User login
Improving your approach to nasal obstruction
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
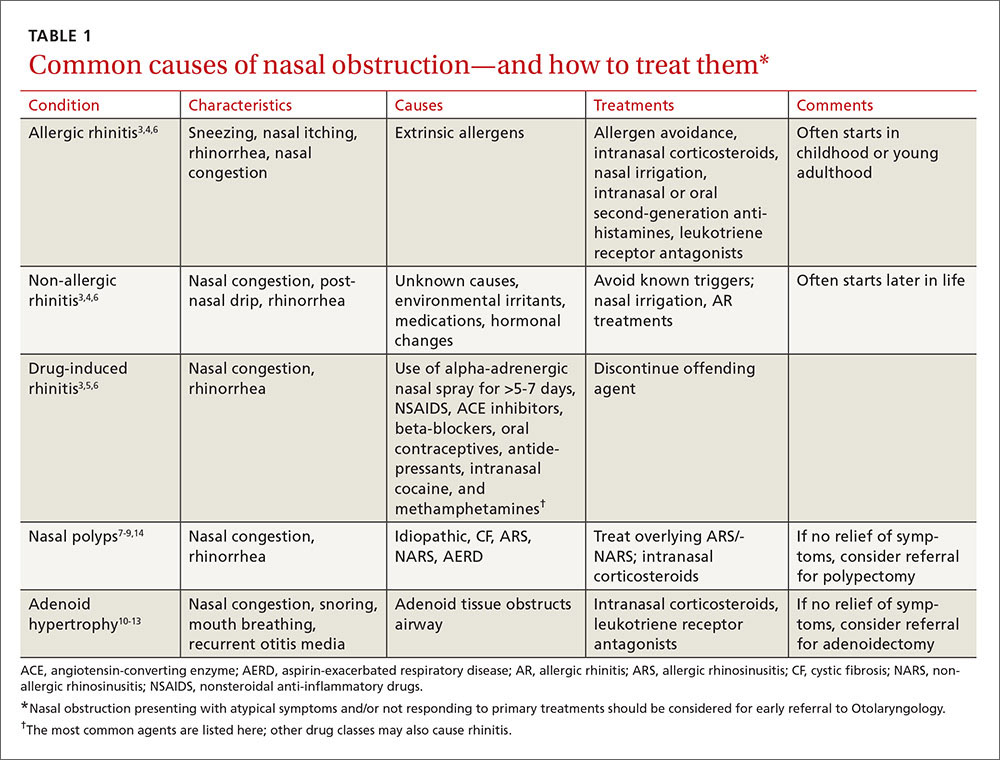
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).
Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
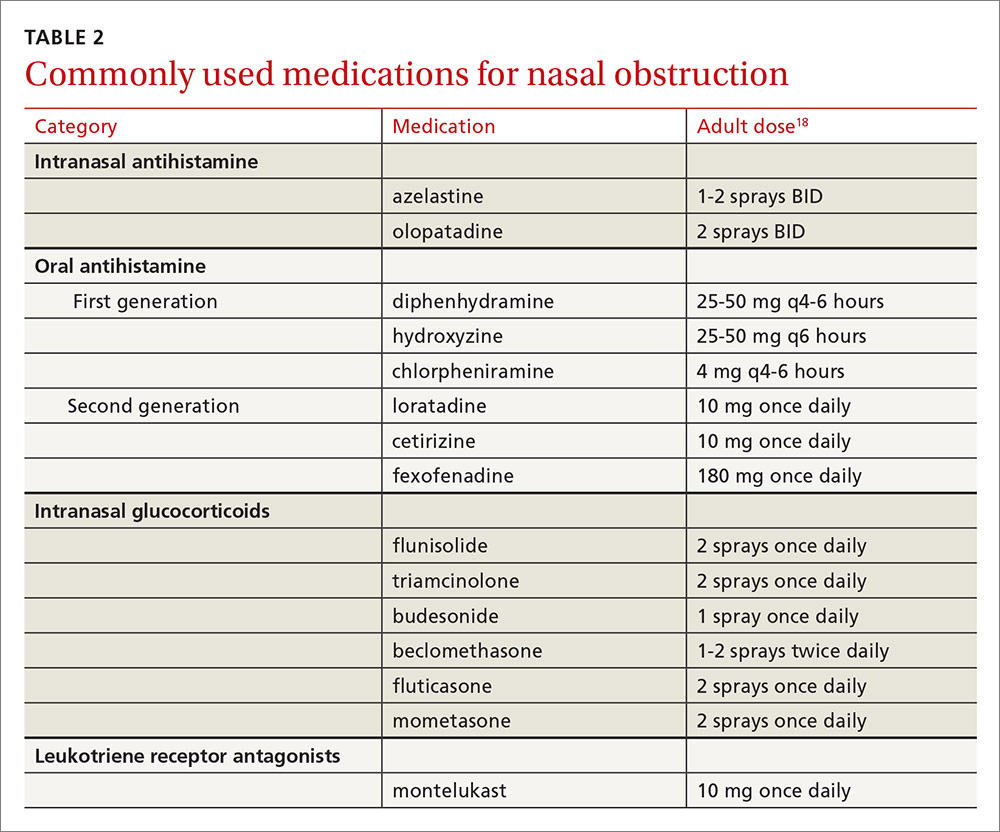
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.
Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).

Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.

Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
Nasal obstruction is one of the most common reasons that patients visit their primary care providers.1,2 Often described by patients as nasal congestion or the inability to adequately breathe out of one or both nostrils during the day and/or night, nasal obstruction commonly interferes with a patient’s ability to eat, sleep, and function, thereby significantly impacting quality of life. Overlapping presentations can make discerning the exact cause of nasal obstruction difficult.
To improve diagnosis and treatment, we review here the evidence-based recommendations for the most common causes of nasal obstruction: rhinitis, rhinosinusitis (RS), drug-induced nasal obstruction, and mechanical/structural abnormalities (TABLE 13-14).

Rhinitis/rhinosinusitis: It all begins with inflammation
Sneezing, rhinorrhea, nasal congestion, and nasal itching are complaints that signal rhinitis, which affects 30 to 60 million people in the United States annually.3 Rhinitis can be allergic, non-allergic, infectious, hormonal, or occupational in nature. All forms of rhinitis share inflammation as the cause of the nasal obstruction. The most common form is allergic rhinitis (AR), which includes seasonal AR and perennial AR. Seasonal AR is typically caused by outdoor allergens and waxes and wanes with pollen seasons. Perennial AR is caused mostly by indoor allergens, such as dust mites, molds, cockroaches, and pet dander; it persists all or most of the year.6 Causes of non-allergic rhinitis (NAR) include environmental irritants such as cigarette smoke, perfume, and car exhaust; medications; and hormonal changes,6 but most causes of NAR are unknown.3,6
While AR can begin at any age, most people develop symptoms in childhood or as young adults, whereas NAR tends to begin later in life. Nasal itching can help to distinguish AR from NAR. NAR symptoms tend to be perennial and include postnasal drainage. If symptoms persist longer than 12 weeks despite treatment, the condition becomes known as chronic rhinosinusitis (CRS).
Treatment of rhinitis: Tiered and often continuous
Treatment of AR and NAR is similar and multitiered beginning with the avoidance of irritants and/or allergens whenever possible, moving on to pharmacotherapy, and, at least for AR, ending with allergen immunotherapy. Treatment is often an ongoing process and typically requires continuous therapy as opposed to treatment on an as-needed basis.3 It is unnecessary to perform allergy testing before making a presumed diagnosis of NAR and starting treatment.6
Intranasal corticosteroids. Currently, intranasal glucocorticosteroids (INGCs) are the most effective monotherapy for AR and NAR and have few adverse effects when used at prescribed doses.3,4 For mild to intermittent symptoms, begin with the maximum dosage of an INGC for the patient’s age and proceed with incremental reductions to identify the lowest effective dose.3 If INGCs alone are ineffective, studies have shown that the addition of an intranasal second-generation antihistamine can be of some benefit.3,4 In fact, an INGC and an intranasal antihistamine—along with saline nasal irrigation—is recommended for both AR and NAR resistant to single therapy.3,6,15 If intranasal antihistamines are not an option, oral therapy can be initiated.
Start with second-generation antihistamines and consider LRAs. For oral therapy, start with second-generation antihistamines (loratadine, cetirizine, fexofenadine). First-generation antihistamines (diphenhydramine, hydroxyzine, chlorpheniramine), although widely available at relatively low cost, can cause several significant adverse effects including sedation, impaired cognitive function, and agitation in children.3,4 Because second-generation antihistamines have fewer adverse effects, they are recommended as first-line therapy when oral antihistamine therapy is desired, such as for nasal congestion, sneezing, and itchy, watery eyes.
Of note: A 2014 meta-analysis found that a leukotriene receptor antagonist (LRA) (montelukast) had efficacy similar to oral antihistamines for symptom relief in AR, and that LRAs may be better suited to nighttime symptoms (difficulty falling asleep, nighttime awakenings, congestion on awakening), while antihistamines may provide better relief of daytime symptoms (pruritus, rhinorrhea, sneezing).16 Although further head-to-head, double-blind randomized controlled trials (RCTs) are needed to confirm the results and investigate possible gender differences in symptom response, consider an LRA for first-line therapy in patients with AR who have predominantly nighttime symptoms.
What about pregnant women and the elderly?
It is important to consider teratogenicity when selecting medications for pregnant patients, especially during the first trimester.3 Nasal cromolyn has the most reassuring safety profile in pregnancy. Cetirizine, chlorpheniramine, loratadine, diphenhydramine, and tripelennamine may be used in pregnancy. The US Food and Drug Administration considers them to have a low risk of fetal harm, based on human data, whereas it views many other antihistamines as probably safe, based on limited or no human data. Most INGCs are not expected to cause fetal harm, but limited human data are available. Avoid prescribing oral decongestants to women who are in the first trimester of pregnancy due to the risk of gastroschisis in newborns.17
Elderly patients represent another population for which adverse effects must be carefully considered. Allergies in individuals >65 years of age are uncommon. Rhinitis in this age group is often secondary to cholinergic hyperactivity, alpha-adrenergic hyperactivity, or rhinosinusitis. Given elderly patients’ increased susceptibility to the potential adverse central nervous system (CNS) and anticholinergic effects of antihistamines, non-sedating medications are recommended. Oral decongestants also should be used with caution in this population, not only because of CNS effects, but also because of heart and bladder effects3 (TABLE 218).
For drug-induced rhinitis, stop the offending drug and consider an INGC
Several types of medications, both oral and inhaled, are known to cause rhinitis. The use of alpha-adrenergic decongestant sprays for more than 5 to 7 days can induce rebound congestion on withdrawal, known as rhinitis medicamentosa.3 Repeated use of intranasal cocaine and methamphetamines can also result in rebound congestion. Oral medications that can result in rhinitis or congestion include angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, nonsteroidal anti-inflammatory drugs (NSAIDS), oral contraceptives, and even antidepressants.3
The treatment for drug-induced rhinitis is termination of the offending agent. INGCs can be used to help decrease inflammation and control symptoms once the offending agent is discontinued.

Mechanical/structural causes of obstruction are wide-ranging
Mechanical/structural causes of nasal obstruction range from foreign bodies to anatomical variations including nasal polyps, a deviated septum, adenoidal hypertrophy, foreign bodies, and tumors. Because more than one etiology may be at work, it is best to first treat any non-mechanical causes of obstruction, such as ARS or NARS.
Nasal polyposis often requires both a medical and surgical approach
Nasal polyps are benign growths arising from the mucosa of the nasal sinuses and nasal cavities and affecting up to 4% of the population.7 Their etiology is unclear, but we do know that nasal polyps result from underlying inflammation.7 Uncommon in children outside of those affected by cystic fibrosis,7 nasal polyposis can be associated with disease processes such as AR and sinusitis. Polyps are also associated with clinical syndromes such as aspirin-exacerbated respiratory disease (AERD) syndrome, which involves upper and lower respiratory tract symptoms in patients with asthma who have taken aspirin or other NSAIDs.9
Symptoms vary with the location and size of the polyps, but generally include nasal congestion, alteration in smell, and rhinorrhea. The goals of treatment are to restore or improve nasal breathing and olfaction and prevent recurrence.8 This often requires both a medical and surgical approach.
Topical corticosteroids are effective at reducing both the size of polyps and associated symptoms (rhinorrhea, rhinitis).8 And research has shown that steroids reduce the need for both primary and repeat surgical polypectomies.4 Other treatments to consider prior to surgery (if no symptom reduction occurs with INGCs) include systemic (oral) corticosteroids, intra-polyp steroid injections, macrolide antibiotics, and nasal washes.7,14
When symptoms of polyposis are refractory to medical management, functional endoscopic sinus surgery (FESS) is the surgical procedure of choice.3 In addition to refractory symptoms, indications for FESS include the need to correct anatomic deformities believed to be contributing to the persistence of disease and the need to debulk advanced nasal polyposis.3 The principal goal is to restore patency to the ostiomeatal unit.3
Several studies have reported a high success rate for FESS in improving the symptoms of CRS.3,19-23 In a 1992 study, for example, 98% of patients reported improvement following surgery,19 and in a follow-up report approximately 6 years later, 98% of patients continued to report subjective improvement.22
For septal etiologies, consider septoplasty
Deviation of the nasal septum is a common structural etiology for nasal obstruction arising primarily from congenital, genetic, or traumatic causes.24 Turbulent airflow from the septal deviation often causes turbinate hypertrophy, which creates (or exacerbates) the obstructive symptoms from the septal deviation.25
Septoplasty is the most common ear, nose, and throat operation in adults.26 Reduction of nasal symptoms has been reported in up to 89% of patients who receive this surgery, according to one single-center, non-randomized trial.27 Currently, at least one multicenter, randomized trial is underway that aims to develop evidence-based guidelines for septoplasty.26
Septal perforation is another etiology that can present with nasal obstruction symptoms. Causes include traumatic perforation, inflammatory or collagen vascular diseases, infections, overuse of vasoconstrictive medications, and malignancy.28,29 A careful inspection of the nasal septum is necessary to identify a perforation; this may require nasal endoscopy.
Anterior, rather than posterior, perforations are more likely to cause symptoms of nasal obstruction. Posterior perforations rarely require treatment unless malignancy is suspected, in which case referral for biopsy is recommended. Anterior perforations are treated initially with avoidance of any causative agent if, for example, the problem is drug- or medication-induced, and then with humidification and emollients.28,29
For anterior perforations, septal silicone buttons can be used for recalcitrant symptoms. However, observational studies indicate that for long-term symptom resolution, silicone buttons are effective in only about one-third of patients.29
For patients with persistent symptoms despite the above measures, surgical repair with various flap techniques is an option. A meta-analysis of case studies involving various techniques concluded that there is a wide variety of options, and that surgeons must weigh factors such as the characteristics and etiology of the perforation and their own experience and expertise when choosing from among available methods.30 Additional good quality research is necessary before clear recommendations regarding technique can be made.
Adenoid hypertrophy: Consider corticosteroid nasal drops
Adenoid hypertrophy is a common cause of chronic nasal obstruction in children. Although adenoidectomy is commonly performed to correct the problem, current evidence regarding the efficacy of the procedure is inconclusive.10 Evidence demonstrates corticosteroid nasal drops significantly reduce symptoms of nasal obstruction in children and may provide an effective alternative to surgical resection.18 Studies have also demonstrated that treatment with oral LRAs significantly reduces adenoid size and nasal obstruction symptoms.12,13
Foreign bodies: Don’t forget “a mother’s kiss”
Foreign bodies are the most common cause of nasal obstruction in the pediatric population. There is a paucity of high-quality evidence on removal of these objects; however, a number of retrospective reviews and case series support that most objects can be removed in the office or emergency department without otolaryngologic referral.31,32
Techniques for removal include positive pressure, which is best used for smooth or soft objects. Positive pressure techniques include having the patient blow their own nose or having a parent use a mouth-to-mouth–type blowing technique (ie, the “mother’s kiss” method).32 Refer patients to Otolaryngology if the obstruction involves:31
- objects not easily visualized by anterior rhinoscopy
- chronic or impacted objects
- button batteries or magnets
- penetrating or hooked objects
- any object that cannot be removed during an initial attempt.
Nasal tumors: More common in older men
Nasal tumors occur most often in the nasal cavity itself and are more common in men ≥60 years.33 There is no notable racial predominance.33 Other risk factors include human papillomavirus (HPV) infection, tobacco smoke, and occupational exposure to inhaled wood dust, glues, and adhesives.34-37
Benign tumors occurring in the nasal cavity are a diverse group of disorders, including inverted papillomas, squamous papillomas, pyogenic granulomas, and other less common lesions, all of which typically present with nasal obstruction as a symptom. Many of these lesions cause local tissue destruction or have a high incidence of recurrence. These tumors are treated universally with nasoendoscopic resection.38
Malignant nasal tumors are rare but serious causes of nasal obstruction, making up 3% of all head and neck cancers.39 Most nasal cancers present when they are locally advanced and cause unilateral nasal obstruction, lacrimation, and epistaxis. These symptoms are typically refractory to initial medical management and present as CRS. This diagnosis should be suspected in certain patient groups, such as those who have been exposed to wood dust (eg, construction workers or those who work in wood mills).36
Computed tomography is the gold standard imaging method for CRS; however, if nasal cancer is suspected, referral for biopsy and histopathologic examination is necessary for a final diagnosis.39 Because of the nonspecific nature of their initial presentation, many nasal tumors are at an advanced stage and carry a poor prognosis by the time they are diagnosed.39
CORRESPONDENCE
Margaret A. Bayard, MD, MPH, FAAFP, Naval Hospital Camp Pendleton, 200 Mercy Circle, Camp Pendleton, CA 92005; [email protected].
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
1. Centers for Disease Control and Prevention. CDC’s FastStats on Ambulatory Care Use and Physician Office Visits. Selected patient and provider characteristics for ambulatory care visits to physician offices and hospital outpatient and emergency departments: United States, 2009-2010. Available at: http://www.cdc.gov/nchs/data/ahcd/combined_tables/AMC_2009-2010_combined_web_table01.pdf. Accessed November 9, 2016.
2. US Census Bureau. Table 158. Visits to office-based physician and hospital outpatient departments, 2006. Available at: http://www2.census.gov/library/publications/2006/compendia/statab/126ed/tables/07s0158.xls. Accessed November 13, 2016.
3. Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103-S115.
4. Wallace DV, Dykewicz MS. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1-S84.
5. Krouse J, Lund V, Fokkens W, et al. Diagnostic strategies in nasal congestion. Int J Gen Med. 2010;3:59-67.
6. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108:S2-S8.
7. Newton JR, Ah-See KW. A review of nasal polyposis. Ther Clin Risk Manag. 2008;4:507-512.
8. Badia L, Lund V. Topical corticosteroids in nasal polyposis. Drugs. 2001;61:573-578.
9. Fahrenholz JM. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:113-124.
10. van den Aardweg MT, Schilder AG, Herket E, et al. Adenoidectomy for recurrent or chronic nasal symptoms in children. Cochrane Database Syst Rev. 2010;CD008282.
11. Demirhan H, Aksoy F, Ozturan O, et al. Medical treatment of adenoid hypertrophy with “fluticasone propionate nasal drops”. Int J Pediatr Otorhinolaryngol. 2010;74:773-776.
12. Shokouhi F, Jahromi AM, Majidi MR, et al. Montelukast in adenoid hypertrophy: its effect on size and symptoms. Iran J Otorhinolaryngol. 2015;27:433-448.
13. Goldbart AD, Greenberg-Dotan S, Tai A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130:e575-e580.
14. Moss WJ, Kjos KB, Karnezis TT, et al. Intranasal steroid injections and blindness: our personal experience and a review of the past 60 years. Laryngoscope. 2015;125:796-800.
15. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis. Laryngoscope. 2013;123:53-56.
16. Xu Y, Zhang J, Wang J. The efficacy and safety of selective H1-antihistamine versus leukotriene receptor antagonist for seasonal allergic rhinitis: a meta-analysis. PLoS One. 2014;9:e112815.
17. Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
18. Lexi-Comp Online. Available at: online.lexi.com/crlsql/servlet/crlonline. Accessed November 9, 2016.
19. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope.1992;102:1-18.
20. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev. 2006;CD004458.
21. Chambers DW, Davis WE, Cook PR, et al. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504-510.
22. Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151-157.
23. Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158-161.
24. Aziz T, Biron VL, Ansari K, et al. Measurement tools for the diagnosis of nasal septal deviation: a systematic review. J Otolaryngol Head Neck Surg. 2014;43:11.
25. Grutzenmacher S, Robinson DM, Grafe K, et al. First findings concerning airflow in noses with septal deviation and compensatory turbinate hypertrophy—a model study. ORL J Otorhinolaryngol Relat Spec. 2006;68:199-205.
26. van Egmond MMHT, Rovers MM, Hendriks CTM, et al. Effectiveness of septoplasty versus non-surgical management for nasal obstruction due to deviated nasal septum in adults: study protocol for a randomized controlled trial. Trials. 2015;16:500.
27. Gandomi B, Bayat A, Kazemei T. Outcomes of septoplasty in young adults: the Nasal Obstruction Septoplasty Effectiveness study. Am J Otolaryngol. 2010;31:189-192.
28. Døsen L, Have R. Silicone button in nasal septal perforation. Long term observations. Rhinology. 2008;46:324-327.
29. Kridel RW. Considerations in the etiology, treatment and repair of septal perforations. Facial Plast Surg Clin North Am. 2004;12:435-450.
30. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007;121:419-426.
31. Mackle T, Conlon B. Foreign bodies of the nose and ears in children. Should these be managed in the accident and emergency setting? Int J Pediatr Otorhinolaryngol. 2006;70:425-428.
32. Cook S, Burton M, Glasziou P. Efficacy and safety of the “mother’s kiss” technique: a systematic review of case reports and case series. CMAJ. 2012;184:E904-E912.
33. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
34. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999:13:435-438.
35. Luce D, Gerin M, Leclerc A, et al. Sinonasal cancer and occupational exposure to formaldehyde and other substances. Int J Cancer. 1993;53:224-231.
36. Mayr SI, Hafizovic K, Waldfahrer F, et al. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010;83;631-638.
37. Syrjänen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174-181.
38. Wood JW, Casiano RR. Inverted papillomas and benign nonneoplastic lesions of the nasal cavity. Am J Rhinol Allergy. 2012;26:157-163.
39. Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging. 2012;12:136-152.
PRACTICE RECOMMENDATIONS
› Consider intranasal corticosteroids for patients with nasal polyps, as they are effective at reducing the size of the polyps and associated symptoms of obstruction, rhinorrhea, and rhinitis. A
› Prescribe intranasal corticosteroids for patients with adenoid hypertrophy. A
› Refer patients with chronic refractory rhinosinusitis for functional endoscopic sinus surgery. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A look at the burden of opioid management in primary care
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
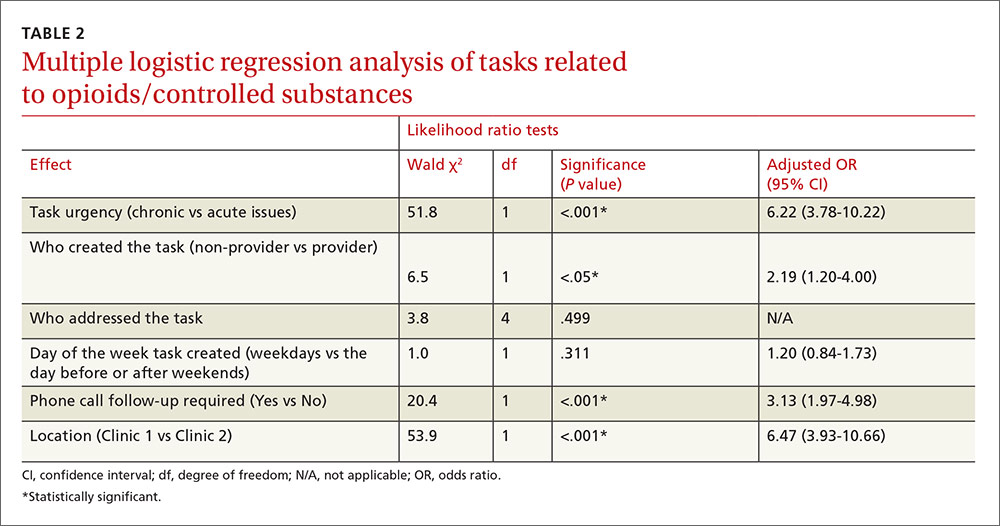
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.
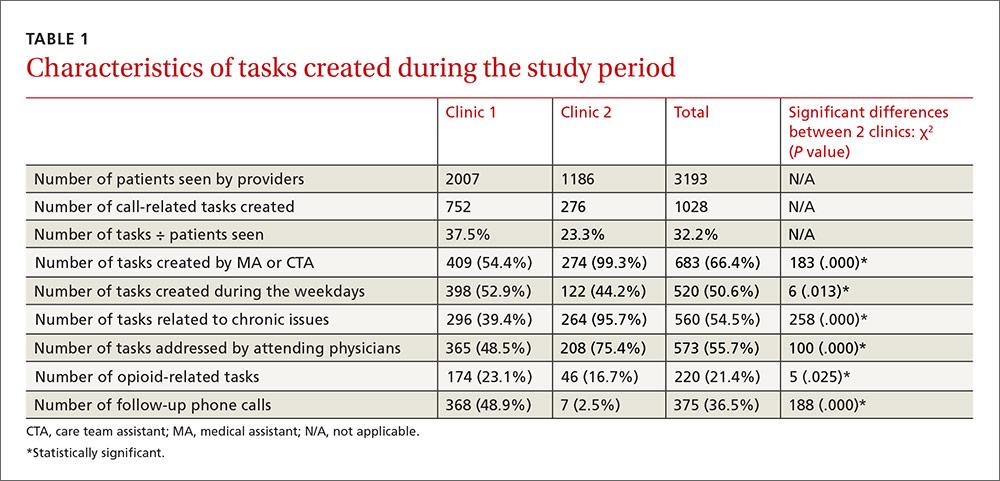
Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.
DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.
Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
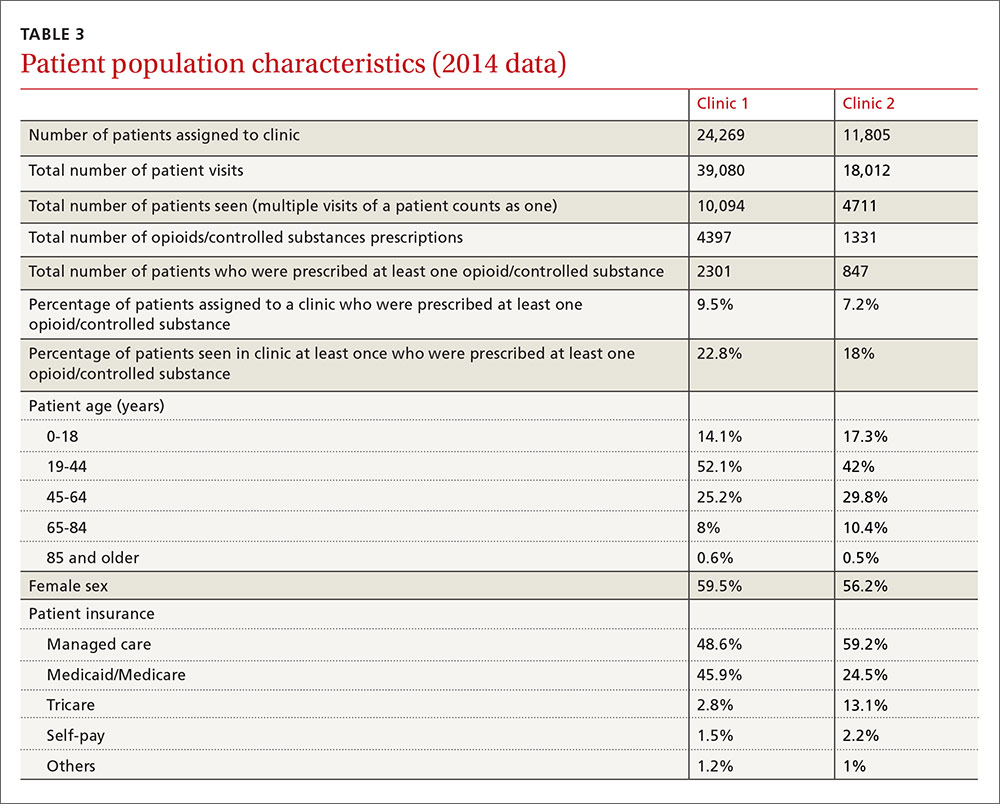
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected].
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.

Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.

DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.

Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected].
ABSTRACT
Purpose Pain management with opioids in primary care is challenging. The objective of this study was to identify the number of opioid-related tasks in our clinics and determine whether opioid-related tasks occur more often in a residency setting.
Methods This was a retrospective observational review of an electronic health record (EHR) system to evaluate tasks related to the use of opioids and other controlled substances. Tasks are created in the EHR when patients call the clinic; the task-box system is a means of communication within the EHR. The study setting was 2 university-based family medicine clinics. Clinic 1 has faculty and resident providers in an urban area. Clinic 2 has only faculty providers in a suburban area. We reviewed all tasks recorded in November 2010.
Results A total of 3193 patients were seen at the clinics. In addition, 1028 call-related tasks were created, 220 of which (21.4%) were opioid-related. More than half of the tasks were about chronic (ongoing) patient issues. More than one‑third of the tasks required follow-up phone calls. Multiple logistic regression analysis showed more opioid-related tasks in the residency setting (Clinic 1) compared with the nonresidency setting (Clinic 2), (23.1% vs 16.7%; P<.001). However, multiple logistic regression analysis did not show any correlations between opioid-related tasks and who addressed the tasks or the day tasks were created.
Conclusions Primary care physicians prescribe significant amounts of opioids. Due to the nature of opioid use and abuse, a well-planned protocol customized to the practice or institution is required to streamline this process and decrease the number of unnecessary phone calls and follow-ups.
Pain management with opioids in primary care is challenging,1,2 and many physicians find it unsatisfying and burdensome.3 More than 60 million patient visits for chronic pain occur annually in the United States, consuming large amounts of time and resources.4 Contributing to the challenge is the need to ensure patient safety and satisfaction, as well as staff satisfaction with pain management.5-8 Opioid-related death is a major cause of iatrogenic mortality in the United States:9,10 From 1999 to 2006, fatal opioid-involved intoxications more than tripled from 4000 to 13,800.7
At issue for many providers, as well as patients and staff, is dissatisfaction with current systems in place for managing chronic non-cancer pain with opioids.2,3,8,11 In developing this study, we decided to focus on the systems aspect of care with 2 primary outcome measures in mind. Specifically, we sought to identify the tasks related to managing opioids and other controlled substances in 2 primary care clinics in a university-based family medicine program and to determine what proportion of all routine tasks in these 2 clinics could be attributed to opioid-related issues. With our secondary outcome measures, we sought to compare the number of opioid-related tasks in the residency setting with those in a nonresidency setting, and to identify factors that might be associated with an increase in the number of opioid-related tasks.
METHODS
Setting and design
We conducted a retrospective observational pilot study reviewing our electronic health record (EHR) system (Allscripts TouchWorks) at 2 of our outpatient family medicine clinics at the University of Colorado. When patients call the clinics, or when patient-care-related concerns need to be addressed, an electronic task message is created and sent to the appropriate task box for staff or provider response. The task box system is how staff and providers communicate within the EHR. Each provider has a personal task box, and there are other task boxes in the system (eg, triage, medication refill) for urgent and non-urgent patient care issues.
For example, when a patient calls to request a refill, a medical assistant (MA), care team assistant (CTA), or nurse will create a task for the medication refill box. If the task is urgent, it is marked with a red asterisk and a triage provider will address the task that same day. Non-urgent triage tasks will be addressed by the patient’s primary care provider within 2 to 3 days. Depending on the issue at hand, the task may or may not require phone calls to the patient, pharmacy, or insurance company.
Clinic 1, in urban Denver, has 13 physicians (many of them part-time clinical faculty), one nurse practitioner (NP), one physician assistant (PA), and 18 family medicine residents. Clinic 2, in a suburb of Denver, has 5 physicians (only one is part-time) and one nurse practitioner. Clinic 1 is divided into 3 pods, and each has the same number of attending physicians, residents, and MAs, and either a PA or NP.
We reviewed, one by one, all tasks created from November 1 to 30, 2010. One of the study’s investigators categorized each task according to the following descriptors: who created the task, who addressed the task, what day of the week the task was created, urgency of the task, whether the task required a follow-up phone call, and whether the task was related to opioid/controlled-substance issues. The task was categorized as acute if the issue was related to a condition that had been present for fewer than 3 weeks. Chronic tasks were created for conditions present for ≥3 weeks. At the time the study was completed, our EHR had no portal through which we could communicate with patients.
ANALYSIS
We conducted statistical analyses with the IBM SPSS, version 22.0 (SPSS, Inc, Chicago, Illinois). We used descriptive statistics to examine the frequency and percentage for all variables. We used a chi-squared (χ2) test to assess the differences between the 2 clinics, and used a binary multiple logistic regression model to determine possible factors related to opioid-related tasks. P values <.05 were considered statistically significant. The Colorado Multiple Institutional Review Board approved this study.
RESULTS
Clinics 1 and 2, respectively, saw 2007 and 1186 patients during the study period (TABLE 1). The additional 1028 tasks generated by phone calls were almost equally distributed among the 3 pods of Clinic 1 (290, 202, and 260) and Clinic 2 (276). For data analysis, we compared Clinic 1 with Clinic 2 and also compared the 3 pods of Clinic 1 individually with Clinic 2. Both approaches produced similar results.

Most tasks (54% for Clinic 1 and 99% for Clinic 2) were created by MAs and CTAs. At Clinic 1, tasks were also created by residents (17%), PA/NPs (8%), attending physicians (7%), and others/clinical nurses (14%). Tasks at Clinic 1 were addressed by attending physicians (49%), residents (25%), PA/NPs (25%), and others (1%). At Clinic 2, tasks were addressed by attending physicians (75%) and PA/NPs (25%). Approximately half of the tasks (51%) in both clinics were created during weekdays, compared with the day after weekends/holidays (28%), the day before weekends/holidays (17%), and during weekends/holidays (4%). Chronic patient issues, acute patient issues, and other issues accounted for 54%, 29%, and 17% of tasks, respectively. Follow-up phone calls to patients, pharmacies, or others occurred in 37% of tasks. Two hundred twenty tasks (21%) in the clinics combined were related to opioids and controlled substances.
Multiple logistic regression analysis of data from both clinics (TABLE 2) showed more opioid-related tasks in Clinic 1 compared with Clinic 2 (P<.001), and that these tasks were more often related to chronic issues than to acute issues (P<.001). Tasks created by MAs, CTAs, clinical nurses, and others were more likely to be opioid-related compared with the tasks created by attending physicians, residents, NPs, or a PA (25% vs 15%; P<.05). Compared with non-opioid-related tasks, opioid-related tasks required more follow-up phone calls (P<.001). Follow-up phone calls to pharmacies occurred more often with opioid-related tasks than with non-opioid tasks (11% vs 5%), while follow-up phone calls to patients occurred more often for non-opioid related tasks than opioid-related tasks (28% vs 18%). No correlations with task creation were found for who addressed the opioid-related task or the day the task was created.

DISCUSSION
This study demonstrated that our process of handling patient issues related to opioids accounts for a large proportion of all tasks. Dealing with tasks is time consuming, not only for attending physicians and residents but also for clinic nurses and staff. Almost a quarter of clinic tasks were opioid related. As has been shown in previous studies,5-8 chronic pain management with opioids is an unsatisfying task for staff and care providers at our clinics. We also found that tasks created by non-providers were more likely to be opioid-related than were tasks created by providers. This is most likely due to the fact that non-providers cannot write prescriptions and they have to ask providers for further reviews.

Khalid et al found that, compared with attending physicians, residents had more patients on chronic opioids who displayed concerning behaviors, including early refills and refills from multiple providers.13 The higher number of part-time providers at Clinic 1 in our study may have also caused insufficient continuity of care at that site. Nevertheless, this model of practice is used in many academic primary care institutions.4 Another possible reason for the difference could be a lack of resident training on current guidelines for managing opiates for chronic pain.3,13,14 Again, this was a pilot study and we drew no solid conclusion about the reasons for differences between these 2 clinics.
It is obvious, however, that we spend a significant amount of time and resources dealing with chronic pain management. Our institution created an opioid/controlled-substance patient registry about 3 years ago. The data for 2014 showed that 22.8% and 18% of patients seen at least once at Clinic 1 and Clinic 2, respectively, were prescribed opioids/controlled substances (TABLE 3).
Possible solutions to reduce tasks related to opioid management. For both small and large practices, one way to reduce the number of tasks related to opioid management and, therefore, the time allocated to completing those tasks, would be to have a clear protocol to follow.3,4,8,11,14,15 The protocol may include the creation of an opioid/controlled-substance registry and the development and implementation of clinical decision support programs.
We also recommend the dissemination of tools for clinical management at the point of care. These can include a controlled-substance risk assessment tool for aberrant behaviors, a controlled-substance informed consent form, a functional and quality-of-life assessment, electronic clinical-note templates in the EHR, urine drug screening, and routine use of existing state pharmacy prescription drug monitoring programs. Also essential would be the provision of routine educational programs for clinicians regarding chronic pain management based on existing evidence and guidelines. (See “Opioids for chronic pain: The CDC’s 12 recommendations.”) It has been demonstrated that an EHR opioid dashboard or an EHR-based protocol improved adherence to guidelines for prescribing opiates.16
This study has several limitations. First, this was a small pilot study completed over a short period of time, although we believe the findings are likely representative of the prescribing practices in the 2 clinics we evaluated. Second, it was a retrospective study, which was appropriate for evaluating our questions. Third, we were unable to account for other factors that could potentially confound the results, including, but not limited to, the amount of time allocated to each task, and the total number of patients at each clinic who were on opioids for management of chronic pain during the study period. However, due to our recent addition of an opioid/controlled-substance patient registry, we were able to add information for the year 2014 (TABLE 3). Multi-center large scale studies are required to evaluate this further.
ACKNOWLEDGEMENTS
We thank Dr. Corey Lyon for his editorial assistance.
CORRESPONDENCE
Morteza Khodaee, MD, AFW Family Medicine Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected].
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
1. Smith BH, Torrance N. Management of chronic pain in primary care. Curr Opin Support Palliat Care. 2011;5:137-142.
2. Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307:1377-1378.
3. Leverence RR, Williams RL, Potter M, et al; PRIME Net Clinicians. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
4. Watkins A, Wasmann S, Dodson L, et al. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487-489.
5. Brown J, Setnik B, Lee K, et al. Assessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care setting. J Opioid Manag. 2011;7:467-483.
6. Duensing L, Eksterowicz N, Macario A, et al. Patient and physician perceptions of treatment of moderate-to-severe chronic pain with oral opioids. Curr Med Res Opin. 2010;26:1579-1585.
7. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12:S26-S35.
8. Wenghofer EF, Wilson L, Kahan M, et al. Survey of Ontario primary care physicians’ experiences with opioid prescribing. Can Fam Physician. 2011;57:324-332.
9. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
10. Hartrick CT, Gatchel RJ, Conroy S. Identification and management of pain medication abuse and misuse: current state and future directions. Expert Rev Neurother. 2012;12:601-610.
11. Wiedemer NL, Harden PS, Arndt IO, et al. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573-584.
12. Colburn JL, Jasinski DR, Rastegar DA. Long-term opioid therapy, aberrant behaviors, and substance misuse: comparison of patients treated by resident and attending physicians in a general medical clinic. J Opioid Manag. 2012;8:153-160.
13. Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16:480-487.
14. Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1-E8.
15. Clark LG, Upshur CC. Family medicine physicians’ views of how to improve chronic pain management. J Am Board Fam Med. 2007;20:479-482.
16. Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573-579.
Does your patient really need testosterone replacement?
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3
Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
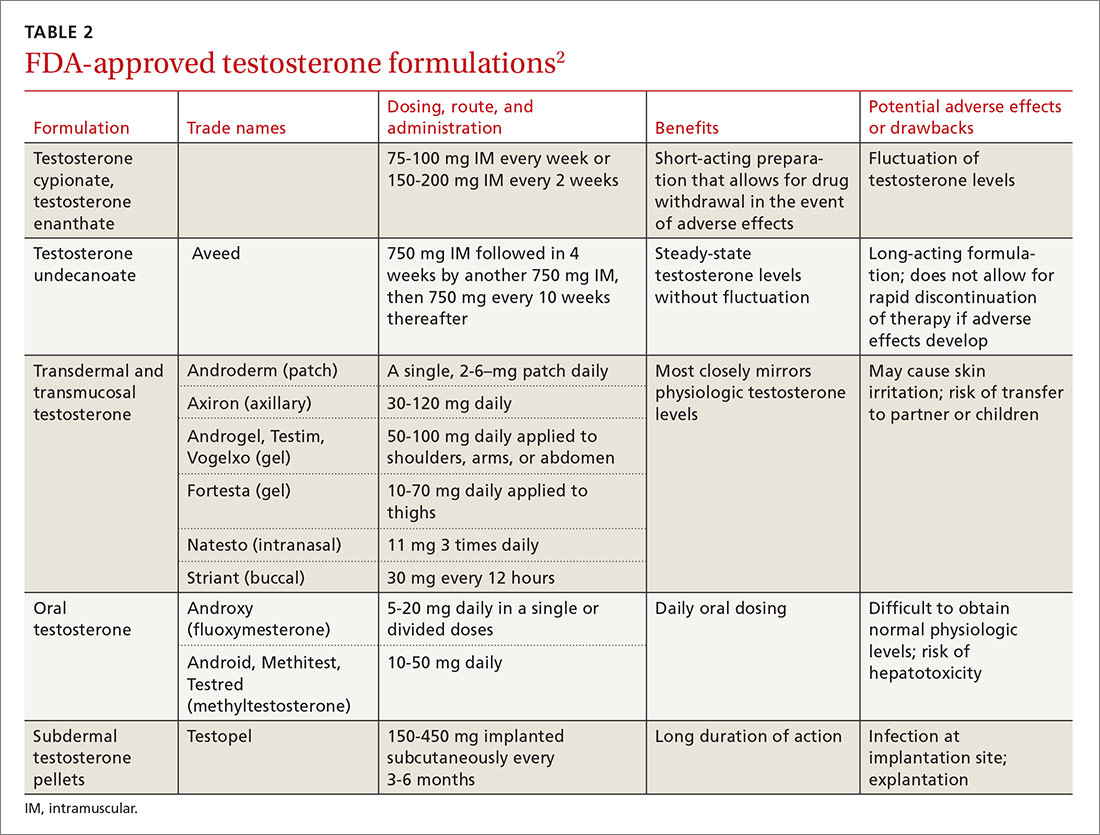
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; [email protected].
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3

Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.

Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; [email protected].
Over the past decade, androgen replacement prescriptions for men ≥40 years of age have increased 3-fold, according to one study.1 While one could argue this trend represents greater attention to an underdiagnosed problem, the study of prescription claims for almost 11 million men found that a quarter of them did not have a testosterone level documented in the 12 months prior to receiving treatment.1
At the same time, sales of testosterone products totaled about $2.4 billion dollars in 2013, a number projected to top $4 billion by 2017.2 The increase in prescribing is thought to be due, at least in part, to direct-to-consumer marketing techniques encouraging patients to seek medical attention if they are experiencing non-specific symptoms, such as fatigue and lack of energy, because their “problem” could be due to low testosterone.
Testosterone begins to decrease after age 40
The Endocrine Society defines “androgen deficiency” as low serum testosterone (generally <280 ng/dL for healthy young men) along with signs and symptoms of hypogonadism, including decreased sexual function; loss of axillary and/or pubic hair; low bone mineral density; loss of motivation and/or concentration; poor mood or depression; decline in cognitive function; and loss of muscle strength and work capacity (TABLE 1).3

Primary vs secondary hypogonadism. Primary (or hypogonadotropic) hypogonadism results when the testes fail to produce adequate testosterone in the presence of normal serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels. Secondary hypogonadism is pituitary or hypothalamic in origin. Patients with primary hypogonadism will have high LH and FSH levels, whereas patients with secondary hypogonadism will have low or normal LH and FSH levels.4 The Endocrine Society recommends checking LH and FSH levels in all patients with hypogonadism to differentiate the primary from the secondary type.3 Patients with late onset primary hypogonadism do not require any further evaluation. In young men, it is important to consider Klinefelter syndrome. This diagnosis can be determined with a karyotype. In patients with secondary hypogonadism, checking serum iron, prolactin, and other pituitary hormones, and getting a magnetic resonance imaging scan of the sella turcica may be indicated. This will rule out infiltrative diseases, such as hemochromatosis, prolactinoma, and hypothalamic or pituitary neoplasm.
Testosterone is present in the body in 3 forms: free testosterone, albumin-bound testosterone, and testosterone bound to sex hormone-binding globulin (SHBG). In young healthy men, only 1% to 2% of testosterone is free, about 40% is albumin-bound and readily dissociates to free testosterone, and the remainder is tightly bound to SHBG, which does not readily dissociate and is therefore biologically unavailable.5 The amount of SHBG increases with age, decreasing the amount of bioavailable testosterone.
Serum levels of testosterone remain approximately stable until about age 40. After age 40, total levels of testosterone decrease by 1% to 2% annually, and serum free testosterone levels decrease by 2% to 3% annually.6 Testing of free testosterone levels is recommended when a patient falls in the low normal range of total testosterone (see below).
Testosterone screening: How and for whom?
The Endocrine Society, consistent with the American Urological Association and the European Association of Urology, recommends against screening the general population for testosterone deficiency, fearing overdiagnosis and treatment of asymptomatic men.3,7,8
The Endocrine Society’s recommendation for targeted screening states that for men with chronic diseases (eg, diabetes mellitus, end-stage renal disease, and chronic obstructive lung disease), measurement of testosterone may be indicated by symptoms such as sexual dysfunction, unexplained weight loss, weakness, or mobility limitation. The recommendation also states that in men with other conditions (eg, pituitary mass, human immunodeficiency virus (HIV)-associated weight loss, low-trauma fracture, or treatment with medications that affect testosterone production), measurement of testosterone may be indicated, regardless of symptoms.3 The United States Preventive Services Task Force does not have any specific recommendations regarding screening for hypogonadism in men.
Start with total serum testosterone
Measuring total serum testosterone should be the initial test for suspected testosterone deficiency. Testosterone levels vary throughout the day, peaking in the morning. As a result, levels should generally be measured before 10 am.
Lab values to watch for. Again, the lower limit of the normal testosterone range in healthy young men is 280 to 300 ng/dL, but may vary depending on the laboratory or assay used.3 If the level is abnormal (<280 ng/dL), repeat the test at least a month later prior to initiating testosterone replacement.3 For men with values in the low normal range and clinical symptoms, obtain levels of free testosterone to confirm the diagnosis.
Patients with chronic diseases, such as obesity, diabetes mellitus, liver disease, nephrotic syndrome, or thyroid disease, are more likely to have an increase in SHBG. For these patients, check free testosterone levels in the setting of symptoms and a low-to-normal total testosterone level.9 If a patient has symptoms of hypogonadism and a total testosterone level in the low normal range, as well as a free testosterone level that is less than the lower limit of normal for a laboratory (typically around 50 ng/dL), it is reasonable to offer testosterone replacement.
Medications such as glucocorticoids and opioids can affect testosterone levels, as can acute or subacute illness.10 Therefore, do not measure testosterone levels while a patient is receiving these medications, and wait until a patient has recovered from being ill before doing any testing.
Temper your response with older men. Many men >65 years old may have testosterone levels below the normal range for healthy, young counterparts. This decline is of uncertain clinical significance; it remains unclear if lower levels in older men result in health problems. Some have suggested establishing age-adjusted normal values, and recommend not initiating testosterone replacement therapy in older men until serum levels are below 200 ng/dL, rather than 280 ng/dL, which is the generally accepted lower limit for younger populations.3,11,12
Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.

Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17
Testosterone replacement raises issues of abuse and CV risk
On October 25, 2016, the US Food and Drug Administration (FDA) approved class-wide labeling changes for all prescription testosterone products, alerting prescribers to the agent’s abuse potential and the serious cardiac and mental health adverse outcomes that have been reported as a result of such abuse. In addition, the FDA is revising the Abuse and Dependence section to include new safety information regarding the risks associated with abuse of testosterone and other anabolic androgenic steroids.18
Prior to this announcement, the FDA had mandated in 2015 that product labels include information about a possible increased risk of myocardial infarction (MI) and stroke in people using testosterone. This warning was based on 2 published studies that showed increased cardiovascular risk.19,20 However, a third larger study showed no increase in risk.21 All 3 of these studies were retrospective and had methodologic limitations, including differing baseline testosterone levels, insufficient documentation of baseline levels, and inadequate monitoring of response to therapy.
A recent statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology in response to the older FDA warning cites the need for randomized controlled trials (RCTs) to elucidate whether an association exists between testosterone replacement and cardiovascular risk.22
Of note, researchers have shown that androgen deprivation therapy (ADT) in patients with prostate cancer impacts cardiovascular risk factors (ie, it increases body fat and decreases lean body mass, increases total cholesterol, and increases insulin resistance and risk of diabetes). ADT may also be associated with increased cardiovascular mortality, although data are conflicting.23
Investigators have shown that testosterone replacement positively affects certain risk factors for cardiovascular disease (CVD) including increasing lean muscle mass and improving laboratory values associated with the metabolic syndrome.24 A large retrospective cohort study of male veterans with documented low total testosterone levels who received their medical care at the Veterans Health Administration (VHA) found that those who received testosterone replacement and achieved normal testosterone levels had lower all-cause, cardiovascular, and stroke mortality than controls.21 The men who did not achieve normal testosterone levels also had lower all-cause mortality (but significantly less than those with normalization of serum testosterone levels), but no change in stroke or cardiovascular mortality.
Since this study was retrospective, there were significant limitations, including unknown baseline characteristics of patients in each group. The CVD risks associated with testosterone therapy in middle-aged and older men should be discussed by physicians and their patients on an individual basis. Some experts believe that patients who have had an MI, revascularization, or a stroke within the past 6 months are not good candidates for replacement therapy.25
Until there are better data from prospective RCTs, it may be prudent to make sure that traditional CVD risk factors including smoking, hypertension, hyperlipidemia, and diabetes have been assessed and are appropriately managed in men prescribed testosterone replacement.
Testosterone helps with ED in certain cases
Testosterone deficiency is associated with sexual dysfunction in men, including decreased libido and erectile dysfunction (ED). About 20% to 40% of men with ED will have low testosterone, although replacement does not always improve the condition.2
Current guidelines do not recommend testosterone replacement to treat ED or sexual dysfunction in the absence of a low serum testosterone level and recommend evaluating for other causes of sexual problems in men.3 In one study, men who did not have documented hypogonadism received testosterone replacement therapy for sexual dysfunction including ED or ejaculator dysfunction. These patients saw no improvement in symptoms.26
CORRESPONDENCE
J. Andrew Hoover, MD, Department of Family and Community Medicine, Lancaster General Hospital, 540 North Duke Street, Lancaster, PA 17604; [email protected].
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
1. Baillargeon J, Urban RJ, Ottenbacher KJ, et al. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466.
2. PL Detail-Document #311005. The use of testosterone and the aging male. Pharmacist’s Letter/Prescriber’s Letter. October 2015.
3. Bhasin S, Cunningham GR, Hayes FJ, et al. Therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95:2536-2559.
4. Tajar A, Forti G, O’Neill TW, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810-1818.
5. Kaufman J, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833-876.
6. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598.
7. American Urological Association. AUA position statement on testosterone therapy. Available at: https://www.auanet.org/education/testosterone-therapy.cfm. Accessed October 24, 2016.
8. Dohle GR, Arver S, Bettocchi C, et al. European Association of Urology. Guidelines on male hypogonadism. 2015. Available at: http://uroweb.org/wp-content/uploads/18-Male-Hypogonadism_LR1.pdf. Accessed October 24, 2016.
9. Tanna MS, Schwartzbard A, Berger JS, et al. Management of hypogonadism in cardiovascular patients: what are the implications of testosterone therapy on cardiovascular morbidity? Urol Clin North Am. 2016;43:247-260.
10. Matsumoto AM. The testis. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. 4th ed. New York, NY: McGraw-Hill; 2001:635-705.
11. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016:374:611-624.
12. Loughlin KR, Klap J. Selective use of testosterone replacement therapy. J Urol. 2016;196:1340-1341.
13. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639-650.
14. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335-1339.
15. Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500-4510.
16. PL Detail-Document #311005. Comparison of testosterone products. Pharmacists’s Letter/Prescriber’s Letter. October 2015.
17. Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol. 2015;7:378-387.
18. US Food and Drug Administration. Testosterone and other anabolic androgenic steroids (AAS): FDA statement - Risks associated with abuse and dependence. Available at: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm526151.htm. Accessed October 26, 2016.
19. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836.
20. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone-therapy prescription in men. PLoS One. 2014;9:e85805.
21. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715.
22. Goodman N, Guay A, Dandona P, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of testosterone and cardiovascular risk. Endocr Pract. 2015;21:1066-1073.
23. Albert SG, Morley JE. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: a systematic review. Clin Endocrinol (Oxf). 2016;85:436-443.
24. Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association. Endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833-840.
25. Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545-577.
26. O’Carroll R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry. 1984;145:146-151.
PRACTICE RECOMMENDATIONS
› Confirm suspected hypogonadism by getting 2 serum testosterone levels at least one month apart prior to initiating testosterone replacement therapy. B
› Consider testosterone replacement therapy when there is both laboratory and clinical evidence of hypogonadism. B
› Offer testosterone replacement to older men (≥65 years) with hypogonadism only after talking to them about the risks and benefits. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antibiotic stewardship: The FP’s role
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.
CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7
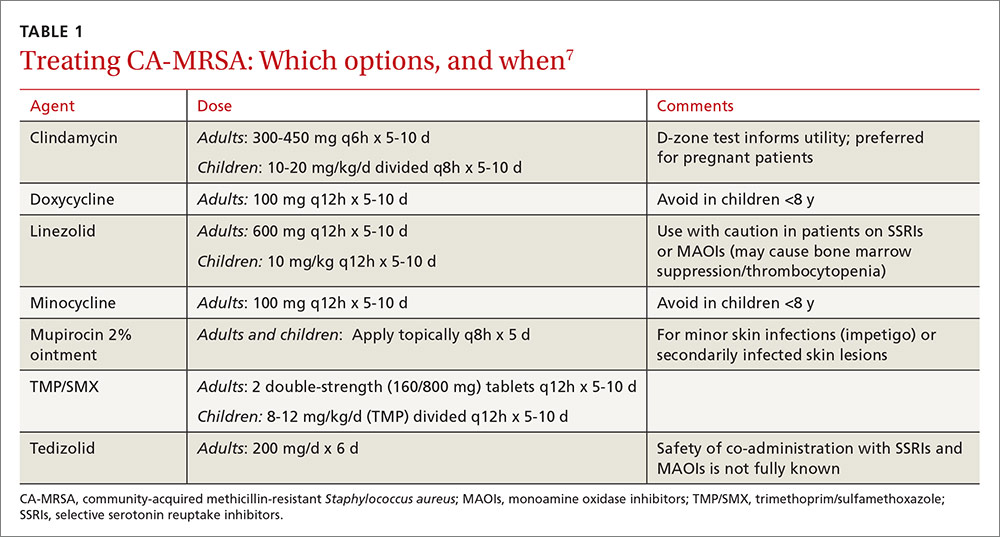
Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13
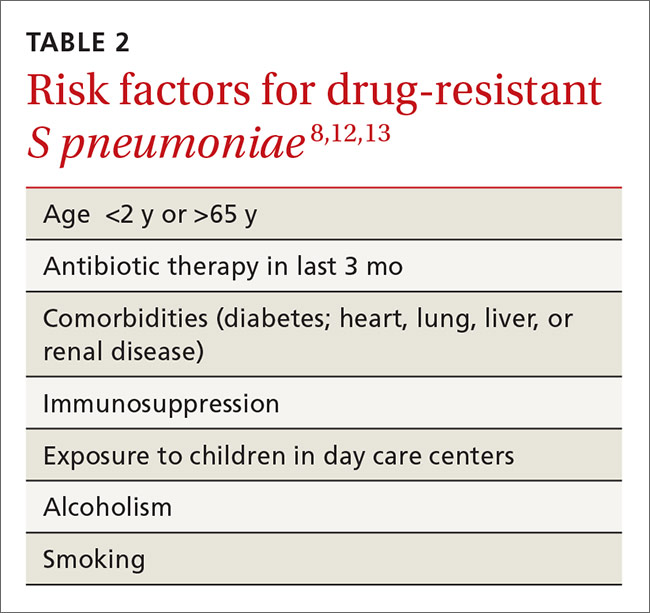
Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19
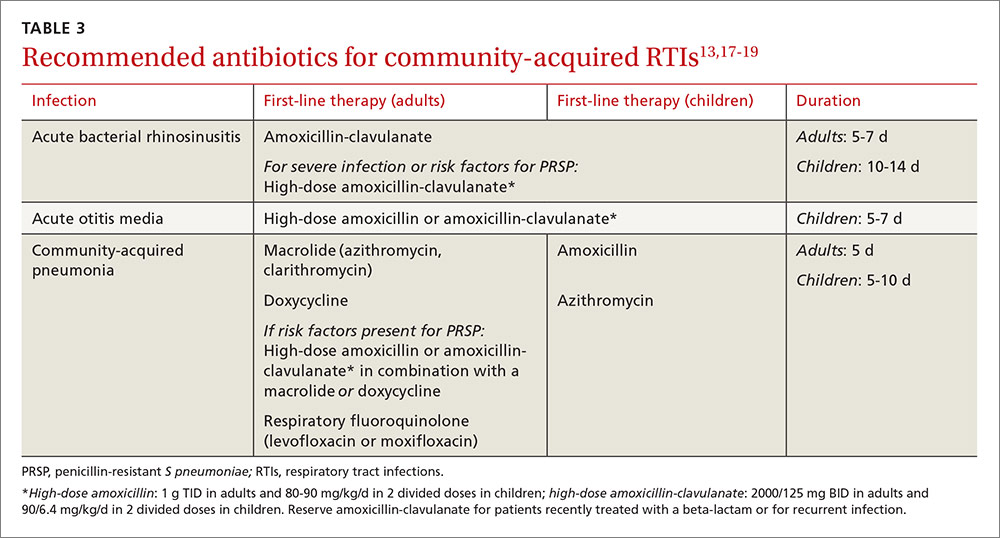
High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1
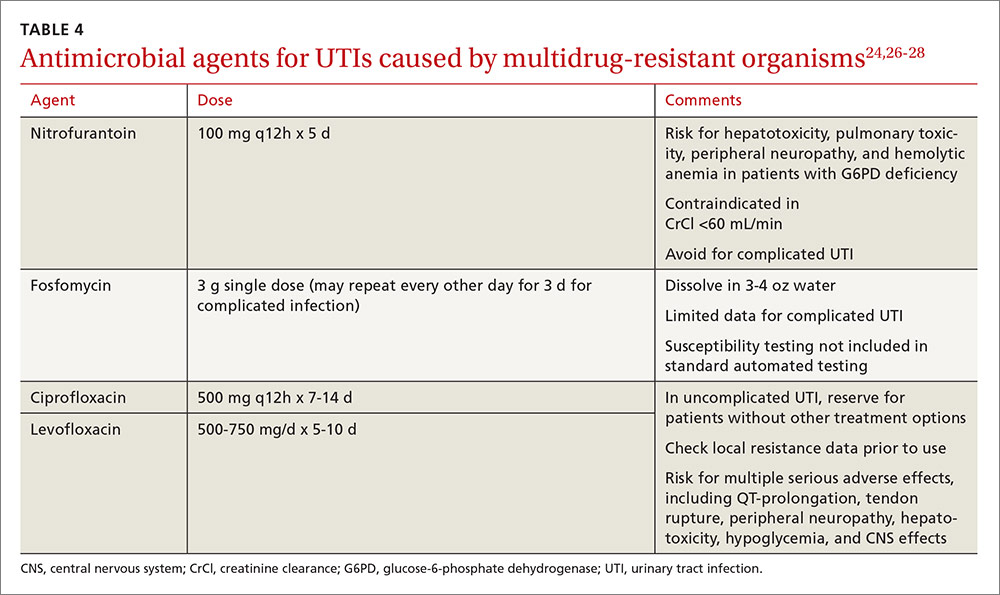
Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.

CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7

Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13

Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19

High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1

Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
Any use of antibiotics can promote the development of drug resistance, but antibiotic misuse is far more likely to lead to superinfections, allergic reactions, and adverse events. These are pressing concerns in ambulatory care, as well as in inpatient settings, but efforts to address unnecessary and inappropriate use of antibiotics have been more focused on the latter.1
A US study published in 2013 found that, in one year alone, nearly 260 million courses of antibiotics were prescribed to patients in ambulatory care. Patients <2 years or >65 years of age had the highest antibiotic prescription rates, and practitioners in family medicine were the most likely to prescribe them.2 Yet evidence suggests that more than half of all community-based antibiotic prescriptions may be unnecessary or inappropriate—the combined result of patient, physician, and health system factors.3 The continuing shift of medical services from acute care to community-based settings has contributed to an increase in antibiotic-resistant infections in the community, as well.
Skin infections caused by Staphylococcus aureus, respiratory infections caused by Streptococcus pneumoniae, urinary tract infections (UTIs) caused by Escherichia coli and other Enterobacteriaceae, and sexually transmitted diseases such as gonorrhea are recent examples of community-transmitted infections for which increases in antibiotic resistance rates have been reported.4 It is crucial for primary care physicians to know when watchful waiting is appropriate, when antibiotics are indicated, and, when needed, which antimicrobials are most likely to be effective. The case studies, text, and tables that follow can be used as a guide.

CASE 1 ›
Ear tugging, fever, and upper respiratory symptoms
Two-year-old Daniel is brought in to see his family physician. His mother reports that he has been tugging at his right ear for the past 24 hours. Daniel also has upper respiratory symptoms and an axial temperature of 101.9°F. He had one episode of vomiting, which kept him out of day care today. The patient’s past medical history is significant for surgery to repair an atrial septal defect 15 months ago.
Daniel takes no medication, has no drug allergies, and his immunizations are up to date. He was given a course of antibiotics to treat otitis media 2 months ago. A physical exam reveals an irritable but normally developing child. An otoscopic exam reveals reddened tympanic membranes bilaterally with normal mobility.
CASE 2 ›
Dysuria and urethral discharge
Twenty-year-old Jon F visits his FP and reports painful urination and purulent urethral discharge of 3 days’ duration. He denies having flank pain. When asked about his sexual history, Mr. F acknowledges having had unprotected intercourse with a sex worker less than 2 weeks ago.
The patient’s past medical history is unremarkable. He reports smoking marijuana occasionally, but denies other recreational drug use.
He tested negative for human immunodeficiency virus (HIV) 18 months ago, but says he has used condoms inconsistently since then. A physical exam reveals normal vital signs, with no sores or rashes. His chest, heart, musculoskeletal, abdominal, and rectal exams are normal. A genital exam reveals a normal circumcised penis, with reddened meatus and purulent discharge; the scrotum and testes are normal and without lesions, tenderness, or masses.
If Daniel and Jon F were your patients, would you prescribe antibiotics for them—and if so, what drugs would you select?
How to manage community-acquired MRSA
Methicillin-resistant S aureus (MRSA) is a common antimicrobial-resistant pathogen found in health care settings and in the community. While most community-acquired infections are minor and involve the skin and soft tissues, community-acquired (CA)-MRSA can cause pneumonia, thrombophlebitis, and necrotizing fasciitis.5
Identifying patients at risk
Individuals who share personal items, such as toothbrushes or razors, or have physical contact with anyone who is infected with or who carries the multidrug-resistant pathogen (eg, those spending time in crowded spaces like prisons, schools, or day care centers) are at increased risk for CA-MRSA. So, too, are intravenous (IV) drug users, men who have sex with men, individuals who have repeated skin trauma or contact with animals, and those of Native American, African-American, and Pacific Islander descent.5
MRSA resistance mechanisms include expression of altered penicillin-binding proteins, which have a reduced affinity for beta-lactam antibiotics. MRSA isolates may also be resistant to macrolides, aminoglycosides, fluoroquinolones, and clindamycin. CA-MRSA is not typically associated with the same degree of multidrug resistance as hospital-acquired strains. Instead, CA-MRSA usually produces a toxin known as Panton-Valentine leukocidin (PVL), which leads to leukocyte destruction and local tissue necrosis.6
Uncomplicated CA-MRSA skin and soft-tissue infections usually respond to incision and drainage, provided the patient (or parent) is instructed in and adheres to the recommended hygiene and wound care provisions. Antimicrobial agents are generally reserved for patients who have extensive disease involving multiple infection sites and/or systemic symptoms, purulent cellulitis without drainable foci, or septic phlebitis, or failed to respond to incision and drainage alone. Extreme age is a reasonable indication for antibiotics as well. Patients with more serious conditions, such as pneumonia, thrombophlebitis, or necrotizing fasciitis, require high acuity care and IV antibiotics.7
First-line empiric therapy in the outpatient setting should be either trimethoprim/sulfamethoxazole (TMP/SMX) or a long-acting tetracycline (TABLE 1).7 The ideal adult dose of TMP/SMX is 2 double-strength tablets (160/800 mg) twice daily for 5 to 10 days, as treatment failures are more prevalent with lower doses. Tetracyclines should be avoided in patients younger than 8 years.7

Clindamycin is an option in settings in which isolates can be tested to ensure a lack of inducible resistance; it is also the preferred agent for pregnant women.7 Topical mupirocin may be suitable for children with minor skin infections or secondarily infected skin lesions. Oral linezolid and tedizolid can also be used to treat CA-MRSA, but cost and the potential for drug interaction may prohibit their use. Linezolid inhibits PVL toxin production, however, and may be useful in more serious infections, such as necrotizing fasciitis.7
Strategies for preventing recurrent infection include personal and environmental hygiene measures for patients and close contacts. Decolonization strategies such as application of intranasal mupirocin 2% and washing with chlorhexidine soap may also be considered.7
How to respond to drug-resistant S pneumoniae
CASE 1 › Daniel
Daniel’s physician recommends observation, analgesics, and follow-up in 24 to 48 hours if his symptoms do not improve. Two days later, his mother brings him back to the office with worsening upper respiratory symptoms. Within the last 24 hours, she reports, Daniel’s axial temperatures have ranged from 101.5 to 102.9°F. He is drinking water and eating popsicles, she adds, but refusing to eat solid food. An otoscopic exam reveals moderately bulging tympanic membranes and poor mobility, with purulent fluid behind the membranes. The rest of the physical exam is non-contributory.
S pneumoniae is a gram-positive diplococcus frequently implicated in sinusitis, acute otitis media, and community-acquired pneumonia among outpatients and in meningitis among hospitalized patients. S pneumoniae is a virulent strain of streptococcus associated with increased morbidity and mortality in both young children and the elderly.8
The emergence of drug resistance
For years, penicillins, cephalosporins, and macrolides were used to treat community-acquired S pneumoniae infections, but penicillin-resistant S pneumoniae (PRSP) emerged in the 1990s. It results from alterations in penicillin-binding proteins, with the degree of resistance dependent on the particular proteins affected and the binding affinity of the beta-lactam agent used. S pneumoniae resistance to macrolides and clindamycin has also emerged.8
In 2010, a report on a large registry of S pneumoniae isolates revealed antibiotic resistance rates of approximately 5% for penicillin, 3% for cephalosporins, 30% for macrolides, and 20% for TMP/SMX. Levofloxacin resistance in S pneumoniae is rare (<1%).9
Penicillin resistance has been associated with poor clinical outcomes in patients with PRSP meningitis who were treated with standard doses of penicillin or cephalosporins.10 The impact of penicillin resistance on clinical outcomes in non-meningeal infections, however, is less clear.8 Macrolide and fluoroquinolone resistance has been associated with worse clinical outcomes and treatment failures.9 Fluoroquinolone use has been linked to an increased risk for adverse events and to Clostridium difficile (C difficile)-associated diarrhea.11 Recent antibiotic use is a major risk factor for developing a drug-resistant S pneumoniae infection. Additional risk factors are listed in TABLE 2.8,12,13

Is it bacterial? Distinguishing viral from bacterial etiologies in upper respiratory tract infections is challenging but critical in determining whether antibiotics are indicated. Use rapid diagnostic assays, whenever possible, to differentiate between them and to identify resistant bacteria.14 Prescribing antibiotics for respiratory infections only when necessary is crucial, as high rates of antimicrobial use is correlated with increasing resistance to S pneumoniae.15,16
What the societies call for
Practice guidelines from the Infectious Diseases Society of America (IDSA) for acute bacterial rhinosinusitis recommend starting antibiotics only when the following criteria are met:
- persistent symptoms that have not improved for ≥10 days
- severe symptoms have been present for ≥3 to 4 days
- “double sickening,” in which symptoms initially improve, but subsequently worsen.17
The acute otitis media guideline, developed by the American Academy of Pediatricians and the American Academy of Family Physicians, takes a similar approach. The guideline recommends observation with close follow-up in patients ≥24 months of age without severe signs or symptoms (eg, otalgia lasting >48 hours, temperature ≥102.2°F in the past 48 hours).18 When antibiotics are needed for respiratory tract infections, the selection of agents should be based on the site of infection, the latest evidence, and an assessment of patient-specific factors, including risk for PRSP (TABLE 3).13,17-19

High-dose amoxicillin has become the mainstay when PRSP is a concern, as the high doses have been found to overcome this type of penicillin resistance. Fluoroquinolones and cephalosporins are typically reserved for selected cases in which drug allergies or resistance to first-line agents is a concern.
CASE 1 › Daniel
The physician prescribes high-dose amoxicillin (80 mg/kg/d taken in 2 divided doses for 5 days) for Daniel, whose exposure at day care and recent antibiotic use put him at increased risk for PRSP. The doctor stresses the importance of giving the medication to Daniel for the full 5 days, even if his symptoms resolve. He encourages the patient’s mother to give him bedtime analgesics and yogurt with active cultures while he takes the amoxicillin to reduce the likelihood of diarrhea. He also schedules a follow-up visit in a week.
Treating drug-resistant UTIs
The vast majority of community-acquired UTIs are caused by E coli, followed by other Enterobacteriaceae such as Klebsiella pneumoniae and Proteus mirabilis.1 Since 2000, E coli resistance to commonly used outpatient antimicrobials has increased, particularly to ciprofloxacin, TMP/SMX, and narrow spectrum beta-lactams (amoxicillin and cephalexin).20-22 The prevalence of extended-spectrum-beta-lactamase (ESBL)-producing Enterobacteriaceae among urinary pathogens is also rising.1
How best to manage uncomplicated cystitis
Treating uncomplicated cystitis is challenging for a number of reasons, including increasing gram-negative resistance, a lack of surveillance data describing local outpatient resistance rates, and limited reliable oral options. UTIs caused by resistant organisms, such as ESBL-producing Enterobacteriaceae, are associated with recent antibiotic use, recurrent UTIs, recent hospitalization, advanced age, multiple comorbidities, hemodialysis, recent international travel, and urinary catheterization.23,24 Urine cultures and susceptibilities should be included in an assessment of patients with any of these risk factors and used to inform antibiotic selection.24 First-line treatment options for uncomplicated cystitis include nitrofurantoin, TMP/SMX (in regions where the uropathogen resistance is <20%), and fosfomycin.25
Multidrug-resistant pathogens
Nitrofurantoin, fosfomycin, and in some instances, fluoroquinolones, are options for treating multidrug-resistant uropathogens, as guided by susceptibility results (TABLE 4).24,26-28 IV antibiotics may be necessary for patients who have severe infections or live in long-term care facilities.24,29 UTIs caused by carbapenem-resistant Enterobacteriaceae are fortunately still rare in outpatient settings.30 There is a lack of high-quality evidence describing best practices for treating infections caused by ESBL-producing bacteria in the community; therefore, antimicrobial selection should be based on infection severity and patient-specific factors.1

Neisseria gonorrhoeae is multidrug-resistant
CASE 2 › Jon F
Mr. F’s physician suspects gonorrhea, although chlamydia cannot be ruled out, and orders a urethral culture, first-catch urine test using nucleic acid amplification, syphilis, and HIV tests. The physician administers ceftriaxone 250 mg IM and azithromycin 1 g PO, instructs the patient to contact all sex partners within the last 60 days, and emphasizes the importance of using latex condoms consistently.
Three days later, Jon returns, complaining that his symptoms have not improved. The urethral culture shows a gram-negative oxidase-positive diplococcus and testing confirms N gonorrhoeae. The lab results also reveal a high cephalosporin minimal inhibitory concentration, negative tests for chlamydia and HIV, and a non-reactive rapid plasma reagin.
Gonococcal infections are the second most common communicable disease reported in the United States, with some 820,000 new cases annually.31 Sites of infection include the cervix, urethra, and rectum, and less commonly, the pharynx, conjunctiva, joints, meninges, and endocardium. Those at risk for gonorrhea are: 31
- women who are sexually active and <25 years of age
- any woman with a new sex partner
- any individual with multiple sex partners or a partner with concurrent partners and/or a known sexually transmitted infection (STI)
- an individual who has a history of inconsistent condom use in non-monogamous relationships
- sex workers.
SIDEBAR
Combatting antibiotic resistance: A call to action for FPs
- Use the best available evidence to determine when antibiotics are indicated.
- Develop and use “antibiograms”—tools that use data from patient specimens to identify patterns of antibiotic susceptibility and guide appropriate empiric antibiotic selection.
- Use rapid diagnostic assays to differentiate between viral and bacterial infections and identify resistant bacteria.
- Add antibiotic use to the list of metrics your practice uses to assess clinician performance.
- Adopt standard, clear language to explain to patients and parents when—and why—antibiotics are not indicated.
- Support public health surveillance efforts by learning and adhering to reporting requirements in your community.
The difficulty of treating gonorrhea
Cephalosporins are now the only class of antimicrobials with reliable activity against N gonorrhoeae. The Centers for Disease Control and Prevention (CDC) no longer recommends fluoroquinolones due to increasing resistance. However, cefixime-resistant strains of gonorrhea and treatment failures have been reported, and the drug is no longer recommended for gonorrhea treatment.31
Combination therapy with one dose of ceftriaxone 250 mg IM and one dose of azithromycin 1 g orally is recommended by the CDC,32 as combination therapy improves efficacy and delays the development of resistance. Azithromycin is preferred over doxycycline as the second agent. That’s both because of a higher prevalence of tetracycline resistance among circulating gonococci and azithromycin’s activity against Chlamydia trachomatis, as patients with gonococcal infections are frequently co-infected with this organism.31
Suspected treatment failures are more likely caused by re-infection than drug resistance. If resistance is suspected, however, physicians should seek guidance from an infectious diseases specialist or the CDC on repeat cultures, susceptibility testing, and antimicrobial therapy.
Two treatment regimens have demonstrated efficacy against cephalosporin-resistant N gonorrhoeae:31,32
- A single dose of gemifloxacin 320 mg PO plus azithromycin 2 g PO or
- a single dose of gentamicin 240 mg IM plus azithromycin 2 g PO.
CASE 2 › Jon F
Mr. F’s physician consults with an infectious disease specialist at the local hospital, who confirms his suspicion that the patient has been infected with antibiotic-resistant N gonorrhoeae. The physician administers gentamicin 240 mg IM plus azithromycin 2 g orally, and warns the patient that he may experience gastrointestinal adverse effects. The physician reports the case, as required, to the local health department.
Antibiotic stewardship: What’s being done…
Efforts to improve antibiotic use in the community setting include a variety of strategies, including academic detailing (ie, evidence-based promotion of drug therapies rather than promotion by manufacturer representatives), patient education, clinical decision support, multi-faceted programs using a combination of interventions, and local and national campaigns.33-36 Recent examples of successful campaigns include the American Board of Internal Medicine Foundation’s Choosing Wisely initiative (choosingwisely.org) and the CDC’s Get Smart program (cdc.gov/getsmart/community/index.html).
Get Smart promotes appropriate antibiotic use by prescribers and aims to decrease demand for antibiotics by patients and promote adherence to prescribed antibiotic regimens. Studies have found that incorporating treatment algorithms and clinical decision support systems into existing electronic medical record systems has led to more appropriate prescribing.37-39
The most effective interventions target both patients/parents and prescribers, provide evidence-based prescribing prompts, require prescribers to justify antibiotic use, and involve clinicians in their design. Future directions should include collaboration with municipal or regional public health organizations to identify community-wide critical infections and resistance trends and strategies that use behavioral interventions to address inappropriate prescribing.40
…and what you can do
Family physicians are key to determining the outcome of the war against antibiotic resistance. See “Combatting antibiotic resistance: A call to action for FPs” on the previous page for specific interventions you can initiate without delay.
The tide will turn only through modification of both prescriber and patient behavior and formalized programs in our communities. Education about appropriate use needs to be included in medical school curricula and continue in the clinic setting through education of physicians in training, medical students, and office staff.41 Become an advocate by promoting the principles of optimal antibiotic stewardship as outlined by the recent IDSA Guidelines for Implementing an Antibiotic Stewardship Program.42 Go to http://cid.oxfordjournals.org/content/62/10/1197.long to learn more.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy Practice & Administration, School of Pharmacy, University of Saint Joseph, 229 Trumbull Street, Hartford, CT 06103; [email protected].
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
2. Hicks DO, Taylor TH. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461-1462.
3. Fleming-Dutra KE, Hersh, A, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
4. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed October 31, 2016.
5. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
6. Chambers HF. Methicillin-resistant Staphylococcus aureus: mechanisms of resistance and implications for treatment. Postgrad Med. 2001;109:43-50.
7. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;5:285-292.
8. Couch KA, Geide T. ASHP therapeutic position statement on strategies for identifying and preventing pneumococcal resistance. Am J Health-Syst Pharm. 2014;71:417-424.
9. Centers for Disease Control and Prevention (2010) ABCs report: Streptococcus pneumoniae. Available at: www.cdc.gov/abcs/reports-findings/survreports/spneu10.html. Accessed October 28, 2016.
10. Pallares R, Fenoll A, Linares J for the Spanish Pneumococcal Infection Study Network. The epidemiology of antibiotic resistance in Streptococcus pneumoniae and the clinical relevance of resistance to cephalosporins, macrolides, and fluoroquinolones. Int J Antimicrob Agents. 2003;22(suppl):S15-S24.
11. US Food and Drug Administration. The benefits and risks of systemic fluoroquinolone antibacterial drugs for the treatment of acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis in patients who have chronic obstructive pulmonary disease (ABECB-COPD), and uncomplicated urinary tract infections (uUTI). 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed October 28, 2016.
12. Nuorti JP, Butler JC, Farley NM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342:681-689.
13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl):S27-S72.
14. Gwaltney JM Jr, Wiesinger BA, Patrieb JT. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233.
15. Diekema DJ, Brueggemann AB, Doern GV. Antimicrobial-drug use and changes in resistance in Streptococcus pneumoniae. Emerg Infect Dis. 2000;6:552-556.
16. Hicks LA, Chien YW, Taylor TH Jr, et al. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631-639.
17. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e1-e41.
18. Lieberthal AS, Caroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999.
19. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.
20. Sanchez GV, Adams SJ, Baird AM, et al. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000–10. J Antimicrob Chemother. 2013:68:1838-1841.
21. Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736-738.
22. Gupta K, Hooten TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
23. Ben-Ami R, Rodrıguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase–producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690.
24. Walker E, Lyman A, Gupta K, et al. Clinical management of an increasing threat: outpatient urinary tract infections due to multidrug-resistant uropathogens. Clin Infect Dis. 2016;63:960-965.
25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
26. Gupta K, Bhadelia N. Management of urinary tract infections from multidrug-resistant organisms. Infect Dis Clin N Am. 2014;28:49-59.
27. Neuner EA, Sekeres J, Hall GS, et al. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56:5744-5748.
28. Tasbakan MI, Pullukcu H, Sipahi OR, et al. Nitrofurantoin in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infection. Int J Antimicrob Agents. 2012;40:554-556.
29. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;268:1897-1902.
30. Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA. 2015;314:1479-1487.
31. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. 2015;64:1-137.
32. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
33. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901-904.
34. Belongia EA, Sullivan BJ, Chyou, PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108;575-583.
35. Gjelstad S, Hoye S, Straand J, et al. Improving antibiotic prescribing in acute respiratory tract infections: cluster randomised trial from Norwegian general practice (prescription peer academic detailing [Rx-PAD] study). BMJ. 2013;347:f4403.
36. Huttner B, Harbarth S. ‘Antibiotics are not automatic anymore’’—the French national campaign to cut antibiotic overuse. PLoS Med. 2009;6: e1000080.
37. Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
38. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.
40. Meeker D, Linder JA, Fox CR, et al
41. Schwartz BS, Armstrong WS, Ohl CA, et al. Create allies, IDSA stewardship commitments should prioritize health professions learners. Clin Infect Dis. 2015; 61:1626-1627.
42. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program:guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:1-27.
PRACTICE RECOMMENDATIONS
› Manage uncomplicated cutaneous abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus with incision and drainage alone. A
› Treat upper respiratory infections associated with drug-resistant Streptococcus pneumoniae with high-dose amoxicillin, which has been found to overcome penicillin resistance. A
› Administer dual therapy with ceftriaxone and azithromycin to patients with gonococcal infections. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Mental Health: A Forgotten Facet of Primary Care
One of the biggest disparities in health care today is the separate treatment of mind and body, despite their known integration.1 While mental and behavioral health conditions are frequently diagnosed and treated within primary care settings, fragmentation persists between the mental and physical health care systems—creating barriers in the quality, outcome, and efficiency of care.2 Since half of Americans with mental health conditions go without essential care, reform of our nation’s mental health system is a priority issue for NPs and PAs and the patients we serve.
Some progress has been made to implement change—the Now Is the Time initiative, launched in 2013, increased federal funding for behavioral health care workforce training in an effort to support more providers in mental and substance use disorder treatment. The Affordable Care Act (ACA) has worked to improve behavioral health coverage for Americans in three ways: ending insurance company discrimination based on pre-existing conditions, requiring health insurance coverage for mental and substance use disorder services, and expanding mental health parity. This has improved coverage and access to mental and substance abuse services for more than 60 million Americans.3 In January 2016, President Obama proposed a $500 million investment to increase access to mental health care.4 The most recent presidential election creates an uncertain future for
Regardless, more work has to be done to guarantee that Americans have the access they need. Sadly, even with these advancements in behavioral health coverage, only about half of children and less than half of adults with diagnosable mental health disorders get the treatment they need.4 A 2016 report from the Rural Health Research Center revealed that more than 15 million Americans face behavioral health issues without access to the necessary care.5
Psychiatric providers (like most other specialists) tend to be located in urban areas, limiting access in rural areas and even some underserved urban communities. Only 43% of family physicians in this country provide mental health care.6 The team-based care that NPs and PAs provide has great potential for bridging this gap in mental health coverage.
NPs and PAs are an important but underutilized resource for improving mental health care access—but how can primary care NPs and PAs work to enhance the delivery of mental health care in our country? In the preprofessional area, it would be prudent to entice qualified individuals in the mental health field—particularly those who are licensed clinical social workers, licensed professional counselors, or marriage and family therapists—into NP and PA programs with preference.
Clinical rotations in behavioral health (BH)/psychiatry should be encouraged—even mandated—in professional education. We should ensure this content is taught in the didactic portion of NP/PA professional education, as well as bolstering psychiatric pharmacology in coursework.
Postprofessional education should encourage primary care NPs and PAs to gain additional self-directed education in BH/psychiatry. This can be achieved via a focused psychiatry “boot camp” (for PAs following the CAQ blueprint, found at www.nccpa.net/psychiatry) or a competency-based online postprofessional certificate in BH/psychiatry (such as—shameless plug—the one offered at my institution; www.atsu.edu/postgraduate-certificate-in-psychiatry-and-behavioral-health-online).7,8
This psychiatric background is fundamental throughout primary care but is crucial in community health centers, correctional health care centers, and Veterans Administration hospitals. Of course, in order to make a difference, we must remove the barriers that prevent psychiatric NPs and PAs from being considered mental health providers and adjust reimbursement accordingly.
Do you have ideas on how to increase the knowledge base of primary care NPs and PAs and enhance the provision of mental health services in this country? Will the political change in leadership in January 2017 increase opportunities to make a difference in mental health care? Please share your thoughts by contacting me at [email protected].
1. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, eds. Primary Care: America’s Health in a New Era. Washington, DC: Institute of Medicine; 1996.
2. Simon GE, Katon WJ, VonKorff M, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry. 2001;158(10): 1638-1644.
3. Enomoto K. Improving access to mental health services - President’s FY 2017 Budget proposes new investments to increase access. http://abilitychicagoinfo.blogspot.com/2016/02/improving-access-to-mental-health.html. Accessed November 3, 2016.
4. Enomoto K. Improving access to mental health services. www.hhs.gov/blog/2016/02/09/improving-access-mental-health-services.html. Accessed November 3, 2016.
5. Rural Health Research Center. Supply and distribution of the behavioral health workforce in rural America. http://depts.washington.edu/fammed/rhrc/wp-content/uploads/sites/4/2016/09/RHRC_DB160_Larson.pdf. Accessed November 3, 2016.
6. Miller BF, Druss B. The role of family physicians in mental health care delivery in the United States: implications for health reform. J Am Board Fam Med. 2013;26(2): 111-113.
7. National Commission on Certification of Physician Assistants. Psychiatry CAQ. www.nccpa.net/psychiatry. Accessed November 3, 2016.
8. A.T. Still University. Postgraduate certificate in psychiatry and behavioral health online. www.atsu.edu/postgraduate-certificate-in-psychiatry-and-behavioral-health-online. Accessed November 3, 2016.
One of the biggest disparities in health care today is the separate treatment of mind and body, despite their known integration.1 While mental and behavioral health conditions are frequently diagnosed and treated within primary care settings, fragmentation persists between the mental and physical health care systems—creating barriers in the quality, outcome, and efficiency of care.2 Since half of Americans with mental health conditions go without essential care, reform of our nation’s mental health system is a priority issue for NPs and PAs and the patients we serve.
Some progress has been made to implement change—the Now Is the Time initiative, launched in 2013, increased federal funding for behavioral health care workforce training in an effort to support more providers in mental and substance use disorder treatment. The Affordable Care Act (ACA) has worked to improve behavioral health coverage for Americans in three ways: ending insurance company discrimination based on pre-existing conditions, requiring health insurance coverage for mental and substance use disorder services, and expanding mental health parity. This has improved coverage and access to mental and substance abuse services for more than 60 million Americans.3 In January 2016, President Obama proposed a $500 million investment to increase access to mental health care.4 The most recent presidential election creates an uncertain future for
Regardless, more work has to be done to guarantee that Americans have the access they need. Sadly, even with these advancements in behavioral health coverage, only about half of children and less than half of adults with diagnosable mental health disorders get the treatment they need.4 A 2016 report from the Rural Health Research Center revealed that more than 15 million Americans face behavioral health issues without access to the necessary care.5
Psychiatric providers (like most other specialists) tend to be located in urban areas, limiting access in rural areas and even some underserved urban communities. Only 43% of family physicians in this country provide mental health care.6 The team-based care that NPs and PAs provide has great potential for bridging this gap in mental health coverage.
NPs and PAs are an important but underutilized resource for improving mental health care access—but how can primary care NPs and PAs work to enhance the delivery of mental health care in our country? In the preprofessional area, it would be prudent to entice qualified individuals in the mental health field—particularly those who are licensed clinical social workers, licensed professional counselors, or marriage and family therapists—into NP and PA programs with preference.
Clinical rotations in behavioral health (BH)/psychiatry should be encouraged—even mandated—in professional education. We should ensure this content is taught in the didactic portion of NP/PA professional education, as well as bolstering psychiatric pharmacology in coursework.
Postprofessional education should encourage primary care NPs and PAs to gain additional self-directed education in BH/psychiatry. This can be achieved via a focused psychiatry “boot camp” (for PAs following the CAQ blueprint, found at www.nccpa.net/psychiatry) or a competency-based online postprofessional certificate in BH/psychiatry (such as—shameless plug—the one offered at my institution; www.atsu.edu/postgraduate-certificate-in-psychiatry-and-behavioral-health-online).7,8
This psychiatric background is fundamental throughout primary care but is crucial in community health centers, correctional health care centers, and Veterans Administration hospitals. Of course, in order to make a difference, we must remove the barriers that prevent psychiatric NPs and PAs from being considered mental health providers and adjust reimbursement accordingly.
Do you have ideas on how to increase the knowledge base of primary care NPs and PAs and enhance the provision of mental health services in this country? Will the political change in leadership in January 2017 increase opportunities to make a difference in mental health care? Please share your thoughts by contacting me at [email protected].
One of the biggest disparities in health care today is the separate treatment of mind and body, despite their known integration.1 While mental and behavioral health conditions are frequently diagnosed and treated within primary care settings, fragmentation persists between the mental and physical health care systems—creating barriers in the quality, outcome, and efficiency of care.2 Since half of Americans with mental health conditions go without essential care, reform of our nation’s mental health system is a priority issue for NPs and PAs and the patients we serve.
Some progress has been made to implement change—the Now Is the Time initiative, launched in 2013, increased federal funding for behavioral health care workforce training in an effort to support more providers in mental and substance use disorder treatment. The Affordable Care Act (ACA) has worked to improve behavioral health coverage for Americans in three ways: ending insurance company discrimination based on pre-existing conditions, requiring health insurance coverage for mental and substance use disorder services, and expanding mental health parity. This has improved coverage and access to mental and substance abuse services for more than 60 million Americans.3 In January 2016, President Obama proposed a $500 million investment to increase access to mental health care.4 The most recent presidential election creates an uncertain future for
Regardless, more work has to be done to guarantee that Americans have the access they need. Sadly, even with these advancements in behavioral health coverage, only about half of children and less than half of adults with diagnosable mental health disorders get the treatment they need.4 A 2016 report from the Rural Health Research Center revealed that more than 15 million Americans face behavioral health issues without access to the necessary care.5
Psychiatric providers (like most other specialists) tend to be located in urban areas, limiting access in rural areas and even some underserved urban communities. Only 43% of family physicians in this country provide mental health care.6 The team-based care that NPs and PAs provide has great potential for bridging this gap in mental health coverage.
NPs and PAs are an important but underutilized resource for improving mental health care access—but how can primary care NPs and PAs work to enhance the delivery of mental health care in our country? In the preprofessional area, it would be prudent to entice qualified individuals in the mental health field—particularly those who are licensed clinical social workers, licensed professional counselors, or marriage and family therapists—into NP and PA programs with preference.
Clinical rotations in behavioral health (BH)/psychiatry should be encouraged—even mandated—in professional education. We should ensure this content is taught in the didactic portion of NP/PA professional education, as well as bolstering psychiatric pharmacology in coursework.
Postprofessional education should encourage primary care NPs and PAs to gain additional self-directed education in BH/psychiatry. This can be achieved via a focused psychiatry “boot camp” (for PAs following the CAQ blueprint, found at www.nccpa.net/psychiatry) or a competency-based online postprofessional certificate in BH/psychiatry (such as—shameless plug—the one offered at my institution; www.atsu.edu/postgraduate-certificate-in-psychiatry-and-behavioral-health-online).7,8
This psychiatric background is fundamental throughout primary care but is crucial in community health centers, correctional health care centers, and Veterans Administration hospitals. Of course, in order to make a difference, we must remove the barriers that prevent psychiatric NPs and PAs from being considered mental health providers and adjust reimbursement accordingly.
Do you have ideas on how to increase the knowledge base of primary care NPs and PAs and enhance the provision of mental health services in this country? Will the political change in leadership in January 2017 increase opportunities to make a difference in mental health care? Please share your thoughts by contacting me at [email protected].
1. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, eds. Primary Care: America’s Health in a New Era. Washington, DC: Institute of Medicine; 1996.
2. Simon GE, Katon WJ, VonKorff M, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry. 2001;158(10): 1638-1644.
3. Enomoto K. Improving access to mental health services - President’s FY 2017 Budget proposes new investments to increase access. http://abilitychicagoinfo.blogspot.com/2016/02/improving-access-to-mental-health.html. Accessed November 3, 2016.
4. Enomoto K. Improving access to mental health services. www.hhs.gov/blog/2016/02/09/improving-access-mental-health-services.html. Accessed November 3, 2016.
5. Rural Health Research Center. Supply and distribution of the behavioral health workforce in rural America. http://depts.washington.edu/fammed/rhrc/wp-content/uploads/sites/4/2016/09/RHRC_DB160_Larson.pdf. Accessed November 3, 2016.
6. Miller BF, Druss B. The role of family physicians in mental health care delivery in the United States: implications for health reform. J Am Board Fam Med. 2013;26(2): 111-113.
7. National Commission on Certification of Physician Assistants. Psychiatry CAQ. www.nccpa.net/psychiatry. Accessed November 3, 2016.
8. A.T. Still University. Postgraduate certificate in psychiatry and behavioral health online. www.atsu.edu/postgraduate-certificate-in-psychiatry-and-behavioral-health-online. Accessed November 3, 2016.
1. deGruy F. Mental health care in the primary care setting. In: Donaldson MS, Yordy KD, Lohr KN, Vanselow NA, eds. Primary Care: America’s Health in a New Era. Washington, DC: Institute of Medicine; 1996.
2. Simon GE, Katon WJ, VonKorff M, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry. 2001;158(10): 1638-1644.
3. Enomoto K. Improving access to mental health services - President’s FY 2017 Budget proposes new investments to increase access. http://abilitychicagoinfo.blogspot.com/2016/02/improving-access-to-mental-health.html. Accessed November 3, 2016.
4. Enomoto K. Improving access to mental health services. www.hhs.gov/blog/2016/02/09/improving-access-mental-health-services.html. Accessed November 3, 2016.
5. Rural Health Research Center. Supply and distribution of the behavioral health workforce in rural America. http://depts.washington.edu/fammed/rhrc/wp-content/uploads/sites/4/2016/09/RHRC_DB160_Larson.pdf. Accessed November 3, 2016.
6. Miller BF, Druss B. The role of family physicians in mental health care delivery in the United States: implications for health reform. J Am Board Fam Med. 2013;26(2): 111-113.
7. National Commission on Certification of Physician Assistants. Psychiatry CAQ. www.nccpa.net/psychiatry. Accessed November 3, 2016.
8. A.T. Still University. Postgraduate certificate in psychiatry and behavioral health online. www.atsu.edu/postgraduate-certificate-in-psychiatry-and-behavioral-health-online. Accessed November 3, 2016.
Diagnosis & assessment of pain: Refining your approach
Pediatric update: 2 vaccine changes and the latest word on media time
VIDEO: No difference between PPI and H2RA for low-dose aspirin gastroprotection
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
Data source: A 270-subject, double-blind, randomized trial in Hong Kong and Japan.
Disclosures: The Research Grant Council of Hong Kong funded the work. The lead investigator has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
In lung cancer screening, patient education works
A counseling and shared decision-making visit improved patient knowledge of the eligibility criteria, benefits, and potential risks of lung cancer screening via a low-radiation chest CT scan.
The Center for Medicare & Medicaid Services has added the type of visit addressed in this study to Medicare’s preventive services benefits for individuals meeting certain criteria, but no previous study had looked at how the implementation of such a visit impacted a patient’s knowledge and understanding.
The researchers noted significant improvement in all questions before and after a counseling session (P = .03 to P less than .0001). Those improvements lessened at 1 month, but were still higher than precounseling scores.
The percentages of participants who knew the age criteria for lung cancer screening before counseling, immediately after counseling, and 1 month after counseling, for example, were 8.8% (11 patients), 59.2% (74 patients), and 21.4% (24 patients), respectively. The percentage of participants able to identify at least one of the potential hazards of screening increased by a similar amount immediately after receiving counseling, as did the percentage of participants able to identify the age criteria for lung cancer screening immediately after receiving counseling. The percentages of patients able to identify at least one of the potential hazards of screening were 38.4% before counseling and 90.4% immediately after receiving counseling. One month following counseling, the percentage of patients with such knowledge remained fairly high, dropping to 78.6%.
“Showing the value of these visits is very important to encourage policymakers and payers to continue to support the counseling and shared decision-making visit,” Peter J. Mazzone, MD, MPH, who led the study, said in an interview.
These types of conversations are important because of the uncertainties surrounding lung cancer screening, which leads to about a 20% reduction in mortality risk. That translates to the need to screen about 250 people to save 1 life. “I think the public sometimes doesn’t realize that the effectiveness of some of these preventive screenings may not be as large as they think they are,” said David Grossman, vice chair of the US Preventive Services Task Force and a senior investigator at the Group Health Research Institute, Seattle, who was not involved in the study.
The researchers developed a centralized counseling and shared decision-making visit that included a narrated slide show and individualized risk assessment. They approached 423 consecutive patients who had been identified by their primary care provider or a specialist as potential candidates for screening. Of those 423 patients, 125 agreed to participate in the study (Chest. 2016 Nov 1. doi: 10.1016/j.chest.2016.10.027).
The session delivered expected improvements in patient knowledge, but there were some surprises. “The starting point of knowledge was perhaps less than we would have anticipated, and the gains, though very substantial, weren’t perfect,” said Dr. Mazzone, who is also director of the lung cancer screening program at the Cleveland Clinic.
The drop in knowledge at 1 month suggests that the information needs to be reinforced, possibly each time patients come in for an annual screening visit, Dr. Mazzone added.
Counseling sessions can also help convince patients to quit smoking, if tobacco use is a concern. “It’s not appropriate to screen for lung cancer without making a commitment to try to quit,” said Dr. Grossman.
No funding source was disclosed. Dr. Mazzone and Dr. Grossman reported having no financial disclosures.
A counseling and shared decision-making visit improved patient knowledge of the eligibility criteria, benefits, and potential risks of lung cancer screening via a low-radiation chest CT scan.
The Center for Medicare & Medicaid Services has added the type of visit addressed in this study to Medicare’s preventive services benefits for individuals meeting certain criteria, but no previous study had looked at how the implementation of such a visit impacted a patient’s knowledge and understanding.
The researchers noted significant improvement in all questions before and after a counseling session (P = .03 to P less than .0001). Those improvements lessened at 1 month, but were still higher than precounseling scores.
The percentages of participants who knew the age criteria for lung cancer screening before counseling, immediately after counseling, and 1 month after counseling, for example, were 8.8% (11 patients), 59.2% (74 patients), and 21.4% (24 patients), respectively. The percentage of participants able to identify at least one of the potential hazards of screening increased by a similar amount immediately after receiving counseling, as did the percentage of participants able to identify the age criteria for lung cancer screening immediately after receiving counseling. The percentages of patients able to identify at least one of the potential hazards of screening were 38.4% before counseling and 90.4% immediately after receiving counseling. One month following counseling, the percentage of patients with such knowledge remained fairly high, dropping to 78.6%.
“Showing the value of these visits is very important to encourage policymakers and payers to continue to support the counseling and shared decision-making visit,” Peter J. Mazzone, MD, MPH, who led the study, said in an interview.
These types of conversations are important because of the uncertainties surrounding lung cancer screening, which leads to about a 20% reduction in mortality risk. That translates to the need to screen about 250 people to save 1 life. “I think the public sometimes doesn’t realize that the effectiveness of some of these preventive screenings may not be as large as they think they are,” said David Grossman, vice chair of the US Preventive Services Task Force and a senior investigator at the Group Health Research Institute, Seattle, who was not involved in the study.
The researchers developed a centralized counseling and shared decision-making visit that included a narrated slide show and individualized risk assessment. They approached 423 consecutive patients who had been identified by their primary care provider or a specialist as potential candidates for screening. Of those 423 patients, 125 agreed to participate in the study (Chest. 2016 Nov 1. doi: 10.1016/j.chest.2016.10.027).
The session delivered expected improvements in patient knowledge, but there were some surprises. “The starting point of knowledge was perhaps less than we would have anticipated, and the gains, though very substantial, weren’t perfect,” said Dr. Mazzone, who is also director of the lung cancer screening program at the Cleveland Clinic.
The drop in knowledge at 1 month suggests that the information needs to be reinforced, possibly each time patients come in for an annual screening visit, Dr. Mazzone added.
Counseling sessions can also help convince patients to quit smoking, if tobacco use is a concern. “It’s not appropriate to screen for lung cancer without making a commitment to try to quit,” said Dr. Grossman.
No funding source was disclosed. Dr. Mazzone and Dr. Grossman reported having no financial disclosures.
A counseling and shared decision-making visit improved patient knowledge of the eligibility criteria, benefits, and potential risks of lung cancer screening via a low-radiation chest CT scan.
The Center for Medicare & Medicaid Services has added the type of visit addressed in this study to Medicare’s preventive services benefits for individuals meeting certain criteria, but no previous study had looked at how the implementation of such a visit impacted a patient’s knowledge and understanding.
The researchers noted significant improvement in all questions before and after a counseling session (P = .03 to P less than .0001). Those improvements lessened at 1 month, but were still higher than precounseling scores.
The percentages of participants who knew the age criteria for lung cancer screening before counseling, immediately after counseling, and 1 month after counseling, for example, were 8.8% (11 patients), 59.2% (74 patients), and 21.4% (24 patients), respectively. The percentage of participants able to identify at least one of the potential hazards of screening increased by a similar amount immediately after receiving counseling, as did the percentage of participants able to identify the age criteria for lung cancer screening immediately after receiving counseling. The percentages of patients able to identify at least one of the potential hazards of screening were 38.4% before counseling and 90.4% immediately after receiving counseling. One month following counseling, the percentage of patients with such knowledge remained fairly high, dropping to 78.6%.
“Showing the value of these visits is very important to encourage policymakers and payers to continue to support the counseling and shared decision-making visit,” Peter J. Mazzone, MD, MPH, who led the study, said in an interview.
These types of conversations are important because of the uncertainties surrounding lung cancer screening, which leads to about a 20% reduction in mortality risk. That translates to the need to screen about 250 people to save 1 life. “I think the public sometimes doesn’t realize that the effectiveness of some of these preventive screenings may not be as large as they think they are,” said David Grossman, vice chair of the US Preventive Services Task Force and a senior investigator at the Group Health Research Institute, Seattle, who was not involved in the study.
The researchers developed a centralized counseling and shared decision-making visit that included a narrated slide show and individualized risk assessment. They approached 423 consecutive patients who had been identified by their primary care provider or a specialist as potential candidates for screening. Of those 423 patients, 125 agreed to participate in the study (Chest. 2016 Nov 1. doi: 10.1016/j.chest.2016.10.027).
The session delivered expected improvements in patient knowledge, but there were some surprises. “The starting point of knowledge was perhaps less than we would have anticipated, and the gains, though very substantial, weren’t perfect,” said Dr. Mazzone, who is also director of the lung cancer screening program at the Cleveland Clinic.
The drop in knowledge at 1 month suggests that the information needs to be reinforced, possibly each time patients come in for an annual screening visit, Dr. Mazzone added.
Counseling sessions can also help convince patients to quit smoking, if tobacco use is a concern. “It’s not appropriate to screen for lung cancer without making a commitment to try to quit,” said Dr. Grossman.
No funding source was disclosed. Dr. Mazzone and Dr. Grossman reported having no financial disclosures.
FROM CHEST
Key clinical point:
Major finding: Failure to identify potential harm dropped from 61.6% to 21.4%.
Data source: Prospective study of 125 patients.
Disclosures: No funding source was disclosed. Dr. Mazzone and Dr. Grossman reported having no financial disclosures.
Ultrasound effective in diagnosing occult hernia in women
ORLANDO – Ultrasound appears to be an effective tool in diagnosing occult hernia in women with unexplained chronic pelvic pain, according to a retrospective cohort study of 96 women.
“As gynecologists, we are likely to see those patients with chronic pelvic pain in our clinic before other specialists due to the location of their pain,” said Joelle Aoun, MD, an ob.gyn. in the division of minimally invasive gynecologic surgery at Henry Ford Health System in Detroit. “So it’s very important to recognize women with a high clinical suspicion for occult hernia and evaluate them in order to prevent a delayed diagnosis and prolonged suffering.”
Hernias can be more difficult to diagnose in women than in men, Dr. Aoun said at the meeting sponsored by AAGL, and the literature offers conflicting findings since most hernia studies are conducted in men or with mixed gender cohorts.
Dr. Aoun and her coinvestigators conducted a retrospective cohort study from January 2005 to July 2016, identifying 96 women with chronic pelvic pain and focal inguinal tenderness. Protruding fat or visceral tissue on physical exam or observed visually led clinicians to suspect a hernia. A single sonographer performed the musculoskeletal ultrasound.
Investigators diagnosed an occult hernia in more than half of the patients (51 women) based on the physical exam and ultrasound findings. Diagnoses included inguinal, femoral, Spigelian, and umbilical hernias.
All women with an ultrasound-diagnosed hernia were referred to general surgery. A majority – 69% of women – underwent surgical exploration. The remaining 31% of women who declined tended to have lower pain scores, Dr. Aoun said. Surgeons confirmed the hernia diagnosis in 97% of the women, or 34 out of the 35 women who had consented to surgery.
The group with a hernia was older and more likely to have arthritis, but otherwise did not differ significantly from the nonhernia cohort.
“We believe musculoskeletal ultrasound is valuable as an initial imaging modality due to its high predictive value, low cost, and noninvasiveness,” Dr. Aoun said.
Chronic pelvic pain is not uncommon, affecting approximately 15% of women during their reproductive years often with significant impacts on quality of life, workplace productivity, and health care utilization, Dr. Aoun said. This presentation also accounts for about 10% outpatient gynecology consultations and approximately 40% of laparoscopies in the United States, she added.
Dr. Aoun and her colleagues are planning a subsequent study of all the women who opted not to undergo surgery to determine their follow-up pain profiles based on chart review and phone interviews.
Dr. Aoun reported having no relevant financial disclosures.
ORLANDO – Ultrasound appears to be an effective tool in diagnosing occult hernia in women with unexplained chronic pelvic pain, according to a retrospective cohort study of 96 women.
“As gynecologists, we are likely to see those patients with chronic pelvic pain in our clinic before other specialists due to the location of their pain,” said Joelle Aoun, MD, an ob.gyn. in the division of minimally invasive gynecologic surgery at Henry Ford Health System in Detroit. “So it’s very important to recognize women with a high clinical suspicion for occult hernia and evaluate them in order to prevent a delayed diagnosis and prolonged suffering.”
Hernias can be more difficult to diagnose in women than in men, Dr. Aoun said at the meeting sponsored by AAGL, and the literature offers conflicting findings since most hernia studies are conducted in men or with mixed gender cohorts.
Dr. Aoun and her coinvestigators conducted a retrospective cohort study from January 2005 to July 2016, identifying 96 women with chronic pelvic pain and focal inguinal tenderness. Protruding fat or visceral tissue on physical exam or observed visually led clinicians to suspect a hernia. A single sonographer performed the musculoskeletal ultrasound.
Investigators diagnosed an occult hernia in more than half of the patients (51 women) based on the physical exam and ultrasound findings. Diagnoses included inguinal, femoral, Spigelian, and umbilical hernias.
All women with an ultrasound-diagnosed hernia were referred to general surgery. A majority – 69% of women – underwent surgical exploration. The remaining 31% of women who declined tended to have lower pain scores, Dr. Aoun said. Surgeons confirmed the hernia diagnosis in 97% of the women, or 34 out of the 35 women who had consented to surgery.
The group with a hernia was older and more likely to have arthritis, but otherwise did not differ significantly from the nonhernia cohort.
“We believe musculoskeletal ultrasound is valuable as an initial imaging modality due to its high predictive value, low cost, and noninvasiveness,” Dr. Aoun said.
Chronic pelvic pain is not uncommon, affecting approximately 15% of women during their reproductive years often with significant impacts on quality of life, workplace productivity, and health care utilization, Dr. Aoun said. This presentation also accounts for about 10% outpatient gynecology consultations and approximately 40% of laparoscopies in the United States, she added.
Dr. Aoun and her colleagues are planning a subsequent study of all the women who opted not to undergo surgery to determine their follow-up pain profiles based on chart review and phone interviews.
Dr. Aoun reported having no relevant financial disclosures.
ORLANDO – Ultrasound appears to be an effective tool in diagnosing occult hernia in women with unexplained chronic pelvic pain, according to a retrospective cohort study of 96 women.
“As gynecologists, we are likely to see those patients with chronic pelvic pain in our clinic before other specialists due to the location of their pain,” said Joelle Aoun, MD, an ob.gyn. in the division of minimally invasive gynecologic surgery at Henry Ford Health System in Detroit. “So it’s very important to recognize women with a high clinical suspicion for occult hernia and evaluate them in order to prevent a delayed diagnosis and prolonged suffering.”
Hernias can be more difficult to diagnose in women than in men, Dr. Aoun said at the meeting sponsored by AAGL, and the literature offers conflicting findings since most hernia studies are conducted in men or with mixed gender cohorts.
Dr. Aoun and her coinvestigators conducted a retrospective cohort study from January 2005 to July 2016, identifying 96 women with chronic pelvic pain and focal inguinal tenderness. Protruding fat or visceral tissue on physical exam or observed visually led clinicians to suspect a hernia. A single sonographer performed the musculoskeletal ultrasound.
Investigators diagnosed an occult hernia in more than half of the patients (51 women) based on the physical exam and ultrasound findings. Diagnoses included inguinal, femoral, Spigelian, and umbilical hernias.
All women with an ultrasound-diagnosed hernia were referred to general surgery. A majority – 69% of women – underwent surgical exploration. The remaining 31% of women who declined tended to have lower pain scores, Dr. Aoun said. Surgeons confirmed the hernia diagnosis in 97% of the women, or 34 out of the 35 women who had consented to surgery.
The group with a hernia was older and more likely to have arthritis, but otherwise did not differ significantly from the nonhernia cohort.
“We believe musculoskeletal ultrasound is valuable as an initial imaging modality due to its high predictive value, low cost, and noninvasiveness,” Dr. Aoun said.
Chronic pelvic pain is not uncommon, affecting approximately 15% of women during their reproductive years often with significant impacts on quality of life, workplace productivity, and health care utilization, Dr. Aoun said. This presentation also accounts for about 10% outpatient gynecology consultations and approximately 40% of laparoscopies in the United States, she added.
Dr. Aoun and her colleagues are planning a subsequent study of all the women who opted not to undergo surgery to determine their follow-up pain profiles based on chart review and phone interviews.
Dr. Aoun reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Key clinical point:
Major finding: About 53% of women with chronic pelvic pain were diagnosed with an occult hernia following physical exam and ultrasound. Surgeons confirmed the hernia diagnosis in 34 out of 35 patients who consented to surgery.
Data source: A retrospective cohort study of 96 women with chronic pelvic pain and focal inguinal tenderness.
Disclosures: Dr. Aoun reported having no relevant financial disclosures.