User login
Differentiating ADHD and bipolar disorder
FDA sunscreen guidance calls for maximal use trials
The Food and Drug Administration has released final industry guidance for nonclinical sunscreen manufacturers, but comments from a top agency official bring into question the pace of progress for getting the products to market.
A 2014 law, the Sunscreen Innovation Act, was intended to speed approval of new sunscreen active ingredients; one impetus for its passage was that no new sunscreen active ingredient has been approved since the late 1990s.
During that time, eight applications for new sunscreen active ingredients languished at the agency, even though many, such as UV filters bisoctrizole and bemotrizinol, are widely available across Europe and elsewhere. The law gave the FDA 1 year to review the backlog of applications for the ingredients and gave the agency 18 months to act on any sunscreen ingredient application submitted after the law went into effect.
“The FDA has issued proposed sunscreen orders identifying data we believe is necessary for the agency to make a positive [generally regarded as safe and effective] determination on those within the [law’s] required time frame, but has yet to receive the additional data we requested” from sunscreen ingredient manufacturers, Theresa M. Michele, MD, director of the FDA’s Division of Nonprescription Drug Products, wrote in a blog post.
To help, the agency has just released the final two of four industry guidance documents. The first addresses the agency’s current thinking on what constitutes a generally safe and effective sunscreen ingredient, while the second outlines data submission procedures. Previous guidance addressed procedural matters.
Specifically, the FDA wants to see evidence that these ingredients aren’t toxic over time, something that requires clinical trials in humans.
“The FDA and industry are essentially at a standstill because industry feels that there is a significant amount of resources they have to [invest] in order to comply with these testing regulations,” Dr. Lim said in an interview. “Industry is not willing to do it.”
In Europe and many other countries, sunscreens are considered cosmetics. The FDA considers them over-the-counter drugs and holds them to a higher approval standard. Although the 2014 law allows the FDA to review 5 years or more of marketing history for ingredients that are in use outside the United States, current approval standards require human absorption data derived from maximal usage trials to determine the risk of chronic exposure to products applied over large areas of the body, Dr. Michele said in a statement. “It is the same standard used by the FDA for all topically applied drugs, and especially for drugs that are used routinely over the course of one’s life.”
Dr. Lim said that the AAD is working as a neutral party to help both sides arrive at a compromise, and that a meeting between FDA and industry officials is scheduled for early 2017. However, he said that he thinks it is “not likely” that the FDA would ever relax its approval process to view sunscreens at the level of cosmetics as is done abroad, since that would mean different sets of standards for the same class of product. “Knowing how the FDA works, this is not going to happen,” Dr. Lim said.
“The FDA is committed to helping ensure that sunscreens are safe and effective for U.S. consumers, but we need data to move forward,” Dr. Michele wrote.
Dr. Lim disclosed that he is a consultant for Pierre Fabre and an investigator for Allergan, Estee Lauder, and Ferndale.
[email protected]
On Twitter @whitneymcknight
The Food and Drug Administration has released final industry guidance for nonclinical sunscreen manufacturers, but comments from a top agency official bring into question the pace of progress for getting the products to market.
A 2014 law, the Sunscreen Innovation Act, was intended to speed approval of new sunscreen active ingredients; one impetus for its passage was that no new sunscreen active ingredient has been approved since the late 1990s.
During that time, eight applications for new sunscreen active ingredients languished at the agency, even though many, such as UV filters bisoctrizole and bemotrizinol, are widely available across Europe and elsewhere. The law gave the FDA 1 year to review the backlog of applications for the ingredients and gave the agency 18 months to act on any sunscreen ingredient application submitted after the law went into effect.
“The FDA has issued proposed sunscreen orders identifying data we believe is necessary for the agency to make a positive [generally regarded as safe and effective] determination on those within the [law’s] required time frame, but has yet to receive the additional data we requested” from sunscreen ingredient manufacturers, Theresa M. Michele, MD, director of the FDA’s Division of Nonprescription Drug Products, wrote in a blog post.
To help, the agency has just released the final two of four industry guidance documents. The first addresses the agency’s current thinking on what constitutes a generally safe and effective sunscreen ingredient, while the second outlines data submission procedures. Previous guidance addressed procedural matters.
Specifically, the FDA wants to see evidence that these ingredients aren’t toxic over time, something that requires clinical trials in humans.
“The FDA and industry are essentially at a standstill because industry feels that there is a significant amount of resources they have to [invest] in order to comply with these testing regulations,” Dr. Lim said in an interview. “Industry is not willing to do it.”
In Europe and many other countries, sunscreens are considered cosmetics. The FDA considers them over-the-counter drugs and holds them to a higher approval standard. Although the 2014 law allows the FDA to review 5 years or more of marketing history for ingredients that are in use outside the United States, current approval standards require human absorption data derived from maximal usage trials to determine the risk of chronic exposure to products applied over large areas of the body, Dr. Michele said in a statement. “It is the same standard used by the FDA for all topically applied drugs, and especially for drugs that are used routinely over the course of one’s life.”
Dr. Lim said that the AAD is working as a neutral party to help both sides arrive at a compromise, and that a meeting between FDA and industry officials is scheduled for early 2017. However, he said that he thinks it is “not likely” that the FDA would ever relax its approval process to view sunscreens at the level of cosmetics as is done abroad, since that would mean different sets of standards for the same class of product. “Knowing how the FDA works, this is not going to happen,” Dr. Lim said.
“The FDA is committed to helping ensure that sunscreens are safe and effective for U.S. consumers, but we need data to move forward,” Dr. Michele wrote.
Dr. Lim disclosed that he is a consultant for Pierre Fabre and an investigator for Allergan, Estee Lauder, and Ferndale.
[email protected]
On Twitter @whitneymcknight
The Food and Drug Administration has released final industry guidance for nonclinical sunscreen manufacturers, but comments from a top agency official bring into question the pace of progress for getting the products to market.
A 2014 law, the Sunscreen Innovation Act, was intended to speed approval of new sunscreen active ingredients; one impetus for its passage was that no new sunscreen active ingredient has been approved since the late 1990s.
During that time, eight applications for new sunscreen active ingredients languished at the agency, even though many, such as UV filters bisoctrizole and bemotrizinol, are widely available across Europe and elsewhere. The law gave the FDA 1 year to review the backlog of applications for the ingredients and gave the agency 18 months to act on any sunscreen ingredient application submitted after the law went into effect.
“The FDA has issued proposed sunscreen orders identifying data we believe is necessary for the agency to make a positive [generally regarded as safe and effective] determination on those within the [law’s] required time frame, but has yet to receive the additional data we requested” from sunscreen ingredient manufacturers, Theresa M. Michele, MD, director of the FDA’s Division of Nonprescription Drug Products, wrote in a blog post.
To help, the agency has just released the final two of four industry guidance documents. The first addresses the agency’s current thinking on what constitutes a generally safe and effective sunscreen ingredient, while the second outlines data submission procedures. Previous guidance addressed procedural matters.
Specifically, the FDA wants to see evidence that these ingredients aren’t toxic over time, something that requires clinical trials in humans.
“The FDA and industry are essentially at a standstill because industry feels that there is a significant amount of resources they have to [invest] in order to comply with these testing regulations,” Dr. Lim said in an interview. “Industry is not willing to do it.”
In Europe and many other countries, sunscreens are considered cosmetics. The FDA considers them over-the-counter drugs and holds them to a higher approval standard. Although the 2014 law allows the FDA to review 5 years or more of marketing history for ingredients that are in use outside the United States, current approval standards require human absorption data derived from maximal usage trials to determine the risk of chronic exposure to products applied over large areas of the body, Dr. Michele said in a statement. “It is the same standard used by the FDA for all topically applied drugs, and especially for drugs that are used routinely over the course of one’s life.”
Dr. Lim said that the AAD is working as a neutral party to help both sides arrive at a compromise, and that a meeting between FDA and industry officials is scheduled for early 2017. However, he said that he thinks it is “not likely” that the FDA would ever relax its approval process to view sunscreens at the level of cosmetics as is done abroad, since that would mean different sets of standards for the same class of product. “Knowing how the FDA works, this is not going to happen,” Dr. Lim said.
“The FDA is committed to helping ensure that sunscreens are safe and effective for U.S. consumers, but we need data to move forward,” Dr. Michele wrote.
Dr. Lim disclosed that he is a consultant for Pierre Fabre and an investigator for Allergan, Estee Lauder, and Ferndale.
[email protected]
On Twitter @whitneymcknight
Proton Pump Inhibitor-Associated Hypomagnesemia: A Retrospective Case-Control Study
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
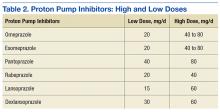
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
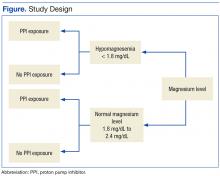
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
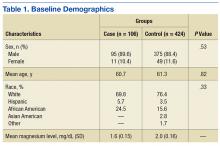
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
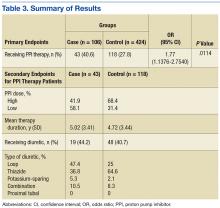
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
In the U.S., proton pump inhibitors (PPIs) are one of the best-selling drug classes—more than $9 billion were spent on PPIs in 2012.1 These medications, available both by prescription and over-the-counter (OTC), are used to treat a variety of gastrointestinal conditions, including heartburn, gastroesophageal reflux disease, and peptic ulcer disease.1
Proton pump inhibitors are generally recognized as safe and effective. In 2011, however, the FDA reviewed Adverse Event Reporting System (AERS) reports, medical literature, and periodic safety updates and issued a safety communication outlining the risk for hypomagnesemia with prolonged PPI use.2 The FDA focused on 53 cases: 30 AERS cases, 15 in the literature, and 8 reported both through AERS and in the literature. The majority involved PPI use that continued for 1 year or longer, but in some cases hypomagnesemia developed after only 3 months. Labeling for prescription PPIs was updated with information about the hypomagnesemia risk, but labeling for the OTC drugs was not affected, as the FDA stated there is little risk with OTC use, and the label already indicated that use should be limited to 14 days at a time and up to 3 courses within 1 year.
Magnesium is an important intracellular cation that plays a role in multiple cellular activities. Low levels of magnesium can lead to a wide variety of adverse events (AEs), including vomiting, diarrhea, cramps, convulsions, bradycardia, and even death.3,4 The mechanism of PPI-associated hypomagnesemia is yet to be established but could be related to, as has been proposed, altered intestinal absorption of magnesium with long-term PPI use.4
Results from investigations of PPI-associated hypomagnesemia have been inconclusive. In a study of PPI-associated AEs reported to the FDA, Luk and colleagues estimated that 1% of patients who experienced an AE reported hypomagnesemia and concluded that all PPIs are associated with hypomagnesemia, but the risk varies. Of the 6 PPIs that have been FDA approved, esomeprazole was associated with the lowest risk, pantoprazole with the most. Results also suggested that the risk was higher for elderly and male patients.
In another study of prior PPI use and its effects on magnesium levels among 11,490 intensive care unit admissions, Danziger and colleagues found that the association of PPI use and hypomagnesemia was limited to patients who concomitantly received a diuretic, and use of a histamine 2 receptor antagonist was not associated with hypomagnesemia.3 A third cross-sectional study of 402 adults with hypomagnesemia on hospital admission found no association between outpatient PPI regimens and hypomagnesemia.5 Other studies designed to investigate PPI-associated hypomagnesemia were limited by short-term PPI use, small samples, concurrent diseases, and confounding variables (eg, history of alcoholism).6,7
Need for Present Study
The evidence needed to establish the incidence of PPI-associated hypomagnesemia is limited. Hypomagnesemia can lead to serious AEs, as just outlined, and is a common indication for hospitalization.8 The hypomagnesemia rate is about 12% in hospitalized patients and sharply higher (60%-65%) in those who are critically ill. Proton pump inhibitor-associated hypomagnesemia is preventable, and monitoring parameters can be recommended to patients undergoing long-term therapy.
Ajumobi and colleagues found that 13,713 (23.4%) of 58,605 patients treated at a VA center over a 12-month period were receiving a PPI.9 Gawron and colleagues found that many veterans had been prescribed a PPI and were receiving high total daily doses for the treatment of gastroesophageal reflux disease.10 The majority of patients received a 90-day or longer supply and showed minimal evidence of step-down therapy or cessation of PPI therapy.
In the present study, the authors investigated the rate of PPI-associated hypomagnesemia in a veteran population at a facility where the majority of PPIs were by prescription, not OTC. The Captain James A. Lovell Federal Health Care Center (FHCC) is a combined DoD and VA facility where veterans and active military members and their dependents receive medical care and prescription drugs.
This study’s primary objective was to determine the rate of PPI-induced hypomagnesemia. The secondary objective was to identify any clinical factors (eg, PPI dose and therapy duration, concomitant use of a diuretic) that might further increase the risk of hypomagnesemia.
Methods
After the study protocol was approved by the Lovell FHCC institutional review board, the authors retrospectively compared patients with a low magnesium level (case group) with patients with a normal magnesium level (control group). In each group, the authors identified patients who underwent PPI therapy and those who did not (Figure).
Study inclusion criteria were low magnesium level (< 1.8 mg/dL) within the past 5 years for veterans in the case group and normal magnesium level (1.8-2.4 mg/dL) within the past 5 years for veterans in the control group. Exclusion criteria were nonveterans and no prior magnesium level for a veteran.
Patients were assigned in a ratio of 1 (case group) to 4 (control group) and were added only after confirmation that multiple magnesium levels had been recorded (January 2008-January 2013).
Patients who met the inclusion criteria were enrolled in the study. Patient’s Computerized Patient Record System charts were reviewed for demographics (sex, age, race); magnesium level; active order for PPI during same period magnesium level was drawn; PPI name, dose, and therapy duration; and concomitant use of a diuretic (yes or no) and, if yes, type of diuretic.
To assess a significance criterion (α) of 0.05 and a power of 80% 1,375 patients in a 1:4 ratio (275 cases, 1,100 controls) were required in order to detect a difference in rates of hypomagnesemia between patients who received a PPI and those who did not. Primary outcome data are reported as percentages and calculated odds ratios (ORs). Significance of ORs was determined with 95% confidence intervals (CIs). Secondary outcomes were PPI dose and therapy duration and concomitant use of a diuretic. Descriptive statistics were used for secondary outcomes.
Results
Five hundred thirty charts (106 cases, 424 controls) were included and reviewed. Table 1 lists the baseline demographics. There were no statistically significant differences in age, sex, or race between the case and control groups. Mean (SD) magnesium level was 1.6 (0.15)
The authors assessed for other clinical factors that might concurrently or
Discussion
One of the most widely prescribed classes of medications, PPIs are often regarded as safe and effective and therefore continued as long-term therapy. Results of this study showed an association of PPI use and hypomagnesemia—thereby adding to the literature. Results for the secondary objective suggest that the association does not necessarily depend on PPI dose, but, given that a statistical analysis of the difference between the case and control groups was not conducted, the statistical significance is unknown.
Although the hypomagnesemia rate remains undetermined, the results of this NNH study suggest a rate higher than previously proposed. Other investigators have estimated the rate of PPI-associated hypomagnesemia at 1%, which does not correlate well with the NNH often calculated in this study. For 2 possible reasons, the poor correlation may be attributable to underreporting of hypomagnesemia: Magnesium levels are not commonly checked with a basic metabolic panel, and many patients who are mildly hypomagnesemic remain asymptomatic.
Future research directions include determining whether the risk for hypomagnesemia is related to patient status (eg, inpatient vs outpatient) and performing statistical analyses on the secondary objective to determine the clinical significance of potential risk factors. Other research directions might involve assessing PPI discontinuation rates in a hypomagnesemic population and assessing outcomes such as hospitalizations and AEs (eg, seizure, tetany, arrhythmia).
Limitations
This study had several limitations. First was the overall design. Study results described only a potential association of PPI use and hypomagnesemia, not definitive cause and effect. Results also depended on an assumed, previously reported rate of PPI-associated hypomagnesemia and a rate of exposure to PPIs, as these data were taken into account in the overall study design. In addition, patient adherence to prescribed therapy and accuracy of medication history were assumed from the medication and dispensing history, as not all medications obtained outside the Lovell FHCC were accurately documented. There also was an external validity limitation in that older men make up the typical FHCC patient population. Last, as inherent to all studies that use objective measures, there was the potential for laboratory magnesium level reporting errors.
Conclusion
The study results identified an association of PPI use and hypomagnesemia in a VA patient population of older men. More studies need to be conducted with non-VA patient populations to further assess the incidence of PPI-associated hypomagnesemia.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.
1. Consumers Union. Consumer Reports Best Buy Drugs: Using the proton pump inhibitors to treat heartburn and stomach acid reflux, comparing effectiveness, safety, and price. http://www.consumer reports.org/health/resources/pdf/best-buy-drugs/PPIsUpdate-FINAL.pdf. Updated July 2013. Accessed November 4, 2016.
2. U.S. Food and Drug Administration. FDA drug safety communication: low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs). http://www.fda.gov/Drugs /DrugSafety/ucm245011.htm. Updated April 7, 2016. Accessed November 4, 2016.
3. Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692-699.
4. Luk CP, Parsons R, Lee YP, Hughes JD. Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us? Ann Pharmacother. 2013;47(6):773-780.
5. Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730-737.
6. Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. QJM. 2010;103(6):387-395.
7. Faulhaber GA, Ascoli BA, Lubina A, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras (1992). 2013;59(3):276-279.
8. Yu ASL. Causes of hypomagnesemia. UpToDate. http://www.uptodate.com/contents/causes-of-hypomagnesemia. Updated February 4, 2016. Accessed November 4, 2016.
9. Ajumobi AB, Vuong R, Ahaneku H. Analysis of nonformulary use of PPIs and excess drug cost in a Veterans Affairs population. J Manag Care Pharm. 2012;18(1):63-67.
10. Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930-937.
Exploring the function of self-criticism
A Stroke of Bad (Pot)luck
ANSWER
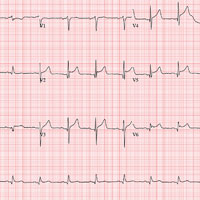
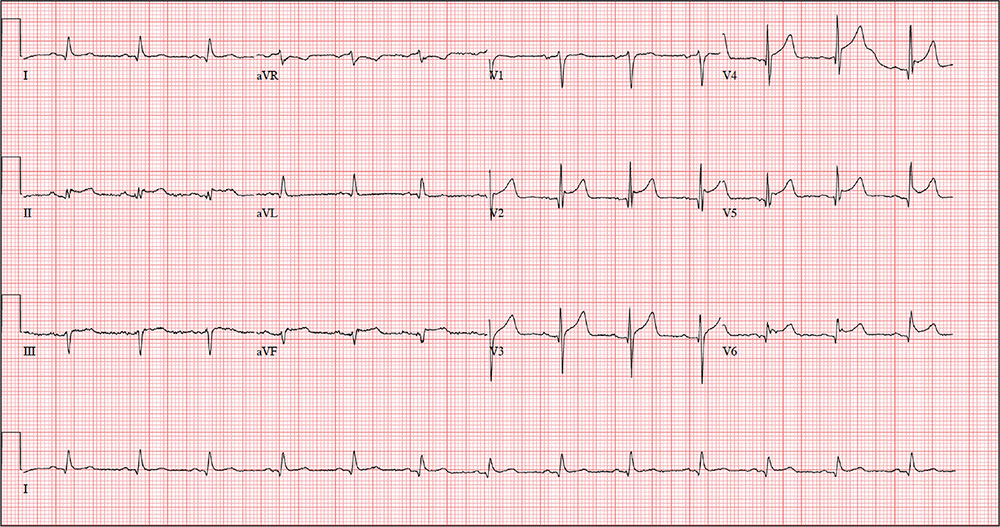
The correct interpretation includes normal sinus rhythm, acute ST elevation anterior myocardial infarction (STEMI), and inferolateral injury. Sinus rhythm is signified by a P wave for every QRS complex and a QRS complex for every P wave at a rate between 60 and 100 beats/min.
A STEMI is defined as new ST elevations at the J point in at least two contiguous leads—in women, 1.5 mm in leads V2 and V3 and 1 mm in all other leads; in men, 2 mm (those 40 and older) or 2.5 mm (those younger than 40) in leads V2 and V3 and 1 mm in all other leads. The anterior location is evidenced by the significant Q waves in leads I, V2, V3, and V4. T-wave inversions in these leads are absent due to the STEMI.
Inferolateral injury is identified by the Q waves in leads I, aVL, V5, and V6. Pseudo Q waves are seen in leads II, III, and aVF.
Troponin levels were significant for an acute myocardial infarction (MI). Cardiac cauterization confirmed an occlusion of the proximal left anterior descending coronary artery, and significant stenosis of the first obtuse marginal branch of the circumflex coronary artery. The patient’s lack of chest pain represents a common presentation of an MI in women, particularly those with a history of diabetes.
ANSWER
The correct interpretation includes normal sinus rhythm, acute ST elevation anterior myocardial infarction (STEMI), and inferolateral injury. Sinus rhythm is signified by a P wave for every QRS complex and a QRS complex for every P wave at a rate between 60 and 100 beats/min.
A STEMI is defined as new ST elevations at the J point in at least two contiguous leads—in women, 1.5 mm in leads V2 and V3 and 1 mm in all other leads; in men, 2 mm (those 40 and older) or 2.5 mm (those younger than 40) in leads V2 and V3 and 1 mm in all other leads. The anterior location is evidenced by the significant Q waves in leads I, V2, V3, and V4. T-wave inversions in these leads are absent due to the STEMI.
Inferolateral injury is identified by the Q waves in leads I, aVL, V5, and V6. Pseudo Q waves are seen in leads II, III, and aVF.
Troponin levels were significant for an acute myocardial infarction (MI). Cardiac cauterization confirmed an occlusion of the proximal left anterior descending coronary artery, and significant stenosis of the first obtuse marginal branch of the circumflex coronary artery. The patient’s lack of chest pain represents a common presentation of an MI in women, particularly those with a history of diabetes.
ANSWER
The correct interpretation includes normal sinus rhythm, acute ST elevation anterior myocardial infarction (STEMI), and inferolateral injury. Sinus rhythm is signified by a P wave for every QRS complex and a QRS complex for every P wave at a rate between 60 and 100 beats/min.
A STEMI is defined as new ST elevations at the J point in at least two contiguous leads—in women, 1.5 mm in leads V2 and V3 and 1 mm in all other leads; in men, 2 mm (those 40 and older) or 2.5 mm (those younger than 40) in leads V2 and V3 and 1 mm in all other leads. The anterior location is evidenced by the significant Q waves in leads I, V2, V3, and V4. T-wave inversions in these leads are absent due to the STEMI.
Inferolateral injury is identified by the Q waves in leads I, aVL, V5, and V6. Pseudo Q waves are seen in leads II, III, and aVF.
Troponin levels were significant for an acute myocardial infarction (MI). Cardiac cauterization confirmed an occlusion of the proximal left anterior descending coronary artery, and significant stenosis of the first obtuse marginal branch of the circumflex coronary artery. The patient’s lack of chest pain represents a common presentation of an MI in women, particularly those with a history of diabetes.
An active 74-year-old woman becomes nauseated and diaphoretic while cleaning up her church’s Sunday evening potluck. She shrugs it off, attributing it to something she ate, but while preparing to go home, she abruptly becomes short of breath and complains she can’t catch her breath. Two coworkers describe her appearance as pale with “complete loss of color” and insist on driving her to the local emergency department (ED).
When she arrives 10 minutes later, her shortness of breath is resolving. She denies chest pain, vomiting, and diarrhea. Despite receiving oxygen via nasal cannula, she still appears pale. An ECG and laboratory tests are ordered.
The patient’s medical history is remarkable for type 2 diabetes, hypothyroidism, obesity, and hypertension. Her surgical history is remarkable for a cholecystectomy and a hysterectomy 40 years ago, following the delivery of her third child.
The patient is a retired elementary school principal. She drinks alcohol socially and has never smoked. She lost her husband to colon cancer several years ago and now lives by herself in her home. She has three adult children who are all alive and well. Her mother died of heart failure, and her father was killed in an automobile accident.
Her current medication list includes metformin, levothyroxine, and hydrochlorothiazide. She states she has tried other medications for her diabetes and hypertension but prefers her current regimen. She is allergic to radiographic contrast media, with a documented near-anaphylactic reaction in the past.
Physical exam reveals a pleasant, cooperative, but apprehensive woman who appears her stated age. She wears corrective lenses and bilateral hearing aids. Her neck veins are not distended, and there is no evidence of thyromegaly. Respirations are rapid and shallow, with few crackles in both bases bilaterally but no rhonchi or rales. Her cardiac exam reveals a regular rate and rhythm with a soft, grade II, mid-systolic murmur at the left upper sternal border without radiation. There are no extra heart sounds or rubs. The abdomen is soft and nontender with well-healed surgical scars. The peripheral pulses are full and equal in both upper and lower extremities. The neurologic exam is grossly intact.
The review of systems shows that she has gained 14 pounds over the past four months, has been less physically active, and feels like she is “slowing down.” She also says her hearing and eyesight aren’t what they used to be, and she has chronic constipation. The remainder of her review of systems is unremarkable.
The patient’s weight is 218 lb and her height, 64 in. Vital signs include a blood pressure of 148/
110 mm Hg; pulse, 80 beats/min and regular; O2 saturation, 98% on 4 L O2 via nasal prongs; and respiratory rate, 20 breaths/min-1.
An ECG is obtained, and blood specimens for labwork—including a complete chemistry profile, complete blood count, thyroid function studies, and troponin levels—are collected. The ECG shows a ventricular rate of 80 beats/min; PR interval, 162 ms; QRS duration, 106 ms; QT/QTc intervals, 370/426 ms; P axis, 51°; R axis, –20°; and T axis, 70°. What is your interpretation of this ECG?
The Liver Meeting 2016 debrief – key abstracts
BOSTON – Amid a plethora of quality research, several abstracts stood out at the annual meeting of the American Association for the Study of Liver Diseases, Arun J. Sanyal, MD, said during the final debrief.
He focused first on nonalcoholic fatty liver disease (NAFLD), which has lacked rigorous studies of disease evolution. Consequently, “current therapeutic development is based on small retrospective data sets with heterogenous populations,” Dr. Sanyal said. Therefore, he and his associates correlated serial biopsies with clinical data (abstract 37). The results confirmed the waxing and waning nature of NAFLD and linked regressing or progressive fibrosis to several factors, including NAFLD Disease Activity score (NAS). NAFLD and nonalcoholic steatohepatitis (NASH) are “not two different diseases, it’s the same disease,” Dr. Sanyal said. “Establishing disease activity as a driver of disease progression is highly relevant for development of noninvasive biomarkers, and also gives us a foundation for the development of clinical trials in this space.”
Several studies of NASH biomarkers yielded notable results at the meeting. In the largest study to date of circulating microRNAs as markers of NASH, (LB2) the miRNAs 34a, 122a, and 200a distinguished patients with and without NAS scores of at least 4 and at least stage 2 fibrosis with areas under the receiver operating characteristic curve (AUROC) between 0.59 and 0.80. “MicroRNAs appear promising, but likely need to be combined with additional biomarkers,” Dr. Sanyal said.
He also noted a study (abstract 40) in which metabolomics of liquid biopsies comprehensively evaluated NAFLD, including fibrosis stage, with AUROCs up to 0.95. Metabolomics “holds promise as a diagnostic tool that can be operationalized for point-of-care testing,” he said.
When it comes to NAFLD, hepatologists “often struggle with what to tell our patients about alcohol,” Dr. Sanyal said. To help clarify the issue, abstract 31 compared NAFLD patients who did or did not report habitually consuming up to two drinks a day in formal prospective questionnaires. After adjustment for baseline histology, abstainers and modest drinkers did not differ on any measure of histologic change, except that abstainers had a greater decrease in steatosis on follow-up biopsy. These findings negate several retrospective studies by suggesting that alcohol consumption does not positively affect the trajectory of NAFLD, Dr. Sanyal concluded.
Many new compounds for treating NASH are in early development, he noted. Among those further along the pipeline, the immunomodulator and CCR2/CCR5 inhibitor cenicriviroc (CVC) missed its primary endpoint (improved NAS and no worsening of fibrosis) but was associated with significantly improved fibrosis without worsening of NASH in the phase 2b CENTAUR study (LB1).
“We also saw highly promising evidence for the effects of ASK1 [apoptosis signal regulating kinase] inhibition on hepatic fibrosis and disease activity in NASH,” Dr. Sanyal added. In a randomized phase II trial (LB3), the ASK1 inhibitor GS-4997 was associated with significant improvement in fibrosis without worsening of NASH when given in combination with simtuzumab, and also improved liver stiffness and magnetic resonance imaging–estimated proton density fat fraction (MRI-PDFF). “These very promising and exciting results need confirmation in more advanced, placebo-controlled trials,” Dr. Sanyal said.
Studies of alcohol use disorders of the liver confirmed that prednisolone has marginal benefits, that the benefits of steroids in general are offset by sepsis, and that pentoxifylline produced no mortality benefit, Dr. Sanyal noted. In studies of primary biliary cirrhosis, the farnesoid-X receptor agonist obeticholic acid (OCA), which was approved by the Food and Drug Administration in 2016, was associated with significantly improved AST to Platelet Ratio Index (APRI) and liver stiffness measures by transient elastography at doses of 10 mg or titrated from 5 mg to 10 mg, with or without ursodeoxycholic acid (abstract 209). In another study, patients with PBC who received norUDCA, a side chain–shortened version of UDCA, experienced decreases in serum ALP levels that were dose dependent and differed significantly from trends in the placebo group (abstract 210).
In another study, the investigational ileal bile acid transporter inhibitor GSK2330672 was associated with significant reductions in itch, compared with placebo, and with lower serum bile acids among pruritic PBC patients (abstract 205). Treatment was associated with diarrhea, but it was usually mild and transient.
Dr. Sanyal concluded by reviewing several studies of cirrhosis and hepatic encephalopathy. In a prospective randomized controlled trial (abstract 247), lactulose with albumin significantly outperformed lactulose monotherapy for reversing hepatic encephalopathy, reducing hospital stays, and preventing mortality, especially sepsis-related death.
In another multicenter, 24-week, phase IV open-label study (abstract 248), 25% of patients experienced breakthrough hepatic encephalopathy when treated with rifaximin monotherapy, compared with only 14% of patients who received both rifaximin and lactulose.
Finally, in a phase II trial (abstract 2064), rifaximin immediate-release (40 mg) significantly outperformed placebo in terms of cirrhosis-related mortality, hospitalizations for cirrhosis, and breakthrough hepatic encephalopathy. The takeaways? “Use albumin with lactulose for acute hepatic encephalopathy,” Dr. Sanyal said. “Rifaximin with lactulose is better than rifaximin alone for secondary prophylaxis, and rifaximin immediate-release may decrease the need for hospitalization and the first bout of hepatic encephalopathy.”
The Liver Meeting next convenes October 20-24, 2017, in Washington, D.C.
Dr. Sanyal disclosed ties to Genfit, NewCo, Akarna, Elsevier, UptoDate, Novartis, Pfizer, Lilly, Astra Zeneca, and a number of other companies.
BOSTON – Amid a plethora of quality research, several abstracts stood out at the annual meeting of the American Association for the Study of Liver Diseases, Arun J. Sanyal, MD, said during the final debrief.
He focused first on nonalcoholic fatty liver disease (NAFLD), which has lacked rigorous studies of disease evolution. Consequently, “current therapeutic development is based on small retrospective data sets with heterogenous populations,” Dr. Sanyal said. Therefore, he and his associates correlated serial biopsies with clinical data (abstract 37). The results confirmed the waxing and waning nature of NAFLD and linked regressing or progressive fibrosis to several factors, including NAFLD Disease Activity score (NAS). NAFLD and nonalcoholic steatohepatitis (NASH) are “not two different diseases, it’s the same disease,” Dr. Sanyal said. “Establishing disease activity as a driver of disease progression is highly relevant for development of noninvasive biomarkers, and also gives us a foundation for the development of clinical trials in this space.”
Several studies of NASH biomarkers yielded notable results at the meeting. In the largest study to date of circulating microRNAs as markers of NASH, (LB2) the miRNAs 34a, 122a, and 200a distinguished patients with and without NAS scores of at least 4 and at least stage 2 fibrosis with areas under the receiver operating characteristic curve (AUROC) between 0.59 and 0.80. “MicroRNAs appear promising, but likely need to be combined with additional biomarkers,” Dr. Sanyal said.
He also noted a study (abstract 40) in which metabolomics of liquid biopsies comprehensively evaluated NAFLD, including fibrosis stage, with AUROCs up to 0.95. Metabolomics “holds promise as a diagnostic tool that can be operationalized for point-of-care testing,” he said.
When it comes to NAFLD, hepatologists “often struggle with what to tell our patients about alcohol,” Dr. Sanyal said. To help clarify the issue, abstract 31 compared NAFLD patients who did or did not report habitually consuming up to two drinks a day in formal prospective questionnaires. After adjustment for baseline histology, abstainers and modest drinkers did not differ on any measure of histologic change, except that abstainers had a greater decrease in steatosis on follow-up biopsy. These findings negate several retrospective studies by suggesting that alcohol consumption does not positively affect the trajectory of NAFLD, Dr. Sanyal concluded.
Many new compounds for treating NASH are in early development, he noted. Among those further along the pipeline, the immunomodulator and CCR2/CCR5 inhibitor cenicriviroc (CVC) missed its primary endpoint (improved NAS and no worsening of fibrosis) but was associated with significantly improved fibrosis without worsening of NASH in the phase 2b CENTAUR study (LB1).
“We also saw highly promising evidence for the effects of ASK1 [apoptosis signal regulating kinase] inhibition on hepatic fibrosis and disease activity in NASH,” Dr. Sanyal added. In a randomized phase II trial (LB3), the ASK1 inhibitor GS-4997 was associated with significant improvement in fibrosis without worsening of NASH when given in combination with simtuzumab, and also improved liver stiffness and magnetic resonance imaging–estimated proton density fat fraction (MRI-PDFF). “These very promising and exciting results need confirmation in more advanced, placebo-controlled trials,” Dr. Sanyal said.
Studies of alcohol use disorders of the liver confirmed that prednisolone has marginal benefits, that the benefits of steroids in general are offset by sepsis, and that pentoxifylline produced no mortality benefit, Dr. Sanyal noted. In studies of primary biliary cirrhosis, the farnesoid-X receptor agonist obeticholic acid (OCA), which was approved by the Food and Drug Administration in 2016, was associated with significantly improved AST to Platelet Ratio Index (APRI) and liver stiffness measures by transient elastography at doses of 10 mg or titrated from 5 mg to 10 mg, with or without ursodeoxycholic acid (abstract 209). In another study, patients with PBC who received norUDCA, a side chain–shortened version of UDCA, experienced decreases in serum ALP levels that were dose dependent and differed significantly from trends in the placebo group (abstract 210).
In another study, the investigational ileal bile acid transporter inhibitor GSK2330672 was associated with significant reductions in itch, compared with placebo, and with lower serum bile acids among pruritic PBC patients (abstract 205). Treatment was associated with diarrhea, but it was usually mild and transient.
Dr. Sanyal concluded by reviewing several studies of cirrhosis and hepatic encephalopathy. In a prospective randomized controlled trial (abstract 247), lactulose with albumin significantly outperformed lactulose monotherapy for reversing hepatic encephalopathy, reducing hospital stays, and preventing mortality, especially sepsis-related death.
In another multicenter, 24-week, phase IV open-label study (abstract 248), 25% of patients experienced breakthrough hepatic encephalopathy when treated with rifaximin monotherapy, compared with only 14% of patients who received both rifaximin and lactulose.
Finally, in a phase II trial (abstract 2064), rifaximin immediate-release (40 mg) significantly outperformed placebo in terms of cirrhosis-related mortality, hospitalizations for cirrhosis, and breakthrough hepatic encephalopathy. The takeaways? “Use albumin with lactulose for acute hepatic encephalopathy,” Dr. Sanyal said. “Rifaximin with lactulose is better than rifaximin alone for secondary prophylaxis, and rifaximin immediate-release may decrease the need for hospitalization and the first bout of hepatic encephalopathy.”
The Liver Meeting next convenes October 20-24, 2017, in Washington, D.C.
Dr. Sanyal disclosed ties to Genfit, NewCo, Akarna, Elsevier, UptoDate, Novartis, Pfizer, Lilly, Astra Zeneca, and a number of other companies.
BOSTON – Amid a plethora of quality research, several abstracts stood out at the annual meeting of the American Association for the Study of Liver Diseases, Arun J. Sanyal, MD, said during the final debrief.
He focused first on nonalcoholic fatty liver disease (NAFLD), which has lacked rigorous studies of disease evolution. Consequently, “current therapeutic development is based on small retrospective data sets with heterogenous populations,” Dr. Sanyal said. Therefore, he and his associates correlated serial biopsies with clinical data (abstract 37). The results confirmed the waxing and waning nature of NAFLD and linked regressing or progressive fibrosis to several factors, including NAFLD Disease Activity score (NAS). NAFLD and nonalcoholic steatohepatitis (NASH) are “not two different diseases, it’s the same disease,” Dr. Sanyal said. “Establishing disease activity as a driver of disease progression is highly relevant for development of noninvasive biomarkers, and also gives us a foundation for the development of clinical trials in this space.”
Several studies of NASH biomarkers yielded notable results at the meeting. In the largest study to date of circulating microRNAs as markers of NASH, (LB2) the miRNAs 34a, 122a, and 200a distinguished patients with and without NAS scores of at least 4 and at least stage 2 fibrosis with areas under the receiver operating characteristic curve (AUROC) between 0.59 and 0.80. “MicroRNAs appear promising, but likely need to be combined with additional biomarkers,” Dr. Sanyal said.
He also noted a study (abstract 40) in which metabolomics of liquid biopsies comprehensively evaluated NAFLD, including fibrosis stage, with AUROCs up to 0.95. Metabolomics “holds promise as a diagnostic tool that can be operationalized for point-of-care testing,” he said.
When it comes to NAFLD, hepatologists “often struggle with what to tell our patients about alcohol,” Dr. Sanyal said. To help clarify the issue, abstract 31 compared NAFLD patients who did or did not report habitually consuming up to two drinks a day in formal prospective questionnaires. After adjustment for baseline histology, abstainers and modest drinkers did not differ on any measure of histologic change, except that abstainers had a greater decrease in steatosis on follow-up biopsy. These findings negate several retrospective studies by suggesting that alcohol consumption does not positively affect the trajectory of NAFLD, Dr. Sanyal concluded.
Many new compounds for treating NASH are in early development, he noted. Among those further along the pipeline, the immunomodulator and CCR2/CCR5 inhibitor cenicriviroc (CVC) missed its primary endpoint (improved NAS and no worsening of fibrosis) but was associated with significantly improved fibrosis without worsening of NASH in the phase 2b CENTAUR study (LB1).
“We also saw highly promising evidence for the effects of ASK1 [apoptosis signal regulating kinase] inhibition on hepatic fibrosis and disease activity in NASH,” Dr. Sanyal added. In a randomized phase II trial (LB3), the ASK1 inhibitor GS-4997 was associated with significant improvement in fibrosis without worsening of NASH when given in combination with simtuzumab, and also improved liver stiffness and magnetic resonance imaging–estimated proton density fat fraction (MRI-PDFF). “These very promising and exciting results need confirmation in more advanced, placebo-controlled trials,” Dr. Sanyal said.
Studies of alcohol use disorders of the liver confirmed that prednisolone has marginal benefits, that the benefits of steroids in general are offset by sepsis, and that pentoxifylline produced no mortality benefit, Dr. Sanyal noted. In studies of primary biliary cirrhosis, the farnesoid-X receptor agonist obeticholic acid (OCA), which was approved by the Food and Drug Administration in 2016, was associated with significantly improved AST to Platelet Ratio Index (APRI) and liver stiffness measures by transient elastography at doses of 10 mg or titrated from 5 mg to 10 mg, with or without ursodeoxycholic acid (abstract 209). In another study, patients with PBC who received norUDCA, a side chain–shortened version of UDCA, experienced decreases in serum ALP levels that were dose dependent and differed significantly from trends in the placebo group (abstract 210).
In another study, the investigational ileal bile acid transporter inhibitor GSK2330672 was associated with significant reductions in itch, compared with placebo, and with lower serum bile acids among pruritic PBC patients (abstract 205). Treatment was associated with diarrhea, but it was usually mild and transient.
Dr. Sanyal concluded by reviewing several studies of cirrhosis and hepatic encephalopathy. In a prospective randomized controlled trial (abstract 247), lactulose with albumin significantly outperformed lactulose monotherapy for reversing hepatic encephalopathy, reducing hospital stays, and preventing mortality, especially sepsis-related death.
In another multicenter, 24-week, phase IV open-label study (abstract 248), 25% of patients experienced breakthrough hepatic encephalopathy when treated with rifaximin monotherapy, compared with only 14% of patients who received both rifaximin and lactulose.
Finally, in a phase II trial (abstract 2064), rifaximin immediate-release (40 mg) significantly outperformed placebo in terms of cirrhosis-related mortality, hospitalizations for cirrhosis, and breakthrough hepatic encephalopathy. The takeaways? “Use albumin with lactulose for acute hepatic encephalopathy,” Dr. Sanyal said. “Rifaximin with lactulose is better than rifaximin alone for secondary prophylaxis, and rifaximin immediate-release may decrease the need for hospitalization and the first bout of hepatic encephalopathy.”
The Liver Meeting next convenes October 20-24, 2017, in Washington, D.C.
Dr. Sanyal disclosed ties to Genfit, NewCo, Akarna, Elsevier, UptoDate, Novartis, Pfizer, Lilly, Astra Zeneca, and a number of other companies.
AT THE LIVER MEETING 2016
A Noninvasive Mechanical Treatment to Reduce the Visible Appearance of Cellulite
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).
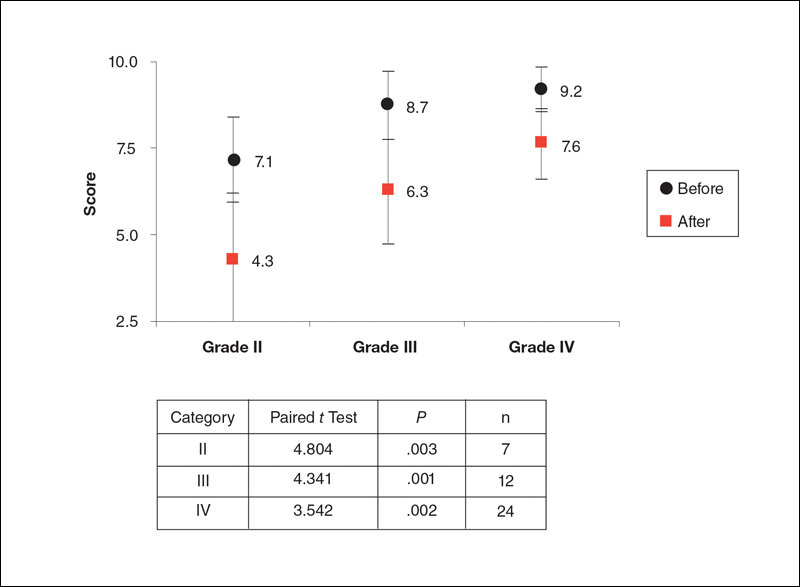
Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).
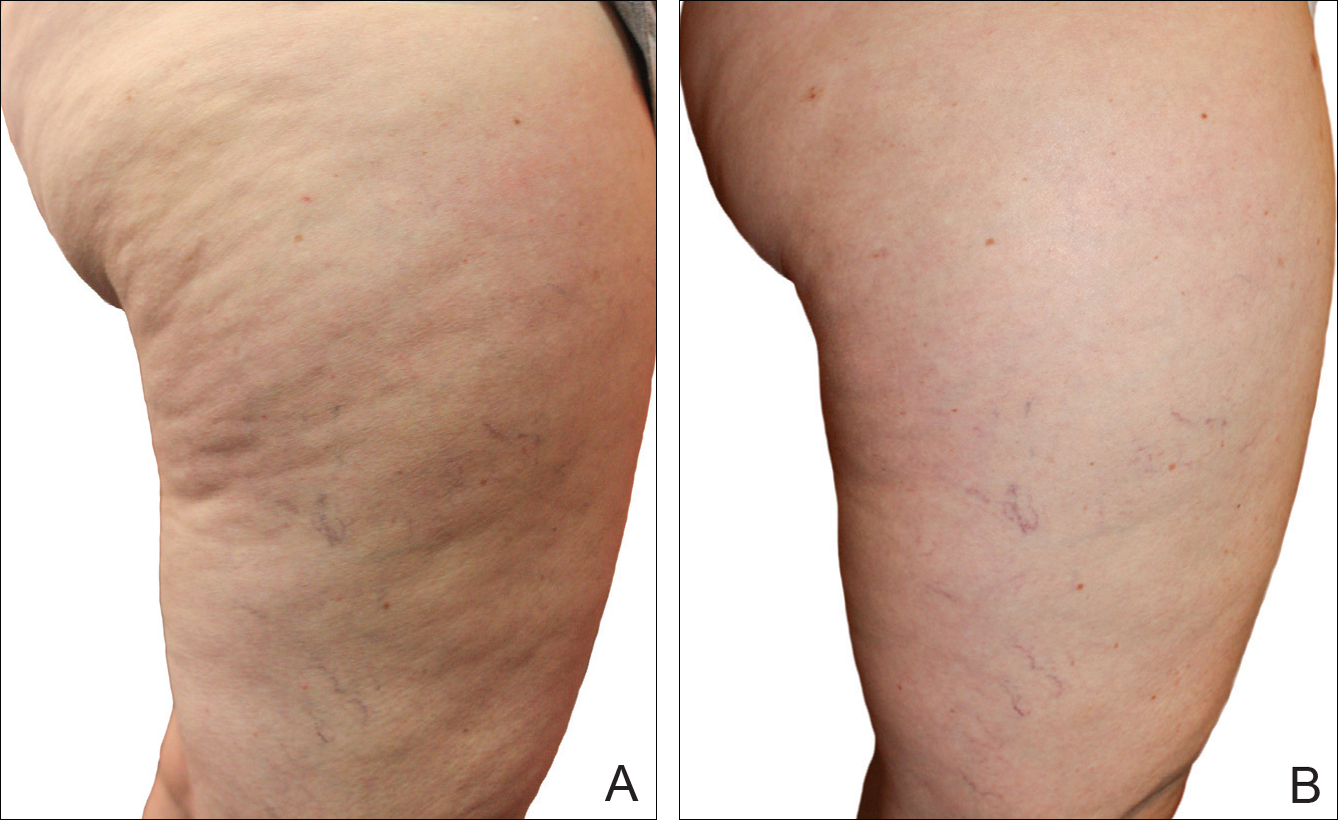
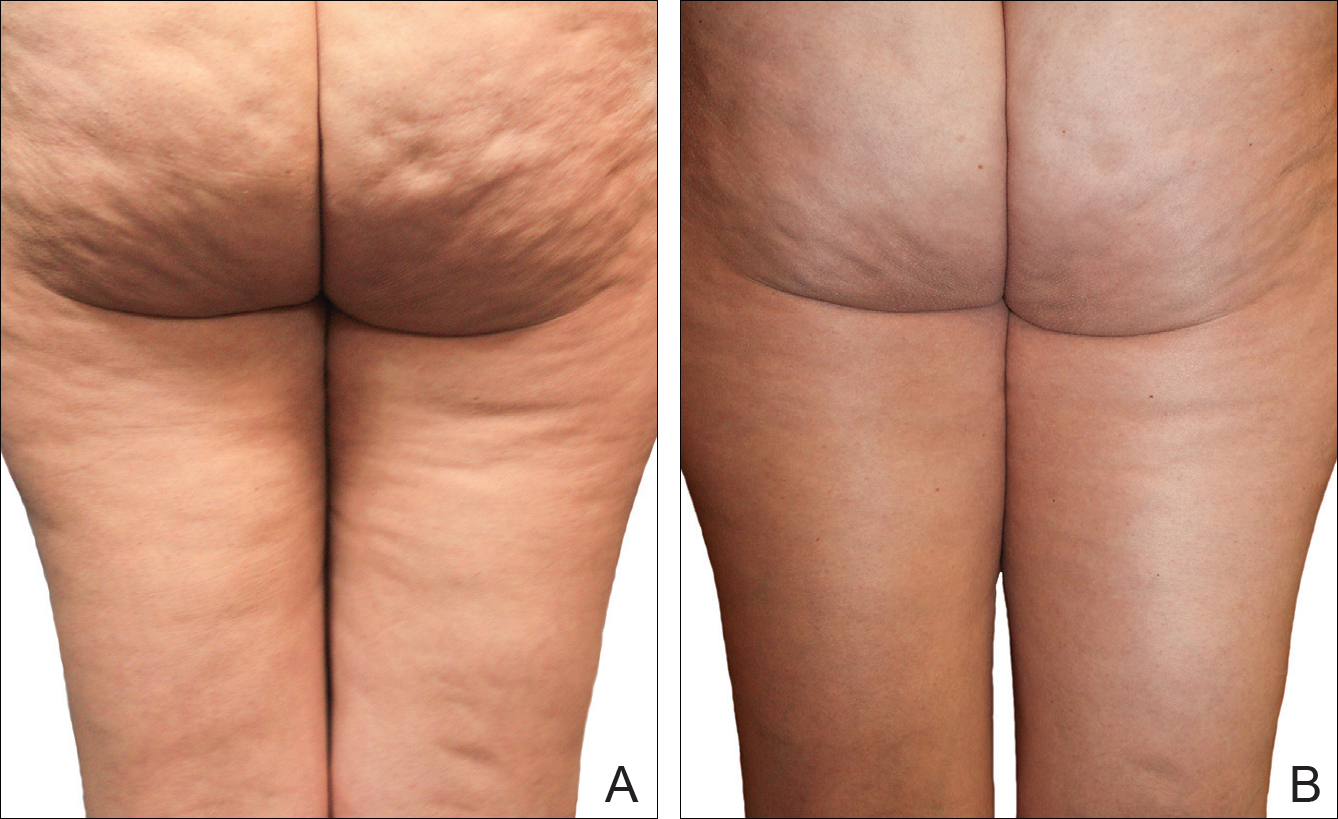
The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).

Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).


The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).

Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).


The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
Practice Points
- Several cellulite treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.
- The noninvasive mechanical treatment for women with cellulite evaluated in this study showed a strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population.
Spot Psoriatic Arthritis Early in Psoriasis Patients
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
A Potpourri of Things to Do Correctly
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
Practice Points
- A biopsy is not separately reportable when another procedure was done at the same site on the same day (eg, shave, excision, destruction).
- Use site-specific biopsy codes when their localization is more precise than the general skin biopsy.
- A simple nail clipping for culture or periodic acid-Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
Physicians Must Encourage HPV Vaccine
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.