User login
Second course of rifaximin edges out placebo in IBS-D trial
Patients with diarrhea-predominant irritable bowel syndrome (IBS-D) who responded to rifaximin but relapsed after completing treatment were significantly more likely to respond to a second course of the antibiotic than to placebo, according to a report in the December issue of Gastroenterology (2016 Aug 5. doi: 10.1053/j.gastro.2016.08.003).
A total of 38% of patients who received a second course of rifaximin met the primary endpoint in the randomized double-blinded trial, compared with 31.5% of the placebo group (P = .03), Dr. Anthony Lembo of Beth Israel Deaconess Medical Center, Boston, and his associates wrote in Gastroenterology. “Although this study had a positive outcome, questions remain regarding the role of nonsystemic antibiotics in the long term, particularly when patients with IBS-D may require years of symptom management,” they added. “Further research is needed to better understand the treatment algorithm in patients who may lose responsiveness to rifaximin.”
Rifaximin (Xifaxan) has been approved in the United States for treating IBS-D since 2015. The agent is an oral, minimally absorbed, broad-spectrum antibiotic that targets the gastrointestinal tract and has rarely been linked to “clinically relevant” antibiotic resistance, the researchers said. However, pivotal IBS-D trials had not investigated the durability of response to rifaximin or the efficacy and safety of repeat treatment, they noted. Therefore, they followed 1,074 patients with IBS-D who had responded to an open-label 2-week course of rifaximin dosed orally at 550 mg three times daily. By definition, these responders had met a combined primary endpoint that included at least a 30% decrease in abdominal pain and at least a 50% decrease in the frequency of loose stools during at least 2 of 4 weeks of follow-up.
In all, 692 (64%) responders relapsed up to 18 weeks after finishing the first rifaximin course, the investigators said. They randomly assigned 636 of these relapsers to double-blinded treatment with either placebo or a second course of rifaximin. In all, 125 of 328 patients (38.1%) in the rifaximin group again met the combined primary endpoint, compared with 97 of 308 patients (31.5%) in the placebo group (P = .03). Repeat rifaximin treatment also significantly outperformed placebo in terms of the individual abdominal pain endpoint (51% versus 42%, respectively; P = .02), but not the stool consistency endpoint (52% versus 50%).
“Adverse event rates were low and similar between groups,” the researchers said. Patients who received a second course of rifaximin most commonly developed nausea (3.7%), upper respiratory infection (3.7%), urinary tract infection (3.4%), and nasopharyngitis (3.0%). Four patients (1%) in each treatment group developed serious adverse events, none of which were deemed treatment related. One patient developed Clostridium difficile colitis 37 days after completing the second course of rifaximin. However, this patient had a past history of C. difficile infection, had tested negative for C. difficile toxins A and B at enrollment, and had completed a 10-day course of cefdinir for a urinary tract infection immediately before developing C. difficile colitis.
Salix Pharmaceuticals makes rifaximin and funded the study. Dr. Lembo and his coinvestigators disclosed ties to Salix.
Patients with diarrhea-predominant irritable bowel syndrome (IBS-D) who responded to rifaximin but relapsed after completing treatment were significantly more likely to respond to a second course of the antibiotic than to placebo, according to a report in the December issue of Gastroenterology (2016 Aug 5. doi: 10.1053/j.gastro.2016.08.003).
A total of 38% of patients who received a second course of rifaximin met the primary endpoint in the randomized double-blinded trial, compared with 31.5% of the placebo group (P = .03), Dr. Anthony Lembo of Beth Israel Deaconess Medical Center, Boston, and his associates wrote in Gastroenterology. “Although this study had a positive outcome, questions remain regarding the role of nonsystemic antibiotics in the long term, particularly when patients with IBS-D may require years of symptom management,” they added. “Further research is needed to better understand the treatment algorithm in patients who may lose responsiveness to rifaximin.”
Rifaximin (Xifaxan) has been approved in the United States for treating IBS-D since 2015. The agent is an oral, minimally absorbed, broad-spectrum antibiotic that targets the gastrointestinal tract and has rarely been linked to “clinically relevant” antibiotic resistance, the researchers said. However, pivotal IBS-D trials had not investigated the durability of response to rifaximin or the efficacy and safety of repeat treatment, they noted. Therefore, they followed 1,074 patients with IBS-D who had responded to an open-label 2-week course of rifaximin dosed orally at 550 mg three times daily. By definition, these responders had met a combined primary endpoint that included at least a 30% decrease in abdominal pain and at least a 50% decrease in the frequency of loose stools during at least 2 of 4 weeks of follow-up.
In all, 692 (64%) responders relapsed up to 18 weeks after finishing the first rifaximin course, the investigators said. They randomly assigned 636 of these relapsers to double-blinded treatment with either placebo or a second course of rifaximin. In all, 125 of 328 patients (38.1%) in the rifaximin group again met the combined primary endpoint, compared with 97 of 308 patients (31.5%) in the placebo group (P = .03). Repeat rifaximin treatment also significantly outperformed placebo in terms of the individual abdominal pain endpoint (51% versus 42%, respectively; P = .02), but not the stool consistency endpoint (52% versus 50%).
“Adverse event rates were low and similar between groups,” the researchers said. Patients who received a second course of rifaximin most commonly developed nausea (3.7%), upper respiratory infection (3.7%), urinary tract infection (3.4%), and nasopharyngitis (3.0%). Four patients (1%) in each treatment group developed serious adverse events, none of which were deemed treatment related. One patient developed Clostridium difficile colitis 37 days after completing the second course of rifaximin. However, this patient had a past history of C. difficile infection, had tested negative for C. difficile toxins A and B at enrollment, and had completed a 10-day course of cefdinir for a urinary tract infection immediately before developing C. difficile colitis.
Salix Pharmaceuticals makes rifaximin and funded the study. Dr. Lembo and his coinvestigators disclosed ties to Salix.
Patients with diarrhea-predominant irritable bowel syndrome (IBS-D) who responded to rifaximin but relapsed after completing treatment were significantly more likely to respond to a second course of the antibiotic than to placebo, according to a report in the December issue of Gastroenterology (2016 Aug 5. doi: 10.1053/j.gastro.2016.08.003).
A total of 38% of patients who received a second course of rifaximin met the primary endpoint in the randomized double-blinded trial, compared with 31.5% of the placebo group (P = .03), Dr. Anthony Lembo of Beth Israel Deaconess Medical Center, Boston, and his associates wrote in Gastroenterology. “Although this study had a positive outcome, questions remain regarding the role of nonsystemic antibiotics in the long term, particularly when patients with IBS-D may require years of symptom management,” they added. “Further research is needed to better understand the treatment algorithm in patients who may lose responsiveness to rifaximin.”
Rifaximin (Xifaxan) has been approved in the United States for treating IBS-D since 2015. The agent is an oral, minimally absorbed, broad-spectrum antibiotic that targets the gastrointestinal tract and has rarely been linked to “clinically relevant” antibiotic resistance, the researchers said. However, pivotal IBS-D trials had not investigated the durability of response to rifaximin or the efficacy and safety of repeat treatment, they noted. Therefore, they followed 1,074 patients with IBS-D who had responded to an open-label 2-week course of rifaximin dosed orally at 550 mg three times daily. By definition, these responders had met a combined primary endpoint that included at least a 30% decrease in abdominal pain and at least a 50% decrease in the frequency of loose stools during at least 2 of 4 weeks of follow-up.
In all, 692 (64%) responders relapsed up to 18 weeks after finishing the first rifaximin course, the investigators said. They randomly assigned 636 of these relapsers to double-blinded treatment with either placebo or a second course of rifaximin. In all, 125 of 328 patients (38.1%) in the rifaximin group again met the combined primary endpoint, compared with 97 of 308 patients (31.5%) in the placebo group (P = .03). Repeat rifaximin treatment also significantly outperformed placebo in terms of the individual abdominal pain endpoint (51% versus 42%, respectively; P = .02), but not the stool consistency endpoint (52% versus 50%).
“Adverse event rates were low and similar between groups,” the researchers said. Patients who received a second course of rifaximin most commonly developed nausea (3.7%), upper respiratory infection (3.7%), urinary tract infection (3.4%), and nasopharyngitis (3.0%). Four patients (1%) in each treatment group developed serious adverse events, none of which were deemed treatment related. One patient developed Clostridium difficile colitis 37 days after completing the second course of rifaximin. However, this patient had a past history of C. difficile infection, had tested negative for C. difficile toxins A and B at enrollment, and had completed a 10-day course of cefdinir for a urinary tract infection immediately before developing C. difficile colitis.
Salix Pharmaceuticals makes rifaximin and funded the study. Dr. Lembo and his coinvestigators disclosed ties to Salix.
FROM GASTROENTEROLOGY
Key clinical point: A second course of rifaximin may be merited in patients with diarrhea-predominant irritable bowel syndrome.
Major finding: In all, 38% of patients who received a second course of the antibiotic met the primary endpoint, compared with 31.5% of those who received placebo (P = .03),
Data source: A randomized, double-blind, phase III trial of 692 patients with IBS-D who relapsed after initially responding to a 2-week course of rifaximin.
Disclosures: Salix Pharmaceuticals, maker of rifaximin, funded the study. Dr. Lembo and his coinvestigators disclosed ties to Salix.
Yoga holds up to medications, walking for irritable bowel syndrome
Yoga may be a feasible and safe add-on therapy for patients with irritable bowel syndrome, based on a systematic review of six randomized controlled trials of 273 patients published in the December issue of Clinical Gastroenterology and Hepatology.
Yoga significantly outperformed no treatment and resembled pharmacologic therapies for IBS on measures of bowel symptoms, anxiety, and quality of life, said Dania Schumann of the University of Duisburg-Essen, Essen, Germany.
“Yoga also seems to be equally effective as a walking program in improving patient-reported outcomes,” she and her coinvestigators wrote. But “wide methodological heterogeneity” and a “mostly unclear risk of bias,” precluded a direct recommendation for yoga in IBS, they said. Nonetheless, “its practice need not be discouraged in this patient population, especially when [patients] believe that it benefits their health, quality of life, or IBS-related comorbidities.”
Experts have increasingly emphasized the role of stress, psychological disorders, and the bidirectional gut-brain axis in IBS, the reviewers noted. Because yoga had been found to cut stress and improve psychological functioning in past studies, they hypothesized that it also might improve IBS symptoms. By searching MEDLINE/Pubmed, the Cochrane Library, CAM-QUEST, CAMbase, and IndMED for studies of IBS and yoga, they identified 93 records, including six randomized controlled trials from India, the United States, and Canada. One trial defined IBS based on Rome I criteria, another used Rome II criteria, three used Rome III criteria, and the sixth trial relied solely on clinical and laboratory measures. Patients ranged in age from 14 to 44 years (median, 32 years), and most were female. They were allowed to continue their usual IBS care (Clin Gastroenterol Hepatol. 2016 Apr 22. doi: 10.1016/j.cgh.2016.04.026). Two trials compared 9-12 weeks of yoga with pharmacologic therapies. In one study, yoga and loperamide were associated with similar improvements in bowel symptoms, state anxiety, gastric motility, and other measures of autonomic reactivity. The second study found no significant differences in the colonic myoelectrical effects of yoga, placebo, and a regimen of psyllium husk, propantheline, and diazepam.
Three studies compared 4-12 weeks of Iyengar or hatha yoga with usual IBS care. Yoga outperformed standard care on measures of IBS symptoms, quality of life, psychological distress, and fatigue in two trials. The third study found a benefit for yoga after wait-listed controls joined the yoga intervention and the researchers combined their data with the other yoga group.
The sixth trial compared yoga with a walking program and found similar effects. Yoga was associated with significant improvements in abdominal pain, visceral sensitivity, and GI symptoms, while walking improved gastrointestinal symptoms, negative affect, and state anxiety. But at 6-month follow-up, walkers had fewer gastrointestinal symptoms than did the yoga group, perhaps because a walking program is easier to maintain at home, the reviewers noted.
Only one trial adequately performed adequate blinding during outcome assessments, and several others were at high risk of performance bias, reporting bias, and attrition bias, the reviewers said. The trials also did not adequately describe methods to randomize patients or conceal group allocations, and “selective reporting and high dropout rates [were] an issue,” they added.
Adverse events related to yoga included three cases of temporarily aggravated lower back pain and one fall after a participant slipped and hit his knee while in a headstand. However, only two trials assessed adverse events, the reviewers noted. “Future studies should ensure rigorous reporting of adverse events, and the correct use of terminology,” they said.
Because meditation, breathing exercises, and yoga seem to improve both stress and IBS symptoms, researchers should consider these practices when studying patients with “an increased gastrointestinal response to stress,” the reviewers concluded. “So far, the recent global guidelines of the World Gastroenterology Organization on IBS consider sufficient physical activity and relaxation techniques to be appropriate nonpharmacologic approaches.”
The reviewers did not report funding sources. They had no relevant conflicts of interest.
Yoga may be a feasible and safe add-on therapy for patients with irritable bowel syndrome, based on a systematic review of six randomized controlled trials of 273 patients published in the December issue of Clinical Gastroenterology and Hepatology.
Yoga significantly outperformed no treatment and resembled pharmacologic therapies for IBS on measures of bowel symptoms, anxiety, and quality of life, said Dania Schumann of the University of Duisburg-Essen, Essen, Germany.
“Yoga also seems to be equally effective as a walking program in improving patient-reported outcomes,” she and her coinvestigators wrote. But “wide methodological heterogeneity” and a “mostly unclear risk of bias,” precluded a direct recommendation for yoga in IBS, they said. Nonetheless, “its practice need not be discouraged in this patient population, especially when [patients] believe that it benefits their health, quality of life, or IBS-related comorbidities.”
Experts have increasingly emphasized the role of stress, psychological disorders, and the bidirectional gut-brain axis in IBS, the reviewers noted. Because yoga had been found to cut stress and improve psychological functioning in past studies, they hypothesized that it also might improve IBS symptoms. By searching MEDLINE/Pubmed, the Cochrane Library, CAM-QUEST, CAMbase, and IndMED for studies of IBS and yoga, they identified 93 records, including six randomized controlled trials from India, the United States, and Canada. One trial defined IBS based on Rome I criteria, another used Rome II criteria, three used Rome III criteria, and the sixth trial relied solely on clinical and laboratory measures. Patients ranged in age from 14 to 44 years (median, 32 years), and most were female. They were allowed to continue their usual IBS care (Clin Gastroenterol Hepatol. 2016 Apr 22. doi: 10.1016/j.cgh.2016.04.026). Two trials compared 9-12 weeks of yoga with pharmacologic therapies. In one study, yoga and loperamide were associated with similar improvements in bowel symptoms, state anxiety, gastric motility, and other measures of autonomic reactivity. The second study found no significant differences in the colonic myoelectrical effects of yoga, placebo, and a regimen of psyllium husk, propantheline, and diazepam.
Three studies compared 4-12 weeks of Iyengar or hatha yoga with usual IBS care. Yoga outperformed standard care on measures of IBS symptoms, quality of life, psychological distress, and fatigue in two trials. The third study found a benefit for yoga after wait-listed controls joined the yoga intervention and the researchers combined their data with the other yoga group.
The sixth trial compared yoga with a walking program and found similar effects. Yoga was associated with significant improvements in abdominal pain, visceral sensitivity, and GI symptoms, while walking improved gastrointestinal symptoms, negative affect, and state anxiety. But at 6-month follow-up, walkers had fewer gastrointestinal symptoms than did the yoga group, perhaps because a walking program is easier to maintain at home, the reviewers noted.
Only one trial adequately performed adequate blinding during outcome assessments, and several others were at high risk of performance bias, reporting bias, and attrition bias, the reviewers said. The trials also did not adequately describe methods to randomize patients or conceal group allocations, and “selective reporting and high dropout rates [were] an issue,” they added.
Adverse events related to yoga included three cases of temporarily aggravated lower back pain and one fall after a participant slipped and hit his knee while in a headstand. However, only two trials assessed adverse events, the reviewers noted. “Future studies should ensure rigorous reporting of adverse events, and the correct use of terminology,” they said.
Because meditation, breathing exercises, and yoga seem to improve both stress and IBS symptoms, researchers should consider these practices when studying patients with “an increased gastrointestinal response to stress,” the reviewers concluded. “So far, the recent global guidelines of the World Gastroenterology Organization on IBS consider sufficient physical activity and relaxation techniques to be appropriate nonpharmacologic approaches.”
The reviewers did not report funding sources. They had no relevant conflicts of interest.
Yoga may be a feasible and safe add-on therapy for patients with irritable bowel syndrome, based on a systematic review of six randomized controlled trials of 273 patients published in the December issue of Clinical Gastroenterology and Hepatology.
Yoga significantly outperformed no treatment and resembled pharmacologic therapies for IBS on measures of bowel symptoms, anxiety, and quality of life, said Dania Schumann of the University of Duisburg-Essen, Essen, Germany.
“Yoga also seems to be equally effective as a walking program in improving patient-reported outcomes,” she and her coinvestigators wrote. But “wide methodological heterogeneity” and a “mostly unclear risk of bias,” precluded a direct recommendation for yoga in IBS, they said. Nonetheless, “its practice need not be discouraged in this patient population, especially when [patients] believe that it benefits their health, quality of life, or IBS-related comorbidities.”
Experts have increasingly emphasized the role of stress, psychological disorders, and the bidirectional gut-brain axis in IBS, the reviewers noted. Because yoga had been found to cut stress and improve psychological functioning in past studies, they hypothesized that it also might improve IBS symptoms. By searching MEDLINE/Pubmed, the Cochrane Library, CAM-QUEST, CAMbase, and IndMED for studies of IBS and yoga, they identified 93 records, including six randomized controlled trials from India, the United States, and Canada. One trial defined IBS based on Rome I criteria, another used Rome II criteria, three used Rome III criteria, and the sixth trial relied solely on clinical and laboratory measures. Patients ranged in age from 14 to 44 years (median, 32 years), and most were female. They were allowed to continue their usual IBS care (Clin Gastroenterol Hepatol. 2016 Apr 22. doi: 10.1016/j.cgh.2016.04.026). Two trials compared 9-12 weeks of yoga with pharmacologic therapies. In one study, yoga and loperamide were associated with similar improvements in bowel symptoms, state anxiety, gastric motility, and other measures of autonomic reactivity. The second study found no significant differences in the colonic myoelectrical effects of yoga, placebo, and a regimen of psyllium husk, propantheline, and diazepam.
Three studies compared 4-12 weeks of Iyengar or hatha yoga with usual IBS care. Yoga outperformed standard care on measures of IBS symptoms, quality of life, psychological distress, and fatigue in two trials. The third study found a benefit for yoga after wait-listed controls joined the yoga intervention and the researchers combined their data with the other yoga group.
The sixth trial compared yoga with a walking program and found similar effects. Yoga was associated with significant improvements in abdominal pain, visceral sensitivity, and GI symptoms, while walking improved gastrointestinal symptoms, negative affect, and state anxiety. But at 6-month follow-up, walkers had fewer gastrointestinal symptoms than did the yoga group, perhaps because a walking program is easier to maintain at home, the reviewers noted.
Only one trial adequately performed adequate blinding during outcome assessments, and several others were at high risk of performance bias, reporting bias, and attrition bias, the reviewers said. The trials also did not adequately describe methods to randomize patients or conceal group allocations, and “selective reporting and high dropout rates [were] an issue,” they added.
Adverse events related to yoga included three cases of temporarily aggravated lower back pain and one fall after a participant slipped and hit his knee while in a headstand. However, only two trials assessed adverse events, the reviewers noted. “Future studies should ensure rigorous reporting of adverse events, and the correct use of terminology,” they said.
Because meditation, breathing exercises, and yoga seem to improve both stress and IBS symptoms, researchers should consider these practices when studying patients with “an increased gastrointestinal response to stress,” the reviewers concluded. “So far, the recent global guidelines of the World Gastroenterology Organization on IBS consider sufficient physical activity and relaxation techniques to be appropriate nonpharmacologic approaches.”
The reviewers did not report funding sources. They had no relevant conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Yoga may be an appropriate adjunctive therapy for patients with irritable bowel syndrome.
Major finding: Yoga outperformed no treatment on measures of gastrointestinal symptoms, anxiety, and quality of life, and was comparable to standard medications and a walking program.
Data source: A systematic review of six randomized controlled trials of 273 patients with irritable bowel syndrome.
Disclosures: The reviewers did not report funding sources. They had no relevant conflicts of interest.
Self-criticism and self-compassion: Risk and resilience
Once thought to only be associated with depression, self-criticism is a transdiagnostic risk factor for diverse forms of psychopathology.1,2 However, research has shown that self-compassion is a robust resilience factor when faced with feelings of personal inadequacy.3,4
Self-critical individuals experience feelings of unworthiness, inferiority, failure, and guilt. They engage in constant and harsh self-scrutiny and evaluation, and fear being disapproved and criticized and losing the approval and acceptance of others.5 Self-compassion involves treating oneself with care and concern when confronted with personal inadequacies, mistakes, failures, and painful life situations.6,7
Although self-criticism is destructive across clinical disorders and interpersonal relationships, self-compassion is associated with healthy relationships, emotional well-being, and better treatment outcomes.
Recent research shows how clinicians can teach their patients how to be less self-critical and more self-compassionate. Neff6,7 proposes that self-compassion involves treating yourself with care and concern when being confronted with personal inadequacies, mistakes, failures, and painful life situations. It consists of 3 interacting components, each of which has a positive and negative pole:
- self-kindness vs self-judgment
- a sense of common humanity vs isolation
- mindfulness vs over-identification.
Self-kindness refers to being caring and understanding with oneself rather than harshly judgmental. Instead of attacking and berating oneself for personal shortcomings, the self is offered warmth and unconditional acceptance.
Humanity involves recognizing that humans are imperfect, that all people fail, make mistakes, and have serious life challenges. By remembering that imperfection is part of life, we feel less isolated when we are in pain.
Mindfulness in the context of self-compassion involves being aware of one’s painful experiences in a balanced way that neither ignores and avoids nor exaggerates painful thoughts and emotions.
Self-compassion is more than the absence of self-judgment, although a defining feature of self-compassion is the lack of self-judgment, and self-judgment overlaps with self-criticism. Rather, self-compassion provides several access points for reducing self-criticism. For example, being kind and understanding when confronting personal inadequacies (eg, “it’s okay not to be perfect”) can counter harsh self-talk (eg, “I’m not defective”). Mindfulness of emotional pain (eg, “this is hard”) can facilitate a kind and warm response (eg, “what can I do to take care of myself right now?”) and therefore lessen self-blame (eg, “blaming myself is just causing me more suffering”). Similarly, remembering that failure is part of the human experience (eg, “it’s normal to mess up sometimes”) can lessen egocentric feelings of isolation (eg, “it’s not just me”) and over-identification (eg, “it’s not the end of the world”), resulting in lessened self-criticism (eg, “maybe it’s not just because I’m a bad person”).
Depression
Several studies have found that self-criticism predicts depression. In 3 epidemiological studies, “feeling worthless” was among the top 2 symptoms predicting a depression diagnosis and later depressive episodes.10 Self-criticism in fourth-year medical students predicted depression 2 years later, and—in males—10 years later in their medical careers better than a history of depression.11 Self-critical perfectionism also is associated with suicidal ideation and lethality of suicide attempts.12
Self-criticism has been shown to predict depressive relapse and residual self-devaluative symptoms in recovered depressed patients.13 In one study, currently depressed and remitted depressed patients had higher self-criticism and lower self-compassion compared with healthy controls. Both factors were more strongly associated with depression status than higher perfectionistic beliefs and cognitions, rumination, and maladaptive emotional regulation.14
Self-criticism and response to treatment. In the National Institute of Mental Health Treatment of Depression Collaborative Research Program,15 self-critical perfectionism predicted a poorer outcome across all 4 treatments (cognitive-behavioral therapy [CBT], interpersonal psychotherapy [IPT], pharmacotherapy plus clinical management, and placebo plus clinical management). Subsequent studies found that self-criticism predicted poorer response to CBT16 and IPT.17 The authors suggest that self-criticism could interfere with treatment because self-critical patients might have difficulty developing a strong therapeutic alliance.18,19
Anxiety disorders
Self-criticism is common across psychiatric disorders. In a study of 5,877 respondents in the National Comorbidity Survey (NCS), self-criticism was associated with social phobia, findings that were significant after controlling for current emotional distress, neuroticism, and lifetime history of mood, anxiety, and substance use disorders.20 Further, in a CBT treatment study, baseline self-criticism was associated with severity of social phobia and changes in self-criticism predicted treatment outcome.21 Self-criticism might be an important core psychological process in the development, maintenance, and course of social phobia. Patients with social anxiety disorder have less self-compassion than healthy controls and greater fear of negative evaluation.
In the NCS, self-criticism was associated with posttraumatic stress disorder (PTSD) even after controlling for lifetime history of affective and anxiety disorders.20 Self-criticism predicted greater severity of combat-related PTSD in hospitalized male veterans,22 and those with PTSD had higher scores on self-criticism scales than those with major depressive disorder.23 In a study of Holocaust survivors, those with PTSD scored higher on self-criticism than survivors without PTSD.24 Self-criticism also distinguished between female victims of domestic violence with and without PTSD.25
Self-compassion could be a protective factor for posttraumatic stress.26 Combat veterans with higher levels of self-compassion showed lower levels of psychopathology, better functioning in daily life, and fewer symptoms of posttraumatic stress.27 In fact, self-compassion has been found to be a stronger predictor of PTSD than level of combat exposure.28
In an early study, self-criticism scores were higher in patients with panic disorder than in healthy controls, but lower than in patients with depression.29 In a study of a mixed sample of anxiety disorder patients, symptoms of generalized anxiety disorder were associated with shame proneness.30 Consistent with these results, Hoge et al31 found that self-compassion was lower in generalized anxiety disorder patients compared with healthy controls with elevated stress. Low self-compassion has been associated with severity of obsessive-compulsive disorder.32
Eating disorders
Self-criticism is correlated with eating disorder severity.33 In a study of patients with binge eating disorder, Dunkley and Grilo34 found that self-criticism was associated with the over-evaluation of shape and weight independently of self-esteem and depression. Self-criticism also is associated with body dissatisfaction, independent of self-esteem and depression. Dunkley et al35 found that self-criticism, but not global self-esteem, in patients with binge eating disorder mediated the relationship between childhood abuse and body dissatisfaction and depression. Numerous studies have shown that shame is associated with more severe eating disorder pathology.33
Self-compassion seems to buffer against body image concerns. It is associated with less body dissatisfaction, body preoccupation, and weight worries,36 greater body appreciation37 and less disordered eating.37-39 Early decreases in shame during eating disorder treatment was associated with more rapid reduction in eating disorder symptoms.40
Interpersonal relationships
Several studies have shown that self-criticism has negative effects on interpersonal relationships throughout life.5,41,42
- Self-criticism at age 12 predicted less involvement in high school activities and, at age 31, personal and social maladjustment.43
- High school students with high self-criticism reported more interpersonal problems.44
- Self-criticism was associated with loneliness, depression, and lack of intimacy with opposite sex friends or partners during the transition to college.45
- In a study of college roommates,46 self-criticism was associated with increased likelihood of rejection.
- Whiffen and Aube47 found that self-criticism was associated with marital dissatisfaction and depression.
- Self-critical mothers with postpartum depression were less satisfied with social support and were more vulnerable to depression.48
Self-compassion appears to enhance interpersonal relationships. In a study of heterosexual couples,49 self-compassionate individuals were described by their partners as being more emotionally connected, as well as accepting and supporting autonomy, while being less detached, controlling, and verbally or physically aggressive than those lacking self-compassion. Because self-compassionate people give themselves care and support, they seem to have more emotional resources available to give to others.
See the Box examining the evidence on the role of self-compassion in borderline personality disorder and non-suicidal self-injury.
Achieving goals
Powers et al50 suggest that self-critics approach goals based on motivation to avoid failure and disapproval, rather than on intrinsic interest and personal meaning. In studies of college students pursuing academic, social, or weight loss goals, self-criticism was associated with less progress to that goal. Self-criticism was associated with rumination and procrastination, which the authors suggest might have focused the self-critic on potential failure, negative evaluation from others, and loss of self-esteem. Additional studies showed the deleterious effects of self-criticism on college students’ progress on obtaining academic or music performance goals and on community residents’ weight loss goals.51
Not surprisingly, self-compassion is associated with successful goal pursuit and resilience when goals are not met52 and less procrastination and academic worry.53 Self-compassion also is associated with intrinsic motivation, goals based on mastery rather than performance, and less fear of academic failure.54
How self-criticism and self-compassion develop
Studies have explored the impact of early relationships with parents and development of self-criticism. Parental overcontrol and restrictiveness and lack of warmth consistently have been identified as parenting styles associated with development of self-criticism in children.55 One study found that self-criticism fully mediated the relationship between childhood verbal abuse from parents and depression and anxiety in adulthood.56 Reports from parents on their current parenting styles are consistent with these studies.57 Amitay et al57 states that “[s]elf-critics’ negative childhood experiences thus seem to contribute to a pattern of entering, creating, or manipulating subsequent interpersonal environments in ways that perpetuate their negative self-image and increase vulnerability to depression.” Not surprisingly, self-criticism is associated with a fearful avoidant attachment style.58 Review of the developmental origins of self-criticism confirms these factors and presents findings that peer relationships also are important factors in the development of self-criticism.59,60
Early positive relationships with caregivers are associated with self-compassion. Recollections of maternal support are correlated with self-compassion and secure attachment styles in adolescents and adults.61 Pepping et al62 found that retrospective reports of parental rejection, overprotection, and low parental warmth was associated with low self-compassion.
Benefits of self-compassion
A growing body of research suggests that self-compassion is strongly linked to mental health. Greater self-compassion consistently has been associated with lower levels of depression and anxiety,3 with a large effect size.4 Of course, central to self-compassion is the lack of self-criticism, but self-compassion still protects against anxiety and depression when controlling for self-criticism and negative affect.6,63 Self-compassion is a strong predictor of symptom severity and quality of life among individuals with anxious distress.64
The benefits of self-compassion stem partly from a greater ability to cope with negative emotions.6,63,65 Self-compassionate people are less likely to ruminate on their negative thoughts and emotions or suppress them,6,66 which helps to explain why self-compassion is a negative predictor of depression.67
Self-compassion also enhances positive mind states. A number of studies have found links between self-compassion and positive psychological qualities, such as happiness, optimism, wisdom, curiosity, and exploration, and personal initiative.63,65,68,69 By embracing one’s suffering with compassion, negative states are ameliorated when positive emotions of kindness, connectedness, and mindful presence are generated.
Misconceptions about self-compassion
A common misconception is that abandoning self-criticism in favor of self-compassion will undermine motivation70; however, research indicates the opposite. Although self-compassion is negatively associated with maladaptive perfectionism, it is not correlated with self-adopted performance standards.6 Self-compassionate people have less fear of failure54 and, when they do fail, they are more likely to try again.71 Breines and Chen72 found in a series of experimental studies that engendering feelings of self-compassion for personal weaknesses, failures, and past transgressions resulted in more motivation to change, to try harder to learn, and to avoid repeating past mistakes.
Another common misunderstanding is that self-compassion is a weakness. In fact, research suggests that self-compassion is a powerful way to cope with life challenges.73
Although some fear that self-compassion leads to self-indulgence, there is evidence that self-compassion promotes health-related behaviors. Self-compassionate individuals are more likely to seek medical treatment when needed,74 exercise for intrinsic reasons,75 and drink less alcohol.76 Inducing self-compassion has been found to help people stick to their diets77 and quit smoking.78
Self-compassion interventions
Individuals can develop self-compassion. Shapira and Mongrain79 found that adults who wrote a compassionate letter to themselves once a day for a week about the distressing events they were experiencing showed significant reductions in depression up to 3 months and significant increases in happiness up to 6 months compared with a control group who wrote about early memories. Albertson et al80 found that, compared with a wait-list control group, 3 weeks of self-compassion meditation training improved body dissatisfaction, body shame, and body appreciation among women with body image concerns. Similarly, Smeets et al81 found that 3 weeks of self-compassion training for female college students led to significantly greater increases in mindfulness, optimism, and self-efficacy, as well as greater decreases in rumination compared with a time management control group.
The Box6,70,82-86 describes rating scales that can measure self-compassion and self-criticism.

Mindful self-compassion (MSC), developed by Neff and Germer,87 is an 8-week group intervention designed to teach people how to be more self-compassionate through meditation and informal practices in daily life. Results of a randomized controlled trial found that, compared with a wait-list control group, participants using MSC reported significantly greater increases in self-compassion, compassion for others, mindfulness, and life satisfaction, and greater decreases in depression, anxiety, stress, and emotional avoidance, with large effect sizes indicated. These results were maintained up to 1 year.
Compassion-focused therapy (CFT) is designed to enhance self-compassion in clinical populations.88 The approach uses a number of imagery and experiential exercises to enhance patients’ abilities to extend feelings of reassurance, safeness, and understanding toward themselves. CFT has shown promise in treating a diverse group of clinical disorders such as depression and shame,8,89 social anxiety and shame,90 eating disorders,91 psychosis,92 and patients with acquired brain injury.93 A group-based CFT intervention with a heterogeneous group of community mental health patients led to significant reductions in depression, anxiety, stress, and self-criticism.94 See Leaviss and Utley95 for a review of the benefits of CFT.
Fears of developing self-compassion
It is important to note that some people can access self-compassion more easily than others. Highly self-critical patients could feel anxious when learning to be compassionate to themselves, a phenomenon known as “fear of compassion”96 or “backdraft.”97 Backdraft occurs when a firefighter opens a door with a hot fire behind it. Oxygen rushes in, causing a burst of flame. Similarly, when the door of the heart is opened with compassion, intense pain could be released. Unconditional love reveals the conditions under which we were unloved in the past. Some individuals, especially those with a history of childhood abuse or neglect, are fearful of compassion because it activates grief associated with feelings of wanting, but not receiving, affection and care from significant others in childhood.
Clinicians should be aware that anxiety could arise and should help patients learn how to go slowly and stabilize themselves if overwhelming emotions occur as a part of self-compassion practice. Both CFT and MSC have processes to deal with fear of compassion in their protocols,98,99 with the focus on explaining to individuals that although such fears may occur, they are a normal and necessary part of the healing process. Individuals also are taught to focus on the breath, feeling the sensations in the soles of their feet, or other mindfulness practices to ground and stabilize attention when overwhelming feelings arise.
Clinical interventions
Self-compassion interventions that I (R.W.) find most helpful, in the order I administer them, are:
- exploring perceived advantages and disadvantages of self-criticism
- presenting self-compassion as a way to get the perceived advantages of self-criticism without the disadvantages
- discussing what it means to be compassionate for someone else who is suffering, and then asking what it would be like if they treated themselves with the same compassion
- exploring patients’ misconceptions and fears of self-compassion
- directing patients to the self-compassion Web site to get an understanding of what self-compassion is and how it differs from self-esteem
- taking an example of a recent situation in which the patient was self-critical and exploring how a self-compassionate response would differ.
Asking what they would say to a friend often is an effective way to get at this. In a later therapy session, self-compassionate imagery is a useful way to get the patient to experience self-compassion on an emotional level. See Neff100 and Gilbert98 for other techniques to enhance self-compassion.
1. Shahar B, Doron G, Ohad S. Childhood maltreatment, shame-proneness and self-criticism in social anxiety disorder: a sequential mediational model. Clin Psychol Psychother. 2015:22(6):570-579.
2. Kannan D, Levitt HM. A review of client self-criticism in psychotherapy. J Psychother Integr. 2013;23(2):166-178.
3. Barnard LK, Curry JF. Self-compassion: conceptualizations, correlates, and interventions. Rev Gen Psychol. 2011;15(4):289-303.
4. MacBeth A, Gumley A. Exploring compassion: a meta-analysis of the association between self-compassion and psychopathology. Clin Psychol Rev. 2012;32(6):545-552
5. Blatt SJ, Zuroff DC. Interpersonal relatedness and self-definition: two prototypes for depression. Clin Psychol Rev. 1992;12(5):527-562.
6. Neff KD. The development and validation of a scale to measure self-compassion. Self Identity. 2003;2(2):223-250.
7. Neff KD. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self Identity. 2003;(2)2:85-101.
8. Gilbert P, Procter S. Compassionate mind training for people with high shame and self-criticism: overview and pilot study of a group therapy approach. Clin Psychol Psychother. 2006;13(6):353-379.
9. Dunkley DM, Zuroff DC, Blankstein KR. Specific perfectionism components versus self-criticism in predicting maladjustment. Pers Individ Dif. 2006;40(4):665-676.
10. Murphy JM, Nierenberg AA, Monson RR, et al. Self-disparagement as feature and forerunner of depression: Mindfindings from the Stirling County Study. Compr Psychiatry. 2002;43(1):13-21.
11. Brewin CR, Firth-Cozens J. Dependency and self-criticism as predictors of depression in young doctors. J Occup Health Psychol. 1997;2(3):242-246.
12. Fazaa N, Page S. Dependency and self-criticism as predictors of suicidal behavior. Suicide Life Threat Behav. 2003;33(2):172-185.
13. Teasdale JD, Cox SG. Dysphoria: self-devaluative and affective components in recovered depressed patients and never depressed controls. Psychol Med. 2001;31(7):1311-1316.
14. Ehret AM, Joormann J, Berking M. Examining risk and resilience factors for depression: the role of self-criticism and self-compassion. Cogn Emot. 2015;29(8):1496-1504.
15. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
16. Rector NA, Bagby RM, Segal ZV, et al. Self-criticism and dependency in depressed patients treated with cognitive therapy or pharmacotherapy. Cognit Ther Res. 2000;24(5):571-584.
17. Marshall MB, Zuroff DC, McBride C, et al. Self-criticism predicts differential response to treatment for major depression. J Clin Psychol. 2008;64(3):231-244.
18. Zuroff DC, Blatt SJ, Sotsky SM, et al. Relation of therapeutic alliance and perfectionism to outcome in brief outpatient treatment of depression. J Consult Clin Psychol. 2000;68(1):114-124.
19. Whelton WJ, Greenberg LS. Emotion in self-criticism. Pers Individ Dif. 2005;38(7):1583-1595.
20. Cox BJ, Fleet C, Stein MB. Self-criticism and social phobia in the US national comorbidity survey. J Affect Disord. 2004;82(2):227-234.
21. Cox BJ, Walker JR, Enns MW, et al. Self-criticism in generalized social phobia and response to cognitive-behavioral treatment. Behav Ther. 2002;33(4):479-491.
22. McCranie EW, Hyer LA. Self-critical depressive experience in posttraumatic stress disorder. Psychol Rep. 1995;77(3 pt 1):880-882.
23. Southwick SM, Yehuda R, Giller EL Jr. Characterization of depression in war-related posttraumatic stress disorder. Am J Psychiatry. 1991;148(2):179-183.
24. Yehuda R, Kahana B, Southwick SM, et al. Depressive features in Holocaust survivors with post-traumatic stress disorder. J Traumatic Stress. 1994;7(4):699-704.
25. Sharhabani-Arzy R, Amir M, Swisa A. Self-criticism, dependency and posttraumatic stress disorder among a female group of help-seeking victims of domestic violence in Israel. Pers Individ Dif. 2005;38(5):1231-1240.
26. Beaumont E, Galpin A, Jenkins P. ‘Being kinder to myself’: a prospective comparative study, exploring post-trauma therapy outcome measures, for two groups of clients, receiving either cognitive behaviour therapy or cognitive behaviour therapy and compassionate mind training. Counselling Psychol Rev. 2012;27(1):31-43.
27. Dahm KA. Mindfulness and self-compassion as predictors of functional outcomes and psychopathology in OEF/OIF veterans exposed to trauma. https://repositories.lib.utexas.edu/handle/2152/21635. Published August 2013. Accessed November 8, 2016.
28. Hiraoka R, Meyer EC, Kimbrel NA, et al. Self-compassion as a prospective predictor of PTSD symptom severity among trauma-exposed US Iraq and Afghanistan war veterans. J Trauma Stress. 2015;28(2):127-133.
29. Bagby RM, Cox BJ, Schuller DR, et al. Diagnostic specificity of the dependent and self-critical personality dimensions in major depression. J Affect Disord. 1992;26(1):59-63.
30. Hedman E, Ström P, Stünkel A, et al. Shame and guilt in social anxiety disorder: effects of cognitive behavior therapy and association with social anxiety and depressive symptoms. PLoS One. 2013;8(4):e61713. doi: 10.1371/journal.pone.0061713.
31. Hoge EA, Hölzel BK, Marques L, et al. Mindfulness and self-compassion in generalized anxiety disorder: examining predictors of disability. Evid Based Complement Alternat Med. 2013;2013:576258. doi: 10.1155/2013/576258.
32. Wetterneck CT, Lee EB, Smith AH, et al. Courage, self-compassion, and values in obsessive-compulsive disorder. J Contextual Behav Sci. 2013;2(3-4):68-73.
33. Kelly AC, Carter JC. Why self-critical patients present with more severe eating disorder pathology: The mediating role of shame. Br J Clin Psychol. 2013;52(2):148-161.
34. Dunkley DM, Grilo CM. Self-criticism, low self-esteem, depressive symptoms, and over-evaluation of shape and weight in binge eating disorder patients. Behav Res Ther. 2007;45(1):139-149.
35. Dunkley DM, Masheb RM, Grilo CM. Childhood maltreatment, depressive symptoms, and body dissatisfaction in patients with binge eating disorder: the mediating role of self-criticism. Int J Eat Disord. 2010;43(3):274-281.
36. Wasylkiw L, MacKinnon AL, MacLellan AM. Exploring the link between self-compassion and body image in university women. Body Image. 2012;9(2):236-245.
37. Ferreira C, Pinto-Gouveia J, Duarte C. Self-compassion in the face of shame and body image dissatisfaction: implications for eating disorders. Eat Behavs. 2013;14(2):207-210.
38. Kelly AC, Carter JC, Zuroff DC, et al. Self-compassion and fear of self-compassion interact to predict response to eating disorders treatment: a preliminary investigation. Psychother Res. 2013;23(3):252-264.
39. Webb JB, Forman MJ. Evaluating the indirect effect of self-compassion on binge eating severity through cognitive-affective self-regulatory pathways. Eat Behavs. 2013;14(2):224-228.
40. Kelly AC, Carter JC, Borairi S. Are improvements in shame and self-compassion early in eating disorders treatment associated with better patient outcomes? Int J Eat Disord. 2014;47(1):54-64.
41. Wiseman H, Raz A, Sharabany R. Depressive personality styles and interpersonal problems in young adults with difficulties in establishing long-term romantic relationships. Isr J Psychiatry Rel Sci. 2007;44(4):280-291.
42. Besser A, Priel B. A multisource approach to self-critical vulnerability to depression: the moderating role of attachment. J Pers. 2003;71(4):515-555.
43. Zuroff DC, Koestner R, Powers TA. Self-criticism at age 12: a longitudinal-study of adjustment. Cognit Ther Res. 1994;18(4):367-385.
44. Fichman L, Koestner R, Zuroff DC. Depressive styles in adolescence: Assessment, relation to social functioning, and developmental trends. J Youth Adolesc. 1994;23(3):315-330.
45. Wiseman H. Interpersonal relatedness and self-definition in the experience of loneliness during the transition to university. Personal Relationships. 1997;4(3):285-299.
46. Mongrain M, Lubbers R, Struthers W. The power of love: mediation of rejection in roommate relationships of dependents and self-critics. Pers Soc Psychol Bull. 2004;30(1):94-105.
47. Whiffen VE, Aube JA. Personality, interpersonal context and depression in couples. J Soc Pers Relat. 1999;16(3):369-383.
48. Priel B, Besser A. Dependency and self-criticism among first-time mothers: the roles of global and specific support. J Soc Clin Psychol. 2000;19(4):437-450.
49. Neff KD, Beretvas SN. The role of self-compassion in romantic relationships. Self Identity. 2013;12(1):78-98.
50. Powers TA, Koestner R, Zuroff DC. Self-criticism, goal motivation, and goal progress. J Soc Clin Psychol. 2007;26(7):826-840.
51. Powers TA, Koestner R, Zuroff DC, et al. The effects of self-criticism and self-oriented perfectionism on goal pursuit. Pers Soc Psychol Bull. 2011;37(7):964-975.
52. Hope N, Koestner R, Milyavskaya M. The role of self-compassion in goal pursuit and well-being among university freshmen. Self Identity. 2014;13(5):579-593.
53. Williams JG, Stark SK, Foster EE. Start today or the very last day? The relationships among self-compassion, motivation, and procrastination. Am J Psychol Res. 2008;4(1):37-44.
54. Neff KD, Hseih Y, Dejittherat K. Self-compassion, achievement goals, and coping with academic failure. Self Identity. 2005;4(3):263-287.
55. Campos RC, Besser A, Blatt SJ. The mediating role of self-criticism and dependency in the association between perceptions of maternal caring and depressive symptoms. Depress Anxiety. 2010;27(12):1149-1157.
56. Sachs-Ericsson N, Verona E, Joiner T, et al. Parental verbal abuse and the mediating role of self-criticism in adult internalizing disorders. J Affect Disord. 2006;93(1-3):71-78.
57. Amitay OA, Mongrain M, Fazaa N. Love and control: self-criticism in parents and daughters and perceptions of relationship partners. Pers Individ Dif. 2008;44(1):75-85.
58. Zuroff DC, Fitzpatrick DK. Depressive personality styles: implications for adult attachment. Pers Individ Dif. 1995;18(2):253-265.
59. Kopala-Sibley DC, Zuroff DC. The developmental origins of personality factors from the self-definitional and relatedness domains: a review of theory and research. Rev Gen Psychol. 2014;18(3):137-155.
60. Kopala-Sibley DC, Zuroff DC, Leybman MJ, et al. Recalled peer relationship experiences and current levels of self-criticism and self-reassurance. Psychol Psychother. 2013;86(1):33-51.
61. Neff KD, McGehee P. Self-compassion and psychological resilience among adolescents and young adults. Self Identity. 2010;9(3):225-240.
62. Pepping CA, Davis PJ, O’Donovan A, et al. Individual differences in self-compassion: the role of attachment and experiences of parenting in childhood. Self Identity. 2015;14(1):104-117.
63. Neff KD, Rude SS, Kirkpatrick KL. An examination of self-compassion in relation to positive psychological functioning and personality traits. J Res Pers. 2007;41(4):908-916.
64. Van Dam NT, Sheppard SC, Forsyth JP, et al. Self-compassion is a better predictor than mindfulness of symptom severity and quality of life in mixed anxiety and depression. J Anxiety Disord. 2011;25(1):123-130.
65. Heffernan M, Quinn MT, McNulty SR, et al. Self-compassion and emotional intelligence in nurses. Int J Nursing Practice. 2010;16(4):366-373.
66. Neff KD, Kirkpatrick KL, Rude SS. Self-compassion and adaptive psychological functioning. J Res Pers. 2007;41(1):139-154.
67. Krieger T, Altenstein D, Baettig I, et al. Self-compassion in depression: associations with depressive symptoms, rumination, and avoidance in depressed outpatients. Behav Ther. 2013;44(3):501-513.
68. Breen WE, Kashdan TB, Lenser ML, et al. Gratitude and forgiveness: convergence and divergence on self-report and informant ratings. Pers Individ Dif. 2010;49(8):932-937.
69. Hollis-Walker L, Colosimo K. Mindfulness, self-compassion, and happiness in non-meditators: A theoretical and empirical examination. Pers Individ Dif. 2011;50(2):222-227.
70. Gilbert P, McEwan K, Matos M, et al. Fears of compassion: development of three self-report measures. Psychol Psychother. 2011;84(3):239-255.
71. Neely ME, Schallert DL, Mohammed SS, et al. Self-kindness when facing stress: the role of self-compassion, goal regulation, and support in college students’ well-being. Motiv Emot. 2009;33(1):88-97.
72. Breines JG, Chen S. Self-compassion increases self-improvement motivation. Pers Soc Psychol Bull. 2012;38(9):1133-1143.
73. Allen AB, Leary MR. Self-compassion, stress, and coping. Soc Pers Psychol Compass. 2010;4(2):107-118.
74. Terry ML, Leary MR. Self-compassion, self-regulation, and health. Self Identity. 2011;10(3):352-362.
75. Magnus CMR, Kowalski KC, McHugh TF. The role of self-compassion in women’s self-determined motives to exercise and exercise-related outcomes. Self Identity. 2010;9(4):363-382.
76. Brooks M, Kay-Lambkin F, Bowman J, et al. Self-compassion amongst clients with problematic alcohol use. Mindfulness. 2012;3(4):308-317.
77. Adams CE, Leary MR. Promoting self-compassionate attitudes toward eating among restrictive and guilty eaters. J Soc Clin Psychol. 2007;26(10):1120-1144.
78. Kelly AC, Zuroff DC, Foa CL, et al. Who benefits from training in self-compassionate self-regulation? A study of smoking reduction. J Soc Clin Psychol. 2010;29(7):727-755.
79. Shapira LB, Mongrain M. The benefits of self-compassion and optimism exercises for individuals vulnerable to depression. J Posit Psychol. 2010;5(5):377-389.
80. Albertson ER, Neff KD, Dill-Shackleford KE. Self-compassion and body dissatisfaction in women: a randomized controlled trial of a brief meditation intervention. Mindfulness. 2015;6(3):444-454.
81. Smeets E, Neff K, Alberts H, et al. Meeting suffering with kindness: effects of a brief self-compassion intervention for female college students. J Clinical Psychol. 2014;70(9):794-807.
82. Blatt SJ, D’Afflitti JP, Quinlan DM. Depressive experiences questionnaire. New Haven, CT: Yale University Press; 1976.
83. Weissman AN, Beck AT. Development and validation of the dysfunctional attitude scale: a preliminary investigation. Paper presented at: 62nd Annual Meeting of the Association for Advanced Behavior Therapy; March 27-31, 1978; Toronto, Ontario, Canada.
84. Gilbert P, Clarke M, Hempel S, et al. Criticizing and reassuring oneself: an exploration of forms, styles and reasons in female students. Br J Clin Psychol. 2004;43(pt 1):31-50.
85. Baião R, Gilbert P, McEwan K, et al. Forms of self-criticising/attacking & self-reassuring scale: psychometric properties and normative study. Psychol Psychother. 2015;88(4):438-452.
86. Neff KD. The self-compassion scale is a valid and theoretically coherent measure of self-compassion. Mindfulness. 2016;7(1):264-274.
87. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clinical Psychol. 2013;69(1):28-44.
88. Gilbert P. Introducing compassion-focused therapy. Adv Psychiatr Treat. 2009;15(3):199-208.
89. Kelly AC, Zuroff DC, Shapira LB. Soothing oneself and resisting self-attacks: the treatment of two intrapersonal deficits in depression vulnerability. Cognit Ther Res. 2009;33(3):301-313.
90. Boersma K, Hakanson A, Salomonsson E, et al. Compassion focused therapy to counteract shame, self-criticism and isolation. A replicated single case experimental study of individuals with social anxiety. J Contemp Psychother. 2015;45(2):89-98.
91. Gale C, Gilbert P, Read N, et al. An evaluation of the impact of introducing compassion focused therapy to a standard treatment programme for people with eating disorders. Clin Psychol Psychother. 2014;21(1):1-12.
92. Braehler C, Gumley A, Harper J, et al. Exploring change processes in compassion focused therapy in psychosis: results of a feasibility randomized controlled trial. Br J Clin Psychol. 2013;52(2):199-214.
93. Ashworth F, Clarke A, Jones L, et al. An exploration of compassion focused therapy following acquired brain injury. Psychol Psychother. 2014;88(2):143-162.
94. Judge L, Cleghorn A, McEwan K, et al. An exploration of group-based compassion focused therapy for a heterogeneous range of clients presenting to a community mental health team. Int J Cogn Ther. 2012;5(4):420-429.
95. Leaviss J, Utley L. Psychotherapeutic benefits of compassion-focused therapy: an early systematic review. Psychol Med. 2015;45(5):927-945.
96. Gilbert P, McEwan K, Gibbons L, et al. Fears of compassion and happiness in relation to alexithymia, mindfulness, and self‐criticism. Psychol Psychother. 2012;85(4):374-390.
97. Germer CK, Neff KD. Cultivating self-compassion in trauma survivors. In: Follette VM, Briere J, Rozelle D, et al, eds. Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York, NY: Guilford Press; 2015:43-58.
98. Gilbert P. Compassion focused therapy: the CBT distinctive features series. London, United Kingdom: Routledge; 2010.
99. Germer C, Neff K. The mindful self-compassion training program. In: Singer T, Bolz M, eds. Compassion: bridging theory and practice: a multimedia book. Leipzig, Germany: Max-Planck Institute; 2013:365-396.
100. Neff K. Self-compassion: the proven power of being kind to yourself. New York, NY: HarperCollins; 2015.
Once thought to only be associated with depression, self-criticism is a transdiagnostic risk factor for diverse forms of psychopathology.1,2 However, research has shown that self-compassion is a robust resilience factor when faced with feelings of personal inadequacy.3,4
Self-critical individuals experience feelings of unworthiness, inferiority, failure, and guilt. They engage in constant and harsh self-scrutiny and evaluation, and fear being disapproved and criticized and losing the approval and acceptance of others.5 Self-compassion involves treating oneself with care and concern when confronted with personal inadequacies, mistakes, failures, and painful life situations.6,7
Although self-criticism is destructive across clinical disorders and interpersonal relationships, self-compassion is associated with healthy relationships, emotional well-being, and better treatment outcomes.
Recent research shows how clinicians can teach their patients how to be less self-critical and more self-compassionate. Neff6,7 proposes that self-compassion involves treating yourself with care and concern when being confronted with personal inadequacies, mistakes, failures, and painful life situations. It consists of 3 interacting components, each of which has a positive and negative pole:
- self-kindness vs self-judgment
- a sense of common humanity vs isolation
- mindfulness vs over-identification.
Self-kindness refers to being caring and understanding with oneself rather than harshly judgmental. Instead of attacking and berating oneself for personal shortcomings, the self is offered warmth and unconditional acceptance.
Humanity involves recognizing that humans are imperfect, that all people fail, make mistakes, and have serious life challenges. By remembering that imperfection is part of life, we feel less isolated when we are in pain.
Mindfulness in the context of self-compassion involves being aware of one’s painful experiences in a balanced way that neither ignores and avoids nor exaggerates painful thoughts and emotions.
Self-compassion is more than the absence of self-judgment, although a defining feature of self-compassion is the lack of self-judgment, and self-judgment overlaps with self-criticism. Rather, self-compassion provides several access points for reducing self-criticism. For example, being kind and understanding when confronting personal inadequacies (eg, “it’s okay not to be perfect”) can counter harsh self-talk (eg, “I’m not defective”). Mindfulness of emotional pain (eg, “this is hard”) can facilitate a kind and warm response (eg, “what can I do to take care of myself right now?”) and therefore lessen self-blame (eg, “blaming myself is just causing me more suffering”). Similarly, remembering that failure is part of the human experience (eg, “it’s normal to mess up sometimes”) can lessen egocentric feelings of isolation (eg, “it’s not just me”) and over-identification (eg, “it’s not the end of the world”), resulting in lessened self-criticism (eg, “maybe it’s not just because I’m a bad person”).
Depression
Several studies have found that self-criticism predicts depression. In 3 epidemiological studies, “feeling worthless” was among the top 2 symptoms predicting a depression diagnosis and later depressive episodes.10 Self-criticism in fourth-year medical students predicted depression 2 years later, and—in males—10 years later in their medical careers better than a history of depression.11 Self-critical perfectionism also is associated with suicidal ideation and lethality of suicide attempts.12
Self-criticism has been shown to predict depressive relapse and residual self-devaluative symptoms in recovered depressed patients.13 In one study, currently depressed and remitted depressed patients had higher self-criticism and lower self-compassion compared with healthy controls. Both factors were more strongly associated with depression status than higher perfectionistic beliefs and cognitions, rumination, and maladaptive emotional regulation.14
Self-criticism and response to treatment. In the National Institute of Mental Health Treatment of Depression Collaborative Research Program,15 self-critical perfectionism predicted a poorer outcome across all 4 treatments (cognitive-behavioral therapy [CBT], interpersonal psychotherapy [IPT], pharmacotherapy plus clinical management, and placebo plus clinical management). Subsequent studies found that self-criticism predicted poorer response to CBT16 and IPT.17 The authors suggest that self-criticism could interfere with treatment because self-critical patients might have difficulty developing a strong therapeutic alliance.18,19
Anxiety disorders
Self-criticism is common across psychiatric disorders. In a study of 5,877 respondents in the National Comorbidity Survey (NCS), self-criticism was associated with social phobia, findings that were significant after controlling for current emotional distress, neuroticism, and lifetime history of mood, anxiety, and substance use disorders.20 Further, in a CBT treatment study, baseline self-criticism was associated with severity of social phobia and changes in self-criticism predicted treatment outcome.21 Self-criticism might be an important core psychological process in the development, maintenance, and course of social phobia. Patients with social anxiety disorder have less self-compassion than healthy controls and greater fear of negative evaluation.
In the NCS, self-criticism was associated with posttraumatic stress disorder (PTSD) even after controlling for lifetime history of affective and anxiety disorders.20 Self-criticism predicted greater severity of combat-related PTSD in hospitalized male veterans,22 and those with PTSD had higher scores on self-criticism scales than those with major depressive disorder.23 In a study of Holocaust survivors, those with PTSD scored higher on self-criticism than survivors without PTSD.24 Self-criticism also distinguished between female victims of domestic violence with and without PTSD.25
Self-compassion could be a protective factor for posttraumatic stress.26 Combat veterans with higher levels of self-compassion showed lower levels of psychopathology, better functioning in daily life, and fewer symptoms of posttraumatic stress.27 In fact, self-compassion has been found to be a stronger predictor of PTSD than level of combat exposure.28
In an early study, self-criticism scores were higher in patients with panic disorder than in healthy controls, but lower than in patients with depression.29 In a study of a mixed sample of anxiety disorder patients, symptoms of generalized anxiety disorder were associated with shame proneness.30 Consistent with these results, Hoge et al31 found that self-compassion was lower in generalized anxiety disorder patients compared with healthy controls with elevated stress. Low self-compassion has been associated with severity of obsessive-compulsive disorder.32
Eating disorders
Self-criticism is correlated with eating disorder severity.33 In a study of patients with binge eating disorder, Dunkley and Grilo34 found that self-criticism was associated with the over-evaluation of shape and weight independently of self-esteem and depression. Self-criticism also is associated with body dissatisfaction, independent of self-esteem and depression. Dunkley et al35 found that self-criticism, but not global self-esteem, in patients with binge eating disorder mediated the relationship between childhood abuse and body dissatisfaction and depression. Numerous studies have shown that shame is associated with more severe eating disorder pathology.33
Self-compassion seems to buffer against body image concerns. It is associated with less body dissatisfaction, body preoccupation, and weight worries,36 greater body appreciation37 and less disordered eating.37-39 Early decreases in shame during eating disorder treatment was associated with more rapid reduction in eating disorder symptoms.40
Interpersonal relationships
Several studies have shown that self-criticism has negative effects on interpersonal relationships throughout life.5,41,42
- Self-criticism at age 12 predicted less involvement in high school activities and, at age 31, personal and social maladjustment.43
- High school students with high self-criticism reported more interpersonal problems.44
- Self-criticism was associated with loneliness, depression, and lack of intimacy with opposite sex friends or partners during the transition to college.45
- In a study of college roommates,46 self-criticism was associated with increased likelihood of rejection.
- Whiffen and Aube47 found that self-criticism was associated with marital dissatisfaction and depression.
- Self-critical mothers with postpartum depression were less satisfied with social support and were more vulnerable to depression.48
Self-compassion appears to enhance interpersonal relationships. In a study of heterosexual couples,49 self-compassionate individuals were described by their partners as being more emotionally connected, as well as accepting and supporting autonomy, while being less detached, controlling, and verbally or physically aggressive than those lacking self-compassion. Because self-compassionate people give themselves care and support, they seem to have more emotional resources available to give to others.
See the Box examining the evidence on the role of self-compassion in borderline personality disorder and non-suicidal self-injury.

Achieving goals
Powers et al50 suggest that self-critics approach goals based on motivation to avoid failure and disapproval, rather than on intrinsic interest and personal meaning. In studies of college students pursuing academic, social, or weight loss goals, self-criticism was associated with less progress to that goal. Self-criticism was associated with rumination and procrastination, which the authors suggest might have focused the self-critic on potential failure, negative evaluation from others, and loss of self-esteem. Additional studies showed the deleterious effects of self-criticism on college students’ progress on obtaining academic or music performance goals and on community residents’ weight loss goals.51
Not surprisingly, self-compassion is associated with successful goal pursuit and resilience when goals are not met52 and less procrastination and academic worry.53 Self-compassion also is associated with intrinsic motivation, goals based on mastery rather than performance, and less fear of academic failure.54
How self-criticism and self-compassion develop
Studies have explored the impact of early relationships with parents and development of self-criticism. Parental overcontrol and restrictiveness and lack of warmth consistently have been identified as parenting styles associated with development of self-criticism in children.55 One study found that self-criticism fully mediated the relationship between childhood verbal abuse from parents and depression and anxiety in adulthood.56 Reports from parents on their current parenting styles are consistent with these studies.57 Amitay et al57 states that “[s]elf-critics’ negative childhood experiences thus seem to contribute to a pattern of entering, creating, or manipulating subsequent interpersonal environments in ways that perpetuate their negative self-image and increase vulnerability to depression.” Not surprisingly, self-criticism is associated with a fearful avoidant attachment style.58 Review of the developmental origins of self-criticism confirms these factors and presents findings that peer relationships also are important factors in the development of self-criticism.59,60
Early positive relationships with caregivers are associated with self-compassion. Recollections of maternal support are correlated with self-compassion and secure attachment styles in adolescents and adults.61 Pepping et al62 found that retrospective reports of parental rejection, overprotection, and low parental warmth was associated with low self-compassion.
Benefits of self-compassion
A growing body of research suggests that self-compassion is strongly linked to mental health. Greater self-compassion consistently has been associated with lower levels of depression and anxiety,3 with a large effect size.4 Of course, central to self-compassion is the lack of self-criticism, but self-compassion still protects against anxiety and depression when controlling for self-criticism and negative affect.6,63 Self-compassion is a strong predictor of symptom severity and quality of life among individuals with anxious distress.64
The benefits of self-compassion stem partly from a greater ability to cope with negative emotions.6,63,65 Self-compassionate people are less likely to ruminate on their negative thoughts and emotions or suppress them,6,66 which helps to explain why self-compassion is a negative predictor of depression.67
Self-compassion also enhances positive mind states. A number of studies have found links between self-compassion and positive psychological qualities, such as happiness, optimism, wisdom, curiosity, and exploration, and personal initiative.63,65,68,69 By embracing one’s suffering with compassion, negative states are ameliorated when positive emotions of kindness, connectedness, and mindful presence are generated.
Misconceptions about self-compassion
A common misconception is that abandoning self-criticism in favor of self-compassion will undermine motivation70; however, research indicates the opposite. Although self-compassion is negatively associated with maladaptive perfectionism, it is not correlated with self-adopted performance standards.6 Self-compassionate people have less fear of failure54 and, when they do fail, they are more likely to try again.71 Breines and Chen72 found in a series of experimental studies that engendering feelings of self-compassion for personal weaknesses, failures, and past transgressions resulted in more motivation to change, to try harder to learn, and to avoid repeating past mistakes.
Another common misunderstanding is that self-compassion is a weakness. In fact, research suggests that self-compassion is a powerful way to cope with life challenges.73
Although some fear that self-compassion leads to self-indulgence, there is evidence that self-compassion promotes health-related behaviors. Self-compassionate individuals are more likely to seek medical treatment when needed,74 exercise for intrinsic reasons,75 and drink less alcohol.76 Inducing self-compassion has been found to help people stick to their diets77 and quit smoking.78
Self-compassion interventions
Individuals can develop self-compassion. Shapira and Mongrain79 found that adults who wrote a compassionate letter to themselves once a day for a week about the distressing events they were experiencing showed significant reductions in depression up to 3 months and significant increases in happiness up to 6 months compared with a control group who wrote about early memories. Albertson et al80 found that, compared with a wait-list control group, 3 weeks of self-compassion meditation training improved body dissatisfaction, body shame, and body appreciation among women with body image concerns. Similarly, Smeets et al81 found that 3 weeks of self-compassion training for female college students led to significantly greater increases in mindfulness, optimism, and self-efficacy, as well as greater decreases in rumination compared with a time management control group.
The Box6,70,82-86 describes rating scales that can measure self-compassion and self-criticism.

Mindful self-compassion (MSC), developed by Neff and Germer,87 is an 8-week group intervention designed to teach people how to be more self-compassionate through meditation and informal practices in daily life. Results of a randomized controlled trial found that, compared with a wait-list control group, participants using MSC reported significantly greater increases in self-compassion, compassion for others, mindfulness, and life satisfaction, and greater decreases in depression, anxiety, stress, and emotional avoidance, with large effect sizes indicated. These results were maintained up to 1 year.
Compassion-focused therapy (CFT) is designed to enhance self-compassion in clinical populations.88 The approach uses a number of imagery and experiential exercises to enhance patients’ abilities to extend feelings of reassurance, safeness, and understanding toward themselves. CFT has shown promise in treating a diverse group of clinical disorders such as depression and shame,8,89 social anxiety and shame,90 eating disorders,91 psychosis,92 and patients with acquired brain injury.93 A group-based CFT intervention with a heterogeneous group of community mental health patients led to significant reductions in depression, anxiety, stress, and self-criticism.94 See Leaviss and Utley95 for a review of the benefits of CFT.
Fears of developing self-compassion
It is important to note that some people can access self-compassion more easily than others. Highly self-critical patients could feel anxious when learning to be compassionate to themselves, a phenomenon known as “fear of compassion”96 or “backdraft.”97 Backdraft occurs when a firefighter opens a door with a hot fire behind it. Oxygen rushes in, causing a burst of flame. Similarly, when the door of the heart is opened with compassion, intense pain could be released. Unconditional love reveals the conditions under which we were unloved in the past. Some individuals, especially those with a history of childhood abuse or neglect, are fearful of compassion because it activates grief associated with feelings of wanting, but not receiving, affection and care from significant others in childhood.
Clinicians should be aware that anxiety could arise and should help patients learn how to go slowly and stabilize themselves if overwhelming emotions occur as a part of self-compassion practice. Both CFT and MSC have processes to deal with fear of compassion in their protocols,98,99 with the focus on explaining to individuals that although such fears may occur, they are a normal and necessary part of the healing process. Individuals also are taught to focus on the breath, feeling the sensations in the soles of their feet, or other mindfulness practices to ground and stabilize attention when overwhelming feelings arise.
Clinical interventions
Self-compassion interventions that I (R.W.) find most helpful, in the order I administer them, are:
- exploring perceived advantages and disadvantages of self-criticism
- presenting self-compassion as a way to get the perceived advantages of self-criticism without the disadvantages
- discussing what it means to be compassionate for someone else who is suffering, and then asking what it would be like if they treated themselves with the same compassion
- exploring patients’ misconceptions and fears of self-compassion
- directing patients to the self-compassion Web site to get an understanding of what self-compassion is and how it differs from self-esteem
- taking an example of a recent situation in which the patient was self-critical and exploring how a self-compassionate response would differ.
Asking what they would say to a friend often is an effective way to get at this. In a later therapy session, self-compassionate imagery is a useful way to get the patient to experience self-compassion on an emotional level. See Neff100 and Gilbert98 for other techniques to enhance self-compassion.
Once thought to only be associated with depression, self-criticism is a transdiagnostic risk factor for diverse forms of psychopathology.1,2 However, research has shown that self-compassion is a robust resilience factor when faced with feelings of personal inadequacy.3,4
Self-critical individuals experience feelings of unworthiness, inferiority, failure, and guilt. They engage in constant and harsh self-scrutiny and evaluation, and fear being disapproved and criticized and losing the approval and acceptance of others.5 Self-compassion involves treating oneself with care and concern when confronted with personal inadequacies, mistakes, failures, and painful life situations.6,7
Although self-criticism is destructive across clinical disorders and interpersonal relationships, self-compassion is associated with healthy relationships, emotional well-being, and better treatment outcomes.
Recent research shows how clinicians can teach their patients how to be less self-critical and more self-compassionate. Neff6,7 proposes that self-compassion involves treating yourself with care and concern when being confronted with personal inadequacies, mistakes, failures, and painful life situations. It consists of 3 interacting components, each of which has a positive and negative pole:
- self-kindness vs self-judgment
- a sense of common humanity vs isolation
- mindfulness vs over-identification.
Self-kindness refers to being caring and understanding with oneself rather than harshly judgmental. Instead of attacking and berating oneself for personal shortcomings, the self is offered warmth and unconditional acceptance.
Humanity involves recognizing that humans are imperfect, that all people fail, make mistakes, and have serious life challenges. By remembering that imperfection is part of life, we feel less isolated when we are in pain.
Mindfulness in the context of self-compassion involves being aware of one’s painful experiences in a balanced way that neither ignores and avoids nor exaggerates painful thoughts and emotions.
Self-compassion is more than the absence of self-judgment, although a defining feature of self-compassion is the lack of self-judgment, and self-judgment overlaps with self-criticism. Rather, self-compassion provides several access points for reducing self-criticism. For example, being kind and understanding when confronting personal inadequacies (eg, “it’s okay not to be perfect”) can counter harsh self-talk (eg, “I’m not defective”). Mindfulness of emotional pain (eg, “this is hard”) can facilitate a kind and warm response (eg, “what can I do to take care of myself right now?”) and therefore lessen self-blame (eg, “blaming myself is just causing me more suffering”). Similarly, remembering that failure is part of the human experience (eg, “it’s normal to mess up sometimes”) can lessen egocentric feelings of isolation (eg, “it’s not just me”) and over-identification (eg, “it’s not the end of the world”), resulting in lessened self-criticism (eg, “maybe it’s not just because I’m a bad person”).
Depression
Several studies have found that self-criticism predicts depression. In 3 epidemiological studies, “feeling worthless” was among the top 2 symptoms predicting a depression diagnosis and later depressive episodes.10 Self-criticism in fourth-year medical students predicted depression 2 years later, and—in males—10 years later in their medical careers better than a history of depression.11 Self-critical perfectionism also is associated with suicidal ideation and lethality of suicide attempts.12
Self-criticism has been shown to predict depressive relapse and residual self-devaluative symptoms in recovered depressed patients.13 In one study, currently depressed and remitted depressed patients had higher self-criticism and lower self-compassion compared with healthy controls. Both factors were more strongly associated with depression status than higher perfectionistic beliefs and cognitions, rumination, and maladaptive emotional regulation.14
Self-criticism and response to treatment. In the National Institute of Mental Health Treatment of Depression Collaborative Research Program,15 self-critical perfectionism predicted a poorer outcome across all 4 treatments (cognitive-behavioral therapy [CBT], interpersonal psychotherapy [IPT], pharmacotherapy plus clinical management, and placebo plus clinical management). Subsequent studies found that self-criticism predicted poorer response to CBT16 and IPT.17 The authors suggest that self-criticism could interfere with treatment because self-critical patients might have difficulty developing a strong therapeutic alliance.18,19
Anxiety disorders
Self-criticism is common across psychiatric disorders. In a study of 5,877 respondents in the National Comorbidity Survey (NCS), self-criticism was associated with social phobia, findings that were significant after controlling for current emotional distress, neuroticism, and lifetime history of mood, anxiety, and substance use disorders.20 Further, in a CBT treatment study, baseline self-criticism was associated with severity of social phobia and changes in self-criticism predicted treatment outcome.21 Self-criticism might be an important core psychological process in the development, maintenance, and course of social phobia. Patients with social anxiety disorder have less self-compassion than healthy controls and greater fear of negative evaluation.
In the NCS, self-criticism was associated with posttraumatic stress disorder (PTSD) even after controlling for lifetime history of affective and anxiety disorders.20 Self-criticism predicted greater severity of combat-related PTSD in hospitalized male veterans,22 and those with PTSD had higher scores on self-criticism scales than those with major depressive disorder.23 In a study of Holocaust survivors, those with PTSD scored higher on self-criticism than survivors without PTSD.24 Self-criticism also distinguished between female victims of domestic violence with and without PTSD.25
Self-compassion could be a protective factor for posttraumatic stress.26 Combat veterans with higher levels of self-compassion showed lower levels of psychopathology, better functioning in daily life, and fewer symptoms of posttraumatic stress.27 In fact, self-compassion has been found to be a stronger predictor of PTSD than level of combat exposure.28
In an early study, self-criticism scores were higher in patients with panic disorder than in healthy controls, but lower than in patients with depression.29 In a study of a mixed sample of anxiety disorder patients, symptoms of generalized anxiety disorder were associated with shame proneness.30 Consistent with these results, Hoge et al31 found that self-compassion was lower in generalized anxiety disorder patients compared with healthy controls with elevated stress. Low self-compassion has been associated with severity of obsessive-compulsive disorder.32
Eating disorders
Self-criticism is correlated with eating disorder severity.33 In a study of patients with binge eating disorder, Dunkley and Grilo34 found that self-criticism was associated with the over-evaluation of shape and weight independently of self-esteem and depression. Self-criticism also is associated with body dissatisfaction, independent of self-esteem and depression. Dunkley et al35 found that self-criticism, but not global self-esteem, in patients with binge eating disorder mediated the relationship between childhood abuse and body dissatisfaction and depression. Numerous studies have shown that shame is associated with more severe eating disorder pathology.33
Self-compassion seems to buffer against body image concerns. It is associated with less body dissatisfaction, body preoccupation, and weight worries,36 greater body appreciation37 and less disordered eating.37-39 Early decreases in shame during eating disorder treatment was associated with more rapid reduction in eating disorder symptoms.40
Interpersonal relationships
Several studies have shown that self-criticism has negative effects on interpersonal relationships throughout life.5,41,42
- Self-criticism at age 12 predicted less involvement in high school activities and, at age 31, personal and social maladjustment.43
- High school students with high self-criticism reported more interpersonal problems.44
- Self-criticism was associated with loneliness, depression, and lack of intimacy with opposite sex friends or partners during the transition to college.45
- In a study of college roommates,46 self-criticism was associated with increased likelihood of rejection.
- Whiffen and Aube47 found that self-criticism was associated with marital dissatisfaction and depression.
- Self-critical mothers with postpartum depression were less satisfied with social support and were more vulnerable to depression.48
Self-compassion appears to enhance interpersonal relationships. In a study of heterosexual couples,49 self-compassionate individuals were described by their partners as being more emotionally connected, as well as accepting and supporting autonomy, while being less detached, controlling, and verbally or physically aggressive than those lacking self-compassion. Because self-compassionate people give themselves care and support, they seem to have more emotional resources available to give to others.
See the Box examining the evidence on the role of self-compassion in borderline personality disorder and non-suicidal self-injury.

Achieving goals
Powers et al50 suggest that self-critics approach goals based on motivation to avoid failure and disapproval, rather than on intrinsic interest and personal meaning. In studies of college students pursuing academic, social, or weight loss goals, self-criticism was associated with less progress to that goal. Self-criticism was associated with rumination and procrastination, which the authors suggest might have focused the self-critic on potential failure, negative evaluation from others, and loss of self-esteem. Additional studies showed the deleterious effects of self-criticism on college students’ progress on obtaining academic or music performance goals and on community residents’ weight loss goals.51
Not surprisingly, self-compassion is associated with successful goal pursuit and resilience when goals are not met52 and less procrastination and academic worry.53 Self-compassion also is associated with intrinsic motivation, goals based on mastery rather than performance, and less fear of academic failure.54
How self-criticism and self-compassion develop
Studies have explored the impact of early relationships with parents and development of self-criticism. Parental overcontrol and restrictiveness and lack of warmth consistently have been identified as parenting styles associated with development of self-criticism in children.55 One study found that self-criticism fully mediated the relationship between childhood verbal abuse from parents and depression and anxiety in adulthood.56 Reports from parents on their current parenting styles are consistent with these studies.57 Amitay et al57 states that “[s]elf-critics’ negative childhood experiences thus seem to contribute to a pattern of entering, creating, or manipulating subsequent interpersonal environments in ways that perpetuate their negative self-image and increase vulnerability to depression.” Not surprisingly, self-criticism is associated with a fearful avoidant attachment style.58 Review of the developmental origins of self-criticism confirms these factors and presents findings that peer relationships also are important factors in the development of self-criticism.59,60
Early positive relationships with caregivers are associated with self-compassion. Recollections of maternal support are correlated with self-compassion and secure attachment styles in adolescents and adults.61 Pepping et al62 found that retrospective reports of parental rejection, overprotection, and low parental warmth was associated with low self-compassion.
Benefits of self-compassion
A growing body of research suggests that self-compassion is strongly linked to mental health. Greater self-compassion consistently has been associated with lower levels of depression and anxiety,3 with a large effect size.4 Of course, central to self-compassion is the lack of self-criticism, but self-compassion still protects against anxiety and depression when controlling for self-criticism and negative affect.6,63 Self-compassion is a strong predictor of symptom severity and quality of life among individuals with anxious distress.64
The benefits of self-compassion stem partly from a greater ability to cope with negative emotions.6,63,65 Self-compassionate people are less likely to ruminate on their negative thoughts and emotions or suppress them,6,66 which helps to explain why self-compassion is a negative predictor of depression.67
Self-compassion also enhances positive mind states. A number of studies have found links between self-compassion and positive psychological qualities, such as happiness, optimism, wisdom, curiosity, and exploration, and personal initiative.63,65,68,69 By embracing one’s suffering with compassion, negative states are ameliorated when positive emotions of kindness, connectedness, and mindful presence are generated.
Misconceptions about self-compassion
A common misconception is that abandoning self-criticism in favor of self-compassion will undermine motivation70; however, research indicates the opposite. Although self-compassion is negatively associated with maladaptive perfectionism, it is not correlated with self-adopted performance standards.6 Self-compassionate people have less fear of failure54 and, when they do fail, they are more likely to try again.71 Breines and Chen72 found in a series of experimental studies that engendering feelings of self-compassion for personal weaknesses, failures, and past transgressions resulted in more motivation to change, to try harder to learn, and to avoid repeating past mistakes.
Another common misunderstanding is that self-compassion is a weakness. In fact, research suggests that self-compassion is a powerful way to cope with life challenges.73
Although some fear that self-compassion leads to self-indulgence, there is evidence that self-compassion promotes health-related behaviors. Self-compassionate individuals are more likely to seek medical treatment when needed,74 exercise for intrinsic reasons,75 and drink less alcohol.76 Inducing self-compassion has been found to help people stick to their diets77 and quit smoking.78
Self-compassion interventions
Individuals can develop self-compassion. Shapira and Mongrain79 found that adults who wrote a compassionate letter to themselves once a day for a week about the distressing events they were experiencing showed significant reductions in depression up to 3 months and significant increases in happiness up to 6 months compared with a control group who wrote about early memories. Albertson et al80 found that, compared with a wait-list control group, 3 weeks of self-compassion meditation training improved body dissatisfaction, body shame, and body appreciation among women with body image concerns. Similarly, Smeets et al81 found that 3 weeks of self-compassion training for female college students led to significantly greater increases in mindfulness, optimism, and self-efficacy, as well as greater decreases in rumination compared with a time management control group.
The Box6,70,82-86 describes rating scales that can measure self-compassion and self-criticism.

Mindful self-compassion (MSC), developed by Neff and Germer,87 is an 8-week group intervention designed to teach people how to be more self-compassionate through meditation and informal practices in daily life. Results of a randomized controlled trial found that, compared with a wait-list control group, participants using MSC reported significantly greater increases in self-compassion, compassion for others, mindfulness, and life satisfaction, and greater decreases in depression, anxiety, stress, and emotional avoidance, with large effect sizes indicated. These results were maintained up to 1 year.
Compassion-focused therapy (CFT) is designed to enhance self-compassion in clinical populations.88 The approach uses a number of imagery and experiential exercises to enhance patients’ abilities to extend feelings of reassurance, safeness, and understanding toward themselves. CFT has shown promise in treating a diverse group of clinical disorders such as depression and shame,8,89 social anxiety and shame,90 eating disorders,91 psychosis,92 and patients with acquired brain injury.93 A group-based CFT intervention with a heterogeneous group of community mental health patients led to significant reductions in depression, anxiety, stress, and self-criticism.94 See Leaviss and Utley95 for a review of the benefits of CFT.
Fears of developing self-compassion
It is important to note that some people can access self-compassion more easily than others. Highly self-critical patients could feel anxious when learning to be compassionate to themselves, a phenomenon known as “fear of compassion”96 or “backdraft.”97 Backdraft occurs when a firefighter opens a door with a hot fire behind it. Oxygen rushes in, causing a burst of flame. Similarly, when the door of the heart is opened with compassion, intense pain could be released. Unconditional love reveals the conditions under which we were unloved in the past. Some individuals, especially those with a history of childhood abuse or neglect, are fearful of compassion because it activates grief associated with feelings of wanting, but not receiving, affection and care from significant others in childhood.
Clinicians should be aware that anxiety could arise and should help patients learn how to go slowly and stabilize themselves if overwhelming emotions occur as a part of self-compassion practice. Both CFT and MSC have processes to deal with fear of compassion in their protocols,98,99 with the focus on explaining to individuals that although such fears may occur, they are a normal and necessary part of the healing process. Individuals also are taught to focus on the breath, feeling the sensations in the soles of their feet, or other mindfulness practices to ground and stabilize attention when overwhelming feelings arise.
Clinical interventions
Self-compassion interventions that I (R.W.) find most helpful, in the order I administer them, are:
- exploring perceived advantages and disadvantages of self-criticism
- presenting self-compassion as a way to get the perceived advantages of self-criticism without the disadvantages
- discussing what it means to be compassionate for someone else who is suffering, and then asking what it would be like if they treated themselves with the same compassion
- exploring patients’ misconceptions and fears of self-compassion
- directing patients to the self-compassion Web site to get an understanding of what self-compassion is and how it differs from self-esteem
- taking an example of a recent situation in which the patient was self-critical and exploring how a self-compassionate response would differ.
Asking what they would say to a friend often is an effective way to get at this. In a later therapy session, self-compassionate imagery is a useful way to get the patient to experience self-compassion on an emotional level. See Neff100 and Gilbert98 for other techniques to enhance self-compassion.
1. Shahar B, Doron G, Ohad S. Childhood maltreatment, shame-proneness and self-criticism in social anxiety disorder: a sequential mediational model. Clin Psychol Psychother. 2015:22(6):570-579.
2. Kannan D, Levitt HM. A review of client self-criticism in psychotherapy. J Psychother Integr. 2013;23(2):166-178.
3. Barnard LK, Curry JF. Self-compassion: conceptualizations, correlates, and interventions. Rev Gen Psychol. 2011;15(4):289-303.
4. MacBeth A, Gumley A. Exploring compassion: a meta-analysis of the association between self-compassion and psychopathology. Clin Psychol Rev. 2012;32(6):545-552
5. Blatt SJ, Zuroff DC. Interpersonal relatedness and self-definition: two prototypes for depression. Clin Psychol Rev. 1992;12(5):527-562.
6. Neff KD. The development and validation of a scale to measure self-compassion. Self Identity. 2003;2(2):223-250.
7. Neff KD. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self Identity. 2003;(2)2:85-101.
8. Gilbert P, Procter S. Compassionate mind training for people with high shame and self-criticism: overview and pilot study of a group therapy approach. Clin Psychol Psychother. 2006;13(6):353-379.
9. Dunkley DM, Zuroff DC, Blankstein KR. Specific perfectionism components versus self-criticism in predicting maladjustment. Pers Individ Dif. 2006;40(4):665-676.
10. Murphy JM, Nierenberg AA, Monson RR, et al. Self-disparagement as feature and forerunner of depression: Mindfindings from the Stirling County Study. Compr Psychiatry. 2002;43(1):13-21.
11. Brewin CR, Firth-Cozens J. Dependency and self-criticism as predictors of depression in young doctors. J Occup Health Psychol. 1997;2(3):242-246.
12. Fazaa N, Page S. Dependency and self-criticism as predictors of suicidal behavior. Suicide Life Threat Behav. 2003;33(2):172-185.
13. Teasdale JD, Cox SG. Dysphoria: self-devaluative and affective components in recovered depressed patients and never depressed controls. Psychol Med. 2001;31(7):1311-1316.
14. Ehret AM, Joormann J, Berking M. Examining risk and resilience factors for depression: the role of self-criticism and self-compassion. Cogn Emot. 2015;29(8):1496-1504.
15. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
16. Rector NA, Bagby RM, Segal ZV, et al. Self-criticism and dependency in depressed patients treated with cognitive therapy or pharmacotherapy. Cognit Ther Res. 2000;24(5):571-584.
17. Marshall MB, Zuroff DC, McBride C, et al. Self-criticism predicts differential response to treatment for major depression. J Clin Psychol. 2008;64(3):231-244.
18. Zuroff DC, Blatt SJ, Sotsky SM, et al. Relation of therapeutic alliance and perfectionism to outcome in brief outpatient treatment of depression. J Consult Clin Psychol. 2000;68(1):114-124.
19. Whelton WJ, Greenberg LS. Emotion in self-criticism. Pers Individ Dif. 2005;38(7):1583-1595.
20. Cox BJ, Fleet C, Stein MB. Self-criticism and social phobia in the US national comorbidity survey. J Affect Disord. 2004;82(2):227-234.
21. Cox BJ, Walker JR, Enns MW, et al. Self-criticism in generalized social phobia and response to cognitive-behavioral treatment. Behav Ther. 2002;33(4):479-491.
22. McCranie EW, Hyer LA. Self-critical depressive experience in posttraumatic stress disorder. Psychol Rep. 1995;77(3 pt 1):880-882.
23. Southwick SM, Yehuda R, Giller EL Jr. Characterization of depression in war-related posttraumatic stress disorder. Am J Psychiatry. 1991;148(2):179-183.
24. Yehuda R, Kahana B, Southwick SM, et al. Depressive features in Holocaust survivors with post-traumatic stress disorder. J Traumatic Stress. 1994;7(4):699-704.
25. Sharhabani-Arzy R, Amir M, Swisa A. Self-criticism, dependency and posttraumatic stress disorder among a female group of help-seeking victims of domestic violence in Israel. Pers Individ Dif. 2005;38(5):1231-1240.
26. Beaumont E, Galpin A, Jenkins P. ‘Being kinder to myself’: a prospective comparative study, exploring post-trauma therapy outcome measures, for two groups of clients, receiving either cognitive behaviour therapy or cognitive behaviour therapy and compassionate mind training. Counselling Psychol Rev. 2012;27(1):31-43.
27. Dahm KA. Mindfulness and self-compassion as predictors of functional outcomes and psychopathology in OEF/OIF veterans exposed to trauma. https://repositories.lib.utexas.edu/handle/2152/21635. Published August 2013. Accessed November 8, 2016.
28. Hiraoka R, Meyer EC, Kimbrel NA, et al. Self-compassion as a prospective predictor of PTSD symptom severity among trauma-exposed US Iraq and Afghanistan war veterans. J Trauma Stress. 2015;28(2):127-133.
29. Bagby RM, Cox BJ, Schuller DR, et al. Diagnostic specificity of the dependent and self-critical personality dimensions in major depression. J Affect Disord. 1992;26(1):59-63.
30. Hedman E, Ström P, Stünkel A, et al. Shame and guilt in social anxiety disorder: effects of cognitive behavior therapy and association with social anxiety and depressive symptoms. PLoS One. 2013;8(4):e61713. doi: 10.1371/journal.pone.0061713.
31. Hoge EA, Hölzel BK, Marques L, et al. Mindfulness and self-compassion in generalized anxiety disorder: examining predictors of disability. Evid Based Complement Alternat Med. 2013;2013:576258. doi: 10.1155/2013/576258.
32. Wetterneck CT, Lee EB, Smith AH, et al. Courage, self-compassion, and values in obsessive-compulsive disorder. J Contextual Behav Sci. 2013;2(3-4):68-73.
33. Kelly AC, Carter JC. Why self-critical patients present with more severe eating disorder pathology: The mediating role of shame. Br J Clin Psychol. 2013;52(2):148-161.
34. Dunkley DM, Grilo CM. Self-criticism, low self-esteem, depressive symptoms, and over-evaluation of shape and weight in binge eating disorder patients. Behav Res Ther. 2007;45(1):139-149.
35. Dunkley DM, Masheb RM, Grilo CM. Childhood maltreatment, depressive symptoms, and body dissatisfaction in patients with binge eating disorder: the mediating role of self-criticism. Int J Eat Disord. 2010;43(3):274-281.
36. Wasylkiw L, MacKinnon AL, MacLellan AM. Exploring the link between self-compassion and body image in university women. Body Image. 2012;9(2):236-245.
37. Ferreira C, Pinto-Gouveia J, Duarte C. Self-compassion in the face of shame and body image dissatisfaction: implications for eating disorders. Eat Behavs. 2013;14(2):207-210.
38. Kelly AC, Carter JC, Zuroff DC, et al. Self-compassion and fear of self-compassion interact to predict response to eating disorders treatment: a preliminary investigation. Psychother Res. 2013;23(3):252-264.
39. Webb JB, Forman MJ. Evaluating the indirect effect of self-compassion on binge eating severity through cognitive-affective self-regulatory pathways. Eat Behavs. 2013;14(2):224-228.
40. Kelly AC, Carter JC, Borairi S. Are improvements in shame and self-compassion early in eating disorders treatment associated with better patient outcomes? Int J Eat Disord. 2014;47(1):54-64.
41. Wiseman H, Raz A, Sharabany R. Depressive personality styles and interpersonal problems in young adults with difficulties in establishing long-term romantic relationships. Isr J Psychiatry Rel Sci. 2007;44(4):280-291.
42. Besser A, Priel B. A multisource approach to self-critical vulnerability to depression: the moderating role of attachment. J Pers. 2003;71(4):515-555.
43. Zuroff DC, Koestner R, Powers TA. Self-criticism at age 12: a longitudinal-study of adjustment. Cognit Ther Res. 1994;18(4):367-385.
44. Fichman L, Koestner R, Zuroff DC. Depressive styles in adolescence: Assessment, relation to social functioning, and developmental trends. J Youth Adolesc. 1994;23(3):315-330.
45. Wiseman H. Interpersonal relatedness and self-definition in the experience of loneliness during the transition to university. Personal Relationships. 1997;4(3):285-299.
46. Mongrain M, Lubbers R, Struthers W. The power of love: mediation of rejection in roommate relationships of dependents and self-critics. Pers Soc Psychol Bull. 2004;30(1):94-105.
47. Whiffen VE, Aube JA. Personality, interpersonal context and depression in couples. J Soc Pers Relat. 1999;16(3):369-383.
48. Priel B, Besser A. Dependency and self-criticism among first-time mothers: the roles of global and specific support. J Soc Clin Psychol. 2000;19(4):437-450.
49. Neff KD, Beretvas SN. The role of self-compassion in romantic relationships. Self Identity. 2013;12(1):78-98.
50. Powers TA, Koestner R, Zuroff DC. Self-criticism, goal motivation, and goal progress. J Soc Clin Psychol. 2007;26(7):826-840.
51. Powers TA, Koestner R, Zuroff DC, et al. The effects of self-criticism and self-oriented perfectionism on goal pursuit. Pers Soc Psychol Bull. 2011;37(7):964-975.
52. Hope N, Koestner R, Milyavskaya M. The role of self-compassion in goal pursuit and well-being among university freshmen. Self Identity. 2014;13(5):579-593.
53. Williams JG, Stark SK, Foster EE. Start today or the very last day? The relationships among self-compassion, motivation, and procrastination. Am J Psychol Res. 2008;4(1):37-44.
54. Neff KD, Hseih Y, Dejittherat K. Self-compassion, achievement goals, and coping with academic failure. Self Identity. 2005;4(3):263-287.
55. Campos RC, Besser A, Blatt SJ. The mediating role of self-criticism and dependency in the association between perceptions of maternal caring and depressive symptoms. Depress Anxiety. 2010;27(12):1149-1157.
56. Sachs-Ericsson N, Verona E, Joiner T, et al. Parental verbal abuse and the mediating role of self-criticism in adult internalizing disorders. J Affect Disord. 2006;93(1-3):71-78.
57. Amitay OA, Mongrain M, Fazaa N. Love and control: self-criticism in parents and daughters and perceptions of relationship partners. Pers Individ Dif. 2008;44(1):75-85.
58. Zuroff DC, Fitzpatrick DK. Depressive personality styles: implications for adult attachment. Pers Individ Dif. 1995;18(2):253-265.
59. Kopala-Sibley DC, Zuroff DC. The developmental origins of personality factors from the self-definitional and relatedness domains: a review of theory and research. Rev Gen Psychol. 2014;18(3):137-155.
60. Kopala-Sibley DC, Zuroff DC, Leybman MJ, et al. Recalled peer relationship experiences and current levels of self-criticism and self-reassurance. Psychol Psychother. 2013;86(1):33-51.
61. Neff KD, McGehee P. Self-compassion and psychological resilience among adolescents and young adults. Self Identity. 2010;9(3):225-240.
62. Pepping CA, Davis PJ, O’Donovan A, et al. Individual differences in self-compassion: the role of attachment and experiences of parenting in childhood. Self Identity. 2015;14(1):104-117.
63. Neff KD, Rude SS, Kirkpatrick KL. An examination of self-compassion in relation to positive psychological functioning and personality traits. J Res Pers. 2007;41(4):908-916.
64. Van Dam NT, Sheppard SC, Forsyth JP, et al. Self-compassion is a better predictor than mindfulness of symptom severity and quality of life in mixed anxiety and depression. J Anxiety Disord. 2011;25(1):123-130.
65. Heffernan M, Quinn MT, McNulty SR, et al. Self-compassion and emotional intelligence in nurses. Int J Nursing Practice. 2010;16(4):366-373.
66. Neff KD, Kirkpatrick KL, Rude SS. Self-compassion and adaptive psychological functioning. J Res Pers. 2007;41(1):139-154.
67. Krieger T, Altenstein D, Baettig I, et al. Self-compassion in depression: associations with depressive symptoms, rumination, and avoidance in depressed outpatients. Behav Ther. 2013;44(3):501-513.
68. Breen WE, Kashdan TB, Lenser ML, et al. Gratitude and forgiveness: convergence and divergence on self-report and informant ratings. Pers Individ Dif. 2010;49(8):932-937.
69. Hollis-Walker L, Colosimo K. Mindfulness, self-compassion, and happiness in non-meditators: A theoretical and empirical examination. Pers Individ Dif. 2011;50(2):222-227.
70. Gilbert P, McEwan K, Matos M, et al. Fears of compassion: development of three self-report measures. Psychol Psychother. 2011;84(3):239-255.
71. Neely ME, Schallert DL, Mohammed SS, et al. Self-kindness when facing stress: the role of self-compassion, goal regulation, and support in college students’ well-being. Motiv Emot. 2009;33(1):88-97.
72. Breines JG, Chen S. Self-compassion increases self-improvement motivation. Pers Soc Psychol Bull. 2012;38(9):1133-1143.
73. Allen AB, Leary MR. Self-compassion, stress, and coping. Soc Pers Psychol Compass. 2010;4(2):107-118.
74. Terry ML, Leary MR. Self-compassion, self-regulation, and health. Self Identity. 2011;10(3):352-362.
75. Magnus CMR, Kowalski KC, McHugh TF. The role of self-compassion in women’s self-determined motives to exercise and exercise-related outcomes. Self Identity. 2010;9(4):363-382.
76. Brooks M, Kay-Lambkin F, Bowman J, et al. Self-compassion amongst clients with problematic alcohol use. Mindfulness. 2012;3(4):308-317.
77. Adams CE, Leary MR. Promoting self-compassionate attitudes toward eating among restrictive and guilty eaters. J Soc Clin Psychol. 2007;26(10):1120-1144.
78. Kelly AC, Zuroff DC, Foa CL, et al. Who benefits from training in self-compassionate self-regulation? A study of smoking reduction. J Soc Clin Psychol. 2010;29(7):727-755.
79. Shapira LB, Mongrain M. The benefits of self-compassion and optimism exercises for individuals vulnerable to depression. J Posit Psychol. 2010;5(5):377-389.
80. Albertson ER, Neff KD, Dill-Shackleford KE. Self-compassion and body dissatisfaction in women: a randomized controlled trial of a brief meditation intervention. Mindfulness. 2015;6(3):444-454.
81. Smeets E, Neff K, Alberts H, et al. Meeting suffering with kindness: effects of a brief self-compassion intervention for female college students. J Clinical Psychol. 2014;70(9):794-807.
82. Blatt SJ, D’Afflitti JP, Quinlan DM. Depressive experiences questionnaire. New Haven, CT: Yale University Press; 1976.
83. Weissman AN, Beck AT. Development and validation of the dysfunctional attitude scale: a preliminary investigation. Paper presented at: 62nd Annual Meeting of the Association for Advanced Behavior Therapy; March 27-31, 1978; Toronto, Ontario, Canada.
84. Gilbert P, Clarke M, Hempel S, et al. Criticizing and reassuring oneself: an exploration of forms, styles and reasons in female students. Br J Clin Psychol. 2004;43(pt 1):31-50.
85. Baião R, Gilbert P, McEwan K, et al. Forms of self-criticising/attacking & self-reassuring scale: psychometric properties and normative study. Psychol Psychother. 2015;88(4):438-452.
86. Neff KD. The self-compassion scale is a valid and theoretically coherent measure of self-compassion. Mindfulness. 2016;7(1):264-274.
87. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clinical Psychol. 2013;69(1):28-44.
88. Gilbert P. Introducing compassion-focused therapy. Adv Psychiatr Treat. 2009;15(3):199-208.
89. Kelly AC, Zuroff DC, Shapira LB. Soothing oneself and resisting self-attacks: the treatment of two intrapersonal deficits in depression vulnerability. Cognit Ther Res. 2009;33(3):301-313.
90. Boersma K, Hakanson A, Salomonsson E, et al. Compassion focused therapy to counteract shame, self-criticism and isolation. A replicated single case experimental study of individuals with social anxiety. J Contemp Psychother. 2015;45(2):89-98.
91. Gale C, Gilbert P, Read N, et al. An evaluation of the impact of introducing compassion focused therapy to a standard treatment programme for people with eating disorders. Clin Psychol Psychother. 2014;21(1):1-12.
92. Braehler C, Gumley A, Harper J, et al. Exploring change processes in compassion focused therapy in psychosis: results of a feasibility randomized controlled trial. Br J Clin Psychol. 2013;52(2):199-214.
93. Ashworth F, Clarke A, Jones L, et al. An exploration of compassion focused therapy following acquired brain injury. Psychol Psychother. 2014;88(2):143-162.
94. Judge L, Cleghorn A, McEwan K, et al. An exploration of group-based compassion focused therapy for a heterogeneous range of clients presenting to a community mental health team. Int J Cogn Ther. 2012;5(4):420-429.
95. Leaviss J, Utley L. Psychotherapeutic benefits of compassion-focused therapy: an early systematic review. Psychol Med. 2015;45(5):927-945.
96. Gilbert P, McEwan K, Gibbons L, et al. Fears of compassion and happiness in relation to alexithymia, mindfulness, and self‐criticism. Psychol Psychother. 2012;85(4):374-390.
97. Germer CK, Neff KD. Cultivating self-compassion in trauma survivors. In: Follette VM, Briere J, Rozelle D, et al, eds. Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York, NY: Guilford Press; 2015:43-58.
98. Gilbert P. Compassion focused therapy: the CBT distinctive features series. London, United Kingdom: Routledge; 2010.
99. Germer C, Neff K. The mindful self-compassion training program. In: Singer T, Bolz M, eds. Compassion: bridging theory and practice: a multimedia book. Leipzig, Germany: Max-Planck Institute; 2013:365-396.
100. Neff K. Self-compassion: the proven power of being kind to yourself. New York, NY: HarperCollins; 2015.
1. Shahar B, Doron G, Ohad S. Childhood maltreatment, shame-proneness and self-criticism in social anxiety disorder: a sequential mediational model. Clin Psychol Psychother. 2015:22(6):570-579.
2. Kannan D, Levitt HM. A review of client self-criticism in psychotherapy. J Psychother Integr. 2013;23(2):166-178.
3. Barnard LK, Curry JF. Self-compassion: conceptualizations, correlates, and interventions. Rev Gen Psychol. 2011;15(4):289-303.
4. MacBeth A, Gumley A. Exploring compassion: a meta-analysis of the association between self-compassion and psychopathology. Clin Psychol Rev. 2012;32(6):545-552
5. Blatt SJ, Zuroff DC. Interpersonal relatedness and self-definition: two prototypes for depression. Clin Psychol Rev. 1992;12(5):527-562.
6. Neff KD. The development and validation of a scale to measure self-compassion. Self Identity. 2003;2(2):223-250.
7. Neff KD. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self Identity. 2003;(2)2:85-101.
8. Gilbert P, Procter S. Compassionate mind training for people with high shame and self-criticism: overview and pilot study of a group therapy approach. Clin Psychol Psychother. 2006;13(6):353-379.
9. Dunkley DM, Zuroff DC, Blankstein KR. Specific perfectionism components versus self-criticism in predicting maladjustment. Pers Individ Dif. 2006;40(4):665-676.
10. Murphy JM, Nierenberg AA, Monson RR, et al. Self-disparagement as feature and forerunner of depression: Mindfindings from the Stirling County Study. Compr Psychiatry. 2002;43(1):13-21.
11. Brewin CR, Firth-Cozens J. Dependency and self-criticism as predictors of depression in young doctors. J Occup Health Psychol. 1997;2(3):242-246.
12. Fazaa N, Page S. Dependency and self-criticism as predictors of suicidal behavior. Suicide Life Threat Behav. 2003;33(2):172-185.
13. Teasdale JD, Cox SG. Dysphoria: self-devaluative and affective components in recovered depressed patients and never depressed controls. Psychol Med. 2001;31(7):1311-1316.
14. Ehret AM, Joormann J, Berking M. Examining risk and resilience factors for depression: the role of self-criticism and self-compassion. Cogn Emot. 2015;29(8):1496-1504.
15. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
16. Rector NA, Bagby RM, Segal ZV, et al. Self-criticism and dependency in depressed patients treated with cognitive therapy or pharmacotherapy. Cognit Ther Res. 2000;24(5):571-584.
17. Marshall MB, Zuroff DC, McBride C, et al. Self-criticism predicts differential response to treatment for major depression. J Clin Psychol. 2008;64(3):231-244.
18. Zuroff DC, Blatt SJ, Sotsky SM, et al. Relation of therapeutic alliance and perfectionism to outcome in brief outpatient treatment of depression. J Consult Clin Psychol. 2000;68(1):114-124.
19. Whelton WJ, Greenberg LS. Emotion in self-criticism. Pers Individ Dif. 2005;38(7):1583-1595.
20. Cox BJ, Fleet C, Stein MB. Self-criticism and social phobia in the US national comorbidity survey. J Affect Disord. 2004;82(2):227-234.
21. Cox BJ, Walker JR, Enns MW, et al. Self-criticism in generalized social phobia and response to cognitive-behavioral treatment. Behav Ther. 2002;33(4):479-491.
22. McCranie EW, Hyer LA. Self-critical depressive experience in posttraumatic stress disorder. Psychol Rep. 1995;77(3 pt 1):880-882.
23. Southwick SM, Yehuda R, Giller EL Jr. Characterization of depression in war-related posttraumatic stress disorder. Am J Psychiatry. 1991;148(2):179-183.
24. Yehuda R, Kahana B, Southwick SM, et al. Depressive features in Holocaust survivors with post-traumatic stress disorder. J Traumatic Stress. 1994;7(4):699-704.
25. Sharhabani-Arzy R, Amir M, Swisa A. Self-criticism, dependency and posttraumatic stress disorder among a female group of help-seeking victims of domestic violence in Israel. Pers Individ Dif. 2005;38(5):1231-1240.
26. Beaumont E, Galpin A, Jenkins P. ‘Being kinder to myself’: a prospective comparative study, exploring post-trauma therapy outcome measures, for two groups of clients, receiving either cognitive behaviour therapy or cognitive behaviour therapy and compassionate mind training. Counselling Psychol Rev. 2012;27(1):31-43.
27. Dahm KA. Mindfulness and self-compassion as predictors of functional outcomes and psychopathology in OEF/OIF veterans exposed to trauma. https://repositories.lib.utexas.edu/handle/2152/21635. Published August 2013. Accessed November 8, 2016.
28. Hiraoka R, Meyer EC, Kimbrel NA, et al. Self-compassion as a prospective predictor of PTSD symptom severity among trauma-exposed US Iraq and Afghanistan war veterans. J Trauma Stress. 2015;28(2):127-133.
29. Bagby RM, Cox BJ, Schuller DR, et al. Diagnostic specificity of the dependent and self-critical personality dimensions in major depression. J Affect Disord. 1992;26(1):59-63.
30. Hedman E, Ström P, Stünkel A, et al. Shame and guilt in social anxiety disorder: effects of cognitive behavior therapy and association with social anxiety and depressive symptoms. PLoS One. 2013;8(4):e61713. doi: 10.1371/journal.pone.0061713.
31. Hoge EA, Hölzel BK, Marques L, et al. Mindfulness and self-compassion in generalized anxiety disorder: examining predictors of disability. Evid Based Complement Alternat Med. 2013;2013:576258. doi: 10.1155/2013/576258.
32. Wetterneck CT, Lee EB, Smith AH, et al. Courage, self-compassion, and values in obsessive-compulsive disorder. J Contextual Behav Sci. 2013;2(3-4):68-73.
33. Kelly AC, Carter JC. Why self-critical patients present with more severe eating disorder pathology: The mediating role of shame. Br J Clin Psychol. 2013;52(2):148-161.
34. Dunkley DM, Grilo CM. Self-criticism, low self-esteem, depressive symptoms, and over-evaluation of shape and weight in binge eating disorder patients. Behav Res Ther. 2007;45(1):139-149.
35. Dunkley DM, Masheb RM, Grilo CM. Childhood maltreatment, depressive symptoms, and body dissatisfaction in patients with binge eating disorder: the mediating role of self-criticism. Int J Eat Disord. 2010;43(3):274-281.
36. Wasylkiw L, MacKinnon AL, MacLellan AM. Exploring the link between self-compassion and body image in university women. Body Image. 2012;9(2):236-245.
37. Ferreira C, Pinto-Gouveia J, Duarte C. Self-compassion in the face of shame and body image dissatisfaction: implications for eating disorders. Eat Behavs. 2013;14(2):207-210.
38. Kelly AC, Carter JC, Zuroff DC, et al. Self-compassion and fear of self-compassion interact to predict response to eating disorders treatment: a preliminary investigation. Psychother Res. 2013;23(3):252-264.
39. Webb JB, Forman MJ. Evaluating the indirect effect of self-compassion on binge eating severity through cognitive-affective self-regulatory pathways. Eat Behavs. 2013;14(2):224-228.
40. Kelly AC, Carter JC, Borairi S. Are improvements in shame and self-compassion early in eating disorders treatment associated with better patient outcomes? Int J Eat Disord. 2014;47(1):54-64.
41. Wiseman H, Raz A, Sharabany R. Depressive personality styles and interpersonal problems in young adults with difficulties in establishing long-term romantic relationships. Isr J Psychiatry Rel Sci. 2007;44(4):280-291.
42. Besser A, Priel B. A multisource approach to self-critical vulnerability to depression: the moderating role of attachment. J Pers. 2003;71(4):515-555.
43. Zuroff DC, Koestner R, Powers TA. Self-criticism at age 12: a longitudinal-study of adjustment. Cognit Ther Res. 1994;18(4):367-385.
44. Fichman L, Koestner R, Zuroff DC. Depressive styles in adolescence: Assessment, relation to social functioning, and developmental trends. J Youth Adolesc. 1994;23(3):315-330.
45. Wiseman H. Interpersonal relatedness and self-definition in the experience of loneliness during the transition to university. Personal Relationships. 1997;4(3):285-299.
46. Mongrain M, Lubbers R, Struthers W. The power of love: mediation of rejection in roommate relationships of dependents and self-critics. Pers Soc Psychol Bull. 2004;30(1):94-105.
47. Whiffen VE, Aube JA. Personality, interpersonal context and depression in couples. J Soc Pers Relat. 1999;16(3):369-383.
48. Priel B, Besser A. Dependency and self-criticism among first-time mothers: the roles of global and specific support. J Soc Clin Psychol. 2000;19(4):437-450.
49. Neff KD, Beretvas SN. The role of self-compassion in romantic relationships. Self Identity. 2013;12(1):78-98.
50. Powers TA, Koestner R, Zuroff DC. Self-criticism, goal motivation, and goal progress. J Soc Clin Psychol. 2007;26(7):826-840.
51. Powers TA, Koestner R, Zuroff DC, et al. The effects of self-criticism and self-oriented perfectionism on goal pursuit. Pers Soc Psychol Bull. 2011;37(7):964-975.
52. Hope N, Koestner R, Milyavskaya M. The role of self-compassion in goal pursuit and well-being among university freshmen. Self Identity. 2014;13(5):579-593.
53. Williams JG, Stark SK, Foster EE. Start today or the very last day? The relationships among self-compassion, motivation, and procrastination. Am J Psychol Res. 2008;4(1):37-44.
54. Neff KD, Hseih Y, Dejittherat K. Self-compassion, achievement goals, and coping with academic failure. Self Identity. 2005;4(3):263-287.
55. Campos RC, Besser A, Blatt SJ. The mediating role of self-criticism and dependency in the association between perceptions of maternal caring and depressive symptoms. Depress Anxiety. 2010;27(12):1149-1157.
56. Sachs-Ericsson N, Verona E, Joiner T, et al. Parental verbal abuse and the mediating role of self-criticism in adult internalizing disorders. J Affect Disord. 2006;93(1-3):71-78.
57. Amitay OA, Mongrain M, Fazaa N. Love and control: self-criticism in parents and daughters and perceptions of relationship partners. Pers Individ Dif. 2008;44(1):75-85.
58. Zuroff DC, Fitzpatrick DK. Depressive personality styles: implications for adult attachment. Pers Individ Dif. 1995;18(2):253-265.
59. Kopala-Sibley DC, Zuroff DC. The developmental origins of personality factors from the self-definitional and relatedness domains: a review of theory and research. Rev Gen Psychol. 2014;18(3):137-155.
60. Kopala-Sibley DC, Zuroff DC, Leybman MJ, et al. Recalled peer relationship experiences and current levels of self-criticism and self-reassurance. Psychol Psychother. 2013;86(1):33-51.
61. Neff KD, McGehee P. Self-compassion and psychological resilience among adolescents and young adults. Self Identity. 2010;9(3):225-240.
62. Pepping CA, Davis PJ, O’Donovan A, et al. Individual differences in self-compassion: the role of attachment and experiences of parenting in childhood. Self Identity. 2015;14(1):104-117.
63. Neff KD, Rude SS, Kirkpatrick KL. An examination of self-compassion in relation to positive psychological functioning and personality traits. J Res Pers. 2007;41(4):908-916.
64. Van Dam NT, Sheppard SC, Forsyth JP, et al. Self-compassion is a better predictor than mindfulness of symptom severity and quality of life in mixed anxiety and depression. J Anxiety Disord. 2011;25(1):123-130.
65. Heffernan M, Quinn MT, McNulty SR, et al. Self-compassion and emotional intelligence in nurses. Int J Nursing Practice. 2010;16(4):366-373.
66. Neff KD, Kirkpatrick KL, Rude SS. Self-compassion and adaptive psychological functioning. J Res Pers. 2007;41(1):139-154.
67. Krieger T, Altenstein D, Baettig I, et al. Self-compassion in depression: associations with depressive symptoms, rumination, and avoidance in depressed outpatients. Behav Ther. 2013;44(3):501-513.
68. Breen WE, Kashdan TB, Lenser ML, et al. Gratitude and forgiveness: convergence and divergence on self-report and informant ratings. Pers Individ Dif. 2010;49(8):932-937.
69. Hollis-Walker L, Colosimo K. Mindfulness, self-compassion, and happiness in non-meditators: A theoretical and empirical examination. Pers Individ Dif. 2011;50(2):222-227.
70. Gilbert P, McEwan K, Matos M, et al. Fears of compassion: development of three self-report measures. Psychol Psychother. 2011;84(3):239-255.
71. Neely ME, Schallert DL, Mohammed SS, et al. Self-kindness when facing stress: the role of self-compassion, goal regulation, and support in college students’ well-being. Motiv Emot. 2009;33(1):88-97.
72. Breines JG, Chen S. Self-compassion increases self-improvement motivation. Pers Soc Psychol Bull. 2012;38(9):1133-1143.
73. Allen AB, Leary MR. Self-compassion, stress, and coping. Soc Pers Psychol Compass. 2010;4(2):107-118.
74. Terry ML, Leary MR. Self-compassion, self-regulation, and health. Self Identity. 2011;10(3):352-362.
75. Magnus CMR, Kowalski KC, McHugh TF. The role of self-compassion in women’s self-determined motives to exercise and exercise-related outcomes. Self Identity. 2010;9(4):363-382.
76. Brooks M, Kay-Lambkin F, Bowman J, et al. Self-compassion amongst clients with problematic alcohol use. Mindfulness. 2012;3(4):308-317.
77. Adams CE, Leary MR. Promoting self-compassionate attitudes toward eating among restrictive and guilty eaters. J Soc Clin Psychol. 2007;26(10):1120-1144.
78. Kelly AC, Zuroff DC, Foa CL, et al. Who benefits from training in self-compassionate self-regulation? A study of smoking reduction. J Soc Clin Psychol. 2010;29(7):727-755.
79. Shapira LB, Mongrain M. The benefits of self-compassion and optimism exercises for individuals vulnerable to depression. J Posit Psychol. 2010;5(5):377-389.
80. Albertson ER, Neff KD, Dill-Shackleford KE. Self-compassion and body dissatisfaction in women: a randomized controlled trial of a brief meditation intervention. Mindfulness. 2015;6(3):444-454.
81. Smeets E, Neff K, Alberts H, et al. Meeting suffering with kindness: effects of a brief self-compassion intervention for female college students. J Clinical Psychol. 2014;70(9):794-807.
82. Blatt SJ, D’Afflitti JP, Quinlan DM. Depressive experiences questionnaire. New Haven, CT: Yale University Press; 1976.
83. Weissman AN, Beck AT. Development and validation of the dysfunctional attitude scale: a preliminary investigation. Paper presented at: 62nd Annual Meeting of the Association for Advanced Behavior Therapy; March 27-31, 1978; Toronto, Ontario, Canada.
84. Gilbert P, Clarke M, Hempel S, et al. Criticizing and reassuring oneself: an exploration of forms, styles and reasons in female students. Br J Clin Psychol. 2004;43(pt 1):31-50.
85. Baião R, Gilbert P, McEwan K, et al. Forms of self-criticising/attacking & self-reassuring scale: psychometric properties and normative study. Psychol Psychother. 2015;88(4):438-452.
86. Neff KD. The self-compassion scale is a valid and theoretically coherent measure of self-compassion. Mindfulness. 2016;7(1):264-274.
87. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clinical Psychol. 2013;69(1):28-44.
88. Gilbert P. Introducing compassion-focused therapy. Adv Psychiatr Treat. 2009;15(3):199-208.
89. Kelly AC, Zuroff DC, Shapira LB. Soothing oneself and resisting self-attacks: the treatment of two intrapersonal deficits in depression vulnerability. Cognit Ther Res. 2009;33(3):301-313.
90. Boersma K, Hakanson A, Salomonsson E, et al. Compassion focused therapy to counteract shame, self-criticism and isolation. A replicated single case experimental study of individuals with social anxiety. J Contemp Psychother. 2015;45(2):89-98.
91. Gale C, Gilbert P, Read N, et al. An evaluation of the impact of introducing compassion focused therapy to a standard treatment programme for people with eating disorders. Clin Psychol Psychother. 2014;21(1):1-12.
92. Braehler C, Gumley A, Harper J, et al. Exploring change processes in compassion focused therapy in psychosis: results of a feasibility randomized controlled trial. Br J Clin Psychol. 2013;52(2):199-214.
93. Ashworth F, Clarke A, Jones L, et al. An exploration of compassion focused therapy following acquired brain injury. Psychol Psychother. 2014;88(2):143-162.
94. Judge L, Cleghorn A, McEwan K, et al. An exploration of group-based compassion focused therapy for a heterogeneous range of clients presenting to a community mental health team. Int J Cogn Ther. 2012;5(4):420-429.
95. Leaviss J, Utley L. Psychotherapeutic benefits of compassion-focused therapy: an early systematic review. Psychol Med. 2015;45(5):927-945.
96. Gilbert P, McEwan K, Gibbons L, et al. Fears of compassion and happiness in relation to alexithymia, mindfulness, and self‐criticism. Psychol Psychother. 2012;85(4):374-390.
97. Germer CK, Neff KD. Cultivating self-compassion in trauma survivors. In: Follette VM, Briere J, Rozelle D, et al, eds. Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York, NY: Guilford Press; 2015:43-58.
98. Gilbert P. Compassion focused therapy: the CBT distinctive features series. London, United Kingdom: Routledge; 2010.
99. Germer C, Neff K. The mindful self-compassion training program. In: Singer T, Bolz M, eds. Compassion: bridging theory and practice: a multimedia book. Leipzig, Germany: Max-Planck Institute; 2013:365-396.
100. Neff K. Self-compassion: the proven power of being kind to yourself. New York, NY: HarperCollins; 2015.
When to use an anticonvulsant to treat alcohol withdrawal
Alcohol withdrawal is an uncomfortable and potentially life-threatening condition that must be treated before patients can achieve sobriety. Benzodiazepines remain the first-line treatment for alcohol withdrawal; however, these agents could:
- exacerbate agitation
- interact adversely with other medications, particularly opioids
- be unsafe for outpatients at risk of drinking again.
Off-label use of anticonvulsants could reduce these risks. In our emergency department, we routinely use these agents as monotherapy for patients discharging to outpatient detoxification or as adjunctive treatment for patients who require admission for severe withdrawal (Table1,2).
Gabapentin is safe for patients with liver disease and has few drug–drug interactions.1 Dosages of at least 1,200 mg/d seems to be comparable to lorazepam for alcohol withdrawal and could help prevent relapse after the withdrawal period.1 Many patients report that gabapentin helps them sleep. Gabapentin could cause gastrointestinal upset or slight dizziness; patients with severe renal disease might require dosage adjustments.
Carbamazepine. In a randomized double-blind trial, carbamazepine was superior to lorazepam in preventing rebound withdrawal symptoms and reducing post-treatment drinking, although both agents were effective in decreasing withdrawal symptoms.2 Avoid this agent in patients with serum liver enzymes 3 times higher than normal values
Divalproex with as-needed benzodiazepines reduces the duration of withdrawal and risk of medical complications.3 Avoid using divalproex in patients with thrombocytopenia, leukopenia, or severe liver disease.
1. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
2. Malcom R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on a single versus multiple previous alcohol withdrawals in an outpatient randomized trial. J Gen Int Med. 2002;17(5):349-355.
3. Eyer F, Schreckenberg M, Adorjan K, et al. Carbamazepine and valproate as adjuncts in the treatment of alcohol withdrawal syndrome: a retrospective cohort study. Alcohol Alcohol. 2011;46(2):177-184.
Alcohol withdrawal is an uncomfortable and potentially life-threatening condition that must be treated before patients can achieve sobriety. Benzodiazepines remain the first-line treatment for alcohol withdrawal; however, these agents could:
- exacerbate agitation
- interact adversely with other medications, particularly opioids
- be unsafe for outpatients at risk of drinking again.
Off-label use of anticonvulsants could reduce these risks. In our emergency department, we routinely use these agents as monotherapy for patients discharging to outpatient detoxification or as adjunctive treatment for patients who require admission for severe withdrawal (Table1,2).
Gabapentin is safe for patients with liver disease and has few drug–drug interactions.1 Dosages of at least 1,200 mg/d seems to be comparable to lorazepam for alcohol withdrawal and could help prevent relapse after the withdrawal period.1 Many patients report that gabapentin helps them sleep. Gabapentin could cause gastrointestinal upset or slight dizziness; patients with severe renal disease might require dosage adjustments.
Carbamazepine. In a randomized double-blind trial, carbamazepine was superior to lorazepam in preventing rebound withdrawal symptoms and reducing post-treatment drinking, although both agents were effective in decreasing withdrawal symptoms.2 Avoid this agent in patients with serum liver enzymes 3 times higher than normal values
Divalproex with as-needed benzodiazepines reduces the duration of withdrawal and risk of medical complications.3 Avoid using divalproex in patients with thrombocytopenia, leukopenia, or severe liver disease.
Alcohol withdrawal is an uncomfortable and potentially life-threatening condition that must be treated before patients can achieve sobriety. Benzodiazepines remain the first-line treatment for alcohol withdrawal; however, these agents could:
- exacerbate agitation
- interact adversely with other medications, particularly opioids
- be unsafe for outpatients at risk of drinking again.
Off-label use of anticonvulsants could reduce these risks. In our emergency department, we routinely use these agents as monotherapy for patients discharging to outpatient detoxification or as adjunctive treatment for patients who require admission for severe withdrawal (Table1,2).
Gabapentin is safe for patients with liver disease and has few drug–drug interactions.1 Dosages of at least 1,200 mg/d seems to be comparable to lorazepam for alcohol withdrawal and could help prevent relapse after the withdrawal period.1 Many patients report that gabapentin helps them sleep. Gabapentin could cause gastrointestinal upset or slight dizziness; patients with severe renal disease might require dosage adjustments.
Carbamazepine. In a randomized double-blind trial, carbamazepine was superior to lorazepam in preventing rebound withdrawal symptoms and reducing post-treatment drinking, although both agents were effective in decreasing withdrawal symptoms.2 Avoid this agent in patients with serum liver enzymes 3 times higher than normal values
Divalproex with as-needed benzodiazepines reduces the duration of withdrawal and risk of medical complications.3 Avoid using divalproex in patients with thrombocytopenia, leukopenia, or severe liver disease.
1. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
2. Malcom R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on a single versus multiple previous alcohol withdrawals in an outpatient randomized trial. J Gen Int Med. 2002;17(5):349-355.
3. Eyer F, Schreckenberg M, Adorjan K, et al. Carbamazepine and valproate as adjuncts in the treatment of alcohol withdrawal syndrome: a retrospective cohort study. Alcohol Alcohol. 2011;46(2):177-184.
1. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
2. Malcom R, Myrick H, Roberts J, et al. The effects of carbamazepine and lorazepam on a single versus multiple previous alcohol withdrawals in an outpatient randomized trial. J Gen Int Med. 2002;17(5):349-355.
3. Eyer F, Schreckenberg M, Adorjan K, et al. Carbamazepine and valproate as adjuncts in the treatment of alcohol withdrawal syndrome: a retrospective cohort study. Alcohol Alcohol. 2011;46(2):177-184.
Managing clozapine-induced neutropenia and agranulocytosis
Mr. S, age 43, has schizophrenia and been stable on clozapine for 6 years after several other antipsychotic regimens failed. Mr. S also has a history of hypertension, dyslipidemia, and gastroesophageal reflux disorder. His medication regimen includes clozapine, 400 mg/d, lisinopril, 20 mg/d, atorvastatin, 40 mg/d, omeprazole, 40 mg/d, and a multivitamin. During routine blood monitoring, Mr. S shows a significant drop in absolute neutrophil count (ANC) (750/µL) (reference range, 1,500 to 8,000 µL). Mr. S , who is African American, has no history of benign ethnic neutropenia (BEN) or ANC <1,000/µL. While reviewing his chart, clinicians note that Mr. S had an ANC of 1,350/µL3 years earlier in 2013. Because a complete workup reveals no other cause for this lab abnormality, we determine that is clozapine-induced. Mr. S’s physician asks about treatment options that would allow him to stay on clozapine.
Because of clozapine’s efficacy in treatment-resistant schizophrenia, many psychiatrists aim to
Clozapine-induced neutropenia
Clozapine was approved in 1989 for managing treatment-resistant schizophrenia after demonstrating better efficacy than chlorpromazine.1 However, the adverse effects of neutropenia (white blood cell count [WBC] <3,000/μL) and agranulocytosis (ANC <500/μL3) leading to death were reported in later studies.2,3 One study in the United Kingdom and Ireland reported a prevalence of 2.9% for neutropenia and 0.8% for agranulocytosis among patients taking clozapine.3 Because of this risk, the FDA mandated WBC and ANC monitoring before initiating clozapine and periodically thereafter. In October 2015, the Risk Evaluation and Mitigation Strategies program for clozapine updated recommended ANC levels and eliminated WBC monitoring. ANC monitoring frequencies are summarized in the Table.1
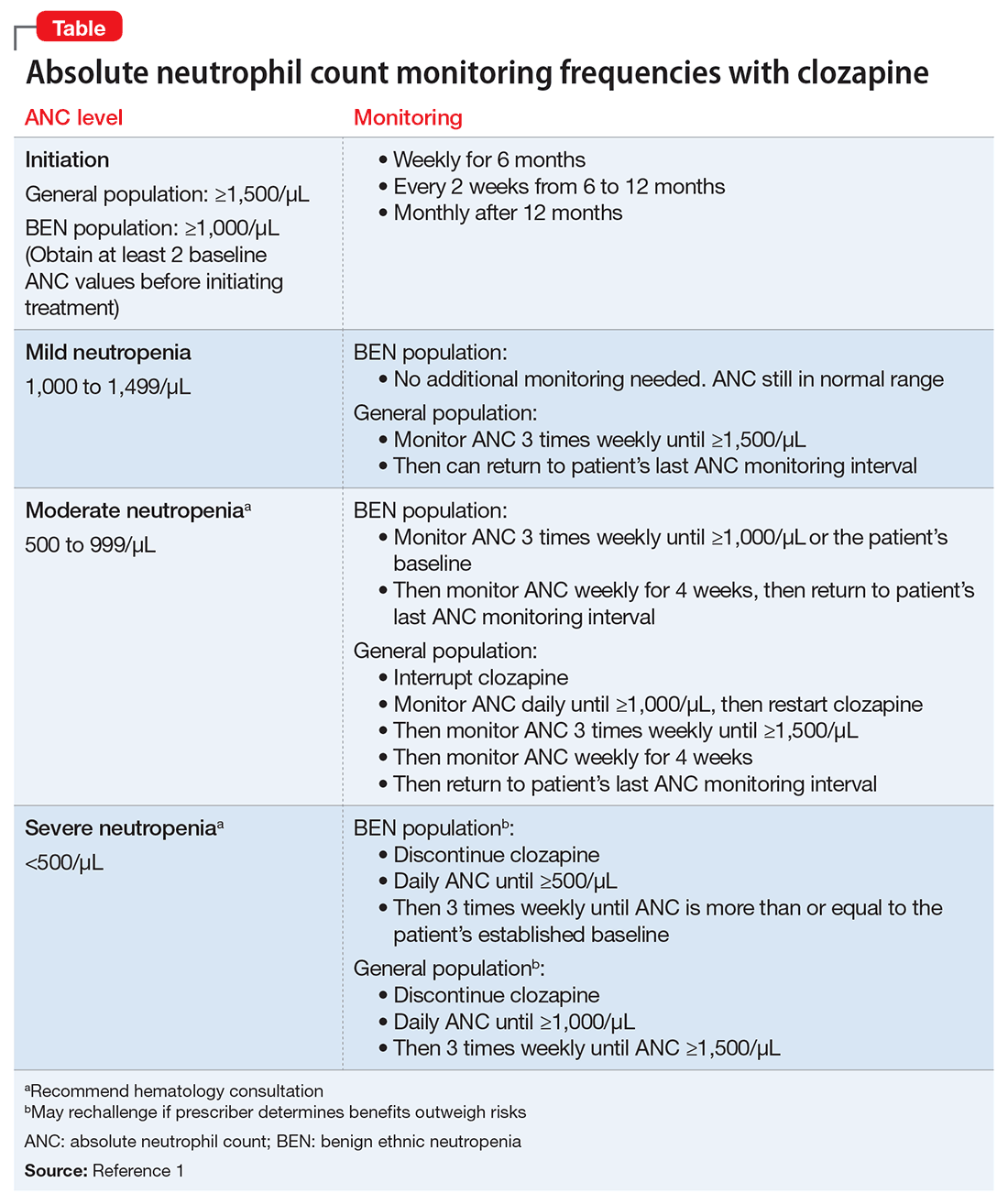
The exact mechanism of clozapine-induced neutropenia is unknown, although it is possible it stems from the drug’s effect on white blood cell precursors.2 Neutropenia typically appears within 3 months of clozapine initiation; however, delayed cases have been reported. Additionally, the risk is higher in certain patient populations (African heritage, Yemenite, West Indians, and Arab). Patients with a lower ANC at clozapine initiation and advanced age appear to be at higher risk.2
Filgrastim
The use of granulocyte-colony stimulating factor, such as filgrastim, often is viewed as a “rescue” treatment. Filgrastim’s mechanism of action is related to neutrophil production and proliferation. Several articles from the 1990s reported efficacy in the short-term management of low WBC or ANC. However, few articles, mainly case reports, have looked at long-term use of these agents. One article examined 3 patients, average age 45, who developed neutropenia during clozapine treatment.4 Filgrastim at an average dosage of 0.6 to 0.9 mg/week was used successfully. The dosage was reduced to 0.3 mg/week in 1 patient, although neutropenia returned.
Because of the lack of literature regarding long-term therapy, it is recommended to consider short-term treatment with filgrastim to normalize ANC after a severe drop in a symptomatic patient. Physicians also must consider the potential barriers to filgrastim treatment including adverse effects, such as allergic reactions, bone pain, and thrombocytopenia, and high cost.
Adjunctive lithium
Lithium could cause leukocytosis, which could balance neutropenia induced by clozapine. One of the largest studies evaluating lithium therapy with clozapine-induced neutropenia and agranulocytosis studied 25 patients taking clozapine with a previous “red result” (WBC <3,000/μL, ANC <1,500/μL, or platelets <50,000/μL).3 Lithium treatment was started before or simultaneously with the reinitiation of clozapine in most patients; the remaining patients started treatment at a later date. Only 1 of 25 patients experienced a repeat “red result.” The average lithium level was 0.54 mEq/L.
It is important to remember that initiating adjunctive lithium carries risk. Adverse effects include gastrointestinal upset, tremors, polyuria, polydipsia, and nephrotoxicity.
Additionally, there is risk that lithium simply masks the preliminary states of neutropenia leading to a more severe agranulocytosis without warning.3 Again, the mechanism of action of clozapine-induced neutropenia is thought to be related to the drug’s effect on WBC precursors. The mechanism of lithium-induced leukocytosis is unknown, therefore it’s possible that lithium will not protect a patient from clozapine-induced neutropenia or agranulocytosis, and can lead to serious adverse events.
When deciding whether to rechallenge a patient on clozapine who had a prior episode of moderate or severe neutropenia or agranulocytosis, a risk vs benefit discussion is necessary. One study found that 20 of 53 patients (38%) experienced a repeat dyscrasia when rechallenged.5 Of these patients, most experienced a lower ANC that presented faster and took longer to resolve.5 If a patient has experienced true agranulocytosis, the recommendation is to not rechallenge clozapine.
Related Resources
• Clozapine REMS Program. www.clozapinerems.com.
• Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48,49.
• Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
Drug Brand Names
Atorvastatin • Lipitor
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fligrastim • Neupogen
Lisinopril • Prinivil
Lithium • Eskalith, Lithobid
Omeprazole • Prilosec
1. Clozapine [package insert]. North Wales, PA: TEVA Pharmaceuticals USA; 2015.
2. Lundblad W, Azzam PN, Gopalan P, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;8(8):537-543.
3. Kanaan RA, Kerwin RW. Lithium and clozapine rechallenge: a retrospective case analysis. J Clin Psychiatry. 2006;67(5):756-760.
4. Hägg S, Rosenius S, Spigset O. Long-term combination treatment with clozapine and filgrastim in patients with clozapine-induced agranulocytosis. Int Clin Psychopharmacol. 2003;18(3):173-174.
5. Dunk LR, Annan LJ, Andrews CD. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry. 2006;188:255-263.
Mr. S, age 43, has schizophrenia and been stable on clozapine for 6 years after several other antipsychotic regimens failed. Mr. S also has a history of hypertension, dyslipidemia, and gastroesophageal reflux disorder. His medication regimen includes clozapine, 400 mg/d, lisinopril, 20 mg/d, atorvastatin, 40 mg/d, omeprazole, 40 mg/d, and a multivitamin. During routine blood monitoring, Mr. S shows a significant drop in absolute neutrophil count (ANC) (750/µL) (reference range, 1,500 to 8,000 µL). Mr. S , who is African American, has no history of benign ethnic neutropenia (BEN) or ANC <1,000/µL. While reviewing his chart, clinicians note that Mr. S had an ANC of 1,350/µL3 years earlier in 2013. Because a complete workup reveals no other cause for this lab abnormality, we determine that is clozapine-induced. Mr. S’s physician asks about treatment options that would allow him to stay on clozapine.
Because of clozapine’s efficacy in treatment-resistant schizophrenia, many psychiatrists aim to
Clozapine-induced neutropenia
Clozapine was approved in 1989 for managing treatment-resistant schizophrenia after demonstrating better efficacy than chlorpromazine.1 However, the adverse effects of neutropenia (white blood cell count [WBC] <3,000/μL) and agranulocytosis (ANC <500/μL3) leading to death were reported in later studies.2,3 One study in the United Kingdom and Ireland reported a prevalence of 2.9% for neutropenia and 0.8% for agranulocytosis among patients taking clozapine.3 Because of this risk, the FDA mandated WBC and ANC monitoring before initiating clozapine and periodically thereafter. In October 2015, the Risk Evaluation and Mitigation Strategies program for clozapine updated recommended ANC levels and eliminated WBC monitoring. ANC monitoring frequencies are summarized in the Table.1

The exact mechanism of clozapine-induced neutropenia is unknown, although it is possible it stems from the drug’s effect on white blood cell precursors.2 Neutropenia typically appears within 3 months of clozapine initiation; however, delayed cases have been reported. Additionally, the risk is higher in certain patient populations (African heritage, Yemenite, West Indians, and Arab). Patients with a lower ANC at clozapine initiation and advanced age appear to be at higher risk.2
Filgrastim
The use of granulocyte-colony stimulating factor, such as filgrastim, often is viewed as a “rescue” treatment. Filgrastim’s mechanism of action is related to neutrophil production and proliferation. Several articles from the 1990s reported efficacy in the short-term management of low WBC or ANC. However, few articles, mainly case reports, have looked at long-term use of these agents. One article examined 3 patients, average age 45, who developed neutropenia during clozapine treatment.4 Filgrastim at an average dosage of 0.6 to 0.9 mg/week was used successfully. The dosage was reduced to 0.3 mg/week in 1 patient, although neutropenia returned.
Because of the lack of literature regarding long-term therapy, it is recommended to consider short-term treatment with filgrastim to normalize ANC after a severe drop in a symptomatic patient. Physicians also must consider the potential barriers to filgrastim treatment including adverse effects, such as allergic reactions, bone pain, and thrombocytopenia, and high cost.
Adjunctive lithium
Lithium could cause leukocytosis, which could balance neutropenia induced by clozapine. One of the largest studies evaluating lithium therapy with clozapine-induced neutropenia and agranulocytosis studied 25 patients taking clozapine with a previous “red result” (WBC <3,000/μL, ANC <1,500/μL, or platelets <50,000/μL).3 Lithium treatment was started before or simultaneously with the reinitiation of clozapine in most patients; the remaining patients started treatment at a later date. Only 1 of 25 patients experienced a repeat “red result.” The average lithium level was 0.54 mEq/L.
It is important to remember that initiating adjunctive lithium carries risk. Adverse effects include gastrointestinal upset, tremors, polyuria, polydipsia, and nephrotoxicity.
Additionally, there is risk that lithium simply masks the preliminary states of neutropenia leading to a more severe agranulocytosis without warning.3 Again, the mechanism of action of clozapine-induced neutropenia is thought to be related to the drug’s effect on WBC precursors. The mechanism of lithium-induced leukocytosis is unknown, therefore it’s possible that lithium will not protect a patient from clozapine-induced neutropenia or agranulocytosis, and can lead to serious adverse events.
When deciding whether to rechallenge a patient on clozapine who had a prior episode of moderate or severe neutropenia or agranulocytosis, a risk vs benefit discussion is necessary. One study found that 20 of 53 patients (38%) experienced a repeat dyscrasia when rechallenged.5 Of these patients, most experienced a lower ANC that presented faster and took longer to resolve.5 If a patient has experienced true agranulocytosis, the recommendation is to not rechallenge clozapine.
Related Resources
• Clozapine REMS Program. www.clozapinerems.com.
• Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48,49.
• Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
Drug Brand Names
Atorvastatin • Lipitor
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fligrastim • Neupogen
Lisinopril • Prinivil
Lithium • Eskalith, Lithobid
Omeprazole • Prilosec
Mr. S, age 43, has schizophrenia and been stable on clozapine for 6 years after several other antipsychotic regimens failed. Mr. S also has a history of hypertension, dyslipidemia, and gastroesophageal reflux disorder. His medication regimen includes clozapine, 400 mg/d, lisinopril, 20 mg/d, atorvastatin, 40 mg/d, omeprazole, 40 mg/d, and a multivitamin. During routine blood monitoring, Mr. S shows a significant drop in absolute neutrophil count (ANC) (750/µL) (reference range, 1,500 to 8,000 µL). Mr. S , who is African American, has no history of benign ethnic neutropenia (BEN) or ANC <1,000/µL. While reviewing his chart, clinicians note that Mr. S had an ANC of 1,350/µL3 years earlier in 2013. Because a complete workup reveals no other cause for this lab abnormality, we determine that is clozapine-induced. Mr. S’s physician asks about treatment options that would allow him to stay on clozapine.
Because of clozapine’s efficacy in treatment-resistant schizophrenia, many psychiatrists aim to
Clozapine-induced neutropenia
Clozapine was approved in 1989 for managing treatment-resistant schizophrenia after demonstrating better efficacy than chlorpromazine.1 However, the adverse effects of neutropenia (white blood cell count [WBC] <3,000/μL) and agranulocytosis (ANC <500/μL3) leading to death were reported in later studies.2,3 One study in the United Kingdom and Ireland reported a prevalence of 2.9% for neutropenia and 0.8% for agranulocytosis among patients taking clozapine.3 Because of this risk, the FDA mandated WBC and ANC monitoring before initiating clozapine and periodically thereafter. In October 2015, the Risk Evaluation and Mitigation Strategies program for clozapine updated recommended ANC levels and eliminated WBC monitoring. ANC monitoring frequencies are summarized in the Table.1

The exact mechanism of clozapine-induced neutropenia is unknown, although it is possible it stems from the drug’s effect on white blood cell precursors.2 Neutropenia typically appears within 3 months of clozapine initiation; however, delayed cases have been reported. Additionally, the risk is higher in certain patient populations (African heritage, Yemenite, West Indians, and Arab). Patients with a lower ANC at clozapine initiation and advanced age appear to be at higher risk.2
Filgrastim
The use of granulocyte-colony stimulating factor, such as filgrastim, often is viewed as a “rescue” treatment. Filgrastim’s mechanism of action is related to neutrophil production and proliferation. Several articles from the 1990s reported efficacy in the short-term management of low WBC or ANC. However, few articles, mainly case reports, have looked at long-term use of these agents. One article examined 3 patients, average age 45, who developed neutropenia during clozapine treatment.4 Filgrastim at an average dosage of 0.6 to 0.9 mg/week was used successfully. The dosage was reduced to 0.3 mg/week in 1 patient, although neutropenia returned.
Because of the lack of literature regarding long-term therapy, it is recommended to consider short-term treatment with filgrastim to normalize ANC after a severe drop in a symptomatic patient. Physicians also must consider the potential barriers to filgrastim treatment including adverse effects, such as allergic reactions, bone pain, and thrombocytopenia, and high cost.
Adjunctive lithium
Lithium could cause leukocytosis, which could balance neutropenia induced by clozapine. One of the largest studies evaluating lithium therapy with clozapine-induced neutropenia and agranulocytosis studied 25 patients taking clozapine with a previous “red result” (WBC <3,000/μL, ANC <1,500/μL, or platelets <50,000/μL).3 Lithium treatment was started before or simultaneously with the reinitiation of clozapine in most patients; the remaining patients started treatment at a later date. Only 1 of 25 patients experienced a repeat “red result.” The average lithium level was 0.54 mEq/L.
It is important to remember that initiating adjunctive lithium carries risk. Adverse effects include gastrointestinal upset, tremors, polyuria, polydipsia, and nephrotoxicity.
Additionally, there is risk that lithium simply masks the preliminary states of neutropenia leading to a more severe agranulocytosis without warning.3 Again, the mechanism of action of clozapine-induced neutropenia is thought to be related to the drug’s effect on WBC precursors. The mechanism of lithium-induced leukocytosis is unknown, therefore it’s possible that lithium will not protect a patient from clozapine-induced neutropenia or agranulocytosis, and can lead to serious adverse events.
When deciding whether to rechallenge a patient on clozapine who had a prior episode of moderate or severe neutropenia or agranulocytosis, a risk vs benefit discussion is necessary. One study found that 20 of 53 patients (38%) experienced a repeat dyscrasia when rechallenged.5 Of these patients, most experienced a lower ANC that presented faster and took longer to resolve.5 If a patient has experienced true agranulocytosis, the recommendation is to not rechallenge clozapine.
Related Resources
• Clozapine REMS Program. www.clozapinerems.com.
• Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48,49.
• Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
Drug Brand Names
Atorvastatin • Lipitor
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fligrastim • Neupogen
Lisinopril • Prinivil
Lithium • Eskalith, Lithobid
Omeprazole • Prilosec
1. Clozapine [package insert]. North Wales, PA: TEVA Pharmaceuticals USA; 2015.
2. Lundblad W, Azzam PN, Gopalan P, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;8(8):537-543.
3. Kanaan RA, Kerwin RW. Lithium and clozapine rechallenge: a retrospective case analysis. J Clin Psychiatry. 2006;67(5):756-760.
4. Hägg S, Rosenius S, Spigset O. Long-term combination treatment with clozapine and filgrastim in patients with clozapine-induced agranulocytosis. Int Clin Psychopharmacol. 2003;18(3):173-174.
5. Dunk LR, Annan LJ, Andrews CD. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry. 2006;188:255-263.
1. Clozapine [package insert]. North Wales, PA: TEVA Pharmaceuticals USA; 2015.
2. Lundblad W, Azzam PN, Gopalan P, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;8(8):537-543.
3. Kanaan RA, Kerwin RW. Lithium and clozapine rechallenge: a retrospective case analysis. J Clin Psychiatry. 2006;67(5):756-760.
4. Hägg S, Rosenius S, Spigset O. Long-term combination treatment with clozapine and filgrastim in patients with clozapine-induced agranulocytosis. Int Clin Psychopharmacol. 2003;18(3):173-174.
5. Dunk LR, Annan LJ, Andrews CD. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry. 2006;188:255-263.
Suicidal and asking for money for food
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
CASE Suicidal and hungry
Mr. L, age 59, attempts suicide by taking approximately 20 acetaminophen tablets of unknown dosage. He immediately comes to the emergency department where blood work reveals a 4-hour acetaminophen level of 94.8 μg/mL (therapeutic range, 10 to 30 μg/mL; toxic range, >150 μg/mL); administration of N-acetylcysteine is unnecessary. Mr. L is admitted to general medical services for monitoring and is transferred to our unit for psychiatric evaluation and management.
During our initial interview, Mr. L, who has a developmental disability, is grossly oriented and generally cooperative, reporting depressed mood with an irritable affect. He is preoccupied with having limited funds and repeatedly states he is worried that he can’t buy food, but says that the hospital could help by providing for him. Mr. L states that his depressed mood is directly related to his financial situation and, that if he had more money, he would not be suicidal. He cites worsening visual impairment that requires surgery as an additional stressor.
On several occasions, Mr. L states that the only way to help him is to give him $600 so that he can buy food and pay for medical treatment. Mr. L says he does not feel supported by his family, despite having a sister who lives nearby.
What would you include in the differential diagnosis for Mr. L?
a) major depressive disorder (MDD)
b) depression secondary to a medical condition
c) neurocognitive disorder
d) adjustment disorder with depressive features
e) factitious disorder
The authors’ observations
Our differential diagnosis included MDD, adjustment disorder, neurocognitive disorder, and factitious disorder. He did not meet criteria for MDD because he did not have excessive guilt, loss of interest, change in sleep or appetite, psychomotor dysregulation, or change in energy level. Although suicidal behavior could indicate MDD, the fact that he immediately walked to the hospital after taking an excessive amount of acetaminophen suggests that he did not want to die. Further, he attributed his suicidal thoughts to environmental stressors. Similarly, we ruled out adjustment disorder because he had no reported or observed changes in mood or anxiety. Although financial difficulties might have overwhelmed his limited coping abilities, he took too much acetaminophen to ensure that he was hospitalized. His motivation for seeking hospitalization ruled out factitious disorder. Mr. L has a developmental disability, but information obtained from collateral sources ruled out an acute change to cognitive functioning.
HISTORY Repeated admissions
Mr. L has a history of a psychiatric hospitalization 3 weeks prior to this admission. He presented to an emergency department stating that his blood glucose was low. Mr. L was noted to be confused and anxious and said he was convinced he was going to die. At that time, his thought content was hyper-religious and he claimed he could hear the devil. Mr. L was hospitalized and started on low-dosage risperidone. At discharge, he declined referral for outpatient mental health treatment because he denied having a mental illness. However, he was amenable to follow up at a wellness clinic.
Mr. L has worked at a local supermarket for 19 years and has lived independently throughout his adult life. After he returned to the community, he was repeatedly absent from work, which further exacerbated his financial strain. He attended a follow-up outpatient appointment but reported, “They didn’t help me,” although it was unclear what he meant.
Between admissions to our hospital, Mr. L had 2 visits to an emergency department, the first time saying he felt depressed and the second reporting he attempted suicide by taking 5 acetaminophen tablets. On both occasions he requested placement in a residential facility but was discharged home after an initial assessment. Emergency room records indicated that Mr. L stated, “If you cannot give me money for food, then there is no use and I would rather die.”
What is the most likely DSM-5 diagnosis for Mr. L?
a) schizophrenia
b) malingering
c) brief psychotic disorder
d) dependent personality disorder
The authors’ observations
Malingering in DSM-5 is defined as the “intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives.”1 These external incentives include financial compensation, avoiding military duties, evading criminal charges, and avoiding work, and are collectively considered as secondary gain. Although not considered a diagnosis in the strictest sense, clinicians must differentiate malingering from other psychiatric disorders. In the literature, case reports describe patients who feigned an array of symptoms including those of posttraumatic stress disorder, paraphilias, cognitive dysfunction, depression, anxiety, and psychosis.2-5
In Mr. L’s case, malingering presented as suicidal behavior with an inadvertently high fatality risk. Notably, Mr. L came to an emergency room a few days before this admission after swallowing 5 acetaminophen tablets in a suicide attempt, which did not lead to a medical or psychiatric hospitalization. In an attempt to ensure admission, Mr. L then took a potentially lethal dose of 20 acetaminophen tablets. In our assessment and according to his statements, the primary motivation for the suicide attempt was to obtain reliable food and housing. Mr. L’s developmental disability might have contributed to a relative lack of understanding of the consequences of his actions. In addition, poor overall communication and coping skills led to an exaggerated response to psychosocial stressors.
Malingering and suicide attempts
Few studies have investigated malingering in regards to suicide and other psychiatric emergencies. In a study of 227 consecutive psychiatric emergencies assessed for evidence of malingering, 13% were thought to be feigned or exaggerated.6 Interestingly, the most commonly reported secondary gain was food and shelter, similar to Mr. L. This study did not report the types of psychiatric emergencies, therefore suicidal actions associated with malingering could not be evaluated.
In another study, 40 patients hospitalized for suicidal ideation (n = 29, 72%) or suicidal gestures (n = 11, 28%) in a large, urban tertiary care center were evaluated for malingering by anonymous report of feigned or exaggerated symptoms.7 Most of these patients were diagnosed with a mood disorder (28%) and/or an adjustment disorder (53%). Four (10%) admitted to malingering. Among the malingerers, reasons for feigning illness included:
- wanting to be hospitalized
- wanting to make someone angry or feel sorry
- gaining access to detoxification programs
- getting treatment for emotional problems.
Interestingly, an analysis of demographic factors associated with malingering reveals an association with suicide attempts but not persistent suicidal ideations. This could be because of selection bias; patients who reported a suicide attempt might be more likely to be hospitalized.
A follow-up study8 evaluated 50 additional consecutive psychiatric inpatients admitted to the same tertiary care hospital for suicide risk. Unlike the previous study, a larger proportion of these patients had made a suicide attempt (n = 21, 42%) and a greater number had made a previous suicide attempt (n = 33, 66%). Primary mood disorders comprised most of the psychiatric diagnoses (n = 28, 56%). In this study, the exact nature of the suicide gestures was not documented, leaving open the question of lethality of the attempts. These studies do not suggest that those who malinger are not at risk for suicide, only that these patients tend to exaggerate the severity of their ideations or behaviors.
OUTCOME Reluctantly discharged
We contact Mr. L’s siblings, who offer to provide temporary housing and financial support and assist him with medical needs. This abated Mr. L’s suicidal ideation; however, he wishes to remain in the hospital with the goals of obtaining eyeglasses and dentures. We explain that psychiatric hospitalization is no longer indicated and he is discharged.
Which of the following is the most effective management strategy for malingering?
a) direct confrontation of the malingering patient
b) immediate discharge once malingering is identified
c) evaluation for possible comorbid psychiatric conditions
d) neuropsychiatric consultation
The authors’ observations
The challenges of treating patients who malinger include clinician uncertainty in making the diagnosis and high variability in occurrence across settings (Table 1). Current estimates indicate that 4% to 8% of medical and psychiatric cases not involved in litigation or compensation have an element of feigned symptoms.3,9 The rate could be higher in specific circumstances such as medicolegal disputes and criminal cases.10
The societal impact of malingering is significant. Therefore, identifying these patients is an important clinical intervention that can have a wide impact.11 However, it is also important to acknowledge that genuine psychiatric illness could be comorbid with malingering. Although differentiating a patient’s true from feigned symptoms can be difficult, it is critical to carefully evaluate the patient in order to provide the best treatment.
It seems that physicians can detect malingering, but documentation often is not provided. In the Rissmiller et al study,7 all 4 cases of malingering were identified retrospectively by study psychiatrists; however, none of their medical records included documentation of malingering, a finding also reported in the Yates et al study.6 Also concerning, the clinicians suspected malingering in some patients who were not feigning symptoms, suggesting that a relatively high threshold is necessary for making the diagnosis.
How to help patients who malinger
Identifying malingering in patients with obvious secondary gain is important to prevent exposure to potential adverse effects of medication and unnecessary use of medical resources. In addition, obtaining collateral information, records from previous admissions or outpatient treatment, and psychological testing adds to the body of evidence suggesting malingering. We also recommend a comprehensive psychosocial evaluation to identify the presence of secondary gain.
Management of malingering (Table 2) includes building a strong therapeutic alliance, exploring reasons for feigning symptoms, open discussion of inciting external factors such as interpersonal conflict or difficulties at work, and/or confrontation.10 In addition, supportive psychotherapy might help strengthen coping mechanisms and problem solving strategies, thereby removing the need for secondary gain.12 Additionally, face-saving mechanisms that allow the patient to discard their feigned symptoms, or enable the person to alter his (her) history, could be to his benefit. Lastly, and importantly, clinicians should focus efforts on ruling out or effectively treating comorbid psychiatric conditions.
From a risk management standpoint, include all available data to support the malingering diagnosis in your progress notes and discharge summaries. A clinician seeking to discharge a patient suspected of malingering who is still endorsing suicidal or homicidal intent will benefit from administrative review, including legal counsel to mitigate risk, and be more confident discharging somebody assessed to be malingering.
We recognize that certain patients could trigger countertransference reactions that impel clinicians to take on a significant caretaking role. Patients skillful at deception could manifest a desire to rescue or save them. In these instances, clinicians should examine why and how these feelings have come about, particularly if there is evidence that the individual could be attempting to use the interaction to achieve secondary gain. Awareness of these feelings could help with the diagnostic formulation. Moreover, a clinician who has such strong feelings might be tempted to abet a patient in achieving the secondary gain, or protect him (her) from the natural consequences of individual’s deception (eg, not discharging a hospitalized patient). This is counter-therapeutic and reinforces maladaptive behaviors and coping processes.13
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington DC: American Psychiatric Association; 2013.
2. Fedoroff JP, Hanson A, McGuire M, et al. Simulated paraphilias: a preliminary study of patients who imitate or exaggerate paraphilic symptoms and behaviors. J Forensic Sci. 1992;37(3):902-911.
3. Mittenberg W, Patton C, Canyock EM, et al. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002;24(8):1094-1102.
4. Waite S, Geddes A. Malingered psychosis leading to involuntary psychiatric hospitalization. Australas Psychiatry. 2006;14(4):419-421.
5. Hall RC, Hall RC. Malingering of PTSD: forensic and diagnostic consideration, characteristics of malingerers and clinical presentations. Gen Hosp Psychiatry. 2006;28(6):525-535.
6. Yates BD, Nordquist CR, Shultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
7. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicidal ideators and attempters. Crisis. 1998;19(2):62-66.
8. Rissmiller D, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric inpatients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
9. Sullivan K, Lange RT, Dawes S. Methods of detecting malingering and estimated symptom exaggeration base rates in Australia. Journal of Forensic Neuropsychology. 2007;4(4):49-70.
10. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
11. Chafetz M, Underhill J. Estimated costs of malingered disability. Arch Clin Neuropsychol. 2013;28(7):633-639.
12. Peebles R, Sabel
13. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
We can work it out: Should I hire my patient?
Dear Dr. Mossman,
Each month, I see my patient, Mr. R, for a 15-minute medication management appointment. At his latest visit, Mr. R mentioned his financial difficulties. He also observed that our office needed to have some carpentry work done—not a surprise, because he’s known in our area as one of the best carpenters around. He suggested that I hire him as payment for the next 6 appointments. What risks might I encounter if I oblige him?
Submitted by “Dr. Z”
Nearly 29 million Americans are uninsured,1 and even more have trouble accessing mental health care.2 Many psychiatrists struggle to provide affordable services while remaining financially viable.3,4 For outpatients with limited means to pay for care, spacing appointments to fit their budgets might compromise treatment.5 Simply not charging patients poses its own clinical and ethical challenges.6-8
As a result, some mental health professionals make barter arrangements to help their patients enter or continue treatment. To answer Dr. Z’s question on whether exchanging services might be a way to arrange matters with some patients, we explore:
- the idea of bartering for psychiatric treatment
- related ethical and legal considerations
- when and in what situations bartering might be appropriate.
Think of what I’m saying: Bartering for treatment
“Barter” refers to exchanging commodities, products, or services of equivalent value without using money.9 In 2010, Nevada Republican Senate candidate Sue Lowden encouraged barter for health care and harkened back to an earlier time where “they would bring a chicken to the doctor; they would say ‘I’ll paint your house.’”10
Such payment arrangements have been encouraged as health care has become increasingly commoditized.11-13 This happens through both direct barter between physician and patient and barter exchanges. Barter exchange systems have been set up on Web sites (as of 2013, at least 400 such online exchanges were available14), local communities,11,15 and social programs. For example, through the “Swapping Guns for Therapy” program, psychologists in California gave free or reduced-fee care for people who traded in their guns.16
Try to see it my way: A prevailing view of barter
Several psychiatrists recommend against bartering for treatment, for a variety of reasons.7,8,17-19 Simon18 argues that a stable fee policy is part of a proper therapeutic framework, and money is “the only acceptable medium of exchange when receiving payment from patients.” Emotional distress and the power differential inherent in treatment might prevent a patient from making an accurate assessment of the value of the bartered goods or services,7,8,17,18,20 which could lead to future claims of undue influence from trading goods or services below market value.17 To avoid the possibility of exploitating the patient, Simon18 recommends that the psychiatrist’s professional fee be “the only material benefit received from the patient.”
The American Psychiatric Association’s code of ethics states that “it is not ethical to switch a doctor–patient relationship to an employer–employee one … and, in most cases, such an arrangement would be unethical.”21 In some therapeutic settings, employing a patient risks inappropriate self-disclosure and intrusion.16
More than other physicians, psychiatrists pay special attention to professional boundaries, the technical term for the “edge of appropriate behavior,” within which safe, effective care can occur.22,23 Although some boundary crossings can be harmless and even constructive, repeated boundary crossings are the forerunners to improper behavior, including sexual relationships with patients.24-26
Out of concern that bartering could become the first step down a slippery ethical slope toward patient exploitation, mental health clinicians have deemed the practice “ethically troubling,”19 said it did “not usually work out well,”7 and declared it “so fraught with risks for both parties that it seem[ed] illogical to even consider it as an option.”27
While I see it your way: What barter proponents say
Reports of bartering for chickens28 and purchasing fuel from a patient in remote Alaska29 show that not all physicians agree and why they feel that professional codes of ethics reflect an urban bias.28,29 In many rural areas and small towns, access to mental health services is limited, and patients often interact with their doctors outside of clinical encounters.23,29-31
Bartering can benefit a physician’s practice by:
- reducing the need to discount services
- eliminating bureaucratic burdens of traditional insurance arrangements
- facilitating development of a patient base
- allowing patients choice and flexibility in seeking medical care.6,16,32
Bartering could confer certain clinical benefits, such as:
- enhancing trust and empathy32
- encouraging patients to make their needs known constructively6
- modeling financial self-care6
- helping the doctor to feel fairly compensated for providing thoughtful care6
- acknowledging the patient’s cultural values15,33
- affirming that patients and doctors both produce things of value.16
I have always thought: Other ethical models
An ethical approach to bartering that requires careful thought and respect for the patient’s needs appears consistent with a primary goal of treatment: “to increase the capacity of individuals to make more rational choices in their lives and to be relatively freer from disabling conflicts.”20 Some authors criticize slippery-slope arguments and strict-rule ethical approaches as being too rigid, limiting, or risk-averse.22,26,34 In Table 1,6,8,16,18,27,29-31,35-37 we list several factors that might weigh for or against a decision to enter into a barter arrangement as payment for care.
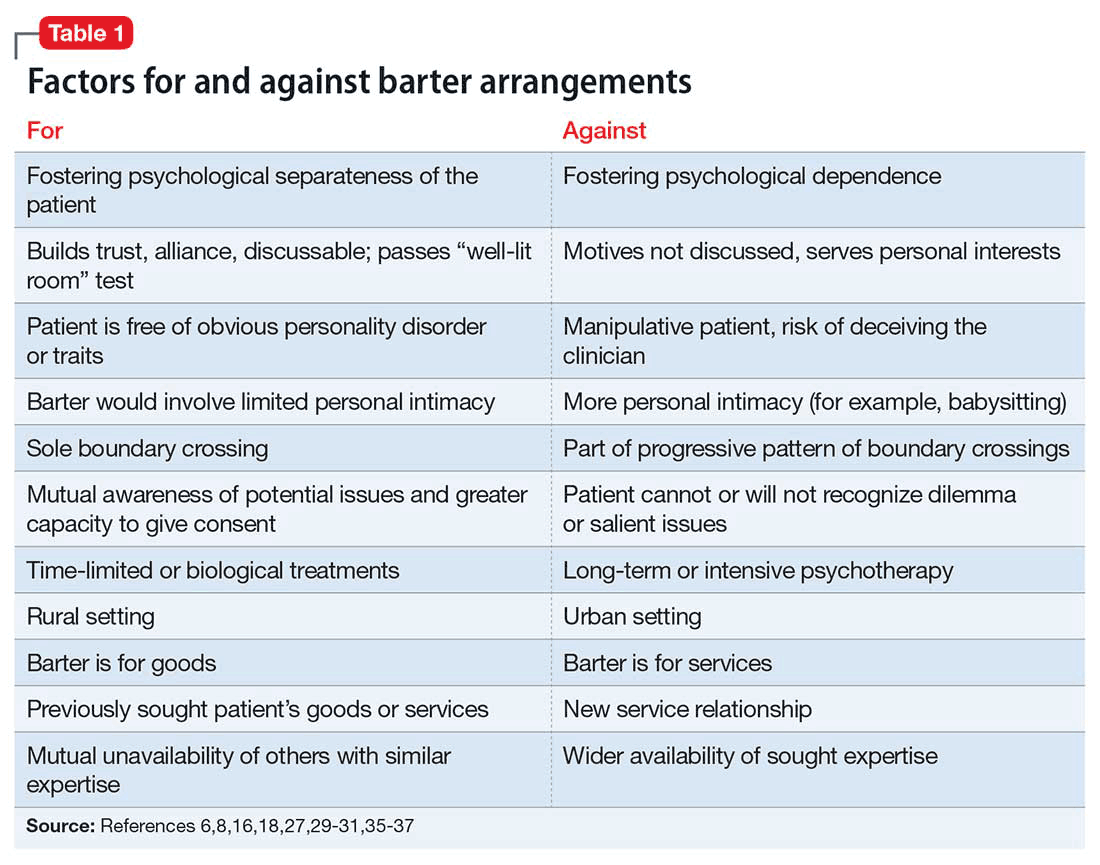
In a similar manner, Martinez33,38 proposed a graded-risk framework that encourages examination of potential harms and benefits of a decision, potential coercive or exploitative elements, the clinician’s intentions and aspiration to professional ideals, and the context of the decision. Within this framework, some bartering arrangements might be encouraged and, perhaps, even obligatory because of the potential benefits to the patient; other arrangements (eg, trading psychotherapy for menial services) might be unjustifiable. Martinez38 argues that this approach fosters mutual decision-making with patients, discourages physician paternalism, and “demands that we struggle with the particulars with each case.”
Gottlieb’s decision-making model35 recognizes that trying to avoid all dual relationships is unrealistic and not all dual relationships are exploitative. Instead, a clinician must assess 3 dimensions of current and proposed relationships:
- the degree of power differential
- the duration of treatment
- the clarity of termination.
The decision-making process also requires involvement of the patient, who if “unable to recognize the dilemma or is unwilling to consider the issues before deciding, should be considered at risk, and the contemplated relationship rejected.”35
So I will ask you once again: Dr. Z’s decision
In the case of Dr. Z and Mr. R, a barter arrangement might work in the sense of permitting and sustaining good care. Mr. R suggested the idea and might not be able to afford care without it. Nothing in Dr. Z’s description suggests that Mr. R has personality characteristics or other conditions that would compromise his ability to give informed consent or to understand the nuances of a barter arrangement. Dr. Z is not providing a treatment (eg, psychodynamic therapy) that a barter arrangement could contaminate. That the arrangement would be circumscribed limits the effect of a power differential, as would its brief duration and defined termination endpoint. Dr. Z’s letter to the authors also shows his willingness to seek consultation.
There’s a chance that we may fall apart: Reasons for caution
Martinez’s graded-risk approach recognizes reasons for caution:
- the risk of harm to the patient or doctor–patient relationship
- the uncertain benefit to the patient
- the blurring of Dr. Z’s self-interest and Mr. R’s needs
- some ambiguity about possible exploitation.
Dr. Z and Mr. R have not discussed the value of Mr. R’s work—which might create a rift between them—and despite Mr. R’s reputation, other carpenters are available. Future med-check appointments will give them little time to explore and discuss the meanings of the barter.
Any proposed barter arrangement creates some clinical perils that can be particularly salient in mental health treatment. Patients could view themselves as “special” or entitled to enhanced access to the doctor because of exchanged services, which could take a toll on the doctor.39 The physician’s objectivity might diminish, and the business aspect of their relationship could make both parties less comfortable when discussing sensitive information relevant to treatment.31,40 Also, the suggested barter is for services to be provided at Dr. Z’s office, where confidentiality may be breeched and transference issues could arise.
A medical malpractice claim states that a doctor has breached a duty of care to a patient such that harm (or “damages”) resulted.41 Should Dr. Z and Mr. R’s barter agreement turn sour and harm follow, Mr. R could sue for recovery of damages based of a claim of duress, undue influence, or other aspects of the doctor–patient power differential.27,42,43 Given the published views we have described, a psychiatrist who barters also may be viewed as violating state regulations that measure the standard of care against generally accepted practice.
Only time will tell if I am right or I am wrong
If you face a situation similar to Dr. Z’s and want to consider a barter arrangement, you can take several steps to mitigate potential risk to your patient and ensure competent care (Table 25,6,15,16,32,35,39,40,44-47). One of the most important steps is to seek ongoing consultation, both before and after a decision to barter. Ideally, the consulting colleague would know you and your circumstances and would have sufficient clinical grasp of the patient to make an informed assessment of risks and benefits.35 This consultation, as well as your own rationale for acting on recommendations, should be thoroughly documented in the patient’s records.26,44,45
Certain types of barter should be off limits, including:
- trading prescription drugs for goods or services
- trading for services that tie into the success of one’s business (eg, business advising or marketing)16
- offering treatment in exchange for illegal or ethically unacceptable services.48
Beyond ethical considerations are some practical issues. The Internal Revenue Service has specific rules regarding taxation of bartered goods and services, which must be included as taxable income.46 If possible, an independent agent should appraise the traded goods or services before the agreement.6 When working in a group practice, the clinician might have to figure out how to allocate the received goods or services such as shared overhead costs.28 Preferably, the patient’s goods or services should be provided before care is delivered.16 If not, the duration of services rendered should be limited, and either party should have the option to disengage from the relationship if one feels dissatisfied.16
A written contract, discussed ahead of time, can be a sound way to summarize the terms of the arrangement. Both sides also should consider what would happen if an injury occurred.16 Finally, you must adhere to any relevant state laws regarding payment for services, particularly if the patient has health insurance.32
If the bartering arrangement does not work, you should take an open and non-defensive approach. If you believe you have made a mistake, consider apologizing.45
1. Kaiser Commission on Medicaid and the Uninsured. Key facts about the uninsured population. http://kff.org/uninsured/fact-sheet/key-facts-about-the-uninsured-population. Published September 29, 2016. Accessed October 7, 2016.
2. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed October 7, 2016.
3. Insel T. Director’s blog: the paradox of parity. May 30, 2014. https://www.nimh.nih.gov/about/director/2014/the-paradox-of-parity.shtml. Published May 30, 2014. Accessed October 7, 2016.
4. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
5. What do you do when patients cannot pay? Psychiatry (Edgmont). 2009;6(5):51-52.
6. Hill M. Barter: ethical considerations in psychotherapy. Women Ther. 2000;22(3):81-91.
7. Simon RI. Commentary: treatment boundaries—flexible guidelines, not rigid standards. J Am Acad Psychiatry Law. 2001;29(3):287-289.
8. Simon RI, Williams IC. Maintaining treatment boundaries in small communities and rural areas. Psychiatr Serv. 1999;50(11):1440-1446.
9. Compact edition of the Oxford English dictionary. New York, NY: Oxford University Press; 1971:171.
10. Coolican JP. Sue Lowden stands by health care plan. Las Vegas Sun. http://lasvegassun.com/news/2010/apr/20/sue-lowden-draws-fire-repeating-health-care-barter. Published April 20, 2010. Accessed September 20, 2016.
11. Consumer Reports. Barter sometimes allow patients to pay for health care they otherwise could not afford. Washington Post. https://www.washingtonpost.com/national/health-science/barter-sometimes-allow-patients-to-pay-for-health-care-they-otherwise-could-not-afford/2013/09/30/e7e5a55e-069d-11e3-88d6-d5795fab4637_story.html. Published September 20, 2013. Accessed September 27, 2016.
12. Ellis B. MediBid auction site lets doctors bid for patients. CNN Money. http://money.cnn.com/2014/01/09/pf/insurance/medibid. Published January 9, 2014. Accessed September 23, 2016.
13. Ambrosino B. Surgery for sale: the ethics of health care bartering in a social media marketplace. http://hub.jhu.edu/2014/01/16/hopkins-ethicist-ponders-medibid. Published January 16, 2014. Accessed September 23, 2016.
14. Thomas C. When patients barter for health care. https://ethicalnag.org/2013/07/30/barter. Published July 30, 2016. Accessed October 9, 2016.
15. Syme G. Fetters or freedom: dual relationships in counselling. Int J Adv Counselling. 2006;28(1):57-69.
16. Zur O. Bartering in psychotherapy and counselling: complexities, case studies and guidelines. New Therapist. 2008;58:18-26.
17. Simon RI. The psychiatrist as a fiduciary: avoiding the double agent role. Psychiatric Annals. 1987;17(9):622-626.
18. Simon RI. Treatment boundary violations: clinical, ethical, and legal considerations. Bull Am Acad Psychiatry Law. 1992;20(3):269-288.
19. Walker R, Clark JJ. Heading off boundary problems: clinical supervision as risk management. Psychiatr Serv. 1999;50(11):1435-1439.
20. Malmquist CP, Norman MT. Psychiatrist-patient boundary issues following treatment termination. Am J Psychiatry. 2001;158(7):1010-1018.
21. American Psychiatric Association. The opinions of the ethics committee on the principles of medical ethics, with annotations especially applicable to psychiatry. https://www.psychiatry.org/psychiatrists/practice/ethics. Published 2016. Accessed October 4, 2016.
22. Gutheil TG, Gabbard GO. Misuses and misunderstandings of boundary theory in clinical and regulatory settings. Am J Psychiatry. 1998;155(3):409-414.
23. Crowden A. Professional boundaries and the ethics of dual and multiple overlapping relationships in psychotherapy. Monash Bioeth Rev. 2008;27(4):10-27.
24. Gabbard GO. Commentary: boundaries, culture, and psychotherapy. J Am Acad Psychiatry Law. 2001;29(3):284-286.
25. Kroll J. Boundary violations: a culture-bound syndrome. J Am Acad Psychiatry Law. 2001;29(3):274-283.
26. Gottlieb MC, Younggren JN. Is there a slippery slope? Considerations regarding multiple relationships and risk management. Professional Psychology: Research and Practice. 2009;40(6):564-557.
27. Woody RH. Bartering for psychological services. Professional Psychology: Research and Practice. 1998;29(2):174-178.
28. Bartering for medical care. MGMA Connex. 2008;8(6):11.
29. Roberts LW, Battaglia J, Epstein RS. Frontier ethics: mental health care needs and ethical dilemmas in rural communities. Psychiatr Serv. 1994;50(4):497-503.
30. Endacott R, Wood A, Judd F, et al. Impact and management of dual relationships in metropolitan, regional and rural mental health practice. Aust N Z J Psychiatry. 2006;40(11-12):987-994.
31. Scopelliti J, Judd F, Grigg M, et al. Dual relationships in mental health practice: issues for clinicians in rural settings. Aust N Z J Psychiatry. 2004;38(11-12):953-959.
32. Ayers AA. Bartering basics for the urgent care operator. http://www.alanayersurgentcare.com/Linked_Files/2013_Articles/Ayers_UCAOA_Bartering_%20Basics_2012_01_09.pdf. Accessed September 23, 2016.
33. Savin D, Martinez R. Cross-cultural boundary dilemmas: a graded-risk assessment approach. Transcult Psychiatry. 2006;42(2):243-258.
34. Glass LL. The gray areas of boundary crossings and violations. Am J Psychother. 2003;57(4):429-444.
35. Gottlieb MC. Avoiding exploitive dual relationships: a decision-making model. Psychotherapy (Chic). 1993;30(1):41-48.
36. Lane JA. The ethical implications of bartering for mental health services: examining interdisciplinary ethical standards. http://pdxscholar.library.pdx.edu/coun_fac/36. Published 2012. Accessed October 17, 2016.
37. Miller RD, Maier GJ. Nonsexual boundary violations: sauce for the gander. J Psychiatry Law. 2002;30(3):309-329.
38. Martinez R. A model for boundary dilemmas: ethical decision-making in the patient-professional relationship. Ethical Hum Sci Serv. 2000;2(1):43-61.
39. Salmon K, Klijnsma M. Boundary issues: employing patients as staff? Br J Gen Pract. 2009;59(558):56-57.
40. College of Physicians and Surgeons Ontario. Hiring patients may compromise physician-patient relationship. Dialogue. 2015;3:47.
41. Bal S. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467(2):339-347.
42. What puts a psychiatrist at risk for a malpractice lawsuit? Psychiatry (Edgmont). 2009;6(8):38-39.
43. Geis v Landau, 117 Misc2d 396 (NY Misc 1983).
44. Nisselle P. Danger zone. When boundaries are crossed in the doctor-patient relationship. Aust Fam Physician. 2000;29(6):541-544.
45. Pope KS, Keith-Spiegel P. A practical approach to boundaries in psychotherapy: making decisions, bypassing blunders, and mending fences. J Clin Psychol. 2008;64(5):638-652.
46. IRS Publication 17. https://www.irs.gov/publications/p17/ch12.html. Published 2015. Accessed October 5, 2016.
47. Epstein RS, Simon RI. The exploitation index: an early warning indicator of boundary violations in psychotherapy. Bull Menninger Clin. 1990;54(4):450-465.
48. Skutch J. Savannah doctor accused of trading drugs for sex with strippers. Augusta Chronicle. http://chronicle.augusta.com/news/crime-courts/2013-01-31/savannah-doctor-accused-trading-drugs-sex-strippers. Published January 31, 2013. Accessed October 16, 2016.
Dear Dr. Mossman,
Each month, I see my patient, Mr. R, for a 15-minute medication management appointment. At his latest visit, Mr. R mentioned his financial difficulties. He also observed that our office needed to have some carpentry work done—not a surprise, because he’s known in our area as one of the best carpenters around. He suggested that I hire him as payment for the next 6 appointments. What risks might I encounter if I oblige him?
Submitted by “Dr. Z”
Nearly 29 million Americans are uninsured,1 and even more have trouble accessing mental health care.2 Many psychiatrists struggle to provide affordable services while remaining financially viable.3,4 For outpatients with limited means to pay for care, spacing appointments to fit their budgets might compromise treatment.5 Simply not charging patients poses its own clinical and ethical challenges.6-8
As a result, some mental health professionals make barter arrangements to help their patients enter or continue treatment. To answer Dr. Z’s question on whether exchanging services might be a way to arrange matters with some patients, we explore:
- the idea of bartering for psychiatric treatment
- related ethical and legal considerations
- when and in what situations bartering might be appropriate.
Think of what I’m saying: Bartering for treatment
“Barter” refers to exchanging commodities, products, or services of equivalent value without using money.9 In 2010, Nevada Republican Senate candidate Sue Lowden encouraged barter for health care and harkened back to an earlier time where “they would bring a chicken to the doctor; they would say ‘I’ll paint your house.’”10
Such payment arrangements have been encouraged as health care has become increasingly commoditized.11-13 This happens through both direct barter between physician and patient and barter exchanges. Barter exchange systems have been set up on Web sites (as of 2013, at least 400 such online exchanges were available14), local communities,11,15 and social programs. For example, through the “Swapping Guns for Therapy” program, psychologists in California gave free or reduced-fee care for people who traded in their guns.16
Try to see it my way: A prevailing view of barter
Several psychiatrists recommend against bartering for treatment, for a variety of reasons.7,8,17-19 Simon18 argues that a stable fee policy is part of a proper therapeutic framework, and money is “the only acceptable medium of exchange when receiving payment from patients.” Emotional distress and the power differential inherent in treatment might prevent a patient from making an accurate assessment of the value of the bartered goods or services,7,8,17,18,20 which could lead to future claims of undue influence from trading goods or services below market value.17 To avoid the possibility of exploitating the patient, Simon18 recommends that the psychiatrist’s professional fee be “the only material benefit received from the patient.”
The American Psychiatric Association’s code of ethics states that “it is not ethical to switch a doctor–patient relationship to an employer–employee one … and, in most cases, such an arrangement would be unethical.”21 In some therapeutic settings, employing a patient risks inappropriate self-disclosure and intrusion.16
More than other physicians, psychiatrists pay special attention to professional boundaries, the technical term for the “edge of appropriate behavior,” within which safe, effective care can occur.22,23 Although some boundary crossings can be harmless and even constructive, repeated boundary crossings are the forerunners to improper behavior, including sexual relationships with patients.24-26
Out of concern that bartering could become the first step down a slippery ethical slope toward patient exploitation, mental health clinicians have deemed the practice “ethically troubling,”19 said it did “not usually work out well,”7 and declared it “so fraught with risks for both parties that it seem[ed] illogical to even consider it as an option.”27
While I see it your way: What barter proponents say
Reports of bartering for chickens28 and purchasing fuel from a patient in remote Alaska29 show that not all physicians agree and why they feel that professional codes of ethics reflect an urban bias.28,29 In many rural areas and small towns, access to mental health services is limited, and patients often interact with their doctors outside of clinical encounters.23,29-31
Bartering can benefit a physician’s practice by:
- reducing the need to discount services
- eliminating bureaucratic burdens of traditional insurance arrangements
- facilitating development of a patient base
- allowing patients choice and flexibility in seeking medical care.6,16,32
Bartering could confer certain clinical benefits, such as:
- enhancing trust and empathy32
- encouraging patients to make their needs known constructively6
- modeling financial self-care6
- helping the doctor to feel fairly compensated for providing thoughtful care6
- acknowledging the patient’s cultural values15,33
- affirming that patients and doctors both produce things of value.16
I have always thought: Other ethical models
An ethical approach to bartering that requires careful thought and respect for the patient’s needs appears consistent with a primary goal of treatment: “to increase the capacity of individuals to make more rational choices in their lives and to be relatively freer from disabling conflicts.”20 Some authors criticize slippery-slope arguments and strict-rule ethical approaches as being too rigid, limiting, or risk-averse.22,26,34 In Table 1,6,8,16,18,27,29-31,35-37 we list several factors that might weigh for or against a decision to enter into a barter arrangement as payment for care.

In a similar manner, Martinez33,38 proposed a graded-risk framework that encourages examination of potential harms and benefits of a decision, potential coercive or exploitative elements, the clinician’s intentions and aspiration to professional ideals, and the context of the decision. Within this framework, some bartering arrangements might be encouraged and, perhaps, even obligatory because of the potential benefits to the patient; other arrangements (eg, trading psychotherapy for menial services) might be unjustifiable. Martinez38 argues that this approach fosters mutual decision-making with patients, discourages physician paternalism, and “demands that we struggle with the particulars with each case.”
Gottlieb’s decision-making model35 recognizes that trying to avoid all dual relationships is unrealistic and not all dual relationships are exploitative. Instead, a clinician must assess 3 dimensions of current and proposed relationships:
- the degree of power differential
- the duration of treatment
- the clarity of termination.
The decision-making process also requires involvement of the patient, who if “unable to recognize the dilemma or is unwilling to consider the issues before deciding, should be considered at risk, and the contemplated relationship rejected.”35
So I will ask you once again: Dr. Z’s decision
In the case of Dr. Z and Mr. R, a barter arrangement might work in the sense of permitting and sustaining good care. Mr. R suggested the idea and might not be able to afford care without it. Nothing in Dr. Z’s description suggests that Mr. R has personality characteristics or other conditions that would compromise his ability to give informed consent or to understand the nuances of a barter arrangement. Dr. Z is not providing a treatment (eg, psychodynamic therapy) that a barter arrangement could contaminate. That the arrangement would be circumscribed limits the effect of a power differential, as would its brief duration and defined termination endpoint. Dr. Z’s letter to the authors also shows his willingness to seek consultation.
There’s a chance that we may fall apart: Reasons for caution
Martinez’s graded-risk approach recognizes reasons for caution:
- the risk of harm to the patient or doctor–patient relationship
- the uncertain benefit to the patient
- the blurring of Dr. Z’s self-interest and Mr. R’s needs
- some ambiguity about possible exploitation.
Dr. Z and Mr. R have not discussed the value of Mr. R’s work—which might create a rift between them—and despite Mr. R’s reputation, other carpenters are available. Future med-check appointments will give them little time to explore and discuss the meanings of the barter.
Any proposed barter arrangement creates some clinical perils that can be particularly salient in mental health treatment. Patients could view themselves as “special” or entitled to enhanced access to the doctor because of exchanged services, which could take a toll on the doctor.39 The physician’s objectivity might diminish, and the business aspect of their relationship could make both parties less comfortable when discussing sensitive information relevant to treatment.31,40 Also, the suggested barter is for services to be provided at Dr. Z’s office, where confidentiality may be breeched and transference issues could arise.
A medical malpractice claim states that a doctor has breached a duty of care to a patient such that harm (or “damages”) resulted.41 Should Dr. Z and Mr. R’s barter agreement turn sour and harm follow, Mr. R could sue for recovery of damages based of a claim of duress, undue influence, or other aspects of the doctor–patient power differential.27,42,43 Given the published views we have described, a psychiatrist who barters also may be viewed as violating state regulations that measure the standard of care against generally accepted practice.
Only time will tell if I am right or I am wrong
If you face a situation similar to Dr. Z’s and want to consider a barter arrangement, you can take several steps to mitigate potential risk to your patient and ensure competent care (Table 25,6,15,16,32,35,39,40,44-47). One of the most important steps is to seek ongoing consultation, both before and after a decision to barter. Ideally, the consulting colleague would know you and your circumstances and would have sufficient clinical grasp of the patient to make an informed assessment of risks and benefits.35 This consultation, as well as your own rationale for acting on recommendations, should be thoroughly documented in the patient’s records.26,44,45
Certain types of barter should be off limits, including:
- trading prescription drugs for goods or services
- trading for services that tie into the success of one’s business (eg, business advising or marketing)16
- offering treatment in exchange for illegal or ethically unacceptable services.48
Beyond ethical considerations are some practical issues. The Internal Revenue Service has specific rules regarding taxation of bartered goods and services, which must be included as taxable income.46 If possible, an independent agent should appraise the traded goods or services before the agreement.6 When working in a group practice, the clinician might have to figure out how to allocate the received goods or services such as shared overhead costs.28 Preferably, the patient’s goods or services should be provided before care is delivered.16 If not, the duration of services rendered should be limited, and either party should have the option to disengage from the relationship if one feels dissatisfied.16
A written contract, discussed ahead of time, can be a sound way to summarize the terms of the arrangement. Both sides also should consider what would happen if an injury occurred.16 Finally, you must adhere to any relevant state laws regarding payment for services, particularly if the patient has health insurance.32
If the bartering arrangement does not work, you should take an open and non-defensive approach. If you believe you have made a mistake, consider apologizing.45
Dear Dr. Mossman,
Each month, I see my patient, Mr. R, for a 15-minute medication management appointment. At his latest visit, Mr. R mentioned his financial difficulties. He also observed that our office needed to have some carpentry work done—not a surprise, because he’s known in our area as one of the best carpenters around. He suggested that I hire him as payment for the next 6 appointments. What risks might I encounter if I oblige him?
Submitted by “Dr. Z”
Nearly 29 million Americans are uninsured,1 and even more have trouble accessing mental health care.2 Many psychiatrists struggle to provide affordable services while remaining financially viable.3,4 For outpatients with limited means to pay for care, spacing appointments to fit their budgets might compromise treatment.5 Simply not charging patients poses its own clinical and ethical challenges.6-8
As a result, some mental health professionals make barter arrangements to help their patients enter or continue treatment. To answer Dr. Z’s question on whether exchanging services might be a way to arrange matters with some patients, we explore:
- the idea of bartering for psychiatric treatment
- related ethical and legal considerations
- when and in what situations bartering might be appropriate.
Think of what I’m saying: Bartering for treatment
“Barter” refers to exchanging commodities, products, or services of equivalent value without using money.9 In 2010, Nevada Republican Senate candidate Sue Lowden encouraged barter for health care and harkened back to an earlier time where “they would bring a chicken to the doctor; they would say ‘I’ll paint your house.’”10
Such payment arrangements have been encouraged as health care has become increasingly commoditized.11-13 This happens through both direct barter between physician and patient and barter exchanges. Barter exchange systems have been set up on Web sites (as of 2013, at least 400 such online exchanges were available14), local communities,11,15 and social programs. For example, through the “Swapping Guns for Therapy” program, psychologists in California gave free or reduced-fee care for people who traded in their guns.16
Try to see it my way: A prevailing view of barter
Several psychiatrists recommend against bartering for treatment, for a variety of reasons.7,8,17-19 Simon18 argues that a stable fee policy is part of a proper therapeutic framework, and money is “the only acceptable medium of exchange when receiving payment from patients.” Emotional distress and the power differential inherent in treatment might prevent a patient from making an accurate assessment of the value of the bartered goods or services,7,8,17,18,20 which could lead to future claims of undue influence from trading goods or services below market value.17 To avoid the possibility of exploitating the patient, Simon18 recommends that the psychiatrist’s professional fee be “the only material benefit received from the patient.”
The American Psychiatric Association’s code of ethics states that “it is not ethical to switch a doctor–patient relationship to an employer–employee one … and, in most cases, such an arrangement would be unethical.”21 In some therapeutic settings, employing a patient risks inappropriate self-disclosure and intrusion.16
More than other physicians, psychiatrists pay special attention to professional boundaries, the technical term for the “edge of appropriate behavior,” within which safe, effective care can occur.22,23 Although some boundary crossings can be harmless and even constructive, repeated boundary crossings are the forerunners to improper behavior, including sexual relationships with patients.24-26
Out of concern that bartering could become the first step down a slippery ethical slope toward patient exploitation, mental health clinicians have deemed the practice “ethically troubling,”19 said it did “not usually work out well,”7 and declared it “so fraught with risks for both parties that it seem[ed] illogical to even consider it as an option.”27
While I see it your way: What barter proponents say
Reports of bartering for chickens28 and purchasing fuel from a patient in remote Alaska29 show that not all physicians agree and why they feel that professional codes of ethics reflect an urban bias.28,29 In many rural areas and small towns, access to mental health services is limited, and patients often interact with their doctors outside of clinical encounters.23,29-31
Bartering can benefit a physician’s practice by:
- reducing the need to discount services
- eliminating bureaucratic burdens of traditional insurance arrangements
- facilitating development of a patient base
- allowing patients choice and flexibility in seeking medical care.6,16,32
Bartering could confer certain clinical benefits, such as:
- enhancing trust and empathy32
- encouraging patients to make their needs known constructively6
- modeling financial self-care6
- helping the doctor to feel fairly compensated for providing thoughtful care6
- acknowledging the patient’s cultural values15,33
- affirming that patients and doctors both produce things of value.16
I have always thought: Other ethical models
An ethical approach to bartering that requires careful thought and respect for the patient’s needs appears consistent with a primary goal of treatment: “to increase the capacity of individuals to make more rational choices in their lives and to be relatively freer from disabling conflicts.”20 Some authors criticize slippery-slope arguments and strict-rule ethical approaches as being too rigid, limiting, or risk-averse.22,26,34 In Table 1,6,8,16,18,27,29-31,35-37 we list several factors that might weigh for or against a decision to enter into a barter arrangement as payment for care.

In a similar manner, Martinez33,38 proposed a graded-risk framework that encourages examination of potential harms and benefits of a decision, potential coercive or exploitative elements, the clinician’s intentions and aspiration to professional ideals, and the context of the decision. Within this framework, some bartering arrangements might be encouraged and, perhaps, even obligatory because of the potential benefits to the patient; other arrangements (eg, trading psychotherapy for menial services) might be unjustifiable. Martinez38 argues that this approach fosters mutual decision-making with patients, discourages physician paternalism, and “demands that we struggle with the particulars with each case.”
Gottlieb’s decision-making model35 recognizes that trying to avoid all dual relationships is unrealistic and not all dual relationships are exploitative. Instead, a clinician must assess 3 dimensions of current and proposed relationships:
- the degree of power differential
- the duration of treatment
- the clarity of termination.
The decision-making process also requires involvement of the patient, who if “unable to recognize the dilemma or is unwilling to consider the issues before deciding, should be considered at risk, and the contemplated relationship rejected.”35
So I will ask you once again: Dr. Z’s decision
In the case of Dr. Z and Mr. R, a barter arrangement might work in the sense of permitting and sustaining good care. Mr. R suggested the idea and might not be able to afford care without it. Nothing in Dr. Z’s description suggests that Mr. R has personality characteristics or other conditions that would compromise his ability to give informed consent or to understand the nuances of a barter arrangement. Dr. Z is not providing a treatment (eg, psychodynamic therapy) that a barter arrangement could contaminate. That the arrangement would be circumscribed limits the effect of a power differential, as would its brief duration and defined termination endpoint. Dr. Z’s letter to the authors also shows his willingness to seek consultation.
There’s a chance that we may fall apart: Reasons for caution
Martinez’s graded-risk approach recognizes reasons for caution:
- the risk of harm to the patient or doctor–patient relationship
- the uncertain benefit to the patient
- the blurring of Dr. Z’s self-interest and Mr. R’s needs
- some ambiguity about possible exploitation.
Dr. Z and Mr. R have not discussed the value of Mr. R’s work—which might create a rift between them—and despite Mr. R’s reputation, other carpenters are available. Future med-check appointments will give them little time to explore and discuss the meanings of the barter.
Any proposed barter arrangement creates some clinical perils that can be particularly salient in mental health treatment. Patients could view themselves as “special” or entitled to enhanced access to the doctor because of exchanged services, which could take a toll on the doctor.39 The physician’s objectivity might diminish, and the business aspect of their relationship could make both parties less comfortable when discussing sensitive information relevant to treatment.31,40 Also, the suggested barter is for services to be provided at Dr. Z’s office, where confidentiality may be breeched and transference issues could arise.
A medical malpractice claim states that a doctor has breached a duty of care to a patient such that harm (or “damages”) resulted.41 Should Dr. Z and Mr. R’s barter agreement turn sour and harm follow, Mr. R could sue for recovery of damages based of a claim of duress, undue influence, or other aspects of the doctor–patient power differential.27,42,43 Given the published views we have described, a psychiatrist who barters also may be viewed as violating state regulations that measure the standard of care against generally accepted practice.
Only time will tell if I am right or I am wrong
If you face a situation similar to Dr. Z’s and want to consider a barter arrangement, you can take several steps to mitigate potential risk to your patient and ensure competent care (Table 25,6,15,16,32,35,39,40,44-47). One of the most important steps is to seek ongoing consultation, both before and after a decision to barter. Ideally, the consulting colleague would know you and your circumstances and would have sufficient clinical grasp of the patient to make an informed assessment of risks and benefits.35 This consultation, as well as your own rationale for acting on recommendations, should be thoroughly documented in the patient’s records.26,44,45
Certain types of barter should be off limits, including:
- trading prescription drugs for goods or services
- trading for services that tie into the success of one’s business (eg, business advising or marketing)16
- offering treatment in exchange for illegal or ethically unacceptable services.48
Beyond ethical considerations are some practical issues. The Internal Revenue Service has specific rules regarding taxation of bartered goods and services, which must be included as taxable income.46 If possible, an independent agent should appraise the traded goods or services before the agreement.6 When working in a group practice, the clinician might have to figure out how to allocate the received goods or services such as shared overhead costs.28 Preferably, the patient’s goods or services should be provided before care is delivered.16 If not, the duration of services rendered should be limited, and either party should have the option to disengage from the relationship if one feels dissatisfied.16
A written contract, discussed ahead of time, can be a sound way to summarize the terms of the arrangement. Both sides also should consider what would happen if an injury occurred.16 Finally, you must adhere to any relevant state laws regarding payment for services, particularly if the patient has health insurance.32
If the bartering arrangement does not work, you should take an open and non-defensive approach. If you believe you have made a mistake, consider apologizing.45
1. Kaiser Commission on Medicaid and the Uninsured. Key facts about the uninsured population. http://kff.org/uninsured/fact-sheet/key-facts-about-the-uninsured-population. Published September 29, 2016. Accessed October 7, 2016.
2. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed October 7, 2016.
3. Insel T. Director’s blog: the paradox of parity. May 30, 2014. https://www.nimh.nih.gov/about/director/2014/the-paradox-of-parity.shtml. Published May 30, 2014. Accessed October 7, 2016.
4. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
5. What do you do when patients cannot pay? Psychiatry (Edgmont). 2009;6(5):51-52.
6. Hill M. Barter: ethical considerations in psychotherapy. Women Ther. 2000;22(3):81-91.
7. Simon RI. Commentary: treatment boundaries—flexible guidelines, not rigid standards. J Am Acad Psychiatry Law. 2001;29(3):287-289.
8. Simon RI, Williams IC. Maintaining treatment boundaries in small communities and rural areas. Psychiatr Serv. 1999;50(11):1440-1446.
9. Compact edition of the Oxford English dictionary. New York, NY: Oxford University Press; 1971:171.
10. Coolican JP. Sue Lowden stands by health care plan. Las Vegas Sun. http://lasvegassun.com/news/2010/apr/20/sue-lowden-draws-fire-repeating-health-care-barter. Published April 20, 2010. Accessed September 20, 2016.
11. Consumer Reports. Barter sometimes allow patients to pay for health care they otherwise could not afford. Washington Post. https://www.washingtonpost.com/national/health-science/barter-sometimes-allow-patients-to-pay-for-health-care-they-otherwise-could-not-afford/2013/09/30/e7e5a55e-069d-11e3-88d6-d5795fab4637_story.html. Published September 20, 2013. Accessed September 27, 2016.
12. Ellis B. MediBid auction site lets doctors bid for patients. CNN Money. http://money.cnn.com/2014/01/09/pf/insurance/medibid. Published January 9, 2014. Accessed September 23, 2016.
13. Ambrosino B. Surgery for sale: the ethics of health care bartering in a social media marketplace. http://hub.jhu.edu/2014/01/16/hopkins-ethicist-ponders-medibid. Published January 16, 2014. Accessed September 23, 2016.
14. Thomas C. When patients barter for health care. https://ethicalnag.org/2013/07/30/barter. Published July 30, 2016. Accessed October 9, 2016.
15. Syme G. Fetters or freedom: dual relationships in counselling. Int J Adv Counselling. 2006;28(1):57-69.
16. Zur O. Bartering in psychotherapy and counselling: complexities, case studies and guidelines. New Therapist. 2008;58:18-26.
17. Simon RI. The psychiatrist as a fiduciary: avoiding the double agent role. Psychiatric Annals. 1987;17(9):622-626.
18. Simon RI. Treatment boundary violations: clinical, ethical, and legal considerations. Bull Am Acad Psychiatry Law. 1992;20(3):269-288.
19. Walker R, Clark JJ. Heading off boundary problems: clinical supervision as risk management. Psychiatr Serv. 1999;50(11):1435-1439.
20. Malmquist CP, Norman MT. Psychiatrist-patient boundary issues following treatment termination. Am J Psychiatry. 2001;158(7):1010-1018.
21. American Psychiatric Association. The opinions of the ethics committee on the principles of medical ethics, with annotations especially applicable to psychiatry. https://www.psychiatry.org/psychiatrists/practice/ethics. Published 2016. Accessed October 4, 2016.
22. Gutheil TG, Gabbard GO. Misuses and misunderstandings of boundary theory in clinical and regulatory settings. Am J Psychiatry. 1998;155(3):409-414.
23. Crowden A. Professional boundaries and the ethics of dual and multiple overlapping relationships in psychotherapy. Monash Bioeth Rev. 2008;27(4):10-27.
24. Gabbard GO. Commentary: boundaries, culture, and psychotherapy. J Am Acad Psychiatry Law. 2001;29(3):284-286.
25. Kroll J. Boundary violations: a culture-bound syndrome. J Am Acad Psychiatry Law. 2001;29(3):274-283.
26. Gottlieb MC, Younggren JN. Is there a slippery slope? Considerations regarding multiple relationships and risk management. Professional Psychology: Research and Practice. 2009;40(6):564-557.
27. Woody RH. Bartering for psychological services. Professional Psychology: Research and Practice. 1998;29(2):174-178.
28. Bartering for medical care. MGMA Connex. 2008;8(6):11.
29. Roberts LW, Battaglia J, Epstein RS. Frontier ethics: mental health care needs and ethical dilemmas in rural communities. Psychiatr Serv. 1994;50(4):497-503.
30. Endacott R, Wood A, Judd F, et al. Impact and management of dual relationships in metropolitan, regional and rural mental health practice. Aust N Z J Psychiatry. 2006;40(11-12):987-994.
31. Scopelliti J, Judd F, Grigg M, et al. Dual relationships in mental health practice: issues for clinicians in rural settings. Aust N Z J Psychiatry. 2004;38(11-12):953-959.
32. Ayers AA. Bartering basics for the urgent care operator. http://www.alanayersurgentcare.com/Linked_Files/2013_Articles/Ayers_UCAOA_Bartering_%20Basics_2012_01_09.pdf. Accessed September 23, 2016.
33. Savin D, Martinez R. Cross-cultural boundary dilemmas: a graded-risk assessment approach. Transcult Psychiatry. 2006;42(2):243-258.
34. Glass LL. The gray areas of boundary crossings and violations. Am J Psychother. 2003;57(4):429-444.
35. Gottlieb MC. Avoiding exploitive dual relationships: a decision-making model. Psychotherapy (Chic). 1993;30(1):41-48.
36. Lane JA. The ethical implications of bartering for mental health services: examining interdisciplinary ethical standards. http://pdxscholar.library.pdx.edu/coun_fac/36. Published 2012. Accessed October 17, 2016.
37. Miller RD, Maier GJ. Nonsexual boundary violations: sauce for the gander. J Psychiatry Law. 2002;30(3):309-329.
38. Martinez R. A model for boundary dilemmas: ethical decision-making in the patient-professional relationship. Ethical Hum Sci Serv. 2000;2(1):43-61.
39. Salmon K, Klijnsma M. Boundary issues: employing patients as staff? Br J Gen Pract. 2009;59(558):56-57.
40. College of Physicians and Surgeons Ontario. Hiring patients may compromise physician-patient relationship. Dialogue. 2015;3:47.
41. Bal S. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467(2):339-347.
42. What puts a psychiatrist at risk for a malpractice lawsuit? Psychiatry (Edgmont). 2009;6(8):38-39.
43. Geis v Landau, 117 Misc2d 396 (NY Misc 1983).
44. Nisselle P. Danger zone. When boundaries are crossed in the doctor-patient relationship. Aust Fam Physician. 2000;29(6):541-544.
45. Pope KS, Keith-Spiegel P. A practical approach to boundaries in psychotherapy: making decisions, bypassing blunders, and mending fences. J Clin Psychol. 2008;64(5):638-652.
46. IRS Publication 17. https://www.irs.gov/publications/p17/ch12.html. Published 2015. Accessed October 5, 2016.
47. Epstein RS, Simon RI. The exploitation index: an early warning indicator of boundary violations in psychotherapy. Bull Menninger Clin. 1990;54(4):450-465.
48. Skutch J. Savannah doctor accused of trading drugs for sex with strippers. Augusta Chronicle. http://chronicle.augusta.com/news/crime-courts/2013-01-31/savannah-doctor-accused-trading-drugs-sex-strippers. Published January 31, 2013. Accessed October 16, 2016.
1. Kaiser Commission on Medicaid and the Uninsured. Key facts about the uninsured population. http://kff.org/uninsured/fact-sheet/key-facts-about-the-uninsured-population. Published September 29, 2016. Accessed October 7, 2016.
2. National Alliance on Mental Illness. A long road ahead: achieving true parity in mental health and substance use care. https://www.nami.org/About-NAMI/Publications-Reports/Public-Policy-Reports/A-Long-Road-Ahead/2015-ALongRoadAhead.pdf. Published April 2015. Accessed October 7, 2016.
3. Insel T. Director’s blog: the paradox of parity. May 30, 2014. https://www.nimh.nih.gov/about/director/2014/the-paradox-of-parity.shtml. Published May 30, 2014. Accessed October 7, 2016.
4. Bishop TF, Press MJ, Keyhani S, et al. Acceptance of insurance by psychiatrists and the implications for access to mental health care. JAMA Psychiatry. 2014;71(2):176-181.
5. What do you do when patients cannot pay? Psychiatry (Edgmont). 2009;6(5):51-52.
6. Hill M. Barter: ethical considerations in psychotherapy. Women Ther. 2000;22(3):81-91.
7. Simon RI. Commentary: treatment boundaries—flexible guidelines, not rigid standards. J Am Acad Psychiatry Law. 2001;29(3):287-289.
8. Simon RI, Williams IC. Maintaining treatment boundaries in small communities and rural areas. Psychiatr Serv. 1999;50(11):1440-1446.
9. Compact edition of the Oxford English dictionary. New York, NY: Oxford University Press; 1971:171.
10. Coolican JP. Sue Lowden stands by health care plan. Las Vegas Sun. http://lasvegassun.com/news/2010/apr/20/sue-lowden-draws-fire-repeating-health-care-barter. Published April 20, 2010. Accessed September 20, 2016.
11. Consumer Reports. Barter sometimes allow patients to pay for health care they otherwise could not afford. Washington Post. https://www.washingtonpost.com/national/health-science/barter-sometimes-allow-patients-to-pay-for-health-care-they-otherwise-could-not-afford/2013/09/30/e7e5a55e-069d-11e3-88d6-d5795fab4637_story.html. Published September 20, 2013. Accessed September 27, 2016.
12. Ellis B. MediBid auction site lets doctors bid for patients. CNN Money. http://money.cnn.com/2014/01/09/pf/insurance/medibid. Published January 9, 2014. Accessed September 23, 2016.
13. Ambrosino B. Surgery for sale: the ethics of health care bartering in a social media marketplace. http://hub.jhu.edu/2014/01/16/hopkins-ethicist-ponders-medibid. Published January 16, 2014. Accessed September 23, 2016.
14. Thomas C. When patients barter for health care. https://ethicalnag.org/2013/07/30/barter. Published July 30, 2016. Accessed October 9, 2016.
15. Syme G. Fetters or freedom: dual relationships in counselling. Int J Adv Counselling. 2006;28(1):57-69.
16. Zur O. Bartering in psychotherapy and counselling: complexities, case studies and guidelines. New Therapist. 2008;58:18-26.
17. Simon RI. The psychiatrist as a fiduciary: avoiding the double agent role. Psychiatric Annals. 1987;17(9):622-626.
18. Simon RI. Treatment boundary violations: clinical, ethical, and legal considerations. Bull Am Acad Psychiatry Law. 1992;20(3):269-288.
19. Walker R, Clark JJ. Heading off boundary problems: clinical supervision as risk management. Psychiatr Serv. 1999;50(11):1435-1439.
20. Malmquist CP, Norman MT. Psychiatrist-patient boundary issues following treatment termination. Am J Psychiatry. 2001;158(7):1010-1018.
21. American Psychiatric Association. The opinions of the ethics committee on the principles of medical ethics, with annotations especially applicable to psychiatry. https://www.psychiatry.org/psychiatrists/practice/ethics. Published 2016. Accessed October 4, 2016.
22. Gutheil TG, Gabbard GO. Misuses and misunderstandings of boundary theory in clinical and regulatory settings. Am J Psychiatry. 1998;155(3):409-414.
23. Crowden A. Professional boundaries and the ethics of dual and multiple overlapping relationships in psychotherapy. Monash Bioeth Rev. 2008;27(4):10-27.
24. Gabbard GO. Commentary: boundaries, culture, and psychotherapy. J Am Acad Psychiatry Law. 2001;29(3):284-286.
25. Kroll J. Boundary violations: a culture-bound syndrome. J Am Acad Psychiatry Law. 2001;29(3):274-283.
26. Gottlieb MC, Younggren JN. Is there a slippery slope? Considerations regarding multiple relationships and risk management. Professional Psychology: Research and Practice. 2009;40(6):564-557.
27. Woody RH. Bartering for psychological services. Professional Psychology: Research and Practice. 1998;29(2):174-178.
28. Bartering for medical care. MGMA Connex. 2008;8(6):11.
29. Roberts LW, Battaglia J, Epstein RS. Frontier ethics: mental health care needs and ethical dilemmas in rural communities. Psychiatr Serv. 1994;50(4):497-503.
30. Endacott R, Wood A, Judd F, et al. Impact and management of dual relationships in metropolitan, regional and rural mental health practice. Aust N Z J Psychiatry. 2006;40(11-12):987-994.
31. Scopelliti J, Judd F, Grigg M, et al. Dual relationships in mental health practice: issues for clinicians in rural settings. Aust N Z J Psychiatry. 2004;38(11-12):953-959.
32. Ayers AA. Bartering basics for the urgent care operator. http://www.alanayersurgentcare.com/Linked_Files/2013_Articles/Ayers_UCAOA_Bartering_%20Basics_2012_01_09.pdf. Accessed September 23, 2016.
33. Savin D, Martinez R. Cross-cultural boundary dilemmas: a graded-risk assessment approach. Transcult Psychiatry. 2006;42(2):243-258.
34. Glass LL. The gray areas of boundary crossings and violations. Am J Psychother. 2003;57(4):429-444.
35. Gottlieb MC. Avoiding exploitive dual relationships: a decision-making model. Psychotherapy (Chic). 1993;30(1):41-48.
36. Lane JA. The ethical implications of bartering for mental health services: examining interdisciplinary ethical standards. http://pdxscholar.library.pdx.edu/coun_fac/36. Published 2012. Accessed October 17, 2016.
37. Miller RD, Maier GJ. Nonsexual boundary violations: sauce for the gander. J Psychiatry Law. 2002;30(3):309-329.
38. Martinez R. A model for boundary dilemmas: ethical decision-making in the patient-professional relationship. Ethical Hum Sci Serv. 2000;2(1):43-61.
39. Salmon K, Klijnsma M. Boundary issues: employing patients as staff? Br J Gen Pract. 2009;59(558):56-57.
40. College of Physicians and Surgeons Ontario. Hiring patients may compromise physician-patient relationship. Dialogue. 2015;3:47.
41. Bal S. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467(2):339-347.
42. What puts a psychiatrist at risk for a malpractice lawsuit? Psychiatry (Edgmont). 2009;6(8):38-39.
43. Geis v Landau, 117 Misc2d 396 (NY Misc 1983).
44. Nisselle P. Danger zone. When boundaries are crossed in the doctor-patient relationship. Aust Fam Physician. 2000;29(6):541-544.
45. Pope KS, Keith-Spiegel P. A practical approach to boundaries in psychotherapy: making decisions, bypassing blunders, and mending fences. J Clin Psychol. 2008;64(5):638-652.
46. IRS Publication 17. https://www.irs.gov/publications/p17/ch12.html. Published 2015. Accessed October 5, 2016.
47. Epstein RS, Simon RI. The exploitation index: an early warning indicator of boundary violations in psychotherapy. Bull Menninger Clin. 1990;54(4):450-465.
48. Skutch J. Savannah doctor accused of trading drugs for sex with strippers. Augusta Chronicle. http://chronicle.augusta.com/news/crime-courts/2013-01-31/savannah-doctor-accused-trading-drugs-sex-strippers. Published January 31, 2013. Accessed October 16, 2016.
Are you neuroprotecting your patients? 10 Adjunctive therapies to consider
Are you ‘neuroprotecting’ your patients?
Fortunately, many studies have demonstrated that in addition to controlling clinical symptoms, antidepressants, mood stabilizers, and atypical antipsychotics all have neuroprotective effects, including stimulating neurogenesis, preventing apoptosis, and increasing neurotrophins. However, more needs to be done to protect the brain’s gray and white matter and to prevent negative neuroplasticity and disconnectivity of brain circuits that often are documented in patients with psychotic and mood disorders.
There are, in fact, many off-label supplements with strong neuroprotective effects that psychiatrists can use as an adjunct to standard evidence-based pharmacotherapy. These agents generally are safe and well tolerated and often are sold over the counter, but are not covered by insurance. However, considering the disability that often is associated with schizophrenia, treatment-resistant depression, or psychotic mania, it is reasonable to consider using these agents, many of which are supported by studies published in peer-reviewed journals. However, because they are widely available and not proprietary and large, expensive registration trials such as the ones conducted by the pharmaceutical industry are not done, none is likely to receive FDA approval. Therefore, it is up to psychiatrists and nurse practitioners to use them judiciously in patients at risk for neurotoxicity.
Here are 10 agents with neuroprotective effects supported by published data that can be considered as add-on to the standard treatments in an effort to mitigate neurotoxicity and protect the brain from the destructive processes that accompany acute episodes of psychosis, mania, and depression.
Omega-3 fatty acids have been shown in several studies to help reduce psychopathology of psychosis, mania, or depression when used as an adjunctive agent.1 It appears to be more effective in the early stages of psychiatric disorders than in the chronic phase. It has anti-inflammatory, anti-oxidant, and anti-apoptotic effects; activates cell-signaling pathways; and prevents synaptic loss as well as neuronal and glial death.2
N-acetylcysteine (NAC) is a powerful antioxidant that increases glutathione, which is produced in the mitochondria. Schizophrenia is associated with mitochondrial dysfunction with low levels of glutathione, which puts the brain at risk for neurodegeneration caused by high levels of free radicals produced during psychosis. Adding NAC to antipsychotics during acute psychotic episodes—especially the first episode—can significantly reduce the neurotoxic effects of reactive oxygen and nitrogen species, also known as free radicals.3 In studies of traumatic brain injury in rats, NAC reduced brain edema, neuroinflammation, blood–brain barrier permeability, and apoptosis.4
Minocycline. This antibiotic has been studied extensively as an adjunctive treatment in schizophrenia and has proven to have several neuroprotective effects including anti-inflammatory, anti-oxidant, and anti-apoptotic, and reduces glutamate excitotoxicity.5 Several studies have documented its usefulness in acute psychotic episodes.
Vitamin D. Because of its vital role in neurodevelopment (neuronal differentiation, axonal connectivity), vitamin D deficiency has been associated with several psychiatric disorders including autism, schizophrenia, depression, and Alzheimer’s disease.6 Measure serum levels of vitamin D in patients with psychotic and mood disorders and implement supplementation if it is low—and it often is in these patients.
Nicotine is neuroprotective against glutamate excitotoxicity and it also inhibits apoptosis.7 However, it should never be administered via cigarettes, which are loaded with hundreds of toxic substances! It can be administered via patches or nicotine gum, which are usually used to help in smoking cessation. Nicotine also also can have a pro-cognitive effect.
Melatonin. Many people associate melatonin with sleep. However, it has multiple neuroprotective effects by being an antioxidant, protecting mitochondrial integrity, and modulating the immune system, as well as attenuating microglial activation, which triggers neuroinflammation and oxidative stress. It also protects against cellular senescence, which is due to inflammation and reactive oxygen species. Furthermore, melatonin is useful in ameliorating the metabolic syndrome, which is associated with neurotoxic effects on brain tissue caused by the pro-inflammatory effects of peri-omental fat in obesity.8 Adjunctive melatonin could be helpful in patients with schizophrenia or depression who suffer from metabolic syndrome.
Erythropoietin is a hormone produced by the kidneys to promote the formation of red blood cells. It is a potent neuroprotective cytokine that promotes neuronal survival via anti-apoptotic effects. It protects against glutamate and nitrous oxide toxicity9 and haloperidol-induced neuronal death.10 It is clinically used (since FDA approval in 1989) in severe anemia due to chronic kidney disease or chemotherapy, as well as in inflammatory bowel disease. It does have some “black-box” warnings so its use should be limited.
Cox-2 inhibitors. This is a well-known class of anti-inflammatory drugs, which are FDA-approved for pain and inflammation. Studies of adjunctive use of cox-2 inhibitors in acute psychosis show that these drugs accentuate the efficacy of antipsychotic medications.11 The reason is that acute psychosis is associated with neuro-inflammation, which leads to neurotoxicity.
Lithium. Dosages to treat mania are usually 900 to 1500 mg/d. However, in minute (homeopathic) dosages as low as 1 mg/d, lithium has been shown to prevent progression of amnestic mild cognitive impairment to full dementia.12 This interesting observation suggests that lithium not only induces neurogenesis and increases gray matter volume,13 but may be neuroprotective against amyloid neurotoxicity. The effects of very low doses of lithium in depression and schizophrenia have not been studied yet.
Caffeine. Yes, the good old brew people seek all day is neuroprotective and prevents mood and memory dysfunction caused by stress.14 Caffeine should be avoided in patients with anxiety disorders, but it may be helpful for the brains of patients with mood or psychotic disorders. Caffeine reverses synaptic dysfunction in the circuits of the hippocampus caused by chronic unpredictable stress (quite common among our psychiatric patients).
The above interventions may be helpful for some patients but not others. Practitioners should consider using 1 or more of those adjunctive neuroprotective agents in patients who are at risk for neurodegenerative changes secondary to recurrences of acute and severe psychosis or mood episodes. Although clinicians cannot monitor brain structural integrity, they can assess the rate of symptomatic improvement and degree of functional restoration in their patients. Until a cure is found, these little steps—taken cautiously and judiciously—could help alleviate our patients’ suffering and the risk of neurotoxicity associated with their serious psychiatric disorder.
1. Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: possible stage-specific effects. Ann Clin Psychiatry. 2015;27(4):289-296.
2. Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;7(5-6):287-293.
3. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
4. Chen G, Shi J, Hu Z, et al. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458
5. Dean OM, Data-Franco J, Giorlando F, et al. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391-401.
6. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47-64.
7. Akaike A, Tkada-Takatori Y, Kume T, et al. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40(1-2):211-216.
8. Cardinali DP, Hardeland R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies [published online May 11, 2016]. Neuroendocrinology. doi:10.1159/000446543.
9. Yamasaki M, Mishima HK, Yamashita H, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050(1-2):15-26.
10. Pilllai A, Dhandapani KM, Pillai BA, et al. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology. 2008;33(8):1942-1951.
11. Müller N, Myint AM, Weidinger E, et al. Anti-inflammatory treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:146-153.
12. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
13. Dong BT, Tu GJ, Han YX, et al. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol. 2015;8(3):2473-2483.
14. Kaster MP, Machado NJ, Silva HB, et al. Caffeine acts through neuronal adenosine A24 receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112(25):7833-7838.
Are you ‘neuroprotecting’ your patients?
Fortunately, many studies have demonstrated that in addition to controlling clinical symptoms, antidepressants, mood stabilizers, and atypical antipsychotics all have neuroprotective effects, including stimulating neurogenesis, preventing apoptosis, and increasing neurotrophins. However, more needs to be done to protect the brain’s gray and white matter and to prevent negative neuroplasticity and disconnectivity of brain circuits that often are documented in patients with psychotic and mood disorders.
There are, in fact, many off-label supplements with strong neuroprotective effects that psychiatrists can use as an adjunct to standard evidence-based pharmacotherapy. These agents generally are safe and well tolerated and often are sold over the counter, but are not covered by insurance. However, considering the disability that often is associated with schizophrenia, treatment-resistant depression, or psychotic mania, it is reasonable to consider using these agents, many of which are supported by studies published in peer-reviewed journals. However, because they are widely available and not proprietary and large, expensive registration trials such as the ones conducted by the pharmaceutical industry are not done, none is likely to receive FDA approval. Therefore, it is up to psychiatrists and nurse practitioners to use them judiciously in patients at risk for neurotoxicity.
Here are 10 agents with neuroprotective effects supported by published data that can be considered as add-on to the standard treatments in an effort to mitigate neurotoxicity and protect the brain from the destructive processes that accompany acute episodes of psychosis, mania, and depression.
Omega-3 fatty acids have been shown in several studies to help reduce psychopathology of psychosis, mania, or depression when used as an adjunctive agent.1 It appears to be more effective in the early stages of psychiatric disorders than in the chronic phase. It has anti-inflammatory, anti-oxidant, and anti-apoptotic effects; activates cell-signaling pathways; and prevents synaptic loss as well as neuronal and glial death.2
N-acetylcysteine (NAC) is a powerful antioxidant that increases glutathione, which is produced in the mitochondria. Schizophrenia is associated with mitochondrial dysfunction with low levels of glutathione, which puts the brain at risk for neurodegeneration caused by high levels of free radicals produced during psychosis. Adding NAC to antipsychotics during acute psychotic episodes—especially the first episode—can significantly reduce the neurotoxic effects of reactive oxygen and nitrogen species, also known as free radicals.3 In studies of traumatic brain injury in rats, NAC reduced brain edema, neuroinflammation, blood–brain barrier permeability, and apoptosis.4
Minocycline. This antibiotic has been studied extensively as an adjunctive treatment in schizophrenia and has proven to have several neuroprotective effects including anti-inflammatory, anti-oxidant, and anti-apoptotic, and reduces glutamate excitotoxicity.5 Several studies have documented its usefulness in acute psychotic episodes.
Vitamin D. Because of its vital role in neurodevelopment (neuronal differentiation, axonal connectivity), vitamin D deficiency has been associated with several psychiatric disorders including autism, schizophrenia, depression, and Alzheimer’s disease.6 Measure serum levels of vitamin D in patients with psychotic and mood disorders and implement supplementation if it is low—and it often is in these patients.
Nicotine is neuroprotective against glutamate excitotoxicity and it also inhibits apoptosis.7 However, it should never be administered via cigarettes, which are loaded with hundreds of toxic substances! It can be administered via patches or nicotine gum, which are usually used to help in smoking cessation. Nicotine also also can have a pro-cognitive effect.
Melatonin. Many people associate melatonin with sleep. However, it has multiple neuroprotective effects by being an antioxidant, protecting mitochondrial integrity, and modulating the immune system, as well as attenuating microglial activation, which triggers neuroinflammation and oxidative stress. It also protects against cellular senescence, which is due to inflammation and reactive oxygen species. Furthermore, melatonin is useful in ameliorating the metabolic syndrome, which is associated with neurotoxic effects on brain tissue caused by the pro-inflammatory effects of peri-omental fat in obesity.8 Adjunctive melatonin could be helpful in patients with schizophrenia or depression who suffer from metabolic syndrome.
Erythropoietin is a hormone produced by the kidneys to promote the formation of red blood cells. It is a potent neuroprotective cytokine that promotes neuronal survival via anti-apoptotic effects. It protects against glutamate and nitrous oxide toxicity9 and haloperidol-induced neuronal death.10 It is clinically used (since FDA approval in 1989) in severe anemia due to chronic kidney disease or chemotherapy, as well as in inflammatory bowel disease. It does have some “black-box” warnings so its use should be limited.
Cox-2 inhibitors. This is a well-known class of anti-inflammatory drugs, which are FDA-approved for pain and inflammation. Studies of adjunctive use of cox-2 inhibitors in acute psychosis show that these drugs accentuate the efficacy of antipsychotic medications.11 The reason is that acute psychosis is associated with neuro-inflammation, which leads to neurotoxicity.
Lithium. Dosages to treat mania are usually 900 to 1500 mg/d. However, in minute (homeopathic) dosages as low as 1 mg/d, lithium has been shown to prevent progression of amnestic mild cognitive impairment to full dementia.12 This interesting observation suggests that lithium not only induces neurogenesis and increases gray matter volume,13 but may be neuroprotective against amyloid neurotoxicity. The effects of very low doses of lithium in depression and schizophrenia have not been studied yet.
Caffeine. Yes, the good old brew people seek all day is neuroprotective and prevents mood and memory dysfunction caused by stress.14 Caffeine should be avoided in patients with anxiety disorders, but it may be helpful for the brains of patients with mood or psychotic disorders. Caffeine reverses synaptic dysfunction in the circuits of the hippocampus caused by chronic unpredictable stress (quite common among our psychiatric patients).
The above interventions may be helpful for some patients but not others. Practitioners should consider using 1 or more of those adjunctive neuroprotective agents in patients who are at risk for neurodegenerative changes secondary to recurrences of acute and severe psychosis or mood episodes. Although clinicians cannot monitor brain structural integrity, they can assess the rate of symptomatic improvement and degree of functional restoration in their patients. Until a cure is found, these little steps—taken cautiously and judiciously—could help alleviate our patients’ suffering and the risk of neurotoxicity associated with their serious psychiatric disorder.
Are you ‘neuroprotecting’ your patients?
Fortunately, many studies have demonstrated that in addition to controlling clinical symptoms, antidepressants, mood stabilizers, and atypical antipsychotics all have neuroprotective effects, including stimulating neurogenesis, preventing apoptosis, and increasing neurotrophins. However, more needs to be done to protect the brain’s gray and white matter and to prevent negative neuroplasticity and disconnectivity of brain circuits that often are documented in patients with psychotic and mood disorders.
There are, in fact, many off-label supplements with strong neuroprotective effects that psychiatrists can use as an adjunct to standard evidence-based pharmacotherapy. These agents generally are safe and well tolerated and often are sold over the counter, but are not covered by insurance. However, considering the disability that often is associated with schizophrenia, treatment-resistant depression, or psychotic mania, it is reasonable to consider using these agents, many of which are supported by studies published in peer-reviewed journals. However, because they are widely available and not proprietary and large, expensive registration trials such as the ones conducted by the pharmaceutical industry are not done, none is likely to receive FDA approval. Therefore, it is up to psychiatrists and nurse practitioners to use them judiciously in patients at risk for neurotoxicity.
Here are 10 agents with neuroprotective effects supported by published data that can be considered as add-on to the standard treatments in an effort to mitigate neurotoxicity and protect the brain from the destructive processes that accompany acute episodes of psychosis, mania, and depression.
Omega-3 fatty acids have been shown in several studies to help reduce psychopathology of psychosis, mania, or depression when used as an adjunctive agent.1 It appears to be more effective in the early stages of psychiatric disorders than in the chronic phase. It has anti-inflammatory, anti-oxidant, and anti-apoptotic effects; activates cell-signaling pathways; and prevents synaptic loss as well as neuronal and glial death.2
N-acetylcysteine (NAC) is a powerful antioxidant that increases glutathione, which is produced in the mitochondria. Schizophrenia is associated with mitochondrial dysfunction with low levels of glutathione, which puts the brain at risk for neurodegeneration caused by high levels of free radicals produced during psychosis. Adding NAC to antipsychotics during acute psychotic episodes—especially the first episode—can significantly reduce the neurotoxic effects of reactive oxygen and nitrogen species, also known as free radicals.3 In studies of traumatic brain injury in rats, NAC reduced brain edema, neuroinflammation, blood–brain barrier permeability, and apoptosis.4
Minocycline. This antibiotic has been studied extensively as an adjunctive treatment in schizophrenia and has proven to have several neuroprotective effects including anti-inflammatory, anti-oxidant, and anti-apoptotic, and reduces glutamate excitotoxicity.5 Several studies have documented its usefulness in acute psychotic episodes.
Vitamin D. Because of its vital role in neurodevelopment (neuronal differentiation, axonal connectivity), vitamin D deficiency has been associated with several psychiatric disorders including autism, schizophrenia, depression, and Alzheimer’s disease.6 Measure serum levels of vitamin D in patients with psychotic and mood disorders and implement supplementation if it is low—and it often is in these patients.
Nicotine is neuroprotective against glutamate excitotoxicity and it also inhibits apoptosis.7 However, it should never be administered via cigarettes, which are loaded with hundreds of toxic substances! It can be administered via patches or nicotine gum, which are usually used to help in smoking cessation. Nicotine also also can have a pro-cognitive effect.
Melatonin. Many people associate melatonin with sleep. However, it has multiple neuroprotective effects by being an antioxidant, protecting mitochondrial integrity, and modulating the immune system, as well as attenuating microglial activation, which triggers neuroinflammation and oxidative stress. It also protects against cellular senescence, which is due to inflammation and reactive oxygen species. Furthermore, melatonin is useful in ameliorating the metabolic syndrome, which is associated with neurotoxic effects on brain tissue caused by the pro-inflammatory effects of peri-omental fat in obesity.8 Adjunctive melatonin could be helpful in patients with schizophrenia or depression who suffer from metabolic syndrome.
Erythropoietin is a hormone produced by the kidneys to promote the formation of red blood cells. It is a potent neuroprotective cytokine that promotes neuronal survival via anti-apoptotic effects. It protects against glutamate and nitrous oxide toxicity9 and haloperidol-induced neuronal death.10 It is clinically used (since FDA approval in 1989) in severe anemia due to chronic kidney disease or chemotherapy, as well as in inflammatory bowel disease. It does have some “black-box” warnings so its use should be limited.
Cox-2 inhibitors. This is a well-known class of anti-inflammatory drugs, which are FDA-approved for pain and inflammation. Studies of adjunctive use of cox-2 inhibitors in acute psychosis show that these drugs accentuate the efficacy of antipsychotic medications.11 The reason is that acute psychosis is associated with neuro-inflammation, which leads to neurotoxicity.
Lithium. Dosages to treat mania are usually 900 to 1500 mg/d. However, in minute (homeopathic) dosages as low as 1 mg/d, lithium has been shown to prevent progression of amnestic mild cognitive impairment to full dementia.12 This interesting observation suggests that lithium not only induces neurogenesis and increases gray matter volume,13 but may be neuroprotective against amyloid neurotoxicity. The effects of very low doses of lithium in depression and schizophrenia have not been studied yet.
Caffeine. Yes, the good old brew people seek all day is neuroprotective and prevents mood and memory dysfunction caused by stress.14 Caffeine should be avoided in patients with anxiety disorders, but it may be helpful for the brains of patients with mood or psychotic disorders. Caffeine reverses synaptic dysfunction in the circuits of the hippocampus caused by chronic unpredictable stress (quite common among our psychiatric patients).
The above interventions may be helpful for some patients but not others. Practitioners should consider using 1 or more of those adjunctive neuroprotective agents in patients who are at risk for neurodegenerative changes secondary to recurrences of acute and severe psychosis or mood episodes. Although clinicians cannot monitor brain structural integrity, they can assess the rate of symptomatic improvement and degree of functional restoration in their patients. Until a cure is found, these little steps—taken cautiously and judiciously—could help alleviate our patients’ suffering and the risk of neurotoxicity associated with their serious psychiatric disorder.
1. Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: possible stage-specific effects. Ann Clin Psychiatry. 2015;27(4):289-296.
2. Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;7(5-6):287-293.
3. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
4. Chen G, Shi J, Hu Z, et al. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458
5. Dean OM, Data-Franco J, Giorlando F, et al. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391-401.
6. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47-64.
7. Akaike A, Tkada-Takatori Y, Kume T, et al. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40(1-2):211-216.
8. Cardinali DP, Hardeland R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies [published online May 11, 2016]. Neuroendocrinology. doi:10.1159/000446543.
9. Yamasaki M, Mishima HK, Yamashita H, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050(1-2):15-26.
10. Pilllai A, Dhandapani KM, Pillai BA, et al. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology. 2008;33(8):1942-1951.
11. Müller N, Myint AM, Weidinger E, et al. Anti-inflammatory treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:146-153.
12. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
13. Dong BT, Tu GJ, Han YX, et al. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol. 2015;8(3):2473-2483.
14. Kaster MP, Machado NJ, Silva HB, et al. Caffeine acts through neuronal adenosine A24 receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112(25):7833-7838.
1. Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: possible stage-specific effects. Ann Clin Psychiatry. 2015;27(4):289-296.
2. Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;7(5-6):287-293.
3. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
4. Chen G, Shi J, Hu Z, et al. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458
5. Dean OM, Data-Franco J, Giorlando F, et al. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391-401.
6. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47-64.
7. Akaike A, Tkada-Takatori Y, Kume T, et al. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40(1-2):211-216.
8. Cardinali DP, Hardeland R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies [published online May 11, 2016]. Neuroendocrinology. doi:10.1159/000446543.
9. Yamasaki M, Mishima HK, Yamashita H, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050(1-2):15-26.
10. Pilllai A, Dhandapani KM, Pillai BA, et al. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology. 2008;33(8):1942-1951.
11. Müller N, Myint AM, Weidinger E, et al. Anti-inflammatory treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:146-153.
12. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
13. Dong BT, Tu GJ, Han YX, et al. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol. 2015;8(3):2473-2483.
14. Kaster MP, Machado NJ, Silva HB, et al. Caffeine acts through neuronal adenosine A24 receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112(25):7833-7838.
The merits of buprenorphine for pregnant women with opioid use disorder; A possible solution to the ‘shrinking’ workforce
The merits of buprenorphine for pregnant women with opioid use disorder
Several medication-assisted treatments (MATs) for opioid use disorder were highlighted in “What clinicians need to know about treating opioid use disorder,” (Evidence-Based Reviews,
The landmark study, the Maternal Opioid Treatment: Human Experimental Research, a 2010 multicenter randomized controlled trial compared buprenorphine with methadone in pregnant women with opioid use disorder. The results revealed that neonates exposed to buprenorphine needed 89% less morphine to treat neonatal abstinence syndrome (NAS), 43% shorter hospital stay, and 58% shorter duration of medical treatment for NAS compared with those receiving methadone. Other advantages of buprenorphine over methadone are lower risk of overdose, fewer drug–drug interactions, and the option of receiving treatment in an outpatient setting, rather than a licensed treatment program, such as a methadone maintenance treatment program, which is more tightly controlled.1-3
The previous recommendation was to consider buprenorphine for patients who refused methadone or were unable to take it, or when a methadone treatment program wasn’t available. This study highlighted some clear advantages for treating this subpopulation with methadone instead of buprenorphine: only 18% of patients receiving methadone discontinued treatment, compared with 33% of those receiving buprenorphine,1-3 and methadone had a lower risk of diversion.3 The accepted practice has been to recommend methadone treatment for patients with mental, physical, or social stressors because of the structure of opioid treatment programs (OTP) (also known as methadone maintenance treatment programs). However, buprenorphine can be dispensed through an OTP, following the same stringent rules and regulations.4
The single agent, buprenorphine—not buprenorphine/naloxone—is recommended to prevent prenatal exposure to naloxone. It is thought that exposure to naloxone in utero might produce hormonal changes in the fetus.1,5 O'Connor et al5 noted methadone’s suitability during breast-feeding because of its low concentration in breast milk. Buprenorphine is excreted at breast milk to plasma ratio of 1:1, but because of buprenorphine’s poor oral bioavailability, infant exposure has little impact on the NAS score, therefore it’s suitable for breast-feeding mothers.5
Adegboyega Oyemade, MD, FAPA
Addiction Psychiatrist
Kaiser Permanente
Baltimore, Maryland
References
1. Lori W. Buprenorphine during pregnancy reduces neonate distress. https://www.drugabuse.gov/news-events/nida-notes/2012/07/buprenorphine- during-pregnancy-reduces-neonate-distress. Published July 6, 2016. Accessed September 9, 2016.
2. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320-2331.
3. ACOG Committee on Health Care for Underserved Women; American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obest Gynecol. 2012;119(5):1070-1076.
4. Addiction Treatment Forum. Methadone vs. buprenorphine: how do OTPs and patients make the choice? http://atforum.com/2013/11/methadone-vs-buprenorphine-how-do-otps-and-patients-make-the-choice. Published November 15, 2013. Accessed September 9, 2016.
5. O’Connor A, Alto W, Musgrave K, et al. Observational study of buprenorphine treatment of opioid-dependent pregnancy women in a family medicine residency: reports on maternal and infant outcomes. J Am Board Fam Med. 2011;24(2):194-201.
A possible solution to the 'shrinking' workforce
I would like to offer another pragmatic, easy, and quick solution for dealing with the shrinking psychiatrist workforce (The psychiatry workforce pool is shrinking. What are we doing about it? From the Editor.
The United States is short approximately 45,000 psychiatrists.1 Burnout—a silent epidemic among physicians—is prevalent in psychiatry. What consumes time and leads to burn out? “Scut work.”
There are thousands of unmatched residency graduates.2 Most of these graduates have clinical experience in the United States. Psychiatry residency programs should give these unmatched graduates 6 months of training in psychiatry and use them as our primary workforce. These assistant physicians could be paired with 2 to 3 psychiatrists to perform the menial tasks, including, but not limited to, phone calls, prescriptions, prior authorizations, chart review, and other clinical and administrative paper work. This way, psychiatrists can focus on the interview, diagnoses, and treatment, major medical decision-making, and see more patients.
Employing assistant physicians to provide care has been suggested as a solution.3 Arkansas, Kansas, and Missouri have passed laws that allow medical school graduates who did not match with a residency program to work in underserved areas with a collaborating physician.
Because 1 out of 4 individuals have a mental illness and more of them are seeking help because of increasing awareness and the Affordable Care Act, the construct of “assistant physicians” could ease psychiatrists’ workload allowing them to deliver better care to more people.
Maju Mathew Koola, MD
Associate Professor
Department of Psychiatry and Behavioral Sciences
George Washington University
School of Medicine and Health Sciences
Washington, DC
References
1. Carlat D. 45,000 More psychiatrists, anyone? Psychiatric Times. http://www.psychiatrictimes.com/articles/45000-more-psychiatrists-anyone-0.Published August 3, 2010. Accessed November 11, 2016.
2. The Match: National Resident Matching Program. results and data: 2016 main residency match. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-and-Data-2016.pdf. Published April 2016. Accessed November 11, 2016.
3. Miller JG, Peterson DJ. Employing nurse practitioners and physician assistants to provide access to care as the psychiatrist shortage continues. Acad Psychiatry. 2015;39(6):685-686.
Concerns in psychiatry
The August 2016 editorial (Unresolved questions about the specialty lurk in the cortext of psychiatrists.
Denis F. Darko, MD
CEO
NeuroSci R&D Consultancy, LLC
Maple Grove, Minnesota
The merits of buprenorphine for pregnant women with opioid use disorder
Several medication-assisted treatments (MATs) for opioid use disorder were highlighted in “What clinicians need to know about treating opioid use disorder,” (Evidence-Based Reviews,
The landmark study, the Maternal Opioid Treatment: Human Experimental Research, a 2010 multicenter randomized controlled trial compared buprenorphine with methadone in pregnant women with opioid use disorder. The results revealed that neonates exposed to buprenorphine needed 89% less morphine to treat neonatal abstinence syndrome (NAS), 43% shorter hospital stay, and 58% shorter duration of medical treatment for NAS compared with those receiving methadone. Other advantages of buprenorphine over methadone are lower risk of overdose, fewer drug–drug interactions, and the option of receiving treatment in an outpatient setting, rather than a licensed treatment program, such as a methadone maintenance treatment program, which is more tightly controlled.1-3
The previous recommendation was to consider buprenorphine for patients who refused methadone or were unable to take it, or when a methadone treatment program wasn’t available. This study highlighted some clear advantages for treating this subpopulation with methadone instead of buprenorphine: only 18% of patients receiving methadone discontinued treatment, compared with 33% of those receiving buprenorphine,1-3 and methadone had a lower risk of diversion.3 The accepted practice has been to recommend methadone treatment for patients with mental, physical, or social stressors because of the structure of opioid treatment programs (OTP) (also known as methadone maintenance treatment programs). However, buprenorphine can be dispensed through an OTP, following the same stringent rules and regulations.4
The single agent, buprenorphine—not buprenorphine/naloxone—is recommended to prevent prenatal exposure to naloxone. It is thought that exposure to naloxone in utero might produce hormonal changes in the fetus.1,5 O'Connor et al5 noted methadone’s suitability during breast-feeding because of its low concentration in breast milk. Buprenorphine is excreted at breast milk to plasma ratio of 1:1, but because of buprenorphine’s poor oral bioavailability, infant exposure has little impact on the NAS score, therefore it’s suitable for breast-feeding mothers.5
Adegboyega Oyemade, MD, FAPA
Addiction Psychiatrist
Kaiser Permanente
Baltimore, Maryland
References
1. Lori W. Buprenorphine during pregnancy reduces neonate distress. https://www.drugabuse.gov/news-events/nida-notes/2012/07/buprenorphine- during-pregnancy-reduces-neonate-distress. Published July 6, 2016. Accessed September 9, 2016.
2. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320-2331.
3. ACOG Committee on Health Care for Underserved Women; American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obest Gynecol. 2012;119(5):1070-1076.
4. Addiction Treatment Forum. Methadone vs. buprenorphine: how do OTPs and patients make the choice? http://atforum.com/2013/11/methadone-vs-buprenorphine-how-do-otps-and-patients-make-the-choice. Published November 15, 2013. Accessed September 9, 2016.
5. O’Connor A, Alto W, Musgrave K, et al. Observational study of buprenorphine treatment of opioid-dependent pregnancy women in a family medicine residency: reports on maternal and infant outcomes. J Am Board Fam Med. 2011;24(2):194-201.
A possible solution to the 'shrinking' workforce
I would like to offer another pragmatic, easy, and quick solution for dealing with the shrinking psychiatrist workforce (The psychiatry workforce pool is shrinking. What are we doing about it? From the Editor.
The United States is short approximately 45,000 psychiatrists.1 Burnout—a silent epidemic among physicians—is prevalent in psychiatry. What consumes time and leads to burn out? “Scut work.”
There are thousands of unmatched residency graduates.2 Most of these graduates have clinical experience in the United States. Psychiatry residency programs should give these unmatched graduates 6 months of training in psychiatry and use them as our primary workforce. These assistant physicians could be paired with 2 to 3 psychiatrists to perform the menial tasks, including, but not limited to, phone calls, prescriptions, prior authorizations, chart review, and other clinical and administrative paper work. This way, psychiatrists can focus on the interview, diagnoses, and treatment, major medical decision-making, and see more patients.
Employing assistant physicians to provide care has been suggested as a solution.3 Arkansas, Kansas, and Missouri have passed laws that allow medical school graduates who did not match with a residency program to work in underserved areas with a collaborating physician.
Because 1 out of 4 individuals have a mental illness and more of them are seeking help because of increasing awareness and the Affordable Care Act, the construct of “assistant physicians” could ease psychiatrists’ workload allowing them to deliver better care to more people.
Maju Mathew Koola, MD
Associate Professor
Department of Psychiatry and Behavioral Sciences
George Washington University
School of Medicine and Health Sciences
Washington, DC
References
1. Carlat D. 45,000 More psychiatrists, anyone? Psychiatric Times. http://www.psychiatrictimes.com/articles/45000-more-psychiatrists-anyone-0.Published August 3, 2010. Accessed November 11, 2016.
2. The Match: National Resident Matching Program. results and data: 2016 main residency match. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-and-Data-2016.pdf. Published April 2016. Accessed November 11, 2016.
3. Miller JG, Peterson DJ. Employing nurse practitioners and physician assistants to provide access to care as the psychiatrist shortage continues. Acad Psychiatry. 2015;39(6):685-686.
Concerns in psychiatry
The August 2016 editorial (Unresolved questions about the specialty lurk in the cortext of psychiatrists.
Denis F. Darko, MD
CEO
NeuroSci R&D Consultancy, LLC
Maple Grove, Minnesota
The merits of buprenorphine for pregnant women with opioid use disorder
Several medication-assisted treatments (MATs) for opioid use disorder were highlighted in “What clinicians need to know about treating opioid use disorder,” (Evidence-Based Reviews,
The landmark study, the Maternal Opioid Treatment: Human Experimental Research, a 2010 multicenter randomized controlled trial compared buprenorphine with methadone in pregnant women with opioid use disorder. The results revealed that neonates exposed to buprenorphine needed 89% less morphine to treat neonatal abstinence syndrome (NAS), 43% shorter hospital stay, and 58% shorter duration of medical treatment for NAS compared with those receiving methadone. Other advantages of buprenorphine over methadone are lower risk of overdose, fewer drug–drug interactions, and the option of receiving treatment in an outpatient setting, rather than a licensed treatment program, such as a methadone maintenance treatment program, which is more tightly controlled.1-3
The previous recommendation was to consider buprenorphine for patients who refused methadone or were unable to take it, or when a methadone treatment program wasn’t available. This study highlighted some clear advantages for treating this subpopulation with methadone instead of buprenorphine: only 18% of patients receiving methadone discontinued treatment, compared with 33% of those receiving buprenorphine,1-3 and methadone had a lower risk of diversion.3 The accepted practice has been to recommend methadone treatment for patients with mental, physical, or social stressors because of the structure of opioid treatment programs (OTP) (also known as methadone maintenance treatment programs). However, buprenorphine can be dispensed through an OTP, following the same stringent rules and regulations.4
The single agent, buprenorphine—not buprenorphine/naloxone—is recommended to prevent prenatal exposure to naloxone. It is thought that exposure to naloxone in utero might produce hormonal changes in the fetus.1,5 O'Connor et al5 noted methadone’s suitability during breast-feeding because of its low concentration in breast milk. Buprenorphine is excreted at breast milk to plasma ratio of 1:1, but because of buprenorphine’s poor oral bioavailability, infant exposure has little impact on the NAS score, therefore it’s suitable for breast-feeding mothers.5
Adegboyega Oyemade, MD, FAPA
Addiction Psychiatrist
Kaiser Permanente
Baltimore, Maryland
References
1. Lori W. Buprenorphine during pregnancy reduces neonate distress. https://www.drugabuse.gov/news-events/nida-notes/2012/07/buprenorphine- during-pregnancy-reduces-neonate-distress. Published July 6, 2016. Accessed September 9, 2016.
2. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320-2331.
3. ACOG Committee on Health Care for Underserved Women; American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obest Gynecol. 2012;119(5):1070-1076.
4. Addiction Treatment Forum. Methadone vs. buprenorphine: how do OTPs and patients make the choice? http://atforum.com/2013/11/methadone-vs-buprenorphine-how-do-otps-and-patients-make-the-choice. Published November 15, 2013. Accessed September 9, 2016.
5. O’Connor A, Alto W, Musgrave K, et al. Observational study of buprenorphine treatment of opioid-dependent pregnancy women in a family medicine residency: reports on maternal and infant outcomes. J Am Board Fam Med. 2011;24(2):194-201.
A possible solution to the 'shrinking' workforce
I would like to offer another pragmatic, easy, and quick solution for dealing with the shrinking psychiatrist workforce (The psychiatry workforce pool is shrinking. What are we doing about it? From the Editor.
The United States is short approximately 45,000 psychiatrists.1 Burnout—a silent epidemic among physicians—is prevalent in psychiatry. What consumes time and leads to burn out? “Scut work.”
There are thousands of unmatched residency graduates.2 Most of these graduates have clinical experience in the United States. Psychiatry residency programs should give these unmatched graduates 6 months of training in psychiatry and use them as our primary workforce. These assistant physicians could be paired with 2 to 3 psychiatrists to perform the menial tasks, including, but not limited to, phone calls, prescriptions, prior authorizations, chart review, and other clinical and administrative paper work. This way, psychiatrists can focus on the interview, diagnoses, and treatment, major medical decision-making, and see more patients.
Employing assistant physicians to provide care has been suggested as a solution.3 Arkansas, Kansas, and Missouri have passed laws that allow medical school graduates who did not match with a residency program to work in underserved areas with a collaborating physician.
Because 1 out of 4 individuals have a mental illness and more of them are seeking help because of increasing awareness and the Affordable Care Act, the construct of “assistant physicians” could ease psychiatrists’ workload allowing them to deliver better care to more people.
Maju Mathew Koola, MD
Associate Professor
Department of Psychiatry and Behavioral Sciences
George Washington University
School of Medicine and Health Sciences
Washington, DC
References
1. Carlat D. 45,000 More psychiatrists, anyone? Psychiatric Times. http://www.psychiatrictimes.com/articles/45000-more-psychiatrists-anyone-0.Published August 3, 2010. Accessed November 11, 2016.
2. The Match: National Resident Matching Program. results and data: 2016 main residency match. http://www.nrmp.org/wp-content/uploads/2016/04/Main-Match-Results-and-Data-2016.pdf. Published April 2016. Accessed November 11, 2016.
3. Miller JG, Peterson DJ. Employing nurse practitioners and physician assistants to provide access to care as the psychiatrist shortage continues. Acad Psychiatry. 2015;39(6):685-686.
Concerns in psychiatry
The August 2016 editorial (Unresolved questions about the specialty lurk in the cortext of psychiatrists.
Denis F. Darko, MD
CEO
NeuroSci R&D Consultancy, LLC
Maple Grove, Minnesota
Wart's That Lesion?
A 10-year-old girl is referred to dermatology for evaluation of an asymptomatic “wart” that manifested on her thigh several years ago. At first, the lesion grew rapidly, but it has since stabilized. It remains unaffected despite multiple treatments with liquid nitrogen.
According to her parents (with confirmation from her medical record), the patient is otherwise quite healthy.
EXAMINATION
A spindle-shaped, pinkish red, 1.2 x 0.7-cm nodule with a smooth surface and rounded borders is located on the patient’s distal left anterior thigh. The lesion itself is quite firm but nontender to the touch, and its long axis is parallel to transverse local skin tension lines. The patient’s type II skin is otherwise unremarkable.
After consultation with the patient and her family, the lesion is excised under local anesthesia with 5-mm margins in an elliptical pattern to yield favorable lines of closure. The sample is sent to pathology.
What is the diagnosis?
DISCUSSION
The pathology report showed both epithelioid and spindle cells with scattered apoptotic cells seen at the dermoepidermal junction, with no atypia. The margins were clear. Such findings are consistent with a diagnosis of Spitz nevus.
Prior to the 1950s, lesions with these clinical and histologic features were believed to be melanoma and therefore termed juvenile melanoma. But their unique morphologic and histologic architecture—and a lack of signs of progressive or metastatic disease in affected patients—prompted suspicion that they were not really malignant. A pathologist named Sophie Spitz conducted a retrospective study that revealed no cancerous activity in any of the patients. Her findings, on a superficial level, eventually led to the elimination of the term “juvenile melanoma”—but more importantly, they eradicated a great deal of unnecessary, and often mutilating, surgery.
The features of the case patient’s lesion—color, texture, shape, and initial rapid growth —are typical of Spitz nevi, as are the location and the age of the patient. However, the range of morphologic presentations includes darker lesions (some almost black) and those of varying vertical depth and appearance (ie, intradermal, compound, junctional).
A note of caution, however: Although Spitz nevi are benign, true melanomas can present with “Spitzoid” morphologic and histologic features (they’re even called “Spitzoid melanomas”). Key distinguishing features can only be identified via microscopic examination of the lesion’s full vertical structure. So, for safety’s sake, the standard of care for Spitz nevi is to excise them totally, with clear, deep, wide margins, whenever possible.
The case patient had her sutures removed 10 days after surgery. Her ongoing care will include a biannual check for signs of recurrence.
TAKE-HOME LEARNING POINTS
• Until the mid-1950s, what we now refer to as Spitz nevi were called “juvenile melanoma” and were treated as such, with radical and often disfiguring surgeries.
• Spitz nevi are usually seen in children. They can be red, pink, or even black, and often have a history of recent growth and an elliptiform shape.
• Excision with totally clear margins is the correct approach, along with pathologic examination.
• Spitz nevi have histologic features suggestive of melanoma, and some melanomas have “Spitzoid” features. Therefore, all cases must be handled with great care.
A 10-year-old girl is referred to dermatology for evaluation of an asymptomatic “wart” that manifested on her thigh several years ago. At first, the lesion grew rapidly, but it has since stabilized. It remains unaffected despite multiple treatments with liquid nitrogen.
According to her parents (with confirmation from her medical record), the patient is otherwise quite healthy.
EXAMINATION
A spindle-shaped, pinkish red, 1.2 x 0.7-cm nodule with a smooth surface and rounded borders is located on the patient’s distal left anterior thigh. The lesion itself is quite firm but nontender to the touch, and its long axis is parallel to transverse local skin tension lines. The patient’s type II skin is otherwise unremarkable.

After consultation with the patient and her family, the lesion is excised under local anesthesia with 5-mm margins in an elliptical pattern to yield favorable lines of closure. The sample is sent to pathology.
What is the diagnosis?
DISCUSSION
The pathology report showed both epithelioid and spindle cells with scattered apoptotic cells seen at the dermoepidermal junction, with no atypia. The margins were clear. Such findings are consistent with a diagnosis of Spitz nevus.
Prior to the 1950s, lesions with these clinical and histologic features were believed to be melanoma and therefore termed juvenile melanoma. But their unique morphologic and histologic architecture—and a lack of signs of progressive or metastatic disease in affected patients—prompted suspicion that they were not really malignant. A pathologist named Sophie Spitz conducted a retrospective study that revealed no cancerous activity in any of the patients. Her findings, on a superficial level, eventually led to the elimination of the term “juvenile melanoma”—but more importantly, they eradicated a great deal of unnecessary, and often mutilating, surgery.
The features of the case patient’s lesion—color, texture, shape, and initial rapid growth —are typical of Spitz nevi, as are the location and the age of the patient. However, the range of morphologic presentations includes darker lesions (some almost black) and those of varying vertical depth and appearance (ie, intradermal, compound, junctional).
A note of caution, however: Although Spitz nevi are benign, true melanomas can present with “Spitzoid” morphologic and histologic features (they’re even called “Spitzoid melanomas”). Key distinguishing features can only be identified via microscopic examination of the lesion’s full vertical structure. So, for safety’s sake, the standard of care for Spitz nevi is to excise them totally, with clear, deep, wide margins, whenever possible.
The case patient had her sutures removed 10 days after surgery. Her ongoing care will include a biannual check for signs of recurrence.
TAKE-HOME LEARNING POINTS
• Until the mid-1950s, what we now refer to as Spitz nevi were called “juvenile melanoma” and were treated as such, with radical and often disfiguring surgeries.
• Spitz nevi are usually seen in children. They can be red, pink, or even black, and often have a history of recent growth and an elliptiform shape.
• Excision with totally clear margins is the correct approach, along with pathologic examination.
• Spitz nevi have histologic features suggestive of melanoma, and some melanomas have “Spitzoid” features. Therefore, all cases must be handled with great care.
A 10-year-old girl is referred to dermatology for evaluation of an asymptomatic “wart” that manifested on her thigh several years ago. At first, the lesion grew rapidly, but it has since stabilized. It remains unaffected despite multiple treatments with liquid nitrogen.
According to her parents (with confirmation from her medical record), the patient is otherwise quite healthy.
EXAMINATION
A spindle-shaped, pinkish red, 1.2 x 0.7-cm nodule with a smooth surface and rounded borders is located on the patient’s distal left anterior thigh. The lesion itself is quite firm but nontender to the touch, and its long axis is parallel to transverse local skin tension lines. The patient’s type II skin is otherwise unremarkable.

After consultation with the patient and her family, the lesion is excised under local anesthesia with 5-mm margins in an elliptical pattern to yield favorable lines of closure. The sample is sent to pathology.
What is the diagnosis?
DISCUSSION
The pathology report showed both epithelioid and spindle cells with scattered apoptotic cells seen at the dermoepidermal junction, with no atypia. The margins were clear. Such findings are consistent with a diagnosis of Spitz nevus.
Prior to the 1950s, lesions with these clinical and histologic features were believed to be melanoma and therefore termed juvenile melanoma. But their unique morphologic and histologic architecture—and a lack of signs of progressive or metastatic disease in affected patients—prompted suspicion that they were not really malignant. A pathologist named Sophie Spitz conducted a retrospective study that revealed no cancerous activity in any of the patients. Her findings, on a superficial level, eventually led to the elimination of the term “juvenile melanoma”—but more importantly, they eradicated a great deal of unnecessary, and often mutilating, surgery.
The features of the case patient’s lesion—color, texture, shape, and initial rapid growth —are typical of Spitz nevi, as are the location and the age of the patient. However, the range of morphologic presentations includes darker lesions (some almost black) and those of varying vertical depth and appearance (ie, intradermal, compound, junctional).
A note of caution, however: Although Spitz nevi are benign, true melanomas can present with “Spitzoid” morphologic and histologic features (they’re even called “Spitzoid melanomas”). Key distinguishing features can only be identified via microscopic examination of the lesion’s full vertical structure. So, for safety’s sake, the standard of care for Spitz nevi is to excise them totally, with clear, deep, wide margins, whenever possible.
The case patient had her sutures removed 10 days after surgery. Her ongoing care will include a biannual check for signs of recurrence.
TAKE-HOME LEARNING POINTS
• Until the mid-1950s, what we now refer to as Spitz nevi were called “juvenile melanoma” and were treated as such, with radical and often disfiguring surgeries.
• Spitz nevi are usually seen in children. They can be red, pink, or even black, and often have a history of recent growth and an elliptiform shape.
• Excision with totally clear margins is the correct approach, along with pathologic examination.
• Spitz nevi have histologic features suggestive of melanoma, and some melanomas have “Spitzoid” features. Therefore, all cases must be handled with great care.