recommended five servings of fruit and vegetables each day, parents can serve fruit with breakfast every day and offer vegetables and/or fruit as a snack. Including side salads and a frozen vegetable with dinner will add two more servings, and children can munch on chopped veggies while parents prepare dinner. Offering fruit as a dessert provides another opportunity to bump up kids’ fruit and veggie intake, Dr. Parks said.
To manage the risk of unhealthy eating when out and about, Dr. Parks recommends planning ahead by packing a snack such as yogurt, fruit and vegetables, a sandwich or wrap, and water.
She described the “apple test” for determining whether someone is eating because of boredom or stress or because of actual hunger.“The next time you are thinking about a mini meal or second helpings at a meal, ask yourself, ‘Would I eat an apple instead?’ ” Dr. Parks said. “If the answer is no, then you probably are not really hungry and just need to get away from food.”
Other things people can consider when about to eat something are whether they are actually hungry and whether a distraction such as the television is contributing to distracted eating. “People may eat when they’re happy, sad, or bored,” Dr. Parks said, noting that outside messages such as commercials, advertisements, and passing restaurants may make someone feel like eating even if they don’t need sustenance at that moment. “Consider whether you really are hungry before you eat,” she said.
Avoiding emotional eating and using mindfulness
Additionally, parents and children can avoid emotional eating by skipping the food when they feel angry, tired, nervous, bored, or sad, instead choosing activities such as journaling, taking a walk, listening to music, reading a book, or taking deep breaths while thinking pleasant thoughts. It’s only time to eat if you physically feel hungry, your stomach is rumbling, you are not craving some specific sweet or salty food, or it’s a meal or snack time (or at least 2.5-4 hours since the last time you ate).
Dr. Parks also reviewed ways that mindfulness may help reduce the risk of obesity by reducing stress, enhancing a person’s ability to regulate their everyday behaviors, and teaching individuals to accept discomfort. Another stress reduction strategy is repeated use of “4-7-8 breathing,” which begins with exhalation while the mouth is closed. Then, inhale through the nose for 4 seconds, hold the breath for 7 seconds and slowly exhale out the mouth for 8 seconds.
Reducing the risk of obesity from stress comes from learning to manage stress. Clinicians can play a role in helping both parents and children learn strategies to manage and cope with stress in the short term while developing resilience over the longer term and reducing the likelihood of poor eating and emotional eating.
Dr. Parks reported no disclosures.
Survey: More than half of gynecologic oncologists altered morcellation practices
ORLANDO – Following the Food and Drug Administration’s 2014 warning against use of laparoscopic uterine power morcellation, 13% of gynecologic oncologists decreased their use of the technique and another 39% discontinued it altogether, according to survey responses from 199 members of the Society of Gynecologic Oncology.
“This really gives a snapshot about how gynecologic oncologists feel about power morcellation in light of the FDA warning,” said Kerac N. Falk, MD, a resident at the Icahn School of Medicine at Mount Sinai, New York.
About 41% of gynecologic oncologists changed their surgical technique to minimally invasive without power morcellation. Another 20% of respondents who previously used power morcellation have switched to laparotomy.
A more rigorous informed consent process, better attention to patient selection, and enhanced protocols are positive effects emerging since the FDA Safety Communication was issued in April 2014, Dr. Falk said at the meeting sponsored by AAGL.
The 34-item survey included questions about demographics, institutional policies, and attitudes before and after the FDA warning. Among the respondents, 65% were men. Both “early” and “very seasoned” surgeons participated in the survey. The majority of the respondents were moderate- to high-volume surgeons.
Men were significantly more likely to decrease or discontinue use of power morcellation, compared with women (P = .0015). Region of practice, years in practice, or institution type did not significantly influence changes in practice. “Most said it was not a personal choice, but more about patient choice or an institutional policy change,” Dr. Falk said.
There is still a role for power morcellation in carefully selected patients, Dr. Falk added.
In July 2014, AAGL issued a statement in response to the FDA warning, stating that “we should improve but not abandon power morcellation, and that power morcellation with appropriate informed consent should remain available to appropriately screened, low-risk women.” In addition, the American College of Obstetricians and Gynecologists stated, “Although the worsening of an occult malignancy as a result of power morcellation is, of course, tragic, we believe that an approach that combines deliberate patient selection criteria with robust informed consent will help protect women from a negative outcome, while maintaining access to morcellation for women who would benefit from it.”
Dr. Falk is not a coauthor on the study. He presented the findings on behalf of a colleague unable to attend the meeting. Dr. Falk reported having no relevant financial disclosures.
ORLANDO – Following the Food and Drug Administration’s 2014 warning against use of laparoscopic uterine power morcellation, 13% of gynecologic oncologists decreased their use of the technique and another 39% discontinued it altogether, according to survey responses from 199 members of the Society of Gynecologic Oncology.
“This really gives a snapshot about how gynecologic oncologists feel about power morcellation in light of the FDA warning,” said Kerac N. Falk, MD, a resident at the Icahn School of Medicine at Mount Sinai, New York.
About 41% of gynecologic oncologists changed their surgical technique to minimally invasive without power morcellation. Another 20% of respondents who previously used power morcellation have switched to laparotomy.
A more rigorous informed consent process, better attention to patient selection, and enhanced protocols are positive effects emerging since the FDA Safety Communication was issued in April 2014, Dr. Falk said at the meeting sponsored by AAGL.
The 34-item survey included questions about demographics, institutional policies, and attitudes before and after the FDA warning. Among the respondents, 65% were men. Both “early” and “very seasoned” surgeons participated in the survey. The majority of the respondents were moderate- to high-volume surgeons.
Men were significantly more likely to decrease or discontinue use of power morcellation, compared with women (P = .0015). Region of practice, years in practice, or institution type did not significantly influence changes in practice. “Most said it was not a personal choice, but more about patient choice or an institutional policy change,” Dr. Falk said.
There is still a role for power morcellation in carefully selected patients, Dr. Falk added.
In July 2014, AAGL issued a statement in response to the FDA warning, stating that “we should improve but not abandon power morcellation, and that power morcellation with appropriate informed consent should remain available to appropriately screened, low-risk women.” In addition, the American College of Obstetricians and Gynecologists stated, “Although the worsening of an occult malignancy as a result of power morcellation is, of course, tragic, we believe that an approach that combines deliberate patient selection criteria with robust informed consent will help protect women from a negative outcome, while maintaining access to morcellation for women who would benefit from it.”
Dr. Falk is not a coauthor on the study. He presented the findings on behalf of a colleague unable to attend the meeting. Dr. Falk reported having no relevant financial disclosures.
ORLANDO – Following the Food and Drug Administration’s 2014 warning against use of laparoscopic uterine power morcellation, 13% of gynecologic oncologists decreased their use of the technique and another 39% discontinued it altogether, according to survey responses from 199 members of the Society of Gynecologic Oncology.
“This really gives a snapshot about how gynecologic oncologists feel about power morcellation in light of the FDA warning,” said Kerac N. Falk, MD, a resident at the Icahn School of Medicine at Mount Sinai, New York.
About 41% of gynecologic oncologists changed their surgical technique to minimally invasive without power morcellation. Another 20% of respondents who previously used power morcellation have switched to laparotomy.
A more rigorous informed consent process, better attention to patient selection, and enhanced protocols are positive effects emerging since the FDA Safety Communication was issued in April 2014, Dr. Falk said at the meeting sponsored by AAGL.
The 34-item survey included questions about demographics, institutional policies, and attitudes before and after the FDA warning. Among the respondents, 65% were men. Both “early” and “very seasoned” surgeons participated in the survey. The majority of the respondents were moderate- to high-volume surgeons.
Men were significantly more likely to decrease or discontinue use of power morcellation, compared with women (P = .0015). Region of practice, years in practice, or institution type did not significantly influence changes in practice. “Most said it was not a personal choice, but more about patient choice or an institutional policy change,” Dr. Falk said.
There is still a role for power morcellation in carefully selected patients, Dr. Falk added.
In July 2014, AAGL issued a statement in response to the FDA warning, stating that “we should improve but not abandon power morcellation, and that power morcellation with appropriate informed consent should remain available to appropriately screened, low-risk women.” In addition, the American College of Obstetricians and Gynecologists stated, “Although the worsening of an occult malignancy as a result of power morcellation is, of course, tragic, we believe that an approach that combines deliberate patient selection criteria with robust informed consent will help protect women from a negative outcome, while maintaining access to morcellation for women who would benefit from it.”
Dr. Falk is not a coauthor on the study. He presented the findings on behalf of a colleague unable to attend the meeting. Dr. Falk reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Key clinical point:
Major finding: Among 199 members of the Society of Gynecologic Oncology, 39% said they suspended use of power morcellation, and 13% decreased their use of the technique.
Data source: A survey sent to all members of the Society of Gynecologic Oncology with responses from 199 members.
Disclosures: Dr. Falk reported having no relevant financial disclosures.
Evaluating Fontan failure risk after arrhythmia
People who have undergone the Fontan procedure have been known to be prone to developing arrhythmias, but few studies have evaluated their prognosis, so researchers from Australia and New Zealand analyzed results of more than 1,000 patients with Fontan circulation and found that two-thirds did not have any arrhythmia at 20 years, and that, among those who did have arrhythmias, almost three-quarters survived 10 years.
“After the first onset of an arrhythmia, close surveillance of ventricular function is required,” Thomas A. Carins, MD, and his colleagues reported (J Thorac Cardiovasc Surg. 2016;152:1355-63). They analyzed data from 1,034 patients who had Fontan procedures from 1975 to 2014 in the Australia and New Zealand Fontan Registry. “The development of an arrhythmia is associated with a heightened risk of subsequent failure of the Fontan circulation,” they wrote.
The study aimed to determine the type of arrhythmias Fontan patients had and what impact that had on long-term outcomes. The most common Fontan approach used in study patients was the extracardiac conduit (555), followed by the lateral tunnel approach (269) and atriopulmonary (210). Those who had the extracardiac Fontan were least likely to develop an arrhythmia, with a hazard ratio of 0.23 (P less than .001), which Dr. Carins and his coauthors noted was in line with previous reports of arrhythmias occurring in patients who had undergone the atriopulmonary connection (Circulation. 2004;109:2319-25; J Thorac Cardiovasc Surg. 1998;115:499-505).
Overall, 195 patients in the study developed arrhythmia, with 162 having tachyarrhythmia, 74 having bradyarrhythmia and 41 having both. “At 20 years, freedom from any arrhythmia, tachyarrhythmia, and bradyarrhythmia was 66%, 69%, and 85%, respectively,” the researchers said.
The following outcomes occurred after the first onset of arrhythmia – tachyarrhythmia in 153 patients and bradyarrhythmia in 42: Thirty-three died; 12 had heart transplants, 30 had a Fontan correction to an extracardiac conduit, three had a Fontan takedown, 12 developed enteropathy, and 25 developed New York Heart Association class III or IV symptoms. Eighty-four patients reached the composite endpoint of Fontan failure.
After they developed arrhythmias, most patients in all three Fontan procedure groups remained free from Fontan failure at 10 years: 67% in the extracardiac conduit group; 54% in the lateral tunnel group; and 51% in the atriopulmonary group.
Medical management of up to four medications was the preferred initial treatment for those with tachyarrhythmias (86%); 101 patients had a single episode of tachyarrhythmia at follow-up intervals of four to 13 years (7.6 year median). “Those who experienced a single versus multiple episodes of tachyarrhythmia showed comparable freedom from Fontan failure at 15 years,” noted Dr. Carins and his coauthors – with rates of 34% and 33%, respectively. Of the 74 patients with bradyarrhythmias, 66 received pacemakers.
“Survival after the onset of an arrhythmia was surprisingly good with 67% and 84% of patients alive at 10 years after the onset of a tachyarrhythmia and bradyarrhythmia, respectively,” the study authors said. “There was no association between occurrence of arrhythmia and survival.”
About 40% of the patients with a tachyarrhythmia or bradyarrhythmia in the study had reduced ventricular function at 10 years after onset, the researchers wrote. “Although the assessment of ventricular function in this study was clearly subjective, we nonetheless believe that these findings suggest that the onset of an arrhythmia is associated with a progressive deterioration in cardiac function,”they noted.
Coauthor Andrew Bullock, MBBS, disclosed receiving consulting fees from Actelion. Dr. Cairns and other coauthors had no financial relationships to disclose.
When interpreting the data that the Australian and New Zealand researchers analyzed, one must be cautious about viewing arrhythmia as an early indicator for Fontan revision, Mark E. Alexander, MD, of Boston Children’s Hospital and Harvard Medical School, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152:1364-5).
The outcome of a Fontan revision after an arrhythmia “becomes self-fulfilling,” Dr. Alexander said. He questioned what the revision procedure would be when the initial operation was an extracardiac Fontan. “The complex risks of that procedure continue to keep decisions regarding Fontan revisions challenging,” he said. He also noted the study did not analyze the association of ventricular function and arrhythmias “in a substantive way.”
And Dr. Alexander did not interpret the study results as an endorsement of the extracardiac Fontan or a rejection of the lateral tunnel approach. The early adoption of the extracardiac Fontan by the groups the authors represented is itself a limitation of the study, he said. Challenges with follow-up of extracardiac techniques in this and other studies “limit our ability to declare a ‘victor’ in that debate,” he said. “It does remind the electrophysiologist that he or she needs to master the techniques of entering the pulmonary venous atrium in these patients.”
The precision of calculating risk after an operation grows weaker with time, he said, and at 15-20 years morbidity starts to increase and follow-up becomes “more diffuse,” Dr. Alexander said. “That reality means we look forward to this group continuing to enhance our understanding of how our changing management decisions can aid our patients.”
Dr. Alexander had no financial relationships to disclose.
When interpreting the data that the Australian and New Zealand researchers analyzed, one must be cautious about viewing arrhythmia as an early indicator for Fontan revision, Mark E. Alexander, MD, of Boston Children’s Hospital and Harvard Medical School, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152:1364-5).
The outcome of a Fontan revision after an arrhythmia “becomes self-fulfilling,” Dr. Alexander said. He questioned what the revision procedure would be when the initial operation was an extracardiac Fontan. “The complex risks of that procedure continue to keep decisions regarding Fontan revisions challenging,” he said. He also noted the study did not analyze the association of ventricular function and arrhythmias “in a substantive way.”
And Dr. Alexander did not interpret the study results as an endorsement of the extracardiac Fontan or a rejection of the lateral tunnel approach. The early adoption of the extracardiac Fontan by the groups the authors represented is itself a limitation of the study, he said. Challenges with follow-up of extracardiac techniques in this and other studies “limit our ability to declare a ‘victor’ in that debate,” he said. “It does remind the electrophysiologist that he or she needs to master the techniques of entering the pulmonary venous atrium in these patients.”
The precision of calculating risk after an operation grows weaker with time, he said, and at 15-20 years morbidity starts to increase and follow-up becomes “more diffuse,” Dr. Alexander said. “That reality means we look forward to this group continuing to enhance our understanding of how our changing management decisions can aid our patients.”
Dr. Alexander had no financial relationships to disclose.
When interpreting the data that the Australian and New Zealand researchers analyzed, one must be cautious about viewing arrhythmia as an early indicator for Fontan revision, Mark E. Alexander, MD, of Boston Children’s Hospital and Harvard Medical School, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152:1364-5).
The outcome of a Fontan revision after an arrhythmia “becomes self-fulfilling,” Dr. Alexander said. He questioned what the revision procedure would be when the initial operation was an extracardiac Fontan. “The complex risks of that procedure continue to keep decisions regarding Fontan revisions challenging,” he said. He also noted the study did not analyze the association of ventricular function and arrhythmias “in a substantive way.”
And Dr. Alexander did not interpret the study results as an endorsement of the extracardiac Fontan or a rejection of the lateral tunnel approach. The early adoption of the extracardiac Fontan by the groups the authors represented is itself a limitation of the study, he said. Challenges with follow-up of extracardiac techniques in this and other studies “limit our ability to declare a ‘victor’ in that debate,” he said. “It does remind the electrophysiologist that he or she needs to master the techniques of entering the pulmonary venous atrium in these patients.”
The precision of calculating risk after an operation grows weaker with time, he said, and at 15-20 years morbidity starts to increase and follow-up becomes “more diffuse,” Dr. Alexander said. “That reality means we look forward to this group continuing to enhance our understanding of how our changing management decisions can aid our patients.”
Dr. Alexander had no financial relationships to disclose.
People who have undergone the Fontan procedure have been known to be prone to developing arrhythmias, but few studies have evaluated their prognosis, so researchers from Australia and New Zealand analyzed results of more than 1,000 patients with Fontan circulation and found that two-thirds did not have any arrhythmia at 20 years, and that, among those who did have arrhythmias, almost three-quarters survived 10 years.
“After the first onset of an arrhythmia, close surveillance of ventricular function is required,” Thomas A. Carins, MD, and his colleagues reported (J Thorac Cardiovasc Surg. 2016;152:1355-63). They analyzed data from 1,034 patients who had Fontan procedures from 1975 to 2014 in the Australia and New Zealand Fontan Registry. “The development of an arrhythmia is associated with a heightened risk of subsequent failure of the Fontan circulation,” they wrote.
The study aimed to determine the type of arrhythmias Fontan patients had and what impact that had on long-term outcomes. The most common Fontan approach used in study patients was the extracardiac conduit (555), followed by the lateral tunnel approach (269) and atriopulmonary (210). Those who had the extracardiac Fontan were least likely to develop an arrhythmia, with a hazard ratio of 0.23 (P less than .001), which Dr. Carins and his coauthors noted was in line with previous reports of arrhythmias occurring in patients who had undergone the atriopulmonary connection (Circulation. 2004;109:2319-25; J Thorac Cardiovasc Surg. 1998;115:499-505).
Overall, 195 patients in the study developed arrhythmia, with 162 having tachyarrhythmia, 74 having bradyarrhythmia and 41 having both. “At 20 years, freedom from any arrhythmia, tachyarrhythmia, and bradyarrhythmia was 66%, 69%, and 85%, respectively,” the researchers said.
The following outcomes occurred after the first onset of arrhythmia – tachyarrhythmia in 153 patients and bradyarrhythmia in 42: Thirty-three died; 12 had heart transplants, 30 had a Fontan correction to an extracardiac conduit, three had a Fontan takedown, 12 developed enteropathy, and 25 developed New York Heart Association class III or IV symptoms. Eighty-four patients reached the composite endpoint of Fontan failure.
After they developed arrhythmias, most patients in all three Fontan procedure groups remained free from Fontan failure at 10 years: 67% in the extracardiac conduit group; 54% in the lateral tunnel group; and 51% in the atriopulmonary group.
Medical management of up to four medications was the preferred initial treatment for those with tachyarrhythmias (86%); 101 patients had a single episode of tachyarrhythmia at follow-up intervals of four to 13 years (7.6 year median). “Those who experienced a single versus multiple episodes of tachyarrhythmia showed comparable freedom from Fontan failure at 15 years,” noted Dr. Carins and his coauthors – with rates of 34% and 33%, respectively. Of the 74 patients with bradyarrhythmias, 66 received pacemakers.
“Survival after the onset of an arrhythmia was surprisingly good with 67% and 84% of patients alive at 10 years after the onset of a tachyarrhythmia and bradyarrhythmia, respectively,” the study authors said. “There was no association between occurrence of arrhythmia and survival.”
About 40% of the patients with a tachyarrhythmia or bradyarrhythmia in the study had reduced ventricular function at 10 years after onset, the researchers wrote. “Although the assessment of ventricular function in this study was clearly subjective, we nonetheless believe that these findings suggest that the onset of an arrhythmia is associated with a progressive deterioration in cardiac function,”they noted.
Coauthor Andrew Bullock, MBBS, disclosed receiving consulting fees from Actelion. Dr. Cairns and other coauthors had no financial relationships to disclose.
People who have undergone the Fontan procedure have been known to be prone to developing arrhythmias, but few studies have evaluated their prognosis, so researchers from Australia and New Zealand analyzed results of more than 1,000 patients with Fontan circulation and found that two-thirds did not have any arrhythmia at 20 years, and that, among those who did have arrhythmias, almost three-quarters survived 10 years.
“After the first onset of an arrhythmia, close surveillance of ventricular function is required,” Thomas A. Carins, MD, and his colleagues reported (J Thorac Cardiovasc Surg. 2016;152:1355-63). They analyzed data from 1,034 patients who had Fontan procedures from 1975 to 2014 in the Australia and New Zealand Fontan Registry. “The development of an arrhythmia is associated with a heightened risk of subsequent failure of the Fontan circulation,” they wrote.
The study aimed to determine the type of arrhythmias Fontan patients had and what impact that had on long-term outcomes. The most common Fontan approach used in study patients was the extracardiac conduit (555), followed by the lateral tunnel approach (269) and atriopulmonary (210). Those who had the extracardiac Fontan were least likely to develop an arrhythmia, with a hazard ratio of 0.23 (P less than .001), which Dr. Carins and his coauthors noted was in line with previous reports of arrhythmias occurring in patients who had undergone the atriopulmonary connection (Circulation. 2004;109:2319-25; J Thorac Cardiovasc Surg. 1998;115:499-505).
Overall, 195 patients in the study developed arrhythmia, with 162 having tachyarrhythmia, 74 having bradyarrhythmia and 41 having both. “At 20 years, freedom from any arrhythmia, tachyarrhythmia, and bradyarrhythmia was 66%, 69%, and 85%, respectively,” the researchers said.
The following outcomes occurred after the first onset of arrhythmia – tachyarrhythmia in 153 patients and bradyarrhythmia in 42: Thirty-three died; 12 had heart transplants, 30 had a Fontan correction to an extracardiac conduit, three had a Fontan takedown, 12 developed enteropathy, and 25 developed New York Heart Association class III or IV symptoms. Eighty-four patients reached the composite endpoint of Fontan failure.
After they developed arrhythmias, most patients in all three Fontan procedure groups remained free from Fontan failure at 10 years: 67% in the extracardiac conduit group; 54% in the lateral tunnel group; and 51% in the atriopulmonary group.
Medical management of up to four medications was the preferred initial treatment for those with tachyarrhythmias (86%); 101 patients had a single episode of tachyarrhythmia at follow-up intervals of four to 13 years (7.6 year median). “Those who experienced a single versus multiple episodes of tachyarrhythmia showed comparable freedom from Fontan failure at 15 years,” noted Dr. Carins and his coauthors – with rates of 34% and 33%, respectively. Of the 74 patients with bradyarrhythmias, 66 received pacemakers.
“Survival after the onset of an arrhythmia was surprisingly good with 67% and 84% of patients alive at 10 years after the onset of a tachyarrhythmia and bradyarrhythmia, respectively,” the study authors said. “There was no association between occurrence of arrhythmia and survival.”
About 40% of the patients with a tachyarrhythmia or bradyarrhythmia in the study had reduced ventricular function at 10 years after onset, the researchers wrote. “Although the assessment of ventricular function in this study was clearly subjective, we nonetheless believe that these findings suggest that the onset of an arrhythmia is associated with a progressive deterioration in cardiac function,”they noted.
Coauthor Andrew Bullock, MBBS, disclosed receiving consulting fees from Actelion. Dr. Cairns and other coauthors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The development of arrhythmia is associated with a heightened risk of failure of Fontan circulation after a Fontan procedure.
Major finding: At 20 years, freedom from any arrhythmia was 66%, and after the onset of any arrhythmia freedom from Fontan failure was 55%.
Data source: 1,034 patients who had undergone a Fontan procedure from 1975 to 2014 as recorded in the Australian and New Zealand Fontan Registry.
Disclosures: Coauthor Andrew Bullock, MBBS, reported receiving consulting fees from Actelion. All other others have no financial relationships to disclose.
Tinea Capitis Caused by Trichophyton rubrum Mimicking Favus
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.
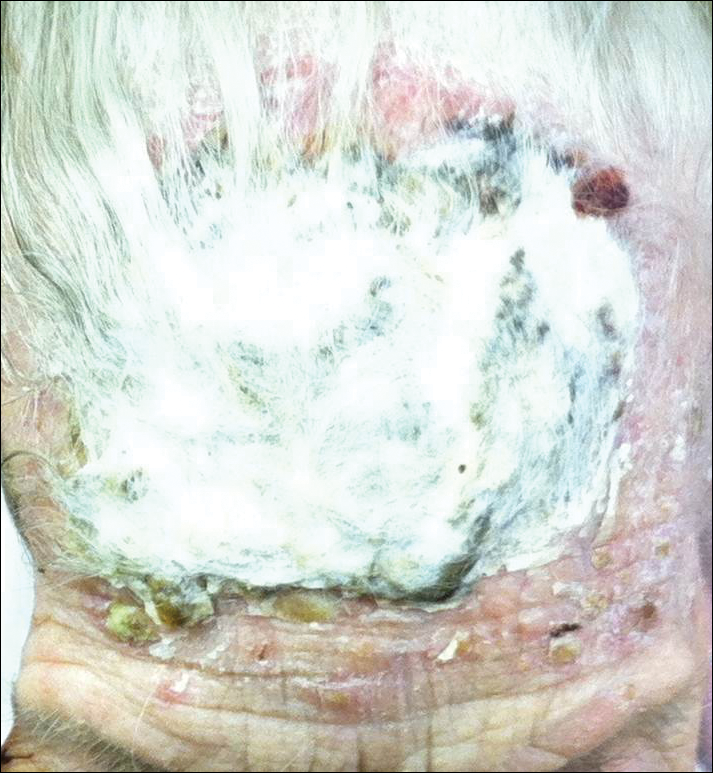
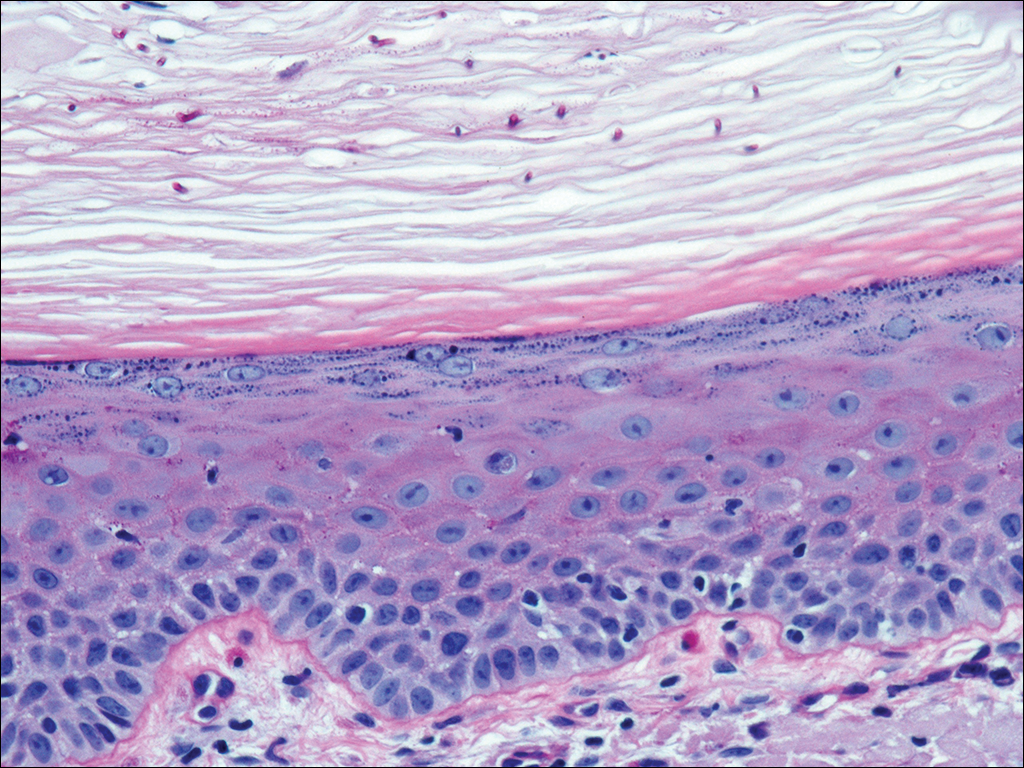
At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).

Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.


At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).


Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.


At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).


Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
Practice Points
- Although favus is uncommonly seen in developed countries, it still exists and can mimick other conditions, notably cutaneous malignancies.
- Favus may affect the skin and nails in addition to the hair.
- The lesions of favus may persist for many years.